- 1Department of Digestive Surgery, Xijing Hospital of Digestive Diseases, The Fourth Military Medical University, Xi'an, China
- 2State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi'an, China
- 3Department of General Surgery, Chinese People's Liberation Army Central Theater Command General Hospital, Wuhan, China
- 4School of Clinical Medicine, Yan'an University, Yan'an, China
- 5School of Clinical Medicine, Xi'an Medical University, Xi'an, China
- 6Department of General Surgery, The Southern Theater Air Force Hospital, Guangzhou, China
- 7Department of Preventive Health Care, The First Affiliated Hospital of Air Force Medical University, Xi'an, China
- 8Department of Experiment Surgery, The First Affiliated Hospital of Air Force Medical University, Xi'an, China
Background: Colorectal cancer (CRC) is a major global health concern, with obesity rates rising and an observed obesity paradox where higher body mass index (BMI) is linked to better outcomes in certain patient groups. This study aims to explore how age and tumor stage modify the association between BMI and mortality risk in CRC patients.
Materials and methods: This retrospective cohort study included 4,114 CRC patients who underwent surgery between December 2013 and December 2019. Patients were categorized by BMI, age, and TNM stage. Multivariate Cox regression models and Kaplan-Meier survival analyses were used to assess the impact of BMI on mortality risk, adjusting for potential confounders such as age, sex, and cancer stage.
Results: Higher BMI was associated with lower mortality risk across the study population. Specifically, the protective effect of higher BMI was most pronounced in patients aged 65 and older and in those with Stage III disease. The multivariate Cox regression analysis revealed that each unit increase in BMI was associated with a 7% decrease in mortality risk. The Kaplan-Meier survival analysis showed significant survival benefits for higher BMI in patients aged 65 and older and in Stage III patients.
Conclusions: Higher BMI is associated with lower mortality risk in colorectal cancer patients, particularly in those aged 65 and older and those with Stage III disease. These findings highlight the importance of considering BMI, age, and TNM stage jointly in clinical practice for CRC patients.
1 Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer worldwide and the second leading cause of cancer-related deaths (1). With the improvement in living standards, the incidence of obesity has significantly increased, leading to a higher number of obese patients diagnosed with colorectal cancer (2, 3). The relationship between obesity and colorectal cancer is complex and paradoxical (4). While obesity is a known risk factor for the development of CRC, recent studies have shown that higher BMI may be associated with better outcomes in certain patient populations, a phenomenon known as the “obesity paradox” (5–7).
Numerous studies have investigated the relationship between BMI and patient prognosis in colorectal cancer, but consensus has not been reached (8). Some studies suggest that higher BMI is associated with increased mortality risk, while others indicate a protective effect of higher BMI on survival outcomes (3, 4, 9, 10). Age and tumor stage are important factors that can influence the relationship between BMI and patient prognosis (4, 11, 12). However, few studies have comprehensively explored how age and tumor stage modify the association between BMI and mortality risk in colorectal cancer patients.
Therefore, this study aims to investigate the age- and stage- modified associations between BMI and the mortality risk in CRC. By conducting a retrospective cohort study, we will analyze the combined effects of BMI, age, and TNM stage on mortality risk. This research will provide valuable insights for developing more personalized treatment strategies and improving the prognosis of colorectal cancer patients.
2 Materials and methods
2.1 Study design and participants
This retrospective cohort study included colorectal cancer patients who underwent surgery at the Department of Digestive Surgery, First Affiliated Hospital of the Air Force Medical University, and the Department of General Surgery, Shaanxi Provincial People's Hospital, from December 2013 to December 2019. Inclusion criteria: (1) Pathologically diagnosed with colorectal adenocarcinoma, staged T1-4a (pT1-4a), N0/N+, M0 per the American Joint Committee on Cancer (AJCC) Eighth Edition Cancer Staging Manual; (2) Underwent radical surgery (R0 resection); (3) Had complete clinical and pathological data; (4) Had complete follow-up information; (5) Post-operative adjuvant chemotherapy was administered according to the NCCN guidelines: patients with Stage II disease plus high-risk features and all patients with Stage III disease were offered adjuvant therapy. Exclusion criteria: (1) Other concurrent malignancies; (2) Preoperative chemoradiotherapy; (3) Metabolic diseases (hypothyroidism, Cushing's syndrome, polycystic ovary syndrome, diabetes, or metabolic syndrome); (4) Long-term use of glucocorticoids, insulin, or GLP-1 receptor agonists. This study complies with the Declaration of Helsinki and was conducted in accordance with the protocol approved by the ethics committee of the First Affiliated Hospital of the Air Force Military Medical University, with the ethics protocol number: KY20232232-F-1. All participants provided informed consent and were informed about the study's purpose and data confidentiality.
2.2 Clinical data collection
We collected patients' baseline and pathological data. Baseline data included gender, age, and BMI at diagnosis. Age groups were Early-onset (< 50), Intermediate-onset (50 ≤ age < 64), and Late-onset (≥65) (13). BMI categories were underweight (< 18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30) per WHO criteria. Pathological data included tumor location, differentiation degree (well, moderately, poorly), DNA mismatch repair status (dMMR/pMMR), vascular invasion (negative/positive), number of harvested lymph nodes, and TNM stage (I, II, III). Tumor locations were right-sided colon cancer (cecum to hepatic flexure), left-sided colon cancer (splenic flexure to rectosigmoid junction), and rectal cancer. Follow-up was conducted via outpatient visits and phone calls. Patients had follow-ups every 3 months for the first 2 years, then every 6 months thereafter. Overall survival (OS) was calculated from the date of initial surgery to death or last follow-up. The primary study endpoint was overall survival, and the censoring date for follow-up was 30 June 2024.
2.3 Statistical analysis
Quantitative data following a normal distribution are presented as the mean ± standard deviation (x ± s), with intergroup comparisons conducted using the independent samples t-test. For quantitative data with a skewed distribution, the median (Q1, Q3) is used, and intergroup comparisons are performed using the Mann-Whitney U test. Categorical data are described using absolute numbers and percentages, with intergroup comparisons made via the chi-square test. To assess the overall impact of BMI on mortality risk, hazard ratio (HR) curves are plotted for the entire study population. Additionally, HR curves are separately plotted for different TNM stages and age groups to compare the differential effects of BMI on mortality risk. The Kaplan-Meier method was employed to estimate survival probabilities, and log-rank tests were used to compare survival differences across groups. Cox proportional hazards regression models were utilized to assess the relationship between BMI and mortality risk, with adjustments for potential confounding variables such as age, sex, and cancer stage. Stratified survival analyses were conducted to explore variations in the impact of BMI on mortality risk across different age and stage subgroups. All analyses were performed using R 4.2.1 and SPSS 24.0. P-values were only calculated for the primary hypotheses (e.g., associations between BMI, age, stage, and mortality risk in Cox models). No p-values were reported for baseline characteristic comparisons to avoid multiplicity issues. A two-tailed p-value < 0.05 was considered statistically significant.
