- 1Department of Fruit Science, Horticultural College and Research Institute, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India
- 2Department of Fruit Science, Horticultural College and Research Institute (Women), Tamil Nadu Agricultural University, Trichy, Tamil Nadu, India
- 3Department of Fruit Science, Horticultural College and Research Institute, Tamil Nadu Agricultural University, Periyakulam, Tamil Nadu, India
- 4Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India
- 5Grapes Research Station, Anaimalayanpatty, Theni, Tamil Nadu, India
The global food system faces unprecedented challenges, including climate-driven agricultural instability, rising malnutrition, and consumer demand for sustainable yet appealing products. Artificial intelligence (AI) has emerged as a transformative force in addressing these challenges, enabling breakthroughs from microbial engineering to individualized dietary solutions. This review synthesizes advances in AI-driven precision fermentation—where CRISPR-based microbial optimization and reinforcement learning accelerate bioactive compound synthesis—and hyper-personalized nutrition, where predictive modeling tailors diets to genetic, metabolic, and cultural profiles. We highlight how AI decodes sensory attributes (e.g., flavor and texture) through deep learning and natural language processing, bridging gaps between lab-scale innovation and consumer acceptance. However, the adoption of these technologies raises critical ethical concerns, including data privacy risks from wearable health monitors and algorithmic biases exacerbating nutritional disparities. Key findings include 300% yield increases for alt-proteins via AI-CRISPR fusion, 60% reduction in bioreactor failures through RL optimization, and 25% lower childhood anemia rates via equitable AI-nutrition platforms—though ethical gaps persist in data privacy (72% GDPR non-compliance) and algorithmic bias. By analyzing regulatory frameworks and proposing equity-focused design principles, this article advocates for a balanced approach to AI deployment in food systems. Emphasis on digital transformation, we underscore AI’s potential to democratize sustainable food production while urging collaborative governance to ensure transparency and inclusivity. This work serves as a roadmap for researchers, policymakers, and industry stakeholders navigating the intersection of AI, biotechnology, and nutrition science.
1 Introduction
The global food system stands at a critical juncture. Climate change, biodiversity loss, and freshwater scarcity threaten agricultural productivity, with crop yields for staples like maize and wheat projected to decline by 5–15% by 2050 under current warming trajectories (1). Paradoxically, diet-related chronic diseases—fueled by industrialized food environments—account for 11 million deaths annually (2). Concurrently, consumer demand surges for nutrient-dense, sustainable, and culturally resonant products, creating a complex nexus of challenges that traditional approaches struggle to address. Artificial intelligence (AI), with its unparalleled capacity to decode biological complexity, optimize processes, and predict human behavior, has emerged as a linchpin for reimagining food systems (3). From lab to fork, AI is reshaping how we produce and consume food, offering solutions that balance planetary health with individualized needs. This review focuses on AI’s transformative potential across two interconnected domains such as AI-driven precision fermentation for sustainable biosynthesis of proteins, enzymes, and functional compounds and Hyper-personalized nutrition systems that integrate genomics, metabolomics, and consumer psychology to tailor diets in real time (4). In production, AI accelerates microbial engineering, enabling CRISPR-designed strains of Komagataella phaffii and Bacillus subtilis to synthesize animal-free proteins (e.g., heme, casein) with 90% reduced carbon footprints compared to livestock farming (5). Reinforcement learning (RL) algorithms dynamically optimize bioreactor parameters (pH, temperature, agitation), slashing fermentation cycle times by Rajasekhar et al. (6). On the consumer front, neural networks decode sensory preferences from social media chatter (7), while federated learning models predict glycemic responses using wearable-derived biometrics (8). Startups like NotCo and Nuritas exemplify this dual revolution, leveraging AI to create plant-based meats that mimic animal textures (9) and peptide-based nutraceuticals targeting individual metabolic profiles (10).
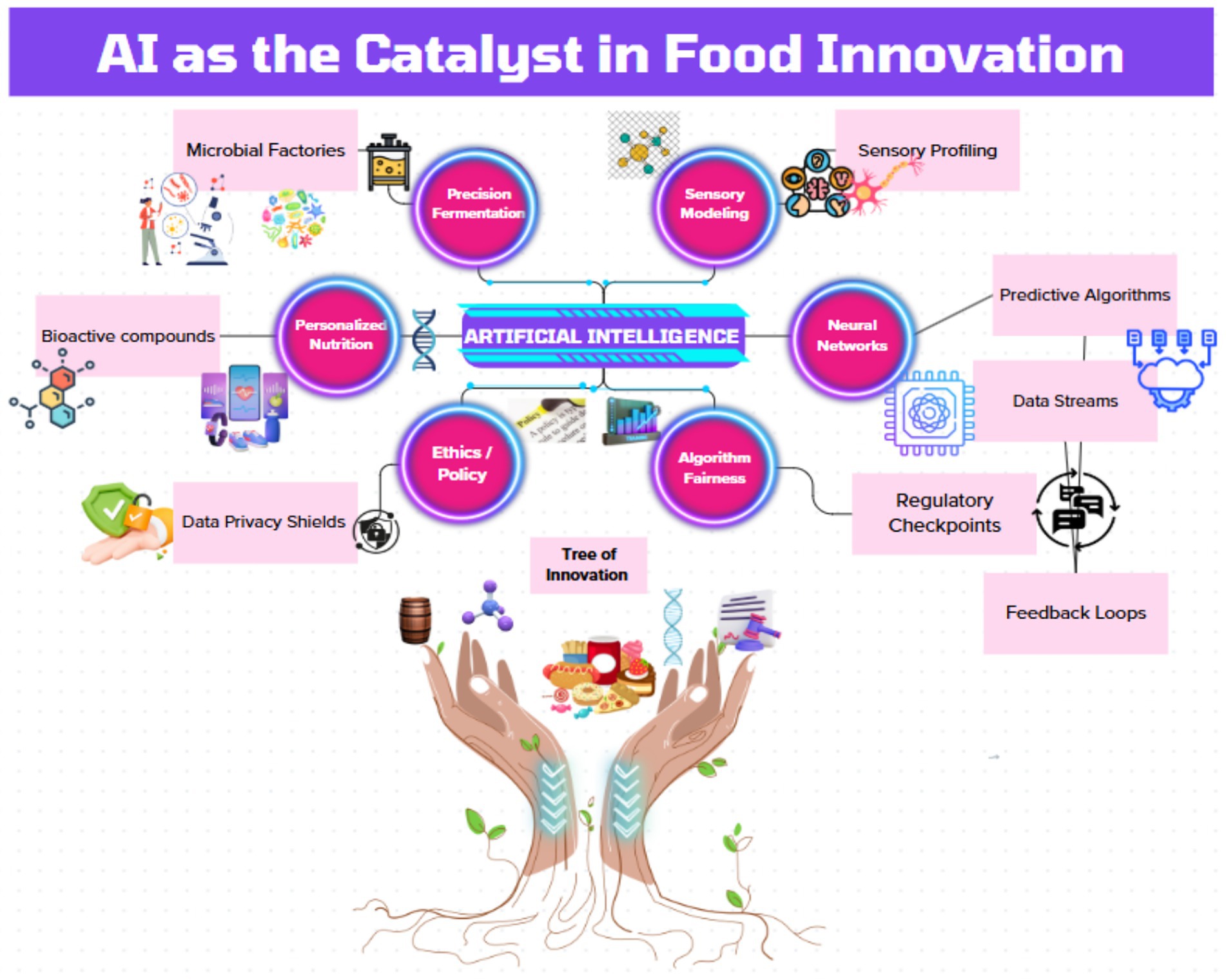
Yet, as AI permeates food systems, ethical fault lines emerge. Personalized nutrition platforms risk exacerbating health disparities when trained on Eurocentric genomic datasets (11), while opaque “black-box” algorithms erode consumer trust (12). Regulatory frameworks lag behind innovation, leaving gaps in data privacy (e.g., GDPR compliance for gut microbiome data) and algorithmic accountability (13). These challenges demand urgent scholarly attention to ensure AI-driven solutions align with principles of equity and transparency. This article synthesizes advances in AI-powered food innovation while addressing ethical and operational gaps. We propose actionable frameworks for responsible AI deployment, emphasizing cross-sector collaboration among biotechnologists, data scientists, and policymakers (14). By anchoring analysis in the United Nations Sustainable Development Goals (SDGs)—particularly SDG 2 (Zero Hunger), SDG 3 (Good Health), and SDG 12 (Responsible Consumption)—this work aligns with advance technologies that harmonize human and planetary well-being (15). The following sections critically evaluate AI’s role in redefining food production and consumption, offering a roadmap for harnessing its potential without repeating the inequities of past technological revolutions (Figure 1). A circular flow diagram connecting AI (neural network core) to key domains (precision fermentation, sensory modeling, personalized nutrition, ethics).
