- 1Faculty of Dentistry, Zarqa University, Zarqa, Jordan
- 2Institute of Basic Medical Sciences, Khyber Medical University, Peshawar, Pakistan
- 3Department of Biological and Health Sciences, Pak-Austria Fachhochschule Institute of Applied Sciences and Technology, Haripur, Pakistan
- 4Institute of Health Sciences, Khyber Medical University, Swat, Pakistan
- 5Department of Visceral Surgery and Medicine, Bern University Hospital, Bern, Switzerland
- 6Maurice Müller Laboratories, Department for Biomedical Research, University of Bern, Bern, Switzerland
Malnutrition, encompassing undernutrition, micronutrient deficiencies, and overnutrition, remain a pervasive global health challenge. This underprivileged condition contributes significantly to worldwide morbidity and mortality and causes profound impairments in growth, development, immune function, and metabolic health. Understanding the underlying biological mechanisms is critical, and animal models are indispensable tools for dissecting these complex pathways and for evaluating potential nutritional interventions under controlled conditions that are infeasible in humans. This literature review comprehensively examines rodent models and explores other diverse animal models used to investigate malnutrition, ranging from invertebrates (e.g., Drosophila) and fish (zebrafish) to mammals (piglets and non-human primates). We highlight how each model has yielded mechanistic insights into malnutrition-induced pathophysiology, i.e., from altered metabolic signaling to immune dysfunction and critically evaluate their strengths and limitations in replicating the multifactorial nature of human malnutrition. Key considerations include the extent to which each model mimics human nutritional deficits or excesses, appropriate developmental stages, species-specific metabolic differences, and the influence of comorbid factors such as infection or gut microbiome alterations. We emphasize translational relevance by identifying where animal-derived findings align with clinical observations and where they diverge, underscoring the challenges in extrapolating preclinical results to human disease. Overall, this review provides a comprehensive resource to guide researchers in selecting appropriate animal models and interpreting their findings, with the ultimate goal of enhancing the translation of preclinical insights into improved strategies to address malnutrition.
1 Introduction
Malnutrition, encompassing both undernutrition and over nutrition, stands as a prevalent global health challenge. Understanding the underlying mechanisms of malnutrition is of paramount importance because it lays the foundation for the creation of interventions that are not only effective but also precisely targeted. In this context, animal models-based research serves as indispensable tools to unravel underlying mechanisms involved in malnutrition. Animal models allow researchers to investigate complex physiological processes, metabolic pathways, and long-term effects of nutritional deficiencies or excesses in controlled environments. These models enable the study of specific nutrient interactions, gene expression changes, and systemic responses to malnutrition that would be challenging or unethical to examine in human subjects. By replicating various forms of malnutrition in animals, scientists can elucidate the underlying molecular and cellular mechanisms, identify potential biomarkers, and test novel therapeutic approaches. Furthermore, animal studies provide valuable insights into the developmental consequences of malnutrition, particularly during critical growth periods, which can inform strategies for preventing and mitigating long-term health impacts on humans. However, it is essential to acknowledge the inherent limitations of animal models. The physiological differences between animals and humans, as highlighted in the literature, necessitate careful interpretation and extrapolation of findings. It is currently accepted that several factors such as species-specific metabolic rates, digestive systems, and immune responses, can influence the manifestation and progression of malnutrition. Therefore, a critical evaluation of each model’s relevance to human conditions is paramount.
This review provides a comprehensive exploration of animal models that are currently employed in the study of malnutrition, with a specific focus on highlighting their individual strengths, limitations and translational potential offering a unique resource over existing literature by integrating recent omics and microbiome insights to guide evidence-based interventions for global health challenges.
2 Malnutrition overview
2.1 Malnutrition definition and types
Malnutrition refers to the deficiencies or excesses in nutrient intake, an imbalance of essential nutrients, or impaired nutrient utilization. Over the last two decades, the definition of malnutrition has gradually evolved leaving a significant impact on advancing the understanding of malnutrition at both academic and clinical levels (1). Generally, the definition of malnutrition is limited to the deficiencies, excesses, or imbalances of energy and/or nutrients of an individual (2). While The Lancet has covered various aspects of malnutrition at length, the comprehensive definition from the World Health Organization (WHO) addresses both undernutrition and over nutrition (2). WHO categorize malnutrition into three broad categories: (a) undernutrition including stunting, wasting and underweight (b) micronutrients malnutrition encompassing deficiency or excess of one or more micronutrients such as vitamins and minerals, and (c) overweight including obesity and diet-related, non-communicable diseases (such as heart disease, stroke, diabetes, and some cancers) (3). Throughout this review, ‘malnutrition’ serves as the overarching term encompassing undernutrition (e.g., stunting, wasting), over nutrition (e.g., obesity), and micronutrient imbalances, with specific sub-terms used for precision.
2.2 Global burden of malnutrition
Malnutrition is still a global issue of public health concerns despite a gradual decline in prevalence over the last three decades. According to the latest WHO estimates, around 2.5 billion adults across the world were overweight and 390 million were underweight in 2022. Malnutrition was also common in children under 5 years of age with approximately 149 million stunted, 45 million wasted and 37 million overweight or obese children globally, during the same period. Malnutrition consequences are devastating, especially in children with around 45 million under five affected by wasting worldwide (4). The burden of malnutrition is greatest in low and middle-income countries with South Asia and Sub-Saharan Africa being the hardest hit region of the world. It is estimated that around two-thirds of the 150.2 million stunted children reside in these two regions only (5). More recently, low-income and middle-income countries are faced with a rising problem of the double burden of malnutrition. The phenomena, characterized by the coexistence of under and overnutrition, at the same time, affect one third of all LMICs posing significant threat to the health and well-being of the masses. Another closely associated problem is the adverse impact of recent poly-crises (existence of more than one major crises at the same time such as war, climate change, pandemic, inflation and food insecurity) on chronic malnutrition which will likely further exacerbate the existing situation (6). Successful tackling of malnutrition problem require evidence based, cost effective, and sustainable interventions.
2.3 Impact of malnutrition on health
Malnutrition has a significant impact human health at every stage of life. During early life, malnutrition contributes to nearly 45% deaths in children under five years of age, largely by weakening immune defenses and increasing vulnerability to infections (7). Beyond immediate mortality risk, malnutrition in early life has severe short-term effects on growth, development, and overall health and wellbeing. A child who is malnourished experiences impaired physical growth (manifesting as low weight-for-age and height-for-age), recurrent illnesses due to immune suppression, and delayed cognitive and motor development (8). For example, undernourished children exhibit higher rates of developmental delay and infections such as pneumonia and diarrheal disease. If acute malnutrition is not treated on time, these early insults can become chronic, leading to stunting, a state of irreversible growth failure associated with long-term deficits in cognition and school performance. Indeed, poor nutrition during the first 1,000 days of life (from conception through toddlerhood) can cause permanent reductions in a child’s cognitive ability and educational attainment. A recent systematic review confirmed that childhood undernutrition is strongly associated with impaired neurodevelopment, lower IQ and academic achievement, and behavioral problems (9). In turn, these deficits translate to diminished productivity and economic potential in adulthood (10). In adolescents and adults, chronic undernutrition leads to muscle wasting, weakness, and fatigue, which reduces work capacity and quality of life. The immune system is compromised at all ages, making malnourished individuals of any age more susceptible to diseases such as tuberculosis and other infections (11). Pregnant women who are malnourished are more likely to give birth to low-birth-weight infants, perpetuating an intergenerational cycle of poor health. The long-term consequences of early-life malnutrition extend into adulthood in myriad ways. Childhood stunting has “long-lasting physiologic effects” and is associated with increased risks of adult-onset conditions such as obesity, type 2 diabetes, and cardiovascular disease (12). These findings support the concept of developmental programming, whereby early nutritional deprivation permanently alters metabolism and organ function. For example, survivors of severe famine in utero or early childhood have shown higher rates of hypertension, glucose intolerance, and other metabolic disorders in mid-life (13). Such outcomes are thought to arise when a body conditioned to scarcity (undernutrition) is later exposed to energy sufficiency or excess, leading to a mismatch that overwhelms homeostatic capacity.
Importantly, malnutrition involves not only macronutrient deprivation (protein-calorie undernutrition) but often multiple micronutrient deficiencies. Lacking essential vitamins and minerals produces classic syndromes that compound health problems. Iron deficiency, for instance, causes anemia and fatigue, while vitamin A deficiency leads to night blindness and impairs immunity, increasing infection risk. In malnourished children, deficiencies of zinc and vitamin D contribute to poor immune function, stunted bone growth (rickets in children), and greater infection severity (14–16). Iodine deficiency in pregnancy can result in cretinism – severe, irreversible neurodevelopmental impairment in the child, underscoring how specific nutrient deficits during critical periods devastate human capital (17). These insights emphasize that malnutrition’s effects span from molecular deficits (vitamins, minerals) to whole-body dysfunction, affecting virtually every organ system. In summary, malnutrition in early life impairs growth, immune defense, and cognitive development in the short term, and sets the stage for chronic health issues and socio-economic disadvantages in the long term.
3 Animal models in nutrition research
3.1 Historical context of animal models in nutritional science
The use of animal models in nutrition research dates back to the early 19th century when François Magendie, in 1816, demonstrated that dogs fed on a diet devoid of protein (nitrogen) could not sustain life thus establishing the essential role of dietary proteins in health (18). By the late 19th century, animal model-based research played a pivotal role in identifying certain unknown dietary factors later known as vitamins for their crucial role in health and disease. For example, Christiaan Eijkman, in 1890, observed that chickens fed only polished rice developed neuropathy (resembling human beriberi), which was cured by restoring rice bran, leading to the discovery of thiamine (vitamin B₁) (19). In 1907, Axel Holst and Theodor Frölich induced scurvy in guinea pigs, one of the few animals like humans, that require dietary vitamin C, thereby validating a model for vitamin C deficiency (20). Similarly, dogs were used in the 1920s to identify niacin as the missing nutrient in pellagra (“black tongue” in dogs) (21). Rats quickly became a mainstay of early 20th-century nutrition science, leading to the discovery of fat-soluble and water-soluble vitamins by carefully controlled feeding studies (22). These foundational experiments established animal models as indispensable tools in defining malnutrition not just as caloric deprivation but as specific nutrient deficiencies.
