- 1Exercise Science School, Beijing Sport University, Beijing, China
- 2College of Physical Education, Hunan Normal University, Changsha, China
- 3School of Physical Education, University of Science and Technology Beijing, Beijing, China
Objective: Obesity is a major global public health challenge, and Vitamin D deficiency is prevalent among obese individuals. This study aimed to evaluate whether Vitamin D supplementation enhances the effectiveness of exercise-induced weight loss in overweight or obese adults by integrating transcriptomic analysis and meta-analysis.
Methods: Transcriptomic data from the GEO and GTEx databases were integrated for differential gene expression analysis, Gene Ontology (GO)/Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment, and Gene Set Enrichment Analysis (GSEA). Currently, clinical transcriptomic data regarding the effect of Vitamin D on exercise intervention outcomes in overweight/obese adults is limited. To address this gap, this study utilized cold-induced skeletal muscle shivering as a surrogate model to explore its potential molecular mechanisms. A meta-analysis of eight randomized controlled trials (RCT) involving 481 participants, was conducted to assess the combined effects of exercise and Vitamin D supplementation on body composition and metabolic parameters, with subgroup analyses by age and exercise type.
Results: Transcriptomic analysis revealed abnormal expression of Vitamin D metabolism-related genes in skeletal muscle of obese individuals, with enrichment in pathways such as lipid digestion and absorption. Post-intervention, Vitamin D response pathways were significantly upregulated. The meta-analysis showed that combined intervention had a significant effect on waist circumference (mean difference [MD] = −1.48, 95% CI: −2.02 to −0.94, p < 0.05). Subgroup analysis indicated that improvements in body weight and Body Mass Index (BMI) were more pronounced among older adults and those undergoing aerobic exercise.
Conclusion: This study, through integrated high-throughput transcriptomic analysis and meta-analysis, systematically demonstrates that Vitamin D supplementation may enhance skeletal muscle metabolic responsiveness to exercise in overweight or obese adults. The effect appears especially significant in older populations and within aerobic exercise contexts. These findings suggest that Vitamin D supplementation could serve as a synergistic strategy in exercise-based weight loss programs for targeted populations. Future research should focus on individual Vitamin D status, optimization of exercise modalities, and validation of underlying mechanisms to support personalized and precise interventions.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/.
1 Introduction
Vitamin D is a fat-soluble vitamin that plays a vital role in maintaining bone health and regulating calcium-phosphorus metabolism. It also participates in numerous physiological functions such as immune modulation, anti-inflammatory activity, and antioxidant defense. In recent years, accumulating evidence has shown a close association between Vitamin D deficiency and obesity. Obesity is frequently associated with deficiencies in various micronutrients, with Vitamin D deficiency being the most prevalent (1), which may be attributed to its fat-soluble nature leading to sequestration in adipose tissue, chronic systemic inflammation, and reduced outdoor activity. Therefore, the high global prevalence of Vitamin D deficiency may be one of the key contributors to the rising obesity rates worldwide (2).
Vitamin D deficiency has been linked to multiple metabolic disorders, including dyslipidemia, insulin resistance, and type 2 diabetes (3, 4), all of which are common complications of obesity. Systematic reviews and meta-analyses have suggested that cholecalciferol supplementation may reduce body mass index (BMI) and waist circumference in overweight and obese individuals to a certain extent. However, data on more specific body composition metrics such as body fat percentage and waist-to-hip ratio remain limited (5).
In recent years, researchers have increasingly focused on the non-skeletal effects of Vitamin D, particularly its potential benefits on muscle function. Vitamin D deficiency is associated with muscle weakness, reduced exercise performance, and increased risk of falls, whereas supplementation may enhance muscle strength, improve repair processes, and boost endurance and overall physical function (6). Consequently, it has been proposed that Vitamin D supplementation may potentiate the effects of exercise interventions in improving weight and metabolic outcomes among overweight or obese adults, especially by synergistically optimizing body composition. At the mechanistic level, transcriptomic technology offers new insights into the potential synergy between Vitamin D and exercise. By analyzing gene expression changes under different conditions, transcriptomics can reveal how Vitamin D and exercise jointly regulate pathways in skeletal muscle, adipose tissue, and inflammation. Previous studies have suggested that Vitamin D may modulate signaling pathways such as AMPK, PPARγ, and NF-κB, thereby influencing lipid metabolism, energy homeostasis, and immune function (7–11). Thus, transcriptomics not only facilitates mechanistic understanding but may also serve as a novel molecular biomarker tool for evaluating intervention responses. Although existing studies provide preliminary evidence for the potential value of Vitamin D in obesity management, numerous unresolved issues remain, including optimal dosing, intervention duration, and the ideal combination model with exercise. Therefore, the current study systematically integrates transcriptomic data and clinical trial evidence to evaluate whether Vitamin D supplementation enhances the weight loss effects of exercise in overweight or obese adults.
Compared to previous studies, this review provides a more comprehensive evaluation of the role of Vitamin D supplementation in exercise-induced weight loss in overweight and obese populations. Given the heterogeneity in participant age, exercise modalities, and Vitamin D dosing across studies, we incorporated both transcriptomic and meta-analytic approaches to enhance scientific rigor. This review primarily focuses on the effects of Vitamin D supplementation on body composition and metabolic health in overweight/obese individuals, and further compares its effects on plasma 25-hydroxyvitamin D [25(OH)D] levels when combined with or without exercise. By integrating high-throughput molecular data with clinical evidence, we aim to provide a theoretical and practical framework for developing personalized obesity interventions based on a synergistic “nutrition–exercise–molecular target” strategy, especially for individuals seeking to improve their health through scientifically grounded methods.
2 Methods
2.1 Transcriptomic data acquisition and analysis
The transcriptomic RNA-seq data of skeletal muscle from 30 obese adults were downloaded from the GEO database [GSE271452, 15 pre-intervention and 15 post-intervention samples, BGISEQ-500 sequencing platform, data type: TPM (Transcripts Per Million)] (12). Additionally, RNA-seq data of 396 normal adult skeletal muscle samples were obtained from the GTEx database1 (Illumina HiSeq 2000/2500 sequencing platform, data type: TPM).
RNA-Seq data were subjected to normalization, including the removal of low-expression genes with an average expression below 1 across all samples. The Wilcoxon test was applied to compare gene expression differences between the control and experimental groups. Log2 fold change (logFC) and median differences were calculated, and p-values were adjusted using the False Discovery Rate (FDR) method, selecting genes with |logFC| > 1 and FDR < 0.05. These steps ensured data standardization and improved the reliability and accuracy of differential analysis results. ComBat was used to adjust the TPM data from both groups for batch effects, followed by Principal Component Analysis (PCA) to validate the effectiveness of batch effect correction. Subsequently, differentially expressed genes, metabolite abundance, and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were conducted to extract key data. DEGs were identified based on a fold change greater than 0.5 or less than −0.5, with an adjusted p-value < 0.1 (13). Differential expression analysis was conducted using R software (version 4.3.3), and p-values were adjusted for multiple comparisons using the q-value method.
2.2 Registration and public involvement
This systematic review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42024589772 (14). The literature search followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (15). As a secondary analysis of published data, this study did not involve direct patient or public participation.
2.3 Search strategy
A comprehensive search was conducted in the PubMed, Web of Science, Cochrane Library, EMBASE, and Scopus databases for RCT published up to November 2024. The reference lists of the screened articles were examined to identify additional relevant studies. Search terms included both controlled vocabulary and free-text keywords related to adults, overweight/obesity, vitamin D, and exercise (e.g., exercise, physical training, physical activity, fitness training, aerobic training, or resistance training), and were adapted for each individual database. Specific keywords used for the search are provided in Supplementary Table S1, and the complete electronic search strategies for all databases are detailed in Supplementary Table S2.
