- 1Institut für Biostatistik und Informatik in Medizin und Alternsforschung, Universitätsmedizin Rostock, Rostock, Germany
- 2Conway Institute of Biomolecular and Biomedical Research, School of Medicine, University College Dublin, Dublin, Ireland
Dietary components substantially influence aging-related health outcomes through the interaction with the gut microbiome. In this narrative review, we compiled human dietary intervention trials with varying complexities: from simple modifications like the addition of herbs and spices, nuts and beans, to whole-diet patterns such as the calorie-restricted high-polyphenol Green-Mediterranean diet. We show that the addition of fiber- and polyphenol-rich foods consistently enrich short-chain fatty acid (SCFA) producing bacteria such as Faecalibacterium, Eubacterium, Roseburia, and Blautia, and modulate various plasma and fecal metabolites, including increased levels of propionic acid when combining nuts with caloric restriction, increased visceral fat loss mediated by urolithins, and enhanced anti-inflammatory effects, potentially due to synergistic action between SCFAs and polyphenol metabolites. Furthermore, we highlight that relatively simple dietary modifications can produce meaningful microbiome and metabolite shifts, particularly in elderly and metabolically compromised populations, where the microbiome may be more responsive to intervention, and intervention effects are more pronounced. When added to strategies like caloric restriction, these foods can help preserve microbial diversity, maintain beneficial taxa, and enhance anti-inflammatory effects. These insights can inform the development of microbiome-targeted dietary strategies for improving health in high-risk populations.
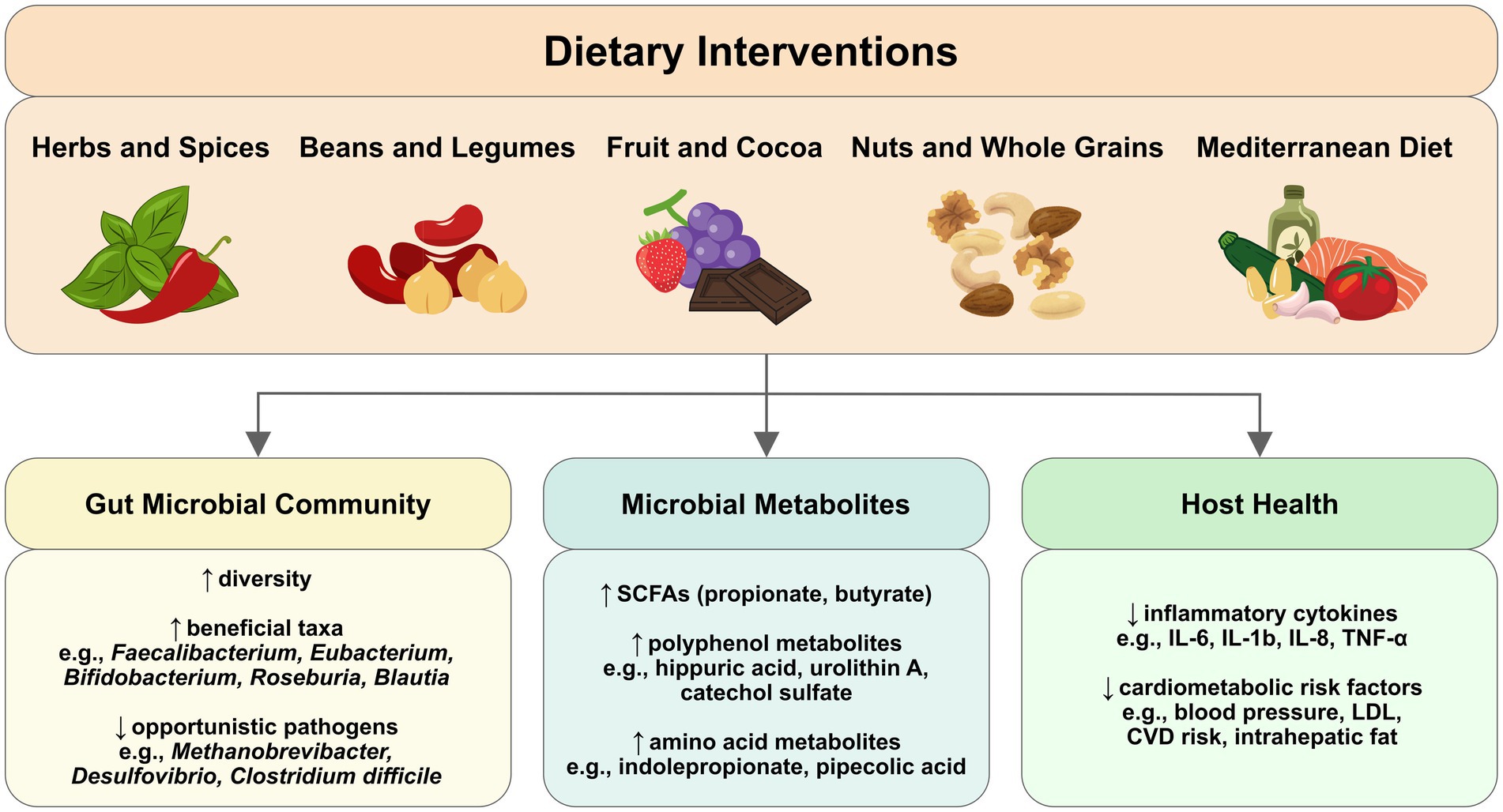
Graphical Abstract. Dietary interventions can modulate gut microbial composition and metabolites that influence host health outcomes. Gut microbiome-targeting interventions can range from simple food additions to more complex dietary patterns, influencing gut microbial composition, diversity, metabolite production, and resulting health benefits. Arrows show the progression from dietary intake through microbial changes to host health outcomes. ↑ indicates increase; ↓ indicates decrease.
1 Background: dietary patterns, microbial modulation, and intervention potential
Large-scale, population-based epidemiological studies show that dietary habits strongly influence the risk of disease, mortality, and disability, and the likelihood of healthy aging (1, 2). The quantity and types of nutrients affect metabolic and aging-related pathways (3), and shape the gut microbiome, including microbial diversity, composition, and metabolite production. These microbial changes closely correlate with health status and age-related physiological decline, both reflecting and potentially contributing to deteriorating health (4, 5). Differences in microbial composition and microbially produced metabolites have been consistently identified across a broad range of non-communicable diseases: in cardiovascular disease (6), hypertension (7), metabolic syndrome (8) and diabetes (9), chronic kidney disease (10), colorectal cancer (11), frailty (12), age-related macular degeneration (13) and Alzheimer’s disease (14). This knowledge, together with the widespread prevalence of high-risk dietary habits, i.e., high intake of sodium, meat, sugar, and trans fats, and low consumption of vegetables, whole grains, fruits, nuts, low-fat dairy, and seeds (1, 2) have driven the development of microbiome-targeted dietary interventions.
Such interventions tend to be more effective when they include fiber- and polyphenol-rich foods, which are selectively fermented by gut microbes into health-promoting metabolites such as short-chain fatty acids (SCFAs) and phenolic acids. These metabolites contribute to gut barrier integrity, immune modulation, and metabolic regulation. This fermentation process underlies, at least in part, the health benefits commonly associated with fiber- and polyphenol-rich foods. For example, in patients with Parkinson’s disease, diets high in fiber have been associated with anti-inflammatory SCFA producers and reduced neuroinflammation, while higher sugar intake correlates with potentially pathogenic bacteria (15).
Among health-promoting dietary strategies, interventions enriched in polyphenols, including the addition of specific foods (e.g., spices, legumes, and nuts) as well as whole-diet patterns such as the Mediterranean and Green-Mediterranean diets, can modulate the microbial community and enhance the production of beneficial microbial metabolites (16–18). This review focuses on these dietary interventions, exploring their effects on the gut microbiome, host metabolite profiles and gut barrier integrity, and the mechanisms by which these effects are mediated.
2 Microbial metabolism of dietary components
Fermentable polysaccharides, polyphenols, residual peptides and amino acids, and biopolymers that consist of polysaccharides bound to phenolic acids, can be broken down by the colonic microbiota in a series of biochemical reactions, involving different species of microorganisms and intermediary products. The metabolic products from microbial fermentation have metabolic, immunomodulatory, and neurological effects (19), modifying the local gut environment and host health. The composition (and potential benefits) of the metabolic output is determined by availability and composition of substrates present for fermentation, and the functional capacity of the microbiota to break down these components.
2.1 Microbial fermentation of dietary fibers and SCFA-mediated host effects
Dietary fibers are complex carbohydrates that escape digestion in the upper gastrointestinal tract and reach the colon, where they serve as substrates for microbial fermentation. Fermentable fibers include a broad range of plant-derived polysaccharides, such as pectin, arabinoxylan, beta-glucans, fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), inulin, xyloglucans, and resistant starches (20, 21). These fibers differ in solubility, structure, and fermentability, and have been studied for their influence on gut microbial composition, particularly on fiber-degrading taxa, and the concentration of microbial metabolites (22). While humans lack the enzymes required to break down plant cell wall components, the majority of gut microbes have the functional capacity to do so, and to use them as carbon and energy source (23, 24). The main fermentation end products from fiber are short-chain fatty acids (SCFAs), which have been extensively studied because of their role in metabolic health (25). SCFAs are organic acids containing two to six carbon atoms, with acetate (C2), propionate (C3), and butyrate (C4) being the most abundant, and produced in a ratio of approximately 3:1:1 (26). The key SCFAs, their microbial producers, and host-relevant functions are summarized in Table 1.
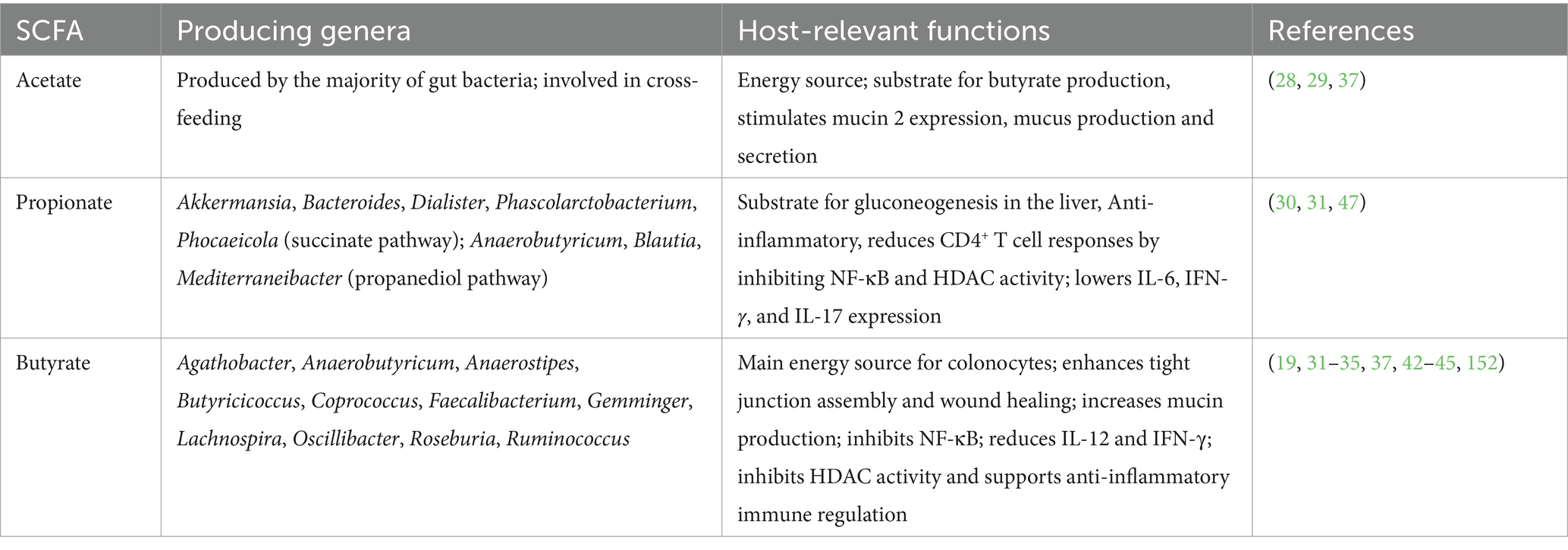
Table 1. Overview of major short-chain fatty acids (SCFAs) produced through microbial fermentation of dietary fibers in the colon, associated bacterial genera, and reported effects on host metabolism, gut barrier integrity, and immune function.
