- 1Department of Orthopedics, The Second Affiliated Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 2School of Biomedical Sciences, The University of Western Australia, Perth, WA, Australia
- 3Shenzhen Institute of Advanced Technology Chinese Academy of Sciences, Shenzhen University of Advanced Technology, Shenzhen, China
Osteoarthritis (OA) is the most common chronic joint disease worldwide. Increasing studies have confirmed that obesity, metabolic status and gut microbiota imbalance can promote the occurrence of OA through the “metabolism-inflammation-oxidative stress” network, which is closely related to daily nutrition or dietary intake. The key nutrients with therapeutic effects mainly exert anti-inflammatory, anti-oxidative and chondro-protective effects. Among them, ω-3 polyunsaturated fatty acids and polyphenols are important components of anti-inflammatory diets, while collagen peptides, vitamin D, calcium, probiotics, glucosamine, chondroitin and hyaluronic acid are commonly used in clinical practice as important nutritional support treatments or preventive measures for OA to promote cartilage repair. In terms of dietary patterns, the Mediterranean diet (MD) rich in various nutrients can be used as the basic pattern for OA patients due to its anti-inflammatory and anti-oxidative properties and good clinical effects. Based on MD and evidence from clinical studies, this review constructs a four-level progressive nutritional plan for OA patients with the goals of relieving pain, delaying cartilage degeneration, improving function, and reducing the need for drugs and surgical intervention. We have also proposed customized nutritional management strategies for several special OA populations to reduce the occurrence of nutrition-related adverse events. Collectively, systematic nutritional intervention is expected to become the third major treatment alongside physical and drug therapy, enabling more OA patients to avoid adverse effects caused by repeated drug use and potential risks associated with surgery and prosthesis replacement.
Introduction
Osteoarthritis (OA) is often misperceived as an inevitable wear-and-tear disease of aging. Yet, the startling reality is that metabolic and nutritional factors, many of which are modifiable, are now recognized as primary drivers of its pathogenesis, contributing to a global burden that afflicts over 300 million people (1). This redefinition of OA from a purely mechanical to a metabolic-inflammatory disorder opens transformative avenues for management, positioning nutritional intervention not merely as supportive care, but as a cornerstone of disease-modifying strategy.
OA stands as the most common chronic joint disease and a leading cause of disability globally (2–4). Its hallmark pathological features including cartilage degradation, subchondral bone sclerosis, osteophyte formation and synovial inflammation, are fueled by a complex interplay of inflammatory cascades, oxidative stress, and mechanical stress imbalance (5–7). While non-modifiable risks like age, gender, and genetics are well-known, emerging evidence underscores that obesity, metabolic syndrome, and gut dysbiosis are potent, modifiable risk factors, intricately linked to daily dietary patterns (8, 9).
Capitalizing on this understanding, nutritional interventions target OA through a multi-tiered approach: (1) Upstream regulation using anti-inflammatory nutrients (e.g., omega-3 fatty acids, polyphenols) to quell systemic inflammation and oxidative stress (10); (2) Downstream protection with cartilage-building substrates (e.g., collagen peptides, vitamin D) to enhance matrix integrity (11, 12); and (3) Systemic intervention through dietary modulations (e.g., weight loss, glycemic control) to ameliorate metabolic drivers and joint loading (13).
As the evidence base solidifies, the future of OA care lies in personalization, tailoring nutritional plans based on genetics, gut microbiota, and individual metabolic profiles. Therefore, this review will critically synthesize the pathophysiological links between nutrition and OA, evaluate the evidence for key nutrients and dietary patterns, and propose a framework for implementing personalized nutrition strategies to reshape the clinical management of this debilitating disease.
The pathological association between nutrition and OA
Obesity and OA: intertwined pathways of inflammation, mechanics, and diet
Obesity is a critical risk factor for OA, driven by a synergy of mechanical overload and adipose tissue-mediated systemic inflammation. Beyond simple biomechanics, where each 1 kg of weight gain multiplies knee joint load by 3–4 kg, accelerating cartilage wear and activating pathogenic pathways like Wnt/β-catenin, the endocrine function of fat is paramount (14, 15).
The pathological role of adipose tissue extends beyond mere energy storage. Pro-inflammatory adipokines (e.g., leptin, resistin) and adipose-derived exosomes (carrying miRNAs like miR-3074-5p) create a chronic inflammatory joint microenvironment by polarizing synovial macrophages toward an M1 phenotype and inhibiting anti-inflammatory adiponectin (16–18). This paradigm explains the high prevalence of OA in non-weight-bearing joints (e.g., hands) among obese individuals, a finding that cannot be attributed to mechanical load alone. Furthermore, obesity-related metabolic dysfunction exacerbates cartilage damage. Elevated palmitate induces chondrocyte apoptosis via endoplasmic reticulum stress (ERS), while a high-fat diet promotes the formation of advanced glycation end products (AGEs), which upregulate MMPs through the RAGE receptor (19–21).
Crucially, these pathways are fueled by specific dietary risk factors. The chronic consumption of a Western-style diet, which is rich in saturated fats, refined carbohydrates, and processed foods, directly promotes weight gain and establishes a state of meta-inflammation. This dietary pattern not only expands adipose tissue mass but also fundamentally alters its secretory profile, increasing the release of the very adipokines and exosomes that drive joint destruction.
However, a key counterargument persists. Not all obese individuals develop OA, and not all OA patients are obese. This suggests significant heterogeneity, likely mediated by genetic predispositions, specific adipose tissue distribution (e.g., visceral vs. subcutaneous), and other pathological factors.
Oxidative stress and cartilage damage
Oxidative stress is also an important mechanism causing cartilage damage. Oxidative stress mainly damages cartilage homeostasis through excessive accumulation of reactive oxygen species (ROS). The specific mechanisms are as follows. (1) ROS directly attacks the mitochondria of chondrocytes, causing leakage of the electron transport chain (ETC) and further generating superoxide anion (O₂−), which forms a vicious cycle, inducing collapse of mitochondrial membrane potential and ATP synthesis disorder. (2) ROS activates NF-κB and MAPK signaling pathways, upregulating the expression of MMPs (MMP-13, MMP-3) and inflammatory factors (IL-1β, TNF-α), thereby accelerating the degradation of type II collagen (Col-II) and proteoglycans. (3) ROS induces DNA oxidative damage (such as formation of 8-OHdG) and lipid peroxidation (MDA increase), triggering chondrocyte apoptosis through Bax/Bcl-2 imbalance and activation of caspase-3. (4) Furthermore, the oxidative stress imbalance, caused by excessive ROS production, impairs the antioxidant defense system by directly damaging antioxidant enzymes (such as SOD and GPx) and depleting key antioxidant molecules like glutathione, leading to fragmentation of hyaluronic acid in the extracellular matrix and abnormal collagen cross-linking, thereby reducing the mechanical properties of cartilage (6, 22, 23). The implication of this self-perpetuating cycle is that oxidative stress is not merely a bystander but an active driver of OA progression, creating a feed-forward loop where inflammation begets more oxidative stress, and vice versa.
The gut-joint axis: microbial dysbiosis and OA
Emerging evidence firmly establishes a link between gut microbiota dysbiosis and OA progression, primarily mediated by microbial metabolites via the “gut-joint axis.” The core protective mechanism involves short-chain fatty acids (SCFAs) like butyrate and propionate, produced from dietary fiber fermentation by commensals such as Bacteroides and Faecalibacterium prausnitzii. SCFAs activate GPCRs (GPR43/41) and inhibit histone deacetylases (HDAC), promoting regulatory T-cell (Treg) differentiation while suppressing Th17 cell polarization within the joint, thereby restoring immune homeostasis and reducing pro-inflammatory cytokine release. Concurrently, SCFAs enhance intestinal barrier integrity by upregulating tight junction proteins (e.g., occludin), which reduces systemic lipopolysaccharide (LPS) leakage and inhibits TLR4/NF-κB-driven synovial inflammation (24, 25). The significance of specific microbial shifts, such as an increased Firmicutes/Bacteroidetes ratio, extends beyond a mere descriptive biomarker. It implies a fundamental depletion of beneficial SCFA-producing taxa, creating a permissive environment for systemic inflammation and oxidative stress to accelerate chondrocyte apoptosis. Furthermore, the expansion of pathobionts like Peptostreptococcus can generate detrimental metabolites (e.g., indole derivatives), which directly upregulate cartilage-degrading MMP-13 (26–28).
However, several counterarguments and challenges temper the translational optimism. First, the causal direction in human studies remains ambiguous: it is unclear whether gut dysbiosis is a driver of OA or a consequence of its associated factors, such as reduced physical activity, diet changes, or chronic medication use. Second, the microbial landscape exhibits considerable inter-individual variation, suggesting that a universal “OA microbiome” signature may not exist, and interventions would likely require personalization (29). Finally, while rodent models demonstrate compelling proof-of-concept (e.g., SCFA supplementation ameliorates OA), the translatability of these findings to human physiology, with its greater complexity and longer disease course, is a significant hurdle that future research must overcome (30). Therefore, while maintaining gut microbiota stability represents a promising nutritional intervention strategy, its application necessitates a nuanced understanding of these complexities.
The synergistic effect of nutritional-related metabolic syndrome on OA
Beyond obesity alone, the constellation of nutrition-related metabolic syndrome (MetS) components including insulin resistance, dyslipidemia, and hyperglycemia, synergistically exacerbates OA progression through interconnected pathways. This creates a deleterious metabolic milieu that accelerates joint damage. Hyperglycemia drives the accumulation of advanced glycation end products AGEs, which activate the RAGE/MAPK signaling axis, thereby inhibiting Col-II synthesis and promoting a catabolic state in chondrocytes (31). Concurrently, insulin resistance and resultant hyperinsulinemia impair chondrocyte homeostasis via the PI3K/Akt pathway, leading to suppressed FOXO1 transcription factor activity. This dual dysfunction reduces glucose uptake and antioxidant defense while paradoxically activating mTORC1 to promote cellular senescence (32, 33).
The critical implication of these findings is that OA should be reconceptualized, in a significant subset of patients, as a systemic metabolic disorder rather than a purely localized joint disease. This paradigm shift underscores that therapeutic strategies must target the underlying metabolic dysfunction, not just symptomatic pain relief.
However, a key counterargument challenges a direct causative role. The clinical correlation between MetS and OA is robust, yet not all individuals with MetS develop severe OA, and vice versa. This indicates substantial phenotypic heterogeneity and suggests that metabolic factors likely act as potent disease accelerants in genetically or biomechanically susceptible individuals, rather than being universal initiators (34). Furthermore, the relative contribution of each metabolic abnormality (e.g., hyperglycemia vs. dyslipidemia) to joint damage remains poorly defined, complicating the development of targeted nutritional interventions. Disentangling these individual effects represents a crucial future direction for personalized medicine in OA.
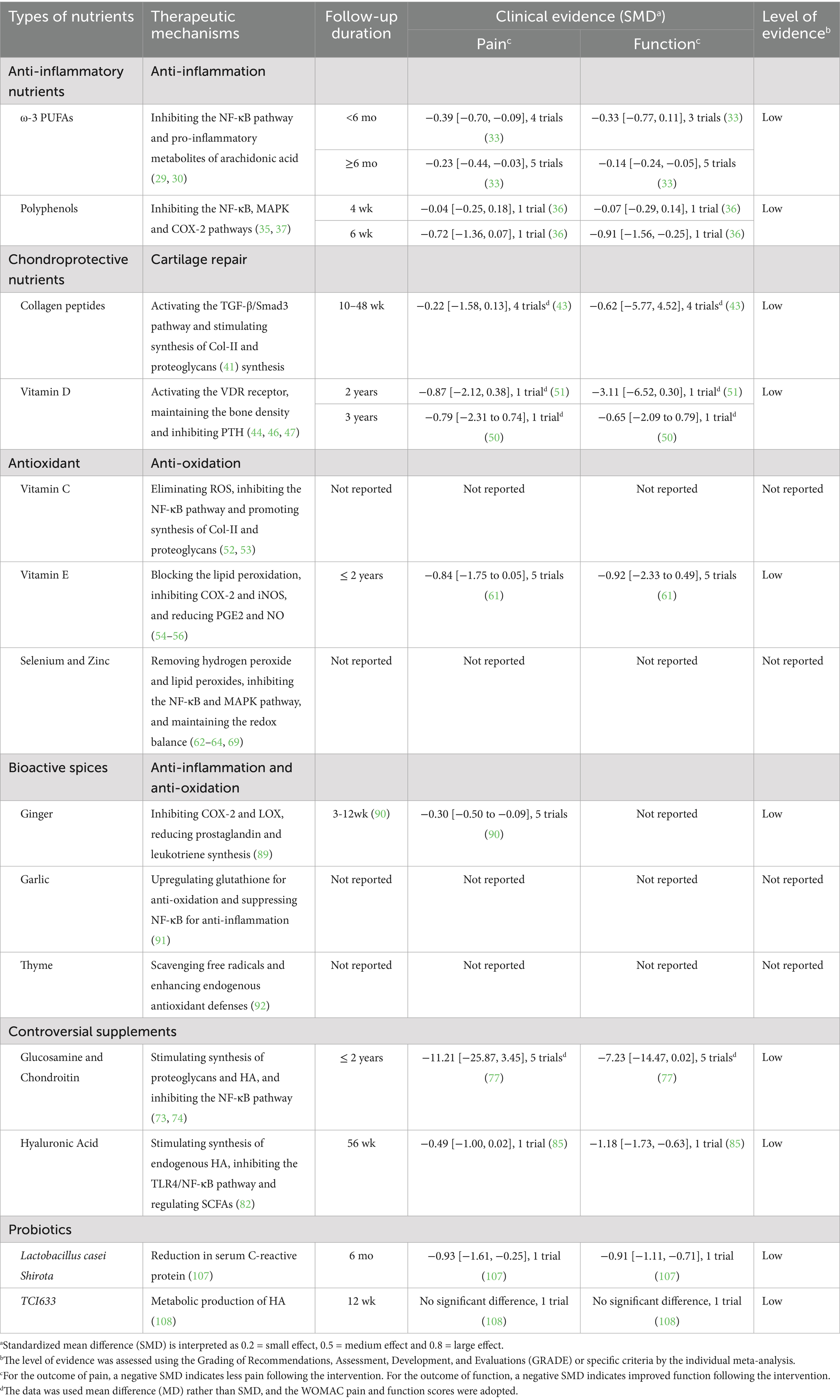
In conclusion, OA pathogenesis is propelled by a metabolically dysregulated milieu, where obesity, oxidative stress, gut dysbiosis, and broader metabolic syndrome converge to create a self-perpetuating cycle of inflammation and tissue damage (Figure 1). Future research must therefore pivot toward integrated, multi-targeted nutritional strategies that simultaneously address these interconnected pathways, moving beyond single-nutrient approaches to achieve personalized OA management (35).
