- 1Department of Ultrasound, Jilin Cancer Hospital, Changchun, Jilin, China
- 2Department of Anesthesiology, Jilin Cancer Hospital, Changchun, Jilin, China
1 Introduction
Type 2 Diabetes Mellitus (T2DM) is a chronic metabolic disorder characterized by elevated blood glucose levels resulting from insulin resistance and relative insulin deficiency. It is one of the most prevalent endocrine disorders worldwide, driven by a combination of genetic, environmental, and lifestyle factors (1, 2). T2DM is associated with a range of systemic complications, including cardiovascular disease, neuropathy, nephropathy, and retinopathy, which significantly impact patient quality of life (3, 4). The pathogenesis of T2DM involves complex metabolic dysregulation, notably disturbances in glucose and lipid metabolism, often exacerbated by poor dietary habits, sedentary lifestyles, and obesity (5–7).
Osteoarthritis (OA) is a degenerative joint disease characterized by the progressive breakdown of articular cartilage, subchondral bone remodeling, and synovial inflammation (8). It is the most common form of arthritis, primarily affecting weight-bearing joints such as the knees, hips, and hands (9). OA leads to pain, stiffness, reduced mobility, and diminished quality of life, imposing a significant socioeconomic burden. Recent research recognizes the role of metabolic and inflammatory processes in its development (10, 11).
Emerging evidence indicates a significant link between diabetes and osteoarthritis (OA), suggesting that metabolic disturbances inherent to diabetes may contribute to joint degeneration (12, 13). The association is multifaceted, involving systemic inflammation, oxidative stress, and altered cellular metabolism within joint tissues (14, 15). Hyperglycemia and insulin resistance can induce inflammatory pathways that exacerbate cartilage breakdown and impair repair mechanisms (16). Additionally, diabetes-related metabolic changes, such as dyslipidemia and mitochondrial dysfunction, can directly affect chondrocyte viability and function (17). Obesity, a common comorbidity of diabetes, further amplifies joint stress and inflammatory mediators, accelerating OA progression. Recent research highlights that early-life hyperglycemic environments may epigenetically predispose individuals to increased susceptibility to OA later in life, partly through mitochondrial impairment and disrupted cellular homeostasis in cartilage (18). Understanding the intricate relationship between diabetes-induced metabolic abnormalities and joint health is essential for developing integrated therapeutic strategies aimed at preventing and managing osteoarthritis in diabetic populations.
2 Key factors contributing to nutritional dysregulation in T2DM
Nutritional metabolic dysregulation refers to the disruption of normal metabolic processes resulting from imbalances in nutrient intake, absorption, and utilization. It encompasses a spectrum of metabolic disturbances characterized by abnormal glucose metabolism, lipid abnormalities, and inflammatory responses. In the context of T2DM, this dysregulation manifests as impaired insulin signaling, increased hepatic glucose production, and dyslipidemia, which collectively contribute to systemic metabolic imbalance (19, 20). Such disturbances not only impair energy homeostasis but also set the stage for chronic low-grade inflammation and tissue damage, thereby influencing the development and progression of comorbid conditions such as osteoarthritis. Several interconnected factors drive nutritional metabolic dysregulation in individuals with T2DM. Central among these is insulin resistance, where cells become less responsive to insulin, leading to elevated blood glucose levels (21, 22). Excessive caloric intake, particularly diets high in refined carbohydrates and saturated fats, exacerbates this resistance and promotes adiposity (23). Obesity, especially visceral fat accumulation, acts as both a cause and consequence of metabolic disturbances, secreting pro-inflammatory cytokines that further impair insulin sensitivity (24). Additionally, altered lipid metabolism results in elevated triglycerides and low HDL cholesterol, compounding the metabolic imbalance (25).
3 Pathophysiological mechanisms linking T2DM and osteoarthritis
3.1 Inflammation and its role in OA with T2DM
Chronic low-grade inflammation is a hallmark shared by both T2DM and osteoarthritis (26, 27). In T2DM, nutritional metabolic dysregulation, characterized by hyperglycemia and dyslipidemia, promotes the activation of inflammatory pathways within adipose tissue, pancreatic islets, and other metabolic organs (28). This systemic inflammatory state leads to increased circulating pro-inflammatory cytokines, such as tumor necrosis factor-alpha, interleukins, and C-reactive protein, which can infiltrate joint tissues (29). In osteoarthritis, these inflammatory mediators contribute to cartilage degradation, synovial inflammation, and subchondral bone changes. The persistent inflammatory milieu not only accelerates joint tissue breakdown but also exacerbates insulin resistance, creating a vicious cycle that links metabolic dysregulation with joint degeneration (30). The interplay of inflammatory processes thus serves as a central mechanism connecting T2DM and osteoarthritis pathogenesis (Figure 1).
