Abstract
Background:
Sarcopenia is a systemic disorder characterized by the progressive loss of skeletal muscle mass and function; however, its impact on the treatment outcomes of patients with esophageal cancer remains inconclusive. We aimed to evaluate the impact of sarcopenia and dynamic changes in skeletal muscle during treatment on neoadjuvant immunochemotherapy (NICT) efficacy and prognosis in patients with locally advanced ESCC.
Methods:
We retrospectively included 272 patients with locally advanced ESCC who received NICT. We calculated the skeletal muscle index (SMI) and its rate of change (ΔSMI%) from CT images at the L3 vertebral level obtained before and after treatment. Sarcopenia was defined as an SMI < 52.4 cm2/m2 in men and <38.5 cm2/m2 in women, and a ΔSMI% < −2.8% was designated as excessive skeletal muscle loss.
Results:
The prevalence of sarcopenia increased from 50.9% before treatment to 55.1% at therapy completion. Pre-NICT sarcopenia correlated with tumor progression (p = 0.02) and was associated with a significantly lower pathological complete response (pCR) in patients who had sarcopenia than in those without (14.7% vs. 25.0%, p = 0.04). Patients with tumor progression had a significantly lower SMI than those in the disease-control group (41.6 ± 7.24 vs. 48.71 ± 8.39, p = 0.04). In a subgroup analysis of excessive skeletal muscle loss, these patients experienced higher hematologic toxicity (leukopenia: 33.4% vs. 20.9%, p = 0.04; anemia: 70.7% vs. 50.6%, p = 0.01) and lower pCR rate (12.0% vs. 22.8%, p = 0.05). After a median follow-up of 20.4 months, sarcopenia before or after NICT did not significantly affect overall survival (OS) or disease-free survival (DFS) (p > 0.05). Conversely, excessive skeletal muscle loss during treatment emerged as an independent prognostic factor for OS in multivariate analysis (HR = 0.47; 95% CI, 0.25–0.91; p = 0.03); however, it was not associated with DFS (p = 0.22).
Conclusion:
Treatment-induced excessive skeletal muscle loss may serve as a predictive marker for NICT toxicity and short-term survival in patients with locally advanced ESCC, highlighting the need for dynamic nutritional monitoring to optimize treatment tolerance.
Introduction
Esophageal squamous cell carcinoma (ESCC) is a malignancy that poses a significant threat to human health (1). The advent of immune checkpoint inhibitors (ICIs) has made neoadjuvant immunochemotherapy (NICT) an important treatment strategy for patients with locally advanced ESCC (2–4). However, a considerable number of patients demonstrate primary resistance to ICIs and may even experience disease progression (2, 5). Currently, programmed death-ligand 1 (PD-L1) remains the only Food and Drug Administration-approved predictive biomarker for immunotherapy; however, concerns exist regarding its high testing costs and variable accuracy (6). Multiple studies have shown that even PD-L1–negative patients benefit from immunotherapy (2, 7, 8). Therefore, identifying novel predictive markers that are more cost-effective and reliable is urgently required.
Sarcopenia is a systemic disorder characterized by the progressive loss of skeletal muscle mass and function and is closely associated with malnutrition, reduced physical activity, and chronic inflammation (9–11). This condition is recognized as an independent risk factor for poor prognosis across various malignancies (12–14). In patients with ESCC presenting primarily with dysphagia, the reported incidence of sarcopenia ranges from 44.0–74.2%; nonetheless, its treatment-related risks remain frequently underestimated (9, 15–17). Notably, esophageal cancer patients face a risk of nutritional decline from the time of diagnosis, as tumor-mediated metabolic competition, swallowing impairment, and treatment toxicity collectively accelerate muscle loss (18). The impact of sarcopenia on the treatment outcomes of patients with esophageal cancer has been investigated in several studies; however, their findings remain inconclusive. In some studies, it was suggested that sarcopenia is associated with poor prognosis (12–14), while in others, conflicting findings were reported (18–20). Moreover, skeletal muscle mass represents a continuously changing, dynamic parameter. Nevertheless, most current studies rely on cross-sectional assessments at a single time point, and the potential value of dynamic changes in muscle mass during treatment is overlooked. Furthermore, existing evidence primarily pertains to neoadjuvant chemoradiotherapy, with limited studies on immunotherapy.
In this study, we evaluated the effects of sarcopenia and dynamic changes in skeletal muscle mass during treatment on therapeutic response and survival outcomes in patients with locally advanced ESCC undergoing NICT. We further identified independent risk factors for sarcopenia, anticipating that these findings will provide valuable insights for risk stratification and individualized treatment planning for patients with ESCC.
Materials and methods
Patients
We retrospectively analyzed the clinical records of patients with esophageal cancer who received neoadjuvant therapy followed by surgical resection at the Affiliated Hospital of North Sichuan Medical College between 2020 and 2024. The institutional ethics committee approved the study (File Number: 2025ER240-1), which was conducted in accordance with the 2013 Declaration of Helsinki; written informed consent was waived owing to its retrospective design. Inclusion criteria were: (i) age 18–80 years and a preoperative diagnosis of locally advanced, resectable ESCC staged at least cT3 or N+; (ii) receipt of at least two cycles of NICT at our institution, followed by minimally invasive McKeown esophagectomy. Exclusion criteria included: (i) prior antitumor treatments or distant metastases; (ii) palliative resection or exploratory surgery only; (iii) lack of abdominal CT images before or after treatment, or incomplete clinical records. Pathological staging was determined following the 8th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system. Figure 1 shows the patient selection process.
Figure 1
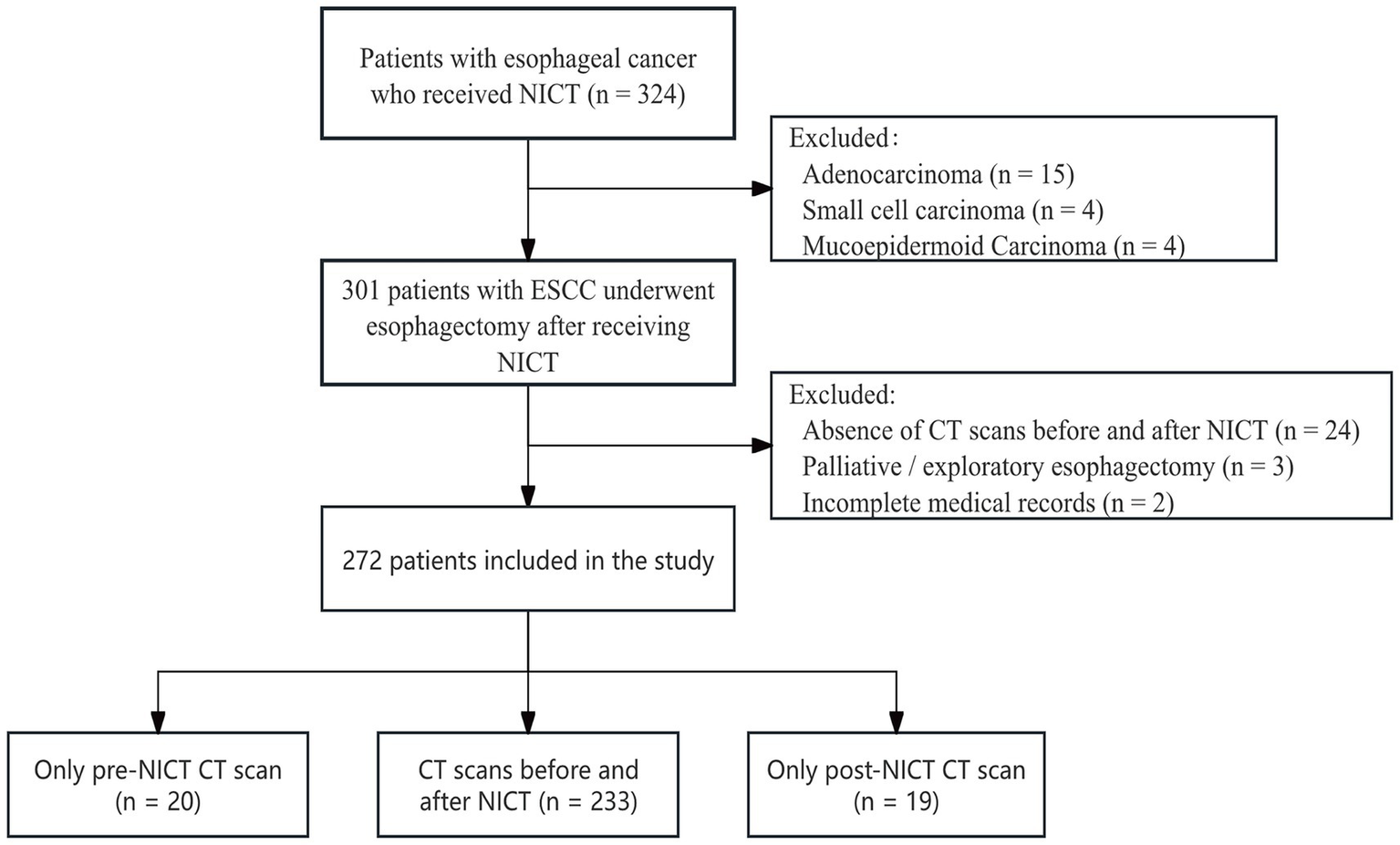
Flow chart of the study.
