Abstract
Introduction:
The purpose of this research was to examine the relationship between metabolic obesity phenotypes and preterm birth (PTB) as well as the impact of obesity and metabolic abnormalities on PTB.
Methods:
A total of 20,259 pregnant singleton women participated in prospective birth cohort research conducted in China. Obesity metabolic phenotypes were categorized using pre-pregnancy body mass index (BMI) and metabolic state. Any delivery before 37 full weeks of gestation, as determined by the best obstetric estimate available, was considered PTB.
Results:
As the number of metabolically unfavorable components grows, so does the risk of developing PTB. Compared to women with a metabolically healthy normal weight, those who are normal weight and overweight (including obese) with metabolically unwell had an increased chance of having PTB (adjusted OR: 1.33 and 1.62, respectively). Additionally, additive interaction analysis revealed a significant interaction between overweight and metabolic unhealthiness for PTB risk (RERI = 0.41, AP = 0.24, SI = 2.22). People who are overweight and metabolically unwell have a 0.41 relative excess risk (which accounts for 24%) of PTB, and their combined risk is 2.22 times higher than that of those who are exposed to either risk alone.
Conclusion:
PTB risks are increased by metabolic abnormalities and overweight (including obese), and there are notable interaction effects between metabolic abnormalities and overweight (including obese) and PTB.
1 Introduction
Preterm birth (PTB), defined by the World Health Organization (WHO) as delivery before 37 completed weeks of gestation, represents a global clinical and public health challenge (1). It is associated with long-term adverse health effects in children and remains the leading cause of neonatal mortality and infant death (2). About 85% of these births are moderate (32–33 weeks) to late preterm babies (34–36 weeks), 10% are very preterm babies (28–31 weeks), and 5% are extremely preterm babies (<28 weeks) (3). Every year, about one million babies die from prematurity, and many survivors are disabled (4).
Numerous studies have demonstrated a link between obesity and preterm birth (5–7). Obesity can exacerbate the physiological inflammation associated with pregnancy (8). This inflammatory state, which is linked to both advanced maternal age and obesity, is a well-established risk factor for preterm birth (9). Additionally, common metabolic dysregulation, such as dyslipidemia, may be linked to inflammation and infection, and elevated inflammatory proteins may result in hypercholesterolemia, which may be linked to blood clot development and result in pregnancy issues such as placental abruption, which can exacerbate PTB (10, 11). Obesity is known as one of the most important public health concerns with a steadily increasing prevalence around the world, which is a well-known risk factor for many aspects of morbidity and mortality (12, 13), as well as causing a high economic burden to society (14). Current estimates suggest that by 2038 about 38% of the world's population is expected to be obese (15). In parallel with the increase in the general population, the prevalence of overweight and obesity also has increased in pregnant women (16). However, not all obese individuals show an equal health risk (17), some individuals may appear obesity but have normal metabolic conditions. They are defined as metabolically healthy obesity (MHO), a condition in which, despite the significant excess weight, traditional risk factors as insulin resistance, dyslipidemia, and hypertension are not present (18–20). Based on different metabolic conditions and body types, the population can be divided into metabolically unhealthy obesity (MUO), metabolically unhealthy normal weight (MUNW), and seven other types of obesity metabolic phenotypes.
Unfortunately, there are currently few thorough investigations on the relationship between obesity's metabolic abnormalities and pregnancy complications, with the majority of research on the metabolic phenotypes of obesity concentrating on cardiovascular disorders (21–23). In order to enrich this part of the research, we conducted a prospective birth cohort study among Chinese pregnant women. Our main goals are to quantify the separate and combined contributions of obesity and metabolic abnormalities to these pregnancy problems, as well as to clarify the relationship between metabolic obesity phenotypes and the incidence of PTB.
2 Methods
2.1 Study population
This prospective population-based cohort study was conducted to examine the relationship between metabolic obesity phenotypes and PTB. It was based on the Fujian Birth Cohort Study (FJBCS), which was initiated in November 2019 at the Fujian Maternal and Child Health Hospital in Fujian, China. As of June 2023, there were 25,538 patients with confirmed pregnancy outcomes. The final study requirements were met by 20,259 identified maternal mothers after excluding women who had diabetes or hypertension before conception or at baseline, had unclear pregnancy outcomes, had abortions, and had missed pre-pregnancy body mass index (BMI) data (Supplementary Figure S1). A comparison of the basic characteristics between participants and non-participants had shown in Supplementary Table S1. Due to the large sample size, the between-group differences were statistically significant, though the actual differences in baseline characteristics across populations remained within acceptable limits. The ethics committee of Fujian Provincial Maternal and Child Health Hospital authorized all study procedures (approval number: 2017KR-030), and each participant signed a written informed consent form. Additionally, we verified that every study was conducted in compliance with the Declaration of Helsinki.
2.2 Measurement of pre-pregnancy characteristics
In a face-to-face interview, participants completed a questionnaire about sociodemographic [maternal age, pre-pregnancy weight, ethnicity, assisted reproduction, gravidity, parity, marital status, income, work, inter-pregnancy interval, the season of delivery (spring, summer, autumn, and winter), and educational attainment] and lifestyle factors (history of smoking and alcohol consumption) when they were 10–12 weeks pregnant. The year of delivery (2019, 2020, 2021, and 2022) was obtained from the hospital information registry. Pre-pregnancy weight and height measurements were used to determine body mass index (BMI, kg/m2). An automated sphygmomanometer was used to measure the diastolic blood pressure (DBP) and systolic blood pressure (SBP). The enzymatic electrode method was used to measure the levels of fasting plasma glucose (FPG). Standardized enzymatic assays were used to examine serum lipid profiles, which included triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 (Apo-A1), and apolipoprotein B (Apo-B).
2.3 Definition of metabolic obesity phenotypes
The BMI of the study population is determined using the Working Group On Obesity in China established criteria (24), with “underweight” being defined as having a BMI of less than 18.5 kg/m2, “normal” as having a BMI between 18.5 and 24.0 kg/m2, “overweight” as having a BMI between 24.0 and less than 28.0 kg/m2, and “obese” as having a BMI greater than 28.0 kg/m2. We also performed sensitivity analyses using the WHO international BMI cut-offs. If a woman had any one of the following, she was said to have a metabolic factor: at the first prenatal booking, (i) the SBP was greater than 130 mmHg; (ii) the DBP was greater than 85 mmHg; (iii) the non-fasting capillary glucose was greater than 6.8 mmol/L; or (iv) dyslipidemia, which is defined as TC greater than 5.44 mmol/L, TG greater than 1.8 mmol/L, LDL-C greater than 3.96 mmol/L, HDL-C less than 1.04 mmol/L, or an apoB/apoA1 (apoB/apoA1) ratio greater than 0.8 (25). Metabolically healthy underweight (MHUW), metabolically unhealthy underweight (MUUW), metabolically healthy normal weight (MHNW), metabolically unhealthy normal weight (MUNW), metabolically healthy overweight (including obese; MHO), and metabolically unhealthy overweight (including obese; MUO) were the six groups into which all study participants were divided after combining their body weight phenotypes and metabolic conditions (2).
2.4 Definition of preterm birth
The PTB is defined as any birth before 37 completed weeks of gestation determined by the best available obstetric estimate, which mostly relied on an early pregnancy ultrasound in combination with the last menstrual period (LMP) (26): gestational age was determined using a combination of the last menstrual period (LMP) date and early ultrasound examination. For participants who underwent an ultrasound examination in early pregnancy ( ≤ 14 weeks), the measurement of crown-rump length was used as the primary method for estimating gestational age. For those without an early ultrasound scan, self-reported LMP date was used. After excluding iatrogenic preterm birth (such as that caused by placental abruption, placenta accreta, cervical cerclage, pulmonary hypertension, eclampsia, fetal distress, etc.), a sensitivity analysis was conducted with spontaneous preterm birth as the study outcome.
