- 1College of Physical Education, Wuha City Polytechnnic, Wuhan, China
- 2College of Arts and Physical Education, Nanchang Normal College of Applied Technology, Nanchang, China
- 3Faculty of Health Service, Naval Medical University, Shanghai, China
- 4College of Physical Education, Yangzhou University, Yangzhou, China
- 5College of Physical Education, Jiangxi Normal University, Nanchang, China
Background: This study aimed to explore the association between daily stair climbing and the risk of hyperuricemia and to investigate the potential mediating role of blood lipid biomarker levels in this association.
Methods: This study is a prospective cohort study from the UK Biobank, including 14,635 participants. Participants were categorized based on their self-reported daily stair climbing habits and the presence of hyperuricemia, which was defined as serum uric acid concentration > 420 μmol/L for men and > 360 μmol/L for women. Blood lipid biomarker levels were assessed as potential mediators. We used a mediation analysis framework to estimate the direct and indirect effects of daily stair climbing on hyperuricemia risk. All analyses were conducted using R Studio version 4.2.3. Statistical significance was defined as a two-sided p-value of < 0.05.
Results: Overall, compared to the no stair climbing group, with full adjustment, we observed a significant negative correlation between participants who climbed 160 to 200 steps of stairs daily and hyperuricemia; the HRs were 0.70 (95% CI: 0.51–0.95, p = 0.024). Mediation analysis revealed a significant indirect effect of stair climbing (160–200 steps/day) on the risk of hyperuricemia, mediated through high-density lipoprotein cholesterol and triglyceride levels, with mediation proportions of 27.6 and 21.8%, respectively (p < 0.001).
Conclusion: Daily stair climbing is associated with a reduced risk of hyperuricemia, and this relationship may be partially mediated by alterations in high-density lipoprotein cholesterol and triglyceride levels. These findings suggest that promoting daily physical activity, such as stair climbing, may be an effective strategy for managing uric acid levels and reducing the risk of hyperuricemia.
Introduction
Hyperuricemia—characterized by elevated serum uric acid (SUA)—is an emerging metabolic risk factor for gout, cardiovascular diseases, and chronic kidney disease (1, 2). Its pathogenesis involves disrupted uric acid metabolism, modulated by diet habits (3), renal function (4), genetic predisposition (5, 6), and lifestyle factors such as physical activity (7–10). Among these, physical inactivity is a modifiable risk factor that has been suggested to contribute to the pathophysiology of hyperuricemia. Regular physical activity is known to benefit cardiovascular health, improve metabolic function, and enhance renal health, potentially mitigating the risk of developing hyperuricemia (11). Stair climbing is a common, low-cost, and easily accessible form of exercise that engages multiple muscle groups, improves cardiovascular function, and can be incorporated into daily routines without the need for specialized equipment (12–15). While its cardiovascular benefits are established (16–18), stair climbing’s role in SUA levels and its potential role in preventing or managing hyperuricemia remain to be fully understood. Thus, investigating the relationship between stair climbing and hyperuricemia could provide valuable insights into the role of simple, everyday physical activities in metabolic health.
Several studies have explored the broader relationship between physical activity and hyperuricemia. It was reported that higher levels of physical activity were inversely associated with serum uric acid concentrations (7), suggesting that more active individuals may have a lower risk of developing hyperuricemia. Stair climbing, being a more routine and less deliberate activity, may serve as a proxy for general physical activity in the daily lives of individuals and could provide a unique insight into how everyday movements influence metabolic outcomes. Moreover, a growing body of evidence suggests that physical activities, even those that occur in short bursts throughout the day, can have significant impacts on hyperuricemia or metabolic health (7, 19–21). Stair climbing, as an activity that can be performed in short intervals and incorporated seamlessly into daily routines, may be a particularly effective form of exercise for improving metabolic health outcomes in the general population.
This study aims to investigate the potential association between daily stair climbing and hyperuricemia risk in the UK Biobank cohort. We also aim to explore whether the potential benefits of stair climbing are more pronounced in certain subgroups of the population, such as those with unhealthy diets or sedentary lifestyles. By leveraging the extensive data from the UK Biobank, we provided a deeper understanding of how a simple, daily activity, such as stair climbing, can contribute to the prevention of hyperuricemia, potentially informing public health strategies aimed at reducing the burden of this condition.
Methods
Data source and study population
The UK Biobank is a population-based prospective cohort comprising over 500,000 participants aged 37–73 years recruited across the United Kingdom between 2006 and 2010. Detailed techniques for this research are available in a previous study (22). The study protocol was approved by the National Health Service (NHS) National Research Ethics Service (Ref: 11/NW/0382), with all participants providing written informed consent. Detailed information about the UK Biobank cohort is available on their website.1 Our study was conducted under UK Biobank application number 75732.
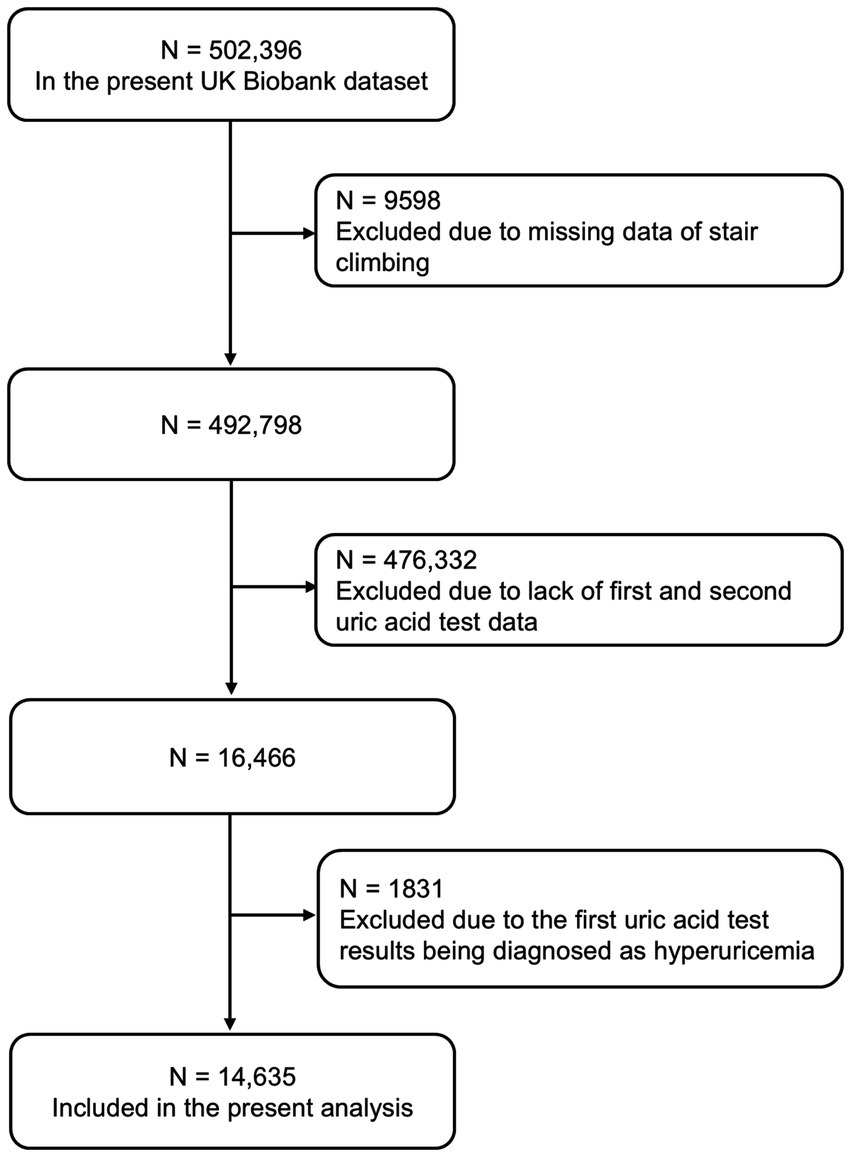
We excluded individuals with missing data on stair climbing (n = 9,598), with unavailable uric acid measurements in phase I and phase II (n = 476,332), and subjects with pre-existing hyperuricemia at baseline (n = 1831). Finally, we included 14,635 participants in the present study (Figure 1).
