- Montana Center for Work Physiology and Exercise Metabolism, University of Montana, Missoula, MT, United States
The influence of protein intake on insulin resistance, has garnered an increasing amount of interest over the past few decades. Increased provisions of dietary protein during weight loss helps preserve skeletal muscle, which as the largest organ in the human body, is responsible for 80% of insulin-stimulated glucose disposal. The postprandial influence of essential amino acids (EAAs) either alone or as part of intact proteins are regulated through leucine-induced activation of mammalian target of rapamycin (mTOR) that serves to promote muscle protein synthesis and maintain skeletal muscle. High protein diets and/or EAA supplementation have also been demonstrated to improve satiety and augment mitochondrial function, which may have an indirect or direct influence on insulin sensitivity. On the other hand, chronic elevations in postabsorptive concentrations of branched chains amino acids (BCAAs) have been associated with chronic activation of the mTOR pathway, impairing insulin action. It appears that causal links between BCAAs and the pathogenesis of insulin resistance are reliant on chronic hyperinsulinemia and nutrient overload that foster chronic lipotoxicity. Conversely, postprandial elevations in EAAs leverage sensing as an anabolic mediator to facilitate muscle remodeling, augment satiety and improve metabolic regulation.
Introduction
The influence of amino acids on insulin resistance is controversial. In this mini-review, we describe the differences between acute postprandial and chronic postabsorptive elevations in amino acids on metabolism. While environmental stress, physical stress and growth factors (ie., hormones, nutrients, and energy) influence signaling networks responsible for growth and proliferation, clinical outcomes also seem largely dependent on the energy status and metabolic health of the individual.
Weight loss and improved insulin sensitivity: impact of high protein diet
Hypocaloric dietary interventions promote favorable changes in metabolic risk factors across a wide range of cohorts (1–5). Reducing energy intake promotes improvements in glucose metabolism in individuals with type 2 diabetes (6). While the impact of hypocaloric dietary interventions on the mitigation of insulin resistance is strong, dietary-induced weight loss can lead to muscle loss (7, 8). Based on conclusions from systematic reviews and meta-analyses, increased dietary protein during weight loss seems to preserve lean mass, accelerate the loss of fat mass, and improve cardiometabolic outcomes (9–13).
In a clinical trial that compared the influence of high-protein (i.e., 800 kcal, 45% protein, 35% carbohydrate, 20% fat) and high carbohydrate (i.e., 800 kcal, 20% protein, 60% carbohydrate, 20% fat) diets, the high protein diet promoted greater retention of fat free mass, improvements in insulin-stimulated glucose disposal (derived from euglycemic, hyperinsulinemic clamp method) and reduced 3-methylhistidine excretion (a marker of protein breakdown) compared to the high carbohydrate diet (14). Obese, insulin-resistant female participants who adhered to a hypocaloric high protein diet compared to a Mediterranean diet for only 3 weeks (15) demonstrated reductions in insulin resistance and fasting plasma insulin. Two-fold greater reductions in insulin resistance were described in participants with early-onset type 2 diabetes who followed a hypocaloric high protein diet (35% protein of caloric intake) compared to a hypocaloric standard protein diet (i.e., 18% protein of caloric intake) (9). Greater reductions in fat mass and insulin resistance have also been reported in overweight and obese women following a similar hypocaloric high protein (i.e., 35% protein of caloric intake) diet compared to a hypocaloric standard protein diet (i.e., 20% protein of caloric intake) (16).
When we consider that skeletal muscle is responsible for ~80% of insulin-stimulated glucose disposal and plays a key role in the etiology of insulin resistance (17, 18), modest elevations in dietary protein that preserve muscle mass during dietary-induced weight loss seem logical. The increased provision of dietary protein and EAAs in the context of a hypocaloric diet also fosters the preferential loss of adipose tissue that is directly proportional to the increased energetic demand linked to increased muscle protein synthesis (19). Based on the studies discussed above, mitigating the loss of skeletal muscle is a critical component of dietary interventions aimed at achieving reductions in body weight (20). Otherwise, caloric restriction-induced negative energy balance will result in 10–35% reductions in skeletal muscle, linked to reductions in functional capacity (7). Under these circumstances, muscle atrophy is especially problematic in older adults, leading to increased risk of falls, morbidity and mortality (21).
Bed rest and insulin resistance: impact of high protein diet
Short-term bed rest (i.e., 10 days) promotes the onset of hepatic and peripheral insulin resistance in older, otherwise healthy adults as determined by multi-stage hyperinsulinemic, euglycemic clamp methodologies (22). Presentation of these metabolic abnormalities occurs in conjunction with the bed rest- induced loss of skeletal muscle and rapid decrements in functional parameters (19, 23). Even in young healthy adults, 3 days of bed rest resulted in a 45% decline in insulin-stimulated leg glucose uptake, coupled with a 43% reduction in whole-body protein and myofibrillar protein synthesis, and a 3% reduction in leg muscle volume (24). These detrimental alterations in metabolism and muscle remodeling reflect a reduction in energy demand and lack of mechanical stress to the skeletal muscle, resulting in perturbations in mitochondrial lipid metabolism (25). Glycogen and lipid intermediates accumulate under these conditions, which seems to foster elevations in fasting plasma insulin and insulin resistance (26). In the face of acute cessation of physical activity or bed rest, muscle atrophy, a decline in functional capability and the concomitant development of metabolic abnormalities are somewhat predictable, albeit unfortunate outcomes (22, 23, 27).