3 Results
3.1 Demographic characteristics
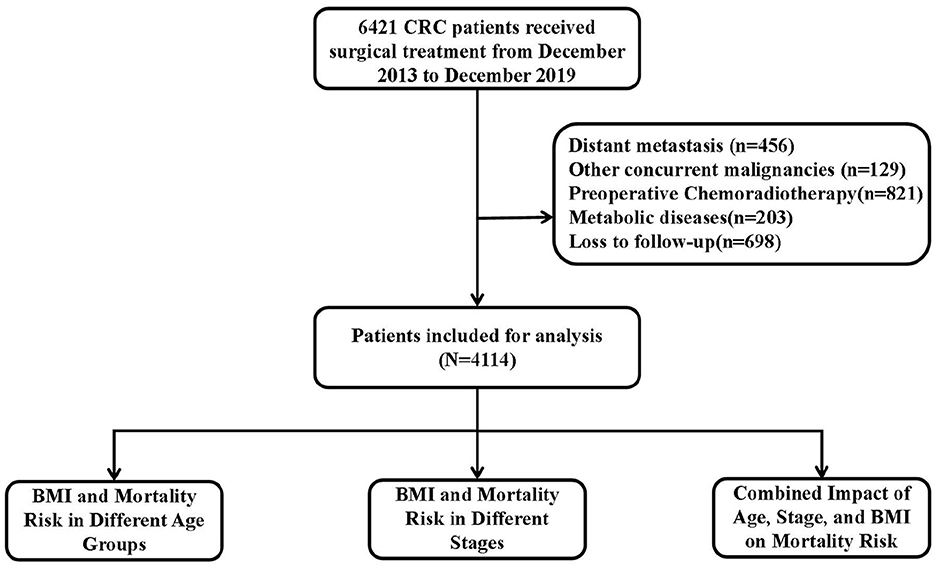
This study enrolled 6,421 CRC patients confirmed by pathology, 2,307 patients were excluded for the following reasons: distant metastasis (n = 456), other concurrent malignancies (n = 129), preoperative chemoradiotherapy (n = 821), metabolic diseases (n = 203), and loss to follow-up (n = 698), and 4,114 were finally analyzed after screening. Figure 1 shows the study flowchart. Among the included patients, 59.6% were male and 40.4% were female; 22.8% had right-colon cancer, 24.9% left-colon cancer, and 53.3% rectal cancer. The mean age at diagnosis was 61.1 years (SD = 12.1). The proportions of patients in stages I, II, and III were 18.3%, 37.9%, and 43.8%, respectively. The follow-up time in our study ranged from 2 to 95 months, with a median follow-up of 42 months. We categorized patients based on BMI, age, and stage and analyzed the baseline characteristics of each group.