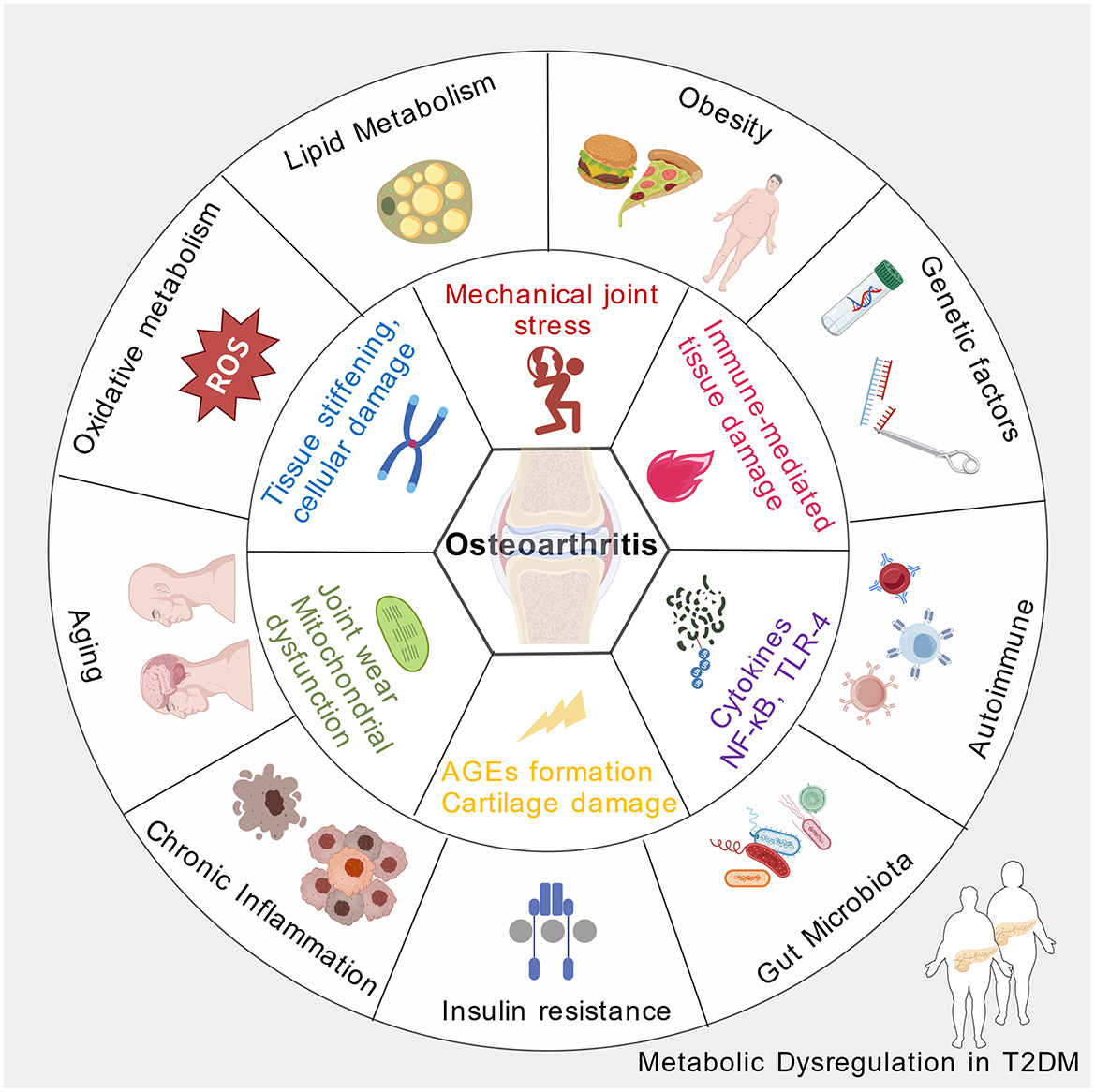
Figure 1. The pathophysiological mechanisms of osteoarthritis induced by nutritional disorders secondary to type 2 diabetes (Created with https://BioGDP.com (57)).
