- 1Department of Anesthesiology, Chi Mei Medical Center, Tainan, Taiwan
- 2School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan
- 3Department of Anesthesiology, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
- 4Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan
- 5Department of Nutrition, Chi Mei Medical Center, Tainan, Taiwan
- 6Department of Anesthesiology, Chi Mei Medical Center, Liouying, Tainan, Taiwan
Background: Vitamin D deficiency (VDD) may contribute to venous thromboembolism (VTE) through effects on coagulation and endothelial function, but existing studies show inconsistent results. We investigated the association between VDD and VTE risk using a large matched cohort design.
Methods: We conducted a retrospective matched cohort study using the TriNetX database, including patients aged ≥45 years with serum 25-hydroxyvitamin D (25(OH)D) measurements between 2010 and 2023. VDD was defined as serum 25(OH)D < 20 ng/mL, while controls had levels ≥30 ng/mL. After 1:1 propensity score matching, the final cohort comprised 69,845 patients in each group. Primary outcomes were deep vein thrombosis (DVT) and pulmonary embolism (PE) occurring 3–12 months after the index date. Secondary outcomes included all-cause mortality and intensive care unit (ICU) admission.
Results: During one-year follow-up, VDD was significantly associated with increased risk of DVT (Hazard ratio [HR] 1.62, 95% confidence interval [CI]: 1.37–1.92; p < 0.001) and PE (HR 1.62, 95% CI: 1.34–1.96; p < 0.001) compared to controls. The association persisted over 2 years with modest attenuation (DVT: HR 1.49; PE: HR 1.61). A dose–response relationship was observed, with vitamin D insufficiency (20–30 ng/mL) showing intermediate risk levels (DVT: HR 1.36; PE: HR 1.43). VDD was also associated with higher mortality (HR 2.20, 95% CI: 1.99–2.43) and ICU admission risks (HR 1.47, 95% CI: 1.33–1.62). Subgroup analyses revealed consistent associations across demographic groups, with diabetes mellitus significantly modifying the DVT association.
Conclusion: Vitamin D deficiency is independently associated with increased VTE risk in a dose-dependent manner, with effects extending to mortality and healthcare utilization. These findings support vitamin D optimization for VTE prevention, though randomized trials are needed to establish causality.
1 Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), imposes a substantial global health burden, affecting one to two individuals per 1,000 person-years in Europe and the United States (1, 2). Approximately 20% of individuals with VTE die within 1 year—often due to the underlying cause, and survivors frequently experience long-term complications, contributing to significant morbidity, healthcare burden, and mortality (1, 3–5). As efforts to prevent thrombotic events continue to evolve, a deeper understanding of multifactorial risk profiles is essential to identify modifiable factors and improve patient outcomes. Among the emerging risk factors, vitamin D deficiency (VDD) has garnered considerable attention owing to its potential mechanistic role in thrombosis and its widespread prevalence across diverse populations (6–8). VDD may contribute to a prothrombotic state through several mechanisms: impairment of endothelial integrity, upregulation of tissue factor expression, downregulation of anticoagulant proteins such as thrombomodulin, and dysregulation of the fibrinolytic system through altered plasminogen activator inhibitor-1 levels (6–8). These mechanistic insights have prompted numerous observational studies investigating the association between vitamin D status and VTE risk (9–13). Vitamin D also plays an important role in modulating the immune system. It enhances innate immunity and regulates adaptive immune responses by reducing pro-inflammatory cytokines and supporting immune homeostasis (14, 15). These immunomodulatory effects may reduce vascular inflammation and contribute to protection against venous thrombosis.
Our previous meta-analysis identified a significant association between low serum vitamin D levels and an elevated risk of VTE (16). However, the majority of the included studies were cross-sectional in nature, with only three longitudinal investigations (12, 13, 17) offering temporal evidence to support a potential causal relationship. A key limitation in the existing literature is the variability in the definitions of VDD, with cutoff values ranging from less than 20 ng/mL to less than 30 ng/mL, contributing to substantial heterogeneity in exposure classification (16). While meta-analysis (16) offers preliminary directional insight, substantial methodological variability across studies, including differences in study design, exposure assessment, outcome definition, and confounder adjustment, limits the robustness and generalizability of the pooled estimates. These limitations highlight the need for more methodologically rigorous research that minimizes design heterogeneity, adopts standardized definitions of vitamin D status, and uses appropriate statistical approaches to elucidate the relationship between vitamin D and VTE risk.
Based on established biological mechanisms and emerging clinical evidence, we hypothesized that VDD would be independently associated with increased VTE risk in a dose-dependent manner. The primary aim of this study was to conduct a comprehensive matched cohort analysis using a large-scale electronic health records database to quantify the association between VDD and VTE risk while controlling for confounding variables through propensity score matching.
2 Methods
2.1 Data sources and ethical statement
The TriNetX platform comprises electronic health records from over 150 million patients, with data contributed by healthcare organizations primarily in the United States, but also from Europe, Latin America, Asia-Pacific, and the Middle East and Africa regions (18). The database has been extensively utilized in published research to support a broad range of clinical studies (19–21). The TriNetX platform provides access to real-world clinical data, including demographics, diagnoses, procedures, medications, and laboratory results, enabling large-scale epidemiological research. The study protocol adhered to the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Chi Mei Medical Center (IRB number: 11310-E04). Given the use of de-identified data from an existing database, the requirement for individual informed consent was waived in accordance with the applicable regulations.
2.2 Study design and population
We conducted a matched cohort study including patients aged ≥ 45 years who had serum 25-hydroxyvitamin D [25(OH)D] measurements recorded between January 2010 and December 2023. Individuals aged over 45 years were selected for this study because the incidence of VTE and VDD increases substantially with age, particularly after midlife. Focusing on this age group improves the relevance and generalizability of our findings to populations at higher risk for both conditions. The index date was defined as the first documented measurement of either VDD [serum 25(OH)D < 20 ng/mL] for the VDD group or adequate vitamin D levels [serum 25(OH)D ≥ 30 ng/mL] for the control group. The control group consisted of patients with adequate vitamin D levels but were not healthy volunteers; rather, they were real-world patients without prior VTE. Both groups were well-matched for comorbidities through propensity score matching.
To ensure consistent vitamin D status and minimize misclassification bias, all patients were required to have a confirmatory measurement within their respective vitamin D category during a second measurement taken 3–12 months after the index date. Patients in the VDD group were excluded if any 25(OH)D measurement exceeded 20 ng/mL during the confirmation period. Similarly, for the control group, patients were excluded if any 25(OH)D measurement fell below 30 ng/mL during the same 3–12 month confirmation window. This bilateral exclusion criterion maintained group integrity by ensuring that the patients remained in their designated vitamin D status category throughout the confirmation period. The follow-up period was defined as 3–12 months after the index date. This washout period of 3 months was implemented to exclude VTE events that might have occurred due to acute conditions present at the time of vitamin D measurement, thereby focusing on the chronic effects of VDD on thrombotic risk.
2.3 Exclusion criteria
Patients were systematically excluded if they had any of the following conditions documented before the follow-up period (i.e., 3–12 months after the index date): a history of DVT or PE; HIV infection; organ transplantation; hemiplegia, hemiparesis, paraplegia, or quadriplegia; current hormone replacement therapy; or use of oral contraceptives.
Additionally, patients were excluded if they had any of the following conditions documented within 3 months prior to the follow-up period: pregnancy, lower extremity surgery, or cerebral infarction or hemorrhage. These exclusion criteria were designed to eliminate patients with established risk factors for VTE that could confound the association with VDD.
2.4 Data collection and propensity score matching
Patient characteristics extracted from the database included demographic variables (age, body mass index categories, sex, and race/ethnicity), comorbidities, laboratory values, and medications. Comorbidities assessed included essential hypertension, lipid disorders, diabetes mellitus, neoplasms, chronic kidney disease, ischemic heart disease, nicotine dependence, liver disease, heart failure, cerebrovascular disease, gout, alcohol-related disorders, COVID-19, rheumatoid arthritis, malnutrition, long-term steroid use, and systemic lupus erythematosus.
