- 1Department of General Surgery, Lanzhou University Second Hospital, Lanzhou University Second Clinical Medical College, Lanzhou, China
- 2Department of Dermatology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
Introduction: Animal and clinical studies have demonstrated a range of potential health benefits associated with cinnamon. However, its effects on metabolic parameters such as blood glucose, blood pressure, lipid profiles, and body weight in patients with metabolic diseases remain controversial. To systematically assess the current evidence, we conducted an umbrella review of meta-analyses to comprehensively evaluate the impact of cinnamon supplementation on metabolic outcomes in patients with metabolic diseases.
Methods: A systematic search was performed in PubMed, Embase, Web of Science, Scopus, and the Cochrane Library to identify relevant systematic reviews and meta-analyses of randomized placebo-controlled trials investigating cinnamon supplementation in individuals with metabolic diseases. The methodological quality and strength of evidence were assessed using AMSTAR 2 tool (A MeaSurement Tool to Assess systematic Reviews, version 2).
Results: A total of 21 meta-analyses comprising 139 comparisons, were included for qualitative synthesis. The findings indicate that cinnamon supplementation is significantly associated with improvements in fasting blood glucose and lipid profiles, with more pronounced effects observed in patients with diabetes and metabolic syndrome. Subgroup analyses suggest that higher doses (>1.5 g/day) and shorter intervention durations (≤2 months) may enhance these benefits. Additionally, cinnamon shows potential in modulating insulin resistance, antioxidant capacity, and blood pressure regulation.
Conclusion: These results underscore the promising role of cinnamon as an adjunctive therapy for metabolic diseases. Future research should focus on well-designed randomized controlled trials with extended follow-up periods to further confirm its efficacy and elucidate underlying mechanisms, thereby providing robust evidence for clinical and public health applications.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251010073, identifier: CRD420251010073.
1 Introduction
Metabolic diseases comprise a group of conditions characterized by disturbances in glucose, lipid, or protein metabolism (1). Common examples include type 2 diabetes, hypertension, obesity, hyperlipidemia, hyperuricemia/gout, non-alcoholic fatty liver disease, and metabolic syndrome (1). In recent years, the prevalence of metabolic diseases has been rising continuously and has reached alarming levels, posing a significant global public health burden (2). Currently, over 890 million adults worldwide are diagnosed with obesity, and more than 589 million are living with diabetes (3, 4).
Cinnamomum, a genus in the Lauraceae family, is widely used not only as a culinary spice but also in traditional herbal medicine (5, 6). The spice cinnamon is obtained from plants of the genus Cinnamomum. Preclinical and clinical studies have demonstrated that cinnamon possesses diverse pharmacological properties, including antioxidant, anti-inflammatory, antitumor, immunomodulatory, antidiabetic, and lipid-lowering effects (7, 8).
Although numerous studies have reported beneficial metabolic outcomes associated with cinnamon supplementation in individuals with metabolic diseases (9–11), findings across trials remain inconsistent. Therefore, an umbrella review is warranted to systematically evaluate and synthesize evidence from existing systematic reviews and meta-analyses. This study aims to assess the overall effects of cinnamon on metabolic outcomes in patients with metabolic diseases. Additionally, we seek to explore whether the effectiveness of cinnamon varies according to dosage, duration of intervention, or underlying disease type, thereby providing more robust evidence to guide clinical practice and future research.
2 Materials and methods
This umbrella review was prospectively registered with PROSPERO (CRD420251010073). The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12).
2.1 Literature search strategy
A comprehensive search was conducted in PubMed, Web of Science, Embase, Scopus, and the Cochrane Library up to March 2025 to identify systematic reviews and meta-analyses investigating the effects of cinnamon supplementation on metabolic diseases. The search terms included: (“Cinnamomum zeylanicum” OR “Cinnamomum verum” OR “Cinnamon” OR “Cinnamons”) AND (“Systematic Review” OR “Meta-Analysis” OR “systematic literature review” OR “meta-analysis”). During study selection, only studies conducted in patients with metabolic diseases—including diabetes, metabolic syndrome, polycystic ovary syndrome (PCOS), Non-Alcoholic Fatty Liver Disease (NAFLD), hypertension and related diseases—were included. No language restrictions were applied. Relevant studies were identified through screening of titles, abstracts, and full texts. Non-English articles meeting the inclusion criteria were included, with data extraction assisted by translation tools (e.g., DeepL, ChatGPT) when necessary.
2.2 Eligibility and inclusion/exclusion criteria
Inclusion criteria were as follows:
(1) Adults aged ≥18 years with diagnosed metabolic diseases (e.g., diabetes, PCOS, NAFLD, metabolic syndrome, hypertension), (2) The intervention involved supplementation with cinnamon or cinnamon extract, with cinnamon used as the sole intervention, either as a dietary supplement or a culinary spice, (3) Placebo-controlled comparisons, (4) At least one outcome reported among: fasting blood glucose (FBG), glycated hemoglobin (HbA1c), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), total cholesterol (CHOL), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), systolic blood pressure (SBP), diastolic blood pressure (DBP), body weight (BW), or body mass index (BMI), (5) Meta-analyses reporting effect sizes (MD, WMD, or SMD) with 95% confidence intervals (CIs).
Exclusion criteria: (1) Systematic reviews without quantitative synthesis, (2) Meta-analyses lacking effect sizes with 95% CIs or with incomplete data, (3) Studies using cinnamon combined with other supplements, (4) Meta-analyses based solely on observational studies.
2.3 Data extraction, quality assessment, and publication bias
We used EndNote software to remove duplicate records during the study selection process. Two reviewers independently extracted data, with a third reviewer verifying accuracy. Disagreements were resolved by consultation with a fourth researcher. Duplicate records were removed and references were managed using EndNote software during the study selection process. Extracted data included: first author, year of publication, number of included studies, study design, population characteristics, intervention details, outcome measures, total sample size, effect estimates with 95% CIs, heterogeneity statistics (I2), and statistical models used. For primary studies within each meta-analysis, we extracted author, sample size, outcome type, group sample sizes, and pre/post means with SDs for further re-analysis.
Methodological quality was assessed using the AMSTAR-2 tool. Publication bias was evaluated with Egger's test, and studies with significant bias were adjusted using the Trim and Fill method. Sensitivity analyses were performed to assess the robustness of the findings (13).
2.4 Statistical analysis
Statistical analyses were performed using Stata (version 15.0) and R Studio (version 4.3.2). Except where specified, two-tailed p-values < 0.05 were considered statistically significant.
For all included studies, we extracted mean changes and SDs for outcomes before and after intervention in both intervention and control groups to estimate overall mean differences as effect sizes. When not directly reported, mean changes were calculated as post-intervention minus baseline values, SDs were computed as √[(baseline SD2 + endpoint SD2) – 2R × baseline SD × endpoint SD] assuming a correlation coefficient (R) of 0.5. If only SE was reported (14), SD was calculated as SE × √n (n = sample size per group). For studies reporting medians with ranges or 95% CIs, means and SDs were estimated using standard formulas. All meta-analyses were synthesized using standardized mean differences (SMDs).
For each eligible meta-analysis, both fixed- and random-effects models were applied to calculate pooled SMDs with 95% CIs (15). Studies with incomplete data were excluded to ensure accuracy. Between-study heterogeneity was assessed via I2 and its 95% CI (16, 17). Prediction intervals (PIs) under random-effects models were also computed to assess the likely range of true effects in future studies (18).
Publication bias was assessed using Egger's test, with p < 0.05 suggesting small-study effects (19). To explore sources of heterogeneity, subgroup analyses were conducted based on cinnamon dose, intervention duration, and disease type. Adverse events related to cinnamon supplementation were also summarized.
Excess significance bias (20, 21) was evaluated by comparing the observed number of significant studies (O, p < 0.05) with the expected number (E). The E-value was calculated as the sum of statistical power across all included studies, with power estimated based on the effect size from the largest study in each meta-analysis using a non-central t-distribution. A p-value < 0.10 was considered indicative of excess significance bias.
2.5 Assessment of evidence credibility
The strength of evidence was classified according to the following criteria (22–24): (1) p < 10−6 in random-effects meta-analysis, (2) total sample size >1,000, (3) p < 0.05 in the largest individual study, (4) I2 < 50%, (5) no evidence of small-study effects, (6) 95% PI excluding the null value, (7) no excess significance bias. Associations meeting all seven criteria were considered convincing. Evidence was deemed highly suggestive if criteria (1–3) were met, suggestive if only p ≤ 0.001 and sample size >1,000 were satisfied, weak if only p ≤ 0.05 was met, and non-significant if p > 0.05. The results of the Evidence Credibility assessment are presented in Tables 2 and 3 and Figures 3–13.
2.6 Overlap assessment and strategy for handling overlapping meta-analyses
Corrected Covered Area (CCA) was used to quantify overlap between included meta-analyses (25):
where Nr is the total number of primary study occurrences (including duplicates), Ns is the number of unique studies, and R is the number of meta-analyses. Overlap was categorized as slight (0%−5%), moderate (6%−10%), high (11%−15%), or very high (>15%) (25). This helps identify redundancy and risk of bias from duplicate evidence.
For high overlap (CCA ≥ 6%), two strategies were used (26–28): (1) selecting the most recent, comprehensive, or methodologically robust meta-analysis (via AMSTAR-2), (2) extracting all relevant primary studies for a de novo meta-analysis. When overlap was slight (CCA ≤ 5%), existing pooled estimates were directly used (29).
