- Department of Orthopedics, The First Affiliated Hospital of Soochow University, Orthopedic Institute of Soochow University, Suzhou Medical College of Soochow University, Suzhou, Jiangsu, China
Osteopenia and osteoporosis (OP) are serious public health concerns that impose substantial health and economic burdens on the global population. Lactoferrin (Lf) is a natural iron-binding glycoprotein that exhibits numerous biological functions. This review summarized the role of circulating Lf and related biomarkers in maintaining bone health. Lf may protect against OP through various mechanisms, including the osteoprotegerin/receptor activator of nuclear factor κB ligand/receptor activator of nuclear factor κB, bone morphogenetic protein signaling pathway, liver–bone axis, insulin-like growth factor 1 signaling pathway, autophagy, and gut microbiota. Moreover, the peptides derived from Lf and Lf-based nanoformulations or biomaterials show potential in preventing OP. Overall, this review supports the potential application of Lf for OP.
Introduction
Osteopenia and osteoporosis (OP) refer to conditions characterized by decreased bone mineral density (BMD); however, they differ in the severity of bone loss. Osteopenia represents a milder form of bone loss than OP. The global prevalence of osteopenia and OP is estimated to reach 40.4% and 19.7%, respectively (1), resulting in a substantial health and financial burden. OP can lead to fragile fractures, which can lead to disability and even death in older adults (2, 3).
Lactoferrin (Lf) is primarily found in milk. It is a natural iron-binding glycoprotein with a molecular weight of approximately 78 kDa and consists of >690 amino acids. Lf supplementation has been reported to be beneficial against various diseases, including obesity, type 2 diabetes, atherosclerosis, non-alcoholic liver disease, alcoholic liver disease, and some cancers (4, 5). Studies have reported the positive effect of Lf on OP. To obtain a comprehensive understanding of the association between Lf and OP, this review summarizes the protective effects and underlying mechanisms of Lf treatment in OP.
Association between Lf and bone health
Endogenous Lf is present in serum, neutrophils, and saliva. Although reference values for serum Lf levels have not been established in the general population, most studies have indicated circulating Lf concentrations of ~500 ng/ml (6–10); however, some studies have reported that Lf circulates at concentrations as low as 500 pg/ml or as high as 3,500 ng/ml (11, 12). Lf levels in biological samples are associated with several diseases, including inflammatory bowel disease (13), Alzheimer's disease, allergic rhinitis (9), and rheumatoid arthritis (11). Lf is a potential biomarker for these diseases; however, studies on the association between serum Lf and bone health are limited, and their interactions remain unclear. In a cross-sectional study, although there was no association between serum Lf concentration and BMD or N-terminal propeptide of type 1 precollagen (P1NP), a positive correlation of Lf with parathyroid hormone and β-crosslaps (β-CTx) was observed in older women (14). Specifically, circulating Lf was associated with bone resorption markers (14). Hanna et al. evaluated the saliva Lf levels in patients with OP and in healthy controls without OP (15). The results indicated that, although not statistically significant, Lf levels decreased in both unstimulated and stimulated saliva from OP patients compared with the control group (15); however, this was a preliminary analysis with no adjustments performed. The predictive value of Lf in OP still requires investigation through large-scale studies.
Studies on the effects of exogenous Lf supplementation for OP have been conducted primarily in cells or animals. Although Bharadwaj et al. found that a milk ribonuclease-enriched Lf supplement could restore the balance of bone turnover within a short period in postmenopausal women (16), the study failed to isolate Lf and report BMD. Therefore, direct evidence from clinical trials remains lacking. Low research priority and limited market attention might be two important reasons. On the one hand, more studies on OP mostly focused on bisphosphonates, denosumab, and hormonal therapy (17), and Lf was regarded as a relatively low priority in scientific resource allocation. On the other hand, Lf had a smaller market scale than medicines or other classical nutrients (e.g., 247 million for Lf in 2025 vs. 1.3 billion for vitamin D in 2022), resulting in insufficient support for clinical trials (18, 19). Moreover, cohort studies on the associations between Lf intake and BMD were also hard to conduct. Since almost all Lf intake was from milk, Lf intake inevitably coincided with increased calcium intake. Consequently, even though calcium intake could be adjusted to some extent by statistical methods, the confounding factor cannot be entirely eliminated.
Endogenous Lf is also present in breast milk, saliva, and neutrophils (4, 20). Immunohistochemical analyses have indicated that fetal osteoblasts (OBs) exhibit Lf immunoreactivity, whereas adult OBs do not (21, 22). In the fetus, Lf was detected up to the 18th week of gestation and disappeared after the 30th week (22, 23). Thus, Lf may be involved in bone growth regulation during the early phases (23) but not as an optional biomarker for OP in adults. In addition, Lf may be expressed in osteocartilagineous tumors, chondroblastomas, chondromyxoid fibromas, and osteoid osteomas but not in osteosarcomas, chondrosarcomas, ossifying fibromas, osteochondroma, and enchondromas, which may reflect a mature phenotype of these tumors (21, 23, 24).
Effects of Lf on osteoblasts and osteoclasts
Bone remodeling is tightly regulated through crosstalk between bone-forming OBs and bone-resorbing osteoclasts (OCs) (25). Compared with OCs, more in vitro studies have focused on the effects of Lf on OBs. Nagashima et al. found that human recombinant Lf promotes MC3T3-E1 cell differentiation and calcification (26). Another study indicated that Lf mediates the enhanced osteogenesis of adipose-derived stem cells (27). Mechanistically, the mitogen-activated protein kinase (MAPK) signaling pathway (28) and BCL2-Beclin1 signaling-mediated autophagy (29) participate in OB formation. In contrast, Owen et al. compared the anabolic effects of five compounds on OBs. No effect on osteogenic differentiation was observed, and even a high dose of Lf (1 mg/ml) produced an adverse effect (30). Furthermore, certain Lf-derived peptide fractions (fragment residues 624 to 632, also called LPF-C, and amino acids 97–122 from the N-terminus) also induce OB proliferation (31, 32), which warrants further investigation.
Some studies have indicated that Lf not only promotes OBs but also inhibits OC development (33–35). However, Lf does not alter bone resorption in calvarial organ culture, which suggests that Lf does not affect mature OC function (34). In other words, Lf is able to affect immature OCs but not mature ones. Lorget et al. found that Lf inhibited osteoclastogenesis and bone resorption through a mechanism independent of the osteoprotegerin/receptor activator of nuclear factor κB ligand/receptor activator of nuclear factor κB (OPG/RANKL/RANK) (36).
Many factors might lead to discrepancies in the effects of Lf on OBs or OCs, including dose, source, and intervention time. Additionally, cell type might also be an important reason. For example, Lf at the same dose could promote differentiation and calcification in MC3T3-E1 cells (26) but not in human mesenchymal progenitor-derived OBs (30). Iron saturation might further play a role, since some researchers observed that the biological effects of Lf varied with iron saturation levels in other diseases (37). Meanwhile, we should hold a cautious attitude toward the results from the cell-based studies due to the inherent limitations in their evidence hierarchy.
Effects of the Lf forms on bone
Most studies on Lf have primarily focused on the bovine or human form. Generally, they appear to exhibit comparable activity (38); however, it should be emphasized that the activities are not always interchangeable, because their modes of intestinal receptor recognition is inconsistent (39).
