- 1Department of Vascular Surgery, Ningbo Medical Center Lihuili Hospital, Ningbo University, Ningbo, China
- 2Department of Clinical Nutrition, Shanghai Deji Hospital, Qingdao University, Shanghai, China
Background: Accumulating data have elucidated a significant association between insulin resistance (IR) and the risk of cardiovascular diseases. The estimated glucose disposal rate (eGDR), a quantitative indicator of glucose metabolism, has been increasingly recognized as a reliable clinical indicator of IR. Atherosclerotic cardiovascular disease (ASCVD) is a type of cardiovascular disease caused by atherosclerosis. However, the association and potential mechanisms between eGDR and ASCVD risk remain unclear.
Methods: We conducted cross-sectional and retrospective cohort studies using data from the two health examination centers of Qingdao University Affiliated Shanghai Deji Hospital and Ningbo Medical Center Lihuili Hospital to horizontally and longitudinally evaluate the potential relationship and explore potential mechanisms between eGDR and the risk of ASCVD.
Results: A significant negative linear association between eGDR and ASCVD risk was observed in our study. After adjusting for some covariates, each unit increment in eGDR was associated with a 16.1% decrease in odds ratio of ASCVD (OR = 0.839, 95% CI: 0.806–0.894) in the cross-sectional study and a 12.4% decrease in hazard ratio of ASCVD (HR = 0.876, 95% CI: 0.788, 0.974) in the retrospective cohort study. This negative association was robust in most subgroups and various sensitivity analyses. Furthermore, eGDR presented greater predictive performances for ASCVD diagnosis and new-onset ASCVD risk compared with TyG, HOMA-IR, and METS-IR. In addition, AIP, VAI, CMI, LAP, and BMI had partial mediation effects on the association between eGDR and ASCVD risk.
Conclusion: This study elucidates the significant negative dose-response linear relationship of eGDR with ASCVD risk and the partial mediation effects of AIP, VAI, CMI, LAP, and BMI, highlighting the significance of enhancing glucose-utilizing capacity in decreasing the risk of ASCVD.
1 Introduction
Atherosclerotic cardiovascular disease (ASCVD), which constitutes a spectrum of vascular pathologies including coronary, cerebral, and peripheral arterial manifestations, has epidemiologically emerged as the leading global burden of cardiovascular disease and fatal outcomes worldwide (1, 2). In the USA, ASCVD accounts for 28–43% of mortality in cardiovascular diseases (3, 4). Some studies have demonstrated that hypertension, hyperlipidemia, smoking, and diabetes are established risk factors in improving the occurrence and progression of ASCVD risk. However, there are no exact etiological mechanisms for the occurrence and progression of ASCVD. In addition, recent evidence has demonstrated that insulin resistance (IR) may be an important risk factor for increasing the risk of ASCVD (5). The study of Shi et al. demonstrated that HOMA-IR and METS-IR, well-established biomarkers of IR, exhibit a significant dose-response relationship with ASCVD risk (6). However, the potential mechanisms of the association between IR and ASCVD remain incompletely elucidated, and it is imperative to further explore possible mechanisms using large population research.
Insulin resistance (IR) represents a fundamental endocrine dysfunction wherein target tissues demonstrate impaired sensitivity to physiological insulin action at both receptor and post-receptor levels, constituting a core metabolic disorder in numerous chronic diseases (7, 8). This metabolic disorder is primarily manifested through impaired glucose homeostasis, particularly in skeletal muscle and adipose tissue, resulting from defective insulin-stimulated glucose uptake and utilization (9, 10). The progression of IR has been shown to involve multifactorial etiology, with genetic predisposition and environmental determinants synergistically contributing to its pathogenesis through complex gene-environment interactions (11, 12). The estimated glucose disposal rate (eGDR), calculated by waist circumference and hypertension status, hemoglobin A1c (HbA1c), has emerged as a validated surrogate marker for IR (13, 14). When eGDR levels increased, glucose utilization ability is enhanced, which suggests a low risk of IR. Therefore, eGDR demonstrates significant clinical utility and predictive value in the risk of IR (15, 16). Currently, some evidence has elucidated a significant negative dose-dependent association between high eGDR levels and incident adverse cardiovascular disease, particularly myocardial infarction and ischemic stroke. However, the relationship of eGDR with ASCVD risk remains unclear. In addition, some epidemiological studies have demonstrated that obesity status is linked with both eGDR and ASCVD (17–19). For example, Geng et al. demonstrate that eGDR was negatively associated with WWI, as an obesity related index, in two cohort studies in China and the UK (20). In addition, Makhmudova et al. used UK Biobank data to explore the association between visceral obesity and risk of ASCVD, and their findings reveal that high visceral obesity increased the risk of ASCVD (21). Therefore, based on the above evidence, we speculate that obesity may be a possible mediating factor in the association of eGDR with ASCVD risk. However, no studies have yet investigated the potential mediating effect of obesity in the association of eGDR with ASCVD risk.
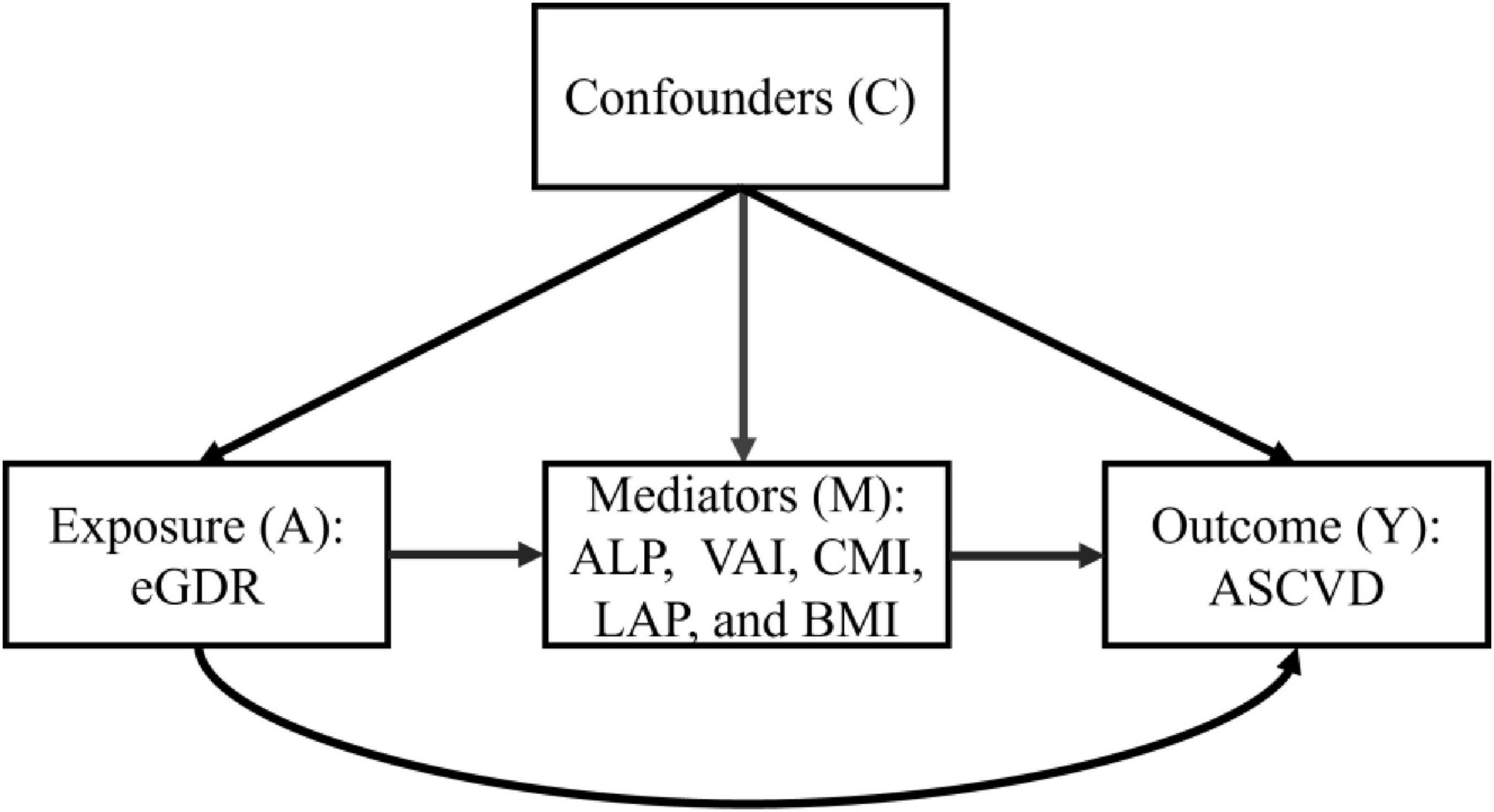
To elucidate this relationship and potential mechanisms, we conducted cross-sectional and retrospective cohort studies using population health examination data from the health examination centers of Qingdao University Affiliated Shanghai Deji Hospital and Ningbo Medical Center Lihuili Hospital to horizontally and longitudinally examine the association of eGDR with ASCVD risk and investigate the potential mediating effects of five obesity-related biomarkers, including the atherogenic index of plasma (AIP), visceral adiposity index (VAI), cardiometabolic index (CMI), lipid accumulation product (LAP), and body mass index (BMI).
2 Materials and methods
2.1 Study design and participants
We designed a cross-sectional study using population health examination data from June 1, 2011, to June 1, 2012, in the two health examination centers of Qingdao University Affiliated Shanghai Deji Hospital and Ningbo Medical Center Lihuili Hospital to horizontally explore the association of eGDR and the risk of ASCVD. Furthermore, we also conducted a retrospective cohort study by following up four times with individuals without ASCVD from June 6, 2012, to December 31, 2024 to longitudinally explore the association of eGDR and new-onset ASCVD risk. The average follow-up time for these individuals was 8.2 years. Individuals with newly diagnosed ASCVD were the primary outcome in our retrospective cohort study. This cross-sectional and retrospective cohort studies were approved by the Ethics Committee of the Qingdao University Affiliated Shanghai Deji Hospital (SHDJ-2025-10) and Ningbo Medical Center Lihuili Hospital (NBLHLH-2025-15). Each individual voluntarily signed informed consent documents in accordance with the Declaration of Helsinki principles.
