Abstract
Background:
The relationship between changes in alcohol consumption and hepatic steatosis among alcohol consumers remains poorly understood. This study aimed to evaluate the association between changes in alcohol consumption and hepatic steatosis in a large population-based cohort of alcohol consumers.
Methods:
This study included 33,427 participants with reported alcohol consumption, categorized as mild, moderate, or heavy at baseline and imaging visits. Hepatic steatosis was assessed via magnetic resonance (MR) imaging during the imaging visit.
Results:
9,131 (27.3%) participants were diagnosed with hepatic steatosis at imaging visit. After adjusting for confounders, mild drinkers who progressed to moderate (aOR 1.26, 95% CI 1.10–1.44) or heavy drinking (aOR 1.70, 95% CI 1.12–2.57) had elevated odds of hepatic steatosis compared to stable mild drinkers. Moderate drinkers who maintained moderate drinking (aOR 1.36, 95% CI 1.21–1.53) or progressed to heavy drinking (aOR 2.27, 95% CI 1.84–2.79) also showed increased risk compared to those who transitioned to mild drinking. Conversely, heavy drinkers who transitioned to moderate (aOR 0.58, 95% CI 0.47–0.72) or mild drinking (aOR 0.34, 95% CI 0.25–0.45) had significantly lower odds compared to stable heavy drinkers. Stratified analyses revealed that males, individuals under 65 years, those with higher BMI, and hypertensive patients were more susceptible to hepatic steatosis with increased alcohol consumption.
Conclusion:
Increasing alcohol intake raises the odds of hepatic steatosis, while reducing intake lowers the odds. Public health strategies should focus on decreasing alcohol consumption to alleviate the burden of hepatic steatosis.
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease, has emerged as a leading global cause of chronic liver disease and a major driver of cirrhosis worldwide (1–3). Concurrently, the COVID-19 pandemic has exacerbated high-risk alcohol consumption, significantly increasing the prevalence of alcohol-related liver disease (ALD) and alcohol-related cirrhosis, particularly in the United States (4–6). This dual burden of metabolic and alcohol-related liver diseases has created a complex epidemiological landscape, with alcohol-related cirrhosis now surpassing other etiologies as the primary indication for liver transplantation among U.S. adults (7, 8). These trends underscore the urgent need for comprehensive strategies to address the growing burden of both MASLD and ALD in this evolving era of liver disease management.
Among individuals with MASLD, concurrent moderate to heavy alcohol consumption is associated with accelerated liver fibrosis progression and more rapid disease advancement. While excessive alcohol intake is a well-established risk factor for hepatic steatosis (9), the relationship between low-to-moderate alcohol consumption and liver health remains controversial. Some studies suggest a potential protective effect, indicating that low-to-moderate alcohol intake may be associated with reduced risks of hepatic steatosis and secondary liver disease. Notably, studies conducted in Japan have shown that moderate alcohol consumption may protect against the development of fatty liver disease (FLD) (10, 11). This geographical variation is supported by a meta-analysis by Roerecke et al. (12) which identified beneficial effects of low alcohol consumption in Japan based on robust epidemiological evidence, although no such association was found in other regions. Further reinforcing this perspective, Dunn et al. (13) reported that moderate alcohol consumption correlated with a lower prevalence of steatohepatitis, hepatocellular ballooning, and liver fibrosis. Similarly, Unalp-Arida and Ruhl (14) found that moderate alcohol consumption was associated with lower controlled attenuation parameter (CAP) levels, while heavy drinking showed no correlation with fatty liver severity. However, contrasting evidence highlights the potential risks of alcohol consumption. Multiple studies have demonstrated that alcohol intake significantly increases the risk of chronic liver diseases, with daily drinking and non-meal-associated consumption posing particularly high risks. Additionally, moderate alcohol consumption has been linked to progressive liver fibrosis, with diabetic patients displaying heightened susceptibility to advanced fibrosis even at moderate drinking levels (15–19). These conflicting findings underscore the complex relationship between alcohol consumption and liver health in patients with MASLD.
To the best of our knowledge, there are currently no large population-based cohort studies that have evaluated the impact of changes in alcohol consumption status on hepatic steatosis. Given the lack of relevant data and the conflicting findings regarding the effect of concurrent alcohol use on the risk of liver disease progression, our objective was to assess the impact of changes in alcohol consumption status on hepatic steatosis.
2 Materials and methods
2.1 Study design and participants
This cross-sectional study was performed using the data obtained from the UK Biobank (UKB) dataset (reference number 170239), a large prospective cohort study that recruited over 500,000 participants aged 37–73 years across England, Scotland, and Wales between 2006 and 2010 (20). During the baseline visit, participants underwent an initial assessment, which included information from questionnaires, verbal interviews, physical measurements, and biological samples. In April 2014, a subset of 100,000 participants was invited to participate in the initial phase of a multimodal imaging visit, during which they underwent abdominal magnetic resonance imaging (MRI) (21). Participants who completed the alcohol consumption questionnaire and provided information on their current alcohol intake were included in this study. Of the 502,178 participants, we excluded those who without full sets of Liver MultiScan® data (n = 460,731), those with missing data for proton density fat fraction (PDFF) (n = 928), those lacking alcohol consumption data at baseline (n = 6,839) or alcohol consumption data at the imaging visit (n = 232), and those diagnosed with viral hepatitis (n = 21). Ultimately, the analytic sample comprised 33,427 participants (Figure 1).
