- 1School of Public Health, Bengbu Medical University, Bengbu, China
- 2School of Psychology, Shenzhen University, Shenzhen, China
Background: Ultra-processed food (UPF) intake and sedentary behavior (SB) are recognized as significant contributors to depressive symptoms. However, the joint effects of these two factors on depressive symptoms remain insufficiently explored. This study aimed to investigate the independent and combined associations of UPF intake and SB with depressive symptoms in a young population.
Methods: Cross-sectional analyses were conducted using baseline survey data from the Shenzhen Youth Health Cohort. UPF intake, SB, and depressive symptoms were self-reported via questionnaires. Multifactorial logistic regression models were employed to assess the independent and joint associations between UPF intake, SB, and depressive symptoms.
Results: A total of 1,461 participants were included, with 610 (41.8%) reporting depressive symptoms and 851 (58.2%) without. Compared with the group with the lowest UPF supply ratio (Q1), the risk of depressive symptoms was significantly higher in the third (Q3) and fourth (Q4) quartiles, with odds ratios (95% CI) of 1.60 (1.16–2.21) and 2.05 (1.48–2.85), respectively. Similarly, individuals with SB exceeding 8 h per day had a significantly increased risk of depressive symptoms compared to those with SB of less than 4 h per day (OR = 1.75, 95% CI: 1.25–2.44). In the combined analysis, participants with high UPF intake and sedentary time ≥6 h per day exhibited the highest risk of depressive symptoms compared to those with low UPF intake and sedentary time <6 h per day (OR = 2.31, 95% CI: 1.62–3.31).
Conclusion: Higher intake of UPF and increased SB were significantly associated with depressive symptoms in the young population. Additionally, the combination of high UPF intake and sedentary time exceeding 6 h per day was associated with a further heightened risk of depressive symptoms.
1 Introduction
Depressive symptoms represent early or mild forms of depression, where the severity and duration typically do not meet the diagnostic criteria for clinical depression. Individuals may experience transient sadness, loss of interest, feelings of hopelessness, and anxiety (1). While depressive symptoms may gradually resolve over time or with changes in environmental circumstances, persistent symptoms pose a risk not only for the development of depression but also for other issues (2), such as suicidal tendencies (3). According to a report released by the Institute of Psychology at the Chinese Academy of Sciences in 2023 (4), the prevalence of depressive symptoms in the adult population was 10.6%, with the highest rates observed in the youth demographic, particularly among those aged 18–24 and 25–34 years. Within these age groups, the detection rates were notably high at 24.1 and 12.3%, respectively, significantly surpassing those in other age brackets.
Concurrent with socioeconomic development, shifts in lifestyle behaviors—particularly diet and SB—have emerged as novel contributors to the onset of depressive symptoms. As diet plays a pivotal role in mental health (5, 6), the relationship between ultra-processed food (UPF) intake and depressive symptoms has garnered growing scientific and public attention. Multiple studies have demonstrated that high UPF consumption is associated with an elevated risk of depressive symptoms compared to lower UPF intake (7–9). However, research examining the association between UPF intake and depressive symptoms specifically within youth populations remains limited. On the other hand, sedentary behavior (SB) is an established health risk factor independent of physical activity (10). Previous research has indicated that SB is positively correlated with an increased risk of depressive symptoms (11). Furthermore, studies have shown that sedentary time exceeding 6 h per day significantly increases the risk of depressive symptoms when compared to sedentary time of less than 2 h per day (12).
Health risk behaviors are often synergistic, with combinations of two or more behaviors frequently linked to a higher risk of chronic diseases than each behavior individually (13). Evidence suggests that UPF intake and SB may mutually reinforce one another, with individuals who spend more time sedentary more likely to consume higher amounts of UPF (14), and UPF intake potentially promoting SB (15). However, most existing research has focused on the effects of individual factors on depressive symptoms, leaving the combined influence of UPF intake and SB largely unexplored.
Thus, utilizing data from the Shenzhen youth population, this study aims to investigate the independent and joint associations of UPF intake, SB, and depressive symptoms within this demographic.
2 Methods
2.1 Sample source
This study is based on a baseline survey conducted within the Shenzhen Youth Population Health Cohort. The primary aim of the cohort is to identify potential risk and protective factors for disease, including social characteristics, behavioral performance, lifestyle choices, health literacy, and psychological traits. Additionally, the cohort seeks to describe the pathways of health changes and their underlying mechanisms from a multidimensional perspective, while providing a scientific foundation for the development of targeted health strategies that account for the unique health ecology of Shenzhen. Participants were recruited between December 2023 and October 2024 from the physical examination centers of two tertiary hospitals in Longhua District, Shenzhen. A cross-sectional survey was conducted through the use of QR codes and electronic questionnaires. The study received approval from the Medical Committee of the Science and Technology Ethics Committee of Tsinghua University (Ethics No. 20230065) prior to commencement.
2.2 Inclusion and exclusion criteria
(1) Inclusion Criteria: Participants aged 18–35 years who provided signed informed consent.
(2) Exclusion Criteria: Individuals with a history of major metabolic or serious illnesses (e.g., cancer, stroke); individuals with cognitive impairments preventing cooperation; individuals with missing dietary data; and those with extreme total energy intake, defined as energy intake below the 2.5th percentile or above the 97.5th percentile of the sex-specific distribution in our sample, a method commonly used in nutritional epidemiological studies (16). The final cohort comprised 1,461 participants.
2.3 UPF intake assessment
Dietary intake was assessed using a self-administered Food Frequency Questionnaire (FFQ), developed by the research team. The questionnaire was based on the 6th edition of the Chinese Food Composition Table (Standard Edition) and the NOVA Food Classification System (17). The FFQ was adapted from the instrument used in the China Health and Nutrition Examination Survey cohort study (18). It includes 10 food categories: staple foods, legumes, vegetables, fruits, meat, eggs, dairy, alcoholic beverages and drinks, snacks, and condiments. The Cronbach’s α coefficient for the FFQ in this study was 0.974.
UPF intake was evaluated using the UPF energy supply ratio (19), which is calculated as:
Participants were then categorized into quartiles based on the UPF energy supply ratio: Q1 (UPF energy supply ratio <23.2%), Q2 (23.2–38.8%), Q3 (38.8–53.3%), and Q4 (>53.3%).
2.4 Assessment of SB
SB over the previous 7 days was assessed using the International Physical Activity Questionnaire (IPAQ) short form (20). Based on prior studies, SB was classified into four categories: <4 h/day, 4–6 h/day, 6–8 h/day, and >8 h/day (21).