3.2 Rationale for using animal models in malnutrition studies
Animal models remain crucial in malnutrition research because they allow controlled mechanistic investigations that would be impossible or unethical in humans. Current knowledge about nutrient functions, requirements, and interactions is based largely on animal studies under defined diets. In contrast to human studies, animal experiments can isolate one variable (e.g., protein intake or a single micronutrient) and rigorously test its effect on physiology, thereby revealing causal relationships (23). For example, experimental models have been used to demonstrate how protein or micronutrient deficiencies impair immune development and host defense, or how early-life undernutrition can program long-term metabolic outcomes (24, 25). With animals, researchers can perform frequent sampling of tissues and observe effects on organ systems (brain, liver, immune organs, etc.) across the lifespan or even across generations in a relatively short time. Such studies have provided mechanistic insight into the links between malnutrition and outcomes like stunted growth, cognitive deficits, and altered immunity. Another key rationale is translational research: animal models serve as a bridge to human applications. Promising nutritional interventions (therapeutic diets, micronutrient supplements, microbiome therapies, etc.) can be first tested for efficacy and safety in animals (26). Indeed, many therapeutic foods and supplement strategies were optimized in animal trials before being applied clinically. The ability to control experimental conditions tightly (genetics, environment, diet composition) in animal models yields high-quality data that, with careful interpretation, can guide human studies (27). In sum, animal models offer a level of experimental precision and the opportunity for invasive analysis (e.g., organ histology, gene expression) that together provide invaluable insight into the mechanisms of malnutrition and potential interventions.
3.3 Ethical considerations and regulatory frameworks
The use of animals in malnutrition research carries important ethical obligations. Researchers must ensure that studies are justified and humane, adhering to the 3Rs principle: Replacement, Reduction, Refinement. Replacement means using non-animal alternatives whenever possible, for instance, cell cultures or computer models, although for integrated whole-body nutritional effects, animal models are often still necessary. Reduction refers to using the minimum number of animals required to achieve scientific objectives, employing good experimental design and statistical power analysis to avoid waste. Refinement involves minimizing pain and distress for the animals: in malnutrition experiments, this can include careful monitoring of body condition, setting humane endpoints (e.g., stopping a dietary restriction if an animal loses a certain percentage of body weight or shows signs of severe illness), and providing supportive care as appropriate. Since severe nutrient deprivation can cause suffering, researchers must balance scientific aims with animal welfare, perhaps by inducing milder degrees of malnutrition or shorter durations if that suffices to model the condition. These 3Rs principles, first proposed by Russell and Burch in 1959, have become enshrined in legislation and guidelines governing animal research (28). Institutional Animal Care and Use Committees (IACUCs) or ethics boards oversee malnutrition studies to ensure compliance with welfare standards and that no feasible alternative exists. Species choice is also an ethical consideration: scientists preferentially use less sentient or simpler organisms (e.g., rodents or fish) in place of higher primates unless the research question absolutely requires a close human analog (29). For example, while a macaque model might yield unique data on cognitive development under malnutrition, a rodent is typically used for initial studies, given the much lower ethical and financial burden. All animal experiments are conducted under licenses or regulations that demand humane housing, feeding, and handling. In practice, implementing ethical frameworks not only protects animals but also improves science: refined techniques and healthy, unstressed animals yield more reliable, interpretable results. In summary, the scientific rationale for animal models must always be weighed against ethical imperatives, and researchers are duty-bound to design malnutrition studies that maximize knowledge gained while minimizing harm to animals.
4 Commonly used animal models for malnutrition research
A wide range of animal species have been employed to model malnutrition, each offering unique advantages for probing specific aspects of undernutrition, overnutrition, or micronutrient deficiencies. Figure 1 provides an overview of commonly used models, spanning rodents to non-mammalian species, along with their key features and research applications. No single model recapitulates human malnutrition in its entirety; instead, each species contributes complementary insights. Below, we discuss how each model has advanced understanding of malnutrition’s effects and identify species-specific strengths and limitations.
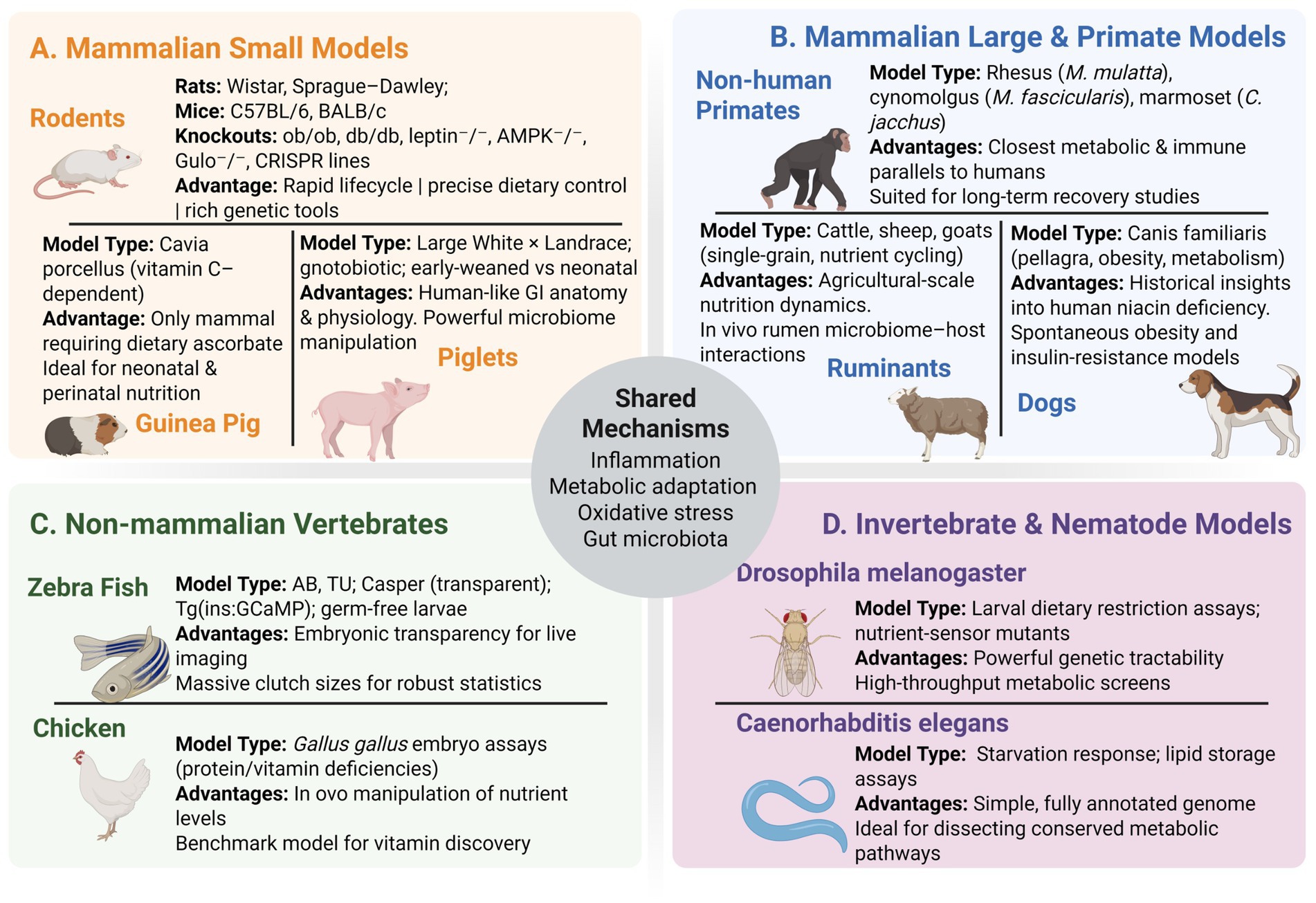
Figure 1. Comprehensive overview of animal models used to study malnutrition, organized by taxonomic group. Mammalian small models (A) include rodents (Wistar, Sprague–Dawley, C57BL/6, BALB/c, and key knock-out lines), guinea pigs (ascorbate-dependent Cavia porcellus), and piglets (conventional, gnotobiotic, early-weaned vs. neonatal), each valued for rapid lifecycles, ascorbate dependency, or human-like gut physiology. Mammalian large and primate models (B) comprise non-human primates (rhesus, cynomolgus, marmoset), dogs (pellagra/obesity models), and ruminants (cattle, sheep, goats), chosen for their translational relevance, historical insights, or rumen microbiome dynamics. Non-mammalian vertebrates (C) cover zebrafish strains (AB, TU, Casper, reporter lines, germ-free larvae) and chicken (Gallus gallus embryo assays), offering live imaging and in-vivo nutrient manipulation. Invertebrate and nematode models (D) include Drosophila melanogaster (dietary restriction assays, nutrient-sensor mutants) and Caenorhabditis elegans (starvation response, lipid storage assays) for high-throughput genetics and conserved metabolic pathway studies. The central hub highlights shared mechanisms: inflammation, metabolic adaptation, oxidative stress, and gut microbiota.
