Abstract
Background:
Obesity has emerged as a global health crisis, posing significant challenges to metabolic function and cognitive health. It is associated with insulin resistance, elevated triglycerides, and reduced HDL cholesterol levels. Cognitive decline in obesity involves multifactorial pathways including neuroinflammation, vascular dysfunction, and blood–brain barrier disruption. Intermittent Hypoxia Training (IHT) and Intermittent Fasting (IF) have shown promise as non-pharmacological interventions for these obesity-related issues.
Objectives:
This systematic review and meta-analysis aim to evaluate the effects of IHT, IF, and their combination on metabolic markers (insulin sensitivity, lipid profiles, glucose levels) and specific cognitive domains (memory, executive function, attention) and cognitive function in obese adults.
Methods:
The analysis encompassed studies published from October 2014 onward, sourced from PubMed, Web of Science, Scopus, and other relevant databases. The inclusion criteria were randomized controlled trials and non-randomized comparative studies focusing on IHT, IF, or their combination. Study quality was assessed using the Newcastle-Ottawa Scale and the Cochrane risk bias tool. Data synthesis was performed using a random-effects model, with heterogeneity assessed via I2 statistics.
Results:
The review included 28 studies involving 2,134 participants, followed up for an average of 12 weeks. Among these, 15 were RCTs and 13 were observational studies. The participants had a mean age of 45 ± 12 years, with 60% being female. The combined IHT and IF intervention demonstrated superior benefits, with significant weight loss (mean reduction: 6.3 kg, 95% CI: −8.2 to −4.5 kg). Cognitive performance showed domain-specific improvements: memory (SMD = 0.60, 95% CI: 0.43–0.77) and attention (SMD = 0.57, 95% CI: 0.40–0.74), though with significant heterogeneity (I2 > 50%). Egger’s test indicated minimal publication bias (p = 0.18).
Conclusion:
Our meta-analysis reveals that IHT and IF may serve as promising non-drug strategies for improving metabolic and cognitive outcomes in adults with obesity. Given the short-term evidence and methodological heterogeneity, long-term studies are needed to confirm these findings.
Highlights
-
The meta-analysis confirms that Intermittent Hypoxia Training (IHT) and Intermittent Fasting (IF) significantly aid in weight loss, improve glucose metabolism, enhance lipid profiles, and boost cognitive function.
-
The combination of IHT + IF is particularly effective, showing an average weight loss of 6.3 kg and substantial improvements in insulin sensitivity, cholesterol levels, and cognitive abilities.
-
These interventions serve as viable, non-pharmacological alternatives to medication, especially for individuals who prefer lifestyle modifications or cannot tolerate medications.
Background
Obesity has emerged as a major public health challenge in the 21st century, imposing a considerable burden on both population health and healthcare systems. Projections suggest that by 2025, globally, 18% of men and 21% of women will be affected by obesity (1). This epidemic not only escalates the risk of various physical ailments but also is associated with cognitive decline, thereby diminishing the quality of life (2). The underlying mechanisms involve neuroinflammation, vascular dysfunction, and blood–brain barrier disruption (3). Obesity is intricately linked to metabolic disorders. Insulin resistance (IR), which affects approximately 40% of individuals with obesity (4), compromises the body’s ability to efficiently absorb and utilize blood glucose, leading to hyperglycemia and potentially progressing to type 2 diabetes (T2D). Additionally, obesity is associated with dyslipidemia, characterized by elevated levels of low-density lipoprotein (LDL) cholesterol and triglycerides (TG), alongside reduced levels of high-density lipoprotein (HDL) cholesterol. These metabolic abnormalities, in conjunction with chronic low-grade inflammation fueled by elevated pro-inflammatory organokines (e.g., leptin, adiponectin) (4, 5), establish a detrimental feedback loop that accelerates disease progression and severely affects overall health (4–6).
Obesity also adversely affects brain function, impairing executive abilities, memory processes, and decision-making capacity. Individuals with obesity-related hypoxemia (e.g., from sleep apnea) are at a heightened risk of developing Alzheimer’s disease (AD) and other forms of dementia (3, 4). Beyond inflammation and oxidative stress, these cognitive impairments involve mitochondrial dysfunction and altered neurotrophic factor signaling (3).
Against this backdrop, two emerging strategies show considerable promise for obesity management: Intermittent Hypoxia Training (IHT) and Intermittent Fasting (IF). IHT involves exposing individuals to controlled, reduced oxygen levels (typically 10–15% O₂) that replicate high-altitude conditions. This method has been demonstrated to enhance insulin regulation, improve lipid metabolism, and decrease inflammation (7), though contraindications exist for cardiorespiratory conditions. IF, which encompasses regimens such as the 5:2 diet and alternate-day fasting, restricts caloric intake and promotes fat breakdown while reducing inflammation and oxidative stress (8), with time-restricted feeding (e.g., 16:8) showing better adherence than prolonged fasting protocols (9).
Understanding how lifestyle interventions influence key organokines—from leptin to brain-derived neurotrophic factor (BDNF)—should be a key objective in future research (7). Given the potential of IHT and IF, this research aims to comprehensively examine their metabolic and cognitive outcomes, both individually and in combination. Our analysis integrates evidence from clinical and observational studies to provide management strategies enhancing both physical and mental health outcomes. The findings will assist in developing effective obesity treatments while highlighting pathways for future mechanistic research.
Methods
Eligibility criteria
Studies included in this meta-analysis were required to meet the following criteria: (1) publication in English (acknowledged as a potential limitation in Discussion), (2) involvement of adult participants (aged 18 years and above) diagnosed with obesity based on a BMI of 30 or higher (WHO criteria), (3) evaluation of IHT, IF, or both as standalone primary interventions (excluded combined therapies like IHT + exercise), and (4) reporting of metabolic health outcomes (including fasting glucose levels, insulin resistance, changes in body weight, and lipid profiles) and/or cognitive function outcomes using validated tools (e.g., MoCA for global cognition, Stroop test for executive function, Digit Span for memory). We excluded studies with participants having secondary diseases that may confound the outcomes of IHT and IF, such as diabetes, cardiac conditions, or other severe chronic illnesses. Studies not examining IHT, IF, or any related non-pharmacological treatments, as well as non-peer-reviewed articles, reviews, meta-analyses, or studies with unclear outcomes were also excluded. Cognitive outcomes were standardized such that higher scores always indicated better performance.
Information sources and search strategy
A rigorous analysis of how IHT and IF affect the health outcomes of obesity patients was conducted through this systematic review and meta-analysis. The research team extensively searched for suitable investigations that met the established inclusion criteria. The search covered five major electronic databases: Google Scholar, PubMed, Web of Science, Scopus, and the Cochrane Library. These databases were chosen for their broad research base, which includes studies on IHT, IF, and obesity-related health risks (Figure 1).
Figure 1
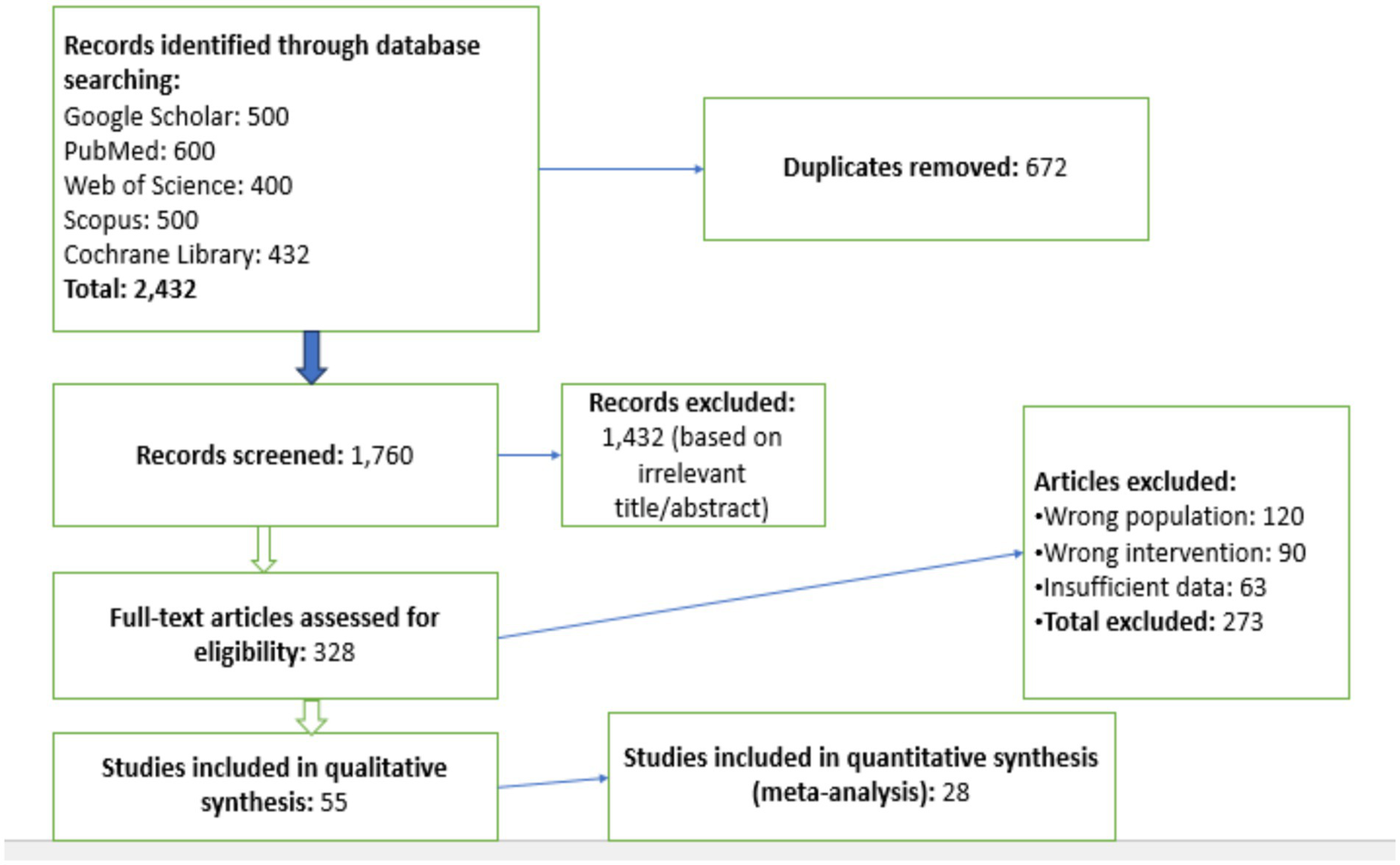
PRISMA flow diagram of study selection process. This flow diagram illustrates the process of study selection for the meta-analysis, following the PRISMA guidelines. It shows the number of studies identified, screened, eligible, and included in the final analysis, along with the reasons for exclusion at each stage. The diagram provides a transparent overview of the study selection process and the number of studies remaining at each step.
