- 1Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The Fifth Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Guangdong Second Traditional Chinese Medicine Hospital, Guangzhou, China
Aerobic exercise capacity is a critical determinant of endurance performance and overall health. Natural medicinal plant polysaccharides (NMPPs) have emerged as promising bioagents to enhance aerobic capacity through multi-target mechanisms. This review summarizes the effects of NMPPs on improving aerobic capacity, including oxygen supply and utilization in skeletal muscle, as well as the storage and metabolism of energy substrates. Additionally, we discuss the structural specificity related to their bioactivities. Furthermore, the mechanisms by which NMPPs enhance aerobic capacity encompass anti-fatigue properties, antioxidative effects, anti-inflammatory actions, immunomodulation, and modulation of gut microbiota. However, although there are many in vitro evidences, clinical translation requires standardized human trials and deeper exploration of structure-activity relationships. NMPPs represent a safe, multi-mechanistic alternative to conventional strategies, offering novel solutions for improving athletic performance and health resilience.
1 Introduction
Aerobic exercise capacity refers to the body's ability to efficiently produce energy via aerobic metabolism during sustained exercise to maintain muscle contractions. Its key physiological indicators are VO2 max and lactate threshold (1). In competitive sports, excellent aerobic exercise ability is closely related to the endurance of athletes (2). Highly trained male runners exhibited enhanced running economy relative to amateur-level runners (3). Aerobic exercise is an effective non-pharmacological intervention to improve cardiorespiratory fitness (CRF) (2). Enhanced aerobic capacity has been associated with diminished morbidity, decrease risk, and improved quality of life for cardiovascular diseases. Epidemiological studies indicate that long - term inactivity can cause a marked decrease in cardiopulmonary function (VO2 max). Around 28%−35% of adults have a VO2 max below the health threshold (< 35 ml/kg/min). The primary interventions currently employed to enhance aerobic exercise capacity involve specific training methodologies, including high-volume training (HVT), threshold training (THR), high-intensity interval training (HIIT), as well as nutritional supplements such as creatine. However, the practical implementation of these established training regimens often faces challenges related to poor adherence in the general population, primarily due to the substantial time commitment, perceived discomfort, or difficulty associated with maintaining high training volumes or intensities (4). Furthermore, conventional nutritional supplements may interfere with exercise adaptability and yield only limited improvements in CRF (5). Critically, even when adhered to, improvements in aerobic capacity achieved through these common interventions are frequently reported as modest and not consistently sustained (6). Therefore, exploring novel, safe, and bioactive strategies that can effectively augment the adaptive response to exercise training represents a crucial research imperative.
NMPPs are novel natural origin nutritional agents, and have the potential for multi-target physiological regulation to enhancing aerobic exercise capacity. NMPPs are biopolymers formed by the connection of more than 10 monosaccharides through glycosidic bonds and are widely found in the roots, stems, or fruits of medicinal plants such as Astragalus membranaceus, Lycium barbarum, and Ganoderma lucidum, etc. They are attracting increasing attention due to their diverse bioactivities, including antioxidative properties, anti-inflammatory effects, immune regulation, and so on. Their activity is closely related to the molecular properties, including monosaccharide composition, degree of branching, and spatial conformation (7). Recent studies have revealed that NMPPs exert anti-fatigue effects through pathways such as scavenging free radicals induced by exercise, regulating energy metabolism, and inhibiting inflammatory cascade reactions (8–10), which is closely related to aerobic exercise performance. Although current studies in this area are not as extensive as those examining other functions, the implications for improving athletic performance remain significant. This review aims to summarize the effects and mechanisms of NMPPs in improving aerobic exercise capacity, fully exploring the potential of polysaccharides to provide new solutions for enhancing aerobic exercise capacity.
2 Classification and biological activities of polysaccharides from natural medicinal plants
2.1 The classification of natural medicinal plant polysaccharides
NMPPs can be classified by different criteria, including resources, solubility, extraction methods, and structural characteristics. Among these, structural classification is one of the most significant determinants of bioactivity. The molecular structures encompass various factors, including molecular weight, monosaccharide compositions, structural characterization, types of modification of natural polysaccharides and conformational characterization (9). Among these structural features, functional groups significantly influence their bioactivities by interacting with biological receptors and regulating signaling pathways. This interaction occurs through alterations in the charge distribution, spatial conformation, and hydrophilicity/hydrophobicity of the polysaccharides. Here, NMPPs with five common functional groups are discussed.
2.1.1 Sulfated polysaccharides
Sulfated polysaccharides (SPs) are naturally occurring anionic polymers primarily composed of cellulose and hemicellulose, characterized by sulfate ester groups () as a class of bioactive macromolecules in plant systems (11, 12). Latest studies have provided an update on the structural chemistry of the major sulfate polysaccharides, including the galactans (e.g., agarans and carrageenans), ulvans, and fucans. SPs have demonstrated numerous beneficial bioactivities, including antioxidant, antidiabetic, hypoglycemic, anti-inflammatory, immunomodulatory, antiviral, and anticancer effects (13). The sulfated Morinda citrifolia (14) and Chinese yam (15) polysaccharide showed the good antioxidant activity, and them up to Vc level. The SPs from Orchis chusua D. Don maintained moderately stable antioxidant and probiotic ability. Among the various bioactivities, the modulation of adaptive immunity by SPs through multiple mechanisms has been the most extensively studied. Polysaccharides from Sea buckthorn leaves (SBLPs) are sulfated polysaccharide containing uronic acid. SBLPs showed antioxidant activity and immunological activity in vitro, also had the activity of immune stimulation on RAW264.7 cell (16). SPs promote dendritic cell maturation and antigen presentation to initiate T cell responses, directly regulate T cell activation, proliferation, and differentiation while balancing T cell subsets (e.g., Th1, Th2, Th17, and Treg), stimulate B cell activation and antibody production, enhance NK cell cytotoxicity, and induce cytokine secretion (e.g., ILs, IFNs, and TNF-α) to coordinate immune responses. These combined mechanisms enhance pathogen/tumor clearance, suggesting SPs hold significant promise as adjuvants in vaccine formulations (17).
Chemical modification can improve the physicochemical and functional properties of SPs. Platycodon grandiflorum roots polysaccharides (PGPs) exhibited specific antioxidant activities through Sephacryl S-100 column elution (18). The sulfated derivative polysaccharide from Siraitia grosvenorii had the ability to scavenge DPPH radicals, hydroxyl radicals and superoxide anions, and the scavenging power tended to increase with the increase in polysaccharide concentration (19). Natural Lycium barbarum seed dreg polysaccharides by sulfation showed the highest ABTS radical scavenging and reducing power while showed better DPPH radical scavenging effect than natural polysaccharides (20). Sulfated plumula nelumbinis polysaccharide significantly increase the proliferation of RAW264.7 macrophages and improve the activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) based on cell model of H2O2-induced oxidative damage (21). However, most studies on biological effects of SPs have been conducted in vitro or in animal models. Therefore, further research involving human subjects is imperative to confirm these effects.
2.1.2 Acetylated polysaccharides
The acetylated polysaccharides are characterized by that the sugar unit hydroxyl is replaced by an acetyl group (-OCOCH3). In nature, acetylated polysaccharides are extensively distributed in plants, microorganism, and animals (22). Acetyls play an essential role in polysaccharide behaviors for various biological activities (23). In recent years, a large number of studies have shown that polysaccharides exhibit excellent antioxidant and immune activities in the presence of acetyl groups. The antioxidant activity of Litchi pericarp polysaccharide (24), Chinese yam polysaccharide (15), Cyclocarya paliurus leaves polysaccharides (25) and pumpkin polysaccharides (26) were improved after acetylation modification. Introducing acetyls into polysaccharides could significantly modify their physicochemical properties and change their biological activities, such as solubility and water-solubility. After acetylation, bitter gourd polysaccharides (27), cyperus esculentus polysaccharides (28) exhibited stronger antioxidant, anticoagulant, and immune activity. The acetylated polysaccharide from Orchis chusua D. Don displayed the best proliferation effects on Bifidobacterium adolescentis (29). Arabinose (Ara) and galactose (Gal) contents were changed, and the antioxidant activity of Cyclocarya paliurus polysaccharide (CPP0.1) was subsequently increased (30). Acetylated polysaccharides of Cyclocarya paliurus polysaccharide have immunomodulatory effects on murine macrophage RAW264.7 (31). At the molecular level, pectic polysaccharide from Cucurbita moschata Duch likely activates macrophages mechanistically through TLR4- and CR3-dependent signaling pathways, involving coordinated activation of both NF-κB and MAPKs cascades (32).
2.1.3 Carboxymethylated polysaccharides
Carboxymethylated polysaccharides contain carboxymethyl (-OCH2COOH), which can significantly enhance water solubility of native polysaccharides. This modification enables structural diversity as well as providing additional bioactivities. Carboxymethylated polysaccharides were found to exhibit antioxidant activity, anti-tumor activity, immunomodulatory activity and antibacterial activity (33). Among them, antioxidant activity has been studied more extensively. The carboxymethylated polysaccharides extracted from peony seed dreg maintained moderately stable antioxidant ability (34). Carboxymethylated Morinda citrifolia polysaccharide (14) and carboxymethylated polysaccharide from Chinese yam (15) showed the good antioxidant activity, and them up to Vc level. Carboxymethylated polysaccharide from Orchis chusua D. Don (29), carboxymethylated cushaw polysaccharide (35) and carboxymethylated cucumber polysaccharide (36) exhibit better ability to scavenge superoxide anions and hydroxyl radicals. In addition, carboxymethylation modification of relevant high degree of substitution can enhance the dendritic cells maturation-inducing function of polysaccharide from the seeds of Plantago asiatica L (37).
Carboxymethylation could effectively increase the antioxidant activities of the polysaccharide. Carboxymethylated polysaccharide from Garcinia mangostana rind showed stronger activity compared to the other three chemical modification (38). Carboxymethylated polysaccharides from blackcurrant fruits (CRNPs) possessed stronger scavenging activities on radicals (hydroxyl and superoxide radicals) and better anti-lipid peroxidation activities, as well as better protection effects on erythrocyte hemolyses in vitro compared with polysaccharide extracted from blackcurrant fruits (RNP). The activities of CRNPs were significantly enhanced with the increase of the degree of substitution (DS) (203). One study showed that both of exopolysaccharide LEP-1b and its carboxymethylated derivative CLEP-1b from a Lachnum sp ameliorated physical fatigue and extended exhaustive swimming time. Moreover, CLEP-1b demonstrated dose-dependent enhancement of anti-fatigue effects, most notably at 200 mg/kg (39). This suggests that the carboxymethylated polysaccharides can be exploited as a potential healthcare compound to combat fatigue and to boost strength. Even though many studies showed that carboxymethylation could enhance the bioactivities, the mechanisms by which the carboxylate group contributes to these bioactivities remain unclear. Further exploration of the interaction between carboxymethyl polysaccharides and body molecules will facilitate the targeted production of functional polysaccharides.
2.1.4 Phosphorylated polysaccharides
Phosphorylated polysaccharides contain phosphate groups covalently attached to their saccharide units through ester bonds (), imparting unique charge and functional properties (40). Phosphorylation can reduce viscosity, improve the water solubility and biological activity of natural polysaccharides. Therefore, phosphorylated polysaccharides have attracted increasing attention owing to their antioxidant, antitumor, antiviral, immunomodulatory, and hepatoprotective effects (41). Phosphoric Onion polysaccharides (42), phosphorylated Morinda citrifolia polysaccharide (14) and phosphorylated polysaccharide from Chinese yam (15) have a good antioxidant activity, and the activity was similar to that of Vc positive control. Moreover, phosphorylated polysaccharide from Orchis chusua D. Don (SP-P) was demonstrated the highest scavenging ability on hydroxyl radical and growth-promoting activity on Lactobacillus Bulgaricus (29).
Due to their diverse bioactivities and structural modifications, phosphorylated polysaccharides are increasingly studied as targets of phosphorylation modification. The phosphorylation modification product of polysaccharide from purple sweet potato (PPSP) could significantly enhances the scavenging effects on hydroxyl radicals and superoxide anions. Additionally, it could also improve the anti-lipid peroxidation ability (43). The phosphorylated polysaccharides from peony seed dreg exhibited maximum hydroxyl radical scavenging activity and ferrous ion chelating ability as compared to native polysaccharides (34). Phosphorylated Cyclocarya paliurus polysaccharide (P-CP) significantly boosted its ability to protect cells from hydrogen peroxide-induced oxidative damage compared to the native polysaccharide (44). Phosphorylation of polysaccharide from Sanchi (Panax notoginseng) flower (45), phosphorylation modification effectively enhances Abrus cantoniensis Polysaccharides (ACP) (46), phosphorylated pumpkin polysaccharide (47), phosphorylated polysaccharides from native ginseng (48), phosphorylated garlic polysaccharide (49), phosphorylated cushaw polysaccharides (50), the antioxidant activity of phosphorylated polysaccharides is several times stronger than the unphosphorylated polysaccharide. The phosphorylated derivatives of Amana edulis polysaccharide possess higher reducing power compared with the native compound (51). Phosphorylated Radix Cyathulae officinalis Kuan polysaccharides (PRCPs) have been extensively studied in immunoregulatory activity. PRCPs not only enhance humoral immunity by elevating serum immunoglobulin levels (IgG, IgA, IgM) and promoting splenocyte proliferation, but also strengthen cellular immunity through macrophage phagocytosis activation, cytokine modulation (IFN-γ, IL-2,−4,−5,−6,−10), and T-cell subpopulation regulation (52). Furthermore, PRCPs acts as a potent adjuvant to boost vaccine efficacy by facilitating dendritic cell maturation and amplifying pathogen-specific antibody responses (53, 54). The efficacy of a drug is closely related to its structure. Although phosphorylated polysaccharides are highly active and have a wide range of effects, the structure-activity relationship and mechanism of action have not been studied extensively.
2.1.5 Amino polysaccharides
Amino polysaccharides contain amino groups, typically derived from amino sugar monomers. Chitosan is a typical and relatively extensively studied amino polysaccharide. Chitosan was widely used in food and pharmaceutical industry due to its multidimensional properties, such as biocompatibility, biodegradability, antibacterial properties and non-toxicity, muco-adhesivity, adsorption properties, etc., and thus they can be widely used in variety of areas (55–57). Chitosan and its nanocomposites have applications in drug delivery (58, 59) and carrier for fertilizer (60). Animal experiments have shown that the amino sugar/curdlan hybrid materials are promising as a new type of polysaccharide immunoadjuvants useful for cancer chemotherapy (61). The function of polysaccharides in the medical field and the mechanism of interaction with body molecules need to be further explored and studied.
It is postulated that bioactivities of polysaccharides and physicochemical properties are directly or indirectly regulated by their structure (62). By introducing groups into the polysaccharide chain through physical, biological and chemical molecular modifications, the functions and effects of polysaccharides can be better exerted (63).
2.2 The biological activity of polysaccharides from natural medicinal plants
2.2.1 Anti-fatigue activity
The anti-fatigue activity of NMPPs is a multifaceted process involving direct enhancement of energy metabolism, reduction of fatigue-associated biomarkers, and activation of antioxidant defense systems, often through synergistic or multi-target mechanisms. Polysaccharides from Zingiber officinale (ZOPA) significantly improve glycogen storage in gastrocnemius muscles while regulating energy metabolism and reducing metabolic waste accumulation, thereby delaying fatigue onset (64). Dendrobium officinale polysaccharide (EPDO) extends forced swimming time in mice by downregulating blood lactic acid (BLA) and urea nitrogen (BUN) levels, coupled with elevated SOD activity, highlighting its dual role in metabolic regulation and oxidative stress mitigation (65). These effects are further exemplified by okra polysaccharides, which prolong swimming endurance by increasing hepatic and muscle glycogen reserves while suppressing BUN and BLA accumulation (66).
The structural specificity of NMPPs significantly influences anti-fatigue potency. Bupleurum chinense DC polysaccharide BCP-2 showed superior fatigue-alleviating effects compared with BCP-1, attributable to distinct backbone compositions and branching patterns. Both contain oligogalacturonides, but BCP-1 features a backbone of 4-β-Galp and 4,6-β-Glcp with C4-branching, whereas BCP-2 consists of 3,5-α-Araf residues branched at C3 (67). Acidic polysaccharides from Panax ginseng (e.g., WGPA-A and WSGP-S3) exhibit superior activity compared with neutral counterparts, attributed to their higher sulfate content and molecular conformation (68, 69). Such structure-activity relationships suggest that targeted modifications of polysaccharide physicochemical properties could optimize their anti-fatigue potential.
NMPPs-mediated antioxidant activity can enhance exercise capacity. Polysaccharides from Gynostemma pentaphyllum (GPP) prolong exercise endurance in mice by scavenging excess ROS and preserving skeletal muscle glycogen, highlighting their potential to counteract exercise-induced oxidative stress (70). Acerola cold-water soluble polysaccharides (ACWS) exert anti-fatigue effects in vivo, likely via ROS neutralization and energy metabolism optimization (71). Polygonatum sibiricum polysaccharide (PSP) exhibits remarkable antioxidant and anti-aging properties by reducing ROS levels and increasing antioxidant enzyme activities in skeletal muscle tissue. However, direct studies on NMPPs in post-exercise recovery remain limited, direct evidence linking polysaccharide-mediated antioxidant effects to post-exercise recovery remains sparse.
Emerging evidence also implicates gut microbiota modulation and neuroendocrine regulation in polysaccharide-mediated fatigue resistance. Astragalus polysaccharides (APs) alleviates chronic fatigue syndrome by restoring gut microbial homeostasis and metabolite profiles, while Phragmites rhizoma polysaccharide suppresses hypothalamus-pituitary-adrenal axis hyperactivation to mitigate stress-induced fatigue (72, 73). These findings collectively highlight the diverse mechanisms through which plant polysaccharides combat fatigue, ranging from molecular-level metabolic adjustments to systemic physiological adaptations, positioning them as promising candidates for improving aerobic exercise capacity and post-exercise recovery.
