Abstract
In recent years, interest in the impact of diet on the skin has increased significantly, and dietary interventions are now considered an essential part of managing certain dermatological conditions. Among them, the ketogenic diet (KD) has gained particular attention. KD is a high-fat, very low-carbohydrate, and moderate-protein dietary approach that induces ketosis, defined by serum ketone body concentrations exceeding 0.5 mmol/L. Although initially developed for the treatment of drug-resistant epilepsy in children, its applications have broadened over time. Current evidence on KD and the skin remains limited, focusing mainly on inflammatory skin diseases such as acne, psoriasis, and hidradenitis suppurativa (HS), as well as cutaneous melanoma. In this review, we summarize the existing data on these conditions and discuss the molecular mechanisms underlying the potential effects of KD, including anti-inflammatory and antioxidant pathways, modulation of signaling cascades, and interactions with the gut microbiota. Finally, we also address the reported adverse effects of KD on the skin.
1 Introduction
Numerous scientific studies have confirmed the role of diet in maintaining the proper functioning of the human body and in the prevention and treatment of various diseases, including obesity, diabetes, and cardiovascular disease (1–3). As the level of education in society increases, so too does awareness of the impact of diet on the skin (4). Dietary factors can have both positive and adverse effects on the skin (5). A balanced and varied diet provides the skin with adequate nutrients, which play a key role in maintaining the integrity of the skin barrier and promoting skin health (6). Proper nutrition improves hydration and elasticity and delays skin aging (7). An unhealthy diet plays a key role in the development of many skin conditions, including inflammatory skin diseases (8). Certain dietary patterns, particularly those diets high in saturated fatty acids (SFAs), simple sugars, and highly processed foods, may exacerbate inflammation in conditions such as acne vulgaris, psoriasis, atopic dermatitis, and hidradenitis suppurativa (HS) (5, 9, 10). Some dietary factors may also contribute to premature skin aging and the development of skin cancer (11, 12). Dietary interventions are currently considered an important element in the treatment of skin conditions. They can be used as a preventive measure or adjuvant therapy for chronic skin conditions (11, 12). Several dietary patterns, including the Mediterranean diet (MD), intermittent fasting, the ketogenic diet (KD), and gluten-free and vegan diets, are recognized for their skin-benefiting effects. These diets differ primarily in their macronutrient composition (13). Among these, KD is gaining popularity due to promising results in the treatment of various skin diseases (13, 14). KD is a low-carbohydrate dietary regimen that has gained widespread public recognition, particularly in the treatment of drug-resistant epilepsy in children and rapid fat reduction (15, 16). Widely promoted in the media, it has become one of the most commonly used weight loss diets worldwide (16). Although its mechanisms of action are not fully understood, KD's contemporary applications extend beyond its original use (17). Recent reports indicate that KD may also have a potential therapeutic role in supporting the treatment of selected skin conditions, most notably inflammatory dermatoses such as acne, psoriasis, hidradenitis suppurativa, as well as cutaneous melanoma (14, 18–21). As the available evidence is still limited, this review focuses on these diseases while placing particular emphasis on the molecular mechanisms—such as modulation of inflammatory pathways, promotion of weight loss, strengthening of antioxidant defense against reactive oxygen species (ROS), and alterations in the gut microbiota—which may provide broader insights into the potential effects of KD on skin health (14, 18–20).
2 History of the ketogenic diet
The earliest reports on the use of a low-carbohydrate diet to reduce the severity of symptoms, especially epileptic seizures, date back to antiquity (22). Hippocrates claimed that the body of an epilepsy patient should be purified. He used fasting similar to KD for this purpose (22–25). These early ancient practices laid the foundations for the development of the modern KD pattern (23, 25, 26). Nevertheless, the core concept of KD emerged in the 1860s (26). Entrepreneur William Banting pioneered the low-carbohydrate diet (27). Banting, who struggled with obesity and related complications, limited his carbohydrate intake on the advice of Dr. William Harvey (27). The first references to this diet appeared in medical literature in 1864 (28). However, this model differed significantly from contemporary KD standards. The proposed weight loss diet excluded specific carbohydrate products, such as bread, butter, milk, potatoes, and sugar (28). In 1869, William Banting published the fourth edition of his book, “Letter on Corpulence,” which proclaimed the high effectiveness of a low-carbohydrate diet in reducing body fat (29). The low-carbohydrate diet gained wide public acceptance (28, 29).
In 1911, French doctors Guelpe and Marie published the first scientific report on the benefits of intermittent fasting in epilepsy (30). Another breakthrough in the popularization of KD came in 1921 (16). Dr. Rollin Woodyatt, an American endocrinologist, observed that starvation caused blood glucose levels to decrease and acetone and β-hydroxybutyric acid levels to increase (16, 31, 32). At the same time, another American physician, Russell Wilder, concluded that a low-carbohydrate diet was a more beneficial alternative to radical fasting. He recommended a low-carbohydrate diet as a treatment for epileptic seizures, especially in children (16, 32–34). Wilder also introduced the term “ketogenic diet” into general use, referring to a diet high in fat and low in carbohydrates (28).
In 1925, Dr. Mynie Gustav Peterman, a pediatrician and Wilder's colleague, developed the classic KD, which consisted of 1 g of protein per kilogram of the child's body weight, 10–15 g of carbohydrates per day, with fats making up the remaining energy requirements (31, 35). Peterman confirmed the effectiveness of KD in the treatment of epileptic seizures in children (31, 36).
Advances in the pharmaceutical industry and technologies, particularly the discovery of phenytoin in 1938, laid the foundation for the development of effective anti-seizure medications. Drug therapy replaced the use of KD over the following decades (28, 37, 38). KD enjoyed a resurgence in popularity in the 1990s (39). In 1994, Dateline National Broadcasting Company (NBC) television aired a report on the case of Charlie Abrahams, the son of American film director and screenwriter Jim Abrahams. The boy, who had epilepsy, was treated with KD, which successfully reduced his epileptic seizures (39, 40). KD has once again been found to have application in the treatment of chronic neurological diseases (37).
3 Ketogenic diet
The World Health Organization (WHO) recommends that adults limit their total fat intake to less than 30% of their daily energy intake (41). In the diets of children aged 2 years and over and of adults, fats should mainly be derived from unsaturated fatty acids (41). It is recommended that saturated fats account for no more than 10% of daily energy intake and trans fats for no more than 1% (41). It is also recommended that free sugars be limited to less than 10% of total energy intake and that adults consume vegetables and fruits of at least 400 g/day, as well as naturally occurring fiber of at least 25 g/day (41).
KD significantly deviates from standard healthy nutrition recommendations. KD is a dietary model primarily based on high-fat foods, combined with a minimal intake of carbohydrates (42, 43). Compared to other low-carbohydrate diets, KD is characterized by the strictest carbohydrate restriction (42). Overall, the diet is predominantly fat-based, with a moderate proportion of protein and only a small fraction of carbohydrates (44). The goal of this diet is to induce ketosis. Ketosis is a metabolic state in which the concentration of ketone bodies exceeds 0.5 mmol/L (42, 45). The upper limit of ketosis is considered to be 3 mmol/L (42, 45, 46).
Several common variants of KD exist, differing in the macronutrient ratios (47, 48). These include the standard ketogenic diet (SKD), the targeted ketogenic diet (TKD), the cyclical ketogenic diet (CKD), and the high-protein ketogenic diet (HPKD). The different variations of KD are selected to suit the individual patient's needs and therapeutic goals (Table 1) (47–49).
Table 1
| Type of ketogenic diet | Nutritional composition | Goal and benefits | References |
|---|---|---|---|
| Standard ketogenic diet (SKD) | The most common type of ketogenic diet; it typically consists of about 70–75% fat, 20–25% protein, and 5–10% carbohydrates | Most commonly recommended for the treatment of epilepsy and weight loss | (47, 48, 259) |
| Targeted ketogenic diet (TKD) | Provides additional carbohydrates during exercise | Recommended for physically active individuals | (32, 47, 49) |
| Cyclical ketogenic diet (CKD) | Involves periods of higher carbohydrate intake with periods of low carbohydrate intake | Recommended for individuals who engage in intensive exercise; it increases energy levels during training | (47, 49, 260) |
| High-protein ketogenic diet (HPKD) | Involves a higher protein intake, usually consisting of 60% fat, 35% protein, and 5% carbohydrates | Recommended for athletes to reduce body weight while maintaining muscle mass | (47, 260) |
Characteristics of the ketogenic diet types.
Other KD types are also used for therapeutic purposes, such as the very low energy ketogenic therapy (VLEKT), formerly referred to as very low-calorie ketogenic diet (VLCKD), the modified Atkins diet (MAD), the medium-chain triglyceride ketogenic diet (MCTKD), and the low glycemic index treatment (LGIT) (50, 51). These dietary approaches share the principles of the traditional KD but differ in total energy supply, macronutrient distribution, and degree of restrictiveness. MAD, MCTKD, and LGIT are generally less restrictive, more palatable, and easier to follow in the long term (52, 53). VLEKT represents a medically supervised intervention primarily recommended for patients with obesity and metabolic or inflammatory conditions (54).
The VLEKT therapy was introduced by the “KetoNut” expert panel of the Italian Society of Nutraceuticals (SINut) and the Italian Association of Dietetics and Clinical Nutrition (ADI). VLEKT is characterized by a very low total energy intake (650–800 kcal per day), carbohydrate restriction to less than 30 g/day, fat intake of around 20 g/day with a preference for sources of unsaturated fats such as extra-virgin olive oil, and protein intake of 0.8–1.2 g/kg of reference body weight (i.e., calculated based on the patient's height) (54). The updated nomenclature was introduced to reduce confusion in the literature and to emphasize the therapeutic framework of this intervention, rather than merely its caloric restriction (54).
MAD was developed in 2003 as an alternative to the conventional KD, particularly in the context of treating drug-resistant epilepsy (55). It recommends a carbohydrate intake of 10–20 g/day (48, 55). MAD does not require limiting calories, fluids, or protein (56). In MAD, the ratio of fats to carbohydrates and proteins is 1:1 or 2:1 (51).
MCTKD was developed in 1971 by Huttenlocher et al. (57, 58). In MCTKD, medium-chain triglycerides (MCTs) are digested and metabolized much faster than long-chain triglycerides (LCTs) (59). MCTs enter the liver directly via the portal vein and are rapidly converted to ketones (59). In MCTKD, MCTs provide up to 60% of total energy intake. Consequently, MCTKD is easier to adhere to and maintain long-term than the classic KD (59). The primary natural sources of MCTs are coconut oil, which contains high amounts of lauric acid (C12:0), and palm kernel oil, which includes caprylic acid (C8:0) and capric acid (C10:0) (59, 60). Literature reports indicate that the use of MCTKD is not without adverse reactions. Gastrointestinal side effects, such as vomiting, diarrhea, or bloating, are often reported during MCTKD use (51, 58).
LGIT recommends consuming less than 50 g, or 40–60 g, of low glycemic index (GI) carbohydrates per day, which accounts for 10% of total energy intake (36, 52, 53, 61). In LGIT, proteins account for about 20–30% and fats for 60% of daily energy intake (53, 61). This KD variety prevents blood glucose spikes after a meal, which may reduce the risk of epileptic seizures (36).
KD is not a universal diet; therefore, to achieve health benefits, the patient's individual needs must be considered (62). KD can affect lipid levels, electrolytes, and renal and hepatic function (63). Thus, it should be introduced under close medical supervision, taking into account food allergies and intolerances (62, 64). It is common practice, especially in children with drug-resistant epilepsy, to introduce KD in a hospital setting (64). The classic hospital KD protocol involves a 24–48-h fast to induce metabolic ketosis, after which the KD ratio is gradually increased, starting with a 1:1 ratio (1 g of fat per 1 g of protein and carbohydrates), until a 4:1 ratio is achieved (61, 64, 65). Once KD has been fully implemented, the patient continues the diet in an outpatient setting (52). Hospitalization is particularly recommended in cases requiring intensive caregiver training, monitoring of epileptic status, and strict metabolic control in infants (65). Another proposed option does not require hospitalization, and the KD ratio is increased weekly, starting at 1:1, until a 4:1 ratio is achieved (61, 65). Forgoing fasting in this option improves dietary tolerance, reducing the acute side effects of rapid ketosis (61, 65). The International Ketogenic Diet Study Group (IKDSG) recommends thorough laboratory diagnosis before and during KD to ensure optimal safety and effectiveness (52, 62, 66). The laboratory tests include a complete blood count with platelet count, blood glucose levels, electrolytes (potassium, sodium, and chloride), liver and kidney function tests (albumin, creatinine, and blood urea nitrogen), fasting lipid profile, vitamin D levels, a serum acylcarnitine profile, and a urinalysis (52, 62, 66). In the case of epilepsy, it is additionally recommended to perform electroencephalography (EEG) and 3 Tesla (3T) magnetic resonance imaging (MRI) of the head. Patients with suspected heart disease are additionally recommended to have an echocardiogram (ECG) (52, 67).
According to IKDSG guidelines, KD use is strictly contraindicated in patients with a deficiency of carnitine (primary), carnitine palmitoyltransferase (CPT) 1 or 2, carnitine translocase, pyruvate carboxylase, medium-chain acyl-coenzyme A (CoA) dehydrogenase (MCAD), long-chain acyl-CoA dehydrogenase (LCAD), short-chain acyl-CoA dehydrogenase (SCAD), and disorders of fatty acid β-oxidation and porphyria. Further, KD should not be used in patients with chronic kidney disease, liver failure, hypertriglyceridemia-induced acute pancreatitis, heart failure, especially NYHA Class IV, eating disorders, respiratory failure, as well as in the elderly, and during pregnancy and lactation (52, 62, 63, 67, 68).
KD is widely used as an adjuvant therapy for many diseases. It is a well-established therapeutic approach for the treatment of drug-resistant epilepsy, particularly in children. Research findings indicate that after 3 months of KD use, the frequency of epileptic seizures decreases by at least 50% (69, 70). It has also been applied successfully in patients with overweight and obesity (71). Meta-analyses of clinical trials indicate that KD leads to faster weight loss in the short term, with optimal results usually achieved within the first 3–6 months (71, 72). A key aspect of KD compared to other weight loss diets is its ability to regulate hunger and satiety (71, 73). KD has also emerged as a promising dietary intervention for treating insulin resistance and type 2 diabetes (74). Scientific evidence shows that it reduces fasting glucose and glycated hemoglobin (HbA1c) levels in patients with type 2 diabetes (75). KD also shows potential in the prevention and treatment of cardiovascular disease (76). This diet usually leads to an increase in HDL cholesterol and a decrease in triglycerides (76, 77). However, there is evidence that it may also increase LDL cholesterol in some patients, which increases the risk of cardiovascular disease. KD has also shown promising effects on Alzheimer's disease (AD) and Parkinson's disease (PD) (78). Studies suggest that KD may support cognitive function in early AD and motor function in PD patients (78). There is preliminary evidence that it may benefit individuals with autism spectrum disorder (ASD) (79). Numerous scientific studies also confirm the beneficial effects of KD in treating certain types of cancer (39, 80, 81). The key anti-cancer mechanism of KD is carbohydrate reduction, which leads to lower glucose levels and slower proliferation of cancer cells (39, 82). KD is also used in glucose transporter 1 (GLUT1) deficiency syndrome to provide an alternative source of energy for the brain in conditions of impaired glucose access (83). It is also a beneficial dietary intervention in polycystic ovary syndrome (PCOS) (84). In addition, KD's effect on reducing oxidative stress and inflammation has also been confirmed (85). The role of KD in reducing the severity of symptoms in many skin conditions and its beneficial effects on the skin are also gaining recognition (14, 18–20). The therapeutic potential of KD is shown in Figure 1, whereas the key mechanisms specifically related to skin health are summarized in Table 2.
Figure 1
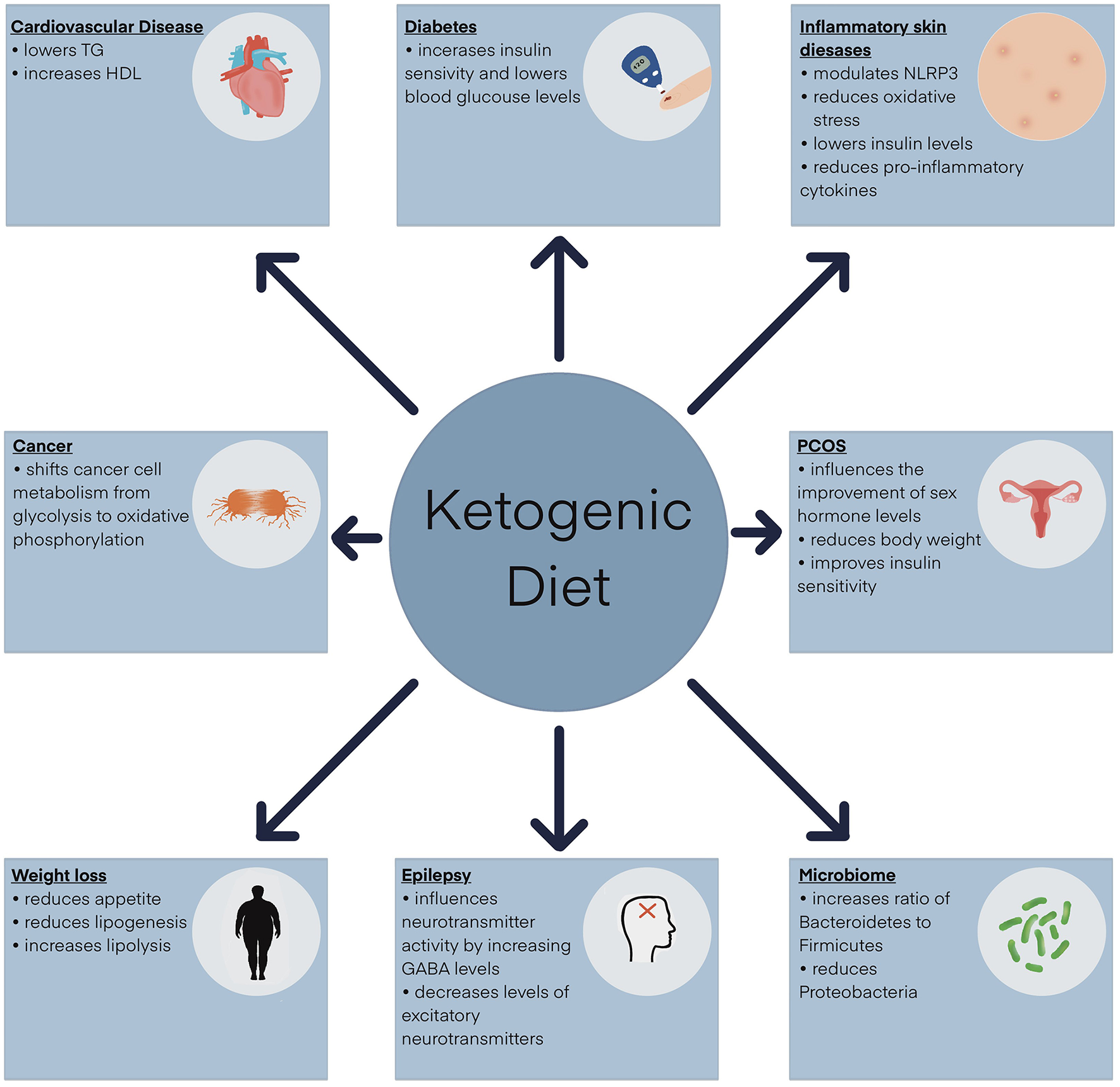
Clinical application of the ketogenic diet. GABA, γ-aminobutyric acid; HDL, high-density lipoprotein; NLRP3, NOD-like receptor pyrin domain-containing protein 3 inflammasome; TG, triglycerides.
