Abstract
Background:
Non-alcoholic fatty liver disease (NAFLD) is a common health challenge worldwide and urgently requires effective therapeutic interventions. Flavonoids, a diverse group of plant-derived polyphenols, have demonstrated multifaceted biological functions, like anti-inflammatory, antioxidant and metabolic regulatory capacities, suggesting their potential utility in the management of NAFLD.
Objective:
This systematic review and meta-analysis aims to synthesize evidence from randomized controlled trials (RCTs) that examined the effects of flavonoid supplementation (including specific subclasses) in patients with NAFLD on liver function, markers of inflammation, lipid profiles, anthropometric measures and insulin resistance.
Methods:
This study rigorously adhered to the Cochrane Handbook for Systematic Reviews of Interventions (Version 6.5, 2024) and the PRISMA guidelines. A substantial literature review was obtained from PubMed, OVID, Web of Science Core Collection, Embase, and Cochrane Library databases, including studies published through December 2024.
Results:
The analysis incorporated 25 RCTs involving 1,689 participants with NAFLD. Flavonoids intervention significantly decreased aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), total cholesterol (TC), steatosis score, body mass index (BMI), triglycerides (TG), fasting blood sugar (FBS), insulin levels and augmented the quantitative insulin sensitivity check index (QUICKI) levels. However, no significant alterations were observed in gamma-glutamyl transferase (GGT), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), body weight (WT), waist-to-height ratio (WHtR), hip circumference (HC), Waist Circumference (WC). Moreover, the impact on hepatic steatosis and fibrosis scores was non-significant.
Conclusion:
Flavonoids exhibit potential therapeutic benefits in mitigating liver enzyme levels, lipid profiles, enhancing insulin sensitivity among NAFLD patients. Nonetheless, their influence on inflammatory markers, and fibrosis scores appears to be limited. Future investigations should focus on assessing the long-term security and effectiveness of flavonoid supplementation in managing NAFLD and exploring their synergistic potential in combination with other therapeutic strategies.
Systematic review registration:
1 Introduction
NAFLD is a spectrum disorder defined by ≥5% hepatic macrovesicular steatosis after excluding secondary etiologies (e.g., drugs, monogenic disorders) and excessive alcohol use. NAFLD can progress from simple steatosis to more severe conditions such as non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis and eventually hepatocellular carcinoma (HCC) (1). Since June 2023, NAFLD has been redefined as metabolic dysfunction-associated steatotic liver disease (MASLD), which emphasizes the importance of metabolic dysfunction in the pathogenesis (2). Global epidemiological trends reveal expanding overweight/obesity rates, driven by significant shifts in diet and lifestyle. This surge correlates with rising prevalence of chronic illnesses associated with obesity, including cardiovascular disease (CVD), metabolic syndrome, type 2 diabetes (T2DM) and NAFLD (3, 4). Latest evidence from the Global Burden of Disease (GBD) study reveals a concerning escalation in the prevalence of NAFLD, with the global number of affected patients doubling over the three-decade period from 1990 to 2021 (5). NAFLD affects 30.05% of the population worldwide, and it is predicted that 33.5% of adults aged ≥15 years will have NAFLD by 2030 (6, 7).
NAFLD is a clinically diverse disease influenced by environment, diet, and genetics, with its specific pathogenesis still unclear. NAFLD pathogenesis is classically attributed to the “two-hit” hypothesis: First hit – Adipose insulin resistance drives lipolysis and free fatty acid flux, overwhelming hepatic uptake to cause steatosis; Second hit – Prolonged lipid toxicity induces ER stress, mitochondrial damage, and oxidative injury, triggering inflammation and progression to NASH. Adipose dysfunction (reduced adiponectin, pro-inflammatory cytokines) further fuels insulin resistance and inflammation in a self-amplifying loop (8).
NAFLD/NASH currently lacks approved pharmacotherapies, with clinical management primarily focused on lifestyle modifications such as Mediterranean diets rich in Flavonoids (9). Flavonoids—a structurally diverse class of plant-derived phenolic compounds—show particular promise. Flavonoids demonstrate multi-target bioactivities by modulating a range of signaling pathways. Their antioxidant activity involves direct free radical scavenging, chelation of metal ions (e.g., Fe2+, Cu2+), and enhancement of endogenous antioxidant enzymes (e.g., SOD, CAT, GPx) through the Nrf2/ARE pathway. Furthermore, flavonoids support cardiovascular health by improving lipid metabolism—notably reducing ox-LDL and triglycerides—promoting endothelial NO release, and suppressing platelet activation. They also exhibit anti-diabetic effects via stimulation of insulin secretion, activation of insulin signaling pathways (including PI3K/Akt and AMPK), and inhibition of gluconeogenic enzymes such as PEPCK and G-6-Pase (10). Flavonoids, due to these characteristics, have shown promise in clinical interventions for multiple metabolic disorders, such as CVD, NAFLD and neurodegenerative diseases (11–13).
Over 5,000 identified flavonoids (classified into 7 subgroups: flavones, isoflavones, flavanols, etc.) demonstrate multi-target effects in NAFLD through antioxidation, anti-inflammation, and metabolic regulation properties (14, 15). Despite preclinical evidence supporting flavonoids’ multi-target effects in NAFLD, clinical translation remains limited by inconsistent efficacy reports and mechanistic ambiguity across trials, particularly regarding their impacts on metabolic parameters. The heterogeneity may partially stem from variations in flavonoid subtypes and dosage regimens. Existing meta-analyses may not encompass the latest clinical trials, particularly involving novel formulations. By integrating the most recent data, this systematic review and meta-analysis provides an updated and comprehensive efficacy assessment, and synthesizes RCTs to delineate subclass-specific benefits, dose–response relationships, and biomarker correlations, providing evidence-based guidance for integrating flavonoids into precision nutrition strategies against NAFLD.
2 Materials and methods
To ensure methodological rigor and transparency, this systematic review and meta-analysis strictly followed the Cochrane Handbook for Systematic Reviews of Interventions (Version 6.5, 2024) (16) and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). The protocol of the study has been prospectively submitted to the PROSPERO international registry (Registration ID: CRD420251001203).
2.1 Search strategy and selection criteria
A systematic search of the literature was carried out in 5 databases (PubMed, OVID, Web of Science Core Collection, Embase, Cochrane Library) to identify studies published before December 2024. The search strategy incorporated both Medical Subject Headings (MeSH) terms and keywords associated with NAFLD, flavonoids, and therapeutic interventions. The selection criteria were limited by language and publication type (Appendix A1). Furthermore, a manual examination of reference lists from both included studies and pertinent review articles was undertaken to locate additional qualifying literature.
2.2 Inclusion and exclusion criteria
Two independent reviewers (Qianqian Liu and Haodi Luan) employed the PICOS framework to filter articles that examined the efficacy of flavonoid supplements on health outcomes. The inclusion criteria were: (1) Adult patients diagnosed with NAFLD; (2) Flavonoids supplementation for at least 4 weeks; (3) Presence of a placebo or control group for comparison; (4) Only randomized controlled trials (RCTs) were included. (5) Studies published in English. Trials were excluded if they failed to meet the following criteria: (1) review articles, case reports, conference proceedings, non-randomized controlled trials, observational studies, and animal studies; (2) lack of data availability; (3) did not report on the specified outcome measures of interest. Inconsistencies were reconciled by consensus among the reviewers or, if necessary, in consultation with a third reviewer (Zhijiao Duan).
2.3 Study selection and data extraction
The study implemented a systematic literature screening and data extraction protocol: two reviewers (Haodi Luan and Zhijiao Duan) conducted dual-blind title/abstract screening, resolving discrepancies through consensus discussions with third-reviewer arbitration (Qianqian Liu) when needed, followed by full-text assessments. Independent parallel data extraction by trained researchers (Haodi Luan and Zhijiao Duan) utilized a standardized form capturing study design, demographics, intervention parameters, outcome metrics, and quantitative data (pre/post means ± SDs), supplemented by tripartite verification—third-researcher validation and author communications with customized data templates.
2.4 Risk of bias assessment
The quality of the included studies was assessed using the Cochrane Risk of Bias Tool. Two reviewers (Jing Ai and Yan Wang) independently assessed the risk of bias. Divergent assessments underwent structured group deliberation with all research team members to establish consensus-based ratings, ensuring inter-rater reliability and minimizing subjective interpretation errors.
2.5 Statistical analysis
The Statistical analyses were performed using STATA, version 16(Stata Corp, College Station, TX). To assess the effects of flavonoid supplementation on health outcomes, we calculated the standard deviations (SD) and mean differences between the intervention and control groups. These data were then used to determine the standardized mean difference (SMD) with 95% confidence intervals (CIs), applying a random-effects model for analysis. A random-effects model was applied for all meta-analyses, as we anticipated substantial clinical and methodological heterogeneity across studies due to variations in flavonoid subtypes, dosages, intervention durations, and participant characteristics. This model provides a more conservative estimate of the effect size and is appropriate when studies are not functionally identical, allowing for generalization beyond the included studies. Heterogeneity among the included studies was evaluated using I2 statistics, the interpretation of I2 values was as follows: 0% indicated no heterogeneity, <25% represented mild heterogeneity, 25–50% suggested moderate heterogeneity, and >50% reflected substantial heterogeneity across studies (18). To further investigate potential influencing factors, we conducted subgroup analyses based on key study characteristics, such as flavonoids type, flavonoids dose.
To evaluate potential publication bias, we conducted funnel plot visualization and quantified asymmetry using Egger’s regression test (Appendix A2). To address missing data, we used multiple imputation techniques to estimate missing values, ensuring robustness in our findings. To assess result stability, we conducted sensitivity analyses that systematically excluded studies with high risk of bias and re-analyzed the data (Appendix A2). All statistical analyses were performed using two-sided tests, with a significance threshold set at p < 0.05.
3 Results
3.1 Search results
Our literature search initially yielded 3,019 publications (see attached document for the detailed search strategy). Following duplicate removal, we screened 1991 records based on their titles and abstracts. Of these, 47 articles underwent full-text assessment for eligibility. After careful evaluation, our review and meta-analysis incorporated 25 eligible studies. The PRISMA flow diagram provides a comprehensive overview of the exclusion process and reasons for article exclusion at each stage (Figure 1).
