- 1Center for Reproductive Medicine, Guangdong Women and Children Hospital, Guangzhou, China
- 2Women and Children’s Hospital, Southern University of Science and Technology, Shenzhen, China
- 3School of Public Health, Sun Yat-sen University, Guangzhou, China
Background: Methylenetetrahydrofolate reductase (MTHFR) regulates folate metabolism and homocysteine (Hcy) methylation. Impaired folate metabolism and vitamin D deficiency are both closely associated with female reproductive disorders, but their specific roles and relationship remain largely unknown. This study aimed to investigate the relationship between MTHFR polymorphisms and vitamin D status and to examine the mediating effect of Hcy.
Methods: A total of 6,344 infertile patients were included in this retrospective study. Multivariable logistic regression and multiple linear regression models, and stratified analyses were used to investigate the relationship between MTHFR polymorphisms (C677T and A1298C) and vitamin D status. Smooth curve fitting model and spearman correlation analysis were used to explore the correlation between Hcy levels and vitamin D status. Mediation analyses were performed to examine the direct and indirect effects of MTHFR polymorphisms on vitamin D status.
Results: The risk of vitamin D deficiency and serum Hcy levels were significantly higher in patients with MTHFR677CT and TT compared with CC (p < 0.001 for both). In multivariate regression models, MTHFR677CT and TT were positively associated with vitamin D deficiency compared with CC. No significant differences were found for A1298C polymorphism. Smooth curve fitting models showed that serum Hcy was linearly correlated with both 25(OH)D levels (p-nonlinear = 0.063) and prevalence of vitamin D deficiency (p-nonlinear = 0.261). In mediation analyses using logistic regression models, Hcy mediated 15.8 and 41.6% of the associations between 677CT and TT (versus CC) and vitamin D deficiency, respectively.
Conclusion: The effect of C677T polymorphism on vitamin D status can be explained jointly by a direct association between C677T polymorphism and vitamin D, and an indirect association mediated by Hcy.
Introduction
Methylenetetrahydrofolate reductase (MTHFR) in a key enzyme in the folate pathway, responsible for folate metabolism and homocysteine methylation (1). C677T and A1298C are the two most common polymorphisms of MTHFR gene (2, 3). Mutations at these two loci reduce MTHFR enzyme activity, resulting in disruption of the conversion of 5,10-methylenetetrahydrofolate to 5-methylenetetrahydrofolate and cysteine to methionine, thereby causing disturbances in folate and homocysteine status (4). The MTHFR C677T mutation results in the conversion of alanine to valine at position 677, leading to a decrease in MTHFR enzyme activity, with only about 30% of the enzyme activity retained in homozygous TT genotype (3). Similarly, the A1298C polymorphism changes glutamate to alanine at position 1,298, resulting in a lesser reduction in enzyme activity (5).
Folate deficiency or MTHFR gene defects exhibit DNA hypomethylation and abnormal biochemical and/or phenotypic changes in animal models (6, 7), cell culture (8) and humans (9–12). Currently, MTHFR gene testing is widely used for clinical diagnosis of folate metabolism capacity and to provide a reference for the use of medications such as folic acid before or during pregnancy. Many studies have reported an association between MTHFR C677T and A1298C polymorphisms and recurrent pregnancy loss (RPL) (13–16). In addition, MTHFR C677T and A1298C polymorphisms have been associated with a variety of pregnancy-related complications (17–19), such as preeclampsia. The relationship between MTHFR polymorphisms and IVF/ICSI outcomes has also been explored, but with conflicting results (20–22). MTHFR polymorphisms are associated with female reproductive health, but their specific roles and mechanisms remain largely unknown.
Mutations in MTHFR gene result in elevated homocysteine (Hcy) levels (4). Hcy is an intermediate metabolite of methionine, and its elevation is associated with pro-oxidative and pro-thrombotic states that induce endothelial dysfunction, thus damaging various organs and tissues (23, 24). Although MTHFR polymorphisms and/or hyperhomocysteinemia are strongly associated with cardiovascular risk and adverse pregnancy outcomes, studies have shown that lowering Hcy levels by increasing folic acid intake is not necessarily effective in reducing the risk of adverse outcomes, or may even yield opposite results, suggesting that other factors may interfere with folate metabolism pathway and influence its downstream effects (25–28).
Vitamin D deficiency is a health concern for women worldwide and is associated with cardiovascular disease, cancer and all-cause mortality (29, 30), as well as adverse pregnancy outcomes and female reproductive diseases (31–34). However, the role of vitamin D and the mechanism by which it is associated with these disease are unclear. Vitamin D works by activating the vitamin D receptor, which regulates the transcription of target genes responsible for various biological processes (35–37). In addition to its well-known role in calcium balance and bone health, vitamin D regulates cell proliferation and differentiation, apoptosis, angiogenesis, anti-inflammation, immunomodulation, and multiple metabolic pathways (38–40).
The relationship between folate metabolism and vitamin D remains poorly understood. Previous studies have suggested that folate may affect bone health, which may be related to vitamin D function (41), and that there may be an association between MTHFR polymorphisms and bone mineral density (42). In vitamin D-deficient mice, supplementation with folic acid, vitamin B12 and vitamin D together may improve learning and memory performance more than vitamin D alone (43). Studies have shown an inverse relationship between 25-hydroxyvitamin D [25(OH)D] and Hcy levels in the general population (44), and that both vitamin D deficiency and hyperhomocysteinemia are risk factors for cardiovascular disease (23, 30). One study has investigated the effect of MTHFR C677T polymorphism on serum vitamin D and Hcy levels in women with RPL, but this study had a small sample size (n = 837) and included only women with RPL, which may not be generalizable to other populations (45).
We hypothesized that MTHFR polymorphisms may be associated with vitamin D status; however, no studies have examined the specific nature of this relationship or quantified the strength of the association. Therefore, we genotyped the MTHFR C677T and A1298C polymorphisms in infertile women and investigated their relationship with vitamin D status, aiming to better understand how and to what extent the MTHFR polymorphisms affect vitamin D status and hopefully providing a theoretical basis for individualized treatment of infertility.
Methods
Study design and participants
Infertile patients undergo a comprehensive infertility evaluation at our fertility center, including infections, endocrinology, metabolism, ultrasound, and other tests related to pregnancy preparation/infertility including MTHFR gene polymorphisms, homocysteine and vitamin D, as well as semen analysis. All patients are informed of the benefits and costs of each test, and it is up to the patient to decide whether or not to undergo infertility-related tests. The study was approved by the Institutional Review Board of Guangdong Women and Children Hospital. Given the retrospective design, the requirement for informed consent was waived in accordance with institutional and national ethical guidelines. The study included infertile patients who underwent a comprehensive infertility assessment between January 2019 and May 2024, which included testing for MTHFR gene polymorphisms, serum Hcy and 25(OH)D levels. In this study, we included infertile patients who had started taking folic acid supplements in preparation for pregnancy. We excluded patients from the study if they met any of the following criteria: use of hormone therapy; vitamin D and calcium therapy within 3 months; uterine abnormalities; other medical conditions including kidney disease, hypertension, diabetes and tumors; and missing core data. According to international guideline recommendations (46–48), patients were divided into two groups according to the criteria of serum vitamin D deficiency: < 50 nmoL/L group and ≥ 50 nmoL/L group.
Measurement of biochemical parameters
Blood samples were collected from patients at their first visit to the fertility clinic to assess biochemical parameters. All tests were performed by our clinical laboratory in a timely manner. Serum 25(OH)D, anti-mullerian hormone (AMH), and fasting insulin were assessed using chemiluminescence. Serum Hcy, fasting glucose, triglyceride and total cholesterol were measured by enzymatic method. Serum low-density lipoprotein, high-density lipoprotein, and hemoglobin were detected using colorimetric method.
MTHFR gene polymorphisms
DNA extraction kit (Magen, Guangzhou, China) was used to extract genomic DNA according to the manufacturer’s instructions. Genotypes for the MTHFR C677T and A1298C loci were determined by fluorescence quantitative polymerase chain reaction, as previously reported (19). MTHFR C677C, C677T and T677T were determined as wild-type (CC), heterozygous (CT) and homozygous (TT), respectively; MTHFR A1298A, A1298C and C1298C were determined as wild-type (AA), heterozygous (AC) and homozygous (CC), respectively.
Statistical analysis
Statistical software package (SPSS, version 22.0) and R software (version 4.3) were used to perform the analyses. Kolmogorov–Smirnov test was used to determine whether the continuous variables were normally distributed. Continuous variables were expressed as mean with standard deviation or median with interquartile range, and comparisons of differences between two groups were made using Student’s t-test or Mann–Whitney U-test, and comparisons of differences between three groups were made using one-way ANOVA or Kruskal-Wallis test, as appropriate. Categorical variables were presented as number with percentage and compared by Pearson’s chi-square test or Fisher’s exact test. p-value < 0.05 was considered statistically significant.
