- 1Department of Clinical Nutrition, Longgang Centre Hospital of Shenzhen, Shenzhen, Guangdong, China
- 2Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Department of Clinical Nutrition, The General Hospital of Western Theater Command, Chengdu, Sichuan, China
- 4Department of Rehabilitation Medicine, The General Hospital of Western Theater Command, Chengdu, Sichuan, China
- 5Department of Health Management Center & Institute of Health Management, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
Background: A plateau hypoxic environment can increase the physiological burden on athletes. Although nutritional interventions have been recognized as a potential strategy to improve plateau acclimatization, evidence in support of specific dietary patterns is still lacking. This study compared the effects of different dietary interventions on cardiopulmonary fitness during plateau exercise through systematic evaluation and network meta-analysis methods.
Methods: This study systematically reviewed relevant literature up to June 2025 and included 20 randomized controlled trials (RCTs) conducted at altitudes above 1,500 meters involving healthy participants aged 16 years and above who engaged in physical activities. The primary outcomes included cardiopulmonary indicators [maximal oxygen uptake (VO2max), heart rate (HR)], blood biomarkers [peripheral oxygen saturation (SpO2), hematocrit (HCT)], and subjective perception indicators [rating of perceived exertion (RPE)]. For each outcome, the pooled effects of each intervention compared to others were estimated. Mean difference (MD) or standardized mean difference (SMD) with 95% Credible Intervals (95% CrI) were calculated. The Surface Under the Cumulative Ranking Curve (SUCRA) was used to rank the dietary interventions and quantify their relative effectiveness. In addition, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was applied to assess the quality of evidence.
Results: A total of 20 randomized controlled trials involving 329 participants were included, evaluating eight dietary interventions. Moderate-quality evidence indicated that carbohydrate supplementation significantly improved the percentage of maximal oxygen uptake (VO2max) compared to placebo (SMD = 1.13, 95% CrI: 0.25 to 2.05) and reduced RPE scores (MD = −0.77, 95% CrI: −1.83 to −0.09). Moderate-quality evidence indicated that carbohydrate supplementation combined with glutamine ranked highest in improving SpO2 (SUCRA 84.54%) and RPE (SUCRA 69.37%), while iron supplementation showed the highest SUCRA rankings for HR (56.54%) and HCT (66.67%). However, these interventions did not demonstrate statistically significant advantages. Notably, the observed increase in VO2max exceeded the minimally clinically important difference (MCID) of 1.0 ml/kg/min reported in previous studies, suggesting that the effect of carbohydrate supplementation on VO2max may have clinical relevance.
Conclusions: Differences exist in the effects of different dietary interventions on cardiopulmonary fitness during altitude exercise. The current network meta-analysis indicates that carbohydrate-based strategies show beneficial effects, with carbohydrate plus glutamine supplementation demonstrating greater advantages in SpO2 and RPE, while carbohydrate alone is more supported in improving VO2max. Therefore, carbohydrate-based strategies may serve as effective options to promote altitude acclimatization, whereas iron supplementation may have potential benefits in improving HCT and HR.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD420251069629, identifier: CRD420251069629.
1 Introduction
In recent years, a growing number of people have been engaging in physical activity at high altitudes for purposes such as work, recreation, and sports (1). Particularly in the field of sports, interest in understanding the effects of altitude on physical performance has significantly increased since the 1968 Olympic Games were held at an altitude of over 2,000 meters (2). At present, high-altitude training has become a crucial method for enhancing endurance in athletic disciplines. The unique geographical and climatic conditions at high altitudes lead to a series of physiological adaptations in the human body. As altitude increases, the oxygen content in the air decreases, creating a hypoxic environment that induces physiological responses to enhance oxygen transport and utilization. Specifically, the body stimulates the expression of erythropoietin (EPO) through hypoxia-inducible factors (HIFs) in the kidneys. Once released into the bloodstream, EPO promotes the differentiation of erythroid progenitor cells in the bone marrow into mature red blood cells. In addition, hypoxia can increase capillary density and enhance mitochondrial efficiency. Together, these adaptations improve the oxygen-carrying capacity of the blood (3, 4). Studies have shown that once athletes adapt to high-altitude conditions, hypoxia regulation may benefit and protect the cardiovascular system, increase maximal oxygen uptake (VO2max), and thereby improve athletic performance (5).
Nutritional supplementation is crucial for high-altitude training. The International Olympic Committee (IOC) Nutrition Expert Group recommends that athletes increase their intake of energy, carbohydrate (CHO), iron, fluids, and antioxidant-rich foods during high-altitude training (6). Acute exposure to high altitudes for training imposes significant stress on various physiological and metabolic processes. A review on high-altitude nutrition, hydration and supplementation pointed out that prolonged stay at high altitude often leads to an imbalance between energy expenditure and intake, highlighting the importance of both macronutrient and micronutrient supplementation (7). This perspective is supported by several empirical studies. For example, a study on elite runners training at a 2,100-meter altitude camp found that daily supplementation with 200 mg of elemental iron significantly increased red blood cell mass following high-altitude exposure (8). Similarly, research by Oliver et al. showed that participants in high-altitude expeditions who received CHO supplementation experienced significantly lower Rated Perceived Exertion (RPE) and improved physical performance compared to the placebo group (9).
At present, there is no definitive evidence identifying which dietary intervention is most effective in improving cardiopulmonary fitness and physical performance during high-altitude exercise. Existing studies have primarily focused on the following nutritional interventions: nitrates, CHO, CHO combined with glutamine, high-protein diets, d-aspartic acid, iron, antioxidant-rich foods, rhodiola crenulata-and cordyceps sinensis (RC) (10–17). The aim of this study is to rank the effectiveness of different dietary and therapeutic interventions used to enhance cardiopulmonary function under high-altitude conditions by analyzing changes in cardiopulmonary indicators [(VO2max), heart rate (HR)], blood biomarkers [peripheral oxygen saturation (SpO2), hematocrit (HCT)], and RPE. The findings are intended to provide evidence-based guidance for athletes and high-altitude activity enthusiasts to optimize their nutrition and training strategies in such environments.
2 Methods
2.1 Search strategy
This systematic review and network meta-analysis followed the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (18). We conducted a comprehensive search across multiple online databases, including PubMed (Medline), Embase, and Web of Science–Science Citation Index, to identify potential studies. Additionally, manual searches were performed on preprint platforms (medRxiv and Research Square) as well as other databases such as CINAHL, China National Knowledge Infrastructure (CNKI), Wanfang Data, and Scielo. Although Scopus and ScienceDirect are also widely used in systematic reviews, they were not included in the present search. Scopus has substantial overlap with PubMed, Embase, and Web of Science, which were already comprehensively searched, while ScienceDirect primarily serves as a publisher-specific database and provides limited additional coverage beyond these sources. Therefore, the exclusion of these databases is unlikely to have materially affected the comprehensiveness of our literature search. Besides, to supplement the academic database searches, we also explored gray literature sources including ProQuest Dissertations and Theses, EThOS, and ClinicalTrials.gov. The search terms used to identify eligible studies included combinations of keywords such as (“dietary intervention” OR “nutritional supplementation” OR “nutrient supplementation” OR “dietary strategy” OR “macronutrient” OR “micronutrient”), (“high altitude” OR “plateau” OR “highland” OR “mountain” OR “hypoxia” OR “low oxygen”), and (“exercise performance” OR “physical performance” OR “physical training” OR “endurance training” OR “strength training”). Validated filters were applied to identify randomized controlled trials (RCTs). The search covered literature published from the inception of each database up to June 2025. The study protocol was registered in PROSPERO (CRD420251069629) and strictly followed throughout the review process.
2.2 Eligibility criteria
This study included randomized controlled trials (RCTs) involving healthy participants aged 16 years or older who engaged in physical activities (such as hiking, long-distance running, mountaineering, or cycling) at high-altitude regions above 1,500 meters (19). Healthy participants were defined as individuals without a history of acute mountain sickness, without cardiovascular, metabolic, pulmonary diseases, or other chronic conditions, and not taking concomitant medications that could affect exercise performance or altitude adaptation (20). All included studies were required to implement clearly defined dietary interventions and report at least one of the following outcome indicators: VO2max, HR, SpO2, HCT, or RPE. Exclusion criteria were as follows: (1) non-RCT study designs; (2) animal or in vitro experiments; (3) participants with severe chronic diseases (e.g., cardiovascular disease or chronic mountain sickness) or acute infections; (4) interventions not involving nutritional components (e.g., pure pharmacological or oxygen therapy); (5) studies with missing key data or inaccessible full texts; and (6) duplicate publications.
2.3 Screening and data extraction
The literature screening and data extraction were conducted independently by two reviewers (Y.X., D.M.). After removing duplicates, the titles, abstracts, and full texts were reviewed in strict accordance with the inclusion and exclusion criteria. A standardized data collection form was designed and developed using Microsoft Excel. The extracted information included: basic publication details (title, authors, year, and country); study design; participant characteristics (sample size, age); intervention details (altitude exposure model, altitude level, type of physical activity, type and dosage of dietary intervention, and duration); outcome indicators (VO2max, HR, SpO2, HCT, and RPE). For studies with missing data, we contacted the corresponding authors via email to request the original data. If no response was received, the study was excluded.
2.4 Quality assessment
This study employed the Cochrane Risk of Bias 2.0 tool (ROB 2.0) to rigorously assess the methodological quality of the included RCTs (21). Two independent reviewers (Y.X. and B.W.) conducted a cross-assessment of each study across the following seven key domains: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessors; (5) completeness of outcome data; (6) selective outcome reporting; (7) other potential sources of bias. Each trial was ultimately classified as having a “low risk of bias,” “unclear risk of bias,” or “high risk of bias.” All risk of bias assessment results were documented in the study characteristics table using Review Manager software (version 5.4).
