- Department of Pediatrics, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
Objective: This retrospective study aimed to investigate the predictive value of novel albumin-associated nutritional inflammation markers, including the C-reactive protein-albumin-lymphocyte (CALLY) index, C-reactive protein to albumin ratio (CAR), neutrophil-to-albumin ratio (NAR), neutrophil percentage-to-albumin ratio (NPAR) and prognostic nutritional index (PNI), for intravenous immunoglobulin (IVIG) resistance in Kawasaki disease (KD) patients.
Methods: We conducted a retrospective analysis of clinical data from pediatric patients diagnosed with KD and admitted to our hospital between January 2012 and November 2023. Data were analyzed using univariate analysis, binary logistic regression analysis, and receiver operating curve (ROC) analysis.
Results: The study included 716 children with KD, and 78 of them (10.9%) were diagnosed with IVIG-resistant KD. CAR, NAR and NPAR were positively correlated with IVIG resistance, while the CALLY index and PNI showed negative correlations. The area under the ROC curve (AUC) values of the CALLY index, CAR, NAR, NPAR and PNI were 0.725, 0.700, 0.580, 0.717 and 0.712.
Conclusion: Our findings suggest that these novel nutritional inflammation indexes could be useful tools for predicting IVIG resistance in KD patients, potentially guiding timely intensified therapy. Compared to NAR, the CALLY index, CAR, NPAR, and PNI demonstrate stronger predictive performance for intravenous immunoglobulin (IVIG) resistance in Kawasaki disease and may hold greater potential for clinical application.
1 Introduction
Kawasaki disease (KD) is a systemic vasculitis primarily affecting medium-sized arteries, especially the coronary arteries, and is a major cause of acquired heart disease in children in developed countries (1, 2). Combining high-dose intravenous immunoglobulin (IVIG) therapy with aspirin has been established as the first-line therapy for KD and can effectively reduce the occurrence rate of coronary artery lesions (CALs), from 20 to 25% to approximately 2–4% (3). Nevertheless, about 10–20% of KD patients are unresponsive to IVIG therapy, increasing their risk of CALs (4). Thus, early prediction of IVIG resistance is crucial, as these patients may benefit from prompt intensified therapy.
While the cause of KD is not yet understood, systemic inflammatory responses are pivotal in its pathogenesis and progression. Albumin, once primarily seen as an indicator of nutritional status, is now also recognized as a protein in the acute inflammatory response (5). Increasing evidence highlights the interconnection between inflammation and nutrition. Serum albumin levels were found to have a negative correlation with inflammation. Studies have indicated that hypoalbuminemia is commonly observed in patients with KD during the acute phase, primarily resulting from increased vascular permeability and serum albumin leakage, highlighting changes in albumin-derived markers (6–9). In recent years, several novel inflammatory and nutritional indices have been proposed, including the C-reactive protein-albumin-lymphocyte (CALLY) index, C-reactive protein to albumin ratio (CAR), neutrophil-to-albumin ratio (NAR), neutrophil percentage-to-albumin ratio (NPAR) and prognostic nutritional index (PNI). These markers integrate multiple clinical evaluation parameters and provide more valuable information than individual markers alone (10–12). These inflammatory factors were initially mostly used to assess the prognosis of various cancers. Such as in 2021, the CALLY index was first applied to the prognosis research of hepatocellular carcinoma, and its correlation was confirmed (13). In 2016, NPAR was first applied to assess the prognosis of rectal cancer (14). Currently, various albumin-derived nutritional inflammation indices have been linked to the risk and severity of several diseases, such as tumors, as well as neurological, gastrointestinal, and respiratory diseases (15–18). Their affordability and widespread accessibility in daily clinical practice have increased their popularity, as a higher ratio frequently suggests a poor prognosis. However, to our knowledge, there are few studies on CAR, NPAR, and PNI, and no studies on the CALLY index and NAR in KD patients. While prior studies have examined individual inflammatory markers (e.g., CRP), this is the first to evaluate composite nutritional-inflammatory indexes (CALLY, CAR, NAR, NPAR, and PNI) in predicting IVIG resistance. Therefore, our study aimed to determine if albumin-derived markers (CALLY index, CAR, NAR, NPAR, and PNI) integrating inflammation and nutrition could be the significant predictors for IVIG resistance in KD patients.
2 Patients and methods
2.1 Participants
We retrospectively reviewed the clinical records of 716 pediatric patients with KD hospitalized at Mianyang Central Hospital between January 2012 and November 2023. Two experienced pediatricians, including at least one KD specialist, confirmed the diagnosis of complete and incomplete KD as per the 2017 American Heart Association guidelines (1). Two experienced pediatricians which with more than 5 years of clinical experience in pediatrics independently assessed each case. If both pediatricians reached the same conclusion, the patient was either included or excluded accordingly. In cases of disagreement, a third pediatrician was consulted to make the final determination. Complete KD diagnosis required ≥5 days of fever and ≥4 of the following clinical features: oral changes, extremity changes, rash, cervical lymphadenopathy, and bilateral bulbar conjunctival injection without exudate. Incomplete KD diagnosis included prolonged unexplained fever, <4 principal clinical features, and supportive laboratory or echocardiographic findings. All patients received IVIG (2 g/kg) intravenously and aspirin (30–50 mg/kg) orally. IVIG resistance was characterized by a persistent fever exceeding 38 °C at 36 h post-initial IVIG dose, or a recurrent fever accompanied by at least one primary clinical symptom of KD within 2 weeks, typically between 2 and 7 days post-treatment. KD shock syndrome (KDSS) was characterized by a ≥ 20% decrease in baseline systolic blood pressure or clinical signs of hypoperfusion (19).
Inclusion criteria included: (1) confirmed diagnosis of KD; (2) patients with age of 28 days or more and 16 years or less. Exclusion criteria included: (1) patients without IVIG therapy during hospitalization; (2) patients lacking complete data; (3) patients treated with glucocorticoid, other immunosuppressive drugs, or IVIG at other medical facilities; (4) patients with recurrent KD.
Data regarding the demographics, clinical indicators, and laboratory parameters were obtained from the hospital’s electronic records. All laboratory indicators were collected for assessment during the acute febrile phase prior to IVIG treatment. In line with previous studies (10, 16–18, 20), we calculated the CALLY index, CAR, NAR, NPAR and PNI using the following formulas:
CALLY index = albumin × lymphocyte count/(CRP × 10).
CAR = CRP/albumin.
NAR = neutrophil count/albumin.
NPAR = neutrophil percentage/albumin.
PNI = albumin+5 × lymphocyte count.
The Ethics Committee of Mianyang Central Hospital approved this study, with informed consent being waived (No. S20250312-01). This study was in line with the Helsinki declaration.
2.2 Statistical analysis
Normally distributed continuous variables are presented as mean ± standard deviation (SD) and analyzed between groups using Student’s t-test. Non-normally distributed variables are expressed as median (interquartile range, IQR) and compared between groups using the Mann–Whitney U test. Categorical data are presented as numbers (%) and analyzed using the Chi-square test or Fisher’s exact test. Binary logistic regression analysis was employed for conducting multivariate analysis by using variables of p < 0.1 in univariate analysis. To avoid collinearity, albumin-based markers were incorporated and assessed in the separate statistical models. The predictive ability of each novel nutritional inflammation marker for identifying IVIG resistance was assessed by the receiver operating characteristic (ROC) curve. Statistical analyses were conducted with SPSS 22.0, considering a p-value <0.05 as significant.
