- Department of Gastroenterology, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, Beijing, China
Background: Sarcopenia affects treatment outcomes in patients with Crohn's disease (CD), yet research focusing on pediatric populations is limited. This study aimed to determine the prevalence of sarcopenia among Chinese children with CD and to evaluate its impact on biologic therapy by analyzing body composition parameters based-on computed tomography enterography (CTE).
Methods: Pediatric CD patients who underwent CTE and received infliximab (IFX) treatment between 2022 and 2025 were enrolled. Clinical, laboratory, and radiological data were collected. CTE was utilized to assess body composition. The control group consisted of children without inflammatory bowel disease (non-IBD) who underwent abdominal CT scans.
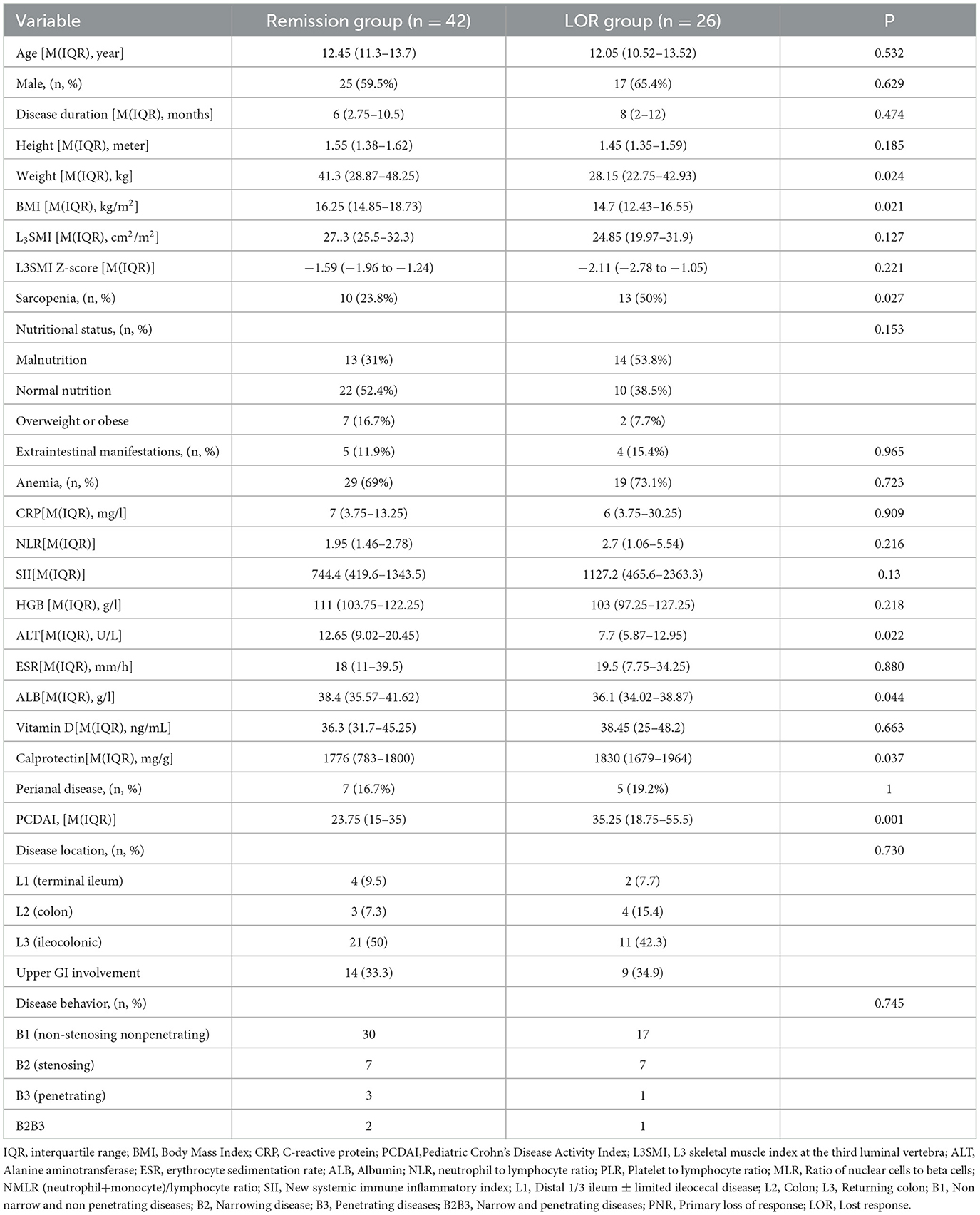
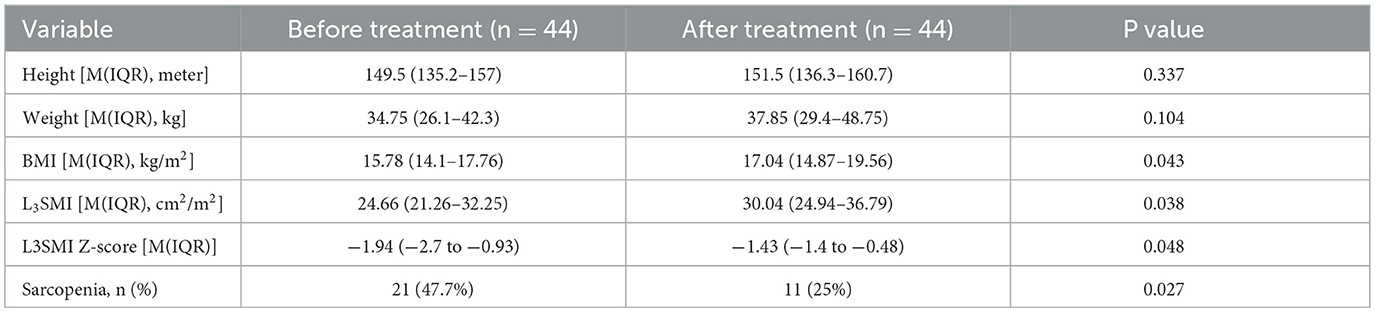
Results: A total of 68 children with CD (mean age 11.89 years) were included. The prevalence of sarcopenia was significantly higher in the CD group compared to the 136 controls (33.8% vs. 10.2%, P = 0.001). Body mass index (BMI) was identified as the only protective factor against sarcopenia (OR 0.734, 95% CI 0.578–0.932, P = 0.005). Among CD patients, those with loss of response (LOR) to IFX had a significantly higher incidence of sarcopenia than those in remission (50% vs. 23.8%, P = 0.027). After treatment with infliximab combined with total exclusive enteral nutrition (EEN) or partial enteral nutrition (PEN) in 44 children, follow-up CTE showed a significant reduction in sarcopenia prevalence (47.7% vs. 25%, P = 0.027).
