- 1School of Physical Education, Guizhou Normal University, Guiyang, China
- 2Key Laboratory of Brain Function and Brain Disease Prevention and Treatment of Guizhou Province, Guiyang, China
The intricate interactions between gut microbiota and cognitive function have become a forefront topic at the convergence of neuroscience and nutrition. This review systematically evaluates the bidirectional relationship between dietary phytochemicals and gut microbiota, highlighting their potential mechanisms for promoting cognitive health. The review begins by describing how gut microbiota dysbiosis can contribute to cognitive decline by transmitting gut-derived signals to the central nervous system via the gut–brain axis. Subsequently, the discussion focuses on how phytochemicals act as modulators of gut microbiota composition and undergo microbial-mediated metabolic transformation. Special attention is paid to four key microbial-derived metabolites—urolithins, sulforaphane, equol, and hesperidin—that exhibit neuroprotective effects through antioxidative, anti-inflammatory, neuroprotective, and metabolic regulatory pathways. Furthermore, the review examines how individual variability in gut microbiota composition influences the efficiency of phytochemical biotransformation and underscores the implications for precision nutrition interventions. Emerging evidence indicates that the synergistic regulation of the gut–brain axis by dietary phytochemicals and gut microbiota offers a robust theoretical basis for developing novel strategies to preserve cognitive function. Future research should further clarify the molecular mechanisms underlying specific microbe–phytochemical interactions and accelerate the clinical translation of personalized nutrition strategies.
1 Introduction: the complex interplay between diet, gut microbiota, and cognition
Progressive cognitive decline is a hierarchical pathological process characterized by gradual alterations at the molecular, cellular, and systemic levels (Figure 1). Ultimately, these changes manifest as impaired bodily function and reduced overall health status (1–3). Clinically, affected individuals frequently present with memory impairment, deficits in attention and executive function, diminished language and information processing abilities, as well as a spectrum of neuropsychiatric symptoms. Collectively, these deficits undermine independence in activities of daily living (4–6).
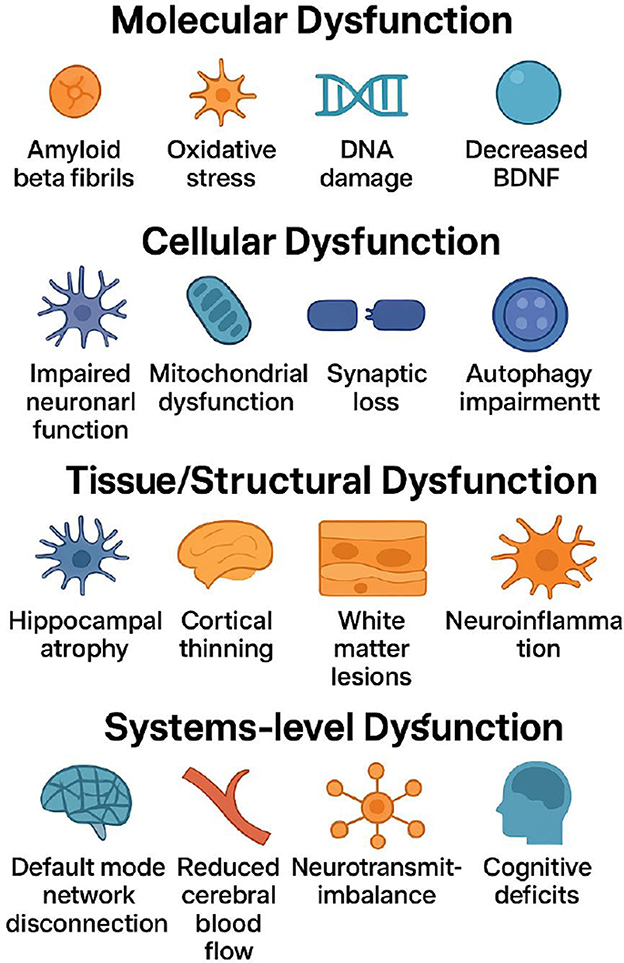
Figure 1. Multilevel pathological mechanisms of progressive cognitive decline: from molecular to cellular, tissue, and systems levels (The process initiates at the molecular level with amyloid-β fibril formation, oxidative stress, DNA damage, and reduced BDNF levels that trigger cellular injury. These molecular disruptions progress to cellular dysfunction, characterized by impaired neuronal function, mitochondrial failure, synaptic loss, and autophagy impairment that compromise fundamental neural processes. Continued damage leads to tissue/structural dysfunction, including hippocampal atrophy, cortical thinning, white-matter lesions, and neuroinflammation with blood-brain barrier disruption, reflecting macroscopic brain pathology. The cascade culminates in system-level dysfunction, where large-scale network disconnection (e.g., default mode network), reduced cerebral blood flow, neurotransmitter imbalances, and manifest cognitive deficits represent clinical endpoints.).
In the past decade, extensive research has identified the gut microbiota as a central regulator of brain aging and cognitive function (7). Disruptions in the composition, diversity, or functionality of the microbiota—referred to as “dysbiosis”—have been strongly linked to adverse cognitive outcomes (8, 9). Beyond taxonomic alterations, dysbiosis also reshapes the spectrum of microbiota-derived metabolites. These metabolites influence brain health by modulating neuroinflammation, synaptic plasticity, and mitochondrial function (10–12). Notably, older adults who retain a “youthful” microbial profile tend to show better-preserved cognitive function and produce a greater diversity of beneficial metabolites (11, 13), highlighting the substantial potential for targeting the gut ecosystem in cognitive health interventions.
Diet is the most influential and modifiable external factor in shaping the composition and function of the gut microbiota. Systematic reviews have shown that dietary patterns rich in whole grains, fruits, vegetables, and other fiber-dense plant-based foods—including, but not limited to, the Mediterranean and DASH diets—while minimizing their intake of refined sugars and saturated fats, are associated with a significantly lower risk of developing neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease (14, 15). A common characteristic of these dietary patterns is their abundance of phytochemicals—diverse, non-nutritive secondary metabolites derived from plants. Phytochemicals exert their effects both directly on the host and indirectly via microbiota-mediated biotransformation, producing bioactive metabolites that act along the gut–brain axis (16).
Polyphenols are a representative example: the gut microbiota can degrade their complex structures into smaller, highly bioavailable derivatives, thereby enhancing the integrity of the intestinal barrier and mitigating neuroinflammation and oxidative stress (17). Similarly, phytochemicals such as curcumin and resveratrol modulate the gut microbial ecosystem by increasing beneficial taxa and suppressing opportunistic pathogens, together creating a neuroprotective environment that supports brain homeostasis (18, 19).
This review aims to comprehensively synthesize the bidirectional communication between dietary phytochemicals and the gut microbiota, elucidating the molecular mechanisms by which these interactions support cognitive health. Specifically, we address the following key questions:
How does the gut microbiota regulate cognitive function through the gut–brain axis?
In what ways do phytochemicals reprogram the composition and metabolic activity of the microbiota?
Through which pathways do microbiota-derived phytochemical metabolites exert neuroprotective effects?
Addressing these questions will not only deepen our understanding of the biological foundations of cognitive decline but also provide a theoretical framework for the development of precision nutrition and microbiota-targeted strategies to prevent or delay cognitive impairment.
2 Gut microbiota and cognitive function
2.1 The role of gut microbiota in health and cognition
As the largest and most intricate microbial ecosystem in the human body, the gut microbiota is composed of approximately 3.8 × 1013 microorganisms. Its composition and function are shaped by a multitude of factors—including host genetics, dietary patterns, lifestyle, and disease status—resulting in pronounced inter-individual heterogeneity and dynamic shifts across the human lifespan (20, 21). Through the neuroendocrine, immune, and metabolic axes of the gut–brain axis, the gut microbiota establishes a multi-layered, bidirectional communication network with the central nervous system. This network exerts profound effects on brain development, information processing, and the aging process (22, 23).
2.1.1 Metabolic signaling: SCFAs and the blood–brain barrier
From a metabolic perspective, short-chain fatty acids (SCFAs)—including acetate, propionate, and butyrate—serve as key signaling molecules bridging the gut and the brain. Butyrate-producing bacteria, notably from the phylum Firmicutes, ferment dietary fiber to generate these metabolites (24). SCFAs contribute to central nervous system health by activating G protein-coupled receptors (GPR41/43), inhibiting histone deacetylase (HDAC) activity, and upregulating tight junction proteins. These processes reinforce blood–brain barrier (BBB) integrity, regulate microglial maturation, and suppress lipopolysaccharide (LPS)-mediated neuroinflammation, ultimately supporting hippocampus-dependent memory and spatial navigation (25, 26). Importantly, lower plasma SCFA levels have been strongly associated with reduced cognitive scores in patients with Alzheimer's disease (AD), supporting their potential as both biomarkers and intervention targets (27, 28).
2.1.2 Neurotransmitter modulation: serotonin and GABA
In addition to metabolic signaling, the gut microbiota plays a pivotal role in neurotransmitter synthesis and regulation. Approximately 95% of serotonin (5-hydroxytryptamine, 5-HT) is synthesized in intestinal enterochromaffin cells, a process that is dependent on aromatic amino acid decarboxylase signals secreted by certain Bacillus species (29). Gut-derived 5-HT influences vagal nerve activity, platelet serotonin release, and energy metabolism, thereby indirectly impacting mood and cognition. Its precursor, 5-hydroxytryptophan (5-HTP), can cross the BBB and be converted to 5-HT in the brain, directly promoting synaptic plasticity (30, 31).