Figure 1
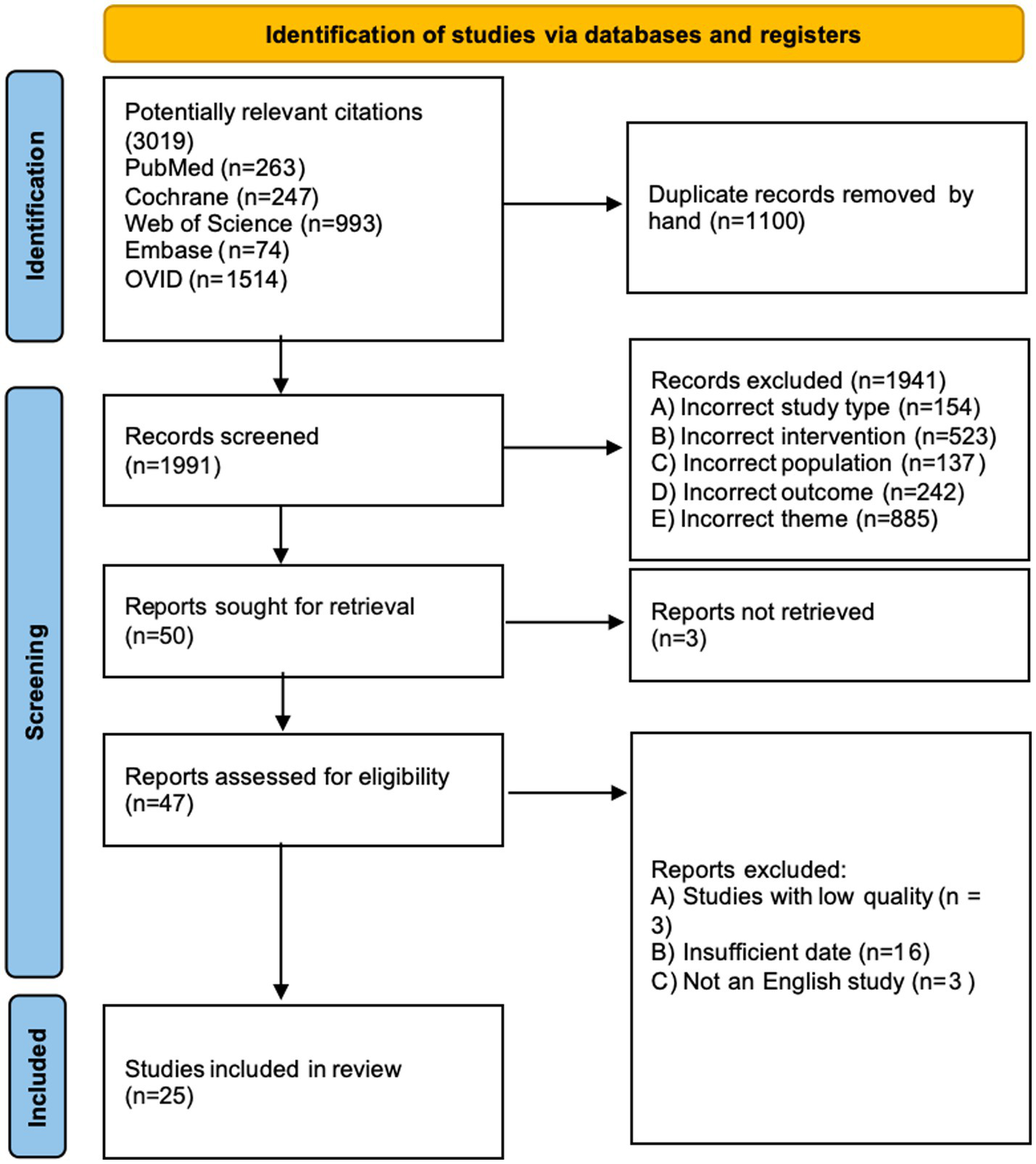
Prisma flow diagram of study selection process.
3.2 Characteristics of included studies
This meta-analysis included 25 RCTs investigating the effects of nine flavonoid—quercetin, genistein, naringenin, hesperidin, anthocyanin, pueraria, isoflavones, catechin, and silymarin—on patients with NAFLD or NASH. Flavonoid dosages varied from 32 to 1,080.6 mg per day, with intervention periods ranging from 4 to 24 weeks, and the most frequent duration was 12 weeks (n = 10). The RCTs were conducted across multiple countries, including Iran (n = 17), China (n = 3), Pakistan (n = 3), Turkey (n = 1), Spain (n = 1). Detailed study characteristics are presented in Table 1.
Table 1
| Flavonoids | Author | Design | Country | Population (IG/CG) |
Mean age | Gender (M/F) | Intervention | Duration | Relevant outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | IG | CG | ||||||||
| Quercetin | Li et al.2024 (35) | Double-BlindRCT, Crossover | China | 41/41 | 39.2 ± 11.7 | 40.1 ± 9.1 | I:27/14 | C:27/14 | Quercetin 500 mg | Placebo | 12 weeks | WT, HbA1c, ALT TG, LDL-C |
|
| Hosseinikia et al.2020 (36) | Double-Blind RCT, Parallel |
Iran | 39/39 | 43.4 ± 11.1 | 45.9 ± 9.2 | I:15/24 | C:13/26 | Quercetin 500 mg | Placebo | 12 weeks | TNF-α, GGT, TG, TC LDL, HDL, BMI, Hs-CRP, WHR |
||
| Pasdar et al.2020 (37) | Double-Blind RCT, Pilot |
Iran | 39/39 | 43.46 ± 11.13 | 45.89 ± 9.16 | I:15/24 | C:13/26 | Quercetin 500 mg | Placebo | 12 weeks | RBC, MCHC | ||
| Genistein | Amanat et al.2018 (38) | Double-Blind RCT, Parallel |
Iran | 41/41 | 44.22 ± 11.80 | 42.94 ± 9.55 | I:30/11 | C:31/10 | Genistein 250 mg | Placebo | 8 weeks | FBS, HOMA-IR, IL-6, TNF-α WHR, TG, BMI, LDL-C, HDL-C, AST, ALT |
|
| Naringenin | Namkhah et al.2021 (39) | Double-Blind RCT, Parallel |
Iran | 22/22 | 44.7 ± 10.7 | 47 ± 9.4 | I:12/10 | C:13/9 | Naringenin 200 mg | Placebo | 4 weeks | NFS, WT, BMI, TG, TC, LDL, HDL, WC | |
| Hesperidin | Yari et al.2020 (40) | Open-labeled Parallel |
Iran | 22/21 | 45.82 ± 11.69 | 46.11 ± 11.63 | I:11/11 | C:10/11 | Hesperidin1,000 mg + Lifestyle modification | Lifestyle modification | 12 weeks | HOMA-IR, CRP, TNF-α, NF-κB, Fibrosis score、Steatosis score FLI | |
| Anthocyanin | Sangsefidi et al. 2021 (41) | Double-Blind RCT, Parallel |
Iran | 25/25 | 41.48 ± 9.53 | 42.68 ± 9.96 | I:12/13 | C:11/14 | Anthocyanin 32 mg | Placebo | 12 weeks | ALT, AST, CK-18, Fibrosis score, Steatosis score | |
| Yarhosseini et al. 2021 (42) | Double-Blind RCT, Parallel |
Iran | 25/25 | 41.4 ± 9.5 | 42.6 ± 9.9 | I:12/13 | C:11/14 | Anthocyanin 32 mg | Placebo | 12 weeks | WC, HC, WHR | ||
| Izadi et al. 2020 (43) | Double-Blind RCT, Parallel |
Iran | 30/31 | 43.3 ± 10.2 | 42.8 ± 10.6 | I:17/13 | C:19/12 | Anthocyanin 250 mg | Placebo | 8 weeks | TG, ALT, AST, TC, LDL-C, HDL-C, WT, BMI, WC | ||
| Zhang et al. 2014 (44) | Double-Blind RCT, Parallel |
China | 37/37 | 44.9 ± 7.5 | 46.9 ± 7.7 | I:19/18 | C:20/17 | Anthocyanin 320 mg | Placebo | 12 weeks | ALT, AST, TG, TC, LDL, HDL-C, BMI, CK-18, HOMA-IR, WHR | ||
| Bayram et al. 2024 (45) | RCT, Parallel | Turkey | 22/22 | 43.90 ± 10.44 | 43.40 ± 12.46 | I:10/12 | C:10/12 | Anthocyanin 350 mg | Placebo | 8 weeks | ALT, AST, ALP, GGT, TG, TC, LDL, HDL, FBG, HbA1c, HOMA-IR, CRP, BMI, WC, HC | ||
| Ghanbari et al. 2024 (46) | Double-Blind RCT, Parallel |
Iran | 25/25 | 43.52 ± 8.12 | 44.88 ± 10.14 | I:10/15 | C:14/11 | Grape Seed Extract 520 mg | Placebo | 8 weeks | ALT, AST, TG, TC, LDL-c, HDL-c, BMI, QUICK, HOMA-IR | ||
| Pueraria | Li et al. 2024 (47) | Triple-blind RCT, Parallel |
China | 60/61 | 56.2 ± 10 | 58.1 ± 9.6 | I:30/30 | C:31/30 | Silybin 138.0 mg, Puerarin 68.4 mg, Salvianolic acid 65.4 mg | Placebo | 24 weeks | FIB-4, HOMA-IR, WT, BMI, WC, WHR, CRP, IL-6, FIB-4, APRI, NFS | |
| Isoflavones | Tehrani et al. 2024 (48) | Double-Blind RCT, Parallel |
Iran | 25/21 | F:51.93 ± 11.15, M:47.60 ± 14.98 |
F:52.09 ± 5.73 M:46.0 ± 14.10 |
I:10/15 | C:10/11 | Soy isoflavones 100 mg | Placebo | 12 weeks | ALT, AST, Steatosis score, Fibrosis score, GGT, FGF-21, WT, BMI, WC, WHR | |