Conclusion: Sarcopenia is common in Chinese children with CD and adversely affects the efficacy of biological therapy. CTE is a valuable tool for assessing sarcopenia in this population. Early detection and intervention may improve clinical outcomes for children with CD.
1 Introduction
Crohn's disease (CD) is a chronic inflammatory disease of the gastrointestinal tract that can lead to progressive intestinal damage and disability (1). The incidence of CD among children in middle-income countries is increasing annually (2). Due to reduced food intake, intestinal malabsorption, chronic protein loss through the feces, and increased energy demands from hypermetabolism (3, 4), children with CD may suffer from growth disorders, malnutrition, and sarcopenia, even during remission (5). In 2014, the Asian Working Group for Sarcopenia defined sarcopenia as an age-related decline in skeletal muscle mass as well as muscle function (defined by muscle strength or physical performance) (6). Sarcopenia has been associated with older age, but it is also a known complication of various chronic diseases, such as cirrhosis, intestinal disorders, chronic kidney disease, neurodegenerative diseases, and malignant tumors (7–17). Sarcopenia has been observed in both children and adult with CD (18–28). Sarcopenia affects the prognosis of CD and is recognized as a risk factor for surgery, hospitalization, and postoperative complications in patients with CD (29).
The primary challenge in defining sarcopenia in children lies in lacking a standardized definition and diagnostic criteria (30, 31). Children's skeletal muscle mass can be assessed using various methods, including bioelectrical impedance analysis (BIA) (21), dual-energy X-ray absorptiometry (DXA) (17), computed tomography (CT) (17), and magnetic resonance imaging (MRI) (19). However each technique exhibits considerable variability and lacks established reference standards. The total skeletal muscle area measured by CT or MRI at the L3-L4 lumbar spine levels is regarded as the gold standard for assessing skeletal muscle mass (6). CT enterography (CTE) is a routine diagnostic procedure for children with CD, and can be utilized to evaluate their skeletal muscle mass without the need for additional tests. However, pediatric studies using CTE to assess muscle mass in CD patients are limited, and there are regional and racial variations in health standard reference values (31).
The number of relevant studies on sarcopenia in children with CD is limited, and there is a lack of longitudinal follow-up examining the interaction between sarcopenia and CD treatment. Therefore, this study aims to investigate the incidence of sarcopenia in Chinese children with CD using CT-based body composition parameters, observe the interaction between sarcopenia and CD treatment, and determine whether sarcopenia affects the response to biologic therapies.
2 Materials and methods
2.1 Study population
This study is a retrospective analysis conducted from January 2022 to February 2025, involving patients diagnosed with CD at the Gastroenterology Department of Beijing Children's Hospital. The inclusion criteria were as follows: (1) age under 18 years; (2) diagnosis of CD based on the revised Porto criteria (32); (3) completion of a CTE examination within 3 months prior to CD diagnosis; (4) received standard infliximab (IFX) treatment with a follow-up period exceeding 12 months; (5) availability of complete clinical data and clear CTE images suitable for body composition evaluation. For the control group, inclusion criteria included: (1) healthy children who underwent an abdominal CT scan at our center; (2) normal abdominal CT scan results; (3) sex and age were matched to the CD group; and (4) absence of chronic conditions such as cancer, chronic inflammatory diseases, infections, or malnutrition.
2.2 Data collection
We collected demographic and clinical data of CD patients from electronic medical records. The data included age, gender, body weight, height, body mass index (BMI), disease duration, extraintestinal manifestations (EIM), perianal lesions, Paris classification (33), Pediatric Crohn's Disease Activity Index (PCDAI) (34), disease activity, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), fecal calprotectin (FC) and response to IFX.
2.3 Definitions
BMI is calculated by dividing weight (kg) by the square of height (m). Based on the results of a blood routine examination, calculate the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), neutrophil+monocyte/lymphocyte ratio (NMLR). The new systemic immune inflammation index (SII) was calculated using the formula platelet count × neutrophil count/lymphocyte count.
Primary non-response (PNR) refers to the lack of response to IFX treatment in children with CD by the 14th week of therapy; These patients do not achieve clinical remission or sufficient improvement and subsequently discontinue IFX. Secondary loss of response (SLOR) describes the discontinuation of IFX maintenance therapy following disease recurrence after an initial positive response, which may involve dose escalation (either increasing the dose or shortening the dosing interval). In this study, loss of response (LOR) encompasses both PNR and SLOR.
The induction dose of IFX was 5 mg/kg, administered intravenously at weeks 0, 2, and 6. During the maintenance period, IFX was given intravenously every 4 to 8 weeks.
The L3 Skeletal Muscle Index (L3SMI) is calculated by dividing the total cross-sectional area of all skeletal muscles at the L3 vertebral body level, including the psoas major, erector spinae, quadratus lumborum, transversus abdominis, external oblique, and internal oblique, measured via CT or MRI by the square of the individual's height in meters. The L3SMI Z-score was evaluated using previously published online tools (34) (https://square.umin.ac.jp/ped-muscle-calc/index.html, accessed on January 10, 2025). Sarcopenia was defined as an L3SMI Z-score less than −2 standard deviation, according to previous studies (16, 20).
2.4 Skeletal muscle area
CTE examination was performed following the standard imaging protocol. At the level of the third lumbar vertebra, the boundaries of skeletal muscles were manually outlined using ImageJ software version 1.54p (35). Throughout the study, the boundaries of skeletal muscles were determined by trained radiologists. Pre-defined radiation attenuation ranges are used to demarcate muscle (-29 Hounsfield units to +150 Hounsfield units) (see Figure 1). Calculate the skeletal muscle area (SMA) in square centimeters from the pixel count using ImageJ.
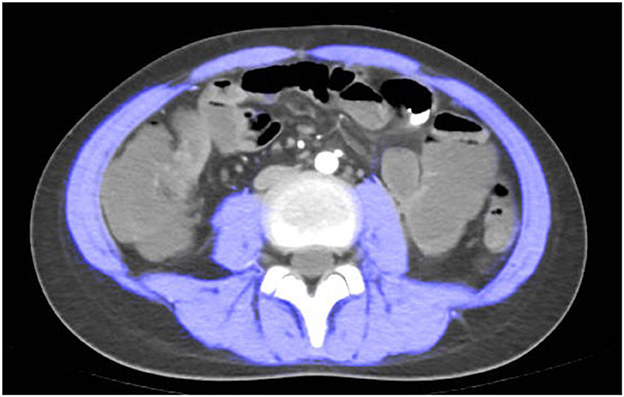
Figure 1. Computed tomography (CT) scan measured at spinal level L3. a patient with sarcopenia (Male, height = 1.55 m, L3SMI 28.67, L3SMI Z-score−2.423).
