- 1Department of Endocrinology and Metabolism, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of Anatomy, School of Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Diabetic gastroparesis (DGP), a prevalent manifestation of diabetic autonomic neuropathy, is characterized by impaired gastrointestinal motility and delayed emptying. The pathogenesis of DGP is multifactorial, with chronic hyperglycemia serving as a key contributor through its induction of oxidative stress, inflammatory responses, immune dysregulation, and gut microbiota imbalance. These pathological processes collectively induce injury to interstitial cells of Cajal (ICCs) and gastric smooth muscle cells (GSMCs), accompanied by neuromuscular dysfunction, ultimately resulting in gastroparesis and its associated clinical symptoms. In the management of DGP, traditional Chinese medicine (TCM) offers distinct advantages, including demonstrated efficacy, favorable safety profiles, personalized treatment approaches, excellent patient tolerance, diverse dosage forms, and multi-target therapeutic effects. While the precise mechanisms remain to be fully elucidated, emerging evidence suggests that TCM may exert its beneficial effects through the protection of ICCs and GSMCs, modulation of brain-gut peptide dysregulation and neuropathy, regulation of inflammatory and immune responses, attenuation of oxidative stress, and restoration of gut microbiota homeostasis. Notably, current evidence supporting TCM for DGP leans on preclinical data with a focus on positive outcomes, whereas clinical evidence is sparse and largely limited to Chinese cohorts, reflecting the need for more clinical data and multi-cohort studies. This review comprehensively summarizes recent advances in understanding the mechanisms underlying TCM-mediated prevention and treatment of DGP, intending to provide a scientific foundation for expanding the clinical application of TCM in DGP management.
1 Introduction
Diabetic gastroparesis (DGP) is a common autonomic neuropathy of diabetes, characterized by delayed gastric emptying (GE) in the absence of mechanical outlet obstruction and other etiologies that may delay GE. Its main clinical symptoms include early satiety, nausea, vomiting, bloating, abdominal pain, diarrhea, and constipation, which further exacerbate impaired glucose regulation and malnutrition, thereby significantly affecting patients’ quality of life (1). Bharucha et al. (2) review indicated that up to 50% of patients with moderately controlled type 1 and type 2 diabetes mellitus (DM) have GE. The exact pathogenesis of DGP has not been fully elucidated, but current research suggests its pathological processes involve multiple factors: structural and functional damage to interstitial cells of Cajal (ICCs) and gastric smooth muscle cells (GSMCs); abnormal secretion of brain-gut peptides and neuropathy; inflammatory responses and immune dysregulation; oxidative stress; and gut microbiota imbalance. It is worth noting that these pathogenic factors are not only closely associated with chronic hyperglycemia but also interact with each other to jointly promote the occurrence and progression of DGP (3, 4).
Based on the above pathogenesis, common treatment methods for DGP in Western medicine include gastroprokinetic agents, antiemetics, antacids, antibiotics, gastric electrical stimulation, and new surgical treatments represented by gastric per-oral endoscopic myotomy (2, 5). However, these methods may lead to drug resistance, poor efficacy, and other adverse effects such as arrhythmia, hyperprolactinemia, bleeding and ulceration, which limit their clinical application in patients with DGP (2, 6). Therefore, there is a need to identify safer and more effective therapeutic strategies to treat DGP.
Traditional Chinese Medicine (TCM) has a 2000-year-long history in managing gastrointestinal disorders, such as refractory nausea, vomiting, and abdominal distension. According to TCM theory, DGP can be classified into different categories based on its clinical symptoms, such as “Xiao Ke disease” (wasting-thirst disease) combined with “Pi Man” (abdominal distension), “Wei Tong” (epigastric pain) and “Ou Tu” (vomiting). The fundamental pathogenesis of DGP resides in the spleen-stomach dysfunction, defined by imparied transportation and transformation, which induces phlegm turbidity, blood stasis, and “Qi” (the vital energy that circulates throughout the body) stagnation (7). Based on this theoretical framework, modern Chinese medicine scholars have proposed new perspectives. For instance, Professor Cui Shusheng suggests that the primary disease locations in DGP are the spleen, stomach, and intestines, with significant connections to the liver, lungs, and kidneys. In the early stage, disharmony in the descending function of the lungs and stomach leads to fluid injury and dryness-heat, warranting treatment by regulating the lungs and stomach simultaneously to moisten dryness and unblock the intestines. In the intermediate stage, impaired liver Qi flow triggers upward stomach Qi counterflow, which is treated by soothing the liver, regulating the spleen, harmonizing the stomach, and guiding the counterflow downwards. In the late stage, there is a dual deficiency of “Qi” and “Yin” (the nourishing, cooling, and grounding aspect of the body that counters the active, warm, and outward energy of “Yang”), coexisting with blood stasis and turbidity. At this stage, treatment focuses on tonifying “Qi,” nourishing “Yin,” eliminating stasis, and resolving turbidity (8). Professor Tong Xiaolin argues that DGP can be divided into an acute phase and a remission phase. The acute phase primarily involves the inversion of stomach “Qi,” “Yang” (the active, warm, and stimulating energy that drives bodily processes) deficiency in the spleen and stomach, with the main therapy focus on relieving symptoms such as vomiting and stomach distention. Commonly used TCM herbs include Xiaobanxia Decoction, Zhizhu Decoction. During the remission phase, the most common TCM syndrome patterns include cold deficiency in the “Middle Jiao” (the middle region of the body associated with the spleen and stomach, responsible for digestion, absorption, and the transformation of food into energy and nutrients to sustain bodily nourishment and vitality), and spleen-kidney “Yang” decline. The treatment principles focus on warming “Yang” to dissipate cold and boosting “Qi” to strengthen the spleen, with representative prescriptions such as Fuzi Lizhong Decoction and Xuanfu Daizhe Decoction (9). Beyond the oral administration of TCM herbal formulations, acupuncture and moxibustion exert notable bidirectional regulatory effects in gastrointestinal disorders, effectively restoring balance to both hyperactive and weakened digestive functions. Zusanli (ST-36), a primary acupoint for gastric disorders, regulates spleen-stomach function and promotes gastrointestinal motility. Sanyinjiao (SP-6), where the three “Yin” meridians converge, strengthens the spleen, harmonizes the stomach, and nourishes the “Yin” to moisten the intestines. Liangmen (ST-21), adjacent to the stomach, can relieve gastric reflux and dissolve food stagnation. Combined acupuncture of Zusanli, Liangmen, Sanyinjiao acupoints and so on, synergistically enhances spleen-stomach harmony and promotes “Qi” circulation to relieve stagnation (10).
In recent years, accumulating evidence has demonstrated that based on the theory of syndrome differentiation and treatment in TCM, the application of TCM therapies, including oral herbal formulations, external acupuncture and moxibustion, or a combination of internal and external treatments, can effectively alleviate the key symptoms of DGP, such as nausea, vomiting, abdominal distension, and pain. Notably, TCM interventions are characterized by minimal invasiveness, high safety, and low recurrence rates, underscoring their significant potential in DGP management (11–14). However, from a pathophysiological perspective, the mechanisms underlying the therapeutic efficacy of TCM in DGP remain incompletely elucidated, which has limited its evidence-based validation and broader clinical adoption. To the best of our knowledge, this review represents the first comprehensive analysis to systematically elucidate the multifaceted mechanisms of TCM in DGP treatment, including the protection of ICCs and GSMCs, and the modulation of brain-gut peptides dysregulation and neuropathy, the regulation of inflammatory and immune responses, inhibition of oxidative stress, and improvement of gut microbiota imbalance. This work not only deepens our understanding of the therapeutic potential of TCM in treating DGP but also provides a scientific basis for further clinical application. By integrating TCM theories with modern biomedical research, this review bridges the gap between empirical knowledge and scientific validation, offering a holistic perspective on the therapeutic mechanisms of TCM in treating DGP.
2 Mechanisms of TCM in treating DGP
TCM ameliorates diabetic gastroparesis through multiple mechanisms: alleviating interstitial cells of Cajal (ICCs) injury, regulating brain-gut peptides and neuropathy, attenuating gastric smooth muscle cells (GSMCs) damage, modulating inflammation/immunity, inhibiting oxidative stress, and restoring gut microbiota homeostasis, thereby improving gastrointestinal motility (Table 1).
2.1 Reducing ICCs injury
The interstitial cells of Cajal (ICCs) are specialized mesenchymal cells located throughout the gastrointestinal tract. As pacemaker cells, ICCs are responsible for generating and propagating slow-wave electrical potentials, which are critical for coordinating the rhythmic contractions of GI smooth muscle (15). ICCs also serve as critical intermediaries between enteric neurons and smooth muscle cells (SMCs). They facilitate the transmission of both excitatory and inhibitory signals that govern smooth muscle activity (16). The impairment or loss of ICCs disrupts antral peristaltic function, resulting in diminished gastric motility and subsequent delays in gastric emptying (17). Studies have also identified a significant decrease in the number of ICCs and ultrastructural alterations, including outer membrane rupture, intracellular mitochondria shrinkage, membrane wrinkling, cristae disappearance in the gastric tissue of rats with DGP (18, 19). Moreover, studies have demonstrated a significant loss of ICCs in DGP patients compared to controls (20). These ICCs impairments were observed in the stomach, jejunum, and colon of individuals with diabetic gastroenteropathy, irrespective of diabetes type (type 1 or type 2) (21). Therefore, reducing ICCs injury can be an important target for preventing and treating DGP.
It has been confirmed that TCM interventions can effectively improve DGP by decreasing ICCs injury (Figure 1). Banxia Xiexin Decoction (BXD) is a classical prescription of traditional Chinese medicine that is composed of seven key herbs: Pinellia ternate (Thunb.) Makino (Banxia), Scutellaria baicalensis Georgi (Huangqin), Zingiber officinale Roscoe (Shengjiang), Panax ginseng C. A. Mey. (Rensheng), Coptis chinensis Franch. (Huanglian), Ziziphus jujuba Mill. (Dazao), and Glycyrrhiza uralensis Fisch (Gancao). Research has demonstrated that BXD can improve gastric motility in DGP mice by inhibiting the expression of advanced glycation end products (AGEs) and advanced glycation product receptors (RAGE), while promoting neuronal nitric oxide synthase (nNOS) expression to regulate ICCs proliferation (22). Additionally, research has also found that the ethyl acetate extract of the TCM herb Salsola collina (EES) can significantly enhance gastric emptying in DGP rats. This effect is mediated by improving the number and function of ICCs through the upregulating the c-Kit/SCF signaling pathway (23). The tyrosine-protein kinase kit (c-Kit) serves as a critical regulator of ICCs, mediating both their development and functional integration within the gastrointestinal tract. Through its activation by stem cell factor (SCF), c-Kit facilitates ICC formation and promotes the establishment of functional gap junctions between ICCs and SMCs, thereby maintaining coordinated visceral motility (24–26). Autophagy is a highly conserved, lysosome-dependent degradation pathway that transports intracellular proteins, damaged organelles, and other cellular components to lysosomes for breakdown and recycling, playing a critical role in preserving normal cellular function and ensuring a stable intracellular environment essential for cell survival (17). Excessive autophagy has been implicated in the pathogenesis of ICCs damage and dysfunction, contributing to diabetic gastroenteropathy and intestinal dysmotility under chronic hyperglycemic conditions (27). The level of P62 typically shows an inverse correlation with autophagic flux, as it is degraded during active autophagy (28). Atg5 has a critical function in autophagosome-lysosome fusion, while Beclin1 expression is widely regarded as a key biomarker of autophagy activation; therefore, Atg5 and Beclin1 proteins are upregulated during autophagy activity (29, 30). Research has shown that emodin, as the active component of the TCM herb Rheum palmatum L. (Dahuang), can upregulate the expression of c-Kit and P62 proteins, downregulate the expression of autophagy-related proteins Beclin1 and Atg5, thereby inhibiting excessive autophagy of colonic ICCs and improving colonic motility and intestinal defecation disorders in DGP rats (31).
