Abstract
Background:
Postoperative pneumonia (POP) remains a serious complication following esophagectomy for esophageal cancer (EC) patients, contributing to increased morbidity, mortality, and healthcare costs. This study aimed to evaluate whether preoperative prognostic nutritional index (PNI) could be an independent predictor of POP in EC patients.
Methods:
This study included 200 EC patients who underwent esophagectomy between January 2021 to December 2022. Receiver operating characteristic (ROC) curve analysis was conducted to assess the predictive ability of preoperative PNI for POP. Univariate and multivariate logistic regression analyses were used to identify risk factors for POP among EC patients. A predictive nomogram model was conducted. The performance of the nomogram model was evaluated by the AUC curve, calibration curve and decision curve analysis (DCA).
Results:
Two hundred EC patients receiving esophagectomy were included finally, and 73 (36.5%) cases developed POP. ROC curve analysis showed that preoperative PNI predicted the occurrence of POP with an AUC value of 0.602 at a cut-off value of 49.6; the sensitivity, specificity, and Youden index was 64.38%, 63.78%, 0.2716, respectively. Univariate logistic regression analysis showed that male, aged ≥60 years old, TNM stage III, tumor location, hospital stay time >16 days, WBC counts >5.62 × 109/L, neutrophil counts >3.52 × 109/L, monocyte counts >0.40 × 109/L, and preoperative PNI ≤ 49.6 were risk factors for POP. Multivariate logistic regression analysis indicated that tumor location, hospital stay time >16 days, WBC counts >5.62 × 109/L, monocyte counts >0.40 × 109/L, and preoperative PNI ≤ 49.6 were significant risk factors for POP among EC patients receiving esophagectomy. A nomogram model was established. The ROC curve incorporating PNI showed an excellent discrimination in detecting POP with an AUC value of 0.831 (95% CI: 0.772–0.890). The calibration curve suggested that the predicted results of this nomogram model exhibited a good concordance with the actual results. The DCA indicated that this nomogram model achieved net benefits for predicting POP.
Conclusion:
Preoperative PNI is a significant predictive factor for the occurrence of POP in EC patients. The nomogram model incorporating preoperative PNI shows good accuracy and clinical practicality in predicting the occurrence of POP among EC patients.
1 Introduction
Esophageal cancer (EC), the seventh most common cancer globally, remains a formidable clinical challenge, with over 600,000 new cases and 540,000 deaths annually (1, 2). Despite advances in treatment, the prognosis of EC remains poor, with low five-year survival rates, underscoring the need for better risk stratification and perioperative management (3, 4). Up to now, surgery remains the most effective treatment for EC patients. Various postoperative complications for EC patients included anastomotic leakage, pneumonia, recurrent laryngeal nerve injury, gastric emptying disorders, anastomotic stenosis, chylothorax, etc. Postoperative pneumonia (POP) was one of the most common postoperative complications of EC. The incidence of POP in patients receiving esophagectomy ranged from 14.60 to 39.26% (5). POP was reported to be associated with prolonged hospitalization, increased mortality, and elevated healthcare costs (6). Therefore, early prediction of POP is important for surgeons to make suitable clinical decisions. Identifying predictive markers for patients with POP is urgently necessary.
Esophagectomy could lead to weight loss, inadequate food intake, and nutrition issues (7). Preoperative nutritional status was associated with an increased risk of postoperative adverse events and a poor prognosis for EC patients (8). A pre-treatment nutritional assessment is recommended according to the nutrition guidelines (7, 9). The prognostic nutritional index (PNI), derived from serum albumin and lymphocyte counts, has gained recognition as a biomarker integrating nutritional and immune status of patients. PNI was regarded as a nutritional marker to evaluate the malnutrition status of patients with EC (10). A host of studies demonstrated that PNI was associated with the occurrence of postoperative complications and the survival of EC patients after esophagectomy (11–16). However, its role in predicting POP after esophagectomy, a common complication with significant clinical and economic consequences, remains unclear. Therefore, this study aimed to investigate the association between preoperative PNI and the risk of POP in patients with EC. By elucidating this relationship, we determined to inform targeted preoperative optimization and reduce the burden of this life-threatening complication.
2 Methods and materials
2.1 Study design and setting
This study was a retrospective observational analysis. Patients who underwent esophagectomy were included from The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University between January 2021 to December 2022. Inclusion Criteria were as follows: 1. EC patients aged 40–90 years old; 2. EC patients received radical esophagectomy; 3. TNM stage was from I to III. EC patients with the following conditions were excluded: 1. patients with incomplete clinical data; 2. patients refused to participate in this study; 3. patients received preoperative use of anti-infective drugs; 4. infections occurred in other organs after surgery; 5. EC patients not undergoing surgical treatment. Written informed consent was provided by every patient. This study was approved by the Ethics committee of Huaian No.1 People’s Hospital (KY-2025-097-01). This study conformed to the Declaration of Helsinki guidelines.
2.2 Definition of POP
POP was diagnosed within 30 days after operation in one or both lungs infection according to the following items: (1) chest computed tomography scans or X-rays showing relevant imaging presentation, such as infiltrative lesions; (2) respiratory symptoms including fever, cough, and difficulty breathing were shown; (3) signs of lung consolidation were presented, such as pulmonary moist rales (17–19). Surgeons documented pneumonia occurrence through clinical assessments and radiological investigations.
2.3 Data collection
Age, sex, drinking, smoking, body mass index (BMI), diabetes mellitus, tumor node metastasis (TNM) stage, hypertension, tumor location, and hospital stay time were collected. Preoperative laboratory results including monocyte, white blood cell (WBC), neutrophil, platelet, albumin, and lymphocyte were extracted from electronic medical records. The PNI calculation required the following formula: PNI = albumin (g/L) + 5 × lymphocyte count (109/L).
2.4 Statistical analysis
The continuous variables were presented as the means ± standard deviations (SDs), or medians with interquartile ranges (IQRs), depending on the data distribution patterns. Categorical variables were displayed as frequencies and percentages. The independent t-test or Mann–Whitney U test was used to analyze continuous variables, and the chi-square test or Fisher’s exact test was used to evaluate categorical variables for group comparisons. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive ability of PNI for POP. This study involved univariate and multivariate logistic regression analyses to detect independent pneumonia predictors. The ROC curve, decision curve analysis (DCA), and calibration plots were used to evaluate the nomogram model. Odds ratio (OR) and 95% confidence intervals (CIs) were calculated. A p-value lower than 0.05 was considered statistically significant. SPSS version 22.0, GraphPad Prism 8, and R software 4.1.3 were used.