2.5 Statistical analysis
Categorical variables are represented as frequency and percentage (%). Continuous variables are expressed as mean ± standard deviation or as median with interquartile range (IQR, lower and upper quartiles). Fisher's exact test was used to compare categorical variables, while t-tests or the Mann-Whitney U test were applied to compare continuous variables. Relevant indicators between clinical characteristics and sarcopenia/LOR were identified through univariate analysis, and followed by binary logistic regression analysis. A two-sided P-value < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS 26.0 (SPSS, Inc., Chicago, IL, USA). The Kaplan Meier curves generated with GraphPad Prism 10 (GraphPad Software, CA, USA).
2.6 Ethical approval
This study was approved by the Medical Ethics Committee of Beijing Children's Hospital in China (Approval Number: 2024-Y-073-D). Given the retrospective nature of the study, the requirement for informed consent was waived.
3 Results
3.1 Patient population
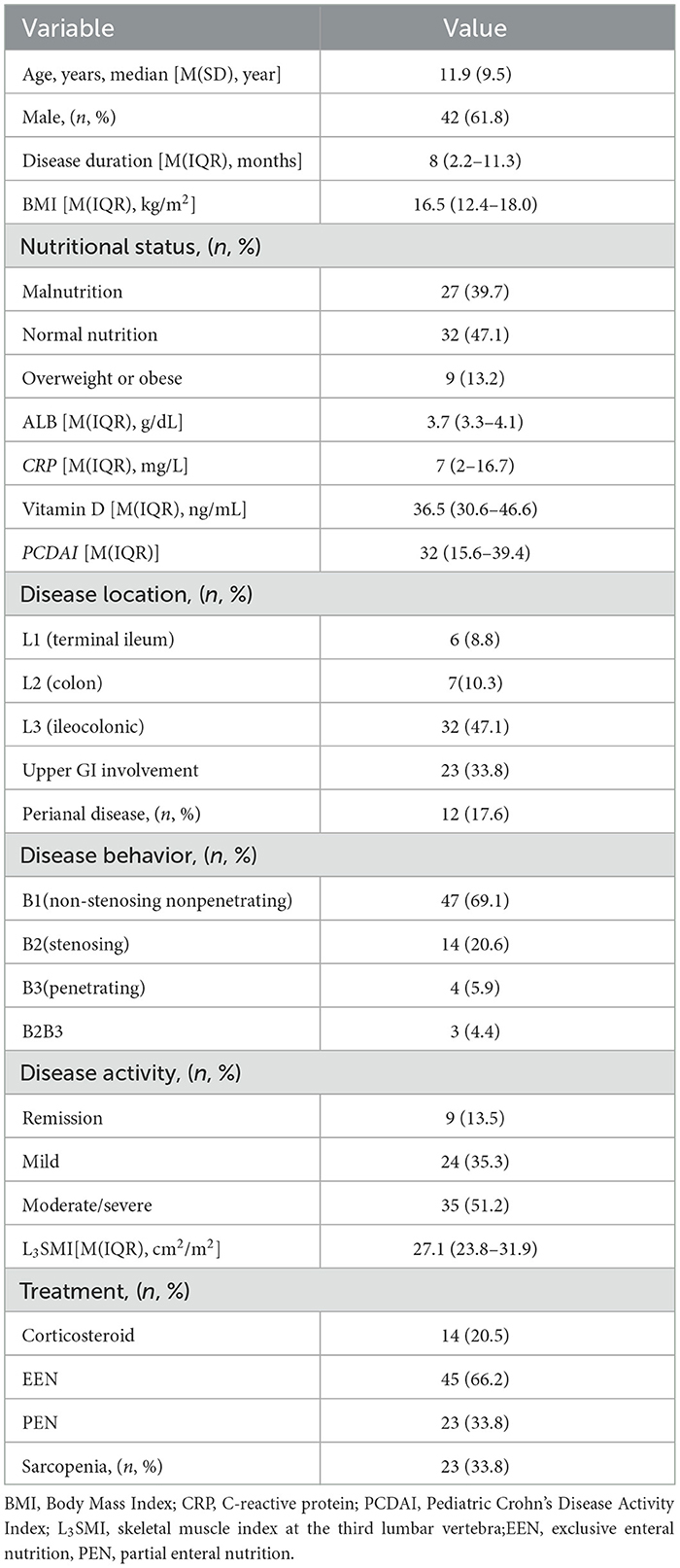
A total of 128 patients with CD completed CTE. Of these, 68 patients met the inclusion criteria and were included in this study. The mean age was 11.9 years (SD 9.5). Forty-two (61.8%) patients were boys. The median disease duration was 8 months (IQR, 2.3–11.3 months). Thirty-two patients (47.1%) had an ileo-colonic localization. 66.2% of patients received exclusive enteral nutrition (EEN), while 33.8% received partial enteral nutrition (PEN). Demographic data are presented in Table 1. Based on the L3SMI Z-score, 23 patients (33.8%) were diagnosed with sarcopenia. Two patients exhibited sarcopenia during the remission period. One in nine overweight or obese patients had sarcopenia, while six out of 32 children with normal nutritional status had sarcopenia.
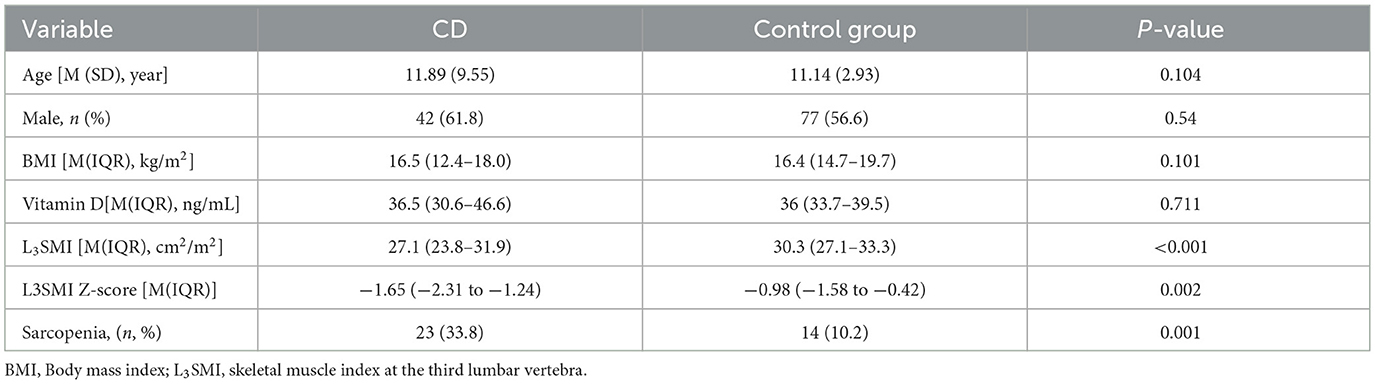
A total of 136 healthy children were included in the control group, all of whom underwent abdominal CT due to digestive foreign bodies, back pain or acute trauma. There were no significant differences in age, gender, BMI, or Vitamin D between the control group and the CD group (P > 0.05, Table 2). However, the L3SMI and L3SMI Z-score in the CD group were significantly lower than those in the control group (P < 0.001 and P = 0.002, respectively). Additionally, the incidence of sarcopenia was significantly higher in the CD group compared to the control group (33.8% vs. 10.2%, P = 0.001).