The ability to generate SCFAs is functionally redundant across taxonomically distinct bacteria (27): acetate is formed by the vast majority of gut bacteria while butyrate and propionate are produced by subsets of bacteria that form functionally distinct groups, and multiple products can be generated by the same species (28).
Cross-feeding also takes place; e.g., acetate can increase butyrate production, noted between acetate-producing Bifidobacterium and butyrate-producing Faecalibacterium (29). Examples of propionate-producing genera are Akkermansia, Bacteroides, Dialister, Phascolarctobacterium, and Phocaeicola via primarily the succinate pathway, and Anaerobutyricum (formerly Eubacterium halii), Blautia and Mediterraneibacter via the propanediol pathway (30, 31).
Butyrate is mostly formed by genera of the highly oxygen-sensitive anaerobic Clostridia families Lachnospiraceae and Ruminococcaceae, and examples include Agathobacter, Anaerobutyricum, Anaerostipes, Butyricicoccus, Coprococcus, Faecalibacterium, Gemminger, Lachnospira, Oscillibacter, Roseburia and Ruminococcus (31–33).
SCFAs serve as signaling molecules, energy supply, and regulators of metabolism (e.g., insulin sensitivity and fat storage), the immune system, and the gut barrier.
Butyrate provides 70% of the energy requirement of the colonic epithelium (34), facilitates tight junction assembly and promotes wound healing of the intestinal epithelium (35, 36). Butyrate (and acetate) can stimulate mucin 2 (Muc2) expression, mucus production and secretion (37).
SCFAs exert their functions by signaling through surface-expressed (free fatty acid) G-protein coupled receptors on epithelial cells, fat cells, and immune cells (38) or via histone deacetylase (HDAC) inhibition (39). Both signaling routes can regulate T-cell differentiation to induce IL-10-producing regulatory T-cells (40, 41). Butyrate is especially associated with intestinal and immuno-modulatory functions (19, 42, 43), due to its inhibitory effect on NF-κB (44) as well as IL-12 and IFN-γ (45), which play a role in chronic low-grade inflammation (46).
Similarly, propionate was shown to be inversely regulated by fasting and refeeding, and to reduce inflammatory CD4+ T cell responses by inhibiting NF-κB activity and histone deacetylase activity, leading to lower levels of IL-6, IFN-γ, and IL-17 (47).
2.2 Protein fermentation and health-relevant microbial metabolites
While our focus is on fiber- and polyphenol-rich dietary interventions, it is important to understand the complete metabolic landscape of the colon, where the availability and type of substrates change along its length, influencing microbial metabolism and the resulting health effects.
In the first part of the large intestine (the proximal colon), microbes primarily ferment carbohydrates, the amount of which gradually decreases toward the descending colon (48), where microbes are specialized to harvest energy from residual peptides and proteins, yielding a more diverse array of metabolic products compared to the fermentation of dietary fibers (23).
Of these metabolites, branched-chain fatty acids (BCFAs) have gained attention because of their association with metabolic imbalances and poor colonic health (49–51). BCFA levels are influenced by diet, and reduced when changing from a Western to a Mediterranean diet, and negatively correlate to butyrate- and acetate generating bacteria, and microbial diversity (52).
Metabolite profiles of prediabetic individuals can feature increased levels of BCFAs (53). Prevotella copri and Bacteroides vulgatus are two examples of microbes that were found to drive the association between BCFA synthesis and insulin resistance (53). Besides metabolic imbalances, there is also a link between BCFAs and cancer development. A study found that BCFAs produced by Clostridium symbiosum led to increased cholesterol synthesis via mTORC1, in turn activating hedgehog signaling, resulting in increased colorectal cancer stemness and tumor growth in mice (54). Other (unfavorable) metabolites from amino acids include the uremic toxin p-cresol, produced from the fermentation of tyrosine, and associated with chronic kidney disease (55), generated by, e.g., Clostridium difficile (56).
2.3 Fiber-dependent generation of beneficial aromatic amino acid metabolites
Some amino acid metabolites are thought to benefit human health. The effects on colonic and systemic health have been noted for tryptophan metabolite indolepropionate and phenylalanine-metabolite phenylpropionate. Indolepropionate can be produced by members of the Clostridium genus or by a two-step transamination and reduction reaction by lactic acid bacteria and Bifidobacterium (57). Several Clostridium species were found to generate indolepropionate: C. sporogenes (58), three strains of C. cadaveris (59), the toxin-producing C. botulinum (60), as well as Peptostreptococcus anaerobius CC14N (59). An important link between the generation of beneficial tryptophan metabolites and dietary fiber intake was established by Qi et al., who found that the intake of fiber-rich foods was most strongly associated with indolepropionate concentrations (50). Interestingly, a study deciphered a mechanism by which specific tryptophan metabolites were generated, which was independent of abundances of tryptophan-metabolizing gut bacteria, but regulated in a substrate-specific manner of metabolic pathways that involved different species and cross-feeding mechanisms (57). In more detail, in an in vitro experiment, indole-producing E. coli and indolepropionate-producing C. sporogenes were shown to compete for tryptophan, and fiber-degrading Bacteroides thetaiotaomicron moved the scale in favor of C. sporogenes, by cross-feeding monosaccharides to E. coli, making more tryptophan available to C. sporogenes (57). The results suggest that fermentable fibers can regulate indole production as a beneficial tryptophan metabolite (57), which is in line with studies that found positive associations of indolepropionate with fiber intake (61) but also with polyphenols (62). Finally, it was shown that fiber-mediated regulation of indole generation was not limited to particular species or communities, but is more of a typical phenomenon taking place in the human gut microbiota (57). Indolepropionate concentrations were found to be inversely associated with T2DM risk (50), and lower concentrations were present in patients with heart failure with preserved ejection fraction (HFpEF) in two separate cohorts (63). Indolepropionate is an ligand of aryl hydrocarbon receptors (AhR), and engages with other receptors including pregnane X receptor (PXR) and Toll-like Receptor 4 (63, 64). In a mouse model of HFpEF it was shown that indolepropionate supplementation attenuated diastolic dysfunction, oxidative stress and inflammation by enhancing the nicotinamide adenine dinucleotide salvage pathway, suppression of NNMT (nicotinamide N-methyltransferase) expression, and restoration of nicotinamide, NAD+/NADH, and SIRT3 levels (63). Indolepropionate was also shown to upregulate occludin, which is a tight junction protein (63), thereby strengthening the gut barrier and decreasing intestinal permeability (59). A similar protective action was observed for Bacteroides fragilis-derived phenylpropionate in pigs, activating AhR signaling and maintaining the integrity of the intestinal epithelial barrier (65). These examples show how microbial amino acid fermentation can result in metabolites with either beneficial or unfavorable effects on host health, as summarized in Table 2.
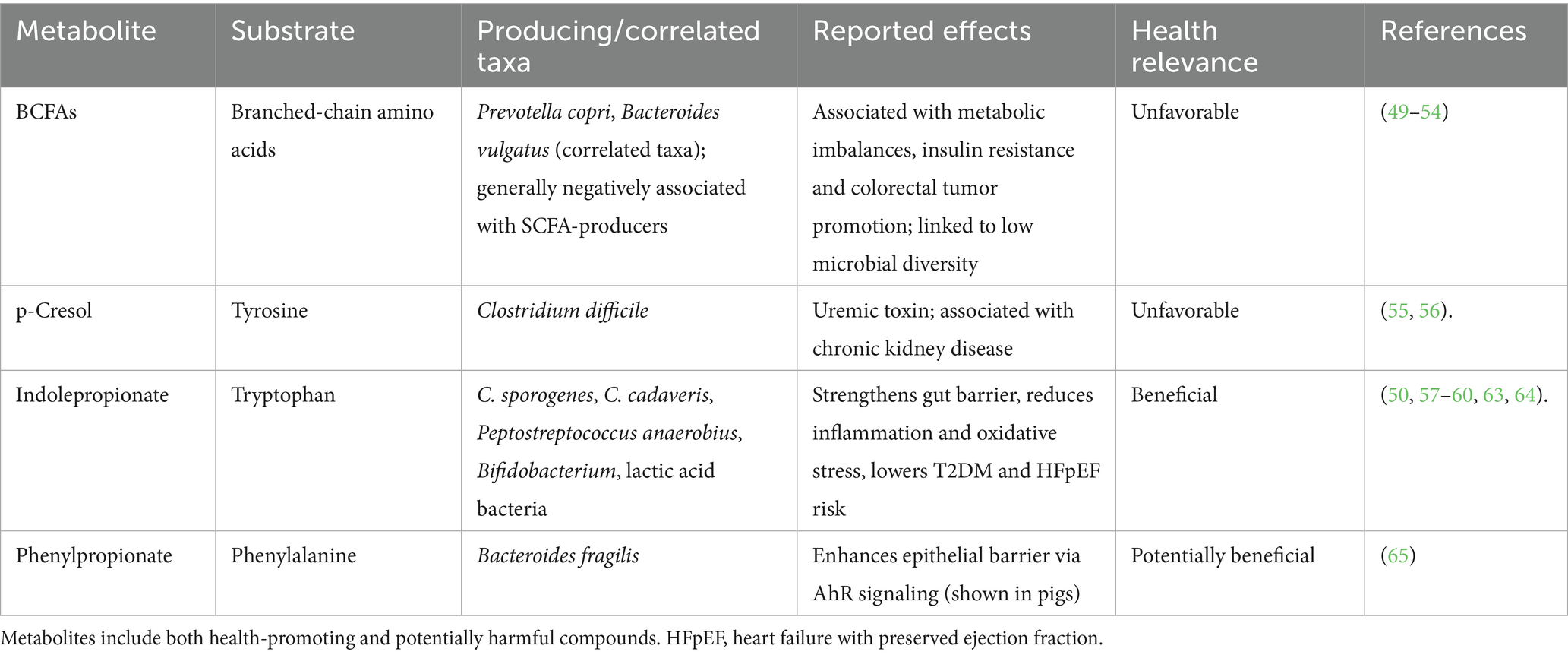
Table 2. Selected microbial metabolites derived from amino acid fermentation, their substrates, examples of known producers, and host effects as described in the text.
2.4 Microbial metabolism of dietary polyphenols
Polyphenols are a diverse group of bioactive plant compounds long recognized for their antioxidative capacity and health benefits (66). Most polyphenols have low bioavailability and require microbial transformation for better bioaccessibility (67–69). There are different classes of polyphenols, such as flavonoids (including flavonols and flavanols), ellagitannins, isoflavones and lignans, differing in chemical structure, complexity and origin, that are converted to phenolic acids by the microbiota (70). Proanthocyanidins are oligomeric flavanols, which are themselves a subclass of flavonoids (71), and are abundant in foods like spices, cocoa, beans, legumes, nuts, berries, grains, tea and fruits (72). Flavonols, on the other hand, are broken down by microbiota-mediated ring-fission, resulting in low molecular weight phenolic catabolites including benzoic acid, phenylacetic acid and phenylpropionic acid, hippuric acid, and valerolactones (70, 72, 73).
Phenolic compounds can modulate gut microbial composition and barrier integrity. For example, mice supplemented with epigallocatechin gallate, a polyphenol found in tea, featured increased Faecalibacterium, Bifidobacterium and Akkermansia abundances and butyrate production and an anti-inflammatory effect as well as an enhanced gut barrier function in a model of inflammatory bowel disease (IBD) (74).
As described above, fiber played an important role in the generation of indolepropionate from tryptophan. The presence of fiber is often necessary for the beneficial action of polyphenols as well, as described below, but fiber is not a mandatory mediator in this case. For example, a fiber-free diet combined with polyphenols indicated that polyphenol-supplementation can suppress mucus barrier degradation (75). In this experiment, mice fed a high-fat/high-sucrose (HFHS) diet were supplemented with a cranberry-rich extract or water. Akkermansia abundances then reached 30% relative abundance upon the HFHS diet supplemented with the cranberry extract, associated with a number of protective effects, including reduced weight gain and visceral obesity and blunted oxidative stress and inflammation compared to the HFHS control group. A potential explanation for the protective effect of polyphenols was indicated by the significantly higher expression of mucus-encoding Muc2 and tumor suppressing transcription factor Krüppel-like factor 4 (Klf4) in the colon (76) compared to HFHS controls. Significantly higher Muc2 expression points toward the higher production of mucin, thereby maintaining the mucus lining and providing the fermentation substrate for Akkermansia (75).
