- 1Renal Division, Department of Medicine, Heilongjiang Academy of Chinese Medicine Sciences, Harbin, China
- 2Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Diabetic kidney disease (DKD) is one of the leading causes of end-stage renal disease worldwide, and lipid metabolism disorder is a key factor in accelerating its progression. Among them, the impaired fatty acid oxidation (FAO) function of renal tissue constitutes one of the core pathological links of lipid metabolism disorders. In DKD, impaired FAO function can directly lead to lipid accumulation, mitochondrial stress, and trigger an inflammatory cascade, thereby promoting the occurrence and development of glomerulosclerosis and renal tubular injury. However, the efficacy of current DKD treatment strategies is still limited. Natural compounds (such as polyphenols, phenolic acids, alkaloids, glycosides, and carotenoids) have shown potential in renal protection due to their multi-target and multi-pathway characteristics. Studies have shown that regulating the FAO process in the context of lipid metabolism disorders is a crucial mechanism by which natural compounds can exert anti-DKD effects. It is worth noting that peroxisome proliferator-activated receptors (PPARs) are core transcription factors that regulate FAO. Specifically, these active ingredients can upregulate the expression of their downstream target genes by activating the PPAR signaling pathway (especially PPARα), thereby improving FAO function, correcting abnormal lipid metabolism, and ultimately delaying the progression of renal pathological mechanisms such as inflammation and fibrosis. The above findings provide an essential scientific basis for the development of new, safe, and effective DKD therapeutic drugs.
1 Introduction
Diabetic Kidney Disease (DKD), one of the most common microvascular complications of diabetes, affects approximately 30–40% of diabetic patients who will ultimately develop the condition during the later stages of their disease (1, 2). DKD is characterized by persistent proteinuria and progressive renal dysfunction, with its pathological changes including renal enlargement, thickening of the basement membrane in the glomeruli, cell damage in the podocytes, as well as destruction of glomerular and tubular structures. Ultimately, it can progress to end-stage renal disease (3, 4). The pathogenesis of DKD involves multiple factors, such as the activation of inflammatory responses, imbalance of oxidative stress, and dysregulation of lipid metabolism, among which the nephrotoxicity caused by lipid accumulation has been confirmed as a key driving factor accelerating renal fibrosis (5–7). At present, the treatment of DKD focuses on comprehensive management strategies, including blood glucose control, blood pressure management, blood lipid regulation, and diet and lifestyle interventions (8). As of now, the drugs approved in the FDA database for delaying the progression of DKD mainly focus on RAAS inhibitors, SGLT2 inhibitors, and non-steroidal MRAs. They can improve glomerular permeability and enhance insulin sensitivity, with apparent renal and cardiovascular protective effects, and have become a standard component in the treatment of DKD (9). Among them, dapagliflozin and empagliflozin have their indications expanded to the CKD population (10). Finerenone, as the first approved non-steroidal MRA, has new capabilities in anti-inflammation and anti-fibrosis (11). Although these drugs have been proven to play a stable role in the treatment of DKD and are widely used in clinical practice, these treatments still have certain limitations (12).
Lipid metabolism disorder is not only an important driving factor for systemic diseases such as hypercholesterolemia, hypertriglyceridemia, diabetes, and atherosclerosis, but also plays a key role in the occurrence and development of DKD (13). The progression of DKD is characterized by significant lipid disorders, which are characterized by abnormal accumulation of lipids such as free fatty acids, diacylglycerols, and ceramides in kidney tissue, thereby triggering lipotoxicity. Hypoxia in the renal microenvironment inhibits fatty acid oxidation and promotes lipid synthesis through the HIF-1α pathway, while chronic inflammation further aggravates lipid metabolism disorders through inflammatory cytokines, inflammasomes and macrophage polarization (14). In addition, lipid metabolism may also involve mechanisms such as cell ferroptosis, lipid metabolism reprogramming, and immune regulation of intestinal microbiota (15). The damage to renal tubular epithelial cells (TECs) is the core link in the progression of DKD. Its intracellular lipid accumulation can inhibit mitochondrial fatty acid oxidation (FAO), resulting in reduced ATP synthesis and cell energy failure, thereby accelerating the deterioration of renal function. In podocytes, lipid deposition can directly induce cytoskeletal rearrangement, shedding, and apoptosis, thereby accelerating the process of fibrosis (16). The process of FAO is crucial in lipid metabolism, providing energy for cell function and participating in the inflammatory response of cells (17). In addition, the role of FAO in DKD has been confirmed by research (18, 19). Mitochondrial FAO is the main pathway of fatty acid (FA) degradation and is essential for maintaining human energy homeostasis. At the same time, FAO is the main energy source for the heart, skeletal muscle, and kidney. Inhibition of FAO can destroy the balance between the synthesis, uptake and consumption of fatty acids (FA) in renal cells through transcriptional regulation (such as STAT6-PPARα axis), destruction of renal microenvironment homeostasis (hypoxia, inflammation), and triggering mitochondrial dysfunction, eventually leading to abnormal lipid accumulation and renal injury (20, 21). Peroxisome proliferator-activated receptors (PPARs) belong to the ligand-activated nuclear receptor family and are key regulators of FAO. They can upregulate the expression of FAO-related genes such as carnitine palmitoyl transferase I (CPT1) and medium-chain acyl-CoA dehydrogenase (MCAD). PPARγ dominates lipid storage and homeostasis, while PPARα promotes fatty acid decomposition (22). Recent studies have found that renal lipid metabolism disorders in diabetic patients can aggravate insulin resistance and glomerulosclerosis. PPARα agonists can reduce lipid accumulation by activating the FAO pathway, thereby improving renal injury (23). Intervention with PPARα agonists, such as fenofibrate and pemafibrate, has been shown to restore the expression levels of FAO-related genes [e.g., carnitine palmitoyltransferase 1A (CPT1A), acyl-CoA oxidase 1 (ACOX1)] in animal models, while simultaneously improving mitochondrial homeostasis and alleviating renal injury (24, 25). On the other hand, although PPARγ agonists like pioglitazone exert reno-protective effects by improving mitochondrial function, reducing oxidative stress levels, and alleviating renal tubulointerstitial fibrosis, direct evidence regarding their ability to directly restore FAO function in renal tubular cells remains relatively limited (26). Notably, activation of both PPARα and PPARγ pathways via PPARα/γ dual agonists (e.g., tesaglitazar) or the combination of fenofibrate and pioglitazone can exhibit synergistically enhanced reno-protective effects, specifically including reducing proteinuria excretion, inhibiting renal inflammatory responses, and delaying the progression of fibrosis (27, 28). In summary, these findings clearly highlight the value of PPARα and PPARγ as key therapeutic targets for regulating renal lipid metabolism disorders in DKD. Targeting PPAR subtypes or combined regulation is expected to be a potential strategy to enhance DKD metabolic damage and pathological progression.