To investigate the relationship between MTHFR gene polymorphisms and vitamin D levels in infertile women, we used logistic regression models to investigate the effect of MTHFR polymorphisms (C677T and A1298C) on vitamin D deficiency and multiple linear regression models to investigate the effect of MTHFR polymorphisms (C677T and A1298C) on serum 25(OH)D levels. The selection of model covariates was screened according to a priori clinical and epidemiological knowledge combined with a directed acyclic graph (DAG) (Supplementary Figure S1). Variables were included if they had a p value < 0.05 in comparisons stratified by vitamin D levels or MTHFR genotypes, or if they were deemed clinically relevant to vitamin D status (e.g., body mass index (BMI), type of infertility). Two models were built based on DAG when Hcy was the mediating variable. In model 1, minimal sufficient adjusted variables included age, BMI, AMH, type of infertility and causes of infertility to estimate the total effect of MTHFR polymorphisms on vitamin D status. In model 2, age, BMI, AMH, type of infertility, causes of infertility, hemoglobin and season of blood collection were adjusted confounders to estimate the total effect of MTHFR polymorphisms on vitamin D status. To assess the relationships between serum 25(OH)D levels or vitamin D deficiency and serum Hcy levels, smooth curve fitting models were constructed. We used generalized linear model and generalized additive model to explore potential association between vitamin D status and Hcy levels and tested for nonlinearity using maximum likelihood method. The correlation coefficients between Hcy and 25(OH)D were calculated by Spearman correlation analysis for the total population and for different MTHFR C677T genotypes. To ensure data integrity, we included only patients with complete data for the key variables (MTHFR genotypes, serum 25(OH)D levels, and Hcy levels). Missing values for other covariates were imputed using the median.
Mediation analysis
To examine the direct and indirect effects (via Hcy) of MTHFR polymorphisms on vitamin D status, mediation analyses were performed. We used the classic framework of mediation analysis, based on the three-step approach proposed by Baron and Kenny (49), to assess direct and indirect effects. The following three models were constructed:
1. Total effect of MTHFR polymorphisms on vitamin D status:
Here, Y represents vitamin D status, X represents the MTHFR polymorphisms, C denotes a set of covariates controlled for in the model, β1 is the coefficient of the total effect of MTHFR polymorphisms on Vitamin D, and ϵ is the residual error term of the model.
2. Effect of MTHFR polymorphisms on Hcy:
In this model, M represents Hcy levels, and β3 is the coefficient estimating the effect of MTHFR polymorphisms on Hcy. C represents a set of covariates controlled for in the model.
3. Direct effect of MTHFR polymorphisms on vitamin D status (controlling for Hcy):
Here, β’1 represents the direct effect of MTHFR polymorphisms on Vitamin D status, and β5 denotes the effect of Hcy on vitamin D status, controlling for covariates in C.
Based on the mediation analysis framework, the indirect effect of MTHFR polymorphisms on Vitamin D status (via Hcy) and the mediation proportion can be calculated using the following formula:
For continuous outcome variables:
For binary outcome variables:
In addition, we calculated the total effect as: ; and the direct effect as:
To assess the significance of the indirect effect, we used the bootstrap method, sampling 1,000 times to compute the standard errors and 95% confidence intervals for the indirect effect and mediation proportion. This procedure ensures the robustness and accuracy of the results.
Results
Patient characteristics and blood biochemicals stratified according to 25(OH)D levels
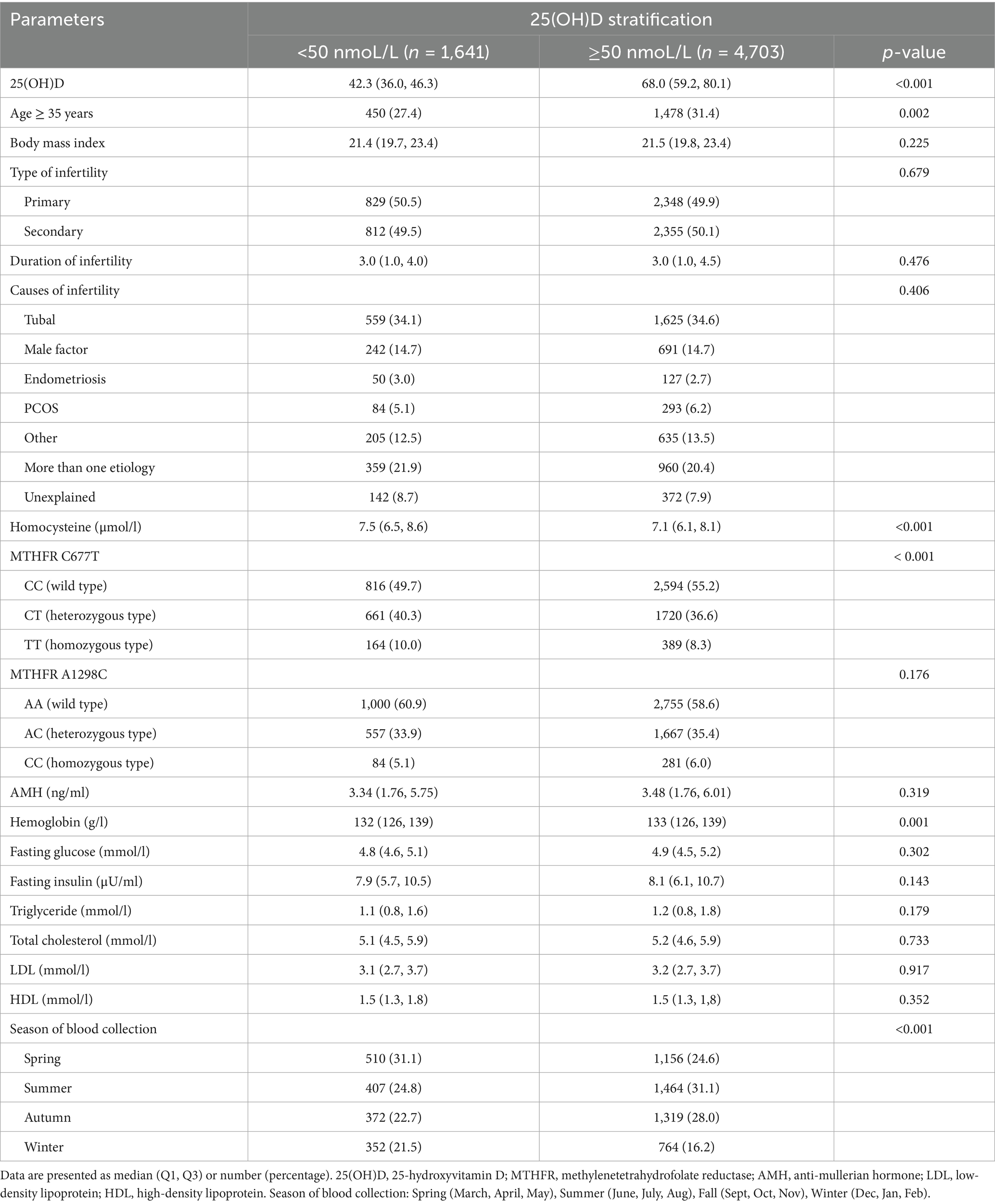
A total of 6,344 infertile patients who met the inclusion criteria were included in this study. To explore the features associated with vitamin D deficiency, we divided all patients into two groups according to the threshold of vitamin D deficiency (< 50 nmoL/L). Except for age, Hcy, MTHFR C677T genotype, hemoglobin and season of blood collection, other characteristics of the two groups were comparable (Table 1). The proportion of those aged ≥ 35 years in the 25(OH)D ≥ 50 nmoL/L group was significantly higher than in the < 50 nmoL/L group (31.4% vs. 27.4%; p = 0.002). Notably, the MTHFR C677T genotypes were significantly different between the two groups (p < 0.001), whereas the MTHFR A1298C genotypes were comparable between the two groups (p = 0.176). In addition, Hcy levels were significantly higher in the 25(OH)D < 50 nmoL/L group than in the ≥ 50 nmoL/L group (median 7.5 vs. 7.1 μmoL/L; p < 0.001), and hemoglobin levels were significantly lower in the 25(OH)D < 50 nmoL/L group than in the ≥ 50 nmoL/L group (median 132 vs. 133 g/L; p = 0.001). There was a significant difference in the season of blood collection between the two groups (p < 0.001), suggesting that the prevalence of vitamin D deficiency tends to be higher in winter and spring. BMI, type of infertility, duration of infertility, causes of infertility, AMH, and a range of metabolic indicators were comparable between the two groups (Table 1).