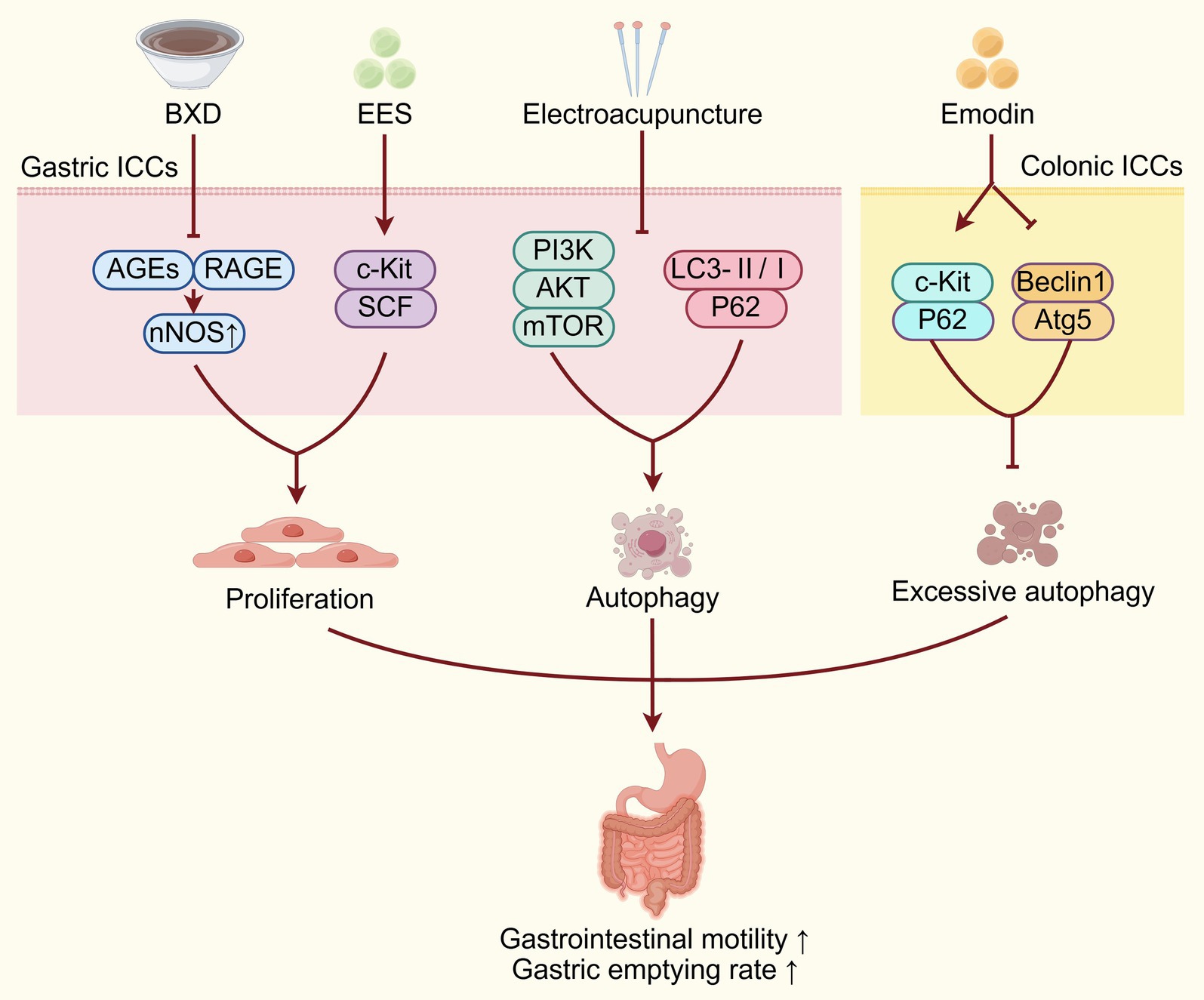
Figure 1. TCM improves DGP by reducing ICCs injury. TCM interventions act on gastric/colonic ICCs via diverse pathways (AGEs/RAGE, c-Kit/SCF, PI3K/Akt/mTOR, autophagy-related proteins) to regulate proliferation, autophagy, and inhibit excessive autophagy, improving DGP by alleviating ICCs injury. BXD, Banxia Xiexin Decoction; AGEs, advanced glycation end products; RAGE, receptor for advanced glycation end products; c-Kit, tyrosine-protein kinase kit; SCF, stem cell factor; ICCs, interstitial cells of Cajal.
However, autophagy exerts a complex and multifaceted role in the pathogenesis of gastrointestinal motility disorders, as both excessive and insufficient autophagy can act as key contributors to the disease (32). Zhang et al. (33) found that electroacupuncture (EA) at Zusanli, Sanyinjiao, and Liangmen acupoints can promote ICCs autophagy through downregulating the PI3K/Akt/mTOR signaling pathway, thereby improving gastric emptying. The specific mechanism is that EA can suppress the type I PI3K expression, thereby inhibiting phosphatidylinositol (3,4,5)- trisphosphate (PIP3) generation, Akt phosphorylation, and mTOR activation. Through this pathway, EA ultimately enhances autophagy-related gene expression and downstream substrate activity via inhibition of the mTOR-ubiquitin-proteasome pathway to promote cellular autophagy (33). Additionally, electroacupuncture at acupoints such as Zusanli, Sanyinjiao, and Liangmen has also been shown to enhance the autophagy of ICCs and reduce their apoptosis rate. This is evidenced by a decrease in the autophagic flux blockage marker—microtubule-associated protein 1A/1B-light chain 3 (LC3)-II/I ratio, and a reduction in the expression of P62 protein, thereby improving gastric motility in DGP rats (17). Apoptosis, a form of programmed cell death, is critical for regulating cell proliferation and tissue homeostasis. However, the precise mechanism linking autophagy and apoptosis in ICCs remains elusive. In conclusion, TCM interventions such as BXD, EES, emodin and electroacupuncture can ameliorate ICCs injury by regulating proliferation, autophagy and apoptosis, thereby exerting therapeutic effects on DGP.
However, most current studies on these TCM interventions for ICCs protection in DGP are limited by small sample sizes, lack of blinding, and potential bias. The possible reason may lies in standardizing TCM formulations and designing blinded trials for acupuncture, highlighting the need for larger-scale randomized controlled trials (RCTs) in future research.
2.2 Modulation of brain-gut peptides dysregulation and neuropathy
Brain-gut peptides constitute a class of highly bioactive molecules that are widely distributed across both the gastrointestinal tract and nervous system, serving as critical regulators of gastrointestinal motility through both excitatory and inhibitory mechanisms (34). Among these regulatory factors, ghrelin and gastrin (GAS) function as potent stimulators of gastrointestinal activity. They enhance digestive juice secretion and promoting smooth muscle contraction to facilitate the propulsion of gastric and intestinal conten while augmenting overall motility (35). Similarly, substance P (SP) and acetylcholine (ACh) act as excitatory neurotransmitters. They significantly contribute to gastrointestinal smooth muscle contraction, intestinal peristalsis, and luminal propulsion (36, 37). Conversely, inhibitory mediators such as somatostatin (SS) and cholecystokinin (CCK) suppress exocrine pancreatic secretion and bile production, consequently attenuating smooth muscle contractility and delaying gastrointestinal emptying (38). Vasoactive intestinal peptide (VIP) serves as an important inhibitory neurotransmitter that modulates gastrointestinal motility by affecting normal intestinal contraction and spontaneous pyloric contraction (23). Additional neurotransmitters, including 5-hydroxytryptamine (5-HT), dopamine (DA), and norepinephrine (NE), further contribute to motility regulation through complex interactions with enteric neurons and direct effects on gastric smooth muscle (39).
The sophisticated regulation of brain-gut peptide secretion and function is mediated by integrated neural networks encompassing the enteric nervous system (ENS), autonomic nervous system, and central nervous system, which operates through multi-layered control mechanisms (13, 40). In the context of diabetes mellitus, chronic hyperglycemia and its associated pathological consequences, including oxidative stress, systemic inflammation, tissue ischemia, and AGE accumulation, collectively contribute to the development of diabetic neuropathy. This manifests through multiple mechanisms: enteric plexus neuronal apoptosis, nerve demyelination, axonal degeneration, disruption of neuronal calcium homeostasis, loss of NOS-containing neurons, and impaired synaptic transmission between extrinsic autonomic neurons and SMCs (41–43). Clinical and experimental evidence supports the association between delayed gastric emptying and neuronal degeneration. This relationship is exemplified in ENS ablation murine models, which exhibit hypoganglionosis, a marked reduction of nNOS-expressing neurons in the gastric antrum, and delayed gastric emptying (44). These findings are further corroborated by clinical studies in human patients with DGP, where full-thickness gastric biopsies reveal significant reductions in circular muscle enteric nerve fibers (45). Mechanistic studies conducted in DGP murine models have revealed that activation of transient receptor potential vanilloid type 1 (TRPV1) channels in gastrointestinal vagal afferent fibers can stimulate nitric oxide (NO) release through modulation of nNOS, resulting in fundic relaxation and consequent improvement of delayed gastric emptying (46). Collectively, these findings highlight the fundamental role of brain-gut peptide dysregulation and neuropathic changes in the pathogenesis of DGP.
TCM can effectively improve DGP by modulating brain-gut peptides and neuropathy (Figure 2). For instance, the ethyl acetate extract of Salsola collina (EES) can significantly improve gastric emptying in DGP rats. Its mechanism is not only associated with regulating the number and function of ICCs, which serve as the mediator of neuromuscular transmission in the gastrointestinal tract, but also with modulating of brain-gut peptides levels, such as increasing serum ghrelin and GAS levels, while inhibiting the secretion of SS and VIP (23). Similarly, studies have shown that emodin enhances gastrointestinal motility by reducing excessive autophagy in ICCs and increasing serum SP while suppressing VIP concentrations (31). The combined employment of classic TCM herbs Coptis chinensis Franch. (Huanglian) and Pinellia ternate (Thunb.) Makino (Banxia) has specific effects on nerotransmitters. It increase the levels of SP, glucagon like peptide-1 (GLP-1), and 5-HT, while decreasing DA and NE levels. Further mechanism exploration has shown that the TCM intervention leads to opposing regulation of the MAPK/p70S6K/S6 signalling pathway in both the stomach and brain, thereby promoting gastric motility (39). Berberine, the main active ingredient of the TCM herb Huanglian, has been shown to promote the release of Ach in the intermuscular plexus of the gastric fundus smooth muscle by acting on calcium channels. This action helps improve gastric fundus contraction disorders and alleviate vacuolar lesions in the neuronal cells of the gastric fundus muscle plexus, thereby promoting gastric emptying in DGP rats (47). Moreover, Wan et al. (48, 49)have discovered that Jinqi Zhizhu Decoction (JZD), which consists of TCM herbs such as Hedysarum polybotrys Hand-Mazz. (Hongqi), Citrus aurantium L. (Zhike), Atractylodes macrocephala Koidz. (Baizhu), Coptis chinensis Franch. (Huanglian), Pinellia ternate (Thunb.) Makino (Banxia), and Endothelium Corneum Gigeriae Galli (Jineijin), could effectively reduce the gastric residual rate in DGP rats. Mechanism studies have discovered that he formula can increase serum MTL, plasma DA, ACh, 5-HT, GAS and SP levels. Furthermore, Chaiqin Wendan Decoction (CWD), a traditional Chinese herbal formulation composed of Bupleurum chinense DC. (Chaihu), Citrus reticulata Blanco. (Chenpi), Citrus aurantium L. (Zhishi), Ziziphus jujuba Mill. (Dazao), Bambusa tuldoides Munro. (Zhuru), Poria cocos (Schw.) Wolf (Fuling), Atractylodes macrocephala Koidz. (Baizhu), Paeonia lactiflora Pall. (Baishao), Salvia miltiorrhiza Bunge. (Danshen), Scutellaria baicalensis Georgi. (Huangqin), Pinellia ternate (Thunb.) Makino (Banxia), and Glycyrrhiza uralensis Fisch. ex-DC. (Gancao). This formula has shown therapeutic efficacy in DGP patients. Mechanistically, the formula can enhance gastric motility through increasing the serum MTL and GAS levels, while decreasing the CCK levels (50). Electroacupuncture has also demonstrated its efficacy in treating DGP by modulating brain-gut peptides dysregulation. For instance, Han Xu’s study revealed that electroacupuncture stimulation at the Zusanli acupoint (ST36) can upregulate key neurotransmitters, including nNOS, ChAT, and protein gene product 9.5 (PGP9.5) in gastric antrum tissues. This, in turn, helps reduce the loss of neurotransmitters and neurons in the ENS, significantly improving gastric motility in DGP rats (51). Both point moxibustion and electroacupuncture at the Zusanli, Sanyinjiao, and Liangmen acupoints can enhance the gastric emptying rate and intestinal propulsion in DGP rats. This effect is achieved by increasing the expression of eNOS mRNA and decreasing the expression of Angiotensin II (ATII) mRNA in the gastric antrum. Under pathological conditions, ATII can promote endothelial cells to secrete vasoconstrictor peptides and reduce eNOS activity, leading to gastrointestinal endothelial dysfunction and motility disorders (52). Additionally, electroacupuncture at the Zusanli, Sanyinjiao, and Liangmen acupoints can enhance gastric emptying by increasing the expression of ghrelin and GHSR mRNA in the gastric antrum (53). In summary, TCM treatment including Banxia Xiexin decoction, Jinqi Zhizhu Decoction, Chaiqin Wendan Decoction, the Huanglian-Banxia drug pair, EES, berberine, as well as point moxibustion and electroacupuncture, can help alleviate DGP by modulating brain-gut peptides and neuropathy.
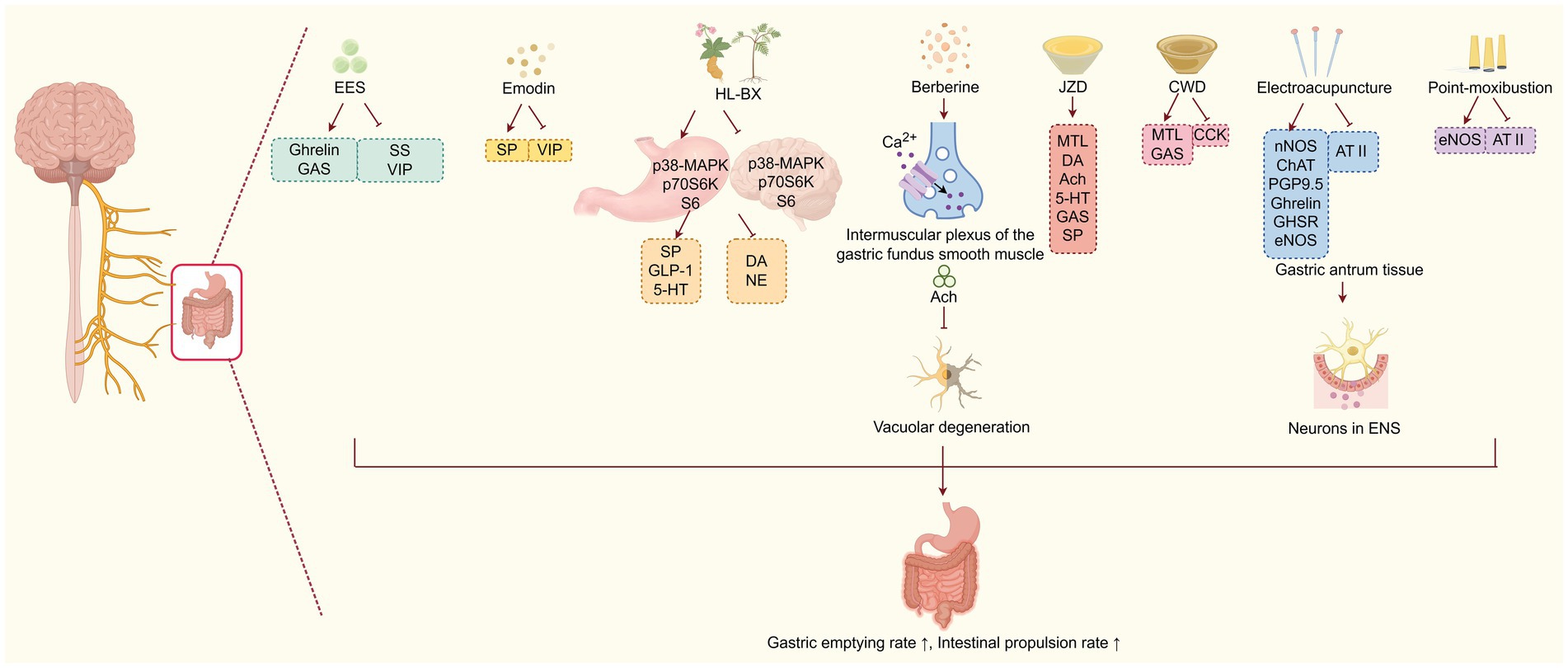
Figure 2. TCM improves DGP by modulating brain-gut peptides and neuropathy. Various TCM interventions (herbs, acupuncture, moxibustion) regulate brain-gut peptides and neural pathways, which suppress vacuolar degeneration and reduce the loss of neurons in the enteric nervous system (ENS), ultimately enhancing gastric emptying and intestinal propulsion.