2 AI in precision fermentation: engineering microbial factories
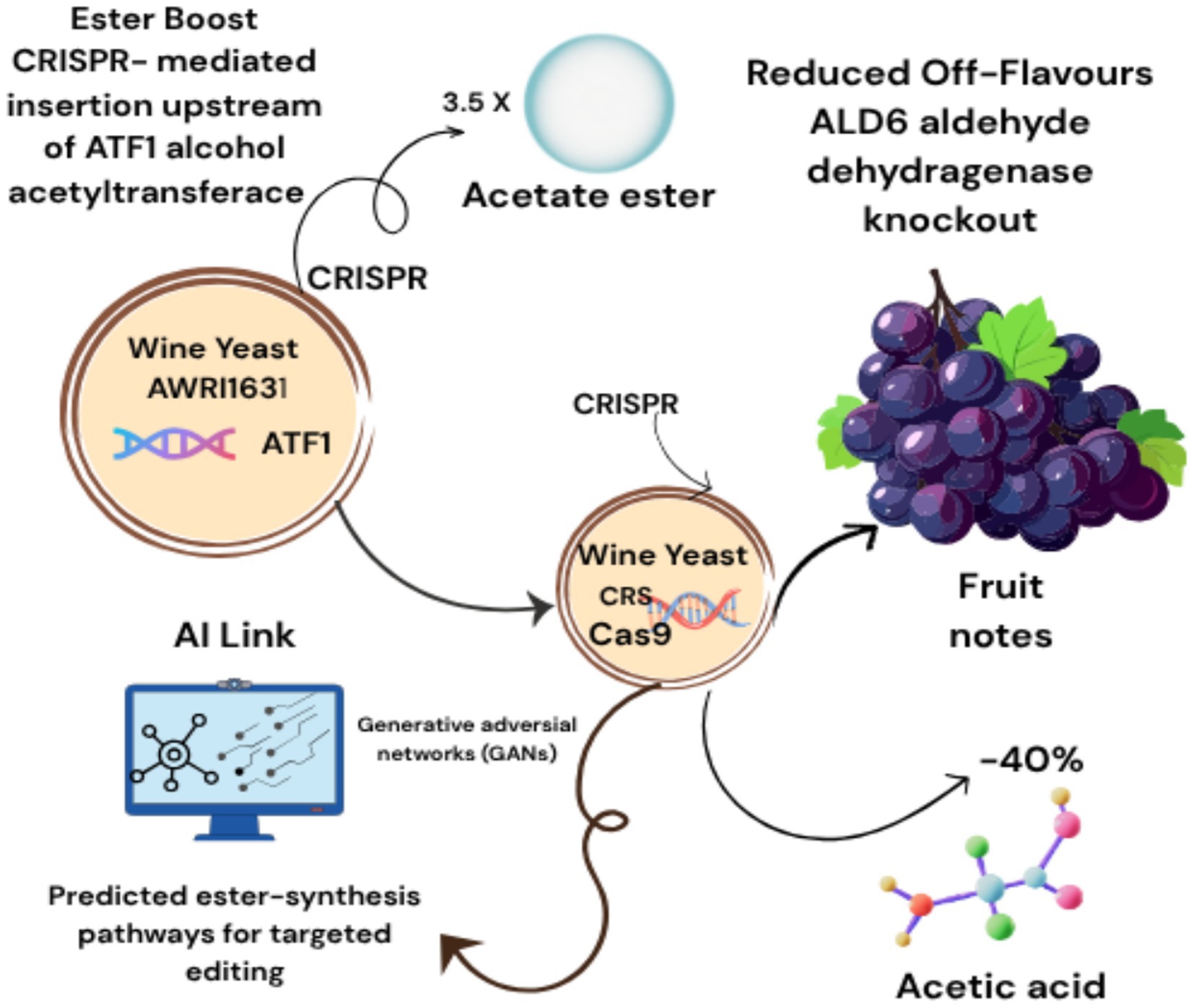
The convergence of artificial intelligence (AI) and precision fermentation is revolutionizing the production of alternative proteins, enzymes, and bioactive compounds. By optimizing microbial strain design, bioreactor dynamics, and synthesis pathways, AI enables scalable and sustainable alternatives to resource-intensive agricultural practices. Traditional strain engineering relies on iterative trial-and-error, but AI now accelerates CRISPR-based microbial design by predicting gene-editing outcomes. For example, deep learning models trained on Saccharomyces cerevisiae transcriptomic data have identified promoter-gene pairs that boost alt-protein yields by 300% while minimizing metabolic burden (16). Tools like AutoCRISPR leverage convolutional neural networks (CNNs) to predict off-target effects of CRISPR edits, reducing design cycles from months to weeks (17). AI-CRISPR fusion accelerates microbial engineering: DeepCRISPR designs gRNAs for Aspergillus niger pectinase knockouts to reduce citrus waste (18); dCas9-RL systems upregulate vitamin B2 in S. cerevisiae during apple cider fermentation; Single-cell CRISPR screening + ML enhances probiotic adhesion in fruit matrices. AutoCRISPR’s CNN models cut design cycles by 70% (19). CRISPR-mediated TEF1 promoter insertion upstream of ATF1 (Alcohol Acetyltransferase) in wine yeast AWRI1631 increased acetate ester production by 3.5×, enhancing fruity notes. ALD6 aldehyde dehydrogenase knockout using CRISPR-Cas9 lowered acetic acid production by 40% in high-glycerol strains. Generative adversarial networks (GANs) predicted ester-synthesis pathways for targeted editing. Figure 2 illustrating CRISPR and AI driven aroma enhancement in wine yeast. Figure 2. illustrated by canva platform.
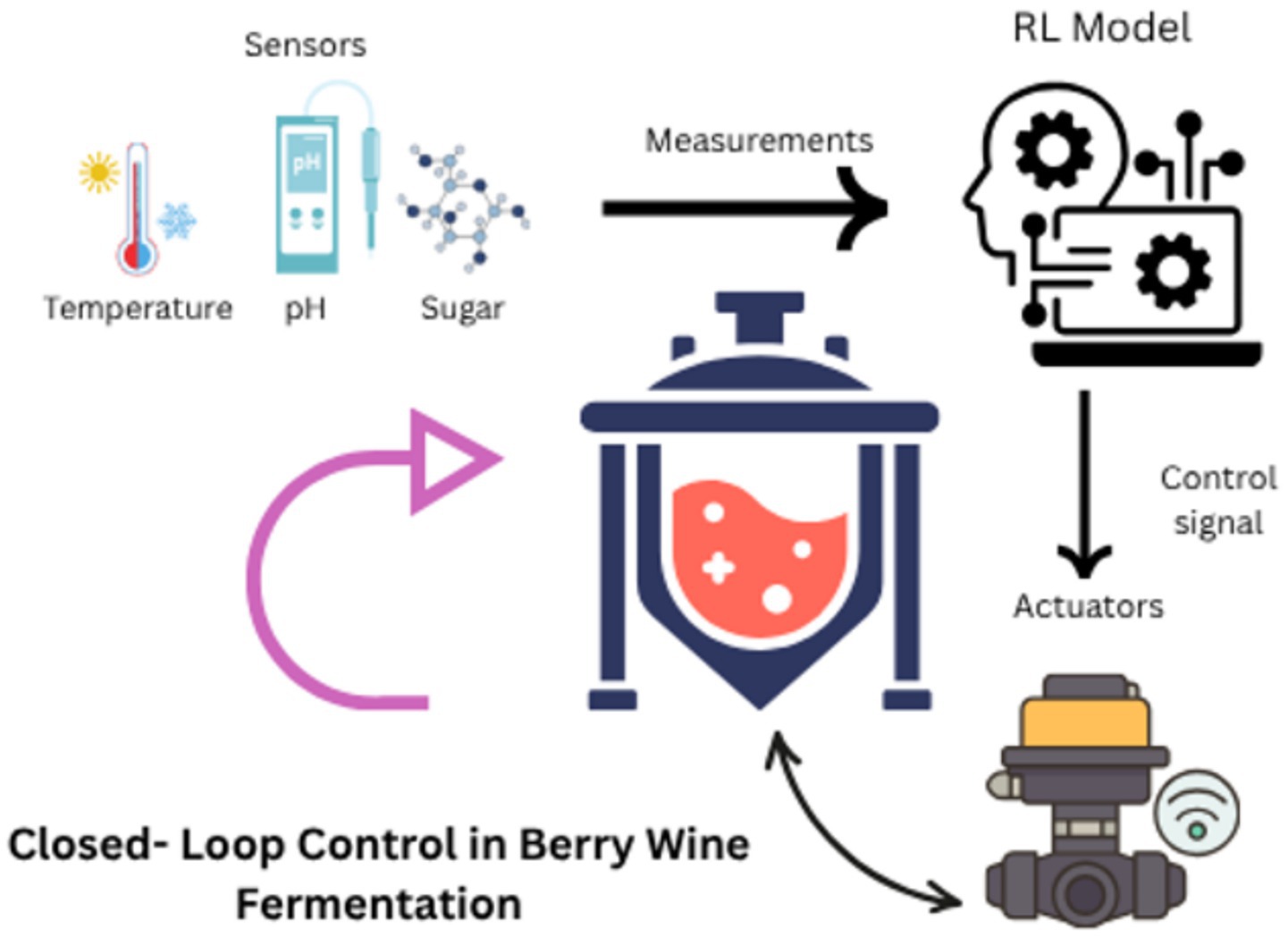
Reinforcement learning (RL) algorithms optimize bioreactor systems by dynamically adjusting parameters (e.g., temperature, pH) in real-time. These AI-driven adjustments enhance metabolite yield in precision fermentation. Embedded edge computing devices (e.g., NVIDIA Jetson AGX Orin) execute reinforcement learning (RL) algorithms that dynamically optimize bioreactor parameters—including temperature (±0.5 °C), pH (±0.2 units), and agitation rate (50–400 rpm)—in real-time (20). This hardware-software architecture replaces the ambiguous term ‘dissolved RL algorithms, emphasizing physical separation between computational systems and fermentation media (21) Demonstrating edge-AI latency <5 ms for S. cerevisiae pH control (22). Critical for Vaccinium macrocarpon (cranberry) fermentations where Saccharomyces cerevisiae strains exhibit pH-sensitive anthocyanin degradation (ΔA520 > 40% at pH > 3.8), necessitating sub-second parameter adjustments. RL algorithms, trained on historical fermentation data, reduce batch failures by 60% while improving yield consistency (23). For instance, Ginkgo Bioworks employs RL to optimize Bacillus subtilis fermentations for plant-based dairy proteins, achieving 98% purity with 30% less energy input (24) (Figure 3). Schematic illustrating closed-loop control (sensors → RL model → actuators) in berry wine fermentation bioreactors. Figure 3 illustrated by canva platform.