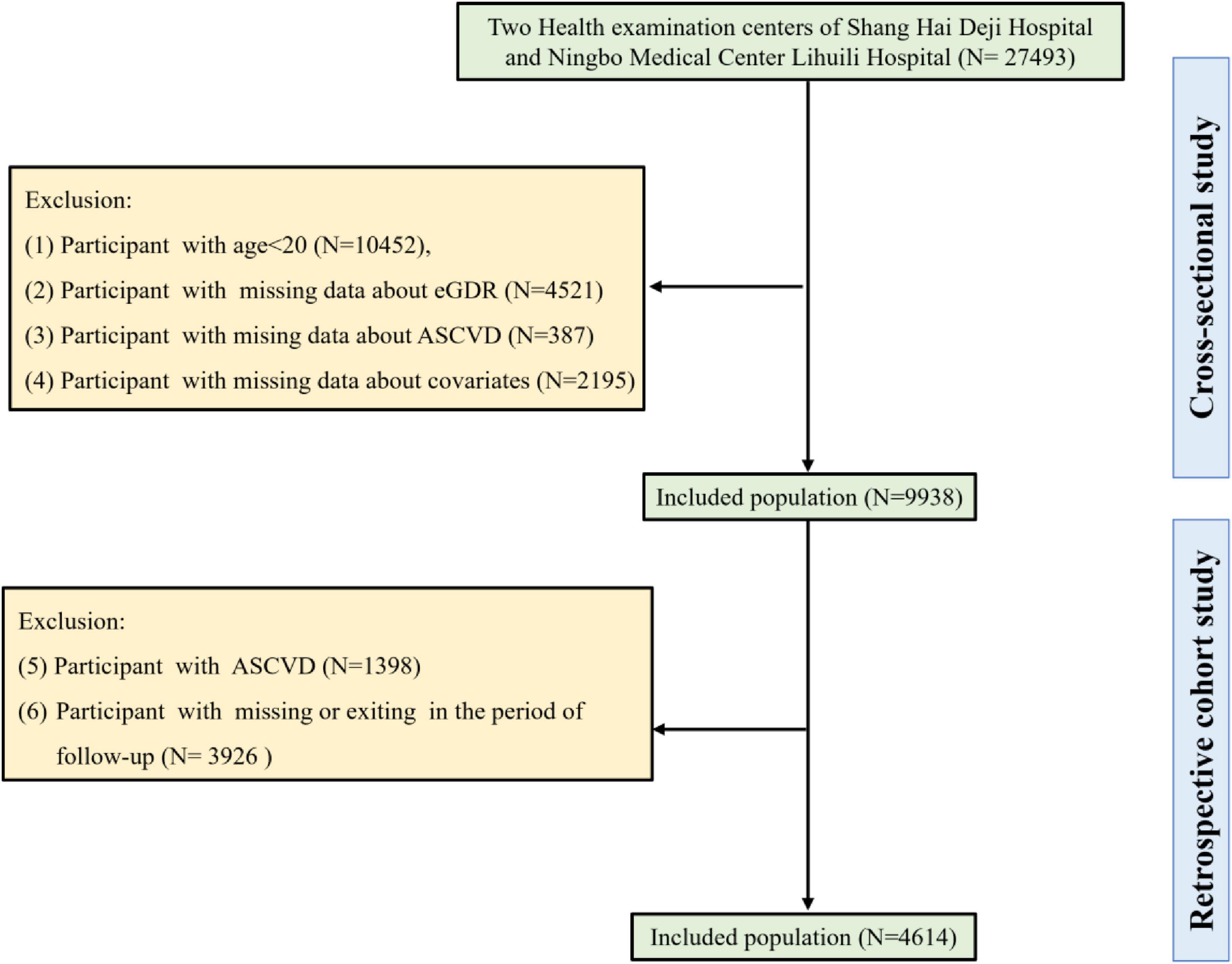
Our cross-sectional study initially included 27,493 individuals from two health examination centers of Qingdao University Affiliated Shanghai Deji Hospital and Ningbo Medical Center Lihuili Hospital. According to the exclusion criteria of our cross-sectional study, individuals under 20 years of age (N = 10,452), those with missing eGDR data (N = 4,521), missing ASCVD data (N = 387), and missing covariate information (N = 2,195) were excluded. Finally, 9,938 individuals were included in our cross-sectional study. In addition, 4,614 participants were included in our retrospective cohort study after excluding individuals with ASCVD (N = 1,398), and those with missing or exiting in the period of follow-up (N = 3,926). A detailed flowchart (Figure 1) delineated the participant selection process.
2.2 Measurement of the eGDR
The eGDR was calculated using a multivariable-adjusted algorithm: 21.158− (0.09 × WC) − (3.407 × HT) − (0.551 × HbA1c); incorporating: WC (waist circumference in cm), HT (hypertension status defined as 1 = present, 0 = absent), and HbA1c (glycated hemoglobin measured in %) (22).
2.3 Definition of ASCVD
Following the American Heart Association guidelines and established clinical criteria, ASCVD was defined as: participants with physician-diagnosed cardiovascular conditions, including coronary artery disease, angina pectoris, myocardial infarction, cerebrovascular accident, congestive heart failure, and stroke (23).
2.4 Measurement of AIP, VAI, CMI, LAP, and BMI
The related obesity indices were calculated as the following formulas, respectively:
AIP = log10 [TG (mg/dL)/HDL-C (mg/dL)].
VAI (male) = [WC (cm)/39.68 + (1.88 × BMI)] × [TG (mmol/L)/1.03] × [1.31/HDL − C (mmol/L)].
VAI (female) = [WC (cm)/36.58 + (1.89 × BMI)] × [TG (mmol/L)/0.81] × [1.52/HDL − C (mmol/L)].
CMI = [TG (mmol/L)/HDL-C (mmol/L)] × [WC (cm)/height (cm)],
LAP (male) = [WC (cm) − 65] × [TG (mg/dL)], LAP (female) = [WC (cm) − 58] × [TG (mg/dL)], BMI = [weight (kg)/height2 (m)] (24–28).
2.5 Covariates
Our study included the following sociodemographic, lifestyle, and clinical biomarker covariates. Sociodemographic and lifestyle covariates included age (<60 years, ≥ 60 years), gender (female and male), education levels (less than high school, high school, more than college), and poverty index ratio (PIR) (< 1.0, 1.0–3.0, and ≥ 3.0), body mass index (BMI) (< 18.5, 18.5–24.9, 25–29.9, ≥ 30 kg/m2), smoking status (never/former/current smoker) and drinking status (never/former/current drinker), physical activity (vigorous/moderate/low level). Current smoker was regarded as participants with a lifetime cumulative smoking of ≥ 100 cigarettes, and were still smoking at the time of the survey. Former smoker was regarded as participants with a lifetime cumulative smoking of ≥ 100 cigarettes, but had quit smoking at the time of the survey. Never smoker was regarded as participants with a lifetime cumulative smoking of ≤ 100 cigarettes (29). Current drinker was determined as participants who have consumed any alcoholic beverage within the past 12 months. Former drinker was determined as participants who previously drank regularly but have abstained from alcohol for more than 12 months. Never drinker was determined as participants with a lifetime alcohol consumption < 12 drinks (30). Vigorous activity refers to engaging in vigorous physical activity at least 3 days per week, accumulating a total of at least 1,500 MET-minutes per week. Moderate activity refers to engaging in at least 30 min of moderate-intensity activity or brisk walking on at least 5 days per week. Low-level activity refers to those who do not meet the standards for “moderate” or “Vigorous” levels (31, 32). Hypertension was defined as participants with three times average systolic blood pressure (SBP) of ≥ 130 and a diastolic blood pressure (DBP) of ≥ 80 mmHg, or diagnosed by a physician, or usage of lower blood pressure medicine, which was categorized into yes or no. Diabetes was defined as participants with fasting glucose ≥ 126 mg/dL or HbA1c ≥ 6.5%, or diagnosis by a physician, or usage of lower blood glucose medicine, which was categorized into yes or no (33, 34). Clinical biomarkers covariates included waist circumference (WC), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), glycated hemoglobin A1c (HbA1c), fast glucose, C-reactive protein (CRP), uric acid (UA), total cholesterol (TC), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), and lactate dehydrogenase (LDH).
2.6 Statistical analysis
In this study, statistical analysis was performed on the baseline characteristics of the participants, using Student’s t-test for continuous variables and χ2 tests for categorical variables. The eGDR was evaluated as both continuous and categorical variables, with quartile-based stratification (Q1–Q4) applied for categorical analyses. To assess potential associations between eGDR and ASCVD risk, we conducted multivariable logistic regression models, a multivariable Cox proportional hazards regression model, and restricted cubic spline (RCS) regression models. The predictive performance of eGDR for ASCVD diagnosis was evaluated using receiver operating characteristic (ROC) curve analysis, C-statistic index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI). The predictive value of eGDR for new-onset ASCVD risk was evaluated using a time-dependent receiver operating characteristic (Time-dependent ROC) curve, C-index, NRI, and IDI. To evaluate the robustness of the association of eGDR with ASCVD risk, we conducted subgroup analyses and sensitivity analyses, such as using multiple imputations for missing covariate data, deleting participants with hypertension, diabetes, and chronic kidney disease, depression, and cancer, and using Fine-Gray competing-risk models that included deaths of non-ASCVD as competing events. We used the mediation analysis repeated bootstrap sampling method (N = 5,000) to explore the mediation effect of obesity-related indices (AIP, VAI, CMI, LAP, and BMI). The causal framework was shown in Figure 2. To control the potential type I error, multiple testing corrections were performed through the Bonferroni correction method. All statistical analyses were performed using R software (version 4.3.3). P < 0.05 was used as the statistical significance criterion.
