- Department of Biotechnology, Thapar Institute of Engineering and Technology, Patiala, Punjab, India
Lean Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD) is a substantial challenge in India, manifesting in individuals with normal BMI and indicative of a ‘metabolically obese normal weight’ phenotype. This review delineates the unique gut microbial signatures that characterize Indian lean MASLD, distinguishing it from obese MASLD. Principal modifications encompass substantial decreases in Faecalibacterium (particularly F. prausnitzii), Ruminococcus, Lactobacillus, Lachnospira, and Subdoligranulum, alongside an increase in pro-inflammatory Escherichia-Shigella. Dysbiotic patterns in India are influenced by factors such as fiber-deficient diets rich in refined carbohydrates, visceral obesity, insulin resistance, and genetic predispositions, including the PNPLA3 rs738409 polymorphism. Microbial alterations can contribute to disease by compromising gut barrier integrity, facilitating endotoxemia, and affecting the generation of beneficial metabolites. The combination signature of increased Escherichia-Shigella and decreased Lachnospira/Subdoligranulum exhibits significant diagnostic accuracy for detecting lean MASLD in the Indian population. These findings highlight lean MASLD as a mechanistically unique condition necessitating customized diagnostic and treatment approaches beyond standard weight management.
1 Introduction
Non-alcoholic Fatty Liver Disease (NAFLD) encompasses a wide range of hepatic disorders, beginning with simple hepatic steatosis (non-alcoholic fatty liver; NAFL), characterized by the buildup of lipids in the liver. This may evolve to Non-alcoholic Steatohepatitis (NASH), a more severe variant marked by inflammation and hepatocellular damage, frequently resulting in advanced liver conditions such as fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) (1). The terminology related to fatty liver disease has recently evolved, introducing the unifying terms Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD) (2) and Metabolic Dysfunction-associated Steatohepatitis (MASH) (3). This reclassification transcends the prior focus on alcohol use, clearly recognizing the robust metabolic processes of the condition (4). The diagnosis of MASLD necessitates evidence of hepatic steatosis, characterized by above 5% fat accumulation in hepatocytes, together with the presence of at least one of five cardiometabolic risk factors. The criteria encompass overweight or obesity, prediabetes or diabetes mellitus, raised triglycerides, and low high-density lipoprotein levels (4). This change in terminology and diagnostic standards expands the clinical viewpoint, promoting a multidisciplinary approach for patient management. The extensive clinical scope, especially pertinent in India given the significant prevalence of diabetes and prediabetes, emphasizes the growing necessity for a comprehensive, multidisciplinary approach to diagnosis and management.
The role of gut microbiota in the pathogenesis of MASLD is well established (5, 6). Nevertheless, studying the gut microbiota composition in lean MASLD patients is crucial, as their condition exemplifies a notable metabolic contradiction that contests the traditional obesity-focused concept of hepatic disease causation (7). Given that these patients do not possess the primary contributor to insulin resistance and hepatic steatosis (excess adiposity), the progression of their disease clearly suggests the involvement of alternate, significant pathogenic pathways. Investigating their microbiome could allow understanding the microbial contributions from the overwhelming effects of obesity, potentially identifying unique microbial signatures, pathways (e.g., specific bile acid alterations, endotoxin production, or metabolite generation), or dysbiosis patterns specifically driving liver injury in this phenotype. This knowledge could be essential for comprehending fundamental disease mechanisms beyond adiposity, developing targeted diagnostic biomarkers for lean individuals who are frequently underdiagnosed, and formulating innovative microbiome-modulating therapeutics effective across all NAFLD subtypes, particularly for this high-risk group where conventional weight-loss recommendations are less relevant.
2 The emerging phenotype of lean MASLD
Lean MASLD denotes the occurrence of fatty liver disease in persons who do not satisfy the criteria for overweight or obesity, generally characterized by a Body Mass Index (BMI) below 25 kg/m2 among Asian populations (8). Notwithstanding their normal BMI, these patients often display modest yet considerable changes in body composition, including increased total body and regional adiposity, especially in visceral and deep subcutaneous adipose tissues. Standard anthropometric measurements frequently fail to appropriately identify concerns related to fat compartmentalization. This phenotype is commonly referred to as ‘metabolically obese normal weight’ (MONW) (8). Lean MASLD is an escalating global health issue, with an estimated prevalence of 4.1%, and is particularly prevalent among Asian populations (9). In India, the incidence of MASLD in lean individuals is significant, as evidenced by a biopsy-based study indicating that 33.7% of lean liver donors were affected by MASLD (10). The prevalence of MASLD in South Asia varies significantly, ranging from 9 to 45% (11). Research in India indicates that lean MASLD patients may exhibit markedly abnormal metabolic profiles, such as dyslipidemia and impaired glucose metabolism, similar to those found in obese individuals, despite having lower fasting plasma glucose and insulin resistance levels (12). In fact, it was suggested that central obesity is an independent factor influencing advanced fibrosis in lean individuals with MASLD (13). The significant occurrence of lean MASLD in India, despite ostensibly normal BMI, underscores the ‘metabolically obese normal weight’ phenotype and it was suggested that MASLD definition is more suitable to lean NAFLD patients than MAFLD (14). This suggests that BMI alone is an inadequate measure of metabolic health, particularly in Asian populations, and that underlying fat distribution, especially visceral adiposity, and hereditary factors are significant contributors to the disease (4). This discovery contests traditional obesity-focused perspectives of MASLD, highlighting the necessity for more refined diagnostic and risk stratification methodologies. In these settings, the gut microbiome may function as a significant diagnostic or therapeutic target, irrespective of manifest obesity.