MTHFR C677T and A1298C polymorphisms in infertile women
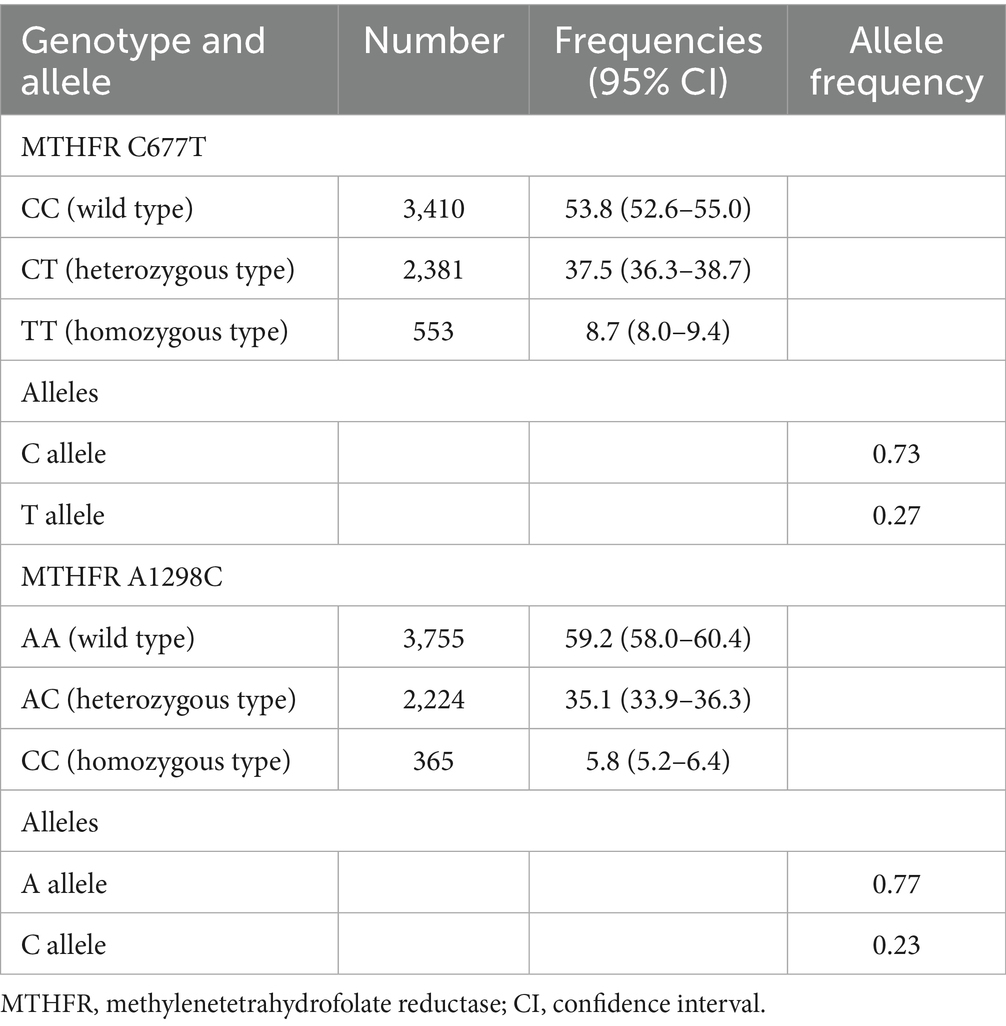
Table 2 summarizes the MTHFR C677T and A1298C genotypes and allele frequencies in infertile women. For MTHFR C677T, the prevalence of CC, CT and TT genotypes was 53.8, 37.5 and 8.7%, respectively, and the allele frequency was 0.73 for the C allele and 0.27 for the T allele. For MTHFR A1298C, the prevalence of AA, AC and CC genotypes was 59.2, 35.1 and 5.8%, respectively, and the allele frequency was 0.77 for the A allele and 0.23 for the C allele.
Patient characteristics and blood biochemicals of MTHFR C677T and A1298C polymorphisms
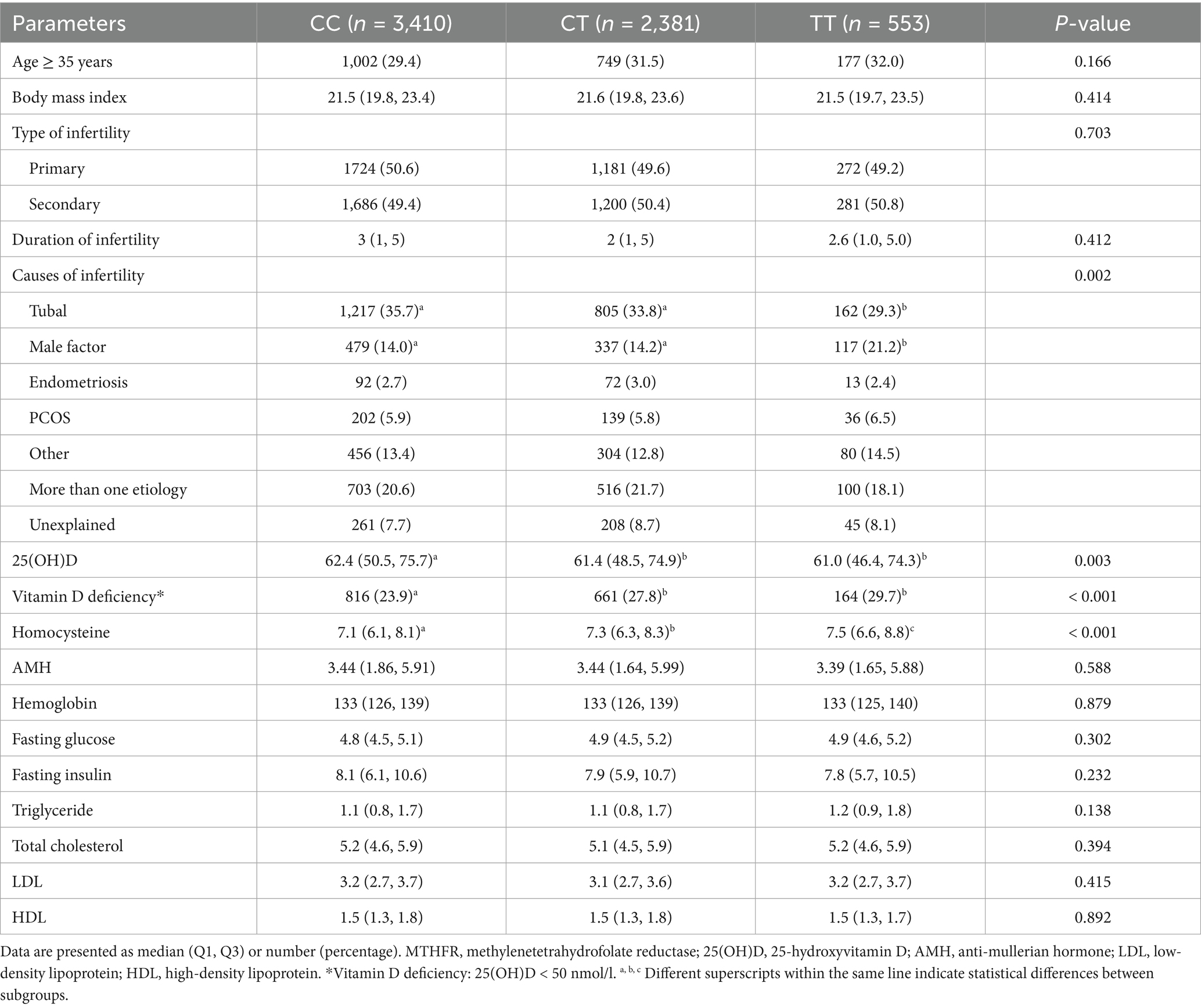
We compared the clinical features and biochemical indicators of the MTHFR C677T genotypes and A1298C genotypes, respectively. Table 3 summarizes patient characteristics and biochemical parameters grouped by MTHFR C677T genotypes. Age, BMI, type of infertility, duration of infertility, AMH, hemoglobin, and a range of metabolic indicators did not differ among the three groups. Remarkably, patients with 677CT and TT genotypes had a significantly higher risk of vitamin D deficiency than those with CC genotype (27.8 and 29.7% vs. 23.9; p < 0.001). In addition, there were significant differences in the causes of infertility and Hcy levels between different C677T genotypes. As for MTHFR A1298C polymorphism, all of the above parameters, including vitamin D status and Hcy levels, did not differ significantly between A1298C genotypes (Supplementary Table S1).
Similarly, Figure 1 shows the effects of MTHFR C677T and A1298C polymorphisms on serum Hcy and 25(OH)D levels. In terms of C677T, Hcy levels in TT variant were significantly higher than those in CC and CT variants, and Hcy levels in CT variant were significantly higher than in CC variant. The 25(OH)D levels were significantly lower in TT and CT variants than in CC variant. In addition, A1298C polymorphisms did not affect Hcy and 25(OH)D levels (Figure 1).