2.5 Assessment of depressive symptoms
Depressive symptoms were assessed using the Patient Health Questionnaire-9 (PHQ-9) for the previous 2 weeks. The PHQ-9 comprises 9 items, each scored on a 4-point Likert scale, with a total score range of 0–27 (22). Higher scores indicate greater severity of depressive symptoms. A score of 0–4 indicates no depressive symptoms, 5–9 suggests mild depressive symptoms, 10–14 indicates moderate depressive symptoms, 15–19 reflects moderately severe depressive symptoms, and 20–27 suggests severe depressive symptoms. For this study, a PHQ-9 score of ≥5 was considered indicative of depressive symptoms (23).
2.6 Covariates
The general questionnaire collected information on several covariates, including demographic characteristics (age, gender, education level, income status), mental health status (history of mental illness, medication use for mental health conditions), lifestyle behaviors (smoking, alcohol consumption, sleep duration, physical activity), and body mass index (BMI, kg/m2). Educational attainment was categorized into three levels: high school and below, college or bachelor’s degree, and master’s degree or above. Income status was classified as insufficient, balanced, or sufficient. Physical activity levels were grouped into low, medium, and high categories.
2.7 Statistical analysis
Normality tests indicated that all continuous variables were non-normally distributed. Non-normally distributed continuous variables were presented as medians (interquartile range), with differences between groups assessed using the Wilcoxon rank sum test and Kruskal-Wallis test. Categorical variables were reported as frequencies (proportions), and differences between groups were evaluated using the χ2-test. Multifactorial logistic regression models were applied to assess the associations between UPF intake, sedentary behavior, and depressive symptoms. The models were as follows: Model 1 (unadjusted); Model 2 (adjusted for age, gender, education level, income status, BMI, history of mental illness, and history of medication use for mental health conditions); and Model 3 (further adjusted for smoking, alcohol consumption, physical activity, sleep duration, and energy intake, based on Model 2). A three-node (10th, 50th, and 90th percentiles) Restricted Cubic Splines (RCS) model was employed to evaluate the dose–response relationship between UPF intake, SB, and depressive symptoms.
Joint analyses were conducted by combining UPF intake (low: UPF supply ratio ≤ 38.8%, high: UPF supply ratio >38.8%) and SB (sedentary time <6 h/day; sedentary time ≥ 6 h/day), with the reference group consisting of those with low UPF intake and sedentary time <6 h/day. The interaction between UPF intake and SB in relation to depressive symptoms was further examined. Finally, subgroup and sensitivity analyses were performed to assess the robustness of the findings. Statistical analyses were conducted using SPSS (version 23.0) and R (version 4.3.2), with a significance level set at α = 0.05 and two-sided tests.
3 Results
3.1 Participant characteristics
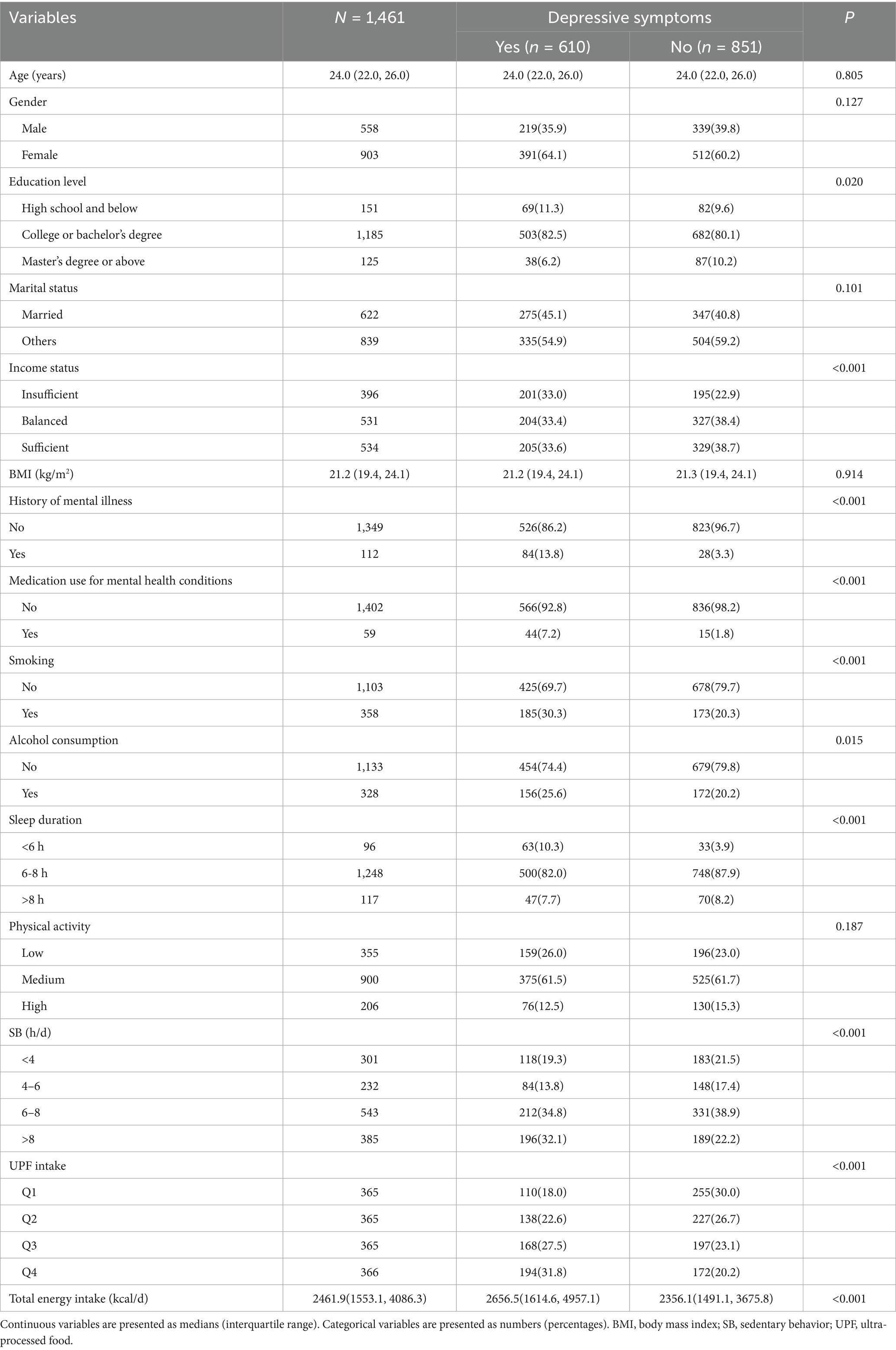
As shown in Table 1, a total of 1,461 participants were included in this study, of which 610 (41.8%) reported depressive symptoms, and 851 (58.2%) did not. Energy intake was significantly higher in participants with depressive symptoms compared to those without (p < 0.001). Compared with individuals without depressive symptoms, those with depressive symptoms were more likely to have a college or bachelor’s degree, insufficient income, a history of mental illness and psychiatric medication use, smoking habits, alcohol consumption, sleep duration of less than 6 h, SB exceeding 8 h per day, and higher proportions in the Q3 and Q4 UPF energy supply ratio groups. No statistically significant differences were observed between depressive symptoms and factors such as age, gender, marital status, BMI, and physical activity levels (p > 0.05).
