- 1Department of Rehabilitation Medicine, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2The Second Clinical School of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3School of Medicine of Southeast University, Nanjing, Jiangsu, China
- 4Eight-Year Medical Doctor Program, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Department of Gerontology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu, China
- 6Department of Gerontology, Suzhou Municipal Hospital, Suzhou, Jiangsu, China
- 7Department of Critical Care Medicine, Cancer Hospital of Shantou University Medical College, Shantou, Guangdong, China
Background: The Controlled Nutritional Status (CONUT) metric has demonstrated effectiveness as a prognostic indicator for acute and chronic diseases in addition to other wasting conditions. However, its association with sarcopenia in elderly hospitalized patients remains insufficiently explored. Our study objectives included the assessment of the potential of CONUT score to predict sarcopenia onset.
Methods: Our study was a single center retrospective cohort study. Patients from the Department of Geriatrics of the First People’s Hospital of Kunshan were recruited for this study. Multiple indicators related to nutrition and sarcopenia, including CONUT, Prognostic Nutritional Index (PNI), triglyceride–total cholesterol–body weight index (TCBI), Geriatric Nutritional Risk Index (GNRI), and handgrip strength (HGS). Spearman’s and Pearson’s correlation were calculated to assess the associations between nutritional indices and sarcopenia-related indicators. The demographic characteristics, physical examination findings and laboratory parameters were included in univariate logistic regression. Based on the results of univariate logistic regression and theoretical analysis, variables were selected for multivariate logistic regression in order to identify risk factors for sarcopenia.
Results: A total of 236 elderly hospitalized patients were included. Malnutrition was prevalent in patients with sarcopenia. The optimal CONUT cut-off values were defined as >4 for males and >3 for females, dividing patients into high CONUT (n = 140, 59.32%) and low CONUT (n = 96, 40.58%) groups. Patients in the high CONUT group had lower levels of albumin, prealbumin, hemoglobin, and total lymphocyte count. Multivariate logistic regression analysis showed that a high CONUT score was an independent risk factor for sarcopenia (OR:1.814, 95% CI: 1.019–3.255, p = 0.044). Male sex and low iron level were also demonstrated to be associated with sarcopenia.
Conclusion: CONUT score is an independent risk factor for sarcopenia and may serve as a practical indicator for sarcopenia risk screening in elderly hospitalized patients.
1 Introduction
Sarcopenia is a progressive, widespread skeletal muscle disorder (1–4), affecting approximately 10% of adults aged ≥60 years globally and predicted to impact over 500 million people worldwide by 2050 (4). The severe consequences of sarcopenia adversely affect the quality of life of older individuals, place greater demands on families, and pose a significant public health challenge. The current management of sarcopenia focuses primarily on resistance exercise and nutritional interventions (5, 6). Given the absence of specific pharmacotherapies for sarcopenia, identifying its modifiable risk factors is critical for developing targeted preventive strategies. Sarcopenia is positively associated with various disorders, including diabetes and its complications, osteoporosis, anorexia nervosa, cardiovascular disease, respiratory disease, arthritis, and metabolic disorders (7–11). However, current interventions have been ineffective, highlighting the need to explore new, easy-to-intervene risk factors.
Maintaining the nutritional status is essential for good health and skeletal muscle maintenance. Nutritional status monitoring is an effective approach for disease prediction (12, 13). Previous research has demonstrated a direct relationship between insufficient nutrition and the development of sarcopenia (6, 14). The symptoms of sarcopenia can be improved by supplementation with whey protein, essential amino acids, and vitamin D (15). Leucine-rich proteins, which possess specific synthetic functions, can fundamentally conserve muscles and impede the progression of skeletal sarcopenia (16–18). The European Society of Parenteral and Enteral Nutrition (ESPEN) also argues that moderate protein consumption can prevent illnesses.
Regarding the diagnosis of malnutrition, there remains no validated gold standard, and existing methods are generally inadequate for routine clinical practice (19, 20). Accordingly, we evaluated the association between the Controlling Nutritional Status (CONUT) score, a simple and readily calculable clinical metric, and sarcopenia. The CONUT score is constructed using three biomarkers: serum albumin, total lymphocyte count, and total cholesterol (TC) levels, and it serves as a practical indicator of a patient’s nutritional and immune status (21). Specifically, albumin is a well-recognized marker of systemic protein reserves, total lymphocyte count reflects the integrity of cellular immune function. Additionally, TC can indirectly reflect energy metabolism status, as reduced TC levels are often associated with insufficient energy intake or increased energy expenditure in elderly populations (21). The CONUT score correlates with the functional status reflected by these biomarkers: a higher score, accompanied by lower levels of the three nutrients, is associated with a greater decline in protein reserve, immune function, and energy metabolism. To date, this score has shown promising predictive value across various clinical contexts.
Compared to other nutritional indicators such as PNI and GNRI, CONUT offers unique advantages. It comprehensively integrates three key dimensions including protein status, immune function, and energy metabolism, thus providing a more holistic assessment of nutritional status, while also being simpler to calculate. Initially developed and validated in surgical and oncology departments, the CONUT score was designed to predict acute deterioration during hospitalization (21–24). However, recent analyses have demonstrated its significant prognostic relevance in diverse clinical conditions, including chronic diseases, cancer, and cardiac disorders (19, 25–27). Despite the well-established predictive value of the CONUT score for numerous conditions, research reports on its association with sarcopenia remain relatively limited.
The purpose of this study was therefore to examine the association between nutritional indicators, such as CONUT score, and sarcopenia development. Additionally, this study aimed to explore the risk factors for the development of sarcopenia, which would provide support for effective clinical prediction of sarcopenia.
2 Materials and methods
2.1 Participants
This is a single-centre, retrospective cohort study. A total of 236 patients from the Department of Geriatrics at First People’s Hospital of Kunshan were retrospectively recruited for this study. The inclusion criteria were as follows: (1) age over 60 years and (2) complete information about the clinicopathological diagnosis. The exclusion criteria included (1) age less than 60 years; (2) unclear diagnosis; (3) lack of information about the clinicopathological diagnosis; (4) recent or current use of drugs that cause muscle damage, such as statins; (5) limb impairment that prevents physical tests, such as grip strength, four-metre step test, 5-time chair stand test, balance test, and others; and (6) refusal or legal incapacity to provide informed consent. Patients with missing data were excluded, the overall missing data rate was <5%.
2.2 Assessment of sarcopenia
The Asian Working Group for Sarcopenia (AWGS) criteria proposed in 2019 (5) were used to diagnose sarcopenia in this study. The diagnostic criteria for sarcopenia in these guidelines include decreased grip strength and/or usual stride speed, as well as decreased skeletal muscle mass. Grip strength was measured using a CAMRY EH101 electronic hand dynamometer on the dominant hand. Patients with a usual step speed of less than 1.0 m/s on a 6-metre walk test were classified as having a positive result (5). Skeletal muscle mass (ASM) was measured by DXA scanning, following the established criteria. Skeletal muscle mass was measured using the Relative Skeletal Muscle Mass Index (RSMI) (ASM/height2), which is also a prerequisite for the diagnosis of sarcopenia. Measurements were taken from stable patients without severe inflammation.