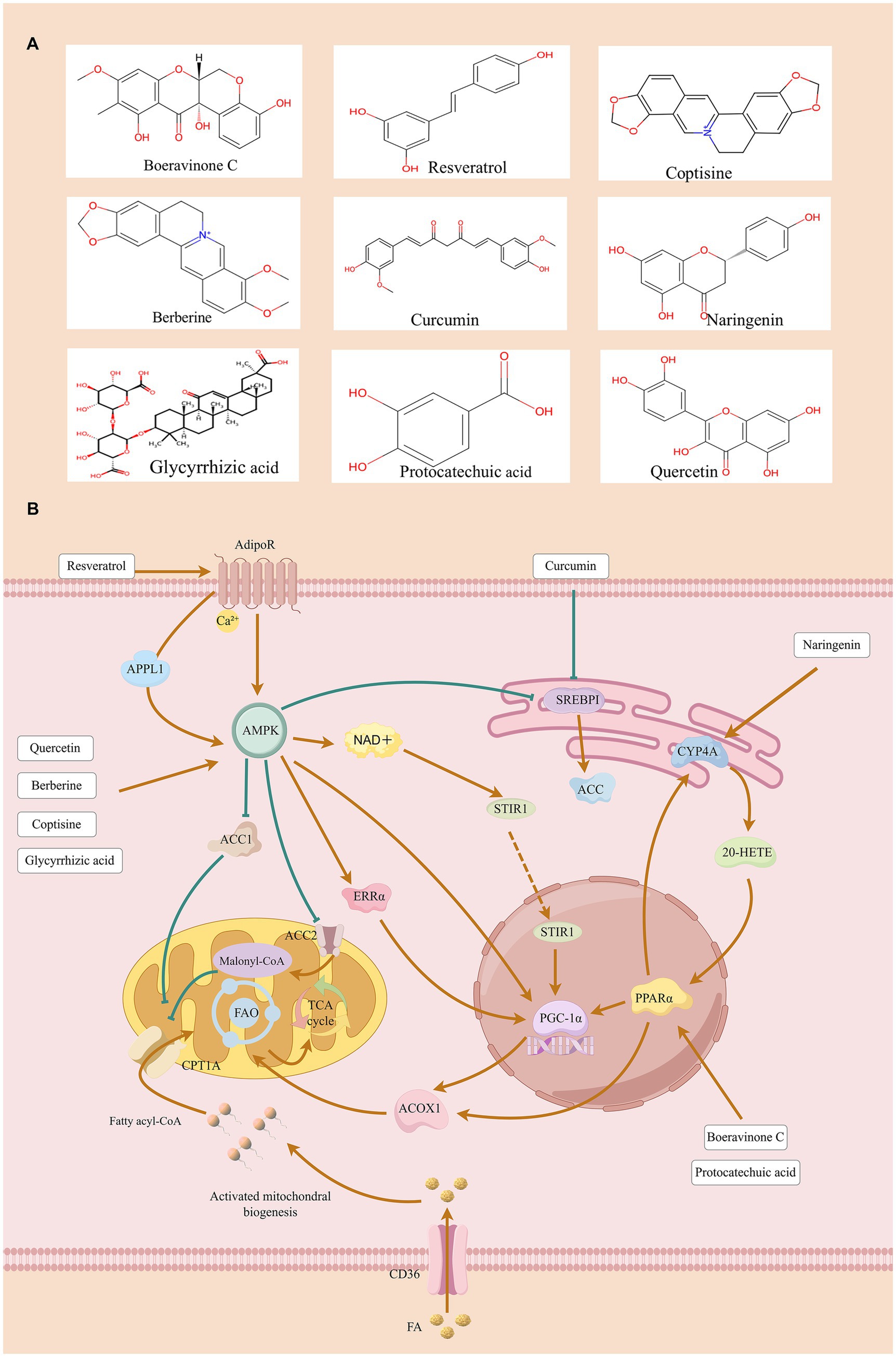
Natural compounds have structurally clear chemical formulas, play a crucial role in drug discovery, and often exhibit multi-target synergistic effects in disease treatment (29). Natural compounds account for a significant portion of drugs approved by the FDA. For instance, in the field of oncology, half of the small-molecule drugs approved over the past 60 years are either natural compounds or directly derived from natural products (30). Modern pharmacological research and clinical trials have initially confirmed the potential of natural compounds in the treatment of DKD (31, 32). These active ingredients have a variety of pharmacological activities such as anti-inflammatory, anti-oxidative stress, anti-fibrosis, and regulation of lipid metabolism. They can inhibit pathological processes such as ferroptosis and autophagy, thereby improving the pathological state of DKD (33–35). It is worth noting that natural components can also correct lipid metabolism disorders in DKD by specifically regulating the activity of FAO key enzymes and related upstream and downstream signaling pathways (36). Therefore, this article aims to summarize the molecular mechanism of natural active ingredients improving DKD by targeting the FAO process to promote the discovery of DKD treatment. The relevant core mechanisms are summarized in Table 1 and shown in Figure 1. We believe that although there are deficiencies in research design and clinical trials, naturally active ingredients have a good prospect in the clinical transformation of DKD treatment.
2 Natural compounds regulating FAO to improve DKD
In this review, we searched PubMed for articles published from inception to June 29, 2025. Literature search was performed according to the following search terms (DKD or DN or Diabetic nephropathy or Diabetic kidney disease) and (singular or herbs or Natural ingredients or Natural produce or Natural compounds) and (PPARs or PPAR or FAO or Oxidation of fatty acid). Our inclusion criteria mainly include: Cell or animal experimental studies in the DKD model; Intervention treatment using natural active ingredients; and only retrieve English literature. The time range is from the establishment of the PubMed database to June 2025; Only experimental studies are included, excluding reviews and commentaries. A total of 10 studies were eligible, involving 9 different natural active ingredients. Use ClinicalTrials.gov, International Clinical Trials Registry Platform (ICTRP), China Clinical Trial Registry (ChiCTR), and European Union Clinical Trials Register (EUCTR) to monitor the clinical trials involved. We summarized the natural compounds used to treat DKD by regulating FAO, and divided them into polyphenols, phenolic acids, alkaloids, glycosides, and carotenoids according to their structural characteristics.
2.1 Polyphenols
Polyphenols are a class of natural compounds that are widely present in food. They are characterized by the presence of at least two phenolic groups associated with more or less complex structures, usually with high molecular weight (37). Research shows that polyphenols can exert anti-inflammatory, antioxidant, and lipid metabolism-regulating effects by inhibiting the JNK and NF-κB inflammatory signaling pathways, as well as activating the AKT/AS160/GLUT4 pathway, thereby improving metabolic function disorders (38). Epidemiological studies indicate that consuming high levels of natural polyphenols in the diet is linked to a decreased risk of various chronic illnesses, such as cardiovascular disease, type 2 diabetes, and chronic kidney disease (39). Notably, recent studies have demonstrated that polyphenols can regulate PPARα to influence energy storage, adipogenesis, upregulation of fatty acid metabolism, and β-oxidation processes in tissues. They can also engage PPARβ/δ to participate in the uptake and β-oxidation of fatty acids (FA) in muscle and white adipose tissue. Additionally, PPARγ mediates energy storage-adipogenesis, glucose metabolism, and inflammatory responses in white adipose tissue (WAT) and macrophages. The synergistic effects of the aforementioned PPAR subtypes collectively ameliorate the state of renal lipid metabolism disorders in patients with DKD (40).
Resveratrol (RV) is a common polyphenolic compound found in various plants such as grapes and berries, playing an essential role in the treatment of kidney diseases. Mechanism studies have shown that RV inhibits the SphK1-NF-κB inflammatory cascade and enhances LC3-II/Beclin1-mediated autophagic flux by activating the SIRT1 signaling pathway, thereby exerting a renal protective effect (41). At the level of lipid metabolism, RV can regulate the process of fat accumulation, promote fatty acid oxidation and lipolysis, and inhibit a variety of renal injury and fibrosis processes (42). However, resveratrol exhibits characteristics common to most natural active ingredients in terms of pharmacokinetics, namely low bioavailability and rapid metabolism (43). A double-blind trial of patients with type 2 diabetes and coronary heart disease confirmed that RV can upregulate the expression levels of PPARγ and SIRT1 in peripheral blood mononuclear cells (PBMCs) (44). In the DKD model, RV activates the AMPK/SIRT1/PPARα signaling axis by activating adiponectin receptors (AdipoR1/AdipoR2), induces AMPK phosphorylation, and enhances SIRT1/PGC-1α signaling, thereby regulating the downstream effector molecule PPARα/ERR-1α/SREBP1. Finally, RV protects the kidney by improving oxidative stress, inhibiting apoptosis, and increasing circulating adiponectin levels (45, 46). Although the vast majority of animal studies support that resveratrol can ameliorate renal pathology in DKD mice, conflicting results exist regarding its effects on key signaling pathways (AMPK/SIRT1). In dose-related studies, the effect of resveratrol exhibits a U-shaped curve, which supports the need for more refined dose–response clinical studies in the future (47).
Turmeric is a kind of Curcuma plant that is widely planted in Southeast Asia. The main active ingredient is curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) (48). As a lipophilic polyphenol classified as curcuminoids, it has anti-inflammatory and antioxidant effects (49). Recent studies have shown that curcumin can alleviate inflammation in chronic kidney disease (CKD) by reducing chemokine 2 (CCL-2), interferon-γ (IFN-γ), and interleukin-4 (IL-4) and regulating lipid peroxidation (50). Preclinical data from animal models and Phase I clinical studies conducted on human volunteers and cancer patients indicate that the systemic bioavailability of orally administered curcumin is low and exhibits rapid metabolic rates (51). A meta-analysis related to curcumin showed that curcumin treatment can significantly reduce serum creatinine (Scr), total cholesterol (TC), and fasting blood glucose (FBG) in patients with DKD, demonstrating potential efficacy (52). But it also found no statistically significant impact on indicators such as BUN and proteinuria, suggesting that its efficacy may be limited to specific pathological processes (52). Curcumin treatment of the db/db mice model can effectively reduce chemokine ligand CXCL16, sterol regulatory element binding protein 1 (SREBP1), SREBP2, and adipose differentiation-related protein (ADRP), and upregulate the expression of PPARγ in renal cortex. Studies have shown that curcumin can improve lipid metabolism disorders in podocytes and reduce glomerular lipid deposition through the CXCL16-PPARγ signaling pathway, thereby reducing the pathological damage of DKD (53).