3.2 Association of UPF intake and SB with depressive symptoms
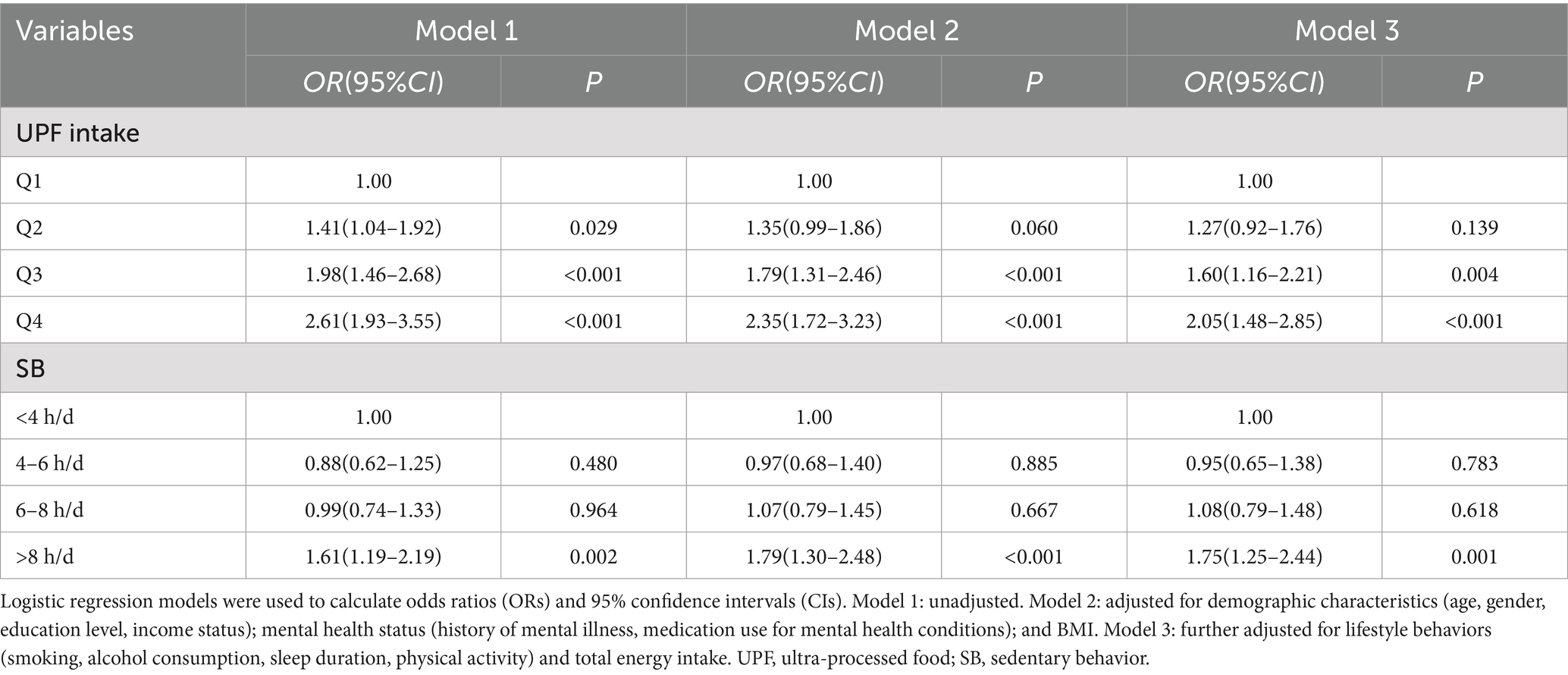
Logistic regression models were employed to analyze the associations between UPF intake, SB, and depressive symptoms. In the unadjusted model (Table 2), the risk of depressive symptoms increased across the higher quartiles of UPF intake when compared to the lowest quartile (Q1). The odds ratios (ORs) with 95% confidence intervals (CIs) for Q2, Q3, and Q4 were 1.41 (1.04–1.92), 1.98 (1.46–2.68), and 2.61 (1.93–3.55), respectively. After adjusting for confounding factors, including age, gender, education level, income status, BMI, history of mental illness, medication use for psychiatric conditions, smoking, alcohol consumption, physical activity, sleep duration, and total energy intake, the risk remained significantly higher in the Q3 and Q4 groups compared to the Q1 group, with ORs of 1.60 (1.16–2.21) and 2.05 (1.48–2.85), respectively. No significant association was observed between the Q2 group and depressive symptoms. Restricted Cubic Splines (RCS) analysis (Figure 1A) revealed no nonlinear dose–response relationship between UPF intake and depressive symptom risk (P for nonlinear = 0.449).