3 Results
3.1 Patient characteristics
A total of 200 patients with EC undergoing esophagectomy were included in this study (Figure 1). Seventy-three EC patients developed POP, and the incidence rate was 36.5%. Most of studies reported lower incidence of POP in EC patients (<36.5%) (19–23), but some showed higher incidence of POP (>36.5%) (24). Table 1 presents the clinicopathological characteristics of EC patients, comparing those in the non-pneumonia group (n = 127) with those in the pneumonia group (n = 73). Significant differences were observed in several variables. Male patients were more prevalent in the pneumonia group (78.1%) compared to the non-pneumonia group (63.8%) (p = 0.035). Patients aged 60 years or older were more common in the non-pneumonia group (89.8%) than in the pneumonia group (78.1%) (p = 0.024). TNM stage III was more frequently observed in the pneumonia group (47.9%) compared to the non-pneumonia group (32.3%) (p = 0.028). Tumor location also differed significantly between the pneumonia group and the non-pneumonia group (p = 0.015). Laboratory findings revealed that patients in the pneumonia group had significantly higher WBC counts (>5.62 × 109/L, 65.8% vs. 40.2%, p < 0.001), neutrophil counts (>3.52 × 109/L, 58.9% vs. 43.3%, p = 0.034), and monocyte counts (>0.40 × 109/L, 65.8% vs. 34.6%, p < 0.001). Additionally, the pneumonia group had a lower PNI (PNI ≤ 49.6, 63.0% vs. 35.4%, p < 0.001). Hospital stay time was notably longer in the pneumonia group, with 56.2% of patients staying more than 16 days compared to 29.1% in the non-pneumonia group (p < 0.001). Other variables including BMI, smoking, drinking, hypertension, diabetes mellitus, platelet count, lymphocyte count, and albumin levels did not show statistically significant differences between the two groups.
Figure 1
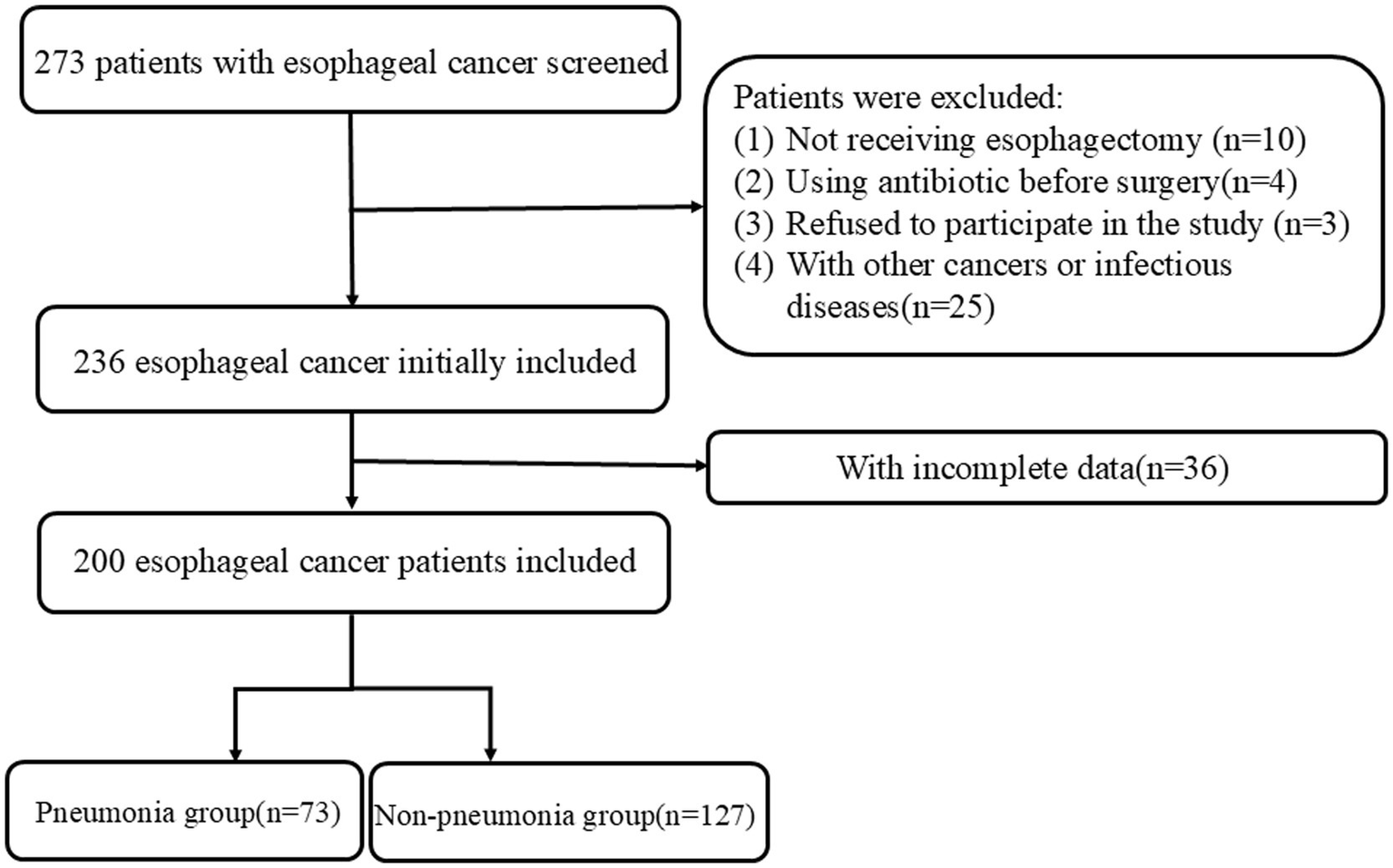
Flow diagram of enrolled patients.
