- 1King Abdullah International Medical Research Center-WR, King Saud bin Abdulaziz University for Health Sciences, Ministry of National Guard for Health Affairs, Jeddah, Saudi Arabia
- 2Bioscience, Biological and Environmental Sciences and Engineering Division, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia
- 3Department of Pharmacy Practices, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
- 4Department of Clinical Biochemistry, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
- 5Gastroenterology Unit, Department of Medicine, King Abdulaziz Medical City, Jeddah, Saudi Arabia
- 6Anatomic Pathology Division, Children’s Hospital of Eastern Ontario, University of Ottawa, Ottawa, ON, Canada
- 7Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada
Metabolic Associated Steatosis Liver Disease (MASLD) and its advanced form, Metabolic Associated Steatohepatitis (MASH), represent growing global health concerns closely linked to obesity, type 2 diabetes mellitus (T2DM), and metabolic syndrome. The gut microbiome has emerged as a key modulator in MASLD pathogenesis through the gut–liver axis, influencing hepatic fat accumulation, inflammation, and fibrosis via microbial metabolites and immune responses. Dysbiosis–characterized by altered microbial diversity and composition–contributes to hepatic lipid dysregulation, systemic inflammation, and impaired bile acid signaling. Metabolites such as short-chain fatty acids (SCFAs), trimethylamine-N-oxide (TMAO), and ethanol play critical roles in disease progression. Recent innovations in precision medicine, including microbiome profiling, metabolomics, and genomics, offer promising diagnostic and therapeutic strategies. Targeted probiotics, fecal microbiota transplantation (FMT), and personalized dietary interventions are under investigation for modulating the gut microbiome. This systematic review, conducted in accordance with PRISMA 2020 guidelines, is the first to comprehensively integrate both animal and human studies on MASLD/MASH-related gut microbiome alterations. It uniquely synthesizes microbial taxa, functional metabolites, and region-specific patterns–including data from underrepresented MENA populations. Eligible studies from PubMed, Scopus, and Web of Science evaluated microbial composition, metabolite profiles, and associations with steatosis, inflammation, and fibrosis. The findings underscore the diagnostic and therapeutic potential of microbiome modulation and emphasize the need for longitudinal, mechanistically driven studies. This systematic review is the first to integrate both animal and human studies on MASLD/MASH-related gut microbiome alterations. Unlike previous reviews, it uniquely emphasizes microbial taxa, functional metabolites, and region-specific patterns, including underrepresented MENA populations. By synthesizing findings from diverse cohorts, this review highlights diagnostic and therapeutic opportunities while identifying persistent gaps in longitudinal data, regional representation, and multi-omics integration.
Introduction
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) has become a common concern in public health, impacting around 25%–30% of adults worldwide (1, 2). According to World Health Organization (WHO) estimates, the growing prevalence of MASLD reflects the rising trends in obesity and type 2 diabetes mellitus (T2DM) worldwide, highlighting the disease’s importance on a global health scale. MASLD is characterized by the accumulation of fat in the liver without heavy alcohol consumption and can range from simple hepatic steatosis to more severe forms such as Metabolic Dysfunction-Associated Steatohepatitis (MASH) fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC) (2, 3). The increasing incidence of MASLD underscores the urgent need for public health measures because it has become the most common chronic liver condition worldwide, paralleling trends in obesity and T2DM that negatively affect metabolic health (4). The widespread presence of MASLD can be seen in its high rates in different regions, each influenced by lifestyle, diet, and metabolic health. In Western Europe and North America, high rates of obesity and metabolic syndrome contribute to a prevalence of approximately 25%–30% among adults (2). In the United Kingdom, the prevalence of MASLD has increased due to obesity and metabolic syndrome, leading to higher rates of MASH, a more severe form of the condition that could result in liver failure. In North America, MASLD is a primary reason for liver transplants, stressing the critical necessity for action. The Middle East and North Africa (MENA) region is experiencing alarmingly high rates of MASLD, primarily driven by the increasing prevalence of obesity and T2DM. The prevalence of MASLD in Saudi Arabia is projected to increase from 25.8% in 2017 to 31.7% by 2030, reflecting a 48% rise in the number of cases, reaching an estimated 12.5 million individuals. Similarly, in the United Arab Emirates (UAE), the prevalence is anticipated to reach 30.2% by 2030, corresponding to a 46% increase in cases, totaling approximately 372,000. These trends highlight the critical need for targeted public health interventions to address the region’s escalating metabolic health burden (5).
In East Asia, there is a growing occurrence of metabolic disorders due to urbanization and changes in diet, resulting in increased prevalence rates. In China, around 29.2% of adults are affected by MASLD, while in South Korea, the rate is approximately 30%, and younger populations in Japan are experiencing a higher incidence than in the past (1). In South Asia, especially India, MASLD rates reflect these trends because of the high occurrence of T2DM and changing eating habits (1, 4). These worldwide trends emphasize the immediate necessity for local measures to control the disease’s rise and lessen its effects on public health. It is crucial to address metabolic health, dietary habits, and obesity through specific strategies tailored to each region to lessen the impact of MASLD on healthcare systems globally. Emerging evidence suggests that artificial sweeteners may influence gut microbiota composition, with potential metabolic and inflammatory consequences relevant to MASLD progression. Recent evaluations by IARC/WHO have also raised questions regarding long-term health effects, including carcinogenicity, which warrant further investigation in the context of liver disease (6, 7).
These regional and global trends in MASLD prevalence are closely intertwined with rising rates of metabolic disorders–particularly type 2 diabetes mellitus (T2DM), which shares overlapping pathophysiological pathways with liver steatosis. The connection between MASLD and T2DM highlights a reciprocal relationship (8). Nearly 70% of individuals with T2DM are estimated to also have MASLD, where insulin resistance (IR) and hyperglycemia worsen hepatic fat accumulation, while MASLD contributes to systemic IR and pancreatic β-cell dysfunction, increasing the risk of T2DM (9, 10). However, not all individuals with T2DM develop MASLD, and not all MASLD patients progress to T2DM. Still, their coexistence significantly amplifies the risk of complications such as fibrosis and cirrhosis (8, 11).
Beyond traditional metabolic risk factors, growing attention has turned to the gut microbiome as a central modulator of MASLD through its influence on hepatic inflammation, lipid metabolism, and systemic insulin resistance. The gut microbiome is being increasingly acknowledged as a significant contributor to the development of MASLD, with the gut-liver axis becoming a key mechanism. Dysbiosis (microbial imbalance) in the gut microbiota can result in liver inflammation (hepatitis or “portitis”), fibrosis, and other metabolic disturbances. Understanding these fundamental processes is essential for the development of effective microbiome-targeted therapies. Recent research indicates that altering the composition of gut bacteria can improve liver health, suggesting that modulating the microbiome may serve as a promising therapeutic strategy for managing MASLD and MASH (11–14).
The gut microbiome is mechanistically relevant to MASLD due to its direct and indirect interactions with hepatic metabolism. Gut-derived bacterial metabolites–including SCFAs, ethanol, ammonia, and trimethylamine-N-oxide (TMAO)–influence hepatic fat accumulation, insulin sensitivity, and inflammatory signaling. Dysbiosis also disrupts gut barrier integrity, leading to increased translocation of microbial components such as lipopolysaccharide (LPS) into the portal circulation, which promotes hepatic inflammation and fibrosis. Through the gut–liver axis, these mechanisms link intestinal microbial composition to the pathogenesis and progression of MASLD. The specific mechanisms by which the gut microbiome influences liver disease pathogenesis are described in detail in the sections below.
To address the growing burden of MASLD, comprehensive global public health strategies are required. These should include lifestyle changes, genetic screening, and microbiome-focused treatments. Region-specific interventions tailored to local dietary habits, lifestyle factors, and genetic predispositions are essential to prevent the rapid increase of MASLD and to reduce its impact on global health (15, 16). By implementing these strategies through coordinated efforts, the healthcare community can better manage the escalating public health issue, improve individual outcomes, and alleviate the strain on healthcare systems worldwide (17).
This systematic review aims to comprehensively evaluate current evidence on the role of gut microbiome dysbiosis in MASLD/MASH pathogenesis, integrating findings from animal models and clinical studies. Detailed insights into how gut dysbiosis may contribute to liver inflammation and fibrosis are vital for developing future microbiome-targeted treatments, highlighting the importance of integrating these insights into therapeutic strategies. Despite the rapidly rising prevalence of MASLD in MENA countries, few microbiome-focused reviews have addressed regional microbial patterns, dietary drivers, or therapeutic opportunities in these populations. Furthermore, existing reviews often separate animal and human findings. To bridge this gap, our systematic review integrates both preclinical and clinical studies, demonstrates MENA-specific insights, and explores microbial function and metabolite pathways relevant to MASLD progression.
Gut microbiome dysbiosis in MASLD/MASH
The gut microbiota is diverse, consisting of various bacterial species classified by genus, family, order, and phyla. The composition of an individual’s gut microbiota differs from person to person. It is shaped early in life and influenced by factors such as birth gestation, type of delivery, feeding methods, and weaning. External factors like antibiotic use also play a role. Despite this variability, most healthy adults share a core set of bacterial species. For instance, Escherichia coli is commonly found in many individuals. The dominant bacterial groups in the adult gut are Bacteroidetes and Firmicutes, while Actinobacteria, Proteobacteria, and Verrucomicrobia are present in smaller amounts. In addition to bacteria, the gut also contains methanogenic archaea (mainly Methanobrevibacter smithii), eukaryotes (primarily yeasts), and viruses (mostly bacteriophages) (18, 19). Although these elements are consistently found in the gut, identifying a core set of species-level phylotypes has revealed as standard microbes such as Faecalibacterium prausnitzii, Roseburia intestinalis, and Bacteroides uniformis. However, these species may comprise less than 0.5% of the microbial population in some individuals (20, 21). Several factors can negatively impact the beneficial gut flora, including antibiotic use, psychological and physical stress, radiation, changes in gastrointestinal (GIT) peristalsis, and dietary modifications (22). The balance of gut microbiota is influenced by various host factors such as lifestyle, diet, medications, hygiene, health, and genetics. This imbalance, or dysbiosis, may contribute to the onset of immune, metabolic, neurodegenerative, psychological, and other infectious diseases, including “long COVID-19” (23–26).
The liver and microbiome interact closely via the portal vein, which transports gut-derived substances to the liver, while bile acids and antibodies from the liver provide feedback to the intestine. Bile acids engage with nuclear receptors to regulate metabolism and play a key role in controlling gut microbiota. This interaction occurs at the gut mucosal barrier, where intestinal epithelial cells maintain gut balance by keeping gut microbiota separate from the host’s immune cells (27) (Figure 1). Figure 1 presents the structural organization of the intestinal barrier and illustrates how its disruption contributes to the development and progression of MASLD and MASH through increased microbial translocation and systemic inflammation. The interface between the liver and the microbiome is the gut mucosal barrier, which is made up of intestinal epithelial cells that maintain gut homeostasis by segregating gut microbiota and host immune cells. The mucus barrier and diet influence microbiota composition, impacting health by preventing harmful microbiota-epithelium contact that could trigger inflammation. The barrier also nourishes and stabilizes microbiota, helping them remain despite digestive movement. A gut vascular barrier (GVB) blocks bacteria from entering the portal circulation and reaching the liver. However, certain pathogenic bacteria and possibly some pathobionts have developed ways to bypass this barrier. This barrier may become compromised in some pathological conditions, such as MASLD and MASH. The microbiota shapes the entire intestinal barrier through the coordinated activity of structural components (such as mucus and epithelial cells), immune cells (including intraepithelial and lamina propria cells), and soluble mediators (like IgA and antimicrobial peptides). Alterations in any of these elements can disrupt the intestinal barrier. Additionally, the microbiota can influence the effectiveness of treatments by interacting with the immune system (27). Communication between the gut and liver occurs through bile acids, with the liver producing bile that shapes the gut microbiome, while gut microbiota modulates bile acid composition. The gut-liver axis, the bidirectional interaction, is essential for maintaining physiological health. In conditions like MASLD, which ranges from simple fatty liver to more severe stages like MASH with fibrosis, gut dysbiosis plays a significant role. Factors like obesity, diet, and metabolic syndrome contribute to changes in the gut microbiota, increasing intestinal permeability and allowing harmful metabolites such as lipopolysaccharide (LPS) to enter the liver, worsening liver injury. Microbial metabolites like ethanol, phenylacetate, and TMAVA are linked to the progression of MASLD, while metabolites derived from tryptophan may help reduce liver inflammation. Additionally, patients with MASLD or MASH often exhibit altered bile acid profiles, disrupting liver function (28) (Figure 1).