Moreover, the gut microbiota sustains cerebral chemical homeostasis by regulating the supply of neurotransmitter precursors. Certain strains of Lactobacillus are capable of producing γ-aminobutyric acid (GABA), while Bifidobacterium species enhance glutamate decarboxylase activity to accelerate GABA synthesis. Additionally, shifts in microbial metabolism of indole derivatives and disturbances in the tryptophan–kynurenine pathway have been strongly associated with cognitive impairments resembling depressive phenotypes (32, 33).
2.1.3 Bile acid signaling and the gut–brain axis
Bile acid metabolism represents another critical route within the gut–brain axis. Gut microbes facilitate the transformation of primary bile acids into secondary bile acids via 7α-dehydroxylation. These secondary metabolites interact with the farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5), modulating neuroinflammation, mitochondrial function, and BDNF–CREB signaling, thereby contributing to memory consolidation and emotional stability (34, 35). Recent animal studies further demonstrate that interactions among gut microbiota, bile acids, and TGR5 receptors significantly influence host metabolism and intestinal barrier function, suggesting novel avenues for therapeutic intervention (36).
2.1.4 Clinical and translational evidence
Collectively, the gut microbiota shapes central nervous system function and cognitive performance through multiple, interconnected pathways—including SCFA–BBB integrity, 5-HT–neuroplasticity, GABA–synaptic inhibition, and secondary bile acid–receptor signaling.
However, there are inconsistencies across clinical and animal studies regarding specific microbial taxa–function relationships. For example, some studies have observed reduced abundance of beneficial genera such as Lactobacillus and Bifidobacterium and increased levels of potential pathogens in patients with AD and mild cognitive impairment (MCI) (37, 38). Conversely, a recent meta-analysis of ten randomized controlled trials (n = 419) found no significant improvement in total Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA) scores after 8–24 weeks of probiotic supplementation (39). These discrepancies may be attributable to heterogeneity in disease stage, the probiotic strains or combinations used, dosage, and duration of follow-up—highlighting the need for personalized approaches to probiotic interventions.
Before a robust causal chain linking specific microbiota changes, metabolic signaling, and cognitive outcomes can be established, more rigorous and standardized multi-omics studies are essential to improve evidence quality and enhance clinical translatability.
2.2 Interactions among dysbiosis, cognitive decline, and inflammation
Dysbiosis refers to a disrupted state of gut microbial composition and function and is closely linked to both cognitive decline and chronic inflammation (40, 41). The underlying mechanisms involve several key pathways.
2.2.1 Intestinal barrier disruption and systemic inflammation
Under homeostatic conditions, short-chain fatty acids (SCFAs) induce the secretion of MUC2 and maintain the expression of tight junction proteins such as ZO-1 and Claudin-1, thereby preserving intestinal barrier integrity (42). When microbial abundance and metabolic profiles are altered, mucin-producing bacteria (e.g., mucinase-positive Bacteroides) decrease, while pro-inflammatory Gram-negative bacteria increase, resulting in greater barrier permeability. Consequently, exogenous lipopolysaccharides (LPS) enter the circulation, activating the TLR4–MyD88–NF-κB signaling cascade and inducing the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α (43). These cytokines can cross the blood–brain barrier (BBB) and activate microglia, leading to dendritic spine retraction, reduced long-term potentiation (LTP), and ultimately, impairments in learning and memory (44). Recent in vivo studies have further confirmed that gut-derived LPS from the Enterobacteriaceae family significantly upregulates hippocampal TNF-α mRNA in mice within 48 h and prolongs escape latency in the Morris water maze test (45).
2.2.2 Neurotransmitter metabolic disruption—The serotonin pathway
Dysbiosis reduces the production of metabolites such as butyrate, thereby inhibiting intestinal tryptophan hydroxylase 1 (TPH1) activity and impeding peripheral serotonin (5-HT) synthesis (46). Simultaneously, a pro-inflammatory environment diverts tryptophan metabolism toward the kynurenine pathway, resulting in the accumulation of neurotoxic metabolites such as quinolinic acid and 3-hydroxykynurenine (47). This dual effect not only depletes serotonin precursors but also suppresses the synthesis of GABA and acetylcholine, weakening synaptic plasticity and emotional regulation (48, 49). In human studies, a lower plasma 5-HTP/kynurenine ratio is significantly correlated with reduced MoCA scores, underscoring the mediating role of the metabolic axis in cognitive decline (50).
2.2.3 Mitochondrial dysfunction and oxidative stress
Microbial-derived metabolites, such as indole-3-propionic acid, activate the PGC-1α-NRF1 pathway and promote neuronal mitochondrial biogenesis. Under dysbiotic conditions, these protective signals are diminished, resulting in reduced ATP production and increased reactive oxygen species (ROS) accumulation—creating a vicious cycle of mitochondrial injury and oxidative stress (51, 52). Recent multi-omics studies have shown that patients with AD exhibit reduced fecal levels of butyrate-producing bacteria such as Butyrivibrio, which correlates closely with lower plasma mitochondrial DNA copy number and poorer verbal fluency (53). In neonatal hypoxia-ischemia models, antibiotic-induced dysbiosis significantly amplifies hippocampal ROS levels and worsens long-term cognitive deficits (54).
Although animal models and some open-label clinical studies consistently suggest that modulation of gut microbiota through probiotics, prebiotics, or fecal microbiota transplantation (FMT) can reduce inflammatory burden and confer cognitive benefits (55), high-quality randomized controlled trials (RCTs) have yet to yield consistent conclusions. A recent systematic review and meta-analysis including 10 RCTs (n = 778) reported an overall standardized mean difference (SMD) of ≈0.52 for probiotics on global cognition, albeit with substantial heterogeneity (I2 = 68%) (56). Conversely, another meta-analysis focusing on populations with mild cognitive impairment and Alzheimer's disease (n = 419) observed no significant improvement in MMSE or MoCA scores, indicating that intervention duration, strain composition, and disease stage may be decisive factors for efficacy (39). Regarding interventions, the first multicenter, double-blind phase II FMT trial (GUT-PARFECT) significantly improved motor symptoms in Parkinson's disease patients but failed to yield statistically significant benefits on cognitive scales (57).
Overall, current research remains limited by sample sizes generally < 100, follow-up periods of ≤ 12 weeks, a lack of uniform cognitive assessment tools and time points, and insufficient integration of metagenomic, metabolomic, and neuroimaging data. These methodological shortcomings hinder the construction of a clear “microbe–metabolite–brain phenotype” causal chain (58). Such limitations help explain discrepancies in clinical evidence and highlight the urgent need for multi-omics tracking and long-term randomized trial designs in future research.
3 Key mechanisms linking dietary phytochemicals and cognitive function
3.1 Classification, sources, and bioactivities of phytochemicals
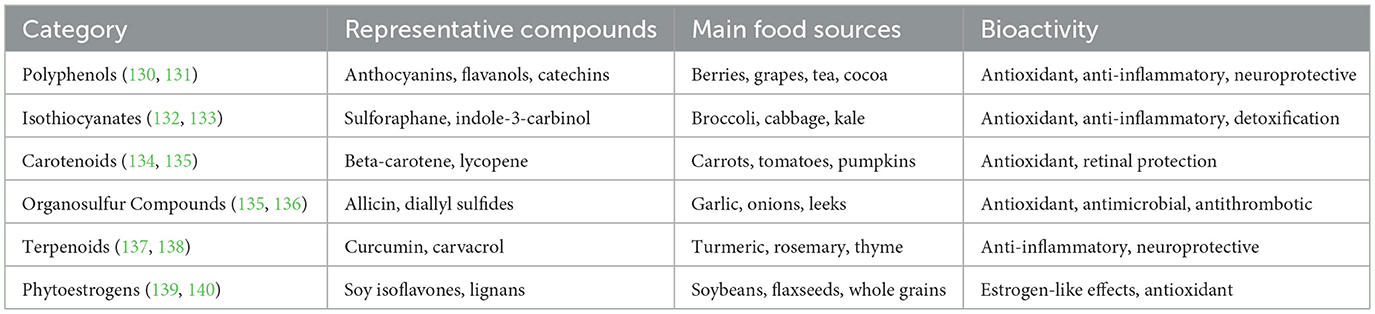
Phytochemicals are non-essential, bioactive trace compounds widely found in plant-based foods. To date, over 100,000 types have been identified, with the major groups including polyphenols, carotenoids, isothiocyanates, organosulfur compounds, terpenoids, and phytoestrogens (59). Although these compounds are not essential nutrients for humans, they have attracted significant attention due to their antioxidant, anti-inflammatory, and chronic disease prevention properties. Their fate in the human body is determined by factors such as molecular size, lipophilicity, blood–brain barrier (BBB) permeability, chemical stability, and, crucially, gut microbial metabolism (60, 61). Many parent molecules, due to their polarity or steric hindrance, have difficulty crossing the BBB. However, after transformation by microbial-specific enzymes into sulfate or glucuronide conjugates, these metabolites can be detected in brain tissue and often exhibit enhanced neuroactivity (62, 63). The classification, typical dietary sources, and representative bioactivities of major dietary phytochemicals are summarized in Table 1.