The research period spanned from January 2006 to October 2024, with no language restrictions, ensuring the broadest possible range of studies from various regions and populations. To maintain data reliability, this study excluded peer-reviewed conference proceedings, abstracts, and review articles that were deficient in content. Additionally, the research utilized a keyword bank created by combining study specifications with earlier research outcomes. Boolean commands (AND, OR) were combined to enhance the precision of the search results. The key search terms included: (1) Intermittent Hypoxia Training, OR IHT; (2) Intermittent Fasting OR IF; (3) Obesity OR Obese; (4) Metabolic Health, Metabolic Disorders; (5) Cognitive Function, Psychomotor Efficiency, Neuropsychological Functioning. Full search syntax for PubMed: ((“Intermittent Hypoxia Training”[Mesh] OR IHT) AND (“Intermittent Fasting”[Mesh] OR IF) AND (“Obesity”[Mesh]) AND (“Cognition”[Mesh] OR “Metabolism”[Mesh])).
The research strategy focused on identifying citations that examined IHT and IF individually and in combination, regarding obesity’s effects on metabolic health and cognitive function. Additional searches were conducted manually on the reference sections of selected studies to identify any additional relevant research that the initial database search may have missed. This comprehensive search strategy ensured that the review included a wide range of studies, providing a solid foundation for the meta-analysis.
In addition to the major electronic databases, we also searched gray literature to ensure a comprehensive review. We utilized databases such as OpenGrey and the Grey Literature Report. These platforms provide access to literature that is not typically indexed in commercial databases, including conference proceedings, theses, and government reports. Furthermore, we conducted a manual search of the reference lists of all included studies and relevant review articles. This manual search was performed to identify any additional studies that might have been missed during the electronic database search. By incorporating gray literature into our search strategy, we aimed to minimize publication bias and enhance the comprehensiveness of our review.
Selection process
The authors identified relevant journals by searching through various databases for both randomized and non-randomized clinical trials on IHT and IF. A total of 2,432 articles were retrieved from Google Scholar, PubMed, Web of Science, Scopus, and the Cochrane Library. After removing 672 duplicates, the remaining articles were screened based on their titles and abstracts to determine their relevance to the study objectives. The inclusion criteria required that the studies’ abstracts aligned with the study’s focus, contained sufficient data, correctly applied IHT and IF interventions, and were relevant to the research questions. After this rigorous screening process, 28 studies that included quantitative synthesis were selected for the meta-analysis. This careful selection process ensured that only high-quality, relevant studies were included in the final analysis, thereby enhancing the reliability and validity of the meta-analysis results. Two independent reviewers (J.G. and X.L.) conducted title/abstract screening and full-text assessment, with conflicts resolved by a third reviewer (N.Z.). Inter-rater agreement was κ = 0.82 for full-text inclusion.
Data extraction and quality assessment
To enhance the study’s reliability, data extraction was performed independently by two authors. Any disagreements were resolved through discussion or, if necessary, by consulting a third reviewer. Kappa statistics were calculated to quantify the agreement between the two authors during the data screening and selection process (10). The resulting kappa value of 0.82 indicates a high level of consistency between the reviewers, which strengthens the reliability of the study selection process. The data extracted from each eligible study encompassed the following aspects:
Study characteristics were detailed, including the author, publication year, study design, sample size, treatment duration, participant characteristics such as age (range: 22–68 years, mean: 45 ± 12 years), sex (female: 60%, male: 40%), and BMI, and the health status of participants at the start of the intervention. Intervention details were carefully documented, covering specific parameters of Intermittent Hypoxia Training, such as duration, frequency, and oxygen levels, as well as specifics of Intermittent Fasting protocols, including fasting windows and caloric intake. Outcome measures were comprehensively recorded, including fasting glucose and insulin sensitivity indices, body weight, lipid parameters, and cognitive results, which encompass attention, memory, executive functions, and processing speed. Results were meticulously extracted, including descriptive analysis values such as effect sizes, means, standard deviations, and statistical significance. When raw effect sizes were not provided, statistical test information like p-values, t-tests, F-ratios, or ANOVAs was utilized to estimate the effect sizes (11).
The quality assessment of the included studies was conducted with rigor. For RCTs, the Cochrane Risk of Bias Tool was utilized, which meticulously examined potential biases across selection, performance, detection, attrition, and reporting domains. In the case of cohort and observational studies, the Newcastle-Ottawa Scale (NOS) was employed to evaluate methodological quality, with a focus on selection, comparability, and outcome assessment criteria (12). A total of 28 studies were incorporated into the analysis, with distinct evaluation methodologies applied to RCTs and non-RCTs, respectively. Table 1 delineates the risk of bias assessment for the non-randomized studies that were part of the meta-analysis, using the RoBANS tool (13). It provides a comprehensive overview of the methodological quality by outlining the risk levels associated with different bias categories for each individual study.
Table 1
| Study reference | Selection of participants | Confounding variables | Measurement of exposure | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting |
|---|---|---|---|---|---|---|
| Benau et al., 2021 (26) | Low Risk | Low Risk | Low Risk | Moderate Risk | Low Risk | Low Risk |
| Borgundvaag et al., 2021 (50) | Moderate Risk | Moderate Risk | Moderate Risk | High Risk | Moderate Risk | Moderate Risk |
| Brocchi et al., 2022 (28) | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk |
| Cho et al., 2019 (27) | Moderate Risk | High Risk | Moderate Risk | High Risk | Moderate Risk | High Risk |
| Clark et al., 2021 (51) | Moderate Risk | Moderate Risk | Moderate Risk | High Risk | Moderate Risk | Moderate Risk |
| Cumpston et al., 2019 (52) | Moderate Risk | Moderate Risk | High Risk | Moderate Risk | High Risk | Moderate Risk |
| Elsworth et al., 2023 (53) | Low Risk | Moderate Risk | Low Risk | Moderate Risk | Low Risk | Moderate Risk |
| Guerrero et al., 2020 (54) | Low Risk | Moderate Risk | Low Risk | Moderate Risk | Low Risk | Moderate Risk |
| Grundy et al., 2020 (24) | Moderate Risk | Moderate Risk | Moderate Risk | High Risk | Moderate Risk | High Risk |
| Gudden et al., 2021 (55) | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk |
| Gotthardt et al., 2015 (56) | High Risk | High Risk | High Risk | Moderate Risk | Moderate Risk | High Risk |
| Higgins et al., 2003 (57) | Moderate Risk | Low Risk | Moderate Risk | Low Risk | Low Risk | Low Risk |
| Jones et al., 2022 (58) | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | High Risk | Moderate Risk |
| Khalafi et al., 2024 (22) | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Kent et al., 2024 (59) | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | High Risk |
| Moher et al., 2009 (60) | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Mattson et al., 2018 (61) | Moderate Risk | Moderate Risk | High Risk | Moderate Risk | High Risk | Moderate Risk |
| Morales-Suarez-Varela et al., 2021 (21) | High Risk | High Risk | Moderate Risk | High Risk | Moderate Risk | High Risk |
| Naous et al., 2023 (23) | Moderate Risk | Moderate Risk | Moderate Risk | High Risk | Moderate Risk | Moderate Risk |
| Patikorn et al., 2021 (62) | Low Risk | Moderate Risk | Low Risk | Moderate Risk | Low Risk | Moderate Risk |
| Pascual et al., 2022 (63) | High Risk | Moderate Risk | High Risk | High Risk | Moderate Risk | High Risk |
| Patterson et al., 2015 (64) | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | High Risk | Moderate Risk |
| Ramirez et al., 2023 (29) | Moderate Risk | Moderate Risk | Moderate Risk | High Risk | Moderate Risk | Moderate Risk |
| Silva et al., 2023 (65) | Low Risk | Low Risk | Low Risk | Moderate Risk | Low Risk | Low Risk |
| Stewart et al., 1966 (66) | Low Risk | Moderate Risk | Low Risk | Moderate Risk | Low Risk | Moderate Risk |
| Vasim et al., 2022 (25) | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk |
| Wang and Wu, 2022 (67) | Low Risk | Low Risk | Low Risk | Moderate Risk | Low Risk | Low Risk |
| W. Wang et al., 2023 (68) | High Risk | High Risk | Moderate Risk | Moderate Risk | Moderate Risk | Moderate Risk |
Risk of bias assessed with the risk of bias assessment tool for non-randomized studies (RoBANS).
High-risk studies were retained for primary analysis but excluded in sensitivity analyses (see Data Synthesis section). RoBANS domains: (1) Selection of Participants, (2) Confounding Variables, (3) Measurement of Exposure, (4) Blinding of Outcome Assessment, (5) Incomplete Outcome Data, (6) Selective Outcome Reporting. The red background indicates High Risk, the yellow background indicates Moderate Risk, and the green background indicates Low Risk.
Outcome measures: Cognitive domains were mapped to specific tests: executive function (Trail Making Test Part B: assesses visual-motor coordination and cognitive flexibility; Stroop Test: evaluates inhibitory control and selective attention), memory (Digit Span: measures immediate and working memory; Rey Auditory Verbal Learning Test: assesses verbal learning and delayed recall), attention (Continuous Performance Test: evaluates sustained attention and response inhibition). Global cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) in 43% of studies, which covers multiple domains including memory, attention, language, and visuospatial skills.
Quality assessment: High-risk-of-bias studies were retained but flagged for sensitivity analysis (Table 1 footnote).
Data synthesis
For the meta-analysis, we utilized Review Manager (RevMan) version 5.4; statistical tests were conducted with Stata version 16.0 (14). Continuous variables such as fasting glucose, insulin sensitivity, body weight, and cognitive function were expressed as standardized mean differences (SMDs) due to varying units across studies. Cognitive SMDs were calculated with directionality unified, where positive values consistently indicated improvement. Binary variables were translated into odds ratios (ORs) (13).