Table 2
| Mechanism | Potential effects on the skin | References |
|---|---|---|
| Antioxidant effects | Activation of Nrf2 and AMPK pathways; increased antioxidant/detoxifying enzymes; modulation of NAD+/NADH balance and SIRT activity; protection against ROS-induced skin damage (studied mainly in acne, psoriasis, melanoma, HS) | (19, 20, 124, 125, 141) |
| Anti-inflammatory effects | Suppression of NLRP3 inflammasome; reduction of pro-inflammatory cytokines (IL-1β, IL-6, IL-17, IL-18, IL-23); attenuation of inflammatory mediators (studied mainly in acne, psoriasis, hidradenitis suppurativa) | (18, 19, 21) |
| Regulation of signaling pathways | Modulation of AKT/mTOR, FOXO1, SREBP1, and NF-κB, as well as activation of AMPK (studied mainly in acne, psoriasis, and cutaneous melanoma) | (18, 19, 21) |
| Gut microbiota modulation | Restoration of gut–skin axis balance; reduction of dysbiosis-associated inflammation and barrier dysfunction (studied mainly in acne, psoriasis, and hidradenitis suppurativa) | (21, 158, 163, 165, 214, 218, 261) |
| Metabolic effects | Improved insulin sensitivity; reduction of IGF-1 and insulin signaling; increased IGFBP-3 and SHBG; inhibition of SREBP-1–mediated lipogenesis; weight loss benefits (notably in psoriasis and HS); alterations in lipid metabolism, including increased sphingomyelins (cutaneous melanoma) | (19, 21, 222) |
Molecular mechanisms by which the ketogenic diet (KD) may influence skin health.
AMPK, adenosine 5′-monophosphate-activated protein kinase; AKT, protein kinase B; FOXO1, forkhead box protein O1; HS, hidradenitis suppurativa; IGF-1, insulin-like growth factor; IGFBP-3, insulin-like growth factor-binding protein 3; IL-1β, interleukin-1β; IL-6, interleukin 6; IL-17, interleukin 17; IL-18, interleukin 18; IL-23, interleukin 23; mTOR, mechanistic target of rapamycin; NAD+/NADH, nicotinamide adenine dinucleotide (oxidized/reduced form); NF-κB, nuclear factor kappa B; NLRP3, NOD-like receptor pyrin domain-containing protein 3; Nrf2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; SHBG, sex hormone-binding globulin; SIRT, sirtuin; SREBP1, sterol regulatory element-binding transcription factor 1.
4 Ketogenesis and ketone bodies
The proper functioning of the human body is dependent on the energy provided by food (86). Carbohydrates are the primary, but not the only, source of energy necessary for the proper functioning of the brain, central nervous system, and red blood cells (87, 88). The energy substrate with a key role in the human body is glucose (89). Increased blood glucose concentration induces an increase in the production of a polypeptide hormone called insulin (90, 91). Its main task is to regulate glucose to optimal levels for the body (90, 92). Insufficient carbohydrate intake of less than 50 g/day leads to reduced insulin secretion (71, 93). The human body's inability to extract energy from carbohydrates prompts it to use alternative sources of energy (93). The initial stage involves an increase in the concentration of glucagon, a hormone produced by the pancreas (94). Glucagon stimulates the release of glucose from glycogen stores (95, 96). Once these are depleted, the enzymatic process of gluconeogenesis begins. The primary role of this process is to convert amino acids, glycerol, and lactic acid into glucose (96). Nevertheless, gluconeogenesis can only last for a total of 3 days in the human body. At this point, the body's stored fat tissue becomes the next energy source, initiating ketogenesis (35, 97). The breakdown of fatty acids in the liver leads to an increase in the production of ketone bodies. Ketone bodies serve as an alternative energy source for various tissues and organs in the human body. Naturally, the human body continuously produces a small amount of ketone bodies, which can then be used to produce energy (98). However, elevated ketone levels are observed in patients with type 1 diabetes, as well as during intense physical exercise, alcohol abuse, or very restrictive diets (99). Depending on their concentration, ketone bodies can have both beneficial and harmful effects. Their elevated concentration, usually exceeding 7 mmol/L, is observed during fasting. However, a concentration exceeding 25 mmol/L are often found in patients with type 1 diabetes (100). When insulin deficiency is severe, blood glucose levels rise, leading to increased ketone body production. This condition, known as diabetic ketoacidosis, can lead to hazardous complications that are harmful to the patient's health and may even be life-threatening (101). The most serious of these are sepsis and cerebral edema, which can lead to shock and coma (102). There is also a distinction between alcoholic ketoacidosis associated with chronic alcohol abuse and starvation ketoacidosis resulting from a starvation diet (99, 103).
Ketones are a class of organic compounds containing a carbonyl group (C=O) connected to two hydrocarbon groups (R). These molecules are highly soluble in water, so their transport to various tissues does not require the involvement of lipoproteins (104, 105). Ketone bodies in the human body include acetone, acetoacetate (AcAc), and β-hydroxybutyrate (BHB). They have not only metabolic but also signaling functions. All of the compounds mentioned above are formed during ketogenesis (104).
Ketogenesis occurs in the liver, specifically, in hepatocytes, through the mitochondrial β-oxidation of fatty acids. It is a complex mechanism. It involves the conversion of acetyl-CoA to ketone bodies: AcAc, BHB, and acetone. In the first step of ketogenesis, the two acetyl-CoA molecules react with each other. Under the influence of thiolase (acetyl-CoA acetyltransferase 1, ACAT1), CoA-SH is detached from one molecule of acetyl-CoA, and acetoacetyl-CoA (AcAc-CoA) is formed. AcAc-CoA then combines with another acetyl-CoA molecule to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction is catalyzed by 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2). Subsequently, 3-hydroxy-methylglutaryl-CoA lyase (HMGCL) cleaves HMG-CoA in an irreversible reaction into acetyl-CoA and the first ketone body, AcAc. AcAc is converted in a reversible reaction catalyzed by D-β-hydroxybutyrate dehydrogenase (BDH1), leading to the formation of BHB, or undergoes non-enzymatic decarboxylation with the formation of acetone (Figure 2) (104, 105).
Figure 2
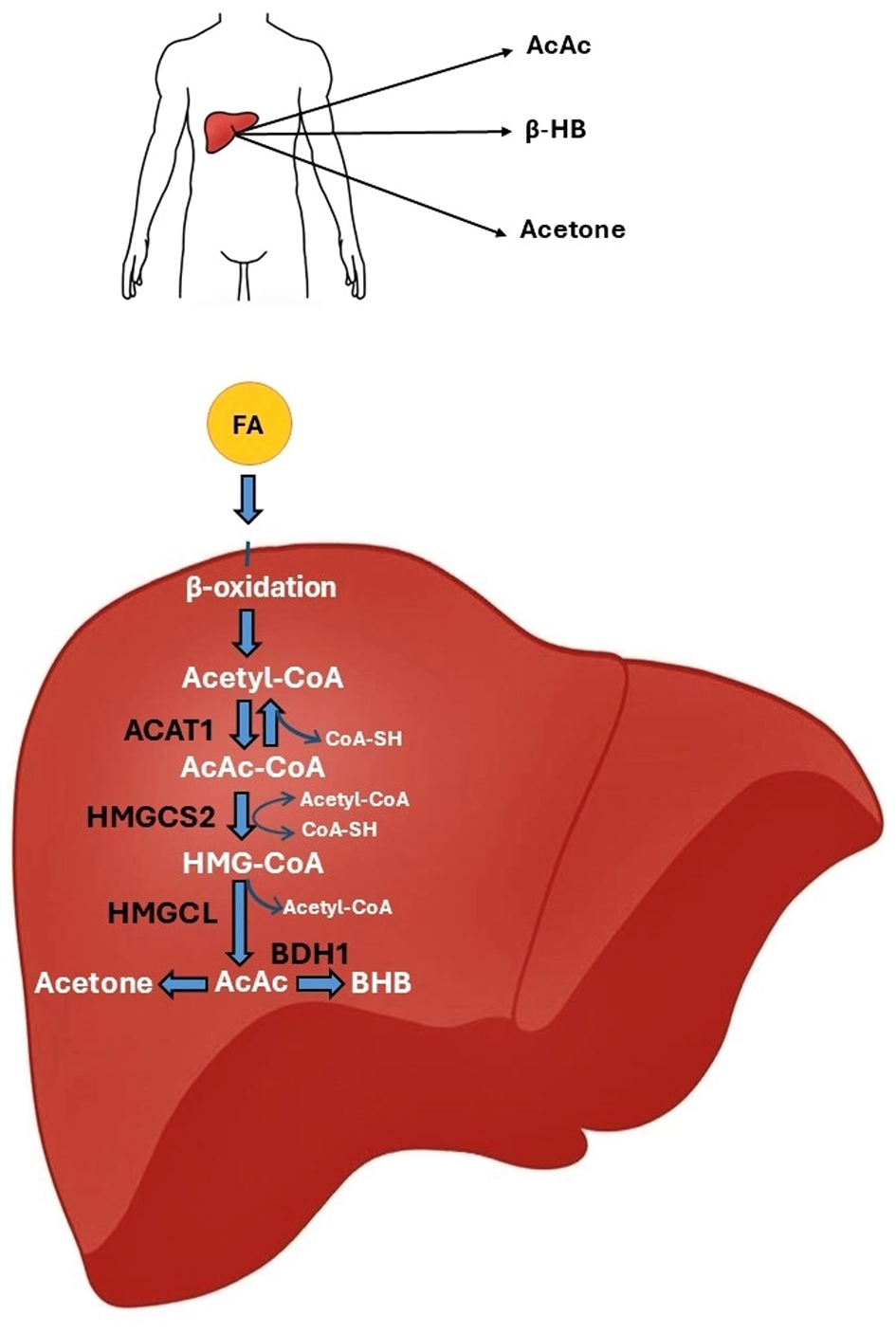
The ketone body synthesis pathway. In the mitochondria of hepatocytes, fatty acids undergo β-oxidation, which converts them into acetyl-CoA molecules. The action of the ACAT1 enzyme results in the fusion of two acetyl-CoA molecules to form AcAc-CoA. AcAc-CoA combines with another acetyl-CoA molecule under the action of the HMGCS2 enzyme to form HMG-CoA. The HMGCL enzyme breaks down HMG-CoA into AcAc. AcAc can be converted to BHB by the BDH1 enzyme or decarboxylated to acetone. AcAc, acetylacetone; ACAT1, acetyl-CoA acetyltransferase 1; BDH1, D-β-hydroxybutyrate dehydrogenase; BHB, β-hydroxybutyrate; HMGCL, 3-hydroxy-methylglutaryl-CoA lyase; HMGCS2, 3-hydroxy-3-methylglutaryl-CoA synthase 2.
5 Antioxidant potential of the ketogenic diet
The skin is the largest organ of the human body, one that is particularly susceptible to oxidative stress because of its constant exposure to external factors (106, 107). Oxidative stress is defined as an imbalance between the production of ROS and the antioxidant capacity of the body (106). ROS are highly reactive molecules such as hydrogen peroxide (H2O2), superoxide anion radical (), hydroxyl radical (OH), and singlet oxygen (1O2) (108). Antioxidants are a group of chemical compounds that neutralize ROS (109). Antioxidants can be divided into endogenous antioxidants, such as antioxidant enzymes: superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), as well as exogenous antioxidants, which mainly include non-enzymatic antioxidants such as vitamin C, vitamin E, carotenoids, ubiquinone, and flavonoids (110). An imbalance between oxidation and antioxidant protection damages cellular structures like proteins, lipids, and DNA, which impairs their function (111, 112). Several studies have shown that oxidative stress is involved in the pathogenesis of inflammatory skin diseases, such as atopic dermatitis, acne, allergic dermatitis, and psoriasis (113). It also contributes to accelerated skin aging and can lead to the development of skin cancer (114).
Many factors are known to induce oxidative stress in the skin. These include environmental factors, such as ultraviolet (UV) radiation and environmental pollutants, including ozone and cigarette smoke (115, 116). Other oxidative stress triggers include a poor diet, low physical activity, prolonged stress, and intrinsic factors, such as mitochondrial dysfunction leading to reduced adenosine 5′-triphosphate (ATP) production, inflammation, and hypoxia (106, 117). Oxidative stress affects both the structure and function of the skin. It leads to changes in the structure of skin matrix proteins, such as collagen and elastin, and consequently, to loss of skin elasticity and firmness, the formation of wrinkles, and pigmentation disorders (116–118). Oxidative stress is also responsible for impaired immune responses in the skin (119). In the context of inflammatory skin diseases, oxidative stress can disrupt the skin's hydrolipid barrier, damage keratinocyte DNA, oxidize lipids in the stratum corneum, and induce the production of pro-inflammatory cytokines—interleukin-1β (IL-1β) and interleukin 33 (IL-33)—in the dermis, promoting inflammation and exacerbating the disease (120).
As an exogenous source of antioxidants, the diet plays a significant role in protecting against oxidative stress. Dietary antioxidants neutralize reactive oxygen species, maintain redox balance, and prevent skin disease (121). Current literature suggests that KD supports the body's ability to combat oxidative stress by producing ketone bodies, which may alleviate the symptoms of many skin conditions linked to oxidative stress (14). There are two potential mechanisms by which KD may enhance the body's natural antioxidant capacity. The first mechanism is that ketones stimulate nuclear factor erythroid 2-related factor 2 (Nrf2) (14, 19, 122). Nrf2 plays a key role in protecting cells from oxidative stress-induced damage (14, 19). Under physiologic conditions, Nrf2 is present in the cytosol in an inactive form as a complex with the inhibitory protein Keap1. Keap1 plays a vital role in regulating the ubiquitination of Nrf2 and, at a later stage, its degradation (19, 123). An animal study showed that rats on a ketogenic diet during metabolic adaptation (approximately 3 weeks) exhibited increased production of H2O2 originating from the hippocampus mitochondria and 4-hydroxynonenal (4-HNE), a product of lipid peroxidation. These reactive molecules have a signaling function, modifying the Keap1 protein, which leads to Nrf2 activation (124). Nrf2 is released from the Nrf2–Keap1 system and then translocates to the cell nucleus, where it combines with small musculoaponeurotic fibrosarcoma (sMAF) proteins to form heterodimers. The Nrf2–sMAF heterodimer binds to the antioxidant response element (ARE), which leads to the transcription of genes encoding antioxidant and detoxification enzymes such as SOD, CAT, GPX, heme oxygenase 1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), which protect cells from oxidative stress and toxins (19, 124–126) (Figure 3).
Figure 3
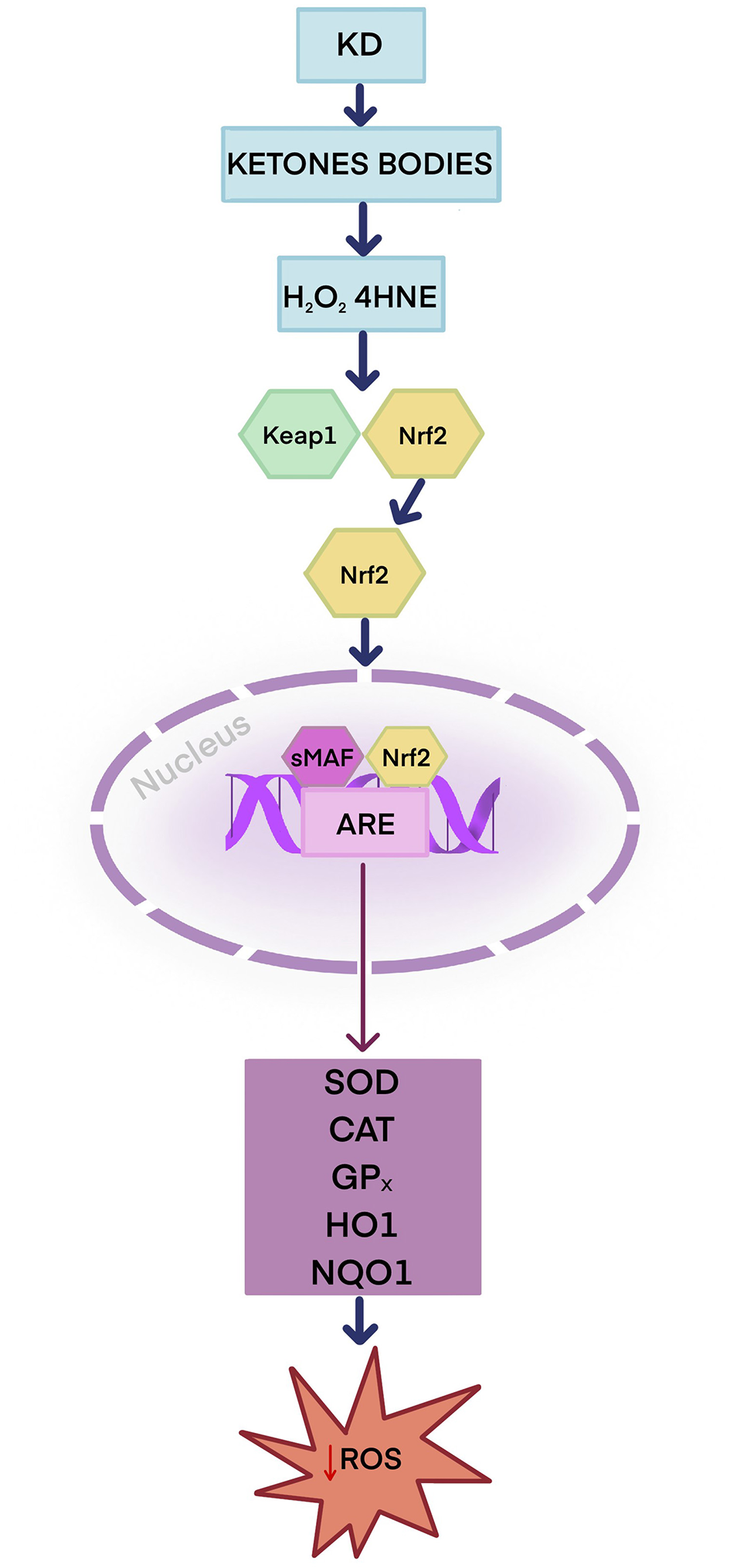
Potential antioxidant role of the ketogenic diet—Keap1–Nrf2–ARE antioxidant response pathway. The ketogenic diet produces ketone bodies that enhance the production of H2O2 and 4-HNE. H2O2 and 4-HNE activate Nrf2. Nrf2 is released from the Nrf2–Keap1 complex and travels to the cell nucleus, where it forms heterodimers with sMAF proteins, allowing it to bind to ARE sequences in DNA. Nrf2 activates antioxidant and detoxification enzymes: SOD, CAT, GPX, HO-1, and NQO1. ARE, antioxidant response element; CAT, catalase; GPX, glutathione peroxidase; 4-HNE, 4-hydroxynonenal; H2O2, Hydrogen peroxide; HO-1, heme oxygenase; KD, ketogenic diet; Keap 1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; NQO1, NAD(P)H quinone dehydrogenase 1; SOD, superoxide dismutase; sMAF, small musculoaponeurotic fibrosarcoma protein.