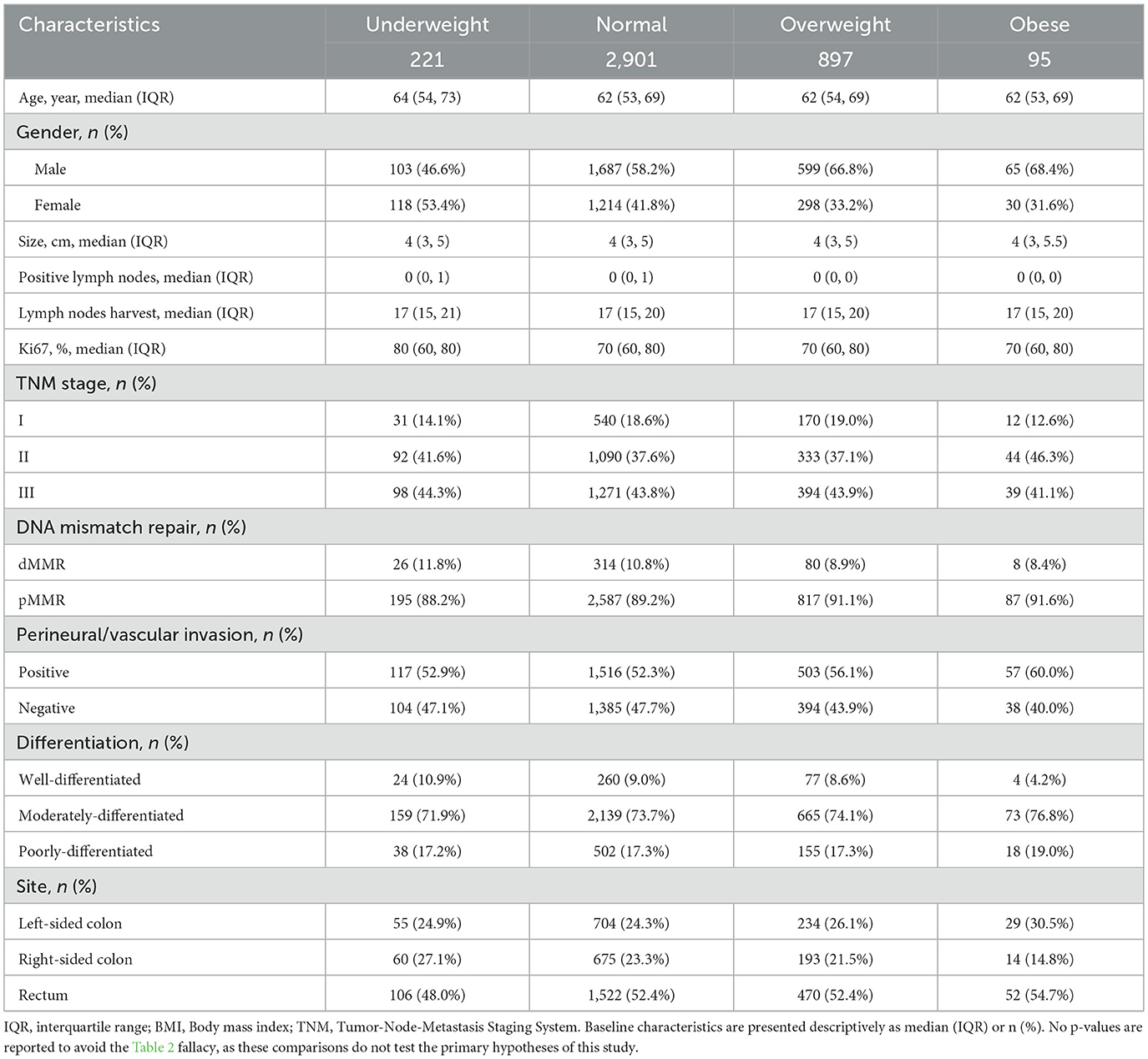
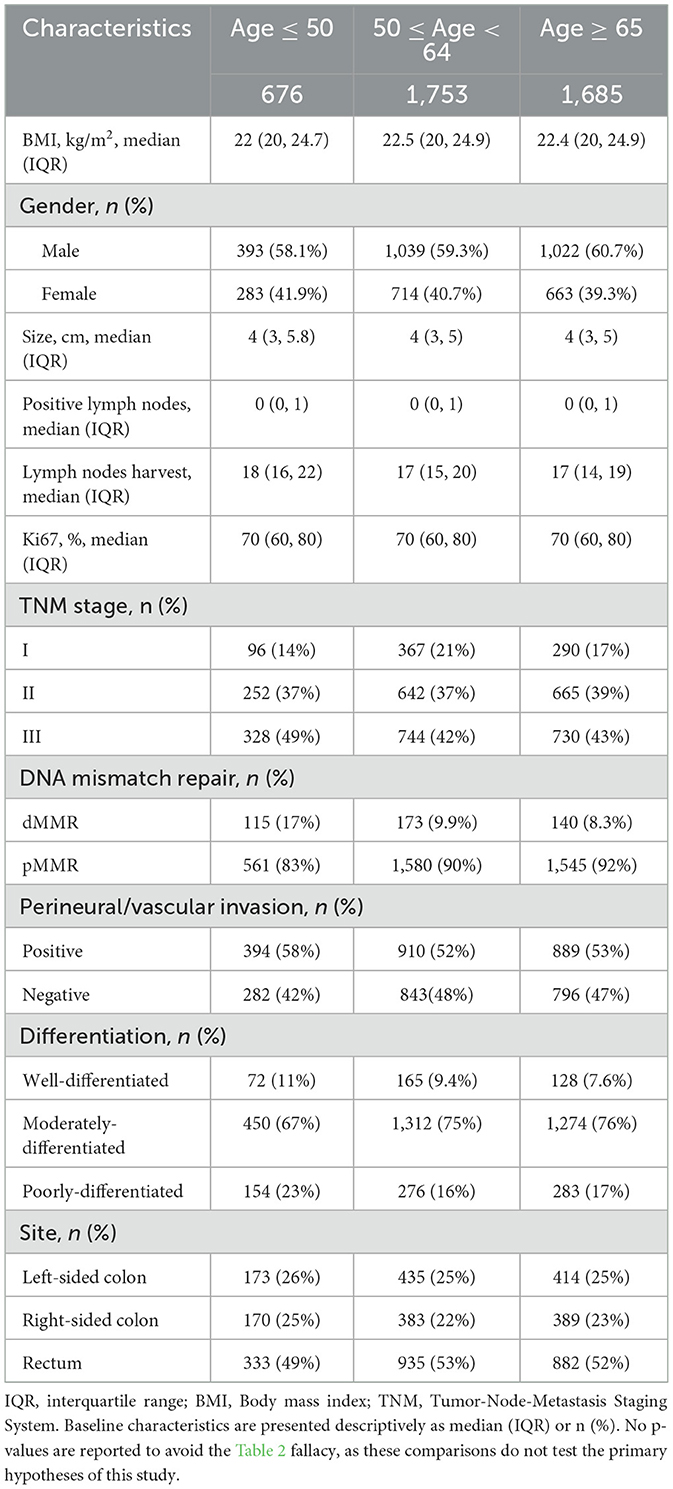
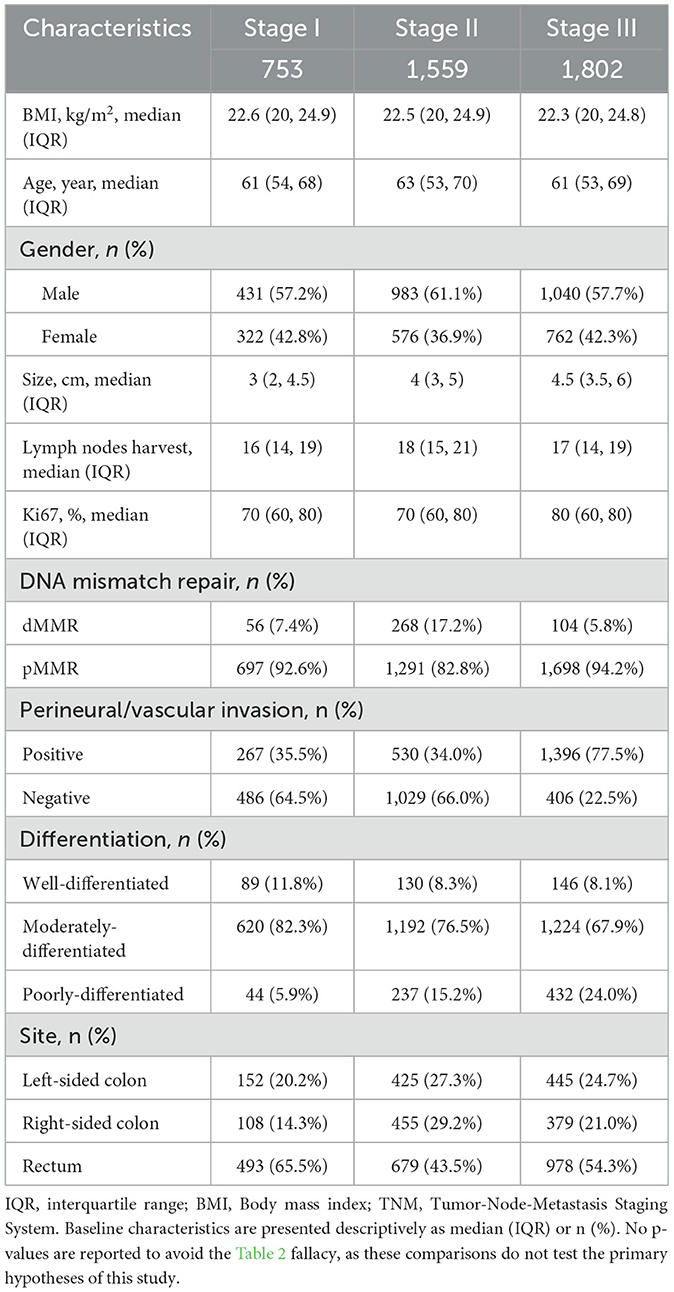
BMI stratification showed a progressive increase in male representation from underweight (46.6%) to obese categories (68.4%), with generally similar distributions of other variables (Table 1). In the baseline characteristic analysis by age groups, younger patients (< 50 years) had lower median BMI (22.0 kg/m2), larger tumors (median 4.0 cm), higher dMMR prevalence (17%), and more lymph node involvement (median 1 positive node) compared to older groups, while elderly patients (≥65 years) had fewer harvested lymph nodes (median 17 vs. 18 in < 50 y; Table 2). Stage stratification demonstrated Stage III patients had substantially higher rates of vascular invasion (77.5% vs. 34–35% in early stages) and poor differentiation (24.0% vs. 5.9–15.2%), with decreasing rectal cancer prevalence from Stage I (65.5%) to Stage II (43.5%; Table 3).
