- 1Department of Dietetics and Nutrition, University of Kansas Medical Center, Kansas City, KS, United States
- 2Alzheimer’s Disease Research Center, University of Kansas, Fairway, KS, United States
- 3Kansas University Diabetes Institute, University of Kansas Medical Center, Kansas City, KS, United States
- 4Kansas Center for Metabolism and Obesity Research, University of Kansas Medical Center, Kansas City, KS, United States
- 5Department of Neurology, University of Kansas Medical Center, Kansas City, KS, United States
- 6Department of Health, Sport, and Exercise Sciences, University of Kansas, Lawrence, KS, United States
Objective: To investigate the potential muscular benefits of an eight-week creatine monohydrate (CrM) supplementation in patients with Alzheimer’s disease (AD).
Methods: This single-arm pilot trial, conducted at the University of Kansas Medical Center in Kansas City, examined the intervention-associated changes in muscle strength, muscle size, and neuromuscular junction (NMJ) integrity following 8 weeks of CrM supplementation (20 g/day) in 20 participants with AD. All participants completed handgrip-strength measurements on the dominant hand (highest of three trials in kg of force). Ten participants completed lower body strength assessment via leg dynamometry at three velocities (1.05 rad∙s−1, 2.10 rad∙s−1, 3.14 rad∙s−1), with peak torque (in Newton-meters) recorded over five repetitions. Eighteen participants completed muscle size assessment by ultrasound measurement of cross-sectional area (mCSA, cm2) in the rectus femoris and vastus medialis, as well as muscle thickness (cm) in the rectus femoris, vastus medialis, and vastus lateralis. NMJ integrity was assessed in 19 participants by measuring plasma C-terminal agrin fragment (CAF) levels. All assessments were measured at baseline and 8 weeks.
Results: Following 8 weeks of CrM, mean hand-grip strength increased by 1.9 kg from baseline (p = 0.02). Lower leg strength did not change for any velocity among the ten participants who completed leg dynamometry. mCSA (n = 18) increased from baseline in the rectus femoris (p = 0.03) and vastus medialis (p = 0.01), but muscle thickness (n = 18) did not change in the rectus femoris (p = 0.41), vastus medialis (p = 0.37), nor vastus lateralis (p = 0.17). Subcutaneous fat (n = 18) decreased in the rectus femoris region (p = 0.006) and vastus lateralis region (p = 0.003), with no change in the vastus medialis region (p = 0.52). Mean CAF (n = 19) values did not change (p = 0.46).
Conclusion: This eight-week pilot trial suggests that 20 g/day of CrM may provide modest skeletal muscle benefits in patients with AD. These data provide preliminary evidence to warrant further investigation of the potential for CrM to prevent AD-related decline in muscle function.
Clinical trial registration: ClinicalTrials.gov, identifier NCT05383833.
1 Introduction
The loss of physical function is common in Alzheimer’s disease (AD) and often presents as diminished muscle mass and strength (1). Changes in muscle strength and size appears to be not only a consequence of AD, limiting mobility and promoting frailty, but also a contributor to its risk and progression (1, 2). In an AD mouse model, neuromuscular dysfunction emerged before cognitive impairment (3), indicating that skeletal muscle changes occur early in the disease process and may be an underappreciated therapeutic target.
For instance, in older adults with mild cognitive impairment due to AD, six months of resistance training increased muscle strength and integrity, improved cognition, and attenuated brain amyloid burden and atrophy (4). In AD mouse models, direct manipulation of skeletal muscle (5) and stimulation of anabolic pathways that drive hypertrophy (6) also improved cognition, further implicating muscle as a modifiable node in the disease cascade. Thus, interventions that enhance muscle and strength may not only slow functional decline in AD but may also have broader disease-modifying potential, as suggested by preclinical studies (5, 6).
Creatine monohydrate (CrM) has substantial evidence for enhancing muscle strength and size (7–14), and our recent pilot trial suggests CrM may be associated with benefits in AD patients (7). The basis for these effects lies in creatine’s (Cr) role as an organic compound found primarily in skeletal muscle (15) that stores high-energy phosphates in the form of phosphocreatine (PCr) and helps maintain intracellular energy flux (16, 17). CrM supplementation increases intramuscular creatine stores, thereby expanding the capacity for PCr formation and ATP regeneration during high-intensity muscle contractions. This enhanced energy availability can support greater force production and facilitate the energy-demanding processes of muscle protein synthesis, potentially leading to improvements in muscle strength and size (8–13). In older adults, CrM supplementation has been shown to improve strength and function (12, 14); however, its effects on skeletal muscle have not been investigated in the context of AD.
The purpose of this single-arm pilot study was to investigate our hypothesis that 8 weeks of CrM supplementation improves muscle strength, size, and neuromuscular junction integrity (NMJ) in AD.
2 Materials and methods
2.1 Creatine to augment bioenergetics in Alzheimer’s study and participants
The Creatine to Augment Bioenergetics in Alzheimer’s study (7, 18) allocated 20 participants with a clinical diagnosis of probable AD-dementia (19) to the 20 g/day CrM intervention. As the primary outcome of the CABA trial was feasibility, a single-arm design was employed to assess tolerability, compliance, and preliminary efficacy signals to inform future randomized controlled trials. Participants were 60–90 years old, were on a stable dose of AD-related medications (e.g., donepezil or memantine) for at least 30 days, had a study partner, scored ≥17 on the Mini-Mental State Exam (MMSE) (20), spoke English as the primary language, and had the ability to perform leg strength exercises. Exclusion criteria included insulin-dependent diabetes, chemotherapy or radiation within the past 5 years, a recent cardiac event (e.g., myocardial infarction), diagnosis of another neurodegenerative disease, inability to undergo MRI, and participation in a clinical trial or investigational drug or therapy within 30 days of screening. Participants were encouraged to maintain regular dietary intake and physical activity levels during the study. The study protocol was approved by the University of Kansas Medical Center Institutional Review Board, and all participants provided informed consent in accordance with institutional guidelines.