2.5 Health effects of microbially derived polyphenol metabolites
Ellagitannins and their hydrolysis product ellagic acid, found, e.g., in walnuts, berries, pomegranates, pecans and almonds, are known to be metabolized by Gordonibacter and Ellagibacter, certain Lachnospiraceae members and Enterocloster species into urolithins (77, 78). Enterocloster species harbor a urolithin C dehydroxylase operon, recently identified as a key mechanism for converting urolithin C to urolithin A, potentially explaining inter-individual differences in urolithin A production (78). Urolithin A has attracted particular interest due to its broad health benefits. It has been shown to increase lifespan in model organisms such as C. elegans and mice by enhancing mitophagy, improving cellular function, and alleviating systemic inflammation (79, 80). In a mouse model of colitis, urolithin A (derived from caffeic and ellagic acid) was associated with reduced inflammation and improved gut barrier function (81). In humans, urolithin A acts as a direct AhR ligand, repressing the transcription of pro-inflammatory mediators, including IL6 and prostaglandin-endoperoxide synthase 2 (PTGS2) (82–84). Additional studies have reported its role in enhancing mitophagy, improving mitochondrial function and reducing inflammatory responses (85) as well as its association with increased loss of visceral fat (86).
Urolithin A supplementation (1,000 mg) in elderly individuals improved muscle endurance and reduced inflammatory biomarkers while being safe and well tolerated (87).
Polyphenol-rich foods, including strawberries, can enhance urolithin A production and increase abundances of SCFA-producing bacteria. For instance, daily consumption of 500 g strawberries for nine days significantly increased urinary urolithin A and plasma antioxidant capacity (88), and a 10-week dietary intervention based on strawberries increased diversity and Faecalibacterium and Prevotella abundances (16).
Hippurate is another microbially derived metabolite that has been positively associated with gut microbial diversity, polyphenol metabolism, and diets high in fruit and whole grains (89, 90). Elevated hippurate levels have been linked to reduced risk of metabolic syndrome and increased visceral fat loss (86, 89, 91). Additionally, hippurate correlates with higher abundance of Faecalibacterium prausnitzii and lower levels of Ruminococcus and Eubacterium, suggesting a specific role in metabolic health regulation (89). Elevated blood hippurate has also been associated with increased adipose tissue expression of neuroglobin, a neuroprotective oxygen-binding protein primarily expressed in brain neurons (89, 92).
These two microbially derived metabolites exemplify the diverse host benefits of polyphenol metabolism by the gut microbiota.
2.6 Microbial breakdown of polyphenol-bound dietary fiber
The microbial fermentation of plant fibers can release bioactive phenolic compounds from the plant matrix. Many polyphenols are associated with dietary fibers through hydrophobic interactions, hydrogen bonding via hydroxyl groups, or covalent ester bonds; they may also accumulate inside vacuoles or become integrated in the plant cell wall (93, 94).
Polyphenol-bound dietary fibers, such as those in whole-grain cereals, have been experimentally shown to have higher antioxidant capacity than polyphenols alone (95). In grains like wheat, corn, rice and rye, the primary structural polysaccharide is arabinoxylan, a pentose sugar polymer, composed of a xylose backbone with arabinose side chains (96, 97). The phenolic compound ferulic acid is ester-linked to these arabinose side chains where it contributes to the structural integrity of the grain cell wall by crosslinking adjacent arabinoxylan polymers (98). The bran, which includes the aleurone and outer grain layers, contains approximately 90% of whole grain phenolic acids, primarily ferulic acid. Most of it is concentrated in the aleurone, which also stores proteins, phytate, and inorganic nutrients (98, 99). Upon reaching the colon, the antioxidant fiber is fermented by gut microbes, for example Bacteroides species can cleave the ester bond linking ferulic acid to arabinoxylan, releasing ferulic acid for absorption (100).
2.7 Synergy between phenolic acids and SCFAs
Zheng et al., demonstrated that phenolic acid metabolites and SCFAs can act synergistically to reduce inflammation in Caco-2 cells, a commonly used model of the intestinal epithelial barrier (73). The study showed that the combination of butyrate with any of three phenolic metabolites (phenyl acetic acid, benzoic acid, and phenyl propionic acid) at physiologically relevant concentrations significantly reduced IL-8 production more effectively than the individual compounds alone. The anti-inflammatory synergy was mediated through the downregulation of IL-8, TNF-α, and VCAM-1, at both gene and protein expression levels (73). The findings suggest that SCFAs and polyphenol metabolites may work synergistically to restore gut homeostasis by reducing inflammation (73).
3 Influence of aging and diet on the gut environment
The human gut and its microbiota undergo progressive changes with aging. These include a decline in commensal bacteria, an increase in potentially pathogenic species and greater interindividual variability in microbiota composition. These shifts are accompanied by local changes in the gut environment, including reduced capacity to maintain barrier integrity and an altered inflammatory profile.
3.1 Aging of the gut barrier
Studies have shown that impaired barrier function is associated with thinning of the mucus layer, increased intestinal permeability, and, in older individuals, a slower turnover of the intestinal epithelium. These changes are influenced by both dietary factors and the host microbiota (101, 102). The gut barrier plays a critical role in preventing the translocation of microbes, endotoxins and food antigens into systemic circulation, a function essential for avoiding inflammation and sepsis (103, 104).
Fecal microbiota transfer studies highlight the role of the microbiota in barrier function and host aging. When microbiota from aged mice are transferred to young mice, the young mice exhibit increased intestinal permeability along with inflammation in the central nervous system and retina, and altered cytokine signaling (103). Conversely, transferring microbiota from young to old mice can reverse these effects (103).
In humans, intestinal permeability is often assessed using plasma zonulin levels, a physiological modulator of tight junctions secreted by intestinal epithelial cells in response to dietary or microbial stimuli (105, 106). Zonulin lowers the expression of tight junctions and thereby increases intestinal permeability. Elevated zonulin levels have been linked to aging, frailty, chronic obstructive pulmonary disease (107, 108), arthritis (106) and cognitive impairment, particularly during the progression to Alzheimer’s disease (AD) (109). In AD, changes in gut barrier integrity have been suggested as a potential trigger before AD onset (109). Furthermore, microbial encroachment into the inner mucus layer is a feature observed in dysglycemia, independent of obesity (110). Zonulin levels also correlate with metabolic and microbial profiles. Higher zonulin is associated with increased waist circumference, fat mass, and systemic inflammation (111). In contrast, lower zonulin levels are linked to greater microbial diversity, particularly higher abundances of butyrate-producing Faecalibacterium prausnitzii, and reduced abundances of Bacteroidaceae, Veillonellaceae, Bacteroides, and Blautia (112). These microbial differences are associated with healthier dietary patterns, including greater intake of fiber, omega-3 polyunsaturated fatty acids, vitamins, and minerals (112).
3.2 The essential role of dietary fiber in gut homeostasis
Studies in both model organisms and humans have shown dietary fiber is essential for maintaining a homeostatic relationship between host and microbiota.
This becomes especially evident when examining the consequences of fiber deprivation on gut microbiome composition and barrier integrity. In mice, fiber-deprivation leads to significant compositional changes in both the small and large intestinal microbiota (113–115). In the small intestine, a decline in segmented filamentous bacteria has been observed, coinciding with impaired intestinal Th17 and intraepithelial T-cell development and enhanced susceptibility to infection. These alterations were also observed in the offspring, but could only be reversed through fecal microbiota transplantation, not with a high-fiber diet alone (115).
In the large intestine, low-fiber diets are associated with higher abundances of glycan-degrading microbes, particularly Akkermansia muciniphila, and intestinal barrier dysfunction (101, 113, 116).
Low-fiber intake has also been linked to increased abundances of IgE-coated commensals, reduced mucus thickness, exacerbated allergic responses and increased inflammation (116). In addition, antitumor immunity is adversely affected by low-fiber intake (117).
Desai et al. demonstrated that both chronic and intermittent fiber-deficiency shifted the microbiota from saccharolytic fermentation to use of host-derived mucus glycans as a fermentation substrate. This shift was characterized by a rapid expansion of glycan-degrading bacteria such as Akkermansia muciniphila and Bacteroides caccae, alongside a reduction in polysaccharide-fermenting species, without changing plasma levels of propionate or butyrate. The resulting thinning of the mucus layer favored pathogen expansion, increased inflammation and morbidity (113).
Holmberg et al. further showed that fecal microbiota transplantation from individuals on a low-fiber diet failed to induce adequate mucus production in mice. However, increasing fiber intake by 14 g significantly shifted the microbial composition and restored commensal-mucus interaction, mediated by Blautia through its production of acetate and propionate (101).
Although A. munciniphila is often considered a beneficial genus due to its propionate-generating ability, recent evidence points to a context-dependent role. Derosa et al. found that baseline A. munciniphila abundance was a negative predictor of 12-month survival in non-small cell lung cancer patients undergoing immune checkpoint inhibitor therapy (118). Patients with A. muciniphila abundances below 4.8% (considered normal) had the longest median survival (27 months), while those with levels above 4.8% had the shortest (8 months). Those patients with no detectable A. muciniphila had a median survival of 16 months after treatment (118). Those in the normal range also exhibited higher microbial diversity (Shannon Index) compared to patients with high or absent A. muciniphila levels (118). This represents a clear example of a U-shaped association of a microbial biomarker, in which extremes in one direction or the other are associated with the lowest benefits, or even with negative outcomes.
4 Dietary interventions: microbiome modulation, microbial diversity, and baseline composition
The microbial response to dietary interventions is partly determined by the initial composition and diversity of the gut microbiota, and the lead-in diet (52). Given the high interindividual variability in microbiomes, responses vary widely. For example, individuals with low abundances of fiber-degrading taxa such as Prevotella are more likely to experience an increase in butyrate-producing bacteria (31). In a short-term Mediterranean diet intervention, participants with lower initial diversity exhibited greater variability in microbial response (52). However, even individuals with higher diversity show changes in metabolites profiles that deviated from their initial state, highlighting the importance of considering the metabolome when attempting to define a healthy microbiome and to evaluate intervention outcomes (119).
In the following sections, we will review dietary intervention studies in elderly individuals and those with ongoing disease processes such as prediabetes, obesity, and metabolic syndrome. In these conditions, lower abundances of fiber-degrading bacteria are common (3, 22, 25, 120). We focus on clinical trials involving polyphenol-rich foods, high-fiber and complex-fiber interventions, and whole-diet interventions, organized by increasing complexity: from simple additions of single foods or food groups (sections 4.2–4.6), to food reformulation strategies (section 4.5.3), whole dietary patterns (sections 4.8–4.9), and finally personalized nutrition approaches (section 4.10). We begin with a contrasting example of fiber restriction (section 4.1) to highlight the importance of fiber for maintaining a healthy gut microbiome. This progression allows us to examine how interventions of varying complexity can achieve similar beneficial outcomes through shared mechanisms of increasing SCFA production and polyphenol metabolism. Where relevant, we will refer to previous sections on how dietary components are processed by the microbiome and their influence on host physiology, drawing from observational studies, in vitro research, and animal models.
4.1 High-protein, low-carbohydrate diets and microbiome effects
Before examining interventions beneficial for health and the microbiome, the following example aims to address the unintended effects on the host and microbiome of a high-protein, low-carbohydrate weight-loss diet. In a clinical trial, 80 overweight or obese postmenopausal women followed an 8-week very-low-calorie diet based on a meal replacement shake (121). The shake contained skimmed milk powder, milk protein, sodium caseinate, maltodextrin, canola and sunflower vegetable oils, artificial sweeteners, and was fortified with minerals, vitamins and inulin. The intervention led to weight loss and transient improvements in glucose regulation. Gut microbiota shifted significantly, with increased abundance of Akkermansia, and reduced abundances of plant-metabolizing genera Roseburia, Ruminococcus, Eubacterium, which are known to produce SCFAs (see section 2.1). Levels of SCFAs acetate, butyrate and valerate were significantly reduced (121). To explore the role of the microbiome, human fecal samples from trial participants were transferred into mice. This reproduced weight loss but revealed an unexpected enrichment of pathogenic C. difficile post-intervention, despite similar baseline abundances pre-intervention. This suggested a diminished capacity of the post-diet to restrict pathogen growth (121). These findings underscore how a complex change in diet composition can lead to multifaceted effects on host biomarkers, involving changes in the microbiome and its metabolic output.
4.2 High-polyphenol dietary interventions
4.2.1 The MaPLE trial: substitution of low- with high-polyphenol foods
A randomized controlled trial, the MaPLE trial, evaluated the substitution of low-polyphenol- with high polyphenol-foods in elderly with increased intestinal permeability. Over 8 weeks, participants consumed three daily portions of polyphenol-rich foods, e.g., berries, blood orange, pomegranate juice, green tea, apple and dark chocolate, providing a broad spectrum of polyphenols including proanthocyanidins, tannins and flavonols. The intervention resulted in lower plasma zonulin and fecal calprotectin levels, lower blood pressure, increased abundances of SCFA-producing bacteria, and modulation of multiple cytokines and metabolites (122–124).
