Abstract
Introduction:
From ancient times, donkey milk (DM) has attracted much attention due to its particular properties. Donkey milk is characterized by a high level of lactose and low levels of protein and fat. However, studies investigating lipid changes in DM during lactation are limited.
Methods:
The lipid profile of DM at different lactation stages was analyzed using lipidomics in this study. Milk samples were collected from six lactating Dezhou donkeys on days 1, 30, 60, 90, 120, 150, and 180 of lactation.
Results:
A total of 1,552 lipids were identified, belonging to 5 major categories and 21 subclasses. Glycerophospholipids (GP, 62.11%) and sphingolipids (SP, 22.94%) were the predominant categories. At the subclass level, phosphatidylcholine (PC, 27.26%), phosphatidylethanolamine (PE, 17.59%), and phosphatidylserine (PS, 6.77%) were the most abundant lipids. Significant variations in the levels of GP, SP, glycerolipids (GL), and free fatty acids (FA) were observed across lactation stages. Colostrum was rich in GP and SP, whereas mature milk contained more GL. Regarding FA, mature milk had a higher content of linolenic acid than colostrum, while colostrum was richer in carnitines. Fourteen lipids were identified as potential biomarkers for distinguishing between DM from different lactation stages.
Discussion:
These results indicate that the lactation stage significantly affects the lipid composition of DM. This study provides a detailed lipidomic profile of DM, which will facilitate its further development and utilization.
1 Introduction
From ancient times, donkey milk (DM) has attracted much attention due to its particular properties. Donkey milk is characterized by a high level of lactose and low levels of protein and fat (1, 2). Milk lipids are important sources of energy for infants (3, 4). Lipids also have additional physiological roles, including cell membrane composition and involvement in cell signaling (5). The fat content of DM ranges from 0.3 to 1.8%, which is lower than that of buffaloes, cows, goats, sheep, and yaks (4, 6, 7). Owing to its low fat content, DM can be part of a low-calorie diet or act as a source of food for the elderly (8, 9). Moreover, similar to human milk, DM is rich in unsaturated fatty acids (UFAs) and essential fatty acids (FAs) (4, 6). DM has low fat content and a favorable FA composition, making it a potential functional food ingredient (9). Research has shown that DM can modulate glucose and lipid metabolism, which enhance the antioxidant and anti-inflammatory abilities in animals (7, 10).
Milk fat is a highly variable milk component, and its composition varies greatly among different animals (4); it is also influenced by many other factors, such as diet, individual differences, daily rhythms, lactation stage, and season (11, 12). For example, increasing the proportion of fresh herbage in the diet increased the milk fat and polyunsaturated fatty acid (PUFA) contents (11). Furthermore, Ren et al. (13) reported that dietary roughage significantly affected the lipid and volatile organic compound contents of DM. The fat content and FA composition also vary with lactation stage. Some studies have shown that the content of milk fat and saturated FAs (SFAs) decreases during lactation, whereas the content of UFAs increases (14, 15). Season also has an effect on milk fat composition. In winter, DM had a lower percentage of short-chain SFAs but higher levels of long-chain SFAs and monounsaturated FAs (MUFAs); in the cool season, it contained lower C18:0 and higher palmitoleic, oleic, and vaccenic acids (15).
Lipids can be classified into eight main categories: glycerolipid (GL), glycerophospholipid (GP), sphingolipid (SP), sterol lipid (ST), fatty acyl (FA), polyketide (PK), prenolipid (PR), and saccharolipid (SL) (44). FA, GL, GP, and SP are the primary lipid categories in milk (3). The compositions of GL, FA, GP, and SP in DM have been previously investigated (8, 16–18); however, studies investigating lipid changes in DM during lactation are limited. Therefore, this study employed lipidomics technology to analyze differential lipids in DM across different lactation periods.
2 Materials and methods
2.1 Milk samples collection
Donkey milk was collected from six healthy lactating Dezhou donkeys at different days in milk (DIM): 1 (A), 30 (B), 60 (C), 90 (D), 120 (E), 150 (F), and 180 (G). The lactating donkeys were from a farm in Liaocheng City, China. The donkeys had free access to water and were fed the same diet consisting of grass hay (available ad libitum) supplemented with 2 kg of concentrate per donkey per day. The donkeys were milked by hand twice daily at 11 am and 3 pm. The foals were separated from their dams for 4 h prior to milking. The collected milk samples were rapidly frozen in liquid nitrogen and stored at −80 °C until analysis.
2.2 Lipid extraction
A volume of 100 μl of milk sample was mixed with 0.75 ml of methanol in a glass tube and vortexed. Then, 2.5 ml of methyl tertiary-butyl ether (MTBE) was added, and the mixture was incubated in a shaker for 1 h at room temperature. Afterwards, 0.625 ml of MS-grade water was added. After 10 min of incubation, the sample was centrifuged for 10 min at 1,000 × g. The upper (organic) phase was collected, and the lower phase was re-extracted using the method described above. The combined organic phases were dried and then reconstituted in 100 μl of isopropanol (45). Finally, the extracts were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
2.3 UHPLC-MS/MS analysis
The Vanquish UHPLC system (Thermo Fisher, Germany) coupled with an Orbitrap Q Exactive™ HF mass spectrometer (Thermo Fisher, Germany) was used for UHPLC-MS/MS analyses. Separation was achieved using a Thermo Accucore C30 column (150 × 2.1 mm, 2.6 μm) maintained at 40 °C with a flow rate of 0.35 ml/min. The mobile phase consisted of (A) acetonitrile/water (60:40, v/v) containing 10 mM ammonium acetate and 0.1% formic acid, and (B) acetonitrile/isopropanol (10:90, v/v) containing 10 mM ammonium acetate and 0.1% formic acid. The following gradient program was applied: 70% A (2 min), 57% A (5 min), 45% A (5.1 min), 30% A (11 min), 1% A (16 min), and 70% A (18.1 min).
The Q Exactive™ HF mass spectrometer was operated in both positive and negative ionization modes with the following parameters: sheath gas flow rate, 40 psi; sweep gas flow rate, 10 L/min; auxiliary gas flow rate, 10 L/min (positive mode) or 7 L/min (negative mode); spray voltage, 3.5 kV; capillary temperature, 320 °C; heater temperature, 350 °C; S-lens RF level, 50; scan range, m/z 114–1,700; automatic gain control target, 3e6; normalized collision energy, 22, 24, 28 eV; injection time, 100 ms; isolation window, 1 m/z; automatic gaincontrol target for MS2, 2e5; dynamic exclusion, 6 s.
2.4 Data search
The raw data files generated by UHPLC-MS/MS were processed using Compound Discoverer 3.1 (CD3.1, Thermo Fisher) to perform peak alignment, peak picking, and quantitation for each metabolite. The main parameters were set as follows: retention time tolerance, 0.2 min; mass tolerance, 5 ppm; signal intensity tolerance, 30%; signal-to-noise ratio, 3; and minimum intensity, 100,000. Subsequently, the peak intensities were normalized to the total spectral intensity. The normalized data was used to predict the molecular formula based on additive ions, molecular ion peaks and fragment ions. And then peaks were matched with the Lipidmaps and Lipidblast database to obtained the accurate qualitative and relative quantitative results. Statistical analyses were performed using R (version 3.4.3) and Python (version 2.7.6). When the data were not normally distributed, normal transformations were attempted using the area normalization method.
2.5 Data analysis
Principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) were performed using MetaX. The statistical significance (P-value) was calculated using a t-test. Metabolites were considered differential if they met the following criteria: VIP > 1, P < 0.05, and [fold change (FC) ≥2 or FC ≤ 0.5]. For clustering heatmaps, the data were normalized to z-scores based on the intensity areas of the differential metabolites and were visualized using the Pheatmap package in R.