2.2 CrM intervention
Participants consumed 20 g of powdered CrM (Life Extension Inc., United States) daily for 8 weeks, divided into two 10-gram doses, mixed into beverages of the participant’s choice. This two ×10 g dosing regimen was selected because it has been shown to be safe (21) and to minimize participant and study partner burden, as managing fewer daily doses would be easier for patients with AD and their study partners. To support adherence, research dietitians contacted the study partner weekly, and study partners completed a daily CrM tracker.
2.3 Physical-activity assessment
Baseline physical activity was measured with the two-item Stanford Brief Activity Survey (SBAS) (20), as physical activity may affect muscle strength and size. Study partners selected one statement that best described the participant’s usual on-the-job (or daily routine) activity and one that best described their leisure-time activity during the past year, each ranging from sedentary to vigorous. Responses were cross-referenced on the SBAS color-coded scoring table to classify each participant into one of five overall activity categories: inactive, light, moderate, hard, or very hard.
2.4 Muscle strength, size, and body composition acquisition
All muscular and body composition assessments were measured at baseline and 8 weeks.
2.4.1 Handgrip strength
Handgrip strength was measured on the participant’s dominate hand using a calibrated Jamar hand dynamometer, a validated method for assessing upper body strength (22, 23). While seated in a chair with their feet flat on the floor, participants squeezed the dynamometer with maximal effort for at least 3 seconds per trial while the study team provided verbal encouragement. Each participant completed three sets, with at least 1 minute of rest between sets. The highest force value (kg) recorded across the three trials was used as the participant’s maximal handgrip strength.
2.4.2 Leg muscle strength
A sub-sample of the last 10 participants to participate in CABA completed leg strength testing as described in detail by Herda et al. (24). Participants performed maximal isokinetic contractions of the right leg extensors using a calibrated Biodex isokinetic dynamometer (Biodex Corp., Shirley, NY), with the hip positioned at a 90° angle. Each participant completed five maximal contractions at three velocities (1.05 rad∙s−1, 2.10 rad∙s−1, 3.14 rad∙s−1), with 5 minutes of rest between each velocity. Study personnel provided verbal encouragement to elicit maximal effort and speed during each trial. Torque, position, and velocity signals were recorded using the Biodex system. Dynamometer signals were sampled at 2000 Hz, and torque data were low-pass filtered with a 10 Hz cutoff. Peak torque (Newton-meters; Nm) was calculated as the highest 0.25 s epoch from each contraction using custom-written software (LabVIEW 2019, National Instruments, Austin, TX). For each velocity, the highest peak torque value was used for analysis.
2.4.3 Leg muscle morphology
Leg extensor morphology was assessed with B-mode ultrasonography (Logiq e, GE Healthcare, Chicago, IL) following Herda et al. (24). With participants supine, panoramic transverse images were captured at standardized landmarks: rectus femoris (50% patella to greater trochanter distance), vastus lateralis (40% lateral-epicondyle to anterior superior iliac spine), and vastus medialis (20% medial-epicondyle to anterior superior iliac spine). A custom foam-padded probe guide ensured perpendicular sweeps while minimal pressure and ample gel prevented compression. ImageJ (National Institutes of Health, Bethesda, MD) was used to trace muscle cross-sectional area (mCSA, cm2) and extract mean echo intensity (mEI, grayscale score from 0 to 255) from the same region; muscle and subcutaneous-fat thickness were measured with the straight-line tool, as per Cleary et al. (25). Two participants did not complete this assessment. Figure 1 shows a representative rectus femoris image.
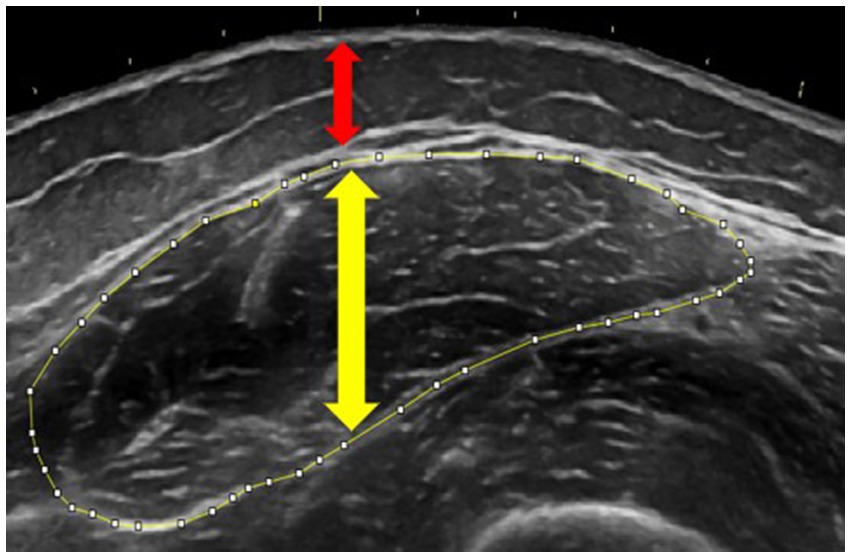
Figure 1. Ultrasonography image demonstrating measurements of muscle cross-sectional area (outlined in yellow), muscle thickness (yellow arrow), and subcutaneous adipose thickness (red arrow) of the rectus femoris. Tracing was performed using ImageJ software.
2.4.4 Anthropometrics and body composition
Height and weight were measured using a calibrated stadiometer and digital scale. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Waist circumference was measured using a standard tape measure (26). Body composition was assessed using bioelectrical impedance analysis (BIA) (Bodystat Quadscan 4,000) (27). Percent lean body mass was used as the primary measure of body composition.