Notably, participants with higher zonulin levels were also those with higher BMI and insulin resistance (123). Calprotectin levels (a biomarker used to evaluate gut inflammation) correlated with age, insulin, HOMA index, CRP, IL-6 and TNF-α (123, 124). Analysis of the serum metabolome showed that the intervention increased the microbially-produced polyphenol metabolites hippuric acid (see section 2.4), catechol sulfate, 2-methylpyrogallol sulfate and HPPA-S (3-(3-hydroxyphenyl) propanoic acid sulfate) (122). Furthermore, the theophylline metabolites 3-methylxanthine and 7-methylxanthine increased in concentration, while deoxycarnitine, hydroxyhexanoylcarnitine and asparagine were reduced.
Theobromine, a diet-derived metabolite from cocoa, was positively correlated with SCFA-producing genera such as Roseburia, Butyricicoccus, Faecalibacterium, and Lactonifactor, and negatively with Methanobrevibacter, and Desulfovibrio. Faecalibacterium and members of the Ruminococcaceae family increased significantly and inversely correlated with inflammation markers (123). These important findings support that microbially metabolized plant compounds can reinforce gut barrier integrity and reduce systemic inflammation in elderly.
4.2.2 Adding herbs and spices to a Western dish
Another approach to increasing polyphenol intake was tested by adding herbs and spices to a Western-style dish. In a randomized cross-over controlled trial involving obese individuals at risk for cardiovascular disease, this simple dietary addition induced favorable shifts in microbiota composition and host health markers (125). A medium dose (3.3 g) and high dose (6 g) of spice significantly altered community composition (beta-diversity) while the medium dose also increased alpha-diversity (125). Notable increases in Faecalibacterium, Ruminococcus and the genus UCG005 from the Ruminococcaceae family were observed in the high-dose spice intervention along with reduction in 24-h systolic blood pressure (125, 126).
A secondary analysis found that the medium-dose spice diet significantly reduced fasting plasma IL-6 and postprandial IL-1β, IL-8 and TNF-α and modulated monocyte function (127). In another study in healthy Singaporen males, spice intake was associated with higher Bifidobacterium abundances and increased levels of the phenolic acids cinnamic acid and phenylacetic acid (see section 2.4) (128).
4.3 Interventions with legumes
4.3.1 Bean-based intervention in high-risk obese patients
Colorectal cancer development is strongly influenced by lifestyle factors and adiposity (11, 129, 130). In the BEGONE trial, high-risk obese patients with a history of precancerous colonic polyps or colon cancer participated in a bean-enriched dietary intervention. Participants consumed one cup of beans (providing 16 g dietary fiber and 14 g of protein) alongside their usual diet. This intervention significantly increased the abundances of Faecalibacterium, Eubacterium, and Bifidobacterium, diversity, and induced broad changes in the host metabolome (131) (Table 3). Metabolic changes included reduced indole, increased levels of in pipecolic acid, the methyl group donor SAM (S-Adenosylmethionine), and trigonelline (131). These metabolites that increased upon the intervention have been associated with anti-inflammatory and potentially anti-aging effects. Pipecolic acid has been shown to ameliorate LPS-induced inflammation in mice and is associated with frequent exercise (132). Trigonelline, a microbial-derived catabolite of niacin and NAD+ precursor, is reduced in sarcopenia and is considered a potential therapeutic for age-related muscle decline (133). Furthermore, a reduction in interleukin-10 receptor-α and an increase in fibroblast-growth factor-19 (FGF19) were observed. FGF19 controls bile acid and glucose homeostasis, and is associated with increased glucose uptake and energy expenditure (134).
4.3.2 Calorie-restricted, legume-enriched diet in prediabetics
A 16-week, calorie-restricted, legume-enriched randomized clinical trial was conducted in 127 Chinese prediabetic individuals, aged 45 to 75 years (135). Participants in the intervention group replaced two daily meals with dishes composed of 100 g of legumes (mixed beans, red kidney beans, or chickpeas), a soy-based meat alternative, vegetables, and a low glycemic index (GI) carbohydrate source (rice or noodles), along with herbs, spices, and vegetable oil. The intervention led to a 40% reduction in total and saturated fat intake, a 33% reduction in salt intake, and an increase in fiber intake of 17 g/day. In addition, participants in the intervention group exhibited significantly greater reductions in LDL cholesterol, total cholesterol, and HbA1c over time compared to controls, indicating improved lipid and glycemic regulation. Each participant provided six stool samples (weeks 0, 2, 4, 8, 12, and 16) for metagenomic sequencing. Taxonomic responses included increases in Clostridia (e.g., Eubacterium rectale, Roseburia faecis, Roseburia hominis) and Bifidobacterium, and decreases in Ruminococcus (R. gnavus, R. torques, R. lactaris), Bacteroides (B. massiliensis, B. stercoris), and Bilophila wadsworthia. About half the taxa were significantly correlated with dietary fiber intake. Positive correlations were observed for E. rectale and Bifidobacterium, and negative correlations (and decreasing abundances) for R. torques, R. lactaris, and R. gnavus. Interestingly, microbial species enrichment and depletion peaked by the second week and remained stable thereafter. Increased plasma metabolites included methylcysteine and pipecolic acid, the organic acid aconitic acid, and indolepropionic acid, which might be produced in higher amounts due to the increased availability of dietary fiber (see section 2.3). Numerous fecal metabolites decreased upon the intervention, including benzoic acid, 2-furoic acid, various carnitines, amino acids, bile acids and fatty acids. Considering SCFAs, only plasma acetic acid was significantly increased, and negatively correlated with HbA1c, LDL-C, TC and TC/HDL-C ratio (135).
4.4 Dietary fiber from grains and food reformulation
4.4.1 Corn-based fiber intervention
A 2024 study investigated the effects of different types of corn flour: whole-grain, refined-grain, and a mix of refined grain with corn bran, on lipid markers in 36 adults with LDL cholesterol above 110 mg/dL (136). The combination of refined flour and corn bran, which contained the highest levels of ferulic acid (see Section 2.6), significantly reduced LDL cholesterol over time, with reductions greater than 5% observed in approximately 70% of participants. Meanwhile, the whole-grain flour significantly increased the abundance of butyrate-producing Agathobaculum (136), see Section 2.1.
4.4.2 Whole-grain intervention
In contrast, an 8-week whole-grain vs. refined-grain intervention in individuals at risk for metabolic syndrome did not alter community composition, yet it significantly reduced body weight and levels of the inflammatory markers IL-6, CRP and IL-1β (137). In addition, the intervention increased plasma butyrate and urinary excretion of several metabolites, including the alkylresorcinol DHPPA-glucuronide, 2-aminophenol-sulfate (a microbial degradation product of wheat and rye benzoxazinoids), and the phenol metabolites pyrocatechol-sulfate and pyrocatechol-glucuronide (137).
4.4.3 Food reformulation: Fiber-enriched products
Fiber enrichment of convenience foods is a promising strategy to increase fiber intake (138). In a two-week trial, a sourdough-croissant enriched with a diverse fiber blend (from wheat, rye, poppy seeds, walnuts, flax seeds, and others, totaling 4.8 g fiber per 100 g) was compared to a conventional croissant (1.3 g dietary fiber per 100 g) for effects on metabolic variables, the gut microbiome and appetite (139). Exclusion criteria included high physical activity and the habitual intake of fruit and vegetables.
The fiber-enriched croissant increased urinary levels of urolithin A sulfate (see section 2.5), likely due to the addition of walnuts. The intervention also led to reduced levels of dipeptidyl peptidase-4 (DPPIV) and a slight decrease in fasting blood glucose (139). Urolithin A is known to inhibit DPPIV (140), which degrades incretins - hormones that provoke insulin secretion. Inhibition of DPPIV can therefore improve insulin sensitivity (141).
4.5 Interventions with nuts
In a recent study involving healthy individuals, the consumption of 23 g of walnuts twice daily increased alpha diversity, and the abundances of 13 genera (Table 3): Roseburia, Rothia, Parasutterella, Lachnospiraceae UCG-004, Butyricicoccus, Bilophila, Eubacterium eligens, Lachnospiraceae UCG-001, Gordonibacter, Paraprevotella, Lachnospira, Ruminococcus torques, and Sutterella (142). Notably, the intervention significantly increased urolithin A and iso-urolithin A, which were significantly associated with several taxa including Gordonibacter, Angelakisella, Defluviitaleaceae UCG-011, and Anaerovoracaceae Family XIII (142). These findings suggest that polyphenols in walnuts likely contributed to the observed rise in urolithins (see Section 2.5).
In another study with nuts, 57 g almonds consumed daily for 8 weeks increased alpha-diversity and reduced Bacteroides fragilis compared to an isocaloric cracker snack (143). A separate energy-restricted study found that a nut-enriched breakfast, compared to a nut-free breakfast, significantly reduced ghrelin levels, suggesting a role in appetite regulation among individuals at risk for cardiometabolic disease (144).
4.6 Caloric restriction combined with nuts in overweight women
Two 8-week randomized controlled trials tested the effects of caloric restriction (CR) with or without the addition of nuts in women with high BMI and increased waist circumference and at least one other cardiometabolic risk factor.
In the first trial, participants consumed 30 g cashews and 15 g Brazil nuts daily. Both CR groups (with and without nuts) showed significant changes in beta-diversity, indicating a shift in community composition. However, only the CR-only group exhibited a reduction in alpha-diversity and a significant increase in the Firmicutes-to-Bacteroidetes ratio, changes that were not observed when nuts were added to the diet (145). A reduction in alpha-diversity points to a less stable microbial community.
Fecal SCFA analysis showed that CR and nuts significantly increased fecal propionic acid, and reduced acetate (145). Lactulose-secretion was used to measure intestinal permeability, which did not differ due to treatment, but lactulose secretion was significantly lower in the CR-nut-group compared to CR at the end of the intervention (145). Abundances that differed after the intervention versus baseline included increased Ruminococcus and UCG-002 in the CR-nut group and increased abundances of Blautia, and Ruminococcaceae genera UCG-005 and UCG-002, and a reduction in Ruminococcus in the CR group. Intestinal permeability correlated with fat-loss, IL-8 and Ruminococcus abundances (145). These findings suggest that the addition of nuts to caloric restriction may preserve community stability and support beneficial microbial taxa.
A second trial tested the effect of 8 g of Brazil nuts daily combined with CR to CR alone. The CR-nut group showed significantly decreased levels of CRP, TNF, IL-1β and IL-8 indicating an anti-inflammatory effect of the nut-enriched intervention (146).
4.7 Mediterranean diet interventions
The NU-AGE randomized controlled trial examined the effects of a one-year Mediterranean (Med) diet intervention in elderly subjects from five countries (Italy, United Kingdom, France, Netherlands, Poland) (147). Increased intake of fibers from vegetables, fruits and whole grains, plant proteins from legumes, polyunsaturated fatty acids from fish and vitamins and reduced fat, alcohol, salt and sugar intake consistently modulated microbiome composition across countries.
Greater adherence to the Med diet was associated with improved cognitive function, reduced frailty and lower inflammation. Key microbial responders included SCFA-producing taxa (see section 2.1) such as Faecalibacterium prausnitzii, Roseburia hominis, Eubacterium species (E. rectale, E. eligens, E. xylanophilum), as well as Bacteroides thetaiotaomicron, Prevotella copri and Anaerostipes hadrus. These taxa were negatively associated with inflammatory markers hsCRP and IL-17 and measures associated with increased frailty; and were further negatively associated with lower production of secondary bile acids and the uremic toxin p-cresol (147). Prior studies have similarly shown reductions in p-cresol following increased fiber intake (55).
The CORDIOPREV trial evaluated the long-term effects of a Med diet versus a low-fat diet in patients with established coronary heart disease over a seven-year follow-up (8, 148). The Med diet was superior in reducing the risk of major cardiovascular events (148). The analysis of fecal samples from a subgroup of 106 male participants revealed distinct baseline microbial compositions among individuals with and without metabolic syndrome. Those with metabolic syndrome had lower abundances of Bacteroides, Prevotella, Roseburia, Ruminococcus, and Faecalibacterium, and higher abundances of Streptococcus and Clostridia (8). Interestingly, the intervention only altered microbial composition in those with metabolic syndrome, and not in individuals without metabolic syndrome independent of obesity (8). Most of the compositional differences in participants with metabolic syndrome were reversed after follow-up 2 years later in addition to significantly decreased TG levels in both dietary intervention groups (8). Nutritional intake patterns shifted during the trial: the Med diet group increased their intake of olive oil, nuts and oily fish, and reduced consumption of carbohydrates and saturated fatty acids. The low-fat group increased carbohydrate consumption and reduced fat-intake; both groups reduced their intake of red and processed meats and carbonated drinks. Both groups increased dietary fiber consumption by 2.3 g (Med) and 3.2 g (low-fat) per 1,000 kcal (8, 148).
