Abstract
Objectives:
This network meta-analysis aimed to evaluate the comparative effectiveness of different exercise and nutritional interventions on muscle strength, skeletal muscle mass, and physical function in older adults with sarcopenia diagnosed according to the European Working Group on Sarcopenia in Older People and Asian Working Group for Sarcopenia criteria.
Methods:
We systematically searched PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials up to July 2025. A Bayesian random-effects network meta-analysis was performed, with additional subgroup and meta-regression analyses. Certainty of evidence was assessed using the Confidence in Network Meta-Analysis (CINeMA) framework, and interventions were ranked according to their relative effectiveness and certainty of evidence. This study was registered in PROSPERO (CRD420251124534).
Results:
A total of 35 randomised controlled trials involving 2,331 participants were included. Exercise combined with nutritional supplementation was the most effective intervention for improving handgrip strength (MD = 3.69, 95% CrI 0.72 to 5.10; SUCRA 99.04%), gait speed (MD = 0.11, 95% CrI 0.03 to 0.17; SUCRA 87.12%), and appendicular skeletal muscle mass index (ASMI) (MD = 0.35, 95% CrI 0.19 to 0.49; SUCRA 99.82%), with improvements in handgrip strength and ASMI significantly greater than those achieved with Exercise or Nutrition alone. Exercise alone improved handgrip strength, gait speed, and ASMI, whereas protein supplementation alone improved handgrip strength and gait speed but had no significant effect on ASMI. Subgroup analyses indicated that resistance training with protein supplementation produced the most significant improvements in handgrip strength and gait speed. In contrast, resistance training with protein and vitamin D supplementation was most effective for improving ASMI. Meta-regression analysis did not identify any significant sources of heterogeneity.
Conclusion:
These findings support combined exercise and nutritional interventions as a preferred treatment option for improving muscle strength, muscle mass, and physical function in older adults with sarcopenia. However, the overall certainty of the evidence ranged from low to very low. In particular, multicomponent exercise programmes centred on resistance training and combined with protein supplementation may offer superior benefits for enhancing muscle strength and physical function.
1 Introduction
Sarcopenia is an age-related condition characterised by loss of muscle mass, decline in muscle strength, and deterioration in physical function (1, 2). It is associated with an increased risk of adverse clinical outcomes, including disability, falls, and mortality (1, 2). It is common among older adults, particularly those aged 65 years and above. Depending on the diagnostic criteria and population background, prevalence estimates vary widely from 10 to 50%, and even the most conservative estimates suggest that 5–10% of the general population is affected (3, 4). This burden represents a persistent and substantial challenge for public health systems and the allocation of healthcare resources (5).
Currently, no pharmacological therapy has been proven in clinical practice to have definitive efficacy against sarcopenia (6). International guidelines consistently recommend non-pharmacological interventions as the preferred approach, with particular emphasis on the role of modifiable factors such as exercise and nutrition in prevention and reversal (2, 7). These strategies are considered feasible to implement and carry significant public health relevance (6). Among them, resistance training is recognised as one of the most effective interventions for increasing muscle strength and mass and is supported by high-quality evidence (2, 5, 7). Its effects are primarily mediated through mechanical loading that activates the mTORC1 signalling pathway, thereby enhancing muscle protein synthesis, while also improving motor unit recruitment and neuromuscular coordination to promote gains in strength and function (8). However, because current resistance training guidelines are primarily based on machine- or free-weight exercises, standardized programmes may lack key components that facilitate functional transfer, and the resulting strength gains may not fully translate into functional improvements (9). In fact, functional recovery requires not only strength but also neuromuscular control, balance, and coordination, and therefore resistance training alone is often insufficient to provide comprehensive benefits for individuals with functional limitations (10). For individuals with functional limitations, resistance training alone may not provide comprehensive benefits (11). In clinical settings, resistance training is frequently incorporated into multicomponent programmes that include aerobic and balance training to facilitate the translation of strength gains into functional improvements (10). In parallel, Protein supplementation is a key nutritional strategy to enhance muscle protein synthesis after exercise and is recommended in international consensus statements as an important adjunctive therapy (7). Leucine-rich proteins can directly stimulate mTORC1 signalling and provide the substrates required for protein accretion, thereby amplifying the post-exercise anabolic response and partially overcoming age-related anabolic resistance (12). Although protein supplementation is often combined with exercise in clinical practice to reinforce training effects, the proposed synergistic effect remains debated. It has not yet been confirmed by high-quality evidence (5, 7, 13).
In addition, with the growing diversity of exercise and nutritional interventions, and their various combinations, it remains unclear which strategies provide the greatest clinical benefits for sarcopenia, particularly in terms of skeletal muscle mass, muscle strength, and physical performance. However, direct head-to-head comparisons between different interventions are scarce, and previous reviews have often been unable to determine the relative effectiveness of exercise, nutrition, or their combination. A further major challenge lies in the lack of universally accepted diagnostic criteria, with many primary studies using inconsistent or even non-standard definitions. This has led to systematic differences in participants’ functional status and disease severity across studies, thereby undermining the interpretability and generalisability of the findings (12, 13). As a result, this methodological heterogeneity has also constrained the conclusions of earlier meta-analyses, which often pooled trials with disparate definitions and thus provided evidence of limited clinical applicability.
To address this limitation, the present study employed a network meta-analysis to compare and synthesise the available interventions systematically. However, in network meta-analyses, ensuring comparability of disease severity and functional status across study populations is a prerequisite for minimising between-study heterogeneity and ensuring the reliability of intervention rankings (14). To address this methodological challenge and strengthen clinical applicability, the present study included only randomised controlled trials that applied the most internationally recognised and clinically relevant consensus definitions for sarcopenia, namely the EWGSOP and AWGS criteria (15, 16). Within this standardised diagnostic framework, we synthesised the best available evidence to compare and rank the relative effects of different exercise and nutritional interventions on skeletal muscle mass, muscle strength, and physical performance in older adults with sarcopenia. We aimed to generate robust evidence-based recommendations to guide clinical practice.
2 Methods
2.1 Protocol and registration
The protocol for this systematic review and network meta-analysis was registered in PROSPERO (CRD420251124534). The study followed the PRISMA 2020 guidelines for systematic reviews and meta-analyses and the PRISMA-NMA extension for network meta-analyses (17, 18).
2.2 Search strategy and study selection
We searched PubMed, Web of Science, the Cochrane Central Register of Controlled Trials (CENTRAL), and Embase for randomised controlled trials (RCTs) published from database inception to July 2025. The search strategy combined Medical Subject Headings (MeSH) and free-text keywords, with Boolean operators (AND, OR) applied to ensure comprehensive coverage. An example of a core search string was: Sarcopenia[MeSH Terms] AND (Resistance training[MeSH Terms] OR Whey Proteins[MeSH Terms] OR beta-hydroxyisovaleric acid[MeSH Terms] OR Amino Acids, Essential[MeSH Terms] OR Leucine[MeSH Terms] OR Amino Acids, Branched-Chain[MeSH Terms] OR Amino Acids[MeSH Terms]). The complete search strategies for each database are provided in Appendix 1. For study selection, three reviewers (GS, BW, and LX) independently screened titles, abstracts, and full texts according to predefined eligibility criteria. Disagreements were resolved through consultation with a fourth reviewer (YE). To minimise the risk of missing relevant studies, we also screened the reference lists of included articles and related systematic reviews.
2.3 Eligibility criteria
Eligibility was assessed using the PICOS framework (Participants, Interventions, Comparators, Outcomes, and Study design) (19). Studies were included if they met all of the following criteria: (1) Participants were adults aged ≥65 years with sarcopenia diagnosed according to consensus definitions from either the European Working Group on Sarcopenia in Older People (EWGSOP) or the Asian Working Group for Sarcopenia (AWGS). (2) Interventions consisted of structured exercise programmes centred on resistance training, protein-based supplementation, or a combination of the two. (3) Control groups received health education, usual care, or placebo. (4) At least one of the following primary outcomes was reported: handgrip strength, gait speed, or appendicular skeletal muscle mass index (ASMI). These outcomes are widely recommended in international consensus statements as core measures of muscle strength, physical performance, and skeletal muscle mass (2). (5) Study design was restricted to RCTs.