2.2.2 Antioxidant activity
The antioxidant activity of NMPPs plays a pivotal role in mitigating oxidative stress through direct free radical scavenging, activation of endogenous antioxidant pathways, and modulation of gut microbiota. Garlic polysaccharide (GP), an inulin-type fructan, exhibits potent direct antioxidant activity by neutralizing reactive oxygen species (ROS) (74). Artemisia ordosica polysaccharide (AOP) demonstrates dose-dependent free radical scavenging capacity in vitro and enhances systemic antioxidant defenses in vivo by upregulating GSH-Px and SOD activities in rats (75).
Indirect antioxidant mechanisms often involve immunomodulation and gut microbiota regulation. APs enhance antioxidant responses in coral trout by improving intestinal morphology and modulating microbial communities, which synergistically reduce oxidative stress (74, 76). Mulberry leaf polysaccharide reverses cyclophosphamide-induced intestinal damage in chicks by restoring gut microbiota balance and enhancing immune-antioxidant crosstalk (77). Xylo-oligosaccharides combined with γ-irradiated APs amplify antioxidant capacity in broilers through microbiota-driven immunometabolic adaptations (78).
Notably, NMPPs frequently exhibit synergistic antioxidant effects. Date seed polysaccharide-derived selenium nanoparticles (MPS-NPs) display dual antioxidant and antibacterial properties, suggesting ROS scavenging and pathogen inhibition jointly alleviate oxidative damage (79). Additionally, LBP improves sub-health conditions in mice by simultaneously boosting antioxidant enzymes, immune function, and anti-fatigue activity, indicating interconnected pathways (80). Antioxidant capacity is a critical contributor to anti-fatigue efficacy, particularly given the excessive free radical generation during prolonged exercise. Lycium barbarum polysaccharide (LBP) and Panax ginseng acidic polysaccharide (WGPA) exhibit potent antioxidant effects, enhancing SOD activity and alleviating oxidative damage (81, 82). Notably, Polygonatum kingianum polysaccharides (PKPs) ameliorate fatigue by activating the NRF2/HO-1/NQO1 pathway, which synergistically enhances antioxidant defenses and mitochondrial biogenesis via AMPK/PGC-1α/TFAM signaling (83). This dual modulation of redox balance and energy metabolism underscores the potential of polysaccharides to accelerate post-exercise recovery by counteracting exercise-induced oxidative stress.
2.2.3 Anti-inflammatory effects
The anti-inflammatory properties of NMPPs have been extensively investigated, demonstrating therapeutic potential in diverse inflammatory diseases through modulation of inflammatory signaling pathways and immune cell functions. In hepatic fibrosis models, GLP further inhibits hepatic stellate cell activation and extracellular matrix deposition by targeting TGF-β/Smad signaling, while regulating inflammation-, apoptosis-, and cell cycle-related proteins (84). APs mitigate lipopolysaccharide (LPS)-induced systemic inflammation by blocking NF-κB/MAPK signaling (85).
Notably, the anti-inflammatory effects of polysaccharides are closely linked to their regulation of the gut microenvironment. Rattan Pepper polysaccharide alleviates dextran sulfate sodium (DSS)-induced intestinal inflammation and depressive behavior via bidirectional modulation of the microbiota-gut-brain axis, mediated by gut microbiota remodeling and reduced inflammatory mediators (e.g., IL-1β, IL-6) (86). Likewise, Abelmoschus manihot polysaccharide enhances intestinal mucus barrier integrity by promoting the abundance of Akkermansia muciniphila, thereby attenuating intestinal inflammation (87). Additionally, Ephedrae Herba polysaccharide suppresses ovalbumin (OVA)-induced asthmatic airway inflammation by restoring the Th1/Th2 and Th17/Treg immune balance (88).
At the molecular level, polysaccharides predominantly exert anti-inflammatory effects by inhibiting key pathways such as NF-κB and TLR4. RG-I pectin-like polysaccharide from Rosa chinensis alleviates non-alcoholic steatohepatitis-related inflammation and fibrosis by disrupting HMGB1/TLR4/NF-κB signaling (89). Colon-targeted modified ginseng polysaccharides significantly reduce pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and suppress NF-κBp65/TRAF6 signaling (90). Hippophae rhamnoides polysaccharides (HRP) further protect intestinal barrier function by upregulating tight junction proteins (occludin, claudin-1) and mitigating inflammatory mediators (91). NF-κB, as a transcription factor, plays an important role in the regulation of proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-8 (92). Moreover, Moreover, immune adaptations was related to physical improvement (93).
2.2.4 Immunomodulation
NMPPs mediate diverse immunomodulatory activities via direct cellular interactions, indirect signaling cascades, and synergistic effects. Direct immunomodulation is often mediated by enhancing immune cell activity or cytokine production. Sophora cassia polysaccharides significantly enhance B-cell and T-cell lymphocyte proliferation, indicating their potential to strengthen physical immunity (94). Hippophae rhamnoides polysaccharide (HRP) and Apocynum venetum flower polysaccharide (AVFP) demonstrate strong immune-enhancing effects in vitro and in vivo, likely by activating macrophages and lymphocytes (91, 95). A fructan-type GP further upregulates immune responses in macrophages and immunosuppressed mice, suggesting its role in restoring immune homeostasis (96). Additionally, polysaccharides from Areca catechu L. inflorescence effectively modulate immune responses in peripheral blood and spleen, emphasizing their systemic immunoregulatory capacity (97).
Indirect immunomodulation frequently involves interactions with the gut microbiota and intestinal immunity. Dendrobium officinale polysaccharide (DOP) and APs regulate macrophage and lymphocyte functions while improving intestinal barrier integrity and microbiota composition, thereby amplifying immune defenses (76, 98). Yupingfeng polysaccharides enhance intestinal health in Macrobrachium rosenbergii by fortifying immunity, barrier function, and microbial balance in low-fishmeal diets (99). Floccularia luteovirens polysaccharides activate the immune system in immunosuppressed mice by reshaping gut microbiota and fecal metabolites (100).
Synergistic mechanisms combining antioxidant and immunomodulatory effects are also prominent. Mulberry leaf polysaccharide alleviates cyclophosphamide-induced intestinal damage and growth inhibition in chicks by simultaneously boosting antioxidant capacity, immune regulation, and microbiota modulation (77). APs enhances coral trout growth and immunity by improving antioxidant responses and intestinal microbiota (101). Notably, xylo-oligosaccharides and γ-irradiated APs synergistically enhance immune responses and antioxidant capacity in broilers, highlighting the interplay between redox balance and immune activation (78). Additionally, Ficus carica polysaccharide (FCPs) mitigate oxidative stress via ROS scavenging while enhancing hepatic glucose metabolism and dendritic cell-driven IL-6/IL-12 production, positioning it as a multifunctional phytochemical candidate for metabolic-immune axis modulation (102).
Furthermore, polysaccharides such as Ephedra sinica polysaccharide (ESP) and Rehmannia glutinosa polysaccharide (RGP) modulate mucosal immunity by reducing pro-inflammatory cytokines, protecting intestinal barriers, and balancing microbiota-immune crosstalk, offering potential strategies to mitigate exercise-induced gastrointestinal stress (103, 104). These findings underscore the multifaceted immunomodulatory roles of plant polysaccharides, positioning them as promising candidates for improving exercise resilience through immune and metabolic optimization.
2.2.5 Modulation of gut microbiota
The modulation of gut microbiota represents a key mechanism by which natural medicinal plant polysaccharides influence host health, acting through both direct microbial interactions and indirect host-mediated pathways. Zingiber officinale derived polysaccharides (ZOPA and ZOPA-1) directly enhance intestinal flora diversity, alter microbial abundance, and regulate short chain fatty acid (SCFA) concentrations, potentially mediating anti-fatigue effects through the gut-muscle axis (64). Ethanol precipitated polysaccharides from Dendrobium officinale (EPDO-60) restores oxidative-antioxidative balance and accelerates fatigue metabolite clearance by reshaping gut microbial community structure (65). These direct modulatory effects are often coupled with indirect mechanisms. Green radish polysaccharides and vinegar-processed Schisandra chinensis polysaccharide ameliorate hyperlipidemia and type 2 diabetes by promoting SCFAs production and regulating microbial composition, thereby improving metabolic homeostasis (105, 106). SCFAs, as critical metabolites, not only enhance intestinal barrier function but also serve as energy substrates for skeletal muscles, suggesting a potential link to aerobic exercise recovery by mitigating exercise-induced energy depletion.
Structural specificity also dictates functional outcomes. Steaming duration alters Polygonatum cyrtonema polysaccharide (PCP) molecular weight and monosaccharide composition, thereby diversifying their digestion, absorption, and fermentation characteristics by gut microbiota (107). Xylo-oligosaccharides combined with γ-irradiated APs synergistically enhance antioxidant capacity and microbiota composition in broilers (78), emphasizing the importance of structural optimization for targeted efficacy.
Some polysaccharides exert synergistic effects through multi-target pathways. PKPs alleviate fatigue by simultaneously activating NRF2/HO-1/NQO1 and AMPK/PGC-1α/TFAM signaling pathways while modulating gut microbiota (83). Similarly, APs improve chronic fatigue syndrome by regulating gut microbiota and metabolites, highlighting a microbiota-metabolite-axis crosstalk (73). This dual regulation underscores the interconnected roles of microbial balance and host signaling in enhancing physiological resilience.
Antioxidant and anti-inflammatory activities further link gut microbiota modulation to aerobic exercise adaptation. MPS-NPs exhibit dose-dependent antioxidant and antibacterial effects (79), while acid-assisted Asparagus cochinchinensis polysaccharides protect against neurodegeneration via the microbiota-gut-brain axis (108). Since intense exercise generates excessive ROS, polysaccharides with ROS-scavenging properties, such as AOP (75), may accelerate post-exercise recovery by neutralizing oxidative stress.
Emerging evidence suggests cross-tissue communication mediated by gut microbiota. PCPY-1, a homogeneous polysaccharide from Polygonatum cyrtonema, alleviates fatigue in exhausted mice by enhancing osteocalcin-mediated bone-muscle crosstalk, thereby promoting muscle energy metabolism and ATP generation (109). This highlights the potential of polysaccharides to bridge gut microbial modulation with systemic energy regulation, a mechanism highly relevant to aerobic endurance. Nevertheless, translational studies are required to validate these findings in exercise models, particularly regarding fatigue mitigation and performance enhancement.
2.2.6 Hypoglycemic and hypolipidemic effects
Natural medicinal plant polysaccharides counteract hyperglycemia and hyperlipidemia by targeting metabolic pathways directly and leveraging gut microbiota-dependent mechanisms, with synergistic actions increasingly recognized. Direct regulation of glucose and lipid metabolism is often mediated by targeting key signaling pathways. Rhizoma Ligustici Chuanxiong polysaccharides (RLMP) enhance hepatic glucose uptake and suppress oxidative stress by activating the PI3K/Akt/GLUT-4 pathway, thereby reducing hyperglycemia in diabetic models (110). PSP improve insulin sensitivity, reduce glycated serum protein levels, and normalize lipid metabolism in T2DM mice, directly alleviating hyperglycemia and dyslipidemia (111). Astragalus membranaceus polysaccharides (AMP) further demonstrate direct anti-diabetic effects by restoring pancreatic β-cell function and inhibiting hepatic gluconeogenesis (112).
Due to the limited intestinal absorption of high-molecular-weight polysaccharides, their systemic benefits frequently rely on gut microbiota-mediated metabolic reprogramming. Ulva lactuca polysaccharides mitigate aging-associated hyperglycemia in diabetic mice by reshaping gut microbiota composition and promoting the production of SCFAs, which enhance insulin signaling and suppress systemic inflammation (113). Ficus carica polysaccharides, obtained via ultrasound-assisted extraction, ameliorate oxidative stress and immunomodulatory imbalances, likely through microbiota-dependent pathways involving bacterial metabolite interactions (102). Green radish polysaccharides alleviate diet-induced hyperlipidemia by enriching beneficial gut bacteria (e.g., Lactobacillus) and stimulating SCFA production, which suppresses hepatic lipid accumulation (106). These studies underscore the pivotal role of gut microbiota in translating polysaccharide structures into metabolic benefits.
Notably, certain polysaccharides exhibit synergistic mechanisms by combining direct metabolic effects with microbiota-dependent regulation. Okra polysaccharides alleviate T2DM by directly activating the PI3K/AKT/GSK3β-Nrf2 pathway to reduce oxidative stress while simultaneously modulating gut microbial ecology to improve glucose homeostasis (114). Such multi-target actions highlight the potential of polysaccharides to address complex metabolic disorders through complementary pathways. Although current research focuses on diabetes and hyperlipidemia, the antioxidant and anti-inflammatory properties of these polysaccharides may indirectly enhance aerobic exercise capacity. Exercise-induced oxidative stress and inflammation could be mitigated by polysaccharide-mediated free radical scavenging, potentially accelerating post-exercise recovery. However, more studies are needed to further explore the cross-mechanism interactions.
2.2.7 Antitumor activity
Multifaceted antitumor properties of NMPPs arise from direct cytostatic/cytotoxic actions, indirect immune modulation and signaling pathway interference, and synergistic interplay often involving gut microbiota. A growing body of evidence highlights their ability to directly inhibit tumor cell proliferation and induce apoptosis. DOP suppress MNNG-induced precancerous lesions by regulating the Wnt/β-catenin pathway and altering endogenous metabolites (115). Similarly, APs inhibit tumor progression in prostate, liver, and non-small-cell lung cancers by suppressing cell growth, invasion, and enhancing apoptosis, while also improving chemosensitivity and immunity (116). Crocus sativus petal polysaccharides further demonstrate antitumor efficacy by remodeling the tumor microenvironment (TME) (117).
Indirect mechanisms often involve immune enhancement and gut microbiota modulation. Glycyrrhiza polysaccharides activate γδT cell-mediated antitumor responses via the TLRs/NF-κB pathway and gut microbiota interactions in murine models (118). A cold-water extracted polysaccharide-protein complex from Grifola frondosa exerts antitumor effects in H22 tumor-bearing mice by activating TLR4-NF-κB signaling and modifying gut microbiota composition (119). Codonopsis polysaccharides synergize with doxorubicin (DOX) to amplify tumor-killing effects and immune regulation (120).
Notably, gut microbiota-mediated pathways are a recurring theme. Reviews underscore that natural polysaccharides target gut microbiota to exert antitumor effects (121, 122). This aligns with broader evidence that polysaccharides modulate microbial metabolites, which in turn influence systemic immunity and inflammation—processes also critical in mitigating oxidative stress.
2.2.8 Other activities
In addition to the well-documented bioactivities, natural medicinal plant polysaccharides exhibit diverse physiological effects, including anti-aging, and antidepressant activities, often mediated through gut microbiota modulation. Similarly, a water-soluble polysaccharide from Ginkgo biloba leaves exerts antidepressant activity by regulating the gut microbiome and its associated metabolites, suggesting a potential role in alleviating psychological stress through microbiota-gut-brain axis interactions (123).
These findings underscore the systemic and multifaceted roles of polysaccharides in promoting holistic health. While direct evidence linking these activities to aerobic exercise enhancement remains limited, their capacity to improve metabolic regulation, reduce oxidative damage, and modulate neuropsychiatric states may indirectly support exercise performance and recovery. Nevertheless, further studies are warranted to explore these potential cross-domain benefits and elucidate the mechanistic connections between polysaccharide modulation and aerobic capacity optimization.
NMPPs exhibit multifaceted bioactivities through multi-target and multi-pathway mechanisms, with their structural features dictating functional specificity. Through systematic induction and analysis, we have unveiled the extensive sources and diverse functions of these polysaccharides. Closely related to physiological foundations, these pathways enable medicinal plant polysaccharides to significantly ameliorate pathological states and enhance physiological conditions. These findings not only highlight their potential as supplements but also underscore their vast application value in modern medicine. Consequently, the role of medicinal plant polysaccharides as multifunctional natural supplements in the prevention and treatment of a variety of diseases cannot be overlooked, and their research and application prospects are promising. The biological activity of polysaccharides from natural medicinal plants is shown in Figure 1.