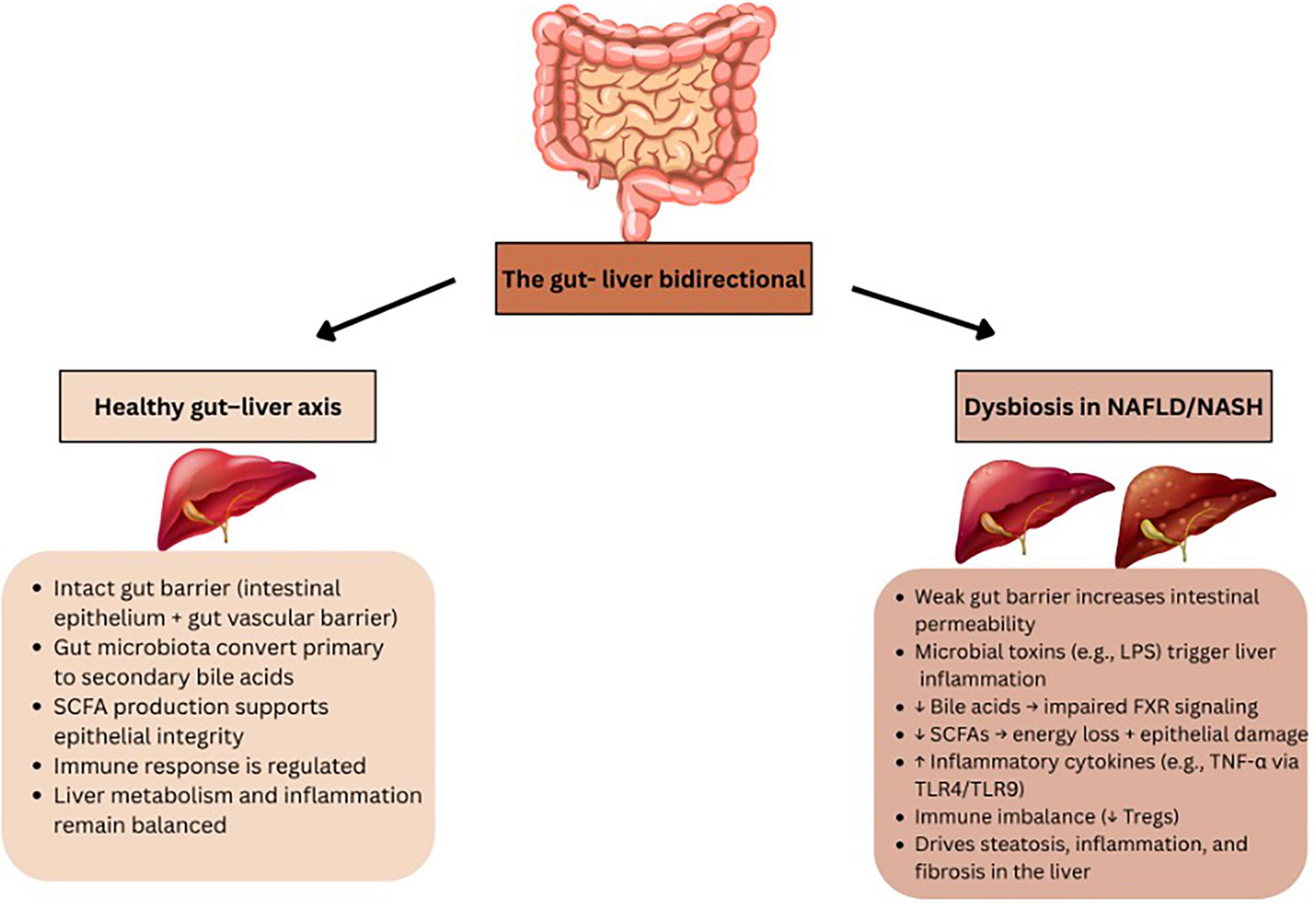
Figure 1. Overview of the gut–liver axis in health and disease. The healthy gut–liver axis involves a stable gut barrier, regulated immune responses, and microbial production of SCFAs and bile acid metabolism, which together support liver homeostasis. In contrast, dysbiosis in NAFLD/NASH leads to barrier dysfunction, increased intestinal permeability, translocation of microbial products (e.g., LPS), decreased SCFA and bile acid production, immune imbalance, and activation of pro-inflammatory pathways, ultimately promoting hepatic steatosis, inflammation, and fibrosis.
Bile acids play a crucial role in shaping the gut microbiota through a bidirectional interaction, where the microbiota influences bile acid metabolism, and bile acids, in turn, affect the microbiota. After being converted into secondary bile acids, they signal through the farnesoid X receptor (FXR) in the intestinal epithelium, enhancing the epithelial barrier, repairing damage to the gut vascular barrier, and regulating aspects of metabolic syndrome. However, studies in mice with FXR knockouts have yielded mixed results, indicating that FXR may have different roles in the development of NASH (27). The Pathogen-associated molecular patterns (PAMPs) play a role in liver damage in MASLD. Inflammasome deficiency-induced changes in the gut microbiota lead to hepatic steatosis and inflammation through the portal circulation, triggering toll-like receptors (TLRs) such as TLR4 and TLR9 agonists. This process increases necrosis factor-alpha (TNF-α) production in the liver, intensifying inflammation. Postbiotics, such as short-chain fatty acids (SCFAs) like acetate, propionate, and butyrate, are produced during the breakdown of dietary fibers. These substances affect the intestinal epithelial barrier, immune system, and microbiota. SCFAs, in particular, influence immune cell differentiation, including T regulatory cells, and enhance the microbicidal activity of macrophages. Additionally, postbiotics can impact the balance between brown and white adipose tissue. The thickness and integrity of the mucus barrier, which covers the intestinal epithelium, can vary depending on the gut segment (27).
Methods
We conducted a systematic literature search in PubMed, Scopus, and Web of Science databases up to March 2025. Keywords included “MASLD,” “MASH,” “gut microbiota,” “microbiome dysbiosis,” “gut-liver axis,” “microbial metabolites,” “animal models,” and “human studies.” Boolean operators and MeSH terms were used where applicable. As shown in Figure 2, the study selection process followed the PRISMA 2020 guidelines, detailing records identified, screened, and included in the final analysis.

Figure 2. PRISMA 2020 flow diagram. PRISMA 2020 flow diagram outlining the study identification, screening, eligibility assessment, and inclusion process for this systematic review. A total of 92 studies were included in the final analysis following exclusion of duplicates and irrelevant records.
Registration and protocol
This review was not registered in a public database such as PROSPERO.
Inclusion criteria
• Original studies (animal or human) examining the role of gut microbiota in MASLD/MASH.
• Studies reporting gut microbiome composition, metabolite profiling, or intervention outcomes.
• Peer-reviewed articles in English.
Exclusion criteria
• Editorials, conference abstracts, case reports without mechanistic data.
• Studies focusing exclusively on viral hepatitis or alcohol-related liver disease.
Search strategy
The search strategy included the following terms: (“MASLD” OR “Metabolic Associated Steatosis Liver Disease” OR “MASH” OR “Metabolic Associated Steatohepatitis” OR “NAFLD”) AND (“gut microbiota” or “microbiome” OR “dysbiosis” OR “gut-liver axis” OR “microbial metabolites” OR “animal models” OR “human studies).
Study selection
Titles and abstracts were screened independently by two reviewers. Full texts of potentially eligible studies were assessed for inclusion. Discrepancies were resolved through (29).
Data extraction and synthesis
Data were extracted using a standardized form, capturing: study design, model used, microbiome changes, metabolites involved, and liver-related outcomes. Findings were synthesized narratively and tabulated for clarity.
Despite the growing number of studies examining the gut microbiome in MASLD, the majority of included human research was cross-sectional. This limits our ability to understand temporal microbial shifts or determine causality in disease progression. Longitudinal studies–particularly those that evaluate microbiome dynamics in relation to steatosis, fibrosis, or clinical interventions–remain sparse. The lack of prospective data remains a key gap in the field, constraining the development of microbiome-based diagnostics or therapeutic monitoring tools.
Risk of bias assessment
Risk of bias was not formally assessed in this narrative synthesis. Included studies were appraised based on study design, sample size, and relevance to the research objectives.
PRISMA flow diagram
(Reference the flowchart: A PRISMA flow diagram illustrating the study selection process is presented in Figure 2).
Results: evidence linking dysbiosis to the progression of MASLD/MASH
Animal studies
Animal studies have played a crucial role in clarifying the mechanisms by which gut microbiota influence the progression of MASLD/MASH. These studies frequently employs to replicate human disease conditions, offering valuable insights into the impact of changes in gut microbiota on liver health. The relationship between observations in animal models and human patients is essential. However, animal models can replicate specific features of human disease, they also possess limitations that need to be recognized.
Animal models are essential for comprehending the pathophysiology of diseases such as MASLD/MASH. The classification of these models includes induced and spontaneous types. Induced models arise from targeted interventions, whereas spontaneous models develop naturally within specific strains (30, 31). For instance, rodents are often utilized because of their genetic and physiological similarities to humans, enabling the observation of the effects of gut microbiota changes on liver health (32). Studies show that changes in gut microbiota can result in heightened intestinal permeability, allowing LPS to enter the bloodstream, which initiates inflammation and plays a role in liver damage (33). This mechanism has been documented in numerous animal studies, underscoring the promise of microbiota-targeted therapies for addressing MASLD/MASH.
Nonetheless, although these animal models offer significant insights, it is crucial to acknowledge their constraints. Variations in metabolic processes, anatomical structures, and the progression of diseases between animals and humans can influence how findings can be applied across species. For instance, specific therapeutic interventions that show efficacy in animal models might not produce comparable outcomes in human clinical trials because of these discrepancies (31, 32). A thorough assessment of how animal model findings relate to human conditions is essential for progressing therapeutic approaches. The subsequent sections will explore the use of various animal models in gut microbiome research, as outlined in Tables 1A, B.
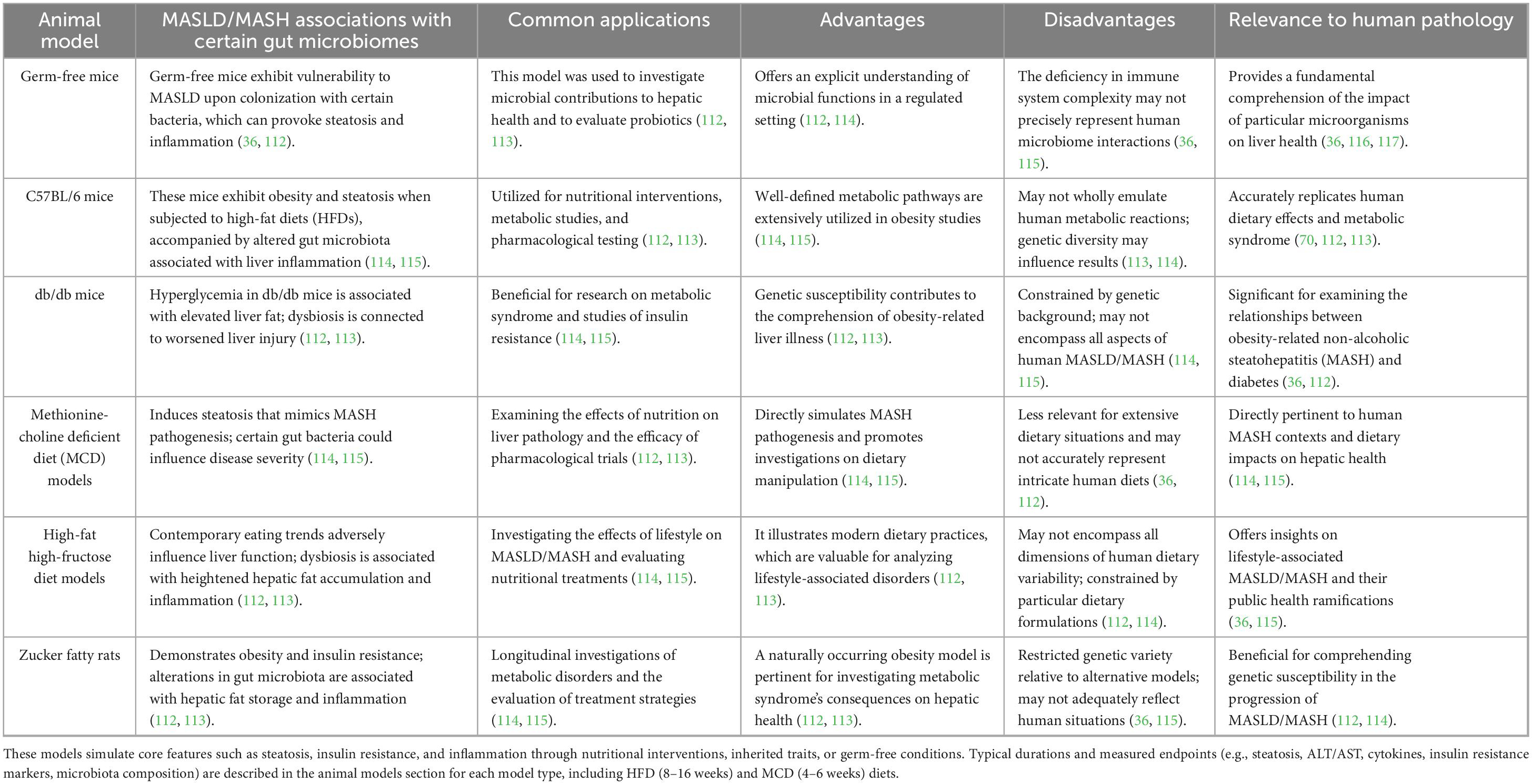
Table 1A. Dietary, genetic, and gnotobiotic models of MASLD/MASH: commonly used dietary, genetic, and gnotobiotic animal models in MASLD and MASH research.

Table 1B. Translational and pharmacological models of MASLD/MASH: commonly used translational and pharmacological animal models, including Sprague-Dawley rats and non-human primates, that offer physiological relevance and experimental flexibility for simulating MASLD/MASH features and evaluating interventions.
Studies on germ-free (GF) mice
Germ-free mice have played a crucial role in clarifying the influence of gut microbiota on liver pathology related to MASLD/MASH. GF mice that are colonized with microbiota from patients with obesity or MASLD show a greater accumulation of hepatic fat and inflammation when compared to those that remain uncolonized. These finidings indicate the microbial communities can directly impact liver disease. For example, specific strains of Firmicutes or Bacteroidetes have demonstrated an ability to worsen steatosis and inflammation in these models (34–36). Moreover, fecal microbial transplantation (FMT) studies have validated this connection. GF mice that were given microbiota from MASH patients show hepatic steatosis and inflammatory changes, underscoring the causal role of gut microbiota in the development of MASLD (35–37). The results indicate that changes in gut microbiota composition are not just linked to MASLD/MASH but could play a significant role in its progression (Tables 1A, B).
High-fat diet (HFD) models
High-fat diet models are extensively utilized to investigate MASLD/MASH because they effectively replicate the metabolic characteristics of the disease. Providing rodents with diets rich in fat (usually comprising 45%–75% of total caloric intake) results in notable alterations in gut microbiota composition, which is associated with elevated hepatic lipids and insulin resistance. For instance, C57BL/6 mice subjected to high-fat diets exhibit obesity and demonstrate increased levels of pro-inflammatory cytokines like TNF-α and interleukin-6 (IL-6), which are associated with liver inflammation and damage (35, 37). The dysbiosis caused by high-fat diets is marked by a reduction in beneficial bacteria such as Lactobacillus and a rise in pathogenic bacteria like Escherichia coli. This change plays a role in metabolic endotoxemia and liver inflammation, strengthening the link between gut microbiota and the advancement of liver disease (34, 36). HFD models typically require 8–16 weeks of dietary intervention to induce hepatic steatosis, inflammation, and early fibrosis. Common readouts include histological grading of steatosis, serum liver enzymes (ALT, AST), inflammatory cytokines (e.g., TNF-α, IL-6), insulin resistance indices, and gut microbiota profiling via 16S rRNA sequencing or metagenomics. Research shows that microbial signatures linked to high-fat diets can increase intestinal permeability, facilitating bacterial translocation that worsens liver inflammation (Tables 1A, B) (37, 38).