Despite a rise in the prevalence among the general population, lean MASLD is frequently underdiagnosed in India owing to various factors. The therapeutic focus on obesity as a principal risk factor results in the neglect of cases in individuals with a normal BMI, even though data indicate that lean MASLD accounts for 9.7% of all MASLD cases (15). Secondly, restricted availability of non-invasive diagnostic techniques (e.g., transient elastography) in primary care environments impedes early identification of such conditions in the non-obese individuals (16). Third, asymptomatic progression and clinician unawareness lead to missed diagnosis, especially in lean individuals with metabolic disorders such as diabetes (4). Ultimately, regional variations in healthcare infrastructure and inadequate incorporation of lean-specific screening in guidelines increase underdiagnosis (17, 18). Overcoming these obstacles necessitates comprehensive diagnostic criteria and improved clinician education regarding metabolic disorders beyond obesity.
3 Gut microbial signatures in Indian lean MASLD
3.1 Distinct microbial profiles
Recent studies repeatedly demonstrate that lean MASLD patients possess distinct gut microbial signatures and display considerably different gut microbial diversity, a metric of microbial community dissimilarity, in comparison to both obese MASLD patients and healthy controls (12). This indicates that the pathogenic mechanisms behind lean MASLD may be fundamentally different from those in obese MASLD (19). In general, MASLD patients may exhibit an elevation in Proteobacteria at the phylum level (20) and changed Firmicutes/Bacteroidetes ratios (21); however, specific microbial alterations are more severe and distinctive in lean individuals. The persistent identification of unique gut microbial signatures in lean MASLD patients, in contrast to obese MASLD patients, strongly substantiates the argument that lean MASLD is not simply a less severe variant of the obese condition, but rather a distinct mechanistic phenotype (9). This indicates that diagnostic indicators and therapy techniques designed for obese MASLD may not be immediately applicable or ideally successful for lean persons, requiring customized approaches that address their distinct microbial profiles.
3.2 Key microbial taxa
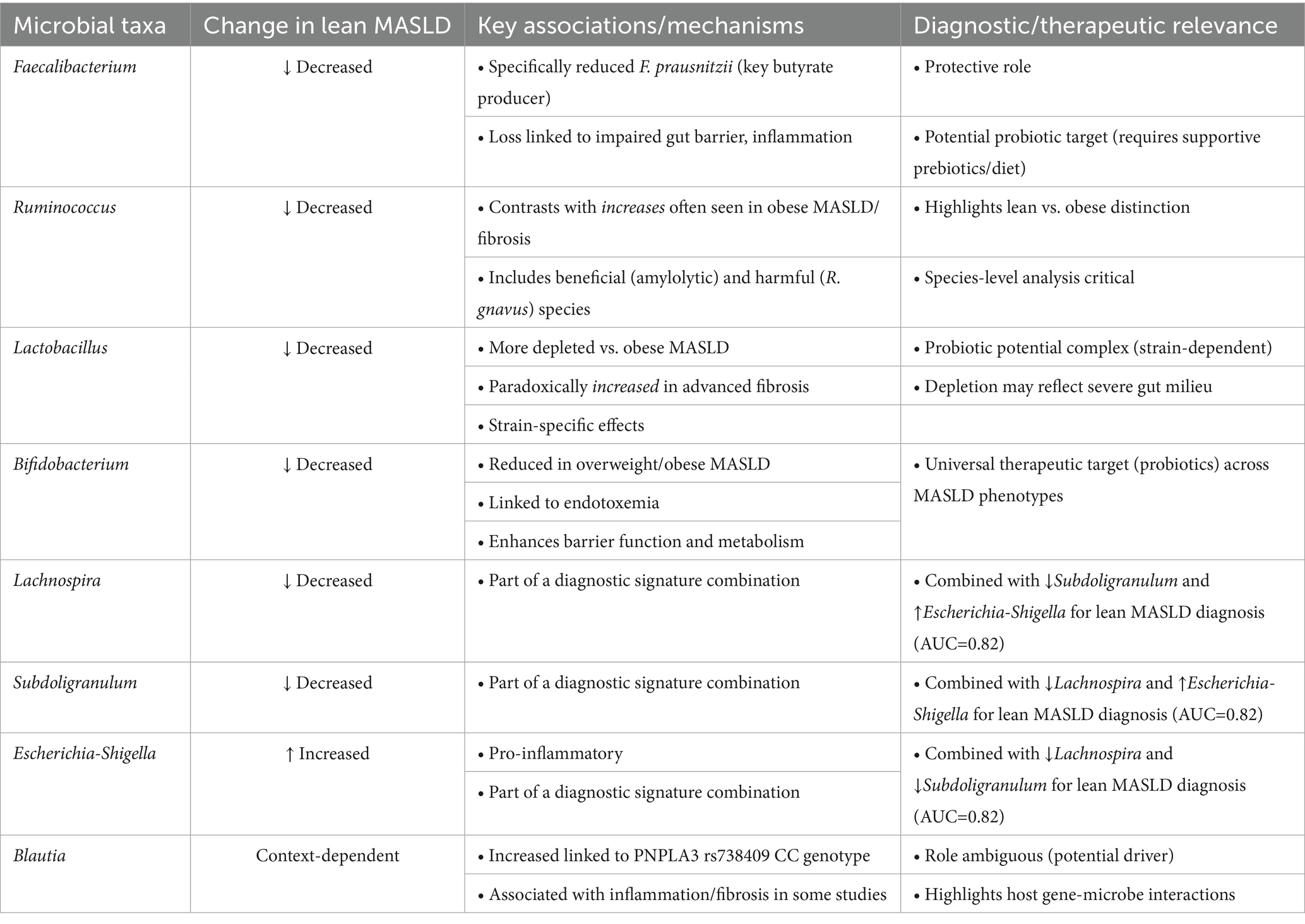
Numerous significant microbial genera and species have been associated with lean MASLD, with particular findings relevant to the Indian population (Table 1).