By influencing metabolic pathways, KD can also indirectly modulate NAD+/NADH levels, which play an important role in maintaining redox homeostasis (19, 127). NAD+ is an electron carrier in glycolysis and the Krebs cycle, where it is converted into the reduced form NADH, which is essential for ATP production (127, 128). NAD+ also plays an important role in DNA repair, serving as a substrate for poly-(ADP-ribose) polymerase (PARP) enzymes that are involved in the response to DNA damage (127–130). PARP, and PARP-1 in particular, uses NAD+ to add ADP-ribose residues and target proteins to DNA (127–129). This modification leads to the remodeling of chromatin structure and the recruitment of DNA repair proteins, such as X-ray repair cross-complementing protein 1 (XRCC1), DNA ligases, and DNA polymerases (129, 130). In addition, NAD+ activates sirtuins (127–130). Sirtuins represent a family of seven NAD+-dependent enzymes (SIRT1–SIRT7) (129). Sirtuins, particularly SIRT1 and SIRT3, protect cells from oxidative stress by increasing the activity of antioxidant enzymes, including superoxide dismutase 2 (SOD2) and CAT (127, 131, 132). In addition, SIRT1 reduces oxidative stress and inflammation by inhibiting the nuclear factor kappa B (NF-κB) signaling pathway, resulting in decreased production of inflammatory cytokines, including IL-1β, interleukin-6 (IL-6), tumor necrosis factor (TNF-α), and ROS (133–135). In contrast, SIRT3 and SIRT5 improve mitochondrial function, as manifested by reduced ROS production during oxidative phosphorylation (136). With age, NAD+ levels decrease while NADH levels increase, leading to metabolic disorders, mitochondrial dysfunction, oxidative stress, and accelerated aging (137). A disrupted NAD+/NADH balance may contribute to the development of many diseases, including cardiovascular and neurodegenerative disorders such as Alzheimer's and Parkinson's disease, metabolic disorders such as type 2 diabetes, and inflammatory skin conditions such as acne and psoriasis (135–137). It has been reported that the NAD+/NADH ratio is more important for maintaining good health than NAD+ alone (138, 139). KD may increase the NAD+/NADH ratio through various mechanisms. Firstly, restricted carbohydrate intake in KD leads to a reduction in glycolysis, the primary pathway of glucose catabolism, which is responsible for the production of NADH (140). Restriction of glycolysis results in reduced NADH production, which may contribute to an increase in the NAD+/NADH ratio (141, 142). By inhibiting glycolysis, KD reduces oxidative stress due to decreased ROS production (122, 143). Secondly, ketone bodies are metabolized in mitochondria, resulting in less NAD+ consumption and an increase in the NAD+/NADH ratio in the cytoplasm (144). However, further studies are needed to clarify the exact mechanism by which KD affects NAD+/NADH levels. Current knowledge is largely derived from preclinical studies investigating the biological processes involved, with a lack of clinical trials confirming these effects in human subjects.
6 The ketogenic diet and the gut microbiota
The gut microbiota, also known as the gut microbiome, is a collection of diverse species of bacteria, fungi, and protozoa that colonize the human gastrointestinal tract from birth (145). It is estimated that the gut microbiota comprises about 100 trillion microorganisms (145). These bacteria are part of the Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia families, with Firmicutes and Bacteroidetes being predominant (145–147). A qualitative or quantitative disturbance of the microbiota colonizing the gut is known as dysbiosis (146–148). Dysbiosis impairs the intestinal barrier, increasing its permeability and allowing bacteria and lipopolysaccharides (LPS) from the gut to translocate into the systemic circulation, leading to systemic inflammation (148). The gut microbiota plays an important role in the function of the immune system and metabolic activity (148–150). It is responsible for the breakdown of complex and undigested polysaccharides, particularly mucin, resistant starch, and oligosaccharides, which the human body cannot metabolize on its own (151, 152). Subsequently, the gut microbiota produces short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate, which affect intestinal homeostasis (147, 153). It also plays a key role in the synthesis of vitamins, including vitamins K and B12 (153).
Studies indicate that the type of delivery significantly impacts the composition of newborns' gut microbiota (154). It has been found that the gut microbiota of babies born naturally is rich in Bifidobacteria, Lactobacillus, and Bacteroides (155). In contrast, babies delivered by cesarean section have poorer gut microbiota, dominated by Staphylococcus, Streptococcus, and Clostridium, and a significant deficiency of Bifidobacteria (155). It is believed that the first 2 years of life are crucial for the development of the immune system and the formation of the gut microbiome (156). Various factors, including lifestyle, diet, stress, antibiotics, and bacterial infections, can also modulate the gut microbiota (147, 157). Among these, diet has the most significant impact on the gut microbiota (157). Dietary interventions can modulate both the composition and function of the gut microbiota (157). Poor dietary habits, particularly a diet high in saturated fats and refined carbohydrates, lead to changes in the composition of the gut microbiome. This increases the Firmicutes/Bacteroidetes ratio, which is associated with obesity (158, 159). In contrast, foods rich in dietary fiber increase the diversity of the gut microbiota and the production of metabolites, including SCFAs, which have anti-inflammatory effects (160, 161).
The literature highlights an important relationship between the gut microbiota and the skin, referred to as the gut–skin axis (162). A growing body of evidence suggests that many skin diseases, such as acne vulgaris, psoriasis, atopic dermatitis, and HS, may result from an imbalance of the gut microbiota (162). Nevertheless, these studies often report varied and inconsistent findings (162). Several studies in patients with psoriasis and psoriatic arthritis have observed an increased abundance of Firmicutes in the gut microbiota (162). Firmicutes are Gram-positive bacteria involved in fat production and storage (162). An increased ratio of Firmicutes to Bacteroidetes results in a weakened intestinal mucosal barrier, which promotes the passage of bacteria into the bloodstream and leads to chronic inflammation. This is due to a change in carbohydrate metabolism, specifically an increased production of the pro-inflammatory acetate and a decreased production of butyrate, which strengthens the intestinal barrier and has anti-inflammatory effects (162). Recent reports indicate that acne may also result from changes in the gut microbiota (21, 162–164). However, studies on the Firmicutes/Bacteroidetes ratio in acne are inconclusive. Two authors demonstrated a reduced Firmicutes/Bacteroidetes ratio in acne patients (163, 164). Also shown was an increase in Proteobacteria, whose significant numbers damage the intestinal barrier, enhancing inflammation. Another study found a high Firmicutes/Bacteroidetes ratio in acne patients before antibiotic therapy (164). Discordant research findings may be attributed to varied research methodologies and characteristics of the control group, including differences in age, sex, and socioeconomic factors. Further research is needed to evaluate the impact of the gut microbiota on inflammatory skin diseases.
KD may modulate the gut microbiota, which may be vital in some inflammatory skin diseases. Several studies indicated that KD increases the Bacteroidetes/Firmicutes ratio (165, 166). Bacteroidetes increase lipid metabolism, which is associated with fat loss (165). Obesity is a known risk factor for some inflammatory skin conditions, such as psoriasis and HS (167, 168). By increasing Bacteroidetes, KD may lead to weight loss and alleviate the symptoms of the above diseases (162, 165). Bacteroidetes also produce SCFAs, which may reduce systemic inflammation typical of inflammatory skin diseases (162). SCFAs, especially butyrate, modulate the immune system by activating G-protein-coupled receptors (GPCRs), particularly G-protein-coupled receptor 43 (GPR43) and G-protein-coupled receptor 109A (GPR109A) (169). GPCRs promote the differentiation of regulatory T cells (Tregs) by activating mTOR and inhibiting the histone deacetylase (HDAC) pathway (169). Tregs are responsible for inhibiting immune responses by reducing the severity of chronic inflammation. SCFAs inhibit the NF–κB pathway and reduce the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, which can alleviate skin symptoms in psoriasis, acne, or HS (169). Another study found that KD also reduces the amount of Proteobacteria, which are linked to inflammation (170). Although KD may potentially modulate the composition of the gut microbiota, current clinical evidence in dermatological patients is minimal, and the majority of the data come from studies conducted in individuals with obesity or epilepsy. Further well-designed randomized controlled trials in dermatological populations are needed to assess the long-term impact of this intervention on skin diseases.
7 Ketogenic diet in inflammatory skin diseases
7.1 Acne
Acne vulgaris is one of the most common chronic inflammatory skin diseases. It is estimated to affect approximately 9.4% of the world population (171, 172). Acne predominantly appears during adolescence, but is also increasingly common in adulthood (173). The clinical manifestations of acne are comedonal, papular, nodular, and cystic lesions, which can result in scarring, post-inflammatory hyperpigmentation, or, less commonly, hypopigmentation (172). Acne vulgaris lesions most commonly affect the face, neck, back, and chest (172). A significant seborrhea is also observed in acne (172).
The etiopathogenesis of acne is complex and multifactorial. It is most commonly associated with increased sebum production in the sebaceous glands, abnormal hyperkeratinization of the hair follicles and their excessive colonization by C. acnes, and the release of inflammatory mediators in the skin (172–174). Other causes include genetic predisposition, hormonal factors, poor skin care, and even diet (172). The skin condition of acne patients has also been shown to be significantly dependent on the gut microbiota (175). The gut microbiota plays an important role in the pathophysiology of acne vulgaris, potentially via interaction with the mTOR pathway (21, 174). Increased mTOR activity conditions excessive keratinocyte proliferation and increased lipogenesis in sebocytes, ultimately leading to sebum overproduction (21). Metabolites derived from the gut microbiota regulate various metabolic functions, such as proliferation and lipid metabolism, which are mediated by the mTOR pathway (21). The mTOR pathway regulates the intestinal barrier, which can, in turn, influence the composition of the intestinal microbiota (21). Excessive activation of mTOR can also lead to increased intestinal permeability and dysbiosis (21). Recent data indicate that oxidative stress also significantly impacts the course of acne vulgaris (21, 176, 177). Overproduction of ROS is involved in the pathogenesis of acne vulgaris via various pathways, including activation of mTOR, peroxisome proliferator-activated receptors (PPARs), and toll-like receptors (TLRs) (21, 176–178). ROS induce inflammation by increasing the production of pro-inflammatory cytokines, especially IL-1, IL-8, and TNF-α (176, 177). NOD-like receptor pyrin domain-containing protein 3 (NLRP3) may be involved in the pathogenesis of acne vulgaris (179). Studies have shown that the NLRP3 inflammasome is also involved in other skin diseases, such as psoriasis, urticaria, and bullous pemphigoid (179). The leading acne-associated bacterium, C. acnes, stimulates the immune system through NLRP3 activation (180). Subsequently, NLRP3 leads to the activation of caspase 1. This enzyme mediates the inflammatory response by producing pro-inflammatory cytokines, such as IL-1β and IL-18, which contribute to the development of acne vulgaris (180).
The importance of diet in the course and treatment of acne vulgaris is also increasingly emphasized; however, research findings are still limited and inconclusive. Recent reports indicate that high blood insulin levels exacerbate acne (21). Foods with a high glycemic index (GI) cause a sharp rise in glucose levels, leading to a sudden surge in insulin release (181). Elevated insulin levels cause an increase in insulin-like growth factor (IGF-1) and a decrease in insulin-like growth factor-binding protein 3 (IGFBP-3) (19–21). This results in excessive keratinocyte and sebocyte proliferation, as well as decreased activity of the retinoic acid receptor (RAR) and retinoid X receptor (RXR) in the skin, causing increased comedogenicity and higher sebum production (19, 182). In addition, increased levels of insulin and IGF-1 reduce the synthesis of sex hormone-binding globulin (SHBG) (19). SHBG is a protein produced in the liver that binds to testosterone, dihydrotestosterone (DHT), and estradiol (183). Low SHBG levels cause an increase in free testosterone (fT) in the blood, which is converted into DHT by the enzyme 5-α-reductase (184). DHT is responsible for increased sebum production by the sebaceous glands and exacerbates acne lesions (21, 185, 186).
The effect of KD on acne is a growing area of research. It has been speculated that KD may alleviate symptoms of acne vulgaris and even completely suppress them due to its anti-inflammatory and antioxidant properties (21). Ketone bodies exert their anti-inflammatory effects through different mechanisms. BHB is the leading and most biologically active ketone body produced during KD use (21, 187). It plays a significant role in promoting moderate mitochondrial stress, which activates Nrf2, adenosine 5′-monophosphate-activated protein kinase (AMPK), and SIRT1 and SIRT3, all of which are involved in anti-inflammatory and antioxidant responses (21, 188). This activation may further contribute to the inhibition of forkhead box protein O1 (FOXO1) and the reduction of ROS (189). In addition, BHB inhibits NLRP3 activation, resulting in a reduced release of inflammatory factors, particularly IL-1β and IL-18, by inhibiting caspase-1 activation (21). Several studies have shown that the ketogenic diet, especially VLEKT, can reduce acne lesions by lowering insulin levels (21, 176, 190). This contradicts the Western pattern diet, which stimulates insulin secretion with only a slight increase in blood glucose levels (21, 176). KD lowers insulin, causing a decrease in IGF-1, which consequently leads to an increase in IGFBP-3 (21). Furthermore, a decrease in insulin and IGF-1 levels is associated with an increase in SHGB (21). An increase in SHGB reduces free testosterone and sebum production (21, 191). A decrease in IGF-1 leads to reduced activity of the protein kinase B (AKT)/mTOR pathway, which in turn activates FOXO1 (21, 192). FOXO1 inhibits sterol regulatory element-binding transcription factor 1 (SREBP1) (193). SREBP1 plays an important role in lipid metabolism. A reduction in SREBP1 activity results in less sebum production and reduced acne lesions (Figure 4) (194). A study evaluating the effectiveness of a 45-day VLEKT in improving acne in 31 young women with moderate acne and grade I obesity showed a reduction in markers of oxidative stress, such as reactive oxygen metabolite derivatives (dROMs), and a decrease in pro-inflammatory metabolites, including trimethylamine N-oxide (TMAO). The study indicates that the VLEKT may be a potential therapeutic tool for treating skin diseases associated with oxidative stress and inflammation (176). However, further research is needed to evaluate the interaction mechanism between ketones and NLRP3 and determine its efficacy and safety as a treatment option for acne vulgaris (13, 19, 182). There is still no reliable evidence confirming VLEKT's effectiveness in treating acne vulgaris. Existing studies are mostly preliminary, involve small cohorts, and lack long-term randomized controlled trials, which limits the strength of current conclusions.
