Abstract
Liver diseases pose a serious threat to human health, necessitating the development of safe and effective preventive and therapeutic strategies. Gardenia fruit (GF), the mature fruit of Gardenia jasminoides Ellis, has been widely used in both food and medicinal applications. Over time, GF and its major bioactive constituents, the iridoids, have demonstrated significant potential in the prevention and treatment of various liver diseases. This review first summarizes the structural characteristics and pharmacological activities of the major iridoids in GF from a phytochemical perspective. It then focuses on the therapeutic effects of GF extracts against non-alcoholic fatty liver disease, cholestatic liver disease, acute liver injury, and liver fibrosis. Furthermore, the review provides a comprehensive examination of the multi-target mechanisms by which iridoids mediate their hepatoprotective effects. These mechanisms include the regulation of lipid metabolism, attenuation of cholestasis, suppression of inflammation and oxidative stress, amelioration of mitochondrial dysfunction, modulation of autophagy, as well as anti-fibrotic, anti-hepatocarcinogenic, and detoxification activities. Among these, the inhibition of inflammation and oxidative stress is highlighted as a primary mechanism of action. In addition, this review critically evaluates the current limitations associated with the use of GF and its iridoids in liver disease treatment and discusses potential directions for future research. The aim of this review is to provide theoretical foundations and scientific guidance for the further research and development of GF-based therapeutic agents.
1 Introduction
The liver is an essential organ in the human body, playing a critical role in detoxification, metabolism regulation, protein synthesis, bile production, and energy storage (1). Various factors, including viruses, bacteria, chemical substances, and medications, can cause different degrees of liver injury (2). Prolonged exposure to these toxic elements can precipitate the development and progression of various liver diseases, such as viral hepatitis, alcoholic liver diseases (ALD), non-alcoholic fatty liver diseases (NAFLD), and autoimmune liver diseases (AILD) (3). If these initial pathological changes are not addressed promptly and effectively, they may progressively worsen over time, ultimately leading to irreversible severe liver conditions like cirrhosis and hepatocellular carcinoma (HCC). In the past few decades, liver diseases and their related complications have become a major global health issue, causing more than two million deaths each year, which represents about 4% of worldwide mortality (4). Therefore, identifying safe and effective treatments to prevent or reverse liver diseases is essential for addressing this significant public health concern.
Gardenia fruit (GF), the mature fruit of Gardenia jasminoides Ellis, is referred to as “Zhizi” in Chinese, “Cape Jasmine” in Korean, and “Sanshishi” in Japanese, and was initially documented in the Shennong Herbal Classic (5). In traditional Chinese medicine (TCM), GF has been utilized for its properties in clearing heat and purging fire, promoting diuresis to clear heat, and cooling the blood while detoxifying (6). As a clinically important hepatoprotective and cholagogic agent, GF is frequently utilized in various classic formulations, such as Zhi Zi Da Huang Tang (7), Yin Chen Hao Tang (8), Qushi Huayu Decoction (9) and Zhizi Baipi Soup (10) for the treatment of liver diseases. Studies in pharmacology have shown that GF has multiple effects, such as reducing inflammation (11), antidepressant (12), antiviral (13), anti-thrombotic effect (14), and hepatoprotective activities (15). Notably, GF has been officially acknowledged by China's Ministry of Health as part of the initial group of medicinal and edible resource varieties. In China and East Asia, GF is widely used both as a functional food supplement, which are incorporated as food ingredients and dietary supplements (16). Moreover, in both United States and the European Union, GF is commercially turned into concentrated fruit juices, either on its own or mixed with other fruits, for various uses (17). Phytochemical studies have demonstrated that iridoids (IGs) are characteristic constituents of GF and are generally considered the functional components responsible for its pharmacological activities (18). Importantly, IGs exhibit considerable potential in the prevention and treatment of liver diseases.
To date, a considerable body of research has explored the role of iridoids derived from GF in the prevention and treatment of liver diseases. These studies underscore the significant potential of these compounds and provide a scientific foundation for identifying the constituents responsible for their hepatoprotective effects. Nonetheless, the existing literature is fragmented and lacks a systematic review and synthesis. Previous reviews have predominantly concentrated on the phytochemical composition and general pharmacological properties of GF, with insufficient focus on the hepatoprotective mechanisms of its iridoids. Consequently, these reviews are not comprehensive and do not adequately reflect recent advancements in the field. To address this deficiency, we conducted an exhaustive search of databases, including PubMed, ScienceDirect, Elsevier and Google Scholar and CNKI, to collect all pertinent literature published up to 2024 concerning the use of GF and its active iridoid components in liver diseases prevention and treatment. This review is the first to systematically summarize the therapeutic effects and underlying mechanisms of iridoids in liver diseases, thereby establishing a solid scientific foundation for the future development of GF- or iridoid-based hepatoprotective drugs or functional foods.
2 IGs in GF
IGs are active compounds commonly found in the Scrophulariaceae, Pyrolaceae, Oleaceae, Labiatae, Rubiaceae, and Gentianaceae families (19). These compounds typically occur as glycosides with a glucose unit attached at C-1. IGs are structurally divided into two main types: carbocyclic iridoids, which have a cyclopentane ring connected to a dihydropyran unit, and secoiridoids, formed by the breaking of the cyclopentane ring. IGs generally have significant bitter properties. For instance, iridoid compounds, the primary element of gentian root, serve as a crucial ingredient in the creation of bitter medications and also enhance the secretion of gastric juice and bile. Therefore, they are used in traditional medicine to treat liver diseases (20).
Since the initial discovery of geniposide and gardenoside in the 1960s, researchers have identified many other IGs from GF, such as geniposidic acid, genipin, and genipin-1-β-gentiobioside (21, 22). Among these, geniposide stands out as a key IG, with its content ranging from about 3.18%−6.32% in the whole fruit and reaching up to 7.68% in the seeds (23, 24). The geniposide content in GF exhibits considerable variability across different geographical regions. Shang et al. (25) reported that the highest geniposide concentration was found in samples from Hunan province (34.64 ± 0.45 mg/g), followed by those from Jiangxi (33.10 ± 0.36 mg/g), Anhui (30.73 ± 0.41 mg/g), Sichuan (27.96 ± 0.45 mg/g), and Henan (27.88 ± 0.37 mg/g) provinces. Furthermore, Xu et al. (26) documented significant variations in the concentrations of 12 representative components across 40 samples, with geniposide levels ranging from 37.92 to 72.23 mg/g and the total content of seven iridoids varying between 59.93 and 94.31 mg/g. A more recent investigation quantified 13 major chemical constituents in GF. This study revealed that the concentrations of six iridoids—geniposide, genipin, shanzhiside, geniposidic acid, genipin 1-gentiobioside, and deacetylasperulosidic acid methyl ester—achieved maximum values of 104.63 ± 17.68, 0.27 ± 0.01, 3.22 ± 0.09, 0.412 ± 0.02, 19.08 ± 0.48, and 2.98 ± 0.70 mg/g, respectively (27). Notably, geniposide can be enzymatically converted into genipin—a compound utilized as a natural red/blue colorant in the food industry upon reaction with different amino acids—through the action of β-D-glucosidase from gut microbiota. Pharmacological studies have demonstrated that IGs exhibit notable multifaceted biological activities, including hepatoprotective (28), anti-inflammatory (29), neuroprotective (30), antitumor (31), hypoglycemic and hypolipidemic activities (32). The diverse pharmacological effects of IGs are primarily attributed to modifications such as epoxidation and hydroxylation of their fundamental structure, as well as esterification of aromatic acids derived from the shikimic acid pathway (33). The IGs components in GF are compiled and summarized in Table 1, with their chemical structures illustrated in Figure 1.
Table 1
| No | Compound name | Formula | Molecular weight | References |
|---|---|---|---|---|
| 1 | disperoside A | C34H46O20 | 774.26 | (34) |
| 2 | disperoside B | C34H46O20 | 774.26 | (34) |
| 3 | 6′-nicotinoyloxygeniposide | C23H27NO11 | 493.16 | (34) |
| 4 | geniposide | C17H24O10 | 388.14 | (34) |
| 5 | genipin-gentiobioside | C23H34O15 | 550.19 | (34) |
| 6 | 10-O-trans-sinapoylgeniposide | C28H34O14 | 594.19 | (34) |
| 7 | lippianoside B | C27H32O13 | 564.54 | (34) |
| 8 | 6′-O-trans-p-coumaroylgeniposide | C26H30O12 | 534.17 | (34) |
| 9 | 6′-O-trans-sinapoylgeniposide | C28H34O14 | 594.19 | (34) |
| 10 | 6-O-methyldeacetylasperulosidic acid methyl ester | C18H26O11 | 418.15 | (34) |
| 11 | 6-O-methylscandoside methyl ester | C18H26O11 | 418.15 | (34) |
| 12 | 2′-O-decanoylgardoside | C26H40O11 | 528.26 | (34) |
| 13 | 2′-O-trans-p-coumaroylgardoside | C25H28O12 | 520.16 | (34) |
| 14 | 2′-O-(4-methoxycinnamoyl)mussaenosidic acid | C25H30O12 | 522.17 | (34) |
| 15 | euphrasin | C21H16O4 | 212.10 | (34) |
| 16 | campsinol | C21H16O4 | 212.10 | (34) |
| 17 | genipin 1-O-β-D-isomaltoside | C23H34O15 | 550.19 | (35) |
| 18 | genipin 1,10-di-O-β-D-glucopyranoside | C23H34O15 | 550.19 | (35) |
| 19 | scandoside methyl ester | C17H24O11 | 404.13 | (35) |
| 20 | deacetylasperulosidic acid methyl ester | C17H24O11 | 404.13 | (35) |
| 21 | gardenoside | C17H24O11 | 404.13 | (35) |
| 22 | geniposidic acid | C16H22O10 | 374.12 | (36) |
| 23 | gardaloside | C16H22O9 | 358.13 | (37) |
| 24 | ixoroside | C16H24O9 | 360.14 | (37) |
| 25 | shanzhiside | C16H24O11 | 392.13 | (37) |
| 26 | 6″-O-trans-sinapoylgenipin gentiobioside | C34H44O19 | 756.25 | (38) |
| 27 | 6″-O-trans-p-coumaroylgenipin gentiobioside | C32H40O17 | 696.23 | (38) |
| 28 | 6″-O-trans-cinnamoylgenipin gentiobioside | C32H40O16 | 680.23 | (38) |
| 29 | 6′-O-trans-p-coumaroylgeniposidic acid | C25H28O12 | 520.16 | (38) |
| 30 | 10-O-succinoylgeniposide | C21H28O13 | 488.15 | (38) |
| 31 | 6′-O-acetylgeniposide | C19H26O11 | 430.15 | (38) |
| 32 | 10-O-acetylgeniposide | C19H26O11 | 430.15 | (38) |
| 33 | 11-(6-O-trans-sinapoylglucopyranosyl)gardendiol | C27H34O13 | 566.20 | (38) |
| 34 | 10-(6-O-trans-sinapoylglucopyranosyl)gardendiol | C27H34O13 | 566.20 | (38) |
| 35 | 6″-O-trans-feruloylgenipin gentiobioside | C33H42O18 | 726.24 | (39) |
| 36 | 2′-O-trans-caffeoylgardoside | C25H28O13 | 536.15 | (39) |
| 37 | jasmigeniposide B | C28H36O14 | 596.21 | (39) |
| 38 | jasmigeniposide A | C33H40O18 | 724.22 | (39) |
| 39 | genipin 1,10-di-O-α-L-rhamnoside | C23H34O13 | 518.20 | (40) |
| 40 | genipin 1,10-di-O-β-D-xylopyranoside | C21H30O13 | 490.17 | (40) |
| 41 | genipin 1-O-α-L-rhamnoside | C17H24O9 | 372.14 | (40) |
| 42 | genipin 1-O-β-D-xylopyranoside | C16H22O9 | 358.13 | (40) |
| 43 | 6-O-methylscandoside methyl ester | C18H26O11 | 418.15 | (41) |
| 44 | 8-O-methylmonotropein methyl ester | C18H26O11 | 418.15 | (41) |
| 45 | gardoside | C16H22O10 | 374.12 | (41) |
| 46 | gardenamide | C11H13NO4 | 223.08 | (42) |
| 47 | 6α-butoxygeniposid | C21H32O11 | 460.19 | (42) |
| 48 | 6β-butoxygeniposid | C21H32O11 | 460.19 | (42) |
| 49 | 6″-O-p-cis-coumaroylgenipin gentiobioside | C32H40O17 | 696.23 | (42) |
| 50 | gardenate A | C12H18O6 | 258.11 | (43) |
| 51 | 2-hydroxyethyl gardenamide A | C13H17NO5 | 267.11 | (43) |
| 52 | gardenal-I | C11H14O4 | 210.09 | (44) |
| 53 | gardenal-II | C11H16O4 | 212.10 | (44) |
| 54 | gardenal-III | C11H14O4 | 212.10 | (44) |
| 55 | 6-α-methoxy geniposide | C18H26O11 | 418.15 | (44) |
| 56 | lamalbidic acid | C15H22O12 | 394.11 | (44) |
| 57 | genipin | C11H14O5 | 226.08 | (45) |
| 58 | gardoside methyl ester | C17H24O10 | 388.14 | (46) |
| 59 | 6″-O-trans-caffeoylgenipin gentiobioside | C32H40O18 | 712.22 | (47) |
| 60 | genipin 1-O-β-D-apiofuranosyl (1 → 6) β-D-glucopyranoside | C22H32O14 | 520.18 | (47) |
| 61 | genipin 1-O-α-D-xylopyranosyl (1 → 6) β-D-glucoopyranoside | C22H32O14 | 520.18 | (47) |
| 62 | genameside A | C17H26O12 | 422.14 | (48) |
| 63 | genameside B | C17H26O12 | 422.14 | (48) |
| 64 | 7α,8β-epoxy-8α-dihydrogeniposide | C17H24O11 | 404.13 | (49) |
| 65 | 8-epiapodantheroside | C17H24O10 | 388.14 | (49) |
| 66 | 6′-O-trans-sinapoyl gardoside | C27H32O14 | 580.18 | (49) |
| 67 | mussaenosidic acid | C16H24O10 | 376.14 | (50) |
| 68 | deacetylasperulosidic acid | C16H22O11 | 390.12 | (50) |
| 69 | shanzhisidemethyl ester | C17H26O11 | 406.15 | (45) |
| 70 | 2′-O-cis-coumaroylgardoside | C25H28O12 | 520.16 | (51) |
| 71 | 6′-O-caffeoylioxide | C25H26O14 | 550.13 | (51) |
| 72 | 10-O-(4″-O-methylsuccinoyl)geniposide | C22H30O13 | 502.17 | (52) |
| 73 | 7-deoxygardoside | C16H22O9 | 358.13 | (53) |
| 74 | tarenninoside C | C24H26O14 | 538.13 | (53) |
| 75 | 10-O-caffeoyl deacetyl daphylloside | C26H30O14 | 566.16 | (53) |
| 76 | 7-deoxy-8-epiloganic acid | C16H24O9 | 360.14 | (53) |
| 77 | secologanoside | C16H22O11 | 390.12 | (53) |
Chemical information of iridoids from gardenia fruit.
