- 1Department of Anesthesiology, Chi Mei Medical Center, Tainan, Taiwan
- 2School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan
- 3Department of Anesthesiology, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
- 4Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan
- 5Department of Anesthesiology, Chi Mei Medical Center, Liouying, Tainan, Taiwan
Background: Obstructive sleep apnea (OSA) is a global health concern associated with cardiovascular and metabolic complications. Vitamin D deficiency (VDD) is common and may contribute to OSA pathophysiology; however, most evidence is cross-sectional. This study investigated whether sustained VDD is associated with an increased risk of developing OSA.
Methods: This retrospective cohort study utilized data from the TriNetX federated research network (2010–2023) to investigate the relationship between VDD and incident OSA in adults aged ≥ 18 years. Patients were classified based on sustained serum 25-hydroxyvitamin D levels, with deficiency defined as ≤20 ng/mL and sufficiency as ≥30 ng/mL. To ensure an accurate exposure status, all included individuals underwent confirmatory vitamin D measurements within 3–12 months. After 1:1 propensity score matching, patients were followed for up to 5 years for new-onset OSA diagnoses, with a three-month washout period after vitamin D assessment to mitigate reverse causality and enhance causal inference.
Results: Analysis of 126,563 matched pairs demonstrated that OSA risk was significantly higher in the VDD group than in the controls (5.7% vs. 4.4%; HR 1.26, 95% CI 1.21–1.30; p < 0.001). This association demonstrated temporal consistency across 1-, 3-, and 5-year follow-ups. A clear dose–response relationship emerged, with severe VDD [≤10 ng/mL] conferring greater risk (HR 1.39, 95% CI 1.26–1.53; p < 0.001). Subgroup analyses revealed significant effect modification: stronger associations in women versus men (HR 1.32 vs. 1.19; P for interaction: 0.003), younger versus older adults (HR 1.45 vs. 1.15; P for interaction <0.001), and overweight/obese versus normal-weight individuals (HR 1.27 vs. 1.02; P for interaction <0.001).
Conclusion: Sustained VDD was independently associated with OSA risk, with temporal consistency and dose–response relationships supporting potential causality. The effects were most pronounced in women, younger adults, and overweight/obese individuals, suggesting that targeted assessment and intervention strategies may be warranted in these high-risk subgroups.
1 Introduction
Obstructive sleep apnea (OSA) is a prevalent and potentially life-threatening sleep disorder affecting an estimated 936 million adults aged 30–69 years worldwide, contributing significantly to global morbidity and healthcare costs (1, 2). Anatomical factors are fundamental to OSA pathogenesis. Dempsey et al. established that variations in upper airway structure and collapsibility play a central role in sleep-disordered breathing, even in non-clinical populations (3). Characterized by repetitive upper airway collapse during sleep, OSA leads to intermittent hypoxemia, sleep fragmentation, and an increased risk of cardiovascular disease, metabolic dysfunction, and neurocognitive impairment (4–8). The economic burden of OSA extends far beyond direct treatment costs, with recent analyses indicating that OSA-related comorbidities and consequences impose healthcare expenditures exceeding €30 billion annually in developed countries, underscoring its substantial impact (9). Recent findings by Gong et al. revealed that body mass index (BMI) serves as a mediator in the link between serum vitamin D levels and sleep deprivation, emphasizing the need to consider metabolic status when assessing the influence of nutritional factors on sleep (10). While traditional risk factors include obesity, male sex, and advancing age (11–14), emerging evidence suggests that nutritional deficiencies may contribute to OSA pathogenesis through mechanisms involving upper airway muscle function, inflammatory pathways, and respiratory control (15–19).
Vitamin D deficiency (VDD), defined as 25-hydroxyvitamin D levels below 20 ng/mL (20), affects approximately 35% of people globally and has been implicated in various respiratory conditions beyond its classical role in bone metabolism (21–24). Mechanistically, vitamin D receptors are widely distributed throughout the respiratory muscles, and VDD may impair upper airway dilator muscle function, increase systemic inflammation, and alter immune responses that could predispose to OSA development (19, 25). While evidence suggests an association between vitamin D status and OSA risk, most studies have been cross-sectional in design, failing to establish temporal relationships and providing limited evidence for causality (26–28). Recent prospective evidence from the UK Biobank demonstrated an inverse association between serum 25-hydroxyvitamin D concentrations and new-onset OSA, particularly in individuals with obesity (29). However, significant knowledge gaps remain, including limited population diversity, uncertain dose–response relationships across different VDD severities, and incomplete understanding of effect modification by demographic and clinical factors beyond obesity. Additionally, while the UK Biobank study provided valuable long-term evidence with a 12-year follow-up (29), it lacked shorter-term outcome assessments that could inform immediate public health intervention strategies.
Given the high prevalence of both VDD and OSA, establishing whether VDD is a modifiable risk factor for OSA development has significant clinical and public health implications. This multi-institutional retrospective study utilized the TriNetX federated health research network to examine the association between sustained VDD and subsequent OSA development in a large, well-characterized patient population using rigorous propensity score matching. We hypothesized that patients with confirmed VDD would demonstrate an increased risk of developing OSA compared to vitamin D-sufficient controls, with potential dose–response relationships and differential effects across patient subgroups.
2 Methods
2.1 Data sources and ethical statement
This multi-institutional retrospective study used the TriNetX database to identify eligible patients across multiple healthcare networks. TriNetX is a global federated health research network that provides access to de-identified electronic health records from participating healthcare organizations. It has been widely used in previous studies across various medical disciplines, including research on perioperative outcomes, chronic disease management, and health disparities (30–33). The database maintains patient privacy through robust de-identification processes that comply with applicable privacy regulations while preserving data utility for research purposes. The study protocol adhered to the ethical principles outlined in the Declaration of Helsinki for medical research involving human participants. The research protocol was reviewed and approved by the Institutional Review Board of Chi Mei Medical Center (IRB number: 11310-E04), which waived the requirement for informed consent owing to the retrospective nature of the study and the use of de-identified data.
2.2 Inclusion criteria
The study period encompassed January 1, 2010, through December 31, 2023, and included patients who were 18 years of age or older at the time of enrollment. Patients were eligible for inclusion if they were identified as having VDD, defined as 25-hydroxyvitamin D [25(OH)D] levels less than 20 ng/mL at their first measurement (VDD group). The index date was defined as the date of the first documented VDD. To ensure that patients had sustained VDD rather than transient low levels, a confirmatory 25(OH)D measurement showing continued deficiency was required within 3 to 12 months following the initial test. Similarly, the control group consisted of patients with vitamin D sufficiency, defined as 25(OH)D levels greater than 30 ng/mL, who also required a second confirmatory measurement within the same 3–12 month timeframe to ensure sustained adequate vitamin D status.
2.3 Exclusion criteria
Patients were excluded if they had any pre-existing sleep disorders at baseline, including insomnia (ICD-10: G47.0), hypersomnia (G47.1), circadian rhythm sleep disorders (G47.2), or sleep apnea (G47.3). Additional exclusion criteria included congenital conditions that could predispose to sleep disorders: other specified congenital malformation syndromes affecting multiple systems (Q87), other congenital malformations of the skull and face bones (Q75), cleft lip and cleft palate (Q35–Q37), and diseases of the myoneural junction and muscle (G70–G73). Patients with advanced kidney disease, including end-stage renal disease (N18.6), chronic kidney disease stage 4 (N18.4), and chronic kidney disease stage 5 (N18.5), were excluded. Other exclusions included acromegaly and pituitary gigantism (E22.0), disorders of the nose and nasal sinuses (J34), and malignant neoplasms of the head, face, and neck (C76.0). Patients who died during the follow-up period between the index date and the study endpoint were also excluded from the analysis. The timeframe for the exclusion criteria is illustrated in Figure 1.