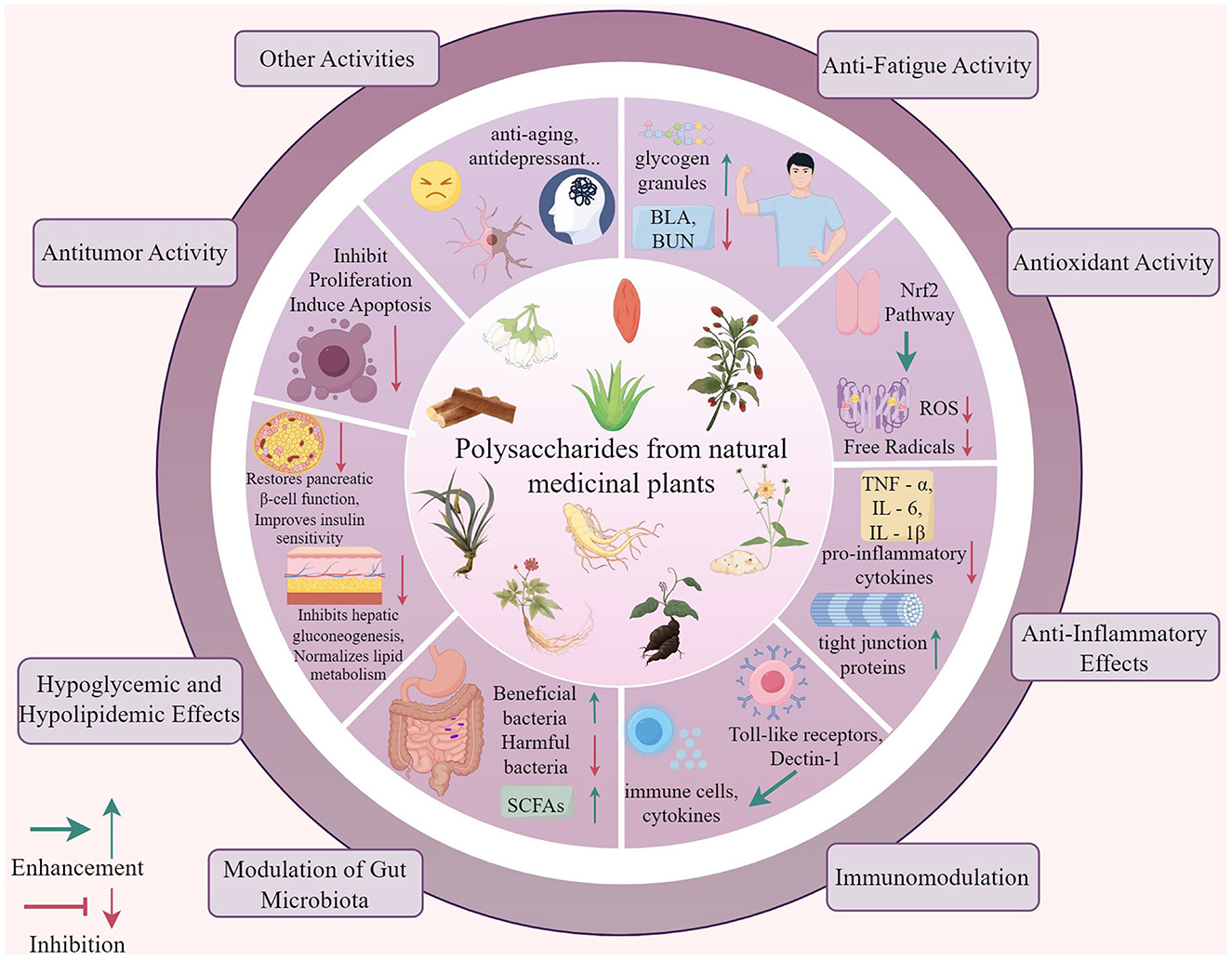
Figure 1. The biological activities of natural medicinal plant polysaccharides. The biological activity of polysaccharides from natural medicinal plants including anti-fatigue activity, antioxidant activity, anti-Inflammatory effects, immunomodulation, modulation of gut microbiota, hypoglycemic and hypolipidemic effects, antitumor activity, other activities like anti-aging and antidepressant activities.
Table 1 provides a summary of the study on classification and biological activities of NMPPs.
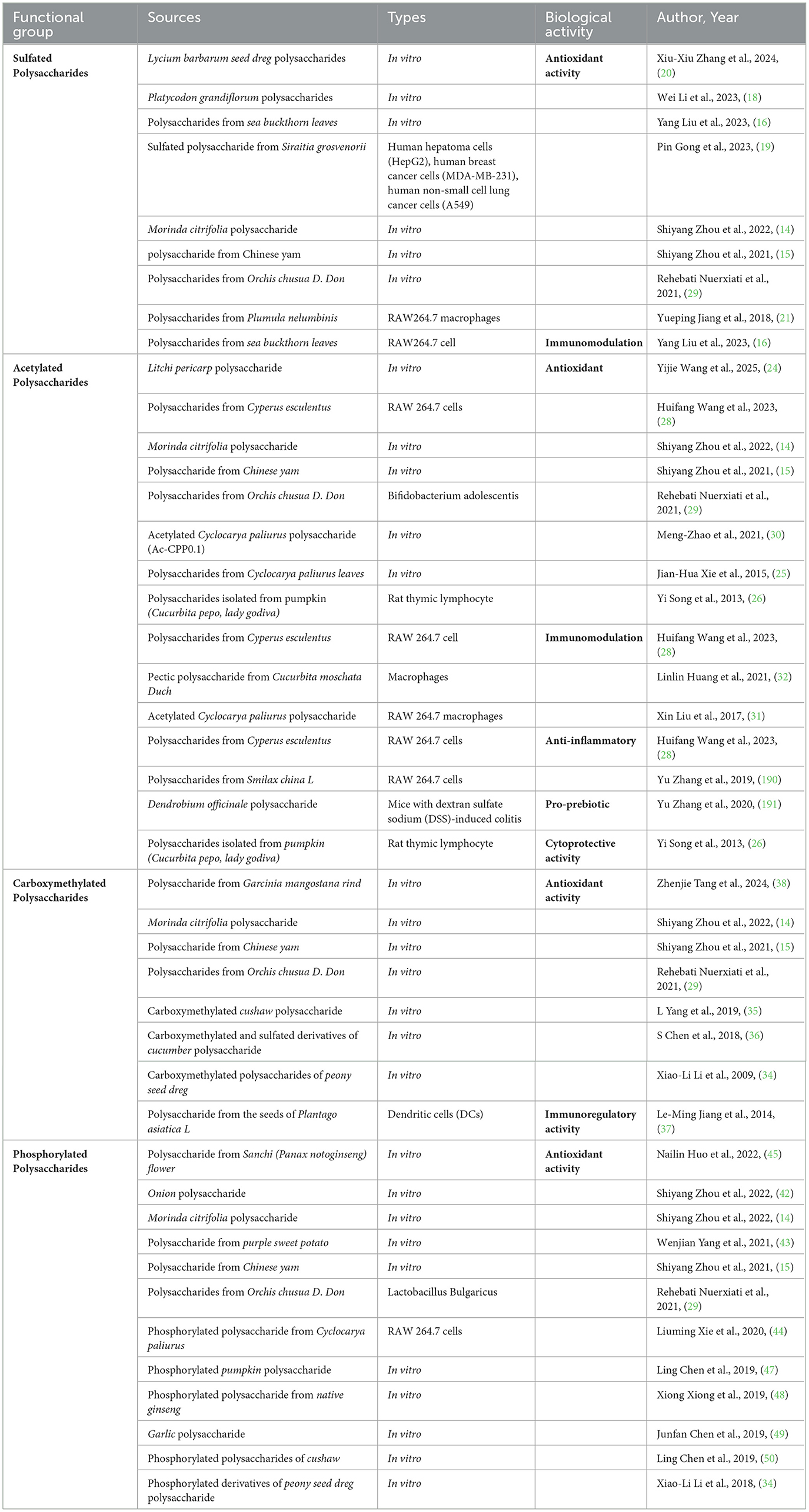
Table 1. Study on classification and biological activities of natural medicinal plant polysaccharides.
3 Aerobic exercise capacity and its influencing factors
The biological foundation of aerobic exercise capacity lies in the aerobic metabolic energy supply capacity. The direct energy source for skeletal muscle contraction during exercise is adenosine triphosphate (ATP), the resynthesis of which during this process primarily relies on aerobic metabolic pathways. The prerequisite for aerobic metabolic energy supply is oxygen availability. Consequently, any factor influencing oxygen supply or utilization within the organism can affect human aerobic metabolic energy production.
3.1 Oxygen supply capacity in skeletal muscle during exercise
The oxygen supply capacity of skeletal muscle during exercise depends on inspired oxygen concentration and cardiopulmonary function. VO2 max is an important variable that sets the upper limit for endurance performance and limited by the ability of the cardiorespiratory system to deliver oxygen to the exercising muscles (1). Increased vascular endothelial growth factor (VEGF) can induce angiogenesis, increase capillary density improve oxygen utilization, while elevated myoglobin further optimizes intracellular oxygen diffusion and improve blood supply to coronary arteries and skeletal muscle, then enhance the effect of endurance training (124). The increase of lactate and reactive oxygen species such as NO in the tissue can also cause vasodilation and increase the supply of blood and oxygen. Physiological levels of ROS are essential for skeletal muscle force production, while excess ROS induces contractile dysfunction. Muscle contraction activity increases the production of ROS through mitochondria, NADPH oxidase and other pathways (125).
3.2 Oxygen utilization capacity in skeletal muscle during exercise
During aerobic exercise, skeletal muscle utilizes oxygen, supporting energy substrate metabolism during contraction. Mitochondria generate ATP through the aerobic oxidation of carbohydrates, lipids and amino acids. Their oxidative capacity depends on mitochondrial density, cristae membrane surface area and the activity of aerobic enzymes (e.g. citrate synthase). Mitochondrial biogenesis is usually regulated by a variety of factors. PGC-1α is considered the main factor in regulating mitochondrial biogenesis, integrity and function, in cooperation with downstream nuclear transcription cofactors, such as nuclear respiratory factor-1 and−2 (NRF-1 and NRF-2) (126). PGC-1α expression is higher in tissues and organs with high energy metabolic load, such as heart, skeletal muscle and adipose tissue (127).
Mild heat shock plays an important role in metabolic remodeling by activating C2C12 muscle cell line mitochondrial biogenesis through the AMPK-SIRT1-PGC-1α axis (128). Notably, diverse exercise modalities similarly engage mitochondrial adaptation pathways, albeit with varying efficacy and molecular emphasis. Endurance training can enhance mitochondrial respiratory function in skeletal muscle by promoting the secretion of 12S rRNA-c (MOTS-c) and activating the AMPK/PGC-1α pathway (129). Endurance exercise for 8 weeks increased PGC-1α in the gastrocnemius muscle of rats, accompanied by increased mitochondrial biosynthesis and increased ratio of slow muscle fibers to fast muscle fibers (130). NOX2 and muscle/endothelial NOX4 can mediate skeletal muscle adaptation to endurance exercise through mitochondrial biogenesis and adaptive gene networks regulated by reactive oxygen species (131). HIIT can enhance the synthesis of mitochondrial proteins through DNA promoter region methylation, thereby improving mitochondrial respiration and aerobic capacity in skeletal muscle (132).
Antioxidant capacity plays an important role in maintaining mitochondrial function, which improves the body's ability to use oxygen and improve aerobic exercise capacity. Endurance exercise training increases the abundance of key antioxidant enzymes in the trained muscles, nuclear factor erythroid 2-related factor (Nrf2) signaling pathway is responsible for many of the exercise-induced changes in muscle antioxidant capacity (133).
3.3 Storage and metabolism of energy substances in skeletal muscle
Glucose and fat serve as two primary energy sources for prolonged exercise through aerobic metabolism, while the ability of skeletal muscles to oxidize glucose and fatty acids is also a crucial factor affecting aerobic endurance. Glycogen reserves in muscles and the liver can directly influence the magnitude of aerobic metabolic capacity and the duration of energy supply. The higher the glycogen content in muscles, the greater the potential for aerobic energy supply. Increased glycogen reserves in the liver help maintain stable blood glucose levels during prolonged exercise, thereby enhancing athletic performance. In addition, the ability of fat mobilization and liver utilization of glycerol, ketone bodies and some amino acids for gluconeogenesis are enhanced during long-term exercise, which also plays a very important role in improving long-term endurance exercise ability. Thus dietary intake during training influences the intensity and duration of exercise that athletes can sustain, ultimately affecting endurance performance. During exercise, supplementation with carbohydrates and other energy substrates can increase glycogen reserves in muscles. Strategic manipulation of carbohydrate and protein intake may optimize training adaptations, whereas excessive carbohydrate availability and antioxidants could blunt responsiveness (134). Plant polysaccharides have been shown to reduce fatigue and enhance athletic performance such as strength and endurance by improving energy metabolism (135, 136).
Emerging evidence suggests that fatty acids may regulate muscle lipid metabolism as signaling molecules through transcriptional mechanisms mediated by PPAR activation, NAD-dependent SIRT1 stimulation, and the AMPK signaling pathway (137). Exercise-induced cathecholamine release in skeletal muscle enhances the browning of white adipose tissue, promoting fat mobilization and improving aerobic metabolism (138). In addition, lactic acid produced by glycolysis may promote muscle metabolism toward lipid/glutamine oxidation through AMPK-PPAR activation, and enhance insulin sensitivity mediated by PI3K-AKT (139). Long-term intake of lactic acid and long-term high-intensity training can reduce body fat by increasing fat oxidation through lactic acid (140).
During exercise, exercise orchestrates a complex endocrine cascade that dynamically regulates energy substrate mobilization and utilization across tissues. The secretion of stress hormones such as adrenaline and catecholamine increases, and the release of muscle factors and cytokines from active muscles also play a hormone-like metabolic regulation role during long-term exercise, including stimulating glycogen breakdown in the liver and stimulating fat breakdown in adipose tissue. exercise-induced release of irisin from skeletal muscle enhances browning of white adipose tissue, indirectly supporting aerobic metabolism via systemic lipid mobilization (138). Exercise-induced activation of skeletal muscle p38γ stimulates interleukin-15 (IL-15) secretion, which signals to the motor cortex to enhance locomotor activity, forming a muscle-brain axis (141). IL-15, a skeletal muscle-derived myokine elevated during exercise, exhibits dual metabolic roles: pharmacological doses enhance systemic insulin sensitivity and lipid oxidation, while elevated interstitial muscle levels suggest autocrine/paracrine regulation of glucose homeostasis and oxidative metabolism, necessitating further investigation into dose-dependent signaling mechanisms (142). IL-6 increased GLUT4 expression in muscle and that this phenomenon may play a role in the post-exercise enhancement of insulin sensitivity in skeletal muscle (143).
4 Effects of polysaccharides from common natural medicinal plants on aerobic exercise capacity
4.1 Ginseng polysaccharides
Ginseng polysaccharides derived from the roots of Panax ginseng are well-studied for their medicinal properties including anti-fatigue effects. Studies have shown that the properties of polysaccharides affect their effects on improving aerobic exercise capacity. Water-soluble polysaccharides isolated from Ginseng (WGP) is an active component extracted from ginseng, which possesses a variety of pharmacological activities. WGPN (Neutral Ginseng Polysaccharide) and WGPA (Acidic Ginseng Polysaccharide) are two distinct fractions of WGP, respectively representing neutral and acidic polysaccharides. The anti-fatigue effects were evaluated using the forced swim test (FST), and serum biochemical parameters reduced immobility in the FST, but WGPA showed significant effects at lower doses compared to WGP and WGPN. Additionally, the FST-induced changes indicative of fatigue—such as decreased glucose (GLU) and GSH-Px levels, and increased creatine phosphokinase (CK), lactic dehydrogenase (LDH), and malondialdehyde (MDA) levels—were effectively mitigated by the respective doses of WGP, WGPN, and WGPA (69). It is worth noting that another study further fractionated WGPA into two components, WGPA-A and WGPA-N, using anion-exchange chromatography. In the forced swimming test, WGPA and WGPA-A were able to prolong the swimming time, whereas WGPA-N could not. Additionally, the levels of MDA and LDH in the serum were increased, while the levels of SOD and GSH-Px were decreased. Interestingly, the structural degeneration of mitochondria was ameliorated (81). WSGP-S3 is an acidic heteropolysaccharide extracted from steamed ginseng via ultrafiltration. In the anti-fatigue activity assays, WSGP-S3 significantly extended the exhaustive swimming time of fatigued mice. It also elevated the levels of liver and muscle glycogen, as well as the activities of SOD, catalase (CAT), and GSH-Px. Furthermore, it reduced the levels of BLA, BUN, and MDA compared to the control group. Additionally, WSGP-S3 promoted spleen cell proliferation in fatigued mice (68). The ginseng acidic polysaccharide APs-1 prolonged fatigue exhaustive swimming time, reduced BLA, LDH, and BUN levels, enhanced SOD and CAT activities, mitigated MDA-induced oxidative damage, increased CK activity, regulated glycolysis, and alleviated muscle fiber contraction (144). POL (Polysaccharide), OLI (Oligosaccharide), and WAT (Aqueous Extract) are different active components extracted from Codonopsis pilosula. Weight-loaded swimming test showed that, compared with the control treatment, only POL treatment significantly prolonged the swimming time of the mice. POL groups had the strongest hypoxia tolerance, followed by the OLI and WAT groups (145).
4.2 Lycium barbarum polysaccharide
LBP is one of the most extensively studied functional polysaccharides and has demonstrated various biological activities, including antioxidant, anti-fatigue, mitochondrial-enhancing, and energy metabolism-regulating properties. Recently, many studies have focused on its capacity to improve aerobic exercise performance through mechanistic insights into oxidative stress mitigation, mitochondrial function optimization, and innovative formulations for practical applications. In the exercise test, the rats treated with LBP showed a significantly prolonged time to exhaustion during running, along with a significant decrease in MDA levels and a significant increase in SOD and GSH-Px levels. These results indicate that LBP can effectively prevent oxidative damage following intense exercise (192).
In addition, LBP could enhance antioxidant ability in sub-health mice and showed anti-fatigue ability in sub-health mice (80). LBP-4a is a polysaccharide fraction purified from Lycium barbarum. After 4 weeks of treatment with LBP-4a in model mice prepared using compound factors such as forced swimming tests, sleep deprivation, and wrapping restraint stress tests, it was found that LBP-4a treatment reduced skeletal muscle damage and MDA levels, while enhancing SOD and GSH-Px activities compared to the model group. Additionally, LBP-4a increased mitochondrial membrane potential and calcium ion (Ca2+) levels in skeletal muscle mitochondria, with the high-dose group showing better effects than the low-dose group (146).
With the further research on LBP, it was gradually developed for practical application. LBP1-SeNPs are selenium nanoparticles (SeNPs) prepared using Lycium barbarum polysaccharide (LBP1) with a molecular weight of 92,441 Da as the stabilizer and capping agent. The high-dose group of LBP1-SeNPs exhibited the longest exhaustion swimming time, which was significantly greater than both the control group and the positive group. All tested dose groups of LBP1-SeNPs showed a significant increase in exhaustion swimming time compared to the control group, demonstrating that LBP1-SeNPs could be developed as a potential anti-fatigue nutritional supplement (147). Lycium barbarum polysaccharide effervescent tablets (LBPT) are effervescent tablets formulated by mixing LBP with excipients and help patients who have difficulty swallowing conventional tablets or capsules. Animal experiments showed that LBP and LBPT significantly increased the exhaustive swimming time in rats. LBP and LBPT improved biochemical markers in rat serum, such as lactic acid and creatine kinase, enhanced the antioxidant capacity of rat muscle, and reversed the decrease in serum glucose, ATP and glycogen content caused by exercise. Transmission electron microscopy showed that LBP and LBPT increased the density of mitochondria in rat liver (82). This suggests that more stable forms of polysaccharides can be mined and convenient dosage forms can be made for more people in the future.
