- Institute of Traditional Chinese Medicine of Sichuan Academy of Chinese Medicine Sciences, Chengdu, Sichuan, China
Herbal polysaccharides (HPs), as a category of macromolecular compounds, exhibits unique prebiotic benefits, including antioxidant and immunomodulatory effects, and regulatory effects on the gut microbiota. Particularly, the gut microbiota, often referred to as the ‘forgotten organ’ and the ‘second human genome’, plays a pivotal role in human health. In vitro fermentation techniques, including simulated gastrointestinal digestion and fermentation models, have emerged as effective tools for studying the gut microbiota and its relation to diseases. In vitro fermented polysaccharides can mimic the intestinal environment in vivo, which is crucial for understanding the mechanisms of polysaccharide metabolism, clarifying their metabolic pathways, and elucidating their prebiotic activity. By integrating high-impact research from the past 5 years, this review examines the structural changes of HPs during in vitro fermentation, their microbial metabolic mechanisms, and their prebiotic activity, addressing a current gap by integrating the ‘structure-microbe-function’ relationship within this field. The findings provide a theoretical basis for the effective utilization of traditional herbs, and offer insights for the development of novel functional products. Future studies should combine dynamic fermentation models with stratified clinical trials to accelerate the translational application of herbal polysaccharides.
1 Introduction
Herbal plants, revered since antiquity as a cornerstone of global ethnomedicine, constitute an invaluable biocultural heritage (1, 2). Herbs of different origins, structures and forms are rich in a variety of active ingredients, including polysaccharides, polyphenols, flavonoids, saponins, alkaloids and volatile oils (3–5). Notably, polysaccharide-rich herbs usually have excellent antioxidant, hypoglycemic, hypolipidemic, immunomodulatory, and gut microbiota-regulating properties (6, 7). As an important class of biomolecules, polysaccharides are widely found in various types of organisms and play a key role in life activities (8). With the continuous development of sugar science in recent years, polysaccharides have shown great value in food, medicine, cosmetics and other industries. Especially in the pharmaceutical field, herbal polysaccharides (HPs) have become an important raw material for the research and development of new drugs and health products due to their rich biological activity (9, 10).
As a key component of the human microecosystem, gut microbiota is of great significance to host health (11). It is involved in many physiological processes such as food digestion, nutrient absorption, immune regulation, and its imbalance is closely related to the development of a variety of diseases such as obesity, diabetes mellitus, cardiovascular disease and neurodegenerative diseases (12, 13). Studies have shown that polysaccharides and gut microbiota have a complex and mutual relationship. On the one hand, the gut microbiota can metabolize polysaccharides, produce short-chain fatty acids (SCFAs) and other beneficial metabolites, which can regulate intestinal pH, promote intestinal peristalsis, and also enhance immunity by regulating immune cell function (14). On the other hand, polysaccharides can be used as prebiotics to selectively promote the growth and reproduction of beneficial bacteria while inhibiting the growth of harmful bacteria, thus maintaining the balance of gut microbiota (15).
In vitro fermentation of polysaccharides is a process of in vitro using microorganisms that mimic the intestinal environment in vivo. This process is usually carried out in a fermentation system containing a specific microbial community, and the metabolic changes of polysaccharides under the action of microorganisms are investigated by controlling the conditions such as temperature, pH, and substrate concentration (16, 17). In vitro fermentation is of great significance, as it helps to understand the metabolic mechanism of polysaccharides in the intestinal tract, clarifies the pathways and products of polysaccharides utilized by microorganisms, and provides a theoretical basis for the interpretation of polysaccharides’ prebiotic activity and their effects on intestinal health (18, 19). In addition, in vitro fermentation technology has become an effective means of screening polysaccharides with specific functions due to its highly controllable and reproducible nature (20).
Therefore, this review aims to explore the in vitro fermentation characteristics and prebiotic potential of HPs. It focuses on summarizing the metabolic processes of HPs in simulated in vitro fermentation, their effects on microbial communities, and the associated biological effects. By investigating various in vitro fermentation models of different polysaccharides and identifying those with potential prebiotic and immunomodulatory activities, this review provides a theoretical foundation for the development of novel functional products.
2 Review methodology
This narrative review was designed to rapidly map the recent landscape of HP in-vitro fermentation research rather than to perform a quantitative meta-analysis. To ensure transparency we nevertheless adopted a structured search strategy. Web of Science Core Collection and PubMed were interrogated from January 2014 to October 2024 (last search 20 October 2024) with the following string: (herbal polysaccharide* OR “plant polysaccharide*” OR “medicinal mushroom polysaccharide*”) AND (“in vitro fermentation” OR “fecal fermentation” OR “gut microbiota fermentation”) AND (SCFA* OR “short-chain fatty acid*” OR “prebiotic*” OR “microbiome”), restricted to English-language articles published in JCR Q1–Q3 journals. After duplicate removal, full-text evaluation yielded 134 eligible studies, including 41 highly cited or hotspot papers. Due to the evident methodological heterogeneity, the data are descriptive rather than statistical. Additionally, this review confirms that all evidence is based on in vivo animal studies with appropriate controls or in vitro/ex vivo experiments of equivalent quality.
3 Acquisition of HPs in fermentation and their relation to gut microbiota
3.1 Chemical structure and classification of HPs
In the early days, people discovered the medicinal value of certain HPs through traditional medicine and practical experience (21), and used them for disease treatment, which provided important directions for subsequent scientific exploration (22, 23). With the development of science and technology, researchers have employed chemical analytical methods to separate and identify polysaccharides, gradually clarifying their chemical structures and compositions (24). Studies on the polysaccharides of common herbal plants have revealed their structural characteristics, including monosaccharide composition and glycosidic bond types (25). The chemical structure of HPs is complex and diverse, primarily composed of monosaccharides linked by glycosidic bonds (26). Factors such as monosaccharide composition, glycosidic bond types, degree of branching, and molecular weight collectively determine the structure and function of polysaccharides. Based on monosaccharide composition, HPs can be classified into homopolysaccharides and heteropolysaccharides. Homopolysaccharides are composed of a single type of monosaccharide, such as starch, which is polymerized from glucose monomers; heteropolysaccharides consist of two or more types of monosaccharides (27–29). From a functional perspective, they can be divided into storage polysaccharides and structural polysaccharides, with the former exemplified by starch and the latter by cellulose in plant cell walls (30). HPs with different structures exhibit diverse biological activities. Polysaccharides with specific glycosidic bonds and branching structures may be more readily recognized and utilized by gut microbiota, thereby exerting prebiotic activity (31). In contrast, certain polysaccharides containing special functional groups may possess stronger antioxidant and immunomodulatory activities. Accumulating evidence indicates that the biological activity of polysaccharides is closely linked to their structural characteristics, particularly the types of functional groups (sulfate, acetyl, carboxyl, amino) which significantly influence antioxidant and immunomodulatory activities (32). For instance, sulfated polysaccharides (such as those derived from certain algae or fungi) exhibit stronger antioxidant activity due to their sulfate groups enhancing free radical scavenging capacity (33). Furthermore, acetylation modifications can enhance immunomodulatory activity by altering the polysaccharide’s hydrophobicity and spatial conformation, thereby improving its binding affinity to immune cell surface receptors (34).
3.2 Extraction, isolation and purification of HPs
The extraction and purification of HPs are crucial for obtaining high purity and high activity polysaccharides. Common extraction methods include water extraction, acid extraction, alkali extraction, enzymatic extraction, ultrasound - assisted extraction, and microwave - assisted extraction (35–37). Water extraction is simple and low-cost but may have low efficiency. Acid and alkali extraction can increase the extraction rate but may damage the polysaccharide structure. Enzymatic extraction is mild and specific, avoiding polysaccharide structural damage. Ultrasound and microwave - assisted methods accelerate polysaccharide release through physical effects, improving extraction efficiency (38). Purification often involves column chromatography, ultrafiltration, and precipitation. Column chromatography, such as ion - exchange and gel - permeation chromatography, separates and purifies polysaccharides based on charge and molecular weight (39). Ultrafiltration uses suitable membranes to remove impurities, and precipitation methods like ethanol precipitation achieve preliminary purification (40). Wu et al. demonstrated that compared to traditional hot water extraction of Acidic Polysaccharides from Lotus Leaves, deep eutectic solvent-assisted extraction (LLP-D) reduced the molecular weight of the main component from 12.9 × 104 Da to 4.0 × 104 Da and significantly increased the esterification degree. These structural alterations rendered LLP-D more readily fermentable by gut microbiota in vitro, as evidenced by substantial enhancements in antioxidant, hypoglycemic, and immunostimulatory activities (41). These studies demonstrate that extraction processes not only determine polysaccharide yield but also enhance fermentability and functional activity through a dual mechanism of “shearing-functional group modification”.
In identification, chromatography and spectrometry are widely used. Size - exclusion chromatography (SEC) and asymmetric flow field - flow fractionation (AF4) separate polysaccharides by hydrodynamic volume (42, 43). Combined with multi - angle laser light scattering (MALLS) and refractive index (RI) detectors, they provide information on molecular weight distribution, size, branching, and conformation (44). Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) analyzes oligosaccharides released after periodate oxidation and hydrolysis, distinguishing different polysaccharides (45, 46). These technologies have greatly advanced the study of HPs’ structure and function. Combining different extraction and purification techniques can effectively enhance the purity and activity of HPs.
3.3 Mechanism of HPs fermentation by gut microbiota
Numerous studies have shown that HPs possess a wide range of biological activities, including anti-inflammatory, anti-tumor, immunomodulatory, hypoglycemic and hypolipidemic biological activities, and exhibit broad application prospects in the fields of medicine and food (Figure 1) (47). Microbial fermentation can significantly influence polysaccharide purity while regulating their structure, thereby exerting a substantial impact on their biological activity (48). On one hand, fermentation degrades non-sugar impurities such as proteins, lipids, and pigments in plant raw materials, indirectly enhancing the relative purity of polysaccharides (49). On the other hand, microorganisms may synthesize new metabolites during their metabolic processes, such as extracellular polysaccharides, pigments, or organic acids (50, 51). The interaction of HPs with specific microorganisms or gut microbiota during fermentation is the central mechanism for the structural and functional changes of the polysaccharides (52). The secretion of CAZymes, the production of organic acids and the attack of free radicals work together to determine the final properties of HPs during fermentation (53). First, microorganisms secrete carbohydrate-active enzymes (CAZymes) that alter the structure and function of HPs by specifically hydrolyzing or modifying glycosidic bonds (54). The CAZymes family consists of glycoside hydrolases, polysaccharide cleaving enzymes, and pectin methylesterases, among others, whose activity is influenced by fermentation pH, temperature, and substrate properties. These enzymes reduce the molecular weight of polysaccharides by cleaving glycosidic bonds or removing side-chain groups, altering their solubility, viscosity and biological activity (55, 56). Secondly, organic acids (lactic acid, acetic acid) produced by bacterial fermentation promote the hydrolysis of HPs by lowering the environmental pH, altering their molecular weight and functional properties (57). Different microorganisms produce different types of organic acids, resulting in polysaccharides exhibiting different structural and functional changes during fermentation (58). Additionally, free radicals generated during fermentation lead to polysaccharide breakage and structural changes by attacking either glycosidic bonds or side chains groups of polysaccharides (59). Free radical action may expose additional active sites that enhance the antioxidant or immunomodulatory capacity of polysaccharides, and may also lead to cross-linking of polysaccharides to form more stable network structures (60, 61). At the molecular level, metagenomic evidence from Bacteroides ovatus suggests that the loci Bov_0483–0486 encode enzymes able to cleave (1–5)-α-L-Araf linkages, while NADH-oxidase-generated radicals have been proposed (but not yet directly demonstrated in herbal-polysaccharide fermentation) to introduce aldehyde groups that may contribute to antioxidant activity (62, 63).