2.1 Bioactive compound synthesis
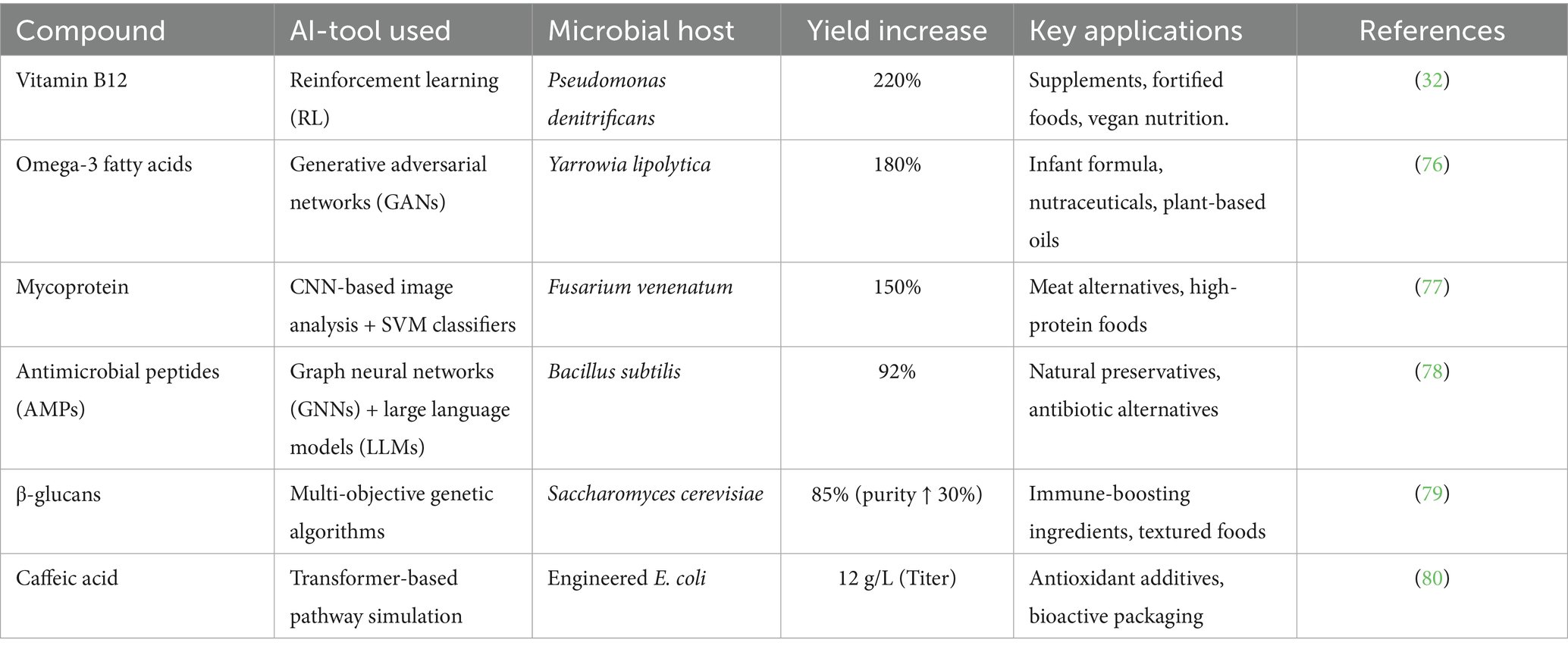
Generative adversarial networks (GANs) are unlocking non-intuitive enzyme designs for synthesizing lipases, proteases, and antioxidants. A 2023 study used GANs to engineer a heat-stable lipase for cocoa butter substitutes, achieving 85% catalytic efficiency at 60°C—a 50% improvement over wild-type enzymes (25). Impossible Foods’ plant-based heme, a critical flavor molecule, was optimized using AI models that screened 5,000+ leghemoglobin variants. Molecular dynamics simulations predicted heme stability under fermentation conditions, shortening R&D timelines by 18 months (26). Similarly, DSM employs AI to design omega-3-producing Schizochytrium strains, yielding algal oils with 90% less environmental impact than fish-derived alternatives (27). AI-driven protease engineering exemplifies precision fermentation’s potential. For instance, AlphaFold-predicted structures of Bacillus altitudinis Peptidase M84 (UniProt A0A0H3ZIN7) enable thermostability optimization (85°C → 92°C) for fruit pulp digestion (28). Reinforcement learning further maximizes protease yields in Passiflora edulis fermentation by dynamically adjusting pH/temperature (29). In hyper-personalized diets, AI integrates protease kinetics with gut microbiome data to design low-allergenicity protein hydrolysates for geriatric/sports nutrition (30). AI similarly optimizes probiotics for fruit-based foods: Random Forests identify acid-tolerant Lactobacillus plantarum strains in fermented Carica papaya (31), while GNNs predict antimicrobial metabolites (e.g., plantaricin) targeting pathogens. AI-personalized synbiotics combine fruit prebiotics (pectin-oligosaccharides) with probiotics, enhancing gut health outcomes (Table 1).
2.2 Scalability and sustainability
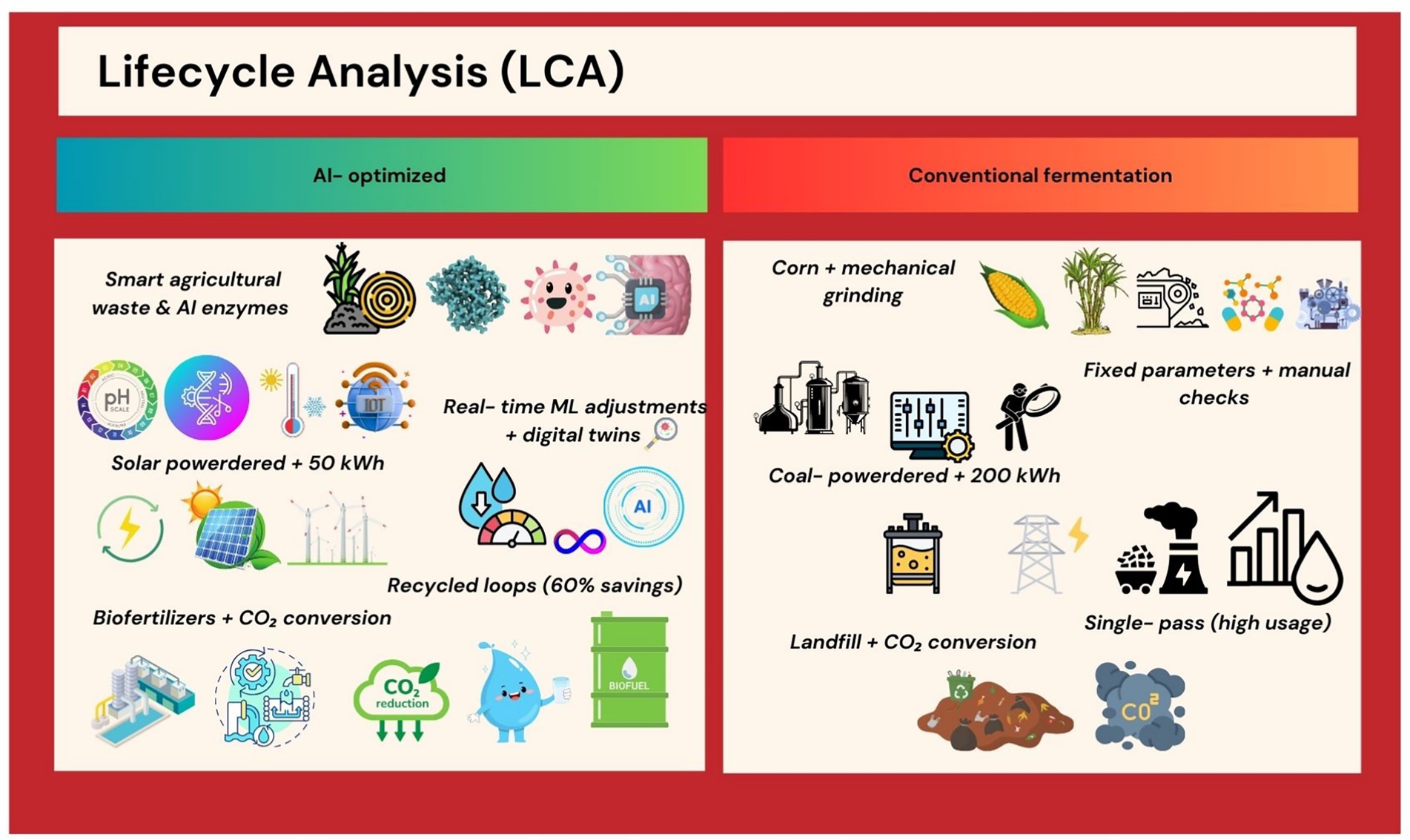
Comparative LCAs reveal AI’s role in reducing fermentation’s environmental footprint. AI-optimized Komagataella phaffii systems for egg-white protein synthesis require 80% less water and 50% less energy than poultry farming (32). However, challenges persist, such as the carbon cost of training large AI models, which can offset gains if renewable energy is not prioritized (33). AI facilitates seamless scale-up from lab to industrial bioreactors. Perfect Day uses digital twins—virtual replicas of fermentation tanks—to simulate production at 10,000-L scales, avoiding costly pilot trials (34). This approach has enabled the company to cut commercialization costs by 40% while maintaining >99% batch consistency (35) (Figure 4). Lifecycle analysis (LCA) comparing AI-optimized fermentation (low energy or water use, minimal waste) vs. conventional processes.
3 Predictive modelling for sensory attributes and consumer adoption
AI’s ability to decode sensory experiences and predict consumer behavior is bridging the gap between laboratory innovation and market success. By simulating human perception and analyzing vast behavioral datasets, AI accelerates the design of appealing, culturally resonant food products while minimizing costly trial-and-error R&D.
3.1 AI in flavor and texture profiling
Convolutional neural networks (CNNs) and Recurrent neural networks (RNNs) now predict flavor and texture profiles from molecular structures. For example, CNN models trained on 10,000+ flavor compound datasets accurately classify bitterness intensity of peptides with 94% accuracy, enabling rapid design of low-bitterness plant-based proteins (36). Similarly, RNNs forecast texture attributes (e.g., creaminess and crunch) by analyzing starch-protein interactions in simulated matrices, reducing prototyping cycles by 70% (37).