Resistance exercise has been demonstrated to mitigate the loss of skeletal muscle and the development of insulin resistance during bed rest (28). Physical activity is not always practical or well-tolerated in clinical settings (29), so dietary approaches to address the bed rest-induced loss of skeletal muscle and dysregulation of metabolism during bed rest have been investigated (30). Specialized approaches using branched-chain amino acids or individual EAAs have been effective in maintaining muscle protein synthesis and functional capability (31–33). Supplementation of ß -hydroxy-ß -methylbutyrate (HMB), a metabolite of leucine seems to promote mitochondrial function (i.e., OXPHOS complex II protein and total OXPHOS content) (34). Additional studies are needed to confirm these results and evaluate the protective influence of protein and/or EAA consumption against bed rest-induced muscle atrophy and the sequelae of insulin resistance (35).
Amino acid supplementation, molecular mechanisms and metabolic health
Clinical evidence supports the role of EAA supplementation as a tool to improve metabolic health (36–39). EAA supplementation results in elevated mitochondrial biogenesis, reduced oxidative damage, enhanced muscle protein synthesis, physical capacity, reduced body weight, and improved immune function. A pharmaceutical therapy that promoted these types of systemic improvements would be heralded in the pursuit of health and longevity. It is therefore important to delineate the physiological mechanisms responsible for beneficial alterations in organ health that provide the above-stated EAA-related benefits.
To gain a better understanding of how these molecular mechanisms affect metabolic health, we should consider how the dietary consumption of EAAs influences metabolism in the muscle, liver, and adipose tissue. A sedentary lifestyle is commonly characterized by muscle atrophy, excessive accumulation of adipose tissue, and infiltration of fatty acids in the liver (40). The aging process itself further contributes to these metabolic abnormalities, partly due to the concomitant presentation of anabolic resistance, which makes maintaining a metabolically healthy balance between muscle and fat even more challenging. While increased physical activity is a powerful tool in the struggle against these abnormalities (41), only 20% of the adult population meets the guidelines recommended by the US Centers for Disease Control (42). EAAs, branched chain amino acids (BCAAs), or more specifically, leucine, have been consistently demonstrated to improve mitochondrial biogenesis in clinical and pre-clinical studies without any change in physical activity (36). The mechanistic underpinnings by which dietary consumption of amino acids mitigates pathogenic metabolic sequelae are then important.
We know that amino acids, particularly leucine, transiently activate the mammalian target of rapamycin complex 1 (mTORC1), Yin-Yang 1 (YY1), and peroxisome proliferator-activated receptor-gamma coactivator-1-α (PGC-1α) (36) (Figure 1). Working cohesively with nuclear respiratory factors (NRF-1,2), mitochondrial transcription factor A (TFAM), and sirtuins (SIRT1/3), these signaling intermediates are responsible for the promotion of mitochondrial biogenesis (43, 44). On the other hand, reduced hepatic expression of PGC-1α has been closely linked to increased intrahepatic lipid in humans (Figure 1), whereas hepatic PGC-1α ablation in mice leads to compromised mitochondrial metabolism, which ultimately serves to rapidly induce hepatic steatosis (45). Perturbations in PGC-1α also contributes to the accumulation of ceramide in skeletal muscle, which has been implicated in the pathogenesis of peripheral insulin resistance (46). On the other hand, elevations in the expression of PGC-1α alleviate insulin resistance in skeletal muscle (47, 48).
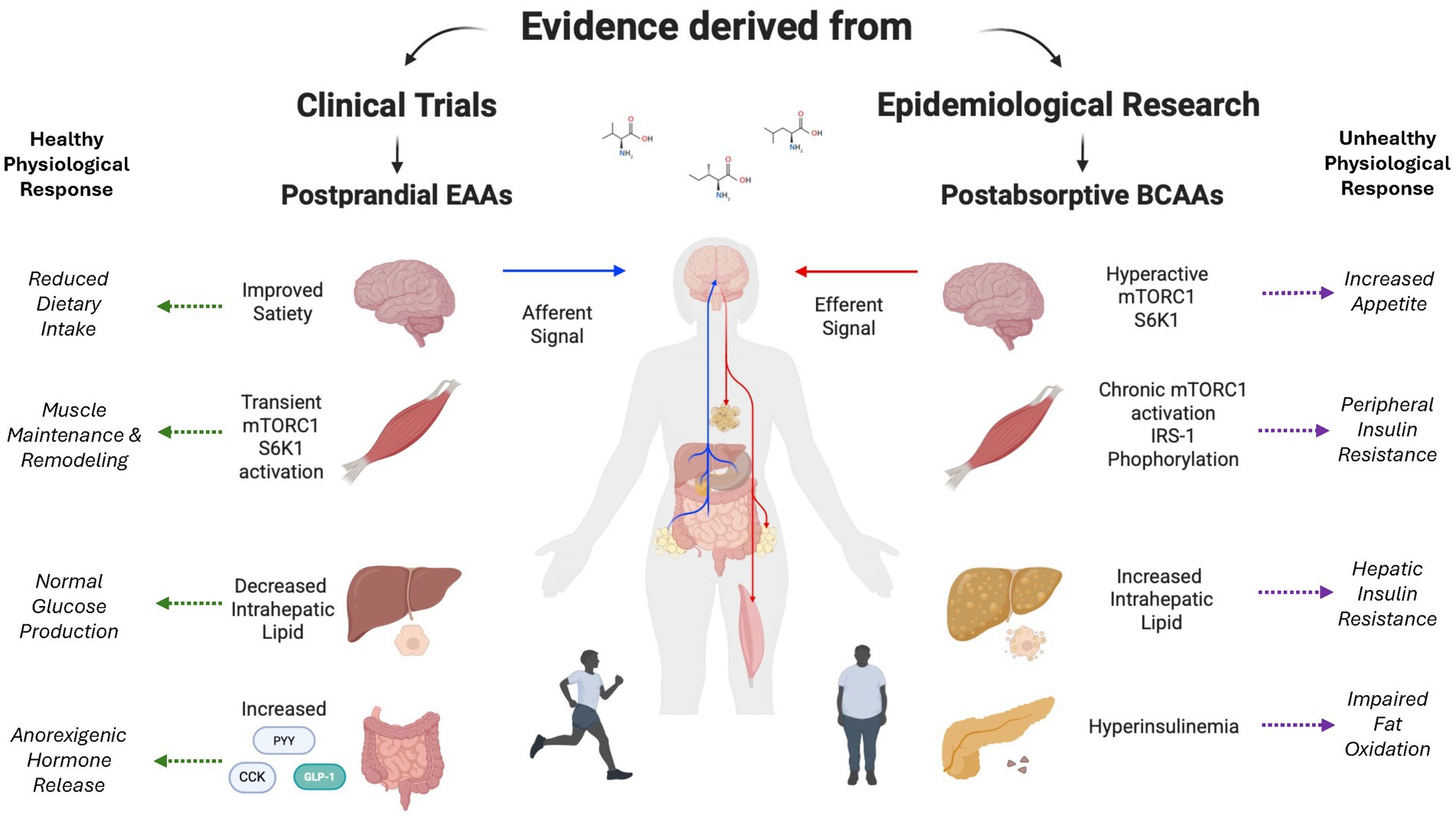
Figure 1. Dichotomous role of amino acids on insulin resistance. Impact of acute alterations in postprandial plasma EAA concentrations compared to chronic elevations in plasma BCAA concentrations on central and peripheral organ metabolism. Created with BioRender.com.