Exclusion criteria were: (1) Participants with sarcopenia secondary to specific health conditions such as cancer, diabetes, stroke, HIV, chronic obstructive pulmonary disease, chronic kidney disease, liver cirrhosis, other severe illnesses, or recent organ transplantation. (2) Studies published in languages other than English. (3) Studies without sufficient data for analysis. (4) Full-text articles unavailable from databases or other sources.
2.4 Data extraction
Data were extracted independently by two reviewers (RT and YJ) using a predesigned standardized Excel spreadsheet (Microsoft Excel, 2019; Microsoft Corporation, Redmond, WA, USA) and verified by a third reviewer (JW). Extracted data included: study characteristics (first author, year, country, diagnostic criteria), population characteristics (age, sample size), intervention characteristics (duration, type of exercise, nutritional supplementation details), and outcome data (mean and standard deviation for all continuous variables). Where data were missing, up to three email requests were sent to corresponding authors at three-week intervals.
2.5 Measures of treatment effect
Treatment effects were expressed as mean differences (MD) with standard deviations (SD). When SDs were not reported, they were calculated from standard errors, 95% confidence intervals, p values, or t statistics (20). For studies lacking SDs of change scores, we estimated these using the following formula:
Where the correlation coefficient (r) was set at 0.5, reflecting a moderate level of test–retest reliability commonly accepted in the literature (20). This assumption balances potential variability between pre- and post-intervention measurements to enhance robustness.
2.6 Quality assessment of evidence
The risk of bias for each included trial was assessed using the Cochrane Risk of Bias tool for randomised trials (ROB 2.0), covering random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting (21). A study was considered to have an overall low risk of bias if all domains were rated as low risk (score 1). If at least one domain was rated as high risk, the overall risk of bias was considered high (score 3). In all other cases, the risk of bias was considered to raise some concerns (score 2). Two reviewers independently performed the assessments, and disagreements were resolved through discussion.
The certainty of evidence was evaluated using the CINeMA framework, considering six domains: within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence (22, 23). These domains address potential systematic errors within individual studies, the impact of selective reporting and publication bias, the relevance of evidence to the research question, the degree of uncertainty in effect estimates, consistency across study results, and the agreement between direct and indirect evidence.
2.7 Minimally contextualised framework
A minimally contextualised framework was applied to grade imprecision, using the control group as the reference and classifying interventions according to the magnitude of effect and certainty of evidence (24). An effect size of zero was taken as the threshold for no effect. Interventions were categorised as among the most effective if the credible interval did not include zero. The point estimate was clearly away from the null, and intermediate if the point estimate was away from the null. Still, the credible interval was close to or overlapped zero, and among the least effective if the credible interval included zero and the point estimate was close to zero (25, 26). Certainty of evidence was further classified as high or moderate versus low or very low based on the GRADE framework to guide interpretation (24).
To assess clinical relevance, we referred to previously established minimal important differences (MID) for key sarcopenia outcomes: 5.0 kg for handgrip strength (27) and 0.10 m/s for gait speed (28).
2.8 Statistical analysis
The network meta-analysis was conducted in R (version 4.3.3; R Foundation for Statistical Computing, Vienna, Austria) using the multinma package within a Bayesian framework. The treatment network was depicted with nodes representing interventions and edges representing head-to-head comparisons. Treatment effects were estimated using MCMC methods, with random-effects models fitted to account for between-study heterogeneity (29, 30). MD with 95% credible intervals (CrIs) were used as the measure of effect for all outcomes, applying consistent units or scales across studies. Heterogeneity was quantified using τ2, interpreted as low (<0.04), low to moderate (0.04–0.16), moderate to high (0.16–0.36), or high (>0.36) (31, 32). Global inconsistency was assessed by comparing model fit between the consistency model and the unrelated mean effects model, using residual deviance, deviance information criterion (DIC), and the effective number of parameters (pD).^33 Local inconsistency was examined using the node-splitting method, which compares direct and indirect evidence; a p-value below 0.05 was considered to indicate significant inconsistency (33).
To rank interventions, the surface under the cumulative ranking curve (SUCRA) was calculated using the MetaInsight tool (version 6.4.0; University of Leicester, Leicester, UK). SUCRA values quantify the overall probability of each intervention being the best option, enabling the identification of the most effective approach (34). Meta-regression analyses were conducted to explore potential effect modifiers, including age, proportion of male participants, sample size, intervention duration, baseline BMI, baseline handgrip strength, and baseline ASMI, and to test the robustness of the results. Publication bias was assessed by visual inspection of funnel plots generated using the netmeta package and further evaluated with Egger’s test.
3 Results
3.1 Literature selection and study characteristics
The systematic search identified 3,996 potential records. After removal of duplicates, 3,423 articles remained for title and abstract screening. Of these, 74 articles were retrieved for full-text review. In total, 35 studies met the inclusion criteria and were included in the systematic review and meta-analysis, comprising 2,331 participants with a mean age of 74.95 (SD 5.58) years. The whole selection process is presented in Figure 1, detailed characteristics of included studies are provided in Appendix 2, and the complete search strategies are reported in Appendix 1.
Figure 1
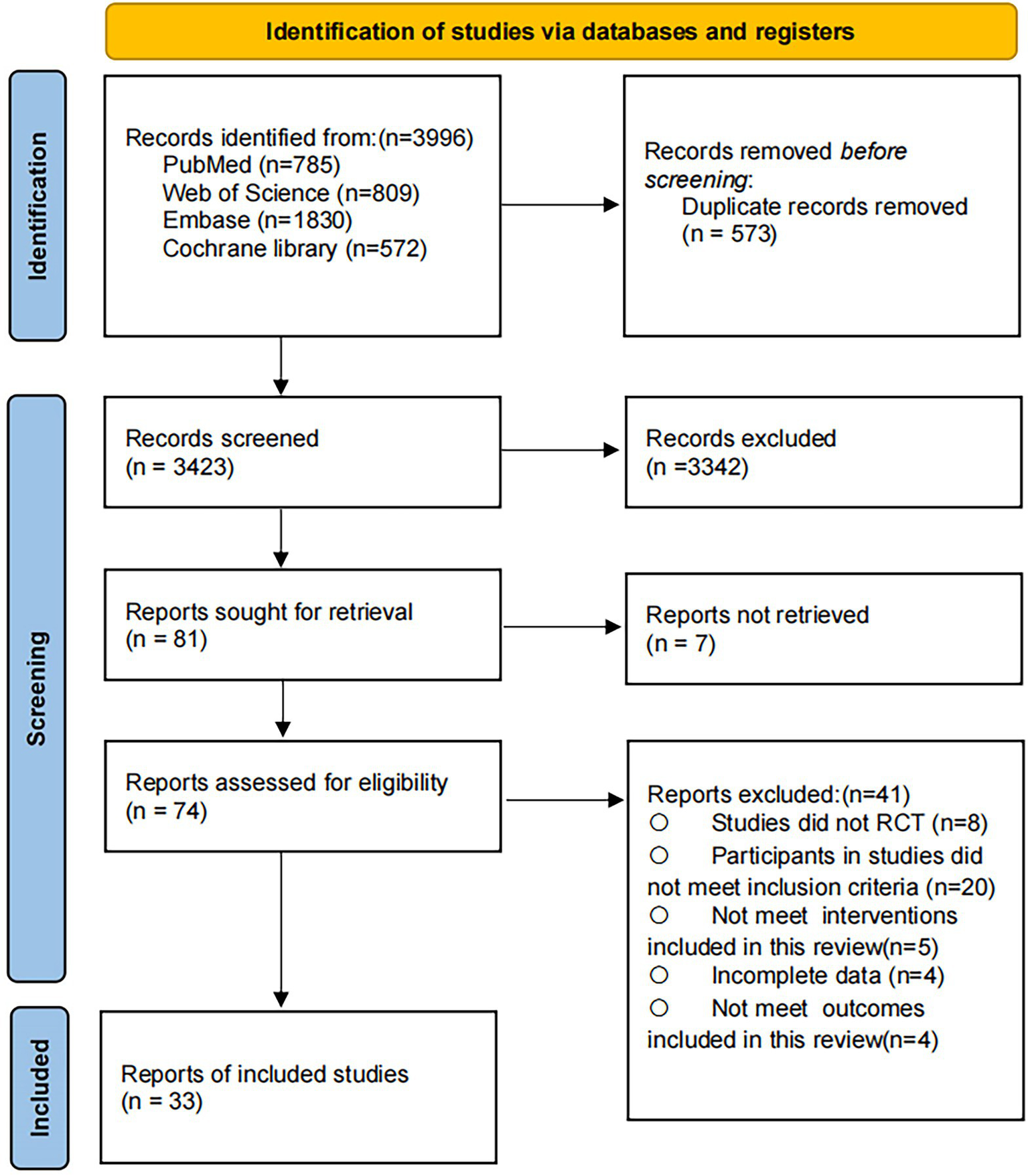
PRISMA flow diagram of the search process for studies.