3 Results
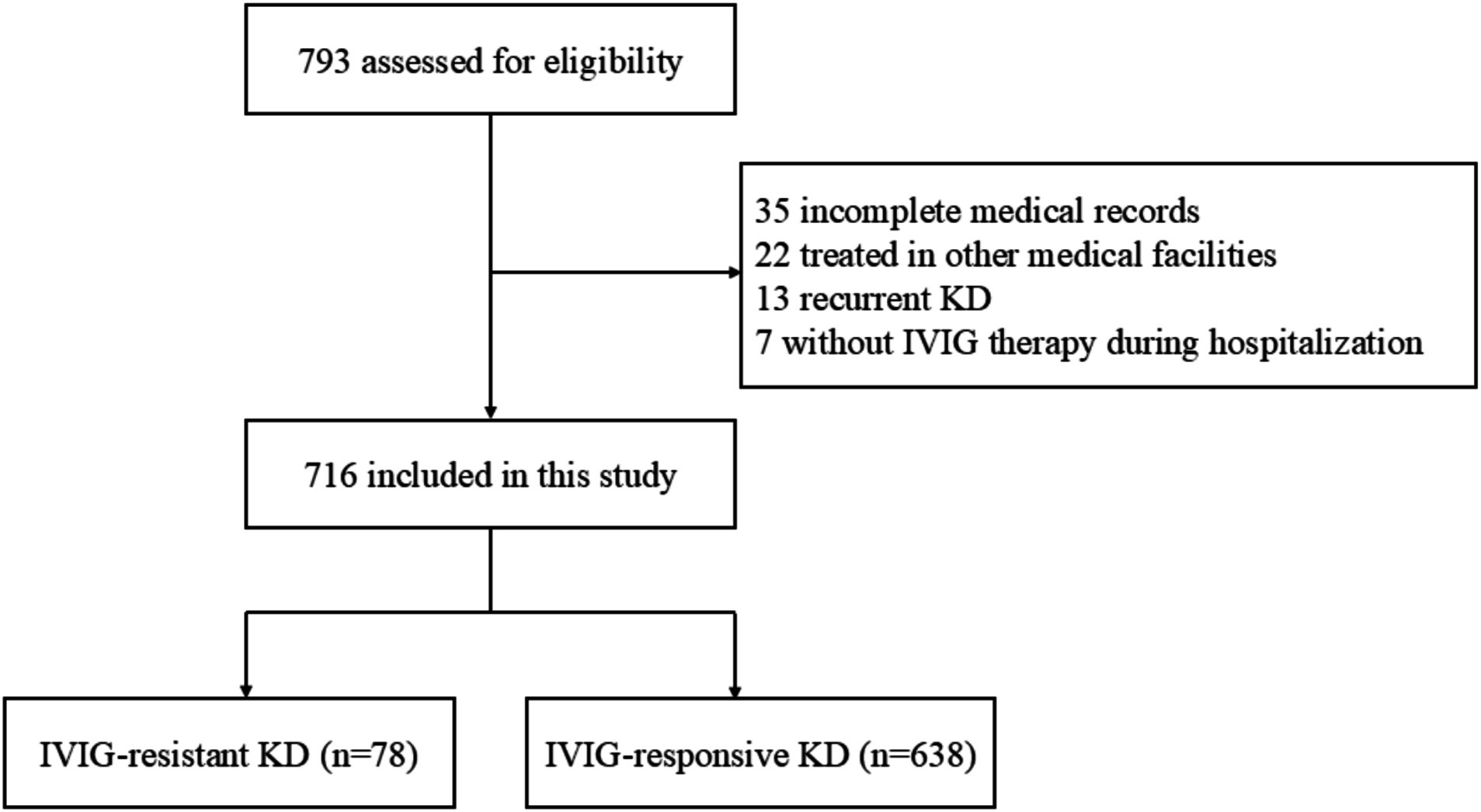
After excluding 77 cases based on the predefined exclusion criteria (Figure 1), 716 patients with KD were enrolled, consisting of 429 (59.9%) males and 287 (40.1%) females. The median age was 2.2 (1.2, 3.7) years old. Among the included individuals, 78 (10.9%) and 638 (89.1%) were diagnosed with IVIG-resistant KD and IVIG-responsive KD, respectively.
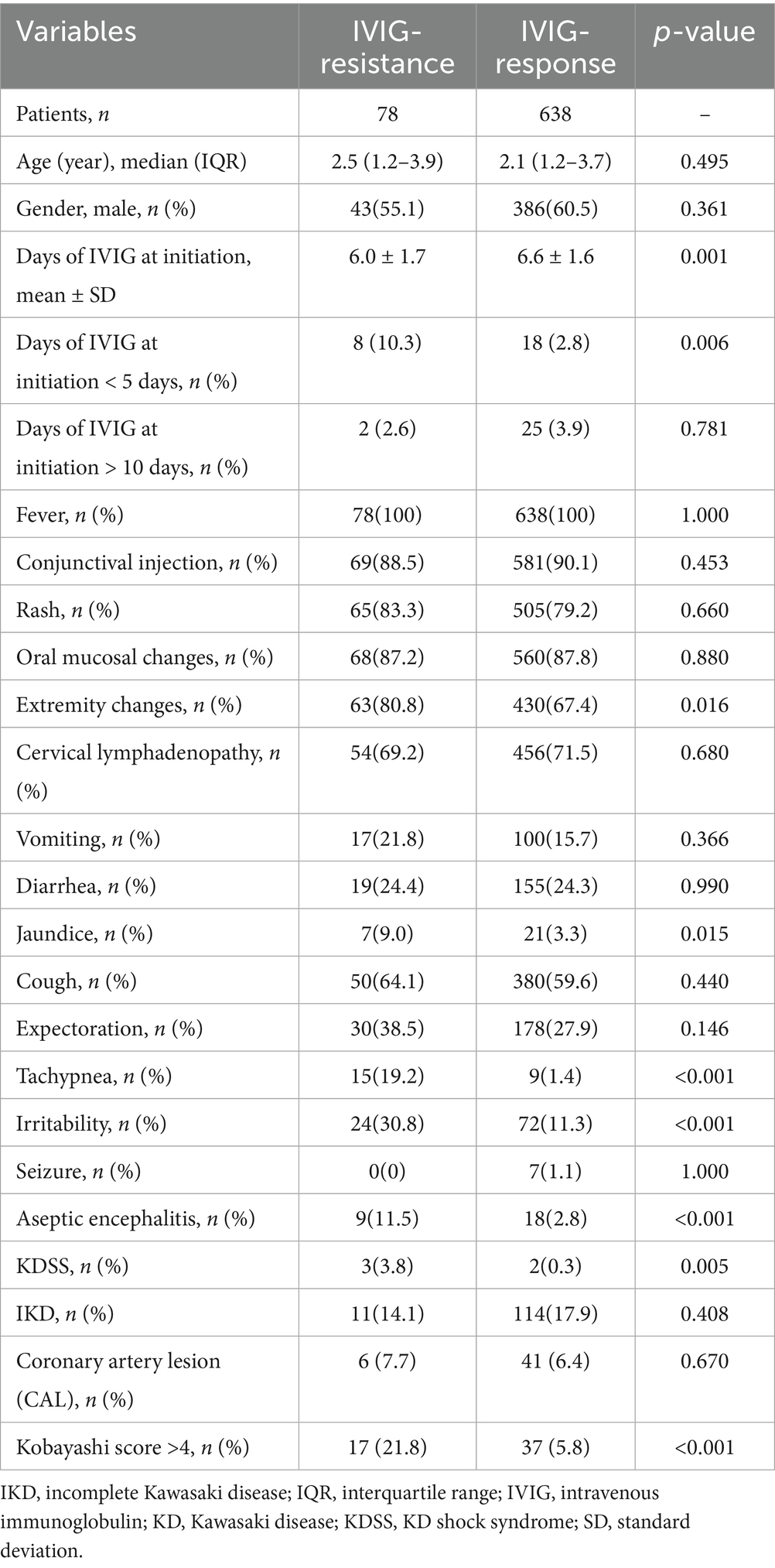
As shown in Table 1, no significant differences were observed between the IVIG-responsive group and IVIG-resistant group regarding sex, age, the occurrence of incomplete KD, and the other four typical clinical manifestations of KD, except extremity changes (p > 0.05). The IVIG-resistant group showed a significantly higher incidence of extremity changes, irritability, jaundice, tachypnea, aseptic meningitis, and KDSS than the IVIG-responsive group (all p < 0.05).
