- 1Department of Interdisciplinary Medicine, University of Bari “Aldo Moro”, Bari, Italy
- 2INBB National Institute for Biostructure and Biosystems, Rome, Italy
- 3Department of Translational Biomedicine and Neuroscience (DiBraiN), University of Bari “Aldo Moro”, Bari, Italy
Background: Abdominal obesity is a major global health burden, driving risk for cardiovascular disease, type 2 diabetes, and cancer. The Mediterranean Diet (MedDiet), recognized for its cardiometabolic benefits, emphasizes Extra-Virgin Olive Oil (EVOO) as a primary fat source. We previously validated the Chrono Med Diet Score (CMDS), an index integrating dietary quality and chrono-nutritional principles, and demonstrated its associations with abdominal adiposity and cancer incidence. Although EVOO is central to the MedDiet, mechanisms related to its specific contributions to metabolic health remain partial. In the present study, we investigated the relationship between consistent EVOO intake frequency, MedDiet adherence (CMDS), and anthropometric outcomes.
Methods: We analyzed data from 16,273 adults (46.5% male) who completed the CMDS-based online survey since April 2023. Data included age, sex, height, weight, waist circumference (WC), Body Mass Index (BMI) and dietary/lifestyle information. EVOO intake frequency was categorized as: sporadic (<3 days/week), frequent (≥3 but <6 days/week), or regular (≥6 days/week), based on ~25 g/day (~2 tablespoons). Statistical analyses included Student’s t-tests, ANOVA with Bonferroni correction, mediation analysis, and multivariable logistic regression adjusting for confounders.
Results: Significant sex differences were observed in age, BMI, WC, and CMDS. Participants with regular EVOO intake were significantly older (55.9 ± 8.1 years) than sporadic (53.9 ± 7.1) and frequent (54.1 ± 7.7) consumers (p = 0.0019) yet showed more favorable anthropometrics. Compared to sporadic intake, regular intake was associated with significantly lower BMI (24.7 ± 3.0 vs. 26.6 ± 2.9, p < 0.001) and WC (89.1 ± 6.7 cm vs. 99.4 ± 9.1 cm, p < 0.0001), with consistent results across sexes (p < 0.0001 for both). Mediation analysis revealed that EVOO’s effect on WC was significantly mediated by CMDS (β = −0.83, p < 0.0001), accounting for 61.9% of the total effect. A direct association also persisted after adjusting for CMDS (β = −0.59, p < 0.0001). In logistic regression, non-regular EVOO intake was associated with substantially higher odds of abdominal obesity (Odds Ratio 5.1; 95% Confidence Interval: 3.3–6.8; p < 0.0001).
Conclusion: In this large cohort, regular EVOO consumption, while defining higher CMDS adherence, is independently associated with lower BMI and WC. EVOO exerts a dual role in metabolic health, both mediating and independently enhancing the relationship between chrono-Mediterranean diet adherence and reduced abdominal obesity. Non-regular EVOO intake emerges as a strong risk factor for visceral adiposity, irrespective of overall diet quality.
1 Introduction
Abdominal obesity, characterized by excess fat accumulation around abdominal organs, is a primary driver of Metabolic syndrome (MetS) and is strongly associated with increased risks of cardiovascular disease, type 2 diabetes, cancers, and overall mortality (1–4). Abdominal fat is metabolically active, secreting inflammatory cytokines that contribute to systemic low-grade chronic inflammation and insulin resistance, further perpetuating metabolic dysfunction (5, 6). Consequently, identifying effective lifestyle strategies, to mitigate and reduce abdominal obesity, particularly dietary interventions, is a critical public health priority (7). Given its clinical importance, the accurate assessment of abdominal obesity is crucial. Waist circumference (WC) is the most widely used and accepted anthropometric parameter for directly quantifying central adiposity and visceral fat accumulation (8). Complementing this, Body Mass Index (BMI) serves as a standard measure for assessing overall body weight relative to height, providing a broader context of general adiposity (9). Together, these non-invasive measurements are fundamental tools in both clinical practice and epidemiological research for identifying individuals at high cardiometabolic risk.
The Mediterranean diet (MedDiet) has emerged as a cornerstone dietary pattern associated with numerous health benefits (10). Characterized by high consumption of fruits, vegetables, legumes, whole grains, nuts, fish, extra-virgin olive oil (EVOO), with moderate poultry intake and low red/processed meat and sweets consumption (11), the MedDiet has demonstrated efficacy in improving MetS components (12). EVOO itself represents the main source of fat in the MedDiet (13). It is constituted by a high content of monounsaturated fatty acids (MUFA), mainly oleic acid, a variable but significant amount of polyunsaturated fatty acids (PUFA), as well as minor amounts of antioxidant micronutrients like polyphenols, squalene, lignans, phenyl-ethyl alcohols and secoiridoids (14, 15). Oleic acid (18,1 n-9) accounts for approximately 49 to 83% of total fatty acids (FA) in EVOO (16), and its consumption has been linked to improved pancreatic and hepatic secretory activities, as well as reduced risk for development of gastric-duodenal ulcers (17). Previously, our research group has demonstrated the significant interaction of MUFAs with plasma lipids and lipoprotein compositions, ultimately resulting in reduced inflammation, coagulation and oxidative stress, and a significant improvement of glucose homeostasis and blood pressure (15, 18). These findings were further confirmed by health claims by the Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), which stated that oleic acid contained in EVOO, together with polyphenols, significantly contributes to the maintenance of normal blood cholesterol levels (19, 20). Beyond their effects on cholesterol, these bioactive polyphenols, such as hydroxytyrosol and oleocanthal, are known to exert potent anti-inflammatory and antioxidant effects by modulating key cellular signaling pathways involved in metabolic health. Experimental studies have demonstrated their ability to reduce markers of oxidative stress and inflammation, providing a strong mechanistic basis for the role of EVOO in preventing metabolic diseases linked to visceral adiposity (21, 22).
To build on the significant beneficial role of MedDiet and its key components on global health, our research group has validated the Chrono Med Diet Score (CMDS), an easy-to-use questionnaire, which considers lifestyle habits and a modern chrono-nutritional approach, in addition to information on food consumption. The CMDS consists of 11 food categories, and takes into account the time of farinaceous products intake and physical activity (23). EVOO is reputed a standalone factor, opposed to other sources of fat like butter or margarine. In previous studies, we highlighted that CMDS is associated not only with MedDiet adherence but also with several conditions, including abdominal obesity, dyslipidemia, glucose intolerance, increased cardiovascular risk, and gastrointestinal cancer (24).
Despite the recognized benefits of MedDiet and EVOO, gaps remain in understanding the specific contribution of EVOO consumption patterns within the context of overall chrono-dietary adherence. Specifically, the extent to which regular EVOO intake influences anthropometric outcomes like BMI and WC, independently or in conjunction with adherence to broader chrono-dietary principles (like CMDS), needs clarification. Furthermore, understanding the pathways through which these associations occur is crucial. In the present study, we aimed to investigate the associations between habitual EVOO intake frequency, adherence to the MedDiet, and key anthropometric measures in a large population sample.