3.2 Risk of bias and certainty of evidence
Overall, 15 studies (42.9%) were judged to be at low risk of bias, 14 (40%) at some concerns, and 6 (17.1%) at high risk of bias (Figure 2). Risk of bias assessments for each trial are provided in Appendix 3. To evaluate global consistency within the network, the goodness-of-fit of the consistency and inconsistency models was compared. Across all outcomes, the difference in the deviance information criterion (DIC) between the two models was less than 5, indicating comparable fit and no statistically significant evidence of global inconsistency (Appendix 4). In local consistency assessment, the node-splitting approach identified discrepancies between direct and indirect evidence for specific comparisons, leading to downgrading of the certainty for those specific outcomes (Appendix 5). Using the CINeMA framework, the certainty of evidence for all pairwise comparisons was rated, with most classified as “very low” to “low” (Appendix 8). The results of the minimally contextualised framework are summarised in Table 1. Funnel plot analysis did not reveal evidence of asymmetry, suggesting no substantial publication bias (Appendix 9).
Figure 2
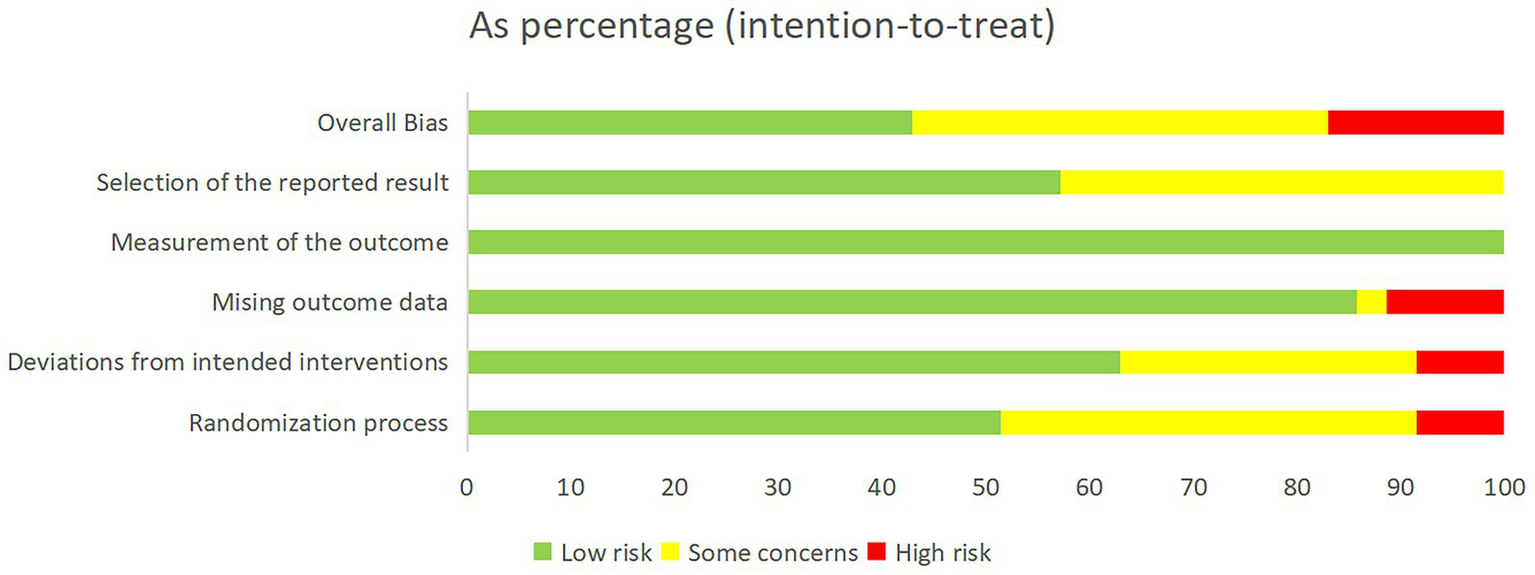
Overall risk of bias is presented as a percentage of each risk of bias item across all included studies.
Table 1
| Outcome | Certainty of evidence | Group | Intervention | Intervention vs Control | SUCRA |
|---|---|---|---|---|---|
| Handgrip strength | Low certainty (low to very low certainty evidence) | Category 2: among the most effective | Exercise + Nutrition | 3.69 (0.72; 5.10) | 99.04 |
| Category 1: intermediately effective | Exercise | 2.21 (0.6; 3.37) | 54.85 | ||
| Nutrition | 1.95 (0.69; 3.32) | 45.95 | |||
| Gait speed | Low certainty (low to very low certainty evidence) | Category 2: among the most effective | Exercise + Nutrition | 0.11 (0.03; 0.17) | 87.12 |
| Category 1: intermediately effective | Nutrition | 0.08 (0.03; 0.14) | 60.33 | ||
| Exercise | 0.07 (0.02; 0.12) | 52.34 | |||
| Appendicular skeletal muscle mass index | Low certainty (low to very low certainty evidence) | Category 2: among the most effective | Exercise + Nutrition | 0.35 (0.19; 0.49) | 99.82 |
| Category 1: intermediately effective | Exercise | 0.16 (0.03; 0.28) | 54.06 | ||
| Category 0: among the least effective | Nutrition | 0.11 (−0.02; 0.23) | 44.66 |
The results of the minimally contextualised framework.
3.3 Muscle strength
Thirty studies involving 1954 participants reported changes in handgrip strength (Figure 3). Low- or very low-certainty evidence indicated that Exercise + Nutrition (MD = 3.69, 95% CI 0.72 to 5.10; SUCRA 99.04%), exercise alone (MD = 2.21, 95% CI 0.60 to 3.37; SUCRA 54.85%), and nutrition alone (MD = 1.95, 95% CI 0.69 to 3.32; SUCRA 45.95%) all significantly improved handgrip strength. However, although all three interventions reached statistical significance, none of their effect estimates reached the predefined MID threshold of 5 kg, indicating limited clinical significance. Nevertheless, further comparisons showed that Exercise + Nutrition was significantly more effective than either Exercise or Nutrition alone.