3.2 Multivariate Cox regression analysis
A multivariate Cox regression analysis was performed to assess the relationships between key prognostic factors and mortality risk, adjusting for potential confounders. Each unit increase in BMI was associated with a 7% decrease in mortality risk (HR = 0.933, 95% CI: 0.911–0.956, P < 0.001). Mortality risk increased by 2.5% per year of age (HR = 1.025, 95% CI: 1.018–1.031, P < 0.001). Tumor size was linked to mortality risk, with a 7.2% increase per cm (HR = 1.072, 95% CI: 1.030–1.116, P = 0.001). Each additional positive lymph node was associated with an 11% increase in mortality risk (HR = 1.110, 95% CI: 1.084–1.136, P < 0.001). Conversely, each additional harvested lymph node was associated with a 2.4% decrease in mortality risk (HR = 0.976, 95% CI: 0.961–0.991, P = 0.002). Patients with dMMR had a 32% lower mortality risk than those with pMMR (HR = 0.680, 95% CI: 0.507–0.912, P = 0.01). Compared to stage I, stage III CRC was associated with an 80.3% increase in mortality risk (HR = 1.803, 95% CI: 1.378–2.359, P < 0.001). Poorly differentiated tumors carried a 23.1% higher mortality risk than well-differentiated ones (HR = 1.231, 95% CI: 1.024–1.476, P < 0.001). Other variables, including gender, S100, CD34, and tumor location, were not significantly associated with mortality risk (Table 4).
3.3 Combined effects of BMI, age, and stage on risk of mortality
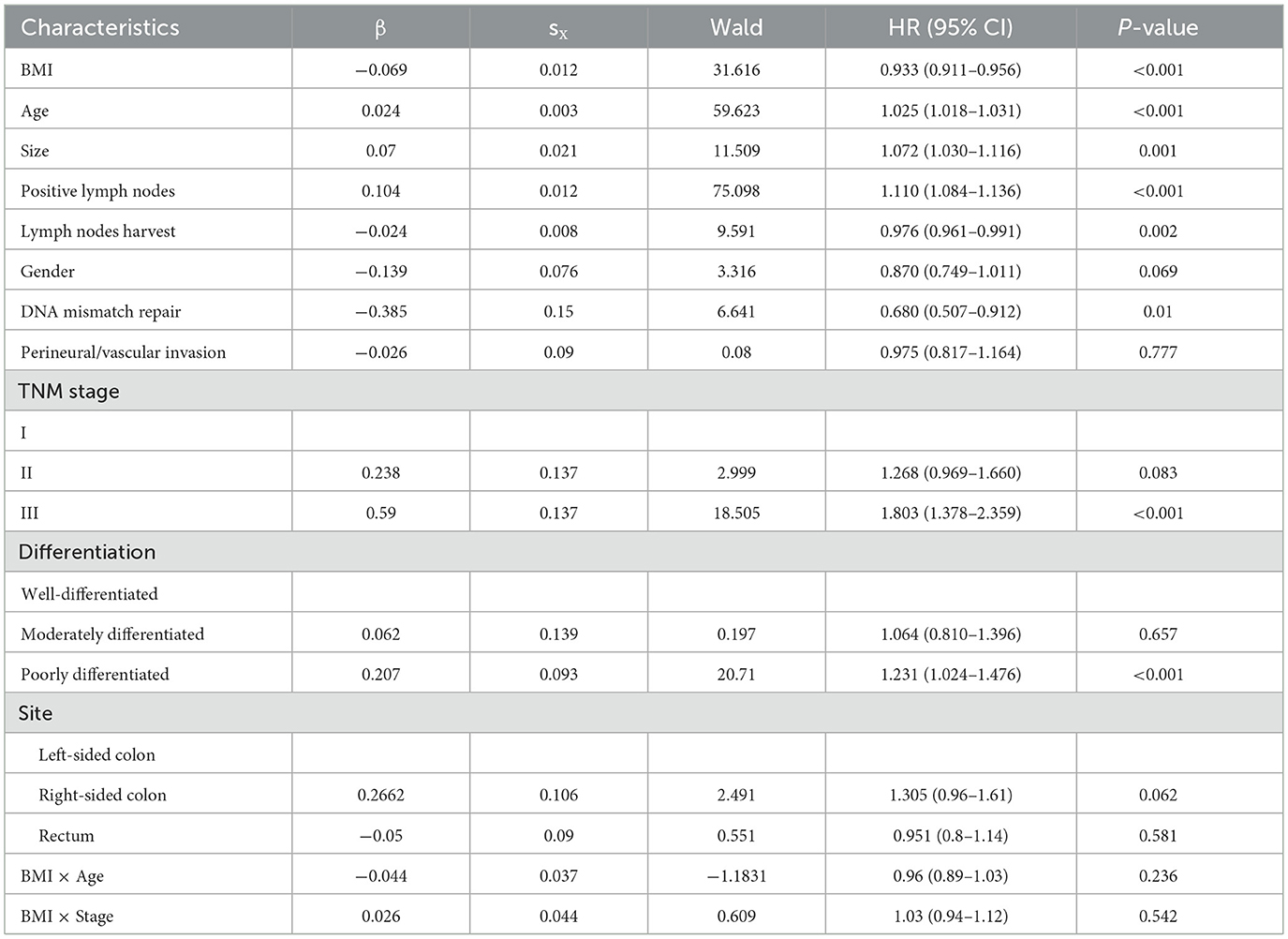
To evaluate the impact of BMI, age and stage on the risk of mortality in patients with colorectal cancer. Higher BMI is associated with lower mortality risk across the entire study population (Figure 2A). In patients aged 65 and older, the hazard ratio (HR) decreases sharply with increasing BMI, while in those younger than 50 and aged 50–64, the decline in HR is less steep (Figure 2B). In Stage III, there's a clear downward trend in HR with increasing BMI, Stage II shows a similar but less pronounced trend, and in Stage I, the HR decreases with higher BMI but the curve flattens at higher BMI values (Figure 2C).