Quercetin [Ka(3,3′,4′,5,7-Pentahydroxyflavone)] is a representative substance of flavonols (54). It is widely found in many plants and is known for its significant antioxidant, anti-inflammatory, and anti-diabetic properties (55). Studies have shown that quercetin can activate adipose tissue signal transduction, promote fatty acid β-oxidation, and reduce lipid deposition by up-regulating the expression of PPARα (56). It also down-regulated the expression of PPARγ and inhibited the activity of inflammatory factors mediated by the MAPK signaling pathway (57). Quercetin exhibits low bioavailability and extensive first-pass metabolism in pharmacokinetics. Animal studies have shown that after intravenous injection of quercetin in rats, it is widely distributed in the kidneys, liver, heart, and brain tissues (58). Only one clinical trial has shown that quercetin can reduce blood pressure in patients with stage 1 hypertension (59). We have noted that there are inconsistencies in the conclusions of existing literature regarding the mechanism of quercetin in relation to the PPAR signaling pathway. An early biochemical study demonstrated that quercetin and related lipoxygenase inhibitors can bind to the ligand-binding domain of PPAR, thereby inhibiting PPAR-mediated transcriptional processes in primary keratinocytes. This finding suggests that PPAR may be directly antagonized under certain conditions (60). In contrast, multiple subsequent studies on metabolic tissues and disease models have shown that quercetin can activate the LKB1-AMPK-SIRT1 signaling axis, thereby promoting FAO and upregulating the expression of PPARα-regulated targets (e.g., CPT1) (56, 61, 62). Furthermore, a study using a DKD model reported that the combination of Dasatinib and quercetin (DQ combination) can improve renal FAO function. Subsequent mechanistic studies, via molecular docking and genetic manipulation, have confirmed that the DQ combination promotes the FAO process by specifically upregulating the expression of key FAO enzymes CPT1A and ACOX1—through targeted activation of PPARα. Meanwhile, this combination also significantly reduces the expression of myofibroblast activation marker (α-SMA) and extracellular matrix proteins, providing experimental evidence for the involvement of the DQ combination in the restoration of PPARα-dependent FAO function in renal tissues (63). The above findings suggest that the effect of quercetin on PPARs appears to be dependent on the specific cellular or tissue context: in certain non-metabolic cells, it exhibits an inhibitory effect on PPARs, whereas in renal tissues, it promotes FAO by activating AMPK/PPARα-related pathways.
Naringenin (2, 3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a flavonoid compound widely found in the peel of citrus fruits (64). Its core biological functions include alleviating oxidative stress, improving lipid metabolism disorders, and thus exerting renal protective effects (65). Existing studies have shown that the compound can significantly improve the disease phenotype of mice models of hyperuricemia and acute kidney injury by reducing serum uric acid levels and alleviating pathological changes of renal fibrosis (66, 67). Molecular mechanism studies further revealed that naringenin inhibited the expression of SREBP-1c through activation of the AMPK signaling pathway, thereby reducing adipogenesis, while up-regulating the expression of PPARα and promoting fatty acid oxidation, thereby improving metabolic disorders and renal lipid metabolism (68–70). Research has found that the bioavailability of naringenin in rat and dog models is 44.1 and 34.4% respectively, indicating a significant first-pass effect; while in humans, it shows a longer half-life (approximately 48 h) and a notable gender difference in single-dose administration (71). Currently, the 10 registered clinical trials are insufficient to provide clear conclusions, mainly limited by the lack of pharmacokinetic data on naringenin, poor chemical stability, and complex metabolism (72). In studies related to diabetic models, the therapeutic effects of naringenin have shown contradictions, with conflicting conclusions reported across different studies. On one hand, high-dose naringenin can significantly reduce blood glucose levels, and its hypoglycemic effect is comparable to that of insulin (73). On the other hand, a study by Xulu demonstrated that naringin (50 mg/kg) exerts no significant hypoglycemic effect on streptozotocin (STZ)-induced type 1 diabetic rats, but only improves their dyslipidemia and atherosclerosis index (74). Despite the aforementioned controversies, the therapeutic value of naringenin in DKD has been validated by multiple experiments. Specifically, it can effectively reduce proteinuria levels and alleviate the degree of renal fibrosis in model animals. Naringenin can directly up-regulate PPARα/PPARγ protein expression through activation of the CYP4A-20-HETE pathway, and at the same time inhibit the activity of pro-fibrotic factors, such as transforming growth factor-β (TGF-β), and interleukin-1 (IL-1), and ultimately improve the state of lipid metabolism disorders (75).
Oxybaphus himalaicus Edgew is a traditional Tibetan medicine approved by the Chinese government. There are still a few studies on modern pharmacology, and the main components and mechanism of action are not completely clear (76). Existing studies have shown that the ethanol extract of this plant can improve DKD by regulating the PPAR signaling pathway (77). Notably, Boeravinone C, a novel natural monomer isolated from O. himalaicus, showed bidirectional regulation in DKD mice models and human renal tubular epithelial cells (HK-2) experiments: On the one hand, it activates PPARα transcription factor, enhances FAO ability, and reduces renal tubular lipid accumulation; on the other hand, it inhibits the NF-κB inflammatory pathway and reduces the inflammatory response. The above synergistic effect finally reduces the apoptosis and fibrosis process of renal tubular cells and improves the pathological damage of DKD (78).
Polyphenols can regulate the FAO process of renal tubular cells in DKD by activating PPARα/γ nuclear receptors; at the same time, they can reduce oxidative stress injury and inhibit inflammatory response, thereby delaying the process of renal fibrosis.
2.2 Phenolic acids
Phenolic acids are a type of natural phenolic compounds found in various plant-based foods, existing in forms such as amides, esters, or glycosides, and possessing multiple biological activities. Experiments have shown that phenolic acids can regulate lipid metabolism in liver cells by modulating the AMPK, SREBP1, and ACC signaling pathways, and exert anti-inflammatory effects by regulating the TLR, NF-κB, and NLRP3 pathways (79). Epidemiological studies show that phenolic acids have properties including but not limited to anticancer, cardioprotective, anti-inflammatory, immunomodulatory, and anti-obesity effects (80). Existing studies have shown that phenolic acids can improve renal fibrosis in the DKD model mice and regulate autophagy, oxidative stress, and related lipid metabolism disorders, particularly through the FAO process.
Protocatechuic acid (PCA), also known as 3,4-dihydroxybenzoic acid, is a phenolic acid compound that naturally exists in a variety of vegetables and fruits. It is also the main active ingredient of traditional Chinese medicine, such as Salvia miltiorrhiza and Hibiscus (81). Mechanism studies have shown that PCA improves metabolic disorders by activating the AMPK/SIRT1 signaling pathway, enhancing mitochondrial biogenesis, and inhibiting NF-κB-mediated inflammatory response (82). Its renal protective effect has been confirmed in a variety of experimental models, which can effectively exert multiple effects such as anti-inflammatory, anti-oxidation, and anti-bacterial (81). Pharmacokinetic studies show that PCA has difficulty penetrating the skin barrier and the blood–brain barrier, with a moderate plasma protein binding rate, but good metabolic stability (83). In the rat model of kidney injury, the absorption of PCA was accelerated and its elimination was slowed, indicating that renal function impairment altered its pharmacokinetic characteristics (84). Existing research has not involved human clinical trials related to the treatment of kidneys with protocatechuic acid, focusing only on rat models. It was found that PCA could restore the mRNA expression levels of PPARα and PPARγ in renal tissue, reduce the concentrations of advanced glycation end product receptor (RAGE), advanced glycation end product (AGEs) and glycated albumin, and promote the reconstruction of PPARγ expression in diabetic mice, improve renal metabolic homeostasis and reduce DKD damage (85). Furthermore, multiple studies have demonstrated that PCA can increase the level of FAO in metabolic tissues: specifically, dietary supplementation with PCA enhances the protein abundance and enzymatic activity of CPT1 in the liver and adipose tissue, and relevant mechanistic studies have been conducted in hepatic cell models (86, 87). However, to date, no studies have directly evaluated whether PCA can restore or enhance FAO function in renal tubular cells or DKD models. Given the core regulatory role of renal tubular FAO in the pathophysiological progression of DKD, in-depth exploration of the tissue-specific regulatory effect of PCA on FAO in renal tissues holds significant research value and feasibility, with potential prospects for clinical translation. Finally, although PCA is considered to be a relatively safe natural phenolic acid PCA is considered a relatively safe natural phenolic acid, systematic toxicological studies, long-term safety evaluations, and research on drug metabolism interactions (CYP/UGT) are still required before its application in DKD.
2.3 Alkaloid
Alkaloids are secondary metabolites widely found in plants, characterized by unique cyclic structures containing nitrogen atoms and extensive biological activity, playing an indispensable role in the human body and the field of medicine. Under alkaline conditions, alkaloids can exhibit lipid membrane permeability and are often used as various clinical drug formulations to treat diseases such as cancer, rheumatoid arthritis, and cardiovascular issues (88). Research has found that alkaloids such as sanguinarine can affect cell proliferation and invasion activity through the JAK/STAT signaling pathway (89). Papaverine can upregulate the levels of cAMP and cGMP in cells, relaxing vascular smooth muscle (90). Eriocitrin can influence platelet activation by regulating the PI3K/Akt/GSK3β signaling pathway (91). In recent years, research has found that berberine, as an alkaloid, can play a role in the treatment of DKD by affecting lipid metabolism.