Data were analyzed by one-way ANOVA in GraphPad Prism 6 software. Data are presented as mean ± SD. A statistically significant difference is defined as P < 0.05.
3 Results
3.1 Lipid classes of DM
A total of 1,552 lipids, spanning five categories and 21 subclasses, were detected in DM (Figure 1). The composition by category was as follows: 964 glycerophospholipids (GP, 62.11%), 356 sphingolipids (SP, 22.94%), 125 glycerolipids (GL, 8.05%), 106 fatty acyls (FA, 6.83%), and 1 sterol lipid (ST, 0.06%). The identified lipid subclasses consisted of 423 glycerophosphatidylcholine (PC) (27.26%), 273 glycerophosphatidylethanolamine (PE) (17.59%), 105 glycerophosphatidylserine (PS) (6.77%), 52 phosphatidic acid (PA) (3.35%), 47 glycerophosphatidylinositol (PI) (3.03%), 41 glycerophosphatidylglycerol (PG) (2.64%), 18 CL (1.16%), 3 hemi-bis(monoacylglycerol)phosphate (HBMP) (0.19%), 2 bis (monoacylglycerol) phosphate ester (BMP) (0.13%), 199 sphingomyelin (SM) (12.82%), 108 ceramide (Cer) (6.96%), 41 hexosylceramide (HexCer) (2.64%), 7 monosialodihexosylganglioside (GM) (0.45%), 1 sulfatides hexosyl-ceramide (SHexCer) (0.06%), 84 triacylglycerol (TAG) (5.41%), 38 diacylglycerol (DAG) (2.45%), 2 diacylglyceryl trimethylhomoserine (DGTS) (0.13%), 1 glucuronosyldiacylglycerol (GlcADG) (0.06%), 86 free fatty acid (FA) (5.54%), 20 acylcarnitine (Acar) (1.29%), and 1 cholesteryl esters (CE) (0.06%).
Figure 1
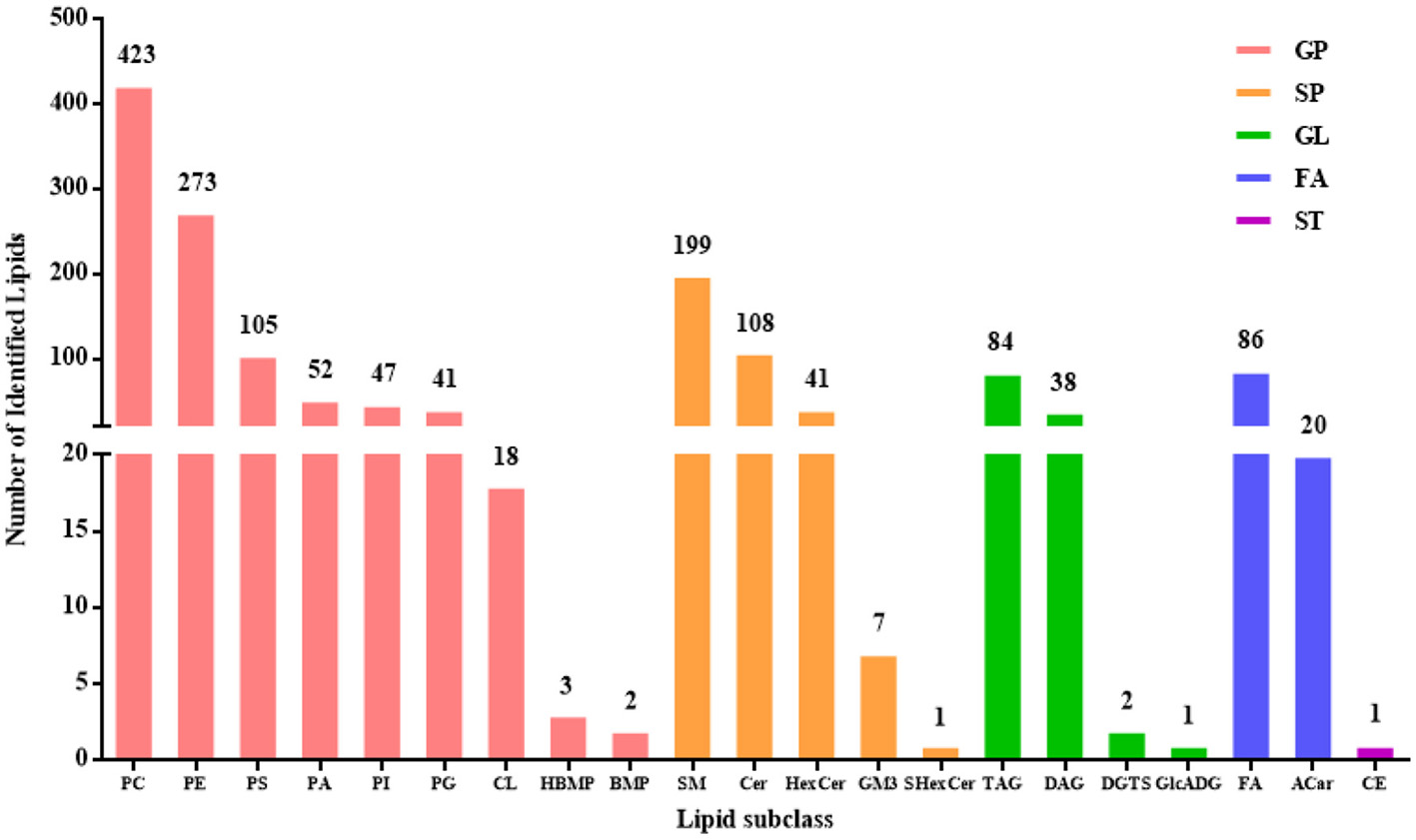
The numbers of lipids identified in DM.
3.2 Lipid profiles of DM among different lactation stages
The data from DM samples at different lactation stages were analyzed using PCA and PLS-DA. Figure 2 shows the PCA results for all milk samples from 1 to 180 DIM. Figure 3 displays the PCA and PLS-DA score plots of milk lipids from Group A (1 DIM) and Group B (30 DIM). The lactation stage had a significant effect on the DM lipid profile. The contents of GP and SP in colostrum were significantly higher than those in mature milk (P < 0.05; Figure 4). Similarly, the relative content of GL in DM at 120 DIM was significantly higher than that at 1 DIM and 30 DIM (P < 0.05; Figure 4).