2.5 Statistical analyses
The median (interquartile range) was used to characterize skewed continuous data, whereas mean ± SD was used to express all regularly distributed continuous variables. Frequencies (%) were used to represent categorical variables. To check for differences between the various groups of obesity metabolic phenotypes, we employed the Kruskal–Wallis H test (skewed distribution), One-Way ANOVA test (normal distribution), or Chi-square (categorical variables) test.
Using multivariable logistic regression analysis, the adjusted odds ratio (aOR) and 95% confidence interval (CI) for the beginning of PTB were evaluated. Known to affect metabolic status or be linked to PTB, covariates were chosen beforehand and modified in the logistic regression models. Maternal age, ethnicity, gravidity, parity, assisted reproduction, alcohol and tobacco use, marital status, and educational attainment were among these factors. Missing covariates were addressed using imputation by chained equations.
Pre-specified stratified analyses were based on subgroup characteristics (parity, pregnancy type, and maternal age). To check for multiplicative interaction between subgroups, the likelihood ratio test was employed. Furthermore, additive interaction was evaluated using two indices: the relative excess risk due to interaction (RERI), and the attributable proportion (AP) owing to interaction and verified RERI, AP, and the SI by using the delta method. If the 95% CIs for RERI and AP do not overlap 0, the interaction between them is considered statistically significant. Additionally, the estimated OR for PTB of a logistic model with a metabolic abnormalities-BMI interaction term was utilized to create an interaction spline with four knots using the R package “interaction RCS.” The R Statistical Software (Version 4.2.2, http://www.R-project.org, The R Foundation) were used for all analyses. Statistical significance was defined as a two-sided P value <0.05.
3 Results
3.1 Baseline characteristics
Table 1 lists the baseline demographic and clinical characteristics stratified by obesity metabolic phenotypes among 20,259 women during their first pregnancy. The participation rate among eligible women was 75%. Those who defined as MUO were more likely to be older and to drink alcohol than those who had a typical pregnancy. Compared to women with other metabolic phenotypes of obesity, individuals with MUO had lower levels of HDL (1.7 ± 0.4 mmol/L) but greater pre-pregnancy BMI (26.8 ± 6.7 kg/m2), blood pressure (SBP/DBP: 122.0 ± 10.9/73.9 ± 9.2 mmHg), FPG (5.2 ± 1.3 mmol/L), TC (6.5 ± 1.3 mmol/L), and TG [3.8 (3.0–5.0) mmol/L].
Table 1
| Maternal characteristics | Total (20,259) | Obesity metabolic phenotype | P value | |||
|---|---|---|---|---|---|---|
| MHUW/MHNW (12,030) | MUUW/MUNW (5,196) | MHO (1,459) | MUO (1,574) | |||
| Maternal age, years | 30.3 ± 3.9 | 29.9 ± 3.8 | 30.7 ± 4.0 | 30.9 ± 4.0 | 31.4 ± 4.2 | <0.001 |
| Ethnicity-Han, n (%) | 19,823 (97.8) | 11,774 (97.9) | 5,084 (97.8) | 1,428 (97.9) | 1,537 (97.6) | 0.634 |
| Educational-University level, n(%) | 14,537 (71.9) | 8,812 (73.3) | 1,001 (68.7) | 3,693 (71.2) | 1,031 (65.6) | <0.001 |
| Marriage, n (%) | 19,231 (94.9) | 11,368 (94.5) | 4,941 (95.1) | 1,405 (96.3) | 1,517 (96.4) | 0.001 |
| Smoking, n (%) | 410 (2.0) | 229 (1.9) | 93 (1.8) | 45 (3.1) | 43 (2.7) | 0.005 |
| Alcohol status, n (%) | 16,235 (80.1) | 9,610 (79.9) | 4,209 (81) | 1,164 (79.8) | 1,252 (79.5) | <0.001 |
| Income | <0.001 | |||||
| <0.9 w (monthly) | 8,364 (41.3) | 4,989 (39.7) | 408 (43.6) | 2,407 (42.6) | 560 (50.1) | |
| ≥0.9 w (monthly) | 11,895 (58.7) | 7,565 (60.3) | 527 (56.4) | 3,246 (57.4) | 557 (49.9) | |
| Work (employees or technical staff) | 14,759 (72.9) | 9,326 (74.3) | 679 (72.6) | 4,032 (71.3) | 722 (64.6) | <0.001 |
| Pregnancy interval (year) | <0.001 | |||||
| <1 | 8,870 (43.8) | 5,850 (46.6) | 369 (39.5) | 2,303 (40.7) | 348 (31.2) | |
| 1–2 | 2,630 (13.0) | 1,564 (12.5) | 125 (13.4) | 740 (13.1) | 201 (18) | |
| ≥2 | 8,759 (43.2) | 5,140 (40.9) | 441 (47.2) | 2,610 (46.2) | 568 (50.9) | |
| Assisted reproduction, n (%) | 1,424 (7.0) | 684 (5.7) | 489 (9.4) | 95 (6.5) | 156 (9.9) | <0.001 |
| Gravidity, n(%) | <0.001 | |||||
| 1 | 8,870 (43.8) | 5,652 (47) | 2,159 (41.6) | 567 (38.9) | 492 (31.3) | |
| 2 | 6,143 (30.3) | 3,608 (30) | 1,538 (29.6) | 451 (30.9) | 546 (34.7) | |
| ≥3 | 5,246 (25.9) | 2,770 (23) | 1,499 (28.8) | 441 (30.2) | 536 (34.1) | |
| Parity, n(%) | <0.001 | |||||
| 0 | 12,124 (59.8) | 7,565 (62.9) | 2,976 (57.3) | 807 (55.3) | 776 (49.3) | |
| 1 | 7,293 (36.0) | 4,026 (33.5) | 1,986 (38.2) | 573 (39.3) | 708 (45) | |
| ≥2 | 842 (4.2) | 439 (3.6) | 234 (4.5) | 79 (5.4) | 90 (5.7) | |
| Pre-pregnancy BMI, kg/m2 | 21.2 ± 4.1 | 20.1 ± 2.0 | 20.7 ± 2.0 | 26.5 ± 8.0 | 26.8 ± 6.7 | <0.001 |
| SBP, mmHg | 114.5 ± 11.1 | 111.8 ± 9.8 | 118.6 ± 12.1 | 114.6 ± 9.8 | 122.0 ± 10.9 | <0.001 |
| DBP, mmHg | 69.3 ± 9.9 | 67.5 ± 7.9 | 72.2 ± 13.1 | 69.5 ± 7.9 | 73.9 ± 9.2 | <0.001 |
| FPG, mmol/L | 5.0 ± 1.2 | 5.0 ± 1.1 | 5.1 ± 1.2 | 5.1 ± 1.3 | 5.2 ± 1.3 | <0.001 |
| TC, mmol/L | 6.6 ± 1.2 | 6.5 ± 1.1 | 7.0 ± 1.4 | 6.1 ± 1.1 | 6.5 ± 1.3 | <0.001 |
| TG, mmol/L | 3.2 (2.5, 4.1) | 3.0 (2.4, 3.8) | 3.6 (2.8, 4.7) | 3.1 (2.5, 3.8) | 3.8 (3.0, 5.0) | <0.001 |
| HDL, mmol/L | 1.8 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.3 | 1.7 ± 0.4 | <0.001 |
| LDL, mmol/L | 3.6 ± 1.0 | 3.6 ± 0.9 | 3.8 ± 1.2 | 3.3 ± 0.9 | 3.5 ± 1.1 | <0.001 |
| Apo A1, g/L | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.3 | 1.4 ± 0.3 | 1.5 ± 0.3 | <0.001 |
| Apo B, g/L | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.9 ± 0.2 | <0.001 |
| Apo B/Apo A1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.2 | <0.001 |
| Hypertensive, n (%) | 2,057 (10.2) | 0 (0) | 1,539 (29.6) | 0 (0) | 518 (32.9) | <0.001 |
| Hyperglycemia, n (%) | 360 (1.8) | 0 (0) | 229 (4.5) | 0 (0) | 131 (8.4) | <0.001 |
| Hyperlipidemia, n (%) | 5,231 (25.8) | 0 (0) | 3,965 (76.3) | 0 (0) | 1,266 (80.4) | <0.001 |
| Metabolic unhealthy, n (%) | 13,489 (66.6) | 12,030 (100) | 0 (0) | 1,459 (100) | 0 (0) | <0.001 |
| Preterm birth, n (%) | 1,000 (4.9) | 516 (4.3) | 287 (5.5) | 72 (4.9) | 125 (7.9) | <0.001 |
Demographic, metabolic and clinical variables in pregnant women by obesity metabolic phenotype group.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Apo A1, apolipoprotein A1; Apo B, apolipoprotein B; MHUW, metabolically healthy underweight; MUUW, metabolically unhealthy underweight; MHNW, metabolically healthy normal weight; MUNW, metabolically unhealthy normal weight; MHO, metabolically healthy overweight (including obese); MUO, metabolically unhealthy overweight (including obese). Continuous variables with normal distributions are expressed as the mean-standard deviation, whereas those with non-normal distributions are presented as the median and interquartile range.