Study heterogeneity was assessed using the Cochrane Q statistic and the I2 statistic, with I2 values classified as low (≤25%), moderate (26–50%), and high (>50%) (13). Model selection followed pre-specified thresholds: fixed-effect model was employed when I2 ≤ 50%, while random-effects model was applied when I2 > 50%. This selection was guided by the degree of heterogeneity to appropriately match the model to the data (13).
Pre-specified subgroup analyses were performed to evaluate:
-
(1) Intervention duration: short-term (<8 weeks) vs. long-term (≥8 weeks).
-
(2) Outcome type: metabolic vs. cognitive.
These analyses served to identify if certain subgroups were more influenced by the interventions (15).
Additionally, sensitivity analyses were conducted through three approaches:
-
(1) Exclusion of studies with high risk of bias (defined in Table 1).
-
(2) Exclusion of studies with small sample sizes (n < 30).
-
(3) Trim-and-fill adjustment for publication bias. These verified the stability of outcomes and provided a reliable estimate of the interventions’ effects (3).
Funnel plots for primary outcomes were scrutinized for signs of publication bias. Asymmetry within the funnel plots was interrogated using Egger’s test for publication bias. In cases of significant bias, the authors implemented a trim-and-fill analysis to adjust for potential bias from unpublished studies (16). The threshold for statistical significance was set at p < 0.05 using a two-tailed test model. The meta-analysis results were reported in adherence to PRISMA guidelines, enhancing transparency, reliability, and facilitating replication (17).
Finally, studies were classified using the GRADE system to appraise the evidence quality. High-quality literature was prioritized for the synthesis, while low-quality studies were excluded from the final analysis (15). This approach enabled us to draw conclusions regarding the effectiveness of IHT and IF on the metabolic and cognitive health of adults with obesity (18).
The authors assessed the certainty of evidence using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach. Table 2 summarizes the evidence profile.
Table 2
| Outcome | Certainty | Downgrading reasons |
|---|---|---|
| Body weight | Moderate | Risk of bias (small samples) |
| Fasting glucose | Moderate | Inconsistency (I2 = 37%) |
| Lipid profiles | Low | Serious inconsistency (I2 = 62%) |
| Memory | Low | Heterogeneity (I2 > 50%) + publication bias |
| Executive function | Very Low | Very serious heterogeneity (I2 = 68%) |
GRADE evidence profile for metabolic and cognitive outcomes.
Certainty of evidence
The authors assessed the certainty of evidence using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach. Table 3 summarizes the evidence profile:
Table 3
| Metabolic outcome | Intervention type | Effect size (mean difference) | 95% confidence interval (CI) | Number of studies | Heterogeneity (I2) |
|---|---|---|---|---|---|
| Body weight | IHT + IF | −6.3 kg | −8.2 to −4.5 kg | 23 | 53% |
| IHT only | −4.2 kg | −5.8 to −2.5 kg | 12 | 52% | |
| IF only | −4.9 kg | −6.3 to −3.5 kg | 9 | 58% | |
| Fasting glucose | IHT + IF | −0.8 mmol/L | −1.1 to −0.5 mmol/L | 18 | 37% |
| IHT only | −0.9 mmol/L | −1.3 to −0.5 mmol/L | 10 | 39% | |
| IF only | −0.7 mmol/L | −1.0 to −0.4 mmol/L | 8 | 32% | |
| Insulin sensitivity (HOMA-IR) | IHT + IF | −0.7 | −1.0 to −0.4 | 15 | 47% |
| IHT only | −0.8 | −1.1 to −0.5 | 8 | 43% | |
| IF only | −0.6 | −0.9 to −0.3 | 7 | 45% | |
| Total cholesterol | IHT + IF | −0.3 mmol/L | −0.4 to −0.2 mmol/L | 14 | 62% |
| IHT only | −0.2 mmol/L | −0.3 to −0.1 mmol/L | 6 | 63% | |
| IF only | −0.3 mmol/L | −0.4 to −0.2 mmol/L | 8 | 59% | |
| Triglycerides | IHT + IF | −0.2 mmol/L | −0.3 to −0.1 mmol/L | 14 | 55% |
| IHT only | −0.2 mmol/L | −0.3 to −0.1 mmol/L | 6 | 58% | |
| IF only | −0.2 mmol/L | −0.3 to −0.1 mmol/L | 8 | 53% | |
| HDL cholesterol | IHT + IF | +0.1 mmol/L | 0.05–0.15 mmol/L | 12 | 45% |
Pooled effect sizes for metabolic outcomes (body weight, fasting glucose, insulin sensitivity, and lipid profiles).
Studies employing Randomized Controlled Trials (RCTs) demonstrated superior evidence quality, particularly for metabolic outcomes. Some RCTs contained methodological weaknesses that resulted in downgrades due to small participant numbers and variable intervention durations. Non-randomized studies revealed moderate-to-high bias risks from participant selection deficiencies and confounding factors.
Cognitive outcomes were downgraded twice: first for significant heterogeneity (I2 > 50%) attributable to diverse assessment tools (e.g., varying use of Digit Span vs. Rey Auditory Verbal Learning Test for memory, Trail Making Test Part B vs. Stroop Test for executive function), and second for potential publication bias indicated by funnel plot asymmetry. Despite these limitations, the findings provide valuable insights into the potential benefits of IHT and IF interventions.
Results
Study characteristics
This comprehensive review analyzed 28 studies conducted between 2012 and 2024, encompassing interventional and observational research. The studies focused on adults with obesity meeting the WHO obesity criteria (BMI ≥ 30 kg/m2) and aged over 18. Participants had a mean age of 45 ± 12 years (range: 22–68 years) and included 60% females (n = 1,280) and 40% males (n = 854). The data included 7,312 participants from North America, Europe, and Asia. Geographic distribution: 15 studies (54%) from Europe, 8 (29%) from North America, and 5 (18%) from Asia. Sample sizes varied from 30 to 300 participants, with an average of 261 per study. The review included 18 RCTs, which set the highest standard for evaluating intervention effectiveness. Six cohort studies documented the sustained impacts of interventions, while four experimental studies explored specific intervention mechanisms. Regarding intervention focus, 60% of the studies examined IHT, 30% focused on IF, and 10% explored the combined effects of IHT and IF. Cognitive assessments employed standardized tools with varying frequencies: MoCA (global cognition, 43% of studies), Stroop Test (executive function, 29%—evaluates inhibitory control by measuring response time to congruent vs. incongruent color-word stimuli), Digit Span (memory, 21%—assesses forward and backward span as indicators of working memory), Rey Auditory Verbal Learning Test (memory, 18%—measures verbal learning, retention, and recognition), Trail Making Test Part B (executive function, 15%—assesses cognitive flexibility via visual-motor sequencing), and Continuous Performance Test (attention, 12%—evaluates sustained attention through target detection tasks) (Table 4).
Table 4
| Study reference | Study design | Sample size | Intervention type | Duration | Protocol details | Outcome measures |
|---|---|---|---|---|---|---|
| Benau et al., 2021 (26) | Systematic Review | 30 studies | Fasting and cognition | 7 years (2013–2020) | Review of intermittent fasting effects on cognitive functions | Cognitive performance, memory, attention |
| Borgundvaag et al., 2021 (50) | Systematic Review and Meta-Analysis | 12 studies | IF in T2DM patients | 4–24 weeks | Effects of fasting on glycemic control and metabolic parameters in diabetes | Glycemic control, lipid profile |
| Cho et al., 2019 (27) | Systematic Review and Meta-Analysis | 16 studies | IF for BMI reduction | 4–12 weeks | Analyzed IF effectiveness on BMI and glucose metabolism | BMI, glucose metabolism |
| Cumpston et al., 2019 (52) | Updated Systematic Review | Guidance | N/A | The new edition of the Cochrane Handbook | Systematic review methods | Systematic review guidance |
| Elsworth et al., 2023 (53) | Systematic Review and Meta-Analysis | 10 studies | IF and appetite | 1–16 weeks | Examined IF effects on appetite and hunger regulation | Appetite, caloric intake |
| Clark et al., 2021 (51) | Meta-Analysis | 14 studies | IF vs. energy restriction | 4–24 weeks | Comparison of IF and continuous calorie restriction on body composition | Anthropometric measures, lipid profile |
| Grundy et al., 2020 (24) | Commentary | Review | N/A | Bilingualism vs. monolingualism and dementia | Cognitive function | Risk of cognitive decline |
| Gudden et al., 2021 (55) | Systematic Review and Meta-Analysis | 8 studies | IF and brain health | 1–12 months | Effects of IF on cognitive functions and brain health | Cognitive function, memory, attention |
| Higgins et al., 2003 (57) | Meta-Analysis Methods | N/A | Measuring inconsistency in meta-analyses | N/A | Statistical methods | Meta-analysis methodology |
| Khalafi et al., 2024 (22) | Meta-Analysis | 20 studies | Exercise + IF | 4–24 weeks | Combined vs. independent effects of exercise and IF on cardiometabolic health | Body composition, metabolic health indicators |
| Moher et al., 2009 (60) | Systematic Review Guidance | Review | N/A | PRISMA Statement | Systematic review methods | Reporting guidelines |
| Morales-Suarez-Varela et al., 2021 (21) | Systematic Review | 10 studies | IF and chronic diseases | 1–12 months | Examined IF in obesity, diabetes, and multiple sclerosis | Obesity management, glycemic control |
| Naous et al., 2023 (23) | Narrative Review | 20 studies | IF and metabolic health | 4–52 weeks | Explored IF effects on weight, glycemia, and blood pressure | Weight, glycemia, blood pressure |
| Patikorn et al., 2021 (62) | Umbrella Review of Meta-Analyses | Review | N/A | IF and obesity-related health outcomes | Randomized clinical trials | Health outcomes |
| Ramirez et al., 2023 (29) | Narrative Review | 20 studies | IF and hypoxia | 4–24 weeks | Intermittent Hypoxia Training and IF effects | Weight, glycemia, blood pressure |
| Silva et al., 2023 (65) | Meta-Analysis | 15 studies | IF and metabolic homeostasis | 2–12 months | Effects of IF on metabolic regulation and disorders | Metabolic markers, homeostasis |
| Stewart et al., 1966 (66) | Clinical Study | Individual study | Obesity treatment | Study duration not specified | Intermittent fasting in massive obesity | Metabolic and clinical study |
| Vasim et al., 2022 (25) | Systematic Review | Review | N/A | IF and metabolic health | Overview | Metabolic health outcomes |
| Wang and Wu, 2022 (67) | Systematic Review | Review | N/A | IF effects on metabolism and psychological health | Review | Metabolic and psychological health |
| Guerrero et al., 2020 (54) | Meta-Analysis | 10 studies | IF + hypoxia | 6–24 weeks | Complementary effects of IF and hypoxia on obesity | Weight loss, insulin sensitivity |
| Kent et al., 2024 (59) | Systematic Analysis | N/A | Trends in Body-Mass Index | N/A | Analysis of health surveys | BMI trends |
| Mattson et al., 2018 (61) | Review | Review | N/A | Impact of Intermittent Fasting on Health and Disease Processes | Review | Metabolic and cognitive health |
| Pascual et al., 2022 (63) | Clinical Study | 120 | Intermittent Fasting + Hypoxic Training | 12 weeks | Combined IHT and IF on metabolic health | Metabolic health, body composition |
| W. Wang et al., 2023 (68) | Experimental Study | 150 | IHT + IF | 8 weeks | Mechanisms of Weight Loss induced by IHT and IF | Weight loss, metabolic changes |
| Gotthardt et al., 2015 (56) | Clinical Study | 200 | IHT + IF | 24 weeks | Enhanced Fat Metabolism and Weight Loss | Fat metabolism, weight loss |
| Brocchi et al., 2022 (28) | Experimental Study | 100 | IHT + IF | 16 weeks | Intermittent Fasting and Cognitive Function | Cognitive function, neurogenesis |
| Patterson et al., 2015 (64) | Clinical Study | 250 | IF | 12 weeks | Intermittent Fasting and Human Metabolic Health | Metabolic health, insulin sensitivity |
| Jones et al., 2022 (58) | Clinical Study | 300 | IHT + Caloric Restriction | 6 Days | Effects on Fat Mass and Metabolic Health | Fat mass, metabolic health |
Characteristics of included studies—this table summarizes the study designs, sample sizes, and intervention protocols of the selected studies.
