- 1Faculty of Health Sciences, Department of Nutrition and Dietetics, Mehmet Akif Ersoy University, Burdur, Türkiye
- 2Faculty of Health Sciences, Department of Nutrition and Dietetics, Çankırı Karatekin University, Çankırı, Türkiye
Background: Chronic fatigue is a persistent state of physical, emotional, and cognitive exhaustion that does not resolve with rest. Behavioral and environmental factors may contribute to the onset and course of chronic fatigue. In this context, we aimed to examine the predictive roles of obesogenic environment and hedonic appetite in chronic fatigue.
Method: We conducted the study on 505 Turkish adults aged 18–65. Participants completed a questionnaire form containing questions about demographic characteristics, dietary habits, the Chalder Fatigue Scale (CFS), the Power of Food Scale (PFS), and the Assessment of Obesogenic Environment Assessment Scale (AOES).
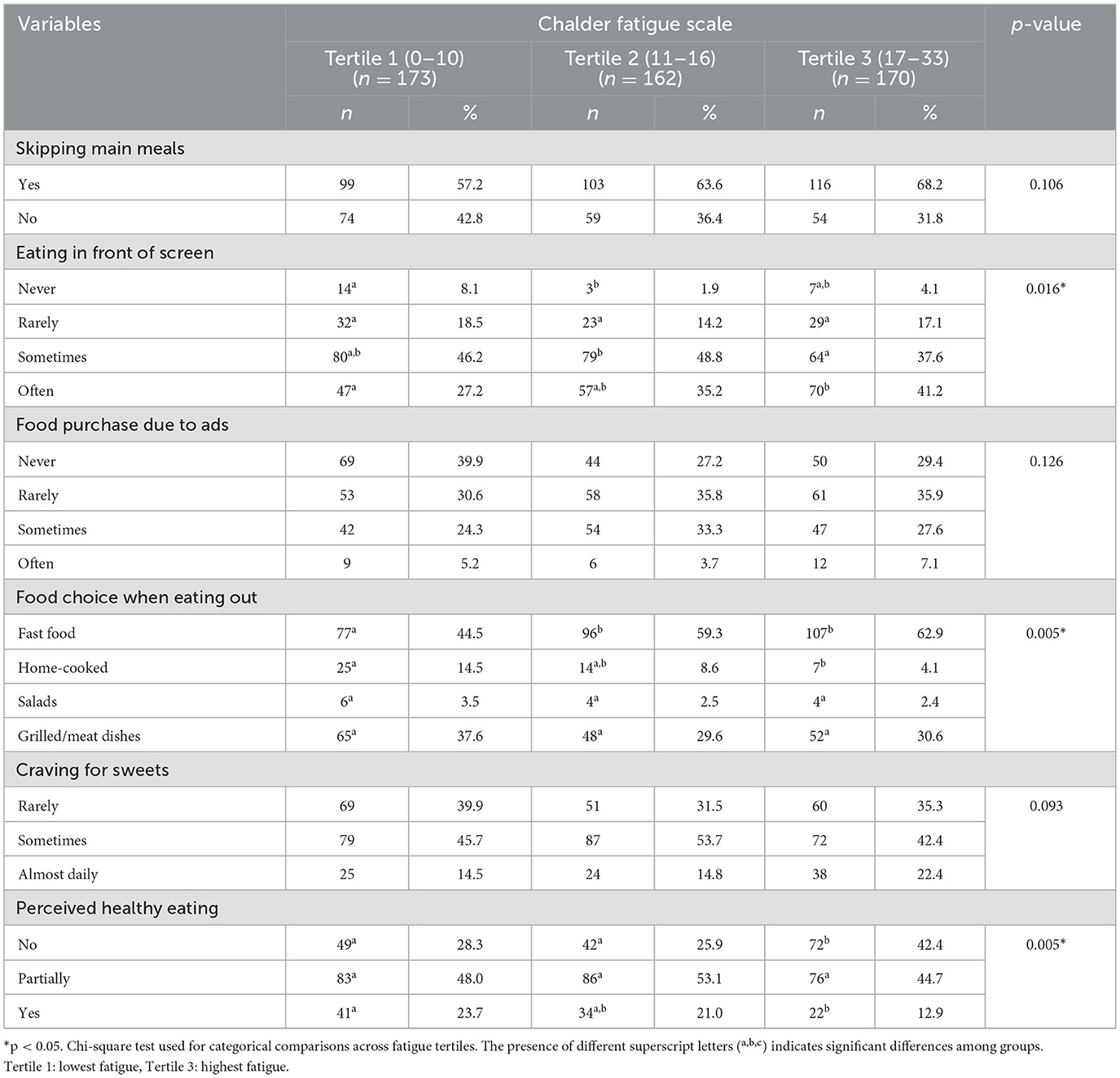
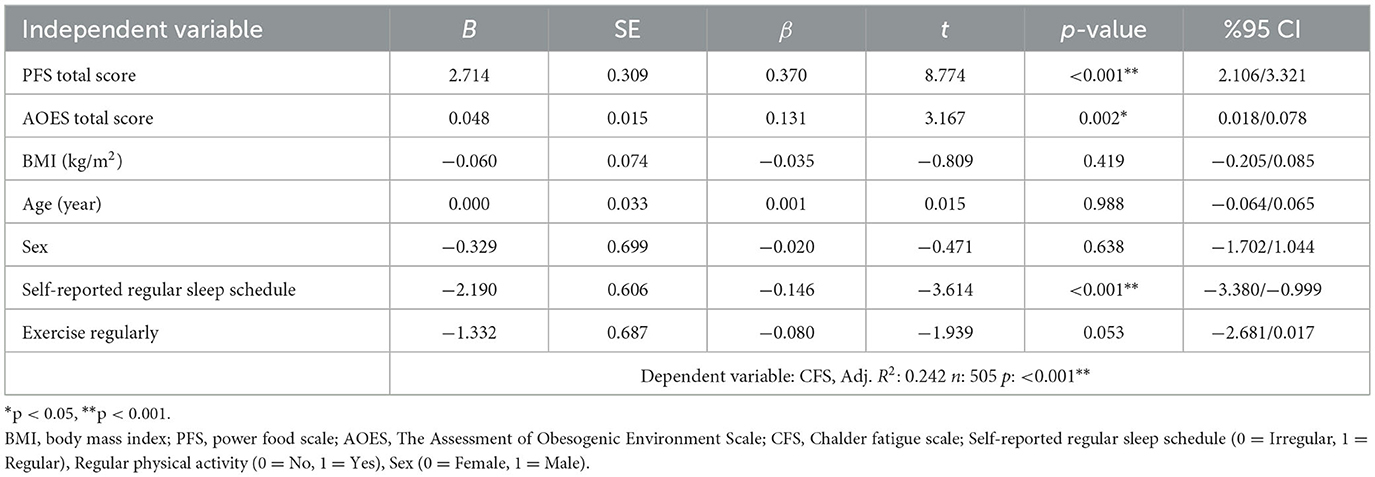
Results: The proportion of women is higher in the third tertile (highest fatigue) than in the first tertile (p = 0.014). The proportion of those who exercise regularly in the third tertile is lower compared to the first and second tertiles (p = 0.001). The percentage of participants who are sedentary for more than 4 h per day is lower in the first tertile than in the second and third tertiles (p = 0.007). The proportion of individuals who often eat in front of a screen is higher in the third tertile (41.2%) than in the first tertile (27.2%; p = 0.016). The proportion of individuals in the first tertile of fatigue level who prefer fast food when eating out is 44.5%, which is lower than that of the second and third tertiles (p < 0.05). As hedonic appetite (β = 0.370, p < 0.001) and exposure to obesogenic environment (β = 0.131, p = 0.002) levels increase, fatigue symptoms also increase. It was found that individuals with high levels of chronic fatigue had a more obesogenic environment in terms of physical, social, and economic aspects; however, they were environmentally advantaged in terms of cultural factors and access to experts (p < 0.05). Participants who self-report having a regular sleep schedule exhibit significantly lower levels of fatigue (β = −0.146, p < 0.001).