| Catechin | Pezeshki et al. 2016 (49) | Double-Blind RCT, Parallel |
Iran | 35/36 | None | None | I:16/19 | C:16/20 | Catechins 3.45 mg Caffeine 11.375 mg, GC:25.66 mg, EGC:15.38 mg, EC:29.305 mg, EGCG:157.145 mg, GCG:11.45 mg, ECG:13.235 mg | Placebo | 90 days | AST, ALT, ALP, WT, BMI | |
| Silymarin | Hashemi et al. 2009 (50) | Double-Blind RCT, Parallel |
Iran | 50/50 | 39.28 ± 11.117 | 39.0 ± 10.70 | I:28/22 | C:29/21 | Silymarin 280 mg | Placebo | 24 weeks | AST, ALT, FBS, TG, TC, LDL, HDL, BMI | |
| Solhi et al.2014 (51) | RCT, Parallel | Iran | 33/31 | 43.6 ± 8.3 | 39.36 ± 10.5 | I:19/14 | C:19/12 | Silymarin 210 mg | Placebo | 8 weeks | AST, ALT | ||
| Anushiravani et al. 2019 (52) | Double-Blind RCT, Parallel |
Iran | 30/30 | None | None | None | None | Silymarin 140 mg | Placebo | 12 weeks | BMI, WC, TG, TC, LDL-C, HDL-C, FBS | ||
| Aller et al. 2015 (53) | RCT, Parallel | Spain | 18/18 | None | None | None | None | Silymarin 1080.6 mg + Vitamin E 72 mg + Lifestyle modification | Lifestyle modification | 12 weeks | BMI, WC, GGT, FLI, NFS, ALT, AST, TG, FBS, HOMA-IR, WT | ||
| Memon et al. 2015 (54) | Double-Blind RCT, Parallel |
Pakistan | 31/33 | 49.0 ± 9.70 | 48 ± 8.9 | I:21/12 | C:21/10 | Silymarin 280 mg | Placebo | 3 months | ALT, AST, TG, TC, LDL, HDL | ||
| Rangboo et al. 2016 (55) | Double-Blind RCT, Parallel |
Iran | 30/30 | 47.27 ± 8.12 | 49.83 ± 12.79 | I:21/9 | C:21/9 | 2,700 mg Cynara scolymus extract | Placebo | 2 months | ALT, AST, TG, TC, LDL-C, HDL-C, FBS | ||
| Shaikh et al. 2021 (56) | RCT, Parallel | Pakistan | 100/100 | None | None | None | None | Silymarin 400 mg | Placebo | 12 weeks | AST, ALT | ||
| Mirhashemi et al. 2022 (57) | Double-Blind RCT, Parallel |
Iran | 27/25 | 37.81 ± 9.93 | 38.08 ± 10.01 | None | None | 560 mg Silymarin+Lifestyle modification | Placebo +Lifestyle modification | 8 weeks | AST, ALT, AST/ALT, BMI, Fib-4, NFS | ||
| Atarodi et al. 2022 (58) | Double-Blind RCT, Parallel |
Iran | 27/29 | 36.46 ± 10.00 | 37.52 ± 8.94 | I:9/18 | C:8/21 | Silymarin 140 mg | Placebo | 4 weeks | ALT, AST, ALP, TB, DB, TG, TC, LDL-C, HDL-C, BMI | ||
| Hafiza et al. 2024 (59) | RCT, Parallel | Pakistan | I1:16 I2:16 I3:16 C:16 |
None | None | I1:7/9 I2:6/10 I3:8/8 |
C:8/8 | I1: Silymarin 200 mg I2: Silymarin 300 mg I3: Silymarin 400 mg |
Placebo | 3 months | ALT, AST, ALP, CRP, ESR, BMI | ||
Characteristics of all trials included in the present meta-analysis.
AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; ALP, Alkaline Phosphatase; GGT, G-Glutamyl-Transferase, BMI, Body Mass Index; WT, Weight; HC, Hip Circumference; WC, Waist Circumference; WHR, Waist-to-Hip Ratio; CRP; C-Reactive Protein; TNF-α:tumor necrosis factor; IL-6, Interleukin-6; CK-18, Cytokeratin-18; LDL-C, Low-Density lipo-Protein Cholesterol; TC, Total Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; TG, Triglycerides; FBS, Fasting Blood Sugar; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; QUICKI, Quantitative Insulin Sensitivity Check Index; IG, Intervention group; CG, Control group; I, Intervention; C, Control.
3.3 Risk of bias assessment
We assessed the risk of bias in all RCTs using the Review Manager, with a visual summary and graphical representation provided in Figures 2, 3. This systematic review included a total of 25 randomized controlled trials (RCTs). The overall risk of bias, as assessed by the Cochrane Risk of Bias tool, was low. Regarding specific domains: for randomization, 20 trials were rated as low risk and 5 as unclear; for allocation concealment, 19 were low risk and 6 were unclear. Blinding of participants and personnel was low risk in 19, high risk in 2, and unclear in 4 trials. Blinding of outcome assessment was low risk in 15 and unclear in 10 trials. The risks of bias from incomplete outcome data and selective reporting were low for the majority of included studies. The risk of other biases was high in 8 and low in 17 studies.
Figure 2
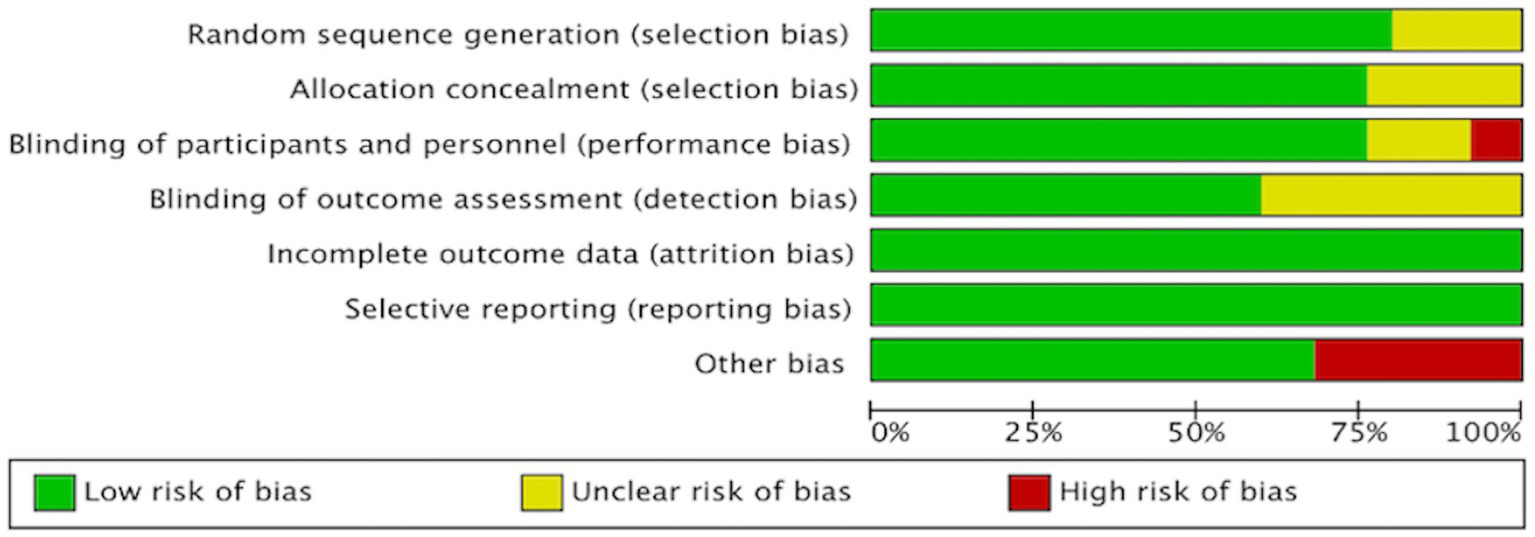
Risk of bias assessment of the included studies.
Figure 3
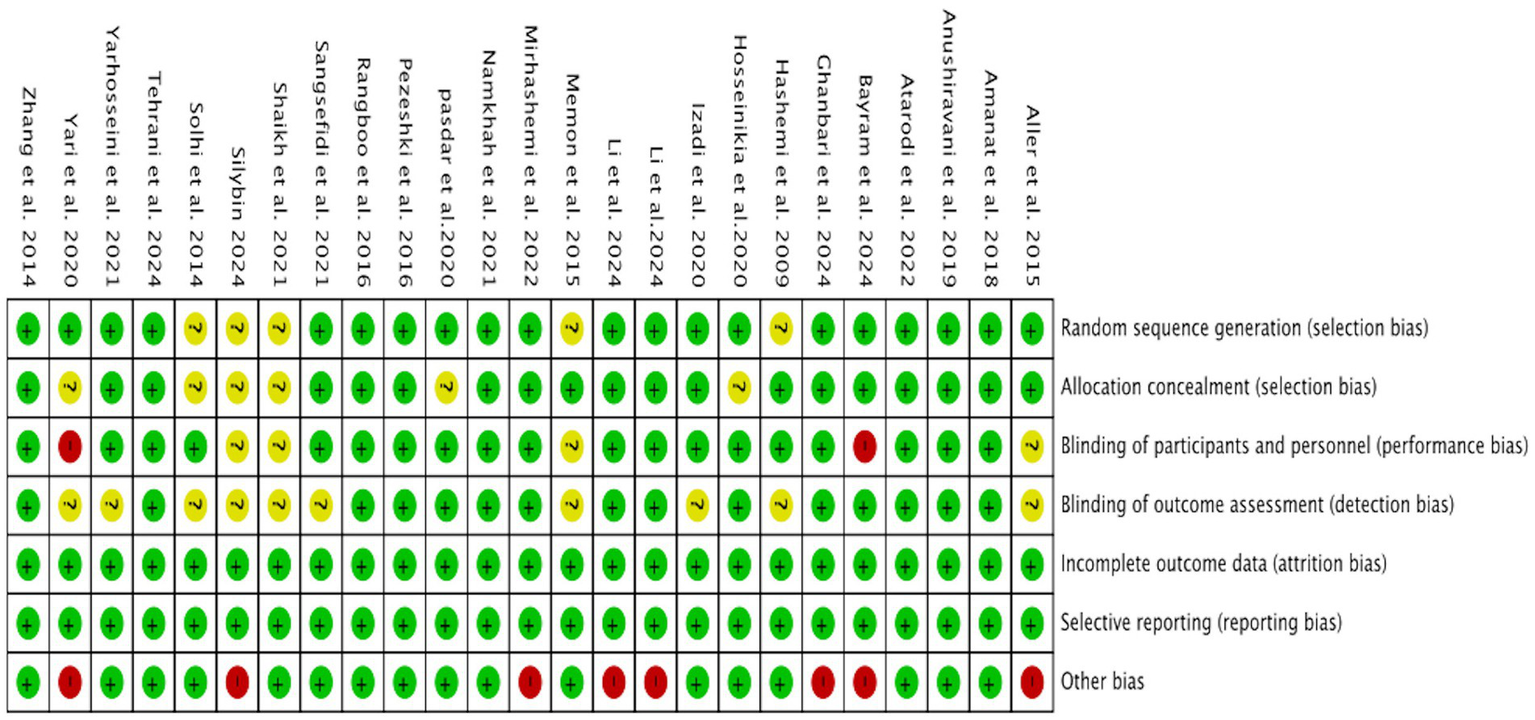
Risk of bias assessment of the included studies.