Models of methionine-choline deficient diet (MCD)
Methionine-choline deficient diet is a prominent model for the swift induction of MASH in rodent studies. Mice on this diet exhibit notable hepatic inflammation and fibrosis within weeks, primarily attributed to disrupted lipid metabolism caused by a lack of choline. Research indicates that MCD feeding results in significant changes in gut microbiota composition, significantly an increase in Proteobacteria, which correlates with inflammatory responses and worsens liver pathology (39, 40). The MCD model shows a notable rise in pro-inflammatory cytokines like IL-1β and TNF-α in the intestines, suggesting a connection between gut inflammation and the advancement of liver disease (40, 41). MCD diet models generally run for 4–6 weeks and produce rapid-onset fibrosis and inflammation, with outcomes including liver histopathology, metabolic profiles, and microbial community composition. These duration ranges and endpoints are representative of standard MASLD/MASH experimental protocols. The swift emergence of MASH-like characteristics facilitates the investigation of early treatment options and underscores the influence of gut microbiota on liver inflammation modulation (Tables 1A, B).
Genetic models
Genetically modified models, such as the db/db and ob/ob mice, serve as essential tools for elucidating the progression of MASLD and MASH, given their inherent metabolic disturbances including obesity and insulin resistance. When subjected to dietary challenges, such as high-fat or high-fructose regimens, these models exhibit exacerbated hepatic pathology alongside pronounced alterations in gut microbiota composition. For example, ob/ob mice exposed to a high-fructose diet develop marked hepatic steatosis, which is paralleled by shifts in gut microbial populations that promote inflammatory pathways (42). These findings underscore the synergistic relationship between genetic susceptibility and environmental influences in shaping the gut-liver axis, ultimately contributing to liver disease progression (Tables 1A, B).
Combination models
Models that integrate genetic predisposition with dietary interventions provide a more comprehensive framework for studying MASLD/MASH pathogenesis. The KK-Ay mouse model, which possesses a genetic inclination toward obesity and type 2 diabetes, demonstrates severe hepatic injury and microbiota dysbiosis when challenged with a high-fat diet (39). Notably, these combination models more accurately replicate the multifactorial nature of human disease, revealing intricate interactions between gut microbial dysbiosis, compromised intestinal barrier function, and systemic inflammation (41). Such models provide valuable insights into the complex metabolic networks and inflammatory pathways that underlie disease progression, thereby highlighting potential therapeutic targets (Tables 1A, B).
The included animal models reflect various mechanisms by which gut microbiome alterations can influence hepatic steatosis, inflammation, and fibrosis in MASLD and MASH. A detailed comparison is provided in (Tables 1A, B). Typical durations for these models range from 4 to 6 weeks in MCD and germ-free studies to 8–16 weeks in HFD or genetic models. Across studies, key parameters measured include liver steatosis (via histology), serum liver enzymes (ALT/AST), pro-inflammatory cytokines (e.g., TNF-α, IL-6), insulin resistance markers, and gut microbial composition. By clarifying key pathophysiological mechanisms, they not only enhance our understanding of the gut-liver axis but also serve as indispensable platforms for the identification and validation of novel therapeutic strategies. Continued research leveraging these models will be vital for advancing translational efforts aimed at mitigating the global burden of liver disease.
While rodent models such as high-fat diet (HFD)-fed mice have been instrumental in uncovering microbiome–liver interactions, they do not fully recapitulate the complexity of human MASLD/MASH pathophysiology. Differences in diet, bile acid metabolism, immune responses, and microbial ecosystems between mice and humans pose translational challenges. To bridge this gap, future studies may benefit from incorporating humanized mouse models–such as germ-free mice colonized with human microbiota–as well as integrating multi-omics approaches (e.g., metagenomics, metabolomics, transcriptomics) to capture host–microbiome interactions more holistically. These strategies can improve the relevance of preclinical findings and enhance their applicability to human MASLD progression and treatment.
Human studies
Altered microbial taxa in MASLD and MASH
Recent studies have thoroughly examined the connection between gut microbiota and MASLD. Rau et al. identified distinct gut microbial profiles in individuals with MASH, characterized by an abundance of SCFA-producing bacteria like Fusobacterium and Prevotella. Elevated concentrations of fecal short-chain fatty acids, especially acetate and propionate, correlated with enhanced pro-inflammatory immune responses, marked by an increased Th17/rTreg cell ratio. The results underscore the importance of microbial metabolites in managing low-grade inflammation, which contributes to liver inflammation and fibrosis, thus positioning the gut-liver axis as a crucial factor in the advancement of MASLD (43).
Recent studies have explored the connection between gut microbiota and MASLD. Rau et al. (43) identified distinct microbial profiles in individuals with NASH, marked by an abundance of SCFA-producing bacteria such as Fusobacterium and Prevotella. Elevated levels of short-chain fatty acids, particularly acetate and propionate, were linked to increased pro-inflammatory immune responses, including a higher Th17/rTreg cell ratio. These findings highlight the role of microbial metabolites in driving low-grade inflammation, which contributes to liver inflammation and fibrosis, positioning the gut-liver axis as a key factor in the progression of MASLD (43).
Similarly, Iino et al. (44) demonstrated that patients with MASLD exhibited diminished levels of Faecalibacterium, a beneficial bacteria known for producing SCFAs. This observation implies that a decrease in anti-inflammatory SCFAs such as butyrate could potentially facilitate the progression of the disease. The findings indicate that focusing on gut microbiota via dietary or probiotic interventions could serve as a viable management approach for MASLD (44). Astbury et al. provided insights into microbial diversity, revealing that patients with MASH, especially those with cirrhosis, showed a notable decrease in gut microbial diversity. The rise in Collinsella abundance was associated with higher triglycerides and cholesterol levels, whereas a reduction in Ruminococcaceae could potentially worsen the inflammatory conditions in MASH (45).
Boursier et al. further investigated the association between gut dysbiosis and M severity, revealing that the abundance of Bacteroides correlated with advanced fibrosis (F ≥ 2). At the same time, levels of Prevotella and Ruminococcus varied with disease stages. The observed microbial alterations were associated with metabolic pathways involving carbohydrates, lipids, and amino acids, indicating their possible role as biomarkers for disease severity and as targets for therapy (46).
Lanthier et al. conducted a study utilizing 16S rRNA sequencing, revealing a decreased abundance of Clostridium sensu stricto in patients with fibrosis, alongside an increase in Enterobacteriaceae and Escherichia/Shigella. The observed changes in the microbiome correlated with liver stiffness and muscle dysfunction, suggesting possible avenues for therapeutic intervention (47). Alferink et al. noted a decrease in microbial diversity among patients with MASLD who displayed steatosis, highlighting a relationship between specific bacterial genera, such as Coprococcus and Ruminococcus gauvreaui, and alterations in metabolic pathways, especially regarding bile acid synthesis and biotin metabolism. The results demonstrate that the variety and functionality of gut microbiota are essential in developing MASLD (48).
In a similar vein, Loomba et al. utilized whole-genome sequencing to identify microbial signatures that distinguish advanced fibrosis from less severe stages of MASLD, underscoring the potential of microbiome-based diagnostics for early disease detection (49). Oh et al. demonstrated the effectiveness of microbial and metabolomic signatures as diagnostic tools for cirrhosis, achieving high accuracy when integrated with clinical markers such as serum albumin and aspartate aminotransferase (AST) levels (50). Furthermore, Da Silva et al. emphasized changes in the gut microbiome, noting a decrease in Ruminococcus and Faecalibacterium prausnitzii, which occurred independently of obesity and insulin resistance. The observed changes were associated with modified metabolite profiles, including increased levels of propionate and 2-hydroxybutyrate, which further suggest a role for gut dysbiosis in the progression of MASLD and MASH (51).
Hoyles et al. established a connection between the enrichment of Proteobacteria and a decrease in microbial diversity, which is associated with hepatic inflammation and changes in lipid metabolism. Interventions like fecal microbiota transplants have shown promise in reducing steatosis and associated metabolic disturbances (52). Behary et al. investigated dysbiosis in MASLD-cirrhosis and its advancement to HCC, demonstrating that immune modulation induced by gut microbiota promotes T-cell suppression, which plays a role in disease progression (53). Caussy et al. identified a collection of microbial traits that distinguish MASLD-cirrhosis from non-cirrhosis, showcasing significant diagnostic precision and emphasizing the microbiome as a non-invasive biomarker for liver disease (54).
Ponziani et al. demonstrated a connection between gut dysbiosis and elevated inflammatory markers, alongside changes in bacterial composition, such as an increase in Bacteroides and a decrease in Akkermansia, in patients with MASLD-related HCC (55). Yang et al. noted a reduction of microbial diversity along with notable alterations in gut microbiota and metabolites, especially lipid molecules, in the context of metabolic-associated steatosis liver disease (MASLD). The results underscore possible pathways for diagnosis and treatment (56). León-Mimila et al. illustrated connections between metabolites derived from the microbiome, such as TMAO, and MASLD, especially among diabetic patients, highlighting the interaction of metabolic and microbiome elements in the advancement of the disease (57). Subsequent investigations, such as those conducted by Chen et al. and Nimer et al., explored bile acid metabolism in MASLD and MASH, revealing modified profiles and their links to fibrosis, inflammation, and genetic predispositions (58, 59). Lastly, Puri et al. and Lee et al. provided insights into the alterations in primary and secondary bile acids, alongside variations in microbial diversity associated with the severity of fibrosis, presenting further biomarkers for disease progression (60). The expanding collection of evidence underscores the complex interactions among the gut microbiome, its metabolites, and the development of MASLD/MASH, presenting new possibilities for diagnostics and treatment strategies.
Recent tissue-specific microbiome analyses have expanded our understanding of MASLD pathogenesis beyond stool-based data. In a 2024 metagenomic study of liver and adipose samples from MASLD patients, taxa such as Enterococcus, Granulicatella, and members of the Morganellaceae family were enriched in hepatic tissue and correlated with pro-inflammatory gene signatures, histological steatohepatitis, and fibrosis severity (61). These findings support a growing model where tissue-resident microbiota may contribute directly to liver inflammation and immune modulation in MASLD (Figure 3). In a recent large-scale meta-analysis, Nychas et al. (62) identified a robust set of gut microbial signatures for NAFLD across multiple international cohorts using harmonized metagenomic datasets. Taxa such as Acinetobacter, Faecalibacterium, and Enterococcus were consistently associated with NAFLD severity and fibrosis progression. These reproducible microbial markers support the clinical utility of gut microbiome profiling for MASLD stratification and diagnosis (62).
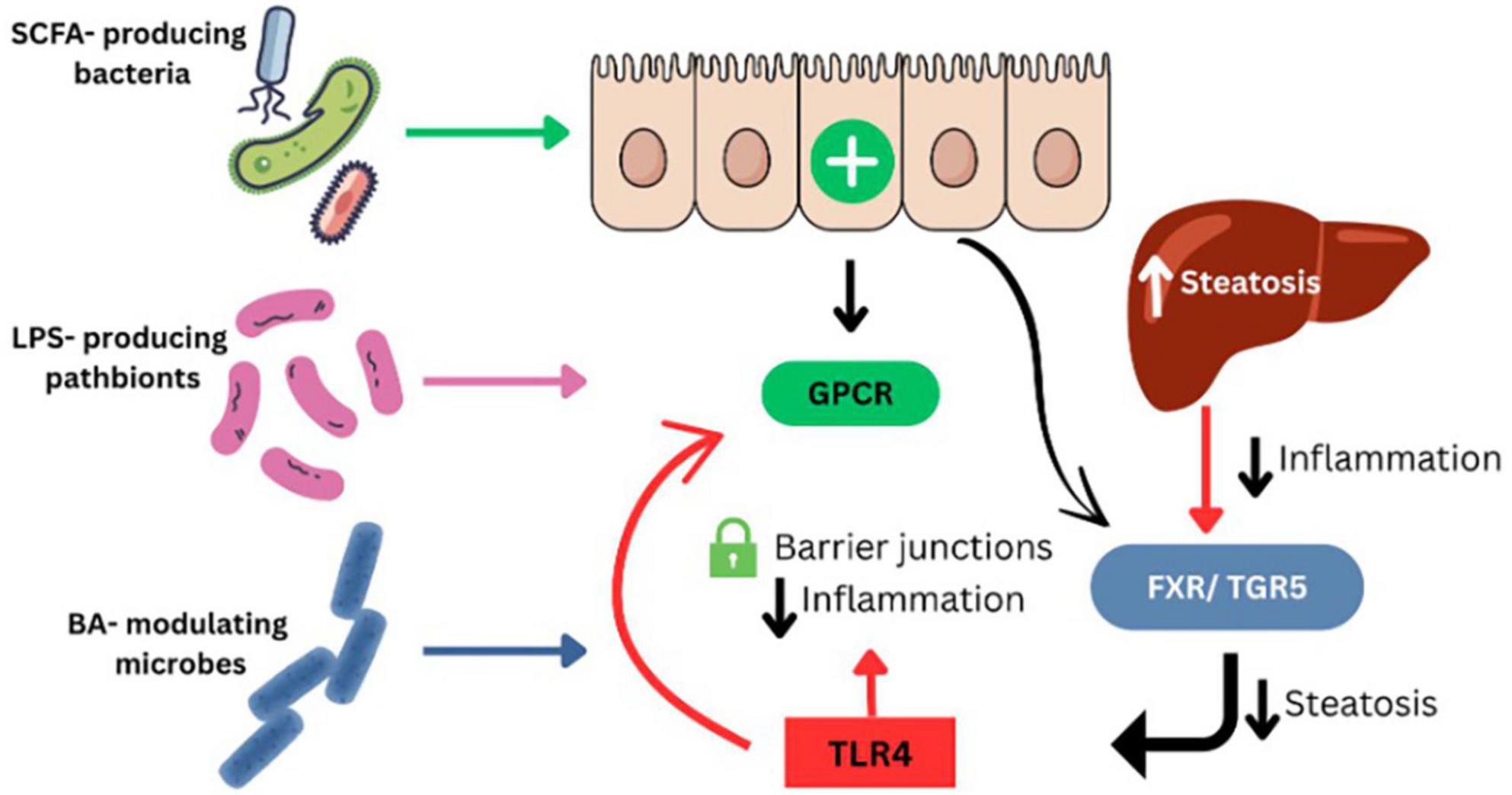
Figure 3. Gut microbiome-derived mechanisms contributing to MASLD pathogenesis. SCFA-producing bacteria enhance intestinal barrier integrity and reduce inflammation via activation of GPCR signaling. LPS-producing pathobionts increase gut permeability and trigger hepatic inflammation through TLR4. Bile acid–modulating microbes influence hepatic metabolism and inflammation through FXR and TGR5 signaling pathways. Disruption of these microbial functions promotes steatosis, immune dysregulation, and liver injury in MASLD.