2.4 Study selection: inclusion and exclusion criteria
The study participants included adults who were overweight (BMI ≥ 25 kg/m2) or obese (BMI ≥ 30 kg/m2), aged over 18 years (with no upper age limit). These individuals underwent physical activity interventions and took Vitamin D supplements. Participants with obesity-related comorbidities such as type 2 diabetes, hypertension, dyslipidemia, metabolic syndrome, liver diseases (e.g., NAFLD/NASH), and osteoarthritis were not excluded. The interventions included exercise training programs or other measures designed to promote physical activity, in combination with Vitamin D supplementation. The exercise training could be aerobic, resistance, mixed, or high-intensity interval training, in any combination of these types. Exercise training could be supervised, partially supervised, or unsupervised. Vitamin D supplementation was administered in either liquid and/or solid forms. The control groups included those who received only exercise interventions, placebo (e.g., stretching), or Vitamin D supplementation alone. The primary outcomes assessed included changes in weight, BMI, body fat percentage, blood glucose control, and lipid metabolism markers (e.g., triglycerides (TG), High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL)), among other metabolic health indicators. Only RCT were included, with the following types of studies excluded: (a) trials conducted in pregnant women; (b) studies focusing solely on dietary patterns, single food interventions, or exercise-only interventions; (c) studies centered on primary prevention of weight gain/obesity; (d) exercise interventions combined with other treatments (e.g., medications).
2.5 Data extraction
Data were independently extracted by two reviewers using a standardized form, including study characteristics, participant demographics, intervention details (such as Vitamin D dosage, regimen, and duration), and supervision information (Table 1). Outcome measures included body composition parameters (body weight, BMI, body fat percentage, waist circumference) and metabolic markers (TG, fasting glucose, HDL, LDL, and 25(OH)D). In the event of discrepancies between the two reviewers during data extraction, the differences will first be discussed, and the original literature will be reviewed or consulted with field experts for clarification. If consensus cannot be reached, a third researcher with relevant expertise will be invited to adjudicate. For cases of missing or unclear data, the research team will proactively contact the original authors to obtain the missing information, ensuring the completeness and accuracy of the data.
2.6 Data synthesis and statistical analysis
Meta-analyses were performed using RevMan 5.4. Weighted mean differences (WMDs) or standardized mean differences (SMDs) were calculated for each outcome. Depending on heterogeneity (I2 statistic), a fixed-effect model was used for I2 < 50%, and a random-effects model for I2 ≥ 50% (16). To further explore the variability in intervention effects, we adopted 60 years as the threshold for the elderly group (<60 years vs. ≥60 years), in accordance with high-quality studies and systematic reviews. This age cutoff aligns with international standards and effectively reflects age-related physiological and cognitive changes (17, 18). Additionally, subgroup analyses were conducted based on exercise types (aerobic, resistance, and multimodal), with statistical tests performed (19). Robustness was evaluated using one-by-one sensitivity analyses. Publication bias was assessed through visual inspection of funnel plots, and Egger’s test was performed if asymmetry was evident and the number of studies exceeded 10.
2.7 Risk of bias assessment
Study quality was independently assessed by two reviewers using the Cochrane Risk of Bias Tool (20). The evaluation covered random sequence generation, allocation concealment, blinding, completeness of outcome data, and selective reporting (21). Disagreements were resolved by a third reviewer.
3 Results
3.1 Transcriptomic analysis
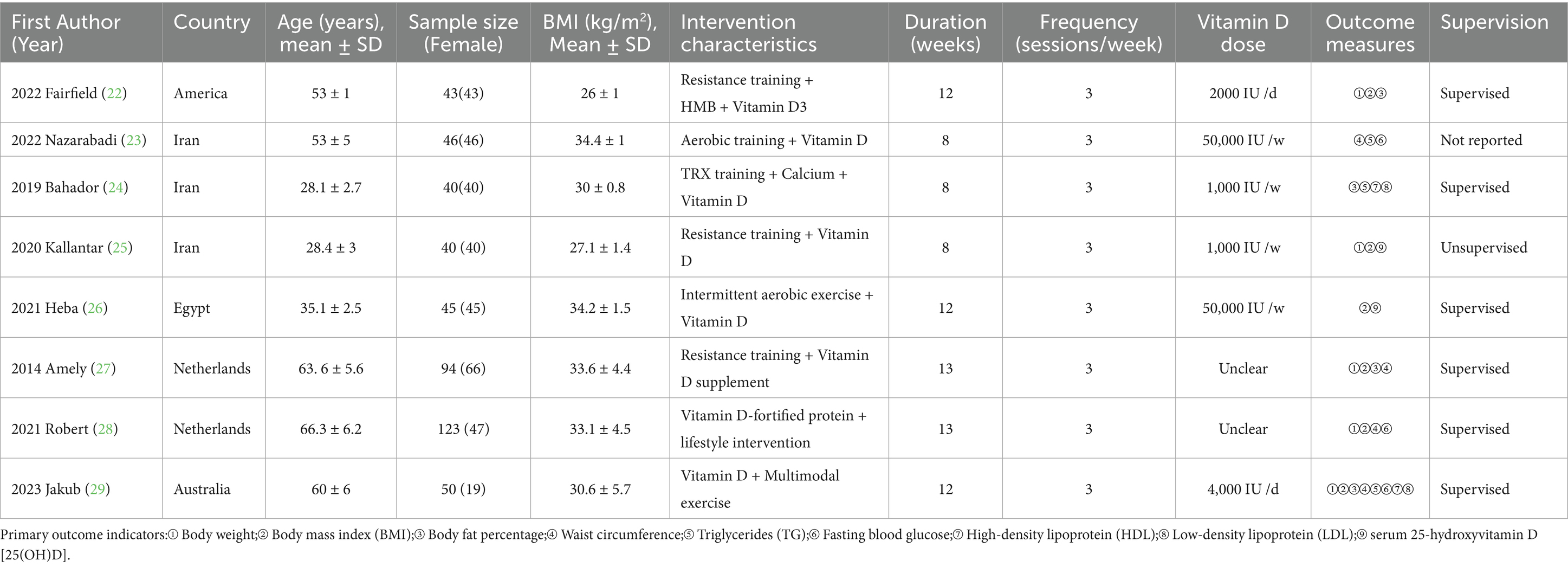
Due to the lack of healthy control samples in the GSE271452 dataset, transcriptomic data from skeletal muscle tissue in the GTEx database were integrated for batch effect correction and normalization (Figures 1A,B). Differential expression analysis identified 36 genes that were significantly differentially expressed between obese and healthy adult skeletal muscle samples (Figure 1C). KEGG pathway enrichment analysis revealed that these genes were significantly enriched in several metabolism-related pathways (Figure 1D), including fat and protein digestion and absorption, pancreatic secretion, and fatty acid metabolism. Although these pathways were not directly enriched in Vitamin D digestion or absorption, their functional alterations may indirectly impair Vitamin D metabolism and bioavailability. For example, reduced lipid absorption efficiency could decrease the uptake of fat-soluble vitamins such as Vitamin D, and changes in genes related to pancreatic function and protein digestion may affect gut physiology, further exacerbating Vitamin D deficiency. GO (Gene Ontology) functional annotation supported this hypothesis, showing that obese individuals exhibited significant alterations in gene expression related to Vitamin D-associated biological processes, such as calcium ion transport, hormone metabolism, immune response, inflammation regulation, and cellular structural maintenance (Figure 1E). These mechanistic alterations may collectively contribute to impaired Vitamin D metabolism in obese populations, increasing the risk of metabolic syndrome and its related complications. Therefore, Vitamin D supplementation strategies for individuals with obesity should be tailored with consideration of their distinct molecular expression profiles.