2.3 Data collection
Demographic data, physical examination findings, medical history, and serum laboratory test results were retrospectively collected. The demographic data included age, sex, and body mass index (BMI). Physical examination findings included relative skeletal muscle mass index (RSMI), handgrip strength (HGS), a four-metre step test, a 5-time chair stand test, and balance test. Technical term abbreviations such as RSMI and HGS were explained upon first use. The medical history included hypertension, diabetes mellitus, osteoporosis, COPD, atherosclerosis, cancer, neuromuscular disorders, and renal failure. Additionally, serum laboratory test results were collected, comprising of serum albumin concentration (ALB), prealbumin (PA), hemoglobin (Hb), transferrin (TRF), serum total cholesterol (TC), triglyceride (TG), total peripheral lymphocyte (TLC), fasting blood glucose (FBG), iron (Fe), zinc (Zn), and calcium (Ca). A manual screening evaluation was conducted to collect clinical data.
For the four-metre step test, a speed of 1 m/s or lower was deemed positive, whereas anything higher was classified as negative. During the balance trials, patients were asked to stand in three different positions: feet side by side, one heel at the midpoint of the other foot, and feet in a line, heel to toe. A positive result was determined if balance could not be maintained for more than 10 s in any of the three specified positions. For the 5-time chair stand test, patients were required to stand up from a chair with a standard height of 43 cm and cross their arms over their chest 5 times. A total time of 12 s or more was considered a positive result. These tests are based on the AWGS Consensus on the Diagnosis and Treatment of Sarcopenia, which was updated in 2019 (5).
The PNI is computed by adding 10 times the ALB in grams per litre (g/L) to 0.005 times the total lymphocyte count per cubic millimeter (/mm3) (28). The triglyceride–TCBI was determined by multiplying the values for serum total cholesterol (TC) in milligrams per deciliter (mg/dL), triglyceride (TG) in mg/dL, and body weight in kilograms (/kg), and then dividing the product by 1,000 (29). The Geriatric Nutritional Risk Index (GNRI) was calculated as follows: GNRI = 1.487 × ALB (g/L) + 41.7 × weight/ideal weight (kg). The ideal body weight was calculated as: ideal bodyweight = 22 × square of height (m) (30).
2.4 Controlling nutritional status score cut-off value
The CONUT score is commonly used to evaluate nutritional status. The CONUT score was determined by combining ALB, TLC, and TC. These three indicators were combined to derive the corresponding CONUT scores, as illustrated in Supplementary Table 1. A score of less than 2 on the CONUT scale indicates normal nutritional status, while scores ranging between 2 and 4 indicate mild malnutrition. Scores between 5 and 8 indicate moderate malnutrition, and a score of 9 or above indicate severe malnutrition.
2.5 Statistical analysis
SPSS version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Categorical variables are presented as numbers and percentages, and continuous variables are presented as medians, first quartiles, and third quartiles. Cut-off values commonly used in clinical practice were employed to convert continuous variables, such as BMI, ALB, Hb, and FBG, into categorical variables. The optimal cut-off values for PNI, TCBI, and GNRI were calculated using Receiver Operating Characteristic (ROC) analysis. As the study population was elderly, and some indicators, such as TLC, were different from those of the normal population, the median was used as the cut-off value. Considering the differences in CONUT scores between males and females, the cut-off values were divided based on the median CONUT scores for males and females, respectively. This approach was chosen to account for potential physiological differences in nutritional and immune markers between sexes that could influence baseline CONUT scores. Hence, patients were categorized into two groups based on the cut-off value: those with high CONUT scores and those with low CONUT scores.