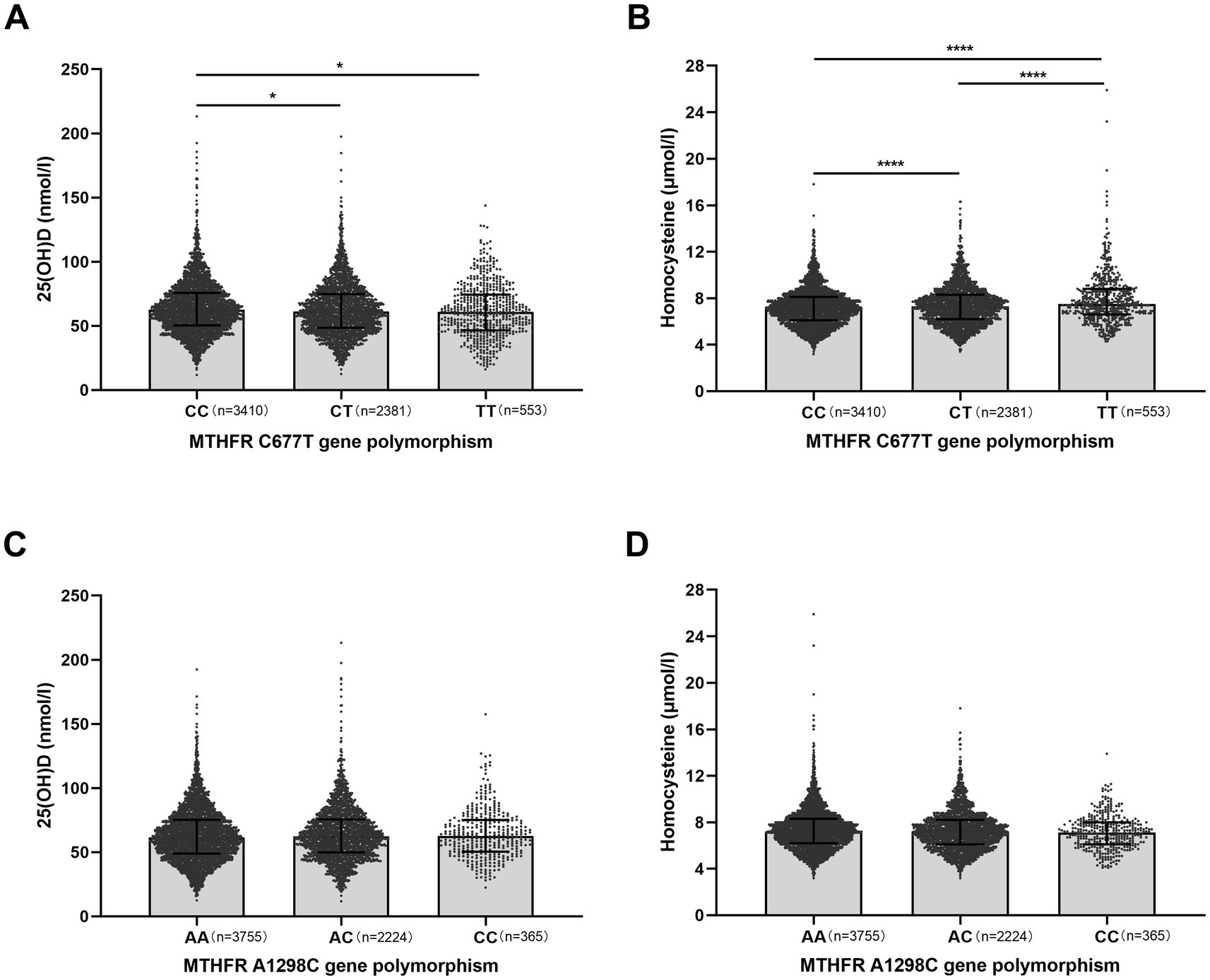
Figure 1. Effects of methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms on serum 25(OH)D and homocysteine (Hcy) levels in infertile patients. (A,B) Effect of MTHFR C677T genotype (CC, CT, TT) on serum 25(OH)D (nmol/l) and Hcy (μmol/l) levels. CC, wild-type; CT, heterozygous; TT, homozygous. (C,D) Effect of MTHFR A1298C genotype (AA, AC, CC) on serum 25(OH)D and Hcy levels. AA, wild-type; AC, heterozygous; CC, homozygous. Data are shown as median with interquartile range. *p < 0.05, ****p < 0.0001.
Association between MTHFR polymorphisms and vitamin D status
Multivariate logistic regression analyses were performed to assess the effect of MTHFR C677T and A1298C polymorphisms on vitamin D deficiency (Table 4). Based on prior knowledge and recommendation of DAG (Supplementary Figure S1), a set of covariates were selected and adjusted in the multivariate models. When Hcy was the mediating variable, two models were built based on DAG. In model 1, after controlling for age, BMI, AMH, type of infertility and causes of infertility, MTHFR 677CT (adjusted OR, 1.225; 95% CI, 1.087–1.380) and TT (adjusted OR, 1.355; 95% CI, 1.110–1.654) were positively associated with the risk of vitamin D deficiency compared with CC. In model 2, MTHFR 677CT (adjusted OR, 1.229; 95% CI, 1.089–1.386) and TT (adjusted OR, 1.355; 95% CI, 1.109–1.657) were also significantly associated with the risk of vitamin D deficiency compared with CC. With regard to MTHFR A1298C, there were no significant effects of different polymorphisms on vitamin D deficiency in the two logistic regression models (Table 4).
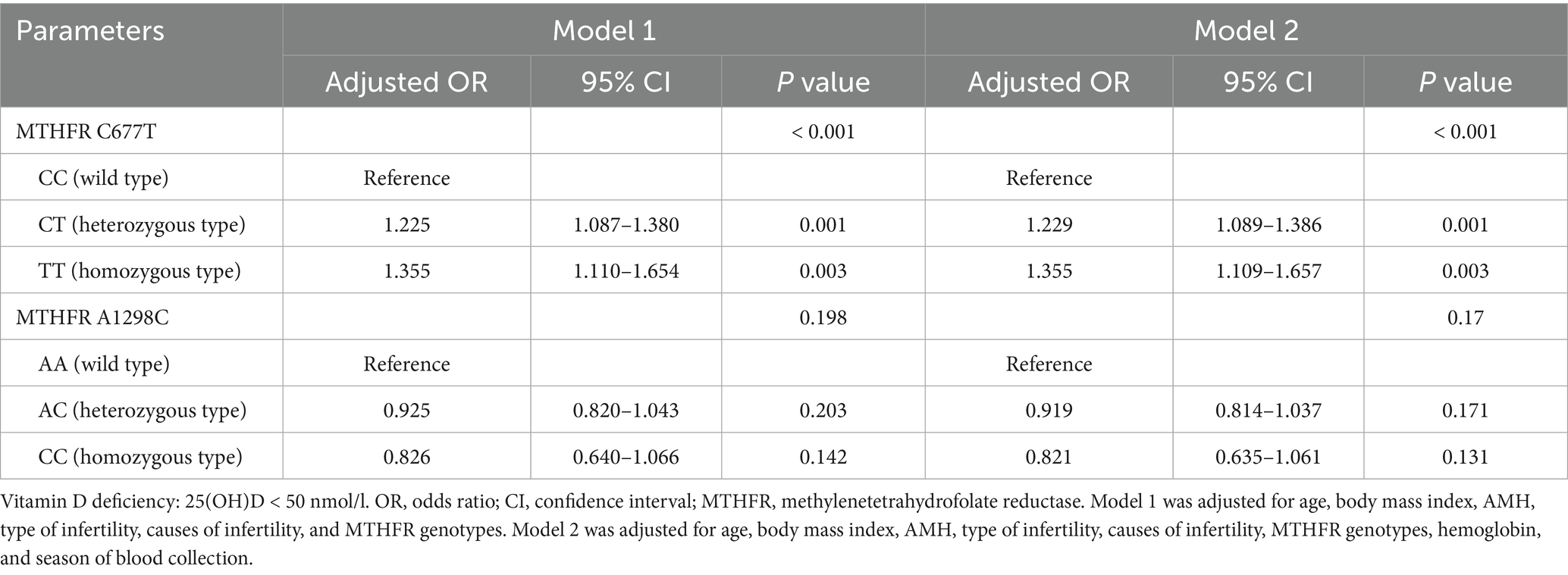
Table 4. Multivariable logistic regression analysis for the effect of MTHFR polymorphisms on vitamin D deficiency.
Multiple linear regression analyses were used to assess the effect of MTHFR C677T and A1298C polymorphisms on serum 25(OH)D levels (Table 5). In model 1, MTHFR 677CT (B, −1.371; 95%CI, −2.448, −0.293) and TT (B, −2.799; 95%CI, −4.652, −0.946) were negatively correlated with serum 25(OH)D levels compared to CC. In model 2, MTHFR C677T genotypes had a similar effect on 25(OH)D levels. As well, the MTHFR A1298C genotypes had no significant effect on 25(OH)D levels in either model.
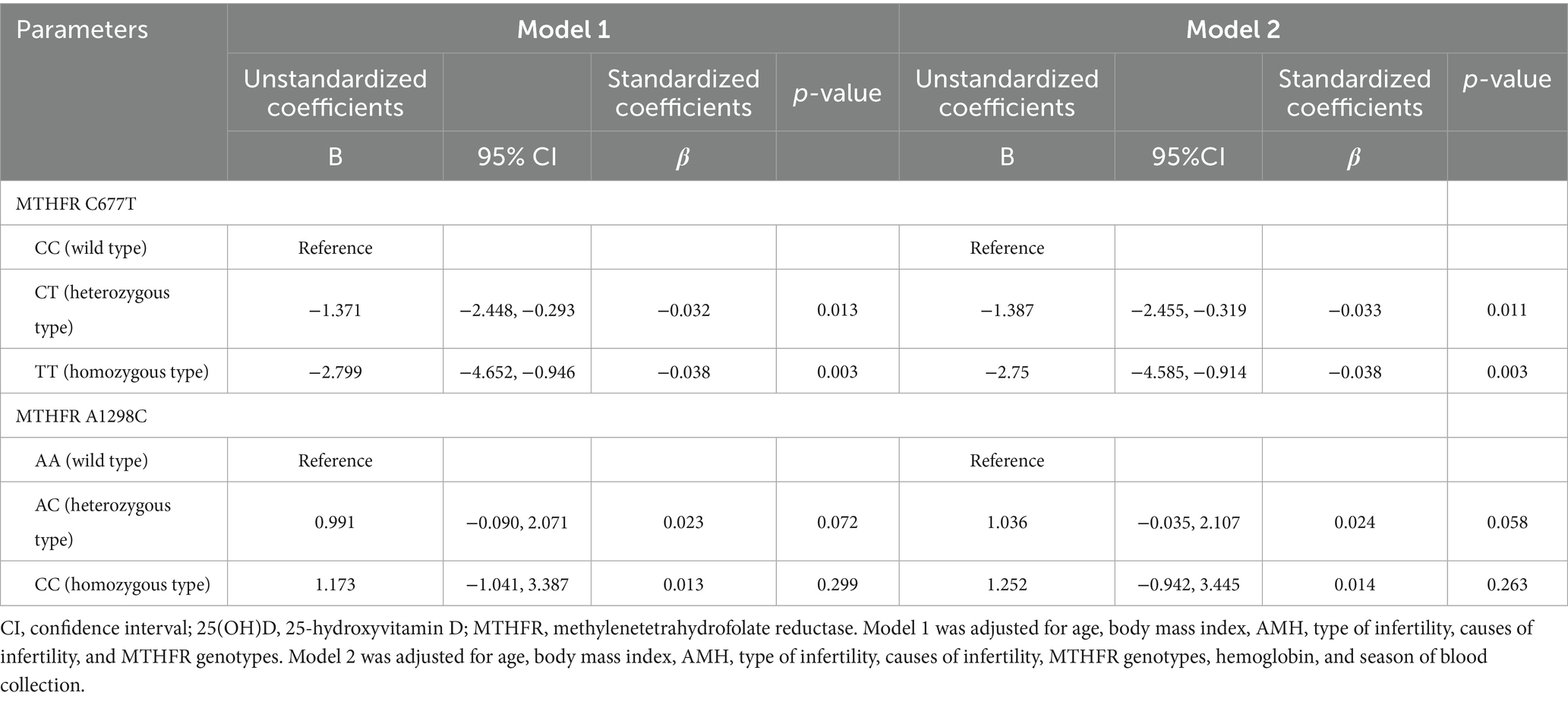
Table 5. Multiple linear regression analysis for the effect of MTHFR polymorphisms on serum 25(OH)D levels.
