- 1Department of Emergency Medicine, The Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 2Second School of Clinical Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 3Department of Gynecology, Guiyang Maternal and Child Health Care Hospital, Guiyang, Guizhou, China
Background: This systematic review and meta-analysis seeks to extensively estimate the interrelation between vitamin D (VD) and clinical results among both pediatric and adult sepsis patients.
Methods: A search was implemented through four databases (PubMed, Embase, Cochrane Library, and Web of Science) up to February 2025. Meta-analysis was implemented utilizing Stata 15 and Meta-Disc software.
Results: Thirty-nine studies were included, encompassing 1,208 pediatric and neonatal sepsis patients, and 60,566 adult sepsis patients. The results showed that the average VD level in neonates with sepsis was 12.99 (95% CI: 8.11, 17.87), and the average VD level in children was 24.84 (95% CI: 21.34, 28.33). Their VD levels were considerably lower relative to healthy individuals or those without sepsis, with statistical distinction (p < 0.05). The aggregated prevalence of VD deficiency and insufficiency was 54%. When VD levels were <30 ng/mL, the aggregate prevalence of deficiency and insufficiency was the highest at 76%. A considerable interrelation between VD deficiency and mortality was identified, contrasted with the control group (p < 0.05). Among adults with sepsis, the average VD level was 17.12 (95% CI: 14.19, 20.05). Relative to the healthy cohort, VD levels substantially declined, with statistical distinction (p < 0.05); relative to those without sepsis, there was no statistical distinction in VD levels (p = 0.05). The pooled prevalence of VD deficiency and insufficiency was 55%. The deficiency of VD was considerably correlated with both the incidence and mortality of sepsis (p < 0.001). Supplementation with VD did not reduce the length of ICU stay (p = 0.67), but it can considerably reduce the risk of death (p < 0.05). The sensitivity and specificity of VD to forecast mortality among adult sepsis patients were 81 and 31%, respectively.
Conclusion: Vitamin D status in both pediatric and adult sepsis individuals was predominantly in a deficient state, and the prevalence of VD deficiency and insufficiency is relatively high. VD deficiency was considerably linked to elevated mortality among pediatric sepsis individuals and also the incidence and mortality of adult sepsis individuals. VD may serve as a valuable biomarker to forecast mortality among adult sepsis individuals.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420250651346, CRD420250651346
1 Introduction
Sepsis is featured as a fatal organ failure originating from a dysregulated immune response to infection, which induces a systemic hyperinflammatory state. Severe sepsis can progress to septic shock, which is linked to an in-hospital mortality exceeding 40% (1). Sepsis and septic shock pose a serious global health threat and are principal causes of demise in intensive care units (ICU) (2). Recent data show that approximately 11 million deaths occur annually due to sepsis, accounting for nearly 20% of global mortality (3). The Surviving Sepsis Campaign Guidelines (SSCG) and the Japanese Sepsis Society clinical guidelines (J-SSCG) are high-impact guidelines established in recent years. Both of them advocate for early detection, effective control of infection sources, appropriate antimicrobial treatment, and adequate organ assistance to mitigate the burden of sepsis (4, 5). Therefore, it’s urgent to identify effective biomarkers and immune modulatory treatments to estimate the effect on the early diagnosis, therapeutic intervention, and prognosis of sepsis patients.
Recently, vitamin D (VD) has garnered particular attention for its crucial function in the immune system. VD can protect cells from harmful signals by suppressing inflammatory responses (6). It not only regulates innate and adaptive immune responses but also improves tolerance in immune reactions (7). Genetic evidence indicates a considerable causal interrelation between VD status and immune cells (8). Proteomics studies illustrated a negative interrelation between VD status and five immunoglobulins (JCHAJN, IGHV4-28, GHV4-34, IGHM, and IGLV2-11) (9). Nevertheless, the implication of VD for sepsis remains controversial. VD supplementation had no discernible implication on sepsis individuals’ mortality, as indicated by a recent network meta-analysis (10). However, previous meta-analyses have displayed a close interconnection between VD status and both the incidence and mortality of sepsis among both children and adults (11–13). Importantly, no studies have implemented a meta-analysis to estimate the forecasting capability of VD for the incidence and mortality of sepsis patients.
Therefore, our aim is to determine the interrelation between VD status and the risk of the occurrence and mortality of sepsis, and the predictive value of VD from observational studies, based on the guideline directions of early identification, rational intervention, and prognostic value, through systematic reviews and meta-analyses. This research calculates the pooled prevalence of VD deficiency and assesses VD status among sepsis individuals by combining means and standard deviations. This research also determines the relevance of supplementing VD on the length of ICU stay and mortality among sepsis individuals from randomized controlled trials (RCTs). Furthermore, considering age-related differences in sepsis patients, this study comprehensively assesses the relevance of VD in both pediatric and adult populations.
2 Methods
The design, implementation, and reporting of meta-analysis results in this research adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (14) (Appendix S1). This research was registered on the PROSPERO website1. The registration number was CRD420250651346.
2.1 Searching strategies
Related studies in PubMed, Embase, Cochrane Library, and Web of Science were searched by two researchers (AB), who were trained in systematic review approaches. The search terms were designed by combining medical subject headings (MeSH) terms and free terms. The search covered literature from database inception to February 2025. The search terms were as follows: VD OR vd OR 25-HydroxyVD AND sepsis OR Bloodstream Infection OR Septicemia. The specific search strategy is depicted in Supplementary Table S1. Reference lists from pertinent publications or reviews were manually searched as supplementary sources. Endnote 21 was leveraged to manage the retrieved references.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
(i) The research subjects were individuals diagnosed with sepsis, regardless of age. The sepsis diagnosis must meet the criteria of SIRS, Sepsis-3, ICD-9, positive blood cultivation, or medical records indicating sepsis. The severity of sepsis consisted of severe sepsis and septic shock. In the control group, the “healthy cohort” was defined as individuals without any active or major chronic diseases, including known risk factors for sepsis. The “non-sepsis population” consisted of patients hospitalized for other reasons (e.g., elective surgery, stable chronic disease, or non-infectious acute conditions) but confirmed after assessment not to have sepsis.
(ii) The intervention or exposure factor involved VD supplementation or exposure to high levels of VD. VD deficiency was identified as a 25(OH)D level <20 ng/mL; insufficiency was identified as a 25(OH)D level of 20–30 ng/mL (15); severe deficiency was identified as a 25(OH)D level <12 ng/mL (16, 17); Due to the lack of established age specific vitamin D thresholds in sepsis patients of different age groups, the above uniform threshold values were applied to all included neonatal and pediatric populations.
(iii) The control group was not supplemented with VD or was exposed to inadequate levels of VD.
(iv) Outcome measures included VD levels in sepsis individuals, the prevalence of VD deficiency and insufficiency, the association and predictive value of VD with the incidence or mortality risk of sepsis, and the influence of VD supplementation on length of ICU stay and mortality in sepsis patients.
(v) Study types encompassed cohort studies, cross-sectional studies, case–control studies, and RCTs.
2.2.2 Exclusion criteria
(i) Review articles, clinical registration protocols, guidelines, and other types of studies.
(ii) Studies lacking complete data on odds ratios (OR), relative risks (RR), hazard ratios (HR), or their 95% confidence intervals.
(iii) Duplicated publications.
2.3 Literature screening
Two researchers (A and B) separately screened the retrieved literature records utilizing pre-established inclusion and exclusion criteria. If dissents occurred, a third researcher (C) was engaged to resolve the issue. EndNote 21 was utilized as the reference management tool for this study, inputting the initial search results. Both researchers independently searched for duplicate records, screened the titles and abstracts of the retrieved articles, and further appraised eligible studies by downloading and reading the complete text.
2.4 Data collection process
Two researchers (A and B) independently carried out the data extraction process and subsequently organized and collated the extracted data. The subsequent data information was obtained from studies that complied with the inclusion criteria: first author, publication year, study type, country/region, age, sample size, sepsis type, diagnostic criteria for sepsis, type of VD, and outcome measures [(i) Mean and standard deviation were extracted to assess VD status and length of ICU stay among sepsis individuals; (ii) The number of events and total number of participants were extracted to appraise the prevalence of VD deficiency; (iii) Binary variables were extracted to estimate the interrelation between VD and mortality in sepsis patients; (iv) Both multivariable-adjusted and unadjusted results were extracted to appraise the link between VD and the incidence and mortality of sepsis; (v) For diagnostic tests, true positives, false negatives, true negatives, false positives, sensitivity, and specificity were extracted to estimate the predictive value of VD for the incidence and mortality of sepsis]. Any disputes were addressed by discussing with a third investigator (C).
2.5 Quality appraisal
Two researchers (A and B) separately estimated the quality and methodological rigor of the included articles. The Newcastle–Ottawa scale (NOS) was leveraged to appraise the quality of cohort studies and case–control studies (18). The scale encompassed three domains: the selection of research groups, the comparability of the exposed (case) group and the control group achieved by adjusting for confounding factors, and the ascertainment of outcomes of interest. Articles with a score of 7 or more were rated as high quality. A score of 4–6 denoted moderate quality, and 0–3 signified low quality. Cross-sectional studies were appraised by leveraging the Agency for Healthcare Research and Quality (AHRQ) assessment tool (19). This tool contained 11 items. A score of 8–11 denoted high quality, 4–7 indicated moderate quality, and 0–3 denoted low quality. For RCT studies, the Cochrane risk of bias tool (RoB2.0) was leveraged (20). This tool assessed the risk of bias (RoB) in five domains: (i) bias originating from randomization process; (ii) bias originating from deviations from the intended interventions; (iii) bias originating from missing outcome data; (iv) bias in outcomes measurement; (v) bias in the reporting of results. In case of any disagreements, a third investigator (C) would be consulted to make the final decision.
2.6 Statistical analyses
Statistical analysis was implemented utilizing Stata 15 and Meta-Disc 1.4 software. The I2 statistic and Cochran Q test were leveraged to estimate the heterogeneity of the included studies. I2 of 25–50% denoted low heterogeneity; I2 of 50–75% signified moderate heterogeneity, and I2 > 75% denoted high heterogeneity (21). p < 0.05 or I2 > 50% signified significant heterogeneity between studies, and a random-effects model was leveraged. Otherwise, a fixed-effects model was applied. Sensitivity analysis was implemented utilizing the leave-one-out method for outcomes with more than five included studies, in order to ascertain the stability of the results (22). Additionally, subgroup analysis was conducted by the degree of VD deficiency and control groups (healthy individuals, non-sepsis groups) to estimate the relevance of these characteristics on the outcomes and whether they were sources of heterogeneity. For meta-analyses including over 10 studies, potential publication bias was appraised. The Egger’s test was leveraged to further estimate publication bias (23). For diagnostic tests, Spearman correlation analysis was implemented utilizing Meta-Disc 1.4 software to assess threshold effects, where a strong positive correlation indicated the possibility of a threshold effect. If no threshold effect was found, data were combined for further analysis. A bivariate mixed-effects model was leveraged to summarize the effect sizes, including the summary sensitivity (SSEN) and summary specificity (SSPE). Deeks’ funnel plot was utilized to examine publication bias.
3 Results
3.1 Literature screening
This study initially retrieved 2,890 publications, of which 872 were from PubMed, 1724 from Embase, 188 from Cochrane, and 106 from Web of Science. After excluding duplicates (n = 369) and removing others for various reasons (n = 153), we reviewed the titles and abstracts of 2,368 publications. After excluding 2,133 studies, 235 articles were reviewed fully. Twenty-one articles were eliminated owing to the unavailability of their whole texts, and we examined 214 publications in detail. Finally, 39 eligible articles were incorporated into this analysis (Supplementary Figure S1).
3.2 Research characteristics
This study divided the baseline information into two parts based on age characteristics: neonates and children, and adults.