3.2 Comparison between CD patients with sarcopenia and those without sarcopenia
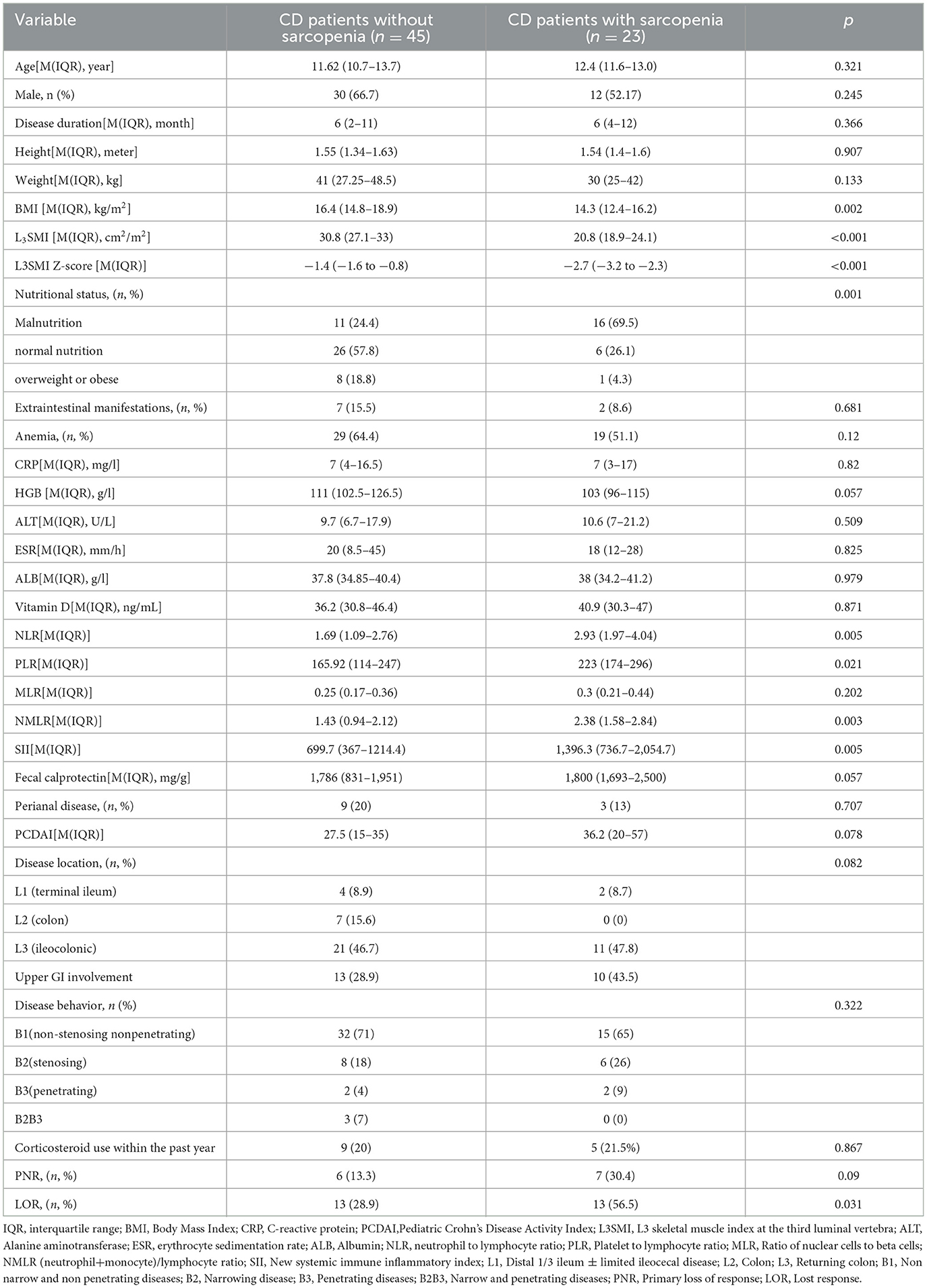
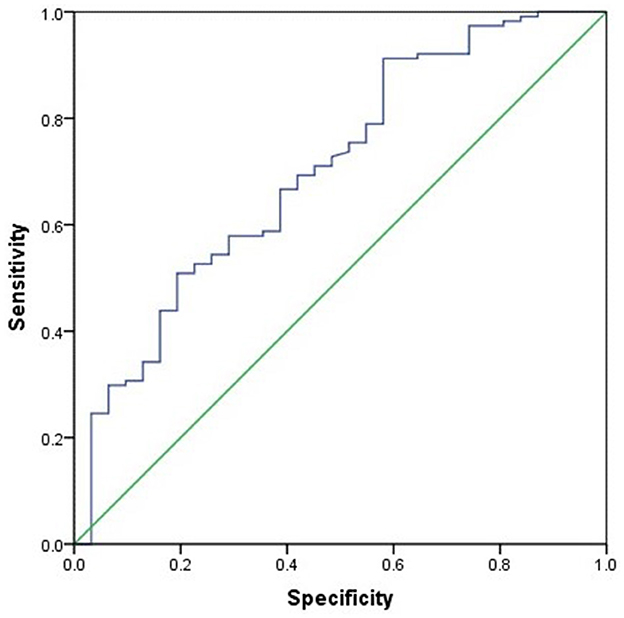
The differences between CD patients with sarcopenia and those without sarcopenia are presented in Table 3. Compared to CD patients without sarcopenia, those with sarcopenia had significantly lower BMI, L3SMI, L3SMI Z-score, and proportions of normal nutrition and malnutrition (P ≤ 0.001). NLR, PLR, NMLR, and SII were significantly higher (P < 0.05), and the proportion of LOR to IFX treatment was also greater (P = 0.031) (Table 3). There was no statistically significant difference between the two groups in terms of corticosteroid use within 1 year prior to CD diagnosis and baseline vitamin D levels (measured before CD diagnosis). Subsequently, age, gender, BMI, NLR, PLR, NMLR, and SII were included in a binary logistic regression analysis, which identified that BMI (OR 0.734, 95% CI 0.578–0.932) as the sole protective factor against sarcopenia (P = 0.005). BMI showed the best performance in distinguishing the presence of sarcopenia, with an AUC of 0.731 (95% CI 0.60–0.85, P = 0.002). The optimal BMI cutoff value was 13.84, with a sensitivity of 79.1% and a specificity of 52.3% (see Figure 2).