Figure 1
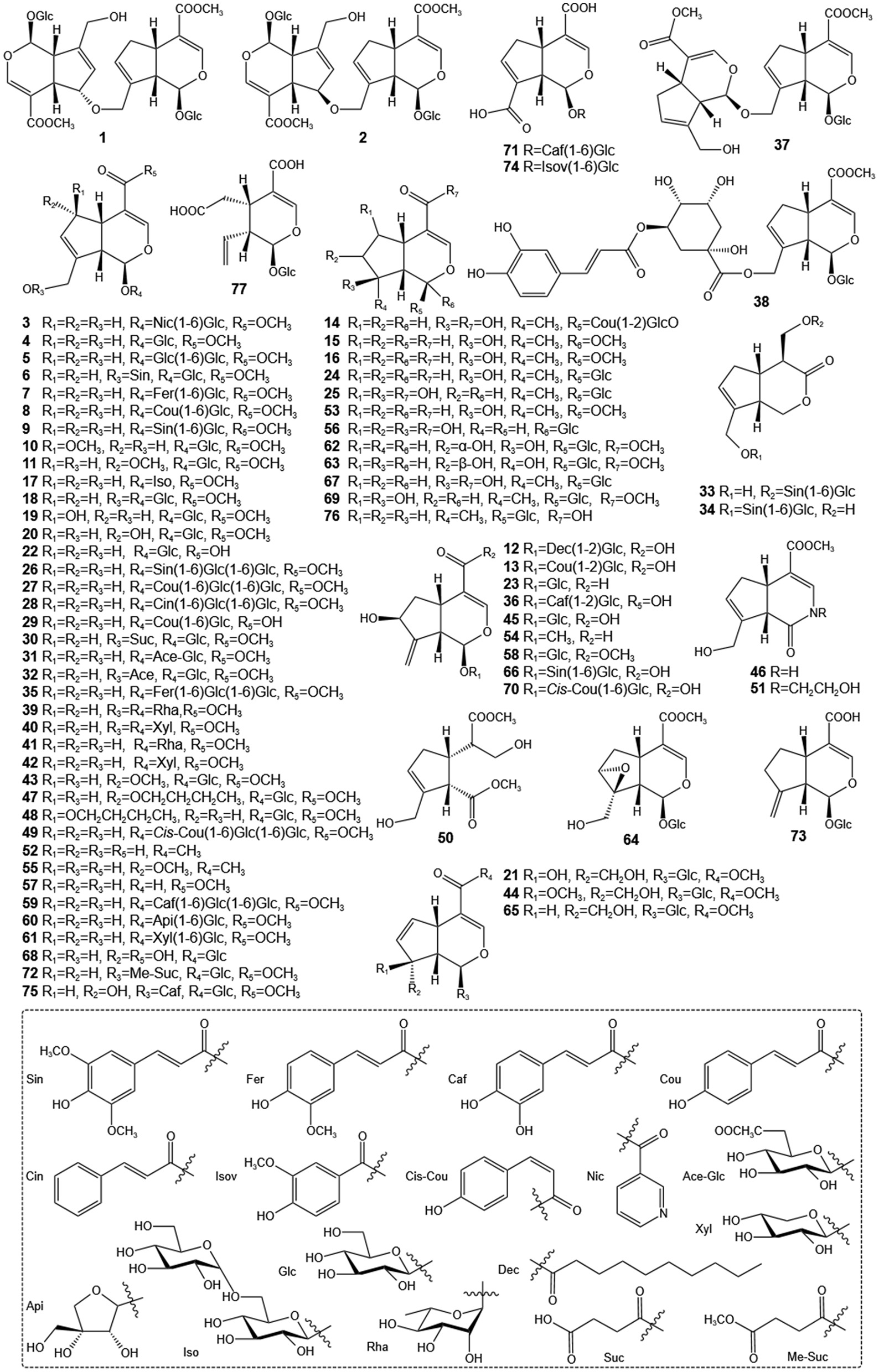
Structures of iridoids of GF.
3 GF is utilized in treating various liver diseases
3.1 Nonalcoholic fatty liver disease (NAFLD)
NAFLD is a prevalent disorder characterized by an abnormal buildup of fat in the liver, progressing from simple fatty liver to non-alcoholic steatohepatitis (NASH), potentially resulting in fibrosis, cirrhosis, and liver cancer (54). It is closely associated with metabolic syndrome, obesity, type 2 diabetes, and dyslipidemia, making it a major global health issue (55). The complex causes of NAFLD include genetic factors, insulin resistance, adipose tissue dysfunction, and oxidative stress (56). Recent research suggested that gut microbiota and its metabolites play a role in NAFLD's development and progression, with dysbiosis potentially causing liver inflammation and damage (57). Moreover, endothelial cell dysfunction is also linked to NAFLD progression and may increase cardiovascular disease risk (58). Although there is no FDA-approved medication for NAFLD, managing the condition primarily relies on diet and weight loss. New treatments are being developed to target specific metabolic pathways to change the course of the disease.
GF, a traditional Chinese medicine clinically utilized for NAFLD treatment, significantly improved metabolic and hepatic parameters in high-fat diet (HFD)-induced models. Current research has shown that relative to the non-treated HFD controls, GF (25, 50, 100 mg/kg) administration significantly lowered concentrations of serum total cholesterol (TC), lipoprotein cholesterol, triglycerides (TG), ALT, AST, LDH, free fatty acids (FFA), glucose, and insulin, concurrently reducing hepatic TG, TC, and malondialdehyde (MDA) levels (59). Furthermore, the aqueous extract of GF (28 mg/kg) modulate key liver injury biomarkers and signaling pathways—including mTOR, 8-hydroxy2′-deoxyguanosine (8-OHdG), TGF-β, ERK1/2 phosphorylation, and oxidative stress markers (60). In addition, the crude extract of crocin from GF (100 and 200 mg/kg) also attenuated hyperglycemia, dyslipidemia, and hepatic oxidative stress, while beneficially restructuring gut microbiota in HFD-fed rats by reducing the Firmicutes/Bacteroidetes ratio and enriching Akkermansia, Bacteroides, and Lactobacillus (61). Notably, GF-containing TCM formulas effectively treat NAFLD in clinical and preclinical settings. Yin Zhi Huang (YZH, 10 and 30 ml/kg daily) reduces diet-induced obesity and liver fat by inhibiting AMPK/SREBP-1-related lipogenesis and boosting AMPK/ACC/CPT1A-driven mitochondrial β-oxidation (62). Likewise, Qushi Huayu Decoction (QHD) reduces liver lipogenesis through XBP1s-dependent pathways, circumventing the regulatory influence of SREBP1 and ChREBP (63).
3.2 Cholestatic liver disease (CLD)
CLD involves conditions affecting the bile ducts, caused by primary or secondary injuries (64). Its multifactorial etiology includes immune, genetic, and environmental factors. CLD progression varies, often involving ductular reaction, hepatic fibrosis, bile acid accumulation, inflammatory infiltration, and potential intestinal barrier impairment (65). Bile acids are crucial for cholesterol elimination, and disruptions in their synthesis and transport can lead to CLD by causing toxic substance retention (66). It has been reported that the aqueous extract of GF (21 and 42 mg/kg) alleviated alpha-naphthylisothiocyanate (ANIT)-induced hepatotoxicity and cholestasis in rats. This protective effect was associated with the upregulation of Cyp8b1 expression, which inhibited BA synthesis in the liver, promoted BA excretion via the intestinal-fecal route, and enhanced the enterohepatic circulation of Bas (67). At the same time, the GF aqueous extract (900 mg/kg) restored disturbances in primary BA biosynthesis, glycerophospholipid metabolism, tryptophan metabolism, and arachidonic acid metabolism caused by ANIT administration (68). In addition, Yinchenhao Decoction (YCHD), a famous traditional Chinese formula containing GF (6, 9 and 12 g/kg), has been shown to modulate the expression of metabolic enzymes and transporters under cholestatic conditions (69). Notably, herbal formulations follow specific compatibility principles, as interactions between herbs can lead to either synergistic or antagonistic effects. Indeed, studies have demonstrated that the combination of rhubarb and gardenia exerts synergistic effects in ANIT-induced cholestatic rats at both pharmacodynamic and pharmacokinetic levels (70).
3.3 Acute liver injury (ALI)
ALI is a clinical syndrome characterized by significant hepatic damage, involving extensive infiltration of inflammatory cells, structural destruction, and functional abnormalities of the liver. This condition can be precipitated by various etiological factors, including excessive alcohol consumption, viral infections, medication overdoses, and acute exposure to toxins, potentially progressing to liver failure in severe cases (71). Previous research has indicated that treatment with 50% ethanol extract of GF (880 mg/kg) may mitigate acetaminophen (APAP)-induced hepatotoxicity, likely due to its anti-inflammatory and antioxidant properties (72). Additionally, Liu et al. (73) reported that YCHT (8 g/kg) provides liver protection by influencing metabolic pathways and changes in gut microbiota during liver damage caused by CCl4. Moreover, GF aqueous extracts (112.5, 225 and 450 mg/ml) have demonstrated efficacy in reducing Bacillus Calmette-Guérin (BCG)- and lipopolysaccharide (LPS)-induced immunological liver injury in murine models (74). Furthermore, Zhizi Baipi Soup (ZBS) and its simplified formulations containing Zhizi exhibit significant protective effects against concanavalin A (Con A)-induced immunological liver injury in mice (75).
3.4 Liver fibrosis (LF)
LF is a significant pathological state marked by the overaccumulation of extracellular matrix (ECM) proteins, leading to the disruption of normal liver architecture and function. LF, which can arise from various types of liver damage, may advance to cirrhosis, liver failure, and HCC, creating a major global health challenge (76). Central to this fibrogenic process is the activation of hepatic stellate cells (HSCs), which undergo transdifferentiation into myofibroblasts and become the primary source of excessive ECM deposition (77). Previously, Shin et al. (78). reported that the aqueous extracts of GF (200 mg/kg) mitigate thioacetamide-induced LF in mice through the AMPK/SIRT1/NF-κB and Nrf2 signaling pathway. Similarly, GF root aqueous extracts (5, 10 and 20 mg/kg) also conferred protection against CCl4-induced LF in rats, potentially through mechanisms involving enhanced ECM degradation and inhibition of lipid peroxidation (79). In addition, YCHD (3.15 g/kg) alleviated dimethylnitrosamine (DMN)-induced LF by modulating enzymes involved in bile acid metabolism and inhibiting chenodeoxycholic acid (CDCA)-induced HSC proliferation and activation via the TGF-β1/Smad/ERK signaling pathway (80). Furthermore, synergistic approaches show promise: Co-treatment with GF and Silymarin effectively ameliorated oxidative stress-driven LF in a thioacetamide mice model, attributed to the regulation of hepatic sirtuin1 activity (81). Additionally, Zhizi Bopi Decoction and its individual components have also been reported to exhibit significant, albeit varying degrees of, anti-fibrotic effects on the liver (82).
4 The role and mechanism of iridoids from GF in the treatment of liver diseases
4.1 Effects of iridoids on hepatic lipid metabolism
4.1.1 Nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway
The Nrf2 signaling pathway is crucial for defending cells against oxidative stress and inflammation. As a transcription factor, Nrf2 moves to the nucleus upon activation and binds with the antioxidant response element in gene promoters, triggering the production of antioxidant and protective enzymes (83, 84). Normally, Kelch-like ECH-associated protein 1 (Keap1) regulates this pathway by holding Nrf2 in the cytoplasm and promoting its degradation. However, during oxidative stress, Nrf2 detaches from Keap1, allowing the activation of genes that detoxify and eliminate harmful oxidants and electrophiles (85). As early as 2019, Shen et al. (86) found that geniposide (50, 75 and 100 mg/kg) exerts protective effects against lipid accumulation by enhancing antioxidant and anti-inflammatory capacities, which is at least partly attributed to its inhibition of the Nrf2/HO-1 pathway.
4.1.2 Adenosine monophosphate-activated protein kinase (AMPK) signaling pathway
AMPK serves as a pivotal cellular energy sensor essential for maintaining energy homeostasis. Upon activation by an elevated AMP/ATP ratio during energy stress, AMPK coordinates metabolic changes by enhancing catabolic pathways that generate ATP and inhibiting anabolic processes that consume ATP (87, 88). Importantly, AMPK regulates lipid, glucose, and protein metabolism, autophagy, and mitochondrial function, underscoring its critical role in addressing metabolic disorders such as NAFLD (89). It is noteworthy that in addition to the Nrf2/HO-1 pathway, geniposide also suppresses the AMPK pathway to inhibit lipid accumulation (86). Additionally, a combination of geniposide, Peanut Skin Extract (PSE), and Isoquercitrin has been shown to significantly reduce body and liver weight, ameliorate hepatic steatosis, and improve liver function markers in mice. These effects are primarily mediated through regulation of the AMPK/ACC/CPT1 and AMPK/ULK1/LC3B signaling pathways (90).
4.1.3 MicroRNAs (miRNAs)
miRNAs are small noncoding RNAs, usually around 22 nucleotides long, that are essential for regulating gene expression after transcription. These molecules are highly conserved across species and are involved in a broad range of biological activities, such as cell growth, differentiation, development, and programmed cell death (91). The abnormal regulation of miRNAs has been linked to the development of several diseases, including cancer, cardiovascular diseases, and neurodegenerative disorders (92). It has been reported that genipin (5 and 20 mg/kg) reduces HFD-induced hyperlipidemia and liver steatosis in mice by modulating the miR-142a-5p/SREBP-1c pathway (93). At the same time, the researchers further found that genipin (20 mg/kg) demonstrated wide-ranging benefits in ameliorating metabolic disorders and sperm dysfunction caused by a high-fat diet in male mice by regulating miR-132 in a tissue-specific manner (94). These findings highlight the significant roles of iridoids and miRNAs in preventing and treating hepatic lipid metabolism issues.
4.1.4 Intestinal microbiota
The intestinal microbiota is integral to the maintenance of gastrointestinal homeostasis and overall health. This intricate ecosystem, comprising bacteria, viruses, fungi, and protozoa, inhabits the human gut and participates in different bodily functions such as digestion, metabolism, and immune response. The composition and diversity of the intestinal microbiota are modulated by factors such as diet, genetics, and environmental exposures, with perturbations in this microbial community having significant implications (95). The gut-liver axis represents a pivotal pathway through which the gut microbiota exerts influence on hepatic lipid metabolism, and disruptions within this axis are associated with metabolic disorders such as NAFLD and obesity (96, 97). Notably, geniposide-based combination therapies demonstrate efficacy in regulating hepatic lipid metabolism through intestinal microbiota modulation. This is evidenced by Peng et al. (98) who reported that a geniposide and chlorogenic acid combination ameliorates NASH in HFD mice, partly via protection of gut barrier function. Recent study further confirmed that this combination reduces blood lipids and hepatic lipid accumulation, lowers serum ALT/AST levels and liver weight index, ameliorates intestinal dysbiosis, and modulates intestinal and serum bile acid metabolism in murine NASH models (99).
4.1.5 Pyroptosis
Pyroptosis, an inflammatory form of programmed cell death mediated by gasdermin proteins, functions as a vital defense mechanism against microbial infections by activating inflammasomes—multiprotein complexes responsible for detecting pathogens and cellular stress. This process initiates the activation of inflammatory caspases, leading to the elimination of infected cells and the recruitment of immune effectors to enhance immune responses (100, 101). Nevertheless, dysregulated pyroptosis can contribute to inflammatory pathology and tissue damage, highlighting its dual roles in health and disease (102). Relevant results showed that, in both in vitro (10, 25 and 50 μM) and in vivo (40 mg/kg) models of NAFLD, gardenoside attenuates lipid accumulation, enhances cell viability, reduces ROS, and suppresses pyroptosis via downregulation of pyroptosis markers (NLRP3, ASC, caspase-1 p20, GSDMD-N, IL-1β) and reduced CTCF/DPP4 expression (103). While genipin's role in hepatic lipid metabolism regulation is established, its relationship with pyroptosis remains undefined. Study demonstrated that genipin attenuates HFD-induced liver damage and inhibits UCP2-mediated pyroptosis in both HFD-fed mice (5 and 20 mg/kg) and free fatty acid (FFA)-treated hepatocytes (20 μM) models exhibiting marked pyroptotic activation (104).
4.1.6 Apoptosis
Apoptosis is a crucial programmed cell death process that maintains homeostasis in multicellular organisms by removing unfit or damaged cells without causing inflammation. It is vital from development through adulthood, involving complex molecular pathways governed by Bcl-2 proteins, caspases, and their inhibitors (105). Recent studies suggested that geniposide (900 μg/ml) influences global DNA methylation levels and modulates the expression of P53, Bcl-2, and Akt, thereby collectively inhibiting apoptosis in human hepatocytes (106).
4.2 Effects of iridoids on cholestasis
4.2.1 Bile acids (BAs) homeostasis
BAs are crucial for lipid digestion, absorption, and act as signaling molecules in metabolic regulation. Their homeostasis involves liver synthesis, gut microbiota modification, and intestinal reabsorption, essential for metabolic and immune balance (107). Disruptions can cause diseases like liver disorders and metabolic syndrome. BA regulation involves pathways and receptors like farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5), which influence glucose, lipid, and energy metabolism (108). FXR is particularly important for BA synthesis and transport, with its deficiency potentially leading to metabolic disorders (109). Notably, among the iridoids of GF, geniposide is the primary compound that affects cholestasis by modulating BA metabolism. In one recent study, geniposide (50 mg/kg) regulated cholesterol metabolism by modulating the liver-gut crosstalk of bile acids mediated by FXR in C57BL/6 and ApoE-/- mice. This effect promoted hepatic BA synthesis and ileal BA excretion by regulating the enterohepatic circulation of BAs (110). In addition, in cases of cholestatic liver injury, geniposide (50 and 100 mg/kg) consistently regulates BA transporters in an FXR-dependent manner. It alleviates sclerosing cholangitis by upregulating canalicular efflux transporters (BSEP, MRP2, MDR1, MDR2) to enhance biliary BA secretion, while suppressing hepatic BA synthesis through the downregulation of CYP7A1 (111). For ANIT-induced cholestasis, geniposide (25, 50 and 100 mg/kg) restores BA homeostasis by dual modulation of uptake and efflux: it represses basolateral OATP2-mediated uptake while inducing canalicular BSEP and basolateral OSTβ-dependent excretion, shifting BA elimination to urine. These actions occur alongside activation of hepatoprotective nuclear receptors (FXR, PXR, SHP), without altering detoxification enzymes (CYP3A2, UGT1A1, SULT2A1) (112, 113).