Figure 4
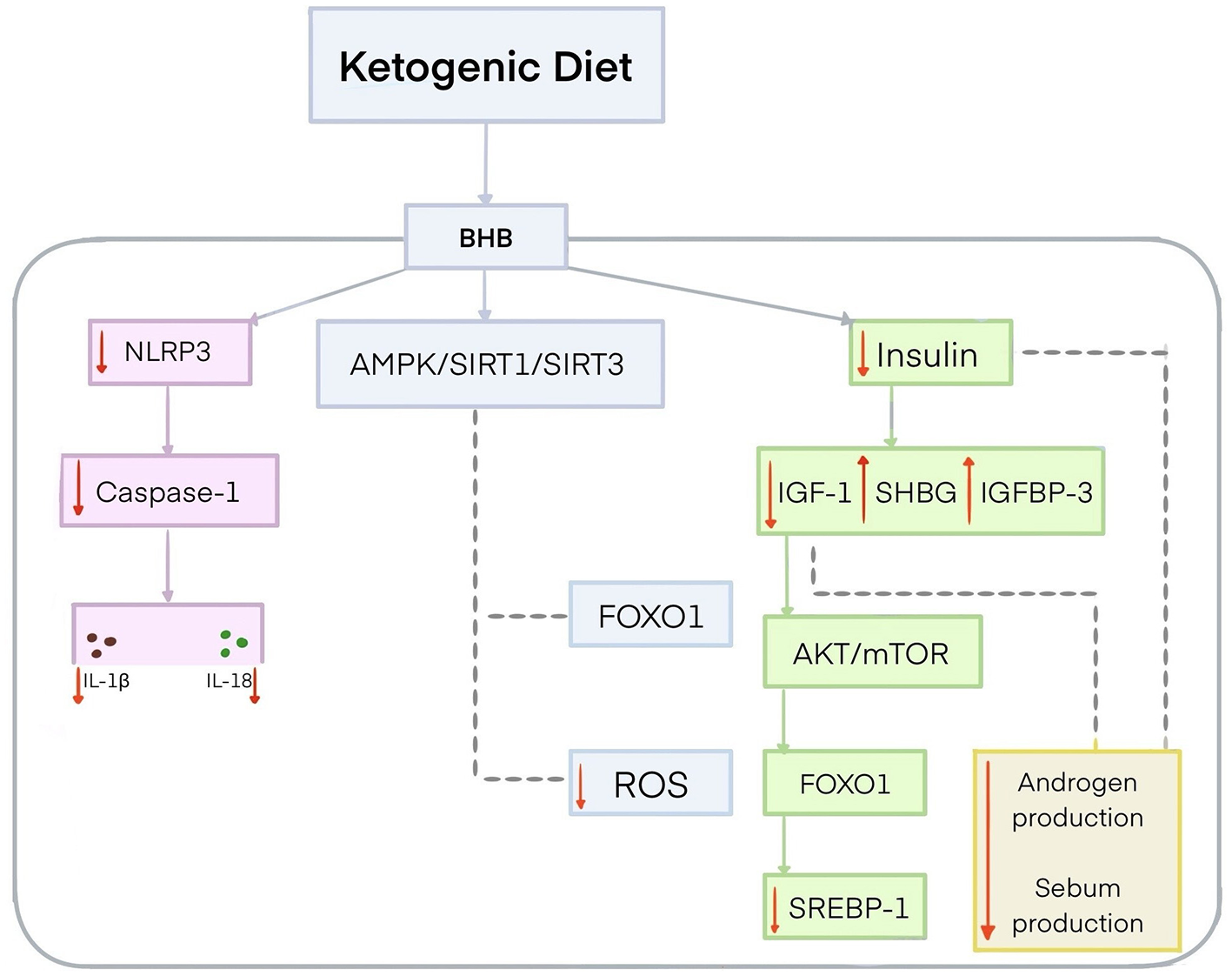
Potential mechanism for the effect of the ketogenic diet on acne. The ketogenic diet produces BHB, which inhibits the activity of NLRP3 and thereby reduces the pro-inflammatory cytokines IL-1β and IL-18 by inhibiting caspase-1 activation. BHB activates AMPK/SIRT1/SIRT3 signaling, leading to FOXO1 inhibition and reduced ROS levels. BHB contributes to lower insulin levels, leading to a reduction in IGF-1 and an increase in IGFBP-3. The reduction of insulin and IGF-1 increases SHBG concentrations, resulting in reduced androgen activity and lower sebum production. Decreased IGF-1 concentrations lead to AKT/mTOR inhibition and FOXO1 activation. FOXO1 inhibits SREBP1. AKT, protein kinase B; AMPK, adenosine 5′-monophosphate-activated protein kinase; FOXO1, forkhead box protein O1; IGF-1, insulin-like growth factor; IGFBP-3, insulin-like growth factor-binding protein 3; IL-1β, interleukin-1β; IL-18, interleukin 18; mTOR, mechanistic target of rapamycin; NLRP3, NOD-like receptor pyrin domain-containing protein 3; SHGB, sex hormone-binding globulin; SIRT 1, sirtuin 1; SIRT 3, sirtuin 3; SREBP1, sterol regulatory element-binding transcription factor 1.
7.2 Psoriasis
Psoriasis is a chronic, non-infectious, systemic inflammatory disease of autoimmune origin. It affects approximately 2–3% of the world's population (195). According to available data, it is most commonly observed in Caucasians and Scandinavians, while Asian and African populations are less affected (196). Psoriasis affects both men and women, although it usually develops earlier in women (197, 198). It can develop at any age, but is most common in people aged 15–30 (199). Typical sites of psoriatic lesions include the scalp, elbows, knees, hands, feet, and the lumbosacral region (197, 200).
The typical symptoms of psoriasis are skin lesions resulting from epidermal hyperproliferation, increased angiogenesis, and accumulation of inflammatory cells (201). However, the clinical symptoms of psoriasis may vary and depend on its type. The most common is plaque psoriasis (202, 203). The classic clinical manifestations of plaque psoriasis are well-demarcated, erythematous, scaly plaques covered with silvery scales (202, 203). Another form is pustular psoriasis, characterized by numerous pustular lesions filled with pus (202). Pustular psoriasis has two variants—generalized and localized palmoplantar (202). The most severe variant of psoriasis is the generalized form, which is often accompanied by systemic symptoms (202).
The pathogenesis of psoriasis is multifactorial and is still not fully understood. Immunological factors play a prominent role in the development of this disease (204). Psoriasis involves uncontrolled activation of T helper cells (Th1) and (Th17), resulting in the overproduction of pro-inflammatory cytokines, such as IL-1, IL-6, IL-23, IL-22, interferon alpha (IFN-α), and TNF-α (204–206). Genetic factors also play a key role in the pathogenesis of psoriasis (204, 207). The human leukocyte antigen (HLA-Cw6) is considered one of the strongest alleles determining the development of this disease (199, 207). It affects the activation of T cells, especially CD8+ T cells, which then recognize HLA-Cw6 antigen presentation (208). In turn, T cells produce inflammatory cytokines, such as IL-17 and IL-23, which exacerbate skin inflammation (206, 209). The presence of HLA-Cw6 affects the course and severity of psoriasis, as well as comorbidities. Some studies indicate that HLA-Cw6 in patients with psoriasis may be associated with better biologic treatment efficacy, particularly with IL-23 and IL-17 inhibitors (207). Environmental factors also contribute to the development of psoriasis (195, 199). These include a history of infection, the use of certain drugs, especially TNF-α inhibitors, smoking, alcohol, and stress (195, 199).
The relationship between psoriasis and obesity is bidirectional. Chronic systemic inflammation is believed to be the key pathomechanism underlying this relationship (167, 201). Numerous studies have shown that obesity is a risk factor for psoriasis, affecting its course and the Psoriasis Area and Severity Index (PASI) (205, 210). Excess visceral fat leads to increased production of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-17, as well as the following adipokines: leptin, visfatin, and resistin, which exacerbate inflammation (13, 205). A more severe course of this disease was found in obese patients compared to normal-weight patients (203, 205, 210). Obesity also negatively affects the efficacy of biologic treatment in patients with psoriasis (205). A second direction of the interrelationship between psoriasis and obesity indicates that patients with psoriasis have elevated levels of inflammatory cytokines, particularly TNF-α, which can lead to metabolic abnormalities, including insulin resistance, and may consequently lead to the development of obesity (211). Clinical trial meta-analyses have shown that weight loss resulting from dietary interventions and increased physical activity in patients with psoriasis significantly reduces the severity of disease symptoms (212, 213).
Diet plays a significant role in the course of psoriasis (13, 214, 215). Dietary factors can be an effective part of psoriasis therapy, helping to alleviate disease symptoms and modulating systemic inflammation and the gut microbiota (214). One study showed that KD and MD may benefit patients with psoriasis (205). KD's potential role in alleviating psoriasis symptoms may be due to several mechanisms. The first relates to weight reduction, which is important as obesity is one of the factors contributing to psoriasis exacerbation (201, 205). Another mechanism may be the anti-inflammatory effect of KD. KD reduces inflammatory cytokines, such as IL-6, IL-17, and IL-23, which have a significant role in the etiopathogenesis of psoriasis (201, 205). The last relates to the antioxidant effects of KD. It has been suggested that ketone bodies produced while on a KD may reduce ROS, which is associated with increased inflammation (205).
One clinical trial evaluated the effects of KD on obesity, psoriasis, and psoriatic arthritis (205). It included 26 patients who were randomly assigned a KD or MD for 8 weeks. This was followed by a 6-week washout period. After its completion, patients switched to the other diet for another 8 weeks. The study assessed various clinical and biochemical markers, including body weight, body mass index (BMI), waist circumference (WC), total fat mass, and visceral fat, as well as such inflammatory markers as the PASI and the Disease Activity Index of Psoriatic Arthritis (DAPSA). Both diets were found to have a beneficial effect on reducing body weight, total fat mass, visceral fat, BMI, and WC. With regard to these parameters, KD was found to be slightly more effective than MD. The study also showed that KD affected inflammatory markers. Patients at the start of the study had moderate psoriasis with a mean PASI of 5.09 ± 5.73. After KD treatment, the PASI was 3.15 ± 4.88. This diet significantly reduced the PASI score (p = 0.04) compared to the baseline. In contrast, the PASI score after MD did not change significantly (3.82 ± 3.93, p = 0.278 vs. baseline). The difference between diets was statistically significant (p = 0.038). Inflammatory cytokines, such as IL-6, IL-17, and IL-23, also decreased after KD. In contrast, MD showed no significant changes in inflammatory markers (205).
Another study evaluated the effect of VLEKT on patients with psoriasis (195). The study enrolled 30 patients aged 18–65 with obesity and plaque psoriasis. Study participants were on a VLEKT for 4 weeks. Clinical assessments covered body weight, BMI, waist and hip circumference, visceral fat, PASI, Dermatology Life Quality Index (DLQI), visual analog scale (VAS) for itch ratings, and measurement of the following inflammatory cytokines: IL-1β, IL-2, IL-4, interferon (IFN-γ), and TNF-α. VLEKT resulted in significant weight loss and improved PASI (by 50%), DLQI, and VAS scores for pruritus and pain. There was also an improvement in the levels of metabolites closely associated with psoriasis, particularly, an increase in folic acid, vitamin B12, calcium, and bilirubin. Reduced concentrations of inflammatory cytokines, including IL-2 and IL-1β, were also reported. The other cytokines tested—IL-4, TNF-α, and INF-γ—did not show any significant changes, probably due to the short study period. VLEKT also increased L-glutamine and glutamate levels, and decreased L-alanine, L-leucine, and choline. Changes in their levels may serve as potential biomarkers for the early diagnosis and prognosis of this disease. Although the findings of both studies are promising for treating psoriasis, they involved a limited number of participants and were conducted over a short period. Further studies with a larger patient population and evaluation of the long-term impact of the VLEKT on patients with psoriasis are required. At present, there is still no high-quality randomized controlled trial confirming the efficacy of VLEKT in psoriasis, which highlights the need for more robust clinical evidence (195).
7.3 Hidradenitis suppurativa
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic inflammatory disease mainly affecting areas with a high concentration of apocrine glands (216). The etiology of this disease is not fully understood. It is suggested that it may be caused by immune disorders, hormonal imbalances, genetic predisposition, obesity, smoking, and environmental factors, including diet (216). Inflammatory cytokines, such as IL-17 and TNF-α, are particularly important in the pathogenesis of HS (217). In addition, dysbiosis may also contribute to the development and clinical severity of HS (218). An imbalance of the gut microbiota can lead to immune activation and inflammation, particularly through an increase in pro-inflammatory lipopolysaccharides (LPS), which results in increased production of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 (218). Changes in the gut microbiome during HS can also lead to reduced production of anti-inflammatory SCFAs, which in turn inhibit pattern recognition receptors (PRRs) involved in pathogen recognition and reduce the production of IL-10 and TGF-β (218). Patients with more severe HS are also found to have elevated levels of TMAO, which is associated with inflammation and cardiometabolic risk (216, 219). TMAO may be a potential biomarker of disease progression (219). HS usually emerges in adolescence and is recurrent (216). It is much more common in women, especially those between the second and third decades of life (219). HS symptoms include painful nodules and abscesses that can lead to the development of fistulas and scarring (216).
Diet plays a significant role in the course and treatment of HS (216). Many studies indicate that specific foods can exacerbate or alleviate the symptoms of this disease (216, 220). Dietary factors exacerbating HS include dairy products, foods with a high glycemic index, and brewer's yeast (220). Obesity is considered one of the important risk factors for this disease, possibly through systemic inflammation (216). A correlation has been found between high BMI and HS symptom severity (216). The introduction of dietary modifications may lead to weight reduction and alleviate disease symptoms (220). Research suggests that VLEKT has therapeutic potential in treating HS symptoms, particularly through its anti-inflammatory effects, reduction of body fat, and changes in the gut microbiota composition, which modulate systemic inflammation and oxidative stress (216).
A pilot study evaluating the effect of a 28-day VLEKT on clinical HS severity in 12 overweight and obese women revealed weight loss and a reduction in HS severity as measured by the Sartorius score (216). The Sartorius score is a clinical tool designed to assess the severity of HS. It involves counting individual skin lesions, including fistulas, abscesses, nodules, and scars in seven anatomical regions. It also measures the greatest distance between two similar lesions in each anatomical region, such as axillae, buttocks, genitalia, and others, on both the left and right sides of the body (216). There was also a decrease in metabolic markers, including TMAO, which is associated with improved gut microbiota, oxidized low-density lipoprotein (OxLDL), and decreased oxidative stress, as evidenced by reduced dROM levels. Although VLEKT has therapeutic potential in reducing HS symptoms, further studies on a larger population and randomized controlled trials are necessary to confirm the effectiveness of VLEKT in improving HS symptoms. Existing evidence is limited to preliminary observations from small cohorts and short follow-up, which does not allow for firm clinical conclusions (216).
8 The ketogenic diet and skin cancer
Recent literature indicates that KD may be a promising adjuvant therapy for many malignant tumors. It has been shown that KD may have anti-tumor potential for several cancer types, including colorectal, brain, breast, and pancreatic cancer (39, 81, 82). The majority of the studies suggest that KD may counteract the Warburg effect (20, 82). The Warburg effect is a metabolic process in which tumor cells convert glucose to lactate, even in the presence of oxygen, which promotes tumor progression (20, 82). The potential mechanism of KD is to reduce the availability of glucose to tumor cells, depriving them of energy, inhibiting their proliferation, and tumor growth (13, 20, 82). Recent reports indicate that the reverse Warburg effect may also occur in the tumor formation process, whereby tumor cells undergo oxidative phosphorylation (OXPHOS) instead of glycolysis (221). However, the reverse Warburg effect does not apply to all tumors (221). Recent evidence suggests that KD may also be beneficial in skin cancers, particularly melanocyte-derived melanoma (222).
Cutaneous melanoma is a rapidly growing malignant skin tumor with a propensity to metastasize (223). It develops from neuroectodermal melanocytes (224). Melanocytes are located in the basal layer of the epidermis and synthesize melanin, which plays a key role in photoprotection against UV radiation (224). It has the highest mortality rate compared to other skin tumors, such as basal cell carcinoma and cutaneous squamous cell carcinoma (223, 224). Cutaneous melanoma at an advanced stage is resistant to standard cancer treatment (225). UV radiation is considered one of the most important inducers of cutaneous melanoma (223, 224). Other causes include a genetic predisposition, a high number of pigmented nevi, and a fair complexion (223, 224).
The literature emphasizes the potential impact of dietary choices on the risk of developing cutaneous melanoma (225). A clinical control study conducted on a group of 273 patients with cutaneous melanoma and a control group of 269 healthy individuals evaluated the impact of an anti-inflammatory diet on the risk of developing cutaneous melanoma (225). A study shows that participants who followed an anti-inflammatory diet, incorporating at least eight anti-inflammatory food products, had a lower risk of melanoma. The odds ratio (OR) for the association between an inflammatory diet and melanoma risk was 0.29 with a 95% confidence interval (CI) of 0.17–0.49. The study also included genetic analysis, which focused mainly on the −765 G > C polymorphism in the cyclooxygenase-2 (COX2) gene. COX2 is an enzyme that plays an important role in the synthesis of prostaglandins involved in inflammatory responses (225). The regulation of COX2 expression is complex, involving transcriptional and post-transcriptional mechanisms influenced by cytokines, growth factors, and pro-inflammatory stimuli (225). Overexpression of COX2 is reported in many types of cancer, including cutaneous melanoma, which contributes to tumor progression and angiogenesis (225). It was found that this polymorphism did not increase the risk of melanoma (225).
One in vivo study assessed the effect of KD on the progression and metastatic development of cutaneous melanoma (222). The study was conducted in a mouse model with BRAF and NRAS mutations, as well as wild-type melanoma xenografts and allografts of highly metastatic melanoma (222). The BRAF gene is located on chromosome band 7q34. This gene encodes the serine/threonine kinase B-Raf. BRAF plays a vital role in the mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK) signaling pathway, which influences cell growth and proliferation (226). The BRAF mutation, in particular the valine to glutamic acid mutation (V600E), is associated with many types of cancer, especially cutaneous melanoma. It leads to the activation of the MAPK pathway, which conditions the uncontrolled proliferation of tumor cells (226). The NRAS gene encodes the N-ras protein (227). NRAS mutations in melanoma occur mainly at codon 61, with Q61R considered the most common (227). They activate the RAS/MAPK pathway, which promotes cell proliferation and survival (227). A study on the effect of KD on the skin used immunocompetent (mutation-free) and immunocompromised mice (BRAF V600E, NRAS Q61R) (222). KD was shown to slow tumor growth in immunocompromised mice that had genetically and metabolically differentiated melanoma xenografts, indicating that it can inhibit tumor progression regardless of the genetic basis of the tumors. Reductions in metastasis were also found in immunocompetent mice with highly metastatic melanoma allografts, indicating that KD may not only slow tumor growth but also reduce the translocation of tumor cells to other sites. KD has also been shown to cause changes in amino acid metabolism, in particular, a reduction in levels of α-aminoadipic acid, a key biomarker of cancer (222). These changes were also observed in tumor xenografts. KD also contributed to increased sphingomyelin levels and enhanced hydroxylation of sphingomyelins and acylcarnitines, which determine changes in cell membrane composition. The increase in sphingomyelins can interfere with signaling pathways, including phosphatidylinositol 3-kinase (PI3K)/AKT and MAPK, inhibiting cancer cell proliferation and growth (222).
The role of KD as an adjuvant therapy for cutaneous melanoma appears promising (222). However, current evidence is mainly derived from preclinical studies, and further investigations, particularly rigorous clinical trials in humans, are necessary to clarify the mechanisms involved and to evaluate their efficacy and safety in patients with cutaneous melanoma.
9 Systemic complications of KD
The initial stage of KD is often accompanied by short-lived symptoms, commonly referred to as “keto flu,” including fatigue, gastrointestinal disturbances, and headache, which usually resolve within a few days (228, 229). Patients may also experience a characteristic breath odor due to acetone formation (228, 230).
Studies indicate that KD can be used in both short and long terms (48). However, long-term use may be associated with several complications, the most common of which is nutrient deficiency (231). Nutritional deficiency disorders can impair the immune system, increasing susceptibility to infection and impairing wound healing (232).
Another serious complication associated with the use of KD involves potential changes in the lipid profile, particularly an increase in low-density lipoprotein (LDL) cholesterol (48). Long-term use of KD may also increase the risk of kidney stones (231, 233). The incidence of nephrolithiasis in patients on KD ranges from 3 to 10% (233).