3.3 Predictors of Response to IFX Treatment
After a one-year follow-up, 26 children who received IFX treatment were found to have LOR. The incidence of sarcopenia in LOR patients was significantly higher than that in remission group (50% vs. 23.8%, P = 0.027) (Figure 3). Baseline weight, BMI, ALT, and ALB levels were significantly lower in LOR patients than in those in remission (P < 0.05), while FC and PCDAI levels were higher in the LOR (P < 0.05) (see Table 4). Binary logistic regression analysis identified that PCDAI as an independent risk factor for loss of response to IFX treatment (OR=1.066, 95% CI 1.027–1.107, P = 0.001).
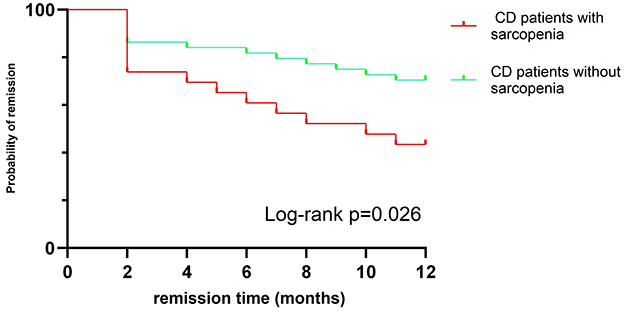
Figure 3. Kaplan–Meier curve showing different rates of remission between sarcopenia CD patients and No-sarcopenia CD patients.
3.4 Dynamic changes in body composition before and after treatment
All patients received IFX and EEN/PEN treatment. Among them, 44 patients required re-evaluation with CTE for disease assessment, with an average interval of 203.5 days between evaluations. After treatment, there were no significant changes in height or weight, however, BMI, L3SMI, and the L3SMI Z-score increased significantly (P < 0.05). Additionally, the proportion of patients with sarcopenia decreased significantly, from 47.7% to 25% (P = 0.027) (see Table 5).
4 Discussion
This study demonstrates that sarcopenia is commonly observed in children with CD in China, with a significantly higher incidence compared to control children. Children with CD and sarcopenia show poorer nutritional status and increased inflammation-related markers, which are associated with a higher rate of non-response to the biologic therapy IFX. Treatment with IFX can improve the nutritional status of these children and reduce the incidence of sarcopenia.
Sarcopenia is more common among the elderly (36); however, recent studies have shown that secondary sarcopenia also frequently occurs in adults and children with various diseases, including endocrine disorders, tumors, liver diseases, and inflammatory bowel disease, etc. (7–28). The prevalence of sarcopenia in adults with IBD ranges from approximately 2% to 30% (37–39), while in pediatric CD, it ranges from 23.5% to 81% (18, 23). Consistent with other pediatric studies, our research also found sarcopenia in children with CD, with the incidence of 33.8%. Variations in incidence rates may be attributed to differences in study populations and the criteria used to assess skeletal muscle index. Additionally, we observed sarcopenia in healthy children in China, with an incidence rate of 10.2%, which may be related to reduced physical activity due to prolonged sedentary behavior and academic stress. Similar to Gülşen's findings (5), we also found sarcopenia during the remission phase of CD. Therefore, it is important to pay attention to the nutritional status of children with CD even during remission.
In addition to malnutrition, sarcopenia can also occur in patients with a normal BMI or obesity. A meta-analysis of 50 studies involving 86,285 individuals aged 60 years and older found that sarcopenic obesity has a prevalence of 11% among the Asian–Oceanic population (40). Naruse et al. reported that 9.6% (10 out 104) of CD patients had sarcopenic obesity (41). Although the exact cause remains unknown, but factors such as steroid therapy and lack of physical activity can lead to significant changes in body weight and contribute to sarcopenic obesity in IBD patients (42). In our study, we also identified seven patients with normal BMI or obesity who had sarcopenia, which suggests patients with a normal BMI or obesity can also develop sarcopenia, indicating that assessing the nutritional status of CD patients should not rely solely on BMI.
Currently, there is limited research on the risk factors associated with sarcopenia (43). A meta-analysis shows that gender, low BMI, age, and low albumin levels significantly affect the occurrence of sarcopenia in adult patients with CD (44). In this study, univariate analysis revealed that BMI, NLR, PLR, NMLR, and SII were linked to sarcopenia; however, binary logistic regression analysis identified low BMI as the sole risk factor. Low BMI is associated with malnutrition, which in turn increases the incidence of sarcopenia. No significant associations were found between gender, age, low albumin levels, and sarcopenia.
The diagnosis of adult sarcopenia is based on decline in skeletal muscle mass combined with low muscle strength and/or reduced physical performance (45). However, there is currently no unified definition or diagnostic criteria for sarcopenia in children (30, 31). Most studies diagnose pediatric sarcopenia using skeletal muscle mass indicators, such as the total psoas muscle area measured by CT or MRI (46). The total skeletal muscle area assessed by CT or MRI at the L3-L4 lumbar spine levels is considered the gold standard for measuring skeletal muscle mass (45). Although CT and MRI are expensive and lack portability, they are the fundamental methods for diagnosing CD and do not require additional examinations, making them more suitable than other methods for evaluating the skeletal muscle mass in CD patients. Compared to MRE, CTE is more appropriate for diagnosing and assessing pediatric CD in developing countries; therefore, CTE was used to evaluate skeletal muscle mass in this study. Reference standards for skeletal muscle mass vary by race and region. Based on CT scans, some researchers have reported pediatric reference values for the total lumbar muscle area in South Korea (47), Europe,and America (48), as well as reference values for the abdominal skeletal muscle compartments in Asian (49); Using DXA, Mi et al. reported pediatric reference values for skeletal muscle mass in China (50). Our study primarily relies on CTE to assess skeletal muscle area. Due to the lack of CT-based reference values for abdominal skeletal muscle area in Chinese children, Kudo et al.'s (49) standards were used in this study. Although some data on healthy children were collected in our study, the sample size was insufficient to develop a standard reference curve. Therefore, further multicenter studies are necessary to increase the sample size and improve CT-based reference values for abdominal skeletal muscle area in Chinese children. Muscle strength measurement in children primarily focuses on grip strength, with only one study addressing muscle strength in children (51). Given the challenges in assessing strength and function in young children, most pediatric studies do not include this information. As our study is retrospective, no muscle strength assessments were conducted. Future prospective studies will incorporate muscle strength measurements.