Figure 1
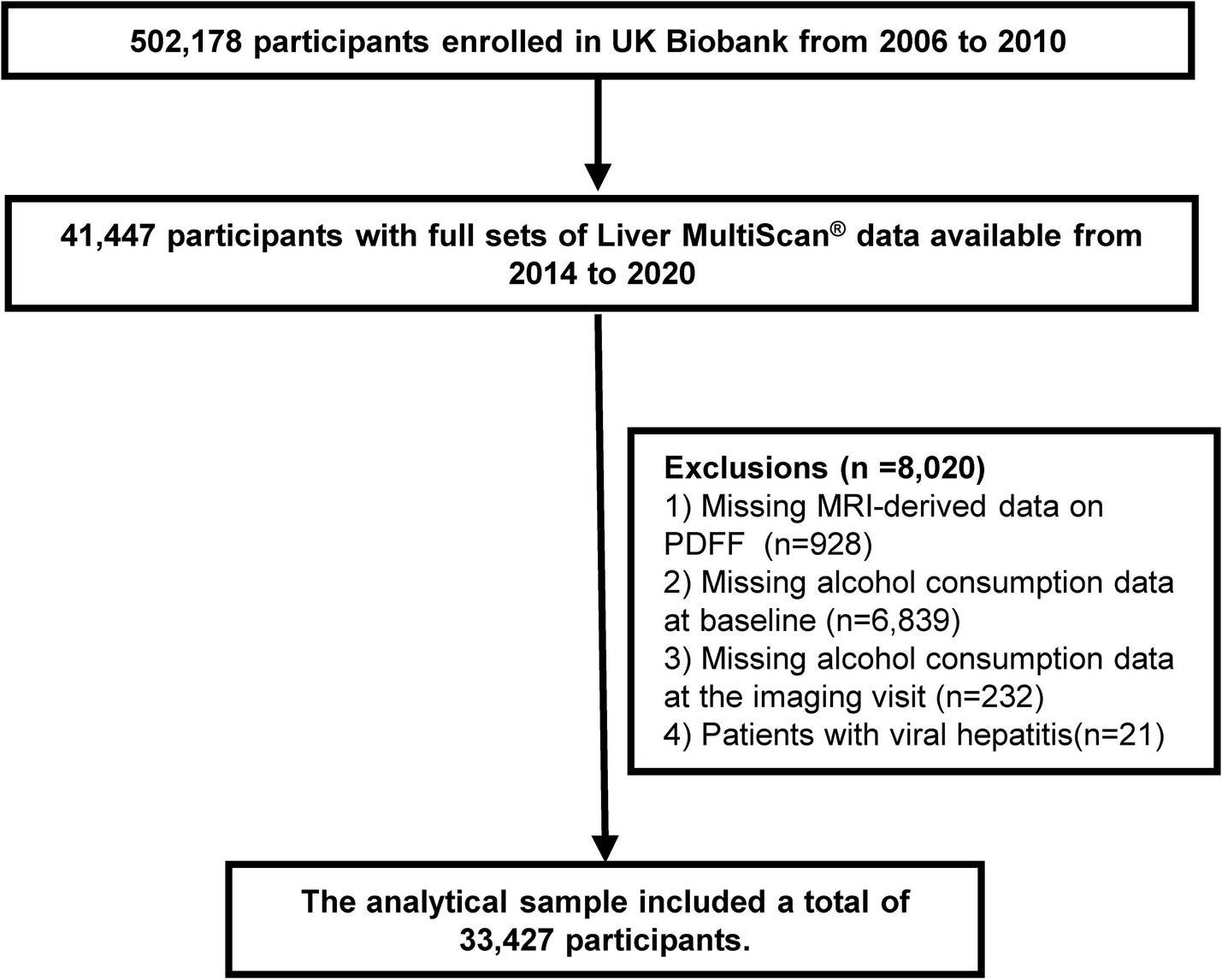
Flowchart of participant selection. MRI, magnetic resonance imaging; PDFF, proton density fat fraction.
The UK Biobank study received approval from the North West Multicenter Research Ethics Committee (Ref: 11/NW/0382), and all participants provided written informed consent. Information regarding the methods, data availability, and access procedures for the UK Biobank can be found on the study’s website.1
2.2 Exposure assessment
Alcohol consumption patterns were assessed using a validated questionnaire that systematically evaluated both the frequency and quantity of various alcoholic beverages consumed by participants. Data collection took place at two key time points: the baseline visits (2006–2010) and the subsequent imaging visits (2014). The assessment included standardized measurements for multiple beverage types, such as beer, cider, champagne, white wine, red wine, spirits, and other alcoholic drinks. In this study, participants quantified their average alcohol intake using standardized units, reporting it as weekly consumption for regular drinkers and monthly consumption for occasional drinkers.
Weekly and monthly alcohol consumption was calculated by analyzing the reported frequency and quantity of each beverage type, which were then converted into daily grams of alcohol. The calculations considered the average alcohol by volume (ABV) for each drink along with standard conversion factors to translate beverage units into grams of pure alcohol. The UK Biobank defined alcohol units as follows: a pint or can of beer, lager, or cider is two units; a single shot of spirits (25 mL) is one unit; and a standard glass of wine (175 mL) is two units. Total weekly units were calculated by summing the reported consumption across all categories. For participants reporting monthly intake, the values were converted to weekly units by dividing by 4.3. Finally, weekly units were divided by 7 to determine daily alcohol consumption (22).
Alcohol intake can be broadly classified as mild (up to 20 g per day for women and 30 g for men), moderate (21–39 g per day for women and 31–59 g for men), or heavy (≥40 g per day for women and ≥60 g for men) (23).
2.3 Hepatic steatosis definition
Liver MRI scans were conducted following a standardized protocol during the imaging visit. Details of the liver MRI and analysis protocols have been described previously (21, 24). Participants were scanned using a Siemens 1.5 Tesla MAGNETOM Aera scanner (Siemens Healthineers, Erlangen, Germany) with a 6-min dual-echo Dixon Vibe protocol. This procedure produced a volumetric dataset that separated water and fat from the neck to the knee. Body composition analyses were performed using AMRA Profiler Research (AMRA Medical AB, Linköping, Sweden) (25). The average liver PDFF was calculated from nine regions of interest, which were carefully selected to avoid inhomogeneities, major blood vessels, and bile ducts. The presence of steatosis was evaluated using MRI-derived PDFF measurements, which have demonstrated reliability and accuracy in quantifying liver fat content. Hepatic steatosis was defined as a PDFF of ≥5%, consistent with established cut-off values for diagnosing this condition (26, 27).
2.4 Covariates
In our analyses, we incorporated demographic data from the UK Biobank dataset, including age, sex, and body mass index (BMI) at both baseline and the imaging visit as potential covariates. BMI ≥30 kg/m2 is defined as obesity (28). Furthermore, we conducted a supplementary evaluation of blood biomarkers, including high-density lipoprotein (HDL), low-density lipoprotein (LDL), glycated hemoglobin (HbA1c), triglycerides, and albumin. Additionally, we performed a comprehensive analysis of previously identified risk factors, such as the use of medications for hypertension, diabetes, and dyslipidemia (29). We included metabolic syndrome (MetS) as an important covariate in our analysis. MetS is defined as if participants had three or more of the following criteria (30): (1) abdominal obesity: WC >102 cm for males and >88 cm for females; (2) elevated blood pressure: systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg, or taking prescription for hypertension; (3) hypertriglyceridemia: triglyceride (TG) ≥150 mg/dL; (4) low high density lipoprotein cholesterol (HDLC): HDL-C <40 mg/dL for males and <50 mg/dL for females; and (5) elevated blood glucose: fasting glucose ≥100 mg/dL or taking insulin or diabetic pills to lower blood sugar.