3 Results
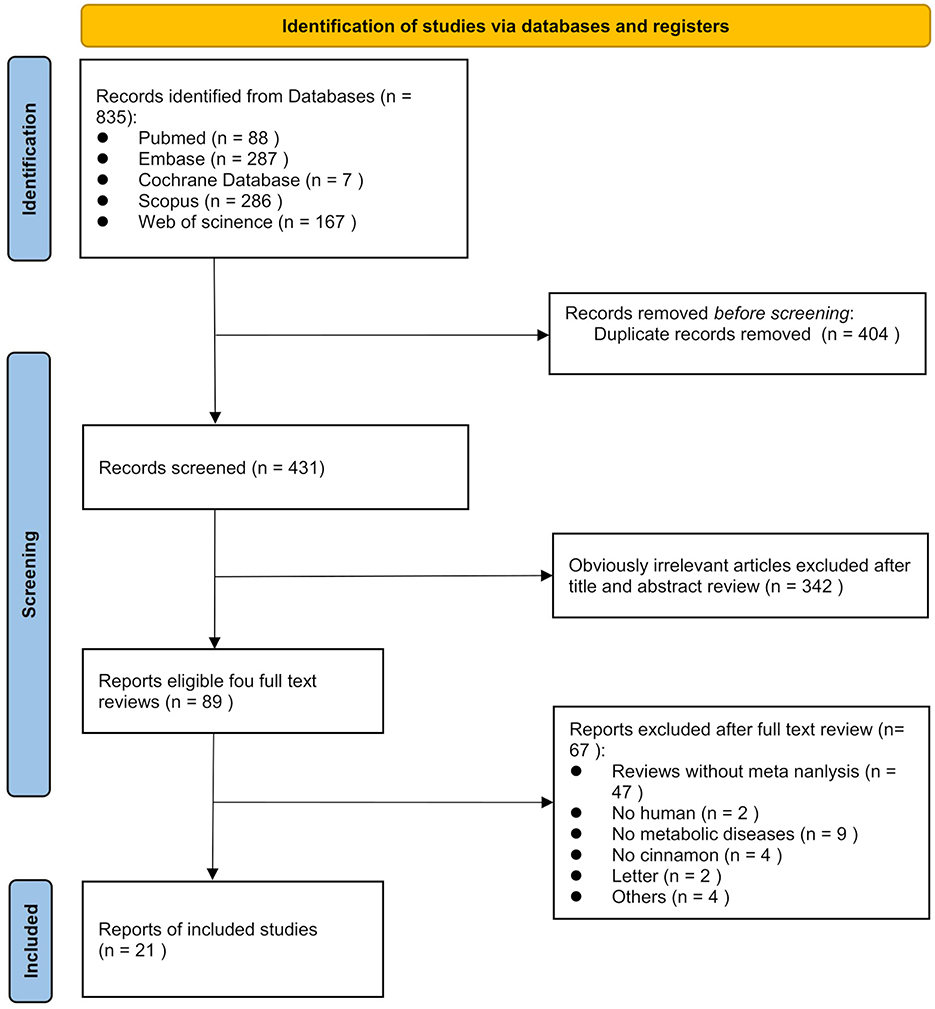
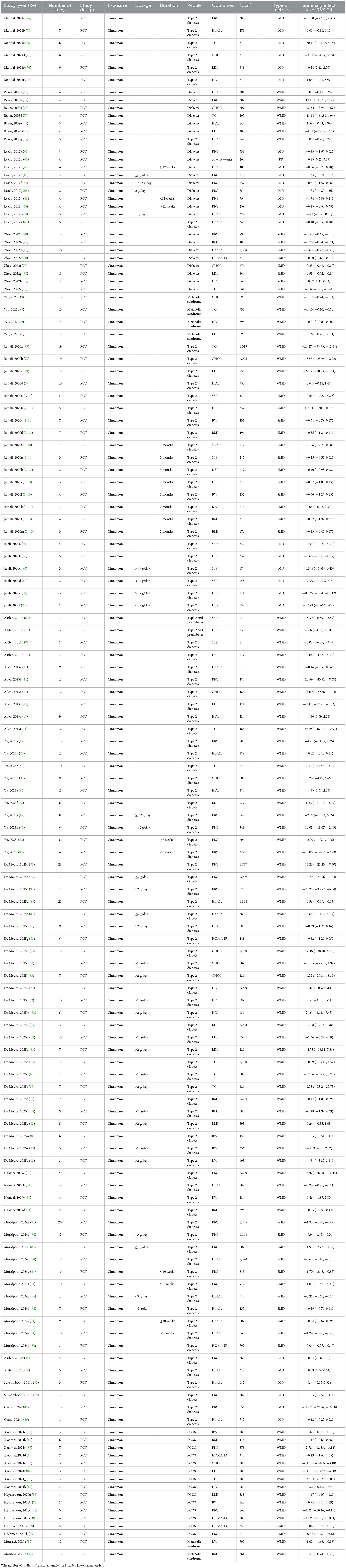
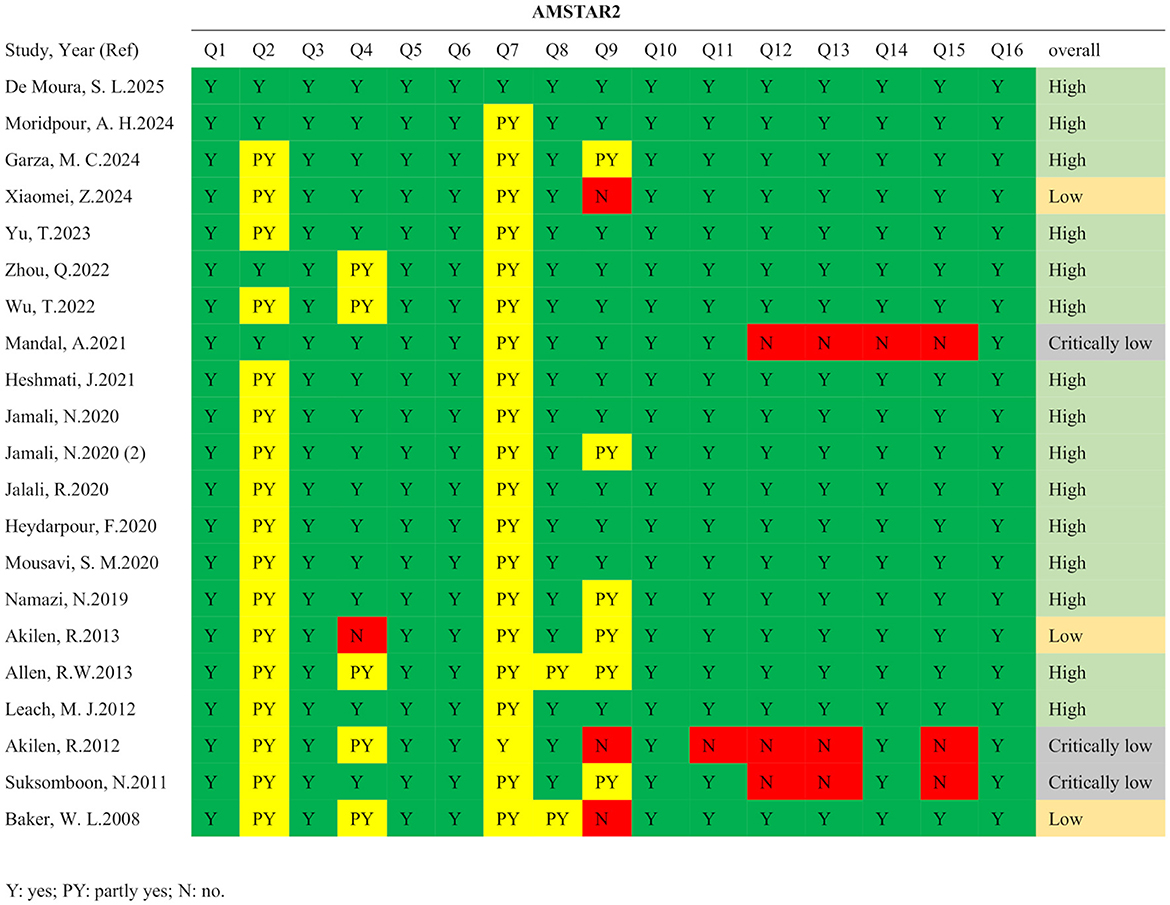
According to the PRISMA guidelines, the literature screening process of this study is presented in Figure 1. A total of 835 records were initially identified through systematic searches of the selected electronic databases. After screening the titles, abstracts, and full texts, and excluding studies that did not meet the inclusion criteria, 21 meta-analyses comprising 139 comparisons were ultimately included. The detailed characteristics of the included studies are summarized in Table 1. The standardized mean differences (SMDs) under the random-effects model, corresponding p-values, and heterogeneity measures from the included meta-analyses are presented in Table 2. These publications were dated from 2008 to 2025. The cinnamon supplementation dose in the included studies ranged from 0.12 to 6 grams per day, with intervention durations varying from 1.5 to 12 months. Regarding the methodological quality assessment, the AMSTAR 2 tool was used to evaluate all included studies. Among the 21 meta-analyses, 15 were rated as high quality, three as low quality, and three as critically low quality (Figure 2).
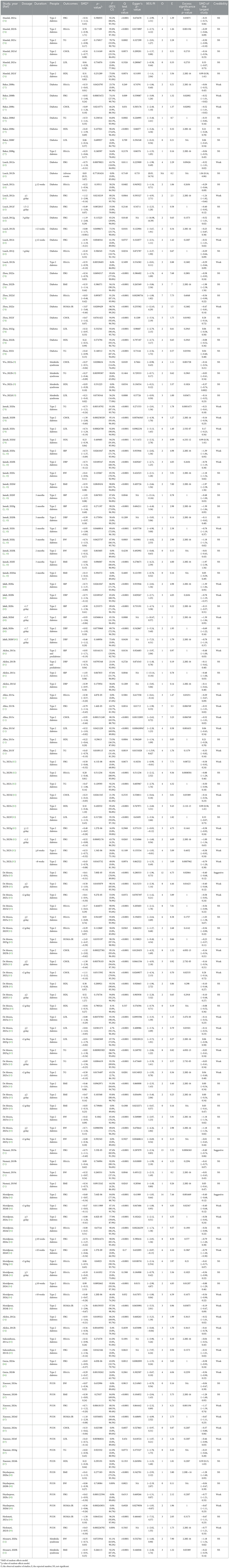
Table 2. Effect estimates, evidence credibility, risk of bias, and heterogeneity assessment in the included meta-analyses.
3.1 Cinnamon and FBG outcomes
Fifteen comparisons evaluated the effect of cinnamon supplementation on FBG in patients with metabolic diseases. The pooled analysis showed a significant reduction (SMD = −0.61, 95% CI: −0.70 to −0.52) with no heterogeneity (I2 = 0%, p = 0.8743), suggesting high consistency (Figure 3). However, 13 comparisons (86.7%) exhibited substantial heterogeneity (I2 > 50%), possibly due to variation in dosage, intervention duration, and population characteristics. Egger's test indicated marginal funnel plot asymmetry (bias = −1.00, p = 0.051), and two comparisons showed small-study effects (p < 0.05). Excess significance bias was observed in 10 comparisons (66.7%). After imputing four missing studies via the trim-and-fill method, the effect remained significant (SMD = −0.58, 95% CI: −0.66 to −0.51), supporting the robustness of the findings.
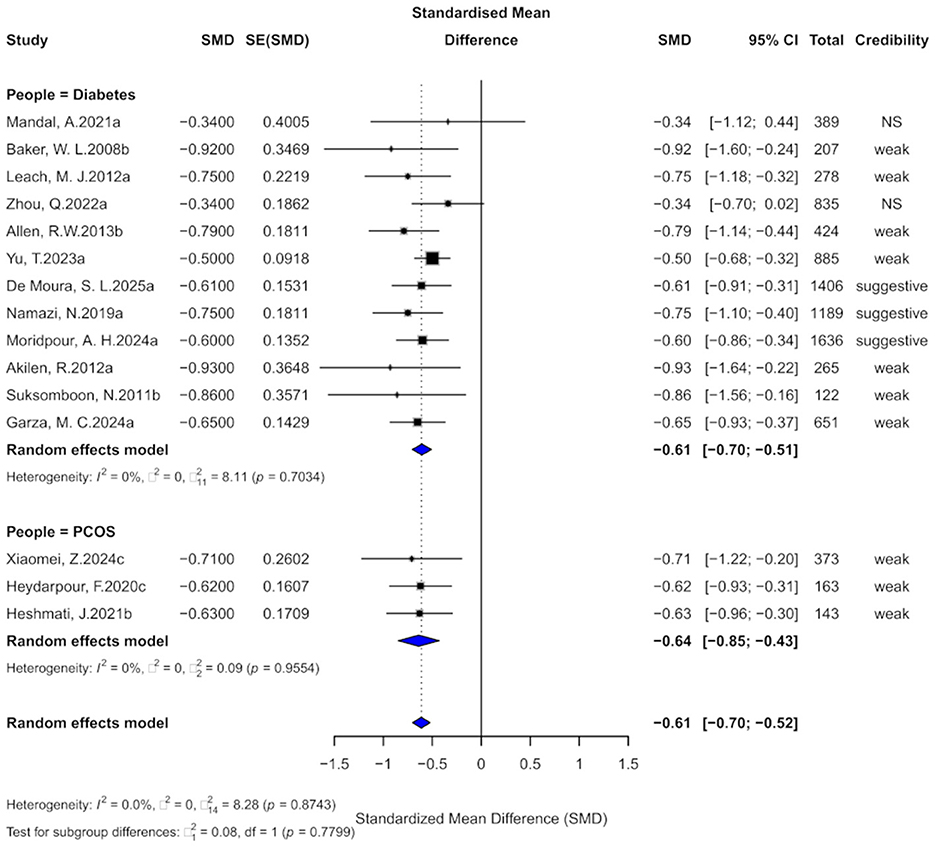
Figure 3. Forest plot of the effect of cinnamon supplementation on FBG in patients with metabolic diseases.