3.2 The odds ratios (ORs) for preterm birth based on the body mass index, metabolic status, and metabolic components
Analysis revealed a significant association between metabolic health status and the risk of preterm birth: among 20,259 participants, compared to metabolically healthy women, the risk of preterm birth increases by 28% in women with one metabolically unhealthy component (aOR = 1.28, 95% CI: 1.11–1.47, P = 0.001), and the risk increases by 86% in women with two metabolically unhealthy components (P < 0.001). It is worth noting that the impact of hyperlipidemia on the risk of preterm birth is particularly significant, with an aOR of 1.45 (95% CI: 1.26–1.66, P < 0.001) and the risk of preterm birth in metabolically unhealthy women 34% higher than in metabolically healthy women (P < 0.001). Interestingly, the analysis showed that the effects of having three metabolically unhealthy number components on the occurrence of PTB were not statistically significant (Table 2). Similar results were also observed in the analysis of spontaneous preterm birth (Supplementary Table S2).
Table 2
| Variable | Total, n | PTB, n (%) | Crude | Adjusted | ||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |||
| Body mass index | ||||||
| Underweight | 3,114 | 135 (4.3) | 0.91 (0.75–1.1) | 0.34 | 0.99 (0.82–1.19) | 0.892 |
| Normal weight | 14,112 | 668 (4.7) | 1 (Ref) | 1 (Ref) | ||
| Overweight (including obese) | 3,033 | 197 (6.5) | 1.40 (1.19–1.65) | <0.001 | 1.33 (1.12–1.57) | 0.001 |
| Metabolic components | ||||||
| Hypertensive | ||||||
| No | 18,202 | 877 (4.8) | 1 (Ref) | 1 (Ref) | ||
| Yes | 2,057 | 123 (6) | 1.26 (1.03–1.53) | 0.021 | 1.23 (1.01–1.5) | 0.036 |
| Hyperlipidemia | ||||||
| No | 15,028 | 657 (4.4) | 1 (Ref) | 1 (Ref) | ||
| Yes | 5,231 | 343 (6.6) | 1.53 (1.34–1.76) | <0.001 | 1.45 (1.26–1.66) | <0.001 |
| Hyperglycemia | ||||||
| No | 19,574 | 950 (4.9) | 1 (Ref) | 1 (Ref) | ||
| Yes | 360 | 22 (6.1) | 1.28 (0.82–1.97) | 0.273 | 1.14 (0.74–1.77) | 0.548 |
| Number of metabolically unhealthy components | ||||||
| 0 | 13,489 | 588 (4.4) | 1 (Ref) | 1 (Ref) | ||
| 1 | 5,930 | 340 (5.7) | 1.33 (1.16–1.53) | <0.001 | 1.28 (1.11–1.47) | <0.001 |
| 2 | 802 | 68 (8.5) | 2.03 (1.56–2.64) | <0.001 | 1.86 (1.43–2.42) | <0.001 |
| 3 | 38 | 4 (10.5) | 2.58 (0.91–7.3) | 0.074 | 2.22 (0.78–6.29) | 0.134 |
| Metabolic status | ||||||
| Metabolically healthy | 13,489 | 588 (4.4) | 1 (Ref) | 1 (Ref) | ||
| Metabolically unhealthy | 6,770 | 412 (6.1) | 1.42 (1.25–1.62) | <0.001 | 1.34 (1.18–1.53) | <0.001 |
The odds ratios (ORs) for PTB according to the body mass index, metabolic components, and metabolic status.
Models were adjusted for age, ethnicity, education, marriage, smoking, alcohol status, gravidity, parity, assisted reproduction, income, work and inter-pregnancy. PTB, preterm birth; OR, odds ratio; aOR, adjusted odds ratio.
3.3 The association of obesity metabolic phenotypes with preterm birth
Compared to MHNW women, MUO women showed the highest risk of developing PTB after full adjustment, with aOR of 1.85 (95% CI: 1.19–2.89). The risks of developing PTB were significantly increased by metabolically unhealthy which showed that women with MUNW had 43% higher PTB chances, with aORs of 1.43 (95% CI: 1.03–1.99) than MHNW. However, compared to MHNW women, the preterm birth risk for MHUW, MHO, and MUUW women was not observed to be statistically significant (Figure 1).