2 Materials and methods
2.1 Study participants and data collection
The CMDS public survey was available online in both English and Italian for the general population since publication of the original study in April 2023 (25). The survey included some mandatory questions, such as age, height, weight. Following these inputs, participants could proceed with questions related to their dietary and lifestyle habits. Upon completion of the questionnaire, the system calculated an individual CMDS score and provided personalized health recommendations according to the results. No incentives were offered for the survey participation. Each participant was given a unique identifier based on internet protocol address, which was not collected, to avoid multiple inputs from the same subject in the online database. Only geographical area was registered for the purpose of the study, proving that 94.1% of the recorded input came from the European area. All responses were filled anonymously and on a voluntary basis. The survey was conducted in accordance with EU Regulation 2016/679 on the protection of natural persons about the processing of personal data (GDPR) (26). By completing the survey, all participants gave their written informed consent to participate in the present study. No personal data were stored following survey completion. The investigation was performed in accordance with the World Medical Association’s Declaration of Helsinki (27) and did not include any experiment involving human or biological human samples, nor research on identifiable human data. Since all responses were provided anonymously and on a voluntary basis, ethics committee approval was not required. Results were reported according to the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) statement (28). The research study has been approved by Italian Ministry of University and Research (concession decree no. 1550 of October 11th, 2022) under the project PNRR-NRRP 2022; PE00000003 entitled “ON Foods - Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods.”
2.2 Clinical and anthropometric assessment
Height was recorded in meters (m) and weight in kilograms (kg). BMI was calculated as weight divided by the square of height (kg/m2). Participants were categorized based on BMI as overweight (BMI 25.0–29.9 kg/m2) or obese (BMI ≥ 30.0 kg/m2) (29). For WC measurement, instructions taken from standard protocols were provided via the survey website; participants were advised to measure horizontally midway between the lowest rib margin and the superior iliac crest. Consistent with the study population being primarily European, abdominal obesity was defined according to the International Diabetes Federation (IDF) 2006 criteria for Europids as positive when above 94 cm in males and 80 cm in females (30, 31).
2.3 Chrono med diet score
The CMDS has been validated by our research group as a reliable score for evaluating adherence to the MedDiet, as well as its association with abdominal adiposity. Eleven food categories are evaluated to determine the overall score based on daily to weekly intake: (1) fruit, (2) vegetables and nuts, (3) legumes, (4) farinaceous products (i.e., bread, pasta, cookies), (5) grain cereals, (6) fish, (7) meat and meat products, (8) milk and dairy products, (9) EVOO, (10) butter, margarine and lard, and (11) alcohol intake. Two additional categories (time of farinaceous product intake and physical activity) were added to characterize the eating habits and overall metabolic health status. EVOO intake was assessed and categorized based on the frequency of consuming a reference amount, defined as approximately two tablespoons (25 grams) per day. Participants were classified into three groups. Sporadic intake was defined as consuming the reference amount of EVOO on fewer than 3 days per week. Frequent Intake was defined as consuming the reference amount on at least three but fewer than 6 days per week. Lastly, Regular Intake was defined as consuming the reference amount of EVOO on at least 6 days per week (23). The overall CMDS score ranged from −13 to 25 points, considering a cut-off of 13 points to discriminate MedDiet adherence (Supplementary Figure 1).
2.4 Statistical analysis
Descriptive statistical analyses of study sample were performed, and results were expressed as mean±standard deviation (SD). Comparisons of socio-demographic and anthropometric variables between two groups were conducted with the Student t-test for continuous variables. Comparisons between more than two groups were performed via one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test, where appropriate. To quantify the extent to which EVOO participated in the transmittance of change from adherence to the MedDiet to the increase in WC, a mediation analysis was performed. Furthermore, a logistic univariate model was tested to estimate the relationship between the exposure factor (EVOO) and the outcome of interest (abdominal obesity). Potentially confounding variables in the assessment of the causal effect were accounted for in a multivariable logistic regression. Results were expressed as Odds Ratio (OR) with their relative 95% confidence interval (95% CI) and graphically plotted in a forest plot. All statistical analyses were performed using the NCSS 2025 Statistical Software, version 25.0.2 (NCSS, LLC, Kaysville, Utah, United States) and GraphPad Prism, version 10.4.2 (GraphPad Software; San Diego, United States).
3 Results
3.1 Baseline characteristics of the study population
A total of 20,784 individuals were examined. Only subjects who declared to be 18 years old or older were included in the present study. Subsequently, 1,561 entries were removed due to incorrect or nonsensical data entry (e.g., text in numerical fields). An additional 1,671 participants were excluded based on outlier analysis, which identified clinically implausible anthropometric values. This resulted in a dataset of 17,552 potentially eligible participants. From this group, 1,279 individuals were excluded from the present analysis due to missing waist circumference data. Thus, the final analytical sample comprised 16,273 participants (7,561 males and 8,712 females). A flow diagram detailing participant selection is provided in Supplementary Figure 2.