Figure 2
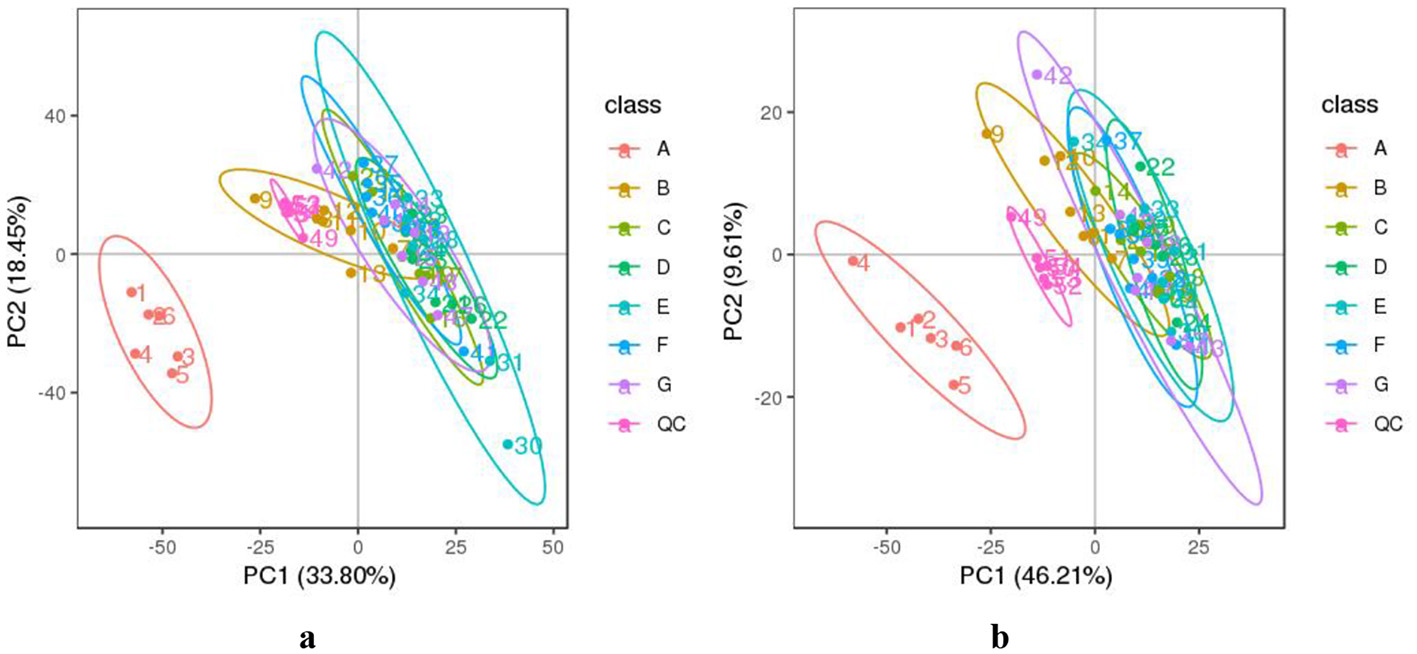
The PCA analysis of DM samples at different lactation stages. (a) The negative and (b) positive ion mode. The horizontal axis PC1 and vertical axis PC2 represent the scores of the principal components ranked first and second, respectively. Different colored dots represent samples from different groups. Ellipses represent a 95% confidence interval. QC, quality control. The milk samples were taken from six lactating donkeys at seven different lactation stages (DIM): 1 (A), 30 (B), 60 (C), 90 (D), 120 (E), 150 (F), and 180 (G).
Figure 3
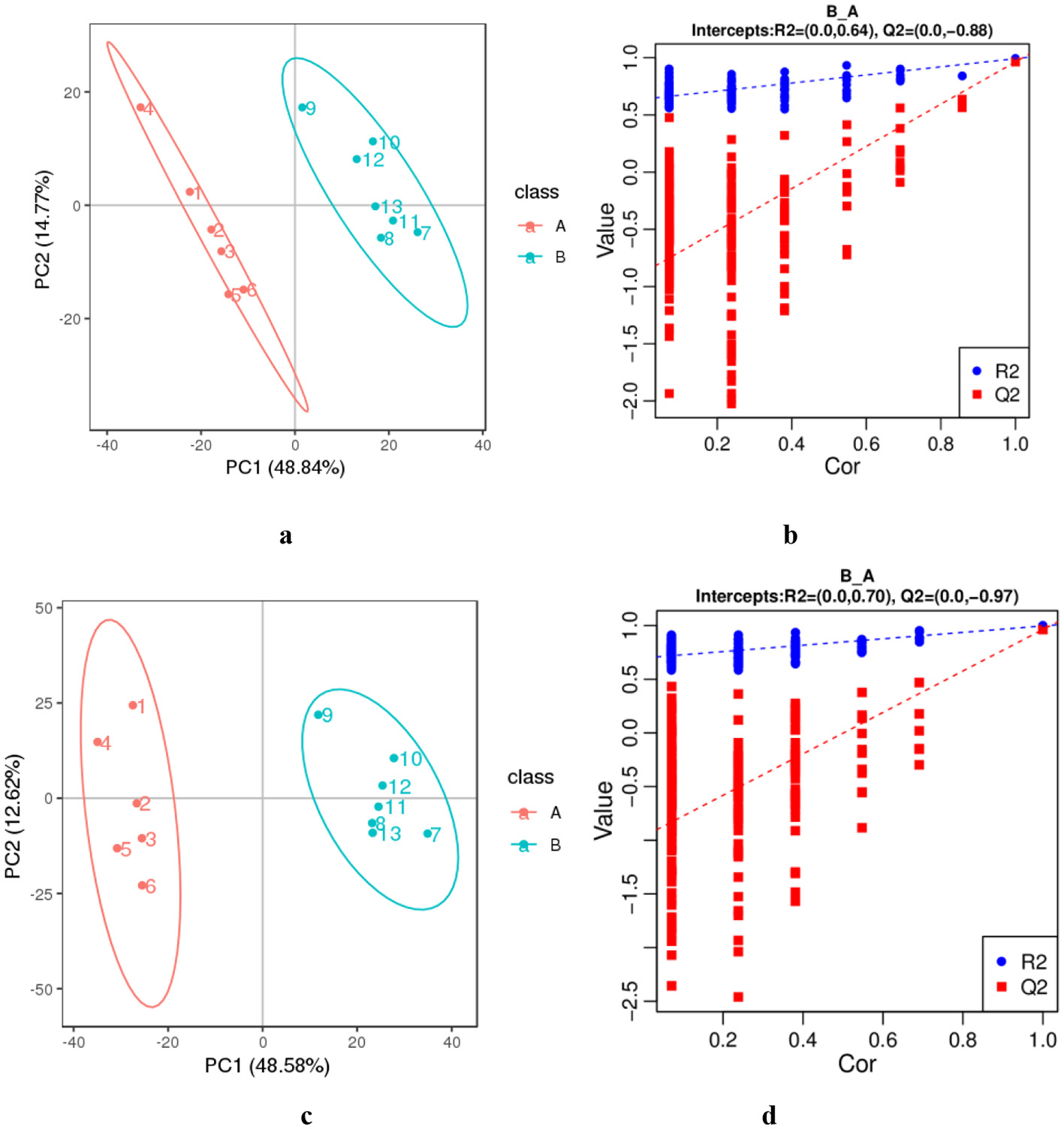
The PCA (a, c) and PLS-DA (b, d) of lipids from colostrum (A) and mature milk (B). (a, b) The negative ion mode. (c, d) The positive ion mode. The horizontal axis PC1 and vertical axis PC2 represent the scores of the principal components ranked first and second, respectively. Ellipses represent a 95% confidence interval. The horizontal axis in the validation plots chart represents the correlation between the random group Y and the original group Y, while the vertical axis represents the scores of R2 and Q2.