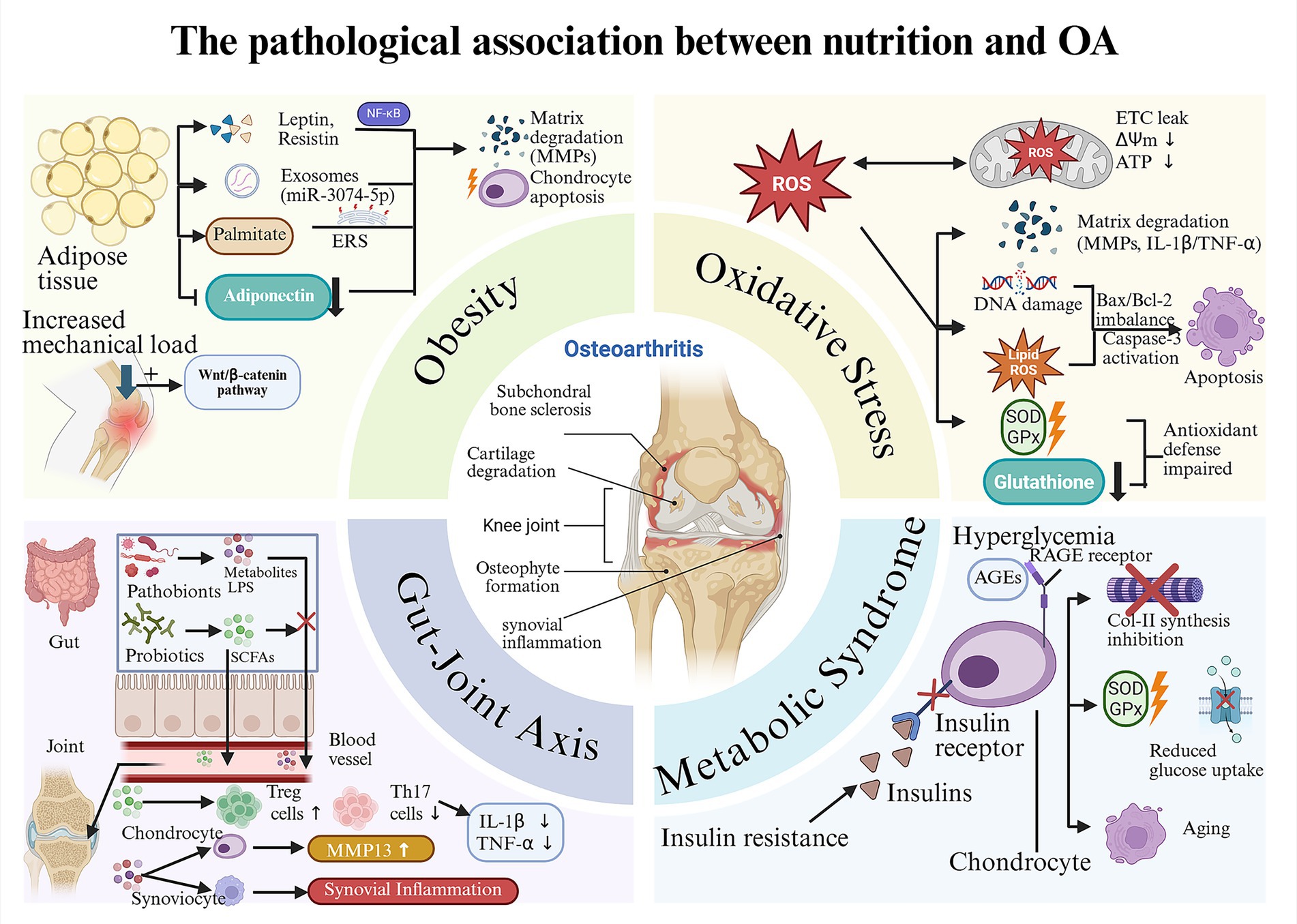
Figure 1. The pathological association between nutrition and OA: “metabolism-inflammation-oxidative stress” axis. Obesity: High-fat/high-sugar diets promote the proliferation and secretion profile alteration of adipose tissue, which further leads to the degradation of the cartilage matrix and the apoptosis of chondrocytes. The increased mechanical load caused by obesity can also accelerate cartilage wear. Oxidative stress: Unhealthy dietary habits such as high-fat/high-sugar diets can also cause cartilage damage by activating oxidative stress, which is specifically divided into the mitochondrial pathway, the inflammatory pathway, the apoptotic pathway and the antioxidant defense impairment pathway. Gut-joint axis: The imbalance between beneficial and harmful bacteria in the gut can lead to a decline in the defense capacity induced by SCFAs, subsequently causing a large amount of harmful substances (such as LPS) to enter the bloodstream. These harmful substances can cause inflammatory damage within the joint cavity. Metabolic Syndrome: Metabolic syndrome (taking insulin resistance and hyperglycemia as examples) can cause cartilage damage through interconnected pathways (the AGEs-RAGE pathway and various effects triggered by the dysfunction of insulin receptors).
Evidence-based effects of key nutrients
After understanding the pathological connection between nutrients and OA and discovering the significant role of the “metabolism-inflammation-oxidative stress” network in it, this study explores key nutrients that can prevent or treat OA through evidence-based medicine and reveals the mechanisms by which they exert preventive or therapeutic effects (Table 1).
Anti-inflammatory nutrients
ω-3 polyunsaturated fatty acids
ω-3 polyunsaturated fatty acids (ω-3 PUFAs) are a type of essential fatty acids with multiple double bonds. They are named because the first double bond is located on the third carbon atom from the methyl end. The main components include α-linolenic acid (ALA, from plants), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA, from marine sources) (36). ALA is mainly found in flaxseeds, chia seeds, and walnuts. It needs to be converted into EPA and DHA through enzymatic reactions in the human body, but the conversion rate is relatively low (about 5–10%) (37). Fish oil (such as salmon and mackerel) and algae can directly provide bioactive forms of EPA and DHA (38). By inhibiting the NF-κB pathway and competitively antagonizing pro-inflammatory metabolites of arachidonic acid (such as PGE₂ and LTB₄), they exert anti-inflammatory and cartilage-protective effects (39, 40). Beyond anti-inflammation, ω-3 PUFAs also exhibit potent antioxidant properties by activating the Nrf2 signaling pathway (41). The combined anti-inflammatory and antioxidant effects converge to achieve chondroprotection (Figure 2). Flaxseeds, as a plant-based source of ALA, are more suitable for vegetarians. However, their anti-inflammatory effects are often inferior to direct supplementation of EPA/DHA due to the limited conversion efficiency (37). A randomized controlled trial (RCT) demonstrated that after 6 months of supplementation with 1.2 g/d of EPA + DHA for patients with knee OA, the WOMAC pain score decreased by 23% (p = 0.02), and the synovial inflammation assessed by MRI was relieved (42). A systematic review (including 9 RCTs) confirmed that ω-3 PUFAs could alleviate the pain symptoms of OA patients (p = 0.002) and improve joint function (SMD = −0.21) (43). The consistent symptom-modifying effect across trials strongly implies that ω-3 PUFAs are a viable dietary strategy for managing OA-related pain, particularly for individuals seeking or requiring adjunctive approaches to pharmacotherapy.
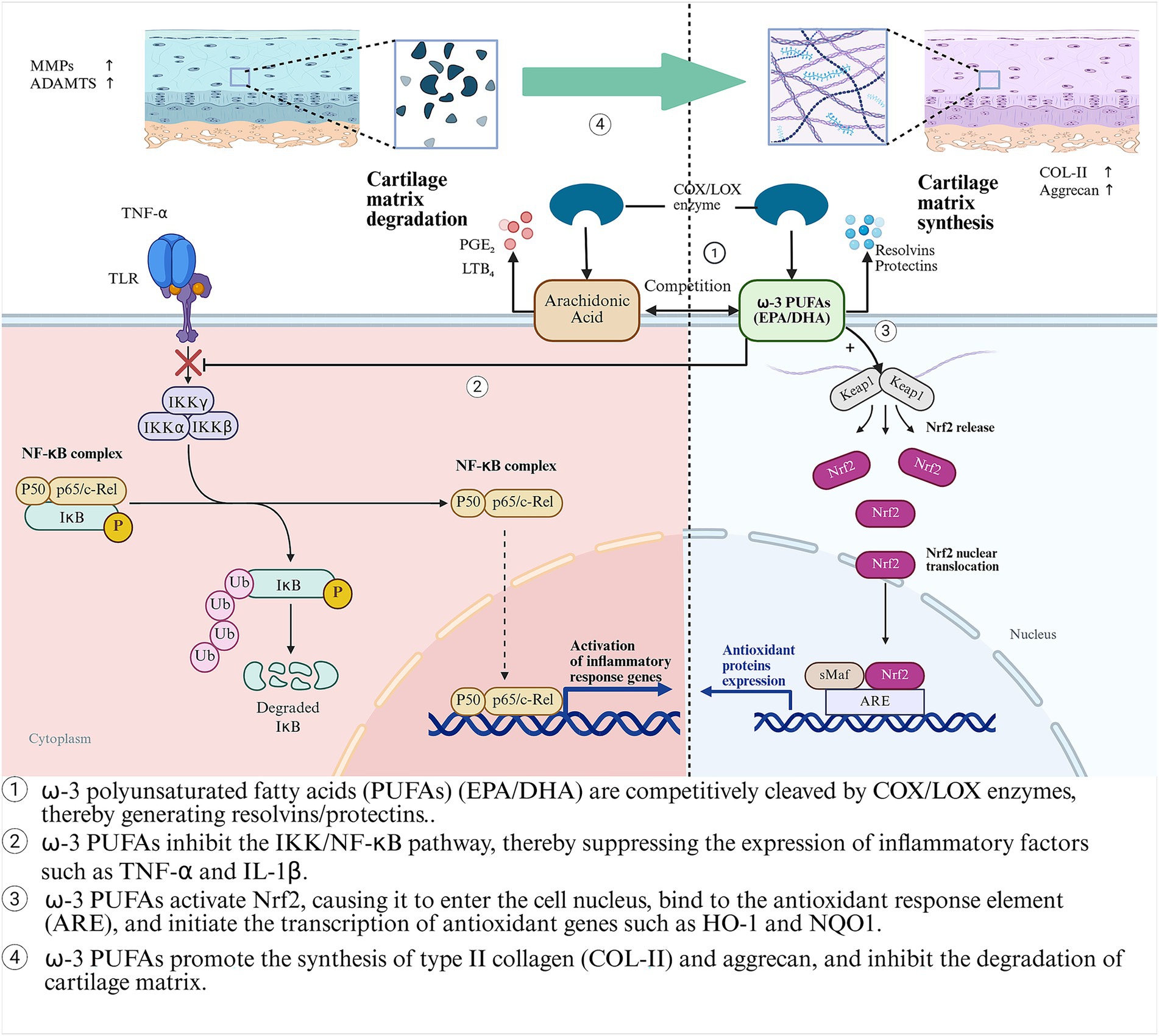
Figure 2. The anti-inflammatory, antioxidant and cartilage-protective mechanisms of ω-3 PUFAs (EPA/DHA) in OA.
However, significant heterogeneity in EPA/DHA dosing (0.5–3 g/d) and ratios across studies, short trial durations (typically ≤6 months), and a lack of robust structural outcome data (e.g., joint space narrowing) have precluded their formal recommendation as a standard adjunctive treatment in clinical guidelines (44). This evidence gap highlights a critical disconnect between compelling biological mechanisms and the level of proof required for widespread clinical implementation. Therefore, future research must prioritize large-scale, long-term (≥2 years) RCTs employing standardized EPA/DHA formulations and doses targeted to achieve specific tissue or plasma levels. These studies should explicitly address whether ω-3 PUFAs offer disease-modifying effects by incorporating advanced imaging biomarkers of cartilage integrity. Ultimately, defining the optimal dosing regimen and identifying patient subgroups most likely to respond will be key to translating this promising nutritional intervention into personalized OA care.
Polyphenols
Polyphenols are a type of natural organic compounds widely present in plants. They possess multiple phenolic hydroxyl structures and are mainly classified into flavonoids, phenolic acids, curcuminoids, and stilbenes. Curcumin is the main active component in turmeric roots (belonging to the curcuminoid category). Studies have shown that it can reduce the release of pro-inflammatory factors by inhibiting the NF-κB and COX-2 pathways, and activate Nrf2 to enhance the activity of antioxidant enzymes (45). Even more, some polyphenols can act as plant estrogens or SERMs to alleviate the cartilage degeneration associated with menopausal OA (46). A double-blind RCT showed that after 8 weeks of oral administration of 1.5 g/d curcumin (containing piperine) to patients with knee OA, the WOMAC pain score significantly decreased (p = 0.03). The effect was comparable to that of ibuprofen and was safer (47). Resveratrol is a type of stilbenes polyphenol that can be found in grape skins, berries, and peanuts. It inhibits the NF-κB and MAPK signaling pathways by activating the SIRT1 deacetylase, reduces ROS production, and delays chondrocyte apoptosis (48). Clinical studies have shown that a 500 mg daily dose of resveratrol for 3 months can significantly reduce the VAS score, but the improvement in joint structure is limited (49).
The compelling safety profile of these polyphenols, especially when compared to non-steroidal anti-inflammatory drugs (NSAIDs), implies their prime utility as long-term adjunctive therapies or for individuals intolerant to conventional pharmacotherapy. However, a central counterargument revolves around the issue of low oral bioavailability, particularly for curcumin. The promising effects observed in vitro often require sophisticated formulations (e.g., with piperine, nanoparticles) to be partially translated in humans, raising questions about the efficacy of simple dietary intake or standard extracts (50). Consequently, future research directions must extend beyond efficacy confirmation. The priority lies in optimizing delivery systems to enhance bioavailability and conducting longer-term trials with structural outcomes as primary endpoints. Furthermore, exploring synergistic effects, either between different polyphenols or with conventional treatments, and identifying biomarkers to predict individual responses are crucial steps toward personalized nutrition strategies for OA.
Chondro-protective nutrients
Collagen peptide
Collagen peptides are small molecular fragments of collagen (with a molecular weight of approximately 2–5 kDa) obtained through enzymatic processing. They mainly come from the cartilage, bones, and skin of cows, fish, or chickens, and have high bioavailability. The potential mechanisms of collagen peptides in the prevention or treatment of OA are as follows. (1) Stimulate chondrocytes to synthesize Col-II and proteoglycans, while inhibiting the activities of MMP-13 and ADAMTS-5, and reducing matrix degradation. (2) Promote cartilage repair by activating the TGF-β/Smad3 signaling pathway. (3) Inhibit the release of synovial inflammatory factors (51). Although there are not many studies on the relevant mechanisms, collagen peptides have still gained popularity among some patients with OA. A RCT showed that daily supplementation with 10.78 g of collagen peptide (COLLinstant®) for 6 months could significantly reduce the VAS pain score of knee OA patients (an average reduction of 43.6%, p < 0.001) and improve joint mobility (p < 0.02) (52). A meta-analysis involving 5 RCTs (with a total of 519 participants) indicated that the collagen peptide group showed significant superiority over the placebo group in terms of pain relief (WMD: −16.57, p < 0.001) and stiffness improvement (−0.41, p = 0.01), but the long-term impact on joint function still requires further verification (53). The consistent symptom-modifying effect, coupled with an excellent safety profile, positions collagen peptides as a well-tolerated adjunct to core OA management strategies like exercise and anti-inflammatory diets. However, a key counterargument hinges on the lack of robust evidence for disease-modifying effects. Most trials are of short duration and utilize varied sources and doses of collagen peptides (e.g., 8–12 g/d), and crucially, they lack convincing imaging data demonstrating a halt in cartilage loss or joint space narrowing (52). This suggests that while collagen peptides are effective for symptom control, their ability to structurally alter OA progression remains an open and critical question. Therefore, future research must prioritize long-term (≥2-year) RCTs with standardized, well-characterized preparations, using quantitative MRI biomarkers of cartilage volume or composition as primary endpoints. Additionally, exploring potential synergies with other anabolic agents (e.g., vitamin D) and identifying patient subgroups most likely to respond (e.g., based on baseline collagen turnover markers) will be essential for advancing personalized nutrition protocols in OA.