Microbiome-derived metabolites and host pathophysiology
Microbial metabolites such as SCFAs and bile acids play key roles in host metabolism, gut barrier integrity, and hepatic inflammation (Figure 3). Because these mechanisms are more thoroughly characterized in human studies, we refer the reader to the Section “Human Studies” for a detailed explanation of their roles in MASLD and MASH (Table 2).
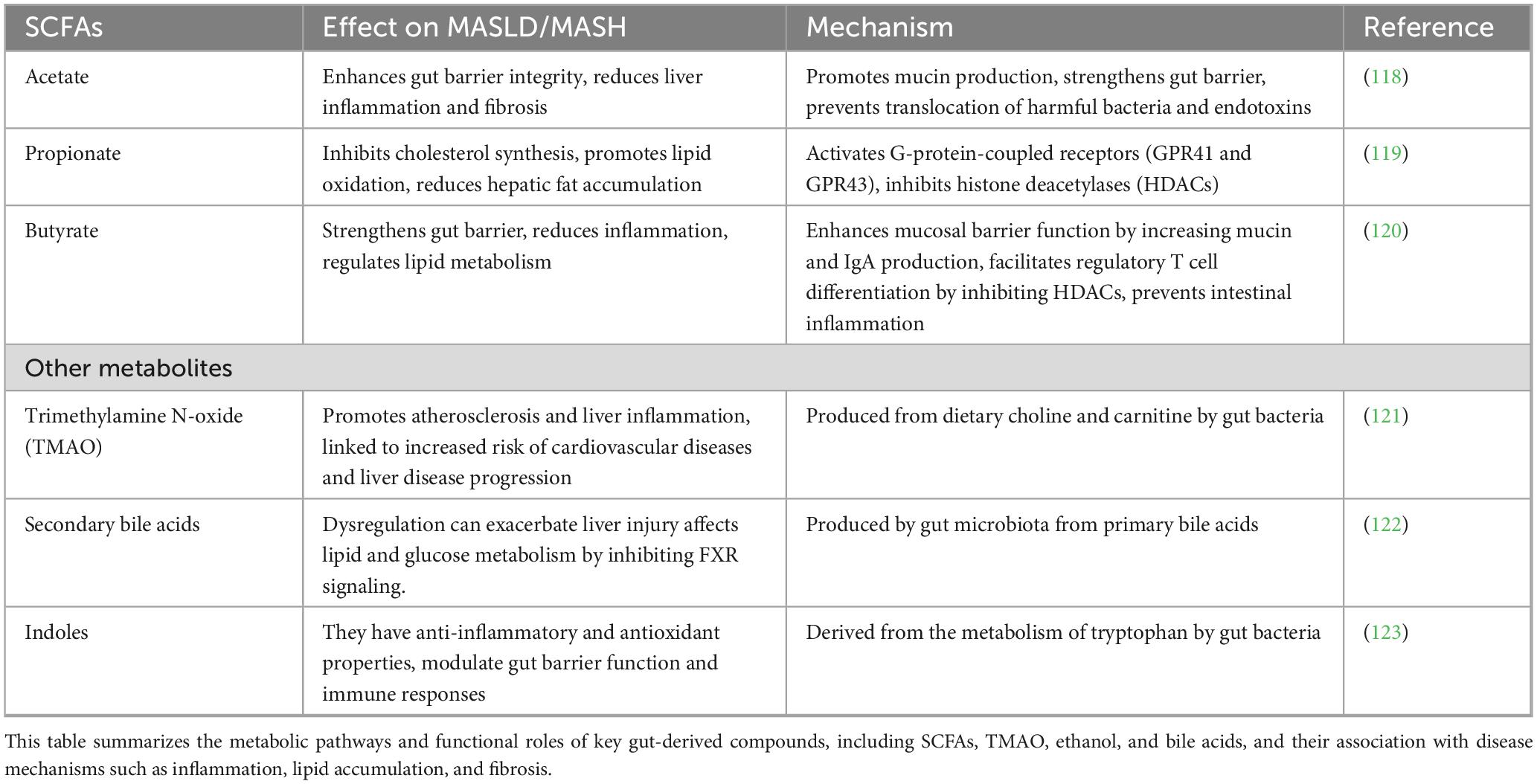
Table 2. Role of short-chain fatty acids (SCFAs) and other gut microbial metabolites in MASLD/MASH pathogenesis.
The majority of human studies included in this review were cross-sectional, limiting the ability to assess temporal shifts in gut microbial composition or determine causality. While these studies provide valuable associations between microbiome profiles and MASLD/MASH severity, they do not allow for longitudinal tracking of microbiome changes during disease progression or treatment response. This reflects a methodological gap that constrains interpretation of dynamic microbiome–host interactions in MASLD.
A significant limitation in the current MASLD microbiome literature is the lack of longitudinal human studies. Most data are derived from cross-sectional cohorts, which provide only a snapshot in time and cannot establish whether gut microbiome alterations are causes, consequences, or correlates of disease. This design limitation hampers our understanding of how microbial patterns evolve from early steatosis to advanced fibrosis or MASH, and how they respond to interventions such as dietary modification, weight loss, or probiotic supplementation. Without prospective follow-up, it is difficult to identify predictive microbial markers or determine the durability of microbiome modulation. Future research should prioritize longitudinal and interventional studies to address these gaps and enable microbiome-based stratification and therapy in MASLD.
Discussion
Innovative techniques in gut microbiome analysis
Multiple complementary approaches have been developed to study the gut microbiome in MASLD/MASH, ranging from culture-independent high-throughput sequencing to culture-based innovations. These include targeted sequencing (e.g., 16S rRNA, 18S rRNA, ITS), untargeted methods such as shotgun metagenomics and metagenomic next-generation sequencing (mNGS), and complementary omics such as transcriptomics, proteomics, metabolomics, and culturomics. Each technique varies in resolution, functional insights, and limitations, and can be combined to generate a holistic understanding of microbial communities and their interactions with the host. Table 3 summarizes the key methodologies, their primary applications, advantages, and limitations in the context of MASLD research.
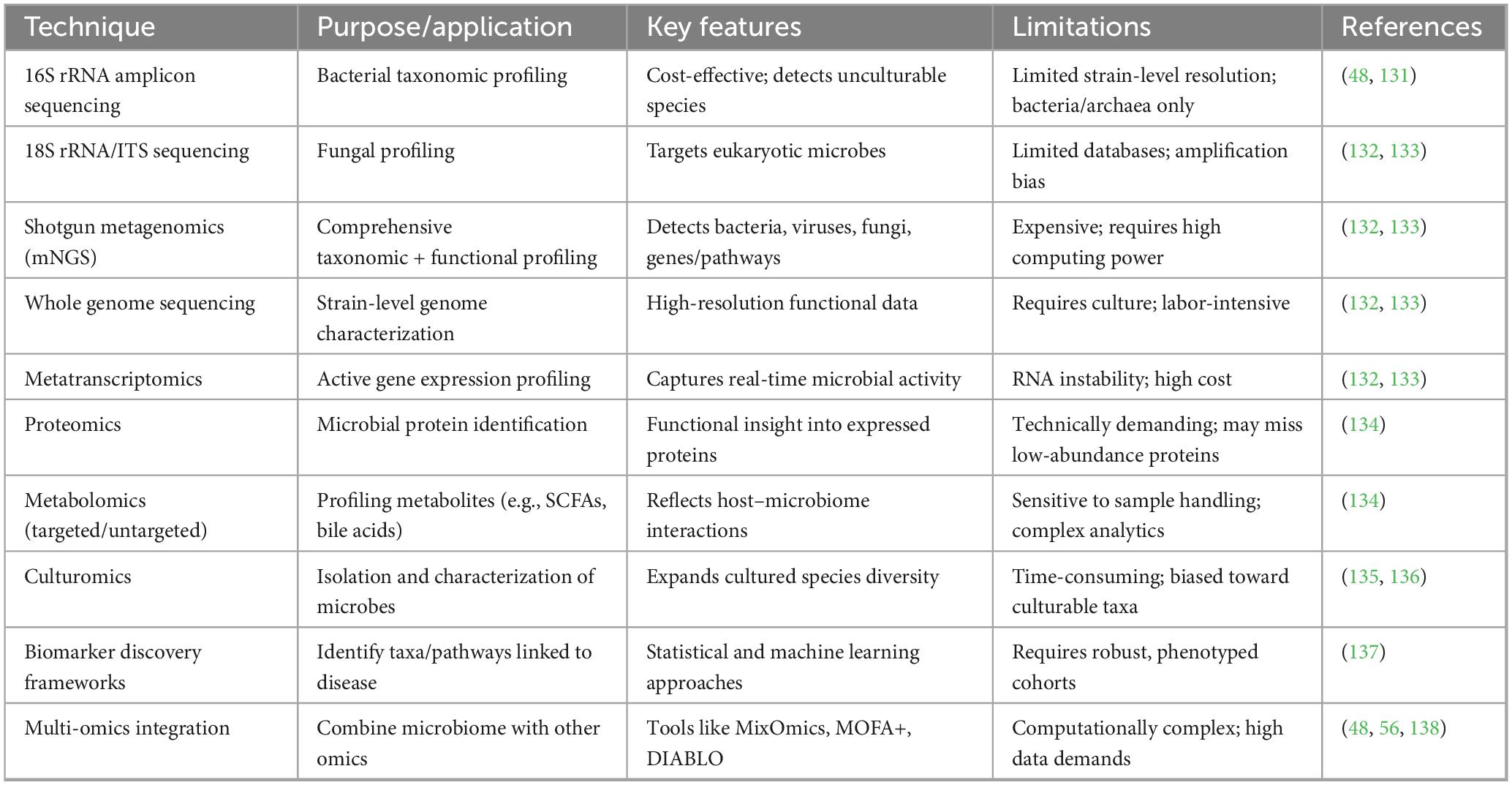
Table 3. Innovative techniques for gut microbiome analysis in MASLD/MASH research, including their primary applications, key methdological features and limitations.
Microbial taxa correlated with MASLD and MASH: a tabular analysis
The intricate relationship between the gut microbiome and the liver in MASLD and MASH encompasses several microbial taxa and their corresponding metabolites. Recent advancements in sequencing technologies have enabled more thorough investigations of the gut metagenome, yielding fresh insights into the specific microbial species and roles implicated in liver disorders (63). One of the most regularly observed modifications in the gut microbiome occurs in patients with MASLD and MASH. A substantial alteration is the elevated ratio of Firmicutes to Bacteroidetes, associated with improved energy extraction from food and changes in bile acid metabolism, both of which are contributing factors to the development of fatty liver disease (64, 65).
Disease stage-specific microbiome signatures
Certain genera within the Firmicutes phylum have been linked to MASLD and MASH. Ruminococcus is frequently present in greater quantities in MASLD patients, corresponding with increased fibrosis severity. Ruminococcus species generate acetate, a substrate that can enhance hepatic lipogenesis, consequently promoting fat storage in the liver (34, 37). Certain genera within the Firmicutes phylum have been linked to MASLD and MASH. Ruminococcus is frequently present in greater quantities in MASLD patients, corresponding with increased fibrosis severity. Ruminococcus species generate acetate, a substrate that can enhance hepatic lipogenesis, consequently promoting fat storage in the liver (34, 37). In contrast, specific advantageous bacterial taxa are reduced in patients with MASLD and MASH.
The role of Faecalibacterium prausnitzii in MASLD and MASH–particularly its depletion and involvement in SCFA-mediated anti-inflammatory pathways–is summarized in Table 4. The Proteobacteria phylum, particularly the Enterobacteriaceae family, has been identified as enriched in individuals with MASLD and MASH. These gram-negative bacteria are distinguished by their synthesis of LPS, which can trigger inflammatory responses that worsen liver disease. An elevated presence of Escherichia coli, a constituent of the Enterobacteriaceae family, is associated with higher liver inflammation and fibrosis severity in patients with MASH (66, 67). This indicates that dysbiosis within this bacterial group may be crucial in the pathogenesis of liver disorders. A notable group is the Bacteroidetes phylum, especially the genus Bacteroides. Although the total quantity of Bacteroidetes is frequently diminished in MASLD patients, specific species, such as Bacteroides vulgatus, have shown a favorable correlation with the severity of MASLD. This link may arise from its activity fostering intestinal inflammation and impairing barrier integrity, potentially resulting in heightened translocation of bacteria and their byproducts into the bloodstream, exacerbating hepatic inflammation (68, 69). Interstingly, while several studies indicate a reduction in Bacteroidetes among MASLD patients, others suggest that particular species may proliferate as the severity of the disease intensifies (36). Conversely, individuals with MASLD and MASH typically exhibit a reduction in members of the Actinobacteria phylum, especially the species Bifidobacterium. Bifidobacterium species are often seen as advantageous due to their capacity to generate short-chain fatty acids such as acetate and lactate, which can then be transformed into butyrate by other intestinal bacteria. Butyrate is recognized for its preventive properties regarding intestinal health and may assist in alleviating the progression of liver disease (67, 69). Particular microbial taxa have been associated with specific phases of MASLD development. Lactobacillus species are typically more prevalent in patients with mild steatosis, but genera like Oscillospira and Dorea are enriched in individuals with MASH. This indicates that distinct bacterial populations may assume specific roles during the different stages of liver disease (43, 70). The association between gut microbial taxa and MASLD and MASH is intricate and encompasses numerous metabolites that affect liver function and disease advancement. Specific bacteria in the gut microbiota can generate ethanol via fermentation, leading to oxidative stress and hepatic damage. The presence of Desulfovibrio piger, a hydrogen sulfide-producing species, is higher in MASH patients, potentially worsening intestinal inflammation and impairing intestinal barrier function (71–73).

Table 4. Microbial taxa associated with MASLD and MASH: correlations, proposed mechanisms, and functional roles.
The intricate relationships among specific microbial taxa, their metabolites, and host factors play a significant role in the pathophysiology of liver disorders (16, 38). Comprehending the intricate connections between the gut microbiota and the pathogenesis of MASLD/MASH presents prospects for the development of innovative therapeutic strategies aimed at the gut microbiome for the prevention and treatment of these hepatic disorders (64, 74). Potential solutions encompass the utilization of probiotics, prebiotics, and other microbiome-modulating therapies to reestablish a healthy gut microbial equilibrium and enhance liver health (16, 74).