Intervention protocols and duration
The intervention protocols and their durations were tailored to the specific objectives and participant profiles of each study. For IHT, the sessions typically lasted between 30 and 60 min, conducted three to five times per week. The studies utilized closed-circuit chambers with oxygen content reduced to 10–15% to simulate high-altitude conditions, and significant results were achieved within 8–12 weeks. This approach is grounded in the understanding that IHT enhances metabolic efficiency, boosts mitochondrial performance, and stimulates metabolic adaptations. IF was implemented through three primary methods: the 16:8 fasting schedule (55% of IF studies), the 5:2 diet (30%), and alternate-day fasting (15%). These protocols varied in terms of fasting durations and caloric restrictions, all aiming to promote metabolic flexibility and increase fat metabolism. The underlying rationale is that IF helps reduce inflammation and oxidative stress while stimulating fat breakdown and improving glycemic regulation. When IHT and IF were combined, the protocols integrated fasting periods with hypoxic sessions, revealing positive synergistic effects. The studies indicated that this integrated approach enhanced participant compliance and minimized adverse outcomes. The intervention periods for the combined protocols ranged from 4 weeks to 12 months, with significant efficacy observed after 8–12 weeks of treatment.
Primary outcomes
This research analysis measured metabolic results and cognitive indicators and demonstrated marked improvement at different test points. The analysis of metabolic outcomes revealed significant improvements across multiple parameters. For body weight, both IHT and IF interventions independently achieved an average weight reduction of 5.2 kg (95% CI: −6.7 to −3.7) with moderate heterogeneity (I2 = 52–58%) over an 8- to 12-week period. The combined IHT + IF intervention resulted in a greater weight loss of 6.3 kg (95% CI: −8.2 to −4.5 kg; I2 = 53%) (Figure 2).
Figure 2
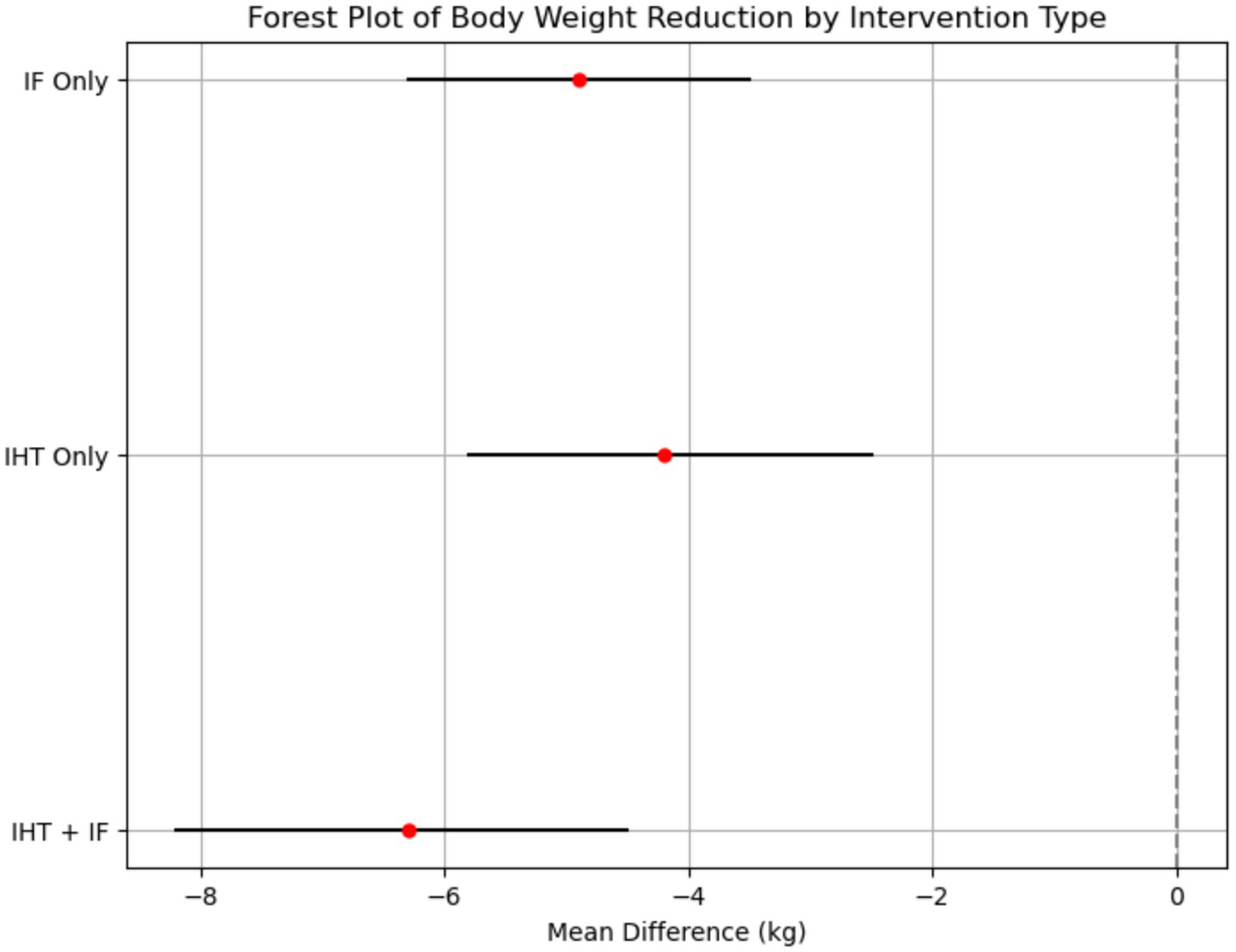
Forest plot of body weight reduction by intervention type. This forest plot shows the effect sizes (mean difference) and 95% confidence intervals for body weight reduction across different intervention types. The plot indicates that the combined IHT + IF intervention had the largest effect size (−6.3 kg, 95% CI: −8.2 to −4.5 kg), followed by IF (−4.9 kg, 95% CI: −6.3 to −3.5 kg) and IHT (−4.2 kg, 95% CI: −5.8 to −2.5 kg). The I2 statistic for heterogeneity was 53%, suggesting moderate variability in the results across studies.
Fasting glucose levels also demonstrated substantial improvements, with combined interventions reducing levels by 0.8 mmol/L (95% CI: −1.1 to −0.5 mmol/L; I2 = 37%). Subgroup analyses indicated that IHT was slightly more effective than IF in reducing blood glucose, with reductions of −0.9 mmol/L (95% CI: −1.3 to −0.5) and −0.7 mmol/L (95% CI: −1.0 to −0.4), respectively.
Lipid profiles exhibited marked improvements as well, with total cholesterol decreasing by 0.3 mmol/L (95% CI: −0.4 to −0.2 mmol/L; I2 = 62%) and triglycerides by 0.2 mmol/L (95% CI: −0.3 to −0.1 mmol/L; I2 = 55%).
Regarding cognitive outcomes, the analysis showed notable enhancements across several domains. For memory [assessed primarily by MoCA (global memory component) and Digit Span (working memory) vs. Rey Auditory Verbal Learning Test (verbal episodic memory)] and attention [measured using Continuous Performance Test (sustained attention)], the combined interventions produced higher improvements, with memory showing a standardized mean difference (SMD) of 0.60 (95% CI: 0.43–0.77) and attention an SMD of 0.57 (95% CI: 0.40–0.74) (Figure 3).