Figure 1
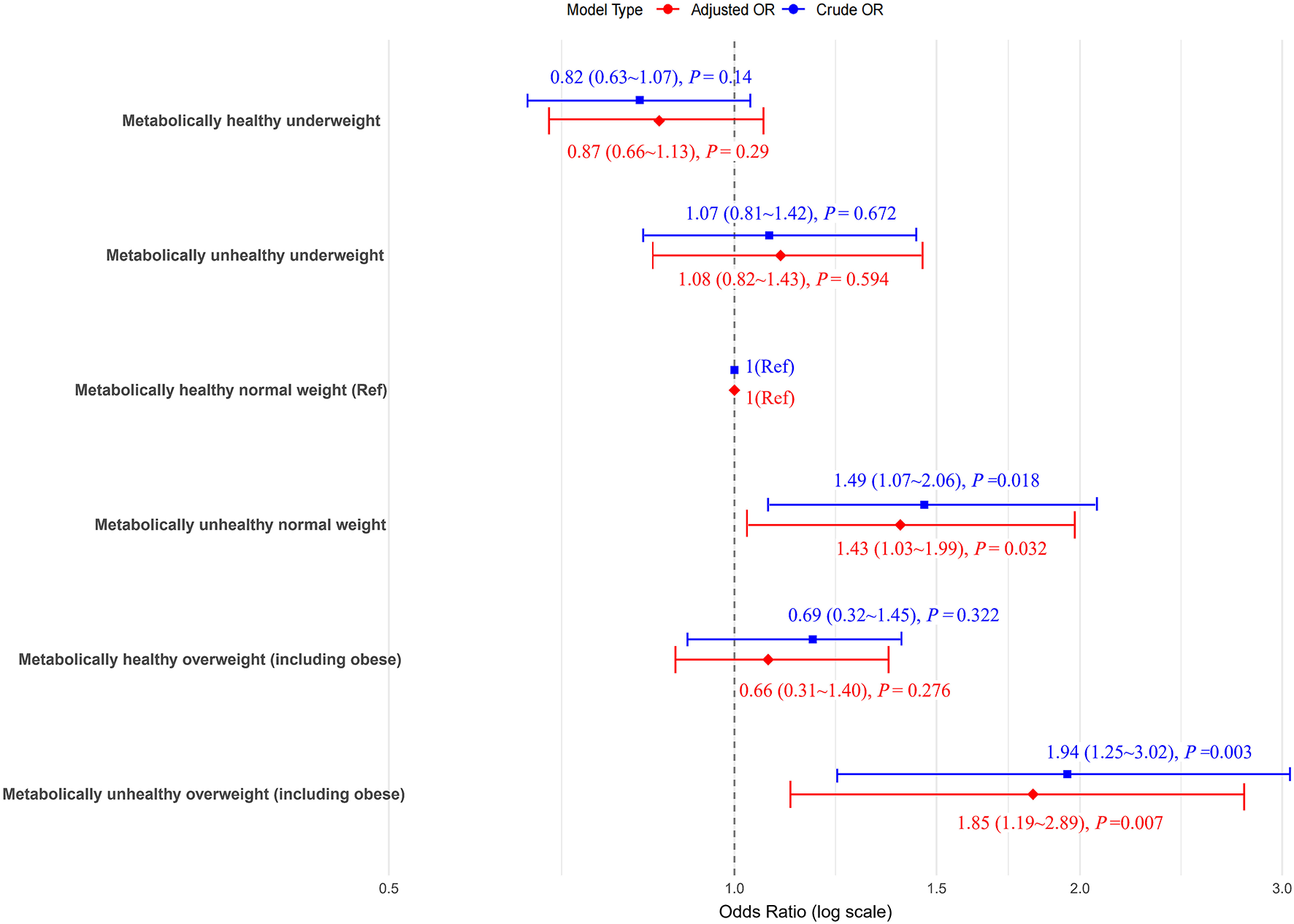
The odds ratios (ORs) for preterm birth (PTB) according to the metabolic body weight phenotypes. Models adjusted for age, ethnicity, education, marriage, smoking, alcohol status, gravidity, parity, assisted reproduction, income, work and inter-pregnancy. The blue icons in the model type are boxes and the red ones are diamonds.
3.4 Association of metabolic phenotypes with preterm birth across body weight categories
In women with normal weight, the risk of preterm birth is higher in metabolically unhealthy women compared to metabolically healthy individuals, with an aOR of 1.33 (95% CI: 1.13–1.57). Similarly, in overweight (including obese) women, the incidence of preterm birth is higher in metabolically unhealthy women compared to metabolically healthy women, with an aOR of 1.62 (P = 0.002; Table 3). Analysis of spontaneous preterm birth revealed similar results (Supplementary Table S3).
Table 3
| Variable | Total, n | PTB, n (%) | Crude | Adjusted | ||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | aOR (95%CI) | P value | |||
| Metabolically healthy underweight | 2,446 | 110 (4.5) | 1 (Ref) | 1 (Ref) | ||
| Metabolically unhealthy underweight | 668 | 25 (3.7) | 0.83 (0.53–1.29) | 0.397 | 0.81 (0.52–1.26) | 0.35 |
| Metabolically healthy normal weight | 9,584 | 406 (4.2) | 1 (Ref) | 1 (Ref) | ||
| Metabolically unhealthy normal weight | 4,528 | 262 (5.8) | 1.39 (1.18–1.63) | <0.001 | 1.33 (1.13–1.57) | <0.001 |
| Metabolically healthy overweight (including obese) | 1,459 | 72 (4.9) | 1 (Ref) | 1 (Ref) | ||
| Metabolically unhealthy overweight (including obese) | 1,574 | 125 (7.9) | 1.66 (1.23–2.24) | 0.001 | 1.62 (1.19–2.19) | 0.002 |
Relationship of metabolic phenotypes and PTB in different body weight phenotypes.
Models were adjusted for age, ethnicity, education, marriage, smoking, alcohol status, gravidity, parity, assisted reproduction, income, work and inter-pregnancy. OR, odds ratio; aOR, adjusted odds ratio.
3.5 Interaction analysis of the effects of body weight status and metabolic phenotypes on preterm birth
Significant additive interactions and multiplicative interactions were found between body weight status and metabolic phenotypes on the risk of PTB (Table 4, Figure 2). Three measures of additive interaction between being overweight (including obese) and metabolically unhealthy (RERI, AP) showed a relative excess risk of 0.41, with an AP of 0.24 (95% CI: 0.01–0.46), indicating that the combined effect of these two risk factors may result in a 24% increased risk of PTB (Table 4, Figure 2). According to the interaction spline, metabolic problems were linked to PTB across all BMI ranges, however the relationship was noticeably stronger at higher BMI values (Figure 3).
Table 4
| Measures | OR/Estimates | 95% CI | P value |
|---|---|---|---|
| Obesity metabolic phenotype | |||
| MHNW | 1 (Ref) | ||
| MUNW | 1.24 | 1.07–1.44 | <0.001 |
| MHO | 1.09 | 0.85–1.41 | 0.51 |
| MUO | 1.76 | 1.43–2.16 | <0.001 |
| Subgroup analysis | |||
| Metabolically unhealthy on PTB | |||
| Normal weight | 1.24 | 1.07–1.44 | <0.001 |
| Overweight | 1.60 | 1.18–2.16 | <0.001 |
| Overweight on PTB | |||
| Metabolically healthy | 1.09 | 0.85–1.41 | 0.49 |
| Metabolically unhealthy | 1.41 | 1.13–1.75 | <0.001 |
| Interaction analysis | |||
| Multiplicative interaction | 1.29 | 0.92–1.80 | 0.14 |
| Additive interaction | |||
| RERI | 0.41 | 0.00–0.85 | 0.03 |
| AP | 0.24 | 0.01–0.46 | 0.02 |
| SI | 2.22 | 0.76–6.48 | <0.001 |
Interaction analysis of the effects of overweight (including obese) and metabolically unhealthy on PTB.
Multiplicative interaction of the effects of overweight (including obese) and metabolically unhealthy on preterm birth was assessed by including the main effects of them and the product term in the model and the Pinteraction was represented by the P-value of product term. RERI, AP were used to indicate additive interaction of overweight (including obese) and metabolically unhealthy on preterm birth. Models were adjusted for age, ethnicity, education, marriage, smoking, alcohol status, gravidity, parity, assisted reproduction, income, work and inter-pregnancy. MHNW, metabolically healthy normal weight; MUNW, normal weight with metabolic abnormalities; MHO, overweight (including obese) without metabolic abnormalities; MUO, overweight (including obese) with metabolic abnormalities.
