- 1Department of Physical Education, Ludong University, Yantai, China
- 2Faculty of Educational Studies, Universiti Putra Malaysia, Serdang, Malaysia
- 3Department of Physical Education, Soongsil University, Seoul, Republic of Korea
- 4Department of Sports Teaching and Research, Lanzhou University, Lanzhou, China
This meta-analysis aimed to evaluate the effects of Rhodiola rosea L. (RR) supplementation on endurance performance and key physiological biomarkers, including oxidative stress, muscle damage, inflammation, and metabolic markers. A systematic search of Web of Science, PubMed, Scopus, EBSCO, and CNKI identified randomized controlled trials published up to March 20, 2025. Random-effects meta-analyses were conducted using R software, and methodological quality was appraised using the Physiotherapy Evidence Database scale. Additionally, subgroup analyses assessed the moderating effects of daily RR dosage, training duration, and training status. A total of 26 randomized controlled trials were included, involving 668 healthy participants with a mean age of 22.0 ± 10.7 years. The mean intervention duration was 33 days, with outcome assessments conducted from immediate post-exercise through 24-hour follow-up. The results indicated that RR supplementation significantly improved endurance-related outcomes, including VO2max (11 studies; ES = 0.32, p < 0.01), time to exhaustion (TTE; 7 studies; ES = 0.38, p < 0.05), and time trial performance (TTP; 5 studies; ES = −0.40, p < 0.05). Antioxidant capacity was enhanced, with increases in total antioxidant capacity (TAC; 6 studies; ES = 0.59, p < 0.05) and superoxide dismutase (SOD; 7 studies; ES = 1.16, p < 0.01), alongside reductions in malondialdehyde (MDA; 6 studies; ES = −1.21, p < 0.001). RR also reduced creatine kinase (CK; 9 studies; ES = −0.84, p < 0.01) and lactate levels (LA; 7 studies; ES = −0.87, p < 0.05), indicating improved metabolic efficiency. No significant effects were observed on inflammatory markers, including interleukin-6 (IL-6) and C-reactive protein (CRP). Subgroup analyses indicated greater VO2max improvements at doses >600 mg/day, with trained individuals exhibiting lower CK levels and more pronounced reductions in CK at early follow-up assessments (≤15 min) post-exercise. In conclusion, RR supplementation is an effective ergogenic aid for enhancing endurance performance and improving physiological biomarkers related to oxidative stress, muscle damage, and metabolic efficiency, though heterogeneity across studies warrants cautious interpretation.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, PROSPERO CRD42024619014.
1 Introduction
Rhodiola rosea L. (RR) is a perennial herb native to cold, high-altitude regions of the Northern Hemisphere, traditionally used in Tibetan, Russian, and Chinese medicine to enhance physical and mental well-being (1–3). As a recognized adaptogen, RR is widely included in dietary supplements for its ability to improve physiological responses to physical and psychological stress. Its key bioactive compounds, rosavin and salidroside, have been reported to reduce fatigue, enhance energy metabolism and oxygen utilization, and mitigate oxidative stress (4–6). These properties have garnered increasing attention regarding RR's potential to enhance endurance capacity, recovery, and long-term training adaptations (7–9).
Multiple mechanisms have been proposed to elucidate the ergogenic effects of RR supplementation on endurance performance, recovery, and long-term training adaptations. Evidence from rodent studies underscores RR's capacity to significantly enhance endurance performance (10–12). This enhancement is attributed to the multifaceted modulation of various physiological processes, including the reduction of muscle damage, alleviation of oxidative stress, suppression of inflammatory responses, and optimization of energy metabolism (13–15). Specifically, RR has been shown to reduce key markers of muscle damage (e.g., lactate dehydrogenase and creatine kinase [CK]), while modulating oxidative stress (e.g., lowering malondialdehyde [MDA] levels and enhancing the activity of antioxidant enzymes such as superoxide dismutase [SOD] and catalase) (5, 16). Moreover, its antioxidative properties counteract free radical-mediated cellular injury, promoting efficient post-exercise repair (5). RR supplementation also demonstrates potent anti-inflammatory effects, significantly reducing inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α), thereby alleviating exercise-induced inflammation and expediting recovery (13, 15). Additionally, by enhancing energy metabolism and lactate clearance, RR supplementation improves mitochondrial function and oxygen utilization, effectively delaying fatigue and bolstering endurance capacity (4, 6). These beneficial effects are likely mediated by its ability to stimulate mitochondrial biogenesis, improve ATP synthesis efficiency, and optimize muscle metabolic pathways (10, 17). In summary, these findings underscore the robust capacity of RR supplementation to enhance endurance performance and recovery through integrative mechanisms targeting muscle damage, oxidative stress, inflammation, and energy efficiency, supported by robust evidence from rodent studies.
Despite the significant benefits demonstrated in animal studies, human research results remain inconsistent. Some studies suggest that RR supplementation can enhance endurance performance, such as extending time to exhaustion (TTE), improving VO2max, and affecting related biomarkers (e.g., reducing muscle damage, alleviating oxidative stress, and decreasing inflammation) (9, 18, 19). However, other studies have failed to observe these effects (20–22). Shanely et al. (22) reported no significant differences between the RR supplementation and control groups in marathon performance, and interleukin levels among marathon runners. Parisi et al. (21) found that RR supplementation did not significantly affect time to TTE or VO2max in trained male athletes compared to the control group, although it did lower lactate and CK levels. Similarly, Jówko et al. (20) observed no improvements in endurance capacity or hormonal levels in healthy male students, but reported a significant increase in total antioxidant capacity (TAC).
Although two systematic reviews have assessed the impacts of RR supplementation on exercise performance and related biomarkers (6, 7), the studies included in these reviews were limited, and neither employed meta-analysis techniques. This lack of statistical rigor restricts the ability to draw robust conclusions. These reviews primarily focused on overall exercise performance, while the majority of the primary studies have concentrated on endurance performance. Given the discrepancies in existing literature, this study aims to conduct a comprehensive meta-analysis to systematically evaluate the effects of RR supplementation on human endurance performance and related biomarkers. It is hypothesized that RR supplementation will significantly enhance endurance performance compared to a placebo or control group.
2 Materials and methods
This systematic review was conducted in accordance with the PRISMA guidelines for systematic reviews (52) and meta-analyses and is registered with Prospero (registration number: CRD42024619014).