4.2.2 Bile salt export pump (BSEP)
BSEP, a canalicular ATP-binding cassette (ABC) transporter, plays a crucial role in hepatobiliary bile acid secretion (114). By actively exporting bile salts from hepatocytes into bile canaliculi, BSEP sustains the enterohepatic circulation and prevents the accumulation of cytotoxic bile acids within the liver (115). Dysfunction of BSEP is a pivotal factor in the pathogenesis of cholestatic liver disease (116). Early research by Wu et al. (117) demonstrated that geniposide (150 mg/kg) significantly upregulates both mRNA and protein expression of the BSEP in cholestatic rats. This effect is mediated primarily through the activation of the FXR pathway. Specifically, geniposide promotes FXR binding to the BSEP promoter and facilitates the recruitment of the transcriptional co-activators PGC-1α and CARM1. This coordinated action markedly enhances BSEP transcription. In another investigation conducted by Chen et al. (118), geniposidic acid (25, 50 and 150 mg/kg) demonstrates dose-dependent hepatoprotective effects against ANIT-induced cholestasis and liver injury. Geniposidic acid pretreatment effectively restores bile flow, normalizes bile acid and bilirubin levels, reduces serum markers of liver damage (GOT, GPT, γ-GT, TB, DB, TBA), and mitigates histopathological damage. Importantly, geniposidic acid counteracts the ANIT-induced downregulation of FXR, BSEP, and Mrp2, significantly upregulating their mRNA expression.
4.2.3 STAT3 and NF-κB signaling
NF-κB controls the transcription of genes linked to immune response, inflammation, cell fate, and activity (119). When NF-κB is activated, it leads to an increase in inflammatory factors like TNF-α, IL-1β, and IL-6, and TNF-α can subsequently activate NF-κB signaling (120). STAT3 is a cytoplasmic transcription factor that is part of the STAT family, which stands for signal transducers and activators of transcription. STAT3 activation can occur through various cytokines and growth factors, such as the typical IL-6. Consequently, NF-κB and STAT3 signaling are closely linked and play a significant role in hepatotoxicity (121). In an early study, Chen et al. (122) found that geniposide (50 mg/kg) significantly alleviated ANIT-induced cholestasis and liver injury by normalizing the expression of genes involved in bile acid metabolism and transport. Furthermore, geniposide reduced the levels of TNF-α and suppressor of cytokine signaling 3 (SOCS3), while also inhibiting the activation and expression of STAT3 and NF-κB.
4.3 Effects of iridoids on liver fibrosis (LF)
4.3.1 Oxidative stress and flammatory
Oxidative stress and inflammation are critical factors driving the progression of hepatic fibrosis. Chronic inflammation exacerbates fibrosis severity by activating HSCs, which subsequently secrete pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 to promote fibrogenesis (123). Concurrently, oxidative stress contributes to fibrotic pathogenesis through HSC activation and collagen deposition (123, 124). Emerging research highlights the potential of iridoids in mitigating these processes. Specifically, Lamiophlomis Herba demonstrates protective effects against LF, inflammation, and oxidative stress. These actions are partially mediated by its bioactive constituent shanzhiside methyl ester (SME), which significantly downregulates key fibrotic markers including fibronectin, collagen isoforms (Col1a1, Col3a1, Col-IV), α-SMA, laminin (LN), and procollagen type II (PC-II) (125). At the same time, research conducted by Ge et al. (126) demonstrated that SME upregulates antioxidant genes (Nqo1, Ho1) while simultaneously downregulating inflammatory genes (Il-6, Il-18), resulting in a subsequent decrease in the expression of genes and proteins related to the extracellular matrix, such as Col1a1, Col3a1, LN, α-Sma, PC-III, and Col-IV. In addition, geniposide (50 mg/kg) has been found to ameliorates CCl4-induced LF in mice by suppressing oxidative stress and inflammation. This is evidenced by enhanced activities of antioxidant enzymes (SOD, GSH-Px), reduced MDA levels, and reduced production of pro-inflammatory cytokines (IL-6, IL-1β, TNF-α) in liver tissue (127). Further study suggested that geniposide (25 and 50 mg/kg) restored BA profiles and suppress NLRP3 inflammasome activation, thereby alleviating LF in BDL mice, highlighting its potential for treating cholestatic liver fibrosis (128).
4.3.2 Sonic hedgehog (Shh) signaling pathway
The Shh signaling pathway plays a vital role in embryonic development, tissue structuring, and the upkeep of stem cells. It regulates cell growth, specialization, and programmed cell death across different tissues (129). Notably, recent evidence suggests that Shh signaling plays a role in the activation of HSCs, indicating its potential as a novel therapeutic target for LF (130). In this regard, geniposide has demonstrated the ability to inhibit activated HSC-T6 cells, decreasing their viability with IC50 values of 77.11 and 42.88 μM at 24 and 48 h, respectively, and causing cell cycle arrest at the G2/M phase, and prominently suppressing the Shh signaling pathway (131).
4.3.3 Transforming growth factor-β (TGF-β) signaling pathway
HSCs, which are nonparenchymal perisinusoidal cells located in the liver, have various functions and are crucial in the development of LF (77). TGF-β1 acts as a master regulator of fibrogenesis, promoting HSC activation. This activation occurs primarily through TGF-β1-induced phosphorylation of Smad2/3, leading to the formation and nuclear translocation of Smad complexes. Within the nucleus, these complexes upregulate key profibrotic genes, including those encoding α-SMA and COL1A1 (132). Significantly, geniposide exhibits strong antifibrotic properties by inhibiting LX-2 cell proliferation and reducing the expression of COL1A1, fibronectin, α-SMA, and TGF-β1/Smad signaling proteins (20 μM). These effects are confirmed in vivo (40 mg/kg), where geniposide significantly reduces CCl4-induced LF, HSC activation, and TGF-β1/Smad protein expression in mice (133).
4.4 Effects of iridoids on inflammation
4.4.1 NOD-like receptor protein 3 (NLRP3) inflammasome
The NLRP3 inflammasome, the most extensively characterized inflammasome, is a cytoplasmic multiprotein complex composed of apoptosis-associated speck-like protein (ASC) and the effector pro-caspase-1 (134). The activation of NLRP3 is triggered by different stimuli, such as pathogens and non-infectious damage, primarily through the production of ROS from mitochondria or the activation and deubiquitination of cathepsin B (135). When stimulated, NLRP3 connects with ASC to form the inflammasome complex, resulting in the activation of caspase-1, which then proteolytically activates IL-1β and IL-18 (136). Notably, NLRP3 inflammasome activation is implicated in cholestatic liver injury, as observed in patients and murine models (137, 138). Critically, iridoids from GF targeting NLRP3 exhibit therapeutic potential against liver pathologies. Geniposidic acid (25, 50 and 100 mg/kg) acts as a covalent NLRP3 inhibitor that directly binds NLRP3, suppressing bile acid-induced inflammation in hepatocytes/macrophages and attenuating cholestatic injury in ANIT-induced models (139). In addition, genipin (25, 50 and 100 mg/kg) inhibits necroptosis-mediated NLRP3 activation by reducing RIP1/RIP3 necrosome formation, thereby suppressing caspase-1 cleavage and IL-1β/IL-18 release in GalN/LPS-induced hepatotoxicity (140). Furthermore, gardenoside combined with 6,7-Dimethoxycoumarin and Rhein directly attenuates hepatic NLRP3 inflammasome activation, ameliorating pathological features of NAFLD (141). These findings highlight the therapeutic potential of structurally related iridoids in modulating NLRP3-driven liver pathologies.
4.4.2 Nuclear factor-κB (NF-κB) signaling pathway
The NF-κB signaling pathway is essential for controlling immune and inflammatory responses and is crucial in various physiological and pathological conditions, such as cancer, metabolic disorders, and neurodegenerative diseases (142, 143). NF-κB comprises a family of transcription factors that, upon activation, translocate to the nucleus to regulate the expression of genes involved in inflammation, immune response, cell proliferation, and survival (144). According to Liang et al. (145), gardenoside (10 and 20 μM) helps reduce cellular steatosis in HepG2 cells that have been induced with free fatty acids (FFA), representing a model of hepatic steatosis. This effect is associated with reduced supernatant levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) and the inhibition of NF-κB activation. Another investigation revealed that genipin (100 μM) can reduce inflammation in liver cells by inhibiting NF-κB activation, which in turn reduces the levels of important inflammatory mediators such as inducible nitric oxide synthase (iNOS) and TNF-α (146).
4.4.3 Phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway
The PI3K/Akt/mTOR signaling pathway is a critical regulator of diverse cellular processes, including inflammation. Upon activation, Akt rapidly transmits signals to mTOR; the subsequent activation of mTOR exerts anti-inflammatory and anti-apoptotic effects (147, 148). Recent research has established a strong association between dysregulation of this pathway and the pathogenesis of liver diseases (149, 150). Supporting its therapeutic relevance, studies as early as 2017 demonstrated that geniposide (5, 10 and 20 mg/kg) protects rats against hepatic ischemia/reperfusion (I/R) injury. This protective effect is mediated, at least in part, through the suppression of inflammation and apoptosis via activation of the PI3K/Akt/mTOR signaling pathway, highlighting its potential as a modulator of this cascade (147).
4.4.4 Methyl-CpG binding protein 2 (MeCP2) signaling pathway
MeCP2 orchestrates diverse aspects of gene expression regulation, encompassing transcriptional activation and repression, RNA splicing, chromatin remodeling, and modulation of chromatin architecture (151). Dysregulation of MeCP2 has been implicated in liver disease pathogenesis. Emerging evidence indicates that MeCP2 plays a central role in the activation of HSCS (152). Notably, studies using mice model of CCl4-induced acute liver injury (20 mg/kg) and LPS-treated THP-1 cells (80 μM) suggest that geniposide may function as an effective modulator of the MeCP2-Hedgehog signaling axis during inflammatory pathogenesis (153).
4.4.5 Mitogen-activated protein kinase (MAPK) signaling pathway
MAPK signaling pathway represents a critical intracellular cascade that regulates fundamental cellular processes—including proliferation, differentiation, and stress responses (154). This pathway comprises three principal subfamilies: extracellular signal-regulated kinases 1/2 (ERK1/2), c-Jun N-terminal kinases (JNK), and p38 MAPK. Upon extracellular stimulation, phosphorylation of ERK, JNK, and p38 activates these kinases to mediate inflammatory cascades (155). Within this mechanistic framework, geniposide (20 and 40 mg/kg) demonstrates hepatoprotective and regenerative effects in rat liver models, primarily through suppression of MAPK signaling. Crucially, geniposide specifically inhibits p38 MAPK phosphorylation (p-p38), thereby attenuating inflammatory pathway activation and subsequent hepatocellular apoptosis (156).
4.5 Effects of iridoids on oxidative stress
Oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) production and endogenous antioxidant defenses, represents a central pathophysiological mechanism in the pathogenesis and progression of diverse liver diseases (157). Under pathological conditions, excessive hepatic ROS generation overwhelms both enzymatic and non-enzymatic antioxidant systems. Consequently, oxidative stress constitutes a key underlying driver in chronic liver disease (CLD) of various etiologies and is critically implicated in hepatocarcinogenesis (158). Currently, numerous studies have demonstrated that geniposide offers protective effects against various chemically induced liver injuries, such as those caused by acetaminophen (APAP, 10, 30 and 100 mg/kg) (159), tripterygium glycosides (TG, 20, 40 and 80 mg/kg) (159), and ethanol (20, 40 and 80 mg/kg) (160, 161), mainly by modulating oxidative stress pathways. Its mechanisms include: (a) restoration of redox homeostasis: elevating hepatic glutathione (GSH) levels and enhancing the activities of key antioxidant enzymes, including glutathione peroxidase (GPx), glutathione S-transferase (GST), superoxide dismutase (SOD), and catalase (CAT), across multiple injury models (159–161). (b) Suppression of pro-oxidant drivers: downregulating cytochrome P450 2E1 (CYP2E1) expression (thereby reducing APAP bioactivation) and attenuating lipid peroxidation [as evidenced by reduced malondialdehyde (MDA) and lipid peroxidation (LPO) products] (159–161). (c) Transcriptional regulation: upregulating the expression of antioxidant genes, such as CuZn-SOD and catalase (CAT), particularly in models of alcohol-induced liver injury (161). (d) Systemic metabolic rebalancing: ameliorating ethanol-induced disturbances in systemic metabolism, notably within amino acid metabolism pathways and oxidative stress biomarkers (162).
4.6 Effects of iridoids on autophagy
Autophagy is a critical catabolic process essential for maintaining hepatic homeostasis. It achieves this by degrading misfolded proteins, damaged organelles, and lipid droplets (163). This pathway is primarily regulated by autophagy-related genes (ATG), alongside key signaling pathways including mTOR, AMPK, and PI3K/AKT (164). Furthermore, the microtubule-associated protein 1A/1B light chain 3 (LC3) plays a pivotal role in autophagosome formation and governs the expression of lysosomal membrane proteins (LAMP-1, LAMP-2, RAB7), as well as facilitating autophagosome-lysosome fusion. Impairments in autophagic function are strongly associated with various forms of liver injury, while enhancing autophagy activation offers a potential strategy to mitigate disease progression (165). In this context, genipin (1, 2.5 and 5 mg/kg) effectively protects against sepsis-induced liver injury in CLP models by restoring impaired hepatic autophagic flux. It reduces liver damage, lowers serum aminotransferases and pro-inflammatory cytokines, and improves survival. Mechanistically, genipin upregulates the Atg12-Atg5 conjugate, restores Atg3 expression, normalizes LC3-II and p62 levels, and enhances lysosomal function by recovering LAMP-2 and Rab7 expression (166).
4.7 Effects of iridoids on mitochondrial
Mitochondria, which are crucial for ATP production and maintaining cellular balance, experience major dysfunction during reperfusion injury, leading to energy failure, excessive ROS production, calcium overload, and eventually cell death (167). Mitochondrial quality control (QC) maintains peak performance by coordinating biogenesis, fission and fusion dynamics, and mitophagy (168). Critically, the interplay between mitochondrial QC and oxidative stress constitutes a key pathogenic determinant in ischemic diseases (169). As early as 2017, the research of Shin et al. (170) indicated that genipin (100 mg/kg) ameliorated hepatic ischemia/reperfusion (IR) injury by restoring mitochondrial QC. Specifically, genipin reversed IR-induced mitochondrial dysfunction and oxidative stress through coordinated regulation of key QC pathways: it suppressed pathological fission (via Drp1, PINK1) while concurrently restoring compromised mitochondrial biogenesis (PGC-1α, NRF1, TFAM), mitophagy (Parkin), fusion (Mfn2), and energy-sensing pathways (SIRT1, p-AMPK).
4.8 Effects of iridoids on HCC
HCC is a predominant contributor to global cancer-related mortality, representing a substantial burden on healthcare systems (171). Surgical resection remains the primary treatment modality for patients with early to intermediate-stage HCC. Nonetheless, postoperative recurrence is observed in up to 70% of patients within 5 years, significantly undermining the long-term prognosis following hepatectomy (172, 173). Recent research has highlighted the therapeutic potential of iridoids derived from GF in the management of HCC. For instance, geniposide (30 mg/kg in vivo or 200 μg/ml in vitro) has been demonstrated to directly target the TLR4/MyD88 signaling pathway, thereby inhibiting VEGF expression and angiogenesis independently of HIF-1α (174). Additionally, genipin (25 and 50 mg/kg) exhibits anti-tumor activity through multiple mechanisms, including the activation of PPARγ, which impedes CCR2-mediated macrophage infiltration into the postoperative liver and reduces recurrence, as well as the inhibition of IRE1α in tumor-associated macrophages (175). Moreover, the combination of geniposide and paeoniflorin has been shown to enhance the anti-liver cancer effects of sorafenib by attenuating the activation of the NF-κB/HIF-2α/SerpinB3 pathway (176).