3.2.1 Faecalibacterium
A reduction of Faecalibacterium is associated with the pathophysiology of MASLD as demonstrated in a BMI- and sex-matched study of MASLD patients within a large study cohort (22). This association was irrespective of BMI and adiposity. However, a prospective pilot investigation revealed that lean NASH patients had a 3-fold reduction in the abundance of Faecalibacterium compared to control groups (19). Reduced Faecalibacterium, particularly its predominant strain F. prausnitzii, is noted in overall MASLD participants compared to healthy controls, with further reductions in individuals with more severe fibrosis (stage 3–4) (23). F. prausnitzii is a prominent butyrate-producing bacterium (24). Butyrate is recognized for its anti-inflammatory characteristics and its function in preserving gut barrier integrity (25). Preclinical studies suggest that F. prausnitzii supplementation enhances glucose homeostasis, inhibits hepatic lipid accumulation, mitigates liver damage and fibrosis, and restores compromised gut barrier function in mouse models of MASLD (26). Nonetheless, one study observed that oral gavage of F. prausnitzii did not ameliorate diet-induced steatohepatitis in mice, despite its correlation with MASLD severity in humans (23). The persistent finding of diminished Faecalibacterium in lean NASH and more severe MASLD, along with its established function as a beneficial butyrate producer, clearly indicates its protective significance. Although why reduced Faecalibacterium levels is observed in Indian lean MASLD patients remains unexplored, it is likely attributable to a fiber-deficient diet (27), coupled with a metabolic milieu marked by pronounced insulin resistance, visceral fat accumulation, and chronic inflammation, despite the absence of overt obesity (28). This establishes a gut milieu unsuitable to F. prausnitzii, while its reduction further intensifies gut barrier impairment and inflammation, accelerating the advancement of MASLD. Genetic predisposition and environmental variables, such as antibiotic usage, could also contribute to this dysbiotic profile (29). Moreover, the divergence between human associations and mouse intervention studies underscores the intricacy of microbiome interventions, merely introducing a beneficial bacterium may be insufficient if the gut environment is not favorable for its colonization or if other factors, such as diet and host genetics, diminish its efficacy. This indicates that future therapies may require the integration of probiotics with prebiotics or alternative dietary adjustments to establish a conducive environment for these advantageous bacteria.
3.2.2 Ruminococcus
Indian lean MASLD patients exhibited a 3-fold reduction in the amount of Ruminococcus (19). In contrast, certain investigations including general MASLD patients indicated elevated amounts of Ruminococcus relative to controls (30). Significantly, the abundance of Ruminococcus has been independently correlated with liver fibrosis (F ≥ 2) (19). Ruminococcus gnavus has been associated with pro-inflammatory markers, inflammation, and fibrosis in patients with MASLD (31). The genus Ruminococcus exhibits significant heterogeneity, encompassing both advantageous and potentially harmful species, hence complicating the understanding of its function (6). R. gnavus is recognized for its impact on host metabolism and its ability to provoke either pro-inflammatory or anti-inflammatory responses (32). The populations are susceptible to nutritional alterations (6). The conflicting results regarding Ruminococcus (reduced in lean NASH, while elevated in general MASLD and correlated with fibrosis) highlight the necessity for species-level differentiation and patient stratification (lean vs. obese, fibrosis stage). This implies that certain Ruminococcus species may have contradictory effects on liver health, or their influence is contingent upon context (e.g., affected by food or other concurrent dysbiosis). General analyses at the genus level may obscure significant specific effects, necessitating additional research to investigate species-specific roles and their interactions within the intricate gut ecosystem. Nevertheless, the reduced abundance of Ruminococcus in Indian lean MASLD patients is likely attributable to a confluence of dietary and metabolic variables. The common diet characterized by high-processed carbohydrates and low-resistant starch (e.g., refined rice and wheat) deprives amylolytic Ruminococcus spp. of their principal fermentable substrates (33). Simultaneously, metabolic dysfunction, marked by visceral fat accumulation, insulin resistance, and systemic inflammation even at reduced BMI, facilitates gut dysbiosis and barrier impairment (34). This milieu promotes pro-inflammatory mucin-degrading Ruminococcus strains (e.g., R. gnavus), which may temporarily proliferate but ultimately lead to pathogenic alterations, while diminishing beneficial fiber-utilizing species essential for butyrate synthesis and gut health.