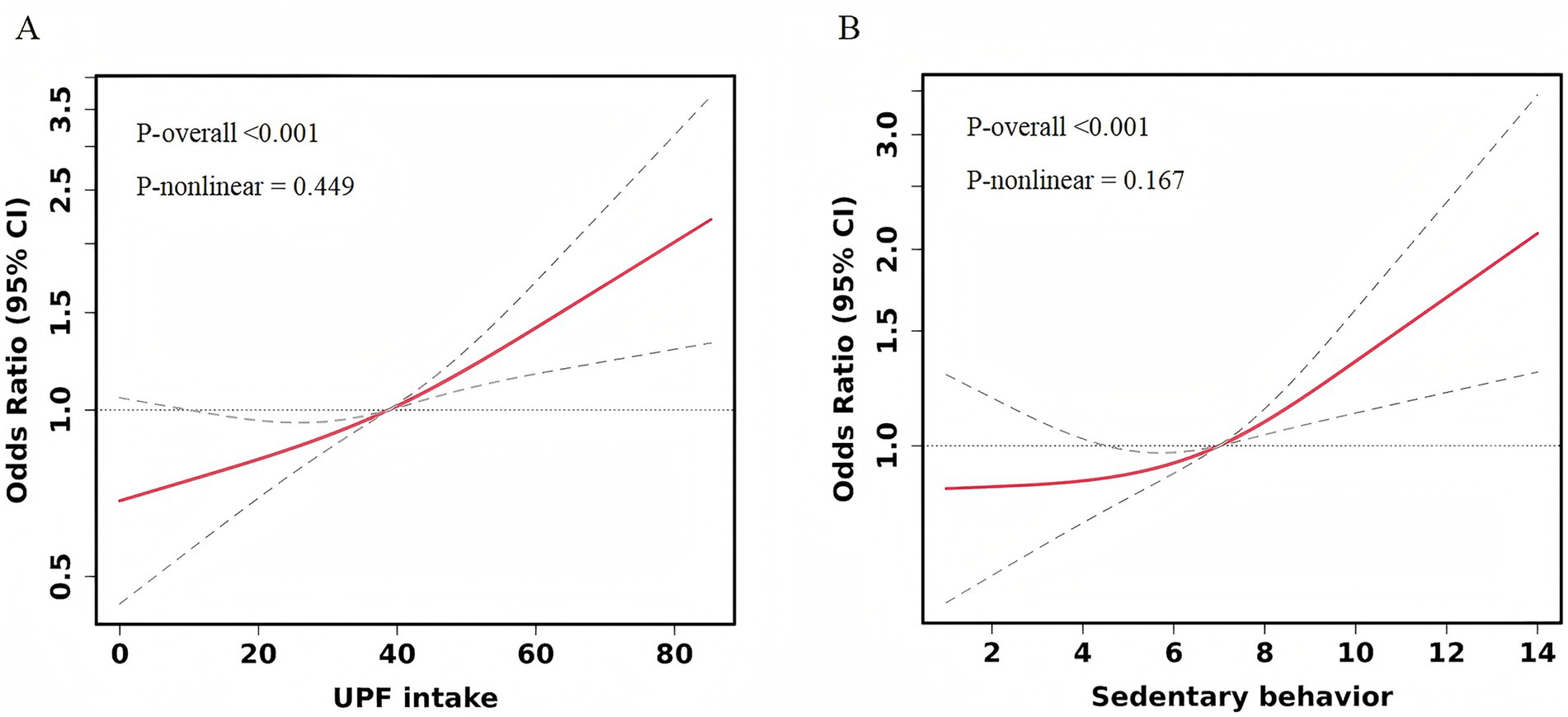
Figure 1. Restricted cubic spline curve for the association between UPF intake, SB, and depressive symptoms. Adjusted for age, gender, education level, income status, BMI, history of mental illness, medication use for psychiatric conditions, smoking, alcohol consumption, physical activity, sleep duration, and total energy intake. (A) UPF intake. (B) Sedentary behavior.
In relation to SB, when not adjusted for confounders, the most sedentary group (sedentary time >8 h/day) had a significantly higher risk of depressive symptoms compared to the least sedentary group (sedentary time <4 h/day), with an OR of 1.61 (95% CI: 1.19–2.19). After adjusting for all covariates, the risk of depressive symptoms remained significantly higher in the highest sedentary time group compared to the lowest sedentary time group, with an OR of 1.75 (95% CI: 1.25–2.44). Sedentary time categories of 4–6 h/day and 6–8 h/day did not show significant associations with depressive symptoms in any of the models, as detailed in Table 2. Additionally, no nonlinear dose–response relationship was observed between SB and depressive symptom risk (P for nonlinear = 0.167) (Figure 1B).
3.3 Combined effects of UPF intake and SB on depressive symptoms
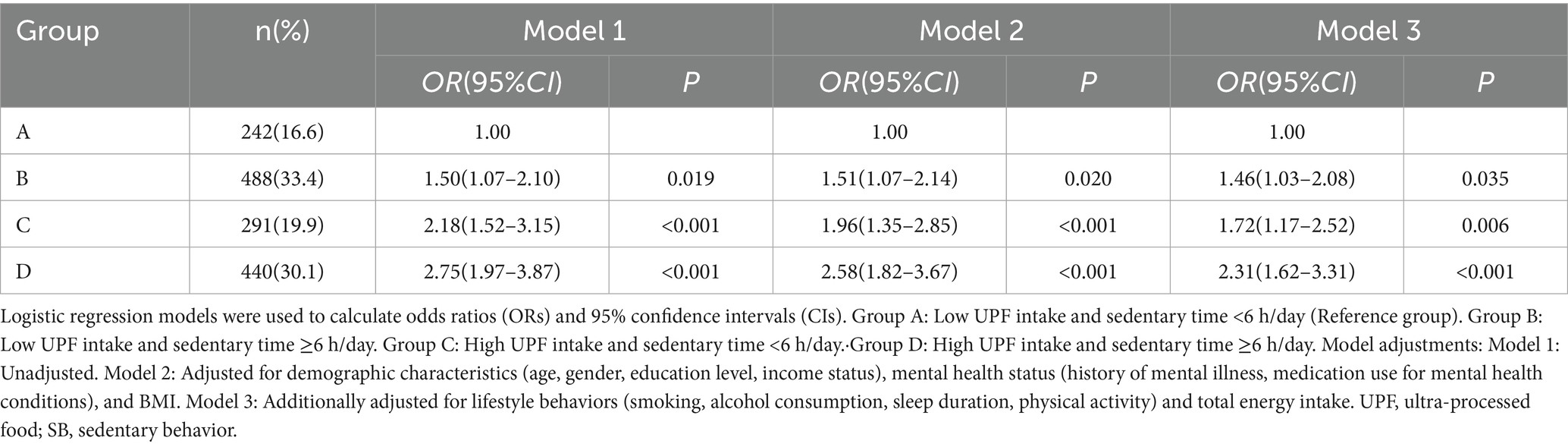
The combined effects of UPF intake and SB were analyzed across four subgroups (Table 3): Group A (low UPF intake and sedentary time <6 h/day, n = 242), Group B (low UPF intake and sedentary time ≥6 h/day, n = 488), Group C (high UPF intake and sedentary time <6 h/day, n = 291), and Group D (high UPF intake and sedentary time ≥6 h/day, n = 440). In Model 3, participants with high UPF intake and sedentary time ≥6 h/day exhibited the greatest risk of depressive symptoms compared to those with low UPF intake and sedentary time <6 h/day, with an OR of 2.31 (95% CI: 1.62–3.31). The risk of depressive symptoms was also significantly increased in the other subgroups, with ORs of 1.46 (95% CI: 1.03–2.08) and 1.72 (95% CI: 1.17–2.52) for Groups B and C, respectively. Additionally, the interaction between UPF intake and SB on depressive symptoms was not statistically significant (Supplementary Table 1).
3.4 Subgroup and sensitivity analysis
Subgroup analyses based on gender (male/female), smoking status (yes/no), and alcohol consumption (yes/no) revealed similar associations between UPF intake and depressive symptoms (Figure 2A). However, no significant associations were observed between SB and depressive symptoms among non-smokers and non-drinkers (Figure 2B). Sensitivity analyses further confirmed the consistency of the results when age (early youth/late youth) and BMI (underweight/normal weight/overweight or obese) were categorized (Supplementary Tables 2, 3).
