Abstract
Background:
Postoperative pneumonia is a common and serious complication following non-cardiac surgery. However, the relationship between preoperative albumin levels and postoperative pneumonia remains unclear in major non-cardiac surgeries, and the cut-off value for predicting postoperative pneumonia has yet to be determined.
Methods:
Data from patients who underwent non-cardiac surgery between January 1, 2019, and December 31, 2022, were extracted from the institutional electronic medical records of Chongqing University Cancer Hospital. The primary exposure of interest was preoperative albumin levels, and the outcome was postoperative in-hospital pneumonia.
Results:
The results revealed a non-linear dose–response relationship between preoperative albumin levels and postoperative pneumonia. A 1 g/L increase in preoperative albumin levels was associated with a 4.4% reduction in adjusted odds ratio (aOR) of postoperative pneumonia (aOR: 0.956, 95% CI: 0.940–0.973, p < 0.001). Patients with preoperative albumin levels <38.9 g/L or between 38.9 and 45.3 g/L had a 67.4 and 30.3% higher OR, respectively, compared to those with levels >45.3 g/L (aOR: 1.674, 95% CI: 1.313–2.135, p < 0.001; and aOR: 1.303, 95% CI: 1.045–1.624, p = 0.019). Subgroup analyses indicated that the risk of developing pneumonia was more pronounced in younger, female, and healthier patients.
Conclusion:
These findings suggest that preoperative albumin levels are significantly associated with the development of postoperative pneumonia in patients undergoing major non-cardiac surgery. A preoperative albumin level of <45.3 g/L may serve as a predictive marker for postoperative pneumonia.
1 Introduction
Postoperative pneumonia is a common and serious complication, with an incidence of 2–3% in non-cardiac surgery (1). It can lead to increased ICU admissions and a higher risk of postoperative morbidity and mortality (2, 3). Studies have shown that postoperative pneumonia can prolong hospitalization by 6–9 days (4), significantly increasing hospitalization costs. Therefore, identifying predictors of postoperative pneumonia may help reduce its incidence.
Albumin is the most abundant protein in human plasma, and a level below 35 g/L is defined as hypoalbuminemia (5). Hypoalbuminemia often develops before surgery due to malnutrition, malignancy, diabetes, and other conditions. Preoperative hypoalbuminemia is an independent risk factor for postoperative complications in lower extremity surgeries (6–10). However, evidence regarding the relationship between preoperative albumin levels and postoperative pneumonia in major non-cardiac surgeries remains limited. To our knowledge, the optimal preoperative albumin level for predicting postoperative pneumonia and whether the clinical threshold of 35 g/L is sufficiently stringent remain unclear.
In this retrospective study, we analyzed data from non-cardiac surgical patients to determine the association between preoperative albumin levels and postoperative in-hospital pneumonia. Additionally, we aimed to identify the cut-off value of preoperative albumin that could predict postoperative in-hospital pneumonia.
2 Methods
2.1 Study design
This retrospective study was approved by the Institutional Ethics Committee of Chongqing University Cancer Hospital, China (approval number: CZLS2024020-A). Due to the anonymized nature of the data and the retrospective design of the study, the requirement for informed consent from patients was waived by the Institutional Ethics Committee of Chongqing University Cancer Hospital.
2.2 Patients
Patients who underwent major non-cardiac surgery between January 1, 2019, and December 31, 2022, were initially enrolled. The inclusion criteria were: age ≥18 years, elective major non-cardiac surgery, surgical duration ≥2 h, and general anesthesia with intubation. The exclusion criteria were: patients with pneumonia or systemic infectious diseases within 1 month before surgery, and those with missing data on preoperative albumin levels or postoperative pneumonia diagnosis.
2.3 Data collection
The institutional electronic medical records of Chongqing University Cancer Hospital were reviewed from January 1, 2019, to December 31, 2022, using a validated automated data search software. Data from surgical patients who met the inclusion criteria were extracted based on clinical relevance and findings from previous studies. All data were collected using a self-designed case report form. The following variables were extracted: patient characteristics [age, sex, body mass index (BMI), ASA classification, smoking status]; and comorbidities including heart disease, hypertension, diabetes, renal disease, liver disease, cancer, stroke, chronic obstructive pulmonary disease (COPD), asthma, thyroid disease. Preoperative laboratory test results: hemoglobin (Hb), fasting glucose, white blood cell count (WBC), and albumin levels. Intraoperative variables: body temperature, mean arterial pressure (MAP), fluid infusion rate, and surgical duration.
2.4 Preoperative albumin measurement
The primary exposure of interest was the preoperative albumin level, which was measured at the central laboratory of Chongqing University Cancer Hospital after hospital admission but before surgery. If multiple measurements were taken before surgery, the result closest to the surgery date was used for analysis. Two definitions of cutoff points for albumin were evaluated: Clinical cutoff points: <35 g/L and ≥35 g/L. Cutoff points based on the first and third tertiles of albumin levels from the restricted cubic spline model.
2.5 Outcome measurement
The outcome of interest was postoperative in-hospital pneumonia. Cases were initially identified based on the diagnosis of postoperative pneumonia and subsequently reviewed to confirm the diagnosis according to the criteria established by the Centers for Disease Control and Prevention of the United States (CDC, 2008) (11).