3 Results
3.1 Baseline demographic characteristics of the participants
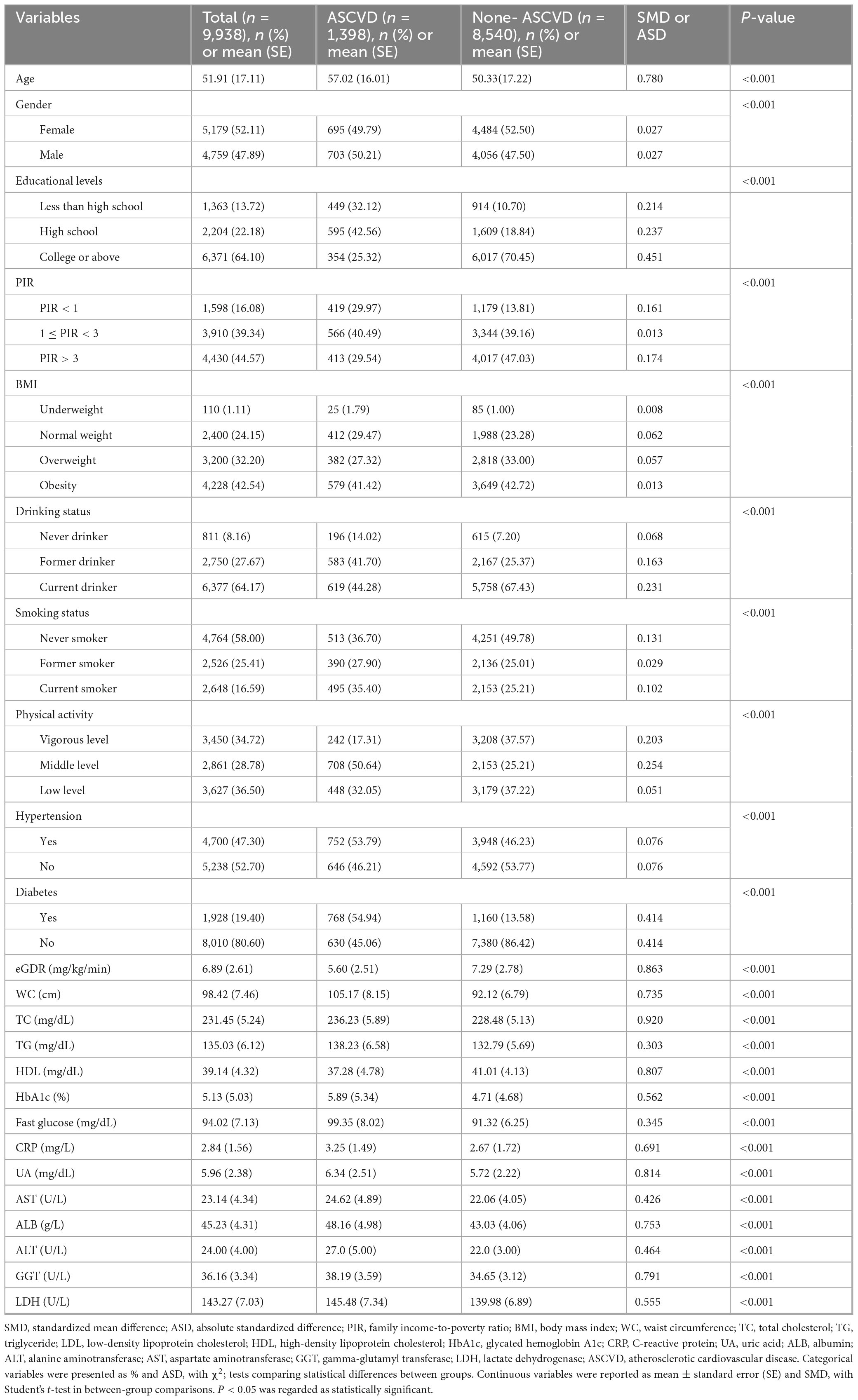
Table 1 and Supplementary Table 1 summarized the baseline characteristics of the participants. As shown in Table 1, this cross-sectional study included 9,938 participants aged ≥ 20 years, among whom 52.11% (n = 5,179) were female, and 14.07% (n = 1,398) met diagnostic criteria for ASCVD. In addition, our retrospective cohort study comprised 4,614 participants, among whom 47.88% (n = 2,209) were female, and 18.80% (n = 868) were new-onset ASCVD (Supplementary Table 1). Significant differences (P < 0.05) were observed across all covariates when comparing participants with ASCVD to those with non-ASCVD in cross-sectional and retrospective cohort studies. In addition, the baseline demographic characteristics of the included and excluded participants were displayed in Supplementary Table 2, and no significant differences (P > 0.05) were observed across most covariates when comparing included participants to those excluded in the retrospective cohort study.
3.2 Association between eGDR and the risk of ASCVD
In the cross-sectional study, after adjusting for some covariates, each standardized unit increase in eGDR was associated with a significantly lower odds of ASCVD, with an odds ratio (OR) of 0.839 (OR = 0.839, 95% CI: 0.806–0.894, p< 0.001). In addition, the highest quartile of the eGDR group was associated with a markedly lower odds of ASCVD (OR = 0.405, 95% CI: 0.276–0.576, p < 0.001) compared with the lowest-quartile referent group after adjusting for some covariates (Table 2). To further explore the association between eGDR and the risk of new-onset ASCVD, we conducted a retrospective cohort study based on a large population survey. Similarly, in our retrospective cohort study, each standardized unit increase in eGDR was associated with a significantly 12.4% lower hazard ratio (HR) of new-onset ASCVD (HR = 0.876, 95% CI: 0.788–0.974, p = 0.001) after adjusting for some covariates. Furthermore, compared with the lowest quartile of the eGDR referent group, the HR of new-onset ASCVD in the highest quartile group markedly lowered by 58.3% (HR = 0.417, 95% CI: 0.325–0.554, p < 0.001) after adjusting for some covariates (Table 2). The RCS curves were conducted to assess the potential non-linear relationship between eGDR and the risk of ASCVD. Additionally, after adjusting for some covariates, RCS curves demonstrated that GDR exhibited a dose-response relationship with OR of ASCVD in the cross-sectional study (P for overall < 0.001, P for nonlinear = 0.136) and the HR of new-onset ASCVD in the retrospective cohort (P for overall < 0.001, P for nonlinear = 0.176) in Figure 3.
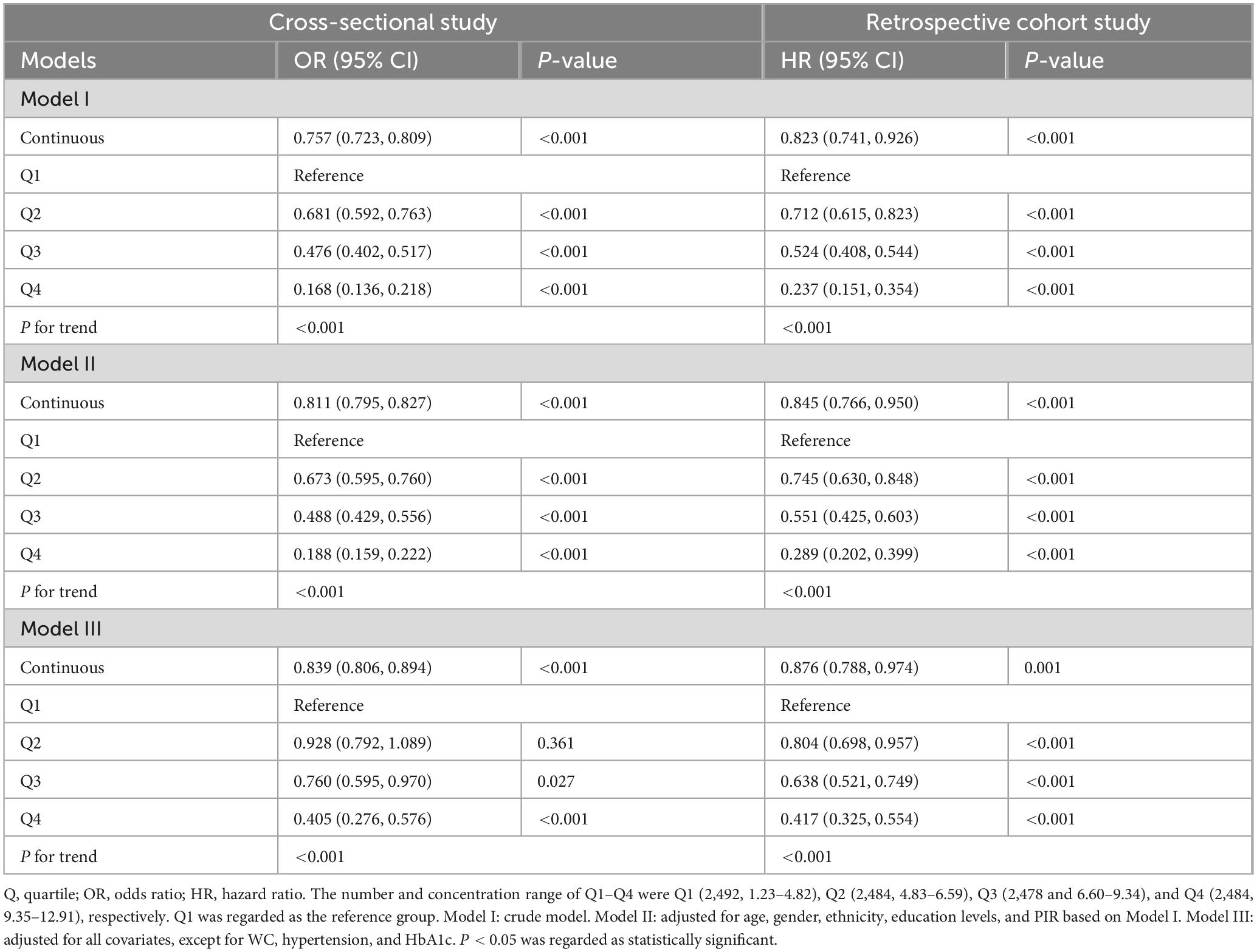
Table 2. Association between eGDR and the risk of ASCVD in cross-sectional and retrospective studies.