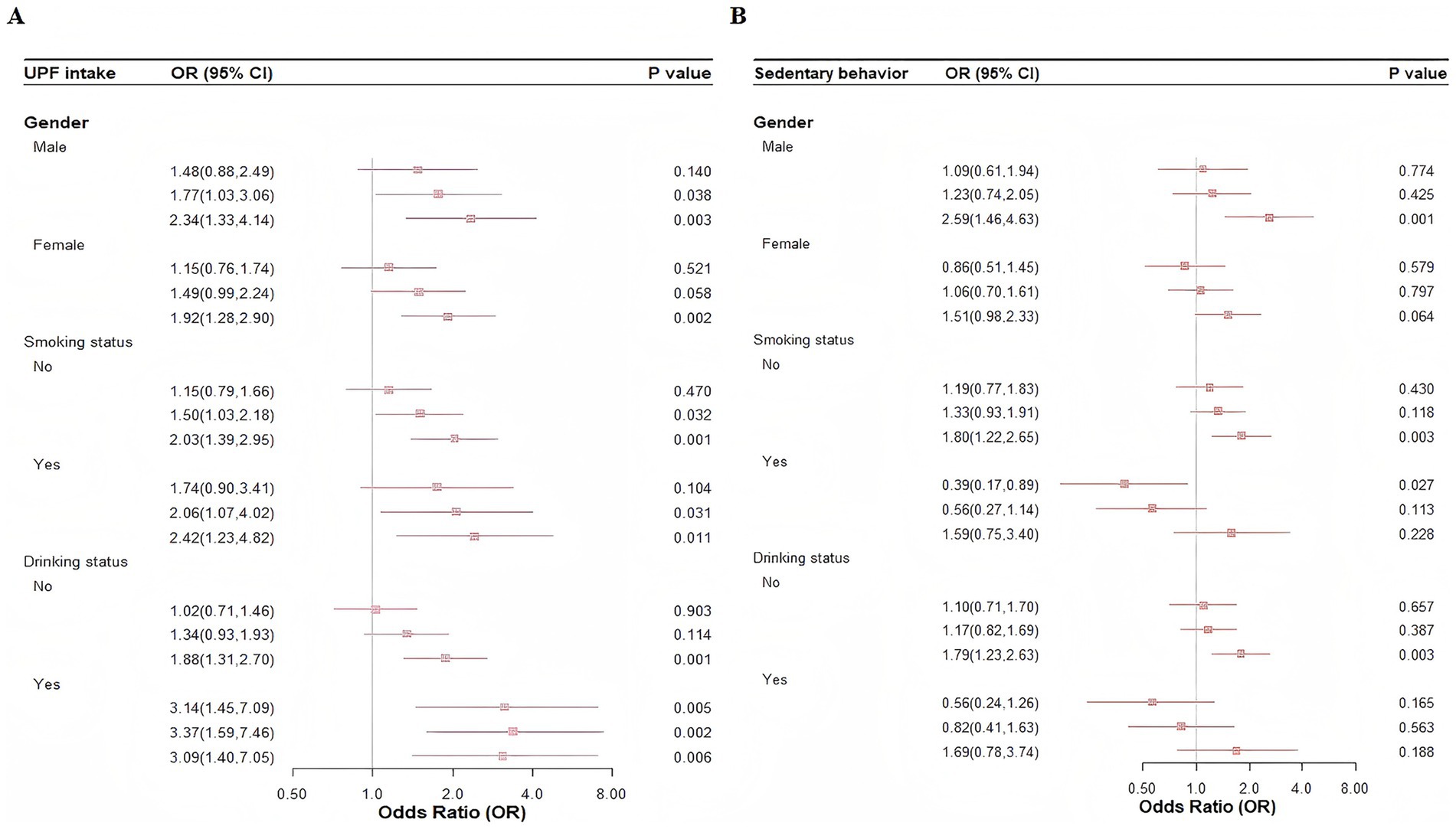
Figure 2. Subgroup analysis of the association between UPF intake, SB, and depressive symptoms. (A) UPF intake. (B) Sedentary behavior.
4 Discussion
Our findings suggest that, in young populations, both higher intake of UPF and increased SB are significantly associated with an elevated risk of depressive symptoms. Furthermore, the combination of high UPF intake and sedentary time exceeding 6 h per day further exacerbates this risk, as demonstrated in the joint analysis.
These results align with several previous epidemiological studies. A cohort study of 26,730 adults revealed that higher UPF intake was positively associated with an increased risk of depressive symptoms (24). Two subsequent cross-sectional studies found that individuals in the highest quartile of UPF consumption had higher odds of developing depressive symptoms compared to those in the lowest quartile (8, 9). However, these studies were primarily conducted in countries such as France (24), the United States (9), Italy (8), Spain (25), and Brazil (26), and mostly involved adult populations. Our study, focusing on young adults in Shenzhen, China, supports these findings and extends the knowledge in this area by demonstrating that higher UPF intake is also associated with an increased risk of depressive symptoms among young adults.
The potential mechanisms underlying the association between UPF intake and depressive symptoms are multifaceted. Firstly, UPF is characterized by high levels of sugar, salt, saturated fat, and energy, while being low in protein, dietary fiber, vitamins, and minerals, resulting in poor nutritional quality (27). Diets with these components can induce dysbiosis, disrupt gut barrier function, and alter neurotransmitter metabolism both in the gut and brain, ultimately affecting brain function and behavior (28). Secondly, various food additives in UPF may contribute to depressive symptoms (29). For example, artificial sweeteners (e.g., aspartame, saccharin) may alter the synthesis and release of neurotransmitters such as dopamine, norepinephrine, and serotonin, which are critical to mental health (30). Additionally, bisphenol A (commonly used in the production of plastic food and beverage containers) disrupts stress-sensitive and endocrine systems, potentially contributing to depressive states later in life (31).
Our study also found that individuals in the highest sedentary time group (>8 h/day) had a significantly increased risk of depressive symptoms compared to those in the lowest sedentary time group (<4 h/day). A previous study involving adolescents aged 12–15 years (N = 67,077) across 30 low- and middle-income countries found that the prevalence of depressive symptoms increased linearly with sedentary time, with behaviors exceeding 1–2 h per day associated with a higher risk of depression, regardless of physical activity levels (32). A separate cross-sectional study conducted in China (N = 11,787) found that nearly 50% of college students with screen time exceeding 4 h per day reported depressive symptoms, with this association being significantly stronger for screen time >4 h/day compared to ≤2 h/day (33).