3.2 Obesity as a common risk factor
Obesity, frequently resulting from nutritional metabolic dysregulation, is a significant shared risk factor for both T2DM and osteoarthritis. Excess adipose tissue, particularly visceral fat, acts as an active endocrine organ secreting adipokines and cytokines that promote systemic inflammation (31). These bioactive molecules impair insulin signaling. Simultaneously, increased body weight imposes mechanical stress on weight-bearing joints, such as the knees and hips, leading to cartilage wear and osteoarthritis (32). Moreover, adipokines like leptin and adiponectin influence cartilage metabolism and synovial inflammation, further linking obesity to joint degeneration (33, 34). Therefore, obesity serves as a nexus where nutritional metabolic dysregulation fosters both metabolic and joint pathologies through inflammatory and biomechanical pathways.
3.3 Nutrient metabolic changes catalyzed OA
Diabetes induces a range of metabolic alterations that predispose individuals to osteoarthritis. Hyperglycemia leads to the formation of advanced glycation end products (AGEs), which accumulate in cartilage and other joint tissues, stiffening the extracellular matrix and impairing its resilience (35, 36). These modifications compromise the biomechanical properties of cartilage, making it more susceptible to damage under normal physiological loads. Additionally, diabetes-associated mitochondrial dysfunction and oxidative stress impair chondrocyte viability and function, disrupting the maintenance of cartilage homeostasis (37, 38). Altered lipid metabolism and insulin resistance further contribute to a pro-degenerative environment within joints (39). Collectively, these metabolic disturbances foster an environment conducive to cartilage breakdown, inflammation, and joint deterioration, thereby linking abnormal nutritional metabolism in diabetes to the pathogenesis of osteoarthritis.
3.4 Gut microbiota dysbiosis triggers OA in T2DM
A healthy gut microbiota maintains intestinal barrier integrity and modulates immune homeostasis. Dysbiosis, characterized by reduced microbial diversity and shifts in microbial populations, can compromise the intestinal barrier, leading to increased permeability. This disruption permits translocation of microbial components such as lipopolysaccharides (LPS) into systemic circulation, triggering low-grade systemic inflammation. Such chronic inflammatory states are central to insulin resistance development in T2DM and contribute to joint tissue degradation in OA. Gut microbiota dysbiosis influences immune cell differentiation and cytokine profiles. An imbalance favoring pro-inflammatory microbial species can skew immune responses toward a Th17 and M1 macrophage phenotype, fostering an environment conducive to tissue inflammation and destruction (40, 41). In the context of OA, this immune dysregulation amplifies cartilage catabolism and synovial inflammation (42). In T2DM, immune activation contributes to pancreatic β-cell dysfunction and peripheral insulin resistance. The shared immune pathways underscore the microbiota's role in bridging metabolic and joint pathologies.
4 Importance of nutritional interventions for T2DM patients with OA
4.1 Anti-inflammatory foods
Nutritional strategies emphasizing anti-inflammatory foods play a crucial role in managing osteoarthritis (OA), especially in patients with comorbid conditions such as T2DM. Diets rich in fruits, vegetables, whole grains, fatty fish, nuts, and seeds provide bioactive compounds like omega-3 fatty acids, antioxidants, and phytochemicals that can modulate inflammatory pathways involved in OA pathogenesis (43). These foods help reduce systemic low-grade inflammation, which is a key contributor to cartilage degradation and joint pain. For instance, omega-3 fatty acids have been shown to inhibit pro-inflammatory cytokines and mediators such as leptin, which is produced by adipose tissue and has been implicated in cartilage breakdown (44). Incorporating such anti-inflammatory foods into daily diets can therefore mitigate joint inflammation, slow disease progression, and improve quality of life for OA patients. Moreover, dietary patterns like the Mediterranean diet, characterized by high intake of plant-based foods and healthy fats, have demonstrated potential in reducing inflammatory markers and supporting joint health (45).
