- 1Laboratory of General Surgery, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 2Department of Proctology, Shanghang Hospital of Traditional Chinese Medicine, Longyan, Fujian Province, China
- 3Department of Neurology, Shanghang County Hospital, Longyan, Fujian Province, China
- 4Center of Hepato-Pancreato-Biliary Surgery, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 5Department of Internal Medicine, Shanghang Hospital of Traditional Chinese Medicine, Longyan, Fujian Province, China
- 6Department of Internal Medicine, Shanghai Seventh People’s Hospital, Shanghai, China
- 7Department of Pharmacy, Shanghang County Hospital, Longyan, Fujian Province, China
- 8National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
Background: Nutritional and inflammatory status have both been implicated in colon cancer risk. The advanced lung cancer inflammation index (ALI) is a composite prognostic index that incorporates body mass index (BMI), serum albumin, and neutrophil-to-lymphocyte ratio (NLR), reflecting both nutritional and systemic inflammatory states. However, its role in predicting colon cancer prevalence in elderly individuals remains unclear.
Methods: We used the ALI as a composite marker reflecting both inflammation status and nutritional health. The ALI is calculated as BMI × serum albumin/NLR, where higher values indicate better nutritional status and lower systemic inflammation. To evaluate the association between ALI and colon cancer prevalence, we conducted multivariate logistic regression, applied an Extreme Gradient Boosting (XGBoost) machine learning model, and performed subgroup analyses. Additionally, a smoothed two-piece logistic regression model was used to identify the ALI threshold predictive of colon cancer.
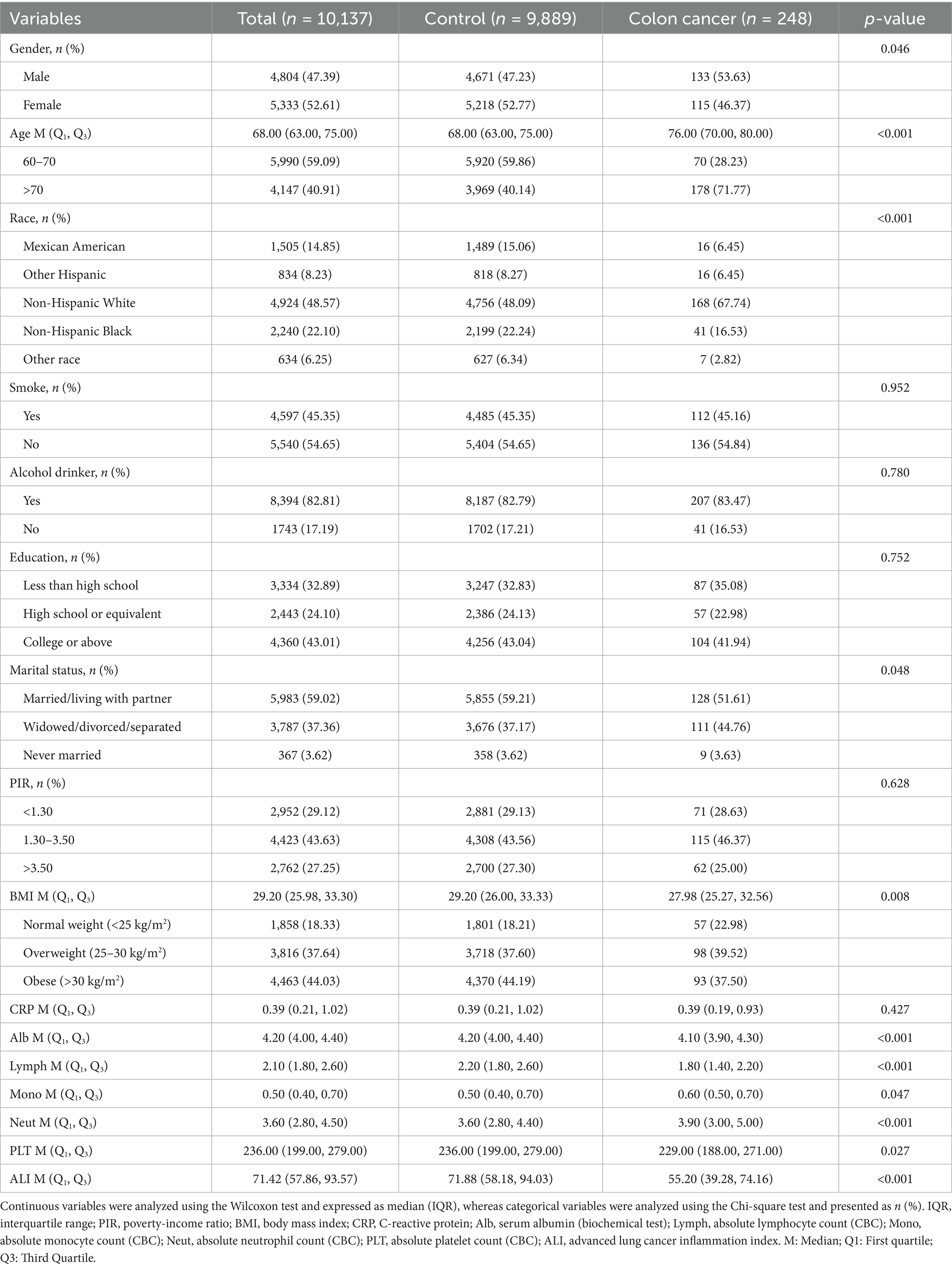
Results: The study included 10,137 elderly participants, with a colon cancer prevalence of 2.45%. The ALI was significantly lower in the colon cancer group compared to those without (p < 0.001). Multivariable logistic regression revealed a significant inverse association between ALI and colon cancer (p < 0.05), with individuals in the highest ALI tertile experiencing a 67% lower risk compared to those in the lowest tertile (p for trend = 0.008). Generalized additive models showed a linear relationship, identifying an inflection point at 4.73 and a predictive threshold of 113.3. Sensitivity analyses confirmed the robustness of these findings, particularly among individuals aged over 70 years, females, unmarried individuals, alcohol consumers, and those with a BMI below 30. In the XGBoost model, ALI demonstrated the highest predictive value for colon cancer (AUC = 0.717), outperforming traditional demographic and clinical variables such as age and BMI. Furthermore, ALI showed a positive association with dietary health status (p < 0.05) but was not significantly related to bowel habits.
Conclusion: This study demonstrated that ALI, a nutritional-inflammation prognostic index, is significantly and inversely associated with the risk of colon cancer in older adults. Thus, ALI may serve as a promising, non-invasive biomarker for risk stratification, particularly among high-risk subgroups such as unmarried females, alcohol consumers, and those with lower BMI. Its strong predictive value, confirmed by machine learning, supports its potential role in personalized prevention. Further studies are required to explore underlying mechanisms, including dietary and microbial factors.