2.1 Data sources and search strategy
A comprehensive literature search was conducted by two independent researchers across multiple databases, including Web of Science, PubMed, Scopus, EBSCO MEDLINE databases, and CNKI, for articles published up to March 20, 2025. The search strategy involved using the following combinations of terms: Rhodiola rosea, Rhodiola, Rosea, Roseroot, Golden root, Arctic root, Rhodioloside, Salidroside, Endurance, Sport, Athletic, Exercise, and Training. Detailed search alerts are documented in Supplementary File 1. The identified articles were managed and screened using EndNote reference management software. Duplicates were first removed using EndNote. The titles and abstracts of the remaining articles were then independently reviewed by the two researchers. Discrepancies between the two researchers were resolved by a third researcher.
2.2 Selection criteria
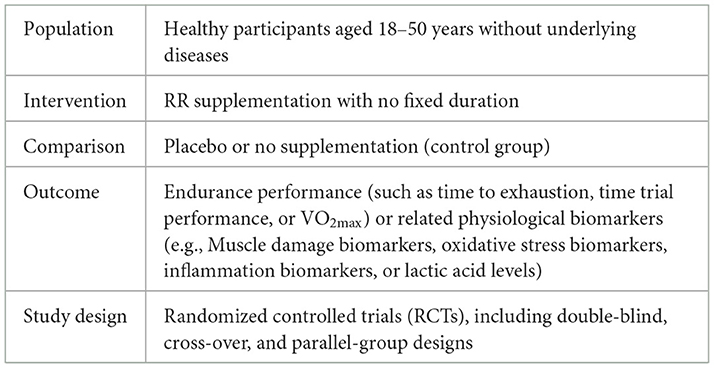
Studies eligible for inclusion were required to meet the following PICOS criteria: healthy participants aged 18–50 years without underlying diseases (P), with RR supplementation of no fixed duration as the intervention (I), compared to a placebo or control group (C). The outcomes (O) included endurance performance (e.g., TTE, TTP, VO2max) and related physiological biomarkers (e.g., muscle damage biomarkers, oxidative stress markers, inflammation markers, lactate levels). Studies were required to be randomized controlled trials (RCTs), including double-blind, cross-over, and parallel-group designs (S). For further details on the PICOS criteria, please refer to Table 1. Exclusion criteria included: (1) studies that did not report specific outcome data; (2) editorial articles, conference abstracts, and reviews; (3) studies with a PEDro scale rating of <5.
2.3 Data extraction
The following information was extracted from the included studies: study source (authors, publication year), study design (parallel or cross-over), participant characteristics (gender, age, sample size of each group), supplementation protocol (type, dosage, duration), exercise intervention or testing methods, and outcome variables (mean and standard deviations). The primary outcome variables included: aerobic performance (VO2max, TTE, time trial performance [TTP]), muscle damage markers (CK), oxidative stress markers (TAC, MDA, and SOD), inflammatory markers (IL-6, and CRP), and metabolic indicators (lactate levels [LA]).
2.4 Quality assessment and risk of bias
The methodological quality of the included studies was assessed using the Physiotherapy Evidence Database scale (PEDro; https://www.pedro.org.au). Following the approach used in previous meta-analyses (23), studies were classified as follows: low quality (≤ 3 points), moderate quality (4–5 points), and high quality (6–10 points). The risk of bias across studies was evaluated using funnel plots and Egger's regression test to assess potential asymmetry and publication bias.
2.5 Data analysis
Meta-analysis was performed using the R packages (R version 4.3.0 with R Studio version 2023.06.1 + 524). The metagen() function from the meta package was used for meta-analyses and subgroup analyses. The standardized mean difference (SMD: Hedges' g) was used to assess the difference between RR supplementation and placebo/control groups. A random-effects model was applied, weighting studies by their standard error to address heterogeneity. Effect sizes were categorized as trivial (< 0.2), small (0.2–0.5), medium (0.5–0.8), and large (>0.8) (24). Heterogeneity was assessed with I2 and τ2, where I2 values of ≤ 25%, 25%−50%, and ≥75% indicate low, medium, and high heterogeneity, respectively. A total of 10 meta-analyses were conducted, assessing the following outcomes: (1) aerobic performance (TTP, TTE, VO2max); (2) muscle damage biomarkers (CK); (3) oxidative stress biomarkers (TAC, MDA, SOD); (4) inflammation biomarkers (IL-6, CRP); and (5) metabolic markers (LA).
Additionally, subgroup analyses were conducted to evaluate the moderating effects of training-related factors, including daily RR dosage, training duration, follow-up time points, training status, and comparator type, on the observed outcomes. These analyses were restricted to cases where at least two subgroups included three or more relatively homogeneous studies. Statistical significance was determined at a threshold of p < 0.05.
3 Results
3.1 Search results and general characteristics of participants and protocols
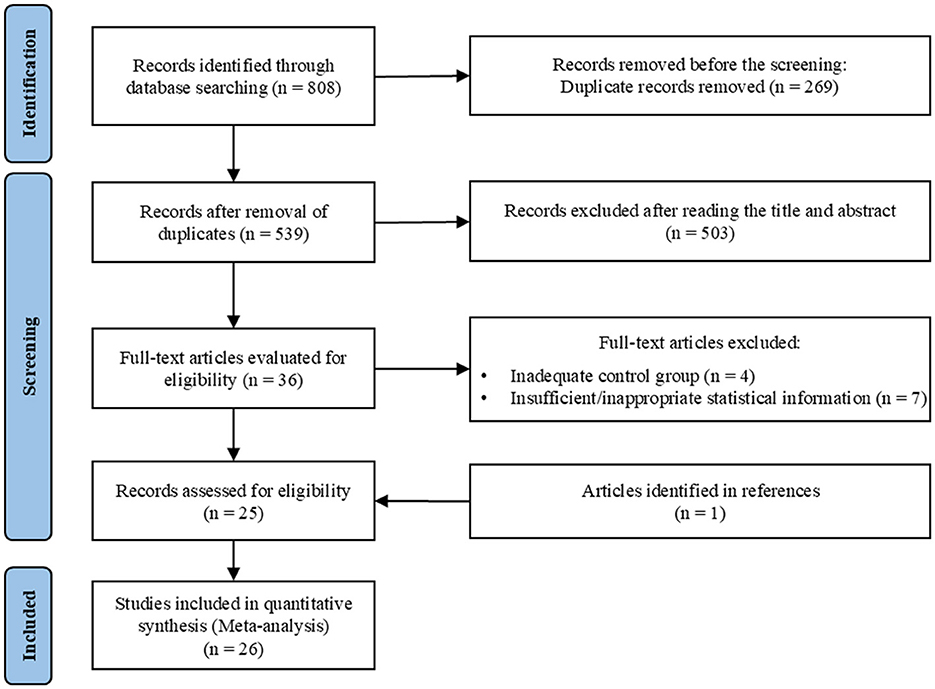
A total of 808 articles were initially identified from various databases: Scopus (n = 199), EBSCO Medical Databases (n = 161), Web of Science (n = 122), PubMed (n = 87), and CNKI (n = 239). After removing duplicates, 539 articles remained for title and abstract screening. Following this, 36 full-text articles were assessed for eligibility. Ultimately, 26 studies were included in the meta-analysis (see Figure 1).
