- 1Emergency Department, Jining No.1 People's Hospital, Jining, Shandong, China
- 2Department of Geriatric Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 3Department of Cardiology, Jining No.1 People's Hospital, Jining, Shandong, China
- 4Institute of Emergency and Critical Care of Jining Medical Research Academy, Jining, Shandong, China
- 5Department of Geriatrics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
1 Introduction
The intersection between metabolic dysfunction and cognitive impairment represents one of the most pressing yet under-addressed challenges in modern medicine. With the global prevalence of diabetes reaching epidemic proportions, currently affecting more than 800 million adults worldwide, the neurological sequelae of this condition—particularly diabetes-associated cognitive dysfunction (DACD)—have emerged as a critical public health concern requiring immediate attention (1). DACD manifests through progressive memory impairment, executive function decline, and accelerated neurodegenerative processes (2, 3). These neurological deficits significantly impair quality of life while simultaneously exacerbating diabetes self-management challenges, thereby establishing a detrimental feedback loop between metabolic dysregulation and cognitive deterioration. Although the mechanisms underlying cognitive dysfunction in patients with diabetes are currently unclear, emerging evidence suggests that the interplay of insulin resistance (IR), chronic neuroinflammation, synaptic dysfunction, and gut-brain axis dysregulation are pivotal drivers of DACD pathogenesis (4–6). Contemporary therapeutic strategies for DACD demonstrate limited efficacy, predominantly emphasizing glycemic management while inadequately addressing the complex pathophysiology of diabetes-induced neural injury. This therapeutic gap highlights the critical need for interventions that simultaneously target the metabolic, inflammatory, and neurodegenerative components of DACD. Accumulating evidence suggests that omega-3 polyunsaturated fatty acids (PUFAs), specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), may represent promising therapeutic candidates for DACD. Table 1 synthesizes preclinical and clinical evidence documenting the pleiotropic neuroprotective mechanisms of omega-3 PUFAs, encompassing therapeutic ingredients, targets of action, and research results.
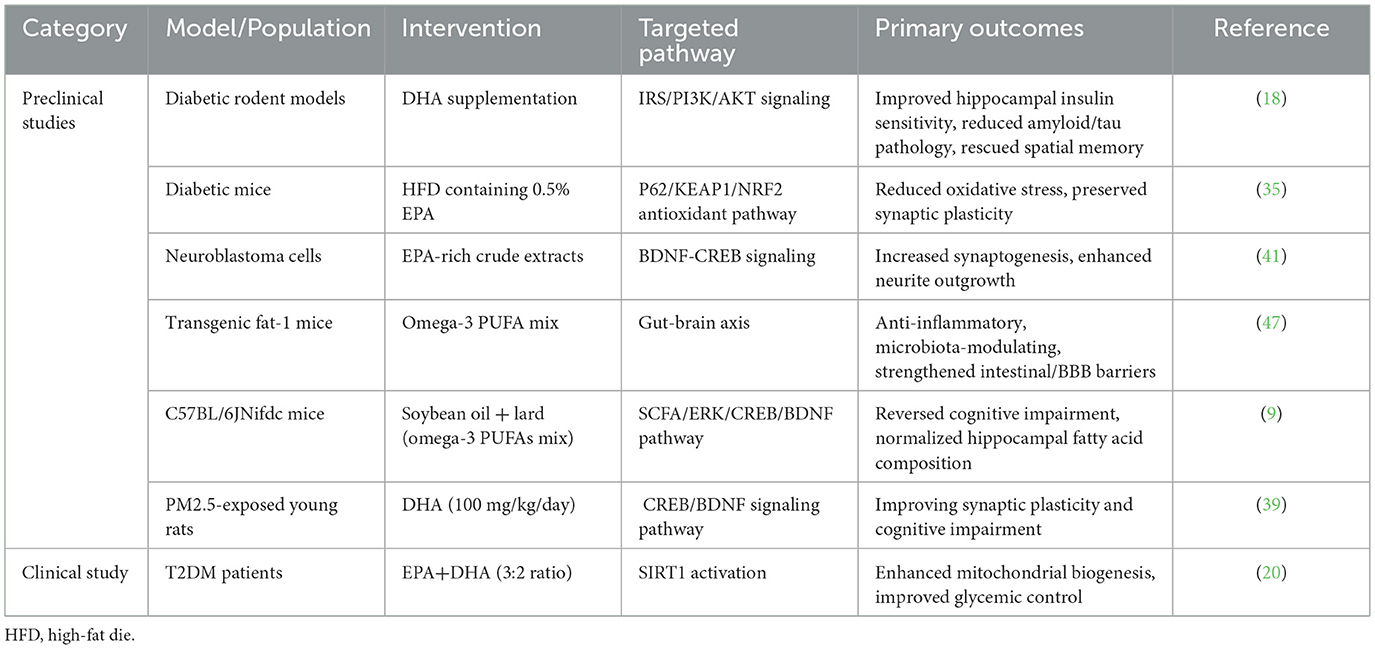
Table 1. Neuroprotective and metabolic benefits of omega-3 PUFAs: evidence from preclinical and clinical studies.