The Mann–Whitney U test was used to examine differences between groups for continuous variables, while for categorical variables, either Pearson’s or Fisher’s exact chi-square test was employed. Spearman’s rank correlation analysis and Pearson’s correlation analysis were performed to examine the correlations between various nutritional scores, including BMI, CONUT, PNI, TCBI, and GNRI in patients with and without sarcopenia. Logistic regression was used to study the risk factors for sarcopenia development and to predict the risk of developing sarcopenia based on nutritional scores. First, univariate analysis of risk factors was conducted. Indicators with a p-value < 0.05 were included in the multivariate logistic regression to estimate the associated odds ratios (OR) and 95% confidence intervals (CI). Two-sided tests were used for all evaluations, and statistical significance was set at p < 0.05.
3 Results
3.1 Patient baseline characteristics
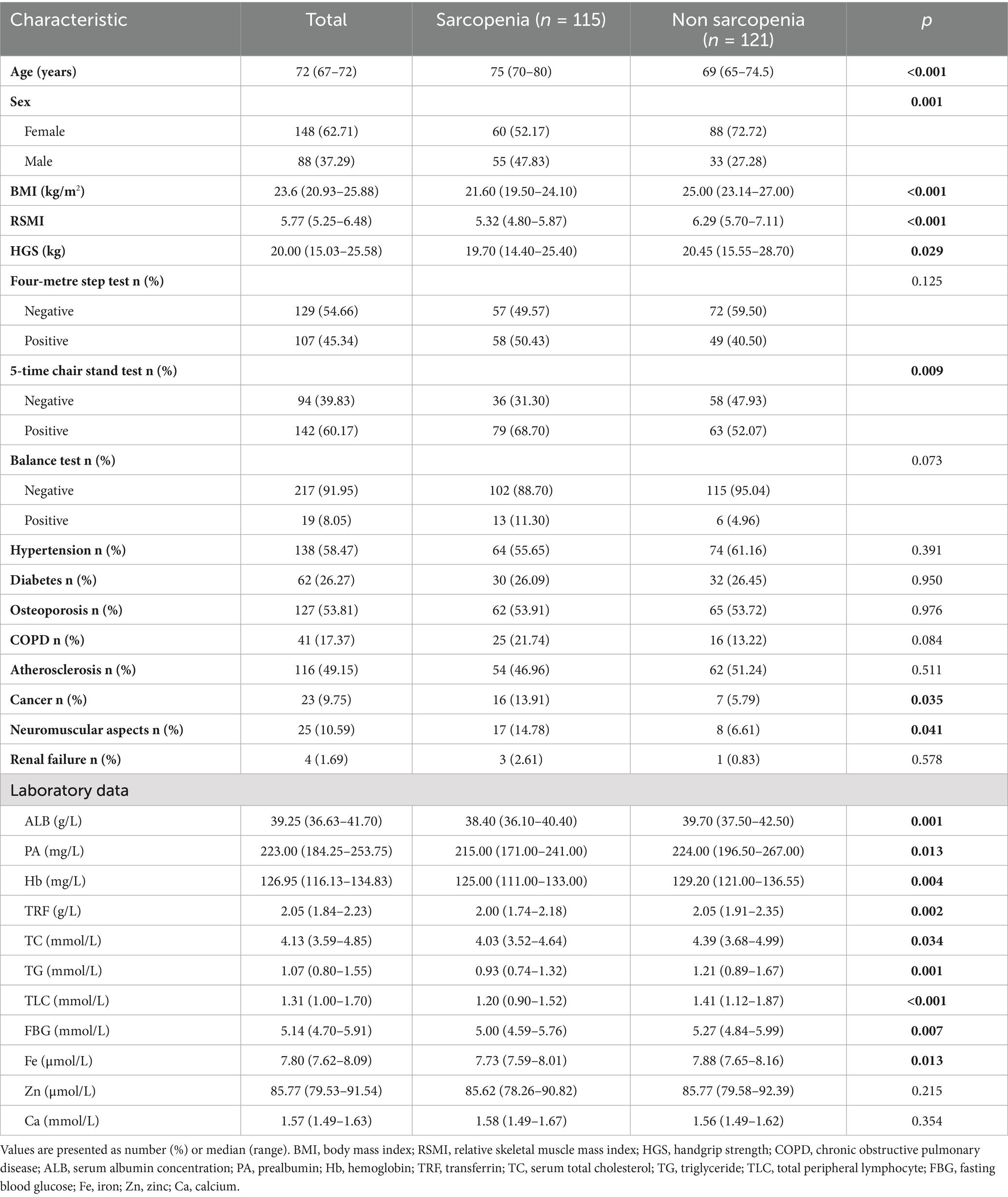
A total of 236 patients were enrolled in this study, and their baseline information is shown in Table 1. The median patient age was 72 years. A total of 148 patients were female (62.71%) and 88 patients were male (37.29%). The median BMI (kg/m2), RSMI, and HGS (kg) were 23.6 kg/m2, 5.77 and 20.00 kg, respectively. All the above indices were higher in non-sarcopenic patients than in sarcopenic patients and were statistically significant (all p < 0.05). The number of positive results in the four-metre step test, sit-to-up test, and balance test were 107 (45.34%), 142 (60.17%), and 19 (8.05%), respectively. Twenty-three (9.75%) of the patients had or have had cancer, which was significantly higher in sarcopenia patients than in non-sarcopenia patients (13.91% vs. 5.79%, p = 0.035). The percentage of neuropsychiatric disorders was also higher in patients with sarcopenia than in the group without sarcopenia (14.78% vs. 6.61%, p = 0.041). Among laboratory parameters, non-sarcopenic patients had significantly higher median levels of albumin (ALB), prealbumin (PA), hemoglobin (Hb), transferrin (TRF), total cholesterol (TC), triglycerides (TG), total lymphocyte count (TLC), fasting blood glucose (FBG), and Fe (μmol/L) than sarcopenic patients (all p < 0.05). Additionally, triglyceride levels in patients with sarcopenia were only three-quarters of those in patients without sarcopenia. Serum prealbumin and hemoglobin levels in both groups were slightly above the lower bounds of normal values, whereas iron levels were lower than the standard range for adults.
3.2 Differences in nutritional scores between sarcopenia and non-sarcopenia patients
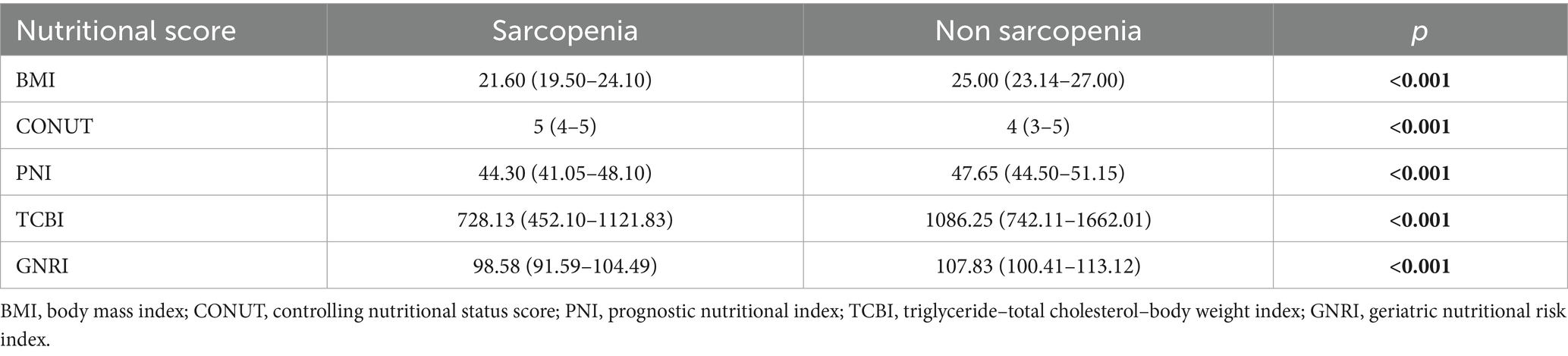
The nutritional status of patients is commonly evaluated using various scores including BMI, CONUT, PNI, TCBI, and GNRI. Table 2 shows the differences in nutritional scores between patients with and without sarcopenia. The results indicated that sarcopenia patients had higher CONUT scores compared to non-sarcopenia patients (5 vs. 4, p < 0.001). Comparing the BMI of the two groups, it was found that the majority of sarcopenic patients had normal levels of adiposity, while a proportion of non-sarcopenic patients were classified as overweight. In contrast, PNI, TCBI, and GNRI were significantly lower in sarcopenic patients than in non-sarcopenic patients (all p < 0.05), consistent with poorer nutritional status in the sarcopenia group.
3.3 Association of nutrition score with sarcopenia assessment
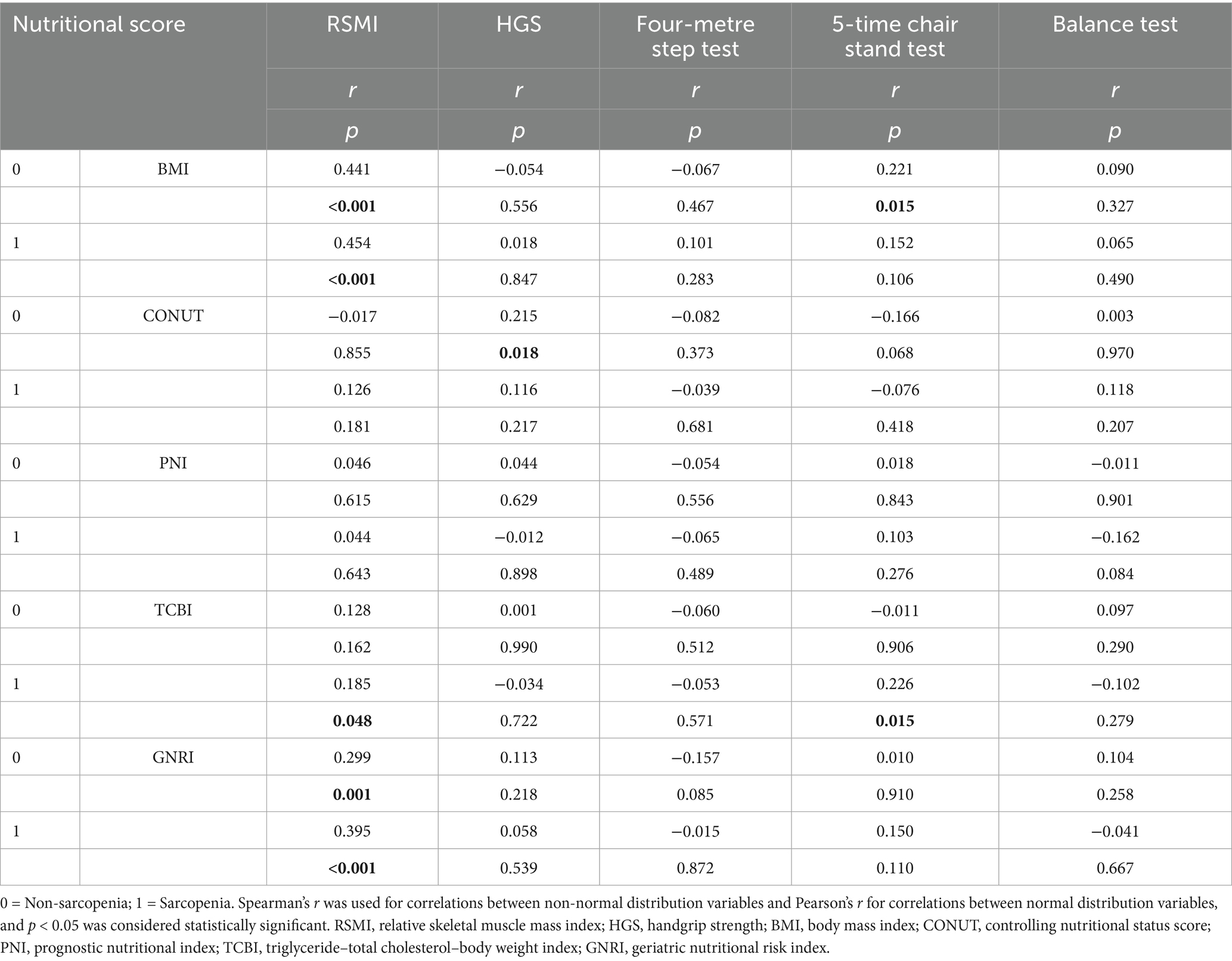
The correlations between the nutritional scores and RSMI, HGS, four-metre step test, 5-time chair stand test and balance test in patients with and without sarcopenia are shown in Table 3; Supplementary Figure 5. The results showed a lack of significant correlation between most nutritional scores and the indices used to assess sarcopenia in both groups. The PNI did not correlate with any of the parameters in either group, however, correlations between BMI, CONUT, TCBI, GNRI, and some of the parameters were evident. In both groups, there was a significant correlation between BMI, GNRI, and RSMI; however, this correlation was higher in patients with sarcopenia than in non-sarcopenia patients.