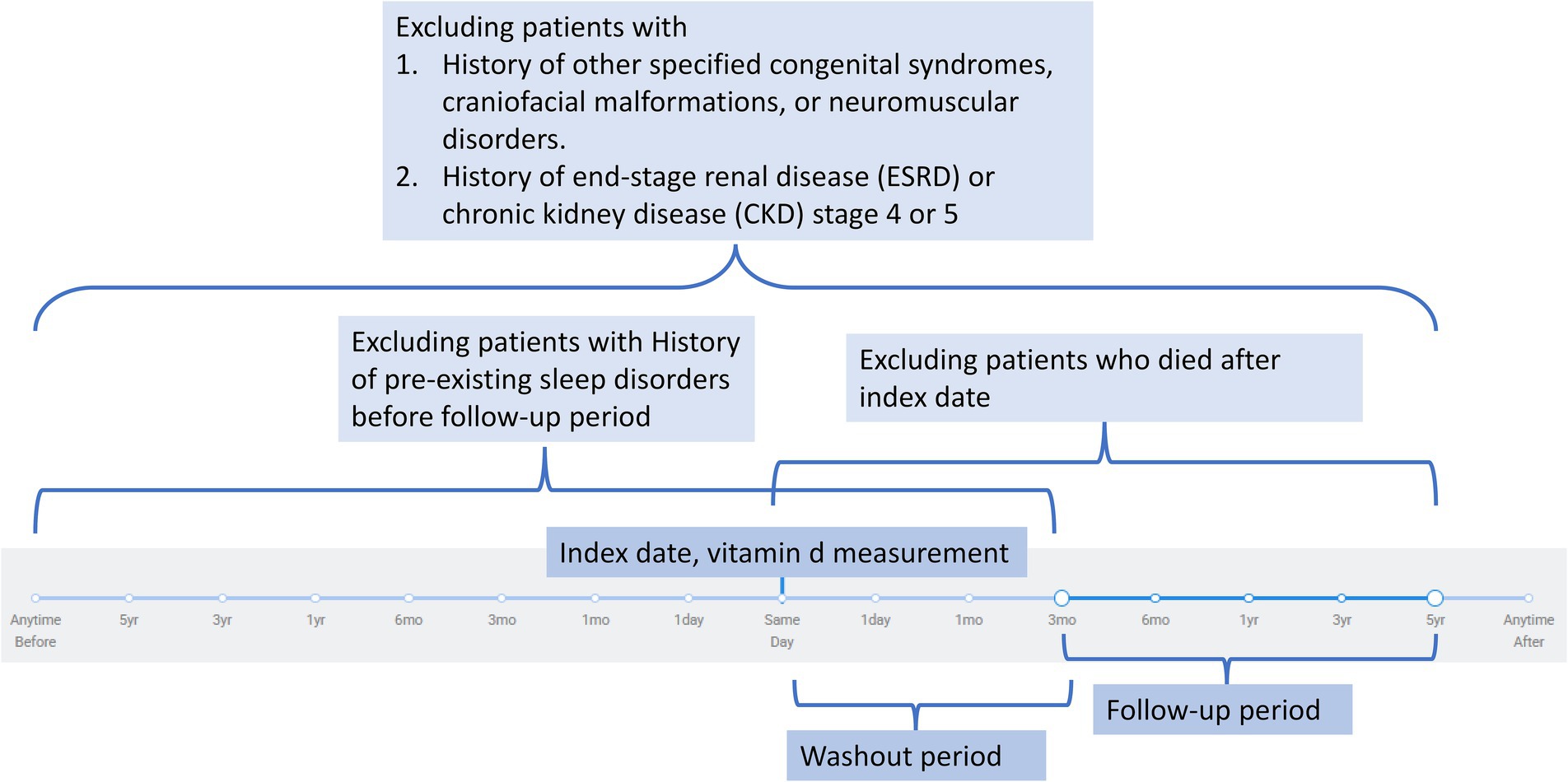
Figure 1. Flowchart illustrating the exclusion criteria and study timeline. Patients were excluded if they had congenital or neuromuscular syndromes, advanced chronic kidney disease (stage 4–5) or end-stage renal disease, pre-existing sleep disorders before follow-up, or death after the index date. The index date was defined as the date of vitamin D measurement. A washout period followed the index date to minimize reverse causality, after which eligible participants were observed during the defined follow-up period.
2.4 Outcomes and follow-up period
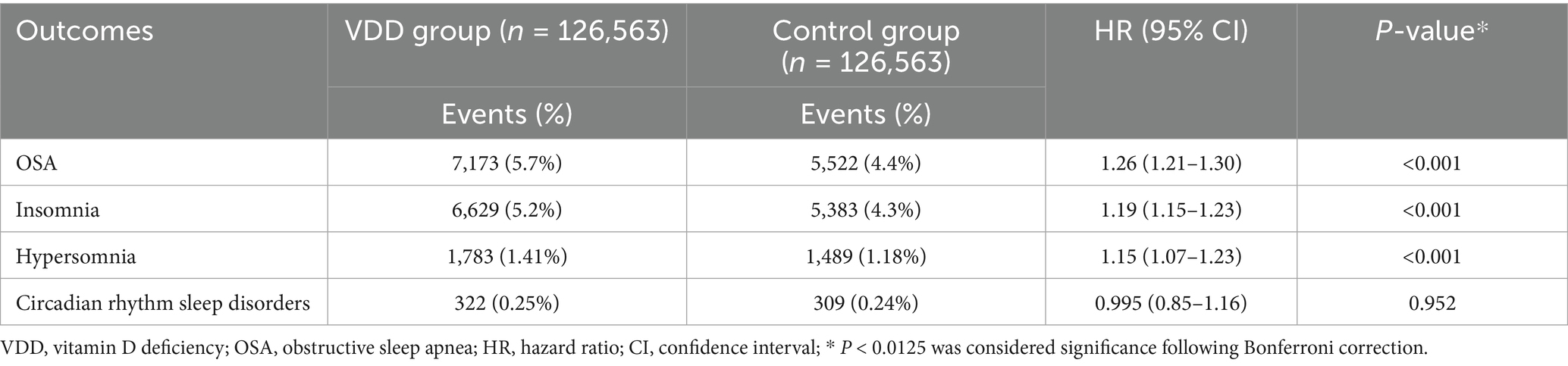
The primary outcome was the development of OSA during the five-year follow-up period after the index date. To minimize bias and reduce misclassification of pre-existing OSA events, a three-month washout period was implemented after the index date, during which any OSA diagnoses were excluded from the analysis. This washout period aimed to better reflect the incident risk attributable to VDD rather than undiagnosed preexisting conditions. The secondary outcomes included the development of other sleep disorders, specifically insomnia (G47.0), hypersomnia (G47.1), and circadian rhythm sleep disorders (G47.2). To assess the short-term effects of VDD, additional analyses were conducted at one-year and three-year follow-up intervals.
2.5 Data collection
The baseline demographic, clinical, and medication characteristics of all eligible patients were extracted from the TriNetX database and are summarized in Table 1. Variables included age; sex; race/ethnicity; comorbidities (e.g., cardiovascular, metabolic, renal, pulmonary, hepatic, and oncologic conditions); lifestyle and nutritional factors (e.g., nicotine dependence, alcohol-related disorders, malnutrition, and obesity); and relevant medication use, including antidiabetic agents, sedatives, and vitamin D supplementation.
2.6 Subgroup analyses
Pre-specified subgroup analyses were conducted to examine the consistency of the primary findings across different patient populations. Subgroups were defined by age (18–50 years versus >50 years), sex (male versus female), presence of diabetes mellitus (yes versus no), presence of hypertension (yes versus no), presence of chronic kidney disease (yes versus no), and BMI categories (BMI < 25 kg/m2 versus BMI ≥ 25 kg/m2). These analyses aimed to identify potential effect modifiers and assess the generalizability of the findings across diverse patient populations.