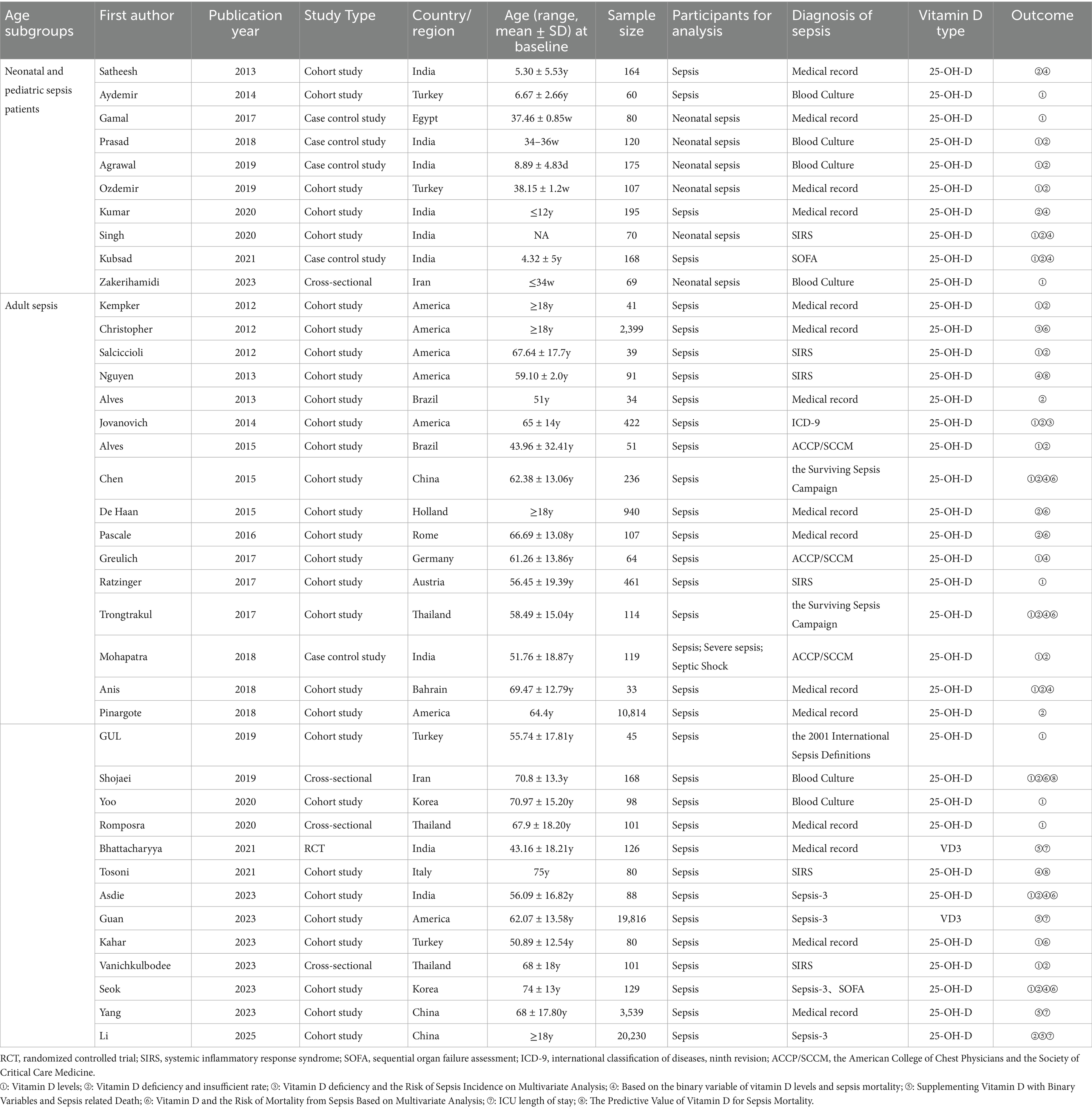
Among neonates and children, 10 studies were included, consisting of five cohort studies (24–28), four case–control studies (29–32), and one cross-sectional study (33). Six studies were carried out in India (24, 27, 28, 30–32), two in Turkey (25, 26), one in Egypt (29), and one in Iran (33). Eight studies reported levels of VD (25, 26, 28–33). Seven studies reported the prevalence of VD deficiency (24, 26–28, 30–32). Four studies reported the risk of mortality due to VD deficiency (24, 27, 28, 32).
In adult sepsis patients, 29 studies were included, comprising 24 cohort studies (34–57), three cross-sectional studies (58–60), one case–control study (61), and one RCT (62). Seven studies were carried out in the United States (34–37, 39, 48, 53), three in China (41, 55, 56), three in Thailand (46, 59, 60), three in India (52, 61, 62), two in Brazil (38, 40), two in Turkey (49, 54), two in South Korea (50, 57), one in the Netherlands (42), one in Romania (43), one in Germany (44), one in Australia (45), one in Bahrain (47), one in Iran (58), and one in Italy (51). Eighteen studies reported levels of VD (34, 36, 39–41, 44–47, 49, 50, 52, 54, 57–61). Seventeen studies reported the prevalence of VD deficiency and insufficiency (34, 36, 38–43, 46–48, 52, 56–58, 60, 61). Two studies reported the VD deficiency and the risk of sepsis onset through multivariate statistical analysis (35, 39). Twelve studies reported VD and the mortality risk of sepsis based on binary variables (37, 41, 44, 46, 47, 51, 52, 57). Four studies reported the VD supplementation and the mortality risk of sepsis (53, 55, 56, 62). Eight studies reported the VD status and the mortality risk of sepsis through multivariate statistical analysis (35, 41–43, 52, 54, 57, 58). Four studies investigated the relevance of VD supplementation on the length of ICU stay in individuals with sepsis (53, 55, 56, 62). Three studies reported the forecasting capability of VD for mortality in individuals with sepsis (37, 58) (Table 1).
3.3 RoB appraisal of included studies
The RoB of cohort studies and case–control studies was appraised utilizing the NOS scale (Supplementary Tables S2, S3). Among the 29 cohort studies, 20 were of high quality and nine were of moderate quality. Among the five case–control studies, one was of high quality and four were of moderate quality. The four cross-sectional studies were assessed for RoB utilizing the AHRQ tool, among which two studies were of high quality and two were of moderate quality (Supplementary Table S4). A RoB assessment of one RCT study was conducted utilizing the ROB2 tool. The RCT was assessed to have a low RoB in domains such as bias originating from the randomization process, bias originating from deviations from the intended interventions, bias originating from missing outcome data, bias in outcome measurement, and bias in the reporting of outcomes (Supplementary Figure S2).
3.4 Neonatal and pediatric sepsis patients
3.4.1 VD levels
Nine studies were included to estimate the VD status among neonates and children having sepsis. The outcomes of the Cochrane Q test (p < 0.001) and the I2 estimate (99.2%) indicated significant heterogeneity. The random-effects model was leveraged for meta-analysis. The results showed that the average VD level in neonates with sepsis was 12.99 (95% CI: 8.11, 17.87), and the average VD level in children was 24.84 (95% CI: 21.34, 28.33) (Figure 1).
Further subgroup analyses were made by control groups comprising healthy individuals or neonates and children without sepsis, based on seven included studies. The Cochrane Q test (p < 0.001) and I2 estimate (97.5%) also displayed considerable heterogeneity. The random-effects meta-analysis indicated that neonates and children suffering from sepsis had considerably diminished VD levels relative to control groups, with a statistical distinction (SMD = −2.29, 95% CI: −3.31, −1.28, p < 0.001). Subgroup analysis revealed that, compared with healthy individuals, the sepsis group had a considerably lower VD level with statistical distinction (SMD = −3.16, 95% CI: −5.33, −1.00, p = 0.004). Additionally, when compared with those without sepsis, the sepsis group also exhibited significantly lower VD levels with a statistical distinction (SMD = −1.83, 95% CI: −3.06, −0.61, p = 0.003).
Furthermore, to figure out the source of heterogeneity, when the control group consisted of healthy individuals, I2 = 98.4%, p < 0.001; and when the control group consisted of individuals without sepsis, I2 = 97%, p < 0.001. This suggests that the control group may not be the primary source of the high heterogeneity (Figure 2).
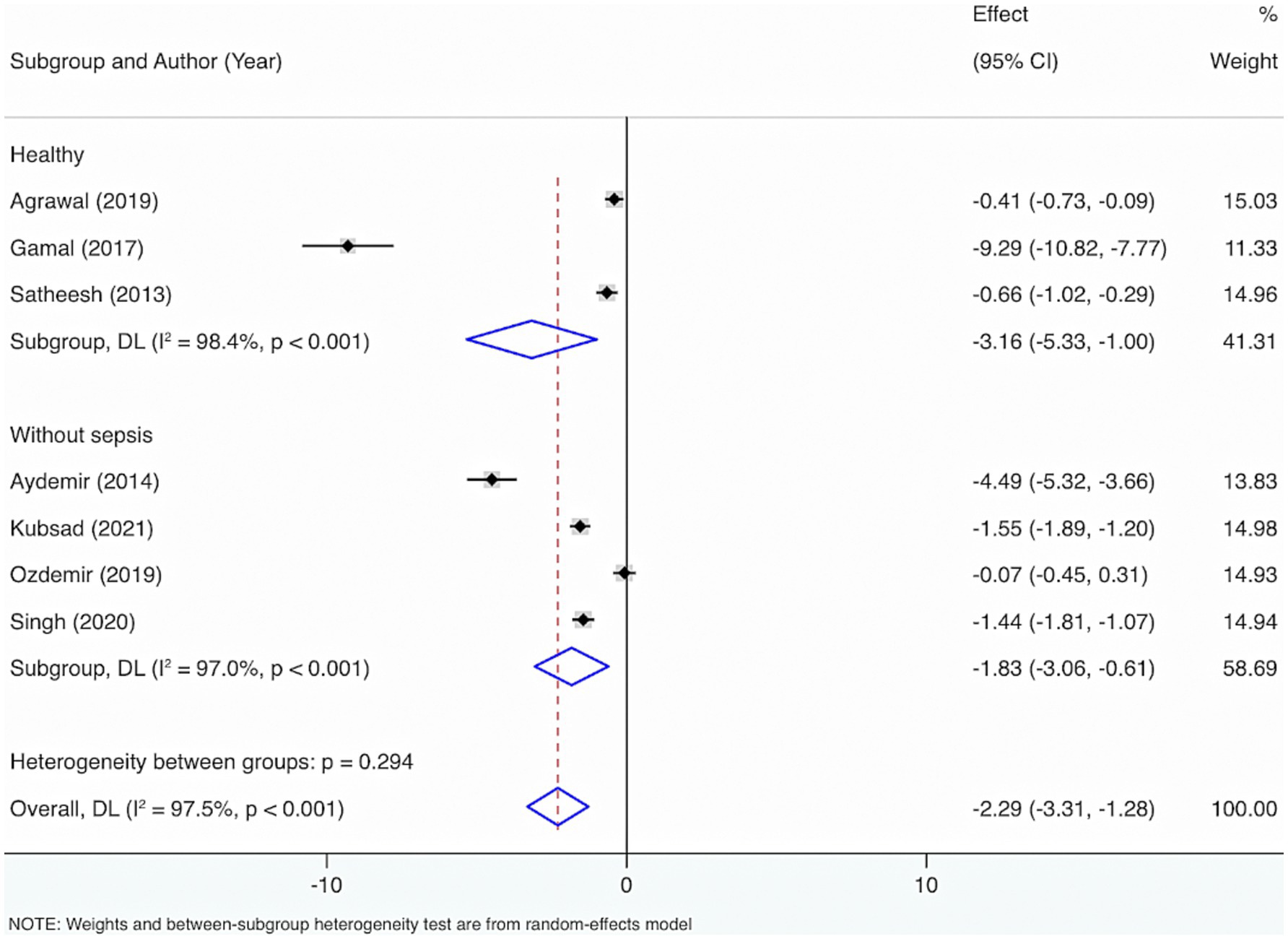
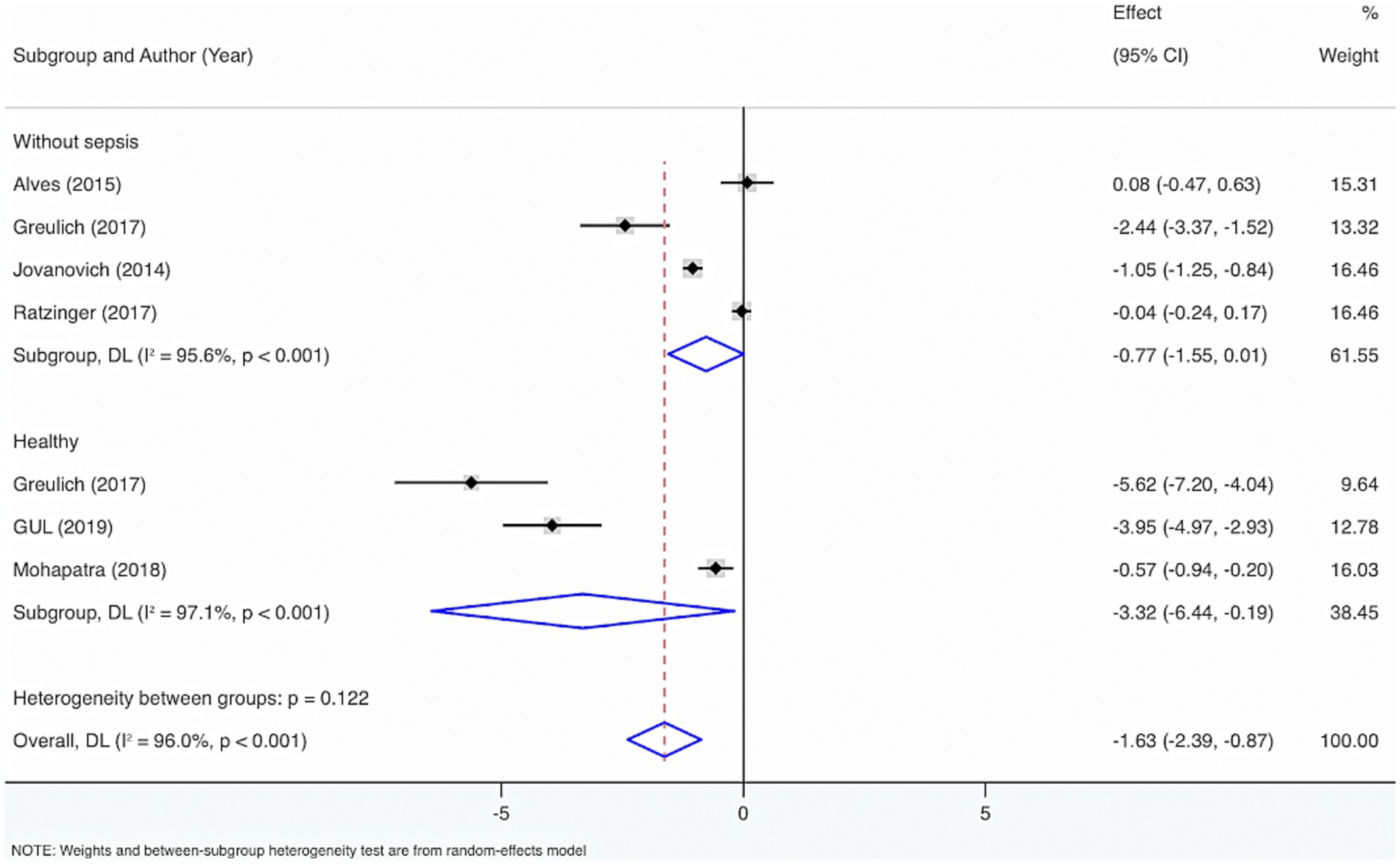
Figure 2. Subgroup analyses were made by control groups comprising healthy individuals or neonates and children without sepsis.