Figure 3
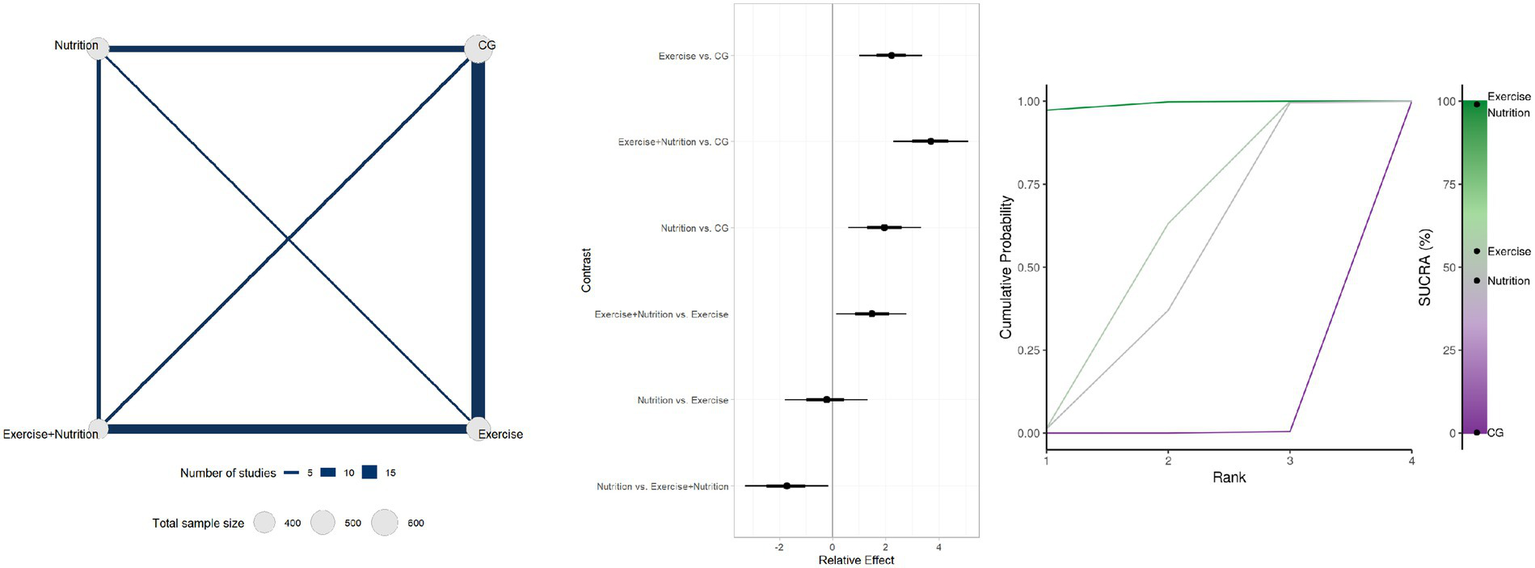
Summary of network meta-analysis results for handgrip strength: evidence network, relative effects, and SUCRA ranking.
3.4 Physical function
Twenty-seven studies involving 1804 participants reported changes in gait speed (Figure 4). Low-certainty evidence indicated that Exercise + Nutrition (MD = 0.11, 95% CrI 0.03 to 0.17; SUCRA 87.12%), Exercise alone (MD = 0.07, 95% CrI 0.02 to 0.12; SUCRA 60.33%), and nutrition alone (MD = 0.08, 95% CrI 0.03 to 0.14; SUCRA 52.34%) all significantly improved gait speed. However, only the effect estimate for Exercise + Nutrition exceeded the predefined MID threshold of 0.10 m/s, while its 95% CrI crossed the threshold, indicating potential clinical relevance. By contrast, although Exercise alone and Nutrition alone reached statistical significance, their effect estimates did not meet the MID, indicating limited clinical significance.
Figure 4
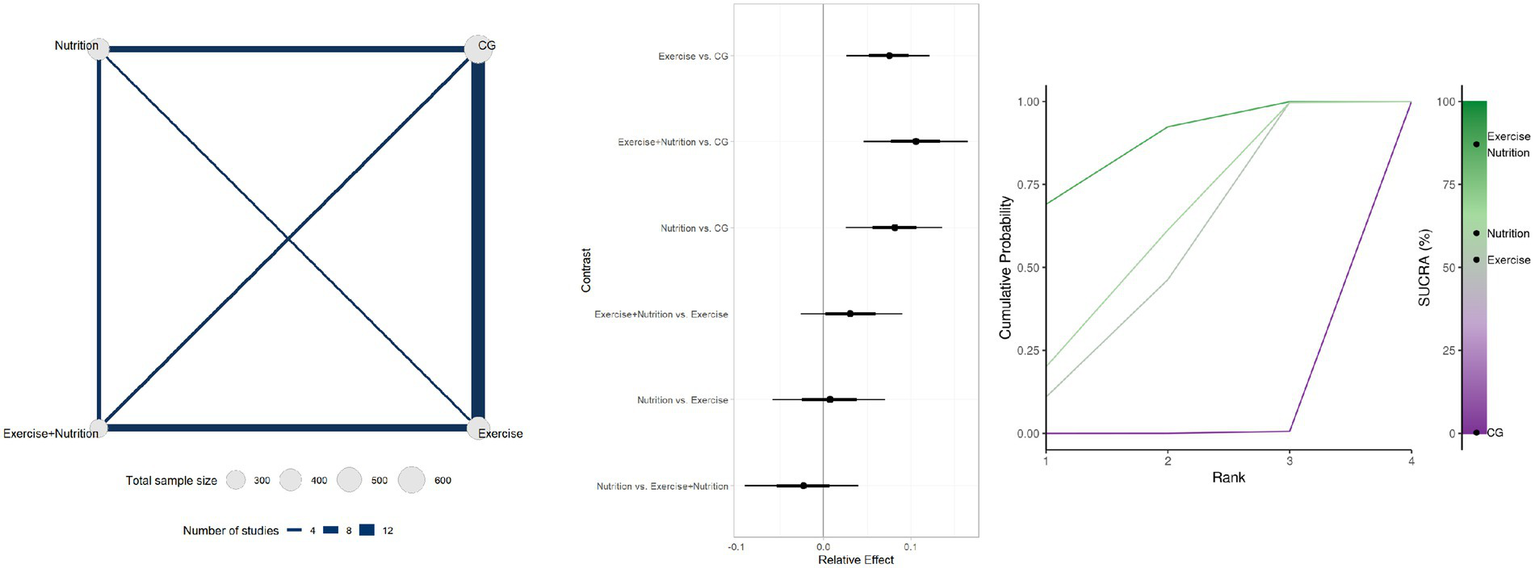
Summary of network meta-analysis results for gait speed: evidence network, relative effects, and SUCRA ranking.
3.5 Muscle mass
Seventeen studies involving 1,137 participants reported changes in appendicular skeletal muscle mass (Figure 5). Low- or very low-certainty indicated that Exercise + Nutrition (MD = 0.35, 95% CrI 0.19 to 0.49; SUCRA 99.82%) and exercise alone (MD = 0.16, 95% CrI 0.03 to 0.28; SUCRA 54.06%) significantly improved muscle mass, whereas nutrition alone (MD = 0.11, 95% CrI − 0.02 to 0.23; SUCRA 44.66%) did not show a significant effect.
