- 1School of Physical Education, Shandong University, Jinan, China
- 2Culture and Tourism College, Guangdong Vocational Academy of Art, Foshan, China
1 Introduction
Metabolic syndrome (MetS) is a multifaceted condition characterized by a cluster of risk factors, including hypertension, elevated fasting blood glucose levels, increased waist circumference (WC), elevated triglyceride (TG) levels, and reduced high-density lipoprotein (HDL) cholesterol levels (1–5). Conventional treatments for MetS typically involve pharmacological interventions and physical activity (6, 7). The former, such as oral hypoglycemic and lipid-lowering drugs, are effective in controlling blood glucose and lipid levels but have some side effects and dependence (8, 9). The latter are helpful for weight control and improving insulin sensitivity, but these methods are often difficult to adhere to in the long term (10). Therefore, exploring more effective alternative treatment options has become an urgent need.
In recent years, the ketogenic diet (KD)—a low-carbohydrate, high-fat nutritional regimen—has attracted considerable attention (11). Specifically, KD promotes fat burning by increasing ketone body (KB) levels [e.g., β-hydroxybutyrate (β-BHB)], improves insulin sensitivity, regulates lipid metabolism, and reduces chronic low-grade inflammatory responses, which in turn alleviates the symptoms of MetS (12, 13). However, most current studies have focused on short-term effects in specific populations, leaving a gap in data from multicenter, large-scale clinical trials that encompass diverse ethnicities, age groups, and lifestyles (14–16). Future research should focus on the effects of the KD on various aspects of MetS, especially its adaptation in different populations. In addition, exploring optimized regimens, such as cyclical KD, and combining them with other therapeutic options may provide more effective avenues for the treatment of MetS.
2 KD strategies for MetS: based on a comprehensive review's perspective
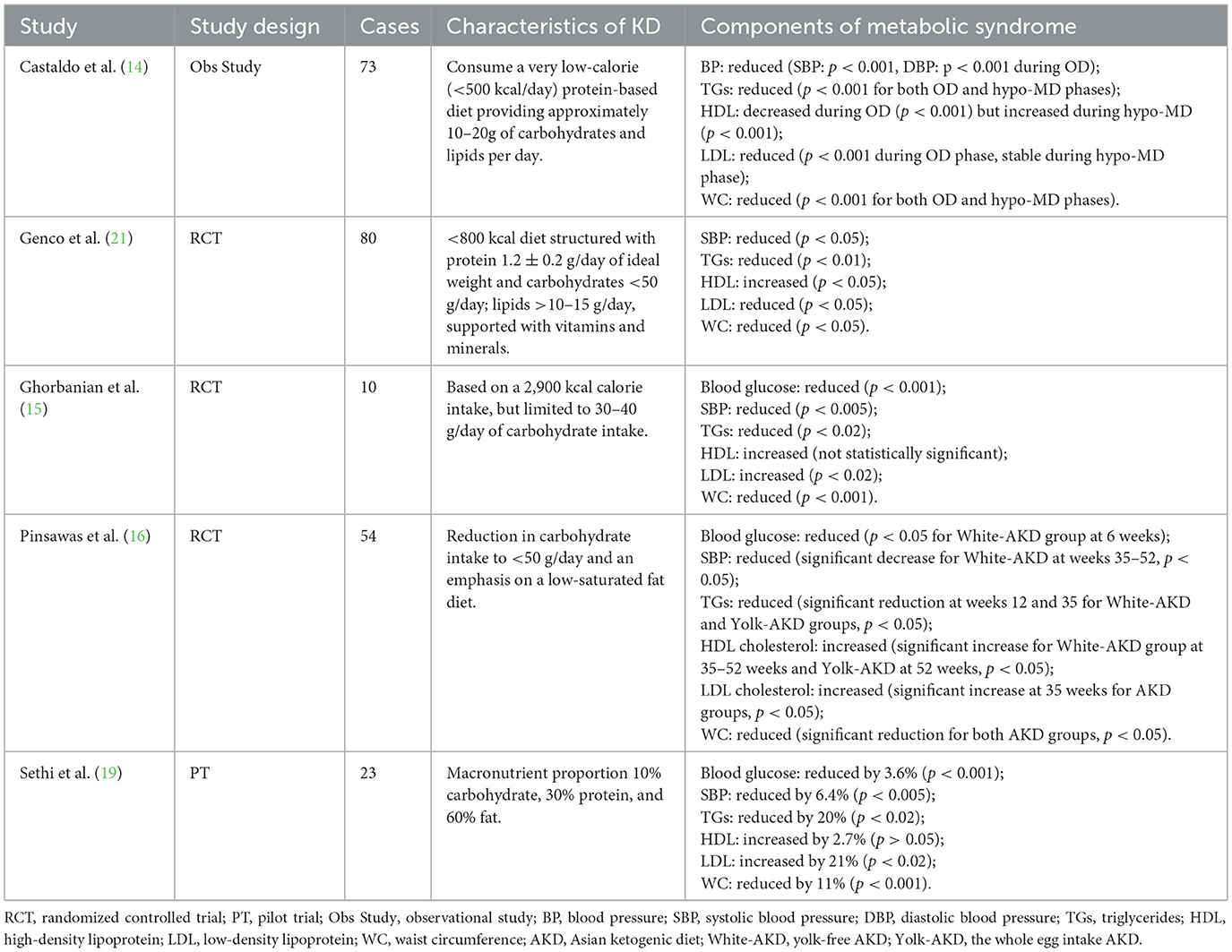
MetS is a group of closely related metabolic abnormalities that typically include obesity, insulin resistance, hyperglycemia, and hyperlipidemia (17). Treatment for MetS does not focus on a single health indicator; instead, it aims to reduce the overall metabolic burden through comprehensive interventions (18). Sethi et al. (19) explored the effects of KD on metabolic health in patients with schizophrenia and bipolar disorder. Over 4 months, the KD led to a 12% reduction in body weight, a 12% decrease in body mass index (BMI), a 13% decrease in WC, and a 36% decrease in visceral fat. Furthermore, homeostatic model assessment for insulin resistance was reduced by 27%, TG levels decreased by 25%, and HDL levels increased by 2.7%. In a retrospective cohort study, Zachos et al. (20) assessed the metabolic health effects of low-fiber carbohydrates in patients with bipolar disorder. In contrast to the effects of KD, an increased intake of low-fiber carbohydrates was associated with an increased prevalence of MetS and higher BMI in this primary cohort. These findings suggest that the KD is highly effective in improving metabolic health, particularly in patients with psychiatric disorders.