Table 1
| Variables | Non-pneumonia group (n = 127) | Pneumonia group (n = 73) | P-value |
|---|---|---|---|
| Sex, n (%) | 0.035* | ||
| Female | 46 (36.2%) | 16 (21.9%) | |
| Male | 81 (63.8%) | 57 (78.1%) | |
| Age, n (%) | 0.024* | ||
| <60 years | 13 (10.2%) | 16 (21.9%) | |
| ≥60 years | 114 (89.8%) | 57 (78.1%) | |
| BMI, n (%) | 0.161 | ||
| ≤23.9 kg/m2 | 78 (61.4%) | 52 (71.2%) | |
| >23.9 kg/m2 | 49 (38.6%) | 21 (28.8%) | |
| Drinking, n (%) | 0.732 | ||
| No | 115 (90.6%) | 65 (89.0%) | |
| Yes | 12 (9.4%) | 8 (11.0%) | |
| Smoking, n (%) | 0.851 | ||
| No | 104 (81.9%) | 59 (80.8%) | |
| Yes | 23 (18.1%) | 14 (19.2%) | |
| Hypertension, n (%) | 0.520 | ||
| No | 94 (74.0%) | 57 (78.1%) | |
| Yes | 33 (26.0%) | 16 (21.9%) | |
| Diabetes mellitus, n (%) | 0.770 | ||
| No | 118 (92.9%) | 67 (91.8%) | |
| Yes | 9 (7.1%) | 6 (8.2%) | |
| TNM stage, n (%) | 0.028* | ||
| I-II | 86 (67.7%) | 38 (52.1%) | |
| III | 41 (32.3%) | 35 (47.9%) | |
| Tumor location, n (%) | 0.015* | ||
| Upper | 12 (9.4%) | 14 (19.2%) | |
| Middle | 83 (65.4%) | 33 (45.2%) | |
| Lower | 32 (25.2%) | 26 (35.6%) | |
| Hospital stay time, n (%) | <0.001* | ||
| ≤16 day | 90 (70.9%) | 32 (43.8%) | |
| >16 day | 37 (29.1%) | 41 (56.2%) | |
| WBC, n (%) | <0.001* | ||
| ≤5.62*109/L | 76 (59.8%) | 25 (34.2%) | |
| >5.62*109/L | 51 (40.2%) | 48 (65.8%) | |
| Neutrophil, n (%) | 0.034* | ||
| ≤3.52*109/L | 72 (56.7%) | 30 (41.1%) | |
| >3.52*109/L | 55 (43.3%) | 43 (58.9%) | |
| Monocyte, n (%) | <0.001* | ||
| ≤0.40*109/L | 83 (65.4%) | 25 (34.2%) | |
| >0.40*109/L | 44 (34.6%) | 48 (65.8%) | |
| Platelet, n (%) | 0.463 | ||
| ≤189.5*109/L | 61 (48.0%) | 39 (53.4%) | |
| >189.5*109/L | 66 (52.0%) | 34 (46.6%) | |
| Lymphocyte, n (%) | 0.317 | ||
| >1.46*109/L | 65 (51.2%) | 31 (42.5%) | |
| ≤1.46*109/L | 62 (48.8%) | 42 (57.5%) | |
| Albumin, n (%) | 0.145 | ||
| ≥42.8 g/L | 71 (55.9%) | 33 (45.2%) | |
| <42.8 g/L | 56 (44.1%) | 40 (54.8%) | |
| PNI, n (%) | <0.001* | ||
| >49.6 | 82 (64.6%) | 27 (37.0%) | |
| ≤49.6 | 45 (35.4%) | 46 (63.0%) |
Clinicopathological characteristics of esophageal cancer patients between non-pneumonia and pneumonia groups.
BMI, body mass index; TNM stage, tumor node metastasis stage; WBC, white blood cell; PNI, prognostic nutritional index. *indicating statistically significant (P < 0.05).
3.2 Predictive ability of PNI for POP
The predictive ability of preoperative PNI was evaluated in this study (Table 2). ROC curve analysis showed that PNI predicted the occurrence of POP with an AUC value of 0.602 at a cut-off value of 49.6. The sensitivity was 64.38%; the specificity was 63.78%; and the Youden index was 0.2716. In conclusion, the predictive ability of preoperative PNI for POP was moderate (Figure 2).
Table 2
| Variables | Cut-off value | Sensitivity % | Specificity % | AUC value | Youden index |
|---|---|---|---|---|---|
| PNI | ≤49.6 | 64.38 | 63.78 | 0.6020 (0.5185–0.6855) | 0.2716 |
Optimal cut-off value of PNI for predicting the postoperative pneumonia.
PNI, prognostic nutritional index.
Figure 2
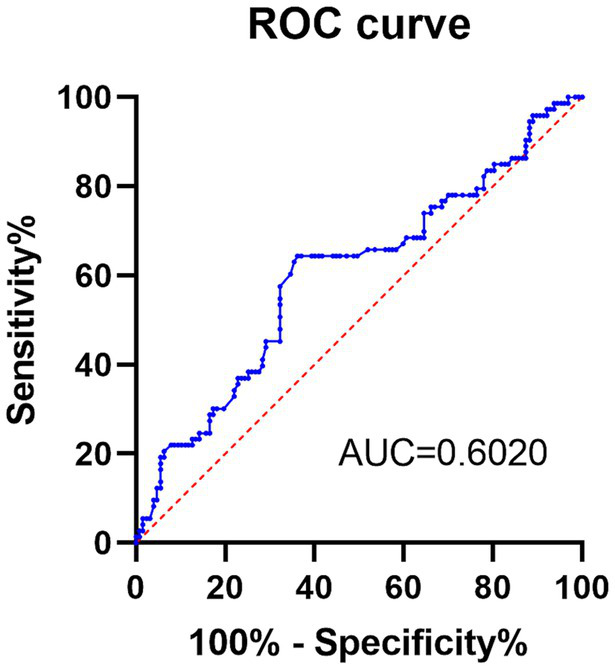
The receiver operating characteristic curve of preoperative PNI in predicting the occurrence of POP.