4.1 Rodent models (mice and rats)
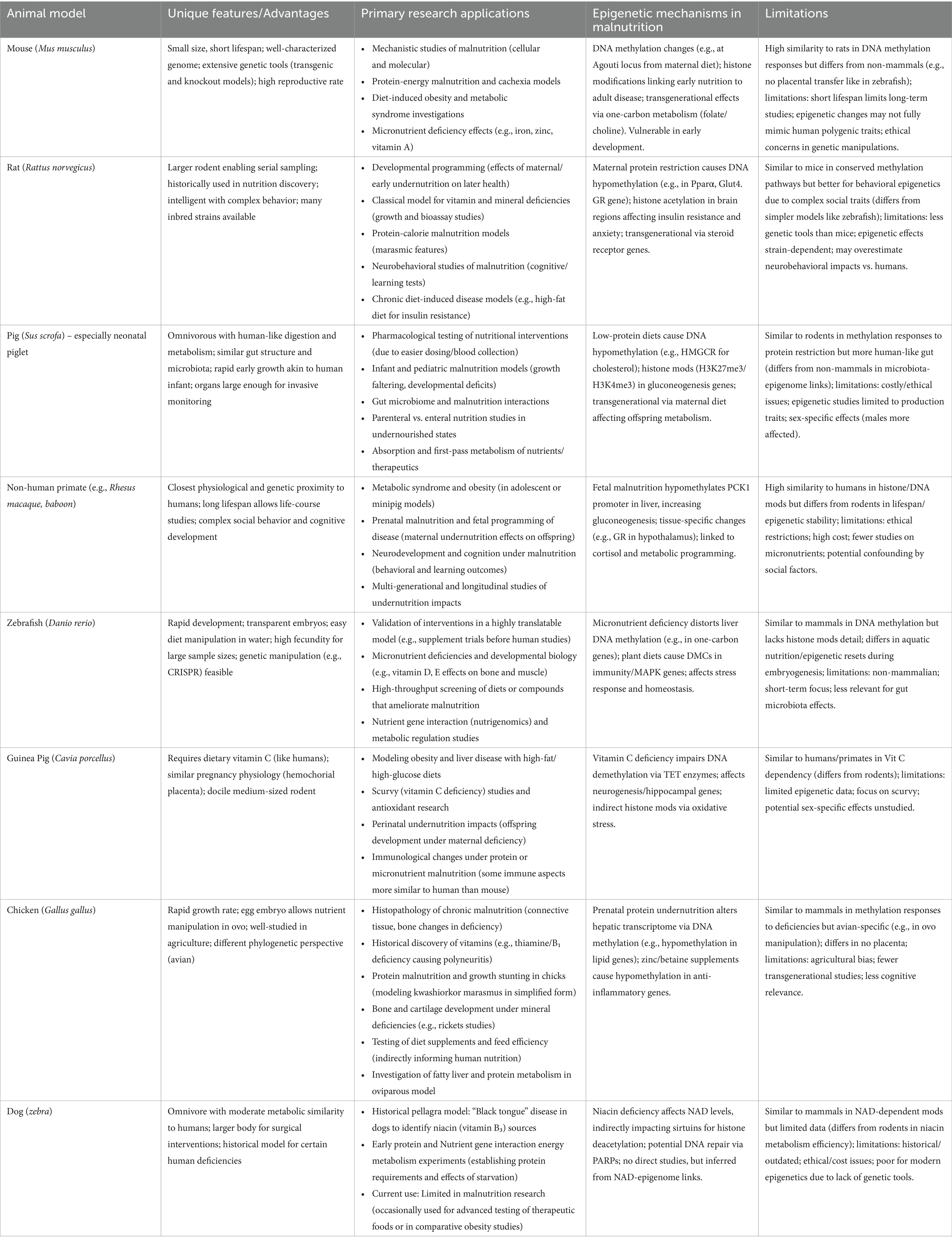
Mice (Mus musculus) and rats (Rattus norvegicus) are the most frequently used laboratory models in nutrition research. Their small size, short reproductive cycles, and relatively low cost allow for large study cohorts and even multigenerational experiments. A particularly powerful feature of mice is their genetic tractability, i-e the availability of inbred strains and modern genomic editing (transgenics, knockouts) enables researchers to dissect gene-nutrient interactions with precision. Rodents share a high degree of physiological and genetic homology with humans, which permits reasonable extrapolation of findings in many cases. For example, mice and rats have been used to elucidate mechanisms of protein-energy malnutrition (PEM) by feeding low-protein or calorie-restricted diets that produce weight loss, stunting, muscle wasting, and immune dysfunction analogous to human (26, 30–32). Such models have confirmed causative links between inadequate protein intake and outcomes like impaired glucose homeostasis and loss of lean body mass. Rodent models have also been central to micronutrient deficiency research: iron-deficient diets in rats (33) induce anemia and cognitive impairment, zinc deficiency in mice (34) leads to growth failure and immune deficits, and vitamin A deficiency (35) in rodents causes vision problems and susceptibility to infection. Notably, many vitamin requirements for humans were first identified by studies in rats. Rodents have further proven invaluable in studying overnutrition and diet-related diseases; high-fat or high-sugar diets in mice can induce obesity, type 2 diabetes, and nonalcoholic fatty liver disease, shedding light on metabolic syndrome under conditions of food excess. Neonatal rodent models (especially mice) are used to mimic early-life malnutrition and have demonstrated how inadequate nutrition during critical windows can disrupt organ development (e.g., brain growth, immune system maturation) and even program metabolic changes that persist into adulthood. Such developmental programming studies in rodents underpin the DOHaD (Developmental Origins of Health and Disease) concept (36). Transgenerational effects have also been examined, for instance, malnourishing pregnant or lactating rodents can produce offspring with long-term deficits, helping to parse how maternal nutrition influences the next generation. Overall, rodent models have contributed to virtually every domain of malnutrition research, from basic nutrient physiology to testing of interventions (such as fortified diets or gut microbiome therapies) in a controlled setting. Table 1 summarizes several mouse models of malnutrition and their characteristics.
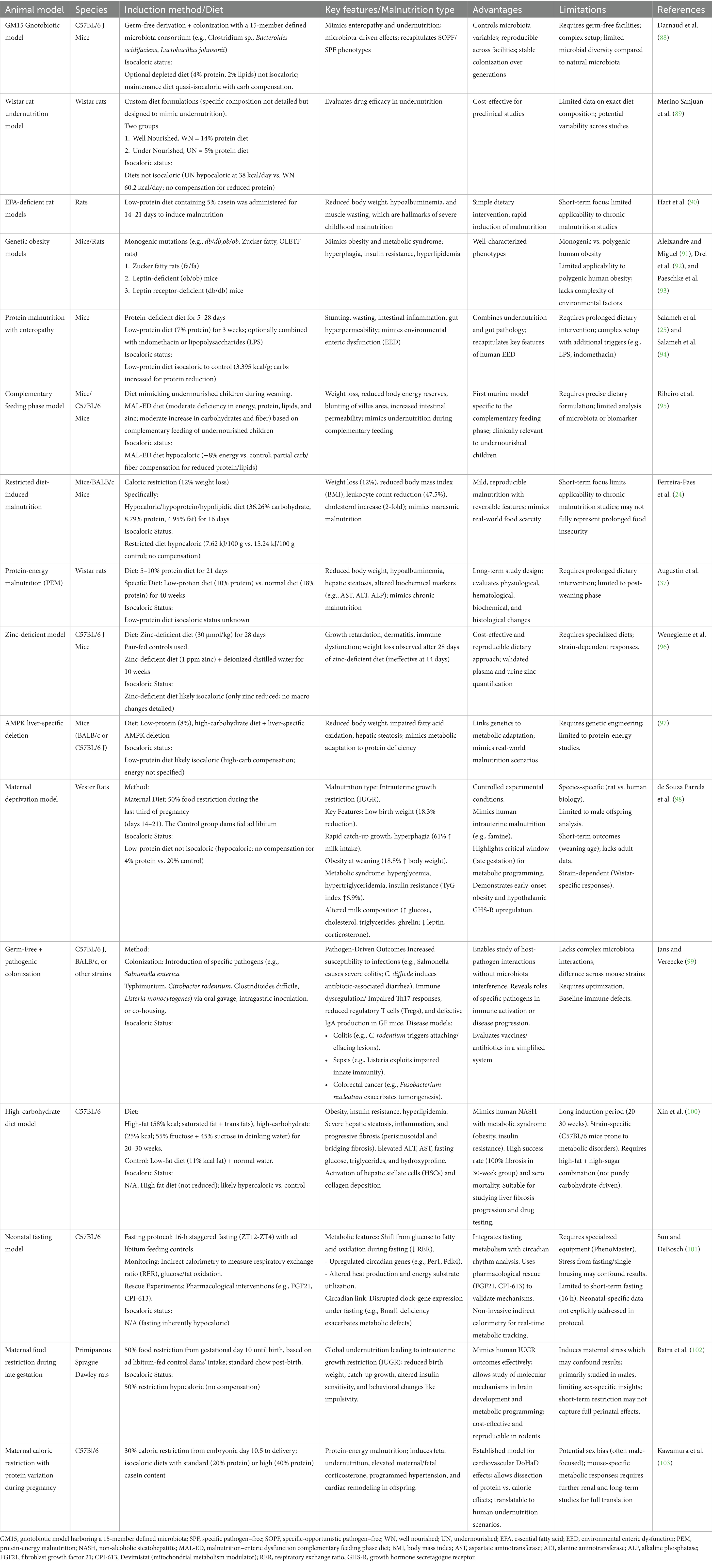
Table 1. Overview of experimentally induced malnutrition and metabolic–immune challenge models in rodents.