2.5 Data cleaning
Before conducting the calculations, we implemented a data cleaning step to ensure the accuracy of our consumption estimates. Specifically, any negative values reported for alcohol consumption—considered to be data entry errors, invalid responses, or instances where patients chose not to answer—were excluded from the analysis. This approach was consistently applied across all alcohol consumption variables to maintain uniformity in data processing.
2.6 Statistical analysis
We presented baseline characteristics in the form of means (standard deviation) or median [interquartile range (IQR)] for quantitative variables and in the form of frequencies [percentages (%)] for categorical variables. Differences in characteristics were compared by using ANOVA tests or the Kruskal–Wallis H test for continuous variables and chi-squared tests for categorical variables.
Multivariable logistic regression models were employed to estimate the association between changes in alcohol consumption and hepatic steatosis, with results reported as odds ratios (ORs) and corresponding 95% confidence intervals (CIs). The analyses included the following adjustments: the crude model adjusted for no covariates. Model 1 adjusted for age and sex. Model 2 adjusted for the same risk factors as Model 1 plus BMI, waist circumference (WC), hypertension (HP), diabetes, hypertension medication, diabetes medication, dyslipidemia medication. Model 3 included all the factors in Model 2, further adjusted for aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), albumin, triglycerides, HDL, HbA1c. Additionally, we further conducted stratified analyses based on demographics and comorbidities, specifically age (<65 years versus ≥65 years), sex (male versus female), BMI (<30 kg/m2 versus ≥30 kg/m2), and HP (yes versus no). In these analyses, models were controlled for all other covariates except those used for stratification to examine the potential modifiers of effect.
We did not use any imputation method for missing data due to the low rate of missing values. All tests were two-tailed, and p < 0.05 was considered significant. Statistical analyses were conducted by using R software (version 4.4.1, The R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Baseline characteristics
According to the inclusion and exclusion criteria, a total of 33,427 participants (50.32% male, mean age: 55.13 ± 7.49 years) were included in the baseline alcohol use status analyses. The baseline characteristics of these participants are presented in Table 1. Among the 33,427 participants, 22,370 (66.92%) were classified as mild alcohol consumers, 8,552 (25.58%) as moderate alcohol consumers, and 2,505 (7.5%) as heavy alcohol consumers. Overall, participants who were classified as heavy alcohol consumers were more likely to be younger, female, and had a higher prevalence of previous and current smoking. Additionally, they exhibited higher levels of BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), WC, as well as elevated platelet counts, glucose, AST, ALT, GGT, triglycerides, and HDL.
Table 1
| Variable | Overall | Mild alcohol consumption | Moderate alcohol consumption | Heavy alcohol consumption | p-value |
|---|---|---|---|---|---|
| Total, n (%) | 33,427 | 22,370 (66.92) | 8,552 (25.58) | 2,505 (7.5) | |
| Baseline age, years, mean (SD) | 55.13 ± 7.49 | 55.09 ± 7.59 | 55.28 ± 7.32 | 54.97 ± 7.21 | 0.082 |
| Sex, male, n (%) | 16,820 (50.32) | 11,117 (49.70) | 4,491 (52.51) | 1,212 (48.38) | <0.001 |
| BMI, kg/m2, mean (SD) | 26.41 ± 4.00 | 26.32 ± 4.05 | 26.44 ± 3.82 | 27.04 ± 4.03 | <0.001 |
| Waist circumference, cm, mean (SD) | 87.94 ± 12.27 | 87.52 ± 12.27 | 88.41 ± 12.10 | 90.06 ± 12.48 | <0.001 |
| Hip circumference, cm, mean (SD) | 101.92 ± 7.75 | 101.80 ± 7.84 | 101.97 ± 7.43 | 102.86 ± 7.88 | <0.001 |
| Smoking status, n (%) | <0.001 | ||||
| Never | 19,746 (59.07) | 14,611 (65.32) | 4,258 (49.79) | 877 (35.01) | |
| Previous | 11,546 (34.54) | 6,649 (29.72) | 3,604 (42.14) | 1,293 (51.62) | |
| Current | 2,073 (6.20) | 1,077 (4.81) | 668 (7.81) | 328 (13.09) | |
| Prefer not to answer | 61 (0.18) | 32 (0.14) | 22 (0.26) | 7 (0.28) | |
| Systolic blood pressure, mmHg, mean (SD) | 137.35 ± 18.74 | 136.49 ± 17.90 | 138.43 ± 18.20 | 141.02 ± 18.64 | <0.001 |
| Diastolic blood pressure, mmHg, mean (SD) | 81.56 ± 10.41 | 80.94 ± 9.97 | 82.34 ± 10.08 | 83.94 ± 10.12 | <0.001 |
| Hypertension, n (%) | 7,475 (22.36) | 4,708 (21.05) | 2,045 (23.91) | 722 (28.82) | <0.001 |
| Diabetes mellitus, n (%) | 1,964 (5.88) | 1,354 (6.05) | 446 (5.22) | 164 (6.55) | 0.007 |
| Antihypertensive medication, n (%) | 2,825 (8.45) | 1,843 (8.24) | 727 (8.50) | 255 (10.18) | 0.004 |
| Glucose-lowering drug, n (%) | 99 (0.30) | 71 (0.32) | 23 (0.27) | 5 (0.20) | 0.510 |
| Lipid-lowering therapy, n (%) | 3,035 (9.08) | 1,933 (8.64) | 858 (10.03) | 244 (9.74) | <0.001 |
| Platelet, 109/L, mean (SD) | 248.60 ± 55.86 | 248.09 ± 55.05 | 249.17 ± 53.10 | 250.79 ± 55.06 | 0.032 |
| Glucose, mmol/L, mean (SD) | 5.00 ± 0.93 | 4.98 ± 0.84 | 5.03 ± 0.88 | 5.09 ± 0.95 | <0.001 |
| AST, U/L, mean (SD) | 25.83 ± 10.00 | 25.55 ± 9.80 | 26.01 ± 8.78 | 26.96 ± 11.01 | <0.001 |
| ALT, U/L, mean (SD) | 23.00 ± 13.73 | 22.67 ± 13.07 | 23.44 ± 13.48 | 24.44 ± 14.39 | <0.001 |
| GGT, U/L, mean (SD) | 34.11 ± 33.50 | 31.71 ± 27.65 | 36.91 ± 35.82 | 45.84 ± 50.86 | <0.001 |
| Albumin, g/L, mean (SD) | 45.50 ± 2.51 | 47.22 ± 4.98 | 47.48 ± 4.99 | 47.56 ± 4.95 | <0.001 |
| Triglycerides, mmol/L, median (IQR) | 1.38 (0.98–2.00) | 1.46 (1.02–1.95) | 1.42 (0.99–1.91) | 1.46 (0.99–2.00) | 0.014 |
| High-density lipoprotein, mmol/L, mean (SD) | 1.50 ± 0.38 | 1.46 ± 0.34 | 1.55 ± 0.36 | 1.64 ± 0.40 | <0.001 |
| Hemoglobin A1c, mmol/L, mean (SD) | 34.92 ± 5.10 | 34.97 ± 5.00 | 34.63 ± 4.69 | 34.58 ± 4.88 | <0.001 |
Baseline characteristics of participants for baseline alcohol consumption status analyses.