Current mechanistic studies mainly focus on: (1) scavenging free radicals and activating the Nrf2–ARE antioxidant pathway (64), (2) inhibiting the NF-κB–MAPK inflammatory cascade (65), (3) modulating neurotransmitter and neurotrophic factor levels (66), and (4) epigenetic reprogramming via histone acetylation and DNA methylation (66). However, systematic comparisons of the pharmacokinetics, dose–response relationships, and BBB permeability among different classes of phytochemicals remain limited (60, 67, 68). This gap has partially constrained the extrapolation of results and their clinical translation.
3.2 The phytochemical–gut microbiota–brain axis: a prebiotic perspective and current evidence
Early research primarily attributed the neuroprotective effects of phytochemicals to their direct antioxidant and anti-inflammatory actions. However, pharmacokinetic data demonstrate that most parent phytochemical molecules exist in plasma and cerebrospinal fluid at extremely low free concentrations, largely in conjugated forms. This indicates that the true “active agents” mediating neuroprotection are likely microbiota-derived metabolites (63). From a prebiotic perspective—defined here as the ability of dietary components to selectively promote the growth and metabolic activity of beneficial gut microbes—phytochemicals serve dual roles: they act as “metabolic substrates” for the microbiota and simultaneously “reshape the microbial ecosystem” by selectively inhibiting or promoting specific taxa (16, 69) (see Figure 2).
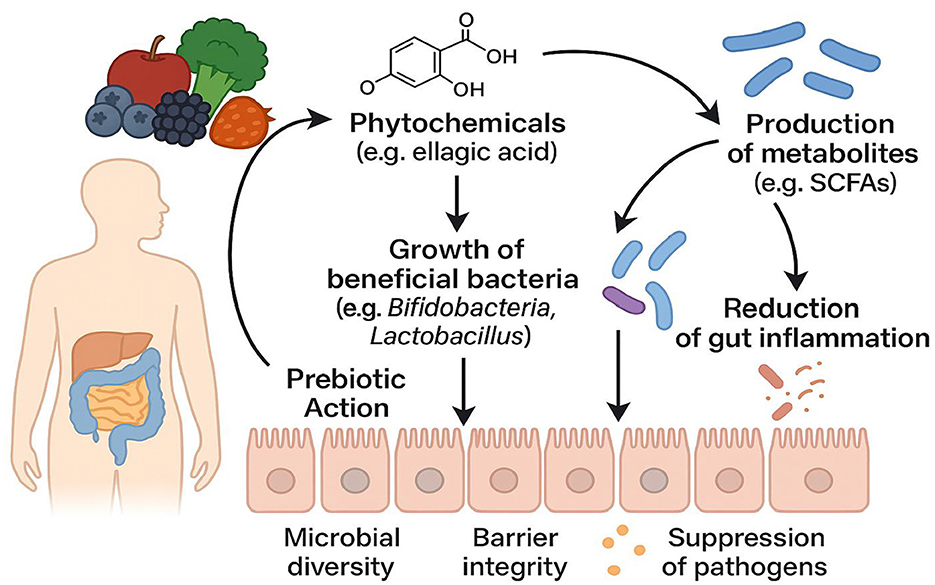
Figure 2. Schematic Illustration of Prebiotic Mechanisms in Phytochemical–Gut Microbiota Interactions (Dietary phytochemicals, such as ellagic acid and related compounds, exert prebiotic effects upon reaching the colon through three principal pathways: (1) to selectively promote the proliferation of beneficial bacteria—such as Bifidobacterium and Lactobacillus—thus enhancing microbial diversity, (2) to enhance the metabolism of beneficial microbes and stimulate the production of functional metabolites (e.g., short-chain fatty acids, SCFAs), which in turn suppress potential pathogens and reduce intestinal inflammation, and (3) to comprehensively strengthen intestinal barrier integrity.).
For example, interventions with berry anthocyanins have been shown in both animal and human studies to increase the abundance of butyrate-producing bacteria and elevate short-chain fatty acid (SCFA) levels, with these changes correlating with improved spatial memory (70). Importantly, phytochemicals may exhibit a “double-edged sword” effect—meaning their benefits and risks depend on dose and duration. For instance, oral curcumin has been shown to ameliorate memory deficits in APPswe/PS1dE9 mice by inhibiting the HMGB1–RAGE/TLR4–NF-κB pathway, yet high doses or prolonged use can reduce hepatic and renal iron content and adversely affect reproductive function (71–73).
Recent evidence from a meta-analysis of 24 trials (N = 2,336) indicates that long-term polyphenol supplementation in adults aged ≥60 produces only mild improvements in immediate recall, with no significant effects on delayed recall or executive function and considerable between-study heterogeneity (I2 > 60%) (74). Similarly, systematic reviews of curcumin interventions report that only two double-blind RCTs have observed meaningful cognitive benefits in populations with cognitive impairment or dementia; most studies in healthy or metabolically abnormal individuals have shown limited advantage (75). Furthermore, a 12-week matcha intervention in Japanese older adults improved emotion recognition and sleep quality but did not yield statistically significant changes in MMSE or MoCA scores (76).
The mechanistic targets of different phytochemicals vary considerably.
Polyphenols primarily enhance synaptic plasticity and antioxidative defenses (77); isothiocyanates are more associated with detoxification and anti-inflammatory pathways (78); and terpenoids (e.g., curcumin) exhibit both anti-inflammatory and epigenetic regulatory effects (79).
Nonetheless, several key challenges remain: the majority of RCTs have relatively short follow-up periods ( ≤ 16 weeks), highly variable intervention doses, delivery matrices (e.g., food vehicles or supplement forms), and marked differences in baseline microbiota composition. This limits the ability to detect cumulative cognitive effects. There is a lack of integrated multi-omics approaches; only a few studies concurrently assess metagenomic and metabolomic changes, making it difficult to establish a robust causal “phytochemical–microbiota metabolism–central phenotype” chain. Variability in individual microbiome backgrounds further complicates efficacy assessment and clinical translation.
Collectively, current evidence suggests that the cognitive benefits of phytochemicals are largely dependent on their biotransformation into low-molecular-weight metabolites by the gut microbiota and the consequent remodeling of microbial community structure. However, the effectiveness of these interventions is limited by factors such as dose, intervention duration, and inter-individual microbiome variability. There is a clear need for long-term, stratified, multi-omics randomized trials to elucidate optimal compound–microbiota matching strategies for cognitive health and advance the clinical translation of precision diet–microbiota interventions, thereby capturing the full complexity of the diet–microbiota–brain axis.
3.3 Molecular mechanisms of microbiota-derived metabolic pathways
3.3.1 Short-chain fatty acid (SCFA) pathway
Butyrate enhances long-term potentiation (LTP) and synaptic plasticity by activating GPR41/43 receptors and inhibiting histone deacetylase (HDAC) activity. These actions result in the downregulation of inflammatory mediators such as IL-1β and TNF-α and the upregulation of hippocampal BDNF expression, ultimately supporting cognitive function and reducing neuroinflammation (9, 80). Notably, in D-galactose-induced accelerated aging models, intervention with citrus flavonoids and hawthorn polysaccharides significantly increases fecal butyrate levels and reverses spatial memory deficits. This highlights a mechanistic chain: polyphenols or polysaccharides, through butyrate-producing bacteria, increase butyrate production, which in turn upregulates BDNF (81). However, most human intervention studies have small sample sizes, short durations, and often lack adequate control for dietary fiber intake in the control group. These limitations may overestimate the true contribution of SCFAs to cognitive improvement (82, 83).
3.3.2 Bile acid signaling pathway
Metabolomic studies reveal that patients with Alzheimer's disease (AD) show a shift characterized by reduced primary bile acids (PBAs) and increased neurotoxic secondary bile acids (SBAs). The severity of this imbalance is negatively correlated with MMSE scores, indicating cognitive decline (84). Animal experiments demonstrate that curcumin can upregulate PBAs and suppress SBAs via the FXR–SHP axis, thereby improving spatial learning and memory (85, 86). Despite these promising findings, clinical RCTs targeting bile acid modulation remain very limited. Furthermore, the strain-specific and dose–response relationships of Ruminococcus and Eubacterium in bile acid transformation have not yet been validated in human populations, limiting the translation to personalized interventions (87, 88). Future research should utilize integrated metagenomic and humanized mouse model approaches to clarify the causal relationship of the microbiota–bile acid–FXR/TGR5–BDNF signaling axis in cognitive health.
3.3.3 Tryptophan–kynurenine metabolic pathway
The gut microbiota determines whether tryptophan is metabolized toward serotonin (5-HT) synthesis or the kynurenine (KYN) branch. Human studies indicate that higher levels of KYN and 3-hydroxykynurenine are negatively correlated with cognitive performance, whereas indole-3-propionate (IPA), which is enriched in centenarians, may exert neuroprotective effects (89). In DSS-induced colitis models, dysbiosis is associated with upregulation of IDO-1/TDO-2 and inhibition of KAT2, resulting in KYN accumulation within the blood–brain barrier and subsequent cognitive impairment (90, 91). These findings underscore that inflammation-driven IDO-1 activation is a key driver of KYN elevation and that the modulation of the microbiota composition–tryptophan metabolic profile may alleviate both gut inflammation and central cognitive disorders, thus representing a promising therapeutic avenue.