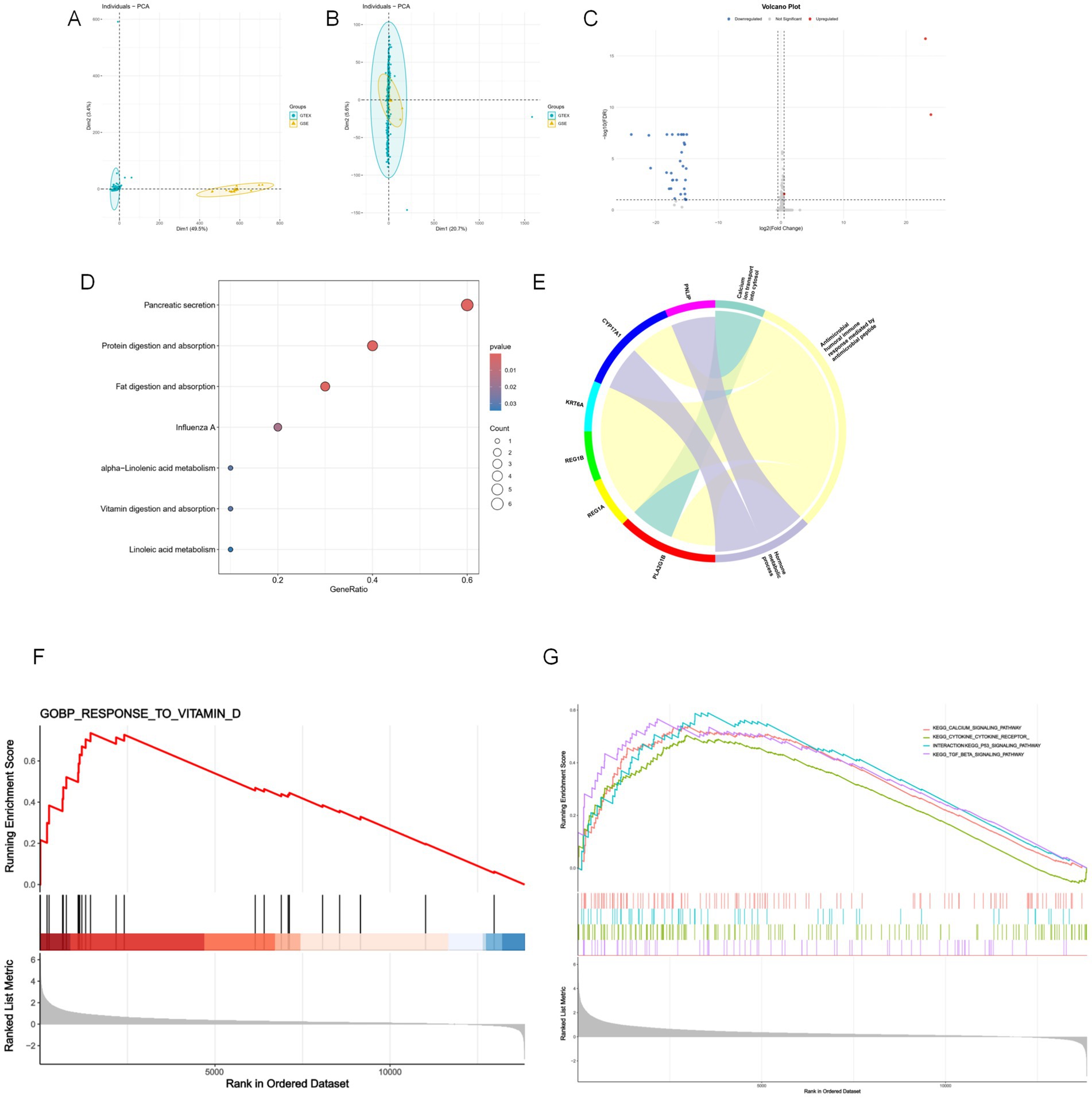
Figure 1. Transcriptomic analysis of GSE271452 and GTEx skeletal muscle data. (A,B) Principal component analysis (PCA) of skeletal muscle transcriptomic data from GSE271452 and GTEx before and after batch effect correction. (C) Volcano plot of differentially expressed genes in merged data (logFC > 0.5 or < −0.5, FDR < 0.1). (D) KEGG pathway enrichment of differentially expressed genes. (E) GO enrichment highlighting Vitamin D metabolism-related pathways and associated genes. (F,G) GSEA results of skeletal muscle transcriptomes in obese adults before and after cold exposure, showing enrichment of Vitamin D response genes and key signaling pathways.
Furthermore, clinical transcriptomic data addressing the key question of whether Vitamin D enhances the effects of exercise interventions remain scarce. To explore potential molecular mechanisms, we employed cold-induced skeletal muscle shivering as a surrogate model. Previous studies have shown that cold exposure can stimulate low-intensity muscle contractions, elevate energy expenditure, and improve obesity-related metabolic outcomes. GSEA (Gene Set Enrichment Analysis) was performed on skeletal muscle transcriptomic data from obese adults before and after cold exposure. The results showed significant enrichment of Vitamin D-responsive genes post-intervention, alongside activation of several KEGG pathways, including calcium signaling, cytokine-cytokine receptor interaction, p53 signaling, and TGF-β signaling (Figures 1F,G). These findings suggest that cold-induced muscle activity may upregulate Vitamin D response genes and downstream pathways, thereby improving skeletal muscle metabolic function, enhancing lipid metabolism, and promoting tissue repair. This provides an important molecular clue for further investigations into how Vitamin D may synergize with exercise interventions to improve metabolic health in obese individuals.
3.2 Meta-analysis results
3.2.1 Literature search
A total of 851 potentially eligible records were identified through systematic database searching. After removing 158 duplicates, full-text screening was conducted for 10 articles to determine eligibility. Of these, 8 studies met the inclusion criteria and were incorporated into both the systematic review and the meta-analysis (22–29). Figure 2 illustrates the flowchart of the study’s search and selection process.
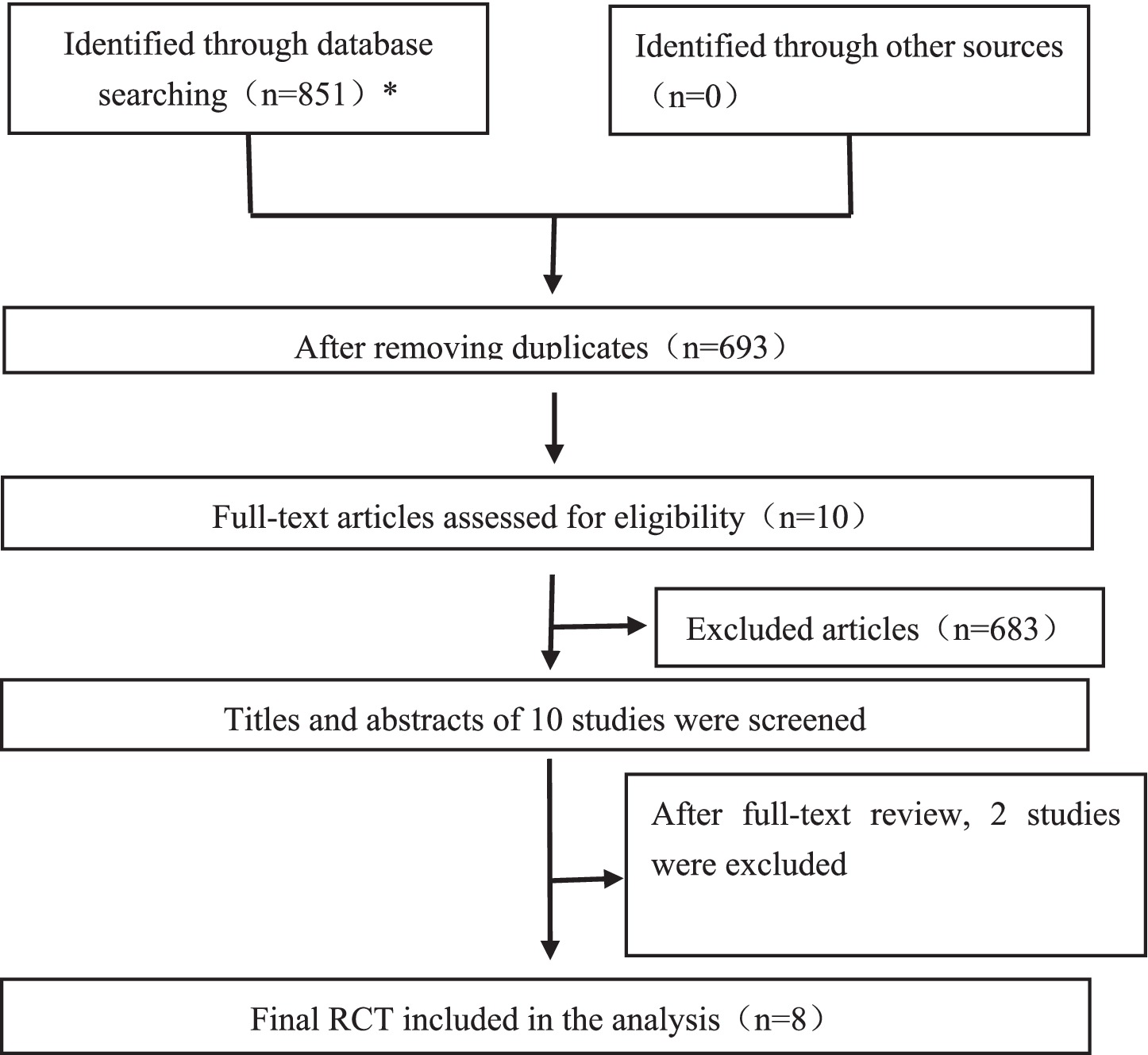
Figure 2. PRISMA flowchart of study selection. The number of studies retrieved from each database was as follows: PubMed (n = 38), Web of Science (n = 90), Cochrane Library (n = 160), Scopus (n = 454), and Embase (n = 109).