Compelling preclinical evidence supports omega-3 PUFAs as biologically rational candidates for DACD management, based on their indispensable roles in both the structural architecture of neural tissue (particularly DHA's enrichment in synaptic membranes) and functional regulation of neuroprotective pathways. DHA accounts for ~30% of total brain phospholipid content, reflecting its fundamental role in preserving neuronal membrane stability and synaptic functionality. Beyond these structural contributions, omega-3 PUFAs exhibit significant biological activity in regulating neural insulin sensitivity, attenuating neuroinflammatory processes, and promoting neuroplastic adaptations—each representing key pathophysiological mechanisms in DACD (7–10). Emerging research has increasingly implicated the gut-brain axis in the pathogenesis of DACD, uncovering novel therapeutic opportunities for omega-3 PUFAs. Experimental evidence demonstrates that these bioactive lipids can modulate intestinal microbial ecology by promoting commensal taxa (e.g., Bifidobacterium, Lactobacillus) and suppressing pathobionts (11, 12). Such microbiota alterations may elevate circulating levels of neuroactive microbial metabolites, particularly short-chain fatty acids, thereby establishing a systemic milieu conducive to neural homeostasis.
Despite robust preclinical evidence, clinical outcomes of omega-3 PUFA supplementation in DACD remain heterogeneous. While certain randomized controlled trials report significant cognitive improvements in diabetic cohorts, others demonstrate only marginal benefits. The observed heterogeneity in clinical outcomes likely stems from variations in study methodologies, including differences in design parameters and population characteristics. Furthermore, growing evidence indicates that genetic factors, particularly APOE polymorphisms, may serve as key determinants of interindividual responsiveness to omega-3 PUFAs interventions. This is because the APOE ε4 genotype alters DHA metabolism when supplemented with omega-3 PUFAs, resulting in a lower plasma response to omega-3 PUFAs in APOE ε4-positive than in APOE ε4-negative individuals. This study systematically evaluated the multi-target mechanism of action of omega-3 PUFAs in DACD and identified key knowledge gaps for future translational research.
2 Omega-3 PUFAs: metabolic maestros in DACD management
Accumulating evidence indicates that IR impairs cerebral insulin signaling, resulting in deficient neuronal glucose uptake and metabolic dysfunction. Central insulin resistance (CIR) represents a defining pathological feature of diabetes that promotes cognitive dysfunction through multifaceted mechanisms including impaired cerebral glucose metabolism, compromised synaptic plasticity, disrupted neurotrophic signaling, and dysregulated energy homeostasis (13, 14). The dysregulation of the insulin receptor substrate/phosphatidylinositol 3-kinase/protein kinase B (IRS/PI3K/AKT) pathway is a direct trigger of IR (15). Contemporary research reveals omega-3 PUFAs, particularly EPA and DHA, as pleiotropic modulators of the metabolic perturbations underlying DACD. Omega-3 PUFAs ameliorate CIR through three complementary mechanisms: (i) modulation of membrane fluidity in neuronal organelles (endoplasmic reticulum and Golgi apparatus), (ii) activation of peroxisome proliferator-activated receptor gamma (PPARγ) receptors with concomitant suppression of endoplasmic reticulum stress, and (iii) restoration of IRS/PI3K/AKT signaling pathways (8, 16, 17). In the experimental rodent model, DHA supplementation restored systemic glucose homeostasis, ameliorated hippocampal insulin sensitivity, and reduced hippocampal amyloid formation and tau phosphorylation, while rescuing hippocampus-dependent spatial memory and cognitive deficits (18). Sirtuin1 (SIRT1) is a conservative nicotinamide adenine dinucleotide (NAD)+-dependent deacetylase that is mainly located in the nucleus, which closely correlates with mitochondrial biogenesis, lipid metabolism, and metabolic fluxes (19). Available evidence suggests that omega-3 PUFAs enhance mitochondrial biogenesis and oxidative metabolism by upregulating SIRT1 expression, which in turn counteracts diabetes-induced cerebral hypometabolism (20, 21). The pleiotropic actions of omega-3 PUFAs extend beyond metabolic regulation to encompass suppression of microglia-dependent neuroinflammation and oxidative stress, addressing multiple pathological cascades in parallel.