Overall, while current evidence suggests that phytochemicals can improve cognition by upstream remodeling of the gut microbiota and its metabolic products, human trials remain limited by small sample sizes, short follow-up periods, and insufficient mechanistic measurements (e.g., metabolite quantification and receptor expression profiling). There is an urgent need for long-term, integrated, and stratified randomized studies to confirm the mediating effects of these pathways across different ages and disease stages and to identify the optimal combinations of compounds, bacterial strains, and dosages for individualized cognitive interventions.
4 Microbial-derived metabolites from dietary phytochemicals and their cognitive benefits
Microbial metabolites derived from dietary phytochemicals play a pivotal role in maintaining cognitive health. The gut microbiota possesses a vast and flexible metabolic network capable of enzymatically transforming a wide range of phytochemicals into unique bioactive metabolites. These microbial-derived metabolites typically exhibit greater bioavailability and distinct biological activities, acting through multiple pathways to influence both the gut and central nervous systems.
As illustrated in Figure 3, the gut microbiota can metabolize polyphenols, sulfur-containing compounds, nitrogen-containing compounds, and carotenoids via processes such as deglycosylation, dehydroxylation, ring cleavage, and decarboxylation, thereby generating small-molecule metabolites (e.g., SCFAs, urolithins, and IPA) with higher bioavailability. These products reinforce BBB integrity, inhibit neuroinflammation, and increase BDNF expression, ultimately supporting cognitive function through the gut–brain axis.
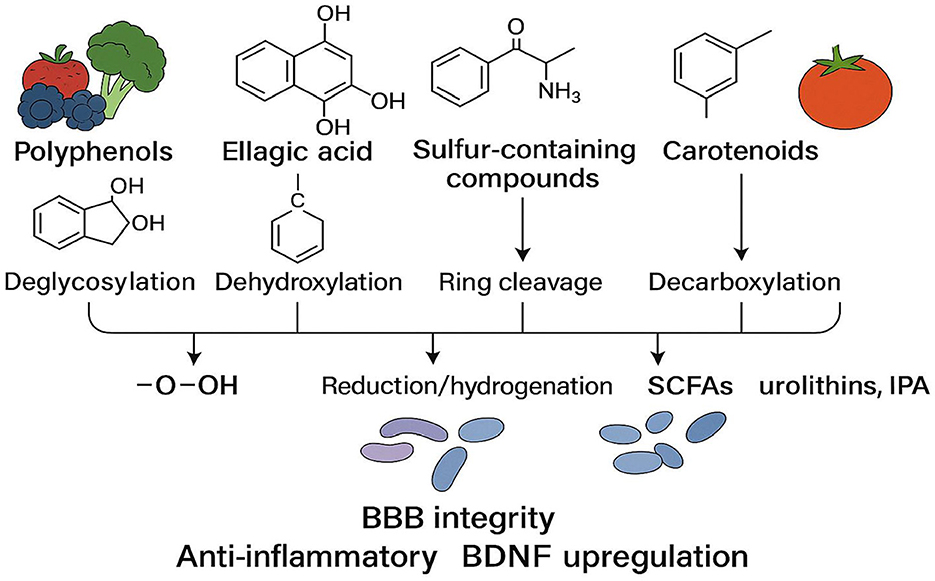
Figure 3. Microbial biotransformation pathways of dietary phytochemicals [Upon catalysis by specific microbial enzyme systems, dietary phytochemicals—ranging from polyphenols, sulfur-containing compounds, nitrogen-containing compounds, to carotenoids (from left to right)—undergo reactions such as deglycosylation, dehydroxylation, ring cleavage, and decarboxylation, resulting in small-molecule metabolites with higher bioavailability. Representative products include short-chain fatty acids (SCFAs), urolithins, and indole-3-propionate (IPA). These metabolites collectively promote synaptic plasticity and neuroprotection by enhancing blood–brain barrier (BBB) integrity, suppressing neuroinflammation, and upregulating brain-derived neurotrophic factor (BDNF).].
This section focuses on four categories of microbial-derived metabolites from phytochemicals that are especially relevant to cognitive function: urolithins, sulforaphane, equol and hesperetin. We discuss their dietary sources, precursor phytochemicals, the bacterial taxa involved in their production, and their mechanisms of action in supporting cognitive health.
4.1 Urolithins: key products of the ellagitannin–ellagic acid pathway
Pomegranate, strawberries, and walnuts are notable dietary sources of ellagitannins. These compounds are hydrolyzed in the upper gastrointestinal tract to release ellagic acid (EA). In the colon, ellagic acid undergoes sequential dehydroxylation, ring opening, and decarboxylation, catalyzed by gut bacteria such as Gordonibacter and Ellagibacter. This microbial transformation leads to the production of urolithin A (Uro-A) and urolithin B (Uro-B) (92, 93). The presence or absence of the ellagic acid decarboxylase gene cluster defines three metabolic phenotypes: UM-0 (non-producers), UM-A (Uro-A predominant), and UM-B (Uro-B and iso-Uro-A predominant), reflecting inter-individual variability in microbial metabolism (94).
Animal studies have shown that oral Uro-A (300 mg·kg−1 for 14 days) significantly improves spatial memory, reduces neuronal apoptosis, and promotes neurogenesis in APP/PS1 mice (95). Pharmacokinetic data indicate that Uro-A is rapidly absorbed, with peak brain concentrations achieved 4 h after a single dose, and is present mainly as glucuronide, sulfate, and methylated derivatives. Brain tissue concentrations (28–35 ng·g−1) are higher than plasma levels, suggesting selective enrichment (96). Uro-A modulates key anti-aging and autophagy pathways (AMPK, SIRT, mTOR), although its direct molecular targets require further elucidation (97). Urolithin C (Uro-C) has also been shown to reduce Aβ1–42 deposition and maintain cholinergic balance in aging models (98).
While preclinical evidence robustly supports the neuroprotective and anti-inflammatory roles of urolithins, human data remain limited and inconsistent. Uro-A is generally recognized as safe (GRAS) by the FDA and exhibits moderate BBB permeability and multi-target mitochondrial benefits (99, 100). However, the absence of large, dose-escalation, and long-term RCTs, particularly those stratified by UM phenotype, is a major gap in clinical translation. Overall, urolithins are promising agents for cognitive protection, but future studies should focus on population stratification and rigorous long-term evaluation.
4.2 Sulforaphane: a neuroregulator in the glucosinolate–isothiocyanate pathway
Cruciferous vegetables such as broccoli and kale are rich in glucoraphanin, which is hydrolyzed by plant myrosinase or microbial β-thioglucosidase to produce sulforaphane (SFN). SFN covalently binds cysteine residues in Keap1, leading to Nrf2 nuclear translocation and activation of ARE-dependent antioxidant and detoxification genes. In addition, SFN reversibly inhibits HDAC through thiol alkylation, increasing histone acetylation and upregulating neurotrophic factors such as BDNF (101, 102).
Twelve-week dietary supplementation with SFN in animal models enhances hippocampal PGC-1α, NRF-1, and TFAM transcription, promotes mitochondrial biogenesis, and mitigates age-related cognitive decline (103). SFN also increases BDNF expression and synaptic plasticity through HDAC inhibition in AD mouse and neuron models (102). Early clinical trials are encouraging: a 12-week, double-blind RCT of 30 mg/day SFN improved spatial orientation and working memory in traumatic brain injury patients (104), while similar interventions in older adults improved overall cognitive performance and selectively benefited processing speed and working memory (105, 106).
However, current studies are limited by small sample sizes, lack of dose-gradient design, and short follow-up durations. Long-term safety and the minimum effective dose remain unclear, emphasizing the need for multi-center RCTs with pharmacokinetic monitoring. In summary, SFN demonstrates strong neuroprotective mechanisms across preclinical and early clinical studies, but definitive large-scale evidence is still needed.
4.3 Equol: the soy isoflavone–gut microbial estrogen pathway
Daidzein, a major soy isoflavone, is metabolized by gut bacteria such as Slackia isoflavoniconvertens into equol. Only 30–50% of Asian adults are equol producers, underlining the critical influence of the gut microbiota phenotype on efficacy (107, 108). Equol supplementation activates the ERβ-PI3K–Akt signaling pathway and improves spatial memory in animal models (109).
Epidemiological evidence from large Japanese cohorts supports a link between soy isoflavone intake and reduced cognitive impairment risk (110, 111), though most studies do not directly assess equol producer status. Cross-sectional studies show that S-equol producers exhibit better cognitive scores and lower MCI prevalence (112), but findings in other populations (e.g., Singaporean, Chinese, and Asian-American) are inconsistent, likely due to lower isoflavone intake and lack of phenotype assessment (113, 114).
Meta-analyses of RCTs suggest that soy isoflavones provide small improvements in global cognition and memory, but with considerable limitations in sample size and follow-up (115, 116). Mechanistically, equol combines estrogenic, antioxidant, and anti-inflammatory properties, affecting vascular function, metabolic homeostasis, and neuroinflammation—all relevant to the prevention of vascular cognitive impairment (VCID). Future research should stratify participants by equol producer status, use long-term, dose-gradient RCTs, and comprehensively monitor estrogen-related adverse effects. Overall, equol represents a microbiota-dependent, multi-modal neuroprotective agent with particular potential in precision interventions.
4.4 Hesperetin/naringenin: citrus flavonoid monomethoxylation products
Citrus flavonoids such as hesperidin and naringin are converted by microbial O-methyltransferase–positive bacteria (e.g., Clostridium orbiscindens) into hesperetin and naringenin. These conversions require microbial cleavage of glycosidic bonds and demethylation (117). Alzheimer's disease (AD) features progressive memory decline, Aβ plaque deposition, tau hyperphosphorylation (via GSK-3β activation), and impaired insulin signaling (118–121). In this pathological context, naringenin has shown anti-inflammatory, antioxidant, anti-apoptotic, and neuroprotective effects (122).