In terms of evidence strength, three comparisons (20%) were rated as “suggestive,” 10 (66.7%) as “weak,” and two (13.3%) as “non-significant.” Only one comparison had a 95% prediction interval excluding the null. At p < 0.05, 93.3% were significant, but only one remained significant at p < 0.000001.
Subgroup analysis showed consistent effects in diabetes (SMD = −0.61, 95% CI: −0.70 to −0.51) and PCOS (SMD = −0.64, 95% CI: −0.85 to −0.43), with no significant subgroup difference (χ2 = 0.08, p = 0.7799).
3.2 Cinnamon and HbA1c outcomes
Twelve comparisons assessed the impact of cinnamon on HbA1c levels. Cinnamon supplementation was associated with a moderate reduction (SMD = −0.26, 95% CI: −0.35 to −0.16), with low heterogeneity (I2 = 9.4%, p = 0.3527; Figure 4). Nevertheless, eight comparisons (66.7%) showed substantial heterogeneity, and 4 (33.3%) showed small-study effects. Egger's test confirmed funnel plot asymmetry (p = 0.0006), and excess significance was observed in five comparisons. After adding 5 imputed studies, the adjusted effect remained significant (SMD = −0.32, 95% CI: −0.44 to −0.21), though heterogeneity increased moderately (I2 = 38.1%, p = 0.0565), suggesting stable yet cautious interpretation.
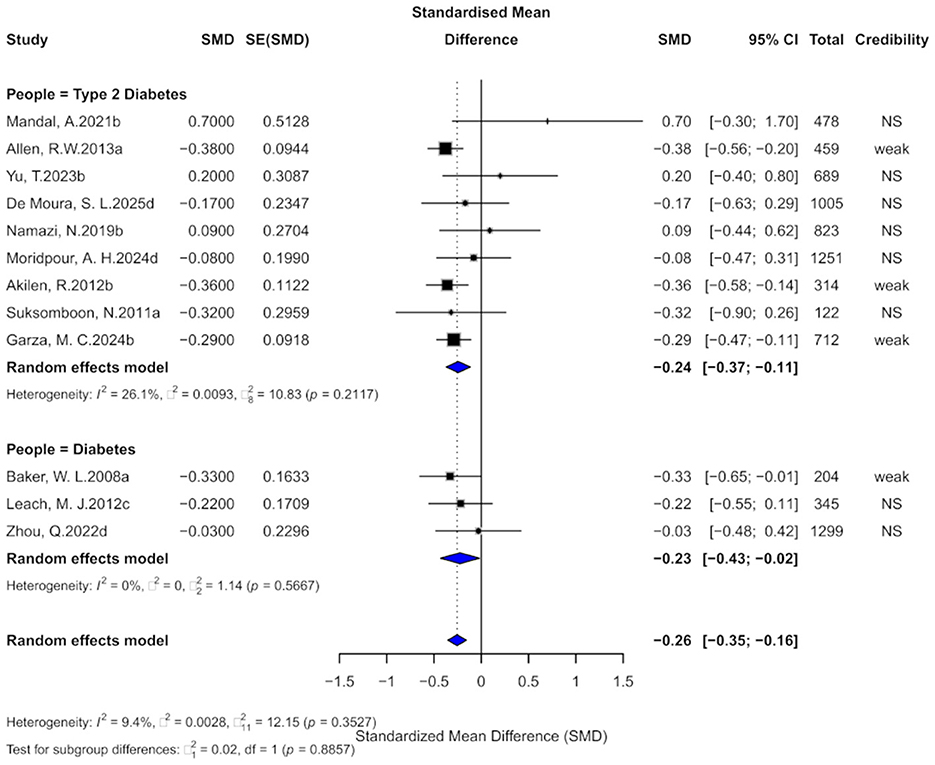
Figure 4. Forest plot of the effect of cinnamon supplementation on HbA1c in patients with metabolic diseases.
Evidence grading showed four comparisons (33.3%) with “weak” evidence, and 8 (66.7%) as “non-significant.” Only two comparisons had prediction intervals excluding the null. Statistically significant results were found in 33.3% at p < 0.05, but only 1 comparison remained significant at p < 0.001.
Subgroup analysis indicated similar reductions in HbA1c among diabetes (SMD = −0.23) and type 2 diabetes (SMD = −0.24), with no significant subgroup difference (χ2 = 0.02, p = 0.8857).
3.3 Cinnamon and HOMA-IR outcomes
Six comparisons examined the effect of cinnamon on HOMA-IR. The pooled effect was significant (SMD = −1.39, 95% CI: −2.14 to −0.64) with high heterogeneity (I2 = 58.2%, p = 0.0352; Figure 5). Egger's test indicated small-study effects (p < 0.05), and one comparison suggested excess significance bias. After trim-and-fill adjustment (four studies imputed), the effect attenuated (SMD = −0.79, 95% CI: −1.89 to 0.30), with the wide confidence interval crossing the null, indicating reduced certainty and the need for cautious interpretation.
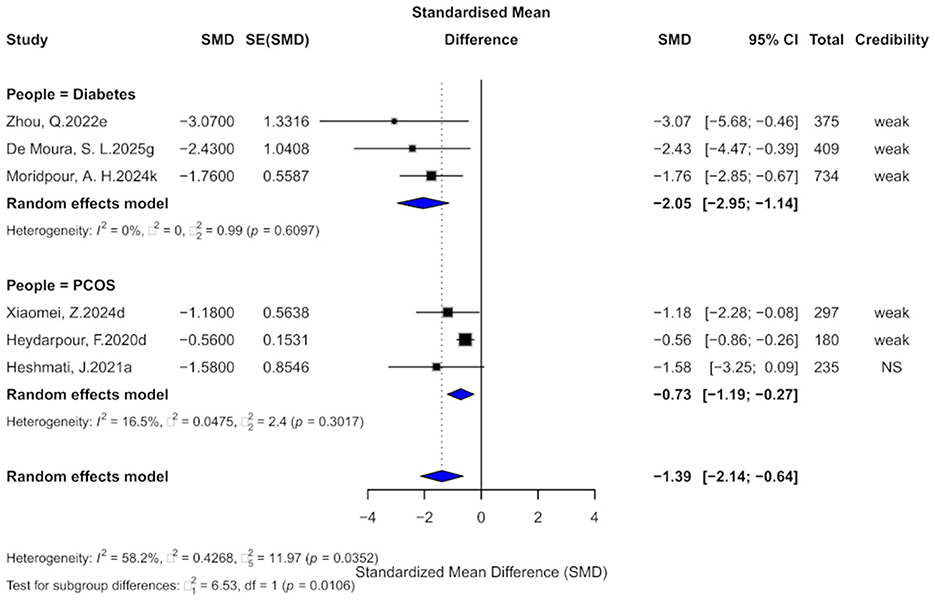
Figure 5. Forest plot of the effect of cinnamon supplementation on HOMA-IR in patients with metabolic diseases.
In terms of evidence, five comparisons (83.3%) provided “weak” and one “non-significant” evidence. Only one had a prediction interval excluding zero. Five comparisons were significant at p < 0.05, but only one at p < 0.001.
Subgroup analysis revealed stronger effects in diabetes (SMD = −2.05, 95% CI: −2.95 to −1.14) than in PCOS (SMD = −0.73, 95% CI: −1.19 to −0.27), with a significant subgroup difference (χ2 = 6.53, p = 0.0106).
3.4 Cinnamon and TG outcomes
Nine comparisons evaluated the effect of cinnamon on TG levels. The pooled analysis showed a significant reduction (SMD = −0.40, 95% CI: −0.55 to −0.25) with low heterogeneity (I2 = 12.7%; Figure 6). However, 88.9% of comparisons had high within-study heterogeneity. Egger's test showed no significant bias (bias = −1.03, p = 0.212), though one comparison had small-study effects and three showed excess significance bias. Trim-and-fill imputation of three studies slightly reduced the effect size (SMD = −0.31, 95% CI: −0.52 to −0.11), with moderate heterogeneity (I2 = 38.9%).
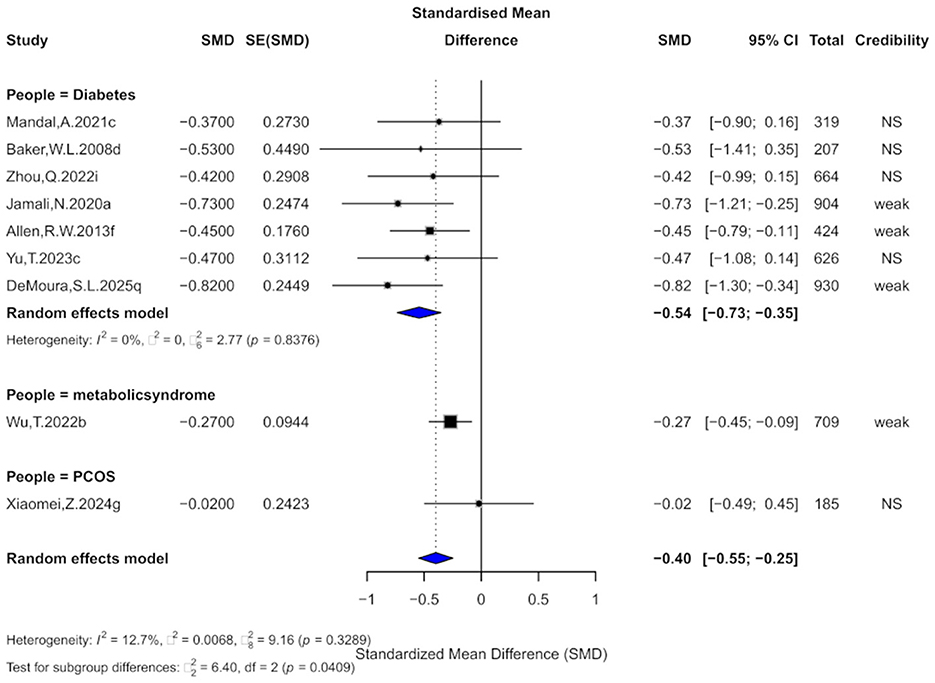
Figure 6. Forest plot of the effect of cinnamon supplementation on TG in patients with metabolic diseases.