3.2.2 Characteristics and risk of bias of included studies
Table 1 summarizes the key characteristics of the eight included studies, published between 2011 and 2023, encompassing a total of 481 participants. Sample sizes ranged from 40 to 123; the proportion of female participants ranged from 38 to 100%. The average age of participants varied from 28 to 66 years, with baseline BMI ranging from 26.1 to 34.2 kg/m2. Exercise interventions lasted between 8 and 13 weeks, conducted three times per week. Vitamin D supplementation dosages varied substantially across studies. Interventions included aerobic, resistance, and multimodal training, most of which were supervised. Additionally, in studies by Fairfield (22) and Heba (26), all participants were Vitamin D deficient at baseline. Detailed risk of bias assessments are illustrated in Figure 3.
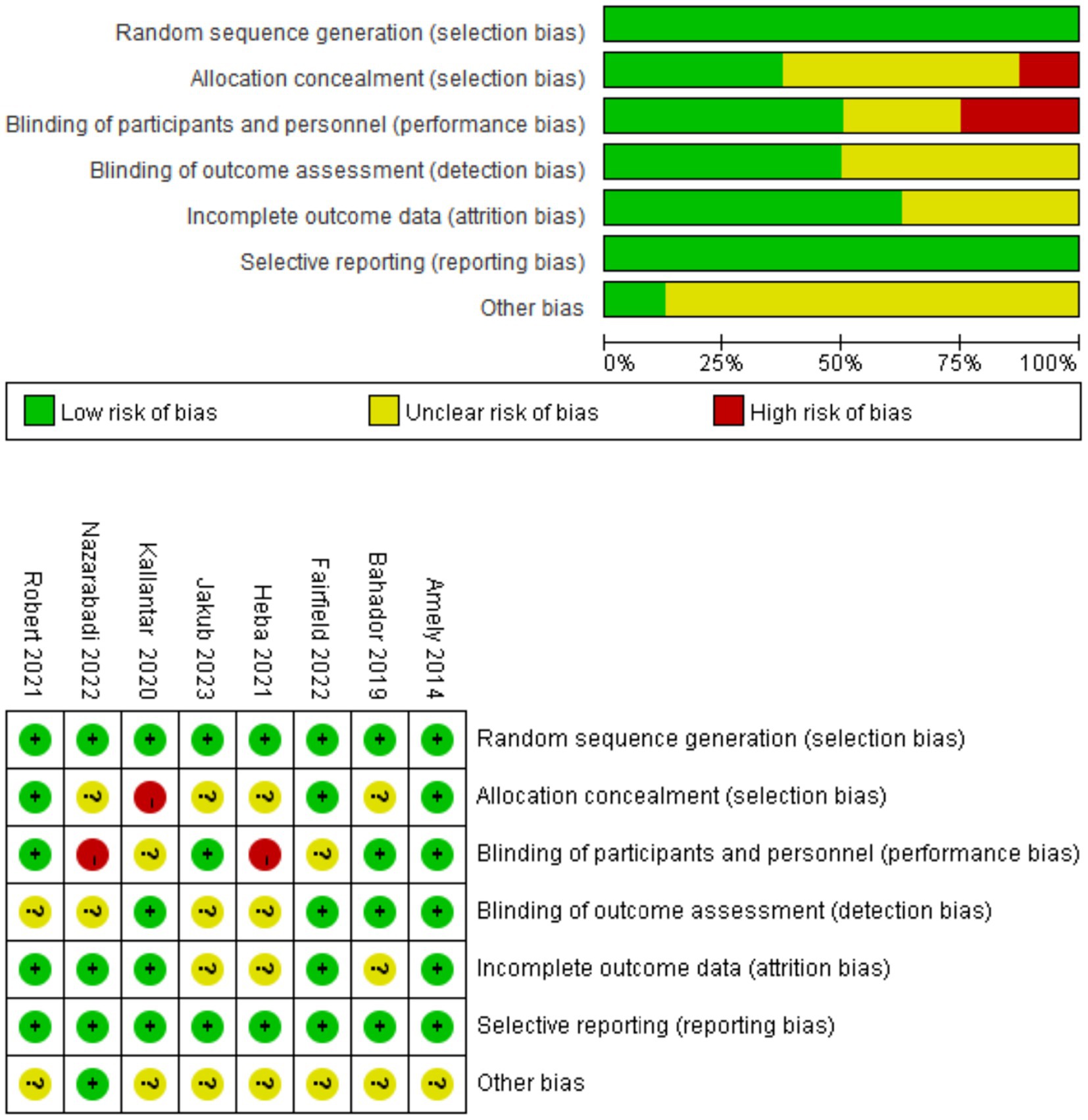
Figure 3. Risk of bias assessment for included studies. All eight included studies were judged to have adequate randomization procedures. Three studies provided details on allocation concealment. Four studies adopted double-blinding, and four described blinded outcome assessments. Most studies reported complete outcome data and showed no evidence of selective reporting.
3.2.3 Effects of exercise combined with Vitamin D supplementation on body composition in overweight/obese adults
Five studies assessed changes in body weight. The random-effects meta-analysis showed that Vitamin D supplementation did not significantly enhance the overall weight reduction effect of exercise [MD = −1.84, 95% CI (−4.85, 1.18)]. However, subgroup analysis revealed that in participants undergoing aerobic exercise, Vitamin D supplementation significantly enhanced weight loss [MD = −2.15, 95% CI (−2.91, −1.38)]. Similar benefits were observed in individuals aged ≥60 years [MD = −2.09, 95% CI (−2.85, −1.33)]. For BMI, the meta-analysis of six studies also showed no overall significant effect [MD = −0.45, 95% CI (−1.54, 0.65)]. Nevertheless, subgroup analysis indicated that Vitamin D supplementation significantly improved BMI reduction when combined with multimodal exercise [MD = −0.50, 95% CI (−0.73, −0.28)], with a similar effect observed in older adults [MD = −0.50, 95% CI (−0.73, −0.28)]. For body fat percentage, results from four studies indicated no significant additive effect of Vitamin D on the reduction induced by exercise [MD = −0.35, 95% CI (−3.33, 2.64)]. In contrast, for waist circumference, data from four studies using a fixed-effects model showed a significant additional reduction with Vitamin D supplementation [MD = −1.48, 95% CI (−2.02, −0.94)] (see Table 2).