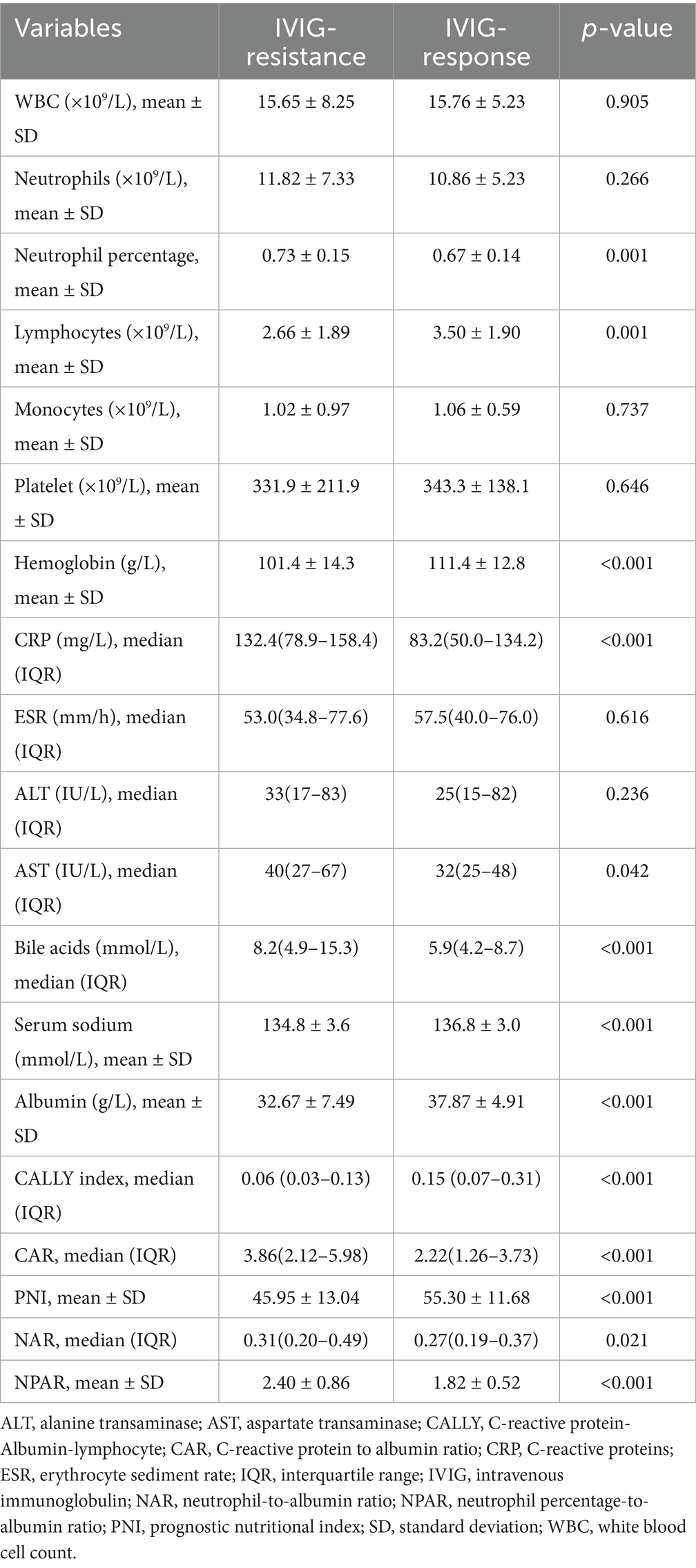
The laboratory findings are shown in Table 2. The IVIG-resistant group exhibited notably elevated CRP, AST and bile acids levels, alongside reduced lymphocytes, hemoglobin, albumin and serum sodium levels compared to the IVIG-responsive group (all p < 0.05). These findings aligned with earlier studies (21–23). Furthermore, it was noted that in the IVIG-resistant group, CAR, NAR and NPAR were significantly higher, whereas the CALLY index and PNI were significantly lower compared to IVIG-responsive group (Table 2 and Figure 2). However, the other laboratory parameters did not differ significantly between the two groups (all p > 0.05).
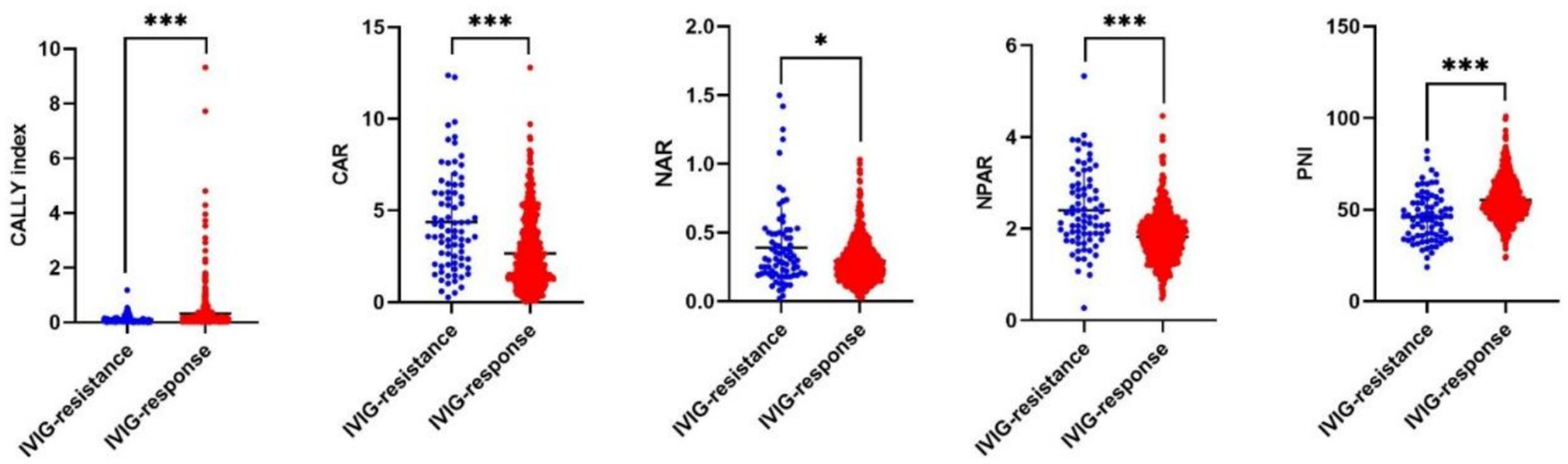
Figure 2. The distribution of 5 novel albumin-associated nutritional inflammation markers in KD patients with IVIG resistance and IVIG response. In IVIG-resistant group, CAR, NAR and NPAR were significantly higher, whereas the CALLY index and PNI were significantly lower compared to IVIG-responsive group.