1 Introduction
Colon cancer is one of the most common malignancies worldwide, with both incidence and mortality rates increasing steadily each year (1). In the United States, this upward trend is particularly evident among older adults, with both the incidence and mortality of colon cancer rising significantly (2). The influence of colon cancer is influenced by multiple factors, including genetics, diet, intestinal inflammation, and intestinal microbiota. Studies have also shown associations between colon cancer and factors such as obesity and marital status (3). This emphasizes the need to distinguish between modifiable (e.g., obesity, physical inactivity, dietary habits) and non-modifiable risk factors (e.g., age, sex, genetics) in developing effective prevention strategies. Inflammation is a key factor in tumor progression (4), as persistent inflammatory responses not only promote tumor cell proliferation but also impair the immune system’s ability to detect and eliminate malignant cells (5). Growing evidence suggests that inflammatory markers may serve as effective predictors of colon cancer risk (6). For example, the systemic immune-inflammation index (SII) and neutrophil-to-lymphocyte ratio (NLR) have been identified as important factors of both the incidence and prognosis of colon cancer (7). In addition, poor nutritional status can contribute to chronic intestinal inflammation, which may further promote cancer cell proliferation (8). Therefore, assessing the combined influence of nutrition and inflammation could offer valuable insight for developing clinical strategies aimed at reducing the risk of colon cancer. The advanced lung cancer inflammation index (ALI) differs from previously established markers by incorporating not only the NLR and albumin (Alb), but also body mass index (BMI) (9), thereby providing a more comprehensive representation of both inflammation and nutritional status in patients with advanced lung cancer (10). This has demonstrated superior prognostic performance compared to other inflammation- and nutrition-based indices (11).
Compared to traditional nutritional risk indices such as the Buzby index or the Geriatric Nutritional Risk Index (GNRI), ALI offers several advantages. First, ALI integrates both inflammatory (NLR) and nutritional (Alb and BMI) components, providing a more comprehensive assessment of patient status. Second, it is simple to calculate using routinely available clinical data. Third, ALI has been validated in various malignancies and chronic diseases as a robust predictor of survival outcomes, making it a clinically practical and prognostically meaningful tool. Emerging evidence also suggests that ALI may serve as a prognostic indicator in breast cancer (12). Previous studies indicated that lower ALI values are associated with poorer survival outcomes in patients with colorectal and gastric cancers, supporting its role as a nutritional prognostic index in these malignancies (13). Importantly, higher ALI values indicate better nutritional status and lower inflammation, which may be protective against cancer risk and progression. Besides oncology, ALI has been widely applied to assess various inflammation- and nutrition-related diseases, including Crohn’s disease, coronary artery disease, and heart failure (14). However, despite its growing clinical relevance, the relationship between ALI and colon cancer remains insufficiently explored. To address this gap, the present study aimed to investigate the association between ALI and colon cancer in elderly individuals in the United States, using data from the 1999–2020 National Health and Nutrition Examination Survey (NHANES).
2 Methods
2.1 Study population
This study used data from the NHANES database, which employs interviews and physical examinations to evaluate the health and nutritional status of adults and children in the U.S. Since 1999, it has been conducted biennially. The survey protocol was approved by the Research Ethics Committee of the National Center for Health Statistics (NCHS) and the Centers for Disease Control and Prevention (CDC). A written informed consent was obtained from all participants or their legal representatives.
For this analysis, we extracted NHANES data from 1999 to 2020 from individuals aged 60 to 85 years. It should be noted that 85 years was not a strict upper inclusion limit; instead, it reflects the maximum age recorded in publicly available NHANES data due to confidentiality constraints. Participants were excluded if they lacked information on cancer diagnosis, nutritional and inflammatory status, education level, marital status, family income, BMI, ethnicity, smoking, alcohol consumption, or other relevant variables, such as C-reactive protein (CRP), AlB, NLR, and other sociodemographic indicators. Additionally, only datasets with a missing data rate below 20% were included. After applying these criteria, a total of 10,137 eligible participants aged 60 years and older were included in the final analysis (Supplementary Figure S1).
2.2 Evaluation of the ALI
ALI was calculated using the formula ALI = BMI × Alb/NLR, where NLR = absolute neutrophil count/absolute lymphocyte count (15). Based on the calculated ALI values, participants were categorized into three groups: low (<61.67), moderate (61.67–84.90), and high (>84.90). These cut-off points correspond to the 33rd and 66th percentiles of the ALI distribution within the study population. Although geriatric-specific nutritional indices like the GNRI have been widely used in older populations, we selected the ALI in this study due to its validated utility across various age groups and its ability to comprehensively reflect both systemic inflammation and nutritional status. In addition, the ALI is a novel and convenient single-index marker that has not yet been reported in the context of colorectal cancer. Given the pivotal role of chronic inflammation in CRC development, the ALI may provide additional clinical insight beyond age-specific nutritional risk scores.
2.3 Assessment of colon cancer
Data were obtained from the Medical Conditions Questionnaire, in which participants were first asked, “Have you ever been told by a doctor or other health professional that you have cancer or any malignancy?” Those who answered “yes” were then asked to specify the type of cancer. Patients who reported only colon cancer (including both primary and isolated tumors) were classified as having colon cancer. In contrast, individuals who reported no history of cancer, a history of other malignancies, or colon cancer combined with other cancer types were categorized as not having colon cancer (16).
2.4 Covariate selection
Based on a review of relevant literature and clinical knowledge, we identified a set of covariates associated with colon cancer to include in our study (Supplementary Figure S2). These covariates included sex (male or female), age (continuous), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other races), smoking status (yes/no), alcohol consumption (yes/no), education level (less than high school, high school or equivalent, and more than high school), BMI (<25 kg/m2, 25–30 kg/m2, and >30 kg/m2), marital status (married/living with partner, widowed/divorced/separated, and never married), and poverty-to-income ratio (PIR) (<1.30, 1.30–3.50, and >3.50). Dietary health status was assessed using the NHANES variable DBQ700, which asked participants to self-assess the overall healthfulness of their diet. Responses were categorized as follows: “Good” (responses 1 = Excellent, 2 = Very good, 3 = Good), “Fair” (response 4), “Poor” (response 5). Participants who responded “Refused,” “Do not know,” or had missing values were excluded. Stool type and defecation frequency were derived from the NHANES variable BHQ060. Chronic constipation was defined as responses 1–2, chronic diarrhea as responses 6–7, and normal stool patterns as responses 3–5. Participants with missing, unknown, or refused answers were excluded. We assessed the normality of continuous variables using the Shapiro–Wilk test and visual inspection of histograms.