Structure-function relationship studies suggest that the differences are minimal for the effects of the various Lf forms on osteogenic activity (40). The iron saturation level of Lf is not a key factor affecting OB function or mitogenic activity in MC3T3-E1 cells (41). In addition, the glycosylated forms and source of Lf do not alter its mitogenic activity (42). Although Wang et al. found that the osteogenic activity of Lf decreased with increasing iron saturation (43), and Zhang et al. found that bovine Lf appears to have more proliferative capacity compared with human Lf (44), the differences may be minor. Studies on Lf in OCs are relatively insufficient, whereas its osteoclastogenic activity appears to be located in the N-lobe of recombinant Lf (38).
Potential mechanisms underlying the effects of Lf on OP
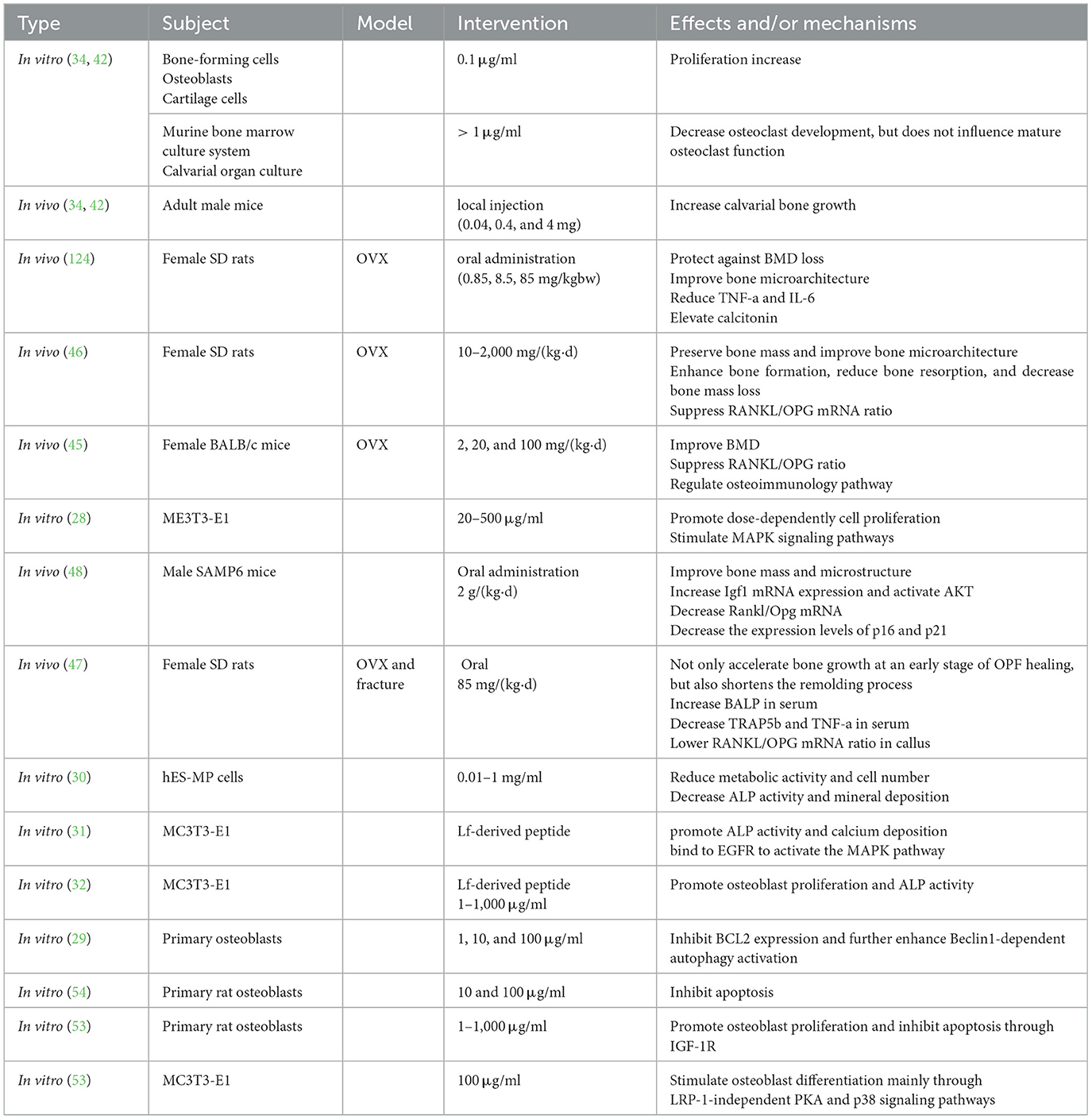
Numerous studies have examined the underlying mechanisms of Lf in OP, as summarized in Table 1. However, many conclusions remain speculative, and the exact mechanisms are poorly understood. A lack of high-quality studies also is an issue. A comprehensive exploration on the mechanisms is necessary for the applications of Lf. Emerging technologies such as spatial metabolomics and single-cell sequencing offer new opportunities for mechanism exploration. Meanwhile, although many pathways are involved in the protective effects of Lf in OP, regulation of the balance between osteoblasts and osteoclasts remains a fundamental mechanism.
OPG/RANKL/RANK signaling pathway
The imbalance between OBs and OCs is a key factor in OP pathogenesis. The OPG/RANKL/RANK system plays an important role in this process. RANKL is expressed by OBs, and it can activate its receptor (RANK) expressed on OCs to promote OC formation. Moreover, the secretory glycoprotein OPG inhibits the effects of RANKL as a decoy receptor. Thus, abnormal alterations in the RANKL/OPG ratio may increase bone resorption and decrease bone formation.
There was direct evidence from animal studies to support that Lf could protect against OP via this signaling pathway. In an estrogen-dependent bone loss model, Fan et al. reported that Lf administration increases BMD in ovariectomized (OVX) female mice, accompanied by a decrease in the RANKL/OPG ratio (45). Similar results were observed in OVX rat models (46, 47). Moreover, the lower RANKL/OPG ratio may be a result of upregulation of IFN-γ, IL-5, and IL-10 (45). Chen et al. also reported that the ameliorative effects of Lf on aging-suppressed osteogenesis through IGF-I signaling were associated with an increased OPG/RANKL ratio at the mRNA level in SAMP6 mice (48).
IGF1 signaling pathway
Insulin-like growth factor 1 (IGF1) is a major mediator of skeletal growth (49). Aging is a major risk factor for OP, and IGF1 may play a crucial role in the development of aging-related OP (50). There is also direct evidence to support the role of IGF1 in the effects of Lf on OP. Chen et al. examined the effects of Lf in a senile OP model (SAMP6 mice) and in senescent OBs (48). The results indicated that Lf could improve bone metabolism and increase Igf1 mRNA expression in vivo. Moreover, Lf improved OB proliferation in an in vitro senescence model (48). Several studies have demonstrated that aging results in oxidative stress in the body, which may contribute to senile OP (51, 52). Lf treatment could inhibit oxidative stress and delay senescence by decreasing p16 and p21 expression levels. Further, knockdown of Igf1 attenuated the effect of Lf on osteogenesis (48), which enhanced the causal inference reliability. Lf-mediated IGF1 upregulation may play a more important role in age-related OP compared with other molecules. Lf may also inhibit apoptosis to promote osteogenesis by upregulating IGF1/IGF1R in vitro (53, 54). Furthermore, knockdown of the IGF1 gene or silencing of IGF1R increases apoptosis in OBs (53, 54). Interestingly, Lf exhibited higher PI3K and RAS phosphorylation levels in IGF1R-silenced OBs, suggesting that Lf might activate PI3K and RAS through an IGF1R-independent pathway (53). Overall, current evidence suggested that Lf may upregulate IGF1 to influence its downstream pathway and directly activate IGF1.