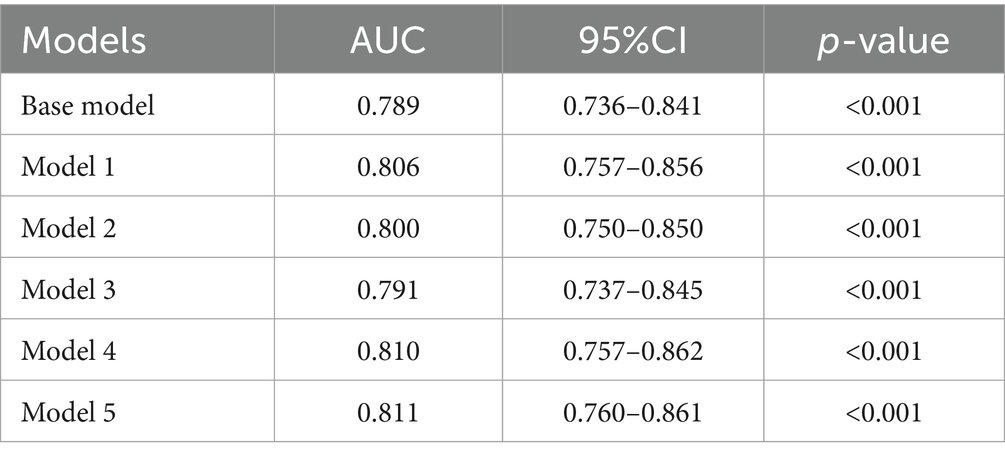
This study investigated the potential role of albumin-based biomarkers in contributing to IVIG responsiveness in KD. The potential predictive values of albumin-based markers including the CALLY index, CAR, NAR, NPAR and PNI, which were shown to be different between the two groups, were assessed in the models. To avoid collinearity, albumin-based markers were incorporated and assessed in the separate statistical models (Supplementary Tables 1–5), Among all the models, variables of Days of IVIG at initiation, Tachypnea, Hemoglobin, and Serum sodium were statistically significant in the binary logistic regression analysis. Therefore, we included the four variables to construct the Base Model, while Models 1–5 were, respectively, formed by incorporating CALLY index, CAR, NAR, NPAR and PNI into the Base Model to compare whether the predictive value of the model increased after the inclusion of these novel nutritional inflammation indexes, respectively. The CALLY index (OR: 0.072, 95% CI: 0.009–0.577, p = 0.013), CAR (OR: 1.207, 95% CI: 1.070–1.361, p = 0.002), NAR (OR: 6.073, 95% CI: 1.610–22.916, p = 0.008), NPAR (OR: 2.535, 95% CI: 1.648–3.898, p < 0.001), and PNI (OR: 0.953, 95% CI: 0.927–0.979, p = 0.001) were independent predictors for IVIG-resistance (Table 3, the brief table and Supplementary Table 6, the complete table).
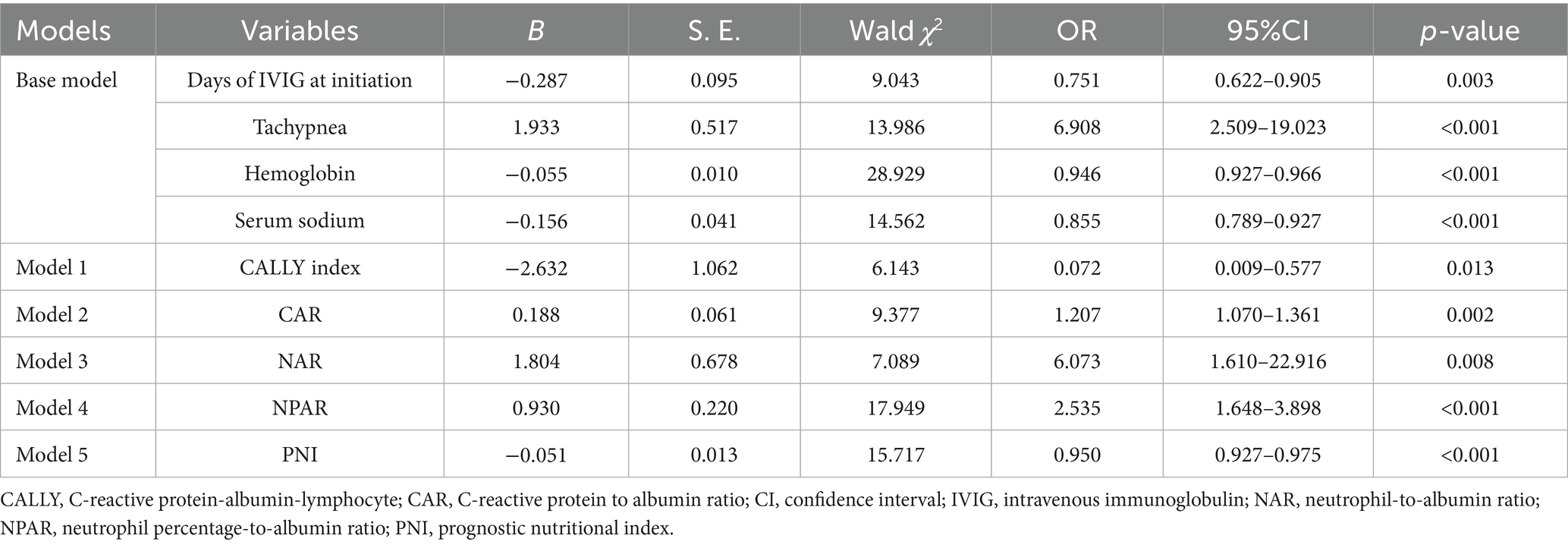
Table 3. Binary logistic regression analysis to evaluate risk factors for IVIG resistance in different models.