10 Adverse effects of the ketogenic diet on the skin
10.1 Prurigo pigmentosa
The benefits of KD for the skin are promising, but with its increasing popularity, prurigo pigmentosa (PP) cases are being reported more frequently (234). PP is a rare inflammatory skin disease (234). This disease was first described in 1971 by Nagashima, a Japanese dermatologist (234, 235). The majority of the cases have been reported in East Asia (234). The disease is also believed to have affected other ethnic groups, but was misdiagnosed due to the doctors' ignorance (236, 237). Today, PP is diagnosed in patients worldwide (234, 238). It affects women much more often than men (234, 239). Typically, the disease emerges in adolescents or young adults (239). Its clinical manifestations include itchy, erythematous papules and patches, and sometimes vesicles arranged in a reticular pattern (234, 235). After the papules resolve, post-inflammatory hyperpigmentation emerges (234, 235). The histopathological picture of PP at an early stage shows a perivascular neutrophilic infiltrate (236, 239). At a later stage, spongiosis and ballooning of keratinocytes are observed (236, 239). A lymphocytic infiltrate and melanophages follow this in the papillary layer of the dermis (235, 238, 239). PP is a recurrent disease (235). The lesions typically develop on the neck, chest, and back (235). The etiology of PP is not fully explained (234, 238). PP has been linked to Still's disease, pregnancy, chafing from clothing, excessive sweating, or Helicobacter pylori (236–238). In addition, it is believed that KD, fasting, untreated diabetes, anorexia nervosa, and bariatric surgery may also induce PP, resulting from increased production of ketone bodies in the liver (236, 237). Some patients with PP have been found to have high levels of ketone bodies in both urine and blood (239). Accumulation of ketone bodies in the blood vessels induces neutrophilic inflammation (239). Several case studies have shown a relationship between KD and PP (Table 3).
Table 3
| Sex/age | Origin | Location of lesions | PP emergence after KD | Treatment | Treatment outcome | Reference |
|---|---|---|---|---|---|---|
| M/21 | Hispanic | Chest and back | 2 months | Oral doxycycline 100 mg twice a day, KD discontinuation | 2 weeks | (234) |
| F/26 | N/A | Chest, back, supraclavicular region | 3 weeks | KD discontinuation | 1 month | (262) |
| F/26 | Saudi Arabia | Chest, back | 3 months | Oral doxycycline 100 mg/d, KD discontinuation | 2 weeks | (238) |
| M/20 | Saudi Arabia | Neck | 2 months | Oral doxycycline 100 mg/d, KD discontinuation |
2 weeks | (238) |
| F/22 | Saudi Arabia | Chest, neck | 4 months | Oral doxycycline 100 mg/d, KD discontinuation | 2 weeks | (238) |
| F/21 | N/A | Chest, inframammary region, neck | 1 week | KD discontinuation | 2 months | (263) |
| M/24 | Caucasian | Neck, chest, back | 9 days | KD discontinuation, topical corticosteroids | Several days | (264) |
| M/16 | N/A | Trunk, proximal limbs | 1 week | KD discontinuation, topical corticosteroids | 15 days | (239) |
| F/16 | Denmark | Back, armpits, abdomen | 2 weeks | Oral tetracycline 500 mg twice a day, topical Betnovate with chinoform, KD discontinuation | 1 month | (265) |
| M/18 | Denmark | Chest, abdomen, back | 2 weeks | Oral tetracycline 500 mg twice a day, topical Betnovate with chinoform, KD discontinuation | 1 month | (265) |
Characteristics of patients with prurigo pigmentosa induced by the KD.
F, female; M, male; N/A, not applicable; KD, ketogenic diet.
In the majority of the cases described, doxycycline 100 mg/day was used to treat PP. However, tetracycline, minocycline, dapsone, and topical corticosteroids are also mentioned as treatment options (234, 235, 237). KD discontinuation and a return to a balanced diet are also necessary. Some patients did not use drug treatment, and PP lesions resolved after KD discontinuation (234). Further studies are needed to clarify the exact mechanisms by which KD influences the development of PP.
10.2 Nutritional deficiencies and skin manifestations in ketogenic interventions
Nutritional deficiencies also negatively affect the appearance of the skin, often causing a number of severe skin conditions (240). In addition to altering appearance, such deficiencies can weaken skin barrier function and resilience (240). Studies indicate that long-term use of KD conditions vitamin C, biotin, and zinc deficiencies (20).
Vitamin C is an important antioxidant that plays a key role in collagen synthesis (241, 242). Its deficiency may lead to impaired wound healing, accelerated skin aging, and, in severe cases, scurvy (241, 243). Several cases of scurvy in children due to KD use have been reported in the literature (64, 244). Until recently, scurvy was considered a historical and forgotten disease; however, recent case reports significantly challenge this assumption (64). Typically, symptoms of scurvy emerge after 1–3 months of significant vitamin C deficiency (245, 246). These include systemic symptoms, such as fatigue, a deteriorated mood, and muscle and joint pain (246, 247). Bleeding gums, anemia, and loss of appetite are also observed (246). Scurvy also affects the skin, particularly causing excessive keratinization of hair follicles, bruising, bloody spots on the skin, and impaired wound healing (245, 246). In a reported case of a 12-year-old girl with cerebral palsy, cystic encephalomalacia, and refractory epilepsy, scurvy was confirmed after long-term KD use (244). The clinical signs were knee swelling, anemia, and bleeding gums. The patient showed intolerance to ready-made ketogenic products, so her primary source of nutrition was blenderized KD in a 2:1 ratio. The patient's KD contained several ingredients, including avocado, chicken breast, double cream, and coconut oil. The diet was supplemented with iodized salt, calcium carbonate, fish oil, and a multivitamin/mineral supplement (Phlexy-vits, Nutricia). The applied KD was shown to provide her with 60–70% of the recommended dietary allowance (RDA) for vitamin C (45 mg/day). Vitamin C was administered at a dose of 500 mg/day for 1 month during the initial stage of scurvy treatment. The dose was then reduced to 250 mg/day. Vitamin C supplementation led to a rapid improvement in symptoms (244). Another report found a case of scurvy following KD use in a 5-year-old girl on the autism spectrum (64). The patient presented with periorbital edema, multiple ecchymotic patches, bleeding gums, poor appetite, weight loss, generalized weakness, and hypochromic anemia. Laboratory tests showed severe vitamin C deficiency. Treatment included supplementation with vitamin C, multivitamins, and elemental iron. The supplementation resulted in a marked improvement (64).
Biotin (vitamin B7) supports keratin production and is important for skin, hair, and nail health (248). Its deficiency, although rare, may present with dermatitis, brittle nails, and hair loss (248). One study assessed the effect of KD on biotin levels using an animal model (249). The study was conducted on 32 male mice divided into four groups: a control (C) group receiving a standard diet with biotin, a biotin-deficient (BD) group on a biotin-free diet, a ketogenic control (KC) group on a ketogenic diet (low-carbohydrate and high-fat) with biotin, and a ketogenic biotin-deficient (KBD) group on a ketogenic diet without biotin. The key comparison was between the KC group (KD with biotin) and the KBD group (KD without biotin), which allowed the researchers to assess whether KD alone increases biotin requirements or accelerates the development of biotin deficiency. The study lasted 9 weeks, during which clinical signs associated with biotin deficiency and biochemical parameters, such as serum and tissue biotin levels (liver, kidney, brain, testes), and biotin-dependent enzyme activity of 3-methylcrotonyl CoA carboxylase (MCC), propionyl-CoA carboxylase (PCC), pyruvate carboxylase (PC) and acetyl-CoA carboxylase (ACC), and blood glucose levels, were evaluated. After 5 weeks, mice in the KBD group showed symptoms of biotin deficiency, including hair loss and dermatitis. The KBD group also had the lowest biotin and fasting glucose levels, likely due to impaired gluconeogenesis. In addition, the expression of MCC, PCC, PC, and AC decreased, indicating metabolic dysfunction (249). Although the results suggest that KD exacerbates biotin deficiency in mice, human clinical studies are needed to confirm these observations (249).
Zinc plays an important role in collagen synthesis and wound healing (250). Its deficiency may lead to skin lesions, impaired wound healing, and increased hair loss (243, 251). Severe zinc deficiency is also associated with acrodermatitis enteropathica, a rare autosomal recessive genetic disorder (252). Acrodermatitis enteropathica causes impaired intestinal absorption of zinc (252). Acquired acrodermatitis enteropathica is also known to be caused by insufficient dietary zinc intake (253). A distinctive sign of the disease is dermatitis, which presents as well-defined erythematous and eczematous plaques, erosions, scabs, and even blisters (254, 255). Diarrhea, conjunctivitis, alopecia, and photophobia are also observed (254, 256). To date, only one case of acrodermatitis enteropathica has been reported among patients using KD—it emerged in an 11-year-old boy with drug-resistant epilepsy (253). The patient presented with mild alopecia and perioral, periorbital, and perigenital eruption. Due to swallowing problems, the patient used a liquid KD formulation containing amino acids and medium- and long-chain triglycerides for 3 months. Despite medical recommendations, the parents did not provide the child with vitamin and mineral supplements. Laboratory tests showed a significant zinc deficiency (15 mcg/dL) compared to the normal range (50.0–120.0 mcg/dL). In turn, biopsy of skin lesions showed superficial epidermal necrosis with dyskeratotic keratinocytes. Treatment included discontinuing KD, administering zinc gluconate 50 mg/day, and emollients. Following treatment, symptoms improved after three days and completely resolved within a month (253).
An appropriately composed KD can minimize side effects (257). Although KD significantly limits carbohydrate intake, it maintains the minimum carbohydrate supply necessary for gluconeogenesis (48). A key element of KD is the avoidance of highly processed foods. Vegetable oils, particularly olive oil, coconut oil, rice oil, MCTs, and avocado oil, are the best sources of fats (48, 257). Brazil nuts, macadamia nuts, walnuts, hazelnuts, and almonds, which are rich in essential fatty acids (EFAs), are also particularly recommended as part of KD (257). Fatty fish, such as mackerel, salmon, eel, and sardines, also contain healthy fats (257). Meat is an equally important part of KD, as a key source of protein and fat. Pork, beef, and poultry are good choices (257). Eggs are also a particularly recommended animal product (257). Dairy products used in KD include full-fat cheeses (Gorgonzola, Mascarpone, Cheddar, Brie, Robiola), full-fat yogurts without added sugar, cream, and butter (48, 257). KD also relies heavily on vegetables, particularly cruciferous and leafy green vegetables, tomatoes, aubergines, broccoli, and mushrooms (48). Recommended fruits include avocados, strawberries, lemons, blueberries, raspberries, coconuts, and olives (48). Permitted beverages on KD are water, tea, and coffee (258). Popular KD meals include omelets, scrambled eggs, salads, beef steaks, and chicken with vegetables (48). The food industry also offers a wide range of ready-made ketogenic products, such as biscuits, focaccia, crackers, and desserts, which vary in nutritional composition (257). Liquid KD formulas with the classic 4:1 ketogenic ratio (4 g of fat per 1 g of protein and carbohydrates combined) are also available (257). These are usually enriched with essential fatty acids, dietary fiber, and vitamins. They are recommended for infants, children, and adults. Another food product for special medical purposes is a powder ketogenic formula for preparing a liquid mixture (257). The formula has a high-fat content with a ketogenic ratio of 4:1 or 3:1 and is fortified with a vitamin and mineral complex, essential fatty acids, and sometimes MCTs (257). For infants, when dissolved in water, this product provides a complete meal tailored to their nutritional needs. Studies indicate that a powder ketogenic formula is an effective, safe, and well-tolerated adjuvant therapy for drug-resistant epilepsy in pediatric patients (257).
11 Study limitations and future prospects
The research findings to date on the effects of KD on the skin indicate potential benefits. However, they have several important limitations. The majority of the available studies include only short-term observations (a few weeks), making it impossible to assess the long-term effects of KD on the skin. Another crucial methodological shortcoming is the small size of the study groups (n < 50). The limited sample size significantly hinders the interpretation of the results. It may result in increased statistical errors due to low test power. A major limitation of many studies investigating the effect of KD on the skin is the lack of randomization, which significantly reduces their scientific value. The lack of standardization in the ketogenic protocols used in research on skin conditions is also a considerable impediment to the synthesis of results. The macronutrient ratios used in studies vary, which makes it difficult to compare their effects on skin processes. Despite promising reports on KD's effects on the skin, a key direction for future research should be to clarify the specific molecular mechanisms and signaling pathways in different layers and cells of the skin. At the same time, randomized clinical trials are needed to precisely determine the optimal timing of dietary intervention and standardize the dietary protocol for macronutrient ratios and calorie intake. They should also include an analysis of potential interactions with conventional treatments and an assessment of KD's long-term safety in skin conditions. A key practical limitation is the difficulty of maintaining long-term adherence to ketogenic interventions, which may reduce their feasibility and sustainability in dermatological practice. Moreover, nutritional deficiencies associated with prolonged KD use (e.g., vitamin C, biotin, and zinc) can impair skin integrity, highlighting the importance of careful monitoring. Importantly, future studies should investigate whether responses to different KD variants vary by sex or age, as these factors may influence both metabolic and dermatological outcomes. A further promising direction is the integration of nutrigenomic approaches to personalize ketogenic interventions, with the potential to optimize efficacy and minimize risks. Addressing these gaps will be essential for establishing evidence-based guidelines for the safe and practical application of ketogenic therapies in dermatology.
12 Summary
This review summarizes current knowledge on the effects of KD on the skin, particularly its potential therapeutic role in inflammatory skin diseases, such as acne vulgaris, psoriasis, hidradenitis suppurativa, and skin cancer. In addition, the impact of KD on the gut microbiota and its potential adverse effects, such as PP and nutrient deficiencies, were also presented. KD is characterized by a dietary pattern that predominates in fats, has a minimal amount of carbohydrates, and a moderate intake of protein. It has been successfully used to treat drug-resistant epilepsy in children for more than a century. KD is also finding applications in reducing obesity and treating insulin resistance, type 2 diabetes, neurodegenerative diseases, and cardiovascular disorders. There is evidence that this diet can also alleviate symptoms of inflammatory skin conditions through its anti-inflammatory and antioxidant effects, as well as modulation of the gut microbiota. The antioxidant potential of KD may be due to its impact on the Nrf2 pathway. Activation of the Nrf2 pathway leads to the induction of antioxidant and detoxifying enzymes, significantly reducing oxidative stress. KD can also reduce oxidative stress by increasing the NAD+/NADH ratio. It is speculated that KD's anti-inflammatory mechanism may be due to the reduction of blood insulin levels, inhibition of the NF–κB pathway, and NLRP3, which blocked the production of pro-inflammatory cytokines. Scientific evidence suggests that KD may alter the composition of the gut microbiota, reducing pro-inflammatory Proteobacteria and increasing the ratio of Bacteroidetes to Firmicutes, which could potentially be beneficial in inflammatory skin diseases. Recent reports indicate that KD may also be an adjuvant therapy for cutaneous melanoma. Despite the potential benefits of KD for skin health, randomized clinical trials are needed to evaluate its efficacy and long-term effects, as well as the exact mechanisms by which it affects the skin.
Statements
Author contributions
NC: Writing – original draft, Conceptualization, Data curation, Visualization. MM: Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was provided by the Medical University of Białystok, Poland (Grant No.: B.SUB.25.250).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Dal N Bilici S . Dietary modulations in preventing cardiometabolic risk in individuals with type 2 diabetes. Curr Nutr Rep. (2024) 13:412–21. 10.1007/s13668-024-00541-z
2.
Billingsley HE Heiston EM Bellissimo MP Lavie CJ Carbone S . Nutritional aspects to cardiovascular diseases and type 2 diabetes mellitus. Curr Cardiol Rep. (2024) 26:73–81. 10.1007/s11886-023-02018-x
3.
Jiménez-Cortegana C Iglesias P Ribalta J Vilariño-García T Montañez L Arrieta F et al . Nutrients and dietary approaches in patients with type 2 diabetes mellitus and cardiovascular disease: a narrative review. Nutrients. (2021) 13:4150. 10.3390/nu13114150
4.
Agrawal S Tomecki KJ . Diet and skin: a primer. Cutis. (2020) 106:278–9. 10.12788/cutis.0131
5.
Ahmed IA Mikail MA . Diet and skin health: the good and the bad. Nutrition. (2024) 119:112350. 10.1016/j.nut.2023.112350
6.
Parke MA Perez-Sanchez A Zamil DH Katta R . Diet and skin barrier: the role of dietary interventions on skin barrier function. Dermatol Pract Concept. (2021) 11:e2021132. 10.5826/dpc.1101a132
7.
Geng R Kang SG Huang K Tong T . Boosting the photoaged skin: the potential role of dietary components. Nutrients. (2021) 13:1691. 10.3390/nu13051691
8.
Gürtler A Laurenz S . The impact of clinical nutrition on inflammatory skin diseases. J Dtsch Dermatol Ges. (2022) 20:185–202. 10.1111/ddg.14683
9.
Melnik BC . Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. (2015) 8:371–88. 10.2147/CCID.S69135
10.
Coêlho LF Casaro MB Ribeiro WR Mendes E Murata G Xander P et al . A short-term high-sugar diet is an aggravating factor in experimental allergic contact dermatitis. Heliyon. (2023) 9:e21225. 10.1016/j.heliyon.2023.e21225
11.
Sardana K Sachdeva S . Role of nutritional supplements in selected dermatological disorders: a review. J Cosmet Dermatol. (2022) 21:85–98. 10.1111/jocd.14436
12.
Katta R Desai SP . Diet and dermatology: the role of dietary intervention in skin disease. J Clin Aesthet Dermatol. (2014) 7:46–51.
13.
Mansilla-Polo M Piquero-Casals J Morgado-Carrasco D . Popular diets and skin effects: a narrative review. Actas Dermosifiliogr. (2024) 115:T374–86. 10.1016/j.ad.2023.10.044
14.
Fomin DA McDaniel B Crane J . The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. (2017) 28:484–7. 10.1080/09546634.2016.1276259
15.
Lin M Gong J Wu L Lin X Zhang Y Lin W et al . ADCY3: the pivotal gene in classical ketogenic diet for the treatment of epilepsy. Front Cell Neurosci. (2024) 18:1305867. 10.3389/fncel.2024.1305867
16.
McGaugh E Barthel B . A review of ketogenic diet and lifestyle. Mo Med. (2022) 119:84–8.
17.