Due to the significant difference in age between the two vitamin D strata, we divided all patients into two subgroups: age < 35 years and ≥35 years, and separately performed multivariable logistic regression analyses (Supplementary Table S2). In multivariable analysis of patients aged < 35 years, MTHFR 677CT (adjusted OR, 1.227; 95% CI, 1.064–1.414) and TT (adjusted OR, 1.421; 95% CI, 1.121–1.802) were positively associated with the risk of vitamin D deficiency compared with CC. However, the effect of C677T on vitamin D deficiency was not significant in multivariable analysis of patients aged ≥ 35 years. For A1298C polymorphism, the 1,298 AC (heterozygous) genotype was negatively associated with vitamin D deficiency in the younger subgroup, whereas in the old subgroup, the A1298C polymorphism was not associated with vitamin D deficiency (Supplementary Table S2).
Association between MTHFR polymorphisms, vitamin D deficiency and Hcy
The smooth curve fitting showed a linear correlation between serum Hcy and 25(OH)D levels (p-overall < 0.001; p-nonlinear = 0.063) (Figure 2A). Moreover, serum Hcy was linearly associated with the risk of vitamin D deficiency (p-overall < 0.001; p-nonlinear = 0.261) (Figure 2B). The correlation coefficients between serum Hcy and 25(OH)D levels were calculated by spearman correlation analysis for the total population and for different MTHFR C677T genotypes (Supplementary Figure S2). A negative correlation between Hcy and 25(OH)D levels was observed in the total population (R = −0.137, p < 0.001). A greater negative correlation between Hcy and 25(OH)D levels was observed in the TT genotype population compared to total, CC and CT genotype populations (Supplementary Figure S2).
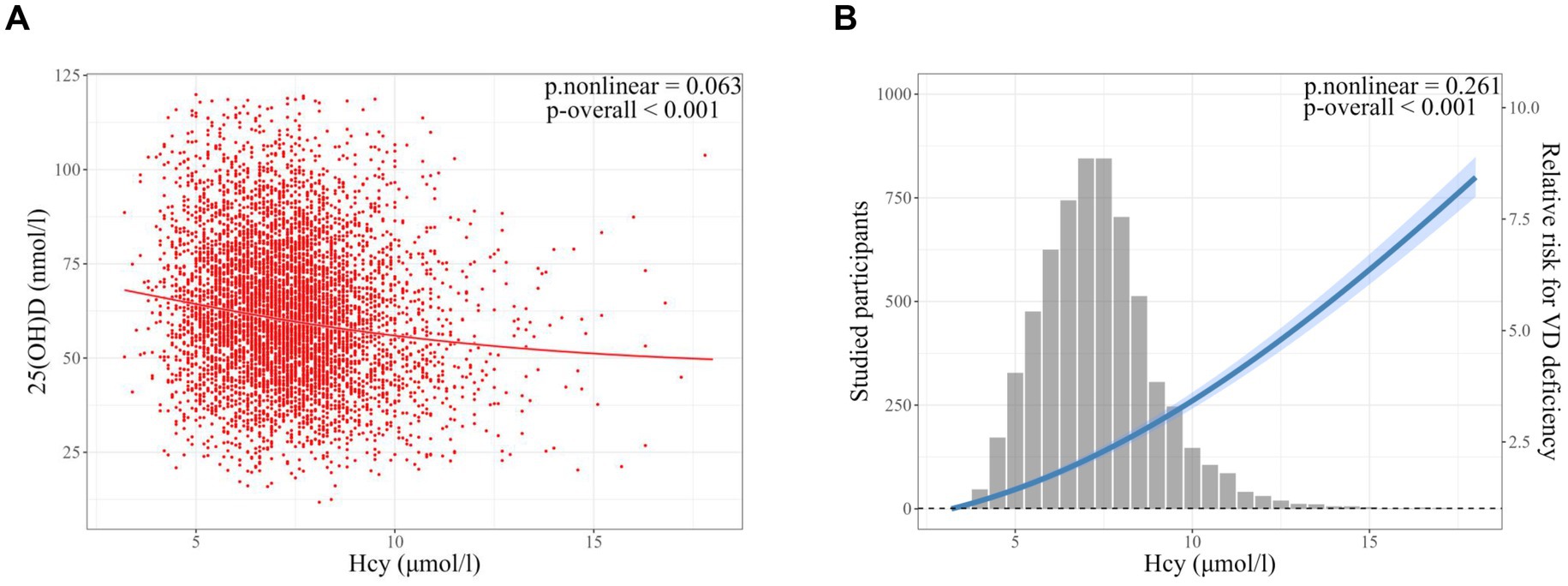
Figure 2. Smooth curve fitting models of the correlation between serum homocysteine (Hcy) and vitamin D (VD) status in infertile patients. (A) Smooth curve fitting of the correlation between serum Hcy (μmol/l) and 25(OH)D (nmol/l) levels. (B) Smooth curve fitting of the correlation between serum Hcy and vitamin D deficiency (25(OH)D < 50 nmoL/L). Models were adjusted for age, body mass index, AMH, type of infertility, causes of infertility, MTHFR C677T genotype, hemoglobin, and season of blood collection. Sample size: n = 6,344. Hcy, homocysteine; VD, vitamin D. p-overall, p-value for model test; p-nonlinear, p-value for nonlinear test.
Hypothesizing that MTHFR C677T may affect vitamin D status through the homocysteine metabolic pathway, we used mediation analysis to partition the total effect of C677T on vitamin D status into a direct effect and an indirect effect mediated by Hcy. Figure 3 shows the mediating effect of Hcy on the association between C677T genotypes and vitamin D deficiency. The total effect of CT vs. CC on vitamin D deficiency was significant (OR, 1.23; 95% CI, 1.09, 1.39). After controlling for Hcy, the direct effect of CT vs. CC on vitamin D deficiency was dominant (OR, 1.20; 95% CI, 1.06, 1.35). The indirect effect of CT vs. CC on vitamin D deficiency mediated by Hcy was also significant (OR, 1.03; 95% CI, 1.01, 1.05), with mediation proportion of 15.8% (95% CI, 6.4, 23.3%). Furthermore, the total effect of TT vs. CC on vitamin D deficiency was more significant (OR, 1.36; 95% CI, 1.11, 1.66). Of note, the direct effect of TT versus CC on vitamin D deficiency was not statistically significant (OR, 1.21; 95% CI, 0.98, 1.48), although the confidence interval indicates a trend toward a positive association. The indirect effect mediated by Hcy was remarkable (OR, 1.12; 95% CI, 1.07, 1.16), with mediation proportion of 41.6% (95% CI, 21.5, 56.3%) (Figure 3). In addition, when analyzing 25(OH)D levels as the outcome, we observed consistent associations (Supplementary Figure S3). Hcy mediated 20.4% of the effect of CT vs. CC on 25(OH)D levels and 39.9% of the effect of TT vs. CC on 25(OH)D levels.