2.5 Synthesis methods
Network meta-analyses based on both Bayesian and frequentist approaches were conducted using R version 4.4.2. A graphical network plot was used to illustrate the comparative relationships among different dietary interventions, where each node represented a dietary strategy with its size proportional to the number of included studies, and the thickness of edges reflected the number of trials with direct comparisons. For continuous outcomes, standardized mean differences (SMDs) were calculated when measurement units were inconsistent across studies, and mean differences (MDs) were used when units were consistent. Effect estimates were reported with 95% credible intervals (CrIs) under the Bayesian framework. Random-effects models were applied, and model fitting was optimized via four-chain Monte Carlo simulations to generate posterior samples. At least 20,000 adaptive iterations were set to ensure model convergence, followed by 50,000 sampling iterations (22). Interventions were ranked using the Surface Under the Cumulative Ranking (SUCRA) curve, with values expressed as percentages ranging from 0 to 100%. Higher SUCRA values indicated better rankings of intervention effectiveness (23). SUCRA results were visualized using STATA version 17. Besides, in this study, between-study heterogeneity was assessed using the between-study variance (τ2, tau-squared). A τ2 value greater than 0.36 was considered indicative of substantial heterogeneity, in line with previously suggested thresholds (24). To test the robustness of the results, sensitivity analyses were performed by excluding studies with high risk of bias. The purpose of these analyses was to evaluate whether the exclusion of potentially influential studies significantly altered the overall estimates or rankings.
2.6 Publication bias and inconsistency analysis
Publication bias was assessed by comparing the funnel plots and performing Egger's test for each outcome. A symmetric distribution in the funnel plots combined with a non-significant Egger's test result (P > 0.05) indicated a low risk of publication bias. All analyses were conducted using the netmeta package in R software (version 4.4.2). Additionally, inconsistency was evaluated by comparing the Deviance Information Criterion (DIC) values between the consistency and inconsistency models (25). The model with the lower DIC was preferred. A DIC difference of ≥3 between the two models was considered to indicate a meaningful difference (26).
2.7 Confidence in estimates
We assessed the quality of evidence using the CINeMA (Confidence in Network Meta-Analysis) platform, which is based on the GRADE (Grading of Recommendations Assessment, Development and Evaluation) framework. CINeMA evaluates each comparison across six domains—within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence—and integrates direct and indirect evidence to determine the overall confidence in network meta-analysis estimates. According to this approach, evidence from RCTs starts at “high” confidence but may be downgraded due to these limitations, resulting in ratings of “moderate,” “low,” or “very low.”
3 Results
3.1 Search and selection
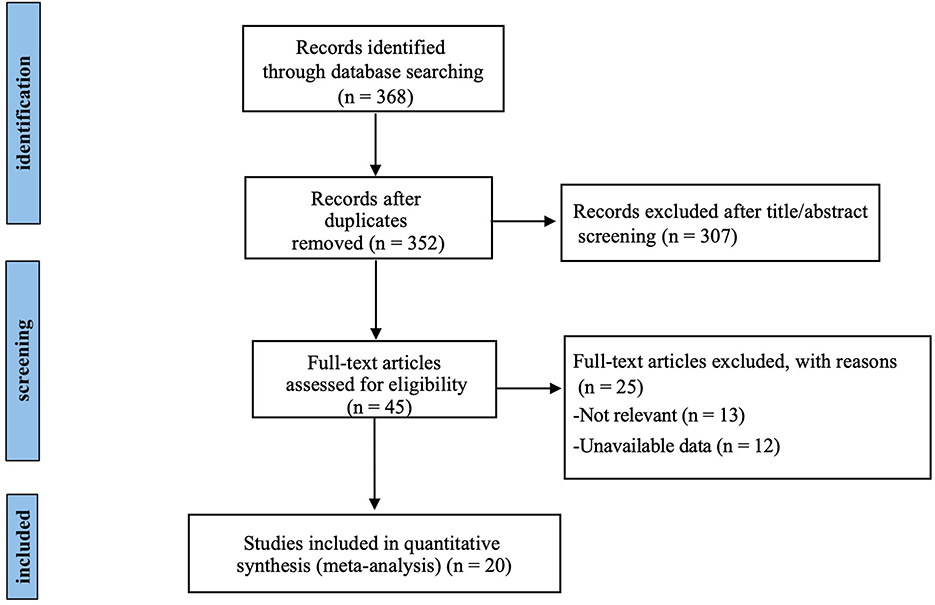
A total of 368 relevant studies were identified through systematic searching, and duplicate records were removed. The remaining 45 studies were assessed based on their titles and abstracts. After a full-text review, 13 studies were excluded because they did not align with the objectives of the meta-analysis, and 12 studies were excluded due to the absence of the outcome measures of interest. Ultimately, 20 RCTs with 329 participants were included (Figure 1).
3.2 Study characteristics
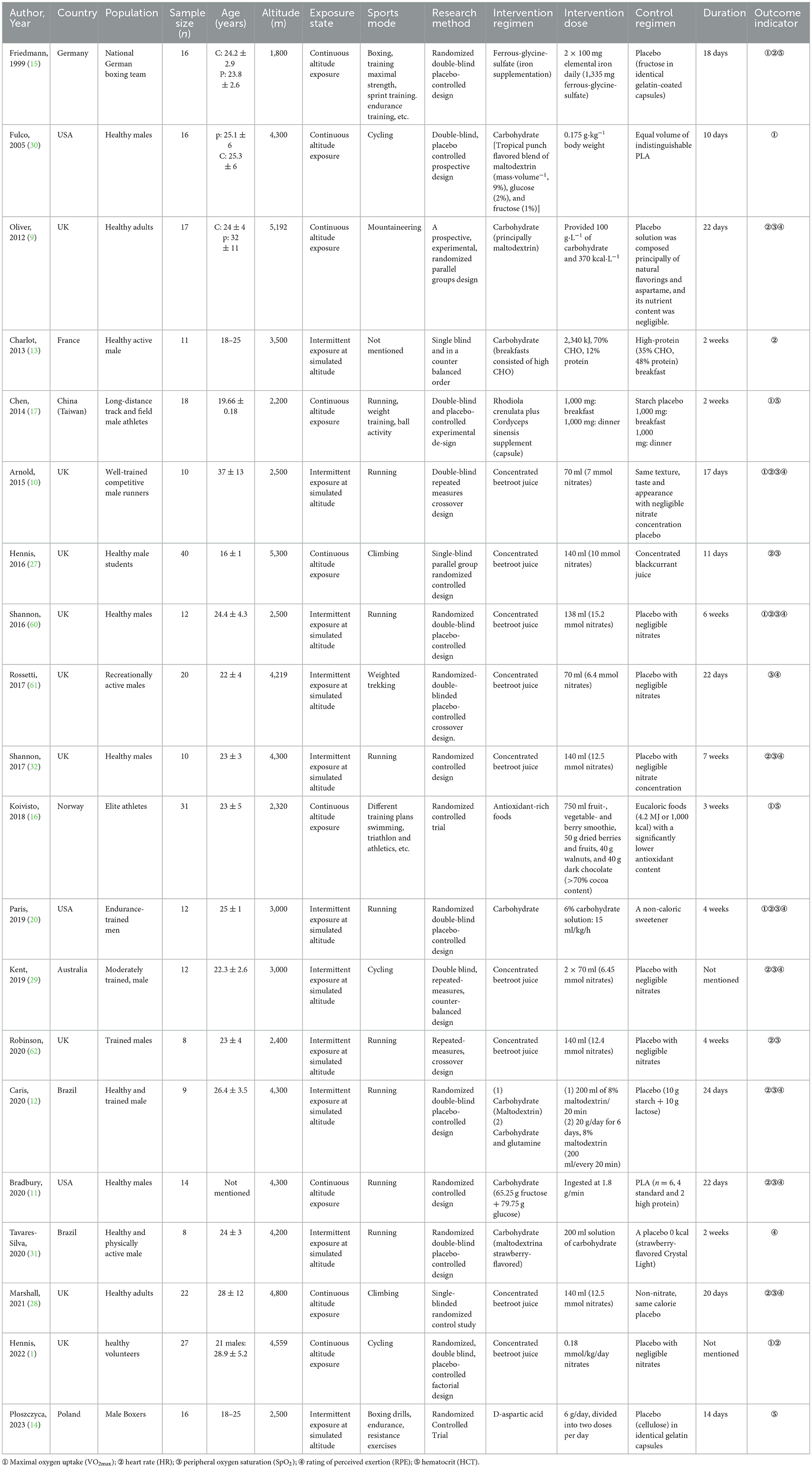
The baseline characteristics of the quantitative and qualitative studies are detailed in Table 1. This meta-analysis included 20 studies published between 1999 and 2023, involving eight types of dietary interventions. The proportion of participants in each dietary intervention was as follows: nitrate (48.94%), CHO (26.44%), antioxidant-rich foods (9.42%), CHO combined with glutamine (5.47%), RC (5.47%), d-aspartic acid (4.86%), iron (4.86%), and high-protein (2.74%). The study duration ranged from 10 days to 7 weeks.