Autophagy
Autophagy is an evolutionarily conserved intracellular “self-eating” process that contributes to the onset and progression of osteopenia and OP. Aging, estrogen deficiency, and high-fat diets can trigger the adipogenic differentiation of mesenchymal stem cells (MSCs) and BMD reduction (55). In addition, the activation of autophagy is correlated with the osteogenic differentiation of MSCs (56). Estrogen can inhibit apoptosis induced by serum deprivation of osteoblasts, which may be partly achieved by promoting autophagy (57). Autophagy also plays a role in signaling pathways, which is significant to osteogenesis. For example, autophagy upregulation is considered one reason for the IGF1-simulated osteogenic differentiation of osteoblasts (58). Direct evidence indicates that Lf can inhibit B-cell lymphoma 2 (BCL2) expression in osteoblasts, further enhancing Beclin1-dependent autophagy activation, which may positively influence osteoblast formation (29). To further investigate the role of BLC2 in Lf-promoted autophagy in OBs, the researchers upregulated BCL2 expression, and it reversed the Lf-induced autophagy promotion. A similar phenomenon also occurred after Beclin1 silencing (29). Regrettably, all data were obtained from in vitro studies. This gap necessitates future validation through well-designed animal experiments.
Bone morphogenetic protein signaling pathway
Bone morphogenetic proteins (BMPs) are cytokines belonging to the transforming growth factor-β (TGF-β) superfamily (59). In particular, BMP2 is considered the gold standard for bone regeneration (60), and it is an osteogenic factor approved by the FDA for clinical use (61). Mouse models generated by suppressing BMP signaling in OBs exhibit osteopenia phenotypes (62–64), further demonstrating the osteogenic role of BMPs in promoting OB differentiation. However, hyperactivated BMP signaling is a risk factor for heterotopic ossification, which is a major side effect of BMP treatment (65). At the molecular level, although the mechanisms were not fully elucidated, there are several potential pathways for promoting osteogenesis by BMPs (59): (1) positively regulating Runx2; (2) crosstalk between BMP and WNT signaling; (3) inducing the expression of osteogenesis-related transcription factors; and (4) positively regulating mammalian target of rapamycin (mTOR) activity. The low-density lipoprotein receptor-related protein (LRP) may also be a receptor for both WNT and Lf (66, 67). BMP signaling appears to have dual effects on bone formation, manifested as antagonizing osteogenesis in OB progenitors, negatively regulating mineralization, and collagen maturation (68–70). This may be the result of WNT antagonist expression induced by the BMP receptor and reduced β-catenin activation (71, 72). Li et al. hypothesized that BMP antagonizes bone formation by inhibiting WNT/β-catenin signaling (59). Alternatively, BMP signaling may promote OC differentiation (59). Nonetheless, the sophisticated interactions between BMPs and WNT/β-catenin and the relationships of BMPs to OB-OC coupling warrant further exploration.
The direct effects of Lf on BMPs were not reported; however, indirect evidence suggests regulatory effects of Lf on the BMP signaling pathway. For example, Lf hydrolysate from the N-lobe promoted OB differentiation in a BMP-dependent manner in vitro, and it could promote osteogenic effects through increasing BMP2 production in OVX rats (73). However, it has not been confirmed that the Lf segment can be generated in the digestive tracts and absorbed into the blood. It remains unknown whether orally administered Lf can exert its bioactivity in this manner.
Liver–bone axis
The liver is the central metabolic organ of the body, and it plays an important role in bone homeostasis. Approximately 40% of individuals with OP have other chronic conditions, including chronic liver injury. The physical distance between the liver and bone limits their direct interaction; however, the liver can communicate the bone by secreting signaling molecules. Lu et al. reported that dysregulation of the liver–bone axis promoted the progression of hepatic osteodystrophy (74). In the liver–bone axis, hepatokine lecithin-cholesterol acyltransferase (LCAT) promotes reverse cholesterol transport from the bone to the liver, whereas its loss may exacerbate the bone loss phenotype. Many studies have described the protective effects of Lf against liver injury (75), and our studies have also shown that Lf can prevent ethanol-induced liver injury in mice (76–78). Although no direct evidence has been established, the present data suggest that the liver–bone axis may be a mechanism for the protection of Lf against OP.
Gut microbiota
Although there was no direct evidence to support that the effects of Lf on OP depend on the gut microbiota, interactions between the gut microbiota and OP have recently been a subject of interest for researchers (79, 80). Gut microbiota dysbiosis has been observed in patients with OP (81–83). However, studies using germ-free or antibiotic-treated mice have produced conflicting results regarding the effects of gut microbiota on bone (84–87). The results were questioned by some scholars, who argued that data from germ-free animals may not be applicable to individuals with normal gut microbiota, and that the unintended effects of antibiotics could not be avoided (80). Understanding the complex association between gut microbiota and OP remains challenging. Moreover, fecal microbiota transplantation is not considered an effective option for the treatment of OP because of the harmful bacteria present in the transplant material (80). Therefore, supplementation with one or several probiotics may be a feasible strategy. Lactobacillus and Bifidobacteria are two conventional probiotics, and several studies have confirmed their beneficial effects on bone remodeling (88–93). In addition, Akkermansia, as a representative of “next-generation probiotics,” also exhibited a positive effect on BMD (94, 95). However, these findings are mainly derived from preclinical studies. Nilsson et al. conducted a well-designed trial to assess the effects of Lactobacillus on bone loss (88). However, this trial included only older women, thus limiting the generalizability to the general population. Moreover, the small sample size also limited the reliability of the findings.
Theoretically, a “Lf-gut microbiota-metabolites-bone” regulatory axis may exist. The aforementioned three bacteria are closely associated with Lf supplementation. An increased abundance of Lactobacillus has been observed in individuals treated with Lf (96). The growth-promoting effects of Lf on Bifidobacteria have also been reported (97). Interestingly, the pepsin hydrolysate of bovine Lf showed stronger bifidogenic effects than natural bovine Lf on some strains of Bifidobacteria (98). Thus, Lf peptides may represent the active bifidogenic form of Lf (97). Furthermore, bifidogenic effects may be achieved by Lf-binding proteins localized at the poles of bifidobacterial cells (99). Furthermore, oral Lf may increase Akkermansia abundance in the gut microbiota (100). This was also confirmed in our experiments (76, 78). The regulation of gut microbiota on bone was likely mediated through their metabolites, of which short-chain fatty acids (SCFAs) were widely recognized as an important candidate (101). Among SCFAs, propionate can only be generated by a few specific bacterial strains (102). Coincidentally, Akkermansia can generate propionate (103), which might be a mechanistic reason for the effects of Lf on OP. While germ-free or antibiotic-treated mice may not represent optimal models, they remain an appropriate choice to verify the relationship among Lf, OP, and gut microbiota. Although the crosstalk between gut microbiota and bone has been reported (80, 101), the influence of Lf on OP through gut microbiota remains theoretical and requires further evidence.
Lf peptides
Lf is nearly completely degraded in the stomach; however, some fragments resist further digestion (104, 105). Therefore, the biological activity of Lf may depend on its peptide fragments. Lactoferricin (Lfcin) and lactoferrampin (Lfampin) are two Lf fragments of interest that exhibit antimicrobial effects (106). Moreover, the anticancer and immunomodulatory effects of Lfcin have been reported (107, 108). Whether Lfcin or Lfampin influences bone metabolism has not yet been established.