4.2 Weight management and its importance
Effective weight management is fundamental in controlling osteoarthritis symptoms and progression, particularly in obese patients. Excess body weight increases mechanical load on weight-bearing joints, accelerating cartilage wear and tear (46). Beyond mechanical stress, adipose tissue secretes adipokines such as leptin and pro-inflammatory cytokines that contribute to systemic inflammation and joint degradation (47). Nutritional interventions aimed at achieving and maintaining a healthy weight can significantly reduce joint pain, improve function, and potentially delay the need for surgical interventions. Studies have shown that even modest weight loss can decrease the load on affected joints and lower systemic inflammation, thereby alleviating OA symptoms (48). For patients with T2DM, weight reduction also improves insulin sensitivity and reduces metabolic inflammation, creating a synergistic benefit for joint health (49). Therefore, integrating calorie-controlled, nutrient-dense diets with physical activity is essential for comprehensive OA management in this population (43).
4.3 Supplements and nutraceuticals
Nutritional supplements and nutraceuticals offer additional avenues for managing OA, particularly in addressing inflammation and cartilage repair. Omega-3 fatty acids, glucosamine, chondroitin sulfate, and certain plant-derived compounds like oleanolic acid have been investigated for their potential to modulate inflammatory responses and support joint integrity (50, 51). For example, omega-3 supplementation has been associated with reduced joint pain and stiffness, likely through its anti-inflammatory effects (52, 53). Similarly, emerging evidence suggests that nutraceuticals targeting leptin signaling and systemic inflammation can be beneficial, especially in obese OA patients where metabolic factors exacerbate joint degeneration (54). Personalized nutritional approaches, possibly guided by genetic information, may optimize the efficacy of these supplements in managing OA symptoms. However, it is important to note that while supplements can complement dietary strategies, they should be used under professional guidance to ensure safety and appropriate dosing (55). Overall, integrating nutraceuticals into a comprehensive nutritional plan can enhance joint health and improve functional outcomes in patients with OA and T2DM.
5 Conclusion
Nutritional metabolic dysregulation plays significant role in the development and progression of osteoarthritis among individuals with T2DM. The interconnected mechanisms—such as chronic inflammation, obesity-related biomechanical stress, and altered metabolic pathways—contribute to joint degeneration beyond traditional wear-and-tear models. Addressing metabolic disturbances through targeted nutritional interventions and lifestyle modifications can potentially mitigate osteoarthritis risk and improve patient outcomes. Recognizing the bidirectional relationship between metabolic health and joint integrity emphasizes the importance of a comprehensive approach to managing T2DM and osteoarthritis concurrently.
There is a pressing need to develop and evaluate innovative therapeutic strategies that address the metabolic underpinnings of osteoarthritis in T2DM. Future studies should explore the efficacy of targeted nutritional interventions, such as personalized diets rich in anti-inflammatory and insulin-sensitizing nutrients, alongside pharmacological agents that modulate metabolic pathways. Additionally, emerging therapies like microbiota transplantation, prebiotics, probiotics, and metabolic modulators warrant rigorous clinical testing to assess their potential in restoring metabolic balance and preventing joint degeneration (56). Integrating multi-omics data can facilitate the identification of biomarkers for treatment response, enabling more precise and effective management approaches.
To better understand the temporal relationship between nutritional metabolic dysregulation and osteoarthritis development, comprehensive longitudinal cohort studies are essential. These studies should track metabolic parameters, dietary patterns, microbiota profiles, and joint health indicators over extended periods. Such research will help clarify causative links, identify early biomarkers of joint degeneration, and determine critical windows for intervention. Moreover, stratifying participants based on metabolic phenotypes can reveal differential risks and inform personalized prevention strategies. Ultimately, these insights will guide clinical practices aimed at early detection and targeted management of osteoarthritis in individuals with T2DM.