3.4 Liver function effects of flavonoid supplementation
Totally, 11 RCTs evaluated flavonoid supplementation on ALT and AST in 631 NAFLD patients. Respectively, For ALT, pooled data from the trials demonstrated that flavonoids intervention reduced ALT levels in comparison with the control group (SMD = −0.50, 95% CI: −0.82 to −0.17, p = 0.003), with substantial heterogeneity (I2 = 75.20%, p < 0.001) (Figure 4A). In a subset analysis, silymarin significantly reduced ALT concentrations (Appendix A3, Figure 1.1) and silybin were effective in reducing ALT concentrations at doses of ≥2000 mg (Appendix A3, Figure 1.3). A pronounced reduction in ALT levels was observed with anthocyanin intervention within a period of fewer than 12 weeks (Appendix A3, Figure 1.2). For AST, the intervention group exhibited a decrease in levels compared to the control group (SMD = −0.37, 95% CI: −0.70 to −0.03, p = 0.034), with substantial heterogeneity (I2 = 77.3%, p < 0.001) (Figure 4B). The efficacy of anthocyanin in reducing AST levels was noted in subgroup analysis (Appendix A3, Figure 2.1). Similarly, silymarin reduced AST levels at higher dosage thresholds of ≥2000 mg (Appendix A3, Figure 2.2).
Figure 4
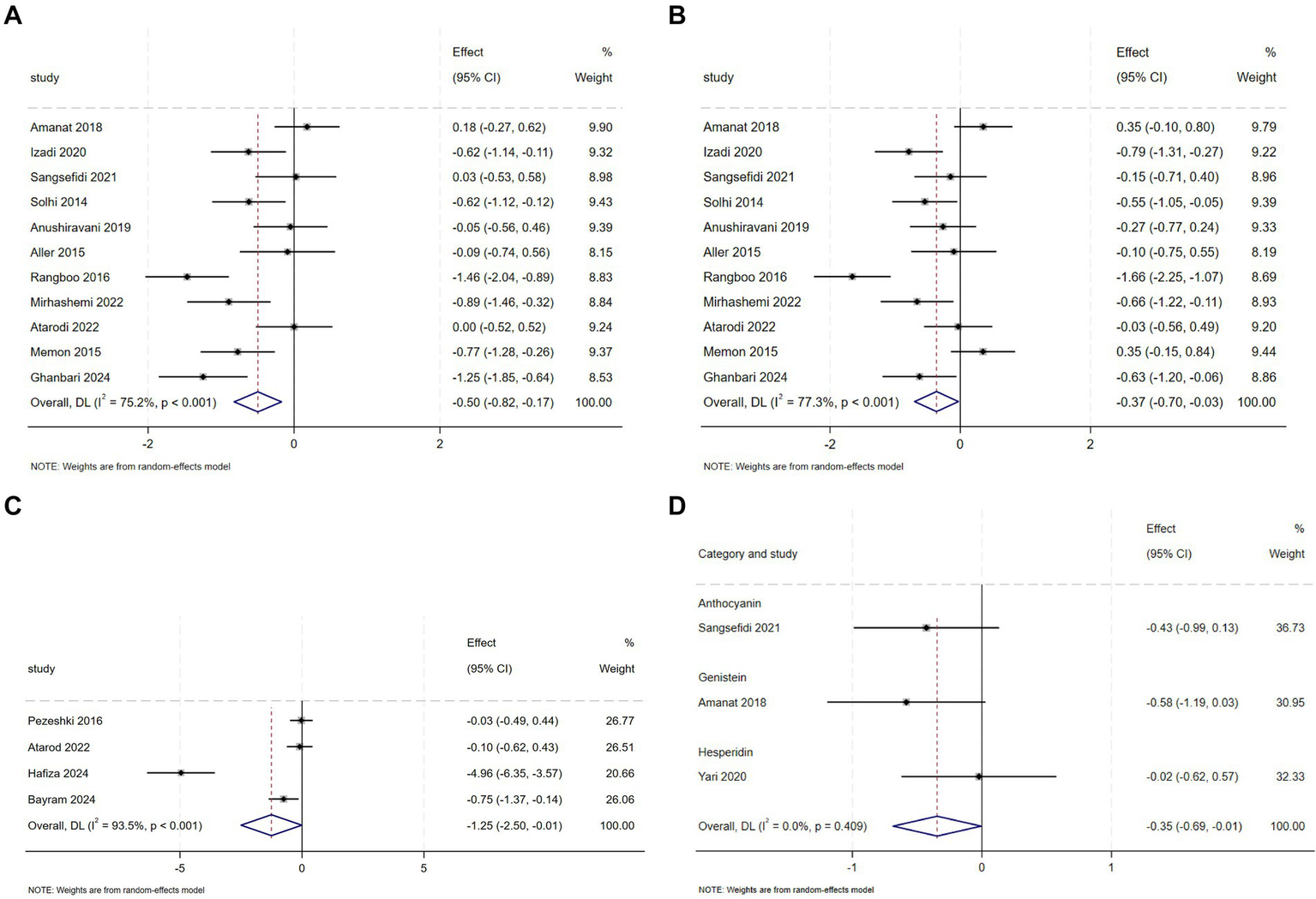
(A) Effect of flavonoid on ALT (B) Effect of flavonoid on AST (C) Effect of flavonoid on ALP (D) Effect of flavonoid on Steatosis score.
Meta-analysis of 4 RCTs showed that flavonoid intervention markedly reduced ALP levels compared to placebo (SMD = −1.25, 95% CI: −2.50 to −0.01, p = 0.048), however, there was considerable heterogeneity between the studies (I2 = 93.5%, p < 0.001) (Figure 4C). Subgroup analyses uncovered that specific flavonoid subtype—namely anthocyanin was effective in lowering ALP levels (Appendix A3, Figure 3.1). Geographical stratification showed significant reductions in studies conducted in Pakistan and Turkey compared to other regions (Appendix A3, Figure 3.2).
Pooled RCT analysis revealed no statistically meaningful reduction in GGT levels following flavonoids intervention compared to controls (SMD = −0.37, 95% CI: −0.89 to 0.16, p = 0.173), with high heterogeneity between studies (I2 = 60.7%, p = 0.079) (Appendix A4, Figure 1). Finally, when pooled data from these studies were analysed, there were no significant differences in hepatic steatosis and fibrosis between placebo and intervention groups (Appendix A4, Figures 2–5), with the exception of the Steatosis score (Figure 4D).
3.5 Effects on FBS and insulin resistance with flavonoid supplementation
Compared to the placebo group, the intervention group showed a perceptible decline in FBS, as indicated by comprehensive analysis (SMD = −0.26, 95% CI: −0.50 to −0.02, p = 0.037; 12 = 35.5%, p = 0.157) (Figure 5B) and Insulin (SMD = −0.54, 95% CI: −0.86 to −0.22, p = 0.001; 12 = 43.5%, p = 0.132) (Figure 5C), with moderate heterogeneity observed in both analyses. Subgroup analyses demonstrated that anthocyanin supplementation exerted a more pronounced effect on FBS reduction, whereas hesperidin, genistein, and anthocyanins decreased insulin concentrations (Appendix A3, Figures 9, 10).