3 Omega-3 PUFAs: simultaneously targeting neuroinflammatory and oxidative pathways
Emerging research reveals a complex interplay between CIR and neuroinflammation in DACD. Chronic hyperglycemia fundamentally alters microglial functional phenotypes, converting these central nervous system immune sentinels from their resting surveillance state to damaging M1-like inflammatory effectors. This polarization is characterized by increased secretion of damaging cytokines (IL-1β, TNF-α) and activation of nuclear factor kappa-B (NF-κB) signaling and the NOD like receptor family pyrin domain containing 3 (NLRP3) inflammasome, leading to elevated levels of IL-6 and IL-18 (22, 23). The diabetic milieu fosters a deleterious neuroimmune cycle where microglia-mediated inflammation and CIR mutually reinforce each other, ultimately impairing neuronal bioenergetics via transcriptional repression of glycolytic machinery (5, 24, 25). Preclinical evidence from the diabetic mouse model reveals this reciprocal relationship, demonstrating significant upregulation of pro-inflammatory M1 marker concurrent with downregulation of neuroprotective M2 marker, findings that correlate strongly with observed cognitive impairments (26). Furthermore, in db/db mice, the hyperglycemic environment of diabetes stimulates microglia to produce reactive oxygen species and activates the NF-κB/NLRP3 signaling pathway, leading to the production of NLRP3 inflammasome (e.g., IL1β, IL6, and IL18), which in turn mediates cognitive dysfunction (27). Oxidative stress is a major pathogenic culprit that leads to metabolic anomalies, as well as neurodegeneration and aging. Accumulating preclinical and clinical studies suggest that oxidative stress contributes to neuronal loss and synaptic disruption through impairment of mitochondrial homeostasis in the brain, ultimately resulting in cognitive dysfunction (28, 29).
Omega-3 PUFAs, particularly DHA and EPA, exhibit multifaceted neuroprotective effects in DACD by modulating interconnected inflammatory and oxidative pathways. First, omega-3 PUFAs mitigate chronic low-grade neuroinflammation associated with hyperglycemia by inhibiting the release of proinflammatory cytokines (e.g., IL-1β, TNF-α) and NF-κB inflammatory signaling pathway transduction (30, 31). Second, they inhibit inflammasome activation (e.g., NLRP3) by reducing oxidative stress and mitochondrial dysfunction, critical drivers of neuronal damage in diabetes (32). Third, omega-3 PUFAs regulate microglia-mediated synaptic pruning and plasticity by suppressing 12/15-lipoxygenase (LOX)/12-HETE signaling, thereby preventing excessive phagocytosis of synaptic elements during neurodevelopment (33). Additionally, omega-3 PUFAs are indispensable components of cell membranes and play an important role in maintaining the membrane structural integrity and fluidity of immune and neuronal cells (31). Moreover, DHA integrates into neuronal membranes, stabilizing lipid rafts and suppressing microglial overactivation triggered by advanced glycation end products (34). Finally, omega-3 PUFAs, particularly EPA, mitigate DACD by suppressing oxidative stress through activation of the P62/KEAP1/NRF2 antioxidant pathway, which reduces reactive oxygen species generation and subsequent neuronal damage (35). In conclusion, omega-3 PUFAs represent a promising dietary intervention targeting the neuroinflammatory-oxidative axis in DACD.
4 Omega-3 PUFAs: synaptic architects in diabetic cognitive protection
Emerging as a neural epicenter of DACD, the hippocampus exhibits signature neurodegenerative changes—particularly synaptic diminution and dendritic retraction-that correlate strongly with clinical disease progression. The function of the hippocampus depends on communication among neurons, and the synapse is the basic information-processing unit that mediates neuronal communication. Preclinical investigations in diabetic models reveal marked synaptic alterations, characterized by diminished spine density, downregulation of synaptic scaffolding proteins (postsynaptic density protein-95, microtubule-associated protein 2), and ultrastructural abnormalities including truncated postsynaptic densities and expanded synaptic clefts (36, 37). These pathological changes correlate strongly with cognitive deficits observed in db/db mice. Omega-3 PUFAs, notably DHA which comprises ~30% of brain phospholipid content, exhibit significant neuroprotective effects at synaptic sites. Experimental studies demonstrate their ability to enhance long-term potentiation, maintain synaptic ultrastructural integrity, and increase expression of activity-regulated cytoskeleton-associated protein, a critical molecular mediator of synaptic plasticity and memory consolidation processes (38, 39). Phosphatidylserine is predominantly localized on the cytoplasmic side of neuronal cell membranes, in facilitating the action of signaling proteins that underpin neuronal survival, neurite growth, and synaptogenesis. DHA orchestrates neural membrane homeostasis through dual actions-stimulating phosphatidylserine-dependent signaling hubs for neurodevelopment while structurally optimizing bilayer fluidity for synaptic transmission efficiency (40). This bifunctional capacity underlies its essential role in neuronal circuit formation and function.
Transcending their membrane-stabilizing functions, omega-3 PUFAs emerge as master regulators of neurotrophic signaling networks, orchestrating complex interactions between growth factor systems and synaptic efficacy pathways. Dietary supplementation with DHA enhances brain-derived neurotrophic factor (BDNF) expression via cAMP response element-binding protein-dependent transcriptional activation, thereby promoting synaptogenesis and supporting cognitive processes (41, 42). DHA-derived neuroprotectin D1 provides additional protection by balancing Bcl-2/Bax ratios to inhibit apoptosis and promote autophagy of damaged organelles. DHA can also clear damaged mitochondria and alleviate mitochondrial dysfunction through PINK1/Parkin mediated mitophagy and also increases acetylcholine and γ-aminobutyric acid levels while decreasing glutamate levels, ultimately counteracting the synaptotoxic effects of diabetes (43, 44). Additionally, omega-3 PUFAs as master regulators of a gut-brain circuit, stimulating short-chain fatty acids (SCFA) production that bridges microbial metabolism to neuronal plasticity. Notably, these synaptic alterations may be further modulated by gut-derived inflammatory signals, as discussed in the following gut-brain axis section.