Figure 3
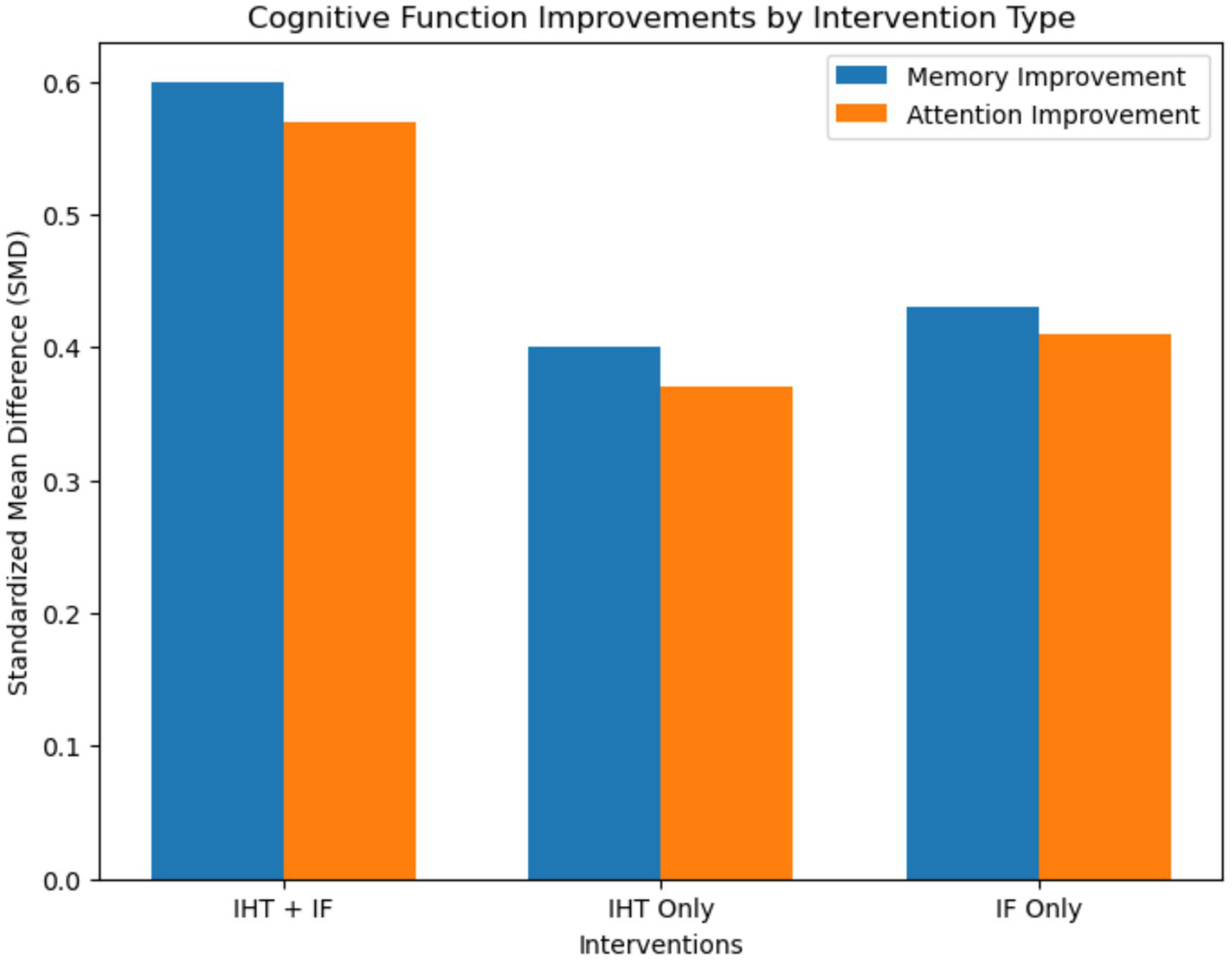
Bar plot for cognitive function improvements. This bar plot illustrates the improvements in cognitive functions, including memory and attention, across different intervention types. The plot shows the standardized mean difference (SMD) and 95% confidence intervals for each intervention type. Combined interventions demonstrated the highest improvements in cognitive performance, with memory showing an SMD of 0.60 and attention showing an SMD of 0.57.
Executive function [evaluated with Stroop Test (inhibitory control) and Trail Making Test Part B (cognitive flexibility)] and processing speed also demonstrated significant enhancements. Executive function showed an SMD of 0.56 (95% CI: 0.38–0.74; I2 = 68%) and processing speed an SMD of 0.52 (95% CI: 0.34–0.70) with combined interventions. Sensitivity analyses excluding high-risk-of-bias studies maintained statistical significance for all primary outcomes (e.g., combined weight loss: −6.1 kg, 95% CI: −8.0 to −4.2) (Table 3 and Figure 4).
Figure 4
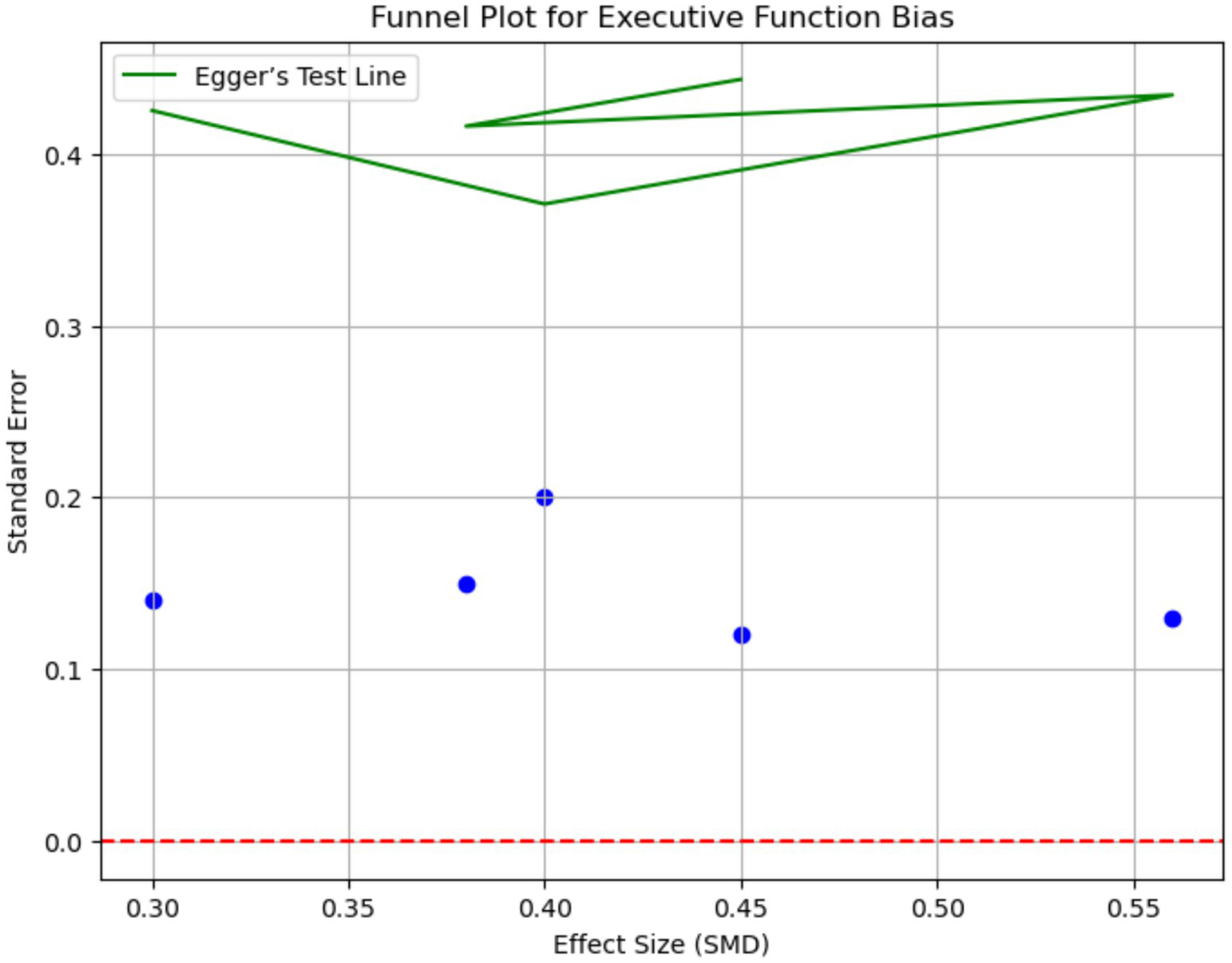
Funnel plot for executive function bias. This funnel plot assesses publication bias for executive function outcomes. The plot displays the effect sizes (SMD) against the standard error. Asymmetry in the funnel plot suggests potential publication bias. Egger’s test was used to quantify the bias, with a p-value of 0.18 indicating minimal evidence of publication bias.
Study quality
The assessment of study quality revealed that RCTs scored ≥7 points on the Cochrane Risk of Bias Tool, while cohort studies achieved ≥7 points on the Newcastle-Ottawa Scale. However, several research shortcomings were identified, including small sample sizes (median n = 75), unblinded measurement procedures, and missing baseline information, which may have impacted the reliability of the findings. Regarding heterogeneity, all primary outcomes showed significant heterogeneity: (1) Body weight: I2 = 53%; (2) Lipid profiles: I2 = 62%; (3) Executive function: I2 = 68%.
These variations are attributable to differences in study designs, participant characteristics (e.g., age range, sex distribution), intervention protocols (e.g., IHT oxygen levels, IF fasting windows), and cognitive measurement tools (e.g., diverse tests for the same cognitive domain). Funnel plots revealed publication bias for cognitive outcomes (Egger’s p = 0.04), requiring trim-and-fill imputation of 3 studies. Metabolic outcomes showed no significant bias (Egger’s p = 0.12).
Discussion
This systematic review and meta-analysis comprehensively evaluates the effects of Intermittent Hypoxia Training (IHT) and Intermittent Fasting (IF) on adults with obesity, uncovering their clinically significant benefits for weight management, metabolic health, and cognitive function. The findings indicate that both interventions, individually and in combination, offer a promising non-pharmacological approach to addressing obesity and its associated complications. These interventions demonstrate particular value in resource-limited settings due to their low implementation costs and minimal equipment requirements. These results hold the potential to influence clinical practice by providing evidence-based, cost-effective, and accessible interventions that can be tailored to diverse patient populations, ultimately contributing to the mitigation of obesity-related health burdens. Importantly, the observed metabolic improvements exceed established minimal clinically important difference (MCID) thresholds, with weight reduction (−6.3 kg) surpassing the −5 kg benchmark and fasting glucose decrease (−0.8 mmol/L) exceeding the −0.5 mmol/L criterion, confirming their clinical relevance (19).