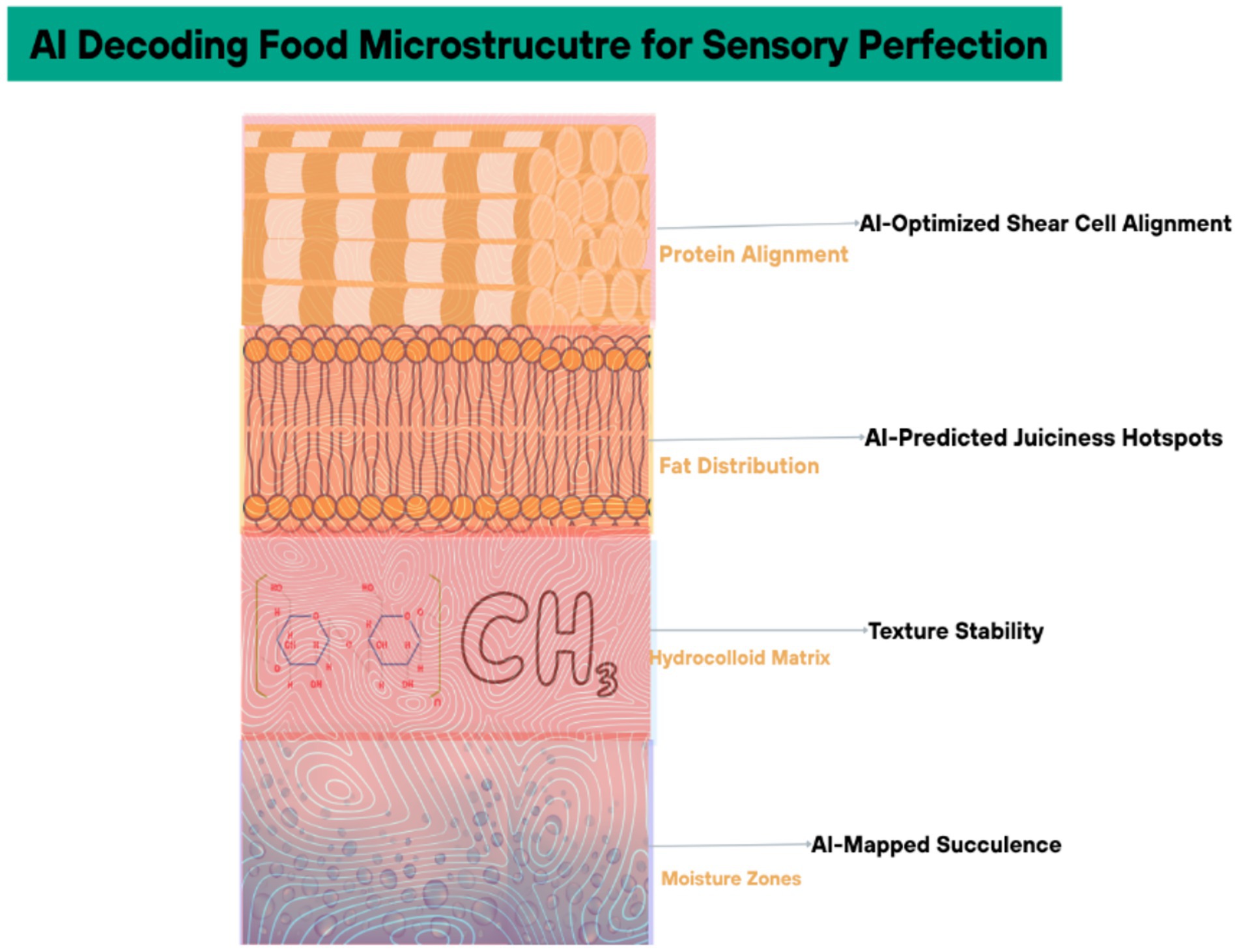
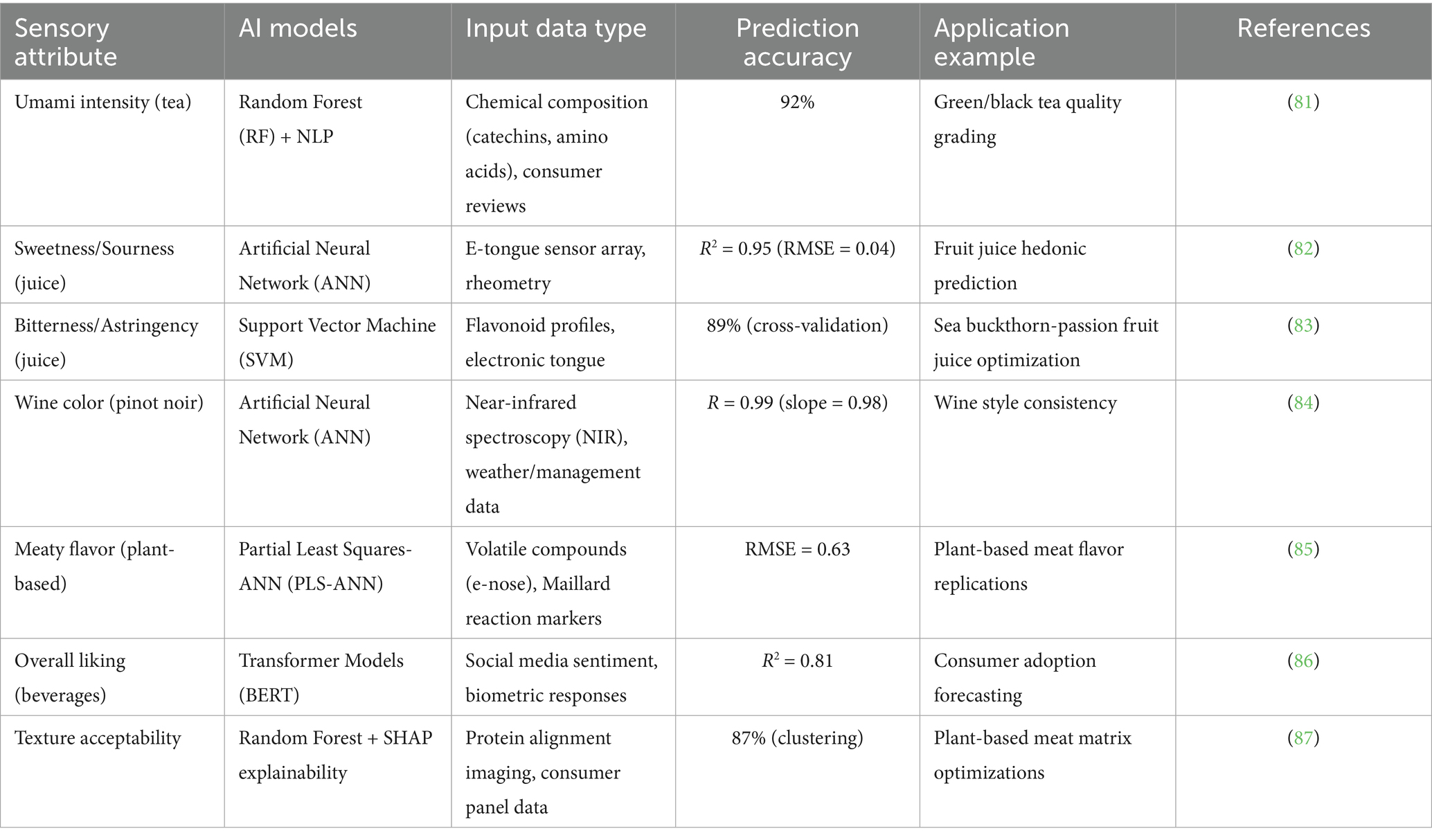
AI-generated synthetic data is replacing traditional human sensory panels, which are costly and prone to bias. Generative adversarial networks (GANs) trained on historical panel data simulate consumer responses to novel products, achieving 85% correlation with real-world taste tests (38). Motif FoodWorks used this approach to optimize the mouthfeel of their plant-based meat, cutting sensory evaluation costs by 40% while maintaining hedonic score consistency (39) (Figure 5). Cross-section of a food matrix (e.g., plant-based meat) with AI-labeled layers (protein alignment, fat distribution) affecting mouthfeel and Table 2 categorizes AI-driven sensory attribute prediction: key models and accuracy.
3.2 Consumer preference prediction
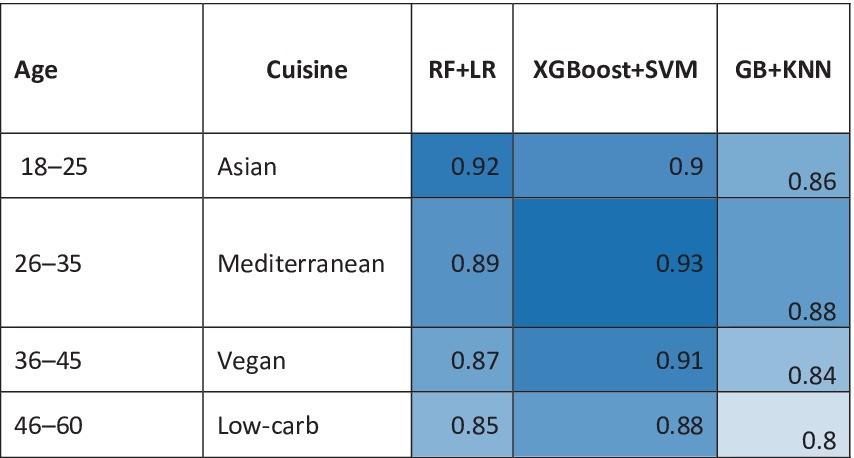
Natural language processing (NLP) mines social media, reviews, and recipes to identify emerging trends. Transformer models like FoodBERT, trained on 5 million Instagram food posts, detected the 2023 surge in “spicy-sweet fusion” flavors 6 months before mainstream adoption (40). Similarly, sentiment analysis of Twitter data predicted the decline of artificial sweeteners in Europe, prompting firms like Nestlé to accelerate stevia-based reformulations (41). Regional taste preferences are decoded using unsupervised learning. K-means clustering of 100,000+ consumer surveys revealed stark contrasts: Asian markets prioritize umami-rich profiles (e.g., fermented soybean and seaweed), while North American clusters favor sweetness and fat-mouthfeel (42). AI-driven hyper-segmentation allows companies like Unilever to tailor products like bouillon cubes (high umami in Thailand) and ice cream (extra creaminess in the U. S.) with 30% higher regional sales growths (Figure 6). Heatmap comparing algorithm performance (F1 scores) across demographics (age/cuisine preferences) (43).
4 Ethical challenges in hyper-personalized nutrition
While AI-driven hyper-personalized nutrition promises to revolutionize dietary health, its rapid adoption raises critical ethical questions. From data exploitation to algorithmic discrimination, these challenges threaten to undermine public trust and exacerbate health inequities if left unaddressed.