Regarding evidence strength, 44.4% were rated “weak,” and the rest “non-significant.” No comparisons reached “suggestive” level, and all prediction intervals included the null. Only 44.4% were significant at p < 0.05, just one remained significant at p < 0.001.
Subgroup analysis showed the greatest TG reduction in diabetes (SMD = −0.54), followed by metabolic syndrome (SMD = −0.27), with no effect in PCOS (SMD = −0.02). Subgroup differences were significant (χ2 = 6.4, p = 0.0409), suggesting population-dependent effects.
3.5 Cinnamon and CHOL outcomes
Nine comparisons evaluated the effect of cinnamon supplementation on CHOL levels. Pooled analysis demonstrated a significant reduction in CHOL (SMD = −0.56, 95% CI: −0.79 to −0.33), although heterogeneity was substantial (I2 = 66.6%; Figure 7). Notably, 77.8% of comparisons exhibited considerable within-study heterogeneity (I2 > 50%). Three comparisons showed evidence of small-study effects, and four indicated excess significance bias. Egger's test revealed significant publication bias (bias = −3.18, p = 0.002). After imputing four potentially missing studies using the trim-and-fill method, the effect size slightly attenuated (SMD = −0.31, 95% CI: −0.70 to 0.08), and heterogeneity increased slightly (I2 = 77.1%).
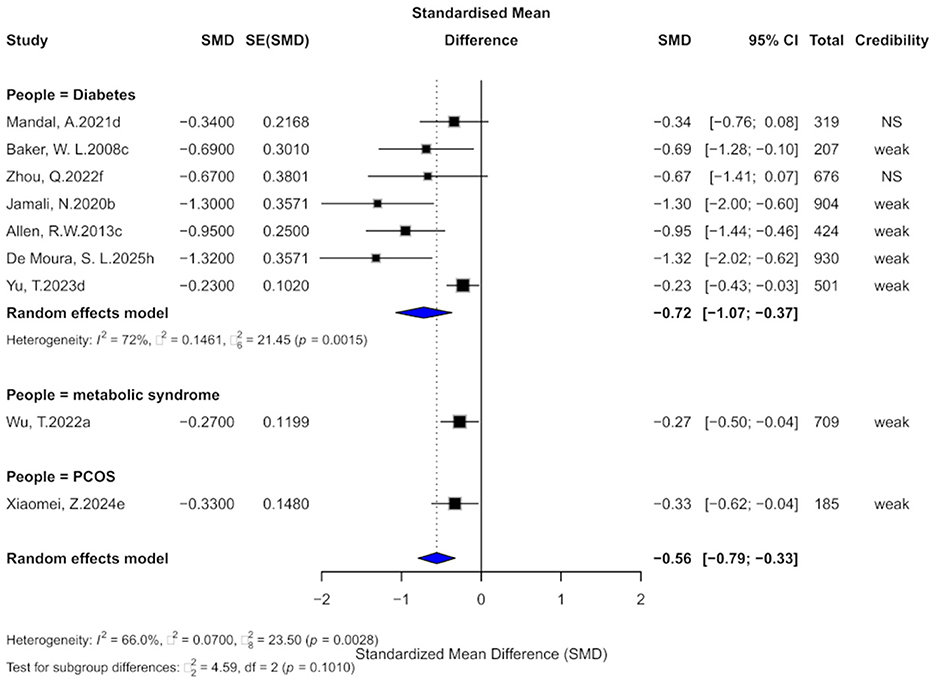
Figure 7. Forest plot of the effect of cinnamon supplementation on CHOL in patients with metabolic diseases.
Regarding the strength of evidence, 77.8% of comparisons were rated as “weak,” and the remaining as “non-significant,” with none reaching the level of “suggestive” or higher. All 95% prediction intervals included the null value. In terms of statistical significance, 77.8% of comparisons achieved p < 0.05, and three remained significant at the stricter threshold of p < 0.001.
Subgroup analyses showed beneficial effects across diabetic (SMD = −0.72), metabolic syndrome (SMD = −0.27), and PCOS populations (SMD = −0.33), with no significant difference between subgroups (χ2 = 4.59, p = 0.1010).
3.6 Cinnamon and HDL outcomes
Nine comparisons evaluated the effect of cinnamon supplementation on HDL levels. The pooled analysis showed a negligible and non-significant effect (SMD = 0.02, 95% CI: −0.10 to 0.14), with no observed heterogeneity across studies (I2 = 0%; Figure 8). The current evidence does not support a significant impact of cinnamon supplementation on HDL levels in patients with metabolic diseases. In terms of evidence grading, all comparisons were classified as “non-significant,” with none reaching the “weak” or higher level.
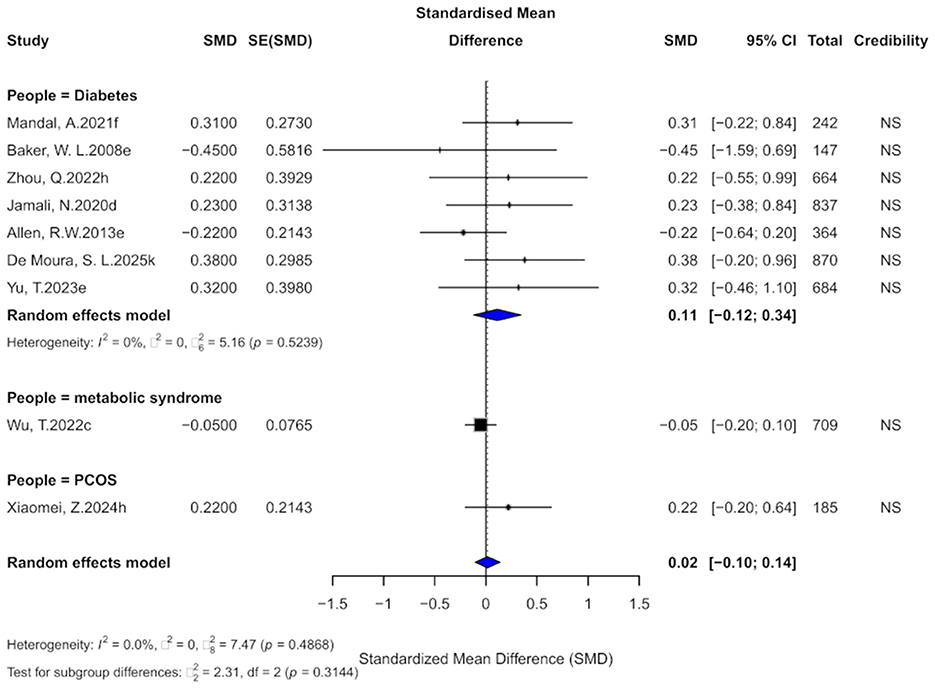
Figure 8. Forest plot of the effect of cinnamon supplementation on HDL in patients with metabolic diseases.
3.7 Cinnamon and LDL outcomes
Nine comparisons assessed the effect of cinnamon supplementation on LDL levels. The pooled analysis indicated a statistically significant reduction (SMD = −0.31, 95% CI: −0.51 to −0.10), with moderate heterogeneity (I2 = 59.3%; Figure 9). Among the included studies, 66.7% exhibited substantial within-study heterogeneity (I2 > 50%). One study showed evidence of small-study effects, and three studies demonstrated excess significance bias. Egger's test did not indicate significant publication bias (bias = −2.34, p = 0.067), though the p-value was near the significance threshold. After applying the trim-and-fill method and imputing four potentially missing studies, the effect size became non-significant (SMD = −0.15, 95% CI: −0.41 to 0.12), with a slight increase in heterogeneity (I2 = 65.5%).
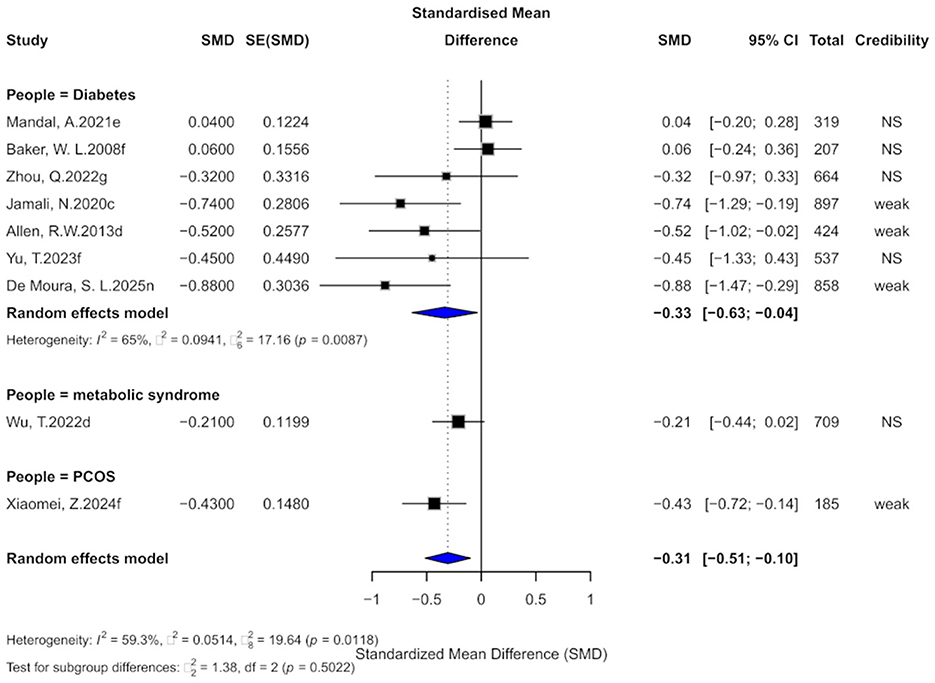
Figure 9. Forest plot of the effect of cinnamon supplementation on LDL in patients with metabolic diseases.
Regarding the level of evidence, 44.4% of comparisons were rated as “weak,” while the rest were “non-significant,” with none reaching the “suggestive” or higher level. All 95% prediction intervals included the null value. Statistically, 44.4% of comparisons were significant at p < 0.05, but none remained significant at the stricter threshold of p < 0.001.
Subgroup analyses revealed significant effects in patients with diabetes (SMD = −0.33) and PCOS (SMD = −0.43), whereas no meaningful effect was observed in those with metabolic syndrome. However, no significant subgroup differences were found (χ2 = 1.38, p = 0.5022).
3.8 Cinnamon and SBP outcomes
Three studies evaluated the effect of cinnamon supplementation on systolic blood pressure (SBP). The pooled analysis demonstrated a significant reduction in SBP (SMD = −0.79, 95% CI: −1.18 to −0.40), with very low overall heterogeneity (I2 = 0%; Figure 10). However, substantial within-study heterogeneity was observed across all included studies (I2 > 50%). No small-study effects were detected, but two studies exhibited evidence of excess significance bias. Due to the limited number of studies (k = 3), Egger's regression test could not be reliably performed. The trim-and-fill analysis did not suggest substantial funnel plot asymmetry, however, given the small sample size, the power to detect publication bias was limited, and the findings should be interpreted with caution.