Figure 5
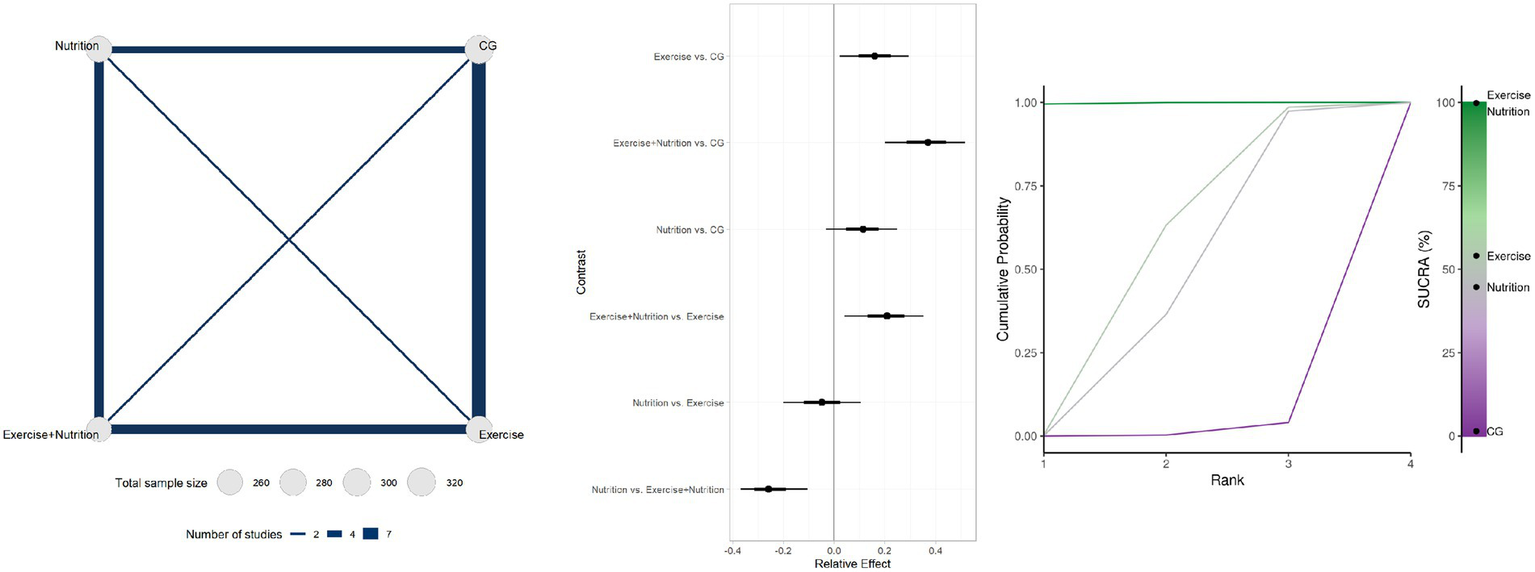
Summary of network meta-analysis results for ASMI: evidence network, relative effects, and SUCRA ranking.
3.6 Subgroup analysis
The subgroup analyses indicated that resistance and balance training plus protein supplementation (RBT + Pro), resistance training plus protein and vitamin D supplementation (RT + Pro + VD), and RBT alone were the most effective interventions for improving handgrip strength (Figure 6A). Among these, the mean effect of RBT + Pro exceeded the predefined MID threshold of 5 kg. However, the lower bound of the confidence interval did not entirely surpass this threshold, suggesting potential clinical relevance. In addition, protein plus vitamin D supplementation (Pro + VD) alone also led to significant improvements in handgrip strength, although its clinical significance remains uncertain. For gait speed, RBT + Pro, aerobic and resistance training combined (ARBT), and RBT alone were the most effective interventions (Figure 6B). Both the mean effect and lower bound of the confidence interval for RBT + Pro and ARBT exceeded the predefined MID threshold of 0.10 m/s, indicating clear clinical relevance. In contrast, although the mean effect of RBT also exceeded the MID threshold, its lower bound did not, suggesting only potential clinical relevance. Regarding ASMI, RT + Pro + VD, RT alone, and Pro + VD were the most effective interventions (Figure 6C). Notably, interventions including aerobic training (AT) did not demonstrate significant improvements in ASMI, suggesting limited benefits for muscle mass and highlighting the need for further high-quality evidence (Appendix 6).
Figure 6
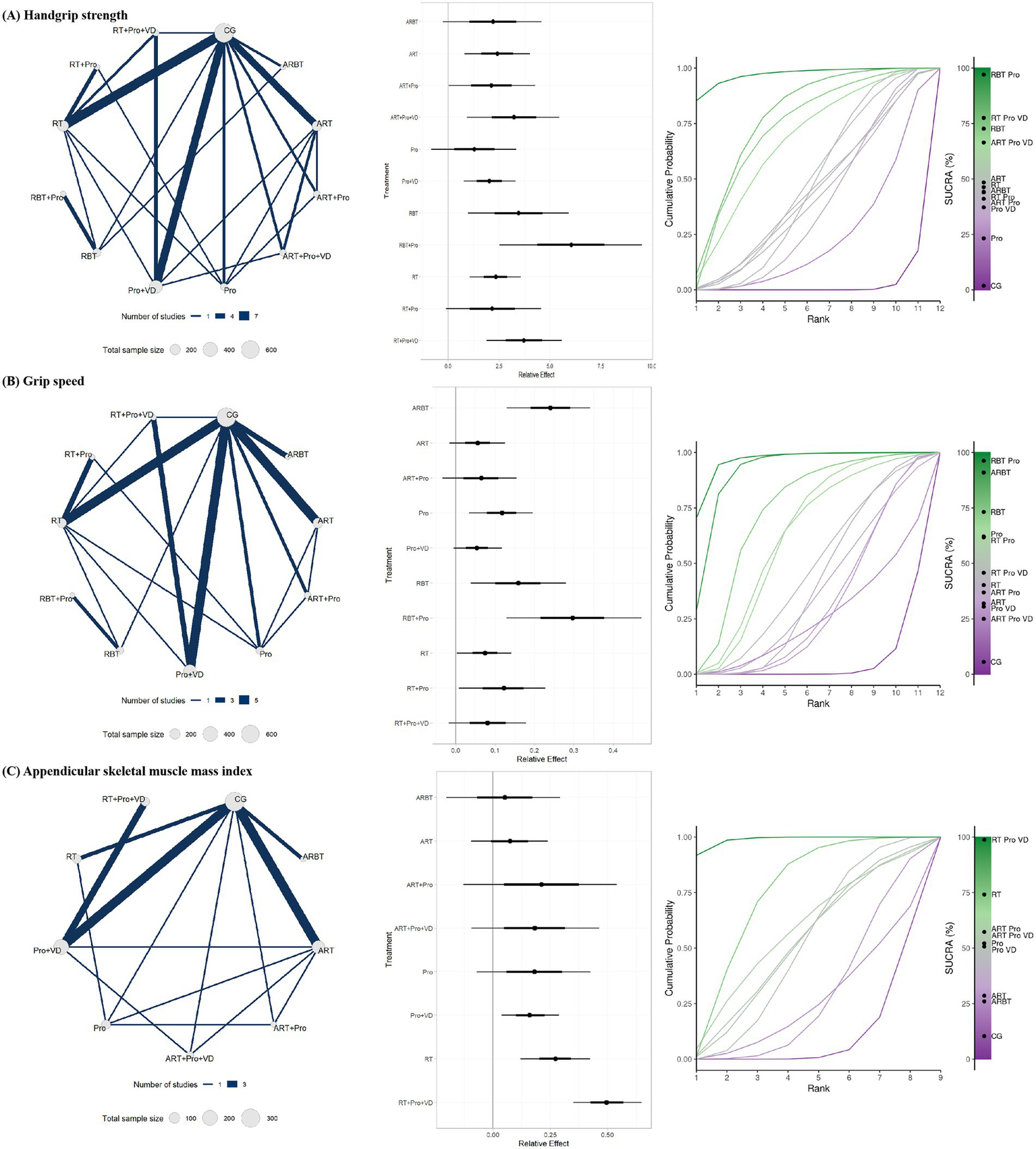
Subgroup analysis results for (A) handgrip strength, (B) gait speed, and (C) ASMI: evidence network, relative effects, and SUCRA rankings.
3.7 Meta-regressions
To further explore the robustness of the findings, meta-regression analyses were conducted to assess potential sources of heterogeneity across all outcome measures. Covariates included mean age, proportion of male participants, sample size, intervention duration, baseline BMI, baseline handgrip strength, and baseline ASMI. No significant associations were observed between these covariates and effect sizes (p > 0.05), suggesting that these factors were unlikely to be major contributors to heterogeneity (Appendix 10).
3.8 Sensitivity analysis
Two sensitivity analyses were performed to evaluate the robustness of the network meta-analysis results. First, all potential sources of heterogeneity were adjusted to their median values, and the models were re-estimated. Second, studies at high risk of bias were excluded, and the primary outcomes were reanalysed. In both cases, results remained consistent with the primary analyses, with only minimal changes in key effect estimates and no substantive differences in effect direction or magnitude, supporting the stability of our conclusions (Appendix 11).