3.4.2 Prevalence of VD deficiency and insufficiency
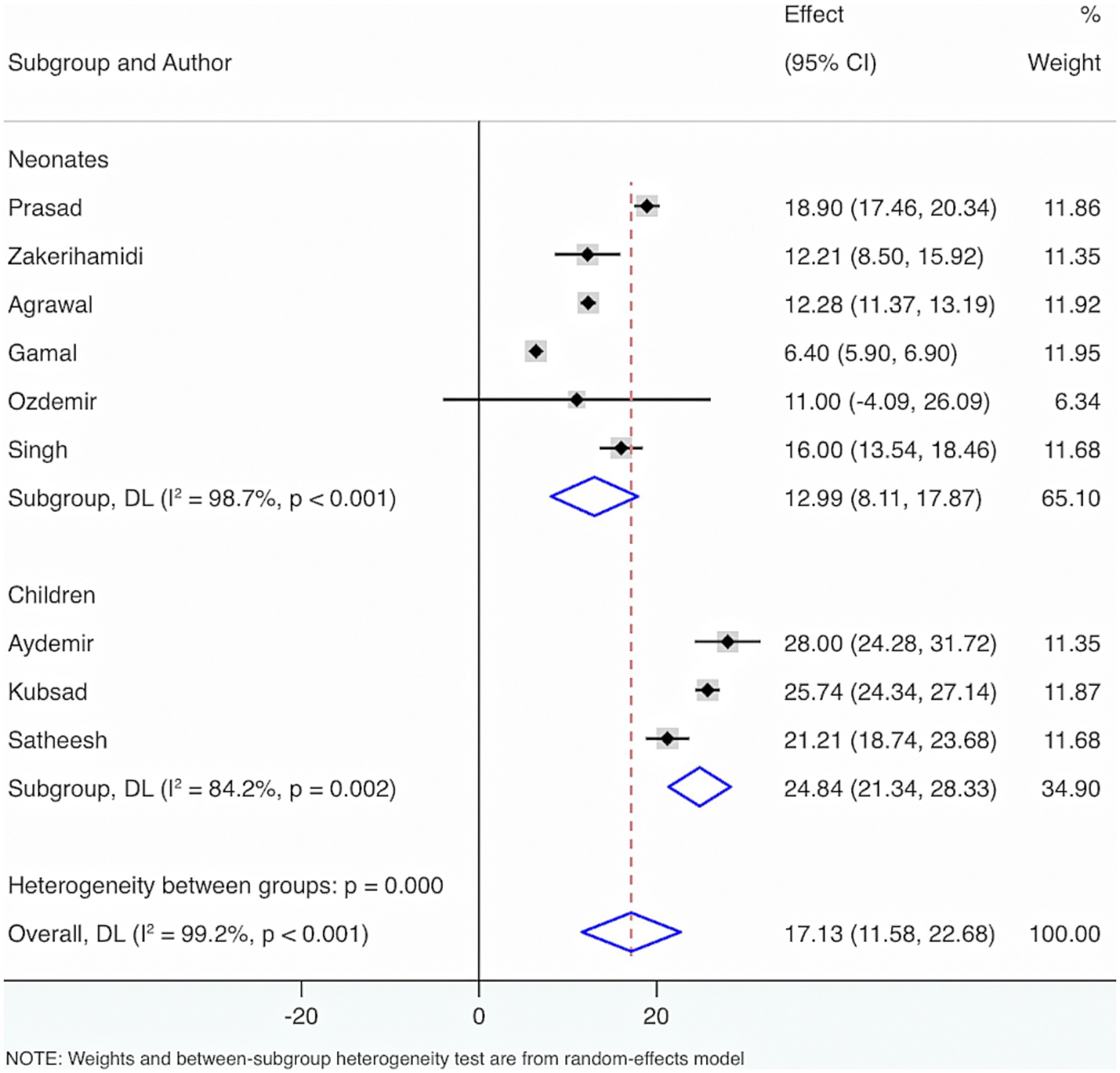
Seven studies were included. The results from the Cochrane Q test (p < 0.001) and I2 estimate (97.6%) signified considerable heterogeneity. Meta-analysis utilizing a random-effects model displayed that the overall prevalence of VD deficiency and insufficiency among neonatal and pediatric sepsis patients was 54% (95% CI: 37, 72%, p < 0.001) (Figure 3).
Subgroup analysis grounded in the characteristics of deficiency and insufficiency displayed that the aggregated prevalence of VD deficiency was 54% (95% CI: 31, 77%, p < 0.001). The overall prevalence of insufficiency was 43% (95% CI: 16, 70%, p = 0.002). The overall prevalence of either deficiency or insufficiency was 76% (95% CI: 66, 86%, p < 0.001).
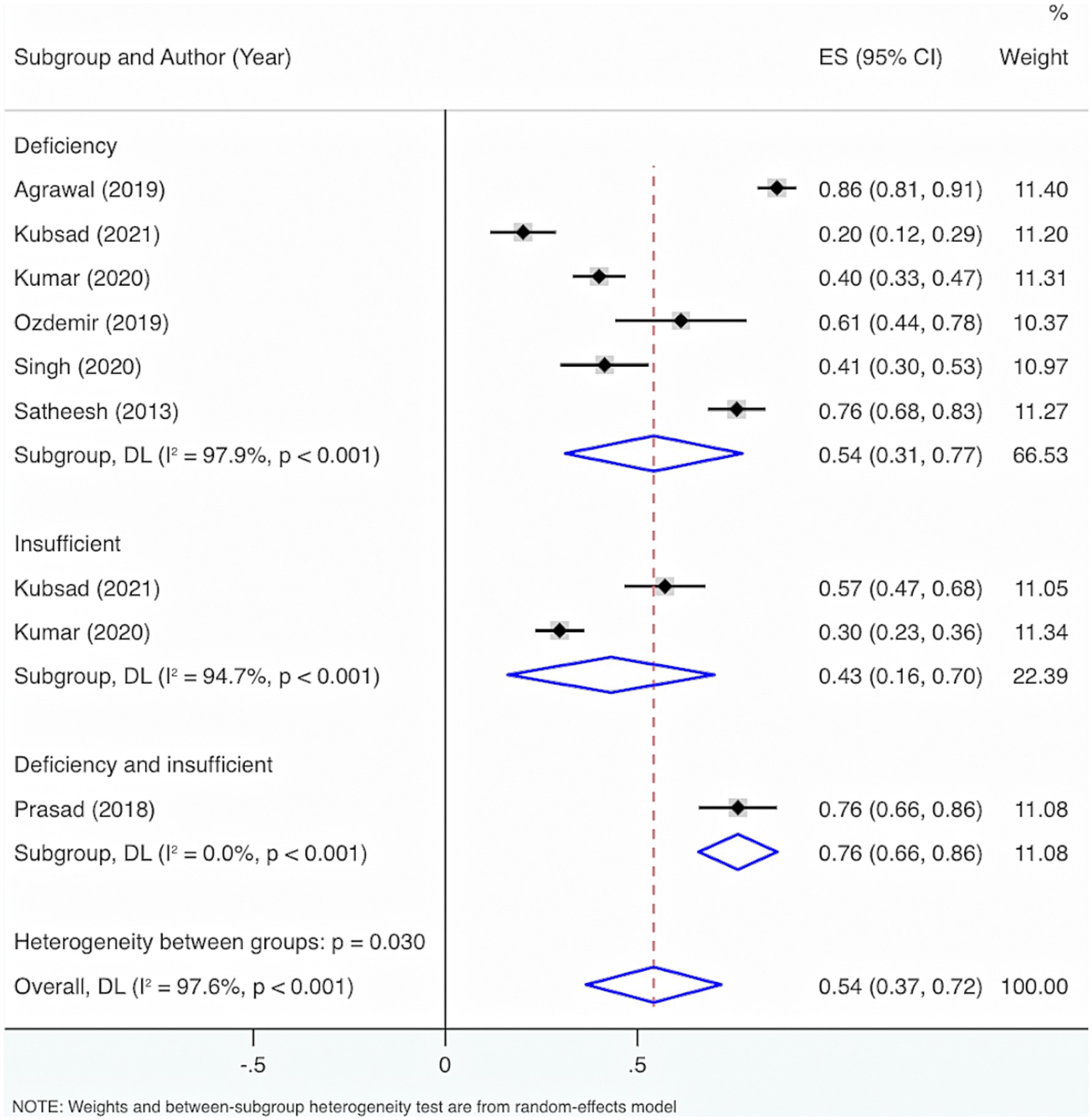
Subgroup analysis grounded in VD levels displayed that when VD levels were <30 ng/mL, <12 ng/mL, or ≤20 ng/mL, the overall prevalence of deficiency and insufficiency was relatively high, at 76% (95% CI: 66, 86%, p < 0.001), 61% (95% CI: 44, 78%, p < 0.001), and 54% (95% CI: 26, 86%, p < 0.001), respectively (Figure 4).
Further exploration of heterogeneity sources showed that in the features of deficiency and insufficiency, the heterogeneity result for deficiency was I2 = 97.9%, p < 0.001, for insufficiency was I2 = 94.7%, p < 0.001, and for deficiency or insufficiency was I2 = 0%, p < 0.001. Consequently, this may not be the principal cause of the high heterogeneity. The heterogeneity results were I2 = 0%, p < 0.001 for VD levels <30 ng/mL; I2 = 94.7%, p < 0.001 for VD levels of 20–29.9 ng/mL; I2 = 98.7%, p < 0.001 for VD levels ≤20 ng/mL; I2 = 0%, p < 0.001 for VD levels <12 ng/mL; I2 = 0%, p < 0.001 for VD levels <11 ng/mL; I2 = 0%, p < 0.001 for VD levels <10 ng/mL. Therefore, VD level was also not the main reason for the high heterogeneity.