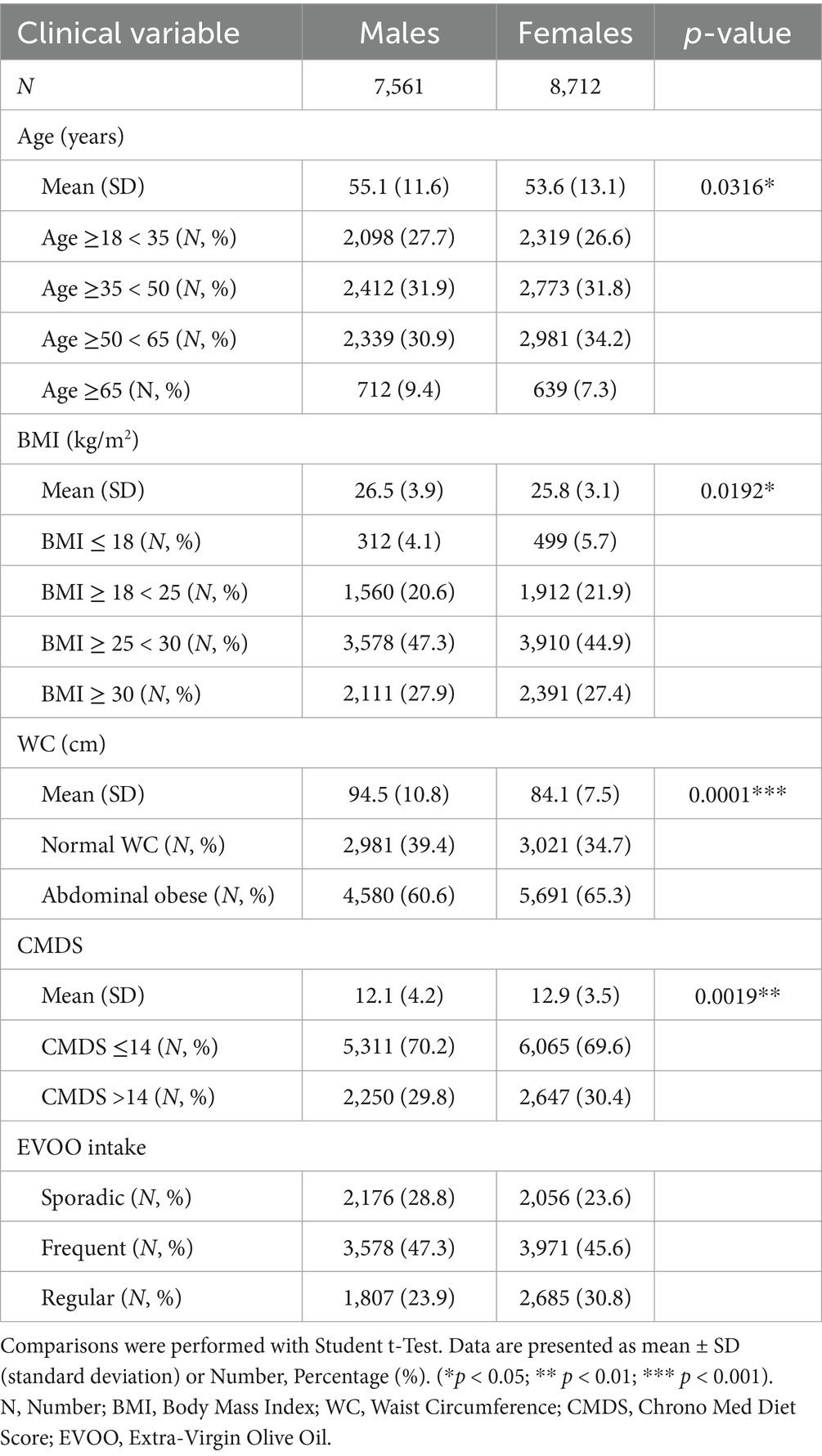
Detailed baseline clinical characteristics stratified by sex are presented in Table 1. Males exhibited a slightly higher mean age (55.1 ± 11.6 years vs. 53.6 ± 13.1 years for females, p = 0.0316) and significantly higher mean BMI (26.5 ± 3.9 kg/m2 vs. 25.8 ± 3.1 kg/m2, p = 0.0192) and WC (94.5 ± 10.8 cm vs. 84.1 ± 7.5 cm, p < 0.001). Conversely, females showed significantly higher mean CMDS (12.9 ± 3.5 vs. 12.1 ± 4.2 for males, p = 0.0019). Abdominal obesity was prevalent in both sexes, identified in 60.6% of males and 65.3% of females. Differences in EVOO consumption frequency were also noted, with regular intake reported more often by females (30.8%) than males (23.9%).
3.2 Sporadic EVOO consumption identifies obese and younger subjects
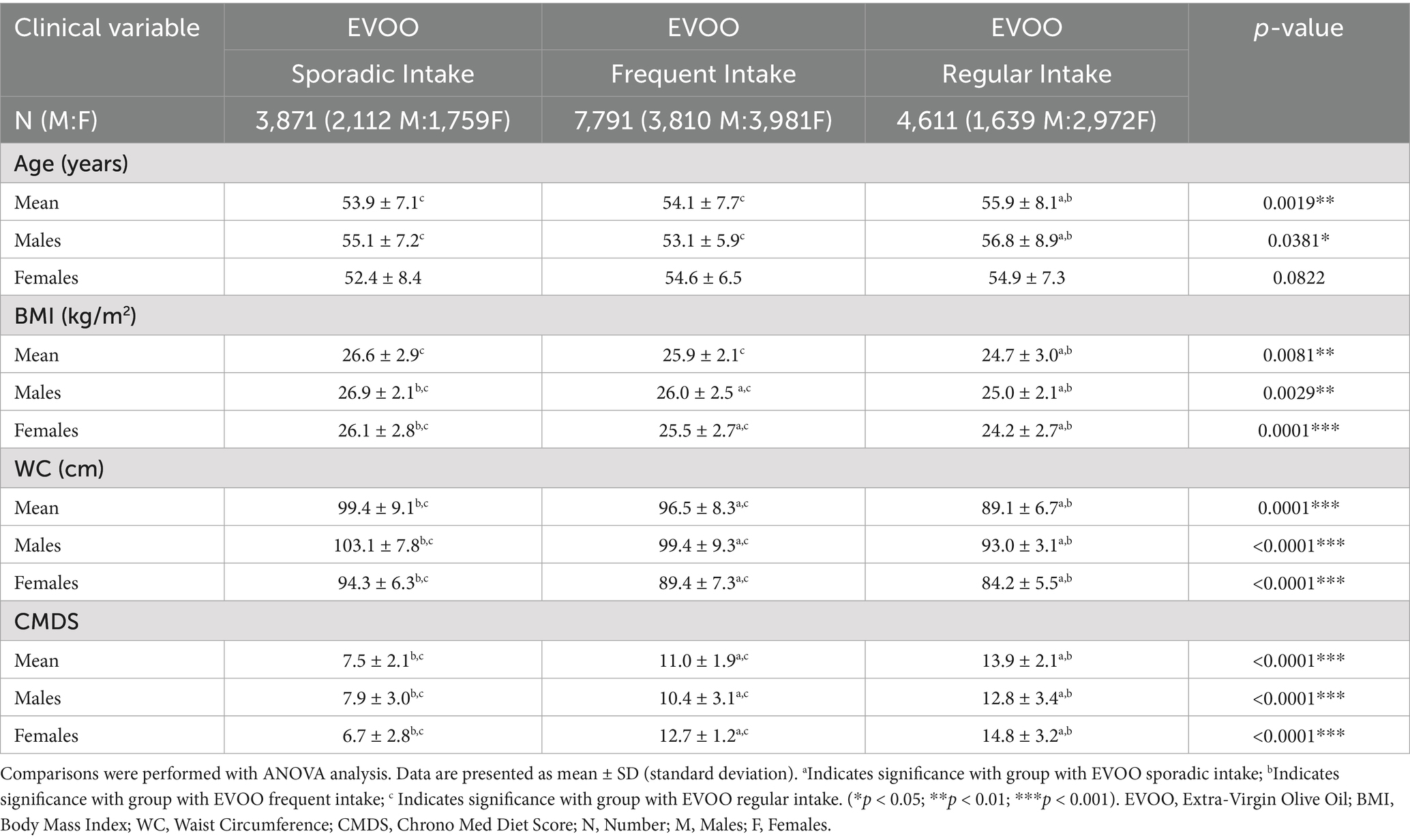
To deepen our analysis on the potential role of EVOO in the definition of anthropometric measurements, we divided our population based on the referred EVOO intake into three categories (Table 2). First, we observed that individuals reporting regular EVOO consumption were significantly (overall ANOVA p = 0.0019, F-ratio = 14.4) older on average (55.9 ± 8.1) compared to both the sporadic (53.9 ± 7.1) and frequent intake groups (54.1 ± 7.7). Nevertheless, a significant inverse association was observed between EVOO intake frequency and BMI (overall ANOVA p = 0.0081, F-ratio = 7.8). Mean BMI in the regular intake group (24.7 ± 3.0) was significantly lower than both the frequent (25.9 ± 2.1) and sporadic intake groups (26.6 ± 2.9). This pattern was observed and significant within both male (p = 0.0029) and female subgroups (p < 0.001). Similarly, WC showed a significant inverse gradient with EVOO intake frequency (overall ANOVA p < 0.0001, F-ratio = 283.4). The regular intake group had the lowest mean WC (89.1 ± 6.7), significantly lower than the frequent (96.5 ± 8.3) and sporadic intake groups (99.4 ± 9.1). This association remained highly significant when analyzed separately for males (p < 0.0001) and females (p < 0.0001). Given the significant role of EVOO in the overall scoring system of the CMDS, we confirmed a strong positive association between EVOO intake frequency and CMDS adherence (overall ANOVA p < 0.0001, F-ratio = 503.2). Mean CMDS scores increased progressively as EVOO intake improved from sporadic (7.5 ± 2.1) to frequent (11.0 ± 1.9), finally to regular (13.9 ± 2.1), both in the overall population and when stratified by sex.