4.3 Polygonatum sibiricum polysaccharide
Polygonatum sibiricum polysaccharide (PSP), derived from the rhizome of Polygonatum sibiricum Redouté, is a bioactive heteropolysaccharide distinguished by its unique monosaccharide composition (e.g., fructose, arabinose) and structural features. PSP effectively alleviated oxidative stress and mitochondrial dysfunction caused by D-gal in C2C12 myotubes, preserving mitochondrial integrity and reducing MAM formation. Additionally, PSP lowered intracellular Ca2+ levels by modulating calcium-related proteins, as confirmed by GO analysis of DEGs. In aged mice, PSP increased muscle mass, enhanced grip strength and hanging time, and reduced ROS levels while boosting antioxidant enzyme activities in skeletal muscle tissue (148).
Polygonatum cyrtonema Hua polysaccharide (PCP), another bioactive heteropolysaccharide extracted from the rhizome of Polygonatum cyrtonema Hua, is characterized by its complex structure composed of glucose, mannose, galactose, and highly branched configurations. PSP and PCP are not identical but distinct bioactive compounds derived from the Polygonatum genus. While both belong to the Liliaceae family and share structural similarities (e.g., glucose-rich heteropolysaccharides), their monosaccharide composition, glycosidic linkages, and bioactivity profiles differ due to species-specific genetic and metabolic variations. Pharmacological studies indicate PCP exhibits stronger mitochondrial function modulation and AMPK pathway activation compared to PSP, reflecting divergent mechanisms in enhancing aerobic capacity. In weight-loaded swimming test, PCP remarkably prolonged the exhaustive swimming time of mice, decreased serum levels of lactic acid (LA), blood urea nitrogen (BUN), SOD, GSH-Px and MDA, and increased the contents of liver glycogen, muscle glycogen and muscle ATP (149). It is worth noting that the swimming time and rotarod time in the high-dose group of PCP were significantly prolonged, increasing by 73 and 64%, respectively. The activities of CAT, GSH-Px and SOD in serum increased by 53.56, 37.69, and 53.67%, respectively, while the levels of MDA, lactic acid, and BUN decreased by 22.90, 17.48, and 24.61%, respectively (150). In the exhaustive swimming mouse model and the co-culture system of BMSCs/C2C12 cells, homogeneous polysaccharide (PCPY-1) from Polgonatum cyrtonema after structure characterization significantly stimulated BMSC differentiation into osteoblasts as determined by the protein expressions of osteogenic markers BMP-2, phosphor-Smad1, RUNX2, and osteocalcin. Meanwhile, PCPY-1 remarkably enhanced myoblast energy metabolism by upregulating osteocalcin release and GPRC6A protein expression; the phosphorylation levels of CREB and HSL; the mRNA levels of GLUT4, CD36, FATP1, and CPT1B, and ATP production in vitro and in vivo (109).
4.4 Maca (Lepidium meyenii Walp) polysaccharide
Maca (Lepidium meyenii Walp.), a traditional medicinal plant native to the Andes, is rich in bioactive polysaccharides characterized by heterogeneous structural features, including distinct monosaccharide compositions and glycosidic linkages. Studies have demonstrated the anti-fatigue effects of Maca polysaccharides using the exhaustive swimming test and biochemical indexes (151), more in-depth research has further researched that the best dose of polysaccharide to exert effect. One study showed low-dose maca polysaccharides group (150 mg/kg/day) had the significant anti-fatigue activity (152). Another study revealed that mice treated with high-dose MP (100 mg/kg bw/day) exhibited significantly elongated swimming durations and accelerated average swimming speeds, along with improved serous biochemical parameters (153). This suggests that more in-depth studies could focus on the range between 100 and 150 mg/kg/day to explore the optimal dose of Maca polysaccharide to exert anti-fatigue.
Structural distinctions critical for polysaccharide function. Two fractions of polysaccharides, MPs-1 and MPs-2, were extracted from Lepidium meyenii Walp.(maca) using water and purified with DEAE-52 and Sephadex G-100 columns. MPs-2 (6.7 kDa) diverges from MPs-1 (7.6 kDa) in monosaccharide composition lacking xylose with a glucose-dominated ratio (1:1.3:36.8 vs. 1:1.7:3.3:30.5) and glycosidic linkage heterogeneity (mixed α/β-pyranose vs. exclusively α-configured). Both MPs-1 and MPs-2 have dose-dependent positive effects on fatigue-related parameters, with MPs-2 showing a better anti-fatigue effect than MPs-1 (154).
4.5 Astragalus polysaccharides
APs, primarily extracted from the roots of Astragalus membranaceus or Astragalus mongholicus, are heteropolysaccharides with diverse monosaccharide compositions (e.g., glucose, galactose, arabinose) and highly branched structures. APs plays a positive regulatory role in the proliferation and differentiation of sheep skeletal muscle satellite cells (SMSCs) (155). An optimal APs dose promotes growth, enhances antioxidant activity, supports immune function, and improves intestinal microbiota in coral trout (101). Moreover, there have been studies on the efficacy and mechanism of APs for Chronic fatigue syndrome (CFS) from the perspective of the gut-brain axis, APs could increase the SCFAs content by regulating the gut microbiota, and SCFAs (especially butyrate) can further regulate the oxidative stress and inflammation in the brain, thus alleviating CFS. This study provides a reference to further explore the efficacy of APs and the role of SCFAs in the central nervous system (73).
4.6 Other polysaccharides
Emerging evidence highlights that polysaccharides from diverse medicinal plants enhance aerobic exercise capacity by modulating energy metabolism, augmenting antioxidant defenses, and reducing fatigue-related biomarkers. Dioscorea opposita polysaccharides (PYB-1, PYB-2) prolong swimming endurance in mice via increased hepatic glycogen, elevated superoxide dismutase (SOD) and GSH-Px activities, and reduced malondialdehyde (MDA) levels (156). Similarly, Zingiber officinale (ZOPA, ZOPA-1), Paris polyphylla (PPPm-1), and Gynostemma pentaphyllum (GPP1-a) polysaccharides enhance skeletal muscle energy metabolism, improve contraction dynamics, and mitigate lactic acid accumulation (64, 70, 157). Polysaccharides from Dendrobium officinale (EPDO-60), Bupleurum chinense (BCP-2), Apple pomace (PAP), and Cassiae semen reduce BUN, LDH and oxidative stress while boosting glycogen reserves (65, 67, 94, 158). Okra (AEP-1, AEP-2), Corn silk, and Mentha haplocalyx (MHa) polysaccharides enhance ATPase activities (Na+-K+-ATP, Ca2+-ATP) and glycogen retention (66, 159, 160), while Portulaca oleracea alleviate exercise-induced fatigue markers (BUN, CK, MDA) and improve anti-fatigue effects (161). Structural features (e.g., molecular weight, purity) critically influence efficacy, as exemplified by PYB-1 had stronger free-radical scavenging activity than PYB-2 (156). These findings underscore the potential of natural polysaccharides as multi-target agents for enhancing exercise endurance through synergistic metabolic and antioxidative pathways.
Despite promising findings, several critical gaps persist in understanding the therapeutic potential of natural medicinal plant polysaccharides for enhancing aerobic exercise capacity. Current studies predominantly rely on animal models and acute exercise protocols, limiting translational relevance to chronic exercise adaptations and human physiology. Secondly, in terms of structure-activity relationships (SARs), exploring more specific structural features (such as linkage patterns, degree of branching, and molecular weight heterogeneity) could help enhance the effects of different polysaccharides. Furthermore, exploring a more precise dose-response relationship and stipulating a standardized protocol would be helpful for the practical application of polysaccharides. Additionally, long-term safety profiles, pharmacokinetic behavior, and bioavailability of polysaccharides in exercise contexts are poorly understood, hindering clinical translation. Future research should couple with advanced structural characterization techniques (e.g., NMR, AFM) to refine SAR models. Human trials are essential to validate preclinical findings, while formulation innovations, such as nanoencapsulation or synergistic combinatorial therapies, could enhance stability, targeting, and efficacy. Addressing these gaps will bridge mechanistic knowledge and practical applications, advancing polysaccharide-based strategies for optimizing aerobic performance.
The polysaccharides from natural medical plants on aerobic exercise capacity is shown in Table 2.
5 Mechanisms of natural medicinal plant polysaccharides in enhancing aerobic exercise capacity
Natural medicinal plant polysaccharides enhanced aerobic exercise capacity through various mechanisms. The major mechanisms include microbiota modulation, energy metabolism regulation, antioxidation, anti-inflammation and immunity regulation.
5.1 Modulation of gut microbiota
5.1.1 Modulation of gut microbiota composition
The composition and function of intestinal microbiota are important in energy metabolism homeostasis, and polysaccharides from natural medicinal plants can improve aerobic exercise ability by regulating the composition of gut microbiota. Many studies have shown that the structure, abundance, diversity in the intestine mediate the effect of polysaccharides from natural medicinal plants on aerobic exercise ability. Polysaccharides such as ZOPA and ZOPA-1 have been shown to modulate the intestinal flora of mice, increasing diversity, altering abundance, regulating short-chain fatty acid concentrations, and enhancing antioxidant capacity through the gut-muscle axis, extending the time of exhaustive swimming in mice (64). Moreover, Ginseng polysaccharides could regulate the gut microbiota composition and promote M2 macrophage polarization by modulating TLR4/MYD88 signaling (90).
Changes in the beneficial and harmful bacteria in the intestinal flora are also important factors for the influence of NMPPs on aerobic exercise ability through the intestinal muscle axis. Polysaccharides from Dendrobium officinale (EPDO-60) modulates gut microbiota community structure by increasing proportions of Bacteroidetes and Firmicutes and abundance of Lactobacillus and Bifidobacterium in gut microbiota, thereby enhancing redox homeostasis and accelerating fatigue-related metabolite clearance (65). HRP promotes beneficial bacteria like Clostridia_UCG-014, Lachnospiraceae and suppressed pathogens such as Atopostipes, Desulfobacterot, which is crucial in HRP-mediated immunity regulation via TRAF6/NF-κB signaling, up-regulated the expression of occludin, claudin-1, and zona occludens-1 (ZO-1) (91). The GRP supplement modulated the Firmicutes/Mycobacteria ratio and Blautia spp., significantly reducing oxidative stress and inflammation in the liver of mice fed a high-fat diet (106). PKPs can reduce the number of harmful bacteria (g_Escherichia-Shigella and g_Helicobacter), while increasing the production of antioxidant bacteria (g_norank_f_Muribaculaceae) and short-chain fatty acids (SCFA), and significantly enhance exhaustive swimming time (83).
5.1.2 Metabolic regulation of gut microbiota
In addition to directly affecting the structure of intestinal flora, polysaccharides from natural medicinal plants can also improve aerobic exercise ability through the metabolites of intestinal flora. SCFAs are significant metabolites produced by gut microbiota during the fermentation of NMPP. A study showed that APs induced SCFAs generation and consequently reversing Nrf2/NF-κB signaling dysregulation in the brain-gut axis, which may be a key mechanism for ameliorating chronic fatigue syndrome-related metabolic disturbances (73). SCFAs also enhanced by ACP to modulate the microbe-gut-brain axis, increasing the lactic acid levels in colon contents, the abundance of Bacteroides while reducing Firmicutes and other harmful bacteria (108). Furthermore, multiple polysaccharides (e.g., FAPs, PKPs) enhanced SCFA production, linking microbial SCFA production to muscle energy optimization and improving gut barrier integrity and energy homeostasis (83, 162). MPS-NPs maintained Firmicutes/Bacteroidetes ratio and upregulated SCFA biosynthesis, such as butyrate production, which plays a critical role in maintaining gut health and energy homeostasis, supporting gut barrier integrity and muscle ATP production (79). Butyrate also mediated antioxidant and anti-inflammatory effects in neurons, linking gut ecology to systemic immunity (73). These findings position the gut as a metabolic orchestrator of muscle resilience (163). The enhanced SCFAs by AVFP is correlated with the increased beneficial bacteria, reduced harmful species, modulated immune responses through NF-κB signaling pathway, and improved the host ability to resist oxidative stress (95). In addition, studies have found that DOP increases taurine and decreases 2-hydroxybutyric acid, while promoting the growth of probiotics such as Dubosiella, Bifidobacterium, and Akkermansia, which also have beneficial effects on improving aerobic exercise capacity (164).
5.2 Energy metabolism in mitochondria and skeletal muscles
Energy metabolism, particularly in mitochondria and skeletal muscles, is another key area where NMPPs exert their effects. In skeletal muscle, the activation of AMPK can initiate the oxidative metabolic program of mitochondria (165, 166). AMPK is a crucial molecular target for skeletal muscle fiber type transformation (167), and can also perceive cellular energy status through direct interactions with ATP, ADP, and AMP (168). Many studies showed that natural medicinal palnt polysaccharides can ameliorate exercise-induced fatigue by rectifying mitochondrial dysfunction, restoring energy metabolism homeostasis, and counteracting oxidative stress. For example, APs-1 activated AMPK signaling to enhance mitochondrial biogenesis and glucose uptake, alleviated muscle fiber contraction (144). Additionally, APs can enhance autophagy and suppressing inflammation and oxidative stress in myocardial tissues through AMPK signaling pathway, thereby protecting against overexercise-induced injury (169). More profoundly, polysaccharides like BCP-2 have been shown to alleviate physical fatigue by regulating the AMPK and Nrf2 signaling pathways in skeletal muscles, thereby enhancing mitochondrial biogenesis and antioxidant defenses (67). LBP and LBPT enhanced biochemical markers (e.g., lactic acid, creatine kinase) and antioxidant capacity in rat serum and muscles, and reversed exercise-induced decreases in serum glucose, ATP, and glycogen, while regulating energy metabolism through the AMPK/PGC-1α pathway (82). LBP also modulated glucose and lipid metabolism, improved skeletal muscle atrophy via AMPK/PINK1/Parkin-mediated mitophagy, and repaired mitochondrial structure and function (170). Furthermore, PKPs promoted energy metabolism by upregulating the expression of AMPK/PGC-1α/TFAM signaling pathway proteins (83). GP increased antioxidant enzyme activity (SOD, GSH-Px, and CAT) and restored ATPase activity by activating the AMPK/PGC-1α pathway (74). APs accelerated sheep skeletal muscle stem cell (SMSC) differentiation by upregulating miR-133a, a microRNA that enhances MAPK/ERK activity and myoblast fusion (155). PCP stimulated neurogenesis through the CREB/BDNF/Akt pathway and augmented glucose uptake via AMPK-TXNIP modulation, synergistically supporting anti-fatigue effects (150). In addition, there are some pathways that are less well studied. PSP attenuated age-associated mitochondrial dysfunction by activating the PI3K/Akt/mTOR pathway, which promoted protein synthesis and reduced muscle atrophy (171). PKPs can promote energy metabolism by upregulating the expression of AMPK/PGC-1α/TFAM signaling proteins, while changing gut flora, and significantly improve various physiological indicators related to fatigue (83).
In addition to directly acting on energy metabolism, NMPPs also play a role in regulating energy metabolism by regulating hormones and enzymes. For example, Polygonatum cyrtonema polysaccharides (PCP) enhanced osteocalcin expression via the BMP-2/Smad1/Runx2 axis, activated p-CREB and p-HSL in skeletal muscle, mediated crosstalk between skeletal and muscular systems to regulate energy metabolism, improving lipid mobilization and ATP generation (109, 149). Additionally, Gavage with panax ginseng polysaccharides (PGP) for 10 days daily could inhibit the formation of MDA in the brain of chronic hypoxia model mice, increase the levels of ATP, ADP, TAP and AEC in hepatocytes, increase CK activities, the ratio of ATP/ADP and ATP/AMP in skeletal muscle, and protect mitochondria by inhibiting mitochondrial swelling and improving energy metabolism (172). Okra polysaccharide enhanced succinate dehydrogenase (SDH) and ATPase activity, accelerating ATP regeneration and reducing CK leakage (66). An acid polysaccharide from Mentha haplocalyx (MHa) promoted mitochondrial biosynthesis by activating AMPK, increased liver glycogen and muscle glycogen storage, and increased the activity of Ca2+-Mg2+-ATPase and Na+-K+-ATPase, significantly increasing the swimming time of mice at exhaustion (160). In addition, polysaccharides from natural medicinal plants can improve motor ability by regulating the central nervous system. Polygonatum sibiricum polysaccharide (PSP) increased hemoglobin levels, decreased LA, BUN and CK levels in the blood; inhibited the increase of 5-HT concentration and tryptophan hydroxylase (TPH2) expression induced by exercise, and prolonged the fatigue time during treadmill exercise (173). Acanthopanax polysaccharide (ACP) can reduce the activation level of ROS-NLRP3 in substantia nigra striatum and improve the motor ability of Parkinson's disease mice (174).
Plant polysaccharides can improve aerobic exercise ability by increasing energy metabolism substrates such as muscle glycogen and liver glycogen. Selenium-enriched Lycium barbarum polysaccharide (LBP1-SeNPs) extended swimming endurance via enhancing muscle glycogen reserves and GSH-Px activity (147). Maca polysaccharides could increase liver glycogen (LG) content in mice, had the significant anti-fatigue activity (152). Apple pomace polysaccharide (PAP) upregulated hepatic glycogen synthase activity, elevating liver and muscle glycogen stores while inhibiting BUN and LDH accumulation, which contributes to their anti-fatigue activity (158). Furthermore, Ziziphus polysaccharide (PPPm-1) reduced glycogen depletion during exhaustive exercise, concurrently suppressing lactate accumulation and promoting Ca2+-mediated muscle contraction efficiency (157).