3.2.3 Lactobacillus
Supplementation of probiotic Lactobacillus has proved to ameliorate diet-induced NASH in animal models (35). Lean MASLD patients demonstrated a higher depletion in Lactobacillus relative to overweight and obese NASH patients (19). Liver fibrosis (≥ F2) correlated with a heightened prevalence of Lactobacilli, while obese MASLD patients exhibited an enrichment of Lactobacilli (19). Patients with general MASLD exhibit elevated levels of Lactobacillus in comparison to healthy controls (5). Nevertheless, these diverse associations, numerous Lactobacillus strains are acknowledged as probiotics that confer advantageous outcomes in rodent models of MASLD, such as enhancing insulin sensitivity, mitigating inflammation, and decreasing lipogenesis (21). Particular strains such as L. acidophilus have shown preventive potential against MASLD-related HCC by generating valeric acid and enhancing gut barrier integrity (36). L. plantarum can mitigate redox-induced hepatocellular injury (37), and inflammation associated with MASLD (38). Probiotic treatment with several Lactobacillus spp. has demonstrated efficacy in ameliorating hepatic steatosis and diminishing liver inflammation (21). The apparently contradicting results regarding Lactobacillus (deficiency in lean MASLD but relatively elevated levels in fibrotic and obese MASLD) further indicate species- or strain-specific effects. Although remains unexplored, this is likely attributable to differing metabolic and nutritional interactions. Lean NASH in Indians is significantly linked to the ‘thin-fat’ phenotype, characterized by elevated visceral adiposity and insulin resistance despite a lower BMI, which induces severe gut inflammation and barrier impairment, resulting in a more adverse microenvironment for commensal lactobacilli compared to certain obese conditions (8, 39). Moreover, dietary elements specific to this population, such as elevated consumption of antimicrobial spices (e.g., turmeric, chili) and widespread intake of refined carbohydrates, may more effectively inhibit Lactobacillus than diets linked to obesity-related MASLD. Ironically, some Lactobacillus spp. may exacerbate inflammation in metabolic disorders, perhaps resulting in increased suppression within the markedly inflammatory lean MASLD environment (40). Although numerous Lactobacillus strains are recognized as probiotics that positively influence liver function, an over-proliferation of specific species or a dysbiosis within the genus may exacerbate disease, particularly in cases of severe fibrosis (41). This suggests that merely enhancing Lactobacillus proliferation may not consistently yield advantages, a detailed comprehension of particular strains and their metabolic roles is essential for successful probiotic applications.
3.2.4 Bifidobacterium
Patients with overweight MASLD demonstrated reduced abundance of Bifidobacterium (19). Within the wider South Asian population, diminished cecal Bifidobacterium levels have been linked to increased endotoxemia, which contributes to diabetes, obesity, and MASLD. Bifidobacterium spp. are acknowledged as advantageous probiotics (42). They are recognized for their ability to repair intestinal barrier integrity, diminish endotoxemia, and enhance lipid metabolism and insulin sensitivity. B. animalis subsp. Lactis V9 has demonstrated the capacity to lower liver transaminases, diminish TLR4 and TLR9 levels, and mitigate liver inflammation (43). The persistent observation of diminished Bifidobacterium in overweight NASH and its correlation with endotoxemia in South Asians underscores its essential function in preserving gut barrier integrity and mitigating systemic inflammation. This underscores that Bifidobacterium depletion is a prevalent dysbiotic characteristic among various MASLD phenotypes (lean, overweight, obese) and indicates that Bifidobacterium-containing probiotics may serve as a universally advantageous therapeutic approach, especially in the Indian context where its levels are observed to be diminished.
3.2.5 Blautia
In a recent study, Blautia exhibited a substantial rise in persons possessing the PNPLA3 rs738409 CC genotype throughout a 4-year duration (44). Several investigations involving general MASLD patients have indicated elevated levels of Blautia in comparison to controls (30). Nonetheless, alternative therapies, such as inulin administration, have demonstrated the capacity to down-regulate Blautia abundance, indicating a potentially adverse impact in some situations (45). The significance of Blautia in obesity and liver disease is seen as controversial, with certain research indicating advantageous effects while others recognize it as a contributing component (46). Blautia has been demonstrated to induce liver inflammation and hepatic fibrosis in murine models (47). The conflicting results regarding Blautia underscore the intricacy of analyzing microbial alterations. The distinct correlation between Blautia elevation and the PNPLA3 CC genotype indicates a gene-microbe interaction that may affect disease progression. This underscores that the influence of a certain genus can be significantly context-dependent, shaped by host genetics, co-existing microbial species, and unique eating habits. It warns against oversimplified categorization of ‘good’ and ‘bad’ (48) and advocates for a more refined comprehension of strain-specific functions and their interactions with host variables.
3.2.6 Other signatures
A study conducted in India comparing lean and non-lean MASLD patients revealed a notable increase of Escherichia-Shigella and a reduction of Lachnospira and Subdoligranulum especially in the lean MASLD subgroup (12). The amalgamation of these bacterial genera showed significant diagnostic precision (AUC of 0.82) in differentiating lean from non-lean MASLD patients (12). The identification of Escherichia-Shigella enrichment and Lachnospira/Subdoligranulum depletion as distinct signatures for Indian lean MASLD is a significant finding, offering concrete, regionally pertinent microbial targets that may function as diagnostic biomarkers or therapeutic intervention points. The elevated diagnostic precision indicates significant potential for clinical utilization. Finally, gut microbial diversity has emerged as a critical indicator of overall metabolic health, where a depleted diversity is generally indicative of metabolic disease. In fact, patients with MASLD show a marked reduction in the gut microbiota diversity compared to healthy cohorts (49–51). However, whether the gut microbial diversity of lean MASLD patients, especially in the case of the Indian cohort, follows a similar trend of reduction, requires further study.