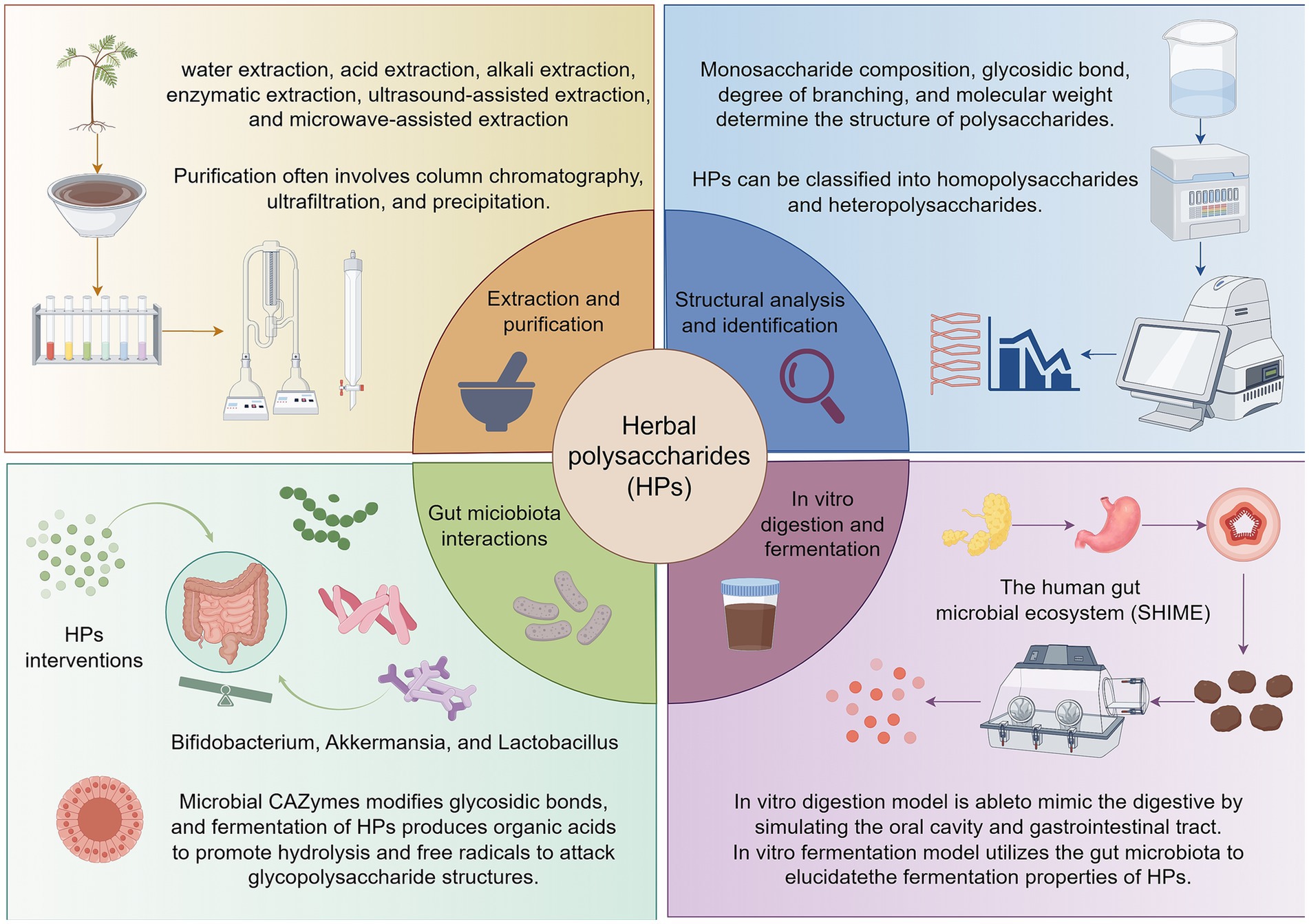
Figure 1. Overview of the extraction, structural characterization, and in vitro digestion-fermentation processes of HPs. Herbal polysaccharides are extracted using various methods including water, acid, alkali, enzymatic, ultrasound-assisted, and microwave-assisted extraction. These polysaccharides are primarily composed of monosaccharides linked by glycosidic bonds, with structural diversity influenced by monosaccharide composition, glycosidic bond types, degree of branching, and molecular weight. In vitro digestion and fermentation models are essential tools for evaluating the functional properties of HPs. The simulated gastrointestinal digestion model mimics the oral, gastric, and intestinal phases of human digestion, allowing for the assessment of structural changes and digestibility. The in vitro fermentation model utilizes gut microbiota to investigate the metabolic fate of HPs, including their effects on microbial composition and metabolite production. Created by Figdraw.com.
3.4 In vitro digestion characteristics of HPs
In vitro modeling is an important tool for studying the biological activity and mechanism of action of HPs. Commonly used in vitro models include simulated gastrointestinal digestion model and in vitro fermentation model (64). Among them, the simulated gastrointestinal tract digestion model is able to dynamically track the structural and property changes of HPs at different stages of digestion by simulating the digestive environments of the oral cavity, stomach and small intestine (65). It was found that the digestive behaviors of different HPs in the gastrointestinal tract were significantly different: some polysaccharides were depolymerized in the gastric acid environment, the molecular weight was reduced, and the content of reducing sugars was significantly increased; while others could pass through the gastrointestinal tract in one piece and finally reach the large intestine (66, 67).
Specifically, salivary amylase hydrolyzes most HPs weakly in the oral phase, and thus these polysaccharides usually enter the stomach intact (68). In the stomach, the synergistic effect of the gastric acid environment and pepsin may lead to depolymerization of some polysaccharides, but this process varies depending on the polysaccharide species. The type of glycosidic bond, degree of branching, molecular weight distribution, and presence of substituent groups of polysaccharides are closely related to the digestive process (69, 70). For example, α-glycosidic bonds are more easily hydrolyzed by human digestive enzymes than β-glycosidic bonds, while highly branched structures or tight folding of polysaccharide chains may enhance their resistance to digestion (71). Studying the in vitro digestion process of HPs not only helps to reveal their metabolic fate in the human body, but also provides a scientific basis for evaluating their nutritional value, biological activity and potential functional applications (Figure 2).
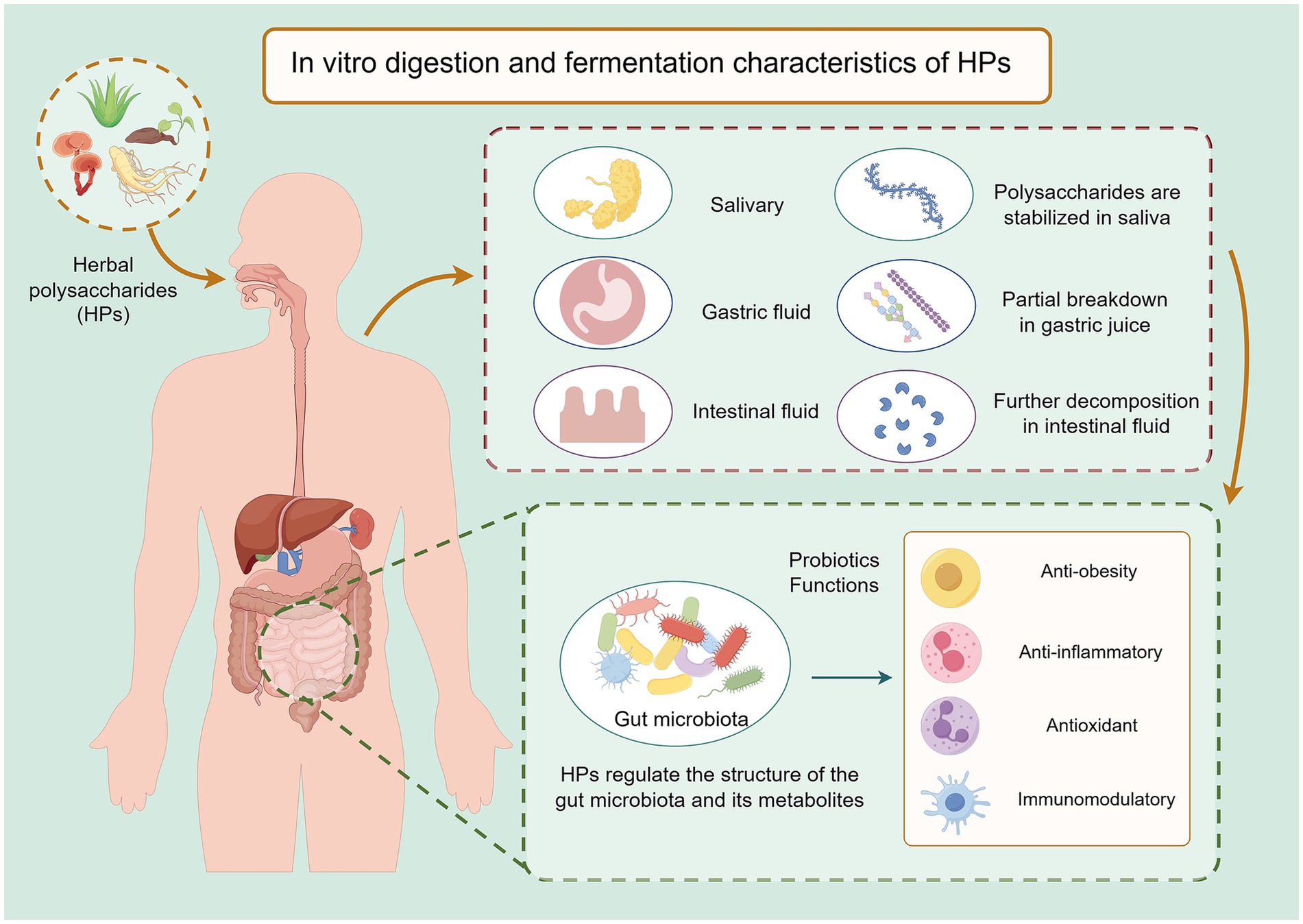
Figure 2. Schematic illustration of the enhancement of biological activity of HPs during in vitro digestion and fermentation. During digestion, HPs undergo partial depolymerization in the gastric and intestinal environments, leading to reduced molecular weight and increased solubility. Subsequent fermentation by gut microbiota further degrades HPs into smaller oligosaccharides or monosaccharides, and promotes the production of beneficial metabolites such as SCFAs. These structural and metabolic transformations not only improve the bioaccessibility of HPs but also expose additional functional groups, thereby amplifying their antioxidant, anti-inflammatory, and immunomodulatory properties. Created by Figdraw.com.