Notably, existing studies in this area often suffer from small sample sizes and insufficient blinding, which may introduce bias. This is partly due to the complexity of measuring brain-gut peptide levels across diverse cohorts, and future research should prioritize well-powered, blinded RCTs to validate these findings.
2.3 Attenuating GSMCs damage
Gastric emptying is modulated not only by the nervous system and brain-gut peptides, but also involves coordinated interactions among GSMCs, ICCs, and various immune cell populations (19). GSMCs are key components of the gastric wall that mediate the rhythmic contractions and relaxations of the stomach. Since reduced propulsive contractions in the gastric antrum can impair the grinding, mixing, and expulsion of food into the duodenum, the proper function of these specialized cells is crucial for proper gastric emptying (54). Numerous studies have shown that prolonged exposure to hyperglycemic conditions induces apoptosis in GSMCs, resulting in significant alterations to gastric smooth muscle morphology and contractile function, which collectively contribute to the pathogenesis of DGP (55–58). Therefore, attenuating GSMCs damage may represent an important therapeutic target for the treatment of diabetic gastroparesis.
Emerging evidence has shown that TCM improves DGP by attenuating GSMCs damage (Figure 3). Research has shown that Hedysari Radix Polysaccharide (HRP), isolated from dried roots of the classic TCM herb Hedysarum polybotrys Hand-Mazz. (Hong qi), can help protect gastric antral SMCs from apoptosis and promote gastric smooth muscle contraction by activating the IGF-1/PI3K/AKT signalling pathway, thereby alleviating DGP (59). Additionally, HRP has been displayed to decrease the mRNA and protein expressions of NLRP3, Caspase-1, GSDMD, and IL-1β in gastric antrum tissue, and thus inhibit the pyroptosis of GSMCs and promote the gastric emptying (60). Pyroptosis is a caspase-dependent, inflammatory programmed cell death mediated by Gasdermin proteins. The inflammatory cascade triggered by the persistent high glucose and high-fat conditions of diabetes may lead to the pyroptosis of GSMCs in the gastric antrum, ultimately resulting in decreased gastric motility and impaired gastric emptying (61). Higenamine, a plant alkaloid first isolated in 1976 from the TCM herb Aconitum carmichaelii Debeaux. (fuzi) root and the main active ingredient in Fuzi Lizhong Decoction, has been shown to inhibit the apoptosis of GSMCs in DGP rats by activating the β2-AR/PI3K/AKT pathway (62). Furthermore, Wan et al. (49) demonstrated that Jinqi Zhizhu Decoction (JZD) effectively restored ultrastructural integrity in GSMCs of DGP rats, ameliorating multiple pathological alterations, including: (i) disrupted intercellular junctions, (ii) basement membrane fragmentation, (iii) organelle depletion, (iv) mitochondrial degeneration, and (v) cytoplasmic vacuolization in the gastric antral region. It also promoted the contraction of gastric smooth muscle by upregulating the RhoA/ROCK (Ras homolog family member A/Rho-associated kinase) signalling pathway in gastric tissue (48). In addition, low-intensity pulsed ultrasound stimulation (LIPUS) at acupoint ST36 (Zusanli) enhances the contractile ability of GSMCs in DGP rats by regulating the RhoA/ROCK and MALAT1/miR-449a/DLL1 signalling pathways, ultimately improving the gastric emptying (63). Studies have shown that in gastric smooth muscle tissue, activated RhoA can enhance MLC20 phosphorylation by binding to the MYPT1 subunit of its downstream effector molecule ROCK and Myosin Light Chain Phosphatase (MLCP), thereby promoting gastric smooth muscle contraction (64). Moreover, mechanistic studies have revealed that MALAT1 can promote phenotypic switching of GSMCs under high-glucose conditions through the miR-449a/DLL1 axis, thereby maintaining cell survival (65). In summary, TCM approaches such as oral administration of Hedysari Radix Polysaccharide, higenamine, Jinqi Zhizhu Decoction, and LIPUS, can protect GSMCs from apoptosis, pyroptosis, and other forms of injury by targeting multiple signalling pathways, showing therapeutic potential in improving gastrointestinal motility of DGP.
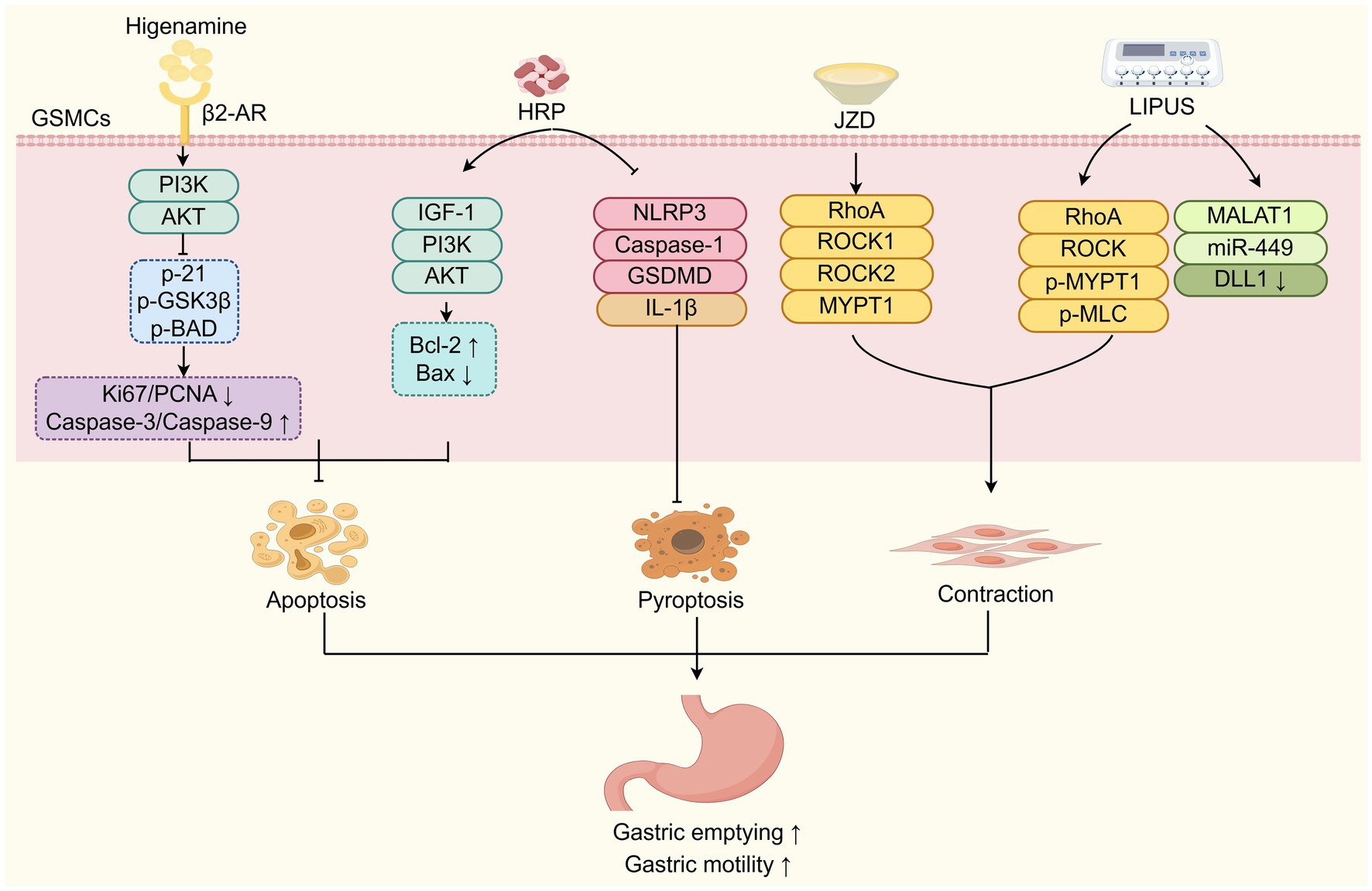
Figure 3. TCM improves DGP by attenuating GSMCs damage. TCM components (HRP, Higenamine, JZD) and LIPUS protect GSMCs from damage (apoptosis, pyroptosis) and enhance contraction via diverse signaling pathways to improve DGP. HRP: Hedysari Radix Polysaccharide; JZD: Jinqi Zhizhu Decoction; LIPUS: low-intensity pulsed ultrasound stimulation; GSMCs: gastric smooth muscle cells.
Nevertheless, current research on TCM-mediated GSMCs protection in DGP is constrained by small sample sizes and lack of blinding, with bias risks arising from inconsistent outcome measures, Future studies should focus on standardized protocols and multi-center blinded trials to enhance evidence reliability.
2.4 Regulating inflammation and immunity
There is accumulating evidence that demonstrates a significant association between elevated systemic inflammatory cytokines, such as IL-6, IL-8, IL-10, IFN-γ, and TNF-α and impaired gastrointestinal motility (66–68). Mechanistic studies reveal that pro-inflammatory cytokines, particularly TNF-α, IL-1β, and IL-6, contribute to hyperglycemia-induced enteric neuronal apoptosis and disruption of the ICC network both structurally and functionally (67, 68). In contrast, IL-10 has been demonstrated to enhance gastric emptying in diabetic mice by upregulating the anti-inflammatory cytokine heme oxygenase 1 (HO-1) levels and restoration of ICC network integrity (69). Reduced IL-10 levels may exacerbate the detrimental effects of pro-inflammatory cytokines on intestinal mucosa, promoting localized inflammation, cytotoxic responses, and subsequent immune dysregulation (70). Clinical trials and experimental studies further support these findings. Gastric biopsies from both DGP patients and animal models consistently show a decrease in anti-inflammatory macrophages, along with upregulated pro-inflammatory macrophage-associated gene expression. This suggests that macrophage-mediated immune imbalance and inflammatory responses play a crucial role in the development of delayed gastric emptying in diabetes (71, 72). Given these pathophysiological mechanisms, TCM interventions may present a promising therapeutic strategy for DGP.
Multiple studies have demonstrated that TCM improves DGP by regulating inflammation and immunity (Figure 4). Yang et al. (73) study found that Banxia Xiexin Decoction can reduce the levels of inflammatory factors such as IL-6, IL-8, and TNF-α. It also upregulates the levels of the anti-inflammatory factor IL-10, as well as immune-related indicators like IgG, SIgA, CD4, CD8, and the CD4/CD8 ratio in the intestinal mucosa. CD8 molecules are mainly expressed in cytotoxic T lymphocytes (CTLs) and play an essential role in cellular immunity, while CD4 molecules assist B cells in antibody production and can also induce inhibition of T cell functions by secreting cytokines. The CD4/CD8 ratio serves as a key indicator of a stable immune environment in the body, with any increase or decrease reflecting changes in immune function (73). Immunoglobulin G (IgG) is the primary protective immunoglobulin in the human body, preventing the invasion of foreign pathogenic microorganisms and antigens. In contrast, SIgA is the major immunoglobulin in the intestinal mucosa that defends against the invasion of commensal bacteria and pathogens, playing a crucial role in maintaining intestinal mucosal homeostasis. A local depletion or reduction of SIgA can lead to recurrent intestinal infections, which stimulate pro-inflammatory cytokines including IL-6, IL-8, and TNF-α, ultimately impairing gastrointestinal motility and gastric emptying (74, 75). These effects reverse pathological changes in the intestinal mucosal muscle layer of DGP rats, including atrophy, necrosis, submucosal degeneration, and inflammatory cell infiltration, thereby improving gastrointestinal motility and gastric emptying (73). Huang et al. (76) research demonstrated that the Spleen-Strengthening and Stomach-Regulating Plaster for umbilical application combined with the oral administration of the modified Sini Hewei Anshen Decoction (SHAD). SHAD includes the TCM herbs such as Bupleurum chinense DC. (Chaihu), Paeonia lactiflora Pall. (Baishao), Citrus aurantium L. (Zhike), Atractylodes macrocephala Koidz. (Baizhu), Citrus reticulata Blanco (Chenpi), Aucklandia costus Falc. (Muxiang), Panax ginseng C. A. Mey. (Renshen), Dioscorea opposita Thunb. (Shanyao), Polygala tenuifolia Willd. (Yuanzhi), Acorus tatarinowii Schott. (Shichangpu), and Glycyrrhiza uralensis Fisch. ex DC. (Gancao). This combined intervention effectively improved the Gastrointestinal Clinical Symptom Index (GCSI) score and gastric emptying time in patients with DGP associated by anxiety due to liver-stomach disharmony. These improvements were achieved by reducing the serum levels of inflammatory factors such as TNF-α and IL-6 (76). Chaihu Shugan San, a TCM decoction consisting of Paeonia lactiflora Pall. (Baishao), Bupleurum chinense DC. (Chaihu), Citrus aurantium L. (Zhike), Cyperus rotundus L. (Xiangfu), Citrus reticulata Blanco. (Chenpi), Ligusticum chuanxiong Hort. (Chuanxiong), and Glycyrrhiza uralensis Fisch. ex-DC. (Gancao), is a classical prescription to promote Qi and blood circulation. Guo et al. (77) have discovered that Chaihu Shugan San decoction in combination with acupuncture at specific acupoints (Zusanli and Guanyuan as main acupoints, Zhongwan and Fenglong as auxiliary acupoints), can invigorate the spleen and stomach, and soothe the liver and relieve depression. Mechanism exploration has found that this combined TCM intervention can reduce serum TNF-α and IL-6 levels, while increasing IL-10 levels to increase gastric motility and improves the clinical symptoms of DGP patients. Similarly, Xiao et al. (78) discovered that electroacupuncture at Zusanli, Liangmen, and Sanyinjiao acupoints could significantly reduce the levels of IL-18, IL-1β and the NLRP3 inflammasome in gastric antrum tissue, leading to a significant increase in gastric emptying rate and small intestinal propulsion rate in DGP rats. Additionally, electroacupuncture at these acupoints could repair the pathological damage in the gastric antrum tissues and improve gastric emptying. Mechansitically, this intervention has been found to inhibit NLRP3/caspase-1/GSDMD pathway to block pyroptosis in DGP murine model (79). In summary, these studies demonstrate that TCM approaches, such as Banxia Xiexin Decoction, Sini Hewei Anshen Decoction, Chaihu Shugan San, Spleen-Strengthening and Stomach-Regulating Plaster for umbilical application, and electroacupuncture at specific acupoints, can effectively reduce inflammatory factors, modulate immune responses, enhance gastrointestinal motility, and thereby prevent and treat DGP.