Another 52-week study assessed the effects of an Asian KD (AKD) on individuals with MetS (16). Participants were randomly assigned to three groups: the whole egg intake AKD group (Yolk-AKD), the yolk-free AKD group (White-AKD), and a balanced low-caloric diet group (BLC). The results showed that the AKD group experienced significant improvements in weight, WC, and insulin sensitivity compared to the BLC group, with the Yolk-AKD group exhibiting the most pronounced weight loss. HDL cholesterol levels increased significantly in the AKD group, while TG levels decreased, indicating that AKD is efficacious in improving blood lipid profiles. Although low-density lipoprotein (LDL) increased, the improvement in HDL level helped to balance overall blood lipids. Furthermore, the AKD group exhibited lower levels of inflammation-related hormones, including a significant reduction in interleukin-6, tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1, underscoring its positive impact on metabolic health.
It is worth noting that a single dietary intervention has obvious limitations in understanding the comparative analysis of the KD with other dietary interventions, especially in the context of MetS. Therefore, in order to show the differences and advantages of the KD vs. other dietary regimens in improving various aspects of MetS, Castaldo et al. (14) investigated the effects of combining a KD with a Mediterranean diet for the treatment of obesity. The results showed that the KD resulted in significant reductions in body weight and abdominal fat, along with improvements in blood glucose levels, lipid profiles, and liver function. While the Mediterranean diet phase also improved metabolic health to some extent compared to KD, a rebound in blood glucose and lipid levels was observed, indicating lower resilience. Overall, the combined approach of the ketogenic and Mediterranean diets proved highly effective in enhancing body weight reduction and metabolic health and lowering cardiovascular risks in patients with obesity. Subsequently, Genco et al. (21) examined the impact of combining a very low-calorie KD with an intragastric balloon (Orbera) on weight loss outcomes in patients with obesity. The study included 80 patients with obesity who were randomly assigned to two groups after 4 months from the start of the study: one group followed a KD with an intragastric balloon (Group A), while the other group adhered to a low-calorie diet (LCD) with an intragastric balloon (Group B). The results revealed that Group A experienced significantly greater weight loss than Group B (8 kg vs. 3 kg), with a total weight loss of 19 kg in Group A compared to 12 kg in Group B (p < 0.05). This study demonstrated that an intervention program combining a KD with an intragastric balloon not only enhances weight loss but also has important clinical significance in improving various metabolic indices in MetS, suggesting its potential in the comprehensive management of MetS.