4.9 Others
Numerous studies confirm the therapeutic benefits of GF and its components, but high doses can cause liver toxicity (177–179). Geniposide, a major GF component, shows dose-dependent liver toxicity in rats, making it a suspected primary toxicant (180–183). However, new evidence points to genipin as the main toxicant. Luo et al. (179) found that pyrimidine, purine, amino acid metabolism, and pantothenate and CoA biosynthesis were disrupted in HepG2 cells treated with genipin, which suggests the potential hepatotoxicity of genipin. Furthermore, Wang et al. (184) found genipin's LD50 in mice to be 510 mg/kg, showing dose-dependent liver toxicity at 125, 250, and 500 mg/kg. Proteomic analysis suggested this toxicity involves impaired UDP-glucuronosyltransferase (UGT) and cytochrome P450 (CYP450) enzyme function. Supporting this, Huang et al. (185) implicated genipin's covalent binding to cellular proteins, particularly its inhibition of drug-metabolizing enzymes, in GF-induced hepatotoxicity. They confirmed that direct covalent binding and metabolic activation mediate genipin -induced CYP450 inactivation. Notably, Gao et al. (186) linked geniposide hepatotoxicity to its metabolite genipin, attributed to genipin's reactive hemiacetal structure. Exposure of the C-1 hydroxyl group facilitates covalent binding, specifically phase II conjugation with lysine residues, forming genipin-lysine (GP-LYS) adducts. These adducts contribute to cellular oxidative stress and subsequent hepatotoxic cascades.
5 Conclusions and future prospects
GF serves not only as a TCM but also as an innovative food resource with extensive application potential. With the ongoing advancements in modern medicine, a growing body of research has highlighted the indispensable role of GF in the treatment of liver diseases. Among its constituents, iridoids are identified as the primary active compounds responsible for its hepatoprotective properties, thereby reinforcing the medicinal value of GF. Therefore, this review systematically summarized 77 types of iridoids that have been isolated and identified from GF to establish the material basis of their hepatoprotective activity (Figure 1, Table 1). Then, preclinical animal experiments have confirmed that GF exerts significant therapeutic effects on various liver pathological models, such as NAFLD, CLD, ALI, and LF (Figure 2, Table 2). Finally, through data analysis, it was revealed that iridoids in GF exert hepatoprotective effects via multiple pathways, including regulation of lipid metabolism, cholestasis, fibrosis, inflammatory response, oxidative stress, autophagy balance, mitochondrial function, and hepatocarcinogenesis (Figures 3, 4, Table 3).
Figure 2
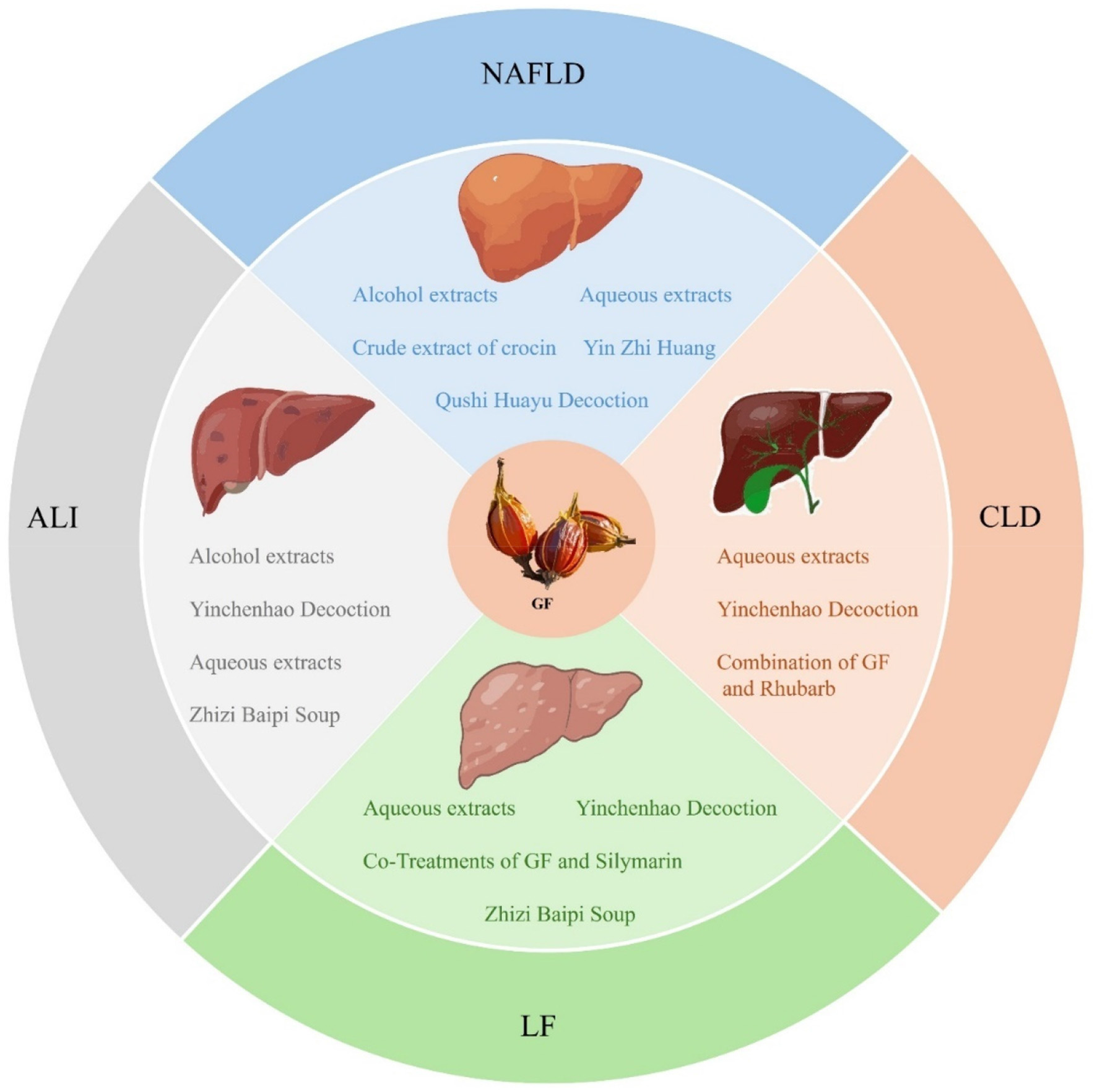
The therapeutic effects of GF extracts against NAFLD, CLD, ALI, and LF.
Table 2
| Liver diseases | Models | Extract/medicines containing GF | Dosage | Effects | References |
|---|---|---|---|---|---|
| NAFLD | HFD-induced SD rat model | Alcohol extracts | 25, 50, 100 mg/kg, 6 weeks | TC↓, TG↓, FFA↓, ALT↓, AST↓, LDH↓, MDA↓, SREBP-1c↓, FAS↓, PPARα↑, PPARγ↓, AMPK↑, CPT-1↑ | (59) |
| NAFLD | HFD-induced C57BL/six mice model | Aqueous extracts | 28 mg/kg/100 μl/day, 8 weeks | mTOR↓, 8-OHdG↓, TGF-β↓, p-ERK↓ | (60) |
| NAFLD | HFD-induced SD rat model | Crude extract of crocin | 100, 200 mg/kg, 12 weeeks | TLR4↓, Myd88↓, NF-κB↓ | (61) |
| NAFLD | HFD-induced C57BL/6J mice model | Yin Zhi Huang | 10, 30 ml/kg, 16 weeks | TG↓, TC↓, ALT↓, AST↓, p-AMPK/AMPK↑, SREBP-1↓, FAS↓, ACC↑, CPT1A↑, PPAR-α↑ | (62) |
| NAFLD | HFD-induced C57BL/six mice model | Qushi Huayu decoction | 0.465, 0.93, 1.86 g of crude drug/ml, 10 ml/kg body weight, daily, 2 weeks | XBP1s↓, p-IRE1α↓, ACC1↓, ACC2↓, FAS↓, SCD1↓, GPAT1↓, AGPAT↓, PAP↓, DGAT2↓ | (63) |
| CLD | ANIT-induced SD rat model | Aqueous extracts | 21, 42 mg/kg, 5 days | Cyp8b1↑, Fxr↑, Ntcp↑, Oatp1↑, Hepatic TaurineBA↓, Fecal PBA Excretion↑ | (67) |
| CLD | ANIT -induced SD rat model | Aqueous extracts | 4 g/kg, 7 days | Hepatic TaurineBA↓, Fecal PBA Excretion↑, MDA↓, TBA↓ TBIL↓, SOD↑ | (68) |
| CLD | ANIT-induced SD rat model | Yinchenhao decoction | 6, 9, 12 g/kg | TBA↓, UGT1A1↓, MRP2↓, BSEP↓, OCT1↓, NTCP↓, MDR1, OATP1A2/4↑, MATE1↓ | (69) |
| CLD | ANIT-induced SD rat model | Combination of GF and Rhubarb | 2 g/kg 7 days | TBIL↓, DBIL↓, ALT↓, AST↓ | (70) |
| ALI | APAP-induced ICR mice model | Alcohol extracts | 0.44, 0.88 g/kg | ALT↓, AST↓, TNF-α↓, IL-6↓, IL-1β↓, GSH↓ MDA↓, CYP2E1↓ | (72) |
| ALI | CCl4-induced SD rat model | Yinchenhao decoction | 8 g/kg, 10 days | Firmicutes↓, bacteroidetes↓, ALT/AST↓ | (73) |
| ALI | BCG and LPS-induced ICR mice. | Aqueous extracts | 112.5, 225, 450 mg/ml, 10 days | ALT↓,AST↓, IL-2 ↑, IL-10↓, IL-4↓, TNF-γ↓ | (74) |
| ALI | Con A-induced ICR mice model | Zhizi Baipi soup | 20 mg/kg, 7 days | ALT↓, AST↓, SOD↑, MDA↓, IFN-γ↓, TNF-α↓, NF-κB-p65↓, NF-κB-p-]p65↓, IL-4↑, IL-6↑ | (75) |
| LF | TAA-induced C57BL/six mice model | Aqueous extracts | 200 mg/kg, 8 weeks | IL-1β↓, TNF-α↓, NF-κB p38↓, α-SMA↓, SIRT1↑, p-AMPKα↑, p-LKB1↑, NOX2 ↓, Nrf2/HO-1↑ | (78) |
| LF | CCl4-induced SD rat model | Aqueous extracts | 5, 10 and 20 g/kg, 4 weeks | AST/ALT↓, SOD↑, MDA↓, GSH↑, GSH-Px↑, α-SMA↓, ColIV↓ | (79) |
| LF | DMN-induced SD rat model | Yinchenhao decoction | 3.15 g/kg, 4 weeks | ALT↓, AST↓, ALP↓, TBA↓, TBIL↓Hepatic TaurineBA↓, Fecal PBA Excretion↑, BSEP↓, α-SMA↓, TGF-β1↓, p-Smad3↓, p-ERK1/2↓ |
(80) |
| LF | TAA-induced C57BL/six mice model | Co-treatments of GF and Silymarin | 50, 100 mg/kg, 8 weeks | 4-HNE↓, 8-OHdG↓, ROS↓, MDA↓, GSH↑, SOD↑, TNF-α↓, IL-1β↓, IL-6↓, α-SMA↓, ColT1↓, ColT3↓, TGF-β1R↓, Smad2↓, Smad3↓, Nrf2↑, HO-1↑, Sirtuin1↑ | (81) |
| LF | CCl4-induced ICR mice model | Zhizi Baipi soup | 2 weeks | Collagen I↓, α-SMA↓, TGF-β↓ | (82) |
GF is utilized in treating various liver diseases.
Figure 3
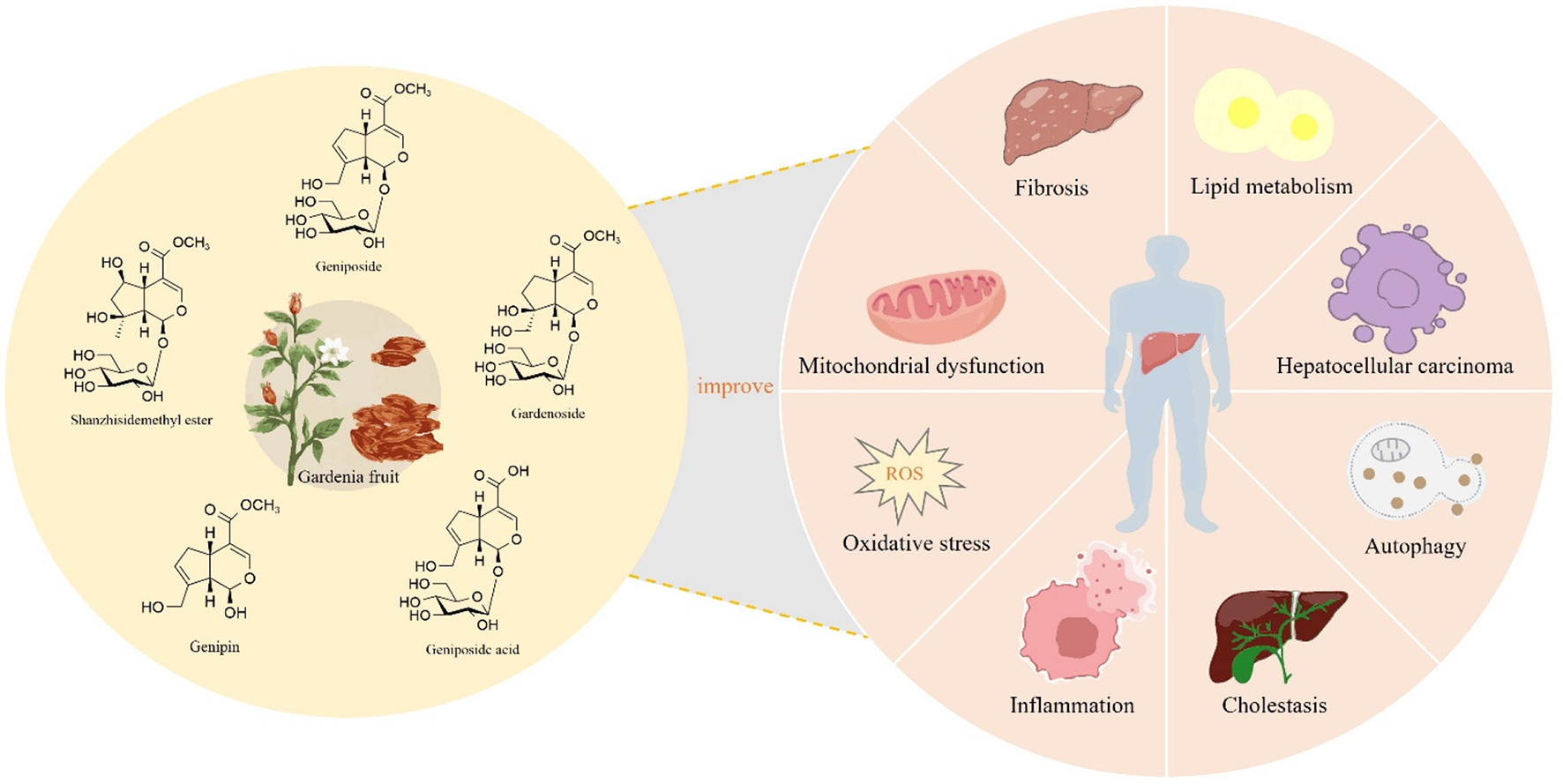
Iridoids in GF exert hepatoprotective effects, potentially through the modulation of lipid metabolism, cholestasis, inflammation, oxidative stress, mitochondrial function, autophagy, fibrosis, and hepatocarcinogenesis.