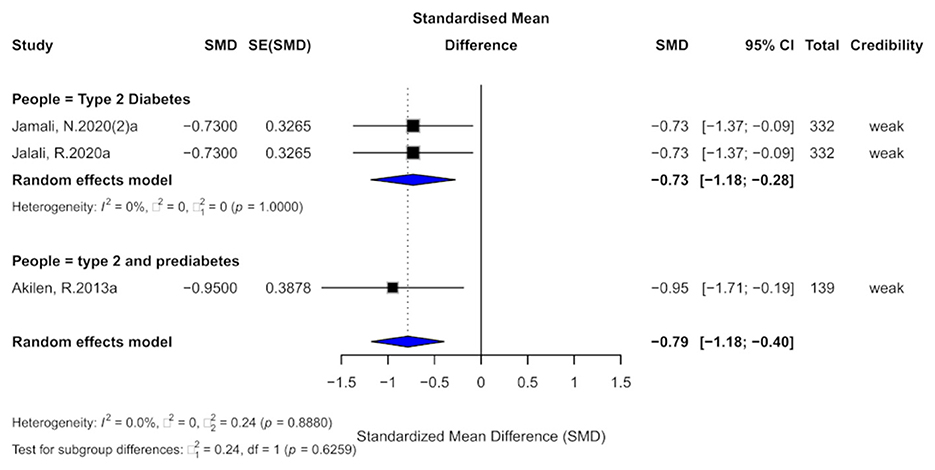
Figure 10. Forest plot of the effect of cinnamon supplementation on SBP in patients with metabolic diseases.
In terms of the credibility of evidence, all three studies were classified as providing “weak” evidence. The 95% prediction intervals for all studies included the null value, and although all results were statistically significant at the p < 0.05 level, none reached the threshold of high significance (p < 0.001).
3.9 Cinnamon and DBP outcomes
Three studies assessed the effect of cinnamon supplementation on DBP. The pooled analysis showed a significant reduction in DBP (SMD = −0.52, 95% CI: −0.81 to −0.23), with very low overall heterogeneity (I2 = 0%; Figure 11). However, two individual studies exhibited substantial within-study heterogeneity (I2 > 50%). No small-study effects were detected across the three studies, although one study demonstrated excess significance bias. The trim-and-fill analysis imputed two potentially missing studies, suggesting the possibility of publication bias. Due to the limited number of included studies (k = 3), Egger's regression test lacked sufficient power and was therefore not reliably performed. Overall, the current evidence is limited, and the findings should be interpreted with caution.
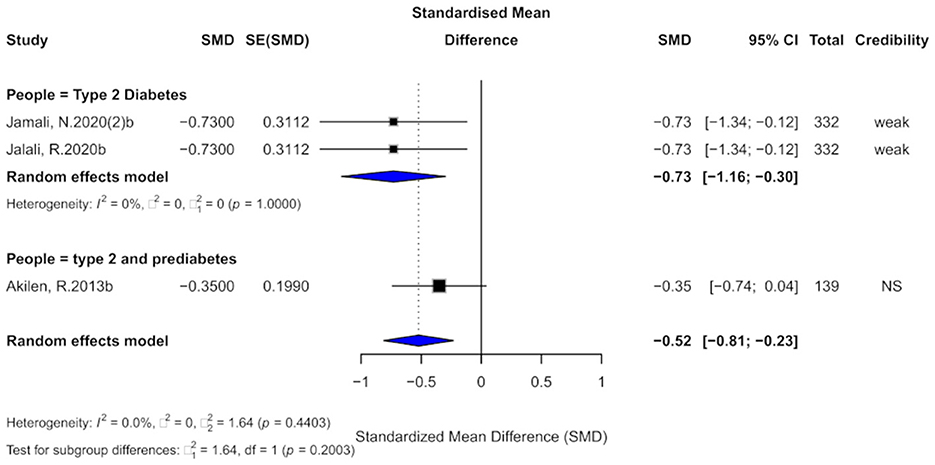
Figure 11. Forest plot of the effect of cinnamon supplementation on DBP in patients with metabolic diseases.
In terms of evidence credibility, two studies provided “weak” evidence, while one was classified as “non-significant.” The 95% prediction intervals for all studies included the null value. Although all three studies reported statistically significant results at p < 0.05, none reached the threshold of high significance (p < 0.001).
3.10 Cinnamon and BW outcomes
Six comparisons evaluated the effect of cinnamon supplementation on BW, yielding a small but statistically significant effect (SMD = −0.21, 95% CI: −0.37 to −0.04), with low heterogeneity across studies (I2 = 0%; Figure 12). In terms of evidence grading, all comparisons were classified as “non-significant,” with none reaching the “weak” or higher level. Therefore, the current evidence does not support a significant effect of cinnamon supplementation on BW in patients with metabolic diseases.
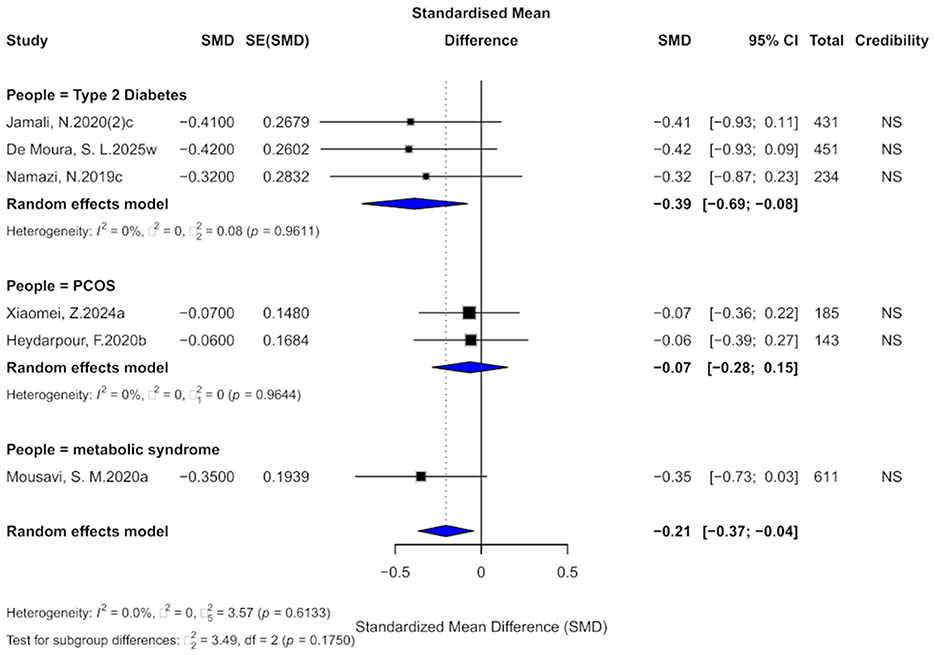
Figure 12. Forest plot of the effect of cinnamon supplementation on BW in patients with metabolic diseases.
3.11 Cinnamon and BMI outcomes
Seven comparisons assessed the impact of cinnamon supplementation on BMI, revealing a moderate and statistically significant reduction (SMD = −0.38, 95% CI: −0.59 to −0.17), with low heterogeneity observed across studies (I2 = 0%; Figure 13). Regarding evidence grading, all comparisons were categorized as “non-significant,” with none reaching the “weak” or higher level. Thus, current evidence does not provide strong support for a meaningful effect of cinnamon supplementation on BMI in individuals with metabolic diseases.
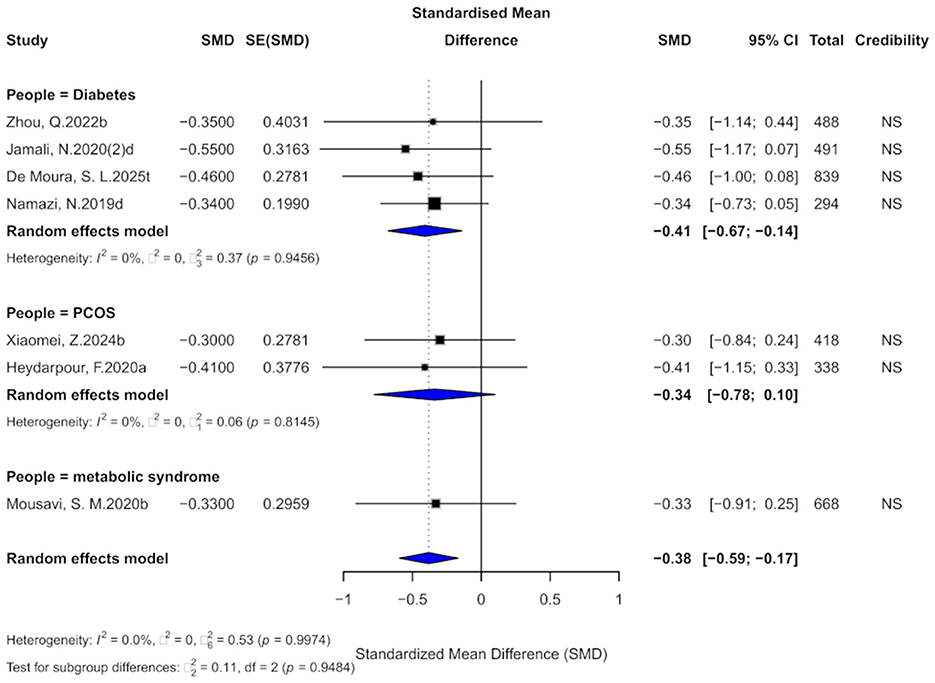
Figure 13. Forest plot of the effect of cinnamon supplementation on BMI in patients with metabolic diseases.
3.12 Cinnamon and adverse events outcomes
Among the 21 meta-analyses included in this study, only one reported on adverse events associated with cinnamon supplementation, with a relative risk of 0.83 (95% CI: 0.22–3.07). The level of evidence was classified as “non-significant,” and no definitive conclusions can be drawn at this time.
3.13 Re-estimation of effect sizes and credibility ceiling analysis results
Nr, Ns, and R were 209, 45, and 21, respectively, yielding a CCA value of 18.2%, which indicates a high degree of overlap among the included meta-analyses. Given the substantial redundancy, excluding overlapping reviews could have resulted in the omission of important studies and introduced selection bias. Therefore, we extracted and synthesized all relevant original studies from the included meta-analyses to conduct a reanalysis, aiming to provide a more comprehensive and less biased assessment of the current evidence.