Assessment of hyperuricemia diseases
Hyperuricemia is a metabolic disease caused by an increase in uric acid levels in the blood, due to the metabolic disorder of a substance called purine in the human body. The reference value of serum uric acid concentration was > 420 for men and > 360 for women (23). The end of follow-up was defined as the earliest occurrence of hyperuricemia until 03 October 2017, whichever occurred first.
Assessment of stair climbing
Stair climbing exposure was assessed through self-reported frequency using the following standardized question: “At home, during the last 4 weeks, about how many times a DAY do you climb a flight of stairs?” (approximately 10 steps). The daily number of steps climbed (steps/day) was determined by multiplying the frequency of climbing one flight of stairs per day by 10 steps. Then, daily stair climbing was classified into six groups: none, 10–50, 60–100, 110–150, 160–200, and over 200 steps/day. Participants with missing stair climbing data were excluded from all analyses.
Other covariates
We considered sociodemographic factors (age, sex, ethnicity, Townsend deprivation index, education qualification, and employment), lifestyle factors (smoking status, alcohol drinker status, sleep duration, sedentary time, vegetable and fruit intake, and total physical activity), and health conditions [body mass index (BMI)] as potential confounders. The vegetable and fruit intake was converted into proportions according to the guidelines on the NHS website (24). The definition and category of covariates are presented in Table 1.
Statistical analysis
Baseline characteristics were stratified by daily stair climbing categories and presented as counts (percentages) for categorical variables or mean ± standard deviation (SD) for continuous variables. We used Cox proportional hazards regression models to predict the association between daily stair climbing and incident hyperuricemia. Hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs) were calculated, and the proportional hazards assumption was validated using Schoenfeld residuals. Person years were calculated from baseline until the end of follow-up. Three incremental models were constructed using the no stair climbing group as reference: Model 1 was adjusted for age and sex; Model 2 was adjusted for Model 1 plus sociodemographic factors (ethnicity, socioeconomic status, education, and employment status); and Model 3 was adjusted for Model 2 plus lifestyle factors (cigarette smoking, vegetable and fruit intake, alcohol consumption, body mass index, sedentary behavior, sleep duration, and total physical activity).
To quantify the indirect (mediated) effect of blood lipid biomarkers, we followed the guidelines by Preacher and Hayes (25) and utilized the mediation package in R to compute the indirect effect. The total effect, direct effect, and indirect effect were estimated as follows: total effect (TE): The total association between stair climbing (X) and hyperuricemia risk (Y), without considering the mediator; direct effect (DE): The effect of stair climbing (X) on hyperuricemia risk (Y), controlling for blood lipid biomarker levels; and indirect effect (IE): The portion of the effect of stair climbing (X) on hyperuricemia risk (Y) that is mediated through blood lipid biomarker levels (M). The mediation proportion was calculated as IE/TE × 100%. Sensitivity analyses by removing participants with missing values of covariates were conducted to test the robustness of our main findings. R Studio version 4.2.3 was used for analyses. Statistical significance was defined as a two-sided p-value of < 0.05.
Results
Population characteristic
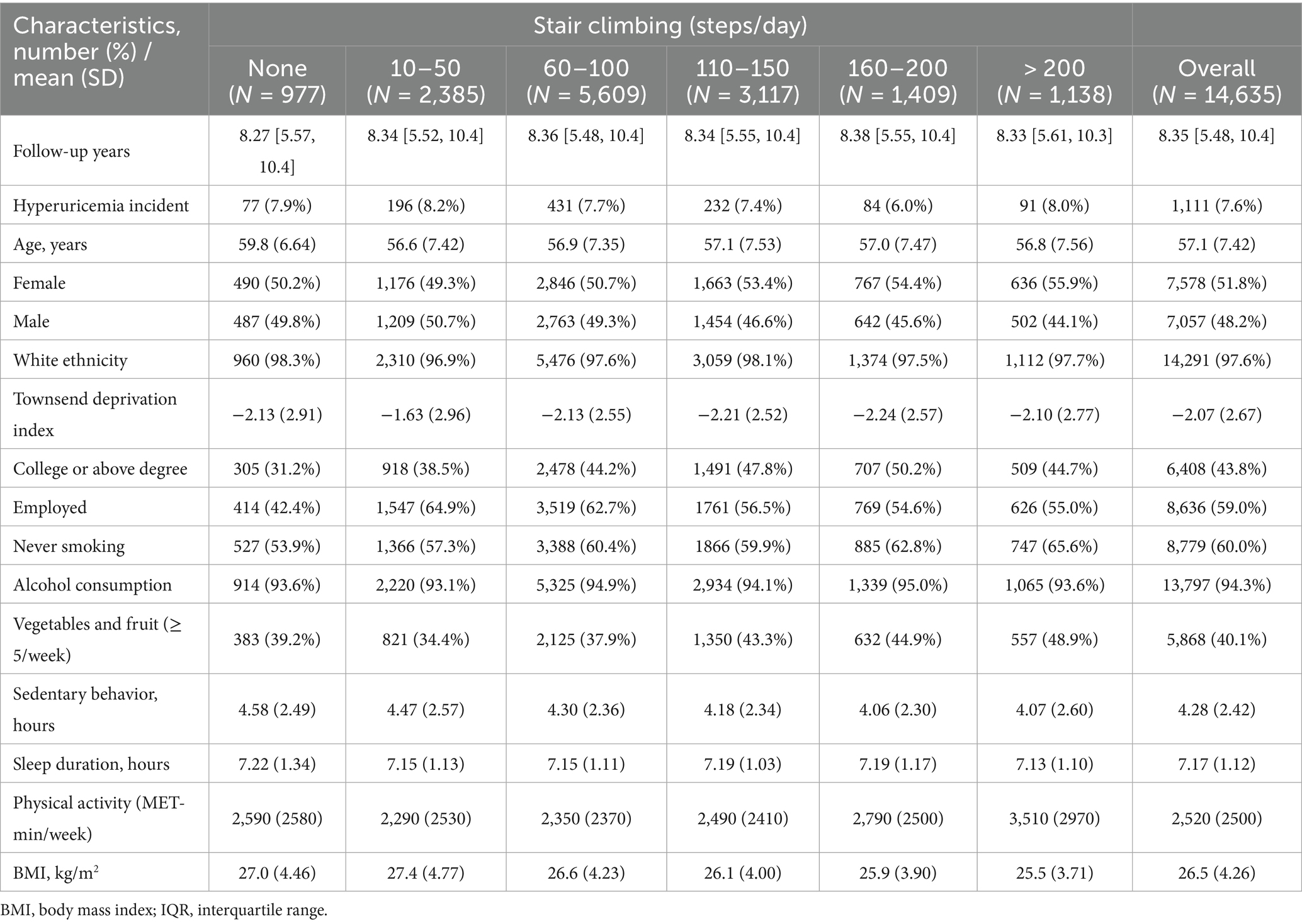
Table 1 presents the baseline characteristics of participants stratified by daily stair climbing levels. Our analysis included 14,635 participants (mean age 57.1 years; 51.8% female). During a mean follow-up period of 8.35 years, a total of 1,111 incident hyperuricemia cases were documented. Participants with college degrees or higher education were most prevalent in the 160–200 steps/day group. Currently employed individuals showed higher stair climbing frequency. Over 90% of participants reported alcohol consumption. Vegetable and fruit intake (≥ 5 servings/week) increased with daily stair climbing volume. Daily stair climbing was inversely associated with sedentary time, sleep duration, and BMI but positively associated with overall physical activity levels.