2.6 Statistical analysis
Continuous variables were presented as median and interquartile range (IQR), while categorical variables were presented as frequencies and percentages [n (%)]. A restricted cubic spline model was used to evaluate the dose–response relationship between preoperative albumin levels and postoperative pneumonia. Preoperative albumin levels were analyzed as both continuous and categorized variables based on clinical criteria (<35 g/L and ≥35 g/L) and the first and third tertiles of preoperative albumin levels. Patients with missing data on preoperative albumin levels or postoperative pneumonia diagnosis were excluded from the final analysis. For other variables with missing values (<5% of data), the missing values were imputed using the median of each cohort, and all data were included in subsequent multivariable analyses.
Odds ratios (OR) and 95% confidence intervals (95% CI) for the risk of postoperative pneumonia were calculated using logistic regression. Both unadjusted and adjusted models (adjusted for all variables listed in Table 1) were analyzed. Subgroup analyses were conducted by stratifying the population based on age (<65 years, ≥65 years), sex (male, female), ASA classification (<III, ≥III), smoking status (present, absent), and surgical duration (<3 h, ≥3 h). Sensitivity analysis was conducted after excluding patients with hemoglobin <100 g/L.
Table 1
| Variable | All patients (n = 28,044) | AL ≥ 45.3 g/L (n = 7,044) | 38.9 g/L ≤ ALB<45.3 g/L (n = 14,069) | ALB < 38.9 g/L (n = 6,931) |
|---|---|---|---|---|
| Preoperative ALB (g/L) | 42.1 (38.9, 45.4) | 47.5 (46.3, 49.2%) | 42.1 (40.6, 43.7) | 36.6 (34.6, 37.9) |
| Age (year) | 52 (45, 62) | 49 (41, 56) | 52 (46, 62) | 57 (49, 67) |
| Gender | ||||
| Male | 9,277 (33.08%) | 1,737 (24.66%) | 4,537 (32.19) | 3,003 (43.33%) |
| Female | 18,767 (66.92%) | 5,307 (75.34%) | 9,532 (67.62%) | 3,928 (56.67%) |
| BMI | 23.4 (21.4, 25.7) | 23.4 (21.4, 25.6) | 23.7 (21.7, 26) | 23 (20.9, 25.3) |
| ASA | ||||
| I | 343 | 211 (3.00%) | 121 (0.86%) | 11 (0.16%) |
| II | 16,556 | 5,124 (72.74%) | 8,605 (61.05%) | 2,827 (40.79%) |
| III | 10,749 | 1,683 (23.89%) | 5,219 (30.02%) | 3,847 (55.50%) |
| IV | 382 | 25 (0.35%) | 122 (0.87%) | 235 (3.39%) |
| V | 14 | 1 (0.01%) | 2 (0.01%) | 11 (0.16%) |
| Smoking | 3,591 (12.8%) | 670 (9.51%) | 1,793 (12.72%) | 1,128 (16.27%) |
| Comorbidity | ||||
| Heart disease | 663 (2.36%) | 116 (1.65%) | 327 (2.32%) | 220 (3.17%) |
| Hypertension | 4,055 (14.46%) | 896 (12.72%) | 2,151 (12.26%) | 1,008 (14.54%) |
| DM | 1,573 (5.61%) | 332 (4.71%) | 767 (5.44%) | 474 (6.84%) |
| Renal disease | 127 (0.45%) | 16 (0.23%) | 57 (0.40%) | 54 (0.78%) |
| Liver disease | 785 (2.8%) | 177 (2.51%) | 345 (2.45%) | 263 (3.79%) |
| Cancer | 558 (1.99%) | 115 (1.63%) | 254 (1.80%) | 189 (2.73%) |
| Stroke | 298 (1.06%) | 43 (0.61%) | 134 (0.95%) | 121 (1.75%) |
| COPD | 97 (0.35%) | 15 (0.21%) | 39 (0.28%) | 43 (0.62%) |
| Asthma | 142 (0.51%) | 35 (0.50%) | 81 (0.57%) | 26 (0.38%) |
| Thyroid disease | 385 (1.37%) | 97 (1.38%) | 181 (1.28%) | 107 (1.54%) |
| Preoperative Hb (g/L) | 129 (117, 139) | 135 (126, 145) | 130 (120, 140) | 119 (105, 130) |
| Glucose (mmol/L) | 4.83 (4.38, 5.39) | 4.94 (4.52, 5.44) | 4.83 (4.38, 5.38) | 4.69 (4.22, 5.31) |
| WBC (*109/L) | 5.78 (4.76, 7.07) | 5.8 (4.84, 7) | 5.71 (4.72, 6.93) | 5.92 (4.76, 7.49) |
| Intraoperative temperature (°C) | 36.4 (36.2, 36.5) | 36.4 (36.2, 36.5) | 36.4 (36.2, 36.5) | 36.3 (36.2, 36.5) |
| MAP (mmHg) | 80.8 (75.9, 86.1) | 80.3 (75.4, 85.6) | 81.1 (76.1, 86.4) | 80.9 (76, 86.1) |
| Infusion rate (mL/kg/h) | 11.5 (9.0, 14.7) | 11.5 (9.0, 14.9) | 11.3 (8.8, 14.4) | 11.8 (9.3, 15.2) |
| Surgical duration | 135 (95, 205) | 120 (86, 181) | 135 (95, 205) | 156 (105, 240) |
| Postoperative pneumonia | 898 (3.2%) | 109 (1.55%) | 403 (2.86%) | 386 (5.57%) |
The clinical characteristics of patients undergoing non-cardiac surgery.