Baseline characteristics are represented as mean (standard deviation), median (interquartile range) or N (%).
BMI, body mass index; IQR, interquartile range; SD, standard deviation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase.
In the imaging follow-up session, conducted after a median follow-up period of 9.2 years, a significant proportion of participants exhibited notable changes in their alcohol consumption patterns. Among the 33,427 participants, 25,181 (75.33%) were classified as mild alcohol consumers, 6,449 (19.29%) as moderate alcohol consumers, and 1,797 (5.38%) as heavy alcohol consumers. The baseline characteristics of participants during the imaging visit were similar to those reported in Supplementary Table S4.
3.2 Association of baseline alcohol consumption with hepatic steatosis
Supplementary Tables S2, S3 illustrate the association between baseline alcohol consumption and hepatic steatosis. Of the 33,427 participants, 9,131 (27.3%) were diagnosed with hepatic steatosis. After adjusting for confounders, participants with heavy and moderate alcohol consumption displayed significantly higher odds of developing hepatic steatosis compared to those with mild alcohol consumption, with adjusted aORs of 1.41 (95% CI: 1.31–1.52) and 2.60 (95% CI: 2.30–2.93), respectively.
3.3 Association between changes in alcohol consumption with hepatic steatosis
Table 2 presents the number and percentage of changes in alcohol consumption status during the imaging follow-up period. Among participants classified as having mild alcohol consumption at baseline, 1,734 participants (7.75%) progressed to moderate or heavy drinking. Among those categorized as moderate drinkers at baseline, 4,081participants (47.72%) transitioned to mild drinking, while 620 participants (7.25%) advanced to heavy drinking. Additionally, among participants identified as heavy drinkers at baseline, 464participants (18.52%) downgraded to mild drinking, and 1,016 participants (40.56%) shifted to moderate drinking. Figure 2 and Supplementary Table S4 shows the association between changes in alcohol consumption with hepatic steatosis. In comparison with stable mild participants, mild participants who progressed to moderate status or heavy status showed significantly elevated odds of developing hepatic steatosis, the aORs were 1.26 (95% CI: 1.10–1.44) and 1.70 (95% CI: 1.12–2.57), respectively. In addition, in comparison with participants who transitioned to mild status, moderate participants who stable moderate or progressed to heavy status showed significantly elevated odds of developing hepatic steatosis, the aORs were 1.36 (95% CI: 1.21–1.53) and 2.27 (95% CI: 1.84–2.79), respectively. In contrast, significantly decreased odds of developing hepatic steatosis were observed in the heavy participants who transitioned to moderate/mild status when compared with stable heavy participants (heavy to moderate, aOR 0.58, 95% CI: 0.47–0.72; heavy to mild, aOR 0.34, 95% CI: 0.25–0.45).
Table 2
| Baseline status | The imaging visit status | Changes in alcohol consumption status | n (%) |
|---|---|---|---|
| Mild | Mild | Stable mild | 20,636 (92.25) |
| Mild | Moderate | Mild to moderate | 1,582 (7.07) |
| Mild | Heavy | Mild to heavy | 152 (0.68) |
| Moderate | Mild | Moderate to mild | 4,081 (47.72) |
| Moderate | Moderate | Stable moderate | 3,851 (45.03) |
| Moderate | Heavy | Moderate to heavy | 620 (7.25) |
| Heavy | Mild | Heavy to mild | 464 (18.52) |
| Heavy | Moderate | Heavy to moderate | 1,016 (40.56) |
| Heavy | Heavy | Stable heavy | 1,025 (40.92) |
Number and percentage of the changes in alcohol consumption status.
The median follow-up time between baseline and the imaging visit was 9.2 years.
Figure 2
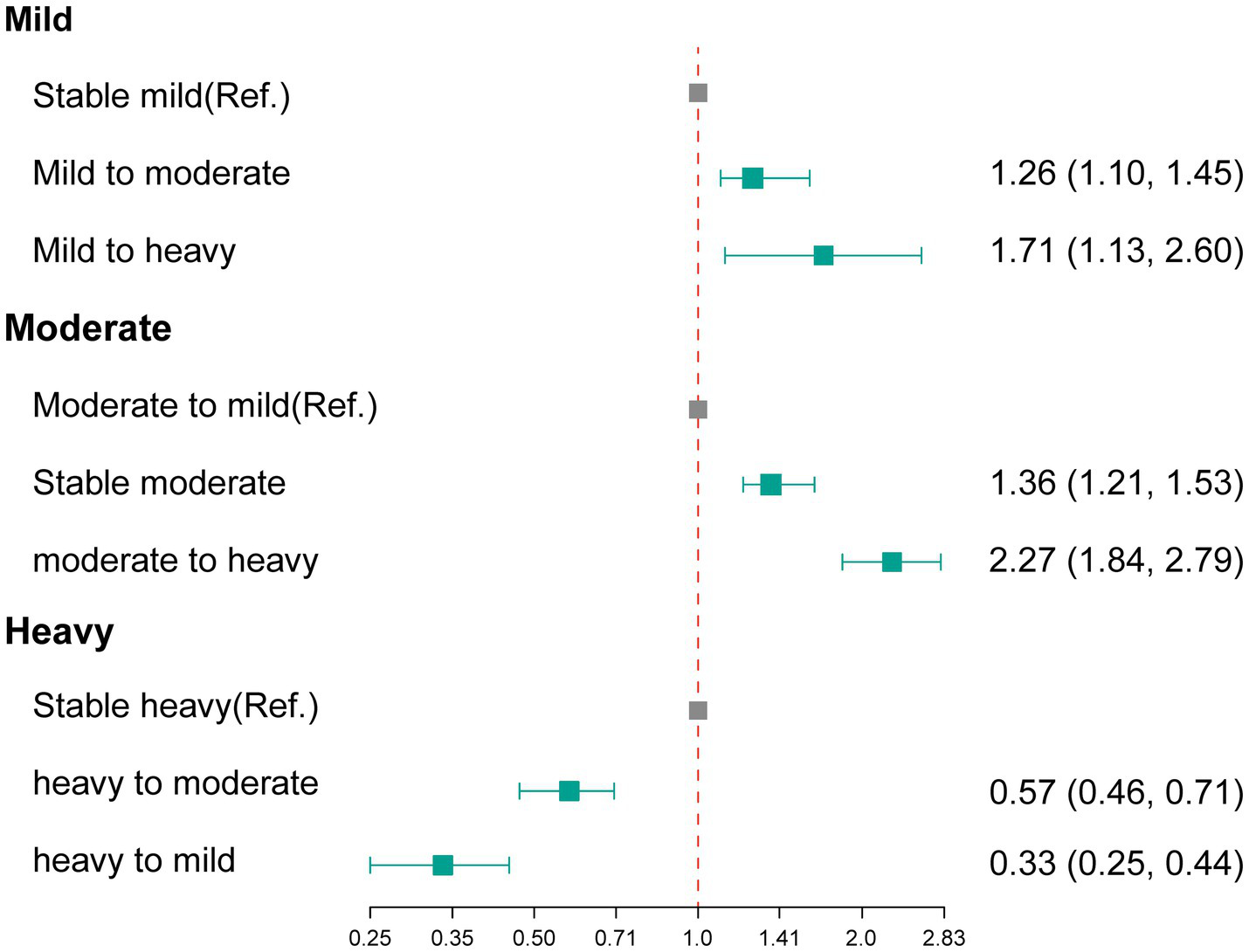
The association between changes in alcohol consumption with hepatic steatosis. Ref., reference group.