Figure 2
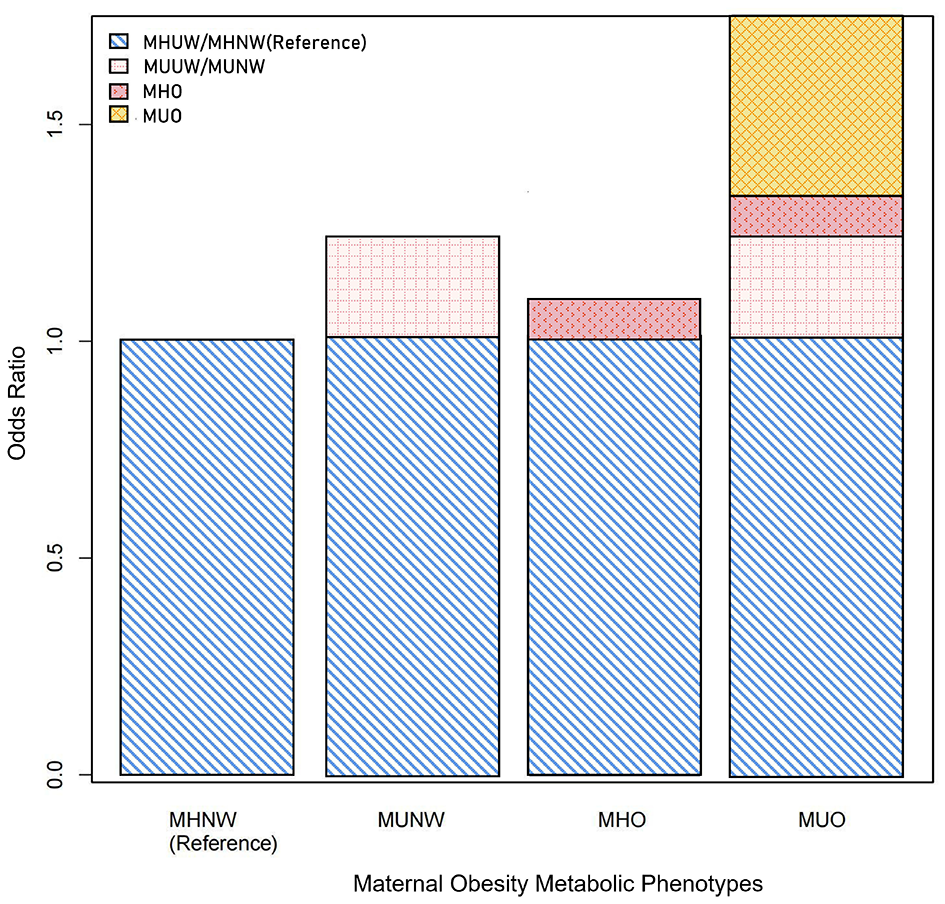
Additive interactions between overweight (including obese) and metabolically unhealthy on the risk of preterm birth (PTB). Models were adjusted for age, ethnicity, education, marriage, smoking, alcohol status, gravidity, parity, assisted reproduction, income, work and inter-pregnancy.
Figure 3
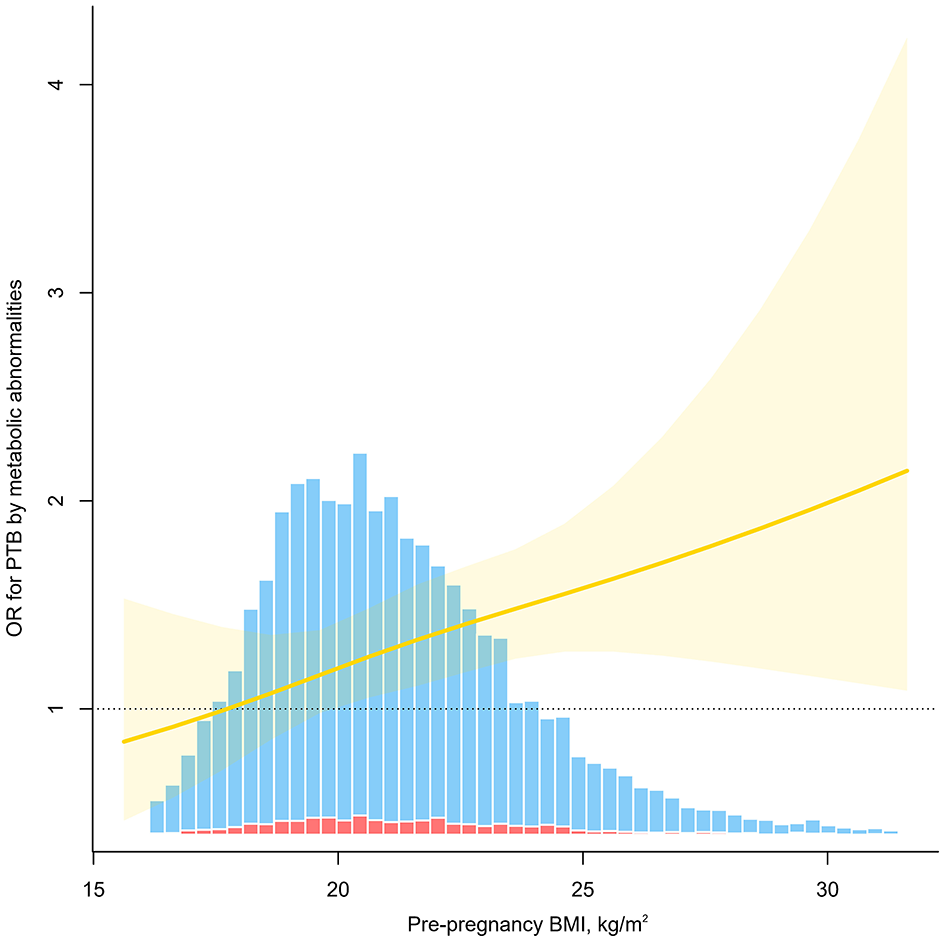
Odds ratio (OR) for preterm birth (PTB) by metabolic abnormalities as a function of pre-pregnancy BMI. The solid red line indicates the estimated OR, the yellow shading indicates the 95% confidence interval, and the bar diagram indicates the distribution of the population, where the red columns are for those who developed PTB and the blue columns are for those who did not. Models were adjusted for age, ethnicity, education, marriage, smoking, alcohol status, gravidity, parity, assisted reproduction, income, work and inter-pregnancy.
3.6 Subgroup analysis and sensitivity analyses
Additional subgroup analyses concerning the role of maternal age, parity and assisted reproduction, the year of delivery and the season of delivery are presented and shown in the Figure 4. Interestingly, the interaction tests showed that these stratification variables had no modification effects (all interaction P-values >0.05), indicating that the observed associations between metabolic obesity indicators and PTB were unaffected by the mother's age, reproductive history, or method of conception. We also performed supplementary sensitivity analyses using the WHO international BMI cut-offs to define obesity (Supplementary Tables S4–S7), and revealed pattern consistent results.