3.3 Subject characteristics
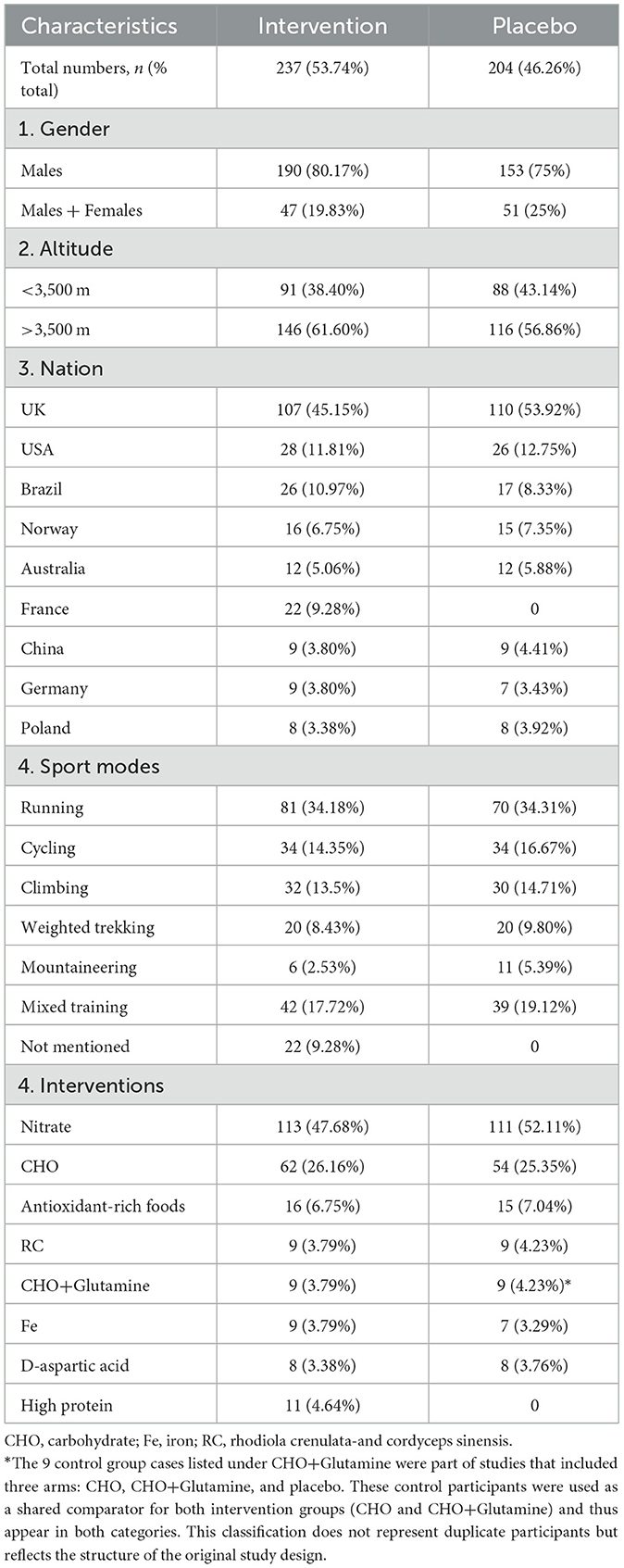
A total of 329 independent participants were included in this meta-analysis. Due to the use of a crossover design in some studies, all participants underwent both the intervention phase and the control phase, resulting in 441 participant data points, with 53.74% from the intervention group and 46.26% from the control group. The study population was predominantly male, with 80.17% in the intervention group and 75% in the control group. Regarding altitude distribution, 40.59% of the participant data points were from high-altitude areas above 3,500 meters. Among the different study locations, the highest proportion of participant data points came from the UK (49.20%). In terms of exercise modalities, running was the most common form of exercise, with 34.18% in the intervention group and 34.31% in the control group (Table 2).
3.4 Risk of bias
Bias risk analysis of the 20 included studies was conducted using the RoB 2 tool (Figure 2). Five studies showed some high-risk points [Charlot et al. (13); Cheng, (17); Hennis et al. (27); Hennis et al. (1); Marshall et al. (28)]. Additionally, four studies had issues with random sequence generation [Charlot et al. (13); Cheng, (17); Kent et al. (29); Paris et al. (20)]. Fourteen studies had problems with allocation concealment [Bradbury et al. (11); Caris and Thomatieli-Santos (12); Charlot et al. (13); Cheng, (17); Friedmann et al. (15); Fulco et al. (30); Hennis et al. (1); Kent et al. (29); Koivisto et al. (16); Oliver et al. (9); Paris et al. (20); Płoszczyca et al. (14); Robison, (62); Tavares-Silva et al. (31)]. Seven studies had issues with blinding of participants and researchers [Bradbury et al. (11); Charlot et al. (13); Hennis et al. (27); Koivisto et al. (16); Marshall et al. (28); Płoszczyca et al. (14); Shannon et al. (32)]. Six studies had problems with blinding of outcome assessors [Bradbury et al. (11); Charlot et al. (13); Kent et al. (29); Marshall et al. (28); Robison, (62); Shannon et al. (32)].
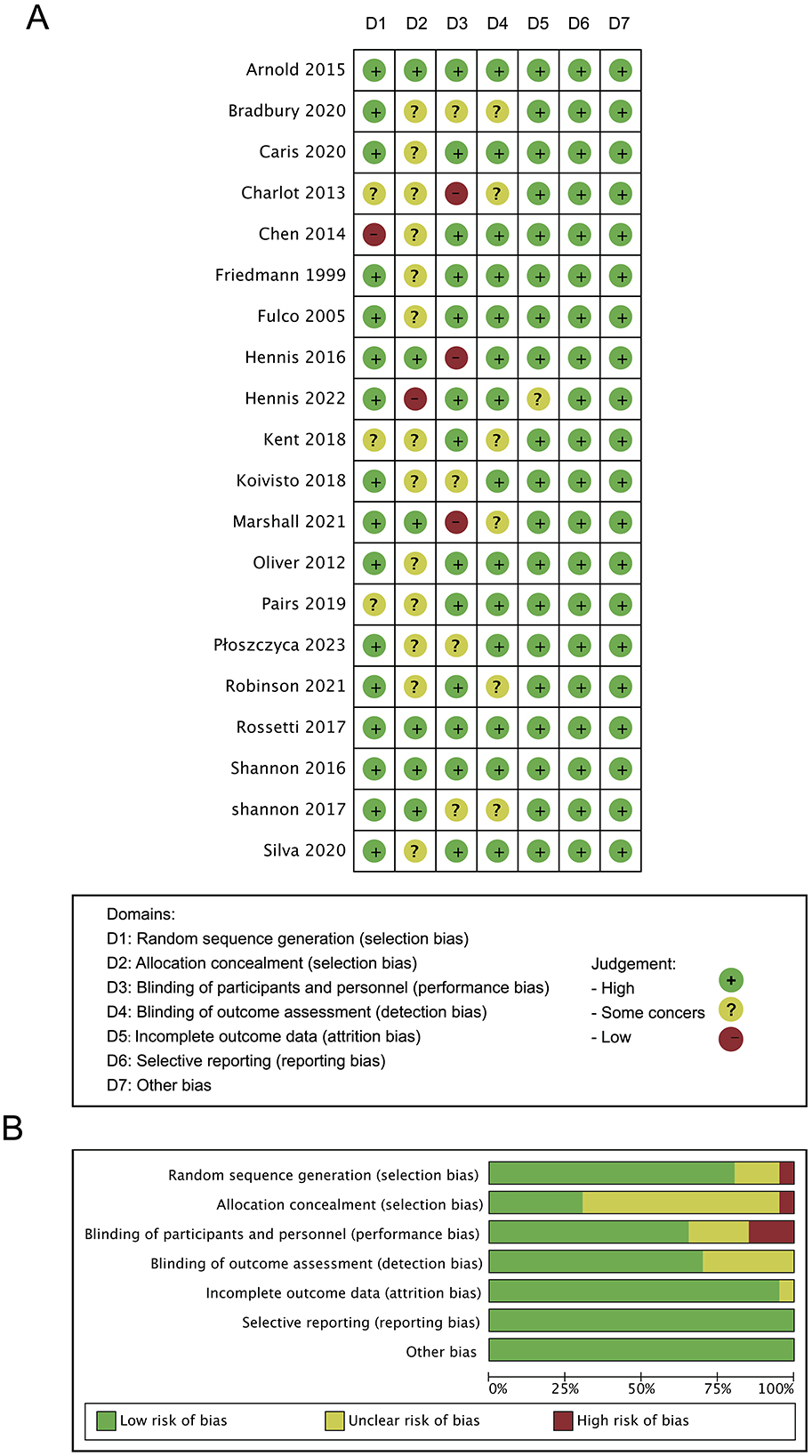
Figure 2. Risk of bias (ROB) analysis highlighting results in all domains examined within the nine identified studies (A) and overall risk of bias for included studies (B).
3.5 Quantitative synthesis
Figure 3 shows the network interventions for each outcome. The thickness of the lines corresponds to the number of studies, and the size of the nodes corresponds to the number of included treatment options. For RPE, SpO2, and HR, the networks contained at least one closed loop, enabling the assessment of inconsistency. In contrast, the VO2max and HCT networks consisted only of star-shaped structures centered on the placebo, with no closed loops available for inconsistency checks.