2.5 Statistical analysis
The analyzed data were weighted following the guidelines provided by NCHS. Due to the marked imbalance between the number of colon cancer cases and controls, we applied NHANES-recommended sampling weights in all analyses to reduce potential bias. In addition, sensitivity analyses including subgroup analyses and propensity score matching (PSM) were conducted to further address group imbalance and assess the robustness of our findings. Participants were divided into two groups based on the presence or absence of colon cancer. Continuous variables are presented as medians with interquartile ranges (IQRs), and categorical variables are expressed as counts and percentages. Nonparametric tests were used to compare continuous variables, while chi-square tests were applied to compare categorical variables. The ALI values were log-transformed using base 10 logarithm (log₁₀) to normalize their distribution and analyzed both as a continuous variable and as tertiles in regression models. Logistic regression models were employed to examine the association between ALI and colon cancer, and a linear trend test was conducted to evaluate the consistency of this relationship across tertiles. All multivariable models were adjusted for key covariates based on clinical relevance and data availability. These included demographic variables (age, sex, race/ethnicity, marital status, education level, and family income-to-poverty ratio [PIR]); lifestyle factors (smoking status and alcohol consumption); anthropometric indicators (BMI); and inflammatory/nutritional markers (serum albumin and C-reactive protein [CRP]). To further explore the relative contribution of various factors to colon cancer incidence, we applied an Extreme Gradient Boosting (XGBoost) machine learning algorithm. The model was trained with a learning rate of 0.3, a maximum tree depth of 8, and 500 trees to optimize performance while minimizing overfitting. Hyperparameter tuning was performed via 10-fold cross-validation (17, 18). The predictive performance of each variable was evaluated using the area under the receiver operating characteristic curve (AUC), mean squared error (MSE), and root mean squared error (RMSE). To interpret the model’s internal logic and assess the contribution of each predictor, we used Shapley Additive Explanations (SHAP) values. These values provide an interpretable summary of variable importance by quantifying each feature’s impact on model output across all permutations. To evaluate the potential nonlinear relationship between ALI and colon cancer, a restricted cubic spline (RCS) curve was generated. Based on this curve, the inflection point was identified using a recursive algorithm, and a two-piece linear regression model was constructed accordingly. Additionally, the relationship between ALI and intestinal health status was explored to gain further insight into the potential mechanisms underlying the association between ALI and colon cancer.
To confirm the reliability of our findings, we conducted a series of sensitivity analyses. First, individuals with other malignant tumors were excluded, and a preliminary analysis was performed to assess potential bias introduced by these participants. Second, subgroup analyses were conducted based on key covariates and their interactions with ALI, with adjustments made for potential confounders. Third, PSM at a 1:1 ratio was performed to balance covariate distributions between the colon cancer group and the control group. Matching variables included sex, age, race/ethnicity, educational level, marital status, family income, and BMI. Following PSM, the matched study population was reanalyzed to verify the robustness of the observed associations. Furthermore, to assess the discriminatory ability of ALI in identifying individuals with colon cancer, we constructed a receiver operating characteristic (ROC) curve and calculated the area under the curve (AUC). This analysis served to validate the predictive performance of ALI in our model beyond its statistical significance in regression. All statistical analyses were performed using R software. A p-value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
A total of 10,137 participants were included in this study, among whom 248 were diagnosed with colon cancer, accounting for 2.45%, as shown in Table 1. Compared with the control group, participants with colon cancer were more likely to be older, of non-Hispanic White ethnicity, married or living with a partner, overweight, and to have a lower level of Alb, absolute lymphocyte count, absolute monocyte count, neutrophil/lymphocyte ratio, and low platelet count (p < 0.05). The ALI value was significantly lower in the colon cancer group [median (IQR): 55.20 (39.28, 74.16)] compared to the control group [median (IQR): 71.88 (58.18, 94.03)] (p < 0.001), suggesting that a lower ALI is associated with poor nutrition and higher systemic inflammation.
3.2 Correlation between ALI and colon cancer incidence
After log transformation, ALI was analyzed both as a continuous and a categorical variable. The association between log ALI and the incidence of colon cancer was analyzed using logistic regression, as presented in Table 2. A significant inverse relationship was observed between ALI and colon cancer across all models, with odds ratios in Model 1 (0.03 [0.02–0.05]), Model 2 (0.05 [0.03–0.07]), and Model 3 (0.05 [0.03–0.07]). Sensitivity analysis using tertile-based categorization of log-transformed ALI further supported this finding. Participants were categorized into tertiles based on the empirical distribution of log ALI values: T1 (<2.95), T2 (2.95–3.45), and T3 (>3.45). In both Models 2 and 3, individuals in T3 had approximately a 67% lower risk of colon cancer compared to those in T1 (Model 3: OR = 0.33, 95% CI: 0.17–0.65; P-value for trend = 0.008).
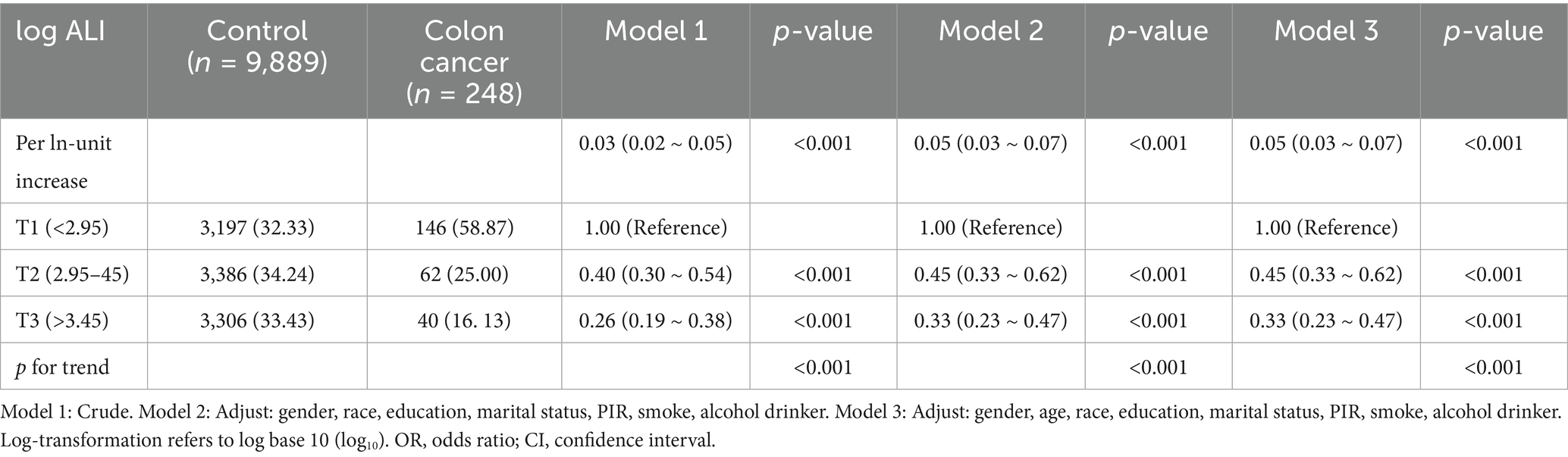
Table 2. Association of advanced log ALI among the US participants aged 60 to 85 years, NHANES, 1999 to 2020.
3.3 Exploration of nonlinear relationships
Using generalized additive models (GAM) and smoothed curve fitting, we examined the relationship between ALI and colon cancer and found a linear association after adjusting for all variables (nonlinear p-value < 0.001; log-likelihood ratio test p < 0.001) (Supplementary Figure S3). A comparison between the linear regression model and the two-stage linear regression model (Supplementary Table S1) indicated that the two-stage regression model provided a better fit for the data. Using the recursive method and two-stage regression model, the inflection point was identified as 4.73, and the corresponding ALI threshold was calculated as 113.3, which may represent a potential cut-off value for reducing the risk of colon cancer.