4 Conclusion
Lean MASLD is a unique and escalating difficulty, especially among the Indian population, as traditional BMI-based diagnoses of obesity may obscure underlying metabolic abnormalities (Figure 1). The pathogenesis of lean MASLD could be closely associated with gut dysbiosis, marked by distinct microbial changes, including diminished Faecalibacterium, Ruminococcus, and Lactobacillus in lean MASLD, as well as a reduction of Lachnospira and Subdoligranulum and an increase of Escherichia-Shigella in Indian lean MASLD. These microbial alterations lead to liver damage by compromising gut barrier integrity, elevating endotoxemia, and modifying the synthesis of microbial metabolites. The advancement of lean MASLD is significantly affected by a complex interaction of genetic factors, particularly the PNPLA3 rs738409 (G/G) genotype, which can directly change the composition of the gut microbiome. Concurrent lifestyle modifications, such as the embrace of Western food patterns abundant in refined carbs and unbalanced fats, together with sedentary behaviors, aggravate gut dysbiosis and influence the distinct metabolic characteristics of lean MASLD in India. The unique pathophysiological pathways present in lean MASLD require customized diagnostic and treatment strategies. Microbiome-targeted therapies, including probiotics, prebiotics, and Fecal Microbiota Transplantation, exhibit significant potential, especially due to their proven effectiveness in non-obese individuals. These solutions provide a tailored approach for addressing MASLD in a demographic where conventional weight-loss therapies may be less effective. Future research should emphasize larger, methodologically designed studies targeting the Indian population to clarify specific microbial signatures, their functional aspects, and the long-term effectiveness of microbiome-modulating strategies in the prevention and treatment of lean MASLD.
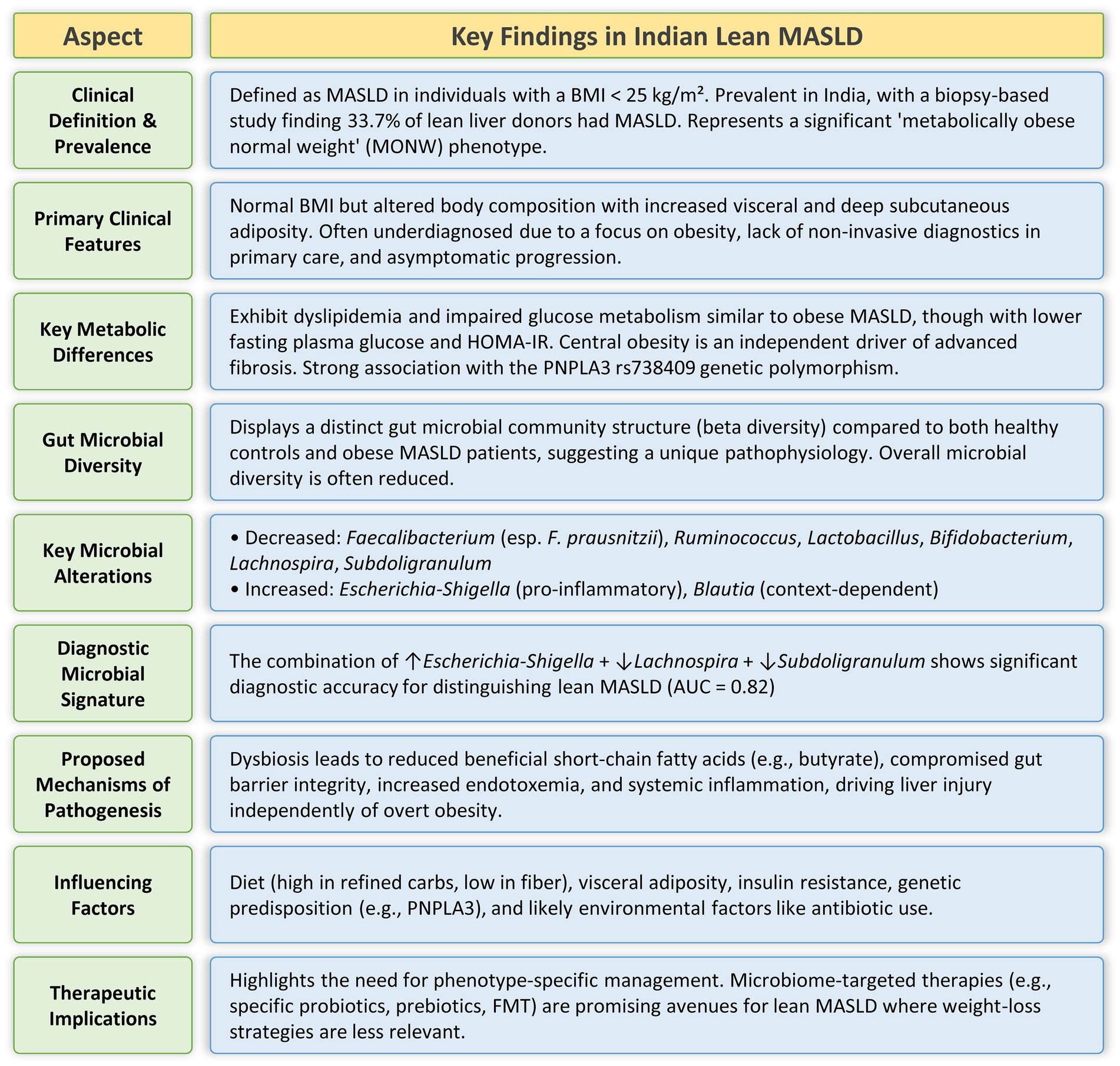
Figure 1. Summary of the major clinical, metabolic, and gut microbial characteristics of the Indian lean MASLD phenotype.