Author contributions
CW: Writing – original draft. XY: Writing – original draft. XZ: Writing – original draft. ML: Writing – original draft. WY: Writing – original draft. SZ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xing JS, Bai ZM. Is testicular dysgenesis syndrome a genetic, endocrine, or environmental disease, or an unexplained reproductive disorder? Life Sci. (2018) 194:120–9. doi: 10.1016/j.lfs.2017.11.039
2. Venetsanopoulou AI, Alamanos Y, Voulgari PV, Drosos AA. Epidemiology and risk factors for rheumatoid arthritis development. Mediterr J Rheumatol. (2023) 34:404–13. doi: 10.31138/mjr.301223.eaf
3. Parsamanesh N, Moossavi M, Bahrami A, Butler AE, Sahebkar A. Therapeutic potential of curcumin in diabetic complications. Pharmacol Res. (2018) 136:181–93. doi: 10.1016/j.phrs.2018.09.012
4. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. (2020) 18:117–24. doi: 10.2174/1570161117666190502103733
5. Wang Y, Xu H, Zhou X, Chen W, Zhou H. Dysregulated bile acid homeostasis: unveiling its role in metabolic diseases. Med Rev. (2021) 4:262–83. doi: 10.1515/mr-2024-0020
6. Kim E, Jeon S. The impact of phytochemicals in obesity-related metabolic diseases: focus on ceramide metabolism. Nutrients. (2023) 15:703. doi: 10.3390/nu15030703
7. Ayeleso AO, Matumba MG, Ntambi JM, Mukwevho E. Chapter 17 - insights into the metabolism of lipids in obesity and diabetes. In:Ntambi JM, , editor. Lipid Signaling and Metabolism. London: Academic Press (2020). p. 345–57. doi: 10.1016/B978-0-12-819404-1.00017-8
8. Kim JR, Yoo JJ, Kim HA. Therapeutics in osteoarthritis based on an understanding of its molecular pathogenesis. Int J Mol Sci. (2018) 19:674. doi: 10.3390/ijms19030674
9. Matsushita I, Motomura H, Seki E, Kimura T. Radiographic changes and factors associated with subsequent progression of damage in weight-bearing joints of patients with rheumatoid arthritis under TNF-blocking therapies-three-year observational study. Mod Rheumatol. (2017) 27:570–5. doi: 10.1080/14397595.2016.1227235
10. Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. (2010) 22:533–7. doi: 10.1097/BOR.0b013e32833b4682
11. Akgun Y, Akgün D, Akgun I. The future of osteoarthritis management: plasma exchange as a new generation treatment. Cureus. (2025) 17:e83889. doi: 10.7759/cureus.83889
12. Frey N, Hügle T, Jick SS, Meier CR, Spoendlin J. Type II diabetes mellitus and incident osteoarthritis of the hand: a population-based case-control analysis. Osteoarthritis Cartilage. (2016) 24:1535–40. doi: 10.1016/j.joca.2016.04.005
13. Alenazi AM, Alothman S, Alshehri MM, Rucker J, Waitman LR, Wick J, et al. The prevalence of type 2 diabetes and associated risk factors with generalized osteoarthritis: a retrospective study using ICD codes for clinical data repository system. Clin Rheumatol. (2019) 38:3539–47. doi: 10.1007/s10067-019-04712-0
14. Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. (2016) 148:183–93. doi: 10.1016/j.lfs.2016.02.002
15. Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PI Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. (2016) 473:4527–50. doi: 10.1042/BCJ20160503C
16. Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. (2013) 4:37. doi: 10.3389/fendo.2013.00037
17. Makrecka-Kuka M, Liepinsh E, Murray AJ, Lemieux H, Dambrova M, Tepp K, et al. Altered mitochondrial metabolism in the insulin-resistant heart. Acta Physiol. (2020) 228:e13430. doi: 10.1111/apha.13430
18. Perdijk O, Butler A, Macowan M, Chatzis R, Bulanda E, Grant RD, et al. Antibiotic-driven dysbiosis in early life disrupts indole-3-propionic acid production and exacerbates allergic airway inflammation in adulthood. Immunity. (2024) 57:1939–54.e7. doi: 10.1016/j.immuni.2024.06.010
19. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. (2015) 6:456–80. doi: 10.4239/wjd.v6.i3.456
20. Sparks DL, Chatterjee C. Purinergic signaling, dyslipidemia and inflammatory disease. Cell Physiol Biochem. (2012) 30:1333–9. doi: 10.1159/000343322