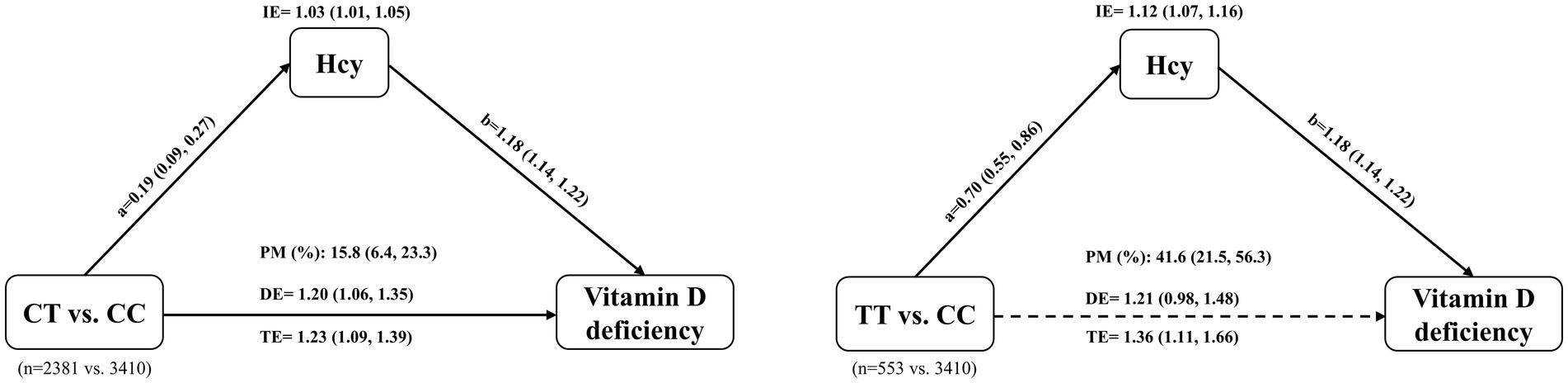
Figure 3. Mediation effect of homocysteine (Hcy) in the association between MTHFR C677T genotype and vitamin D deficiency. The total effect (TE) of the C677T genotype on vitamin D deficiency is partitioned into a direct effect (DE) and an indirect effect (IE) mediated through Hcy. Path ‘a’ represents the estimated change in Hcy levels for the CT or TT genotype compared with the CC genotype. Path ‘b’ represents the estimated change in the risk of vitamin D deficiency for each unit increase in Hcy. The dotted arrow indicates that the direct effect was not statistically significant. The Models were adjusted for age, BMI, AMH, type of infertility, causes of infertility, hemoglobin, and season of blood collection. IE, indirect effect; DE, direct effect; TE, total effect; PM, proportion mediated; CC, wild type; CT, heterozygous type; TT, homozygous type.
Discussion
MTHFR regulates folate metabolism and homocysteine methylation (1), which is strongly associated with female reproductive health. Both folate deficiency (50–52) and vitamin D deficiency (31–34) are closely related to adverse pregnancy outcomes and female reproductive disorders. In this study, we investigated whether two genetic MTHFR polymorphisms (C677T and A1298C) are associated with an increased risk of vitamin D deficiency, and further examined the mediation effects of Hcy.
We found that infertile patients with heterozygous 677CT and homozygous TT genotypes were significantly more likely to be vitamin D deficient than wild-type patients. The risk of vitamin D deficiency and serum Hcy levels were significantly higher in females with heterozygote (677CT) and homozygote (677TT) compared with wild type. There were no significant differences found for A1298C polymorphism. Because correlations have been found between C677T and vitamin D, between C677T and Hcy, and between Hcy and vitamin D, we employed mediation analysis to decompose the total effect of C677T on vitamin D status into a direct effect and an indirect effect mediated by Hcy. The total effect of CT vs. CC on vitamin D deficiency consisted of direct and indirect effects, with 15.8% mediated by Hcy. Intriguingly, the direct effect of TT vs. CC on vitamin D deficiency was not significant, whereas the indirect effect mediated by Hcy was evident, with a mediation proportion of 41.6%. This suggests that for TT genotype, the mediating role of Hcy dominates the total effect of TT vs. CC on vitamin D deficiency.
There is a lack of evidence regarding the relationship between MTHFR polymorphisms and vitamin D status. Our results showed that C677T polymorphism was significantly associated with vitamin D deficiency in infertile women. Conversely, studies have shown no significant difference in serum 25(OH)D levels between MTHFR C677T genotypes in perimenopausal women (53) and healthy young men (54). Consistent with our findings, Ota et al. (45) found that serum 25(OH)D levels were lower in TT genotype than in wild-type in patients with RPL. However, their study had a small sample size (n = 837), included only women with RPL, and may not be generalizable to other populations. They did not analyze the relationship between MTHFR 1298 polymorphism and vitamin D status. Besides, they did not address the specific relationship between MTHFR polymorphisms, Hcy, and vitamin D and the extent of their correlation.
Many studies have found that MTHFR C677T gene polymorphism is associated with Hcy levels in various populations (4, 45, 55), whereas the correlation between A1298C polymorphism and Hcy levels is not as significant as that of C677T (56, 57), and our results are in line with previous studies. Previous studies have found a negative correlation between 25(OH)D and Hcy levels in the general population and in women PRL (44, 45), and both vitamin D deficiency and hyperhomocysteinemia are risk factors for atherosclerotic disease (23, 30) and adverse pregnancy outcomes (31, 58). Consistent with previous studies, our results showed that serum 25(OH)D levels were inversely correlated with Hcy levels, with a higher degree of negative correlation in the 677TT genotype population than in the CC genotype and total populations. These studies showed that vitamin D deficiency and elevated Hcy coexist in different populations and diseases.
To our knowledge, this is the first study with a large sample size in infertile women to demonstrate a significant correlation between MTHFR C677T polymorphisms and vitamin D status, while also quantifying the mediating role of Hcy. MTHFR polymorphisms, hyperhomocysteinemia, and vitamin D deficiency have all been reported as risk factors for infertility and adverse pregnancy outcomes (25–28, 31–34). Our findings reveal that the C677T polymorphism is significantly associated with an increased risk of vitamin D deficiency in infertile women, with part of this effect mediated by elevated Hcy levels. These results have potential clinical implications. Given the critical roles of folate and vitamin D in female reproduction, screening for MTHFR C677T variants may be valuable in infertility evaluations. Early identification of individuals with impaired folate metabolism may guide personalized interventions, such as supplementation with active folate and vitamin D. In addition, Hcy may serve as a modifiable intermediary target to improve reproductive outcomes. Our mediation analysis suggests both direct and indirect effects of C677T on vitamin D status, with the indirect effect through Hcy particularly pronounced in TT carriers. These findings underscore the complex interplay between genetic, metabolic, and nutritional factors in reproductive health and highlight the need for further prospective studies to validate targeted intervention strategies.
Our study highlights the possible interrelationship between folate metabolism and vitamin D. On the one hand, folate metabolism pathway may influence vitamin D status. Previous studies showed that vitamin D biosynthesis in the skin was related to folate metabolism (59) and that folic acid supplementation significantly increased vitamin D levels in eggs of laying hens, but had no effect on vitamin A and vitamin E levels (60). Although these findings may not be directly applicable to humans, they provide preliminary mechanistic insight that warrants further investigation in human studies. On the other hand, vitamin D may affect folate metabolism pathway and downstream functions. Vitamin D has been reported to reduce Hcy levels in cell culture in vitro (61) and in overweight reproductive women (62), suggesting that vitamin D may regulate gene expression of enzymes involved in homocysteine metabolism. Nonetheless, the exact mechanism of the association between folate metabolism pathway and vitamin D is unknown and needs further study.
Some studies have shown that increasing the dose of folic acid may be ineffective or even harmful to the mother and offspring (27, 28), and vitamin D supplementation was not beneficial in several clinical trails (63, 64), but the underlying reasons for these negative results are not known. Previous research has shown that vitamin D regulatory pathways have much in common with folate metabolism-related pathways, including inflammation, immunomodulation, and various metabolic processes (38–40). From this, we hypothesized that folic acid supplementation alone may not fully ameliorate disorders associated with abnormal folate metabolism, including cardiovascular disease and female reproductive disorders, and that combined supplementation with folic acid and vitamin D may be more beneficial in population with C677T polymorphism, as they may synergistically affect folate metabolism pathway and downstream function. So far, there have been no studies on combined folic acid and vitamin D supplements in humans, and future research is warranted. Routine monitoring of vitamin D and homocysteine enables personalized supplementation strategies by identifying subclinical deficiencies, guiding genotype-specific interventions, and preventing metabolic cascades detrimental to fertility. This dual biomarker approach based on mechanistic insights, transforms pre-conception care from reactive nutrient replacement to proactive, precise metabolic optimization, ultimately improving reproductive success and perinatal health.
There are some limitations of this study. Due to the retrospective nature of the study, some patient characteristics—such as smoking habits, physical activity, and the dose and duration of folic acid supplementation—could not be obtained. Although the infertile women in this study had initiated folic acid supplementation in preparation for pregnancy, variations in the dose and timing may have introduced confounding that could not be accounted for in this analysis. This limitation may also affect the accuracy of the estimated mediation effects. Future prospective studies with standardized collection of supplement usage data are warranted to clarify these relationships more precisely. In addition, the relationship between MTHFR polymorphisms and serum 25(OH)D levels was investigated in infertile women, not in normal fertile or menopausal women, so our results may not be generalizable to unselected populations. Moreover, there was a significant difference in age between the two vitamin D strata in this study, which is consistent with our previous report (34). To further exclude the interference of age, all patients were divided into two subgroups based on age threshold. In the younger subgroup, MTHFR C677T polymorphism was positively associated with vitamin D deficiency, whereas in the older subgroup, C677T polymorphism was not associated with vitamin D deficiency. In agreement with our findings, a previous study found that serum 25(OH)D3 levels did not differ between MTHFR C677T genotypes in perimenopausal women (45–58 years) (53). Besides, the 1,298 AC (heterozygous) genotype was negatively correlated with vitamin D deficiency in the younger subgroup, while the A1298C polymorphism was not correlated with vitamin D deficiency in the old subgroup. Therefore, the effect of MTHFR polymorphisms on vitamin D deficiency needs to be interpreted separately according to different age groups. However, we have no clear explanation for this phenomenon and speculate that the effect of MTHFR polymorphisms on vitamin D status is likely to be greater in younger population, whereas in older population, vitamin D status may be related to more factors other than MTHFR polymorphisms. We hypothesize that folic acid and/or vitamin D supplementation may be more effective in correcting the potential adverse effects of MTHFR polymorphisms in younger population compared to older population. Caution should be exercised in interpreting our findings and, given these limitations, external validation of the study is needed in the future.