Author contributions
PD: Funding acquisition, Writing – original draft, Formal analysis, Resources, Visualization, Validation, Conceptualization, Project administration, Writing – review & editing, Investigation, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding received from the Indian Council of Medical Research (IIRPIG-2024-01-00034) is thankfully acknowledged.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pandey, H, Goel, P, Srinivasan, VM, Tang, DWT, Wong, SH, and Lal, D. Gut microbiota in non-alcoholic fatty liver disease: pathophysiology, diagnosis, and therapeutics. World J Hepatol. (2025) 17:106849. doi: 10.4254/wjh.v17.i6.106849
2. Chan, WK, Chuah, KH, Rajaram, RB, Lim, LL, Ratnasingam, J, and Vethakkan, SR. Metabolic dysfunction-associated Steatotic liver disease (MASLD): a state-of-the-art review. J Obes Metab Syndr. (2023) 32:197–213. doi: 10.7570/jomes23052
3. Patel, RH, Parikh, C, Upadhyay, H, Sonaiya, S, Ramnath, P, Singh, S, et al. Resmetirom in the management of metabolic dysfunction-associated steatohepatitis (MASH): a comprehensive review of current evidence and therapeutic potential. Cureus. (2024) 16:e74772. doi: 10.7759/cureus.74772
4. Zargar, AH, Bhansali, A, Majumdar, A, Maheshwari, A, Bhattacharyya, A, Dasgupta, A, et al. Management of metabolic dysfunction-associated steatotic liver disease (MASLD)-an expert consensus statement from Indian diabetologists' perspective. Diabetes Obes Metab. (2025) 27:3–20. doi: 10.1111/dom.16496
5. Schwenger, KJ, Clermont-Dejean, N, and Allard, JP. The role of the gut microbiome in chronic liver disease: the clinical evidence revised. JHEP Rep. (2019) 1:214–26. doi: 10.1016/j.jhepr.2019.04.004
6. Brandl, K, and Schnabl, B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr Opin Gastroenterol. (2017) 33:128–33. doi: 10.1097/MOG.0000000000000349
7. Xu, R, Pan, J, Zhou, W, Ji, G, and Dang, Y. Recent advances in lean NAFLD. Biomed Pharmacother. (2022) 153:113331. doi: 10.1016/j.biopha.2022.113331
8. Das, K, and Chowdhury, A. Lean NASH: distinctiveness and clinical implication. Hepatol Int. (2013) 7:806–13. doi: 10.1007/s12072-013-9477-5
9. Haag, M, Winter, S, Kemas, AM, Tevini, J, Feldman, A, Eder, SK, et al. Circulating metabolite signatures indicate differential gut-liver crosstalk in lean and obese MASLD. JCI Insight. (2025) 10:943. doi: 10.1172/jci.insight.180943
10. Kuchay, MS, Martínez-Montoro, JI, Choudhary, NS, Fernández-García, JC, and Ramos-Molina, B. Non-alcoholic fatty liver disease in lean and non-obese individuals: current and future challenges. Biomedicine. (2021) 9:346. doi: 10.3390/biomedicines9101346
11. Pati, GK, and Singh, SP. Nonalcoholic fatty liver disease in South Asia. Euroasian J Hepatogastroenterol. (2016) 6:154–62. doi: 10.5005/jp-journals-10018-1189
12. Anirvan, P, Khan, ZH, Bhuyan, P, Dixit, S, Dash, R, Mishra, P, et al. Gut microbiota and genetic polymorphisms appear to drive disease expression of nonalcoholic fatty liver disease in lean individuals. J Clin Exp Hepatol. (2025) 15:102503. doi: 10.1016/j.jceh.2025.102503
13. De, A, Bhagat, N, Mehta, M, Singh, P, Rathi, S, Verma, N, et al. Central obesity is an independent determinant of advanced fibrosis in lean patients with nonalcoholic fatty liver disease. J Clin Exp Hepatol. (2025) 15:102400. doi: 10.1016/j.jceh.2024.102400
14. De, A, Bhagat, N, Mehta, M, Taneja, S, and Duseja, A. Metabolic dysfunction-associated steatotic liver disease (MASLD) definition is better than MAFLD criteria for lean patients with NAFLD. J Hepatol. (2024) 80:e61–2. doi: 10.1016/j.jhep.2023.07.031
15. Prasad, M, Bhardwaj, N, Gupta, E, and Thomas, SS. Prevalence and predictors for lean fatty liver disease in general population attending a COVID-19 vaccination center in a tertiary care hospital in India. Euroasian J Hepato Gastroenterol. (2024) 14:145–50. doi: 10.5005/jp-journals-10018-1438
16. Rajan, V, Das, A, Venkatachalam, J, Lohani, K, and Lahariya, C. Managing metabolic dysfunction-associated steatotic liver disease (MASLD) in primary care settings: a review. Prevent Med. (2025) 2:183–91. doi: 10.4103/PMRR.PMRR_159_24