Associations between daily stair climbing and risk of hyperuricemia disease
The independent associations between daily stair climbing and incident hyperuricemia are presented in Table 2. After full adjustment for potential confounders, participants who climbed 160–200 steps/day demonstrated a significantly lower risk of hyperuricemia compared to non-climbers (HRs = 0.70, 95% CI: 0.51–0.95, p = 0.024).
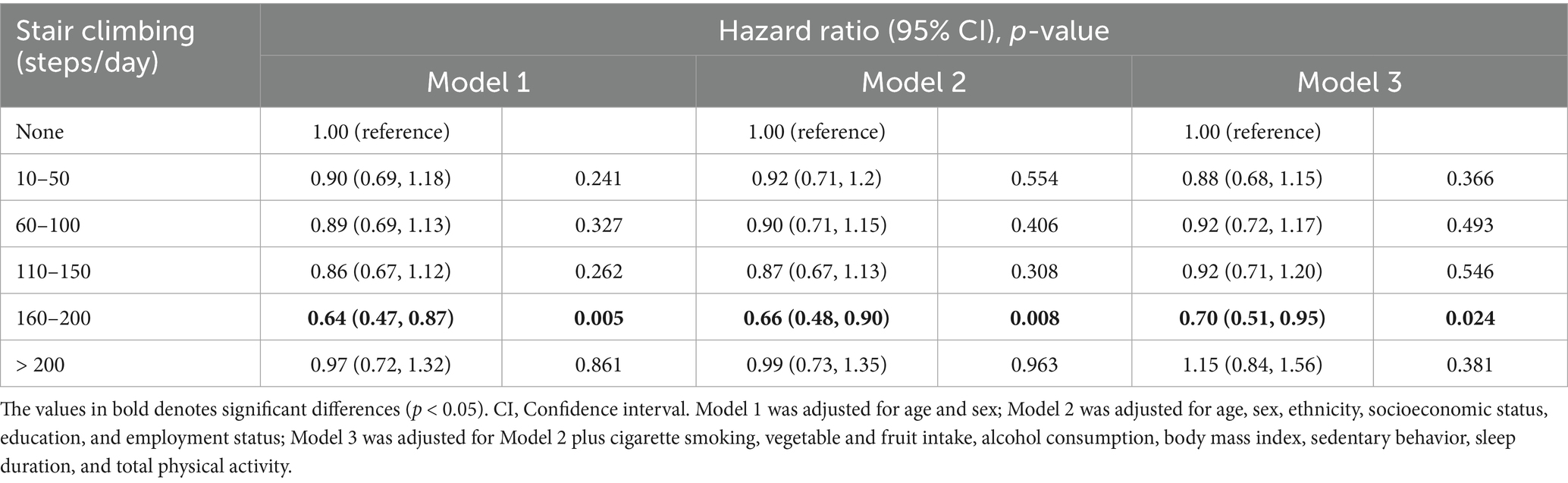
Table 2. Association between daily stair climbing and risk of hyperuricemia disease in 14,635 participants.
Secondary analysis
The results of the subgroup analyses are shown in Table 3. Significant inverse associations between daily stair climbing (160–200 steps/day) and hyperuricemia risk were observed only in participants aged 45–65 years (HRs = 0.67, 95% CI: 0.47–0.96, p = 0.028) and those with normal BMI (HRs = 0.42, 95% CI: 0.21–0.85, p = 0.015). Specifically, women demonstrated reduced hyperuricemia risk at 60–100 steps/day (HRs = 0.64, 95% CI: 0.43–0.94, p = 0.022), whereas men showed a benefit at 160–200 steps/day (HRs = 0.63, 95% CI: 0.41–0.97, p = 0.035). Notably, among all subgroup analyses, only BMI demonstrated a significant interaction effect on the inverse association between stair climbing and hyperuricemia risk (p for interaction = 0.034). However, these subgroup findings should be interpreted with caution due to the wide confidence intervals reflecting limited statistical power due to low hyperuricemia incidence (7.6%).
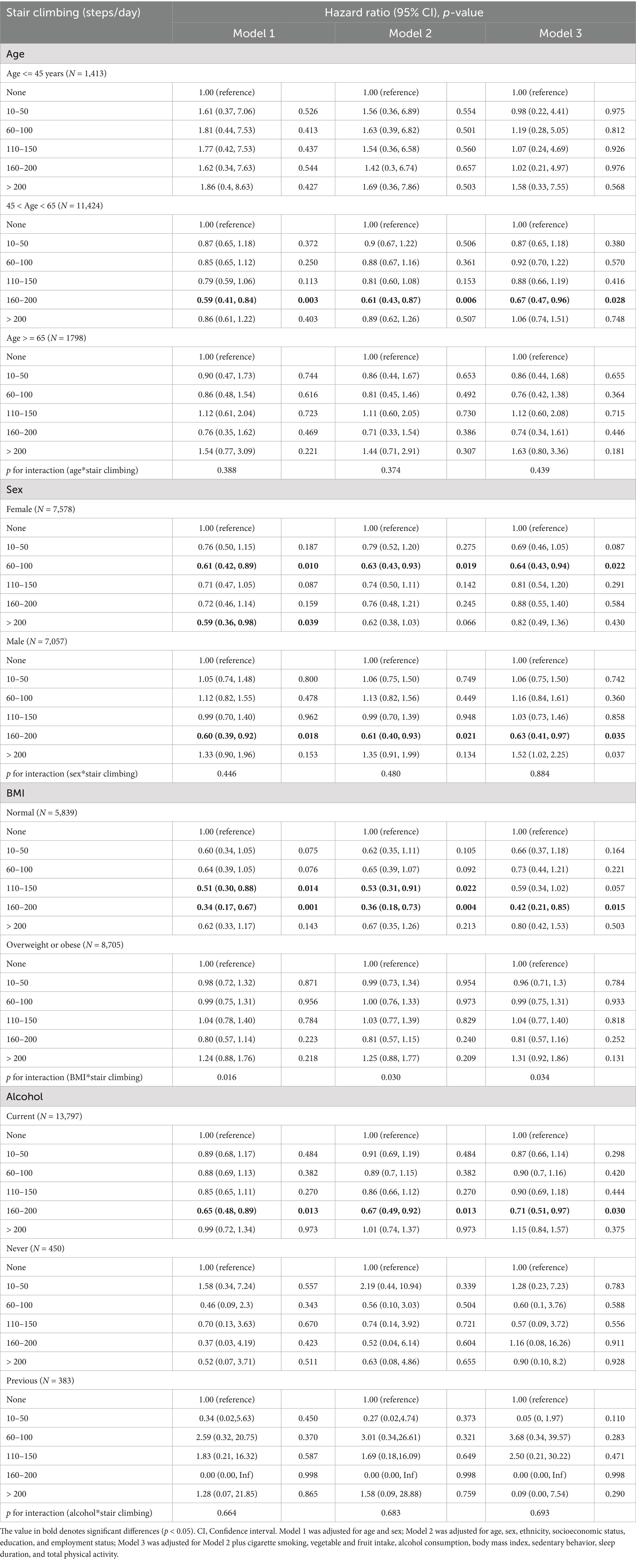
Table 3. Subgroup analyses of associations between daily stair climbing and risk of hyperuricemia disease.