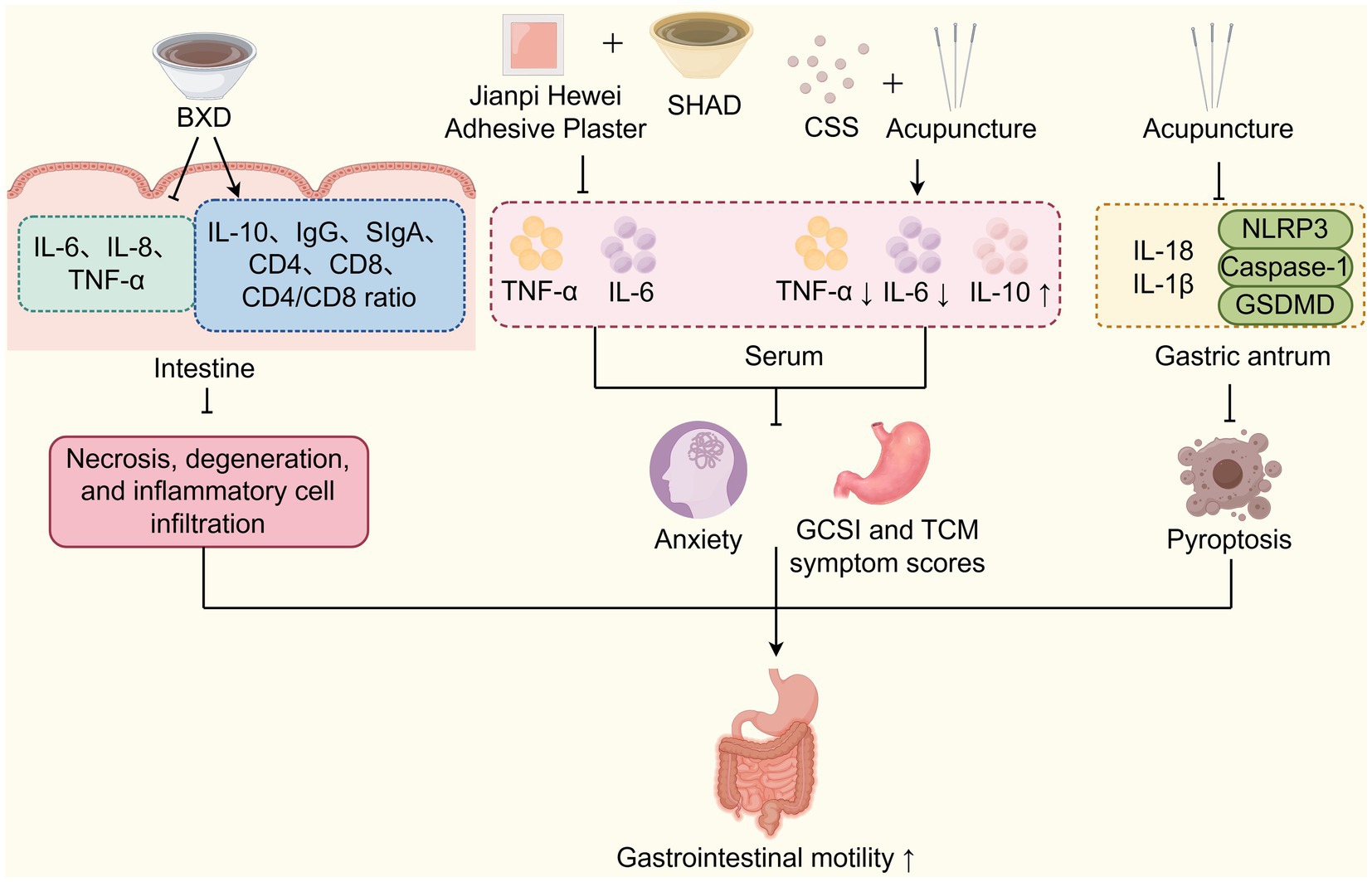
Figure 4. TCM improves DGP by regulating inflammation and immunity. TCM interventions (BXD, Jianpi Hewei Adhesive Plaster, SHAD, CSS, and acupuncture) regulate inflammation, immunity, and pyroptosis via cytokine modulation to improve DGP-related gastrointestinal motility. SHAD: Sini Hewei Anshen Decoction; CSS: Chaihu Shugan San.
However, most of these studies have small sample sizes and lack adequate blinding, leading to potential bias. This is partly because of the difficulty in blinding umbilical plaster and acupuncture interventions, and future work should explore innovative blinding strategies alongside larger cohort studies.
2.5 Inhibiting oxidative stress
Oxidative stress refers to an imbalance between the production of oxygen free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), and the body’s antioxidant defense system, leading to tissue damage. While contributing to the development of various complications of diabetes, oxidative stress also triggers gastric mucosal damage and gastric motility disorders, playing a significant role in the onset and progression of DGP (54, 80, 81). Impaired formation of the ICCs network and extracellular matrix remodeling of GSCMs due to oxidative stress have been identified as key causes of DGP (54, 82). In DGP rats, gastric motility is impaired, with increased levels of malondialdehyde (MDA), a marker of oxidative stress in gastric tissue, and decreased levels of serum superoxide dismutase (SOD), an antioxidant enzyme (83). Additionally, a decline in HO-1-positive macrophages has been reported in rat models of DGP, suggesting that increased oxidative stress and failure of the antioxidant response may play an important role in the pathogenesis of DGP (84).
Through the inhibition of oxidative stress, multiple kinds of TCM compounds can attenuate diabetic gastroparesis (Figure 5). Guo et al. (85) have found that Hedysari Radix Polysaccharide (HRP) can reduce the levels of Kelch-like ECH-associated protein 1 (Keap1) mRNA while increasing the levels of Nuclear factor erythroid 2-related factor 2 (Nrf2), HO-1, thioredoxin (Trx) mRNA, glutathione S-transferase (GST), and quinone oxidoreductase 1 (NQO1) proteins. Consequently, HRP reduces the levels of ROS, MDA and 8-hydroxydeoxyguanosine (8-OHdG), while enhancing the levels of SOD in DGP rats. These findings have suggested that HRP plays a crucial role in DGP therapeutics by inhibiting oxidative stress, repairing small intestinal mucosal damage, and improving gastric emptying and small intestinal propulsion rates. Alpinia officinarum Hance (Gaoliangjiang) and P. cablin (Blanco) Benth (Guanghuoxiang) are TCM plants that have been used as natural remedies for managing diabetes and gastrointestinal issues. According to TCM theory, their therapeutic mechanisms involve the regulation of spleen-stomach meridians and promoting gastric motility. A recent study has demonstrated that A. officinarum Hance-P. cablin (Blanco) Benth (AP) drug pair can inhibit the GSMCs apoptosis by reducing oxidative stress levels through downregulating the AGE/RAGE axis, thereby increasing the gastric emptying rate (86). Curcumin, the main active ingredient of turmeric isolated from the TCM plant Curcuma Longa L. (Jianghuang), has been shown to inhibit the ICCs apoptosis and improve gastric emptying through attenuating oxidative stress and nuclear factor kappa B (NF-κB) activation in gastric tissues of DGP rats (87). Furthermore, recent studies have demonstrated that antioxidant compounds, such as curcumin and cinnamaldehyde, an active constituent derived from the TCM herb Cinnamomum cassia Presl. (Rougui), can ameliorate inflammation and oxidative stress associated with obesity-induced chronic diabetes. This therapeutic effect subsequently restores nitrergic (NO-mediated) neural regulation of gastric motility and emptying function. The underlying mechanisms involve: (i) upregulation of Nrf2 expression and GSH/GSSG ratio, regulating oxidative stress marker genes expression (Txnip, Als2, Epx, and Mpo); (ii) downregulation of Toll-like receptor 4 (TLR4) signalling and suppression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6); (iii) restoration of nNOS cofactor BH4 synthesis via GCH-1 upregulation; and (iv) enhancement of nNOSα protein expression, dimerization, and soluble guanylate cyclase (sGC) activity. These effects were observed in wild-type mice, but not in Nrf2 knockout (Nrf2 KO) mice (88). The study conducted by Hui et al. (1) revealed that atractylenolide-1, one of the primary bioactive compounds of TCM herb Atractylodes macrocephala Koidz (Baizhu), can regulate oxidative stress responses and protect ICCs from apoptosis, and restore the gastric tissue network structure in the DGP rat model by activating the SCF/c-kit signalling pathway. Moreover, electroacupuncture combined with “Zhuang” medicinal thread moxibustion, a unique moxibustion practice of the Zhuang ethnic group in China, was found to inhibit oxidative stress-induced damage to DGP rat model. This was achieved through the upregulation of expression of HO-1, Nrf2, PGC-1α proteins and mRNA, and promotion of Nrf2 nuclear translocation in gastric antrum tissue (89). Acupuncture targeting the Baihui (GV20), Shenting (GV24), Zhongwan (CV12), Zusanli (ST36), Hegu (LI14), and Taichong (LR3) acupoints, in combination with oral use of domperidone, has been shown to reduce the serum levels of ROS, MDA, TNF-α, IL-6 and IL-1β levels, while increasing the SOD activity, thereby inhibiting oxidative stress and inflammatory responses. As a result, it alleviates clinical symptoms and improves the gastric emptying rate in DGP patients diagnosed with a liver stagnation and spleen deficiency pattern (90). These findings suggest that TCM approaches, including the oral administration of Hedysari Radix Polysaccharide, curcumin, cinnamaldehyde, oral or external use of A. officinarum Hance-P. cablin (Blanco) Benth drug pair, as well as electroacupuncture and moxibustion, can effectively treat DGP by reducing oxidative stress levels, decreasing inflammation, and mitigating damage to ICCs, GSMCs, and gastric smooth muscle.
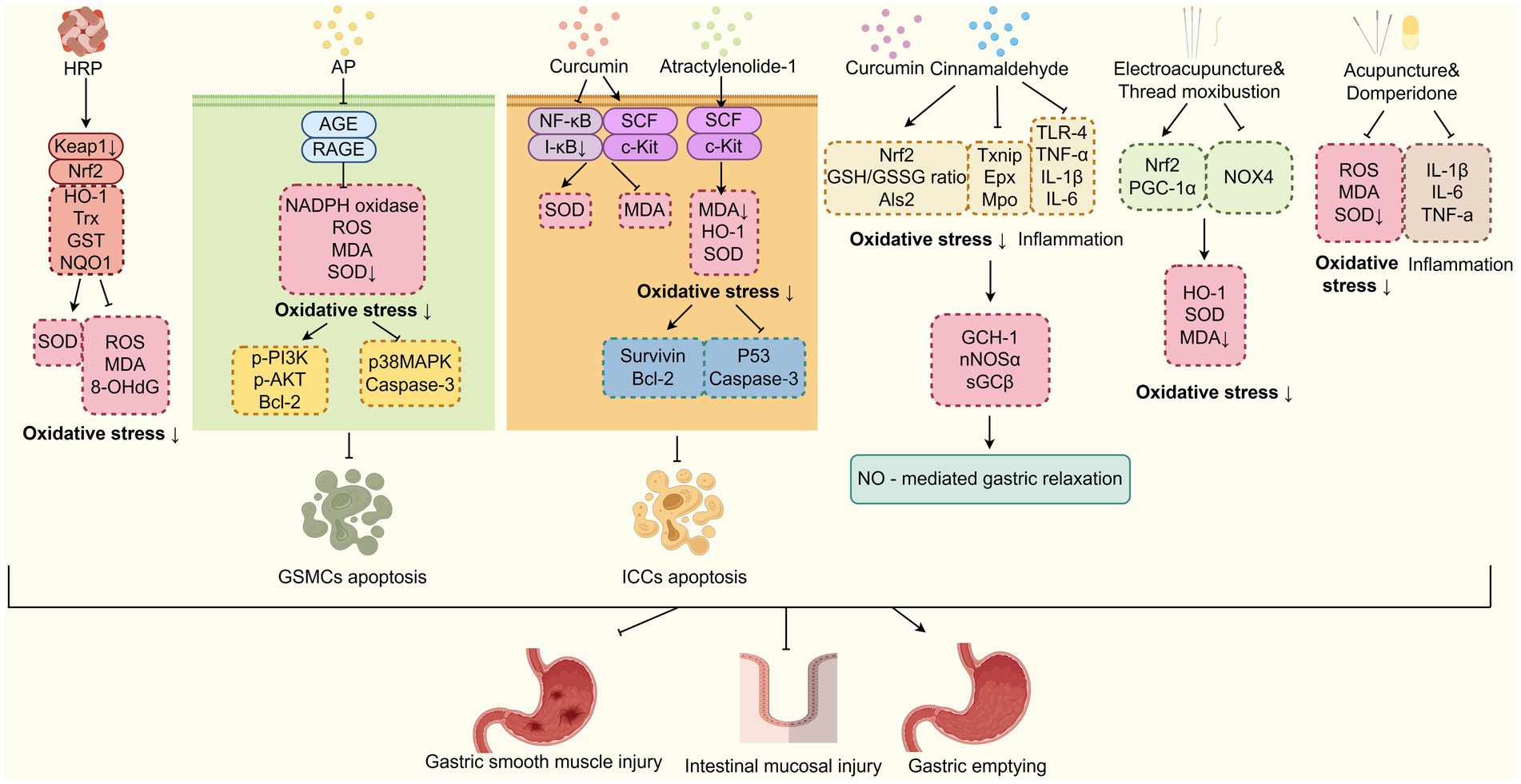
Figure 5. TCM improves DGP by inhibiting oxidative stress. TCM-based interventions (HRP, AP, curcumin, etc.) mitigate DGP. They inhibit oxidative stress via pathways, such as Nrf2, AGE/RAGE, and NF-κB, to reduce apoptosis of GSMCs/ICCs and improve gastric function. HRP, Hedysari Radix Polysaccharide; AP, Alpinia officinarum Hance-P. cablin (Blanco) Benth; Nrf2, Nuclear factor erythroid 2-related factor 2.