4 Discussion
To our knowledge, this is the first network meta-analysis to include only RCTs in which sarcopenia was diagnosed according to the EWGSOP or AWGS consensus criteria. This methodological approach minimises the population heterogeneity and selection bias introduced by the use of inconsistent or self-defined diagnostic criteria in previous reviews. A total of 35 RCTs involving 2,331 older adults with sarcopenia were included, allowing direct comparisons of the relative efficacy of Exercise, Nutrition, and their combination under a unified diagnostic framework. The outcomes examined were muscle mass (ASMI), muscle strength (handgrip strength), and physical function (gait speed). The results showed that Exercise, Nutrition, and combined interventions all significantly outperformed control groups in improving handgrip strength and gait speed. Although nutritional interventions alone produced significant improvements in handgrip strength and gait speed, no significant effect on ASMI was observed. By contrast, combined exercise and nutrition interventions significantly improved both handgrip strength and ASMI compared with either modality alone. To further examine differences among intervention types, we performed subgroup analyses. RBT + Pro emerged as the most effective multicomponent strategy for improving handgrip strength and gait speed, whereas RT + Pro+VD was most effective for improving ASMI. These findings provide more precise comparative evidence for the development of evidence-based prescriptions, offering directly applicable guidance for clinicians, rehabilitation professionals, and policymakers seeking to optimise intervention strategies for sarcopenia.
In our analysis, combined exercise and nutrition was the most effective strategy for improving outcomes in sarcopenia. However, meta-analyses by Wu et al. (35) and Yan et al. (13) reported no significant differences between combined exercise plus protein supplementation and either intervention alone for most key outcomes. In Yan et al.’s (13) analysis, protein supplementation alone showed no significant improvement in handgrip strength, gait speed, or ASMI. This discrepancy may relate to the inclusion of only female participants in their analysis, given that postmenopausal hormonal changes can affect protein metabolism and utilisation. Notably, Yan et al. (13) reported in subgroup analyses that RT and RT combined with nutrition were most effective for improving handgrip strength, gait speed, knee extension strength, and appendicular skeletal muscle mass, which is only partly consistent with our findings. In addition, Yang et al. (36) also reported that combined exercise and nutrition significantly improved handgrip strength, skeletal muscle mass index, gait speed, and five-times sit-to-stand performance in older adults with sarcopenia. However, these improvements did not reach clinical significance, and intervention effects appeared to vary by age, BMI, and living environment. This highlights the need for future research to pay greater attention to population differences and to explore more targeted and clinically translatable intervention strategies. Building on this, our study further provided a more refined classification of exercise and nutritional interventions. For instance, we confirmed the potential benefits of combined protein and vitamin D supplementation. Although previous systematic reviews have reported that vitamin D alone may confer minimal or no benefit for muscle strength and mass, raising serum 25-hydroxyvitamin D from deficient or insufficient levels to sufficiency may be a prerequisite for optimising the anabolic effects of protein or amino acid supplementation (37, 38). Our findings suggest that Pro + VD improved handgrip strength more than protein alone, even when combined with RT, RBT, or aerobic and resistance training (ART). Three studies similarly reported greater improvements in handgrip strength with whey protein plus vitamin D compared with whey protein alone, in both healthy older adults (39) and older adults with sarcopenia (40, 41), irrespective of RT status. However, effects on gait speed and ASMI were minimal. Taken together, current evidence suggests that vitamin D supplementation alone is unlikely to improve muscle strength or function substantially. However, when combined with protein, it may enhance muscle anabolism and strength, warranting consideration as a supportive nutritional strategy in selected populations such as patients with osteoporosis receiving antiresorptive therapy. However, supplementation should be administered gradually, and high-dose or bolus regimens should be avoided, as excessive vitamin D intake has been associated with an increased risk of falls (42, 43). Moreover, the potential risks of protein supplementation require consideration. In patients with chronic kidney disease, prolonged high protein intake may accelerate renal function decline; therefore, consumption exceeding 1.3 g/kg/day should be avoided, with individualized adjustments made under clinical supervision (44). Given that patients with chronic kidney disease are at increased risk of developing sarcopenia, protein intake should be carefully balanced to promote muscle anabolism while minimizing renal burden (45).
Existing evidence consistently supports RT as the cornerstone of sarcopenia management. However, guidelines offer limited recommendations on which resistance-based multicomponent intervention should be prioritised (5, 7). Our findings suggest that multicomponent resistance-based interventions offer advantages over RT alone, particularly for individuals with functional limitations. These patients often struggle with complex, multi-joint, closed-chain movements that demand intermuscular coordination and postural control (46), and restricted joint range of motion can further reduce muscle engagement and neuromuscular activation, thereby limiting gains in muscle mass, strength, and power, and reducing the translation of these gains into functional improvements (46, 47). Incorporating BT into RT programmes may therefore enhance postural stability and movement control, indirectly boosting RT effectiveness and accelerating recovery of muscle function and overall fitness (48). Consistently, Shen et al. (49) concluded that adding balance training (BT) to RT, with or without nutritional supplementation, was the most effective approach for improving most measures of muscle strength and physical function, a conclusion in agreement with our results. It is noteworthy that, among all exercise modalities assessed, only ARBT achieved clinically meaningful improvements in gait speed. This may reflect the multifactorial nature of gait speed, which depends on the integration of cardiorespiratory endurance, coordination, and balance, making it more reliant on aerobic capacity (50). While aerobic plus resistance training, commonly termed concurrent training, has been reported to have an “interference effect” that could attenuate gains in muscle strength and mass (51, 52), no significant adverse effects were observed in our analysis. Nevertheless, the magnitude of improvements in muscle strength and mass was smaller than for RT-based combinations without aerobic training. However, concurrent ART may be particularly appropriate for the management of sarcopenic obesity, as the integration of both modalities facilitates the reduction of fat mass and the enhancement of muscle mass, thereby promoting a more favourable balance between catabolic and anabolic processes (53). In clinical practice, multicomponent interventions combining RT and BT may be preferable for older adults with sarcopenia who have poor baseline function, balance deficits, or restricted joint mobility, offering both safety and functional benefits. For patients needing improvements in gait speed and endurance, low- to moderate-intensity aerobic training may be incorporated alongside strength-based programmes, with close monitoring of muscle strength and mass to mitigate potential interference effects. Such individualised, progressive, multicomponent approaches could maximise real-world intervention benefits while reducing fall risk.
Beyond RT-based multicomponent programmes, growing attention has been directed toward novel training modalities that may further mitigate muscle loss and functional decline (54). Among these, recent studies have reported that high-intensity interval training (HIIT) can improve muscle strength, functional capacity, and lean mass, with effects that are at least comparable to, and in some cases superior to, those of continuous aerobic exercise (55, 56). These findings suggest that HIIT may help counteract age-related declines in muscle mass and function (55, 56). However, the evidence remains limited, as few high-quality trials are available and almost all have been conducted in robust older adults, making it difficult to generalise the results to populations with sarcopenia (57, 58). Because individuals with sarcopenia are typically more frail and functionally impaired, careful evaluation of the safety and tolerability of HIIT is required before it can be broadly recommended in clinical practice. For patients unable to perform conventional exercise, alternative modalities such as electrical muscle stimulation and whole-body vibration have also been explored (53, 59). Although some studies have suggested potential benefits of these surrogate modalities in sarcopenic populations, the evidence remains sparse and inconclusive. Future research should prioritise large, well-designed trials in sarcopenic populations to establish the efficacy and safety of HIIT and to define the role of emerging exercise surrogates, such as electrical muscle stimulation and whole-body vibration, within multimodal management strategies.