Probiotics, prebiotics, and synbiotics in MASLD/MASH management: insights from current studies
Probiotics enhance gut microbiota composition, reduce inflammatory cytokines, and stabilize mucosal immune function in MASLD patients. The therapeutic effects of these interventions are partly mediated through SCFA production, gut barrier support, and inflammation regulation, as detailed in the Section “Human Studies” and Table 5.
Prebiotics improve metabolic health by enhancing the growth of beneficial microbes such as Bifidobacterium and Lactobacillus. Their benefits are similarly linked to SCFA-mediated effects and improved microbial balance, which support liver health as summarized earlier.
Synbiotics combine probiotics and prebiotics to synergistically modulate the gut microbiome. These synergistic effects contribute to gut–liver axis improvement and metabolic regulation, supported by the microbial mechanisms detailed in Table 5.
Fecal microbiota transplantation (FMT) for MASLD and MASH: assessing its therapeutic potential and constraints
Fecal microbiota transplantation (FMT) has emerged as a novel therapeutic approach for gastrointestinal and metabolic disorders, including MASLD and MASH. FMT involves the transfer of fecal material from a healthy donor to a recipient, intending to restore a balanced gut microbiome (75). This procedure has gained attention due to its potential to improve metabolic health by addressing dysbiosis–a common feature in individuals with MASLD and MASH. Numerous studies have demonstrated the efficacy of FMT in patients with liver diseases, particularly in restoring gut microbiota diversity and improving liver function. A groundbreaking study conducted in Saudi Arabia highlighted the potential benefits of FMT for patients with MASH (76). In this clinical trial, patients who received FMT exhibited significantly improved liver function tests, metabolic profiles, and overall gut health compared to the control group. Similar findings have been observed globally. For instance, a systematic review by Gu X et al. (77) analyzed multiple clinical trials and reported that FMT resulted in significant reductions in liver enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), indicating improved liver function. Furthermore, improvements in insulin sensitivity and reductions in body weight were also noted, suggesting that FMT may exert systemic metabolic benefits.
The mechanisms through which FMT exerts its therapeutic effects on liver diseases are multifaceted. One primary mechanism is the restoration of microbial diversity. Individuals with MASLD often exhibit reduced microbial diversity and altered gut microbiota composition, characterized by an overrepresentation of pathogenic bacteria and a deficiency of beneficial species (78). FMT has been shown to restore this diversity, leading to the re-establishment of a healthy gut microbiome. Moreover, FMT enhances gut barrier function, critical in preventing the translocation of harmful bacteria and their byproducts into the bloodstream. Dysbiosis can lead to increased intestinal permeability, allowing bacterial endotoxins, such as LPS, to enter circulation and trigger systemic inflammation (79, 80). By restoring healthy microbiota, FMT can improve gut barrier integrity, reducing the risk of inflammation and liver injury. Additionally, FMT has been shown to modulate immune responses in the gut and liver. A study by (81) reported that FMT could significantly reduce inflammatory markers in patients with MASH. This immunomodulatory effect is crucial in mitigating the chronic inflammation associated with MASLD and MASH, ultimately leading to improved liver health.
Despite the promising results associated with FMT, several challenges and limitations must be considered. One significant concern is the variability in donor microbiota. The composition of the donor microbiome can significantly influence the efficacy of FMT. For example, differences in dietary habits, lifestyle factors, and genetics can lead to variations in microbial composition between donors, potentially affecting treatment outcomes (82). Moreover, the risk of transmission of infectious agents during FMT is a critical concern. While rigorous screening protocols for donors are implemented to minimize this risk, there remains a potential for transmission of pathogens that may not be detected during screening (83). Reports of adverse events following FMT, such as infections and gastrointestinal complications, emphasize the need for careful donor selection and monitoring of recipients. Another limitation of FMT is the lack of standardization in protocols. Variations in the fecal processing, preparation, and administration method can lead to inconsistent results across studies. A consensus on best practices for FMT is essential for establishing its clinical efficacy and safety (84). Despite these challenges, ongoing research continues to explore the potential of FMT in managing MASLD and MASH. Future studies should focus on identifying optimal donor characteristics and developing standardized protocols to enhance the safety and efficacy of FMT. Additionally, investigating the long-term effects of FMT on liver health and metabolic outcomes is crucial to understanding its role in managing MASLD/MASH.
Furthermore, the exploration of alternative microbiome-based therapies, such as targeted probiotics or microbial consortia, may provide safer and more controlled options for modulating the gut microbiome without the risks associated with FMT. A recent study by (85) demonstrated the potential of specific probiotic formulations to mimic the beneficial effects of FMT, suggesting that these therapies may serve as viable alternatives.
The application of FMT in managing MASLD and MASH is not confined to any specific region. Studies from Asia, Europe, and North America have explored its efficacy, highlighting the importance of diverse microbiomes across populations. For example, a study in Japan demonstrated significant improvements in liver function and metabolic parameters in patients with MASH following FMT (64).
In conclusion, FMT represents a promising therapeutic strategy for managing MASLD and MASH, with evidence supporting its efficacy in improving liver function and metabolic health. However, challenges related to donor variability, safety concerns, and the need for standardized protocols must be addressed. Ongoing research and clinical trials will be essential in uncovering the full potential of FMT and alternative microbiome-based therapies in managing these increasingly prevalent liver diseases.
Dietary interventions and gut microbiome dynamics: implications for MASLD and MASH therapy
Dietary interventions play a pivotal role in managing MASLD and MASH as they significantly influence the composition and function of the gut microbiome. The gut microbiota, comprising trillions of microorganisms, is crucial for maintaining metabolic health and homeostasis. Dietary patterns, particularly those rich in fiber, healthy fats, and low in refined sugars, can profoundly affect gut microbial diversity and functionality, leading to improved liver health outcomes (86). Research has shown that the gut microbiome can influence liver health through several mechanisms. These effects are partly mediated through microbial metabolites such as SCFAs, which are discussed in Table 2.
Recent studies have further emphasized the complex relationship between dietary factors and the gut microbiome in the development and management of MASLD and MASH. Dietary patterns rich in fiber and polyphenols have been shown to enhance the growth of beneficial microbial taxa such as Akkermansia muciniphila and Faecalibacterium prausnitzii, which are inversely associated with hepatic fat accumulation and systemic inflammation (36). These dietary-induced microbial shifts lead to increased production of SCFAs and improved gut barrier integrity, thereby reducing endotoxemia and liver inflammation. Adherence to a green-Mediterranean diet, characterized by high intake of plant-based foods and limited animal proteins, has been linked to favorable changes in gut microbiota composition and significant reductions in intrahepatic fat, independent of weight loss (87). Furthermore, gut dysbiosis has been implicated in the pathogenesis of MASLD/MASH through mechanisms involving increased intestinal permeability and translocation of microbial-derived endotoxins, which activate inflammatory pathways in the liver (37). These findings highlight the crucial role of the gut-liver axis and underscore the importance of dietary strategies that restore microbial balance to support liver health in MASLD/MASH patients.
Emerging therapeutic strategies for metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) increasingly center on incretin-based agents, particularly dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists, such as tirzepatide, and GLP-1/glucagon receptor co-agonists like survodutide. These agents have garnered attention due to their potential to target the metabolic dysfunctions that underpin the pathogenesis of MASLD/MASH (88).
Tirzepatide, a novel dual GIP and GLP-1 receptor agonist, has shown promising efficacy in modulating biomarkers associated with non-alcoholic steatohepatitis (NASH) among individuals with type 2 diabetes. In a 26-week randomized, placebo-controlled trial, tirzepatide significantly reduced serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), cytokeratin-18 (K-18), and procollagen type III (Pro-C3), while concurrently increasing levels of adiponectin, a marker of insulin sensitivity and anti-inflammatory activity. The reductions in K-18 and Pro-C3 were both dose-dependent and statistically significant, suggesting that tirzepatide may attenuate hepatic injury and fibrogenesis. These findings support the therapeutic potential of tirzepatide in the management of MASLD/MASH through metabolic and anti-inflammatory mechanisms (88).
Survodutide, a novel GLP-1/glucagon receptor co-agonist, is currently undergoing clinical evaluation for its therapeutic potential in metabolic dysfunction-associated steatohepatitis (MASH). Interim results from a phase 2 randomized clinical trial have demonstrated that survodutide significantly reduces hepatic steatosis, body weight, and markers of hepatic fibrosis, while also improving metabolic parameters. The agent exhibited a safety profile comparable to other incretin-based therapies, with good overall tolerability. These preliminary findings underscore survodutide’s potential as a promising pharmacologic candidate for the treatment of MASH, particularly in individuals with concomitant obesity or features of metabolic syndrome, where metabolic modulation plays a central role in disease progression (89).
Increasing dietary fiber intake is one of the most effective strategies for improving gut health and managing MASLD/MASH. Dietary fibers are fermented in the colon, leading to the production of SCFAs, which play a crucial role in modulating inflammation and improving gut barrier function (90). A recent study found that higher fiber intake was associated with increased fecal SCFA concentrations and improved liver health in patients with MASLD (91). The intake of omega-3 and omega-6 fatty acids has been shown to impact liver health positively. Omega-3 fatty acids, found in fatty fish and certain plant oils, possess anti-inflammatory properties and have been associated with improved liver function (92). Research indicates that increasing omega-3 intake can reduce liver fat and improve insulin sensitivity in individuals with MASLD (93). A study conducted in Saudi population demonstrated that participants who consumed more omega-3-rich foods showed significant improvements in liver enzyme levels and reduced hepatic fat (94).
A systematic review highlighted that increasing antioxidant-rich foods in the diet is associated with improved liver health and reduced inflammation in MASLD patients (95). This effect may be particularly important in the context of populations in Asia and the Gulf region, where dietary habits can be tailored to include more antioxidant-rich foods. Personalized nutrition is an emerging concept that recognizes the unique dietary needs of individuals based on their genetic, environmental, and microbiome profiles. Research suggests that tailored dietary interventions can significantly enhance the management of MASLD/MASH by considering individual differences in gut microbiota composition and metabolic responses (17). A study conducted in the Gulf region demonstrated that personalized dietary recommendations based on microbiome analysis resulted in improved metabolic outcomes and liver health in patients with MASLD (96). This approach holds great promise for optimizing dietary interventions and enhancing patient adherence to dietary recommendations.
Environmental factors, gut microbiome, dietary influences, and lifestyle impacts on MASLD in the MENA region
The Middle East and North Africa (MENA) region has rapidly urbanized in recent decades, triggering significant shifts toward sedentary lifestyles, increased obesity, and higher type 2 diabetes (T2D) prevalence. The MENA region’s T2D rate of approximately 12.3% surpasses the global average of 9.3%, and predictions estimate a further increase of 25% by 2030 and 51% by 2045 due to continued urbanization and dietary shifts (97). Urban populations in Saudi Arabia, for example, show T2D prevalence rates as high as 32%, reflecting the direct impact of urban lifestyles on metabolic health (98). Obesity prevalence in Saudi adults has notably reached around 41%, directly correlating with MASLD risk, making the condition highly prevalent, estimated to affect approximately 44% of adults over 20 years (97) (Table 6).
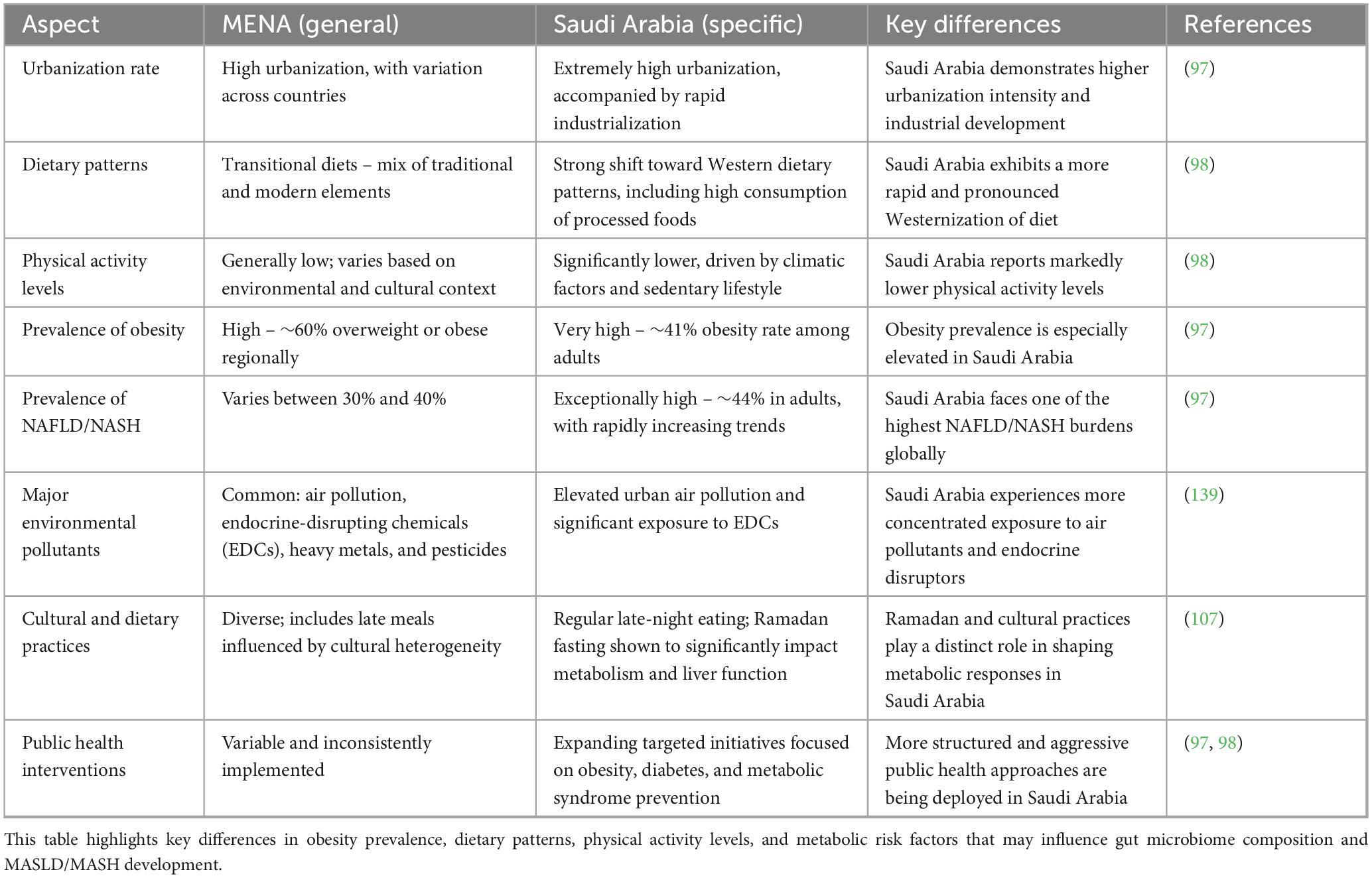
Table 6. Comparison of epidemiological and lifestyle factors between the MENA region and Saudi Arabia.