Figure 5
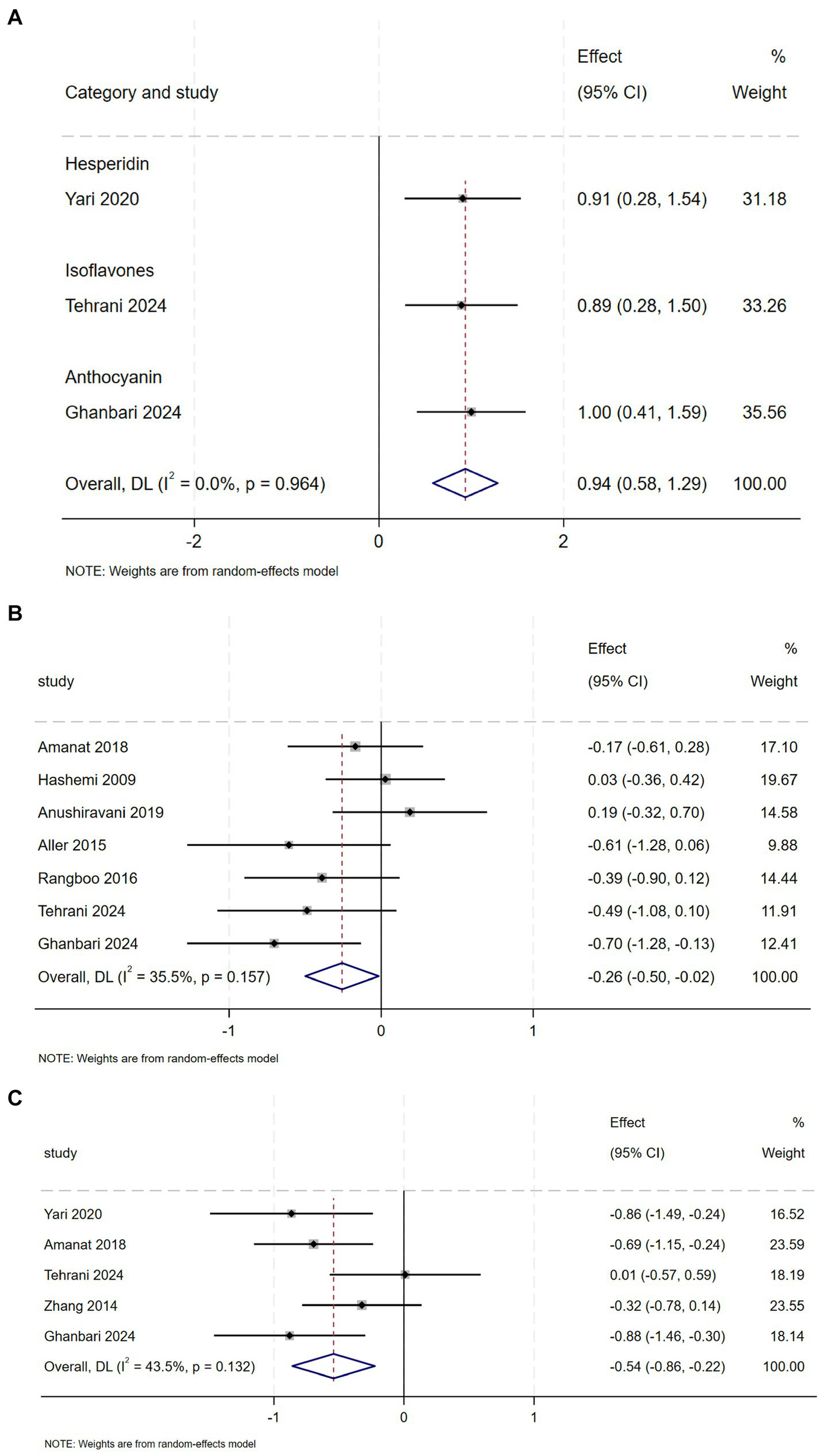
(A) Effect of flavonoid on QUICKI (B) Effect of flavonoid on FBS (C) Effect of flavonoid on Insulin.
And compared with the control group, QUICKI values were higher in the intervention group (SMD = 0.94, 95% CI: 0.58 to 1.29, p = 0.00), with Mild heterogeneity (12 = 0.0%, p = 0.964) (Figure 5A). However, pooled analysis of RCTs revealed no statistically significant reduction in HOMA-IR level following flavonoids intervention compared to control (Appendix A4, Figure 6).
3.6 Effects on anthropometric indices with flavonoid supplementation
Pooled data from 15 clinical trials (909 NAFLD patients) showed that flavonoids led to a more appreciable decrease in BMI compared to controls (SMD = −0.30, 95% CI: −0.50 to −0.10, p = 0.003), with moderate heterogeneity (I2 = 54.1%, p = 0.007) (Figure 6). Hesperidin was associated with reduced BMI in subgroup analyses (Appendix A3, Figure 5.1). Geographical stratification showed significant reductions in studies conducted in Pakistan and Iran compared to Spain (Appendix A3, Figure 5.2). In the evaluation of HC (3 studies, n = 178), combined results indicated no statistically significant effect of flavonoids versus placebo (SMD = −0.22, 95% CI: −0.58 to 0.13, p = 0.218), with substantial heterogeneity (I2 = 28.8%, p = 0.245) (Appendix A4, Figure 7).
Figure 6
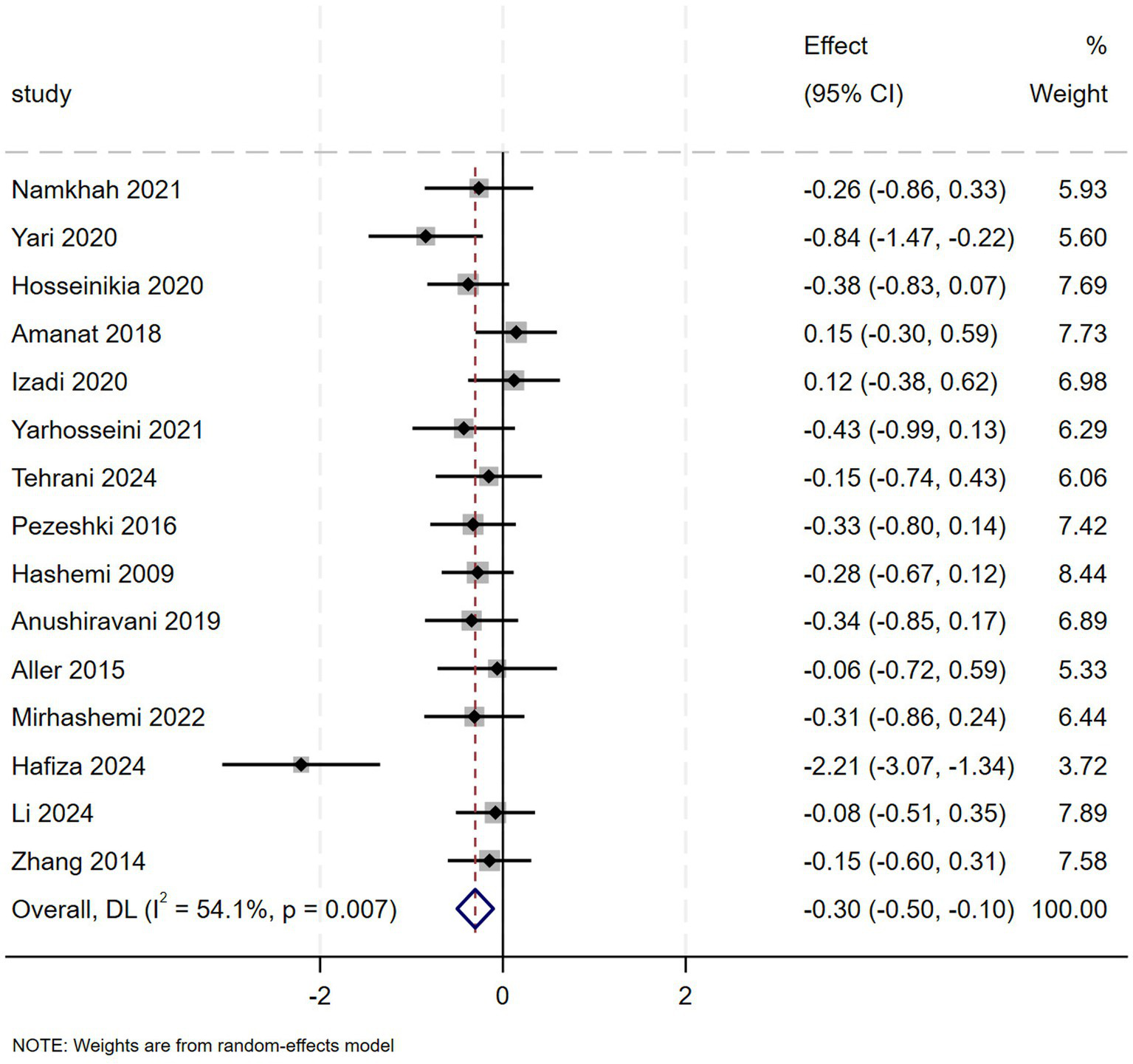
Effect of flavonoid on BMI.
Comparing flavonoids supplementation with placebo in 10 studies revealed no meaningful change in WC (SMD = −0.06, 95% CI: −0.22 to 0.10, p = 0.471), with mild heterogeneity (I2 = 0.0%, p = 0.515) (Appendix A4, Figure 8). However, subgroup analysis revealed that hesperidin reduced WC concentration (Appendix A3, Figure 6). Meta-analysis of available trials (5 for WHtR, 10 for WT) demonstrated that flavonoid intervention had no statistically significant impact on WHtR (Appendix A4, Figure 9) and WT (Appendix A4, Figure 10). And subgroup analyses showed no significant differences based on type or dose categories. (Appendix A3, Figures 7, 8).
3.7 Effects on inflammatory markers with flavonoid supplementation
Totally, 2, 1, 2, and 1 studies evaluated the efficacy of flavonoids on Hs-CRP, IL-6, TNF-a, and CK-18, respectively. Pooling data revealed that flavonoids supplementation showed no significant change in inflammatory markers (Appendix A4, Figure 11). However, subgroup analysis revealed that genistein significantly reduced inflammatory markers (Appendix A3, Figure 11.1). And flavonoids supplementation showed significant change in IL-6 (Appendix A3, Figure 11.2).
3.8 Effects on lipid profile with flavonoid supplementation
Comparing flavonoid supplementation with placebo in 9 studies indicated no substantial change in LDL (SMD = −0.28, 95% CI: −0.57 to 0.01, p = 0.059), with substantial heterogeneity (I2 = 65.9%, p = 0.003) (Appendix A4, Figure 12). However, subgroup analysis revealed that anthocyanin and isoflavones and decrease LDL concentrations (Appendix A3, Figure 15.1). In our meta-analysis of 7 trials (n = 463 NAFLD patients), flavonoid intervention demonstrated no significant effect on HDL levels (Appendix A4, Figure 13). And subgroup analyses showed no significant differences based on type or dose categories (Appendix A3, Figure 14).
TC was evaluated in 9 studies with 573 NAFLD patients. The meta-analysis revealed a statistically significant reduction in TC (SMD = 0.39, 95% CI: −0.67 to −0.10, p = 0.009), though substantial heterogeneity was observed (I2 = 65.6%, p = 0.003) (Figure 7A). Subgroup analysis demonstrated that anthocyanin and isoflavones complementation contributed to a marked decrease in TC levels (Appendix A3, Figure 12.1) and silybin supplementation for <12 weeks was associated with a more marked decrease in TC levels (Appendix A3, Figure 12.2).