17. Mohan, V, Joshi, S, Kant, S, Shaikh, A, Sreenivasa Murthy, L, Saboo, B, et al. Prevalence of metabolic dysfunction-associated steatotic liver disease: mapping across different Indian populations (MAP study). Diab Ther. (2025) 16:1435–50. doi: 10.1007/s13300-025-01748-1
18. Ramesh, PR, Krishnan, P, Prabu, S, Srinivasan, V, and Niranjan, V. Diagnosis and management of metabolic dysfunction-associated steatotic liver disease in south Asians-a clinical review. Obesity Pillars. (2024) 12:100142. doi: 10.1016/j.obpill.2024.100142
19. Duarte, SMB, Stefano, JT, Miele, L, Ponziani, FR, Souza-Basqueira, M, Okada, L, et al. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: a prospective pilot study. Nutr Metab Cardiovasc Dis. (2018) 28:369–84. doi: 10.1016/j.numecd.2017.10.014
20. Zhou, J, Tripathi, M, Sinha, RA, Singh, BK, and Yen, PM. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. (2021) 7:11. doi: 10.20517/2394-5079.2020.134
21. Ren, TY, Li, XY, and Fan, JG. Probiotics for treatment of nonalcoholic fatty liver disease: it is worth a try. Clin Mol Hepatol. (2021) 27:83–6. doi: 10.3350/cmh.2020.0298
22. Iino, C, Endo, T, Mikami, K, Hasegawa, T, Kimura, M, Sawada, N, et al. Significant decrease in Faecalibacterium among gut microbiota in nonalcoholic fatty liver disease: a large BMI- and sex-matched population study. Hepatol Int. (2019) 13:748–56. doi: 10.1007/s12072-019-09987-8
23. Münte, E, Viebahn, G, Khurana, A, Fujiki, J, Nakamura, T, Lang, S, et al. Faecalibacterium prausnitzii is associated with disease severity in MASLD but its supplementation does not improve diet-induced Steatohepatitis in mice. Microorganisms. (2025) 13:675. doi: 10.3390/microorganisms13030675
24. Parsaei, M, Sarafraz, N, Moaddab, SY, and Ebrahimzadeh Leylabadlo, H. The importance of Faecalibacterium prausnitzii in human health and diseases. New Microbes New Infect. (2021) 43:100928. doi: 10.1016/j.nmni.2021.100928
25. Dey, P. Targeting gut barrier dysfunction with phytotherapies: effective strategy against chronic diseases. Pharmacol Res. (2020) 161:105135. doi: 10.1016/j.phrs.2020.105135
26. Shin, JH, Lee, Y, Song, EJ, Lee, D, Jang, SY, Byeon, HR, et al. Faecalibacterium prausnitzii prevents hepatic damage in a mouse model of NASH induced by a high-fructose high-fat diet. Front Microbiol. (2023) 14:1123547. doi: 10.3389/fmicb.2023.1123547
27. Narayan, S, Lakshmipriya, N, Vaidya, R, Bai, MR, Sudha, V, Krishnaswamy, K, et al. Association of dietary fiber intake with serum total cholesterol and low density lipoprotein cholesterol levels in urban Asian-Indian adults with type 2 diabetes. Indian J Endocrinol Metab. (2014) 18:624–30. doi: 10.4103/2230-8210.139215
28. Bilic-Curcic, I, Berkovic, MC, Virovic-Jukic, L, and Mrzljak, A. Shifting perspectives–interplay between non-alcoholic fatty liver disease and insulin resistance in lean individuals. World J Hepatol. (2021) 13:80–93. doi: 10.4254/wjh.v13.i1.80
29. Tarantino, G, and Citro, V. Could adverse effects of antibiotics due to their use/misuse be linked to some mechanisms related to nonalcoholic fatty liver disease? Int J Mol Sci. (2024) 25:93. doi: 10.3390/ijms25041993
30. Del Chierico, F, Nobili, V, Vernocchi, P, Russo, A, De Stefanis, C, Gnani, D, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. (2017) 65:451–64. doi: 10.1002/hep.28572
31. Gupta, H, Min, BH, Ganesan, R, Gebru, YA, Sharma, SP, Park, E, et al. Gut microbiome in non-alcoholic fatty liver disease: from mechanisms to therapeutic role. Biomedicine. (2022) 10:550. doi: 10.3390/biomedicines10030550
32. Juge, N. Microbe profile: Ruminococcus gnavus: the yin and yang of human gut symbionts. Microbiology. (2023) 169:383. doi: 10.1099/mic.0.001383
33. Leung, C, Rivera, L, Furness, JB, and Angus, PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. (2016) 13:412–25. doi: 10.1038/nrgastro.2016.85
34. Portincasa, P, Khalil, M, Graziani, A, Frühbeck, G, Baffy, G, Garruti, G, et al. Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations? Eur J Intern Med. (2024) 119:13–30. doi: 10.1016/j.ejim.2023.10.002