Vitamin D and calcium
Vitamin D (a fat-soluble vitamin) and calcium (an essential mineral) are crucial nutrients for maintaining bone and joint health. Vitamin D, obtained through skin synthesis and dietary sources (e.g., fatty fish, fortified foods), must undergo a two-step hydroxylation in the liver and kidneys to become its active form, 1,25-dihydroxyvitamin D₃ [1,25(OH)₂D₃]. Crucially, the enzymes facilitating this activation process are magnesium-dependent, underscoring the mineral’s essential role in vitamin D metabolism (54). Calcium is mainly found in dairy products, dark green vegetables and legumes. It is the main component of bones and teeth, and also participates in muscle contraction and nerve signal transmission (55). Vitamin D and calcium exert protective effects on OA by jointly regulating the bone-cartilage homeostasis. (1) Vitamin D, by activating the VDR receptor, inhibits the expression of inflammatory factors in chondrocytes and reduces the activity of MMP-13, while promoting the synthesis of Col-II (56). (2) Calcium maintains the bone density beneath the cartilage and prevents joint mechanical imbalance caused by abnormal bone remodeling. (3) Active vitamin D enhances intestinal calcium absorption (57). The two jointly inhibit the increase of parathyroid hormone (PTH), and reduce the risk of bone resorption and cartilage calcification (58). Clinical studies have shown that patients with OA who have a serum 25(OH)D3 level of less than 15 ng/mL experienced significantly increased pain, especially in male patients (p ≤ 0.003) (59). And the risk of OA progression has increased by more than twice (OR: 2.3; 95% CI: 1.1, 4.5) (60). Therefore, many clinical doctors recommend that patients with OA take appropriate supplements of vitamin D and calcium. The current recommended dosage for patients with OA is 1,000–2000 IU of vitamin D per day to maintain serum 25(OH)D3 at 30 ng/mL or higher (61, 62), and 1,000 mg of calcium per day to optimize bone and cartilage protection (63). However, many studies have found that supplementation of 2000 IU + 1,000 mg/d did not improve the symptoms (pain, stiffness, and limited mobility) of OA or delay the progression of the disease within a certain period (2–3 years) (64, 65). It can be seen that there is heterogeneity in the effect of vitamin D and calcium supplementation on symptom improvement, which may be partly explained by variable magnesium status among participants and the different follow-up time. Therefore, long-term RCTs and precise baseline control are necessary for studying the long-term efficacy and complications of vitamin D and calcium.
Antioxidant
Vitamin C and E
Vitamin C (ascorbic acid) and vitamin E (tocopherol) are two crucial antioxidant nutrients that protect cartilage from oxidative damage by water-soluble and lipid-soluble status, respectively. Vitamin C is widely found in citrus fruits and green leafy vegetables. As a cofactor of proline hydroxylase, it directly promotes the synthesis of Col-II and proteoglycans (66). And by eliminating ROS and inhibiting the NF-κB pathway, it reduces mitochondrial oxidative damage in chondrocytes and the expression of inflammatory factors such as MMP-13 (67). Vitamin E, as a fat-soluble antioxidant, is mainly found in nuts and seed oils. It can protect the integrity of cell membranes by blocking the lipid peroxidation chain reaction (68), and can also inhibit activities of COX-2 and iNOS and reduce the production of PGE2 and NO to alleviate synovial inflammation (69, 70). Clinical studies have shown that high dietary intake of vitamin C (≥ 200 mg/d) can inhibit the progression of OA (OR = 0.3, 95% CI: 0.1–0.6) and reduce knee joint pain (OR = 0.3, 95% CI: 0.1–0.8), but it has no significant effect on preventing the occurrence of OA (71). On the contrary, Peregoy et al. proposed a different conclusion. They believed that supplementing vitamin C had certain positive significance for preventing OA, but it cannot inhibit the progression of OA (72). At present, there is still controversy regarding the effect of vitamin C on OA. Therefore, in clinical practice, most doctors do not emphasize the therapeutic significance of vitamin C supplementation for OA. Studies have shown that combined supplementation of vitamin C (1 gram twice daily) and E (100 mg three times daily) can improve the pain score of OA patients (73). The combined application can significantly enhance the antioxidative performance compared to single use. However, the long-term risks and therapeutic effects of the combined application still need to be further evaluated due to the lack of relevant RCTs. But studies have shown that the long-term use of vitamin E should be carefully considered. It may increase the risk of bleeding by 58% (74), and the therapeutic effect is not significant (75).
Selenium and zinc
Selenium and zinc are essential trace elements for the human body. Selenium is incorporated into glutathione peroxidase (GPx) in the form of selenocysteine, which removes hydrogen peroxide (H₂O₂) and lipid peroxides (such as MDA), reducing oxidative damage to chondrocytes. At the same time, it inhibits the NF-κB pathway and reduces the expression of IL-1β and MMP-13 to protect Col-II. It can also maintain the redox balance of chondrocytes through sequestin P (76–78). The dietary sources of selenium include Brazil nuts, seafood and whole grains. A Cross-Sectional Study showed that patients with lower serum selenium levels (<100 micrograms/L) were more likely to develop OA (OR ≥ 1.24), and there was a dose–response relationship (p = 0.005) (79). The result of a RCT demonstrated that daily supplementation with 200 μg of selenium (selenium-containing methionine) for 12 weeks had a certain positive effect in reducing the ESR level of RA patients [the effect size (95% CI) for ESR: 0.38 (−0.14, 0.89)], but it had no significant effect on reducing the serum CRP level (p = 0.05) (80). Although there are very few RCTs on the application of selenium in OA treatment, selenium supplements are not currently recommended for OA treatment considering the potential adverse effects of long-term use (5 years) (81). Appropriate food intake is supported [55–200 mg/d (maximum 400 mg/d)] (82). Zinc, acting as a cofactor for superoxide dismutase (SOD) and metallothionein (MT), neutralizes superoxide anions (O₂−) and inhibits MAPK inflammatory pathways (83), which helps protect the mitochondrial function of chondrocytes and reduces the degradation of cartilage matrix. It is commonly found in red meat, shellfish and beans. Although antioxidative and anti-inflammatory properties of zinc are more beneficial for the prevention and alleviation of OA theoretically, in fact, current mainstream clinical studies suggested that zinc supplementation and high serum concentrations were more likely to increase the risk of OA [p < 0.001, OR 95% CI = 1.044 (1.021–1.067)] (84, 85). The researchers believed that both zinc deficiency and excess were detrimental to bone health and immune function, and thus can accelerate the progression of OA (86). However, there is still a lack of sufficient evidence regarding the optimal concentration range of zinc and its actual therapeutic effect. Therefore, zinc supplementation is not recommended as a feasible treatment for OA. The dietary intake should be maintained at the recommended levels of 8–11 mg per day for adults (87), with a tolerable upper intake level of 40 mg per day to avoid potential toxicity (88).
Bioactive spices
Beyond isolated nutrients, common culinary spices such as ginger, garlic, and thyme are rich sources of bioactive compounds with demonstrated efficacy in mitigating OA-related pathways. Their pleiotropic effects align perfectly with the metabolism-inflammation-oxidative stress network.
Ginger and its bioactive derivatives
The primary bioactive components, gingerols and shogaols, exhibit potent anti-inflammatory activity by inhibiting COX-2 and lipoxygenase (LOX), thereby reducing prostaglandin and leukotriene synthesis (89). A meta-analysis of randomized controlled trials confirms that ginger extract supplementation confers a modest but statistically significant reduction in knee pain and stiffness compared to placebo, with an effect size comparable to some conventional analgesics (90). The implication of these findings is that ginger represents a viable complementary strategy for OA symptom management, potentially enabling a reduction in conventional analgesic use. However, counterarguments highlight the heterogeneity in ginger preparation (dose, extract type) across studies and its moderate effect size, which may not be clinically meaningful for all patients. Gastrointestinal discomfort is also a noted side effect. Future research should prioritize standardized, high-quality extracts and focus on defining which patient phenotypes respond best to ginger intervention. Crucially, investigations into whether its anti-inflammatory effects translate to long-term disease modification beyond symptomatic relief are warranted.
Garlic and its organosulfur compounds
Garlic, a widely used culinary herb, has been recognized for its medicinal properties, primarily attributed to organosulfur compounds like allicin, diallyl disulfide (DADS), and S-allyl cysteine (SAC). These bioactive constituents mediate antioxidant effects by upregulating cellular glutathione levels and exhibit anti-inflammatory activity through suppression of the NF-κB signaling pathway, thereby potentially protecting chondrocytes from inflammatory and oxidative stress (91). While preclinical evidence for these mechanisms is robust, direct high-quality clinical evidence specifically for OA remains limited. A few pilot studies and investigations in related conditions, such as rheumatoid arthritis, suggest beneficial effects on inflammatory markers, but large-scale, long-term RCTs in OA populations are lacking. The implication of these mechanistic findings is that garlic or its standardized extracts could serve as a dietary component for mitigating OA pathophysiology. However, a significant counterargument revolves around the bioavailability and stability of allicin, which is highly volatile and rapidly metabolized. This raises questions about the consistency and efficacy of common garlic preparations. Future research must focus on employing well-characterized, stable garlic extracts with defined sulfur compound profiles in rigorous clinical trials. Furthermore, exploring potential synergistic effects of garlic with other nutraceuticals (e.g., ginger, turmeric) within the “Metabolism-Inflammation-Oxidative Stress” network represents a promising direction for developing multi-targeted dietary interventions.
Thyme and its bioactive monoterpenes
Thyme, a culinary and medicinal herb from the Lamiaceae family, is rich in bioactive monoterpenes, primarily thymol and carvacrol. These compounds exhibit potent antioxidant activity by directly scavenging free radicals and enhancing endogenous antioxidant defenses (92), which could help mitigate the oxidative stress and catabolic processes central to OA progression. However, a major counterargument is the significant gap between in vitro findings and the scarcity of in vivo or clinical evidence for OA. Challenges include the bioavailability of orally administered compounds and the translation of effective in vitro doses to practical human intake. Future research should prioritize well-designed animal studies to confirm efficacy in a whole-organism context. If promising, subsequent clinical trials should utilize standardized thyme extracts with defined chemical profiles to evaluate their effects on OA symptoms and structural progression, potentially as part of a broader dietary approach.
Controversial supplements
Glucosamine and chondroitin
Glucosamine and Chondroitin are important components of cartilage matrix. Glucosamine, as an amino sugar precursor, has been reported by some studies to stimulate the synthesis of proteoglycans and hyaluronic acid (HA) by chondrocytes, inhibit the activities of MMP-3 and ADAMTS-5, and reduce the degradation of cartilage matrix (93). Chondroitin sulfate reduces the release of IL-1β and TNF-α by competitively inhibiting the NF-κB pathway, while increasing the synthesis of Col-II and glycosaminoglycans (GAGs) (94). The combined use of the two can exert a better protective effect on cartilage (95). A clinical study showed that for patients with knee OA, supplementation with 1,500 mg of glucosamine and 1,200 mg of chondroitin for 6 months resulted in better pain relief for patients with moderate to severe pain (WOMAC ≥ 301) compared to the placebo group (p = 0.002), but there was no significant difference in the overall population (96). The results of a meta-analysis that included 8 RCTs (n = 3,793) showed that combined treatment significantly improved the WOMAC score [MD = −12.04 (−22.33 ~ −1.75); p = 0.02], but there was no significance in the improvement of joint space narrowing [MD = −0.09 (−0.18 ~ −0.00); p = 0.04] and the WOMAC stiffness score [MD = −4.70 (−8.57 ~ −0.83); p = 0.02] (97). Although there are still some studies that affirm the effect of the combined use of glucosamine and chondroitin in alleviating the pain of OA (98), most studies believe that the combined treatment has no value in improving the joint structure of OA patients, and there is significant heterogeneity in terms of symptom relief (99, 100). Therefore, the OARSI guidelines conditionally recommended the use of glucosamine and chondroitin for alleviating symptoms of knee OA (with evidence level B), while the ACR guidelines did not include it in their routine recommendations. Glucosamine and chondroitin have good tolerance and no serious side effects have been observed so far. However, some studies suggest that the long-term side effects need to be further investigated (101). We suggest that under the guidance of a doctor, OA patients can try taking them for 3 to 6 months. If there is no effect, the use should be discontinued. Products with high purity and standard dosage (such as crystalline glucosamine sulfate) should be preferred to optimize potential effects.
Oral hyaluronic acid
Oral hyaluronic acid (HA) is a form of different molecular weights (typically < 500 kDa) HA. It can be partially absorbed through the intestinal tract or metabolized by the gut microbiota into active fragments, which stimulate the synthesis of endogenous HA by synovial cells and chondrocytes, thereby increasing the viscosity of synovial fluid. At the same time, it can inhibit the TLR4/NF-κB pathway to reduce the expression of IL-6, IL-8 and COX-2, and alleviate joint inflammation and oxidative stress. It can also indirectly improve the joint microenvironment by regulating the production of SCFAs (102). The tolerance of HA is good and there are currently no reports of adverse effects (103). A 12-month RCT showed that oral HA could improve the clinical symptoms of OA patients aged 70 and below when combined with muscle strength training (104). Another RCT demonstrated that oral administration of large-molecular-weight HA can alleviate pain and improve function in patients with OA in the short term, and can also reduce the use of NSAIDs and painkillers (105). However, there are few reports on the significance in changing the thickness of cartilage. Currently, there are still many doubts about the treatment mechanisms of oral HA, so intra-articular injection of HA is more recommended in clinical practice to better exert its effect (evidence level Ib) (106).
Probiotics
Probiotics refer to live microorganisms that, when consumed in sufficient quantities, provide benefits to the host’s health. The OA-related strains mainly come from fermented foods or commercial preparations and have the ability to resist stomach acid, bile, and establish a good colonization in the intestines. Their potential mechanisms in the prevention and treatment of osteoarthritis are as follows: (1) By restoring the balance of the intestinal flora, enhancing the integrity of the intestinal barrier, reducing LPS entry into the bloodstream, and lowering systemic low-level inflammation. (2) Inhibiting the NF-κB signaling pathway, down-regulating pro-inflammatory factors such as TNF-α, IL-6, and IL-1β, and reducing serum hs-CRP levels. (3) Inducing the differentiation of Treg, improving the immune microenvironment of the joint synovium, and indirectly slowing down cartilage degradation. Although the mechanism research is still ongoing, probiotics have attracted the attention of OA patients due to the “gut-joint axis” concept. Clinical studies have shown that specific strains, such as Lactobacillus casei Shirota (LcS), can significantly improve the WOMAC pain score (p < 0.01) in patients with OA and reduce the level of hs-CRP (107). The thermophilic Streptococcus TCI633 has shown the potential to delay the progression of OA biomarkers (such as sCTX-II), although this study has limitations due to its short duration and small sample size (108).
At present, there are several controversies in this field. Firstly, the effects of probiotics show significant strain-specificity and individual variability. Not all probiotics are effective for OA, and their efficacy largely depends on the initial intestinal flora composition of the host (109). Secondly, most of the current human studies are still at a preliminary stage. The results (such as the insignificant pain improvement in the TCI633 study) suggest that probiotics may mainly function to delay the pathological process rather than quickly alleviate symptoms. Moreover, the long-term safety of probiotics in vulnerable populations still requires vigilance. Therefore, future research should focus on conducting large-scale, long-term, and targeted RCTs on specific OA phenotypes, with the aim of clarifying whether the effects of probiotics have disease-modifying effects. Key directions include using metagenomics to identify baseline characteristics of the gut microbiota that can predict therapeutic efficacy, developing next-generation synthetic microbiota or probiotic-prebiotic consortia for OA, and in-depth exploration of the safety of inactivated probiotics and their metabolites (postbiotics) (110). At present, it is recommended to choose strains that have evidence-based support and to combine them with a high-fiber diet to maximize their benefits.
Dietary patterns and OA treatment
After understanding the mechanisms and evidence-based effects of key nutrients in preventing or treating OA, dietary patterns rich in beneficial nutrients have also received increasing attention. Next, we will investigate the characteristics of different dietary patterns and their potential impact on OA to assess the potential benefits and risks (Table 2).