3.4 Stratified analysis
Figure 3 and Supplementary Table S5 presents a stratified analysis for the association between changes in alcohol consumption and hepatic steatosis among alcohol consumers, highlighting significant effects of sex, age, BMI, and hypertension status on this relationship. Males exhibited a notably higher risk of transitioning from stable mild to heavy drinking, with an OR of 2.26, while females demonstrated a lower risk in the transition from heavy to mild drinking (OR of 0.24). In terms of age, individuals under 65 showed an increased risk of moving from mild to heavy drinking (OR of 1.81), whereas those aged 65 and older had a relatively lower risk. Furthermore, individuals with a BMI of 30 or higher faced significantly heightened risk in the transition from mild to heavy alcohol consumption (OR of 1.95), and hypertensive patients also displayed an increased risk across various transitions, particularly from mild to heavy drinking (OR of 2.34). These findings suggest that males, younger individuals, those with higher BMI, and patients with hypertension are more susceptible to severe hepatic steatosis as alcohol consumption increases.
Figure 3
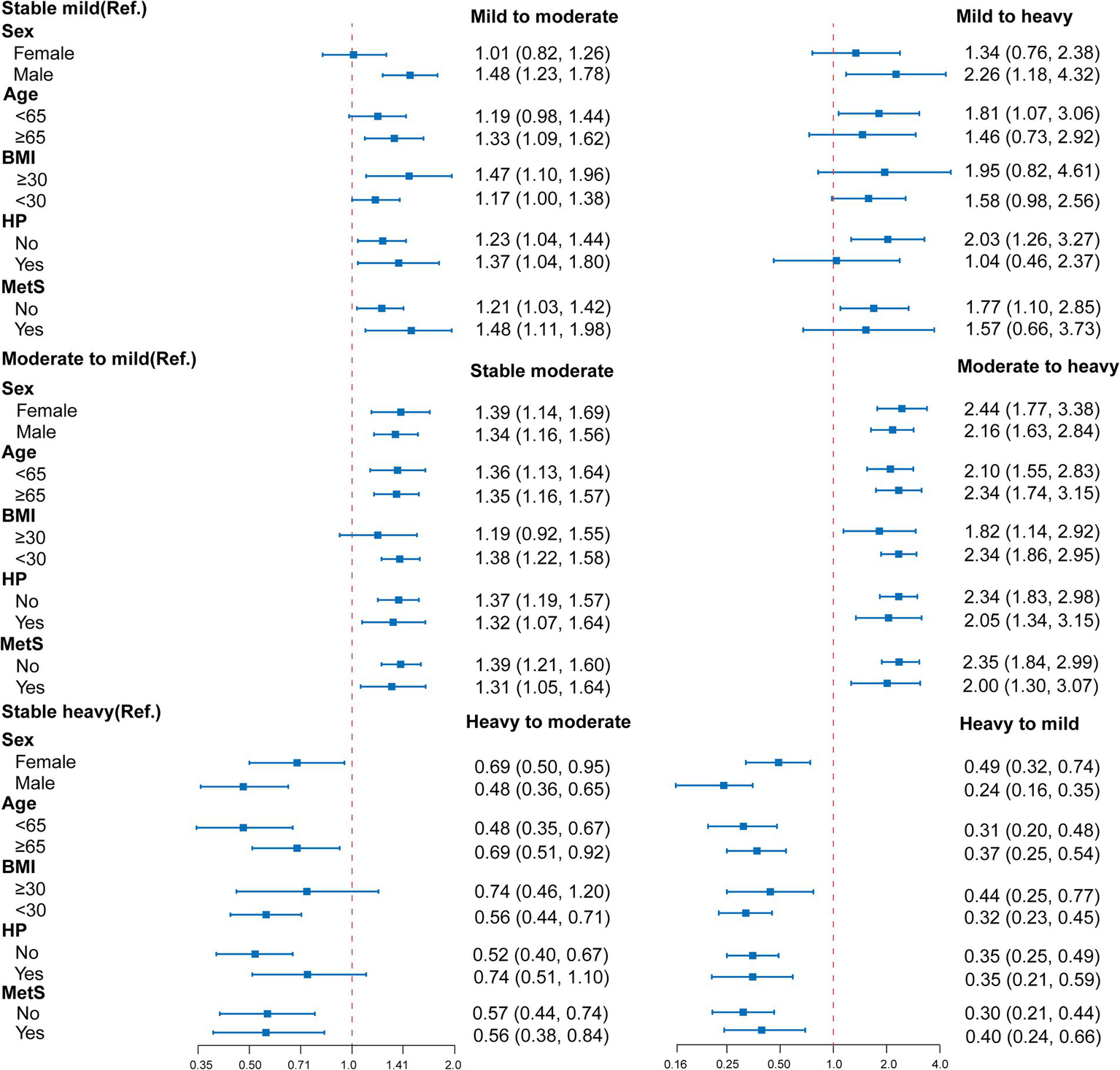
Stratified analysis for the association between changes in alcohol consumption and hepatic steatosis. Multivariable logistic regression models were adjusted for all the factors (age, sex, hypertension, BMI, waist circumference, diabetes mellitus, antihypertensive medication, glucose-lowering drug, and lipid-lowering therapy, AST, ALT, GGT, albumin, triglycerides, HDL, HbA1c and duration of alcohol consumption.) except the stratification factor itself. BMI, body mass index; HP, hypertension; OR, hazard ratio; Ref., reference group.