In our subgroup analyses, no significant associations were found between SB and depressive symptoms among non-smokers and non-drinkers. Several factors may explain this. First, the insufficient sample size in these subgroups could have limited the statistical power to detect a significant effect. Second, chronic smoking may induce depressive symptoms through neuroendocrine and dopaminergic pathways (34), potentially masking the effect of SB (35, 36). Similarly, a J-shaped relationship between alcohol consumption and depressive symptoms has been suggested in existing research, where light or moderate alcohol consumption may have protective effects, while heavy consumption exacerbates depressive symptoms (37–39). These dual influences may reduce the observed relationship between SB and depressive symptoms in these groups.
There are several potential mechanisms explaining the relationship between SB and depressive symptoms. The social withdrawal hypothesis suggests that excessive time spent on passive, non-social activities (e.g., internet use, watching TV, or listening to music) may reduce social engagement, leading to social withdrawal behaviors that are strongly associated with the development of depression (40, 41). Furthermore, SB may mediate depression through inflammatory pathways (42, 43). Research has shown that SB increases C-reactive protein levels, which are associated with the development of depressive symptoms (44).
Additionally, our study observed that the combination of high UPF intake and sedentary time >6 h/day significantly increased the risk of depressive symptoms. However, no significant interaction between UPF intake and SB was found in relation to depressive symptoms. Several studies have proposed mechanisms that could explain this. First, there may be a mutually reinforcing relationship between SB and UPF intake. A previous study have shown that individuals with sedentary time >2 h/day had a higher daily consumption of UPF (42.8%) compared to those with less SB (29.8%) (45). Moreover, increased UPF intake is associated with higher levels of sedentary activities, such as watching TV, playing video games on weekends, and using smartphones during weekdays (46). This mutual reinforcement may further heighten the risk of depressive symptoms. In addition, both UPF intake and SB are linked to inflammatory responses (43, 47), which are a key feature of depression (42), and these factors may work synergistically to increase the risk of depression via inflammatory mechanisms.
Notably, the potential for reverse causality must also be considered, as depressive symptoms may themselves lead to poorer lifestyle choices. Previous studies have shown that depressive symptoms—such as low mood, diminished interest, and lack of energy—can lead individuals to withdraw from physical activity, thereby increasing sedentary behavior (48–51). Concurrently, depressive symptoms are positively correlated with unhealthy dietary patterns, including increased consumption of saturated fats, sugars, and emotional eating behaviors (52). From a physiological perspective, depression activates the hypothalamic–pituitary–adrenal (HPA) axis, leading to increased glucocorticoid release (53), which in turn stimulates appetite—particularly for highly palatable, high-fat, and high-sugar foods such as ultra-processed foods (54, 55). Thus, the associations identified in this study may be partly attributable to the effects of depressive symptoms on behavior. Further longitudinal and intervention studies are needed to clarify the temporal and causal pathways linking UPF intake, sedentary behavior, and depressive symptoms, thereby providing a robust scientific basis for effective mental health promotion strategies among young adults.
5 Strengths and limitations
To the best of our knowledge, this is the first study to examine the association between UPF intake and depressive symptoms in a young population in Shenzhen, China. Furthermore, we explored the combined effects of UPF intake and SB on depressive symptoms. However, several limitations should be considered. First, the study relied on self-reported questionnaires to assess sedentary time, which may have introduced recall bias and potentially affected the accuracy of the findings. Future research should validate these results by incorporating both accelerometer measurements and self-report methods. Second, the study was conducted in Longhua District, Shenzhen, and the sample may not be sufficiently representative of the broader youth population, limiting the generalizability of the findings to other regions or populations. Third, as a cross-sectional study, our design precludes the inference of causality. Longitudinal studies are necessary to confirm the observed associations and establish causal relationships.
6 Conclusion
In conclusion, we found that both higher UPF intake and increased SB were significantly associated with depressive symptoms in the young population. The combination of high UPF intake and sedentary time exceeding 6 h per day was linked to an even greater risk of depressive symptoms. These findings highlight the need for mental health interventions targeting youth to address both UPF consumption and SB. Comprehensive and integrated approaches are essential to mitigate the risk of depressive symptoms in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to Hong Xie, xh@bbmu.edu.cn.
Ethics statement
The studies involving humans were approved by the Medical Committee of the Science and Technology Ethics Committee of Tsinghua University (Ethics No. 20230065). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JR: Formal analysis, Investigation, Methodology, Writing – original draft. LZ: Formal analysis, Methodology, Writing – original draft. YL: Validation, Visualization, Writing – review & editing. SZ: Validation, Visualization, Writing – review & editing. XX: Data curation, Project administration, Writing – review & editing. XC: Project administration, Supervision, Writing – review & editing. HX: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the project “Investigating the Effects and Mechanisms of Ultra-Processed Food Exposure on Depression in Young Adults via the Gut Microbiota-Gut-Brain Axis Pathway” (2024byzd029).
Acknowledgments
The authors thank the staff and the participants of the study for their valuable contributions. The authors thank Yaqin Yang for her valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1675892/full#supplementary-material
References
1. Tackett, JL, and Sharp, C. A developmental psychopathology perspective on personality disorder: introduction to the special issue. J Personal Disord. (2014) 28:1–4. doi: 10.1521/pedi.2014.28.1.1
2. Bulhões, C, Ramos, E, Dias, S, and Barros, H. Depressive symptoms at 13 years as predictors of depression in older adolescents: a prospective 4-year follow-up study in a nonclinical population. Eur Child Adolesc Psychiatry. (2019) 28:595–9. doi: 10.1007/s00787-018-1194-0
3. Tuithof, M, Ten Have, M, Van Dorsselaer, S, Kleinjan, M, Beekman, A, and De Graaf, R. Course of subthreshold depression into a depressive disorder and its risk factors. J Affect Disord. (2018) 241:206–15. doi: 10.1016/j.jad.2018.08.010
4. Fu, XL, Zhang, K, Chen, XF, and Chen, ZY. Report on national mental health development in China (2021–2022). Beijing: Social Sciences Academic Press (2023).