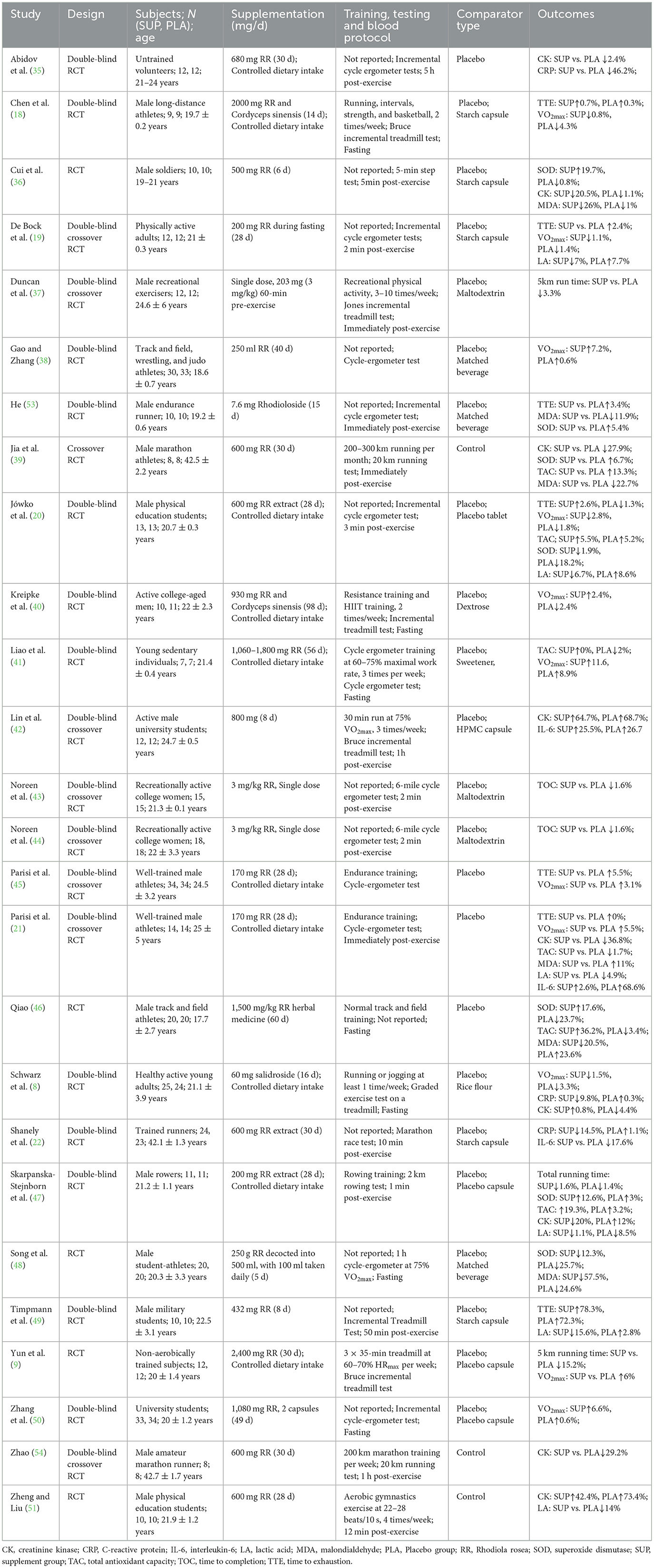
The studies included in the meta-analysis are summarized in Table 2. A total of 668 participants from healthy populations were involved, with ages ranging from 11 to 45 years. Among the 26 studies, 15 focused on male participants, 3 on female participants, and 8 included both male and female. The training durations ranged from 3 days to around 3 months. Follow-up assessments ranged from within 15 min post-exercise to fasting measurements taken the following morning, encompassing both acute and extended recovery phases. The studies included both trained (13 studies) and untrained participants (13 studies). Most studies involved aerobic and endurance training, although some did not provide detailed descriptions of the training protocols. The tests assessed endurance performance and related physiological biomarkers, including TTP (n = 5 groups), TTE (n = 7 groups), VO2max (n = 11 groups), CK (n = 9 groups), TAC (n = 6 groups), MDA (n = 6 groups), SOD (n = 7 groups), IL-6 (n = 3 groups), CRP (n = 3 groups), and LA (n = 7 groups).
3.2 Quality assessment of studies and risk of bias
The quality assessment of the included studies, based on the PEDro scale ratings, is presented in Supplementary File 2. Of the studies included in the meta-analysis, 2 were rated as moderate quality (scoring 4–5 points), while 24 studies were rated as good quality (scoring 6–9 points). The median PEDro score across the studies was 7 out of a possible 10 points. Overall, the high quality of the included studies supports the reliability and robustness of the meta-analysis results. Funnel plots for all outcome measures showed a generally symmetrical distribution, suggesting no significant publication bias (see Supplementary File 3).