4.8 Calorie-restricted, high-polyphenol Mediterranean diet intervention
The DIRECT-PLUS randomized controlled trial integrated multiple anti-aging strategies and included 294 participants with abdominal obesity and dyslipidemia. Participants were randomized into three groups: healthy dietary guidelines, a Med diet, and a polyphenol-rich, low-red/processed meat Green-Med diet. Green-Med and Med diets were calorie-restricted and included daily consumption of 28 g walnuts. Green-Med participants were guided to consume 3–4 cups of green tea and a 500 mL shake composed of Mankai, an aquatic plant rich in protein and polyphenols, which increased the polyphenol content of the intervention by 800 mg gallic acid equivalents (17). Both interventions led to weight loss, improved cardiometabolic markers, body weight, blood pressure and fasting plasma leptin. Further, the interventions resulted in significant compositional changes: Green-Med increased abundances of Prevotella, Bacteroides, Lachnospira and genus DNF00809 from the Eggerthellaceae family and decreased abundances of Dorea, Collinsella and Bifidobacterium (Table 3). Green-Med intervention compositional changes were largely driven by non-core members (17). Interestingly, reduced Bifidobacterium abundances were significantly associated with greater weight loss. In the Med-diet group, Lachnospira, DNF00809 (Eggerthellaceae), Enterorhabdus, Intestimonas and genus UCG-003 from the Erysipelotrichaceae family significantly increased. Changes in metabolic pathways included a stepwise increase in BCAA (branched-chain amino acid) degrading pathways and a decrease in BCAA biosynthesis pathways, and an association analysis revealed that reduced cysteine biosynthesis was linked to, and mediated, weight loss and reduction in CHD risk (17). Positive effects were specifically attributed to the increased polyphenol content in the Green-med group, including the significantly higher reduction in intrahepatic fat compared to the Med diet (149) and higher circulating levels of hippuric acid, as well as Urolithin A (see sections 2.4 and 2.5) that related to greater visceral-adipose tissue reduction, mediated by increased consumption of walnuts and Mankai (86).
The Green-Med diet also impacted biological age, as assessed by DNA methylation age clocks. At the end of the intervention, a ~ 9-month favorable difference between observed and expected methylation age was observed (150). Greater age attenuation was linked to higher intake of Mankai and green tea, which correlated with elevated urinary metabolite levels of hydroxytyrosol, tyrosol, urolithin A, and urolithin C (150).
4.9 Personalized nutrition
A personalized nutrition approach was tested in the post-prandial glucose targeting (PPT) trial in prediabetic individuals. The PPT diet was based on a machine learning algorithm that integrated multiple parameters (meal’s nutrients composition, blood data, anthropometrics, lifestyle and gut microbial features), to predict an individual’s postprandial glycemic response, and then provided recommendations for meals (151). The PPT diet was compared to a Mediterranean diet intervention (participants were encouraged to include whole-wheat bread and grains, legumes, fruits, vegetables, olive oil, fish, poultry and low-fat dairy products in their diet) (151). While both dietary interventions enriched Faecalibacterium species, the PPT diet had a more pronounced effect on the gut microbiome and metabolites than the control diet, including increased richness and diversity, and a significant decrease in human cell shedding. Further, the PPT diet increased Flavonifractor plautii, Roseburia hominis, Ruthenibacterium lactatiformans and three strains of Faecalibacterium prausnitzii (151) (Table 3).
5 Discussion
In this review, we summarized clinical dietary intervention trials conducted in elderly individuals and those at elevated risk for chronic diseases. We focused on fiber- and polyphenol-rich dietary strategies that aim to modulate the gut microbiota to improve host health. Across diverse interventions, we found consistent evidence that even small dietary changes can beneficially shift microbial composition and function, most often reflected in increased abundances of SCFA producers and altered metabolite profiles.
We chose to focus on clinical trials in high-risk populations, where abundances of SCFA producers are generally lower, and intervention effects tend to be more pronounced (51, 121). This is relevant due to several reasons: Western diets typically fall short of recommended fiber intake levels, and our population is aging, intensifying the need to adjust dietary intake to match protective effects against chronic diseases like heart disease and diabetes. Furthermore, reduced microbial resistance to pathogens is often linked to increased intestinal permeability and systemic inflammation, both of which tend to increase with age. This was reflected in the high-protein, low-carbohydrate trial in obese women, where reduced fiber intake led to a bloom of Akkermansia, a glycan-degrading bacterium, reduced levels of SCFAs and the emergence of C. difficile following fecal microbiota transfer into mice (121). In contrast, the MaPLE trial demonstrated that polyphenol-rich foods can strengthen the intestinal barrier in elderly living in a residential care facility, particularly in those with elevated permeability at baseline. Zonulin and calprotectin levels were reduced, SCFA producers increased, and circulating cytokines were modulated (124). These findings align with the evidence in mice that polyphenols can help preserve gut barrier integrity by suppressing mucus degradation and regulating tight junctions (75).
Simple dietary changes like the addition of herbs and spices can change microbiome composition, diversity and metabolites substantially. Individuals at risk for CVD showed increased abundances of SCFA producers like Faecalibacterium and Ruminococcus, and reduced blood pressure and inflammatory cytokine levels (125, 126). In healthy individuals, spices led to increased Bifidobacterium abundances and cinnamic and phenylacetic acid levels (128). Spices included cinnamon, black pepper, ginger, coriander, parsley and more, suggesting that, besides fibers and “whole” polyphenol-rich foods, polyphenol-rich spices can meaningfully impact the microbial fermentation products and host immune response.
We also reviewed two trials where legumes were the primary intervention food: the BEGONE trial (beans) and the study by Wu et al. (135). Both included obese participants, either with prediabetes or a history of colorectal cancer or polyps, and showed increases in SCFA producers, such as Faecalibacterium, Eubacterium and Bifidobacterium, and increased microbial metabolites such as pipecolic acid and indolepropionate (131, 135). Both of these metabolites have been linked to reduced inflammatory responses and derive from microbial amino acid metabolism, potentially with the involvement of Bifidobacterium (57).
While the Mediterranean diet is well established for its cardiometabolic and cognitive benefits (8, 147), similar shifts in microbiome and metabolites can also be achieved with the addition of individual foods like nuts, legumes, and spices, which are often more accessible and easier to implement. These foods contain overlapping classes of fermentable fibers and polyphenols, and frequently result in increased SCFA producers such as Faecalibacterium, Roseburia, and Ruminococcus. These positive microbial responses were seen across multiple trials.
From a public health perspective, food reformulation may be one of the most scalable strategies to improve microbiome-related health outcomes. Trials using fiber-enriched croissants, bran-enriched flours, or whole-grains consistently showed improvements in markers such as LDL cholesterol, fasting glucose, SCFA production, and microbial metabolites like urolithin A (136, 137, 139). These findings support the idea that microbiota-targeted benefits can be achieved through modifying familiar foods, without requiring drastic dietary changes.
The Green-Med diet from the DIRECT-PLUS trial provides an example of a more complex dietary strategy, combining caloric restriction with higher polyphenol intake from walnuts, green tea, and Mankai. Compared to a calorie-restricted Med diet with walnuts, the Green-Med group showed more pronounced changes in microbial composition, SCFA metabolism, and liver fat reduction, as well as a measurable reduction in biological age as assessed by methylation age (17, 149, 150). This points to the potential of multi-component interventions to synergistically target microbiota-mediated health pathways.
A limitation in comparing across trials is the variability in study design, intervention duration, and lead-in diets. Short-term microbial responses are known to be influenced by habitual diet and microbial community diversity (52). In addition, differences in sequencing methods (16S versus metagenomic sequencing) can limit the comparability of microbiota outcomes across studies. Furthermore, in our review, there is a lack of direct head-to-head comparisons between different populations (e.g., young vs. elderly, metabolically healthy vs. compromised) receiving identical interventions. Our conclusion that intervention effects are more pronounced in elderly and metabolically compromised populations is based on synthesis across studies rather than direct comparative trials. These populations typically show lower baseline abundances of beneficial bacteria and compromised barrier function, which may explain their greater responsiveness to dietary interventions. Future studies directly comparing intervention responses across different populations would strengthen these observations.
Additionally, future studies that directly compare complex, multi-component interventions like the Green-Med diet to simpler, more implementable strategies such as replacing low-polyphenol with high-polyphenol foods would be interesting. The goal should not only be to maximize the intervention effect, but also to evaluate real-world applicability. A personalized approach based on baseline microbiota composition or host metabolic profiles may further enhance intervention effectiveness.
Gut microbial dysbiosis becomes more apparent with advancing age and chronic disease. However, the reviewed trials show that the microbiome remains responsive to dietary modulation. Improving dietary patterns by increasing the intake of fiber- and polyphenol-rich foods, such as beans, nuts, legumes, spices, berries, and tea, can help rebalance host-microbiota interactions and reduce modifiable risk factors. These strategies hold promise both for prevention and for targeted metabolic support, particularly in high-risk populations.
Author contributions
FM: Conceptualization, Writing – original draft, Writing – review & editing. AO-M: Visualization, Writing – review & editing. GF: Writing – review & editing. IB: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
GF is a consultant to BlueZoneTech GmbH, who distribute supplements. GF received a grant for partially funding a PhD student from Biovis, who offer lab testing, specifically related to microbiomes.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Afshin, A, Sur, PJ, Fay, KA, Cornaby, L, Ferrara, G, Salama, JS, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2019) 393:1958–72. doi: 10.1016/S0140-6736(19)30041-8
2. Tessier, AJ, Wang, F, Korat, AA, Eliassen, AH, Chavarro, J, Grodstein, F, et al. Optimal dietary patterns for healthy aging. Nat Med. (2025) 31:1644–52. doi: 10.1038/s41591-025-03570-5
3. Longo, VD, and Anderson, RM. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell. (2022) 185:1455–70. doi: 10.1016/j.cell.2022.04.002
4. Araújo, JR, Marques, C, Rodrigues, C, Calhau, C, and Faria, A. The metabolic and endocrine impact of diet-derived gut microbiota metabolites on ageing and longevity. Ageing Res Rev. (2024) 100:102451. doi: 10.1016/j.arr.2024.102451
5. Wilmanski, T, Diener, C, Rappaport, N, Patwardhan, S, Wiedrick, J, Lapidus, J, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. (2021) 3:274–86. doi: 10.1038/s42255-021-00348-0
6. Jie, Z, Xia, H, Zhong, SL, Feng, Q, Li, S, Liang, S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. (2017) 8:845. doi: 10.1038/s41467-017-00900-1
7. Calderón-Pérez, L, Gosalbes, MJ, Yuste, S, Valls, RM, Pedret, A, Llauradó, E, et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci Rep. (2020) 10:6436. doi: 10.1038/s41598-020-63475-w
8. Haro, C, García-Carpintero, S, Rangel-Zúñiga, OA, Alcalá-Díaz, JF, Landa, BB, Clemente, JC, et al. Consumption of two healthy dietary patterns restored microbiota Dysbiosis in obese patients with metabolic dysfunction. Mol Nutr Food Res. (2017) 61:1700300. doi: 10.1002/mnfr.201700300
9. Gaike, AH, Paul, D, Bhute, S, Dhotre, DP, Pande, P, Upadhyaya, S, et al. The gut microbial diversity of newly diagnosed diabetics but not of prediabetics is significantly different from that of healthy nondiabetics. mSystems. (2020) 5:e00578–19. doi: 10.1128/mSystems.00578-19
10. Gao, B, Jose, A, Alonzo-Palma, N, Malik, T, Shankaranarayanan, D, Regunathan-Shenk, R, et al. Butyrate producing microbiota are reduced in chronic kidney diseases. Sci Rep. (2021) 11:23530. doi: 10.1038/s41598-021-02865-0
11. Qin, Y, Tong, X, Mei, WJ, Cheng, Y, Zou, Y, Han, K, et al. Consistent signatures in the human gut microbiome of old- and young-onset colorectal cancer. Nat Commun. (2024) 15:3396. doi: 10.1038/s41467-024-47523-x
12. Zhang, Y, Zhu, Y, Guo, Q, Wang, W, and Zhang, L. High-throughput sequencing analysis of the characteristics of the gut microbiota in aged patients with sarcopenia. Exp Gerontol. (2023) 182:112287. doi: 10.1016/j.exger.2023.112287
13. Zysset-Burri, DC, Keller, I, Berger, LE, Largiadèr, CR, Wittwer, M, Wolf, S, et al. Associations of the intestinal microbiome with the complement system in neovascular age-related macular degeneration. NPJ Genom Med. (2020) 5:34. doi: 10.1038/s41525-020-00141-0
14. Cattaneo, A, Cattane, N, Galluzzi, S, Provasi, S, Lopizzo, N, Festari, C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. (2017) 49:60–8. doi: 10.1016/j.neurobiolaging.2016.08.019
15. Kwon, D, Zhang, K, Paul, KC, Folle, AD, Del Rosario, I, Jacobs, JP, et al. Diet and the gut microbiome in patients with Parkinson’s disease. NPJ Parkinsons Dis. (2024) 10:89. doi: 10.1038/s41531-024-00681-7
16. Meiners, F, Hinz, B, Boeckmann, L, Secci, R, Sueto, S, Kuepfer, L, et al. Computational identification of natural senotherapeutic compounds that mimic dasatinib based on gene expression data. Sci Rep. (2025) 14:6286. doi: 10.1038/s41598-024-55870-4
17. Rinott, E, Meir, AY, Tsaban, G, Zelicha, H, Kaplan, A, Knights, D, et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. (2022) 14:29. doi: 10.1186/s13073-022-01015-z
18. Sandhu, AK, Miller, MG, Thangthaeng, N, Scott, TM, Shukitt-Hale, B, Edirisinghe, I, et al. Metabolic fate of strawberry polyphenols after chronic intake in healthy older adults. Food Funct. (2018) 9:96–106. doi: 10.1039/C7FO01843F
19. Kamada, N, Chen, GY, Inohara, N, and Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. (2013) 14:685–90. doi: 10.1038/ni.2608
20. Dworkin, M, and Falkow, S. The prokaryotes: A handbook on the biology of bacteria. 3rd ed. New York, London: Springer (2006). 1 p.