LFP-C (FKSETKNLL) is a peptide from bovine Lf hydrolysates generated through pepsin digestion. Its osteogenesis activity was demonstrated in vitro (31). Molecular docking suggested that the osteogenesis of LFP-C may result from its binding to the key domain (Lys13-Thr15-Gln16-Leu17-Gly18-Asp22) of the epidermal growth factor receptor (EGFR), which activates the MAPK pathway; however, biological validation has not been performed (31).
The LP2 peptide (RKVRGPPVSCIKRDSPIQ) from human Lf has self-assembly properties and skeletal bioavailability. LP2 stimulates OB differentiation through a BMP-dependent mechanism and osteoblastic production of OPG. Moreover, the subcutaneous administration of LP2 accelerates bone healing and bone formation in vivo (73); however, the underlying molecular mechanisms were not examined. Because of the low resistance of Lf to digestion, Lf peptides may exhibit a higher simulation effect than Lf, particularly in vitro. Additionally, the active form of Lf may be its digestive hydrolysate rather than the intact molecule. Therefore, using intact Lf for in vitro experiments may not fully simulate the real in vivo effects, and the peptides may have higher research value for exploring the underlying mechanisms of Lf.
Optimal Lf doses for humans
The main source of Lf is milk in the daily diet. In milk, the Lf contents mainly concentrate on 0.1~0.2 mg/ml (109, 110). Thus, an adult can ingest 50-100 mg per day through a regular diet. In a rat study, oral administration of Lf at 2 g/kgBW/d (equivalent to 20 g/d for an adult) for 13 weeks did not produce adverse effects (111). Another study also found that a daily intake of up to 9 g Lf is safe for humans (112). Due to its proven safety, Lf has been approved to be added to infant formula in many countries (113), and FAO and WHO recommend the level of Lf supplementation is 500 mg/kg in infant formula (114). Moreover, for adults, an expert consensus indicated that the recommended daily supplementation of Lf is 200–600 mg (115). It should be noted that these doses are intended for the general population. To date, there is still no evidence-based recommended Lf dose for OP prevention and treatment. Although some studies indicated that excessive doses of Lf may have potential negative effects (78, 116), this concern is likely of limited practical relevance. Due to the high cost of Lf supplements, excessive intake is virtually impossible in the real world.
Clinical applicability of Lf
So far, most evidence for the beneficial effects of Lf on bone metabolism derives from preclinical studies, and the clinical trials are markedly lacking. Although no clinical trial has directly focused on Lf and OP, one randomized controlled trial investigated milk ribonuclease-enriched Lf on bone turnover markers. In this trial, milk ribonuclease-enriched Lf supplementation displayed positive effects on serum bone turnover biomarkers in postmenopausal women aged 40–60 years (16). However, it cannot be confirmed that the changes were attributed to Lf; meanwhile, the study was performed in a special population, and BMD was not determined, which might limit its generalizability and reliability. Despite these limitations, Lf is still remains a promising agent for OP protection due to its broad biological activities and high safety (5, 117). In the future, large-scale randomized controlled trials are required to validate the efficacy of Lf in OP. Of course, Lf, as a natural food component, is not as therapeutically effective as conventional pharmaceuticals. Lf may serve as a preventive agent or adjunctive therapy rather than a primary therapeutic agent in clinical practices.
Lf-based nanoformulations or biomaterials
Due to the poor oral bioavailability of Lf, some groups have developed different formulations for bone health. Lf-embedded type 1 collagen membranes retain their pro-calcification effects during osteogenic differentiation in vitro (118). Injectable scaffolds are also considered efficient Lf delivery systems. Kim et al. reported that Lf-loaded porous polymicrospheres promote osteogenic differentiation by controlling Lf release (119). Liposomes are another important delivery system (120, 121). Recently, these topics have been thoroughly reviewed by other scholars (122, 123) and are beyond the scope of this review. Therefore, we have not discussed them in detail in this review.
Conclusion and future outlook
Several mechanisms have been reported to explain the effects of Lf on OP (Figure 1); however, these mechanisms are primarily derived from cell or animal experiments. The lack of clinical evidence remains the largest pain point for the applications of Lf. It is valuable to conduct a randomized, placebo-controlled, double-blinded trial to assess the efficacy of Lf. In this trial, BMD is a more valuable outcome besides serum biomarkers such as ALP, β-CTx, and TP1NP. Considering that the immediate effects of Lf (as a natural food component) may be weak, a long-term intervention is recommended. And other proteins without medical effects can be selected as a placebo. It should be noted that we need to maintain a “cautiously” optimistic attitude until the effectiveness of Lf on OP is confirmed. Moreover, the mechanisms through which lactoferrin regulates these pathways remain a “black box,” and deconstructing this box is a research topic that warrants further exploration.
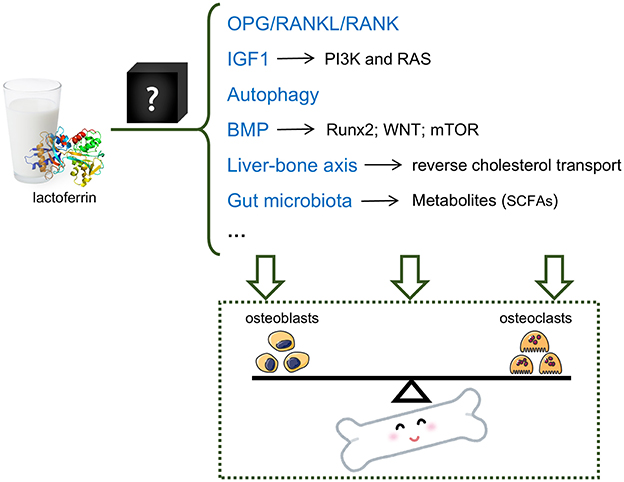
Figure 1. Potential mechanisms of the OP-protective effect of lactoferrin. Lactoferrin may regulate the balance between osteoblasts and osteoclasts via multiple mechanisms, including but not limiting OPG/RANKL/RANK pathway, IGF1 pathway, autophagy, BMP pathway, liver-bone axis and gut microbiota.