Notably, current studies on TCM inhibiting oxidative stress in DGP are limited by small samples and lack of blinding, with bias risks from variable oxidative stress marker detection methods. Future research should adopt standardized assays and large-scale blinded RCTs to strengthen evidence.
2.6 Regulating gut microbiota imbalance
The gut microbiota refers to the microorganisms present in the human digestive system, including up to 1,000 species of bacteria and trillions of individual microorganisms. Under normal circumstances, the gut microbiota plays important roles in digestion and absorption, immune protection, and nutrient synthesis (91). It is closely involved in the absorption, secretion, and metabolism of the digestive tract. When the balance of the gut microbiota is disrupted, it can lead to the development of various diseases. Studies have found a strong link between diabetes and the gut microbiota (92, 93). Individuals with diabetes often exhibit decreased levels of beneficial probiotics such as Lactobacillus and Bifidobacterium, while pathogenic and potentially harmful bacteria, including Gram-negative bacteria and Enterobacter, become more abundant. This microbial imbalance can disrupt intestinal barrier function, increase mucosal permeability and trigger systemic inflammation, leading to increased endotoxin production and gastrointestinal dysfunction, contributing to the development of DGP (92–94). In addition, the gut microbiota, including Bifidobacterium, Bacteroides, and Prevotella, can break down polysaccharides that are difficult for the human body to absorb, generating short-chain fatty acids. These short-chain fatty acids can activate G protein-coupled receptors 41 and 43, promoting the secretion of 5-HT, which works in coordination with the ENS, muscles, and the network of ICCs to regulate the gastrointestinal secretion and intestinal motility (39, 74, 95). Dysbiosis of the gut microbiota can also affect the secretion of GLP-1, bile acid metabolism, vitamin B12 synthesis, TLR4/NF-κB signalling pathway, thereby exacerbating insulin resistance, glucose tolerance abnormalities, intestinal inflammatory response, and oxidative stress, ultimately leading to delayed gastric emptying (74). Therefore, regulating the gut microbiota imbalance may be a novel target for the treatment of DGP.
TCM improves DGP by restoration of gut microbiota homeostasis (Figure 6). For example, Xu et al. (73, 96) discovered that in DGP murine model, gut microbiota imbalance elevated pro-inflammatory cytokines, disruption of the intestinal mucosal immune barrier, and gastrointestinal motility dysfunction. BXD was found to reverse these conditions by increasing the abundance of beneficial bacteria such as Bacteroides, Bifidobacterium, Enterococcus, and Lactobacillus, while reducing the number of pathogenic bacteria Enterobacter. Additionally, as mentioned above, BXD enhances serum levels of IgA, IL-10, CD4, CD8, and CD4/CD8 ratio levels, while reducing serum endotoxin, intestinal IgG, IL-6, IL-8, and TNF-α levels. These findings suggest that BXD improves gastrointestinal motility through multiple mechanisms, including alleviating intestinal tissue inflammation, repairing the mucosal immune barrier, and regulating gut microbiota imbalance. Alpinia officinarum Hance (AOH), a plant from the Zingiberoside family rich in diarylheptanoids and flavonoids, has been widely used in TCM for thousands of years. Research has shown that it can significantly accelerate gastrointestinal propulsion rate in DGP rats by rebalancing the gut microbiota, such as increasing the proportion of Firmicutes and the relative abundance of Proteobacteria and Lachnospiraceae_NK4A136_group, reducing the relative abundance of Bacteroidota (97). FoxiangSan, formulated by National TCM Master Professor Lv Renhe, comprises Citrus medica L.var.socdactylis Swingle (Foshou), Citrus medica L. (Xiangyuan), Cyperus rotundus L. (Xiangfu), Citrus Reticulatae Pericarpium (Chenpi), Inula japonica Thunb. (Xuanfuhua), Lindera aggregata (Sims) Kosterm. (Wuyao), and Pseudostellaria heterophylla (Miq.) Pax ex Pax et Hoffm. (Taizishen), has been demonstrated to improve gastric emptying in DGP rats by decreasing the abundance of Bacteroidetes, Actinobacteria, and Helicobacter pylori, increasing the abundance of Firmicutes and Lachnospiraceae (98). Research has shown that Actinobacteria play an essential role in maintaining the balance of gut microbiota (99, 100). Furthermore, Helicobacter pylori infection was found in 74.6% of patients with DGP, significantly higher than in the diabetic-only and normal control groups (101). Thus, FoxiangSan may enhance gastric emptying in diabetes by regulating the levels of Helicobacter pylori and Actinobacteria. Electroacupuncture on acupoints such as Zhongwan (CV12), Tianshu (ST25), Zhongfu (LU1), bilateral Zhangmen (LR13), bilateral Taibai (SP3), bilateral Taiyuan (LU9), bilateral Zusanli (ST36), and bilateral Shangjuxu (ST37), based on the TCM theory of “ascending lucidity and descending turbidity,” were found to upregulate the relative abundance of beneficial bacteria (Proteobacteria, Olsenella and Christensenellaceae R-7), and inhibit the relative abundance of pathogenic bacteria,(Pseudomonas and Alkalibacterium) in patients with diabetic gastrointestinal dysfunction (102). Olsenella, a genus of Gram-positive, anaerobic, non-spore-forming bacteria belonging to the family Atopobiaceae (phylum Actinobacteria), is closely associated with gastrointestinal immune function (103). Christensenellaceae R-7 is a strain within the family Christensenellaceae (phylum Firmicutes). As a probiotic factor, it shows a significant negative correlation with inflammatory reactions and metabolic diseases (104). Therefore, the “increasing clearance and reducing turbidity” approach may enhance the gastrointestinal inflammatory immune response by boosting the abundance of certain probiotics, thus alleviating gastrointestinal dysfunction in diabetic patients. In conclusion, these studies suggest that TCM, such as Banxia Xiexin decoction, FoxiangSan, and electroacupuncture can effectively treat DGP by promoting the abundance of beneficial gut microbiota and suppressing harmful microbiota. It should be noted that BDX, FoxiangSan, and AOH exert distinct regulatory effects on the relative abundance of Bacteroidota, with BDX upregulating its abundance while FoxiangSan and AOH downregulate it. Bacteroidota plays a critical role in modulating gastric emptying through multiple mechanisms, including impacting short-chain fatty acid production, bile acid metabolism, and gut immune function (105–107). Previous studies have demonstrated variable effects of Bacteroidota on either promoting or delaying gastric emptying, which may be attributed to differences in microbial composition and host microenvironmental factors (107, 108). Consequently, future research should integrate metagenomic analyses with functional validation studies, including bacterial strain colonization experiments, to further elucidate the precise mechanisms through which TCM regulate the gut microbiota for the treatment of DGP.
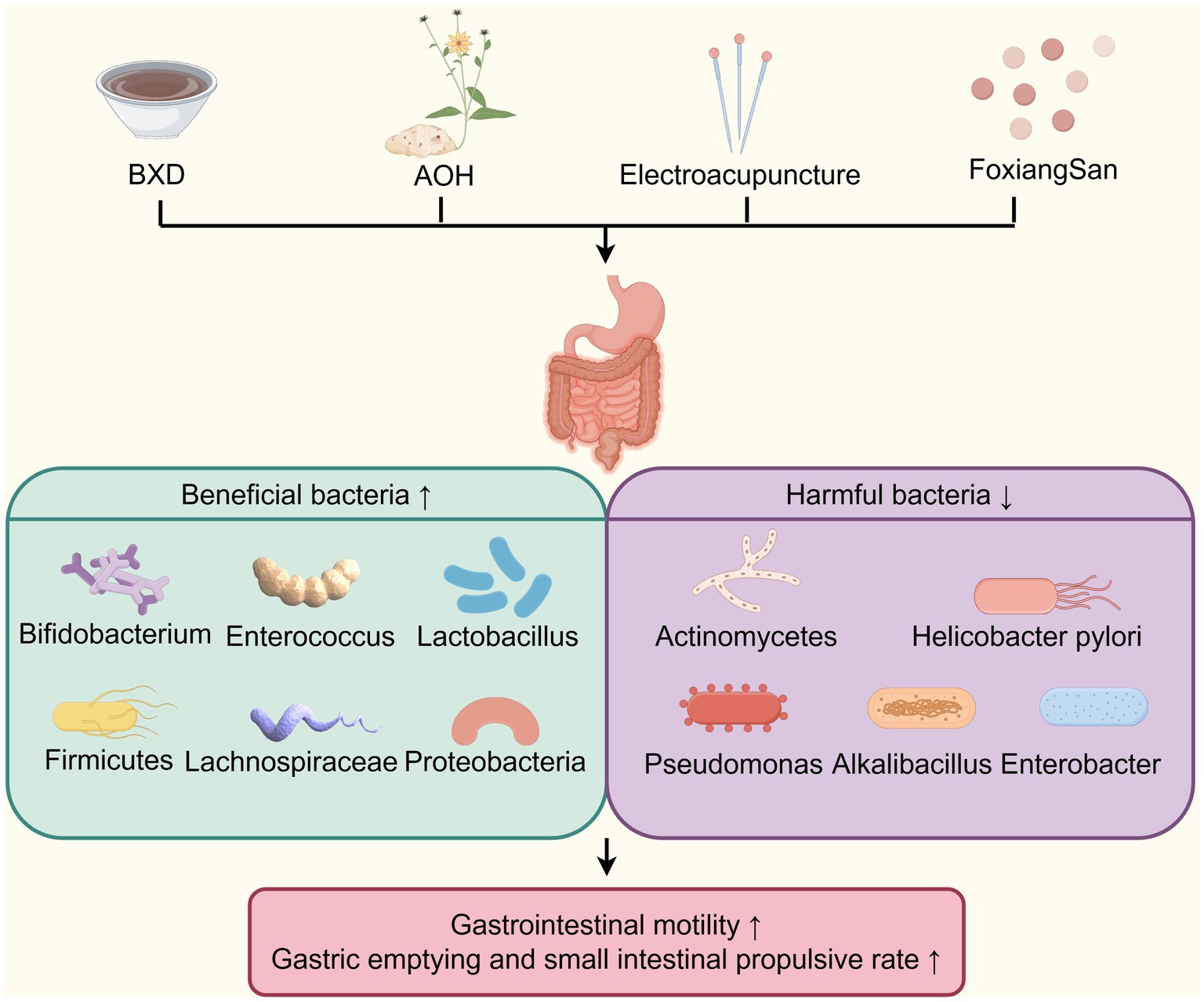
Figure 6. TCM improves DGP by restoration of gut microbiota homeostasis. TCM (BXD, AOH, Electroacupuncture and FoxiangSan) improves DGP by restoring gut microbiota homeostasis, enriching beneficial bacteria (e.g., Bifidobacterium) and depleting harmful ones (e.g., Helicobacter pylori) to enhance gastrointestinal motility. AOH, Alpinia officinarum Hance.
Additionally, most existing studies on TCM regulating gut microbiota in DGP have small sample sizes and lack blinding, introducing potential bias. This is due in part to the high cost of metagenomic analyses in large cohorts, and future work should balance depth of microbial analysis with larger sample sizes to improve result generalizability.
3 Conclusion and perspective
DGP, a common manifestation of diabetic autonomic neuropathy, is characterized by impaired gastric motility and delayed gastric emptying. Affected patients frequently present with symptoms such as postprandial fullness, early satiety, chronic nausea, and other gastrointestinal disturbances, all of which substantially diminish their quality of life. Current therapeutic approaches, including prokinetic medications and surgical interventions, primarily focus on restoring gastric motility and managing symptoms, with limited attention to the disease’s underlying pathogenesis. Their efficacy remains suboptimal, often accompanied by adverse effects and high recurrence rates in certain patient populations. Therefore, there is an urgent need for treatment alternatives that are more targeted to the underlying mechanisms of DGP, and that are safer, more effective, and sustainable. In this review, based on the available evidence and the latest research, we comprehensively evaluated the impact of TCM in both DGP patients and model animals, as well as the possible mechanisms involved. It has been demonstrated that TCM holds considerable promise in treating DGP, with multifaceted strategies including the oral administration of TCM decoctions, active ingredients from medicinal herbs, and external therapies including acupuncture and moxibustion. These approaches target multiple mechanisms, including the regulation of ICCs and GSMCs injury, brain-gut peptide dysregulation and neuropathy, inflammatory and immune responses, oxidative stress, and gut microbiota imbalance.