3.4 Relationship between CONUT and clinicopathological parameters
This study found a difference in CONUT scores between men and women. We calculated the median CONUT scores of 4 and 3 for the male and female groups, respectively, and used these as cut-off values. Based on these cut-off values, we divided the 236 patients into High CONUT and low CONUT score groups and summarized the clinical data of both groups in Table 4. A comparison of the nutritional scores of the PNI, TCBI, and GNRI between the two groups demonstrated higher values for all three indicators in the low CONUT group compared to the high CONUT score group (p < 0.05). This suggests that the nutritional status of patients in the high CONUT group was poorer than that of patients in the low CONUT group, which is consistent with the concept that increasing CONUT values indicating a worse nutritional status. Regarding serological testing, patients in the high CONUT score group exhibited lower levels of albumin, prealbumin, hemoglobin, and serum lymphocyte counts than those in the low CONUT score group (p < 0.05). This indicates that patients in the high CONUT score group may experience compromised protein synthesis and immunity, as well as a heightened likelihood of developing anemia.
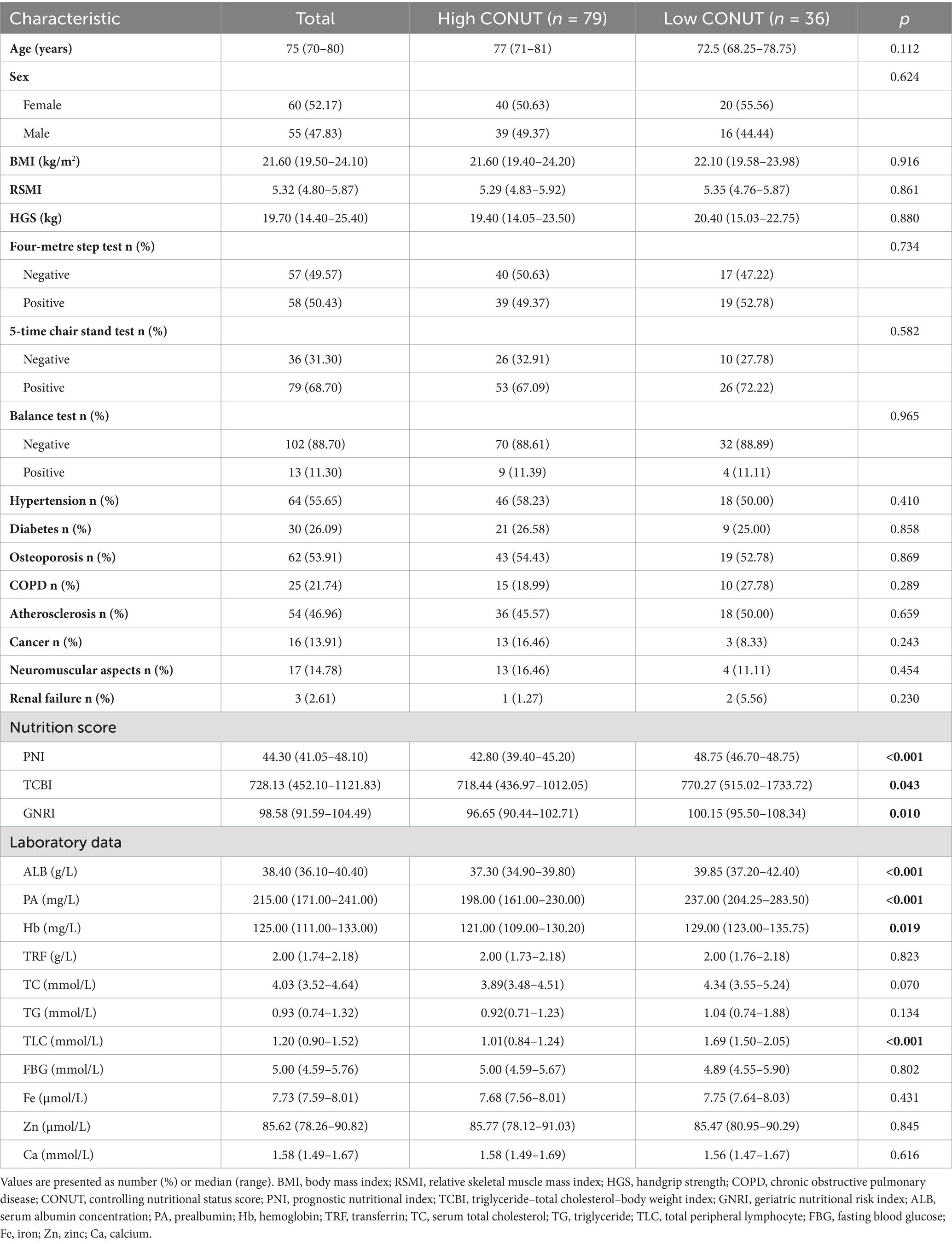
Table 4. The relationship between the CONUT and clinical characteristics of patients with sarcopenia.