5 Omega-3 PUFAs: guardians of the gut-brain axis in diabetes
The gut-brain axis represents a sophisticated bidirectional communication network linking gut microbiota with brain function through immune, neuroendocrine, and metabolic pathways. Diabetes-associated gut dysbiosis orchestrates a dual-hit mechanism: SCFAs depletion undermines epithelial integrity while lipopolysaccharide and toll-like receptor 4 engagement activate pro-inflammatory NF-κB cascades, establishing a systemic-to-neural inflammatory axis (45, 46). Dysbiosis of gut microbiota can also induce deficits in synaptic plasticity through the ER stress-mediated PERK signaling pathway (47). These pathological changes correlate strongly with the microbial alterations and intestinal damage observed in diabetic patients. Omega-3 PUFAs, especially EPA and DHA, emerge as potent modulators of this axis. Omega-3 PUFAs modulate gut microbiota diversity, enriching beneficial taxa (e.g., Bifidobacterium, Lactobacillus) and increasing SCFAs production, which enhances intestinal barrier integrity (11, 48). SCFAs, particularly butyrate, cross the BBB to suppress microglial activation and upregulate BDNF expression. Omega-3 PUFAs and DHA also up-regulated expression of the intestinal tight junction protein occludin and zonula occluden-1, improved the intestinal barrier functions and repaired BBB damage (44). Moreover, omega-3-derived endocannabinoids interact with gut vagal afferents to regulate appetite and glucose homeostasis, creating a holistic approach to metabolic and cognitive protection in diabetes (46, 49).
6 Discussion with future perspectives
Omega-3 PUFAs, especially DHA and EPA, exhibit pleiotropic therapeutic effects against DACD through multiple complementary mechanisms (Figure 1). These essential fatty acids (i) restore cerebral insulin sensitivity via IRS/PI3K/AKT signaling pathway activation, (ii) attenuate neuroinflammation by promoting PPARγ-mediated microglial polarization toward the neuroprotective M2 phenotype, and (iii) enhance synaptic plasticity through BDNF upregulation. Furthermore, their modulation of the gut-brain axis—marked by elevated butyrate production and SCFAs-dependent strengthening of the blood-brain barrier-contributes to systemic anti-inflammatory effects. At the molecular level, DHA demonstrates amyloid-lowering properties while EPA-derived specialized pro-resolving mediators actively resolve inflammation, synergistically protecting hippocampal integrity.