Conclusion: Our findings show that obesogenic environments, irregular sleep schedules, and hedonic appetite are associated with fatigue perception and may serve as its predictors. Strategies to improve physical, social, and economic aspects of obesogenic environments may help prevent chronic fatigue, while addressing hedonic appetite through psychotherapy could also be beneficial. Effective prevention and management of chronic fatigue can strengthen long-term societal resilience and support overall wellbeing.
Introduction
Obesogenic environments refer to aspects of the social environment that contribute to the prevalence of obesity by influencing dietary habits and physical activity levels. Obesogenic factors include the density of fast-food restaurants, the availability of processed food products, food advertising, and social norms (1). Additionally, endocrine-disrupting chemicals are among the factors that contribute to an obesogenic environment. Among these chemicals known as obesogens are phthalates and bisphenol A, which are found in food packaging and plastic containers, as well as pesticides used in agriculture. Obesogens can alter insulin sensitivity and disrupt metabolism and endocrine regulation, resulting in an increase in both the number and size of adipocytes (2). Environmental aspects have strong effects on health and disease development over time, as they contribute to a dietary intake high in energy, fat, and sugar and to metabolic processes that increase body adiposity. Furthermore, the obesogenic environment may exacerbate chronic inflammation, which is important in the pathophysiology of obesity-related diseases (3).
The concept of the exposome has emerged in order to holistically understand the environmental factors that contribute to obesity and other health problems (4). This concept encompasses both external and internal factors, including climate change, air pollution, the urban environment, green spaces, the home environment, lifestyle, pesticides, medications, access to food, economic status, the microbiome, and inflammation (5). The evaluation of the exposome, which refers to the lifelong effects of various risk factors on human health, is very challenging and is therefore generally investigated at specific time points. Achieving a complete assessment of the exposome is currently unattainable, yet even partial evaluations still yield valuable information (6). In this study, the evaluation of particular obesogenic environmental factors among community-dwelling adults is expected to provide meaningful contributions to the field of exposome research.
Environmental conditions can significantly direct individuals' eating behaviors and lead to hedonic nutrition. Hedonic eating refers to the increase in the urge to eat and the loss of control over eating due to the sensory properties of food and social experiences, despite the absence of physical hunger (7). It stimulates the brain's reward system, leading to the frequent and excessive consumption of tasty foods (8). The obesogenic environment can promote hedonic consumption because individuals have easier access to unhealthy food options (9, 10). This non-homeostatic eating pattern leads to an increase in body weight and changes in the neural systems that control behavior. Over time, one becomes accustomed to energy-dense foods, and the cycle that leads to the development of obesity continues (11).
The effects of factors such as the obesogenic environment and hedonic appetite are not limited to metabolic outcomes but can also shape an individual's overall perception of fatigue. Fatigue is defined as a decrease in performance as a result of physical, emotional, and mental exertion. It manifests after periods of activity with physiological changes and improves with rest. However, sometimes, independent of effort, a lack of motivation, a decrease in physical and cognitive capacity can be observed, and quality of life is negatively affected (12). This type of fatigue is chronic. Fatigue symptoms that do not improve with rest and develop without a clear cause, accompanied by memory and concentration deficiencies, are referred to as “chronic fatigue” (13). It has been reported that individuals with chronic fatigue adopt a healthy lifestyle less frequently (14). Unhealthy lifestyle and obesity have also been associated with fatigue and reduced functionality (15). Evidence shows that the interaction between lifestyle and fatigue is bidirectional. The etiology of fatigue perception that does not improve with rest is complex. It can be caused by the interaction of multiple factors, including genetics, the immune system, psychological conditions, adrenal systems, sleep, and nutrition (16). Eating a healthy diet, having a healthy environment, and regulating environmental factors are possible. The aim of this study is to examine the association of modifiable risk factors, particularly the obesogenic environment and hedonic food intake, with symptoms of chronic fatigue in community-dwelling adults.
Research questions
1. Do adults' fatigue levels differ significantly according to their demographic characteristics and lifestyle habits?
2. Do adults' fatigue levels differ significantly according to their dietary habits?
3. Is there a significant relationship between adults' exposure to obesogenic factors, hedonic appetite, and fatigue levels?
4. What are the variables that predict adults' fatigue levels?
Method
The study is cross-sectional and was conducted with 505 adults living in Turkey between January and May 2025. According to a sensitivity analysis conducted using G*Power software (N = 505, α = 0.05, 1-β = 0.80, m = 7), the minimum detectable effect size in the multiple linear regression model was found to be f2 ≈ 0.029 (R2 ≈ 0.029). This result indicates that the sample size is sufficient to detect small but meaningful effects.
Those who volunteered to participate, had internet access, were literate enough to understand the survey, and were between the ages of 18 and 65 were included. Those who are pregnant, breastfeeding, have metabolic disorders, or have been diagnosed with other medical conditions that could confound the results were not included in the study. The online survey prepared in Turkish was distributed through social media platforms using the snowball sampling method. In the first section of the questionnaire, information about the study was provided, and individuals who checked the consent box voluntarily participated in the study. The survey includes sections on participants' demographic characteristics and dietary habits, as well as the Chalder Fatigue Scale, the Nutritional Power Scale, and the Obesogenic Environment Assessment Scale for Adults.
Chalder Fatigue Scale (CFS)
Initially developed as a 14-item scale to assess the severity of fatigue, the scale was later revised to an 11-item version (17, 18). The Turkish validity and reliability study was conducted by Adin et al. in 2022 (19). The scale includes two sub-dimensions: physical fatigue (CFS-PF) and mental fatigue (CFS-MF). The physical fatigue subdimension assesses physical symptoms such as lack of energy, muscle weakness, malaise, fatigue, somnolence, and difficulty initiating daily activities (items 1–7). In contrast, the mental fatigue subdimension evaluates cognitive symptoms, including difficulties with attention and concentration, slips of the tongue during speech, trouble finding the appropriate word, and memory problems (items 8–11). The total score ranges from 0 to 33. Low scores reflect a lower level of fatigue. Cronbach's α values were reported as 0.862 for CFS-PF, 0.704 for CFS-MF, and 0.863 for the total CFS score, respectively (19).