Figure 4
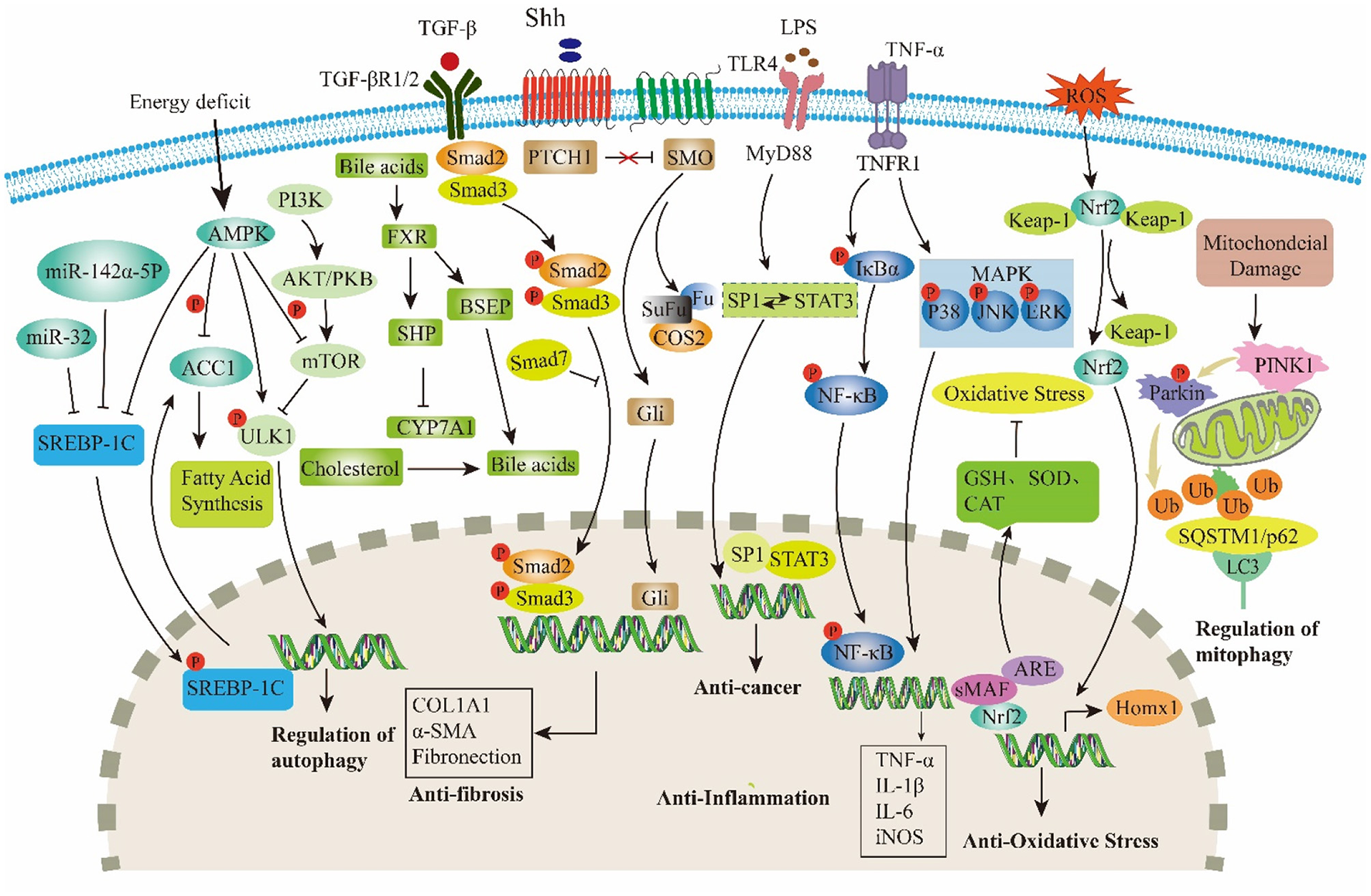
Major targets and signaling pathways modulated by iridoids in GF.
Table 3
| Experimental model | Iridoids | Dosage | Molecular mechanisms | References |
|---|---|---|---|---|
| Nrf2−/−C57BL/six mice; OA and PA-induced HepG2 cells | Geniposide | 50, 75 and 100 mg/kg | Inhibit Nrf2/AMPK/mTOR signaling pathway | (86) |
| HFD-induced ICR mice model | Mixture of peanut skin extract, geniposide, and isoquercitrin | / | Regulate the TLR4/NF-κB, AMPK/ACC/CPT1, and AMPK/UKL1/LC3B signaling pathways | (90) |
| HFD-induced obese mice model | Genipin | 5 and 20 mg/kg | Regulation miR-142a-5p/SREBP-1c axis | (93) |
| HFD-induced obese mice model | Genipin | 20 mg/kg | Inhibit miR-132 expression | (94) |
| HFD-induced C57BL/six mice model | Combination of geniposide and chlorogenic acid | / | Reduce the signaling of gut-derived lipopolysaccharide (LPS); inhibit RhoA/ROCK signaling | (98) |
| HFD-induced C57BL/six mice model; FXR−/− mice | Combination of geniposide and chlorogenic acid | / | Improve the gutmicrobiome; activate FXR signaling | (99) |
| PA and LPS-induced AML12 cells model; HFD-induced C57BL/6J mice model | Gardenoside | 10, 25 and 50 μM in vitro and 40 mg/kg in vivo | Negative-regulate pyroptosis markers (NLRP3, ASC, caspase-1 p20, GSDMD-N, IL-1β); reduce CTCF/DPP4 expression | (103) |
| HFD-induced obese mice model; Hepatocytes treated by free fatty acids |
Genipin | / | Inhibit UCP2-mediated pyroptosis. | (104) |
| Hepatocytes treated by H2O2 | Geniposide | 900 μg/ml | Regulate the global DNA methylation level; inhibit the expression of P53, Bcl-2 and Akt | (106) |
| HFD-induced C57BL/6 and ApoE−/− mice model; HepG2 cells and Caco2 cells |
Geniposide | 50 mg/kg | Regulate the liver-gut crosstalk of bile acids mediated by FXR; promote hepatic bile acid synthesis and ileal bile acid excretion via regulating the enterohepatic | (110) |
| DDC-induced sclerosing cholangitis mice model | Geniposide | 50 and 100 mg/kg | Inhibit expressions of CK19 and Ki67; up-regulate canalicular efflux transporters (BSEP, MRP2, MDR1, MDR2); reduce CYP7A1 mRNA expression | (111) |
| ANIT-induced IC rat model | Geniposide | 25, 50 and 100 mg/kg | Modulate the bile secretion pathway and the glutathione pathway; regulate the expression of ABCG5, NCEH1, OAT3, and GST | (112) |
| ANIT-induced IC rat model | Geniposide | / | Reduce basolateral bile acids uptake via repression of OATP2; decrease Bile acids biosynthesis through down-regulation of CYP7A1, CYP8B1, and CYP27A1; enhance mRNA level of basolateral transporter OSTβ; activate FXR, PXR, and SHP. | (113) |
| ANIT-induced ICR mice model; The HepG2 cell line |
Geniposide | 150 mg/kg | Promote the expression of BSEP through activate FXR and Nrf2 signaling pathway. | (117) |
| ANIT-induced liver injury with acute intrahepatic cholestasis SD rat model | Geniposidic acid | 25, 50 and 150 mg/kg | Up-regulate mRNA expression of FXR, BSEP and Mrp2 | (118) |
| ANIT-induced liver injury ICR mice model | Geniposide | 50 mg/kg | Inhibit STAT3 and NF-κB signaling pathway | (122) |
| SD rats and HSC-T6 cells | shanzhiside methyl ester | / | Inhibit Nrf2/NF-κB signaling pathway | (126) |
| CCl4-induced C57BL/six mice model | Geniposide | 50 mg/kg | Reduce oxidative stress and inflammatory respose, inhibit apoptosis and modulate overall metabolism | (127) |
| C57BL/six mice model | Geniposide | 25 and 50 mg/kg | Active SIRT1/FXR signaling | (128) |
| CCL4-induced C57BL/six mice model HSC-T6 cells |
Geniposide | / | Inhibit Shh signaling, inhibits the activation of HSC | (131) |
| TGF-β1-induced LX-2 cell CCl4-induced BALB/c mice model |
Geniposide | 40 mg/kg in vivo | Inhibit TGF-β1/Smad signaling | (133) |
| ANIT-induced C57BL/six mice NLRP3−/− mice |
Geniposidic acid | 25, 50 and 100 mg/kg | Inhibit NLRP3 inflammasome activation | (139) |
| GalN/LPS-induced ICR mice model | Genipin | 25, 50 and 100 mg/kg | Inhibit necroptosis-mediated inflammasome signaling | (140) |
| HFD-induced rats model | Gardenoside combined with 6,7-Dimethoxycoumarin and Rhein | / | Inhibit the NLRP3 inflammasome | (141) |
| FFA- induced HepG2 hepatocytes | Gardenoside | 10 and 20 μM | Inhibit NF-κB activity | (145) |
| IL-1β-stimulated hepatocytes | Genipin | 100 μM | Inhibit NF-κB activity | (146) |
| Hepatic IRI injury (abdominal surgery without occlusion to produce an ischemia) | Geniposide | / | Inhibit PI3K/Akt/mTOR signaling pathway. | (147) |
| CCl4-induced C57BL/six mice model LPS-treated THP-1 cells |
Geniposide | / | Inhibit MeCP2-Hedgehog signaling axis | (153) |
| CCl4-induced rats models | Geniposide | 20 and 40 mg/kg | Inhibit p38 MAPK signaling pathway | (156) |
| APAP-induced C57BL/six mice model | Geniposide | 20, 40 and 80 mg/kg | Inhibit CYP 2E1 and attenuate the exhaustion of GSH and accumulation of MDA Inhibit TLR 4/NF-κB signaling pathway | (159) |
| Tripterygium glycosides (TG)-induced Kunming mice model | Geniposide | 20, 40 and 80 mg/kg | Promote GSH, GST, GPX, SOD, CAT | (160) |
| Alcohol-induced Kunming mice model | Geniposide | 20, 40 and 80 mg/kg | Promote GSH, GST, GPX, CuZn-SOD and CAT Levels | (161) |
| Alcohol-induced konmin mice model | Geniposide | / | Improve abnormal metabolism, alleviate disorders related to amino acid metabolism and oxidative stress | (162) |
| sepsis-induced mice | Genipin | 1, 2.5 and 5 mg/kg | Restore impaired autophagic flux | (166) |
| C57BL/six mice (ischemia/reperfusion) | Genipin | 100 mg/kg | Modulating mitochondrial quality control | (170) |
| Orthotopic mouse model Human HCC cell line PLC/PRF/5 cells and HUVECs |
Geniposide | 30 mg/kg in vivo or 200 μg/ml in vitro | Inhibit TLR4/MyD88 signaling pathway | (174) |
| The C57BL/six mice, BALB/c nude mice and NOD-SCID mice Luciferase-tagged MHCC-97L cells |
Genipin | 25 and 50 mg/kg | Activate PPARγ | (175) |
| H22 hepatoma tumor-bearing mouse model H22 cell line |
Mixture of geniposide, paeoniflorin combined with sensitized sorafenib | / | Activate NF-κB/HIF-2α/SerpinB3 signaling pathway | (176) |
| SD rats HepG2 cells |
Genipin | / | Distrubed pyrimidine, purine, amino acid metabolism, and CoA biosynthesis | (179) |
| ANIT-induced Kunming mice model | Genipin | 125, 250, and 500 mg/kg | Impaire UDP-glucuronosyltransferase system and cytochrome P450 enzyme activity. | (184) |
| Male SD rats | Genipin | / | Induce P450 inactivation | (185) |
| C57BL/six mice HepG2 cells |
Geniposide | / | attributed to genipin's reactive hemiacetal structure | (186) |
The role and mechanism of iridoids from GF in the treatment of liver diseases.
Despite the progress in existing research, several challenges and difficulties persist: (1) the majority of studies on GF extracts are predominantly based on in vitro cell experiments and rodent models, with a notable lack of human clinical evidence. To elucidate the efficacy and safety of GF in promoting human health, there is an urgent need for high-quality clinical trials that can provide robust scientific support. (2) Although GF exhibits diverse biological activities and holds significant medicinal value, making it a promising candidate for health diets and drug development, its current applications in health-related fields remain relatively limited. The development of functional foods and health products derived from GF is still in its nascent stages, necessitating further exploration of its potential value. (3) The number and types of iridoids isolated from GF are limited, particularly with respect to low-abundance analogs. To facilitate the efficient enrichment and large-scale preparation of these compounds, advanced technologies such as synthetic biology and biocatalysis should be employed to enhance structural diversity and functional research. (4) Current research on GF iridoids has predominantly focused on major constituents such as geniposide, genipin, gardenoside, geniposidic acid, and shanzhiside methyl ester. These compounds are not only present in relatively high abundances but also more readily isolated and quantified, thereby facilitating comprehensive pharmacological evaluation both in vitro and in vivo. In contrast, studies on minor or low-abundance iridoids have largely been restricted to phytochemical identification and structural elucidation, with limited subsequent investigation into their biological activities. We propose that the scarcity of conclusive pharmacological data on these low-abundance compounds may stem from the practical challenges associated with obtaining sufficient quantities of purified material—particularly for in vivo studies. Moreover, it is plausible that these minor iridoids exhibit distinct pharmacokinetic profiles or higher bioactivity, potentially contributing to therapeutic effects through unique mechanisms. However, validation of these hypotheses remains challenging due to the limited availability of purified compounds and the current inadequacy of sensitive analytical methodologies for tracing their metabolic fate and tissue distribution. Further research is essential to elucidate the contributions of these understudied iridoids to the hepatoprotective effects of GF and to clarify their underlying molecular mechanisms. (5) Despite the extensive application of GF in clinical practice, its safety profile remains incompletely characterized, raising concerns about potential risks associated with its use. Recent studies have indicated that high doses of GF may induce hepatotoxicity and nephrotoxicity. For instance, Li et al. (177) demonstrated that rats administered GF for 12 weeks exhibited impaired liver and kidney function following long-term or high-dose exposure. Furthermore, Luo et al. (179) reported that GF treatment resulted in significant plasma biochemical alterations and histopathological changes in the liver. Additionally, it disrupted multiple metabolic pathways, including purine and amino acid metabolism in the liver, and pyrimidine, primary bile acid, amino acid, pantothenate, and CoA biosynthesis pathways in the plasma. Further evidence suggests that geniposide and its aglycone metabolite genipin are the principal compounds responsible for the observed hepatotoxicity of GF (178). According to TCM principles, the synergistic combination of herbs can enhance therapeutic efficacy while reducing toxicity (187). Consequently, relying solely on the toxicity assessment of individual components, such as GF extract, to evaluate the overall safety of the herb is scientifically unsound and may lead to inaccuracies and concealed safety risks. In recent years, there has been a gradual increase in reports of hepatotoxicity linked to geniposide and genipin, underscoring the necessity of exploring emerging technologies, such as nano-sustained release systems, to mitigate their toxic effects and enhance clinical safety. (6) This review primarily examines the hepatoprotective properties of GF and its iridoid constituents. Notably, gardenia flowers and leaves also possess a variety of chemical compounds akin to those present in the fruit, suggesting the potential presence of novel active iridoids (188, 189). Consequently, these plant parts represent promising candidates for the development of new hepatoprotective drugs and merit further exploration. In summary, addressing the identified challenges could significantly expand the applicability of GF iridoids within both the pharmaceutical and food sectors.
Statements
Author contributions
QC: Conceptualization, Data curation, Writing – original draft. XD: Conceptualization, Data curation, Writing – original draft. AL: Data curation, Formal analysis, Investigation, Writing – original draft. QLi: Data curation, Formal analysis, Investigation, Writing – original draft. QLu: Data curation, Formal analysis, Investigation, Writing – original draft. YC: Data curation, Formal analysis, Investigation, Writing – original draft. RW: Data curation, Formal analysis, Investigation, Writing – original draft. LM: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 82460759), the Science & Technology Plan of Zunyi [ZSKHHZ(2023)168], the Xin Miao Funding of Zunyi Medical University [QKPTRC(2021)1350-004], and the Undergraduate Innovation and Entrepreneurship Projects of Zunyi Medical University (Project Nos. 2024106610891, ZYDC202402144, ZYDC202402157).
Acknowledgments
The authors express their gratitude for the support and contributions of all participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GLOSSARY
TC, total cholesterol; TG, triglyceride; FFA, free fatty acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; MDA, malonaldehyde; SREBP-1C, sterol regulatory element binding protein-1c; FAS, Fas cell surface death receptor; PPARα, peroxisome proliferator-activated receptor α; PPARγ, peroxisome proliferator-activated receptor gamma; AMPK, adenosine5′-monophosphate (AMP)-activated protein kinase; p-AMPK, phospho5′AMP-activated protein kinase; CPT-1, carnitine palmitoyltransferase-1; mTOR, mammalian target of rapamycin; 8-OhdG, 8-hydroxy2′-deoxyguanosine; TGF-β, transforming growth factor-β; p-ERK, phosphorylated extracellular signal-regulated kinase; TLR4, toll-like receptor-4; Myd88, myeloid differentiation primary response gene 88; NF-κB, nuclear factor kappa-B; CPT1A, carnitine palmitoyltransferase 1A; p-IRE1α, phosphorylated inositol-requiring enzyme 1α; GPAT1, glycerol-3-phosphate acyltransferase 1; AGPAT, 1-acylglycerol-3-phosphate O-acyltransferase; PAP, prostatic acid phosphatase; DGAT2, diacylglycerol-O-acyltransferase homolog 2 Cyp8b1; Fxr, farnesoid X receptor; Ntcp, sodium taurocholate cotransporting polypeptide; Oatp1, organic anion transporting polypeptide 1; TBA, total bile acid; TBIL, total bilirubin; SOD, superoxide dismutase; HO-1, heme oxygenase 1; Nrf2, nuclear factor erythroid 2-related factor 2; Smad3, mothers against decapentaplegic homolog 3; p-Smad3, phospho- mothers against decapentaplegic homolog 3; Smad2, mothers against DPP homolog 2; ColT3, tumor (T3); α-SMA, α-smooth muscle actin; IL-2, interleukin-2; IL-6, interleukin-6; IL-4, interleukin-4; IL-10, interleukin-10; IL-1β, interleukin-1 beta; TNF-α, tumor necrosis factor-alpha; GSH, glutathione; ROS, reactive oxygen species; 4-HNE, 4-hydroxynonenal; p-ERK1/2, phosphorylated extracellular regulated kinase 1/2; BSEP, bile salt export pump; ALP, alkaline phosphatase; ColIV, collage typeIV; NOX2, NADPH oxidase 2; SIRT1, silent mating type information regulation 2 homolog-1; p38, P38 mitogen-activated protein kinase; IFN-γ, interferon-gamma; TNF-γ, tumor necrosis factor-γ; CYP2E1, cytochrome P450 2E1; DBIL, directbilirubin; MATE1, multidrug and toxin extrusion protein 1; MDR1, multidrug resistance 1; OATP1A2, organic anion transporting polypeptide 1A2; MRP2, multidrug resistance-associated protein 2; UGT1A1, uridine diphosphate-glucuronosyltransferase 1A1; FDA, food and drug administration; CCl4, carbon tetrachloride.