Figure 4
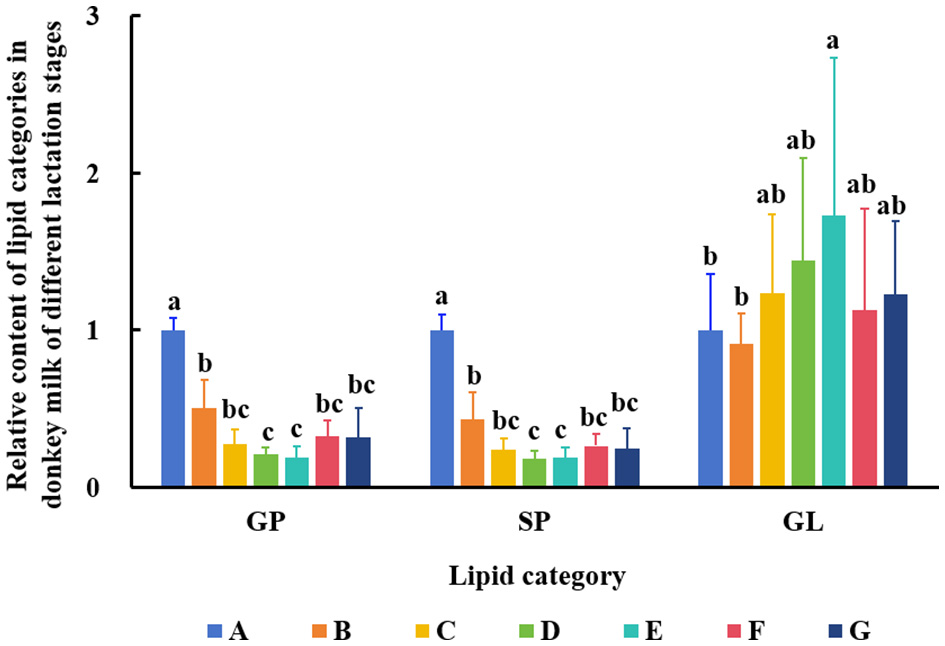
Changes of GP, SP, and GL in DM among different lactation stages. The milk samples were taken from six lactating donkeys at seven different lactation stages (DIM): 1 (A), 30 (B), 60 (C), 90 (D), 120 (E), 150 (F), and 180 (G). The significant differences (P < 0.05) were represented in lowercase letters.
3.3 Differential lipid analysis in DM from different lactation stages
The numbers of differential lipids in DM from different lactation periods are displayed in Table 1 (VIP > 1 and P < 0.05). The comparison between mature milk and colostrum (B vs. A) revealed 964 differential compounds, comprising 432 up-regulated and 532 down-regulated lipids. Among the up-regulated lipids, the main categories were 67 types of GPs, 26 types of SPs, and 16 types of GLs. The down-regulated lipids included 248 types of GPs, 135 types of SPs, 11 types of FAs, and 16 types of GLs. Figure 5 shows the top 20 differential lipids in both positive and negative ion modes between mature milk and colostrum, with six lipids increased and 34 lipids decreased.
Table 1
| Compared samples | Total identified | Differential lipid | Increased lipids | Decreased lipids |
|---|---|---|---|---|
| B vs. A pos | 1,610 | 668 | 386 | 282 |
| C vs. A pos | 734 | 351 | 383 | |
| D vs. A pos | 737 | 300 | 437 | |
| E vs. A pos | 714 | 250 | 464 | |
| F vs. A pos | 728 | 382 | 346 | |
| G vs. A pos | 724 | 354 | 370 | |
| B vs. A neg | 796 | 296 | 46 | 250 |
| C vs. A neg | 349 | 18 | 331 | |
| D vs. A neg | 390 | 35 | 355 | |
| E vs. A neg | 378 | 20 | 358 | |
| F vs. A neg | 350 | 11 | 339 | |
| G vs. A neg | 370 | 22 | 348 |
Numbers of differential lipids in DM at different lactation stages.
Figure 5
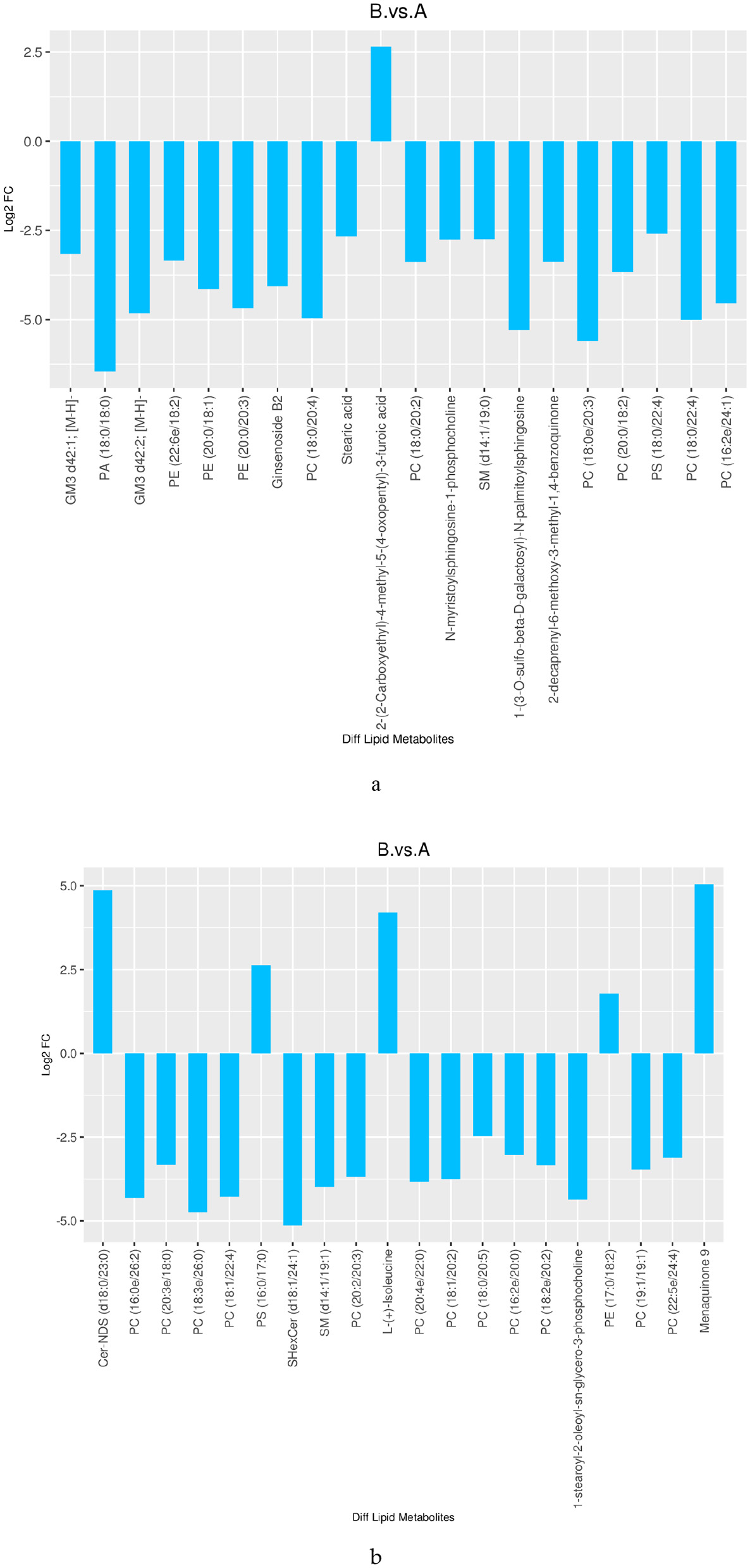
Differences in lipid between mature milk and colostrum (TOP 20) [(a) neg; (b) pos].
Figure 6 shows the clustering heatmap of differential lipids in DM across different lactation periods (1–180 DIM). Supplementary Table S1 lists the top 20 most significantly differential lipids. Fourteen lipid species were identified as potential biomarkers (VIP > 1 and P < 0.05) for distinguishing DM from early, middle, and late lactation (Table 2). Furthermore, four differential lipids were found between colostrum and mid- to late-lactation milk (90–180 DIM), and seven differential lipids were found between colostrum and mature milk (30–180 DIM).