Laboratory parameters included hemoglobin A1c levels (≥7%), serum albumin (≥3.5 g/dL), hemoglobin levels (≥12 mg/dL), and estimated glomerular filtration rate (>60 mL/min/1.73m2). Medication use included ACE inhibitors, anticoagulants, platelet aggregation inhibitors, insulin and analogs, and angiotensin II receptor blockers.
To minimize confounding and selection bias, 1:1 propensity score matching was performed using a greedy matching algorithm without replacement. The propensity score was calculated using logistic regression, incorporating all collected baseline characteristics. Matching was performed with a caliper width of 0.1 standard deviations of the logit of the propensity score. The adequacy of matching was assessed using standardized mean differences, with values <0.1 considered indicative of adequate balance between groups.
2.5 Outcome definitions
The primary outcome was the development of VTE (i.e., DVT or PE) during the 3–12 month follow-up period after the index date. Secondary outcomes included all-cause mortality and intensive care unit admission during the same period. To assess the durability of the association and evaluate long-term effects, we expanded the follow-up period to 3–24 months after the index date in a separate analysis. This extended follow-up allows for the examination of whether the increased thrombotic risk associated with VDD persists over a longer time horizon, providing insight into the clinical significance and sustained impact of vitamin D status on cardiovascular outcomes.
2.6 Assessment of vitamin D insufficiency
To evaluate the dose–response relationship and assess whether milder degrees of VDD confer increased thrombotic risk, we conducted a separate analysis comparing patients with vitamin D insufficiency [serum 25(OH)D 20–30 ng/mL] to those with adequate vitamin D levels [serum 25(OH)D ≥ 30 ng/mL]. This analysis utilized the same inclusion and exclusion criteria, matching methodology, and follow-up periods (3–12 months) as the primary analysis, allowing for the assessment of a potential gradient effect of vitamin D status on VTE risk.
2.7 Subgroup analyses
Pre-specified subgroup analyses were performed to assess whether patient characteristics modified the association between VDD and VTE risk. Subgroups were defined by sex (male vs. female), age categories (>65 years vs. 45–65 years), diabetes mellitus status (present vs. absent), chronic kidney disease status (present vs. absent), and COVID-19 history (present vs. absent).
2.8 Statistical analysis
All statistical analyses were performed using the TriNetX analytics platform. Continuous variables are presented as means with standard deviations, while categorical variables are presented as counts with percentages. The association between VDD and study outcomes was assessed using Cox proportional hazards regression models, with the results reported as hazard ratios (HRs) with 95% confidence intervals (CIs). To account for multiple comparisons across the four primary outcome measures (DVT, PE, mortality, and ICU admission), we applied Bonferroni correction, with statistical significance defined as p < 0.0125 (0.05/4). In subgroup analyses, we set the threshold for statistical significance at p < 0.05 when testing for interactions. We determined whether subgroups differed significantly by examining the p-value for interaction, rather than simply comparing the CIs of estimates within each subgroup. To identify independent predictors of DVT and PE at 1-year follow-up, we performed multivariate Cox proportional hazards regression analyses including VDD and relevant baseline variables (e.g., age, sex, diabetes, chronic kidney disease, etc.). Results were presented as adjusted hazard ratios (aHRs) with 95% confidence intervals. All statistical tests were two-sided, and the analyses followed the intention-to-treat principle based on the vitamin D status at the index date.
3 Results
3.1 Patient selection and baseline characteristics
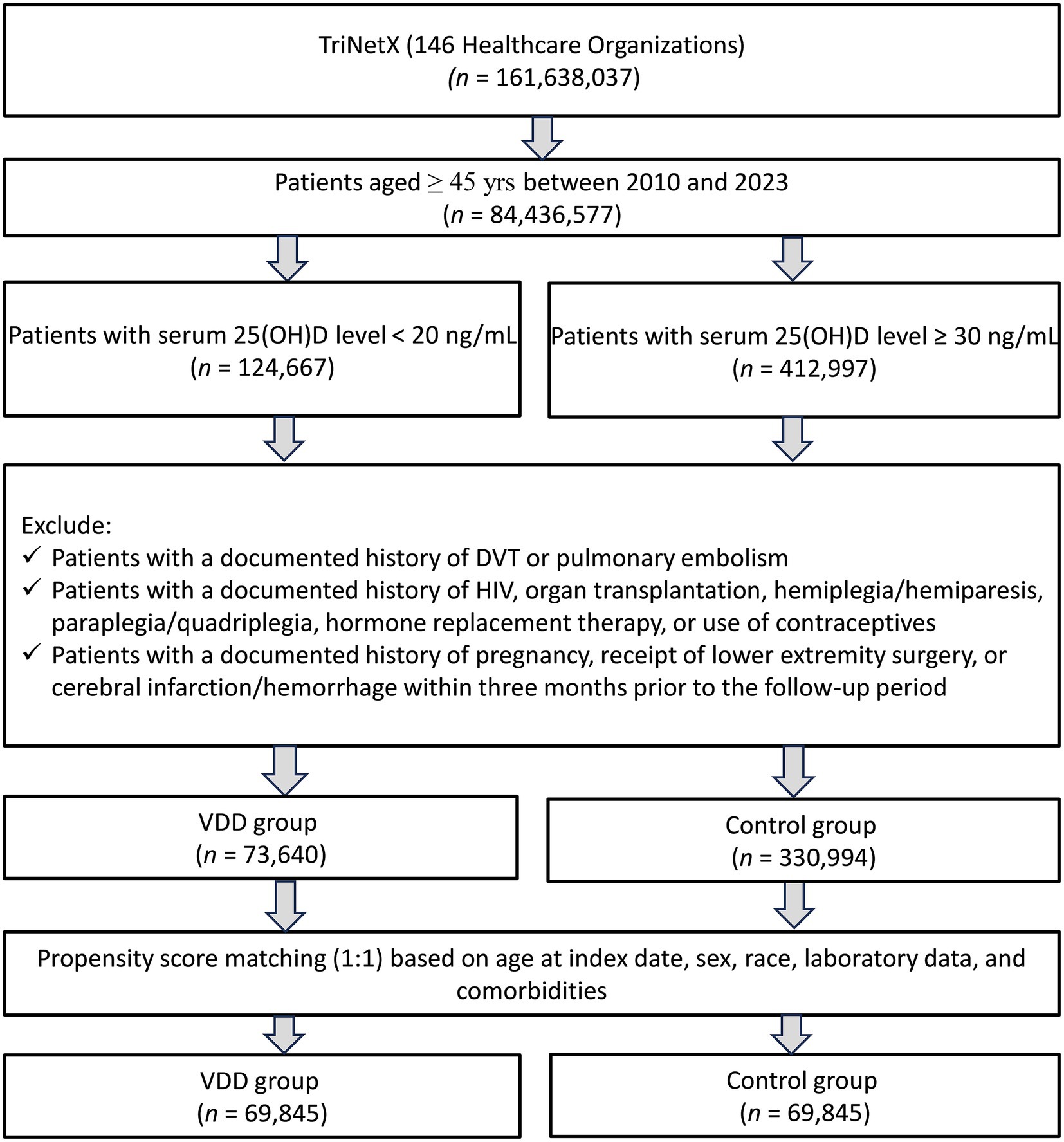
A total of 161,638,037 patients from 146 healthcare organizations in the TriNetX database were screened. We included patients aged ≥45 years with available serum 25 (OH) D levels between 2010 and 2023. After applying these criteria, 124,667 patients with VDD and 412,997 control patients (serum 25(OH)D level ≥30 ng/mL) were identified. After applying the exclusion criteria, 73,640 patients with VDD and 330,994 patients with adequate vitamin D levels were included in the unmatched cohort. Following 1:1 propensity score matching, the final matched cohort comprised of 69,845 patients in each group, with a total of 139,690 patients (Figure 1).