In vitro digestion and fermentation models provide rapid, controlled, and ethically acceptable preliminary screening for how HPs are metabolized by the human gut microbiota. However, they cannot replicate the dynamic absorption, mucus layer, host immune feedback, or circadian variability present in the human gut. Therefore, the antitumor, anti-obesity, and immunomodulatory effects demonstrated in in vitro studies are considered preliminary. All in vitro fermentation data must undergo sequential validation in animal models, pharmacokinetic studies, and stratified human trials before translating to clinical outcomes.
4 In vitro fermentation characteristics of HPs
In vitro fermentation modeling utilizes intestinal microbiota to elucidate the fermentation properties of polysaccharides and their subsequent effects on the gut microbiota (Figure 3). This experimental approach enables the investigation of polysaccharide-induced changes in SCFA production and microbial community structure, thereby providing a foundation for understanding the metabolism of HPs within the gastrointestinal tract and their prebiotic activity. When assessing the prebiotic potential of HPs, the design of in vitro fermentation experiments is of paramount importance (72). Specifically, various types and concentrations of HPs are typically employed as fermentation substrates, with blank control groups and positive control groups supplemented with known prebiotics (inulin or oligofructose) being established during the experimental process. The fermentation characteristics and their impact on the metabolic activity of the intestinal microbiota can be systematically evaluated by monitoring key fermentation indicators, such as pH, SCFA content, and gas production (73). Concurrently, high-throughput 16S rRNA gene sequencing or metagenomic sequencing can be utilized to comprehensively analyze the effects of polysaccharides on the growth and abundance of diverse bacterial populations (74).
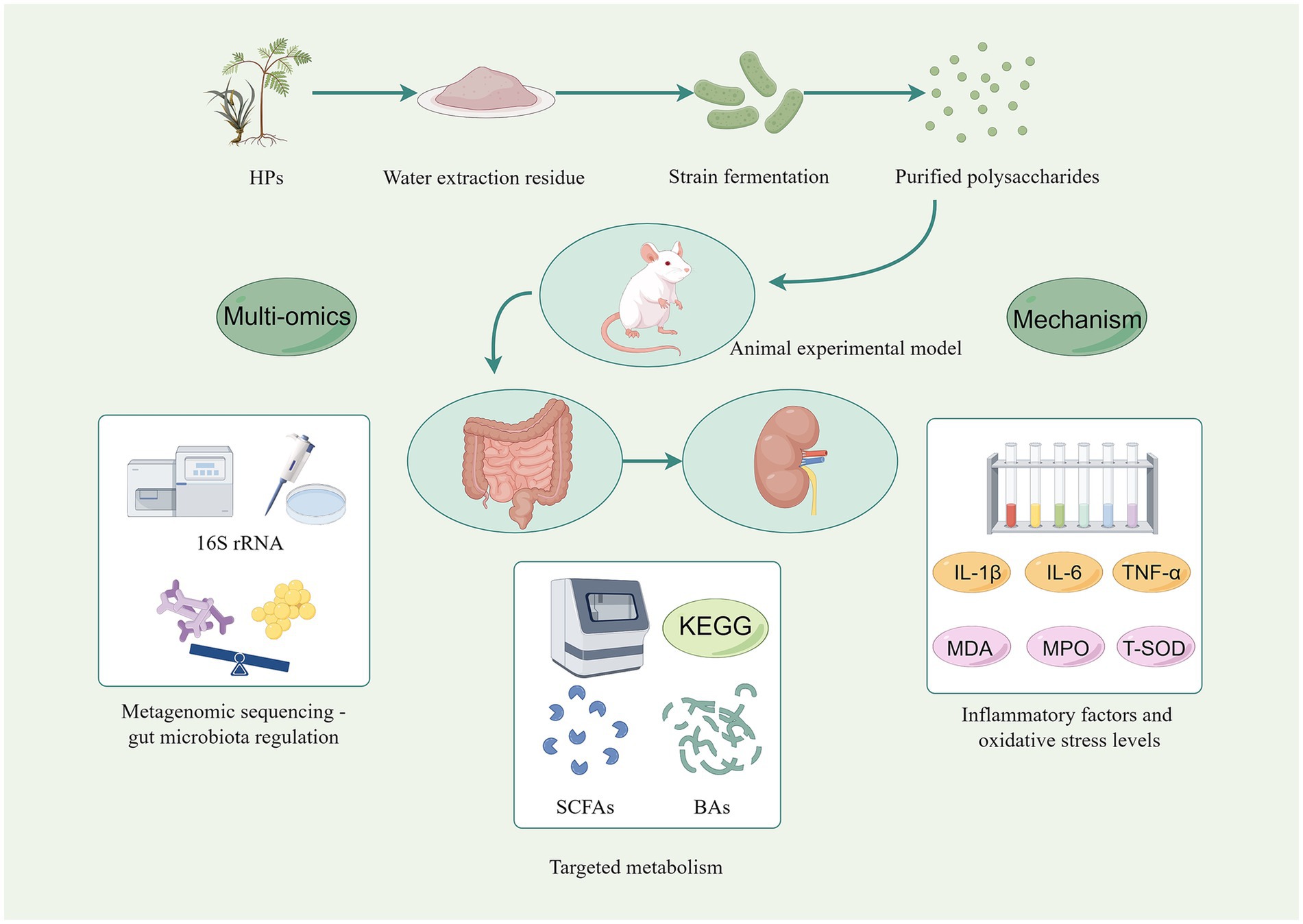
Figure 3. Multi-omics framework for investigating the functional transformation of HPs through microbial fermentation. Water-extracted HPs is subjected to either monostrains (Lactobacillus, Bacteroides) or complex fecal inocula under anaerobic in vitro conditions. Multi-omics tools are then integrated: 16S rRNA and metagenomic sequencing track real-time shifts in microbial community structure and CAZymes; targeted metabolomics quantify SCFAs, bile acids and oxidative-stress markers (MDA, MPO, T-SOD); transcriptomics/proteomics link HP-derived metabolites to host epithelial responses (IL-1β, IL-6, TNF-α, ZO-1, occludin). Created by Figdraw.com.
4.1 Fermentation characteristics of HPs with special strains
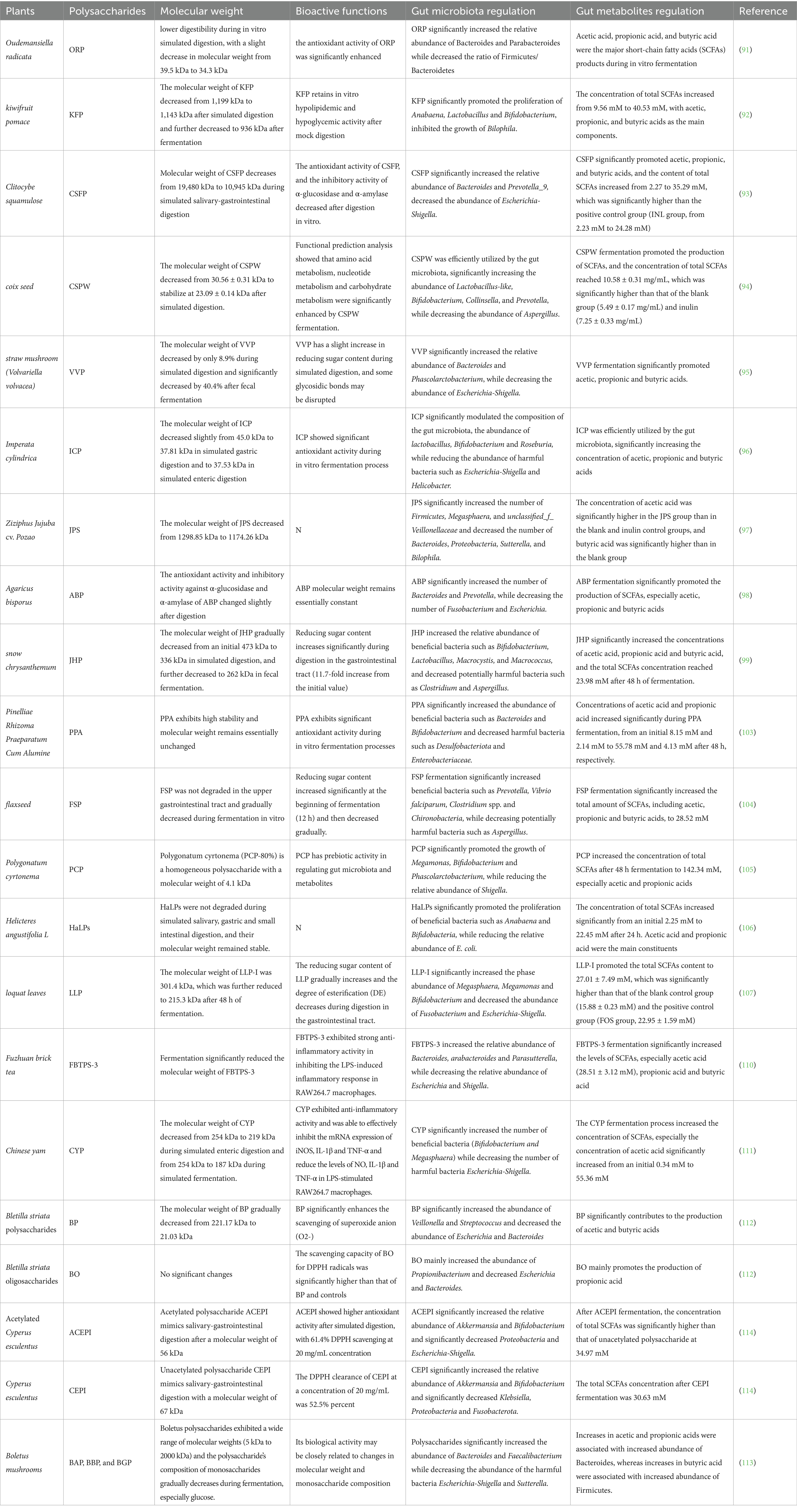
During in vitro fermentation of HPs, specific strains such as Bifidobacterium, Lactobacillus, Akkermansia muciniphila, and Roseburia exhibit significant polysaccharide degradation capacity (75, 76). These strains promote the production of SCFAs by secreting enzyme systems such as polysaccharide lyase and glycoside hydrolase, which degrade complex polysaccharides into low molecular weight fragments or monosaccharides (77). This fermentation pathway not only enhances the prebiotic function of polysaccharides, but also significantly improves the immunomodulatory potential of polysaccharides by regulating the structure of the gut microbiota and the production of metabolites (Table 1).