Data were presented as median (interquartile) or n (%). ALB, albumin; BMI, body mass index; DM, diabetes mellitus; COPD, chronic obstruct pulmonary disease; Hb, hemoglobin; WBC, white blood cell; MAP, mean artery pressure.
To assess the predictive efficacy of preoperative albumin levels for postoperative pneumonia, receiver operating characteristic (ROC) curves were constructed using preoperative albumin levels (as continuous or categorized variables) along with other independent risk factors identified from multivariate regression analysis. The area under the ROC curve (AUC) was calculated using logistic regression models.
Statistical analyses were performed using Stata 16 (Stata Corp) and R software 4.12 (R Foundation for Statistical Computing). All tests were two-sided, and a p-value < 0.05 was considered statistically significant.
3 Results
Data from 22,249 patients who underwent elective major non-cardiac surgery at Chongqing University Cancer Hospital between January 2019 and December 2022 were initially available. After excluding patients with missing preoperative albumin values (n = 150), those with infectious diseases within 1 month before surgery (n = 220), and those with missing diagnostic data for pneumonia (n = 75), a total of 21,804 patients were included in the final analysis.
The characteristics of all patients and the three cohorts based on the first and third tertiles of preoperative albumin levels are presented in Table 1. The median age of the patients was 52 years, and the median preoperative albumin level was 42.1 g/L. Among all patients, 898 (3.2%) developed postoperative pneumonia. Patients with lower preoperative albumin levels were more likely to be smokers and to have comorbidities such as heart disease, hypertension, diabetes, stroke, renal disease, liver disease, and chronic obstructive pulmonary disease (COPD). They were also more likely to develop postoperative pneumonia.
When preoperative albumin was treated as a continuous variable in a multivariable logistic regression model, a 1 g/L increase in preoperative albumin level was associated with a 4.4% reduction in the adjusted odds ratio (aOR) (aOR: 0.956, 95% CI: 0.940–0.973, p < 0.001). Patients were categorized into three tertiles based on their preoperative albumin levels. Those with preoperative albumin levels <38.9 g/L or between 38.9 and 45.3 g/L exhibited a 67.4 and 30.3% higher aOR, respectively, compared to those with levels >45.3 g/L (aOR: 1.674, 95% CI: 1.313–2.135, p < 0.001; and aOR: 1.303, 95% CI: 1.045–1.624, p = 0.019) (Table 2).
Table 2
| Albumin | Unadjusted OR (95%CI) | p-value | Adjusted OR (95%CI) | p-value |
|---|---|---|---|---|
| Continuous variable (increase 1 g/L) | 0.900 (0.888, 0.912) | 0.000 | 0.956 (0.940, 0.973) | 0.000 |
| Clinical cut-off values (g/L) | ||||
| <35 | 3.251 (2.732, 3.869) | 0.000 | 1.551 (1.261, 1.907) | 0.000 |
| ≥35 | 1.000 (reference) | 1.000 (reference) | ||
| Cut-off values (g/L) based on tertiles | ||||
| <38.9 | 3.752 (3.026, 4.654) | 0.000 | 1.658 (1.301, 2.114) | 0.000 |
| 38.9–45.3 | 1.876 (1.515, 2.323) | 0.000 | 1.293 (1.037, 1.613) | 0.022 |
| ≥45.3 | 1.000 (reference) | 1.000 (reference) | ||
The association between preoperative albumin and postoperative pneumonia.
A non-linear dose–response relationship between preoperative albumin levels and postoperative pneumonia was observed using a restricted cubic spline model. Similar associations were found in subgroup analyses (Figure 1). The subgroup analyses further revealed that the risk of developing pneumonia was more pronounced in younger, female, and healthier patients at lower preoperative albumin levels (Figure 2).