3.3 Meta-analysis results
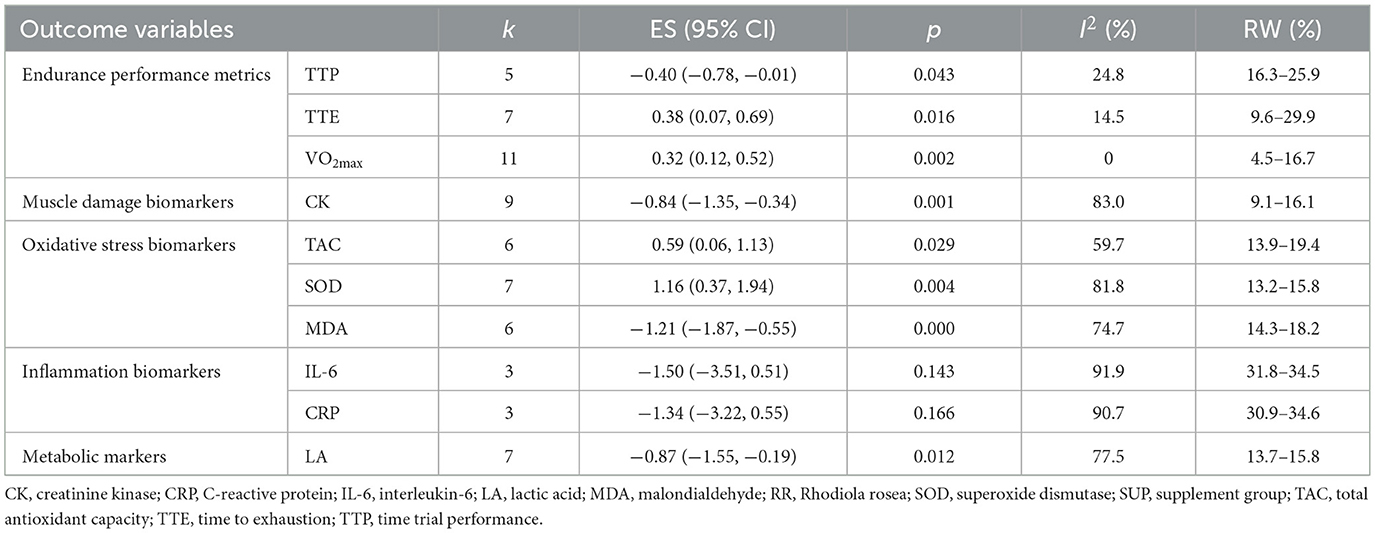
The overall effects of RR supplementation on endurance performance and related biomarkers are shown in Table 3, with forest plots displayed in Supplementary File 4. The findings indicated that RR supplementation produced significant improvements in endurance performance compared to placebo or control groups. Specifically, VO2max was significantly increased (ES = 0.32, 95% CI [0.12, 0.52], p < 0.01), TTE was prolonged (ES = 0.38, 95% CI [0.07, 0.69], p < 0.05), and TTP improved (ES = −0.40, 95% CI [−0.78, −0.01], p < 0.05).
Regarding muscle damage, RR supplementation led to a significant reduction in CK levels (ES = −0.84, 95% CI [−1.35, −0.34], p < 0.01). Furthermore, RR supplementation significantly modulated oxidative stress markers, enhancing TAC (ES = 0.59, 95% CI [0.06, 1.13], p < 0.05) and SOD levels (ES = 1.16, 95% CI [0.37, 1.94], p < 0.01), while simultaneously reducing MDA levels (ES = −1.21, 95% CI [−1.87, −0.55], p < 0.001), suggesting improved antioxidant activity and reduced oxidative damage.
Additionally, RR supplementation resulted in a significant reduction in post-exercise LA levels (ES = −0.87, 95% CI [−1.55, −0.19], p < 0.05). However, no significant effects were observed for the inflammatory biomarkers IL-6 (ES = −1.50, 95% CI [−3.51, 0.51], p = 0.143) and CRP (ES = −1.34, 95% CI [−3.22, 0.55], p = 0.166), compared to control groups.
3.4 Subgroup analysis results
The moderating effects of training factors on the impact of RR supplementation on endurance performance and related biomarkers are summarized in Table 4. The analysis revealed that RR's effect on VO2max was significantly influenced by daily dosage, with higher doses (exceeding 600 mg/d) resulting in more substantial improvements compared to lower doses. Follow-up time points also significantly moderated the effect of RR supplementation on CK levels. Early follow-up assessments (≤ 15 min) were associated with markedly greater reductions in CK levels, whereas this effect diminished at later follow-up intervals. Additionally, training status was found to significantly moderate the effect of RR supplementation on muscle damage markers, particularly CK, with trained individuals exhibiting notably lower CK levels than their untrained counterparts. No other factors were found to significantly moderate the effects of RR supplementation on endurance performance or related physiological indicators.
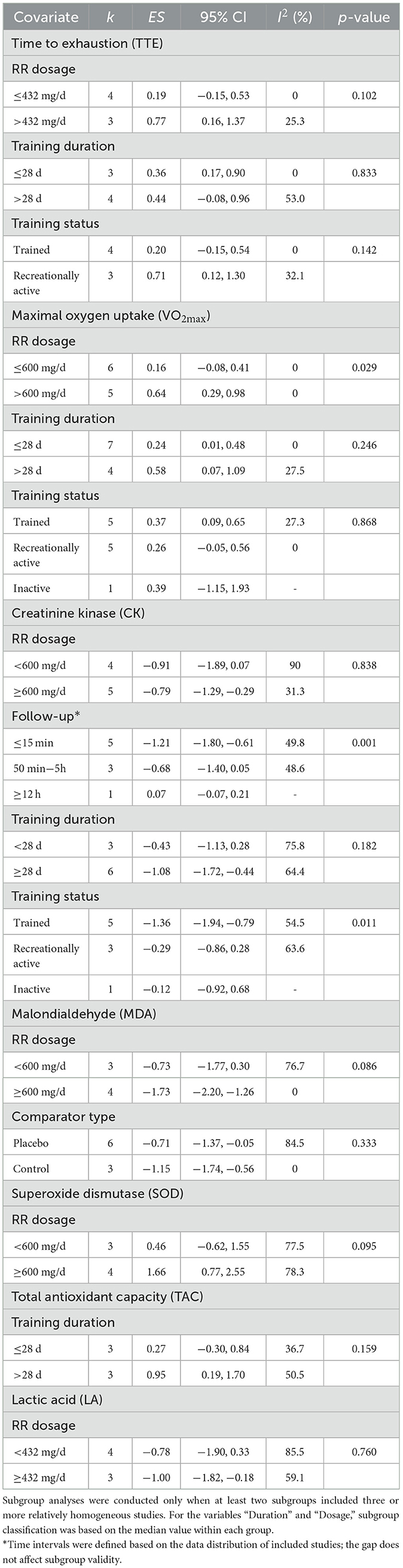
Table 4. Moderation analysis of individual and training factors on the effects of LL-BFR vs. HLR on maximal strength, muscle power, and jump performance.