References
1.
Almeida JI Tenreiro MF Martinez-Santamaria L Guerrero-Aspizua S Gisbert JP Alves PM et al . Hallmarks of the human intestinal microbiome on liver maturation and function. J Hepatol. (2022) 76:694–725. 10.1016/j.jhep.2021.10.015
2.
Mou Y Liao W Li Y Wan L Liu J Luo X et al . Glycyrrhizin and the related preparations: an inspiring resource for the treatment of liver diseases. Am J Chin Med. (2024) 52:315–54. 10.1142/S0192415X24500149
3.
Wang R Tang R Li B Ma X Schnabl B Tilg H . Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. (2020) 18:4–17. 10.1038/s41423-020-00592-6
4.
Devarbhavi H Asrani SK Arab JP Nartey YA Pose E Kamath PS . Global burden of liver disease: 2023 update. J Hepatol. (2023) 79:516–37. 10.1016/j.jhep.2023.03.017
5.
Jin C Zongo AW-S Du H Lu Y Yu N Nie X et al . Gardenia (Gardenia jasminoides Ellis) fruit: a critical review of its functional nutrients, processing methods, health-promoting effects, comprehensive application and future tendencies. Crit Rev Food Sci Nutr. (2023) 65:165–92. 10.1080/10408398.2023.2270530
6.
Li M Chen S Luo K Li X Wang R Yang J et al . Geniposide from gardeniae fructus exerts antipyretic effect in febrile rats through modulating the TLR4/NF-κB signaling pathway. J Ethnopharmacol. (2024) 326:117934. 10.1016/j.jep.2024.117934
7.
Wang S Li X Niu Y Liu Y Zhu Y Lu X et al . Identification and screening of chemical constituents with hepatoprotective effects from three traditional Chinese medicines for treating jaundice. J Sep Sci. (2016) 39:3690–9. 10.1002/jssc.201600437
8.
Zhu YW Li D Ye TJ Qiu FJ Wang XL Yan XF et al . The study of Yin-Chen-Hao-Tang preventing and treating alcoholic fatty liver disease through PPAR signaling pathway based on network pharmacology and RNA-Seq transcriptomics. Evid Based Complement Alternat Med. (2021) 2021:8917993. 10.21203/rs.3.rs-818720/v1
9.
Sun Q Wang X Xin X An Z Hu Y Feng Q . Qushi Huayu decoction attenuated hepatic lipid accumulation via JAK2/STAT3/CPT-1A-related fatty acid β-oxidation in mice with non-alcoholic steatohepatitis. Pharm Biol. (2022) 60:2124–33. 10.1080/13880209.2022.2134898
10.
Wu M Zhang H Li R . Determination of glycyrrhetinic acid in ZhiziBaipi soup by HPLC. West China J Pharm Sci. (2006) 21:399–400. 10.3321/j.issn:1001-5302.2005.15.024
11.
Zhang X Su X Yu X Zhang X Guo X Hou G et al . Preparative separation of iridoid glucosides and crocins from gardeniae fructus using sequential macroporous resin column chromatography and evaluation of their anti-inflammatory and antioxidant activities. J Chromatogr B. (2023) 1229:123887. 10.1016/j.jchromb.2023.123887
12.
Ren L Tao W Zhang H Xue W Tang J Wu R et al . Two standardized fractions of Gardenia jasminoides ellis with rapid antidepressant effects are differentially associated with BDNF up-regulation in the hippocampus. J Ethnopharmacol. (2016) 187:66–73. 10.1016/j.jep.2016.04.023
13.
Guo S Bao L Li C Sun J Zhao R Cui X . Antiviral activity of iridoid glycosides extracted from fructus gardeniae against influenza A virus by PACT-dependent suppression of viral RNA replication. Sci Rep. (2020) 10:1897. 10.1038/s41598-020-58443-3
14.
Zhang H-y Liu H Yang M Wei S-f . Antithrombotic activities of aqueous extract from Gardenia jasminoides and its main constituent. Pharm Biol. (2012) 51:221–5. 10.3109/13880209.2012.717088
15.
Wang L Chen S Liu S Biu AM Han Y Jin X et al . A comprehensive review of ethnopharmacology, chemical constituents, pharmacological effects, pharmacokinetics, toxicology, and quality control of gardeniae fructus. J Ethnopharmacol. (2024) 320:117397. 10.1016/j.jep.2023.117397
16.
Bryś M Urbańska K Olas B . Novel findings regarding the bioactivity of the natural blue pigment genipin in human diseases. Int J Mol Sci. (2022) 23:902. 10.3390/ijms23020902
17.
Neri-Numa IA Pessoa MG Paulino BN Pastore GM . Genipin: a natural blue pigment for food and health purposes. Trends Food Sci Technol. (2017) 67:271–9. 10.1016/j.tifs.2017.06.018
18.
Chang R Liu J Luo Y Huang T Li Q Wen J et al . Isoflavones' effects on pharmacokinetic profiles of main iridoids from gardeniae fructus in rats. J Pharm Anal. (2020) 10:571–80. 10.1016/j.jpha.2019.11.004
19.
Wang C Gong X Bo A Zhang L Zhang M Zang E et al . Iridoids: research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules. (2020) 25:287. 10.3390/molecules25020287
20.
de Carvalho Meirelles G Bridi R von Poser GL . Iridoids as a potential hepatoprotective class: a review. Mini Rev Med Chem. (2023) 23:452–79. 10.2174/1389557522666220816130158
21.
Shan M Yu S Yan H Guo S Xiao W Wang Z et al . A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules. (2017) 22:1689. 10.3390/molecules22101689
22.
Chen L Li M Yang Z Tao W Wang P Tian X et al . Gardenia jasminoides ellis: ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J Ethnopharmacol. (2020) 257:112829. 10.1016/j.jep.2020.112829
23.
Chyau C-C Chiu C-Y Hsieh H-L Hsieh DW-C Hsieh C-R Chang C-H et al . High-purity preparation of enzyme transformed trans-crocetin reclaimed from gardenia fruit waste. Plants. (2022) 11:281. 10.3390/plants11030281
24.
Jin C Wang L Liu X Lu Y Yu N Nie X et al . Health oil preparation from gardenia seeds by aqueous enzymatic extraction combined with puffing pre-treatment and its properties analysis. Food Sci Biotechnol. (2023) 32:2043–55. 10.1007/s10068-023-01319-9
25.
Shang YF Zhang YG Cao H Ma YL Wei ZJ . Comparative study of chemical compositions and antioxidant activities of Zhizi fruit extracts from different regions. Heliyon. (2019) 5:e02853. 10.1016/j.heliyon.2019.e02853
26.
Xu J Zhou R Luo L Dai Y Feng Y Dou Z . Quality evaluation of decoction pieces of gardeniae fructus based on qualitative analysis of the HPLC fingerprint and triple-Q-TOF-MS/MS combined with quantitative analysis of 12 representative components. J Anal Methods Chem. (2022) 2022:2219932. 10.1155/2022/2219932
27.
Qian H Hu Y Wang Z Ren A Zhang H Chu S et al . Comprehensive quality evaluation of different types of gardeniae fructus (Zhizi) and Shuizhizi based on LC-MS/MS. Front Plant Sci. (2024) 15:1346591. 10.3389/fpls.2024.1346591
28.
Zou Y-D Ma X-X Du S-N Qi P-X He F-Y Yang Z-Y et al . Sweritranslactone D, a hepatoprotective novel secoiridoid dimer with tetracyclic lactone skeleton from heat-transformed swertiamarin. Fitoterapia. (2021) 151:104879. 10.1016/j.fitote.2021.104879
29.
Liu W Wu P Song Z Nie F Zhang L Lee D et al . Iridoids from patrinia heterophylla and their anti-inflammatory activity. Phytochemistry. (2023) 212:113720. 10.1016/j.phytochem.2023.113720
30.
Wang H Zhou X-M Wu L-Y Liu G-J Xu W-D Zhang X-S et al . Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J Neuroinflamm. (2020) 17:188. 10.1186/s12974-020-01863-9
31.
Yoshikawa H Matsumoto T Kitagawa T Okayama M Ohta T Yoshida T et al . Anti-proliferative effects of iridoids from Valeriana fauriei on cancer stem cells. Int J Mol Sci. (2022) 23:14206. 10.3390/ijms232214206
32.
Kang J Guo C Thome R Yang N Zhang Y Li X et al . Hypoglycemic, hypolipidemic and antioxidant effects of iridoid glycosides extracted from corni fructus: possible involvement of the PI3K–Akt/PKB signaling pathway. RSC Adv. (2018) 8:30539–49. 10.1039/C8RA06045B
33.
Tian J Qin S Han J Meng J Liang A . A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of fructus gardeniae (Zhi-zi). J Ethnopharmacol. (2022) 289:114984. 10.1016/j.jep.2022.114984
34.
Cao Y-G Ren Y-J Liu Y-L Wang M-N He C Chen X et al . Iridoid glycosides and lignans from the fruits of Gardenia jasminoides eills. Phytochemistry. (2021) 190:112893. 10.1016/j.phytochem.2021.112893
35.
Chen QC Zhang WY Youn U Kim H Lee I Jung H-J et al . Iridoid glycosides from gardeniae fructus for treatment of ankle sprain. Phytochemistry. (2009) 70:779–84. 10.1016/j.phytochem.2009.03.008
36.
Kim HJ Kim EJ Seo SH Shin CG Jin C Lee YS . Vanillic acid glycoside and quinic acid derivatives from gardeniae fructus. J Nat Prod. (2006) 69:600–3. 10.1021/np050447r
37.
Chang WL Wang HY Shi LS Lai JH Lin HC . Immunosuppressive iridoids from the fruits of Gardenia jasminoides. J Nat Prod. (2005) 68:1683–5. 10.1021/np0580816
38.
Yu Y Xie ZL Gao H Ma WW Dai Y Wang Y et al . Bioactive iridoid glucosides from the fruit of Gardenia jasminoides. J Nat Prod. (2009) 72:1459–64. 10.1021/np900176q
39.
Li H-B Yu Y Wang Z-Z Dai Y Gao H Xiao W et al . Iridoid and bis-iridoid glucosides from the fruit of Gardenia jasminoides. Fitoterapia. (2013) 88:7–11. 10.1016/j.fitote.2013.03.025
40.
Shu P Yu M Zhu H Luo Y Li Y Li N et al . Two new iridoid glycosides from gardeniae fructus. Carbohydr Res. (2021) 501:108259. 10.1016/j.carres.2021.108259
41.
Yang L Peng K Zhao S Chen L Qiu F . Monoterpenoids from the fruit of Gardenia jasminoides ellis (Rubiaceae). Biochem Syst Ecol. (2013) 50:435–7. 10.1016/j.bse.2013.06.012
42.
Koichi M Rie O Kyoko F Masao K . Studies of the constituents of gardenia species. I. monoterpenoids from gardeniae fructus. Chem Pharm Bull. (1998) 46:295–1300. 10.1248/cpb.46.1295
43.
Machida K Oyama K Ishii M Kakuda R Yaoita Y Kikuchi M . Studies of the constituents of gardenia species. II terpenoids from gardeniae fructus. Chem Pharm Bull. (2000) 48:746–8. 10.1248/cpb.48.746
44.
Rao AS Chary JS Merugu R . Iridoids from Gardenia jasminoides ellis. Int J Chemtech Res. (2013) 5:418–21.
45.
Wu X Zhou Y Yin F Mao C Li L Cai B et al . Quality control and producing areas differentiation of gardeniae fructus for eight bioactive constituents by HPLC–DAD–ESI/MS. Phytomedicine. (2014) 21:551–9. 10.1016/j.phymed.2013.10.002
46.
Cai CJ Zhang ZL Zuo YM Zhu YY Luo GM Zhang J . Study on the iridoid chemical constituents of Gardenia jasminoides. Lishizhen Med Mater Med Res. (2013) 24:342–3. 10.3969/j.issn.008-0805.2013.02.041
47.
Peng K Yang L Zhao S Chen L Zhao F Qiu F . Chemical constituents from the fruit of Gardenia jasminoides and their inhibitory effects on nitric oxide production. Bioorg Med Chem Lett. (2013) 23:1127–31. 10.1016/j.bmcl.2012.11.099
48.
Ono M Ueno M Masuoka C Ikeda T Nohara T . Iridoid glucosides from the fruit of Genipa americana. Chem Pharm Bull. (2005) 53:1342–4. 10.1248/cpb.53.1342
49.
Wang L Liu S Zhang X Xing J Liu Z Song F et al . A strategy for identification and structural characterization of compounds from Gardenia jasminoides by integrating macroporous resin column chromatography and liquid chromatography-tandem mass spectrometry combined with ion-mobility spectrometry. J Chromatogr A. (2016) 1452:47–57. 10.1016/j.chroma.2016.05.026
50.
Fu Z Ling Y Li Z Chen M Sun Z Huang C . HPLC-Q-TOF-MS/MS for analysis of major chemical constituents of Yinchen–Zhizi herb pair extract. Biomed Chromatogr. (2013) 28:475–85. 10.1002/bmc.3057
51.
Li H-B Ma J-F Mei Y-D Liu L-X Cao Z-y Shi D-F et al . Two new iridoid glycosides from the fruit of Gardenia jasminoides. Nat Prod Res. (2020) 36:186–92. 10.1080/14786419.2020.1775227
52.
Akihisa T Watanabe K Yamamoto A Zhang J Matsumoto M Fukatsu M . Melanogenesis inhibitory activity of monoterpene glycosides from gardeniae fructus. Chem Biodivers. (2012) 9:1490–9. 10.1002/cbdv.201200030
53.
Ma JF . Study on the Main Chemical Components of Traditional Chinese Medicine Gardenia jasminoides. (2019) 2020. 10.27167/d.cnki.gjinu.2019.000824
54.
Pouwels S Sakran N Graham Y Leal A Pintar T Yang W et al . Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. (2022) 22:63. 10.1186/s12902-022-00980-1
55.
Godoy-Matos AF Silva Júnior WS Valerio CM . NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. (2020) 12:60. 10.1186/s13098-020-00570-y
56.
Buzzetti E Pinzani M Tsochatzis EA . The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. (2016) 65:1038–48. 10.1016/j.metabol.2015.12.012
57.
Wu J Wang K Wang X Pang Y Jiang C . The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. (2020) 12:360–73. 10.1007/s13238-020-00814-7
58.
Nasiri-Ansari N Androutsakos T Flessa C-M Kyrou I Siasos G Randeva HS et al . Endothelial cell dysfunction and nonalcoholic fatty liver disease (NAFLD): a concise review. Cells. (2022) 11:2511. 10.3390/cells11162511
59.
Tang Z Li L Xia Z . Exploring anti-nonalcoholic fatty liver disease mechanism of gardeniae fructus by network pharmacology, molecular docking, and experiment validation. ACS Omega. (2022) 7:25521–31. 10.1021/acsomega.2c02629
60.
Lee KP Kim K Yoon E Baek S Ahn S-h . Pharmacological systemic analysis of gardenia fructus against non-alcoholic fatty liver disease and validation of animal models. Phys Act Nutr. (2022) 26:39–45. 10.20463/pan.2022.0006
61.
Lv C Liu X Chen S Yi Y Wen X Li T et al . Extract of Gardenia jasminoides ellis attenuates high-fat diet-induced glycolipid metabolism disorder in rats by targeting gut microbiota and TLR4/Myd88/NF-κB pathway. Antioxidants. (2024) 13:293. 10.3390/antiox13030293
62.
Yao Q Li S Cheng X Zou Y Shen Y Zhang S . Yin Zhi Huang, a traditional Chinese herbal formula, ameliorates diet-induced obesity and hepatic steatosis by activating the AMPK/SREBP-1 and the AMPK/ACC/CPT1A pathways. Ann Transl Med. (2020) 8:231. 10.21037/atm.2020.01.31
63.