3.3 Risk factors of POP among EC patients
The univariate logistic regression analysis for identifying risk factors of POP in EC patients is summarized in Table 3. Male was associated with a significantly higher risk of POP compared to female (OR, 2.023; 95% CI, 1.043–3.923; p = 0.037). Patients aged 60 years or older had a lower risk compared to those under 60 years (OR, 0.406; 95% CI, 0.183–0.902; p = 0.027). Advanced TNM stage (stage III vs. I–II) was significantly associated with an increased risk of POP (OR, 1.932; 95% CI, 1.070–3.489; p = 0.029). Tumor location in the middle esophagus, compared to the upper esophagus, was associated with a reduced risk of POP (OR, 0.341; 95% CI, 0.143–0.814; p = 0.015). Prolonged hospital stay time (>16 days) was strongly associated with an increased risk of POP (OR, 3.117; 95% CI, 1.710–5.680; p < 0.001). Elevated WBC counts (>5.62 × 109/L) were linked to a higher risk of POP (OR, 2.861; 95% CI, 1.571–5.212; p = 0.001), as were elevated neutrophil counts (>3.52 × 109/L; OR, 1.876; 95% CI, 1.047–3.363; p = 0.035) and monocyte counts (>0.40 × 109/L; OR, 3.622; 95% CI, 1.976–6.639; p < 0.001). A lower PNI (PNI ≤ 49.6) was associated with an increased risk of POP (OR, 3.105; 95% CI, 1.707–5.647; p < 0.001).
Table 3
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Sex | ||
| Male vs. female | 2.023(1.043, 3.923) | 0.037* |
| Age | ||
| ≥60 years vs. < 60 years | 0.406(0.183, 0.902) | 0.027* |
| BMI | ||
| >23.9 kg/m2 vs. ≤23.9 kg/m2 | 0.643(0.346, 1.195) | 0.163 |
| Drinking | ||
| Yes vs. no | 1.179(0.458, 3.034) | 0.732 |
| Smoking | ||
| Yes vs. No | 1.073(0.513, 2.243) | 0.851 |
| Hypertension | ||
| Yes vs. No | 0.800(0.404, 1.581) | 0.520 |
| Diabetes mellitus | ||
| Yes vs. No | 1.174(0.400, 3.442) | 0.770 |
| TNM stage | ||
| III vs. I-II | 1.932(1.070, 3.489) | 0.029* |
| Tumor location | ||
| Middle vs. upper | 0.341(0.143, 0.814) | 0.015* |
| Lower vs. upper | 0.696(0.275, 1.763) | 0.445 |
| Hospital stay time | ||
| >16 day vs. ≤16 day | 3.117(1.710, 5.680) | 0.000* |
| WBC | ||
| >5.62*109/L vs. ≤5.62 *109/L | 2.861(1.571, 5.212) | 0.001* |
| Neutrophil | ||
| >3.52 *109/L vs. ≤3.52*109/L | 1.876(1.047, 3.363) | 0.035* |
| Monocyte | ||
| >0.40*109/L vs. ≤0.40*109/L | 3.622(1.976, 6.639) | 0.000* |
| Platelet | ||
| >189.5*109/L vs. ≤189.5*109/L | 0.806(0.453, 1.434) | 0.463 |
| Lymphocyte | ||
| ≤1.46*109/L vs. >1.46*109/L | 1.343(0.753, 2.396) | 0.318 |
| Albumin | ||
| <42.8 g/L vs. ≥42.8 g/L | 1.537(0.861, 2.742) | 0.146 |
| PNI | ||
| ≤49.6 vs. >49.6 | 3.105(1.707, 5.647) | 0.000* |
Univariate logistic regression analyses for predicting postoperative pneumonia.
BMI, body mass index; TNM stage, tumor node metastasis stage; WBC, white blood cell; PNI, prognostic nutritional index. *Indicating statistically significant (P < 0.05).
Further multivariate logistic regression analysis was conducted (Table 4). EC in the middle esophagus was associated with a reduced risk of POP compared to the upper esophagus (OR, 0.182; 95% CI, 0.057–0.579; p = 0.004), and tumors in the lower esophagus also showing a reduced risk (OR, 0.251; 95% CI, 0.070–0.903; p = 0.034). Hospital stay time >16 days was strongly associated with an increased risk of POP (OR, 3.417; 95% CI, 1.647–7.089; p = 0.001). Elevated WBC count (>5.62 × 109/L) was a significant risk factor for POP (OR, 3.827; 95% CI, 1.353–10.827; p = 0.011), as was elevated monocyte count (>0.40 × 109/L; OR, 3.006; 95% CI, 1.384–6.529; p = 0.005). Preoperative PNI ≤ 49.6 was also a significant risk factor for POP, with an odds ratio of 4.659 (95% CI, 2.149–10.103; p < 0.001). Moreover, other variables, including sex, age, TNM stage, and neutrophil counts, were not significantly associated with the risk of POP. In total, tumor location, hospital stay time >16 days, WBC count >5.62 × 109/L, monocyte count >0.40 × 109/L, and low PNI ≤ 49.6 were significant risk factors for POP among EC patients receiving esophagectomy.
Table 4
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Sex | ||
| Male vs. female | 1.616(0.665, 3.929) | 0.289 |
| Age | ||
| ≥60 years vs. < 60 years | 0.566(0.207, 1.549) | 0.268 |
| TNM stage | ||
| III vs. I-II | 1.160(0.557, 2.416) | 0.692 |
| Tumor location | ||
| Middle vs. upper | 0.182(0.057, 0.579) | 0.004* |
| Lower vs. upper | 0.251(0.070, 0.903) | 0.034* |
| Hospital stay time | ||
| >16 day vs. ≤16 day | 3.417(1.647, 7.089) | 0.001* |
| WBC | ||
| >5.62*109/L vs. ≤5.62 *109/L | 3.827(1.353, 10.827) | 0.011* |
| Neutrophil | ||
| >3.52 *109/L vs. ≤3.52*109/L | 0.570(0.212, 1.535) | 0.266 |
| Monocyte | ||
| >0.40*109/L vs. ≤0.40*109/L | 3.006(1.384, 6.529) | 0.005* |
| PNI | ||
| ≤49.6 vs. >49.6 | 4.659(2.149, 10.103) | 0.000* |
Multivariate logistic regression analyses for predicting postoperative pneumonia.
TNM stage, tumor node metastasis stage; WBC, white blood cell; PNI, prognostic nutritional index. *Indicating statistically significant (P < 0.05).