Mediation analysis
To further explore the association between daily stair climbing and the risk of hyperuricemia, a mediation analysis was conducted to explore the mediating effect of blood lipid biomarkers. The mediating role of blood lipid biomarkers in the association between daily stair climbing and hyperuricemia risk is shown in Figure 2 and Table 4. The biomarkers of high-density lipoprotein cholesterol and triglyceride significantly mediated the association between daily stair climbing and hyperuricemia risk, with a proportion of mediation of 27.6 and 21.8%, respectively (p < 0.001).
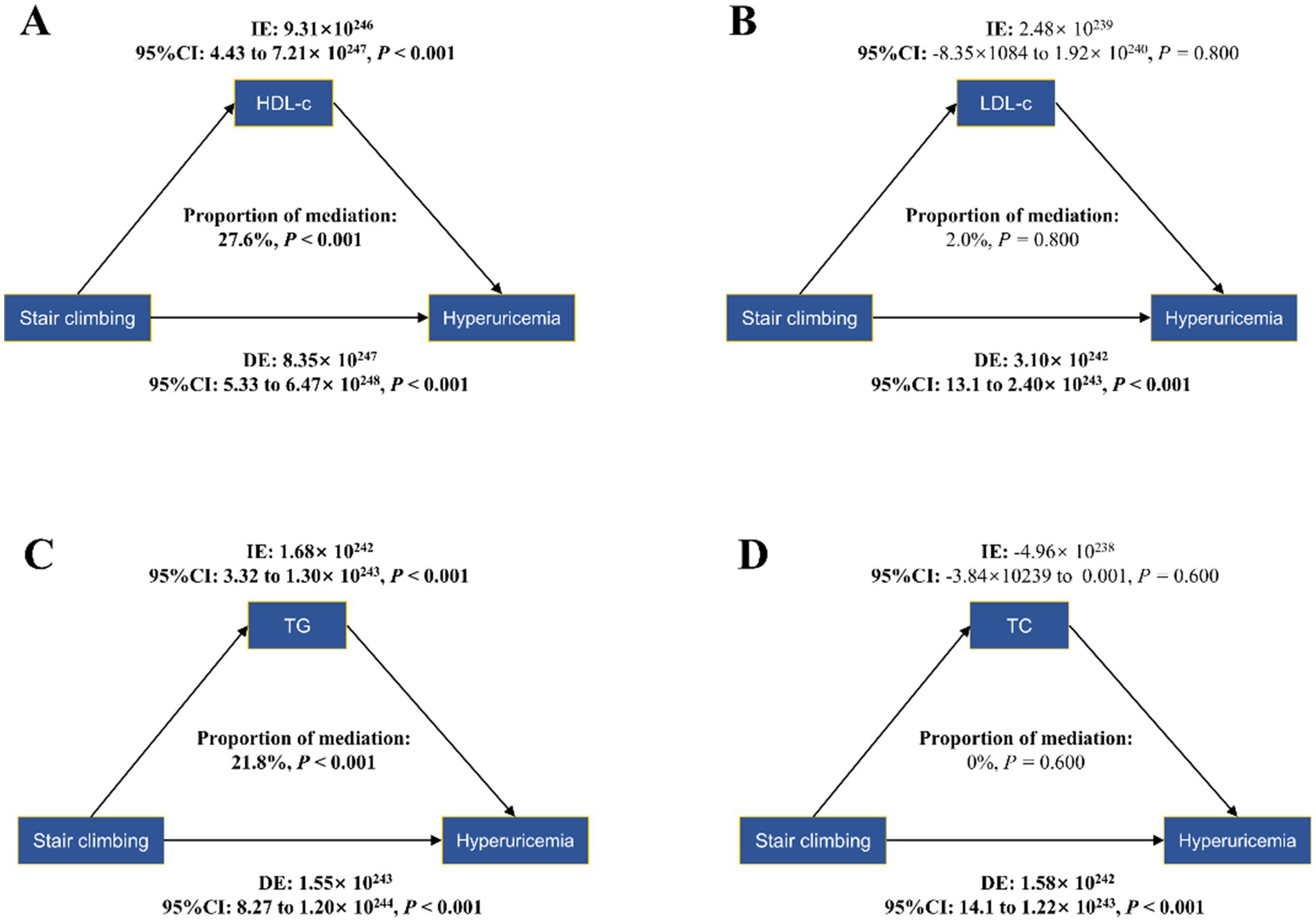
Figure 2. Mediation effects of lipid profiles in the stair-climbing-hyperuricemia association: high-density lipoprotein cholesterol (A), low-density lipoprotein cholesterol (B), triglyceride (C), and total cholesterol (D). The figure presents bootstrap mediation analyses examining potential mediating effects of serum lipids on the association between daily stair climbing (160–200 steps/day) and hyperuricemia incidence. Models were adjusted for age and sex, ethnicity, socioeconomic status, education, and employment status, cigarette smoking, vegetable and fruit intake, alcohol consumption, body mass index, sedentary behavior, sleep duration, and total physical activity. CI, confidence interval; indirect effect (IE), estimated effect through the mediator; direct effect (DE), the estimate of the direct effect; proportion of mediation = IE/DE + IE, ratio expressed as percentage.