21. Tessneer KL, Jackson RM, Griesel BA, Olson AL. Rab5 activity regulates glut4 sorting into insulin-responsive and non-insulin-responsive endosomal compartments: a potential mechanism for development of insulin resistance. Endocrinology. (2014) 155:3315–28. doi: 10.1210/en.2013-2148
22. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. (2008) 31:S262–8. doi: 10.2337/dc08-s264
23. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. (2010) 91:502–9. doi: 10.3945/ajcn.2008.26285
24. Villarroya F, Cereijo R, Gavaldà-Navarro A, Villarroya J, Giralt M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J Intern Med. (2018) 284:492–504. doi: 10.1111/joim.12803
25. Lee SE, Schulze K, Stewart CP, Cole RN, Wu LS, Eroglu A, et al. Plasma proteome correlates of lipid and lipoprotein: biomarkers of metabolic diversity and inflammation in children of rural Nepal. J Lipid Res. (2019) 60:149–60. doi: 10.1194/jlr.P088542
26. Alzamil H. Elevated serum TNF-A is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J Obes. (2020) 2020:5076858. doi: 10.1155/2020/5076858
27. Varra FN, Varras M, Varra VK, Theodosis-Nobelos P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options (review). Mol Med Rep. (2024) 29:95. doi: 10.3892/mmr.2024.13219
28. Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, Lindstad T, et al. Coordinated regulation of nutrient and inflammatory responses by stamp2 is essential for metabolic homeostasis. Cell. (2007) 129:537–48. doi: 10.1016/j.cell.2007.02.049
29. Szarka A, Rigó Jr J, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. (2010) 11:59. doi: 10.1186/1471-2172-11-59
30. Xu H, Li H, Woo SL, Kim SM, Shende VR, Neuendorff N, et al. Myeloid cell-specific disruption of period1 and period2 exacerbates diet-induced inflammation and insulin resistance. J Biol Chem. (2014) 289:16374–88. doi: 10.1074/jbc.M113.539601
31. Karaskova E, Velganova-Veghova M, Geryk M, Foltenova H, Kucerova V, Karasek D. Role of adipose tissue in inflammatory bowel disease. Int J Mol Sci. (2021) 22:4226. doi: 10.3390/ijms22084226
32. McBride J, Zhang S, Wortley M, Paquette M, Klipple G, Byrd E, et al., editors. Neural network analysis of gait biomechanical data for classification of knee osteoarthritis. In: Proceedings of the 2011 Biomedical Sciences and Engineering Conference: Image Informatics and Analytics in Biomedicine. IEEE: Knoxville, TN, USA (2011). doi: 10.1109/BSEC.2011.5872315
33. de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. (2012) 20:846–53. doi: 10.1016/j.joca.2012.05.002
34. Dozio E, Corsi MM, Ruscica M, Passafaro L, Steffani L, Banfi G, et al. Adipokine actions on cartilage homeostasis. Adv Clin Chem. (2011) 55:61–79. doi: 10.1016/B978-0-12-387042-1.00004-6
35. Saudek DM, Kay J. Advanced glycation endproducts and osteoarthritis. Curr Rheumatol Rep. (2003) 5:33–40. doi: 10.1007/s11926-003-0081-x
36. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. (2013) 93:137–88. doi: 10.1152/physrev.00045.2011
37. Coleman MC, Ramakrishnan PS, Brouillette MJ, Martin JA. Injurious loading of articular cartilage compromises chondrocyte respiratory function. Arthritis Rheumatol. (2016) 68:662–71. doi: 10.1002/art.39460
38. Sun K, Wu Y, Zeng Y, Xu J, Wu L, Li M, et al. The role of the sirtuin family in cartilage and osteoarthritis: molecular mechanisms and therapeutic targets. Arthritis Res Ther. (2022) 24:286. doi: 10.1186/s13075-022-02983-8
39. Shin KC, Hwang I, Choe SS, Park J, Ji Y, Kim JI, et al. Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation. Nat Commun. (2017) 8:1087. doi: 10.1038/s41467-017-01232-w
40. Hanidziar D, Koulmanda M. Inflammation and the balance of treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant. (2010) 15:411–5. doi: 10.1097/MOT.0b013e32833b7929
41. Zheng Y, Wei K, Jiang P, Zhao J, Shan Y, Shi Y, et al. Macrophage polarization in rheumatoid arthritis: signaling pathways, metabolic reprogramming, and crosstalk with synovial fibroblasts. Front Immunol. (2024) 15:1394108. doi: 10.3389/fimmu.2024.1394108