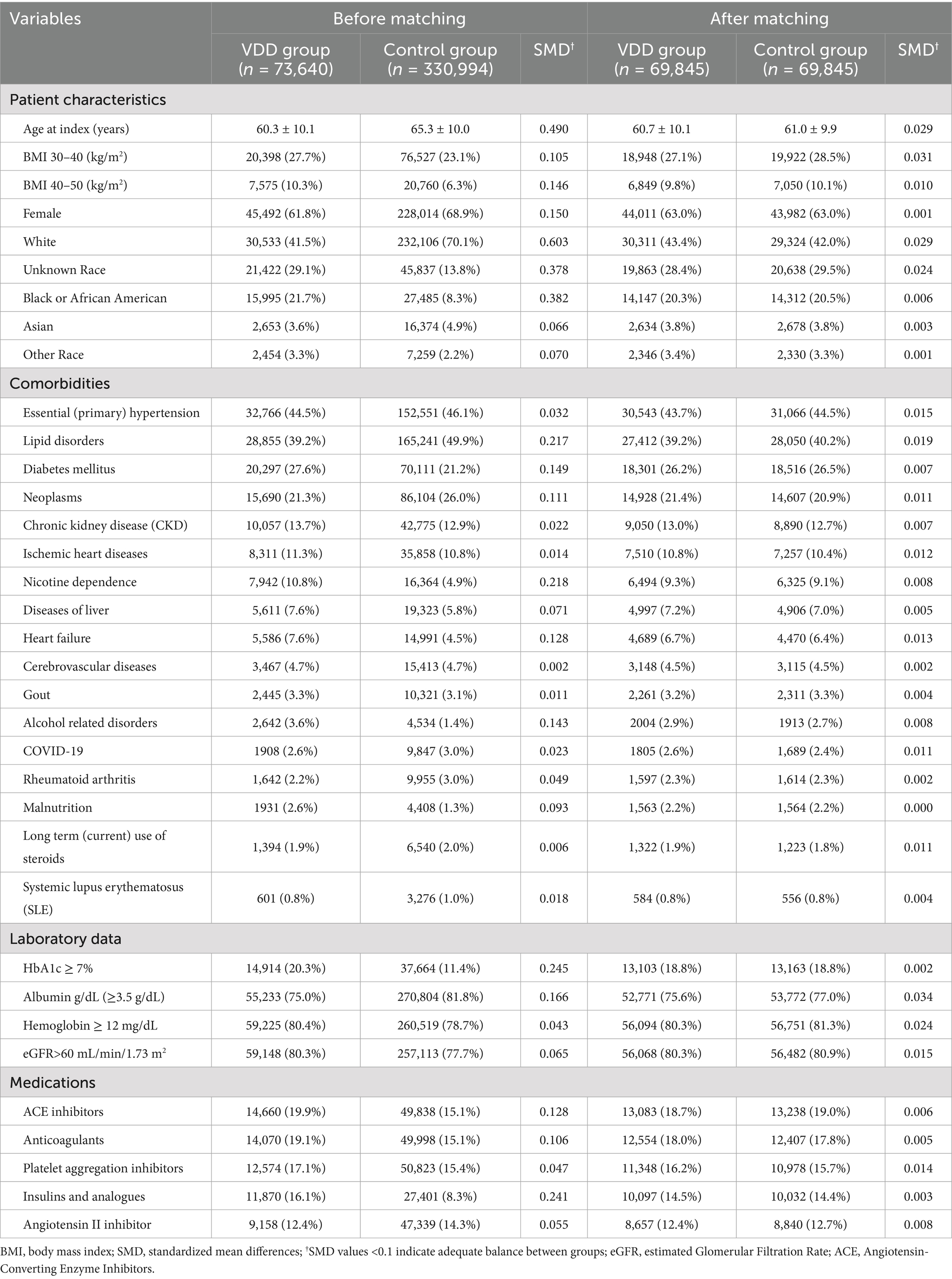
Before matching, significant differences were observed between the VDD and control groups in terms of multiple baseline characteristics (Table 1). Patients with VDD were younger (60.3 ± 10.1 vs. 65.3 ± 10.0 years), had higher rates of obesity, and were less likely to be female (61.8% vs. 68.9%) compared to the control group. Racial distribution differed substantially, with VDD being more prevalent among Black or African American patients (21.7% vs. 8.3%) and less common among White patients (41.5% vs. 70.1%). The comorbidity profiles also differed significantly before matching. Patients with VDD have higher rates of diabetes mellitus, nicotine dependence, heart failure, and alcohol-related disorders. They also demonstrated poorer glycemic control and lower albumin levels. After propensity score matching, excellent balance was achieved across all measured variables (i.e., standardized mean differences <0.1 for all characteristics). The matched cohort had a mean age of 60.7 ± 10.1 years in the VDD group and 61.0 ± 9.9 years in the control group, with similar distributions of sex, race, comorbidities, laboratory values, and medications between groups.
3.2 Outcomes
3.2.1 Risk of VTE at 1-year follow-up
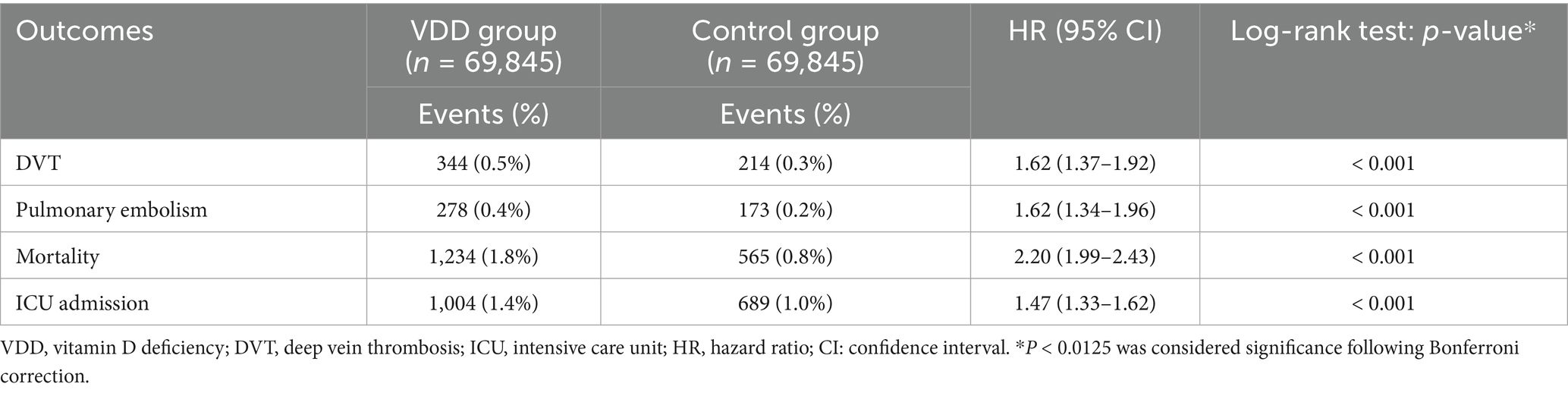
During the first year of follow-up, individuals with VDD exhibited a significantly higher risk of VTE than matched controls (Table 2). The incidence of DVT was 0.5% in the vitamin D-deficient cohort versus 0.3% among controls (HR 1.62, p < 0.001). PE was likewise more frequent in the deficiency group, occurring in 0.4% versus 0.2% of patients (HR 1.62, p < 0.001). Additionally, risks of mortality (HR 2.20, p < 0.001) and ICU admission (HR 1.47, p < 0.001) at 1 year were higher in those with VDD than in the control group. All reported associations remained statistically significant following Bonferroni correction (p < 0.0125).