Figure 4
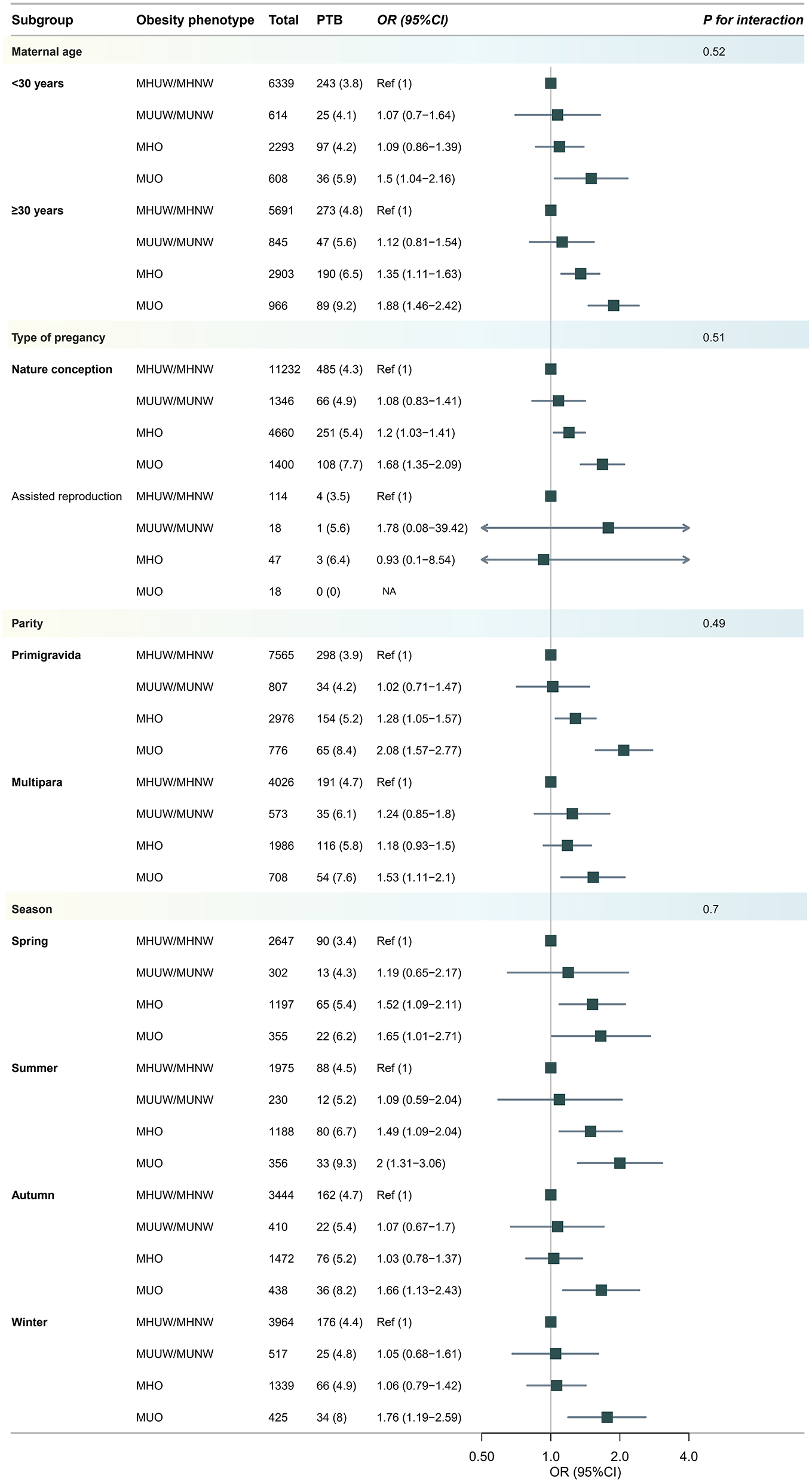
Subgroup analysis for risk of developing preterm birth (PTB) according to body weight phenotype. Models were adjusted for age, ethnicity, education, marriage, smoking, alcohol status, gravidity, parity, assisted reproduction, income, work and inter-pregnancy.
4 Discussion
The present study showed that women who were overweight, including obese, were more likely to develop PTB than women who were normal weight, and that people with metabolic abnormalities were more likely to develop PTB even if they had the same weight phenotype. We also found that there was an additive and multiplicative interaction between PTB and metabolic abnormalities and overweight.
Pregnant women who are obese are at greater risk of a variety of pregnancy-related complications compared with women of normal BMI (27). Several studies have shown that the risk of PTB is positively related to obesity. A large prospective cohort study from China showed that compared to women with normal weight, women with overweight or obesity before pregnancy had an increased risk of preterm birth. In addition, the greatest risk of extremely preterm birth was observed in obese women (7). Ju et al. (28) found that overall maternal obesity (BMI ≥ 30.0 kg/m2) and extreme obesity (BMI ≥ 40.0 kg/m2), were both associated with higher rates of prematurity after controlling for other confounders including maternal race, age, socioeconomic status, and smoking during pregnancy. Obesity can enhance the inflammatory status induced by pregnancy (29), which is characterized by a state of chronic inflammation, oxidative stress, and dysregulated adipokine secretion and will promotes the release of damage-associated molecular patterns (DAMPs). These DAMPs amplify uterine and chorioamnionic inflammation via inflammasome activation, potentially leading to intra-amniotic inflammation, which increase the risk of spontaneous extremely preterm birth. In addition, Obesity compromises placental function through multiple interconnected pathways: chronic inflammation and oxidative stress directly damage placental cells and impair nutrient exchange; epigenetic alterations (e.g., DNA methylation) disrupt gene expression patterns critical for placental development; and dysregulation of metabolic mediators (e.g., leptin, apelin) disrupts vascular tone and angiogenesis, while maternal vascular malperfusion is a well-established risk factor for indicated preterm birth (30). Collectively, these mechanisms may lead to PTB (31).
Numerous diseases are directly linked to metabolic issues, which raises the risk of developing a variety of ailments. Despite having a normal BMI, poor metabolic health still raises the risk of many diseases. Numerous studies have linked metabolic abnormalities to both poor pregnancy outcomes and chronic (32–34) and cardiovascular disease (35, 36). Numerous studies have demonstrated that women with a history of hypertension illnesses during pregnancy are more likely to have cardiovascular disease later in life than those with normotensive pregnancies (37). A study including more than 600,000 women in Norway showed that hypertension during pregnancy was associated with an increased risk of subsequent CVD in comparison with normotensive pregnancy and the highest risk was observed when hypertension was combined with small-for-gestational-age infants and/or preterm delivery (38). Inflammation and infection may help explain the association between metabolic abnormalities and PTB. For example, the lipid changes could relate to infammation and infection, and that hypertriglyceridemia may be considered part of the innate immunity and that increased inflammatory proteins may cause hypercholesterolemia. Studies have shown that high cholesterol may contribute to the formation of blood clots, which can lead to complications during pregnancy, such as placental abruption, which can promote PTB (11). It is noteworthy that our results imply that the development of PTB is significantly influenced by the metabolic state prior to pregnancy. PTB is more common in metabolically unhealthy women than in those who are metabolically healthy, and the risk of PTB rises as the number of metabolically unfavorable components rises. This demonstrates the significance of metabolic status as a risk factor for obstetric issues and validates the results of other previous studies (1, 39–41). Meanwhile, among individuals with metabolic abnormalities, the risk of PTB is significantly increased for both normal-weight and overweight women, which illustrates the importance of the metabolic screening in normal-weight women and to reduce the risk of metabolic abnormalities by improving lifestyle habits, such as maintaining a balanced diet and engaging in regular physical activity. For high-risk individuals with metabolic abnormalities, medications like metformin can be used to lower the risk of developing metabolic diseases.
In this sizable population cohort study, we also evaluated the combined impact of obesity and metabolic disorders on PTB. Compared with MHNW women, we discovered that MUNW and MUO women were more likely to have PTB, which was same as the previous study (25). Among overweight women, PTB was much more common in those with metabolic problems. It is worth noting that among individuals with metabolic abnormalities, the risk of PTB is significantly increased for both normal-weight and overweight women. Interaction analysis shows that overweight and metabolic abnormalities have both additive and multiplicative interactions on the occurrence of PTB which demonstrated that the additive interaction accounts for 24% of PTB events in those exposed to both risk factors (metabolically unhealthy and overweight).