5.3 Antioxidation
Polysaccharides can also effectively improve the antioxidant capacity and consequently improve the aerobic exercise capacity. PKPs can improve intestinal flora and energy metabolism, and enhance exercise ability by activating NRF2/HO-1 signaling pathway to reduce oxidative stress (83). The Keap1-Nrf2/ARE signaling pathway is often implicated in these antioxidant effects, as seen with HWE-JGLR, which enhances antioxidant function in broilers (175). In exhaustive swimming experiments with mice, both crude ZOPA and purified ZOPA-1 exhibited significant anti-fatigue effects, which may improve antioxidant capacity through the activation of the Keap1-Nrf2/ARE and AMPK/PGC-1α signaling pathways (64). Moreover, LBP protected retinal cells against light-induced damage by upregulating Nrf2 and thioredoxin reductase 1 (TrxR1), thereby neutralizing oxygen free radicals and reducing mitochondrial oxidative stress (176). The improvement of antioxidant capacity and the alleviation of oxidative stress by LBP may be achieved through Nrf2/HO-1 pathway (82). LBP1-SeNPs have been found to relieve fatigue by increasing glycogen reserves and enhancing antioxidant enzyme levels (147). LBP-4a (146) and total polysaccharides (MSP) from Millettiae speciosae Champ. Leguminosae (177) can reduce lipid peroxidation and increases antioxidant enzymes in skeletal muscle, improving calcium homeostasis and mitochondrial function.
The increase of antioxidant enzyme activity is also an important factor for the improvement of aerobic exercise ability of NMPPs. Lycium barbarum polysaccharide (LBP-4a) can enhance the anti-fatigue ability of subhealthy mice by reducing the lipid peroxidation level in skeletal muscle tissue, increasing the activity of SOD and GSH-Px, and improving the imbalance of intracellular calcium homeostasis (146). In a study on the mechanism of ginsenoside acid polysaccharide WGPA in the prevention of chronic fatigue syndrome (CFS), it was found that oral administration of WGPA for 15 days in mice could increase serum SOD and GSH-Px, improve mitochondrial structural degeneration, and prolong forced swimming time (81). RGP treatment activated the Nrf2/Keap1 pathway and significantly increased the activity of antioxidant enzymes (178). Similarly, APs enhances total antioxidant capacity, SOD, and GSH-Px activities in heart, kidney, and liver while lowering MDA levels, thereby reducing oxidative stress (76). Other compounds, such as CYP, ameliorated cisplatin-induced muscle atrophy by restoring ATP content, reducing oxidative markers (MDA, LDH), and elevating antioxidant enzymes (SOD) (179). Polysaccharides from S. cassiae have demonstrated strong anti-fatigue activity by ameliorating the levels of antioxidant enzymes such as SOD and GSH-Px while reducing markers of oxidative stress like malondialdehyde (MDA) (94). PCP has been shown to reduce oxidative stress markers and increase antioxidant enzyme activities, thereby exerting anti-fatigue properties (149). Ziyang green tea selenium-polysaccharide (Se-TP) (180) can alleviate exercise fatigue and prolong the time of exhaustion by increasing the content of GSH-PX, SOD and CAT, reducing the level of MDA, and increasing the content of muscle glycogen. PSP can effectively reduce the increase of Ca2+ concentration by regulating calcium-related proteins in muscle cells, reduce oxidative stress and mitochondrial dysfunction, increase muscle mass and improve grip strength and hanging time in old mice (148).
Notably, molecular weight is a key factor affecting their free radical scavenging activity and the effectiveness of enhancing athletic performance. For example, both WSGP-S3 (molecular weight 2.03 × 104) and WSGP-G3 (molecular weight 4.86 × 104) are acidic heteropolysaccharides extracted from steamed ginseng using ultrafiltration methods. However, only WSGP-S3 can extend the swimming endurance time of fatigued mice by increasing the activity of superoxide dismutase, catalase, and glutathione peroxidase, while reducing malondialdehyde levels (68). PYB-1 (molecular weight 145 kDa) and PYB-2 (molecular weight 11 kDa) are both polysaccharides from yam bulbils, but PYB-1 exhibits stronger free radical scavenging activity and can extend the intermittent swimming time of mice more effectively (156). A study compared the effects of Hippophae rhamnoides polysaccharides (HRWP), Lycium barbarum polysaccharides (LBWP), Lycium ruthenicum polysaccharides (LRWP) and Nitraria tangutorum polysaccharides (NTWP), and found that they can all inhibit glucose, SOD and GSH-Px in the liver and heart of mice induced by FST, showing anti-motor fatigue activity, and LBWP and NTWP are far better than HRWP and LRWP at the same dose (181).
5.4 Anti-inflammation
The polysaccharides of natural medicinal plants play a role in improving aerobic exercise ability by regulating inflammation and maintaining the stability of cell function. For example, HRP may regulate the expression of TRAF6 /NF-κB signaling pathway by affecting the diversity of intestinal microbiota and inhibiting the levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β), up-regulated the expression of occludin, claudin-1, and zona occludens-1 (ZO-1) (91). GNP suppresses the expression of COX-2 and iNOS by blocking the MAPK/NF-κB signaling pathway (182). LBP combined with aerobic exercise can reduce the inflammatory factor related indicators of liver LPS/TLR4/NF-κB signaling pathway and improve liver inflammation in NAFLD (183).
5.5 Immunomodulation
The polysaccharides of natural medicinal plants can have a positive effect on aerobic exercise ability by improving immune function and enhancing fatigue recovery (184, 185). In vivo experiments have shown that DOP can enhance the production of sIgA and alleviate cyclophosphamide-induced immunosuppression by increasing immune organ indices, promoting immunoglobulin secretion, and boosting the number of immune cells (186). Polygonatum polysaccharide can enhance the immune regulatory activity, significantly improve the spleen index and thymus index, increase the expression of IL-2, IFN-γ, IgA and IgM, and increase the CD4+/CD8+ ratio, with significant immune regulatory effect (187). Polysaccharide AVFP regulates NF-κB-mediated immune responses by modulating the expression of Bcl3, Lbp, and Cebpd, suggesting its broad-spectrum immunoregulatory activity and ability to enhance the body's resistance to oxidative stress (95). Dietary APs has been shown to enhance immune functions in mice, increasing white blood cell and lymphocyte counts, upregulated genes associated with antioxidant defense and leukocyte proliferation (76). Moreover, APs improved growth performance, enhanced the antioxidant capacity and immune regulation of Plectropomus leopardus by regulating the expression of genes related to antioxidant enzymes and immune response via dose-dependent modulation (101). In vitro experiments have shown that Hemerocallis citrina Borani polysaccharide (HCBP1-1) can significantly promote the secretion of NO, TNF-α, IL-1β, and IL-6 in RAW264.7 cells, as well as the expression of NF-κB p65, demonstrating excellent immune-enhancing activity (188). Barbary wolfberry polysaccharides can increase the phagocytic ability and NO release in RAW264.7 cells by 23 and 76%, respectively, showing the strongest immune-enhancing activity (189).
In summary, NMPPs enhance aerobic exercise capacity through a multifaceted approach involving microbiota modulation, energy metabolism, metabolic regulation, antioxidation, inflammation and immunity, and organ crosstalk. These mechanisms collectively contribute to improved exercise performance, reduced fatigue, and enhanced recovery, making polysaccharides a promising area of research for athletes and individuals seeking to improve their physical endurance. The mechanisms of NMPPs in enhancing aerobic exercise capacity is shown in Figure 2.
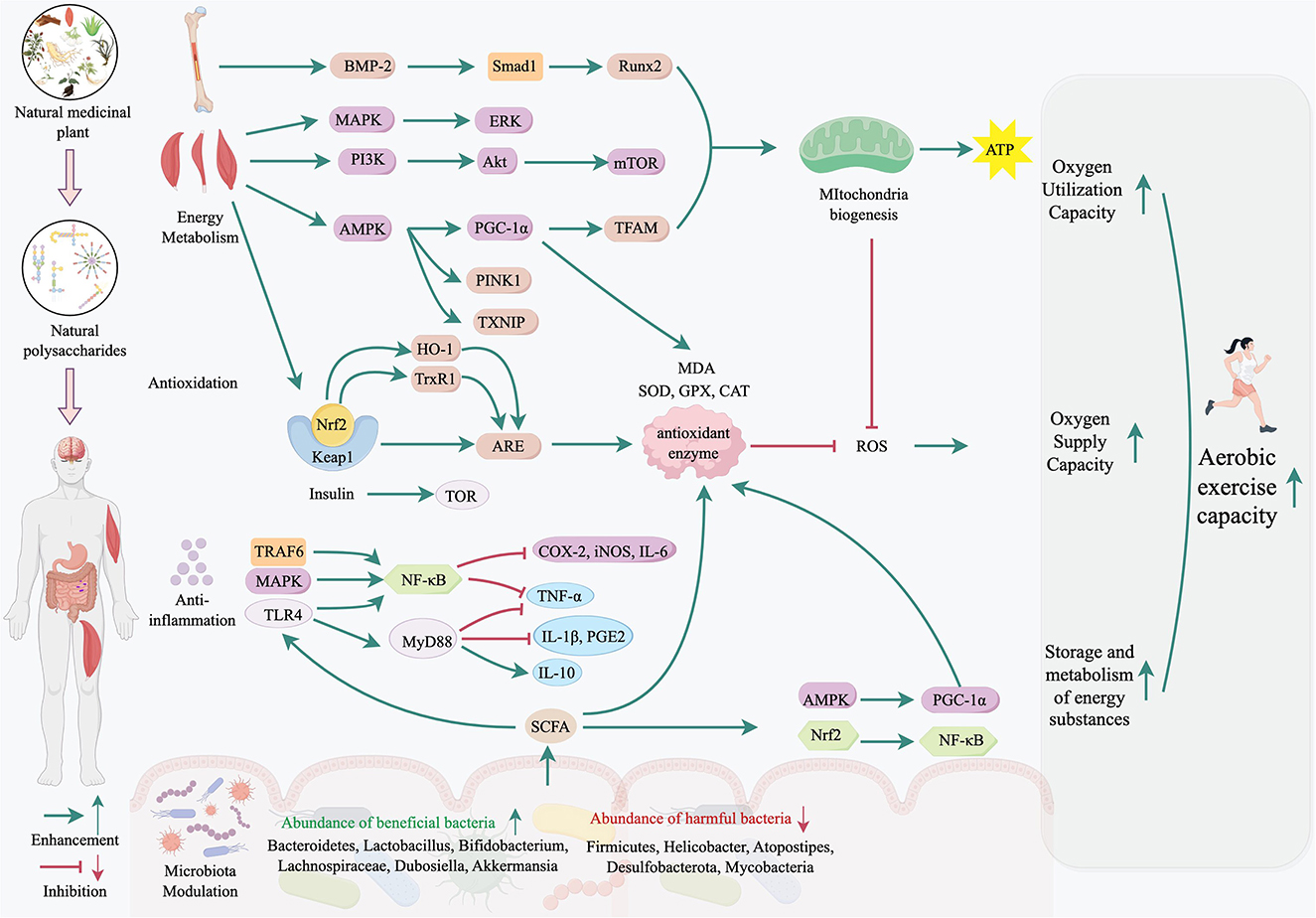
Figure 2. The mechanisms of NMPPs in enhancing aerobic exercise capacity NMPPs can enhance aerobic exercise capacity through the mechanisms of gut microbiota, energy metabolism in skeletal muscle mitochondria, antioxidation, anti-inflammation and immunomodulation.
6 Summary and perspective
In conclusion, NMPPs have emerged as highly promising bioactive compounds with significant potential to enhance aerobic exercise capacity. A wealth of research evidence has been comprehensively reviewed, unraveling the intricate mechanisms through which NMPPs exert their beneficial effects. NMPPs modulate gut microbiota to optimize energy harvest and intestinal homeostasis, fine-tune energy metabolism to boost mitochondrial function and fuel utilization, bolster antioxidant defenses to mitigate exercise-induced oxidative damage, and regulate inflammatory and immune responses to promote recovery and reduce fatigue. These multifaceted actions collectively result in improved aerobic exercise performance, delayed onset of fatigue, and enhanced post-exercise recovery. In addition, the structural diversity of NMPPs imparts them with unique bioactive properties. These structural features dictate their interactions with biological systems, influencing bioavailability, metabolic processing, and the specific signaling pathways they activate or suppress. Thus, a deeper understanding of the structure—activity relationships of NMPPs is crucial for harnessing their full potential in sports nutrition and exercise physiology.
While the existing body of research has laid a solid foundation for our understanding of NMPPs in the context of aerobic exercise capacity enhancement, several limitations and challenges warrant attention. The heterogeneity across experimental models, including variations in animal species, exercise protocols, and outcome measures, complicates the direct comparison of results and the derivation of generalized conclusions. Many exercise programs that test aerobic capacity use forced swimming to exhaustion. Moreover, most of the indicators observed in animal models are the improvement of fatigue state, and there are few studies on direct promotion in physiological health state. Furthermore, most studies have been confined to preclinical models, with limited clinical trials in humans. This gap raises questions about the expansion from preclinical findings to human athletic performance and the potential species—specific differences in the metabolic processing and bioactivities of NMPPs. Additionally, the long-term safety, optimal dosing regimens, and potential synergies or antagonisms between different NMPPs or with other nutrients remain to be elucidated.
Future research endeavors should focus on addressing these limitations through rigorous trials employing standardized protocols and well-defined NMPP preparations. Advanced omics technologies could be leveraged to dissect the molecular mechanisms underpinning the effects of NMPPs on aerobic exercise capacity at a systems level. Furthermore, exploring structure—activity relationships using sophisticated analytical techniques may pave the way for the rational design of NMPP—based supplements with enhanced efficacy and specificity. As the field progresses, NMPPs hold the promise of becoming a cornerstone in sports nutrition, offering athletes and physically active individuals a natural, safe, and effective strategy to optimize their aerobic exercise capabilities and overall athletic performance.
Author contributions
MX: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Visualization. WC: Writing – review & editing. WL: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Basic and Applied Basic Research Foundation of Guangdong Province (2022A1515220151).
Acknowledgments
All the figures in this manuscript were drawn on www.figdraw.com website.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor YHM declared a past co-authorship with the author MX.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRF, cardiorespiratory fitness; HVT, high-volume training; THR, threshold training; HIIT, high-intensity interval training; EPO, erythropoietin; NMPPs, natural medicinal plant polysaccharides; SPs, sulfated polysaccharides; BLA, blood lactic acid; SOD, superoxide dismutase; ROS, reactive oxygen species; GSH-Px, glutathione peroxidase; LPS, lipopolysaccharide; DSS, dextran sulfate sodium; SCFA, short chain fatty acid; ATP, adenosine triphosphate; VEGF, vascular endothelial growth factor; NRF-1 and NRF-2, nuclear respiratory factor-1 and−2; Nrf2, nuclear factor erythroid 2-related factor; IL-15, interleukin-15; FST, forced swim test; GLU, glucose; CK, creatine phosphokinase; LDH, lactic dehydrogenase; MDA, malondialdehyde; CAT, catalase; LA, lactic acid, BUN, blood urea nitrogen; SDH, succinate dehydrogenase; TPH2, tryptophan hydroxylase; LG, liver glycogen; TrxR1, thioredoxin reductase 1; LBP, Lycium barbarum polysaccharide; PKPs, Polygonatum kingianum polysaccharides; GP, Garlic polysaccharide; AOP, Artemisia ordosica polysaccharide; APs, Astragalus polysaccharides; PSP, Polygonatum sibiricum polysaccharide; DOP, Dendrobium officinale polysaccharide; PCP, Polygonatum cyrtonema polysaccharide.