42. Mukherjee A, Das B. The role of inflammatory mediators and matrix metalloproteinases (MMPS) in the progression of osteoarthritis. Biomater Biosyst. (2024) 13:100090. doi: 10.1016/j.bbiosy.2024.100090
43. van Zonneveld SM, van den Oever EJ, Haarman BCM, Grandjean EL, Nuninga JO, van de Rest O, et al. An anti-inflammatory diet and its potential benefit for individuals with mental disorders and neurodegenerative diseases-a narrative review. Nutrients. (2024) 16:2646. doi: 10.3390/nu16162646
44. Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem. (2008) 49:133–43. doi: 10.1007/978-1-4020-8831-5_5
45. Cena H, Calder PC. Defining a healthy diet: evidence for the role of contemporary dietary patterns in health and disease. Nutrients. (2020) 12:334. doi: 10.3390/nu12020334
46. Vijayan S, Margesan T. Hormonal imbalance in obesity and arthritis: points of contact. Curr Rheumatol Rev. (2025) 21:182–93. doi: 10.2174/0115733971293288240313090945
47. Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. (2017) 18:1649. doi: 10.3390/ijms18081649
48. Jin X, Gibson AA, Gale J, Schneuer F, Ding D, March L, et al. Does weight loss reduce the incidence of total knee and hip replacement for osteoarthritis-A prospective cohort study among middle-aged and older adults with overweight or obesity. Int J Obes. (2021) 45:1696–704. doi: 10.1038/s41366-021-00832-3
49. Kolodziejski PA, Leciejewska N, Chmurzynska A, Sassek M, Szczepankiewicz A, Szczepankiewicz D, et al. 30-day spexin treatment of mice with diet-induced obesity (DIO) and type 2 diabetes (T2dm) increases insulin sensitivity, improves liver functions and metabolic status. Mol Cell Endocrinol. (2021) 536:111420. doi: 10.1016/j.mce.2021.111420
50. Jerosch J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: outlook on other nutrient partners especially omega-3 fatty acids. Int J Rheumatol. (2011) 2011:969012. doi: 10.1155/2011/969012
51. Henrotin Y, Marty M, Mobasheri A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas. (2014) 78:184–7. doi: 10.1016/j.maturitas.2014.04.015
52. Norling LV, Perretti M. The role of omega-3 derived resolvins in arthritis. Curr Opin Pharmacol. (2013) 13:476–81. doi: 10.1016/j.coph.2013.02.003
53. Souza PR, Norling LV. Implications for eicosapentaenoic acid- and docosahexaenoic acid-derived resolvins as therapeutics for arthritis. Eur J Pharmacol. (2016) 785:165–73. doi: 10.1016/j.ejphar.2015.05.072
54. Leon-Cabrera S, Solís-Lozano L, Suárez-Álvarez K, González-Chávez A, Béjar YL, Robles-Díaz G, et al. Hyperleptinemia is associated with parameters of low-grade systemic inflammation and metabolic dysfunction in obese human beings. Front Integr Neurosci. (2013) 7:62. doi: 10.3389/fnint.2013.00062
55. Bailey RL. Current regulatory guidelines and resources to support research of dietary supplements in the United States. Crit Rev Food Sci Nutr. (2020) 60:298–309. doi: 10.1080/10408398.2018.1524364
56. Bock PM, Martins AF, Schaan BD. Understanding how pre- and probiotics affect the gut microbiome and metabolic health. Am J Physiol Endocrinol Metab. (2024) 327:E89–e102. doi: 10.1152/ajpendo.00054.2024
Keywords: type 2 diabetes mellitus, nutritional metabolic dysregulation, inflammation, obesity, osteoarthritis, nutritional interventions
Citation: Wang C, Yu X, Zeng X, Li M, Yang W and Zhou S (2025) Nutritional metabolic dysregulation in T2DM as a catalyst for osteoarthritis pathogenesis. Front. Nutr. 12:1676056. doi: 10.3389/fnut.2025.1676056
Received: 30 July 2025; Accepted: 10 September 2025;
Published: 29 September 2025.
Edited by:
Qingqing Yin, Shandong Provincial Hospital Affiliated to Shandong First Medical University, ChinaReviewed by:
Xiangwei Meng, Zibo Central Hospital, ChinaDunyi Qi, Affiliated Hospital of Xuzhou Medical University, China
Copyright © 2025 Wang, Yu, Zeng, Li, Yang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Zhou, emhvdXNodWpsc3pseXlAMTYzLmNvbQ==
 Chengyan Wang1
Chengyan Wang1 Shu Zhou
Shu Zhou