3.3 EVOO intake as a mediator and independent factor for abdominal obesity
To further examine whether EVOO consumption could be considered a significant mediator in the relationship between CMDS adherence and WC, we performed a mediation analysis (Figure 1; Supplementary Table 1). We observed that EVOO intake indeed is a significant mediator (indirect effect coefficient = −0.8293, p < 0.0001), highlighting that part of the association between higher adherence to MedDiet and lower WC operates through EVOO intake. The analysis suggested this mediated pathway accounts for a substantial portion (61.98%) of the overall MedDiet-WC relationship. Furthermore, to determine if EVOO intake had an association with WC independent of its link with the CMDS, we looked at the direct effect of EVOO consumption on WC after controlling for CMDS. Results showed a highly significant direct effect (−0.5912, p < 0.0001), indicating that regular EVOO intake also was a standalone parameter in the definition of lower waist circumference, even when the overall CMDS score was accounted for.
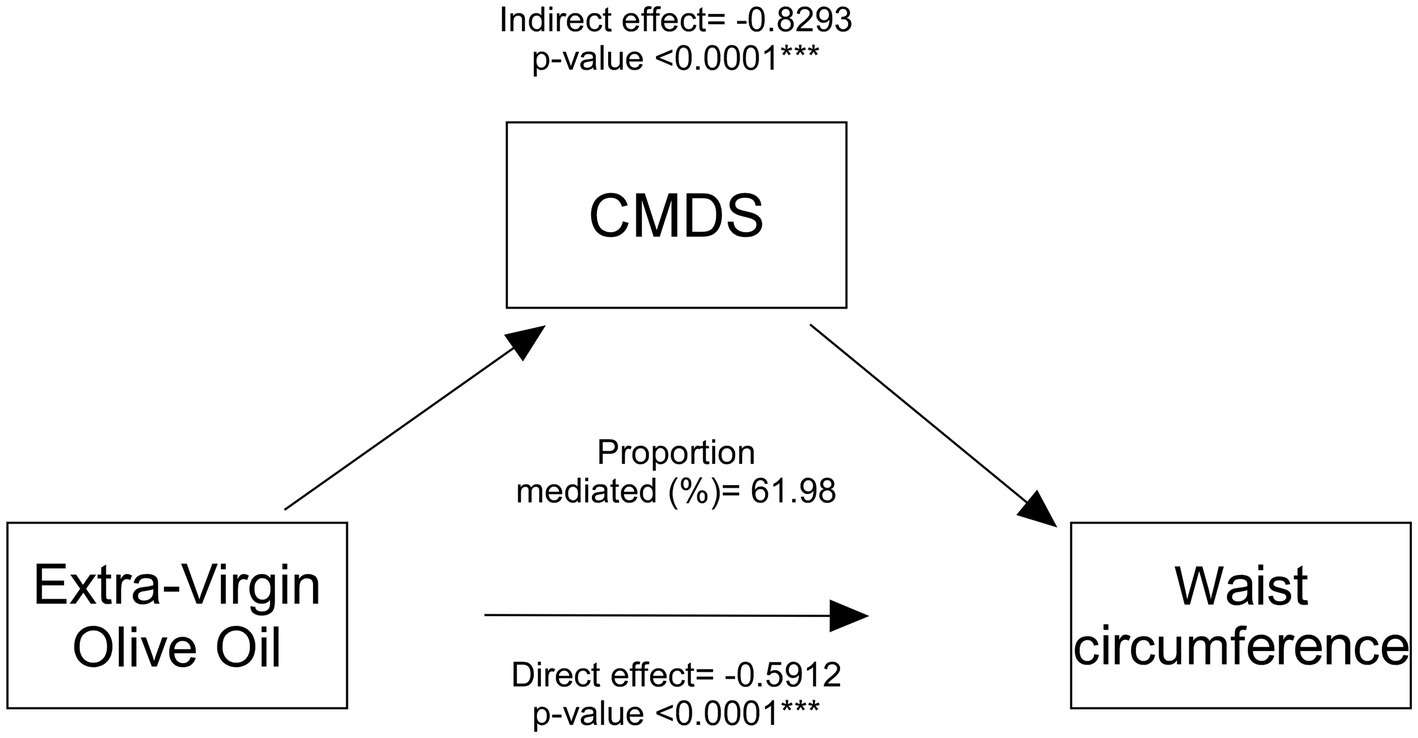
Figure 1. Mediation analysis of EVOO intake effect on waist circumference. Mediation model of the direct effect of Extra-Virgin Olive Oil (EVOO) intake on waist circumference and the indirect effect operating through the Chrono Med Diet Score (CMDS). Coefficients and p-values for direct and indirect paths are shown.
3.4 Individuals with poor EVOO intake are at major risk of obesity
Based on the results of the mediation analysis, and given the significance of potentially confounding variables, we performed a logistic regression, adjusted for age, sex, and overall CMDS score, to analyze the risk for abdominal obesity based on EVOO intake patterns (Figure 2). Compared to individuals reporting regular EVOO intake, those with non-regular intake (sporadic and frequent combined) had significantly higher ORs of being classified as having abdominal obesity (OR = 5.1; 95% CI = 3.3–6.8; p < 0.0001).