Assessment of skeletal muscle loss and definition of sarcopenia
We quantified changes in patients’ skeletal muscle mass, using the Skeletal Muscle Index (SMI) and semi-automatically delineated regions of interest (ROIs) in muscle tissue, defined by Hounsfield unit thresholds of −29 to +150 HU, using the SliceOmatic software. We calculated SMI as the total cross-sectional area of all skeletal muscles at the L3 level on pre- and post-treatment abdominal CT scans (cm2) divided by height squared (m2). Using large-scale population data (21), we defined sarcopenia as an SMI < 52.4 cm2/m2 in men and <38.5 cm2/m2 in women. Changes in SMI before and after NICT were expressed as a percentage: ΔSMI% = (SMI_post-NICT – SMI_pre-NICT)/SMI_pre-NICT × 100%. Supplementary Figure 1 presents a single patient’s CT images showing normal muscle mass pre-NICT and sarcopenia post-NICT.
Neoadjuvant therapy regimens and surgery
The NICT regimen comprised a platinum agent [80 mg/m2 intravenously (IV) on day 1] combined with albumin-bound paclitaxel (200 mg/m2 IV on day 2), followed by an ICI (200 mg IV on day 3). All patients underwent at least two treatment cycles, with an inter-cycle interval of more than 3 weeks. Upon completing NICT, a multidisciplinary team assessed tumor resectability. For patients deemed suitable for curative resection, a minimally invasive three-incision approach (right thoracic, upper abdominal, and left cervical) was employed, together with a 2.5-field lymphadenectomy (22). Postoperatively, we scheduled follow-up visits every 3 months for the first 2 years and every 6 months thereafter. Nutritional interventions were not standardized in this study; in routine practice, patients with dysphagia underwent dietitian assessment and, if indicated, received oral nutritional supplements or nasogastric tube feeding.
Endpoints
The primary endpoints were overall survival (OS) and disease-free survival (DFS). We used a Cox proportional hazards regression model to adjust for clinical covariates and evaluate their prognostic significance. OS was defined as the interval between surgery and death from any cause, while DFS was defined as the interval between surgery and first recurrence or death from any cause. Secondary endpoints comprised neoadjuvant treatment-related adverse events, treatment response rate, pathological complete response (pCR), and postoperative complication rate. We employed a multivariate logistic regression model to identify independent risk factors for sarcopenia before and after neoadjuvant therapy. Post-treatment responses were classified following the Response Evaluation Criteria in Solid Tumors (RECIST) as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD); CR, PR, and SD were collectively defined as disease control (DC). Treatment-related adverse events (TRAEs) were graded following the guidelines in version 5.0 of the National Cancer Institute’s Common Terminology Criteria for Adverse Events.
Statistical analysis
Continuous variables that are normally distributed are expressed as mean ± standard deviation (SD). Non-normally distributed variables are expressed as median (interquartile range [IQR]). We compared continuous variables between groups using the Student’s t-test or the Wilcoxon rank-sum test, depending on their distribution. The chi-square test or Fisher’s exact test was used to compare categorical variables. OS and DFS were estimated using the Kaplan–Meier method; between-group differences were assessed with the log-rank test, and survival curves were plotted in R version 4.3.2. The Kolmogorov–Smirnov (K–S) test indicated that ΔSMI% followed a normal distribution (p < 0.001). We used the Maxstat package in R to determine the optimal ΔSMI% cutoff for OS discrimination. A threshold of −2.8% was identified (p < 0.05), and ΔSMI% < −2.8% was defined as excessive skeletal muscle loss. Statistical analyses were performed using SPSS version 25.0 and R version 4.3.2. Two-sided p-values < 0.05 were considered statistically significant.
Results
Baseline characteristics
We included 272 patients with ESCC treated with NICT followed by surgical resection, with imaging data available for 253 patients before neoadjuvant therapy and for 252 patients after treatment. The baseline clinical characteristics of all patients are shown in Supplementary Table 1. The median age of the patients was 65 years, and the median BMI was 22.9 (IQR 20.8–24.9); most patients were male (73.2%) and reported a history of smoking (52.9%) or alcohol consumption (46.7%). Tumors were located predominantly in the middle third of the esophagus (71.3%) and in the lower third (16.5%). Prior to NICT, clinical staging was predominantly stage III (44.5%), followed by stage II (36.8%).
Characteristics of sarcopenia and multivariate analysis
Table 1 shows the clinicopathological characteristics of patients with sarcopenia versus those without sarcopenia before and after neoadjuvant therapy. Prior to NICT, 129 patients (50.9%) had sarcopenia, which increased to 139 patients (55.1%) at treatment completion. Supplementary Figure 2 shows that, according to RECIST criteria, those without sarcopenia did not experience PD before or after treatment, and those achieving DC had significantly higher SMI values than did the patients with PD (pre-treatment: 48.71 ± 8.39 vs. 41.60 ± 7.24, p = 0.04; post-treatment: 48.43 ± 8.71 vs. 39.57 ± 9.36, p = 0.02). Additionally, the pCR rate was higher in patients without sarcopenia before treatment (25.0% vs. 14.7%; p = 0.04), although the difference was not significant after treatment (p = 0.081 and p = 0.197).