Figure 1
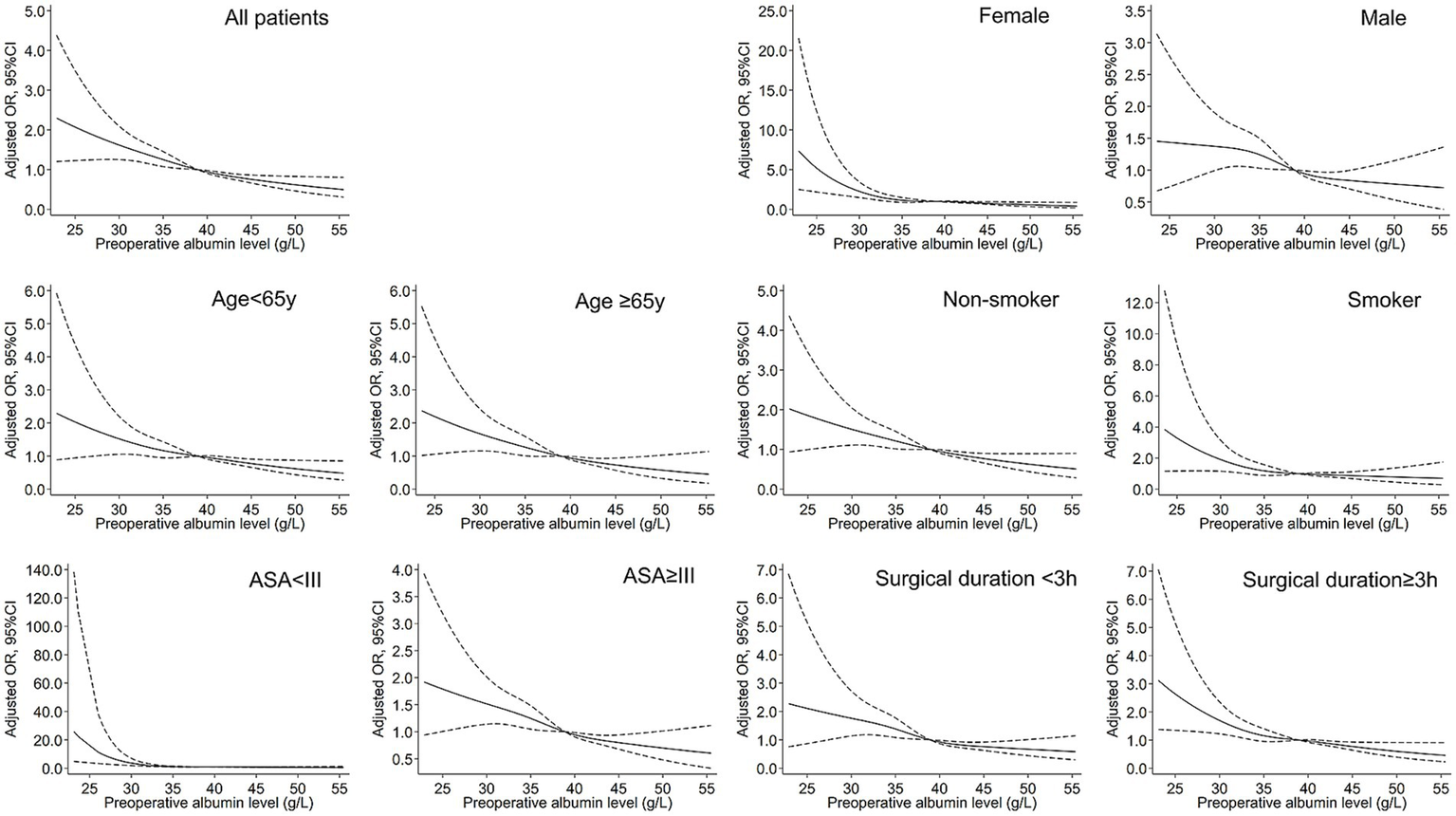
Restrictive cubic spline (RCS) models for the association between preoperative albumin and postoperative pneumonia. The solid black lines represent adjusted ORs, and the dashed lines represent upper and lower limit of 95% CI.
Figure 2
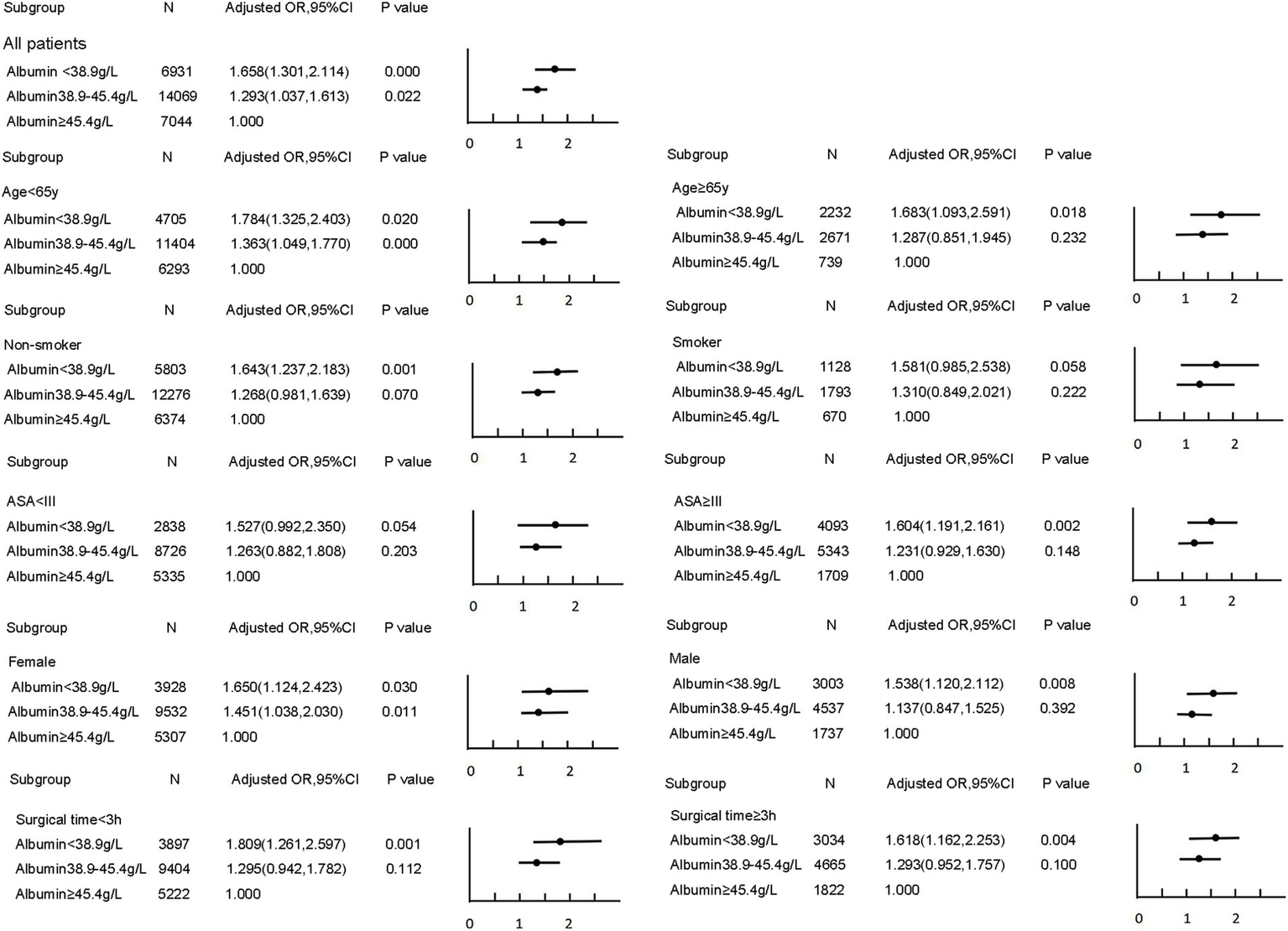
Subgroup analyses for the association between preoperative albumin and postoperative pneumonia.
Multivariate logistic regression identified nine independent risk factors for postoperative pneumonia, including lower preoperative albumin level, older age, male, smoker, ASA > II, combined heart disease, more fluid infusion, longer surgical duration and absence of hypertension (Table 3). When preoperative albumin was incorporated as a continuous variable into the multivariable logistic regression model along with the other eight variables, the aOR was 0.960, and the AUC was 0.780. When preoperative albumin was included as a categorized variable based on the three tertiles, the AUC was 0.779 (Figure 3). Sensitivity analysis further revealed that albumin was an independent risk factor