The Power of Food Scale (PFS)
The scale was developed by Lowe et al. as a 15-item measure to assess hedonic hunger (20). The Turkish validity and reliability study was conducted by Ulker et al. in 2021 (21). The Turkish version of the scale consists of 13 items on a 5-point Likert type and is divided into three sub-dimensions (food available, food present, and food taste). Individuals' enjoyment of eating, their tendency to seek food at unexpected times, and the intensity of their thoughts about food are measured through the food available subdimension. The food present subdimension encompasses the desire to eat triggered by seeing or smelling food, difficulty in refraining from consuming favorite high-calorie foods, constantly contemplating delicious foods, and the inclination to consume certain foods even if they are harmful. The food taste subdimension includes the excitement and anticipation experienced before tasting a preferred food, a strong focus on flavor while eating, interest in foods recommended or described by others, and the desire for foods to be maximally tasty and enjoyable. Items are rated from 1 to 5 (strongly disagree: 1, disagree: 2, neutral: 3, agree: 4, strongly agree: 5). Three sub-dimensions and the total scale score are calculated by summing the item scores and dividing by the number of items. Both subscale scores and the total scale score range from 1 to 5. An increase in the score indicates a higher tendency toward hedonic hunger. The Cronbach's alpha for the PFS scale was calculated as 0.922; the corresponding values for the “food available,” “food present,” and “food taste” subscales were reported as 0.849, 0.797, and 0.82 (21).
The Assessment of Obesogenic Environment Scale (AOES)
The scale aims to evaluate the environment that leads to the development of obesity in individuals. Developed by Kesik and Saglam (22), a validity and reliability study was conducted in Turkish. It is a 7-point Likert type and contains 32 items. It consists of four sub-dimensions: factors and opportunities related to the physical environment, cultural determinants and access to experts, social determinants and their effects, and economic determinants and their effects. The sub-dimension regarding factors and opportunities related to the physical environment consists of 10 items, with the 7th and 8th items being reverse scored. They examine access to healthy foods, meal services, gyms, safe walking areas, and the influence of long work or study hours. The sub-dimension of cultural determinants and access to experts evaluates the social, cultural, and economic factors that influence individuals' healthy eating and physical activity behaviors. This sub-dimension consists of a total of 10 items, with only the 7th item being scored directly, while all other items are reverse scored. It covers the presence of active individuals in one's social circle, cultural attitudes, media and popular figures influence, access to professionals, affordability and availability of healthy foods and equipment, and exposure to unhealthy food advertisements. The sub-dimension of social determinants and their effects evaluates individuals' healthy eating and physical activity behaviors in the context of their social environment, family, peer groups, societal perceptions, and media influence. This sub-dimension consists of a total of eight items, with only the 6th item being reverse scored. The items cover factors such as body image perceptions shaped by social media, the view that healthy eating is boring, attitudes of family and friends toward physical activity, the influence of societal discourse, eating habits at home, the effect of internet use on physical activity, and the food options provided in educational or work environments. The economic determinants and effects sub-dimension consists of four items, and none of the items are reverse-scored. This sub-dimension covers factors such as the affordability of healthy foods and sports equipment, access to paid physical activity opportunities, and the impact of financial constraints on healthy lifestyle choices. It is scored between 32 and 224. Low scores indicate healthy factors, while high scores indicate obesogenic factors. In the validation and reliability study of the scale, the test-retest Cronbach's alpha value was reported as 0.815, and the intraclass correlation coefficient was 0.687. The Cronbach's alpha for internal consistency was 0.761. These results demonstrate that the scale has good reliability, moderate test-retest consistency, and very good internal consistency (22).
Statistical analysis
The IBM SPSS (Statistical Package for the Social Sciences) 25.0 package program was used to evaluate the data. The normality of the data distribution was assessed by examining skewness and kurtosis coefficients, as well as by performing the Shapiro–Wilk test. Parametric tests were used in the analyses because the variables met the normality assumption. Mean, standard deviation, number, and percentage values were used for descriptive statistics. Because the Chalder Fatigue Scale lacks a cutoff point for the total score, participants were divided into tertiles based on their scores. This method, commonly used in scales without a standard cutoff point, was chosen to compare participants with low, moderate, and high fatigue levels. General characteristics, scale scores, and dietary habits were analyzed according to these tertiles using the chi-square test and one-way ANOVA. Different superscript letters indicate statistically significant differences between tertiles based on the Bonferroni post-hoc test (p < 0.05). Tertiles sharing at least one common letter do not differ significantly from each other. The associations between scale scores, age, and body mass index were assessed using the Pearson correlation analysis. Multiple linear regression analysis was used to explore the predictors of fatigue scores. Significance was evaluated at the p < 0.05 level.
Results
Table 1 presents the distribution of scale scores and general characteristics of the participants according to tertiles of fatigue level. As fatigue tertiles increased, the total PFS score and its sub-dimensions—food present and food taste—showed a statistically significant progressive increase, with each tertile differing significantly from the others (p < 0.001 for all, Bonferroni post-hoc). For food availability, post-hoc comparisons indicated that tertile 1 scored significantly lower than both tertile 2 and tertile 3, while no significant difference was observed between Tertiles 2 and 3. The total AOES score in the third tertile according to fatigue level is higher than in the first and second tertiles (p < 0.001). Post-hoc comparisons indicated that, for AOES-P and AOES-E, tertile 1 scored significantly lower than both tertile 2 and tertile 3, while no significant difference was observed between tertiles 2 and 3. For AOES-S, tertile 3 had significantly higher scores than tertile 1 and 2. Participants in the first tertile of the fatigue scale had significantly higher AOES-C scores than those in the second and third tertiles (p < 0.001). Regarding age, post-hoc analysis revealed that participants in Tertile 1 were significantly older than those in tertile 3, while tertile 2 did not differ significantly from either group (p = 0.035). When examining the sex distribution according to the level of fatigue, the proportion of women in the third tertile is higher than in the first tertile (p = 0.014). The proportion of those who exercise regularly in the third tertile is lower compared to the first and second tertiles (p = 0.001). In the first tertile, the proportion of people who are sedentary for more than 4 h per day is lower than in the second and third tertiles (p = 0.007). In the third tertile, the percentage of people who self-reported having a regular sleep schedule is lower than in the first and second tertiles (p < 0.001). In other variables (smoking, alcohol consumption, age, BMI), no statistically significant difference was found according to the level of fatigue (p > 0.05).