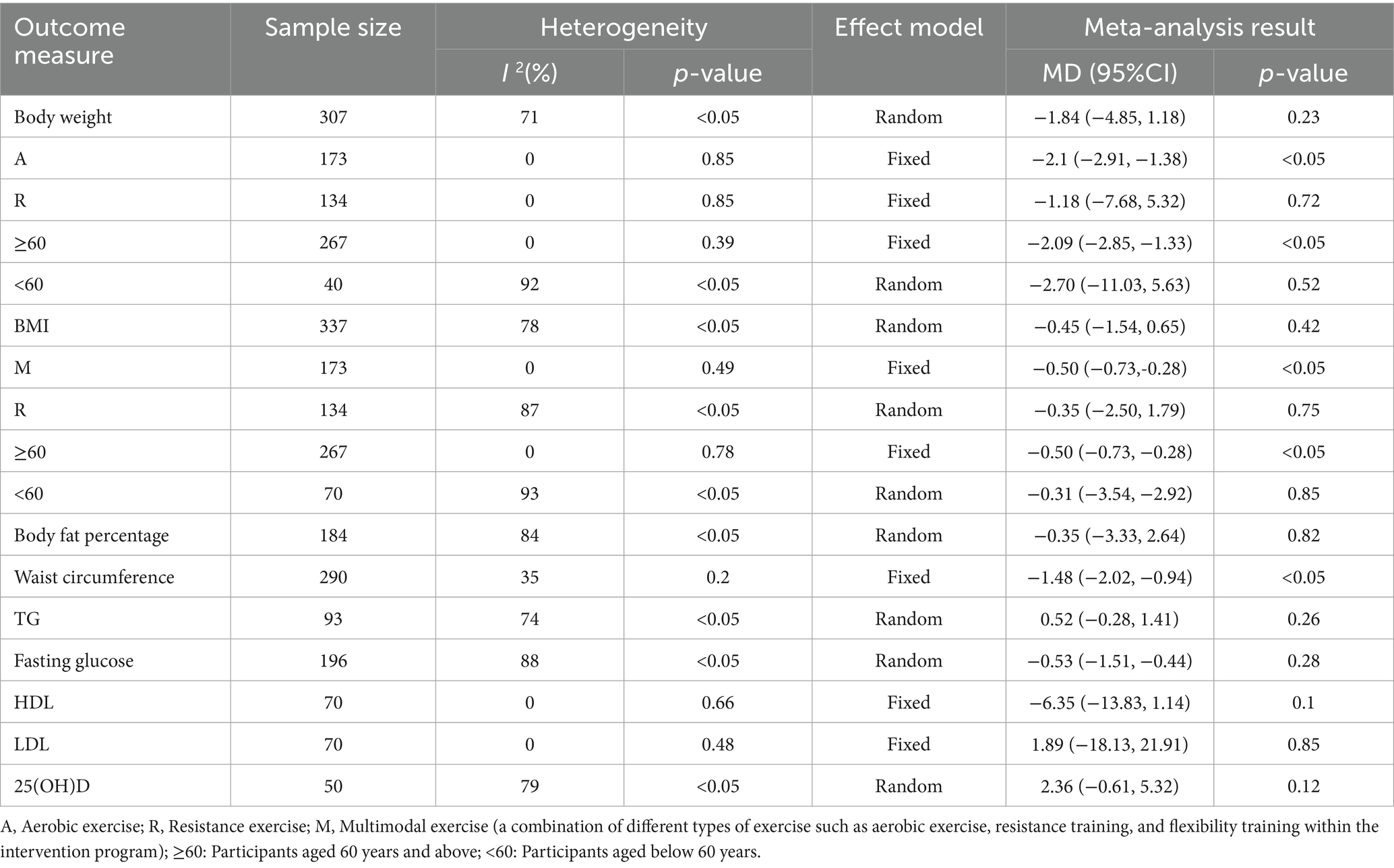
Table 2. Meta-analysis results on the effects of exercise combined with Vitamin D supplementation in overweight or obese adults.
3.2.4 Effects of exercise combined with Vitamin D supplementation on metabolic markers
In terms of metabolic parameters, meta-analysis of three studies showed that Vitamin D supplementation did not significantly enhance the effect of exercise in reducing triglyceride (TG) levels [MD = 0.52, 95% CI (−0.28, 1.41)]. Likewise, no significant difference was found for fasting glucose [MD = −0.53, 95% CI (−1.51, 0.44)]. HDL and LDL analyses were based on two studies. The results showed no significant improvement in HDL [MD = −6.35, 95% CI (−13.83, 1.14)] or reduction in LDL [MD = 1.89, 95% CI (−18.13, 21.91)] with combined intervention. Regarding Vitamin D status, two studies assessed 25(OH)D concentrations. Meta-analysis showed that exercise combined with supplementation did not significantly increase 25(OH)D levels compared to supplementation alone [MD = 2.36, 95% CI (−0.61, 5.32)] (Table 2).
3.2.5 Publication bias assessment
Funnel plots were used to assess publication bias across all outcome variables. No evidence of publication bias was observe (Supplementary Figures S1–S9).
4 Discussion
Vitamin D is widely used in weight management among obese populations due to its role in chronic disease prevention and metabolic regulation (30). Exercise, as a safe and multifaceted intervention, can improve energy metabolism, mood states, and disease risk (31–33). In recent years, increasing research has attempted to incorporate Vitamin D supplementation into exercise programs to enhance their intervention effects. Transcriptomic analysis provides molecular-level evidence for this hypothesis. By integrating data from GSE271452 and GTEx skeletal muscle samples, 36 differentially expressed genes were identified, and enrichment analysis indicated that Vitamin D metabolic disturbances in obesity are primarily mediated through pathways related to lipid digestion and absorption, pancreatic secretion, calcium ion transport, and hormone metabolism. These disturbances may affect nutrient absorption in the gut and exacerbate Vitamin D deficiency. In selecting the experimental model, this study used cold-induced muscle shivering to simulate exercise intervention. Although this approach offers advantages in terms of ethics and control, its physiological mechanisms differ from those of real exercise interventions. Cold-induced energy metabolism regulation involves two mechanisms: shivering thermogenesis through muscle shivering and non-shivering thermogenesis mediated by brown adipose tissue (34, 35). In contrast, voluntary exercise is a more complex physiological adaptation process, involving active muscle contraction, cardiovascular system responses, and long-term metabolic reprogramming (36). The cold-induced muscle shivering model showed that, after intervention, genes related to Vitamin D response in the skeletal muscle of obese participants were significantly upregulated, activating metabolic pathways such as p53, TGF-β, and calcium signaling. This suggests that Vitamin D may be involved in energy metabolism regulation by modulating muscle remodeling and mitochondrial function. These findings are consistent with previous studies on Vitamin D’s role in regulating muscle synthesis, lipid metabolism, and insulin sensitivity (37–43), further supporting its potential mechanistic role in combined interventions. Although this model offers some insights, the indirect connection to actual exercise physiology needs further clarification in future studies, particularly concerning how Vitamin D might regulate muscle remodeling (44–46), mitochondrial function (47–49), and calcium signaling (50, 51) in the context of energy metabolism. Such explanations may help better understand the mechanisms underlying the role of Vitamin D in exercise interventions.
The results of the meta-analysis showed that, although Vitamin D combined with exercise did not demonstrate consistent improvements in weight, BMI, or body fat percentage, it did show certain advantages in reducing waist circumference (30). Subgroup analysis further revealed that the combined intervention was more effective in controlling weight and BMI in older adults (≥60 years) and in participants engaging in aerobic exercise, suggesting that individual characteristics and exercise modalities may modulate intervention effects. Additionally, previous studies have indicated that Vitamin D deficiency is strongly associated with obesity, metabolic syndrome, and cardiovascular disease, particularly in women and individuals with abdominal obesity (2, 52). Although calcitriol supplementation can modestly reduce BMI and waist circumference (53), its direct impact on weight remains limited. Combined interventions may work through multiple mechanisms, such as enhancing Vitamin D receptor (VDR) expression (54), modulating leptin levels (55), and improving gut microbiota and bile acid metabolism (40), thereby synergistically promoting fat metabolism. However, the results on whether exercise significantly elevates serum 25(OH)D levels remain inconsistent. Some studies suggest that endurance or mixed training may be effective, while resistance training alone has limited effects (56–59). Given that Vitamin D in obese populations is primarily stored in adipose tissue with limited bioavailability (60), combined interventions may be of greater clinical significance for this group (22, 26, 61). At the same time, Vitamin D levels are influenced by various factors such as diet, supplement intake, skin color, and sun exposure (62, 63). Therefore, future research should focus on variables such as VDR expression, baseline Vitamin D status, exercise intensity, and intervention duration, and use standardized, long-term follow-up RCT to further clarify the adaptability and mechanistic basis of “Vitamin D + exercise” interventions in different populations.