Table 1
| Variables | Total (n = 253) | Pre-NICT sarcopenia | p-value | Total (n = 252) | Post-NICT sarcopenia | p-value | ||
|---|---|---|---|---|---|---|---|---|
| No (n = 124) | Yes (n = 129) | No (n = 113) | Yes (n = 139) | |||||
| Age, median, years [IQR] | 67(59–70) | 66(58–69) | 67(60–72) | 0.048 | 67(60–71) | 66(58–69) | 68(61–72) | 0.023 |
| BMI, median, [IQR] | 22.9(20.8–24.9) | 24.2(22.5–25.9) | 21.5(19.9–23.5) | <0.001 | 22.7(20.7–24.7) | 24.1(22.4–25.8) | 21.4(19.9–23.5) | <0.001 |
| Sex | ||||||||
| Male | 188(74.3%) | 79(63.7%) | 109(84.5%) | <0.001 | 188(74.6%) | 76(67.3%) | 112(80.6%) | 0.016 |
| Female | 65(25.7%) | 45(36.3%) | 20(15.5%) | 64(25.4%) | 37(32.7%) | 27(19.4%) | ||
| Smoking | ||||||||
| No | 119(47%) | 73(58.9%) | 46(35.7%) | <0.001 | 115(45.6%) | 57(50.4%) | 58(41.7%) | 0.167 |
| Yes | 134(53%) | 51(41.1%) | 83(64.3%) | 137(54.4%) | 56(49.6%) | 81(58.3%) | ||
| Drinking | ||||||||
| No | 133(52.6%) | 79(63.7%) | 54(41.9%) | <0.001 | 131(52.0%) | 65(57.5%) | 66(47.5%) | 0.113 |
| Yes | 120(47.4%) | 45(36.3%) | 75(58.1%) | 121(48.0%) | 48(42.5%) | 73(52.5%) | ||
| Hypertension | ||||||||
| No | 201(79.4%) | 94(75.8%) | 107(82.9%) | 0.160 | 199(79.0%) | 84(74.3%) | 115(82.7%) | 0.104 |
| Yes | 52(20.6%) | 30(24.2%) | 22(17.1%) | 53(21.0%) | 29(25.7%) | 24(17.3%) | ||
| Diabetes | ||||||||
| No | 237(93.7%) | 113(91.1%) | 124(96.1%) | 0.103 | 236(93.7%) | 105(92.9%) | 131(94.2%) | 0.668 |
| Yes | 16(6.3%) | 11(8.9%) | 5(3.9%) | 16(6.3%) | 8(7.1%) | 8(5.8%) | ||
| Cardiopathy | ||||||||
| No | 239(94.5%) | 116(93.5%) | 123(95.3%) | 0.531 | 238(94.4%) | 105(92.9%) | 133(95.7%) | 0.341 |
| Yes | 14(5.5%) | 8(6.5%) | 6(4.7%) | 14(5.6%) | 8(7.1%) | 6(4.3%) | ||
| COPD | ||||||||
| No | 226(89.3%) | 112(90.3%) | 114(88.4%) | 0.615 | 224(88.9%) | 100(88.5%) | 124(89.2%) | 0.858 |
| Yes | 27(10.7%) | 12(9.7%) | 15(11.6%) | 28(11.1%) | 13(11.5%) | 15(10.8%) | ||
| Tumor location | ||||||||
| Upper | 32(12.6%) | 20(16.1%) | 12(9.3%) | 0.087 | 30(11.9%) | 12(10.6%) | 18(12.9%) | 0.721 |
| Middle | 180(71.1%) | 89(71.8%) | 91(70.5%) | 181(71.8%) | 84(74.3%) | 97(69.8%) | ||
| Lower | 41(16.2%) | 15(12.1%) | 26(20.2%) | 41(16.3%) | 17(15%) | 24(17.3%) | ||
| Clinical TNM stage | ||||||||
| II | 93(36.8%) | 50(40.3%) | 43(33.3%) | 0.472 | 183(72.6%) | 87(77.0%) | 96(69.1%) | 0.353 |
| III | 112(44.3%) | 53(42.7%) | 59(45.7%) | 57(22.6%) | 22(19.5%) | 35(31.4%) | ||
| IVA | 48(19.0%) | 21(16.9%) | 27(20.9%) | 12(4.8%) | 4(3.5%) | 8(5.8%) | ||
| Clinical response | ||||||||
| CR + PR | 159(62.8%) | 76(61.3%) | 83(64.3%) | 0.026* | 162(64.3%) | 75(66.4%) | 87(62.6%) | 0.081* |
| SD | 88(34.8%) | 48(38.7%) | 40(31.0%) | 84(33.3%) | 38(33.6%) | 46(33.1%) | ||
| PD | 6(2.4%) | 0 | 6(4.7%) | 6(2.4%) | 0 | 6(2.4%) | ||
| No. of LNs harvested, median, [IQR] | 19(13–26) | 19(13–26) | 20(15–26) | 0.660 | 19(13–26) | 18(13–25) | 21(13–27) | 0.297 |
| Pathological CR | ||||||||
| pCR | 50(19.8%) | 31(25.0%) | 19(14.7%) | 0.040 | 49(19.4%) | 26(23.0%) | 23(16.5%) | 0.197 |
| Non-pCR | 203(80.2%) | 93(75.0%) | 110(85.3%) | 203(80.6%) | 87(77.0%) | 116(83.5%) | ||
| ypTNM stage | ||||||||
| I | 108(42.7%) | 54(53.5%) | 54(41.9%) | 0.996 | 107(42.5%) | 49(43.4%) | 58(41.7%) | 0.859 |
| II | 36(14.2%) | 17(13.7%) | 19(14.7%) | 34(13.5%) | 14(12.4%) | 20(14.4%) | ||
| IIIA | 36(14.2%) | 17(13.7%) | 19(14.7%) | 37(14.7%) | 16(14.2%) | 21(15.1%) | ||
| IIIB | 54(21.3%) | 27(21.8%) | 27(20.9%) | 57(22.6%) | 28(24.8%) | 29(20.9%) | ||
| IVA | 19(7.5%) | 9(7.3%) | 10(7.8%) | 17(6.7%) | 6(5.3%) | 11(7.9%) | ||
Clinical characteristics.
NICT, neoadjuvant immunochemotherapy; BMI, body mass index; CR, complete response; PR, partial response; PD, progression disease; SD, stale disease; pCR, pathological complete response; LN, lymph node, *P-values were derived from Fisher’s exact test.
In the multivariable binary logistic regression (forest plot in Figure 2), age (OR 1.05; 95% CI, 1.01–1.10; p = 0.02), BMI (OR 0.64; 95% CI, 0.56–0.73; p = 0.01), and male sex (OR 2.61; 95% CI, 1.02–6.71; p = 0.04), were identified as independent predictors of pre-NICT sarcopenia. Furthermore, age (OR 1.06; 95% CI, 1.02–1.11; p = 0.01), BMI (OR 0.65; 95% CI, 0.57–0.74; p = 0.01), male sex (OR 4.32; 95% CI, 1.63–11.46; p = 0.01), and clinical N0 stage (OR 0.52; 95% CI, 0.28–0.96; p = 0.03) emerged as independent predictors of post-NICT sarcopenia.
Figure 2
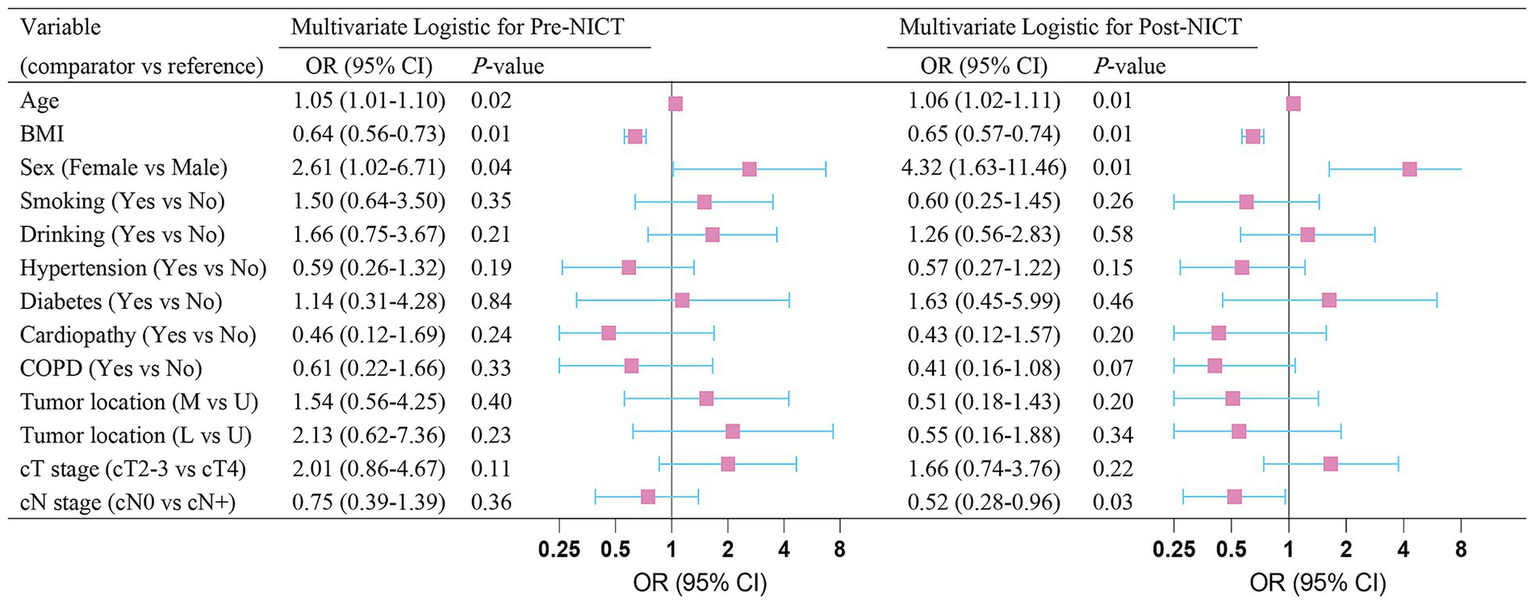
Multivariate logistic-regression analysis of sarcopenia before and after neoadjuvant immunochemotherapy.