Figure 7
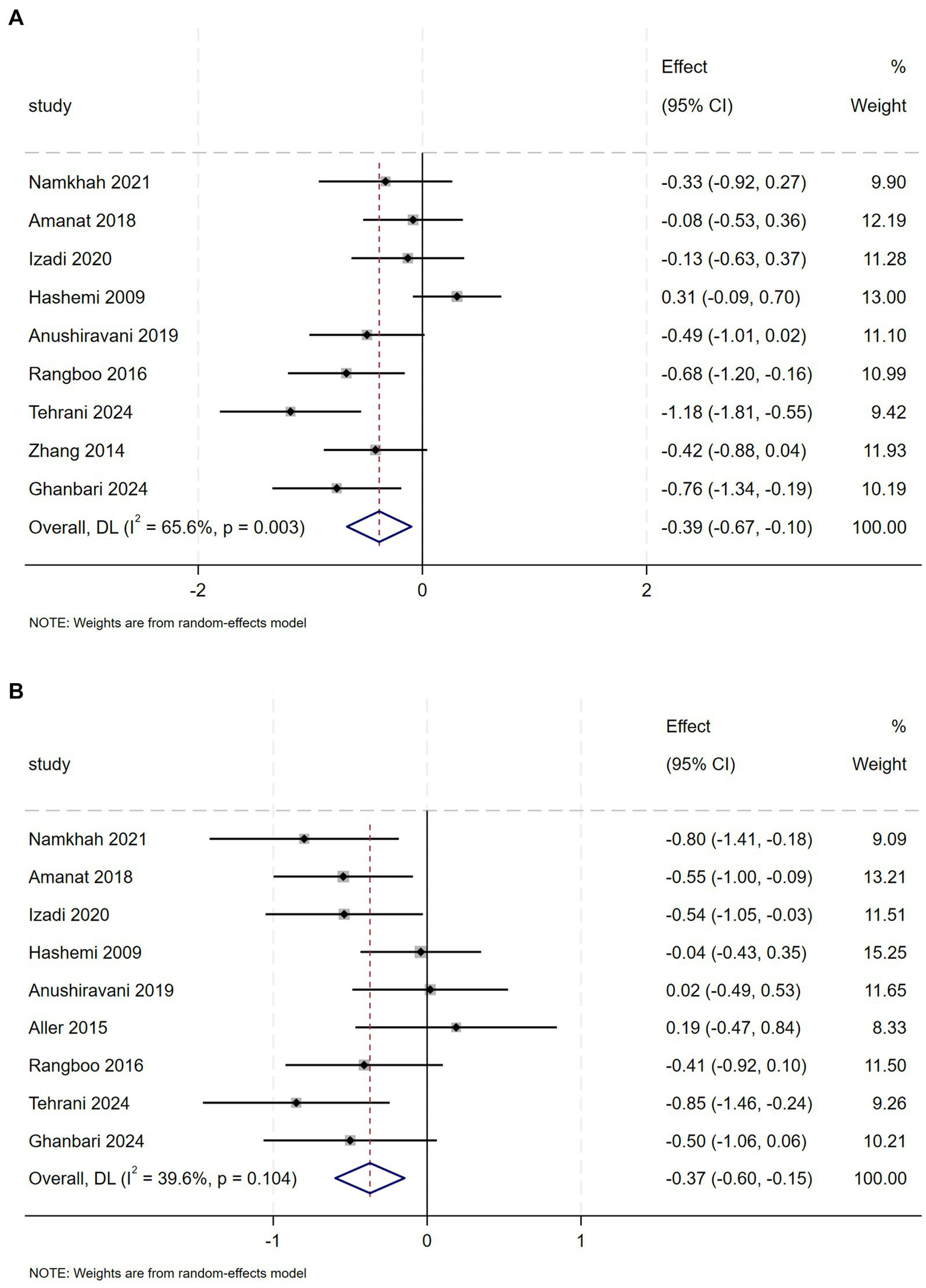
(A) Effect of flavonoid on TC (B) Effect of flavonoid on TG.
Nine trials investigated the effectiveness of flavonoids intervention on TG in 535 patients with NAFLD. Meta-analysis revealed a relevant effect of flavonoids intervention on TG (SMD = −0.37, 95% CI: −0.60 to −0.15, p = 0.001), with moderate heterogeneity (I2 = 39.6%, p = 0.104) (Figure 7B). Regarding flavonoids classes, subgroup analysis demonstrated that naringenin, genistein, anthocyanin and isoflavones significantly decreased TG levels (Appendix A3, Figure 13).
4 Discussion
NAFLD represents a major worldwide health burden, yet current pharmacological treatment options remain inadequate and effective pharmacological treatments are still limited. Flavonoids mitigate NAFLD through gut-liver axis modulation and multi-target mechanisms. They enrich beneficial bacteria (e.g., Lactobacillus, Bifidobacterium), inhibit pathogens, and enhance gut barrier function, reducing endotoxemia and systemic inflammation. Concurrently, flavonoids activate AMPK/Nrf2 pathways—suppressing SREBP-1c-mediated lipogenesis and boosting antioxidant defenses (SOD, CAT)—while inhibiting NF-κB to attenuate inflammation (TNF-α, IL-6). PPAR-α activation promotes fatty acid β-oxidation. Improved insulin sensitivity further alleviates steatosis and oxidative stress. Synergistic effects with conventional drugs enhance therapeutic efficacy against NAFLD (19). Specifically, quercetin downregulates SREBP-1c and FAS through the AMPK/SIRT1 signaling pathway to inhibit lipogenesis (20). EGCG enhances its affinity for PPARα and CPT-1 via its galloyl group, thereby promoting mitochondrial fatty acid β-oxidationx (21). Meanwhile, proanthocyanidins increase the abundance of Akkermansia, restore the intestinal barrier, and alleviate LPS-TLR4-mediated hepatic inflammation (22).
A comprehensive insight into the efficacy of flavonoids in the treatment of NAFLD and their potential as adjunctive therapies is provided by our updated systematic review and meta-analysis.
The meta-analysis encompassing 25 controlled trials found that flavonoid treatment could significantly reduce ALT, AST, ALP, BMI, TC, TG, FBS, insulin levels, Steatosis score and there was significant increase in QUICKI. To assess the robustness of these core findings against potential biases, we conducted pre-specified sensitivity analyses (by sequentially excluding each study). Reassuringly, the direction and statistical significance of the pooled estimates for these objective biochemical outcomes (e.g., ALT, AST, TG, TC, Insulin, QUICKI) remained stable, indicating that these beneficial effects are robust and unlikely to be driven solely by performance or detection bias present in some included studies. However, there was no significant change in GGT, LDL, HDL, WT, HC, WHtR, HOMA-IR, markers of inflammation, levels of hepatic steatosis and fibrosis.
When significant heterogeneity was observed among the included studies (I2 > 50%), subgroup analyses were conducted to explore potential sources of variation. Variables such as flavonoid subtype, intervention dosage, duration of treatment, country, age, and sex were examined iteratively to identify specific factors contributing to heterogeneity. The full analytical procedures and detailed results are provided in Appendix A3.
Our analysis revealed that flavonoids supplementation significantly reduced ALT, AST and ALP levels, indicating improved liver function. These findings align with prior meta-analysis examining flavonoid interventions in NAFLD patients (13, 23). Specifically, silymarin demonstrated notable efficacy in reducing these liver enzymes. High-dose silybin (≥2000 mg) produces the greatest reduction in ALT and AST levels. These findings are consistent with prior meta-analytic evidence confirming silymarin’s hepatoprotective effects in NAFLD, including significant improvements in ALT and AST levels, reductions in hepatic steatosis, and decreased liver stiffness (24). While anthocyanin significantly lowers ALT, AST, and ALP in the short term (<12 weeks), making it suitable for patients requiring rapid improvement in liver enzymes. However, the impact on GGT was less pronounced, suggesting that while flavonoids can improve certain aspects of liver function, their effects may be more limited in other areas. In addition, Flavonoid intervention showed no significant improvement in non-invasive fibrosis scores (NFS, FIB-4), likely due to insufficient intervention duration, the inherently slow progression of liver fibrosis, and the limited sensitivity of serum biomarkers for early-stage detection.
Flavonoids supplementation significantly reduce FBS and insulin levels, and increase QUICKI, anthocyanins demonstrated a more pronounced effect in reducing FBS, while improvements in insulin levels were associated with various flavonoids such as hesperidin, genistein, and anthocyanins. Similarly, a meta-analysis reported that interventions rich in flavanols significantly reduced insulin levels and HOMA-IR, with consistent results across different subgroups (25). However, a meta-analysis showed that consumption of flavonoids did not lead to significant changes in insulin, HOMA-IR, and FBS levels in patients with NAFLD (23). These discrepancies may stem from differences in baseline metabolic profiles, as well as variations in study design (e.g., flavonoids dosage, intervention duration, or participant characteristics).
Flavonoids showed a significant reduction in BMI, particularly with hesperidin supplementation, hesperidin monotherapy can simultaneously reduce BMI and waist circumference, with particularly notable efficacy in addressing central obesity, in line with a prior meta-analysis investigating the impact of hesperidin in patients with obesity (26). Notably, hesperidin stimulates the secretion of cholecystokinin (CCK)—a key appetite-regulating hormone—in enteroendocrine STC-1 cells. This CCK-mediated mechanism contributes to its anti-obesity effects by promoting satiety and suppressing appetite (27). However, no significant effects were observed on WC, WHtR, or WT. This suggests that while flavonoids may help in weight management to some extent, their impact on overall body composition may be limited.
Despite no significant overall change in inflammatory markers, subgroup analysis revealed that genistein significantly reduced inflammatory markers. This finding contrasts with a previous meta-analysis, which reported significant reductions in TNF-α and NF-κB levels following flavonoids supplementation in NAFLD patients (23). The discrepancy between these results may be attributed to differences in study inclusion - our analysis incorporated 13 additional studies that were not considered in the prior meta-analysis. Genistein, the major soy isoflavone, possesses anticancer, antioxidant, and anti-inflammatory properties, along with tyrosine kinase inhibition (28, 29). Ji et al. demonstrated that genistein alleviates NASH in HFD-fed rats by improving liver function, reduced thiobarbituric acid-reactive substances (TBARS), TNF-α, and IL-6 levels, and inhibiting IκB-α/NF-κB and JNK pathways (30). Although genistein showed a potential reduction in IL-6 in a subgroup analysis, the sample size was too small to support conclusive findings. Flavonoid intervention demonstrates no significant overall effect on inflammatory mediators such as Hs-CRP, IL-6, and TNF-α. This may be attributed to their upstream antioxidant and metabolic roles within the inflammatory cascade, combined with the low baseline inflammation levels in the studied populations, which left limited room for improvement. Furthermore, methodological limitations, including the small number of studies reporting each outcome, inconsistent assay platforms, and single time-point measurement, further reduce statistical power and increase heterogeneity.