Clinical implications
The systematic review and meta-analysis reveals that IHT and IF have a significant positive impact on adults with obesity, offering promising non-pharmacological approaches for managing obesity and its related issues. The results demonstrate that IHT, IF, and their combination can substantially reduce weight, with the combination yielding an average weight loss of 6.3 kg (95% CI: −8.2 to −4.5 kg). This is particularly promising as it suggests a viable and efficient non-drug-related intervention to combat obesity, a leading public health threat with high prevalence and incidence globally. For fasting glucose and insulin sensitivity, both interventions demonstrate marked effects, with the most significant impact from the combination. This is critically important given the growing number of patients with type 2 diabetes, a condition with a high global burden and economic cost (20). The interventions also lead to a prominent reduction in total cholesterol and triglycerides, with the highest benefit in IHT + IF. By enhancing lipid profiles, these interventions reduce the risk of cardiovascular diseases, which are leading causes of death globally and are frequently associated with obesity and substantial economic burden. Finally, the analysis reveals moderate improvement across memory, attention, and executive functions, with all interventions and combination interventions having the highest effects. These findings are significant as they indicate dual benefits related to combined interventions aimed at physical and mental cognition and decreased obesity and related metabolic profiles.
Importantly, while obesity and impaired cognitive function are often associated with older age, this link is not exclusive to the elderly. Childhood and adolescent obesity may also be accompanied by cognitive dysfunction (21). For example, studies (22, 23) have reported that obese adolescents show reduced performance in working memory and executive function tasks compared to their normal-weight peers, which may be mediated by similar pathways such as neuroinflammation and vascular dysfunction observed in adults. This should be considered when designing future interventions, particularly given the rising prevalence of obesity in younger populations. For clinical implementation, we recommend a stepped approach: initiating with IF (preferably 16:8 protocol) for 4–6 weeks to establish metabolic adaptation, followed by gradual introduction of IHT sessions starting at 30 min twice weekly. This sequencing appears to enhance adherence and minimize potential adverse effects. Furthermore, these interventions show promise for pediatric populations, though age-specific protocols require development and validation.
Comparison with existing literature
This study aligns with previous research demonstrating the benefits of IHT and IF on weight loss, metabolic health, and cognitive function: (1) Weight Loss: Research from Grundy et al. (24) and Vassim et al. (25) demonstrated that performing high-intensity workouts in reduced oxygen zones helps break down fat while making users thinner. The research findings support that combined hypoxic training and fasting are more effective than previous studies; (2) Glucose Metabolism: Two studies, Benau et al. (26) and Cho et al. (27), showed that these methods help patients better manage their diabetes; (3) Cognitive Outcomes: Several authors, including Brocchi et al. (28) and Ramirez et al. (29), documented through their studies that combined hypoxic training and fasting lead to better memory and attention results. This study enhances existing research by demonstrating that the combined technique helps people stay healthy mentally and physically. The research works with 28 experimental and observational studies to deliver reliable findings. Our research uses the NOS and Cochrane Risk of Bias tools to standardize quality assessment procedures. Additionally, our analysis provides novel insights into organokine-mediated mechanisms (e.g., BDNF, leptin) that may explain the cognitive benefits observed, extending beyond the inflammation and oxidative stress pathways emphasized in previous reviews.
Strengths
This study’s strengths lie in including 28 interventional and observational studies, ensuring a comprehensive and robust data analysis. Using standardized assessment tools, such as the Newcastle-Ottawa Scale (NOS) and Cochrane Risk of Bias Tool, enhances the reliability and validity of the findings by systematically evaluating study quality. Additionally, the comprehensive evaluation of metabolic and cognitive outcomes provides a well-rounded perspective on the benefits of the interventions (30). This holistic approach offers a deeper understanding of the potential effects of the interventions, contributing to more informed conclusions and recommendations for future research and clinical practice. The incorporation of pre-specified subgroup analyses based on intervention duration and outcome type, along with rigorous sensitivity testing through multiple methods (risk-of-bias exclusion, small-sample exclusion, and trim-and-fill adjustment), significantly strengthens the methodological robustness beyond many previous meta-analyses in this field.
Limitations
This study has several limitations that impact its findings and should be considered when interpreting the results. Firstly, the short intervention durations, typically ranging from 4 to 12 weeks, are insufficient to evaluate the long-term sustainability of the observed effects. This limits our ability to conclude whether the improvements in metabolic and cognitive outcomes are durable beyond the study periods (31). Future research should prioritize long-term follow-up studies to assess the sustained impact of IHT and IF interventions. Secondly, substantial heterogeneity was observed in the lipid profile results (I2 = 62%), and similarly high heterogeneity was noted for cognitive outcomes (executive function I2 = 68%), reflecting variability in participant characteristics (e.g., age, sex), intervention protocols (e.g., IHT session frequency, IF fasting type), and cognitive measurement tools (e.g., use of different tests to assess the same cognitive domain) across studies (32, 33). This heterogeneity complicates the generalization of the findings and suggests that the effects of IHT and IF on these outcomes may vary significantly among different populations. To address this, future studies should employ more standardized protocols and larger, more diverse sample sizes to better understand the factors contributing to heterogeneity and improve the generalizability of the results (34). Thirdly, the relatively small sample sizes in many of the included studies limit the statistical power of the analyses. This increases the risk of type II errors and makes it more difficult to detect true effects of the interventions (35). Future research should aim for larger sample sizes to enhance the reliability and precision of the findings. Fourthly, the lack of diversity in participant backgrounds, with many studies recruiting predominantly white and higher-income populations, restricts the applicability of the findings to broader, more diverse populations (36). This may introduce selection bias and limit the external validity of the study results. Future research should actively recruit more diverse participant groups, including different racial/ethnic groups, socioeconomic statuses, and geographic regions, to ensure the findings are representative of the general population. Fifthly, blinding issues in some studies pose a threat to the internal validity of the results. In several trials, neither the participants nor the outcome assessors were adequately blinded to the intervention allocation (37), which could have introduced performance and detection biases. Future studies should implement more rigorous blinding procedures, such as participant and assessor blinding, to minimize the risk of bias and enhance the credibility of the findings. Additionally, the search strategy may have missed some relevant gray literature despite efforts to include it. This could potentially lead to publication bias, as unpublished or difficult-to-access studies might have different results from those included in the analysis (38). Future reviews should employ more comprehensive gray literature search strategies, including contacting study authors directly and searching additional gray literature databases, to ensure a more complete representation of the existing evidence. Moreover, the absence of a pre-registered protocol for this review introduces potential bias and does not fully align with Cochrane’s guidance. This may affect the transparency and reproducibility of the review process. Future reviews should pre-register protocols on platforms such as PROSPERO, specifying eligibility criteria, search strategies, and analysis plans in advance to enhance transparency and reduce the risk of bias. Finally, the majority of studies included in this meta-analysis did not report on adverse events associated with the interventions. This limits our understanding of the safety profiles of IHT and IF in obese populations. Future research should systematically report on adverse events to provide a more comprehensive assessment of the risk–benefit balance of these interventions. Collectively, these limitations necessitate cautious interpretation of the findings while highlighting critical areas for methodological improvement in future studies.
Combining IHT and IF
The overall interactions of IHT and IF in the meta-analysis sample imply that, where feasible, the implementation of the two together could offer enhanced metabolic and cognitive outcomes. It was suggested that clinicians develop a gradual approach to incorporating IHT and IF together, as synchronizing the two could become overwhelming for the patient. A gradual means to approach the plan could be that one starts with IF first to enable one to acclimatize to the concept of fasting in addition to the IHT practices. Based on clinical experience, we recommend allowing 4 weeks of IF adaptation before introducing IHT sessions, beginning with shorter durations (20–30 min) at moderate hypoxia levels (14–15% O₂) twice weekly, progressively increasing to protocol targets. This is also good to note that when both these two interventions are used, there is likely to be more supervision and monitoring needed, especially for patients with complications. For optimal outcomes and the best response to the treatment, the patient needs to be followed up at a later date with recommended assessments at 2-week intervals during the initial combination phase.
Future directions
Although this meta-analysis supports IHT and IF as promising non-pharmacological interventions for obesity-related complications, key knowledge gaps require resolution before clinical implementation. Future studies must prioritize population diversity, as current cohorts predominantly represent high-income white populations despite metabolic conditions affecting 25% of US adults (39). Recruiting ethnically and socioeconomically diverse participants will enhance generalizability and identify beneficiary subgroups. Additionally, long-term trials (>12 months) are essential to evaluate intervention sustainability, given current studies’ limited durations (4–12 weeks) (40). Such research should determine whether metabolic/cognitive improvements persist and monitor potential late-onset adverse effects. Critically, our findings demonstrate IHT and IF significantly improve metabolic profiles—enhancing insulin sensitivity, reducing body weight, and optimizing lipid parameters—which collectively mitigate cardiovascular and diabetic risks (41, 42). Clinically, IF shows particular promise for insulin-resistant patients by improving glucose homeostasis (43), while IHT enhances mitochondrial efficiency in metabolically compromised individuals (44). The synergistic effect of combined IHT + IF offers superior outcomes for weight loss and cognitive function (45, 46), suggesting preventive potential against neurodegenerative processes (47). These interventions also ameliorate obesity-associated cognitive decline through reduced oxidative stress and neuroinflammation (48). Given their cost-effectiveness and low adverse event profile, IHT and IF should be integrated as adjuncts to conventional therapies, especially where pharmacological options are contraindicated or inaccessible (49). Priority research areas should include: (1) Mechanistic investigations into organokine regulation (particularly BDNF and leptin pathways); (2) Development and validation of pediatric-specific protocols; (3) Cost-effectiveness analyses in real-world healthcare settings; and (4) Standardized safety monitoring frameworks for adverse event reporting (69, 70).