The perturbed regulation of PGC-1α activation has been directly linked to several clinical problems. For example, disruptions in the PGC-1α/TFAM signaling pathway during pregnancy increase the risk of metabolic diseases in offspring (49). In patients suffering from diabetic kidney disease, mitochondrial dysfunction represents a hallmark of the clinical condition whereby PGC-1α activation is suppressed, potentially leading to the worsening of insulin resistance (50). Evidence from pre-clinical studies supports the role of PGC-1α and/or SIRT1 activation on the amelioration of lipotoxicity implicated in the pathogenesis of insulin resistance (51). PGC-1α also plays an important role in mitigating the detrimental impact of oxidative stress (52), which is directly connected to lipid abnormalities, atherosclerosis, hypertension, and increased risk of type 2 diabetes (53).
Physical activity, cold exposure, and/or caloric restriction are known to positively influence the regulatory influence of PGC-1α on mitochondrial biogenesis (54). Dietary-induced alterations in EAA availability, more specifically leucine, may also activate signaling pathways that augment mitochondrial biogenesis, potentially suppressing the development of insulin resistance even during periods of inactivity (55). Dietary leucine promotes browning of white adipose tissue and fatty acid oxidation in adipose tissue through the adenosine 5′-monophosphate-activated protein kinase (AMPK)-silent information regulator of transcription 1 (SIRT-1)-PGC-1α axis. In a somewhat similar fashion, dietary leucine activates PGC-1α through SIRT1-AMPK signals in skeletal muscle, increasing mitochondrial biogenesis, enhancing fatty acid and improving insulin sensitivity (55).
Therefore, the beneficial influence of EAAs on metabolic health may be derived from the augmentation of mitochondrial biogenesis that would not occur otherwise without increased physical activity (19, 23, 37, 38, 56, 57). While these changes appear to impact body composition and occur across organ systems in several clinical studies (58, 59), the EAA-induced activation of PGC-1α and the beneficial alterations in mitochondrial function may be the common denominator.
Amino acids, satiety and metabolic health
The satiety cascade is regulated by a complex interplay between sensory factors, gastrointestinal influences via gastric distension and alterations in gut-derived peptides, and alterations in nutrient status/energy balance (60). The impact of these regulatory factors on food intake is consistent with the aminostatic theory whereby amino acids promote satiety (61) (Figure 1). In the context of dietary-induced elevations in amino acid concentrations, the regulation of nutrient intake may be directly influenced by the intrinsic need to maintain the effective remodeling of skeletal muscle. Dietary intervention studies linking amino acid concentrations to satiety support the theory (62, 63). On the other hand, pre-clinical studies suggest more comprehensive regulation, including the roles of glucoreceptors in the intestine and liver, playing a significant role (64). The influence of amino acids on satiety involves a complex network of central and peripheral nutrient sensing systems (65). While leucine-induced alterations in forebrain-hindbrain circuitry represent a central regulatory element that reduces nutrient consumption via negative feedback loops (66), divergent alterations in circulating anorexigenic hormones/orexigenic hormones elicited by dietary leucine highlight the importance of peripheral factors (67) (Figure 1).
Demonstrating the importance of peripheral regulation, high-protein diets have been demonstrated to elicit elevations in anorexigenic hormones while at the same time suppressing orexigenic hormones. Where dietary protein suppresses orexigenic hormones like ghrelin, anorexigenic hormones like GLP-1, cholecystokinin (CCK), and peptide YY are increased (Figure 1). For example, protein intake promotes GLP-1, which not only promotes satiety but has also been tied to enhancements in ß-cell function and glycemic status, which is logically consistent with the efficacy of GLP-1 as a therapeutic tool in the fight against metabolic disease (68). The amino acids phenylalanine and l-tryptophan are influential in promoting CCK secretion (69), which in turn serves to delay gastric emptying, promote satiety, and reduce dietary intake (70). In fact, the intravenous delivery of CCK reduces food intake, potentially influenced by the activation of CCK1 receptors that enhance satiety (71). While the specific amino acids responsible for the peptide YY response to protein have not been identified (72), acute dietary feeding of protein promotes the release of peptide YY that has a direct impact on improved satiation (73). Pre-clinical data also suggest that chronic elevations in dietary protein also increase plasma peptide YY and peptide YY expression (73).