2.7 Additional analyses
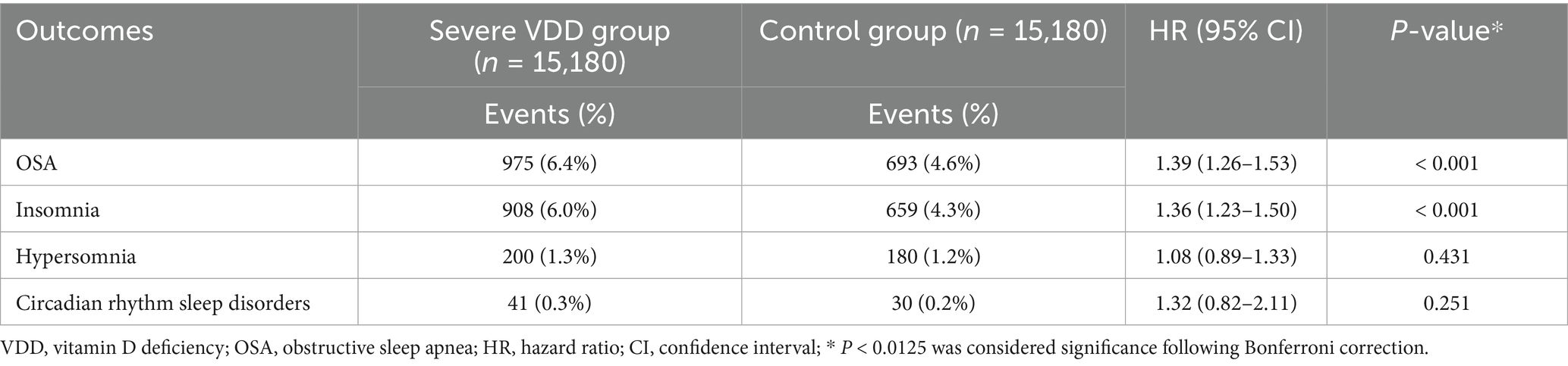
To further characterize the relationship between vitamin D status and sleep disorders, we evaluated the impact of severe VDD, defined as 25(OH)D levels less than 10 ng/mL, on OSA incidence rates. The rationale for this analysis was to determine whether profound VDD conferred an additional risk beyond moderate deficiency.
2.8 Statistical analysis
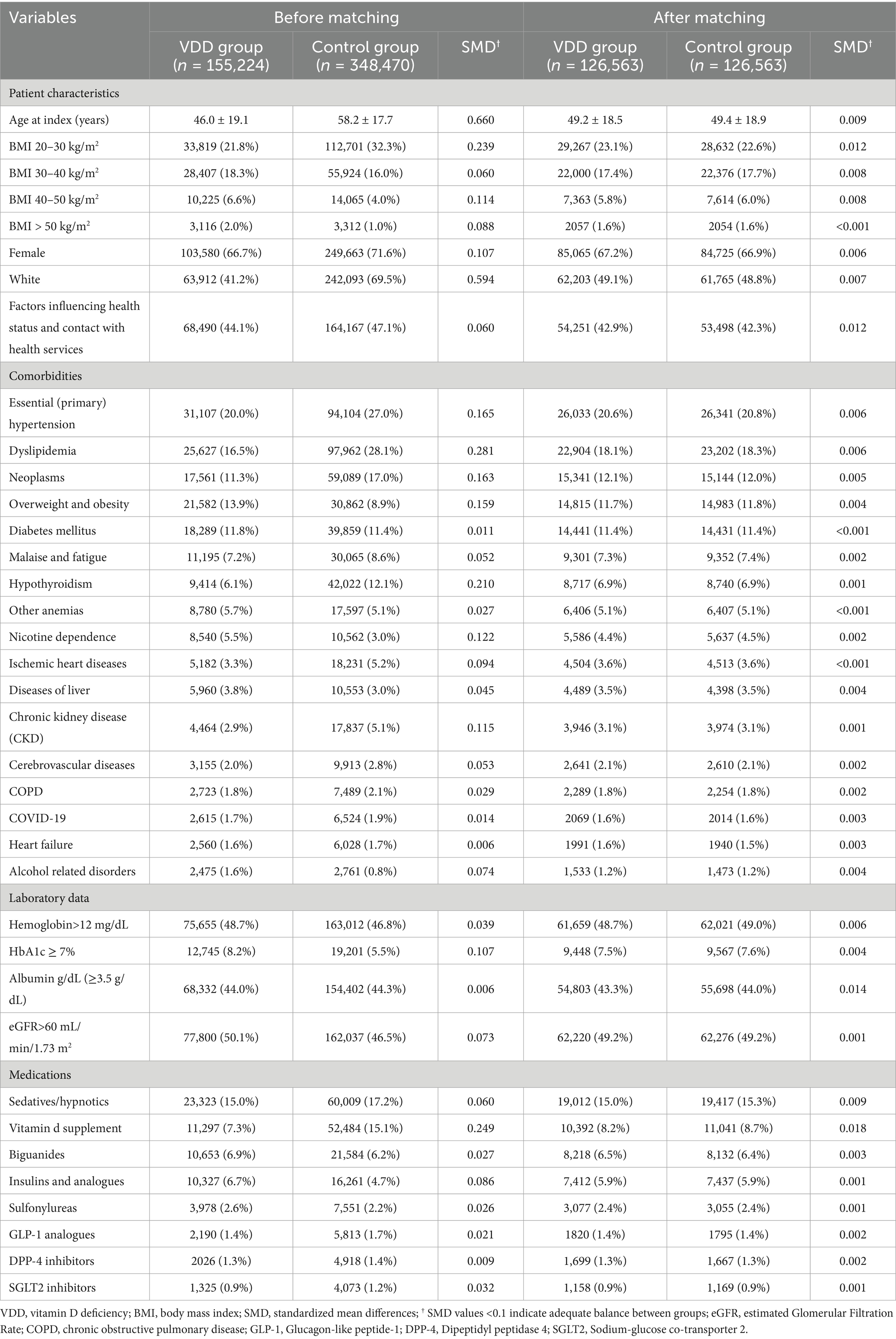
Continuous variables are presented as mean ± standard deviation, while categorical variables are expressed as frequencies and percentages. To address potential selection bias and ensure balanced comparison groups, one-to-one propensity score matching was performed using a caliper width of 0.1 standard deviations of the logit of the propensity score. The propensity score model included all baseline demographic characteristics, comorbidities, and medications as covariates. Matching was conducted without replacement using a greedy nearest-neighbor algorithm to optimize the balance between groups. Baseline characteristics between the VDD and control groups were compared using standardized mean differences (SMD), with SMD values less than 0.1 indicating adequate balance between matched groups.
Time-to-event analyses were conducted using Kaplan–Meier survival curves, and Cox proportional hazards regression models were employed to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the development of OSA and other sleep disorders. The proportional hazard assumption was assessed using Schoenfeld residuals to ensure model validity. Multivariate Cox proportional hazards models were constructed to identify independent risk factors for new-onset OSA, adjusting for relevant baseline characteristics. To account for multiple comparisons across the four primary outcomes, Bonferroni correction was applied, and statistical significance was set at p < 0.0125 (0.05/4 outcomes). All statistical analyses were performed using the built-in analytical tools of the TriNetX platform, ensuring the consistency and reproducibility of results across the federated network.
3 Results
3.1 Patient selection and baseline characteristics
The patient selection process is illustrated in Figure 2. After applying exclusion criteria (e.g., pre-existing sleep disorders, advanced kidney disease, congenital abnormalities, and follow-up mortality), we identified an initial cohort of 155,224 patients with documented VDD and 348,470 patients with sufficient vitamin D levels. Propensity score matching was performed using a caliper width of 0.1 standard deviations without replacement. The effectiveness of the matching procedure is illustrated in Figure 3, which demonstrates a substantial overlap between the density functions of the matched groups. After applying 1:1 propensity score matching, each well-balanced cohort comprised 126,563 individuals.