Moreover, in this study’s meta-analysis, we observed high I2 values for some outcome measures, suggesting significant heterogeneity across studies. This heterogeneity may stem from differences in demographic characteristics such as age, gender distribution, baseline Vitamin D status, and methodological differences such as exercise modalities (e.g., aerobic, resistance, or multimodal training), intervention duration, frequency, and supplementation doses (ranging from 1,000 IU/d to 50,000 IU/w). Higher doses of Vitamin D may have a more significant impact on muscle function and fat metabolism, but this was not consistently reflected in the current analysis (29, 64, 65). To reduce bias due to heterogeneity, we used a random-effects model for the summary estimate and further explored the moderating effects of individual characteristics and intervention modalities through subgroup analysis. Notably, subgroup results showed that Vitamin D combined with exercise had a more significant impact on weight and BMI improvement in older adults (≥60 years) and those participating in aerobic exercise, suggesting that age has a clear physiological boundary, which is often used in previous studies to distinguish populations with significant differences in metabolic sensitivity. However, the studies included in the current analysis used multiple different serum 25(OH)D cutoffs (25 nmol/L, 50 nmol/L, and 75 nmol/L), and some studies cited multiple thresholds within the same paper (29), which limited the feasibility of this subgroup analysis. Although theoretically, both interventions may synergistically affect fat generation, reduce inflammation, and show potential in improving metabolic indicators, high-quality RCT are currently lacking to confirm their advantages in weight or body fat reduction (66–68). As such studies increase, especially with standardized reporting of Vitamin D status, this suggestion will have significant potential to refine target populations and intervention strategies.
In terms of transcriptomic analysis, although batch effects between the GSE and GTEx databases were corrected using the ComBat algorithm, and the comparability of the integrated data was verified through PCA, there remain certain differences in sample size and individual characteristics (such as BMI levels, gender composition, etc.), which may influence the robust identification of differentially expressed genes. Particularly in the exploration of mechanisms using cold-induced muscle shivering as a model for exercise intervention, while this model does not fully replicate the metabolic activation mechanisms of voluntary exercise, it offers a certain degree of substitutability in terms of controllability, ethical applicability, and biological energy mobilization. Therefore, it can serve as an approximate research tool to study the involvement of Vitamin D in skeletal muscle metabolic remodeling. It is also worth noting that some of the included studies did not provide detailed information on Vitamin D formulation, bioavailability, intervention duration, or adherence to supplementation, limiting the in-depth analysis of the dose–response relationship. Furthermore, the lack of baseline 25(OH)D status reports restricts the evaluation of sensitivity in Vitamin D deficiency subgroups. Future research should focus on intervention stratification based on individual nutritional status, particularly in individuals with low or deficient Vitamin D levels, by combining multimodal exercise interventions (i.e., composite regimens involving various types of exercise). This approach could enhance the comparability and external validity of intervention outcomes.
The clinical significance of this study lies in the finding that Vitamin D supplementation can amplify the weight loss effects induced by exercise, particularly in improving waist circumference and promoting reductions in weight and BMI. These benefits are especially pronounced in older adults and overweight or obese individuals undergoing aerobic exercise interventions. Our findings suggest that combining exercise with Vitamin D supplementation may represent an effective strategy for optimizing weight management and metabolic health. Therefore, future studies should further define the optimal exercise protocols, explore the ideal dosage and duration of Vitamin D supplementation, and examine its effects on other metabolic markers, such as blood glucose and lipid profiles, to enhance its clinical efficacy in improving body composition and metabolic health. Additionally, the integration of multi-omics data and nutritional behavior monitoring technologies to systematically elucidate the mechanisms underlying the combined effects of Vitamin D and exercise, as well as individual response differences, will provide stronger empirical support for the development of precise and effective exercise-nutrition combined interventions.
5 Conclusion
This study systematically evaluated the effects of Vitamin D supplementation on exercise-based interventions in overweight and obese adults, and explored potential underlying mechanisms using transcriptomic data. Transcriptomic analysis revealed that Vitamin D-related gene expression in skeletal muscle is significantly altered in individuals with obesity, primarily through pathways involving fat absorption, pancreatic function, calcium transport, and hormone metabolism. Additionally, a cold-induced muscle shivering model indicated significant enrichment of Vitamin D-responsive genes and their downstream pathways following intervention, suggesting a potential role in muscle metabolic regulation. Meta-analysis results showed that combining exercise with Vitamin D supplementation significantly improved waist circumference reduction, with more pronounced effects on weight and BMI observed in older adults and those engaging in aerobic training—supporting a possible synergistic effect. However, no significant enhancements were observed in glucose or lipid metabolism, which may be due to individual differences in Vitamin D metabolism, baseline levels, or intervention protocols. In summary, Vitamin D supplementation may enhance exercise-induced benefits by improving skeletal muscle metabolism and adaptive responses, thereby synergistically supporting weight-loss interventions. Future research should focus on personalized Vitamin D status, intervention intensity and duration, and molecular validation, aiming to develop more precise and effective nutrition-exercise strategies for overweight and obese populations.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found in the NCBI GEO repository: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE271452 and the GTEX repository: https://gtexportal.org/home/downloads/adult-gtex/bulk_tissue_expression.
Author contributions
TP: Data curation, Funding acquisition, Writing – original draft. ZZ: Data curation, Investigation, Writing – original draft. WL: Data curation, Writing – original draft. JZ: Investigation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Fundamental Research Funds for the Central Universities (Grant No. 2025023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1664960/full#supplementary-material
Footnotes
References
1. Via, M. The malnutrition of obesity: micronutrient deficiencies that promote diabetes. ISRN Endocrinol. (2012) 2012:103472. doi: 10.5402/2012/103472
2. Karampela, I, Sakelliou, A, Vallianou, N, Christodoulatos, G-S, Magkos, F, and Dalamaga, M. Vitamin D and obesity: current evidence and controversies. Curr Obes Rep. (2021) 10:162–80. doi: 10.1007/s13679-021-00433-1
3. Zhu, T, Zhao, J, Zhuo, S, Hu, Z, Ouyang, S, Wunier, YS, et al. High fat diet and high cholesterol diet reduce hepatic vitamin D-25-hydroxylase expression and serum 25-hydroxyvitamin D3 level through elevating circulating cholesterol, glucose, and insulin levels. Mol Nutr Food Res. (2021) 65:e2100220. doi: 10.1002/mnfr.202100220
4. Gnudi, L, Fountoulakis, N, Panagiotou, A, Corcillo, A, Maltese, G, Rife, MF, et al. Effect of active vitamin-D on left ventricular mass index: results of a randomized controlled trial in type 2 diabetes and chronic kidney disease. Am Heart J. (2023) 261:1–9. doi: 10.1016/j.ahj.2023.03.003
5. López-Muñoz, P, Torres-Costoso, AI, Fernández-Rodríguez, R, Guzmán-Pavón, MJ, de Arenas-Arroyo, SN, Basco-López, JÁ, et al. Effect of vitamin D supplementation on fatigue in multiple sclerosis: a systematic review and meta-analysis. Nutrients. (2023) 15:2861. doi: 10.3390/nu15132861
6. Takeuchi, I, Yoshimura, Y, Shimazu, S, Jeong, S, Yamaga, M, and Koga, H. Effects of branched-chain amino acids and vitamin D supplementation on physical function, muscle mass and strength, and nutritional status in sarcopenic older adults undergoing hospital-based rehabilitation: a multicenter randomized controlled trial. Geriatr Gerontol Int. (2019) 19:12–7. doi: 10.1111/ggi.13547
7. Greene, NP, Fluckey, JD, Lambert, BS, Greene, ES, Riechman, SE, and Crouse, SF. Regulators of blood lipids and lipoproteins? PPARδ and AMPK, induced by exercise, are correlated with lipids and lipoproteins in overweight/obese men and women. Am J Physiol Endocrinol Metab. (2012) 303:E1212–21. doi: 10.1152/ajpendo.00309.2012
8. Rajakumar, K, Yan, Q, Khalid, AT, Feingold, E, Vallejo, AN, Demirci, FY, et al. Gene expression and cardiometabolic phenotypes of vitamin D-deficient overweight and obese black children. Nutrients. (2019) 11:2016. doi: 10.3390/nu11092016
9. Hoseini, R, Damirchi, A, and Babaei, P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. (2017) 36:54–9. doi: 10.1016/j.nut.2016.06.010
10. Chang, E, and Kim, Y. Vitamin d insufficiency exacerbates adipose tissue macrophage infiltration and decreases AMPK/SIRT1 activity in obese rats. Nutrients. (2017) 9:338. doi: 10.3390/nu9040338
11. Diniz, TA, de Lima Junior, EA, Teixeira, AA, Biondo, LA, da Rocha, LAF, Valadão, IC, et al. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. (2021) 266:118868. doi: 10.1016/j.lfs.2020.118868
12. Sellers, AJ, VAN Beek, SMM, Hashim, D, Baak, R, Pallubinsky, H, Moonen-Kornips, E, et al. Cold acclimation with shivering improves metabolic health in adults with overweight or obesity. Nat Metab. (2024) 6:2246–53. doi: 10.1038/s42255-024-01172-y
13. Rapaport, F, Khanin, R, Liang, Y, Pirun, M, Krek, A, Zumbo, P, et al. Comprehensive evaluation of differential gene expression analysis methods for RNA-seq data. Genome Biol. (2013) 14:R95. doi: 10.1186/gb-2013-14-9-r95
14. Booth, A, Clarke, M, Ghersi, D, Moher, D, Petticrew, M, and Stewart, L. An international registry of systematic-review protocols. Lancet. (2011) 377:108–9. doi: 10.1016/S0140-6736(10)60903-8
15. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2024) 6:e1000097. doi: 10.1371/journal.pmed.1000097
16. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
17. Dykiert, D, Der, G, Starr, JM, and Deary, IJ. Age differences in intra-individual variability in simple and choice reaction time: systematic review and meta-analysis. PLoS One. (2012) 7:e45759. doi: 10.1371/journal.pone.0045759
18. Rupprecht, M, Wagenpfeil, S, Schöpe, J, Vieth, R, Vogt, T, and Reichrath, J. Meta-analysis of European clinical trials characterizing the healthy-adult serum 25-hydroxyvitamin D response to vitamin D supplementation. Nutrients. (2023) 15:3986. doi: 10.3390/nu15183986