Conclusion
In summary, both direct and Hcy-mediated pathways may contribute to the link between MTHFR C677T polymorphisms and vitamin D deficiency in infertile women. We hypothesize that elevated Hcy and vitamin D deficiency in infertile patients with MTHFR C677T polymorphism may together contribute to the development of diseases associated with abnormal folate metabolism. Further research into the mechanisms underlying the interrelationship between folate metabolism and vitamin D is needed, and clinical trials of combined folate and vitamin D supplementation are urgently required.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Guangdong Women and Children Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This is a retrospective study, and the written informed consent was waived by the Institutional Review Board of Guangdong Women and Children Hospital.
Author contributions
RZ: Writing – review & editing, Funding acquisition, Project administration, Resources, Formal analysis, Writing – original draft, Conceptualization, Methodology, Data curation, Software, Investigation, Validation. ZZ: Writing – original draft, Methodology. ZW: Investigation, Writing – review & editing. MD: Project administration, Writing – review & editing, Validation. LH: Data curation, Writing – review & editing. SW: Writing – review & editing, Project administration, Investigation. XZ: Writing – review & editing, Supervision, Visualization. FL: Writing – review & editing, Supervision, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the Natural Science Foundation of Guangdong Province, China (2025A1515010073).
Acknowledgments
The authors thank all the staff of the Reproductive Medicine Center of Guangdong Women and Children’s Hospital for their cooperation and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1644302/full#supplementary-material
References
1. Schalinske, KL, and Smazal, AL. Homocysteine imbalance: a pathological metabolic marker. Advances Nutr. (2012) 3:755–62. doi: 10.3945/an.112.002758
2. Goyette, P, Sumner, JS, Milos, R, Duncan, AM, Rosenblatt, DS, Matthews, RG, et al. Human methylenetetrahydrofolate reductase: isolation of cDNA mapping and mutation identification. Nat Genet. (1994) 7:551. doi: 10.1038/ng0894-551a
3. Frosst, P, Blom, HJ, Milos, R, Goyette, P, Sheppard, CA, Matthews, RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. (1995) 10:111–3. doi: 10.1038/ng0595-111
4. Engbersen, AM, Franken, DG, Boers, GH, Stevens, EM, Trijbels, FJ, and Blom, HJ. Thermolabile 5,10-methylenetetrahydrofolate reductase as a cause of mild hyperhomocysteinemia. Am J Hum Genet. (1995) 56:142–50.
5. van der Put, NM, Gabreëls, F, Stevens, EM, Smeitink, JA, Trijbels, FJ, Eskes, TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. (1998) 62:1044–51. doi: 10.1086/301825
6. Kim, YI, Pogribny, IP, Basnakian, AG, Miller, JW, Selhub, J, James, SJ, et al. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. (1997) 65:46–52. doi: 10.1093/ajcn/65.1.46
7. Chen, Z, Karaplis, AC, Ackerman, SL, Pogribny, IP, Melnyk, S, Lussier-Cacan, S, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. (2001) 10:433–43. doi: 10.1093/hmg/10.5.433
8. Kimura, M, Umegaki, K, Higuchi, M, Thomas, P, and Fenech, M. Methylenetetrahydrofolate reductase C677T polymorphism, folic acid and riboflavin are important determinants of genome stability in cultured human lymphocytes. J Nutr. (2004) 134:48–56. doi: 10.1093/jn/134.1.48
9. Jacob, RA, Gretz, DM, Taylor, PC, James, SJ, Pogribny, IP, Miller, BJ, et al. Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J Nutr. (1998) 128:1204–12. doi: 10.1093/jn/128.7.1204
10. Stern, LL, Mason, JB, Selhub, J, and Choi, SW. Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prevent. (2000) 9:849–53.
11. Friso, S, Choi, SW, Girelli, D, Mason, JB, Dolnikowski, GG, Bagley, PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA. (2002) 99:5606–11. doi: 10.1073/pnas.062066299
12. Castro, R, Rivera, I, Ravasco, P, Camilo, ME, Jakobs, C, Blom, HJ, et al. 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C->T and 1298A->C mutations are associated with DNA hypomethylation. J Med Genet. (2004) 41:454–8. doi: 10.1136/jmg.2003.017244
13. Nelen, WL, Steegers, EA, Eskes, TK, and Blom, HJ. Genetic risk factor for unexplained recurrent early pregnancy loss. Lancet (London, England). (1997) 350:861. doi: 10.1016/S0140-6736(97)24038-9
14. Nair, RR, Khanna, A, and Singh, K. MTHFR C677T polymorphism and recurrent early pregnancy loss risk in north Indian population. Reproduct Sci. (2012) 19:210–5. doi: 10.1177/1933719111417888
15. Chen, H, Yang, X, and Lu, M. Methylenetetrahydrofolate reductase gene polymorphisms and recurrent pregnancy loss in China: a systematic review and meta-analysis. Arch Gynecol Obstet. (2016) 293:283–90. doi: 10.1007/s00404-015-3894-8
16. Xu, Y, Ban, Y, Ran, L, Yu, Y, Zhai, S, Sun, Z, et al. Relationship between unexplained recurrent pregnancy loss and 5,10-methylenetetrahydrofolate reductase polymorphisms. Fertil Steril. (2019) 111:597–603. doi: 10.1016/j.fertnstert.2018.11.011
17. Li, WX, Dai, SX, Zheng, JJ, Liu, JQ, and Huang, JF. Homocysteine metabolism gene polymorphisms (MTHFR C677T, MTHFR A1298C, MTR A2756G and MTRR A66G) jointly elevate the risk of folate deficiency. Nutrients. (2015) 7:6670–87. doi: 10.3390/nu7085303
18. Wu, W, Luo, D, Ruan, X, Gu, C, Lu, W, Lian, K, et al. Polymorphisms in gene MTHFR modify the association between gestational weight gain and adverse birth outcomes. Front Nutr. (2022) 9:919651. doi: 10.3389/fnut.2022.919651
19. Gu, C, Wu, W, Lai, K, Li, H, Wu, L, Lu, W, et al. Maternal pre-pregnancy BMI, MTHFR polymorphisms, and the risk of adverse pregnancy outcomes in pregnant women from South China: a retrospective cohort study. BMC Pregnancy Childbirth. (2023) 23:295. doi: 10.1186/s12884-023-05605-6
20. Haggarty, P, McCallum, H, McBain, H, Andrews, K, Duthie, S, McNeill, G, et al. Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet (London, England). (2006) 367:1513–9. doi: 10.1016/S0140-6736(06)68651-0
21. Dobson, AT, Davis, RM, Rosen, MP, Shen, S, Rinaudo, PF, Chan, J, et al. Methylenetetrahydrofolate reductase C677T and A1298C variants do not affect ongoing pregnancy rates following IVF. Hum Reprod. (2007) 22:450–6. doi: 10.1093/humrep/del396
22. Chen, L, Chen, H, Wang, X, Wei, B, Wu, Z, Chen, S, et al. Association of homocysteine with IVF/ICSI outcomes stratified by MTHFR C677T polymorphisms: a prospective cohort study. Reprod Biomed Online. (2021) 43:52–61. doi: 10.1016/j.rbmo.2021.04.009
23. Lentz, SR. Mechanisms of homocysteine-induced atherothrombosis. J Thrombosis Haemostasis. (2005) 3:1646–54. doi: 10.1111/j.1538-7836.2005.01364.x
24. Dudman, NP, Temple, SE, Guo, XW, Fu, W, and Perry, MA. Homocysteine enhances neutrophil-endothelial interactions in both cultured human cells and rats in vivo. Circ Res. (1999) 84:409–16. doi: 10.1161/01.RES.84.4.409
25. Lange, H, Suryapranata, H, De Luca, G, Börner, C, Dille, J, Kallmayer, K, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. (2004) 350:2673–81. doi: 10.1056/NEJMoa032845
26. Perera, N, Rudland, VL, Simmons, D, and Price, SAL. Folate supplementation in women with pre-existing diabetes. Nutrients. (2023) 15:1879. doi: 10.3390/nu15081879
27. Ledowsky, C, Mahimbo, A, Scarf, V, and Steel, A. Women taking a folic acid supplement in countries with mandatory food fortification programs may be exceeding the upper tolerable limit of folic acid: a systematic review. Nutrients. (2022) 14:2715. doi: 10.3390/nu14132715
28. Cochrane, KM, and Karakochuk, CD. Current evidence and controversies related to folate supplementation during pregnancy. J Obstetrics Gynaecol. (2024) 46:102566. doi: 10.1016/j.jogc.2024.102566
29. Hossein-nezhad, A, and Holick, MF. Vitamin D for health: a global perspective. Mayo Clin Proc. (2013) 88:720–55. doi: 10.1016/j.mayocp.2013.05.011