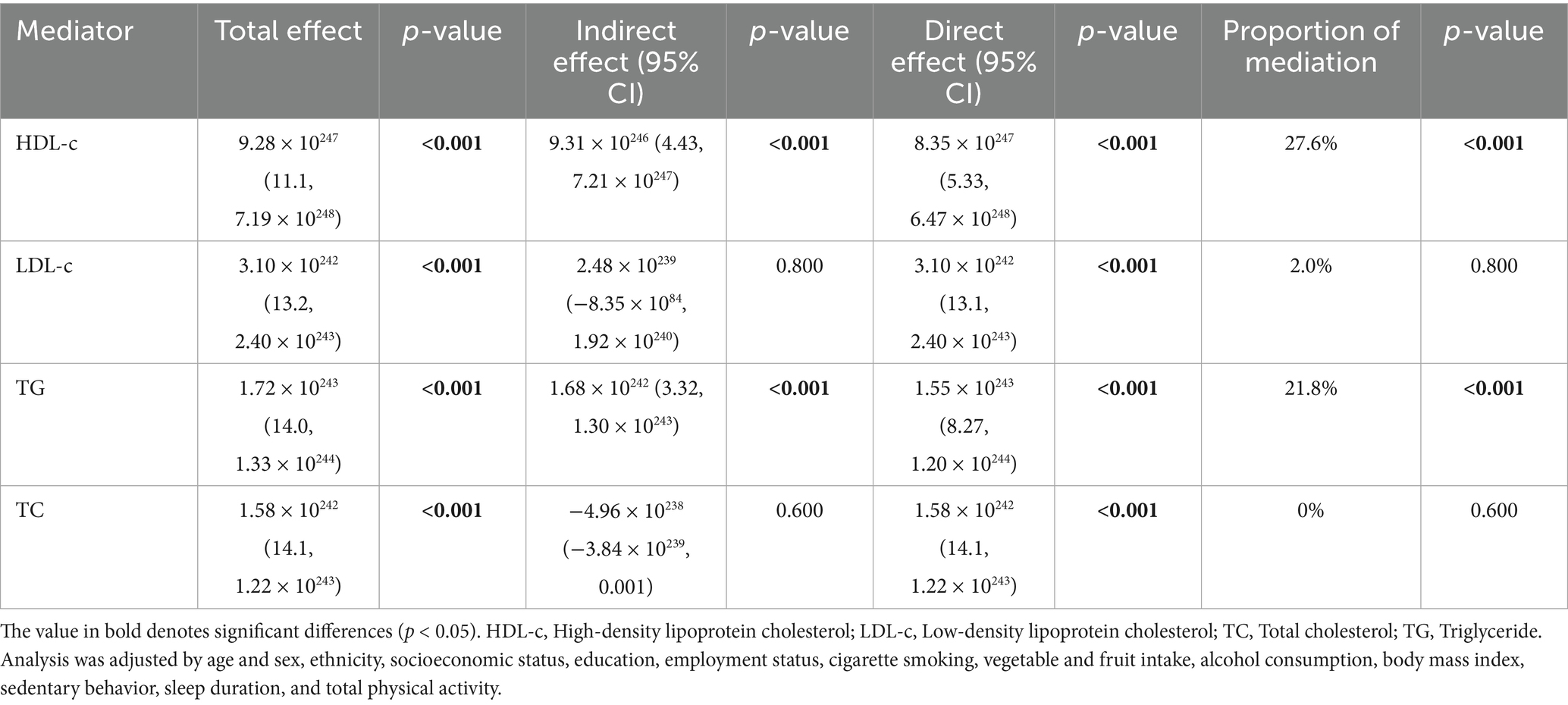
Table 4. Mediation analysis of lipid biomarkers between stair climbing (160–200 steps/day) and hyperuricemia.
Discussion
In this study, we observed a significant association between climbing 160–200 steps of stairs daily and a reduced risk of hyperuricemia. The relationship between stair climbing and decreased hyperuricemia risk was particularly pronounced in specific subgroups, including participants aged 45–65 years, male individuals, those with a normal BMI, and those with a history of alcohol consumption. Additionally, this effect was mediated by the levels of HDL-c and triglyceride, while the mediation effects of total cholesterol and LDL-c were not significant.
While various forms of exercise have been studied, our findings emphasize the potential benefits of stair climbing in a range of 160–200 steps per day. Hyperuricemia is closely associated with obesity, insulin resistance, and metabolic syndrome, all of which exacerbate uric acid accumulation in the bloodstream (26–28). Moreover, climbing stairs might enhance basal metabolic rate and insulin sensitivity (29), leading to increased caloric expenditure and a reduction in visceral fat, as well as protect against the metabolic syndrome (14). However, these effects remain hypothetical in our cohort as these parameters were not directly assessed. In addition, no significant association was observed in the high-exposure group (>200 steps/day), which may reflect that excessive stair climbing could induce joint stress in susceptible individuals, potentially counteracting its metabolic benefits—a phenomenon analogous to the uric acid fluctuations documented in marathon runners (30). Our results are consistent with previous studies that have suggested physical activity as a protective factor against hyperuricemia (7, 9–11, 31–33). Although the intensity and duration of exercise vary among studies, our findings demonstrated that climbing 160–200 steps of stairs per day can have a meaningful impact on lowering the risk of hyperuricemia. To translate our findings into practice, multi-level interventions are needed. For instance, placing eye-catching signage near elevators may nudge behavior change. Coupling this with mobile app tracking (e.g., recording stair counts via accelerometers) could reinforce adherence through self-monitoring.
For participants aged 45–65 years, the relationship between stair climbing and reduced hyperuricemia risk may be attributed to the fact that this age group is more likely to experience a decline in muscle mass and an increase in body fat, which can exacerbate the development of hyperuricemia (34). Stair climbing, as a weight-bearing and moderate-intensity exercise, may help mitigate these changes by improving muscle strength (35, 36), enhancing metabolic rate (37), and promoting better lipid metabolism (29, 38), thereby lowering serum uric acid levels. The stronger association found in male participants might be explained by sex differences in uric acid metabolism (39). Men typically have higher serum uric acid levels than women due to differences in renal clearance, hormonal influences, and body composition (40–44). Regular physical activity has been shown to improve renal function and uric acid excretion (45–48), which could be particularly beneficial for men in this age range, who are at a higher risk for hyperuricemia and gout. Among participants with a normal BMI, the beneficial effects of stair climbing on hyperuricemia risk could be related to the fact that these individuals are less likely to have obesity-related metabolic disturbances (49), which can contribute to elevated uric acid levels. Normal BMI individuals may experience more pronounced improvements in metabolic function and uric acid clearance from physical activity such as stair climbing, without the confounding effects of obesity. Alcohol, particularly beer and spirits, is known to increase uric acid production and decrease renal excretion (50). However, physical activity has been shown to improve the clearance of uric acid through the kidneys (51–54) and reduce the inflammatory response (55–57). Therefore, engaging in regular stair climbing may help attenuate the hyperuricemic effects of alcohol consumption by enhancing renal excretion and mitigating inflammation.
The relationship between stair climbing and hyperuricemia reduction is likely multifactorial, and our study offers further insight into the possible mechanisms involved. Although the mediation effects of HDL-c and triglyceride were modest, their involvement underscores the complex interplay between lipid metabolism and uric acid homeostasis. The role of HDL-c in this mediation process is consistent with its known protective effects on cardiovascular health (58–60) and metabolic function (61). HDL-c is believed to facilitate the removal of excess cholesterol from peripheral tissues, a mechanism that may also help in lowering uric acid levels and reducing the risk of hyperuricemia. Similarly, triglyceride levels, which are often elevated in metabolic dysfunction, may contribute to the pathogenesis of hyperuricemia (62–64). The relationship between physical activity and lipid metabolism is well-documented, with regular exercise known to improve lipid profiles (65–67). Our findings align with this body of evidence, suggesting that the beneficial effects of stair climbing on lipid metabolism could, in part, underlie the observed reduction in hyperuricemia risk.