2.5 NMJ integrity measurement
NMJ degeneration is a feature of AD (28) that can be assessed by measuring C-terminal agrin fragment (CAF), a biomarker that may also predict physical function (28). Thus, fasting plasma CAF levels were quantified in duplicate using an enzyme-linked immunosorbent assay (#ab216945, Abcam, Cambridge, United Kingdom). Per manufacturer protocol, 50 μL of 4-fold diluted plasma or standard solution was added to pre-coated wells, followed by 50 μL of the CAF antibody cocktail. The plate was incubated at room temperature on a plate shaker set to 400 rpm for 1 hour. After incubation, the wells were washed three times with 1 × Wash Buffer PT. Next, 100 μL of TMB development solution was added, and the plate was incubated in the dark for 8 minutes at 400 rpm. Following this, 100 μL of stop solution was added, and the plate was shaken for 1 minute to ensure thorough mixing. Absorbance was measured at 450 nm using an MR-9600 Accuris Smartreader 96 (Benchmark Scientific, Sayreville, NJ). CAF concentrations were determined by interpolating the absorbance values from a standard curve and adjusting for the 4-fold dilution factor. CAF analysis was not completed for one participant.
2.6 Statistical analysis
The primary objective of this study was to investigate whether 8 weeks of CrM supplementation was associated with improvement in muscle strength, size, and NMJ integrity in patients with AD. Continuous data are expressed as mean ± standard deviations, and categorical data are reported as frequencies and percentages. We used paired sample t-tests to analyze mean changes in all measures from baseline to 8 weeks. We used linear mixed models, including the interaction of time and sex with subject ID as a random effect, to explore sex-based differences in muscle strength (handgrip and leg), muscle ultrasonography, percent lean body mass, and CAF measurements. All statistical analyzes were performed using R software (version 4.1.1; R Foundation, Vienna, Austria). A two-sided p-value of less than 0.05 was considered statistically significant.
3 Results
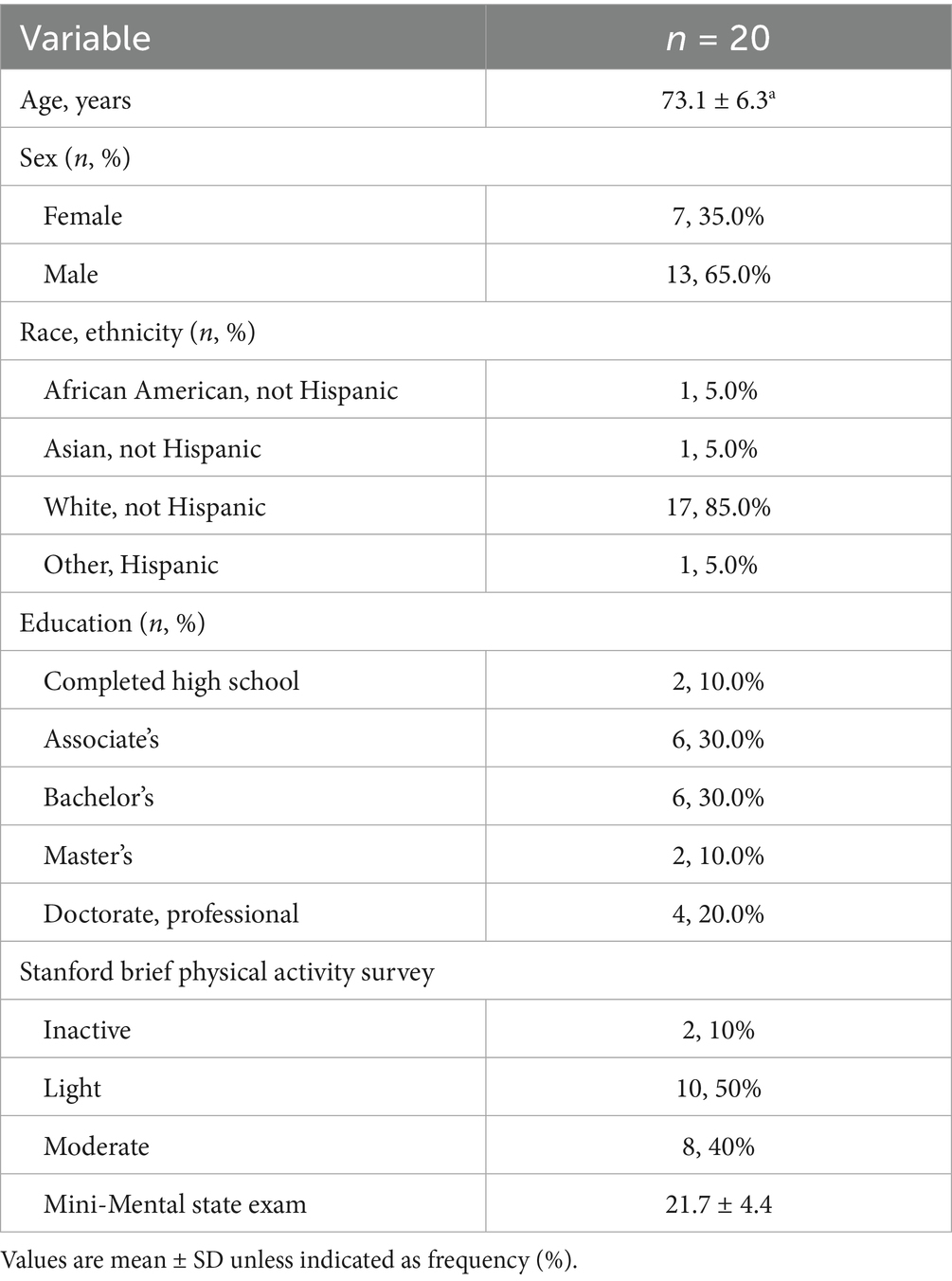
Twenty participants diagnosed with dementia due to probable AD (73.1 ± 6.3 years) completed the CABA study. The CrM intervention was well-tolerated with no withdrawals due to adverse events and excellent adherence, with 19 of 20 participants (95%) achieving ≥80% compliance and mean self-reported dose compliance of 90.0% based on daily study partner tracker logs, as detailed here (7). Baseline demographic characteristics are presented in Table 1. All outcomes are presented in Table 2.