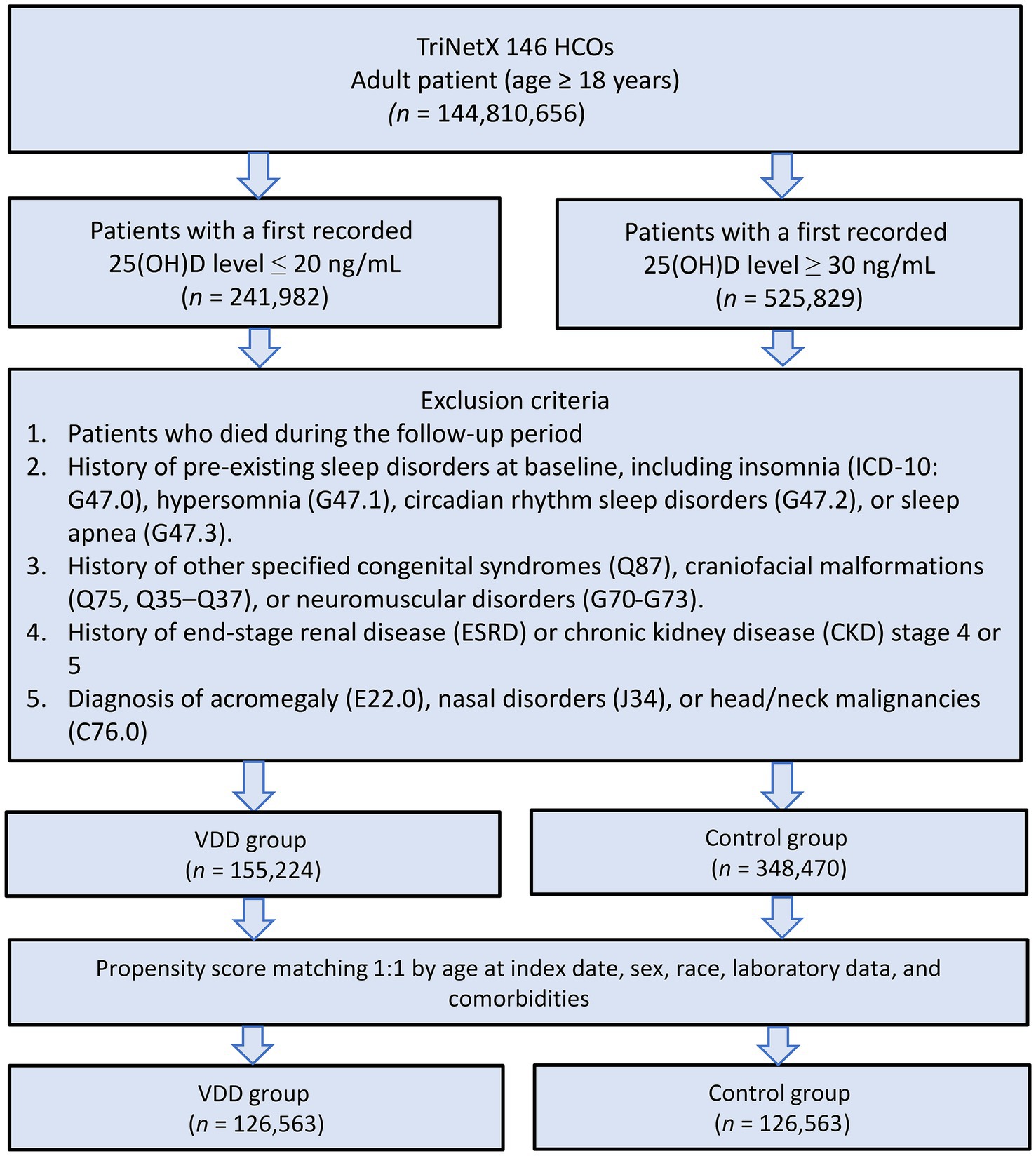
Figure 2. Patient selection flowchart from the TriNetX database. The flowchart illustrates the systematic exclusion process applied to identify eligible patients with vitamin D deficiency (VDD) and vitamin D sufficiency (control group). HCOs, Healthcare Organizations.
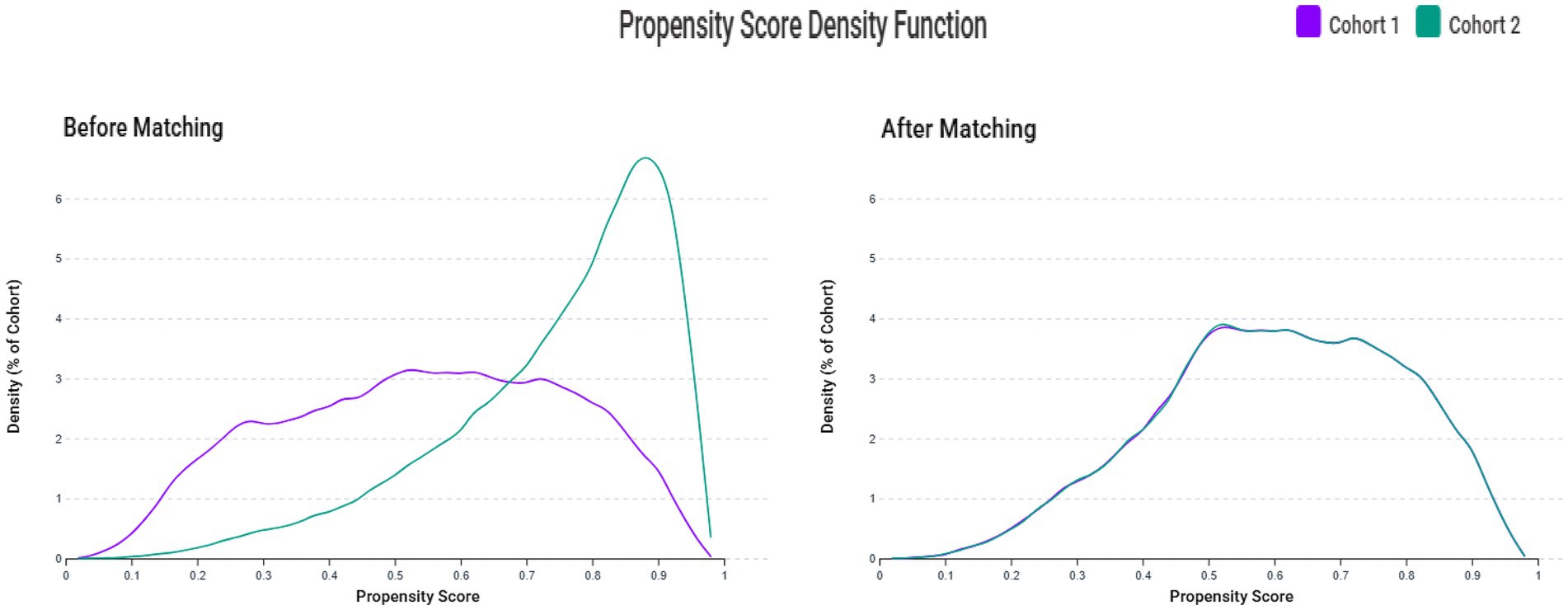
Figure 3. Propensity score density distributions before and after matching. The left panel shows propensity score distributions before matching, demonstrating substantial overlap but different distribution patterns between the vitamin D deficiency group (Cohort 1) and control group (Cohort 2). The right panel shows propensity score distributions after 1:1 matching with a caliper width of 0.1 standard deviations, demonstrating excellent overlap and balance between groups.
Table 1 presents the detailed baseline characteristics before and after matching. Before matching, the VDD group tended to be younger (46.0 ± 19.1 vs. 58.2 ± 17.7 years) and showed a higher prevalence of overweight/obesity and nicotine dependence, while the control group had higher rates of cardiovascular and metabolic comorbidities (e.g., hypertension and lipid disorders). After matching, the two groups were nearly identical in age (49.2 ± 18.5 vs. 49.4 ± 18.9 years), sex distribution (67.2% vs. 66.9% female), and racial composition (49.1% vs. 48.8% White). Balance was also achieved across a wide range of clinical conditions including diabetes (11.4% in both groups), hypertension (20.6% vs. 20.8%), chronic kidney disease (3.1% in both groups), and chronic obstructive pulmonary disease (1.8% in both groups). In addition, medication use (e.g., sedatives/hypnotics) was well balanced, reducing the risk of confounding due to pharmacological factors.
3.2 Outcomes
3.2.1 Risk of outcomes at 5-year follow-up
The primary analysis demonstrated a significant association between VDD and sleep disorders (Table 2). OSA showed the strongest association, with a 5.7% incidence in the VDD group versus 4.4% in controls, corresponding to a 26% increased risk (HR 1.26, 95% CI 1.21–1.30; p < 0.001). The secondary outcomes further supported the link between VDD and sleep disruption. Insomnia incidence rose from 4.3 to 5.2% (HR 1.19, 95% CI 1.15–1.23; p < 0.001), and hypersomnia from 1.18 to 1.41% (HR 1.15, 95% CI 1.07–1.23; p < 0.001). In contrast, circadian rhythm disorders showed no association with vitamin D status (0.25% vs. 0.24%; HR 0.995, 95% CI 0.85–1.16; p = 0.952).
3.2.2 Risk of outcomes at 1-year and 3-year follow-up
Temporal analyses revealed consistent associations across the follow-up intervals (Table 3). The risk of OSA was evident early (HR 1.25, 95% CI 1.17–1.33; p < 0.001 at 1 year) and remained stable at 3 years (HR 1.28, 95% CI 1.23–1.33; p < 0.001), reinforcing the likelihood of a causal relationship rather than a chance finding. Insomnia showed similar persistence, with significant associations at 1 year (HR 1.22, 95% CI 1.14–1.30; p < 0.001) and 3 years (HR 1.19, 95% CI 1.14–1.24; p < 0.001). In contrast, the link between VDD and hypersomnia attenuated over time, declining from a strong association at 1 year (HR 1.25, 95% CI 1.10–1.43; p = 0.001) to a weaker but still significant effect at 3 years (HR 1.10, 95% CI 1.02–1.19; p = 0.020). Consistently, circadian rhythm sleep disorders showed no significant association with vitamin D status at either the 1-year or 3-year follow-up.