3.4 Sensitivity analysis
Multiple sensitivity analyses were performed to evaluate the reliability of the findings. First, excluding participants with other malignant tumors from the control group yielded results consistent with the primary analysis (Supplementary Table S2). Second, subgroup analyses demonstrated that higher ALI levels were significantly associated with a reduced risk of colon cancer across various subpopulations, supporting a potential protective effect. The protective effect of ALI was more pronounced among individuals aged over 70 years, females, unmarried individuals, those who consumed alcohol, and those with a BMI <30. The presence of potential interactions among several subgroups indicates that the protective effect of ALI on colon cancer may be modulated by individual metabolic status, lifestyle factors, and socioeconomic conditions. (Figure 1). Subgroup analyses were conducted to examine whether the association between ALI and colon cancer varied across different strata (e.g., age, sex, BMI). Figure 1 presents the odds ratios and 95% confidence intervals of ALI for colon cancer risk across these subgroups. These comparisons are exploratory and should not be interpreted as indicating causal relationships between ALI and the stratifying variables. After performing PSM, 468 participants were retained in this analysis, with 234 individuals in both the colon cancer and control groups. Differences in variables between the groups were noted before and after PSM (Supplementary Figure S4). Regardless of model adjustment, ALI remained significantly associated with colon cancer (Supplementary Table S3).
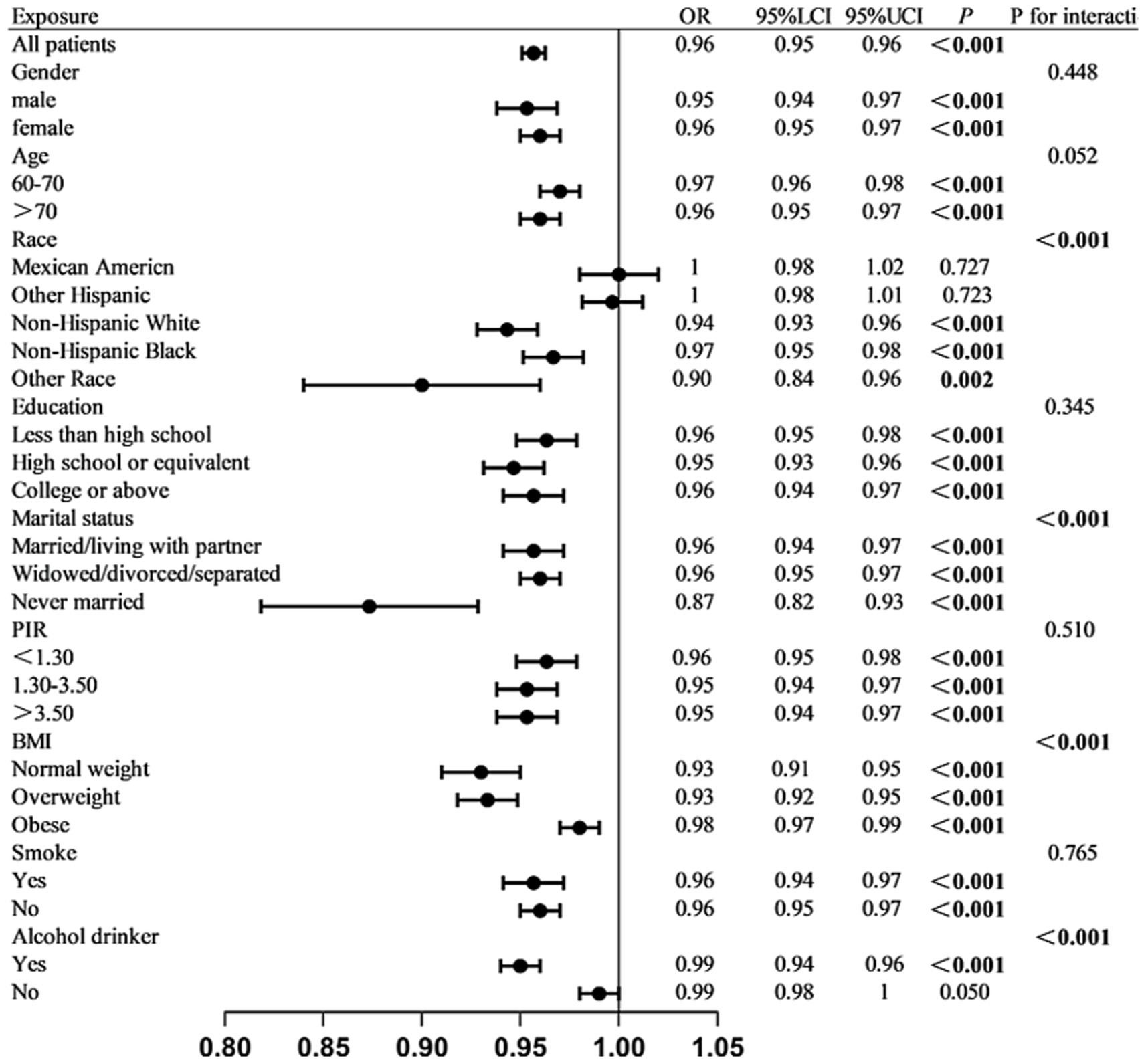
Figure 1. Stratified association between log₁₀-transformed ALI and colon cancer across subgroups. Multivariable logistic regression analyses were conducted in various subgroups to assess the association between ALI and colon cancer. Estimates were adjusted for all covariates listed in the Methods section unless otherwise specified. Interaction p-values are provided for subgroup comparisons.
3.5 Predictive analysis via XGBoost model
Among all the variables analyzed in this study, ALI showed the strongest predictive ability for colon cancer incidence, with an area under the curve (AUC) of 0.717 (Figure 2). Age ranked second with an AUC of 0.712, followed by BMI with an AUC of 0.549. Using the XGBoost machine learning model, the final prediction showed a mean square error (MSE) of 0.08, indicating minimal deviation between predicted and observed values. Additionally, the root mean square error (RMSE) was 0.28, further demonstrating the model’s high predictive accuracy. Figure 3 summarizes the SHAP analysis results of the XGBoost model. ALI emerged as the most influential predictor of colon cancer incidence, followed by age and BMI. The SHAP summary plot provides an interpretable visualization of the contribution of each variable to model predictions. The importance matrix and SHAP dependence plot further confirmed the prominent role of ALI in the model. These findings reinforce the robust predictive value of ALI and highlight its potential utility in early risk stratification for colon cancer.