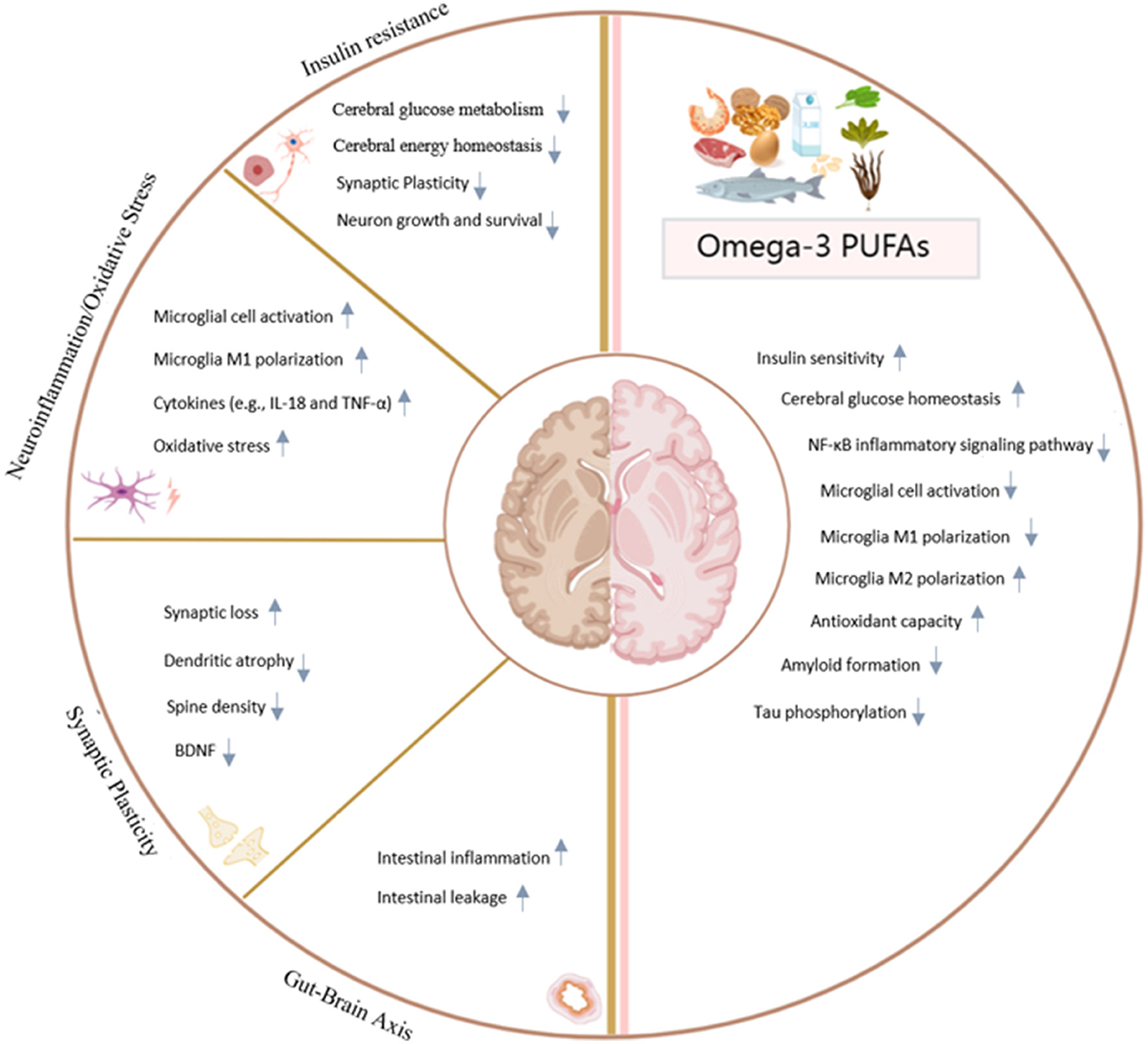
Figure 1. Regulatory mechanism of omega-3 PUFAs on diabetic-associated cognitive dysfunction. Created in MedPerr.com.
Current evidence suggests that diabetic patients may benefit from regular consumption of omega-3 PUFAs-rich foods, though the optimal frequency and dose still need to be confirmed. Although plant-based omega-3 PUFAs have limited capacity to convert into bioactive EPA/DHA, supplementation with marine-derived omega-3 PUFAs shows preliminary promise, particularly for neuroprotection with higher DHA proportions, but ideal ratios remain under investigation. Genetic factors (e.g., APOE ε4) may influence dosing needs, and monitoring tools such as the omega-3 PUFAs index are still in the exploratory phase, awaiting the establishment of reliable thresholds based on randomized controlled trials. Therefore, recommended protocols should be personalized based on emerging evidence and clinical context.
While our findings demonstrate the therapeutic potential of omega-3 PUFAs for DACD, several key challenges must be addressed in future clinical translation. First, the optimal EPA:DHA ratio and dosage regimen remain to be established, particularly considering the metabolic heterogeneity of T2DM populations and potential pharmacodynamic interactions with glucose-lowering medications. Second, standardized cognitive assessment protocols sensitive to early DACD progression are urgently needed, as current diagnostic criteria lack specificity for diabetes-related cognitive decline. Third, the development of validated biomarkers—including erythrocyte omega-3 PUFAs indices, neuroimaging parameters, and inflammatory markers—will be critical for demonstrating target engagement and dose-response relationships in clinical trials. Finally, genetic variations (e.g., APOE ε4), baseline nutritional status, comorbidities, and concomitant medication use must be considered to achieve personalized treatment in different populations. Addressing these challenges through multidisciplinary collaborations will accelerate the development of evidence-based omega-3 PUFAs interventions for DACD prevention and management.