Consistent with the importance of central regulation, leucine-induced activation of mTORC1 in the hypothalamus represents a crucial step in the central signaling cascade that influences satiety. Intracerebroventricular leucine administration reduces dietary intake, whereas administration of rapamycin ameliorates leucine-induced satiety (74). Downstream from mTOR, S6K1 activation has been demonstrated to reduce energy intake and mitigate metabolic perturbations, such as increased fat deposition and the presentation of insulin resistance, even during high-fat feeding in mice (75). High protein diets may also activate the noradrenergic-adrenergic neuronal pathway in the brainstem nucleus of the solitary tract and the meloanocortin neurons of the hypothalamic arcuate nucleus (75).
In addition to the impact of protein or amino acid intake on gut derived hormones that influence satiety, Skov et al., suggested that the inclusion of additional dietary protein also contributes to improved compliance to dietary interventions (76), ensuring negative energy balance required for weight loss that promotes improvements in insulin sensitivity (77). Given the multifaceted benefits of dietary protein on gut-derived hormones, it is not altogether surprising that evidence from dietary interventions support the role of high-protein diets in successful weight management, glucose homeostasis, and lipid metabolism (Figure 1).
Controversial aspects of dietary protein and amino acids on the development of insulin resistance
A wide range of dietary interventions and/or EAA supplementation studies suggest definitive metabolic benefits (2, 9, 11–13, 15, 16, 19). On the other hand, elevations in fasting levels of BCAAs, sulfur amino acids, tyrosine, and phenylalanine have been closely linked to insulin resistance (78–80). These associations between fasting BCAAs and insulin resistance appear to strengthen over time (81), and BCAAs have even been suggested as potential biomarkers for predicting the risk of developing type 2 diabetes (82, 83) or for additional evidence of type 2 diabetes (84). Based on the results of a randomized controlled crossover trial, reductions in dietary consumption of BCAAs lowered meal-induced insulin secretion and postprandial insulin sensitivity as derived from the mixed meal tolerance test (85). However, direct measurements of hepatic and/or peripheral insulin sensitivity in these same studies were not affected by the reduction in dietary intake of BCAAs, suggesting the variations in splanchnic compared to peripheral glucoregulatory hormone concentrations between the mixed meal tolerance test and the clamp, respectively.
Insulin-mediated clearance of BCAA is impaired in obese individuals and seems to worsen in individuals with type 2 diabetes (86). Lower mitochondrial oxidation of BCAAs and reduced whole-body leucine oxidation rates were implicated in the chronic elevation of BCAAs (86). Hyperinsulinemia and nutrient overload may represent a physically inactive—hyperphagic phenotype. For example, chronic post-absorptive elevations in BCAAs have been linked to “hyperactive” mTOR and S6K1 signaling, impairing insulin action due to IRS-1 serine phosphorylation (80). 3-Hydroxyisobutyric acid, a catabolic intermediate of valine directly promotes fatty acid transport in skeleltal muscle, contributing to insulin resistance (87). Isoleucine also leads to increased muscle lipid deposition that has been linked to insulin resistance (88). Nevertheless, the causal link between BCAAs and the pathogenesis of insulin resistance is not clear. Inconclusive results from studies utilizing dietary supplementation of BCAAs, EAAs, and/or protein suggest the presence of multiple factors (i.e., source of intact protein, baseline physical activity, status of energy balance, etc) that play important roles, either negating the influence of fasting BCAAs or reducing their relevance to disease (89, 90) (Figure 1). Longitudinal clinical studies are needed to elucidate the reasons behind inconclusive results.
Conclusion
The dichotomous role of protein, EAAs, and BCAAs in insulin resistance and metabolic health can be perplexing. A growing body of evidence supports the idea that high-protein diets and EAA supplementation support muscle retention, mitochondrial function, and insulin sensitivity, especially during periods of caloric restriction and/or physical inactivity. However, chronically elevated fasting plasma concentrations of BCAAs, under conditions of nutrient excess and hyperinsuliunemia, have been linked to overactivation of mTOR and S6K1 pathways, which may lead to systemic insulin resistance (Figure 1). These seemingly contradictory findings suggest that the relationships between dietary protein and a particular phenotype (ie., timing, context, culture, and metabolic status) may be responsible for the controversy surrounding dietary protein and metabolic health. This nuanced conundrum underscores the need for further investigation and discovery in amino acid metabolism. As we develop deeper insights into the timing of nutrient delivery, the state of energy balance, and cellular signaling, we should utilize amino acid-based strategies to offer targeted therapeutic potential in the prevention and management of insulin resistance and metabolic disease.