An in vitro study found that the monosaccharide composition of Tetrastigma hemsleyanum polysaccharide (THDP2) shifted to galactose: mannose: fucose (12.6:2.3:1.0) after fermentation in Sanghuangporus sanghuang, with a significant decrease in molecular weight to 1,230 kDa, and the main chain structure was transformed to α-1,2-D-galactose and α-1,2-D-mannose. This structural reprogramming significantly modulated tumor metabolites, suggesting that the fermentation process may activate the immunomodulatory pathway by exposing more bioactive sites or changing the conformation (78). Another study showed that longan fruit pulp polysaccharide (LP) was fermented to increase solubility by 86.59% and decrease apparent viscosity by 81.67%, while enhancing immunomodulatory and prebiotic activities. This change was closely related to the degradation of the polysaccharide molecular chain, and the low molecular weight fragments produced during fermentation were more easily utilized by the gut microbiota, thus promoting the generation of SCFAs (79). Furthermore, Polygonatum kingianum polysaccharide (PKPS) fermented by Lactobacillus paracasei showed that the low molecular weight fractions PS2-4 (10–50 kDa) prolonged the lifespan by 20.70% and significantly enhanced DPPH free radical scavenging and α-glucosidase inhibitory activities in the Cryptobacterium hidradenii nematode model (80). This suggests that fermentation may activate the antioxidant, hypoglycemic and anti-aging functions of polysaccharides by introducing new functional groups or changing the conformation. Flammulina velutipes polysaccharides (FVP), as heteropolysaccharides with molecular weights up to 9.52 × 103 kDa, exhibited significant anti-degradation properties in in vitro fermentation, while demonstrating intestinal health protective effects by regulating the composition of gut microbiota to promote bifidobacterial and lactic acid bacteria proliferation and metabolite profiles (81).
Interestingly, several studies have collectively shown that the fermentation process not only changes the molecular structure of polysaccharides, but polysaccharide degradation is synergistically utilized with commensal bacteria and activity is enhanced in a coupled digestion-fermentation system. Brassica rapa L. polysaccharide (BRP2-2) showed a significant decrease in molecular weight within 24 h of in vitro fermentation, suggesting that it is efficiently utilized by intestinal microorganisms. BRP2-2 revealed the molecular interaction mechanism between microorganisms and polysaccharides by up-regulating 143 genes (five independent polysaccharide utilization sites, PULs, and two clusters of carbohydrate-activating enzymes, CAZymes) in response to BRP2-2-induced degradation processes (82). Similarly, Lactobacillus fermentum polysaccharides (LPF) pretreated with Lactobacillus fermentum decreased the molecular weight and promoted the utilization efficiency of Bifidobacterium, Megalobacterium, and Prevotella while producing higher concentrations of acetic acid and butyric acid. This synergistic effect suggests that fermentation lowers the threshold of polysaccharide utilization and optimizes the metabolic network of the bacteria (83). Furthermore, the presence of glucosamine (GlcN) in the monosaccharide composition of Nostoc commune Vauch. polysaccharides (NCVP) after in vitro digestion was hypothesized to be related to the synergistic effect of HCl and digestive enzymes. SCFAs produced by subsequent fermentation further promoted the proliferation of Bifidobacterium and Bacteroidetes, suggesting that the coupled process of digestion and fermentation may enhance the antioxidant and prebiotic activity of polysaccharides by exposing hidden active moieties or generating novel oligosaccharides (84).
In addition, with the widespread application of omics technologies such as metagenomics, metabolomics, and transcriptomics, the strain-specific functions of beneficial bacteria like Lactobacillus and Bifidobacterium. in fermented foods have gradually been revealed (85). Through metagenomic analysis of 19,699 food-derived LAB (FLAB) strains globally, Jin et al. identified significant differences in amino acid synthesis, SCFA metabolism, and secondary metabolite production among strains belonging to genera such as Limosilactobacillus, Lactococcus, and Streptococcus (86). Furthermore, Wu et al. employed a multi-omics approach to reveal that Limosilactobacillus reuteri synergistically interacts with galacto-oligosaccharides (GOS) to enrich Bacteroides acidifaciens and promote its synthesis of pentadecanoic acid (87). This process inhibits the NF-κB signaling pathway and enhances tight junction protein expression. Multi-omics evidence has clearly demonstrated that the enrichment of beneficial bacteria can translate into health benefits for the host.
4.2 Fermentation characteristics of HPs from animal manure
4.2.1 Fermented HPs are accompanied by a decrease in molecular weight
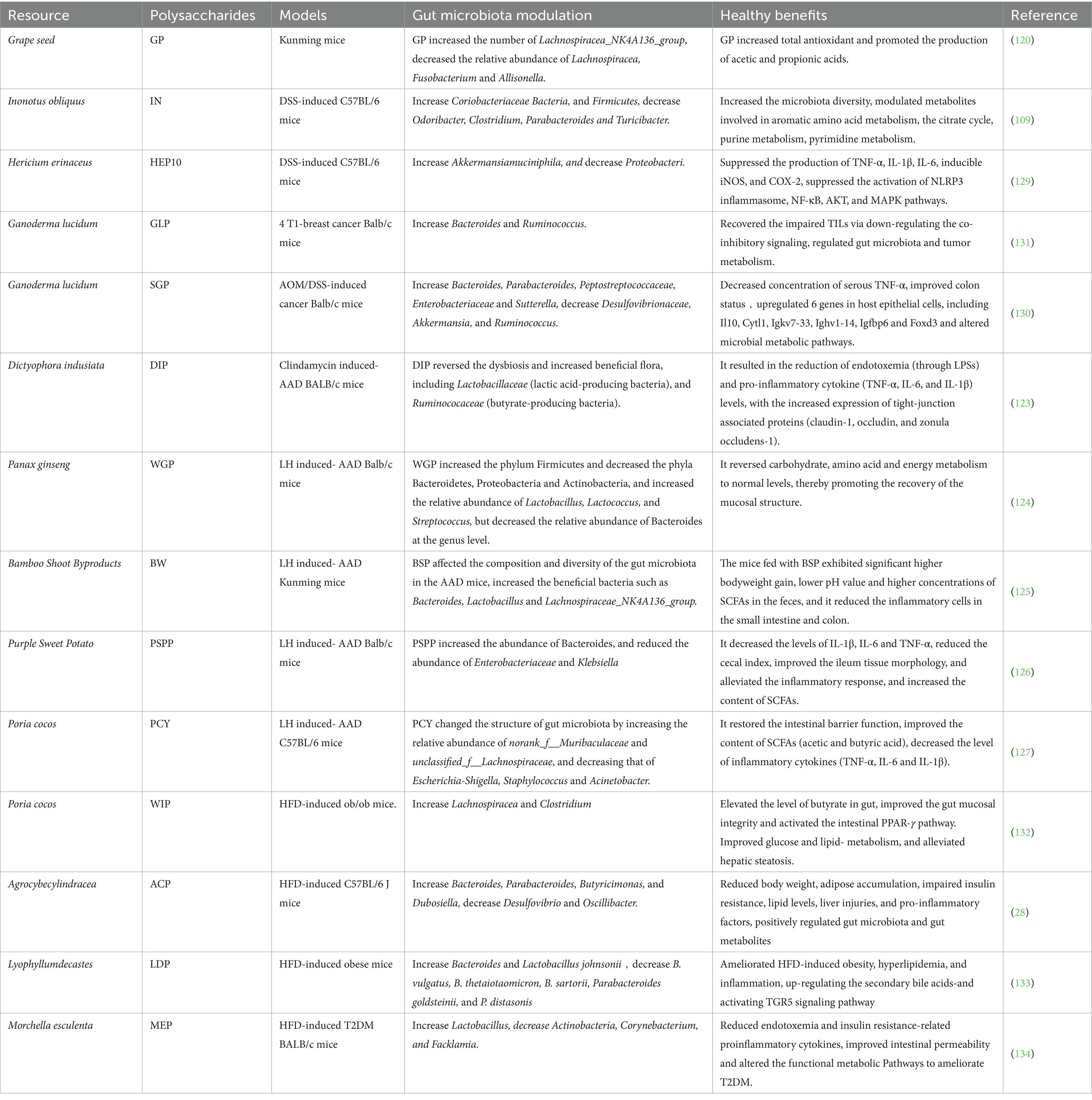
During in vitro fermentation, the complex interactions between animal fecal microbial communities and plant-derived polysaccharides are a key link in the regulation of intestinal microecology and host health. Polysaccharides synergistically enhance their antioxidant, anti-inflammatory and prebiotic potentials through multiple pathways during fermentation, including dynamic adjustment of molecular weight, structural optimization of the gut microbiota, and precise regulation of SCFAs (88). HPs are often accompanied by significant changes in molecular weight during in vitro fermentation. Polysaccharides undergo depolymerization and rearrangement under the action of microbial enzymes, and the decrease in molecular weight not only increases their accessibility, but also exposes potential bioactive sites, laying a structural foundation for subsequent functional activation (Table 2). Interestingly, existing research indicates that the effects of microbial fermentation on HPs extend beyond mere molecular weight reduction (89). In certain cases, microorganisms may secrete extracellular polysaccharides (EPS) during fermentation or repolymerize low-molecular-weight sugar units through mechanisms such as glycosyltransferases, resulting in increased polysaccharide molecular weight (90).
Oudemansiella radicata polysaccharide (ORP) showed a slight decrease in molecular weight from 39.5 kDa to 34.3 kDa in simulated digestion in vitro, along with a significant increase in antioxidant activity, presumably related to the decrease in molecular weight and the formation of polysaccharide-protein complexes. During in vitro fermentation, ORP was effectively utilized by the gut microbiota, significantly increasing the relative abundance of Bacteroides and Parabacteroides while decreasing the ratio of Firmicutes/Bacteroidetes, suggesting a potential anti-obesity effect (91). In an in vitro simulation of digestion and fermentation, the molecular weight of kiwifruit pomace polysaccharide (KFP) decreased from 1,199 kDa to 936 kDa, with a decrease in the content of reducing sugars and a change in the composition of monosaccharides (92). KFP significantly promoted the proliferation of Bacteroides, Lactobacillus, and Bifidobacterium, and at the same time inhibited the growth of harmful bacteria Bilophila growth and regulated the balance of gut microbiota. Guo et al. also demonstrated that the molecular weight of scaly cupuacin polysaccharide (CSFP) decreased from 19.48 kDa to 10.945 kDa and the content of reducing sugars increased during simulated salivary-gastrointestinal digestion (93). CSFP significantly increased the relative abundance of Bacteroides and Prevotella_9, decreased the abundance of Escherichia-Shigella and improved gut flora structure. Coix lacryma polysaccharide (CSPW) showed that the molecular weight of CSPW decreased from 30.56 ± 0.31 kDa to stabilize at 23.09 ± 0.14 kDa after simulated digestion. CSPW was efficiently utilized by the gut microbiota, which significantly increased the numbers of Lactobacillus filamentosus-like bacteria, Bifidobacterium bifidum, Collinsella spp. and Prevotella spp., while decreasing the abundance of the phylum Methanogerminae (94). Furthermore, a study has investigated the dynamic changes of physicochemical properties of the grass mushroom polysaccharide (VVP) and its regulation of gut microbiota and metabolites by simulating the salivary-gastrointestinal digestion and in vitro fecal fermentation models (95). The results showed that VVP basically remained stable during gastrointestinal digestion, and the molecular weight only decreased by 8.9%, entering the colon as an indigestible polysaccharide. During in vitro fermentation, reducing sugars typically increase initially during hydrolysis before decreasing as they are absorbed by microorganisms. VVP is progressively degraded by gut microbiota, with a significant 40.4% reduction in molecular weight and a marked decrease in reducing sugar content, indicating its effective utilization by gut microbiota.