Author contributions
CC: Conceptualization, Writing – original draft, Writing – review & editing. YY: Writing – original draft, Writing – review & editing. PL: Writing – review & editing. YG: Writing – original draft. DS: Conceptualization, Writing – review & editing. SL: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Shandong Provincial Youth Innovation Team Development Plan of Colleges and Universities (Grant No. 2022KJ193) and the Young Elite Sponsorship Program of Shandong Provincial Medical Association (Grant No. 2023_LC_0133).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI Statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou B, Rayner AW, Gregg EW, Sheffer KE, Carrillo-Larco RM, Bennett JE, et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet. (2024) 404:2077–93. doi: 10.1016/S0140-6736(24)02317-1
2. Chen YN, Qin JW, Tao LY, Liu ZZ, Huang J, Liu WL, et al. Effects of Tai Chi Chuan on cognitive function in adults 60 years or older with type 2 diabetes and mild cognitive impairment in china: a randomized clinical trial. Jama Netw Open (2023) 6:e237004. doi: 10.1001/jamanetworkopen.2023.7004
3. Corpas R, Griñán-Ferré C, Rodríguez-Farré E, Pallàs M, Sanfeliu C. Resveratrol induces brain resilience against alzheimer neurodegeneration through proteostasis enhancement. Mol Neurobiol. (2019) 56:1502–16. doi: 10.1007/s12035-018-1157-y
4. Zhao X, Lu J, Zhang J, Liu C, Wang H, Wang Y, et al. sleep restriction promotes brain oxidative stress and inflammation, and aggravates cognitive impairment in insulin-resistant mice. Psychoneuroendocrinology. (2024) 166:107065. doi: 10.1016/j.psyneuen.2024.107065
5. Li Q, Zhao YJ, Guo HY Li Q, Yan CY Li YS, et al. Impaired lipophagy induced-microglial lipid droplets accumulation contributes to the buildup of trem1 in diabetes-associated cognitive impairment. Autophagy. (2023) 19:2639–56. doi: 10.1080/15548627.2023.2213984
6. Zhang ZT, Deng SM, Chen C, He QH, Peng XW, Liang QF, et al. Pterostilbene could alleviate diabetic cognitive impairment by suppressing tlr4/nf-Kb pathway through microbiota-gut-brain axis. Phytother Res. (2023) 37:3522–42. doi: 10.1002/ptr.7827
7. Yu SY, Xie QY, Tan WF, Hu MJ, Xu GL, Zhang X, et al. Different ratios of Dha/Epa reverses insulin resistance by improving adipocyte dysfunction and lipid disorders in Hfd-induced Ir mice. Food Funct. (2023) 14:1179–97. doi: 10.1039/D2FO02686D
8. Gao X, Du L, Randell E, Zhang HJ Li KL, Li D. Effect of different phosphatidylcholines on high fat diet-induced insulin resistance in mice. Food Funct. (2021) 12:1516–28. doi: 10.1039/D0FO02632H
9. Shi RJ, Tian XY, Ji AD, Zhang TY, Xu HA, Qi ZS, et al. A mixture of soybean oil and lard alleviates postpartum cognitive impairment via regulating the brain fatty acid composition and Scfa/Erk(1/2)/Creb/Bdnf pathway. Nutrients. (2024) 16:2641. doi: 10.3390/nu16162641
10. Rogero MM, Leao MD, Santana TM, Pimentel MVDB, Carlini GCG, da Silveira TFF, et al. Potential benefits and risks of Omega-3 fatty acids supplementation to patients with covid-19. Free Radical Bio Med. (2020) 156:190–9. doi: 10.1016/j.freeradbiomed.2020.07.005
11. Liu JS, Huang HX, Yang Q, Zhao JX, Zhang H, Chen W, et al. Dietary supplementation of N-3 lcpufas prevents salmonellosis in a murine model. J Agr Food Chem. (2020) 68:128–37. doi: 10.1021/acs.jafc.9b05899
12. Kaliannan K, Donnell SO, Murphy K, Stanton C, Kang C, Wang B, et al. Decreased tissue Omega-6/Omega-3 fatty acid ratio prevents chemotherapy-induced gastrointestinal toxicity associated with alterations of gut microbiome. Int J Mol Sci. (2022) 23:5322. doi: 10.3390/ijms23105332
13. Rebelos E, Bucci M, Karjalainen T, Oikonen V, Bertoldo A, Hannukainen JC, et al. Insulin resistance is associated with enhanced brain glucose uptake during euglycemic hyperinsulinemia: a large-scale pet cohort. Diabetes Care. (2021) 44:788–94. doi: 10.2337/dc20-1549
14. Kellar D, Craft S. Brain Insulin resistance in Alzheimer's Disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. (2020) 19:758–66. doi: 10.1016/S1474-4422(20)30231-3
15. Cheng FE, Han L, Xiao Y, Pan CY Li YL, Ge XH, et al. D-Inositol ameliorates high fat diet-induced hepatic steatosis and insulin resistance via Pkcε-Pi3k/Akt pathway. J Agr Food Chem. (2019) 67:5957–67. doi: 10.1021/acs.jafc.9b01253