4 Discussion
Our comprehensive analysis of a large UK-based prospective study showed that participants who were heavy drinkers at baseline but reduced their alcohol consumption during the imaging follow-up period experienced significantly lower odds of developing hepatic steatosis. In contrast, participants who were mild drinkers at baseline but increased their alcohol consumption during the imaging follow-up period demonstrated significantly higher odds of developing hepatic steatosis. This association remained consistent across a series of sensitivity analyses. This study is the first to investigate the impact of changes in alcohol consumption over time on hepatic steatosis in a large population cohort. Our findings suggest that assessing alcohol consumption and implementing early interventions may help reduce the risk of developing hepatic steatosis, particularly among participants with heavy or moderate alcohol consumption.
The relationship between alcohol consumption and hepatic steatosis in population-based studies has been inconsistent, as highlighted by previous research (31). For instance, a cross-sectional study involving 4,009 participants from northeastern Germany demonstrated a significant increase in the risk of hepatic steatosis associated with higher average daily alcohol intake in both men and women, particularly among those who were overweight or obese. Notably, heavy alcohol consumption exacerbated this risk in individuals with excess body weight (32). In contrast, a longitudinal study of 5,297 Japanese participants (3,773 men and 1,524 women) suggested that mild to moderate alcohol consumption—and even slightly higher levels in men—may confer long-term protection against fatty liver for the majority of individuals (10). The study by Hagström et al. (33) also showed that low to moderate lifetime alcohol consumption is associated with less advanced stages of fibrosis in non-alcoholic fatty liver disease. Our findings reveal a clear dose–response relationship between alcohol intake and hepatic steatosis among drinkers, indicating that the risk of hepatic steatosis escalates as alcohol consumption increases. Conversely, a reduction in alcohol intake was associated with a decreased likelihood of developing hepatic steatosis. These results are consistent with the findings reported by Bedogni et al. (34). Collectively, our study strengthens the evidence that elevated alcohol consumption is a potential risk factor for hepatic steatosis.
The complex mechanisms underlying the bidirectional relationship between alcohol consumption and hepatic steatosis remain incompletely understood. The liver serves as the central hub for systemic lipid metabolism, processing free fatty acids (FAs) derived from multiple sources including glycolysis, autophagy, and adipose tissue lipolysis. These FAs are subsequently metabolized through β-oxidation for energy production, incorporated into cellular membranes, or esterified into triglycerides within hepatocytes. The synthesized triglycerides are then either packaged into very low-density lipoproteins (VLDLs) for systemic distribution or serve as precursors for primary bile acid synthesis, which facilitates dietary lipid emulsification and absorption. This intricate metabolic network is tightly regulated by hormonal signals, nuclear receptors, and intracellular signaling pathways, maintaining hepatic lipid homeostasis under physiological conditions. However, disruption of these regulatory mechanisms can lead to pathological lipid accumulation in hepatocytes, resulting in fatty liver disease. Additionally, changes in gut microbiota [such as reduced levels of short-chain fatty acids (SCFAs) and decreased bacterial diversity] play a key role in the development and progression of fatty liver disease (35). Alcohol exerts multifaceted effects on hepatic lipid flux through both direct and indirect mechanisms. During alcohol consumption, blood alcohol concentrations can reach millimolar levels (9), triggering a cascade of metabolic alterations. Ethanol metabolism generates reduced NADH, significantly increasing the hepatic NADH:NAD+ ratio, which simultaneously inhibits fatty acid β-oxidation while promoting fatty acid esterification (36). Furthermore, the primary ethanol metabolite acetaldehyde disrupts lipid homeostasis by upregulating key lipogenic genes, including Srebf1, Fasn, and Acc1, through enhanced nuclear translocation and subsequent transcriptional activation of their target genes (37, 38). These alcohol-induced metabolic perturbations collectively promote intrahepatocytic triglyceride accumulation, ultimately driving the pathogenesis of hepatic steatosis. These mechanistic insights are consistent with our study findings, which demonstrate a dose-dependent relationship between alcohol consumption and the risk of hepatic steatosis development.
Abstinence is the most critical intervention for treating hepatic steatosis. However, the improvement in hepatic steatosis after the cessation of alcohol consumption is inconsistent; some patients may still progress to cirrhosis or experience liver decompensation even while remaining abstinent (37, 39). Research conducted by Schonfeld et al. (40) identified the hepatic demethylases lysine demethylase (KDM) 5B and KDM5C as important epigenetic regulators of the liver’s response to alcohol. These enzymes hinder the resolution of liver fibrosis after alcohol cessation, partly by suppressing liver X receptor (LXR) activity. Additionally, Kang et al. (41) performed a mouse model study that demonstrated a gradual decrease in the expression of lipogenic genes following ethanol withdrawal. They also found that hepatic NAD+ levels were rapidly restored after stopping ethanol intake, reaching levels comparable to those in the no-ethanol control group by the third week of the withdrawal period. This evidence suggests that liver steatosis significantly diminishes after abstaining from alcohol. Furthermore, abstaining from alcohol can effectively restore lipid metabolism in the liver of mice, reversing liver damage and inflammation through enhanced metabolic reprogramming (42, 43). Data from our study indicate that among drinkers, a reduction in alcohol consumption significantly lowers the likelihood of developing hepatic steatosis. This highlights that abstaining from alcohol or reducing intake is an effective strategy for managing hepatic steatosis.
To the best of our knowledge, this study is the first to investigate the relationship between changes in alcohol consumption and hepatic steatosis. The strengths of our research include the utilization of a large longitudinal cohort dataset, which features high-quality data collected through standardized methods and multiple measurements, enabling us to control for a comprehensive range of confounding variables. However, there are several limitations to note. Firstly, the cross-sectional design of the study restricts our ability to infer causality regarding the effects of changes in alcohol consumption on hepatic steatosis, making it difficult to determine the direction of the relationship between these two factors. Secondly, we did not conduct liver biopsies, which are considered the gold standard for diagnosing fatty liver disease. Nevertheless, our study employed MRI to evaluate hepatic fat content, which yields reliable results. Thirdly, although the adapted measurements have been validated, alcohol consumption was assessed using self-reported data, which may introduce recall bias and misclassification. Fourthly, the UK Biobank participants tend to be ethnically and racially homogeneous, which limits the generalizability of our findings; further studies are needed to validate our results in more diverse populations. Fifthly, we did not assess the relationship between alcohol consumption and factors such as meal composition, total caloric intake, types of beverages (e.g., beer, wine), or educational level. Lastly, there may be unmeasured confounders, particularly those that are not identifiable through routine health screenings, such as stress or other mental health factors.