4.1 Limitations and future directions
This study has several limitations. First, most comparisons were graded as “very low” to “low” certainty, primarily due to high heterogeneity. Although meta-regressions were conducted to explore potential sources of heterogeneity, no definitive explanatory factors were identified; hence, results should be interpreted cautiously. Second, in subgroup analyses, some interventions were represented by only a few studies, resulting in sparse network connections, which may have reduced statistical power and increased uncertainty in effect estimates. Third, due to the limited number of eligible studies, whey protein, amino acids, and HMB supplementation were combined into a single “protein supplementation” category for analysis. While this improved feasibility, it may have obscured differences in mechanisms between supplement types.
Future research should reduce heterogeneity and strengthen the certainty of evidence by adopting unified diagnostic criteria (e.g., the latest EWGSOP/AWGS) alongside standardized reporting and implementation protocols that clearly specify training intensity, frequency, volume, and progression. Large-scale, multicenter randomized controlled trials are warranted to increase statistical power, enhance external validity, and improve the connectivity of the evidence network, particularly for combined multicomponent exercise and nutritional interventions, which may provide more reliable comparative evidence. Moreover, direct head-to-head trials are needed to compare different forms of protein supplementation (e.g., whey, leucine, EAA, BCAA, HMB), with prespecified stratification according to baseline protein or vitamin D status, sex, and functional capacity. Finally, future studies should broaden outcome assessment to include clinically relevant endpoints such as falls, functional independence, and quality of life, rather than focusing solely on muscle mass and strength, thereby generating evidence with greater clinical applicability and translational value.
5 Conclusion
These findings support combined exercise and nutritional interventions as a preferred treatment option for improving muscle strength, muscle mass, and physical function in older adults with sarcopenia. However, the overall certainty of the evidence ranged from low to very low. In particular, multicomponent exercise programmes centred on resistance training and combined with protein supplementation may offer superior benefits for enhancing muscle strength and physical function. These findings provide direct guidance for clinicians and rehabilitation professionals in developing evidence-based management strategies for sarcopenia, and highlight the importance of multicomponent interventions in restoring function and delaying frailty in older adults.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RZ: Conceptualization, Data curation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. YD: Methodology, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. QZ: Formal analysis, Funding acquisition, Investigation, Resources, Writing – original draft, Writing – review & editing. JY: Formal analysis, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1685014/full#supplementary-material
References
1.
Yuan S Larsson SC . Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533
2.
Cruz-Jentoft AJ Bahat G Bauer J Boirie Y Bruyère O Cederholm T et al . Sarcopenia: revised european consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
3.
Cruz-Jentoft AJ Landi F Schneider SM Zúñiga C Arai H Boirie Y et al . Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing. (2014) 43:748–59. doi: 10.1093/ageing/afu115
4.
Shafiee G Keshtkar A Soltani A Ahadi Z Larijani B Heshmat R . Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. (2017) 16:21. doi: 10.1186/s40200-017-0302-x
5.
Sayer AA Cooper R Arai H Cawthon PM Ntsama Essomba M-J Fielding RA et al . Sarcopenia. Nat Rev Dis Primers. (2024) 10:68. doi: 10.1038/s41572-024-00550-w
6.
Cruz-Jentoft AJ Sayer AA . Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
7.
Dent E Morley JE Cruz-Jentoft AJ Arai H Kritchevsky SB Guralnik J et al . International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. (2018) 22:1148–61. doi: 10.1007/s12603-018-1139-9
8.
Endo Y Nourmahnad A Sinha I . Optimizing skeletal muscle anabolic response to resistance training in aging. Front Physiol. (2020) 11:874. doi: 10.3389/fphys.2020.00874
9.
Pagan JI Bradshaw BA Bejte B Hart JN Perez V Knowles KS et al . Task-specific resistance training adaptations in older adults: comparing traditional and functional exercise interventions. Front Aging. (2024) 5:1335534. doi: 10.3389/fragi.2024.1335534
10.
Parveen A Parveen S Noohu MM . Effect of concurrent and multicomponent training on balance, fear of falling, and muscle strength in older adults: a review. Sport Sci Health. (2023) 19:733–42. doi: 10.1007/s11332-022-00990-5
11.
Karabay D Emük Y Kaya DÖ . Muscle activity ratios of scapular stabilizers during closed kinetic chain exercises in healthy shoulders: a systematic review. J Sport Rehabil. (2019) 29:1001–18. doi: 10.1123/jsr.2018-0449
12.
Breen L Phillips SM . Skeletal muscle protein metabolism in the elderly: interventions to counteract the “anabolic resistance” of ageing. Nutr Metab. (2011) 8:68. doi: 10.1186/1743-7075-8-68
13.
Yan R Huang W Zhong Y Du X . Comparative effectiveness of exercise, protein supplementation, and combined interventions for sarcopenia management in women: a network meta-analysis. Nutrients. (2025) 17:2392. doi: 10.3390/nu17152392
14.
Cipriani A Higgins JPT Geddes JR Salanti G . Conceptual and technical challenges in network Meta-analysis. Ann Intern Med. (2013) 159:130–7. doi: 10.7326/0003-4819-159-2-201307160-00008
15.
Pedauyé-Rueda B García-Fernández P Maicas-Pérez L Maté-Muñoz JL Hernández-Lougedo J . Different diagnostic criteria for determining the prevalence of sarcopenia in older adults: a systematic review. J Clin Med. (2024) 13:2520. doi: 10.3390/jcm13092520
16.
Dent E Woo J Scott D Hoogendijk EO . Sarcopenia measurement in research and clinical practice. Eur J Intern Med. (2021) 90:1–9. doi: 10.1016/j.ejim.2021.06.003
17.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18.
Hutton B Salanti G Caldwell DM Chaimani A Schmid CH Cameron C et al . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
19.
Liberati A Altman DG Tetzlaff J Mulrow C Gøtzsche PC Ioannidis JPA et al . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
20.
JPT Higgins . Cochrane handbook for systematic reviews of interventions. (2008). Available online at: https://cir.nii.ac.jp/crid/1571980075694747776 (Accessed November 17, 2024).
21.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
22.
Nikolakopoulou A Higgins JPT Papakonstantinou T Chaimani A Del Giovane C Egger M et al . CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. (2020) 17:e1003082. doi: 10.1371/journal.pmed.1003082
23.
Papakonstantinou T Nikolakopoulou A Higgins JPT Egger M Salanti G . CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. (2020) 16:e1080. doi: 10.1002/cl2.1080
24.
Brignardello-Petersen R Izcovich A Rochwerg B Florez ID Hazlewood G Alhazanni W et al . GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ. (2020) 371:m3907. doi: 10.1136/bmj.m3907
25.
Hultcrantz M Rind D Akl EA Treweek S Mustafa RA Iorio A et al . The GRADE working group clarifies the construct of certainty of evidence. J Clin Epidemiol. (2017) 87:4–13. doi: 10.1016/j.jclinepi.2017.05.006
26.
Zeng L Brignardello-Petersen R Hultcrantz M Siemieniuk RA Santesso N Traversy G et al . GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol. (2021) 137:163–75. doi: 10.1016/j.jclinepi.2021.03.026
27.
Bohannon RW . Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci. (2019) 31:75–8. doi: 10.1589/jpts.31.75
28.
Bohannon RW Glenney SS . Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract. (2014) 20:295–300. doi: 10.1111/jep.12158
29.
Welton NJ Sutton AJ Cooper N Abrams KR Ades AE . Evidence synthesis for decision making in healthcare. Chichester, UK: John Wiley & Sons (2012).
30.
Mavridis D Salanti G . A practical introduction to multivariate meta-analysis. Stat Methods Med Res. (2013) 22:133–58. doi: 10.1177/0962280211432219
31.
Turner RM Davey J Clarke MJ Thompson SG Higgins JP . Predicting the extent of heterogeneity in meta-analysis, using empirical data from the cochrane database of systematic reviews. Int J Epidemiol. (2012) 41:818–27. doi: 10.1093/ije/dys041
32.
da Costa BR Juni P . Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J. (2014) 35:3336–45. doi: 10.1093/eurheartj/ehu424
33.