Author contributions
DL: Writing – original draft. MG: Writing – original draft. ML: Writing – original draft, Investigation. HZ: Writing – original draft, Investigation. XZ: Writing – original draft, Supervision. QG: Supervision, Writing – original draft. HY: Writing – review & editing, Funding acquisition. QS: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from National Key R&D Program of China, MOST (2023YFC2509900), National Nature Science Foundation of China (82404246, 82172485), the Orthopedic Medical Innovation Center of Jiangsu (CXZX202209), Key Laboratory of Orthopedics of Suzhou (SZS2022017), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Acknowledgments
The authors thank Scientific Compass (https://www.shiyanjia.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xiao PL, Cui AY, Hsu CJ, Peng R, Jiang N, Xu XH, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. (2022) 33:2137–53. doi: 10.1007/s00198-022-06454-3
2. Mousavibaygei SR, Bisadi A, ZareSakhvidi F. Outdoor air pollution exposure, bone mineral density, osteoporosis, and osteoporotic fractures: a systematic review and meta-analysis. Sci Total Environ. (2023) 865:161117. doi: 10.1016/j.scitotenv.2022.161117
3. Afarideh M, Sartori-Valinotti JC, Tollefson MM. Association of sun-protective behaviors with bone mineral density and osteoporotic bone fractures in US adults. JAMA Dermatol. (2021) 157:1437–46. doi: 10.1001/jamadermatol.2021.4143
4. Wang B, Timilsena YP, Blanch E, Adhikari B. Lactoferrin: structure, function, denaturation and digestion. Crit Rev Food Sci Nutr. (2019) 59:580–96. doi: 10.1080/10408398.2017.1381583
5. Demir R, Saritas S, Bechelany M, Karav S. Lactoferrin: properties and potential uses in the food industry. Int J Mol Sci. (2025) 26:1404. doi: 10.3390/ijms26041404
6. Moreno-Navarrete JM, Ortega FJ, Bassols J, Castro A, Ricart W, Fernandez-Real JM. Association of circulating lactoferrin concentration and 2 nonsynonymous LTF gene polymorphisms with dyslipidemia in men depends on glucose-tolerance status. Clin Chem. (2008) 54:301–9. doi: 10.1373/clinchem.2007.095943
7. Moreno-Navarrete JM, Ortega FJ, Bassols J, Ricart W, Fernandez-Real JM. Decreased circulating lactoferrin in insulin resistance and altered glucose tolerance as a possible marker of neutrophil dysfunction in type 2 diabetes. J Clin Endocrinol Metab. (2009) 94:4036–44. doi: 10.1210/jc.2009-0215
8. Fernandez-Real JM, Garcia-Fuentes E, Moreno-Navarrete JM, Murri-Pierri M, Garrido-Sanchez L, Ricart W, et al. Fat overload induces changes in circulating lactoferrin that are associated with postprandial lipemia and oxidative stress in severely obese subjects. Obesity. (2010) 18:482–8. doi: 10.1038/oby.2009.266
9. Choi GS, Shin SY, Kim JH, Lee HY, Palikhe NS, Ye YM, et al. Serum lactoferrin level as a serologic biomarker for allergic rhinitis. Clin Exp Allergy. (2010) 40:403–10. doi: 10.1111/j.1365-2222.2009.03414.x
10. Vengen IT, Dale AC, Wiseth R, Midthjell K, Videm V. Lactoferrin is a novel predictor of fatal ischemic heart disease in diabetes mellitus type 2: long-term follow-up of the HUNT 1 study. Atherosclerosis. (2010) 212:614–20. doi: 10.1016/j.atherosclerosis.2010.06.008
11. Caccavo D, Sebastiani GD, Di Monaco C, Guido F, Galeazzi M, Ferri GM, et al. Increased levels of lactoferrin in synovial fluid but not in serum from patients with rheumatoid arthritis. Int J Clin Lab Res. (1999) 29:30–5. doi: 10.1007/s005990050059
12. Santos-Silva A, Rebelo I, Castro E, Belo L, Catarino C, Monteiro I, et al. Erythrocyte damage and leukocyte activation in ischemic stroke. Clin Chim Acta. (2002) 320:29–35. doi: 10.1016/S0009-8981(02)00039-6
13. Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. (2015) 110:802–19. doi: 10.1038/ajg.2015.120
14. Chailurkit L, Kruavit A, Rajatanavin R, Ongphiphadhanakul B. The relationship of fetuin-A and lactoferrin with bone mass in elderly women. Osteoporos Int. (2011) 22:2159–64. doi: 10.1007/s00198-010-1439-3
15. Sobczak-Jaskow H, Kochanska B, Drogoszewska B. Composition and properties of saliva in patients with osteoporosis taking antiresorptive drugs. Int J Environ Res Public Health. (2023) 20:4294. doi: 10.3390/ijerph20054294
16. Bharadwaj S, Naidu AG, Betageri GV, Prasadarao NV, Naidu AS. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos Int. (2009) 20:1603–11. doi: 10.1007/s00198-009-0839-8
17. Morin SN, Feldman S, Funnell L, Giangregorio L, Kim S, McDonald-Blumer H, et al. Clinical practice guideline for management of osteoporosis and fracture prevention in Canada: 2023 update. CMAJ. (2023) 195:E1333–E48. doi: 10.1503/cmaj.221647
18. Hu C, Shen W, Xia Y, Yang H, Chen X. Lactoferrin: current situation and future prospects. Food Biosci. (2024) 62:105183. doi: 10.1016/j.fbio.2024.105183
19. MarketandMarket. Vitamin D Market: Global Forecast to 2027. (2022). Available online at: https://www.marketsandmarkets.com/Market-Reports/vitamin-d-market-22034298.html (Accessed November, 2022).
20. Okubo K, Kamiya M, Urano Y, Nishi H, Herter JM, Mayadas T, et al. Lactoferrin suppresses neutrophil extracellular traps release in inflammation. EBioMedicine. (2016) 10:204–15. doi: 10.1016/j.ebiom.2016.07.012
21. Ieni A, Barresi V, Grosso M, Rosa MA, Tuccari G. Lactoferrin immuno-expression in human normal and neoplastic bone tissue. J Bone Miner Metab. (2009) 27:364–71. doi: 10.1007/s00774-009-0044-z
22. Antonio I, Valeria B, Maddalena G, Giovanni T. Immunohistochemical evidence of lactoferrin in human embryo-fetal bone and cartilage tissues. Cell Biol Int. (2010) 34:845–9. doi: 10.1042/CBI20090358
23. Ieni A, Barresi V, Grosso M, Speciale G, Rosa MA, Tuccari G. Does lactoferrin behave as an immunohistochemical oncofetal marker in bone and cartilage human neoplasms? Pathol Oncol Res. (2011) 17:287–93. doi: 10.1007/s12253-010-9311-5
24. Ieni A, Barresi V, Grosso M, Rosa MA, Tuccari G. Immunolocalization of lactoferrin in cartilage-forming neoplasms. J Orthop Sci. (2009) 14:732–7. doi: 10.1007/s00776-009-1396-x
25. Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-osteoclast communication and bone homeostasis. Cells. (2020) 9:2073. doi: 10.3390/cells9092073
26. Nagashima D, Ishibashi Y, Kawaguchi S, Furukawa M, Toho M, Ohno M, et al. Human recombinant lactoferrin promotes differentiation and calcification on MC3T3-E1 cells. Pharmaceutics. (2022) 15:60. doi: 10.3390/pharmaceutics15010060
27. Chang Y, Ping A, Chang C, Betz VM, Cai L, Ren B. Lactoferrin mediates enhanced osteogenesis of adipose-derived stem cells: innovative molecular and cellular therapy for bone repair. Int J Mol Sci. (2023) 24:1749. doi: 10.3390/ijms24021749
28. Liu M, Fan F, Shi P, Tu M, Yu C, Yu C, et al. Lactoferrin promotes MC3T3-E1 osteoblast cells proliferation via MAPK signaling pathways. Int J Biol Macromol. (2018) 107:137–43. doi: 10.1016/j.ijbiomac.2017.08.151
29. Ke D, Wang X, Lin Y, Wei S. Lactoferrin promotes the autophagy activity during osteoblast formation via BCL2-Beclin1 signaling. Mol Biol Rep. (2022) 49:259–66. doi: 10.1007/s11033-021-06866-0
30. Owen R, Bahmaee H, Claeyssens F, Reilly GC. Comparison of the anabolic effects of reported osteogenic compounds on human mesenchymal progenitor-derived osteoblasts. Bioengineering. (2020) 7:12. doi: 10.3390/bioengineering7010012
31. Shi P, Fan F, Chen H, Xu Z, Cheng S, Lu W, et al. bovine lactoferrin-derived peptide induced osteogenesis via regulation of osteoblast proliferation and differentiation. J Dairy Sci. (2020) 103:3950–60. doi: 10.3168/jds.2019-17425
32. Wen P, Zhang W, Wang P, Zhang Y, Zhang W, Zhao Y, et al. Osteogenic effects of the peptide fraction derived from pepsin-hydrolyzed bovine lactoferrin. J Dairy Sci. (2021) 104:3853–62. doi: 10.3168/jds.2020-19138
33. Montesi M, Panseri S, Iafisco M, Adamiano A, Tampieri A. Coupling hydroxyapatite nanocrystals with lactoferrin as a promising strategy to fine regulate bone homeostasis. PLoS ONE. (2015) 10:e0132633. doi: 10.1371/journal.pone.0132633
34. Cornish J. Lactoferrin promotes bone growth. Biometals. (2004) 17:331–5. doi: 10.1023/B:BIOM.0000027713.18694.91
35. Naot D, Grey A, Reid IR, Cornish J. Lactoferrin–a novel bone growth factor. Clin Med Res. (2005) 3:93–101. doi: 10.3121/cmr.3.2.93
36. Lorget F, Clough J, Oliveira M, Daury MC, Sabokbar A, Offord E. Lactoferrin reduces in vitro osteoclast differentiation and resorbing activity. Biochem Biophys Res Commun. (2002) 296:261–6. doi: 10.1016/S0006-291X(02)00849-5
37. Zhang Z, Lu M, Chen C, Tong X, Li Y, Yang K, et al. Holo-lactoferrin: the link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics. (2021) 11:3167–82. doi: 10.7150/thno.52028
38. Naot D, Palmano K, Cornish J. Lactoferrin—A Potential Anabolic Intervention in Osteoporosis; Osteoporosis. London: InTech (2012).