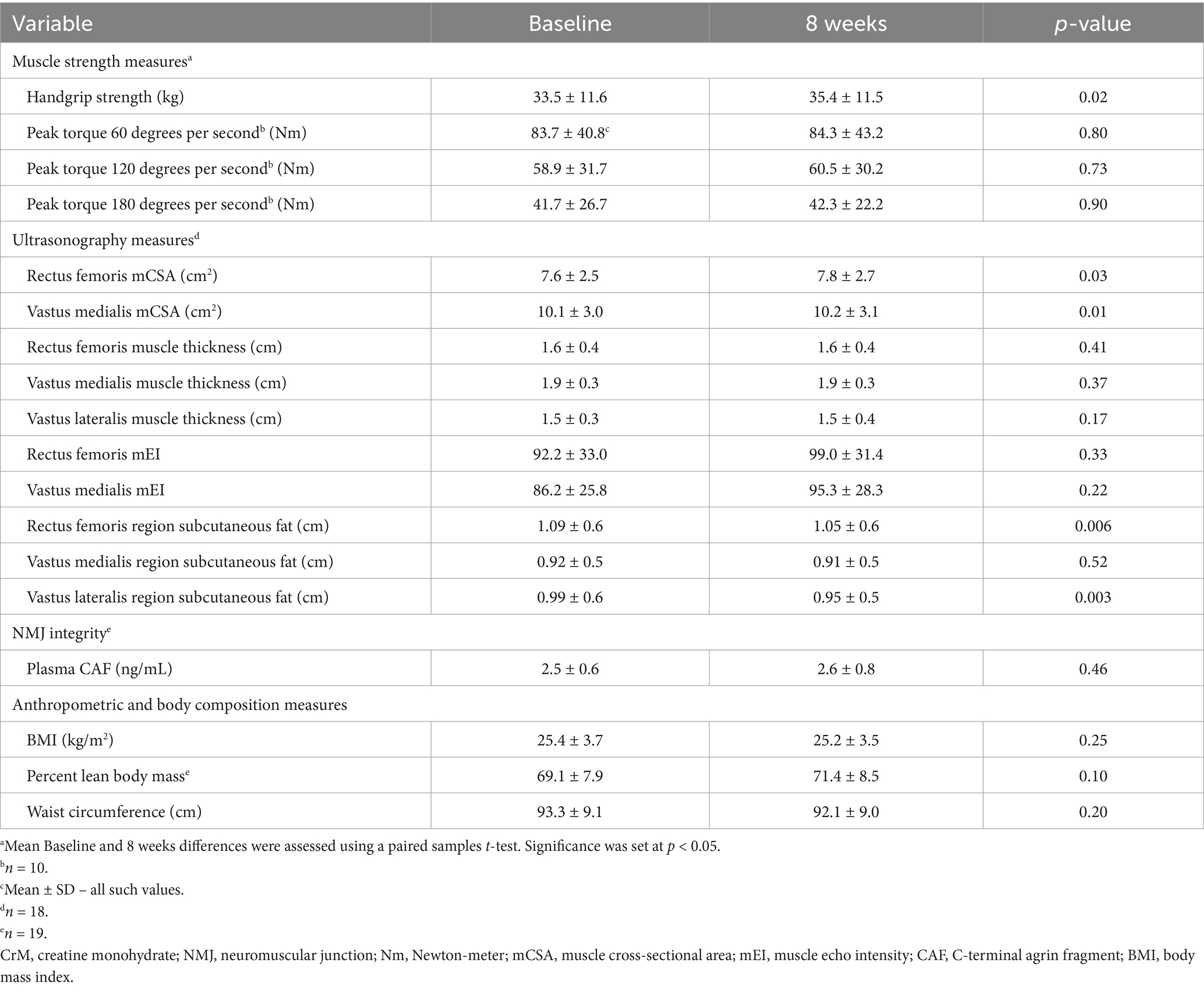
Table 2. Effects of 8 weeks of CrM supplementation on muscle strength, muscle ultrasonography, neuromuscular junction integrity, and body composition.
3.1 Muscle strength
Handgrip strength increased from baseline to 8 weeks (33.5 ± 11.6 kg vs. 35.4 ± 11.5 kg, p = 0.02). Figure 2 illustrates mean changes in handgrip strength from baseline to 8 weeks.

Figure 2. Time point comparisons and individual changes in handgrip strength after 8 weeks of creatine monohydrate supplementation. Boxplots display strength values at baseline and after 8 weeks, with individual trajectories overlaid. Line color reflects baseline physical activity level: orange for inactive, green for light-intensity, and purple for moderate-intensity. Solid lines with circular markers represent male participants, while dashed lines with triangular markers represent female participants. * p < 0.05.
Due to mechanical issues with the leg dynamometer, only ten CABA participants completed leg dynamometry to measure leg strength. Peak torque did not change for any of the three velocities tested: (1.05 rad∙s−1: 83.7 ± 40.8 Nm vs. 84.3 ± 43.2 Nm, p = 0.80; 2.10 rad∙s−1: 58.9 ± 31.7 Nm vs. 60.5 ± 30.2 Nm, p = 0.73; 3.14 rad∙s−1: 41.7 ± 26.7 Nm vs. 42.3 ± 22.2 Nm, p = 0.90). There were no differences by sex for changes in either hand or leg strength, despite males having higher baseline strength than females.
3.2 Leg extensor ultrasonography
Eighteen CABA participants underwent ultrasonography to measure leg muscle size at baseline and 8 weeks. mCSA increased in the rectus femoris (7.6 ± 2.5 cm2 vs. 7.8 ± 2.7 cm2, p = 0.03) and vastus medialis (10.1 ± 3.0 cm2 vs. 10.2 ± 3.1 cm2, p = 0.01).