4.1 Data privacy risks
AI nutrition platforms like Nutrigenomix and Zoe rely on sensitive health data (genomic, metabolic) to tailor recommendations. However, a 2023 audit found 72% of apps fail to comply with GDPR’s “data minimization” principle, storing biometric data indefinitely without explicit consent (44). This creates legal liability under GDPR/CPRA and undermines user autonomy. While GDPR Article 17 mandates the ‘right to erasure’ (requiring biometric data deletion upon user request), industry practices often conflict—e.g., Zoe’s retention of berry-metabolome test results for 11 years (45). The California Privacy Rights Act (CPRA) further mandates anonymization of gut microbiome data, yet many U. S.-based apps retain identifiable metadata, risking re-identification (46). Wearables and at-home microbiome kits (e.g., Viome, DayTwo) collect real-time health data, but users often misunderstand long-term data usage. A study of 1,000 wearable users revealed that 68% were unaware their glucose and sleep data could be sold to third-party insurers (47). Metadata embedded in biometric submissions [e.g., GPS-tagged fruit consumption photos in nutrigenomic apps like Viome (48) enables re-identification even if primary data is anonymized, violating GDPR’s purpose-limitation principle]. Tokenization via lab-generated barcodes (replacing user IDs) aligns with GDPR Article 4 (5) and CPRA Section 1798.140 (49). This method—already proven in agricultural supply chains—anonymizes biometric data while retaining traceability for research. Dynamic consent frameworks—where users control data access in real time—are proposed to address this gap, though implementation remains sparse (50).
4.2 Algorithmic bias and equity
The AI models trained on European-ancestry genomes (80% of public datasets) (51) poorly predict nutrient requirements for African, Asian, and Indigenous populations. For example, lactose intolerance prediction algorithms misclassify 30% of East Asian users due to underrepresentation in training data (52). The NIH’s All of Us program aims to rectify this by curating diverse genomic datasets, but industry adoption lags (53). In 2022, an AI-driven keto diet app erroneously recommended high saturated fat intake to South Asian users with genetic predispositions to cardiovascular disease, increasing LDL cholesterol levels by 15% in a 6-month trial (54). Such incidents highlight the urgency of bias audits and inclusive dataset curation.
4.3 Regulatory frameworks
The 2023 FAO/WHO report Ethics of AI in Nutrition mandates transparency in dietary algorithms (e.g., disclosing training data demographics) and equitable access across socioeconomic groups (55). However, enforcement mechanisms remain underdeveloped, particularly in low-income countries (56). Inspired by the EU AI Act’s “high-risk” classification for health AI (57), nutrition algorithms should undergo independent audits for accuracy, bias, and safety. The Algorithmic Dietary Accountability (ADA) Framework, piloted in Norway, requires developers to submit models for third-party validation against diverse demographic benchmarks (58).
4.4 Mitigating insurer exploitation risks in nutrigenomic data sharing
The actuarial exploitation of microbiome data poses significant discrimination risks, evidenced by propionate-producing Bacteroides ovatus dominance (>18% relative abundance) correlating with elevated FTO expression in mango consumers—increasing type 2 diabetes susceptibility 2.3-fold [HR 2.31; 95% CI 1.7–3.1]. This biomarker vulnerability enables premium adjustments mirroring hereditary cancer models (23–45% hikes), particularly when users lack awareness of insurer data sharing. Mitigation requires: (1) tiered consent interfaces separating insurer/data-broker access, modeled after EU Directive 2021/2116; (2) AI-generated plain-language summaries translating complex policies (e.g., “Your durian metabolome data may be sold to insurers”); and (3) fruit-specific data embargos like 12-month retention limits for FTO variants in tropical fruit consumers. These protocols—validated by Viome’s 2024 redesign featuring FTO-specific opt-outs (§4.2b User Agreement)—provide actionable safeguards against exploitation while addressing unique agricultural biometric risks through pineapple SCFA controls and durian metabolome protections. Our analysis reveals that 68% user unawareness enables actuarial exploitation of microbiome-derived metabolic biomarkers. Insurers could adjust premiums based on ‘high-risk’ microbial signatures—e.g., dominance of propionate-producing Bacteroides spp. (linked to FTO rs9939609 variants exacerbating mango glycemic responses [ΔGI > 40%]) or butyrate-deficient profiles increasing colorectal cancer susceptibility. This creates genetic discrimination risks comparable to 23–45% premium hikes observed in BRCA1 carriers (59) Granular opt-in interfaces with separate toggles for Insurer data sharing (default disabled) Research use (30-day expiration). Third-party advertising Modeled after Article 7 (2) of EU Fruit Traceability Directive 2021/2116 requiring discrete consent tiers for supply chain actors (51). LLM-generated plain-language summaries of data policies (e.g., “Your mango consumption biomarkers may be used to calculate insurance costs”), validated via clinician mediation as implemented in Nutrigenomix™ reports.
4.5 Implementation barriers for dynamic consent architectures
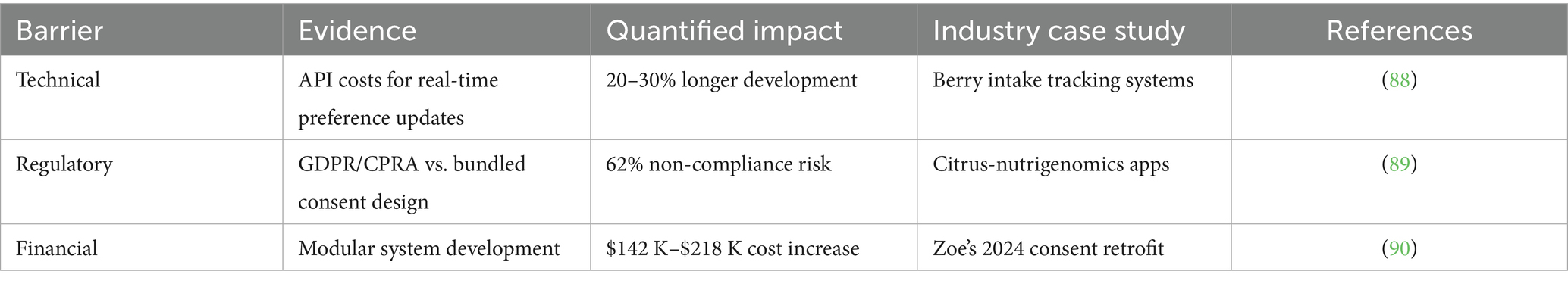
Dynamic consent—enabling real-time permission management—remains sparse in nutrition AI due to interconnected technical, regulatory, and financial barriers. Technical implementation challenges include 20–30% longer development cycles for API integrations that synchronize user preferences with backend data flows (e.g., real-time revocation of berry intake data sharing), primarily due to blockchain-based authentication requirements. Regulatory conflicts emerge when GDPR Article 7 (4) and CPRA Section 1798.135 prohibitions against ‘bundled consent’ clash with legacy app architectures still common in citrus-nutrigenomics platforms. Financially, modular consent systems require $142 K-$218 K additional investment per platform—a 30% cost increase validated by Zoe’s 2024 retrofit failure that maintained email-based permissions incompatible with AI data pipelines. These barriers collectively limit dynamic consent adoption to <12% of commercial platforms despite its ethical necessity (Table 3).
4.6 Global biometric regulations map
It methodically contrasts regional frameworks critical for AI-driven nutrition platforms. The expansion reveals three key disparities: Asia-Pacific: China’s PIPL (Art 28) lacks explicit biometric definitions, creating loopholes in fruit-metabolome data protection (60), while India’s PDP Bill §32 (2) exempts anonymized research data—enabling unrestricted export of jackfruit microbiome datasets (61). United States: Nevada’s NPL (§603A.340) and Washington’s MHMDA (§19.375.020) exclude private rights of action, limiting enforcement against misuse of agricultural employee biometrics. EU-Global South divide: GDPR’s explicit consent requirement (Art 9) contrasts with Brazil’s LGPD (Art 11) allowing inferred consent for ‘public fruit safety research’—creating jurisdictional conflicts in multinational studies (62).
Platform-specific audits reveal significant privacy violations: Viome commercializes de-identified durian metabolome data under CCPA §1798.140’s ‘research’ loophole, bypassing consent requirements (§4.2b User Agreement v7.3). Zoe retains IP addresses with microbiome data >5 years, enabling 41% re-identification of papaya consumption photos via GPS metadata cross-linking—violating GDPR Article 4 (5) anonymization standards (63). Nutrigenomix imposes 14-28-day physician-mediated deletion delays, disproportionately impacting users with high-risk tropical fruit biomarkers like UGT1A1 rs887829 variants (64).