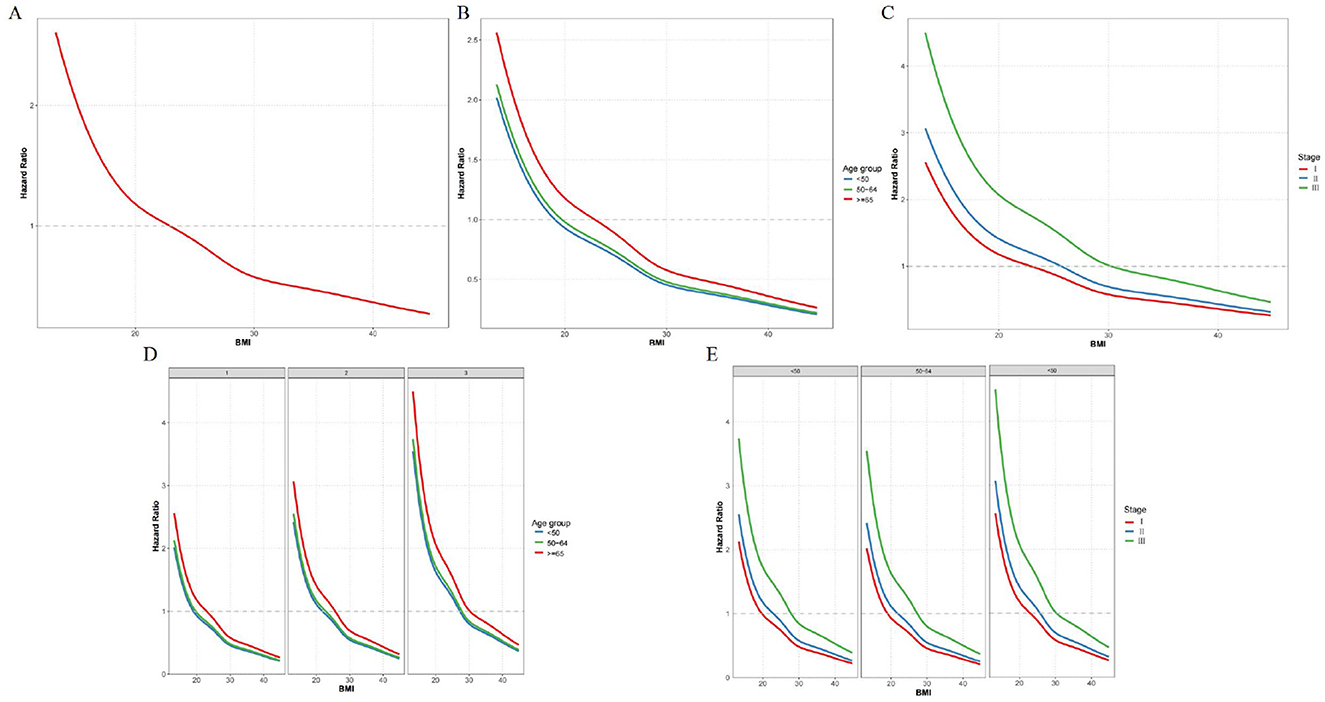
Figure 2. Combined effects of BMI, age, and stage on mortality risk. (A) BMI and mortality risk; (B) BMI and mortality risk by age group; (C) BMI and mortality risk by stage; (D) BMI and mortality risk by TNM stage stratified by age group; (E) BMI and mortality risk by age group stratified by TNM stage.
Further stratified analysis by both age and stage reveals more detailed patterns. In Stage I, the decline in HR with increasing BMI is most pronounced in patients aged 65 and older, while younger patients (< 50) and those aged 50–64 show a less steep decline. In Stage II, all age groups show a moderate decline in HR with higher BMI, but the slope is steeper in the ≥65 group than in the younger groups. In Stage III, the decline in HR with increasing BMI is most pronounced in the oldest age group (≥65; Figure 2D). In Stage I, the HR decreases sharply with increasing BMI in patients aged 65 and older, while the decline is less steep in younger patients (< 50) and those aged 50–64. In Stage II, all age groups show a moderate decline in HR with higher BMI, but the slope is steeper in the ≥65 group than in the younger groups. In Stage III, the HR decreases with increasing BMI, but the curve flattens at higher BMI values across all age groups (Figure 2E).
3.4 Effects of BMI, age and stage on overall survival
The impact of BMI on overall survival in colorectal cancer patients varies across different age groups and TNM stages. Higher BMI is associated with better overall survival across the entire study population (p < 0.0001; Figure 3A). In patients younger than 50, there is no significant difference in overall survival between BMI groups (p = 0.17; Figure 3B). In the 50–64 age group, higher BMI is associated with significantly better overall survival (p = 0.041; Figure 3C). For those 65 and older, there is a significant association between higher BMI and better survival (p < 0.0001; Figure 3D). In Stage I patients, there is no significant difference in survival between BMI groups (p = 0.12; Figure 3E). Stage II patients show no significant difference in survival between BMI groups (p = 0.095; Figure 3F). In Stage III patients, higher BMI is associated with significantly better survival (p < 0.0001; Figure 3G).
Figure 3. (A) K-M curves for overall survival across different BMI groups. (B) K-M curves for overall survival by BMI groups in patients aged < 50. (C) K-M curves for overall survival by BMI groups in patients aged 50–64. (D) K-M curves for overall survival by BMI groups in patients aged ≥65. (E) K-M curves for overall survival by BMI groups in Stage I patients. (F) K-M curves for overall survival by BMI groups in Stage II patients. (G) K-M curves for overall survival by BMI groups in Stage III patients.
4 Discussion
This study investigated the combined effects of BMI, age, and TNM stage on mortality risk in CRC patients. The primary findings were that higher BMI was associated with lower mortality risk across the entire study population, and this relationship was modified by age and TNM stage. Specifically, the protective effect of higher BMI was most pronounced in patients aged 65 and older, as well as in those with Stage III.
The relationship between BMI and CRC prognosis has been extensively studied, yet consensus remains elusive (14, 15). Our findings contribute to the ongoing debate about the “obesity paradox” in CRC. Some previous studies have reported similar protective effects of higher BMI on survival outcomes (10, 16, 17). For instance, a study by Jonathan et al. found that higher BMI was associated with better overall survival in CRC patients, particularly in those with Stage III disease (18). This is consistent with our results showing the most significant survival benefit in Stage III patients. Another study by Bette et al. also observed a survival advantage for overweight and obese CRC patients, suggesting that the obesity paradox might be a real phenomenon in specific patient populations (18, 19).