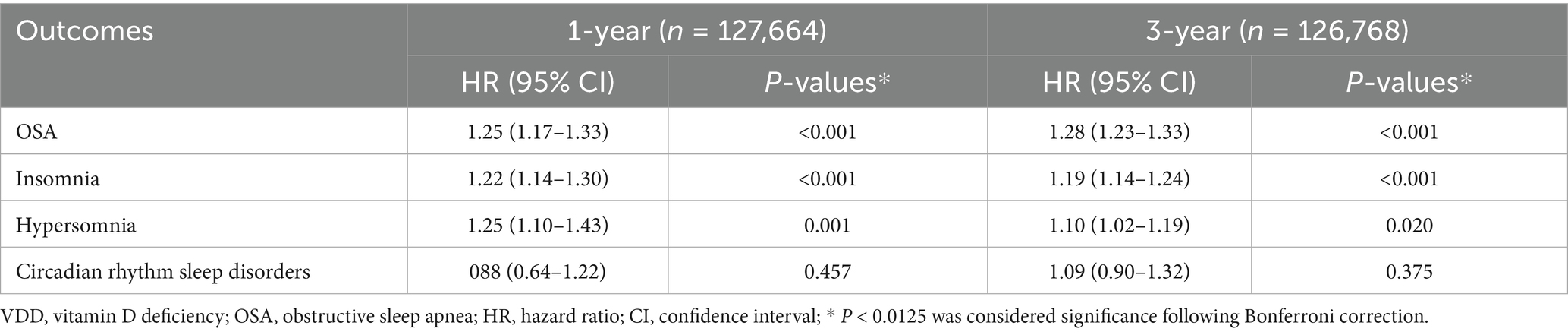
Table 3. Analyses of the association between vitamin D deficiency (VDD) and outcomes at 1- and 3-year follow-up.
3.3 Association between severe vitamin D deficiency and 5-year outcomes
Analysis of severe VDD demonstrated a clear dose–response relationship, supporting biological plausibility (Table 4). Among 15,180 matched pairs, individuals with severe deficiency (25(OH)D < 10 ng/mL) exhibited a significantly higher risk of OSA than those with moderate deficiency (HR 1.39, 95% CI 1.26–1.53; p < 0.001). This 39% increase in hazard notably exceeded the 26% risk associated with moderate deficiency, indicating a graded risk pattern. Similarly, severe deficiency was associated with increased insomnia risk (HR 1.36, 95% CI 1.23–1.50; p < 0.001). In contrast, no significant associations were observed for hypersomnia or circadian rhythm disorders, suggesting that these conditions may involve distinct pathophysiological pathways.
3.4 Subgroup analyses
The effect modification analysis revealed clinically meaningful variations in the association between VDD and OSA risk (Table 5). Notably, sex differences were pronounced, as women exhibited a 32% increased risk of OSA (HR 1.32, 95% CI 1.27–1.38) compared to a 19% increase in men (HR 1.19, 95% CI 1.12–1.25; p for interaction = 0.003), suggesting potential interactions between vitamin D status and sex-specific factors in OSA pathogenesis. Age-stratified analysis demonstrated an inverse relationship with vitamin D sensitivity. Younger adults (18–50 years) experienced a 45% elevated risk (HR 1.45, 95% CI 1.36–1.55), whereas older individuals showed only a modest 15% increase (HR 1.15, 95% CI 1.11–1.20; p for interaction < 0.001), indicating possible age-related variations in vitamin D responsiveness or the influence of competing risk factors. BMI has emerged as a strong effect modifier. No significant association was observed among normal-weight individuals (HR 1.02, 95% CI 0.94–1.13; p = 0.562), whereas overweight and obese patients demonstrated a 27% increased risk (HR 1.27, 95% CI 1.22–1.31; p < 0.001; p for interaction < 0.001), suggesting that VDD may amplify OSA risk primarily in the context of obesity-related mechanisms.
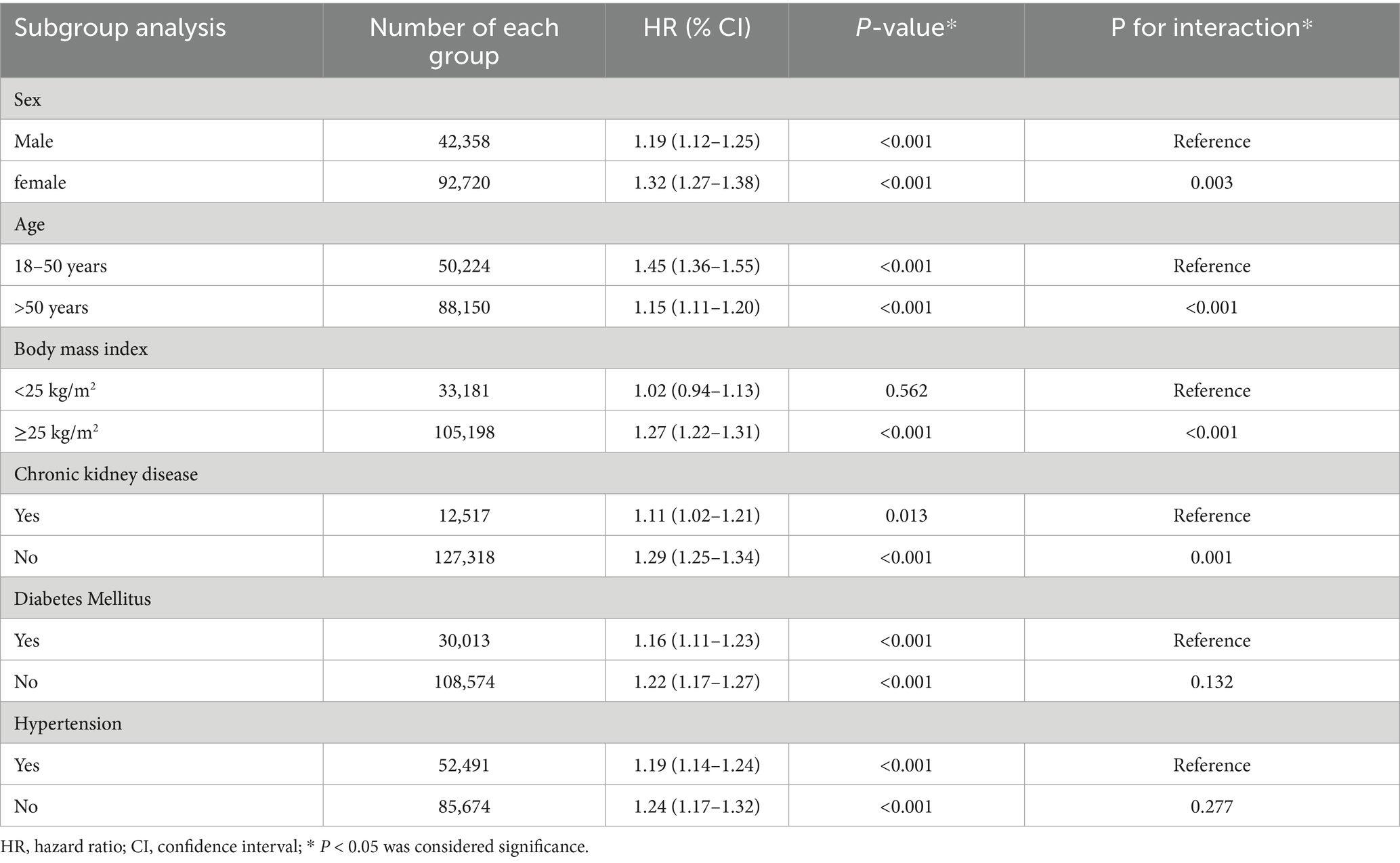
Table 5. Subgroup analyses of association between vitamin D deficiency and risk of obstructive sleep apnea (OSA) at 5-year follow-up.