5 Conclusion
Our findings demonstrate a significant association between changes in alcohol consumption and hepatic steatosis, highlighting that early interventions to modify drinking behaviors may represent an effective strategy for preventing hepatic steatosis.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the UK Biobank study received approval from the North West Multicenter Research Ethics Committee (Ref: 11/NW/0382). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SW: Conceptualization, Funding acquisition, Writing – original draft. HL: Conceptualization, Writing – original draft. ZL: Writing – original draft. FL: Methodology, Writing – review & editing. ZT: Software, Writing – review & editing. PZ: Methodology, Writing – review & editing. CD: Resources, Writing – review & editing. SQ: Resources, Writing – review & editing. TW: Data curation, Funding acquisition, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Guangxi Natural Science Foundation’s Joint Special Fund for Active Health and Frequent Incidence of Common Diseases (Grant No. Guike-2024GXNSFAA010298), the Health Commission of Guangxi Zhuang Autonomous Region in Western Medicine (Grant No. Z-A20240078], National Natural Science Foundation of China (Grant No. 72464004), Natural Science Foundation of Guangxi, China (Grant No. 2023GXNSFAA026397), Innovation Project of Health Economic and Social Development Research Center (Grant No. 2025RWB07), Science and Technology Project of Guangxi Disease Prevention and Control (Grant No. XJKKJ2025YB007), the Innovation Project of Guangxi Graduate Education (Grant No. JGY2024090).
Acknowledgments
This research has been conducted using the UK Biobank Resource under Application Number 170239.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1647225/full#supplementary-material
- ABV
Alcohol by volume
- ALD
Alcohol-related liver disease
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BMI
Body mass index
- CAP
Controlled attenuation parameter
- CI
Confidence interval
- DBP
Diastolic blood pressure
- FLD
Fatty liver disease
- GGT
Gamma-glutamyl transferase
- HbA1c
Glycated hemoglobin
- HDL
High-density lipoprotein
- HP
Hypertension
- LDL
Low-density lipoprotein
- OR
Hazard ratio
- IQR
Interquartile range
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- MRI
Magnetic resonance imaging
- PDFF
Proton density fat fraction
- SBP
Systolic blood pressure
- SD
Standard deviation
- UK
Biobank United Kingdom Biobank
- WC
Waist circumference
Glossary
Footnotes
References
1.
Rinella ME Lazarus JV Ratziu V Francque SM Sanyal AJ Kanwal F et al . A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) 79:1542–56. doi: 10.1016/j.jhep.2023.06.003
2.
Younossi Z Anstee QM Marietti M Hardy T Henry L Eslam M et al . Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
3.
GBD 2017 Cirrhosis Collaborators . The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:245–66. doi: 10.1016/S2468-1253(19)30349-8
4.
Wong T Dang K Ladhani S Singal AK Wong RJ . Prevalence of alcoholic fatty liver disease among adults in the United States, 2001–2016. JAMA. (2019) 321:1723–5. doi: 10.1001/jama.2019.2276
5.
Hirode G Saab S Wong RJ . Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. (2020) 3:e201997. doi: 10.1001/jamanetworkopen.2020.1997
6.
Julien J Ayer T Tapper EB Barbosa C Dowd WN Chhatwal J . Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: a modeling study. Hepatology. (2022) 75:1480–90. doi: 10.1002/hep.32272
7.
Wong RJ Singal AK . Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014-2019. JAMA Netw Open. (2020) 3:e1920294. doi: 10.1001/jamanetworkopen.2019.20294
8.
Younossi ZM Stepanova M Ong J Trimble G AlQahtani S Younossi I et al . Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. (2021) 19:580–589.e5. doi: 10.1016/j.cgh.2020.05.064
9.
You M Arteel GE . Effect of ethanol on lipid metabolism. J Hepatol. (2019) 70:237–48. doi: 10.1016/j.jhep.2018.10.037
10.
Moriya A Iwasaki Y Ohguchi S Kayashima E Mitsumune T Taniguchi H et al . Roles of alcohol consumption in fatty liver: a longitudinal study. J Hepatol. (2015) 62:921–7. doi: 10.1016/j.jhep.2014.11.025
11.
Yamada T Fukatsu M Suzuki S Yoshida T Tokudome S Joh T . Alcohol drinking may not be a major risk factor for fatty liver in Japanese undergoing a health checkup. Dig Dis Sci. (2010) 55:176–82. doi: 10.1007/s10620-008-0693-0
12.
Roerecke M Nanau R Rehm J Neuman M . Ethnicity matters: a systematic review and meta-analysis of the non-linear relationship between alcohol consumption and prevalence and incidence of hepatic steatosis. EBioMedicine. (2016) 8:317–30. doi: 10.1016/j.ebiom.2016.04.023
13.
Dunn W Sanyal AJ Brunt EM Unalp-Arida A Donohue M McCullough AJ et al . Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol. (2012) 57:384–91. doi: 10.1016/j.jhep.2012.03.024
14.
Unalp-Arida A Ruhl CE . Transient elastography-assessed hepatic steatosis and fibrosis are associated with body composition in the United States. Clin Gastroenterol Hepatol. (2022) 20:e808–30. doi: 10.1016/j.cgh.2021.02.009
15.
Ekstedt M Franzen LE Holmqvist M Bendtsen P Mathiesen UL Bodemar G et al . Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. (2009) 44:366–74. doi: 10.1080/00365520802555991
16.
Blomdahl J Nasr P Ekstedt M Kechagias S . Moderate alcohol consumption is associated with advanced fibrosis in non-alcoholic fatty liver disease and shows a synergistic effect with type 2 diabetes mellitus. Metabolism. (2021) 115:154439. doi: 10.1016/j.metabol.2020.154439
17.
Im PK Millwood IY Kartsonaki C Guo Y Chen Y Turnbull I et al . Alcohol drinking and risks of liver cancer and non-neoplastic chronic liver diseases in China: a 10-year prospective study of 0.5 million adults. BMC Med. (2021) 19:216. doi: 10.1186/s12916-021-02079-1
18.
Simpson RF Hermon C Liu B Green J Reeves GK Beral V et al . Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK million women study. Lancet Public Health. (2019) 4:e41–8. doi: 10.1016/S2468-2667(18)30230-5
19.