Berberine (BBR), also known as berberine, is a quaternary ammonium alkaloid isolated from the traditional Chinese medicine Rhizoma Coptidis, which has a long history of conventional medicine. BBR has been used in the therapeutic intervention of DKD due to its multiple pharmacological effects, including blood glucose lowering, anti-inflammatory, anti-fibrotic, and gut microbiota regulation (92, 93). Studies have confirmed that BBR can improve lipid metabolism disorders by regulating PPARγ, thereby reducing insulin resistance (94). Research shows that berberine has low bioavailability and potential toxicity in pharmacology, and structural modifications are needed to improve its pharmacokinetic properties (95). A phase 4 clinical trial (NCT01697735) indicated that berberine can improve metabolic diseases such as type 2 diabetes and hyperlipidemia. A clinical trial involving 97 subjects showed that the hypoglycemic effect of berberine was similar to that of metformin (96). The existing meta-analysis also confirmed that berberine has a hypoglycemic effect on type 2 diabetic patients with different levels of fasting plasma glucose (FPG) and glycosylated hemoglobin (HbA1c) (97). In recent years, mechanism studies have found that BBR promotes -FAO through dual pathways: On the one hand, by up-regulating the expression of PPARα/CPT1A/ACOX1 pathway, the FAO capacity of renal tubular epithelial cells was enhanced and lipid accumulation was reduced; on the other hand, the AMPK/PGC-1α axis is activated to repair mitochondrial function, thereby promoting PPARα activity. The above synergistic effect further strengthens the FAO process and ultimately alleviates the pathological process of DKD renal tubular injury and fibrosis (98). BBR improves DKD renal tubular lesions by targeting mitochondrial function reconstruction and fibrosis inhibition. A large body of existing in vivo and in vitro studies has confirmed that BBR exerts its effects by promoting the expression related to FAO. However, these research conclusions are mostly based on cell experiments or rodent models, and further exploration and verification are still needed for the evidence supporting the effectiveness of its clinical translation.
Coptisine is a protoberberine isoquinoline alkaloid primarily derived from Coptis chinensis Franch., a traditional Chinese herbal medicine. It exhibits diverse pharmacological activities, including anti-inflammatory effects, metabolic regulation, and anti-cancer properties (99). Pharmacokinetic studies have shown that the oral bioavailability of coptisine in mice is extremely low, approximately 8%, primarily attributed to limited intestinal absorption and extensive first-pass hepatic metabolism. This unfavorable pharmacokinetic property may restrict its systemic exposure and therapeutic efficacy (100). To date, no human clinical trials have evaluated coptisine; existing evidence derives from in vitro, animal, and pharmacokinetic studies. Coptisine has demonstrated renoprotective effects in experimental models of DKD, including attenuation of oxidative stress and inflammation in streptozotocin (STZ)-induced diabetic rats (101, 102). Recent studies have demonstrated that in renal tubular epithelial cells (HK-2), coptisine can activate AMPK, enhance the phosphorylation of ACC, and upregulate the expression of CPT-1, thereby reducing lipid accumulation under high glucose and palmitic acid conditions (103). These findings suggest that coptisine may affect FAO-related pathways and exert a renoprotective effect in DKD.
2.4 Glycoside
Glycosides are natural compounds formed by the connection of lipophilic aglycones and hydrophilic sugar moieties through glycosidic bonds, widely distributed in plants. Glycosides can exert therapeutic effects by regulating key physiological processes such as inflammation, oxidative stress, apoptosis, and fibrosis through pathways like PPARγ/AKT/AMPK and NLRP3/NF-κB (104). Clinically, cardiac glycosides such as digoxin, oleandrin C, and bufalin K have been widely used in the treatment of cardiovascular diseases (105). Recent studies have found that glycosides can play a role in the treatment of DKD by improving podocyte injury, reducing proteinuria, and regulating lipid metabolism.
Glycyrrhizic acid (GA) is a triterpenoid saponin extracted from the roots of Glycyrrhiza uralensis Fisch. It not only has a long history of medicinal use but also has been proven to exert biological effects in multiple fields, including anti-inflammation, antioxidant activity, and anti-diabetic effects (106). Defects in FAO within renal tubular epithelial cells represent a major driving factor underlying lipid accumulation, lipotoxicity, and interstitial fibrosis in chronic kidney disease (CKD) and DKD (107). In recent years, experiments have reported the renal protective effect of GA in DKD. In the db/db mouse model, GA treatment activated the AMPK/SIRT1/PGC-1α signaling pathway, which was accompanied by a reduction in proteinuria and improvement in renal histopathology (108). The activation of AMPK, SIRT1, and PGC-1α has been shown to restore mitochondrial biogenesis and enhance the fatty acid oxidation (FAO) process. This protective effect has been validated in animal experiments and human clinical studies, which can protect the kidney from lipotoxic damage (45, 109). Despite the lack of direct evidence that GA upregulates FAO-related enzymes in the kidney, studies using a hepatocellular model of non-alcoholic fatty liver disease (NAFLD) have shown that GA increases the expression of FAO-related genes, such as PPARα and CPT1A, thereby reducing hepatic steatosis (110). Collectively, these findings suggest that GA may alleviate DKD at least in part by activating the AMPK/SIRT1/PGC-1α axis, which is closely associated with mitochondrial energy metabolism and FAO. However, further mechanistic studies are required to determine whether the renal protective effect of GA in DKD is directly mediated by the restoration of renal FAO.
In summary, glycoside compounds can promote mitochondrial biosynthesis by activating the AMPK/PPARγ/PGC-1α axis, and double block inflammation and fibrosis pathways, significantly reducing early diabetic kidney injury in db/db mice.
3 Discussion
Although significant progress has been made in related research, the specific molecular mechanism of natural compounds intervention in DKD still needs to be further explored. Currently, clinical treatment mainly relies on symptomatic therapy such as glycemic control, blood pressure control, and lipid reduction. However, there is an absence of drugs specifically targeting the pathophysiology of DKD. Moreover, conventional medications have limited effects in delaying or controlling the progression of DKD, cannot completely block disease deterioration, and may also cause hyperkalemia and fluctuations in renal function. The association between FAO and DKD is not yet fully understood, and the relationship with mitochondrial autophagy still needs to be clarified. Existing research lacks studies on the interaction between FAO and key pathways of DKD, and the relevant signaling networks have not been clarified. In clinical practice, FAO modulators have not yet been involved in the research of DKD. Furthermore, fatty acid metabolism is closely related to glucose and lipid disorders, but regulating FAO may lead to metabolic imbalances, necessitating a strict assessment of safety. The chemical composition of natural compounds is inherently complex and unstable, which significantly impacts the reliability and reproducibility of research findings. Most bioactive components derived from natural compounds still lack systematic and comprehensive pharmacokinetic and pharmacodynamic research data, and they generally suffer from the issue of relatively low bioavailability. The pharmacological mechanism is not yet fully understood, and some practical components need further exploration and validation; moreover, the potential renal toxicity risks of these components have not been systematically studied. A large number of mechanistic studies are based on cell or animal models, which have limitations in simulating the pathology of late-stage DKD in humans, and there is a lack of high-quality human clinical trials. DKD is often induced by diabetes and is prone to multiple complications. Future research needs to closely combine pharmacological effects with toxicological assessment, attach great importance to safety in clinical exploration, and examine its potential for multi-target comprehensive regulation.
4 Future prospectus
The aforementioned challenges also highlight new directions for future research, with the goal of advancing the clinical translation of natural compounds targeting FAO in DKD. First, from the perspective of clinical feasibility, it is imperative to establish rigorous and standardized production processes and quality control systems to ensure the stability and reproducibility of bioactive components. Concurrently, the pharmacokinetic (ADME) and pharmacodynamic profiles of these compounds in healthy individuals and patients with DKD at different stages should be fully elucidated. Emerging technologies are expected to enhance bioavailability, improve tissue specificity, and reduce potential toxicity, thereby supporting early-phase clinical trials. Validating molecular targets, signaling pathways, and interaction networks using organoid and disease-mimicking models will lay a solid foundation for subsequent Phase I/II clinical trials, while robust Phase III randomized controlled trials (RCTs) are needed to further clarify efficacy and safety based on these preliminary findings.
Second, systematic, biomarker-based strategies should be employed to link molecular mechanisms with clinical outcomes. Lipidomic profiles and renal FAO enzyme expression levels can serve as pharmacodynamic indicators to evaluate therapeutic efficacy and guide patient stratification. Priority should be given to natural compounds with well-defined FAO-modulating effects for conducting high-quality RCTs, incorporating clinically relevant endpoints and patient-reported outcomes (PROs). Additionally, the combined use of natural compounds with drugs proven to have renoprotective effects (e.g., SGLT2 inhibitors, GLP-1 receptor agonists, or RAAS blockers) may exert synergistic effects in improving lipid metabolism and delaying DKD progression.