Conversely, other studies have reported conflicting results, indicating that higher BMI is associated with increased mortality risk in CRC patients (20). The inverse BMI-mortality association might partly reflect survivor bias—where a lower BMI captures individuals who experienced unintentional weight loss or who had previously lost weight because of occult disease—rather than a causal protective effect of adiposity itself. BMI also functions as a surrogate for fat-free mass and overall cardiorespiratory reserve, so the observed benefit could stem from preserved lean tissue rather than excess fat. Moreover, BMI cannot delineate body-fat percentage, visceral adipose distribution, or metabolic derangements, leading to misclassification of sarcopenic obesity and normal-weight obese phenotypes (21). This discrepancy might be due to differences in study populations, sample sizes, and methodological approaches (22). Our study's strength lies in its large sample size and comprehensive analysis of the interaction between BMI, age, and TNM stage, providing more nuanced insights into this complex relationship.
The mechanisms underlying the obesity paradox in CRC remain unclear, but several hypotheses have been proposed (14). One possible explanation is that higher BMI may be associated with a better nutritional reserve, which could help patients tolerate cancer treatments better and recover from complications more effectively (23, 24). Additionally, adipose tissue might have immunomodulatory effects that influence tumor biology and progression (25, 26). Furthermore, the protective effect of higher BMI could be related to differences in body composition, with higher muscle mass potentially contributing to better outcomes (27).
The modifying effects of age and TNM stage observed in our study might be attributed to biological and physiological differences across age groups and disease stages (28, 29). Older patients (≥65 years) with higher BMI might have a more favorable tumor biology and better prognosis (11). In advanced stages like Stage III, the impact of BMI on survival might be more pronounced due to the greater metabolic demands of the disease and the potential benefits of greater nutritional reserves (30, 31). This relationship was further supported by the analysis of overall survival. Higher BMI was associated with better overall survival across the entire study population (p < 0.0001). In patients aged 65 and older, there was a significant association between higher BMI and better survival (p < 0.0001). In Stage III patients, higher BMI was associated with significantly better survival (p < 0.0001). In our cohort, the relatively small proportion of Stage I cases among patients aged ≤ 50 years may be related to the distinct clinicopathological characteristics of early-onset colorectal cancer, which often presents with non-specific symptoms and exhibits more rapid progression, potentially leading to diagnosis at more advanced stages (13). In the absence of cancer-specific survival (CSS) and disease-free survival (DFS) data, our conclusions are based on all-cause death; future prospective studies with complete cause-of-death ascertainment and DFS data are warranted to determine whether the observed BMI survival benefit persists when CRC-related deaths are analyzed separately and when recurrence-free outcomes are considered.
The demographic characteristics of our study population revealed several notable patterns. Males constituted 59.6% of the study cohort, and the mean age at diagnosis was 61.1 years. These figures align with established epidemiological data indicating that CRC is more prevalent in males and typically diagnosed in older adults (32, 33). This study included CRC patients who had not received neoadjuvant chemoradiotherapy before surgery, as such treatment might influence patients' BMI (34, 35). The study also excluded patients with metabolic diseases and those using medications that could affect BMI, minimizing confounding factors related to BMI's influence on prognosis (36, 37). The proportion of male patients increased with rising BMI, a trend that has been previously documented and may reflect hormonal or metabolic differences between genders (38, 39). The lack of significant differences in other indicators across BMI groups suggests that BMI independently influences mortality risk, irrespective of factors like tumor size or lymph node involvement.
Our multivariate Cox regression analysis provided valuable insights into the relationships between key prognostic factors and mortality risk. Each unit increase in BMI was associated with a 7% decrease in mortality risk, reinforcing the obesity paradox. This finding persisted after adjusting for potential confounders such as age, sex, and cancer stage, indicating a robust association (17). Age emerged as a significant prognostic factor, with mortality risk increasing by 2.5% per year. This underscores the biological impact of aging on cancer progression and survival (40, 41). Tumor size and the number of positive lymph nodes were also strongly associated with mortality risk, highlighting their clinical relevance in risk stratification. Each additional harvested lymph node was associated with a 2.4% decrease in mortality risk, emphasizing the importance of thorough lymph node assessment during surgery (42, 43). The protective effect of dMMR status and the increased risk associated with poorly differentiated tumors further validate the biological significance of tumor genetics and histology in prognosis (44, 45). These findings align with existing knowledge and reinforce the need for comprehensive tumor characterization in clinical practice.
Despite the strengths of this study, including its large sample size and detailed analysis, several limitations should be acknowledged. First, as a retrospective study, it is subject to selection and information biases inherent in this design. Second, detailed information on body composition—such as skeletal muscle mass, visceral and subcutaneous fat distribution—was unavailable, which precluded us from distinguishing sarcopenic from non-sarcopenic obesity and may have obscured the true relationship between adiposity and survival. Third, patients who received neoadjuvant chemoradiotherapy were excluded because such treatment can acutely lower BMI, induce sarcopenia, and independently alter tumor biology and stage, thereby acting as a potential confounder; although this exclusion enhances internal validity, it limits the generalisability of our findings to contemporary populations in whom neoadjuvant therapy is standard. Fourth, data on lifestyle factors (physical activity, diet, smoking) were incomplete and may have introduced residual confounding. Future prospective cohorts that incorporate serial body-composition assessments and comprehensive treatment and lifestyle data in diverse populations are warranted to validate and extend these findings. Fifth, although our cohort was drawn from two high-volume tertiary centers, the study remains restricted to patients who underwent curative-intent surgery for Stage I–III disease. Consequently, non-surgical and metastatic (Stage IV) CRC cases were not represented, which may limit the generalizability of our findings to the broader CRC population.
This study highlights the complex interplay between BMI, age, and TNM stage in influencing mortality risk in CRC patients. The findings suggest that the obesity paradox is not uniform across all patient subgroups and should be interpreted within the context of individual patient characteristics. These results can help inform clinical decision-making and risk stratification, ultimately improving the prognosis and management of CRC patients.