In contrast, the lack of significant mediation by LDL-c and total cholesterol is intriguing. While LDL-c and total cholesterol are traditionally associated with cardiovascular risk (68, 69), our findings suggest that their role in the development of hyperuricemia may not be as direct or pronounced in the context of stair climbing. This finding may indicate that specific lipid fractions, particularly HDL-c and triglycerides, are more directly involved in influencing uric acid metabolism in response to physical activity. Our findings support the hypothesis that daily stair climbing can indirectly influence uric acid levels by improving lipid profiles. This reinforces the importance of incorporating simple, accessible forms of exercise into daily routines as part of a broader strategy for preventing and managing hyperuricemia.
Strengths and limitations
One of the strengths of our study is the focus on a specific physical activity, stair climbing, which offers a practical and accessible form of exercise for individuals at risk of hyperuricemia. It is particularly notable that only a specific range of stair climbing activity (160–200 steps per day) was associated with a significant reduction in risk. This finding suggests that, while any physical activity may have some benefits, there may be an optimal intensity or duration for effectively mitigating hyperuricemia risk.
However, several limitations must be considered. First, this is an observational study, and as such, it cannot establish a causal relationship. The mediation analysis we performed shows a potential link between lipid metabolism and the reduced risk of hyperuricemia, but it does not provide direct evidence of causality. Future research studies should consider randomized controlled trials to confirm the role of stair climbing and its impact on lipid profiles and uric acid levels. Additionally, while we controlled for several confounding variables, there may still be unmeasured factors influencing both stair climbing and hyperuricemia risk, such as dietary habits or genetic predispositions. Although we adjusted for general dietary patterns (fruit/vegetable intake), the lack of specific purine-rich food data represents a notable limitation. Future studies should incorporate detailed dietary assessments, such as food frequency questionnaires, specifically targeting purine sources. The generalizability of our findings may also be limited by the specific population studied. While the UK Biobank provides robust phenotypic and genetic data, its underrepresentation of non-white and younger populations may limit the generalizability of our results. Further research is needed to confirm these results in diverse demographic groups.
Conclusion
In conclusion, our study highlights the potential for daily stair climbing (160–200 steps/day) as an effective strategy for reducing hyperuricemia risk. This association appears partially mediated by improved HDL-c and triglyceride levels, providing valuable insights into the physiological mechanisms underpinning this association. Future experimental studies should validate these findings and clarify the underlying mechanisms.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Detailed information about the UK Biobank cohort is available on their website (www.ukbiobank.ac.uk). All participants have signed informed consent forms, and the application number of UKB in our study was 75,732.
Ethics statement
The studies involving humans were approved by the UK Biobank study was approved by the National Health Service (NHS) National Research Ethics Service (Ref: 11/NW/0382). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WY: Software, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Formal analysis. DL: Investigation, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. WH: Formal analysis, Writing – original draft, Investigation. HJ: Writing – original draft, Validation, Formal analysis. YW: Writing – original draft, Formal analysis, Visualization, Validation, Methodology, Software, Writing – review & editing, Investigation. HQ: Investigation, Formal analysis, Methodology, Data curation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The investigators are grateful to the dedicated participants and all research staff of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Dalbeth, N, Gosling, AL, Gaffo, A, and Abhishek, A. Gout. Lancet. (2021) 397:1843–55. doi: 10.1016/s0140-6736(21)00569-9
2. Du, L, Zong, Y, Li, H, Wang, Q, Xie, L, Yang, B, et al. Hyperuricemia and its related diseases: mechanisms and advances in therapy. Signal Transduct Target Ther. (2024) 9:212. doi: 10.1038/s41392-024-01916-y
3. Danve, A, Sehra, ST, and Neogi, T. Role of diet in hyperuricemia and gout. Best Pract Res Clin Rheumatol. (2021) 35:101723. doi: 10.1016/j.berh.2021.101723
4. Park, JH, Jo, YI, and Lee, JH. Renal effects of uric acid: hyperuricemia and hypouricemia. Korean J Intern Med. (2020) 35:1291–304. doi: 10.3904/kjim.2020.410
5. Major, TJ, Dalbeth, N, Stahl, EA, and Merriman, TR. An update on the genetics of hyperuricaemia and gout. Nat Rev Rheumatol. (2018) 14:341–53. doi: 10.1038/s41584-018-0004-x
6. Ragab, G, Elshahaly, M, and Bardin, T. Gout: An old disease in new perspective - a review. J Adv Res. (2017) 8:495–511. doi: 10.1016/j.jare.2017.04.008
7. Dong, X, Li, Y, Zhang, L, Liu, X, Tu, R, Wang, Y, et al. Independent and interactive effect of sitting time and physical activity on prevalence of hyperuricemia: the Henan rural cohort study. Arthritis Res Ther. (2021) 23:7. doi: 10.1186/s13075-020-02385-8
8. Elmagboul, N, Coburn, BW, Foster, J, Mudano, A, Melnick, J, Bergman, D, et al. Physical activity measured using wearable activity tracking devices associated with gout flares. Arthritis Res Ther. (2020) 22:181. doi: 10.1186/s13075-020-02272-2
9. Hong, R, Huang, J, Xu, C, Zhang, X, Mi, F, Xu, F, et al. Association of Sedentary Behavior and Physical Activity with Hyperuricemia and sex differences: results from the China multi-ethnic cohort study. J Rheumatol. (2022) 49:513–22. doi: 10.3899/jrheum.211180