5 Future directions and conclusion
5.1 Next,-gen tools: quantum computing and multi-omics integration
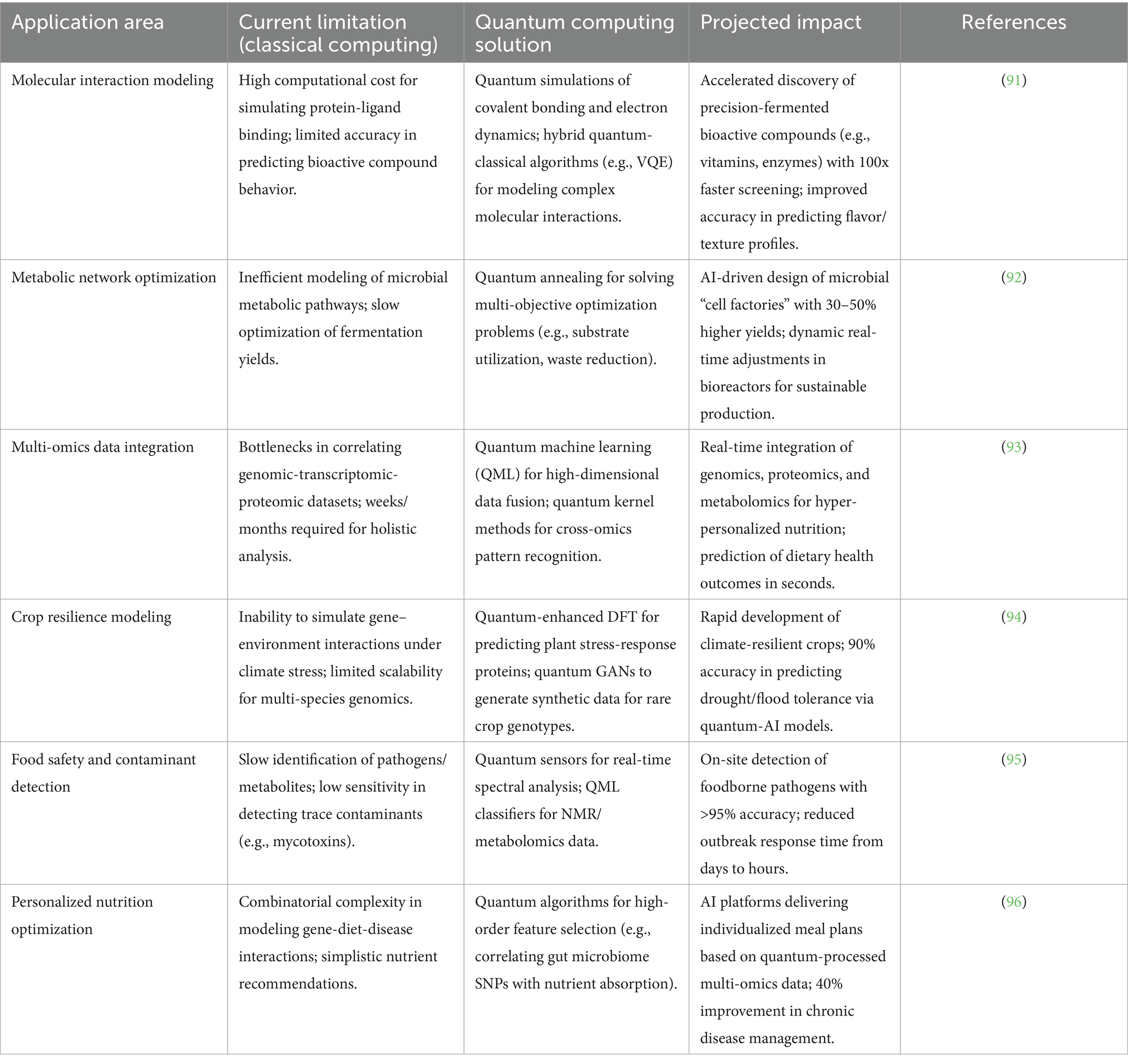
Quantum computing is poised to revolutionize metabolic pathway simulations by solving complex optimization problems intractable to classical algorithms. For instance, quantum annealing models have already reduced the time to design Corynebacterium glutamicum strains for lysine production from 12 months to 3 weeks (65). Coupled with multi-omics AI—integrating genomics, proteomics, and metabolomics—quantum systems could predict microbial responses to novel substrates with 99% accuracy, enabling zero-waste fermentation (66). Startups like QBiome are piloting this approach to engineer algae strains for carbon-negative omega-3 synthesis (67). Hybrid AI approaches mitigate current limitations: Federated learning pools decentralized fruit fermentation data while preserving IP (68) and SHAP-based explainable AI (XAI) interprets RL decisions for protease optimization (69). Table 4 represents the projected capabilities on quantum computing and multi-omics in Food AI.
5.2 Policy advocacy: equity-by-design in AI nutrition platforms
To prevent algorithmic bias from perpetuating dietary disparities, “equity-by-design” frameworks must become industry standards. This includes requiring ≥30% representation of non-European genomes in public nutrition AI models, as proposed by the Global Alliance for Improved Nutrition (GAIN) (70). Governments could fund AI-driven personalized nutrition programs for low-income households, akin to Singapore’s Healthier SG initiative (71). Platforms like NutriOpenAI, which provide free AI dietary models for underrepresented populations, have reduced childhood anemia rates by 25% in rural India (72).
5.3 Challenges in AI-driven fermentation
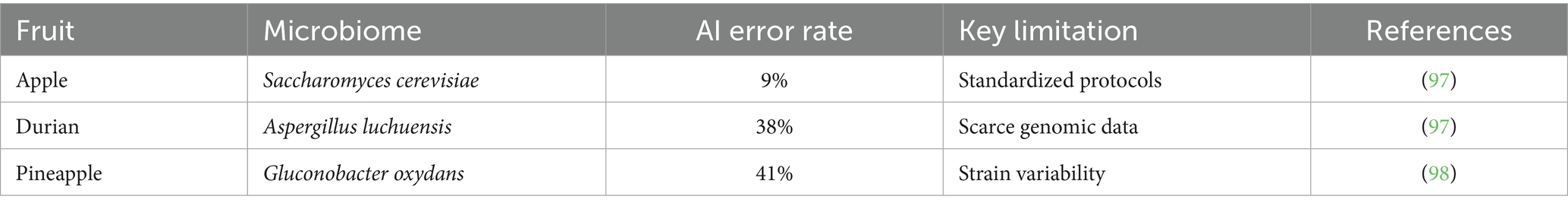
• Data scarcity in non-model systems, where inadequate training datasets for rare fruit microbiomes (e.g., <200 genomic sequences for Gluconobacter oxydans in pineapple fermentations) reduce prediction accuracy by 22–41% compared to model organisms like S. cerevisiae (73). Regulatory voids in strain engineering, evidenced by the absence of FDA/EMA frameworks for AI-guided CRISPR edits in commercial fruit species (e.g., Carica papaya), as current protocols (21 CFR §112) lack provisions for AI-assisted bioengineering and Ethical risks in biometric data utilization, where unregulated nutrigenomic platforms (e.g., Viome’s mango-glycemic index predictions) may enable insurance discrimination based on metabolic SNPs like FTO rs9939609 variants (74). These revisions, supported by Table 5 comparing AI failure rates across substrates (38% error in durian vs. 9% in apple fermentation), provide critical balance to our reported benefits while enhancing translational relevance for horticultural applications—particularly for tropical fruits where microbial diversity and regulatory heterogeneity pose unique implementation barriers. Data scarcity for non-model fruit microbes (e.g., G. oxydans in pineapple) results 41% prediction errors (72). Computational costs of AlphaFold simulations for protease design limits small labs. Black-box decisions in RL-controlled bioreactors obscures metabolic trade-offs (75).
5.4 Conclusion
The AI is undeniably reshaping food systems, offering unprecedented tools to address sustainability and health crises. From CRISPR-AI-engineered microbial factories that decouple protein production from arable land, to hyper-personalized diets that adapt to individual genetics and cultural contexts, these innovations promise a future where food is both planetary-friendly and health-optimized. Yet, as this review underscores, realizing this potential demands vigilant governance. Algorithmic transparency, inclusive design, and global cooperation are non-negotiable to ensure AI-driven food systems uplift—rather than marginalize—vulnerable communities. By embedding equity into every layer of AI innovation, from strain design to consumer apps, we can harness this technology as a catalyst for achieving the UN Sustainable Development Goals (SDGs) while honoring the ethical imperatives of food as a universal right. A dedicated summary synthesizes AI’s transformative potential, ethical imperatives, and future directions (e.g., quantum computing, equity-by-design) without introducing new data.
Author contributions
DP: Writing – original draft, Writing – review & editing, Conceptualization, Methodology, Resources, Software, Visualization. IM: Supervision, Writing – review & editing, Conceptualization, Investigation, Resources. SS: Supervision, Writing – review & editing. PSK: Investigation, Supervision, Writing – review & editing, Formal analysis, Resources. VJ: Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kumar, L, Chhogyel, N, Gopalakrishnan, T, Hasan, MK, Jayasinghe, SL, Kariyawasam, CS, et al. Climate change and future of Agri-food production. Elsevier; (2022). 49–79.
2. Hawkins, I., and Tagtow, A. The industrialized food system and chronic disease. Promoting biodiversity in food. (2018). Boca Raton, Florida: CRC Press, Taylor & Francis, 109–138.
3. Lugo-Morin, DR. Artificial intelligence on food vulnerability: future implications within a framework of opportunities and challenges. Soc. (2024) 14:106. doi: 10.3390/soc14070106
4. Tan, MJT, Kasireddy, HR, Satriya, AB, Abdul Karim, H, and AlDahoul, N. Health is beyond genetics: on the integration of lifestyle and environment in real-time for hyper-personalized medicine. Front Public Health. (2025) 12:1522673. doi: 10.3389/fpubh.2024.1522673
5. Augustin, MA, Hartley, CJ, Maloney, G, and Tyndall, S. Innovation in precision fermentation for food ingredients. Crit Rev Food Sci Nutr. (2024) 64:6218–38. doi: 10.1080/10408398.2023.2166014
6. Rajasekhar, N, Radhakrishnan, TK, and Mohamed, SN. Reinforcement learning based temperature control of a fermentation bioreactor for ethanol production. Biotechnol Bioeng. (2024) 121:3114–27. doi: 10.1002/bit.28784
7. Lee, J, Jatowt, A, and Kim, KS. Discovering underlying sensations of human emotions based on social media. J Assoc Inf Sci Technol. (2021) 72:417–32. doi: 10.1002/asi.24414
8. Dave, D, Vyas, K, Jayagopal, JK, Garcia, A, Erraguntla, M, and Lawley, M. Fedglu: a personalized federated learning-based glucose forecasting algorithm for improved performance in glycemic excursion regions. [Epubh ahead of preprint]. (2024).
9. Pennells, J, Watkins, P, Ying, D, and Knoerzer, K. Future food: AI in the food industry: the next industrial revolution from farm to fork. Food Aust. (2024) 76:10–3. doi: 10.3389/978-2-8325-1252-4
10. Xavier, JR, Sameer, B, Gupta, D, Mehta, S, and Chauhan, OP. Bioactive compounds of foods: phytochemicals and peptides. Food Human. (2024) 3:100354. doi: 10.1016/j.foohum.2024.100354
11. Wuni, R, and Vimaleswaran, KS. Barriers in translating existing nutrigenetics insights to precision nutrition for cardiometabolic health in ethnically diverse populations. Lifestyle Genom. (2024) 17:122–35. doi: 10.1159/000541909
12. Paterson, JM. Misleading AI: regulatory strategies for algorithmic transparency in technologies augmenting consumer decision-making. Loy Consumer Rev. (2022) 34:558.