even after excluding patients with hemoglobin levels below 100 g/L (n = 2,369), the aOR was 0.961 (95%CI: 0.945–0.978, p < 0.001), and the AUC was 0.779 when considered as a continuous variable; and the aOR was 0.740(95%CI: 0.635–0.861, p < 0.001) when considered as a categorized variable and the AUC was 0.778.
Table 3
| Variable | OR (95%CI) | p-value |
|---|---|---|
| Age (year) | 1.027 (1.020, 1.034) | 0.000 |
| Gender (male vs. female) | 1.915 (1.622, 2.261) | 0.000 |
| ASA (III ~ V vs. I ~ II) | 2.019 (1.765, 2.521) | 0.000 |
| Smoker (presence vs. absence) | 1.561 (1.317, 1.850) | 0.000 |
| Heart disease (presence vs. absence) | 1.460 (1.077, 1.978) | 0.015 |
| Hypertension (presence vs. absence) | 0.801 (0.662, 0.970) | 0.023 |
| Preoperative albumin (g/L) | 0.960 (0.946, 0.974) | 0.000 |
| Fluid infusion rate (mL/kg/h) | 1.018 (1.002, 1.034) | 0.026 |
| Surgical duration (min) | 1.002 (1.002, 1.003) | 0.000 |
The independent risk factors from multivariate logistic regression.
OR, odds ratio; 95%CI, 95% confidence intervals; BMI, body mass index; DM, diabetes mellitus; WBC, white blood cell.