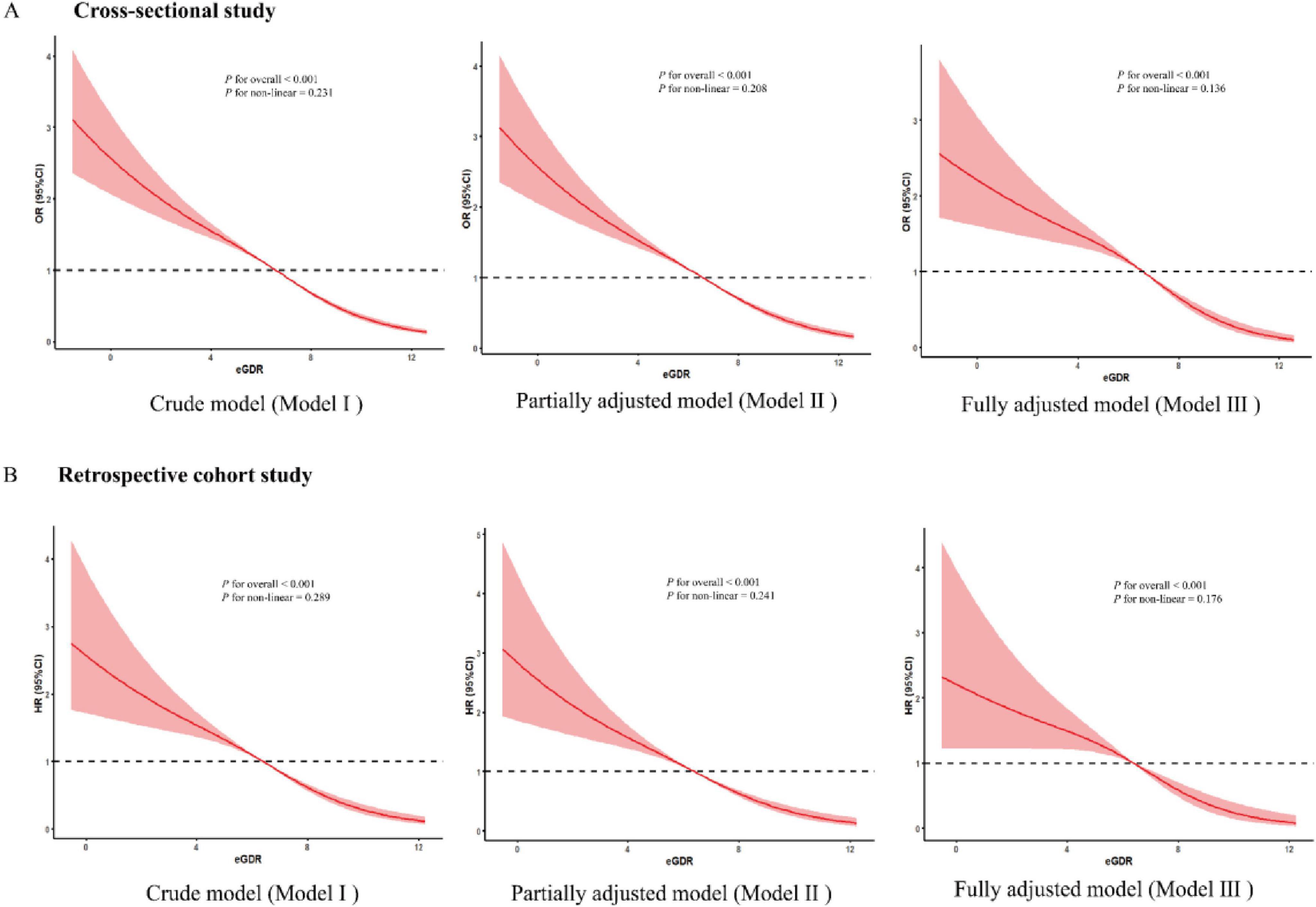
Figure 3. The dose-response association between eGDR and the risk of ASCVD. (A) The dose-response relationship between eGDR and ASCVD of the three models in a cross-sectional study. (B) The dose-response relationship between eGDR and new-onset ASCVD risk of the three models in a retrospective study.
3.3 Subgroup analyses
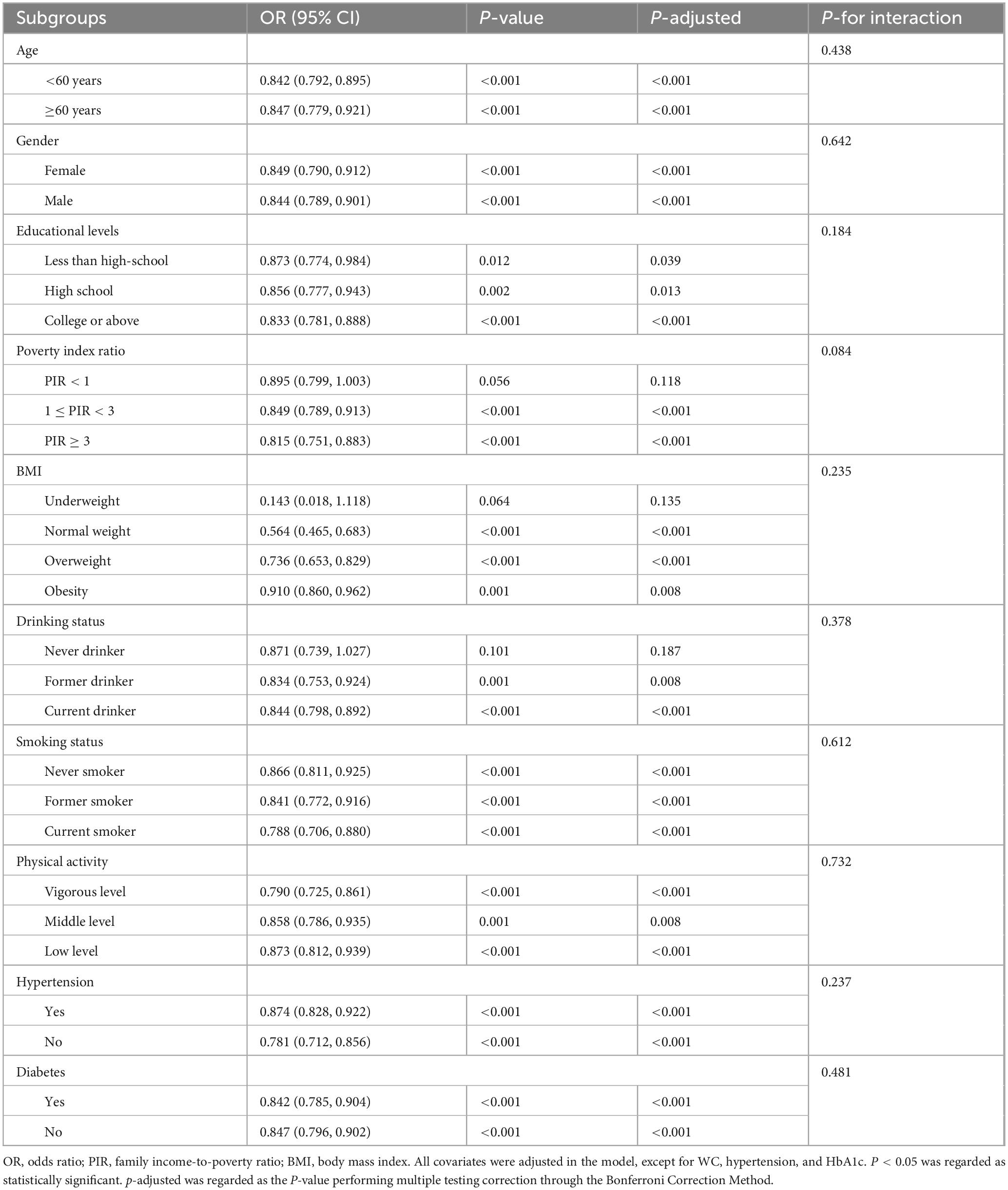
In this study, to validate the consistency of the negative association between eGDR and the risk of ASCVD, we conducted subgroup analyses stratified by key sociodemographic and lifestyle characteristics, including age, gender, ethnicity, etc., in our cross-sectional and retrospective cohort studies. As detailed in Table 3 and Supplementary Table 3, in multivariable-adjusted logistic regression and Cox proportional hazard regression analyses, negative associations between eGDR and the risk of ASCVD persisted across most prespecified subgroups after adjusting for some covariates, indicating a stable effect of eGDR for ASCVD risk regardless of population characteristics. Notably, subgroup analyses also revealed no significant effect modification by these population characteristics on the association between eGDR and the risk of ASCVD (all P for interaction > 0.05).
3.4 Increased discrimination effect of eGDR on the diagnosis of ASCVD in the cross-sectional analysis
Emerging evidence has established that IR-related indicators, such as TyG, HOMA-IR, and METS-IR, were associated with ASCVD (35–40). However, the diagnostic utility of eGDR, as a novel IR-related metric, for ASCVD diagnosis remained unestablished. Therefore, in this study, we employed the receiver operating characteristic (ROC) curve and some related key predictive indicators (C-statistic index, NRI, and IDI) to evaluate the diagnostic performance of eGDR on ASCVD in the cross-sectional analysis. As shown in Figure 4A and Supplementary Table 4, our results revealed that after adjusting for some covariates, all IR-related indicators have a significant diagnostic performance on the ASCVD diagnosis (all P < 0.05). Furthermore, eGDR presented a moderate diagnostic performance for the diagnosis of ASCVD, with the C-statistic index (0.706; 95% CI, 0.685–0.729), and had positive NRI and IDI in comparison with TyG, HOMA-IR, and METS-IR, suggesting the increased discrimination performance of eGDR on ASCVD (all P < 0.05). In addition, the result of the calibration curves also showed that eGDR had greater diagnostic performance compared with TyG, HOMA-IR, and METS-IR (Figure 4B).