3.5 Factors predicting sarcopenia
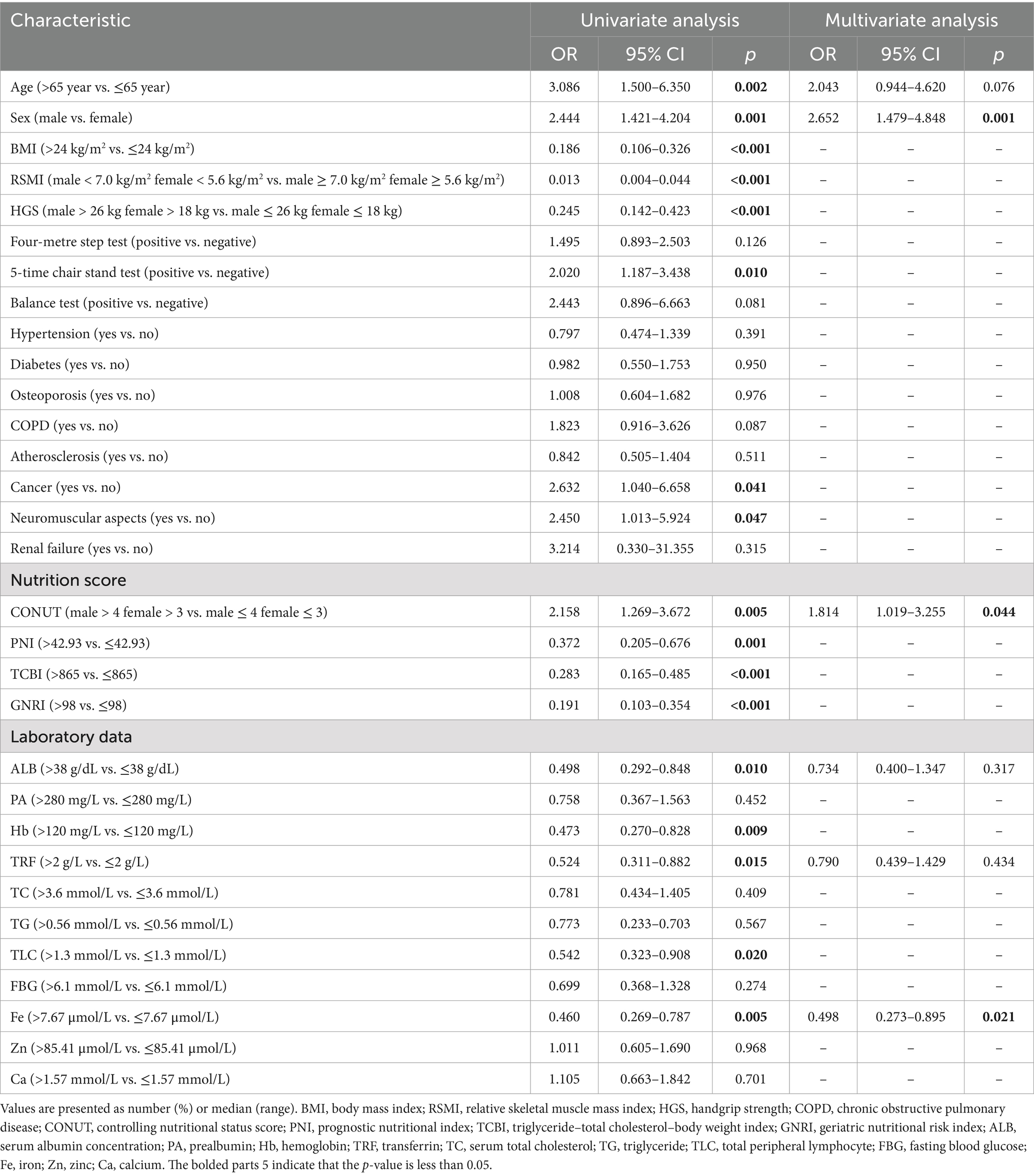
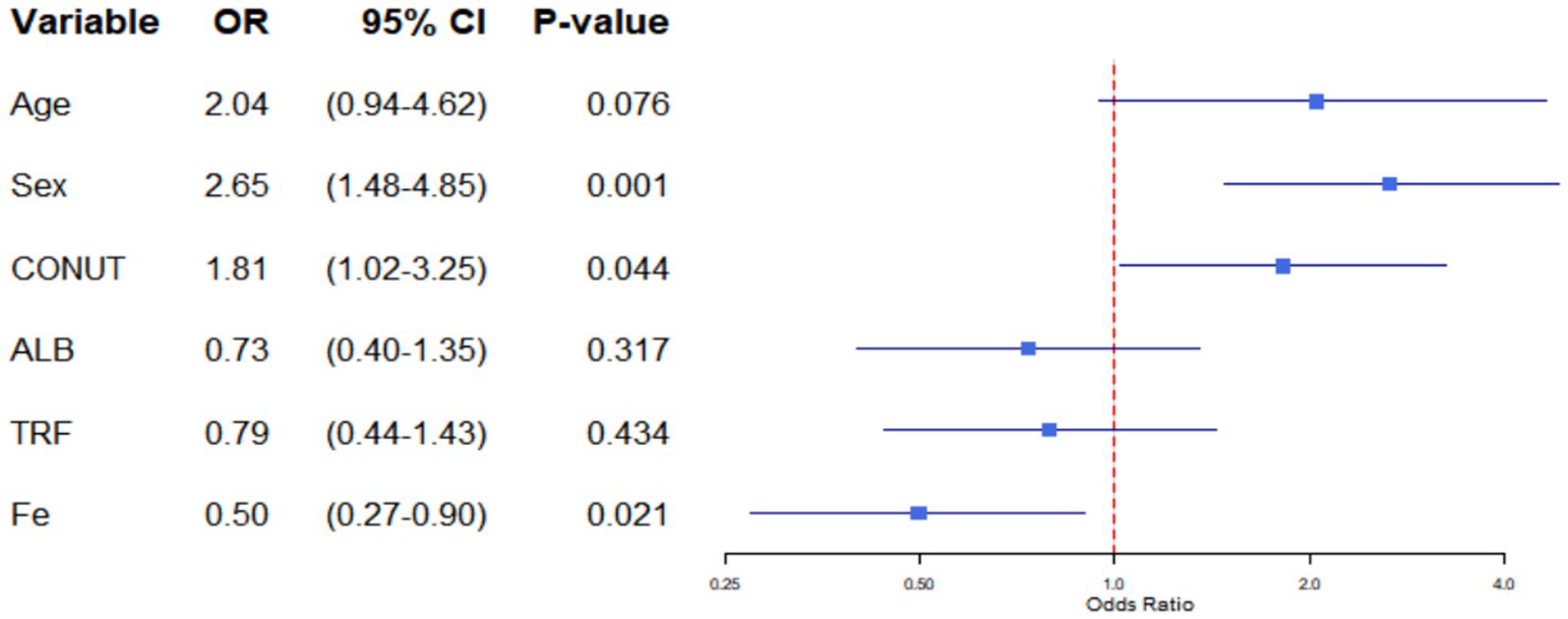
The findings from the univariate and multivariate regression analyses of the 236 patients are displayed in Table 5; Figure 1. In terms of demographics, patients who were male, older (age greater than 65 years), and had a low BMI (BMI ≤ 24 kg/m2) were more likely to develop sarcopenia. On physical examination, low RSMI (<7.0 kg/m2 in male and <5.6 kg/m2 in female), low HGS (<26 kg in men and <18 kg in women), and a positive 5-time chair stand test were positively associated with sarcopenia. However, the results of the balance test and the four-metre step test showed no significant correlation with sarcopenia. Patients with a history of cancer or neuromuscular disease are also more likely to develop sarcopenia. Unexpectedly, no correlation was observed between diabetes, osteoporosis, and sarcopenia. This result differed from that of Izzo et al. (30). In terms of diet, all four commonly used nutritional scores included in this study were associated with sarcopenia. CONUT male > 4, female > 3, PNI ≤ 42.93, TCBI ≤ 865 and GNRI ≤ 98 were found to be risk factors for the development of sarcopenia. Additionally, in terms of laboratory tests, higher ALB, Hb, TRF and TLC were proven to be protective factors for the development of sarcopenia. Similar to the past medical history, fasting blood glucose was not significantly correlated with sarcopenia. Among the trace elements, Fe has been shown to be a risk factor for sarcopenia. However, the Ca and Zn levels were not correlated with sarcopenia.