4 Discussion
This meta-analysis provides strong evidence supporting the positive effects of RR supplementation on endurance performance and related biomarkers compared to placebo or control groups. Significant improvements were observed in VO2max, TTE, and TTP, indicating enhanced endurance capacity. RR supplementation also effectively reduced muscle damage, as evidenced by decreased CK levels, and improved antioxidant capacity, demonstrated by increased TOC and SOD levels and decreased MDA levels. However, no significant effects were observed on inflammatory biomarkers, such as IL-6 and CRP. Subgroup analysis revealed that higher doses of RR (exceeding 600 mg/day) were associated with more substantial improvements in VO2max. Additionally, trained individuals exhibited significantly lower CK levels compared to untrained individuals. In general, the included studies were of good quality, with 24 rated as good and 2 as moderate on the PEDro scale.
4.1 Effects of RR supplementation on endurance performance
As one of the key findings of this study, RR supplementation significantly enhanced VO2max, extended TTE, and reduced TTP compared to the control or placebo groups. These results align with prior systematic reviews (6, 7), which also highlight the beneficial effects of RR on endurance performance. By synthesizing data from multiple studies, this research provides robust statistical evidence, further supporting the potential of RR as an effective performance enhancer.
RR supplementation significantly extended TTE and reduced TTP, indicating its potential to delay fatigue onset and enhance exercise performance. These improvements are likely associated with the optimization of mitochondrial function (25, 26). Previous research suggests that RR supplementation promotes mitochondrial biogenesis and improves ATP synthesis efficiency, thereby enhancing muscle energy supply (25). Moreover, RR may further improve endurance performance by increasing fat oxidation, reducing lactate accumulation, and improving lactate clearance (9, 21). This meta-analysis provides further support by confirming that RR supplementation significantly reduces post-exercise lactate levels, further supporting the idea that RR enhances endurance performance and mitigates exercise-induced fatigue through the optimization of energy metabolism.
While RR supplementation shows promise in enhancing endurance performance, the effects observed across studies have been inconsistent, suggesting that exercise-related factors may significantly influence its effectiveness. Specifically, the study by Jówko et al. (20) did not observe significant improvements in VO2max in healthy males, likely due to the absence of an exercise training component in the intervention. The lack of exercise-induced metabolic stress may have limited the potential benefits of RR supplementation. Similarly, Parisi et al. (21) found no significant effects on VO2max or TTE in trained male athletes. This lack of effect may be attributed to the relatively low daily dose of RR (170 mg/day), which could have been insufficient to induce meaningful physiological changes. Furthermore, Schwarz et al. (8) found no substantial improvements in endurance performance with a lower RR dose (60 mg/day). These findings suggest that the effectiveness of RR supplementation may be sensitive to both the inclusion of exercise training and the specific dosage used. Future studies should explore the interaction between exercise training and RR supplementation, and determine the optimal dosage and duration for significant outcomes.
4.2 Effects of RR supplementation on antioxidant capacity, muscle damage, and inflammation
This meta-analysis indicated that RR supplementation may significantly enhance endurance performance by mitigating oxidative stress and muscle damage. Specifically, RR supplementation resulted in a marked decrease in MDA levels and a concomitant increase in TAC and SOD levels, highlighting its potential role in mitigating oxidative damage and bolstering antioxidant defense mechanisms. Oxidative stress, which frequently arises following intense exercise, leads to an accumulation of free radicals that can impair muscle cell function and delay the recovery process (27). RR supplementation appears to counteract this by enhancing the activity of key antioxidant enzymes, such as SOD and catalase, which neutralize free radicals and protect cellular integrity, thereby optimizing muscle function (28, 29). The observed decline in MDA levels, a reliable marker of lipid peroxidation, further corroborates RR's capacity to alleviate oxidative stress and protect cellular membranes. Similarly, the reduction in CK levels suggests a protective effect against exercise-induced muscle damage. While this meta-analysis did not directly evaluate energy metabolism, research suggests that antioxidants may enhance mitochondrial function and energy production, potentially facilitating more efficient recovery post-exercise (25). In summary, these findings highlight the potential of RR supplementation as a promising intervention for improving endurance performance and expediting recovery by targeting oxidative stress, supporting muscle repair, and enhancing recovery efficiency.
While RR supplementation demonstrated clear benefits in mitigating oxidative stress and muscle damage, its impact on inflammation markers such as IL-6 and CRP did not reach statistical significance. This meta-analysis indicated a trend suggesting RR's potential to reduce inflammation; however, the lack of significant findings is likely due to the limited number of studies (only three for both IL-6 and CRP) and small sample sizes. This underscores the need for further research with larger sample sizes and more rigorous methodologies to better elucidate RR's effects on exercise-induced inflammation. Therefore, although current evidence is promising, it remains inconclusive regarding RR's role in modulating post-exercise inflammatory responses.
4.3 Moderating effects of training factors on RR supplementation efficacy
Subgroup analysis revealed that higher doses of RR supplementation (exceeding 600 mg/day) were significantly associated with improvements in VO2max. This effect may be mediated through RR's ability to enhance antioxidant capacity, thereby mitigating exercise-induced oxidative stress and reducing free radical generation. Evidence suggests that reducing oxidative stress may help preserve mitochondrial structure and function, improve energy production efficiency, and delay muscle fatigue (5, 30). Furthermore, reducing oxidative stress may also improve cardiovascular function, which contributes to the enhancement of VO2max (31). Therefore, dosages exceeding 600 mg/day may offer potential benefits for optimizing endurance performance. However, it is important to note that the optimal dosage could vary based on individual characteristics and specific training contexts. Future research should aim at larger, well-controlled trials to establish the most effective and safe dosage range for diverse populations.