Author contributions
MC: Conceptualization, Writing – original draft, Writing – review & editing. RC: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition, Project administration, Resources, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from the United States Army Medical Research and Development Command (W81XWH-22-9-0014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coker, RH, Williams, RH, Yeo, SE, Kortebein, PM, Bodenner, DL, Kern, PA, et al. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J Clin Endocrinol Metab. (2009) 94:4258–66. doi: 10.1210/jc.2008-2033
2. González-Salazar, LE, Pichardo-Ontiveros, E, Palacios- González, B, Vigil-Martínez, A, Granados-Portillo, O, and Guizar-Heredia, R. Effect of the intake of dietary protein on insulin resistance in subjects with obesity: a randomized controlled clinical trial. Eur J Nutr. (2021) 60:2435–47. doi: 10.1007/s00394-020-02428-5
3. Johnson, ML, Distelmaier, K, Lanza, IR, Irving, BA, Robinson, MM, and Konopka, AR. Mechanisms by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes. (2015) 65:74–84. doi: 10.2337/db15-0675
4. Khalafi, M, Maleki, AH, Symonds, ME, Rosenkranz, SK, Rohani, H, and Ehsanifar, M. The effects of intermittent fasting on body composition and cardiometabolic health in adults with prediabetes or type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. (2024) 26:3820–41. doi: 10.1111/dom.15730
5. McLaughlin, T, Abbasi, F, Lamendola, C, Yee, G, Carter, S, and Cushman, SW. Dietary weight loss in insulin-resistant non-obese humans: metabolic benefits and relationship to adipose tissue cell size. Nutr Metab Cardiovasc Dis. (2019) 29:62–8. doi: 10.1016/j.numecd.2018.09.014
6. Lan, J, Chen, M, Zhang, X, and Yang, J. Effect of dietary carbohydrate intake on glycaemic control and insulin resistance in type 2 diabetes: a systematic review and meta-analysis. Asia Pac J Clin Nutr. (2025) 34:282–97. doi: 10.6133/apjcn.202506_34(3).0003
7. Cava, E, Yeat, NC, and Mittendorfer, B. Preserving healthy muscle with weight loss. Adv Nutr. (2017) 8:511–9. doi: 10.3945/an.116.014506
8. Sanatanasto, AJ, Glynn, NW, Newman, MA, Taylor, CA, Brooks, MM, Goodpaster, BH, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. (2011):516576. doi: 10.1155/2011/516576
9. Marco-Benedi, V, Pérez-Calahorra, S, Bea, AM, Lamiquiz-Moneo, I, Baila-Rueda, L, Cenarro, A, et al. High-protein energy-restricted diets induce greater improvement in glucose homeostasis but not in adipokines compared to standard-protein diets in early-onset diabetic adults with overweight or obesity. Clin Nutr. (2019) 39:1354–63. doi: 10.1016/j.clun.2019.06.005
10. Heather, HJ, Clifton, PM, Astrup, A, Wycherley, TP, Westerterp-Plantenga, MS, Luscombe, ND, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. (2015):1011329S. doi: 10.3945/sjcn.114.084038
11. Kim, JE, O’Connor, LE, Sands, LP, Slebodnik, MB, and Campbell, WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev. (2016) 74:210–24. doi: 10.1093/nutrit/nuv065
12. Santesso, N, Akl, EA, Bianchi, M, Mente, A, Mustafa, R, HeelsAndsdell, D, et al. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr. (2012) 66:780–8. doi: 10.1038/ejcn.2012.37
13. Schwingshackl, L, and Hoffman, G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. (2013) 12:48. doi: 10.1186/1475-2891-12-48
14. Piatti, PM, Monti, F, Fermo, I, Baruffaldi, L, Nassar, R, Santambrogio, G, et al. Hypocaloric high-protein diet improves glucose oxidation and spares lean mass; comparison to hypocaloric high carbohydrate diet. Metabolism. (1994) 43:1481–7. doi: 10.1016/0026-0495(94)90005-1
15. Tettamanzi, F, Bagnardi, V, Louca, P, Nogal, A, Serafina, G, Mambrini, SP, et al. A high protein diet is more effective in improving insulin resistance and glycaemic variability compared to a Mediterranean diet - a cross-over controlled inpatient dietary study. Nutrients. (2021) 13:4380. doi: 10.3390/nu13124380
16. Mateo-Gallego, R, Marco-Benedi, V, Perez-Calahorra, S, Bea, AM, Baila-Rueda, A, Lamiquiz-Moneo, I, et al. Energy-restricted, high-protein diets more effectively impact cardiometabolic profile in overweight and obese women than lower-protein diets. Clin Nutr. (2017) 36:371–9. doi: 10.1016/j.clnu.2016.01.018
17. DeFronzo, RA, and Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. (2009) 32:S157–63. doi: 10.2337/dc09/S302
18. Merz, KE, and Thurmond, DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. (2020) 10:785–809. doi: 10.1002/cphy.c190029
19. Coker, RH, Miller, S, Schutzler, S, Deutz, NEP, and Wolfe, RR. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr J. (2012) 11:105. doi: 10.1186/1475-2891-11-105
20. Bartholomew, CL, Martins, C, and Gower, B. Association between insulin sensitivity and lean mass loss during weight loss. Obesity. (2024) 32:1156–62. doi: 10.1002/oby.24022
21. Moreland, JD, Richardson, JA, Goldsmith, CH, and Clase, CM. Muscle weakness and falls in older adults. J Am Geriatr Soc. (2004) 52:1121–19. doi: 10.1111/j.1532-5415.2004.52310.x