The large sample size and the evaluation of interactions between body weight status and metabolic phenotypes on PTB, as well as a quantitative assessment of the association between obesity and metabolic abnormalities, were the primary strengths of our work. However, the present study has several limitations. First, the pre-pregnancy weight used to calculate BMI is provided by the study population, which may affects the accuracy of the BMI calculation. In addition, the BMI cannot show the distribution of body fat or differentiate between lean and fat mass (42). Second, several subgroup analyses were performed with a limited number of studies, making it difficult to achieve a firm conclusion about the findings. Another potential limitation of this study is its hospital-based design. As the sample was recruited from clinical settings rather than the general community, it is susceptible to Berkson's bias. The case mix and risk factor estimates observed in our study may not be directly generalizable to the wider Chinese population. Future studies employing a population-based design are warranted to validate our findings. It is important to note that the study population is from a region with a relatively low mean BMI. This leaner body-habitus distribution resulted in a limited number of individuals with high body mass index in the cohort, creating a “restricted range of exposure.” Methodologically, this most likely led to an attenuation of the effect size of the association between BMI and preterm birth. Therefore, our study might not have fully captured the stronger association that could exist in a population with a broader BMI spectrum. Future research involving multi-center populations with diverse BMI distributions is needed to validate our findings and quantify this association more accurately. We acknowledge that our study may be subject to residual confounding, and its single-center design may affect generalizability. The use of self-reported BMI could lead to non-differential misclassification, likely biasing results toward the null, while the lack of direct visceral adiposity measures is a recognized constraint. Potential misclassification of metabolic status, also likely non-differential, was also noted. Despite these limitations, we have taken care to contextualize our findings and emphasize that they provide valuable hypothesis-generating insights, underscoring the need for future multi-center studies with more precise measurements to confirm our results. Beside, the diagnosis of metabolic abnormalities in early pregnancy carries a risk of over-diagnosis. Physiological adaptations of early pregnancy, such as hemodilution from plasma volume expansion, vasodilation induced by progesterone, and stimulation of thyroid function by human chorionic gonadotropin (hCG), can alter metabolic parameters away from their pre-pregnancy baselines. These adaptations mean that the most diagnostic criteria we employed (such as blood pressure, glucose), which are based on thresholds derived from non-pregnant populations or fixed cut-offs, may have reduced specificity in the context of pregnancy, erroneously categorizing some normal physiological adaptations as pathological states. Future research aimed at establishing pregnancy-specific and gestational-age-adjusted diagnostic criteria for metabolic parameters would greatly enhance the accuracy of such studies.
In conclusion, our study shows that obesity and metabolic disorders have multiplicative and additive interaction effect on PTB and are linked to an increased risk of PTB. Aside from that, metabolic disorders may make normal-weight women more vulnerable to PTB. Therefore, clinical monitoring and treatment of the pregnant woman's metabolism, together with appropriate risk classification and improved prevention, are necessary in addition to focus on overweight or obesity.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Fujian Provincial Maternal and Child Health Hospital authorized all study procedures (approval number: 2017KR-030). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC: Conceptualization, Data curation, Methodology, Software, Writing – original draft. YM: Data curation, Investigation, Software, Writing – review & editing. QL: Data curation, Investigation, Methodology, Writing – review & editing. QZ: Conceptualization, Data curation, Methodology, Resources, Software, Writing – review & editing. BS: Conceptualization, Data curation, Methodology, Software, Writing – review & editing. ZW: Data curation, Investigation, Methodology, Software, Writing – review & editing. WLiu: Conceptualization, Data curation, Investigation, Software, Writing – review & editing. JL: Conceptualization, Data curation, Investigation, Software, Writing – review & editing. HS: Conceptualization, Data curation, Investigation, Writing – review & editing. HG: Data curation, Investigation, Methodology, Software, Writing – review & editing. WLi: Conceptualization, Investigation, Methodology, Software, Writing – review & editing. YZ: Funding acquisition, Investigation, Methodology, Project administration, Software, Writing – review & editing. HL: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Youth Program, Grant No. 82304156) and the by the Natural Science Foundation of Fujian Province of China (Grant No. 2025J01200). The funder did not contribute to the study's design, collection, analysis, and interpretation of data.
Acknowledgments
The authors are grateful to all of the participants, the staff, and the other study investigators for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1648996/full#supplementary-material
Abbreviations
Apo A1, apolipoprotein A1; Apo B, apolipoprotein B; AP, attributable proportion; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GH, gestational hypertension; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MHUW, metabolically healthy underweight; MUUW, metabolically unhealthy underweight; MHNW, metabolically healthy normal weight; MUNW, metabolically unhealthy normal weight; MHO, metabolically healthy overweight (including obese); MUO, metabolically unhealthy overweight (including obese); OR, odds ratio; PE, preeclampsia; RERI, relative excess risk due to interaction; SBP, systolic blood pressure; SI, synergy index; TG, triglycerides; TC, total cholesterol.
References
1.
Smith CJ Baer RJ Oltman SP Breheny PJ Bao W Robinson JG et al . Maternal dyslipidemia and risk for preterm birth. PloS ONE. (2018) 13:e0209579. 10.1371/journal.pone.0209579
2.
Liu B Xu G Sun Y Du Y Gao R Snetselaar LG et al . Association between maternal pre-pregnancy obesity and preterm birth according to maternal age and race or ethnicity: a population-based study. Lancet Diabetes Endocrinol. (2019) 7:707–14. 10.1016/S2213-8587(19)30193-7
3.
da Fonseca EB Damião R Moreira DA . Preterm birth prevention. Best Pract Res Clin Obstet Gynaecol. (2020) 69:40–9. 10.1016/j.bpobgyn.2020.09.003
4.
Ohuma EO Moller AB Bradley E Chakwera S Hussain-Alkhateeb L Lewin A et al . National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. (2023) 402:1261–71. 10.1016/S0140-6736(23)00878-4
5.
Cnattingius S Villamor E Johansson S Edstedt Bonamy AK Persson M Wikström AK et al . Maternal obesity and risk of preterm delivery. JAMA. (2013) 309:2362–70. 10.1001/jama.2013.6295
6.
Ehrenberg HM Weiner SJ . Maternal obesity, uterine activity, and the risk of spontaneous preterm birth. Obstet Gynecol. (2009) 113:1373–4. 10.1097/AOG.0b013e3181a8673e
7.
Su XJ Huang SJ Li X Du QL . Prepregnancy overweight and obesity are associated with an increased risk of preterm birth in Chinese women. Obes Facts. (2020) 13:237–44. 10.1159/000506688
8.
Stewart FM Freeman DJ Ramsay JE Greer IA Caslake M Ferrell WR . Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. (2007) 92:969–75. 10.1210/jc.2006-2083
9.
Cappelletti M Della Bella S Ferrazzi E Mavilio D Divanovic S . Inflammation and preterm birth. J Leukoc Biol. (2016) 99:67–78. 10.1189/jlb.3MR0615-272RR
10.
Catov JM Bodnar LM Kip KE Hubel C Ness RB Harger G et al . Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. (2007) 197:610. e611-610. e617. 10.1016/j.ajog.2007.04.024
11.
Zhang B Huang R Yang D Chen G Chen Y Han J et al . Combination of colchicine and ticagrelor inhibits carrageenan-induced thrombi in mice. Oxid Med Cell Longev. (2022) 2022:3087198. 10.1155/2022/3087198
12.
Duarsa ABS Widiyanto A Irene Putri S Anulus A Tri Atmojo J Sani Fajriah A . The association between body mass index and all-cause mortality: a meta-analysis. Int J Health Sci. (2022) 6:367–83. 10.53730/ijhs.v6nS4.5521
13.
Ling J Chen S Zahry NR Kao TSA . Economic burden of childhood overweight and obesity: a systematic review and meta-analysis. Obesity Rev. (2023) 24:e13535. 10.1111/obr.13535
14.