Figure 6
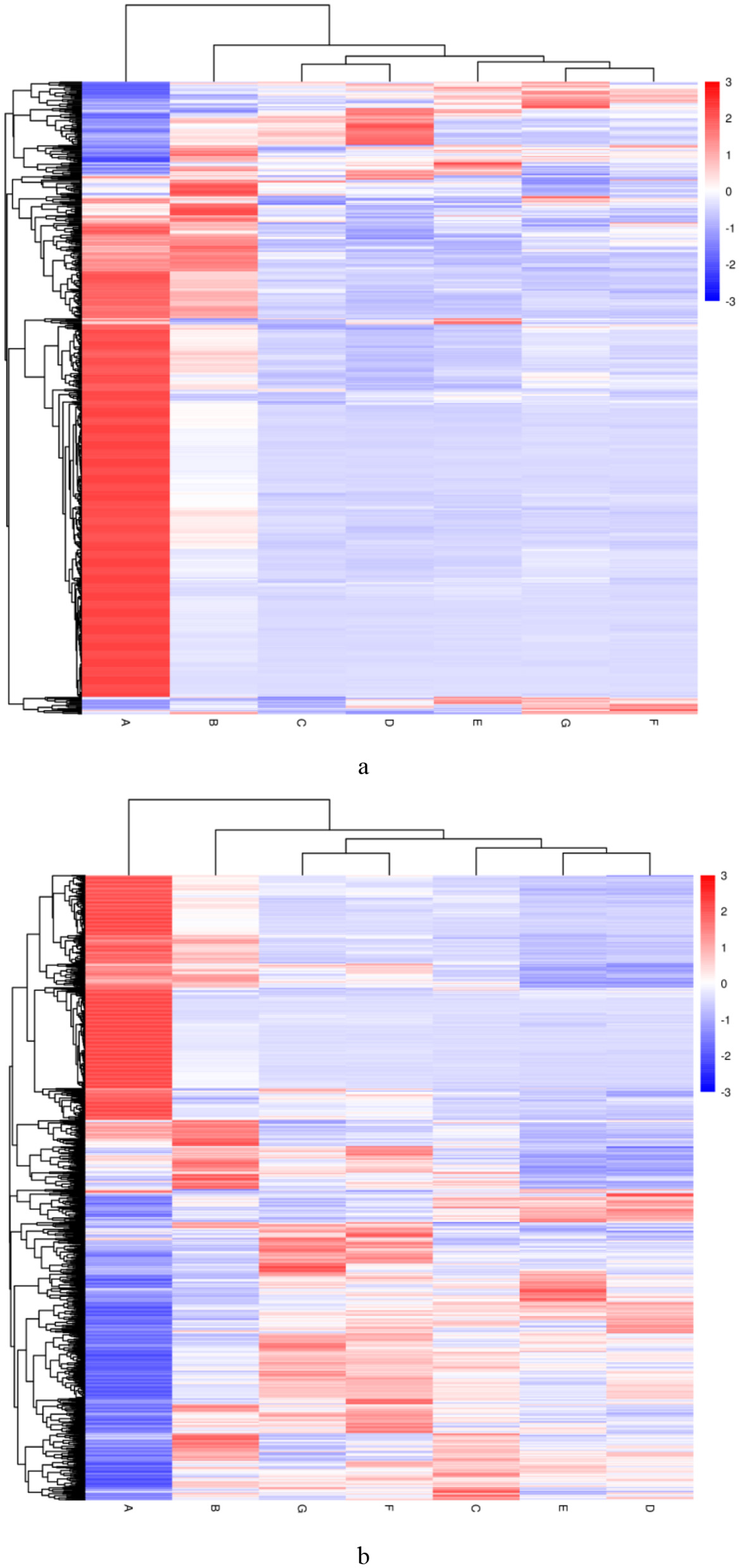
Differential lipid clustering heatmap of DM at different lactation periods. (a) The negative and (b) positive ion mode. Vertical clustering refers to the clustering of samples, while horizontal clustering refers to the clustering of lipids. The shorter the clustering branch, the higher the similarity. The milk samples were taken from six lactating donkeys at seven different lactation stages (DIM): 1 (A), 30 (B), 60 (C), 90 (D), 120 (E), 150 (F), and 180 (G).
Table 2
| Lactation stages | Name | Formula | Molecular weight | RT (min) | FCa |
|---|---|---|---|---|---|
| Early (30–60 DIM) | PC (18:0e/26:4) | C52 H98 N O7 P | 879.70952 | 14.465 | 0.019792 |
| PC (o-18:2(9Z,12Z)/24:0) | C50 H98 N O7 P | 855.70418 | 15.168 | 0.019618 | |
| PC (18:0e/20:3) | C46 H88 N O7 P | 843.63649 | 12.704 | 0.020683 | |
| 1,2-dicapryl-sn-glycero-3-phosphate | C23 H45 O8 P | 480.28712 | 7.108 | 44.94803 | |
| 2-[(5Z,8Z,11Z,14Z,17Z)-eicosapentaenoyl]-sn-glycerol | C23 H36 O4 | 376.25988 | 3.405 | 41.90718 | |
| Mid (90–120 DIM) | SM (d14:1/26:1) | C45 H89 N2 O6 P | 784.64797 | 12.675 | 0.009933 |
| PC (18:0/19:2) | C45 H86 N O8 P | 799.60948 | 11.853 | 0.013871 | |
| SM (d25:2/13:0) | C43 H85 N2 O6 P | 756.61512 | 11.249 | 0.01651 | |
| PC (14:0e/18:1) | C40 H80 N O7 P | 717.56822 | 10.905 | 0.013091 | |
| N-[(2S,3R,4E,6E)-1,3-Dihydroxy-4,6-tetradecadien-2-yl]icosanamide | C34 H65 N O3 | 535.49817 | 7.986 | 74.58051 | |
| Late (150–180 DIM) | GM3 d42:2; [M-H]- | C65 H118 N2 O21 | 1262.82198 | 12.538 | 0.014668 |
| PC (18:0e/20:3) | C46 H88 N O7 P | 843.63649 | 12.704 | 0.020298 | |
| PC (19:0/18:2) | C45 H86 N O8 P | 799.60977 | 11.921 | 0.011914 | |
| PC(P-16:0/16:0) | C40 H80 N O7 P | 717.56726 | 11.653 | 0.008462 | |
| Mid-late (90–180 DIM) | PC (18:3e/22:2) | C48 H88 N O7 P | 821.63212 | 11.76 | 0.016006 |
| SM (d28:2/12:1) | C45 H87 N2 O6 P | 782.63286 | 11.763 | 0.014785 | |
| SM (d25:2/15:1) | C45 H87 N2 O6 P | 782.63193 | 10.9 | 0.010918 | |
| SM (d14:2/12:0) | C31 H61 N2 O6 P | 634.43276 | 3.582 | 0.014429 | |
| Early-mid-late (30–180 DIM) | SHexCer (d18:1/24:1) | C48 H91 N O11 S | 424.30224 | 12.579 | 0.018992 |
| PC (18:0/22:5) | C48 H86 N O8 P | 835.60975 | 11.852 | 0.017121 | |
| PC (19:0/19:0) | C46 H92 N O8 P | 817.67156 | 13.76 | 0.015033 | |
| PC (18:0/20:3) | C46 H86 N O8 P | 811.60917 | 11.879 | 0.011075 | |
| HexCer-NS (d16:1/20:1) | C42 H79 N O8 | 725.58261 | 10.336 | 0.011089 | |
| 1-(3-O-sulfo-beta-D-galactosyl)-N-palmitoylsphingosine | C40 H77 N O11 S | 779.52292 | 9.525 | 0.012098 | |
| PA (18:0/18:0) | C39 H77 O8 P | 704.53635 | 13.597 | 0.008439 |
Differential lipids in DM at different lactation stages compared to colostrum (Top 20).
RT, retention time.
aFC, fold change, mean value of peak area obtained from DM group/mean value of peak area obtained from DC group.