3.4.3 VD deficiency and risk of mortality
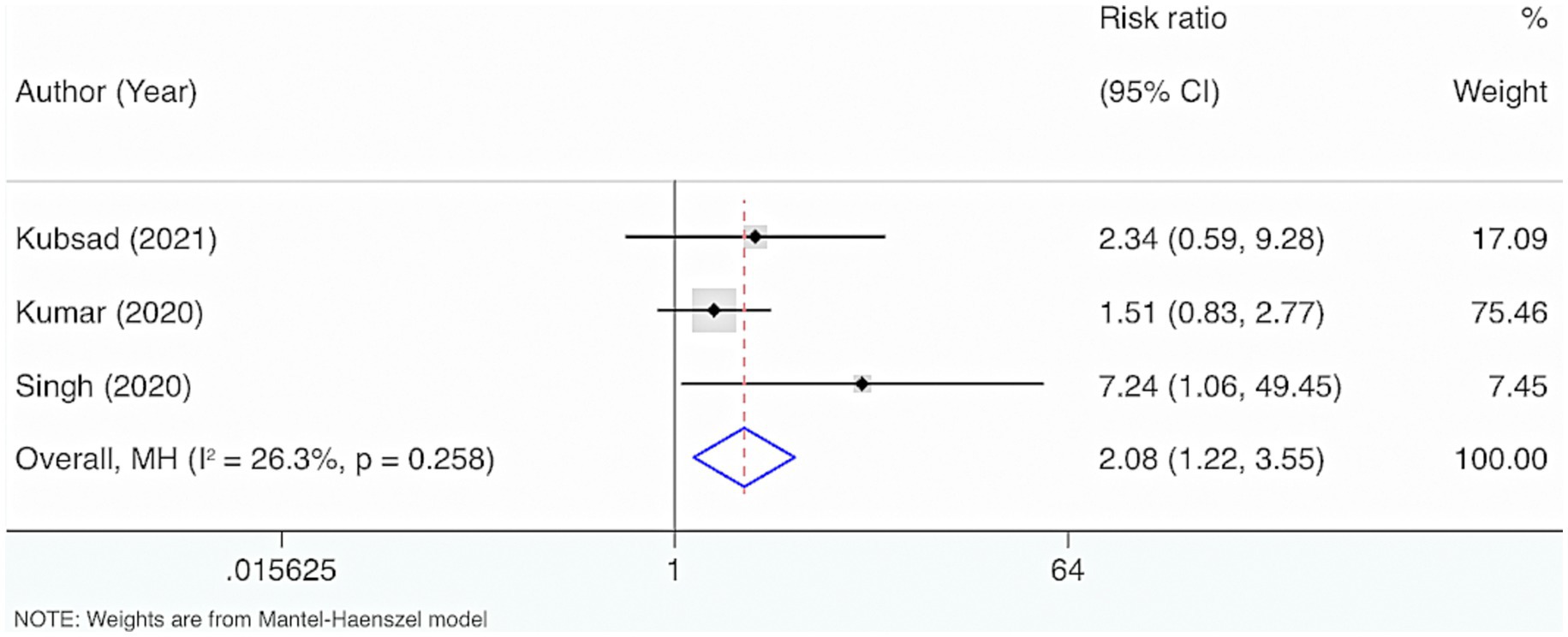
Three studies were included. The results from the Cochrane Q test (p = 0.26) and I2 estimate (26.3%) displayed no significant heterogeneity. Meta-analysis leveraging a fixed-effects model indicated a marked link between the deficiency of VD and mortality in neonatal and pediatric sepsis patients compared to the control group (RR = 2.08, 95% CI: 1.22, 3.55, p = 0.007) (Figure 5).
3.5 Adult sepsis
3.5.1 VD levels
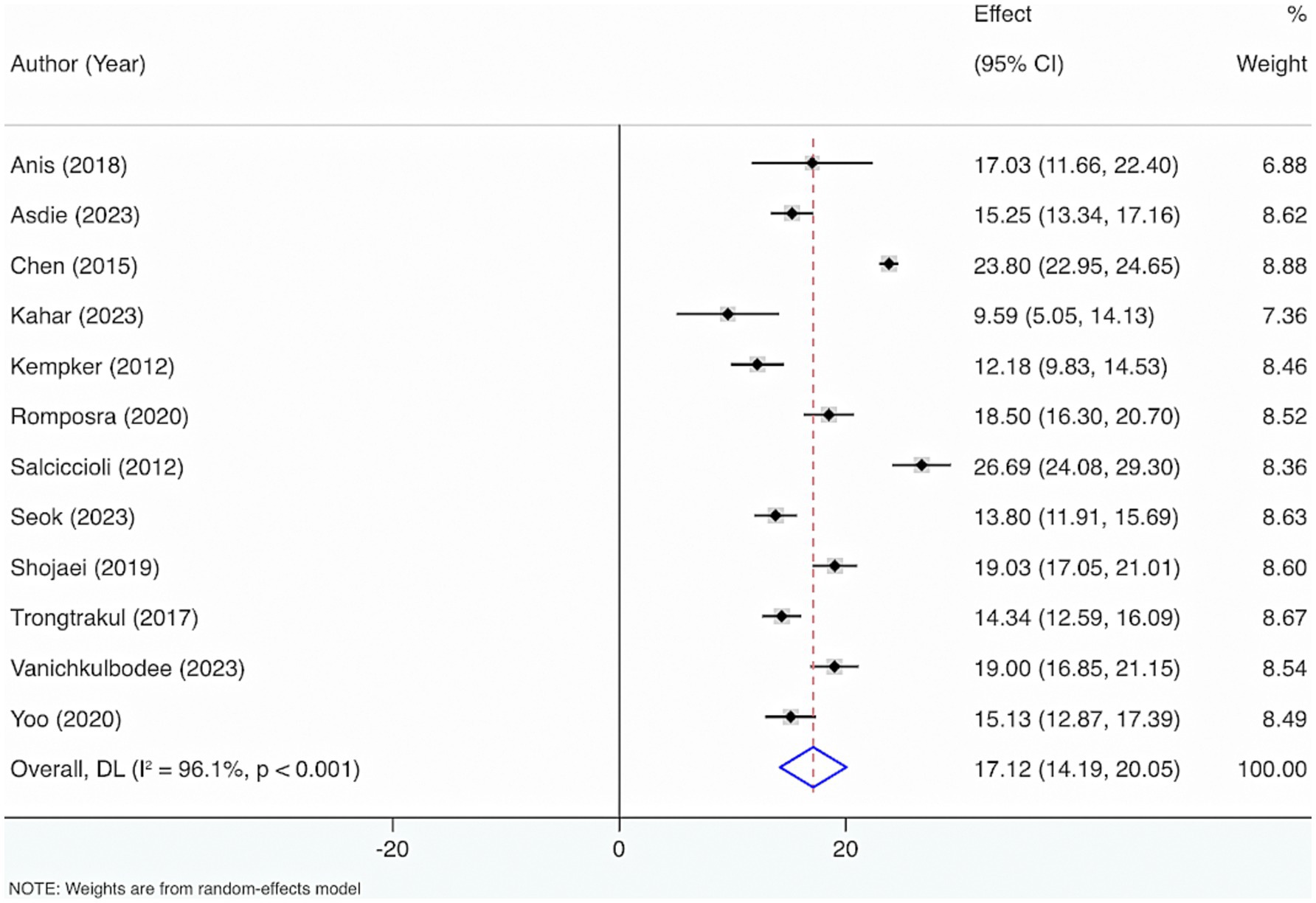
Twelve studies were included to estimate VD levels in adult sepsis individuals. The results from the Cochrane Q test (p < 0.001) and I2 estimate (96.1%) displayed considerable heterogeneity. Meta-analysis leveraging a random-effects model displayed that the average level of VD was 17.12 (95% CI: 14.19, 20.05) (Figure 6).
Seven studies were included to perform subgroup analysis by the control group, encompassing healthy individuals and those without sepsis. The results from the Cochrane Q test (p < 0.001) and I2 estimate (96.0%) showed considerable heterogeneity. Meta-analysis leveraging a random-effects model displayed that VD levels were considerably diminished in sepsis individuals relative to healthy individuals or those without sepsis (SMD = −1.63, 95% CI: −2.39, −0.87, p < 0.001), with considerable statistical distinction. Subgroup analysis showed that compared to healthy individuals, VD levels were considerably lower in adults with sepsis, with statistical differences (SMD = −3.32, 95% CI: −6.44, −0.19, p = 0.04); relative to people without sepsis, there was no statistically distinction in VD levels (SMD = −0.77, 95% CI: −1.55, 0.01, p = 0.05).
In addition, to figure out the source of heterogeneity, when the control group comprised healthy individuals, I2 = 97.1%, p < 0.001. When the control group comprised individuals without sepsis, I2 = 95.6%, p < 0.001. Therefore, the control group may not cause the high heterogeneity (Figure 7).
3.5.2 Prevalence of VD deficiency and insufficiency
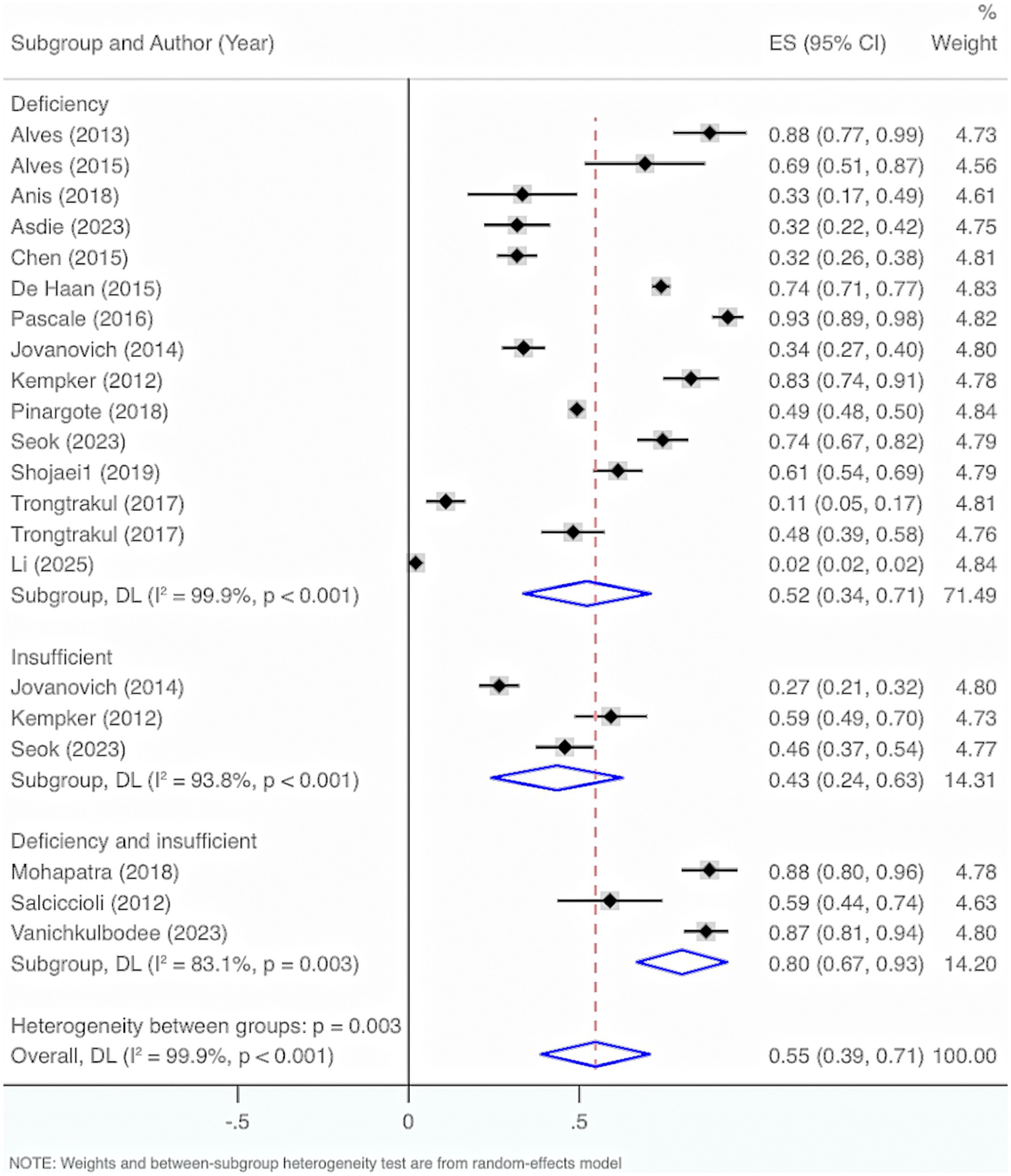
Seventeen studies were included. The results from the Cochrane Q test (p < 0.001) and I2 estimate (99.9%) showed significant heterogeneity. Meta-analysis was executed by leveraging a random-effects model. The results unraveled an overall prevalence of VD deficiency and insufficiency of 55% (95% CI: 39, 71%, p < 0.001).
Subgroup analysis by characteristics of deficiency and insufficiency displayed that the overall prevalence of VD deficiency was 52% (95% CI: 34, 71%, p < 0.001). The overall prevalence of insufficiency was 43% (95% CI: 24, 63%, p < 0.001). The overall prevalence of either deficiency or insufficiency was 80% (95% CI: 67, 93%, p < 0.001).
Further exploration of heterogeneity sources illustrated that in the features of deficiency and insufficiency, the heterogeneity result for deficiency was I2 = 99.9%, p < 0.001, for insufficiency was I2 = 93.8%, p < 0.001, and for deficiency or insufficiency was I2 = 83.1%, p < 0.001. Therefore, this may not be the main cause of the high heterogeneity (Figure 8).