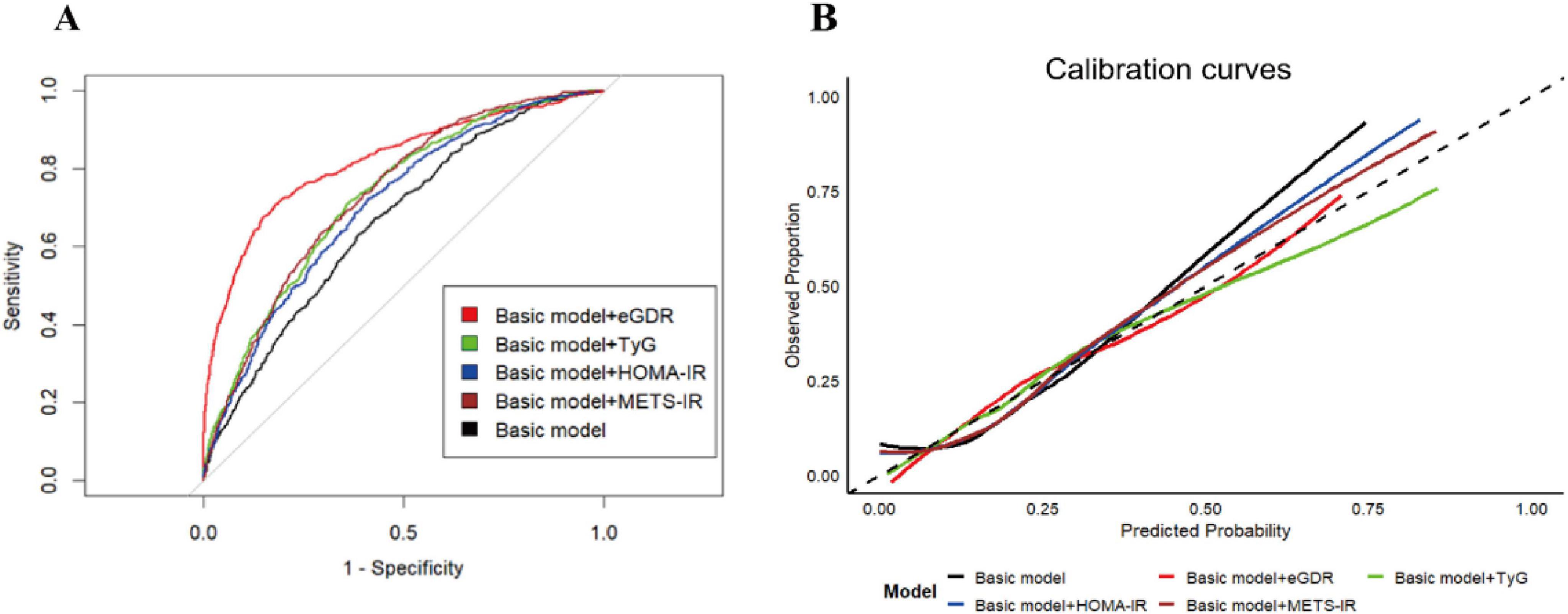
Figure 4. The diagnostic performance of IR-related indicators on the ASCVD diagnosis. The ROC curves (A) and calibration curves (B) for predictive performance of the ASCVD diagnosis. The basic model was adjusted for all covariates, except for WC, hypertension, and HbA1c.
3.5 Increased predictive performance of eGDR on the risk of new-onset ASCVD in the retrospective cohort analysis
Currently, the predictive utility of eGDR, as a novel IR-related metric, for new-onset ASCVD risk remained unclear. Similarly, in our retrospective cohort analysis, we conducted the time-dependent receiver operating characteristic curve with times set at 5 and 10 years (Time-dependent ROC) and used some related key predictive indicators (C-index, NRI, and IDI) to evaluate the predictive performance of eGDR on new-onset ASCVD risk. Time-dependent ROC results demonstrated that eGDR showed a consistently moderate predictive effect on the 5-year risk (AUC = 0.790, 95% CI: 0.778–0.817) and 10-year risk (AUC = 0.822, 95% CI: 0.802–0.844) of new-onset ASCVD (Figures 5A–E). In addition, as presented in Supplementary Table 5, eGDR had the highest C-index of 0.792 (95% CI: 0.766, 0.828) and positive NRI and IDI compared to TyG, HOMA-IR, and METS-IR (all P < 0.05), indicating eGDR improved risk reclassification of new-onset ASCVD. In addition, the result of the calibration curves also revealed that eGDR had greater predictive performance compared with TyG, HOMA-IR, and METS-IR (Figure 5F).
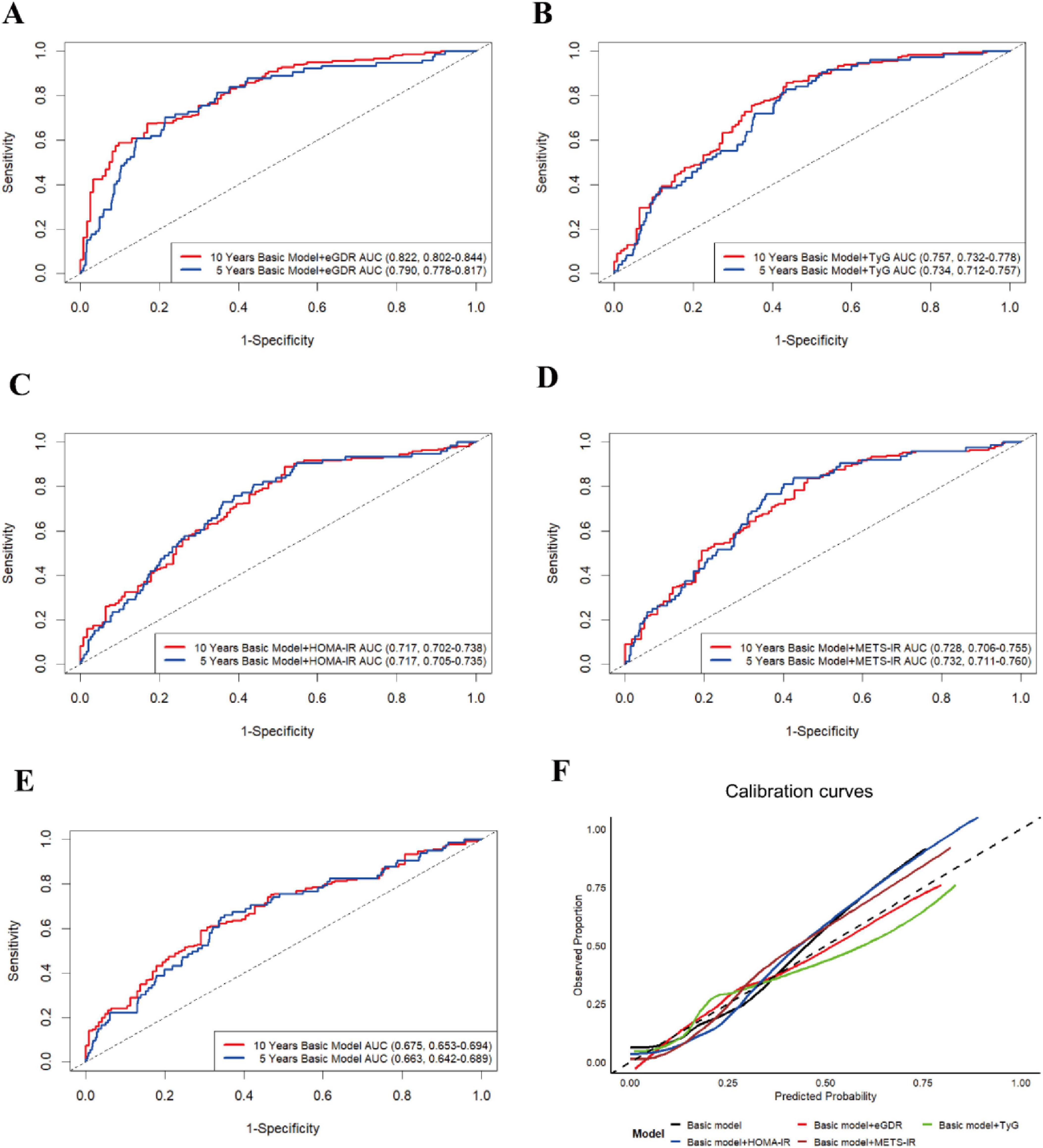
Figure 5. The time-dependent ROC curve (times = 5 and 10 years) for predictive effect of eGDR (A), TyG (B), HOMA-IR (C), METS-IR (D), and basic model (E) on the new-onset ASCVD risk. (F) The calibration curves (10 years) for the predictive performance of the new-onset ASCVD. The basic model was adjusted for all covariates, except for WC, hypertension, and HbA1c.
3.6 The mediation effects of five obesity-related markers
The mediation analysis was conducted to explore the mediation role of five obesity-related indicators (AIP, VAI, CMI, LAP, and BMI) on the negative associations between eGDR and the risk of ASCVD in our cross-sectional and retrospective cohort study through bootstrapped samples. First, we explore the associations between eGDR and AIP, VAI, CMI, LAP, and BMI. As detailed in Supplementary Table 6, a significant negative association of eGDR with AIP, VAI, CMI, LAP, and BMI was observed (all P < 0.05). Then, the association of AIP, VAI, CMI, LAP, and BMI with ASCVD risk was assessed. Our results showed that five related obesity markers were positively associated with the risk of ASCVD in Supplementary Table 7 (all P < 0.05). Finally, the result of the mediation analysis illustrated that mediation effects of AIP, VAI, CMI, LAP, and BMI on the association between eGDR and the risk of ASCVD, with the mediated proportion of 17.40 and 15.86% for AIP, 9.46 and 10.78% for VAI, 14.12 and 12.29% for CMI, 3.25 and 4.56% for LAP, and 12.13 and 13.47% for BMI, in the cross-sectional study and the retrospective cohort study, respectively (Figure 6). These results suggested that the association was partially mediated by obesity. In addition, we performed the exposure-mediator interaction to explore their potential interactive effect, and our results showed that no exposure-mediator interaction was observed in Supplementary Table 8 (all P > 0.05).