Subgroup analysis also revealed a significant difference in CK levels between trained and untrained individuals following RR supplementation, with trained individuals exhibiting lower CK levels. This suggests that individuals with higher baseline fitness levels may derive greater benefits from RR supplementation in reducing exercise-induced muscle damage. Trained individuals typically possess more efficient recovery mechanisms, which may enhance their response to RR, thereby optimizing its effectiveness in mitigating muscle damage and promoting faster recovery (32, 33). Thus, when assessing the potential benefits of RR supplementation, it is essential to consider an individual's training status. Tailoring supplementation strategies to an individual's fitness level could optimize outcomes for both athletes and recreational exercisers.
Moreover, the follow-up time point significantly moderated the effect of RR on CK levels. Measurements taken within 15 min post-exercise showed a more pronounced reduction in CK compared to later time points. This may be explained by the rapid antioxidant and anti-inflammatory effects of RR's active compounds early after exercise, which effectively reduce acute muscle damage and inhibit CK release (5). As time progresses, CK is gradually released and influenced by various physiological processes, diminishing the supplement's protective effects (34). In contrast, intervention duration did not show a significant moderating effect on endurance performance or related biomarkers. This lack of effect may be due to the relatively short durations of most included studies, with the majority lasting < 6 weeks and only three studies exceeding this timeframe. Such limited variation reduces the ability to detect duration-dependent effects. Future research should prioritize monitoring physiological markers within 15 min post-exercise and consider extending intervention duration to better understand RR's effects on muscle recovery and endurance performance.
None of the included studies reported adverse effects related to supplementation. While this suggests a favorable safety profile, underreporting cannot be ruled out. Future studies should explicitly report adverse events to enable a comprehensive evaluation of both efficacy and safety.
4.4 Limitation
This meta-analysis review has certain limitations. First, only one analysis included more than 10 studies, while the rest had fewer, which may limit the precision of effect estimates and increase uncertainty. Second, data constraints prevented the inclusion of key biomarkers such as glutathione peroxidase, thiobarbituric acid reactive substances, and myoglobin, reducing the scope of findings. Lastly, while subgroup analyses were performed to address high heterogeneity, yet residual variability persisted owing to differences in study designs, limited numbers of studies with small sample sizes, and incomplete reporting of training protocols. These limitations may affect the robustness and generalizability of the findings. Future studies should prioritize larger sample sizes, standardized protocol reporting, and comprehensive biomarker assessment.
5 Conclusions
This meta-analysis provides strong evidence supporting the positive effects of RR supplementation on endurance performance, oxidative stress, muscle damage, and metabolic efficiency. RR supplementation significantly improved endurance outcomes, including increased VO2max, prolonged TTE, and reduced TTP. It also enhanced antioxidant capacity, evidenced by increased TAC and SOD levels, and decreased MDA levels. Muscle damage markers, such as CK, were significantly reduced, and lactate levels dropped, suggesting improved metabolic efficiency. However, RR supplementation did not show significant effects on exercise-induced inflammation, as measured by IL-6 and CRP, likely due to the limited number of studies evaluating these outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XW: Formal analysis, Conceptualization, Data curation, Writing – original draft, Methodology, Software. XY: Conceptualization, Methodology, Formal analysis, Writing – original draft, Data curation, Software. ZG: Software, Resources, Writing – original draft, Formal analysis, Data curation. JZ: Writing – original draft, Formal analysis, Resources, Data curation. YL: Methodology, Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1645346/full#supplementary-material
Supplementary File 1 | Search alert.
Supplementary File 2 | Physiotherapy Evidence Database (PEDro) scale ratings.
Supplementary File 3 | Funnel plots.
Supplementary File 4 | Forest plots.
References
1. Durazzo A, Lucarini M, Nazhand A, Coêlho AG, Souto EB, Arcanjo DD, et al. Rhodiola rosea: main features and its beneficial properties. Rendiconti Lincei Scienze Fisiche e Naturali. (2022) 33:71–82. doi: 10.1007/s12210-022-01055-y
2. Gan Z, Fang X, Yin C, Tian Y, Zhang L, Zhong X, et al. Extraction, purification, structural characterization, and bioactivities of the genus Rhodiola L. polysaccharides: a review. Int J Biol Macromol. (2024) 276:133614. doi: 10.1016/j.ijbiomac.2024.133614
3. Konstantinos F, Heun R. The effects of Rhodiola rosea supplementation on depression, anxiety and mood—a systematic review. Global Psychiatry. (2020) 3:72–82. doi: 10.2478/gp-2019-0022
4. Ivanova Stojcheva E, Quintela JC. The effectiveness of Rhodiola rosea L. Preparations in alleviating various aspects of life-stress symptoms and stress-induced conditions—encouraging clinical evidence. Molecules. (2022) 27:3902. doi: 10.3390/molecules27123902
5. Lu Y, Deng B, Xu L, Liu H, Song Y, Lin F, et al. Effects of Rhodiola rosea supplementation on exercise and sport: a systematic review. Front Nutr. (2022) 9:856287. doi: 10.3389/fnut.2022.856287
6. Tinsley GM, Jagim AR, Potter GD, Garner D, Galpin AJ. Rhodiola rosea as an adaptogen to enhance exercise performance: a review of the literature. Br J Nutr. (2024) 131:461–73. doi: 10.1017/S0007114523001988
7. Sanz-Barrio PM, Noreen EE, Gilsanz-Estebaranz L, Lorenzo-Calvo J, Martinez-Ferran M, Pareja-Galeano H. Rhodiola rosea supplementation on sports performance: a systematic review of randomized controlled trials. Phytother Res. (2023) 37:4414–28. doi: 10.1002/ptr.7950
8. Schwarz NA, Stratton MT, Colquhoun RJ, Manganti AM, Sherbourne M, Mourey F, et al. Salidroside and exercise performance in healthy active young adults–an exploratory, randomized, double-blind, placebo-controlled study. J Int Soc Sports Nutr. (2024) 21:2433744. doi: 10.1080/15502783.2024.2433744