21. Stephen, AM, Champ, MMJ, Cloran, SJ, Fleith, M, Van Lieshout, L, Mejborn, H, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. (2017) 30:149–90. doi: 10.1017/S095442241700004X
22. LaBouyer, M, Holtrop, G, Horgan, G, Gratz, SW, Belenguer, A, Smith, N, et al. Higher total faecal short-chain fatty acid concentrations correlate with increasing proportions of butyrate and decreasing proportions of branched-chain fatty acids across multiple human studies. Gut Microb. (2022) 3:e2. doi: 10.1017/gmb.2022.1
23. Canfora, EE, Meex, RCR, Venema, K, and Blaak, EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. (2019) 15:261–73. doi: 10.1038/s41574-019-0156-z
24. Martens, EC, Lowe, EC, Chiang, H, Pudlo, NA, Wu, M, McNulty, NP, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. (2011) 9:e1001221. doi: 10.1371/journal.pbio.1001221
25. Ecklu-Mensah, G, Choo-Kang, C, Maseng, MG, Donato, S, Bovet, P, Viswanathan, B, et al. Gut microbiota and fecal short chain fatty acids differ with adiposity and country of origin: the METS-microbiome study. Nat Commun. (2023) 14:5160. doi: 10.1038/s41467-023-40874-x
26. Martin-Gallausiaux, C, Marinelli, L, Blottière, HM, Larraufie, P, and Lapaque, N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. (2021) 80:37–49. doi: 10.1017/S0029665120006916
27. Rath, S, Rud, T, Karch, A, Pieper, DH, and Vital, M. Pathogenic functions of host microbiota. Microbiome. (2018) 6:174. doi: 10.1186/s40168-018-0542-0
28. Kircher, B, Woltemate, S, Gutzki, F, Schlüter, D, Geffers, R, Bähre, H, et al. Predicting butyrate- and propionate-forming bacteria of gut microbiota from sequencing data. Gut Microbes. (2022) 14:2149019. doi: 10.1080/19490976.2022.2149019
29. Rios-Covian, D, Gueimonde, M, Duncan, SH, Flint, HJ, and De Los Reyes-Gavilan, CG. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis Cox M, editor. FEMS Microbiol Lett. (2015) 362:fnv 176. doi: 10.1093/femsle/fnv176
30. Reichardt, N, Duncan, SH, Young, P, Belenguer, A, McWilliam Leitch, C, Scott, KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. (2014) 8:1323–35. doi: 10.1038/ismej.2014.14
31. Van-Wehle, T, and Vital, M. Investigating the response of the butyrate production potential to major fibers in dietary intervention studies. NPJ Biofilms Microbiomes. (2024) 10:63. doi: 10.1038/s41522-024-00533-5
32. Pryde, SE, Duncan, SH, Hold, GL, Stewart, CS, and Flint, HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. (2002) 217:133–9. doi: 10.1111/j.1574-6968.2002.tb11467.x
33. Vital, M, Karch, A, and Pieper, DH. Colonic butyrate-producing communities in humans: an overview using omics data. mSystems. (2017) 2:e00130–17. doi: 10.1128/mSystems.00130-17
34. De Preter, V, Geboes, KP, Bulteel, V, Vandermeulen, G, Suenaert, P, Rutgeerts, P, et al. Kinetics of butyrate metabolism in the normal colon and in ulcerative colitis: the effects of substrate concentration and carnitine on the β-oxidation pathway: kinetics of butyrate metabolism in ulcerative colitis. Aliment Pharmacol Ther. (2011) 34:526–32. doi: 10.1111/j.1365-2036.2011.04757.x
35. Peng, L, Li, ZR, Green, RS, Holzmanr, IR, and Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
36. Wang, RX, Lee, JS, Campbell, EL, and Colgan, SP. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc Natl Acad Sci USA. (2020) 117:11648–57. doi: 10.1073/pnas.1917597117
37. Paone, P, and Cani, PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. (2020) 69:2232–43. doi: 10.1136/gutjnl-2020-322260
38. Sun, M, Wu, W, Liu, Z, and Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. (2017) 52:1–8. doi: 10.1007/s00535-016-1242-9
39. Hosseinkhani, F, Heinken, A, Thiele, I, Lindenburg, PW, Harms, AC, and Hankemeier, T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes. (2021) 13:1–22. doi: 10.1080/19490976.2021.1882927
40. Park, J, Kim, M, Kang, SG, Jannasch, AH, Cooper, B, Patterson, J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. (2015) 8:80–93. doi: 10.1038/mi.2014.44
41. Singh, N, Gurav, A, Sivaprakasam, S, Brady, E, Padia, R, Shi, H, et al. Activation of Gpr 109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. (2014) 40:128–39. doi: 10.1016/j.immuni.2013.12.007
42. Topping, DL, and Clifton, PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. (2001) 81:1031–64. doi: 10.1152/physrev.2001.81.3.1031
43. Martin, AM, Sun, EW, Rogers, GB, and Keating, DJ. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front Physiol. (2019) 10:428. doi: 10.3389/fphys.2019.00428
44. Lee, C, Kim, BG, Kim, JH, Chun, J, Im, JP, and Kim, JS. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int Immunopharmacol. (2017) 51:47–56. doi: 10.1016/j.intimp.2017.07.023
45. Liu, L, Li, L, Min, J, Wang, J, Wu, H, Zeng, Y, et al. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. (2012) 277:66–73. doi: 10.1016/j.cellimm.2012.05.011
46. Mussbacher, M, Salzmann, M, Brostjan, C, Hoesel, B, Schoergenhofer, C, Datler, H, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. (2019) 10:85. doi: 10.3389/fimmu.2019.00085
47. Han, K, Meadows, AM, Rodman, MJ, Russo, AC, Sharma, R, Singh, K, et al. Propionate functions as a feeding state–dependent regulatory metabolite to counter proinflammatory signaling linked to nutrient load and obesity. J Leukoc Biol. (2024) 115:738–49. doi: 10.1093/jleuko/qiae006
48. Cummings, JH, Pomare, EW, Branch, WJ, Naylor, CP, and Macfarlane, GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. (1987) 28:1221–7. doi: 10.1136/gut.28.10.1221
49. Portune, KJ, Beaumont, M, Davila, AM, Tomé, D, Blachier, F, and Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: the two sides of the coin. Trends Food Sci Technol. (2016) 57:213–32. doi: 10.1016/j.tifs.2016.08.011
50. Qi, Q, Li, J, Yu, B, Moon, JY, Chai, JC, Merino, J, et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: an integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut. (2022) 71:1095–105. doi: 10.1136/gutjnl-2021-324053
51. Russell, WR, Gratz, SW, Duncan, SH, Holtrop, G, Ince, J, Scobbie, L, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. (2011) 93:1062–72. doi: 10.3945/ajcn.110.002188
52. Bourdeau-Julien, I, Castonguay-Paradis, S, Rochefort, G, Perron, J, Lamarche, B, Flamand, N, et al. The diet rapidly and differentially affects the gut microbiota and host lipid mediators in a healthy population. Microbiome. (2023) 11:26. doi: 10.1186/s40168-023-01469-2
53. Pedersen, HK, Gudmundsdottir, V, Nielsen, HB, Hyotylainen, T, Nielsen, T, Jensen, BAH, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. (2016) 535:376–81. doi: 10.1038/nature18646
54. Ren, YM, Zhuang, ZY, Xie, YH, Yang, PJ, Xia, TX, Xie, YL, et al. BCAA-producing Clostridium symbiosum promotes colorectal tumorigenesis through the modulation of host cholesterol metabolism. Cell Host Microbe. (2024) 32:1519–1535.e7. doi: 10.1016/j.chom.2024.07.012
55. Salmean, YA, Segal, MS, Palii, SP, and Dahl, WJ. Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J Ren Nutr. (2015) 25:316–20. doi: 10.1053/j.jrn.2014.09.002
56. Passmore, IJ, Letertre, MPM, Preston, MD, Bianconi, I, Harrison, MA, Nasher, F, et al. Para-cresol production by Clostridium difficile affects microbial diversity and membrane integrity of gram-negative bacteria. PLoS Pathog. (2018) 14:e1007191. doi: 10.1371/journal.ppat.1007191
57. Sinha, AK, Laursen, MF, Brinck, JE, Rybtke, ML, Hjørne, AP, Procházková, N, et al. Dietary fibre directs microbial tryptophan metabolism via metabolic interactions in the gut microbiota. Nat Microbiol. (2024) 9:1964–78. doi: 10.1038/s41564-024-01737-3
58. Wikoff, WR, Anfora, AT, Liu, J, Schultz, PG, Lesley, SA, Peters, EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. (2009) 106:3698–703. doi: 10.1073/pnas.0812874106
59. Dodd, D, Spitzer, MH, Van Treuren, W, Merrill, BD, Hryckowian, AJ, Higginbottom, SK, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. (2017) 551:648–52. doi: 10.1038/nature24661
60. Elsden, SR, Hilton, MG, and Waller, JM. The end products of the metabolism of aromatic amino acids by clostridia. Arch Microbiol. (1976) 107:283–8. doi: 10.1007/BF00425340
61. Tuomainen, M, Lindström, J, Lehtonen, M, Auriola, S, Pihlajamäki, J, Peltonen, M, et al. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr Diabetes. (2018) 8:35. doi: 10.1038/s41387-018-0046-9
62. Peron, G, Meroño, T, Gargari, G, Hidalgo-Liberona, N, Miñarro, A, Lozano, EV, et al. A polyphenol-rich diet increases the gut microbiota metabolite indole 3-propionic acid in older adults with preserved kidney function. Mol Nutr Food Res. (2022) 66:e2100349. doi: 10.1002/mnfr.202100349
63. Wang, YC, Koay, YC, Pan, C, Zhou, Z, Tang, W, Wilcox, J, et al. Indole-3-propionic acid protects against heart failure with preserved ejection fraction. Circ Res. (2024) 134:371–89. doi: 10.1161/CIRCRESAHA.123.322381
64. Venkatesh, M, Mukherjee, S, Wang, H, Li, H, Sun, K, Benechet, AP, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity. (2014) 41:296–310. doi: 10.1016/j.immuni.2014.06.014
65. Hu, J, Chen, J, Xu, X, Hou, Q, Ren, J, and Yan, X. Gut microbiota-derived 3-phenylpropionic acid promotes intestinal epithelial barrier function via AhR signaling. Microbiome. (2023) 11:102. doi: 10.1186/s40168-023-01551-9
66. Zhang, H, and Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. (2016) 8:33–42. doi: 10.1016/j.cofs.2016.02.002
67. Bié, J, Sepodes, B, Fernandes, PCB, and Ribeiro, MHL. Polyphenols in health and disease: gut microbiota, bioaccessibility, and bioavailability. Compounds. (2023) 3:40–72. doi: 10.3390/compounds3010005
68. Gibson, GR, Hutkins, R, Sanders, ME, Prescott, SL, Reimer, RA, Salminen, SJ, et al. Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
69. Rodríguez-Daza, MC, Pulido-Mateos, EC, Lupien-Meilleur, J, Guyonnet, D, Desjardins, Y, and Roy, D. Polyphenol-mediated gut microbiota modulation: toward prebiotics and further. Front Nutr. (2021) 8:689456. doi: 10.3389/fnut.2021.689456
70. Clifford, MN, Ludwig, IA, Pereira-Caro, G, Zeraik, L, Borges, G, Almutairi, TM, et al. Exploring and disentangling the production of potentially bioactive phenolic catabolites from dietary (poly) phenols, phenylalanine, tyrosine and catecholamines. Redox Biol. (2024) 71:103068. doi: 10.1016/j.redox.2024.103068
71. Renard, CMGC, Watrelot, AA, and Le Bourvellec, C. Interactions between polyphenols and polysaccharides: mechanisms and consequences in food processing and digestion. Trends Food Sci Technol. (2017) 60:43–51. doi: 10.1016/j.tifs.2016.10.022
72. Mena, P, Calani, L, Bruni, R, and Del Rio, D. Bioactivation of high-molecular-weight polyphenols by the gut microbiome. in: K Tuohy and D RioDel. Diet-microbe interactions in the gut. Amsterdam, Netherlands: Elsevier, pp. 73–101. (2015).