Polysaccharides with different chemical structures have selective enrichment effects on microbial communities. This structure–function match enables polysaccharides to target and regulate the composition of the flora, inhibiting conditionally pathogenic bacteria while enriching beneficial bacteria such as butyric acid-producing bacteria. Yu et al. showed that Imperata cylindrica polysaccharide (ICP) significantly modulated the composition of the gut microbiota, increasing the abundance of beneficial bacteria such as Lactobacillus, Bifidobacterium and Roseburia, while decreasing the abundance of harmful bacteria such as Escherichia-Shigella and Helicobacter (96). In addition, molecular docking experiments showed that ICP was able to interact with the key proteins TLR2, MUC1 and MMP9 through high-affinity binding sites. A study systematically investigated the role of Ziziphus jujuba cv. pozao polysaccharide (JPS) in the regulation of gut microbiota during digestion and fermentation in vitro. JPS significantly increased the number of Firmicutes, Megasphaera, and unclassified_f_Veillonellaceae and decreased the number of numbers of Bacteroides, Proteobacteria, Sutterella and Bilophila (97). Fu et al. investigated simulated digestion and fecal fermentation modeling and found that Agaricus bisporus polysaccharide (ABP) significantly increased the number of Bacteroides and Prevotella, while decreasing the number of Fusobacterium and Escherichia (98). During fecal fermentation, snow chrysanthemum polysaccharide (JHP) was further degraded into smaller fragments by gut microbiota, while significantly increasing the relative abundance of beneficial bacteria such as Bifidobacterium, Lactobacillus, Macrocystis, and Macrococcus, and decreasing the abundance of potentially harmful bacteria, such as Clostridium and Aspergillus (99).
4.2.2 Fermented HPs promote the production of SCFAs
Notably, HPs produce a variety of metabolites during in vitro fermentation, with the production of SCFAs at the core. Acetic acid, propionic acid and butyric acid play an important role in maintaining intestinal health, as they regulate intestinal pH and inhibit the growth of harmful bacteria, while providing energy to intestinal epithelial cells and promoting the repair and regeneration of intestinal mucosa (100, 101). The analysis of these metabolites can provide insights into the fermentation pathways and mechanisms of polysaccharides, infer the metabolic pathways of polysaccharides under the action of microorganisms, and provide a basis for further research on the prebiotic activity of polysaccharides and their effects on host health (102).
Pinelliae Rhizoma Praeparatum Cum Alumine polysaccharide (PPA) showed high stability during simulated digestion. During in vitro fermentation, PPA significantly increased the abundance of Bacteroides and Bifidobacterium, and the concentrations of acetic acid and propionic acid increased significantly during PPA fermentation, from an initial 8.15 mM and 2.14 mM, respectively, to 55.78 mM and 4.13 mM after 48 h, and these SCFAs play an important role in maintaining intestinal health (103). Flaxseed polysaccharide (FSP) was degraded progressively by the gut microbiota, with a gradual decrease in molecular weight and a first increase and then a decrease in reducing sugar content, which was efficiently utilized by the gut microbiota (104). FSP increased the relative abundance of Prevotella, Vibrio falciparum, Clostridium. and Macrocystis. and decreased the abundance of potentially harmful bacteria such as Ascomycetes. at the same time as it simultaneously and significantly increased the total amount of SCFAs (28.52 mM), including acetic, propionic and butyric acids. Another study showed that the polysaccharide derived from Polygonatum cyrtonema (PCP-80%) had a molecular weight of 4.1 kDa and consisted mainly of fructose in the β-configuration and glucose in the α-configuration. During in vitro fermentation, pcp-80% increased the production of SCFAs to 142.34 mM, suggesting its prebiotic activity (105). Helicteres angustifolia L polysaccharides (HaLPs) significantly promoted the proliferation of beneficial bacteria such as Anabaena and Bifidobacteria, while reducing the relative abundance of potentially harmful bacteria, and facilitated the increase of total SCFAs from an initial level of 2.25 mM to 22.45 mM after 24 h, suggesting their potential to improve intestinal metabolism and promote host health (106). In addition, Wu et al. investigated the dynamics of physicochemical properties of loquat leaf polysaccharides (LLP) and their modulation of gut microbiota and metabolites by simulating salivary-gastrointestinal digestion and in vitro cecal fermentation models. LLP-I increased Megasphaera, Megamonas, and Bifidobacterium, and decreased Fusobacterium and Escherichia-Shigella. LLP-I fermentation significantly contributed to the total SCFAs content of 27.01 ± 7.49 mM, which is important for gut health (107).
4.2.3 Fermented HPs hold potential for enhancing biological activity
Natural polysaccharides are inherently rich in biological activity, but their activity is further enhanced by structural changes and metabolite generation during in vitro simulated digestion and fermentation (108, 109). Specifically, during in vitro digestion and fermentation of polysaccharides, their molecular weight decreases, glycosidic bonds are broken, and the monosaccharide composition may be altered (91). Such structural changes not only increase the solubility and bioavailability of polysaccharides, but also expose more active sites, making it easier for them to interact with biomolecules, thus enhancing bioactivities.
Chen et al. found that Fuzhuan brick tea polysaccharides (FBTPS-3) increased the relative abundance of Bacteroides, Arabacteroides, and Parasutterella, while decreasing the relative abundance of deleterious bacteria (Escherichia and Shigella), and increased the levels of acetic, propionic, and butyric acids (110). In addition, the fermented FBTPS-3 exhibited strong anti-inflammatory activity in inhibiting the LPS-induced inflammatory response in RAW264.7 macrophages. Meanwhile, the molecular weight of Chinese yam polysaccharides (CYP) remained essentially unchanged in gastric juice but was partially degraded in the small intestine and was progressively decomposed during fermentation. CYP significantly increased the number of Bifidobacterium and Megasphaera, while decreasing the number of harmful bacteria Escherichia-Shigella population (111). Morever, CYP had significant anti-inflammatory and intestinal barrier-protecting effects, especially in the LPS-stimulated co-culture model of Caco-2/RAW264.7 cells that effectively suppressed the inflammatory response. In a comparative study, Bletilla striata polysaccharides (BP) and oligosaccharides (BO) exhibited different properties during in vitro mock digestion and fermentation (112). the molecular weight of BP gradually decreased from 221.17 kDa to 21.03 kDa, while BO remained stable during mock digestion. During in vitro fermentation, both BP and BO were efficiently utilized by the gut microbiota, with BP significantly increasing the abundance of Veillonella and Streptococcus, while BO increased the abundance of Propionibacterium. Meanwhile, BP significantly promoted the production of acetic acid and butyric acid, while BO mainly promoted the production of propionic acid. In addition, the BP fermentation broth had a higher scavenging capacity for superoxide anion (O2-), while the BO fermentation broth had a higher scavenging capacity for DPPH free radicals. In a study, polysaccharides BAP, BBP, and BGP were extracted from bovine hepatitis bacteria (B. auripes, B. bicolor, and B. griseus) by subcritical water extraction, and in vitro fermentation modeling was utilized to explore their regulatory effects on gut microbiota and metabolites (113). It was found that B. bovis polysaccharides had a broad molecular weight distribution and a complex monosaccharide composition, with molecular weight gradually decreasing during fermentation and monosaccharides being preferentially utilized by the gut microbiota. BAP improved the composition and structure of gut microbiota, suggesting that it has a significant prebiotic effect and can improve intestinal health by regulating gut microbiota and metabolites. Interestingly, In another comparative study of acetylated polysaccharides, Yuan et al. systematically explored the effects of acetylation of Cyperus esculentus polysaccharides on their physicochemical properties, digestive behavior, and intestinal microbial fermentation characteristics (114). Acetylation significantly reduced the molecular weight of the polysaccharide and enhanced its antioxidant activity. In in vitro fermentation, the acetylated polysaccharide (ACEPI) exhibited faster fermentation kinetics, produced more SCFAs, and significantly reduced pH. Both polysaccharides modulated the gut microbiota, promoting the growth of the beneficial bacteria Akkermansia while inhibiting the harmful bacteria (Proteobacteria and Escherichia-Shigella). Furthermore, ACEPI significantly increased the abundance of Parabacteroides after fermentation, while CEPI promoted the growth of Bifidobacterium. These changes may improve intestinal health and metabolic functions by regulating intestinal metabolites and related protein factors ZO-1 and IL-10, thus demonstrating the potential application of acetylated polysaccharides in functional food development.
Together, these studies show that the fermentation process not only changes the molecular structure of polysaccharides, but also significantly enhances the bioactivity and prebiotic function of polysaccharides by modulating the composition and metabolic activity of the gut microbiota. Collectively, polysaccharides influence microbial interactions and alter the structure and function of microbial communities, which in turn positively affect host health. It is worth noting that experimental results from in vitro fermentation modeling need to be interpreted with caution, as they cannot fully mimic the complex physiological environment of the human body (gut motility, host–microbe interactions). Nevertheless, as an efficient and controllable research tool, the in vitro fermentation model provides important theoretical support for exploring the prebiotic activity of HPs and their underlying mechanisms, and lays the foundation for further animal experiments and human clinical trials.
5 HPs and their prebiotic activity after fermentation
Prebiotics are indigestible food components that selectively stimulate the growth and activity of beneficial flora in the host’s intestinal tract, thereby beneficially affecting host health (115, 116). HPs and their fermented products exhibit significant bioactivities by regulating the composition and metabolism of the gut microbiota, providing new ideas and strategies for the prevention and treatment of a wide range of chronic diseases (Table 3) (27, 117). A large number of studies have formed a consensus that polysaccharides, in addition to increasing the number and types of beneficial bacteria and improving the microecological balance of the intestinal tract, are also able to regulate the metabolic function of the gut microbiota, affecting their digestion and absorption of nutrients as well as the metabolism of harmful substances (118, 119).
After simulated salivary-gastrointestinal digestion and in vitro fecal fermentation, grape seed polysaccharides (GP) extracted by aqueous alcoholic precipitation increased total antioxidant, increased the relative abundance of Lachnospiracea_NK4A136_group (p = 0.008239), decreased the relative abundance of Lachnospiracea relative abundance of Fusobacterium and Allisonella, and promoted acetic and propionic acids (120). In addition, Aloe vera polysaccharides (APs) and soybean polysaccharides (SP) also increased the concentration of SCFAs during in vitro fermentation in a simulator of the human gut microbial ecosystem (SHIME), increasing the concentration of Bifidobacterium and Lactobacillus, while decreasing Escherichia-Shigella and Escherichia coli (121, 122).
The integrity of the intestinal barrier is essential for maintaining intestinal health. HPs are able to enhance the intestinal barrier function and protect the mucosa from damage by regulating the composition and metabolism of gut microbiota. For example, Dictyophora indusiata polysaccharides (DIP) were able to reverse clindamycin- and metronidazole-induced intestinal dysbiosis, increase the number of beneficial flora such as Lactobacillus (lactic acid-producing bacteria) and Rumatococcaceae (butyric acid-producing bacteria), and increase the expression of tight junction-associated proteins, decreasing endotoxemia and proinflammatory cytokine Levels (123). In addition, Panax ginseng (WGP) polysaccharide was able to alleviate lincomycin-induced antibiotic-associated diarrhea (AAD) in mice by increasing the number of Lactobacillus, Lactococcus, and Streptococcus, regulating carbohydrate, amino acid, and energy metabolism, and promoting the restoration of intestinal mucosa (124). Bamboo shoot polysaccharide (BW) affected the composition and diversity of the gut microbiota of AAD mice by increasing beneficial bacteria such as Bacteroidetes mimicus, Lactobacillus and Lachnospiraceae_NK4A136_group (125). Purple Sweet Potato polysaccharide (PSPP) improves gut health in AAD mice by increasing anaplasmosis, decreasing Enterobacteriaceae and Klebsiella pneumoniae, decreasing inflammation levels and cecum index, and improving ileal tissue morphology (126). It is worth noting that Poria cocos (PCY)-derived water-insoluble polysaccharides were also effective in alleviating the symptoms of lincomycin hydrochloride-induced AAD after fermentation. PCY increased the number of Muribaculaceae and Lachnospiraceae, and decreased the number of E. coli-Shigella, Staphylococcus, and Fusobacterium (127).