5 Conclusions
Higher BMI is associated with lower mortality risk in colorectal cancer patients, particularly in those aged 65 and older and those with Stage III disease. These findings highlight the importance of considering BMI, age, and TNM stage jointly in clinical practice for CRC patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of the Air Force Military Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YQ: Writing – original draft. BK: Writing – original draft, Conceptualization. YJ: Data curation, Methodology, Writing – review & editing. ZZ: Data curation, Writing – original draft. BH: Data curation, Writing – original draft. JS: Data curation, Writing – original draft. HM: Investigation, Writing – original draft. SL: Methodology, Writing – original draft. YD: Software, Writing – original draft. QW: Data curation, Writing – original draft. YG: Data curation, Writing – original draft. SQ: Investigation, Writing – original draft. ZT: Conceptualization, Writing – review & editing. JZ: Data curation, Writing – review & editing. YH: Data curation, Writing – review & editing. JL: Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China [82172781], funded by Shaanxi special support plan scientific and technological innovation leader [4457475042BR2200OY], funded by National Clinical Research Center for Digestive Diseases [CBSKL2022ZZ44], and funded by Shaanxi Provincial Health Scientific Research Innovation Team Project [2024TD-06], and Scientific and Technological Innovation Team of Shaanxi Innovation Capability Support Plan (2023-CX-TD-67).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AJCC, American Joint Committee on Cancer; BMI, Body Mass Index; CI, Confidence interval; CRC, Colorectal Cancer; HR, Hazard ratio; IQR, Interquartile range; SD, Standard Deviation; SMDs, Standardized mean differences; TNM stage, Tumor-Node-Metastasis Staging System.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JR, et al. The global syndemic of obesity, undernutrition, and climate change: the lancet commission report. Lancet. (2019) 393:791–846. doi: 10.1016/S0140-6736(19)30310-1
3. Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. (2019) 69:88–112. doi: 10.3322/caac.21499
4. Li H, Boakye D, Chen X, Jansen L, Chang-Claude J, et al. Associations of body mass index at different ages with early-onset colorectal cancer. Gastroenterology. (2022) 162:1088–97.e3. doi: 10.1053/j.gastro.2021.12.239
5. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
6. Loomans-Kropp HA, Umar A. Analysis of body mass index in early and middle adulthood and estimated risk of gastrointestinal cancer. JAMA Netw Open. (2023) 6:e2310002. doi: 10.1001/jamanetworkopen.2023.10002
7. Bailly L, Fabre R, Pradier C, Iannelli A. Colorectal cancer risk following bariatric surgery in a nationwide study of French individuals with obesity. JAMA Surg. (2020) 155:395–402. doi: 10.1001/jamasurg.2020.0089
8. Tu H, McQuade JL, Davies MA, Huang M, Xie K, Ye Y, et al. Body mass index and survival after cancer diagnosis: a pan-cancer cohort study of 114 430 patients with cancer. Innovation. (2022) 3:100344. doi: 10.1016/j.xinn.2022.100344
9. Paragomi P, Zhang Z, Abe SK, Islam MR, Rahman MS, Saito E, et al. Body mass index and risk of colorectal cancer incidence and mortality in Asia. JAMA Netw Open. (2024) 7:e2429494. doi: 10.1001/jamanetworkopen.2024.29494
10. Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. (2016) 2:1137–45. doi: 10.1001/jamaoncol.2016.0732
11. Hsu YJ, Yu YL, Jhuang JR, You JF, Liao CK, Tsai WS, et al. Comparison of laparoscopic and open surgery for colorectal malignancy in obese patients: a propensity score-weighted cohort study. Int J Surg. (2024) 110:4598–607. doi: 10.1097/JS9.0000000000001536
12. Mandic M, Safizadeh F, Niedermaier T, Hoffmeister M, Brenner H. Association of overweight, obesity, and recent weight loss with colorectal cancer risk. JAMA Netw Open. (2023) 6:e239556. doi: 10.1001/jamanetworkopen.2023.9556
13. Gao XH, Li J, Liu LJ, Zheng NX, Zheng K, Mei Z, et al. Trends, clinicopathological features, surgical treatment patterns and prognoses of early-onset versus late-onset colorectal cancer: a retrospective cohort study on 34067 patients managed from 2000 to 2021 in a Chinese tertiary center. Int J Surg. (2022) 104:106780. doi: 10.1016/j.ijsu.2022.106780
14. Li Y, Li C, Wu G, Yang W, Wang X, Duan L, et al. The obesity paradox in patients with colorectal cancer: a systematic review and meta-analysis. Nutr Rev. (2022) 80:1755–68. doi: 10.1093/nutrit/nuac005
15. Mandic M, Li H, Safizadeh F, Niedermaier T, Hoffmeister M, Brenner H. Is the association of overweight and obesity with colorectal cancer underestimated? An umbrella review of systematic reviews and meta-analyses. Eur J Epidemiol. (2023) 38:135–44. doi: 10.1007/s10654-022-00954-6
16. Becerra-Tomás N, Markozannes G, Cariolou M, Balducci K, Vieira R, Kiss S, et al. Post-diagnosis adiposity and colorectal cancer prognosis: a global cancer update programme (CUP Global) systematic literature review and meta-analysis. Int J Cancer. (2024) 155:400–25. doi: 10.1002/ijc.34905
17. Walter V, Jansen L, Hoffmeister M, Ulrich A, Roth W, Bläker H, et al. Prognostic relevance of prediagnostic weight loss and overweight at diagnosis in patients with colorectal cancer. Am J Clin Nutr. (2016) 104:1110–20. doi: 10.3945/ajcn.116.136531
18. Kocarnik JM, Chan AT, Slattery ML, Potter JD, Meyerhardt J, Phipps A, et al. Relationship of prediagnostic body mass index with survival after colorectal cancer: stage-specific associations. Int J Cancer. (2016) 139:1065–72. doi: 10.1002/ijc.30163
19. Caan BJ, Cespedes Feliciano EM, Kroenke CH. The importance of body composition in explaining the overweight paradox in cancer-counterpoint. Cancer Res. (2018) 78:1906–12. doi: 10.1158/0008-5472.CAN-17-3287
20. Simillis C, Taylor B, Ahmad A, Lal N, Afxentiou T, Powar MP, et al. A systematic review and meta-analysis assessing the impact of body mass index on long-term survival outcomes after surgery for colorectal cancer. Eur J Cancer. (2022) 172:237–51. doi: 10.1016/j.ejca.2022.05.020
21. Donini LM, Pinto A, Giusti AM, Lenzi A, Poggiogalle E. Obesity or BMI Paradox? Beneath the tip of the iceberg. Front Nutr. (2020) 7:53. doi: 10.3389/fnut.2020.00053