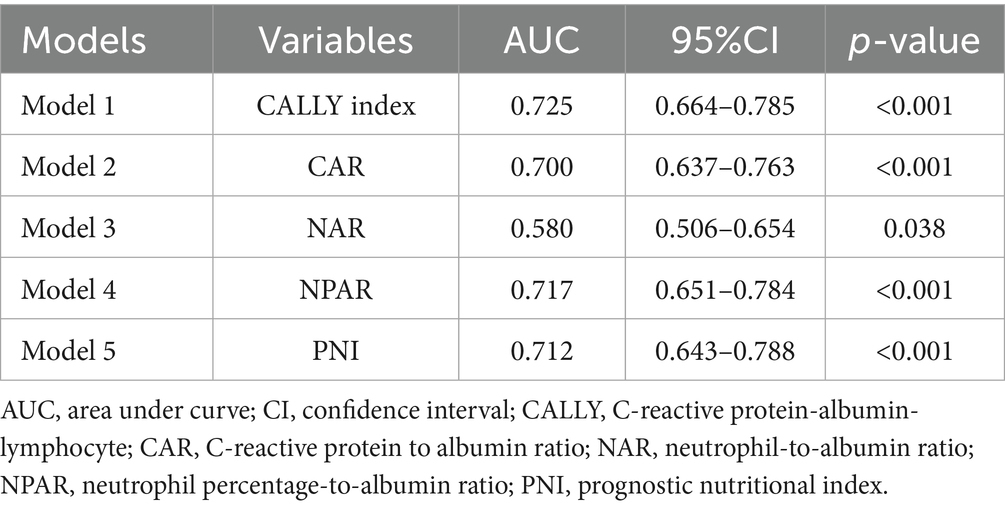
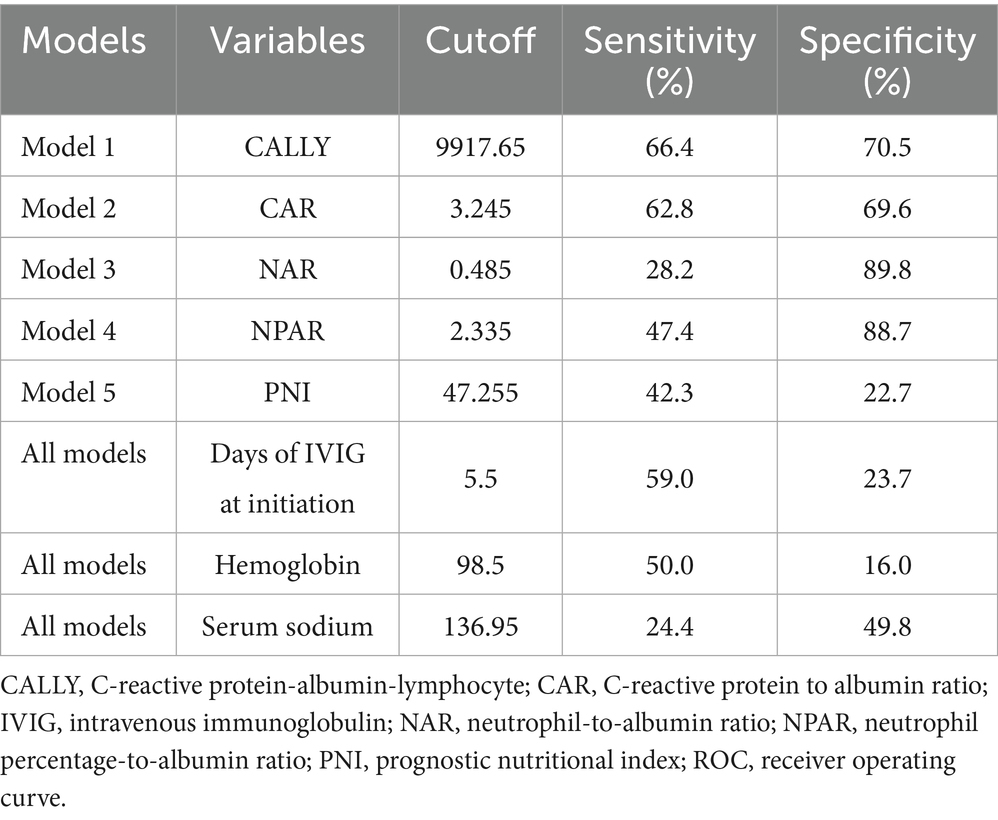
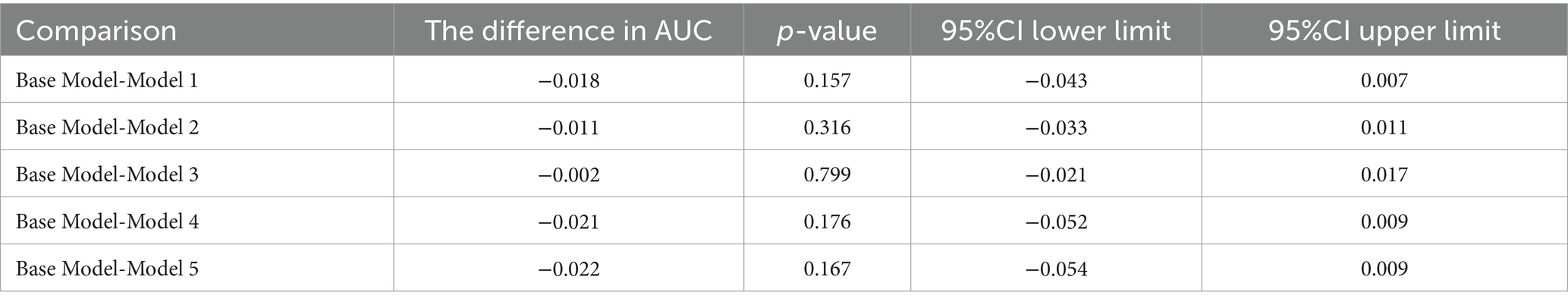
Furthermore, the predictive power of those above albumin-derived markers for IVIG-resistance were analyzed (Tables 4, 5; Figure 3; Supplementary Tables 7–11). ROC curve analyses indicated that the CALLY index, CAR, NAR, NPAR, and PNI are predictive of IVIG-resistance, with AUC values of 0.725 (cut-off value: 0.105, sensitivity: 66.4%, specificity: 70.5%), 0.700 (cut-off value: 3.245, sensitivity: 62.8%, specificity: 69.6%), 0.580 (cut-off value: 0.485, sensitivity: 28.2%, specificity: 89.8%), 0.717 (cut-off value: 2.335, sensitivity: 47.4%, specificity: 88.7%), and 0.712 (cut-off value: 47.255, sensitivity: 42.3%, specificity: 22.7%), respectively. Meanwhile, the AUC values corresponding to the multiple comparison analysis of the predicted probabilities of each model was presented (Table 6 and Figure 4). As shown in Table 6, the AUC values of Models 1–5 have increased compared to those of the Base Model. However, the DeLong test results indicate that there was no statistically significant difference in AUC between the Base Model and Models 1–5 (Table 7).