Baylie T Ayelgn T Tiruneh M Tesfa KH . Effect of ketogenic diet on obesity and other metabolic disorders: narrative review. Diabetes Metab Syndr Obes. (2024) 17:1391–401. 10.2147/DMSO.S447659
18.
Roster K Xie L Nguyen T Lipner SR . Impact of ketogenic and low-glycemic diets on inflammatory skin conditions. Cutis. (2024) 113:75–80. 10.12788/cutis.0942
19.
Mcdaniel B Do BM Fomin DA Crane J . Ketones, the ketogenic diet, and the skin: a review of where we are and where we should go. J Skin. (2018) 2:18–9.
20.
Harb D Abdullah L Kurban M Abbas O . Ketogenic diet and the skin. Skinmed. (2023) 21:315–20.
21.
Barrea L Cacciapuoti S Megna M Verde L Marasca C Vono R et al . The effect of the ketogenic diet on acne: could it be a therapeutic tool?Crit Rev Food Sci Nutr. (2024) 64:6850–69. 10.1080/10408398.2023.2176813
22.
Wheless JW . History of the ketogenic diet. Epilepsia. (2008) 49(Suppl 8):3–5. 10.1111/j.1528-1167.2008.01821.x
23.
McCandless DW . Epilepsy: Animal and Human Correlations. New York, NY: Springer (2014). p. 1–519.
24.
Sharma S Jain P . The ketogenic diet and other dietary treatments for refractory epilepsy in children. Ann Indian Acad Neurol. (2014) 17:253–8. 10.4103/0972-2327.138471
25.
Wheless JW . History and origin of the ketogenic diet. In: StafstromCERhoJM, editors. Epilepsy and the Ketogenic Diet.Totowa, NJ: Humana Press (2004). p. 31–50. 10.1007/978-1-59259-808-3_2
26.
Lane J Fontaine KR . Ketogenic diet and health. OBM Integr Complement Med. (2020) 6:1. 10.21926/obm.icm.2102015
27.
Arora SK McFarlane SI . Review on “Atkins diabetes revolution: the groundbreaking approach to preventing and controlling type 2 diabetes” by Vernon MC and Eberstein JA. Nutr Metab (Lond). (2004) 1:14. 10.1186/1743-7075-1-14
28.
Ivannikova E V Altashina M V Troshina EA . The ketogenic diet: history, mechanism of action, indications and contraindications. Probl Endokrinol (Mosk). (2021) 68:49–72. 10.14341/probl12724
29.
Dashti HM Mathew TC Al-Zaid NS . Efficacy of low-carbohydrate ketogenic diet in the treatment of type 2 diabetes. Med Princ Pract. (2021) 30:223–35. 10.1159/000512142
30.
Höhn S Dozières-Puyravel B Auvin S . History of dietary treatment: Guelpa & Marie first report of intermittent fasting for epilepsy in 1911. Epilepsy Behav. (2019) 94:277–80. 10.1016/j.yebeh.2019.03.018
31.
Zarnowska IM . Therapeutic use of the ketogenic diet in refractory epilepsy: what we know and what still needs to be learned. Nutrients. (2020) 12:1–23. 10.3390/nu12092616
32.
Pietrzak D Kasperek K Rekawek P Piatkowska-Chmiel I . The therapeutic role of ketogenic diet in neurological disorders. Nutrients. (2022) 14:1952. 10.3390/nu14091952
33.
Kim JM . Ketogenic diet: old treatment, new beginning. Clin Neurophysiol Pract. (2017) 2:161–2. 10.1016/j.cnp.2017.07.001
34.
Kossoff E Cervenka M . Ketogenic dietary therapy controversies for its second century. Epilepsy Curr. (2020) 20:125–9. 10.1177/1535759719890337
35.
Yakupova EI Bocharnikov AD Plotnikov EY . Effects of ketogenic diet on muscle metabolism in health and disease. Nutrients. (2022) 14:3842. 10.3390/nu14183842
36.
Gano LB Patel M Rho JM . Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. (2014) 55:2211–28. 10.1194/jlr.R048975
37.
Walczyk T Wick JY . The ketogenic diet: making a comeback. Consult Pharm. (2017) 32:388–96. 10.4140/TCP.n.2017.388
38.
Barañano KW Hartman AL . The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol. (2008) 10:410–9. 10.1007/s11940-008-0043-8
39.
Talib WH Mahmod AI Kamal A Rashid HM Alashqar AMD Khater S et al . Ketogenic diet in cancer prevention and therapy: molecular targets and therapeutic opportunities. Curr Issues Mol Biol. (2021) 43:558–89. 10.3390/cimb43020042
40.
Taylor MK Swerdlow RH Sullivan DK . Dietary neuroketotherapeutics for Alzheimer's disease: an evidence update and the potential role for diet quality. Nutrients. (2019) 11:1910. 10.3390/nu11081910
41.
World Health Organization (WHO) . WHO Updates Guidelines on Fats and Carbohydrates. (2023). Available online at: https://www.who.int/news/item/17-07-2023-who-updates-guidelines-on-fats-and-carbohydrates (Accessed April 19, 2025).
42.
Davis JJ Fournakis N Ellison J . Ketogenic diet for the treatment and prevention of dementia: a review. J Geriatr Psychiatry Neurol. (2021) 34:3–10. 10.1177/0891988720901785
43.
Gołabek KD Regulska-Ilow B . Possible nonneurological health benefits of ketogenic diet: review of scientific reports over the past decade. J Obes. (2022) 2022:7531518. 10.1155/2022/7531518
44.
Harvey KL Holcomb LE Kolwicz SC . Ketogenic diets and exercise performance. Nutrients. (2019) 11:2296. 10.3390/nu11102296
45.
Williams MS Turos E . The chemistry of the ketogenic diet: updates and opportunities in organic synthesis. Int J Mol Sci. (2021) 22:5230. 10.3390/ijms22105230
46.
Cannataro R Petro JL Abrego-Guandique DM Cione E Caroleo MC Kreider RB et al . Ketogenic diet plus resistance training applied to physio-pathological conditions: a brief review. Appl Sci. (2024) 14:5445. 10.3390/app14135445
47.
Shilpa J Mohan V . Ketogenic diets: boon or bane?Indian J Med Res. (2018) 148:251–3. 10.4103/ijmr.IJMR_1666_18
48.
Ashtary-Larky D Bagheri R Bavi H Baker JS Moro T Mancin L et al . Ketogenic diets, physical activity and body composition: a review. Br J Nutr. (2022) 127:1898–920. 10.1017/S0007114521002609
49.
Kysel P Haluzíková D DoleŽalová RP Lanková I Lacinová Z Kasperová BJ et al . The influence of cyclical ketogenic reduction diet vs. nutritionally balanced reduction diet on body composition, strength, and endurance performance in healthy young males: a randomized controlled trial. Nutrients. (2020) 12:1–12. 10.3390/nu12092832
50.
Szendi K Murányi E Hunter N Németh B . Methodological challenges and confounders in research on the effects of ketogenic diets: a literature review of meta-analyses. Foods. (2024) 13:248. 10.3390/foods13020248
51.
Malinowska D Żendzian-Piotrowska M . Ketogenic diet: a review of composition diversity, mechanism of action and clinical application. J Nutr Metab. (2024) 2024:6666171. 10.1155/2024/6666171
52.
Kossoff EH Zupec-Kania BA Auvin S Ballaban-Gil KR Bergqvist AGC Blackford R et al . Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. (2018) 3:175–92. 10.1002/epi4.12225
53.
Pfeifer HH Lyczkowski DA Thiele EA . Low glycemic index treatment: implementation and new insights into efficacy. Epilepsia. (2008) 49(Suppl 8):42–5. 10.1111/j.1528-1167.2008.01832.x
54.
Barrea L Caprio M Grassi D Cicero AFG Bagnato C Paolini B et al . New Nomenclature for the Very Low-Calorie Ketogenic Diet (VLCKD): very low-energy ketogenic therapy (VLEKT). Ketodiets and Nutraceuticals Expert Panels: “KetoNut”, Italian Society of Nutraceuticals (SINut) and the Italian Association of Dietetics and Clinical Nutrition (ADI). Curr Nutr Rep. (2024) 13:552–6. 10.1007/s13668-024-00560-w
55.
Kossoff EH Dorward JL . The modified Atkins diet. Epilepsia. (2008) 49(Suppl 8):37–41. 10.1111/j.1528-1167.2008.01831.x
56.
Zhu H Bi D Zhang Y Kong C Du J Wu X et al . Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. (2022) 7:1–21. 10.1038/s41392-021-00831-w
57.
Li H Wang Y Guo J Zhang P Xu Z Peng K et al . Efficacy and safety of modified medium-chain triglyceride ketogenic diet in patients with drug-resistant epilepsy. Acta Epileptol. (2024) 6:1–9. 10.1186/s42494-024-00150-x
58.
Wells J Swaminathan A Paseka J Hanson C . Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy—a review. Nutrients. (2020) 12:1809. 10.3390/nu12061809
59.
Watanabe S Tsujino S . Applications of medium-chain triglycerides in foods. Front Nutr. (2022) 9:802805. 10.3389/fnut.2022.802805
60.
Duranova H Kuzelova L Fialkova V Simora V Kovacikova E Joanidis P et al . Coconut-sourced MCT oil: its potential health benefits beyond traditional coconut oil. Phytochem Rev. (2024) 24:659–700. 10.1007/s11101-024-09969-1
61.
Verrotti A Iapadre G Di Francesco L Zagaroli L Farello G . Diet in the treatment of epilepsy: what we know so far. Nutrients. (2020) 12:2645. 10.3390/nu12092645
62.
Cervenka MC Wood S Bagary M Balabanov A Bercovici E Brown MG et al . International recommendations for the management of adults treated with ketogenic diet therapies. Neurol Clin Pract. (2021) 11:385–97. 10.1212/CPJ.0000000000001007
63.
Watanabe M Tuccinardi D Ernesti I Basciani S Mariani S Genco A et al . Scientific evidence underlying contraindications to the ketogenic diet: an update. Obes Rev. (2020) 21:e13053. 10.1111/obr.13053
64.
Ahmad SA Al Thobiti TA El Toum M Al Harbi F . Florid scurvy in an autistic child on a ketogenic diet. Pediatr Emerg Care. (2021) 37:E483–4. 10.1097/PEC.0000000000001695
65.
De Giorgis V Tagliabue A Bisulli F Brambilla I Camerini A Cusmai R et al . Ketogenic dietary therapies in epilepsy: recommendations of the Italian League against Epilepsy Dietary Therapy Study Group. Front Neurol. (2023) 14:1215618. 10.3389/fneur.2023.1215618
66.
Schoeler NE Simpson Z Whiteley VJ Nguyen P Meskell R Lightfoot K et al . Biochemical assessment of patients following ketogenic diets for epilepsy: current practice in the UK and Ireland. Epilepsia Open. (2019) 5:73–9. 10.1002/epi4.12371
67.
de Brito Sampaio LP Henriques-Souza AMM Lin K Neri LCL Inuzuka LM Uchôa LIL et al . Ketogenic therapy in childhood and adolescence: recommendations of the Brazilian experts group. Arq Neuropsiquiatr. (2023) 81:597–606. 10.1055/s-0043-1768676
68.
Kossoff EH Zupec-Kania BA Amark PE Ballaban-Gil KR Bergqvist AGC Blackford R et al . Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. (2009) 50:304–17. 10.1111/j.1528-1167.2008.01765.x
69.
Raju KNV Gulati S Kabra M Agarwala A Sharma S Pandey RM . Kalra V. Efficacy of 4:1 (classic) versus 25:1 ketogenic ratio diets in refractory epilepsy in young children: a randomized open labeled study. Epilepsy Res. (2011) 96:96–100. 10.1016/j.eplepsyres.2011.05.005
70.
Pizzo F Collotta AD Di Nora A Costanza G Ruggieri M Falsaperla R . Ketogenic diet in pediatric seizures: a randomized controlled trial review and meta-analysis. Expert Rev Neurother. (2022) 22:169–77. 10.1080/14737175.2022.2030220
71.
Dyńka D Rodzeń Ł Rodzeń M Pacholak-Klimas A Ede G Sethi S et al . Ketogenic diets for body weight loss: a comparison with other diets. Nutrients. (2025) 17:965. 10.3390/nu17060965
72.
Patikorn C Saidoung P Pham T Phisalprapa P Lee YY Varady KA et al . Effects of ketogenic diet on health outcomes: an umbrella review of meta-analyses of randomized clinical trials. BMC Med. (2023) 21:196. 10.1186/s12916-023-02874-y
73.
Gibson AA Seimon RV Lee CMY Ayre J Franklin J Markovic TP et al . Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes Rev. (2015) 16:64–76. 10.1111/obr.12230
74.
Paoli A Bianco A Moro T Mota JF Coelho-Ravagnani CF . The effects of ketogenic diet on insulin sensitivity and weight loss, which came first: the chicken or the egg?Nutrients. (2023) 15:3120. 10.3390/nu15143120
75.
Yuan X Wang J Yang S Gao M Cao L Li X et al . Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. (2020) 10:38. 10.1038/s41387-020-00142-z
76.
Dyńka D Kowalcze K Charuta A Paziewska A . The ketogenic diet and cardiovascular diseases. Nutrients. (2023) 15:3368. 10.3390/nu15153368
77.
Li S Lin G Chen J Chen Z Xu F Zhu F et al . The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr Disord. (2022) 22:34. 10.1186/s12902-022-00947-2
78.
Price S Ruppar TM . Ketogenic therapies in Parkinson's disease, Alzheimer's disease, and mild cognitive impairment: an integrative review. Appl Nurs Res. (2023) 74:151745. 10.1016/j.apnr.2023.151745
79.
Li Q Liang J Fu N Han Y Qin J . A ketogenic diet and the treatment of autism spectrum disorder. Front Pediatr. (2021) 9:650624. 10.3389/fped.2021.650624
80.
Weber DD Aminzadeh-Gohari S Tulipan J Catalano L Feichtinger RG Kofler B . Ketogenic diet in the treatment of cancer – where do we stand?Mol Metab. (2019) 33:102–21. 10.1016/j.molmet.2019.06.026
81.
Egashira R Matsunaga M Miyake A Hotta S Nagai N Yamaguchi C et al . Long-term effects of a ketogenic diet for cancer. Nutrients. (2023) 15:2334. 10.3390/nu15102334
82.
Neha Chaudhary R . Ketogenic diet as a treatment and prevention strategy for cancer: a therapeutic alternative. Nutrition. (2024) 124:112427. 10.1016/j.nut.2024.112427
83.
Klepper J Diefenbach S Kohlschütter A Voit T . Effects of the ketogenic diet in the glucose transporter 1 deficiency syndrome. Prostaglandins Leukot Essent Fatty Acids. (2004) 70:321–7. 10.1016/j.plefa.2003.07.004
84.
Barrea L Verde L Camajani E Cernea S Frias-Toral E Lamabadusuriya D et al . Ketogenic diet as medical prescription in women with polycystic ovary syndrome (PCOS). Curr Nutr Rep. (2023) 12:56–64. 10.1007/s13668-023-00456-1
85.
Ildarabadi A Ali SNMM Rahmani F Mosavari N Pourbakhtyaran E Rezaei N . Inflammation and oxidative stress in epileptic children: from molecular mechanisms to clinical application of ketogenic diet. Rev Neurosci. (2024) 35:473–88. 10.1515/revneuro-2023-0128
86.
Bo S Fadda M Fedele D Pellegrini M Ghigo E Pellegrini N et al . critical review on the role of food and nutrition in the energy balance. Nutrients. (2020) 12:1161. 10.3390/nu12041161
87.
Slavin J Carlson J . Carbohydrates. Adv Nutr. (2014) 5:760–1. 10.3945/an.114.006163
88.
Clemente-Suárez VJ Mielgo-Ayuso J Martín-Rodríguez A Ramos-Campo DJ Redondo-Flórez L Tornero-Aguilera JF . The burden of carbohydrates in health and disease. Nutrients. (2022) 14:3809. 10.3390/nu14183809
89.
Sosicka P Ng BG Freeze HH . Therapeutic monosaccharides: looking back, moving forward. Biochemistry. (2020) 59:3064–77. 10.1021/acs.biochem.9b00565
90.
Wilcox G . Insulin and insulin resistance. Clin Biochem Rev. (2005) 26:19–39.
91.
Komatsu M Takei M Ishii H Sato Y . Glucose-stimulated insulin secretion: a newer perspective. J Diabetes Investig. (2013) 4:511–6. 10.1111/jdi.12094
92.
Rahman MS Hossain KS Das S Kundu S Adegoke EO Rahman MA et al . Role of insulin in health and disease: an update. Int J Mol Sci. (2021) 22:6403. 10.3390/ijms22126403
93.
Gershuni VM Yan SL Medici V . Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep. (2018) 7:97–106. 10.1007/s13668-018-0235-0
94.
Röder PV Wu B Liu Y Han W . Pancreatic regulation of glucose homeostasis. Exp Mol Med. (2016) 48:e219. 10.1038/emm.2016.6
95.
Colberg SR . Why glucagon matters for hypoglycemia and physical activity in individuals with type 1 diabetes. Front Clin Diabetes Healthc. (2022) 3:889248. 10.3389/fcdhc.2022.889248
96.
Miyamoto T Amrein H . Gluconeogenesis: an ancient biochemical pathway with a new twist. Fly (Austin). (2017) 11:218–23. 10.1080/19336934.2017.1283081
97.
Blagosklonny MV . The mystery of the ketogenic diet: benevolent pseudo-diabetes. Cell Cycle. (2019) 18:2157–63. 10.1080/15384101.2019.1644765
98.
Dilliraj LN Schiuma G Lara D Strazzabosco G Clement J Giovannini PP et al . The evolution of ketosis: potential impact on clinical conditions. Nutrients. (2022) 14:3613. 10.3390/nu14173613
99.
Blanco JC Khatri A Kifayat A Cho R Aronow WS . Starvation ketoacidosis due to the ketogenic diet and prolonged fasting - a possibly dangerous diet trend. Am J Case Rep. (2019) 20:1728–31. 10.12659/AJCR.917226
100.
Kanikarla-Marie P Jain SK . Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med. (2016) 95:268–77. 10.1016/j.freeradbiomed.2016.03.020
101.
Chiasson JL Aris-Jilwan N Bélanger R Bertrand S Beauregard H Ékoé JM et al . Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. (2003) 168:859–66.
102.