Despite their utility, rodents have important limitations. They differ from humans in gut anatomy, basal metabolic rate, and lifespan, which can affect how malnutrition manifests. Crucially, standard laboratory rodents are resistant to some clinical features of severe malnutrition, such as kwashiorkor-like edema is typically not observed in rodent models, even when diets are extremely protein-deficient (37). Rodents also synthesize certain vitamins endogenously (e.g., vitamin C), so they do not naturally develop scurvy; researchers must use special strains (such as Gulo-knockout mice) or other workarounds to study vitamin C deficiency. Furthermore, rodent behavior and cognition, while informative, are not as complex as humans’, limiting their use for studying higher-order neurodevelopmental effects (where larger animals might be preferred). Additionally, notable differences exist between the rodent and human gut microbiome, which can influence malnutrition outcomes. Comparative analyses of rodent and human gut microbiomes reveal marked interspecies divergences in taxonomic composition, functional gene repertoires, and metabolite profiles, with implications for translational validity of preclinical models. Human fecal communities are typically enriched in Bacteroides, Ruminococcaceae, and Clostridiales, exhibiting a higher Firmicutes–Bacteroidetes ratio relative to murine and rat counterparts, which often harbor greater proportions of Lactobacillus and Muribaculaceae (38, 39). Metagenomic comparisons indicate that only ~4% of bacterial genes share substantial sequence identity between the two hosts, underscoring functional disparities despite overlapping genera (38). Short-chain fatty acid patterns also differ, with humans and non-human primates showing closer β-diversity clustering than rodents, and mice generally more similar to humans than rats. Moreover, human-derived microbial consortia engraft with variable efficiency in germ-free rodents, with GF rats more faithfully recapitulating donor Clostridiales composition than GF mice, which exhibit selective enrichment of certain Bacteroides phylotypes (40). These differences mean that findings in mice and rats must be translated to humans with caution. Nonetheless, the ease of manipulating and observing rodents under controlled dietary regimens makes them an indispensable first-line model for malnutrition research.
4.2 Porcine models (pigs)
Pigs (Sus scrofa domesticus), especially young piglets, have emerged as valuable models in nutritional research due to their close anatomical and physiological resemblance to humans. Pig gastrointestinal tracts, digestive functions, and dietary patterns (omnivorous) are more similar to humans than those of rodents. Notably, the neonatal piglet’s developmental trajectory parallels that of human infants in many aspects: newborn pigs have a precocial developmental stage with organ maturity and metabolism comparable to a human newborn, and their brain growth spurt occurs in the early postnatal period, analogous to humans (41). These similarities make piglets an excellent model for pediatric undernutrition. Researchers can use infant piglets to study conditions like infantile malnutrition, intrauterine growth restriction, or prematurity under different feeding regimens. For example, feeding piglets a protein-deficient or micronutrient-deficient diet reliably produces symptoms of growth faltering, impaired gut function, and neurodevelopmental delays, closely mirroring human infant malnutrition. One study demonstrated that protein-restricted piglets had altered gut microbiota composition and weakened intestinal barrier function, providing insight into how malnutrition contributes to the cycle of diarrhea and nutrient malabsorption in children (a phenomenon unethical to induce experimentally in human infants) (42, 43). Pigs have also been extensively used to compare enteral vs. parenteral nutrition strategies in malnourished states; because their size permits surgical catheterization and repeated sampling, neonatal piglets are ideal for testing interventions like fortified formulas, probiotics, or therapeutic foods and observing their effects on organ systems (44). The translational relevance of pig studies is high – many findings on nutrient requirements and metabolism in pigs have directly informed clinical nutrition for human babies. Beyond the infant model, growing or adult pigs (including miniature pig breeds) serve as models of metabolic syndrome and obesity, since they can develop diet-induced obesity with metabolic and cardiovascular complications that resemble those in obese humans. Like humans, pigs deposit both subcutaneous and visceral fat and can exhibit insulin resistance, making them useful for studying overnutrition and related chronic diseases (45).
The pig model’s strengths come with some practical and ethical challenges. Pigs are large and costly to house and feed, and their longer lifespan and gestation (relative to rodents) mean experiments are more time- and resource-intensive (46). Group sizes for pig studies are usually smaller, and specialized facilities are needed for their care. Ethically, pigs are intelligent mammals, raising welfare considerations; studies must ensure proper housing and minimize stress (which can otherwise affect nutritional physiology). While piglets adapt well to laboratory rearing, they require skilled care to mimic maternal nutrition (e.g., feeding with artificial sow milk replacer). Another limitation is that even pigs do not fully recapitulate human malnutrition in the context of complex social and environmental factors; for example, infections common in malnourished children are not automatically present in experimental piglets unless introduced. Nonetheless, when it comes to translational fidelity, the pig is considered one of the best models after primates. Swine studies have been instrumental in bridging the gap between rodent findings and human clinical trials, particularly for pediatric nutrition. In summary, porcine models combine physiological similarity to humans with the ability to conduct invasive and longitudinal studies, thereby greatly enhancing our understanding of malnutrition’s impact on growth, gastrointestinal health, and organ development.
4.3 Non-human primate models
Non-human primates (NHPs) such as rhesus macaques (Macaca mulatta) and baboons (Papio spp.) offer the closest approximation to human biology among animal models of malnutrition. Their digestive system, immune responses, endocrinology, and neurodevelopmental processes are highly similar to humans, and they are capable of complex social and behavioral interactions (47). Because of this, NHP models have provided unique insights, particularly in areas where lower animals are too dissimilar, for example, the long-term cognitive and behavioral consequences of early-life malnutrition. Researchers have conducted studies in which pregnant or lactating monkeys are fed nutrient-restricted diets to examine effects on offspring (48). Prenatal malnutrition in rhesus macaques has been shown to result in infants with significant neurodevelopmental impairments and metabolic alterations (49). In one seminal study, protein-energy malnutrition imposed on pregnant rhesus monkeys led to babies that exhibited delayed neural maturation and behavioral abnormalities, highlighting the fetal origins of cognitive deficits (49, 50). Such primate studies reinforce evidence from human cohorts that maternal undernutrition can have lasting effects on progeny (the developmental origins hypothesis), while offering a controlled experimental confirmation of causality. NHPs have also been used to model postnatal malnutrition, for instance, separating infant monkeys from adequate nutrition during the nursing period has helped investigators track the impact on brain growth, immune development, and even epigenetic changes over their lifespan (51). With the ability to follow individuals for years, NHP models uniquely enable life-course studies of malnutrition. These models can span from infancy into adolescence or adulthood over years or even decades, providing longitudinal insights into malnutrition’s life-course effects, closely mirroring the extended human developmental timeline in NHPs. This has shed light on how early stunting or wasting might predispose individuals to later health problems like obesity, diabetes, or impaired immune responses. Additionally, certain aspects of malnutrition-related pathologies are better replicated in primates: for example, rhesus monkeys on an atherogenic diet can develop arterial lesions more similar to human cardiovascular disease than what occurs in rodent models (52).
Despite these advantages, NHP models are used sparingly and only for the most critical translational questions, due to ethical and practical limitations. Non-human primates are sentient, social, and often endangered animals; experiments on them are subject to stringent ethical scrutiny (46). The 3Rs principle strongly encourages using NHPs only when no other species will suffice. Their care is expensive and requires specialized primate facilities and veterinary expertise. Reproductive rates are slow (one offspring at a time, with long gestation), so sample sizes are inherently limited. Moreover, ethical frameworks typically prevent inducing severe malnutrition in primates; studies may use moderate dietary restriction rather than life-threatening undernutrition, to avoid undue harm. These factors mean that while NHP studies provide high relevance, they are not amenable to high-throughput experimentation. Another limitation is timescale: a monkey’s developmental timeline is much longer than a rodent’s, so studies can take years or decades. For example, to observe multigenerational effects (F1, F2 generations) in primates would be impractical. Nonetheless, even a small number of well-designed primate studies have been profoundly influential (53). They’ve confirmed, in a controlled way, phenomena suspected from human data, such as the link between early malnutrition and impaired cognitive function, lending weight to public health arguments for early nutrition interventions (54). In summary, non-human primates are the closest proxy to human malnutrition and fill an important niche in research, but their use is rightly limited to questions where their unique similarity is indispensable.
4.4 Zebrafish models
Over the past two decades, the small tropical zebrafish (Danio rerio) has become a novel model organism in nutritional science, including malnutrition research. Zebrafish offer several experimental advantages: they are small and inexpensive to maintain, have high fecundity (hundreds of offspring per mating), and develop rapidly (organs form within days). Uniquely, zebrafish embryos and larvae are transparent, allowing direct observation of developmental processes and organogenesis under a microscope. Researchers can easily manipulate the nutrient content of the water or feed to which larval zebrafish are exposed, making it straightforward to create models of deficiency or overnutrition. For example, zebrafish larvae raised in water lacking certain micronutrients will exhibit developmental abnormalities relevant to that deficiency, vitamin D deficiency leads to skeletal malformations and poor bone mineralization in developing zebrafish, recapitulating rickets-like features (55). Zebrafish have also been used to study lipid metabolism; diets high in cholesterol or certain fatty acids can induce vascular changes and fat deposition, providing a proxy for studying hyperlipidemia and obesity on a microscopic scale (56, 57). Notably, zebrafish can develop diet-induced obesity and metabolic perturbations when fed a high-calorie diet, including increased adiposity and insulin-resistant phenotypes (58). These models have been utilized in nutrigenomics investigations to examine how gene expression shifts in response to overnutrition or nutrient scarcity. Another area where zebrafish excel is high-throughput intervention testing, large numbers of zebrafish larvae can be arrayed in multi-well plates to screen combinations of diets or therapeutic compounds that might improve outcomes in malnourished conditions. This approach has been applied to identify nutritional supplements that enhance growth or to test probiotics that might mitigate undernutrition effects, with readouts like growth rate, enzyme expression, or bone development assessed rapidly in vivo (59, 60). Additionally, zebrafish have contributed to understanding transgenerational effects of malnutrition: one study showed that parental micronutrient deficiencies in zebrafish distorted DNA methylation patterns in the liver of their offspring, implicating heritable epigenetic changes similar to those observed in mammals (61, 62). Given their genetic tractability (with tools like CRISPR available), zebrafish allow researchers to knock out or modify genes of interest to see how those changes impact nutritional phenotypes, providing a powerful way to link nutrient sensing pathways with specific genes.