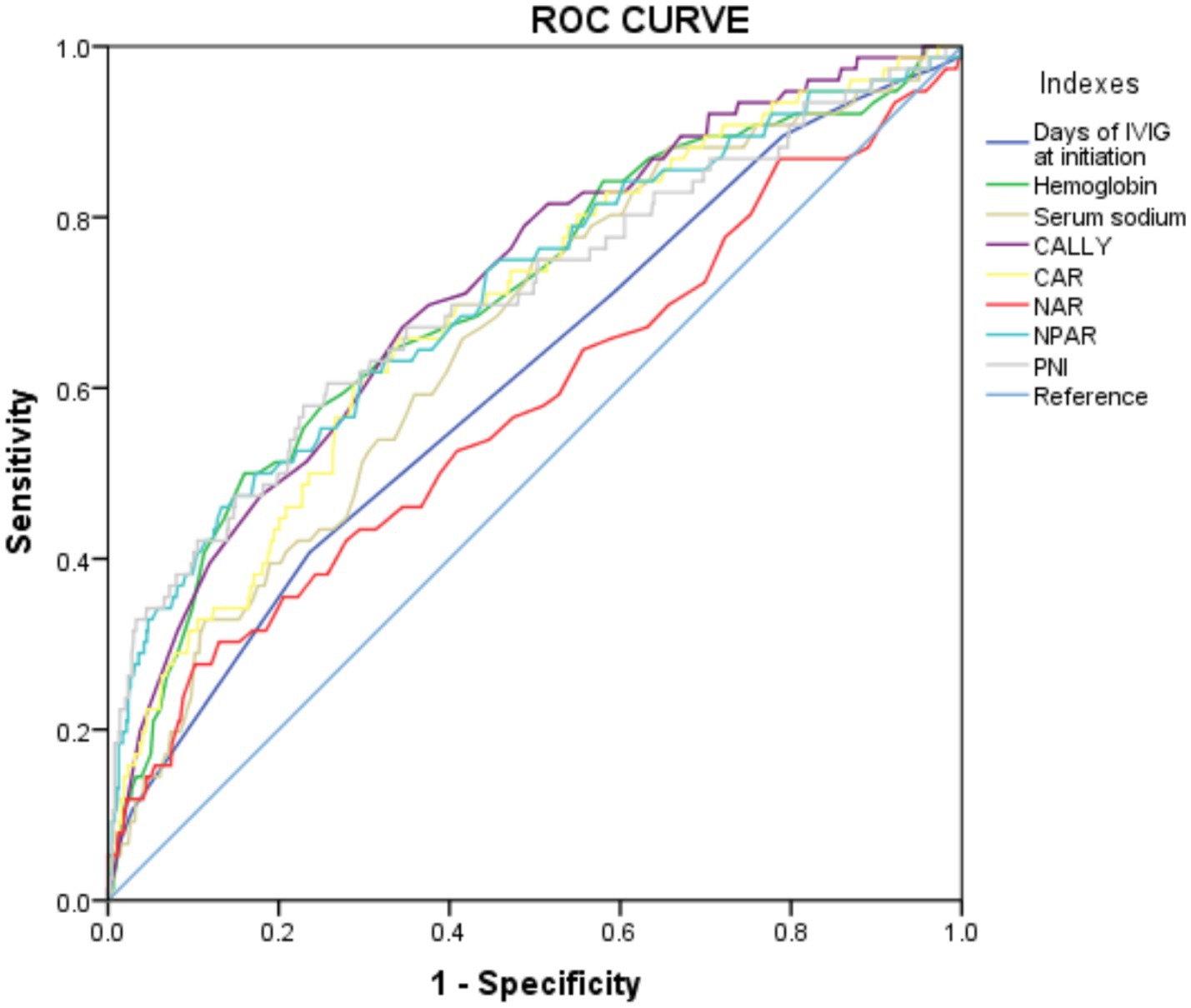
Figure 3. The ROC curve for models in predicting IVIG resistance in the whole cohort. CALLY, C-reactive protein-albumin-lymphocyte; CAR, C-reactive protein to albumin ratio; NAR, neutrophil-to-albumin ratio; NPAR, neutrophil percentage-to-albumin ratio; PNI, prognostic nutritional index.
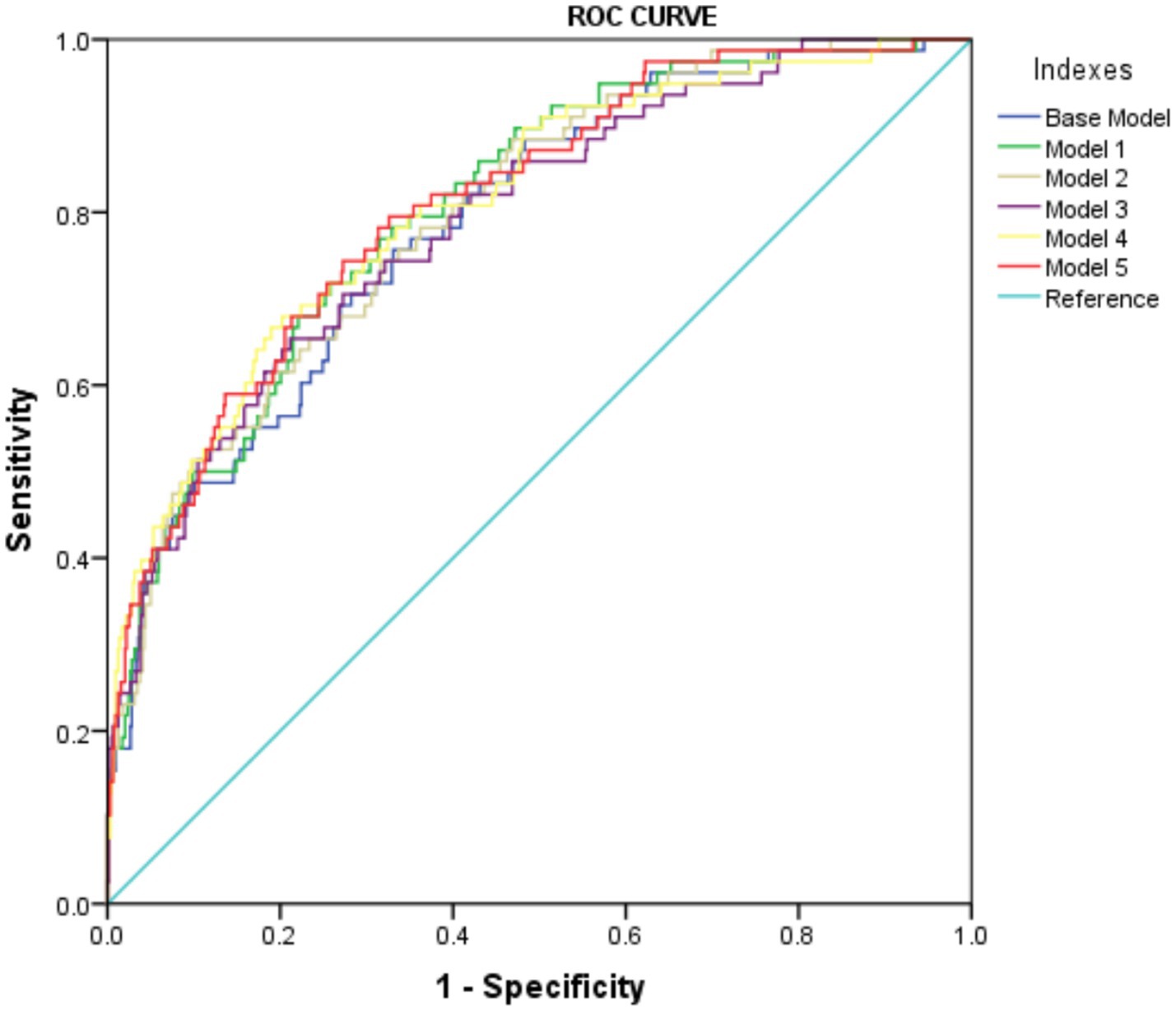
Figure 4. The ROC curve of predicted probabilities in different Models in predicting IVIG resistance.