Variables for multivariate logistic regression were selected by combining the results of univariate logistic regression with theoretical analysis to eliminate confounding factors (Table 5). The multivariate analysis showed that male sex (OR = 2.652, 95% CI: 1.479–4.848, p = 0.001), CONUT male > 4, female > 3 (OR = 1.814; 95% CI: 1.019–3.255; p = 0.044) and Fe ≤ 7.67 mol/L (OR = 0.498; 95% CI: 0.273–0.895; p = 0.021) were independent factors for the development of sarcopenia.
4 Discussion
The risk factors for sarcopenia are currently being investigated. As a result, the effective prediction and prevention of sarcopenia remain a substantial challenge, primarily in the elderly population. This study evaluated the association between novel nutritional indicators and sarcopenia. Our results showed that sarcopenic patients differed from non-sarcopenic patients in terms of BMI and multiple nutritional indices including CONUT, PNI, TCBI, and GNRI. Among these, the CONUT was identified as an independent risk factor for sarcopenia. However, its ability to predict sarcopenia requires further validation (Supplementary Figures 2–4).
Our study demonstrated that a high CONUT score is associated with an increased risk of sarcopenia, though the precise mechanisms underlying this association remain to be fully elucidated. Serum albumin, a key marker of systemic protein reserve, is directly linked to sarcopenia through two pathways supported by sarcopenia-specific observations. First, reduced albumin levels indicate insufficient dietary protein intake or impaired protein utilization—both well-documented risk factors for sarcopenia. A prospective study in community-dwelling older adults showed that hypoalbuminemia (serum albumin <3.8 g/dL) was associated with a 2.3-fold higher risk of incident sarcopenia, likely via limiting muscle protein synthesis (31). Second, hypoalbuminemia disrupts muscle microenvironment integrity by reducing intravascular colloid osmotic pressure, leading to interstitial edema. It might associate with impaired muscle contractility via increased tissue stiffness (32). Also, in patients with sarcopenia, reduced albumin levels and upregulated expression of inflammatory factors further diminish the synthesis of multiple proteins involved in amino acid conservation (33, 34). Notably, these links still require further validation in interventional trials.
Secondly, reduced total lymphocyte count contributes to sarcopenia via inflammatory dysregulation, with direct evidence from sarcopenia cohorts (5, 33, 35, 36). Mechanistically, lymphocyte depletion impairs regulatory T-cell function, which normally restricts excessive pro-inflammatory cytokine release. Previous studies have demonstrated potential causal correlation between IL-10, IP-10, M-CSF, Neutrophil-to-lymphocyte ratio (NLR) and sarcopenia-related traits (37, 38). Thus, reduced lymphocyte counts typically indicate impaired muscle regenerative potential.
Finally, decreased total cholesterol levels reflect caloric depletion and may hinder the synthesis of steroid hormones (39–41). Epidemiological evidence supporting this association comes from a cross-sectional study of 303 adults aged ≥60 years, which demonstrated that serum concentrations of TC, TG, and LDL were significantly lower in the sarcopenia group (p < 0.01) (42). The potential mechanism linking low TC to sarcopenia lies in cholesterol’s role as an essential precursor for testosterone and vitamin D biosynthesis (43–45). Both hormones play non-redundant roles in maintaining skeletal muscle homeostasis: testosterone supports muscle protein synthesis by activating the mTOR signaling pathway, while vitamin D regulates calcium handling in muscle fibers and preserves neuromuscular function.
These findings demonstrate that malnutrition is a potential pathogenic mechanism of sarcopenia. According to Papadopoulou et al. (6), protein and vitamin supplementation is essential for disease prevention. Importantly, leucine-rich proteins play vital roles. The reduction in protein degradation caused by lowering the ubiquitin pathway can be attributed to HMB, a leucine metabolite. This process yields the substrates required for cell membrane repair (46). Furthermore, a prudent dietary pattern may be useful in avoiding sarcopenia. A dietary plan that offers antioxidants may have a constructive impact on muscle sustenance (6). Therefore, we propose the prevention and resolution of sarcopenia from a nutritional perspective with a focus on precision.
Notably, when compared with other frequently employed nutritional indicators, CONUT either demonstrates advantages or exhibits no disadvantages. Hao et al. (47) have found that GNRI is a reliable predictor of sarcopenia in American adults aged 45 and above, but its calculation relies on ideal body weight, which may differ from the actual situation. This dependency makes GNRI vulnerable to confounding in populations with abnormal weight, as obesity and sarcopenia often coexist in older adults, which may obscure the true association between nutrition and sarcopenia. By contrast, the CONUT score is not directly dependent on body weight (unlike BMI, GNRI, and TCBI), thereby avoiding confounding by obesity or weight abnormalities. For example, in our cohort, sarcopenic patients had a significantly lower median BMI (21.60 kg/m2) than non-sarcopenic patients (25.00 kg/m2), but CONUT still independently predicted sarcopenia after adjusting for BMI, confirming its robustness to weight differences. For the PNI, Cheng et al. (48) reported that a higher PNI was associated with a lower incidence of sarcopenia in community-dwelling older adults, but PNI only integrates serum albumin and total lymphocyte count, lacking an indicator for energy metabolism.
In addition, the CONUT score is easy to calculate from a comprehensive blood count, making it an appropriate follow-up test. The preferred CONUT cut-off value differs among various studies (21–24). For gastric cancer, the optimum cut-off is 4 (21), whereas for esophageal cancer, it is 6 (22). However, Dalmiglio et al. (49) chose 3 as the optimal cut-off for predicting the prognosis of patients with advanced thyroid cancer treated with TKI. For patient grouping in this study, we considered a score of 4 for men and 3 for women as the optimal threshold. However, further validation is required to confirm whether this sex-specific CONUT cut-off is applicable to other sarcopenia populations.
Our study did not detect a significant association among diabetes, osteoporosis, and sarcopenia. The underlying reason might be that the elderly hospitalized patients included in our study may be afflicted with multiple acute or chronic conditions. These comorbidities have the potential to obscure the independent impact of diabetes or osteoporosis on sarcopenia. It was further demonstrated that three indicators (age, albumin and transferrin) exhibited statistical significance in univariate logistic regression. However, upon inclusion in multivariate regression, these indicators failed to demonstrate comparable results. Consequently, a multicollinearity analysis was conducted among the variables incorporated into the multivariate regression (Supplementary Table 2). The findings indicated the absence of multicollinearity among the variables, with any observed multicollinearity falling within acceptable limits. It is hypothesised that these three variables may exert confounding effects or mediate actions within the model. However, exploring causal relationships between variables requires a more rigorous experimental design.