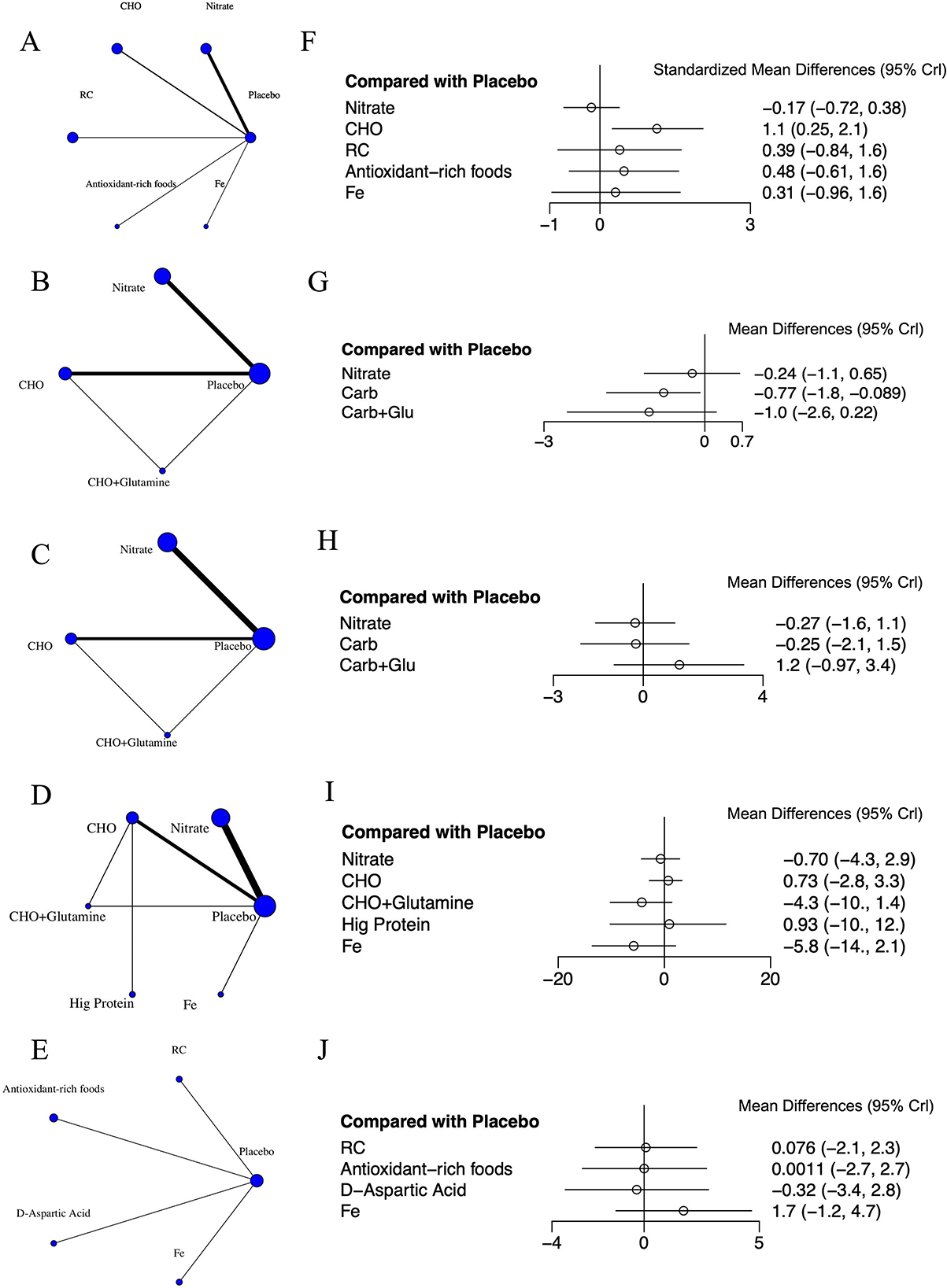
Figure 3. Network meta-analysis of various interventions on cardiopulmonary fitness. (A) Network of interventions for VO2max. (B) Network of interventions for RPE. (C) Network of interventions for SpO2. (D) Network of interventions for HR. (E) Network of interventions for HCT. (F) Forest plot displaying weighted standardized mean difference and 95% credible interval for the effect of various interventions vs. placebo on VO2max levels. (G) Forest plot displaying weighted mean difference and 95% credible interval for the effect of various interventions vs. placebo on RPE levels. (H) Forest plot displaying weighted mean difference and 95% credible interval for the effect of various interventions vs. placebo on SpO2 levels. (I) Forest plot displaying weighted mean difference and 95% credible interval for the effect of various interventions vs. placebo on HR levels. (J) Forest plot displaying weighted mean difference and 95% credible interval for the effect of various interventions vs. placebo on HCT levels. CHO, carbohydrate; Fe, iron.
3.6 Ranking of interventions
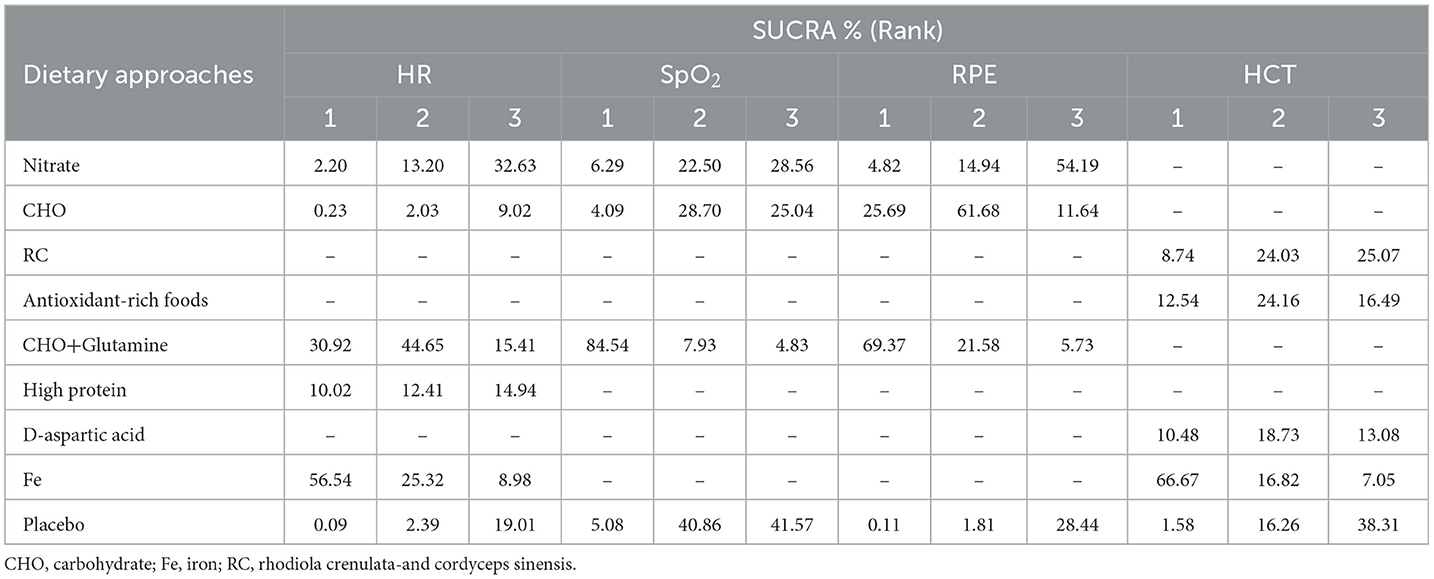
The SUCRA was used to calculate the probability of each dietary intervention being ranked for each outcome. The results are shown in Table 3.
3.7 Results of cardiopulmonary fitness
A total of five cardiopulmonary outcomes (VO2max, HR, SpO2, HCT, and RPE) were analyzed across multiple studies and interventions (Figure 3). CHO supplementation consistently showed favorable effects, demonstrating significant improvements in VO2max compared to both placebo (SMD = 1.13, 95% CrI: 0.25–2.05) and nitrate supplementation (SMD = 1.31, 95% CrI: 0.26–2.38; Figure 4). For RPE, CHO alone also showed superiority over placebo (MD = −0.77, CrI: −1.83 to −0.09; Figure 4). For both RPE and SpO2, CHO combined with glutamine ranked highest in effectiveness (SUCRA: 69.37 and 84.54%, respectively; Figure 5). Besides, iron supplementation ranked highest for improving HR (SUCRA: 56.54%) and HCT (SUCRA: 66.67%; Figure 5).
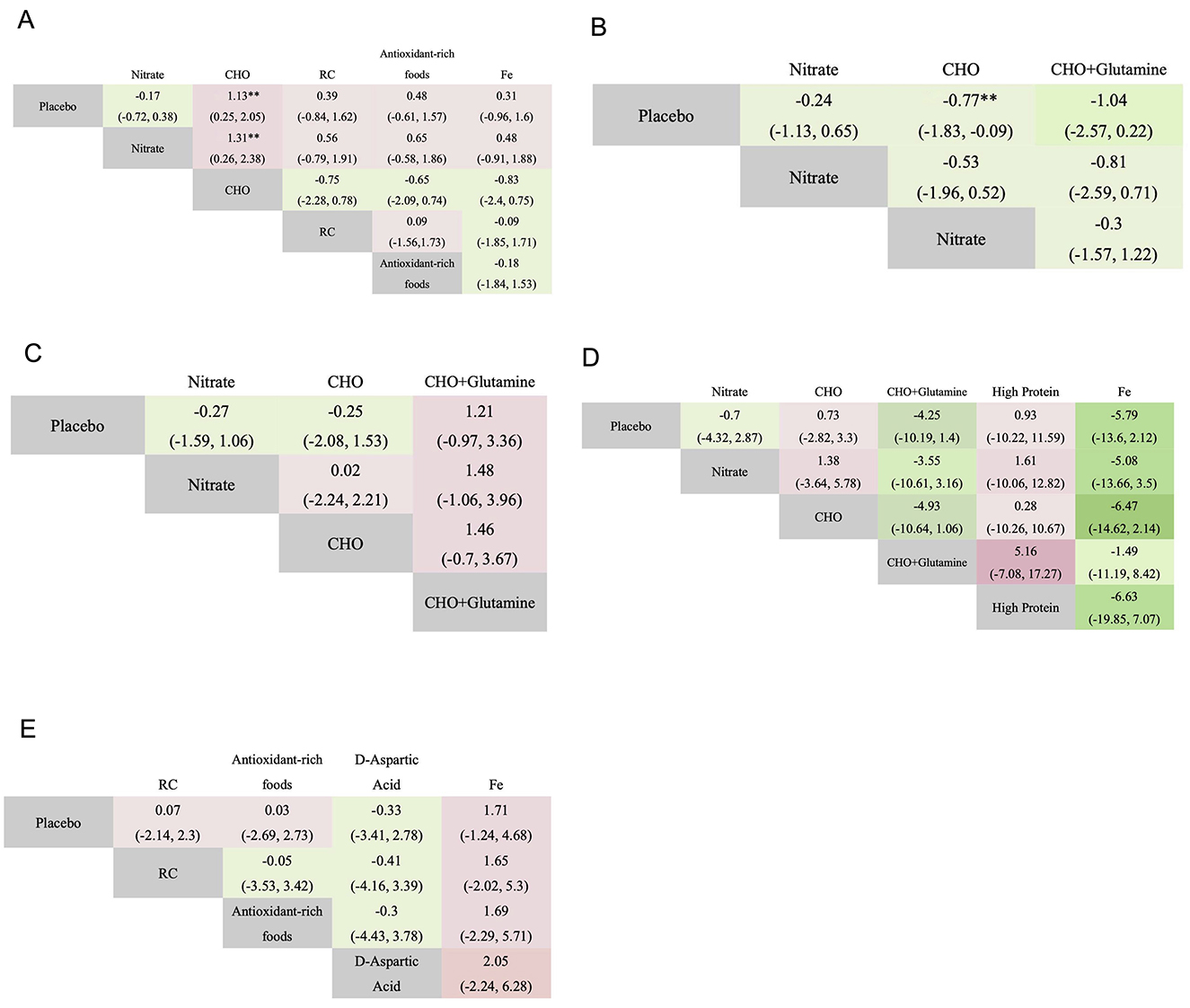
Figure 4. League table displaying pairwise comparisons among various interventions on VO2max (A), RPE (B), SpO2 (C), HR (D), and HCT (E). Statistically significant differences are bolded (**). CHO, carbohydrate; Fe, iron; **P < 0.01.