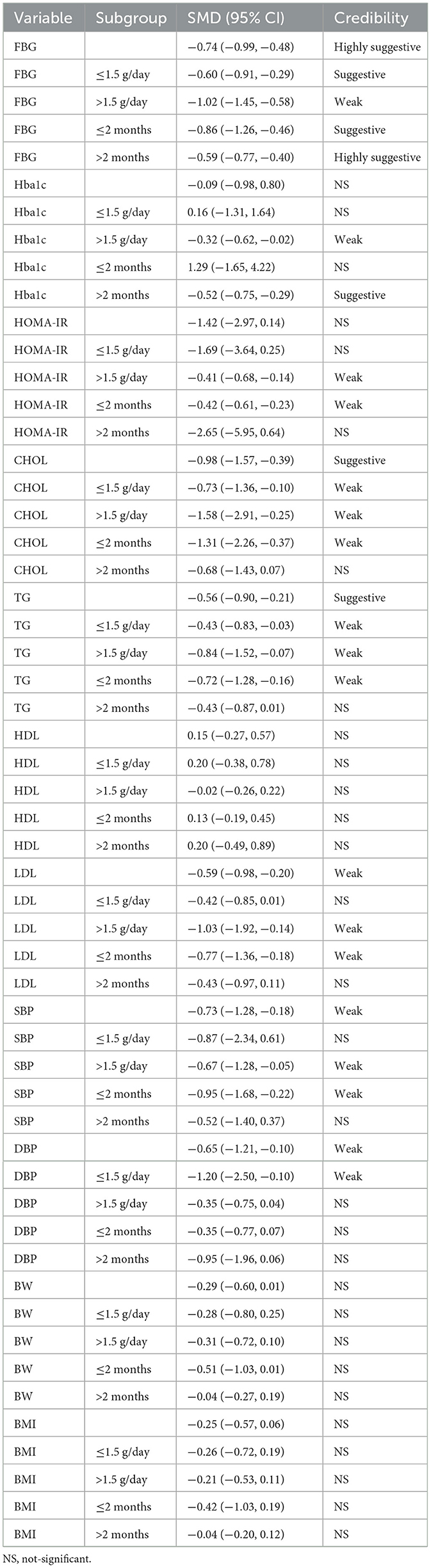
The pooled effect estimates of cinnamon supplementation on various metabolic outcomes in patients with metabolic diseases based on the reanalysis are presented in Table 3. Subgroup analyses were also performed according to the dosage and duration of supplementation, with detailed results shown in Table 3.
4 Discussion
This umbrella review highlights the potential role of cinnamon supplementation as a complementary approach for managing metabolic outcomes in patients with metabolic diseases. While variations in cinnamon form, dosage, intervention duration, and underlying disease conditions may contribute to heterogeneity across studies, the overall evidence suggests that cinnamon could improve glucose metabolism, lipid profiles, and other metabolic parameters. These findings underscore the promise of cinnamon as an adjunctive nutritional strategy, while also emphasizing the need for cautious interpretation.
In terms of glucose metabolism, this study selected FBG, HbA1c, HOMA-IR as the primary evaluation indicators. The results suggest that cinnamon supplementation may improve FBG in patients with metabolic diseases, with the highest level of evidence rated as “suggestive.” Given the substantial overlap among the original studies, we reanalyzed all relevant primary data, which continued to support the beneficial effect of cinnamon on glycemic control, with the evidence level upgraded to “highly suggestive.” Furthermore, higher doses (>1.5 g/day) and shorter intervention durations (≤2 months) were associated with more pronounced improvements, suggesting that short-term, high-dose interventions may yield more clinically meaningful benefits. Although cinnamon supplementation also showed trends toward improvement in HbA1c and HOMA-IR, the supporting evidence for these outcomes was consistently rated as “weak,” and reanalysis of the original data rendered the overall effects non-significant. Therefore, caution is warranted when interpreting the effects of cinnamon on HbA1c and HOMA-IR, and further high-quality studies are required to confirm these findings.
Several existing reviews and original studies have proposed the potential antidiabetic mechanisms of cinnamon (30–34). Purified cinnamon extract (CE) and cinnamon polyphenols (CP) have been shown to upregulate insulin receptor β (IRβ) and glucose transporter 4 (GLUT4) protein expression in 3T3-L1 adipocytes, thereby enhancing insulin signaling and glucose uptake. CP also increases GLUT4 levels, suggesting insulin-like activity and long-term regulation of glucose transport (33–36). Insulin resistance is associated with impaired GLUT4 translocation due to disrupted tyrosine phosphorylation of insulin receptor substrates (IRS) (35, 36). Methylhydroxychalcone polymer (MHCP), a bioactive compound in cinnamon, mimics insulin action by activating the IRS–PI3K pathway, promoting glucose uptake and glycogen synthesis, and inhibiting glycogen synthase kinase-3β (GSK-3β) (33, 35, 37). Moreover, cinnamon suppresses hepatic gluconeogenesis by downregulating phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase, and activates AMP-activated protein kinase (AMPK), leading to improved energy metabolism and upregulation of PPAR-α and PPAR-γ, which help modulate lipid and glucose metabolism (38–40). Additionally, cinnamon may exert glycemic benefits via gastrointestinal mechanisms, such as delaying gastric emptying and glucose absorption, and enhancing cellular glucose utilization (34, 41).
In terms of lipid metabolism, the findings suggest that cinnamon supplementation may lead to modest to moderate improvements in CHOL, TG, and LDL, although the highest level of evidence supporting these effects was rated as “weak.” No significant impact was observed on HDL. Subgroup analyses based on disease type indicated no substantial differences in the effects of cinnamon among patients with diabetes, metabolic syndrome, or PCOS. Following a re-analysis of all original study data, the beneficial effects on CHOL, TG, and LDL were further supported, and the evidence level for some outcomes was upgraded to “suggestive.” Additionally, subgroup analyses by dose and intervention duration showed that higher doses (>1.5 g/day) and shorter intervention periods (≤2 months) were associated with greater improvements in CHOL and TG.
Studies have shown that the lipid-lowering effects of cinnamon are mediated through multiple mechanisms. Firstly, cinnamon inhibits hepatic HMG-CoA reductase activity, thereby reducing endogenous cholesterol synthesis (42). It also promotes lipolysis, potentially by improving insulin resistance and suppressing the overproduction of intestinal apoB48-containing lipoproteins, thus contributing to lipid metabolism regulation (42, 43). Moreover, cinnamon is rich in polyphenolic compounds, which not only inhibit intestinal cholesterol absorption (44), but also upregulate the expression of peroxisome proliferator-activated receptor alpha (PPAR-α) in adipose tissue. This leads to enhanced lipoprotein lipase activity and improved uptake and metabolism of free fatty acids (32, 45, 46). Cinnamon also facilitates lipid metabolism via activation of antioxidant pathways. Animal studies have demonstrated that cinnamon supplementation significantly increases the expression of nuclear factor erythroid 2–related factor 2 (Nrf2) and its downstream effector heme oxygenase-1 (HO-1) (47). In addition, in vitro research indicates that cinnamic acid can inhibit pancreatic lipase activity, thereby reducing the hydrolysis of dietary TG and subsequent intestinal absorption of fatty acids, ultimately contributing to decreased LDL-C and increased HDL-C levels (48, 49). S-(+)-Linalool, a major component of cinnamon, has also been shown to significantly reduce plasma triglyceride (TG) levels and exert anti-adipogenic effects by inhibiting lipid accumulation in 3T3-L1 adipocytes (50).
The meta-analysis in this umbrella review found no statistically significant effects of cinnamon supplementation on BMI and BW, with all seven included outcomes being non-significant. Similarly, after re-extracting and reanalyzing data from the original studies, no significant differences were observed. This finding is consistent with the study by Namazi et al. (51) which also reported no significant improvements in BW or BMI following cinnamon supplementation. However, it contrasts with the meta-analysis by Mousavi et al. (52) which included 12 RCTs and found that cinnamon significantly reduced BW, BMI, waist circumference, and body fat percentage—particularly among individuals aged < 50 years or those with a baseline BMI ≥30 kg/m2. In addition, the umbrella review by Keramati et al. (53) supported the beneficial effects of cinnamon in significantly reducing BW and BMI. These discrepancies may be attributed to methodological differences. Unlike previous studies that commonly used mean difference (MD) or weighted mean difference (WMD) as effect sizes, the present study applied SMD for data synthesis. Moreover, we re-extracted baseline and post-intervention values from the original studies and calculated effect sizes based on pre- and post-intervention changes, rather than using only the final endpoint values. Such methodological distinctions may partly explain the inconsistent results across studies.
In terms of blood pressure regulation, the findings of this study indicate that cinnamon supplementation exerts a moderate to strong lowering effect on both SBP and DBP. However, the quality of evidence was mostly rated as “weak.” After re-extracting and re-analyzing all original study data, the SMDs remained largely unchanged, and the strength of evidence was consistent, suggesting that the conclusions are relatively robust. Subgroup analyses further revealed that the significant reduction in SBP was primarily observed in studies using a daily dose >1.5 g and an intervention duration of no more than 2 months. In contrast, the reduction in DBP was more pronounced in studies using a lower daily dose (≤1.5 g). This dose-response relationship suggests that cinnamon's effects on blood pressure may involve different mechanisms or threshold effects, warranting further investigation.
Oxidative stress plays a critical role in the onset and progression of diabetes and cardiovascular diseases (54). Evidence suggests that cinnamon can enhance the antioxidant status in individuals with metabolic syndrome, attenuate free radical generation (55), and lower plasma malondialdehyde concentrations (56), thereby reducing lipid peroxidation and potentially contributing to blood pressure regulation. With respect to vascular function, cinnamon has been shown to increase serum nitric oxide (NO) levels (57) and promote its production (58), facilitating vasodilation, while also stimulating the release of calcitonin gene-related peptide (CGRP) (59) and improving arterial wall compliance (60). It can suppress vascular smooth muscle cell proliferation (61) and downregulate the transcription and mRNA expression of endothelial factors, leading to reduced expression of vascular cell adhesion molecule-1 (VCAM-1) and SICAM-1 (62). On the metabolic side, cinnamon improves insulin resistance (55), helps maintain normal vascular contractility through modulation of Ca2+ influx (24), and alleviates hyperuricemia (63). Additionally, it may reduce sympathetic nerve activity (64) and mitigate resting tachycardia, neural hyperexcitability, and elevated plasma norepinephrine (64). Collectively, these mechanisms may act synergistically to lower blood pressure, with effects potentially more pronounced in individuals with diabetes or metabolic syndrome.
Due to the limited number of meta-analyses reporting adverse effects of cinnamon, with only one relevant meta-analysis included in this study (65), data re-pooling was not feasible. In the included meta-analysis, two primary studies reported adverse events in participants receiving cinnamon at a dose of 1 g: one case of rash (66) and one case of hives (67). Two other primary studies reported adverse events in the control groups: one case of nausea (68) and one case of mild gastric pain lasting 2 days (69). Overall, adverse events associated with oral cinnamon were infrequent and generally mild. The U.S. Food and Drug Administration (FDA) has classified cinnamon as Generally Recognized As Safe (GRAS). Current evidence indicates that cinnamon is well-tolerated at daily doses up to 6 grams, while higher doses may cause mild and self-limiting gastrointestinal or skin reactions (7, 70, 71). Systematic reviews also support its safety as a dietary component or herbal supplement (7, 65). Human safety data are limited, with most evidence derived from in vitro and animal studies, which suggest that high coumarin content may lead to hepatotoxicity, bleeding risks, allergic reactions, and potential carcinogenicity (72, 73). Overall, cinnamon is considered safe at appropriate doses, but its long-term safety requires further clinical investigation.
Recently, Qin et al. (74) confirmed in a review that cinnamon and its active components exert beneficial effects on multiple parameters related to metabolic syndrome, including insulin sensitivity, blood glucose levels, lipid regulation, antioxidant capacity, inflammation, blood pressure, and weight management, which aligns broadly with the findings of our study. Furthermore, the combined use of cinnamon with a low-carbohydrate ketogenic diet (LCKD) has shown potential in improving glycemic and blood pressure control (75). As a low-cost and readily accessible natural product, cinnamon demonstrates promising clinical application prospects and may serve as a complementary therapy and nutritional intervention. Future well-designed, high-quality clinical trials are warranted to further validate its long-term efficacy, safety, and underlying mechanisms, thereby facilitating its broader application in the management of metabolic diseases.