Hagstrom H Hemmingsson T Discacciati A Andreasson A . Alcohol consumption in late adolescence is associated with an increased risk of severe liver disease later in life. J Hepatol. (2018) 68:505–10. doi: 10.1016/j.jhep.2017.11.019
20.
Sudlow C Gallacher J Allen N Beral V Burton P Danesh J et al . UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
21.
Littlejohns TJ Holliday J Gibson LM Garratt S Oesingmann N Alfaro-Almagro F et al . The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat Commun. (2020) 11:2624. doi: 10.1038/s41467-020-15948-9
22.
Daviet R Aydogan G Jagannathan K Spilka N Koellinger PD Kranzler HR et al . Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat Commun. (2022) 13:1175. doi: 10.1038/s41467-022-28735-5
23.
Rinella ME Neuschwander-Tetri BA Siddiqui MS Abdelmalek MF Caldwell S Barb D et al . AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. (2023) 77:1797–835. doi: 10.1097/HEP.0000000000000323
24.
Linge J Nasr P Sanyal AJ Dahlqvist Leinhard O Ekstedt M . Adverse muscle composition is a significant risk factor for all-cause mortality in NAFLD. JHEP Rep. (2023) 5:100663. doi: 10.1016/j.jhepr.2022.100663
25.
Linge J Borga M West J Tuthill T Miller MR Dumitriu A et al . Body composition profiling in the UK biobank imaging study. Obesity. (2018) 26:1785–95. doi: 10.1002/oby.22210
26.
Caussy C Reeder SB Sirlin CB Loomba R . Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. (2018) 68:763–72. doi: 10.1002/hep.29797
27.
Caussy C Alquiraish MH Nguyen P Hernandez C Cepin S Fortney LE et al . Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. (2018) 67:1348–59. doi: 10.1002/hep.29639
28.
Dicker D Karpati T Promislow S Reges O . Implications of the European Association for the study of obesity’s new framework definition of obesity: prevalence and association with all-cause mortality. Ann Intern Med. (2025) 178:1065–72. doi: 10.7326/ANNALS-24-02547
29.
Marti-Aguado D Calleja JL Vilar-Gomez E Iruzubieta P Rodriguez-Duque JC Del Barrio M et al . Low-to-moderate alcohol consumption is associated with increased fibrosis in individuals with metabolic dysfunction-associated steatotic liver disease. J Hepatol. (2024) 81:930–40. doi: 10.1016/j.jhep.2024.06.036
30.
Grundy SM Brewer HB Jr Cleeman JI Smith SC Jr Lenfant C American Heart Association; National Heart, Lung, and Blood Institute . Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. (2004) 109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6
31.
Boyle M Masson S Anstee QM . The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J Hepatol. (2018) 68:251–67. doi: 10.1016/j.jhep.2017.11.006
32.
Lau K Baumeister SE Lieb W Meffert PJ Lerch MM Mayerle J et al . The combined effects of alcohol consumption and body mass index on hepatic steatosis in a general population sample of European men and women. Aliment Pharmacol Ther. (2015) 41:467–76. doi: 10.1111/apt.13067
33.
Hagstrom H Nasr P Ekstedt M Kechagias S Onnerhag K Nilsson E et al . Low to moderate lifetime alcohol consumption is associated with less advanced stages of fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. (2017) 52:159–65. doi: 10.1080/00365521.2016.1239759
34.
Bedogni G Miglioli L Masutti F Castiglione A Croce LS Tiribelli C et al . Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology. (2007) 46:1387–91. doi: 10.1002/hep.21827
35.
Tarantino G Citro V . What are the common downstream molecular events between alcoholic and nonalcoholic fatty liver?Lipids Health Dis. (2024) 23:41. doi: 10.1186/s12944-024-02031-1
36.
Ontko JA . Effects of ethanol on the metabolism of free fatty acids in isolated liver cells. J Lipid Res. (1973) 14:78–86. doi: 10.1016/S0022-2275(20)39332-9
37.
Osna NA Donohue TM Jr Kharbanda KK . Alcoholic liver disease: pathogenesis and current management. Alcohol Res. (2017) 38:147–61.
38.
You M Fischer M Deeg MA Crabb DW . Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem. (2002) 277:29342–7. doi: 10.1074/jbc.M202411200
39.
Takahashi H Shigefuku R Maeyama S Suzuki M . Cirrhosis improvement to alcoholic liver fibrosis after passive abstinence. BMJ Case Rep. (2014) 2014:bcr2013201618. doi: 10.1136/bcr-2013-201618
40.
Schonfeld M O’Neil M Weinman SA Tikhanovich I . Alcohol-induced epigenetic changes prevent fibrosis resolution after alcohol cessation in miceresolution. Hepatology. (2024) 80:119–35. doi: 10.1097/HEP.0000000000000675
41.
Kang H Kim MB Park YK Lee JY . A mouse model of the regression of alcoholic hepatitis: monitoring the regression of hepatic steatosis, inflammation, oxidative stress, and NAD+ metabolism upon alcohol withdrawal. J Nutr Biochem. (2022) 99:108852. doi: 10.1016/j.jnutbio.2021.108852
42.
Pi A Jiang K Ding Q Lai S Yang W Zhu J et al . Alcohol abstinence rescues hepatic steatosis and liver injury via improving metabolic reprogramming in chronic alcohol-fed mice. Front Pharmacol. (2021) 12:752148. doi: 10.3389/fphar.2021.752148
43.
Thomes PG Rasineni K Yang L Donohue TM Jr Kubik JL McNiven MA et al . Ethanol withdrawal mitigates fatty liver by normalizing lipid catabolism. Am J Physiol Gastrointest Liver Physiol. (2019) 316:G509–18. doi: 10.1152/ajpgi.00376.2018
Summary
Keywords
alcohol consumption, hepatic steatosis, epidemiology study, cross-sectional study, Biobank
Citation
Wei S, Luo H, Liu Z, Liu F, Tang Z, Zhu P, Deng C, Qu S and Wu T (2025) The relationship between changes in alcohol consumption and hepatic steatosis among alcohol consumers: a large-scale population-based Biobank study. Front. Nutr. 12:1647225. doi: 10.3389/fnut.2025.1647225
Received
15 June 2025
Accepted
30 September 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Masoud Rahmati, Aix-Marseille University, France
Reviewed by
Giovanni Tarantino, University of Naples Federico II, Italy
Harsh Kishore, Dayanand Medical College & Hospital, India
Updates
Copyright
© 2025 Wei, Luo, Liu, Liu, Tang, Zhu, Deng, Qu and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxia Deng, dcxgxyz@163.com; Shenhong Qu, shqu@gxams.org.cn; Tengyan Wu, wutengyan@gxmu.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.