16. Lepretti M, Martucciello S, Burgos Aceves MA, Putti R, Lionetti L. Omega-3 fatty acids and insulin resistance: focus on the regulation of mitochondria and endoplasmic reticulum stress. Nutrients. (2018) 10:350. doi: 10.3390/nu10030350
17. Acosta-Montano P, Garcia-Gonzalez V. Effects of dietary fatty acids in pancreatic beta cell metabolism, implications in homeostasis. Nutrients. (2018) 10:393. doi: 10.3390/nu10040393
18. Xu JQ Ni B, Ma CC, Rong S, Gao H, Zhang L, et al. Docosahexaenoic acid enhances hippocampal insulin sensitivity to promote cognitive function of aged rats on a high-fat diet. J Adv Res. (2023) 45:31–42. doi: 10.1016/j.jare.2022.04.015
19. Shao D, Yao CX, Kim MH, Fry J, Cohen RA, Costello CE, et al. Improved mass spectrometry-based activity assay reveals oxidative and metabolic stress as sirtuin-1 regulators. Redox Biol. (2019) 22:101150. doi: 10.1016/j.redox.2019.101150
20. Werida RH, Ramzy A, Ebrahim YN, Helmy MW. Effect of Coadministration of Omega-3 fatty acids with glimepiride on glycemic control, lipid profile, irisin, and sirtuin-1 in Type 2 Diabetes mellitus patients: a randomized controlled trial. Bmc Endocr Disord. (2023) 23:259. doi: 10.1186/s12902-023-01511-2
21. Vargas R, Riquelme B, Fernández J, Alvarez D, Pérez IF, Cornejo P, et al. Docosahexaenoic acid-thyroid hormone combined protocol as a novel approach to metabolic stress disorders: relation to mitochondrial adaptation via liver Pgc-1α and Sirtuin1 activation. Biofactors. (2019) 45:271–8. doi: 10.1002/biof.1483
22. Liu W, Li K, Zheng M, He L, Chen T. Genipin attenuates diabetic cognitive impairment by reducing lipid accumulation and promoting mitochondrial fusion via Fabp4/Mfn1 signaling in microglia. Antioxidants (2022) 12:74. doi: 10.3390/antiox12010074
23. Sood A, Fernandes V, Preeti K, Khot M, Khatri DK, Singh SB. Fingolimod alleviates cognitive deficit in type 2 diabetes by promoting microglial M2 polarization via the Pstat3-Jmjd3 Axis. Mol Neurobiol. (2023) 60:901–22. doi: 10.1007/s12035-022-03120-x
24. Chow HM, Shi M, Cheng AF, Gao YH, Chen GM, Song X, et al. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat Neurosci. (2019) 22:1806–19. doi: 10.1038/s41593-019-0505-1
25. Hu YL, Cao KL, Wang F, Wu WY, Mai WH, Qiu LY, et al. Dual roles of hexokinase 2 in shaping microglial function by gating glycolytic flux and mitochondrial activity. Nat Metab. (2022) 4:1756–74. doi: 10.1038/s42255-022-00707-5
26. Hui Y, Xu ZQ, Li JX, Kuang LY, Zhong YM, Tang YY, et al. Nonenzymatic function of dpp4 promotes diabetes-associated cognitive dysfunction through Igf-2r/Pka/Sp1/Erp29/Ip3r2 pathway-mediated impairment of treg function and m1 microglia polarization. Metabolism. (2023) 138:155340. doi: 10.1016/j.metabol.2022.155340
27. Hu T, Wei JW, Zheng JY, Luo QY, Hu XR, Du Q, et al. Metformin improves cognitive dysfunction through Sirt1/Nlrp3 pathway-mediated neuroinflammation in Db/Db Mice. J Mol Med. (2024) 102:1101–15. doi: 10.1007/s00109-024-02465-1
28. Solanki I, Parihar P, Shetty R, Parihar MS. Synaptosomal and mitochondrial oxidative damage followed by behavioral impairments in streptozotocin induced diabetes mellitus: restoration by malvastrum tricuspidatum. Cell Mol Biol. (2017) 63:94–101. doi: 10.14715/cmb/2017.63.7.16
29. Suresh S, Begum RF, Singh SA, Chitra V. Anthocyanin as a therapeutic in alzheimer's disease: a systematic review of preclinical evidences. Ageing Res Rev. (2022) 76:101595. doi: 10.1016/j.arr.2022.101595
30. Liu BP, Zhang YP, Yang ZY, Liu MJ, Zhang C, Zhao YT, et al. Ω-3 Dpa protected neurons from neuroinflammation by balancing microglia M1/M2 polarizations through inhibiting Nf-Kb/Mapk P38 signaling and activating neuron-Bdnf-Pi3k/Akt pathways. Mar Drugs. (2021) 19:587. doi: 10.3390/md19110587
31. Xia J, Yang LE, Huang CY, Deng SY, Yang ZY, Zhang YP, et al. Omega-3 polyunsaturated fatty acid eicosapentaenoic acid or docosahexaenoic acid improved ageing-associated cognitive decline by regulating glial polarization. Mar Drugs. (2023) 21:398. doi: 10.3390/md21070398
32. Anderson EJ, Thayne KA, Harris M, Shaikh SR, Darden TM, Lark DS, et al. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium pparγ activation? Antioxid Redox Sign. (2014) 21:1156–63. doi: 10.1089/ars.2014.5888
33. Madore C, Leyrolle Q, Morel L, Rossitto M, Greenhalgh AD, Delpech JC, et al. Essential Omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat Commun. (2020) 11:6133. doi: 10.1038/s41467-020-19861-z