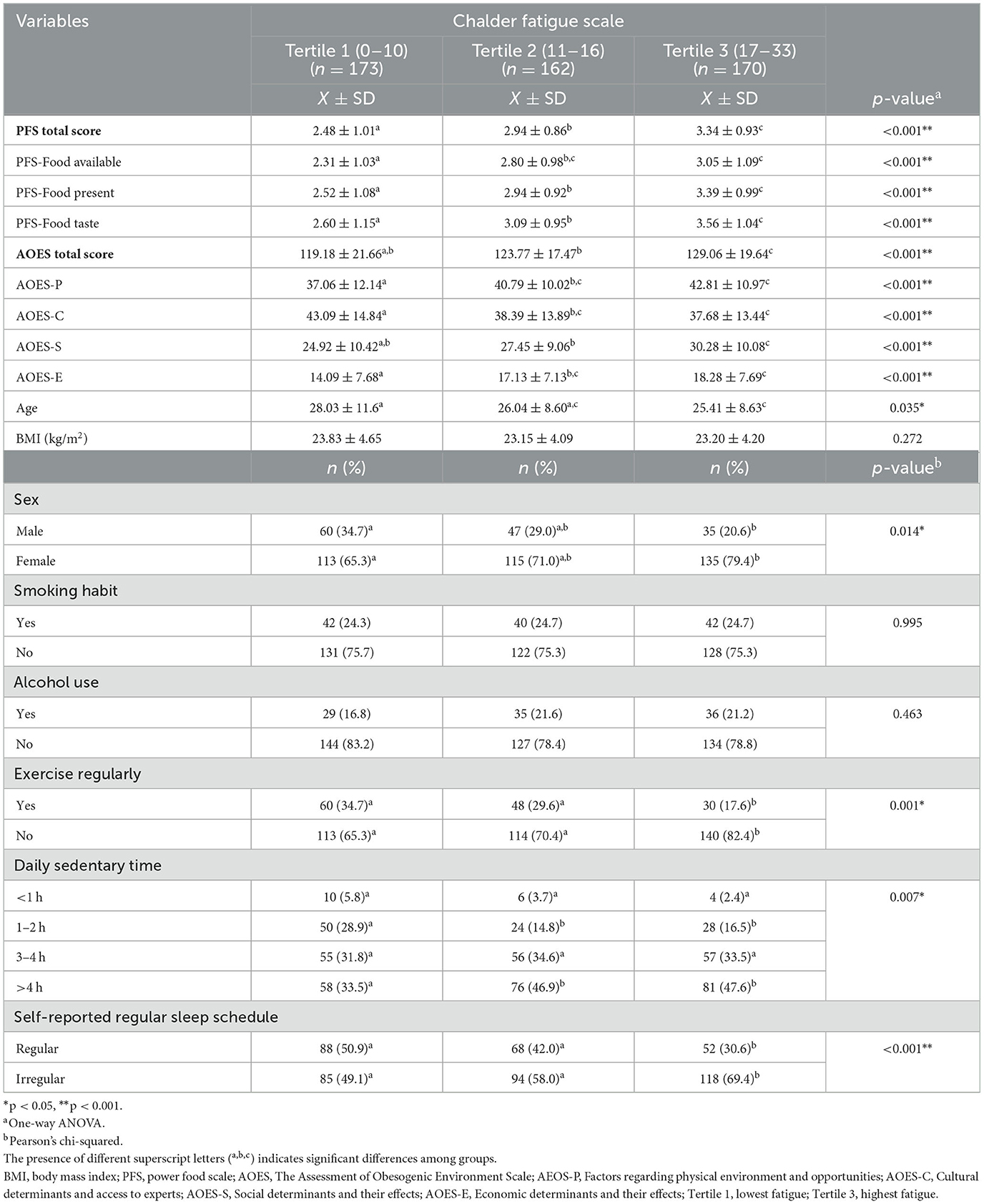
Table 1. Distribution of scale scores and general characteristics according to individuals' fatigue level tertiles.
Table 2 shows the distribution of participants' eating habits according to their levels of fatigue. The proportion of individuals who often eat in front of a screen is higher in the third tertile (41.2%) than in the first tertile (27.2%; p = 0.016). The proportion of individuals in the first tertile of fatigue level who prefer fast food when eating out is 44.5%, which is lower than that of the second and third tertiles (p < 0.05). When eating out, the proportion of those who prefer home-cooked style meals is higher in the first tertile compared to the third tertile (p < 0.05). The proportion of individuals who consider themselves to have a healthy diet is higher in the first tertile (23.7%) than in the third tertile (12.9%; p = 0.005). Skipping main meals, purchasing food based on advertisements, and the frequency of craving sweets did not show a statistically significant difference according to the level of fatigue (p > 0.05).
Figure 1 summarizes the relationships among individuals' scale scores, age, and BMI. Accordingly, the food power scale is positively correlated with mental fatigue (r = 0.333, p < 0.001) and physical fatigue (r = 0.401, p < 0.001) scores. Additionally, the food power scale scores increase as AOES scores increase (r = 0.291, p < 0.001). AOES scores are significantly positively correlated with both physical fatigue scores (r = 0.282) and mental fatigue scores (r = 0.219; p < 0.001). A significant negative correlation was found between age and the total PFS score (r = −0.175, p < 0.001). In addition, a negative correlation was observed between age and mental fatigue score (r = −0.124, p = 0.005), as well as between age and physical fatigue score (r = −0.096, p = 0.032). A positive correlation was found between age and BMI (r = 0.346, p < 0.001).
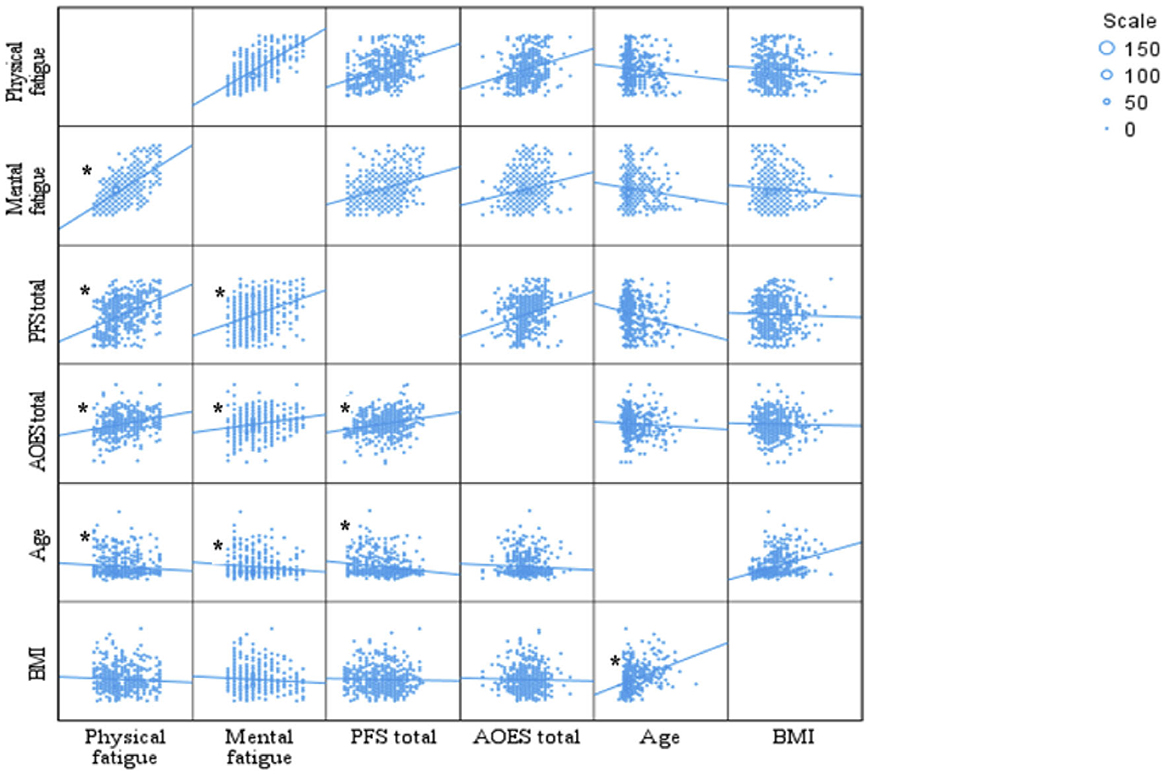
Figure 1. Correlations between scales, age, and BMI. *p < 0.05. Pearson Correlation test. PFS, power food scale; AOES, The Assessment of Obesogenic Environment Scale; BMI, body mass index.