22. Carr PR, Amitay EL, Jansen L, Alwers E, Roth W, Herpel E, et al. Association of BMI and major molecular pathological markers of colorectal cancer in men and women. Am J Clin Nutr. (2020) 111:562–9. doi: 10.1093/ajcn/nqz315
23. Cespedes Feliciano E, Chen WY. Clinical implications of low skeletal muscle mass in early-stage breast and colorectal cancer. Proc Nutr Soc. (2018) 77:382–7. doi: 10.1017/S0029665118000423
24. Assumpção J, Pasquarelli-do-Nascimento G, Duarte M, Bonamino MH, Magalhães KG. The ambiguous role of obesity in oncology by promoting cancer but boosting antitumor immunotherapy. J Biomed Sci. (2022) 29:12. doi: 10.1186/s12929-022-00796-0
25. Lee-Rueckert M, Canyelles M, Tondo M, Rotllan N, Kovanen PT, Llorente-Cortes V, et al. Obesity-induced changes in cancer cells and their microenvironment: Mechanisms and therapeutic perspectives to manage dysregulated lipid metabolism. Semin Cancer Biol. (2023) 93:36–51. doi: 10.1016/j.semcancer.2023.05.002
26. Li B, Sun S, Li JJ, Yuan JP, Sun SR, Wu Q. Adipose tissue macrophages: implications for obesity-associated cancer. Mil Med Res. (2023) 10:1. doi: 10.1186/s40779-022-00437-5
27. Cespedes Feliciano EM, Kroenke CH, Caan BJ. The obesity paradox in cancer: how important is muscle. Annu Rev Nutr. (2018) 38:357–79. doi: 10.1146/annurev-nutr-082117-051723
28. Tian YE, Cropley V, Maier AB, Lautenschlager NT, Breakspear M, Zalesky A. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat Med. (2023) 29:1221–31. doi: 10.1038/s41591-023-02296-6
29. Sassun R, Sileo A, Ng JC, Violante T, Gomaa I, Mandrekar J, et al. Validated integration of tumor deposits in n staging for prognostication in colon cancer. JAMA Surg. (2025) 2025:e246729. doi: 10.1001/jamasurg.2024.6729
30. Zhu Y, Lin X, Zhou X, Prochownik EV, Wang F, Li Y. Posttranslational control of lipogenesis in the tumor microenvironment. J Hematol Oncol. (2022) 15:120. doi: 10.1186/s13045-022-01340-1
31. Tan DJH, Ng CH, Muthiah M, Yong JN, Chee D, Teng M, et al. Rising global burden of cancer attributable to high BMI from 2010 to 2019. Metabolism. (2024) 152:155744. doi: 10.1016/j.metabol.2023.155744
32. GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol. (2022) 7:627–47. doi: 10.1016/S2468-1253(22)00044-9
33. Gupta S, May FP, Kupfer SS, Murphy CC. Birth cohort colorectal cancer (CRC): implications for research and practice. Clin Gastroenterol Hepatol. (2024) 22:455–69.e7. doi: 10.1016/j.cgh.2023.11.040
34. Wang H, Jia H, Gao Y, Zhang H, Fan J, Zhang L, et al. Serum metabolic traits reveal therapeutic toxicities and responses of neoadjuvant chemoradiotherapy in patients with rectal cancer. Nat Commun. (2022) 13:7802. doi: 10.1038/s41467-022-35511-y
35. Daveri E, Vergani B, Lalli L, Ferrero G, Casiraghi E, Cova A, et al. Cancer-associated foam cells hamper protective T cell immunity and favor tumor progression in human colon carcinogenesis. J Immunother Cancer. (2024) 12:e009720. doi: 10.1136/jitc-2024-009720
36. Elmaleh-Sachs A, Schwartz JL, Bramante CT, Nicklas JM, Gudzune KA, Jay M. Obesity management in adults: a review. JAMA. (2023) 330:2000–15. doi: 10.1001/jama.2023.19897
37. Liu HY, Eso AA, Cook N, O'Neill HM, Albarqouni L. Meal timing and anthropometric and metabolic outcomes: a systematic review and meta-analysis. JAMA Netw Open. (2024) 7:e2442163. doi: 10.1001/jamanetworkopen.2024.42163
38. Jha I, McMahon GA, Perugino CA, Katz G, Wallace ZS, Fernandes A, et al. Sex as a predictor of clinical phenotype and determinant of immune response in IgG4-related disease: a retrospective study of patients fulfilling the American college of rheumatology-european league against rheumatism classification criteria. Lancet Rheumatol. (2024) 6:e460–8. doi: 10.1016/S2665-9913(24)00089-4
39. Karlsson T, Hadizadeh F, Rask-Andersen M, Johansson Å, Ek WE. Body mass index and the risk of rheumatic disease: linear and nonlinear mendelian randomization analyses. Arthritis Rheumatol. (2023) 75:2027–35. doi: 10.1002/art.42613
40. Chatsirisupachai K, Lagger C, de Magalhães JP. Age-associated differences in the cancer molecular landscape. Trends Cancer. (2022) 8:962–71. doi: 10.1016/j.trecan.2022.06.007
41. Harper EI, Weeraratna AT. A wrinkle in time: how changes in the aging ECM drive the remodeling of the tumor immune microenvironment. Cancer Discov. (2023) 13:1973–81. doi: 10.1158/2159-8290.CD-23-0505
42. Qiao Y, Zhu J, Han T, Jiang X, Wang K, Chen R, et al. Finding the minimum number of retrieved lymph nodes in node-negative colorectal cancer using real-world data and the SEER database. Int J Surg. (2023) 109:4173–84. doi: 10.1097/JS9.0000000000000746
43. Ning FL, Gu WJ, Dai LZ, Du WY, Zeng YJ, Zhang JK, et al. Identification and initial validation of maximal tumor area as a novel prognostic factor for overall and disease-free survival in patients with resectable colon cancer: a retrospective study. Int J Surg. (2023) 109:3407–16. doi: 10.1097/JS9.0000000000000623
44. Emiloju OE, Sinicrope FA. Neoadjuvant immune checkpoint inhibitor therapy for localized deficient mismatch repair colorectal cancer: a review. JAMA Oncol. (2023) 9:1708–15. doi: 10.1001/jamaoncol.2023.3323
Keywords: colorectal cancer, BMI, age, TNM stage, obesity paradox, mortality risk
Citation: Qiao Y, Kang B, Jiang Y, Zhang Z, Hu B, Song J, Ma H, Liu S, Du Y, Wang Q, Guo Y, Qin S, Tan Z, Zhu J, Huang Y and Li J (2025) Higher BMI reduces mortality in elderly and Stage III colorectal cancer patients: insights from a multicenter cohort study. Front. Nutr. 12:1655707. doi: 10.3389/fnut.2025.1655707
Received: 28 June 2025; Accepted: 21 August 2025;
Published: 19 September 2025.
Edited by:
Gahee Song, Kyung Hee University, Republic of KoreaReviewed by:
Silvio Pires Gomes, University of São Paulo, BrazilWenying Qiao, Capital Medical University, China
Yanjun Gao, Wuhan University, China
Copyright © 2025 Qiao, Kang, Jiang, Zhang, Hu, Song, Ma, Liu, Du, Wang, Guo, Qin, Tan, Zhu, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jipeng Li, amlwZW5nbGkxOTc0QGFsaXl1bi5jb20=; Yi Huang, aHkxOTc3QGZtbXUuZWR1LmNu; Jun Zhu, empzdHlAZm1tdS5lZHUuY24=; Zhaobang Tan, YXVzZ3N0dWR5QDE2My5jb20=
†These authors have contributed equally to this work
 Yihuan Qiao
Yihuan Qiao Boyu Kang
Boyu Kang Yu Jiang1,2†
Yu Jiang1,2† Zecheng Zhang
Zecheng Zhang Jiawei Song
Jiawei Song Jun Zhu
Jun Zhu