Dutt A Saraswat S Thakur N . Effect of increasing body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. Int J Acad Med Pharm. (2023) 5:1383–7. 10.47009/jamp.2023.5.2.290
15.
Reichetzeder C . Overweight and obesity in pregnancy: their impact on epigenetics. Eur J Clin Nutr. (2021) 75:1710–22. 10.1038/s41430-021-00905-6
16.
Hill B Skouteris H Teede HJ Bailey C Baxter JB Bergmeier HJ et al . Health in preconception, pregnancy and postpartum global alliance: international network preconception research priorities for the prevention of maternal obesity and related pregnancy and long-term complications. J Clin Med. (2019) 8:2119. 10.3390/jcm8122119
17.
Genovesi S Antolini L Orlando A Gilardini L Bertoli S Giussani M et al . Cardiovascular risk factors associated with the metabolically healthy obese (MHO) phenotype compared to the metabolically unhealthy obese (MUO) phenotype in children. Front Endocrinol. (2020) 11:27. 10.3389/fendo.2020.00027
18.
Blüher M . Metabolically healthy obesity. Endocr Rev. (2020) 41:bnaa004. 10.1210/endrev/bnaa004
19.
Vukovic R Dos Santos TJ Ybarra M Atar M . Children with metabolically healthy obesity: a review. Front Endocrinol. (2019) 10:865. 10.3389/fendo.2019.00865
20.
Schulze MB Stefan N . Metabolically healthy obesity: from epidemiology and mechanisms to clinical implications. Nat Rev Endocrinol. (2024) 20:633–46. 10.1038/s41574-024-01008-5
21.
Piché M-E Tchernof A Després J-P . Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. (2020) 126:1477–500. 10.1161/CIRCRESAHA.120.316101
22.
Liu X et al . Risk of cardiovascular diseases among different metabolic obesity phenotypes: a prospective observational study. Metab Syndr Relat Disord. (2023) 21:306–13. 10.1089/met.2022.0100
23.
Acharya S Shukla S Wanjari A . Subclinical risk markers for cardiovascular disease (CVD) in metabolically healthy obese (MHO) subjects. J Clin Diagn Res. (2019) 13:OC01–6 10.7860/JCDR/2019/41317.12890
24.
Bei-Fan Z . Cooperative meta-analysis group of working group on obesity in China predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr. (2002) 11:S685–93. 10.1046/j.1440-6047.11.s8.9.x
25.
Pétursdóttir Maack H Larsson A Axelsson O Olovsson M Wikström AK Sundström Poromaa I . Pregnancy in metabolic healthy and unhealthy obese women. Acta Obstet Gynecol Scand. (2020) 99:1640–8. 10.1111/aogs.13929
26.
Chen C Zhang JW Xia HW Zhang HX Betran AP Zhang L et al . Preterm birth in China between 2015 and 2016. Am J Public Health. (2019) 109:1597–604. 10.2105/AJPH.2019.305287
27.
Denison FC Aedla NR Keag O Hor K Reynolds RM Milne A et al . Care of women with obesity in pregnancy. BJOG. (2019) 126:e62–e106. 10.1111/1471-0528.15386
28.
Ju AC Heyman MB Garber AK Wojcicki JM . Maternal obesity and risk of preterm birth and low birthweight in Hawaii PRAMS, 2000–2011. Matern Child Health J. (2018) 22:893–902. 10.1007/s10995-018-2464-7
29.
Kivelä J Sormunen-Harju H Girchenko PV Huvinen E Stach-Lempinen B Kajantie E et al . Longitudinal metabolic profiling of maternal obesity, gestational diabetes, and hypertensive pregnancy disorders. J Clin Endocrinol Metab. (2021) 106:e4372–88. 10.1210/clinem/dgab475
30.
Nijman TA van Vliet EO Benders MJ Mol BW Franx A Nikkels PG et al . Placental histology in spontaneous and indicated preterm birth: a case control study. Placenta. (2016) 48:56–62. 10.1016/j.placenta.2016.10.006
31.
Doshani A Konje JC . Placental dysfunction in obese women and antenatal surveillance. Best Pract Res Clin Obstet Gynaecol. (2023) 91:102407. 10.1016/j.bpobgyn.2023.102407
32.
Koppe L Fouque D Soulage CO . Metabolic abnormalities in diabetes and kidney disease: role of uremic toxins. Curr Diab Rep. (2018) 18:1–9. 10.1007/s11892-018-1064-7
33.
Wang TY Wang RF Bu ZY Targher G Byrne CD Sun DQ et al . Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. (2022) 18:259–68. 10.1038/s41581-021-00519-y
34.
Yun HR Kim H Park JT Chang TI Yoo TH Kang SW et al . Obesity, metabolic abnormality, and progression of CKD. Am J Kidney Dis. (2018) 72:400–10. 10.1053/j.ajkd.2018.02.362
35.
Lee H Lee Y-h Kim SU Kim HC . Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. (2021) 19:2138–47. e2110. 10.1016/j.cgh.2020.12.022
36.
Rotariu D Babes EE Tit DM Moisi M Bustea C Stoicescu M et al . Oxidative stress–Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed Pharmacother. (2022) 152:113238. 10.1016/j.biopha.2022.113238
37.
Brown MC Best KE Pearce MS Waugh J Robson SC Bell R . Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. (2013) 28:1–19. 10.1007/s10654-013-9762-6
38.
Riise H Sulo G Tell GS Igland J Nygård O Iversen A-C et al . Association between gestational hypertension and risk of cardiovascular disease among 617 589 Norwegian women. J Am Heart Assoc. (2018) 7:10. 10.1161/JAHA.117.008337
39.
Sharami SH Gholipour M Milani F Kazemnejad E Heirati SFD Ranjbar ZA . The association between dyslipidemia and preterm birth: a prospective cohort study in the north of Iran. Endocr Metab Immune Disord Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). (2020) 20:227–33. 10.2174/1871530319666190529090517
40.
Ji X Wu C Chen M Wu L Li T Miao Z et al . Analysis of risk factors related to extremely and very preterm birth: a retrospective study. BMC Pregnancy Childbirth. (2022) 22:818. 10.1186/s12884-022-05119-7
41.
Lv Y Xu L He Z Liu X Guo Y . The association between pregnancy levels of blood lipids and the risk of preterm birth. Sci Rep. (2024) 14:10800. 10.1038/s41598-024-61119-x
42.
Rhee E-J . Being metabolically healthy, the most responsible factor for vascular health. Diabetes Metab J. (2018) 42:19. 10.4093/dmj.2018.42.1.19
Summary
Keywords
obesity metabolic phenotypes, metabolism, preterm birth, prospective cohort research, interaction analysis
Citation
Chen J, Miao Y, Li Q, Zhang Q, Sun B, Wu Z, Liu W, Liu J, Shi H, Gao H, Li W, Zhu Y and Li H (2025) Maternal obesity phenotype, metabolic dysfunction, and preterm birth: a prospective birth cohort study. Front. Nutr. 12:1648996. doi: 10.3389/fnut.2025.1648996
Received
18 June 2025
Accepted
29 September 2025
Published
22 October 2025
Volume
12 - 2025
Edited by
Jingya Wang, University of Birmingham, United Kingdom
Reviewed by
Quanfu Zhang, Baoan Women's and Children's Hospital, China
Madhulima Saha, Command Hospital Kolkata, India
Updates
Copyright
© 2025 Chen, Miao, Li, Zhang, Sun, Wu, Liu, Liu, Shi, Gao, Li, Zhu and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibo Li haiboli89@163.comYibing Zhu zybfmc@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.