39. Kawakami H, Lonnerdal B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am J Physiol. (1991) 261:G841–6. doi: 10.1152/ajpgi.1991.261.5.G841
40. Cornish J, Palmano K, Callon KE, Watson M, Lin JM, Valenti P, et al. Lactoferrin and bone; structure-activity relationships. Biochem Cell Biol. (2006) 84:297–302. doi: 10.1139/o06-057
41. Amini AA, Nair LS. Evaluation of the bioactivity of recombinant human lactoferrins toward murine osteoblast-like cells for bone tissue engineering. Tissue Eng Part A. (2013) 19:1047–55. doi: 10.1089/ten.tea.2012.0227
42. Cornish J, Callon KE, Naot D, Palmano KP, Banovic T, Bava U, et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology. (2004) 145:4366–74. doi: 10.1210/en.2003-1307
43. Wang XY, Guo HY, Zhang W, Wen PC, Zhang H, Guo ZR, et al. Effect of iron saturation level of lactoferrin on osteogenic activity in vitro and in vivo. J Dairy Sci. (2013) 96:33–9. doi: 10.3168/jds.2012-5692
44. Zhang JL, Han X, Shan YJ, Zhang LW, Du M, Liu M, et al. Effect of bovine lactoferrin and human lactoferrin on the proliferative activity of the osteoblast cell line MC3T3-E1 in vitro. J Dairy Sci. (2018) 101:1827–33. doi: 10.3168/jds.2017-13161
45. Fan F, Shi P, Liu M, Chen H, Tu M, Lu W, et al. Lactoferrin preserves bone homeostasis by regulating the RANKL/RANK/OPG pathway of osteoimmunology. Food Funct. (2018) 9:2653–60. doi: 10.1039/C8FO00303C
46. Hou JM, Xue Y, Lin QM. Bovine lactoferrin improves bone mass and microstructure in ovariectomized rats via OPG/RANKL/RANK pathway. Acta Pharmacol Sin. (2012) 33:1277–84. doi: 10.1038/aps.2012.83
47. Li W, Hu J, Ji P, Zhu S, Zhu Y. Oral administration of bovine lactoferrin accelerates the healing of fracture in ovariectomized rats. J Bone Miner Metab. (2020) 38:648–57. doi: 10.1007/s00774-020-01105-1
48. Chen XW Li YH, Zhang MJ, Chen Z, Ke DS, Xue Y, Hou JM. Lactoferrin ameliorates aging-suppressed osteogenesis via IGF1 signaling. J Mol Endocrinol. (2019) 63:63–75. doi: 10.1530/JME-19-0003
49. Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, et al. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone. (2013) 56:355–62. doi: 10.1016/j.bone.2013.06.029
50. Lindsey RC, Rundle CH, Mohan S. Role of IGF1 and EFN-EPH signaling in skeletal metabolism. J Mol Endocrinol. (2018) 61:T87–T102. doi: 10.1530/JME-17-0284
52. Almeida M, O'Brien CA. Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci. (2013) 68:1197–208. doi: 10.1093/gerona/glt079
53. Hou JM, Chen EY, Lin F, Lin QM, Xue Y, Lan XH, et al. Lactoferrin induces osteoblast growth through IGF-1R. Int J Endocrinol. (2015) 2015:282806. doi: 10.1155/2015/282806
54. Hou JM, Chen EY, Wei SC, Lin F, Lin QM, Lan XH, et al. Lactoferrin inhibits apoptosis through insulin-like growth factor I in primary rat osteoblasts. Acta Pharmacol Sin. (2014) 35:523–30. doi: 10.1038/aps.2013.173
55. Yin X, Zhou C, Li J, Liu R, Shi B, Yuan Q, et al. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. (2019) 7:28. doi: 10.1038/s41413-019-0058-7
56. Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, et al. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. (2013) 52:524–31. doi: 10.1016/j.bone.2012.10.024
57. Yang YH, Chen K, Li B, Chen JW, Zheng XF, Wang YR, et al. Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER-ERK-mTOR pathway. Apoptosis. (2013) 18:1363–75. doi: 10.1007/s10495-013-0867-x
58. Xi G, Rosen CJ, Clemmons DR, IGF-I. and IGFBP-2 stimulate AMPK activation and autophagy, which are required for osteoblast differentiation. Endocrinology. (2016) 157:268–81. doi: 10.1210/en.2015-1690
59. Wu M, Wu S, Chen W, Li YP. The roles and regulatory mechanisms of TGF-beta and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res. (2024) 34:101–23. doi: 10.1038/s41422-023-00918-9
60. Lowery JW, Rosen V. Bone morphogenetic protein-based therapeutic approaches. Cold Spring Harb Perspect Biol. (2018) 10:022327. doi: 10.1101/cshperspect.a022327
61. Zhou N, Li Q, Lin X, Hu N, Liao JY, Lin LB, et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. (2016) 366:101–11. doi: 10.1007/s00441-016-2403-0
62. Yang C, Yang L, Wan M, Cao X. Generation of a mouse model with expression of bone morphogenetic protein type II receptor lacking the cytoplasmic domain in osteoblasts. Ann N Y Acad Sci. (2010) 1192:286–91. doi: 10.1111/j.1749-6632.2009.05248.x
63. Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest. (2010) 120:2457–73. doi: 10.1172/JCI42285
64. Hu Y, Hao X, Liu C, Ren C, Wang S, Yan G, et al. Acvr1 deletion in osteoblasts impaired mandibular bone mass through compromised osteoblast differentiation and enhanced sRANKL-induced osteoclastogenesis. J Cell Physiol. (2021) 236:4580–91. doi: 10.1002/jcp.30183
65. Zhang J, Zhao Y, Hou X, Chen B, Xiao Z, Han J, et al. The inhibition effects of insulin on BMP2-induced muscle heterotopic ossification. Biomaterials. (2014) 35:9322–31. doi: 10.1016/j.biomaterials.2014.07.056
66. Grey A, Banovic T, Zhu Q, Watson M, Callon K, Palmano K, et al. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol. (2004) 18:2268–78. doi: 10.1210/me.2003-0456
67. Pawaputanon Na Mahasarakham C, Ezura Y, Kawasaki M, Smriti A, Moriya S, Yamada T, et al. BMP-2 enhances Lgr4 gene expression in osteoblastic cells. J Cell Physiol. (2016) 231:887–95. doi: 10.1002/jcp.25180
68. Lim J, Shi Y, Karner CM, Lee SY, Lee WC, He G, et al. Dual function of Bmpr1a signaling in restricting preosteoblast proliferation and stimulating osteoblast activity in mouse. Development. (2016) 143:339–47. doi: 10.1242/dev.126227
69. Zhang Y, McNerny EG, Terajima M, Raghavan M, Romanowicz G, Zhang Z, et al. Loss of BMP signaling through BMPR1A in osteoblasts leads to greater collagen cross-link maturation and material-level mechanical properties in mouse femoral trabecular compartments. Bone. (2016) 88:74–84. doi: 10.1016/j.bone.2016.04.022