This umbrella review has several strengths. First, it comprehensively synthesizes published meta-analyses examining the association between cinnamon supplementation and metabolic outcomes in patients with metabolic diseases, covering a wide range of indicators. Second, a rigorous and systematic search strategy was employed across multiple databases, with study selection and data extraction independently conducted by two researchers, ensuring quality and objectivity. Third, pooled effect sizes for each meta-analysis were recalculated using a random-effects model, alongside assessments of heterogeneity, small-study effects, and excess significance bias, thereby enhancing the reliability of the findings. Fourth, to address overlap among included studies, we reanalyzed all original study data to minimize bias from duplicated data inclusion. Lastly, this review used pre- and post-intervention changes as the basis for data synthesis rather than relying solely on post-intervention values, which better controls for baseline differences.
Nevertheless, several limitations exist. First, due to methodological constraints, only meta-analyses with complete individual study data were included, potentially excluding relevant studies lacking comprehensive data. Second, despite stringent inclusion criteria, residual bias cannot be entirely ruled out, given heterogeneity in patient baseline characteristics, cinnamon varieties, and preparation methods. Finally, some meta-analyses included fewer than 10 studies, which limits the statistical power to detect small-study effects and excess significance bias, complicating the identification of potential sources of bias.
5 Conclusion
Cinnamon supplementation, as a natural metabolic modulator, has been extensively studied for its effects on metabolic disorder-related parameters. This study comprehensively evaluated the associations between cinnamon supplementation and metabolic indicators—including blood glucose, lipid profiles, blood pressure, and body weight—in patients with metabolic syndrome. The results demonstrated that cinnamon supplementation significantly improved fasting blood glucose and lipid levels, particularly among individuals with diabetes and metabolic syndrome. Subgroup analyses indicated that higher doses (>1.5 g/day) and shorter intervention durations (≤2 months) were more likely to yield clinically meaningful improvements. Additionally, cinnamon showed potential benefits in modulating insulin resistance, oxidative stress, and blood pressure regulation. These findings underscore the promising role of cinnamon as an adjunctive therapy and nutritional intervention in managing metabolic diseases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HG: Writing – review & editing, Writing – original draft. LZ: Data curation, Writing – review & editing, Software. QW: Resources, Writing – review & editing, Supervision, Funding acquisition. YF: Resources, Writing – review & editing, Methodology, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by Gansu Provincial Science and Technology Program (24YFFA045, 23JRRA1008, 23JRRA0979); University Faculty Innovation Fund Program (2024B-025); Cuiying Science and Technology Innovation Program (CY2023-QN-B01); and Gansu Clinical Medical Research Center Construction Project (21JR7RA433).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Esteves JV, Stanford KI. Exercise as a tool to mitigate metabolic disease. Am J Physiol Cell Physiol. (2024) 327:C587–98. doi: 10.1152/ajpcell.00144.2024
2. Chew NWS, Ng CH, Tan DJH, Kong G, Lin C, Chin YH, et al. The global burden of metabolic disease: data from 2000 to 2019. Cell Metab. (2023) 35:414–28.e3.
3. IDF Diabetes Atlas. Global Diabetes Data & Statistics (2025). Available online at: https://diabetesatlas.org/ (Accessed August 6, 2025).
4. World Health Organization. Obesity and Overweight (2025). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed August 6, 2025).
5. Kowalska J, Tyburski J, Matysiak K, Jakubowska M, Łukaszyk J, Krzymińska J. Cinnamon as a useful preventive substance for the care of human and plant health. Molecules. (2021) 26:5299. doi: 10.3390/molecules26175299
6. Rao PV, Gan SH. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Alternat Med. (2014) 2014:642942. doi: 10.1155/2014/642942
7. Hajimonfarednejad M, Ostovar M, Raee MJ, Hashempur MH, Mayer JG, Heydari M. Cinnamon: a systematic review of adverse events. Clin Nutr. (2019) 38:594–602. doi: 10.1016/j.clnu.2018.03.013
8. Kawatra P, Rajagopalan R. Cinnamon: mystic powers of a minute ingredient. Pharmacognosy Res. (2015) 7:S1–6. doi: 10.4103/0974-8490.157990
9. Wu T, Huang W, He M, Yue R. Effects of cinnamon supplementation on lipid profiles among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis. Complement Ther Clin Pract. (2022) 49:101625. doi: 10.1016/j.ctcp.2022.101625
10. Jamali N, Jalali M, Saffari-Chaleshtori J, Samare-Najaf M, Samareh A. Effect of cinnamon supplementation on blood pressure and anthropometric parameters in patients with type 2 diabetes: a systematic review and meta-analysis of clinical trials. Diabetes Metab Syndr. (2020) 14:119–25. doi: 10.1016/j.dsx.2020.01.009
11. Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. (2013) 11:452–9. doi: 10.1370/afm.1517
12. Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. (2021) 10:39. doi: 10.1186/s13643-020-01542-z
13. Sui J, Guo J, Pan D, Wang Y, Xu Y, Sun G, et al. The efficacy of dietary intake, supplementation, and blood concentrations of carotenoids in cancer prevention: insights from an umbrella meta-analysis. Foods. (2024) 13:1321. doi: 10.3390/foods13091321
14. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester: John Wiley & Sons (2021). p. 547. doi: 10.1002/9781119558378
15. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002
16. Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. (2007) 335:914–6. doi: 10.1136/bmj.39343.408449.80
17. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
18. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342:d549. doi: 10.1136/bmj.d549
19. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. (2001) 323:101–5. doi: 10.1136/bmj.323.7304.101
20. Ioannidis JPA, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. (2007) 4:245–53. doi: 10.1177/1740774507079441
21. Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, et al. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol. (2013) 11:e1001609. doi: 10.1371/journal.pbio.1001609
22. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. (2015) 350:g7607. doi: 10.1136/bmj.g7607
23. Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JPA. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. (2016) 23:1–9. doi: 10.1016/j.parkreldis.2015.12.008
24. Radua J, Ramella-Cravaro V, Ioannidis JPA, Reichenberg A, Phiphopthatsanee N, Amir T, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry. (2018) 17:49–66. doi: 10.1002/wps.20490
25. Pieper D, Antoine SL, Mathes T, Neugebauer EAM, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. (2014) 67:368–75. doi: 10.1016/j.jclinepi.2013.11.007
26. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. (2015) 13:132–40. doi: 10.1097/XEB.0000000000000055
27. Solmi M, De Toffol M, Kim JY, Choi MJ, Stubbs B, Thompson T, et al. Balancing risks and benefits of cannabis use: umbrella review of meta-analyses of randomised controlled trials and observational studies. BMJ. (2023) 382:e072348. doi: 10.1136/bmj-2022-072348
28. Cooper H, Koenka AC. The overview of reviews: unique challenges and opportunities when research syntheses are the principal elements of new integrative scholarship. Am Psychol. (2012) 67:446–62. doi: 10.1037/a0027119
29. Xie Y, Xu J, Zhou D, Guo M, Zhang M, Gao Y, et al. Micronutrient perspective on COVID-19: umbrella review and reanalysis of meta-analyses. Crit Rev Food Sci Nutr. (2024) 64:6783–801. doi: 10.1080/10408398.2023.2174948
30. Silva ML, Bernardo MA, Singh J, de Mesquita MF. Cinnamon as a complementary therapeutic approach for dysglycemia and dyslipidemia control in type 2 diabetes mellitus and its molecular mechanism of action: a review. Nutrients. (2022) 14:2773. doi: 10.3390/nu14132773
31. Medagama AB. The glycaemic outcomes of cinnamon, a review of the experimental evidence and clinical trials. Nutr J. (2015) 14:108. doi: 10.1186/s12937-015-0098-9
32. Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. (2008) 2008:581348. doi: 10.1155/2008/581348
33. Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys. (2007) 459:214–22. doi: 10.1016/j.abb.2006.12.034
34. Akilen R, Tsiami A, Devendra D, Robinson N. Cinnamon in glycaemic control: systematic review and meta analysis. Clin Nutr. (2012) 31:609–15. doi: 10.1016/j.clnu.2012.04.003
35. Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. (2000) 106:165–9. doi: 10.1172/JCI10582
36. Shepherd PR, Kahn BB. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. N Engl J Med. (1999) 341:248–57. doi: 10.1056/NEJM199907223410406
37. Jarvill-Taylor KJ, Anderson RA, Graves DJ. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J Am Coll Nutr. (2001) 20:327–36. doi: 10.1080/07315724.2001.10719053
38. Kopp C, Singh SP, Regenhard P, Müller U, Sauerwein H, Mielenz M. Trans-cinnamic acid increases adiponectin and the phosphorylation of AMP-activated protein kinase through G-protein-coupled receptor signaling in 3T3-L1 adipocytes. Int J Mol Sci. (2014) 15:2906–15. doi: 10.3390/ijms15022906
39. Leonardini A, Laviola L, Perrini S, Natalicchio A, Giorgino F. Cross-talk between PPARgamma and insulin signaling and modulation of insulin sensitivity. PPAR Res. (2009) 2009:818945. doi: 10.1155/2009/818945
40. Chan SMH, Sun RQ, Zeng XY, Choong ZH, Wang H, Watt MJ, et al. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes. (2013) 62:2095–105. doi: 10.2337/db12-1397
41. Hlebowicz J, Darwiche G, Björgell O, Almér LO. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J Clin Nutr. (2007) 85:1552–6. doi: 10.1093/ajcn/85.6.1552
42. Lee JS, Jeon SM, Park EM, Huh TL, Kwon OS, Lee MK, et al. Cinnamate supplementation enhances hepatic lipid metabolism and antioxidant defense systems in high cholesterol-fed rats. J Med Food. (2003) 6:183–91. doi: 10.1089/10966200360716599
43. Qin B, Polansky MM, Sato Y, Adeli K, Anderson RA. Cinnamon extract inhibits the postprandial overproduction of apolipoprotein B48-containing lipoproteins in fructose-fed animals. J Nutr Biochem. (2009) 20:901–8. doi: 10.1016/j.jnutbio.2008.08.005
44. Shalaby MA, Saifan HY. Some pharmacological effects of cinnamon and ginger herbs in obese diabetic rats. J Intercult Ethnopharmacol. (2014) 3:144–9. doi: 10.5455/jice.20140818050741
45. Shang C, Lin H, Fang X, Wang Y, Jiang Z, Qu Y, et al. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. (2021) 12:12194–220. doi: 10.1039/D1FO01935J
46. Kim SH, Choung SY. Antihyperglycemic and antihyperlipidemic action of Cinnamomi cassiae (cinnamon bark) extract in C57BL/ks db/db mice. Arch Pharm Res. (2010) 33:325–33. doi: 10.1007/s12272-010-0219-0
47. Tuzcu Z, Orhan C, Sahin N, Juturu V, Sahin K. Cinnamon polyphenol extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed rats. Oxid Med Cell Longev. (2017) 2017:1583098. doi: 10.1155/2017/1583098
48. Mnafgui K, Derbali A, Sayadi S, Gharsallah N, Elfeki A, Allouche N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet- induced obese rats. J Food Sci Technol. (2015) 52:4369–77. doi: 10.1007/s13197-014-1488-2
49. Subramaniam S, Subramaniam R, Rajapandian S, Uthrapathi S, Gnanamanickam VR, Dubey GP. Anti-atherogenic activity of ethanolic fraction of Terminalia arjuna bark on hypercholesterolemic rabbits. Evid Based Complement Alternat Med. (2011) 2011:487916. doi: 10.1093/ecam/neq003
50. Cheng BH, Sheen LY, Chang ST. Hypolipidemic effects of S-(+)-linalool and essential oil from Cinnamomum osmophloeum ct. linalool leaves in mice. J Tradit Complement Med. (2018) 8:46–52. doi: 10.1016/j.jtcme.2017.02.002
51. Namazi N, Khodamoradi K, Khamechi SP, Heshmati J, Ayati MH, Larijani B. The impact of cinnamon on anthropometric indices and glycemic status in patients with type 2 diabetes: a systematic review and meta-analysis of clinical trials. Complement Ther Med. (2019) 43:92–101. doi: 10.1016/j.ctim.2019.01.002
52. Mousavi SM, Rahmani J, Kord-Varkaneh H, Sheikhi A, Larijani B, Esmaillzadeh A. Cinnamon supplementation positively affects obesity: a systematic review and dose-response meta-analysis of randomized controlled trials. Clin Nutr. (2020) 39:123–33. doi: 10.1016/j.clnu.2019.02.017
53. Keramati M, Musazadeh V, Malekahmadi M, Jamilian P, Jamilian P, Ghoreishi Z, et al. Cinnamon, an effective anti-obesity agent: evidence from an umbrella meta-analysis. J Food Biochem. (2022) 46:e14166. doi: 10.1111/jfbc.14166
54. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. (2012) 28:539–43. doi: 10.1016/j.nut.2011.08.013
55. Mancini-Filho J, Van-Koiij A, Mancini DA, Cozzolino FF, Torres RP. Antioxidant activity of cinnamon (Cinnamomum zeylanicum, breyne) extracts. Boll Chim Farm. (1998) 137:443–7.