The anti-inflammatory property of the Mediterranean diet and clinical studies
The Mediterranean Diet (MD) is characterized by high intake of olive oil, fish, whole grains, fruits, vegetables and nuts, which exerts anti-inflammatory and antioxidative effects through the synergistic effects of multiple components. The core component, extra virgin olive oil, is rich in oleocanthal, which inhibits COX-1/2 activity (similar to the mechanism of ibuprofen), and reduces the production of PGE₂ and LTB₄. In addition to oleocanthal, the MD is also abundant in various polyphenolic compounds, which confer synergistic benefits by targeting multiple pathways relevant to OA pathogenesis, including inflammation, oxidative stress, and cartilage metabolism (111–116) (Table 3). Omega-3 fatty acids (from fish) downregulate the NF-κB pathway and reduce IL-6 and TNF-α. Dietary fiber promotes the production of SCFAs by gut bacteria, enhancing the anti-inflammatory function of Treg cells (117, 118). Generous use of aromatic herbs and spices like garlic, ginger, and thyme further reduces systemic inflammation and oxidative stress. Clinical studies have shown that strict MD (Q5) can significantly reduce the risk and progression of OA (RR = 0.91; 95% CI: 0.82–0.998) (119). MD can significantly reduce the levels of IL-1β (~47%, p = 0.010) and sCOMP (1 U/L, ~8%, p = 0.014) in the blood of OA patients, and improve their mobility of joint (p < 0.05) (120). When compared with a low-fat diet, MD could better alleviate the pain of OA patients (p = 0.04), which indicates the unique anti-inflammatory property of MD that distinguishes it from general weight loss diets. Considering that weight loss remains the first-line treatment for OA, especially for obese patients (121), and MD, as a basic dietary pattern, can simultaneously achieve weight loss and anti-inflammatory effects, thus, it has certain therapeutic value for OA patients.
However, a key counterargument is that the observed benefits in observational studies may be confounded by the overall healthier lifestyle of MD adherents, who typically exhibit higher physical activity levels and lower rates of smoking. Furthermore, the long-term sustainability of the MD in non-Mediterranean populations poses a significant challenge for widespread implementation, and the specific contribution of its individual components remains difficult to isolate. Therefore, future research should prioritize long-term, randomized controlled trials designed to disentangle the effects of the MD from other healthy behaviors. Key directions include investigating the diet’s impact on structural disease progression using advanced imaging and exploring culturally adapted, supported behavioral interventions to enhance adherence and translate this promising dietary pattern into real-world clinical practice for diverse OA populations. Even more, by conducting a detailed analysis of the effects and proportions of each component in the MD diet, we can make specialized adjustments for different patients to balance the therapeutic effect and the acceptance.
The efficacy of low-calorie diets on obesity-related OA
Low-Calorie Diet (LCD) induces weight loss by restricting daily energy intake (typically 1,200–1,500 kcal), which plays a dual protective role in obesity-related OA. (1) It reduces mechanical load, thereby decreasing cartilage wear and abnormal stress on subchondral bone (13). (2) It regulates metabolic inflammation. The reduction of visceral fat down-regulates pro-inflammatory adipokines (leptin, resistin), up-regulates anti-inflammatory adiponectin, and inhibits the NF-κB pathway and the release of IL-1β and TNF-α in synovial macrophages. Simultaneously, it improves insulin sensitivity and reduces the damage of AGEs to cartilage (122). The IDEA (Intensive Diet and Exercise for Arthritis) trial (n = 454) demonstrated that obese patients with knee OA who received diet-related weight loss combined with exercise for 18 months showed significant improvements in pain (p < 0.05) and function (p < 0.005) (123). In addition to the symptoms improvements, clinical studies have shown that weight loss induced by a low-calorie diet can increase the concentration of adiponectin in the serum (p = 0.0480) and decrease the concentration of COMP (p < 0.0001) (124), which indicated a lower risk of cartilage damage. From an imaging perspective, weight loss was observed to reduce the number of pixels in the cartilage layer on T2 images (p = 0.001), which was associated with the alleviation of pain (p = 0.02) and function (p = 0.03). A weight loss of more than 10% significantly inhibited the progression of OA over the 48-month follow-up period (125). In conclusion, low-calorie diets and weight loss have a good therapeutic effect and have been classified as strong recommended treatments for obesity-related OA. However, the counterargument lies in the challenge of long-term sustainability. While efficacy is clear in controlled trials, real-world adherence to stringent calorie restriction is often poor, with high rates of weight regain. This raises questions about the practicality of LCD as a standalone long-term strategy for most individuals. Furthermore, weight loss alone may not fully resolve the dysregulated metabolic and inflammatory milieu in all patients, particularly those with longstanding OA, indicating the need for complementary anti-inflammatory interventions.
Therefore, future research should pivot toward sustainable dietary patterns that incorporate LCD principles but focus on food quality and satiety (e.g., MD) to enhance long-term adherence. Key directions include investigating the role of body composition changes (preserving muscle mass during weight loss) on clinical outcomes and developing personalized nutritional approaches based on metabolic phenotypes to identify those most likely to benefit from specific dietary interventions. When choosing a low-calorie diet, it is important to supplement an appropriate amount of protein [≥1.2 g/kg/d (126)] and combine functional exercises to maintain muscle strength.
The potential benefits and risks of a vegetarian diet
Vegetarianism (or plant-based diet) is a dietary pattern that mainly consists of foods derived from plants, excluding or strictly limiting animal ingredients such as meat, poultry, and fish. The core foods include vegetables, fruits, whole grains, beans, nuts, and seeds (127). The high intake of fruits, vegetables, legumes and nuts in a vegetarian diet provides abundant polyphenols (such as quercetin) and vitamin C, which inhibit the NF-κB pathway and COX-2 activity, and reduce inflammatory markers such as IL-6 and CRP (128). Meanwhile, the low energy density diet represented by vegetarianism helps with weight loss and reduces the mechanical load on joints (129). A vegetarian diet also reduces the intake of AGEs (such as grilled meats) and minimizes the damage to cartilage caused by the AGEs-RAGE axis (130). Although there are certain benefits, the vegetarian pattern still has some risks and controversies. Firstly, long-term vegetarianism can lead to nutrients deficiencies. Among them, vitamin B12 deficiency causes an increase in homocysteine levels (131), which may exacerbate oxidative stress and apoptosis of chondrocyte. The absorption rate of zinc combined with phytate and plant-based non-heme iron is low, which may weaken the activities of SOD/GPx (132). Strict vegetarians may suffer from EPA/DHA deficiency (133), and they need to rely on the conversion of ALA, which might affect the anti-inflammatory effect of ω-3 PUFAs. Secondly, the lack of essential amino acids (such as lysine) in plant proteins may reduce the synthesis of cartilage matrix (134). Currently, multiple RCT studies have shown that the vegetarian diet can significantly improve the WOMAC scores (p ≤ 0.0001) and reduce inflammatory indices in the short term (from 4 months to 1 year) (135, 136). However, long-term follow-up studies have revealed that this dietary pattern increased the risk of osteoporosis and fractures (137, 138), which is likely to accelerate the progression of OA. Therefore, it is recommended to adopt an elastic vegetarian diet (such as a diet mainly consisting of plants with the addition of fish, eggs, and dairy products) for the management of OA, and avoid strict vegetarianism. At the same time, regular monitoring of serum B12, ferritin and zinc levels should be conducted, and supplementation should be provided when necessary.
The regulatory effect of intermittent fasting on metabolism and inflammation
Intermittent Fasting (IF) is a periodic energy intake restriction pattern, which includes alternate eating (5:2 pattern, restricting calorie intake to 500–600 kcal on 2 days per week) and fasting periods (such as 16:8 pattern, fasting for 16 h each day). IF may exert therapeutic effects through the following three mechanisms. One is metabolic regulation. During fasting periods, insulin secretion is reduced, the AMPK pathway is activated, and glucose uptake and fatty acid oxidation are increased, which will improve insulin resistance and obesity-related OA (139). At the same time, fasting induces the expression of key genes for autophagy (ATG5/LC3), removes damaged mitochondria and misfolded proteins, and inhibits apoptosis of chondrocytes (140, 141). The second effect is anti-inflammation. Beyond reducing visceral fat and suppressing the NF-κB pathway (142), emerging evidence suggests IF may directly inhibit the activation of the NLRP3 inflammasome, a key sensor of metabolic stress that drives the production of IL-1β (143). Thirdly, by enhancing the activity of antioxidant enzymes (SOD, GPx), IF can reduce the generation of ROS in mitochondria and block the oxidative cascade reaction (144). At present, there are not many reports on the use of IF in the treatment of OA (145). Therefore, the actual efficacy and long-term safety are not definite. However, some studies have shown that for patients with type II diabetes and a history of taking antidiabetic medications, the use of IF should be avoided, as there is a risk of causing hypoglycemia (146). In a word, it is not recommended to use IF to achieve weight control and OA treatment, especially for patients with diabetes, eating disorders, malnutrition, sarcopenia, pregnancy, and adolescent OA.
Nutrition management for special OA populations
Although many nutrients and dietary patterns can exert certain therapeutic or preventive effects on OA by regulating metabolism, inflammation and oxidation, and maintaining cartilage homeostasis, the application of them still requires individualized adjustments to avoid the occurrence of other nutrition-related adverse events. Therefore, in the following content, we will provide customized nutrition management strategies for special OA populations.
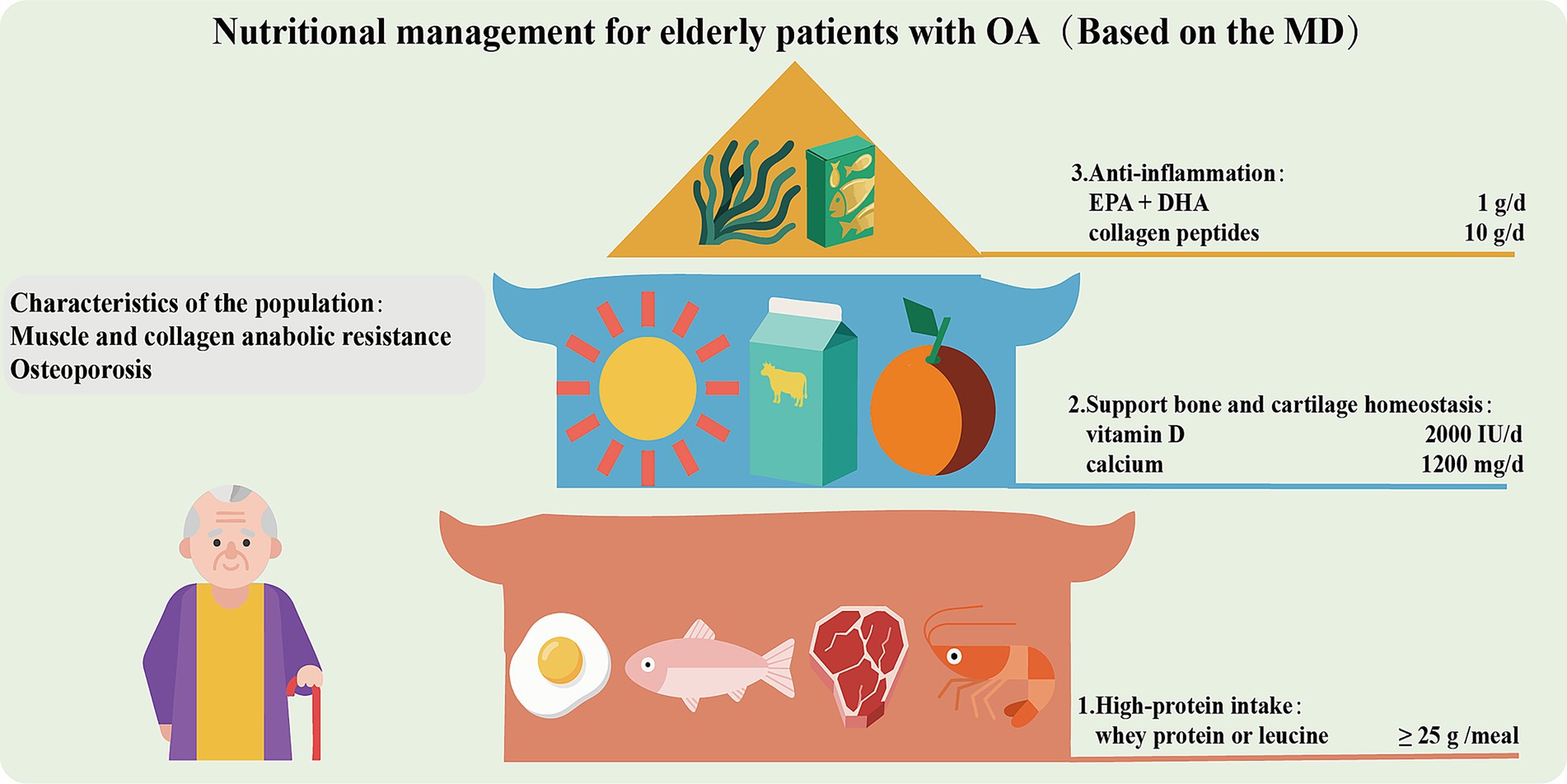
Nutritional management for elderly patients with OA
In elderly patients with OA, the decline in growth hormone and insulin-like growth factor 1, and the impaired differentiation of osteoblasts lead to resistance in muscle and collagen synthesis, and osteoporosis (147). The frequent pain and limited mobility lead to a significant reduction in physical activity, resulting in long-term deficiencies of vitamin D and calcium. Moreover, the reduced mechanical load stimulation and the use of NSAIDs further inhibit the synthesis of muscle protein and collagen (148), and exacerbate the destruction of bone trabecular microstructure. The above pathological factors necessitate that the nutritional strategy for elderly patients with OA should focus on anti-inflammation, muscle protection and metabolic regulation (Figure 3). Firstly, it is emphasized that high protein intake (1.2–1.5 g/kg/d) is of great importance (149). Prioritize the intermittent supplementation of whey protein or foods rich in leucine (such as eggs and fish), with each meal containing at least 25 grams to promote muscle synthesis and prevent sarcopenia, which will help prevent the progression of OA. Secondly, it is necessary to enhance the intake of vitamin D (2000 IU/day) and calcium (1,200 mg/day) to maintain serum 25(OH)D3 more than 30 ng/mL, which will support the stability of bone and cartilage. Additionally, ω-3 PUFAs (EPA + DHA 1 g/day) and collagen peptides (10 g/day) are also recommended to promote cartilage matrix synthesis. Overall, the MD (≥ 30 mL of extra virgin olive oil per day and ≥ 2 servings of fish per week) should be the basic diet for elderly patients with OA. High AGEs diets (such as grilled red meat) should be restricted. Calorie intake should be more than 30 kcal/kg/day. Obese patients should moderately limit calories (~500 kcal/day), but the total should not be too low to avoid malnutrition. Regular monitoring of renal function (eGFR) and muscle strength (grip strength or walking speed) is necessary. Patients with advanced kidney disease should adjust protein intake (150).