The relative contents of some FAs in DM across different lactation periods are shown in Figure 7. The results revealed that the lactation stage had a significant effect on the levels of α-linolenic acid (ALA), persin, tuliposide A, decanoic acid, lauric acid, ascorbyl palmitate, and carnitine (P < 0.05). The changes in persin, tuliposide A, decanoic acid, and lauric acid during lactation were similar, increasing initially and then decreasing (P < 0.05). The relative content of ascorbyl palmitate in DM increased with the lactation period and was highest in the late stage (P < 0.05). In contrast, the relative content of carnitine was highest in colostrum.
Figure 7
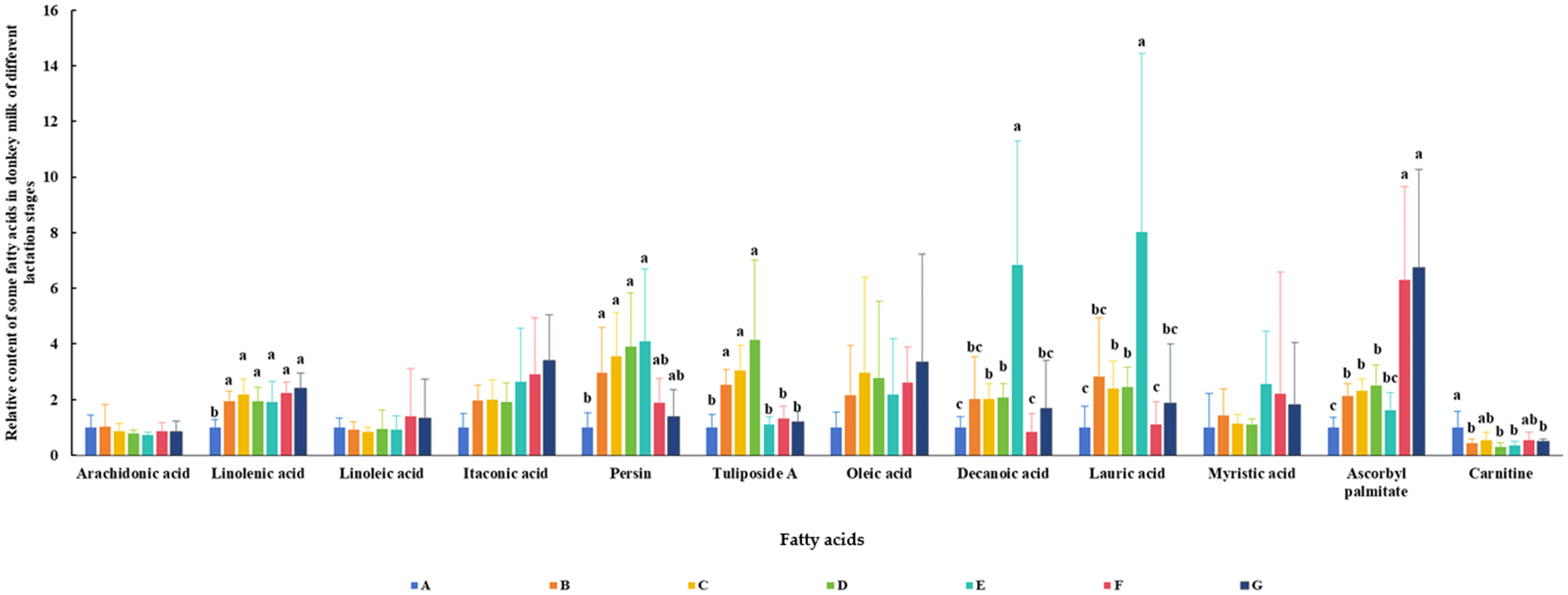
Relative content of some fatty acids in DM of different lactation periods. The milk samples were taken from six lactating donkeys at seven different lactation stages (DIM): 1 (A), 30 (B), 60 (C), 90 (D), 120 (E), 150 (F), and 180 (G). The significant differences (P < 0.05) were represented in lowercase letters.
4 Discussion
The lipid composition of DM was investigated in this study. A total of 1,552 lipids were detected, spanning five categories and 21 subclasses. GP (62.11%) and SP (22.94%) were the predominant lipids, with PC (27.26%), PE (17.59%), and PS (6.77%) as the major subclasses. In contrast, Li et al. (19) identified 335 lipids from 13 subclasses in DM using UHPLC-QTOF-MS-based quantitative lipidomics, reporting TG, SM, DG, and PE as the main lipids. Another study by Ren et al. (13) detected 1,841 lipids from 29 subclasses using LC-MS, categorized into GL (64.86%), GP (17.71%), SP (8.47%), ST (2.18%), FA (1.14%), and derivatized lipids (5.64%). The dominant subclasses were TG (55.19%), PC (8.69%), DG (8.20%), and PE (5.98%). Li et al. (20) analyzed lipids in donkey colostrum (3 DIM) and mature milk (90 DIM) using LC-MS, identifying 1,774 lipids from six categories and 30 subclasses. TG accounted for 61.95%, followed by DG (7.67%), PC (6.09%), and PE (5.19%). Previous studies have indicated that TG is the most abundant lipid in milk, exceeding 50% in human, donkey, horse, buffalo, cow, goat, sheep, and yak milk (4, 12, 21). Our results differ from these findings, which may be attributed to factors such as animal species and lactation stage. For instance, camel milk contains high levels of PE, PC, and SM (over 30% collectively) (4), while pig milk is rich in PC, SM, and PE (over 53%) (4), aligning more closely with our results. Furthermore, the milk samples in this study included colostrum (1 DIM) and mature milk (30–180 DIM), unlike the studies by Ren et al. (13) and Li et al. (20). The higher fat content and GP proportion in colostrum may explain the discrepancies. Additionally, differences in lipid detection methods across studies could also contribute to the varying results. It should be noted that the composition and content of lipids in DM vary with different diets (22). The changes in lipids in DM may also be caused by dietary differences. Moreover, there were only six lactating donkeys per group in this study, and the individual variations among them may have influenced the results. In future research, it is necessary to increase the sample size and reduce individual differences to improve the generalizability of research results.
The lactation stage had a significant effect on the lipid composition of DM. Li et al. (19) reported 60 differential lipids between donkey mature milk and colostrum, with Hex2Cer, PS, and PI being the main subclasses that differed. In this study, we identified 964 differential lipids between mature milk (30 DIM) and colostrum (1 DIM), of which 432 were increased and 532 were decreased. The GP, SP, GL, and FA categories showed significant differences across lactation periods. Donkey colostrum is rich in GP and SP. These lipids are not only components of cell membranes but also promote infant brain and nerve development and modulate inflammatory responses (23). SM and PC, which are sources of choline, are beneficial for the development of the central nervous system in infants (12, 24). Cer and SM are involved in cell proliferation, differentiation, signal transduction, and the development of the infant immune system (25–27). Therefore, donkey colostrum is an ideal source of nutrition for the optimal growth and development of infants. Conversely, mature donkey milk, especially from the mid-lactation stage (90–120 DIM), contained higher levels of GL. This finding is consistent with the conclusions of Ren et al. (13) and Li et al. (19). Furthermore, 14 types of lipids (5 for early lactation, 5 for mid lactation, and 4 for late lactation) were identified as potential biomarkers for distinguishing DM from different lactation stages.