In Table 3, the results of the multiple linear regression analysis conducted with the fatigue level (CFS total score) as the dependent variable are presented. The model is statistically significant (Adj. R2 = 0.242, p < 0.001) and explains 24.2% of the total variance. The total PFS score and the total AOES score are significantly and positively correlated with the CFS score. In other words, as the level of hedonic food craving (β = 0.370, p < 0.001) and exposure to an obesogenic environment (β = 0.131, p = 0.002) increase, the symptoms of fatigue also increase. In contrast, self-reporting having a regular sleep schedule significantly reduces the CFS score (β = −0.146, p < 0.001). Variables such as age, BMI, sex, and regular exercise were not found to be significant predictors of fatigue levels (p > 0.05).
Discussion
Among those with high levels of chronic fatigue, the proportion of women, individuals who do not exercise regularly, those who spend more than 4 h a day in sedentary activities, and those with irregular sleep schedules is higher. The literature has reported that chronic fatigue is predominantly associated with women and has attributed this to hormonal, cultural, and socio-economic differences (13, 23). Additionally, previous studies have reported that, in line with our findings, individuals with chronic fatigue have reduced participation in physical activity and an increase in sedentary behaviors and complaints of insomnia (24, 25). This situation is explained by changes in autonomic activity, alterations in hormone profiles, and the individual's behavioral and psychological responses (26, 27).
In our study, individuals with chronic fatigue appeared to be disadvantaged in terms of physical, social, and economic factors that support regular physical activity and healthy nutrition, while they seemed to be advantaged in terms of cultural factors and access to specialists. This can be interpreted as benefiting individuals with chronic fatigue from Turkish cultural traditions of nutrition and physical activity and from experts, but with a continued lack of social support and environmental disadvantages. Turkey has a rich culinary culture and a tradition of preparing meals at home, both of which support healthy eating (28). However, in Turkey, the culture of physical activity is not as prominent as the culinary culture. Although opportunities for physical activity have increased, an active lifestyle has not yet become a widespread social habit. Individuals experiencing chronic fatigue may be inclined to view culturally accepted healthy eating and physical activity as potential solutions, and they may seek expert advice to support these choices. In a previous study, it was stated that awareness of chronic fatigue may shape individuals' help-seeking behaviors and lifestyle attitudes (29). In another study, it was reported that individuals experiencing chronic fatigue are more likely to adopt a healthy lifestyle compared to healthy individuals (30).
We assessed the relationship of physical environment, social, and economic factors with fatigue holistically using a single scale in our study; in contrast, the literature generally analyzes these relationships separately. One study concluded that pocket parks alleviate mental fatigue among young adults, and that considering environmental features such as plant diversity, vegetation density, and the comfort of resting spaces in the planning and design process is important for enhancing these restorative effects (31). Other studies have found that both screen time and long working hours are associated with mental fatigue (32, 33). Another study, having a low household income was associated with a 53% higher risk of developing chronic fatigue (34). A systematic review has indicated that individuals with chronic fatigue can have multiple physical and psychological needs and often seek support from family, friends, and professionals to cope with the limitations in their daily lives (35). Nonetheless, it should be emphasized that the scale employed to assess obesogenic factors in our study does not encompass environmental pollutants that could influence chronic fatigue. It has been reported that environmental pollutants, known as xenobiotics (e.g., pesticides, industrial chemicals, heavy metals), may contribute to the development of chronic fatigue later in life by impairing immune system function, particularly during early life (36). Other studies have reported that environmental factors such as air, water, noise, and light pollution can also harm cognitive and psychological health (37, 38). Environmental pollution and pollutants are recognized as part of the obesogenic environment. Therefore, it is recommended to develop comprehensive scales in the future to jointly assess environmental pollutants and other obesogenic factors.
Nutrition directly affects the balance of the gut microbiota, the level of oxidative stress, and mitochondrial functions. Disruptions in these physiological processes can lead to chronic inflammation in the body and impairments in energy metabolism, contributing to the development of chronic fatigue syndrome (39). In this study, among individuals with high levels of fatigue, the proportion of those consuming fast food and frequently eating in front of screens increased, while the proportion of those who believed they were eating healthily decreased. Previous studies have also reported that the proportion of individuals with chronic fatigue syndrome who consume unhealthy foods is high and that improving diet quality can alleviate symptoms (30, 40).
In this study, the variables predicting chronic fatigue were examined. Accordingly, age, sex, BMI, and regular exercise did not play a significant predictive role. However, previous studies have shown significant relationships. Female sex, increased body mass index, and inactivity have been reported as risk factors for chronic fatigue (41, 42). Although inactivity is recognized as a risk factor in the literature, several studies have reported that the response to exercise differs in patients with chronic fatigue and that exercising can worsen symptoms (43, 44). While the age variable is not associated with chronic fatigue in line with our findings, it has been reported that increasing age is strongly associated with an increased risk of severe fatigue (45). Despite the significant differences in sex and regular exercise participation according to fatigue levels observed in the descriptive analyses, these variables did not emerge as predictors of chronic fatigue in the regression model. Even though key variables were incorporated into the regression model, the lack of a significant association might be attributed to complex interrelations among these variables or the exclusion of certain influential factors. In this study, variables such as educational level, socioeconomic status, and area of residence, which are likely to be associated with chronic fatigue and may act as confounding factors, were not included in the survey. It is recommended that future studies collect and incorporate these variables into the model. Our finding that sleep schedule is a determinant of chronic fatigue is supported by the literature (46–48). It has been reported that sleep problems may arise in individuals with chronic fatigue due to changes in cytokine profiles (46). In another study, it was determined that individuals with chronic fatigue are more sensitive to sleep delays and disruptions in sleep quality associated with low physical activity levels (47). While sleep disorders manifest as a symptom in individuals with chronic fatigue, they are also considered a contributing factor to the persistence of the condition. It has been stated that the deterioration of sleep quality can lead to a cycle that perpetuates chronic fatigue due to the interactions of biological, psychological, and social factors (48).