Despite these advantages, this review still has several limitations. Firstly, relatively few clinical trials explore the mechanisms of TCM in treating DGP, featuring small sample sizes and predominantly Chinese study populations. This imbalance between robust basic research and weak clinical evidence specifically restricts the clinical promotion of TCM for DGP: it fails to meet international evidence-based medicine standards, limits applicability across diverse ethnic groups, and impedes inclusion in global clinical practice guidelines. To bridge this gap, future studies should prioritize multicenter, large-sample randomized controlled trials, enroll multi-ethnic populations to improve generalizability, integrate syndrome differentiation into study designs for stratified analysis, and conduct long-term follow-ups to verify long-term efficacy and safety.
Secondly, while multiple studies have confirmed that TCM alleviates DGP through diverse mechanisms. For instance, it has been found that Banxia Xiexin Decoction modulates ICCs proliferation, gut microbiota, inflammatory responses, and immune dysregulation simultaneously. Howerver, key gaps persist, particularly regarding the bidirectional regulation of specific gut microbial genera (e.g., Bacteroidota) by different TCMs: Banxia Xiexin Decoction upregulates Bacteroidota abundance, whereas FoxiangSan and Alpinia officinarum Hance downregulate it. This regulatory difference may stem from the functional heterogeneity of Bacteroidota, as a high-impact study revealed that Bacteroidales, the dominant order in Bacteroidota, exhibit strain-specific fitness variations driven by differences in Acyl-CoA thioesterase activity and nucleotide polymorphisms regulating Acyl-CoA transferase, which further determine their sensitivity to metabolites including butyrate and ability to adapt to glycan environments. While we hypothesize that the multi-target effects of TCM, the theory has not yet been confirmed by direct experimental evidence. To clarify the specific role of Bacteroidota in TCM-mediated DGP relief will require functional validation experiments such as strain-level colonization in gnotobiotic mice and metabolomic analysis of Bacteroidota-derived metabolites.
Additionally, it has been demonstrated that the PI3K/AKT pathway exert opposing roles in the TCM treatment of DGP. To be specific, electroacupuncture ameliorates DGP by downregulating the PI3K/AKT/mTOR pathway to enhance ICCs autophagy, while Hedysari Radix Polysaccharide, Higenamine, and the A. officinarum–P. cablin drug pair treat DGP by activating the IGF and β2-AR to upregulate p-PI3K/p-AKT expression, inhibiting GSMCs apoptosis. The opposing regulation of PI3K/AKT in DGP may stem from its multifaceted roles in distinct pathophysiological processes, particularly driven by cell type heterogeneity and spatial compartmentalization of signaling molecules. Single-cell sequencing of gastric muscle tissue from gastroparesis patients reveals that muscularis macrophages (MM) exhibit altered expression of tissue-protective and stromal-activating genes, remodeling the microenvironment of ICCs and GSMCs and modulating their responsiveness to PI3K/AKT regulation. Moreover, AKT activation is strictly compartment-specific: electroacupuncture inhibits plasma membrane-localized AKT in ICCs to suppress mTORC1 and promote autophagy, while TCM components activate nuclear or endosomal AKT in GSMCs to regulate apoptotic transcription factors like FOXO1. This spatial-dependent pattern aligns with findings in gastric adenocarcinoma, where spatial transcriptomics shows cell microenvironment (e.g., tumor-stroma interface) dictates PI3K/AKT activity, supporting that cell localization and microenvironment together shape the pathway’s function in DGP. For example, PI3K/AKT/mTOR activation typically inhibits autophagy but suppresses apoptosis, reflecting dual regulatory effects, and TCM’s multi-target, multi-pathway properties also help explain this differentiation. However, it should be noted that further in-depth research is needed to clarify the exact role of PI3K/AKT in DGP and its potential regulatory network, as current hypothesis relies on indirect evidence from spatial transcriptomics and pathway correlation analyses rather than direct functional validation.
To further address these limitations and promote the application of TCM in the treatment of DGP and expand its global acceptance in evidence-based medicine, further efforts are warrant. In the context of current Western treatments for DGP, prokinetics (e.g., domperidone) often relieve symptoms but carry risks of extrapyramidal effects, gastric electrical stimulation (GES) is invasive and effective only in select patients, and surgery is reserved for severe, refractory cases. To tackle these drawbacks, TCM can serve as a complementary or alternative option, offering advantages of multi-target mechanism regulation, favorable safety profiles, and ability to address both symptoms and underlying pathogenesis, especially for patients intolerant to Western drugs or ineligible for invasive interventions. Among TCM’s multiple therapeutic mechanisms for DGP, ICCs protection may act as the core driver for improving gastric emptying, given ICCs’ essential role as gastric motility pacemaker cells, without intact ICCs function, other regulatory effects, such as the brain-gut peptide modulation, struggle to restore normal gastric motility. Gut microbiota regulation, by contrast, exerts a synergistic effect: it enhances ICCs protection indirectly via metabolites like short-chain fatty acids that support ICCs survival and function. Future studies can use ICCs knockout models or gut microbiota depletion approaches to verify this priority and synergistic relationship.
Furthermore, more focus should be put on conducting clinical randomized controlled trials that adhere to the principles of syndrome differentiation and treatment, while exploring the mechanisms of TCM from multiple dimensions. For complex Chinese herbal compounds like Banxia Xiexin Decoction, modern technologies can be leveraged to screen key active components: network pharmacology can construct “component-target-disease” interaction networks to predict core ingredients targeting DGP-related pathways (PI3K/AKT and inflammatory signaling,etc.), while metabolomics can track in vivo absorption, distribution, metabolism, and excretion of compounds to identify bioavailable active metabolites. Additionally, the synergistic mechanism of components, especially scientific validation of the “monarch-minister-adjuvant-courier” theory, requires further research. The future research may include the component knockout/addition experiments in DGP models to verify whether monarch herbs, (e.g., Pinelliae Rhizoma in Banxia Xiexin Decoction) play a core role and minister herbs (e.g., Scutellariae Radix), enhance efficacy or reduce toxicity.
In addition, by using modern techniques such as in situ hybridization, immunohistochemistry, and gene chips, we can explore the mechanism of action of natural products from TCM and study the direct targets of these candidate drugs. Alternatively, we can rely on modern analytical methods and translational models, such as using network pharmacology to analyze the potential targets of natural products from TCM. Moreover, it is essential to utilize high-throughput 16S rRNA gene sequencing and metagenomics to comprehensively characterize how TCM regulate the composition, diversity, and functional potential of the gut microbiota in patients with DGP, and observe whether specific beneficial bacterial genera are enriched or harmful bacterial genera are inhibited. At the same time, metabolomics should be employed to analyze serum, urine, and fecal samples to identify key metabolite changes induced by TCM treatment, such as short-chain fatty acids, tryptophan metabolites, bile acids, neurotransmitters and their precursors. This multi-omics integration approach is indispensable for mapping complex interaction networks. These will provide a necessary pre-clinical basis for the wide application of natural products from traditional Chinese medicine. Additionally, in TCM research on gastroparesis, organoids can mimic gastric tissue properties, and single-cell omics can analyze cellular heterogeneity; their combination facilitates mechanism elucidation. These efforts with novel research techniques will lay a more solid scientific foundation for the development and application of TCM as a complementary or alternative approach to conventional treatments in the prevention and treatment of DGP, ultimately providing patients, particularly those with limited efficacy or adverse effects from previous treatments, with safer and more effective therapeutic options.
Author contributions
HZ: Conceptualization, Formal analysis, Writing – original draft. QY: Validation, Writing – original draft. XF: Formal analysis, Writing – original draft. ZQ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (82304940), the Traditional Chinese Medicine Science and Technology Plan Project of Zhejiang Province (2022ZB102), and the Medical and Health Science and Technology Plan Project of Zhejiang Province (2023KY858).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li, H, Cao, W, Zhang, XB, Zhang, XX, Gu, C, Gu, LM, et al. Atractylenolide-1 alleviates gastroparesis in diabetic rats by activating the stem cell factor/c-kit signaling pathway. Mol Med Rep. (2021) 24:691. doi: 10.3892/mmr.2021.12331
2. Bharucha, AE, Kudva, YC, and Prichard, DO. Diabetic gastroparesis. Endocr Rev. (2019) 40:1318–52. doi: 10.1210/er.2018-00161
3. Jalleh, R, Marathe, CS, Rayner, CK, Jones, KL, and Horowitz, M. Diabetic gastroparesis and Glycaemic control. Curr Diab Rep. (2019) 19:153. doi: 10.1007/s11892-019-1281-8
4. Ohkuma, T, Iwase, M, Fujii, H, Ide, H, Komorita, Y, Yoshinari, M, et al. Defecation frequency and glycemic control in patients with diabetes: the Fukuoka diabetes registry. J Diabetes Complicat. (2021) 35:107751. doi: 10.1016/j.jdiacomp.2020.107751
5. Shakhatreh, M, Jehangir, A, Malik, Z, and Parkman, HP. Metoclopramide for the treatment of diabetic gastroparesis. Expert Rev Gastroenterol Hepatol. (2019) 13:711–21. doi: 10.1080/17474124.2019.1645594
6. Pang, B, Zhou, Q, Li, JL, Zhao, LH, and Tong, XL. Treatment of refractory diabetic gastroparesis: Western medicine and traditional Chinese medicine therapies. World J Gastroenterol. (2014) 20:6504–14. doi: 10.3748/wjg.v20.i21.6504
7. Huang, JK, Cheng, SL, Guan, TT, and Yang, XH. Discussion on the thinking of traditional Chinese medicine treatment of diabetic gastroparesis. Chin J Tradit Chin Med Phar. (2020) 35:304–6.
8. Hu, KX, Liu, DL, and Cui, SS. The experience of Cui Shusheng in stage-based treatment of diabetic gastroparesis. Guid J Tradit Chin Med Pharm. (2021) 27:209–13. doi: 10.13862/j.cnki.cn43-1446/r.2021.07.038
9. Pang, B, Zhou, Q, Li, JL, and Tong, XL. Clinical experience of professor TONG Xiao-lin in treating diabetic gastroparesis. Chin J Tradit Chin Med Phar. (2014) 29:2246–9.
10. Su, ZW, Fu, L, Zheng, HB, and Li, Y. Analysis of the bidirectional regulation effect of ancient acupuncture on intestinal motility based on data mining. Chin J Tradit Chin Med Phar. (2015) 30:2202–6.