The zebrafish model, while innovative, has limitations stemming from its evolutionary distance from humans. As an aquatic ectotherm, the zebrafish’s metabolism and physiology differ in fundamental ways: for example, they lack lungs, have a two-chambered heart, and excrete nitrogenous waste as ammonia into water, factors that make some aspects of malnutrition (like impacts on pulmonary development or precise basal metabolic rate comparisons) hard to translate (63). Their nutrient absorption occurs directly from water as well as from food, which is unlike human oral intake. Moreover, certain diseases related to malnutrition (such as kwashiorkor’s edema or marasmus-related infections) do not manifest in fish. Thus, while zebrafish are superb for studying basic developmental biology and molecular responses to nutrient variation, researchers must be cautious in extrapolating results to human malnutrition without corroboration in mammalian models. Emphasizing the need for translational validation, zebrafish findings (e.g., on nutrient-gene interactions) must be corroborated in mammalian models like rodents or pigs before human application, due to phylogenetic differences in metabolism and epigenetics (64). In practice, zebrafish often complement, rather than replace, rodent studies: a discovery in zebrafish (e.g., a gene that is activated during nutrient stress) can be followed up in mice for validation. Despite these caveats, the zebrafish’s contribution to malnutrition research is growing, especially in the realm of nutrient-gene interactions and rapid screening. As a high-throughput system, it helps narrow down hypotheses that can later be tested in mammals. In summary, zebrafish exemplify how a non-mammalian model can significantly advance our understanding of malnutrition’s molecular underpinnings and potential interventions, even though direct translational leaps require additional confirmation in more human-like systems.
4.5 Other animal models and considerations
In addition to the above models, several other species have historically played important roles in malnutrition research, though their current use varies. Guinea pigs (Cavia porcellus) were critical in early nutrition science, as they share humans’ inability to synthesize vitamin C. guinea pig experiments first proved that diets lacking fresh fruits or vegetables cause scurvy (65, 66), and they remain the standard model for studying vitamin C deficiency. Guinea pigs have also been used in perinatal nutrition studies, since their placentation and gestation have some similarities to humans (they have a hemochorial placenta), making them a model for studying fetal nutrient restriction and its effects on offspring (67). However, guinea pigs are less commonly used today outside of specific contexts like scurvy or some immunological aspects of malnutrition, in part because they are more expensive and less genetically malleable than rodents. Chickens (Gallus gallus) and other avian species provided early evidence linking diet and disease: Eijkman’s chicken model of beriberi was one example (68), and chick growth assays were widely used in the mid-20th century to discover vitamins (such as vitamin K and folate) and minerals by omitting them from feed and observing deficiency signs. Chicks grow rapidly, which can accentuate the effects of nutrient deficits or excesses, and they have been used to study protein malnutrition as well. In modern research, chickens are mainly utilized in agricultural and veterinary nutrition, but lessons from poultry science (e.g., on the effects of protein or mineral deficiencies on skeletal development) have parallels in human biology. Dogs (Canis lupus familiaris) are another historically significant model: aside from Magendie’s 19th-century protein studies, dogs were used in the early 1900s to study pellagra, where a condition called “black tongue” in dogs proved analogous to pellagra and helped identify niacin/vitamin B₃ as the preventive factor (69). Dogs were also subjects in some classic protein and energy metabolism studies due to their larger size, which allowed serial blood sampling and even fistulas for digestive studies. Today, dogs and cats are rarely used for human malnutrition research (they are more common in pet nutrition or as models for diseases like obesity and diabetes), in part due to ethical reasons and the availability of other models. Ruminants (cattle, sheep, goats) have generally been used to address agricultural malnutrition (e.g., poor pasture leading to protein-energy malnutrition in livestock) and to understand basic nutrient cycles, rather than as models for human malnutrition, their specialized digestive systems (foregut fermentation) make their metabolism quite different from humans. One notable historical example is the single-grain experiment in cattle by Stephen Babcock in 1911, which showed that cows fed only corn vs. only wheat had markedly different health outcomes, foreshadowing the discovery of micronutrients. While such large-animal work informed nutrition science, ruminants are not commonly used to emulate human malnutrition per se (70, 71).
Finally, it is worth mentioning that small invertebrate models have contributed to the fundamental understanding of nutrient sensing and energy balance. The fruit fly Drosophila melanogaster and the nematode C. elegans, for instance, have been used to unravel genetic pathways of hunger, fat storage, and metabolic adaptation to undernutrition (72, 73). These organisms offer unparalleled genetic tools and low-cost, high-throughput experimentation, moreover researchers have evolved populations of flies under chronic malnutrition to study adaptive genetic changes (74), and identified nutrient-sensing hormones and neural circuits that often have mammalian counterparts. However, due to their very simple body plans and major physiological differences, invertebrates serve as discovery engines for molecular mechanisms rather than direct models of human malnutrition. Any findings in flies or worms typically require validation in vertebrate models.
In summary, the landscape of animal models for malnutrition research is diverse. Each species, from mouse to monkey to zebrafish, brings a unique lens through which to investigate the complex problem of malnutrition. Researchers choose a model based on the specific question: mice for detailed mechanism and genetics, pigs for translational gut and infant studies, zebrafish for developmental or genomic screening, and so on. By integrating knowledge across these models, the field gains a more complete picture of how nutrient deficiencies or excesses impact living systems. Importantly, scientists remain aware of species-specific limitations and strive to cross-validate important findings in multiple models, as well as in human observational or clinical studies, to bridge any translational gaps. The table below (Table 2) summarizes key animal models, their special features, and examples of how they contribute to malnutrition research.
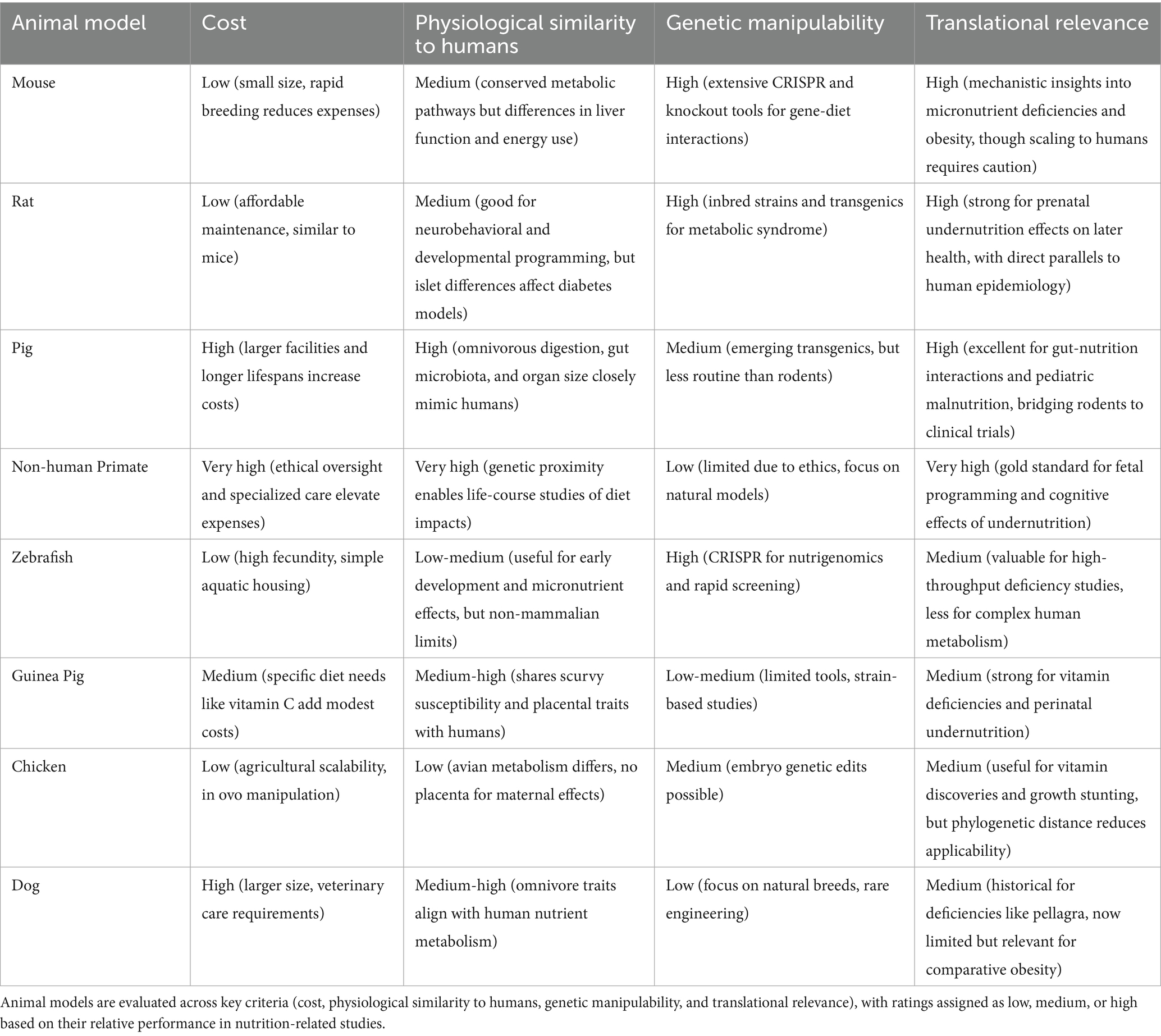
Each model organism above has shed light on different facets of malnutrition. Additionally, Table 3 provides a comparative summary of animal models. Ratings are based on established biomedical literature, reflecting relative assessments (e.g., cost considers housing, breeding, and ethical factors; physiological similarity emphasizes metabolic and digestive parallels to humans) (26, 75–79). By leveraging the unique features of each species, researchers can investigate malnutrition at levels ranging from genes and molecules (mice, zebrafish) to whole-organism physiology and behavior (pigs, primates). The primary applications listed show how each species contributes i-e rodents and zebrafish often uncover mechanisms and potential interventions, whereas pigs and primates strengthen translational relevance. It is through a combination of these models, aligned with ethical use and guided by the 3Rs, that science advances our understanding of malnutrition and informs strategies to alleviate it. Ultimately, findings from animal models must be integrated with human studies to fully bridge the translational gap, but animal research remains a cornerstone for unraveling the complex biology of malnutrition.