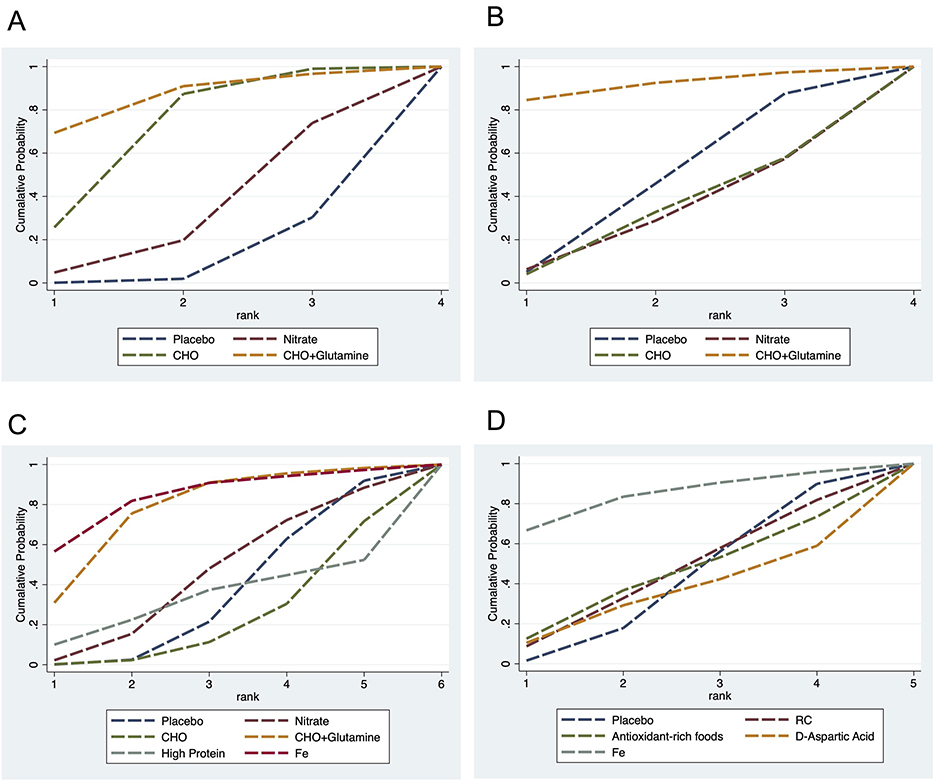
Figure 5. Surface Under the Cumulative Ranking Curve (SUCRA) illustrating the cumulative probability of each intervention being among the best in terms of RPE (A), SpO2 (B), HR (C), and HCT (D) levels. CHO, carbohydrate; Fe, iron.
3.8 Heterogeneity assessment
Between-study heterogeneity was evaluated using the estimated τ2 for each cardiopulmonary outcome. The τ2 values for VO2max (τ2 ≈ 0.12) and RPE (τ2 ≈ 0.33) were below the commonly cited threshold of 0.36, indicating low heterogeneity (24). In contrast, the τ2 values for SpO2 (τ2 ≈ 0.59), HR (τ2 ≈ 2.03), and HCT (τ2 ≈ 0.71) exceeded this threshold, suggesting substantial heterogeneity in these outcomes. These results imply that the studies reporting VO2max and RPE yielded more consistent findings, whereas greater variability was observed among studies evaluating SpO2, HR, and HCT.
3.9 Publication bias and hypothesis of overall consistency between networks
Figure 6 shows the funnel plot for publication bias. The results of the Egger regression test indicate no evidence of publication bias (all egger regression tests for comparisons were >0.05, Table 4). The comparison of DIC values between the consistency and inconsistency models showed that the consistency model was superior for all outcomes (Table 5), with no evidence of inconsistency in the network meta-analysis.
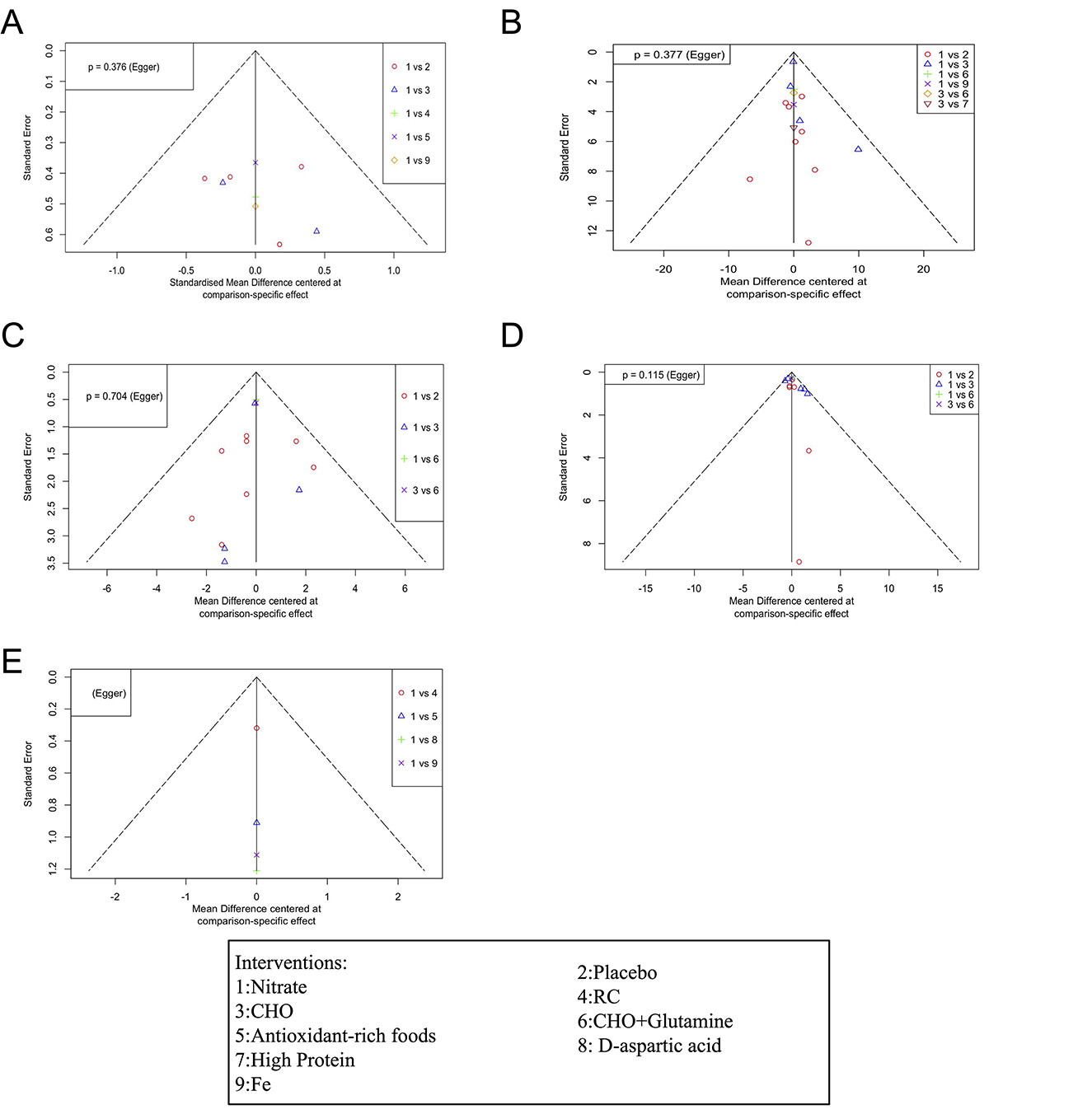
Figure 6. Funnel plot detailing publication bias in the studies reporting the impact of dietary nitrate on VO2max (A), HR (B), SpO2 (C), RPE (D), and HCT (E) levels. CHO, carbohydrate; Fe, iron; RC, rhodiola crenulata- and cordyceps sinensi.
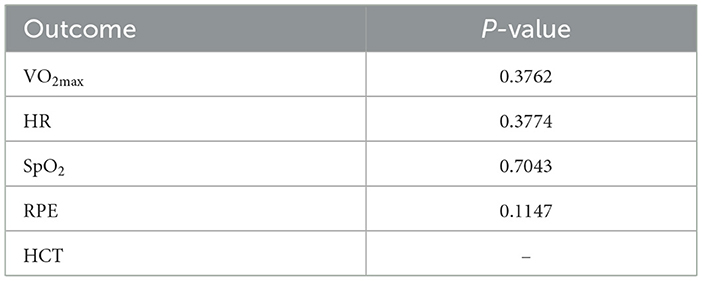
Table 4. Egger's test for publication bias in dietary interventions on cardiopulmonary outcomes at high altitude.
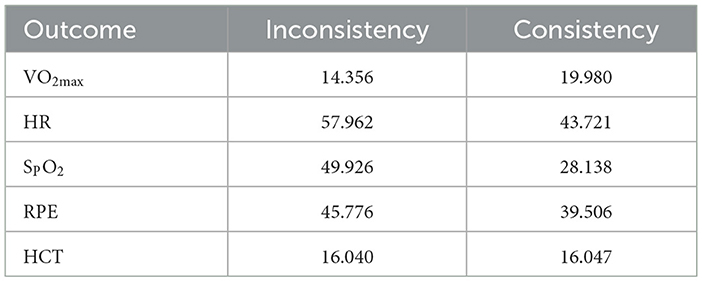
Table 5. Deviance information criterion (DIC) comparison between consistency and inconsistency models for each outcome.