4 Discussion
Research on IVIG-resistant KD has long been a significant challenge for pediatricians. Over the past decades, efforts have been made to identify more effective methods for predicting IVIG resistance. Initially, single factors such as CRP and albumin were used; later, scoring systems incorporating multiple variables, such as the Kobayashi score, were developed. While single biomarkers lack comprehensiveness in capturing the complex inflammatory response during the acute phase of KD, multi-factor scoring systems can be somewhat cumbersome in clinical settings. Therefore, this study aims to explore inflammatory indicators that are both comprehensive and clinically practical for predicting IVIG-resistant KD. This was a relatively large-scale study and, to our knowledge, the first to explore albumin-related biomarkers, including the CALLY index, CAR, NAR, NPAR and PNI, in IVIG-resistant KD. In our study we compared albumin-derived nutritional inflammation indices between two groups and found that CAR, NAR and NPAR were positively correlated with IVIG resistance, while the CALLY index and PNI showed negative correlations. In conclusion, our findings suggest that the CALLY index, CAR, NAR, NPAR and PNI are associated with IVIG resistance risk.
Serum albumin produced by the liver is a negative acute-phase reactant (AFR) that decreases during immune activation, whereas CRP produced by the liver is a positive AFR that increases in inflammation or infection. Neutrophils indicate active inflammation, whereas lymphocytes serve as markers for immune regulation. During an inflammatory response, delayed neutrophil apoptosis and stem cell stimulation by growth factors result in neutrophilia and redistribution within the lymphatic system, while increased lymphocyte apoptosis causes lymphocytopenia (24). Consistent with these findings, our study demonstrated that the IVIG-resistant group exhibited elevated CRP levels and reduced lymphocyte and albumin levels compared to the IVIG-responsive group. The absence of consensus on a singular risk factor and the susceptibility of individual inflammatory parameters to external influences have led to a research focus on combined risk factor indices. These indices are considered potentially more reliable for predicting IVIG resistance in KD patients than individual parameters.
Albumin-derived markers (CALLY index, CAR, NAR, NPAR, and PNI), calculated based on serum albumin, CRP, lymphocyte count, and neutrophils, more comprehensively reflect the nutritional and inflammation status. These markers are crucial for evaluating the severity and predicting outcomes of inflammatory conditions (25–27). The CALLY index, introduced by Liu et al. (28), combines albumin, lymphocyte count and CRP, offering insights into nutritional status, inflammation, and immune function (14). NAR serves as a crucial index that integrates the advantages of neutrophils and albumin to provide a comprehensive assessment of systemic immunity and nutritional status. This study represents the inaugural examination of the CALLY index and NAR within the framework of KD. In our study, we found that the results of the ROC analysis demonstrated that the CALLY index had the highest AUC among the parameters evaluated, indicating that it exhibited superior diagnostic performance. However, NAR demonstrated the least effective performance in predicting IVIG resistance in KD, as indicated by its lowest AUC among the evaluated parameters. It was well recognized that during the acute phase of KD serum albumin levels and lymphocyte counts were typically reduced, whereas CRP levels, neutrophil counts, and the neutrophil percentage were elevated. Among these albumin-derived markers, the three components of the CALLY Indexes show a consistent direction of change, while the other four albumin-derived markers show a consistent direction of change for two of their components. This might be the reason why the CALLY Indexes have the highest predictive value. In the univariate analysis of the components of the five albumin-derived markers, albumin levels, lymphocyte count, CRP levels, and neutrophil percentage demonstrated statistically significant differences between the two patient groups, whereas neutrophil count did not reach statistical significance. This might partially explain the relatively lower predictive value of NAR.
To date, limited research has explored the association between CAR, NPAR, PNI, and KD. A meta-analysis showed that lower PNI or high CAR was associated with the increased risk to develop IVIG resistance (28). NPAR was identified an independent biomarker for IVIG resistance (10). Our findings confirm that CAR, NPAR, and PNI are independent risk factors for IVIG resistance, aligning with previous research. Unlike previous studies, which often analyzed these markers individually, our study comprehensively examined the association of these albumin-derived markers with IVIG-resistant KD. As of the time of writing this manuscript, our study was the first to report the association between CALLY indexes and IVIG-resistant KD. Additionally, only one previous study had investigated the predictive value of NPAR for IVIG-resistant KD, and it was based on a sample size of 438 cases, which is considerably smaller than that of our study (10). This study indicates that PNI, NPAR, and CAR are potential predictive factors for IVIG resistance in KD, a finding that aligns with the results of previous research. In this study, we also calculated the Kobayashi score for each patient. However, its sensitivity for identifying IVIG resistance in Kawasaki disease was only 21.8% (17/78), which was lower than that of other albumin-derived markers evaluated in this study. Therefore, in this study, compared with the Kobayashi score, the albumin-derived markers demonstrate greater potential for clinical application.
This study has certain limitations. First, owing to its retrospective, single-center design and the insufficient sample size of patients, and 35 patients were excluded from the study due to missing data, all of which inevitably led to a certain degree of selection bias in our research process. The potential for bias cannot be completely ruled out, which may reduce the statistical power of our findings. Second, the homogeneity of the study population in terms of racial background may limit the generalizability of our findings. For instance, the Kobayashi score, which has achieved relatively good predictive effects in previous reports, may not perform well in our study due to differences in ethnicity. Therefore, prospective multicenter studies with larger and more diverse racial samples are necessary to confirm these results. The association of hypoalbuminemia (PNI, CAR) with IVIG resistance aligns with KD’s endothelial dysfunction paradigm. For future work, we need to proactively collect data on KD patients in our hospital or from other centers for external validation, so that our research can be better applied in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Mianyang Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consentd for participation from the participants’ legal guardians/next of kin as the study was retrospective.
Author contributions
CY: Formal analysis, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing. DX: Conceptualization, Formal analysis, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. JC: Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. JG: Data curation, Investigation, Software, Supervision, Writing – original draft. Y-NZ: Data curation, Investigation, Software, Supervision, Writing – review & editing. YH: Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XS: Data curation, Funding acquisition, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Project of Sichuan Provincial Health Commission, 24WSXT026; the Youth innovative Scientific Research Project of Sichuan Medical Association, Q2024031; the Scientific Research Project of Mianyang Health Commission, 2024031; the Talent Introduction Project of Mianyang Central Hospital, 2024RCYJ-004; and the Incubation Project of Mianyang Central Hospital, 2019FH07.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1651750/full#supplementary-material
References
1. McCrindle, BW, Rowley, AH, Newburger, JW, Burns, JC, Bolger, AF, Gewitz, M, et al. Diagnosis, treatment, and long-term Management of Kawasaki Disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
2. Kuo, HC. Diagnosis, Progress, and treatment update of Kawasaki disease. Int J Mol Sci. (2023) 24:13948. doi: 10.3390/ijms241813948
3. Galeotti, C, Kaveri, SV, Cimaz, R, Koné-Paut, I, and Bayry, J. Predisposing factors, pathogenesis and therapeutic intervention of Kawasaki disease. Drug Discov Today. (2016) 21:1850–7. doi: 10.1016/j.drudis.2016.08.004
4. Uehara, R, Belay, ED, Maddox, RA, Holman, RC, Nakamura, Y, Yashiro, M, et al. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J. (2008) 27:155–60. doi: 10.1097/INF.0b013e31815922b5