3.5.3 VD deficiency and incidence of sepsis
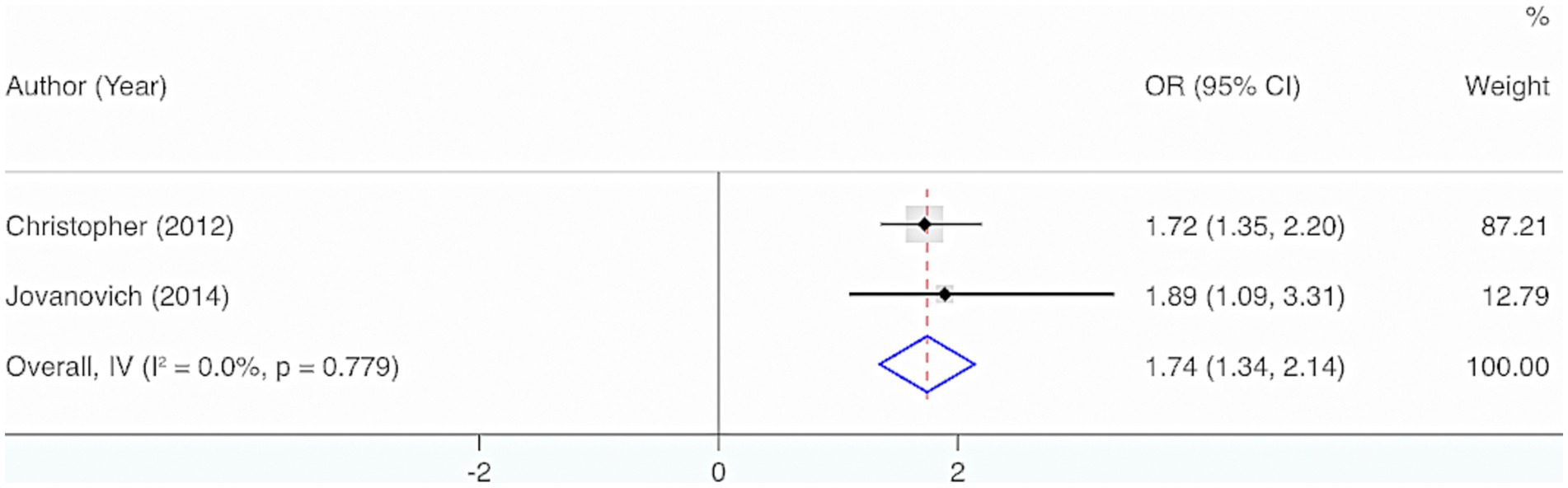
Two studies were included. The results from the Cochrane Q test (p = 0.78) and I2 estimate (0%) displayed no significant heterogeneity. Meta-analysis utilizing a fixed-effects model indicated a considerable interrelation between the deficiency of VD and the incidence of sepsis in adults (OR = 1.74, 95% CI: 1.34, 2.14, p < 0.001) (Figure 9).
3.5.4 VD levels and sepsis mortality
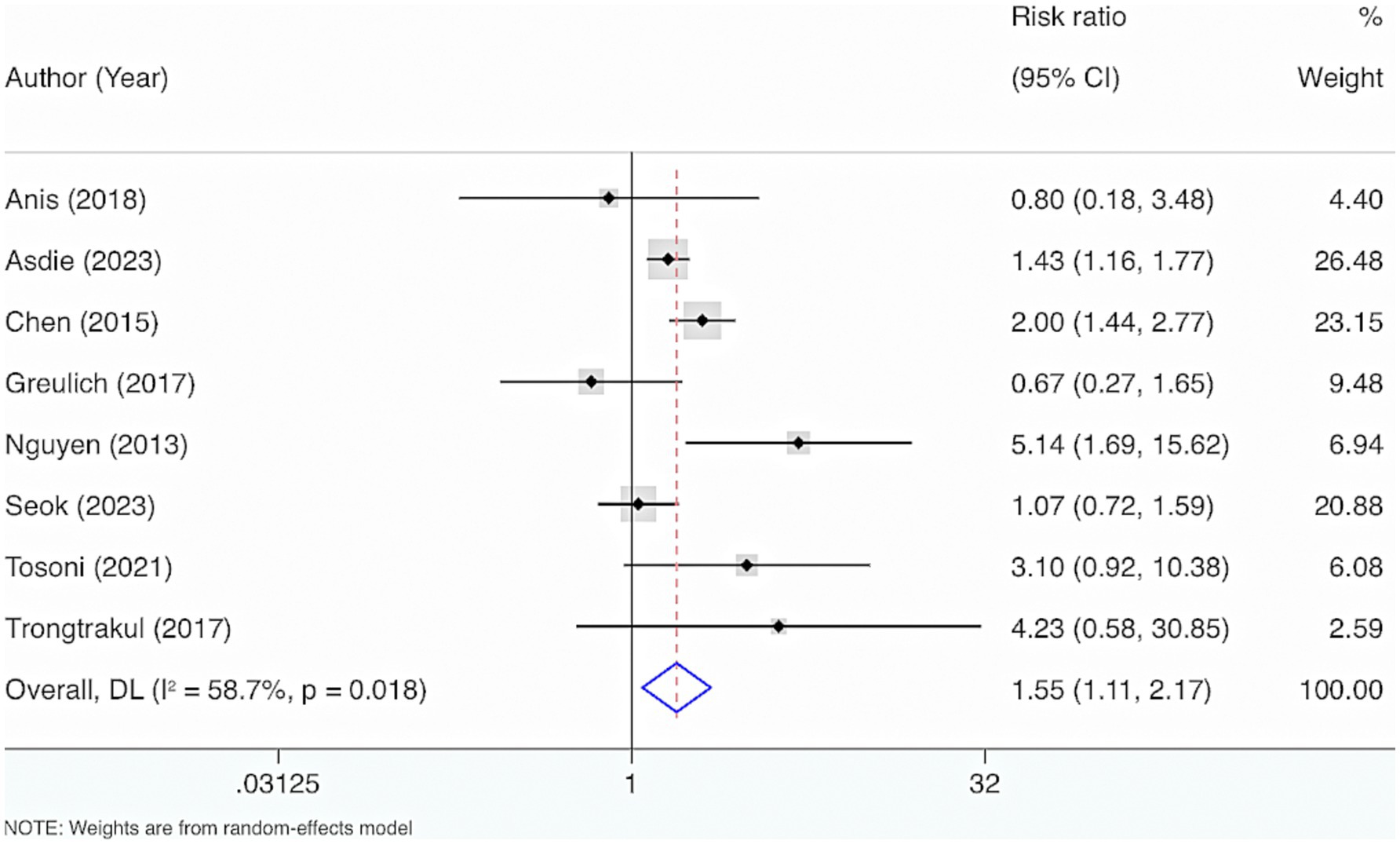
Eight studies were included. The results from the Cochrane Q test (p = 0.02) and I2 estimate (58.7%) displayed considerable heterogeneity. Meta-analysis leveraging a random-effects model displayed a considerable interrelation between reduced VD levels and mortality among adult individuals with sepsis (RR = 1.55, 95% CI: 1.11, 2.17, p = 0.01) (Figure 10).
3.5.5 VD supplementation and mortality of sepsis
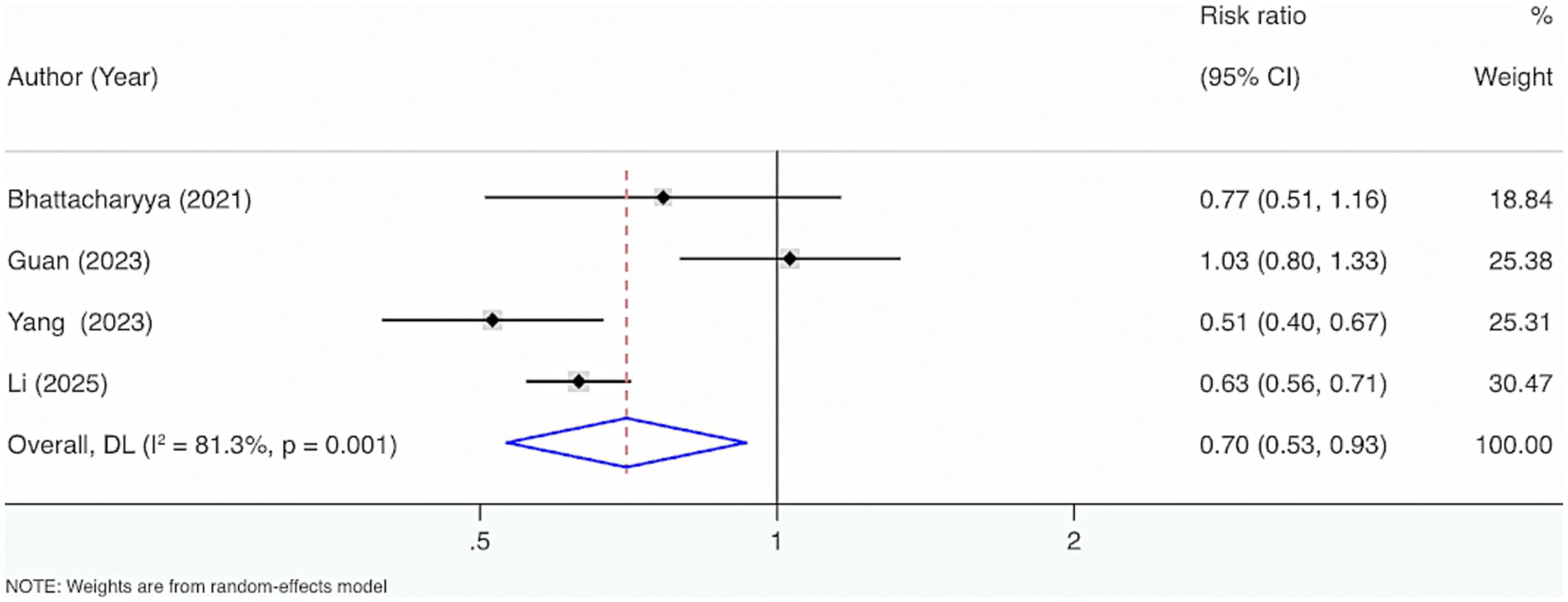
Four studies were included. The results from the Cochrane Q test (p = 0.001) and I2 estimate (81.3%) showed considerable heterogeneity. Meta-analysis leveraging a random-effects model displayed that VD supplementation considerably reduced the occurrence of mortality events (RR = 0.70, 95% CI: 0.53, 0.93, p = 0.01) (Figure 11).