Flavonoids significantly reduced TC, TG levels, with naringenin, genistein, anthocyanin, and isoflavones showing the most significant effects. However, the levels of LDL and HDL were not significantly changed. Tsuda et al. reported that anthocyanins upregulated the expression of key adipogenic and metabolic regulators—including PPARγ, uncoupling protein 2 (UCP2), adipocyte fatty acid-binding protein (aP2), and lipoprotein lipase (LPL) —while also stimulating the secretion of adipocytokines (adiponectin and leptin) in isolated rat adipocytes (31). Naringenin attenuates lipid accumulation through AMPK pathway activation, modulating lipid metabolism genes to reduce TC, TG, and non-HDL-C levels. This dual action alleviates hepatic steatosis and decreases adipocyte size in both HFD-fed Sprague–Dawley rats and 3 T3-L1 adipocytes (32). The absence of significant improvements in lipid markers (LDL-C, HDL-C) and anthropometric measures (WT, HC, WHtR) may reflect either interindividual variability in flavonoid response or the necessity of combined dietary/lifestyle modifications to elicit measurable effects.
Nevertheless, this review has several limitations. First, the overall evidence base remains limited by the number and sample size of available RCTs. Significant heterogeneity was observed across several outcomes, which, despite our subgroup analyses, could not be fully resolved due to the variability in flavonoid subtypes, dosages, and patient characteristics. Second, some included studies had a high or unclear risk of bias, particularly in the domains of blinding (performance and detection bias), which could potentially overestimate the intervention effects. However, as demonstrated by our sensitivity analyses, the exclusion of these studies did not alter the significance of our primary findings for key liver and metabolic parameters, suggesting that the core conclusions are resilient to these potential biases. Finally, the generalizability of findings may be constrained by the geographical concentration of studies (e.g., predominantly Iran). While p-values alone are insufficient to assess clinical efficacy, meaningful improvements in NAFLD management typically require:a BMI reduction of at least 5%, an ALT decrease of ≥17 U/L, a relative MRI-PDFF reduction of ≥30%, a wc reduction of at least 5 cm, and an overall weight loss of 5–10% (1, 33, 34). After converting our pooled SMDs to absolute units, only the ALT reduction (≈17.5 U/L) approximated its threshold; all other translated benefits—BMI, waist circumference, and lipid parameters fell short of these targets. Consequently, flavonoids remain clinically sub-therapeutic as monotherapy and should be employed solely as adjunctive agents, integrated with foundational diet and exercise interventions to achieve robust, guideline-level NAFLD outcomes.
No significant acute toxicity of flavonoids was observed in the studies included in this analysis. However, their long-term safety remains constrained by metabolic complexity (structural heterogeneity and variations in bioavailability) and the lack of robust methods for assessing in vivo oxidative damage (13). These limitations contribute to fragmented data on chronic exposure effects and hinder the objective quantification of metabolic endpoints.
To address these challenges, future research should prioritize: (1) Advanced analytical technologies: The development of high-resolution metabolomics and real-time oxidative stress biomarkers is imperative to elucidate flavonoid absorption, distribution, and excretion dynamics. (2) Large-scale RCTs: Large-scale multicenter randomized controlled trials are crucial to validate long-term efficacy, optimize dosing regimens, and establish safety profiles. Future trials should feature extended durations (≥18 months) and larger cohorts, specifically enrolling patients with liver fibrosis stages F2-F4. Primary endpoints must include objective measures such as histologic improvement (e.g., NASH resolution without fibrosis worsening) or imaging biomarkers (e.g., MRI-PDFF, VCTE-LSM) to generate higher-level evidence. (3) Synergistic therapies: Investigating combinatorial approaches with existing metabolic modulators (e.g., SGLT2 inhibitors or GLP-1 agonists) may enhance therapeutic outcomes in complex disease management.
5 Conclusion
Given the growing global use of phytotherapies, healthcare providers should maintain dual competency in both botanical and conventional treatments. Current evidence indicates flavonoid supplementation improves key NAFLD markers, including ALT, AST, ALP, BMI, TC, TG and FBS, Insulin, QUICKI. While flavonoids show therapeutic potential for NAFLD, critical knowledge gaps remain regarding their long-term safety and clinical efficacy. Additionally, structural heterogeneity among flavonoid subclasses, coupled with inherently poor aqueous solubility and low oral bioavailability, significantly limits their potential. Future NAFLD investigations should focus on three key areas: advanced metabolomic profiling of flavonoid pharmacokinetics, multicenter randomized controlled trials to validate clinical outcomes, and synergistic combination therapies with existing metabolic drugs.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
QL: Writing – original draft, Data curation, Formal analysis, Investigation. HL: Methodology, Data curation, Writing – original draft, Software. ZD: Investigation, Writing–original draft, and Writing–review & editing. JA: Methodology, Validation, Investigation, Writing – original draft. YW: Methodology, Supervision, Writing – review & editing, Resources. PC: Supervision, Conceptualization, Funding acquisition, Methodology, Writing – review & editing, Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1660065/full#supplementary-material
References
1.
Rinella ME Neuschwander-tetri BA Siddiqui MS Abdelmalek MF Caldwell S Barb D et al . AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. (2023) 77:1797–835. doi: 10.1097/hep.0000000000000323
2.
Lazarus J Newsome P Francque S Lazarus JV Newsome PN Francque SM et al . A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. (2023) 79:E93–4. doi: 10.1097/hep.0000000000000696
3.
Huang T Behary J Zekry A . Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern Med J. (2020) 50:1038–47. doi: 10.1111/imj.14709
4.
Cotter T Rinella M . Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. (2020) 158:1851–64. doi: 10.1053/j.gastro.2020.01.052
5.
Wang Y Huang X Ye S Li T Huang Y Cheryala M et al . Global burden of metabolic-associated fatty liver disease: a systematic analysis of global burden of disease study 2021. Chin Med J. (2025). doi: 10.1097/CM9.0000000000003517 (online ahead of print).
6.
Younossi Z Golabi P Paik J Younossi ZM Paik JM Henry A et al . The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. (2023) 77:1335–47. doi: 10.1097/hep.0000000000000004
7.
Estes C Razavi H Loomba R Younossi Z Sanyal AJ . Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. (2018) 67:123–33. doi: 10.1002/hep.29466
8.
Buzzetti E Pinzani M Tsochatzis E . The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. (2016) 65:1038–48. doi: 10.1016/j.metabol.2015.12.012
9.
Del Bo’ C Perna S Allehdan S Rafique A Saad S AlGhareeb F et al . Does the Mediterranean diet have any effect on lipid profile, central obesity and liver enzymes in non-alcoholic fatty liver disease (NAFLD) subjects? A systematic review and meta-analysis of randomized control trials. Nutrients. (2023) 15:2250. doi: 10.3390/nu15102250
10.
Hao B Yang Z Liu H Liu Y Wang S . Advances in flavonoid research: sources, biological activities, and developmental prospectives. Curr Issues Mol Biol. (2024) 46:2884–925. doi: 10.3390/cimb46040181
11.
Zamani M Kelishadi MR Ashtary-Larky D Amirani N Goudarzi K Torki IA et al . The effects of green tea supplementation 391 on cardiovascular risk factors: a systematic review and meta-analysis. Front Nutr. (2023) 9:1084455. doi: 10.3389/fnut.2022.1084455
12.
Nabavi SF Khan H D'onofrio G Šamec D Shirooie S Dehpour AR et al . Apigenin as neuroprotective agent: of mice and men. Pharmacol Res. (2018) 128:359–65. doi: 10.1016/j.phrs.2017.10.008
13.
Ranneh Y Bedir AS Abu-Elsaoud AM Al Raish S . Polyphenol intervention ameliorates non-alcoholic fatty liver disease: an updated comprehensive systematic review. Nutrients. (2024) 16:4150. doi: 10.3390/nu16234150
14.
Nan S Tongfei W Quan G et al . Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. (2022) 383:132531. doi: 10.1016/j.foodchem.2022.132531
15.
Banerjee T Sarkar A Ali S Ali SZ Bhowmik R Karmakar S et al . Bioprotective role of phytocompounds against the pathogenesis of non-alcoholic fatty liver disease to non-alcoholic steatohepatitis: unravelling underlying molecular mechanisms. Planta Med. (2024) 90:675–707. doi: 10.1055/a-2277-4805
16.
Higgins JPT Thomas J Chandler J Cumpston M Li T Page MJ et al . editors. Cochrane handbook for systematic reviews of interventions Version 6.5. London (UK): Cochrane (2024).
17.
Moher D Liberati A Tetzlaff J Altman DG The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18.
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
19.
Li H Liang J Han M Gao Z . Polyphenols synergistic drugs to ameliorate non-alcoholic fatty liver disease via signal pathway and gut microbiota: a review. J Adv Res. (2025) 68:43–62. doi: 10.1016/j.jare.2024.03.004
20.
Yang H Yang T Heng C Zhou Y Jiang Z Qian X et al . Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother Res. (2019) 33:3140–52. doi: 10.1002/ptr.6486
21.
Sun X Dey P Bruno RS Zhu J . EGCG and catechin relative to green tea extract differentially modulate the gut microbial metabolome and liver metabolome to prevent obesity in mice fed a high-fat diet. J Nutr Biochem. (2022) 109:109094. doi: 10.1016/j.jnutbio.2022.109094
22.
Anhê FF Roy D Pilon G Dudonné S Matamoros S Varin TV et al . A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. (2015) 64:872–83. doi: 10.1136/gutjnl-2014-307142
23.
Li L Ji K Du F et al . Does flavonoid supplementation alleviate non-alcoholic fatty liver disease? A systematic review and Meta-analysis of randomized controlled trials. Mol Nutr Food Res. (2023) 67:e2300480. doi: 10.1002/mnfr.202300480
24.