73. Zheng, S, Zhang, H, Liu, R, Huang, CL, Li, H, Deng, ZY, et al. Do short chain fatty acids and phenolic metabolites of the gut have synergistic anti-inflammatory effects? – new insights from a TNF-α-induced Caco-2 cell model. Food Res Int. (2021) 139:109833. doi: 10.1016/j.foodres.2020.109833
74. Wu, Z, Huang, S, Li, T, Li, N, Han, D, Zhang, B, et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. (2021) 9:184. doi: 10.1186/s40168-021-01115-9
75. Anhê, FF, Roy, D, Pilon, G, Dudonné, S, Matamoros, S, Varin, TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. (2015) 64:872–83. doi: 10.1136/gutjnl-2014-307142
76. He, Z, He, J, and Xie, K. KLF4 transcription factor in tumorigenesis. Cell Death Discov. (2023) 9:118. doi: 10.1038/s41420-023-01416-y
77. Iglesias-Aguirre, CE, García-Villalba, R, Beltrán, D, Frutos-Lisón, MD, Espín, JC, Tomás-Barberán, FA, et al. Gut Bacteria involved in Ellagic acid metabolism to yield human Urolithin Metabotypes revealed. J Agric Food Chem. (2023) 71:4029–35. doi: 10.1021/acs.jafc.2c08889
78. Pidgeon, R, Mitchell, S, Shamash, M, Suleiman, L, Dridi, L, Maurice, CF, et al. Diet-derived urolithin a is produced by a dehydroxylase encoded by human gut Enterocloster species. Nat Commun. (2025) 16:999. doi: 10.1038/s41467-025-56266-2
79. Ballesteros-Álvarez, J, Nguyen, W, Sivapatham, R, Rane, A, and Andersen, JK. Urolithin a reduces amyloid-beta load and improves cognitive deficits uncorrelated with plaque burden in a mouse model of Alzheimer’s disease. Geroscience. (2023) 45:1095–113. doi: 10.1007/s11357-022-00708-y
80. Ryu, D, Mouchiroud, L, Andreux, PA, Katsyuba, E, Moullan, N, Nicolet-dit-Félix, AA, et al. Urolithin a induces mitophagy and prolongs lifespan in C. Elegans and increases muscle function in rodents. Nat Med. (2016) 22:879–88. doi: 10.1038/nm.4132
81. Han, D, Wu, Y, Lu, D, Pang, J, Hu, J, Zhang, X, et al. Polyphenol-rich diet mediates interplay between macrophage-neutrophil and gut microbiota to alleviate intestinal inflammation. Cell Death Dis. (2023) 14:656. doi: 10.1038/s41419-023-06190-4
82. DiNatale, BC, Schroeder, JC, and Perdew, GH. Ah receptor antagonism inhibits constitutive and cytokine inducible IL6 production in head and neck tumor cell lines. Mol Carcinog. (2011) 50:173–83. doi: 10.1002/mc.20702
83. Lahoti, TS, John, K, Hughes, JM, Kusnadi, A, Murray, IA, Krishnegowda, G, et al. Aryl hydrocarbon receptor antagonism mitigates cytokine-mediated inflammatory signalling in primary human fibroblast-like synoviocytes. Ann Rheum Dis. (2013) 72:1708–16. doi: 10.1136/annrheumdis-2012-202639
84. Muku, GE, Murray, IA, Espín, JC, and Perdew, GH. Urolithin a is a dietary microbiota-derived human aryl hydrocarbon receptor antagonist. Meta. (2018) 8:86. doi: 10.3390/metabo8040086
85. D’Amico, D, Andreux, PA, Valdés, P, Singh, A, Rinsch, C, and Auwerx, J. Impact of the natural compound Urolithin a on health, disease, and aging. Trends Mol Med. (2021) 27:687–99. doi: 10.1016/j.molmed.2021.04.009
86. Zelicha, H, Kloting, N, Kaplan, A, Yaskolka Meir, A, Rinott, E, Tsaban, G, et al. The effect of high-polyphenol Mediterranean diet on visceral adiposity: the DIRECT PLUS randomized controlled trial. BMC Med. (2022) 20:327. doi: 10.1186/s12916-022-02525-8
87. Liu, S, D’Amico, D, Shankland, E, Bhayana, S, Garcia, JM, Aebischer, P, et al. Effect of urolithin a supplementation on muscle endurance and mitochondrial health in older adults: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2144279. doi: 10.1001/jamanetworkopen.2021.44279
88. Prymont-Przyminska, A, Zwolinska, A, Sarniak, A, Wlodarczyk, A, Krol, M, Nowak, M, et al. Consumption of strawberries on a daily basis increases the non-urate 2, 2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity of fasting plasma in healthy subjects. J Clin Biochem Nutr. (2014) 55:48–55. doi: 10.3164/jcbn.13-93
89. Pallister, T, Jackson, MA, Martin, TC, Zierer, J, Jennings, A, Mohney, RP, et al. Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci Rep. (2017) 7:13670. doi: 10.1038/s41598-017-13722-4
90. Pruss, KM, Chen, H, Liu, Y, Van Treuren, W, Higginbottom, SK, Jarman, JB, et al. Host-microbe co-metabolism via MCAD generates circulating metabolites including hippuric acid. Nat Commun. (2023) 14:512. doi: 10.1038/s41467-023-36138-3
91. Pallister, T, Jackson, MA, Martin, TC, Glastonbury, CA, Jennings, A, Beaumont, M, et al. Untangling the relationship between diet and visceral fat mass through blood metabolomics and gut microbiome profiling. Int J Obes. (2017) 41:1106–13. doi: 10.1038/ijo.2017.70
92. Yu, Z, Cheng, C, Liu, Y, Liu, N, Lo, EH, and Wang, X. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. (2018) 9:945. doi: 10.1038/s41419-018-1007-x
93. González-Aguilar, GA, Blancas-Benítez, FJ, and Sáyago-Ayerdi, SG. Polyphenols associated with dietary fibers in plant foods: molecular interactions and bioaccessibility. Curr Opin Food Sci. (2017) 13:84–8. doi: 10.1016/j.cofs.2017.03.004
94. Otegui, MS. Imaging polyphenolic compounds in plant tissues In: JD Reed, VAP Freitas, and S Quideau, editors. Recent advances in polyphenol research. 1st ed. Hoboken, NJ: Wiley (2021). 281–95.
95. Saura-Calixto, F. Antioxidant dietary fiber product: a new concept and a potential food ingredient. J Agric Food Chem. (1998) 46:4303–6. doi: 10.1021/jf9803841
96. Collins, HM, Burton, RA, Topping, DL, Liao, M, Bacic, A, and Fincher, GB. Review: variability in fine structures of noncellulosic cell wall polysaccharides from cereal grains: potential importance in human health and nutrition. Cereal Chem. (2010) 87:272–82. doi: 10.1094/CCHEM-87-4-0272
97. Izydorczyk, MS, and Biliaderis, CG. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr Polym. (1995) 28:33–48. doi: 10.1016/0144-8617(95)00077-1
98. Parker, ML, Ng, A, and Waldron, KW. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum L. cv. Avalon) grains. J Sci Food Agric. (2005) 85:2539–47. doi: 10.1002/jsfa.2304
99. Lillioja, S, Neal, AL, Tapsell, L, and Jacobs, DR. Whole grains, type 2 diabetes, coronary heart disease, and hypertension: links to the aleurone preferred over indigestible fiber. Biofactors. (2013) 39:242–58. doi: 10.1002/biof.1077
100. Pereira, GV, Abdel-Hamid, AM, Dutta, S, D’Alessandro-Gabazza, CN, Wefers, D, Farris, JA, et al. Degradation of complex arabinoxylans by human colonic Bacteroidetes. Nat Commun. (2021) 12:459. doi: 10.1038/s41467-020-20737-5
101. Holmberg, SM, Feeney, RH, Prasoodanan, PKV, Puértolas-Balint, F, Singh, DK, Wongkuna, S, et al. The gut commensal Blautia maintains colonic mucus function under low-fiber consumption through secretion of short-chain fatty acids. Nat Commun. (2024) 15:3502. doi: 10.1038/s41467-024-47594-w
102. Mihaylova, MM, Cheng, CW, Cao, AQ, Tripathi, S, Mana, MD, Bauer-Rowe, KE, et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell. (2018) 22:769–778.e4. doi: 10.1016/j.stem.2018.04.001
103. Parker, A, Romano, S, Ansorge, R, Aboelnour, A, Le Gall, G, Savva, GM, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. (2022) 10:68. doi: 10.1186/s40168-022-01243-w
104. Hooper, LV, Littman, DR, and Macpherson, AJ. Interactions between the microbiota and the immune system. Science. (2012) 336:1268–73. doi: 10.1126/science.1223490
105. Buckley, A, and Turner, JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. (2018) 10:a029314. doi: 10.1101/cshperspect.a029314
106. Tajik, N, Frech, M, Schulz, O, Schälter, F, Lucas, S, Azizov, V, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. (2020) 11:1995. doi: 10.1038/s41467-020-15831-7
107. Karim, A, Muhammad, T, Ustrana, S, and Qaisar, R. Intestinal permeability marker zonulin as a predictor of sarcopenia in chronic obstructive pulmonary disease. Respir Med. (2021) 189:106662. doi: 10.1016/j.rmed.2021.106662
108. Qi, Y, Goel, R, Kim, S, Richards, EM, Carter, CS, Pepine, CJ, et al. Intestinal permeability biomarker Zonulin is elevated in healthy aging. J Am Med Dir Assoc. (2017) 18:810.e1–4. doi: 10.1016/j.jamda.2017.05.018
109. Boschetti, E, Caio, G, Cervellati, C, Costanzini, A, Rosta, V, Caputo, F, et al. Serum zonulin levels are increased in Alzheimer’s disease but not in vascular dementia. Aging Clin Exp Res. (2023) 35:1835–43. doi: 10.1007/s40520-023-02463-2
110. Chassaing, B, Raja, SM, Lewis, JD, Srinivasan, S, and Gewirtz, AT. Colonic microbiota encroachment correlates with Dysglycemia in humans. Cell Mol Gastroenterol Hepatol. (2017) 4:205–21. doi: 10.1016/j.jcmgh.2017.04.001
111. Mörkl, S, Lackner, S, Meinitzer, A, Mangge, H, Lehofer, M, Halwachs, B, et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur J Nutr. (2018) 57:2985–97. doi: 10.1007/s00394-018-1784-0
112. Mokkala, K, Röytiö, H, Munukka, E, Pietilä, S, Ekblad, U, Rönnemaa, T, et al. Gut microbiota richness and composition and dietary intake of overweight pregnant women are related to serum Zonulin concentration, a marker for intestinal permeability. J Nutr. (2016) 146:1694–700. doi: 10.3945/jn.116.235358
113. Desai, MS, Seekatz, AM, Koropatkin, NM, Kamada, N, Hickey, CA, Wolter, M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. (2016) 167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043
114. Sonnenburg, ED, Smits, SA, Tikhonov, M, Higginbottom, SK, Wingreen, NS, and Sonnenburg, JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. (2016) 529:212–5. doi: 10.1038/nature16504
115. Royer, CJ, Rodriguez-Marino, N, Yaceczko, MD, Rivera-Rodriguez, DE, Ziegler, TR, and Cervantes-Barragan, L. Low dietary fiber intake impairs small intestinal Th17 and intraepithelial T cell development over generations. Cell Rep. (2023) 42:113140. doi: 10.1016/j.celrep.2023.113140