3.2.2 Risk of VTE at 2-year follow-up
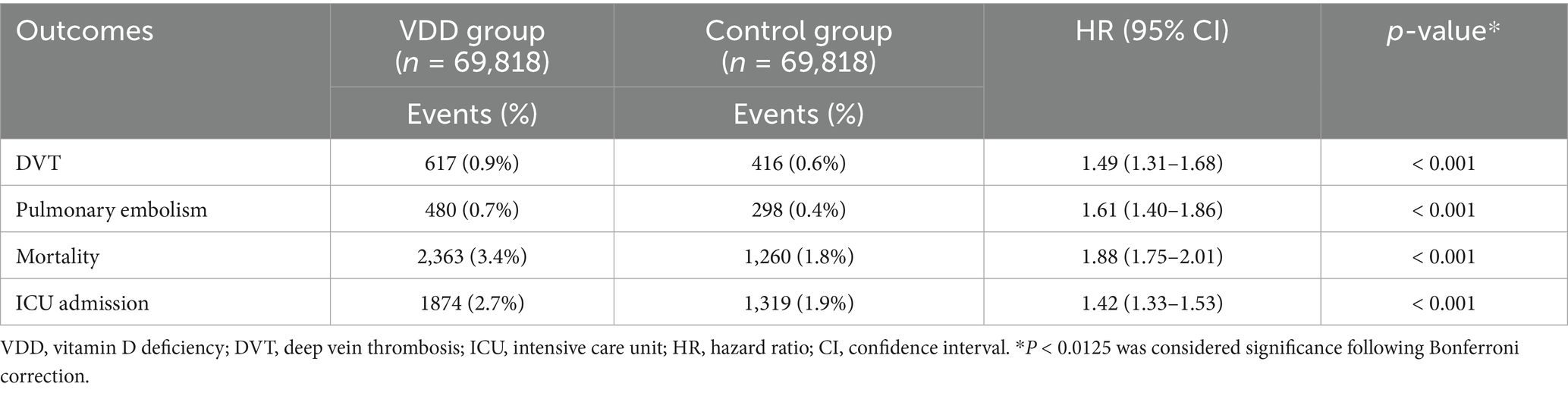
The risk association persisted during the extended follow-up period (3–24 months post-index), which included 69,818 patients in each matched group (Table 3). Despite a modest attenuation in effect sizes over the extended follow-up period, VDD remained significantly linked to a higher risk of VTE. DVT was observed in 0.9% of the deficiency group versus 0.6% of the controls (HR 1.49, p < 0.001). Similarly, PE occurred more frequently in vitamin D-deficient patients (0.7%) than in those with adequate levels (0.4%; HR 1.61, p < 0.001). By the two-year mark, excess risks extended to secondary outcomes as well. The VDD group experienced higher risks of all-cause mortality (HR 1.88, p < 0.001) and ICU admission (HR 1.42, p < 0.001) relative to controls.
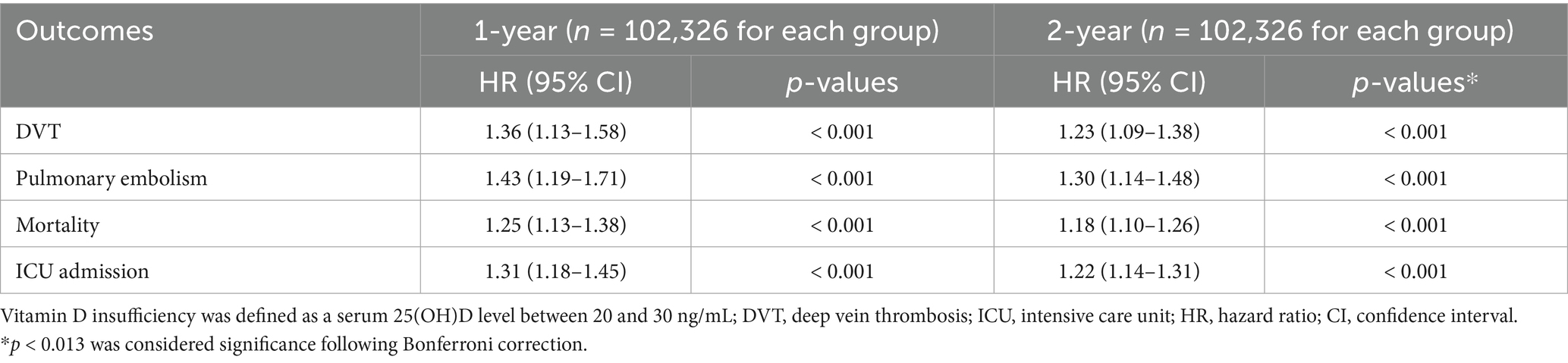
3.3 Association between vitamin D insufficiency and VTE risk
To compare vitamin D insufficiency (20–30 ng/mL) with adequate levels (≥30 ng/mL), 102,326 matched pairs were analyzed (Table 4). The results demonstrated a gradient in risk, as patients with vitamin D insufficiency experienced intermediate rates of adverse outcomes compared to those with deficiency or sufficiency. After 1 year, vitamin D insufficiency was associated with an increased risk of DVT (HR 1.36, p < 0.001) and PE (HR 1.43, p < 0.001). These individuals also had higher risks of mortality (HR 1.25, p < 0.001) and ICU admission (HR 1.31, p < 0.001). The two-year analysis confirmed the persistence of these associations. DVT risk remained elevated (HR 1.23, p < 0.001), as did PE risk (HR 1.30, p < 0.001), mortality (HR 1.18, p < 0.001), and ICU admissions (HR 1.22, p < 0.001).
3.4 Subgroup analyses
Subgroup analyses were conducted to explore potential effect modifiers of the association between VDD and the risk of DVT at 1 year (Table 5). The association remained consistent across sexes, with a similar effect size observed in males (HR 1.57) and females (HR 1.53; p-interaction = 0.887). When stratified by age, the association was stronger in individuals aged 45–65 years (HR 1.80) compared to those over 65 years (HR 1.40), though the interaction was not statistically significant (p-interaction = 0.207). Diabetes mellitus significantly modified the association (p-interaction = 0.022), with a more pronounced effect in patients without diabetes (HR 1.90) than in those with diabetes (HR 1.26). A similar trend was observed for chronic kidney disease (CKD), with a higher risk among those without CKD (HR 1.67) versus those with CKD (HR 1.21), although the interaction did not reach statistical significance (p-interaction = 0.090). History of COVID-19 infection did not significantly influence the association (p-interaction = 0.555), with comparable hazard ratios in patients with (HR 1.40) and without (HR 1.61) prior infection.