3.4 Construction a nomogram model for predicting POP
A nomogram model was established according the results of multivariate logistic regression analysis (Figure 3). The AUC value for this prediction model was 0.831 (95% Cl: 0.772–0.890), indicating that this nomogram model showed a good predictive validity for predicting POP (Figure 4A). The calibration curve of this nomogram model indicated that the nomogram predictive model presented a good consistency between the observational probability and predicted probability in predicting POP (Figure 4B). The DCA indicated that this nomogram model achieved the high clinical net benefit in predicting POP among EC patients (Figure 4C).
Figure 3
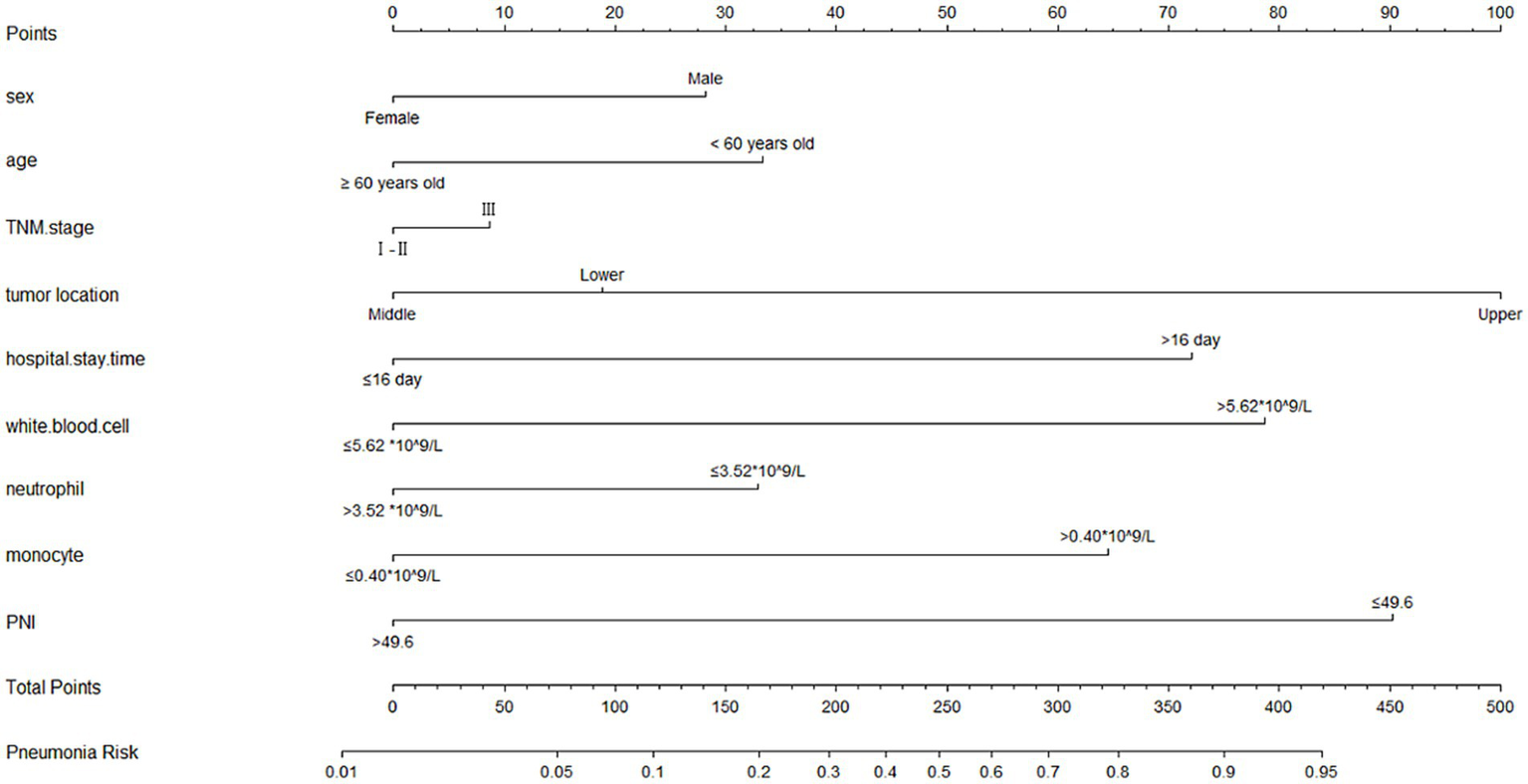
The nomogram for predicting occurrence of POP.
Figure 4
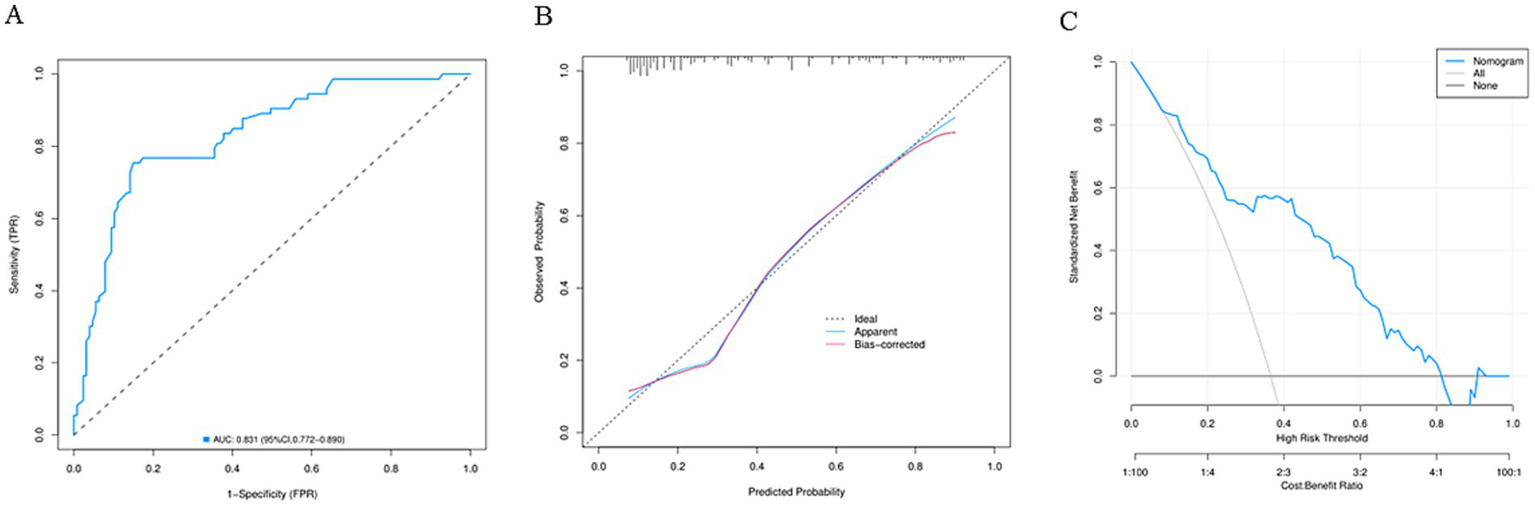
(A) The receiver operating characteristic curve of the nomogram model. (B) The calibration curve of the nomogram model. (C) The decision curve analysis of the nomogram model.