Varadarajan P . Risk factors for mortality in children with diabetic keto acidosis from developing countries. World J Diabetes. (2014) 5:932–8. 10.4239/wjd.v5.i6.932
103.
Noor NM Basavaraju K Sharpstone D . Alcoholic ketoacidosis: a case report and review of the literature. Oxf Med Case Rep. (2016) 2016:31–3. 10.1093/omcr/omw006
104.
Newman JC Verdin E . Ketone bodies as signaling metabolites. Trends Endocrinol Metab. (2014) 25:42–52. 10.1016/j.tem.2013.09.002
105.
Cotter DG Schugar RC Crawford PA . Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. (2013) 304:1060–76. 10.1152/ajpheart.00646.2012
106.
Papaccio F . D'arino A, Caputo S, Bellei B. Focus on the contribution of oxidative stress in skin aging. Antioxidants (Basel). (2022) 11:1121. 10.3390/antiox11061121
107.
Dorf N Maciejczyk M . Skin senescence—from basic research to clinical practice. Front Med (Lausanne). (2024) 11:1484345. 10.3389/fmed.2024.1484345
108.
Pizzino G Irrera N Cucinotta M Pallio G Mannino F Arcoraci V et al . Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. (2017) 2017:8416763. 10.1155/2017/8416763
109.
Turcov D Zbranca-Toporas A Suteu D . Bioactive compounds for combating oxidative stress in dermatology. Int J Mol Sci. (2023) 24:17517. 10.3390/ijms242417517
110.
Korczowska-Łacka I Słowikowski B Piekut T Hurła M Banaszek N Szymanowicz O et al . Disorders of endogenous and exogenous antioxidants in neurological diseases. Antioxidants. (2023) 12:1811. 10.3390/antiox12101811
111.
Serra V Grune T Sitte N Saretzki G Von Zglinicki T . Telomere length as a marker of oxidative stress in primary human fibroblast cultures. Ann N Y Acad Sci. (2000) 908:327–30. 10.1111/j.1749-6632.2000.tb06666.x
112.
Benoit I Burty-Valin E Radman M . A proteome-centric view of ageing, including that of the skin and age-related diseases: considerations of a common cause and common preventative and curative interventions. Clin Cosmet Investig Dermatol. (2023) 16:79–85. 10.2147/CCID.S397751
113.
Khan AQ Agha MV Sheikhan KSAM Younis SM Tamimi M Al Alam M et al . Targeting deregulated oxidative stress in skin inflammatory diseases: an update on clinical importance. Biomed Pharmacother. (2022) 154:113601. 10.1016/j.biopha.2022.113601
114.
Tu Y Quan T . Oxidative stress and human skin connective tissue aging. Cosmetics. (2016) 3:28. 10.3390/cosmetics3030028
115.
Chylińska N Maciejczyk M . Hyaluronic acid and skin: its role in aging and wound-healing processes. Gels. (2025) 11:281. 10.3390/gels11040281
116.
Chen J Liu Y Zhao Z Qiu J . Oxidative stress in the skin: impact and related protection. Int J Cosmet Sci. (2021) 43:495–509. 10.1111/ics.12728
117.
Ogawa F Sato S . Roles of oxidative stress in photoaging and the pathogenesis of systemic sclerosis. Nihon Rinsho Meneki Gakkai Kaishi. (2006) 29:349–58. 10.2177/jsci.29.349
118.
Hussein RS Bin Dayel S Abahussein O El-Sherbiny AA . Influences on skin and intrinsic aging: biological, environmental, and therapeutic insights. J Cosmet Dermatol. (2024) 24:e16688. 10.1111/jocd.16688
119.
Starr JM Starr RJ . Skin aging and oxidative stress. In: PreedyVR, editor. Aging: Oxidative Stress and Dietary Antioxidants.Amsterdam: Academic Press (2014). p. 15–22. 10.1016/B978-0-12-405933-7.00002-0
120.
Nopriyati Novriani R Thaha A Diba S Yahya YF Aryani IA . The role of oxidative stress in atopic dermatitis. Biosci Med Res. (2022) 6:2347–52. 10.37275/bsm.v6i11.603
121.
Haque MA Khaliduzzaman A Asaduzzaman M Pattadar SN Hasan M . Dietary food antioxidants and their radical scavenging activity: a review. Int Food Res J. (2023) 30:63–78. 10.47836/30.1.04
122.
Milder J Patel M . Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. (2012) 100:295–303. 10.1016/j.eplepsyres.2011.09.021
123.
Oh YS Jun HS . Effects of glucagon-like peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci. (2018) 19:26. 10.3390/ijms19010026
124.
Milder JB Liang LP Patel M . Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. (2010) 40:238–44. 10.1016/j.nbd.2010.05.030
125.
Lu Y Yang YY Zhou MW Liu N Xing HY Liu XX Li F . Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. (2018) 683:13–8. 10.1016/j.neulet.2018.06.016
126.
Surh YJ Kundu JK Na HK . Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. (2008) 74:1526–39. 10.1055/s-0028-1088302
127.
Yuan X Liu Y Bijonowski BM Tsai AC Fu Q Logan TM et al . NAD+/NADH redox alterations reconfigure metabolism and rejuvenate senescent human mesenchymal stem cells in vitro. Commun Biol. (2020) 3:774. 10.1038/s42003-020-01514-y
128.
Elamin M Ruskin DN Sacchetti P Masino SA . A unifying mechanism of ketogenic diet action: the multiple roles of nicotinamide adenine dinucleotide. Epilepsy Res. (2020) 167:106469. 10.1016/j.eplepsyres.2020.106469
129.
Ruszkiewicz JA Bürkle A Mangerich A . Fueling genome maintenance: on the versatile roles of NAD+ in preserving DNA integrity. J Biol Chem. (2022) 298:102037. 10.1016/j.jbc.2022.102037
130.
Kauppinen A Suuronen T Ojala J Kaarniranta K Salminen A . Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. (2013) 25:1939–48. 10.1016/j.cellsig.2013.06.007
131.
Ogura Y Kitada M Koya D . Sirtuins and renal oxidative stress. Antioxidants. (2021) 10:1198. 10.3390/antiox10081198
132.
Yu L Li Y Song S Zhang Y Wang Y Wang H et al . The dual role of sirtuins in cancer: biological functions and implications. Front Oncol. (2024) 14:1384928. 10.3389/fonc.2024.1384928
133.
Ji Z Liu GH Qu J . Mitochondrial sirtuins, metabolism, and aging. J Genet Genom. (2022) 49:287–98. 10.1016/j.jgg.2021.11.005
134.
Alqrad MAI El-Agamy DS Ibrahim SRM Sirwi A Abdallah HM Abdel-Sattar E et al . SIRT1/Nrf2/NF-κB signaling mediates anti-inflammatory and anti-apoptotic activities of oleanolic acid in a mouse model of acute hepatorenal damage. Medicina (Lithuania). (2023) 59:1351. 10.3390/medicina59071351
135.
Lee SH Kim HJ Oh GS Lee SB Khadka D Cao W et al . Augmentation of NAD+ by dunnione ameliorates imiquimod-induced psoriasis-like dermatitis in mice. J Inflamm Res. (2022) 15:4623–36. 10.2147/JIR.S372543
136.
Poljšak B Kovač V Špalj S Milisav I . The central role of the NAD+ molecule in the development of aging and the prevention of chronic age-related diseases: strategies for NAD+ modulation. Int J Mol Sci. (2023) 24:2959. 10.3390/ijms24032959
137.
Lautrup S Sinclair DA Mattson MP Fang EF . NAD+ in brain aging and neurodegenerative disorders. Cell Metab. (2019) 30:630–55. 10.1016/j.cmet.2019.09.001
138.
Amjad S Nisar S Bhat AA Shah AR Frenneaux MP Fakhro K et al . Role of NAD+ in regulating cellular and metabolic signaling pathways. Mol Metab. (2021) 49:101195. 10.1016/j.molmet.2021.101195
139.
She J Sheng R Qin ZH . Pharmacology and potential implications of nicotinamide adenine dinucleotide precursors. Aging Dis. (2021) 12:1879–97. 10.14336/AD.2021.0523
140.
Pinto A Bonucci A Maggi E Corsi M Businaro R . Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants. (2018) 7:63. 10.3390/antiox7050063
141.
Elamin M Ruskin DN Masino SA Sacchetti P . Ketone-based metabolic therapy: is increased NAD+ a primary mechanism?Front Mol Neurosci. (2017) 10:377. 10.3389/fnmol.2017.00377
142.
Huang YZ McNamara JO . Inhibiting glycolysis to reduce seizures: how it might work. Nat Neurosci. (2006) 9:1351–2. 10.1038/nn1106-1351
143.
Drabińska N . Current perspective about the effect of a ketogenic diet on oxidative stress – a review. Pol J Food Nutr Sci. (2024) 74:92–105. 10.31883/pjfns/185366
144.
Hsu FY Liou JY Tang FY Sou NL Peng JH Chiang EPI . Ketogenic diet consumption inhibited mitochondrial one-carbon metabolism. Int J Mol. (2022) 23:3650. 10.3390/ijms23073650
145.
Vetrani C Di Nisio A Paschou SA Barrea L Muscogiuri G Graziadio C et al . From gut microbiota through low-grade inflammation to obesity: key players and potential targets. Nutrients. (2022) 14:2103. 10.3390/nu14102103
146.
Magne F Gotteland M Gauthier L Zazueta A Pesoa S Navarrete P et al . The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients?Nutrients. (2020) 12:1474. 10.3390/nu12051474
147.
Acevedo-Román A Pagán-Zayas N Velázquez-Rivera LI Torres-Ventura AC Godoy-Vitorino F . Insights into gut dysbiosis: inflammatory diseases, obesity, and restoration approaches. Int J Mol Sci. (2024) 25:9715. 10.3390/ijms25179715
148.
Salvadori M Rosso G . Update on the gut microbiome in health and diseases. World J Methodol. (2024) 14:89196. 10.5662/wjm.v14.i1.89196
149.
Belkaid Y Hand TW . Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41. 10.1016/j.cell.2014.03.011
150.
Rowland I Gibson G Heinken A Scott K Swann J Thiele I et al . Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. (2017) 57:1–24. 10.1007/s00394-017-1445-8
151.
Glover JS Ticer TD Engevik MA . Characterizing the mucin-degrading capacity of the human gut microbiota. Sci Rep. (2022) 12:8456. 10.1038/s41598-022-11819-z
152.
Fusco W Lorenzo MB Cintoni M Porcari S Rinninella E Kaitsas F et al . Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. (2023) 15:2211. 10.3390/nu15092211
153.
Hadadi N Berweiler V Wang H Trajkovski M . Intestinal microbiota as a route for micronutrient bioavailability. Curr Opin Endocr Metab Res. (2021) 20:100285. 10.1016/j.coemr.2021.100285
154.
Dedikova OV Zakharova IN Kuchina AE Berezhnaya IV Sugian NG . Formation of the infant's intestinal microbiota depending on the delivery method: long-term consequences and correction options: a review. Pediatr Consil Med. (2023) 19:25–9. 10.26442/26586630.2023.1.202092
155.
Coelho GDP Ayres LFA Barreto DS Henriques BD Prado MRMC Passos CMD . Acquisition of microbiota according to the type of birth: an integrative review. Rev Lat Am Enfermagem. (2021) 29:e3446. 10.1590/1518.8345.4466.3446
156.
Pantazi AC Balasa AL Mihai CM Chisnoiu T Lupu VV Kassim MAK et al . Development of gut microbiota in the first 1000 days after birth and potential interventions. Nutrients. (2023) 15:3647. 10.3390/nu15163647
157.
Martinez JE Kahana DD Ghuman S Wilson HP Wilson J Kim SCJ et al . Unhealthy lifestyle and gut dysbiosis: a better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front Endocrinol (Lausanne). (2021) 12:667066. 10.3389/fendo.2021.667066
158.
Hamamah S Amin A Al-Kassir AL Chuang J Covasa M . Dietary fat modulation of gut microbiota and impact on regulatory pathways controlling food intake. Nutrients. (2023) 15:3365. 10.3390/nu15153365
159.
Beam A Clinger E Hao L . Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. (2021) 13:2795. 10.3390/nu13082795
160.
Li Y Huang Y Liang H Wang W Li B Liu T et al . The roles and applications of short-chain fatty acids derived from microbial fermentation of dietary fibers in human cancer. Front Nutr. (2023) 10:1243390. 10.3389/fnut.2023.1243390
161.
Arifuzzaman M Collins N Guo CJ Artis D . Nutritional regulation of microbiota-derived metabolites: implications for immunity and inflammation. Immunity. (2024) 57:14–27. 10.1016/j.immuni.2023.12.009
162.
Widhiati S Purnomosari D Wibawa T Soebono H . The role of gut microbiome in inflammatory skin disorders: a systematic review. Dermatol Reports. (2021) 14:9188. 10.4081/dr.2022.9188
163.
Deng Y Wang H Zhou J Mou Y Wang G Xiong X . Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm Venereol. (2018) 98:783–90. 10.2340/00015555-2968
164.
Thompson KG Rainer BM Antonescu C Florea L Mongodin EF Kang S et al . Minocycline and its impact on microbial dysbiosis in the skin and gastrointestinal tract of acne patients. Ann Dermatol. (2020) 32:21–30. 10.5021/ad.2020.32.1.21
165.
Lim JM Letchumanan V Tan LTH Hong KW Wong SH Ab Mutalib NS et al . Ketogenic diet: a dietary intervention via gut microbiome modulation for the treatment of neurological and nutritional disorders (a narrative review). Nutrients. (2022) 14:3566. 10.3390/nu14173566
166.
Newell C Bomhof MR Reimer RA Hittel DS Rho JM Shearer J . Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. (2016) 7:37. 10.1186/s13229-016-0099-3
167.
Vata D Tarcau BM Popescu IA Halip IA Patrascu AI Gheuca Solovastru DF et al . Update on obesity in psoriasis patients. Life. (2023) 13:1947. 10.3390/life13101947
168.
Storer MA Danesh MJ Sandhu ME Pascoe V Kimball AB . An assessment of the relative impact of hidradenitis suppurativa, psoriasis, and obesity on quality of life. Int J Womens Dermatol. (2018) 4:198–202. 10.1016/j.ijwd.2018.08.009
169.
Xiao X Hu X Yao J Cao W Zou Z Wang L et al . The role of short-chain fatty acids in inflammatory skin diseases. Front Microbiol. (2023) 13:1083432. 10.3389/fmicb.2022.1083432
170.
Rohwer N El Hage R Smyl C Ocvirk S Goris T Grune T et al . Ketogenic diet has moderate effects on the fecal microbiota of wild-type mice. Nutrients. (2023) 15:4629. 10.3390/nu15214629
171.
Tan JKL Bhate K . A global perspective on the epidemiology of acne. Br J Dermatol. (2015) 172:3–12. 10.1111/bjd.13462
172.
Vasam M Korutla S Bohara RA . Acne vulgaris: a review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem Biophys Rep. (2023) 36:101578. 10.1016/j.bbrep.2023.101578
173.
Nandy P Shrivastava T . Exploring the multifaceted impact of acne on quality of life and well-being. Cureus. (2024) 16:e52727. 10.7759/cureus.52727
174.
Akdag N Atli E Zhuri D Güler HS Ürün YG . A study of FoxO1, mTOR, miR-21, miR-29b, and miR-98 expression levels regarding metabolic syndrome in acne vulgaris patients. Cureus. (2024) 16:e56562. 10.7759/cureus.56562
175.
Cao Q Guo J Chang S Huang Z Luo Q . Gut microbiota and acne: a Mendelian randomization study. Skin Res Technol. (2023) 29:e13473. 10.1111/srt.13473
176.
Verde L Frias-Toral E Cacciapuoti S Simancas-Racines D Megna M Caiazzo G et al . Very low-calorie ketogenic diet (VLCKD): a therapeutic nutritional tool for acne?J Transl Med. (2024) 22:322. 10.1186/s12967-024-05119-5
177.
Kardeh S Moein SA Namazi MR Kardeh B . Evidence for the important role of oxidative stress in the pathogenesis of acne. Galen Med J. (2019) 8:e1291. 10.31661/gmj.v8i0.1291
178.
Lvov AN Sidorenko EE . Evaluation of PPARγ expression in skin of acne patients. Med alphabet. (2022) 1:21–3. 10.33667/2078-5631-2022-27-21-23
179.
Wang D Duncan B Li X Shi J . The role of NLRP3 inflammasome in infection-related, immune-mediated and autoimmune skin diseases. J Dermatol Sci. (2020) 98:146–51. 10.1016/j.jdermsci.2020.03.001
180.
Zhu W Wang H-L Bu X-L Zhang J-B Lu Y-G . A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. (2022) 10:645. 10.21037/atm-21-5924
181.
Cacciapuoti S Annunziata MC Megna M Villani A Martora F Fabbrocini G et al . Dietary intervention in acne management: review of the literature and future prospective. J Egypt Women's Dermatol Soc. (2024) 21:83–91. 10.4103/jewd.jewd_46_23
182.
Fomin DA Handfield K . The ketogenic diet and dermatology: a primer on current literature. Cutis. (2020) 105:40–3.
183.
Le TN Nestler JE Strauss JF Wickham EP . Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab. (2012) 23:32–40. 10.1016/j.tem.2011.09.005
184.
Sharma A Welt CK . Practical approach to hyperandrogenism in women. Med Clin North Am. (2021) 105:1099–116. 10.1016/j.mcna.2021.06.008
185.
Lee WJ Jung HD Chi SG Kim BS Lee SJ Kim DW et al . Effect of dihydrotestosterone on the upregulation of inflammatory cytokines in cultured sebocytes. Arch Dermatol Res. (2010) 302:429–33. 10.1007/s00403-009-1019-6
186.
Ebede TL Arch EL Berson D . Hormonal treatment of acne in women. J Clin Aesthet Dermatol. (2009) 2:16–22.
187.
Newman JC Verdin E . β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. (2017) 37:51–76. 10.1146/annurev-nutr-071816-064916
188.
Kolb H Kempf K Röhling M Lenzen-Schulte M Schloot NC Martin S . Ketone bodies: from enemy to friend and guardian angel. BMC Med. (2021) 19:313. 10.1186/s12916-021-02185-0
189.
Saline M Badertscher L Wolter M Lau R Gunnarsson A Jacso T et al . and AKT protein kinases hierarchically phosphorylate the N-terminus of the FOXO1 transcription factor, modulating interactions with 14-3-3 proteins. J Biol Chem. (2019) 294:13106. 10.1074/jbc.RA119.008649
190.