30. Holick, MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18:153–65. doi: 10.1007/s11154-017-9424-1
31. Aghajafari, F, Nagulesapillai, T, Ronksley, PE, Tough, SC, O'Beirne, M, and Rabi, DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. (2013) 346:f1169. doi: 10.1136/bmj.f1169
32. Tabesh, M, Salehi-Abargouei, A, Tabesh, M, and Esmaillzadeh, A. Maternal vitamin D status and risk of pre-eclampsia: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2013) 98:3165–73. doi: 10.1210/jc.2013-1257
33. Tamblyn, JA, Pilarski, NSP, Markland, AD, Marson, EJ, Devall, A, Hewison, M, et al. Vitamin D and miscarriage: a systematic review and meta-analysis. Fertil Steril. (2022) 118:111–22. doi: 10.1016/j.fertnstert.2022.04.017
34. Zhou, R, Zhu, Z, Dong, M, Wang, Z, Huang, L, Wang, S, et al. Nonlinear correlation between serum vitamin D levels and the incidence of endometrial polyps in infertile women. Hum Reprod. (2024) 39:2685–92. doi: 10.1093/humrep/deae241
35. Halder, SK, Goodwin, JS, and Al-Hendy, A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. (2011) 96:E754–62. doi: 10.1210/jc.2010-2131
36. Bikle, DD. Vitamin D: an ancient hormone. Exp Dermatol. (2011) 20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x
37. Zhang, X, Nicosia, SV, and Bai, W. Vitamin D receptor is a novel drug target for ovarian cancer treatment. Curr Cancer Drug Targets. (2006) 6:229–44. doi: 10.2174/156800906776842939
39. Liu, PT, Stenger, S, Li, H, Wenzel, L, Tan, BH, Krutzik, SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science (New York, NY). (2006) 311:1770–3. doi: 10.1126/science.1123933
40. Ylikomi, T, Laaksi, I, Lou, YR, Martikainen, P, Miettinen, S, Pennanen, P, et al. Antiproliferative action of vitamin D. Vitam Horm. (2002) 64:357–406. doi: 10.1016/S0083-6729(02)64010-5
41. Percival, MA, Pasco, JA, Hosking, SM, Williams, LJ, Holloway-Kew, KL, and Hyde, NK. Gestational folate and offspring bone health; the vitamin D in pregnancy study. Calcif Tissue Int. (2021) 108:605–9. doi: 10.1007/s00223-020-00795-z
42. Levasseur, R. Bone tissue and hyperhomocysteinemia. Joint Bone Spine. (2009) 76:234–40. doi: 10.1016/j.jbspin.2008.11.002
43. Wang, L, Zhou, C, Yu, H, Hao, L, Ju, M, Feng, W, et al. Vitamin D, folic acid and vitamin B(12) can reverse vitamin D deficiency-induced learning and memory impairment by altering 27-hydroxycholesterol and S-adenosylmethionine. Nutrients. (2022) 15:132. doi: 10.3390/nu15010132
44. Amer, M, and Qayyum, R. The relationship between 25-hydroxyvitamin D and homocysteine in asymptomatic adults. J Clin Endocrinol Metab. (2014) 99:633–8. doi: 10.1210/jc.2013-3262
45. Ota, K, Takahashi, T, Han, A, Damvaeba, S, Mizunuma, H, and Kwak-Kim, J. Effects of MTHFR C677T polymorphism on vitamin D, homocysteine and natural killer cell cytotoxicity in women with recurrent pregnancy losses. Hum Reprod. (2020) 35:1276–87. doi: 10.1093/humrep/deaa095
46. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health In: AC Ross, CL Taylor, AL Yaktine, and HB Del Valle, editors. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press (US) (2011)
47. Holick, MF, Binkley, NC, Bischoff-Ferrari, HA, Gordon, CM, Hanley, DA, Heaney, RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
48. Prevention and management of osteoporosis. World Health Organization technical report series (2003) 921:1–164.
49. Baron, RM, and Kenny, DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. (1986) 51:1173–82. doi: 10.1037/0022-3514.51.6.1173
50. Lucock, M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. (2000) 71:121–38. doi: 10.1006/mgme.2000.3027
51. Murto, T, Skoog Svanberg, A, Yngve, A, Nilsson, TK, Altmäe, S, Wånggren, K, et al. Folic acid supplementation and IVF pregnancy outcome in women with unexplained infertility. Reprod Biomed Online. (2014) 28:766–72. doi: 10.1016/j.rbmo.2014.01.017
52. Wen, SW, White, RR, Rybak, N, Gaudet, LM, Robson, S, Hague, W, et al. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): double blind, phase III, randomised controlled, international, multicentre trial. BMJ (Clinical research ed). (2018) 362:k3478. doi: 10.1136/bmj.k3478
53. Abrahamsen, B, Madsen, JS, Tofteng, CL, Stilgren, L, Bladbjerg, EM, Kristensen, SR, et al. A common methylenetetrahydrofolate reductase (C677T) polymorphism is associated with low bone mineral density and increased fracture incidence after menopause: longitudinal data from the Danish osteoporosis prevention study. J Bone Miner Res. (2003) 18:723–9. doi: 10.1359/jbmr.2003.18.4.723
54. Abrahamsen, B, Jørgensen, HL, Nielsen, TL, Andersen, M, Haug, E, Schwarz, P, et al. MTHFR c.677C>T polymorphism as an independent predictor of peak bone mass in Danish men--results from the Odense androgen study. Bone. (2006);38(2):215–219.
55. Jacques, PF, Bostom, AG, Williams, RR, Ellison, RC, Eckfeldt, JH, Rosenberg, IH, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. (1996) 93:7–9. doi: 10.1161/01.CIR.93.1.7
56. Weisberg, I, Tran, P, Christensen, B, Sibani, S, and Rozen, R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. (1998) 64:169–72. doi: 10.1006/mgme.1998.2714
57. Weisberg, IS, Jacques, PF, Selhub, J, Bostom, AG, Chen, Z, Curtis Ellison, R, et al. The 1298A→C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. (2001) 156:409–15. doi: 10.1016/S0021-9150(00)00671-7
58. Nelen, WL, Bulten, J, Steegers, EA, Blom, HJ, Hanselaar, AG, and Eskes, TK. Maternal homocysteine and chorionic vascularization in recurrent early pregnancy loss. Hum Reprod. (2000) 15:954–60. doi: 10.1093/humrep/15.4.954
59. Lucock, M, Thota, R, Garg, M, Martin, C, Jones, P, Furst, J, et al. Vitamin D and folate: a reciprocal environmental association based on seasonality and genetic disposition. Am J Hum Biol. (2018) 30:e23166. doi: 10.1002/ajhb.23166
60. Yu, AC, Deng, YH, Long, C, Sheng, XH, Wang, XG, Xiao, LF, et al. High dietary folic acid supplementation reduced the composition of fatty acids and amino acids in fortified eggs. Foods (Basel, Switzerland). (2024) 13:1048. doi: 10.3390/foods13071048
61. Kriebitzsch, C, Verlinden, L, Eelen, G, van Schoor, NM, Swart, K, Lips, P, et al. 1,25-dihydroxyvitamin D3 influences cellular homocysteine levels in murine preosteoblastic MC3T3-E1 cells by direct regulation of cystathionine β-synthase. J Bone Mineral Res. (2011) 26:2991–3000. doi: 10.1002/jbmr.493
62. Al-Bayyari, N, Al-Zeidaneen, S, Hailat, R, and Hamadneh, J. Vitamin D(3) prevents cardiovascular diseases by lowering serum total homocysteine concentrations in overweight reproductive women: a randomized, placebo-controlled clinical trial. Nutr Res. (2018) 59:65–71. doi: 10.1016/j.nutres.2018.07.012
63. Forman, JP, Bischoff-Ferrari, HA, Willett, WC, Stampfer, MJ, and Curhan, GC. Vitamin D intake and risk of incident hypertension: results from three large prospective cohort studies. Hypertension. (2005) 46:676–82. doi: 10.1161/01.HYP.0000182662.82666.37
Keywords: MTHFR polymorphism, vitamin D, homocysteine, infertility, mediation analysis
Citation: Zhou R, Zhu Z, Wang Z, Dong M, Huang L, Wang S, Zhang X and Liu F (2025) Association between MTHFR polymorphisms and vitamin D status in infertile women: a mediation analysis. Front. Nutr. 12:1644302. doi: 10.3389/fnut.2025.1644302
Edited by:
Owen Kelly, Sam Houston State University, United StatesReviewed by:
Zaid Al-Attar, University of Baghdad, IraqAndreea Mirela Caragea, Fundeni Clinical Institute, Romania
Copyright © 2025 Zhou, Zhu, Wang, Dong, Huang, Wang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenghua Liu, bGl1c2hpbmUyMDA2QDE2My5jb20=; Xiqian Zhang, NjUxNTU3MDc1QHFxLmNvbQ==
†ORCID: Fenghua Liu, orcid.org/0000-0002-3860-9466
Xiqian Zhang, orcid.org/0009-0001-5338-8884
 Ruiqiong Zhou
Ruiqiong Zhou Zhenghong Zhu
Zhenghong Zhu Zhaoyi Wang
Zhaoyi Wang Mei Dong1,2
Mei Dong1,2 Li Huang
Li Huang Fenghua Liu
Fenghua Liu