19. Borenstein, M, Hedges, LV, Higgins, JPT, and Rothstein, HR. Introduction to meta-analysis. Hoboken: Wiley (2021).
20. Higgins, JPT, Altman, DG, Gøtzsche, PC, Higgins, JP, Jüni, P, Moher, D, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Higgins, JPT, and Sally, G. Cochrane handbook for systematic reviews of interventions. Chicester, West Sussex, UK: Wiley-Blackwell (2008).
22. Fairfield, WD, Minton, DM, Elliehausen, CJ, Nichol, AD, Cook, TL, Rathmacher, JA, et al. Small-scale randomized controlled trial to explore the impact of β-hydroxy-β-methylbutyrate plus vitamin D3 on skeletal muscle health in middle aged women. Nutrients. (2022) 14:4674. doi: 10.3390/nu14214674
23. Nazarabadi, P, Etemad, Z, Hoseini, R, Nazarabadi, PN, and Moradi, F. Anti-inflammatory effects of a period of aerobic training and vitamin D supplementation in postmenopausal women with metabolic syndrome. Int J Prev Med. (2022) 13:60. doi: 10.4103/ijpvm.IJPVM_312_20
24. Bahador, H, and Taghian, F. The effect of 8 weeks interval exercises and vitamin D supplementation on plasma levels of dipeptidyl peptidase-4 and retinol binding protein 4 in overweight women. IJEM. (2019) 21:73–82.
25. Kallantar, N, and Fatolahi, H. Decrease of plasma TNF-α and CRP levels in response to post-exhaust resistance training and vitamin D supplementation in overweight healthy women. Rom J Diabetes Nutr Metab Dis. (2020) 27:349–56. doi: 10.46389/rjd-2020-1051
26. Heba, AAA, Rodriguez-Sanz, D, Ewidea, M, Al-Hamaky, DMA, Mohamed, MAE-R, and Elerian, AE. Efficacy of vitamin D supplementation in addition to aerobic exercise training in obese women with perceived myalgia: a single-blinded randomized controlled clinical trial. Nutrients. (2021) 13:1819. doi: 10.3390/nu13061819
27. Amely, MV, Verlaan, S, Engberink, MF, Swinkels, S, de Vogel-van n Bosch, J, and Weijs, PJM. A high whey protein–, leucine-, and vitamin D–enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. (2015) 101:279–86. doi: 10.3945/ajcn.114.090290
28. Robert, MG, Pasman, WJ, Bongers, A, Tump, A, van Ginkel, A, Tromp, W, et al. Effect of an enriched protein drink on muscle mass and glycemic control during combined lifestyle intervention in older adults with obesity and type 2 diabetes: a double-blind RCT. Nutrients. (2020) 13:64. doi: 10.3390/nu13010064
29. Jakub, M, Rodriguez, AJ, Cervo, MM, Gandham, A, Xu, CLH, Glavas, C, et al. Vitamin D supplementation and exercise for improving physical function, body composition and metabolic health in overweight or obese older adults with vitamin D deficiency: a pilot randomized, double-blind, placebo-controlled trial. Eur J Nutr. (2023) 62:951–64. doi: 10.1007/s00394-022-03038-z
30. Ródenas Esteve, I, Wanden-Berghe, C, and Sanz-Valero, J. Effects of nutritional status on the multiple sclerosis disease: systematic review. Nutr Hosp. (2018) 35:211–23. doi: 10.20960/nh.1229
31. Swift, DL, Mcgee, JE, Earnest, CP, Carlisle, E, Nygard, M, and Johannsen, NM. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis. (2018) 61:206–13. doi: 10.1016/j.pcad.2018.07.014
32. Oppert, JM, Bellicha, A, VAN Baak, MA, Battista, F, Beaulieu, K, Blundell, JE, et al. Exercise training in the management of overweight and obesity in adults: synthesis of the evidence and recommendations from the European Association for the Study of obesity physical activity working group. Obes Rev. (2021) 22:e13273. doi: 10.1111/obr.13273
33. Lavie, CJ, Arena, R, Swift, DL, Johannsen, NM, Sui, X, Lee, DC, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 117:207–19. doi: 10.1161/CIRCRESAHA.117.305205
34. Dumont, L, Richard, G, Espagnet, R, Frisch, F, Fortin, M, Samson, A, et al. Shivering, but not adipose tissue thermogenesis, increases as a function of mean skin temperature in cold-exposed men and women. Cell Metab. (2025) 37:S1550-4131(25)00305-5. doi: 10.1016/j.cmet.2025.06.010
35. Bell, DG, Tikuisis, P, and Jacobs, I. Relative intensity of muscular contraction during shivering. J Appl Physiol. (1992) 72:2336–42. doi: 10.1152/jappl.1992.72.6.2336
36. Opichka, M, Shute, R, Marshall, K, and Slivka, D. Effects of exercise in a cold environment on gene expression for mitochondrial biogenesis and mitophagy. Cryobiology. (2019) 90:47–53. doi: 10.1016/j.cryobiol.2019.08.007
37. Aarts, E, VAN Groningen, L, Horst, R, Telting, D, van Sorge, A, Janssen, I, et al. Vitamin D absorption: consequences of gastric bypass surgery. Eur J Endocrinol. (2011) 164:827–32. doi: 10.1530/EJE-10-1126
38. Vranić, L, Mikolašević, I, and Milić, S. Vitamin D deficiency: consequence or cause of obesity? Medicina (Kaunas). (2019) 55:541. doi: 10.3390/medicina55090541
39. Cheng, S, Massaro, JM, Fox, CS, Larson, MG, Keyes, MJ, McCabe, EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham heart study. Diabetes. (2010) 59:242–8. doi: 10.2337/db09-1011
40. Lin, HR, Xu, F, Chen, D, Xie, K, Yang, Y, Hu, W, et al. The gut microbiota-bile acid axis mediates the beneficial associations between plasma vitamin D and metabolic syndrome in Chinese adults: a prospective study. Clin Nutr. (2023) 42:887–98. doi: 10.1016/j.clnu.2023.03.022
41. Yu, K, Song, W, Tu, X, Zhou, K, and Prabahar, K. The effect of vitamin D on the lipid profile in individuals with overweight or obesity: a meta-analysis and systematic review of randomized controlled trials. Prostaglandins Other Lipid Mediat. (2025) 176:106938. doi: 10.1016/j.prostaglandins.2024.106938
42. Hu, L, Velu, P, Prabahar, K, Hernández-Wolters, B, Kord-Varkaneh, H, and Xu, Y. Effect of vitamin D supplementation on lipid profile in overweight or obese women: a meta-analysis and systematic review of randomized controlled trials. Nutr Rev. (2025) 83:1657–68. doi: 10.1093/nutrit/nuae226
43. Hao, L, Lu, A, Gao, H, Niu, J, Prabahar, K, Seraj, SS, et al. The effects of vitamin D on markers of glucose and obesity in postmenopausal women: a meta-analysis of randomized controlled trials. Clin Ther. (2023) 45:913–20. doi: 10.1016/j.clinthera.2023.07.009
44. Endo, I, Inoue, D, Mitsui, T, Umaki, Y, Akaike, M, Yoshizawa, T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 144:5138–44. doi: 10.1210/en.2003-0502
45. Girgis, CM, Clifton-Bligh, RJ, Mokbel, N, Cheng, K, and Gunton, JE. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology. (2025) 155:347–57. doi: 10.1210/en.2013-1205
46. Ceglia, L, Niramitmahapanya, S, da Silva Morais, M, Rivas, DA, Harris, SS, Bischoff-Ferrari, H, et al. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. (2025) 98:E1927–35. doi: 10.1210/jc.2013-2820.