4 Discussion
4.1 Interpretation of main findings and comparison with previous studies
Our study demonstrated that preoperative PNI was a significant predictor of POP in patients with EC. This finding aligned with previous studies validating PNI as a reliable predictor of postoperative complications of EC patients. The discriminative ability of PNI confirmed that the utility of PNI in the risk stratification of POP, consistent with studies showing similar predictive performance for postoperative complications (12–14). Up to date, only one study explored the relationship between preoperative PNI and the risk of POP among EC patients receiving esophagectomy, and it showed that preoperative PNI was not a risk factor for POP (25), which was contradictory with this study. Some factors may contribute to this phenomenon. One, this study included EC patients with pneumonia during hospitalization after surgery, while the study by Nishimura et al. enrolled EC patients who occurred over 3 months after esophagectomy (25). Two, the sample sizes differed in these two studies. Three, different surgical methods and postoperative care may be potential reasons. In addition, some studies depicted the association between PNI and the clinical outcomes of pneumonia. A Japanese study indicated that the PNI could predict the risk of POP after lung cancer surgery (26), which was replicated in another study (27). Another Japanese study also showed that PNI was a risk factor for POP after general and digestive surgery (28). A Chinese study found that PNI was associated with mortality of COVID-19 patients (29). A study by Shang et al. showed that PNI was a significant predictor of new-onset pneumonia in peritoneal dialysis patients (30). Three studies indicated that PNI was negatively associated with the mortality of community-acquired pneumonia (31–33). According to these abovementioned studies, we could find a close relationship between PNI and the occurrence of pneumonia.
4.2 Possible mechanisms linking low PNI and the occurrence of POP
The association between low PNI and the occurrence of POP could be attributed to multiple interconnected mechanisms. Malnutrition, reflected by low PNI, impairs protein synthesis and tissue repair while weakening antioxidant defenses (34). Concurrently, lymphopenia compromises cellular immunity function, reducing the ability to combat respiratory pathogens (35). These effects are exacerbated by the systemic inflammatory response to surgery, which further suppresses immune function. The combination of nutritional deficiency and immune dysfunction created a vulnerable state where patients were more susceptible to pulmonary infections (36, 37). This mechanistic understanding was supported by studies showing that patients with low PNI have higher levels of inflammatory markers like CRP and PCT, which correlated with increased infection risk (38, 39). The specific mechanisms linking low PNI and the occurrence of POP requires further studies to explore.
4.3 Risk factors for POP other than PNI
Our analysis confirmed several established risk factors for POP. We found that tumor location, hospital stay time, WBC, and monocyte were also associated with the risk of POP. High level of WBC and monocyte before operation meant high inflammatory state in the body. In addition, the further aggravation of pulmonary inflammation caused by surgery made patients more prone to pulmonary infections. EC patients with longer hospital stay time had a greater likelihood of developing POP after surgery.
4.4 Clinical implications and recommendations
Malnutrition is substantially associated with higher morbidity, disability, delayed recovery, and elevated healthcare costs (40). Continuous monitoring of nutritional status of EC cancer patients is necessary, such as PNI. Routine preoperative PNI assessment should be implemented to identify high-risk patients who may benefit from nutritional optimization and immune support. According to the findings of this study, for patients with PNI ≤ 49.6, targeted interventions such as individualized nutritional therapy and prehabilitation programs may potentially reduce the risk of POP, which needs further studies to validate it. PNI could help clinical clinicians to evaluate risk stratification of POP. Preoperative PNI could help to identify patients with poor nutritional status. A meta-analysis indicated that the mixed nutrition therapy for postoperative esophageal cancer patients could reduce the incidence of postoperative complications including POP (41). Unfortunately, this study did not investigate whether preoperative nutritional intervention could reduce postoperative complications, including POP. We need to develop nutritional interventions to mitigate the adverse events of cancer-related malnutrition. It is pivotal to utilize markers to identify the POP in high-risk patients at an individual level when implementing nutritional interventions. The main challenge is to identify the most effective way to incorporate these markers into established assessment tools, optimizing personalized nutritional therapies for patients with malnutrition. Surprisingly, studies reported that artificial intelligence was a promising tool in healthcare with potential applications in nutritional management, which could help to improve early detection, risk stratification, and personalized nutritional therapies for EC patients with POP (42, 43).
4.5 Limitations of the study
Several limitations should be acknowledged. The retrospective design introduced potential selection bias and limited causal interpretation. Being a single-center study, our findings may have limited generalizability to other practice settings. The sample size, particularly in the pneumonia group, restricted our ability to analyze less common risk factors. Additionally, we could not analyze all potential confounders, such as variations in surgical technique or preoperative pulmonary function. Our study did not investigate whether preoperative nutritional interventions could reduce the incidence of POP in EC patients. These limitations highlighted the need for multicenter prospective studies to validate our findings.