Dias S Welton NJ Sutton AJ Caldwell DM Lu G Ades AE . Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. (2013) 33:641–56. doi: 10.1177/0272989X12455847
34.
Mbuagbaw L Rochwerg B Jaeschke R Heels-Andsell D Alhazzani W Thabane L et al . Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. (2017) 6:79. doi: 10.1186/s13643-017-0473-z
35.
Wu P-Y Huang K-S Chen K-M Chou C-P Tu Y-K . Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: a systematic review and network meta-analysis. Maturitas. (2021) 145:38–48. doi: 10.1016/j.maturitas.2020.12.009
36.
Yang Y Pan N Luo J Liu Y Ossowski Z . Exercise and nutrition for sarcopenia: a systematic review and meta-analysis with subgroup analysis by population characteristics. Nutrients. (2025) 17:2342. doi: 10.3390/nu17142342
37.
Verlaan S Maier AB Bauer JM Bautmans I Brandt K Donini LM et al . Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults - the PROVIDE study. Clin Nutr. (2018) 37:551–7. doi: 10.1016/j.clnu.2017.01.005
38.
Rathmacher JA Pitchford LM Khoo P Angus H Lang J Lowry K et al . Long-term effects of calcium β-hydroxy-β-methylbutyrate and vitamin D3 supplementation on muscular function in older adults with and without resistance training: a randomized, double-blind, controlled study. J Gerontol A Biol Sci Med Sci. (2020) 75:2089–97. doi: 10.1093/gerona/glaa218
39.
Nasimi N Sohrabi Z Nunes EA Sadeghi E Jamshidi S Gholami Z et al . Whey protein supplementation with or without vitamin D on sarcopenia-related measures: a systematic review and meta-analysis. Adv Nutr. (2023) 14:762–73. doi: 10.1016/j.advnut.2023.05.011
40.
Gkekas NK Anagnostis P Paraschou V Stamiris D Dellis S Kenanidis E et al . The effect of vitamin D plus protein supplementation on sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Maturitas. (2021) 145:56–63. doi: 10.1016/j.maturitas.2021.01.002
41.
Chang MC Choo YJ . Effects of whey protein, leucine, and vitamin D supplementation in patients with sarcopenia: a systematic review and meta-analysis. Nutrients. (2023) 15:521. doi: 10.3390/nu15030521
42.
Prokopidis K Giannos P Katsikas Triantafyllidis K Kechagias KS Mesinovic J Witard OC et al . Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:1642–52. doi: 10.1002/jcsm.12976
43.
Cheng S-H Chen K-H Chen C Chu W-C Kang Y-N . The optimal strategy of vitamin D for sarcopenia: a network meta-analysis of randomized controlled trials. Nutrients. (2021) 13:3589. doi: 10.3390/nu13103589
44.
Ikizler TA Burrowes JD Byham-Gray LD Campbell KL Carrero J-J Chan W et al . KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. (2020) 76:S1–S107. doi: 10.1053/j.ajkd.2020.05.006
45.
Duarte MP Almeida LS Neri SGR Oliveira JS Wilkinson TJ Ribeiro HS et al . Prevalence of sarcopenia in patients with chronic kidney disease: a global systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2024) 15:501–12. doi: 10.1002/jcsm.13425
46.
Coelho-Júnior HJ Picca A Calvani R Marzetti E . Prescription of resistance training for sarcopenic older adults: does it require specific attention?Ageing Res Rev. (2022) 81:101720. doi: 10.1016/j.arr.2022.101720
47.
Aartolahti E Lönnroos E Hartikainen S Häkkinen A . Long-term strength and balance training in prevention of decline in muscle strength and mobility in older adults. Aging Clin Exp Res. (2020) 32:59–66. doi: 10.1007/s40520-019-01155-0
48.
Lehmann N Kuhn YA Keller M Aye N Herold F Draganski B et al . Balance training improves postural control and performance-related prefrontal brain activation in healthy older adults: results of a six-month randomized controlled training intervention. Neurobiol Aging. (2025) 154:71–83. doi: 10.1016/j.neurobiolaging.2025.07.001
49.
Shen Y Shi Q Nong K Li S Yue J Huang J et al . Exercise for sarcopenia in older people: a systematic review and network meta-analysis. J Cachexia Sarcopenia Muscle. (2023) 14:1199–211. doi: 10.1002/jcsm.13225
50.
Beaudart C Rolland Y Cruz-Jentoft AJ Bauer JM Sieber C Cooper C et al . Assessment of muscle function and physical performance in daily clinical practice. Calcif Tissue Int. (2019) 105:1–14. doi: 10.1007/s00223-019-00545-w
51.
Wilson JM Marin PJ Rhea MR Wilson SMC Loenneke JP Anderson JC . Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. (2012) 26:2293–307. doi: 10.1519/JSC.0b013e31823a3e2d
52.
Huiberts RO Wüst RCI van der Zwaard S . Concurrent strength and endurance training: a systematic review and meta-analysis on the impact of sex and training status. Sports Med. (2024) 54:485–503. doi: 10.1007/s40279-023-01943-9
53.
Xu S Tu S Hao X Chen X Pan D Liao W et al . Exercise, nutrition, and neuromuscular electrical stimulation for sarcopenic obesity: a systematic review and meta-analysis of management in middle-aged and older adults. Nutrients. (2025) 17:1504. doi: 10.3390/nu17091504
54.
Moretti A Tomaino F Paoletta M Liguori S Migliaccio S Rondanelli M et al . Physical exercise for primary sarcopenia: an expert opinion. Front Rehabil Sci. (2025) 6:1538336. doi: 10.3389/fresc.2025.1538336
55.
Morcillo-Losa JA Díaz-Martínez M d P Ceylan Hİ Moreno-Vecino B Bragazzi NL Párraga Montilla J . Effects of high-intensity interval training on muscle strength for the prevention and treatment of sarcopenia in older adults: a systematic review of the literature. J Clin Med. (2024) 13:1299. doi: 10.3390/jcm13051299
56.
Liu Q-Q Xie W-Q Luo Y-X Li Y-D Huang W-H Wu Y-X et al . High intensity interval training: a potential method for treating sarcopenia. Clin Interv Aging. (2023) 17:857–72. doi: 10.2147/CIA.S366245
57.
Müller DC Boeno FP Izquierdo M Aagaard P Teodoro JL Grazioli R et al . Effects of high-intensity interval training combined with traditional strength or power training on functionality and physical fitness in healthy older men: a randomized controlled trial. Exp Gerontol. (2021) 149:111321. doi: 10.1016/j.exger.2021.111321
58.
Bruseghini P Capelli C Calabria E Rossi AP Tam E . Effects of high-intensity interval training and isoinertial training on leg extensors muscle function, structure, and intermuscular adipose tissue in older adults. Front Physiol. (2019) 10:1260. doi: 10.3389/fphys.2019.01260
59.
Wu S Ning H-T Xiao S-M Hu M-Y Wu X-Y Deng H-W et al . Effects of vibration therapy on muscle mass, muscle strength and physical function in older adults with sarcopenia: a systematic review and meta-analysis. Eur Rev Aging Phys Act. (2020) 17:14. doi: 10.1186/s11556-020-00247-5
Summary
Keywords
sarcopenia, exercise, nutrition, older adults, resistance training
Citation
Zhao R, Dong Y, Zheng Q and Yao J (2025) Exercise and nutrition strategies for sarcopenia in older adults: evidence from a network meta-analysis based on EWGSOP and AWGS criteria. Front. Nutr. 12:1685014. doi: 10.3389/fnut.2025.1685014
Received
13 August 2025
Accepted
30 September 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Jian Sun, Guangzhou Sport University, China
Reviewed by
Antimo Moretti, University of Campania Luigi Vanvitelli, Italy
Ratko Peric, OrthoSport Banja Luka, Bosnia and Herzegovina
Updates
Copyright
© 2025 Zhao, Dong, Zheng and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiwei Yao, 15150550801@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.