Muscle thickness did not change for any muscles: rectus femoris (1.6 ± 0.4 cm vs. 1.6 ± 0.4 cm, p = 0.41; vastus medialis 1.9 ± 0.3 cm vs. 1.9 ± 0.3 cm, p = 0.37; and vastus lateralis 1.5 ± 0.3 cm vs. 1.5 ± 0.4 cm, p = 0.17). Similarly, no significant changes were observed in mEI (rectus femoris: 92.2 ± 33.0 cm vs. 99.0 ± 31.4 cm, p = 0.33; vastus medialis: 86.2 ± 25.8 cm vs. 95.3 ± 28.3 cm, p = 0.22). In contrast, subcutaneous fat decreased in the rectus femoris region (1.09 ± 0.6 cm vs. 1.05 ± 0.6 cm, p = 0.006) and vastus lateralis region (0.99 ± 0.6 cm vs. 0.95 ± 0.5 cm, p = 0.003). No changes were observed in the vastus medialis region (0.92 ± 0.5 cm vs. 0.91 ± 0.5 cm, p = 0.52). There were no differences by sex for changes in all ultrasonography measures, despite males having larger mCSA in the rectus femoris and vastus medialis than females.
3.3 Anthropometrics and body composition
Twenty participants completed BMI and waist circumference measurements at both baseline and the 8-week visit, while 19 completed BIA. BMI did not change from baseline to 8 weeks (25.4 ± 3.7 kg/m2 vs. 25.2 ± 3.5 kg/m2, p = 0.25). Similarly, percent lean body mass showed no significant change (69.1 ± 7.9% vs. 71.4 ± 8.5%, p = 0.10), nor did waist circumference (93.3 ± 9.1 cm vs. 92.1 ± 9.0 cm, p = 0.20). There were no differences by sex for changes in percent lean body mass, despite males having greater percent lean body mass than females.
3.4 NMJ integrity
Nineteen participants completed CAF measurements at both baseline and the 8-week visit. Plasma CAF concentrations did not change form baseline to 8 weeks (2.5 ± 0.6 ng/mL vs. 2.6 ± 0.8 ng/mL, p = 0.46). There were no differences by sex for changes in CAF.
4 Discussion
Results from this pilot study suggest 8 weeks of CrM supplementation is associated with modest improvement in muscle strength and size in patients with AD. This is the first study to test whether CrM may benefit skeletal muscle in AD, and these preliminary gains justify larger, controlled trials to investigate its promise as a low-cost strategy for slowing AD-related decline in muscle health.
We observed associated improvements in upper body muscle strength following 8 weeks of CrM supplementation, with a 1.9 kg (~6%) increase in handgrip strength, a reliable proxy of upper body strength. This improvement is clinically meaningful as handgrip strength is associated with quality of life in older adults (29) and mortality in patients with AD (30). While we are the first to test CrM supplementation in patients with AD, similar handgrip strength improvements have been documented in healthy older adults (10). Although our leg strength data were limited to a subset of participants (n = 10), we did not observe statistically significant changes in leg strength. These results should be interpreted with considerable caution due to the lack of standardized familiarization trials, which are essential for reliable strength measurements in older adults and may have resulted in underestimation of true strength or learning effects (24). While maintaining leg strength over 8 weeks may be clinically important in AD, where progressive muscle weakness is common (1), the methodological limitations prevent us from drawing definitive conclusions about CrM’s effects on lower extremity strength. Nevertheless, the feasibility of conducting isokinetic dynamometry in this population was demonstrated, suggesting potential utility for future studies with proper methodological controls. Together, the improvement in handgrip strength aligns with previous studies showing CrM benefits in other populations and suggest that CrM may help preserve overall muscle function in AD. Future studies should incorporate standardized familiarization protocols and consider combining CrM supplementation with resistance training or cognitive-motor interventions, as this approach improves muscle outcomes more than supplementation alone (8–10, 31) and may be particularly beneficial for optimizing muscle strength and function in patients with AD.
We observed modest but statistically significant associated improvements in mCSA in the rectus femoris (+0.2 cm2) and vastus medialis (+0.1 cm2), muscle groups where larger mCSA are generally associated with greater leg strength (32). However, we did not observe corresponding leg strength improvements in our limited sample. Our findings align with well-documented benefits of CrM for muscle size in other populations (8, 9, 11–13). In addition to these changes in mCSA, we also noted a localized decrease in subcutaneous fat thickness in the rectus femoris and vastus lateralis, despite stable overall body weight and composition. This localized decrease in subcutaneous fat thickness, occurring alongside increases in muscle size, suggests that CrM supplementation may promote favorable changes in muscle-to-fat ratio at the tissue level, independent of overall body composition changes. Although the mCSA increases were small, they may still be clinically relevant as they represent preservation/gains that could help offset the 1–2% annual age-related muscle loss typically seen in older adults (33), which may be accelerated in AD (1). Modest gains in muscle size can improve functional capacity (34) and glucose metabolism (35), which is perturbed in AD (36). Moreover, since mCSA captures only a portion of total muscle volume, a 0.2 cm2 increase could reflect a more substantial hypertrophic response. While we cannot rule out that some mCSA increase is due to CrM-related muscle hydration (37), the concurrent improvement in handgrip strength supports that the increases in mCSA may have functional implications. Taken together, these findings, though based on a brief, eight-week trial, suggest that CrM supplementation may help preserve muscle size and support functional improvements in individuals with AD, a population particularly vulnerable to muscle loss (38–40).
We assessed NMJ integrity using CAF as a biomarker of NMJ degeneration but found no statistically significant changes after 8 weeks of CrM supplementation. This lack of change may reflect that the eight-week intervention duration was too short to observe meaningful changes in NMJ biomarker; NMJ remodeling and regeneration are complex processes that may require months rather than weeks to manifest detectable changes in circulating biomarkers (41). Our patients also showed relatively preserved NMJ integrity compared to other AD cohorts (28) and age-matched healthy controls (42), which may have limited the room for improvement. Additionally, CAF may have inherent variability and sensitivity limitations (43), and CAF levels may be influenced by factors beyond creatine supplementation, such as physical activity, inflammation, or disease progression rates, which could mask potential treatment effects in a heterogeneous AD population. Investigating CrM’s effects on NMJ integrity may require longer intervention periods, larger sample sizes, and more sensitive biomarkers or complementary assessments such as electromyography to capture subtle neuromuscular changes that circulating biomarkers might miss.