Finally, existing evidence demonstrates that enhancing local renal FAO can effectively reduce lipid accumulation, improve mitochondrial function, and alleviate fibrosis (111). However, there is no reliable evidence to support the claim that “upregulating renal FAO alone can restore systemic lipid levels to a healthy state.” In contrast, clinical data indicate that enhanced systemic FAO (particularly in the liver) can significantly improve lipid profiles (112, 113). Therefore, the value of FAO upregulation in DKD primarily lies in its local renoprotective effects, while its role in the systemic circulation remains to be further elucidated. In summary, FAO modulation represents a promising emerging direction for natural compounds-based DKD therapy, but comprehensive validation of its safety, efficacy, and clinical application value through systematic translational research is still required.
5 Conclusion
Our review found that natural compounds targeting FAO-related signaling pathways hold promise as important adjuvant therapeutic agents in the treatment of DKD. Polyphenols, phenolic acids, alkaloids, and glycosides can all affect the progression of DKD. They mainly improve lipid metabolism and deposition by regulating pathways such as PPARα/γ, AMPK/SIRT1/PGC-1α, and their downstream effector proteins, thereby alleviating podocyte injury and the process of fibrosis. In summary, with its multi-target synergistic regulation characteristics, natural compounds can not only directly improve oxidative stress injury and lipid metabolism disorder of DKD, but also restore renal function by regulating the FAO process, which provides a distinctive and potential intervention strategy for DKD treatment.
Author contributions
JS: Writing – original draft. CY: Writing – original draft. ZL: Writing – original draft. XG: Supervision, Writing – review & editing. SL: Supervision, Writing – review & editing. HG: Supervision, Writing – review & editing. YA: Conceptualization, Writing – review & editing. PL: Conceptualization, Funding acquisition, Writing – review & editing. NL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82575000, No. 82274489), Beijing Natural Science Foundation (No.7232326), Fifth Batch of National Traditional Chinese Medicine Excellent Clinical Talents Training Project. (Announcement from the Personnel and Education Department of the National Administration of Traditional Chinese Medicine. No. 2022-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lin, X, Lei, X-Q, Yang, J-K, Jia, J, Zhong, X, Tan, R-Z, et al. Astragalus mongholicus bunge and Panax notoginseng formula (a&P) improves renal mesangial cell damage in diabetic nephropathy by inhibiting the inflammatory response of infiltrated macrophages. BMC Complement Med Ther. (2022) 22:17. doi: 10.1186/s12906-021-03477-x
2. Liu, D, Chen, X, He, W, Lu, M, Li, Q, Zhang, S, et al. Update on the pathogenesis, diagnosis, and treatment of diabetic tubulopathy. Integr Med Nephrol Androl. (2024) 11:e23-00029. doi: 10.1097/imna-d-23-00029
3. Zhao, H, Li, Z, Yan, M, Ma, L, Dong, X, Li, X, et al. Irbesartan ameliorates diabetic kidney injury in db/db mice by restoring circadian rhythm and cell cycle. J Transl Intern Med. (2024) 12:157–69. doi: 10.2478/jtim-2022-0049
4. Bai, Y, Chi, K, Zhao, D, Shen, W, Liu, R, Hao, J, et al. Identification of functional heterogeneity of immune cells and tubular-immune cellular interplay action in diabetic kidney disease. J Transl Intern Med. (2024) 12:395–405. doi: 10.2478/jtim-2023-0130
5. Geng, Y, Dong, Z, Wang, Y, Zhang, P, Tang, J, Li, P, et al. Efficacy of huangkui capsules in the treatment of diabetic kidney disease: a systematic review and using network pharmacology. Integr Med Nephrol Androl. (2023) 10:e00020. doi: 10.1097/imna-d-22-00020
6. Chen, Y, Liu, Y, and Cao, A. The potential of huangqi decoction for treating diabetic kidney disease. Integr Med Nephrol Androl. (2024) 11:e00020. doi: 10.1097/IMNA-D-23-00020
7. Jia, J, Tan, R, Xu, L, Wang, H, Li, J, Su, H, et al. Hederagenin improves renal fibrosis in diabetic nephropathy by regulating Smad3/NOX4/SLC7A11 signaling-mediated tubular cell ferroptosis. Int Immunopharmacol. (2024) 135:112303. doi: 10.1016/j.intimp.2024.112303
8. Huang, W, Chen, Y-Y, Li, Z-Q, He, F-F, and Zhang, C. Recent advances in the emerging therapeutic strategies for diabetic kidney diseases. Int J Mol Sci. (2022) 23:10882. doi: 10.3390/ijms231810882
9. de Boer, IH, Khunti, K, Sadusky, T, Tuttle, KR, Neumiller, JJ, Rhee, CM, et al. Diabetes management in chronic kidney disease: a consensus report by the american diabetes association (ADA) and kidney disease: improving global outcomes (KDIGO). Diabetes Care. (2022) 45:3075–90. doi: 10.2337/dci22-0027
10. Heerspink, HJL, Stefánsson, BV, Correa-Rotter, R, Chertow, GM, Greene, T, Hou, F-F, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. (2020) 383:1436–46. doi: 10.1056/NEJMoa2024816
11. Chen, W, Zheng, L, Wang, J, Lin, Y, and Zhou, T. Overview of the safety, efficiency, and potential mechanisms of finerenone for diabetic kidney diseases. Front Endocrinol. (2023) 14:1320603. doi: 10.3389/fendo.2023.1320603
12. Cai, R, Li, C, Zhao, Y, Yuan, H, Zhang, X, Liang, A, et al. Traditional chinese medicine in diabetic kidney disease: multifaceted therapeutic mechanisms and research progress. Chin Med. (2025) 20:95. doi: 10.1186/s13020-025-01150-w
13. Chen, Y, Chen, M, Zhu, W, Zhang, Y, Liu, P, and Li, P. Morroniside attenuates podocytes lipid deposition in diabetic nephropathy: a network pharmacology, molecular docking and experimental validation study. Int Immunopharmacol. (2024) 138:112560. doi: 10.1016/j.intimp.2024.112560
14. Yu, W, Haoyu, Y, Ling, Z, Xing, H, Pengfei, X, Anzhu, W, et al. Targeting lipid metabolic reprogramming to alleviate diabetic kidney disease: molecular insights and therapeutic strategies. Front Immunol. (2025) 16:1549484. doi: 10.3389/fimmu.2025.1549484
15. Han, Y-Z, Du, B-X, Zhu, X-Y, Wang, Y-Z-Y, Zheng, H-J, and Liu, W-J. Lipid metabolism disorder in diabetic kidney disease. Front Endocrinol. (2024) 15:1336402. doi: 10.3389/fendo.2024.1336402
16. Zhang, X, Wu, W, Li, Y, and Peng, Z. Exploring the role and therapeutic potential of lipid metabolism in acute kidney injury. Ren Fail. (2024) 46:2403652. doi: 10.1080/0886022X.2024.2403652
17. Liu, P, Wang, C, Wang, Y, Zhang, H, Liu, B, and Qiu, X. Zishen qingre tongluo formula improves renal fatty acid oxidation and alleviated fibrosis via the regulation of the TGF-β1/Smad3 signaling pathway in hyperuricemic nephrology rats. Biomed Res Int. (2021) 2021:2793823. doi: 10.1155/2021/2793823
18. Xie, Y, Yuan, Q, Tang, B, Xie, Y, Cao, Y, Qiu, Y, et al. CPT1A protects podocytes from lipotoxicity and apoptosis in vitro and alleviates diabetic nephropathy in vivo. Diabetes. (2024) 73:879–95. doi: 10.2337/db23-0811
19. Jang, H-S, Noh, MR, Kim, J, and Padanilam, BJ. Defective mitochondrial fatty acid oxidation and lipotoxicity in kidney diseases. Front Med. (2020) 7:65. doi: 10.3389/fmed.2020.00065
20. Wang, Y, Liu, T, Wu, Y, Wang, L, Ding, S, Hou, B, et al. Lipid homeostasis in diabetic kidney disease. Int J Biol Sci. (2024) 20:3710–24. doi: 10.7150/ijbs.95216
21. Li, J, Yang, Y, Li, Q, Wei, S, Zhou, Y, Yu, W, et al. STAT6 contributes to renal fibrosis by modulating PPARα-mediated tubular fatty acid oxidation. Cell Death Dis. (2022) 13:66. doi: 10.1038/s41419-022-04515-3