11. Tian, J, Li, M, Liao, J, Li, J, and Tong, X. Chinese herbal medicine banxiaxiexin decoction treating diabetic gastroparesis: a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. (2013) 2013:749495. doi: 10.1155/2013/749495
12. Tian, JX, Li, M, Liao, JQ, Liu, WK, and Tong, XL. Xiangshaliujunzi decoction for the treatment of diabetic gastroparesis: a systematic review. World J Gastroenterol. (2014) 20:561–8. doi: 10.3748/wjg.v20.i2.561
13. Wang, B, Zeng, KW, Hong, ZF, Ti, GX, Wang, LY, Lu, P, et al. Banxia xiexin decoction treats diabetic gastroparesis through PLC-IP(3)-ca(2+)/NO-cGMP-PKG signal pathway. Chin J Integr Med. (2020) 26:833–8. doi: 10.1007/s11655-020-3077-8
14. Wu, T, Yue, R, Li, L, and He, M. Study on the mechanisms of Banxia Xiexin decoction in treating diabetic gastroparesis based on network pharmacology. Interdiscip Sci. (2020) 12:487–98. doi: 10.1007/s12539-020-00389-1
15. Sanders, KM, Ward, SM, and Koh, SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. (2014) 94:859–907. doi: 10.1152/physrev.00037.2013
16. Jin, BC, Ha, SE, Wei, L, Singh, R, Zogg, H, Clemmensen, B, et al. Colonic motility is improved by the activation of 5-HT(2B) receptors on interstitial cells of Cajal in diabetic mice. Gastroenterology. (2021) 161:608–622.e7. doi: 10.1053/j.gastro.2021.04.040
17. Wei, X, Lin, YP, Zhao, DF, Xiao, X, Chen, Q, Chen, S, et al. Electroacupuncture relieves suppression of autophagy in interstitial cells of Cajal of diabetic gastroparesis rats. Can J Gastroenterol Hepatol. (2020) 2020:1–10. doi: 10.1155/2020/7920715
18. Lin, GH, Zhang, JW, Li, LX, Lin, G, Zhang, J, Li, L, et al. Effect of electroacupuncture on gastric interstitial cells of Cajal in a rat model of diabetic gastroparesis. Exp Ther Med. (2016) 11:2489–94. doi: 10.3892/etm.2016.3185
19. Grover, M, Farrugia, G, Lurken, MS, Bernard, CE, Faussone-Pellegrini, MS, Smyrk, TC, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. (2011) 140:1575–1585.e8. doi: 10.1053/j.gastro.2011.01.046
20. Grover, M, Bernard, CE, Pasricha, PJ, Parkman, HP, Gibbons, SJ, Tonascia, J, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol Motil. (2017) 29. doi: 10.1111/nmo.13018
21. Horváth, VJ, Vittal, H, and Ordög, T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. (2005) 5:1528–33. doi: 10.2337/diabetes.54.5.1528
22. Li, LZ, Ding, N, and Yue, RS. The effect of Banxia Xiexin decoction on gastric emptying, gastric tissue AGEs content, and the expression of RAGE and nNOS proteins in a diabetic gastroparesis mouse model. J Tradit Chin Med. (2022) 63:2375–81. doi: 10.13288/j.11-2166/r.2022.24.013.10, [in Chinese]
23. Zhao, XL, Wang, HB, Zhang, ZW, Jin, H, and Gong, YL. Effects of ethyl acetate extract of Salsola collina on brain-gut peptides and interstitial cells of gastric Cajal in rats with diabetic gastroparesis. Iran J Basic Med Sci. (2020) 23:1218–24. doi: 10.22038/ijbms.2020.43521.10223
24. Pathania, S, Pentikainen, OT, and Singh, PK. A holistic view on c-kit in cancer: structure, signaling, pathophysiology and its inhibitors. Biochim Biophys Acta Rev Cancer. (2021) 1876:188631. doi: 10.1016/j.bbcan.2021.188631
25. Chen, X, Meng, X, Zhang, H, Feng, C, Wang, B, Li, N, et al. Intestinal proinflammatory macrophages induce a phenotypic switch in interstitial cells of Cajal. J Clin Invest. (2020) 130:6443–56. doi: 10.1172/JCI126584
26. Yin, J, Liang, Y, Wang, D, Yan, Z, Yin, H, Wu, D, et al. Naringenin induces laxative effects by upregulating the expression levels of c-kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J Mol Med. (2018) 41:649–58. doi: 10.3892/ijmm.2017.3301
27. Zani-Ruttenstock, E, Zani, A, Paul, A, Diaz-Cano, S, and Ade-Ajayi, N. Interstitial cells of Cajal are decreased in patients with gastroschisis associated intestinal dysmotility. J Pediatr Surg. (2015) 50:750–4. doi: 10.1016/j.jpedsurg.2015.02.029
28. Lippai, M, and Lőw, P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. Biomed Res Int. (2014) 2014:832704. doi: 10.1155/2014/832704
29. Xu, ZF, Pan, ZZ, Jin, Y, Gao, Z, Jiang, F, Fu, H, et al. Inhibition of PRKAA/AMPK (Ser485/491) phosphorylation by crizotinib induces cardiotoxicity via perturbing autophagosome-lysosome fusion. Autophagy. (2024) 20:416–36. doi: 10.1080/15548627.2023.2259216
30. Tran, S, Juliani, J, Harris, TJ, Evangelista, M, Ratcliffe, J, Ellis, SL, et al. BECLIN1 is essential for intestinal homeostasis involving autophagy-independent mechanisms through its function in endocytic trafficking. Commun Biol. (2024) 7:209. doi: 10.1038/s42003-024-05890-7
31. Wang, YJ, Dong, N, Zhou, Y, Li, H, Qin, G, Li, H, et al. Effects of Emodin on protein expression related to autophagy of interstitial cells of Cajal in diabetic rats. Chem Pharm Bull. (2023) 71:129–33. doi: 10.1248/cpb.c22-00596
32. Dai, YC, Zheng, L, Zhang, YL, Chen, X, Chen, DL, Wang, LJ, et al. Jianpi Qingchang decoction regulates intestinal motility of dextran sulfate sodium-induced colitis through reducing autophagy of interstitial cells of Cajal. World J Gastroenterol. (2017) 23:4724–34. doi: 10.3748/wjg.v23.i26.4724
33. Zhang, TH, Zhao, ST, Li, XY, Xiao, XJ, Xiao, L, Wei, X, et al. Electroacupuncture promotes gastrointestinal motility by activating autophagy of Cajal interstitial cells via downregulating PI3K/Akt/mTOR signaling pathway in stomach of diabetic gastroparesis rats. Acupunct Res. (2022) 47. doi: 10.13702/j.1000-0607.20211241
34. Gaddipati, KV, Simonian, HP, Kresge, KM, Boden, GH, and Parkman, HP. Abnormal ghrelin and pancreatic polypeptide responses in gastroparesis. Dig Dis Sci. (2006) 51:1339–46. doi: 10.1007/s10620-005-9022-z
35. Sanger, GJ, and Lee, K. Hormones of the gut-brain axis as targets for the treatment of upper gastrointestinal disorders. Nat Rev Drug Discov. (2008) 7:241–54. doi: 10.1038/nrd2444
36. Lan, J, Wang, K, Chen, G, Cao, G, and Yang, C. Effects of inulin and isomalto-oligosaccharide on diphenoxylate-induced constipation, gastrointestinal motility-related hormones, short-chain fatty acids, and the intestinal flora in rats. Food Funct. (2020) 11:9216–25. doi: 10.1039/d0fo00865f
37. Johnson, CD, Barlow-Anacker, AJ, Pierre, JF, Touw, K, Erickson, CS, Furness, JB, et al. Deletion of choline acetyltransferase in enteric neurons results in postnatal intestinal dysmotility and dysbiosis. FASEB J. (2018) 32:4744–52. doi: 10.1096/fj.201701474RR
38. Van den Houte, K, Scarpellini, E, Verbeure, W, Mori, H, Schol, J, Masuy, I, et al. The role of GI peptides in functional dyspepsia and gastroparesis: a systematic review. Front Psych. (2020) 11:172. doi: 10.3389/fpsyt.2020.00172
39. Chen, W, Chen, Q, Huang, JY, Shen, X, Zhang, L, Jiang, G, et al. Huanglian-banxia promotes gastric motility of diabetic rats by modulating brain-gut neurotransmitters through MAPK signaling pathway. Neurogastroenterol Motil. (2024) 36:e14779. doi: 10.1111/nmo.14779
40. Rivera, LR, Poole, DP, Thacker, M, and Furness, JB. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil. (2011) 23:980–8. doi: 10.1111/j.1365-2982.2011.01780.x
41. Dillon, BR, Ang, L, and Pop-Busui, R. Spectrum of diabetic neuropathy: new insights in diagnosis and treatment. Annu Rev Med. (2024) 75:293–306. doi: 10.1146/annurev-med-043021-033114
42. Yu, B, Sun, MM, Wang, ZH, Zhu, B, Xue, J, Yang, W, et al. Effects of stimulating local and distal Acupoints on diabetic gastroparesis: a new insight in revealing acupuncture therapeutics. Am J Chin Med. (2021) 49:1151–64. doi: 10.1142/s0192415x21500555
43. Anitha, M, Vijay-Kumar, M, Sitaraman, SV, Gewirtz, AT, and Srinivasan, S. Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology. (2012) 143:1006–16 e4. doi: 10.1053/j.gastro.2012.06.034
44. Baker, C, Ahmed, M, Cheng, K, Arciero, E, Bhave, S, Ho, WLN, et al. Hypoganglionosis in the gastric antrum causes delayed gastric emptying. Neurogastroenterol Motil. (2020) 32:e13766. doi: 10.1111/nmo.13766
45. Grover, M, Bernard, CE, Pasricha, PJ, Lurken, MS, Faussone‐Pellegrini, MS, Smyrk, TC, et al. Clinical-histological associations in gastroparesis: results from the gastroparesis clinical research consortium. Neurogastroenterol Motil. (2012) 24:531–539, e249. doi: 10.1111/j.1365-2982.2012.01894.x
46. Xu, SY, Liang, SC, Pei, Y, Wang, R, Zhang, Y, Xu, Y, et al. TRPV1 dysfunction impairs gastric Nitrergic neuromuscular relaxation in high-fat diet-induced diabetic gastroparesis mice. Am J Pathol. (2023) 193:548–57. doi: 10.1016/j.ajpath.2023.01.005
47. Hou, CC, Liang, HY, Hao, ZS, and Zhao, D. Berberine ameliorates the neurological dysfunction of the gastric fundus by promoting calcium channels dependent release of ACh in STZ-induced diabetic rats. Saudi Pharm J. (2023) 31:433–43. doi: 10.1016/j.jsps.2023.01.010
48. Wan, SF, Li, YQ, Wang, XL, Shu, C, Wei, ZH, Zhang, L, et al. Effects of Jinqi Zhizhu decoction on gastric motility of GK rats with diabetic gastroparesis. Chin J Inf Tradit Chin Med. (2018) 25:56–60. doi: 10.3969/j.issn.1005-5304.2018.09.014
49. Wan, SF, Li, YQ, Shu, C, Zhang, L, Wang, XL, Wei, ZH, et al. Protecting and repairing effect of Jinqi Zhizhu decoction on smooth muscle of gastric antrum in Goto - Kakizaki rat with diabetic gastroparesis. Chin J Clin Pharmacol. (2018) 34:2327–30. doi: 10.13699/j.cnki.1001-6821.2018.19.024
50. Xu, XY, Ou, XF, Jin, XJ, Zhan, DF, and Li, SC. Effect of modified Chaiqin Wendantang on blood glucose level and gastrointestinal dysfunction in patients with diabetic gastroparesis. Chin J Exp Tradit Med Form. (2020) 26:98–104. doi: 10.13422/j.cnki.syfjx.20201824
51. Han, X, Chen, XY, Wang, X, Gong, M, Lu, M, Yu, Z, et al. Electroacupuncture at ST36 improve the gastric motility by affecting neurotransmitters in the enteric nervous system in type 2 diabetic rats. Evid Based Complement Alternat Med. (2021) 2021:1–13. doi: 10.1155/2021/6666323
52. Liu, L, Wu, XF, Zheng, XN, Guo, X, Yue, ZH, Liu, M, et al. Effect of point-moxibustion and electroacupuncture on the expression of endothelial nitric oxide synthase mRNA and angiotensin2 mRNA in gastric antrum in diabetic gastroparesis rats. Acupunct Res. (2017) 42:240–5. doi: 10.13702/j.1000-0607.2017.03.009
53. Lin, YP, Wan, QQ, Peng, Y, He, FE, and Shen, J. Effect of Electropuncture at "Zusanli(ST36)" on gastrointestinal motility and expression of ghrelin mRNA and growth hormone Secretagogue receptor mRNA in diabetic gastroparesis rats. Acupunct Res. (2015) 40:290–5. doi: 10.13702/j.1000-0607.2015.04.006
54. Song, BH, Zhang, GY, Bao, Y, and Zhang, MH. Involvement of oxidative stress-AMPK-Cx43-NLRP3 pathway in extracellular matrix remodeling of gastric smooth muscle cells in rats with diabetic gastroparesis. Cell Stress Chaperones. (2024) 29:440–55. doi: 10.1016/j.cstres.2024.04.005
55. Zhang, MH, Fang, XS, Guo, JY, and Jin, Z. Effects of AMPK on apoptosis and energy metabolism of gastric smooth muscle cells in rats with diabetic gastroparesis. Cell Biochem Biophys. (2019) 77:165–77. doi: 10.1007/s12013-019-00870-9
56. Zhang, MH, Jiang, JZ, Cai, YL, Piao, LH, and Jin, Z. Significance of dynamic changes in gastric smooth muscle cell apoptosis, PI3K-AKT-mTOR and AMPK-mTOR signaling in a rat model of diabetic gastroparesis. Mol Med Rep. (2017) 16:1530–6. doi: 10.3892/mmr.2017.6764
57. Gong, YY, Zhu, Y, Zhu, BQ, Gong, Y, Zhu, B, Si, X, et al. LncRNA MALAT1 is up-regulated in diabetic gastroparesis and involved in high-glucose-induced cellular processes in human gastric smooth muscle cells. Biochem Biophys Res Commun. (2018) 496:401–6. doi: 10.1016/j.bbrc.2018.01.038
58. Zhang, XZ, Sun, Y, Zhang, MH, and Jin, Z. Insulin-like growth factor-1 inhibits apoptosis of rat gastric smooth muscle cells under high glucose condition via adenosine monophosphate-activated protein kinase (AMPK) pathway. Folia Histochem Cytobiol. (2022) 60:74–88. doi: 10.5603/FHC.a2022.0005
59. Miao, LL, Wan, SF, Zhang, L, Li, RK, Wei, ZH, Zhu, XL, et al. Hedysari radix polysaccharide regulates apoptosis of smooth muscle cells in gastric antrum of rat model of diabetic gastroparesis via IGF-1/PI3K/Akt signaling pathway. Chin J Exp Tradit Med Form. (2024) 30:130–9. doi: 10.13422/j.cnki.syfjx.20231936
60. Zhong, QL, Li, RK, Wan, SF, Zhang, Q, Wei, ZH, Guo, Q, et al. Effects of hedysarum polybotrys polysacchcaide on pyroptosis of smooth muscle cells in gastric antrum tissue of diabetic gastroparesis rats. Chin J Inf Tradit Chin Med. (2023) 30:100–6. doi: 10.19879/j.cnki.1005-5304.202210077