Sarcopenia may be associated with poor prognosis in CD, but there is still controversy. A meta-analysis indicated that sarcopenia is associated with an increased risk of hospitalization and abscess formation in patients with CD. However, it does not appear to significantly influence the need for surgery, loss of biological response, requirement for biological therapy, or the occurrence of surgical site leaks (52). Conversely, some studies has identified sarcopenia as a risk factor for developing LOR in CD patients treated with IFX (53–55). Research on pediatric populations is limited; for instance, Calia et al. found no significant association between sarcopenia and CD outcomes (19), whereas Atalan et al. (22) reported that patients with a psoas index in the lowest quartile had a significantly higher risk of requiring biologic therapy and experiencing disease exacerbation. Our study observed that CD patients with sarcopenia had a higher incidence of LOR and poorer outcomes when treated with IFX, although sarcopenia was not an independent risk factor for LOR. Larger studies are needed to better understand the impact of sarcopenia on CD outcomes in children.
The treatment of sarcopenia involves resistance training, nutritional support, and etiological treatment. In CD, treatment includes enteral nutrition support and immunosuppressive or biologic therapies, which improve both the nutritional status and inflammatory response of children, thereby reducing the incidence of sarcopenia. Subramaniam et al. (28) found that IFX can reverse inflammatory sarcopenia in patients with Crohn's disease. This study also supports that IFX combined with nutritional support can improve the nutritional status of children with CD and reverse sarcopenia. Research has shown that exercise can increase muscle mass in patients with IBD (56); however, due to low self-esteem, body image, and active IBD symptoms, many CD patients have limited physical activity (57). Consequently, some children with CD are unable to fully overcome sarcopenia through medication and nutritional support treatment alone. This study further demonstrated that after one year of treatment, 25% of patients still exhibited sarcopenia, highlighting the importance of incorporating appropriate exercise as part of the treatment plan for children with CD.
As one of the largest PIBD medical centers in China, this study is the first to use CTE to assess the prevalence of sarcopenia in Chinese children with CD and its impact on treatment outcomes Additionally, it longitudinally examines the reciprocal relationship between CD treatment and sarcopenia. However, this study has several limitations: (1) the absence reference standard range for total skeletal muscle area measured by CT/MRI at the L3-L4 lumbar spine levels in Chinese children;(2) its retrospective design, which lacks assessments of muscle strength, muscle function, and physical activity; and (3) a small sample size of CD patients meeting the inclusion criteria, potentially introducing selection bias. Further studies with larger cohorts are needed.
5 Conclusion
Sarcopenia is commonly observed in Chinese children with CD and can influence the effectiveness of biologic therapies. CTE can be assess CD-related sarcopenia in pediatric patients. Sarcopenia may be added to disease activity scores. IFX can improve the nutritional status of children with CD and reverse sarcopenia. However, due to limited awareness of this condition among pediatricians, further research is needed to better understand the relationship between sarcopenia and CD. Enhancing pediatricians' knowledge will facilitate early detection and intervention, ultimately improving the clinical outcomes for children with CD.
Data availability statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available due to privacy reasons. Requests to access these datasets should be directed to Jie Wu, d3VqaWVfMDIyMDI0QDE2My5jb20=.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Beijing Children's Hospital in China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because Due to the retrospective nature of the study, informed consent was waived..
Author contributions
CG: Conceptualization, Data curation, Investigation, Writing – review & editing, Methodology, Writing – original draft, Formal analysis, Visualization. YK: Data curation, Visualization, Writing – original draft, Formal analysis, Methodology, Investigation, Conceptualization, Writing – review & editing. GW: Writing – review & editing, Methodology, Conceptualization, Project administration, Validation, Investigation. JD: Supervision, Validation, Formal analysis, Writing – original draft, Data curation, Resources, Software. CY: Software, Validation, Formal analysis, Supervision, Resources, Data curation, Writing – original draft. JW: Writing – review & editing, Funding acquisition, Conceptualization, Project administration, Validation, Methodology, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the National Key Research and Development Program of China [No. 2023YFC2706503], the National Natural Science Foundation of China (Grant No. 82471746); Beijing Natural Science Foundation (J230009); Beijing Hospitals Authority's Ascent Plan (DFL20221003).