10. Park, DY, Kim, YS, Ryu, SH, and Jin, YS. The association between sedentary behavior, physical activity and hyperuricemia. Vasc Health Risk Manag. (2019) 15:291–9. doi: 10.2147/vhrm.S200278
11. Zhang, T, Liu, W, and Gao, S. Exercise and hyperuricemia: an opinion article. Ann Med. (2024) 56:2396075. doi: 10.1080/07853890.2024.2396075
12. Hong, JY, Li, YJ, Metcalfe, RS, and Chen, YC. Effects of acute and chronic stair-climbing exercise on metabolic health: a systematic review. J Sports Sci. (2024) 42:498–510. doi: 10.1080/02640414.2024.2345414
13. Jenkins, EM, Nairn, LN, Skelly, LE, Little, JP, and Gibala, MJ. Do stair climbing exercise "snacks" improve cardiorespiratory fitness? Appl Physiol Nutr Metab. (2019) 44:681–4. doi: 10.1139/apnm-2018-0675
14. Whittaker, AC, Eves, FF, Carroll, D, Roseboom, TJ, Ginty, AT, Painter, RC, et al. Daily stair climbing is associated with decreased risk for the metabolic syndrome. BMC Public Health. (2021) 21:923. doi: 10.1186/s12889-021-10965-9
15. Koutnik, AP. Stair climbing exercise as a novel health intervention for menopause: cardiovascular and skeletal muscle implications. Menopause. (2018) 25:721–2. doi: 10.1097/gme.0000000000001107
16. Song, Z, Wan, L, Wang, W, Li, Y, Zhao, Y, Zhuang, Z, et al. Daily stair climbing, disease susceptibility, and risk of atherosclerotic cardiovascular disease: a prospective cohort study. Atherosclerosis. (2023) 386:117300. doi: 10.1016/j.atherosclerosis.2023.117300
17. Tomiyama, H. Routine stair climbing for vascular health. Hypertens Res. (2021) 44:1357–8. doi: 10.1038/s41440-021-00701-6
18. Arafa, A, Kokubo, Y, Shimamoto, K, Kashima, R, Watanabe, E, Sakai, Y, et al. Stair climbing and incident atrial fibrillation: a prospective cohort study. Environ Health Prev Med. (2022) 27:10. doi: 10.1265/ehpm.21-00021
19. Atakan, MM, Li, Y, Koşar Ş, N, Turnagöl, HH, and Yan, X. Evidence-based effects of high-intensity interval training on exercise capacity and health: a review with historical perspective. Int J Environ Res Public Health. (2021) 18:201. doi: 10.3390/ijerph18137201
20. Clarke, J, and Janssen, I. Sporadic and bouted physical activity and the metabolic syndrome in adults. Med Sci Sports Exerc. (2014) 46:76–83. doi: 10.1249/MSS.0b013e31829f83a0
21. Huang, CH, and Yen, M. Applying an exercise snack-based health promotion strategy. Hu Li Za Zhi. (2023) 70:78–83. doi: 10.6224/jn.202304_70(2).10
22. Sudlow, C, Gallacher, J, Allen, N, Beral, V, Burton, P, Danesh, J, et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
23. Li, Q, Li, X, Wang, J, Liu, H, Kwong, JS, Chen, H, et al. Diagnosis and treatment for hyperuricemia and gout: a systematic review of clinical practice guidelines and consensus statements. BMJ Open. (2019) 9:e026677. doi: 10.1136/bmjopen-2018-026677
24. Kim, JH, Lim, S, Choi, SH, Kim, KM, Yoon, JW, Kim, KW, et al. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J Gerontol A Biol Sci Med Sci. (2014) 69:1244–52. doi: 10.1093/gerona/glu050
25. Preacher, KJ, and Hayes, AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. (2008) 40:879–91. doi: 10.3758/brm.40.3.879
26. Borghi, C, Agabiti-Rosei, E, Johnson, RJ, Kielstein, JT, Lurbe, E, Mancia, G, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. (2020) 80:1–11. doi: 10.1016/j.ejim.2020.07.006
27. Yanai, H, Adachi, H, Hakoshima, M, and Katsuyama, H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int J Mol Sci. (2021) 22:221. doi: 10.3390/ijms22179221
28. Seifi, N, Nosrati, M, Koochackpoor, G, Aghasizadeh, M, Bahari, H, Namdar, HB, et al. The association between hyperuricemia and insulin resistance surrogates, dietary-and lifestyle insulin resistance indices in an Iranian population: MASHAD cohort study. Nutr J. (2024) 23:5. doi: 10.1186/s12937-023-00904-2
29. Ghosal, AM, and Chandrasekaran, B. Stair-climbing interventions on cardio-metabolic outcomes in adults: a scoping review. J Taibah Univ Med Sci. (2024) 19:136–50. doi: 10.1016/j.jtumed.2023.10.003
30. Kosaki, K, Kumamoto, S, Tokinoya, K, Yoshida, Y, Sugaya, T, Murase, T, et al. Xanthine oxidoreductase activity in marathon runners: potential implications for marathon-induced acute kidney injury. J Appl Physiol (1985). (2022) 133:1–10. doi: 10.1152/japplphysiol.00669.2021
31. Cai, N, Chen, M, Wu, L, Feng, P, Ye, X, Liu, Q, et al. Association between physical activity and the prevalence of gout among patients with type 2 diabetes mellitus and hyperuricemia: a two-center population-based cross-sectional study. Clin Rheumatol. (2024) 43:2955–61. doi: 10.1007/s10067-024-07081-5
32. Hong, R, Huang, J, Xu, C, Zhang, X, Mi, F, Xu, F, et al. Association of Sedentary Behavior and Physical Activity with Hyperuricemia and sex differences: results from the China multi-ethnic cohort study. J Rheumatol. (2022) 49:860. doi: 10.3899/jrheum.211180.C1
33. Zeng, X, Huang, J, Shen, T, Xu, Y, Yan, X, Li, Q, et al. Nonlinear dose-response association of moderate-to-vigorous physical activity with hyperuricemia in US adults: NHANES 2007-2018. PLoS One. (2024) 19:e0302410. doi: 10.1371/journal.pone.0302410
34. Oncel Yoruk, E, Dost, FS, Ontan, MS, Ates Bulut, E, Aydin, AE, and Isik, AT. Hyperuricemia may be associated with muscle wellness in older adults. Int Urol Nephrol. (2023) 55:2981–8. doi: 10.1007/s11255-023-03588-z
35. Lee, JH, Lee, GB, Chung, WY, Wang, JW, and Jang, KM. Stair-climbing training with interferential electrotherapy improves knee muscle strength, dynamic postural stability, pain score, and physical activity in patients with knee osteoarthritis. Diagnostics (Basel). (2024) 14:60. doi: 10.3390/diagnostics14182060
36. Van Roie, E, van Uffelen, J, and Delecluse, C. Stair-climbing versus machine-based resistance exercise to improve muscle power among older adults: a noninferiority trial. J Strength Cond Res. (2024) 39:e496–505. doi: 10.1519/jsc.0000000000005005
37. Bruce, FM, Floyd, WF, and Ward, JS. Oxygen consumption and heart rate during stair climbing. J Physiol. (1967) 191:90p–2p.