70. Zhang H, Zhang Y, Terajima M, Romanowicz G, Liu Y, Omi M, et al. Loss of BMP signaling mediated by BMPR1A in osteoblasts leads to differential bone phenotypes in mice depending on anatomical location of the bones. Bone. (2020) 137:115402. doi: 10.1016/j.bone.2020.115402
71. Liu Z, Tang Y, Qiu T, Cao X, Clemens TL, A. dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. J Biol Chem. (2006) 281:17156–63. doi: 10.1074/jbc.M513812200
72. Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, et al. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res. (2010) 25:200–10. doi: 10.1359/jbmr.090806
73. Pal S, Sayeed M, Kumar A, Verma DP, Harioudh MK, Verma NK, et al. Self-assembling nano-globular peptide from human lactoferrin acts as a systemic enhancer of bone regeneration: a novel peptide for orthopedic application. ACS Appl Mater Interfaces. (2021) 13:17300–15. doi: 10.1021/acsami.1c01513
74. Lu K, Shi TS, Shen SY, Shi Y, Gao HL, Wu J, et al. Defects in a liver-bone axis contribute to hepatic osteodystrophy disease progression. Cell Metab. (2022) 34:441–57. doi: 10.1016/j.cmet.2022.02.006
75. Xiong L, Ren F, Lv J, Zhang H, Guo H. Lactoferrin attenuates high-fat diet-induced hepatic steatosis and lipid metabolic dysfunctions by suppressing hepatic lipogenesis and down-regulating inflammation in C57BL/6J mice. Food Funct. (2018) 9:4328–39. doi: 10.1039/C8FO00317C
76. Li D, He Q, Yang H, Du Y, Yu K, Yang J, et al. Daily dose of bovine lactoferrin prevents ethanol-induced liver injury and death in male mice by regulating hepatic alcohol metabolism and modulating gut microbiota. Mol Nutr Food Res. (2021) 2021:e2100253. doi: 10.1002/mnfr.202100253
77. Li D, Hu Z, He Q, Guo Y, Chong Y, Xu J, et al. Lactoferrin alleviates acute alcoholic liver injury by improving redox-stress response capacity in female C57BL/6J mice. J Agric Food Chem. (2021) 69:14856–67. doi: 10.1021/acs.jafc.1c06813
78. Li DM, Wu YX, Hu ZQ, Wang TC, Zhang LL, Zhou Y, et al. Lactoferrin prevents chronic alcoholic injury by regulating redox balance and lipid metabolism in female C57BL/6J mice. Antioxidants. (2022) 11:1508. doi: 10.3390/antiox11081508
79. Lorenzo J. From the gut to bone: connecting the gut microbiota with Th17 T lymphocytes and postmenopausal osteoporosis. J Clin Invest. (2021) 131:6619. doi: 10.1172/JCI146619
80. Lyu Z, Hu Y, Guo Y, Liu D. Modulation of bone remodeling by the gut microbiota: a new therapy for osteoporosis. Bone Res. (2023) 11:31. doi: 10.1038/s41413-023-00264-x
81. Wang H, Liu J, Wu Z, Zhao Y, Cao M, Shi B, et al. Gut microbiota signatures and fecal metabolites in postmenopausal women with osteoporosis. Gut Pathog. (2023) 15:33. doi: 10.1186/s13099-023-00553-0
82. Li C, Huang Q, Yang R, Dai Y, Zeng Y, Tao L, et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int. (2019) 30:1003–13. doi: 10.1007/s00198-019-04855-5
83. Huang D, Wang J, Zeng Y, Li Q, Wang Y. Identifying microbial signatures for patients with postmenopausal osteoporosis using gut microbiota analyses and feature selection approaches. Front Microbiol. (2023) 14:1113174. doi: 10.3389/fmicb.2023.1113174
84. Uchida Y, Irie K, Fukuhara D, Kataoka K, Hattori T, Ono M, et al. Commensal microbiota enhance both osteoclast and osteoblast activities. Molecules. (2018) 23:1517. doi: 10.3390/molecules23071517
85. Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. (2016) 351:854–7. doi: 10.1126/science.aad8588
86. Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. (2015) 6:7486. doi: 10.1038/ncomms8486
87. Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. (2014) 158:705–21. doi: 10.1016/j.cell.2014.05.052
88. Nilsson AG, Sundh D, Backhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. (2018) 284:307–17. doi: 10.1111/joim.12805
89. Guo M, Liu H, Yu Y, Zhu X, Xie H, Wei C, et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes. (2023) 15:2190304. doi: 10.1080/19490976.2023.2190304
90. Jiang X, Qi X, Xie C. Lactobacillus plantarum LP45 inhibits the RANKL/OPG signaling pathway and prevents glucocorticoid-induced osteoporosis. Food Nutr Res. (2023) 67:9064. doi: 10.29219/fnr.v67.9064
91. Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S, et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int. (2015) 2015:897639. doi: 10.1155/2015/897639
92. Roberts JL, Liu G, Darby TM, Fernandes LM, Diaz-Hernandez ME, Jones RM, et al. Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomed Pharmacother. (2020) 132:110831. doi: 10.1016/j.biopha.2020.110831
93. Wallimann A, Hildebrand M, Groeger D, Stanic B, Akdis CA, Zeiter S, et al. An exopolysaccharide produced by Bifidobacterium longum 35624(R) inhibits osteoclast formation via a TLR2-dependent mechanism. Calcif Tissue Int. (2021) 108:654–66. doi: 10.1007/s00223-020-00790-4
94. Liu JH, Yue T, Luo ZW, Cao J, Yan ZQ, Jin L, et al. Akkermansia muciniphila promotes type H vessel formation and bone fracture healing by reducing gut permeability and inflammation. Dis Model Mech. (2020) 13:043620. doi: 10.1242/dmm.043620
95. Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci. (2021) 8:2004831. doi: 10.1002/advs.202004831
96. Hu P, Zhao F, Zhu W, Wang J. Effects of early-life lactoferrin intervention on growth performance, small intestinal function and gut microbiota in suckling piglets. Food Funct. (2019) 10:5361–73. doi: 10.1039/C9FO00676A
97. Oda H, Wakabayashi H, Yamauchi K, Abe F. Lactoferrin and bifidobacteria. Biometals. (2014) 27:915–22. doi: 10.1007/s10534-014-9741-8
98. Oda H, Wakabayashi H, Yamauchi K, Sato T, Xiao JZ, Abe F, et al. Isolation of a bifidogenic peptide from the pepsin hydrolysate of bovine lactoferrin. Appl Environ Microbiol. (2013) 79:1843–9. doi: 10.1128/AEM.03343-12
99. Rahman MM, Kim WS, Ito T, Kumura H, Shimazaki K. Examination of bovine lactoferrin binding to bifidobacteria. Prikl Biokhim Mikrobiol. (2008) 44:529–32. doi: 10.1134/S0003683808050049
100. Wang S, Zhou J, Xiao D, Shu G, Gu L. Bovine lactoferrin protects dextran sulfate sodium salt mice against inflammation and impairment of colonic epithelial barrier by regulating gut microbial structure and metabolites. Front Nutr. (2021) 8:660598. doi: 10.3389/fnut.2021.660598