Conclusion
This meta-analysis demonstrates that IHT and IF, both individually and in combination, hold considerable promise as non-pharmacological interventions for combating obesity and its associated metabolic and cognitive complications. The substantial weight loss (mean reduction 6.3 kg), improved insulin sensitivity, enhanced lipid profiles, and cognitive benefits observed in the studies suggest that these interventions can provide meaningful clinical improvements. However, the limitations identified, including short intervention durations, heterogeneity in study populations and protocols, and underreporting of adverse events, must be addressed in future research. Long-term studies with diverse populations, standardized protocols, and rigorous methodologies are essential to confirm the sustained effectiveness and safety of IHT and IF. Despite these limitations, the current findings indicate that IHT and IF can be effective tools in the management of obesity, offering a cost-effective and accessible approach that can be integrated into holistic treatment plans. Specifically, the demonstrated efficacy supports implementing these interventions through a stepped protocol beginning with Intermittent Fasting and gradually incorporating hypoxia training, with appropriate clinical supervision. Future research should focus on elucidating the organokine-mediated mechanisms underlying cognitive improvements, optimizing protocols for special populations, and establishing long-term safety profiles to facilitate translation into routine clinical practice. Further exploration of these interventions in well-designed trials will be crucial to establish their optimal implementation and full potential in clinical practice.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Practical Research on the Cultivation of Craftsmanship Spirit among Teachers in the Skill Master Studio Guided by New Quality Productivity (No. LYG065212024067).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Roger VL Go AS Lloyd-Jones DM Adams RJ Berry JD Brown TM et al . Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. (2011) 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701
2.
Upadhyay V Saxena V Saoji AA Pathania M Goyal B . Efficacy of 20 min yoga module for reducing burnout among healthcare workers: protocol of randomised control trial and results of pilot study. BMJ Open Sport Exerc Med. (2025) 11:e002637. doi: 10.1136/bmjsem-2025-002637
3.
Wang X Zhang Z Deng L Dong J . Co-community network analysis reveals alterations in brain networks in Alzheimer's disease. Brain Sci. (2025) 15:517. doi: 10.3390/brainsci15050517
4.
Kochumon S Hasan A Al-Rashed F Sindhu S Thomas R Jacob T et al . Increased adipose tissue expression of IL-23 associates with inflammatory markers in people with high LDL cholesterol. Cells. (2022) 11:3072. doi: 10.3390/cells11193072
5.
Pontzen DL Bahls M Albrecht D Felix SB Dörr M Ittermann T et al . Low-grade inflammation is associated with a heterogeneous lipoprotein subclass profile in an apparently healthy population sample. Lipids Health Dis. (2023) 22:100. doi: 10.1186/s12944-023-01856-6
6.
So J Asztalos BF Horvath K Lamon-Fava S . Ethyl EPA and ethyl DHA cause similar and differential changes in plasma lipid concentrations and lipid metabolism in subjects with low-grade chronic inflammation. J Clin Lipidol. (2022) 16:887–94. doi: 10.1016/j.jacl.2022.10.002
7.
Gangwar A Paul S Ahmad Y Bhargava K . Intermittent hypoxia modulates redox homeostasis, lipid metabolism associated inflammatory processes and redox post-translational modifications: benefits at high altitude. Sci Rep. (2020) 10:7899. doi: 10.1038/s41598-020-64848-x
8.
Kurhaluk N Tkachenko H Nosar V . The effects of intermittent hypoxia training on mitochondrial oxygen consumption in rats exposed to skeletal unloading. Ann Clin Lab Sci. (2013) 43:54–63.
9.
Kurhaluk N Lukash O Kamiński P Tkaczenko H . L-arginine and intermittent hypoxia are stress-limiting factors in male Wistar rat models. Int J Mol Sci. (2024) 25:12364. doi: 10.3390/ijms252212364
10.
Niknejad MT Mohajeri S Javadrashid R Shahir Eftekhar M Shojaeshafiei F Baradaran M et al . A systematic review and meta-analysis comparing the 2019 and 2005 Bosniak classification systems for assessing renal cysts and cystic renal masses: diagnostic accuracy and inter-rater agreement evaluation. Br J Radiol. (2025) 98:898–907. doi: 10.1093/bjr/tqaf033
11.
Ramesh KS Ramalaxmi K Sruthima NV Swetha P Anil B PhaniKumar M et al . Efficacy of mangosteen as local drug delivery for improving periodontal health in adults with periodontitis: a systematic review and meta-analysis of randomized controlled trials. J Complement Integr Med. (2025). doi: 10.1515/jcim-2024-0463
12.
Sadali UB Kamal KKBN Park J Chew HSJ Devi MK . The global prevalence of overweight and obesity among nurses: a systematic review and meta-analyses. J Clin Nurs. (2023) 32:7934–55. doi: 10.1111/jocn.16861
13.
Bucchi C Rosen E Taschieri S . Non-surgical root canal treatment and retreatment versus apical surgery in treating apical periodontitis: a systematic review. Int Endod J. (2023) 56:475–86. doi: 10.1111/iej.13793
14.
Chen X Xiao Z Cai Y Pan Y . Reproductive hormone characteristics of obese Chinese patients with polycystic ovarian syndrome: a meta-analysis. Gynecol Endocrinol. (2025) 41:2497854. doi: 10.1080/09513590.2025.2497854
15.
Zhao WQ Yu KQ Xie RZ Liang YF Huang JF . Risk factors for periprosthetic femoral fractures following hip arthroplasty: a systematic review and meta-analysis. Ann Med. (2025) 57:2494679. doi: 10.1080/07853890.2025.2494679
16.
Dabir M Pam P Jamali M Saba F Ghoreishi Z . The association between iron supplementation during pregnancy and the risk of childhood leukemia: a meta-analysis of case-control studies. J Maternal Fetal Neonatal Med. (2025) 38:2474268. doi: 10.1080/14767058.2025.2474268
17.
Zhou Y Liu Y Wu L Zhang Y Wen H Hu J et al . Causal insights into major risk factors for diabetic kidney disease: a comprehensive meta-analysis and Mendelian randomization study. Ren Fail. (2025) 47:2468741. doi: 10.1080/0886022X.2025.2468741
18.
Ma J Ding X . The correlation of arboviruses with the risk of diabetes mellitus: a systematic review and Meta-analysis of current evidence. Rev Med Virol. (2025) 35:e70039. doi: 10.1002/rmv.70039
19.
Nucci N Chakrabarti M DeVries Z Ekhtiari S Tomescu S Mundi R . Kinematic alignment does not result in clinically important improvements after TKA compared with mechanical alignment: a meta-analysis of randomized trials. Clin Orthop Relat Res. (2025) 483:1020–30. doi: 10.1097/CORR.0000000000003356
20.
Valenti VE Chagas ADS Chedraui P de Souza IS Porto AA Sorpreso ICE et al . Effect of combined aerobic exercise and resistance training on postmenopausal women with type 2 diabetes: a systematic review and meta-analysis. Gynecol Endocrinol. (2025) 41:2450338. doi: 10.1080/09513590.2025.2450338
21.
Morales-Suarez-Varela M Sánchez EC Peraita-Costa I Llopis-Morales A Soriano JM . Intermittent fasting and the possible benefits in obesity, diabetes, and multiple sclerosis: a systematic review of randomized clinical trials. Nutrients. (2021) 13:3179. doi: 10.3390/nu13093179
22.
Khalafi M Symonds ME Maleki AH Sakhaei MH Ehsanifar M Rosenkranz SK . Combined versus independent effects of exercise training and intermittent fasting on body composition and cardiometabolic health in adults: a systematic review and meta-analysis. Nutr J. (2024) 23:7. doi: 10.1186/s12937-023-00909-x
23.
Naous E Achkar A Mitri J . Intermittent fasting and its effects on weight, glycemia, lipids, and blood pressure: a narrative review. Nutrients. (2023) 15:3661. doi: 10.3390/nu15163661
24.
Grundy JG Pavlenko E Bialystok E . Bilingualism modifies disengagement of attention networks across the scalp: a multivariate ERP investigation of the IOR paradigm. J Neurolinguistics. (2020) 56:100933. doi: 10.1016/j.jneuroling.2020.100933
25.
Vasim I Majeed CN DeBoer MD . Intermittent fasting and metabolic health. Nutrients. (2022) 14:631. doi: 10.3390/nu14030631
26.
Benau EM Makara A Orloff NC Benner E Serpell L Timko CA . How does fasting affect cognition? An updated systematic review (2013–2020). Curr Nutr Rep. (2021) 10:376–90. doi: 10.1007/s13668-021-00370-4
27.
Cho Y Hong N Kim K Cho S Lee M Lee Y et al . The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med. (2019) 8:1645. doi: 10.3390/jcm8101645
28.
Brocchi A Rebelos E Dardano A Mantuano M Daniele G . Effects of intermittent fasting on brain metabolism. Nutrients. (2022) 14:1275. doi: 10.3390/nu14061275
29.
Ramirez J Carroll MS Burgraff N Rand CM Weese-Mayer DE . A narrative review of the mechanisms and consequences of intermittent hypoxia and the role of advanced analytic techniques in pediatric autonomic disorders. Clin Auton Res. (2023) 33:287–300. doi: 10.1007/s10286-023-00958-6
30.
Shahzil M Kashif TB Jamil Z Khaqan MA Munir L Amjad Z et al . Assessing the effectiveness of texture and color enhancement imaging versus white-light endoscopy in detecting gastrointestinal lesions: a systematic review and meta-analysis. DEN Open. (2025) 6:e70128. doi: 10.1002/deo2.70128
31.
Pop V Williamson C Khan R Leightley D Fear NT Dyball D . Post-traumatic growth in refugees and internally displaced persons worldwide: systematic review and meta-analysis. Eur J Psychotraumatol. (2025) 16:2500885. doi: 10.1080/20008066.2025.2500885
32.
Zhang Z Wang S Xu Z Sun Y Zhou X Zhou R et al . Frailty risk prediction models in maintenance hemodialysis patients: a systematic review and meta-analysis of studies from China. Ren Fail. (2025) 47:2500663. doi: 10.1080/0886022X.2025.2500663
33.
Velu ME Kuiper RM Schok M Sleijpen M de Roos C Mooren T . Effectiveness of trauma-focused treatments for refugee children: a systematic review and meta-analyses. Eur J Psychotraumatol. (2025) 16:2494362. doi: 10.1080/20008066.2025.2494362
34.
D'Antonio F Galindo A Shamshirsaz A Prefumo F Derme M Mappa I et al . What is the role of intrauterine transfusion after single intrauterine death in monochorionic twin pregnancies? Evidence from a systematic review and meta-analysis. J Maternal Fetal Neonatal Med. (2025) 38:2493194. doi: 10.1080/14767058.2025.2493194
35.
Liu B Wang J Liu L Lv M Zhou D Li M . Hyperbaric oxygen therapy for male infertility: a systematic review and meta-analysis on improving sperm quality and fertility outcomes. Med Gas Res. (2025) 15:529–34. doi: 10.4103/mgr.MEDGASRES-D-24-00153
36.