Treatment-related adverse events and surgical complications
Patients with sarcopenia and those without demonstrated favorable safety profiles and manageable adverse events before and after NICT. As shown in Table 2, among grade 1–2 treatment-related adverse events before (145, 57.3%) and after (143, 56.7%) NICT, anemia was the most frequent, with a significantly higher incidence in the sarcopenia group than in the non-sarcopenia group (65.1% vs. 49.2%, p = 0.012; 63.3% vs. 48.7%, p = 0.024). The incidence of other TRAEs did not significantly differ between groups. In the pre- (30.8%) and post- (31.7%) NICT cohorts, postoperative pulmonary infection was the most common complication, with no significant difference between patients with sarcopenia and those without (p = 0.304; p = 0.972). We found no significant differences between groups in operative time, intraoperative blood loss, or anastomotic leakage (p > 0.05) (see Table 3).
Table 2
| Variables | Total (n = 253) | Pre-NICT sarcopenia | p-value | Total (n = 252) | Post-NICT sarcopenia | P-value | ||
|---|---|---|---|---|---|---|---|---|
| No (n = 124) | Yes (n = 129) | No (n = 113) | Yes (n = 139) | |||||
| Leukopenia | ||||||||
| None | 190(75.1%) | 98(79%) | 92(71.3%) | 0.137 | 187(74.2%) | 90(79.6%) | 97(69.8%) | 0.075 |
| Grade 1–2 | 58(22.9%) | 25(20.2%) | 33(25.6%) | 59(23.4%) | 21(18.6%) | 38(27.3%) | ||
| Grade 3–4 | 5(2.0%) | 1(0.8%) | 4(3.1%) | 6(2.4%) | 2(1.8%) | 4(2.9%) | ||
| Neutropenia | ||||||||
| None | 219(86.6%) | 111(89.5%) | 108(83.7%) | 0.175 | 216(85.7%) | 102(90.3%) | 114(82.0%) | 0.059 |
| Grade 1–2 | 23(9.1%) | 9(7.3%) | 14(10.9%) | 24(9.5%) | 8(7.1%) | 16(11.5%) | ||
| Grade 3–4 | 11(4.3%) | 4(3.2%) | 7(5.4%) | 12(4.8%) | 3(2.7%) | 9(6.5%) | ||
| Anemia | ||||||||
| None | 106(41.9%) | 62(50.0%) | 44(34.1%) | 0.012 | 107(42.5%) | 57(50.4%) | 50(36.0%) | 0.024 |
| Grade 1–2 | 145(57.3%) | 61(49.2%) | 84(65.1%) | 143(56.7%) | 55(48.7%) | 88(63.3%) | ||
| Grade 3–4 | 2(0.8%) | 1(0.8%) | 1(0.8%) | 2(0.8%) | 1(0.9%) | 1(0.7%) | ||
| Thrombocytopenia | ||||||||
| None | 206(81.4%) | 100(80.6%) | 106(82.2%) | 0.757 | 206(81.7%) | 91(80.5%) | 115(82.7%) | 0.652 |
| Grade 1–2 | 45(17.8%) | 23(18.5%) | 22(17.1%) | 44(17.5%) | 21(18.6%) | 23(16.5%) | ||
| Grade 3–4 | 2(0.8%) | 1(0.8%) | 1(0.8%) | 2(0.8%) | 1(0.9%) | 1(0.7%) | ||
| Liver Abnormalities | ||||||||
| None | 195(77.1%) | 93(75.0%) | 102(79.1%) | 0.660 | 199(79.0%) | 85(75.2%) | 114(82.0%) | 0.191 |
| Grade 1–2 | 55(21.7%) | 29(23.4%) | 26(20.2%) | 51(20.2%) | 27(23.9%) | 24(17.3%) | ||
| Grade 3–4 | 3(1.2%) | 2(1.6%) | 1(0.8%) | 2(0.8%) | 1(0.9%) | 1(0.7%) | ||
| Kidney Abnormalities | ||||||||
| None | 239(94.5%) | 114(91.9%) | 125(96.9%) | 0.103 | 240(95.2%) | 105(92.9%) | 135(97.1%) | 0.144 |
| Grade 1–2 | 14(5.5%) | 10(8.1%) | 4(3.1%) | 12(4.8%) | 8(7.1%) | 4(2.9%) | ||
| Grade 3–4 | 0 | 0 | 0 | 0 | 0 | 0 | ||
TRAEs of neoadjuvant therapy.
TRAEs, treatment-related adverse events; NICT, neoadjuvant immunochemotherapy.
Table 3
| Variables | Pre-NICT sarcopenia | Post-NICT sarcopenia | Total (n = 253) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No (n = 124) | Yes (n = 129) | p-value | Total (n = 252) | No (n = 113) | Yes (n = 139) | p-value | |||
| Operation time, median, min, [IQR] | 210(185–240) | 205(185–237) | 210(190–245) | 0.255 | 209(185–240) | 205(185–238) | 213(190–244) | 0.223 | |
| Blood loss, median, ml, [IQR] | 100(60–100) | 100(60–100) | 100(62–100) | 0.520 | 100(10–100) | 100(55–100) | 100(68–100) | 0.640 | |
| Pulmonary infection | |||||||||
| No | 175(69.2%) | 82(66.1%) | 93(72.1%) | 0.304 | 172(68.3%) | 77(68.1%) | 95(68.3%) | 0.972 | |
| Yes | 78(30.8%) | 42(33.9%) | 36(27.9%) | 80(31.7%) | 36(31.9%) | 44(31.7%) | |||
| Anastomotic leakage | |||||||||
| No | 245(96.8%) | 120(96.8%) | 125(96.9%) | 1.000* | 242(96.0%) | 108(95.6%) | 134(96.4%) | 0.738 | |
| Yes | 8(3.2%) | 4(3.2%) | 4(3.1%) | 10(4.0%) | 5(4.4%) | 5(3.6%) | |||
| Gastric emptying disorders | |||||||||
| No | 247(97.6%) | 120(96.8%) | 127(98.4%) | 0.439* | 245(97.2%) | 108(9.6%) | 137(98.6%) | 0.248* | |
| Yes | 6(2.4%) | 4(3.2%) | 2(1.6%) | 7(2.8%) | 5(4.4%) | 2(1.4%) | |||
| Respiratory failure | |||||||||
| No | 251(99.2%) | 122(98.4%) | 129(100%) | 0.239* | 250(99.2%) | 112(99.1%) | 138(99.3%) | 1.000* | |
| Yes | 2(0.8%) | 2(1.6%) | 0 | 2(0.8%) | 1(0.9%) | 1(0.7%) | |||
Complications after surgical treatment.
NICT, neoadjuvant immunochemotherapy, *P-values were derived from Fisher’s exact test.