Extensive studies have demonstrated that prebiotics can degrade complex plant polysaccharides into oligosaccharides or monosaccharides during fermentation by secreting a series of carbohydrate-active enzymes (CAZymes), such as β-galactosidase, α-glucosidase, and arabinase. This process subsequently produces acetic acid, propionic acid, and butyric acid (19). Regarding immunomodulation, SCFAs produced by Lactobacillus and Bifidobacterium through polysaccharide fermentation promote regulatory T cell (Treg) differentiation and suppress Th17 cell-mediated inflammatory responses, thereby maintaining intestinal immune homeostasis. Furthermore, these strains degrade polysaccharides to generate immunologically active oligosaccharide fragments that directly activate macrophages and dendritic cells. This enhances expression of anti-inflammatory factors like IL-10 and IFN-γ, thereby bolstering host immune defense capabilities (128).
Inflammatory response is an important pathological feature of many chronic diseases, and gut microbiota plays a key role in regulating the inflammatory response. HPs and their fermentation products can significantly decrease inflammatory markers and mitigated symptoms by modulating the composition and metabolism of gut microbiota. For example, Inonotus obliquus polysaccharide (IN) significantly ameliorated DSS-induced colitis in a DSS-induced C57BL/6 J mouse model by increasing gut microbial diversity and modulating systemic metabolism (109). In addition, Hericium erinaceus low molecular mass polysaccharide (HEP10) significantly ameliorated DSS-induced colitis by inhibiting the induction of pro-inflammatory cytokines iNOS and cyclooxygenase-2 (COX-2), as well as inhibiting the activation of gut flora-associated NLRP3 inflammasome vesicles (129).
HPs play an important role in immunomodulation and can regulate the body’s immune response through multiple pathways. On the one hand, it can activate immune cells, such as macrophages, T-cells and B-cells, and enhance their phagocytosis, cytokine secretion and immune response. On the other hand, polysaccharides can induce macrophages to release cytokines such as tumor necrosis factor-α and interleukin-1, which can enhance the body’s inflammatory response and immune defense ability. For example, Ganoderma lucidum spore polysaccharide (SGP) was able to ameliorate AOM and DSS-induced male AOM and DSS-induced male by decreasing the concentration of TNF-α, up-regulating the expression of genes such as Il10, Cytl1, Igkv7-33, Ighv1-14, Igfbp6, Foxd3, and altering the metabolic pathways of intestinal microorganisms in the host epithelial cells. Colon cancer in Balb/c mice (130). Furthermore, Ganoderma lucidum polysaccharide (GLP) inhibited colonic inflammation and tumorigenesis by modulating gut microbiota and immune cell function in AOM/DSS-induced C57BL/6 mice, as evidenced by increased production of SCFAs, attenuation of endotoxemia, down-regulation of IL-1β, iNOS, and COX-2 expression, and inhibition of lipopolysaccharide (LPS) induced inflammatory markers and MAPK activation (131). In clinical applications, the use of nutraceuticals or drugs containing HPs in immunocompromised patients can effectively improve the body’s immunity and reduce the risk of infectious diseases.
Accumulating evidence indicates shows that HPs have a positive impact on metabolic health. In terms of blood glucose regulation, some polysaccharides can slow down the digestion and absorption of carbohydrates by inhibiting the activities of α-amylase and α-glucosidase, thus lowering the rise of blood glucose after meals. In lipid regulation, HPs can lower the levels of total cholesterol, triglyceride and LDL cholesterol in blood, and at the same time raise the level of HDL cholesterol, which can help prevent and improve cardiovascular diseases such as atherosclerosis. For example, Agrocybe cylindracea polysaccharide (ACP) reduced the number of Vibrio desulfuricans, increased the abundance of Vibrio parahaemolyticus, and decreased the levels of TNF-α and IL-6 in mice induced by a high-fat diet (28). The water insoluble polysaccharide of Poria cocos (WIP) significantly reduced obesity symptoms by increasing butyric acid-producing bacteria and butyric acid levels, improving intestinal mucosal integrity, activating the intestinal PPAR-γ signaling pathway, and improving glucose and lipid metabolism (132). Lyophyllum decastes polysaccharide (LDP) significantly ameliorated high-fat diet-induced obesity, hyperlipidemia, and inflammation by modulating gut microbiota, increasing the upregulation of secondary bile acids, and activating the TGR5 signaling pathway (133). In addition, Morchella esculenta polysaccharide (MEP) significantly ameliorated high-fat diet-induced diabetes by augmenting Lactobacillus, inhibiting enterococci, and modulating inflammatory cascade responses, including Toll-like receptor 4 (TLR4), COX-2, and NF-κB (134). Without exception, these studies have shown that HPs can have a beneficial impact on overall metabolic health by regulating gut flora and their metabolites and improving energy metabolism and fat storage.
6 Conclusions and outlooks
Emerging evidence highlights a surge of interest in fermented HPs. This review synthesizes current evidence that microbial fermentation, mediated by either specific bacterial strains or complex fecal consortia, remodels the native plant polysaccharides, markedly enhancing and diversifying their biological activities. In individuals with intestinal dysbiosis, supplementation with targeted HPs preferentially promotes the proliferation of beneficial gut commensals, restoring microbial equilibrium and improving overall gastrointestinal health. However, limitations lie in the fact that we have not yet fully integrated the “polysaccharide-enzyme-metabolite-receptor,” nor have we conducted direct validation using gene knockout and germ-free animals. Moreover, the in vivo mechanisms of polysaccharides are complex and influenced by various factors, and it is challenging to deeply investigate their targets and signaling pathways. As researchers continue to explore, new analytical techniques, synthetic methods, and research approaches are opening up greater possibilities for in-depth studies of polysaccharides. Based on the in vitro fermentation characteristics of HPs and their impact on the gut microbiota, there is potential to develop innovative drugs for the treatment of immune-related diseases, metabolic disorders, and cancer. This review examines the impact of fermented HPs on microbial communities and their biological effects, providing a theoretical foundation for the effective utilization of herbal medicines and insights into the development of novel functional products. Future research should combine dynamic fermentation models with stratified population trials to further advance the translation of herbal polysaccharides from laboratory studies to clinical applications.
Author contributions
YoL: Data curation, Conceptualization, Writing – original draft, Methodology, Validation. YW: Data curation, Methodology, Writing – original draft, Supervision. CL: Conceptualization, Writing – original draft, Data curation. GL: Data curation, Conceptualization, Writing – review & editing. JF: Supervision, Methodology, Data curation, Writing – review & editing. YiL: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Basic Research Project of Public Welfare Scientific Research Institutes of Sichuan Province, project number: No. 2023–4-763; Sichuan Provincial Department of Science and Technology, project number: 2024ZHYS0016; The 2023 National Traditional Chinese Medicine Characteristic Technology Inheritance Talent Training Project, project number: [2023]96, T20234832005; and the Provincial-Level Traditional Chinese Medicine Talent Development - Support for Qihuang Scholars Project, project number: [2025]20; the Basic Research Project of Public Welfare Scientific Research Institutes of Sichuan Province, project number: No. 2023–4-764.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guo, X, Luo, W, Wu, L, Zhang, L, Chen, Y, Li, T, et al. Natural products from herbal medicine self-assemble into advanced bioactive materials. Adv Sci. (2024) 11:388. doi: 10.1002/advs.202403388
2. Chu, K, Liu, J, Zhang, X, Wang, M, Yu, W, Chen, Y, et al. Herbal medicine-derived exosome-like nanovesicles: a rising star in cancer therapy. Int J Nanomedicine. (2024) 19:7585–603. doi: 10.2147/ijn.S477270
3. Li, H-Y, Huang, S-Y, Zhou, D-D, Xiong, R-G, Luo, M, Saimaiti, A, et al. Theabrownin inhibits obesity and non-alcoholic fatty liver disease in mice via serotonin-related signaling pathways and gut-liver Axis. J Adv Res. (2023) 52:59–72. doi: 10.1016/j.jare.2023.01.008
4. Nie, Q, Chen, H, Hu, J, Fan, S, and Nie, S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit Rev Food Sci Nutr. (2018) 59:848–63. doi: 10.1080/10408398.2018.1536646
5. Feng, W, Ao, H, Peng, C, and Yan, D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. (2019) 142:176–91. doi: 10.1016/j.phrs.2019.02.024
6. Gong, X, Li, X, Bo, A, Shi, R-Y, Li, Q-Y, Lei, L-J, et al. The interactions between gut microbiota and bioactive ingredients of traditional Chinese medicines: a review. Pharmacol Res. (2020) 157:104824. doi: 10.1016/j.phrs.2020.104824
7. Xue, H, Mei, CF, Wang, FY, and Tang, XD. Relationship among Chinese herb polysaccharide (Chp), gut microbiota, and chronic diarrhea and impact of Chp on chronic diarrhea. Food Sci Nutr. (2023) 11:5837–55. doi: 10.1002/fsn3.3596
8. Zhao, W-x, Wang, T, Zhang, Y-n, Chen, Q, Wang, Y, Xing, Y-q, et al. Molecular mechanism of polysaccharides extracted from Chinese medicine targeting gut microbiota for promoting health. Chin J Integr Med. (2022) 30:171–80. doi: 10.1007/s11655-022-3522-y