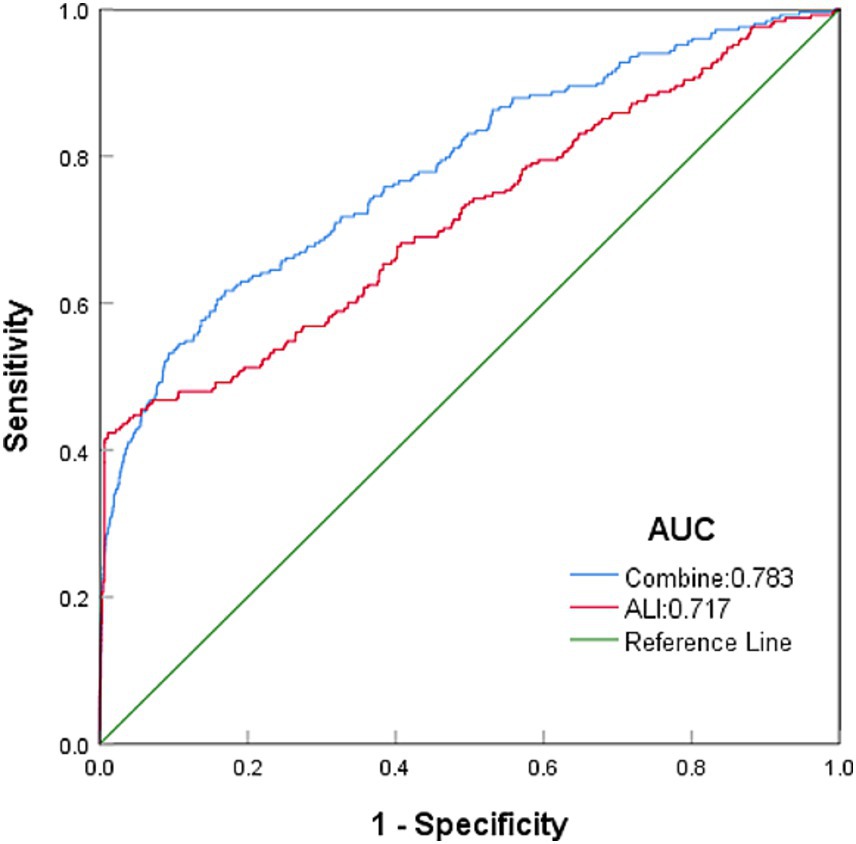
Figure 2. Top predictors of colon cancer identified by the XGBoost model, ranked by AUC. To compare the predictive ability of ALI and other variables, we used the XGBoost machine learning model with 10-fold cross-validation. The AUC values of the top variables are displayed. ALI ranked as the strongest predictor, followed by age and BMI.
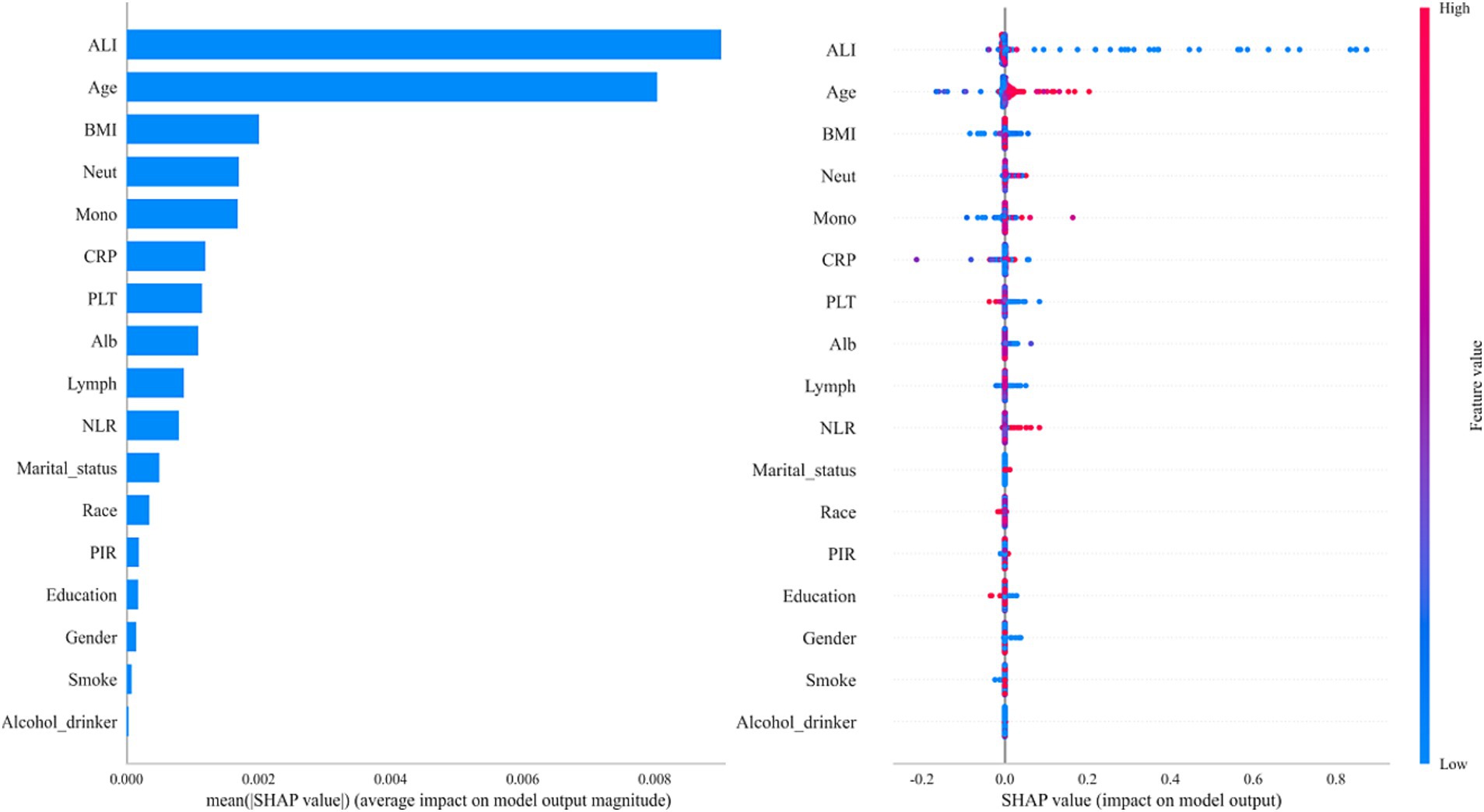
Figure 3. SHAP summary plot demonstrating the contribution of each variable to the XGBoost model. SHAP values were used to interpret the contribution of each feature in the model. The plot illustrates both the magnitude and direction (positive or negative) of each variable’s impact on the predicted colon cancer risk.
3.6 Investigation of potential links
Furthermore, this study also evaluated the relationship between ALI and dietary health status, stool type, and the frequency of defecation per week after full adjustment for confounders. A significant positive association was observed between dietary health status and ALI in both the total population and the control group (Table 3). In contrast, no significant associations were found between ALI and either stool type or weekly defecation frequency in either group (Supplementary Table S4).