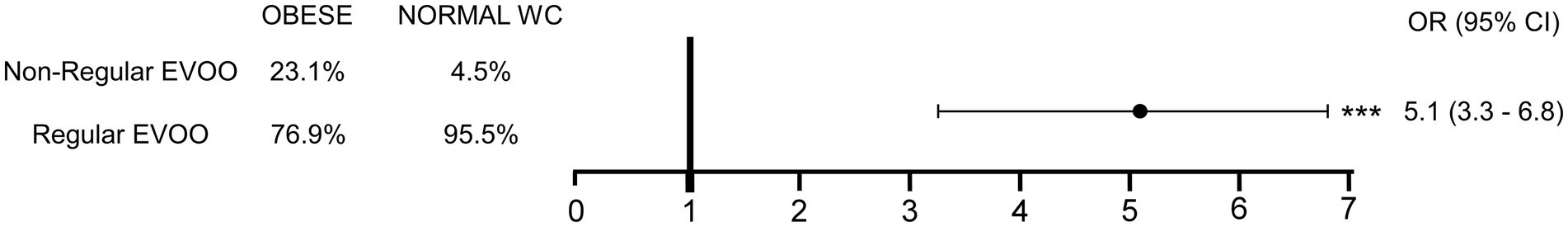
Figure 2. Risk for abdominal obesity according to EVOO intake after adjusting for potentially confounding variables. Contingency table to assess the Odds Ratio (OR) for abdominal obesity development according to EVOO intake and relative OR with 95% confidence interval is shown, following multivariable analysis to account for potential confounding variables. (***p < 0.0001). Extra-Virgin Olive Oil, EVOO; Waist Circumference, WC; Odds Ratio, OR; Confidence Interval, CI.
4 Discussion
This large cross-sectional study provides compelling evidence linking regular EVOO consumption patterns with anthropometric outcomes. Our primary findings indicate that EVOO intake plays a significant role in the determination of WC, both as an independent factor and a mediator in the relationship between adherence to the MedDiet and anthropometric indices. Furthermore, our analysis suggests that non-regular EVOO consumption (2 tablespoons on fewer than 6 days per week) was associated with a striking five-fold increase in the odds of abdominal obesity, even after adjusting for potential confounders.
The observed inverse associations between EVOO intake frequency and anthropometric indices, especially WC, align with the well-documented benefits of the MedDiet, of which EVOO is a central component, on weight management and metabolic health. Indeed, it has been widely suggested that the consumption of a MedDiet rich in olive oil can prevent obesity (32, 33), type 2 diabetes mellitus (34), and MetS. Existing randomized clinical trials on the effects of EVOO on body weight and fat have yielded limited and conflicting results (35–37). Nonetheless, our research group has extensively studied in the last years the benefits of EVOO intake on the whole-body health, specifically analyzing the role of stearoyl-CoA desaturase 1 (SCD1), a crucial enzyme for the endogenous biosynthesis of oleic acid (C18:1, n9) and palmitoleic acid (C16:1, n7) from dietary or de novo synthesized saturated FA (15, 38, 39). Numerous studies have attempted to untangle the complex relationship between SCD1 and the progression of a variety of diseases, including obesity and diabetes (40), cardiovascular diseases (41, 42), and cancer (43). Previously, our group has studied the complex role of SCD1 in modulating hepatic lipid metabolism, inflammation, fibrosis, and susceptibility to liver diseases, highlighting the importance of intestinal MUFA production in maintaining hepatic health (44). Indeed, intestinal Scd1 deletion decreased hepatic MUFA levels, suggesting a direct contribution of gut-derived MUFAs to hepatic FA content. Nonetheless, we found that the absence of SCD1 and endogenously synthesized MUFAs in the intestinal epithelium increased susceptibility to inflammation and colorectal cancer development, which are reversed by dietary oleic acid supplementation (38). This could explain the magnitude of the differences observed between groups based on EVOO consumption, untangling the potential clinical relevance of habitual EVOO intake in relation to central adiposity, a key risk factor for cardiometabolic diseases. These results are in line with previously published data, highlighting the role of MedDiet and its components, especially EVOO, in reducing inflammation, improving lipid profiles, and enhancing insulin sensitivity, all of which can influence body composition (45). Indeed, in the European Prospective Investigation into Cancer and Nutrition (EPIC)-PANACEA prospective cohort, the high adherence to the MedDiet was linked to a decreased possibility to become overweight or obese (46). Moreover, Soriguer et al. followed-up 613 outpatients over 6 years and highlighted a significant decrease in obesity incidence in the group of patients who consumed EVOO instead of sunflower oil (47). Similarly, Guasch-Ferrè et al. found in two separate U. S. prospective cohorts that higher olive oil intake was associated with lower risk of total and cause-specific mortality, and that changing sources of fat from margarine, butter, mayonnaise, and dairy fat to EVOO was associated with lower risk of mortality (48). Our findings on better anthropometrics are part of a broader, though complex, picture of EVOO’s metabolic benefits. This is highlighted by a recent comprehensive meta-analysis of 33 randomized trials, which found that while EVOO consumption did not significantly alter most lipid profiles or blood pressure, it did lead to a significant improvement in insulin sensitivity. This specific effect on glucose metabolism and insulin resistance, which are central to the pathophysiology of abdominal obesity, provides a strong potential mechanism that could underlie the favorable body composition findings we observed in our cohort (49).
Moreover, bioactive polyphenols, such as hydroxytyrosol and oleocanthal, are known to exert potent anti-inflammatory and antioxidant effects by modulating key cellular signaling pathways involved in metabolic health. Experimental studies have demonstrated their ability to reduce markers of oxidative stress and inflammation, providing a strong mechanistic basis for the role of EVOO in preventing metabolic diseases linked to visceral adiposity (21, 22).
Nevertheless, the inherent overlap between MedDiet and EVOO challenging the isolation of EVO effects alone should be acknowledged. Anyhow, various findings suggest olive oil’s own positive impact on weight management. For instance, Buckland et al. demonstrated that regular EVOO use improves the taste of salads, vegetables, and legumes, thereby encouraging the consumption of high-fiber, low-energy-density foods that enhance fullness (50). From a biochemical perspective, the consumption of dietary oleic acid triggers satiety by acting as a molecular sensor that promotes the release of the gut-derived lipid messenger oleoylethanolamide (51). Nonetheless, in a randomized, double-blinded clinical trial by Galvão Cândido et al., fat loss was approximately 80% higher in women consuming an energy-restricted diet containing EVOO compared to a control group, proving again the role of EVOO in energy-restricted programs for obesity treatment (52).
The unique benefits of EVOO are further highlighted when compared to interventions with other vegetable oils, for which results are widely inconsistent. A comprehensive meta-analysis of 25 randomized controlled trials on canola oil found that its consumption led only to a modest decrease in overall body weight and had no significant effect on body fat markers (53). A randomized trial by Oliveira-de-Lira et al. in obese women on a calorie-restricted diet found that different oils produced distinct outcomes. While coconut oil supplementation led to greater reductions in waist circumference and body fat, chia oil was more effective at improving lipid profiles (54). Conversely, in a randomized controlled crossover study, coconut oil had no impact on anthropometric and biochemical findings (55). On this basis, one of the novel aspects of our study is the finding that EVOO is not only a component of diet that mediates a significant part of the MedDiet-WC relationship but can, indeed, be considered as a single active contributor to the benefits associated with MedDiet adherence on WC. The substantial OR linking non-regular EVOO intake to abdominal obesity further highlights the potential protective role of consistent EVOO consumption against this metabolically harmful fat accumulation.