In AD models, naringenin improves spatial learning and memory, modulates the PI3K/AKT/GSK-3β pathway, reduces tau phosphorylation, restores insulin signaling and PPAR-γ activity, and confers both metabolic and neuroprotective benefits (123, 124). In vitro, naringenin protects against Aβ-induced apoptosis in neuronal cells by regulating caspase-3, PI3K/AKT, and GSK-3β (125). Animal studies show that oral naringenin reduces hippocampal lipid peroxidation, neuronal apoptosis, and reverses memory loss; its neuroprotection is partly estrogen receptor–dependent (126). Naringenin also inhibits AChE and BACE1, restoring memory, and targets multiple AD-related enzyme pathways (127–129).
Collectively, current evidence supports naringenin's multi-target protective actions against AD-related cognitive impairment via the modulation of amyloid/tau pathology, PI3K/AKT/GSK-3β and insulin pathways, cholinergic neurotransmission, the CRMP2 axis, and enzyme inhibition, as well as through antioxidative and anti-inflammatory mechanisms. Its estrogenic and metabolic regulatory properties further reinforce its candidacy as a multi-target nutraceutical-pharmaceutical for future clinical evaluation.
5 Conclusion and future perspectives
This review systematically analyzed the complex interplay between dietary phytochemicals and the gut microbiota and how this interaction impacts cognitive health. Based on a comprehensive synthesis of recent research evidence, several key conclusions can be drawn.
Dietary patterns and gut microbial community composition jointly represent major determinants of cognitive function. Through multiple mechanisms, they improve cognitive performance and effectively prevent or delay the onset and progression of age-related neurodegenerative diseases. Phytochemicals in the daily diet play a central role in maintaining gut ecological balance, modulating systemic inflammatory responses, and supporting cognitive health. These bioactive compounds exhibit multidimensional physiological regulatory functions: they synergistically suppress excessive inflammation, promote the development of a diverse and functionally balanced gut microbiota, and establish a robust metabolic signaling network. Collectively, these features constitute the biological foundation for maintaining both gut and cognitive health.
Importantly, there is marked inter-individual variability in the efficiency of microbial metabolite production, largely determined by the unique characteristics of each person's gut microbiota. Evidence indicates that different microbial metabolic phenotypes (e.g., urolithin metabotypes) exert distinct effects on cognitive function, providing an important theoretical basis for the development of personalized nutritional interventions. Bioactive metabolites produced during microbial biotransformation—such as urolithin A, sulforaphane, equol, and hesperetin—generally exhibit higher bioavailability and greater biological activity compared to their parent phytochemicals. These metabolites act as key mediators of the cognitive protective effects associated with plant-based foods.
Crucially, the alleviation of gut dysbiosis and chronic low-grade inflammation—both recognized as central pathological hallmarks of cognitive decline—has emerged as a major strategy for the prevention and intervention of cognitive disorders. By precisely modulating gut–brain axis signaling, plant-derived bioactive compounds optimize neuroimmune function, enhance neural plasticity, and upregulate neurotrophic factor expression, thus providing comprehensive protective mechanisms for cognitive health.
With ongoing advances in precision nutrition and gut microbiome research, the future holds promise for the development of personalized dietary interventions tailored to specific microbial metabolic phenotypes, thereby maximizing the cognitive benefits of phytochemicals. Moreover, integrated studies combining gut microbiome profiling and cognitive function assessment will facilitate a more comprehensive understanding of the diet–microbiota–brain interaction network, supporting the development of more effective and scientifically grounded nutritional strategies for cognitive disorder prevention. At the clinical application level, the development of functional foods enriched in key phytochemicals and specific probiotic formulations may offer new avenues for the protection of cognitive health, particularly for individuals with limited microbial metabolic capacity.
Author contributions
LL: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grant No. 72364006). Additional support was provided by the Guizhou Provincial Basic Research Program [Natural Science; Qiankehe Fund for Basic Research-ZK (2023) General 251].
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. This manuscript utilized artificial intelligence tools to assist with partial text translation during the writing process.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hullinger R, Puglielli L. Molecular and cellular aspects of age-related cognitive decline and Alzheimer's disease. Behav Brain Res. (2017) 322:191–205. doi: 10.1016/j.bbr.2016.05.008
2. Pluvinage JV, Wyss-Coray T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. (2020) 21:93–102. doi: 10.1038/s41583-019-0255-9
3. Shea JM, Villeda SA. Microglia aging in the hippocampus advances through intermediate states that drive activation and cognitive decline. eLife. (2025) 13:RP97671. doi: 10.7554/eLife.97671
4. Alfeo F, Lanciano T, Abbatantuono C, Gintili G, De Caro MF, Curci A, et al. Cognitive, emotional, and daily functioning domains involved in decision-making among patients with mild cognitive impairment: a systematic review. Brain Sci. (2024) 14:278. doi: 10.3390/brainsci14030278
5. Toth C, Tulliani N, Bissett M, Liu KP. The relationship between cognitive function and performance in instrumental activities of daily living in older adults. Br J Occup Ther. (2022) 85:120–9. doi: 10.1177/03080226211008722
6. Elendu C, Amaechi DC, Elendu TC, Ibhiedu JO, Egbunu EO, Ndam AR, et al. Stroke and cognitive impairment: understanding the connection and managing symptoms. Ann Med Surg. (2023) 85:6057–66. doi: 10.1097/MS9.0000000000001441
7. Ling Z, Liu X, Cheng Y, Yan X, Wu S. Gut microbiota and aging. Crit Rev Food Sci Nutr. (2022) 62:3509–34. doi: 10.1080/10408398.2020.1867054
8. Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. (2016) 21:738–48. doi: 10.1038/mp.2016.50
9. Kandpal M, Indari O, Baral B, Jakhmola S, Tiwari D, Bhandari V, et al. Dysbiosis of gut microbiota from the perspective of the gut-brain axis: role in the provocation of neurological disorders. Metabolites. (2022) 12:1064. doi: 10.3390/metabo12111064
10. Hasan A, Scuderi SA, Capra AP, Giosa D, Bonomo A, Ardizzone A, et al. An updated and comprehensive review exploring the gut-brain axis in neurodegenerative disorders and neurotraumas: implications for therapeutic strategies. Brain Sci. (2025) 15:654. doi: 10.3390/brainsci15060654
11. Shen L, Zhao H, Xi Y, Wang Z, Deng K, Gou W, et al. Mapping the gut microbial structural variations in healthy aging within the Chinese population. Cell Rep. (2024) 43:114968. doi: 10.1016/j.celrep.2024.114968
12. Qiao L, Yang G, Wang P, Xu C. The potential role of mitochondria in the microbiota-gut-brain axis: implications for brain health. Pharmacol Res. (2024) 209:107434. doi: 10.1016/j.phrs.2024.107434
13. Sato Y, Atarashi K, Plichta DR, Arai Y, Sasajima S, Kearney SM, et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature. (2021) 599:458–64. doi: 10.1038/s41586-021-03832-5
14. Ellouze I, Sheffler J, Nagpal R, Arjmandi B. Dietary patterns and Alzheimer's disease: an updated review linking nutrition to neuroscience. Nutrients. (2023) 15:3204. doi: 10.3390/nu15143204
15. Grant WB, Blake SM. Diet's role in modifying risk of Alzheimer's disease: history and present understanding. J Alzheimers Dis. (2023) 96:1353–82. doi: 10.3233/JAD-230418
16. Santhiravel S, Bekhit AEA, Mendis E, Jacobs JL, Dunshea FR, Rajapakse N, et al. The impact of plant phytochemicals on the gut microbiota of humans for a balanced life. Int J Mol Sci. (2022) 23:8124. doi: 10.3390/ijms23158124
17. Bié J, Sepodes B, Fernandes PC, Ribeiro MH. Polyphenols in health and disease: gut microbiota, bioaccessibility, and bioavailability. Compounds. (2023) 3:40–72. doi: 10.3390/compounds3010005
18. Augusti PR, Conterato GM, Denardin CC, Prazeres ID, Serra AT, Bronze MR, et al. Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: implications for COVID-19. J Nutr Biochem. (2021) 97:108787. doi: 10.1016/j.jnutbio.2021.108787
19. Di Meo F, Margarucci S, Galderisi U, Crispi S, Peluso G. Curcumin, gut microbiota, and neuroprotection. Nutrients. (2019) 11:2426. doi: 10.3390/nu11102426
20. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7:14. doi: 10.3390/microorganisms7010014
21. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biology. (2016) 14:e1002533. doi: 10.1371/journal.pbio.1002533
22. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. (2015) 28:203–9.