34. Calder PC. Long chain fatty acids and gene expression in inflammation and immunity. Curr Opin Clin Nutr. (2013) 16:425–33. doi: 10.1097/MCO.0b013e3283620616
35. Tian A, Zheng Y, Li H, Zhang Z, Du L, Huang X, et al. Eicosapentaenoic acid activates the P62/Keap1/Nrf2 pathway for the prevention of diabetes-associated cognitive dysfunction. Food Funct. (2024) 15:5251–71. doi: 10.1039/d4fo00774c
36. Liu J, Deng ZF, Yu ZJ, Zhou WP, Yuan Q. The circrna Circ-Nbea participates in regulating diabetic encephalopathy. Brain Res (2022) 1774:147702. doi: 10.1016/j.brainres.2021.147702
37. Li YS, Jiang T, Du MY, He SX, Huang N, Cheng B, et al. Ketohexokinase-dependent metabolism of cerebral endogenous fructose in microglia drives diabetes-associated cognitive dysfunction. Exp Mol Med. (2023) 55:2417–32. doi: 10.1038/s12276-023-01112-y
38. Huguet G, Puig-Parnau I, Serrano JCE, Martin-Gari M, Rodríguez-Palmero M, Moreno-Muñoz JA, et al. Hippocampal neurogenesis and expression are enhanced in high-fat fed prepubertal female pigs by a diet including Omega-3 fatty acids and Cect8242. Eur J Nutr. (2023) 62:2463–73. doi: 10.1007/s00394-023-03165-1
39. Gui JX, Xie MD, Wang LM, Tian B, Liu BK, Chen HS. et al. Protective effects of docosahexaenoic acid supplementation on cognitive dysfunction and hippocampal synaptic plasticity impairment induced by early postnatal Pm25 exposure in young rats. N-S Arch Pharmacol. (2024) 397:6563–75. doi: 10.1007/s00210-024-03028-4
40. Sinclair AJ. Docosahexaenoic acid and the brain- What is its role? Asia Pac J Clin Nutr. (2019) 28:675–88. doi: 10.6133/apjcn.201912_28(4).0002
41. Gite S, Ross RP, Kirke D, Guihéneuf F, Aussant J, Stengel DB, et al. Nutraceuticals to promote neuronal plasticity in response to corticosterone-induced stress in human neuroblastoma cells. Nutr Neurosci. (2019) 22:551–68. doi: 10.1080/1028415X.2017.1418728
42. Agni MB, Hegde PS, Rai P, Sadananda M, Gowda KMD. Astaxanthin and Dha supplementation modulates the maternal undernutrition-induced impairment of cognitive behavior and synaptic plasticity in adult life of offspring's -exploring the molecular mechanism. Mol Neurobiol. (2024) 61:8975–95. doi: 10.1007/s12035-024-04147-y
43. Sun ERY, Zhang J, Deng Y, Wang J, Wu Q, Chen W, et al. Docosahexaenoic acid alleviates brain damage by promoting mitophagy in mice with ischaemic stroke. Oxid Med Cell Longev. (2022) 2022:3119649. doi: 10.1155/2022/3119649
44. Bie NN, Feng XJ Li CJ, Meng M, Wang CL. The protective effect of docosahexaenoic acid on pc12 cells in oxidative stress induced by ho through the Trkb-Erk1/2-Creb pathway. Acs Chem Neurosci. (2021) 12:3433–44. doi: 10.1021/acschemneuro.1c00421
45. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
46. Zhao Z, Li F, Ning J, Peng R, Shang J, Liu H, et al. Novel compound flz alleviates rotenone-induced pd mouse model by suppressing Tlr4/Myd88/Nf-Kappab pathway through microbiota-gut-brain axis. Acta Pharm Sin B. (2021) 11:2859–79. doi: 10.1016/j.apsb.2021.03.020
47. Govindarajulu M, Pinky PD, Steinke I, Bloemer J, Ramesh S, Kariharan T, et al. Gut metabolite tmao induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front Mol Neurosci. (2020) 13:138. doi: 10.3389/fnmol.2020.00138
48. Kaliannan K, Wang B, Li XY, Kim KJ, Kang JX. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep-Uk. (2015) 5:11276. doi: 10.1038/srep11276
Keywords: omega-3 polyunsaturated fatty acids, diabetic-associated cognitive dysfunction, insulin resistance, neuroinflammation, synaptic plasticity, gut-brain axis
Citation: Cui C, Yang Y, Liu P, Gao Y, Song D and Li S (2025) Omega-3 polyunsaturated fatty acids in diabetic-associated cognitive dysfunction: a nutritional therapeutic perspective. Front. Nutr. 12:1651304. doi: 10.3389/fnut.2025.1651304
Received: 21 June 2025; Accepted: 11 August 2025;
Published: 29 August 2025.
Edited by:
Shaohua Wang, Zhongda Hospital, ChinaReviewed by:
Qiaoli Zhai, Uppsala University, SwedenLiyan Huang, Capital Medical University, China
Jianming Wei, Utrecht University, Netherlands
Copyright © 2025 Cui, Yang, Liu, Gao, Song and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daqing Song, c2RxODgxOUAxNjMuY29t; Shangbin Li, bGlzaGFuZ2JpbkAxMjYuY29t
†These authors have contributed equally to this work
 Chunying Cui
Chunying Cui Yan Yang2†
Yan Yang2† Pengfei Liu
Pengfei Liu