In addition, Ghorbanian et al. (15) investigated the effects of a KD and aerobic exercise (AE) on the metabolic health of middle-aged men with MetS. The results revealed that the AE+KD group experienced significant reductions in body weight, BMI, and body fat percentage, alongside substantial decreases in retinol binding protein 4 levels. The AE intervention also led to significant reductions in fatty acid binding protein 5 levels. Furthermore, the AE+KD group demonstrated notable improvements in insulin resistance and increased insulin sensitivity.
3 Potential mechanisms of KD on MetS
Recent studies have shown that dietary patterns with increased protein intake trigger the release of anorexigenic hormones, such as glucagon-like peptide-1 (GLP-1), cholecystokinin (CCK), and peptide YY (PYY), which collectively contribute to reduced appetite. This mechanism is particularly relevant in the management of MetS, where appetite control is essential for weight management. Hall et al. (22) examined the effects of casein and whey proteins on appetite and gastrointestinal hormone secretion. Their findings revealed that whey protein notably elevated plasma amino acid levels, stimulating the secretion of CCK and GLP-1, thereby enhancing satiety. In a similar vein, Lejeune et al. (23) investigated the impact of a high-protein (HP) diet vs. an adequate-protein diet on 24-h satiety, energy expenditure, and substrate metabolism. Although energy intake did not differ substantially between the two diets, energy expenditure and fat oxidation were elevated in the HP diet, suggesting that HP diets may play a crucial role in improving insulin sensitivity and long-term weight management, thereby addressing multiple aspects of MetS.
KD influences blood pressure through the renin-angiotensin-aldosterone (RAA) system. For instance, Belany et al. (24) investigated the effects of a low-calorie, low-fat diet (LFD) compared to a KD on the human body, specifically focusing on aldosterone and renin. The study revealed that after 6 weeks of intervention, aldosterone levels increased significantly in the KD group, while no notable changes were observed in the LFD group. Specifically, aldosterone levels in the KD group increased by 88% and 144%, which may be attributed to the elevated concentration of KBs. Moreover, despite the rise in aldosterone, cardiovascular metabolic risks, including blood pressure and blood glucose levels, remained unaffected in the KD group. Despite elevated aldosterone, blood pressure, and blood glucose levels were not adversely affected in the KD group, suggesting that the KD may have a potentially protective role in the management of MetS, particularly for blood pressure and cardiovascular health.
Evidence from patients with type 2 diabetes mellitus (T2DM) indicates that the stringent restriction of carbohydrates in a KD diminishes the intestinal absorption of monosaccharides, resulting in lowered blood glucose levels and minimized glycemic fluctuations. The trial conducted by Yancy et al. (25) assessed the effects of a low-carbohydrate KD in overweight individuals with T2DM over a 16-week period. The findings revealed substantial improvements in weight, WC, diastolic blood pressure, and glycemic control, as evidenced by reductions in glycated hemoglobin levels. In their review, Bolla et al. (26) explored the role of low-carbohydrate and KDs in managing both type 1 and type 2 diabetes. This finding demonstrates the potential of the KD in the short-term management of MetS, but more clinical studies are needed to validate its long-term effects and safety.