Acknowledgments
We would like to thank all the nurses and doctors in the Department of Gastroenterology of Beijing Children's Hospital for their support of this work and thank all the reviewers for their dedication to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. (2017) 389:1741–55. doi: 10.1016/S0140-6736(16)31711-1
2. Kuenzig M E, Fung S G, Marderfeld L, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. (2022) 162:1147–59. doi: 10.1053/j.gastro.2021.12.282
3. Prieto JMI, Andrade AR, Magro DO, Imbrizi M, Nishitokukado I, Ortiz-Agostinho CL, et al. Nutritional Global Status and Its Impact in Crohn's Disease. J Can Assoc Gastroenterol. (2021) 4:290–5. doi: 10.1093/jcag/gwab006
4. Balestrieri P, Ribolsi M, Guarino MPL, Emerenziani S, Altomare A, Cicala M. Nutritional aspects in inflammatory bowel diseases. Nutrients. (2020), 12:372. doi: 10.3390/nu12020372
5. Ünal NG, Oruç N, Tomey O, Ömer Özütemiz A. Malnutrition and sarcopenia are prevalent among inflammatory bowel disease patients with clinical remission. Eur J Gastroenterol Hepatol. (2021), 33:1367–75. doi: 10.1097/MEG.0000000000002044
6. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
7. Zhang S, Zhang L, Wu H, Li L. The current clinical management of muscle wasting in hemodialysis patients with end-stage renal disease. Int Urol Nephrol. (2025). doi: 10.1007/s11255-025-04563-6
8. Manabe T, Tsuchida W, Kobayashi T, Fujimoto M, Inai T, Kido K, et al. Spatiotemporal and kinematic gait characteristics in older patients with type 2 diabetes mellitus with and without sarcopenia. Sci Rep. (2025) 15:18000. doi: 10.1038/s41598-025-00205-0
9. Hopkins S, Hall J, Saunders H, Bashir R, Lakhter V, Vaidya A, et al. Sarcopenia in patients with chronic thromboembolic pulmonary hypertension. J Cardiovasc Dev Dis. (2025) 12:162. doi: 10.3390/jcdd12050162
10. Pardali EC, Klonizakis M, Goulis DG, Papadopoulou SK, Cholevas C, Giaginis C, et al. Sarcopenia in rheumatic diseases: a hidden issue of concern. Diseases. (2025) 13:134. doi: 10.3390/diseases13050134
11. Zhang Y, Wu Q, Wang Y, Chen Q, Han S, Li B, et al. Systemic inflammation and disruption of the local microenvironment compromise muscle regeneration: critical pathogenesis of autoimmune-associated sarcopenia. Interact J Med Res. (2025) 14:e64456. doi: 10.2196/64456
12. Wang R, Zhu L, Hao J, Lerttanatum N, Chen X, Wu L, et al. Computed tomography-based body composition and systemic inflammation for outcome prediction in patients with resectable esophageal squamous cell carcinoma. J Thorac Dis. (2025) 17:2441–52. doi: 10.21037/jtd-2025-508
13. da Silva NTF, Torres DM, Dos Santos Corrêa MV, Costa RM, Fabro EAN, Aguiar SS, et al. The prevalence of sarcopenia and its associations with weekly energy expenditure and physical activity levels in female patients with breast cancer at the time of diagnosis. Braz J Phys Ther. (2025) 29:101224. doi: 10.1016/j.bjpt.2025.101224
14. Filis P, Papagiannopoulos CK, Markozannes G, Chalitsios CV, Zerdes I, Valachis A, et al. Associations of sarcopenia, sarcopenia components and sarcopenic obesity with cancer incidence: a prospective cohort study of 414,094 participants in UK Biobank. Int J Cancer. (2025) 157:1316–32. doi: 10.1002/ijc.35480
15. Yilmaz E, Arsava EM, Topcuoglu MA. Subclinical atherosclerosis and sarcopenia: a prospective study. Medicine. (2025) 104:e42494. doi: 10.1097/MD.0000000000042494
16. Lewandowski CG, Silveira TT, Moreno YMF, Leite HP. Sarcopenia in children and adolescents with cancer: a systematic review of diagnostic assessment methods Pediatr Blood Cancer. (2025) 72:e31844. doi: 10.1002/pbc.31844
17. Mangus RS, Bush WJ, Miller C, Kubal CA. Severe sarcopenia and increased fat stores in pediatric patients with liver, kidney, or intestine failure. J Pediatr Gastroenterol Nutr. (2017) 65:579–83. doi: 10.1097/MPG.0000000000001651
18. Çakar S, Eren G, Erdur CB, Önder M, Pelek S, Demirtaş D, et al. Are vitamin D levels related to sarcopenia in children with inflammatory bowel disease? J Clin Med. (2025) 14:1548. doi: 10.3390/jcm14051548
19. Calia M, Rebora P, Gandola D, Norsa L, Maino C, Romanchuk A, et al. Investigating sarcopenia in pediatric Crohn's Disease with magnetic resonance enterography: an observational study. Clin Nutr ESPEN. (2025) 68:14–21. doi: 10.1016/j.clnesp.2025.04.027
20. Blagec P, Sara S, Tripalo Batoš A, Trivić MaŽuranić I, Močić Pavić A, Mišak Z, et al. Magnetic resonance imaging can be used to assess sarcopenia in children with newly diagnosed crohn's disease. Nutrients. (2023). 15:3838. doi: 10.3390/nu15173838
21. Boros KK, Veres G, Pintér HK, Richter É, Cseh Á, Dezsofi A, et al. Novel approach to assess sarcopenia in children with inflammatory bowel disease. Front Pediatr. (2024) 12:1204639. doi: 10.3389/fped.2024.1204639
22. Atlan L, Cohen S, Shiran S, Sira LB, Pratt LT, Yerushalmy-Feler A, et al. Sarcopenia is a predictor for adverse clinical outcome in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2021) 72:883–8. doi: 10.1097/MPG.0000000000003091
23. Mager DR, Carroll MW, Wine E, Siminoski K, MacDonald K, Kluthe CL, et al. Vitamin D status and risk for sarcopenia in youth with inflammatory bowel diseases. Eur J Clin Nutr. (2018) 72:623–6. doi: 10.1038/s41430-018-0105-2
24. Palmese F, Del Toro R, Di Marzio G, Cataleta P, Sama MG, Domenicali M, et al. Sarcopenia and Vitamin D Deficiency in Patients with Crohn's Disease: Pathological Conditions That Should Be Linked Together. Nutrients. (2021) 13:1378. doi: 10.3390/nu13041378
25. Binay Safer V, Tasci I, Safer U. Crohn's disease, visceral obesity and sarcopenia. Clin Nutr. (2020) 39:2315–6. doi: 10.1016/j.clnu.2020.05.037
26. Ashton JJ, Green Z, Beattie RM. Beyond bedside measures of malnutrition in paediatric Crohn's disease – Should we be thinking of sarcopenia. Clin Nutr. (2020) 39:1639–42. doi: 10.1016/j.clnu.2020.03.034
27. Grillot J, D'Engremont C, Parmentier AL, Lakkis Z, Piton G, Cazaux D, et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn's disease. Clin Nutr. (2020) 39:3024–30. doi: 10.1016/j.clnu.2020.01.001
28. Subramaniam K, Fallon K, Ruut T, Lane D, McKay R, Shadbolt B, et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn's disease. Aliment Pharmacol Ther. (2015) 41:419–28. doi: 10.1111/apt.13058