Survival outcomes and prognostic factors
After a median follow-up of 20.4 months (95% CI: 19.2–21.6), 61 patients had died. The 1- and 2-year OS rates were 87 and 74%, respectively, whereas the DFS rates were 77 and 62%. Kaplan–Meier curve (Figure 3) shows that OS or DFS did not significantly differ between patients with sarcopenia and those without, either before or after NICT (p > 0.05). To assess the prognostic impact of dynamic skeletal muscle changes during NICT, we analyzed 233 patients with complete pre- and post-treatment imaging to calculate ΔSMI%. We used the Maxstat package in R to determine the optimal ΔSMI% cutoff for OS; a threshold of −2.8% (Figure 4) was used to stratify patients into low (<−2.8%) and high (≥−2.8%) ΔSMI% groups (see Supplementary Table 2 for baseline characteristics). Kaplan–Meier curve (Figure 5) shows that the 2-year OS rate was significantly higher in the ΔSMI% ≥ −2.8% group than in the ΔSMI% < −2.8% group (78% vs. 67%; p = 0.015). However, the 2-year DFS did not significantly differ between the two groups (63% vs. 57%; p = 0.22). Finally, we constructed a Cox proportional hazards regression model to further evaluate the prognostic value of relevant variables (Table 4). In the univariate analysis, age, chronic obstructive pulmonary disease, and ΔSMI% were associated with poorer OS; after multivariate adjustment, ΔSMI% remained an independent predictor of OS (high vs. low: HR 0.47; 95% CI, 0.25–0.91; p = 0.03).
Figure 3
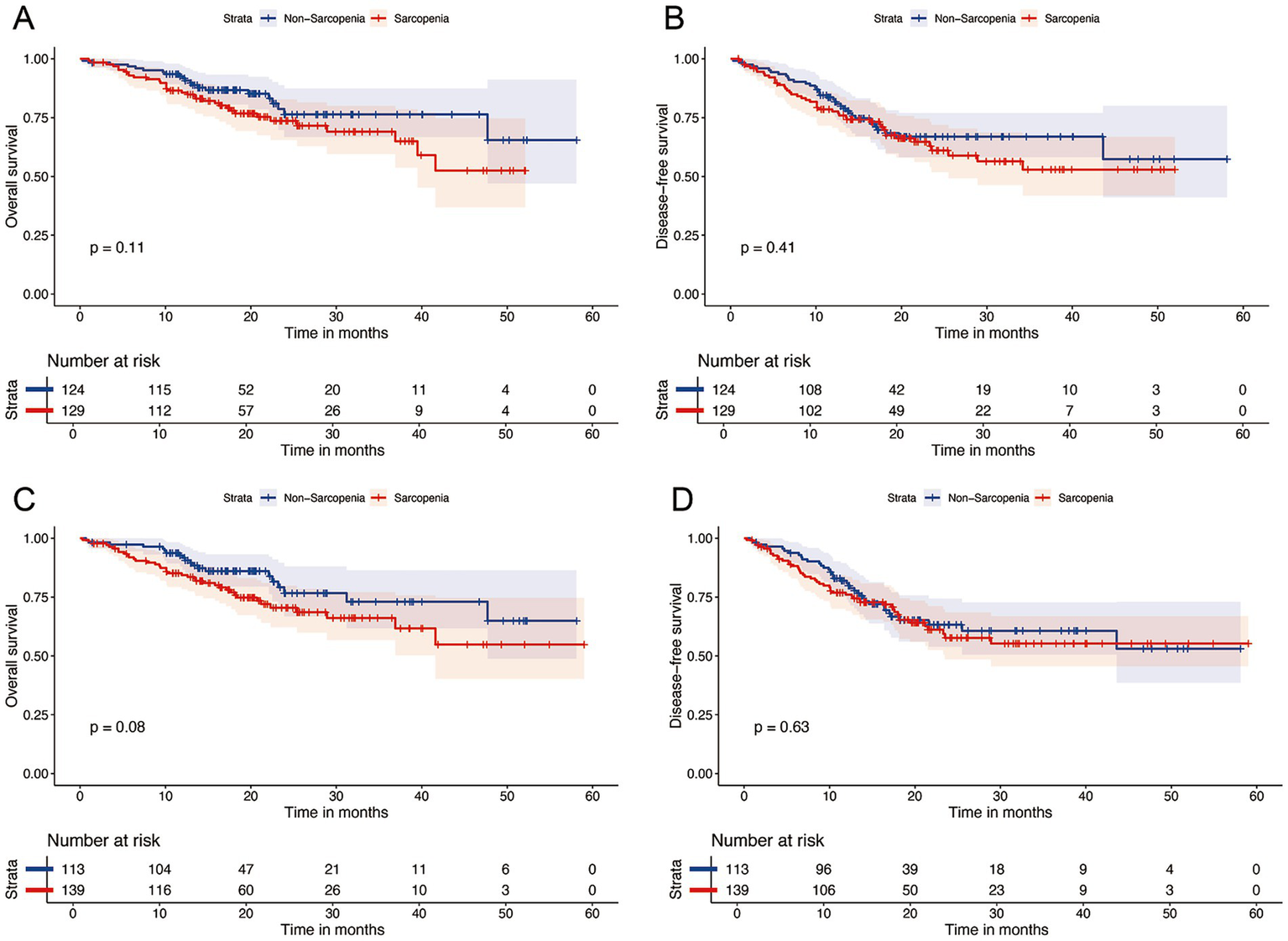
Kaplan–Meier survival analysis of OS (A) and DFS (B) between Pre-NICT sarcopenia and Pre-NICT non-sarcopenia; Kaplan–Meier survival analysis of OS (C) and DFS (D) between Post-NICT sarcopenia and Post-NICT non-sarcopenia.
Figure 4
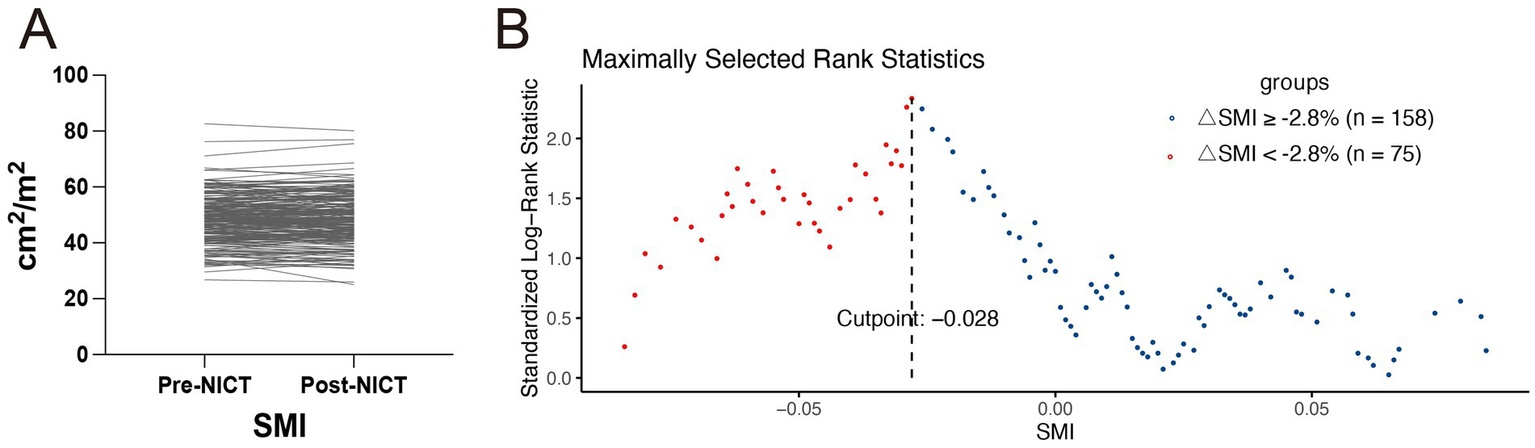
(A) Changes in skeletal muscle index of all patients before and after neoadjuvant immunochemotherapy. (B) The optimal cutoff value for OS determined using the Maxstat package in R software.