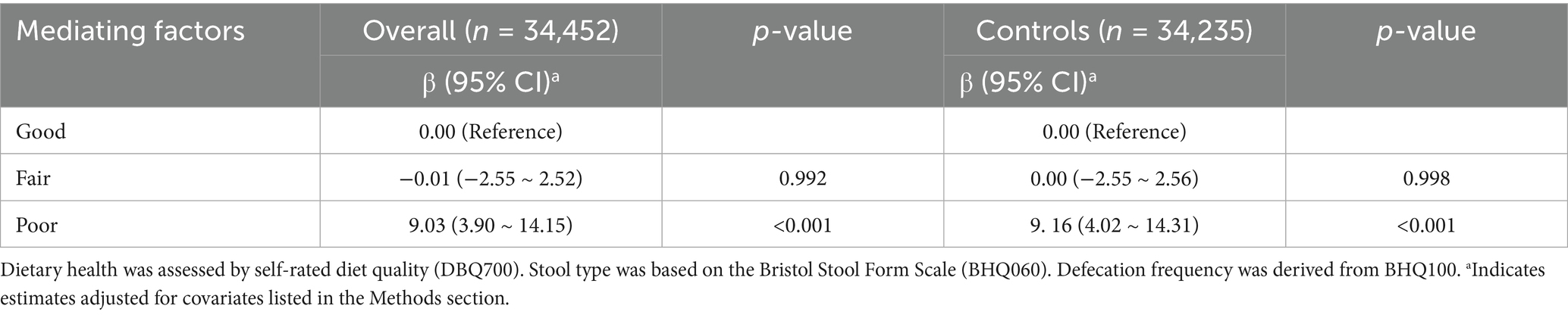
Table 3. The association between ALI and dietary health status in the overall population and control group.
4 Discussion
This study demonstrated a strong and consistent inverse linear association between the ALI and the incidence of colon cancer, indicating that higher ALI levels may have a protective effect. Among all variables examined, ALI emerged as the most significant predictor of colon cancer risk, as confirmed by the XGBoost machine learning model. Furthermore, a significant association between ALI and dietary health status was identified, suggesting a potential association between the nutritional-inflammatory balance and colorectal cancer occurrence. These findings suggest that higher ALI levels and healthy dietary patterns are associated with lower colon cancer risk, though causal relationships cannot be established.
Using a smoothed two-stage logistic regression model, the ALI threshold for colon cancer incidence was identified as 113.3. This threshold determination plays a significant clinical role in developing personalized prevention strategies and implementing early interventions for high-risk populations.Subgroup analyses revealed that higher ALI levels were significantly associated with reduced odds of colon cancer in specific populations, suggesting a potential protective effect. This supports the interpretation that higher ALI values reflect better nutritional and lower inflammatory status. This inverse association was particularly evident among individuals aged over 70 years, females, unmarried individuals, those who consumed alcohol, and those with a BMI below 30. These findings may reflect the stronger influence of nutritional and inflammatory imbalance in populations with limited social support (19), or those experiencing alcohol-related metabolic disturbances (20). Although not all subgroup associations reached statistical significance, the consistent negative trend across groups highlights the potential of ALI as a universal prognostic biomarker.
The ALI demonstrates strong predictive power for colon cancer incidence among the variables analyzed in this study. The XGBoost model identified ALI as one of the top three most influential predictors. As an advanced machine learning algorithm, XGBoost can handle large-scale datasets and uncover complex nonlinear relationships among variables. It has been widely validated in many medical studies for its effectiveness in predicting disease risks, particularly in conditions characterized by multi-factor interactions (21, 22).Further exploration of the relationship between ALI and dietary health status, stool type, and weekly defecation frequency revealed a significantly positive correlation between ALI and dietary health status. This association may be attributed to the impact of diet on the intestinal microbiota, which plays a critical role in colon cancer development. For example, diets high in red meat may promote the growth of certain bacteria, such as Klebsiella, Clostridium, and Staphylococcus, which can produce carcinogens and promote intestinal carcinogenesis (23). High-fat diets have been shown to increase the production of secondary bile acids such as deoxycholic acid (DCA) and lithocholic acid (LCA) (24). Similarly, a high-fructose diet has been shown to alter the proliferation and cell growth induced by the intestinal microbiota in pancreatic and colon cancer cell lines (25), whereas high-sugar diets may disrupt the intestinal microbiota balance and facilitate the translocation of bacteria and toxins to enter the bloodstream, thereby promoting cancer development (26). In contrast, diets rich in dietary fiber, low in fat, and abundant in antioxidants and fermented foods can promote the fermentation of intestinal microbiota to produce short-chain fatty acids (SCFAs) such as acetic acid, propionic acid, and butyric acid (27). These SCFAs improve intestinal barrier function and exhibit anti-inflammatory effects, which may lower colon cancer risk (28). Butyric acid, in particular, is considered a potent anti-cancer substance that regulates proliferation, differentiation, and apoptosis of intestinal epithelial cells (29, 30), and also modulates immune responses while reducing inflammation (31, 32). Therefore, considering the close association between gut microbiota dysbiosis and colon cancer, and the role of a healthy diet in preserving microbial homeostasis, our study suggests that the observed association between ALI and colon cancer may be partly explained by its correlation with healthy dietary patterns and potential links with gut microbiota.
Numerous studies have demonstrated a close association between colon cancer and inflammatory responses, with inflammation contributing to tumor development by activating immune pathways and compromising the integrity of the intestinal barrier (33). Patients with inflammatory bowel disease (IBD) exhibit a significantly higher incidence of colon cancer compared to the general population (34). ALI, as a composite marker inflecting both inflammation status and nutritional health, is relevant not only in various types of cancer but also in influencing the onset and prognosis of colon cancer (35). Several studies have highlighted the predictive value of ALI in the development and progression of multiple types of cancer (36). Maintaining good nutritional status plays a critical role in preserving intestinal barrier function, minimizing inflammatory responses, and thereby reducing colon cancer risk (37, 38). The presence of potential interactions among several subgroups suggests that the observed association between ALI and colon cancer may be influenced by lifestyle factors and socioeconomic conditions.
Traditional colorectal cancer (CRC) screening tools, such as carcinoembryonic antigen (CEA) and the fecal immunochemical test (FIT), focus primarily on tumor markers or occult gastrointestinal bleeding. In contrast, the ALI captures a broader physiological profile by incorporating systemic inflammation and nutritional parameters. Although CEA and FIT remain central to early CRC detection, they provide limited information on host-related factors. The ALI, calculated from routine clinical data (BMI, Alb, and NLR), is non-invasive and low-cost, and may serve as a valuable adjunct for risk evaluation in elderly individuals, who often face malnutrition and chronic inflammatory states.
This study has several strengths and limitations. First, XGBoost effectively handles high-dimensional data by automatically selecting the most predictive variables, thereby eliminating the need for manual variable selection used in traditional statistical methods. This approach enhances model accuracy, reduces the risk of overfitting, and improves generalizability to unseen data.By quantifying the contribution of each variable to the model, XGBoost helps researchers to identify key factors associated with colon cancer incidence and reveals complex nonlinear relationships among variables, offering insights for clinical decision-making, prioritizing high-risk factors, and guiding targeted prevention strategies. The combination of XGBoost with clinical practice provides a new perspective for early prevention and intervention of colon cancer and holds significant implications for public health. Second, determining the ALI threshold using an inflection point enhances the ability to identify individuals at elevated risk more accurately. Early preventive measures, such as routine screening and lifestyle modifications including diet and exercise, can be implemented to reduce colon cancer risk. Clinically, using the ALI threshold allows physicians to assess individual colon cancer risk more precisely and, when combined with other risk factors (e.g., age, sex, family history), to develop personalized health management plans. Third, this study suggests that ALI may influence colon cancer risk through its association with dietary health and the gut microbiota. Unhealthy dietary patterns may disrupt the intestinal microbiota and promote carcinogenesis. Exploring the mediating role of the gut microbiota in the ALI–colon cancer association provides novel insights for cancer prevention. These findings not only promote interdisciplinary collaboration but also offer a potential theoretical basis for public health policy development. Future research is needed to clarify the mechanistic role of the gut microbiota in colon cancer pathogenesis, which may enhance strategies for early diagnosis and prevention. Given the rising global burden of colon cancer, exploring how ALI and dietary health affect cancer development through the microbiota has both scientific relevance and practical value for global cancer prevention and health management.