References
1. Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. (2000) 32:70–84. doi: 10.1097/00005768-200001000-00012
2. Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. (2008) 586:35–44. doi: 10.1113/jphysiol.2007.143834
3. Morgan DW, Baldini FD, Martin PE, Kohrt WM. Ten kilometer performance and predicted velocity at VO2max among well-trained male runners. Med Sci Sports Exerc. (1989) 21:78–83. doi: 10.1249/00005768-198902000-00014
4. Stöggl T, Sperlich B. Polarized training has greater impact on key endurance variables than threshold, high intensity, or high volume training. Front Physiol. (2014) 5:33. doi: 10.3389/fphys.2014.00033
5. Poulsen SK, Due A, Jordy AB, Kiens B, Stark KD, Stender S, et al. Health effect of the New Nordic Diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am J Clin Nutr. (2014) 99:35–45. doi: 10.3945/ajcn.113.069393
6. Eagles AN, Sayers MGL, Bousson M, Lovell DI. Correction to: current methodologies and implications of phase identification of the vertical jump: a systematic review and meta-analysis. Sports Med. (2018) 48:497. doi: 10.1007/s40279-017-0806-z
7. Albeituni SH, Yan J. The effects of β-glucans on dendritic cells and implications for cancer therapy. Anticancer Agents Med Chem. (2013) 13:689–98. doi: 10.2174/1871520611313050003
8. Zhou Y, Chu Z, Luo Y, Yang F, Cao F, Luo F, et al. Dietary polysaccharides exert anti-fatigue functions via the gut-muscle axis: advances and prospectives. Foods. (2023) 12:3083. doi: 10.3390/foods12163083
9. Lei Z, Shi Y, Zou J, Zhang X, Xin B, Guo D, et al. A review of the polysaccharides against fatigue and the underlying mechanism. Int J Biol Macromol. (2024) 275:133601. doi: 10.1016/j.ijbiomac.2024.133601
10. Liu Y, Feng Z, Hu Y, Xu X, Kuang T, Liu Y. Polysaccharides derived from natural edible and medicinal sources as agents targeting exercise-induced fatigue: a review. Int J Biol Macromol. (2024) 256:128280. doi: 10.1016/j.ijbiomac.2023.128280
11. Pliego-Cortés H, Hardouin K, Bedoux G, Marty C, Cérantola S, Freile-Pelegrín Y, et al. Sulfated polysaccharides from seaweed strandings as renewable source for potential antivirals against herpes simplex virus 1. Mar Drugs. (2022) 20:116. doi: 10.3390/md20020116
12. Muthukumar J, Chidambaram R, Sukumaran S. Sulfated polysaccharides and its commercial applications in food industries-a review. J Food Sci Technol. (2021) 58:2453–66. doi: 10.1007/s13197-020-04837-0
13. Jegadeshwari B, Rajaram R. A critical review on pharmacological properties of sulfated polysaccharides from marine macroalgae. Carbohydr Polym (2024) 344:122488. doi: 10.1016/j.carbpol.2024.122488
14. Zhou S, Huang G. Extraction, derivatization, and antioxidant activity of Morinda citrifolia polysaccharide. Chem Biol Drug Des. (2022) 99:603–8. doi: 10.1111/cbdd.14023
15. Zhou S, Huang G, Chen G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. (2021) 361:130089. doi: 10.1016/j.foodchem.2021.130089
16. Liu Y, Ran L, Wang Y, Wan P, Zhou H. Basic characterization, antioxidant and immunomodulatory activities of polysaccharides from sea buckthorn leaves. Fitoterapia. (2023) 169:105592. doi: 10.1016/j.fitote.2023.105592
17. Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. (2011) 9:196–223. doi: 10.3390/md9020196
18. Li W, Zhang Y, Zhao X, Fang L, Yang T, Xie J. Optimization of ultrasonic-assisted extraction of Platycodon grandiflorum polysaccharides and evaluation of its structural, antioxidant and hypoglycemic activity. Ultrason Sonochem. (2023) 100:106635. doi: 10.1016/j.ultsonch.2023.106635
19. Gong P, Wang M, Guo Y, Long H, Wang Z, Cui D, et al. Structure characterization, in vitro antioxidant and anti-tumor activity of sulfated polysaccharide from Siraitia grosvenorii. Foods. (2023) 12:2133. doi: 10.3390/foods12112133
20. Zhang X-X, Zhang W-W, Ni Z-J, Thakur K, Zhang J-G, Khan MR, et al. Effects of different chemical modifications on physicochemical and antioxidation properties of Lycium barbarum seed dreg polysaccharides. Food Chem X. (2024) 22:101271. doi: 10.1016/j.fochx.2024.101271
21. Jiang Y, Zi W, Pei Z, Liu S. Characterization of polysaccharides and their antioxidant properties from Plumula nelumbinis. Saudi Pharm J. (2018) 26:656–64. doi: 10.1016/j.jsps.2018.02.026
22. Li H, Wang Y, Zhao P, Guo L, Huang L, Li X, et al. Naturally and chemically acetylated polysaccharides: structural characteristics, synthesis, activities, and applications in the delivery system: a review. Carbohydr Polym. (2023) 313:120746. doi: 10.1016/j.carbpol.2023.120746
23. Wang X, Wang Z, Shen M, Yi C, Yu Q, Chen X, et al. Acetylated polysaccharides: Synthesis, physicochemical properties, bioactivities, and food applications. Crit Rev Food Sci Nutr. (2024) 64:4849–64. doi: 10.1080/10408398.2022.2146046
24. Wang Y, Huang G. Preparation, structure and properties of litchi pericarp polysaccharide. Sci Rep. (2025) 15:6331. doi: 10.1038/s41598-025-90697-7
25. Xie J-H, Zhang F, Wang Z-J, Shen M-Y, Nie S-P, Xie M-Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr Polym. (2015) 133:596–604. doi: 10.1016/j.carbpol.2015.07.031
26. Song Y, Yang Y, Zhang Y, Duan L, Zhou C, Ni Y, et al. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva). Carbohydr Polym. (2013) 98:686–91. doi: 10.1016/j.carbpol.2013.06.049
27. Chen F, Huang G. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int J Biol Macromol. (2019) 141:14–20. doi: 10.1016/j.ijbiomac.2019.08.239
28. Wang H, Yuan M, Li G, Tao Y, Wang X, Ke S, et al. Chemical characterization, antioxidant and immunomodulatory activities of acetylated polysaccharides from Cyperus esculentus. Food Chem. (2023) 427:136734. doi: 10.1016/j.foodchem.2023.136734
29. Nuerxiati R, Mutailipu P, Abuduwaili A, Dou J, Aisa HA, Yili A. Effects of different chemical modifications on the structure and biological activities of polysaccharides from Orchis chusua D. Don J Food Sci. (2021) 86:2434–44. doi: 10.1111/1750-3841.15734
30. Meng-Zhao null, Yi-Han null, Li J, Qi-An null, Ye X, Xiang-Li null, et al. Structural characterization and antioxidant activity of an acetylated Cyclocarya paliurus polysaccharide (Ac-CPP0.1). Int J Biol Macromol. (2021) 171:112–122. doi: 10.1016/j.ijbiomac.2020.12.201
31. Liu X, Xie J, Jia S, Huang L, Wang Z, Li C. Xie M. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW2647. Int J Biol Macromol. (2017) 98:576–81. doi: 10.1016/j.ijbiomac.2017.02.028
32. Huang L, Zhao J, Wei Y, Yu G, Li F, Li Q. Structural characterization and mechanisms of macrophage immunomodulatory activity of a pectic polysaccharide from Cucurbita moschata Duch. Carbohydr Polym. (2021) 269:118288. doi: 10.1016/j.carbpol.2021.118288
33. Chakka VP, Zhou T. Carboxymethylation of polysaccharides: synthesis and bioactivities. Int J Biol Macromol. (2020) 165:2425–31. doi: 10.1016/j.ijbiomac.2020.10.178
34. Li X-L, Tu X-F, Thakur K, Zhang Y-S, Zhu D-Y, Zhang J-G, et al. Effects of different chemical modifications on the antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int J Biol Macromol. (2018) 112:675–85. doi: 10.1016/j.ijbiomac.2018.01.216
35. Liu Y, Huang G. The antioxidant activities of carboxymethylated cushaw polysaccharide. Int J Biol Macromol. (2019) 121:666–70. doi: 10.1016/j.ijbiomac.2018.10.108
36. Chen S, Huang H, Huang G. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int J Biol Macromol. (2019) 140:1047–53. doi: 10.1016/j.ijbiomac.2019.08.203
37. Jiang L-M, Nie S-P, Zhou H-L, Huang D-F, Xie M-Y. Carboxymethylation enhances the maturation-inducing activity in dendritic cells of polysaccharide from the seeds of Plantago asiatica L. Int Immunopharmacol. (2014) 22:324–31. doi: 10.1016/j.intimp.2014.06.027
38. Tang Z, Huang G. Antioxidant activity of polysaccharide from Garcinia mangostana rind and their derivatives. BMC Complement Med Ther. (2024) 24:283. doi: 10.1186/s12906-024-04594-z
39. Surhio MM, Wang Y, Fang S, Li J, Ye M. Anti-fatigue activity of a Lachnum polysaccharide and its carboxymethylated derivative in mice. Bioorg Med Chem Lett. (2017) 27:4777–80. doi: 10.1016/j.bmcl.2017.07.034
40. Laffargue T, Moulis C, Remaud-Siméon Remaud-Siméon M. Phosphorylated polysaccharides: Applications, natural abundance, and new-to-nature structures generated by chemical and enzymatic functionalisation. Biotechnol Adv. (2023) 65:108140. doi: 10.1016/j.biotechadv.2023.108140
41. Xia S, Zhai Y, Wang X, Fan Q, Dong X, Chen M, et al. Phosphorylation of polysaccharides: a review on the synthesis and bioactivities. Int J Biol Macromol. (2021) 184:946–54. doi: 10.1016/j.ijbiomac.2021.06.149
42. Zhou S, Huang G, Huang H. Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chem. (2022) 388:133000. doi: 10.1016/j.foodchem.2022.133000
43. Yang W, Zhang Y, Tang A, Ruan Q, Huang G. Preparation and antioxidant activity of phosphorylated polysaccharide from purple sweet potato. Chem Biol Drug Des. (2021) 98:828–34. doi: 10.1111/cbdd.13936
44. Xie L, Shen M, Wen P, Hong Y, Liu X, Xie J. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of phosphorylated polysaccharide from Cyclocarya paliurus. Food Chem Toxicol. (2020) 145:111754. doi: 10.1016/j.fct.2020.111754
45. Huo N, Ameer K, Wu Z, Yan S, Jiang G, Ramachandraiah K. Preparation, characterization, structural analysis and antioxidant activities of phosphorylated polysaccharide from Sanchi (Panax notoginseng) flower. J Food Sci Technol. (2022) 59:4603–14. doi: 10.1007/s13197-022-05539-5
46. Lian S, Su J, Fatima I, Zhang Y, Kuang T, Hu H, et al. Revealing the exceptional antioxidant activity of phosphorylated polysaccharides from medicinal Abrus cantoniensis Hance. Int J Biol Macromol. (2024) 278:134532. doi: 10.1016/j.ijbiomac.2024.134532
47. Chen L, Huang G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int J Biol Macromol. (2019) 125:256–61. doi: 10.1016/j.ijbiomac.2018.12.069
48. Xiong X, Huang G, Huang H. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int J Biol Macromol. (2019) 126:842–5. doi: 10.1016/j.ijbiomac.2018.12.266
49. Chen J, Huang G. Antioxidant activities of garlic polysaccharide and its phosphorylated derivative. Int J Biol Macromol. (2019) 125:432–5. doi: 10.1016/j.ijbiomac.2018.12.073
50. Chen L, Huang G. The antioxidant activity of derivatized cushaw polysaccharides. Int J Biol Macromol. (2019) 128:1–4. doi: 10.1016/j.ijbiomac.2019.01.091
51. Griesser J, Burtscher S, Köllner S, Nardin I, Prüfert F, Bernkop-Schnürch A. Zeta potential changing self-emulsifying drug delivery systems containing phosphorylated polysaccharides. Eur J Pharm Biopharm. (2017) 119:264–70. doi: 10.1016/j.ejpb.2017.06.025
52. Feng H, Fan J, Lin L, Liu Y, Chai D, Yang J. Immunomodulatory effects of phosphorylated radix Cyathulae officinalis polysaccharides in immunosuppressed mice. Molecules. (2019) 24:4150. doi: 10.3390/molecules24224150
53. Lin L, Yang J, Yang Y, Zhi H, Hu X, Chai D, et al. Phosphorylation of Radix Cyathula officinalis polysaccharide improves its immune-enhancing activity. J Carbohydr Chem. (2020) 39:50–62. doi: 10.1080/07328303.2019.1700996
54. Feng H, McDonough SP, Fan J, Yang S, Zhao X, Lu Y, et al. Phosphorylated Radix Cyathulae officinalis polysaccharides act as adjuvant via promoting dendritic cell maturation. Molecules. (2017) 22:106. doi: 10.3390/molecules22010106
55. Nasaj M, Chehelgerdi M, Asghari B, Ahmadieh-Yazdi A, Asgari M, Kabiri-Samani S, et al. Factors influencing the antimicrobial mechanism of chitosan action and its derivatives: a review. Int J Biol Macromol. (2024) 277:134321. doi: 10.1016/j.ijbiomac.2024.134321
56. Aghbashlo M, Amiri H, Moosavi Basri SM, Rastegari H, Lam SS, Pan J, et al. Tuning chitosan's chemical structure for enhanced biological functions. Trends Biotechnol. (2023) 41:785–97. doi: 10.1016/j.tibtech.2022.11.009
57. Rafique A, Mahmood Zia K, Zuber M, Tabasum S, Rehman S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: a review. Int J Biol Macromol. (2016) 87:141–54. doi: 10.1016/j.ijbiomac.2016.02.035
58. Narmani A, Jafari SM. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr Polym. (2021) 272:118464. doi: 10.1016/j.carbpol.2021.118464
59. Ali A, Ahmed S. A review on chitosan and its nanocomposites in drug delivery. Int J Biol Macromol. (2018) 109:273–86. doi: 10.1016/j.ijbiomac.2017.12.078
60. Riseh RS, Vazvani MG, Kennedy JF. The application of chitosan as a carrier for fertilizer: a review. Int J Biol Macromol. (2023) 252:126483. doi: 10.1016/j.ijbiomac.2023.126483
61. Kurita K, Matsumura Y, Takahara H, Hatta K, Shimojoh M. Synthesis and macrophage activation of lentinan-mimic branched amino polysaccharides: curdlans having N-Acetyl-d-glucosamine branches. Biomacromolecules. (2011) 12:2267–74. doi: 10.1021/bm200353m
62. Li S, Xiong Q, Lai X, Li X, Wan M, Zhang J, et al. Molecular modification of polysaccharides and resulting bioactivities. Compr Rev Food Sci Food Saf. (2016) 15:237–50. doi: 10.1111/1541-4337.12161
63. Shao P, Qin M, Han L, Sun P. Rheology and characteristics of sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Carbohydr Polym. (2014) 113:365–72. doi: 10.1016/j.carbpol.2014.07.008
64. Jing Y, Li M, Li Y, Ma T, Qu Y, Hu B, et al. Structural characterization and anti-fatigue mechanism based on the gut-muscle axis of a polysaccharide from Zingiber officinale. Int J Biol Macromol. (2024) 283:137621. doi: 10.1016/j.ijbiomac.2024.137621
65. Cai M, Zhu H, Xu L, Wang J, Xu J, Li Z, et al. Structure, anti-fatigue activity and regulation on gut microflora in vivo of ethanol-fractional polysaccharides from Dendrobium officinale. Int J Biol Macromol. (2023) 234:123572. doi: 10.1016/j.ijbiomac.2023.123572
66. Gao H, Zhang W, Wang B, Hui A, Du B, Wang T, et al. Purification, characterization and anti-fatigue activity of polysaccharide fractions from okra (Abelmoschus esculentus (L) Moench). Food Funct. (2018) 9:1088–101. doi: 10.1039/C7FO01821E
67. Jiang P, Ji X, Xia J, Xu M, Hao F, Tong H, et al. Structure and potential anti-fatigue mechanism of polysaccharides from Bupleurum chinense DC. Carbohydr Polym. (2023) 306:120608. doi: 10.1016/j.carbpol.2023.120608
68. Jiao L, Li J, Liu F, Wang J, Jiang P, Li B, et al. Characterisation, chain conformation and antifatigue effect of steamed ginseng polysaccharides with different molecular weight. Front Pharmacol. (2021) 12:712836. doi: 10.3389/fphar.2021.712836
69. Wang J, Li S, Fan Y, Chen Y, Liu D, Cheng H, et al. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C. A Meyer J Ethnopharmacol. (2010) 130:421–3. doi: 10.1016/j.jep.2010.05.027
70. Chi A, Tang L, Zhang J, Zhang K. Chemical composition of three polysaccharides from Gynostemma pentaphyllum and their antioxidant activity in skeletal muscle of exercised mice. Int J Sport Nutr Exerc Metab. (2012) 22:479–85. doi: 10.1123/ijsnem.22.6.479
71. Dallazen JL, Ciapparini PG, Maria-Ferreira D, da Luz BB, Klosterhoff RR, Felipe LPG, et al. Arabinan-rich pectic polysaccharide fraction from Malpighia emarginata fruits alleviates inflammatory pain in mice. Food Res Int. (2024) 176:113743. doi: 10.1016/j.foodres.2023.113743
72. Chung Y, Park T, Yim S, Lee JH, Bang J, Shin Y, et al. Polysaccharide-rich extract of Phragmites rhizome attenuates water immersion stress and forced swimming fatigue in rodent animal model. J Med Food. (2019) 4:355–64. doi: 10.1089/jmf.2018.4218
73. Wei X, Xin J, Chen W, Wang J, Lv Y, Wei Y, et al. Astragalus polysaccharide ameliorated complex factor-induced chronic fatigue syndrome by modulating the gut microbiota and metabolites in mice. Biomed Pharmacother. (2023) 163:114862. doi: 10.1016/j.biopha.2023.114862