23. Lu S, Zhao Q, Guan Y, Sun Z, Li W, Guo S, et al. The communication mechanism of the gut-brain axis and its effect on central nervous system diseases: a systematic review. Biomed Pharmacother. (2024) 178:117207. doi: 10.1016/j.biopha.2024.117207
24. Shin Y, Han S, Kwon J, Ju S, Choi TG, Kang I, et al. Roles of short-chain fatty acids in inflammatory bowel disease. Nutrients. (2023) 15:4466. doi: 10.3390/nu15204466
25. Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. (2020) 11:25. doi: 10.3389/fendo.2020.00025
26. Choe U. Role of dietary fiber and short-chain fatty acids in preventing neurodegenerative diseases through the gut-brain axis. J Funct Foods. (2025) 129:106870. doi: 10.1016/j.jff.2025.106870
27. Marizzoni M, Coppola L, Festari C, Luongo D, Salamone D, Naviglio D, et al. Circulating short chain fatty acids in Alzheimer's disease: a cross-sectional observational study. J Alzheimers Dis. (2025) 106:38–43. doi: 10.1177/13872877251337773
28. Chen X, Wei J, Zhang L, Wang H, Zhang Y, Li Z, et al. Association between plasma short-chain fatty acids and inflammation in human immunodeficiency virus-associated neurocognitive disorder: a pilot study. Lipids Health Dis. (2025) 24:66. doi: 10.1186/s12944-025-02477-x
29. Mandić AD, Woting A, Jaenicke T, Sander A, Sabrowski W, Rolle-Kampcyk U, et al. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci Rep. (2019) 9:1177. doi: 10.1038/s41598-018-38018-z
30. Hinz M, Stein A, Uncini T. 5-HTP efficacy and contraindications. Neuropsychiatr Dis Treat. (2012) 8:323–8. doi: 10.2147/NDT.S33259
31. Hwang YK, Oh JS. Interaction of the vagus nerve and serotonin in the gut-brain axis. Int J Mol Sci. (2025) 26:1160. doi: 10.3390/ijms26031160
32. Averina OV, Zorkina YA, Yunes RA, Kovtun AS, Ushakova VM, Morozova AY, et al. Bacterial metabolites of human gut microbiota correlating with depression. Int J Mol Sci. (2020) 21:9234. doi: 10.3390/ijms21239234
33. Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. (2021) 13:2099. doi: 10.3390/nu13062099
34. Darmanto AG, Yen TL, Jan JS, Linh TTD, Taliyan R, Yang CH, et al. Beyond metabolic messengers: bile acids and TGR5 as pharmacotherapeutic intervention for psychiatric disorders. Pharmacol Res. (2025) 211:107564. doi: 10.1016/j.phrs.2024.107564
35. Zhao X, Zheng I, Huang W, Tang D, Zhao M, Hou R, et al. Research progress on the mechanism of bile acids and their receptors in depression. Int J Mol Sci. (2025) 26:4023. doi: 10.3390/ijms26094023
36. Larabi AB, Masson HLP, Bäumler AJ. Bile acids as modulators of gut microbiota composition and function. Gut Microbes. (2023) 15:2172671. doi: 10.1080/19490976.2023.2172671
37. Liang C, Pereira R, Zhang Y, Rojas OL. Gut microbiome in Alzheimer's disease: from mice to humans. Curr Neuropharmacol. (2024) 22:2314–29. doi: 10.2174/1570159X22666240308090741
38. Murray ER, Kemp M, Nguyen TT. The microbiota-gut-brain axis in Alzheimer's disease: a review of taxonomic alterations and potential avenues for interventions. Arch Clin Neuropsychol. (2022) 37:595–607. doi: 10.1093/arclin/acac008
39. Tripathi S, Kaushik M, Dwivedi R, Tiwari P, Tripathi M, Dada R. The effect of probiotics on select cognitive domains in mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis Rep. (2024) 8:1422–33. doi: 10.1177/25424823241289039
40. Zhang R, Ding N, Feng X, Liao W. The gut microbiome, immune modulation, and cognitive decline: insights on the gut-brain axis. Front Immunol. (2025) 16:1529958. doi: 10.3389/fimmu.2025.1529958
41. Shen Y, Fan N, Ma SX, Cheng X, Yang X, Wang G. Gut microbiota dysbiosis: pathogenesis, diseases, prevention, and therapy. MedComm. (2025) 6:e70168. doi: 10.1002/mco2.70168
42. Dmytriv TR, Storey KB, Lushchak VI. Intestinal barrier permeability: the influence of gut microbiota, nutrition, and exercise. Front Physiol. (2024) 15:1380713. doi: 10.3389/fphys.2024.1380713
43. Guo Q, Li Y, Dai X, Wang B, Zhang J, Cao H. Polysaccharides: the potential prebiotics for metabolic associated fatty liver disease (MAFLD). Nutrients. (2023) 15:3722. doi: 10.3390/nu15173722
44. Mallick R, Basak S, Das RK, Banerjee A, Paul S, Pathak S, et al. Roles of the gut microbiota in human neurodevelopment and adult brain disorders. Front Neurosci. (2024) 18:1446700. doi: 10.3389/fnins.2024.1446700
45. Wei J, Chen A, Huang D, Teng C, Cai D, Wu X, et al. Gut microbiome-derived lipopolysaccharides aggravate cognitive impairment via TLR4-mediated inflammatory signaling in neonatal rats following hypoxic-ischemic brain damage. Brain Behav Immun. (2025) 127:4–24. doi: 10.1016/j.bbi.2025.02.029
46. Cai J, Cheung J, Cheung SWM, Chin KTC, Leung RWK, Lam RST, et al. Butyrate acts as a positive allosteric modulator of the 5-HT transporter to decrease availability of 5-HT in the ileum. Br J Pharmacol. (2024) 181:1654–70. doi: 10.1111/bph.16305
47. Neupane SP, Lien L, Martinez P, Hestad K, Bramness JG. The relationship of alcohol use disorders and depressive symptoms to tryptophan metabolism: cross-sectional data from a Nepalese alcohol treatment sample. Alcohol Clin Exp Res. (2015) 39:514–21. doi: 10.1111/acer.12651
48. Konstanti P, Ligthart K, Fryganas C, Constantinos P, Smidt H, de Vos WM, et al. Physiology of γ-aminobutyric acid production by akkermansia muciniphila. Appl Environ Microbiol. (2024) 90:e0112123. doi: 10.1128/aem.01121-23
49. Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, Chen H, et al. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry Cogn Neurosci Neuroimaging. (2017) 2:38–44. doi: 10.1016/j.bpsc.2016.06.004
50. Missiego-Beltrán J, Beltrán-Velasco AI. The role of microbial metabolites in the progression of neurodegenerative diseases—therapeutic approaches: a comprehensive review. Int J Mol Sci. (2024) 25:10041. doi: 10.3390/ijms251810041
51. Sun Y, Wang X, Li L, Zhong C, Zhang Y, Yang X, et al. The role of gut microbiota in intestinal disease: from an oxidative stress perspective. Front Microbiol. (2024) 15:1328324. doi: 10.3389/fmicb.2024.1328324
52. Das A, De AJ, Mohanty T, Aich P. Role of oxidative stress, gut microbiota and derived metabolites in the etiology and progression of nonalcoholic fatty liver disease. Redox Exp Med. (2023) 2023:9553269. doi: 10.1530/REM-23-0016
53. Zhao H, Qiu X, Wang S, Wang Y, Xie L, Xia X, et al. Multiple pathways through which the gut microbiota regulates neuronal mitochondria constitute another possible direction for depression. Front Microbiol. (2025) 16:1578155. doi: 10.3389/fmicb.2025.1578155
54. Chen A, Teng C, Wei J, Wu X, Zhang H, Chen P, et al. Gut microbial dysbiosis exacerbates long-term cognitive impairments by promoting intestinal dysfunction and neuroinflammation following neonatal hypoxia-ischemia. Gut Microbes. (2025) 17:2471015. doi: 10.1080/19490976.2025.2471015
55. Baldi S, Mundula T, Nannini G, Amedei A. Microbiota shaping—the effects of probiotics, prebiotics, and fecal microbiota transplant on cognitive functions: a systematic review. World J Gastroenterol. (2021) 27:6715–32. doi: 10.3748/wjg.v27.i39.6715
56. Ma M, Li B, Qu Z, Liu S, Li S. Efficacy of probiotics in patients with cognitive impairment: a systematic review and meta-analysis. PLoS ONE. (2025) 20:e0321567. doi: 10.1371/journal.pone.0321567
57. Bruggeman A, Vandendriessche C, Hamerlinck H, De Looze D, Tate DJ, Vuylsteke M, et al. Safety and efficacy of faecal microbiota transplantation in patients with mild to moderate Parkinson's disease (GUT-PARFECT): a double-blind, placebo-controlled, randomised, phase 2 trial. eClinicalMedicine. (2024) 71:102563. doi: 10.1016/j.eclinm.2024.102563
58. Daliri EBM, Ofosu FK, Chelliah R, Lee BH, Oh DH. Challenges and perspectives in developing probiotics for alleviating neurological disorders: a critical review. Biomolecules. (2021) 11:300. doi: 10.3390/biom11020300
59. Yang Y, Ling W. Health benefits and future research of phytochemicals: a literature review. J Nutr. (2025) 155:87–101. doi: 10.1016/j.tjnut.2024.11.007
60. Liu Y, Chen Z, Li A, Liu R, Yang H, Xia X. The phytochemical potential for brain disease therapy and the possible nanodelivery solutions for brain access. Front Oncol. (2022) 12:936054. doi: 10.3389/fonc.2022.936054