To the best of our knowledge, this is the first study that provides novel evidence suggesting that EVOO can be considered a standalone, independent beneficial factor to WC. Its large sample size, which provides high statistical power to detect associations, is also a significant strength. Furthermore, it is one of the first studies to specifically categorize EVOO intake frequency and use robust statistical methods like mediation and multivariable logistic regression to isolate its independent effect within the context of a modern chrono-nutrition score. However, certain limitations must be acknowledged. The cross-sectional design prevents us from inferring causality between EVOO intake and lower abdominal obesity. Additionally, the reliance on self-reported data for both dietary intake and anthropometric measurements introduce potential for recall bias and measurement error. Finally, socio-economic data like annual income, marital status and smoking were missing from the online survey.
Despite these limitations, the findings have potential implications for public health and dietary guidelines. They support the recommendations emphasizing regular EVOO consumption within a healthy dietary pattern, such as the MedDiet, for maintaining a healthy weight and reducing central obesity. Future research should take advantage of longitudinal designs to establish temporality, utilize quantitative dietary assessment methods, include objective measures of adiposity and biomarkers, and ideally conduct randomized controlled trials to confirm the causal effects of regular EVOO intake on anthropometric outcomes and underlying metabolic pathways.
Taken all together, our findings on this large cross-sectional study demonstrate that regular EVOO consumption of is significantly associated with a more favorable anthropometric profile, characterized by lower BMI and WC, and greater adherence to a chrono-Mediterranean dietary pattern. EVOO intake plays a substantial role in the pathway linking adherence to this healthy dietary pattern and reduced central adiposity, while also indicating independent contributions from the broader dietary pattern. Conversely, non-regular EVOO consumption is associated with significantly increased odds of abdominal obesity. Future longitudinal and intervention studies are warranted to confirm these associations and elucidate the underlying mechanisms.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Italian Ministry of University and Research (concession decree no. 1550 of October 11th, 2022) under the project PNRR-NRRP 2022; PE00000003 entitled “ON Foods - Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods.” The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CM: Investigation, Funding acquisition, Resources, Formal analysis, Software, Writing – original draft, Data curation, Methodology. LC: Resources, Data curation, Software, Writing – original draft, Methodology, Funding acquisition, Investigation, Formal analysis. EB: Data curation, Methodology, Writing – review & editing. SaC: Writing – review & editing, Data curation. FN: Writing – review & editing, Data curation, Methodology. SiC: Data curation, Writing – review & editing. AT: Data curation, Writing – review & editing. MA: Data curation, Methodology, Writing – review & editing. MF: Writing – review & editing, Data curation. RG: Writing – review & editing, Funding acquisition, Methodology. EP: Writing – review & editing, Methodology. MC: Methodology, Writing – review & editing. AM: Writing – review & editing, Supervision, Project administration, Visualization, Investigation, Conceptualization, Validation, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2 Investment 1.3—Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union—Next Generation EU; project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, project title “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods” awarded to Antonio Moschetta. It was also funded by AIRC IG 2019 “Regulation of lipid metabolic pathways in the gut liver axis: relevance in hepatocarcinoma” Id 23239 awarded to Antonio Moschetta. It was funded as well by MIUR- PRIN Progetti di Ricerca di Rilevante Interesse Nazionale 2022 “Metabolic hits in the road to colon cancer” Codice progetto n. 2022H9MPZ5 awarded to Antonio Moschetta. This study was also funded by the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2 Investment 1.4—Call for tender No. 3138 of 16/12/2021 of the Italian Ministry of University and Research funded by the European Union—NextGenerationEU; project code: CN00000041, CUP H93C22000430007, project title “National Center for Gene Therapy and Drugs based on RNA Technology” awarded to Antonio Moschetta. It was funded as well by the European Union—Next Generation EU—PNRR M6C2—Investimento 2.1 Valorizzazione e potenziamento della ricerca biomedica del SSN,” project code PNRR-MR1-2022-12376395. CUP H93C22000780006, project title: Italian Autoimmune Liver Disease (IT-AILD) Clinical Research Network (CRN) awarded to Antonio Moschetta. This study was also funded by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3—Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union—NextGeneration EU; Award Number: project code PE0000015, Concession Decree No. 1243 of 2 August 2022 adopted by the Italian Ministry of University and Research, CUP H33C22000680006, project title “Ageing well in an ageing society—A novel public-private alliance to generate socioeconomic, biomedical and technological solutions for an inclusive Italian ageing society– AGE-IT” awarded to Lucilla Crudele. It was also funded by PON “RICERCA E INNOVAZIONE” 2014-2020—Innovazione (D. M. 10 AGOSTO 2021, N. 1062) awarded to Raffaella Maria Gadaleta.