74. Li T, Xie C, Tian Z, Chai R, Ren Y, Miao J, et al. A soluble garlic polysaccharide supplement alleviates fatigue in mice. NPJ Sci Food. (2024) 8:98. doi: 10.1038/s41538-024-00340-4
75. Xing YY, Xu YQ, Jin X, Shi LL, Guo SW, Yan SM, et al. Optimization extraction and characterization of Artemisia ordosica polysaccharide and its beneficial effects on antioxidant function and gut microbiota in rats. RSC Adv. (2020) 10:26151–64. doi: 10.1039/D0RA05063F
76. Xie Y, Wang L, Sun H, Wang Y, Yang Z, Zhang G, et al. Immunomodulatory, antioxidant and intestinal morphology-regulating activities of alfalfa polysaccharides in mice. Int J Biol Macromol. (2019) 133:1107–14. doi: 10.1016/j.ijbiomac.2019.04.144
77. Cheng M, Shi Y, Cheng Y, Hu H, Liu S, Xu Y, et al. Mulberry leaf polysaccharide improves cyclophosphamide-induced growth inhibition and intestinal damage in chicks by modulating intestinal flora, enhancing immune regulation and antioxidant capacity. Front Microbiol. (2024) 15:1382639. doi: 10.3389/fmicb.2024.1382639
78. Wang Q, Wang XF, Xing T, Li JL, Zhu XD, Zhang L, et al. The combined impact of xylo-oligosaccharides and gamma-irradiated astragalus polysaccharides on the immune response, antioxidant capacity, and intestinal microbiota composition of broilers. Poult Sci. (2022) 101:101996. doi: 10.1016/j.psj.2022.101996
79. Subhash A, Bamigbade G, Abdin M, Jarusheh H, Abu-Jdayil B, Liu S-Q, et al. Date seeds polysaccharides as novel capping agents for selenium nanoparticles: synthesis, characterization, stability, biological activities, and gut microbiota modulation. Food Chem. (2025) 470:142746. doi: 10.1016/j.foodchem.2024.142746
80. Rubino CA, Minden HA. An analysis of eye-movements in children with a reading disability. Cortex. (1973) 9:217–20. doi: 10.1016/S0010-9452(73)80030-9
81. Wang J, Sun C, Zheng Y, Pan H, Zhou Y, Fan Y. The effective mechanism of the polysaccharides from Panax ginseng on chronic fatigue syndrome. Arch Pharm Res. (2014) 37:530–8. doi: 10.1007/s12272-013-0235-y
82. Peng Y, Zhao L, Hu K, Yang Y, Ma J, Zhai Y, et al. Anti-fatigue effects of Lycium barbarum polysaccharide and effervescent tablets by regulating oxidative stress and energy metabolism in rats. Int J Mol Sci. (2022) 23:10920. doi: 10.3390/ijms231810920
83. Xu X, Shan M, Chu C, Bie S, Wang H, Cai S. Polysaccharides from Polygonatum kingianum Collett & Hemsl ameliorated fatigue by regulating NRF2/HO-1/NQO1 and AMPK/PGC-1α/TFAM signaling pathways, and gut microbiota. Int J Biol Macromol. (2024) 266:131440. doi: 10.1016/j.ijbiomac.2024.131440
84. Chen C, Chen J, Wang Y, Fang L, Guo C, Sang T, et al. Ganoderma lucidum polysaccharide inhibits HSC activation and liver fibrosis via targeting inflammation, apoptosis, cell cycle, and ECM-receptor interaction mediated by TGF-β/Smad signaling. Phytomedicine. (2023) 110:154626. doi: 10.1016/j.phymed.2022.154626
85. Dong N, Li X, Xue C, Zhang L, Wang C, Xu X, et al. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J Cell Physiol. (2020) 235:5525–40. doi: 10.1002/jcp.29452
86. Chang L, Wang C, Peng J, Song Y, Zhang W, Chen Y, et al. Rattan pepper polysaccharide regulates DSS-induced intestinal inflammation and depressive behavior through microbiota-gut-brain axis. J Agric Food Chem. (2024) 72:437–48. doi: 10.1021/acs.jafc.3c08462
87. Wang Y, Li C, Li J, Zhang S, Zhang Q, Duan J, et al. Abelmoschus manihot polysaccharide fortifies intestinal mucus barrier to alleviate intestinal inflammation by modulating Akkermansia muciniphila abundance. Acta Pharm Sin B. (2024) 14:3901–15. doi: 10.1016/j.apsb.2024.06.002
88. Zhang B, Zeng M, Zhang Q, Wang R, Jia J, Cao B, et al. Ephedrae Herba polysaccharides inhibit the inflammation of ovalbumin induced asthma by regulating Th1/Th2 and Th17/Treg cell immune imbalance. Mol Immunol. (2022) 152:14–26. doi: 10.1016/j.molimm.2022.09.009
89. Jing X, Zhou G, Zhu A, Jin C, Li M, Ding K, et al. RG-I pectin-like polysaccharide from Rosa chinensis inhibits inflammation and fibrosis associated to HMGB1/TLR4/NF-κB signaling pathway to improve non-alcoholic steatohepatitis. Carbohydr Polym. (2024) 337:122139. doi: 10.1016/j.carbpol.2024.122139
90. Huo DY Li YF, Song LJ, Zhang WX Li XD, Zhang J, Ren S, Wang Z, et al. Colon-targeted ginseng polysaccharides-based microspheres for improving ulcerative colitis via anti-inflammation and gut microbiota modulation. Adv Healthc Mater. (2025) 14:e2404122. doi: 10.1002/adhm.202404122
91. Zhao L, Yu J, Liu Y, Liu Y, Zhao Y, Li M-Y. The major roles of intestinal microbiota and TRAF6/NF-κB signaling pathway in acute intestinal inflammation in mice, and the improvement effect by Hippophae rhamnoides polysaccharide. Int J Biol Macromol. (2025) 296:139710. doi: 10.1016/j.ijbiomac.2025.139710
92. Yang K, Zhang Y, Fang F, Wang M, Lin Y-F, Yan B, et al. The structural characteristics, beneficial effects and biological mechanisms of food and medicinal plant polysaccharides on exercise-induced fatigue: a review. Int J Biol Macromol. (2025) 311:144046. doi: 10.1016/j.ijbiomac.2025.144046
93. de Barcellos LAM, Gonçalves WA, Esteves De Oliveira MP, Guimarães JB, Queiroz-Junior CM, Resende CBD, et al. Effect of physical training on exercise-induced inflammation and performance in mice. Front Cell Dev Biol. (2021) 9:625680. doi: 10.3389/fcell.2021.625680
94. Kang C, Liu Y, Chi A, Zhang Z. The anti-fatigue potential of water-soluble polysaccharides of Semen cassiae on BALB/c mice. Cell Mol Biol. (2021) 67:148–54. doi: 10.14715/cmb/2021.67.2.23
95. Zhao Q, Wang J, Liang H, Guo W, Chu Y, Liu L, et al. Prevention of cyclophosphamide-induced immune suppression by polysaccharides from Apocynum venetum flowers via enhancing immune response, reducing oxidative stress, and regulating gut microbiota in mice. Front Pharmacol. (2024) 15:1354794. doi: 10.3389/fphar.2024.1354794
96. Wu J, Yu G, Zhang X, Staiger MP, Gupta TB, Yao H, et al. A fructan-type garlic polysaccharide upregulates immune responses in macrophage cells and in immunosuppressive mice. Carbohydr Polym. (2024) 344:122530. doi: 10.1016/j.carbpol.2024.122530
97. Chen D, Kang Z, Chen H, Fu P. Polysaccharide from Areca catechu L. inflorescence enhances the intestinal mucosal immunity to maintain immune homeostasis. Int J Biol Macromol. (2024) 278:134900. doi: 10.1016/j.ijbiomac.2024.134900
98. Wei W, Li Z-P, Zhu T, Fung H-Y, Wong T-L, Wen X, et al. Anti-fatigue effects of the unique polysaccharide marker of Dendrobium officinale on BALB/c Mice. Molecules. (2017) 22:155. doi: 10.3390/molecules22010155
99. Liu M, Sun C, Zhou Q, Xu P, Wang A, Zheng X, et al. Supplementation of Yupingfeng polysaccharides in low fishmeal diets enhances intestinal health through influencing the intestinal barrier, immunity, and microflora in Macrobrachium rosenbergii. Front Immunol. (2024)1480897. doi: 10.3389/fimmu.2024.1480897
100. Ma H, Mueed A, Liu D, Ali A, Wang T, Ibrahim M, et al. Polysaccharides of Floccularia luteovirens regulate intestinal immune response, and oxidative stress activity through MAPK/Nrf2/Keap1 signaling pathway in immunosuppressive mice. Int J Biol Macromol. (2024) 277:134140. doi: 10.1016/j.ijbiomac.2024.134140
101. Hao X, Lin H, Lin Z, Yang K, Hu J, Ma Z, et al. Effect of dietary astragalus polysaccharides (APS) on the growth performance, antioxidant responses, immunological parameters, and intestinal microbiota of coral trout (Plectropomus leopardus). Microorganisms. (2024) 12:1980. doi: 10.3390/microorganisms12101980
102. Wang W, Liu X, Wang L, Song G, Jiang W, Mu L, et al. Ficus carica polysaccharide extraction via ultrasound-assisted technique: structure characterization, antioxidant, hypoglycemic and immunomodulatory activities. Ultrason Sonochem. (2023) 101:106680. doi: 10.1016/j.ultsonch.2023.106680
103. Yu L, Lin F, Yu Y, Deng X, Shi X, Lu X, et al. Rehmannia glutinosa polysaccharides enhance intestinal immunity of mice through regulating the microbiota. Int J Biol Macromol. (2024) 283:137878. doi: 10.1016/j.ijbiomac.2024.137878
104. Ma Y, Wei X, Peng J, Wei F, Wen Y, Liu M, et al. Ephedra sinica polysaccharide regulate the anti-inflammatory immunity of intestinal microecology and bacterial metabolites in rheumatoid arthritis. Front Pharmacol. (2024) 15:1414675. doi: 10.3389/fphar.2024.1414675
105. Guo X, Su Y, Du Y, Zhang F, Yu W, Ren W, et al. Vinegar-processed Schisandra chinensis polysaccharide ameliorates type 2 diabetes via modulation serum metabolic profiles, gut microbiota, and fecal SCFAs. Int J Biol Macromol. (2025) 294:139514. doi: 10.1016/j.ijbiomac.2025.139514
106. Geng X, Tian W, Zhuang M, Shang H, Gong Z, Li J. Green radish polysaccharides ameliorate hyperlipidemia in high-fat-diet-induced mice via short-chain fatty acids production and gut microbiota regulation. Foods. (2024) 13:4113. doi: 10.3390/foods13244113
107. Wu W, Wang Y, Yi P, Su X, Mi Y, Wu L, et al. Various steaming durations alter digestion, absorption, and fermentation by human gut microbiota outcomes of Polygonatum cyrtonema Hua polysaccharides. Front Nutr. (2024) 11:1466781. doi: 10.3389/fnut.2024.1466781
108. Li R, Wang H, Wang Q, Zhang Z, Wang L. Acid-assisted polysaccharides extracted from Asparagus cochinchinensis protect against Alzheimer's disease by regulating the microbiota-gut-brain axis. Front Nutr. (2024) 11:1496306. doi: 10.3389/fnut.2024.1496306
109. Li X-Y, Jiang C-L, Zheng C, Hong C-Z, Pan L-H, Li Q-M, et al. Polygonatum cyrtonema hua polysaccharide alleviates fatigue by modulating osteocalcin-mediated crosstalk between bones and muscles. J Agric Food Chem. (2023) 71:6468–79. doi: 10.1021/acs.jafc.2c08192
110. Chen S, Wang L, Rong S, Duan Y, Wang H. Extraction, purification, chemical characterization, and in vitro hypoglycemic activity of polysaccharides derived from Rosa laevigata Michx. Int J Biol Macromol. (2024) 279:135116. doi: 10.1016/j.ijbiomac.2024.135116
111. Wang X, Yang M, Shen Y, Zhang Y, Xiu W, Yu S, et al. Structural characterization and hypoglycemic effect of polysaccharides of Polygonatum sibiricum. J Food Sci. (2024) 89:4771–90. doi: 10.1111/1750-3841.17243
112. Song Q, Zou J, Li D, Cheng SW, Li KLS, Yang X, et al. Gastrointestinal metabolism of Astragalus membranaceus polysaccharides and its related hypoglycemic mechanism based on gut microbial transformation. Int J Biol Macromol. (2024) 280:135847. doi: 10.1016/j.ijbiomac.2024.135847
113. Chen Y, Ouyang Y, Chen X, Chen R, Ruan Q, Farag MA, et al. Hypoglycaemic and anti-ageing activities of green alga Ulva lactuca polysaccharide via gut microbiota in ageing-associated diabetic mice. Int J Biol Macromol. (2022) 212:97–110. doi: 10.1016/j.ijbiomac.2022.05.109
114. Liao Z, Zhang J, Liu B, Yan T, Xu F, Xiao F, et al. Polysaccharide from Okra (Abelmoschus esculentus (L) Moench) improves antioxidant capacity via PI3K/AKT pathways and Nrf2 translocation in a type 2 diabetes model. Molecules. (2019) 24:1906. doi: 10.3390/molecules24101906
115. Zhao Y, Li B, Wang G, Ge S, Lan X, Xu G, et al. Dendrobium officinale polysaccharides inhibit 1-methyl-2-nitro-1-nitrosoguanidine induced precancerous lesions of gastric cancer in rats through regulating Wnt/β-catenin pathway and altering serum endogenous metabolites. Molecules. (2019) 24:2660. doi: 10.3390/molecules24142660
116. Yang Q, Meng D, Zhang Q, Wang J. Advances in research on the anti-tumor mechanism of Astragalus polysaccharides. Front Oncol. (2024) 14:1334915. doi: 10.3389/fonc.2024.1334915
117. Tu J, He Y, Zhang H, Wang J, Li Z, Sun H. Anti-tumor effect of Crocus sativus petals polysaccharides by reconstructing tumor microenvironment. Int J Biol Macromol. (2023) 248:125878. doi: 10.1016/j.ijbiomac.2023.125878
118. Chen Y, Li Z, Bai L, Lu B, Peng Y, Xu P, et al. Glycyrrhiza polysaccharides may have an antitumor effect in γδT cells through gut microbiota and TLRs/NF-κB pathway in mice. FEBS Open Bio. (2024) 14:1011–27. doi: 10.1002/2211-5463.13800
119. Zhao J, He R, Zhong H, Liu S, Liu X, Hussain M, et al. A cold-water extracted polysaccharide-protein complex from Grifola frondosa exhibited anti-tumor activity via TLR4-NF-κB signaling activation and gut microbiota modification in H22 tumor-bearing mice. Int J Biol Macromol. (2023) 239:124291. doi: 10.1016/j.ijbiomac.2023.124291
120. Li N, Xiong YX, Ye F, Jin B, Wu JJ, Han MM, et al. Isolation, purification, and structural characterization of polysaccharides from Codonopsis pilosula and their anti-tumor bioactivity by immunomodulation. Pharmaceuticals. (2023) 16:895. doi: 10.3390/ph16060895
121. Liu L, Li M, Yu M, Shen M, Wang Q, Yu Y, et al. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int J Biol Macromol. (2019) 121:743–51. doi: 10.1016/j.ijbiomac.2018.10.083
122. Han S, Luo Y, Hu Z, Li X, Zhou Y, Luo F. Tumor microenvironment targeted by polysaccharides in cancer prevention: expanding roles of gut microbiota and metabolites. Mol Nutr Food Res. (2025) 69:e202400750. doi: 10.1002/mnfr.202400750
123. Chen P, Hei M, Kong L, Liu Y, Yang Y, Mu H, et al. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. (2019) 10:8161–71. doi: 10.1039/C9FO01178A
124. Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, et al. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. (2024) 97:119–29. doi: 10.1152/japplphysiol.00741.2003
125. Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. (2011) 51:942–50. doi: 10.1016/j.freeradbiomed.2010.12.009
126. Viña J, Salvador-Pascual A, Tarazona-Santabalbina FJ, Rodriguez-Mañas L, Gomez-Cabrera MC. Exercise training as a drug to treat age associated frailty. Free Radic Biol Med. (2016) 98:159–64. doi: 10.1016/j.freeradbiomed.2016.03.024
127. Shen SH, Singh SP, Raffaele M, Waldman M, Hochhauser E, Ospino J, et al. Adipocyte-specific expression of PGC1α promotes adipocyte browning and alleviates obesity-induced metabolic dysfunction in an HO-1-dependent fashion. Antioxidants. (2022) 11:1147. doi: 10.3390/antiox11061147
128. Liu C-T, Brooks GA. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol (2012) 112:354–61. doi: 10.1152/japplphysiol.00989.2011
129. Feng Y, Rao Z, Tian X, Hu Y, Yue L, Meng Y, et al. Endurance training enhances skeletal muscle mitochondrial respiration by promoting MOTS-c secretion. Free Radic Biol Med. (2025) 227:619–28. doi: 10.1016/j.freeradbiomed.2024.12.038
130. Li J, Zhang S, Li C, Zhang X, Shan Y, Zhang Z, et al. Endurance exercise-induced histone methylation modification involved in skeletal muscle fiber type transition and mitochondrial biogenesis. Sci Rep. (2024) 14:21154. doi: 10.1038/s41598-024-72088-6
131. Powers SK, Radak Z, Ji LL, Jackson M. Reactive oxygen species promote endurance exercise-induced adaptations in skeletal muscles. J Sport Health Sci. (2024) 13:780–92. doi: 10.1016/j.jshs.2024.05.001
132. Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. (2017) 25:581–92. doi: 10.1016/j.cmet.2017.02.009
133. Powers SK, Lategan-Potgieter R, Goldstein E. Exercise-induced Nrf2 activation increases antioxidant defenses in skeletal muscles. Free Radic Biol Med. (2024) 224:470–8. doi: 10.1016/j.freeradbiomed.2024.07.041
134. Close GL, Hamilton DL, Philp A, Burke LM, Morton JP. New strategies in sport nutrition to increase exercise performance. Free Radic Biol Med. (2016) 98:144–58. doi: 10.1016/j.freeradbiomed.2016.01.016
135. Lin D, Rezaei MJ. Plant polysaccharides and antioxidant benefits for exercise performance and gut health: from molecular pathways to clinic. Mol Cell Biochem. (2025) 480:2827–46. doi: 10.1007/s11010-024-05178-8
136. Mao YH, Wang M, Yuan Y, Weng X, Li LQ, Song AX. The sports performance improving effects of konjac glucomannan with varying molecular weights in overtrained mice. Int J Biol Macromol. (2024) 282:137523. doi: 10.1016/j.ijbiomac.2024.137523