Significant correlations were found among all the scales used in the current study. Accordingly, the obesogenic environment, hedonic eating, mental fatigue, and physical fatigue are interconnected. Additionally, the obesogenic environment and hedonic eating are among the predictors of chronic fatigue. Although the findings do not directly establish causality, the existing literature provides biological mechanisms that could support these relationships. It has been reported that the obesogenic environment leads to low-grade chronic inflammation (3, 49). It has been suggested that chronic inflammation can increase the feeling of fatigue, along with cellular and structural changes in the central nervous system (50). Another study mentioned that prolonged hedonic eating behavior could alter the homeostatic system and lead to metabolic issues (10). Metabolic dysfunctions are seen as a possible mediator of chronic fatigue symptoms (51). Currently, food intake is more strongly influenced by the rewarding qualities of food than by actual hunger. Sensory cues related to food, including texture, smell, appearance, and taste, are intensively processed by the central nervous system, influencing the brain areas involved in food reward (52). The literature suggests that chronic fatigue syndrome and hedonic appetite share certain mechanisms, particularly those involving dysfunction in the brain's reward system. One study found reduced activity in the basal ganglia among individuals with chronic fatigue syndrome, which was associated with their fatigue (53). The basal ganglia are a group of structures deep in the forebrain that play a key role in reward mechanisms. Different nuclei within the basal ganglia, such as the nucleus accumbens, caudate, putamen, and globus pallidus, are involved in different types of reward. Food rewards have been reported to be processed more prominently in the basal ganglia of the right hemisphere (54). Another study found that the extent of changes in the right putamen, pallidus, and caudate was linked to levels of fatigue and motivation (55). The shared mechanisms between food reward and chronic fatigue pave the way for similar treatment approaches. Indeed, neuromodulation methods such as transcranial magnetic stimulation have been reported to be effective in both of these conditions (56, 57).
Strengths and limitations
In this study, which focuses on a topic that has been little researched in the literature, the association between chronic fatigue and both hedonic appetite and obesogenic environments was examined. The evaluation of environmental and behavioral factors as predictors of chronic fatigue enhances the originality and scientific value of the study. However, it has a few limitations. First, the design of the research prevents the establishment of cause-and-effect relationships between the variables. For causal inferences, longitudinal or experimental designs should be preferred in future research. Secondly, a wide audience has been reached through the online survey method, but there are risks to the accuracy of the responses. There may be misunderstandings of the questions and random answering in the responses. Risks have been attempted to be mitigated with closed-ended questions containing clear and simple expressions and anonymity. Thirdly, the snowball sampling method may have led to homogeneity in the sample. Given that those who are more active on social media or more closely linked to the initial participants had a higher chance of being included in the sample, it is possible that the sample is relatively homogeneous in terms of demographic, socioeconomic, or behavioral features. Thus, the results may not be entirely generalizable to the whole adult population in Turkey. Future studies employing probability-based sampling methods are required to increase the generalizability of the results. Fourthy, the lack of data on participants' residential status and educational background can be considered a limitation of the study, as these factors may be related to obesogenic environmental risk. Another limitation of the study is that the data is based on the participants' self-reports. Due to recall bias, participants may have reported their heights and weights inaccurately or incompletely. Additionally, due to social desirability bias, they may have tended to reflect their lack of control over behaviors, environmental challenges, and levels of fatigue more positively. Future studies can improve the interpretation of findings by supporting self-report scales with objective measurements. Clinical interviews and laboratory biomarkers can be used to identify symptoms of chronic fatigue. In addition, Global Positioning System (GPS)-based digital tracking methods may help assess hedonic appetite, the obesogenic environment, and the interaction between them.
Conclusion
According to the results of this study, among Turkish adults with a high perception of fatigue, there is a higher proportion of women, those who do not exercise regularly, those who spend more than 4 h a day in sedentary activities, and those with irregular sleep hours. Additionally, among individuals with a high level of fatigue, the proportion of those consuming fast food and frequently eating in front of a screen has increased, while the proportion of those who believe they eat healthily has decreased. Obesogenic environment, self-reported sleep schedule, and hedonic appetite are determinants of fatigue perception in adults. Additionally, the findings indicate that individuals with chronic fatigue face physical, social, and economic disadvantages in maintaining healthy eating and regular physical activity, and that environmental improvements are needed.
Our results reflect the need for interventions at both the individual and policy levels in the fight against chronic fatigue. At the individual level, to control the obesogenic environment, healthy foods should be kept at home, a sedentary lifestyle should be avoided, and the use of technology and time spent in front of screens should be limited. To manage hedonic appetite, a distinction should be made between emotional hunger and physiological hunger. Psychotherapy support can be sought to effectively combat emotional triggers that increase food intake. Another key factor of chronic fatigue, sleep schedule, can also be improved with various strategies that can be applied at the individual level. Sticking to regular sleep hours, limiting screen use before bed, and ensuring the room is quiet and dark can be effective in reducing levels of fatigue.
At the political level, exposure to an obesogenic environment can be reduced through urban planning. Active living can be supported by increasing green spaces, bike paths, walking, and recreation areas. Additionally, the proliferation of fast-food chains can be controlled in urban planning processes. During nighttime rest hours, noise control and appropriate lighting practices should be observed in cities. Policymakers should be aware of their critical roles in urban planning while addressing sleep schedule and the obesogenic environment in the fight against chronic fatigue. Additionally, in addition to improving physical conditions, initiatives should be undertaken to strengthen social interaction and support networks.