22. Coker, RH, Hays, NP, Williams, RH, Xu, L, Wolfe, RR, and Evans, WJ. Bedrest worsens impairments in fat and glucose metabolism in older, overweight adults. J Gerontol A Biol Sci Med Sci. (2014) 69:363–70. doi: 10.1093/gerona/glt100
23. Coker, RH, Hays, NP, Williams, RH, Wolfe, RR, and Evans, WJ. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci. (2015) 70:91–6. doi: 10.1093/gerona/glu123
24. Shur, N, Simpson, EJ, Crossland, H, Constantin, D, Cordon, SM, Constanti-Teodosiu, D, et al. Bed-rest and exercise mobilization: concurrent adaptations in muscle glucose and protein metabolism. J Cachexia Sarcopenia Muscle. (2024) 15:603–14. doi: 10.1002/jcsm.13431
25. Kenny, HC, Rudwill, F, Breen, L, Salanova, M, Blottner, D, and Heise, T. Bed rest and resistive vibration exercise unveil novel links between skeletal muscle mitochondrial function and insulin resistance. Diabetologia. (2017) 60:1491–501. doi: 10.1007/s00125-017-4298-z
26. Eggelbusch, M, Charlton, BT, Bosutti, A, Ganse, B, Giakoumaki, I, Grootemaat, AE, et al. The impact of bed rest on human skeletal muscle protein metabolism. Cell Rep Med. (2024) 5:101372. doi: 10.1016/j.xcrm2023.101372
27. Kortebein, PM, Ferrando, AA, Lombeida, J, Wolfe, RR, and Evans, WJ. Effects of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. (2007) 297:1772–4. doi: 10.1001/jama.287.16.1772-b
28. Ogawa, M, Belavy, DL, Yoshiko, A, Armbrecht, G, Miokovic, T, Felsenberg, D, et al. Effects of 8 weeks of bed rest with or without resistance exercise intervention on the volume of the muscle tissue and the adipose tissues of the thigh. Phys Rep. (2020) 8:e14560. doi: 10.14814/phy2.14560
29. Covinsky, KE, Palmer, RM, Fortinsky, RH, Counsell, SR, Stewart, AL, and Kresevic, D. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. (2003) 51:451–8. doi: 10.1046/j.1532-5415.2003.51152.x
30. English, KL, and Paddon-Jones, D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. (2010) 13:34–9. doi: 10.1097/MCO.0b013e328333aa66
31. Ferrando, AA, Paddon-Jones, D, Hays, NP, Kortebein, PM, Ronsen, O, Williams, RH, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. (2010) 29:18–23. doi: 10.1016/j.clnu.2009.03.009
32. Mangogna, A, Di Girolamo, FG, Fiotti, N, Vinci, P, Landolfo, M, Mearelli, FL, et al. High-protein diet with excess leucine prevents inactivity-induced insulin resistance in women. Clin Nutr. (2023) 42:2587–7. doi: 10.1016/j.clnu.2023.10.028
33. Stein, TP, Schluter, MD, Leskiw, MJ, and Boden, G. Attenuation of the protein wasting associated with bed rest by branched-chain amino acids. Nutrition. (1999) 15:656–60. doi: 10.1016/S0899-9007(99)00120-3
34. Standley, RA, Distefano, G, Pereira, S, Tan, M, Kelly, OJ, Coen, PM, et al. Effects of ß-hydroxy- ß-methylbutyrate on skeletal muscle mitochondrial content and dynamics, and lipids after 10 days of bed rest in older adults. J Appl Physiol. (2017) 123:1092–100. doi: 10.1152/japplphysiol.00192.2017
35. Hughes, AK, Francis, T, Rooney, J, Pollock, R, and Witard, O. The effect of protein or amino acid provision on immobilization-induced muscle atrophy in healthy adults: a systematic review and meta-analysis. Exp Physiol. (2024) 109:873–88. doi: 10.1113/EP090434
36. Bifari, F, Ruocco, C, Decimo, I, Fumagalli, G, Valerio, A, and Nisoli, E. Amino acid supplements and metabolic health: a potential interplay between intestinal microbiota and systems control. Genes Nutr. (2017) 12:27. doi: 10.1186/s12263-017-0582-2
37. Coker, RH, Deutz, NE, Schutzler, SES, Beggs, M, Miller, S, Wolfe, RR, et al. Nutritional supplementation with essential amino acids and phytosterols may risk for metabolic syndrome and cardiovascular disease in overweight individuals with mild hyperlipidemia. J Endocrinol Diabetes Obes. (2015) 3:1069.
38. Coker, MS, Ladd, KR, Kim, J, Murphy, CJ, DeCort, R, Newcomer, BR, et al. Essential amino acid consumption lowers intrahepatic lipid despite excess alcohol consumption. Nutrients. (2020) 12:254. doi: 10.3390/nu12010254
39. Dillon, EL, Sheffield-Moore, M, Paddon-Jones, D, Gilkison, C, Sanford, AP, Casperson, SL, et al. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. (2009) 94:1630–7. doi: 10.1210/jc.2008-1564
40. Ko, SH, and Jung, Y. Energy metabolism changes and dysregulated metabolism in postmenopausal women. Nutrients. (2021) 13:4556. doi: 10.3390/nu13124556
41. Lachman, ME, Lipsitz, L, Lubben, J, Casteneda-Sceppa, C, and Jette, AM. When adults don’t exercise: behavioral strategies to increase physical activity in sedentary middle-aged and older adults. Innov Aging. (2018) 2:igy007. doi: 10.1093/geroni/igy007
42. Clarke, TC, Norris, T, and Schiller, JS. (2017). Early release of selected estimates based on data from the 2016 National Health Interview Survey Washington, DC: National Center for Health Statistics. Available online at: https://www.cdc.gov/nchs/data/nhis/earlyrelease/Earlyrelease201705.pdf (Accessed 20 July 2025).