3.5 Risk factors for OSA
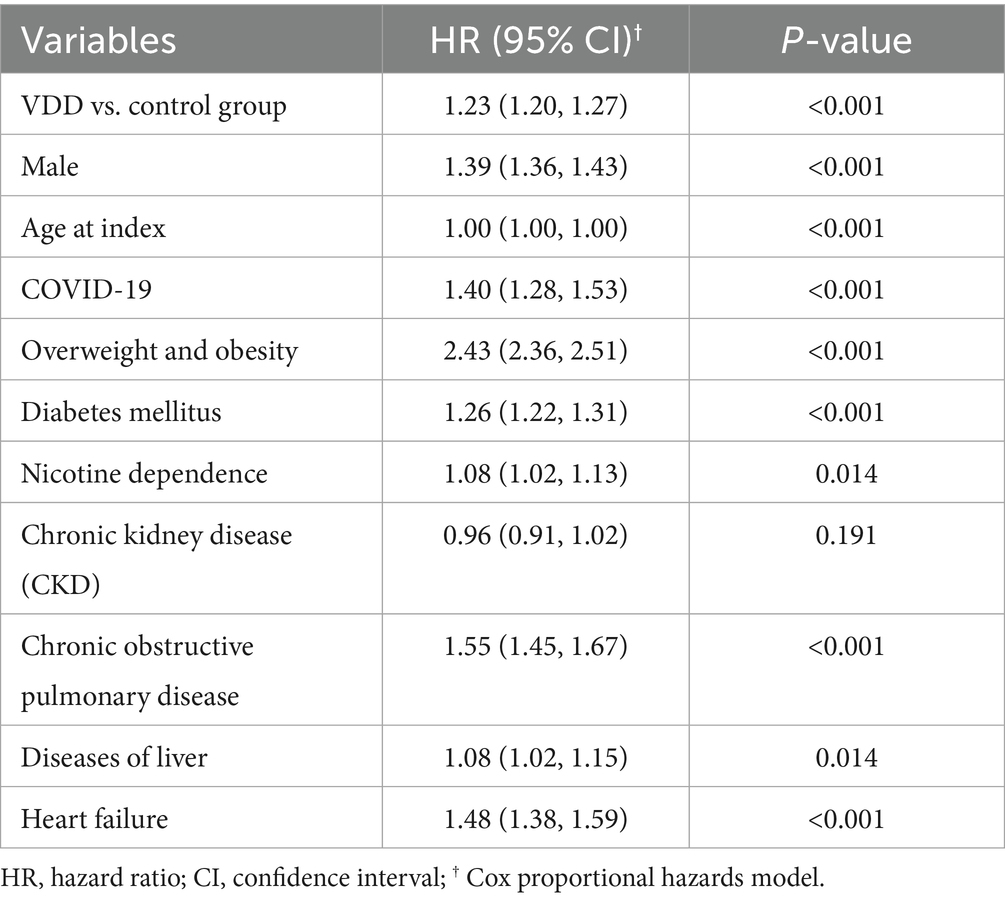
Multivariable modeling confirmed VDD as an independent predictor of OSA (Table 6). Even after adjusting for key confounders, VDD remained significantly associated with increased risk (HR 1.23, 95% CI 1.20–1.27; p < 0.001), supporting its potential pathophysiological role beyond residual confounding. Among covariates, obesity demonstrated the strongest association, more than doubling the risk of OSA (HR 2.43, 95% CI 2.36–2.51; p < 0.001). Respiratory comorbidities also contributed significantly, with COPD increasing the risk by 55% (HR 1.55, 95% CI 1.45–1.67; p < 0.001) and heart failure by 48% (HR 1.48, 95% CI 1.38–1.59; p < 0.001). COVID-19 was identified as an emerging predictor (HR 1.40, 95% CI 1.28–1.53; p < 0.001), possibly reflecting persistent post-infectious respiratory effects. Male sex was also associated with a higher risk (HR 1.39, 95% CI 1.36–1.43; p < 0.001), consistent with known epidemiological trends.
4 Discussion
This large-scale multi-institutional retrospective study demonstrates that patients with sustained VDD faced an increased hazard for OSA development over 5 years. This association demonstrated temporal consistency, a dose–response relationship, and variation across clinically relevant subgroups, supporting a potential causal link. In subgroup analyses, the vitamin D-OSA association was most pronounced in women, younger adults, and individuals with elevated BMI but showed no significant effect in normal-weight individuals. Beyond OSA, VDD is associated with an increased risk of insomnia and hypersomnia, but not circadian rhythm disorders, suggesting specificity in sleep-related pathophysiological mechanisms.
The relationship between vitamin D status and OSA has garnered increasing attention, with accumulating evidence suggesting both cross-sectional associations and potential mechanistic links (26–28). Previous systematic reviews and meta-analyses have consistently demonstrated lower serum 25-hydroxyvitamin D levels in patients with OSA than in controls (26–28). However, most previous studies have been limited by their cross-sectional designs, small sample sizes, and inadequate control for confounding variables, making it difficult to establish temporal relationships or infer causality. Mechanistically, vitamin D support the potential role of vitamin D in OSA pathogenesis is supported by several biological pathways (19, 25). Vitamin D receptors are widely distributed throughout the respiratory muscles, including the upper airway dilator muscles, and deficiency may impair muscle function and reduce upper airway stability during sleep (19, 25). Additionally, vitamin D plays a crucial role in immune regulation and inflammatory pathways, with deficiency associated with increased systemic inflammation that could contribute to upper airway edema and collapsibility (19, 25). The landmark UK Biobank study provided the first large-scale prospective evidence, demonstrating an inverse relationship between serum 25-hydroxyvitamin D concentrations and new-onset OSA over 12 years of follow-up, particularly in individuals with obesity (29).
Our findings demonstrate remarkable temporal consistency in the vitamin D-OSA association across multiple follow-up intervals, suggesting that VDD confers an early and sustained increased risk rather than delayed effects. This consistent temporal pattern supports a potential causal relationship, as random associations are likely to vary across different follow-up periods. The early manifestation of risk within the first year has important clinical implications, suggesting that vitamin D status assessment and potential supplementation may be relevant for short-term OSA prevention strategies. Perhaps the most compelling evidence for a causal relationship comes from our demonstration of a clear dose–response effect. Patients with severe VDD faced a substantially higher OSA risk than those with moderate deficiency, indicating that the relationship is not simply threshold-dependent but exhibits true biological gradation. Such dose–response relationships are considered one of the strongest criteria for establishing causality in observational studies. Our findings align with and substantially extend the UK Biobank results (29), providing novel contributions, including demonstration across diverse healthcare networks, implementation of a three-month washout period to reduce misclassification bias, and requirement for confirmatory vitamin D measurements ensuring sustained deficiency rather than transient low levels.
Our demonstration of significant effect modification across demographic and clinical subgroups represents a novel and potentially paradigm-shifting finding with implications for both mechanistic understanding and clinical practice. The significantly stronger vitamin D-OSA associations in women than in men suggest that vitamin D may interact with hormonal or physiological factors that vary between sexes. Several mechanisms could explain this observation, including higher vitamin D-binding protein levels in women affecting bioavailable vitamin D concentrations, estrogen influences on both vitamin D metabolism and upper airway muscle function, and sex-specific anatomical and physiological determinants of OSA that may be differentially sensitive to vitamin D status (34–36). The striking inverse relationship between age and vitamin D sensitivity represents another novel finding, with younger adults demonstrating a dramatically increased OSA risk compared to older patients. This pattern suggests that VDD may be particularly detrimental during periods of active tissue development and maintenance, with younger individuals potentially having greater vitamin D receptor density and responsiveness. Perhaps the most clinically significant subgroup finding is the complete absence of a vitamin D-OSA association in normal-weight individuals, in contrast with the substantial risk in overweight/obese patients. This interaction suggests that VDD may specifically potentiate obesity-related OSA mechanisms rather than acting as an independent risk factor, with implications including the role of vitamin D in adipose tissue inflammation, metabolic dysfunction, and insulin resistance.