5. Marx, W, Moseley, G, Berk, M, and Jacka, F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. (2017) 76:427–36. doi: 10.1017/S0029665117002026
6. Owen, L, and Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc Nutr Soc. (2017) 76:425–6. doi: 10.1017/S0029665117001057
7. Lane, MM, Gamage, E, Travica, N, Dissanayaka, T, Ashtree, DN, Gauci, S, et al. Ultra-processed food consumption and mental health: a systematic review and Meta-analysis of observational studies. Nutrients. (2022) 14:2568. doi: 10.3390/nu14132568
8. Godos, J, Bonaccio, M, Al-Qahtani, WH, Marx, W, Lane, MM, Leggio, GM, et al. Ultra-processed food consumption and depressive symptoms in a Mediterranean cohort. Nutrients. (2023) 15:504. doi: 10.3390/nu15030504
9. Zheng, L, Sun, J, Yu, X, and Zhang, D. Ultra-processed food is positively associated with depressive symptoms among United States adults. Front Nutr. (2020) 7:600449. doi: 10.3389/fnut.2020.600449
10. Bull, FC, Al-Ansari, SS, Biddle, S, Borodulin, K, Buman, MP, Cardon, G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
11. Huang, Y, Li, L, Gan, Y, Wang, C, Jiang, H, Cao, S, et al. Sedentary behaviors and risk of depression: a meta-analysis of prospective studies. Transl Psychiatry. (2020) 10:26. doi: 10.1038/s41398-020-0715-z
12. Cao, Z, Xu, C, Zhang, P, and Wang, Y. Associations of sedentary time and physical activity with adverse health conditions: outcome-wide analyses using isotemporal substitution model. eClinicalMedicine. (2022) 48:101424. doi: 10.1016/j.eclinm.2022.101424
13. Burke, V, Milligan, RAK, Beilin, LJ, Dunbar, D, Spencer, M, Balde, E, et al. Clustering of health-related behaviors among 18-year-old Australians. Prev Med. (1997) 26:724–33. doi: 10.1006/pmed.1997.0198
14. Werneck, AO, Silva, DR, Malta, DC, Gomes, CS, Souza-Júnior, PR, Azevedo, LO, et al. Associations of sedentary behaviours and incidence of unhealthy diet during the COVID-19 quarantine in Brazil. Public Health Nutr. (2021) 24:422–6. doi: 10.1017/S1368980020004188
15. Oliveira, GAL, Santos Gonçalves, VS, Nakano, EY, and Toral, N. Consumption of ultra-processed foods and low dietary diversity are associated with sedentary and unhealthy eating behaviors: a nationwide study with Brazilian schoolchildren. PLoS One. (2024) 19:e0294871. doi: 10.1371/journal.pone.0294871
16. Zhang, S, Gan, S, Zhang, Q, Liu, L, Meng, G, Yao, Z, et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin chronic low-grade systemic inflammation and health cohort study. Int J Epidemiol. (2022) 51:237–49. doi: 10.1093/ije/dyab174
17. Monteiro, CA, Cannon, G, Levy, RB, Moubarac, J-C, Louzada, ML, Rauber, F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. (2019) 22:936–41. doi: 10.1017/S1368980018003762
18. Popkin, BM, Du, S, Zhai, F, and Zhang, B. Cohort profile: the China health and nutrition survey--monitoring and understanding socio-economic and health change in China, 1989-2011. Int J Epidemiol. (2010) 39:1435–40. doi: 10.1093/ije/dyp322
19. Canhada, SL, Luft, VC, Giatti, L, Duncan, BB, Chor, D, Fonseca, MDJMD, et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian longitudinal study of adult health (ELSA-Brasil). Public Health Nutr. (2020) 23:1076–86. doi: 10.1017/S1368980019002854
20. Bauman, A, Ainsworth, BE, Sallis, JF, Hagströmer, M, Craig, CL, Bull, FC, et al. The descriptive epidemiology of sitting. Am J Prev Med. (2011) 41:228–35. doi: 10.1016/j.amepre.2011.05.003
21. Ekelund, U, Steene-Johannessen, J, Brown, WJ, Fagerland, MW, Owen, N, Powell, KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. (2016) 388:1302–10. doi: 10.1016/S0140-6736(16)30370-1
22. Kroenke, K, Spitzer, RL, and Williams, JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Ghazisaeedi, M, Mahmoodi, H, Arpaci, I, Mehrdar, S, and Barzegari, S. Validity, reliability, and optimal cut-off scores of the WHO-5, PHQ-9, and PHQ-2 to screen depression among university students in Iran. Int J Ment Health Addicti. (2022) 20:1824–33. doi: 10.1007/s11469-021-00483-5
24. Adjibade, M, Julia, C, Allès, B, Touvier, M, Lemogne, C, Srour, B, et al. Prospective association between ultra-processed food consumption and incident depressive symptoms in the French NutriNet-santé cohort. BMC Med. (2019) 17:78. doi: 10.1186/s12916-019-1312-y
25. Contreras-Rodriguez, O, Reales-Moreno, M, Fernández-Barrès, S, Cimpean, A, Arnoriaga-Rodríguez, M, Puig, J, et al. Consumption of ultra-processed foods is associated with depression, mesocorticolimbic volume, and inflammation. J Affect Disord. (2023) 335:340–8. doi: 10.1016/j.jad.2023.05.009
26. De Farias Xavier, DE, De Moraes, RCS, Viana, TAF, Pereira, JKG, Da Costa, PCT, Duarte, DB, et al. Food consumption according to the NOVA food classification and its relationship with symptoms of depression, anxiety, and stress in women. Nutrients. (2024) 16:3734. doi: 10.3390/nu16213734
27. Luiten, CM, Steenhuis, IH, Eyles, H, Ni Mhurchu, C, and Waterlander, WE. Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets. Public Health Nutr. (2016) 19:530–8. doi: 10.1017/S1368980015002177
28. Guo, Y, Zhu, X, Zeng, M, Qi, L, Tang, X, Wang, D, et al. A diet high in sugar and fat influences neurotransmitter metabolism and then affects brain function by altering the gut microbiota. Transl Psychiatry. (2021) 11:328. doi: 10.1038/s41398-021-01443-2
29. Gómez-Donoso, C, Sánchez-Villegas, A, Martínez-González, MA, Gea, A, Mendonça, RDD, Lahortiga-Ramos, F, et al. Ultra-processed food consumption and the incidence of depression in a Mediterranean cohort: the SUN project. Eur J Nutr. (2020) 59:1093–103. doi: 10.1007/s00394-019-01970-1
30. Quines, CB, Rosa, SG, Da Rocha, JT, Gai, BM, Bortolatto, CF, Duarte, MMMF, et al. Monosodium glutamate, a food additive, induces depressive-like and anxiogenic-like behaviors in young rats. Life Sci. (2014) 107:27–31. doi: 10.1016/j.lfs.2014.04.032