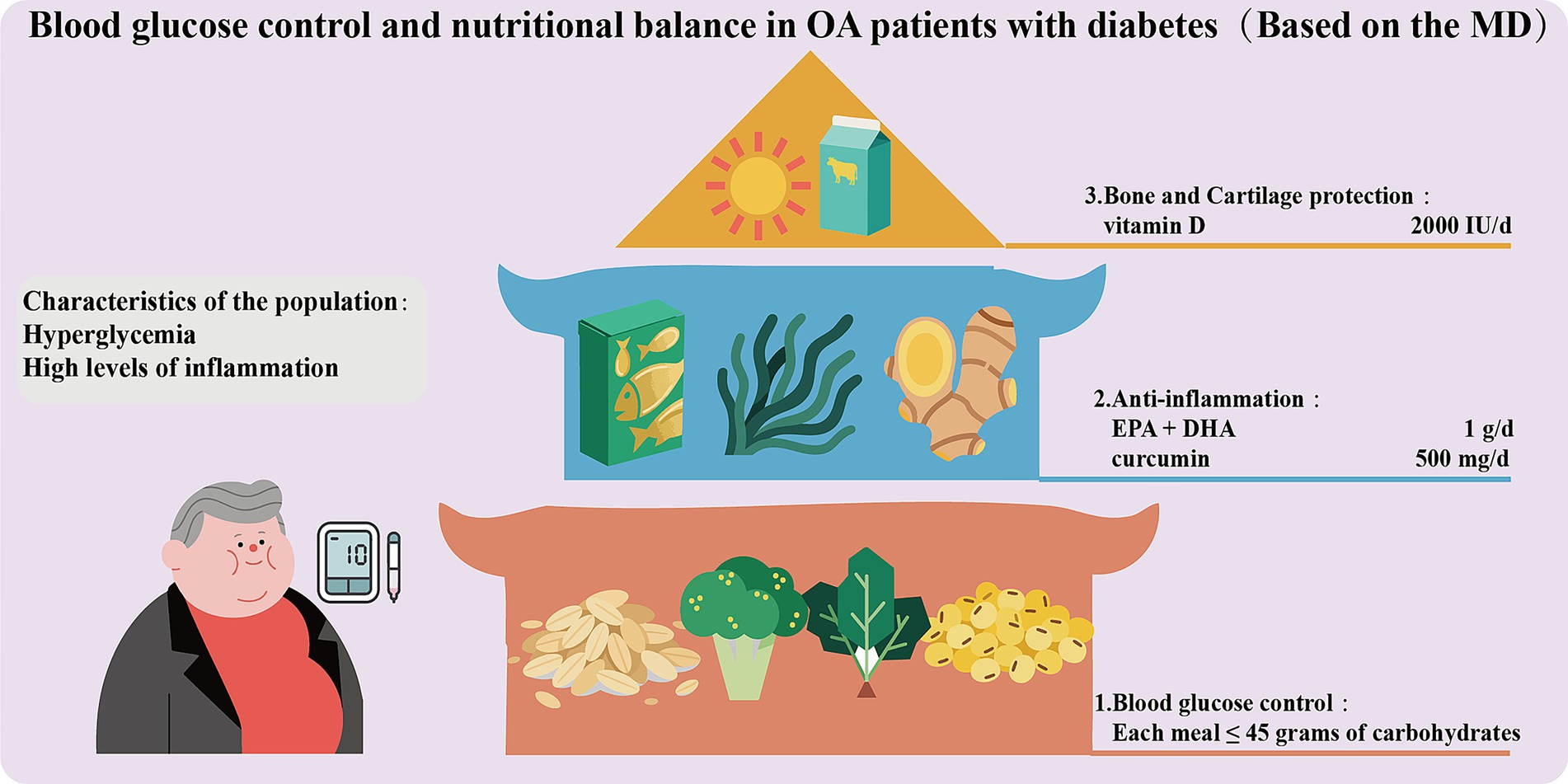
Blood glucose control and nutritional balance in OA patients with diabetes
When OA patients are combined with diabetes, high blood glucose can lead to the accumulation of AGEs (151), which will activate RAGE-dependent inflammatory pathways and disrupt the stability of the cartilage matrix, thereby accelerating the progression of OA. Therefore, these patients need to simultaneously regulate blood glucose and joint inflammation through precise nutritional strategies (Figure 4). Blood glucose control should be given top priority. A low glycemic index (GI) diet consisting of whole grains (oats, quinoa), legumes, and non-starchy vegetables (broccoli, spinach) should be adopted as the main dietary structure. Refined carbohydrates (with a GI > 70) should be restricted to maintain post-meal blood glucose below 7.8 mmol/L (152). Each meal should contain no more than 45 grams of carbohydrates (accounting for 40% of total calories), which should be evenly distributed across the three meals to prevent excessive fluctuations in blood glucose and increased oxidative stress (153). ω-3 PUFAs (EPA + DHA 1 g/day) and polyphenols (curcumin 500 mg/day + piperine) should be recommended to achieve anti-inflammatory effects and improve insulin sensitivity (154). Furthermore, vitamin D (2000 IU per day) can also be appropriately supplemented to protect the cartilage and reduce the risk of joint damage related to diabetic neuropathy (155). Overall, the MD is also applicable to OA patients combined with diabetes. Such patients should avoid foods with high AGEs and strictly limit fructose (such as sugary beverages). HbA1c, vitamin D, and urine microalbumin should be tested every 3 months to monitor for diabetic kidney disease.
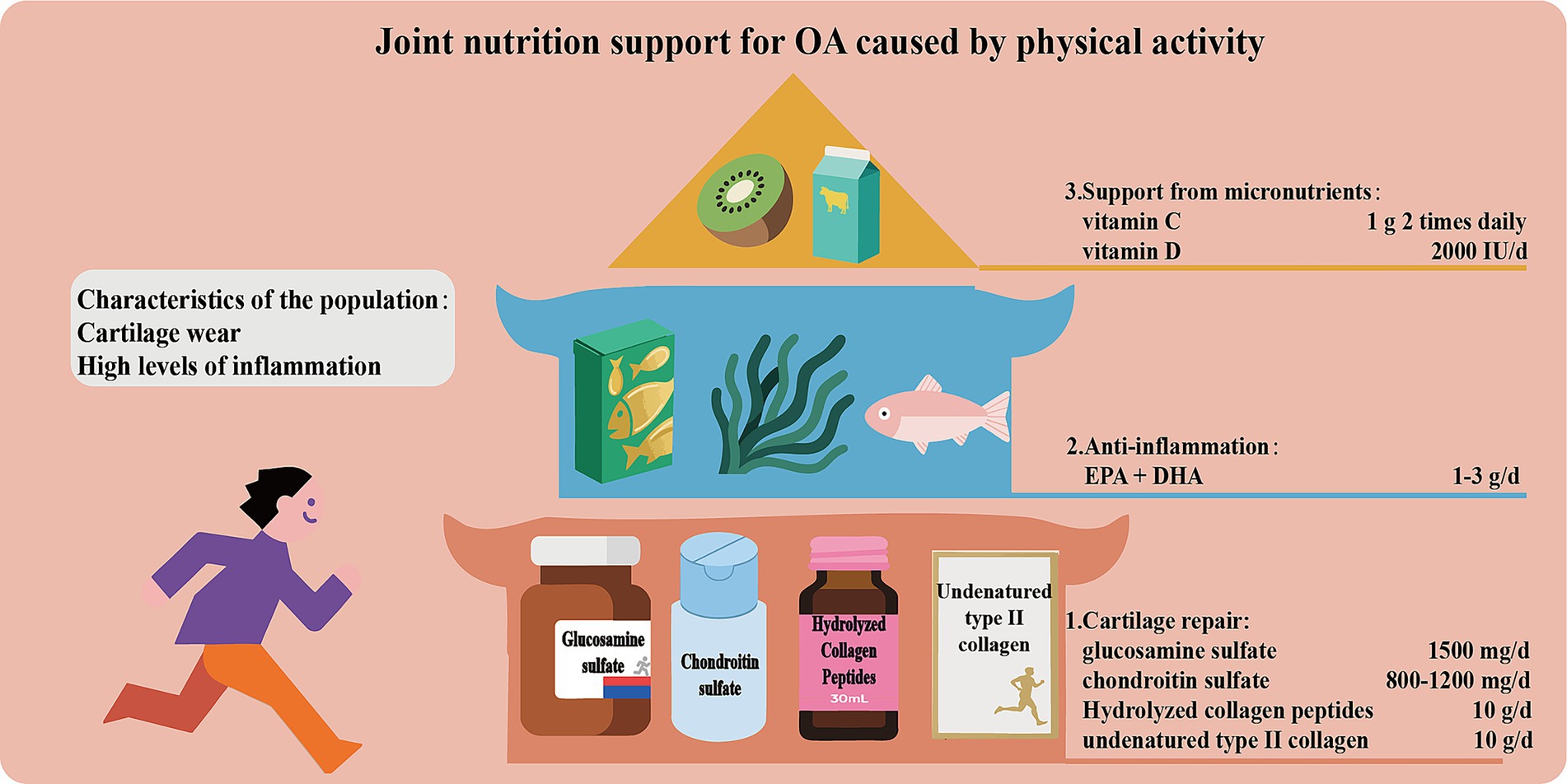
Joint nutrition support for OA caused by physical activity
Due to increased joint load, people who are physically active are prone to cartilage wear and inflammation. Therefore, scientific and reasonable support for cartilage nutrition is of vital importance. The core strategies include the following (Figure 5): (1) Supplement with glucosamine sulfate (1,500 mg/day) and chondroitin sulfate (800–1200 mg/day) as precursors of cartilage matrix to promote cartilage repair. Intake of hydrolyzed collagen peptide (especially Col-II, 10 g/day) or non-denatured Col-II stimulates collagen synthesis and regulates immune response. (2) Supplement with ω-3 PUFAs (EPA + DHA 1–3 g/day) to inhibit the production of pro-inflammatory factors. (3) Ensure the levels of micro-nutrients such as vitamin C and D to support collagen synthesis and bone metabolism.
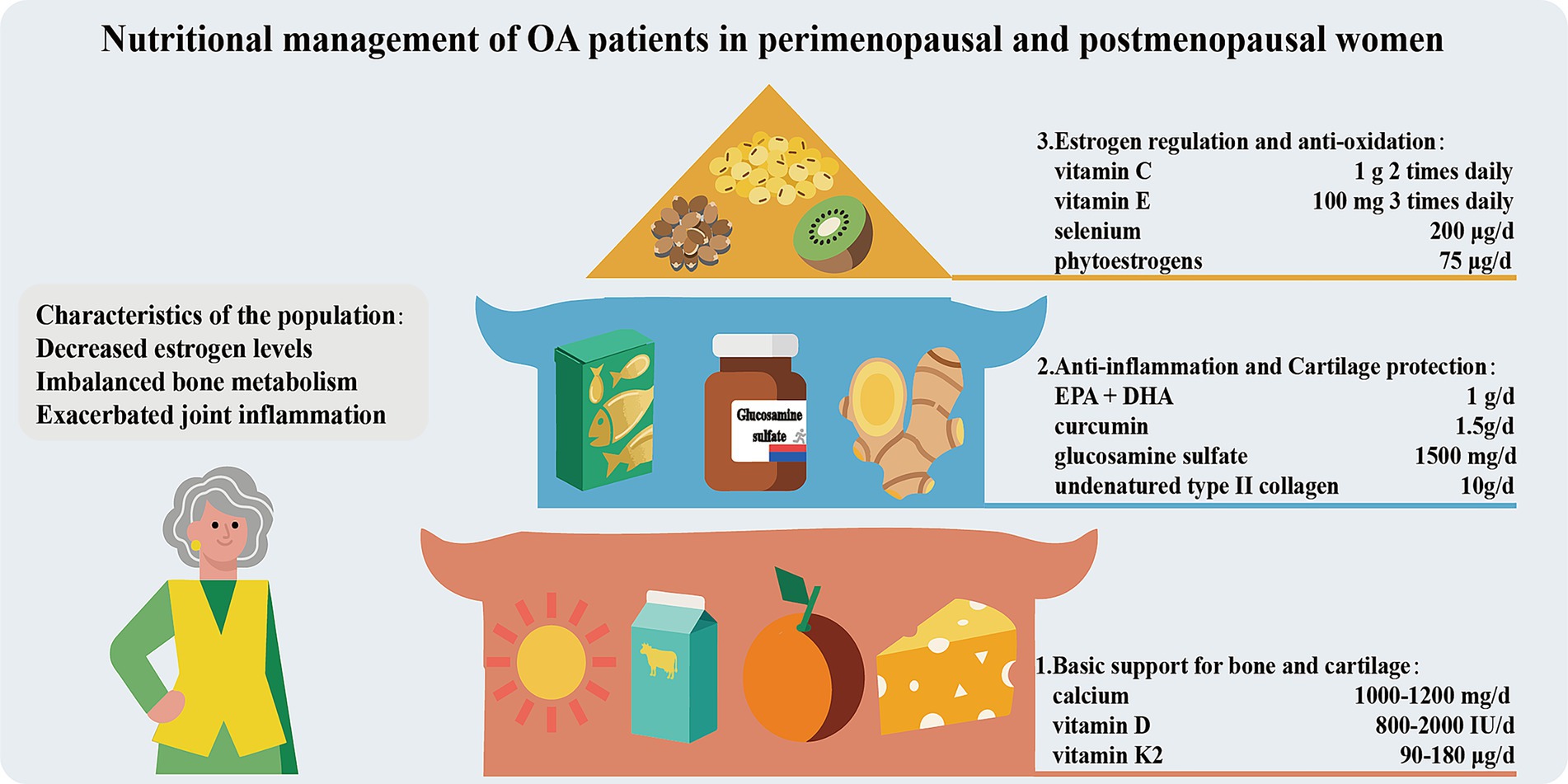
Nutritional management of OA patients in perimenopausal and postmenopausal women
Perimenopausal and postmenopausal women experience a significant decline in estrogen levels, resulting in bone metabolism imbalance (bone resorption > bone formation) and exacerbated joint inflammation. Consequently, the risk of OA increase and symptoms worsen. Therefore, nutritional management should be carried out in three ways (Figure 6). Firstly, basic support for bones and joints should be provided. Adequate calcium (1000–1,200 mg/day, taken in divided doses) and vitamin D (800–2000 IU/day, maintaining blood 25(OH)D3 > 30 ng/mL) intake is the core for maintaining bone density and reducing the risk of fractures (156). Vitamin K2 (MK-7, 90–180 μg per day) can promote the carboxylation of osteocalcin, guiding calcium to be deposited in the bones rather than in soft tissues (157). Secondly, anti-inflammation and cartilage protection should be focused on. Supplementing with ω-3 PUFAs and curcumin can alleviate joint inflammation. Non-denatured Col-II and glucosamine sulfate promote the synthesis of cartilage matrix. Additionally, increasing the intake of foods rich in antioxidants (vitamin C, E, selenium) and phytoestrogens (soy isoflavones, flaxseed lignans) can assist in alleviating menopausal symptoms and oxidative stress damage. Strictly controlling body weight (BMI < 25) to reduce joint load is of crucial importance.
Practical suggestions and future perspectives
After in-depth exploration of nutritional intervention for OA, we have developed a stepwise nutritional intervention plan (Figure 7) based on evidence from clinical studies. This plan aims to relieve pain, delay cartilage degeneration, improve function, and reduce the need for drugs and surgical intervention. It is divided into four progressive steps. The first step (for all OA patients) is following an anti-inflammatory diet (mainly the MD, rich in ω-3 PUFAs, fruits, vegetables, and whole grains), combined with weight loss of 5–10% (for those with a BMI ≥ 25) and low-impact exercises (such as swimming) for 3 months. If the VAS score reduction is less than 20%, move to the next step. The second step is adding joint structure support nutrients, including glucosamine sulfate, chondroitin sulfate, and vitamins D3 and K2. The third step (for insufficient response to the second step) is introducing high-activity cartilage repair components, such as non-denatured Col-II and hydrolyzed collagen peptides (10 g/day). Curcumin should be used to inhibit inflammatory pathways. The fourth step (for refractory/multi-joint OA) is integrate medical nutrition therapy (MNT) based on the previous steps, combined with high-dose ω-3 PUFAs (EPA + DHA 3 g/day) for enhanced anti-inflammation. This step should be based on joint fluid biomarkers (such as COMP, CTX-II), and coordinated with physical therapy (strength training, low-frequency pulsed current stimulation, cold and heat therapy, etc.) and drugs (such as NSAIDs, intra-articular injections). Each step lasts for 3 months. Pain scores, joint function (WOMAC index), and inflammatory markers (hs-CRP) should be assessed dynamically. If effective, maintain or downgrade the step. If ineffective or progressing, upgrade. Liver and kidney function and gastrointestinal tolerance should be monitored throughout the process.