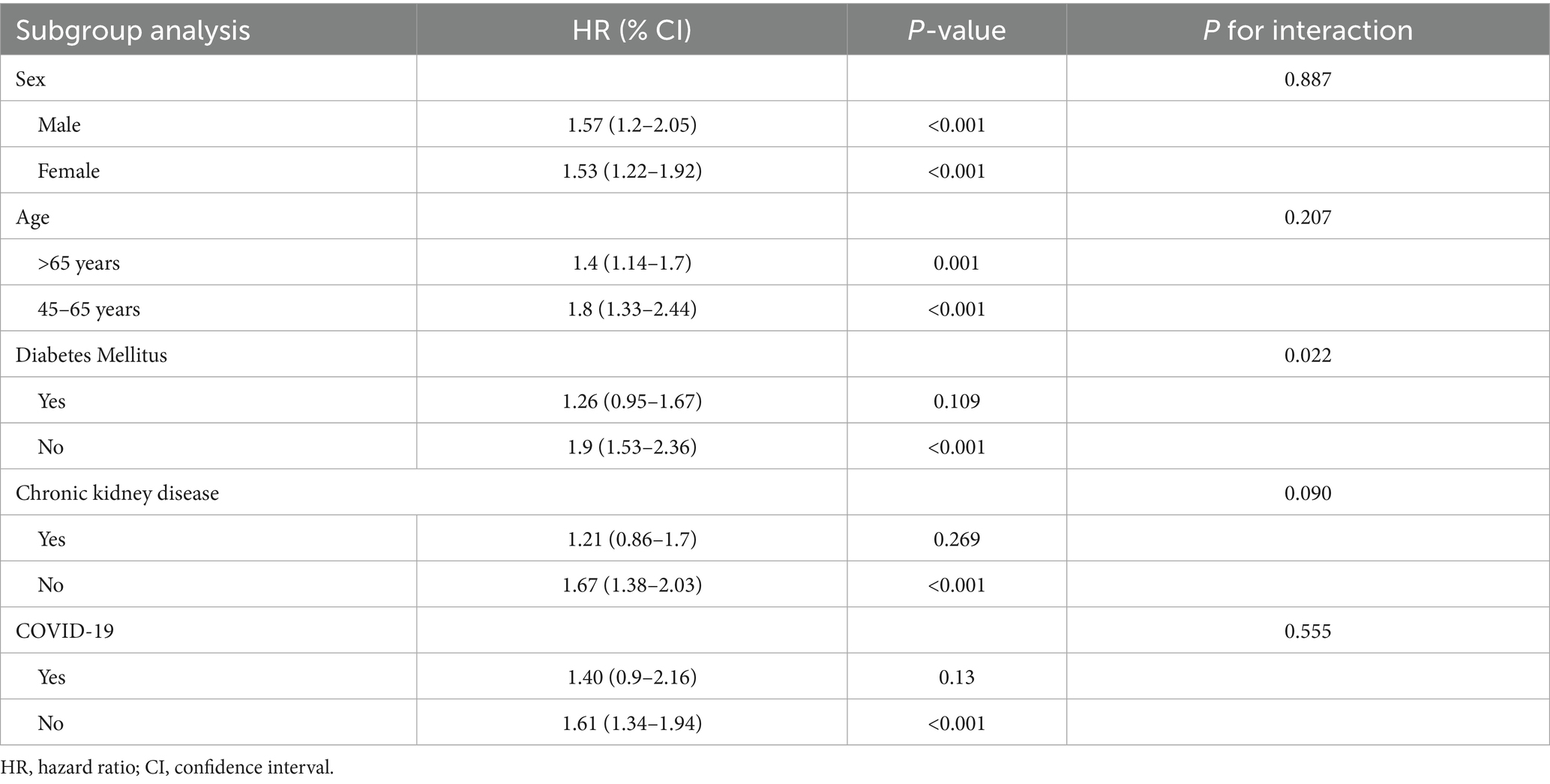
Table 5. Subgroup analysis of association between vitamin D deficiency and risk of deep vein thrombosis at 1-year follow-up.
Regarding PE, the association with VDD remained consistent across subgroups, with no significant effect modification. Notably, patients with a history of COVID-19 displayed a higher risk (HR 2.72, p < 0.001) compared to those without COVID-19 (HR 1.68, p < 0.001), although this difference did not reach statistical significance (p-interaction = 0.198) (Table 6).
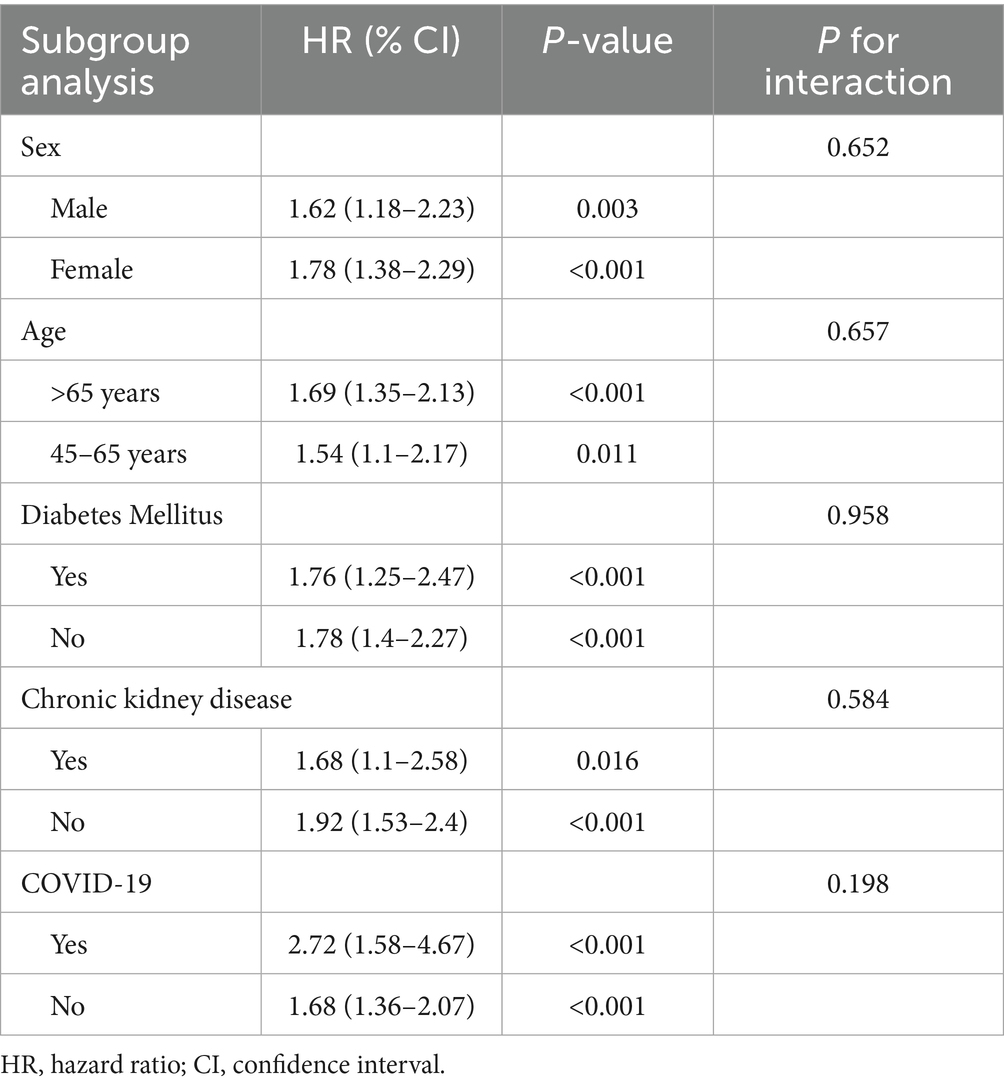
Table 6. Subgroup analysis of association between vitamin D deficiency and risk of pulmonary embolism at 1-year follow-up.