5 Conclusion
In conclusion, our study finds that preoperative PNI as a valuable predictor of POP in EC patients. This strong association between low PNI and the risk of POP, along with the nomogram model’s excellent discriminatory ability, supports incorporating PNI into routine preoperative assessment. These findings underscore the importance of evaluating nutritional status and immune function before surgery and suggest that targeted preoperative optimization may reduce the risk of POP. Future research should focus on validating these results in larger, prospective cohorts and evaluating interventions to improve PNI in high-risk patients.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of the Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CC: Investigation, Methodology, Writing – review & editing, Data curation, Formal analysis, Software, Writing – original draft. CL: Investigation, Methodology, Writing – review & editing, Conceptualization, Project administration, Supervision, Validation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Jiangsu Provincial Medical Key Discipline Cultivation Unit (grand number: JSDW202233).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ilic I Zivanovic Macuzic I Ravic-Nikolic A Ilic M Milicic V . Global burden of esophageal cancer and its risk factors: a systematic analysis of the global burden of disease study 2019. Life. (2024) 15:24. doi: 10.3390/life15010024
2.
Sun L Zhao K Liu X Meng X . Global, regional, and National Burden of esophageal Cancer using the 2019 global burden of disease study. Sci Rep. (2025) 15:3284. doi: 10.1038/s41598-025-86244-z
3.
Jin W Huang K Ding Z Zhang M Li C Yuan Z et al . Global, regional, and National Burden of esophageal Cancer: a systematic analysis of the global burden of disease study 2021. Biomark Res. (2025) 13:3. doi: 10.1186/s40364-024-00718-2
4.
Liu CQ Ma YL Qin Q Wang PH Luo Y Xu PF et al . Epidemiology of esophageal Cancer in 2020 and projections to 2030 and 2040. Thorac Cancer. (2023) 14:3–11. doi: 10.1111/1759-7714.14745
5.
Jiang Y Li Z Jiang W Wei T Chen B . Risk prediction model for postoperative pneumonia in esophageal Cancer patients: a systematic review. Front Oncol. (2024) 14:1419633. doi: 10.3389/fonc.2024.1419633
6.
Schlottmann F Patti MG . Prevention of postoperative pulmonary complications after esophageal Cancer surgery. J Thorac Dis. (2019) 11:S1143–4. doi: 10.21037/jtd.2019.04.57
7.
Kimura Y Gakuhara A Fukuda S Fukuda Y Yoshihara T Koga C et al . Nutritional management during chemotherapy and Chemoradiotherapy for advanced esophageal Cancer. Esophagus. (2025) 22:279–88. doi: 10.1007/s10388-025-01117-8
8.
Cao Y Han D Zhou X Han Y Zhang Y Li H . Effects of preoperative nutrition on postoperative outcomes in esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. (2022) 35:doab028. doi: 10.1093/dote/doab028
9.
Boyle E Elliott JA . Novel nutrition strategies in gastric and esophageal Cancer. Expert Rev Gastroenterol Hepatol. (2025) 19:89–104. doi: 10.1080/17474124.2025.2457444
10.
Kong X Liu P Wang G Sun S Li L . Methods for diagnosing malnutrition in patients with esophageal Cancer, and the association with nutritional and inflammatory indices: a cross-sectional study. Oncol Lett. (2025) 29:223. doi: 10.3892/ol.2025.14969
11.
Aoyama T Atsumi Y Kawahara S Tamagawa H Tamagawa A Maezawa Y et al . The clinical impacts of the prognostic nutritional index for the esophageal Cancer patients who received curative treatment. J Cancer Res Ther. (2024) 20:898–903. doi: 10.4103/jcrt.JCRT_1595_20
12.
Chen C Wang J Li Y . A nomogram model to predict postoperative delirium in esophageal cancer patients undergoing esophagectomy. BMC Cancer. (2025) 25:1082. doi: 10.1186/s12885-025-14478-1
13.
Mijiti M Li D Yan R Yuan T Shen G Zhao D . Development of nomogram for predicting major complications in patients with esophageal cancer in the early postoperative period. BMC Surg. (2023) 23:186. doi: 10.1186/s12893-023-02090-8
14.
Qi Q Song Q Cheng Y Wang N . Prognostic significance of preoperative prognostic nutritional index for overall survival and postoperative complications in esophageal Cancer patients. Cancer Manag Res. (2021) 13:8585–97. doi: 10.2147/CMAR.S333190
15.
Wu M Zhu Y Chen X Wang X Lin X Yan X et al . Prognostic nutritional index predicts the prognosis of patients with advanced esophageal Cancer treated with immune checkpoint inhibitors: a retrospective cohort study. J Gastrointest Oncol. (2023) 14:54–63. doi: 10.21037/jgo-23-48
16.
Zhang G Sun S Dong Z Chunyao H Wang Z Li K et al . Risk factors for unplanned intensive care unit admission after Esophagectomy: a retrospective cohort study of 628 patients with esophageal Cancer. Front Oncol. (2024) 14:1420446. doi: 10.3389/fonc.2024.1420446
17.
Chen B Ke W Li M . A nomogram predicting the risk of postoperative pneumonia after Esophagectomy in esophageal carcinoma. Front Med. (2025) 12:1553163. doi: 10.3389/fmed.2025.1553163
18.
Xu Z Chen C Zhao J Li C Zang B Xiong X . The Cally index as a predictive tool for postoperative pneumonia in esophageal squamous cell carcinoma: a retrospective cohort study. J Inflamm Res. (2025) 18:5463–75. doi: 10.2147/JIR.S517074
19.
Chen J Xiang Q Zheng XJ Jiang XY . Predictive model for postoperative pneumonia in patients with esophageal Cancer after Esophagectomy. Front Oncol. (2025) 15:1529308. doi: 10.3389/fonc.2025.1529308
20.
Kim T Jeon YJ Lee H Kim TH Park SY Kang D et al . Preoperative Dlco and Fev(1) are correlated with postoperative pulmonary complications in patients after Esophagectomy. Sci Rep. (2024) 14:6117. doi: 10.1038/s41598-024-56593-2
21.
Sawai S Nakatani E Sato S Hawke P Mochizuki T Nishida M et al . Peak expiratory flow predicts the occurrence of postoperative pneumonia after Esophagectomy for esophageal Cancer. Dis Esophagus. (2024) 37:doae084. doi: 10.1093/dote/doae084
22.
Nishi S Miki Y Imai T Nambara M Miyamoto H Tamura T et al . The evaluation of sarcopenia before neoadjuvant chemotherapy is important for predicting postoperative pneumonia in patients with esophageal Cancer. Dig Surg. (2023) 40:153–60. doi: 10.1159/000533185
23.