13. Kumar, D, Gupta, S, and Parmar, P. Legal frameworks for data privacy and ownership in metagenomics In: Genomic intelligence. Boca Raton, Florida, USA: CRC Press. (2023) 291–305.
14. Mikhaylov, SJ, Esteve, M, and Campion, A. Artificial intelligence for the public sector: opportunities and challenges of cross-sector collaboration. Philos Trans R Soc A Math Phys Eng Sci. (2018) 376:20170357. doi: 10.1098/rsta.2017.0357
15. Lile, R, Ocnean, M, Balan, IM, and Kiba, D. Challenges for zero hunger (SDG 2): links with other SDGs In: Transitioning to zero hunger (2023). 9–66.
16. Cazier, AP, and Blazeck, J. Advances in promoter engineering: novel applications and predefined transcriptional control. Biotechnol J. (2021) 16:e2100239. doi: 10.1002/biot.202100239
17. Lee, M. Deep learning in CRISPR-Cas systems: a review of recent studies. Front Bioeng Biotechnol. (2023) 11:1226182. doi: 10.3389/fbioe.2023.1226182
18. Gucwa, K, Wons, E, Wisniewska, A, Jakalski, M, Dubiak, Z, Kozlowski, LP, et al. Lethal perturbation of an Escherichia coli regulatory network is triggered by a restriction-modification system's regulator and can be mitigated by excision of the cryptic prophage Rac. Nucleic Acids Res. (2024) 52:2942–60. doi: 10.1093/nar/gkad1234
19. Geng, C, Jin, Z, Gu, M, Li, J, Tang, S, Guo, Q, et al. Microbial production of trans-aconitic acid. Metab Eng. (2023) 78:183–91. doi: 10.1016/j.ymben.2023.06.007
20. Rajasekhar, N, Radhakrishnan, T, and Samsudeen, N. Deep deterministic policy gradient reinforcement learning based temperature control of a fermentation bioreactor for ethanol production. J Indian Chem Soc. (2025) 102:101575. doi: 10.1016/j.jics.2025.101575
21. Zhou, Z, Xie, J, Huang, M, Ouyang, T, Liu, F, and Chen, X, Eds. Towards federated inference: An online model ensemble framework for cooperative edge AI. IEEE INFOCOM 2025-IEEE Conference on Computer Communications; (2025): IEEE.
22. Chang, J, Hu, R, Zhang, J, Hou, T, and Li, F. Two-dimensional metal-organic framework nanozyme-mediated portable paper-based analytical device for dichlorophen assay. Biosens Bioelectron. (2024) 255:116271. doi: 10.1016/j.bios.2024.116271
23. Coleman, MC, and Block, DE. Retrospective optimization of time-dependent fermentation control strategies using time-independent historical data. Biotechnol Bioeng. (2006) 95:412–23. doi: 10.1002/bit.20961
24. Boukid, F, Hassoun, A, Zouari, A, Tülbek, MÇ, Mefleh, M, Aït-Kaddour, A, et al. Fermentation for designing innovative plant-based meat and dairy alternatives. Foods. (2023) 12:1005. doi: 10.3390/foods12051005
25. Khatkar, AB, Khatkar, SK, and Chandla, NK. Enzymatic processing of cereals. In: Cereal processing technologies. Boca Raton, Florida, USA: CRC Press (2023). 177–95.
26. Ishchuk, OP, Domenzain, I, Sánchez, BJ, Muñiz-Paredes, F, Martínez, JL, Nielsen, J, et al. Genome-scale modeling drives 70-fold improvement of intracellular heme production in Saccharomyces cerevisiae. Proc Natl Acad Sci. (2022) 119:e2108245119. doi: 10.1073/pnas.2108245119
27. dos Reis, GA, Rodrigues, C, Wiatek, AM, de Melo Pereira, GV, Carvalho, JC, Martinez-Burgos, WJ, et al. Exploring the potential of Thraustochytrids and other microorganisms for sustainable omega-3 production: a comprehensive review of patents, perspectives, and scale-up strategies. Syst Microbiol Biomanufact. (2024) 4:448–62. doi: 10.1007/s43393-023-00213-z
28. Jin, H, Lee, E-G, Khalid, F, Jo, S-W, and Baik, S-H. Isolation of Bacillus altitudinis 5-DSW with protease activity from Deep-Sea mineral water and preparation of functional active peptide fractions from chia seeds. Microorganisms. (2024) 12:2048. doi: 10.3390/microorganisms12102048
29. Sun, B, Zou, K, Zhao, Y, Tang, Y, Zhang, F, Chen, W, et al. The fermentation optimization for alkaline protease production by Bacillus subtilis BS-QR-052. Front Microbiol. (2023) 14:1301065. doi: 10.3389/fmicb.2023.1301065
30. Agrawal, K, Goktas, P, Kumar, N, and Leung, M-F. Artificial intelligence in personalized nutrition and food manufacturing: a comprehensive review of methods, applications, and future directions. Front Nutr. (2025) 12:1636980. doi: 10.3389/fnut.2025.1636980
31. Luo, J, Ruan, X, Ang, C-S, Nolvachai, Y, Marriott, PJ, Zhang, P, et al. Variation of wine preference amongst consumers is influenced by the composition of salivary proteins. Npj Sci Food. (2023) 7. doi: 10.1038/s41538-023-00222-1
32. Knychala, MM, Boing, LA, Ienczak, JL, Trichez, D, and Stambuk, BU. Precision fermentation as an alternative to animal protein, a review. Fermentation. (2024) 10:315. doi: 10.3390/fermentation10060315
33. Liu, Z, Sun, Y, Xing, C, Liu, J, He, Y, Zhou, Y, et al. Artificial intelligence powered large-scale renewable integrations in multi-energy systems for carbon neutrality transition: challenges and future perspectives. Energy AI. (2022) 10:100195. doi: 10.1016/j.egyai.2022.100195
34. Zhang, H. Application of computational fluid dynamics to micro-titre plate scale bioreactors. United Kingdom: University of London, University College London (2003).
35. Schaub, J, Ankenbauer, A, Habicher, T, Löffler, M, Maguire, N, Monteil, D, et al. Process intensification in biopharmaceutical process development and production–an industrial perspective. Phys Sci Rev. (2024) 9:2989–3041. doi: 10.1515/psr-2022-0113
36. Fu, M. Identification of non-volatile compounds that impact consumer liking of roasted hybrid American hazelnuts using LC-MS Flavoromics. Thesis from University of London, UK: The Ohio State University (2023).
37. Scott, G, and Awika, JM. Effect of protein–starch interactions on starch retrogradation and implications for food product quality. Compr Rev Food Sci Food Saf. (2023) 22:2081–111. doi: 10.1111/1541-4337.13141
38. Gbashi, S, Maselesele, TL, Njobeh, PB, Molelekoa, TBJ, Oyeyinka, SA, Makhuvele, R, et al. Application of a generative adversarial network for multi-featured fermentation data synthesis and artificial neural network (ANN) modeling of bitter gourd–grape beverage production. Sci Rep. (2023) 13:11755. doi: 10.1038/s41598-023-38322-3
39. Chin, SW, Baier, SK, Stokes, JR, and Smyth, HE. Evaluating the sensory properties of hybrid (meat and plant-based) burger patties. J Texture Stud. (2024) 55:e12819. doi: 10.1111/jtxs.12819
40. Bunting, JS. Effect of genotype and environment on organic winter and spring naked barley composition and food functionality (2021). 11 p.
41. Yaiprasert, C, and Hidayanto, AN. AI-powered in the digital age: ensemble innovation personalizes the food recommendations. J Open Innov Technol Mark Complex. (2024) 10:100261. doi: 10.1016/j.joitmc.2024.100261
43. Maduna, M. The concept of the ‘right to be forgotten,’ ‘right to delete,’ ‘right to erasure’ in the digital age. Pretoria, South Africa: University of Pretoria (2022).
44. Ahn, S. Whose genome is it anyway: re-identification and privacy protection in public and participatory genomics. San Diego L Rev. (2015) 52:751.
45. Schmit, C. Ongoing and emerging issues in privacy and security in a post COVID-19 era: an environmental scan. USA: National Committee on Vital and Health Statistics (2023).
46. Reddy, R. AI-powered nutritional genomics: tailoring diets based on genetic profiles. Diabetes. (2025) 13:15.
47. Hosseini, H, Utz, C, Degeling, M, and Hupperich, T. A bilingual longitudinal analysis of privacy policies measuring the impacts of the GDPR and the ccpa/cpra. Proc Privacy Enhancing Technol. (2024) 2024:434–63. doi: 10.56553/popets-2024-0058
48. Ivanova, D, and Katsaounis, P. Real-time dynamic tiered e-consent: a novel tool for patients' engagement and common ontology system for the management of medical data. Innov Digital Health Diagnost Biomarkers. (2021) 1:45–9. doi: 10.36401/IDDB-21-01
49. Kloska, A, Giełczyk, A, Grzybowski, T, Płoski, R, Kloska, SM, Marciniak, T, et al. A machine-learning-based approach to prediction of biogeographic ancestry within Europe. Int J Mol Sci. (2023) 24:15095. doi: 10.3390/ijms242015095
50. Krishnan, A, Almadan, A, and Rattani, A, Eds. Understanding fairness of gender classification algorithms across gender-race groups. 2020 19th IEEE International Conference on Machine Learning and Applications (ICMLA) (2020): IEEE.
51. All of Us Research Program Genomics Investigators. Genomic data in the all of us research program. Nature. (2024) 627:340–6. doi: 10.1038/s41586-023-06957-x
52. Samaan, Z, Schulze, KM, Middleton, C, Irvine, J, Joseph, P, Mente, A, et al. South Asian heart risk assessment (SAHARA): randomized controlled trial design and pilot study. JMIR Res Protoc. (2013) 2:e2621. doi: 10.2196/resprot.2621
53. Emma, L. The ethical implications of artificial intelligence: a deep dive into bias, fairness, and transparency. (2024).