35. Sabirin, F, Lim, SM, Neoh, CF, and Ramasamy, K. Hepatoprotection of probiotics against non-alcoholic fatty liver disease in vivo: a systematic review. Front Nutr. (2022) 9:844374. doi: 10.3389/fnut.2022.844374
36. Lau, HC, Zhang, X, Ji, F, Lin, Y, Liang, W, Li, Q, et al. Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid. EBioMedicine. (2024) 100:104952. doi: 10.1016/j.ebiom.2023.104952
37. Rezgui, R, Walia, R, Sharma, J, Sidhu, D, Alshagadali, K, Ray Chaudhuri, S, et al. Chemically defined Lactobacillus plantarum cell-free metabolites demonstrate cytoprotection in HepG2 cells through Nrf2-dependent mechanism. Antioxidants. (2023) 12:930. doi: 10.3390/antiox12040930
38. Kim, DY, Park, JY, and Gee, HY. Lactobacillus plantarum ameliorates NASH-related inflammation by upregulating L-arginine production. Exp Mol Med. (2023) 55:2332–45. doi: 10.1038/s12276-023-01102-0
39. De, A, Mehta, M, Singh, P, Bhagat, N, Mitra, S, Das, A, et al. Lean Indian patients with non-alcoholic fatty liver disease (NAFLD) have less metabolic risk factors but similar liver disease severity as non-lean patients with NAFLD. Int J Obes. (2023) 47:986–92. doi: 10.1038/s41366-023-01346-w
40. Dey, P, and Ray Chaudhuri, S. Cancer-associated microbiota: from mechanisms of disease causation to microbiota-centric anti-Cancer approaches. Biology. (2022) 11:757. doi: 10.3390/biology11050757
41. Rao, SSC, Rehman, A, Yu, S, and Andino, NM. Brain fogginess, gas and bloating: a link between SIBO, probiotics and metabolic acidosis. Clin Transl Gastroenterol. (2018) 9:162. doi: 10.1038/s41424-018-0030-7
42. Dey, P. Gut microbiota in phytopharmacology: a comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol Res. (2019) 147:104367. doi: 10.1016/j.phrs.2019.104367
43. Campagnoli, LIM, Marchesi, N, Vairetti, M, Pascale, A, Ferrigno, A, and Barbieri, A. Age-related NAFLD: the use of probiotics as a supportive therapeutic intervention. Cells. (2022) 11:827. doi: 10.3390/cells11182827
44. Sato, S, Iino, C, Sasada, T, Furusawa, K, Yoshida, K, Sawada, K, et al. A 4-year cohort study of the effects of PNPLA3 rs738409 genotypes on liver fat and fibrosis and gut microbiota in a non-fatty liver population. Environ Health Prev Med. (2025) 30:17–7. doi: 10.1265/ehpm.24-00365
45. Bao, T, He, F, Zhang, X, Zhu, L, Wang, Z, Lu, H, et al. Inulin exerts beneficial effects on non-alcoholic fatty liver disease via modulating gut microbiome and suppressing the lipopolysaccharide-toll-like receptor 4-Mψ-nuclear factor-κB-nod-like receptor protein 3 pathway via gut-liver Axis in mice. Front Pharmacol. (2020) 11:558525. doi: 10.3389/fphar.2020.558525
46. Chanda, W, Jiang, H, and Liu, SJ. The ambiguous correlation of Blautia with obesity: a systematic review. Microorganisms. (2024) 12:768. doi: 10.3390/microorganisms12091768
47. Yang, M, Qi, X, Li, N, Kaifi, JT, Chen, S, Wheeler, AA, et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat Commun. (2023) 14:228. doi: 10.1038/s41467-023-35861-1
48. Dey, P. Good girl goes bad: understanding how gut commensals cause disease. Microb Pathog. (2024) 190:106617. doi: 10.1016/j.micpath.2024.106617
49. Schnabl, B, Damman, CJ, and Carr, RM. Metabolic dysfunction–associated steatotic liver disease and the gut microbiome: pathogenic insights and therapeutic innovations. J Clin Invest. (2025) 135:423. doi: 10.1172/JCI186423
50. Jiménez-González, C, Alonso-Peña, M, Argos Vélez, P, Crespo, J, and Iruzubieta, P. Unraveling MASLD: the role of gut microbiota, dietary modulation, and AI-driven lifestyle interventions. Nutrients. (2025) 17:1580. doi: 10.3390/nu17091580
Keywords: NAFLD, NASH, MASLD, microbiota, lean, obese, BMI
Citation: Dey P (2025) Gut microbial signatures associated with the Indian lean MASLD phenotype. Front. Nutr. 12:1673517. doi: 10.3389/fnut.2025.1673517
Edited by:
Philippe Gérard, Institut National de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), FranceReviewed by:
Tiziana Maria Mahayri, Academy of Sciences of the Czech Republic (ASCR), CzechiaCopyright © 2025 Dey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Priyankar Dey, cHJpeWFua2FyLmRleUB0aGFwYXIuZWR1; cHJpeWFua2FyZGV5MjhAZ21haWwuY29t
 Priyankar Dey
Priyankar Dey