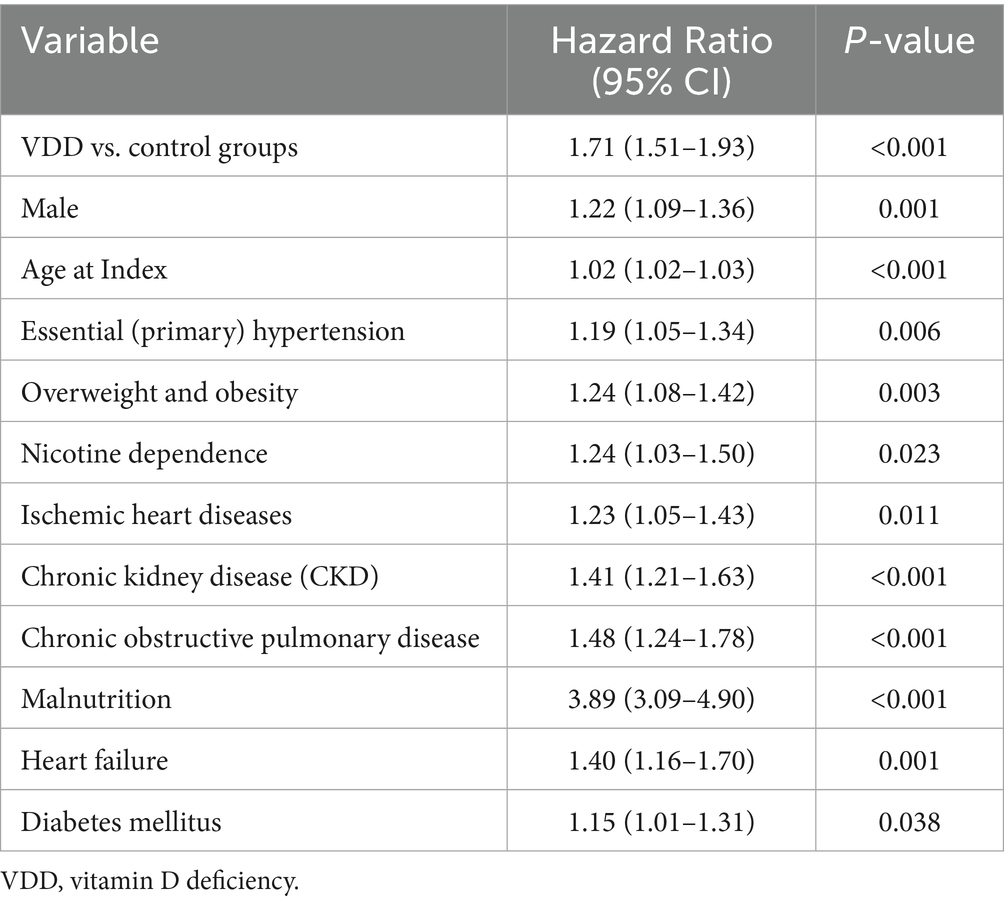
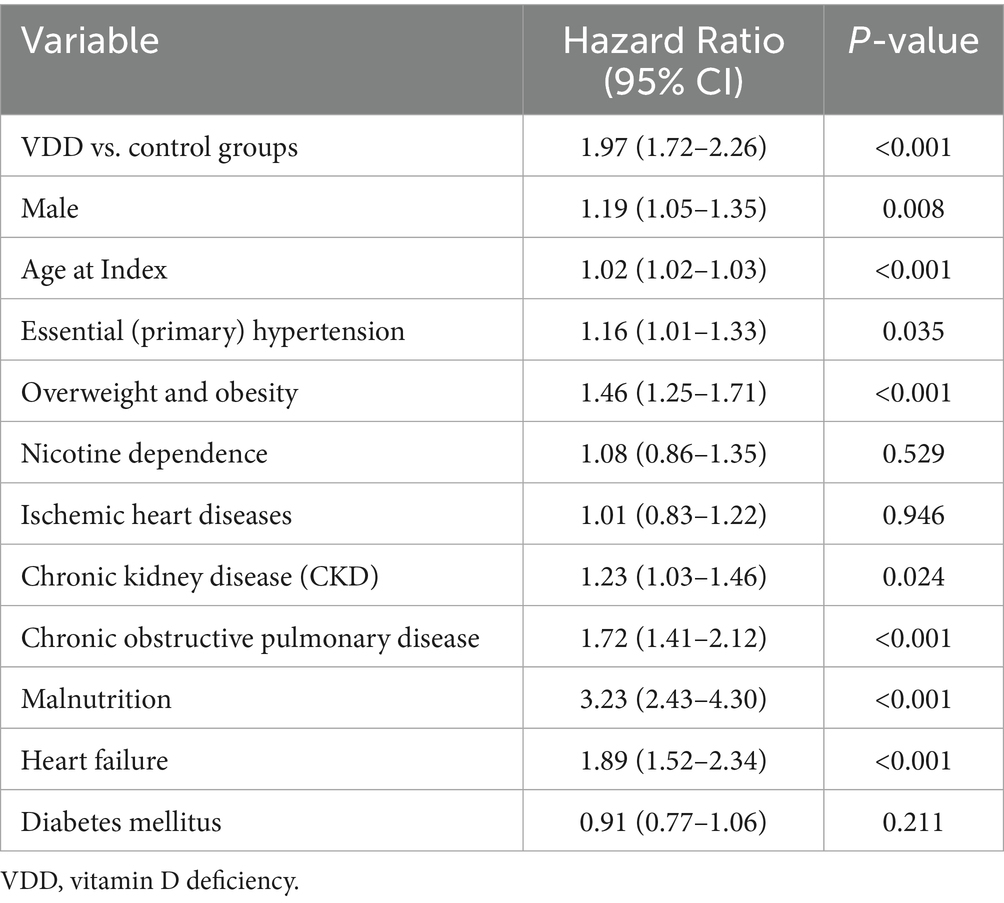
3.5 Multivariate predictors of DVT and PE
In multivariate Cox regression models at 1-year follow-up, VDD remained independently associated with an increased risk of DVT (aHR 1.71, p < 0.001) (Table 7) and PE (aHR 1.97, p < 0.001) (Table 8), after adjusting for age, sex, diabetes, chronic kidney disease, and other relevant covariates.
4 Discussion
This large-scale matched cohort study of 139,690 patients demonstrated a significant association between VDD and an increased risk of VTE. Our findings reveal that individuals with VDD face substantially higher risks of both DVT and PE, with HRs of 1.62 for each condition at one-year follow-up. This association persisted over extended follow-up periods and demonstrated a dose-dependent relationship, with vitamin D insufficiency showing intermediate risk levels between deficiency and sufficiency. Additionally, VDD was associated with increased mortality and intensive care unit admission rates, suggesting broader clinical implications beyond thrombotic events.
The existing literature on VDD and VTE has been characterized by significant methodological limitations and inconsistent findings (16). Most previous investigations have employed cross-sectional designs focusing on specific high-risk populations, including patients undergoing lower-limb surgery, stroke patients, brain injury cases, and spinal cord injury populations (9, 11, 22–24). While these studies have provided valuable insights into vitamin D status in thrombosis-prone populations, their cross-sectional nature limits their ability to establish temporal relationships and causal inference. Only three large longitudinal cohort studies have examined this association in the general population, all published between 2013 and 2014 (12, 13, 17). Notably, the findings from these studies were inconsistent, with two of the three studies failing to demonstrate a significant association between vitamin D status and VTE risk (12, 13). Furthermore, these investigations evaluated long-term associations with follow-up periods ranging from 10.7 to 30 years, which may not provide clinically relevant information for short-term risk stratification and prevention strategies. The extended follow-up periods in these studies may have diluted the observable effects due to changes in vitamin D status over time, lifestyle modifications, and the development of competing risks that could mask the association.
Our findings align with and strengthen the evidence from previous meta-analyses investigating the relationship between vitamin D status and VTE risk (16, 25). Our prior meta-analysis demonstrated a negative relationship between vitamin D levels and VTE risk, with pooled odds ratios of 1.74 (95% CI: 1.37–2.20) for low vitamin D status and hazard ratios of 1.25 (95% CI: 1.07–1.46) from longitudinal studies. Our study corroborates these findings with similar effect sizes. However, our results contrast with those of some earlier longitudinal cohort studies. Two of the three large general population cohort studies from to 2013–2014 found no significant association between vitamin D levels and VTE risk. This discrepancy may be attributed to several factors, including differences in study populations, varying definitions of VDD, extended follow-up periods that may have obscured short-term associations, and potential confounding variables that were not adequately controlled for in earlier studies. The consistency of our findings across both DVT and PE suggests that VDD affects the entire spectrum of VTE rather than being specific to particular manifestations. This supports the biological plausibility of the role of vitamin D in thrombosis through its effects on multiple pathways involved in coagulation, endothelial function, and inflammation.