Tian H Fang Y Liu W Wang J Zhao J Tang H et al . Inhibition on XBP1s-driven lipogenesis by Qushi Huayu decoction contributes to amelioration of hepatic steatosis induced by fructose. J Ethnopharmacol. (2023) 301:115806. 10.1016/j.jep.2022.115806
64.
Nevens F Trauner M Manns MP . Primary biliary cholangitis as a roadmap for the development of novel treatments for cholestatic liver diseases†. J Hepatol. (2023) 78:430–41. 10.1016/j.jhep.2022.10.007
65.
Zhang L Jiang X Shi J Zhang J Shi X Xie Z et al . Isoastragaloside I attenuates cholestatic liver diseases by ameliorating liver injury, regulating bile acid metabolism and restoring intestinal barrier. J Ethnopharmacol. (2024) 335:118649. 10.1016/j.jep.2024.118649
66.
Wang M-Q Zhang K-H Liu F-L Zhou R Zeng Y Chen AL et al . Wedelolactone alleviates cholestatic liver injury by regulating FXR-bile acid-NF-κB/NRF2 axis to reduce bile acid accumulation and its subsequent inflammation and oxidative stress. Phytomedicine. (2024) 122:115124. 10.1016/j.phymed.2023.155124
67.
Qin S Tian J Zhao Y Wang L Wang J Liu S et al . Gardenia extract protects against intrahepatic cholestasis by regulating bile acid enterohepatic circulation. J Ethnopharmacol. (2024) 319:117083. 10.1016/j.jep.2023.117083
68.
Cao Y Li S Zhang Z Zeng M Zheng X Feng W . A metabolomics study on the mechanisms of gardeniae fructus against α-naphthylisothiocyanate-induced cholestatic liver injury. Biomed Chromatogr. (2024) 38:e5961. 10.1002/bmc.5961
69.
Yi Y-X Ding Y Zhang Y Ma N-H Shi F Kang P et al . Yinchenhao decoction ameliorates alpha-naphthylisothiocyanate induced intrahepatic cholestasis in rats by regulating phase II metabolic enzymes and transporters. Front Pharmacol. (2018) 9:510. 10.3389/fphar.2018.00510
70.
Dong L-C Fan Y-X Yu Q Ma J Dong X Li P et al . Synergistic effects of rhubarb-gardenia herb pair in cholestatic rats at pharmacodynamic and pharmacokinetic levels. J Ethnopharmacol. (2015) 175:67–74. 10.1016/j.jep.2015.09.012
71.
Fu J Zhang Z Zhao Y Li X Jiang C He H et al . Acetylcorynoline alleviates acute liver injury via inhibiting TLR4/JNK/NF-?B pathway Based on RNA-seq and molecular docking in vivo and in vitro. Int Immunopharmacol. (2024) 143:113550. 10.1016/j.intimp.2024.113550
72.
Tangpradubkiat P Chayanupatkul M Werawatganone P Somanawat K Siriviriyakul P Klaikeaw N et al . Gardenia jasminoides extract mitigates acetaminophen-induced liver damage in mice. BMC Complement Med Ther. (2024) 24:371. 10.1186/s12906-024-04676-y
73.
Liu F Sun Z Hu P Tian Q Xu Z Li Z et al . Determining the protective effects of Yin-Chen-Hao Tang against acute liver injury induced by carbon tetrachloride using 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal. (2019) 174:567–77. 10.1016/j.jpba.2019.06.028
74.
Chen MJ Wang XY Shen W Ruan F Zhong XM Huang Z . Protective effect of water extract of She medicine Ardisia lindleyana on immune liver injury in mice. Chin J Mod Appl Pharm. (2016) 33:708–11. 10.13748/j.cnki.issn1007-7693.2016.06.07
75.
Yang Y Wu XQ Li XF Li WX Huang C Li J . Protective effects of Zhizi Baipi decoction and compatibility groups containing Gardenia jasminoides on mice with immune liver injury. Chin Pharmacol Bull. (2015) 31:1764–9. 10.3969/j.issn.100-978.2015.12027
76.
Wang L Zhao H Du X Cao Y Wu J Guo P et al . Rosehip (Rosa rugosa Thunb.) ethanol extract ameliorates liver fibrosis through upregulation of the PPAR signaling pathway. J Funct Foods. (2024) 122:106529. 10.1016/j.jff.2024.106529
77.
Bai J Qian B Cai T Chen Y Li T Cheng Y et al . Aloin attenuates oxidative stress, inflammation, and CCl4-induced liver fibrosis in mice: possible role of TGF-β/Smad signaling. J Agric Food Chem. (2023) 71:19475–87. 10.1021/acs.jafc.3c01721
78.
Shin M-R Lee JA Kim M Lee S Oh M Moon J et al . Gardeniae fructus attenuates thioacetamide-induced liver fibrosis in mice via both AMPK/SIRT1/NF-κB pathway and Nrf2 signaling. Antioxidants. (2021) 10:1837. 10.3390/antiox10111837
79.
Dong L Huang X Qin LH Ye FX Jiang LH Huang RB . Study on the effect of Gardenia jasminoides root extract on rats with liver fibrosis induced by carbon tetrachloride. J Chin Med Mat. (2019) 42:897–901. 10.13863/j.issn1001-4454.2019.04.040
80.
Cai F-F Wu R Song Y-N Xiong A-Z Chen X-L Yang M-D et al . Yinchenhao decoction alleviates liver fibrosis by regulating bile acid metabolism and TGF-β/Smad/ERK signalling pathway. Sci Rep. (2018) 8:15367. 10.1038/s41598-018-33669-4
81.
Lee JA Shin M-R Choi J Kim M Park H-J Roh S-S . Co-treatments of gardeniae fructus and silymarin ameliorates excessive oxidative stress-driven liver fibrosis by regulation of hepatic sirtuin1 activities using thioacetamide-induced mice model. Antioxidants. (2022) 12:97. 10.3390/antiox12010097
82.
Qian ZY Li J Huang C Meng XM Ma TT Chen ZL . Therapeutic effects of different combinations of Zhizi Baipi decoction on carbon tetrachloride-induced liver fibrosis in mice. Acta Univ Med Anhui. (2016) 51:68–72. 10.19405/j.cnki.issn1000-1492.2016.01.016
83.
He F Ru X Wen T . NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. (2020) 21:4777. 10.3390/ijms21134777
84.
Xie L Zhou C Wu Y Fu X Zhang G Han X et al . Wenqingyin suppresses ferroptosis in the pathogenesis of sepsis-induced liver injury by activating the Nrf2-mediated signaling pathway. Phytomedicine. (2023) 114. 10.1016/j.phymed.2023.154748
85.
O'Rourke SA Shanley LC Dunne A . The Nrf2-HO-1 system and inflammaging. Front Immunol. (2024) 15:1457010. 10.3389/fimmu.2024.1457010
86.
Shen B Feng H Cheng J Li Z Jin M Zhao L et al . Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J Cell Mol Med. (2020) 24:5097–108. 10.1111/jcmm.15139
87.
Sharma A Anand SK Singh N Dwivedi UN Kakkar P . AMP-activated protein kinase: an energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp Cell Res. (2023) 428:113614. 10.1016/j.yexcr.2023.113614
88.
Huang R Guo F Li Y Liang Y Li G Fu P et al . Activation of AMPK by triptolide alleviates nonalcoholic fatty liver disease by improving hepatic lipid metabolism, inflammation and fibrosis. Phytomedicine. (2021) 92:153739. 10.1016/j.phymed.2021.153739
89.
Lu X Xuan W Li J Yao H Huang C Li J et al . A protects against alcohol-induced liver injury through UQCRC2 to up-regulate mitophagy. Autophagy. (2021) 17:3622–43. 10.1080/15548627.2021.1886829
90.
Yi M Fasina OB Li Y Xiang L Qi J . Mixture of peanut skin extract, geniposide, and isoquercitrin improves the hepatic lipid accumulation of mice via modification of gut microbiota homeostasis and the TLR4 and AMPK signaling pathways. Int J Mol Sci. (2023) 24:16684. 10.3390/ijms242316684
91.
Bartel DP . MicroRNAs: target recognition and regulatory functions. Cell. (2009) 136:215–33. 10.1016/j.cell.2009.01.002
92.
Zhao Y Yu Y Ommati MM Xu J Wang J Zhang J et al . Multiomics analysis revealed the molecular mechanism of miRNAs in fluoride-induced hepatic glucose and lipid metabolism disorders. J Agric Food Chem. (2022) 70:14284–95. 10.1021/acs.jafc.2c03049
93.
Zhong H Chen K Feng M Shao W Wu J Chen K et al . Genipin alleviates high-fat diet-induced hyperlipidemia and hepatic lipid accumulation in mice via miR-142a-5p/SREBP-1c axis. FEBS J. (2017) 285:501–17. 10.1111/febs.14349
94.
Wang L Chen G Wu S Xu Y Guo C Wang M et al . Genipin improves lipid metabolism and sperm parametersin obese mice via regulation of miR-132 expression. Acta Biochim Biophys Sin. (2022), 54:1278–88. 10.3724/abbs.2022120
95.
Cheng Y Ling Z Li L . The intestinal microbiota and colorectal cancer. Front Immunol. (2020) 11:615056. 10.3389/fimmu.2020.615056
96.
Yang S Wei Z Luo J Wang X Chen G Guan X et al . Integrated bioinformatics and multiomics reveal Liupao tea extract alleviating NAFLD via regulating hepatic lipid metabolism and gut microbiota. Phytomedicine. (2024) 132:155834. 10.1016/j.phymed.2024.155834
97.
Xu H Fang F Wu K Song J Li Y Lu X et al . Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome. (2023) 11:1. 10.1186/s40168-023-01709-5
98.
Peng J-h Leng J Tian H-j Yang T Fang Y Feng Q et al . Geniposide and chlorogenic acid combination ameliorates non-alcoholic steatohepatitis involving the protection on the gut barrier function in mouse induced by high-fat diet. Front Pharmacol. (2018) 9:1399. 10.3389/fphar.2018.01399
99.
Li H Xi Y Xin X Feng Q Hu Y . Geniposide plus chlorogenic acid reverses non-alcoholic steatohepatitis via regulation of gut microbiota and bile acid signaling in a mouse model in vivo. Front Pharmacol. (2023) 14:1148737. 10.3389/fphar.2023.1148737
100.
Shi J Gao W Shao F . Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. (2017) 42:245–54. 10.1016/j.tibs.2016.10.004
101.
Wu C Lu W Zhang Y Zhang G Shi X Hisada Y et al . Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity. (2019) 50:1401–11.e4. 10.1016/j.immuni.2019.04.003
102.
Hsu S-K Li C-Y Lin IL Syue W-J Chen Y-F Cheng K-C et al . Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics. (2021) 11:8813–35. 10.7150/thno.62521
103.
Shen T Lei T Chen L Zhu B-B Xu B-L Zhang C-P et al . Gardenoside hinders caspase-1-mediated hepatocyte pyroptosis through the CTCF/DPP4 signaling pathway. Front Physiol. (2021) 12:669202. 10.3389/fphys.2021.669202
104.
Zhong H Liu M Ji Y Ma M Chen K Liang T et al . Genipin reverses HFD-induced liver damage and inhibits UCP2-mediated pyroptosis in mice. Cell Physiol Biochem. (2018) 49:1885–97. 10.1159/000493651
105.
Ulukaya E Acilan C Yilmaz Y . Apoptosis: why and how does it occur in biology?Cell Biochem Funct. (2011) 29:468–80. 10.1002/cbf.1774
106.
Peng X Tan L Song J Lai Y Yu S Xu F et al . Geniposide alleviated hydrogen peroxide-induced apoptosis of human hepatocytes via altering DNA methylation. Food Chem Toxicol. (2023) 182:114158. 10.1016/j.fct.2023.114158
107.
Molinaro A Wahlström A Marschall H-U . Role of bile acids in metabolic control. Trends Endocrinol Metab. (2018) 29:31–41. 10.1016/j.tem.2017.11.002
108.
Fuchs CD Trauner M . Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat Rev Gastroenterol Hepatol. (2022) 19:432–50. 10.1038/s41575-021-00566-7
109.
Su X Gao Y Yang R . Gut microbiota derived bile acid metabolites maintain the homeostasis of gut and systemic immunity. Front Immunol. (2023) 14:1127743. 10.3389/fimmu.2023.1127743
110.
Liu J Li Y Sun C Liu S Yan Y Pan H et al . Geniposide reduces cholesterol accumulation and increases its excretion by regulating the FXR-mediated liver-gut crosstalk of bile acids. Pharmacol Res. (2020) 152:104631. 10.1016/j.phrs.2020.104631
111.
Wen M Liu Y Chen R He P Wu F Li R et al . Geniposide suppresses liver injury in a mouse model of DDC-induced sclerosing cholangitis. Phytother Res. (2021) 35:3799–811. 10.1002/ptr.7086
112.
Zhang J Chen Y Luo G Luo Y . Molecular mechanism of geniposide against ANIT-induced intrahepatic cholestasis by integrative analysis of transcriptomics and metabolomics. Naunyn Schmiedebergs Arch Pharmacol. (2024) 398:765–79. 10.1007/s00210-024-03320-3
113.
Wang L Wu G Wu F Jiang N Lin Y . Geniposide attenuates ANIT-induced cholestasis through regulation of transporters and enzymes involved in bile acids homeostasis in rats. J Ethnopharmacol. (2017) 196:178–85. 10.1016/j.jep.2016.12.022
114.
van Wessel DBE Thompson RJ Gonzales E Jankowska I Sokal E Grammatikopoulos T et al . Genotype correlates with the natural history of severe bile salt export pump deficiency. J Hepatol. (2020) 73:84–93. 10.1016/j.jhep.2020.02.007
115.
Kubitz R Dröge C Stindt J Weissenberger K Häussinger D . The bile salt export pump (BSEP) in health and disease. Clin Res Hepatol Gastroenterol. (2012) 36:536–53. 10.1016/j.clinre.2012.06.006
116.
Soroka CJ Boyer JL . Biosynthesis and trafficking of the bile salt export pump, BSEP: therapeutic implications of BSEP mutations. Mol Aspects Med. (2014) 37:3–14. 10.1016/j.mam.2013.05.001
117.
Wu G Wen M Sun L Li H Liu Y Li R et al . Mechanistic insights into geniposide regulation of bile salt export pump (BSEP) expression. RSC Adv. (2018) 8:37117–28. 10.1039/C8RA06345A
118.
Chen H Huang X Min J Li W Zhang R Zhao W et al . Geniposidic acid protected against ANIT-induced hepatotoxity and acute intrahepatic cholestasis, due to Fxr-mediated regulation of Bsep and Mrp2. J Ethnopharmacol. (2016) 179:197–207. 10.1016/j.jep.2015.12.033
119.
Oeckinghaus A Ghosh S . The NF-B family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. (2009) 1:a000034-a. 10.1101/cshperspect.a000034
120.
Liu T Zhang L Joo D Sun S-C . NF-κB signaling in inflammation. Signal Transduct Target Ther. (2017) 2:17023. 10.1038/sigtrans.2017.23
121.
Abe Y Sano T Otsuka N Ogawa M Tanaka N . PRMT5-mediated methylation of STAT3 is required for lung cancer stem cell maintenance and tumour growth. Commun Biol. (2024) 7:593. 10.1038/s42003-024-06290-7
122.
Tan Z Liu A Luo M Yin X Song D Dai M et al . Geniposide inhibits alpha-naphthylisothiocyanate-induced intrahepatic cholestasis: the downregulation of STAT3 and NFκB signaling plays an important role. Am J Chin Med. (2016) 44:721–36. 10.1142/S0192415X16500397
123.
Hammerich L Tacke F . Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. (2023) 20:633–46. 10.1038/s41575-023-00807-x
124.
Gowifel AMH Khalil MG Nada SA Kenawy SA Ahmed KA Salama MM et al . Combination of pomegranate extract and curcumin ameliorates thioacetamide-induced liver fibrosis in rats: impact on TGF-β/Smad3 and NF-κB signaling pathways. Toxicol Mech Methods. (2020) 30:620–33. 10.1080/15376516.2020.1801926
125.
Chen J Ge J Chen W Zhao Y Song T Fu K et al . UPLC-Q-TOF-MS based investigation into the bioactive compounds and molecular mechanisms of Lamiophlomis Herba against hepatic fibrosis. Phytomedicine. (2023) 121:155085. 10.1016/j.phymed.2023.155085
126.
Ge J Chen W Li M Zhao J Zhao Y Ren J et al . To elucidate the bioactive components of lamiophlomis herba in the treatment of liver fibrosis via plasma pharmacochemistry and network pharmacology. J Pharm Biomed Anal. (2024) 246:116204. 10.1016/j.jpba.2024.116204
127.