Figure 5
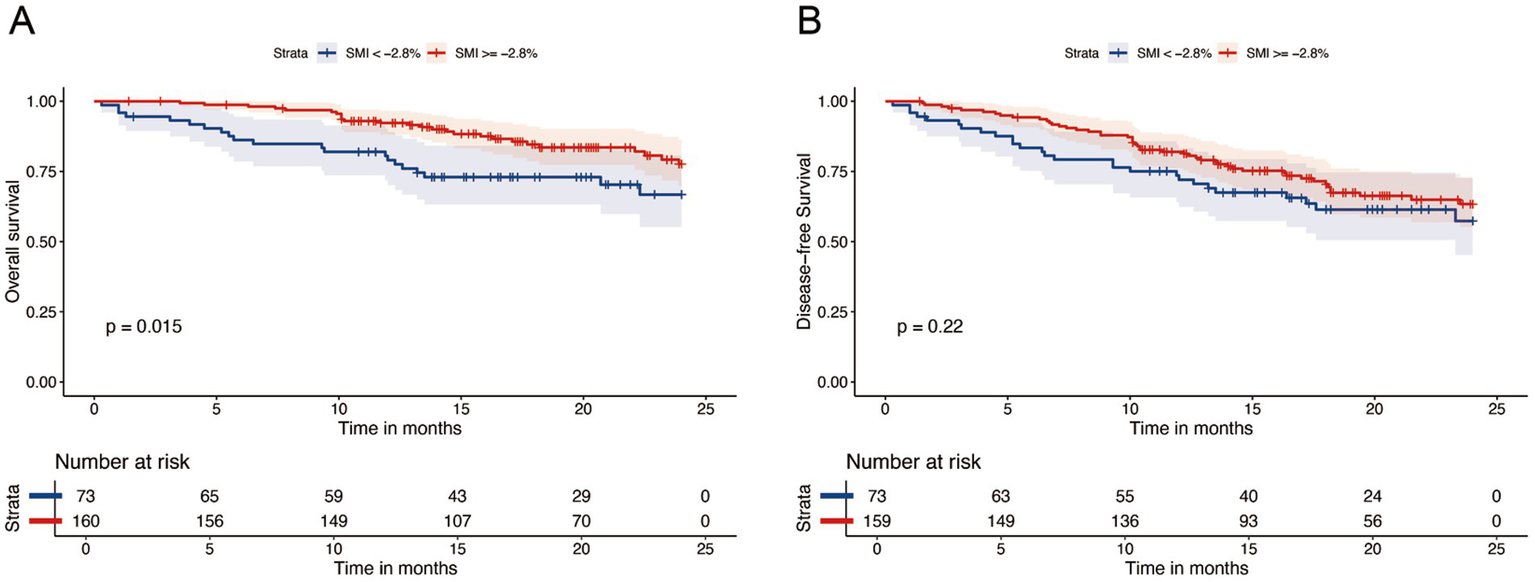
Kaplan–Meier survival analysis of OS (A) and DFS (B) between △SMI ≥ −2.8% and △SMI < −2.8%.
Table 4
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.05 (1.00–1.10) | 0.017 | 1.04 (1.00–1.09) | 0.051 |
| BMI | 0.92 (0.83–1.02) | 0.131 | ||
| Sex (reference: female) | ||||
| Male | 1.32 (0.64–2.73) | 0.456 | ||
| Smoking (reference: no) | ||||
| Yes | 0.92 (0.52–1.64) | 0.786 | ||
| Drinking (reference: no) | ||||
| Yes | 1.02 (0.58–1.80) | 0.95 | ||
| Hypertension (reference: no) | ||||
| Yes | 1.43 (0.76–2.72) | 0.27 | ||
| Diabetes (reference: no) | ||||
| Yes | 1.57 (0.56–4.38) | 0.389 | ||
| Cardiopathy (reference: no) | ||||
| Yes | 2.03 (0.80–5.12) | 0.136 | ||
| COPD (reference: no) | ||||
| Yes | 2.13 (1.03–4.41) | 0.042 | 2.02 (0.97–4.19) | 0.06 |
| Tumor location (reference: upper) | ||||
| Middle | 0.98 (0.41–2.33) | 0.962 | ||
| Lower | 0.86 (0.29–2.56) | 0.788 | ||
| cT stage (reference: cT4) | ||||
| cT2-3 | 0.67 (0.33–1.34) | 0.256 | ||
| cN stage (reference: cN+) | ||||
| N0 | 0.63 (0.34–1.17) | 0.143 | ||
| △SMI (reference: <−2.8%) | ||||
| ≥−2.8% | 0.50 (0.28–0.89) | 0.015 | 0.54 (0.30–0.97) | 0.04 |
| Pre-NICT sarcopenia (reference: no) | ||||
| Yes | 1.45 (0.81–2.62) | 0.214 | ||
| Post-NICT sarcopenia (reference: no) | ||||
| Yes | 1.45 (0.80–2.63) | 0.222 | ||
Univariate and Multivariate COX-regression analysis of risk factors for Overall Survival.
BMI, body mass index; cT, clinical tumor; cN, clinical node; SMI, skeletal muscle index; NICT, neoadjuvant immunochemotherapy.
Comparison between the high-level (△SMI% > −2.8%) group and the low-level (△SMI% < −2.8%) group
Supplementary Table 2 shows that, apart from a significantly higher BMI in the high-level group versus the low-level group (23.2 [IQR 21.3–24.9] vs. 21.6 [19.8–24.5]; p = 0.01), baseline clinical characteristics were otherwise balanced between cohorts. Tumors in the high-level group were predominantly in the mid-esophagus (75.9%) and lower esophagus (15.8%), whereas those in the low-level group were mainly in the mid-esophagus (62.7%) and upper esophagus (21.3%). A significant difference in pCR rates was observed between groups (high vs. low: 22.8% vs. 12.0%; p = 0.05), with the high-level group exhibiting significantly better pathological downstaging after two NICT cycles (p = 0.03). In Supplementary Table 3, besides anemia (p = 0.01), leukopenia incidence also significantly differed between groups (p = 0.04). The high-level group had a lower rate of postoperative pulmonary infection than did the low-level group, although the difference was not statistically significant (28.5% vs. 37.5%; p = 0.17) (see Supplementary Table 4).
Discussion
In this study, we investigated the impact of sarcopenia and skeletal muscle loss on short-term survival outcomes and treatment response in patients with ESCC during NICT. The results showed that excessive skeletal muscle loss during treatment was a negative prognostic factor, whereas sarcopenia diagnosed before or after neoadjuvant therapy was not. Furthermore, excessive muscle loss may help predict inferior treatment response and an increased incidence of hematologic toxicities.
In our cohort, sarcopenia prevalence increased from 50.9% pre-treatment to 55.1% post-treatment, consistent with prior studies (15, 17) and highlighting its high prevalence in esophageal cancer. Nonetheless, the prognostic relevance of sarcopenia in esophageal cancer remains debated (13, 14, 19, 20, 23). In a prior meta-analysis, sarcopenia was linked to worse DFS and OS outcomes (24). Our findings align with recent studies (19, 20, 23), showing that excessive muscle loss during therapy (not static sarcopenia status) was prognostically detrimental. Han et al. (23) found that excessive muscle loss during treatment was significantly associated with worse OS (HR 2.29; 95% CI 1.42–3.73; p = 0.001) and RFS (HR 1.62; 95% CI 1.12–2.35; p = 0.011). Additionally, Xiao et al. (25) reported a non-linear relationship between ΔSMI% during neoadjuvant chemoradiotherapy and survival outcomes (p < 0.05), with ΔSMI% ≥ 12% serving as an independent prognostic factor for OS and DFS (p = 0.04; p = 0.03). These discrepancies may result from variations in measurement techniques and diagnostic thresholds for sarcopenia, as well as patient selection across studies (26). In cases of ICI therapy, Ying et al. (19) reported that patients with positive ΔSMI% had better OS (p = 0.04). Thus, monitoring dynamic muscle loss may be more clinically relevant than that of static sarcopenia status in treatment planning. Our data show that a ΔSMI% threshold of −2.8% may be the most effective predictor of short-term OS under NICT.