However, our study has certain limitations. Firstly, as a single centre retrospective study, the present research is subject to selection bias arising from single centre patient recruitment and information bias stemming from retrospective data collection. This may have consequences for the accuracy and generalizability of our findings. In order to validate the findings in future, it is essential that larger-scale, multicenter prospective studies are conducted. Second, this study focused on the correlation between sarcopenia and nutritional scores (e.g., the CONUT score) in a specific population of older Chinese adults. Nevertheless, as distinct dietary habits and lifestyle patterns across various regions are prominent factors contributing to this disorder, research based on populations from other areas is crucial before generalizing the results of our study.
In conclusion, this study demonstrates that the CONUT score is associated with sarcopenia in elderly hospitalized patients. Additionally, CONUT may serve as a promising indicator for sarcopenia risk assessment. However, additional prospective, multicenter studies are needed to validate its predictive value across diverse populations.
Data availability statement
The datasets presented in this article are not readily available because data privacy policy at our facility, but are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to Zhengli Guo, MzUwODM0NDE2QHFxLmNvbQ==.
Ethics statement
The studies involving humans were approved by The Institutional Review Board (IRB) of First People’s Hospital of Kunshan. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LS: Formal analysis, Investigation, Writing – original draft. YY: Formal analysis, Investigation, Writing – original draft. RY: Investigation, Writing – original draft. BX: Data curation, Writing – review & editing. KZ: Data curation, Writing – review & editing. WZ: Data curation, Writing – original draft. XL: Writing – original draft. YX: Writing – original draft. LL: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. XG: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. ZG: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. MZ: Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Supported in part by Jiangsu Elderly Health Research Project (No. LKM2022058), Kunshan Science Project (No. KS2205), and 2021 Key Youth Medical Science and Technology Innovation Project of Xuzhou Municipal Health Commission, No. XWKYSL20210164.
Acknowledgments
We appreciate the support of First People’s Hospital of Kunshan and the effort of every medical stuff who have treated these patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1669225/full#supplementary-material
References
1. Sayer, AA, and Cruz, J. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. (2022) 51:afac220. doi: 10.1093/ageing/afac220
2. Cruz-Jentoft, AJ, and Sayer, AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
3. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
4. Petermann-Rocha, F, Balntzi, V, Gray, SR, Lara, J, Ho, FK, Pell, JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
5. Chen, L-K, Woo, J, Assantachai, P, Auyeung, T-W, Chou, M-Y, Iijima, K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012
6. Papadopoulou, SK, Papadimitriou, K, Voulgaridou, G, Georgaki, E, Tsotidou, E, Zantidou, O, et al. Exercise and nutrition impact on osteoporosis and sarcopenia-the incidence of Osteosarcopenia: a narrative review. Nutrients. (2021) 13:4499. doi: 10.3390/nu13124499
7. Liu, C, Wong, PY, Chung, YL, Chow, SK, Cheung, WH, and Law, SW. Deciphering the “obesity paradox” in the elderly: a systematic review and meta-analysis of sarcopenic obesity. Obes Rev. (2022) 24:e13534. doi: 10.1111/obr.13534
8. Gao, Q, Hu, K, Yan, C, Zhao, B, Mei, F, and Chen, F. Associated factors of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. Nutrients. (2021) 13:4291. doi: 10.3390/nu13124291
9. Zhang, JZ, Shi, W, Zou, M, Zeng, QS, Feng, Y, and Luo, ZY. Diagnosis, prevalence, and outcomes of sarcopenia in kidney transplantation recipients: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 14:17–29. doi: 10.1002/jcsm.13130
10. Anagnostis, P, Gkekas, NK, Achilla, C, Pananastasiou, G, Taouxidou, P, Mitsiou, M, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int. (2020) 107:453–63. doi: 10.1007/s00223-020-00742-y
11. Ai, Y, Xu, R, and Liu, L. The prevalence and risk factors of sarcopenia in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. (2021) 13:93. doi: 10.1186/s13098-021-00707-7
12. Ran, S, Yao, J, and Liu, B. The association between sarcopenia and cirrhosis: a Mendelian randomization analysis. Hepatobiliary Surg Nutr. (2023) 12:291–3. doi: 10.21037/hbsn-22-632
13. Xing, J, Wan, X, Sang, X, Yang, H, and Du, S. Perioperative management of patients with liver cancer complicated with sarcopenia. Hepatobiliary Surg Nutr. (2022) 11:906–8. doi: 10.21037/hbsn-22-485
14. Papadopoulou, SK. Sarcopenia: a contemporary health problem among older adult populations. Nutrients. (2020) 12:1293. doi: 10.3390/nu12051293
15. Landi, F, Cesari, M, Calvani, R, Cherubini, A, Bari, M, Bejuit, R, et al. The “sarcopenia and physical FRailty IN older people: multi-ComponenT treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res. (2017) 29:89–100. doi: 10.1007/s40520-016-0715-2
16. Ganapathy, A, and Nieves, JW. Nutrition and sarcopenia—what do we know? Nutrients. (2020) 12:1755. doi: 10.3390/nu12061755
17. Devries, MC, McGlory, C, Bolster, DR, Kamil, A, Rahn, M, Harkness, L, et al. Not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J Nutr. (2018) 148:1088–95. doi: 10.1093/jn/nxy091
18. Liao, CD, Chen, HC, Huang, SW, and Liou, TH. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: a systematic review and Meta-regression analysis of randomized trials. Nutrients. (2019) 11:1713. doi: 10.3390/nu11081713
19. Miano, N, Todaro, G, Di Marco, M, Scilletta, S, Bosco, G, Di Giacomo Barbagallo, F, et al. Malnutrition-related liver steatosis, CONUT score and poor clinical outcomes in an internal medicine department. Nutrients. (2024) 16:1925. doi: 10.3390/nu16121925
20. Li, W, Li, M, Wang, T, Ma, G, Deng, Y, Pu, D, et al. Controlling nutritional status (CONUT) score is a prognostic factor in patients with resected breast cancer. Sci Rep. (2020) 10:6633. doi: 10.1038/s41598-020-63610-7
21. Kuroda, D, Sawayama, H, Kurashige, J, Iwatsuki, M, Eto, T, Tokunaga, R, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. (2018) 21:204–12. doi: 10.1007/s10120-017-0744-3
22. Takagi, K, Buettner, S, Ijzermans, JNM, and Wijnhoven, BPL. Systematic review on the controlling nutritional status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res. (2020) 40:5343–9. doi: 10.21873/anticanres.14541