5 Future perspective and emerging trends
The use of animal models in malnutrition research is evolving rapidly. With growing advances in biotechnology, molecular biology, and systems science, new frontiers are emerging that promise to significantly enhance the relevance, precision, and translational value of animal studies in nutrition research. Despite limited recent literature directly addressing emerging trends under this specific framing, current research is converging on several key innovations. Genetically modified rodents and gnotobiotic animals, particularly germ-free mice colonized with human microbiota, are being increasingly employed to dissect host-microbiome interactions that influence nutrient absorption and immune regulation in malnourished states (80). Humanized gnotobiotic mouse models, where germ-free mice are colonized with human gut microbiota have revolutionized our understanding of host-microbiota interactions in the context of malnutrition (81). These models are instrumental in studying how microbial communities influence nutrient absorption, immune development, and metabolic programming (82). Omics technologies, including transcriptomics, metabolomics, and proteomics, are augmenting mechanistic insights by enabling the study of systemic responses to nutrient deficiencies at cellular and molecular levels.
The integration of multi-omics platforms such as, transcriptomics, proteomics, metabolomics, and epigenomics, with animal models has opened new avenues to map the molecular cascades affected by malnutrition. These high-resolution techniques allow for comprehensive profiling of changes at the cellular and systemic levels in response to nutritional deprivation or excess. Systems biology approaches facilitate the construction of interaction networks, enabling the identification of key regulatory nodes and potential therapeutic targets (83). These integrative strategies not only deepen our mechanistic understanding but also support the development of predictive models for nutritional outcomes. Translating omics results from animal models to clinical practice requires cost- and time-effective strategies to bridge preclinical insights with human applications. For instance, multi-omics profiling in rodent models of undernutrition has identified epigenetic biomarkers (e.g., altered methylation) that predict metabolic risks, which can be validated in human cohorts via affordable targeted sequencing panels rather than whole-genome omics (84). Costs have plummeted with next-generation sequencing (now <$1,000/genome), enabling scalable biomarker discovery; AI-driven data integration further accelerates analysis from months to days (85). In malnutrition, this could inform personalized interventions, like micronutrient supplements tailored to gut microbiomics, tested first in pigs for translational fidelity before low-cost human trials. Challenges include validation across diverse populations, but fit-for-purpose animal models (e.g., humanized microbiomes) enhance efficiency.
One of the most promising advancements is the development of genetically modified animal models, particularly using CRISPR/Cas9 and other genome-editing tools. These models allow researchers to study specific gene-nutrient interactions and unravel the role of genetic susceptibilities in the manifestation of malnutrition-related phenotypes (86). Such precision models enable the dissection of nutrient-specific pathways and their downstream physiological consequences, contributing to the formulation of targeted nutritional interventions.
While not animal models per se, advances in 3D organoid cultures and bio-printing technologies offer powerful adjuncts to in vivo studies. These systems, derived from animal or human stem cells, can mimic tissue-specific responses to malnutrition in a controlled environment. Organoids of the intestine, liver, or brain, for instance, allow researchers to study tissue-specific nutrient sensing, absorption, and pathology, complementing whole-animal studies and reducing the reliance on live animals in preliminary investigations (87).
Future directions also prioritize the refinement of ethical and translational considerations, advocating for models that more closely recapitulate human pathophysiology, including the use of non-human primates and 3D organoid systems to complement in vivo findings. Collectively, these innovations promise to deepen our mechanistic understanding of malnutrition, guide biomarker discovery, and inform therapeutic and policy interventions for diverse populations.
Author contributions
SM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HA: Methodology, Writing – original draft, Writing – review & editing. MG: Methodology, Writing – original draft, Writing – review & editing. ZA: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. ZA received funding from the European Research Council Starting Grant [WePredict project number: 949613], the Swiss National Science Foundation [SNSF, grant number: 310030_215675; 31ND30_213452; CRSII—222781; IZSEZ0_229993], the Inselspital, the Swiss Cancer Research Foundation [KFS-5691-08-2022], the Helmut & Horten Foundation [Project ID: 2021-YIG-083], the Kenneth Rainin Foundation, the Ruth & Arthur Scherbarth Stiftung, the Novartis Foundation for Medical-Biological Research, the Edoardo R.-, Giovanni, Giuseppe und Chiarina Sassella-Stiftung, and the Jubiläumsstiftung von Swiss Life. Open access funding by University of Bern.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cederholm, T, Jensen, GL, Correia, M, Gonzalez, MC, Fukushima, R, Higashiguchi, T, et al. Glim criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
2. Elia, M. Defining, recognizing, and reporting malnutrition. Int J Low Extrem Wounds. (2017) 16:230–7. doi: 10.1177/1534734617733902
3. Khaliq, A, Wraith, D, Nambiar, S, and Miller, Y. A review of the prevalence, trends, and determinants of coexisting forms of malnutrition in neonates, infants, and children. BMC Public Health. (2022) 22:879. doi: 10.1186/s12889-022-13098-9
4. WHO. (2024). Malnutrition. Available online at: https://www.who.int/news-room/fact-sheets/detail/malnutrition
5. World Bank Group. (2025). The World Bank and nutrition [online]. World Bank Group. Available: https://www.worldbank.org/en/topic/nutrition/overview#1 [Accessed].
6. WHO. (2024). The World Bank and nutrition. Available online at: https://www.worldbank.org/en/topic/nutrition/overview
7. Tharumakunarajah, R, Lee, A, Hawcutt, DB, Harman, NL, and Sinha, IP. The impact of malnutrition on the developing lung and long-term lung health: a narrative review of global literature. Pulm Ther. (2024) 10:155–70. doi: 10.1007/s41030-024-00257-z
8. Fontaine, F, Turjeman, S, Callens, K, and Koren, O. The intersection of undernutrition, microbiome, and child development in the first years of life. Nat Commun. (2023) 14:3554. doi: 10.1038/s41467-023-39285-9
9. Kirolos, A, Goyheneix, M, Eliasz, MK, Chisala, M, Lissauer, S, Gladstone, M, et al. Neurodevelopmental, cognitive, behavioural and mental health impairments following childhood malnutrition: a systematic review. BMJ Glob Health. (2022) 7:e009330. doi: 10.1136/bmjgh-2022-009330
10. Victora, CG, Adair, L, Fall, C, Hallal, PC, Martorell, R, Richter, L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. (2008) 371:340–57. doi: 10.1016/S0140-6736(07)61692-4
11. Méndez López, LF, González Llerena, JL, Vázquez Rodríguez, JA, Medellín Guerrero, AB, González Martínez, BE, Solís Pérez, E, et al. Dietary modulation of the immune system. Nutrients. (2024) 16:4363. doi: 10.3390/nu16244363
12. Soliman, A, De Sanctis, V, Alaaraj, N, Ahmed, S, Alyafei, F, Hamed, N, et al. Early and long-term consequences of nutritional stunting: from childhood to adulthood. Acta Bio Medica. (2021) 92:e2021168. doi: 10.23750/abm.v92i1.11346
13. Li, Y, Jaddoe, VW, Qi, L, He, Y, Wang, D, Lai, J, et al. Exposure to the chinese famine in early life and the risk of metabolic syndrome in adulthood. Diabetes Care. (2011) 34:1014–8. doi: 10.2337/dc10-2039
14. Magee, PJ, and Mccann, MT. Micronutrient deficiencies: current issues. Proc Nutr Soc. (2019) 78:147–9. doi: 10.1017/S0029665118002677
15. Steele, E, Liu, D, and Omer, E. Managing micronutrient deficiencies in high-risk patients: no small feat! Current Nutrition Reports. (2024) 13:668–78. doi: 10.1007/s13668-024-00552-w
16. Zhang, Y-Y, Chen, B-X, and Wan, Q. Global, regional, and national burden of nutritional deficiencies spanning from 1990 to 2021, with a focus on the impacts observed during the Covid-19 pandemic. Front Nutr. (2025) 12:12–2025. doi: 10.3389/fnut.2025.1535566
17. Gernand, AD, Schulze, KJ, Stewart, CP, West, KP Jr, and Christian, P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. (2016) 12:274–89. doi: 10.1038/nrendo.2016.37
18. Carpenter, KJ. A short history of nutritional science: part 1 (1785–1885). J Nutr. (2003) 133:638–45. doi: 10.1093/jn/133.3.638
19. Carpenter, KJ, and Sutherland, B. Eijkman's contribution to the discovery of vitamins. J Nutr. (1995) 125:155–63. doi: 10.1093/jn/125.2.155
20. Carpenter, KJ. The discovery of vitamin C. Ann Nutr Metab. (2012) 61:259–64. doi: 10.1159/000343121
21. Bryan, CS, and Mull, SR. Pellagra pre-goldberger: Rupert blue, Fleming sandwith, and the “vitamine hypothesis”. Trans Am Clin Climatol Assoc. (2015) 126:20.
22. Mozaffarian, D, Rosenberg, I, and Uauy, R. History of modern nutrition science—implications for current research, dietary guidelines, and food policy. BMJ. (2018) 361:k2392. doi: 10.1136/bmj.k2392
23. Baker, DH. Animal models in nutrition research. J Nutr. (2008) 138:391–6. doi: 10.1093/jn/138.2.391
24. Ferreira-Paes, T, Seixas-Costa, P, and Almeida-Amaral, EE. Validation of a feed protocol in a mouse model that mimics Marasmic malnutrition. Front Vet Sci. (2021) 8:757136. doi: 10.3389/fvets.2021.757136
25. Salameh, E, Morel, FB, Zeilani, M, Déchelotte, P, and Marion-Letellier, R. Animal models of undernutrition and enteropathy as tools for assessment of nutritional intervention. Nutrients. (2019) 11:2233. doi: 10.3390/nu11092233
26. Chalvon-Demersay, T, Blachier, F, Tomé, D, and Blais, A. Animal Models for the Study of the Relationships between Diet and Obesity: A Focus on Dietary Protein and Estrogen Deficiency. Front Nutr. (2017), 4:5. doi: 10.3389/fnut.2017.00005
27. Salter, AM. Animal models in nutrition research. Nutrition Research Methodologies. (2015):265–77. doi: 10.1002/9781119180425.ch18
28. National Center for Replacement, Refinement and Reduction of Animal in Research. (2017). The 3Rs [online]. UK: National Center for replacement, refinement and reduction in of animal in research. Available online at: https://nc3rs.org.uk/who-we-are/3rs
29. Naderi, MM, Sarvari, A, Milanifar, A, Boroujeni, SB, and Akhondi, MM. Regulations and ethical considerations in animal experiments: international laws and Islamic perspectives. Avicenna J Med Biotechnol. (2012) 4:114–20.