31. Rancière, F, Lyons, JG, Loh, VHY, Botton, J, Galloway, T, Wang, T, et al. Bisphenol a and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health. (2015) 14:46. doi: 10.1186/s12940-015-0036-5
32. Vancampfort, D, Stubbs, B, Firth, J, Van Damme, T, and Koyanagi, A. Sedentary behavior and depressive symptoms among 67,077 adolescents aged 12–15 years from 30 low- and middle-income countries. Int J Behav Nutr Phys Act. (2018) 15:73. doi: 10.1186/s12966-018-0708-y
33. Zhang, Y, Wu, X, Tao, S, Li, S, Ma, L, Yu, Y, et al. Associations between screen time, physical activity, and depressive symptoms during the 2019 coronavirus disease (COVID-19) outbreak among Chinese college students. Environ Health Prev Med. (2021) 26:107. doi: 10.1186/s12199-021-01025-0
34. Nisell, M, Nomikos, GG, and Svensson, TH. Nicotine dependence, midbrain dopamine systems and psychiatric disorders. Pharmacol Toxicol. (1995) 76:157–62. doi: 10.1111/j.1600-0773.1995.tb00123.x
35. Munafò, MR, and Araya, R. Cigarette smoking and depression: a question of causation. Br J Psychiatry. (2010) 196:425–6. doi: 10.1192/bjp.bp.109.074880
36. Raffetti, E, Donato, F, Triolo, F, Andersson, F, Forsell, Y, and Galanti, MR. Country differences in the cross-sectional associations between smoking and depressive symptoms in adolescence. Eur J Pub Health. (2022) 32:913–8. doi: 10.1093/eurpub/ckac155
37. An, R, and Xiang, X. Smoking, heavy drinking, and depression among U.S. middle-aged and older adults. Prev Med. (2015) 81:295–302. doi: 10.1016/j.ypmed.2015.09.026
38. Boden, JM, and Fergusson, DM. Alcohol and depression. Addiction. (2011) 106:906–14. doi: 10.1111/j.1360-0443.2010.03351.x
39. Sullivan, LE, Fiellin, DA, and O’Connor, PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. (2005) 118:330–41. doi: 10.1016/j.amjmed.2005.01.007
40. Kraut, R, Patterson, M, Lundmark, V, Kiesler, S, Mukophadhyay, T, and Scherlis, W. Internet paradox: a social technology that reduces social involvement and psychological well-being? Am Psychol. (1998) 53:1017–31. doi: 10.1037/0003-066X.53.9.1017
41. Desjardins, L, Barrera, M, Schulte, F, Chung, J, Cataudella, D, Janzen, L, et al. Predicting social withdrawal, anxiety and depression symptoms in pediatric brain tumor survivors. J Psychosoc Oncol. (2019) 37:22–36. doi: 10.1080/07347332.2018.1535531
42. Kappelmann, N, Arloth, J, Georgakis, MK, Czamara, D, Rost, N, Ligthart, S, et al. Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic correlation and 2-sample Mendelian randomization study. JAMA Psychiatry. (2021) 78:161–70. doi: 10.1001/jamapsychiatry.2020.3436
43. Pinto, AJ, Bergouignan, A, Dempsey, PC, Roschel, H, Owen, N, Gualano, B, et al. Physiology of sedentary behavior. Physiol Rev. (2023) 103:2561–622. doi: 10.1152/physrev.00022.2022
44. Osimo, EF, Baxter, LJ, Lewis, G, Jones, PB, and Khandaker, GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. (2019) 49:1958–70. doi: 10.1017/S0033291719001454
45. Costa, CDS, Flores, TR, Wendt, A, Neves, RG, Assunção, MCF, and Santos, IS. Comportamento sedentário e consumo de alimentos ultraprocessados entre adolescentes brasileiros: Pesquisa Nacional de Saúde do Escolar (PeNSE), 2015. Cad Saude Publica. (2018) 34. doi: 10.1590/0102-311x00021017
46. Machado-Rodrigues, AM, Padez, C, Rodrigues, D, Dos Santos, EA, Baptista, LC, Liz Martins, M, et al. Ultra-processed food consumption and its association with risk of obesity, sedentary behaviors, and well-being in adolescents. Nutrients. (2024) 16:3827. doi: 10.3390/nu16223827
47. Tristan Asensi, M, Napoletano, A, Sofi, F, and Dinu, M. Low-grade inflammation and ultra-processed foods consumption: a review. Nutrients. (2023) 15:1546. doi: 10.3390/nu15061546
48. Arredondo, EM, Lemus, H, Elder, JP, Molina, M, Martinez, S, Sumek, C, et al. The relationship between sedentary behavior and depression among Latinos. Ment Health Phys Act. (2013) 6:3–9. doi: 10.1016/j.mhpa.2012.10.005
49. Azevedo Da Silva, M, Singh-Manoux, A, Brunner, EJ, Kaffashian, S, Shipley, MJ, Kivimäki, M, et al. Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. Eur J Epidemiol. (2012) 27:537–46. doi: 10.1007/s10654-012-9692-8
50. Faulkner, G, and Biddle, SJH. Standing on top of the world: is sedentary behaviour associated with mental health? Ment Health Phys Act. (2013) 6:1–2. doi: 10.1016/j.mhpa.2013.02.003
51. Okely, JA, Čukić, I, Shaw, RJ, Chastin, SF, Dall, PM, Deary, IJ, et al. Positive and negative well-being and objectively measured sedentary behaviour in older adults: evidence from three cohorts. BMC Geriatr. (2019) 19:28. doi: 10.1186/s12877-019-1026-1
52. Whitaker, KM, Sharpe, PA, Wilcox, S, and Hutto, BE. Depressive symptoms are associated with dietary intake but not physical activity among overweight and obese women from disadvantaged neighborhoods. Nutr Res. (2014) 34:294–301. doi: 10.1016/j.nutres.2014.01.007
53. Vreeburg, SA, Hoogendijk, WJG, Van Pelt, J, DeRijk, RH, Verhagen, JCM, Van Dyck, R, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. (2009) 66:617. doi: 10.1001/archgenpsychiatry.2009.50
54. Tasker, JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity. (2006) 14:14. doi: 10.1038/oby.2006.320
Keywords: ultra-processed food, sedentary behavior, depressive symptoms, combined effects, young population
Citation: Ren J, Zhou L, Li Y, Zhang S, Xu X, Chi X and Xie H (2025) The independent and combined associations between the intake of ultra-processed foods, sedentary behavior, and depressive symptoms in young adults. Front. Nutr. 12:1675892. doi: 10.3389/fnut.2025.1675892
Edited by:
Tae-Woon Kim, Gyeongsang National University, Republic of KoreaReviewed by:
Mônica Leila Santana, Federal University of Bahia (UFBA), BrazilMalsoon Shin, Korea University Sejong Campus, Republic of Korea
Copyright © 2025 Ren, Zhou, Li, Zhang, Xu, Chi and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinli Chi, eGlubGljaGlAMTI2LmNvbQ==; Hong Xie, eGhAYmJtdS5lZHUuY24=
†These authors share first authorship
 Jiajia Ren1†
Jiajia Ren1† Lei Zhou
Lei Zhou Xinli Chi
Xinli Chi