61. Figueira I, Garcia G, Pimpão RC, Terrasso AP, Costa I, Almeida AF, et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci Rep. (2017) 7:11456. doi: 10.1038/s41598-017-11512-6
62. Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. (2020) 11:135–57. doi: 10.1080/19490976.2019.1638722
63. Domínguez-López I, López-Yerena A, Vallverdú-Queralt A, Pallàs M, Lamuela-Raventós RM, Pérez M. From the gut to the brain: the long journey of phenolic compounds with neurocognitive effects. Nutr Rev. (2025) 83:e533–46. doi: 10.1093/nutrit/nuae034
64. Corbi G, Conti V, Davinelli S, Scapagnini G, Filippelli A, Ferrara N. Dietary phytochemicals in neuroimmunoaging: a new therapeutic possibility for humans? Front Pharmacol. (2016) 7:364. doi: 10.3389/fphar.2016.00364
65. Fakhri S, Piri S, Moradi SZ, Khan H. Phytochemicals targeting oxidative stress, interconnected neuroinflammatory, and neuroapoptotic pathways following radiation. Curr Neuropharmacol. (2022) 20:836–56. doi: 10.2174/1570159X19666210809103346
66. Naoi M, Inaba-Hasegawa K, Shamoto-Nagai M, Maruyama W. Neurotrophic function of phytochemicals for neuroprotection in aging and neurodegenerative disorders: modulation of intracellular signaling and gene expression. J Neural Transm. (2017) 124:1515–27. doi: 10.1007/s00702-017-1797-5
67. Limanaqi F, Biagioni F, Mastroiacovo F, Polzella M, Lazzeri G, Fornai F. Merging the multi-target effects of phytochemicals in neurodegeneration: from oxidative stress to protein aggregation and inflammation. Antioxidants. (2020) 9:1022. doi: 10.3390/antiox9101022
68. Venkatesan R, Ji E, Kim SY. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: a comprehensive review. Biomed Res Int. (2015) 2015:814068. doi: 10.1155/2015/814068
69. Laparra JM, Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol Res. (2010) 61:219–25. doi: 10.1016/j.phrs.2009.11.001
70. Verediano TA, Stampini Duarte Martino H, Dias Paes MC, Tako E. Effects of anthocyanin on intestinal health: a systematic review. Nutrients. (2021) 13:1331. doi: 10.3390/nu13041331
71. Han Y, Chen R, Lin Q, Liu Y, Ge W, Cao H, et al. Curcumin improves memory deficits by inhibiting HMGB1-RAGE/TLR4-NF-κB signalling pathway in APPswe/PS1dE9 transgenic mice hippocampus. J Cell Mol Med. (2021) 25:8947–56. doi: 10.1111/jcmm.16855
72. Wang Z, Chen F, Li Y, Chaoying L, Wang L, Lu Z, et al. Curcumin promotes spermatogenesis in mice with cryptorchidism by regulating testicular protein O-GlcNAcylation. Front Endocrinol. (2025) 16:1555721. doi: 10.3389/fendo.2025.1555721
73. Chin D, Huebbe P, Frank J, Rimbach G, Pallauf K. Curcumin may impair iron status when fed to mice for six months. Redox Biol. (2014) 2:563–9. doi: 10.1016/j.redox.2014.01.018
74. Farag S, Tsang C, Al-Dujaili EAS, Murphy PN. Effect of polyphenol supplementation on memory functioning in overweight and obese adults: a systematic review and meta-analysis. Nutrients. (2024) 16:474. doi: 10.3390/nu16040474
75. Francis AJ, Sreenivasan C, Parikh A, AlQassab O, Kanthajan T, Pandey M, et al. Curcumin and cognitive function: a systematic review of the effects of curcumin on adults with and without neurocognitive disorders. Cureus. (2024) 16:e67706. doi: 10.7759/cureus.67706
76. Uchida K, Meno K, Korenaga T, Liu S, Suzuki H, Baba Y, et al. Effect of matcha green tea on cognitive functions and sleep quality in older adults with cognitive decline: a randomized controlled study over 12 months. PLoS ONE. (2024) 19:e0309287. doi: 10.1371/journal.pone.0309287
77. Jalouli M, Rahman MA, Biswas P, Rahman H, Harrath AH, Lee IS, et al. Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: a comprehensive review. Front Pharmacol. (2025) 16:1492517. doi: 10.3389/fphar.2025.1492517
78. Kamal RM, Abdull Razis AF, Mohd Sukri NS, Perimal EK, Ahmad H, Patrick R, et al. Beneficial health effects of glucosinolates-derived isothiocyanates on cardiovascular and neurodegenerative diseases. Molecules. (2022) 27:624. doi: 10.3390/molecules27030624
79. Hassan FU, Rehman MS, Khan MS, Ali MA, Javed A, Nawaz A, et al. Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Front Genet. (2019) 10:514. doi: 10.3389/fgene.2019.00514
80. Tang Y, Du J, Wu H, Wang M, Liu S, Tao F. Potential therapeutic effects of short-chain fatty acids on chronic pain. Curr Neuropharmacol. (2024) 22:191–203. doi: 10.2174/1570159X20666220927092016
81. Wu W, Meng T, Han L, Jin F, Han P, Zhou Y. Bridging traditional Chinese medicine and Alzheimer's disease: the pivotal role of gut microbiota in multitarget therapeutic mechanisms. Front Pharmacol. (2025) 16:1630205. doi: 10.3389/fphar.2025.1630205
82. Cherta-Murillo A, Pugh JE, Alaraj-Alshehhi S, Hajjar D, Chambers ES, Frost GS. The effects of SCFAs on glycemic control in humans: a systematic review and meta-analysis. Am J Clin Nutr. (2022) 116:335–61. doi: 10.1093/ajcn/nqac085
83. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012
84. MahmoudianDehkordi S, Arnold M, Nho K, Ahmad S, Jia W, Xie G, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer's disease—an emerging role for gut microbiome. Alzheimers Dement. (2019) 15:76–92. doi: 10.1016/j.jalz.2018.07.217
85. Shao S, Ye X, Su W, Wang Y. Curcumin alleviates Alzheimer's disease by inhibiting inflammatory response, oxidative stress and activating the AMPK pathway. J Chem Neuroanat. (2023) 134:102363. doi: 10.1016/j.jchemneu.2023.102363
86. Varma VR, Wang Y, An Y, Varma S, Bilgel M, Doshi J, et al. Bile acid synthesis, modulation, and dementia: a metabolomic, transcriptomic, pharmacoepidemiologic study. PLoS Med. (2021) 18:e1003615. doi: 10.1371/journal.pmed.1003615
87. Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. (2000) 66:1107–13. doi: 10.1128/AEM.66.3.1107-1113.2000
88. Godlewska U, Bulanda E, Wypych TP. Bile acids in immunity: bidirectional mediators between the host and the microbiota. Front Immunol. (2022) 13:949033. doi: 10.3389/fimmu.2022.949033
89. Yao L, Devotta H, Li J, Lunjani N, Sadlier C, Lavelle A, et al. Dysrupted microbial tryptophan metabolism associates with SARS-CoV-2 acute inflammatory responses and long COVID. Gut Microbes. (2024) 16:2429754. doi: 10.1080/19490976.2024.2429754
90. Niu GY, Zhou Y, Hong H, Wu J, Quan W, Li T, et al. DSS-induced colitis activates the kynurenine pathway in serum and brain by affecting IDO-1 and gut microbiota. Front Immunol. (2023) 13:1089200. doi: 10.3389/fimmu.2022.1089200
91. Zhao LP, Wu J, Quan W, Zhou Y, Hong H, Niu GY, et al. DSS-induced acute colitis causes dysregulated tryptophan metabolism in brain: An involvement of gut microbiota. J Nutr Biochem. (2023) 115:109282. doi: 10.1016/j.jnutbio.2023.109282
92. Tomás-Barberán FA, García-Villalba R, González-Sarrías A, Selma MV, Espín JC. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, health status. J Agric Food Chem. (2014) 62:6535–8. doi: 10.1021/jf5024615
93. Leng P, Wang Y, Xie M. Ellagic acid and gut microbiota: interactions, and implications for health. Food Sci Nutr. (2025) 13:e70133. doi: 10.1002/fsn3.70133
94. Zhang Q, Zhang W, Yuan X, Peng X, Hu G. Urolithin A in central nervous system disorders: therapeutic applications and challenges. Biomedicines. (2025) 13:1553. doi: 10.3390/biomedicines13071553
95. Gong L, Huang D, Shi Y, Liang Z, Bu Q, Wang M, et al. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J Neuroinflamm. (2019) 16:62. doi: 10.1186/s12974-019-1450-3
96. Yi S, Zhang C, Hu J, Meng Y, Chen L, Yu H, et al. Preparation, characterization, and in vitro pharmacodynamics and pharmacokinetics evaluation of PEGylated urolithin A liposomes. AAPS PharmSciTech. (2021) 22:26. doi: 10.1208/s12249-020-01890-y
97. Wojciechowska O, Kujawska M. Urolithin A in health and diseases: prospects for Parkinson's disease management. Antioxidants. (2023) 12:1479. doi: 10.3390/antiox12071479
98. Jayatunga DPW, Hone E, Khaira H, Lunelli T, Singh H, Guillemin GJ, et al. Therapeutic potential of mitophagy-inducing microflora metabolite, urolithin A for Alzheimer's disease. Nutrients. (2021) 13:3744. doi: 10.3390/nu13113744
99. Zhang Y, Jiang L, Su P, Yu T, Ma Z, Liu Y, et al. Urolithin A suppresses tumor progression and induces autophagy in gastric cancer via the PI3K/Akt/mTOR pathway. Drug Dev Res. (2023) 84:172–84. doi: 10.1002/ddr.22021