43. Liang, C, Curry, BJ, Brown, PL, and Zemel, MB. Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. J Nutr Metab. (2014):239750. doi: 10.1155/2014/239750
44. Sun, X, and Zemel, MB. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab (Lond). (2009) 6:26. doi: 10.1186/1743-7075-6-26
45. Arconzo, M, Piccinin, E, Pasculli, E, Cariello, M, Loiseau, N, Bertrand-Michel, J, et al. Hepatic-specific PGC-1α ablation drives fibrosis in MASH model. Liver Int. (2024) 44:2738–52. doi: 10.1111/liv.16052
46. Koves, TR, Ussher, JR, Noland, RC, Slentz, D, Mosedale, M, Ilkayeva, O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. (2008) 7:45–56. doi: 10.1016/j.cmet.2007.10.013
47. Benton, CR, Wright, DC, and Bonen, A. PGC-1α-mediated regulation of gene expression and metabolism: implications for nutrition and exercise interventions. Appl Physiol Nutr Metab. (2008) 33:843–62. doi: 10.1139/H08-074
48. Lukazuk, B, Kurek, K, Miklosz, A, Zendzian-Piotrowska, M, and Chabowski, A. The role of PGC-1α in the development of insulin resistance in skeletal muscle revisited. Cell Physiol Biochem. (2015) 37:2288–96. doi: 10.1159/000438584
49. Jiang, S, Teague, AM, Tryggestad, JB, Aston, CE, Lyons, T, and Chernausek, SD. Effects of maternal diabetes and fetal sex on human placenta mitochondrial biogenesis. Placenta. (2017) 57:26–32. doi: 10.1016/j.placenta.2017.06.001
50. Akhtar, S, and Siragy, HM. Pro-renin receptor suppresses mitochondrial biogenesis and function via AMPK/SIRT-1/PGC-1α pathway in diabetic kidney. PLoS One. (2019) 14:e0225728. doi: 10.1371/journal.pone.0225728
51. Williams, CB, and Gurd, BJ. Skeletal muscle SIRT1 and the genetics of metabolic health: therapeutic activation by pharmaceuticals and exercise. Appl Clin Genet. (2012) 5:81–91. doi: 10.2147/TACG.S31276
52. St-Pierre, J, Drori, S, Uldry, M, Silvaggi, JM, Rhee, J, Jager, S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1α transcriptional coactivators. Cell. (2006) 127:397–408. doi: 10.1016/j.cell.2006.09.024
53. Manzoor, MF, Arif, Z, Mehmood, I, Munir, D, Razzaq, A, and Ali, A. Oxidative stress and metabolic diseases: relevance and therapeutic strategies. Front Nutr. (2022) 9:994309. doi: 10.3389/fnut.2022.994309
54. Fernandez-Marcos, PJ, and Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. (2011) 93:884S–90S. doi: 10.3945/ajcn.110.001917
55. Zhang, L, Fengna, L, Guo, Q, Duan, Y, Wang, W, Zhong, Y, et al. Leucine supplementation: a novel strategy for modulating lipid metabolism and energy homeostasis. Nutrients. (2020) 12:1299. doi: 10.3390/nu12051299
56. Børsheim, E, Bui, QU, Tissier, S, Cree, MG, Rønsen, O, Morio, B, et al. Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition. (2009) 25:281–8. doi: 10.1016/j.nut.2008.09.001
57. Coker, RH, Barati, Z, Murphy, CJ, Bateman, T, Newcomer, BR, Wolfe, RR, et al. Essential amino acid enriched meal replacement improves body composition and physical function in older adults. Clin Nutr ESPEN. (2022) 51:104–11. doi: 10.1016/j.clnesp.2022.07.004
58. Qin, LQ, Xun, P, Bugnowski, D, Daviglus, ML, Van Horn, L, Stamler, J, et al. Higher branched chain amino acid intake is associated with a lower prevlance of being overwight or obese in middle-aged east Asian and Western adults. J Nutr. (2011) 141:249–54. doi: 10.3945/jn.110.128520
59. Tamanna, N, and Mahmood, N. Emerging roles of branched-chain supplementation in human diseases. Int Schu Res Notices. (2014):235619. doi: 10.1155/2014/235619
60. Blundell, J. Making claims: functional foods for managing appetite and weight. Nat Rev Endocrinol. (2010) 6:53–6. doi: 10.1038/nrendo.2009.224
61. Mellinkoff, SM, Frankland, M, Boyle, D, and Greipel, M. Relationship between serum amino acid concentration and fluctuations in appetite. J Appl Physiol. (1956) 8:535–8. doi: 10.1152/jappl.1956.8.5.535
62. Poppitt, SD, McCormack, D, and Buffenstein, R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav. (1998) 64:279–85. doi: 10.1016/S0031-9384(98)00061-4
63. Veldhorst, MA, Nieuwenhuizen, AG, Hochstenbach-Waelen, A, Westerterp, KR, Engelen, MP, Brummer, RJ, et al. Comparison of the effects of a high- and normal casein breakfast on satiety, ‘satiety’ hormones, plasma amino acids and subsequent energy intake. Br J Nutr. (2009) 101:295–303. doi: 10.1017/S00071145080030
64. Niijima, A, Torii, K, and Uneyama, H. Role played by vagal chemical sensors in the hepato-portal region and duodeno-intestinal canal: an electrophysiological study. Chem Senses. (2005) 30 Suppl 1:i178–9. doi: 10.1093/chemse/bjh172
65. Potier, M, Darcel, N, and Tomé,. Protein, amino acids and the control of food intake. Curr Opin Clin Nutr Metab Care. (2009) 12:54–8. doi: 10.1097/MCO.0b013e32831b9e01
66. Blouet, C, Jo, Y-H, Li, X, and Schwartz, GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurol Sci. (2009) 29:83028311. doi: 10.1523/JNEUROSCI.1668—09.2009
67. Moon, J, and Koh, G. Clinical evidence and mechanisms of high-protein-induced weight loss. J Obes Metab Syndr. (2020) 29:166–73. doi: 10.7570/jomes20028
68. Zhang, M, Zhu, L, Wu, G, Zhang, H, Wang, X, and Qi, X. The impacts and mechanisms of dietary proteins on glucose homeostasis and food intake: a pivotal role of gut hormones. Crit Rev Food Sci Nutr. (2024) 64:12744–58. doi: 10.1080/10408398.2023.2256400
69. Wang, Y, Chandra, R, Samsa, LA, Gooch, B, Fee, BE, Cook, JM, et al. Amino acids stimulate cholecystokinin release through the Ca2+ receptor. Am J Physiol Gastrointest Liver Physiol. (2010) 300:G528–37. doi: 10.1152/ajpgi.00387.2010