3.10 Confidence in evidence and sensitivity analyses
We used the CINeMA platform to systematically evaluate the quality of evidence for the outcomes of our network meta-analysis. Overall, most comparisons were rated as low to moderate confidence, mainly due to concerns about within-study bias and imprecision. Several indirect comparisons were further downgraded to very low confidence because of limited sample sizes and wide confidence intervals (Appendix I). To test the robustness of our findings, we conducted sensitivity analyses by excluding five studies judged to be at high risk of bias and re-running the NMA. The resulting league tables, forest plots, and SUCRA values were generally similar to those from the main analyses (Appendix II). Excluding high-risk studies did not materially affect the effect estimates or treatment rankings, thereby supporting the robustness of our main conclusions.
4 Discussion
This study aimed to rank interventions for improving cardiopulmonary fitness and exercise performance at high altitudes and identify which dietary intervention was most beneficial in modulating the outcome measures of the participants. Our network meta-analysis, based on 20 RCTs involving 329 participants, indicated that different dietary interventions exerted distinct effects on exercise performance at high altitude. Carbohydrate-based strategies showed overall benefits, with carbohydrate plus glutamine supplementation demonstrating advantages in SpO2 and RPE, while carbohydrate alone was more consistently supported for VO2max improvement. Iron supplementation ranked highest for HR and HCT, suggesting potential benefits, although these effects did not reach statistical significance. In contrast, interventions such as high-protein, nitrates, antioxidant-rich foods, RC, and d-aspartic acid showed relatively limited effects.
Cardiopulmonary fitness is a core indicator of an individual's exercise performance and adaptability. In high-altitude environments, various physiological factors such as hypoxia significantly affect the autonomic regulation of the heart and lead to a decline in tissue oxygenation, which is reflected by a decrease in SpO2 and an increase in HR (33). A change in HR greater than 2.4% is considered the minimally clinically important difference (MCID), while for SpO2, a widely accepted MCID is approximately ±4 percentage point (34, 35). VO2max is the gold standard for assessing cardiopulmonary health. Its improvement not only contributes to better cardiopulmonary health but also enhances an individual's exercise performance (36). According to previous research, an increase of 1.0 ml/kg/min in VO2max is associated with a 9% reduction in all-cause mortality risk. Therefore, this threshold was adopted in the present study as the MCID to evaluate the clinical relevance of each intervention's effect (37). RPE is an effective tool for evaluating exercise intensity and physiological load during resistance training. A higher score indicates greater physiological strain caused by exercise and higher cardiopulmonary regulatory pressure (38). For the RPE, a change of 1.5 points is generally considered the MCID, indicating a perceptible and clinically relevant change in exertion levels (34).
Additionally, HCT is positively correlated with SpO2 and VO2max. This indicator also reflects an individual's oxygen transport and cardiopulmonary adaptation (39). Therefore, the five outcome indicators included in this study provide a comprehensive assessment system for cardiopulmonary fitness, effectively reflecting an individual's physiological status and adaptability during high-altitude exercise.
The network meta-analysis results of this study confirm that CHO supplementation effectively maintains the oxidation rate of energy substrates during high-altitude exercise, significantly increasing VO2max and reducing RPE scores. When combined with glutamine, this intervention ranked highest for improving SpO2 and RPE. Our findings are consistent with several previous studies. For example, Caris et al. found that, compared to placebo, CHO supplementation or the combination of CHO and glutamine significantly reduced RPE scores (12). High-altitude training exacerbates the challenges of energy metabolism, and athletes face significantly increased physiological loads compared to sea level, such as shortness of breath, gas exchange disturbances, reduced cardiac output, and central fatigue due to hypoxic responses (40). Therefore, practitioners, coaches, and athletes should not overlook the importance of energy in high-altitude training (41). In lowland areas, CHO supplements have become very common among athletes. According to the FAO Food and Nutrition report, CHO-containing foods are considered to have the most significant impact on exercise performance (42). CHO are the main energy substrate for skeletal muscles during prolonged exercise, and as exercise intensity increases, the proportion of energy supplied by CHO significantly rises (43). During exercise lasting more than 2 h, CHO intake, compared to placebo, prevents hypoglycemia, maintains a higher CHO oxidation rate, and enhances endurance (44). Glutamine, a conditionally essential amino acid, is widely used in sports nutrition. It can influence respiratory function through neuroregulatory mechanisms, helping to restore SpO2 (45). The immunoregulatory effects of glutamine maintain immune cell activity, ensuring training continuity, while also delaying exercise-induced fatigue through various mechanisms (46). Furthermore, the hypoxic environment at high altitudes triggers acute immune suppression, and prolonged exercise training further exacerbates this phenomenon, leading to a significant decline in glutamine concentrations in muscles and plasma. This reduction in glutamine leaves immune cells without the energy sources needed for synthesis and nucleotides. As a result, athletes training at high altitudes are at greater risk of infection (47, 48). Studies have shown that the combination of glutamine and CHO improves exercise performance more effectively than glutamine alone (46). The combined supplementation may enhance performance and improve metabolic status after high-intensity training by increasing glycogen reserves, supporting gluconeogenesis, and accelerating recovery (49). Favano et al. (50) supplemented football players undergoing intermittent treadmill training with glutamine peptides and CHO or with CHO alone and observed that compared to CHO supplementation alone, the combination of glutamine and CHO resulted in increased exercise time and distance, while significantly reducing RPE (50). Furthermore, Carvalho-Peixoto et al. (49) found that the combined supplementation of glutamine and CHO effectively reduced blood ammonia accumulation and central fatigue compared to the control group.
The results of the network meta-analysis in this study further confirm that iron supplementation effectively improves tissue oxygen supply by increasing hemoglobin concentration and enhancing blood oxygen-carrying capacity (51). It showed the highest ranking for post-exercise HR and HCT among the interventions assessed. As a core nutrient for oxygen transport and cellular energy metabolism, iron plays a key role in the adaptation process to high-altitude exercise. The hypoxic environment at high altitudes significantly stimulates the secretion of erythropoietin, thereby greatly increasing the body's demand for iron. Athletes, due to exercise-related iron loss pathways (including sweat loss, exercise-induced hemolysis, gastrointestinal bleeding, and menstrual blood loss in female athletes), face a higher risk of iron deficiency (41). Research evidence indicates that daily iron supplementation effectively promotes erythropoiesis during high-altitude adaptation, particularly for athletes with low baseline ferritin levels (52).
Dietary antioxidants are among the most common sports nutrition supplements used to alleviate exercise-induced oxidative stress. Intense exercise and muscle contractions increase the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), promoting oxidative stress in skeletal muscles. Therefore, endurance athletes commonly take antioxidant supplements to minimize exercise-induced oxidative stress, thereby enhancing recovery and improving athletic performance (53). Additionally, at sea level, dietary nitrates are also widely used as a popular supplement among athletes. Nitrate intake significantly increases the concentration of nitrite () in plasma, which is an important substrate to produce nitric oxide (NO) and a biomarker in its circulation. As a key signaling molecule, NO regulates exercise performance through various physiological mechanisms, including enhancing skeletal muscle contraction efficiency, modulating mitochondrial respiration and energy metabolism, and improving local tissue blood flow. These effects synergistically promote oxygen utilization efficiency and metabolic stability, thereby enhancing exercise endurance and overall performance (54, 55). However, in high-altitude environments, the effects of some antioxidants are not as effective. Our study showed that nitrate supplementation did not demonstrate significant advantages across several indicators. Our previous meta-analysis on the effects of nitrate on cardiopulmonary fitness at high altitudes also showed that while it increased serum nitrite levels, it had no effect on cardiopulmonary fitness (56). Hennis' study similarly indicated that dietary nitrate supplementation did not significantly improve exercise efficiency in high-altitude environments (1). Rhodiola rosea and Cordyceps sinensis are widely found in high-altitude areas in the plateau and mountain regions. Both plants are popular traditional medicines in Europe and Asia. Studies have shown that Rhodiola rosea is often used as an ergogenic aid to enhance endurance performance and antioxidant capacity, while Cordyceps supplementation has been shown to stimulate vasodilation, possibly by stimulating NO release, improving tissue oxygen utilization, and thereby having the potential to enhance endurance performance (17). However, when these two supplements were combined, similar to antioxidant food supplementation, the intervention did not show significant effects. The primary reason for this may be the small sample sizes in the related studies, as most interventions were included in the analysis for only 1–2 indicators, which led to reduced statistical power and made it difficult to fully assess their true effects. This finding is consistent with the studies of Koivisto et al. and Chen et al., which showed no significant effect of antioxidant food supplementation or Rhodiola rosea combined with Cordyceps on HCT (16, 17). This result is similar to high-protein supplementation or D-aspartic acid supplementation, where the effects on high-altitude exercise performance were not significant. Protein is typically considered a low-efficiency fuel source, contributing little to the total energy demands of exercise. For example, Macdermid and Stannard found that cyclists on a high-protein diet took significantly longer to complete a time trial compared to those on a low-protein/high-CHO diet (57). D-aspartic acid may improve muscle function by regulating the hypothalamic-pituitary-gonadal axis and increasing plasma testosterone levels, potentially enhancing exercise performance (58). Płoszczyca et al. found that continuous supplementation of d-aspartic acid did not affect testosterone, cortisol, or hematological responses during training in athletes (14). This may be because the shorter duration of D-aspartic acid supplementation was insufficient to observe changes in hormone secretion.