Acknowledgments
We thank the physicians and nurses of the Unità Operativa Complessa Universitaria di Medicina Interna “Cesare Frugoni” of the Azienda Ospedaliero—Universitaria Policlinico di Bari for their help and support during the study. We also thank Roberta Le Donne for her support. We thank Giuseppe Cavallo, Rita Cometa, Eng. Marianna Stranieri, Antonio Perrone and Angela Abrescia as well from NAPS Lab srls Bari for their help in generating virtual and visual version of the score.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1645230/full#supplementary-material
References
1. Abdelaal, M, Le Roux, CW, and Docherty, NG. Morbidity and mortality associated with obesity. Ann Transl Med. (2017) 5:161. doi: 10.21037/atm.2017.03.107
2. Flegal, KM, Kit, BK, Orpana, H, and Graubard, BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. (2013) 309:71–82. doi: 10.1001/jama.2012.113905
3. Powell-Wiley, TM, Poirier, P, Burke, LE, Després, JP, Gordon-Larsen, P, Lavie, CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. (2021) 143:973. doi: 10.1161/CIR.0000000000000973
4. De Matteis, C, Petruzzelli, S, Graziano, G, Novielli, F, Di Buduo, E, Cantatore, S, et al. Improving cardiovascular risk stratification: the role of abdominal obesity in predicting MACEs. Cardiovasc Diabetol. (2025) 24:328. doi: 10.1186/s12933-025-02885-4
5. Kawai, T, Autieri, MV, and Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. (2021) 320:C375–91. doi: 10.1152/ajpcell.00379.2020
6. Crudele, L, Piccinin, E, and Moschetta, A. Visceral adiposity and Cancer: role in pathogenesis and prognosis. Nutrients. (2021) 13:2101. doi: 10.3390/nu13062101
7. Wadden, TA, Tronieri, JS, and Butryn, ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. (2020) 75:235–51. doi: 10.1037/amp0000517
8. Ross, R, Neeland, IJ, Yamashita, S, Shai, I, Seidell, J, Magni, P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. (2020) 16:177–89. doi: 10.1038/s41574-019-0310-7
9. Whitlock, G, Lewington, S, Sherliker, P, Clarke, R, Emberson, J, Halsey, J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. (2009) 373:1083–96. doi: 10.1016/S0140-6736(09)60318-4
10. Guasch-Ferré, M, and Willett, WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. (2021) 290:549–66. doi: 10.1111/joim.13333
11. Trichopoulou, A, and Lagiou, P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. (2009) 55:383–9. doi: 10.1111/j.1753-4887.1997.tb01578.x
12. Di Daniele, N, Noce, A, Vidiri, MF, Moriconi, E, Marrone, G, Annicchiarico-Petruzzelli, M, et al. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget. (2017) 8:8947–79. doi: 10.18632/oncotarget.13553
13. Estruch, R, Ros, E, Salas-Salvadó, J, Covas, MI, Corella, D, Arós, F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. (2018) 378:e34. doi: 10.1056/NEJMoa1800389
14. Bester, D, Esterhuyse, AJ, Truter, EJ, and Van Rooyen, J. Cardiovascular effects of edible oils: a comparison between four popular edible oils. Nutr Res Rev. (2010) 23:334–48. doi: 10.1017/S0954422410000223
15. Piccinin, E, Cariello, M, De Santis, S, Ducheix, S, Sabbà, C, Ntambi, JM, et al. Role of oleic acid in the gut-liver Axis: from diet to the regulation of its synthesis via Stearoyl-CoA desaturase 1 (SCD1). Nutrients. (2019) 11:2283. doi: 10.3390/nu11102283
16. Servili, M, Sordini, B, Esposto, S, Urbani, S, Veneziani, G, Di Maio, I, et al. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants. (2013) 3:1–23. doi: 10.3390/antiox3010001
17. Bermudez, B, Lopez, S, Ortega, AM, Varela, LM, Pacheco, Y, Abia, R, et al. Oleic acid in olive oil: from a metabolic framework toward a clinical perspective. Curr Pharm Des. (2011) 17:831–43. doi: 10.2174/138161211795428957
18. De Santis, S, Cariello, M, Piccinin, E, Sabbà, C, and Moschetta, A. Extra virgin olive oil: lesson from nutrigenomics. Nutrients. (2019) 11:2085. doi: 10.3390/nu11092085
19. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA J. (2011) 9:2033. doi: 10.2903/j.efsa.2011.2033
20. U.S. Food and Drug Administration. FDA completes review of qualified health claim petition for oleic acid and the risk of coronary heart disease. Available online at: https://www.fda.gov/food/cfsan-constituent-updates/fda-completes-review-qualified-health-claim-petition-oleic-acid-and-risk-coronary-heart-disease (Accessed Apr 14, 2025).
21. Da Silva-Araújo, ER, Toscano, AE, Silva, PBP, Dos Santos, P, Junior, J, Gouveia, HJCB, et al. Effects of deficiency or supplementation of riboflavin on energy metabolism: a systematic review with preclinical studies. Nutr Rev. (2025) 83:e332–42. doi: 10.1093/nutrit/nuae041
22. Gouveia, HJC, Urquiza-Martínez, MV, Manhães-de-Castro, R, Costa-de-Santana, BJR, Villarreal, JP, Mercado-Camargo, R, et al. Effects of the treatment with flavonoids on metabolic syndrome components in humans: a systematic review focusing on mechanisms of action. IJMS. (2022) 23:8344. doi: 10.3390/ijms23158344
23. De Matteis, C, Crudele, L, Battaglia, S, Loconte, T, Rotondo, A, Ferrulli, R, et al. Identification of a novel score for adherence to the Mediterranean diet that is inversely associated with visceral adiposity and cardiovascular risk: the Chrono med diet score (CMDS). Nutrients. (2023) 15:1910. doi: 10.3390/nu15081910
24. De Matteis, C, Crudele, L, Gadaleta, RM, Di Buduo, E, Novielli, F, Petruzzelli, S, et al. Low adherence to Mediterranean diet characterizes metabolic patients with gastrointestinal Cancer. Nutrients. (2024) 16:630. doi: 10.3390/nu16050630
25. Chrono Med Diet Score. Online Questionnaire web page. Available online at: http://www.chronomeddiet.org.
26. European Union. EU regulation 2016/679 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing directive 95/46/EC (general data protection regulation). Available online at: https://eur-lex.europa.eu/eli/reg/2016/679/oj.
27. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191. doi: 10.1001/jama.2013.281053
28. Eysenbach, G. Improving the quality of web surveys: the checklist for reporting results of internet E-surveys (CHERRIES). J Med Internet Res. (2004) 6:e34. doi: 10.2196/jmir.6.3.e34
29. Crudele, L, De Matteis, C, Graziano, G, Novielli, F, Petruzzelli, S, Piccinin, E, et al. AST/ALT-to-platelet ratio (AARPRI) predicts gynaecological cancers: a 8-years follow-up study in 653 women. Sci Rep. (2023) 13:17793. doi: 10.1038/s41598-023-44243-y
30. Alberti, KGMM, Zimmet, P, and Shaw, J. Metabolic syndrome--a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
31. De Matteis, C, Cariello, M, Graziano, G, Battaglia, S, Suppressa, P, Piazzolla, G, et al. AST to platelet ratio index (APRI) is an easy-to-use predictor score for cardiovascular risk in metabolic subjects. Sci Rep. (2021) 11:14834. doi: 10.1038/s41598-021-94277-3