3.5.6 Deficiency of VD and mortality of sepsis
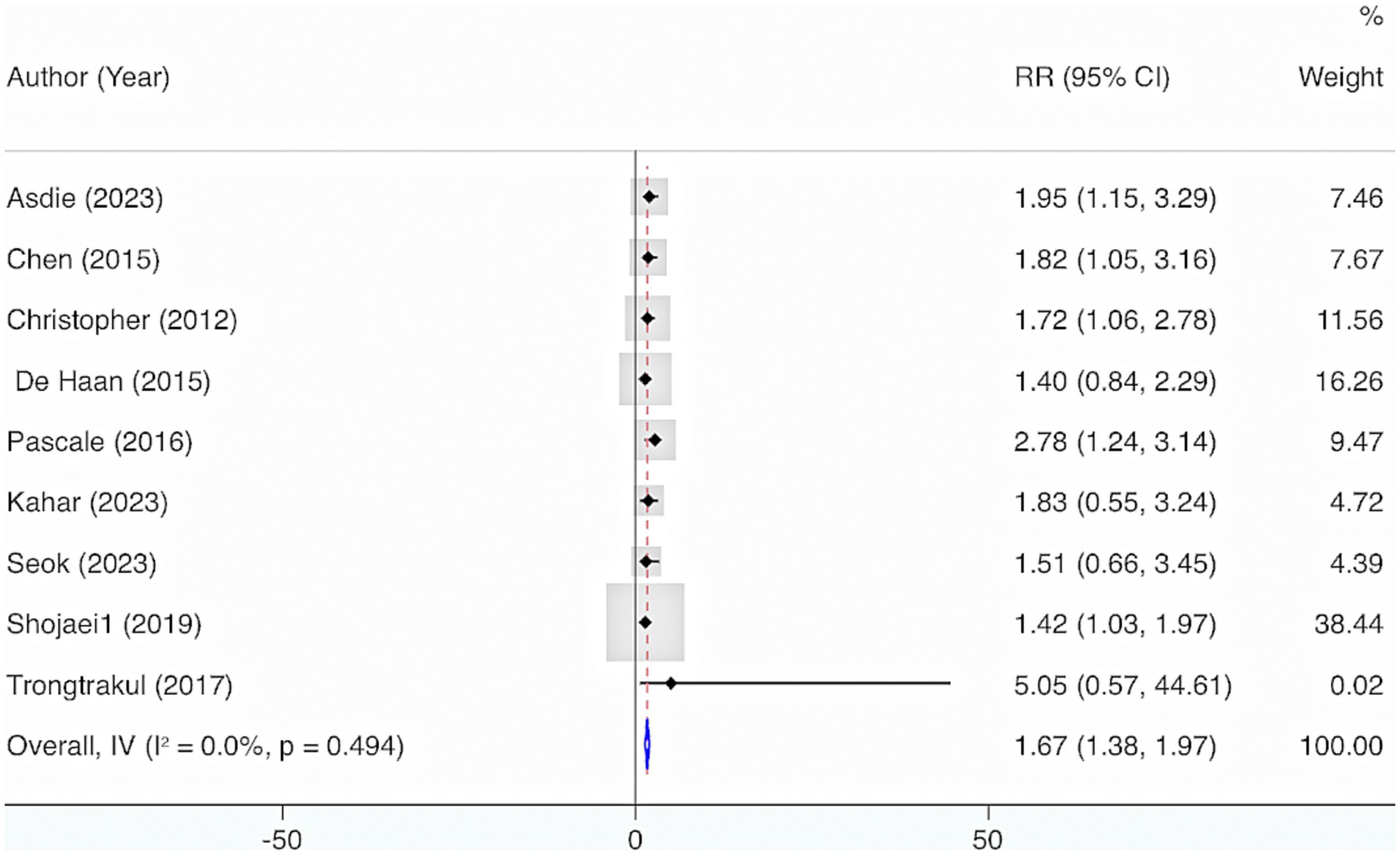
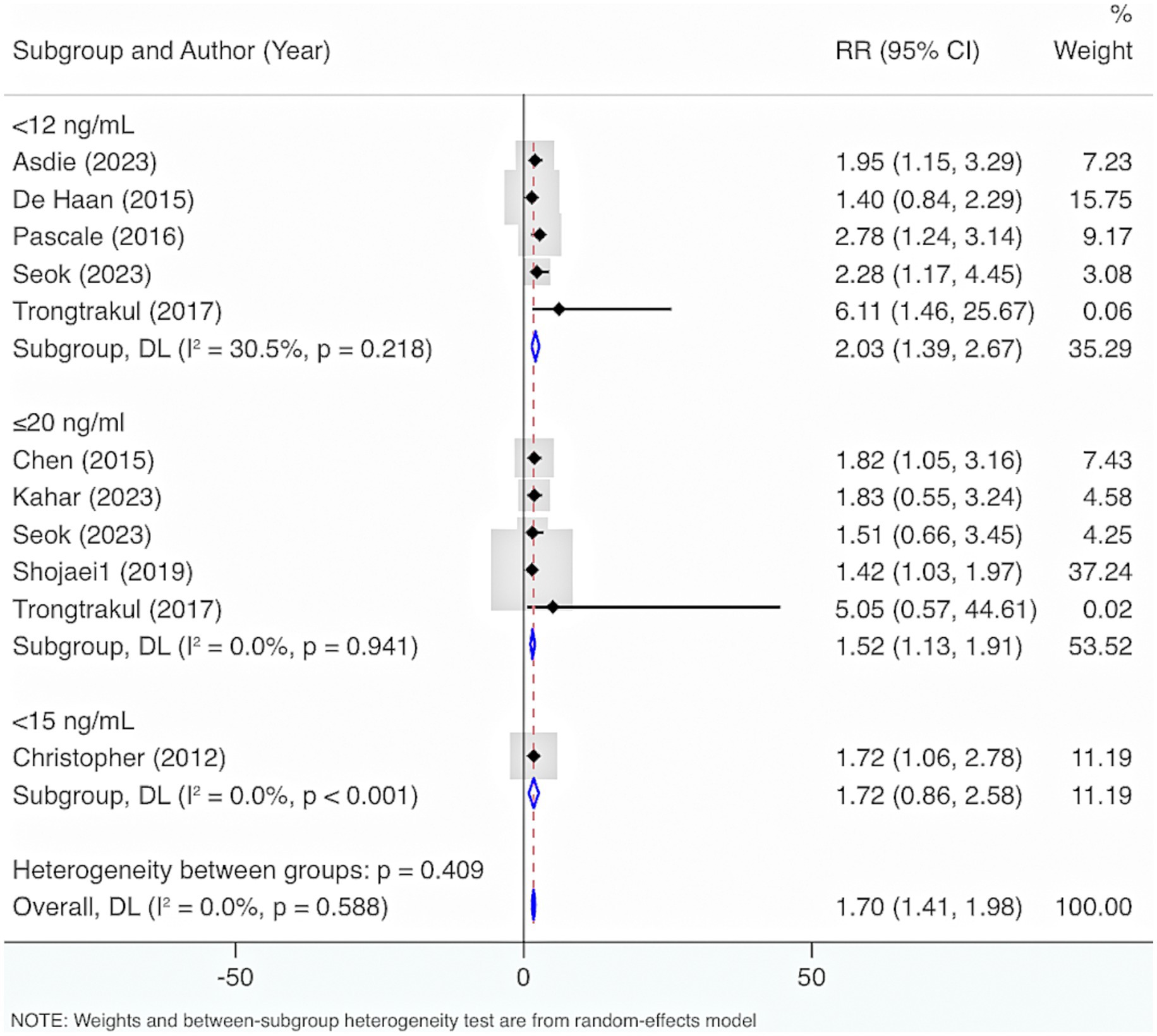
Nine studies were included. The results from the Cochrane Q test (p = 0.49) and I2 estimate (0%) showed no significant heterogeneity. Meta-analysis leveraging a fixed-effects model displayed that deficiency of VD elevated the risk of mortality (RR = 1.67, 95% CI: 1.38, 1.97, p < 0.001) (Figure 12). Further subgroup analysis grounded in levels of VD deficiency illustrated that a more severe deficiency in VD was linked to a higher risk of mortality. When VD levels were <12 ng/mL, the likelihood of mortality was highest (RR = 2.03, 95% CI: 1.39, 2.67, p < 0.001) (Figure 13).
3.5.7 VD supplementation and length of ICU stay
Four studies were incorporated. Results from the Cochrane Q test (p < 0.001) and I2 estimate (89.9%) showed considerable heterogeneity. Meta-analysis leveraging a random-effects model displayed that supplementing VD did not shorten the length of ICU stay (MD = −0.11, 95% CI: −0.62, 0.40, p = 0.67) (Supplementary Figure S3).
3.5.8 Predictive value of VD levels for mortality
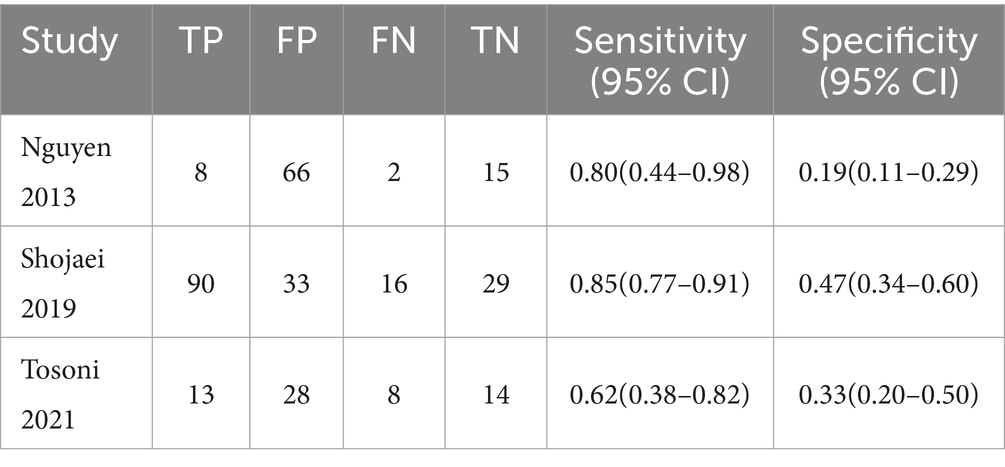
Three studies were included. The combined sensitivity and specificity were 0.81 (95% CI: 0.73, 0.87) and 0.31 (95% CI: 0.25, 0.39). In terms of sensitivity, results from the Cochrane Q test (p = 0.07) and I2 estimate (62%) showed significant heterogeneity. For specificity, results from the Cochrane Q test (p = 0.001) and I2 estimate (85%) showed significant heterogeneity (Table 2).
3.6 Sensitivity analysis and publication bias
Sensitivity analysis was implemented on such outcomes as levels of VD and prevalence of VD deficiency and insufficiency among neonates and pediatric sepsis patients. The leave-one-out method results displayed that excluding any individual research did not substantially affect the main conclusions (Supplementary Figures S4, S5). Sensitivity analysis was implemented on such outcomes as levels of VD, prevalence of VD deficiency and insufficiency among adult sepsis patients, VD levels and the risk of sepsis mortality based on binary variables, and deficiency of VD and mortality of sepsis through multivariable statistical analysis. The results illustrated that excluding any individual research did not substantially affect the main conclusions (Supplementary Figures S6–S9).
Publication bias was estimated for such outcomes as levels of VD and prevalence of VD deficiency and insufficiency among adult sepsis individuals. The funnel plot showed an asymmetric distribution in the analysis of VD levels (Supplementary Figure S10). Egger’s test illustrated potential publication bias (p = 0.03). Furthermore, the trim-and-fill analysis indicated that no missing studies needed to be imputed. This suggests that no funnel plot asymmetry attributable to small-study effects was detected in our analysis, thereby supporting the robustness of the current meta-analytic results (Supplementary Figure S11). The funnel plot also showed an asymmetric distribution in the analysis of the prevalence of VD deficiency and insufficiency (Supplementary Figure S12). Egger’s test illustrated potential publication bias (p < 0.05). Since applying the trim-and-fill method directly to raw proportions can yield illogical results (e.g., values >1), we first performed a logit transformation on the proportions from the original data and conducted the trim-and-fill analysis on the transformed scale. After imputing 10 theoretically missing studies, the pooled effect size was 22.9% (95% CI, 9.3, 56.3%, p = 0.001). This estimate, which is slightly lower than the initial pooled result, suggests that the original findings may have been influenced to some extent by publication bias (Supplementary Figure S13).
4 Discussion
This study is pioneering in comprehensively estimating the VD levels, prevalence of deficiency, interrelation of VD levels with incidence and mortality of sepsis, and forecasting capability of VD among pediatric and adult individuals with sepsis separately. This assessment is conducted in alignment with guidelines on early detection, appropriate intervention, and prognostic value.
This research illustrated that the VD status was relatively deficient among neonates and pediatric individuals with sepsis. The overall prevalence of VD deficiency and insufficiency reached 54%. This result aligns with prior findings (12, 63). Xiao et al. (12) observed that, relative to the control group of healthy individuals, neonates and children suffering from sepsis had decreased VD levels. Yu et al. (63) reported that, compared to neonates and children without sepsis, critically ill infants and children suffering from sepsis had decreased VD levels and more severe deficiency. Workneh Bitew et al. (78) found that the overall prevalence of VD deficiency in neonates with sepsis reached 79.4%. The prevalence of VD deficiency found in our study is relatively lower than that of Workneh Bitew et al. (78). The disparity may arise from differences in the years of publication of the studies included, as most studies we included were published after 2019 (accounting for 78% of the total studies). Improvements in medical care, along with increased awareness of supplementing VD among physicians and parents may induce a reduction in the prevalence of VD deficiency and insufficiency. Additionally, this research illustrated a considerable interrelation between deficiency of VD and mortality among neonates and pediatric sepsis patients. However, previous meta-analyses regarding neonates and pediatric sepsis populations did not report this finding (63, 64). The interrelation between VD deficiency and mortality of neonates and children has been confirmed in other studies. He et al. (65) indicated an independent interrelation between deficiency of VD in children and scores of pediatric mortality risk III. Su and Jia (66) indicated that children with VD deficiency had a significantly elevated likelihood of developing acute mortality and critical mortality relative to those with normal levels of VD, which was 1.77 times that of the normal VD group. This may be due to that VD deficiency could increase the likelihood of respiratory failure and heart failure, thereby increasing mortality (67, 68). Therefore, early identification is recommended for neonates and pediatric sepsis patients with VD deficiency to respond to adverse outcomes in time. Finally, due to the limited number of included studies involving neonatal and pediatric patients with sepsis (n = 10), and the presence of geographically imbalanced distribution (India = 6, Turkey = 2, Egypt = 1, Iran = 1), the interpretation of these findings should be approached with caution, and their generalizability to other regions requires careful consideration.