101. Zheng XQ, Wang DB, Jiang YR, Song CL. Gut microbiota and microbial metabolites for osteoporosis. Gut Microbes. (2025) 17:2437247. doi: 10.1080/19490976.2024.2437247
102. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. (2016) 7:189–200. doi: 10.1080/19490976.2015.1134082
103. Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. (2004) 54:1469–76. doi: 10.1099/ijs.0.02873-0
104. Takeuchi T, Jyonotsuka T, Kamemori N, Kawano G, Shimizu H, Ando K, et al. Enteric-formulated lactoferrin was more effectively transported into blood circulation from gastrointestinal tract in adult rats. Exp Physiol. (2006) 91:1033–40. doi: 10.1113/expphysiol.2006.034876
105. Kuwata H, Yip TT, Yamauchi K, Teraguchi S, Hayasawa H, Tomita M, et al. The survival of ingested lactoferrin in the gastrointestinal tract of adult mice. Biochem J. (1998) 334:321–3. doi: 10.1042/bj3340321
106. Yin C, Wong JH, Ng TB. Recent studies on the antimicrobial peptides lactoferricin and lactoferrampin. Curr Mol Med. (2014) 14:1139–54. doi: 10.2174/1566524014666141015151749
107. Rahman R, Fonseka AD, Sua SC, Ahmad M, Rajendran R, Ambu S, et al. Inhibition of breast cancer xenografts in a mouse model and the induction of apoptosis in multiple breast cancer cell lines by lactoferricin B peptide. J Cell Mol Med. (2021) 25:7181–9. doi: 10.1111/jcmm.16748
108. Ohradanova-Repic A, Prazenicova R, Gebetsberger L, Moskalets T, Skrabana R, Cehlar O, et al. Time to kill and time to heal: the multifaceted role of lactoferrin and lactoferricin in host defense. Pharmaceutics. (2023) 15:1056. doi: 10.3390/pharmaceutics15041056
109. Cheng JB, Wang JQ, Bu DP, Liu GL, Zhang CG, Wei HY, et al. Factors affecting the lactoferrin concentration in bovine milk. J Dairy Sci. (2008) 91:970–6. doi: 10.3168/jds.2007-0689
110. Tsakali E, Chatzilazarou A, Houhoula D, Koulouris S, Tsaknis J, Van Impe J, et al. rapid HPLC method for the determination of lactoferrin in milk of various species. J Dairy Res. (2019) 86:238–41. doi: 10.1017/S0022029919000189
111. Yamauchi K, Toida T, Nishimura S, Nagano E, Kusuoka O, Teraguchi S, et al. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem Toxicol. (2000) 38:503–12. doi: 10.1016/S0278-6915(00)00036-3
112. Hayes TG, Falchook GF, Varadhachary GR, Smith DP, Davis LD, Dhingra HM, et al. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Invest New Drugs. (2006) 24:233–40. doi: 10.1007/s10637-005-3690-6
113. Li W, Liu B, Lin Y, Xue P, Lu Y, Song S, et al. The application of lactoferrin in infant formula: The past, present and future. Crit Rev Food Sci Nutr. (2024) 64:5748–67. doi: 10.1080/10408398.2022.2157792
114. Hao L, Shan Q, Wei J, Ma F, Sun P. Lactoferrin: major physiological functions and applications. Curr Protein Pept Sci. (2019) 20:139–44. doi: 10.2174/1389203719666180514150921
115. Nutrition and Metabolism Management Branch of China International Exchange and Promotive Association for Medical and Health Care. Consensus of Chinese experts on clinical application of lactoferrin. Zhonghua Yu Fang Yi Xue Za Zhi. 2022;56(12):1694-701.
116. Nguyen DN, Jiang P, Stensballe A, Bendixen E, Sangild PT, Chatterton DE. Bovine lactoferrin regulates cell survival, apoptosis and inflammation in intestinal epithelial cells and preterm pig intestine. J Proteomics. (2016) 139:95–102. doi: 10.1016/j.jprot.2016.03.020
117. Tian M, Han YB, Yang GY Li JL, Shi CS, Tian D. The role of lactoferrin in bone remodeling: evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions. Front Endocrinol. (2023) 14:1218148. doi: 10.3389/fendo.2023.1218148
118. Takayama Y, Mizumachi K. Effect of lactoferrin-embedded collagen membrane on osteogenic differentiation of human osteoblast-like cells. J Biosci Bioeng. (2009) 107:191–5. doi: 10.1016/j.jbiosc.2008.09.018
119. Kim SE, Yun YP, Shim KS, Park K, Choi SW, Suh DH. Effect of lactoferrin-impregnated porous poly(lactide-co-glycolide) (PLGA) microspheres on osteogenic differentiation of rabbit adipose-derived stem cells (rADSCs). Colloids Surf B Biointerfaces. (2014) 122:457–64. doi: 10.1016/j.colsurfb.2014.06.057
120. Kawazoe A, Inubushi T, Miyauchi M, Ishikado A, Tanaka E, Tanne K, et al. Orally administered liposomal lactoferrin inhibits inflammation-related bone breakdown without interrupting orthodontic tooth movement. J Periodontol. (2013) 84:1454–62. doi: 10.1902/jop.2012.120508
121. Yamano E, Miyauchi M, Furusyo H, Kawazoe A, Ishikado A, Makino T, et al. Inhibitory effects of orally administrated liposomal bovine lactoferrin on the LPS-induced osteoclastogenesis. Lab Invest. (2010) 90:1236–46. doi: 10.1038/labinvest.2010.80
122. Ong R, Cornish J, Wen J. Nanoparticular and other carriers to deliver lactoferrin for antimicrobial, antibiofilm and bone-regenerating effects: a review. Biometals. (2023) 36:709–27. doi: 10.1007/s10534-022-00455-9
123. Icriverzi M, Dinca V, Moisei M, Evans RW, Trif M, Roseanu A. Lactoferrin in bone tissue regeneration. Curr Med Chem. (2020) 27:838–53. doi: 10.2174/0929867326666190503121546
Keywords: lactoferrin, osteopenia, osteoporosis, bone metabolism, mechanisms
Citation: Li D, Gao M, Li M, Zhao H, Zhou X, Gu Q, Yang H and Shi Q (2025) Role of lactoferrin in osteopenia and osteoporosis. Front. Nutr. 12:1648510. doi: 10.3389/fnut.2025.1648510
Received: 17 June 2025; Accepted: 06 August 2025;
Published: 03 September 2025.
Edited by:
Jailane de Souza Aquino, Federal University of Paraíba, BrazilReviewed by:
Fatemeh Pourteymour Fard Tabrizi, Tabriz University of Medical Sciences, IranAhmed M. Amshawee, Hilla University College, Iraq
Copyright © 2025 Li, Gao, Li, Zhao, Zhou, Gu, Yang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huilin Yang, c3V6aG91c3BpbmVAMTYzLmNvbQ==; Qin Shi, c2hpcWluQHN1ZGEuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Deming Li
Deming Li Maofeng Gao†
Maofeng Gao† Huilin Yang
Huilin Yang Qin Shi
Qin Shi