100. D'Amico D, Olmer M, Fouassier AM, Valdés P, Andreux PA, Rinsch C, et al. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell. (2022) 21:e13662. doi: 10.1111/acel.13662
101. Kaiser S, Di Mascio P, Murphy ME, Sies H. Physical and chemical scavenging of singlet molecular oxygen by tocopherols. Arch Biochem Biophys. (1990) 276:165–70. doi: 10.1016/0003-9861(90)90556-E
102. Kim J, Lee S, Choi BR, Yang H, Hwang Y, Park JH, et al. Sulforaphane epigenetically enhances neuronal BDNF expression and TrkB signaling pathways. Mol Nutr Food Res. (2017) 61:1600194. doi: 10.1002/mnfr.201600194
103. Shimizu S, Kasai S, Yamazaki H, Tatara Y, Mimura J, Engler MJ, et al. Sulforaphane increase mitochondrial biogenesis-related gene expression in the hippocampus and suppresses age-related cognitive decline in mice. Int J Mol Sci. (2022) 23:8433. doi: 10.3390/ijms23158433
104. Liu H, Zimmerman AW, Singh K, Connors SL, Diggins E, Stephenson KN, et al. Biomarker exploration in human peripheral blood mononuclear cells for monitoring sulforaphane treatment responses in autism spectrum disorder. PLoS ONE. (2014) 9:e111262. doi: 10.1038/s41598-020-62714-4
105. Nouchi R, Hu Q, Ushida Y, Suganuma H, Kawashima R. Cognitive training with neurofeedback using NIRS improved cognitive functions in young adults: evidence from a randomized controlled trial. Brain Behavior. (2021) 11:e2424. doi: 10.3390/brainsci12010005
106. Nouchi R, Hu Q, Ushida Y, Suganuma H, Kawashima R. The effects of sulforaphane supplementation on cognitive function in healthy older adults: a randomized, double-blind, placebo-controlled trial. Nutrients. (2022) 14:4106. doi: 10.3389/fnagi.2022.929628
107. Rafii F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites. (2015) 5:56–73. doi: 10.3390/metabo5010056
108. Ko KP, Kim CS, Ahn Y, Park SJ, Kim YJ, Park JK, et al. Plasma isoflavone concentration is associated with decreased risk of type 2 diabetes in Korean women but not men: results from the Korean Genome and epidemiology study. Diabetologia. (2015) 58:726–35. doi: 10.1007/s00125-014-3463-x
109. Sekikawa A, Ihara M, Lopez O, Kakuta C, Lopresti B, Higashiyama A, et al. Effect of S-equol and soy isoflavones on heart and brain. Curr Cardiol Rev. (2019) 15:114–35. doi: 10.2174/1573403X15666181205104717
110. Nakamoto M, Otsuka R, Nishita Y, Tange C, Tomida M, Kato Y, et al. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur J Clin Nutr. (2018) 72:1458–62. doi: 10.1038/s41430-017-0061-2
111. Ozawa M, Ohara T, Ninomiya T, Hata J, Yoshida D, Mukai N, et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: the Hisayama Study. J Am Geriatr Soc. (2014) 62:1224–30. doi: 10.1111/jgs.12887
112. Yoshikata R, Myint K Z, Ohta H. Relationship between equol producer status and metabolic parameters in 743 Japanese women: equol producer status is associated with antiatherosclerotic conditions in women around menopause and early postmenopause. Menopause. (2017) 24:216–24. doi: 10.1097/GME.0000000000000743
113. Talaei M, Feng L, Baum L, Pan A, Yuan JM, Koh WP. Intake of soy products and risk of cognitive impairment: the Singapore Chinese Health Study. Eur J Nutr. (2020) 59:1477–84. doi: 10.1007/s00394-019-02010-8
114. Greendale GA, Huang MH, Leung K, Crawford SL, Gold EB, Wight R, et al. Dietary phytoestrogen intakes and cognitive function during the menopausal transition: results from the study of women's health across the nation phytoestrogen study. Menopause. (2012) 19:894–903. doi: 10.1097/gme.0b013e318242a654
115. Cui C, Birru RL, Snitz BE, Ihara M, Kakuta C, Lopresti B, et al. Effects of soy isoflavones on cognitive function: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2020) 78:134–44. doi: 10.1093/nutrit/nuz050
116. Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Karim R, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. (2016) 86:699–708. doi: 10.1212/WNL.0000000000002980
117. Yang G, Hong S, Yang P, Sun Y, Wang Y, Zhang P, et al. Discovery of an ene-reductase for initiating flavone and flavonol catabolism in gut bacteria. Nat Commun. (2021) 12:790. doi: 10.1038/s41467-021-20974-2
119. Rajmohan R, Reddy PH. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer's disease neurons. J Alzheimers Dis. (2017) 57:975–99. doi: 10.3233/JAD-160612
120. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. (2011) 377:1019–31. doi: 10.1016/S0140-6736(10)61349-9
121. Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. (2018) 14:168–81. doi: 10.1038/nrneurol.2017.185
122. Goyal A, Verma A, Dubey N, Raghav J, Agrawal A. Naringenin: A prospective therapeutic agent for Alzheimer's and Parkinson's disease. J Food Biochem. (2022) 46:e14415. doi: 10.1111/jfbc.14415
123. Islam MR, Al-Imran MIK, Zehravi M, Sweilam SH, Mortuza MR, Gupta JK, et al. Targeting signaling pathways in neurodegenerative diseases: Quercetin's cellular and molecular mechanisms for neuroprotection. Anim Models Exp Med. (2025) 8:798–818. doi: 10.1002/ame2.12551
124. Li S, Zhang Y, Sun Y, Zhang G, Bai J, Guo J, et al. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr Diabetes. (2019) 9:28. doi: 10.1038/s41387-019-0095-8
125. Qiu Q, Zhang N, Lei, X. Effect of naringenin on oxidative stress and Tau protein phosphorylation of Aβ25-35-induced PC12 cell injury. Chinese J Exp Tradit Med Formulae. (2020) 9299.
126. Ghofrani S, Joghataei MT, Mohseni S, Baluchnejadmojarad T, Bagheri M, Khamse S, et al. Naringenin improves learning and memory in an Alzheimer's disease rat model: insights into the underlying mechanisms. Eur J Pharmacol. (2015) 764:195–201. doi: 10.1016/j.ejphar.2015.07.001
127. Zambrano P, Suwalsky M, Jemiola-Rzeminska M, Strzalka K. Studies on the interaction of naringenin with cell membranes and model phospholipid bilayers. Biochim Biophys Acta Biomembr. (2019) 1861:545–51. doi: 10.1016/j.bbamem.2019.03.014
128. Heo HJ, Kim DO, Shin SC, Kim MJ, Kim BG, Shin DH. Effect of antioxidant flavanone, naringenin, from citrus junos on neuroprotection. J Agric Food Chem. (2004) 52:1520–5. doi: 10.1021/jf035079g
129. Tran TH, Vo TT, Vo TQ, Cao TC, Tran TS. Synthesis and evaluation of the acetylcholinesterase inhibitory activities of some flavonoids derived from naringenin. Sci World J. (2021) 2021:4817900. doi: 10.1155/2021/4817900
130. Flanagan E, Müller M, Hornberger M, Vauzour D. Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr Nutr Rep. (2018) 7:49–57. doi: 10.1007/s13668-018-0226-1
131. Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. (2013) 18:1818–92. doi: 10.1089/ars.2012.4581
132. Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev. (2013) 71:709–26. doi: 10.1111/nure.12060
133. Weng JR, Tsai CH, Kulp SK, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. (2008) 262:153–63. doi: 10.1016/j.canlet.2008.01.033
134. Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. (2007) 55:207–16. doi: 10.1016/j.phrs.2007.01.012
135. Stahl W, Sies H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol Biotechnol. (2007) 37:26–30. doi: 10.1007/s12033-007-0051-z
136. Kwak JS, Kim JY, Paek JE, Lee YJ, Kim HR, Park DS, et al. Garlic powder intake and cardiovascular risk factors: a meta-analysis of randomized controlled clinical trials. Nutr Res Pract. (2014) 8:644–54. doi: 10.4162/nrp.2014.8.6.644
137. Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods. (2017) 6:92. doi: 10.3390/foods6100092
138. Afzal A, Oriqat G, Khan MA, Jose J, Afzal M. Chemistry and biochemistry of terpenoids from Curcuma and related species. J Biol Act Prod Nat. (2013) 3:1–55. doi: 10.1080/22311866.2013.782757
139. Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. (2016) 8:754. doi: 10.3390/nu8120754
Keywords: gut microbiota, phytochemicals, cognitive function, gut-brain axis, urolithins, sulforaphane, equol, hesperidin
Citation: Luo L (2025) Promoting cognitive health through the nexus of gut microbiota and dietary phytochemicals. Front. Nutr. 12:1636131. doi: 10.3389/fnut.2025.1636131
Received: 27 May 2025; Accepted: 11 August 2025;
Published: 29 August 2025.
Edited by:
Adnan Amin, Yeungnam University, Republic of KoreaReviewed by:
Khizar Abbas, Bahauddin Zakariya University, PakistanNaeem Ur Rehman, Gomal University, Pakistan
Copyright © 2025 Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Luo, NDYwMDIyODMxQGd6bnUuZWR1LmNu
 Lin Luo
Lin Luo