61. Lin, Y, Hu, YP, Hu, X, Yang, L, Chen, X, Li, Q, et al. Ginsenoside Rb2 improves insulin resistance by inhibiting adipocyte pyroptosis. Adipocytes. (2020) 9:302–12. doi: 10.1080/21623945.2020.1778826
62. An, XX, Long, CL, Deng, XM, Tang, A, Xie, J, Chen, L, et al. Higenamine inhibits apoptosis and maintains survival of gastric smooth muscle cells in diabetic gastroparesis rat model via activating the β2-AR/PI3K/AKT pathway. Biomed Pharmacother. (2017) 95:1710–7. doi: 10.1016/j.biopha.2017.08.112
63. Han, N, Cheng, SD, Jin, Y, Li, GH, Wang, H, and Jin, L. Low-intensity pulsed ultrasound combined with ST36 modulate gastric smooth muscle contractile marker expression via RhoA/Rock and MALAT1/miR-449a/DLL1 signaling in diabetic rats. Neurogastroenterol Motil. (2024) 36:e14843. doi: 10.1111/nmo.14843
64. Amano, M, Nakayama, M, and Kaibuchi, K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken). (2010) 67:545–54. doi: 10.1002/cm.20472
65. Wang, YJ, Wang, Y, Zhu, BQ, Zhu, Y, Jiang, Y, Xiong, W, et al. MALAT1: a pivotal lncRNA in the phenotypic switch of gastric smooth muscle cells via the targeting of the miR-449a/DLL1 Axis in diabetic gastroparesis. Front Pharmacol. (2021) 12:719581. doi: 10.3389/fphar.2021.719581
66. Han, N, Jiang, WJ, Li, GH, Lu, L, Shan, J, Feng, L, et al. Low-intensity pulsed ultrasound at ST36 improves the gastric motility by TNF-α/IKKβ/NF-κB signaling pathway in diabetic rats. J Gastroenterol Hepatol. (2023) 38:2018–26. doi: 10.1111/jgh.16321
67. Sampath, C, Raju, AV, Freeman, ML, Srinivasan, S, and Gangula, PR. Nrf2 attenuates hyperglycemia-induced nNOS impairment in adult mouse primary enteric neuronal crest cells and normalizes stomach function. Am J Physiol Gastrointest Liver Physiol. (2022) 322:G368–82. doi: 10.1152/ajpgi.00323.2021
68. Eisenman, ST, Gibbons, SJ, Verhulst, PJ, Cipriani, G, Saur, D, and Farrugia, G. Tumor necrosis factor alpha derived from classically activated "M1" macrophages reduces interstitial cell of Cajal numbers. Neurogastroenterol Motil. (2017) 29. doi: 10.1111/nmo.12984
69. Choi, KM, Gibbons, SJ, Sha, L, Beyder, A, Verhulst, PJ, Cipriani, G, et al. Interleukin 10 restores gastric emptying, electrical activity, and interstitial cells of Cajal networks in diabetic mice. Cell Mol Gastroenterol Hepatol. (2016) 2:454–67. doi: 10.1016/j.jcmgh.2016.04.006
70. Stein, K, Lysson, M, Schumak, B, Vilz, T, Specht, S, Heesemann, J, et al. Leukocyte-derived Interleukin-10 aggravates postoperative ileus. Front Immunol. (2018) 9:2599. doi: 10.3389/fimmu.2018.02599
71. Bernard, CE, Gibbons, SJ, Mann, IS, Froschauer, L, Parkman, HP, Harbison, S, et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil. (2014) 26:1275–84. doi: 10.1111/nmo.12389
72. Gianluca, C, Simon, JG, Katie, EM, Daniel, SY, Matthew, LT, Seth, TE, et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology. (2018) 8:2122–2136.e12. doi: 10.1053/j.gastro.2018.02.027
73. Yang, X, Yue, RS, Xu, M, Chen, Y, and Zhang, BX. Exploring the effect of Banxia Xiexin Tang on intestinal immune function in DGP model rats from the perspective of "spleen leading suggestion". Shi Zhen Tradit Chin Med. (2019) 30:2078–81. doi: 10.3969/j.issn.1008-0805.2019.09.010
74. Liang, J, and Wang, X. Exploring relationship between intestinal Flora and Diabetic gastroparesis based on "one qi circulation" theory. Chin J Tradit Chin Med. (2022) 37:914–9. doi: 10.16368/j.issn.1674-8999.2022.05.168, [in Chinese]
75. Man, AL, Gicheva, N, and Nicoletti, C. The impact of ageing on the intestinal epithelial barrier and immune system. Cell Immunol. (2014) 289:112–8. doi: 10.1016/j.cellimm.2014.04.001
76. Huang, XQ, Zhou, JH, Chu, XF, Liu, Y, and Du, X. Clinical observation on navel application of Jianpi Hewei adhesive plaster combined with oral use of modified Sini Hewei Anshen decoction for the treatment of diabetic gastroparesis accompanied by anxiety in type 2 diabetes. J Guangzhou Univ Tradit Chin Med. (2024) 41:589–97. doi: 10.13359/j.cnki.gzxbtcm.2024.03.010
77. Guo, LH, Wu, XH, Meng, QQ, Zhang, YL, and Tian, Y. Clinical study on treatment of diabetic gastroparesis with acupuncture and Moxibusiton therapy of "Biaoben matching Acupoints" combined with Chaihu Shugan powder. Chin J Tradit Chin Med Phar. (2023) 41:235–9. doi: 10.13193/j.issn.1673-7717.2023.12.044
78. Xiao, L, Wei, X, Zhang, TH, Li, XY, Yu, XM, Zhao, ST, et al. Effects of Electroacupuncture at ‘Zusanli (ST36)’, ‘Liangmen (ST21)’, and ‘Sanyinjiao (SP6)’ on the NLRP3 Inflammasome and downstream inflammatory factors in rats with diabetic gastroparesis. J Tradit Chin Med. (2023) 64:948–53. doi: 10.13288/j.11-2166/r.2023.09.014
79. Huang, H, Peng, Y, Xiao, L, Wang, J, Xin, Y-h, Zhang, T-h, et al. Electroacupuncture promotes gastric motility by suppressing pyroptosis via NLRP3/caspase-1/GSDMD signaling pathway in diabetic gastroparesis rats. Chin J Integr Med. (2024) 31:448–57. doi: 10.1007/s11655-024-3821-6
80. Da Silva, LM, da Silva, R, Maria-Ferreira, D, Beltrame, OC, da Silva-Santos, JE, and Werner, MFP. Vitamin C improves gastroparesis in diabetic rats: effects on gastric contractile responses and oxidative stress. Dig Dis Sci. (2017) 62:2338–47. doi: 10.1007/s10620-017-4632-9
81. Uppaluri, S, Jain, MA, Ali, H, Shingala, J, Amin, D, Ajwani, T, et al. Pathogenesis and management of diabetic gastroparesis: An updated clinically oriented review. Diabetes Metab Syndr. (2024) 18:102994. doi: 10.1016/j.dsx.2024.102994
82. López-Pingarrón, L, Almeida, H, Soria-Aznar, M, Reyes-Gonzales, MC, Rodríguez-Moratinos, AB, Muñoz-Hoyos, A, et al. Interstitial cells of Cajal and enteric nervous system in gastrointestinal and neurological pathology, relation to oxidative stress. Curr Issues Mol Biol. (2023) 45:3552–72. doi: 10.3390/cimb45040232
83. Smiley, R, Naik, P, McCallum, R, and Showkat Ali, M. Reactive oxygen species overproduction and MAP kinase phosphatase-1 degradation are associated with gastroparesis in a streptozotocin-induced male diabetic rat model. Neurogastroenterol Motil. (2018) 30. doi: 10.1111/nmo.13218
84. Choi, KM, Kashyap, PC, Dutta, N, Stoltz, GJ, Ordog, T, Donohue, TS, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. (2010) 138:2399–409. doi: 10.1053/j.gastro.2010.02.014
85. Guo, Q, Li, YQ, Wan, SF, Wei, ZH, Yang, R, Li, RK, et al. Effects of hedysarum polybotrys polysacchcaide on oxidative stress in diabetic gastroparesis rats. Chin J Inf Tradit Chin Med. (2023) 30:114–20. doi: 10.19879/j.cnki.1005-5304.202203369
86. Zheng, XW, Xue, QR, Wang, YH, Zheng, X, Xue, Q, Wang, Y, et al. A. officinarum Hance - P. cablin (Blanco) Benth drug pair improves oxidative stress, intracellular ca(2+) concentrations and apoptosis by inhibiting the AGE/RAGE axis to ameliorate diabetic gastroparesis: in vitro and in vivo studies. J Ethnopharmacol. (2024) 324:117832. doi: 10.1016/j.jep.2024.117832
87. Jin, QH, Shen, HX, Wang, H, Shou, QY, and Liu, Q. Curcumin improves expression of SCF/c-kit through attenuating oxidative stress and NF-κB activation in gastric tissues of diabetic gastroparesis rats. Diabetol Metab Syndr. (2013) 5:12. doi: 10.1186/1758-5996-5-12
88. Sampath, C, Wilus, D, Tabatabai, M, Freeman, ML, and Gangula, PR. Mechanistic role of antioxidants in rescuing delayed gastric emptying in high fat diet induced diabetic female mice. Biomed Pharmacother. (2021) 137:111370. doi: 10.1016/j.biopha.2021.111370
89. Mai, W, Fan, YS, Miao, FR, and Huang, JW. Effect of electroacupuncture combined with Zhuang-medicine-thread moxibustion on oxidative stress in gastric antrum of diabetic gastroparesis rats. Acupunct Res. (2022) 47:655–64. doi: 10.13702/j.1000-0607.20211273
90. Chen, J, Liang, FX, Wu, S, Chen, ZQ, Chen, B, Zhou, T, et al. Clinical research of acupuncture therapy combined with domperidone in treatment of diabetic gastrparesis of liver stagnation and spleen deficiency. AcupunctRes. (2023) 48:88–94. doi: 10.13702/j.1000-0607.20211215
91. Zhou, BL, Yuan, YT, Zhang, SS, Guo, C, Li, X, Li, G, et al. Intestinal Flora and Disease mutually shape the regional immune system in the intestinal tract. Front Immunol. (2020) 11:575. doi: 10.3389/fimmu.2020.00575
92. Iatcu, CO, Steen, A, and Covasa, M. Gut microbiota and complications of type-2 diabetes. Nutrients. (2021) 14:166. doi: 10.3390/nu14010166
93. Fan, Y, and Pedersen, O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19:55–71. doi: 10.1038/s41579-020-0433-9
94. Caricilli, AM, and Saad, MJ. The role of gut microbiota on insulin resistance. Nutrients. (2013) 5:829–51. doi: 10.3390/nu5030829
95. Yang, X, Yue, RS, Xu, M, Zhang, BX, and Chen, Y. Exploring the mechanism of immune dysfunction caused by intestinal microbiota imbalance in diabetic gastroparesis rats and the interventional effects of Banxia Xiexin decoction. Pharm Clin Chin Mater Med. (2019) 35:17–21. doi: 10.13412/j.cnki.zyyl.2019.02.004
96. Xu, M, Yue, RS, Yang, MY, Yang, X, Wu, TC, and Li, JN. Effects of Banxia Xiexin decoction on intestinal flora and inflammatory factors of diabetic gastroparesis rats. Chin Tradit Herbal Drugs. (2018) 49:3056–61. doi: 10.7501/j.issn.0253-2670.2018.13.015
97. Zheng, XW, Zhang, YX, Tan, YF, Li, Y, Xue, Q, Li, H, et al. Alpinia officinarum Hance extract ameliorates diabetic gastroparesis by regulating SCF/c-kit signaling pathway and rebalancing gut microbiota. Fitoterapia. (2024) 172:105730. doi: 10.1016/j.fitote.2023.105730
98. Huang, JK, Song, YL, Cheng, SL, and Yang, XH. Mechanism of action of FoxiangSan in diabetic gastroparesis: gut microbiota and cAMP/PKA pathway. Heliyon. (2024) 10:e35558. doi: 10.1016/j.heliyon.2024.e35558
99. Li, Z, Lu, G, Li, Z, Wu, B, Luo, E, Qiu, X, et al. Altered Actinobacteria and Firmicutes phylum associated epitopes in patients with Parkinson's disease. Front Immunol. (2021) 12:632482. doi: 10.3389/fimmu.2021.632482
100. Kasanah, N, and Triyanto, T. Bioactivities of Halometabolites from marine Actinobacteria. Biomolecules. (2019) 9:225. doi: 10.3390/biom9060225
101. Huang, J. Analysis of the relationship between Helicobacter pylori infection and diabetic gastroparesis. Chin Med J. (2017) 130:2680–5. doi: 10.4103/0366-6999.218012
102. Lin, WJ, Zheng, S, Yang, XQ, Lu, LH, Liu, WW, and Chen, CY. Clinical efficacy observation of electroacupuncture based on the “ascending lucidity and descending turbidity” theory: a study of gut microbiota in patients with diabetic gastrointestinal dysfunction. Acupunct Res. (2024) 49:1084–91. doi: 10.13702/j.1000-0607.20230690
103. Caballero-Flores, G, Pickard, JM, and Nunez, G. Microbiota-mediated colonization resistance: mechanisms and regulation. Nat Rev Microbiol. (2023) 21:347–60. doi: 10.1038/s41579-022-00833-7
104. Waters, JL, and Ley, RE. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. (2019) 17:83. doi: 10.1186/s12915-019-0699-4
105. Zhang, Y, Wei, XL, Jiang, S, Gao, W, Wang, K, Wang, H, et al. Jianwei Xiaoshi oral liquid attenuates high-calorie diet-induced dyspepsia in immature rats via regulating the pancreatic secretion pathway and maintaining the homeostasis of intestinal microbiota. Chin Med. (2025) 20:6. doi: 10.1186/s13020-024-01052-3
106. Wu, L, Niu, Y, Ren, BY, Wang, S, Song, Y, Wang, X, et al. Naringenin promotes gastrointestinal motility in mice by impacting the SCF/c-kit pathway and gut microbiota. Foods. (2024) 13:2520. doi: 10.3390/foods13162520
107. Campbell, DE, Ly, LK, Ridlon, JM, Hsiao, A, Whitaker, RJ, and Degnan, PH. Infection with Bacteroides phage BV01 alters the host transcriptome and bile acid metabolism in a common human gut microbe. Cell Rep. (2020) 32:108142. doi: 10.1016/j.celrep.2020.108142
Keywords: traditional Chinese medicine, diabetic gastroparesis, therapeutic agent, interstitial cells of Cajal, oxidative stress, gut microbiota homeostasis
Citation: Zhang H, You QH, Feng XH and Qin ZR (2025) Emerging roles of traditional Chinese medicine in the treatment of diabetic gastroparesis. Front. Nutr. 12:1680943. doi: 10.3389/fnut.2025.1680943
Edited by:
Wenrui Xia, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Zhi Jiang, The Second Affiliated Hospital of Guanghzou University of Chinese Medicine, ChinaEclésio Batista De Oliveira Neto, Centro Universitário de Maceió, Brazil
Copyright © 2025 Zhang, You, Feng and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengran Qin, cXpyX3pjbXVAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Hong Zhang
Hong Zhang Qianhui You2†
Qianhui You2†