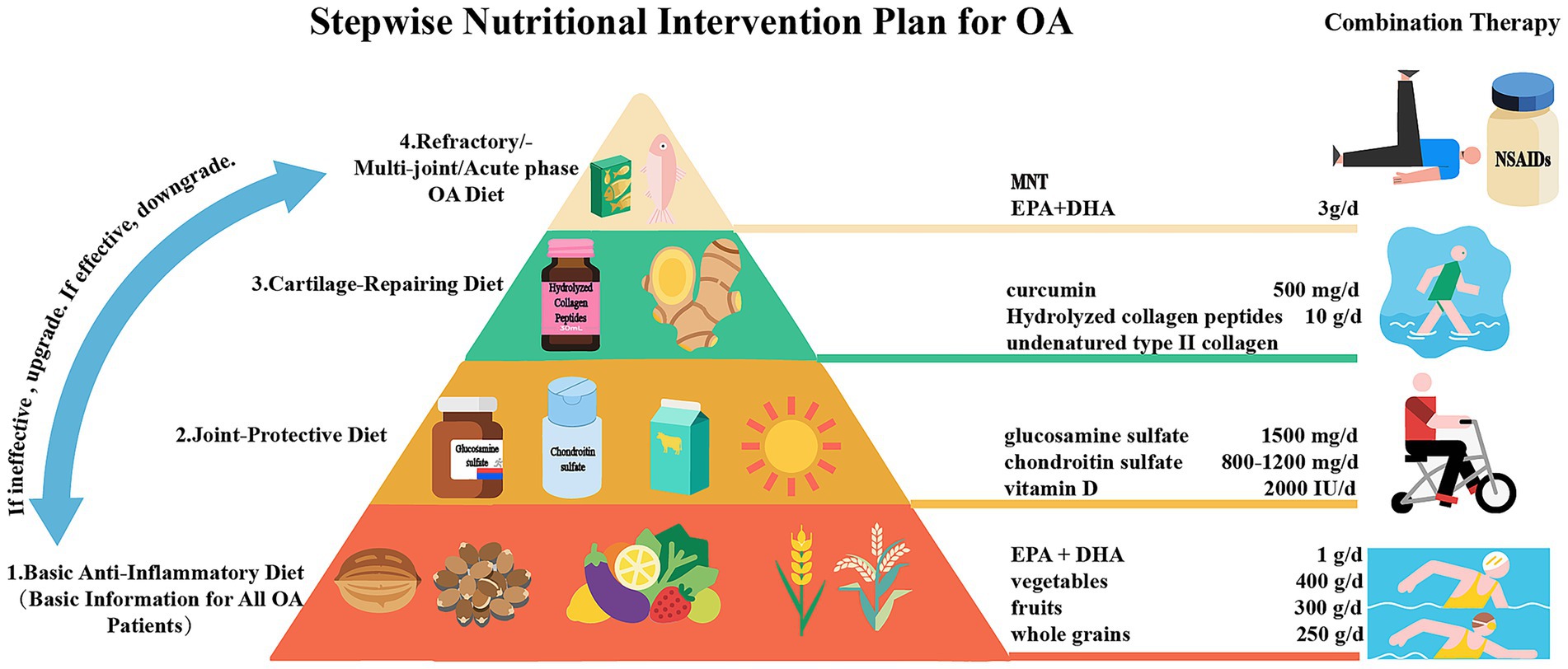
Figure 7. A proposed stepwise nutritional intervention plan for OA patients to relieve pain, delay cartilage degeneration, improve function, and reduce the need for drugs and surgical intervention.
In addition to the stepwise nutritional treatment plan, we have also extended nutritional intervention to the prevention of OA. Primary prevention strategies for OA should target modifiable dietary risk factors that drive obesity and metabolic dysfunction, key contributors to disease onset. These factors predominantly include the frequent consumption of sugar-sweetened beverages, ultra-processed foods, and diets high in saturated fats, which promote weight gain and chronic low-grade inflammation (158). Conversely, emphasizing a pre-emptive anti-inflammatory dietary pattern from early adulthood, such as MD, is advocated. It is also advisable to supplement multivitamins (such as VD, VC, VE, etc.), calcium and probiotics (LcS) in a preventive manner. However, it is not recommended to take them for a long time or in excessive amounts to avoid adverse reactions. These approaches focus on maintaining a healthy weight and mitigating systemic inflammation before the clinical onset of OA. Public health initiatives promoting these approaches, alongside reduced intake of pro-inflammatory foods, represent a cost-effective strategy to lower the population-wide burden of obesity-related OA.
Despite the promising evidence supporting nutritional interventions for OA, several critical limitations in the current research landscape must be acknowledged to contextualize these findings. First, there is considerable heterogeneity in study designs, including wide variations in the dosage, bioavailability, and treatment duration of supplements (e.g., ω-3 PUFAs, collagen peptides), which complicates direct comparison and meta-analysis. Second, many clinical trials are of relatively short duration (often ≤6 months), failing to capture the long-term efficacy and sustainability of interventions on OA progression, a inherently chronic disease. Furthermore, most studies prioritize symptom improvement (e.g., pain reduction) as endpoints, with a pronounced lack of robust evidence demonstrating structural modification (e.g., cartilage preservation via quantitative MRI). The generalizability of findings is also often limited by insufficient consideration of individual differences, such as genetic background, baseline nutritional status, gut microbiota composition, and specific OA phenotypes (e.g., metabolic vs. post-traumatic). Finally, the majority of mechanistic insights are derived from preclinical models, and the translation of these pathways to human pathophysiology remains incompletely elucidated. Addressing these limitations through standardized, long-term, and phenotype-specific randomized controlled trials is imperative for advancing the field of nutritional OA.
We support the importance of a multidisciplinary collaboration model (orthopedics, nutritionists, rheumatology, rehabilitation) in the treatment of OA. Although physical therapy and drug management still dominate OA treatment, the establishment of a stepwise nutritional intervention plan is believed to assist in delaying OA progression, reducing drug use, and avoiding surgical risks. In the future, with the advancement of digital health technology, multi-dimensional data integration and intelligent decision-making will make nutritional intervention more intelligent, customized, and refined. At the same time, with the in-depth research on microbiome-targeted therapy and nutritional epigenetics, the exploration of beneficial and non-beneficial nutrients will be more comprehensive, and the efficiency of nutritional intervention will significantly increase. Nutritional intervention is expected to become the third major treatment modality alongside physical therapy and drug therapy, bringing significant benefits to patients with mild to moderate OA and effectively reducing the proportion of severe OA patients, which allow more people to avoid the adverse effects of repeated drug treatment and the potential risks of surgery and prosthesis replacement.
Conclusion
This review elucidated the significance of the metabolism-inflammation-oxidative stress axis in the occurrence and development of OA, and also dialectically discussed the mechanisms and clinical benefits of beneficial nutrients in the treatment of OA. Among them, ω-3 PUFAs (EPA and DHA) and polyphenols (curcumin) are regarded as key nutrients with potent anti-inflammatory effects, and also the main effect components in the recommended dietary pattern, MD. Collagen peptides, vitamin D, calcium, probiotics, glucosamine and chondroitin are believed to be suitable for supplementation in moderation for the treatment and prevention of OA. By analyzing and comparing the risks and benefits of different dietary patterns, we have developed a stepwise nutritional intervention strategy based on MD for the auxiliary treatment of different types of OA patients and emphasized the importance of comprehensive intervention (physics and medication) in the treatment of OA. Even more, we have specifically tailored the nutritional intervention based on the characteristics of different groups of people. This review will provide new treatment ideas for a large number of OA patients, while avoiding the abuse of drugs and early artificial joint replacement surgeries. It also provides nutritional suggestions and evidence-based data for the prevention of OA. Although there are still some limitations in the current research, we believe that in the future, systematic nutritional intervention is expected to become the third major treatment alongside physical and drug therapy, benefiting more people.
Author contributions
YW: Conceptualization, Investigation, Resources, Writing – original draft, Funding acquisition, Writing – review & editing, Project administration, Data curation, Formal analysis, Methodology. ZC: Visualization, Software, Writing – original draft, Resources, Writing – review & editing, Investigation. YG: Supervision, Writing – review & editing. PS: Supervision, Writing – review & editing. SG: Data curation, Writing – review & editing. MD: Writing – review & editing, Formal analysis. ZT: Resources, Writing – review & editing. YF: Writing – review & editing, Formal analysis. JX: Writing – review & editing, Supervision. CX: Supervision, Writing – review & editing, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Postgraduate Education Innovation program of Shanxi Province (2024SJ155, YW), the central government guiding local funds for scientific and technological development (YDZJSX20231A062, CX), scientific and technological achievements transformation guidance project of Shanxi Province (202204021301067, CX), the National Natural Science Foundation of China (82350710800, 82374470, JX), National Key R&D Program of China (2024YFA0919200, JX), Shenzhen Science and Technology Program (KCXFZ20240903094059020, JX), and Shenzhen Medical Research Fund (B2302005, JX).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Murray, CJL. Findings from the global burden of disease study 2021. Lancet. (2024) 403:2259–62. doi: 10.1016/s0140-6736(24)00769-4
2. Wei, Z, William Brett, R, Jinmin, Z, Weiwei, C, and Jiake, X. Emerging trend in the pharmacotherapy of osteoarthritis. Front Endocrinol (Lausanne). (2019) 10:431. doi: 10.3389/fendo.2019.00431
3. Wei, Z, Hongwei, O, Crispin R, D, and Jiake, X. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. (2016) 4:1–14. doi: 10.1038/boneres.2015.40
4. Kotlarz, H, Gunnarsson, CL, Fang, H, and Rizzo, JA. Osteoarthritis and absenteeism costs: evidence from US National Survey Data. J Occup Environ Med. (2010) 52:263–8. doi: 10.1097/JOM.0b013e3181cf00aa
5. Jenei-Lanzl, Z, Meurer, A, and Zaucke, F. Interleukin-1β signaling in osteoarthritis - chondrocytes in focus. Cell Signal. (2019) 53:212–23. doi: 10.1016/j.cellsig.2018.10.005
6. Lepetsos, P, and Papavassiliou, AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. (2016) 1862:576–91. doi: 10.1016/j.bbadis.2016.01.003
7. De Santis, M, Di Matteo, B, Chisari, E, Cincinelli, G, Angele, P, Lattermann, C, et al. The role of Wnt pathway in the pathogenesis of OA and its potential therapeutic implications in the field of regenerative medicine. Biomed Res Int. (2018) 2018:1–8. doi: 10.1155/2018/7402947
8. Godziuk, K, and Hawker, GA. Obesity and body mass index: past and future considerations in osteoarthritis research. Osteoarthr Cartil. (2024) 32:452–9. doi: 10.1016/j.joca.2024.02.003
9. Hao, X, Shang, X, Liu, J, Chi, R, Zhang, J, and Xu, T. The gut microbiota in osteoarthritis: where do we stand and what can we do? Arthritis Res Ther. (2021) 23:42. doi: 10.1186/s13075-021-02427-9
10. Jerab, D, Blangero, F, da Costa, PCT, de Brito Alves, JL, Kefi, R, Jamoussi, H, et al. Beneficial effects of Omega-3 fatty acids on obesity and related metabolic and chronic inflammatory diseases. Nutrients. (2025) 17:1253. doi: 10.3390/nu17071253
11. Honvo, G, Lengelé, L, Charles, A, Reginster, JY, and Bruyère, O. Role of collagen derivatives in osteoarthritis and cartilage repair: a systematic scoping review with evidence mapping. Rheumatol Ther. (2020) 7:703–40. doi: 10.1007/s40744-020-00240-5
12. Annamalai, R, Sujhithra, A, and Danis Vijay, D. Association between vitamin D and knee osteoarthritis in Indian population: a systematic review and meta-analysis. J Clin Orthop Trauma. (2023) 46:102278. doi: 10.1016/j.jcot.2023.102278
13. Messier, SP, Gutekunst, DJ, Davis, C, and DeVita, P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. (2005) 52:2026–32. doi: 10.1002/art.21139
14. Knarr, BA, Higginson, JS, and Zeni, JA. Change in knee contact force with simulated change in body weight. Comput Methods Biomech Biomed Engin. (2016) 19:320–3. doi: 10.1080/10255842.2015.1018193
15. Chen, L, Zheng, JJY, Li, G, Yuan, J, Ebert, JR, Li, H, et al. Pathogenesis and clinical management of obesity-related knee osteoarthritis: impact of mechanical loading. J Orthop Translat. (2020) 24:66–75. doi: 10.1016/j.jot.2020.05.001
16. Engin, Ayse Basak, and Engin, Atilla (eds) Obesity and lipotoxicity. Cham, Switzerland: Springer International Publishing, (2017) 327–343.