The composition of FAs in DM is more favorable than that of other milks (16, 28). Santillo et al. (29) revealed that, compared with dairy milk, DM has higher long-chain FA (LC-FA) and lower short-chain FA (SC-FA) contents. In addition, DM has a lower SFA content and is richer in PUFAs than cow milk (28). The PUFA content in DM is similar to that in human milk (4, 30). Donkey milk is rich in PUFAs, such as ALA, linoleic acid (LA), and arachidonic acid (ARA) (4). Similarly, in this study, we detected abundant levels of oleic acid, itaconic acid, decanoic acid, persin, tuliposide A, lauric acid, ascorbyl palmitate, carnitine, myristic acid, ARA, ALA, and LA in DM. Many studies have found that these FAs have many biological activities, such as lipid regulation, antioxidant, anti-inflammatory and immune activation, antibacterial, anti-breast cancer, neuroprotective and anti-apoptotic (31–39).
The content of FAs in DM was affected by the lactation stage. The colostrum was rich in carnitine. Compared with colostrum, mature donkey milk contained more ALA. Milk from early- and mid-lactation contained more persin and tuliposide A. The contents of decanoic acid and lauric acid were highest in mid-lactation milk. Donkey milk at the late lactation stage was rich in ascorbyl palmitate. Carnitine is an essential component for fatty acid metabolism and energy production. After birth, an infant's energy demands for movement, growth, differentiation, and maintaining body temperature increase dramatically, which heavily relies on carnitine-mediated fatty acid oxidation (40). Its high concentrations in colostrum help newborns utilize fat as an energy source. The subsequent decrease in concentration reflects the infant's adaptive physiological adjustments to ongoing digestive system development and changing nutritional demands. Furthermore, by facilitating efficient fat metabolism, carnitine ensures a continuous energy supply to the brain, which is fundamental for neural development (40, 41). As a precursor to docosahexaenoic acid (DHA), ALA is the most critical structural fat for infant brain and retinal development (42). Since infants have a limited ability to synthesize DHA endogenously, they rely heavily on ALA provided in breast milk. ALA also possesses anti-inflammatory properties (42, 43). It helps regulate the infant's immune response and prevents excessive inflammation, which is crucial for newborns whose immune systems are not yet fully developed. In addition, ALA can act as pancreatic lipase inhibitors, inhibiting the increase in triglyceride levels in the blood after fat intake, which is beneficial for health and weight management (37). The results of Butt et al. (31) found that persin has an anti-breast cancer effect, which can induce G2-M cell cycle arrest and caspase dependent apoptosis. It has been found that the tuliposides have antibacterial activity (32, 34). Lauric Acid is a well-known antibacterial agent. It also has anti-inflammatory and immune activating effects (35). In study of Sharma et al. (39), the anti-inflammatory, antioxidant, neuroprotective, and anti-apoptotic properties of decanoic acid were observed. The ascorbyl palmitate has antioxidant activities (33). Therefore, DM is an ideal food source for infants and certain patients, as well as a valuable cosmetic ingredient. Specifically, milk from early- and mid-lactation may be more beneficial for patients (e.g., with cancer or enteritis), while DM from mid- and late-lactation is more suitable for use in the cosmetics industry.
In summary, a total of 1,552 lipids were detected in DM, encompassing five lipid categories and 21 subclasses, among which GP and SP were the predominant lipids. The GP, SP, GL, and FA categories varied significantly with the lactation stage. Colostrum was rich in GP and SP, while mature milk contained higher levels of GL. Compared with colostrum, mature donkey milk contained more ALA, whereas colostrum was richer in carnitine. Fourteen lipid species were identified as potential biomarkers for distinguishing DM from different lactation stages. In conclusion, DM contains abundant functional lipids, and the lactation period has a significant effect on its lipid profile. This study contributes to a better understanding of the lipid composition in donkey milk, facilitating its development into high-value nutritional products.
Statements
Data availability statement
The data presented in the study are deposited in the Metabolights repository (https://www.ebi.ac.uk/metabolights), accession number MTBLS12948.
Ethics statement
The animal study was approved by the Animal Care and Use Committee of Liaocheng University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XD: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. ZM: Formal analysis, Writing – review & editing. CW: Supervision, Writing – review & editing, Funding acquisition. MZ: Funding acquisition, Supervision, Writing – review & editing, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Key R&D Program of China (2022YFD1600103 and 2023YFD130200404), the Shandong Provincial Natural Science Foundation (ZR2023MC149), and the Open Project of Liaocheng University Animal Husbandry Discipline (319312105-5).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1662407/full#supplementary-material
References
1.
Zhou M Huang F Du X Liu G Wang C . Fermented Codonopsis pilosula residue improved milk performance of lactating donkeys by enhancing antioxidant capacity and regulating metabolism. Front Vet Sci. (2024) 11:1489480. 10.3389/fvets.2024.1489480
2.
Zhou M Ma Z Du X Wang C Liu G . The milk compositions and blood parameters of lactating Dezhou donkeys changes with lactation stages. Vet Med Sci. (2025) 11:e70269. 10.1002/vms3.70269
3.
Liu Z Rochfort S Cocks B . Milk lipidomics: what we know and what we don't. Prog Lipid Res. (2018) 71:70–85. 10.1016/j.plipres.2018.06.002
4.
Wu Y Sun Y Chen R Qiao Y Zhang Q Li Q et al . Analysis for lipid nutrient differences in the milk of 13 species from a quantitative non-targeted lipidomics perspective. Food Chem X. (2023) 20:101024. 10.1016/j.fochx.2023.101024
5.
Röhrig F Schulze A . The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. (2016) 16:732–49. 10.1038/nrc.2016.89
6.
Gastaldi D Bertino E Monti G Baro C Fabris C Lezo A et al . Donkey's milk detailed lipid composition. Front Biosci – Elite. (2010) 2:537–46. 10.2741/e112
7.
Trinchese G Cavaliere G De Filippo C Aceto S Prisco M Chun JT et al . Human milk and donkey milk, compared to cow milk, reduce inflammatory mediators and modulate glucose and lipid metabolism, acting on mitochondrial function and oleylethanolamide levels in rat skeletal muscle. Front Physiol. (2018) 9:32. 10.3389/fphys.2018.00032
8.
Chiofalo B Dugo P Bonaccorsi IL Mondello L . Comparison of major lipid components in human and donkey milk: new perspectives for a hypoallergenic diet in humans. Immunopharm Immunot. (2011) 33:633–44. 10.3109/08923973.2011.555409
9.
Messias TBON Alves SP Bessa RJB Madruga MS Pacheco MTB Queiroga RCRDE . Fatty acid profile of milk from Nordestina donkey breed raised on Caatinga pasture. J Dairy Res. (2021) 88:205–9. 10.1017/S0022029921000388
10.
Lionetti L Cavaliere G Bergamo P Trinchese G De Filippo C Gifuni G et al . Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and anti-inflammatory defenses in rats. Mol Nutr Food Res. (2012) 56:1596–600. 10.1002/mnfr.201200160
11.
Valle E Pozzo L Giribaldi M Bergero D Gennero MS Dezzutto D et al . Effect of farming system on donkey milk composition. J Sci Food Agr. (2018) 98:2801–8. 10.1002/jsfa.8777
12.
Selvalatchmanan J Rukmini AV Ji S Triebl A Gao L Bendt AK et al . Variability of lipids in human milk. Metabolites. (2021) 11:104. 10.3390/metabo11020104
13.
Ren W Sun M Shi X Wang T Wang Y Wang X et al . Effects of roughage on the lipid and volatile-organic-compound profiles of donkey milk. Foods. (2023) 12:2231. 10.3390/foods12112231
14.
Martemucci G D'Alessandro AG . Fat content, energy value and fatty acid profile of donkey milk during lactation and implications for human nutrition. Lipids Health Dis. (2012) 11:1–14. 10.1186/1476-511X-11-113
15.
Martini M Altomonte I Manica E Salari F . Changes in donkey milk lipids in relation to season and lactation. J Food Compos Anal. (2015) 41:30–4. 10.1016/j.jfca.2014.12.019
16.