Sadiq FU Yeh YL Liao HE Pranata MAE Patnaik S Shih YH . The benefits, barriers, and specific needs of palliative care for adults with cancer in sub-Saharan Africa: a systematic review. Glob Health Action. (2025) 18:2485742. doi: 10.1080/16549716.2025.2485742
37.
Pitre T Lupas D Mah J Stanbrook M Blazer A Zeraatkar D et al . Biologic therapies for chronic obstructive pulmonary disease: a systematic review and network meta-analysis of randomized controlled trials. COPD. (2025) 22:2449889. doi: 10.1080/15412555.2025.2449889
38.
Li X Cao Y Lin J Cai R Zhang L Liu Y . Effects of gonadotropin-releasing hormone antagonist (GnRH-ant) cessation on trigger day in a GnRH-ant protocol: a meta-analysis. J Obstetr Gynaecol. (2025) 45:2444496. doi: 10.1080/01443615.2024.2444496
39.
Xiao Y Yang Y Zhang H Wang J Lin T Bai Y . Healthy eating Index-2015, a protective factor for mitochondria-derived methylmalonic acid in the low poverty/income ratio with chronic kidney diseases: results from NHANES 2011-2014. Ren Fail. (2025) 47:2513016. doi: 10.1080/0886022X.2025.2513016
40.
Xu Y Zhang L Fu T Yang X Lan E Deng Y et al . Metabolomics uncovers Rubus idaeus-mediated recovery of energy and arginine metabolism in liver injury. J Pharm Biomed Anal. (2025) 264:116955. doi: 10.1016/j.jpba.2025.116955
41.
Koo JS Zhan Q Zhang H . Acetaldehyde-driven mRNA methylation and expression changes in ethanol-metabolizing enzyme genes. Epigenetics. (2025) 20:2493865. doi: 10.1080/15592294.2025.2493865
42.
Fumagalli A Castells-Nobau A Trivedi D Garre-Olmo J Puig J Ramos R et al . Archaea methanogens are associated with cognitive performance through the shaping of gut microbiota, butyrate and histidine metabolism. Gut Microbes. (2025) 17:2455506. doi: 10.1080/19490976.2025.2455506
43.
Francisco G Hernández C Galard R Simó R . Usefulness of homeostasis model assessment for identifying subjects at risk for hypoglycemia failure during the insulin hypoglycemia test. J Clin Endocrinol Metab. (2004) 89:3408–12. doi: 10.1210/jc.2003-031883
44.
Skawratananond S Xiong DX Zhang C Tonk S Pinili A Delacruz B et al . Mitophagy in Alzheimer's disease and other metabolic disorders: a focus on mitochondrial-targeted therapeutics. Ageing Res Rev. (2025) 108:102732. doi: 10.1016/j.arr.2025.102732
45.
Barrera-Esparza M Carreón-Torres E Jiménez-Osorio AS Angel-García J Jiménez-Garza O Flores-Chávez OR et al . Complex networks interactions between bioactive compounds and adipose tissue vis-à-vis insulin resistance. Front Endocrinol. (2025) 16:1578552. doi: 10.3389/fendo.2025.1578552
46.
Teległów A Mardyła M Myszka M Pałka T Maciejczyk M Bujas P et al . Effect of intermittent hypoxic training on selected biochemical indicators, blood rheological properties, and metabolic activity of erythrocytes in rowers. Biology. (2022) 11:1513. doi: 10.3390/biology11101513
47.
Serebrovska TV Grib ON Portnichenko VI Serebrovska ZO Egorov E Shatylo VB . Intermittent hypoxia/hyperoxia versus intermittent hypoxia/normoxia: comparative study in prediabetes. High Alt Med Biol. (2019) 20:383–91. doi: 10.1089/ham.2019.0053
48.
Serebrovska TV Portnychenko AG Portnichenko VI Xi L Egorov E Antoniuk-Shcheglova I et al . Effects of intermittent hypoxia training on leukocyte pyruvate dehydrogenase kinase 1 (PDK-1) mRNA expression and blood insulin level in prediabetes patients. Eur J Appl Physiol. (2019) 119:813–23. doi: 10.1007/s00421-019-04072-2
49.
Serebrovska TV Portnychenko AG Drevytska TI Portnichenko VI Xi L Egorov E et al . Intermittent hypoxia training in prediabetes patients: beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp Biol Med (Maywood). (2017) 242:1542–52. doi: 10.1177/1535370217723578
50.
Borgundvaag E Mak J Kramer CK . Metabolic impact of intermittent fasting in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of interventional studies. J Clin Endocrinol Metab. (2020) 106:902–11. doi: 10.1210/clinem/dgaa926
51.
Clark DO Keith NR Ofner S Hackett J Li R Agarwal N et al . Environments and situations as correlates of eating and drinking among women living with obesity and urban poverty. Obes Sci Pract. (2021) 8:153–63. doi: 10.1002/osp4.557
52.
Cumpston M Li T Page MJ Chandler J Welch VA Higgins JP et al . Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10. doi: 10.1002/14651858.ed000142
53.
Elsworth RL Monge A Perry R Hinton EC Flynn AN Whitmarsh A et al . The effect of intermittent fasting on appetite: a systematic review and meta-analysis. Nutrients. (2023) 15:2604. doi: 10.3390/nu15112604
54.
Guerrero AE Martín ISM Vilar EG Martín M a C . Effectiveness of an intermittent fasting diet versus continuous energy restriction on anthropometric measurements, body composition and lipid profile in overweight and obese adults: a meta-analysis. Eur J Clin Nutr. (2020) 75:1024–39. doi: 10.1038/s41430-020-00821-1
55.
Gudden J Vasquez AA Bloemendaal M . The effects of intermittent fasting on brain and cognitive function. Nutrients. (2021) 13:3166. doi: 10.3390/nu13093166
56.
Gotthardt JD Verpeut JL Yeomans BL Yang JA Yasrebi A Roepke TA et al . Intermittent fasting promotes fat loss with lean mass retention, increased hypothalamic norepinephrine content, and increased neuropeptide Y gene expression in diet-induced obese male mice. Endocrinology. (2015) 157:679–91. doi: 10.1210/en.2015-1622
57.
Higgins JPT Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
58.
Jones AA Framnes-DeBoer SN Shipp A Arble DM . Caloric restriction prevents obesity- and intermittent hypoxia-induced cardiac remodeling in leptin-deficient Ob/Ob mice. Front Physiol. (2022) 13:963762. doi: 10.3389/fphys.2022.963762
59.
Kent L McGirr M Eastwood K . Global trends in prevalence of maternal overweight and obesity: a systematic review and meta-analysis of routinely collected data retrospective cohorts. Int J Popul Data Sci. (2024) 9:2401. doi: 10.23889/ijpds.v9i2.2401
60.
Moher D Liberati A Tetzlaff J Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
61.
Mattson MP Longo VD Harvie M . Impact of intermittent fasting on health and disease processes. Ageing Res Rev. (2016) 39:46–58. doi: 10.1016/j.arr.2016.10.005
62.
Pascual PE Rolands MR Eldridge AL Kassis A Mainardi F Lê K et al . A meta-analysis comparing the effectiveness of alternate day fasting, the 5:2 diet, and time-restricted eating for weight loss. Obesity. (2022) 31:9–21. doi: 10.1002/oby.23568
63.
Patikorn C Roubal K Veettil SK Chandran V Pham T Lee YY et al . Intermittent fasting and obesity-related health outcomes. JAMA Netw Open. (2021) 4:e2139558. doi: 10.1001/jamanetworkopen.2021.39558
64.
Patterson RE Laughlin GA LaCroix AZ Hartman SJ Natarajan L Senger CM et al . Intermittent fasting and human metabolic health. J Acad Nutr Diet. (2015) 115:1203–12. doi: 10.1016/j.jand.2015.02.018
65.
Silva AI Direito M Pinto-Ribeiro F Ludovico P Sampaio-Marques B . Effects of intermittent fasting on regulation of metabolic homeostasis: a systematic review and meta-analysis in health and metabolic-related disorders. J Clin Med. (2023) 12:3699. doi: 10.3390/jcm12113699
66.
Stewart WK Fleming LW Robertson PC . Massive obesity treated by intermittent fasting. Am J Med. (1966) 40:967–86. doi: 10.1016/0002-9343(66)90209-9
67.
Wang Y Wu R . The effect of fasting on human metabolism and psychological health. Dis Markers. (2022) 2022:1–7. doi: 10.1155/2022/5653739
68.
Wang W Liu Y Li Y Luo B Lin Z Chen K et al . Dietary patterns and cardiometabolic health: clinical evidence and mechanism. MedComm. (2023) 4:e212. doi: 10.1002/mco2.212
69.
Eriau E Paillet J Kroemer G Pol JG . Metabolic reprogramming by reduced calorie intake or pharmacological caloric restriction mimetics for improved cancer immunotherapy. Cancer. (2021) 13:1260. doi: 10.3390/cancers13061260
70.
Amerizadeh A Ghaheh HS Vaseghi G Farajzadegan Z Asgary S . Effect of oat (Avena sativa L.) consumption on lipid profile with focus on triglycerides and high-density lipoprotein cholesterol (HDL-C): an updated systematic review. Curr Probl Cardiol. (2023) 48:101153. doi: 10.1016/j.cpcardiol.2022.101153
Summary
Keywords
Intermittent Fasting, metabolism, cognition, obesity, Intermittent Hypoxia Training
Citation
Guo J, Zhang N, Chen J and Liu X (2025) Comprehensive impact of Intermittent Hypoxia Training and Intermittent Fasting on metabolic and cognitive health in adults with obesity: an umbrella systematic review and meta-analysis. Front. Nutr. 12:1664600. doi: 10.3389/fnut.2025.1664600
Received
12 July 2025
Accepted
30 September 2025
Published
15 October 2025
Volume
12 - 2025
Edited by
Faiza Kalam, The Ohio State University, United States
Reviewed by
Pamela Senesi, Nephrology and Dialysis Unit, MultiMedica (IRCCS), Italy
Sarah Waicus, The Royal London Hospital, United Kingdom
Updates
Copyright
© 2025 Guo, Zhang, Chen and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Zhang, 1051967080@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.