Although our study did not directly investigate mechanisms, several pathways may explain CrM’s association with improved muscle outcomes. The ATP deficits documented in both skeletal muscle (44) and neurons (36) in AD patients may make this population especially responsive to Cr′s bioenergetic support. Beyond the basic energy metabolism pathways, the concurrent improvements in both muscle size and strength in study suggest activation of anabolic signaling by upregulating muscle protein synthesis (45), downregulating growth-inhibiting proteins such as myostatin (46), and enhancing anabolic signaling through the mammalian target of rapamycin pathway (47) and insulin-like growth factor (48). The antioxidant properties of Cr (49, 50) may be particularly beneficial in AD, where oxidative stress drives both muscle deterioration and neurodegeneration (51, 52), potentially explaining the muscle preservation we observed. Together, these mechanisms may underlie the gains in muscle strength and size observed in this study, though mechanistic studies are needed to determine which pathways are most relevant to CrM’s effects in AD populations.
Our pilot trial suggests that CrM may offer valuable benefits for AD-related functional decline in patients with AD; however, as a single-arm pilot study with limited racial and sex diversity and designed to generate preliminary data rather than provide definitive evidence, these findings should be interpreted cautiously. The lack of a control group, small sample size, and short eight-week duration all limit the strength of our conclusions. Additionally, our leg strength assessment was limited by mechanical issues with the dynamometer, requiring mid-study protocol changes that reduced our sample size and prevented standardized familiarization trials, which are essential for reliable leg strength data in older adults (24). Finally, ultrasonography assessments were conducted without standardized participant hydration protocols, which can affect measurements of mCSA and muscle thickness, and without assessor blinding to participant identity and time point, potentially introducing measurement bias. Future randomized, placebo-controlled trials with larger sample sizes, longer intervention durations of 12–24 weeks, and standardized protocols are needed to confirm these preliminary findings and capture more robust muscle and neuromuscular adaptations.
5 Conclusion
Although our study is limited by its single-arm nature, our study provides preliminary evidence that CrM supplementation is associated with improvements in upper body strength and lower body muscle size in patients with AD. Enhancing skeletal muscle strength and size may help prevent AD-related decline in physical function, potentially slowing functional decline and improving quality of life. As a cost-effective, well-tolerated intervention, CrM represents a promising adjuvant therapeutic strategy that warrants investigation in larger randomized controlled trials to establish its efficacy for preserving physical function in patients with AD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Kansas Medical Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AS: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. DS: Methodology, Supervision, Writing – review & editing. JM: Methodology, Writing – review & editing. AC: Methodology, Writing – review & editing. TH: Methodology, Writing – review & editing. MT: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Alzheimer’s Association (AARG-22-924314), the National Institutes of Health (K01 AG065487 and P30 AG072973), and the National Center for Advancing Translational Sciences of the National Institutes of Health (TL1TR00236 and UL1TR002366). The University of Kansas Medical Center Department of Dietetics and Nutrition provided additional support. Life Extension Inc. donated the creatine monohydrate powder.
Acknowledgments
The authors would like to thank the CABA participants and their study partners for graciously donating their time to this research. We also thank Emma Kelly, Tanu Arora, and Faith Waitsman for their valuable assistance with data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AD, Alzheimer’s disease; ATP, Adenosine triphosphate; BIA, Bioelectrical Impedance Analysis; BMI, Body Mass Index; CABA, Creatine to Augment Bioenergetics in Alzheimer’s; CAF, C-terminal Agrin Fragment; Cr, Creatine; CrM, Creatine Monohydrate; MMSE, Mini-Mental State Examination; mCSA, Muscle Cross-Sectional Area; mEI, Muscle Echo Intensity; NMJ, Neuromuscular Junction; SBAS, Stanford Brief Activity Survey.
References
1. Ogawa, Y, Kaneko, Y, Sato, T, Shimizu, S, Kanetaka, H, and Hanyu, H. Sarcopenia and muscle functions at various stages of Alzheimer disease. Front Neurol. (2018) 9:710. doi: 10.3389/fneur.2018.00710
2. Boyle, PA, Buchman, AS, Wilson, RS, Leurgans, SE, and Bennett, DA. Association of Muscle Strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. (2009) 66:1339–44. doi: 10.1001/archneurol.2009.240
3. Brisendine, MH, and Drake, JC. Early-stage Alzheimer’s disease: are skeletal muscle and exercise the key? J Appl Physiol. (2023) 134:515–20. doi: 10.1152/japplphysiol.00659.2022
4. Broadhouse, KM, Singh, MF, Suo, C, Gates, N, Wen, W, Brodaty, H, et al. Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI. Neuroimage Clin. (2020) 25:102182. doi: 10.1016/j.nicl.2020.102182
5. Nagase, T, and Tohda, C. Skeletal muscle atrophy-induced hemopexin accelerates onset of cognitive impairment in Alzheimer's disease. J Cachexia Sarcopenia Muscle. (2021) 12:2199–210. doi: 10.1002/jcsm.12830
6. Lin, YS, Lin, FY, and Hsiao, YH. Myostatin is associated with cognitive decline in an animal model of Alzheimer's disease. Mol Neurobiol. (2019) 56:1984–91. doi: 10.1007/s12035-018-1201-y
7. Smith, AN, Choi, IY, Lee, P, Sullivan, DK, Burns, JM, Swerdlow, RH, et al. Creatine monohydrate pilot in Alzheimer's: feasibility, brain creatine, and cognition. Alzheimers Dement (N Y). (2025) 11:e70101. doi: 10.1002/trc2.70101
8. Dolan, E, Artioli, GG, Pereira, RMR, and Gualano, B. Muscular atrophy and sarcopenia in the elderly: is there a role for Creatine supplementation? Biomolecules. (2019) 9:642. doi: 10.3390/biom9110642
9. Chilibeck, PD, Kaviani, M, Candow, DG, and Zello, GA. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med. (2017) 8:213–26. doi: 10.2147/OAJSM.S123529
10. Stout, JR, Graves, SB, Cramer, JT, Goldstein, ER, Costa, PB, Smith, AE, et al. Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64-86 years). J Nutr Health Aging. (2007) 11:459–64.