17. Farrag, Y, Farrag, M, Varela-García, M, Torrijos-Pulpón, C, Capuozzo, M, Ottaiano, A, et al. Adipokines as potential pharmacological targets for immune inflammatory rheumatic diseases: focus on rheumatoid arthritis, osteoarthritis, and intervertebral disc degeneration. Pharmacol Res. (2024) 205:107219. doi: 10.1016/j.phrs.2024.107219
18. Li, B, Jin, Y, Zhang, B, Lu, T, Li, J, Zhang, J, et al. Adipose tissue-derived extracellular vesicles aggravate temporomandibular joint osteoarthritis associated with obesity. Clin Transl Med. (2024) 14:e70029. doi: 10.1002/ctm2.70029
19. Tan, L, Harper, LR, Armstrong, A, Carlson, CS, and Yammani, RR. Dietary saturated fatty acid palmitate promotes cartilage lesions and activates the unfolded protein response pathway in mouse knee joints. PLoS One. (2021) 16:e0247237. doi: 10.1371/journal.pone.0247237
20. Tan, L, Carlson, C, and Yammani, R. Dietary fatty acid palmitate induces endoplasmic reticulum stress, chondrocyte death and cartilage lesions in the knee joint of mice. Osteoarthr Cartil. (2020) 28:S68. doi: 10.1016/j.joca.2020.02.103
21. Zhou, M, Zhang, Y, Shi, L, Li, L, Zhang, D, Gong, Z, et al. Activation and modulation of the AGEs-RAGE axis: implications for inflammatory pathologies and therapeutic interventions – a review. Pharmacol Res. (2024) 206:107282. doi: 10.1016/j.phrs.2024.107282
22. Cillero-Pastor, B, Rego-Pérez, I, Oreiro, N, Fernandez-Lopez, C, and Blanco, FJ. Mitochondrial respiratory chain dysfunction modulates metalloproteases −1, −3 and −13 in human normal chondrocytes in culture. BMC Musculoskelet Disord. (2013) 14:235. doi: 10.1186/1471-2474-14-235
23. Ziskoven, C, Jäger, M, Zilkens, C, Bloch, W, Brixius, K, and Krauspe, R. Oxidative stress in secondary osteoarthritis: from cartilage destruction to clinical presentation? Orthop Rev (Pavia). (2010) 2:e23. doi: 10.4081/or.2010.e23
24. Lucas, S, Omata, Y, Hofmann, J, Böttcher, M, Iljazovic, A, Sarter, K, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. (2018) 9:55. doi: 10.1038/s41467-017-02490-4
25. Arora, V, Singh, G, O-Sullivan, IS, Ma, K, Natarajan Anbazhagan, A, Votta-Velis, EG, et al. Gut-microbiota modulation: the impact of the gut-microbiotaon osteoarthritis. Gene. (2021) 785:145619. doi: 10.1016/j.gene.2021.145619
26. Biver, E, Berenbaum, F, Valdes, AM, Araujo de Carvalho, I, Bindels, LB, Brandi, ML, et al. Gut microbiota and osteoarthritis management: an expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res Rev. (2019) 55:100946. doi: 10.1016/j.arr.2019.100946
27. Guss, JD, Ziemian, SN, Luna, M, Sandoval, TN, Holyoak, DT, Guisado, GG, et al. The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthr Cartil. (2019) 27:129–39. doi: 10.1016/j.joca.2018.07.020
28. Zhuang, H, Ren, X, Jiang, F, and Zhou, P. Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis. Mol Med. (2023) 29:17. doi: 10.1186/s10020-023-00614-9
29. Schott, EM, Farnsworth, CW, Grier, A, Lillis, JA, Soniwala, S, Dadourian, GH, et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight. (2018) 3:e95997. doi: 10.1172/jci.insight.95997
30. Boer, CG, Radjabzadeh, D, Medina-Gomez, C, Garmaeva, S, Schiphof, D, Arp, P, et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. (2019) 10:4881. doi: 10.1038/s41467-019-12873-4
31. Li, Q, Wen, Y, Wang, L, Chen, B, Chen, J, Wang, H, et al. Hyperglycemia-induced accumulation of advanced glycosylation end products in fibroblast-like synoviocytes promotes knee osteoarthritis. Exp Mol Med. (2021) 53:1735–47. doi: 10.1038/s12276-021-00697-6
32. Loeser, RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr Cartil. (2009) 17:971–9. doi: 10.1016/j.joca.2009.03.002
33. Chen, J, and Long, F. mTOR signaling in skeletal development and disease. Bone Res. (2018) 6:1. doi: 10.1038/s41413-017-0004-5
34. Courties, A, Sellam, J, and Berenbaum, F. Metabolic syndrome-associated osteoarthritis. Curr Opin Rheumatol. (2017) 29:214–22. doi: 10.1097/bor.0000000000000373
35. Mobasheri, A, Rayman, MP, Gualillo, O, Sellam, J, van der Kraan, P, and Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. (2017) 13:302–11. doi: 10.1038/nrrheum.2017.50
36. Calder, PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. (2015) 1851:469–84. doi: 10.1016/j.bbalip.2014.08.010
37. Burdge, GC, and Calder, PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. (2005) 45:581–97. doi: 10.1051/rnd:2005047
38. Gioxari, A, Kaliora, AC, Marantidou, F, and Panagiotakos, DP. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a systematic review and meta-analysis. Nutrition. (2018) 45:114–124.e4. doi: 10.1016/j.nut.2017.06.023
39. Calder, PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. (2017) 45:1105–15. doi: 10.1042/bst20160474
40. Cordingley, DM, and Cornish, SM. Omega-3 fatty acids for the Management of Osteoarthritis: a narrative review. Nutrients. (2022) 14:3362. doi: 10.3390/nu14163362
41. Yang, Y-C, Lii, CK, Wei, YL, Li, CC, Lu, CY, Liu, KL, et al. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-κB pathways. J Nutr Biochem. (2013) 24:204–12. doi: 10.1016/j.jnutbio.2012.05.003
42. Catherine, LH, March, LM, Aitken, D, Lester, SE, Battersby, R, Hynes, K, et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheum Dis. (2015) 75:23–9. doi: 10.1136/annrheumdis-2014-207169
43. Wen, D, Yi, Z, Yin, E, Lu, R, You, H, Yuan, X, et al. Effect of omega-3 polyunsaturated fatty acids supplementation for patients with osteoarthritis: a meta-analysis. J Orthop Surg Res. (2023) 18:381. doi: 10.1186/s13018-023-03855-w
44. Radcliffe, JE, Thomas, J, Bramley, AL, Kouris-Blazos, A, Radford, BE, Scholey, AB, et al. Controversies in omega-3 efficacy and novel concepts for application. J Nutr Intermed Metab. (2016) 5:11–22. doi: 10.1016/j.jnim.2016.05.002
45. Hewlings, SJ, and Kalman, DS. Curcumin: a review of its effects on human health. Foods. (2017) 6:92. doi: 10.3390/foods6100092
46. Rahaman, SN, Lishadevi, M, and Anandasadagopan, SK. Unraveling the molecular mechanisms of osteoarthritis: the potential of polyphenols as therapeutic agents. Phytother Res. (2025) 39:2038–71. doi: 10.1002/ptr.8455
47. Jian, W, Ming, L, and Yixin, Z. Efficacy and side effect of curcumin for the treatment of osteoarthritis: a meta-analysis of randomized controlled trials. Pak J Pharm Sci. (2019) 32:43–51.
48. Berman, AY, Motechin, RA, Wiesenfeld, MY, and Holz, MK. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. (2017) 1:35. doi: 10.1038/s41698-017-0038-6
49. Hussain, SA, Marouf, BH, Ali, ZS, and Ahmmad, RS. Efficacy and safety of co-administration of resveratrol with meloxicam in patients with knee osteoarthritis: a pilot interventional study. Clin Interv Aging. (2018) 13:1621–30. doi: 10.2147/cia.S172758
50. Anand, P, Kunnumakkara, AB, Newman, RA, and Aggarwal, BB. Bioavailability of curcumin: problems and promises. Mol Pharm. (2007) 4:807–18. doi: 10.1021/mp700113r
51. Bagi, CM, Berryman, ER, Teo, S, and Lane, NE. Oral administration of undenatured native chicken type II collagen (UC-II) diminished deterioration of articular cartilage in a rat model of osteoarthritis (OA). Osteoarthr Cartil. (2017) 25:2080–90. doi: 10.1016/j.joca.2017.08.013
52. Carrillo-Norte, JA, Gervasini-Rodríguez, G, Santiago-Triviño, MÁ, García-López, V, and Guerrero-Bonmatty, R. Oral administration of hydrolyzed collagen alleviates pain and enhances functionality in knee osteoarthritis: results from a randomized, double-blind, placebo-controlled study. Contemp Clin Trials Commun. (2025) 43:101424. doi: 10.1016/j.conctc.2024.101424
53. García-Coronado, J, García-Coronado, JM, Martínez-Olvera, L, Elizondo-Omaña, RE, Acosta-Olivo, CA, Vilchez-Cavazos, F, et al. Effect of collagen supplementation on osteoarthritis symptoms: a meta-analysis of randomized placebo-controlled trials. Int Orthop. (2019) 43:531–8. doi: 10.1007/s00264-018-4211-5
54. Uwitonze, AM, and Razzaque, MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. (2018) 118:181–9. doi: 10.7556/jaoa.2018.037
55. Weaver, CM. Calcium bioavailability and its relation to osteoporosis. Proc Soc Exp Biol Med. (1992) 200:157–60. doi: 10.3181/00379727-200-43409
56. Busa, P, Huang, N, Kuthati, Y, and Wong, C-S. Vitamin D reduces pain and cartilage destruction in knee osteoarthritis animals through inhibiting the matrix metalloprotease (MMPs) expression. Heliyon. (2023) 9:e15268. doi: 10.1016/j.heliyon.2023.e15268
57. Holick, MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. (2011) 12:4–18. doi: 10.2174/138945011793591635
58. Garfinkel, RJ, Dilisio, MF, and Agrawal, DK. Vitamin D and its effects on articular cartilage and osteoarthritis. Orthop J Sports Med. (2017) 5:2325967117711376. doi: 10.1177/2325967117711376
59. Zuo, A, Jia, Q, Zhang, M, Zhou, X, Li, T, and Wang, L. The association of vitamin D with knee osteoarthritis pain: an analysis from the osteoarthritis initiative database. Sci Rep. (2024) 14:30176. doi: 10.1038/s41598-024-81845-6
60. Zhang, FF, Driban, JB, Lo, GH, Price, LL, Booth, S, Eaton, CB, et al. Vitamin D deficiency is associated with progression of knee osteoarthritis. J Nutr. (2014) 144:2002–8. doi: 10.3945/jn.114.193227
61. Holick, MF, Binkley, NC, Bischoff-Ferrari, HA, Gordon, CM, Hanley, DA, Heaney, RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
62. Bruyère, O, Cooper, C, Pelletier, JP, Branco, J, Luisa Brandi, M, Guillemin, F, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of osteoporosis and osteoarthritis (ESCEO). Semin Arthritis Rheum. (2014) 44:253–63. doi: 10.1016/j.semarthrit.2014.05.014
63. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D & Calcium. Dietary reference intakes for calcium and vitamin D (eds A. C. Ross, C. L. Taylor, A. L. Yaktine, and H. B. ValleDel). Washington, D.C., American: National Academies Press (US) Copyright © 2011, National Academy of Sciences. (2011).
64. Arden, NK, Cro, S, Sheard, S, Doré, CJ, Bara, A, Tebbs, SA, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthr Cartil. (2016) 24:1858–66. doi: 10.1016/j.joca.2016.05.020
65. McAlindon, T, LaValley, M, Schneider, E, Nuite, M, Lee, JY, Price, LL, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. (2013) 309:155–62. doi: 10.1001/jama.2012.164487
66. Boyera, N, Galey, I, and Bernard, BA. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int J Cosmet Sci. (1998) 20:151–8. doi: 10.1046/j.1467-2494.1998.171747.x
67. Liu, Z, Ren, Z, Zhang, J, Chuang, CC, Kandaswamy, E, Zhou, T, et al. Role of ROS and Nutritional antioxidants in human diseases. Front Physiol. (2018) 9:477. doi: 10.3389/fphys.2018.00477
68. Zingg, JM. Vitamin E: regulatory role on signal transduction. IUBMB Life. (2019) 71:456–78. doi: 10.1002/iub.1986
69. Jiang, Q, Yin, X, Lill, MA, Danielson, ML, Freiser, H, and Huang, J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci USA. (2008) 105:20464–9. doi: 10.1073/pnas.0810962106
70. Traber, MG, and Stevens, JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. (2011) 51:1000–13. doi: 10.1016/j.freeradbiomed.2011.05.017
71. McAlindon, TE, Jacques, P, Zhang, Y, Hannan, MT, Aliabadi, P, Weissman, B, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. (1996) 39:648–56. doi: 10.1002/art.1780390417
72. Peregoy, J, and Wilder, F. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. (2011) 14:709–15. doi: 10.1017/s1368980010001783
73. Oikonomidis, SA, Simos, YV, Toliopoulos, IK, Verginadis, II, Oikonomidis, AS, Ragos, VN, et al. Vitamin C and E supplementation versus standard meloxicam regimen in the treatment of patients with chronic degenerative arthritis of the knee: a preliminary pilot study. J Musculoskelet Res. (2014) 17:1450003. doi: 10.1142/s0218957714500031
74. Lonn, E, Bosch, J, Yusuf, S, Sheridan, P, Pogue, J, Arnold, JM, et al. Effects of Long-term vitamin E supplementation on cardiovascular events and CancerA randomized controlled trial. JAMA. (2005) 293:1338–47. doi: 10.1001/jama.293.11.1338
75. Mathieu, S, Soubrier, M, Peirs, C, Monfoulet, LE, Boirie, Y, and Tournadre, A. A Meta-analysis of the impact of nutritional supplementation on osteoarthritis symptoms. Nutrients. (2022) 14:1607. doi: 10.3390/nu14081607
76. Zakeri, N, kelishadi, MR, Asbaghi, O, Naeini, F, Afsharfar, M, Mirzadeh, E, et al. Selenium supplementation and oxidative stress: a review. PharmaNutrition. (2021) 17:100263. doi: 10.1016/j.phanu.2021.100263
77. Kang, D, Lee, J, Wu, C, Guo, X, Lee, BJ, Chun, JS, et al. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp Mol Med. (2020) 52:1198–208. doi: 10.1038/s12276-020-0408-y
78. Meng, X, Meng, X, He, Z, Yuan, Y, Fan, Y, Yin, L, et al. Selenium deficiency can promote the expression of VEGF and inflammatory factors in cartilage differentiation and mediates cartilage injury. Biol Trace Elem Res. (2024) 202:4170–9. doi: 10.1007/s12011-023-04003-5
79. Wang, N, Xie, M, Lei, G, Zeng, C, Yang, T, Yang, Z, et al. A cross-sectional study of association between plasma selenium levels and the prevalence of osteoarthritis: data from the Xiangya osteoarthritis study. J Nutr Health Aging. (2022) 26:197–202. doi: 10.1007/s12603-022-1739-2
80. Zamani, B, Taghvaee, F, Akbari, H, Mohtashamian, A, and Sharifi, N. Effects of selenium supplementation on the indices of disease activity, inflammation and oxidative stress in patients with rheumatoid arthritis: a randomized clinical trial. Biol Trace Elem Res. (2024) 202:1457–67. doi: 10.1007/s12011-023-03782-1
81. Rayman, MP, Winther, KH, Pastor-Barriuso, R, Cold, F, Thvilum, M, Stranges, S, et al. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radic Biol Med. (2018) 127:46–54. doi: 10.1016/j.freeradbiomed.2018.02.015
82. Institute of Medicine Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, D.C., American: National Academies Press (US) Copyright 2000 by the National Academy of Sciences. All rights reserved, (2000).
83. Olechnowicz, J, Tinkov, A, Skalny, A, and Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. (2018) 68:19–31. doi: 10.1007/s12576-017-0571-7
84. Gao, T, Chen, T, Ai, C, Gu, Y, Wang, Y, Zhou, X, et al. The causal relationship between zinc and osteoarthritis: a two-sample mendelian randomization study. Biol Trace Elem Res. (2025). doi: 10.1007/s12011-025-04611-3
85. Yang, W-m, Lv, J-f, Wang, Y-y, Xu, Y-m, Lin, J, Liu, J, et al. The daily intake levels of copper, selenium, and zinc are associated with osteoarthritis but not with rheumatoid arthritis in a cross-sectional study. Biol Trace Elem Res. (2023) 201:5662–70. doi: 10.1007/s12011-023-03636-w
86. Tekeoğlu, İ, Şahin, MZ, Kamanlı, A, and Nas, K. The influence of zinc levels on osteoarthritis: a comprehensive review. Nutr Res Rev. (2025) 38:282–93. doi: 10.1017/s0954422424000234
87. Institute of Medicine Panel on Micronutrients. Dietary reference intakes for vitamin a, vitamin K, arsenic, boron, chromium, copper, iodine, Iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C., American: National Academies Press (US) Copyright 2001 by the National Academy of Sciences. All rights reserved, (2001).
88. EFSA Panel on Dietetic Products, N. & Allergies. Scientific opinion on dietary reference values for zinc. EFSA J. (2014) 12:3844. doi: 10.2903/j.efsa.2014.3844
89. Mashhadi, NS, Ghiasvand, R, Askari, G, Hariri, M, Darvishi, L, and Mofid, MR. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. Int J Prev Med. (2013) 4:S36–42.