5. Gabay, C, and Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. (1999) 340:448–54.
6. Yang, P, Meng, L, and Guo, J. Analysis of the clinical characteristics of patients with Kawasaki disease complicated with cholestasis. BMC Pediatr. (2024) 24:777. doi: 10.1186/s12887-024-05278-w
7. Levitt, DG, and Levitt, MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. (2016) 9:229–55. doi: 10.2147/IJGM.S102819
8. Yang, S, Song, R, Zhang, J, Li, X, and Li, C. Predictive tool for intravenous immunoglobulin resistance of Kawasaki disease in Beijing. Arch Dis Child. (2019) 104:262–7. doi: 10.1136/archdischild-2017-314512
9. Lin, MT, Chang, CH, Sun, LC, Liu, HM, Chang, HW, Chen, CA, et al. Risk factors and derived formosa score for intravenous immunoglobulin unresponsiveness in Taiwanese children with Kawasaki disease. J Formos Med Assoc. (2016) 115:350–5. doi: 10.1016/j.jfma.2015.03.012
10. Deng, L, Wang, T, Duan, Y, Liu, B, Jiang, J, Liu, D, et al. Neutrophil percentage-to-albumin ratio is a potential marker of intravenous immunoglobulin resistance in Kawasaki disease. Sci Rep. (2024) 14:15232. doi: 10.1038/s41598-024-66135-5
11. Zhong, X, Xie, Y, Wang, H, Chen, G, Yang, T, and Xie, J. Values of prognostic nutritional index for predicting Kawasaki disease: a systematic review and meta-analysis. Front Nutr. (2024) 11:1305775. doi: 10.3389/fnut.2024.1305775
12. Liu, J, Chen, X, Yang, M, Shen, F, Zhu, F, Jin, J, et al. C-reactive protein to albumin ratio as a prognostic tool for predicting intravenous immunoglobulin resistance in children with Kawasaki disease: a systematic review of cohort studies. Pediatr Rheumatol Online J. (2024) 22:42. doi: 10.1186/s12969-024-00980-6
13. Horstman, IM, Vinke, PC, Suazo-Zepeda, E, Hiltermann, TJN, Heuvelmans, MA, Corpeleijn, E, et al. The association of nutritional and inflammatory biomarkers with overall survival in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. (2024) 15:1764–71. doi: 10.1111/1759-7714.15401
14. Iida, H, Tani, M, Komeda, K, Nomi, T, Matsushima, H, Tanaka, S, et al. Superiority of CRP-albumin-lymphocyte index (CALLY index) as a non-invasive prognostic biomarker after hepatectomy for hepatocellular carcinoma. HPB. (2022) 24:101–15. doi: 10.1016/j.hpb.2021.06.414
15. Tawfik, B, Mokdad, AA, Patel, PM, Li, HC, and Huerta, S. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anti-Cancer Drugs. (2016) 27:879–83. doi: 10.1097/CAD.0000000000000411
16. Su, K, Xiao, S, Wang, M, Wang, K, Fan, Q, Sha, S, et al. Predictive value of albumin to fibrinogen ratio and CALLY index for diagnosis of ulcerative colitis and mucosal healing after Vedolizumab treatment. J Inflamm Res. (2025) 18:589–600. doi: 10.2147/JIR.S500600
17. Chen, X, Li, Y, Chen, H, and Chen, W. Immunoinflammatory markers SIRI and NAR as predictors of respiratory distress syndrome and secondary infections in premature infants. Front Cell Infect Microbiol. (2024) 14:1512884. doi: 10.3389/fcimb.2024.1512884
18. Şahin, O, Güneş, M, and Dönmez, R. C-reactive protein-to-albumin ratio and systemic immune-inflammatory index as potential markers in distinguishing acute cerebellar infarction from benign paroxysmal positional vertigo. Neurosciences. (2025) 30:30–5. doi: 10.17712/nsj.2025.1.20240084
19. Kanegaye, JT, Wilder, MS, Molkara, D, Frazer, JR, Pancheri, J, Tremoulet, AH, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. (2009) 123:e783–9. doi: 10.1542/peds.2008-1871
20. Liu, J, Su, D, Yuan, P, Huang, Y, Ye, B, Liang, K, et al. Prognostic nutritional index value in the prognosis of Kawasaki disease with coronary artery lesions. Front Nutr. (2023) 10:1075619. doi: 10.3389/fnut.2023.1075619
21. Yi, C, Zhou, YN, Guo, J, Chen, J, and She, X. Novel predictors of intravenous immunoglobulin resistance in patients with Kawasaki disease: a retrospective study. Front Immunol. (2024) 15:1399150. doi: 10.3389/fimmu.2024.1399150
22. Wang, Y, Huang, S, Wang, P, Wu, Y, Liu, Y, Pan, Y, et al. Novel predictive scoring system for intravenous immunoglobulin resistance helps timely intervention in Kawasaki disease: the Chinese experience. J Immunol Res. (2023) 2023:1–7. doi: 10.1155/2023/6808323
23. Cheon, EJ, Kim, GB, and Park, S. Predictive modeling of consecutive intravenous immunoglobulin treatment resistance in Kawasaki disease: a nationwide study. Sci Rep. (2025) 15:903. doi: 10.1038/s41598-025-85394-4
24. Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. (2001) 102:5–14.
25. Shi, W, Jiang, Y, Tian, H, Wang, Y, Zhang, Y, Yu, T, et al. C-reactive protein-to-albumin ratio (CAR) and C-reactive protein-to-lymphocyte ratio (CLR) are valuable inflammatory biomarker combination for the accurate prediction of Periprosthetic joint infection. Infect Drug Resist. (2023) 16:477–86. doi: 10.2147/IDR.S398958
26. Hu, Z, Wang, J, Xue, Y, Zhang, Q, Xu, Q, Ji, K, et al. The neutrophil-to-albumin ratio as a new predictor of all-cause mortality in patients with heart failure. J Inflamm Res. (2022) 15:701–13. doi: 10.2147/JIR.S349996
27. Tsunematsu, M, Haruki, K, Taniai, T, Tanji, Y, Shirai, Y, Furukawa, K, et al. The impact of C-reactive protein-albumin-lymphocyte (CALLY) index on the prognosis of patients with distal cholangiocarcinoma following pancreaticoduodenectomy. Ann Gastroenterol Surg. (2022) 7:503–11. doi: 10.1002/ags3.12637
Keywords: Kawasaki disease, C-reactive protein-albumin-lymphocyte index, C-reactive protein to albumin ratio, neutrophil-to-albumin ratio, neutrophil percentage-to-albumin ratio, prognostic nutritional index, intravenous immunoglobulin resistance
Citation: Yi C, Xue D, Chen J, Guo J, Zhou Y-N, Hu Y and She X (2025) Predictive value of novel nutritional inflammation indexes in IVIG-unresponsive Kawasaki disease: a retrospective study. Front. Nutr. 12:1651750. doi: 10.3389/fnut.2025.1651750
Edited by:
Agnieszka Kozioł-Kozakowska, Jagiellonian University Medical College, PolandReviewed by:
Wenqiang Sun, Children's Hospital of Soochow University, ChinaYoshifumi Miyagi, Haibara General Hospital, Japan
Sabyasachi Mohanty, University of Nebraska-Lincoln, United States
Copyright © 2025 Yi, Xue, Chen, Guo, Zhou, Hu and She. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang She, eGlhbmdzaGU2Nzg0QDEyNi5jb20=; Yu Hu, aHV5dTA0NDNAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Cong Yi
Cong Yi Dan Xue†
Dan Xue† Yu Hu
Yu Hu Xiang She
Xiang She