11. Rawson, ES, Wehnert, ML, and Clarkson, PM. Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol Occup Physiol. (1999) 80:139–44. doi: 10.1007/s004210050570
12. Gotshalk, LA, Kraemer, WJ, Mendonca, MA, Vingren, JL, Kenny, AM, Spiering, BA, et al. Creatine supplementation improves muscular performance in older women. Eur J Appl Physiol. (2008) 102:223–31. doi: 10.1007/s00421-007-0580-y
13. Olsen, S, Aagaard, P, Kadi, F, Tufekovic, G, Verney, J, Olesen, JL, et al. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. (2006) 573:525–34. doi: 10.1113/jphysiol.2006.107359
14. Gotshalk, LA, Volek, JS, Staron, RS, Denegar, CR, Hagerman, FC, and Kraemer, WJ. Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc. (2002) 34:537–43. doi: 10.1097/00005768-200203000-00023
15. Wyss, M, and Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol Rev. (2000) 80:1107–213. doi: 10.1152/physrev.2000.80.3.1107
16. Bessman, SP, and Carpenter, CL. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. (1985) 54:831–62. doi: 10.1146/annurev.bi.54.070185.004151
17. Smith, AN, Morris, JK, Carbuhn, AF, Herda, TJ, Keller, JE, Sullivan, DK, et al. Creatine as a therapeutic target in Alzheimer's disease. Curr Dev Nutr. (2023) 7:102011. doi: 10.1016/j.cdnut.2023.102011
18. Taylor, MK, Burns, JM, Choi, IY, Herda, TJ, Lee, P, Smith, AN, et al. Protocol for a single-arm, pilot trial of creatine monohydrate supplementation in patients with Alzheimer's disease. Pilot Feasibility Stud. (2024) 10:42. doi: 10.1186/s40814-024-01469-5
19. McKhann, GM, Knopman, DS, Chertkow, H, Hyman, BT, Jack, CR Jr, Kawas, CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011) 7:263–9. doi: 10.1016/j.jalz.2011.03.005
20. Folstein, MF, Folstein, SE, and McHugh, PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
21. Hersch, SM, Schifitto, G, Oakes, D, Bredlau, AL, Meyers, CM, Nahin, R, et al. The CREST-E study of creatine for Huntington disease: a randomized controlled trial. Neurology. (2017) 89:594–601. doi: 10.1212/WNL.0000000000004209
22. Bohannon, RW. Hand-grip dynamometry provides a valid indication of upper extremity strength impairment in home care patients. J Hand Ther. (1998) 11:258–60. doi: 10.1016/S0894-1130(98)80021-5
23. Trosclair, D, Bellar, D, Judge, LW, Smith, J, Mazerat, N, and Brignac, A. Hand-grip strength as a predictor of muscular strength and endurance. J Strength Cond Res. (2011) 25:S99. doi: 10.1097/01.JSC.0000395736.42557.bc
24. Herda, TJ, Ryan, ED, Kohlmeier, M, Trevino, MA, Gerstner, GR, and Roelofs, EJ. Examination of muscle morphology and neuromuscular function in normal weight and overfat children aged 7-10 years. Scand J Med Sci Sports. (2018) 28:2310–21. doi: 10.1111/sms.13256
25. Cleary, CJ, Herda, TJ, Quick, AM, and Herda, AA. Acute muscle swelling effects of a knee rehabilitation exercise performed with and without blood flow restriction. PLoS One. (2022) 17:e0278540. doi: 10.1371/journal.pone.0278540
26. Norgan, NG. A review of: “anthropometric standardization reference manual”. Edited by T. G. LOHMAN, A. F. ROCHE and R. MARTORELL. (Champaign, IL.: human kinetics books, 1988.) [Pp. vi+ 177.] £28·00. ISBN 087322 121 4. Ergonomics. (1988) 31:1493–4.
27. Verdich, C, Barbe, P, Petersen, M, Grau, K, Ward, L, Macdonald, I, et al. Changes in body composition during weight loss in obese subjects in the NUGENOB study: comparison of bioelectrical impedance vs. dual-energy X-ray absorptiometry. Diabetes Metab. (2011) 37:222–9. doi: 10.1016/j.diabet.2010.10.007
28. Karim, A, Iqbal, MS, Muhammad, T, Ahmad, F, and Qaisar, R. Elevated plasma zonulin and CAF22 are correlated with sarcopenia and functional dependency at various stages of Alzheimer's diseases. Neurosci Res. (2022) 184:47–53. doi: 10.1016/j.neures.2022.08.004
29. Kaczorowska, A, Kozieł, S, and Ignasiak, Z. Hand grip strength and quality of life among adults aged 50-90 years from south West Poland. Sci Rep. (2025) 15:882. doi: 10.1038/s41598-024-84923-x
30. Esteban-Cornejo, I, Ho, FK, Petermann-Rocha, F, Lyall, DM, Martinez-Gomez, D, Cabanas-Sánchez, V, et al. Handgrip strength and all-cause dementia incidence and mortality: findings from the UK biobank prospective cohort study. J Cachexia Sarcopenia Muscle. (2022) 13:1514–25. doi: 10.1002/jcsm.12857
31. Candow, DG, Forbes, SC, Chilibeck, PD, Cornish, SM, Antonio, J, and Kreider, RB. Effectiveness of Creatine supplementation on aging muscle and bone: focus on falls prevention and inflammation. J Clin Med. (2019) 8:488. doi: 10.3390/jcm8040488
32. Kojic, F, Ðurić, S, Ranisavljev, I, Stojiljkovic, S, and Ilic, V. Quadriceps femoris cross-sectional area and specific leg strength: relationship between different muscles and squat variations. PeerJ. (2021) 9:e12435. doi: 10.7717/peerj.12435
33. Wilkinson, DJ, Piasecki, M, and Atherton, PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. (2018) 47:123–32. doi: 10.1016/j.arr.2018.07.005