The mechanisms by which sarcopenia influences ESCC progression and immunotherapy response remain unclear. A growing body of evidence indicates that skeletal muscle not only serves locomotor functions but also acts as an endocrine organ, regulating immune responses via paracrine secretion of myokines (27). In the context of immunotherapy, CD4+ and CD8+ T lymphocytes are the principal effector cells mediating antitumor activity (28). Notably, muscle-derived interleukin-15 (IL-15) has been shown to enhance CD8+T-cell survival and cytotoxicity (29). Conversely, when muscle mass is depleted, interleukin-6 (IL-6) levels become aberrantly elevated (30), which suppresses T-cell activation and proliferation, thereby weakening antitumor immunity. Furthermore, skeletal muscle loss is associated with decreased peripheral CD4+ T-cell counts (28, 31), suggesting a predisposition toward T-cell exhaustion. Together, these findings suggest that sarcopenia may undermine the effectiveness of immune checkpoint inhibitors by disrupting the IL-15/IL-6 balance and exacerbating T-cell dysfunction. In NSCLC immunotherapy settings, sarcopenia has been linked to hyperprogressive disease (32, 33). Moreover, Ying et al. (19) found significantly less muscle loss in the DC group compared with that in the non-DC group (p = 0.03) among patients with ESCC undergoing 3 months of NICT, consistent with our findings. We observed that before NICT, patients with sarcopenia had a significantly lower pCR rate than those without sarcopenia did, with a similar trend in the ΔSMI% < −2.8% subgroup. Based on these results, sarcopenia and its dynamic changes may serve as predictive biomarkers of ICI response.
Declines in skeletal muscle mass have been linked to higher rates of chemotherapy-induced cytotoxicity across various solid tumors (16, 34, 35). A prospective study in patients with NSCLC receiving first-line platinum chemotherapy showed that low muscle mass increased the risk of severe hematologic toxicity by approximately 2.5-fold (35). Furthermore, chemotherapy agents may exacerbate muscle atrophy, creating a vicious cycle (9). However, the literature remains controversial regarding whether sarcopenia increases treatment-related adverse events (36, 37). Our results reveal that excessive muscle loss during treatment significantly increases the risks of leukopenia (p = 0.04) and anemia (p = 0.01). Moreover, several studies (22, 33, 34) indicate that sarcopenia may be a risk factor for major postoperative complications. Zhang et al. (38) reported that, in older patients with ESCC, the sarcopenia group had significantly higher rates of postoperative pneumonia (29.8% vs. 16.9%; p < 0.01) and anastomotic leak (9.5% vs. 3.7%; p < 0.05) than did the non-sarcopenic group. In contrast to expectations, sarcopenia did not significantly predict postoperative complications in our study. Existing evidence on sarcopenia is primarily based on neoadjuvant chemoradiotherapy, and real-world data on the potential impact of ICIs on adverse events have not been recently reported. We observed a higher rate of pulmonary infections in the excessive muscle loss group, although the difference was not statistically significant (37.5% vs. 28.5%; p = 0.17).
This study has some limitations. First, although this clinical study is the largest to date assessing sarcopenia’s impact in ICIs-treated ESCC and the first in which ΔSMI% = −2.8% was proposed as a prognostic cutoff, our findings are based on single-center data and require multicenter prospective validation. Therefore, we defined ΔSMI% < −2.8% as excessive muscle loss to underscore the clinical relevance of treatment-induced sarcopenia. Second, with a median follow-up of 20.4 months, short-term outcomes were addressed; nonetheless, long-term efficacy remains unclear. The lack of detailed documentation of nutritional support (e.g., ONS utilization rates, achievement of caloric targets) may confound interpretations of muscle wasting; future investigations should prospectively standardize nutritional interventions to mitigate such confounding. Finally, no universally accepted method or standard exists for diagnosing sarcopenia. We adopted the common approach of calculating SMI from L3-level CT images, consistent with prior research.
Conclusion
In summary, our findings show that in patients with locally advanced ESCC receiving ICIs, excessive skeletal muscle loss during treatment is associated with poorer short-term survival outcomes. Additionally, regarding therapeutic response and adverse effects, excessive skeletal muscle loss and sarcopenia could serve as predictive biomarkers for the efficacy and toxicity of ICIs. Furthermore, the pivotal role of multidisciplinary team (MDT) -including dietitians- in the perioperative multidisciplinary management of patients with ESCC, particularly in implementing standardized exercise interventions and nutritional support strategies, is highlighted in this study.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Affiliated Hospital of North Sichuan Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because according to national legislation and institutional requirements, written informed consent was not required for participation in this study (File Number: 2025ER240-1).
Author contributions
BX: Writing – original draft, Software, Formal analysis, Visualization, Conceptualization, Writing – review & editing, Methodology. JL: Writing – review & editing, Supervision, Methodology, Formal analysis, Project administration. YZ: Data curation, Investigation, Writing – review & editing. TL: Writing – review & editing, Investigation, Data curation. JX: Writing – review & editing, Methodology, Data curation. HW: Investigation, Writing – review & editing, Data curation. GS: Conceptualization, Supervision, Investigation, Funding acquisition, Project administration, Writing – review & editing. MF: Conceptualization, Resources, Writing – review & editing, Project administration, Investigation, Validation, Formal analysis, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Nanchong City University Science and Technology Strategic Cooperation Special Fund (Grant no. 22SXQT0095) and the Scientific Research Foundation for Advanced Talents, Affiliated Hospital of North Sichuan Medical College (Grant no. 2023GC006).
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1650337/full#supplementary-material
References
1.
Jiang W Zhang B Xu J Xue L Wang L . Current status and perspectives of esophageal cancer: a comprehensive review. Cancer Commun. (2025) 45:281–331. doi: 10.1002/cac2.12645
2.
Sun J-M Shen L Shah MA Enzinger P Adenis A Doi T et al . Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
3.
Li C Zhao S Zheng Y Han Y Chen X Cheng Z et al . Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
4.
Kojima T Shah MA Muro K Francois E Adenis A Hsu C-H et al . Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. (2020) 38:4138–48. doi: 10.1200/JCO.20.01888
5.
Chen Y-X Wang Z-X Jin Y Zhao Q Liu Z-X Zuo Z-X et al . An immunogenic and oncogenic feature-based classification for chemotherapy plus PD-1 blockade in advanced esophageal squamous cell carcinoma. Cancer Cell. (2023) 41:919–932.e5. doi: 10.1016/j.ccell.2023.03.016
6.
Davis AA Patel VG . The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. (2019) 7:278. doi: 10.1186/s40425-019-0768-9
7.
Kelly RJ Ajani JA Kuzdzal J Zander T Van Cutsem E Piessen G et al . Adjuvant Nivolumab in resected esophageal or gastroesophageal junction Cancer. N Engl J Med. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
8.
Luo H Lu J Bai Y Mao T Wang J Fan Q et al . Effect of Camrelizumab vs. placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. (2021) 326:916–25. doi: 10.1001/jama.2021.12836
9.
Fu P Xiao X . Research progress on patients of esophageal cancer complicated with sarcopenia. Br J Hosp Med. (2024) 85:1–15. doi: 10.12968/hmed.2024.0281
10.
Meza-Valderrama D Marco E Dávalos-Yerovi V Muns MD Tejero-Sánchez M Duarte E et al . Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients. (2021) 13:761. doi: 10.3390/nu13030761
11.
Cruz-Jentoft AJ Bahat G Bauer J Boirie Y Bruyère O Cederholm T et al . Sarcopenia: revised european consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
12.
Huo Z Luo S Chong F Tong N Lu Z Zhang M et al . Global leadership initiative in sarcopenia (GLIS)-defined sarcopenia increases the mortality of esophageal cancer patients after esophagectomy: a Chinese real-world cohort study. Nutrition. (2025) 129:112600. doi: 10.1016/j.nut.2024.112600
13.
Tian L Wang Y Che G . Association of preoperative sarcopenia with the risk of anastomotic leakage in surgical esophageal cancer patients: a meta-analysis. Nutr Cancer. (2025) 77:640–7. doi: 10.1080/01635581.2025.2479878
14.
Juez LD Ortega ADC Priego P García Pérez JC Fernández-Cebrián JM Botella-Carretero JI . Impact of sarcopenic obesity on surgical complications and oncologic outcomes of upper gastrointestinal tumors: a systematic review and meta-analysis. Cirugía Española. (2025) 103:182–94. doi: 10.1016/j.cireng.2024.09.005
15.