To the best of our knowledge, this is the first study to systematically assess the ranking of different dietary interventions on high-altitude exercise performance and cardiopulmonary fitness using network meta-analysis methods. The main strength of this study lies in its rigorous methodological design, which comprehensively evaluated multiple key physiological indicators related to high-altitude exercise. The findings clearly identified the three promising nutritional intervention strategies, providing practical guidance for high-altitude trainees. However, there are some limitations. For instance, the overall sample size across the included RCTs was relatively small, with most trials enrolling only a limited number of participants. Such constraints inevitably reduce the statistical power of the analyses and may compromise the stability and precision of the pooled estimates. Under these conditions, both false-negative and false-positive findings become more likely, and some observed effects may reflect random variation rather than consistent clinical benefit. Another key limitation of this review lies in the clinical heterogeneity among the included studies. Specifically, the altitude of exposure varied considerably, ranging from moderate to extreme altitudes, which may have triggered different physiological adaptations. Participants engaged in diverse forms of exercise, such as running, cycling, hiking, and mountaineering, each imposing distinct metabolic and cardiovascular demands. The duration of dietary interventions also varied, from several days to multiple weeks, adding further variability to exposure intensity and adaptation. Additionally, approximately half of the studies were conducted in real high-altitude environments, while the remainder employed simulated hypoxic conditions. These settings differ substantially in terms of environmental stressors such as temperature, terrain, and barometric pressure, which may influence the efficacy of nutritional interventions. Collectively, these variations may have contributed to inconsistencies in effect estimates and complicated the interpretation of outcomes. However, due to the limited number of included studies, formal subgroup analyses could not be performed, representing a constraint in addressing and interpreting potential heterogeneity. Additionally, the current data primarily come from male athletes and active individuals with a training background, which may not fully reflect the impact of gender and training background differences on intervention outcomes. Therefore, the results may be more applicable to trained or active male individuals and may not be fully generalizable to a broader population. Moreover, although SUCRA provides a convenient summary of the relative ranking of different interventions, its interpretation should be approached with caution. SUCRA values are easily influenced by sample size, network structure, and between-study heterogeneity, and they do not directly convey the magnitude or clinical relevance of treatment effects. Some NMAs have attempted to report uncertainty intervals for SUCRA (SUCRA CrIs), but when the analysis is based on only a few small studies, the 95% CrIs are often extremely wide, sometimes spanning the entire range from 0 to 100% (59). This indicates considerable instability and imprecision in the ranking results. Therefore, SUCRA rankings should be regarded as suggestive rather than definitive evidence. Taken together, these limitations mean that the conclusions of the present review should be interpreted with caution, and the overall certainty of evidence is low to moderate. Thus, the effectiveness of targeted dietary interventions in enhancing cardiopulmonary fitness and exercise performance at high altitudes remains a topic of ongoing debate. Future RCTs with larger sample sizes, longer follow-up periods, and more diverse populations (including women and non-athletic individuals), as well as standardized protocols for exercise modality, exposure duration, and supplementation strategies, are urgently needed to confirm and refine these findings, thereby providing more reliable evidence for high-altitude sports nutrition interventions.
5 Conclusion
The eight dietary strategies evaluated across 20 randomized controlled trials involving 329 participants, carbohydrate supplementation significantly improved VO2max and reduced RPE, with the observed increase in VO2max exceeding the MCID, indicating potential clinical relevance. Additionally, carbohydrate combined with glutamine ranked first in improving SpO2 and RPE, while iron supplementation ranked first for enhancing HR and HCT. However, these latter interventions did not demonstrate statistically significant advantages. Moreover, antioxidant-rich foods, nitrates, high-protein diets, and RC showed limited effects. Overall, carbohydrate-based strategies appear to be the most promising for supporting cardiopulmonary function and exercise performance at high altitude. Although the findings provide a reference for high-altitude sports nutrition strategies, the conclusions are limited by the number of studies and sample heterogeneity. Further high-quality studies are needed to validate these findings and optimize nutritional interventions for high-altitude training populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
BW: Methodology, Funding acquisition, Writing – original draft. YX: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. NL: Investigation, Methodology, Writing – original draft. JS: Methodology, Validation, Writing – original draft. BZ: Data curation, Methodology, Writing – original draft. XM: Investigation, Supervision, Writing – review & editing. KZ: Conceptualization, Supervision, Writing – review & editing. CK: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Shenzhen Natural Science Foundation (No. 202389) and Department of Science and Technology of Sichuan Province (No. 2024ZYD0193).
Acknowledgments
Xin Ma, Kaihong Zeng, and Chao Kang are the guarantors of this work and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1658950/full#supplementary-material
References
1. Hennis PJ, Cumpstey AF, O'Doherty AF, Fernandez BO, Gilbert-Kawai ET, Mitchell K, et al. Dietary nitrate supplementation does not alter exercise efficiency at high altitude–further results from the Xtreme Alps study. Front Physiol. (2022) 13:827235. doi: 10.3389/fphys.2022.827235
2. Ramchandani R, Florica IT, Zhou Z, Alemi A, Baranchuk A. Review of athletic guidelines for high-altitude training and acclimatization. High Alt Med Biol. (2024) 25:113–21. doi: 10.1089/ham.2023.0042
3. Deng L, Liu Y, Chen B, Hou J, Liu A, Yuan X. Impact of altitude training on athletes' aerobic capacity: a systematic review and meta-analysis. Life. (2025) 15:305. doi: 10.3390/life15020305
4. Teroujeni SSS. Athlete adaptations to high-altitude training: a behavioral analysis. Health. (2023) 1:110–8. doi: 10.61838/kman.hn.1.2.15
5. de Paula P, Niebauer J. Effects of high altitude training on exercise capacity: fact or myth. Sleep Breath. (2012) 16:233–9. doi: 10.1007/s11325-010-0445-1
6. Koivisto-Mørk AE, Paur I, Paulsen G, Garthe I, Raastad T, Bastani NE, et al. Dietary adjustments to altitude training in elite endurance athletes; impact of a randomized clinical trial with antioxidant-rich foods. Front Sports Act Living. (2020) 2:106. doi: 10.3389/fspor.2020.00106
7. Karpecka-Gałka E, Fraczek B. Nutrition, hydration and supplementation considerations for mountaineers in high-altitude conditions: a narrative review. Front Sports Act Living. (2024) 6:1435494. doi: 10.3389/fspor.2024.1435494
8. Hall R, Peeling P, Nemeth E, Bergland D, McCluskey WT, Stellingwerff T. Single versus split dose of iron optimizes hemoglobin mass gains at 2106 m altitude. Med Sci Sports Exerc. (2019) 51:751. doi: 10.1249/MSS.0000000000001847
9. Oliver SJ, Golja P, Macdonald JH. Carbohydrate supplementation and exercise performance at high altitude: a randomized controlled trial. High Alt Med Biol. (2012) 13:22–31. doi: 10.1089/ham.2011.1087
10. Arnold JT, Oliver SJ, Lewis-Jones TM, Wylie LJ, Macdonald JH. Beetroot juice does not enhance altitude running performance in well-trained athletes. Appl Physiol Nutr Metab. (2015) 40:590–5. doi: 10.1139/apnm-2014-0470
11. Bradbury KE, Berryman CE, Wilson MA, Luippold AJ, Kenefick RW, Young AJ, et al. Effects of carbohydrate supplementation on aerobic exercise performance during acute high altitude exposure and after 22 days of acclimatization and energy deficit. J Int Soc Sports Nutr. (2020) 17:4. doi: 10.1186/s12970-020-0335-2
12. Caris AV, Thomatieli-Santos RV. Carbohydrate and glutamine supplementation attenuates the increase in rating of perceived exertion during intense exercise in hypoxia similar to 4200 m. Nutrients. (2020) 12:3797. doi: 10.3390/nu12123797
13. Charlot K, Pichon A, Richalet J-P, Chapelot D. Effects of a high-carbohydrate versus high-protein meal on acute responses to hypoxia at rest and exercise. Eur J Appl Physiol. (2013) 113:691–702. doi: 10.1007/s00421-012-2472-z
14. Płoszczyca K, Czuba M, Zakrzeska A, Gajda R. The effects of six-gram D-aspartic acid supplementation on the testosterone, cortisol, and hematological responses of male boxers subjected to 11 days of nocturnal exposure to normobaric hypoxia. Nutrients. (2023) 16:76. doi: 10.3390/nu16010076
15. Friedmann B, Jost J, Weller E, Werle E, Eckardt K-U, Bärtsch P, et al. Effects of iron supplementation on total body hemoglobin during endurance training at moderate altitude. Int J Sports Med. (1999) 20:78–85. doi: 10.1055/s-2007-971097
16. Koivisto A, Paulsen G, Paur I, Garthe I, Tønnessen E, Raastad T, et al. Antioxidant-rich foods and response to altitude training: a randomized controlled trial in elite endurance athletes. Scand J Med Sci Sports. (2018) 28:1982–95. doi: 10.1111/sms.13212
17. Chen C-Y, Hou C-W, Bernard JR, Chen C-C, Hung T-C, Cheng L-L, et al. Rhodiola crenulata-and Cordyceps sinensis-based supplement boosts aerobic exercise performance after short-term high altitude training. High Alt Med Biol. (2014) 15:371–9. doi: 10.1089/ham.2013.1114