Figure 3
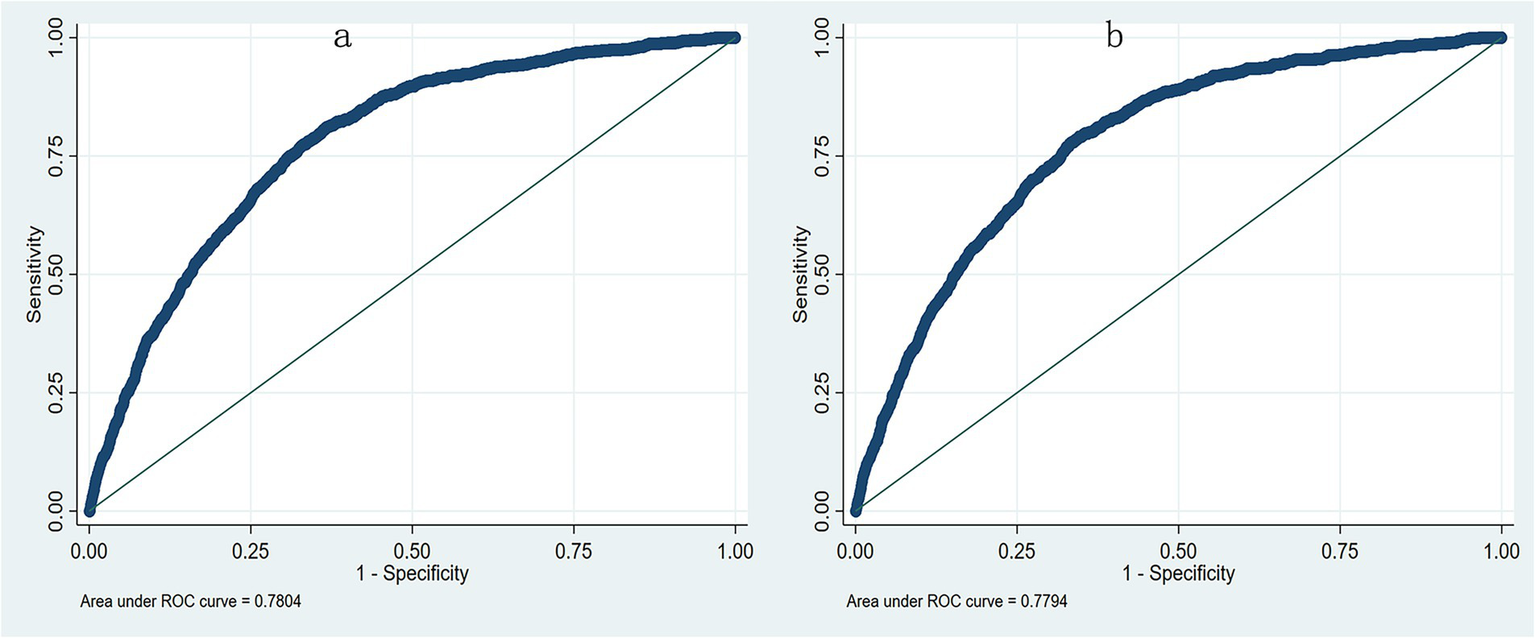
The receiver operating characteristic curve (ROC) from multivariate logistic regression model. (a) The AUC was 0.780 when preoperative albumin as a continuous variable; (b) The AUC was 0.779 when preoperative albumin as a categorized variable.
4 Discussion
This retrospective cohort study demonstrated that preoperative albumin levels were associated with postoperative in-hospital pneumonia in a non-linear response manner in patients undergoing major non-cardiac surgeries. The cut-off points in our study were based on tertiles of preoperative albumin levels (<38.9 g/L, 38.9–45.3 g/L, and >45.3 g/L). Compared with the highest tertile, n albumin level between 38.9 and 45.3 g/L was associated with a 30% increase in risk, and a level <38.9 g/L was associated with a 67% higher risk of postoperative pneumonia.
Preoperative hypoalbuminemia has been associated with postoperative adverse outcomes (9, 12, 13). For instance, Marin et al. (14) analyzed postoperative outcomes in 100 patients undergoing minor esophageal or gastric surgeries and found that patients with normal albumin levels (>35 g/L) had lower mortality. Kudsk et al. (15) conducted a retrospective study of 706 patients undergoing foregut surgery and reported that those with lower preoperative serum albumin levels had a higher incidence of postoperative complications and mortality. Wang et al. (7) identified preoperative hypoalbuminemia as a predictor of postoperative pneumonia through multivariable regression analysis in femoral neck fracture surgery. In a large retrospective study involving 135,008 patients undergoing total joint arthroplasty, preoperative hypoalbuminemia (<35 g/L) was an independent risk factor for postoperative adverse outcomes, including infection, pneumonia, sepsis, and myocardial infarction (9). Bendersky et al. (16) reported that the optimal threshold for preoperative albumin levels to predict postoperative complications in colorectal surgery was 39 g/L. In our study, a preoperative albumin level <45.3 g/L was predictive of postoperative pneumonia, which is higher than the threshold reported by Bendersky.
Malnutrition, often linked with elderly patients, female sex, and comorbidities (17, 18). In our study, patients with lower albumin levels were more likely to have comorbidities such as anemia, heart disease, hypertension, diabetes, stroke, renal disease, liver disease, and COPD. Serum albumin, the most abundant plasma protein, can be influenced by factors such as nutrition, liver dysfunction, malignancy, stress, and comorbidities (e.g., diabetes) (19), and its level serves as an indicator of nutritional status. Low albumin levels can impair immune function, increasing the likelihood of infectious complications after surgery (20).
Our results suggest that the optimal preoperative albumin level (45.3 g/L) for predicting postoperative pneumonia is higher than the clinical cutoff criteria for hypoalbuminemia (<35 g/L). While this statistically derived threshold is higher than the conventional 35 g/L diagnostic level, we suggest it not serve as a strict contraindication to surgery, but as a trigger for preoperative nutritional evaluation. In practice, levels below 45.3 g/L should prompt further assessment using tools such as NRS-2002 and consideration of time-limited nutritional interventions when clinically appropriate.