90. Bartels, EM, Folmer, VN, Bliddal, H, Altman, RD, Juhl, C, Tarp, S, et al. Efficacy and safety of ginger in osteoarthritis patients: a meta-analysis of randomized placebo-controlled trials. Osteoarthr Cartil. (2015) 23:13–21. doi: 10.1016/j.joca.2014.09.024
91. Thakur, P, Dhiman, A, Kumar, S, and Suhag, R. Garlic (Allium sativum L.): a review on bio-functionality, allicin's potency and drying methodologies. S Afr J Bot. (2024) 171:129–46. doi: 10.1016/j.sajb.2024.05.039
92. Youdim, KA, and Deans, SG. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br J Nutr. (2000) 83:87–93. doi: 10.1017/S000711450000012X
93. Bassleer, C, Rovati, L, and Franchimont, P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthr Cartil. (1998) 6:427–34. doi: 10.1053/joca.1998.0146
94. Iovu, M, Dumais, G, and du Souich, P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthr Cartil. (2008) 16:S14–8. doi: 10.1016/j.joca.2008.06.008
95. Bottegoni, C, Muzzarelli, RAA, Giovannini, F, Busilacchi, A, and Gigante, A. Oral chondroprotection with nutraceuticals made of chondroitin sulphate plus glucosamine sulphate in osteoarthritis. Carbohydr Polym. (2014) 109:126–38. doi: 10.1016/j.carbpol.2014.03.033
96. Clegg, DO, Reda, DJ, Harris, CL, Klein, MA, O'Dell, JR, Hooper, MM, et al. Glucosamine, chondroitin Sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. (2006) 354:795–808. doi: 10.1056/NEJMoa052771
97. Meng, Z, Liu, J, and Zhou, N. Efficacy and safety of the combination of glucosamine and chondroitin for knee osteoarthritis: a systematic review and meta-analysis. Arch Orthop Trauma Surg. (2023) 143:409–21. doi: 10.1007/s00402-021-04326-9
98. Hochberg, MC, Martel-Pelletier, J, Monfort, J, Möller, I, Castillo, JR, Arden, N, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. (2016) 75:37–44. doi: 10.1136/annrheumdis-2014-206792
99. Runhaar, J, Rozendaal, RM, van Middelkoop, M, Bijlsma, HJW, Doherty, M, Dziedzic, KS, et al. Subgroup analyses of the effectiveness of oral glucosamine for knee and hip osteoarthritis: a systematic review and individual patient data meta-analysis from the OA trial bank. Ann Rheum Dis. (2017) 76:1862–9. doi: 10.1136/annrheumdis-2017-211149
100. Roman-Blas, JA, Castañeda, S, Sánchez-Pernaute, O, Largo, R, and Herrero-Beaumont, G. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. (2017) 69:77–85. doi: 10.1002/art.39819
101. Goldstein, MR. Glucosamine sulphate and osteoarthritis. Lancet. (2001) 357:1617–8. doi: 10.1016/S0140-6736(00)04753-X
102. Zheng, X, Wang, B, Tang, X, Mao, B, Zhang, Q, Zhang, T, et al. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: a review. Carbohydr Polym. (2023) 299:120153. doi: 10.1016/j.carbpol.2022.120153
103. Oe, M, Tashiro, T, Yoshida, H, Nishiyama, H, Masuda, Y, Maruyama, K, et al. Oral hyaluronan relieves knee pain: a review. Nutr J. (2016) 15:11. doi: 10.1186/s12937-016-0128-2
104. Tashiro, T, Seino, S, Sato, T, Matsuoka, R, Masuda, Y, and Fukui, N. Oral administration of polymer hyaluronic acid alleviates symptoms of knee osteoarthritis: a double-blind, placebo-controlled study over a 12-month period. ScientificWorldJournal. (2012) 2012:167928. doi: 10.1100/2012/167928
105. Arrigo, FGC, Girolimetto, N, Bentivenga, C, Grandi, E, Fogacci, F, and Borghi, C. Short-term effect of a new oral sodium hyaluronate formulation on knee osteoarthritis: a double-blind, randomized, placebo-controlled clinical trial. Dis Markers. (2020) 8:26. doi: 10.3390/diseases8030026
106. Bannuru, RR, Osani, MC, Vaysbrot, EE, Arden, NK, Bennell, K, Bierma-Zeinstra, SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. (2019) 27:1578–89. doi: 10.1016/j.joca.2019.06.011
107. Lei, M, Guo, C, Wang, D, Zhang, C, and Hua, L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: a randomised double-blind, placebo-controlled clinical trial. Benef Microbes. (2017) 8:697–703. doi: 10.3920/bm2016.0207
108. Lyu, JL, Wang, TM, Chen, YH, Chang, ST, Wu, MS, Lin, YH, et al. Oral intake of Streptococcus thermophil us improves knee osteoarthritis degeneration: a randomized, double-blind, placebo-controlled clinical study. Heliyon. (2020) 6:e03757. doi: 10.1016/j.heliyon.2020.e03757
109. Sanders, ME, Merenstein, D, Merrifield, CA, and Hutkins, R. Probiotics for human use. Nutr Bull. (2018) 43:212–25. doi: 10.1111/nbu.12334
110. Salminen, S, Collado, MC, Endo, A, Hill, C, Lebeer, S, Quigley, EMM, et al. The international scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. (2021) 18:649–67. doi: 10.1038/s41575-021-00440-6
111. Parkinson, L, and Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: a review of the beneficial effects on inflammatory disease. Int J Mol Sci. (2014) 15:12323–34. doi: 10.3390/ijms150712323
112. Chen, MP, Yang, SH, Chou, CH, Yang, KC, Wu, CC, Cheng, YH, et al. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm Res. (2010) 59:587–95. doi: 10.1007/s00011-010-0165-9
113. Long, Z, Xiang, W, Li, J, Yang, T, and Yu, G. Exploring the mechanism of resveratrol in reducing the soft tissue damage of osteoarthritis based on network pharmacology and experimental pharmacology. Evid Based Complement Alternat Med. (2021) 2021:1–13. doi: 10.1155/2021/9931957
114. Lv, X, Zhao, XP, Li, WC, Xing, NF, Zong, KQ, Zhai, Y, et al. Anthocyanins and musculoskeletal diseases: mechanisms and therapeutic potential. Front Nutr. (2025) 12:1602034. doi: 10.3389/fnut.2025.1602034
115. Mao, H, Feng, Y, Feng, J, Yusufu, Y, Sun, M, Yang, L, et al. Quercetin-3-O-β-D-glucuronide attenuates osteoarthritis by inhibiting cartilage extracellular matrix degradation and inflammation. J Orthop Translat. (2024) 45:236–46. doi: 10.1016/j.jot.2024.01.007
116. Zhan, J, Yan, Z, Kong, X, Liu, J, Lin, Z, Qi, W, et al. Lycopene inhibits IL-1β-induced inflammation in mouse chondrocytes and mediates murine osteoarthritis. J Cell Mol Med. (2021) 25:3573–84. doi: 10.1111/jcmm.16443
117. Scotece, M, Conde, J, Abella, V, López, V, Francisco, V, Ruiz, C, et al. Oleocanthal inhibits catabolic and inflammatory mediators in LPS-activated human primary osteoarthritis (OA) chondrocytes through MAPKs/NF-κB pathways. Cell Physiol Biochem. (2018) 49:2414–26. doi: 10.1159/000493840
118. Venter, C, Eyerich, S, Sarin, T, and Klatt, KC. Nutrition and the immune system: a complicated tango. Nutrients. (2020) 12:818. doi: 10.3390/nu12030818
119. Veronese, N, Koyanagi, A, Stubbs, B, Cooper, C, Guglielmi, G, Rizzoli, R, et al. Mediterranean diet and knee osteoarthritis outcomes: a longitudinal cohort study. Clin Nutr. (2019) 38:2735–9. doi: 10.1016/j.clnu.2018.11.032
120. Dyer, J, Davison, G, Marcora, SM, and Mauger, AR. Effect of a Mediterranean type diet on inflammatory and cartilage degradation biomarkers in patients with osteoarthritis. J Nutr Health Aging. (2017) 21:562–6. doi: 10.1007/s12603-016-0806-y
121. Duong, V, Oo, WM, Ding, C, Culvenor, AG, and Hunter, DJ. Evaluation and treatment of knee pain: a review. JAMA. (2023) 330:1568–80. doi: 10.1001/jama.2023.19675
122. Zadgaonkar, U. The interplay between adipokines and body composition in obesity and metabolic diseases. Cureus. (2025) 17:e78050. doi: 10.7759/cureus.78050
123. Mihalko, SL, Cox, P, Beavers, DP, Miller, GD, Nicklas, BJ, Lyles, M, et al. Effect of intensive diet and exercise on self-efficacy in overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. Transl Behav Med. (2019) 9:227–35. doi: 10.1093/tbm/iby037
124. Coppock, JA, McNulty, AL, Porter Starr, KN, Holt, AG, Borack, MS, Kosinski, AS, et al. The effects of a 6-month weight loss intervention on physical function and serum biomarkers in older adults with and without osteoarthritis. Osteoarthr Cartil Open. (2023) 5:100376. doi: 10.1016/j.ocarto.2023.100376
125. Gersing, A, Gersing, AS, Solka, M, Joseph, GB, Schwaiger, BJ, Heilmeier, U, et al. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the osteoarthritis initiative. Osteoarthr Cartil. (2016) 24:1126–34. doi: 10.1016/j.joca.2016.01.984
126. Deutz, NE, Bauer, JM, Barazzoni, R, Biolo, G, Boirie, Y, Bosy-Westphal, A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr. (2014) 33:929–36. doi: 10.1016/j.clnu.2014.04.007
127. Melina, V, Craig, W, and Levin, S. Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet. (2016) 116:1970–80. doi: 10.1016/j.jand.2016.09.025
128. Thomas, MS, Calle, M, and Fernandez, ML. Healthy plant-based diets improve dyslipidemias, insulin resistance, and inflammation in metabolic syndrome. A narrative review. Adv Nutr. (2023) 14:44–54. doi: 10.1016/j.advnut.2022.10.002
129. Huang, RY, Huang, CC, Hu, FB, and Chavarro, JE. Vegetarian diets and weight reduction: a Meta-analysis of randomized controlled trials. J Gen Intern Med. (2016) 31:109–16. doi: 10.1007/s11606-015-3390-7
130. Uribarri, J, Woodruff, S, Goodman, S, Cai, W, Chen, X, Pyzik, R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. (2010) 110:911–916.e12. doi: 10.1016/j.jada.2010.03.018
131. Perna, AF, Ingrosso, D, and De Santo, NG. Homocysteine and oxidative stress. Amino Acids. (2003) 25:409–17. doi: 10.1007/s00726-003-0026-8
132. Hunt, JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr. (2003) 78:633S–9S. doi: 10.1093/ajcn/78.3.633S
133. Sarter, B, Kelsey, KS, Schwartz, TA, and Harris, WS. Blood docosahexaenoic acid and eicosapentaenoic acid in vegans: associations with age and gender and effects of an algal-derived omega-3 fatty acid supplement. Clin Nutr. (2015) 34:212–8. doi: 10.1016/j.clnu.2014.03.003
134. Mariotti, F. Vegetarian and plant-based diets in health and disease prevention (ed François Mariotti). San Diego,American: Academic Press, (2017) 621–642.
135. Carlijn, AW, Walrabenstein, W, van der Leeden, M, Turkstra, F, Gerritsen, M, Twisk, JWR, et al. Long-term effectiveness of a lifestyle intervention for rheumatoid arthritis and osteoarthritis: 1-year follow-up of the 'plants for joints' randomised clinical trial. RMD Open. (2024) 10:e004025. doi: 10.1136/rmdopen-2023-004025
136. Walrabenstein, W, Wagenaar, CA, van de Put, M, van der Leeden, M, Gerritsen, M, Twisk, JWR, et al. A multidisciplinary lifestyle program for metabolic syndrome-associated osteoarthritis: the "plants for joints" randomized controlled trial. Osteoarthr Cartil. (2023) 31:1491–500. doi: 10.1016/j.joca.2023.05.014
137. Tong, T, Tong, TYN, Appleby, PN, Armstrong, MEG, Fensom, GK, Knuppel, A, et al. Vegetarian and vegan diets and risks of total and site-specific fractures: results from the prospective EPIC-Oxford study. BMC Med. (2020) 18:353. doi: 10.1186/s12916-020-01815-3
138. Iguacel, I, Miguel-Berges, ML, Gómez-Bruton, A, Moreno, LA, and Julián, C. Veganism, vegetarianism, bone mineral density, and fracture risk: a systematic review and meta-analysis. Nutr Rev. (2018) 77:1–18. doi: 10.1093/nutrit/nuy045
139. Sutton, EF, Beyl, R, Early, KS, Cefalu, WT, Ravussin, E, and Peterson, CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. (2018) 27:1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010
140. Alirezaei, M, Kemball, CC, Flynn, CT, Wood, MR, Whitton, JL, and Kiosses, WB. Short-term fasting induces profound neuronal autophagy. Autophagy. (2010) 6:702–10. doi: 10.4161/auto.6.6.12376
141. Zhang, M-N, Duan, R, Chen, GH, Chen, MJ, Hong, CG, Wang, X, et al. Fasting activates optineurin-mediated mitophagy in chondrocytes to protect against osteoarthritis. Commun Biol. (2025) 8:68. doi: 10.1038/s42003-025-07541-x
142. Varkaneh Kord, H, Tinsley, GM, Santos, HO, Zand, H, Nazary, A, Fatahi, S, et al. The influence of fasting and energy-restricted diets on leptin and adiponectin levels in humans: a systematic review and meta-analysis. Clin Nutr. (2021) 40:1811–21. doi: 10.1016/j.clnu.2020.10.034
143. Neudorf, H, and Little, JP. Impact of fasting & ketogenic interventions on the NLRP3 inflammasome: a narrative review. Biom J. (2024) 47:100677. doi: 10.1016/j.bj.2023.100677
144. Longo, VD, and Mattson, MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. (2014) 19:181–92. doi: 10.1016/j.cmet.2013.12.008
145. Babu, S, Vaish, A, Vaishya, R, and Agarwal, A. Can intermittent fasting be helpful for knee osteoarthritis? J Clin Orthop Trauma. (2021) 16:70–4. doi: 10.1016/j.jcot.2020.12.020
146. Effects of Intermittent Fasting on Health. Aging, and disease. N Engl J Med. (2020) 382:1771–4. doi: 10.1056/NEJMc2001176
147. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2018) 48:16–31. doi: 10.1093/ageing/afy169
148. Atherton, PJ, and Smith, K. Muscle protein synthesis in response to nutrition and exercise. J Physiol. (2012) 590:1049–57. doi: 10.1113/jphysiol.2011.225003
149. Bauer, J, Biolo, G, Cederholm, T, Cesari, M, Cruz-Jentoft, AJ, Morley, JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. (2013) 14:542–59. doi: 10.1016/j.jamda.2013.05.021
150. Obeid, W, Hiremath, S, and Topf, JM. Protein restriction for CKD: time to move on. Kidney360. (2022) 3:1611–5. doi: 10.34067/kid.0001002022
151. Singh, VP, Bali, A, Singh, N, and Jaggi, AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. (2014) 18:1–14. doi: 10.4196/kjpp.2014.18.1.1
152. ElSayed, NA, Aleppo, G, Aroda, VR, Bannuru, RR, Brown, FM, Bruemmer, D, et al. 6. Glycemic targets: standards of care in diabetes—2023. Diabetes Care. (2022) 46:S97–S110. doi: 10.2337/dc23-S006
153. Evert, AB, Dennison, M, Gardner, CD, Garvey, WT, Lau, KHK, MacLeod, J, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. (2019) 42:731–54. doi: 10.2337/dci19-0014
154. Tsitouras, PD, Gucciardo, F, Salbe, AD, Heward, C, and Harman, SM. High omega-3 fat intake improves insulin sensitivity and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm Metab Res. (2008) 40:199–205. doi: 10.1055/s-2008-1046759
155. Sun, X, Yang, X, Zhu, X, Ma, Y, Li, X, Zhang, Y, et al. Association of vitamin D deficiency and subclinical diabetic peripheral neuropathy in type 2 diabetes patients. Front Endocrinol (Lausanne). (2024) 15:1354511. doi: 10.3389/fendo.2024.1354511
156. Weaver, CM, Alexander, DD, Boushey, CJ, Dawson-Hughes, B, Lappe, JM, LeBoff, MS, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. (2016) 27:367–76. doi: 10.1007/s00198-015-3386-5
157. Knapen, MH, Drummen, NE, Smit, E, Vermeer, C, and Theuwissen, E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. (2013) 24:2499–507. doi: 10.1007/s00198-013-2325-6
Keywords: nutritional interventions, osteoarthritis, metabolism, inflammation, oxidative stress, gut-joint axis
Citation: Wang Y, Cao Z, Gao Y, Shao P, Gao S, Dong M, Tian Z, Feng Y, Xu J and Xiang C (2025) Nutritional interventions for osteoarthritis: targeting the metabolism-inflammation-oxidative stress axis—clinical evidence and translational practice. Front. Nutr. 12:1661136. doi: 10.3389/fnut.2025.1661136
Edited by:
Xuchang Zhou, The First Affiliated Hospital of Xiamen University, ChinaReviewed by:
Uzma Amin, Chongqing Medical University, ChinaSyed Nasar Rahaman, Central Leather Research Institute (CSIR), India
Copyright © 2025 Wang, Cao, Gao, Shao, Gao, Dong, Tian, Feng, Xu and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Xiang, Y2h1YW54aWFuZ0BzeG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Yushan Wang
Yushan Wang Zhiyan Cao1†
Zhiyan Cao1† Yingjie Gao
Yingjie Gao Mingjie Dong
Mingjie Dong Yi Feng
Yi Feng