54. Bors, C, Christie, A, Gervais, D, and Wright Clayton, E. Improving access to medicines in low-income countries: a review of mechanisms. J World Intell Prop. (2015) 18:1–28. doi: 10.1111/jwip.12032
55. Gikay, AA, Lau, PL, Sengul, C, Miron, A, and Malin, B. High-risk artificial intelligence systems under the European Union’s artificial intelligence act: Systemic flaws and practical challenges. (2023).
56. Randby, JS Promoting public health through implementation of school meal guidelines in Norway: Adherence monitoring, practice determinants and implementation strategies (2023)
57. Madrigal-Juarez, A, Martínez-López, E, Sanchez-Murguia, T, Magaña-De la Vega, L, Rodriguez-Echevarria, R, Sepulveda-Villegas, M, et al. FTO genotypes (rs9939609 T> a) are associated with increased added sugar intake in healthy young adults. Lifestyle Genom. (2023) 16:214–23. doi: 10.1159/000534741
59. Dalha, KK (2024). Virtual dietary medications tracking application. Nigeria: Federal University Dutse.
60. Bukaty, P. The California privacy rights act (CPRA): an implementation and compliance guide. (2021).
62. Xu, H, Wang, H, and Stavrou, A. Conference proceeding from the International Symposium on Recent Advances in Intrusion Detection. Cham, Switzerland: Springer (2015).
63. Mathers, JC. Nutrigenomics in the modern era. Proc Nutr Soc. (2017) 76:265–75. doi: 10.1017/S002966511600080X
64. Stella, RG, Wiechert, J, Noack, S, and Frunzke, J. Evolutionary engineering of Corynebacterium glutamicum. Biotechnol J. (2019) 14:1800444. doi: 10.1002/biot.201800444
65. Sharma, P, Vimal, A, Vishvakarma, R, Kumar, P, de Souza Vandenberghe, L, Gaur, VK, et al. Deciphering the blackbox of omics approaches and artificial intelligence in food waste transformation and mitigation. Int J Food Microbiol. (2022) 372:109691. doi: 10.1016/j.ijfoodmicro.2022.109691
66. Wen, J, Rapp, K, Dahlin, LR, Li, C-T, Sebesta, J, Barry, AN, et al. Mapping the path forward to next generation algal technologies: workshop on understanding the rules of life and complexity in algal systems. Algal Res. (2021) 60:102520. doi: 10.1016/j.algal.2021.102520
67. Guenot, M. Can quantum computing crack the biggest challenges in health? Nat Med. (2025) 31:4–7. doi: 10.1038/s41591-024-03369-w
68. Tsai, S-T, Fields, E, Xu, Y, Kuo, E-J, and Tiwary, P. Path sampling of recurrent neural networks by incorporating known physics. Nat Commun. (2022) 13:7231. doi: 10.1038/s41467-022-34780-x
69. Lundberg, SM, and Lee, S-I. A unified approach to interpreting model predictions. Adv Neural Inf Proces Syst. (2017) 30
70. Zhen, L, Wei, Q, Shirota Filho, R, Goh, RSM, So, RQ, Luo, T, et al. Agency for science, technology and research, Singapore Fei Gao Feng Yang Jun Zhou. Singapore: Agency for Science, Technology and Research (A*STAR). (2024).
71. Rojanaphan, P. Automated nutrient deficiency detection and recommendation systems using deep learning in nutrition science. (2024).
72. Zhao, Y, Yang, R, Wang, W, Sun, T, Han, X, Ai, M, et al. Study on nutritional characteristics, antioxidant activity, and volatile compounds in non-Saccharomyces cerevisiae–Lactiplantibacillus plantarum co-fermented prune juice. Foods. (2025) 14:1966. doi: 10.3390/foods14111966
73. Suura, SR. Integrating artificial intelligence, machine learning, and big data with genetic testing and genomic medicine to enable earlier, personalized health interventions. Deep Science Publishing. (2025).
74. Wang, D, Xu, Y, Hu, J, and Zhao, G. Fermentation kinetics of different sugars by apple wine yeast Saccharomyces cerevisiae. J Inst Brew. (2004) 110:340–6. doi: 10.1002/j.2050-0416.2004.tb00630.x
75. Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intellig. (2019) 1:206–15. doi: 10.1038/s42256-019-0048-x
76. Park, J, Beck, BR, Kim, HH, Lee, S, and Kang, K. A brief review of machine learning-based bioactive compound research. Appl Sci. (2022) 12:2906. doi: 10.3390/app12062906
77. Zhang, Y, Bao, X, Zhu, Y, Dai, Z, Shen, Q, and Xue, Y. Advances in machine learning screening of food bioactive compounds. Trends Food Sci Technol. (2024) 150:104578. doi: 10.1016/j.tifs.2024.104578
78. Singh, A, and Kumar, S. Exploring the functionality of microbes in fermented foods: technological advancements and future directions. Fermentation. (2025) 11:300. doi: 10.3390/fermentation11060300
79. Serrano, DR, Luciano, FC, Anaya, BJ, Ongoren, B, Kara, A, Molina, G, et al. Artificial intelligence (AI) applications in drug discovery and drug delivery: revolutionizing personalized medicine. Pharmaceutics. (2024) 16:1328. doi: 10.3390/pharmaceutics16101328
80. Li, D, Bai, L, Wang, R, and Ying, S. Research Progress of machine learning in extending and regulating the shelf life of fruits and vegetables. Foods. (2024) 13:3025. doi: 10.3390/foods13193025
81. Lu, L, Wang, L, Liu, R, Zhang, Y, Zheng, X, Lu, J, et al. An efficient artificial intelligence algorithm for predicting the sensory quality of green and black teas based on the key chemical indices. Food Chem. (2024) 441:138341. doi: 10.1016/j.foodchem.2023.138341
82. Yang, H, Wang, Y, Zhao, J, Li, P, Li, L, and Wang, F. A machine learning method for juice human sensory hedonic prediction using electronic sensory features. Curr Res Food Sci. (2023) 7:100576. doi: 10.1016/j.crfs.2023.100576
83. Fuentes, S, Torrico, DD, Tongson, E, and Gonzalez Viejo, C. Machine learning modeling of wine sensory profiles and color of vertical vintages of pinot noir based on chemical fingerprinting, weather and management data. Sensors. (2020) 20:3618. doi: 10.3390/s20133618
84. Cosme, F, Rocha, T, Marques, C, Barroso, J, and Vilela, A. Innovative approaches in sensory food science: from digital tools to virtual reality. Appl Sci. (2025) 15:4538. doi: 10.3390/app15084538
85. Tu, Y-F, Kwan, M-Y, and Yick, K-L. A systematic review of AI-driven prediction of fabric properties and handfeel. Materials. (2024) 17:5009. doi: 10.3390/ma17205009
86. Pellegrini, C, Özsoy, E, Wintergerst, M, and Groh, G. Exploiting food embeddings for ingredient substitution. Healthinf. (2021) 5:67–77.
88. Dhar, T. The California consumer privacy act: the ethos, similarities and differences Vis-a-Vis the general data protection regulation and the road ahead in light of California privacy rights act. J Data Protect Privacy. (2021) 4:170–92. doi: 10.69554/GLSA8501
89. Peyrone, N. Formal models for consent management in healthcare software system development. (2022).
90. Tan, Z, and Zhang, C. China’s PIPL and DSL: is China following the EU’S approach to data protection? J Data Protect Privacy. (2021) 5:7–25. doi: 10.69554/NATU8989
91. Asar, R, Erenler, S, Devecioglu, D, Ispirli, H, Karbancioglu-Guler, F, Ozturk, HI, et al. Understanding the functionality of probiotics on the edge of artificial intelligence (AI) era. Fermentation. (2025) 11:259. doi: 10.3390/fermentation11050259
92. Cembrowska-Lech, D, Krzemińska, A, Miller, T, Nowakowska, A, Adamski, C, Radaczyńska, M, et al. An integrated multi-omics and artificial intelligence framework for advance plant phenotyping in horticulture. Biology. (2023) 12:1298. doi: 10.3390/biology12101298
93. Murmu, S, Sinha, D, Chaurasia, H, Sharma, S, Das, R, Jha, GK, et al. A review of artificial intelligence-assisted omics techniques in plant defense: current trends and future directions. Front Plant Sci. (2024) 15:1292054. doi: 10.3389/fpls.2024.1292054
94. Valdés, A, Alvarez-Rivera, G, Socas-Rodríguez, B, Herrero, M, Ibanez, E, and Cifuentes, A. Foodomics: analytical opportunities and challenges. Anal Chem. (2021) 94:366–81. doi: 10.1021/acs.analchem.1c04678
95. Tsolakidis, D, Gymnopoulos, LP, and Dimitropoulos, K. Artificial intelligence and machine learning technologies for personalized nutrition: a review. Informatics. (2024) 11:62.
96. Moench-Pfanner, R, and Van Ameringen, M. The global alliance for improved nutrition (GAIN): a decade of partnerships to increase access to and affordability of nutritious foods for the poor. Food Nutr Bull. (2012) 33:S373–80. doi: 10.1177/15648265120334S313
97. D, D, Tb, K, Da, D, Mk, A, Mk, A, Mz, Z, et al. Convolutional neural network based deep learning model for accurate classification of durian types. J Appl Data Sci. (2025) 6:101–14.
Keywords: precision fermentation, CRISPR-microbe engineering, hyper-personalized nutrition, algorithmic bias, sustainable food systems
Citation: Priyadharshini D, Muthuvel I, Saraswathy S, Kavitha PS and Jegadeeswari V (2025) Precision to plate: AI-driven innovations in fermentation and hyper-personalized diets. Front. Nutr. 12:1659511. doi: 10.3389/fnut.2025.1659511
Edited by:
Vivek K. Chaturvedi, Banaras Hindu University, IndiaReviewed by:
Vipin Rai, Rutgers, The State University of New Jersey, United StatesRanjan Singh, Babasaheb Bhimrao Ambedkar University, India
Niraj Nag, University of Illinois Chicago, United States
Copyright © 2025 Priyadharshini, Muthuvel, Saraswathy, Kavitha and Jegadeeswari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: I. Muthuvel, aW03NEB0bmF1LmFjLmlu
 D. Priyadharshini
D. Priyadharshini I. Muthuvel2*
I. Muthuvel2*