9. Xiang, S, Lan, Y, Lu, L, Sun, C, Lai, Y, Mai, Z, et al. A novel alternative strategy for monitoring and insight into liver fibrosis progression: the combination of surface-enhanced Raman spectroscopy (Sers) and gut microbiota. Biosens Bioelectron. (2023) 225:225. doi: 10.1016/j.bios.2023.115082
10. Lin, T-L, Lu, C-C, Lai, W-F, Wu, T-S, Lu, J-J, Chen, Y-M, et al. Role of gut microbiota in identification of novel Tcm-derived active metabolites. Protein Cell. (2020) 12:394–410. doi: 10.1007/s13238-020-00784-w
11. Gomes, AC, Hoffmann, C, and Mota, JF. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes. (2018) 9:1–18. doi: 10.1080/19490976.2018.1465157
12. Greten, FR, and Arkan, MC. Gut microbial carcinogen metabolism: another avenue to Cancer. Signal Transduct Target Ther. (2024) 9:297. doi: 10.1038/s41392-024-02015-8
13. Roje, B, Zhang, B, Mastrorilli, E, Kovačić, A, Sušak, L, Ljubenkov, I, et al. Gut microbiota carcinogen metabolism causes distal tissue Tumours. Nature. (2024) 632:1137–44. doi: 10.1038/s41586-024-07754-w
14. Fan, Y, and Pedersen, O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2020) 19:55–71. doi: 10.1038/s41579-020-0433-9
15. Dey, P. Gut microbiota in phytopharmacology: a comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol Res. (2019) 147:367. doi: 10.1016/j.phrs.2019.104367
16. Tan, Y, Li, M, Kong, K, Xie, Y, Zeng, Z, Fang, Z, et al. In vitro simulated digestion of and microbial characteristics in colonic fermentation of polysaccharides from four varieties of Tibetan tea. Food Res Int. (2023) 163:112255. doi: 10.1016/j.foodres.2022.112255
17. Zeibich, L, Schmidt, O, and Drake, HL. Dietary polysaccharides: fermentation potentials of a primitive gut ecosystem. Environ Microbiol. (2019) 21:1436–51. doi: 10.1111/1462-2920.14556
18. Zhang, N, Jin, M, Wang, K, Zhang, Z, Shah, NP, and Wei, H. Functional oligosaccharide fermentation in the gut: improving intestinal health and its determinant factors-a review. Carbohydr Polym. (2022) 284:284. doi: 10.1016/j.carbpol.2021.119043
19. Liu, S, Hu, J, Zhong, Y, Hu, X, Yin, J, Xiong, T, et al. A review: effects of microbial fermentation on the structure and bioactivity of polysaccharides in plant-based foods. Food Chem. (2024) 440:440. doi: 10.1016/j.foodchem.2023.137453
20. Zhang, J, Liu, Q, Gu, F, Liu, Y, Zhou, Y, Li, Y, et al. Effects of Lactobacillus fermentation on Eucheuma Spinosum polysaccharides: characterization and mast cell membrane stabilizing activity. Carbohydr Polym. (2023) 310:120742. doi: 10.1016/j.carbpol.2023.120742
21. Nowakowski, P, Markiewicz-Żukowska, R, Bielecka, J, Mielcarek, K, Grabia, M, and Socha, K. Treasures from the Forest: evaluation of mushroom extracts as anti-Cancer agents. Biomed Pharmacother. (2021) 143:143. doi: 10.1016/j.biopha.2021.112106
22. Khan, I, Huang, G, Li, X, Leong, W, Xia, W, and Hsiao, WLW. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J Funct Foods. (2018) 41:191–201. doi: 10.1016/j.jff.2017.12.046
23. Lai, Y, Fang, Q, Guo, X, Lei, H, Zhou, Q, Wu, N, et al. Effect of polysaccharides from Dictyophora indusiata on regulating gut microbiota and short-chain fatty acids in mice. J Food Meas Charact. (2022) 17:1–11. doi: 10.1007/s11694-022-01596-8
24. Cui, L, Guan, X, Ding, W, Luo, Y, Wang, W, Bu, W, et al. Scutellaria baicalensis Georgi polysaccharide ameliorates Dss-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int J Biol Macromol. (2021) 166:1035–45. doi: 10.1016/j.ijbiomac.2020.10.259
25. Yang, Y, Li, M, Liu, Q, Zhao, Q, Zeng, J, Wang, Q, et al. Starch from Pueraria lobata and the amylose fraction alleviates dextran sodium sulfate induced colitis in mice. Carbohydr Polym. (2023) 302:302. doi: 10.1016/j.carbpol.2022.120329
26. Song, C, Huang, F, Liu, L, Zhou, Q, Zhang, D, Fang, Q, et al. Characterization and prebiotic properties of pectin polysaccharide from Clausena lansium (Lour.) Skeels fruit. Int J Biol Macromol. (2022) 194:412–21. doi: 10.1016/j.ijbiomac.2021.11.083
27. Fang, J, Lin, Y, Xie, H, Farag, MA, Feng, S, Li, J, et al. Dendrobium officinale leaf polysaccharides ameliorated hyperglycemia and promoted gut bacterial associated Scfas to alleviate type 2 diabetes in adult mice. Food Chem. (2022) 13:13. doi: 10.1016/j.fochx.2022.100207
28. Zhu, Z, Huang, R, Huang, A, Wang, J, Liu, W, Wu, S, et al. Polysaccharide from Agrocybe cylindracea prevents diet-induced obesity through inhibiting inflammation mediated by gut microbiota and associated metabolites. Int J Biol Macromol. (2022) 209:1430–8. doi: 10.1016/j.ijbiomac.2022.04.107
29. Luo, Y, Fang, Q, Lai, Y, Lei, H, Zhang, D, Niu, H, et al. Polysaccharides from the leaves of Polygonatum sibiricum red. Regulate the gut microbiota and affect the production of short-chain fatty acids in mice. AMB Express. (2022) 12:35. doi: 10.1186/s13568-022-01376-z
30. Arifuzzaman, M, Won, TH, Li, T-T, Yano, H, Digumarthi, S, Heras, AF, et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature. (2022) 611:578–84. doi: 10.1038/s41586-022-05380-y
31. Fang, Q, Lai, Y, Zhang, D, Lei, H, Wang, F, Guo, X, et al. Gut microbiota regulation and prebiotic properties of polysaccharides from Oudemansiella raphanipes mushroom. World J Microbiol Biotechnol. (2023) 39:167. doi: 10.1007/s11274-023-03616-1
32. Li, Z-W, Du, Z-M, Wang, Y-W, Feng, Y-X, Zhang, R, and Yan, X-B. Chemical modification, characterization, and activity changes of land plant polysaccharides: a review. Polymers. (2022) 14:161. doi: 10.3390/polym14194161
33. Ma, L, Zhang, Q, Lai, Y, Long, X, Wang, F, Feng, B, et al. The sulfated modification of polysaccharide from Poria cocos and its prebiotic effects on gut microbiota. J Sci Food Agric. (2025) 105:7853–67. doi: 10.1002/jsfa.70037
34. Lunin, VV, Wang, H-T, Bharadwaj, VS, Alahuhta, M, Peña, MJ, Yang, J-Y, et al. Molecular mechanism of polysaccharide acetylation by the Arabidopsis Xylan O-acetyltransferase Xoat1. Plant Cell. (2020) 32:2367–82. doi: 10.1105/tpc.20.00028
35. Fang, J, Wang, Z, Wang, P, and Wang, M. Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: a review. Int J Biol Macromol. (2020) 162:1897–905. doi: 10.1016/j.ijbiomac.2020.08.141
36. Nai, J, Zhang, C, Shao, H, Li, B, Li, H, Gao, L, et al. Extraction, structure, pharmacological activities and drug carrier applications of Angelica Sinensis polysaccharide. Int J Biol Macromol. (2021) 183:2337–53. doi: 10.1016/j.ijbiomac.2021.05.213
37. Li, Y, Wang, X, Lv, X, Wang, X, Wang, X, Cui, J, et al. Extractions and rheological properties of polysaccharide from okra pulp under mild conditions. Int J Biol Macromol. (2020) 148:510–7. doi: 10.1016/j.ijbiomac.2020.01.163
38. Song, Z, Xiong, X, and Huang, G. Ultrasound-assisted extraction and characteristics of maize polysaccharides from different sites. Ultrason Sonochem. (2023) 95:95. doi: 10.1016/j.ultsonch.2023.106416
39. Xu, D, Wang, C, Zhuo, Z, Ye, M, and Pu, B. Extraction, purification and antioxidant activity of polysaccharide from cold pressed oil cake of ‘Tengjiao’ seed. Int J Biol Macromol. (2020) 163:508–18. doi: 10.1016/j.ijbiomac.2020.06.207
40. Liu, C, Du, P, Guo, Y, Xie, Y, Yu, H, Yao, W, et al. Extraction, characterization of Aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota. Carbohydr Polym. (2021) 261:261. doi: 10.1016/j.carbpol.2021.117874
41. Wu, D-T, Feng, K-L, Huang, L, Gan, R-Y, Hu, Y-C, and Zou, L. Deep eutectic solvent-assisted extraction, partially structural characterization, and bioactivities of acidic polysaccharides from Lotus leaves. Foods. (2021) 10:330. doi: 10.3390/foods10102330
42. Chen, Z, Wang, D, Gu, S, Wu, N, Wang, K, and Zhang, Y. Size exclusion chromatography and asymmetrical flow field-flow fractionation for structural characterization of polysaccharides: a comparative review. Int J Biol Macromol. (2024) 277:277. doi: 10.1016/j.ijbiomac.2024.134236
43. Pitkänen, L. Potential of size-exclusion chromatography and asymmetric flow field-flow fractionation in separation and characterization of plant polysaccharides. J Chromatogr A. (2025) 1748:1748. doi: 10.1016/j.chroma.2025.465862
44. Zheng, Z, Huang, Q, and Ling, C. Water-soluble yeast Β-Glucan fractions with different molecular weights: extraction and separation by Acidolysis assisted-size exclusion chromatography and their association with proliferative activity. Int J Biol Macromol. (2019) 123:269–79. doi: 10.1016/j.ijbiomac.2018.11.020
45. Gao, X, Lu, Y, Wei, M, Yang, M, Zheng, C, Wang, C, et al. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis of human Milk neutral and Sialylated free oligosaccharides using Girard’s reagent P on-target Derivatization. J Agric Food Chem. (2019) 67:8958–66. doi: 10.1021/acs.jafc.9b02635
46. Tiangpook, S, Nhim, S, Prangthip, P, Pason, P, Tachaapaikoon, C, Ratanakhanokchai, K, et al. Production of a series of Long-chain Isomaltooligosaccharides from maltose by Bacillus subtilis Ap-1 and associated prebiotic properties. Foods. (2023) 12:1499. doi: 10.3390/foods12071499
47. Zhang, T, Yang, Y, Liang, Y, Jiao, X, and Zhao, C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients. (2018) 10:1055. doi: 10.3390/nu10081055
48. Wang, Z, Zheng, Y, Dai, Y, Yang, R, Zhao, R, Sun, G, et al. Effect of probiotic fermentation on the extraction rate and bioactivity of plant-based polysaccharides: a review. Innov Food Sci Emerg Technol. (2024) 98:103863. doi: 10.1016/j.ifset.2024.103863
49. Wang, Z, Zheng, Y, Lai, Z, Kong, Z, Hu, X, Zhang, P, et al. Effect of Saccharomyces cerevisiae Cicc 32883 fermentation on the structural features and antioxidant protection effect of Chinese yam polysaccharide. Foods. (2025) 14:564. doi: 10.3390/foods14040564