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. (2021) 134:103–12. doi: 10.1016/j.jclinepi.2021.02.003
19. Mallet RT, Burtscher J, Richalet J-P, Millet GP, Burtscher M. Impact of high altitude on cardiovascular health: current perspectives. Vascu Health Risk Manage. (2021) 17:317–35. doi: 10.2147/VHRM.S294121
20. Paris HL, Fulton TJ, Chapman RF, Fly AD, Koceja DM, Mickleborough TD. Effect of carbohydrate ingestion on central fatigue during prolonged running exercise in moderate hypoxia. J Appl Physiol. (2019) 126:141–51. doi: 10.1152/japplphysiol.00684.2018
21. Jørgensen L, Paludan-Müller AS, Laursen DR, Savović J, Boutron I, Sterne JA, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. (2016) 5:1–13. doi: 10.1186/s13643-016-0259-8
22. Brooks S. Markov chain Monte Carlo method and its application. J R Stat Soc Ser D. (1998) 47:69–100. doi: 10.1111/1467-9884.00117
23. Yang S, Berdine G. The surface under the cumulative ranking curve. Southwest J Med. (2025) 13:35–8. doi: 10.12746/swjm.v13i55.1445
24. Bo Z, Jian Y, Yan L, Gangfeng G, Xiaojing L, Xiaolan L, et al. Pharmacotherapies for central post-stroke pain: a systematic review and network meta-analysis. Oxid Med Cell Longev. (2022) 2022:3511385. doi: 10.1155/2022/3511385
25. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades A. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. (2013) 33:641–56. doi: 10.1177/0272989X12455847
26. Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network Meta-Analysis for Decision-Making. Chichester: John Wiley & Sons (2018). doi: 10.1002/9781118951651
27. Hennis PJ, Mitchell K, Gilbert-Kawai E, Bountziouka V, Wade A, Feelisch M, et al. Effects of dietary nitrate supplementation on symptoms of acute mountain sickness and basic physiological responses in a group of male adolescents during ascent to Mount Everest Base Camp. Nitric Oxide. (2016) 60:24–31. doi: 10.1016/j.niox.2016.08.007
28. Marshall AR, Rimmer JE, Shah N, Bye K, Kipps C, Woods DR, et al. Marching to the beet: the effect of dietary nitrate supplementation on high altitude exercise performance and adaptation during a military trekking expedition. Nitric Oxide. (2021) 113:70–7. doi: 10.1016/j.niox.2021.05.002
29. Kent GL, Dawson B, McNaughton LR, Cox GR, Burke LM, Peeling P. The effect of beetroot juice supplementation on repeat-sprint performance in hypoxia. J Sports Sci. (2019) 37:339–46. doi: 10.1080/02640414.2018.1504369
30. Fulco CS, Kambis K, Friedlander A, Rock P, Muza S, Cymerman A. Carbohydrate supplementation improves time-trial cycle performance during energy deficit at 4,300-m altitude. J Appl Physiol. (2005) 99:867–76. doi: 10.1152/japplphysiol.00019.2005
31. Tavares-Silva E, Donatto F, Medeiros R, Santos S, Caris A, Thomatieli-Santos R. Carbohydrate supplementation and psychophysiological responses during moderate exercise in hypoxia. J Int Soc Sports Nutr. (2020) 17:3. doi: 10.1186/s12970-019-0331-6
32. Shannon OM, Duckworth L, Barlow MJ, Deighton K, Matu J, Williams EL, et al. Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very-high simulated altitude. Front Physiol. (2017) 8:401. doi: 10.3389/fphys.2017.00401
33. Oliveira ALMB, Rohan PdA, Gonçalves TR, da Silva Soares PP. Effects of hypoxia on heart rate variability in healthy individuals: a systematic review. Int J Cardiovasc Sci. (2017) 30:251–61. doi: 10.5935/2359-4802.20170035
34. Doyle EW, Doyle TL, Bonacci J, Fuller JT. Field-based gait retraining to reduce impact loading using Tibial accelerometers in high-impact recreational runners: a feasibility study. Sensors. (2025) 25:1712. doi: 10.3390/s25061712
35. Balfour-Lynn IM, Prasad SA, Laverty A, Whitehead BF, Dinwiddie R. A step in the right direction: assessing exercise tolerance in cystic fibrosis. Pediatr Pulmonol. (1998) 25:278–84. doi: 10.1002/(SICI)1099-0496(199804)25:4<278::AID-PPUL8>3.0.CO;2-G
36. Crowley E, Powell C, Carson BP, Davies RW. The effect of exercise training intensity on VO2max in healthy adults: an overview of systematic reviews and meta-analyses. Transl Sports Med. (2022) 2022:9310710. doi: 10.1155/2022/9310710
37. Laukkanen JA, Zaccardi F, Khan H, Kurl S, Jae SY, Rauramaa R, et al. Long-Term Change in Cardiorespiratory Fitness and All-Cause Mortality: A Population-Based Follow-Up Study. Mayo Clinic Proceedings. Elsevier (2016). doi: 10.1016/j.mayocp.2016.05.014
38. Lea JW, O'Driscoll JM, Hulbert S, Scales J, Wiles JD. Convergent validity of ratings of perceived exertion during resistance exercise in healthy participants: a systematic review and meta-analysis. Sports Med Open. (2022) 8:2. doi: 10.1186/s40798-021-00386-8
39. Schuler B, Arras M, Keller S, Rettich A, Lundby C, Vogel J, et al. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc Nat Acad Sci USA. (2010) 107:419–23. doi: 10.1073/pnas.0912924107
40. Grocott MPW, Levett DZH, Ward SA. Exercise physiology: exercise performance at altitude. Curr Opin Physiol. (2019) 10:210–8. doi: 10.1016/j.cophys.2019.06.008
41. Stellingwerff T. Nutrition recommendations for altitude training. Sports Sci Exch. (2019) 29:1–5.
42. Joint F, Consultation WE. Carbohydrates in human nutrition (FAO Food and Nutrition Paper 66). Rome, Italy: FAO (1998).
43. Cermak NM, van Loon LJ. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. (2013) 43:1139–55. doi: 10.1007/s40279-013-0079-0
44. Jeukendrup A. A step towards personalized sports nutrition: carbohydrate intake during exercise. Sports Med. (2014) 44:25–33. doi: 10.1007/s40279-014-0148-z
45. Caris AV, Tavares-Silva E, Thomatieli-Santos RV. Effects of carbohydrate and glutamine supplementation on cytokine production by monocytes after exercise in hypoxia: a crossover, randomized, double-blind pilot study. Nutrition. (2020) 70:110592. doi: 10.1016/j.nut.2019.110592
46. Coqueiro AY, Rogero MM, Tirapegui J. Glutamine as an anti-fatigue amino acid in sports nutrition. Nutrients. (2019) 11:863. doi: 10.3390/nu11040863
47. Caris AV, Santos RVT. Performance and altitude: ways that nutrition can help. Nutrition. (2019) 60:35–40. doi: 10.1016/j.nut.2018.09.030
48. Gleeson M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J Nutr. (2008) 138:2045S−9S. doi: 10.1093/jn/138.10.2045S
49. Carvalho-Peixoto J, Alves RC, Cameron L-C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl Physiol Nutr Metab. (2007) 32:1186–90. doi: 10.1139/H07-091
50. Favano A, Santos-Silva PR, Nakano EY, Pedrinelli A, Hernandez AJ, Greve JMD. Peptide glutamine supplementation for tolerance of intermittent exercise in soccer players. Clinics. (2008) 63:27–32. doi: 10.1590/S1807-59322008000100006
51. Buratti P, Gammella E, Rybinska I, Cairo G, Recalcati S. Recent advances in iron metabolism: relevance for health, exercise, and performance. Med Sci Sports Exerc. (2015) 47:1596–604. doi: 10.1249/MSS.0000000000000593
52. Garvican-Lewis LA, Govus AD, Peeling P, Abbiss CR, Gore CJ. Iron supplementation and altitude: decision making using a regression tree. J Sports Sci Med. (2016) 15:204–5.
53. Mason SA, Trewin AJ, Parker L, Wadley GD. Antioxidant supplements and endurance exercise: current evidence and mechanistic insights. Redox Biol. (2020) 35:101471. doi: 10.1016/j.redox.2020.101471
54. Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med. (2014) 44:35–45. doi: 10.1007/s40279-014-0149-y
55. Jones AM. Influence of dietary nitrate on the physiological determinants of exercise performance: a critical review. Appl Physiol Nutr Metab. (2014) 39:1019–28. doi: 10.1139/apnm-2014-0036
56. Kang C, Lin N, Xiong Y, Yang Y, Shi J, Zeng K, et al. Effects of nitrate supplements on cardiopulmonary fitness at high altitude: a meta-analysis of nine randomized controlled trials. PLoS ONE. (2025) 20:e0319667. doi: 10.1371/journal.pone.0319667
57. Macdermid PW, Stannard SR. A whey-supplemented, high-protein diet versus a high-carbohydrate diet: effects on endurance cycling performance. Int J Sport Nutr Exerc Metab. (2006) 16:65–77. doi: 10.1123/ijsnem.16.1.65
58. LaMacchia Z, Williams B, Furst T, Horvath P. Acute D-aspartic acid supplementation does not have an effect on serum testosterone but does have an effect on strength measures in college aged male athletes. Eur J Sports Exerc Sci. (2017) 5:34–41.
59. Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First-and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. (2020) 18:2179–91. e6. doi: 10.1016/j.cgh.2020.01.008
60. Shannon OM, Duckworth L, Barlow MJ, Woods D, Lara J, Siervo M, et al. Dietary nitrate supplementation enhances high-intensity running performance in moderate normobaric hypoxia, independent of aerobic fitness. Nitric Oxide. (2016) 59:63–70. doi: 10.1016/j.niox.2016.08.001
61. Rossetti GM, Macdonald JH, Wylie LJ, Little SJ, Newton V, Wood B, et al. Dietary nitrate supplementation increases acute mountain sickness severity and sense of effort during hypoxic exercise. J Appl Physiol. (2017) 123:983–92. doi: 10.1152/japplphysiol.00293.2017
62. Robinson GP, Killer SC, Stoyanov Z, Stephens H, Read L, James LJ, et al. Influence of dietary nitrate supplementation on high-intensity intermittent running performance at different doses of normobaric hypoxia in endurance-trained males. Int J Sport Nutr Exerc Metab. (2020) 31:1–8. doi: 10.1123/ijsnem.2020-0198
Keywords: high-altitude, nutritional interventions, cardiopulmonary fitness, network meta-analysis, randomized controlled trial
Citation: Wang B, Xiong Y, Lin N, Shi J, Zou B, Ma X, Zeng K and Kang C (2025) Comparative efficacy of different dietary interventions for cardiopulmonary fitness at high altitude: a systematic review and network meta-analysis. Front. Nutr. 12:1658950. doi: 10.3389/fnut.2025.1658950
Received: 03 July 2025; Accepted: 14 October 2025;
Published: 04 November 2025.
Edited by:
Timothy Dean Bryson, Henry Ford Health System, United StatesCopyright © 2025 Wang, Xiong, Lin, Shi, Zou, Ma, Zeng and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Ma, MTM4ODA0MjIzMzZAMTYzLmNvbQ==; Kaihong Zeng, emVuZ2thaWhvbmdAbWVkLnVlc3RjLmVkdS5jbg==; Chao Kang, Q2hhby5rYW5nX3RtbXVAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Bin Wang
Bin Wang Yanle Xiong
Yanle Xiong Ning Lin
Ning Lin Jiaojiao Shi3
Jiaojiao Shi3 Bo Zou
Bo Zou Xin Ma
Xin Ma Chao Kang
Chao Kang