22. Pan, S, Li, Z, Wang, Y, Liang, L, Liu, F, Qiao, Y, et al. A comprehensive weighted gene co-expression network analysis uncovers potential targets in diabetic kidney disease. J Transl Intern Med. (2023) 10:359–68. doi: 10.2478/jtim-2022-0053
23. Njeim, R, Alkhansa, S, and Fornoni, A. Unraveling the crosstalk between lipids and NADPH oxidases in diabetic kidney disease. Pharmaceutics. (2023) 15:1360. doi: 10.3390/pharmaceutics15051360
24. Lakhia, R, Yheskel, M, Flaten, A, Quittner-Strom, EB, Holland, WL, and Patel, V. PPARα agonist fenofibrate enhances fatty acid β-oxidation and attenuates polycystic kidney and liver disease in mice. Am J Physiol Renal Physiol. (2018) 314:F122–31. doi: 10.1152/ajprenal.00352.2017
25. Aomura, D, Harada, M, Yamada, Y, Nakajima, T, Hashimoto, K, Tanaka, N, et al. Pemafibrate protects against fatty acid-induced nephropathy by maintaining renal fatty acid metabolism. Meta. (2021) 11:372. doi: 10.3390/metabo11060372
26. Sun, L, Yuan, Q, Xu, T, Yao, L, Feng, J, Ma, J, et al. Pioglitazone improves mitochondrial function in the remnant kidney and protects against renal fibrosis in 5/6 nephrectomized rats. Front Pharmacol. (2017) 8:545. doi: 10.3389/fphar.2017.00545
27. Cha, DR, Zhang, X, Zhang, Y, Wu, J, Su, D, Han, JY, et al. Peroxisome proliferator activated receptor alpha/gamma dual agonist tesaglitazar attenuates diabetic nephropathy in db/db mice. Diabetes. (2007) 56:2036–45. doi: 10.2337/db06-1134
28. Helmy, MM, Helmy, MW, and El-Mas, MM. Additive renoprotection by pioglitazone and fenofibrate against inflammatory, oxidative and apoptotic manifestations of cisplatin nephrotoxicity: modulation by PPARs. PLoS One. (2015) 10:e0142303. doi: 10.1371/journal.pone.0142303
29. Ancajas, CMF, Oyedele, AS, Butt, CM, and Walker, AS. Advances, opportunities, and challenges in methods for interrogating the structure activity relationships of natural products. Nat Prod Rep. (2024) 41:1543–78. doi: 10.1039/d4np00009a
30. Newman, DJ, and Cragg, GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. (2016) 79:629–61. doi: 10.1021/acs.jnatprod.5b01055
31. Chung, JY-F, Lan, H-Y, and Tang, PM-K. New insights into traditional Chinese medicine in treatment of diabetic nephropathy. Integr Med Nephrol Androl. (2023) 10:e00026. doi: 10.1097/imna-d-22-00026
32. Liu, P, Zhang, J, Wang, Y, Wang, C, Qiu, X, and Chen, D-Q. Natural products against renal fibrosis via modulation of SUMOylation. Front Pharmacol. (2022) 13:800810. doi: 10.3389/fphar.2022.800810
33. Chen, M, Chen, Y, Zhu, W, Yan, X, Xiao, J, Zhang, P, et al. Advances in the pharmacological study of Chinese herbal medicine to alleviate diabetic nephropathy by improving mitochondrial oxidative stress. Biomed Pharmacother. (2023) 165:115088. doi: 10.1016/j.biopha.2023.115088
34. Zhu, W, Chen, M, Wang, Y, Chen, Y, and Zhang, Y Wang, Liu, P, and Li, P Regulation of renal lipid deposition in diabetic nephropathy on morroniside via inhibition of NF-KB/TNF-a/SREBP1c signaling pathway. Chem Biol Interact (2023) 385:110711. doi: 10.1016/j.cbi.2023.110711
35. Zhou, Y, Wu, Q, Wang, X, Li, W, Liu, Q, and Gao, K. Insights into the functional mechanism of diabetic kidney disease treatment with sinensetin based on network pharmacology and molecular docking. Integr Med Nephrol Androl. (2023) 10:e00033. doi: 10.1097/imna-d-22-00033
36. Deng, Y, Zhu, H, Xing, J, Gao, J, Duan, J, Liu, P, et al. The role of natural products in improving lipid metabolism disorder-induced mitochondrial dysfunction of diabetic kidney disease. Front Physiol. (2025) 16:1624077. doi: 10.3389/fphys.2025.1624077
37. Aneklaphakij, C, Saigo, T, Watanabe, M, Naake, T, Fernie, AR, Bunsupa, S, et al. Diversity of chemical structures and biosynthesis of polyphenols in nut-bearing species. Front Plant Sci. (2021) 12:642581. doi: 10.3389/fpls.2021.642581
38. Zhang, S, Xu, M, Zhang, W, Liu, C, and Chen, S. Natural polyphenols in metabolic syndrome: protective mechanisms and clinical applications. Int J Mol Sci. (2021) 22:6110. doi: 10.3390/ijms22116110
39. Gamage, E, Orr, R, Travica, N, Lane, MM, Dissanayaka, T, Kim, JH, et al. Polyphenols as novel interventions for depression: exploring the efficacy, mechanisms of action, and implications for future research. Neurosci Biobehav Rev. (2023) 151:105225. doi: 10.1016/j.neubiorev.2023.105225
40. Enayati, A, Ghojoghnejad, M, Roufogalis, BD, Maollem, SA, and Sahebkar, A. Impact of phytochemicals on PPAR receptors: implications for disease treatments. PPAR Res. (2022) 2022:4714914. doi: 10.1155/2022/4714914
41. Feng, F, Li, S, Shi, J, Chen, W, and Deng, Y. Resveratrol ameliorates streptozotocin induced renal inflammation and promotes autophagy by mediating the SphK1 pathway via Sirt1 in wistar rats. Food Chem Toxicol. (2025) 203:115588. doi: 10.1016/j.fct.2025.115588
42. Zhang, L-X, Li, C-X, Kakar, MU, Khan, MS, Wu, P-F, Amir, RM, et al. Resveratrol (RV): a pharmacological review and call for further research. Biomed Pharmacother. (2021) 143:112164. doi: 10.1016/j.biopha.2021.112164
43. Kapetanovic, IM, Muzzio, M, Huang, Z, Thompson, TN, and McCormick, DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. (2011) 68:593–601. doi: 10.1007/s00280-010-1525-4
44. A, H, G, N, A, F, Ž, R, E, A, F, B, et al. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. (2019) 10:6042–51. doi: 10.1039/c9fo01075k
45. Kim, MY, Lim, JH, Youn, HH, Hong, YA, Yang, KS, Park, HS, et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. (2013) 56:204–17. doi: 10.1007/s00125-012-2747-2
46. Park, HS, Lim, JH, Kim, MY, Kim, Y, Hong, YA, Choi, SR, et al. Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J Transl Med. (2016) 14:176. doi: 10.1186/s12967-016-0922-9
47. Liu, X, Gu, X, Zhang, J, Li, X, Wei, X, Jiang, S, et al. Resveratrol delays the progression of diabetic nephropathy through multiple pathways: A dose-response meta-analysis based on animal models. J Diabetes. (2024) 16:e13608. doi: 10.1111/1753-0407.13608
48. Takahashi, M, Ishiko, T, Kamohara, H, Hidaka, H, Ikeda, O, Ogawa, M, et al. Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) blocks the chemotaxis of neutrophils by inhibiting signal transduction through IL-8 receptors. Mediat Inflamm. (2007) 2007:10767. doi: 10.1155/2007/10767
49. Tan, R-Z, Liu, J, Zhang, Y-Y, Wang, H-L, Li, J-C, Liu, Y-H, et al. Curcumin relieved cisplatin-induced kidney inflammation through inhibiting mincle-maintained M1 macrophage phenotype. Phytomedicine. (2019) 52:284–94. doi: 10.1016/j.phymed.2018.09.210
50. Pivari, F, Mingione, A, Piazzini, G, Ceccarani, C, Ottaviano, E, Brasacchio, C, et al. Curcumin supplementation (meriva®) modulates inflammation, lipid peroxidation and gut microbiota composition in chronic kidney disease. Nutrients. (2022) 14:231. doi: 10.3390/nu14010231
51. Wang, L, Li, W, Cheng, D, Guo, Y, Wu, R, Yin, R, et al. Pharmacokinetics and pharmacodynamics of three oral formulations of curcumin in rats. J Pharmacokinet Pharmacodyn. (2020) 47:131–44. doi: 10.1007/s10928-020-09675-3
52. Jie, Z, Chao, M, Jun, A, Wei, S, and LiFeng, M. Effect of curcumin on diabetic kidney disease: a systematic review and meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Evid Based Complement Alternat Med. (2021) 2021:6109406. doi: 10.1155/2021/6109406