Our investigation makes several novel contributions to the literature. First, this is the largest matched cohort study to date examining the association between VDD and VTE risk, with 139,690 participants providing substantial statistical power to detect clinically meaningful associations. Second, our study design addresses critical limitations of previous research by employing a shorter, more clinically relevant follow-up period (3–12 months) that captures the acute effects of VDD on thrombotic risk while minimizing confounding from temporal changes in vitamin D status. Third, our rigorous propensity score matching methodology ensured balanced comparison groups across multiple demographic, clinical, and laboratory parameters, thereby reducing selection bias and confounding factors that may have influenced previous observational studies. Fourth, we provide evidence of a dose–response relationship by separately analyzing VDD (<20 ng/mL) and insufficiency (20–30 ng/mL), demonstrating graduated risk levels that support a causal relationship. Finally, we provide strong population-based evidence from a large, diverse group of patients, moving beyond the specialized patient groups (patients undergoing lower-limb surgery, stroke patients, brain injury cases, and spinal cord injury populations) that most previous studies have focused on (9, 11, 22–24).
The observed association between VDD and increased mortality risk (HR 2.20) represents a particularly concerning finding that extends beyond primary thrombotic outcomes. This association may reflect multiple pathways through which VDD influences clinical outcomes. The increased mortality could be directly related to the higher incidence of fatal PE in vitamin D-deficient patients (26). However, mortality risk likely encompasses broader mechanisms beyond thrombotic events. VDD has been associated with immune dysfunction, increased susceptibility to infections, cardiovascular disease, and overall frailty, all of which could contribute to an increased mortality risk (27–29). The observed magnitude of the association with mortality (HR 2.20) surpasses that observed for thrombotic events alone, suggesting that VDD may reflect broader impairments in overall health and physiological reserve, or alternatively, that it contributes directly to mortality through multiple overlapping biological pathways. The persistence of elevated mortality risk over the two-year follow-up period (HR 1.88) indicates that VDD represents a sustained risk factor rather than merely an acute marker of poor health status.
Our subgroup analyses provided important insights into the consistency and potential modifiers of the vitamin D-VTE association. For DVT, the association remained robust across sex and age groups, suggesting that VDD represents a universal risk factor, regardless of these demographic characteristics. However, the significant interaction with diabetes mellitus (p-interaction = 0.022) revealed important clinical nuances, with stronger associations observed in patients without diabetes. This interaction may reflect a complex relationship between vitamin D status, glucose metabolism, and coagulation. Patients with diabetes may have multiple competing risk factors for thrombosis, potentially diluting the observable effect of VDD (30, 31). Alternatively, diabetes-related medications or metabolic management strategies may modify the relationship between vitamin D status and thrombotic risk. For PE, the absence of significant effect modification across most subgroups supports the generalizability of our findings. The notably higher risk observed in patients with COVID-19 history (HR 2.72) than in those without (HR 1.68), while not statistically significant for interaction, may reflect the synergistic effects of COVID-19-related hypercoagulability and VDD-associated prothrombotic states.
Several limitations must be acknowledged when interpreting our findings. First, as an observational study, we cannot definitively establish causality despite the temporal sequence and dose–response relationship observed. Residual confounding from unmeasured variables, including genetic factors, dietary patterns, physical activity levels, and seasonal variations in vitamin D synthesis (32, 33), may influence our results. Second, our study relied on electronic health record data, which may be subject to coding inaccuracies, missing data, and selection bias toward patients who seek healthcare more frequently. The requirement for vitamin D testing may introduce a selection bias toward patients with suspected deficiency or those receiving more comprehensive medical evaluation. Third, we did not account for seasonal variations in vitamin D levels, which could influence both exposure classification and outcome risk. Vitamin D status can fluctuate significantly throughout the year, and our confirmation period of 3–12 months may not fully capture these temporal variations. Fourth, we lacked detailed information on vitamin D supplementation use, sun exposure patterns, dietary vitamin D intake, and other factors that could modify the vitamin D status over time. Additionally, we could not assess the potential confounding effects of certain medications that may influence both vitamin D metabolism and the risk of thrombosis. Fifth, our study population was derived from healthcare systems that may not be representative of the broader population, potentially limiting the generalizability of our findings to different demographic groups, geographic regions, or healthcare settings.
5 Conclusion
This large-scale matched cohort study provides evidence that VDD is independently associated with an increased risk of VTE, with a dose-dependent relationship and persistent effects over time. This association extends beyond thrombotic events to include increased mortality and intensive care utilization, suggesting broad clinical implications. Our findings support the biological plausibility of the antithrombotic properties of vitamin D and provide epidemiological evidence that may inform clinical practice guidelines for VTE prevention. Given the modifiable nature of VDD and the substantial public health burden of VTE, these findings warrant serious consideration for prevention strategies. However, randomized controlled trials of vitamin D supplementation are needed to establish causality and determine optimal dosing strategies for VTE prevention. Future research should also investigate the mechanisms underlying the association between vitamin D-VTE association and identify patient populations that might benefit most from vitamin D optimization as part of comprehensive thrombosis prevention strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study protocol was approved by the Institutional Review Board of Chi Mei Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because given the use of de-identified data from an existing database, the requirement for individual informed consent was waived in accordance with the applicable regulations.
Author contributions
K-CH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. L-CC: Data curation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. C-WH: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. J-YW: Methodology, Resources, Software, Writing – original draft, Writing – review & editing. C-HY: Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. C-NH: Methodology, Resources, Software, Writing – original draft, Writing – review & editing. MY: Formal analysis, Software, Validation, Writing – original draft, Writing – review & editing. I-WC: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Chi Mei Medical Center, Tainan, Taiwan, grant number CMFHR112075.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lutsey, PL, and Zakai, NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. (2023) 20:248–62. doi: 10.1038/s41569-022-00787-6
2. Khan, F, Tritschler, T, Kahn, SR, and Rodger, MA. Venous thromboembolism. Lancet. (2021) 398:64–77. doi: 10.1016/S0140-6736(20)32658-1