23. Demir, M, and Demircan, NC. The CONUT score is prognostic in esophageal cancer treated with chemoradiotherapy. Saudi J Gastroenterol. (2023) 29:119–26. doi: 10.4103/sjg.sjg_384_22
24. Peng, L, Meng, C, Li, J, You, C, Du, Y, Xiong, W, et al. The prognostic significance of controlling nutritional status (CONUT) score for surgically treated renal cell cancer and upper urinary tract urothelial cancer: a systematic review and meta-analysis. Eur J Clin Nutr. (2022) 76:801–10. doi: 10.1038/s41430-021-01014-0
25. Jiang, T-T, Zhu, X-Y, Yin, Y-W, Liu, H-J, and Zhang, G-Y. The prognostic significance of malnutrition in older adult patients with acute ischemic stroke. Front Nutr. (2025) 12:12. doi: 10.3389/fnut.2025.1529754
26. Zhang, X, Yang, L, Wang, Z, Wang, H, Nie, S, Zhao, C, et al. Associations between nutritional status and cognitive impairment in older adults: results from the NHANES 2011–2014 cycles. Front Nutr. (2025) 12:12. doi: 10.3389/fnut.2025.1571990
27. Söner, S, Güzel, T, Aktan, A, Kılıç, R, Arslan, B, Demir, M, et al. Predictive value of nutritional scores in non-valvular atrial fibrillation patients: insights from the AFTER-2 study. Nutr Metab Cardiovasc Dis. (2025) 35:103794. doi: 10.1016/j.numecd.2024.103794
28. Yao, N, Hou, Q, and Zhang, S. Prognostic nutritional index, another prognostic factor for extranodal natural killer/T cell lymphoma, nasal type. Front Oncol. (2020) 10:877. doi: 10.3389/fonc.2020.00877
29. Ishiwata, S, Yatsu, S, and Kasai, T. Prognostic effect of a novel simply calculated nutritional index in acute decompensated heart failure. Nutrients. (2020) 12:3311. doi: 10.3390/nu12113311
30. Bouillanne, O, Morineau, G, and Dupont, C. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
31. Kitamura, A, Seino, S, Abe, T, Nofuji, Y, Yokoyama, Y, Amano, H, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. (2021) 12:30–8. doi: 10.1002/jcsm.12651
32. Dull, RO, and Hahn, RG. Hypovolemia with peripheral edema: what is wrong? Crit Care. (2023) 27:206. doi: 10.1186/s13054-023-04496-5
33. Picca, A, Coelho-Junior, HJ, Calvani, R, Marzetti, E, and Vetrano, DL. Biomarkers shared by frailty and sarcopenia in older adults: a systematic review and meta-analysis. Ageing Res Rev. (2022) 73:101530. doi: 10.1016/j.arr.2021.101530
34. Health, Aging and Body Composition StudyVisser, M, Kritchevsky, SB, Newman, AB, Goodpaster, BH, Tylavsky, FA, et al. Lower serum albumin concentration and change in muscle mass: the health, aging and body composition study. Am J Clin Nutr. (2005) 82:531–7. doi: 10.1093/ajcn/82.3.531
35. Al-Dabbagh, S, McPhee, JS, Murgatroyd, C, Butler-Browne, G, Stewart, CE, and Al-Shanti, N. The lymphocyte secretome from young adults enhances skeletal muscle proliferation and migration, but effects are attenuated in the secretome of older adults. Physiol Rep. (2015) 3:e12518. doi: 10.14814/phy2.12518
36. Brook, MS, Wilkinson, DJ, Phillips, BE, Perez-Schindler, J, Philp, A, and Smith, K. Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise. Acta Physiol. (2016) 216:15-41. doi: 10.1111/apha.12532
37. Nie, H, Liu, Y, Zeng, X, and Chen, M. Neutrophil-to-lymphocyte ratio is associated with sarcopenia risk in overweight maintenance hemodialysis patients. Sci Rep. (2024) 14:3669. doi: 10.1038/s41598-024-54056-2
38. Wang, J, Xiang, Y, Wu, L, Zhang, C, Han, B, Cheng, Y, et al. The association between inflammatory cytokines and sarcopenia-related traits: a bi-directional Mendelian randomization study. Eur J Clin Nutr. (2024) 78:1032–40. doi: 10.1038/s41430-024-01486-w
39. Shinchuck, L, and Holick, MF. Vitamin D and rehabilitation: improving functional outcomes. Nutr Clin Pract. (2007) 22:297–304. doi: 10.1177/0115426507022003297
40. Garcia, LA, King, KK, Ferrini, MG, Norris, KC, and Artaza, JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. (2011) 152:2976–86. doi: 10.1210/en.2011-0159
41. Angeline, ME, Gee, AO, Shindle, M, Warren, RF, and Rodeo, SA. The effect of vitamin D deficiency in athletes. Am J Sports Med. (2013) 41:461–4. doi: 10.1177/0363546513475787
42. Jiang, Y, Xu, B, Zhang, K, Zhu, W, Lian, X, Xu, Y, et al. The association of lipid metabolism and sarcopenia among older patients: a cross-sectional study. Sci Rep. (2023) 13:17538. doi: 10.1038/s41598-023-44704-4
43. Widajanti, N, Hadi, U, Soelistijo, SA, Syakdiyah, NH, Rosaudyn, R, and Putra, HBP. The effect of vitamin D supplementation to parameter of sarcopenia in elderly people: a systematic review and meta-analysis. Can Geriatr J. (2024) 27:63–75. doi: 10.5770/cgj.27.694
44. Shigehara, K, Kato, Y, Izumi, K, and Mizokami, A. Relationship between testosterone and sarcopenia in older-adult men: a narrative review. J Clin Med. (2022) 11:6202. doi: 10.3390/jcm11206202
45. Choi, S, Chon, J, Yoo, MC, Shim, GY, Kim, M, Soh, Y, et al. The association of free testosterone with sarcopenic obesity in community-dwelling older men: a cross-sectional study. Medicina. (2024) 60:754. doi: 10.3390/medicina60050754
46. Deutz, NEP, Bauer, JM, Barazzoni, R, Biolo, G, Boirie, Y, Bosy-Westphal, A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr. (2014) 33:929–36. doi: 10.1016/j.clnu.2014.04.007
47. Hao, W, Huang, X, Liang, R, Yang, C, Huang, Z, Chen, Y, et al. Association between the geriatric nutritional risk index and sarcopenia in American adults aged 45 and older. Nutrition. (2025) 131:112628. doi: 10.1016/j.nut.2024.112628
48. Cheng, L, Hu, S, and Wu, Q. Higher prognostic nutritional index is associated with lower incidence of sarcopenia. Geriatr Nurs. (2025) 63:499–504. doi: 10.1016/j.gerinurse.2025.03.046
Keywords: Controlling Nutritional Status (CONUT), sarcopenia, aging, risk factor, nutritional status
Citation: Sun L, Yang Y, Yan R, Xu B, Zhang K, Zhu W, Lian X, Xu Y, Liu L, Gao X, Guo Z and Zhou M (2025) Higher Controlling Nutritional Status (CONUT) score indicates increased risk of sarcopenia in elderly hospitalized patients: a single institution study in China. Front. Nutr. 12:1669225. doi: 10.3389/fnut.2025.1669225
Edited by:
Adriyan Pramono, Diponegoro University, IndonesiaReviewed by:
Botang Guo, Shenzhen University, ChinaSerdar Soner, Diyarbakır Gazi Yaşargil Training and Research Hospital, Türkiye
Copyright © 2025 Sun, Yang, Yan, Xu, Zhang, Zhu, Lian, Xu, Liu, Gao, Guo and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuming Gao, MTQzNjQ3MzkzNEBxcS5jb20=; Zhengli Guo MzUwODM0NDE2QHFxLmNvbQ==; Mingqin Zhou emhvdW1pbmdxaW5zZXVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Leying Sun1,2†
Leying Sun1,2† Ruiyi Yan
Ruiyi Yan Bingqing Xu
Bingqing Xu Yihui Xu
Yihui Xu Xiuming Gao
Xiuming Gao Mingqin Zhou
Mingqin Zhou