34. Skoglund, E, Lundberg, TR, Rullman, E, Fielding, RA, Kirn, DR, Englund, DA, et al. Functional improvements to 6 months of physical activity are not related to changes in size or density of multiple lower-extremity muscles in mobility-limited older individuals. Exp Gerontol. (2022) 157:111631. doi: 10.1016/j.exger.2021.111631
35. Merz, KE, and Thurmond, DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. (2020) 10:785–809. doi: 10.1002/j.2040-4603.2020.tb00136.x
36. Swerdlow, RH, Burns, JM, and Khan, SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimer's Dis. (2010) 20:S265–79. doi: 10.3233/JAD-2010-100339
37. Wu, SH, Chen, KL, Hsu, C, Chen, HC, Chen, JY, Yu, SY, et al. Creatine supplementation for muscle growth: a scoping review of randomized clinical trials from 2012 to 2021. Nutrients. (2022) 14:1255. doi: 10.3390/nu14061255
38. Beeri, MS, Leugrans, SE, Delbono, O, Bennett, DA, and Buchman, AS. Sarcopenia is associated with incident Alzheimer's dementia, mild cognitive impairment, and cognitive decline. J Am Geriatr Soc. (2021) 69:1826–35. doi: 10.1111/jgs.17206
39. Burns, JM, Johnson, DK, Watts, A, Swerdlow, RH, and Brooks, WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. (2010) 67:428–33. doi: 10.1001/archneurol.2010.38
40. Moon, Y, Choi, YJ, Kim, JO, and Han, SH. Muscle profile and cognition in patients with Alzheimer's disease dementia. Neurol Sci. (2018) 39:1861–6. doi: 10.1007/s10072-018-3505-0
41. Arnold, AS, Gill, J, Christe, M, Ruiz, R, McGuirk, S, St-Pierre, J, et al. Morphological and functional remodelling of the neuromuscular junction by skeletal muscle PGC-1α. Nat Commun. (2014) 5:3569. doi: 10.1038/ncomms4569
42. Pratt, J, Motanova, E, Pessanha, L, Narici, M, Boreham, C, and De Vito, G. Plasma C-terminal agrin fragment concentrations across adulthood: reference values and associations with skeletal muscle health. J Cachexia Sarcopenia Muscle. (2024) 15:1501–10. doi: 10.1002/jcsm.13507
43. Pratt, J, De Vito, G, Narici, M, Segurado, R, Pessanha, L, Dolan, J, et al. Plasma C-terminal Agrin fragment as an early biomarker for sarcopenia: results from the Geno fit study. J Gerontol A Biol Sci Med Sci. (2021) 76:2090–6. doi: 10.1093/gerona/glab139
44. Morris, JK, McCoin, CS, Fuller, KN, John, CS, Wilkins, HM, Green, ZD, et al. Mild cognitive impairment and donepezil impact mitochondrial respiratory capacity in skeletal muscle. Function (Oxf). (2021) 2:zqab045. doi: 10.1093/function/zqab045
45. Safdar, A, Yardley, NJ, Snow, R, Melov, S, and Tarnopolsky, MA. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics. (2008) 32:219–28. doi: 10.1152/physiolgenomics.00157.2007
46. Saremi, A, Gharakhanloo, R, Sharghi, S, Gharaati, MR, Larijani, B, and Omidfar, K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol Cell Endocrinol. (2010) 317:25–30. doi: 10.1016/j.mce.2009.12.019
47. Mao, X, Kelty, TJ, Kerr, NR, Childs, TE, Roberts, MD, and Booth, FW. Creatine supplementation upregulates mTORC1 signaling and markers of synaptic plasticity in the dentate gyrus while ameliorating LPS-induced cognitive impairment in female rats. Nutrients. (2021) 13:2758. doi: 10.3390/nu13082758
48. Burke, DG, Candow, DG, Chilibeck, PD, Mac Neil, LG, Roy, BD, Tarnopolsky, MA, et al. Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int J Sport Nutr Exerc Metab. (2008) 18:389–98. doi: 10.1123/ijsnem.18.4.389
49. Barbieri, E, Guescini, M, Calcabrini, C, Vallorani, L, Diaz, AR, Fimognari, C, et al. Creatine prevents the structural and functional damage to mitochondria in myogenic, Oxidatively stressed C2C12 cells and restores their differentiation capacity. Oxidative Med Cell Longev. (2016) 2016:5152029. doi: 10.1155/2016/5152029
50. Sestili, P, Martinelli, C, Colombo, E, Barbieri, E, Potenza, L, Sartini, S, et al. Creatine as an antioxidant. Amino Acids. (2011) 40:1385–96. doi: 10.1007/s00726-011-0875-5
51. Sullivan-Gunn, MJ, and Lewandowski, PA. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. (2013) 13:104. doi: 10.1186/1471-2318-13-104
Keywords: Alzheimer’s disease, creatine monohydrate, muscle strength, non-pharmacological intervention, muscle cross-sectional area, neuromuscular junction
Citation: Smith AN, Sullivan DK, Morris JK, Carbuhn AF, Herda TJ and Taylor MK (2025) Eight weeks of creatine monohydrate supplementation is associated with increased muscle strength and size in Alzheimer’s disease: data from a single-arm pilot study. Front. Nutr. 12:1670641. doi: 10.3389/fnut.2025.1670641
Edited by:
Richard Kreider, Texas A&M University, United StatesReviewed by:
Roberto Cannataro, Magna Græcia University, ItalyTerence Moriarty, University of Northern Iowa, United States
Holly Clarke, MedErgy HealthGroup, Inc., Yardley, United States
Copyright © 2025 Smith, Sullivan, Morris, Carbuhn, Herda and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aaron N. Smith, YXNtaXRoNzFAa3VtYy5lZHU=
 Aaron N. Smith
Aaron N. Smith Debra K. Sullivan
Debra K. Sullivan Jill K. Morris
Jill K. Morris Aaron F. Carbuhn
Aaron F. Carbuhn Trent J. Herda
Trent J. Herda Matthew K. Taylor
Matthew K. Taylor