Thormann M Meyer H-J Wienke A Niehoff J Kröger JR Gutzmer R et al . The prevalence of sarcopenia in patients with solid tumors differs across regions: a systematic review. Nutr Cancer. (2025) 77:102–14. doi: 10.1080/01635581.2024.2401648
16.
Qu J Liu Y Yuan Y Yu Z Ding J He Z et al . Impacts of sarcopenia on adverse events and prognosis in chinese patients with esophageal cancer undergoing chemoradiotherapy. Front Nutr. (2025) 12:1523674. doi: 10.3389/fnut.2025.1523674
17.
Bott R Zylstra J Knight W Whyte GP Lane AM Moss C et al . Prehabilitation of patients with oesophageal malignancy undergoing peri-operative treatment (pre-EMPT): outcomes from a prospective controlled trial. J Surg Oncol. (2025) 131:1508–20. doi: 10.1002/jso.28079
18.
De Felice F Malerba S Nardone V Salvestrini V Calomino N Testini M et al . Progress and challenges in integrating nutritional care into oncology practice: results from a national survey on behalf of the NutriOnc research group. Nutrients. (2025) 17:188. doi: 10.3390/nu17010188
19.
Ying H Chen Y Hong Y Ying K Li S Zhang Y et al . L3-SMI as a predictor of overall survival in oesophageal cancer patients receiving PD-1 inhibitors combined with chemotherapy. Ann Med. (2025) 57:2440114. doi: 10.1080/07853890.2024.2440114
20.
Huang G Zhu J He B Zhou X Wang Y Wu L et al . Prognostic impact of sarcopenia and surgical timing in locally advanced esophageal squamous cell carcinoma receiving neoadjuvant chemoradiotherapy: TIMES study. Ann Surg Oncol. (2025) 32:4140–50. doi: 10.1245/s10434-025-16976-9
21.
Prado CMM Lieffers JR McCargar LJ Reiman T Sawyer MB Martin L et al . Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. (2008) 9:629–35. doi: 10.1016/S1470-2045(08)70153-0
22.
Vashist Y Goyal A Shetty P Girnyi S Cwalinski T Skokowski J et al . Evaluating postoperative morbidity and outcomes of robotic-assisted esophagectomy in esophageal cancer treatment-a comprehensive review on behalf of TROGSS (the robotic global surgical society) and EFISDS (european federation international society for digestive surgery) joint working group. Curr Oncol. (2025) 32:72. doi: 10.3390/curroncol32020072
23.
Yoon HG Oh D Ahn YC Noh JM Pyo H Cho WK et al . Prognostic impact of sarcopenia and skeletal muscle loss during neoadjuvant chemoradiotherapy in esophageal cancer. Cancer. (2020) 12:925. doi: 10.3390/cancers12040925
24.
Fang P Zhou J Xiao X Yang Y Luan S Liang Z et al . The prognostic value of sarcopenia in oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2023) 14:3–16. doi: 10.1002/jcsm.13126
25.
Xiao X Fang P-H Zhou J-F Li X-K Shang Q-X Yang Y-S et al . Impact of skeletal muscle loss and sarcopenia on outcomes of locally advanced esophageal cancer during neoadjuvant chemoradiation. Ann Surg Oncol. (2024) 31:3819–29. doi: 10.1245/s10434-024-14936-3
26.
Kanemura T Takeoka T Sugase T Urakawa S Masuike Y Shinno N et al . Significance of comprehensive analysis of preoperative sarcopenia based on muscle mass, muscle strength, and physical function for the prognosis of patients with esophageal cancer. Ann Surg Oncol. (2024) 31:818–26. doi: 10.1245/s10434-023-14306-5
27.
Afzali AM Müntefering T Wiendl H Meuth SG Ruck T . Skeletal muscle cells actively shape (auto)immune responses. Autoimmun Rev. (2018) 17:518–29. doi: 10.1016/j.autrev.2017.12.005
28.
Ma K Xu Y Cheng H Tang K Ma J Huang B . T cell-based cancer immunotherapy: opportunities and challenges. Sci Bull. (2025) 70:S2095-9273(25)337–8. doi: 10.1016/j.scib.2025.03.054
29.
Takenaka Y Oya R Takemoto N Inohara H . Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle. (2021) 12:1122–35. doi: 10.1002/jcsm.12755
30.
Trestini I Belluomini L Dodi A Sposito M Caldart A Kadrija D et al . Body composition derangements in lung cancer patients treated with first-line pembrolizumab: a multicentre observational study. J Cachexia Sarcopenia Muscle. (2024) 15:2349–60. doi: 10.1002/jcsm.13568
31.
Huang S-W Xu T Zhang C-T Zhou H-L . Relationship of peripheral lymphocyte subsets and skeletal muscle mass index in sarcopenia: a cross-sectional study. J Nutr Health Aging. (2020) 24:325–9. doi: 10.1007/s12603-020-1329-0
32.
Li S Liu Z Ren Y Liu J Lv S He P et al . Sarcopenia was a poor prognostic predictor for patients with advanced lung cancer treated with immune checkpoint inhibitors. Front Nutr. (2022) 9:900823. doi: 10.3389/fnut.2022.900823
33.
Petrova MP Donev IS Radanova MA Eneva MI Dimitrova EG Valchev GN et al . Sarcopenia and high NLR are associated with the development of hyperprogressive disease after second-line pembrolizumab in patients with non-small-cell lung cancer. Clin Exp Immunol. (2020) 202:353–62. doi: 10.1111/cei.13505
34.
Harada T Tsuji T Ueno J Hijikata N Ishikawa A Kotani D et al . Association of sarcopenia with relative dose intensity of neoadjuvant chemotherapy in older patients with locally advanced esophageal cancer: a retrospective cohort study. J Geriatr Oncol. (2023) 14:101580. doi: 10.1016/j.jgo.2023.101580
35.
de Jong C Chargi N Herder GJM van Haarlem SWA van der Meer F van Lindert ASR et al . The association between skeletal muscle measures and chemotherapy-induced toxicity in non-small cell lung cancer patients. J Cachexia Sarcopenia Muscle. (2022) 13:1554–64. doi: 10.1002/jcsm.12967
36.
Lyu J Yang N Xiao L Nie X Xiong J Liu Y et al . Prognostic value of sarcopenia in patients with lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors or immune checkpoint inhibitors. Front Nutr. (2023) 10:1113875. doi: 10.3389/fnut.2023.1113875
37.
Chen N Yu Y Shen W Xu X Fan Y . Nutritional status as prognostic factor of advanced oesophageal cancer patients treated with immune checkpoint inhibitors. Clin Nutr. (2024) 43:142–53. doi: 10.1016/j.clnu.2023.11.030
38.
Zhang M Xiong Y Chen M Xu D Xu K Tian W . Psoas muscle mass index and peak expiratory flow as measures of sarcopenia: relation to outcomes of elderly patients with resectable esophageal cancer. Front Oncol. (2023) 13:1303877. doi: 10.3389/fonc.2023.1303877
Summary
Keywords
sarcopenia, skeletal muscle index, esophageal squamous cell carcinoma, neoadjuvant immunochemotherapy, overall survival
Citation
Xu B, Liu J, Zhang Y, Luo T, Xiong J, Wang H, Shi G and Fu M (2025) Impact of skeletal muscle loss and sarcopenia on outcomes of neoadjuvant immunochemotherapy in esophageal squamous cell carcinoma. Front. Nutr. 12:1650337. doi: 10.3389/fnut.2025.1650337
Received
19 June 2025
Accepted
11 August 2025
Published
12 September 2025
Volume
12 - 2025
Edited by
John Le, University of Alabama at Birmingham, United States
Reviewed by
Natale Calomino, University of Siena, Italy
Matteo Pittacolo, University of Padua, Italy
Updates
Copyright
© 2025 Xu, Liu, Zhang, Luo, Xiong, Wang, Shi and Fu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guidong Shi, 531590883@qq.comMaoyong Fu, fumaoyongmd@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.