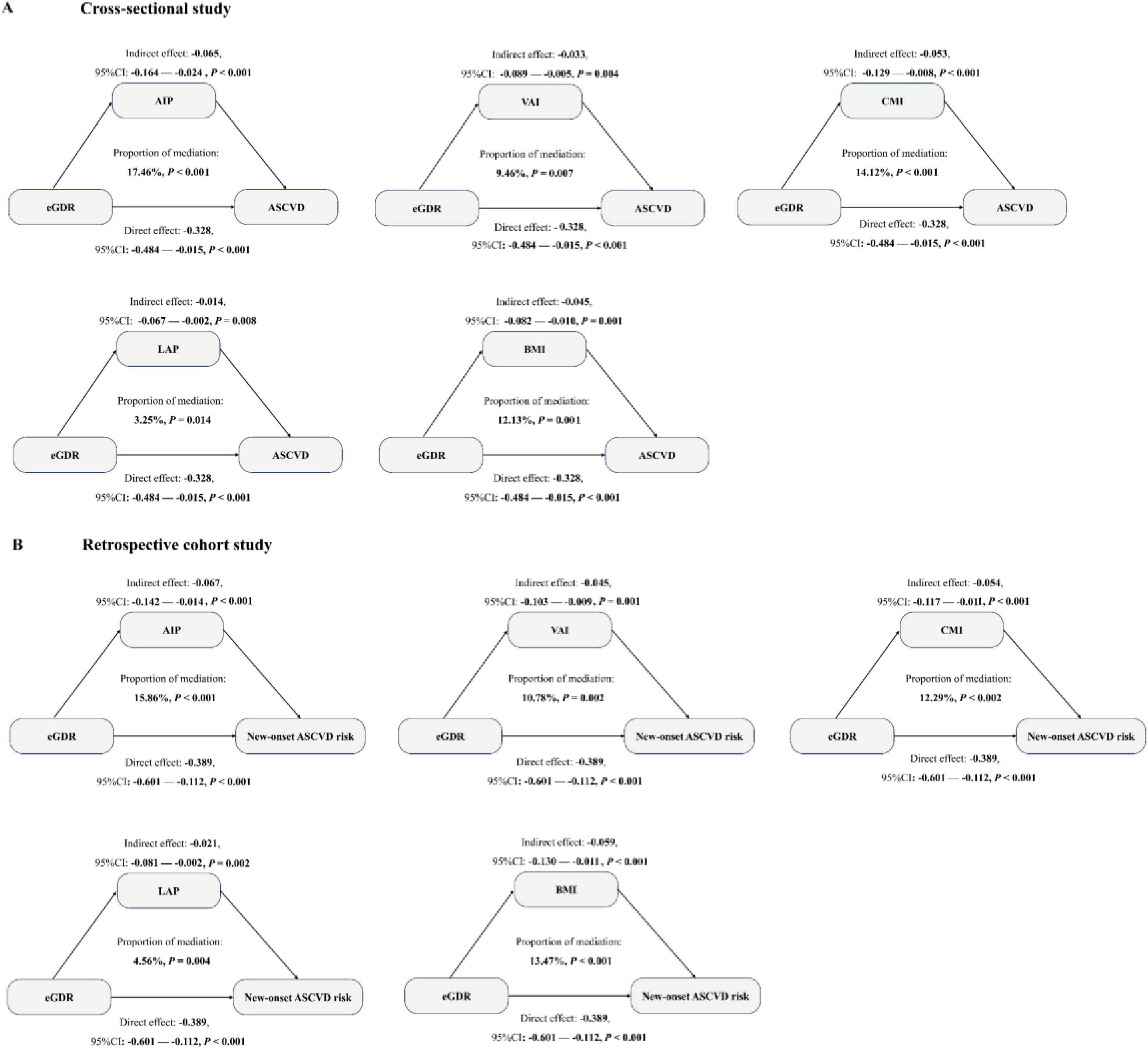
Figure 6. Mediation analysis was used to show the mediation effects of five related obesity indices (AIP, VAI, CMI, LAP, and BMI) in a cross-sectional study (A) and a retrospective cohort study (B). The model was adjusted for all covariates, except for WC, hypertension, and HbA1c.
3.7 Sensitivity analyses
To evaluate the consistency of the observed negative relationship of eGDR with the risk of ASCVD, various sensitivity analyses were performed. Since excessive missing covariate data might impact the association between eGDR and ASCVD risk, we handled these missing covariate data by multiple imputation methods in our cross-sectional and retrospective analysis, and the results demonstrated that the negative association between eGDR and ASCVD risk remained robust after imputation of these missing covariate data (Supplementary Table 9). To avoid the impact of nutritional factors on this association, we have additionally included some nutritional factors, such as energy intake, macronutrient compositions in the diet (intake of saturated fats, refined carbohydrates, and fiber), into our models. Our results showed that the negative association was robust (Supplementary Table 10). To reduce the impact of comorbidity on this relationship, we deleted participants with diabetes, chronic kidney disease, depression, and cancers in the cross-sectional and retrospective cohort analysis. These results also demonstrated that eGDR was negatively associated with the risk of ASCVD (Supplementary Table 12).
Since the applied eGDR equation was first confirmed on type 1 diabetes cohorts and is yet to be completely confirmed in the overall Chinese population. To justify the establishment of the eGDR in the Chinese general population, we added other factors (e.g., BMI) to the formula of eGDR to explore this association. Our findings revealed that this association remains negative (Supplementary Table 11). To minimize the effect of death with non-ASCVD on this association in the retrospective cohort study, we used the Fine-Gray competing-risk model, in which deaths with non-ASCVD were treated as competing-risk events, and our results showed that this association was consistent with the main results after treating death with non-ASCVD as competing-risk events (Supplementary Table 13). To lower the effect of new-onset ASCVD itself on metabolic markers such as HbA1c, blood pressure, and adiposity, leading to lower eGDR values after disease onset. We deleted new-onset ASCVD during our first follow-up to reperform this association between eGDR and new-onset ASCVD risk, and our results showed that this association was consistent with the main results (Supplementary Table 14).
4 Discussion
Epidemiological data have consistently demonstrated a close correlation between abnormal glucose and insulin levels and the risk of cardiovascular disease, such as heart attack, angina, heart failure, and stroke (41). Furthermore, accumulating clinical evidence indicates that elevated levels of IR biomarkers, including TyG, HOMA-IR, and METS-IR, are remarkably linked with increased cardiovascular disease risk. Individuals with cardiovascular disease exhibit markedly higher concentrations of IR-related markers than those without the disease. Mechanically, IR-related markers could increase endothelial dysfunction by activating NF-κB or PI3K/Akt signaling pathways, increasing reactive oxygen species (ROS) production, and secreting proinflammatory cytokines (TNF-α, IL-6, IL-1β), to further improve cardiovascular disease risk. In addition, some IR-related markers also impact the thrombotic status by upregulation of PAI-1 and aggregation of platelets to increase the risk of cardiovascular disease. eGDR is a novel IR indicator that integrates multidimensional metabolic parameters, including WC, hypertension status, and HbA1c, which is more accurate in response to the glucose metabolic levels and cost-effective in clinical practice. Currently, the association of eGDR with the risk of ASCVD remains unclear, and the potential mechanisms are not illustrated.
In this study, we used population health examination data from two health examination centers of Qingdao University Affiliated Shanghai Deji Hospital and Ningbo Medical Center Lihuili Hospital to design cross-sectional and retrospective cohort studies to investigate the relationship of eGDR with the risk of ASCVD. Our results showed that eGDR is negatively and linearly associated with ASCVD risk in the cross-sectional and retrospective cohort studies. And ASCVD risk is lower with eGDR increased after adjusting for some covariates, for example, compared to the lowest quartile of eGDR, the ASCVD risk of the highest quartile of eGDR decreased by 60%, which suggests the potential impact of public health strategies aimed at improving insulin sensitivity across the population. Conversely, the more modest hazard ratio for a single-unit decrease in eGDR reflects the incremental change in risk an individual might expect from a small change in insulin sensitivity. While this per-unit effect is modest, it suggests that achievable improvements in insulin resistance through lifestyle modification could confer meaningful risk reduction at both the individual and population level.
Additionally, this negative relationship maintains consistency in various subgroups, suggesting the association is stable and generalizable. In addition, our results were consistent with findings from Yan et al.’s nationwide prospective cohort study of China, in which eGDR was negatively associated with cardiovascular risk in general adults (42), and these findings were contrasted with Niswender et al., who found that this significant negative association could only be observed in women, and the negative association was not significant in males (43). The observed discrepancy of the association in the study of Niswender et al. likely originates from their methodological limitations, including restricted sample size and inherent design constraints of epidemiological approaches, notably the exclusion of male participants, which may introduce selection bias.
eGDR, as a novel IR-related indicator, is widely used to calculate the glucose metabolic levels in clinical studies due to its being less costly and having a more comprehensive formula for glucose utilization. When eGDR levels increased, the glucose utilization capability improved, which suggests a low risk of developing insulin resistance. Currently, the prediction performance of eGDR on ASCVD diagnosis and the risk of new-onset ASCVD remains unclear. Therefore, in our cross-sectional study, we conducted the ROC curve, calculating the C-statistic index, NRI, and IDI to assess the predictive performance of eGDR for ASCVD diagnosis. Our results showed that compared to TyG, HOMA-IR, and METS-IR, eGDR had a moderate predictive value on ASCVD diagnosis with the positive Δ C-statistic index, NRI, and IDI (all P < 0.05). Additionally, in our retrospective cohort study, we conducted the time-dependent ROC curve (times = 5 and 10 years) and calculated the C-index, NRI, and IDI to evaluate the prediction performance of eGDR on the risk of new-onset ASCVD. Our findings also demonstrated that compared to TyG, HOMA-IR, and METS-IR, eGDR had the greatest predictive effects on the risk of new-onset ASCVD with positive Δ C-index, NRI, and IDI (all P < 0.05). These results suggested eGDR is a more valuable IR biomarker in predicting ASCVD diagnosis and new-onset ASCVD risk, offering novel insights into ASCVD prevention strategies through improving glucose utilization capability and insulin sensitivity.
Currently, the potential mechanisms of the association of eGDR with the risk of ASCVD are not fully clear. Accumulated epidemiological studies have consistently shown a close link between eGDR and obesity (44, 45). For example, in a case-control study from China (N = 418 adults), Yang et.al demonstrated that the prevalence of obesity in adults decreased as eGDR levels increased (46). Furthermore, some studies have demonstrated that obesity status could impact the prevalence and incidence risk of ASCVD. Asztalos et al. revealed that after controlling for some confounding variables, increased BMI levels could independently correlate with an increase in ASCVD risk in a large cohort study from the USA (N = 226,000 middle-aged and elderly populations) (47). Consequently, we hypothesize that obesity may play a vital mediating role in the association between eGDR and the risk of ASCVD. However, there are no studies to explore the mediating effect of related obesity markers in the relationship of eGDR with ASCVD risk. Therefore, in our study, we explored the association between five obesity-related indices (AIP, VAI, CMI, LAP, and BMI) and ASCVD and eGDR and performed mediation analysis to evaluate the role of AIP, VAI, CMI, LAP, and BMI in the association between eGDR and ASCVD risk. Our findings demonstrated that AIP, VAI, CMI, LAP, and BMI were significantly negatively associated with eGDR and positively associated with ASCVD risk. Furthermore, AIP, VAI, CMI, LAP, and BMI had significant mediation effects (all P < 0.05), which suggested eGDR was negatively associated with ASCVD risk by partially mediating obesity status. Some biological evidence has supported our results. Low levels of eGDR indicate insulin resistance, which readily leads to visceral fat accumulation (48). Excessive visceral fat accumulation further triggers adipose tissue dysfunction and lipotoxicity, initiating a complex cascade of events including systemic chronic inflammation, dysregulated adipokine secretion, residual cholesterol accumulation, mitochondrial dysfunction, and endoplasmic reticulum stress (49–52). Ultimately, these processes contribute to the onset and progression of ASCVD.