Barrea L Verde L Annunziata G Antiga E Camajani E Caprio M et al . Medical nutrition therapy in dermatological diseases: a joint consensus statement of the Italian Association of Dietetics and Clinical Nutrition (ADI), the Italian Society of Dermatology and Sexually Transmitted Diseases (SIDeMaST), the Italian Society of Nutraceuticals (SINut), Club Ketodiets and Nutraceuticals “KetoNut-SINut” and the Italian Society of Endocrinology (SIE), Club Nutrition, Hormones and Metabolism. Curr Obes Rep. (2025) 14:42. 10.1007/s13679-025-00630-2
191.
Luque-Luna M Sidro-Sarto M . RF-the role of diet in the management of acne. Actas Dermosifiliogr. (2024) 115:T734–6. 10.1016/j.ad.2023.06.026
192.
Baldwin H Tan J . Effects of diet on acne and its response to treatment. Am J Clin Dermatol. (2020) 22:55–65. 10.1007/s40257-020-00542-y
193.
Deng X Zhang W O-Sullivan IS Williams JB Dong Q Park EA et al . FoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1c. J Biol. Chem. (2012) 287:20132–43. 10.1074/jbc.M112.347211
194.
Gu H An HJ Gwon MG Bae S Zouboulis CC Park KK . The Effects of synthetic SREBP-1 and PPAR-γ decoy oligodeoxynucleotide on acne-like disease in vivo and in vitro via lipogenic regulation. Biomolecules. (2022) 12:1858. 10.3390/biom12121858
195.
Castaldo G Pagano I Grimaldi M Marino C Molettieri P Santoro A et al . Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based Metabolomic study. J Proteome Res. (2021) 20:1509–21. 10.1021/acs.jproteome.0c00646
196.
Armstrong AW Mehta MD Schupp CW Gondo GC Bell SJ Griffiths CEM . Psoriasis prevalence in adults in the United States. JAMA Dermatol. (2021) 157:940–6. 10.1001/jamadermatol.2021.2007
197.
Raharja A Mahil SK Barker JN . Psoriasis: a brief overview. Clin Med. (2021) 21:170173. 10.7861/clinmed.2021-0257
198.
McBride SR Fargnoli MC Fougerousse AC García Bustínduy M Catton L Senturk L et al . Impact of psoriatic disease on women aged 18 to 45: results from a multinational survey across 11 European countries. Int J Womens Dermatol. (2021) 7:697–707. 10.1016/j.ijwd.2021.08.011
199.
Branisteanu DE Pirvulescu RA Spinu AE Porumb EA Cojocaru M Nicolescu AC et al . Metabolic comorbidities of psoriasis (review). Exp Ther Med. (2021) 23:179. 10.3892/etm.2021.11102
200.
Sarac G Koca TT Baglan T . A brief summary of clinical types of psoriasis. North Clin Istanb. (2016) 3:79–82. 10.14744/nci.2016.16023
201.
Barrea L Megna M Cacciapuoti S Frias-Toral E Fabbrocini G Savastano S et al . Very low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: an update for dermatologists and nutritionists. Crit Rev Food Sci Nutr. (2021) 62:398–414. 10.1080/10408398.2020.1818053
202.
Dhabale A Nagpure S . Types of psoriasis and their effects on the immune system. Cureus. (2022) 14:e29536. 10.7759/cureus.29536
203.
Stawczyk M Szczerkowska-Dobosz A Komorowska O Dobosz M Maciejewska-Radomska A Stawczyk LM . The role of diet in psoriasis—a chronic systemic inflammatory disease. Forum Zabur Metab. (2011) 2:205–12.
204.
Kocaaga A Kocaaga M . Psoriasis: an immunogenetic perspective. Glob Med Genet. (2022) 9:82–9. 10.1055/s-0042-1743259
205.
Lambadiari V Katsimbri P Kountouri A Korakas E Papathanasi A Maratou E et al . The effect of a ketogenic diet versus Mediterranean diet on clinical and biochemical markers of inflammation in patients with obesity and psoriatic arthritis: a randomized crossover trial. Int J Mol Sci. (2024) 25:2475. 10.3390/ijms25052475
206.
Sieminska I Pieniawska M Grzywa TM . The immunology of psoriasis—current concepts in pathogenesis. Clin Rev Allergy Immunol. (2024) 66:164–91. 10.1007/s12016-024-08991-7
207.
Morelli M Galluzzo M Madonna S Scarponi C Scaglione GL Galluccio T et al . HLA-Cw6 and other HLA-C alleles, as well as MICB-DT, DDX58, and TYK2 genetic variants associate with optimal response to anti-IL-17A treatment in patients with psoriasis. Expert Opin Biol Ther. (2020) 21:259–70. 10.1080/14712598.2021.1862082
208.
Owczarek W . The role of HLA-Cw6 in psoriasis and psoriatic arthritis. Reumatologia. (2022) 60:303–5. 10.5114/reum.2022.120752
209.
Liu T Li S Ying S Tang S Ding Y Li Y et al . The IL-23/IL-17 pathway in inflammatory skin diseases: from bench to bedside. Front Immunol. (2020) 11:594735. 10.3389/fimmu.2020.594735
210.
Czarnecka A Zabłotna M Purzycka-Bohdan D Nowicki RJ Szczerkowska-Dobosz A . An observational study of 147 psoriasis patients: overweightness and obesity as a significant clinical factors correlated with psoriasis. Medicina (B Aires). (2023) 59:2006. 10.3390/medicina59112006
211.
Agarwal K Das S Kumar R De A . Psoriasis and its association with metabolic syndrome. Indian J Dermatol. (2023) 68:274. 10.4103/ijd.ijd_418_23
212.
Mahil SK McSweeney SM Kloczko E McGowan B Barker JN Smith CH . Does weight loss reduce the severity and incidence of psoriasis or psoriatic arthritis? A critically appraised topic. Br J Dermatol. (2019) 181:946–53. 10.1111/bjd.17741
213.
Upala S Sanguankeo A . Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes. (2015) 39:1197–202. 10.1038/ijo.2015.64
214.
Velutti V Egidi G Gagliardi L Santini SA Anselmi G Aquilanti B et al . Psoriasis and nutrition: a focus on microbiome and microbiota. A review. J Food Nutr Sci. (2021) 3:18–29. 10.1057/jfns000028
215.
Hawkins P Earl K Tektonidis TG Fallaize R . The role of diet in the management of psoriasis: a scoping review. Nutr Res Rev. (2023) 37:296–330. 10.1017/S0954422423000185
216.
Verde L Cacciapuoti S Caiazzo G Megna M Martora F Cavaliere A et al . Very low-calorie ketogenic diet (VLCKD) in the management of hidradenitis suppurativa (acne inversa): an effective and safe tool for improvement of the clinical severity of disease. Results of a pilot study. J Transl Med. (2024) 22:149. 10.1186/s12967-024-04853-0
217.
Saraç Öztürk G Ergun T Peker Eyüboglu I Akkiprik M . Serum high-sensitivity C-reactive protein, tumor necrosis factor-α, interleukin (IL)-1β, IL-17A and IL-23 levels in patients with hidradenitis suppurativa. Cytokine. (2021) 144:155585. 10.1016/j.cyto.2021.155585
218.
Lelonek E Szepietowski JC . Insights into gut microbiome composition in hidradenitis suppurativa: a comprehensive examination of dietary habits and environmental influences. Nutrients. (2024) 16:1776. 10.3390/nu16111776
219.
Barrea L Muscogiuri G Pugliese G de Alteriis G Maisto M Donnarumma M et al . Association of trimethylamine n-oxide (TMAO) with the clinical severity of hidradenitis suppurativa (acne inversa). Nutrients. (2021) 13:1997. 10.3390/nu13061997
220.
Shen AS Johnson JS Kerns ML . Dietary factors and hidradenitis suppurativa. Dermatol Ther (Heidelb). (2023) 13:3007–17. 10.1007/s13555-023-01056-1
221.
Abukwaik R Vera-Siguenza E Tennant D Spill F . p53 orchestrates cancer metabolism: unveiling strategies to reverse the Warburg effect. Bull Math Biol. (2024) 86:124. 10.1007/s11538-024-01346-5
222.
Weber DD Aminzadeh-Gohari S Thapa M Redtenbacher A-S Catalano L Capelôa T et al . Ketogenic diets slow melanoma growth in vivo regardless of tumor genetics and metabolic plasticity. Cancer Metab. (2022) 10:12. 10.1186/s40170-022-00288-7
223.
Lazar AM Costea DO Popp CG Mastalier B . Skin malignant melanoma and matrix metalloproteinases: promising links to efficient therapies. Int J Mol Sci. (2024) 25:7804. 10.3390/ijms25147804
224.
Liu Y Sheikh MS . Melanoma: molecular pathogenesis and therapeutic management. Mol Cell Pharmacol. (2014) 6:228.
225.
Davis LE Shalin SC Tackett AJ . Current state of melanoma diagnosis and treatment. Cancer Biol Ther. (2019) 20:1366–79. 10.1080/15384047.2019.1640032
226.
Smiech M Leszczyński P Kono H Wardell C Taniguchi H . Emerging braf mutations in cancer progression and their possible effects on transcriptional networks. Genes (Basel). (2020) 11:1–14. 10.3390/genes11111342
227.
Eskandarpour M Huang F Reeves KA Clark E Hansson J . Oncogenic NRAS has multiple effects on the malignant phenotype of human melanoma cells cultured in vitro. Int J Cancer. (2009) 124:16–26. 10.1002/ijc.23876
228.
Skartun O Smith CR Laupsa-Borge J Dankel SN . Symptoms during initiation of a ketogenic diet: a scoping review of occurrence rates, mechanisms and relief strategies. Front Nutr. (2025) 12:1538266. 10.3389/fnut.2025.1538266
229.
Di Raimondo D Buscemi S Musiari G Rizzo G Pirera E Corleo D et al . Ketogenic diet, physical activity, and hypertension—a narrative review. Nutrients. (2021) 13:2567. 10.3390/nu13082567
230.
Puchalska P Crawford PA . Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. (2017) 25:262–84. 10.1016/j.cmet.2016.12.022
231.
Batch JT Lamsal SP Adkins M Sultan S Ramirez MN . Advantages and disadvantages of the ketogenic diet: a review article. Cureus. (2020) 12:e9639. 10.7759/cureus.9639
232.
Hajar A Swathi NL Ali A . Immunological insights into nutritional deficiency disorders. In:NayakAMisraS, editors. Causes and Management of Nutritional Deficiency Disorders, vol. 4. Hershey, PA: IGI Global (2024). p. 61–84. 10.4018/979-8-3693-2947-4.ch004
233.
Acharya P Acharya C Thongprayoon C Hansrivijit P Kanduri SR Kovvuru K et al . Incidence and characteristics of kidney stones in patients on ketogenic diet: a systematic review and meta-Analysis. Diseases. (2021) 9:39. 10.3390/diseases9020039
234.
Xiao A Kopelman H Shitabata P Nami N . Ketogenic diet-induced prurigo pigmentosa (the “keto rash”): a case report and literature review. J Clin Aesthet Dermatol. (2021) 14:S29–32.
235.
Böer A Misago N Wolter M Kiryu H Wang XD Ackerman AB . Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. (2003) 25:117–29. 10.1097/00000372-200304000-00005
236.
Böer A Asgari M . Prurigo pigmentosa: an underdiagnosed disease?Indian J Dermatol Venereol Leprol. (2006) 72:405–9. 10.4103/0378-6323.29334
237.
Mufti A Mirali S Abduelmula A McDonald KA Alabdulrazzaq S Sachdeva M et al . Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. (2021) 3:79–87. 10.1016/j.jdin.2021.03.003
238.
Alshaya MA Turkmani MG Alissa AM . Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. (2019) 5:504–7. 10.1016/j.jdcr.2019.03.011
239.
Antoine L Gallais Serezal I Aubin F Puzenat E . Prurigo pigmentosa induced by a ketogenic diet. Eur J Dermatol. (2022) 32:545–6. 10.1684/ejd.2022.4313
240.
Wong CY Chu DH . Cutaneous signs of nutritional disorders. Int J Womens Dermatol. (2021) 7:647–52. 10.1016/j.ijwd.2021.09.003
241.
Gegotek A Skrzydlewska E . Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants. (2022) 11:1993. 10.3390/antiox11101993
242.
Harrison FE May JM . Vitamin C function in the brain: vital role of the ascorbate transporter (SVCT2). Free Radic Biol Med. (2009) 46:719–30. 10.1016/j.freeradbiomed.2008.12.018
243.
Kiani AK Dhuli K Donato K Aquilanti B Velluti V Matera G et al . Main nutritional deficiencies. J Prev Med Hyg. (2022) 63:E93–101. 10.15167/2421-4248/jpmh2022.63.2S3.2752
244.
Alten ED Chaturvedi A Cullimore M Fallon AA Habben L Hughes I et al . No longer a historical ailment: two cases of childhood scurvy with recommendations for bone health providers. Osteoporos Int. (2020) 31:1001–5. 10.1007/s00198-019-05264-4
245.
Tembunde Y Ge S Turney K Driscoll M . Scurvy: a diagnosis not to be missed. Cureus. (2022) 14:e33050. 10.7759/cureus.33050
246.
Gandhi M Elfeky O Ertugrul H Chela HK Daglilar E . Scurvy: rediscovering a forgotten disease. Diseases. (2023) 11:78. 10.3390/diseases11020078
247.
Amisha F Ghanta SN Kumar A Fugere T Malik P Kakadia S . Scurvy in the modern world: extinct or not?Cureus. (2022) 14:e22622. 10.7759/cureus.22622
248.
Patel DP Swink SM Castelo-Soccio L . A review of the use of biotin for hair loss. Skin Appendage Disord. (2017) 3:166–9. 10.1159/000462981
249.
Yuasa M Matsui T Ando S Ishii Y Sawamura H Ebara S et al . Consumption of a low-carbohydrate and high-fat diet (the ketogenic diet) exaggerates biotin deficiency in mice. Nutrition. (2013) 29:1266–70. 10.1016/j.nut.2013.04.011
250.
Molenda M Kolmas J . The role of zinc in bone tissue health and regeneration—a review. Biol Trace Elem Res. (2023) 201:5640–51. 10.1007/s12011-023-03631-1
251.
Prasad AS . Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. (2013) 4:176–90. 10.3945/an.112.003210
252.
Alwadany MM Al Wadani AF Almarri FH Alyami HS Al-Subaie MA . Acrodermatitis enteropathica: a rare case with lifelong implications. Cureus. (2023) 15:e37783. 10.7759/cureus.37783
253.
Osmani S Smidt AC Phan CM Johnson DW . Acquired acrodermatitis enteropathica from a ketogenic diet. JAAD Case Rep. (2021) 9:75–7. 10.1016/j.jdcr.2021.01.016
254.
Del Ciampo IRL Sawamura R Del Ciampo LA . Fernandes MIM. Acrodermatitis enteropathica: clinical manifestations and pediatric diagnosis. Rev Paul Pediatr. (2018) 36:238–41. 10.1590/1984-0462/;2018;36;2;00010
255.
Dev T Sethuraman G . Diagnosis of acrodermatitis enteropathica in resource limited settings. BMJ Case Rep. (2017) 2017:bcr2017220928. 10.1136/bcr-2017-220928
256.
Stiles LI Ferrao K Mehta KJ . Role of zinc in health and disease. Clin Exp Med. (2024) 24:38. 10.1007/s10238-024-01302-6
257.
Leone A De Amicis R Lessa C Tagliabue A Trentani C Ferraris C et al . Food and food products on the Italian market for ketogenic dietary treatment of neurological diseases. Nutrients. (2019) 11:1104. 10.3390/nu11051104
258.
Barrea L Caprio M Camajani E Verde L Perrini S Cignarelli A et al . Ketogenic nutritional therapy (KeNuT)—a multi-step dietary model with meal replacements for the management of obesity and its related metabolic disorders: a consensus statement from the working group of the Club of the Italian Society of Endocrinology (SIE)—diet therapies in endocrinology and metabolism. J Endocrinol Invest. (2024) 47:487–500. 10.1007/s40618-023-02258-2
259.
Zhou C Wang M Liang J He G Chen N . Ketogenic diet benefits to weight loss, glycemic control, and lipid profiles in overweight patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trails. Int J Environ Res Public Health. (2022) 19:10429. 10.3390/ijerph191610429
260.
Kundu S Hossain KS Moni A Zahan MS Rahman MM Uddin MJ . Potentials of ketogenic diet against chronic kidney diseases: pharmacological insights and therapeutic prospects. Mol Biol Rep. (2022) 49:9749–58. 10.1007/s11033-022-07460-8
261.
Yan HM Zhao HJ Guo DY Zhu PQ Zhang CL Jiang W . Gut microbiota alterations in moderate to severe acne vulgaris patients. J Dermatol. (2018) 45:1166–71. 10.1111/1346-8138.14586
262.
Nellore A Maher E Abate M . Prurigo pigmentosa induced by a ketogenic diet. Cureus. (2023) 15:e39498. 10.7759/cureus.39498
263.
Daneshpazhooh M Nikyar Z Hesari KK Rostami E Jamshidi ST Mohaghegh F . Remission of prurigo pigmentosa after breaking ketogenic diet and resuming regular diet. Adv Biomed Res. (2022) 11:70. 10.4103/abr.abr_138_21
264.
Aberer F Wachsmuth N Zunner B Aberer W Moser O . Keto rash following carbohydrate restriction. Korean J Inter Med. (2022) 37:1094–5. 10.3904/kjim.2022.075
265.
Danielsen M Pallesen K Riber-Hansen R Bregnhøj A . A rare case of prurigo pigmentosa in a Danish sibling couple. Case Rep Dermatol. (2023) 15:26–30. 10.1159/000528422
Summary
Keywords
ketogenic diet, skin, acne, psoriasis, hidradenitis suppurativa, skin cancer
Citation
Chylińska N and Maciejczyk M (2025) Effects of the ketogenic diet on skin—potential benefits and risks. Front. Nutr. 12:1686056. doi: 10.3389/fnut.2025.1686056
Received
14 August 2025
Accepted
25 September 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Mingyue Song, South China Agricultural University, China
Reviewed by
Ludovica Verde, University of Naples Federico II, Italy
Arely Vergara-Castañeda, La Salle University of Northwest, Mexico
Updates
Copyright
© 2025 Chylińska and Maciejczyk.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalia Chylińska natalia.chylinska@umb.edu.pl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.