47. Ryan, ZC, Craig, TA, Folmes, CD, Wang, X, Lanza, IR, Schaible, NS, et al. 1α,25-dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J Biol Chem. (2016) 291:1514–28. doi: 10.1074/jbc.M115.684399
48. Latham, CM, Brightwell, CR, Keeble, AR, Munson, BD, Thomas, NT, Zagzoog, AM, et al. Vitamin D promotes skeletal muscle regeneration and mitochondrial health. Front Physiol. (2021) 12:660498. doi: 10.3389/fphys.2021.660498
49. Russo, C, Valle, MS, D'Angeli, F, Surdo, S, and Malaguarnera, L. Resveratrol and vitamin D: eclectic molecules promoting mitochondrial health in sarcopenia. Int J Mol Sci. (2024) 25:7503. doi: 10.3390/ijms25147503
50. Molina, P, Carrero, JJ, Bover, J, Chauveau, P, Mazzaferro, S, and Torres, PU. Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J Cachexia Sarcopenia Muscle. (2017) 8:686–701. doi: 10.1002/jcsm.12218
51. Mcclelland, GB, Craig, PM, Dhekney, K, and Dipardo, S. Temperature- and exercise-induced gene expression and metabolic enzyme changes in skeletal muscle of adult zebrafish (Danio rerio). J Physiol. (2006) 577:739–51. doi: 10.1113/jphysiol.2006.119032
52. Zhao, J, Fu, S, and Chen, Q. Association between the serum vitamin D level and prevalence of obesity/abdominal obesity in women with infertility: a cross-sectional study of the National Health and nutrition examination survey data. Gynecol Endocrinol. (2023) 39:2217251. doi: 10.1080/09513590.2023.2217251
53. Perna, S. Is vitamin D supplementation useful for weight loss programs? A systematic review and meta-analysis of randomized controlled trials. Medicina. (2019) 55:368. doi: 10.3390/medicina55070368
54. Weng, SJ, Yan, DY, Gu, LJ, Chen, L, Xie, Z-J, Wu, Z-Y, et al. Combined treatment with vitamin K2 and PTH enhanced bone formation in ovariectomized rats and increased differentiation of osteoblast in vitro. Chem Biol Interact. (2019) 300:101–10. doi: 10.1016/j.cbi.2019.01.012
55. Wauman, J, and Tavernier, J. Leptin receptor signaling: pathways to leptin resistance. Front Biosci. (2011) 16:2771–93. doi: 10.2741/3885
56. Cui, X, Wang, K, Zhang, J, and Cao, Z-B. Aerobic exercise ameliorates myocardial fibrosis via affecting vitamin D receptor and transforming growth factor-β1 signaling in vitamin D-deficient mice. Nutrients. 15:741. doi: 10.3390/nu15030741
57. Iwamoto, J, Shimamura, C, Takeda, T, Abe, H, Ichimura, S, Sato, Y, et al. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. J Bone Miner Metab. (2004) 22:26–31. doi: 10.1007/s00774-003-0443-5
58. Agergaard, J, Trøstrup, J, Uth, J, Iversen, JV, Boesen, A, Andersen, JL, et al. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men? - a randomized controlled trial. Nutr Metab. 12:32. doi: 10.1186/s12986-015-0029-y
59. Mori, B, Barcellos, JFM, Lima, LER, Zaranza, V, Autran, RG, Camargo, EB, et al. Relationship between vitamin D and physical activity: systematic review and meta-analysis. Braz J Biol. (2022) 82:e263882. doi: 10.1590/1519-6984.263882
60. Wortsman, J, Matsuoka, LY, Chen, TC, Lu, Z, and Holick, MF. Decreased bioavailability of vitamin D in obesity123. Am J Clin Nutr. (2000) 72:690–3. doi: 10.1093/ajcn/72.3.690
61. Mallard, SR, Howe, AS, and Houghton, LA. Vitamin D status and weight loss: a systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. Am J Clin Nutr. (2016) 104:1151–9. doi: 10.3945/ajcn.116.136879
62. Raymond-Lezman, JR, Riskin, SI, and Raymond-Lezman, JR. Benefits and risks of sun exposure to maintain adequate vitamin D levels. Cureus. (2023) 15:8578. doi: 10.7759/cureus.38578
63. Tran, V, Janda, M, Lucas, RM, McLeod, DSA, Thompson, BS, Waterhouse, M, et al. Vitamin D and sun exposure: a community survey in Australia. Curr Oncol. (2023) 30:2465–81. doi: 10.3390/curroncol30020188
64. Vernerová, L, Vokurková, M, Laiferová, NA, Nemec, M, Špiritović, M, Mytiai, O, et al. Vitamin D and its receptor in skeletal muscle are associated with muscle disease manifestation, lipid metabolism and physical fitness of patients with myositis. Arthritis Res Ther. (2025) 27:48. doi: 10.1186/s13075-025-03516-9
65. Gilsanz, V, Kremer, A, Mo, AO, Wren, TA, and Kremer, R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. (2010) 95:1595–601. doi: 10.1210/jc.2009-2309
66. Morris, O, Abdelwahab, M, Abdaziz, A, and Atta, F. Effect of resisted exercise on vitamin D levels in obese insulin resistant patients. NILES J Geriatr Gerontol. (2025) 8:1–12. doi: 10.21608/niles.2024.289891.1089
67. Karadağ, A, and Otu, M. Effect of serum vitamin D levels on weight loss in obese patients doing aerobic exercises: a retrospective study. Med Sci Discov. (2020) 7:400–4. doi: 10.36472/msd.v7i2.346
Keywords: obesity, vitamin D, exercise intervention, transcriptomics, meta-analysis
Citation: Peng T, Zhang Z, Liang W and Zhang J (2025) Can Vitamin D supplementation enhance the effectiveness of exercise-induced weight loss in overweight or obese adults? Evidence from integrated transcriptomic and meta-analysis. Front. Nutr. 12:1664960. doi: 10.3389/fnut.2025.1664960
Edited by:
Kousalya Prabahar, University of Tabuk, Saudi ArabiaReviewed by:
Praveen Devanandan, St. Peter's Institute of Pharmaceutical Sciences Hanamkonda, IndiaKarthik Sankar Babu, Apollo Speciality Hospital, India
S. Sarumathy, SRM Institute of Science and Technology, India
Copyright © 2025 Peng, Zhang, Liang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianhang Peng, MjAyNDExMDA2NkBic3UuZWR1LmNu
 Tianhang Peng
Tianhang Peng Zike Zhang
Zike Zhang Wanyuan Liang
Wanyuan Liang Jiayi Zhang
Jiayi Zhang