53. Chen, Y, Tao, J, He, Y, Hou, X, Fang, J, Huang, J, et al. Curcumin targets CXCL16-mediated podocyte injury and lipid accumulation in diabetic kidney disease treatment. Arch Pharm Res. (2024) 47:924–39. doi: 10.1007/s12272-024-01521-1
54. Deepika,, and Maurya, PK. Health benefits of quercetin in age-related diseases. Molecules. (2022) 27:2498. doi: 10.3390/molecules27082498
55. Tan, R-Z, Wang, C, Deng, C, Zhong, X, Yan, Y, Luo, Y, et al. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting mincle/syk/NF-κB signaling maintained macrophage inflammation. Phytother Res. (2020) 34:139–52. doi: 10.1002/ptr.6507
56. Wang, M, Wang, B, Wang, S, Lu, H, Wu, H, Ding, M, et al. Effect of quercetin on lipids metabolism through modulating the gut microbial and AMPK/PPAR signaling pathway in broilers. Front Cell Dev Biol. (2021) 9:616219. doi: 10.3389/fcell.2021.616219
57. Seo, M-J, Lee, Y-J, Hwang, J-H, Kim, K-J, and Lee, B-Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J Nutr Biochem. (2015) 26:1308–16. doi: 10.1016/j.jnutbio.2015.06.005
58. Yang, L-L, Xiao, N, Li, X-W, Fan, Y, Alolga, RN, Sun, X-Y, et al. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Sci Rep. (2016) 6:35460. doi: 10.1038/srep35460
59. Larson, AJ, Symons, JD, and Jalili, T. Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Adv Nutr. (2012) 3:39–46. doi: 10.3945/an.111.001271
60. Thuillier, P, Brash, AR, Kehrer, JP, Stimmel, JB, Leesnitzer, LM, Yang, P, et al. Inhibition of peroxisome proliferator-activated receptor (PPAR)-mediated keratinocyte differentiation by lipoxygenase inhibitors. Biochem J. (2002) 366:901–10. doi: 10.1042/BJ20020377
61. Dong, J, Zhang, X, Zhang, L, Bian, H-X, Xu, N, Bao, B, et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKα1/SIRT1. J Lipid Res. (2014) 55:363–74. doi: 10.1194/jlr.M038786
62. Gnoni, A, Di Chiara Stanca, B, Giannotti, L, Gnoni, GV, Siculella, L, and Damiano, F. Quercetin reduces lipid accumulation in a cell model of NAFLD by inhibiting de novo fatty acid synthesis through the acetyl-CoA carboxylase 1/AMPK/PP2A axis. Int J Mol Sci. (2022) 23:1044. doi: 10.3390/ijms23031044
63. Guo, X, Wen, S, Wang, J, Zeng, X, Yu, H, Chen, Y, et al. Senolytic combination of dasatinib and quercetin attenuates renal damage in diabetic kidney disease. Phytomedicine. (2024) 130:155705. doi: 10.1016/j.phymed.2024.155705
64. Elsori, D, Pandey, P, Ramniwas, S, Kumar, R, Lakhanpal, S, Rab, SO, et al. Naringenin as potent anticancer phytocompound in breast carcinoma: from mechanistic approach to nanoformulations based therapeutics. Front Pharmacol. (2024) 15:1406619. doi: 10.3389/fphar.2024.1406619
65. Jiang, T, Zhu, F, Gao, X, Wu, X, Zhu, W, and Guo, C. Naringenin loaded fucoidan/polyvinylpyrrolidone nanoparticles protect against folic acid induced acute kidney injury in vitro and in vivo. Colloids Surf B Biointerfaces. (2025) 245:114343. doi: 10.1016/j.colsurfb.2024.114343
66. Yang, B, Xin, M, Liang, S, Huang, Y, Li, J, Wang, C, et al. Naringenin ameliorates hyperuricemia by regulating renal uric acid excretion via the PI3K/AKT signaling pathway and renal inflammation through the NF-κB signaling pathway. J Agric Food Chem. (2023) 71:1434–46. doi: 10.1021/acs.jafc.2c01513
67. Alhazzani, K, Alqarni, NN, Aljerian, K, Raish, M, Aljuffali, L, Alshehri, S, et al. Naringenin mitigates dasatinib-induced kidney damage by modulating antioxidant defense, inflammation, and apoptosis pathways. Int J Med Sci. (2025) 22:110–20. doi: 10.7150/ijms.102088
68. Cai, X, Wang, S, Wang, H, Liu, S, Liu, G, Chen, H, et al. Naringenin inhibits lipid accumulation by activating the AMPK pathway in vivo and vitro. Food Sci Human Wellness. (2023) 12:1174–83. doi: 10.1016/j.fshw.2022.10.043
69. Yu, X, Meng, X, Yan, Y, Wang, H, and Zhang, L. Extraction of naringin from pomelo and its therapeutic potentials against hyperlipidemia. Molecules. (2022) 27:9033. doi: 10.3390/molecules27249033
70. Rigano, D, Sirignano, C, and Taglialatela-Scafati, O. The potential of natural products for targeting PPARα. Acta Pharm Sin B. (2017) 7:427–38. doi: 10.1016/j.apsb.2017.05.005
71. Bai, Y, Peng, W, Yang, C, Zou, W, Liu, M, Wu, H, et al. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front Pharmacol. (2020) 11:364. doi: 10.3389/fphar.2020.00364
72. Noman, AM, Sultan, MT, Raza, H, Imran, M, Hussain, M, Mujtaba, A, et al. An updated review of the anticancer mechanisms and therapeutic potential of naringenin. Food Sci Nutr. (2025) 13:e70626. doi: 10.1002/fsn3.70626
73. Punithavathi, VR, Anuthama, R, and Prince, PSM. Combined treatment with naringin and vitamin C ameliorates streptozotocin-induced diabetes in male wistar rats. J Appl Toxicol. (2008) 28:806–13. doi: 10.1002/jat.1343
74. Xulu, S, and Oroma Owira, PM. Naringin ameliorates atherogenic dyslipidemia but not hyperglycemia in rats with type 1 diabetes. J Cardiovasc Pharmacol. (2012) 59:133–41. doi: 10.1097/FJC.0b013e31823827a4
75. Ding, S, Qiu, H, Huang, J, Chen, R, Zhang, J, Huang, B, et al. Activation of 20-HETE/PPARs involved in Reno-therapeutic effect of naringenin on diabetic nephropathy. Chem Biol Interact. (2019) 307:116–24. doi: 10.1016/j.cbi.2019.05.004
76. Zhan, H, Pu, Q, Long, X, Lu, W, Wang, G, Meng, F, et al. Oxybaphus himalaicus mitigates lipopolysaccharide-induced acute kidney injury by inhibiting TLR4/MD2 complex formation. Antioxidants. (2022) 11:2307. doi: 10.3390/antiox11122307
77. Qu, W, Lan, Y, Cheng, Z, Yuan, H, Zhan, H, Lan, X, et al. Oxybaphus himalaicus alleviates diabetic kidney disease by suppressing the lipid metabolism and inflammation via PPARα signaling. Fitoterapia. (2025) 182:106474. doi: 10.1016/j.fitote.2025.106474
78. Cheng, Z, Zhan, H, Yuan, H, Wang, N, Lan, Y, Qu, W, et al. Boeravinone C ameliorates lipid accumulation and inflammation in diabetic kidney disease by activating PPARα signaling. J Ethnopharmacol. (2025) 342:119398. doi: 10.1016/j.jep.2025.119398
79. Sun, M, Zhang, Z, Xie, J, Yu, J, Xiong, S, Xiang, F, et al. Research progress on the mechanism for improving glucose and lipid metabolism disorders using phenolic acid components from medicinal and edible homologous plants. Molecules. (2024) 29:4790. doi: 10.3390/molecules29204790
80. Afnan,, Saleem, A, Akhtar, MF, Sharif, A, Akhtar, B, Siddique, R, et al. Anticancer, cardio-protective and anti-inflammatory potential of natural-sources-derived phenolic acids. Mol (Basel Switz). (2022) 27:7286. doi: 10.3390/molecules27217286
81. Song, J, He, Y, Luo, C, Feng, B, Ran, F, Xu, H, et al. New progress in the pharmacology of protocatechuic acid: a compound ingested in daily foods and herbs frequently and heavily. Pharmacol Res. (2020) 161:105109. doi: 10.1016/j.phrs.2020.105109
82. Han, L, Yang, Q, Li, J, Cheng, F, Zhang, Y, Li, Y, et al. Protocatechuic acid-ameliorated endothelial oxidative stress through regulating acetylation level via CD36/AMPK pathway. J Agric Food Chem. (2019) 67:7060–72. doi: 10.1021/acs.jafc.9b02647
83. Stojković, DS, Zivković, J, Soković, M, Glamočlija, J, Ferreira, ICFR, Janković, T, et al. Antibacterial activity of veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem Toxicol. (2013) 55:209–13. doi: 10.1016/j.fct.2013.01.005
84. Huang, Y, Zhou, Z, Yang, W, Gong, Z, Li, Y, Chen, S, et al. Comparative pharmacokinetics of gallic acid, protocatechuic acid, and quercitrin in normal and pyelonephritis rats after oral administration of a polygonum capitatum extract. Molecules. (2019) 24:3873. doi: 10.3390/molecules24213873