Our study has several strengths. First, this study shows the moderate predictive efficacies of eGDR for ASCVD diagnosis and the risk of new-onset ASCVD compared with IR-related markers (TyG, HOMA-IR, METS-IR). Second, various potential confounding factors are adjusted to reduce the effect of these covariates on this relationship. Third, various sensitivity analyses are performed to examine the robustness of the results. Finally, this study presents that the obesity-related indices have partial mediation effects on the association between eGDR and ASCVD risk.
There are some limitations to our study. First, in our cross-sectional and retrospective cohort study, some potential confounding factors, such as unmeasured genetic predispositions (e.g., polygenic risk scores for ASCVD), were not adjusted, which may introduce bias in the observed associations. Second, in our retrospective cohort study, some individuals may be lost due to missing, death, etc., during the period of follow-up, which may affect the representativeness of the results. Third, the participants were recruited from health examination centers, which may lead to a “health volunteer bias.” This sample likely over-represents health-conscious individuals with better overall health profiles and access to healthcare, thereby limiting the generalizability of our absolute risk estimates to the broader Chinese adult population. This selection bias could potentially attenuate the observed associations by restricting the range of both risk factors and disease outcomes, or conversely, exaggerate them if the healthiest subset drives the association. Nevertheless, the internal relationships identified within this cohort are still informative. Future research should aim to replicate these findings in more representative, population-based samples to confirm their external validity. Fourth, our study shows the greater predictive effect of eGDR compared with other related IR indices; however, we do not evaluate the predictive performance of eGDR against established ASCVD risk scores such as the China-PAR model. Fifth, the eGDR exposure metric is itself a composite of waist circumference and hypertension statusture research should aim ons by retors for ASCVD. This may create a tautological loop where the predictor and outcome are not sufficiently independent. Sixthly, we revealed the mechanism that obesity partially mediated this association between eGDR and ASCVD. However, there are other factors to affect the association of eGDR with ASCVD. Finally, our study could not reveal the causal relationship of eGDR with the risk of ASCVD. Therefore, in the future, well-designed prospective longitudinal cohort studies and mechanistic animal experiments will be prioritized to establish causal relationships and elucidate the underlying pathophysiological mechanisms.
5 Conclusion
Our cross-sectional and retrospective cohort studies reveal a significant negative dose-response linear relationship between eGDR and the risk of ASCVD. Additionally, compared to TyG, HOMA-IR, and METS-IR, eGDR demonstrates moderate diagnostic capacities for ASCVD diagnosis and predictive capacities for the risk of new-onset ASCVD. In addition, the AIP, VAI, CMI, LAP, and BMI partially mediated the association between eGDR and the risk of ASCVD. In summary, this study elucidates the negative association of eGDR with ASCVD risk, and AIP, VAI, CMI, LAP, and BMI may partially mediate the negative association, advancing the importance of enhancing glucose utilization capability in lowering the risk of cardiovascular disease.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This cross-sectional and retrospective cohort studies were approved by the Ethics Committee of the Qingdao University Affiliated Shanghai Deji Hospital (SHDJ-2025-10) and Ningbo Medical Center Lihuili Hospital (NBLHLH-2025-15). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Funding acquisition. QZ: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. TZ: Conceptualization, Formal analysis, Investigation, Project administration, Writing – review & editing, Data curation. TH: Conceptualization, Data curation, Formal analysis, Project administration, Methodology, Writing – original draft. KY: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing – original draft, Investigation, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Ningbo University (2024BSKY-WZX).
Acknowledgments
We acknowledge with profound appreciation the indispensable contributions of individuals of the two health examination centers of Qingdao University Affiliated Shanghai Deji Hospital and Ningbo Medical Center Lihuili Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1664591/full#supplementary-material
References
1. Wargny M, Goronflot T, Piriou PG, Pouriel M, Bastien A, Prax J, et al. Persistent gaps in the implementation of lipid-lowering therapy in patients with established atherosclerotic cardiovascular disease: a French nationwide study. Diabetes Metab. (2025) 51:101638. doi: 10.1016/j.diabet.2025.101638
2. Kong X, Cai Y, Li Y, Wang P. Causal relationship between apolipoprotein B and risk of atherosclerotic cardiovascular disease: a mendelian randomization analysis. Health Inf Sci Syst. (2025) 13:13. doi: 10.1007/s13755-024-00323-5
3. Alkandari H, Jayyousi A, Shalaby A, Alromaihi D, Subbarao G, ElMohamedy H, et al. Prevalence of atherosclerotic cardiovascular disease in people with type 2 diabetes in the Gulf Region: results from the PACT-MEA study. Public Health. (2025) 242:21–7. doi: 10.1016/j.puhe.2025.02.026
4. Moorthy S, Chen Z, Zhang T, Ponnana SR, Sirasapalli SK, Shivanantham K, et al. The built environment and adverse cardiovascular events in US veterans with cardiovascular disease. Sci Total Environ. (2025) 980:179596. doi: 10.1016/j.scitotenv.2025.179596
5. Semmler G, Balcar L, Wernly S, Völkerer A, Semmler L, Hauptmann L, et al. Insulin resistance and central obesity determine hepatic steatosis and explain cardiovascular risk in steatotic liver disease. Front Endocrinol. (2023) 14:1244405. doi: 10.3389/fendo.2023.1244405
6. Shi Y, Zhang Z, Yang J, Cui S. The association between insulin resistance-related markers and ASCVD with hyperuricemia: results from the 2005 to 2018 National Health and Nutrition Examination Survey. Front Cardiovasc Med. (2025) 12:1583944. doi: 10.3389/fcvm.2025.1583944
7. Yang YX, Xiang JC, Ye GC, Luo KD, Wang SG, Xia QD. Association of insulin resistance indices with kidney stones and their recurrence in a non-diabetic population: an analysis based on NHANES data from 2007-2018. Ren Fail. (2025) 47:2490203. doi: 10.1080/0886022X.2025.2490203
8. Xu L, Chen M, Yan C, Li X, Ni X, Zhou M, et al. Age-specific abnormal glucose metabolism in HIV-positive people on antiviral therapy in China: a multicenter case-control study. Ann Med. (2025) 57:2427910. doi: 10.1080/07853890.2024.2427910
9. Ntetsika T, Catrina SB, Markaki I. Understanding the link between type 2 diabetes mellitus and Parkinson’s disease: role of brain insulin resistance. Neural Regen Res. (2025) 20:3113–23. doi: 10.4103/NRR.NRR-D-23-01910
10. Wang Y, Wang H, Cheng B, Xia J. Associations between triglyceride glucose index-related obesity indices and anxiety: insights from the National Health and Nutrition Examination Survey 2007-2012. J Affect Disord. (2025) 382:443–52. doi: 10.1016/j.jad.2025.04.134
11. Niu J, Rodriguez E, Štambuk T, Trbojević-Akmačić I, Mraz N, Seissler J, et al. Longitudinal study reveals plasma glycans associations with prediabetes/type 2 diabetes in KORA study. Cardiovasc Diabetol. (2025) 24:321. doi: 10.1186/s12933-025-02853-y
12. Zhang Y, Song J, Zhang M, Xiong G, Guo Y, Liu W, et al. Arsenic exposure, genetic susceptibility, lifestyle, and glucose-insulin homeostasis impairment: revealing the association and interaction in a repeated-measures prospective study. Environ Res. (2025) 283:122085. doi: 10.1016/j.envres.2025.122085
13. Chen Y, Zheng L, Zhou Y, Hou Y, Zhou Y, Shen H. The role of cardiovascular disease in the association between estimated glucose disposal rate and chronic kidney disease. Sci Rep. (2025) 15:16034. doi: 10.1038/s41598-025-00359-x
14. Huo G, Yao Z, Yang X, Wu G, Chen L, Zhou D. Association between estimated glucose disposal rate and stroke in middle-aged and older Chinese adults: a Nationwide Prospective Cohort Study. J Am Heart Assoc. (2025) 14:e039152. doi: 10.1161/JAHA.124.039152
15. Elhabashy SA, Abdelhaleem BA, Madkour SS, Kamal CM, Salah NY. Body composition and regional adiposity in adolescents with type 1 diabetes: relation to insulin resistance, glycaemic control and vascular complications. Diabetes Metab Res Rev. (2025) 41:e70041. doi: 10.1002/dmrr.70041
16. Sagar RC, Yates DM, Pearson SM, Kietsiriroje N, Hindle MS, Cheah LT, et al. Insulin resistance in type 1 diabetes is a key modulator of platelet hyperreactivity. Diabetologia. (2025) 68:1544–58. doi: 10.1007/s00125-025-06429-z
17. Giannakopoulou SP, Barkas F, Chrysohoou C, Liberopoulos E, Sfikakis PP, Pitsavos C, et al. Comparative analysis of obesity indices in discrimination and reclassification of cardiovascular disease risk: the ATTICA study (2002-2022). Eur J Intern Med. (2025) 134:96–103. doi: 10.1016/j.ejim.2025.02.007
18. Elshorbagy A, Vallejo-Vaz AJ, Barkas F, Lyons ARM, Stevens CAT, Dharmayat KI, et al. Overweight, obesity, and cardiovascular disease in heterozygous familial hypercholesterolaemia: the EAS FH Studies Collaboration registry. Eur Heart J. (2025) 46:1127–40. doi: 10.1093/eurheartj/ehae791