137. Fritzen AM, Lundsgaard A-M, Kiens B. Tuning fatty acid oxidation in skeletal muscle with dietary fat and exercise. Nat Rev Endocrinol. (2020) 16:683–96. doi: 10.1038/s41574-020-0405-1
138. Li G, Das S. Making a sPLAsh: the expanding repertoire of EV signaling. Cell Metab. (2022) 34:508–10. doi: 10.1016/j.cmet.2022.03.004
139. Heden TD, Chow LS, Hughey CC, Mashek DG. Regulation and role of glycophagy in skeletal muscle energy metabolism. Autophagy. (2022) 18:1078–89. doi: 10.1080/15548627.2021.1969633
140. Jang I, Hwang D, Kyun S, Park HY, Kim J, Lim K. Effect of lactate intake on total fat mass and resting metabolic rate in mice. FASEB Journal. (2022) 36:4. doi: 10.1096/fasebj.2022.36.S1.R4379
141. Folgueira C, Herrera-Melle L, López JA, Galvan-Alvarez V, Martin-Rincon M, Cuartero MI, et al. Remodeling p38 signaling in muscle controls locomotor activity via IL-15. Sci Adv. (2024) 10:eadn5993. doi: 10.1126/sciadv.adn5993
142. Nadeau L, Aguer C. Interleukin-15 as a myokine: mechanistic insight into its effect on skeletal muscle metabolism. Appl Physiol Nutr Metab. (2019) 44:229–38. doi: 10.1139/apnm-2018-0022
143. Ikeda S-I, Tamura Y, Kakehi S, Sanada H, Kawamori R, Watada H. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem Biophys Res Commun. (2016) 473:947–52. doi: 10.1016/j.bbrc.2016.03.159
144. Yu Y, Nie J, Zhao B, Tan J, Lv C, Lu J. Structure characterization and anti-fatigue activity of an acidic polysaccharide from Panax ginseng C. A Meyer J Ethnopharmacol. (2023) 301:115831. doi: 10.1016/j.jep.2022.115831
145. Xie Q, Sun Y, Cao L, Chen L, Chen J, Cheng X, et al. Antifatigue and antihypoxia activities of oligosaccharides and polysaccharides from Codonopsis pilosula in mice. Food Funct. (2020) 11:6352–62. doi: 10.1039/D0FO00468E
146. Zhao R, Cai Y, Shao X, Ma B. Improving the activity of Lycium barbarum polysaccharide on sub-health mice. Food Funct. (2015) 6:2033–40. doi: 10.1039/C4FO01108B
147. Liu G, Yang X, Zhang J, Liang L, Miao F, Ji T, et al. Synthesis, stability and anti-fatigue activity of selenium nanoparticles stabilized by Lycium barbarum polysaccharides. Int J Biol Macromol. (2021) 179:418–28. doi: 10.1016/j.ijbiomac.2021.03.018
148. Chen W, Shen Z, Dong W, Huang G, Yu D, Chen W, et al. Polygonatum sibiricum polysaccharide ameliorates skeletal muscle aging via mitochondria-associated membrane-mediated calcium homeostasis regulation. Phytomedicine. (2024) 129:155567. doi: 10.1016/j.phymed.2024.155567
149. Shen W-D, Li X-Y, Deng Y-Y, Zha X-Q, Pan L-H, Li Q-M, et al. Polygonatum cyrtonema Hua polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling. Int J Biol Macromol. (2021) 175:235–41. doi: 10.1016/j.ijbiomac.2021.01.200
150. Zhang T, Li X-Y, Kuang D-D, Pan L-H, Li Q-M, Luo J-P, et al. Bone-brain communication mediates the amelioration of Polgonatum cyrtonema Hua polysaccharide on fatigue in chronic sleep-deprived mice. Int J Biol Macromol. (2025) 296:139706. doi: 10.1016/j.ijbiomac.2025.139706
151. Tang Y, Zhu Z-Y, Pan L-C, Sun H, Song Q-Y, Zhang Y. Structure analysis and anti-fatigue activity of a polysaccharide from Lepidium meyenii Walp. Nat Prod Res. (2019) 33:2480–9. doi: 10.1080/14786419.2018.1452017
152. Li Y, Xin Y, Xu F, Zheng M, Xi X, Cui X, et al. Maca polysaccharides: extraction optimization, structural features and anti-fatigue activities. Int J Biol Macromol. (2018) 115:618–24. doi: 10.1016/j.ijbiomac.2018.04.063
153. Tang W, Jin L, Xie L, Huang J, Wang N, Chu B, et al. Structural characterization and antifatigue effect in vivo of Maca (Lepidium meyenii Walp) polysaccharide. J Food Sci. (2017) 82:757–64. doi: 10.1111/1750-3841.13619
154. Li J, Sun Q, Meng Q, Wang L, Xiong W, Zhang L. Anti-fatigue activity of polysaccharide fractions from Lepidium meyenii Walp. (maca). Int J Biol Macromol. (2017) 95:1305–11. doi: 10.1016/j.ijbiomac.2016.11.031
155. Su Y, Gao X, Wang Y, Li X, Zhang W, Zhao J. Astragalus polysaccharide promotes sheep satellite cell differentiation by regulating miR-133a through the MAPK/ERK signaling pathway. Int J Biol Macromol. (2023) 239:124351. doi: 10.1016/j.ijbiomac.2023.124351
156. Zhou H-X, Zhang X, Huang R-G, Su T-C. Antifatigue effects and antioxidant activity in polysaccharide fractions from Chinese yam bulbils. Food Sci Nutr. (2024) 12:1218–29. doi: 10.1002/fsn3.3836
157. Hao H, Sha A. Study on anti-fatigue effects and mechanisms of polysaccharide from Paris polyphylla. Dokl Biochem Biophys. (2024) 516:58–65. doi: 10.1134/S1607672924600180
158. Li C, Zhu X, Zhang J, Xu T, Zhang H, Zheng Z, et al. Polysaccharides from apple pomace exhibit anti-fatigue activity through increasing glycogen content. J Food Sci Technol. (2023) 60:283–91. doi: 10.1007/s13197-022-05613-y
159. Zhao H-P, Zhang Y, Liu Z, Chen J-Y, Zhang S-Y, Yang X-D, et al. Acute toxicity and anti-fatigue activity of polysaccharide-rich extract from corn silk. Biomed Pharmacother. (2017) 90:686–93. doi: 10.1016/j.biopha.2017.04.045
160. Ji X, Chen S, Wu Q, Ling M, Tong J, Tong H, et al. An acid polysaccharide from Mentha haplocalyx exerts the antifatigue effect via activating AMPK. Int J Biol Macromol. (2025) 300:140235. doi: 10.1016/j.ijbiomac.2025.140235
161. Xu Z, Shan Y. Anti-fatigue effects of polysaccharides extracted from Portulaca oleracea L. in mice Indian. J Biochem Biophys. (2014) 51:321–5.
162. Yang P, Zhou Q, Zhang Y, Jia M, Li R, Qu Q, et al. Exploring the prebiotic potential of fermented astragalus polysaccharides on gut microbiota regulation in vitro. Curr Microbiol. (2024) 82:52. doi: 10.1007/s00284-024-04035-7
163. Varghese S, Rao S, Khattak A, Zamir F, Chaari A. Physical exercise and the gut microbiome: a bidirectional relationship influencing health and performance. Nutrients. (2024) 16:3663. doi: 10.3390/nu16213663
164. Song Q, Zou J, Cheng SW, Li KSL, Lau DTW, Yang X, et al. Insights into metabolic signatures and regulatory effect of Dendrobium officinale polysaccharides in gut microbiota: a comparative study of healthy and diabetic status. Food Sci Nutr. (2024) 13:e4651. doi: 10.1002/fsn3.4651
165. Xiao L, Liu J, Sun Z, Yin Y, Mao Y, Xu D, et al. AMPK-dependent and -independent coordination of mitochondrial function and muscle fiber type by FNIP1. PLoS Genet. (2021) 17:e1009488. doi: 10.1371/journal.pgen.1009488
166. Garcia-Roves PM, Osler ME, Holmström MH, Zierath JR. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J Biol Chem. (2008) 283:35724–34. doi: 10.1074/jbc.M805078200
167. Röckl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. (2007) 56:2062–9. doi: 10.2337/db07-0255
168. Trefts E, Shaw RJ. AMPK: restoring metabolic homeostasis over space and time. Mol Cell. (2021) 81:3677–90. doi: 10.1016/j.molcel.2021.08.015
169. Tuo X, Deng Z, Huang G, Gong H, Xie H. Astragalus polysaccharide attenuates overexercise-induce myocardial injury via activating AMPK signaling pathway to suppress inflammation and oxidative stress. An Acad Bras Cienc. (2021) 94:e20210314. doi: 10.1590/0001-3765202120210314
170. Ren Y, Wang K, Wu Y, Li J, Ma J, Wang L, et al. Lycium barbarum polysaccharide mitigates high-fat-diet-induced skeletal muscle atrophy by promoting AMPK/PINK1/Parkin-mediated mitophagy. Int J Biol Macromol. (2025) 301:140488. doi: 10.1016/j.ijbiomac.2025.140488
171. Li Y, Liu Z, Yan H, Zhou T, Zheng L, Wen F, et al. Polygonatum sibiricum polysaccharide ameliorates skeletal muscle aging and mitochondrial dysfunction via PI3K/Akt/mTOR signaling pathway. Phytomedicine (2025)156316. doi: 10.1016/j.phymed.2024.156316
172. Li XT, Chen R, Jin LM, Chen HY. Regulation on energy metabolism and protection on mitochondria of Panax ginseng polysaccharide. Am J Chin Med. (2009) 37:1139–52. doi: 10.1142/S0192415X09007454
173. Zhu M, Zhu H, Ding X, Liu S, Zou Y. Analysis of the anti-fatigue activity of polysaccharides from Spirulina platensis: role of central 5-hydroxytryptamine mechanisms. Food Funct. (2020) 11:1826–34. doi: 10.1039/C9FO02804H
174. Han C, Shen H, Yang Y, Sheng Y, Wang J, Li W, et al. Antrodia camphorata polysaccharide resists 6-OHDA-induced dopaminergic neuronal damage by inhibiting ROS-NLRP3 activation. Brain Behav. (2020) 10:e01824. doi: 10.1002/brb3.1824
175. Gao YY, Zhou YH, Liu XP, Di B, He JY, Wang YT, et al. Ganoderma lucidum polysaccharide promotes broiler health by regulating lipid metabolism, antioxidants, and intestinal microflora. Int J Biol Macromol. (2024) 280:135918. doi: 10.1016/j.ijbiomac.2024.135918
176. Tang L, Bao S, Du Y, Jiang Z, Wuliji AO, Ren X, et al. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed Pharmacother. (2018) 103:829–37. doi: 10.1016/j.biopha.2018.04.104
177. Zhao X-N, Liang J-L, Chen H-B, Liang Y-E, Guo H-Z, Su Z-R, et al. Anti-fatigue and antioxidant activity of the polysaccharides isolated from Millettiae speciosae Champ. Leguminosae. Nutrients. (2015) 7:8657–69. doi: 10.3390/nu7105422
178. Ren H, Li K, Min Y, Qiu B, Huang X, Luo J, et al. Rehmannia glutinosa polysaccharides: optimization of the decolorization process and antioxidant and anti-inflammatory effects in LPS-stimulated porcine intestinal epithelial cells. Antioxidants. (2023) 12:914. doi: 10.3390/antiox12040914
179. Wang Y, Liu Y, Zhang Y, Huo Z, Wang G, He Y, et al. Gao W. Effects of the polysaccharides extracted from Chinese yam (Dioscorea opposita Thunb) on cancer-related fatigue in mice. Food Funct. (2021) 12:10602–14. doi: 10.1039/D1FO00375E
180. Chi A, Li H, Kang C, Guo H, Wang Y, Guo F, et al. Anti-fatigue activity of a novel polysaccharide conjugates from Ziyang green tea. Int J Biol Macromol. (2015) 80:566–72. doi: 10.1016/j.ijbiomac.2015.06.055
181. Ni W, Gao T, Wang H, Du Y, Li J, Li C, et al. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J Ethnopharmacol. (2013) 150:529–35. doi: 10.1016/j.jep.2013.08.055
182. Pei Y, Yang S, Xiao Z, Zhou C, Hong P, Qian Z-J. Structural characterization of sulfated polysaccharide isolated from red algae (Gelidium crinale) and antioxidant and anti-inflammatory effects in macrophage cells. Front Bioeng Biotechnol. (2021) 9:794818. doi: 10.3389/fbioe.2021.794818
183. Gao LL, Ma JM, Fan YN, Zhang YN, Ge R, Tao XJ, et al. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int J Biol Macromol. (2021) 183:1379–92. doi: 10.1016/j.ijbiomac.2021.05.066
184. Yang K, Chen Y, Wang M, Zhang Y, Yuan Y, Hou H, et al. The improvement and related mechanism of microecologics on the sports performance and post-exercise recovery of athletes: a narrative review. Nutrients. (2024) 16:1602. doi: 10.3390/nu16111602
185. Nami Y, Barghi A, Shahgolzari M, Salehian M, Haghshenas B. Mechanism of action and beneficial effects of probiotics in amateur and professional athletes. Food Sci Nutr. (2024) 13:e4658. doi: 10.1002/fsn3.4658
186. Nie W, Tong X, Pung C, Li J, Ye H, Huang X. Insights into the relationship between the acetylation of Dendrobium officinale polysaccharides and the ability to promote sIgA secretion. Int J Biol Macromol. (2025) 304:140764. doi: 10.1016/j.ijbiomac.2025.140764
187. Su L-L, Li X, Guo Z-J, Xiao X-Y, Chen P, Zhang J-B, et al. Effects of different steaming times on the composition, structure and immune activity of Polygonatum Polysaccharide. J Ethnopharmacol. (2023) 310:116351. doi: 10.1016/j.jep.2023.116351
188. Ti Y, Zhang Y, Hou Y, Ban Y, Wang X, Li G, et al. Structural analysis and immunological activity of a novel low molecular weight neutral polysaccharide isolated from Hemerocallis citrina Borani. Food Chem. (2025) 469:142566. doi: 10.1016/j.foodchem.2024.142566
189. Liang L, Lin L, Zhao M. Exploration of green preparation strategy for Lycium barbarum polysaccharide targeting Bacteroides proliferative and immune-enhancing activities and its potential use in geriatric foods. Int J Biol Macromol. (2024) 267:131316. doi: 10.1016/j.ijbiomac.2024.131316
190. Zhang Y, Pan X, Ran S, Wang K. Purification, structural elucidation and anti-inflammatory activity in vitro of polysaccharides from Smilax china L. Int J Biol Macromol. (2019) 139:233–43. doi: 10.1016/j.ijbiomac.2019.07.209
191. Zhang Y, Wu Z, Liu J, Zheng Z, Li Q, Wang H, et al. Identification of the core active structure of a Dendrobium officinale polysaccharide and its protective effect against dextran sulfate sodium-induced colitis via alleviating gut microbiota dysbiosis. Food Res Int. (2020) 137:109641. doi: 10.1016/j.foodres.2020.109641
192. Shan X, Zhou J, Ma T, Chai Q. Lycium barbarum polysaccharides reduce exercise-induced oxidative stress. Int J Mol Sci. (2011) 12:1081–8. doi: 10.3390/ijms12021081
193. Zhu H, Xu W, Wang N, Jiang W, Cheng Y, Guo Y, et al. Anti-fatigue effect of Lepidium meyenii Walp. (Maca) on preventing mitochondria-mediated muscle damage and oxidative stress in vivo and vitro. Food Funct. (2021) 12:3132–41. doi: 10.1039/D1FO00383F
194. Li Y-X, Yang Z-H, Lin Y, Han W, Jia S-S, Yuan K. Antifatigue effects of ethanol extracts and polysaccharides isolated from Abelmoschus esculentus. Pharmacogn Mag. (2016) 12:219–24. doi: 10.4103/0973-1296.186341
195. Wang J, Wang X, Xiu W, Li C, Yu S, Zhu H, et al. Ultrasound-assisted preparation of sweet corn cob polysaccharide selenium nanoparticles alleviates symptoms of chronic fatigue syndrome. Food Funct. (2025) 16:133–46. doi: 10.1039/D4FO04195J
196. Tan W, Yu KQ, Liu YY, Ouyang MZ, Yan MH, Luo R, et al. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int J Biol Macromol. (2012) 50:59–62. doi: 10.1016/j.ijbiomac.2011.09.019
197. Zhang H-L, Li J, Li G, Wang D, Zhu L, Yang D. Structural characterization and anti-fatigue activity of polysaccharides from the roots of Morinda officinalis. Int J Biol Macromol. (2009) 44:257–61. doi: 10.1016/j.ijbiomac.2008.12.010
198. Tuo W, Wang S, Shi Y, Cao W, Liu Y, Su Y, et al. Angelica sinensis polysaccharide extends lifespan and ameliorates aging-related diseases via insulin and TOR signaling pathways, and antioxidant ability in Drosophila. Int J Biol Macromol. (2023) 241:124639. doi: 10.1016/j.ijbiomac.2023.124639
199. An Q, Ye X, Han Y, Zhao M, Chen S, Liu X, et al. Structure analysis of polysaccharides purified from Cyclocarya paliurus with DEAE-cellulose and its antioxidant activity in RAW2647 cells. Int J Biol Macromol. (2020) 157:604–15. doi: 10.1016/j.ijbiomac.2019.11.212
200. Yu J, Cao Y, He F, Xiang F, Wang S, Ke W, et al. Polysaccharides from Artemisia argyi leaves: environmentally friendly ultrasound-assisted extraction and antifatigue activities. Ultrason Sonochem. (2024) 107:106932. doi: 10.1016/j.ultsonch.2024.106932
201. Yan F, Hao H. Effects of Laminaria japonica polysaccharides on exercise endurance and oxidative stress in forced swimming mouse model. J Biol Res. (2016) 23:7. doi: 10.1186/s40709-016-0049-4
202. Yen CH, Tsao TH, Huang CU, Yang CB, Kuo CS. Effects of sweet cassava polysaccharide extracts on endurance exercise in rats. J Int Soc Sports Nutr. (2013) 10:18. doi: 10.1186/1550-2783-10-18
Keywords: natural medicinal plant polysaccharides, aerobic exercise capacity, bioactivity mechanisms, structure-activity, anti-fatigue mechanisms
Citation: Xu M, Chen W and Liang W (2025) Effects and mechanisms of polysaccharides from natural medicinal plants on improving aerobic exercise capacity. Front. Nutr. 12:1650499. doi: 10.3389/fnut.2025.1650499
Received: 20 June 2025; Accepted: 30 July 2025;
Published: 01 September 2025.
Edited by:
Yu-Heng Mao, Guangzhou Sport University, ChinaCopyright © 2025 Xu, Chen and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjian Liang, bWFua2luZ2xpYW5nQHFxLmNvbQ==
 Mingxin Xu
Mingxin Xu Weiyu Chen
Weiyu Chen Wenjian Liang
Wenjian Liang