Martini M Altomonte I Salari F . Amiata donkeys: fat globule characteristics, milk gross composition and fatty acids. Ital J Anim Sci. (2014) 13:56–56. 10.4081/ijas.2014.3118
17.
Calvano CD Glaciale M Palmisano F Cataldi TRI . Glycosphingolipidomics of donkey milk by hydrophilic interaction liquid chromatography coupled to ESI and multistage MS. Electrophoresis. (2018) 39:1634–44. 10.1002/elps.201700475
18.
Contarini G Pelizzola V Scurati S Povolo M . Polar lipid of donkey milk fat: phospholipid, ceramide and cholesterol composition. J Food Compos Anal. (2017) 57:16–23. 10.1016/j.jfca.2016.12.013
19.
Li M Li W Wu J Zheng Y Shao J Li Q et al . Quantitative lipidomics reveals alterations in donkey milk lipids according to lactation. Food Chem. (2020) 310:125866. 10.1016/j.foodchem.2019.125866
20.
Li M Sun L Du X Zhao Y Ren W Man L et al . Characterization and discrimination of donkey milk lipids and volatiles across lactation stages. Food Chem X. (2024) 23:101740. 10.1016/j.fochx.2024.101740
21.
Amores G Virto M . Total and free fatty acids analysis in milk and dairy fat. Separations. (2019) 6:14. 10.3390/separations6010014
22.
Chiofalo B Polidori M Costa R Salimei E . Fresh forage in dairy ass's ration: effect on milk fatty acid composition and flavours. Ital J Anim Sci. (2005) 4:433–5. 10.4081/ijas.2005.2s.433
23.
Contarini G Povolo M . Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. (2013) 14:2808–31. 10.3390/ijms14022808
24.
Hernell O Timby N Domellöf M Lönnerdal B . Clinical benefits of milk fat globule membranes for infants and children. J Pediat-Brazil. (2016) 173:S60–5. 10.1016/j.jpeds.2016.02.077
25.
Ratajczak MZ Lee H Wysoczynski M Wan W Marlicz W Laughlin MJ . Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. (2010) 24:976–85. 10.1038/leu.2010.53
26.
Makdissy N Haddad K Mouawad C Popa I Younsi M Valet P . Regulation of srebps by sphingomyelin in adipocytes via a caveolin and Ras-Erk-Mapk-Creb signaling pathway. PLoS ONE. (2015) 10:e0133181. 10.1371/journal.pone.0133181
27.
Quinville BM Deschenes NM Ryckman AE Walia JSA . Comprehensive review: Sphingolipid metabolism and implications of disruption in sphingolipid homeostasis. Int J Mol Sci. (2021) 22:5793. 10.3390/ijms22115793
28.
Medhammar E Wijesinha-Bettoni R Stadlmayr B Nilsson E Charrondiere U Burlingame B . Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J Agric Food Sci. (2012) 92:445–74. 10.1002/jsfa.4690
29.
Santillo A Figliola L Ciliberti MG Caroprese M Marino R Albenzio M . Focusing on fatty acid profile in milk from different species after in vitro digestion. J Dairy Res. (2018) 85:257–62. 10.1017/S0022029918000274
30.
Cimmino F Catapano A Villano I Di Maio G Petrella L Traina G et al . Invited review: human, cow, and donkey milk comparison: focus on metabolic effects. J Dairy Sci. (2023) 106:3072–85. 10.3168/jds.2022-22465
31.
Butt AJ Roberts CG Seawright AA Oelrichs PB Macleod JK Liaw TY et al . A novel plant toxin, persin, with in vivo activity in the mammary gland, induces Bim-dependent apoptosis in human breast cancer cells. Mol Cancer Ther. (2006) 5:2300–9. 10.1158/1535-7163.MCT-06-0170
32.
Shigetomi K Shoji K Mitsuhashi S Ubukata M . The antibacterial properties of 6-tuliposide B. Synthesis of 6-tuliposide B analogues and structure-activity relationship. Phytochemistry. (2010) 71:312–24. 10.1016/j.phytochem.2009.10.008
33.
Bamidele OP Duodu KG Emmambux MN . Encapsulation and antioxidant activity of ascorbyl palmitate with maize starch during pasting. Carbohyd Polym. (2017) 166:202–8. 10.1016/j.carbpol.2017.02.095
34.
Nomura T . Function and application of a non-ester-hydrolyzing carboxylesterase discovered in tulip. Biosci Biotech Bioch. (2017) 81:81–94. 10.1080/09168451.2016.1240608
35.
Joshi S Kaushik V Gode V Mhaskar S . Coconut oil and immunity: what do we really know about it so far?J Assoc Physicians India. (2020) 68:67–72.
36.
Zhu X Guo Y Liu Z Yang J Tang H Wang Y . Itaconic acid exerts anti-inflammatory and antibacterial effects via promoting pentose phosphate pathway to produce ROS. Sci Rep-UK. (2021) 11:18173. 10.1038/s41598-021-97352-x
37.
Li X Morita S Yamada H Koga K Ota W Furuta T et al . Free linoleic acid and oleic acid reduce fat digestion and absorption in vivo as potent pancreatic lipase inhibitors derived from sesame meal. Molecules. (2022) 27:4910. 10.3390/molecules27154910
38.
Alonso-Castro AJ Serrano-Vega R Pérez Gutiérrez S Isiordia-Espinoza MA Solorio-Alvarado CR . Myristic acid reduces skin inflammation and nociception. J Food Biochem. (2022) 46:e14013. 10.1111/jfbc.14013
39.
Sharma H Reeta KH Sharma U Suri V . Decanoic acid mitigates ischemia reperfusion injury by modulating neuroprotective, inflammatory and oxidative pathways in middle cerebral artery occlusion model of stroke in rats. J Stroke Cerebrovasc Dis. (2023) 32:107184. 10.1016/j.jstrokecerebrovasdis.2023.107184
40.
Kepka A Chojnowska S Okungbowa OE Zwierz K . The role of carnitine in the perinatal period. Dev Period Med. (2014) 18:417–25.
41.
Geier DA Geier MR . L-carnitine exposure and mitochondrial function in human neuronal cells. Neurochem Res. (2013) 38:2336–41. 10.1007/s11064-013-1144-7
42.
Saini RK Keum YS . Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance - a review. Life Sci. (2018) 203:255–67. 10.1016/j.lfs.2018.04.049
43.
Miles EA Childs CE Calder PC . Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: a narrative review. Nutrients. (2021) 13:247. 10.3390/nu13010247
44.
Fahy E Subramaniam S Brown HA Glass CK Merrill AH Jr Murphy RC et al . (2005). A comprehensive classification system for lipids. J. Lipid Res. 46, 839–861. 10.1194/jlr.E400004-JLR200
45.
Matyash V Liebisch G Kurzchalia TV Shevchenko A Schwudke D . (2008). Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 5, 1137–1146. 10.1194/jlr.D700041-JLR200
Summary
Keywords
lipids, fatty acids, milk, donkey, lactation stage
Citation
Du X, Ma Z, Wang C and Zhou M (2025) Lipid profile analysis of donkey milk during the lactation. Front. Nutr. 12:1662407. doi: 10.3389/fnut.2025.1662407
Received
09 July 2025
Accepted
29 August 2025
Published
19 September 2025
Volume
12 - 2025
Edited by
Francesca Rigano, University of Messina, Italy
Reviewed by
Ranko S. Romanić, University of Novi Sad, Serbia
Shuangshuang Wang, Zhengzhou University of Light Industry, China
Updates
Copyright
© 2025 Du, Ma, Wang and Zhou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changfa Wang wangchangfa@lcu.edu.cnMiaomiao Zhou zhoumm0329@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.