Chronic fatigue is a multifaceted public health issue. Preventing and managing it can positively impact social wellbeing in a multidimensional way by strengthening societal resilience in the long term, beyond just improving individuals' overall health. Since there may be many unidentified predictors of chronic fatigue, this area is open to further scientific research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical permission were obtained from the Çankırı Karatekin University Health Sciences Ethics Committee to conduct the research (meeting number: 16/23.10.2024). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GH: Methodology, Conceptualization, Supervision, Writing – original draft, Investigation, Writing – review & editing, Visualization, Resources, Data curation, Validation. FT: Formal analysis, Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Corsica JA, Hood MM. Eating disorders in an obesogenic environment. J Am Diet Assoc. (2011) 111:996–1000. doi: 10.1016/j.jada.2011.04.011
2. Mahapatra A, Gupta P, Suman A, Singh RK. Environmental obesogens and human health. In:Heshmati HM, , editor. Hot Topics in Endocrinology and Metabolism. London: IntechOpen (2021). doi: 10.5772/intechopen.96730
3. Martínez-Esquivel A, Trujillo-Silva DJ, Cilia-López VG. Impact of environmental pollution on the obesogenic environment. Nutr Rev. (2022) 80:1787–99. doi: 10.1093/nutrit/nuac003
4. Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. (2005) 14:1847–50. doi: 10.1158/1055-9965.EPI-05-0456
5. Isola S, Murdaca G, Brunetto S, Zumbo E, Tonacci A, Gangemi S. The use of artificial intelligence to analyze the exposome in the development of chronic diseases: a review of the current literature. Informatics. (2024) 11:86. doi: 10.3390/informatics11040086
6. Santos S, Maitre L, Warembourg C, Agier L, Richiardi L, Basagaña X, et al. Applying the exposome concept in birth cohort research: a review of statistical approaches. Eur J Epidemiol. (2020) 35:193–204. doi: 10.1007/s10654-020-00625-4
7. Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav. (2007) 91:432–9. doi: 10.1016/j.physbeh.2007.04.006
8. Mankad M, Gokhale D. Hedonic hunger: eating for desire and not calories. Cardiometry. (2021) 20:160–6. doi: 10.18137/cardiometry.2021.20.160166
9. Stok FM, De Vet E, Wardle J, Chu MT, De Wit J, De Ridder DT. Navigating the obesogenic environment: how psychological sensitivity to the food environment and self-regulatory competence are associated with adolescent unhealthy snacking. Eat Behav. (2015) 17:19–22. doi: 10.1016/j.eatbeh.2014.12.003
10. Yu YH, Vasselli JR, Zhang Y, Mechanick JI, Korner J, Peterli R. Metabolic vs. hedonic obesity: a conceptual distinction and its clinical implications. Obes Rev. (2015) 16:234–47. doi: 10.1111/obr.12246
11. Corbit LH. Effects of obesogenic diets on learning and habitual responding. Curr Opin Behav Sci. (2016) 9:84–90. doi: 10.1016/j.cobeha.2016.02.010
12. Skau S, Sundberg K, Kuhn HG. A proposal for a unifying set of definitions of fatigue. Front Psychol. (2021) 12:739764. doi: 10.3389/fpsyg.2021.739764
13. Faro M, Sàez-Francás N, Castro-Marrero J, Aliste L, de Sevilla TF, Alegre J. Gender differences in chronic fatigue syndrome. Reumatol Clín. (2016) 12:72–7. doi: 10.1016/j.reumae.2015.05.009
14. Bültmann U, Kant I, Kasl SV, Schröer KA, Swaen GM, van den Brandt PA. Lifestyle factors as risk factors for fatigue and psychological distress in the working population: prospective results from the Maastricht Cohort Study. J Occup Environ Med. (2002) 44:116–24. doi: 10.1097/00043764-200202000-00006
15. Flores S, Brown A, Adeoye S, Jason LA, Evans M. Examining the impact of obesity on individuals with chronic fatigue syndrome. Workplace Health Saf. (2013) 61:299–307. doi: 10.3928/21650799-20130617-12
16. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. (2012) 86:741–6.
17. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. (1993) 37:147–53. doi: 10.1016/0022-3999(93)90081-P
18. Cella M, Chalder T. Measuring fatigue in clinical and community settings. J Psychosom Res. (2010) 69:17–22. doi: 10.1016/j.jpsychores.2009.10.007
19. Adin RM, Ceren AN, Salci Y, Fil Balkan A, Armutlu K, Ayhan Kuru Ç. Dimensionality, psychometric properties, and population-based norms of the Turkish version of the Chalder Fatigue Scale among adults. Health Qual Life Outcomes. (2022) 20:161. doi: 10.1186/s12955-022-02074-x
20. Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. (2009) 53:114–8. doi: 10.1016/j.appet.2009.05.016
21. Ulker I, Ayyildiz F, Yildiran H. Validation of the Turkish version of the power of food scale in adult population. Eat Weight Disord. (2021) 26:1179–86. doi: 10.1007/s40519-020-01019-x
22. Kesik S, Saglam D. Development and validity-reliability study of the assessment of the obesogenic environment scale for adults. Gevher Nesibe J Med Health Sci. (2022) 7:1–9. doi: 10.5281/zenodo.6973856
23. Ruiz E, Alegre J, Quintana AG, Aliste L, Blázquez A, de Sevilla TF. Síndrome de fatiga crónica: estudio de una serie consecutiva de 824 casos evaluados en dos unidades especializadas. Revista Clínica Española. (2011) 211:385–90. doi: 10.1016/j.rce.2011.02.013
24. Pajediene E, Bileviciute-Ljungar I, Friberg D. Sleep patterns among patients with chronic fatigue: a polysomnography-based study. Clin Respir J. (2018) 12:1389–97. doi: 10.1111/crj.12667
25. Van der Werf SP, Prins JB, Vercoulen JH, Van der Meer JW, Bleijenberg G. Identifying physical activity patterns in chronic fatigue syndrome using actigraphic assessment. J Psychosom Res. (2000) 49:373–9. doi: 10.1016/S0022-3999(00)00197-5
26. Jackson ML, Bruck D. Sleep abnormalities in chronic fatigue syndrome/myalgic encephalomyelitis: a review. J Clin Sleep Med. (2012) 8:719–28. doi: 10.5664/jcsm.2276
27. Nijs J, Lundberg M. Avoidance behavior towards physical activity in chronic fatigue syndrome and fibromyalgia: the fear for post-exertional malaise. Clin Rheumatol. (2014) 33:151–2. doi: 10.1007/s10067-013-2421-1
28. Batu A, Batu HS. Historical background of Turkish gastronomy from ancient times until today. J Ethnic Foods. (2018) 5:76–82. doi: 10.1016/j.jef.2018.05.002
29. Cho HJ, Menezes PR, Bhugra D, Wessely S. The awareness of chronic fatigue syndrome: a comparative study in Brazil and the United Kingdom. J Psychosom Res. (2008) 64:351–5. doi: 10.1016/j.jpsychores.2007.12.006
30. Goedendorp MM, Knoop H, Schippers GM, Bleijenberg G. The lifestyle of patients with chronic fatigue syndrome and the effect on fatigue and functional impairments. J Hum Nutr Diet. (2009) 22:226–31. doi: 10.1111/j.1365-277X.2008.00933.x
31. Xu J, Qiu B, Zhang F, Zhang J. Restorative effects of pocket parks on mental fatigue among young adults: a comparative experimental study of three park types. Forests. (2024) 15:286. doi: 10.3390/f15020286
32. Naz I, Qamar A. The silent strains and blurred minds: a study of digital screen time, mental fatigue and brain fog among university students. J Reg Stud Rev. (2025) 4:71–9. doi: 10.62843/jrsr/2025.4b111
33. Nagashima S, Suwazono Y, Okubo Y, Uetani M, Kobayashi E, Kido T, et al. Working hours and mental and physical fatigue in Japanese workers. Occup Med. (2007) 57:449–52. doi: 10.1093/occmed/kqm047
34. Hilland GH, Anthun KS. Socioeconomic determinants of myalgic encephalomyelitis/chronic fatigue syndrome in Norway: a registry study. BMC Public Health. (2024) 24:1296. doi: 10.1186/s12889-024-18757-7
35. Drachler MDL, Leite JCDC, Hooper L, Hong CS, Pheby D, Nacul L, et al. The expressed needs of people with chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. BMC Public Health. (2009) 9:458. doi: 10.1186/1471-2458-9-458
36. Dietert RR, Dietert JM. Possible role for early-life immune insult including developmental immunotoxicity in chronic fatigue syndrome (CFS) or myalgic encephalomyelitis (ME). Toxicology. (2008) 247:61–72. doi: 10.1016/j.tox.2008.01.022
37. Jones CL, Haskin O, Younger JW. Association between chronic pain and fatigue severity with weather and air pollution among females with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Int J Environ Res Public Health. (2024) 21:1560. doi: 10.3390/ijerph21121560
38. Batool M, Qurat-ul-Ain SR, Maalik S, Mushtaq S, David M, Saeed K, et al. Pollution and mental health: the hidden psychological impact. In:Abbas RZ, Akhtar R, Arshad J, , editors. One Health in a Changing World. Faisalabad, Pakisthan: Unique Scientific Publisher (2025). p. 120–8.