9. Yun H, Lu B, Su W, Wang J, Zheng J, Wang J, et al. Combined effects of Rhodiola rosea and caffeine supplementation on aerobic endurance and muscle explosiveness: a synergistic approach. Front Nutr. (2024) 11:1335950. doi: 10.3389/fnut.2024.1335950
10. Liu C, Zhao H, Yan Y, Yang W, Chen S, Song G, et al. Synergistic effect of Rhodiola rosea and caffeine supplementation on the improvement of muscle strength and muscular endurance: a pilot study for rats, resistance exercise-untrained and-trained volunteers. Nutrients. (2023) 15:582. doi: 10.3390/nu15030582
11. Qin N, Xie H, Zhao A, Zhang X, Sun Y, Li W, et al. Effects of salidroside on exercise tolerance of mice under high altitude hypoxia environment. J Zhejiang Univ Med Sci. (2022) 51:397–404. doi: 10.3724/zdxbyxb-2022-0158
12. Wang J, Zhang G, Wang D, Yan Y, Yang Q. Effects of nano-Rhodiola rosea combined with treadmill exercise on anti-exercise fatigue in rats. Front Nutr. (2024) 11:1446944. doi: 10.3389/fnut.2024.1446944
13. Drafi F, Bauerova K, Chrastina M, Taghdisiesfejír M, Rocha J, Direito R, et al. Rhodiola rosea L. extract, a known adaptogen, evaluated in experimental arthritis. Molecules. (2023) 28:5053. doi: 10.3390/molecules28135053
14. Pu WL, Zhang MY, Bai RY, Sun LK, Li WH, Yu YL, et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed Pharmacother. (2020) 121:109552. doi: 10.1016/j.biopha.2019.109552
15. Syed RU, Hadi MA, Almarir AM, Alahmari AM, Alremthi YH, Alsagri AAA, et al. Rhodiola rosea L. extract ameliorates ethanol-induced gastric ulcer in rats by alleviating oxidative stress and inflammation via NF-κB pathway inhibition. Curr Plant Biol. (2024) 40:100421. doi: 10.1016/j.cpb.2024.100421
16. Jamioł M, Wawrzykowski J, Dec M, Wilk A, Czelej M. Comparison of various techniques for the extraction. analysis of compounds and determination of antioxidant activities of Rhodiola spp.—a review. Food Rev Int. (2023) 39:467–87. doi: 10.1080/87559129.2021.1918147
17. Bang VMJ, Aranão ALDC, Nogueira BZ, Araújo AC, Bueno PCDS, Barbalho SM, et al. Effects of Rhodiola rosea and panax ginseng on the metabolic parameters of rats submitted to swimming. J Med Food. (2019) 22:1087–90. doi: 10.1089/jmf.2019.0062
18. Chen C-Y, Hou C-W, Bernard JR, Chen C-C, Hung T-C, Cheng L-L, et al. Rhodiola crenulata-and Cordyceps sinensis-based supplement boosts aerobic exercise performance after short-term high altitude training. High Alt Med Biol. (2014) 15:371–9. doi: 10.1089/ham.2013.1114
19. De Bock K, Eijnde BO, Ramaekers M, Hespel P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int J Sport Nutr Exerc Metab. (2004) 14:298–307. doi: 10.1123/ijsnem.14.3.298
20. Jówko E, Sadowski J, Długołecka B, Gierczuk D, Opaszowski B, Cieśliński I, et al. Effects of Rhodiola rosea supplementation on mental performance, physical capacity, and oxidative stress biomarkers in healthy men. J Sport Health Sci. (2018) 7:473–80. doi: 10.1016/j.jshs.2016.05.005
21. Parisi A, Tranchita E, Duranti G, Ciminelli E, Quaranta F, Ceci R, et al. Effects of chronic Rhodiola rosea supplementation on sport performance and antioxidant capacity in trained male: preliminary results. J Sports Med Phys Fitness. (2010) 50:57–63. doi: 10.1016/j.jse.2009.09.003
22. Shanely RA, Nieman DC, Zwetsloot KA, Knab AM, Imagita H, Luo B, et al. Evaluation of Rhodiola rosea supplementation on skeletal muscle damage and inflammation in runners following a competitive marathon. Brain Behav Immun. (2014) 39:204–10. doi: 10.1016/j.bbi.2013.09.005
23. Wang X, Zhang K, bin Samsudin S, bin Hassan MZ, bin Yaakob SSN, et al. Effects of plyometric training on physical fitness attributes in handball players: a systematic review and meta-analysis. J Sports Sci Med. (2024) 23:177. doi: 10.52082/jssm.2024.177
25. Dun Y, Liu S, Zhang W, Xie M, Qiu L. Exercise combined with rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid Med Cell Longev. (2017) 2017:8024857. doi: 10.1155/2017/8024857
26. Hou Y, Tang Y, Wang X, Ai X, Wang H, Li X, et al. Rhodiola Crenulata ameliorates exhaustive exercise-induced fatigue in mice by suppressing mitophagy in skeletal muscle. Exp Ther Med. (2020) 20:3161–73. doi: 10.3892/etm.2020.9072
27. Zhou Z, Chen C, Teo E-C, Zhang Y, Huang J, Xu Y, et al. Intracellular oxidative stress induced by physical exercise in adults: systematic review and meta-analysis. Antioxidants. (2022) 11:1751. doi: 10.3390/antiox11091751
28. Pingitore A, Lima GPP, Mastorci F, Quinones A, Iervasi G, Vassalle C, et al. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nutrition. (2015) 31:916–22. doi: 10.1016/j.nut.2015.02.005
29. Yan Z, Spaulding HR. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. (2020) 32:101508. doi: 10.1016/j.redox.2020.101508
30. Vargas-Mendoza N, Angeles-Valencia M, Morales-González Á, Madrigal-Santillán EO, Morales-Martínez M, Madrigal-Bujaidar E, et al. Oxidative stress, mitochondrial function and adaptation to exercise: new perspectives in nutrition. Life. (2021) 11:1269. doi: 10.3390/life11111269
31. Dubois-Deruy E, Peugnet V, Turkieh A, Pinet F. Oxidative stress in cardiovascular diseases. Antioxidants. (2020) 9:864. doi: 10.3390/antiox9090864
32. Moberg M, Lindholm ME, Reitzner SM, Ekblom B, Sundberg C-J, Psilander N, et al. Exercise induces different molecular responses in trained and untrained human muscle. Med Sci Sports Exer. (2020) 52:1679–90. doi: 10.1249/MSS.0000000000002310
33. Moreno-Villanueva M, Kramer A, Hammes T, Venegas-Carro M, Thumm P, Burkle A, et al. Influence of acute exercise on DNA repair and PARP activity before and after irradiation in lymphocytes from trained and untrained individuals. Int J Mol Sci. (2019) 20:2999. doi: 10.3390/ijms20122999
34. Radišić Biljak V, Lazić A, Nikler A, Pekas D, Saračević A, Trajković N, et al. Post-exercise creatine kinase variability: a literature review. Biochemia Medica. (2025) 35:186–200. doi: 10.11613/BM.2025.020502
35. Abidov M, Grachev S, Seifulla R, Ziegenfuss T. Extract of Rhodiola rosea radix reduces the level of C-reactive protein and creatinine kinase in the blood. Bull Exp Biol Med. (2004) 138:63–4. doi: 10.1023/B:BEBM.0000046940.45382.53
36. Cui J, Wang Y, Zhang X, Ha Z, Wang W, Ma Y, et al. Effects of rholida on the free radical metabolism and serum creatine kinase after exercise at plateau. Space Med Med Eng. (2001) 14:448–51. doi: 10.3969/j.issn.1002-0837.2001.06.013
37. Duncan MJ, Tallis J, Wilson S, Clarke ND. The effect of caffeine and Rhodiola rosea, alone or in combination, on 5-km running performance in men. J Caffeine Res. (2016) 6:40–8. doi: 10.1089/jcr.2015.0025
38. Gao X, Zhang Q. The effects of compound Rhodiola rosea beverage on athletes' physiological function and athletic performance. Northwest Pharm J. (1996) 11:213–4.