Dietary patterns and MASLD
Dietary transitions from traditional nutrient-rich diets toward calorie-dense Western-style diets have significantly influenced metabolic dysfunction and liver health in MENA. Western dietary patterns characterized by high intake of refined sugars, saturated fats, and processed foods have consistently been linked to a 56% increased risk of NAFLD (99). Fructose-rich diets, prevalent in soft drinks and sweetened beverages, particularly exacerbate hepatic steatosis by enhancing lipogenesis and insulin resistance, thus intensifying MASLD severity (99). The Mediterranean diet (MedDiet), characterized by high consumption of fruits, vegetables, whole grains, legumes, nuts, and olive oil, has been linked to numerous health benefits, including improved liver function. Studies indicate that adherence to this diet is associated with lower rates of MASLD and better metabolic profiles. Low-carbohydrate diets, particularly those that restrict refined sugars and grains, have gained popularity in recent years for their potential benefits in managing MASLD/MASH. Research indicates that reducing carbohydrate intake can significantly improve liver fat content and overall metabolic health (100). A study in the Gulf region demonstrated that a low-carbohydrate, high-fat diet resulted in improved liver function markers and reduced hepatic steatosis in patients with (101). Therefore, adherence to the (MedDiet), rich in fiber, polyphenols, and unsaturated fats, significantly improves liver function and reduces steatosis and inflammation in MASLD patients (102). Polyphenols such as resveratrol and curcumin modulate the gut microbiota positively, reducing hepatic inflammation and oxidative stress (102). A meta-analysis highlighted that MedDiet adherence correlates with improved hepatic biomarkers, reduced fibrosis, and lower NAFLD prevalence (103, 104).
Different types of dietary fiber have distinct fermentation profiles that influence SCFA production and thereby modulate MASLD progression. Inulin-type fructans tend to promote acetate and butyrate, whereas resistant starch more selectively enhances butyrate levels. Acetate, while beneficial in moderation, can serve as a substrate for hepatic lipogenesis, potentially exacerbating steatosis, whereas butyrate supports gut barrier function and reduces hepatic inflammation. Therefore, fibers that favor butyrate production–like resistant starch–may offer superior therapeutic benefit in MASLD by enhancing gut–liver axis integrity without promoting lipogenesis. These fermentation-specific effects should be considered in future nutritional intervention strategies (105).
Lifestyle and cultural factors
Physical inactivity, prevalent throughout the MENA region, strongly influences MASLD progression. More than 40% of adults in Arab countries fail to achieve recommended physical activity levels, exacerbating insulin resistance and liver fat accumulation (106). Conversely, moderate exercise consistently demonstrates significant improvements in hepatic steatosis, inflammation, and insulin sensitivity, underscoring the critical role of physical activity in MASLD management (106).
Cultural meal timing, particularly night-time heavy meals and irregular eating patterns, negatively impacts hepatic metabolism and MASLD outcomes. In contrast, intermittent fasting practices, such as Ramadan fasting, positively influence metabolic parameters, weight reduction, and hepatic health, suggesting beneficial circadian rhythm adjustments in MASLD patients (107).
Environmental chemical exposure
Environmental contaminants significantly contribute to MASLD by altering gut microbiota and hepatic metabolism. Chronic exposure to air pollution (PM2.5, NOx) correlates strongly with increased NAFLD prevalence and severity, mediated through systemic inflammation, gut barrier disruption, and endotoxemia (108). Additionally, persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs), such as pesticides, PCBs, BPA, and phthalates, directly exacerbate MASLD via gut microbiota dysbiosis, increased intestinal permeability, oxidative stress, and hepatic inflammation (109). Animal models consistently demonstrate worsened steatosis, insulin resistance, and hepatic inflammation following chronic EDC exposure, indicating significant environmental contribution to MASLD progression (109).
In Saudi Arabia, traditional diets are often high in carbohydrates and saturated fats, contributing to the rising prevalence of MASLD. However, recent shifts toward healthier dietary patterns, such as the Mediterranean diet, have shown promising results in managing liver health (101). Research indicates that adopting such diets can significantly improve liver function and metabolic parameters. Asian countries, particularly East Asian ones, have distinct dietary patterns that influence liver health. The traditional Asian diet, rich in rice, vegetables, and fish, may provide protective effects against MASLD due to its high fiber content and beneficial fatty acid profile (110). Studies from Japan have shown that adherence to traditional dietary practices is associated with lower rates of MASLD and better metabolic health outcomes. Globally, dietary interventions are being recognized as key components in the management of MASLD/MASH. The evidence supports that diets low in saturated fats and high in fruits, vegetables, and whole grains improve liver health and metabolic outcomes. Collaborative efforts across countries can enhance the understanding of dietary influences on liver diseases and facilitate the sharing of effective dietary strategies.
Overall, dietary interventions play a crucial role in modulating the gut microbiome and improving outcomes for individuals with MASLD and MASH. Patients can significantly enhance their liver health and overall well-being by adopting diets rich in fiber, healthy fats, and antioxidants. Personalized dietary strategies based on individual microbiome profiles hold great promise for optimizing treatment outcomes. Ongoing research and collaboration among countries will be essential to further elucidate the relationship between diet, gut microbiota, and liver health.
Regional comparisons and the MENA gap
To our knowledge, this is the first systematic review to synthesize both human and animal MASLD/MASH microbiome data with explicit attention to underrepresented MENA-region populations. Although global studies of MASLD/MASH consistently show reduced microbial diversity, decreased abundance of SCFA producers (e.g., Faecalibacterium, Roseburia), and enrichment of pathobionts like Escherichia, evidence from the MENA region remains sparse. The only identified study from the Arabian Peninsula focused on Arab Kuwaitis, finding Firmicutes and Bacteroidetes as dominant phyla (111). Moreover, recent efforts to expand human gut microbiome references in underrepresented populations (e.g., India, Japan, Korea) reveal significant geographic bias in existing catalogs. Cultural, dietary, and genetic factors–such as limited alcohol consumption, high refined-carbohydrate diets, and consanguinity–may shape region-specific microbiome patterns but are currently underexplored in MASLD research. These gaps highlight the urgent need for high-quality, population-specific studies in Arab and Middle Eastern populations.
Limitations
This review is subject to several limitations. First, the heterogeneity in study designs, inclusion criteria, patient demographics, sample sizes, sequencing platforms, and microbiome analysis methods (e.g., 16S vs. shotgun metagenomics) limits direct comparability across studies. Second, we included only English-language publications, which may have excluded relevant findings reported in other languages. Third, although this review aimed to assess both global and MENA-specific studies, there was a disproportionate representation from non-MENA regions, limiting the ability to perform a robust geographic stratification. The regional microbiome findings from the MENA cohort remain underpowered and should be interpreted with caution. Fourth, this review is based on a narrative synthesis rather than a quantitative meta-analysis, due to methodological variability and inconsistent outcome reporting across studies. Fifth, there was a lack of longitudinal and interventional studies, particularly those that test microbiome modulation strategies (e.g., FMT, synbiotics) in MASLD/MASH. As a result, the ability to infer causality or therapeutic relevance remains limited. Lastly, we acknowledge the absence of formal risk-of-bias assessment using validated tools such as RoB 2 or ROBINS-I, and the lack of protocol registration on platforms such as PROSPERO. While we followed PRISMA 2020 guidelines to ensure transparency, these omissions may limit the reproducibility and critical appraisal of the review.
Conclusion and future directions
This systematic review highlights the significant role of gut microbiome dysbiosis in MASLD/MASH progression. Animal and human studies consistently demonstrate associations between microbial imbalances, metabolite alterations, and liver inflammation and fibrosis. However, future research must address gaps in longitudinal data, standardization of microbiome analysis, and clinical validation of microbiome-targeted therapies. Precision microbiome interventions hold promise, but robust trials are essential for their successful clinical translation.
Advances in high-resolution microbiome analysis – including metagenomics, metabolomics, and integrated multi-omics approaches – have provided valuable insights, yet their translation to clinical practice is hampered by a lack of standardized methodologies and limited longitudinal human studies. Future research should prioritize large-scale, longitudinal cohort studies to clarify microbiome dynamics over time, interventional trials to test microbiome-targeted therapies, and mechanistic studies to establish causality. Moreover, there is a pressing need for the development of validated, non-invasive biomarkers derived from microbiome profiles to aid early diagnosis and disease staging.
Emerging therapies such as probiotics, prebiotics, synbiotics, fecal microbiota transplantation, and precision dietary interventions hold considerable promise. However, their clinical application requires rigorously designed trials with long-term follow-up to assess efficacy, safety, and sustainability. Personalized therapeutic strategies, tailored to individual genetic predispositions, environmental exposures, and microbiome compositions, represent a crucial frontier for future research.
Finally, addressing MASLD and MASH effectively demands a multidisciplinary strategy. Public health initiatives to combat obesity and metabolic syndrome must be integrated with personalized medical interventions targeting the gut-liver axis. By focusing research efforts on these clearly defined priorities, the field can accelerate the translation of microbiome science into meaningful clinical outcomes, ultimately improving patient care and reducing the growing global burden of liver disease.
Author contributions
WB: Methodology, Funding acquisition, Conceptualization, Writing – original draft, Investigation, Resources, Project administration. YB: Investigation, Writing – review & editing. MA: Writing – review & editing, Investigation, Formal analysis. GA: Writing – review & editing, Investigation. SB: Investigation, Writing – review & editing. LA: Writing – review & editing, Investigation. FS: Investigation, Methodology, Supervision, Writing – review & editing. AR: Project administration, Validation, Writing – review & editing, Methodology, Supervision, Resources, Investigation, Formal analysis, Funding acquisition. CS: Project administration, Writing – review & editing, Validation, Resources, Methodology, Investigation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by KAUST- Center of Excellence for Smart-Health (KCSH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chan K, Koh T, Tang A, Quek J, Yong J, Tay P, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10 739 607 individuals. J Clin Endocrinol Metab. (2022) 107:2691–700. doi: 10.1210/clinem/dgac321
2. Asrani S, Devarbhavi H, Eaton J, Kamath P. Burden of liver diseases in the world. J Hepatol. (2019) 70:151–71. doi: 10.1016/j.jhep.2018.09.014
3. Xue W, Zhang J, Zhu Y, Huang W. Identify functional lncRNAs in nonalcoholic fatty liver disease by constructing a ceRNA network. ACS Omega. (2022) 7:22522–30. doi: 10.1021/acsomega.2c01801
4. Dharmalingam M, Yamasandhi P. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J Endocrinol Metab. (2018) 22:421–8. doi: 10.4103/ijem.IJEM_585_17
5. Alswat K, Aljumah A, Sanai F, Abaalkhail F, Alghamdi M, Al Hamoudi W, et al. Nonalcoholic fatty liver disease burden - Saudi Arabia and United Arab Emirates, 2017-2030. Saudi J Gastroenterol. (2018) 24:211–9. doi: 10.4103/sjg.SJG_122_18
6. Riboli E, Beland F, Lachenmeier D, Marques M, Phillips D, Schernhammer E, et al. Carcinogenicity of aspartame, methyleugenol, and isoeugenol. Lancet Oncol. (2023) 24:848–50. doi: 10.1016/S1470-2045(23)00341-8
7. Sergi CM. MASLD and aspartame: are new studies in the horizon? Front Med. (2023) 10:1266918. doi: 10.3389/fmed.2023.1266918
8. Bhatt H, Smith R. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg Nutr. (2015) 4:101–8. doi: 10.3978/j.issn.2304-3881.2015.01.03
9. Padda J, Khalid K, Khedr A, Tasnim F, Al-Ewaidat O, Cooper A, et al. Non-alcoholic fatty liver disease and its association with diabetes mellitus. Cureus. (2021) 13:e17321. doi: 10.7759/cureus.17321
10. Kozlitina J, Smagris E, Stender S, Nordestgaard B, Zhou H, Tybjærg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. (2014) 46:352–6. doi: 10.1038/ng.2901
11. Mitrovic B, Gluvic Z, Obradovic M, Radunovic M, Rizzo M, Banach M, et al. Non-alcoholic fatty liver disease, metabolic syndrome, and type 2 diabetes mellitus: where do we stand today? Arch Med Sci. (2023) 19:884–94. doi: 10.5114/aoms/150639
12. Zhang Y, Wang H, Sang Y, Liu M, Wang Q, Yang H, et al. Gut microbiota in health and disease: advances and future prospects. MedComm. (2020) 5:e70012. doi: 10.1002/mco2.70012
13. Bahitham W, Alghamdi S, Omer I, Alsudais A, Hakeem I, Alghamdi A, et al. Double trouble: how microbiome dysbiosis and mitochondrial dysfunction drive non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Biomedicines. (2024) 12:550. doi: 10.3390/biomedicines12030550
14. Wesam Bahitham AA, Hakeem E, Sendi F, Boubsit A, Alkhayat E, Omer I, et al. The role of microbiome in non-alcoholic fatty liver disease (NAFLD), in microbial ecology. Boca Raton, FL: CRC Press (2023).
15. Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. (2011) 17:3377–89. doi: 10.3748/wjg.v17.i29.3377
16. Duarte S, Stefano J, Oliveira C. Microbiota and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH). Ann Hepatol. (2019) 18:416–21. doi: 10.1016/j.aohep.2019.04.006
17. Iacomino G, Rufián Henares JÁ, Lauria F. Editorial: personalized nutrition and gut microbiota: current and future directions. Front Nutr. (2024) 11:1375157. doi: 10.3389/fnut.2024.1375157
18. Mafra D, Ribeiro M, Fonseca L, Regis B, Cardozo L, Fragoso Dos Santos H, et al. Archaea from the gut microbiota of humans: could be linked to chronic diseases? Anaerobe. (2022) 77:102629. doi: 10.1016/j.anaerobe.2022.102629
19. Sasidharan Pillai S, Gagnon C, Foster C, Ashraf A. Exploring the gut microbiota: key insights into its role in obesity, metabolic syndrome, and type 2 diabetes. J Clin Endocrinol Metab. (2024) 109:2709–19. doi: 10.1210/clinem/dgae499
20. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano G, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7:14. doi: 10.3390/microorganisms7010014
21. Lozupone C, Stombaugh J, Gordon J, Jansson J, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. (2012) 489:220–30. doi: 10.1038/nature11550
22. Hawrelak J, Myers S. The causes of intestinal dysbiosis: a review. Altern Med Rev. (2004) 9:180–97.
23. Hrncir T. Gut Microbiota dysbiosis: triggers, consequences, diagnostic and therapeutic options. Microorganisms. (2022) 10:578. doi: 10.3390/microorganisms10030578
24. Sencio V, Benech N, Robil C, Deruyter L, Heumel S, Machelart A, et al. Alteration of the gut microbiota’s composition and metabolic output correlates with COVID-19-like severity in obese NASH hamsters. Gut Microbes. (2022) 14:2100200. doi: 10.1080/19490976.2022.2100200
25. Iavarone M, D’Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. (2020) 73:1063–71. doi: 10.1016/j.jhep.2020.06.001
26. Zhang J, Zhang Y, Xia Y, Sun J. Microbiome and intestinal pathophysiology in post-acute sequelae of COVID-19. Genes Dis. (2023) 11:100978. doi: 10.1016/j.gendis.2023.03.034
27. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
28. Hsu C, Schnabl B. The gut-liver axis and gut microbiota in health and liver disease. Nat Rev Microbiol. (2023) 21:719–33. doi: 10.1038/s41579-023-00904-3
29. Kim C, Lee M, Yang S, Kim K, Yong D, Kim H, et al. Human reference gut microbiome catalog including newly assembled genomes from under-represented Asian metagenomes. Genome Med. (2021) 13:134. doi: 10.1186/s13073-021-00950-7
30. Tkacs N, Thompson H. From bedside to bench and back again: research issues in animal models of human disease. Biol Res Nurs. (2006) 8:78–88. doi: 10.1177/1099800406289717
31. Khorramizadeh M, Saadat F. Animal models for human disease. Anim Biotechnol. (2020) 26:153–71. doi: 10.1016/B978-0-12-811710-1.00008-2
32. Swearengen J. Choosing the right animal model for infectious disease research. Animal Model Exp Med. (2018) 1:100–8. doi: 10.1002/ame2.12020
33. Picher-Martel V, Valdmanis P, Gould P, Julien J, Dupré N. From animal models to human disease: a genetic approach for personalized medicine in ALS. Acta Neuropathol Commun. (2016) 4:70. doi: 10.1186/s40478-016-0340-5
34. Maestri M, Santopaolo F, Pompili M, Gasbarrini A, Ponziani F. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: effects of current treatments and future strategies. Front Nutr. (2023) 10:1110536. doi: 10.3389/fnut.2023.1110536
35. Chen J, Vitetta L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int J Mol Sci. (2020) 21:5214. doi: 10.3390/ijms21155214
36. Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom A, Verheij J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. (2020) 17:279–97. doi: 10.1038/s41575-020-0269-9
37. Kolodziejczyk A, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. (2019) 11:e9302. doi: 10.15252/emmm.201809302
38. Zhu L, Baker R, Baker S. Gut microbiome and nonalcoholic fatty liver diseases. Pediatr Res. (2015) 77:245–51. doi: 10.1038/pr.2014.157
39. Li H, Toth E, Cherrington N. Asking the right questions with animal models: methionine- and choline-deficient model in predicting adverse drug reactions in human NASH. Toxicol Sci. (2018) 161:23–33. doi: 10.1093/toxsci/kfx253
40. Matthews D, Li H, Zhou J, Li Q, Glaser S, Francis H, et al. Methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis is associated with increased intestinal inflammation. Am J Pathol. (2021) 191:1743–53. doi: 10.1016/j.ajpath.2021.06.010
41. Marcolin E, Forgiarini L, Tieppo J, Dias A, Freitas L, Marroni N. Methionine- and choline-deficient diet induces hepatic changes characteristic of non-alcoholic steatohepatitis. Arq Gastroenterol. (2011) 48:72–9. doi: 10.1590/s0004-28032011000100015
42. Chiba T, Suzuki S, Sato Y, Itoh T, Umegaki K. Evaluation of methionine content in a high-fat and choline-deficient diet on body weight gain and the development of non-alcoholic steatohepatitis in mice. PLoS One. (2016) 11:e0164191. doi: 10.1371/journal.pone.0164191
43. Rau M, Rehman A, Dittrich M, Groen A, Hermanns H, Seyfried F, et al. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur Gastroenterol J. (2018) 6:1496–507. doi: 10.1177/2050640618804444
44. Iino C, Endo T, Mikami K, Hasegawa T, Kimura M, Sawada N, et al. Significant decrease in Faecalibacterium among gut microbiota in nonalcoholic fatty liver disease: a large BMI- and sex-matched population study. Hepatol Int. (2019) 13:748–56. doi: 10.1007/s12072-019-09987-8
45. Astbury S, Atallah E, Vijay A, Aithal G, Grove J, Valdes A. Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes. (2020) 11:569–80. doi: 10.1080/19490976.2019.1681861
46. Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. (2016) 63:764–75. doi: 10.1002/hep.28356
47. Lanthier N, Rodriguez J, Nachit M, Hiel S, Trefois P, Neyrinck A, et al. Microbiota analysis and transient elastography reveal new extra-hepatic components of liver steatosis and fibrosis in obese patients. Sci Rep. (2021) 11:659. doi: 10.1038/s41598-020-79718-9
48. Alferink L, Radjabzadeh D, Erler N, Vojinovic D, Medina-Gomez C, Uitterlinden A, et al. Microbiomics, metabolomics, predicted metagenomics, and hepatic steatosis in a population-based study of 1,355 adults. Hepatology. (2021) 73:968–82. doi: 10.1002/hep.31417
49. Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. (2017) 25: 1054–62.e5. doi: 10.1016/j.cmet.2017.04.001
50. Oh T, Kim S, Caussy C, Fu T, Guo J, Bassirian S, et al. A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metab. (2020) 32: 878–88.e6. doi: 10.1016/j.cmet.2020.06.005
51. Da Silva H, Teterina A, Comelli E, Taibi A, Arendt B, Fischer S, et al. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. (2018) 8:1466. doi: 10.1038/s41598-018-19753-9
52. Hoyles L, Fernández-Real J, Federici M, Serino M, Abbott J, Charpentier J, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. (2018) 24:1070–80. doi: 10.1038/s41591-018-0061-3
53. Behary J, Amorim N, Jiang X, Raposo A, Gong L, McGovern E, et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. (2021) 12:187. doi: 10.1038/s41467-020-20422-7
54. Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. (2019) 10:1406. doi: 10.1038/s41467-019-09455-9
55. Ponziani F, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. (2019) 69:107–20. doi: 10.1002/hep.30036
56. Yang L, Dai Y, He H, Liu Z, Liao S, Zhang Y, et al. Integrative analysis of gut microbiota and fecal metabolites in metabolic associated fatty liver disease patients. Front Microbiol. (2022) 13:969757. doi: 10.3389/fmicb.2022.969757
57. León-Mimila P, Villamil-Ramírez H, Li X, Shih D, Hui S, Ocampo-Medina E, et al. Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes Metab. (2021) 47:101183. doi: 10.1016/j.diabet.2020.07.010
58. Chen J, Zheng M, Liu J, Luo Y, Yang W, Yang J, et al. Ratio of conjugated chenodeoxycholic to muricholic acids is associated with severity of nonalcoholic steatohepatitis. Obesity. (2019) 27:2055–66. doi: 10.1002/oby.22627
59. Nimer N, Choucair I, Wang Z, Nemet I, Li L, Gukasyan J, et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism. (2021) 116:154457. doi: 10.1016/j.metabol.2020.154457
60. Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur P, Cazanave S, et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. (2018) 67:534–48. doi: 10.1002/hep.29359
61. Schwenger K, Copeland J, Ghorbani Y, Chen L, Comelli E, Guttman D, et al. Characterization of liver, adipose, and fecal microbiome in obese patients with MASLD: links with disease severity and metabolic dysfunction parameters. Microbiome. (2025) 13:9. doi: 10.1186/s40168-024-02004-7
62. Nychas E, Marfil-Sánchez A, Chen X, Mirhakkak M, Li H, Jia W, et al. Discovery of robust and highly specific microbiome signatures of non-alcoholic fatty liver disease. Microbiome. (2025) 13:10. doi: 10.1186/s40168-024-01990-y
63. Takeuchi T, Kubota T, Nakanishi Y, Tsugawa H, Suda W, Kwon A, et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature. (2023) 621:389–95. doi: 10.1038/s41586-023-06466-x
64. Tokuhara D. Role of the gut microbiota in regulating non-alcoholic fatty liver disease in children and adolescents. Front Nutr. (2021) 8:700058. doi: 10.3389/fnut.2021.700058
65. Koning M, Herrema H, Nieuwdorp M, Meijnikman A. Targeting nonalcoholic fatty liver disease via gut microbiome-centered therapies. Gut Microbes. (2023) 15:2226922. doi: 10.1080/19490976.2023.2226922
66. Guohong-Liu, Qingxi-Zhao, Hongyun-Wei. Characteristics of intestinal bacteria with fatty liver diseases and cirrhosis. Ann Hepatol. (2019) 18:796–803. doi: 10.1016/j.aohep.2019.06.020
67. Jiang X, Zheng J, Zhang S, Wang B, Wu C, Guo X. Advances in the involvement of gut microbiota in pathophysiology of NAFLD. Front Med. (2020) 7:361. doi: 10.3389/fmed.2020.00361
68. Zhang J, Zhou J, He Z, Li H. Bacteroides and NAFLD: pathophysiology and therapy. Front Microbiol. (2024) 15:1288856. doi: 10.3389/fmicb.2024.1288856
69. Meroni M, Longo M, Dongiovanni P. The role of probiotics in nonalcoholic fatty liver disease: a new insight into therapeutic strategies. Nutrients. (2019) 11:2642. doi: 10.3390/nu11112642
70. Zhang Y, Jiang W, Xu J, Wu N, Wang Y, Lin T, et al. E. coli NF73-1 isolated from NASH patients aggravates NAFLD in mice by translocating into the liver and stimulating M1 polarization. Front Cell Infect Microbiol. (2020) 10:535940. doi: 10.3389/fcimb.2020.535940
71. Kim B, Kim B. Gut microbiota and nonalcoholic fatty liver disease. Kosin Med J. (2023) 38:169–75. doi: 10.7180/kmj.23.138
72. Xia Y, Ren M, Yang J, Cai C, Cheng W, Zhou X, et al. Gut microbiome and microbial metabolites in NAFLD and after bariatric surgery: correlation and causality. Front Microbiol. (2022) 13:1003755. doi: 10.3389/fmicb.2022.1003755
73. Zhou J, Tripathi M, Sinha R, Singh B, Yen P. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. (2021) 7:11. doi: 10.20517/2394-5079.2020.134
74. Gupta H, Min B, Ganesan R, Gebru Y, Sharma S, Park E, et al. Gut microbiome in non-alcoholic fatty liver disease: from mechanisms to therapeutic role. Biomedicines. (2022) 10:550. doi: 10.3390/biomedicines10030550
75. Smits L, Bouter K, de Vos W, Borody T, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. (2013) 145:946–53. doi: 10.1053/j.gastro.2013.08.058
76. Sanai F, Al Khathlan A, Al Fadhli A, Jazzar A, Hashim A, Mansour E, et al. Clinical and economic burden of nonalcoholic steatohepatitis in Saudi Arabia, United Arab Emirates and Kuwait. Hepatol Int. (2021) 15:912–21. doi: 10.1007/s12072-021-10182-x
77. Gu X, Lu Q, Zhang C, Tang Z, Chu L. Clinical application and progress of fecal microbiota transplantation in liver diseases: a review. Semin Liver Dis. (2021) 41:495–506. doi: 10.1055/s-0041-1732319
78. Carter J, Bhattacharya D, Borgerding J, Fiel M, Faith J, Friedman S. Modeling dysbiosis of human NASH in mice: loss of gut microbiome diversity and overgrowth of Erysipelotrichales. PLoS One. (2021) 16:e0244763. doi: 10.1371/journal.pone.0244763
79. Valentin-Cortez FJ, Córdova-Gallardo J, Méndez-Sánchez N. Narrative review of gut microbiota and liver diseases: facts and fictions. Digestive Med Res. (2022) 5:16. doi: 10.21037/dmr-21-85
80. Vijay A, Valdes A. Role of the gut microbiome in chronic diseases: a narrative review. Eur J Clin Nutr. (2022) 76:489–501. doi: 10.1038/s41430-021-00991-6
81. Wong V, Tse C, Lam T, Wong G, Chim A, Chu W, et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis–a longitudinal study. PLoS One. (2013) 8:e62885. doi: 10.1371/journal.pone.0062885
82. Zhu Y, Yang H, Zhang Y, Rao S, Mo Y, Zhang H, et al. Dietary fiber intake and non-alcoholic fatty liver disease: the mediating role of obesity. Front Public Health. (2023) 10:1038435. doi: 10.3389/fpubh.2022.1038435
83. Yadegar A, Bar-Yoseph H, Monaghan T, Pakpour S, Severino A, Kuijper E, et al. Fecal microbiota transplantation: current challenges and future landscapes. Clin Microbiol Rev. (2024) 37:e0006022. doi: 10.1128/cmr.00060-22
84. Drekonja D, Reich J, Gezahegn S, Greer N, Shaukat A, MacDonald R, et al. Fecal microbiota transplantation for clostridium difficile infection: a systematic review. Ann Intern Med. (2015) 162:630–8. doi: 10.7326/M14-2693
85. Merrick B, Allen L, Masirah M, Zain N, Forbes B, Shawcross DL, et al. Regulation, risk and safety of faecal microbiota transplant. Infect Prev Pract. (2020) 2:100069. doi: 10.1016/j.infpip.2020.100069
86. Houttu V, Csader S, Nieuwdorp M, Holleboom A, Schwab U. Dietary interventions in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. (2021) 8:716783. doi: 10.3389/fnut.2021.716783
87. Yaskolka Meir A, Rinott E, Tsaban G, Zelicha H, Kaplan A, Rosen P, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. (2021) 70:2085–95. doi: 10.1136/gutjnl-2020-323106
88. Hartman M, Sanyal A, Loomba R, Wilson J, Nikooienejad A, Bray R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. (2020) 43:1352–5. doi: 10.2337/dc19-1892
89. Sanyal A, Bedossa P, Fraessdorf M, Neff G, Lawitz E, Bugianesi E, et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N Engl J Med. (2024) 391:311–9. doi: 10.1056/NEJMoa2401755
90. Bach Knudsen K. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv Nutr. (2015) 6:206–13. doi: 10.3945/an.114.007450
91. Rau MZ, Knudsen KEB. The effects of dietary fiber on short-chain fatty acid production and liver health. Nutrients. (2020):12:1496–1507.