Yukawa Y Yamashita K Momose K Saito T Tanaka K Makino T et al . A purely laparoscopic approach can reduce the incidence of postoperative pneumonia in esophageal Cancer patients undergoing Esophagectomy. Esophagus. (2025) 22:157–65. doi: 10.1007/s10388-025-01110-1
24.
van der Sluis PC Verhage RJ van der Horst S Wal WM Ruurda JP van Hillegersberg R . A new clinical scoring system to define pneumonia following esophagectomy for cancer. Dig Surg. (2014) 31:108–16. doi: 10.1159/000357350
25.
Nishimura K Miyata K Fukaya M Yokoyama Y Uehara K Yamaguchi J et al . Early volume loss of skeletal muscle after Esophagectomy: a risk for late-onset postoperative pneumonia. Dis Esophagus. (2022) 35:doac019. doi: 10.1093/dote/doac019
26.
Okada S Shimada J Teramukai S Kato D Tsunezuka H Miyata N et al . Risk stratification according to the prognostic nutritional index for predicting postoperative complications after lung cancer surgery. Ann Surg Oncol. (2018) 25:1254–61. doi: 10.1245/s10434-018-6368-y
27.
Park S Ahn HJ Yang M Kim JA Kim JK Park SJ . The prognostic nutritional index and postoperative complications after curative lung Cancer resection: a retrospective cohort study. J Thorac Cardiovasc Surg. (2020) 160:276–285.e1. doi: 10.1016/j.jtcvs.2019.10.105
28.
Baba H Tokai R Hirano K Watanabe T Shibuya K Hashimoto I et al . Risk factors for postoperative pneumonia after general and digestive surgery: a retrospective single-center study. Surg Today. (2020) 50:460–8. doi: 10.1007/s00595-019-01911-9
29.
Wang R He M Yin W Liao X Wang B Jin X et al . The prognostic nutritional index is associated with mortality of Covid-19 patients in Wuhan, China. J Clin Lab Anal. (2020) 34:e23566. doi: 10.1002/jcla.23566
30.
Shang S Huang Y Zhan X Peng F Wang X Wen Y et al . The relationship between the prognostic nutritional index and new-onset pneumonia in peritoneal Dialysis patients. Int Urol Nephrol. (2022) 54:3017–24. doi: 10.1007/s11255-022-03233-1
31.
De Rose L Sorge J Blackwell B Benjamin M Mohamed A Roverts T et al . Determining if the prognostic nutritional index can predict outcomes in community acquired bacterial pneumonia. Respir Med. (2024) 226:107626. doi: 10.1016/j.rmed.2024.107626
32.
Wang G Wang N Liu T Ji W Sun J Lv L et al . Association between prognostic nutritional index and mortality risk in patients with community-acquired pneumonia: a retrospective study. BMC Pulm Med. (2024) 24:555. doi: 10.1186/s12890-024-03373-3
33.
Xie K Zhang C Nie S Kang S Wang Z Zhang X . Prognostic nutritional index (Pni) as an influencing factor for in-hospital mortality in patients with stroke-associated pneumonia: a retrospective study. PeerJ. (2025) 13:e19028. doi: 10.7717/peerj.19028
34.
Cederholm T Bosaeus I . Malnutrition in adults. N Engl J Med. (2024) 391:155–65. doi: 10.1056/NEJMra2212159
35.
Wang Z Zhang W Chen L Lu X Tu Y . Lymphopenia in Sepsis: a narrative review. Crit Care. (2024) 28:315. doi: 10.1186/s13054-024-05099-4
36.
Li D Liu Y Jia Y Yu J Li F Li H et al . Association between malnutrition and stroke-associated pneumonia in patients with ischemic stroke. BMC Neurol. (2023) 23:290. doi: 10.1186/s12883-023-03340-1
37.
Nguyen N Galet C Kurjatko A McGonagill PW . Admission lymphopenia predicts risk of pneumonia and AKI in hospitalized burn injuries. Burns. (2025) 51:1075–81. doi: 10.1016/j.burns.2025.107581
38.
Abe K Choe H Oba M Tezuka T Ike H Kobayashi N et al . Inflammation and nutrition based screening tests for detection of infection in cases of rapid hip destruction. Sci Rep. (2022) 12:3586. doi: 10.1038/s41598-022-07678-3
39.
Nergiz S Ozturk U . The effect of prognostic nutritional index on infection in acute ischemic stroke patients. Medicina. (2023) 59:679. doi: 10.3390/medicina59040679
40.
Wunderle C Urbach K Buchmueller L Randegger S Kaegi-Braun N Laviano A et al . Biomarkers for individualized nutritional therapy in disease-related malnutrition: a narrative review. Am J Clin Nutr. (2025) 122:671–9. doi: 10.1016/j.ajcnut.2025.07.009
41.
Liu S Qiao L Liu Y Liu H Li Y Sun J et al . Efficacy and safety evaluation of mixed nutrition for postoperative esophageal cancer patients in China: a meta-analysis. Front Oncol. (2024) 14:1417765. doi: 10.3389/fonc.2024.1417765
42.
Janssen SM Bouzembrak Y Tekinerdogan B . Artificial intelligence in malnutrition: a systematic literature review. Adv Nutr. (2024) 15:100264. doi: 10.1016/j.advnut.2024.100264
43.
Sguanci M Palomares SM Cangelosi G Petrelli F Sandri E Ferrara G et al . Artificial intelligence in the Management of Malnutrition in Cancer patients: a systematic review. Adv Nutr. (2025) 16:100438. doi: 10.1016/j.advnut.2025.100438
Summary
Keywords
esophageal cancer, prognostic nutritional index, risk factors, nomogram model, predictive factors
Citation
Chen C and Li C (2025) Preoperative prognostic nutritional index as a predictive factor for postoperative pneumonia in esophageal cancer patients undergoing esophagectomy. Front. Nutr. 12:1674518. doi: 10.3389/fnut.2025.1674518
Received
28 July 2025
Accepted
14 October 2025
Published
28 October 2025
Volume
12 - 2025
Edited by
Xuefeng Leng, Sichuan Cancer Hospital, China
Reviewed by
Zhanzhan Li, Central South University, China
Lilong Zhang, Renmin Hospital of Wuhan University, China
Updates
Copyright
© 2025 Chen and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenglin Li, lchl603@njmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.