In adult sepsis patients, VD status was notably deficient, with the overall prevalence of VD deficiency and insufficiency reaching 55%. VD is known for its regulatory function in the immune system and its potential in infection prevention (69, 70). Consequently, in sepsis patients, VD levels are relatively low and the prevalence of deficiency and insufficiency is relatively high. Furthermore, this study observed a considerable interrelation between the deficiency of VD and both the incidence and mortality of sepsis (p < 0.001). Supplementing VD can considerably diminish the risk of mortality (p < 0.05). As an essential micronutrient, VD is crucial in the pathogenesis and mortality of sepsis. VD exerts its effects by binding to VD receptors expressed on T lymphocytes, B lymphocytes, macrophages, and dendritic cells. It specifically modulates both innate and adaptive immune responses, which is vital to maintain immune homeostasis among sepsis patients. Meanwhile, it can prevent severe disease progression and influence prognosis (71, 72). For sepsis patients who are continually challenged by pathogens and uncontrolled immune responses, supplementing VD may be the optimal therapeutic intervention to prevent adverse outcomes. On one hand, VD may restore the levels of serum IL-37, thereby enhancing antimicrobial activity (73); on the other hand, it regulates innate immunity to protect the body from excessive production of inflammatory cytokines (74). Since this outcome was based on only four studies, only one of which was a high-quality RCT, the results may not sufficiently verify the therapeutic effect of vitamin D supplementation on mortality in sepsis patients. This may be attributed to the fact that research in this area is still in its early stages. Despite our comprehensive search strategy, few studies met the eligibility criteria. Furthermore, as the available studies did not provide sufficient intervention details, it was not possible to perform subgroup analyses based on vitamin D dosage, treatment duration, or route of administration. Therefore, future randomized controlled trials should focus on investigating the dose–response relationship of vitamin D supplementation on outcomes in sepsis patients, in order to provide a scientific basis for developing standardized clinical intervention protocols. Lastly, this study also observed that VD had a reliable forecasting ability for mortality in sepsis patients. A recent meta-analysis of multivariable-adjusted follow-up studies has confirmed this finding, showing that severe VD deficiency is independently correlated with an elevated likelihood of mortality in adult individuals with sepsis (11). This may be due to the interaction between VD receptors and related signaling pathways, which enables VD to help maintain the fundamental functions of the heart (75), lungs (76), and kidneys (77) during severe infections.
4.1 Study limitations
Our study has several limitations. First, although most eligible studies were cohort studies, some case–control and cross-sectional studies were also included. Future research could focus exclusively on cohort studies to offer reliable evidence on the long-term effects of VD in individuals with sepsis. Second, there was high heterogeneity in several outcome indicators. Despite conducting subgroup analyses, the sources of heterogeneity were not identified. This may be related to the potential influence of several research characteristics on the outcomes, for instance, the nutritional status of the patients, diagnostic criteria for sepsis, the critical threshold for deficiency of VD, the dose of VD supplementation, and the units of VD measurement. Future research should incorporate more studies and conduct meta-regression to figure out the sources of heterogeneity. Third, this study merged the results of adjusted and unadjusted multivariate analyses, which could make the findings susceptible to residual bias and unadjusted confounding factors. Hence, the results need to be interpreted cautiously. Fourth, the use of a uniform vitamin D deficiency threshold across all pediatric age groups, without distinguishing between neonates and other subgroups, represents another limitation. Given that neonates may have distinct vitamin D metabolic profiles and risk factors, applying a single cut-off value may not accurately reflect the status of all populations. This could be a source of potential heterogeneity and may limit the extension of our conclusions to specific age subgroups. Future studies are urgently needed to establish and validate age-specific thresholds. Fifth, given the well-established physiological differences in vitamin D metabolism between neonates and children, pooling these populations may introduce substantial heterogeneity and could obscure distinct associations within each subgroup. Therefore, the pooled effect estimates should be interpreted with caution, and future studies should report outcomes stratified by these key age groups. Lastly, currently, no research investigates the predictive value of VD for the incidence or mortality of neonatal and individuals with pediatric sepsis. Future studies could focus on this area, preventing adverse outcomes.
5 Conclusion
VD status is deficient among both adult and pediatric sepsis patients, with a high prevalence of deficiency and insufficiency. VD deficiency is significantly linked to mortality in pediatric sepsis. Early supplementation of VD is recommended to prevent adverse outcomes. In adults with sepsis, VD deficiency is tightly linked to both the incidence and mortality. VD supplementation considerably reduces the risk of mortality. However, further large-scale RCTs are required to validate the therapeutic capability of supplementing VD. Interpretation of the results regarding neonatal and pediatric sepsis patients should be made with caution, and their generalizability to other regions requires careful consideration. VD can be a valuable biomarker to forecast mortality in adults with sepsis. Nevertheless, the data in the existing literature are not sufficient to reliably estimate its accuracy. Additional prospective studies are necessitated.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
HZ: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. KL: Formal analysis, Investigation, Methodology, Writing – review & editing. YZ: Conceptualization, Writing – review & editing. JQ: Supervision, Writing – review & editing. GS: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Guizhou Traditional Chinese Medicine Letter [2024] No. 38, and Guizhou Science and Technology Cooperation Foundation-ZK [2024] General 415.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1670083/full#supplementary-material
Footnotes
References
1. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Machado, FR, Cavalcanti, AB, Bozza, FA, Ferreira, EM, Angotti Carrara, FS, Sousa, JL, et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence assessment database, SPREAD): an observational study. Lancet Infect Dis. (2017) 17:1180–9. doi: 10.1016/s1473-3099(17)30322-5
3. Rudd, KE, Johnson, SC, Agesa, KM, Shackelford, KA, Tsoi, D, Kievlan, DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/s0140-6736(19)32989-7
4. Evans, L, Rhodes, A, Alhazzani, W, Antonelli, M, Coopersmith, CM, French, C, et al. Surviving Sepsis campaign: international guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. (2021) 49:e1063–143. doi: 10.1097/ccm.0000000000005337
5. Egi, M, Ogura, H, Yatabe, T, Atagi, K, Inoue, S, Iba, T, et al. The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). Acute Med Surg. (2021) 8:e659. doi: 10.1002/ams2.659
6. Chirumbolo, S, Bjørklund, G, Sboarina, A, and Vella, A. The role of vitamin D in the immune system as a pro-survival molecule. Clin Ther. (2017) 39:894–916. doi: 10.1016/j.clinthera.2017.03.021
7. Ghaseminejad-Raeini, A, Ghaderi, A, Sharafi, A, Nematollahi-Sani, B, Moossavi, M, Derakhshani, A, et al. Immunomodulatory actions of vitamin D in various immune-related disorders: a comprehensive review. Front Immunol. (2023) 14:950465. doi: 10.3389/fimmu.2023.950465
8. Shi, S, Yang, S, Ma, P, Wang, Y, Ma, C, and Ma, W. Genetic evidence indicates that serum micronutrient levels mediate the causal relationships between immune cells and neuropathic pain: a mediation mendelian randomization study. Mol Neurobiol. (2025) 62:8652–69. doi: 10.1007/s12035-025-04805-9
9. Ji, W, Xie, X, Bai, G, Fan, Y, He, Y, Zhang, L, et al. Proteomics reveals that vitamin D deficiency leads to immunoglobulin abnormalities and immune dysregulation in patients with post-COVID-19 condition. J Proteome Res. (2025) 24:1449–61. doi: 10.1021/acs.jproteome.4c01120
10. Safabakhsh, M, Imani, H, Shahinfar, H, Mohammadpour, M, Rohani, P, and Shab-Bidar, S. Efficacy of dietary supplements on mortality and clinical outcomes in adults with sepsis and septic shock: a systematic review and network meta-analysis. Clin Nutr. (2024) 43:1299–307. doi: 10.1016/j.clnu.2024.03.030
11. Li, Y, and Ding, S. Serum 25-hydroxyvitamin D and the risk of mortality in adult patients with Sepsis: a meta-analysis. BMC Infect Dis. (2020) 20:189. doi: 10.1186/s12879-020-4879-1
12. Xiao, D, Zhang, X, Ying, J, Zhou, Y, Li, X, Mu, D, et al. Association between vitamin D status and sepsis in children: a meta-analysis of observational studies. Clin Nutr. (2020) 39:1735–41. doi: 10.1016/j.clnu.2019.08.010
13. de Haan, K, Groeneveld, AB, de Geus, HR, Egal, M, and Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. (2014) 18:660. doi: 10.1186/s13054-014-0660-4
14. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
15. Shang, S, Chen, D, Wei, Y, Zou, S, Chang, Q, Zhou, H, et al. The role of vitamin D and vitamin D receptor in Sepsis. Curr Issues Mol Biol. (2025) 47:500. doi: 10.3390/cimb47070500
16. Fabregat-Bolufer, AB, Escolà-Rodríguez, A, Bedini-Chesa, JL, Casals, G, Morales-Ruiz, M, and Filella, X. Redefining vitamin D status: establishing population-based indirect reference intervals through big data analysis. Clin Chim Acta. (2025) 569:120155. doi: 10.1016/j.cca.2025.120155
17. Amrein, K, Scherkl, M, Hoffmann, M, Neuwersch-Sommeregger, S, Köstenberger, M, Tmava Berisha, A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. (2020) 74:1498–513. doi: 10.1038/s41430-020-0558-y
18. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
19. Shekelle, PG, Ortiz, E, Rhodes, S, Morton, SC, Eccles, MP, Grimshaw, JM, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: how quickly do guidelines become outdated? JAMA. (2001) 286:1461–7. doi: 10.1001/jama.286.12.1461
20. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
21. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Patsopoulos, NA, Evangelou, E, and Ioannidis, JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. (2008) 37:1148–57. doi: 10.1093/ije/dyn065
23. Sterne, JA, and Egger, M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54:1046–55. doi: 10.1016/s0895-4356(01)00377-8
24. Satheesh, P, Verma, S, Singhi, S, and Bansal, A. Prevalence of vitamin D deficiency among children with sepsis, its association with sepsis severity and its outcome in a pediatric ICU. Crit Care. (2013) 17:P35–59. doi: 10.1186/cc12935
25. Aydemir, G, Cekmez, F, Kalkan, G, Fidanci, MK, Kaya, G, Karaoglu, A, et al. High serum 25-hydroxyvitamin D levels are associated with pediatric sepsis. Tohoku J Exp Med. (2014) 234:295–8. doi: 10.1620/tjem.234.295
26. Ozdemir, AA, and Cag, Y. Neonatal vitamin D status and the risk of neonatal sepsis. Pak J Med Sci. (2019) 35:420–5. doi: 10.12669/pjms.35.2.342
27. Kk, M, Das, S, Biswal, N, Parameswaran, N, and Nanda, N. Vitamin D status at admission and its association with mortality in children admitted to the Pediatric intensive care unit. Cureus. (2020) 12:e8413. doi: 10.7759/cureus.8413
28. Singh, P, and Chaudhari, V. Association of early-onset sepsis and vitamin D deficiency in term neonates. Indian Pediatr. (2020) 57:232–4. doi: 10.1007/s13312-020-1757-2