39. Prylińska M, Husejko J, Skierkowska N, Bieniek D, Kożuchowski M, Porada M, et al. Nutritional and dietary interventions for the treatment of Chronic Fatigue Syndrome. J Educ Health Sport. (2018) 8:265–80. doi: 10.5281/zenodo.1307482
40. Haß U, Herpich C, Norman K. Anti-inflammatory diets and fatigue. Nutrients. (2019) 11:2315. doi: 10.3390/nu11102315
41. Thomas N, Gurvich C, Huang K, Gooley PR, Armstrong CW. The underlying sex differences in neuroendocrine adaptations relevant to myalgic encephalomyelitis chronic fatigue syndrome. Front Neuroendocrinol. (2022) 66:100995. doi: 10.1016/j.yfrne.2022.100995
42. van't Leven M, Zielhuis GA, van der Meer JW, Verbeek AL, Bleijenberg G. Fatigue and chronic fatigue syndrome-like complaints in the general population. Eur J Public Health. (2010) 20:251–7. doi: 10.1093/eurpub/ckp113
43. Kindlon T. Do graded activity therapies cause harm in chronic fatigue syndrome? J Health Psychol. (2017) 22:1146–54. doi: 10.1177/1359105317697323
44. Yoshiuchi K, Cook DB, Ohashi K, Kumano H, Kuboki T, Yamamoto Y, et al. A real-time assessment of the effect of exercise in chronic fatigue syndrome. Physiology Behavior. (2007) 92:963–8. doi: 10.1016/j.physbeh.2007.07.001
45. Palacios N, Molsberry S, Fitzgerald KC, Komaroff AL. Different risk factors distinguish myalgic encephalomyelitis/chronic fatigue syndrome from severe fatigue. Sci Rep. (2023) 13:2469. doi: 10.1038/s41598-023-29329-x
46. Mullington JM, Hinze-Selch D, Pollmächer T. Mediators of inflammation and their interaction with sleep: relevance for chronic fatigue syndrome and related conditions. Ann N Y Acad Sci. (2001) 933:201–10. doi: 10.1111/j.1749-6632.2001.tb05825.x
47. Aerenhouts D, Ickmans K, Clarys P, Zinzen E, Meersdom G, Lambrecht L, et al. Sleep characteristics, exercise capacity and physical activity in patients with chronic fatigue syndrome. Disabil Rehabil. (2015) 37:2044–50. doi: 10.3109/09638288.2014.993093
48. Gotts ZM, Newton JL, Ellis JG, Deary V. The experience of sleep in chronic fatigue syndrome: a qualitative interview study with patients. Br J Health Psychol. (2016) 21:71–92. doi: 10.1111/bjhp.12136
49. Filgueiras MDS, Pessoa MC, Bressan J, Vegi ASF, do Carmo AS, de Albuquerque FM, et al. Characteristics of the obesogenic environment around schools are associated with body fat and low-grade inflammation in Brazilian children. Public Health Nutr. (2023) 26:2407–17. doi: 10.1017/S1368980023001696
50. Lee CH, Giuliani F. The role of inflammation in depression and fatigue. Front Immunol. (2019) 10:1696. doi: 10.3389/fimmu.2019.01696
51. Hoel F, Hoel A, Pettersen IK, Rekeland IG, Risa K, Alme K, et al. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight. (2021) 6:e149217. doi: 10.1172/jci.insight.149217
52. Asuku AO, Ayinla MT, Ajibare AJ. Central nervous system regulation of eating and brain functions. In:Mohamed W, Kobeissy F, , editors. Nutrition and Psychiatric Disorders: An Evidence-Based Approach to Understanding the Diet-Brain Connection. Singapore: Springer Nature Singapore (2024). pp. 69–87. doi: 10.1007/978-981-97-2681-3_4
53. Miller AH, Jones JF, Drake DF, Tian H, Unger ER, Pagnoni G. Decreased basal ganglia activation in subjects with chronic fatigue syndrome: association with symptoms of fatigue. PLoS ONE. (2014) 9:e98156. doi: 10.1371/journal.pone.0098156
54. Arsalidou M, Vijayarajah S, Sharaev M. Basal ganglia lateralization in different types of reward. Brain Imaging Behav. (2020) 14:2618–46. doi: 10.1007/s11682-019-00215-3
55. Nakagawa S, Takeuchi H, Taki Y, Nouchi R, Kotozaki Y, Shinada T, et al. Basal ganglia correlates of fatigue in young adults. Sci Rep. (2016) 6:21386. doi: 10.1038/srep21386
56. Sanal-Hayes NE, Slade K, McLaughlin M, Metcalfe P, Berry E, Thornton EJ, et al. Therapeutic use of transcranial magnetic stimulation (TMS) for people with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a scoping review. Fatigue Biomed Health Behav. (2025) 13:262–81. doi: 10.1080/21641846.2025.2513807
Keywords: chronic fatigue, exposome, hedonic appetite, obesogenic environment, Turkish adults
Citation: Helvacı G and Tayhan F (2025) Association of obesogenic environment and hedonic appetite with chronic fatigue in Turkish adults. Front. Nutr. 12:1660721. doi: 10.3389/fnut.2025.1660721
Received: 07 July 2025; Accepted: 22 September 2025;
Published: 13 October 2025.
Edited by:
Yibo Wu, Zhejiang University, ChinaCopyright © 2025 Helvacı and Tayhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gizem Helvacı, Z2hlbHZhY2lAbWVobWV0YWtpZi5lZHUudHI=
†ORCID: Gizem Helvacı orcid.org/0000-0001-8654-9245
Fatma Tayhan orcid.org/0000-0001-8524-9048
 Gizem Helvacı
Gizem Helvacı Fatma Tayhan
Fatma Tayhan