One limitation of this study is the lack of detailed clinical information in the NHANES dataset, such as cancer stage or treatment history. Although we excluded participants with other self-reported malignancies using variable MCQ220 in sensitivity analyses, residual confounding may persist. Additionally, the imbalance in the number of colon cancer cases compared to controls may cause potential estimation bias. To address this, we used weighted logistic regression and conducted multiple sensitivity analyses, including subgroup analyses and propensity score matching, which helped ensure the robustness and reliability of our findings. Another limitation of this study is the lack of direct analysis of dietary patterns and gut microbiota in the study population. Thus, future research should incorporate these factors to explore the underlying mechanisms linking ALI to colon cancer.
5 Conclusion
This study, based on a large nationally representative sample, demonstrated a significant inverse association between ALI and the risk of colon cancer in older adults. ALI, calculated from BMI, serum albumin, and NLR, is a nutritional-inflammation prognostic index that reflects both systemic inflammation and nutritional status. Our findings remained robust across multiple analytical models and subgroups. The predictive value of ALI was further validated using an XGBoost machine learning model, in which ALI emerged as the most important feature.
We also found a positive association between ALI and dietary health status, suggesting a link between nutritional-inflammatory balance and colon cancer risk. However, as gut microbiota data were not assessed in this study, any mechanistic speculation involving microbiome pathways remains premature and requires further investigation.
Collectively, these results indicated that ALI may serve as a promising, non-invasive index for stratifying the risk of prevalent colon cancer in elderly adults. Given its accessibility and clinical interpretability, ALI could be integrated into risk-based screening strategies to support personalized cancer prevention. Future prospective studies incorporating detailed dietary data and gut microbiome profiling are needed to clarify the underlying biological mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the survey protocol, which was approved by the NCHS Ethics Review Board. The approved protocol can be found at this link: https://www.cdc.gov/nchs/nhanes/about/erb.html. Additionally, every participant in the survey supplied written informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study was based on secondary analysis of publicly available and de-identified data from the National Health and Nutrition Examination Survey (NHANES). All NHANES participants provided written informed consent at the time of data collection. The use of this anonymized data does not require additional ethical approval or written informed consent.
Author contributions
HZ: Writing – original draft. YC: Writing – original draft, Data curation. WW: Writing – original draft, Data curation. SL: Formal analysis, Writing – review & editing. YF: Writing – review & editing, Formal analysis. HL: Writing – review & editing, Formal analysis. RL: Writing – review & editing, Formal analysis. JW: Writing – review & editing. MH: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the GuangDong Basic and Applied Basic Research Foundation (no. 2022A1515220130), Science and Technology Program of Guangzhou (2024A04J6489), and Sanming Project of Medicine in Shenzhen (no. SZSM202311002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1642913/full#supplementary-material
References
1. Kocarnik, JM, Compton, K, Dean, FE, Fu, W, Gaw, BL, Harvey, JD, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. (2022) 8:420–44. doi: 10.1001/jamaoncol.2021.6987
2. Xia, C, Dong, X, Li, H, Cao, M, Sun, D, He, S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
3. Lukic, M, Licaj, I, Laaksonen, MA, Weiderpass, E, Borch, KB, and Rylander, C. The burden of colon cancer attributable to modifiable factors—the Norwegian women and Cancer study. Int J Cancer. (2023) 152:195–202. doi: 10.1002/ijc.34237
4. Shah, SC, and Itzkowitz, SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. (2022) 162:715–730.e3. doi: 10.1053/j.gastro.2021.10.035
5. Borgia, M, Dal Bo, M, and Toffoli, G. Role of virus-related chronic inflammation and mechanisms of cancer immune-suppression in pathogenesis and progression of hepatocellular carcinoma. Cancers (Basel). (2021) 13:4387. doi: 10.3390/cancers13174387
6. Huang, L, Hu, Z, Luo, R, Li, H, Yang, Z, Qin, X, et al. Predictive values of the selected inflammatory indexes in colon cancer. Cancer Control. (2022) 29:10732748221091333. doi: 10.1177/10732748221091333
7. Xiang, S, Yang, YX, Pan, WJ, Li, Y, Zhang, JH, Gao, Y, et al. Prognostic value of systemic immune inflammation index and geriatric nutrition risk index in early-onset colorectal cancer. Front Nutr. (2023) 10:1134300. doi: 10.3389/fnut.2023.1134300
8. Sato, R, Oikawa, M, Kakita, T, Okada, T, Abe, T, Yazawa, T, et al. The prognostic value of the prognostic nutritional index and inflammation-based markers in obstructive colorectal cancer. Surg Today. (2020) 50:1272–81. doi: 10.1007/s00595-020-02062-0
9. Mandaliya, H, Jones, M, Oldmeadow, C, and Nordman, IIC. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. (2019) 8:886–94. doi: 10.21037/tlcr.2019.11.20
10. Chen, Y, Guan, M, Wang, R, and Wang, X. Relationship between advanced lung cancer inflammation index and long-term all-cause, cardiovascular, and cancer mortality among type 2 diabetes mellitus patients: NHANES, 1999–2018. Front Endocrinol (Lausanne). (2023) 14:1298345. doi: 10.3389/fendo.2023.1298345
11. Song, M, Zhang, Q, Song, C, Liu, T, Zhang, X, Ruan, G, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. (2022) 13:2504–14. doi: 10.1002/jcsm.13045
12. Gao, X, Qi, J, Du, B, Weng, X, Lai, J, and Wu, R. Combined influence of nutritional and inflammatory status and breast cancer: findings from the NHANES. BMC Public Health. (2024) 24:2245. doi: 10.1186/s12889-024-18533-0
13. Liu, XR, Wang, LL, Zhang, B, Liu, XY, Li, ZW, Kang, B, et al. The advanced lung cancer inflammation index is a prognostic factor for gastrointestinal cancer patients undergoing surgery: a systematic review and meta-analysis. World J Surg Oncol. (2023) 21:81. doi: 10.1186/s12957-023-02982-z