Yang K Chen J Zhang T Yuan X Ge A Wang S et al . Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Immunol. (2022) 13:949746. doi: 10.3389/fimmu.2022.949746
25.
Alshammari N Palma-Duran SA Jiang G González-Sarrías A Pinto P Garcia-Aloy M et al . A systematic review and meta-analysis of randomized controlled trials exploring the role of inter-individual variability on the effect of flavanols on insulin and HOMA-IR. Proc Nutr Soc. (2018) 77:E40. doi: 10.1017/S0029665118000344
26.
Xiong H Wang J Ran Q Lou G Peng C Gan Q et al . Hesperidin: a therapeutic agent for obesity. Drug Des Devel Ther. (2019) 13:3855–66. doi: 10.2147/DDDT.S227499
27.
Kim HY Park M Kim K Lee YM Rhyu MR . Hesperetin stimulates cholecystokinin secretion in enteroendocrine STC-1 cells. Biomol Ther (Seoul). (2013) 21:121–5. doi: 10.4062/biomolther.2012.077
28.
Jaiswal N Akhtar J Singh SP Ahsan F . An overview on genistein and its various formulations. Drug Res (Stuttg). (2019) 69:305–13. doi: 10.1055/a-0797-3657
29.
Yu L Rios E Castro L Liu J Yan Y Dixon D . Genistein: dual role in women's health. Nutrients. (2021) 13:3048. doi: 10.3390/nu13093048
30.
Ji G Yang Q Hao J Guo L Chen X Hu J et al . Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int Immunopharmacol. (2011) 11:762–8. doi: 10.1016/j.intimp.2011.01.036
31.
Tsuda T Ueno Y Aoki H Koda T Horio F Takahashi N et al . Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem Biophys Res Commun. (2004) 316:149–57. doi: 10.1016/j.bbrc.2004.02.031
32.
Cai X Wang S Wang H Liu S Liu G Chen H et al . Naringenin inhibits lipid accumulation by activating the AMPK pathway in vivo and vitro. Food Sci Human Wellness. (2023) 12:1174–83. doi: 10.1016/j.fshw.2022.10.043
33.
Ross R Neeland IJ Yamashita S Shai I Seidell J Magni P et al . Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. (2020) 16:177–89. doi: 10.1038/s41574-019-0310-7
34.
European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity . EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD): Executive Summary AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Diabetologia. (2024) 67:2375–92. doi: 10.1007/s00125-024-06196-3
35.
Li N Cui C Xu J Mi MT Wang J Qin Y . Quercetin intervention reduced hepatic fat deposition in patients with nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled crossover clinical trial. Am J Clin Nutr. (2024) 120:507–17. doi: 10.1016/j.ajcnut.2024.07.013
36.
Hosseinikia M Oubari F Hosseinkia R Tabeshfar Z Salehi MG Mousavian Z et al . Quercetin supplementation in non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled clinical trial. Nutr Food Sci. (2020) 50:1279–93. doi: 10.1108/NFS-10-2019-0321
37.
Pasdar Y Oubari F Zarif MN Abbasi M Pourmahmoudi A Hosseinikia M . Effects of quercetin supplementation on hematological parameters in non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Clin Nutr Res. (2020) 9:11–9. doi: 10.7762/cnr.2020.9.1.11
38.
Amanat S Eftekhari MH Fararouei M Bagheri Lankarani K Massoumi SJ . Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: a randomized, controlled trial. Clin Nutr. (2018) 37:1210–5. doi: 10.1016/j.clnu.2017.05.028
39.
Namkhah Z Naeini F Mahdi Rezayat S Mehdi Yaseri Mansouri S Javad Hosseinzadeh-Attar M . Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial. Int J Clin Pract. (2021) 75:e14852. doi: 10.1111/ijcp.14852
40.
Yari Z Cheraghpour M Alavian SM Hedayati M Eini-Zinab H Hekmatdoost A . The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: an open-labeled randomized controlled trial. Eur J Clin Nutr. (2021) 75:99–111. doi: 10.1038/s41430-020-0679-3
41.
Sangsefidi ZS Yarhosseini F Hosseinzadeh M Ranjbar A Akhondi-Meybodi M Fallahzadeh H et al . The effect of (Cornus mas L.) fruit extract on liver function among patients with nonalcoholic fatty liver: a double-blind randomized clinical trial. Phytother Res. 35:5259–68. doi: 10.1002/ptr.7199
42.
Yarhosseini F Sangouni AA Sangsefidi ZS Hosseinzadeh M Akhondi-Meybodi M Ranjbar A et al . Effect of Cornus mas L. fruit extract on blood pressure, anthropometric and body composition indices in patients with non-alcoholic fatty liver disease: a randomized controlled trial (2023) 56:18–24. doi: 10.1016/j.clnesp.2023.04.018
43.
Izadi F Farrokhzad A Tamizifar B Tarrahi MJ Entezari MH . Effect of sour tea supplementation on liver enzymes, lipid profile, blood pressure, and antioxidant status in patients with non-alcoholic fatty liver disease: a double-blind randomized controlled clinical trial. Phytotherapy Res: PTR. (2021) 35:477–85. doi: 10.1002/ptr.6826
44.
Zhang PW Chen FX Li D Ling WH Guo HH . A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine (Baltimore). (2015) 94:e758. doi: 10.1097/MD.0000000000000758
45.
Bayram HM Iliaz R Gunes FE . Effects of Cornus mas L. on anthropometric and biochemical parameters among metabolic associated fatty liver disease patients: randomized clinical trial. J Ethnopharmacol. (2024) 318:117068. doi: 10.1016/j.jep.2023.117068
46.
Ghanbari P Raiesi D Alboebadi R Zarejavid A Dianati M Razmi H et al . The effects of grape seed extract supplementation on cardiovascular risk factors, liver enzymes and hepatic steatosis in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study. BMC Complement Med Ther. (2024) 24:192. doi: 10.1186/s12906-024-04477-3
47.
Li BY Xi Y Liu YP Wang D Wang C Chen CG et al . Effects of Silybum marianum, Pueraria lobate, combined with Salvia miltiorrhiza tablets on non-alcoholic fatty liver disease in adults: a triple-blind, randomized, placebo-controlled clinical trial. Clin Nutr ESPEN. (2024) 63:2–12. doi: 10.1016/j.clnesp.2024.06.003
48.
Neshatbini Tehrani A Hatami B Helli B Yari Z Daftari G Salehpour A et al . The effect of soy isoflavones on non-alcoholic fatty liver disease and the level of fibroblast growth factor-21 and fetuin a. Sci Rep. (2024) 14:5134. doi: 10.1038/s41598-024-55747-6
49.
Pezeshki A Safi S Feizi A et al . The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int J Prev Med. (2015). doi: 10.4103/2008-7802.173051
50.
Hashemi SJ Eskandar H Sardabi EH . A placebo-controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat Mon. (2009) 9:265–70. doi: 10.1136/gut.2008.174516corr1
51.
Solhi H Ghahremani R Kazemifar AM Hoseini Yazdi Z . Silymarin in treatment of non-alcoholic steatohepatitis: a randomized clinical trial. Casp J Intern Med. (2014) 5:9–15. doi: 10.22088/cjim.5.1.9
52.
Anushiravani A Haddadi N Pourfarmanbar M Mohammadkarimi V . Treatment options for nonalcoholic fatty liver disease: a double-blinded randomized placebo-controlled trial. Eur J Gastroenterol Hepatol. (2019) 31:613–7. doi: 10.1097/MEG.0000000000001369
53.
Aller R Izaola O Gómez S Tafur C González G Berroa E et al . Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci. (2015) 19:3118–24. PMID:
54.
Memon IA Akbar M Bhurgri AN . Effect of silymarin therapy on liver aminotransferase in non-alcoholic fatty liver disease. Med Forum. (2015) 26:46–9.
55.
Rangboo V Noroozi M Zavoshy R et al . The effect of artichoke leaf extract on alanine aminotransferase and aspartate aminotransferase in the patients with nonalcoholic steatohepatitis. Int J Hepatol. (2016) 2016:4030476. doi: 10.1155/2016/4030476
56.
Shaikh KR Shaikh S Ata MA et al . Therapeutic efficacy of silymarin on liver aminotransferases in patients with nonalcoholic fatty liver disease. Rawal Med J. (2021) 46:761.
57.
Mirhashemi SH Hakakzadeh A Yeganeh FE Oshidari B Rezaee SP . Effect of 8 weeks milk thistle powder (silymarin extract) supplementation on fatty liver disease in patients candidates for bariatric surgery. Metabol Open. (2022) 14:100190. doi: 10.1016/j.metop.2022.100190
58.
Atarodi H Pazouki A Gholizadeh B Karami R Kabir A Sadri G et al . Effect of silymarin on liver size and nonalcoholic fatty liver disease in morbidly obese patients: a randomized double-blind clinical trial. J Res Med Sci. (2022) 27:76. doi: 10.4103/jrms.jrms_683_21
59.
Jaffar HM Bader Ul Ain H Tufail T et al . Impact of silymarin-supplemented cookies on liver enzyme and inflammatory markers in non-alcoholic fatty liver disease patients. Food Sci Nutr. (2024) 12:7273–86. doi: 10.1002/fsn3.4348
Summary
Keywords
non-alcoholic fatty liver disease (NAFLD), flavonoids, liver enzymes, insulin resistance, meta-analysis
Citation
Liu Q, Luan H, Duan Z, Ai J, Wang Y and Chen P (2025) Efficacy of flavonoids in non-alcoholic fatty liver disease: an updated systematic review and meta-analysis. Front. Nutr. 12:1660065. doi: 10.3389/fnut.2025.1660065
Received
05 July 2025
Accepted
28 August 2025
Published
15 September 2025
Volume
12 - 2025
Edited by
Dina Keumala Sari, Universitas Sumatera Utara, Indonesia
Reviewed by
Seham Alraish, United Arab Emirates University, United Arab Emirates
Hongcai Li, Northwest A&F University, China
Updates
Copyright
© 2025 Liu, Luan, Duan, Ai, Wang and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Chen, cptxzz@sina.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.