38. Rafiei, H, Omidian, K, Myette-Côté, É, and Little, JP. Metabolic effect of breaking up prolonged sitting with stair climbing exercise snacks. Med Sci Sports Exerc. (2021) 53:150–8. doi: 10.1249/mss.0000000000002431
39. Puig, JG, Mateos, FA, Ramos, TH, Capitán, CF, Michán, AA, and Mantilla, JM. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2). Adv Exp Med Biol. (1986) 195:317–23. doi: 10.1007/978-1-4684-5104-7_54
40. DeBoer, MD, Dong, L, and Gurka, MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and nutrition survey 1999-2006. Metabolism. (2012) 61:554–61. doi: 10.1016/j.metabol.2011.09.003
41. Lee, EH, Kim, JY, and Yang, HR. Sex-specific differences in ectopic fat and metabolic characteristics of paediatric nonalcoholic fatty liver disease. Int J Obes. (2024) 48:486–94. doi: 10.1038/s41366-023-01439-6
42. Li, Q, Lin, F, Gao, Z, Huang, F, and Zhu, P. Sex-specific association between serum uric acid and retinal microvessels. Med Sci Monit. (2019) 25:9973–80. doi: 10.12659/msm.919972
43. Seo, YJ, Shim, YS, Lee, HS, and Hwang, JS. Association of serum uric acid levels with metabolic syndromes in Korean adolescents. Front Endocrinol (Lausanne). (2023) 14:1159248. doi: 10.3389/fendo.2023.1159248
44. Zhang, Y, Cai, M, Dilimulati, D, Lin, Z, Sun, H, Cui, R, et al. Correlation between serum uric acid and body fat distribution in patients with polycystic ovary syndrome. Front Endocrinol (Lausanne). (2021) 12:782808. doi: 10.3389/fendo.2021.782808
45. Kosaki, K, Tanahashi, K, Matsui, M, Akazawa, N, Osuka, Y, Tanaka, K, et al. Sedentary behaviour, physical activity, and renal function in older adults: isotemporal substitution modelling. BMC Nephrol. (2020) 21:211. doi: 10.1186/s12882-020-01869-8
46. Masiero, L, Puoti, F, Bellis, L, Lombardini, L, Totti, V, Angelini, ML, et al. Physical activity and renal function in the Italian kidney transplant population. Ren Fail. (2020) 42:1192–204. doi: 10.1080/0886022x.2020.1847723
47. Villanego, F, Naranjo, J, Vigara, LA, Cazorla, JM, Montero, ME, García, T, et al. Impact of physical exercise in patients with chronic kidney disease: Sistematic review and meta-analysis. Nefrologia (Engl Ed). (2020) 40:237–52. doi: 10.1016/j.nefro.2020.01.002
48. Wilund, KR, Thompson, S, Viana, JL, and Wang, AY. Physical activity and health in chronic kidney disease. Contrib Nephrol. (2021) 199:43–55. doi: 10.1159/000517696
49. Szukiewicz, D. Molecular mechanisms for the vicious cycle between insulin resistance and the inflammatory response in obesity. Int J Mol Sci. (2023) 24:818. doi: 10.3390/ijms24129818
51. Irving, RA, Noakes, TD, Burger, SC, Myburgh, KH, Querido, D, and van Zyl Smit, R. Plasma volume and renal function during and after ultramarathon running. Med Sci Sports Exerc. (1990) 22:581–7. doi: 10.1249/00005768-199010000-00007
52. Ka, T, Yamamoto, T, Moriwaki, Y, Kaya, M, Tsujita, J, Takahashi, S, et al. Effect of exercise and beer on the plasma concentration and urinary excretion of purine bases. J Rheumatol. (2003) 30:1036–42.
53. Takanishi, T, Kimura, N, Ito, T, Morotomi, Y, and Itani, T. The effects of fluid ingestion and its composition on uric acid metabolism during high intensity long term exercise. Nihon Eiseigaku Zasshi. (1998) 53:463–9. doi: 10.1265/jjh.53.463
54. Wołyniec, W, Ratkowski, W, Kasprowicz, K, Małgorzewicz, S, Aleksandrowicz, E, Witek, K, et al. Changes in electrolytes and uric acid excretion during and after a 100 km run. J Biol Regul Homeost Agents. (2018) 32:1205–10.
55. El Assar, M, Álvarez-Bustos, A, Sosa, P, Angulo, J, and Rodríguez-Mañas, L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. (2022) 23:713. doi: 10.3390/ijms23158713
56. Klasson, CL, Sadhir, S, and Pontzer, H. Daily physical activity is negatively associated with thyroid hormone levels, inflammation, and immune system markers among men and women in the NHANES dataset. PLoS One. (2022) 17:e0270221. doi: 10.1371/journal.pone.0270221
57. Metsios, GS, Moe, RH, and Kitas, GD. Exercise and inflammation. Best Pract Res Clin Rheumatol. (2020) 34:101504. doi: 10.1016/j.berh.2020.101504
58. Kunutsor, SK, and Laukkanen, JA. Further proof of a paradoxical relationship between high-density lipoprotein levels and adverse cardiovascular outcomes: are there implications for cardiovascular disease prevention? Eur J Prev Cardiol. (2023) 30:290–2. doi: 10.1093/eurjpc/zwac272
59. Ryan, A, Heath, S, and Cook, P. Dyslipidaemia and cardiovascular risk. BMJ. (2018) 360:k835. doi: 10.1136/bmj.k835
60. Teis, A, Cediel, G, Amigó, N, Julve, J, Aranyó, J, Andrés-Cordón, J, et al. Particle size and cholesterol content of circulating HDL correlate with cardiovascular death in chronic heart failure. Sci Rep. (2021) 11:3141. doi: 10.1038/s41598-021-82861-6
61. Zhang, Q, Ke, Y, and Hong, H. HDL and lipid metabolism. Adv Exp Med Biol. (2022) 1377:49–61. doi: 10.1007/978-981-19-1592-5_4
62. Gou, R, Dou, D, Tian, M, Chang, X, Zhao, Y, Meng, X, et al. Association between triglyceride glucose index and hyperuricemia: a new evidence from China and the United States. Front Endocrinol (Lausanne). (2024) 15:1403858. doi: 10.3389/fendo.2024.1403858
63. Li, Q, Shao, X, Zhou, S, Cui, Z, Liu, H, Wang, T, et al. Triglyceride-glucose index is significantly associated with the risk of hyperuricemia in patients with diabetic kidney disease. Sci Rep. (2022) 12:19988. doi: 10.1038/s41598-022-23478-1
64. Qiu, L, Ren, Y, Li, J, Li, M, Li, W, Qin, L, et al. Nonlinear association of triglyceride-glucose index with hyperuricemia in US adults: a cross-sectional study. Lipids Health Dis. (2024) 23:145. doi: 10.1186/s12944-024-02146-5
65. Muscella, A, Stefàno, E, Lunetti, P, Capobianco, L, and Marsigliante, S. The regulation of fat metabolism during aerobic exercise. Biomolecules. (2020) 10:699. doi: 10.3390/biom10121699
66. Muscella, A, Stefàno, E, and Marsigliante, S. The effects of exercise training on lipid metabolism and coronary heart disease. Am J Physiol Heart Circ Physiol. (2020) 319:H76–h88. doi: 10.1152/ajpheart.00708.2019
67. Ranallo, RF, and Rhodes, EC. Lipid metabolism during exercise. Sports Med. (1998) 26:29–42. doi: 10.2165/00007256-199826010-00003
68. Chen, L, Chen, S, Bai, X, Su, M, He, L, Li, G, et al. Low-density lipoprotein cholesterol, cardiovascular disease risk, and mortality in China. JAMA Netw Open. (2024) 7:e2422558. doi: 10.1001/jamanetworkopen.2024.22558
Keywords: stair climbing, uric acid, blood lipid biomarkers, prospective cohort study, the UK biobank
Citation: Yin W, Luo D, Huang W, Jiang H, Wang Y and Qi H (2025) Lifestyle factors and hyperuricemia risk: a prospective cohort study of 14,635 participants examining the protective role of daily stair climbing. Front. Nutr. 12:1635746. doi: 10.3389/fnut.2025.1635746
Edited by:
Mansoor-Ali Vaali-Mohammed, King Saud University, Saudi ArabiaReviewed by:
Fang Liu, Wuhan University, ChinaDina Khodeer, Suez Canal University, Egypt
Adhila Nazar, Kerala University of Health Sciences, India
Copyright © 2025 Yin, Luo, Huang, Jiang, Wang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yahai Wang, MTE1ODk5MDIwMEBxcS5jb20=; Haixia Qi, cWloYWl4aWFxc21AMTYzLmNvbQ==
†These authors have contributed equally to this work
 Wenkui Yin1,2†
Wenkui Yin1,2† Yahai Wang
Yahai Wang