3. Wendelboe, A, and Weitz, JI. Global Health burden of venous thromboembolism. Arterioscler Thromb Vasc Biol. (2024) 44:1007–11. doi: 10.1161/ATVBAHA.124.320151
4. Grosse, SD, Nelson, RE, Nyarko, KA, Richardson, LC, and Raskob, GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. (2016) 137:3–10. doi: 10.1016/j.thromres.2015.11.033
5. Zuin, M, Rigatelli, G, Temporelli, P, and Bilato, C. Trends in mortality related to venous thromboembolism in the European Union, 2012–2020. Intern Emerg Med. (2024) 19:941–9. doi: 10.1007/s11739-024-03550-6
6. Ojaroodi, AF, Jafarnezhad, F, Eskandari, Z, Keramat, S, and Stanek, A. Recent updates and advances in the association between vitamin D deficiency and risk of thrombotic disease. Nutrients. (2024) 17:90. doi: 10.3390/nu17010090
7. Nie, S, Huang, P, Niu, H, Ding, F, Gong, L, Zou, C, et al. Vitamin D deficiency enhances platelet activation and thrombosis by regulating VDR/Akt pathway based on platelet proteomics. Eur J Pharmacol. (2025) 999:177684. doi: 10.1016/j.ejphar.2025.177684
8. Mohammad, S, Mishra, A, and Ashraf, MZ. Emerging role of vitamin D and its associated molecules in pathways related to pathogenesis of thrombosis. Biomolecules. (2019) 9:9. doi: 10.3390/biom9110649
9. Tao, J, Lou, F, and Liu, Y. The role of vitamin D in the relationship between gender and deep vein thrombosis among stroke patients. Front Nutr. (2021) 8:755883. doi: 10.3389/fnut.2021.755883
10. Andro, M, Delluc, A, Moineau, MP, Tromeur, C, Gouillou, M, Lacut, K, et al. Serum levels of 25(OH)D are not associated with venous thromboembolism in the elderly population. A case-control study. Thromb Haemost. (2016) 115:169–75. doi: 10.1160/TH15-02-0148
11. Moore, M, Goldin, Y, Patel, H, and Greenwald, BD. Low vitamin D level is associated with acute deep venous thrombosis in patients with traumatic brain injury. Brain Sci. (2021) 11:11. doi: 10.3390/brainsci11070849
12. Brodin, E, Lerstad, G, Grimnes, G, Brækkan, SK, Vik, A, Brox, J, et al. Serum levels of vitamin D are not associated with future risk of venous thromboembolism. The Tromsø Study. Thromb Haemost. (2013) 109:885–90. doi: 10.1160/TH12-10-0728
13. Folsom, AR, Roetker, NS, Rosamond, WD, Heckbert, SR, Basu, S, Cushman, M, et al. Serum 25-hydroxyvitamin D and risk of venous thromboembolism: the atherosclerosis risk in communities (ARIC) study. J Thromb Haemost. (2014) 12:1455–60. doi: 10.1111/jth.12665
14. Ghaseminejad-Raeini, A, Ghaderi, A, Sharafi, A, Nematollahi-Sani, B, Moossavi, M, Derakhshani, A, et al. Immunomodulatory actions of vitamin D in various immune-related disorders: a comprehensive review. Front Immunol. (2023) 14:950465. doi: 10.3389/fimmu.2023.950465
15. Fenercioglu, AK. The anti-inflammatory roles of vitamin D for improving human health. Curr Issues Mol Biol. (2024) 46:13514–25. doi: 10.3390/cimb46120807
16. Hung, KC, Yang, SH, Chang, CY, Wang, LK, Lin, YT, Yu, CH, et al. Is circulating vitamin D status associated with the risk of venous thromboembolism? A Meta-analysis of observational studies. Nutrients. (2023) 15:15. doi: 10.3390/nu15051113
17. Brøndum-Jacobsen, P, Benn, M, Tybjaerg-Hansen, A, and Nordestgaard, BG. 25-Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18, 791 participants. J Thromb Haemost. (2013) 11:423–31. doi: 10.1111/jth.12118
18. Ludwig, RJ, Anson, M, Zirpel, H, Thaci, D, Olbrich, H, Bieber, K, et al. A comprehensive review of methodologies and application to use the real-world data and analytics platform tri net X. Front Pharmacol. (2025) 16:1516126. doi: 10.3389/fphar.2025.1516126
19. Chen, I-W, Chang, L-C, Ho, C-N, Wu, J-Y, Tsai, Y-W, Lin, C-M, et al. Association between COVID-19 and the development of chronic kidney disease in patients without initial acute kidney injury. Sci Rep. (2025) 15:1–11. doi: 10.1038/s41598-025-96032-4
20. Hung, CH, Wu, JY, Weng, YS, Hsiao, LW, Liu, YC, and Chiang, IT. Impact of sodium-glucose cotransporter-2 inhibitors on ovarian cancer risk in patients with type 2 diabetes mellitus: a multi-institutional tri NetX study. Diabetes Res Clin Pract. (2025) 222:112109. doi: 10.1016/j.diabres.2025.112109
21. Huang, YJ, Kao, CL, Hung, KC, Lai, YC, Wu, JY, and Chen, IW. Impact of preoperative COVID-19 on postoperative outcomes in patients undergoing bariatric/metabolic surgery: an updated analysis of Trinet X databases. Obes Surg. (2025) 35:1808–17. doi: 10.1007/s11695-025-07850-4
22. Wu, WX, and He, DR. Low vitamin D levels are associated with the development of deep venous thromboembolic events in patients with ischemic stroke. Clin Appl Thromb Hemost. (2018) 24:69s–75s. doi: 10.1177/1076029618786574
23. Ehsanian, R, Timmerman, MA, Wright, JM, McKenna, S, Dirlikov, B, and Crew, J. Venous thromboembolism is associated with lack of vitamin D supplementation in patients with spinal cord injury and Low vitamin D levels. PM R. (2019) 11:125–34. doi: 10.1016/j.pmrj.2018.09.038
24. Hegde, V, Arshi, A, Wang, C, Buser, Z, Wang, JC, Jensen, AR, et al. Preoperative vitamin D deficiency is associated with higher postoperative complication rates in Total knee arthroplasty. Orthopedics. (2018) 41:e489–95. doi: 10.3928/01477447-20180424-04
25. Wan, J, Yuan, J, Li, X, Bao, Y, Hou, Y, Li, Z, et al. Association between serum vitamin D levels and venous thromboembolism (VTE): a systematic review and meta-analysis of observational studies. Complement Ther Med. (2020) 54:102579. doi: 10.1016/j.ctim.2020.102579
26. Gök, G, Karadağ, M, Çinar, T, Nurkalem, Z, and Duman, D. In-hospital and short-term predictors of mortality in patients with intermediate-high risk pulmonary embolism. J Cardiovasc Thorac Res. (2020) 12:321–7. doi: 10.34172/jcvtr.2020.51
27. Ismailova, A, and White, JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord. (2022) 23:265–77. doi: 10.1007/s11154-021-09679-5
28. Liu, L, Chen, C, Lo, K, Huang, J, Yu, Y, Huang, Y, et al. Serum 25-hydroxyvitamin D, frailty, and mortality among the Chinese oldest old: results from the CLHLS study. Nutr Metab Cardiovasc Dis. (2021) 31:2707–15. doi: 10.1016/j.numecd.2021.05.033
29. Agarwal, P, Agarwal, Y, and Hameed, M. Recent advances in association between vitamin D levels and cardiovascular disorders. Curr Hypertens Rep. (2023) 25:185–209. doi: 10.1007/s11906-023-01246-4
30. Ding, C, Guo, C, Du, D, Gong, X, and Yuan, Y. Association between diabetes and venous thromboembolism: a systematic review and meta-analysis. Medicine. (2023) 102:e35329. doi: 10.1097/MD.0000000000035329
31. Jiao, X, Li, Z, An, S, Huang, J, Feng, M, and Cao, G. Does diabetes mellitus increase the incidence of early thrombosis in deep vein following unicompartmental knee arthroplasty: a retrospective cohort study. BMC Geriatr. (2022) 22:448. doi: 10.1186/s12877-022-03153-w
32. Cinar, N, Harmanci, A, Yildiz, BO, and Bayraktar, M. Vitamin D status and seasonal changes in plasma concentrations of 25-hydroxyvitamin D in office workers in Ankara, Turkey. Eur J Intern Med. (2014) 25:197–201. doi: 10.1016/j.ejim.2013.11.004
Keywords: vitamin D deficiency, venous thromboembolism, deep vein thrombosis, pulmonary embolism, cohort study, thrombosis prevention
Citation: Hung K-C, Chang L-C, Hsu C-W, Wu J-Y, Yu C-H, Ho C-N, Yew M and Chen I-W (2025) Association between vitamin D deficiency and risk of venous thromboembolism: a matched cohort study of 139,690 patients. Front. Nutr. 12:1639257. doi: 10.3389/fnut.2025.1639257
Edited by:
Kathryn Hart, University of Surrey, United KingdomReviewed by:
Rachida Raache, University of Science and Technology Houari Boumediene, AlgeriaRivan Virlando Suryadinata, University of Surabaya, Indonesia
Copyright © 2025 Hung, Chang, Hsu, Wu, Yu, Ho, Yew and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: I-Wen Chen, Y2hlbml3ZW5hNjA5MTJAZ21haWwuY29t
 Kuo-Chuan Hung
Kuo-Chuan Hung Li-Chen Chang3
Li-Chen Chang3 Chih-Wei Hsu
Chih-Wei Hsu Jheng-Yan Wu
Jheng-Yan Wu Chia-Hung Yu
Chia-Hung Yu Chun-Ning Ho
Chun-Ning Ho