30. Abubakar, AA, Noordin, MM, Azmi, TI, Kaka, U, and Loqman, MY. The use of rats and mice as animal models in ex vivo bone growth and development studies. Bone Joint Res. (2016) 5:610–8. doi: 10.1302/2046-3758.512.BJR-2016-0102.R2
31. Gąsior, Ł, Pochwat, B, Zaręba-Kozioł, M, Włodarczyk, J, Grabrucker, AM, and Szewczyk, B. Proteomics analysis in rats reveals convergent mechanisms between major depressive disorder and dietary zinc deficiency. Pharmacol Rep. (2025) 77:145–57. doi: 10.1007/s43440-024-00681-7
32. Perlman, RL. Mouse models of human disease: an evolutionary perspective. Evolution Medicine, Public Health. (2016) 2016:170–6. doi: 10.1093/emph/eow014
33. Kamei, A, Watanabe, Y, Ishijima, T, Uehara, M, Arai, S, Kato, H, et al. Dietary iron-deficient anemia induces a variety of metabolic changes and even apoptosis in rat liver: a Dna microarray study. Physiol Genomics. (2010) 42:149–56. doi: 10.1152/physiolgenomics.00150.2009
34. Wong, CP, Rinaldi, NA, and Ho, E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing Il6 promoter demethylation. Mol Nutr Food Res. (2015) 59:991–9. doi: 10.1002/mnfr.201400761
35. Eaton, JS. Ophthalmology of Myodonta: mice, rats, hamsters, gerbils, and relatives In: F Montiani-Ferreira, BA Moore, and G Ben-Shlomo, editors. Wild and exotic animal ophthalmology, vol. 2. Mammals, Cham: Springer International Publishing (2022)
36. Ramírez, V, Bautista, RJ, Frausto-González, O, Rodríguez-Peña, N, Betancourt, ET, and Bautista, CJ. Developmental programming in animal models: critical evidence of current environmental negative changes. Reprod Sci. (2023) 30:442–63. doi: 10.1007/s43032-022-00999-8
37. Augustin, V, Badanthadka, M, Dsouza, V, Kumar, BM, and Shetty, AV. Longitudinal evaluation of developmental protein malnutrition resembling marasmic-kwashiorkor condition in wistar rats. Turk J Pharm Sci. (2024) 21:474–82. doi: 10.4274/tjps.galenos.2023.56736
38. Hugenholtz, F, and De Vos, WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. (2018) 75:149–60. doi: 10.1007/s00018-017-2693-8
39. Nagpal, R, Wang, S, Solberg Woods, LC, Seshie, O, Chung, ST, Shively, CA, et al. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front Microbiol. (2018) 9:2897. doi: 10.3389/fmicb.2018.02897
40. Wos-Oxley, M, Bleich, A, Oxley, APA, Kahl, S, Janus, LM, Smoczek, A, et al. Comparative evaluation of establishing a human gut microbial community within rodent models. Gut Microbes. (2012) 3:234–49. doi: 10.4161/gmic.19934
41. Odle, J, Lin, X, Jacobi, SK, Kim, SW, and Stahl, CH. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu Rev Anim Biosci. (2014) 2:419–44. doi: 10.1146/annurev-animal-022513-114158
42. Fan, P, Liu, P, Song, P, Chen, X, and Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci Rep. (2017) 7:43412. doi: 10.1038/srep43412
43. Yu, D, Zhu, W, and Hang, S. Effects of long-term dietary protein restriction on intestinal morphology, digestive enzymes, gut hormones, and colonic microbiota in pigs. Animals. (2019) 9:180. doi: 10.3390/ani9040180
44. Jeejeebhoy, KN. Enteral and parenteral nutrition: evidence-based approach. Proc Nutr Soc. (2001) 60:399–402. doi: 10.1079/PNS2001103
45. Lunney, JK, Van Goor, A, Walker, KE, Hailstock, T, Franklin, J, and Dai, C. Importance of the pig as a human biomedical model. Sci Transl Med. (2021) 13:eabd5758. doi: 10.1126/scitranslmed.abd5758
47. Huber, HF, Ainsworth, HC, Quillen, EE, Salmon, A, Ross, C, Azhar, AD, et al. Comparative lifespan and healthspan of nonhuman primate species common to biomedical research. Geroscience. (2025) 47:135–51. doi: 10.1007/s11357-024-01421-8
48. Keenan, K, Bartlett, TQ, Nijland, M, Rodriguez, JS, Nathanielsz, PW, and Zürcher, NR. Poor nutrition during pregnancy and lactation negatively affects neurodevelopment of the offspring: evidence from a translational primate model. Am J Clin Nutr. (2013) 98:396–402. doi: 10.3945/ajcn.112.040352
49. Karpf, JA, Sullivan, EL, Roberts, VHJ, Studholme, C, Roberts, CT, and Kroenke, CD. Gestational and early postnatal protein malnutrition disrupts neurodevelopment in rhesus macaques. Cereb Cortex. (2024) 34:bhae462. doi: 10.1093/cercor/bhae462
50. Cheek, DB, Holt, AB, London, WT, Ellenberg, JH, Hill, DE, and Sever, JL. Nutritional studies in the pregnant rhesus monkey—the effect of protein-calorie or protein deprivation on growth of the fetal brain. Am J Clin Nutr. (1976) 29:1149–57. doi: 10.1093/ajcn/29.10.1149
51. Wood, EK, and Sullivan, EL. The influence of diet on metabolism and health across the lifespan in nonhuman primates. Current Opinion Endocrine Metabolic Research. (2022) 24:100336. doi: 10.1016/j.coemr.2022.100336
52. Chiou, KL, Montague, MJ, Goldman, EA, Watowich, MM, Sams, SN, Song, J, et al. Rhesus macaques as a tractable physiological model of human ageing. Philos Trans R Soc Lond Ser B Biol Sci. (2020) 375:20190612. doi: 10.1098/rstb.2019.0612
53. Lewis, D. Biggest ever study of primate genomes has surprises for humanity. Nature. (2023). doi: 10.1038/d41586-023-01776-6
54. Suryawan, A, Jalaludin, MY, Poh, BK, Sanusi, R, Tan, VMH, Geurts, JM, et al. Malnutrition in early life and its neurodevelopmental and cognitive consequences: a scoping review. Nutr Res Rev. (2022) 35:136–49. doi: 10.1017/S0954422421000159
55. Knuth, MM, Stutts, WL, Ritter, MM, Garrard, KP, and Kullman, SW. Vitamin D deficiency promotes accumulation of bioactive lipids and increased endocannabinoid tone in zebrafish. J Lipid Res. (2021) 62:100142. doi: 10.1016/j.jlr.2021.100142
56. Anderson, JL, Carten, JD, and Farber, SA. Chapter 5 - zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol In: HW Detrich, M Westerfield, and LI Zon, editors. Methods in cell biology : Academic Press (2011) 101:111–141. doi: 10.1016/B978-0-12-387036-0.00005-0
57. Jin, Y, Kozan, D, Young, ED, Hensley, MR, Shen, MC, Wen, J, et al. A high-cholesterol zebrafish diet promotes hypercholesterolemia and fasting-associated liver steatosis. J Lipid Res. (2024) 65:100637. doi: 10.1016/j.jlr.2024.100637
58. Williams, MB, and Watts, SA. Current basis and future directions of zebrafish nutrigenomics. Genes Nutr. (2019) 14:34. doi: 10.1186/s12263-019-0658-2
59. Caballero, MV, and Candiracci, M. Zebrafish as screening model for detecting toxicity and drugs efficacy. J Unexplored Med Data. (2018) 3:4. doi: 10.20517/2572-8180.2017.15
60. Ulloa, PE, Iturra, P, Neira, R, and Araneda, C. Zebrafish as a model organism for nutrition and growth: towards comparative studies of nutritional genomics applied to aquacultured fishes. Rev Fish Biol Fish. (2011) 21:649–66. doi: 10.1007/s11160-011-9203-0
61. Adam, AC, Lie, KK, Whatmore, P, Jakt, LM, Moren, M, and Skjærven, KH. Profiling DNA methylation patterns of zebrafish liver associated with parental high dietary arachidonic acid. PLoS One. (2019) 14:e0220934. doi: 10.1371/journal.pone.0220934
62. Skjærven, KH, Jakt, LM, Fernandes, JMO, Dahl, JA, Adam, AC, Klughammer, J, et al. Parental micronutrient deficiency distorts liver Dna methylation and expression of lipid genes associated with a fatty-liver-like phenotype in offspring. Sci Rep. (2018) 8:3055. doi: 10.1038/s41598-018-21211-5
63. Seth, A, Stemple, DL, and Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis Model Mech. (2013) 6:1080–8. doi: 10.1242/dmm.011346
64. Oka, T, Nishimura, Y, Zang, L, Hirano, M, Shimada, Y, Wang, Z, et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. (2010) 10:21. doi: 10.1186/1472-6793-10-21
65. Hess, AF, and Unger, LJ. The scurvy of Guinea pigs: II. Experiments on the effect of the addition of fruits and vegetables to the dietary. J Biol Chem. (1918) 35:487–96.
66. Witkowska, A, Price, J, Hughes, C, Smith, D, White, K, Alibhai, A, et al. The effects of diet on anatomy, physiology and health in the guinea pig. J Animal Health Behavioural Science. (2017) 1:1–6.