29. Gamal, TS, Madiha, A-AS, Hanan, MK, Abdel-Azeem, ME-M, and Marian, GS. Neonatal and maternal 25-OH vitamin D serum levels in neonates with early-onset sepsis. Children. (2017) 4:37. doi: 10.3390/children4050037
30. Prasad, R, Shanataram, B, Kiran, B, and Dsa, S. Vitamin D levels in late pre-term neonates and its association with sepsis. Indian J Public Health Res Dev. (2018) 9:128–33. doi: 10.5958/0976-5506.2018.01327.X
31. Amit, A, Aekta, G, and Jyotsna, S. Role of vitamin-D deficiency in term neonates with late-onset sepsis: a case-control study. J Trop Pediatr. (2019) 65:609–16. doi: 10.1093/tropej/fmz021
32. Kubsad, P, Ravikiran, SR, Bhat, KG, Kamath, N, Kulkarni, V, Manjrekar, PA, et al. Hypovitaminosis D and parathyroid hormone response in critically ill children with sepsis: a case-control study. Indian J Crit Care Med. (2021) 25:923–7. doi: 10.5005/jp-journals-10071-23913
33. Zakerihamidi, M, Boskabadi, H, and Faramarzi, R. Comparison of the level of vitamin D in preterm infected and uninfected infants. Russ J Infect Immun. (2023) 13:754–60. doi: 10.15789/2220-7619-COT-8050
34. Kempker, J, Ziegler, T, Tangpricha, V, Guidot, D, and Martin, G. Vitamin D as a predictor for sepsis in the medical ICU. Chest. (2012) 142:379. doi: 10.1378/chest.1389879
35. Christopher, K, Moromizato, T, Litonjua, A, Braun, A, Gibbons, F, and Giovannucci, E. Association of vitamin D deficiency and the development of sepsis: a registry based cohort study In: Intensive care medicine. ESICM 25th Annual Congress, Lisbon, Portugal. Springer (2012) 38:S8. doi: 10.1007/s00134-012-2683-0
36. Salciccioli, JD, Ginde, AA, Graver, A, Giberson, T, Cristia, C, Cochhi, MN, et al. Assessing vitamin D status in sepsis and association with systemic inflammation. Acad Emerg Med. (2012) 19:S160–1. doi: 10.1186/s12879-016-1877-4
37. Nguyen, HB, Eshete, B, Lau, KW, Sai, A, Villarin, M, and Baylink, D. Serum 1, 25-dihydroxyvitamin D: an outcome prognosticator in human sepsis. PLoS One. (2013) 8:e64348. doi: 10.1371/journal.pone.0064348
38. Alves, F, Azevedo, L, and Machado, F. Vitamin D levels and organ dysfunction in patients with severe sep阿sis and septic shock In: Intensive Care Med. ESICM 26th Annual Congress, Paris, France. Springer (2013) 39:201. doi: 10.1007/s00134-013-3095-5
39. Jovanovich, AJ, Ginde, AA, Holmen, J, Jablonski, K, Allyn, RL, Kendrick, J, et al. Vitamin D level and risk of community-acquired pneumonia and sepsis. Nutrients. (2014) 6:2196–205. doi: 10.3390/nu6062196
40. Alves, FS, Freitas, FGR, Bafi, AT, Azevedo, LCP, and Machado, FR. Serum concentrations of vitamin D and organ dysfunction in patients with severe sepsis and septic shock. Rev Bras Ter Intensiva. (2015) 27:376–82. doi: 10.5935/0103-507X.20150063
41. Chen, Z, Luo, Z, Zhao, X, Chen, Q, Hu, J, Qin, H, et al. Association of vitamin D status of septic patients in intensive care units with altered procalcitonin levels and mortality. J Clin Endocrinol Metab. (2015) 100:516–23. doi: 10.1210/jc.2013-4330
42. Haan, KD. Low serum 25-hydroxyvitamin D at critical care initiation is associated with sepsis and morbidity in Dutch critically ill patients. Crit Care. (2015) 19:P365. doi: 10.1186/cc14445
43. De Pascale, G, Vallecoccia, M, Schiattarella, A, Di Gravio, V, Cutuli, S, Bello, G, et al. Clinical and microbiological outcome in septic patients with extremely low 25-hydroxyvitamin D levels at initiation of critical care. Clin Microbiol Infect. (2016) 22:e7:456. doi: 10.1016/j.cmi.2015.12.015
44. Greulich, T, Regner, W, Branscheidt, M, Herr, C, Koczulla, A, Vogelmeier, C, et al. Altered blood levels of vitamin D, cathelicidin and parathyroid hormone in patients with sepsis—a pilot study. Anaesth Intensive Care. (2017) 45:36–45. doi: 10.1177/0310057X1704500106
45. Ratzinger, F, Haslacher, H, Stadlberger, M, Schmidt, RL, Obermüller, M, Schmetterer, KG, et al. 25 (OH) D and 1, 25 (OH) D vitamin D fails to predict sepsis and mortality in a prospective cohort study. Sci Rep. (2017) 7:40646. doi: 10.1038/srep40646
46. Konlawij, T, and Chookiat, F. Prevalence and association of vitamin D deficiency and mortality in patients with severe sepsis. Int J Gen Med. (2017) 10:415–21. doi: 10.2147/IJGM.S147561
47. Nicolas, E, Naike, B, Guillaume, D, Hafid, AO, and Eric, M. Proceedings of réanimation 2018, the French Intensive Care Society international congress. Ann Intensive Care. (2018) 8:13. doi: 10.1186/s13613-017-0345-7
48. Pinargote, P, Qureshi, R, Salazar, W, Roberts, M, Eaton, C, Snetselaar, L, et al. Prospective association of serum vitamin D level with sepsis-mortality in postmenopausal women: results from the women’s health initiative. Open Forum Infect Dis. (2018) 5:S232–3. doi: 10.1093/ofid/ofy210.647
49. Gul, F, Arslantas, MK, Bilgili, B, Besir, A, and Cinel, I. Serum vitamin D level variation in SIRS, sepsis and septic shock. Marmara Med J. (2019) 32:102–6. doi: 10.5472/marumj.637569
50. Yoo, JW, Jung, YK, Ju, S, Lee, SJ, and Cho, MC. Serum vitamin D binding protein level, but not serum total, bioavailable, free vitamin D, is higher in 30-days survivors than in nonsurvivors with sepsis. Medicine. (2020) 99:e20756. doi: 10.1097/MD.0000000000020756
51. Tosoni, A, Cossari, A, Paratore, M, and Impagnatiello, MGroup IMSS. Delta-procalcitonin and vitamin D can predict mortality of internal medicine patients with microbiological identified sepsis. Medicina. (2021) 57:331. doi: 10.3390/medicina57040331
52. Asdie, RH, Mulya, DP, and Nainggolan, M. Assessment of 28-day survival of patients with sepsis based on vitamin D status: a hospital-based prospective cohort study in Indonesia. Pan Afr Med J. (2023) 45:76. doi: 10.11604/pamj.2023.45.76.36336
53. Guan, J, Shichen, M, Liang, Z, Yu, S, Zhao, M, Zhang, L, et al. Potential benefits of vitamin D for sepsis prophylaxis in critical ill patients. Front Nutr. (2023) 10:1073894. doi: 10.3389/fnut.2023.1073894
54. Kahar, LA, Yusrawati, Y, Jamsari, J, and Maskoen, T. Association between vitamin D levels and mortality in sepsis patients admitted to an intensive care at general hospital Dr. M. Djamil, West Sumatera, Indonesia. Open Access Maced J Med Sci. (2023) 11:122–7. doi: 10.3889/OAMJMS.2023.11162
55. Yang, B, Zhu, Y, Zheng, X, Li, T, Niu, K, Wang, Z, et al. Vitamin D supplementation during intensive care unit stay is associated with improved outcomes in critically ill patients with sepsis: a cohort study. Nutrients. (2023) 15:2924. doi: 10.3390/nu15132924
56. Li, C, Zhao, K, Ren, Q, Chen, L, Zhang, Y, Wang, G, et al. Vitamin D supplementation during intensive care unit stay is associated with improved outcomes in critically ill patients with sepsis: a cohort study. Front Cell Infect Microbiol. (2025) 14:1485554. doi: 10.3389/fcimb.2024.1485554
57. Seok, H, Kim, J, Choi, WS, and Park, DW. Effects of vitamin D deficiency on sepsis. Nutrients. (2023) 15:4309. doi: 10.3390/nu15204309
58. Shojaei, M, Sabzeghabaei, A, Valaei Barhagh, H, and Soltani, S. The correlation between serum level of Vitamin D and outcome of sepsis patients; a cross-cectional study. Arch Acad Emerg Med. (2019) 7:e1.
59. Lahiri, S, Sparrow, N, Mangiacotti, L, Rajput, P, and Koronyo, M. 40th international symposium on intensive care and emergency medicine. Crit Care. (2020) 24:P001. doi: 10.1186/s13054-021-03769-1
60. Vanichkulbodee, A, Romposra, M, Inboriboon, PC, and Trongtrakul, K. Effects of vitamin D insufficiency on sepsis severity and risk of hospitalisation in emergency department patients: a cross-sectional study. BMJ Open. (2023) 13:e064985. doi: 10.1136/bmjopen-2022-064985
61. Mohapatra, N, Sahu, AN, Mishra, SK, Thatoi, PK, and Mohanty, CBK. Association of serum vitamin D levels and severity of sepsis. J Evol Med Dental Sci. (2018) 7:646–9. doi: 10.14260/JEMDS/2018/146
62. Bhattacharyya, A, Subramaniam, R, Baidya, DK, Aggarwal, P, and Wig, N. Effect of early administration of vitamin D on clinical outcome in critically ill sepsis patients: a randomized placebo-controlled trial. Indian J Crit Care Med. (2021) 25:1147–54. doi: 10.5005/jp-journals-10071-23993
63. Yu, W, Ying, Q, Zhu, W, Huang, L, and Hou, Q. Vitamin D status was associated with sepsis in critically ill children: a PRISMA compliant systematic review and meta-analysis. Medicine (Baltimore). (2021) 100:e23827. doi: 10.1097/md.0000000000023827
64. Razavi Khorasani, N, Moazzami, B, Zahedi Tajrishi, F, Mohammadpour, Z, Rouhi, F, Alizadeh-Navaei, R, et al. The association between low levels of vitamin D and clinical outcomes in critically-ill children: a systematic review and Meta-analysis. Fetal Pediatr Pathol. (2020) 39:503–17. doi: 10.1080/15513815.2019.1675832
65. He, M, Cao, T, Wang, J, Wang, C, Wang, Z, and Abdelrahim, MEA. Vitamin D deficiency relation to sepsis, paediatric risk of mortality III score, need for ventilation support, length of hospital stay, and duration of mechanical ventilation in critically ill children: a meta-analysis. Int J Clin Pract. (2021) 75:e13908. doi: 10.1111/ijcp.13908
66. Su, G, and Jia, D. Vitamin D in acute and critically sick children with a subgroup of Sepsis and mortality: a meta-analysis. Nutr Cancer. (2021) 73:1118–25. doi: 10.1080/01635581.2020.1784964
67. Ashoor, TM, Abd Elazim, AEH, Mustafa, ZAE, Anwar, MA, Gad, IA, and Mamdouh Esmat, I. Outcomes of high-dose versus low-dose vitamin D on prognosis of sepsis requiring mechanical ventilation: a randomized controlled trial. J Intensive Care Med. (2024) 39:1012–22. doi: 10.1177/08850666241250319
68. Maiya, S, Sullivan, I, Allgrove, J, Yates, R, Malone, M, Brain, C, et al. Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life-threatening infant heart failure. Heart. (2008) 94:581–4. doi: 10.1136/hrt.2007.119792
69. Prietl, B, Treiber, G, Pieber, TR, and Amrein, K. Vitamin D and immune function. Nutrients. (2013) 5:2502–21. doi: 10.3390/nu5072502
70. Wimalawansa, SJ. Infections and autoimmunity-the immune system and vitamin D: a systematic review. Nutrients. (2023) 15:3842. doi: 10.3390/nu15173842
71. Athanassiou, L, Kostoglou-Athanassiou, I, Koutsilieris, M, and Shoenfeld, Y. Vitamin D and autoimmune rheumatic diseases. Biomolecules. (2023) 13:709. doi: 10.3390/biom13040709
72. Ananta Kahar, L, Yusrawati, Y, Jamsari, J, Maskoen, T, Aribowo, K, and Monika Sari, W. The role of vitamin D binding protein and vitamin D level in mortality of sepsis patients. Rep Biochem Mol Biol. (2023) 12:366–73. doi: 10.61186/rbmb.12.3.366
73. Quraishi, SA, De Pascale, G, Needleman, JS, Nakazawa, H, Kaneki, M, Bajwa, EK, et al. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in Sepsis: a randomized, placebo-controlled trial. Crit Care Med. (2015) 43:1928–37. doi: 10.1097/ccm.0000000000001148
74. Charoenngam, N, and Holick, MF. Immunologic effects of vitamin D on human health and disease. Nutrients. (2020) 12:2097. doi: 10.3390/nu12072097
75. Wang, TJ. Vitamin D and cardiovascular disease. Annu Rev Med. (2016) 67:261–72. doi: 10.1146/annurev-med-051214-025146
76. Dancer, RC, Parekh, D, Lax, S, D'Souza, V, Zheng, S, Bassford, CR, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. (2015) 70:617–24. doi: 10.1136/thoraxjnl-2014-206680
77. Yang, S, Li, A, Wang, J, Liu, J, Han, Y, Zhang, W, et al. Vitamin D receptor: a novel therapeutic target for kidney diseases. Curr Med Chem. (2018) 25:3256–71. doi: 10.2174/0929867325666180214122352
Keywords: sepsis, vitamin D, meta-analysis, clinical outcomes, pediatric and adult patients
Citation: Zhu H, Li K, Zhao Y, Qin J and Song G (2025) Assessment of the influence of vitamin D in patients with sepsis: a systematic review and meta-analysis. Front. Nutr. 12:1670083. doi: 10.3389/fnut.2025.1670083
Edited by:
Denisse Castro-Eguiluz, National Council of Science and Technology (CONACYT), MexicoReviewed by:
Shenglan Shang, General Hospital of Central Theater Command, ChinaEbru Melek Benligül, Tınaztepe University, Türkiye
Copyright © 2025 Zhu, Li, Zhao, Qin and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guolin Song, anprODUyODU1NTVAMTI2LmNvbQ==; Juan Qin, QW50MDAwOTk5QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Hangqi Zhu
Hangqi Zhu Keyi Li1†
Keyi Li1† Juan Qin
Juan Qin