39. Jia Y, Liu X, Tian J, Xiang H, Qin X, Chen A, et al. Research on the effect of salidroside on improving the metabolic pathway changes caused by oxidative stress injury in amateur marathon runners. Nat Prod Res. (2021) 33:837–46. doi: 10.16333/j.1001-6880.2021.5.016
40. Kreipke P, Vince C, Moffatt P, Robert J, Tanner M, Charles J, et al. Effects of concurrent training and a multi-ingredient performance supplement containing Rhodiola rosea and Cordyceps sinensis on body composition, performance, and health in active men. J Dietary Suppl. (2021) 18:597–613. doi: 10.1080/19390211.2020.1822486
41. Liao YH, Chao YC, Sim BYQ, Lin HM, Chen MT, Chen CY, et al. Rhodiola/Cordyceps-based herbal supplement promotes endurance training-improved body composition but not oxidative stress and metabolic biomarkers: a preliminary randomized controlled study. Nutrients. (2019) 11:2357. doi: 10.3390/nu11102357
42. Lin CH, Hsu CC, Lin SW, Hsu MC. Rhodiola rosea does not reduce in vivo inflammatory activity after continuous endurance exercise. Sci Sports. (2019) 34:e155–8. doi: 10.1016/j.scispo.2018.10.006
43. Noreen EE, Buckley JG, Lewis SL. The effects of an acute dose of Rhodiola rosea on exercise performance and cognitive function. J Int Soc Sports Nutr. 6:P14. doi: 10.1186/1550-2783-6-S1-P14
44. Noreen EE, Buckley JG, Lewis SL, Brandauer J, Stuempfle KJ. The effects of an acute dose of Rhodiola rosea on endurance exercise performance. J Strength Cond Res. (2013) 27:839–47. doi: 10.1519/JSC.0b013e31825d9799
45. Parisi A, Ciminelli E, Cerulli C, Quaranta F, Tranchita E. Effect of Rhodiola rosea on endurance exercise performance: a pilot study. NORME PER GLI AUTORI. (2009) 62:149–55. doi: 10.1007/s00421-009-1001-3
46. Qiao Z. Effectts of compound rohoodiola preparation on plasma antioxidant system of track and field athletes. J Beijing Sport Univ. (2009) 32:73–5.
47. Skarpanska-Stejnborn A, Pilaczynska-Szczesniak L, Basta P, Deskur-Smielecka E. The influence of supplementation with Rhodiola rosea L. extract on selected redox parameters in professional rowers. Int J Sport Nutr Exer Metabol. (2009) 19:186–99. doi: 10.1123/ijsnem.19.2.186
48. Song Y, Sun X, Meng C. Effect of hongjintian decoction on serum MDA, SOD, NGF, MBP and BFGF levels of exercise-induced fatigue athletes. Chin J Biochem Pharmaceut. (2015) 35:119–21.
49. Timpmann S, Hackney AC, Tamm M, Kreegipuu K, Unt E, Ööpik V, et al. Influence of Rhodiola rosea on the heat acclimation process in young healthy men. Appl Physiol Nutr Metabol. (2018) 43:63–70. doi: 10.1139/apnm-2017-0372
50. Zhang Z-J, Tong Y, Zou J, Chen P-J, Yu D-H. Dietary supplement with a combination of Rhodiola crenulata and Ginkgo biloba enhances the endurance performance in healthy volunteers. Chin J Integr Med. (2009) 15:177–83. doi: 10.1007/s11655-009-0177-x
51. Zheng S, Liu D. Comparing efficacy of rhodiola rosea, red goji berry, and siraitia grosvenorii in physical recovery after aerobic exercise. Curr Top Nutraceut Res. (2023) 21:326–31. doi: 10.37290/ctnr2641-452X.21:326-331
52. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
53. He Y. The effects of Rhodiola extract on some physiological and biochemical indicators after a single exhaustive exercise (Master's thesis). Liaoning Normal University (2007).
Keywords: roseroot, physical endurance, oxidative stress, muscle damage, inflammation
Citation: Wang X, Yang X, Gao Z, Zeng J and Liu Y (2025) The effect of Rhodiola rosea supplementation on endurance performance and related biomarkers: a systematic review and meta-analysis. Front. Nutr. 12:1645346. doi: 10.3389/fnut.2025.1645346
Received: 11 June 2025; Accepted: 11 August 2025;
Published: 25 September 2025.
Edited by:
Paulina Mazur-Kurach, Akademia Wychowania Fizycznego im. Bronisława Czecha w Krakowie, PolandReviewed by:
Dandara Baia Bonifácio, Universidade Federal de Viçosa, BrazilJimmy Wen, California Northstate University College of Medicine, United States
Copyright © 2025 Wang, Yang, Gao, Zeng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yutong Liu, bGl1eXV0b25nQGx6dS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Xiaolin Wang
Xiaolin Wang Xuezhen Yang
Xuezhen Yang Zhendong Gao
Zhendong Gao Jin Zeng3
Jin Zeng3 Yutong Liu
Yutong Liu