67. Schjoldager, JG, Paidi, MD, Lindblad, MM, Birck, MM, Kjærgaard, AB, Dantzer, V, et al. Maternal vitamin C deficiency during pregnancy results in transient fetal and placental growth retardation in guinea pigs. Eur J Nutr. (2015) 54:667–76. doi: 10.1007/s00394-014-0809-6
68. Pietrzak, K. Christiaan Eijkman (1856-1930). J Neurol. (2019) 266:2893–5. doi: 10.1007/s00415-018-9162-7
70. Carpenter, KJ. A short history of nutritional science: part 2 (1885–1912). J Nutr. (2003) 133:975–84. doi: 10.1093/jn/133.4.975
71. Schneider, HA. Rats, fats, and history. Perspect Biol Med. (1986) 29:392–406. doi: 10.1353/pbm.1986.0003
72. Colombani, J, Raisin, S, Pantalacci, S, Radimerski, T, Montagne, J, and Léopold, P. A nutrient sensor mechanism controls Drosophila growth. Cell. (2003) 114:739–49. doi: 10.1016/S0092-8674(03)00713-X
73. Linford, NJ, Ro, J, Chung, BY, and Pletcher, SD. Gustatory and metabolic perception of nutrient stress in Drosophila. Proc Natl Acad Sci. (2015) 112:2587–92. doi: 10.1073/pnas.1401501112
74. Kawecki, TJ, Erkosar, B, Dupuis, C, Hollis, B, Stillwell, RC, and Kapun, M. The genomic architecture of adaptation to larval malnutrition points to a trade-off with adult starvation resistance in Drosophila. Mol Biol Evol. (2021) 38:2732–49. doi: 10.1093/molbev/msab061
75. Domínguez-Oliva, A, Hernández-Ávalos, I, Martínez-Burnes, J, Olmos-Hernández, A, Verduzco-Mendoza, A, and Mota-Rojas, D. The importance of animal models in biomedical research: current insights and applications. Animals. (2023) 13:1223. doi: 10.3390/ani13071223
76. Leulier, F, Macneil, LT, Lee, WJ, Rawls, JF, Cani, PD, Schwarzer, M, et al. Integrative physiology: at the crossroads of nutrition, microbiota, animal physiology, and human health. Cell Metab. (2017) 25:522–34. doi: 10.1016/j.cmet.2017.02.001
77. Suleiman, JB, Mohamed, M, and Bakar, ABA. A systematic review on different models of inducing obesity in animals: advantages and limitations. J Adv Vet Anim Res. (2020) 7:103–14. doi: 10.5455/javar.2020.g399
78. Farooqi, IS, and Xu, Y. Translational potential of mouse models of human metabolic disease. Cell. (2024) 187:4129–43. doi: 10.1016/j.cell.2024.07.011
79. Mukherjee, P, Roy, S, Ghosh, D, and Nandi, SK. Role of animal models in biomedical research: a review. Lab Anim Res. (2022) 38:18. doi: 10.1186/s42826-022-00128-1
80. Martín, R, Bermúdez-Humarán, LG, and Langella, P. Gnotobiotic rodents: an in vivo model for the study of microbe-microbe interactions. Front Microbiol. (2016) 7:409. doi: 10.3389/fmicb.2016.00409
81. Ridaura, VK, Faith, JJ, Rey, FE, Cheng, J, Duncan, AE, Kau, AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. (2013) 341:1241214. doi: 10.1126/science.1241214
82. Schwarzer, M, Makki, K, Storelli, G, Machuca-Gayet, I, Srutkova, D, Hermanova, P, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. (2016) 351:854–7. doi: 10.1126/science.aad8588
83. Wu, G, and Morris, SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. (1998) 336:1–17. doi: 10.1042/bj3360001
84. García-Giménez, JL, Seco-Cervera, M, Tollefsbol, TO, Romá-Mateo, C, Peiró-Chova, L, Lapunzina, P, et al. Epigenetic biomarkers: current strategies and future challenges for their use in the clinical laboratory. Crit Rev Clin Lab Sci. (2017) 54:529–50. doi: 10.1080/10408363.2017.1410520
85. Babu, M, and Snyder, M. Multi-omics profiling for health. Mol Cell Proteomics. (2023) 22:100561. doi: 10.1016/j.mcpro.2023.100561
86. Valvano, M, Capannolo, A, Cesaro, N, Stefanelli, G, Fabiani, S, Frassino, S, et al. Nutrition, nutritional status, micronutrients deficiency, and disease course of inflammatory bowel disease. Nutrients. (2023) 15:3824. doi: 10.3390/nu15173824
87. Tan, F, Cui, P, Li, S, Tan, Y, and Ma, A. Novel organoids mode evaluating the food nutrition and safety: current state and future prospects. Food Front. (2024) 5:1999–2014. doi: 10.1002/fft2.451
88. Darnaud, M, De Vadder, F, Bogeat, P, Boucinha, L, Bulteau, A-L, Bunescu, A, et al. A standardized gnotobiotic mouse model harboring a minimal 15-member mouse gut microbiota recapitulates Sopf/Spf phenotypes. Nat Commun. (2021) 12:6686. doi: 10.1038/s41467-021-26963-9
89. Merino Sanjuán, M., Catalán Latorre, A., Nacher, A., Miralles-Arnau, S., and Jiménez Torres, N. V. (2011). Animal model of undernutrion for the evaluation of drug pharmacokinetics. Nutricion hospitalaria, 26:1296–1304. doi: 10.1590/S0212-16112011000600016
90. Hart, MH, Grandjean, CJ, Park, JH, Erdman, SH, and Vanderhoof, JA. Essential fatty acid deficiency and postresection mucosal adaptation in the rat. Gastroenterology. (1988) 94:682–7. doi: 10.1016/0016-5085(88)90239-9
91. Aleixandre, A, and Miguel, M. Zucker rats as an experimental model for the study of various diseases. Endocrinol Nutr. (2008) 55:217–22. doi: 10.1016/S1575-0922(08)70670-3
92. Drel, VR, Mashtalir, N, Ilnytska, O, Shin, J, Li, F, Lyzogubov, VV, et al. The leptin-deficient (Ob/Ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. (2006) 55:3335–43. doi: 10.2337/db06-0885
93. Paeschke, S, Winter, K, Bechmann, I, Klöting, N, Blüher, M, Baum, P, et al. Leptin receptor-deficient db/db mice show significant heterogeneity in response to high non-heme iron diet. Front Nutr. (2021) 8:741249. doi: 10.3389/fnut.2021.741249
94. Salameh, E, Jarbeau, M, Morel, FB, Zeilani, M, Aziz, M, Déchelotte, P, et al. Modeling undernutrition with enteropathy in mice. Sci Rep. (2020) 10:15581. doi: 10.1038/s41598-020-72705-0
95. Ribeiro, SA, Braga, EL, Queiroga, ML, Clementino, MA, Fonseca, XM, Belém, MO, et al. A new murine undernutrition model based on complementary feeding of undernourished children causes damage to the Morphofunctional intestinal epithelium barrier. J Nutr. (2024) 154:1232–51. doi: 10.1016/j.tjnut.2024.02.001
96. Wenegieme, TY, Elased, D, Mcmichael, KE, Rockwood, J, Hasrat, K, Ume, AC, et al. Strategies for inducing and validating zinc deficiency and zinc repletion. bioRxiv. (2024). doi: 10.1101/2024.02.28.582542
97. Garcia, D, Hellberg, K, Chaix, A, Wallace, M, Herzig, S, Badur, MG, et al. Genetic liver-specific Ampk activation protects against diet-induced obesity and Nafld. Cell Rep. (2019) 26:192–208.e6. doi: 10.1016/j.celrep.2018.12.036
98. De Souza Parrela, JPS, Borkenhagen, IR, Salmeron, SRF, Lima, TAL, Miranda, GDS, De Oliveira Costermani, H, et al. Intrauterine malnutrition disrupts leptin and ghrelin milk hormones, programming rats. J Endocrinol. (2022) 255:11–23. doi: 10.1530/JOE-21-0427
99. Jans, M, and Vereecke, L. A guide to germ-free and gnotobiotic mouse technology to study health and disease. FEBS J. (2024) 292:1228–51. doi: 10.1111/febs.17124
100. Xin, X, Cai, B-Y, Chen, C, Tian, H-J, Wang, X, Hu, Y-Y, et al. High-trans fatty acid and high-sugar diets can cause mice with non-alcoholic steatohepatitis with liver fibrosis and potential pathogenesis. Nutrition Metabolism. (2020) 17:40. doi: 10.1186/s12986-020-00462-y
101. Sun, J, and Debosch, BJ. Protocol to evaluate fasting metabolism and its relationship to the core circadian clock in mice. Star Protoc. (2025) 6:103660. doi: 10.1016/j.xpro.2025.103660
102. Batra, A, Cuesta, S, Alves, MB, Restrepo, JM, Giroux, M, Laureano, DP, et al. Relationship between insulin and Netrin-1/dcc guidance cue pathway regulation in the prefrontal cortex of rodents exposed to prenatal dietary restriction. J Dev Orig Health Dis. (2023) 14:501–7. doi: 10.1017/S204017442300017X
Keywords: malnutrition mechanism, rodent model, non-human primates, undernutrition, pigs, zebrafish
Citation: Shahzad M, Ahmad HA, Ghani M and Al Nabhani Z (2025) Animal models for understanding the mechanisms of malnutrition: a literature review. Front. Nutr. 12:1655811. doi: 10.3389/fnut.2025.1655811
Edited by:
Matteo Dell'Anno, University of Messina, ItalyReviewed by:
Giovanni Corsetti, University of Brescia, ItalyMariela Chertoff, University of Buenos Aires, Argentina
Copyright © 2025 Shahzad, Ahmad, Ghani and Al Nabhani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziad Al Nabhani, emlhZC5hbG5hYmhhbmlAZGJtci51bmliZS5jaA==
 Muhammad Shahzad
Muhammad Shahzad Habab Ali Ahmad3
Habab Ali Ahmad3 Ziad Al Nabhani
Ziad Al Nabhani