56. Roussel AM, Hininger I, Ziegenfuss TN, Anderson RA. Cinnamon improves the antioxidant variables of people with impaired fasting glucose. J Am Coll Nutr. (2006) 25:443.
57. Amin KA, Abd El-Twab TM. Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: role of atorvastatine and cinnamon. Int J Clin Exp Med. (2009) 2:254–65.
58. Nyadjeu P, Nguelefack-Mbuyo EP, Atsamo AD, Nguelefack TB, Dongmo AB, Kamanyi A. Acute and chronic antihypertensive effects of Cinnamomum zeylanicum stem bark methanol extract in L-NAME-induced hypertensive rats. BMC Complement Altern Med. (2013) 13:27. doi: 10.1186/1472-6882-13-27
59. Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. (2012) 167:13–22. doi: 10.1111/j.1476-5381.2012.02018.x
60. Ranasinghe P, Jayawardena R, Pigera S, Wathurapatha WS, Weeratunga HD, Premakumara GAS, et al. Evaluation of pharmacodynamic properties and safety of Cinnamomum zeylanicum (ceylon cinnamon) in healthy adults: a phase I clinical trial. BMC Complement Altern Med. (2017) 17:550. doi: 10.1186/s12906-017-2067-7
61. Kwon H, Lee JJ, Lee JH, Cho WK, Gu MJ, Lee KJ, et al. Cinnamon and its components suppress vascular smooth muscle cell proliferation by up-regulating cyclin-dependent kinase inhibitors. Am J Chin Med. (2015) 43:621–36. doi: 10.1142/S0192415X1550038X
62. Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun YW, Wung BS. Cinnamaldehyde inhibits the tumor necrosis factor-alpha-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-kappaB activation: effects upon IkappaB and Nrf2. Toxicol Appl Pharmacol. (2008) 229:161–71. doi: 10.1016/j.taap.2008.01.021
63. Rao A, Pandya V, Whaley-Connell A. Obesity and insulin resistance in resistant hypertension: implications for the kidney. Adv Chronic Kidney Dis. (2015) 22:211–7. doi: 10.1053/j.ackd.2014.12.004
64. Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. (2007) 25:909–20. doi: 10.1097/HJH.0b013e328048d004
65. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. (2012) 2012:CD007170. doi: 10.1002/14651858.CD007170.pub2
66. Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med. (2009) 22:507–12. doi: 10.3122/jabfm.2009.05.080093
67. Altschuler JA, Casella SJ, MacKenzie TA, Curtis KM. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care. (2007) 30:813–6. doi: 10.2337/dc06-1871
68. Rosado J. A Study to Determine the Effects of Cinnamon on Blood Glucose and Lipid Levels in Person's With Type-2 Diabetes. (2010). Available online at: https://scholarspace.manoa.hawaii.edu/bitstream/10125/22065/2/uhm_phd_rosado-j_r.pdf (Accessed October 5, 2025).
69. Akilen R, Tsiami A, Devendra D, Robinson N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial. Diabetic Medicine. (2010) 27:1159–67. doi: 10.1111/j.1464-5491.2010.03079.x
70. Hajimonfarednejad M, Nimrouzi M, Heydari M, Zarshenas MM, Raee MJ, Jahromi BN. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: a randomized double-blind placebo controlled clinical trial. Phytother Res. (2018) 32:276–83. doi: 10.1002/ptr.5970
71. Gonçalves LL, Fernandes T, Bernardo MA, Brito JA. Assessment of human health risk of toxic elements due to cinnamon ingestion in the diet. Biol Trace Elem Res. (2019) 189:313–24. doi: 10.1007/s12011-018-1473-0
72. American Diabetes Association, Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American diabetes association. Diabetes Care. (2008) 31 Suppl 1:S61–78. doi: 10.2337/dc08-S061
73. Abraham K, Wöhrlin F, Lindtner O, Heinemeyer G, Lampen A. Toxicology and risk assessment of coumarin: focus on human data. Mol Nutr Food Res. (2010) 54:228–39. doi: 10.1002/mnfr.200900281
74. Qin B, Panickar KS, Anderson RA. Cinnamon: potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J Diabetes Sci Technol. (2010) 4:685–93. doi: 10.1177/193229681000400324
75. Hussain TA, Mathew TC, Dashti AA, Asfar S, Al-Zaid N, Dashti HM. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition. (2012) 28:1016–21. doi: 10.1016/j.nut.2012.01.016
76. Mandal A, Sharma S, Rani R, Ranjan S, Kant R, Mirza A. Impact of cassia bark consumption on glucose and lipid control in type 2 diabetes: an updated systematic review and meta-analysis. Cureus. (2021) 13:e16376. doi: 10.7759/cureus.16376
77. Baker WL, Gutierrez-Williams G, White CM, Kluger J, Coleman CI. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care. (2008) 31:41–3. doi: 10.2337/dc07-1711
78. Zhou Q, Lei X, Fu S, Li Z, Chen Y, Long C, et al. Efficacy of cinnamon supplementation on glycolipid metabolism in T2DM diabetes: a meta-analysis and systematic review. Front Physiol. (2022) 13:960580. doi: 10.3389/fphys.2022.960580
79. Jamali N, Kazemi A, Saffari-Chaleshtori J, Samare-Najaf M, Mohammadi V, Clark CCT. The effect of cinnamon supplementation on lipid profiles in patients with type 2 diabetes: a systematic review and meta-analysis of clinical trials. Complement Ther Med. (2020) 55:102571. doi: 10.1016/j.ctim.2020.102571
80. Jalali R, Mahmoodi M, Moosavian SP, Ferns GA, Sohrabi Z. Cinnamon supplementation improves blood pressure in type 2 diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Clinical Diabetology. (2020) 9:259–66. doi: 10.5603/DK.2020.0021
81. Akilen R, Pimlott Z, Tsiami A, Robinson N. Effect of short-term administration of cinnamon on blood pressure in patients with prediabetes and type 2 diabetes. Nutrition. (2013) 29:1192–6. doi: 10.1016/j.nut.2013.03.007
82. Yu T, Lu K, Cao X, Xia H, Wang S, Sun G, et al. The effect of cinnamon on glycolipid metabolism: a dose-response meta-analysis of randomized controlled trials. Nutrients. (2023) 15:2983. doi: 10.3390/nu15132983
83. de Moura SL, Gomes BGR, Guilarducci MJ, Coelho OGL, Guimarães NS, Gomes JMG. Effects of cinnamon supplementation on metabolic biomarkers in individuals with type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. (2025) 83:249–79. doi: 10.1093/nutrit/nuae058
84. Moridpour AH, Kavyani Z, Khosravi S, Farmani E, Daneshvar M, Musazadeh V, et al. The effect of cinnamon supplementation on glycemic control in patients with type 2 diabetes mellitus: an updated systematic review and dose-response meta-analysis of randomized controlled trials. Phytother Res. (2024) 38:117–30. doi: 10.1002/ptr.8026
85. Suksomboon N, Poolsup N, Boonkaew S, Suthisisang CC. Meta-analysis of the effect of herbal supplement on glycemic control in type 2 diabetes. J Ethnopharmacol. (2011) 137:1328–33. doi: 10.1016/j.jep.2011.07.059
86. Garza MC, Pérez-Calahorra S, Rodrigo-Carbó C, Sánchez-Calavera MA, Jarauta E, Mateo-Gallego R, et al. Effect of aromatic herbs and spices present in the mediterranean diet on the glycemic profile in type 2 diabetes subjects: a systematic review and meta-analysis. Nutrients. (2024) 16:756. doi: 10.3390/nu16060756
87. Xiaomei Z, Xiaoyan F. Effect of cinnamon as a Chinese herbal medicine on markers of cardiovascular risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. (2024) 300:253–61. doi: 10.1016/j.ejogrb.2024.07.032
88. Heydarpour F, Hemati N, Hadi A, Moradi S, Mohammadi E, Farzaei MH. Effects of cinnamon on controlling metabolic parameters of polycystic ovary syndrome: a systematic review and meta-analysis. J Ethnopharmacol. (2020) 254:112741. doi: 10.1016/j.jep.2020.112741
Keywords: cinnamon, metabolic diseases, umbrella, meta-analysis, systematic review
Citation: Gou H, Zhong L, Wei Q and Fan Y (2025) The effects of cinnamon on patients with metabolic diseases: an umbrella review of meta-analyses of randomized controlled trials. Front. Nutr. 12:1683477. doi: 10.3389/fnut.2025.1683477
Received: 11 August 2025; Accepted: 14 October 2025;
Published: 03 November 2025.
Edited by:
Omar Guzmán Quevedo, Higher Technological Institute of Tacambaro, MexicoReviewed by:
Amy Stockert, Ohio Northern University, United StatesMonika Nuffer, University of Colorado Anschutz Medical Campus, United States
Copyright © 2025 Gou, Zhong, Wei and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuya Wei, d2VpcXkyMEBsenUuZWR1LmNu; Yong Fan, ZmFueW9uZzE5NzJAMTYzLmNvbQ==
 Haobo Gou
Haobo Gou Ling Zhong
Ling Zhong Qiuya Wei
Qiuya Wei Yong Fan1*
Yong Fan1*