116. Parrish, A, Boudaud, M, Grant, ET, Willieme, S, Neumann, M, Wolter, M, et al. Akkermansia muciniphila exacerbates food allergy in fibre-deprived mice. Nat Microbiol. (2023) 8:1863–79. doi: 10.1038/s41564-023-01464-1
117. Spencer, CN, McQuade, JL, Gopalakrishnan, V, McCulloch, JA, Vetizou, M, Cogdill, AP, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. (2021) 374:1632–40. doi: 10.1126/science.aaz7015
118. Derosa, L, Routy, B, Thomas, AM, Iebba, V, Zalcman, G, Friard, S, et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. (2022) 28:315–24. doi: 10.1038/s41591-021-01655-5
119. Wilmanski, T, Rappaport, N, Earls, JC, Magis, AT, Manor, O, Lovejoy, J, et al. Blood metabolome predicts gut microbiome α-diversity in humans. Nat Biotechnol. (2019) 37:1217–28. doi: 10.1038/s41587-019-0233-9
120. Sonnenburg, ED, and Sonnenburg, JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. (2014) 20:779–86. doi: 10.1016/j.cmet.2014.07.003
121. Von Schwartzenberg, RJ, Bisanz, JE, Lyalina, S, Spanogiannopoulos, P, Ang, QY, Cai, J, et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature. (2021) 595:272–7. doi: 10.1038/s41586-021-03663-4
122. Peron, G, Gargari, G, Meroño, T, Miñarro, A, Lozano, EV, Escuder, PC, et al. Crosstalk among intestinal barrier, gut microbiota and serum metabolome after a polyphenol-rich diet in older subjects with “leaky gut”: the MaPLE trial. Clin Nutr. (2021) 40:5288–97. doi: 10.1016/j.clnu.2021.08.027
123. Del Bo, C, Bernardi, S, Cherubini, A, Porrini, M, Gargari, G, Hidalgo-Liberona, N, et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: the MaPLE randomised controlled trial. Clin Nutr. (2021) 40:3006–18. doi: 10.1016/j.clnu.2020.12.014
124. Marino, M, Del Bo, C, Martini, D, Perna, S, Porrini, M, Cherubini, A, et al. A (poly)phenol-rich diet reduces serum and faecal calprotectin in older adults with increased intestinal permeability: the MaPLE randomised controlled trial. BMC Geriatr. (2024) 24:707. doi: 10.1186/s12877-024-05272-y
125. Petersen, KS, Anderson, S, Chen See, JR, Leister, J, Kris-Etherton, PM, and Lamendella, R. Herbs and spices modulate gut bacterial composition in adults at risk for CVD: results of a prespecified exploratory analysis from a randomized, crossover, controlled-feeding study. J Nutr. (2022) 152:2461–70. doi: 10.1093/jn/nxac201
126. Petersen, KS, Davis, KM, Rogers, CJ, Proctor, DN, West, SG, and Kris-Etherton, PM. Herbs and spices at a relatively high culinary dosage improves 24-hour ambulatory blood pressure in adults at risk of cardiometabolic diseases: a randomized, crossover, controlled-feeding study. Am J Clin Nutr. (2021) 114:1936–48. doi: 10.1093/ajcn/nqab291
127. Oh, ES, Petersen, KS, Kris-Etherton, PM, and Rogers, CJ. Four weeks of spice consumption lowers plasma proinflammatory cytokines and alters the function of monocytes in adults at risk of cardiometabolic disease: secondary outcome analysis in a 3-period, randomized, crossover, controlled feeding trial. Am J Clin Nutr. (2022) 115:61–72. doi: 10.1093/ajcn/nqab331
128. Khine, WWT, Haldar, S, De Loi, S, and Lee, YK. A single serving of mixed spices alters gut microflora composition: a dose–response randomised trial. Sci Rep. (2021) 11:11264. doi: 10.1038/s41598-021-90453-7
129. Möller, L, Wellmann, I, Stang, A, and Kajüter, H. The epidemiology of colorectal cancer in younger and older patients. Dtsch Arztebl Int. (2023) 120:277–83. doi: 10.3238/arztebl.m2023.0041
130. Vuik, FE, Nieuwenburg, SA, Bardou, M, Lansdorp-Vogelaar, I, Dinis-Ribeiro, M, Bento, MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. (2019) 68:1820–6. doi: 10.1136/gutjnl-2018-317592
131. Zhang, X, Irajizad, E, Hoffman, KL, Fahrmann, JF, Li, F, Seo, YD, et al. Modulating a prebiotic food source influences inflammation and immune-regulating gut microbes and metabolites: insights from the BE GONE trial. EBioMedicine. (2023) 98:104873. doi: 10.1016/j.ebiom.2023.104873
132. Zhang, N, Wang, X, Feng, M, Li, M, Wang, J, Yang, H, et al. Early-life exercise induces immunometabolic epigenetic modification enhancing anti-inflammatory immunity in middle-aged male mice. Nat Commun. (2024) 15:3103. doi: 10.1038/s41467-024-47458-3
133. Membrez, M, Migliavacca, E, Christen, S, Yaku, K, Trieu, J, Lee, AK, et al. Trigonelline is an NAD+ precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nat Metab. (2024) 6:433–47. doi: 10.1038/s42255-024-00997-x
134. Gadaleta, RM, and Moschetta, A. Metabolic messengers: fibroblast growth factor 15/19. Nat Metab. (2019) 1:588–94. doi: 10.1038/s42255-019-0074-3
135. Wu, X, Tjahyo, AS, Volchanskaya, VSB, Wong, LH, Lai, X, Yong, YN, et al. A legume-enriched diet improves metabolic health in prediabetes mediated through gut microbiome: a randomized controlled trial. Nat Commun. (2025) 16:942. doi: 10.1038/s41467-025-56084-6
136. Liedike, B, Khatib, M, Tabarsi, B, Harris, M, Wilson, SL, Ortega-Santos, CP, et al. Evaluating the effects of corn flour product consumption on Cardiometabolic outcomes and the gut microbiota in adults with elevated cholesterol: a randomized crossover. J Nutr. (2024) 154:2437–47. doi: 10.1016/j.tjnut.2024.06.003
137. Roager, HM, Vogt, JK, Kristensen, M, Hansen, LBS, Ibrügger, S, Mærkedahl, RB, et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. (2019) 68:83–93. doi: 10.1136/gutjnl-2017-314786
138. Brandl, B, Rennekamp, R, Reitmeier, S, Pietrynik, K, Dirndorfer, S, Haller, D, et al. Offering Fiber-enriched foods increases Fiber intake in adults with or without Cardiometabolic risk: a randomized controlled trial. Front Nutr. (2022) 9:816299. doi: 10.3389/fnut.2022.816299
139. Barone Lumaga, R, Tagliamonte, S, De Rosa, T, Valentino, V, Ercolini, D, and Vitaglione, P. Consumption of a sourdough-leavened croissant enriched with a blend of fibers influences fasting blood glucose in a randomized controlled trial in healthy subjects. J Nutr. (2024) 154:2976–87. doi: 10.1016/j.tjnut.2024.08.015
140. Les, F, Arbonés-Mainar, JM, Valero, MS, and López, V. Pomegranate polyphenols and urolithin a inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J Ethnopharmacol. (2018) 220:67–74. doi: 10.1016/j.jep.2018.03.029
141. Drucker, DJ, and Nauck, MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. (2006) 368:1696–705. doi: 10.1016/S0140-6736(06)69705-5
142. Liu, H, Birk, JW, Provatas, AA, Vaziri, H, Fan, N, Rosenberg, DW, et al. Correlation between intestinal microbiota and urolithin metabolism in a human walnut dietary intervention. BMC Microbiol. (2024) 24:476. doi: 10.1186/s12866-024-03626-5
143. Dhillon, J, Li, Z, and Ortiz, RM. Almond snacking for 8 wk increases alpha-diversity of the gastrointestinal microbiome and decreases Bacteroides fragilis abundance compared with an isocaloric snack in college freshmen. Curr Dev Nutr. (2019) 3:nzz 079. doi: 10.1093/cdn/nzz079
144. D, MUPR, Paula Silva Caldas, A, Silva AC, SE, Bressan, J, and Hermana Miranda Hermsdorff, H. Nut-enriched energy restricted diet has potential to decrease hunger in women at cardiometabolic risk: a randomized controlled trial (Brazilian nuts study). Nutr Res. (2023) 109:35–46. doi: 10.1016/j.nutres.2022.11.003
145. Kelly Souza Silveira, B, Mayumi Usuda Prado Rocha, D, Stampini Duarte Martino, H, Grancieri, M, Juste Contin Gomes, M, Cuquetto Mantovani, H, et al. Daily cashew and Brazil nut consumption modifies intestinal health in overweight women on energy-restricted intervention: a randomized controlled trial (Brazilian nuts study). J Nutr. (2024) 154:962–77. doi: 10.1016/j.tjnut.2023.12.022
146. Kelly Souza Silveira, B, Silva, AD, Mayumi Usuda Prado Rocha, D, Waskow, K, Stampini Duarte Martino, H, Bressan, J, et al. Brazil nut (Bertholletia excelsa H.B.K.) consumption in energy-restricted intervention decreases Proinflammatory markers and intestinal permeability of women with overweight/obesity: a controlled trial (Brazilian nuts study). J Nutr. (2024) 154:2670–9. doi: 10.1016/j.tjnut.2024.07.016
147. Ghosh, TS, Rampelli, S, Jeffery, IB, Santoro, A, Neto, M, Capri, M, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. (2020) 69:1218–28. doi: 10.1136/gutjnl-2019-319654
148. Delgado-Lista, J, Alcala-Diaz, JF, Torres-Peña, JD, Quintana-Navarro, GM, Fuentes, F, Garcia-Rios, A, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. (2022) 399:1876–85. doi: 10.1016/S0140-6736(22)00122-2
149. Yaskolka Meir, A, Rinott, E, Tsaban, G, Zelicha, H, Kaplan, A, Rosen, P, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. (2021) 70:2085–95. doi: 10.1136/gutjnl-2020-323106
150. Yaskolka Meir, A, Keller, M, Hoffmann, A, Rinott, E, Tsaban, G, Kaplan, A, et al. The effect of polyphenols on DNA methylation-assessed biological age attenuation: the DIRECT PLUS randomized controlled trial. BMC Med. (2023) 21:364. doi: 10.1186/s12916-023-03067-3
151. Shoer, S, Shilo, S, Godneva, A, Ben-Yacov, O, Rein, M, Wolf, BC, et al. Impact of dietary interventions on pre-diabetic oral and gut microbiome, metabolites and cytokines. Nat Commun. (2023) 14:5384. doi: 10.1038/s41467-023-41042-x
Keywords: nutrition, gut microbiome, metabolites, chronic diseases, aging, healthspan, polyphenols, fiber
Citation: Meiners F, Ortega-Matienzo A, Fuellen G and Barrantes I (2025) Gut microbiome-mediated health effects of fiber and polyphenol-rich dietary interventions. Front. Nutr. 12:1647740. doi: 10.3389/fnut.2025.1647740
Edited by:
Hengyi Xu, Nanchang University, ChinaReviewed by:
Nadia Serale, National Research Council (CNR), ItalyXueyan Gu, Jiangxi Normal University, China
Copyright © 2025 Meiners, Ortega-Matienzo, Fuellen and Barrantes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Franziska Meiners, ZnJhbnppc2thLm1laW5lcnNAdW5pLXJvc3RvY2suZGU=
 Franziska Meiners
Franziska Meiners Asiri Ortega-Matienzo1
Asiri Ortega-Matienzo1 Georg Fuellen
Georg Fuellen Israel Barrantes
Israel Barrantes