14. Chen, X, Hong, C, Guo, Z, Huang, H, and Ye, L. Association between advanced lung cancer inflammation index and all-cause and cardiovascular mortality among stroke patients: NHANES, 1999–2018. Front Public Health. (2024) 12:1370322. doi: 10.3389/fpubh.2024.1370322
15. Tu, J, Wu, B, Xiu, J, Deng, J, Lin, S, Lu, J, et al. Advanced lung cancer inflammation index is associated with long-term cardiovascular death in hypertensive patients: National Health and nutrition examination study, 1999–2018. Front Physiol. (2023) 14:1074672. doi: 10.3389/fphys.2023.1074672
16. Guo, F, Wang, M, Guo, X, Pu, L, Sun, M, Li, S, et al. The association between fatty acid intake and breast cancer based on the NHANES and Mendelian randomization study. Cancer Epidemiol. (2021) 73:101966. doi: 10.1016/j.canep.2021.101966
17. Zhang, Z, Ho, KM, and Hong, Y. Machine learning for the prediction of volume responsiveness in patients with oliguric acute kidney injury in critical care. Crit Care. (2019) 23:112. doi: 10.1186/s13054-019-2392-8
18. Livne, M, Boldsen, JK, Mikkelsen, IK, Fiebach, JB, Sobesky, J, and Mouridsen, K. Boosted tree model reforms multimodal magnetic resonance imaging infarct prediction in acute stroke. Stroke. (2018) 49:912–8. doi: 10.1161/STROKEAHA.117.019242
19. Wang, L, Wilson, SE, Stewart, DB, and Hollenbeak, CS. Marital status and colon cancer outcomes in US surveillance, epidemiology and end results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. (2011) 35:417–22. doi: 10.1016/j.canep.2011.04.006
20. Goldwater, D, Karlamangla, A, Merkin, SS, and Seeman, T. Compared to non-drinkers, individuals who drink alcohol have a more favorable multisystem physiologic risk score as measured by allostatic load. PLoS One. (2019) 14:e0223168. doi: 10.1371/journal.pone.0223168
21. Dinh, A, Miertschin, S, Young, A, and Mohanty, SD. A data-driven approach to predicting diabetes and cardiovascular disease with machine learning. BMC Med Inform Decis Mak. (2019) 19:211. doi: 10.1186/s12911-019-0918-5
22. Liu, P, Fu, B, Yang, SX, Deng, L, Zhong, X, and Zheng, H. Optimizing survival analysis of XGBoost for ties to predict disease progression of breast cancer. IEEE Trans Biomed Eng. (2021) 68:148–60. doi: 10.1109/TBME.2020.2996293
23. Farvid, MS, Sidahmed, E, Spence, ND, Mante Angua, K, Rosner, BA, and Barnett, JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. (2021) 36:937–51. doi: 10.1007/s10654-021-00747-w
24. Zeng, H, Umar, S, Rust, B, Lazarova, D, and Bordonaro, M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci. (2019) 20:1214. doi: 10.3390/ijms20051214
25. Proaño-Vasco, A, Baumeister, T, Metwaly, A, Reitmeier, S, Kleigrewe, K, Meng, C, et al. High-fructose diet alters intestinal microbial profile and correlates with early tumorigenesis in a mouse model of Barrett’s esophagus. Microorganisms. (2021) 9:2432. doi: 10.3390/microorganisms9122432
26. Pushpanathan, P, Mathew, GS, Selvarajan, S, Seshadri, KG, and Srikanth, P. Gut microbiota and its mysteries. Indian J Med Microbiol. (2019) 37:268–77. doi: 10.4103/ijmm.IJMM_19_413
27. Hills, RD Jr, Pontefract, BA, Mishcon, HR, Black, CA, Sutton, SC, and Theberge, CR. Gut microbiome: profound implications for diet and disease. Nutrients. (2019) 11:1613. doi: 10.3390/nu11071613
28. Sonnenburg, ED, Smits, SA, Tikhonov, M, Higginbottom, SK, Wingreen, NS, and Sonnenburg, JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. (2016) 529:212–5. doi: 10.1038/nature16504
29. Kaźmierczak-Siedlecka, K, Marano, L, Merola, E, Roviello, F, and Połom, K. Sodium butyrate in both prevention and supportive treatment of colorectal cancer. Front Cell Infect Microbiol. (2022) 12:1023806. doi: 10.3389/fcimb.2022.1023806
30. Che, Y, Chen, G, Guo, Q, Duan, Y, Feng, H, and Xia, Q. Gut microbial metabolite butyrate improves anticancer therapy by regulating intracellular calcium homeostasis. Hepatology. (2023) 78:88–102. doi: 10.1097/HEP.0000000000000313
31. Feitelson, MA, Arzumanyan, A, Medhat, A, and Spector, I. Short-chain fatty acids in cancer pathogenesis. Cancer Metastasis Rev. (2023) 42:677–98. doi: 10.1007/s10555-023-10168-6
32. Liu, G, Tang, J, Zhou, J, and Dong, M. Short-chain fatty acids play a positive role in colorectal cancer. Discov Oncol. (2024) 15:425. doi: 10.1007/s12672-024-00797-0
33. Dolan, RD, Pennel, K, Thompson, J, McKenzie, M, Alexander, P, Richards, C, et al. The relationship between tumour necrosis, systemic inflammation, body composition and survival in patients with colon cancer. BJC Rep. (2025) 3:7. doi: 10.1016/j.bjcre.2024.100007
34. Shahgoli, VK, Noorolyai, S, Ahmadpour Youshanlui, M, Saeidi, H, Nasiri, H, Mansoori, B, et al. Inflammatory bowel disease, colitis, and cancer: unmasking the chronic inflammation link. Int J Color Dis. (2024) 39:173. doi: 10.1007/s00384-023-04550-4
35. Zhang, X, Wang, D, Sun, T, Li, W, and Dang, C. Advanced lung cancer inflammation index (ALI) predicts prognosis of patients with gastric cancer after surgical resection. BMC Cancer. (2022) 22:684. doi: 10.1186/s12885-022-09857-z
36. Xie, H, Huang, S, Yuan, G, Kuang, J, Yan, L, Wei, L, et al. The advanced lung cancer inflammation index predicts short and long-term outcomes in patients with colorectal cancer following surgical resection: a retrospective study. PeerJ. (2020) 8:e10100. doi: 10.7717/peerj.10100
37. Yang, M, Lin, SQ, Liu, XY, Tang, M, Hu, CL, Wang, ZW, et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: from the investigation on nutrition status and clinical outcome of common cancers study. Front Immunol. (2023) 14:1131496. doi: 10.3389/fimmu.2023.1131496
Keywords: inflammation, nutrition, inflammation index of advanced cancer, colon cancer, NHANES
Citation: Zhong H, Chen Y, Wu W, Liu S, Fan Y, Liu H, Lin R, Wan J and He M (2025) Advanced lung cancer inflammation index as a new predictor for colon cancer in elderly patients: an NHANES-based study. Front. Nutr. 12:1642913. doi: 10.3389/fnut.2025.1642913
Edited by:
Aurora Mirabile, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Genlin Lu, Longyou People’s Hospital, ChinaAndreas Antzoulas, General University Hospital of Patras, Greece
Copyright © 2025 Zhong, Chen, Wu, Liu, Fan, Liu, Lin, Wan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Wan, d2FuanVuamllMUAxNjMuY29t; Meifang He, aGVtZWlmYW5nQG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
 Hangyan Zhong
Hangyan Zhong Yisheng Chen3†
Yisheng Chen3† Weigen Wu
Weigen Wu Meifang He
Meifang He