Interestingly, at lower preoperative albumin levels, the risk of pneumonia was more pronounced in younger, female, and generally healthier patients (ASA I-II). Elevated estrogen levels in young women may affect lung epithelial barrier integrity through immunomodulatory pathways. Under hypoalbuminic conditions, this regulation can become disrupted, leading to impaired clearance of lung pathogens (21). Moreover, hypoproteinemia, serving as a stress indicator, may indicate subclinical inflammation or metabolic strain. Unlike chronically ill patients, who may exhibit adapted immune response, healthier patients could experience exaggerated inflammatory reactions following surgical stress and hypoalbuminemia, thereby increasing pneumonia risk (22). Consequently, elective surgery might be deferred until an optimal preoperative albumin level is attained, thereby mitigating the risk of postoperative pneumonia and other potential complications.
Our data analysis further identified surgical duration as an independent risk factor for postoperative pneumonia. Notably, patients in the lower albumin group experienced prolonged surgical durations, which contributed to extended periods of general anesthesia and intraoperative mechanical ventilation. Longer procedures are associated with greater surgical trauma and physiological stress, often necessitating increased administration of opioids, sedatives, and neuromuscular blocking agents. These factors collectively impair respiratory function, ciliary clearance, and immune response, thereby elevating the risk of pulmonary complications. Furthermore, extended operative time may lead to a higher likelihood of prolonged postoperative mechanical ventilation and longer ICU stays, both of which are established risks for developing postoperative hospital-acquirement pneumonia (23, 24). However, our study lacks detailed perioperative data related to medication dosing, ventilation parameters, or precise ICU course. Therefore, residual confounding or unmeasured cross-effects between nutritional status and operative factors cannot be ignored. Further studies incorporating these data are warranted to disentangle these complex interactions.
Several limitations should be acknowledged. First, only in-hospital pneumonia was recorded, thus excluding later-onset cases. Second, this is a retrospective, single-center study, and the generalizability of the results is limited. Third, we did not adjust the risk ratio for certain immunosuppressive conditions such as HIV, chronic wasting diseases, or chemotherapy history (25). Future studies should incorporate these biomarkers of immune function. Additionally, perioperative nutritional support such as albumin infusion or enteral nutrition was not documented, potentially introducing unmeasured confounding. Large-scale, randomized controlled trials are warranted to validate these observations.
In conclusion, preoperative albumin level is significantly associated with postoperative pneumonia in major non-cardiac surgery patients. A threshold of 45.3 g/L appears more predictive than the conventional 35 g/L cutoff. Further research is needed to determine whether preoperative nutritional interventions aim at raising albumin can reduce the incidence of pneumonia and other postoperative complications.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of Chongqing University Cancer Hospital, China. The studies were conducted in accordance with the local legislation and institutional requirements. The requirement for informed consent was waived by the Institutional Ethics Committee of Chongqing University Cancer Hospital due to the anonymized nature of the data.
Author contributions
Q-YP: Writing – review & editing, Software, Resources, Visualization, Funding acquisition, Formal analysis, Writing – original draft. Y-MF: Software, Supervision, Writing – original draft, Data curation, Validation. Y-JY: Data curation, Software, Validation, Writing – original draft, Supervision. H-LL: Funding acquisition, Project administration, Resources, Conceptualization, Writing – review & editing, Methodology, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Chongqing Science and Health Commission Medical Research Project (No. 2021MSXM001) and the Key R&D Project of Chongqing Science and Technology Bureau (No. cstc2020jscx-dxwtBX0010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Fernandez-Bustamante A Frendl G Sprung J Kor DJ Subramaniam B Martinez Ruiz R et al . Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg. (2017) 152:157–66. doi: 10.1001/jamasurg.2016.4065
2.
Walder B Story DA . Postoperative pneumonia: can this important complication be predicted and anticipated?Eur J Anaesthesiol. (2019) 36:87–9. doi: 10.1097/EJA.0000000000000922
3.
Xu J Hu J Yu P Wang W Hu X Hou J et al . Perioperative risk factors for postoperative pneumonia after major oral cancer surgery: a retrospective analysis of 331 cases. PLoS One. (2017) 12:e0188167. doi: 10.1371/journal.pone.0188167
4.
Caparelli ML Shikhman A Jalal A Oppelt S Ogg C Allamaneni S . Prevention of postoperative pneumonia in noncardiac surgical patients: a prospective study using the National Surgical Quality Improvement Program database. Am Surg. (2019) 85:8–14. doi: 10.1177/000313481908500104 PMID:
5.
Gatta A Verardo A Bolognesi M . Hypoalbuminemia. Intern Emerg Med. (2012) 7:193–9. doi: 10.1007/s11739-012-0802-0
6.
Tan Y Jiang L Liu H Pan Z Wang H Chen L . The effect of preoperative hypoalbuminemia on complications after primary hip arthroplasty. J Orthop Surg Res. (2021) 16:562. doi: 10.1186/s13018-021-02702-0
7.
Wang Y Li X Ji Y Tian H Liang X Li N et al . Preoperative serum albumin level as a predictor of postoperative pneumonia after femoral neck fracture surgery in a geriatric population. Clin Interv Aging. (2019) 14:2007–16. doi: 10.2147/CIA.S231736
8.