92. Vell M, Creasy K, Scorletti E, Seeling K, Hehl L, Rendel M, et al. Omega-3 intake is associated with liver disease protection. Front Public Health. (2023) 11:1192099. doi: 10.3389/fpubh.2023.1192099
93. de Oca AP, Julián MT, Ramos A, Puig-Domingo M, Alonso N. Microbiota, fiber, and NAFLD: is there any connection? Nutrients. (2020) 12:3100. doi: 10.3390/nu12103100
94. Alamoudi A, Al-Ghamdi A, Al-Harbi F, Kahtani Al. Omega-3 fatty acids and liver health: a study on the effects of omega-3-rich foods on liver enzyme levels and hepatic fat. J Clin Nutr. (2020) 45.
95. Hadefi A, Arvanitakis M, Trépo E, Zelber-Sagi S. Dietary strategies in non-alcoholic fatty liver disease patients: from evidence to daily clinical practice, a systematic review. United European Gastroenterol J. (2023) 11:663–89. doi: 10.1002/ueg2.12443
96. Aboubakr A, Stroud A, Kumar S, Newberry C. Dietary approaches for management of non-alcoholic fatty liver disease: a Clinician’s guide. Curr Gastroenterol Rep. (2021) 23:21. doi: 10.1007/s11894-021-00827-0
97. Alqahtani S, Abaalkhail F, Alghamdi S, Bzeizi K, Al-Hamoudi W, Paik J, et al. The burden of metabolic dysfunction-associated steatotic liver disease and viral hepatitis in Saudi Arabia. Saudi J Gastroenterol. (2024) 30:310–8. doi: 10.4103/sjg.sjg_62_24
98. Bawazeer N, Alqahtani S, Alzaben A. The association between dietary patterns and socio-demographic and lifestyle characteristics: a sample of Saudi Arabia. Curr Nutr Food Sci. (2021) 9:1046–1057. doi: 10.12944/CRNFSJ.9.3.29
99. Dobbie L, Burgess J, Hamid A, Nevitt S, Hydes T, Alam U, et al. Effect of a low-calorie dietary intervention on liver health and body weight in adults with metabolic-dysfunction associated steatotic liver disease (MASLD) and overweight/obesity: a systematic review and meta-analysis. Nutrients. (2024) 16:1030. doi: 10.3390/nu16071030
100. Varkaneh H, Poursoleiman F, Al Masri M, Alras K, Shayah Y, Masmoum M, et al. Low fat diet versus low carbohydrate diet for management of non-alcohol fatty liver disease: a systematic review. Front Nutr. (2022) 9:987921. doi: 10.3389/fnut.2022.987921
101. Al-Mansour MM, Al-Ghamdi SH. Low-carbohydrate, high-fat diet in the Gulf region and its effects on NAFLD. Gastroenterology. (2021) 160: 1542–51.
102. Hamamah S, Iatcu O, Covasa M. Dietary influences on gut microbiota and their role in metabolic dysfunction-associated steatotic liver disease (MASLD). Nutrients. (2024) 17:143. doi: 10.3390/nu17010143
103. Fogacci F, Giovannini M, Di Micoli V, Grandi E, Borghi C, Cicero A. Effect of supplementation of a butyrate-based formula in individuals with liver steatosis and metabolic syndrome: a randomized double-blind placebo-controlled clinical trial. Nutrients. (2024) 16:2454. doi: 10.3390/nu16152454
104. Pant K, Venugopal S, Lorenzo Pisarello M, Gradilone S. The role of gut microbiome-derived short-chain fatty acid butyrate in hepatobiliary diseases. Am J Pathol. (2023) 193:1455–67. doi: 10.1016/j.ajpath.2023.06.007
105. Münte E, Hartmann P. The role of short-chain fatty acids in metabolic dysfunction-associated steatotic liver disease and other metabolic diseases. Biomolecules. (2025) 15:469. doi: 10.3390/biom15040469
106. Gao Y, Lu J, Liu X, Liu J, Ma Q, Shi Y, et al. Effect of long-term exercise on liver lipid metabolism in chinese patients with NAFLD: a systematic review and meta-analysis. Front Physiol. (2021) 12:748517. doi: 10.3389/fphys.2021.748517
107. Alasmari A, Al-Khalifah A, BaHammam A, Alshiban N, Almnaizel A, Alodah H, et al. Ramadan fasting model exerts hepatoprotective, anti-obesity, and anti-hyperlipidemic effects in an experimentally-induced nonalcoholic fatty liver in rats. Saudi J Gastroenterol. (2023): doi: 10.4103/sjg.sjg_153_23 Online ahead of print.
108. Song Q, Pan J, Pan M, Zheng C, Fan W, Zhen J, et al. Exploring the relationship between air pollution, non-alcoholic fatty liver disease, and liver function indicators: a two-sample Mendelian randomization analysis study. Front Endocrinol. (2024) 15:1396032. doi: 10.3389/fendo.2024.1396032
109. Dolce A, Della Torre S. Sex, nutrition, and NAFLD: relevance of environmental pollution. Nutrients. (2023) 15:2335. doi: 10.3390/nu15102335
110. Nakashita C, Xi L, Inoue Y, Kabura R, Masuda S, Yamano Y, et al. Impact of dietary compositions and patterns on the prevalence of nonalcoholic fatty liver disease in Japanese men: a cross-sectional study. BMC Gastroenterol. (2021) 21:342. doi: 10.1186/s12876-021-01919-x
111. Plummer E, Bulach D, Carter G, Albert M. Gut microbiome of native Arab Kuwaitis. Gut Pathog. (2020) 12:10. doi: 10.1186/s13099-020-00351-y
112. Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. (2012) 18:2300–8. doi: 10.3748/wjg.v18.i19.2300
113. Lau J, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol. (2017) 241:36–44. doi: 10.1002/path.4829
114. Im Y, Hunter H, de Gracia Hahn D, Duret A, Cheah Q, Dong J, et al. A systematic review of animal models of NAFLD finds high-fat, high-fructose diets most closely resemble human NAFLD. Hepatology. (2021) 74:1884–901. doi: 10.1002/hep.31897
115. Flessa C, Nasiri-Ansari N, Kyrou I, Leca B, Lianou M, Chatzigeorgiou A, et al. Genetic and diet-induced animal models for non-alcoholic fatty liver disease (NAFLD) research. Int J Mol Sci. (2022) 23:15791. doi: 10.3390/ijms232415791
116. Wang R, Li H, Yang X, Xue X, Deng L, Shen J, et al. Genetically obese human gut microbiota induces liver steatosis in germ-free mice fed on normal diet. Front Microbiol. (2018) 9:1602. doi: 10.3389/fmicb.2018.01602
117. Chiu C, Ching Y, Li Y, Liu J, Huang Y, Huang Y, et al. Nonalcoholic fatty liver disease is exacerbated in high-fat diet-fed gnotobiotic mice by colonization with the gut microbiota from patients with nonalcoholic Steatohepatitis. Nutrients. (2017) 9:1220. doi: 10.3390/nu9111220
118. Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. (2019) 7:91. doi: 10.1186/s40168-019-0704-8
119. Chambers E, Preston T, Frost G, Morrison D. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep. (2018) 7:198–206. doi: 10.1007/s13668-018-0248-8
120. Wei M, Tu W, Huang G. Regulating bile acids signaling for NAFLD: molecular insights and novel therapeutic interventions. Front Microbiol. (2024) 15:1341938. doi: 10.3389/fmicb.2024.1341938
121. Li Z, Yuan H, Chu H, Yang L. The crosstalk between gut microbiota and bile acids promotes the development of non-alcoholic fatty liver disease. Microorganisms. (2059) 11:2059. doi: 10.3390/microorganisms11082059
122. Zeng F, Su X, Liang X, Liao M, Zhong H, Xu J, et al. Gut microbiome features and metabolites in non-alcoholic fatty liver disease among community-dwelling middle-aged and older adults. BMC Med. (2024) 22:104. doi: 10.1186/s12916-024-03317-y
123. Zhang Z, Zhang H, Chen T, Shi L, Wang D, Tang D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun Signal. (2022) 20:64. doi: 10.1186/s12964-022-00869-5
124. Zhai Q, Wu H, Zheng S, Zhong T, Du C, Yuan J, et al. Association between gut microbiota and NAFLD/NASH: a bidirectional two-sample Mendelian randomization study. Front Cell Infect Microbiol. (2023) 13:1294826. doi: 10.3389/fcimb.2023.1294826
125. Jadhav PA, Thomas A, Nanda R, Chitlange S. Correlation of non-alcoholic fatty liver disease and gut microflora: clinical reports and treatment options. Egyptian Liver J. (2024) 14:21. doi: 10.1186/s43066-024-00327-6
126. Lee G, You H, Bajaj J, Joo S, Yu J, Park S, et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. (2020) 11:4982. doi: 10.1038/s41467-020-18754-5
127. Buzzetti E, Pinzani M, Tsochatzis E. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. (2016) 65:1038–48. doi: 10.1016/j.metabol.2015.12.012
128. Musazadeh V, Assadian K, Rajabi F, Faghfouri A, Soleymani Y, Kavyani Z, et al. The effect of synbiotics on liver enzymes, obesity indices, blood pressure, lipid profile, and inflammation in patients with non-alcoholic fatty liver: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2024) 208:107398. doi: 10.1016/j.phrs.2024.107398
129. Hill C, Guarner F, Reid G, Gibson G, Merenstein D, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
130. Peng L, Li Z, Green R, Holzman I, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
131. Franzosa E, Hsu T, Sirota-Madi A, Shafquat A, Abu-Ali G, Morgan X, et al. Sequencing and beyond: integrating molecular ‘omics’ for microbial community profiling. Nat Rev Microbiol. (2015) 13:360–72. doi: 10.1038/nrmicro3451
132. Jovel J, Patterson J, Wang W, Hotte N, O’Keefe S, Mitchel T, et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front Microbiol. (2016) 7:459. doi: 10.3389/fmicb.2016.00459
133. Sauceda C, Bayne C, Sudqi K, Gonzalez A, Dulai P, Knight R, et al. Stool multi-omics for the study of host-microbe interactions in inflammatory bowel disease. Gut Microbes. (2022) 14:2154092. doi: 10.1080/19490976.2022.2154092
134. Lamichhane S, Sen P, Dickens A, Orešič M, Bertram H. Gut metabolome meets microbiome: a methodological perspective to understand the relationship between host and microbe. Methods. (2018) 149:3–12. doi: 10.1016/j.ymeth.2018.04.029
135. Huang Y, Sheth R, Zhao S, Cohen L, Dabaghi K, Moody T, et al. High-throughput microbial culturomics using automation and machine learning. Nat Biotechnol. (2023) 41:1424–33. doi: 10.1038/s41587-023-01674-2
136. Song H, Kim Y, Kim J, Roh S, Whon T. Advances in culturomics research on the human gut microbiome: optimizing medium composition and culture techniques for enhanced microbial discovery. J Microbiol Biotechnol. (2024) 34:757–64. doi: 10.4014/jmb.2311.11024
137. Long Q, Luo F, Li B, Li Z, Guo Z, Chen Z, et al. Gut microbiota and metabolic biomarkers in metabolic dysfunction-associated steatotic liver disease. Hepatol Commun. (2024) 8:e0310. doi: 10.1097/HC9.0000000000000310
138. Qian X, Zhang H, Li Q, Ma G, Chen Z, Ji X, et al. Integrated microbiome, metabolome, and proteome analysis identifies a novel interplay among commensal bacteria, metabolites and candidate targets in non-small cell lung cancer. Clin Transl Med. (2022) 12:e947. doi: 10.1002/ctm2.947
Keywords: NAFLD, MASLD, NASH, MASH, metabolic syndrome, gut, microbiome, dysbiosis
Citation: Bahitham W, Banoun Y, Aljahdali M, Almuaiqly G, Bahshwan SM, Aljahdali L, Sanai FM, Rosado AS and Sergi CM (2025) “Trust your gut”: exploring the connection between gut microbiome dysbiosis and the advancement of Metabolic Associated Steatosis Liver Disease (MASLD)/Metabolic Associated Steatohepatitis (MASH): a systematic review of animal and human studies. Front. Nutr. 12:1637071. doi: 10.3389/fnut.2025.1637071
Received: 28 May 2025; Accepted: 26 August 2025;
Published: 10 September 2025.
Edited by:
Shafiya Imtiaz Rafiqi, University of Toledo, United StatesReviewed by:
Ankit P. Laddha, University of Connecticut, United StatesY. K. Prabhakar, National Institute of Veterinary Epidemiology And Disease Informatics (ICAR), India
Rathnam Mallesh, University of Denver, United States
Copyright © 2025 Bahitham, Banoun, Aljahdali, Almuaiqly, Bahshwan, Aljahdali, Sanai, Rosado and Sergi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Consolato M. Sergi, Q1NlcmdpQGNoZW8ub24uY2E=; Alexandre S. Rosado, YWxleGFuZHJlLnJvc2Fkb0BrYXVzdC5lZHUuc2E=
†These authors have contributed equally to this work
‡ORCID: Wesam Bahitham, orcid.org/0009-0008-5741-1042; Yusra Banoun, orcid.org/0009-0004-4086-5495; Mutep Aljahdali, orcid.org/0009-0008-4620-8540; Ghufran Almuaiqly, orcid.org/0009-0001-3584-8103; Shahad M. Bahshwan, orcid.org/0009-0000-2534-4035; Linah Aljahdali, orcid.org/0009-0007-6616-4437; Faisal M. Sanai, orcid.org/0000-0002-3830-9236; Alexandre S. Rosado, orcid.org/0000-0001-5135-134
 Wesam Bahitham
Wesam Bahitham Yusra Banoun3‡
Yusra Banoun3‡ Alexandre S. Rosado
Alexandre S. Rosado Consolato M. Sergi
Consolato M. Sergi