Our multivariable analysis identified VDD as an independent predictor of OSA and clarified its relative contribution alongside established risk factors. Obesity has emerged as the dominant risk factor with overwhelming impact, confirming its central role in OSA pathogenesis and reinforcing that weight management remains the most critical intervention for OSA prevention and treatment. Respiratory comorbidities demonstrate a substantial OSA risk, likely reflecting shared pathophysiological mechanisms, including chronic inflammation and altered respiratory mechanics that predispose to upper airway instability during sleep (37, 38). The emergence of COVID-19 as a significant predictor of OSA in our study aligns with recent findings by Lin et al. (39), who demonstrated that individuals with a history of SARS-CoV-2 infection had a substantially elevated risk of developing OSA during a 12-month follow-up period (HR: 1.57). This contemporary association may reflect the long-term respiratory sequelae of COVID-19, including persistent inflammation, upper airway dysfunction, and post-infectious respiratory compromise. Male sex conferred an increased risk, consistent with established epidemiological patterns reflecting anatomical, hormonal, and behavioral differences (14). Despite adjustment for these multiple confounders, VDD retained a significant association, indicating genuine pathophysiological relevance rather than confounding by other risk factors.
Our analysis extends beyond OSA to explore the broader impact of vitamin D on sleep health, revealing disorder-specific associations that offer mechanistic insights. Insomnia showed a consistent association with VDD across all follow-up periods, with stronger links observed in cases of severe deficiency, suggesting a dose–response relationship comparable to that seen with OSA. The vitamin D-insomnia association aligns with growing evidence for the role of vitamin D in sleep regulation through multiple pathways, including vitamin D receptors in brain regions involved in sleep–wake control and vitamin D’s anti-inflammatory effects that may influence sleep quality through modulation of cytokine pathways affecting sleep architecture (40–42). Hypersomnia showed weaker and less consistent associations, with significant effects that diminished over longer follow-up periods, suggesting that VDD may contribute to the early manifestations of excessive daytime sleepiness through mechanisms involving sleep quality and restorative sleep processes. Notably, circadian rhythm sleep disorders showed no significant association with vitamin D status, suggesting that while vitamin D influences sleep quality and respiratory control during sleep, it may not substantially affect the timing and entrainment of circadian rhythms in clinical populations.
Recent critical reviews have highlighted a complex bidirectional relationship between the vitamin D status and OSA development. Schiza et al. (43) emphasized that while there is growing evidence supporting the role of vitamin D in sleep maintenance and regulation, the fundamental question of causality remains unresolved: whether VDD increases OSA risk or OSA itself predisposes to VDD. This uncertainty underscores the importance of our study design with temporal sequencing and washout periods to strengthen causal inference. The meta-analysis by Serafin et al. (44), which excluded patients with comorbidities, reinforces our findings by showing that the vitamin D–OSA association is independent of systemic confounders. Their focus on otherwise healthy populations supports our observation that VDD may be an independent risk factor rather than merely a consequence of OSA-associated comorbidities. Our study extends these findings by demonstrating that sustained VDD precedes OSA development in a large, well-matched cohort, with consistent effects across multiple follow-up intervals.
Several limitations of our study should be acknowledged when interpreting our findings. First, as this was a retrospective observational study using electronic health records, we could not establish definitive causality despite the temporal sequence and dose–response relationships observed. Unmeasured confounding variables, including dietary patterns, physical activity levels, and socioeconomic factors, may influence both vitamin D status and OSA risk. Second, our reliance on ICD-10 diagnostic codes for outcome ascertainment may introduce misclassification bias because OSA diagnosis in clinical practice varies in accuracy and may be influenced by healthcare access, physician awareness, and patient symptom presentation. Third, vitamin D supplementation, although captured as a baseline characteristic, was not incorporated into stratified analyses or adjusted as a time-varying covariate owing to the limitations of the TriNetX dataset, which lacks reliable information on supplement dosage, duration, adherence, and initiation timing. We opted not to exclude patients who had received supplements, as doing so could introduce selection bias and limit generalizability. However, we recognize that the inability to fully account for supplementation introduces residual confounding, and future studies with more granular data on supplementation patterns are warranted. Fourth, although the TriNetX database provides substantial statistical power and diverse populations, it may not be fully representative of all demographic groups or healthcare systems, potentially limiting its generalizability to resource-limited settings. Finally, our study period encompassed the COVID-19 pandemic, which may have affected both healthcare utilization patterns and sleep disorder diagnosis rates.
5 Conclusion
This study demonstrated an independent association between VDD and an increased risk of developing OSA, supported by temporal consistency, dose–response relationships, and biologically plausible effect modification. Our novel finding that vitamin D effects are most pronounced in women, younger adults, and overweight/obese individuals has important implications for personalized risk assessments and targeted intervention strategies. From a clinical perspective, our findings suggest that vitamin D status assessment may be valuable as part of a comprehensive OSA risk evaluation, particularly in women, younger adults, and individuals with an elevated BMI. Future research should focus on randomized controlled trials to definitively establish whether vitamin D supplementation can prevent OSA development, particularly in the high-risk subgroups identified in our analysis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Chi Mei Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because informed consent was not required for this retrospective study, as it involved secondary analysis of pre-existing data without any interventions or direct participant interaction.
Author contributions
K-CH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. T-SY: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. Y-CL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. C-WH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. MY: Methodology, Supervision, Writing – original draft, Writing – review & editing. C-HY: Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. I-WC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Benjafield, AV, Ayas, NT, Eastwood, PR, Heinzer, R, Ip, MSM, Morrell, MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. (2019) 7:687–98. doi: 10.1016/S2213-2600(19)30198-5
2. Young, T, Peppard, PE, and Gottlieb, DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. (2002) 165:1217–39. doi: 10.1164/rccm.2109080
3. Dempsey, JA, Skatrud, JB, Jacques, AJ, Ewanowski, SJ, Woodson, BT, Hanson, PR, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. (2002) 122:840–51. doi: 10.1378/chest.122.3.840
4. Faria, A, Allen, AH, Fox, N, Ayas, N, and Laher, I. The public health burden of obstructive sleep apnea. Sleep Sci. (2021) 14:257–65. doi: 10.5935/1984-0063.20200111
5. Song, SO, He, K, Narla, RR, Kang, HG, Ryu, HU, and Boyko, EJ. Metabolic consequences of obstructive sleep Apnea especially pertaining to diabetes mellitus and insulin sensitivity. Diabetes Metab J. (2019) 43:144–55. doi: 10.4093/dmj.2018.0256
6. Almendros, I, Basoglu, ÖK, Conde, SV, Liguori, C, and Saaresranta, T. Metabolic dysfunction in OSA: is there something new under the sun? J Sleep Res. (2022) 31:e13418. doi: 10.1111/jsr.13418
7. Arnaud, C, Bochaton, T, Pépin, JL, and Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: pathophysiological mechanisms. Arch Cardiovasc Dis. (2020) 113:350–8. doi: 10.1016/j.acvd.2020.01.003
8. Redline, S, Azarbarzin, A, and Peker, Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat Rev Cardiol. (2023) 20:560–73. doi: 10.1038/s41569-023-00846-6
9. Borsoi, L, Armeni, P, Donin, G, Costa, F, and Ferini-Strambi, L. The invisible costs of obstructive sleep apnea (OSA): systematic review and cost-of-illness analysis. PLoS One. (2022) 17:e0268677. doi: 10.1371/journal.pone.0268677
10. Gong, B, Xu, B, Wang, C, and Guo, X. The mediating effect of BMI in serum vitamin D related sleep deprivation based on the NHANES database. Front Nutr. (2025) 12:1571985. doi: 10.3389/fnut.2025.1571985
11. Kirkness, JP, Schwartz, AR, Schneider, H, Punjabi, NM, Maly, JJ, Laffan, AM, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol (1985). (2008) 104:1618–24. doi: 10.1152/japplphysiol.00045.2008
12. de Araujo Dantas, AB, Gonçalves, FM, Martins, AA, Alves, G, Stechman-Neto, J, Corrêa, CC, et al. Worldwide prevalence and associated risk factors of obstructive sleep apnea: a meta-analysis and meta-regression. Sleep Breath. (2023) 27:2083–109. doi: 10.1007/s11325-023-02810-7
13. Thakur, B, Pathak, M, Singh, P, and Padhan, P. Prevalence of obstructive sleep apnea among patients with rheumatoid arthritis and its association with age and body mass index: a systematic review and meta-analysis. Int J Rheum Dis. (2021) 24:1354–61. doi: 10.1111/1756-185X.14178
14. Eckert, DJ, and Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. (2008) 5:144–53. doi: 10.1513/pats.200707-114MG
15. Archontogeorgis, K, Nena, E, and Steiropoulos, P. Roles of vitamins and nutrition in obstructive sleep apnea. Expert Rev Respir Med. (2025) 19:145–63. doi: 10.1080/17476348.2025.2462192
16. Gunes, FE, Agan, K, Aktac, S, Karadeniz, D, Sunter, G, Vural, E, et al. Assessment of impact of dietary patterns on obstructive sleep Apnea patients. Sleep Sci. (2024) 17:e82–9. doi: 10.1055/s-0043-1776745
17. Bouloukaki, I, Tsiligianni, I, Mermigkis, C, Bonsignore, MR, Markakis, M, Pataka, A, et al. Vitamin D deficiency in patients evaluated for obstructive sleep apnea: is it associated with disease severity? Sleep Breath. (2021) 25:1109–17. doi: 10.1007/s11325-020-02142-w
18. Bluher, AE, Kearney, T, Vazifedan, T, and Baldassari, CM. Vitamin D deficiency and Pediatric obstructive sleep Apnea severity. JAMA Otolaryngol Head Neck Surg. (2025) 151:72–7. doi: 10.1001/jamaoto.2024.3737
19. Yao, N, Ma, C, Dou, R, Shen, C, Yuan, Y, Li, W, et al. Exploring the link between vitamin D deficiency and obstructive sleep apnea: a comprehensive review. J Sleep Res. (2024) 33:e14166. doi: 10.1111/jsr.14166
20. Bordelon, P, Ghetu, MV, and Langan, RC. Recognition and management of vitamin D deficiency. Am Fam Physician. (2009) 80:841–6.