85. Lin, C-Y, Tsai, S-J, Huang, C-S, and Yin, M-C. Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice. J Agric Food Chem. (2011) 59:5117–24. doi: 10.1021/jf200103f
86. Xiang, P, Du, Y, Chen, G, Mao, Y, Li, S, Li, Q, et al. Dietary achievable dose of protocatechuic acid, a metabolite of flavonoids, inhibits high-fat diet-induced obesity in mice. Mol Nutr Food Res. (2024) 68:e2300451. doi: 10.1002/mnfr.202300451
87. Sun, R, Kang, X, Zhao, Y, Wang, Z, Wang, R, Fu, R, et al. Sirtuin 3-mediated deacetylation of acyl-CoA synthetase family member 3 by protocatechuic acid attenuates non-alcoholic fatty liver disease. Br J Pharmacol. (2020) 177:4166–80. doi: 10.1111/bph.15159
88. Heinrich, M, Mah, J, and Amirkia, V. Alkaloids used as medicines: structural phytochemistry meets biodiversity-an update and forward look. Molecules. (2021) 26:1836. doi: 10.3390/molecules26071836
89. Huang, L-J, Lan, J-X, Wang, J-H, Huang, H, Lu, K, Zhou, Z-N, et al. Bioactivity and mechanism of action of sanguinarine and its derivatives in the past 10 years. Biomed Pharmacother. (2024) 173:116406. doi: 10.1016/j.biopha.2024.116406
90. Ashrafi, S, Alam, S, Sultana, A, Raj, A, Emon, NU, Richi, FT, et al. Papaverine: a miraculous alkaloid from opium and its multimedicinal application. Molecules. (2023) 28:3149. doi: 10.3390/molecules28073149
91. Huang, C-J, Huang, W-C, Lin, W-T, Shu, L-H, Sheu, J-R, Tran, O-T, et al. Rutaecarpine, an alkaloid from evodia rutaecarpa, can prevent platelet activation in humans and reduce microvascular thrombosis in mice: crucial role of the PI3K/akt/GSK3β signal axis through a cyclic nucleotides/VASP-independent mechanism. Int J Mol Sci. (2021) 22:11109. doi: 10.3390/ijms222011109
92. Gong, L, Wang, R, Wang, X, Liu, J, Han, Z, Li, Q, et al. Research progress of natural active compounds on improving podocyte function to reduce proteinuria in diabetic kidney disease. Ren Fail. (2023) 45:2290930. doi: 10.1080/0886022X.2023.2290930
93. Ma, Z, Zhu, L, Wang, S, Guo, X, Sun, B, Wang, Q, et al. Berberine protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition involving the inactivation of the NLRP3 inflammasome. Ren Fail. (2022) 44:923–32. doi: 10.1080/0886022X.2022.2079525
94. Liu, Y-F, Wang, H-H, Geng, Y-H, Han, L, Tu, S-H, and Wang, H. Advances of berberine against metabolic syndrome-associated kidney disease: regarding effect and mechanism. Front Pharmacol. (2023) 14:1112088. doi: 10.3389/fphar.2023.1112088
95. Utami, AR, Maksum, IP, and Deawati, Y. Berberine and its study as an antidiabetic compound. Biology. (2023) 12:973. doi: 10.3390/biology12070973
96. Yin, J, Xing, H, and Ye, J. Efficacy of berberine in patients with type 2 diabetes. Metab Clin Exp. (2008) 57:712–7. doi: 10.1016/j.metabol.2008.01.013
97. Xie, W, Su, F, Wang, G, Peng, Z, Xu, Y, Zhang, Y, et al. Glucose-lowering effect of berberine on type 2 diabetes: a systematic review and meta-analysis. Front Pharmacol. (2022) 13:1015045. doi: 10.3389/fphar.2022.1015045
98. Rong, Q, Han, B, Li, Y, Yin, H, Li, J, and Hou, Y. Berberine reduces lipid accumulation by promoting fatty acid oxidation in renal tubular epithelial cells of the diabetic kidney. Front Pharmacol. (2022) 12:729384. doi: 10.3389/fphar.2021.729384
99. Lu, Q, Tang, Y, Luo, S, Gong, Q, and Li, C. Coptisine, the characteristic constituent from coptis chinensis, exhibits significant therapeutic potential in treating cancers, metabolic and inflammatory diseases. Am J Chin Med. (2023) 51:2121–56. doi: 10.1142/S0192415X2350091X
100. Yan, Y, Zhang, H, Zhang, Z, Song, J, Chen, Y, Wang, X, et al. Pharmacokinetics and tissue distribution of coptisine in rats after oral administration by liquid chromatography-mass spectrometry. Biomed Chromatogr. (2017) 31:e3918. doi: 10.1002/bmc.3918
101. Zhai, J, Li, Z, Zhang, H, Ma, L, Ma, Z, Zhang, Y, et al. Coptisine ameliorates renal injury in diabetic rats through the activation of Nrf2 signaling pathway. Naunyn Schmiedeberg's Arch Pharmacol. (2020) 393:57–65. doi: 10.1007/s00210-019-01710-6
102. Zhai, J, Li, Z, Zhang, H, Lu, Z, Zhang, Y, Li, M, et al. Coptisine mitigates diabetic nephropathy via repressing the NRLP3 inflammasome. Open Life Sci. (2023) 18:20220568. doi: 10.1515/biol-2022-0568
103. Tao, J, Hao, T-C, Zhang, X-Y, Lu, P, and Yang, Y. Coptisine inhibits lipid accumulation in high glucose- and palmitic acid-induced HK-2 cells by regulating the AMPK/ACC/CPT-1 signaling pathway. Naunyn Schmiedeberg's Arch Pharmacol. (2025) 398:5465–74. doi: 10.1007/s00210-024-03617-3
104. Chen, K, Wei, X, Pariyani, R, Kortesniemi, M, Zhang, Y, and Yang, B. 1H NMR metabolomics and full-length RNA-seq reveal effects of acylated and nonacylated anthocyanins on hepatic metabolites and gene expression in zucker diabetic fatty rats. J Agric Food Chem. (2021) 69:4423–37. doi: 10.1021/acs.jafc.1c00130
105. Ponce, A, Flores-Maldonado, C, and Contreras, RG. Cardiac glycosides: from natural defense molecules to emerging therapeutic agents. Biomolecules. (2025) 15:885. doi: 10.3390/biom15060885
106. Hosseinzadeh, H, and Nassiri-Asl, M. Pharmacological effects of glycyrrhiza spp. and its bioactive constituents: update and review. Phytother res: PTR. (2015) 29:1868–86. doi: 10.1002/ptr.5487
107. Hu, Q, Jiang, L, Yan, Q, Zeng, J, Ma, X, and Zhao, Y. A natural products solution to diabetic nephropathy therapy. Pharmacol Ther. (2023) 241:108314. doi: 10.1016/j.pharmthera.2022.108314
108. Hou, S, Zhang, T, Li, Y, Guo, F, and Jin, X. Glycyrrhizic acid prevents diabetic nephropathy by activating AMPK/SIRT1/PGC-1α signaling in db/db mice. J Diabetes Res. (2017) 2017:2865912. doi: 10.1155/2017/2865912
109. Clark, AJ, and Parikh, SM. Targeting energy pathways in kidney disease: the roles of sirtuins, AMPK, and PGC1α. Kidney Int. (2021) 99:828–40. doi: 10.1016/j.kint.2020.09.037
110. Sun, X, Duan, X, Wang, C, Liu, Z, Sun, P, Huo, X, et al. Protective effects of glycyrrhizic acid against non-alcoholic fatty liver disease in mice. Eur J Pharmacol. (2017) 806:75–82. doi: 10.1016/j.ejphar.2017.04.021
111. Miguel, V, Tituaña, J, Herrero, JI, Herrero, L, Serra, D, Cuevas, P, et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J Clin Invest. (2021) 131:e140695. doi: 10.1172/JCI140695
112. Bougarne, N, Weyers, B, Desmet, SJ, Deckers, J, Ray, DW, Staels, B, et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr Rev. (2018) 39:760–802. doi: 10.1210/er.2018-00064
Keywords: diabetic kidney disease, renal lipid metabolism disorders, natural compounds, fatty acid oxidation, PPARs
Citation: Sun J, Yin C, Li Z, Gao X, Li S, Gao H, An Y, Liu P and Liu N (2025) Natural compounds regulating fatty acid oxidation in the treatment of diabetic kidney disease. Front. Nutr. 12:1669557. doi: 10.3389/fnut.2025.1669557
Edited by:
Manuela Machado, Escola Superior de Biotecnologia - Universidade Católica Portuguesa, PortugalReviewed by:
Adeniyi Adebesin, University of Texas Southwestern Medical Center, United StatesPalash Mitra, Midnapore City College, India
Copyright © 2025 Sun, Yin, Li, Gao, Li, Gao, An, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan An, NDE3NTY3ODZAcXEuY29t; Peng Liu, ZHJsaXVwZW5nQHNpbmEuY24=; Na Liu, eHVhbmZ1aHVhQDEyNi5jb20=
†These authors have contributed equally to this work
 Jianing Sun
Jianing Sun Chengqian Yin
Chengqian Yin Zhe Li1†
Zhe Li1† Peng Liu
Peng Liu