Yang L Bi L Jin L Wang Y Li Y Li Z et al . Geniposide ameliorates liver fibrosis through reducing oxidative stress and inflammatory respose, inhibiting apoptosis and modulating overall metabolism. Front Pharmacol. (2021) 12:772635. 10.3389/fphar.2021.772635
128.
Qin T Hasnat M Wang Z Hassan HM Zhou Y Yuan Z et al . Geniposide alleviated bile acid-associated NLRP3 inflammasome activation by regulating SIRT1/FXR signaling in bile duct ligation-induced liver fibrosis. Phytomedicine. (2023) 118:155405. 10.1016/j.phymed.2023.154971
129.
Gorojankina T . Hedgehog signaling pathway: a novel model and molecular mechanisms of signal transduction. Cell Mol Life Sci. (2016) 73:1317–32. 10.1007/s00018-015-2127-4
130.
Hu L Lin X Lu H Chen B Bai Y . An overview of hedgehog signaling in fibrosis. Mol Pharmacol. (2015) 87:174–82. 10.1124/mol.114.095141
131.
Lin X Li J Xing Y-Q . Geniposide, a sonic hedgehog signaling inhibitor, inhibits the activation of hepatic stellate cell. Int Immunopharmacol. (2019) 72:330–8. 10.1016/j.intimp.2019.04.016
132.
Meng L Lv H Kong Q Li S Jiang N Yu C et al . The combination of paeoniflorin and metformin synergistically inhibits the progression of liver fibrosis in mice. Eur J Pharmacol. (2024) 981:176917. 10.1016/j.ejphar.2024.176917
133.
Zhou LH Chen X . Geniposide inhibits liver fibrosis and hepatic stellate cell activation via the TGF-β1/Smad signaling pathway. Acta Physiologica Sinica. (2022) 74:217–24. 10.13294/j.aps.2022.0019
134.
Dick MS Sborgi L Rühl S Hiller S Broz P . ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun. (2016) 7:11929. 10.1038/ncomms11929
135.
Groß Christina J Mishra R Schneider Katharina S Médard G Wettmarshausen J Dittlein Daniela C et al . K + efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. (2016) 45:761–73. 10.1016/j.immuni.2016.08.010
136.
Paik S Kim JK Silwal P Sasakawa C Jo E-K . An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. (2021) 18:1141–60. 10.1038/s41423-021-00670-3
137.
Cai S-Y Ge M Mennone A Hoque R Ouyang X Boyer JL . Inflammasome is activated in the liver of cholestatic patients and aggravates hepatic injury in bile duct–ligated mouse. Cell Mol Gastroenterol Hepatol. (2020) 9:679–88. 10.1016/j.jcmgh.2019.12.008
138.
Frissen M Liao L Schneider KM Djudjaj S Haybaeck J Wree A et al . Bidirectional role of NLRP3 during acute and chronic cholestatic liver injury. Hepatology. (2021) 73:1836–54. 10.1002/hep.31494
139.
Song M Chen Z Qiu R Zhi T Xie W Zhou Y et al . Inhibition of NLRP3-mediated crosstalk between hepatocytes and liver macrophages by geniposidic acid alleviates cholestatic liver inflammatory injury. Redox Biol. (2022) 55:102404. 10.1016/j.redox.2022.102404
140.
Seo M-J Hong J-M Kim S-J Lee S-M . Genipin protects d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the necroptosis-mediated inflammasome signaling. Eur J Pharmacol. (2017) 812:128–37. 10.1016/j.ejphar.2017.07.024
141.
Zhao T Lun S Yan M Park J Wang S Chen C . 6,7-Dimethoxycoumarin, gardenoside and rhein combination improves non-alcoholic fatty liver disease in rats. J Ethnopharmacol. (2024) 322:117646. 10.1016/j.jep.2023.117646
142.
Guo Q Jin Y Chen X Ye X Shen X Lin M et al . NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. (2024) 9:53. 10.1038/s41392-024-01757-9
143.
Ding B Lin C Liu Q He Y Ruganzu JB Jin H et al . Tanshinone IIA attenuates neuroinflammation via inhibiting RAGE/NF-κB signaling pathway in vivo and in vitro. J Neuroinflamm. (2020) 17:302. 10.1186/s12974-020-01981-4
144.
Zinatizadeh MR Schock B Chalbatani GM Zarandi PK Jalali SA Miri SR . The nuclear factor kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. (2021) 8:287–97. 10.1016/j.gendis.2020.06.005
145.
Liang H Zhang L Wang H Tang J Yang J Wu C et al . Inhibitory effect of gardenoside on free fatty acid-induced steatosis in HepG2 hepatocytes. Int J Mol Sci. (2015) 16:27749–56. 10.3390/ijms161126058
146.
Nakatake R Tsuda T Matsuura T Miki H Hishikawa H Matsushima H et al . Genipin inhibits the induction of inducible nitric oxide synthase through the inhibition of NF-κB activation in rat hepatocytes. Drug Metab Lett. (2017) 10:254–63. 10.2174/1872312810666161020164658
147.
Rong YP Huang HT Liu JS Wei L . Protective effects of geniposide on hepatic ischemia/reperfusion injury. Transplant Proc. (2017) 49:1455–60. 10.1016/j.transproceed.2017.02.063
148.
Pan L Cheng Y Yang W Wu X Zhu H Hu M et al . Nintedanib ameliorates bleomycin-induced pulmonary fibrosis, inflammation, apoptosis, and oxidative stress by modulating PI3K/Akt/mTOR pathway in mice. Inflammation. (2023) 46:1531–42. 10.1007/s10753-023-01825-2
149.
Xiu A-Y Ding Q Li Z Zhang C-Q . Doxazosin Attenuates Liver Fibrosis by Inhibiting Autophagy in Hepatic Stellate Cells via Activation of the PI3K/Akt/mTOR Signaling Pathway. Drug Des Devel Ther. (2021) 15:3643–59. 10.2147/DDDT.S317701
150.
Tian L-Y Smit DJ Jücker M . The role of PI3K/AKT/mTOR signaling in hepatocellular carcinoma metabolism. Int J Mol Sci. (2023) 24:2652. 10.3390/ijms24032652
151.
Lai M Qiu Z Zou S Yang J Long Q Chen H et al . Mecp2 fine-tunes quiescence exit by targeting nuclear receptors. eLife. (2024) 12:RP89912. 10.7554/eLife.89912
152.
Tao H Huang C Yang J-J Ma T-T Bian E-B Zhang L et al . MeCP2 controls the expression of RASAL1 in the hepatic fibrosis in rats. Toxicology. (2011) 290:327–33. 10.1016/j.tox.2011.10.011
153.
Ma T-t Li X-f Li W-x Yang Y Huang C Meng X-m et al . Geniposide alleviates inflammation by suppressing MeCP2 in mice with carbon tetrachloride-induced acute liver injury and LPS-treated THP-1 cells. Int Immunopharmacol. (2015) 29:739–47. 10.1016/j.intimp.2015.08.045
154.
Cargnello M Roux PP . Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. (2011) 75:50–83. 10.1128/MMBR.00031-10
155.
Cai Q Wang Z Zhang R Zhang L Cui S Lin H et al . Huangjia Ruangan granule inhibits inflammation in a rat model with liver fibrosis by regulating TNF/MAPK and NF-κB signaling pathways. Evid Based Complement Alternat Med. (2022) 2022:1–16. 10.1155/2022/8105306
156.
Li C Zhang K Jin X Gao X Lv J Shen J et al . A transcriptomics and network pharmacology approach to elucidate the mechanism of action of geniposide on carbon tetrachloride-induced liver injury in rats. Int Immunopharmacol. (2023) 120:110391. 10.1016/j.intimp.2023.110391
157.
Pignatelli P Menichelli D Pastori D Violi F . Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. (2018) 76:713–22. 10.5603/KP.a2018.0071
158.
Seen S . Chronic liver disease and oxidative stress – a narrative review. Expert Rev Gastroenterol Hepatol. (2021) 15:1021–35. 10.1080/17474124.2021.1949289
159.
Yang S Kuang G Jiang R Wu S Zeng T Wang Y et al . Geniposide protected hepatocytes from acetaminophen hepatotoxicity by down-regulating CYP 2E1 expression and inhibiting TLR 4/NF-κB signaling pathway. Int Immunopharmacol. (2019) 74:105625. 10.1016/j.intimp.2019.05.010
160.
Wang J Miao M Qu L Cui Y Zhang Y . Protective effects of geniposide against tripterygium glycosides (TG)-induced liver injury and its mechanisms. J Toxicol Sci. (2016) 41:165–73. 10.2131/jts.41.165
161.
Wang J Zhang Y Liu R Li X Cui Y Qu L . Geniposide protects against acute alcohol-induced liver injury in mice via up-regulating the expression of the main antioxidant enzymes. Can J Physiol Pharmacol. (2015) 93:261–7. 10.1139/cjpp-2014-0536
162.
Zhang T Zhang A Qiu S Sun H Han Y Guan Y et al . High-throughput metabolomics approach reveals new mechanistic insights for drug response of phenotypes of geniposide towards alcohol-induced liver injury by using liquid chromatography coupled to high resolution mass spectrometry. Mol Biosyst. (2017) 13:73–82. 10.1039/C6MB00742B
163.
Martinez-Lopez N Singh R . Autophagy and lipid droplets in the liver. Annu Rev Nutr. (2015) 35:215–37. 10.1146/annurev-nutr-071813-105336
164.
Cao W Li J Yang K Cao D . An overview of autophagy: mechanism, regulation and research progress. Bull Cancer. (2021) 108:304–22. 10.1016/j.bulcan.2020.11.004
165.
Zhu L Du J Dai Y Shen Y Li H Zhang Q et al . Morinda officinalis iridoid glycosides alleviate methotrexate-induced liver injury in CIA rats by increasing liver autophagy and improving lipid metabolism homeostasis. J Ethnopharmacol. (2024) 333:118486. 10.1016/j.jep.2024.118486
166.
Cho HI Kim SJ Choi JW Lee SM . Genipin alleviates sepsis-induced liver injury by restoring autophagy. Br J Pharmacol. (2016) 173:980–91. 10.1111/bph.13397
167.
Lemasters JJ Theruvath TP Zhong Z Nieminen A-L . Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta Bioenerg. (2009) 1787:1395–401. 10.1016/j.bbabio.2009.06.009
168.
de Castro IP Martins LM Loh SHY . Mitochondrial quality control and Parkinson's disease: a pathway unfolds. Mol Neurobiol. (2010) 43:80–6. 10.1007/s12035-010-8150-4
169.
Ikeda Y Shirakabe A Maejima Y Zhai P Sciarretta S Toli J et al . Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. (2015) 116:264–78. 10.1161/CIRCRESAHA.116.303356
170.
Shin J-K Lee S-M . Genipin protects the liver from ischemia/reperfusion injury by modulating mitochondrial quality control. Toxicol Appl Pharmacol. (2017) 328:25–33. 10.1016/j.taap.2017.05.002
171.
Vogel A Meyer T Sapisochin G Salem R Saborowski A . Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. 10.1016/S0140-6736(22)01200-4
172.
Niu Z-S Wang W-H Niu X-J . Recent progress in molecular mechanisms of postoperative recurrence and metastasis of hepatocellular carcinoma. World J Gastroenterol. (2022) 28:6433–77. 10.3748/wjg.v28.i46.6433
173.
Llovet JM Kelley RK Villanueva A Singal AG Pikarsky E Roayaie S et al . Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7. 10.1038/s41572-020-00240-3
174.
Zhang C Wang N Tan HY Guo W Chen F Zhong Z et al . Direct inhibition of the TLR4/MyD88 pathway by geniposide suppresses HIF-1α-independent VEGF expression and angiogenesis in hepatocellular carcinoma. Br J Pharmacol. (2020) 177:3240–57. 10.1111/bph.15046
175.
Wu J Chan Y-T Lu Y Feng Z Yuan H Xu X et al . Genipin-activating PPARγ impedes CCR2-mediated macrophage infiltration into postoperative liver to suppress recurrence of hepatocellular carcinoma. Int J Biol Sci. (2023) 19:5257–74. 10.7150/ijbs.87327
176.
Li J-F Zheng X-R Zhang H-Y Shen C-M Shen G-x Jiang J-W et al . Effects of sensitized sorafenib with a paeoniflorin and geniposide mixture on liver cancer via the NF-κB-HIF-2α-serpinB3 pathway. Evid Based Complement Alternat Med. (2022) 2022:1–11. 10.1155/2022/1911311
177.
Li C Gao X Gao X Lv J Bian X Lv J et al . Effects of medicine food fructus gardeniae on liver and kidney functions after oral administration to rats for 12 weeks. J Food Biochem. (2021) 45:e13752. 10.1111/jfbc.13752
178.
Li C Lan M Lv J Zhang Y Gao X Gao X et al . Screening of the hepatotoxic components in fructus gardeniae and their effects on rat liver BRL-3A cells. Molecules. (2019) 24:3920. 10.3390/molecules24213920
179.
Luo Y Gao F Chang R Zhang X Zhong J Wen J et al . Metabolomics based comprehensive investigation of gardeniae fructus induced hepatotoxicity. Food Chem Toxicol. (2021) 153:112250. 10.1016/j.fct.2021.112250
180.
Wang Y Feng F . Evaluation of the hepatotoxicity of the Zhi-Zi-Hou-Po decoction by combining UPLC-Q-exactive-MS-based metabolomics and HPLC-MS/MS-based geniposide tissue distribution. Molecules. (2019) 24:511. 10.3390/molecules24030511
181.
Tian J Yi Y Zhao Y Li C Zhang Y Wang L et al . Oral chronic toxicity study of geniposide in rats. J Ethnopharmacol. (2018) 213:166–75. 10.1016/j.jep.2017.11.008
182.
Zeng X Jiang J Liu S Hu Q Hu S Zeng J et al . Bidirectional effects of geniposide in liver injury: preclinical evidence construction based on meta-analysis. J Ethnopharmacol. (2024) 319:117061. 10.1016/j.jep.2023.117061
183.
Liu Y Liu B Shi M Ye T Li H Sergi D . NLRP3 inflammasome activation is involved in geniposide-induced hepatotoxicity. Mediators Inflamm. (2025) 2025:4112856. 10.1155/mi/4112856
184.
Wang S Ge S Chen Y Zhou F Wang J Chen L et al . Acute and subacute hepatotoxicity of genipin in mice and its potential mechanism. Heliyon. (2023) 9:e21834. 10.1016/j.heliyon.2023.e21834
185.
Huang H Zhao Y Huang C Lv N Zhao J Sun S et al . Unraveling a combined inactivation mechanism of cytochrome P450s by genipin, the major reactive aglycone derived from gardeniae fructus. Chem Res Toxicol. (2023) 36:1483–94. 10.1021/acs.chemrestox.3c00102
186.
Gao A Ni Y Chen C Xin W Wang Y Zhang W . Covalent binding of geniposide metabolites to hepatic proteins: a potential mechanism for its hepatotoxicity. Chem Biol Interact. (2025) 408:111411. 10.1016/j.cbi.2025.111411
187.
Luo Y Zhang X Zhang W Yang Q You W Wen J et al . Compatibility with semen sojae praeparatum attenuates hepatotoxicity of gardeniae fructus by regulating the microbiota, promoting butyrate production and activating antioxidant response. Phytomedicine. (2021) 90:153656. 10.1016/j.phymed.2021.153656
188.
Li W Li H Shao Q Liang X Zhou Q Tang Y . Iridoid glycosides from the flowers of Gardenia jasminoides: isolation, characterization, and antioxidant potential. Fitoterapia. (2025) 180:106316. 10.1016/j.fitote.2024.106316
189.
Li Y Wang XL Song JL Qi HY . Chemical constituents of the leaves of Gardenia jasminoides. Chin J Exp Tradit Med Formulae. (2016) 22:3. 10.13422/j.cnki.syfjx.2016130068
Summary
Keywords
gardenia fruit, iridoids, liver disease, pharmacological effects, molecular mechanism
Citation
Cao Q, Du X, Liu A, Li Q, Luo Q, Chen Y, Wang R and Meng L (2025) Iridoids in gardenia fruit: key components in liver disease therapy. Front. Nutr. 12:1667863. doi: 10.3389/fnut.2025.1667863
Received
23 July 2025
Accepted
01 September 2025
Published
25 September 2025
Volume
12 - 2025
Edited by
Weicheng Hu, Yangzhou University, China
Reviewed by
Junzi Wu, Yunnan University of Traditional Chinese Medicine, China
Salome Dini, University of Otago, New Zealand
Updates
Copyright
© 2025 Cao, Du, Liu, Li, Luo, Chen, Wang and Meng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingjie Meng menglj718@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.