70. Liddle, RA. Cholecystokinin In: JH Walsh and GH Dockray, editors. Gut Peptides. New York: Raven (1994). 175–216.
71. Muurahainen, N, Kissileff, HR, Derogatis, AJ, and Pi-Sunyer, FX. Effects of cholecystokinin-octapeptide (CCK-9) on food intake and gastric emptying in man. Physiol Behav. (1988) 44:645–9. doi: 10.1016/0031-Ad9384(88)90330-7
72. Cooper, JA. Factors affecting circulating levels of peptide YY in humans: a comprehensive review. Nutr Res Rev. (2014) 27:186–97. doi: 10.1017/S0954422414000109
73. Batterham, RL, Heffron, H, Kapoor, S, Chivers, JE, Chandarana, K, Herzog, H, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. (2006) 4:223–33. doi: 10.1016/j.cmet.2006.08.001
74. Cota, D, Proulx, K, Smith, KA, Kozma, SC, Thomas, G, Woods, SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. (2006) 312:927–30. doi: 10.1126/science.1124147
75. Blouet, C, Ono, H, and Schwartz, GJ. Mediobasal hypothalamic p70S6 kinase 1 modulates control of energy homeostasis. Cell Metab. (2008) 8:459–67. doi: 10.1016/j.cmet.2008.10.004
76. Skov, AR, Toubro, S, Ronn, B, Holm, L, and Astrup, A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. (1999) 23:528–36. doi: 10.1038/sj.ijo.0800867
77. Clamp, LD, Hume, DJ, Lambert, EV, and Kroff, J. Enhanced insulin sensitivity in successful, weight loss maintainers compared with matched controls with no weight loss history. Nutr Diabetes. (2017) 7:e282. doi: 10.1038/nutd.2017.31
78. Le Couteur, DG, Ribeiro, R, Senior, A, Hsu, B, Hirani, V, Blyth, FM, et al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older men: the concord health and ageing in men project. J Geronotol A Biol Sci Med Sci. (2020) 75:1805–10. doi: 10.1093/Gerona/glz192
79. Nawaz, SS, and Siddiqui, K. The emerging role of branch chain amino acids in the prediction of diabetes: a brief review. Curr Diabetes Rev. (2020) 16:532–7. doi: 10.2174/1573399815666190502113632
80. Newgard, C, An, J, Bain, JR, Muehlbauer, MJ, Stevens, RD, Lien, LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. (2009) 9:311–26. doi: 10.1016/j.cmet.2009.02.002
81. Wang, TJ, Larson, MG, Vasan, RS, Cheng, S, Rhee, EP, McCabe, E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. (2011) 17:448–53. doi: 10.1038/nm.2307
82. Giebertz, P, and Daniel, H. Branched-chain amino acids as biomarkers for diabetes. Curr Opin Nutr Metab Care. (2016) 19:48–54. doi: 10.1097/MCO.0000000000000235
83. Menni, C, Fauman, E, Erte, I, Perry, JRB, Kastenmüller, G, Shin, SY, et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. (2013) 62:4270–6. doi: 10.2337/db13-0570
84. Shah, SH, Svetkey, LP, and Newgard, CB. Branching out for detection of type 2 diabetes. Cell Metab. (2011) 13:491–2. doi: 10.1016/j.cmet.2011.04.003
85. Karusheva, Y, Koessler, T, Strassburger, K, Markgraf, D, Mastrototaro, L, and Jelenik, T. Short-term dietary restriction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am J Clin Nutr. (2019) 110:1098–107. doi: 10.1093/ajcn/nqz191
86. Vanweert, F, de Ligt, M, Hoeks, J, Hesselink, MKC, Schrauwen, P, and Pheilix, E. Elevated plasma branched-chain amino acid levels correlate with type 2 diabetes-related metabolic disturbances. J Clin Endocrinol Metab. (2021) 106:e1827–36. doi: 10.1210/clinem/dgaa751
87. Jang, C, Oh, SF, Wada, S, Rowe, GC, Liu, L, Chan, MC, et al. A branched chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. (2016) 22:421–6. doi: 10.1038/nm.4057
88. Liu, S, Sun, Y, Zhao, R, Wang, Y, Zhang, W, and Pang, W. Isoleucine increases muscle mass through promoting myogenesis and intramyocellular fat deposition. Food Funct. (2020) 12:144–53. doi: 10.1039/D0FO02156C
89. De Bandt, J-P, Coumoul, X, and Barouki, R. Branched-chain amino acids and insulin resistance, from protein supply to dietary-induced obesity. Nutrients. (2022) 5:68. doi: 10.3390/nu15010068
Keywords: metabolism, diet, signaling factors, nutrients, lipotoxicity and nutrition
Citation: Coker MS and Coker RH (2025) Dietary proteins, amino acids and insulin resistance: a mini review. Front. Nutr. 12:1671286. doi: 10.3389/fnut.2025.1671286
Edited by:
Lijuan Sun, Singapore Institute for Clinical Sciences (A*STAR), SingaporeReviewed by:
Gianfranca Carta, University of Cagliari, ItalyKen-Ichi Kobayashi, Notre Dame Seishin University, Japan
Raees Khan, University of Adelaide, Australia
Copyright © 2025 Coker and Coker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert H. Coker, cm9iZXJ0LmNva2VyQHVtb250YW5hLmVkdQ==
 Melynda S. Coker
Melynda S. Coker Robert H. Coker
Robert H. Coker