Gao YC Zhang YW Shi L Gao W Li YJ Chen H et al . What are risk factors of postoperative pneumonia in geriatric individuals after hip fracture surgery: a systematic review and Meta-analysis. Orthop Surg. (2023) 15:38–52. doi: 10.1111/os.13631
9.
Kishawi D Schwarzman G Mejia A Hussain AK Gonzalez MH . Low preoperative albumin levels predict adverse outcomes after Total joint arthroplasty. J Bone Joint Surg Am. (2020) 102:889–95. doi: 10.2106/JBJS.19.00511
10.
Baba H Tokai R Hirano K Watanabe T Shibuya K Hashimoto I et al . Risk factors for postoperative pneumonia after general and digestive surgery: a retrospective single-center study. Surg Today. (2020) 50:460–8. doi: 10.1007/s00595-019-01911-9
11.
Horan TC Andrus M Dudeck MA . CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. (2008) 36:309–32. doi: 10.1016/j.ajic.2008.03.002
12.
Kim S McClave SA Martindale RG Miller KR Hurt RT . Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship?Am Surg. (2017) 83:1220–7. doi: 10.1177/000313481708301123
13.
Haskins IN Baginsky M Amdur RL Agarwal S . Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. (2017) 36:1333–8. doi: 10.1016/j.clnu.2016.08.023
14.
Marin FA Lamônica-Garcia VC Henry MA Burini RC . Grade of esophageal cancer and nutritional status impact on postsurgery outcomes. Arq Gastroenterol. (2010) 47:348–53. doi: 10.1590/s0004-28032010000400006
15.
Kudsk KA Tolley EA DeWitt RC Janu PG Blackwell AP Yeary S et al . Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enteral Nutr. (2003) 27:1–9. doi: 10.1177/014860710302700101
16.
Bendersky V Sun Z Adam MA Rushing C Kim J Youngwirth L et al . Determining the optimal quantitative threshold for preoperative albumin level before elective colorectal surgery. J Gastrointest Surg. (2017) 21:692–9. doi: 10.1007/s11605-017-3370-9
17.
Garcia GH Fu MC Dines DM Craig EV Gulotta LV . Malnutrition: a marker for increased complications, mortality, and length of stay after total shoulder arthroplasty. J Shoulder Elb Surg. (2016) 25:193–200. doi: 10.1016/j.jse.2015.07.034
18.
Ozkalkanli MY Ozkalkanli DT Katircioglu K Savaci S . Comparison of tools for nutrition assessment and screening for predicting the development of complications in orthopedic surgery. Nutr Clin Pract. (2009) 24:274–80. doi: 10.1177/0884533609332087
19.
Rungsakulkij N Tangtawee P Suragul W Muangkaew P Mingphruedhi S Aeesoa S . Correlation of serum albumin and prognostic nutritional index with outcomes following pancreaticoduodenectomy. World J Clin Cases. (2019) 7:28–38. doi: 10.12998/wjcc.v7.i1.28
20.
Weimann A Braga M Carli F Higashiguchi T Hübner M Klek S et al . Espen guideline: clinical nutrition in surgery. Clin Nutr. (2017) 36:623–50. doi: 10.1016/j.clnu.2017.02.013
21.
Dunn SE Perry WA Klein SL . Mechanisms and consequences of sex differences in immune responses. Nat Rev Nephrol. (2024) 20:37–55. doi: 10.1038/s41581-023-00787-w
22.
Ivascu R Torsin LI Hostiuc L Nitipir C Corneci D Dutu M . The surgical stress response and anesthesia: a narrative review. J Clin Med. (2024) 13:3017. doi: 10.3390/jcm13103017
23.
Miskovic A Lumb AB . Postoperative pulmonary complications. Br J Anaesth. (2017) 118:317–34. doi: 10.1093/bja/aex002
24.
Chandler D Mosieri C Kallurkar A Pham AD Okada LK Kaye RJ et al . Perioperative strategies for the reduction of postoperative pulmonary complications. Best Pract Res Clin Anaesthesiol. (2020) 34:153–66. doi: 10.1016/j.bpa.2020.04.011
25.
Munteanu C Schwartz B . The relationship between nutrition and the immune system. Front Nutr. (2022) 9:1082500. doi: 10.3389/fnut.2022.1082500
Summary
Keywords
postoperative pneumonia, albumin, non-cardiac surgery, dose–response, restricted cubic spline
Citation
Pang Q-Y, Feng Y-M, Yang Y-J and Liu H-L (2025) Association between preoperative albumin and postoperative pneumonia in patients undergoing major non-cardiac surgery. Front. Nutr. 12:1642756. doi: 10.3389/fnut.2025.1642756
Received
07 June 2025
Accepted
06 October 2025
Published
23 October 2025
Volume
12 - 2025
Edited by
Giuseppe Fiorentino, Colli Hospital, Italy
Reviewed by
Guangdong Wang, First Affiliated Hospital of Xi’an Jiaotong University, China
Xuebing Xu, Hong Kong University-Shenzhen Hospital, China
Updates
Copyright
© 2025 Pang, Feng, Yang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian-Yun Pang, pqy047417@163.com; Hong-Liang Liu, liuhl75@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.