29. Zhang T, Cao L, Cao T, Yang J, Gong J, Zhu W, et al. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients With Crohn's Disease Undergoing Bowel Resection. JPEN J Parenter Enteral Nutr. (2017) 41:592–600. doi: 10.1177/0148607115612054
30. Ooi PH, Thompson-Hodgetts S, Pritchard-Wiart L, Gilmour SM, Mager DR. Pediatric sarcopenia: a paradigm in the overall definition of malnutrition in children? JPEN J Parenter Enteral Nutr. (2020) 44:407–18. doi: 10.1002/jpen.1681
31. Aljilani B, Tsintzas K, Jacques M, Radford S, Moran GW. Systematic review: Sarcopenia in paediatric inflammatory bowel disease. Clinical Nutrition ESPEN. (2023) 57:647–54. doi: 10.1016/j.clnesp.2023.08.009
32. Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. (2014) 58:795–806. doi: 10.1097/MPG.0000000000000239
33. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. (2011) 17:1314–21. doi: 10.1002/ibd.21493
34. Kudo W, Terui K, Takenouchi A, et al. Establishment of a quantitative assessment model and web-based calculation tool for the skeletal muscle index in children. Clin Nutr ESPEN. (2023) 58:160–4. doi: 10.1016/j.clnesp.2023.09.918
35. Schneider C A, Rasband W S, Eliceiri K W, NIH. Image to ImageJ: 25 years of image analysis. Nat Methods. (2012) 9:671–5. doi: 10.1038/nmeth.2089
36. Chiu W, Kao T, Peng T. Prevalence of sarcopenia in Asian older adults: a comparison of nine diagnostic criteria across different regions. Exp Gerontol. (2025) 202:112721. doi: 10.1016/j.exger.2025.112721
37. Ergenc I, Ismail Basa C, Uzum A, Sahin S, Kani HT, Aslan R, et al. High prevalence of muscle weakness and probable sarcopenia in patients with inflammatory bowel disease. Nutr Clin Pract. (2024) 39:557–67. doi: 10.1002/ncp.11125
38. Bryant RV, Ooi S, Schultz CG, Goess C, Grafton R, Hughes J, et al. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment Pharmacol Ther. (2015) 41:895–906. doi: 10.1111/apt.13156
39. Liu Y, Tian L. Research progress on the predictive role of sarcopenia in the course and prognosis of inflammatory bowel disease. PeerJ. (2023) 11:e16421. doi: 10.7717/peerj.16421
40. Gao Q, Mei F, Shang Y, Hu K, Chen F, Zhao L, et al. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin Nutr. (2021) 40:4633–41. doi: 10.1016/j.clnu.2021.06.009
41. Naruse T, Sato H, Takahashi K, Sato C, Kojima Y, Kawata Y, et al. Association between Clinical Characteristics and Sarcopenia or Sarcopenic Obesity in Crohn's Disease. Intern Med. (2025) 64:1451–8. doi: 10.2169/internalmedicine.4420-24
42. Massironi S, Viganò C, Palermo A, Pirola L, Mulinacci G, Allocca M, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. (2023) 8:579–90. doi: 10.1016/S2468-1253(23)00011-0
43. Yuan S, Larsson SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533
44. Yu Z, Liu Q, Chen Y, Chen D, Pan T, Kong F. Meta analysis of the influencing factors of sarcopenia in patients with Crohn's disease. Am J Med Sci. (2025) 369:605–12. doi: 10.1016/j.amjms.2024.12.010
45. [Guideline for diagnosis and treatment of sarcopenia in China (2024 edition)]. Zhonghua Yi Xue Za Zhi. (2025) 105:181203. doi: 10.3760/cma.j.cn112137-20240724-01701
46. Inoue T, Wakabayashi H, Kawase F, Kokura Y, Takamasu T, Fujiwara D, et al. Diagnostic criteria, prevalence, and clinical outcomes of pediatric sarcopenia: a scoping review. Clin Nutr. (2024) 43:1825–43. doi: 10.1016/j.clnu.2024.06.024
47. Kim J, Lee MJ, Lim HJ, Kwon Y, Han K, Yoon H. Pediatric reference values for total psoas muscle area in Korean children and adolescents. Front Pediatr. (2024) 12:1443523. doi: 10.3389/fped.2024.1443523
48. Lurz E, Patel H, Lebovic G, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle. (2020) 11:405–14. doi: 10.1002/jcsm.12514
49. Kudo W, Terui K, Hattori S, Takenouchi A, Komatsu S, Oita S, et al. Establishment and validation of reference values for abdominal skeletal muscle compartments in children. Clin Nutr. (2023) 42:653–60. doi: 10.1016/j.clnu.2023.02.022
50. Liu J, Yan Y, Xi B, Huang G, Mi J. Skeletal muscle reference for Chinese children and adolescents. J Cachexia Sarcopenia Muscle. (2019) 10:155–64. doi: 10.1002/jcsm.12361
51. Rezende IFB, Conceição-Machado MEP, Souza VS, Santos EMD, Silva LR. Sarcopenia in children and adolescents with chronic liver disease. J Pediatr. (2020) 96:439–46. doi: 10.1016/j.jped.2019.02.005
52. Saleh O, Alshwayyat S, Hares MAL, Shalan S, Alasmar D, Alkurdi O, et al. Evaluating the role of sarcopenia in adverse clinical outcomes for Crohn's disease patients: a systematic review and meta-analysis. Int J Colorectal Dis. (2025) 40:35. doi: 10.1007/s00384-025-04828-7
53. Li S, Wu H, Miao S, Huang C, Zhang Y, Shao X, et al. CT-based body composition parameters predict the loss of response to infliximab in patients with Crohn's disease. Am J Med Sci. (2025) 369:189–96. doi: 10.1016/j.amjms.2024.08.025
54. Liu J, Tang H, Lin T, Wang J, Cui W, Xie C, et al. Sarcopenia assessed by computed tomography or magnetic resonance imaging is associated with the loss of response to biologic therapies in adult patients with Crohn's disease. Clin Transl Sci. (2023) 16:2209–21. doi: 10.1111/cts.13621
55. Fang Y, Fang L, Ye M, Jiang H, Long X, Zhang H, et al. Low muscle mass is associated with efficacy of biologics in Crohn's disease. Clin Nutr. (2024) 43:2354–63. doi: 10.1016/j.clnu.2024.09.003
56. van de Pol N, Visser EH, van Noord D, van der Woude CJ, de Vries AC, de Jonge V, et al. Evaluation of an exercise program in patients with inflammatory bowel disease: a pilot study. Dig Dis Sci. (2025) 70:2097–104. doi: 10.1007/s10620-025-09030-x
Keywords: Crohn's disease, infliximab, computed tomography enterography, body composition, sarcopenia, children
Citation: Guo C, Kong Y, Wang G, Du J, Yu C and Wu J (2025) CT-Based assessment of sarcopenia and its association with biologic treatment outcomes in Chinese Children with Crohn's disease. Front. Nutr. 12:1660731. doi: 10.3389/fnut.2025.1660731
Received: 06 July 2025; Accepted: 29 September 2025;
Published: 20 October 2025.
Edited by:
Artur Delgado, Sao Paulo University, BrazilReviewed by:
Pugazhendhi Srinivasan, University of Kansas Medical Center, United StatesMirella Sherif, Cairo University, Egypt
Copyright © 2025 Guo, Kong, Wang, Du, Yu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Wu, d3VqaWVAYmNoLmNvbS5jbg==
†These authors have contributed equally to this work and share first authorship
 Cheng Guo
Cheng Guo Yan Kong†
Yan Kong†