21. Gaudet, M, Plesa, M, Mogas, A, Jalaleddine, N, Hamid, Q, and Al Heialy, S. Recent advances in vitamin D implications in chronic respiratory diseases. Respir Res. (2022) 23:252. doi: 10.1186/s12931-022-02147-x
22. Zhu, M, Wang, T, Wang, C, and Ji, Y. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. (2016) 11:2597–607. doi: 10.2147/COPD.S101382
23. Hilger, J, Friedel, A, Herr, R, Rausch, T, Roos, F, Wahl, DA, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. (2014) 111:23–45. doi: 10.1017/S0007114513001840
24. Mogire, RM, Mutua, A, Kimita, W, Kamau, A, Bejon, P, Pettifor, JM, et al. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health. (2020) 8:e134–42. doi: 10.1016/S2214-109X(19)30457-7
25. Archontogeorgis, K, Nena, E, Papanas, N, and Steiropoulos, P. The role of vitamin D in obstructive sleep apnoea syndrome. Breathe (Sheff). (2018) 14:206–15. doi: 10.1183/20734735.000618
26. Loh, HH, Lim, QH, Kang, WH, Yee, A, Yong, MC, and Sukor, N. Obstructive sleep apnea and vitamin D: an updated systematic review and meta-analysis. Hormones (Athens). (2023) 22:563–80. doi: 10.1007/s42000-023-00481-3
27. Li, X, He, J, and Yun, J. The association between serum vitamin D and obstructive sleep apnea: an updated meta-analysis. Respir Res. (2020) 21:294. doi: 10.1186/s12931-020-01554-2
28. Neighbors, CLP, Noller, MW, Song, SA, Zaghi, S, Neighbors, J, Feldman, D, et al. Vitamin D and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med. (2018) 43:100–8. doi: 10.1016/j.sleep.2017.10.016
29. Zhang, Y, Zhang, Y, Ye, Z, Zhou, C, Yang, S, Liu, M, et al. Relationship of serum 25-hydroxyvitamin D, obesity with new-onset obstructive sleep apnea. Int J Obes. (2024) 48:218–23. doi: 10.1038/s41366-023-01402-5
30. Chen, IW, Yu, TS, Lai, YC, Yang, CP, Yu, CH, and Hung, KC. Association between vitamin D deficiency and clinical outcome in patients with COVID-19 in the post-omicron phase. Front Nutr. (2025) 12:1583276. doi: 10.3389/fnut.2025.1583276
31. Hung, KC, Chang, LC, Chang, YJ, Ho, CN, Wu, JY, Liu, WC, et al. Vitamin D deficiency and diabetic retinopathy risk in patients with newly diagnosed type 2 diabetes mellitus: a retrospective analysis. Front Nutr. (2025) 12:1614287. doi: 10.3389/fnut.2025.1614287
32. Chen, IW, Chang, LC, Wu, JY, Lai, YC, Chang, YJ, Cheng, WJ, et al. Association between preoperative COVID-19 infection and postoperative outcomes in patients with obstructive sleep Apnea undergoing metabolic surgery: a retrospective analysis. Obes Surg. (2025) 35:2218–26. doi: 10.1007/s11695-025-07900-x
33. Hung, KC, Weng, HL, Lai, YC, Wu, JY, Chang, YJ, Hung, IY, et al. Association between preoperative Anemia and postoperative acute kidney injury in patients undergoing metabolic and bariatric surgery: a multi-institute study. Obes Surg. (2025) 35:1827–37. doi: 10.1007/s11695-025-07854-0
34. Wierzbicka, A, and Oczkowicz, M. Sex differences in vitamin D metabolism, serum levels and action. Br J Nutr. (2022) 128:2115–30. doi: 10.1017/S0007114522000149
35. Martins, FO, and Conde, SV. Gender differences in the context of obstructive sleep Apnea and metabolic diseases. Front Physiol. (2021) 12:792633. doi: 10.3389/fphys.2021.792633
36. Perger, E, Silvestri, R, Bonanni, E, Di Perri, MC, Fernandes, M, Provini, F, et al. Gender medicine and sleep disorders: from basic science to clinical research. Front Neurol. (2024) 15:1392489. doi: 10.3389/fneur.2024.1392489
37. Locke, BW, Lee, JJ, and Sundar, KM. OSA and chronic respiratory disease: mechanisms and epidemiology. Int J Environ Res Public Health. (2022) 19:5473. doi: 10.3390/ijerph19095473
38. McNicholas, WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med. (2009) 180:692–700. doi: 10.1164/rccm.200903-0347PP
39. Lin, WC, Wu, MC, Wang, YH, Lin, CH, and Wei, JC. The prevalence of obstructive sleep apnea syndrome after COVID-19 infection. J Med Virol. (2024) 96:e29392. doi: 10.1002/jmv.29392
40. de Oliveira, DL, Hirotsu, C, Tufik, S, and Andersen, ML. The interfaces between vitamin D, sleep and pain. J Endocrinol. (2017) 234:R23–r36. doi: 10.1530/JOE-16-0514
41. Romano, F, Muscogiuri, G, Di Benedetto, E, Zhukouskaya, VV, Barrea, L, Savastano, S, et al. Vitamin D and sleep regulation: is there a role for vitamin D? Curr Pharm Des. (2020) 26:2492–6. doi: 10.2174/1381612826666200310145935
42. Singh, AK, Kumar, S, Mishra, S, Rajotiya, S, Debnath, S, Raj, P, et al. The effects of vitamin D levels on physical, mental health, and sleep quality in adults: a comprehensive investigation. Front Nutr. (2024) 11:1451037. doi: 10.3389/fnut.2024.1451037
43. Schiza, S, Bouloukaki, I, Kaditis, A, Lombardi, C, and Bonsignore, MR. Vitamin D deficiency: a forgotten aspect in sleep disorders? A critical update. Sleep Med. (2024) 121:77–84. doi: 10.1016/j.sleep.2024.06.023
Keywords: obstructive sleep apnea, vitamin D deficiency, 25-hydroxyvitamin D, sleep disorders, propensity score matching, dose–response relationship
Citation: Hung K-C, Yu T-S, Lai Y-C, Hsu C-W, Yew M, Yu C-H and Chen I-W (2025) Vitamin D deficiency and subsequent risk of obstructive sleep apnea: a multi-institutional retrospective study. Front. Nutr. 12:1651712. doi: 10.3389/fnut.2025.1651712
Edited by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaCopyright © 2025 Hung, Yu, Lai, Hsu, Yew, Yu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: I-Wen Chen, Y2hlbml3ZW5hNjA5MTJAZ21haWwuY29t
 Kuo-Chuan Hung
Kuo-Chuan Hung Ting-Sian Yu3
Ting-Sian Yu3 Yi-Chen Lai
Yi-Chen Lai Chih-Wei Hsu
Chih-Wei Hsu Chia-Hung Yu
Chia-Hung Yu