In addition to this, MetS is usually accompanied by chronic low-grade systemic inflammation that is closely associated with MetS features such as insulin resistance, abdominal fat accumulation, and dyslipidemia (27). It has been found that one of the main mechanisms by which the KD modulates inflammation is by promoting an increase in the level of circulating β-BHB, the primary KB, which can inhibit the inflammatory response through multiple pathways (28). β-BHB inhibits the expression of pro-inflammatory genes (e.g., TNF-α, IL-1β, and NF-κB) by upregulating anti-inflammatory genes, such as NF-κBIA and MAP3K8, thereby reducing the release of inflammatory factors (28–32). In addition, KD directly affects lipid profile and insulin sensitivity by limiting the intake of digestible carbohydrates. Specific mechanisms include decreasing insulin secretion, promoting lipolysis, and increasing KB levels, thereby improving insulin signaling (33, 34). Through these mechanisms, the KD not only helps alleviate inflammation associated with MetS but also significantly improves metabolic indices, such as body weight, blood glucose, blood lipids, and blood pressure, thus providing an important adjunctive role in the treatment of MetS.
4 Discussion
KD, characterized by its low-carbohydrate and high-fat composition, has demonstrated considerable potential in enhancing metabolic health, particularly in areas such as weight management, insulin sensitivity, and lipid regulation (Table 1). Nonetheless, there is ongoing debate regarding whether the KD surpasses traditional dietary approaches in terms of adherence, nutritional adequacy, and lifestyle compatibility. Other dietary approaches, such as the Mediterranean diet, have also shown substantial effectiveness in mitigating the symptoms of MetS, offering potentially greater feasibility for long-term adherence (35, 36). In contrast, the prolonged use of the KD may carry certain risks, particularly regarding nutritional deficiencies and cardiovascular health. Chronic carbohydrate restriction may result in deficiencies of essential micronutrients, such as vitamin C, magnesium, and calcium, thereby increasing the likelihood of diminished bone density and compromised immune function. Therefore, it is crucial to compare the long-term effects of these dietary strategies, with particular attention to adherence and nutritional balance, to guide future research. Specifically, future studies should examine the sustained health outcomes of the KD for individuals with MetS, comparing its safety profile to other dietary alternatives.
Beyond clinical research, recent advancements in molecular biology and epidemiology have provided new insights into the underlying mechanisms of the KD. For instance, studies indicate that the KD may play a critical role in reducing inflammation and enhancing insulin sensitivity by modulating gut microbiota, activating KB receptors, and influencing fatty acid metabolism pathways (37, 38). Additionally, emerging research suggests that the KD may facilitate fat oxidation and weight regulation by optimizing energy metabolism in liver and muscle cells (39, 40). These molecular findings open valuable avenues for future research, particularly in refining the KD approach to minimize adverse effects while maximizing therapeutic outcomes.
Given existing research gaps, several pertinent questions and hypotheses for future investigation into the KD's effects on MetS arise: (1) a comparative analysis of the KD vs. other low-carbohydrate diets in terms of long-term adherence, metabolic health, and cardiovascular risks; (2) the effects of prolonged KD on micronutrient deficiencies, bone health, and cardiovascular function; and (3) the mechanisms by which the KD influences gut microbiota and metabolic pathways in the management of MetS.
Author contributions
JC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the collaborators of this study for their time and effort in our research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wannamethee SG. The metabolic syndrome and cardiovascular risk in the British Regional Heart Study. Int J Obes. (2008) 32:S25–9. doi: 10.1038/ijo.2008.32
2. McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. (2005) 28:385–90. doi: 10.2337/diacare.28.2.385
3. Myers J, Kokkinos P, Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. (2019) 11:1652. doi: 10.3390/nu11071652
5. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
6. Gallardo-Alfaro L, Bibiloni MDM, Mascaró CM, Montemayor S, Ruiz-Canela M, Salas-Salvadó J, et al. Leisure-time physical activity, sedentary behaviour and diet quality are associated with metabolic syndrome severity: the PREDIMED-plus study. Nutrients. (2020) 12:1013. doi: 10.3390/nu12041013
7. López-Murillo LD, González-Ortiz M, Martínez-Abundis E, Cortez-Navarrete M, Pérez-Rubio KG. Effect of Banaba (Lagerstroemia speciosa) on metabolic syndrome, insulin sensitivity, and insulin secretion. J Med Food. (2022) 25:177–82. doi: 10.1089/jmf.2021.0039
8. Wu D, Wang X, Liu Q, Luo X. Comparative efficacy and safety of oral hypoglycaemic drugs as adjunctive therapy in the management of type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. (2025) 27:4354–70. doi: 10.1111/dom.16474
9. Said AH, Abd Rahim IS, Mohamad Zaini NNB, Saiful Nizam NIB. Factors affecting adherence to lipid-lowering drugs: a scoping review. Oman Med J. (2023) 38:e523. doi: 10.5001/omj.2023.67
10. Lopes S, Félix G, Mesquita-Bastos J, Figueiredo D, Oliveira J, Ribeiro F. Determinants of exercise adherence and maintenance among patients with hypertension: a narrative review. Rev Cardiovasc Med. (2021) 22:1271–8. doi: 10.31083/j.rcm2204134
11. Gershuni VM, Yan SL, Medici V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep. (2018) 7:97–106. doi: 10.1007/s13668-018-0235-0
12. Zhou C, Wang M, Liang J, He G, Chen N. Ketogenic diet benefits to weight loss, glycemic control, and lipid profiles in overweight patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trails. Int J Environ Res Public Health. (2022) 19:10429. doi: 10.3390/ijerph191610429
13. Choy KYC, Louie JCY. The effects of the ketogenic diet for the management of type 2 diabetes mellitus: a systematic review and meta-analysis of recent studies. Diabetes Metab Syndr. (2023) 17:102905. doi: 10.1016/j.dsx.2023.102905
14. Castaldo G, Monaco L, Castaldo L, Galdo G, Cereda E. An observational study of sequential protein-sparing, very low-calorie ketogenic diet (Oloproteic diet) and hypocaloric Mediterranean-like diet for the treatment of obesity. Int J Food Sci Nutr. (2016) 67:696–706. doi: 10.1080/09637486.2016.1186157
15. Ghorbanian B, Wong A, Iranpour A. The effect of dietary carbohydrate restriction and aerobic exercise on retinol binding protein 4 (RBP4) and fatty acid binding protein 5 (FABP5) in middle-aged men with metabolic syndrome. Br J Nutr. (2023) 130:553–63. doi: 10.1017/S0007114522003580
16. Pinsawas B, Surawit A, Mongkolsucharitkul P, Pongkunakorn T, Suta S, Manosan T, et al. Asian low-carbohydrate diet with increased whole egg consumption improves metabolic outcomes in metabolic syndrome: a 52-week intervention study. J Nutr. (2024) 154:3331–45. doi: 10.1016/j.tjnut.2024.08.027
17. Lemieux I, Després JP. Metabolic syndrome: past, present and future. Nutrients. (2020) 12:3501. doi: 10.3390/nu12113501
18. Castro-Barquero S, Ruiz-León AM, Sierra-Pérez M, Estruch R, Casas R. Dietary strategies for metabolic syndrome: a comprehensive review. Nutrients. (2020) 12:2983. doi: 10.3390/nu12102983
19. Sethi S, Wakeham D, Ketter T, Hooshmand F, Bjornstad J, Richards B, et al. Ketogenic diet intervention on metabolic and psychiatric health in bipolar and schizophrenia: a pilot trial. Psychiatry Res. (2024) 335:115866. doi: 10.1016/j.psychres.2024.115866
20. Zachos KA, Godin O, Choi J, Jung JH, Aouizerate B, Aubin V, et al. Diet quality and associations with lactate and metabolic syndrome in bipolar disorder. J Affect Disord. (2024) 364:167–77. doi: 10.1016/j.jad.2024.05.167
21. Genco A, Ienca R, Ernesti I, Maselli R, Casella G, Bresciani S, et al. Improving weight loss by combination of two temporary antiobesity treatments. Obes Surg. (2018) 28:3733–7. doi: 10.1007/s11695-018-3448-9
22. Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. (2003) 89:239–48. doi: 10.1079/BJN2002760
23. Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. (2006) 83:89–94. doi: 10.1093/ajcn/83.1.89
24. Belany P, Kackley ML, Zhao S, Kluwe B, Buga A, Crabtree CD, et al. Effects of hypocaloric low-fat, ketogenic, and ketogenic and ketone supplement diets on aldosterone and renin. J Clin Endocrinol Metab. (2023) 108:1727–39. doi: 10.1210/clinem/dgad009
25. Yancy WS, Vernon MC, Westman EC. A pilot trial of a low-carbohydrate, ketogenic diet in patients with type 2 diabetes. Metab Syndr Relat Disord. (2003) 1:239–43. doi: 10.1089/154041903322716723
26. Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L. Low-Carb and Ketogenic diets in type 1 and type 2 diabetes. Nutrients. (2019) 11:962. doi: 10.3390/nu11050962
27. Hertiš Petek T, Homšak E, Svetej M, Marčun Varda N. Metabolic syndrome, inflammation, oxidative stress, and vitamin D levels in children and adolescents with obesity. Int J Mol Sci. (2024) 25:10599. doi: 10.3390/ijms251910599
28. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. (2015) 21:263–9. doi: 10.1038/nm.3804
29. Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL, et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson's disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation. (2015) 12:9. doi: 10.1186/s12974-014-0230-3
30. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. (2013) 339:211–4. doi: 10.1126/science.1227166
31. Huang C, Wang P, Xu X, Zhang Y, Gong Y, Hu W, et al. The ketone body metabolite β-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia. (2018) 66:256–78. doi: 10.1002/glia.23241
32. Qiao G, Lv T, Zhang M, Chen P, Sun Q, Zhang J, et al. β-hydroxybutyrate (β-HB) exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated macrophages in Liza haematocheila. Fish Shellfish Immunol. (2020) 107(Pt B):444–51. doi: 10.1016/j.fsi.2020.11.005
33. Paoli A, Bianco A, Moro T, Mota JF, Coelho-Ravagnani CF. The effects of ketogenic diet on insulin sensitivity and weight loss, which came first: the chicken or the egg? Nutrients. (2023) 15:3120. doi: 10.3390/nu15143120
34. Merovci A, Finley B, Hansis-Diarte A, Neppala S, Abdul-Ghani MA, Cersosimo E, et al. Effect of weight-maintaining ketogenic diet on glycemic control and insulin sensitivity in obese T2D subjects. BMJ Open Diabetes Res Care. (2024) 12:e004199. doi: 10.1136/bmjdrc-2024-004199
35. Quetglas-Llabrés MM, Monserrat-Mesquida M, Bouzas C, García S, Argelich E, Casares M, et al. Impact of adherence to the mediterranean diet on antioxidant status and metabolic parameters in NAFLD patients: a 24-month lifestyle intervention study. Antioxidants. (2024) 13:480. doi: 10.3390/antiox13040480
36. Montemayor S, Mascaró CM, Ugarriza L, Casares M, Llompart I, Abete I, et al. Adherence to mediterranean diet and NAFLD in patients with metabolic syndrome: the FLIPAN study. Nutrients. (2022) 14:3186. doi: 10.3390/nu14153186
37. Koh S, Dupuis N, Auvin S. Ketogenic diet and neuroinflammation. Epilepsy Res. (2020) 167:106454. doi: 10.1016/j.eplepsyres.2020.106454
38. Santangelo A, Corsello A, Spolidoro GCI, Trovato CM, Agostoni C, Orsini A, et al. The influence of ketogenic diet on gut microbiota: potential benefits, risks and indications. Nutrients. (2023) 15:3680. doi: 10.3390/nu15173680
39. Yakupova EI, Bocharnikov AD, Plotnikov EY. Effects of ketogenic diet on muscle metabolism in health and disease. Nutrients. (2022) 14:3842. doi: 10.3390/nu14183842
Keywords: ketogenic diet, ketogenic, metabolic syndrome, mechanisms, strategies
Citation: Chen J and Yao J (2025) Exploring the potential of the ketogenic diet in managing metabolic syndrome: mechanisms, strategies, and future research directions. Front. Nutr. 12:1658691. doi: 10.3389/fnut.2025.1658691
Received: 03 July 2025; Accepted: 11 August 2025;
Published: 29 August 2025.
Edited by:
Shaojie Liu, Frist Affiliated Hospital of Xiamen University, ChinaReviewed by:
Mohsen Khosravi, Zahedan University of Medical Sciences, IranMarta Biesiekierska, Medical University of Lodz, Poland
Copyright © 2025 Chen and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiawei Yao, eWFvandAZ2RkZGMuZWR1LmNu
 Jiping Chen
Jiping Chen Jiawei Yao2*
Jiawei Yao2*