32. Esposito, K, Marfella, R, Ciotola, M, Di Palo, C, Giugliano, F, Giugliano, G, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. (2004) 292:1440. doi: 10.1001/jama.292.12.1440
33. Stefania, DS, Clodoveo, ML, Cariello, M, D’Amato, G, Franchini, C, Faienza, MF, et al. Polyphenols and obesity prevention: critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit Rev Food Sci Nutr. (2021) 61:1804–26. doi: 10.1080/10408398.2020.1765736
34. Perez-Martinez, P, Garcia-Rios, A, Delgado-Lista, J, Perez-Jimenez, F, and Lopez-Miranda, J. Mediterranean diet rich in olive oil and obesity, metabolic syndrome and diabetes mellitus. Curr Pharm Des. (2011) 17:769–77. doi: 10.2174/138161211795428948
35. Estruch, R, Martínez-González, MÁ, Corella, D, Salas-Salvadó, J, Ruiz-Gutiérrez, V, Covas, MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. (2006) 145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004
36. Salas-Salvadó, J, Fernández-Ballart, J, Ros, E, Martínez-González, MA, Fitó, M, Estruch, R, et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. (2008) 168:2449. doi: 10.1001/archinte.168.22.2449
37. McManus, K, Antinoro, L, and Sacks, F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int J Obes. (2001) 25:1503–11. doi: 10.1038/sj.ijo.0801796
38. Ducheix, S, Peres, C, Härdfeldt, J, Frau, C, Mocciaro, G, Piccinin, E, et al. Deletion of Stearoyl-CoA desaturase-1 from the intestinal epithelium promotes inflammation and tumorigenesis, reversed by dietary oleate. Gastroenterology. (2018) 155:1524–1538.e9. doi: 10.1053/j.gastro.2018.07.032
39. Cariello, M, Contursi, A, Gadaleta, RM, Piccinin, E, De Santis, S, Piglionica, M, et al. Extra-virgin olive oil from Apulian cultivars and intestinal inflammation. Nutrients. (2020) 12:1084. doi: 10.3390/nu12041084
40. ALJohani, AM, Syed, DN, and Ntambi, JM. Insights into stearoyl-CoA desaturase-1 regulation of systemic metabolism. Trends Endocrinol Metab. (2017) 28:831–42. doi: 10.1016/j.tem.2017.10.003
41. Matsui, H, Yokoyama, T, Sekiguchi, K, Iijima, D, Sunaga, H, Maniwa, M, et al. Stearoyl-CoA desaturase-1 (SCD1) augments saturated fatty acid-induced lipid accumulation and inhibits apoptosis in cardiac myocytes. PLoS One. (2012) 7:e33283. doi: 10.1371/journal.pone.0033283
42. Bednarski, T, Olichwier, A, Opasinska, A, Pyrkowska, A, Gan, AM, Ntambi, JM, et al. Stearoyl-CoA desaturase 1 deficiency reduces lipid accumulation in the heart by activating lipolysis independently of peroxisome proliferator-activated receptor α. Biochim Biophys Acta. (2016) 1861:2029–37. doi: 10.1016/j.bbalip.2016.10.005
43. Sen, U, Coleman, C, and Sen, T. Stearoyl coenzyme a desaturase-1: multitasker in cancer, metabolism, and ferroptosis. Trends in Cancer. (2023) 9:480–9. doi: 10.1016/j.trecan.2023.03.003
44. Ducheix, S, Piccinin, E, Peres, C, Garcia-Irigoyen, O, Bertrand-Michel, J, Fouache, A, et al. Reduction in gut-derived MUFAs via intestinal stearoyl-CoA desaturase 1 deletion drives susceptibility to NAFLD and hepatocarcinoma. Hepatol Commun. (2022) 6:2937–49. doi: 10.1002/hep4.2053
45. Romaguera, D, Norat, T, Vergnaud, AC, Mouw, T, May, AM, Agudo, A, et al. Mediterranean dietary patterns and prospective weight change in participants of the EPIC-PANACEA project. Am J Clin Nutr. (2010) 92:912–21. doi: 10.3945/ajcn.2010.29482
46. Razquin, C, Martinez, JA, Martinez-Gonzalez, MA, Mitjavila, MT, Estruch, R, and Marti, A. A 3 years follow-up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. Eur J Clin Nutr. (2009) 63:1387–93. doi: 10.1038/ejcn.2009.106
47. Soriguer, F, Almaraz, MC, Ruiz-de-Adana, MS, Esteva, I, Linares, F, García-Almeida, JM, et al. Incidence of obesity is lower in persons who consume olive oil. Eur J Clin Nutr. (2009) 63:1371–4. doi: 10.1038/ejcn.2009.65
48. Guasch-Ferré, M, Li, Y, Willett, WC, Sun, Q, Sampson, L, Salas-Salvadó, J, et al. Consumption of olive oil and risk of total and cause-specific mortality among U.S. adults. J Am Coll Cardiol. (2022) 79:101–12. doi: 10.1016/j.jacc.2021.10.041
49. Morvaridzadeh, M, Cohen, AA, Heshmati, J, Alami, M, Berrougui, H, Zoubdane, N, et al. Effect of extra virgin olive oil on anthropometric indices, inflammatory and Cardiometabolic markers: a systematic review and Meta-analysis of randomized clinical trials. J Nutr. (2024) 154:95–120. doi: 10.1016/j.tjnut.2023.10.028
50. Buckland, G, Bach, A, and Serra-Majem, L. Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev. (2008) 9:582–93. doi: 10.1111/j.1467-789X.2008.00503.x
51. Schwartz, GJ, Fu, J, Astarita, G, Li, X, Gaetani, S, Campolongo, P, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. (2008) 8:281–8. doi: 10.1016/j.cmet.2008.08.005
52. Galvão Cândido, F, Xavier Valente, F, Da Silva, LE, Gonçalves Leão Coelho, O, Gouveia Peluzio, MDC, and Gonçalves Alfenas, RDC. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: a randomized, double-blinded, placebo-controlled clinical trial. Eur J Nutr. (2018) 57:2445–55. doi: 10.1007/s00394-017-1517-9
53. Raeisi-Dehkordi, H, Amiri, M, Humphries, KH, and Salehi-Abargouei, A. The effect of canola oil on body weight and composition: a systematic review and Meta-analysis of randomized controlled clinical trials. Adv Nutr. (2019) 10:419–32. doi: 10.1093/advances/nmy108
54. Oliveira-de-Lira, L, Santos, EMC, De Souza, RF, Matos, RJB, Silva, MCD, Oliveira, LDS, et al. Supplementation-dependent effects of vegetable oils with varying fatty acid compositions on anthropometric and biochemical parameters in obese women. Nutrients. (2018) 10:932. doi: 10.3390/nu10070932
Keywords: extra-virgin olive oil, Mediterranean diet, abdominal obesity, nutrition, waist circumference
Citation: De Matteis C, Crudele L, Di Buduo E, Cantatore S, Novielli F, Cultrera S, Tricase AF, Arconzo M, Florio M, Gadaleta RM, Piccinin E, Cariello M and Moschetta A (2025) Regular extra-virgin olive oil intake independently associates with lower abdominal obesity. Front. Nutr. 12:1645230. doi: 10.3389/fnut.2025.1645230
Edited by:
Omar Guzmán Quevedo, Higher Technological Institute of Tacambaro, MexicoReviewed by:
Yoredy Sarmiento-Andrade, Universidad Técnica Particular de Loja, EcuadorEulália Silva-Araújo, Federal University of Pernambuco, Brazil
Copyright © 2025 De Matteis, Crudele, Di Buduo, Cantatore, Novielli, Cultrera, Tricase, Arconzo, Florio, Gadaleta, Piccinin, Cariello and Moschetta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Moschetta, YW50b25pby5tb3NjaGV0dGFAdW5pYmEuaXQ=
†These authors share first authorship
 Carlo De Matteis
Carlo De Matteis Lucilla Crudele
Lucilla Crudele Ersilia Di Buduo1
Ersilia Di Buduo1 Elena Piccinin
Elena Piccinin Marica Cariello
Marica Cariello Antonio Moschetta
Antonio Moschetta