19. Tong XC, Liu K, Xu JY, Zhang XJ, Xue Y. A negative correlation of low estimated glucose disposal rate with significant liver fibrosis in adults with NAFLD and obesity: results from NHANES 2017-2020. BMC Gastroenterol. (2025) 25:206. doi: 10.1186/s12876-025-03798-y
20. Geng Y, Gu S, Fan Y, Lu X, Zhou Z, Zhang N, et al. Weight-adjusted-waist index, estimated glucose disposal rate, C-reactive protein, and mortality risks among individuals with type 2 diabetes: 2 prospective cohort studies in China and the United Kingdom. Am J Clin Nutr. (2025) 122:1442–51. doi: 10.1016/j.ajcnut.2025.09.018
21. Makhmudova U, Wild B, Williamson A, Steinhagen-Thiessen E, Langenberg C, Eils R, et al. Visceral adipose tissue, aortic distensibility and atherosclerotic cardiovascular risk across body mass index categories. Eur J Prev Cardiol. (2025) [Online ahead of print.]. doi: 10.1093/eurjpc/zwaf447
22. Li X, Zeng Z. Exploring the link between estimated glucose disposal rate and Parkinson’s disease: cross-sectional and mortality analysis of NHANES 2003-2016. Front Aging Neurosci. (2025) 17:1548020. doi: 10.3389/fnagi.2025.1548020
23. Li J, Chen XL, Ou-Yang XL, Zhang XJ, Li Y, Sun SN, et al. Association of tea consumption with all-cause/cardiovascular disease mortality in the chronic kidney disease population: an assessment of participation in the national cohort. Ren Fail. (2025) 47:2449578. doi: 10.1080/0886022X.2025.2449578
24. Zhang H, Shi L, Tian N, Zhu M, Liu C, Hou T, et al. Association of the atherogenic index of plasma with cognitive function and oxidative stress: a population-based study. J Alzheimers Dis. (2025) 105:13872877251334826. doi: 10.1177/13872877251334826
25. Su Z, Cao L, Chen H, Zhang P, Wu C, Lu J, et al. Obesity indicators mediate the association between the aggregate index of systemic inflammation (AISI) and type 2 diabetes mellitus (T2DM). Lipids Health Dis. (2025) 24:176. doi: 10.1186/s12944-025-02589-4
26. Fan W, Guo W, Chen Q. Association between cardiometabolic index and infertility risk: a cross-sectional analysis of NHANES data (2013-2018). BMC Public Health. (2025) 25:1626. doi: 10.1186/s12889-025-22679-3
27. Cui A, Zhuang Y, Wei X, Han S. The association between lipid accumulation products and bone mineral density in U.S. Adults, a nationally representative cross-sectional study. Sci Rep. (2025) 15:16373. doi: 10.1038/s41598-025-00833-6
28. Xu H, Zhang Z, Wang Y. Weight loss methods and risk of depression: evidence from the NHANES 2005-2018 cohort. J Affect Disord. (2025) 380:756–66. doi: 10.1016/j.jad.2025.03.199
29. Oo MZ, Tint SS, Panza A, Pongpanich S, Viwattanakulvanid P, Bodhisane S, et al. Associated factors of smoking behaviors among industrial workers in Myanmar: the role of modifying factors and individual beliefs, guided by the health belief model. Front Public Health. (2025) 13:1655922. doi: 10.3389/fpubh.2025.1655922
30. Chen X, Wu J, Wang Y, He Y, Ye H, Liu J. Depressive symptom trajectories and incident metabolic syndrome in middle-aged and older adults: a longitudinal analysis of the ELSA study. Front Psychiatry. (2025) 16:1666316. doi: 10.3389/fpsyt.2025.1666316
31. Sai-Chuen Hui S, Chin EC, Chan JK, Chan BP, Wan JH, Wong SW. Association of “weekend warrior” and leisure time physical activity patterns with health-related physical fitness: a cross-sectional study. BMC Public Health. (2025) 25:3316. doi: 10.1186/s12889-025-24711-y
32. Pan Y, Zhang W, Dai Y, Liu Y, Gao D, Zhang Y, et al. Association between accelerometer-measured physical activity, genetic risk, and incident type 2 diabetes: a prospective cohort study. Diabetes Obes Metab. (2025) 27:7578–86. doi: 10.1111/dom.70166
33. Hong T, Lian Z, Zhang C, Zhang W, Ye Z. Hypertension modifies the association between serum Klotho and chronic kidney disease in US adults with diabetes: a cross-sectional study of the NHANES 2007-2016. Ren Fail. (2025) 47:2498089. doi: 10.1080/0886022X.2025.2498089
34. Liu C, Yang J, Li H, Deng Y, Dong S, He P, et al. Association between life’s essential 8 and diabetic kidney disease: a population-based study. Ren Fail. (2025) 47:2454286. doi: 10.1080/0886022X.2025.2454286
35. Li S, Liu HH, Zhang Y, Zhang M, Zhang HW, Zhu CG, et al. Association of triglyceride glucose-derived indices with recurrent events following atherosclerotic cardiovascular disease. J Obes Metab Syndr. (2024) 33:133–42. doi: 10.7570/jomes23055
36. Xia X, Chen S, Tian X, Xu Q, Zhang Y, Zhang X, et al. Association of triglyceride-glucose index and its related parameters with atherosclerotic cardiovascular disease: evidence from a 15-year follow-up of Kailuan cohort. Cardiovasc Diabetol. (2024) 23:208. doi: 10.1186/s12933-024-02290-3
37. Hou Q, Qi Q, Han Q, Yu J, Wu J, Yang H, et al. Association of the triglyceride-glucose index with early-onset atherosclerotic cardiovascular disease events and all-cause mortality: a prospective cohort study. Cardiovasc Diabetol. (2024) 23:149. doi: 10.1186/s12933-024-02249-4
38. Elsabaawy M, Naguib M, Abuamer A, Shaban A. Comparative application of MAFLD and MASLD diagnostic criteria on NAFLD patients: insights from a single-center cohort. Clin Exp Med. (2025) 25:36. doi: 10.1007/s10238-024-01553-3
39. Antonio-Villa NE, Juárez-Rojas JG, Posadas-Sánchez R, Reyes-Barrera J, Medina-Urrutia A. Visceral adipose tissue is an independent predictor and mediator of the progression of coronary calcification: a prospective sub-analysis of the GEA study. Cardiovasc Diabetol. (2023) 22:81. doi: 10.1186/s12933-023-01807-6
40. Wu Z, Lan Y, Wu D, Chen S, Jiao R, Wu S. Arterial stiffness mediates insulin resistance-related risk of atherosclerotic cardiovascular disease: a real-life, prospective cohort study. Eur J Prev Cardiol. (2025) 2025:zwaf030. doi: 10.1093/eurjpc/zwaf030
41. Peng W, Li Z, Fu N. Association between eGDR and MASLD and liver fibrosis: a cross-sectional study based on NHANES 2017-2023. Front Med. (2025) 12:1579879. doi: 10.3389/fmed.2025.1579879
42. Yan L, Zhou Z, Wu X, Qiu Y, Liu Z, Luo L, et al. Association between the changes in the estimated glucose disposal rate and new-onset cardiovascular disease in middle-aged and elderly individuals: a nationwide prospective cohort study in China. Diabetes Obes Metab. (2025) 27:1859–67. doi: 10.1111/dom.16179
43. Niswender KD, Fazio S, Gower BA, Silver HJ. Balanced high fat diet reduces cardiovascular risk in obese women although changes in adipose tissue, lipoproteins, and insulin resistance differ by race. Metabolism. (2018) 82:125–34. doi: 10.1016/j.metabol.2018.01.020
44. Gu M, Zhang D, Wu Y, Li X, Liang J, Su Y, et al. Association between brachial-ankle pulse wave velocity, obesity-related indices, and the 10-year incident risk score of atherosclerotic cardiovascular disease: the rural Chinese cohort study. Nutr Metab Cardiovasc Dis. (2025) 35:103791. doi: 10.1016/j.numecd.2024.103791
45. Pavanello C, Ruscica M, Castiglione S, Mombelli GG, Alberti A, Calabresi L, et al. Triglyceride-glucose index: carotid intima-media thickness and cardiovascular risk in a European population. Cardiovasc Diabetol. (2025) 24:17. doi: 10.1186/s12933-025-02574-2
46. Yang D, Zhou J, Garstka MA, Xu Q, Li Q, Wang L, et al. Association of obesity- and insulin resistance-related indices with subclinical carotid atherosclerosis in type 1 diabetes: a cross-sectional study. Cardiovasc Diabetol. (2025) 24:193. doi: 10.1186/s12933-025-02736-2
47. Asztalos BF, Russo G, He L, Diffenderfer MR. Body mass index and cardiovascular risk markers: a large population analysis. Nutrients. (2025) 17:740. doi: 10.3390/nu17050740
48. Han XD, Li YJ, Wang P, Han XL, Zhao MQ, Wang JF, et al. Insulin resistance-varying associations of adiposity indices with cerebral perfusion in older adults: a population-based study. J Nutr Health Aging. (2023) 27:219–27. doi: 10.1007/s12603-023-1894-2
49. Parsaei M, Karimi E, Barkhordarioon A, Yousefi M, Tarafdari A. Lipid accumulation product and visceral adiposity index in polycystic ovary syndrome: a systematic review and meta-analysis of observational studies. Lipids Health Dis. (2025) 24:311. doi: 10.1186/s12944-025-02719-y
50. Shen X, Li M, Li Y, Jiang Y, Niu K, Zhang S, et al. Bazi Bushen ameliorates age-related energy metabolism dysregulation by targeting the IL-17/TNF inflammatory pathway associated with SASP. Chin Med. (2024) 19:61. doi: 10.1186/s13020-024-00927-9
51. Kataoka H, Nitta K, Hoshino J. Visceral fat and attribute-based medicine in chronic kidney disease. Front Endocrinol. (2023) 14:1097596. doi: 10.3389/fendo.2023.1097596
Keywords: atherosclerotic cardiovascular disease, estimated glucose disposal rate, cross-sectional study, retrospective cohort study, mediation analysis
Citation: Wang Z, Zhou Q, Zheng T, Huang T and Yi K (2025) Association between estimated glucose disposal rate and the risk of atherosclerotic cardiovascular disease: insight from cross-sectional and retrospective cohort studies. Front. Nutr. 12:1664591. doi: 10.3389/fnut.2025.1664591
Received: 12 July 2025; Revised: 22 October 2025; Accepted: 17 November 2025;
Published: 10 December 2025.
Edited by:
Serafino Fazio, Federico II University Hospital, ItalyReviewed by:
Shirong Tan, Salk Institute for Biological Studies, United StatesQiying Ye, Florida State University, United States
Copyright © 2025 Wang, Zhou, Zheng, Huang and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kefan Yi, eWtmMjI0NjE4QDE2My5jb20=
 Zixuan Wang1
Zixuan Wang1 Kefan Yi
Kefan Yi