50. Yang, J, Zheng, Y, Yang, Y, Huang, Z, Sun, G, Zhao, R, et al. Effects of microbial fermentation on the anti-inflammatory activity of Chinese yam polysaccharides. Front Nutr. (2025) 11:624. doi: 10.3389/fnut.2024.1509624
51. Yang, Y, Lai, Z, Hu, X, Zhang, P, Zhang, Y, Zheng, Y, et al. Structural characterization and combined immunomodulatory activity of fermented Chinese yam polysaccharides with probiotics. Int J Biol Macromol. (2025) 307:307. doi: 10.1016/j.ijbiomac.2025.142290
52. Tingirikari, JMR. In-vitro prebiotic analysis of microbiota accessible pectic polysaccharides. Curr Microbiol. (2019) 76:1452–60. doi: 10.1007/s00284-019-01781-x
53. Shang, Q, Jiang, H, Cai, C, Hao, J, Li, G, and Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr Polym. (2018) 179:173–85. doi: 10.1016/j.carbpol.2017.09.059
54. Bakky, MAH, Tran, NT, Zhang, M, Zhang, Y, Liang, H, Wang, Y, et al. In vitro fermentation of Gracilaria Lemaneiformis and its sulfated polysaccharides by rabbitfish gut microbes. Int J Biol Macromol. (2023) 246:246. doi: 10.1016/j.ijbiomac.2023.125561
55. Qin, H, Huang, L, Teng, J, Wei, B, Xia, N, and Ye, Y. Purification, characterization, and bioactivity of Liupao tea polysaccharides before and after fermentation. Food Chem. (2021) 353:419. doi: 10.1016/j.foodchem.2021.129419
56. Shen, S, Hong, T, Liu, Z, Liu, S, Ni, H, Jiang, Z, et al. In vitro-simulated intestinal Flora fermentation of Porphyra haitanensis polysaccharides obtained by different assisted extractions and their fermented products against Ht-29 human Colon Cancer cells. Food Funct. (2023) 14:10747–58. doi: 10.1039/d3fo04421a
57. Wang, J, Ma, Y, Xu, X, Huang, G, Zhang, R, Jia, X, et al. Comparison of different Longan polysaccharides during gut Bacteroides fermentation. Food Chem. (2024) 461:461. doi: 10.1016/j.foodchem.2024.140840
58. Min, F-F, Hu, J-L, Nie, S-P, Xie, J-H, and Xie, M-Y. In vitro fermentation of the polysaccharides from Cyclocarya paliurus leaves by human fecal inoculums. Carbohydr Polym. (2014) 112:563–8. doi: 10.1016/j.carbpol.2014.06.027
59. Chen, J, Zou, Y, Zheng, T, Huang, S, Guo, L, Lin, J, et al. The in vitro fermentation of Cordyceps militaris polysaccharides changed the simulated gut condition and influenced gut bacterial motility and translocation. J Agric Food Chem. (2022) 70:14193–204. doi: 10.1021/acs.jafc.2c05785
60. Xiang, G, Sun, H, Chen, Y, Guo, H, Liu, Y, Li, Y, et al. Antioxidant and hypoglycemic activity of tea polysaccharides with different degrees of fermentation. Int J Biol Macromol. (2023) 228:224–33. doi: 10.1016/j.ijbiomac.2022.12.114
61. Zhang, X, Liu, L, Luo, J, and Peng, X. Anti-aging potency correlates with metabolites from in vitro fermentation of edible fungal polysaccharides using human fecal intestinal microflora. Food Funct. (2022) 13:11592–603. doi: 10.1039/d2fo01951e
62. Zheng, J, Hu, B, Zhang, X, Ge, Q, Yan, Y, Akresi, J, et al. Dbcan-Seq update: Cazyme gene clusters and substrates in microbiomes. Nucleic Acids Res. (2023) 51:D557–63. doi: 10.1093/nar/gkac1068
63. Liang, J, Zhang, M, Li, X, Yue, Y, Wang, X, Han, M, et al. Structure and immunomodulatory activity of Lentinus edodes polysaccharides modified by probiotic fermentation. Food Sci Human Wellness. (2024) 13:421–33. doi: 10.26599/fshw.2022.9250036
64. Cheng, Y, Huang, X, Li, L, Liu, L, Zhang, C, Fan, X, et al. Effects of solid fermentation on Polygonatum cyrtonema polysaccharides: isolation, characterization and bioactivities. Molecules. (2023) 28:498. doi: 10.3390/molecules28145498
65. Zhou, W, Yan, Y, Mi, J, Zhang, H, Lu, L, Luo, Q, et al. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. J Agric Food Chem. (2018) 66:898–907. doi: 10.1021/acs.jafc.7b05546
66. Jiang, C, Li, H, Li, J, Zou, G, Li, C, Fang, Z, et al. In vitro simulated digestion and fermentation behaviors of polysaccharides from Pleurotus cornucopiae and their impact on the gut microbiota. Food Funct. (2024) 15:10051–66. doi: 10.1039/d4fo02873b
67. Shen, S, Yang, W, Li, L, Zhu, Y, Yang, Y, Ni, H, et al. In vitro fermentation of seaweed polysaccharides and tea polyphenol blends by human intestinal Flora and Their effects on intestinal inflammation. Food Funct. (2023) 14:1133–47. doi: 10.1039/d2fo03390a
68. Ma, M, Quan, M, Zhang, J, Zhang, A, Gao, P, Shang, Q, et al. In vitro fermentation of polysaccharide from edible alga Enteromorpha clathrata by the gut microbiota of patients with ulcerative colitis. Nutrients. (2023) 15:122. doi: 10.3390/nu15194122
69. Kong, Q, Dong, S, Gao, J, and Jiang, C. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int J Biol Macromol. (2016) 91:867–71. doi: 10.1016/j.ijbiomac.2016.06.036
70. Li, X, Xie, Q, Huang, S, Shao, P, You, L, and Pedisić, S. Digestion & fermentation characteristics of sulfated polysaccharides from Gracilaria chouae using two extraction methods in vitro and in vivo. Food Res Int. (2021) 145:406. doi: 10.1016/j.foodres.2021.110406
71. Li, Q-Y, Dou, Z-M, Chen, C, Jiang, Y-M, Yang, B, and Fu, X. Study on the effect of molecular weight on the gut microbiota fermentation properties of blackberry polysaccharides in vitro. J Agric Food Chem. (2022) 70:11245–57. doi: 10.1021/acs.jafc.2c03091
72. Wang, X, Wang, X, Jiang, H, Cai, C, Li, G, Hao, J, et al. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr Polym. (2018) 195:601–12. doi: 10.1016/j.carbpol.2018.05.003
73. Zhang, X, Aweya, JJ, Huang, Z-X, Kang, Z-Y, Bai, Z-H, Li, K-H, et al. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its Agaro-oligosaccharides by human fecal Inocula and its impact on microbiota. Carbohydr Polym. (2020) 234:115894. doi: 10.1016/j.carbpol.2020.115894
74. Gu, F, Borewicz, K, Richter, B, van der Zaal, PH, Smidt, H, Buwalda, PL, et al. In vitro fermentation behavior of isomalto/malto-polysaccharides using human fecal inoculum indicates prebiotic potential. Mol Nutr Food Res. (2018) 62:232. doi: 10.1002/mnfr.201800232
75. Jang, YJ, Kim, W-K, Han, DH, Lee, K, and Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. (2019) 10:696–711. doi: 10.1080/19490976.2019.1589281
76. Oh, NS, Lee, JY, Kim, Y-T, Kim, SH, and Lee, J-H. Cancer-protective effect of a Synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal Cancer. Gut Microbes. (2020) 12:1785803. doi: 10.1080/19490976.2020.1785803
77. Wang, X, Xue, J, Zhang, R, Li, Y, Li, X, Ding, Y, et al. Prebiotic characteristics of degraded polysaccharides from Acanthopanax senticosus polysaccharide on broilers gut microbiota based on in vitro digestion and fecal fermentation. Poult Sci. (2024) 103:807. doi: 10.1016/j.psj.2024.103807
78. Cheng, J, Wang, Y, Wei, H, He, L, Hu, C, Cheng, S, et al. Fermentation-mediated variations in structure and biological activity of polysaccharides from Tetrastigma hemsleyanum Diels et Gilg. Int J Biol Macromol. (2023) 253:253. doi: 10.1016/j.ijbiomac.2023.127463
79. Huang, F, Hong, R, Zhang, R, Yi, Y, Dong, L, Liu, L, et al. Physicochemical and biological properties of Longan pulp polysaccharides modified by Lactobacillus fermentum fermentation. Int J Biol Macromol. (2019) 125:232–7. doi: 10.1016/j.ijbiomac.2018.12.061
80. Yang, J-J, Zhang, X, Dai, J-F, Ma, Y-G, and Jiang, J-G. Effect of fermentation modification on the physicochemical characteristics and anti-aging related activities of Polygonatum kingianum polysaccharides. Int J Biol Macromol. (2023) 235:235. doi: 10.1016/j.ijbiomac.2023.123661
81. Ye, Z, Yu, L, Zhang, C, Gao, Y, Zhao, J, Narbad, A, et al. Modulation of gut microbiota and metabolites by Flammulina velutipes polysaccharides during in vitro human fecal fermentation: unveiling Bacteroides as a potential primary degrader. Food Chem. (2024) 450:139309. doi: 10.1016/j.foodchem.2024.139309
82. Yi, W, Shi, J, Zhou, W, Wei, J, Sun, Y, Zeng, X, et al. In vitro fermentation of a purified fraction of polysaccharides from the root of Brassica rapa L. by human gut microbiota and its interaction with Bacteroides ovatus. Food Chem. (2025) 473:473. doi: 10.1016/j.foodchem.2025.143109
83. Xia, C, Zhang, R, Jia, X, Dong, L, Ma, Q, Zhao, D, et al. In vitro human gut microbiota fermentation of litchi pulp polysaccharides as affected by Lactobacillus pre-treatment. Food Chem. (2024) 445:734. doi: 10.1016/j.foodchem.2024.138734
84. Li, H, Liu, S, Liu, Y, Li, W, Niu, A, Ren, P, et al. Effects of in vitro digestion and fermentation of Nostoc commune Vauch. Polysaccharides on properties and gut microbiota. Carbohydr Polym. (2022) 281:119055. doi: 10.1016/j.carbpol.2021.119055
85. Jin, H, Quan, K, You, L, Kwok, L-Y, Ma, T, Li, Y, et al. A genomic compendium of cultivated food-derived lactic acid Bacteria unveils their contributions to human health. Sci Bull. (2025) 70:1761–5. doi: 10.1016/j.scib.2024.12.002
86. Arzamasov, AA, Rodionov, DA, Hibberd, MC, Guruge, JL, Kent, JE, Kazanov, MD, et al. Integrative genomic reconstruction reveals heterogeneity in carbohydrate utilization across human gut Bifidobacteria. Nat Microbiol. (2025) 10:2031–47. doi: 10.1038/s41564-025-02056-x
87. Wu, Y, Zhang, X, Liu, X, Zhao, Z, Tao, S, Xu, Q, et al. Galactooligosaccharides and Limosilactobacillus reuteri synergistically alleviate gut inflammation and barrier dysfunction by enriching Bacteroides acidifaciens for Pentadecanoic acid biosynthesis. Nat Commun. (2024) 15:144. doi: 10.1038/s41467-024-53144-1
88. Su, Y, Cheng, S, Ding, Y, Wang, L, Sun, M, Man, C, et al. A comparison of study on intestinal barrier protection of polysaccharides from Hericium Erinaceus before and after fermentation. Int J Biol Macromol. (2023) 233:123558. doi: 10.1016/j.ijbiomac.2023.123558
89. Wang, Z, Zheng, Y, Lai, Z, Hu, X, Wang, L, Wang, X, et al. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: a review. Int J Biol Macromol. (2024) 254:254. doi: 10.1016/j.ijbiomac.2023.127955
90. Wang, Z, Zheng, Y, Zhou, X, Wang, X, Liu, X, Wang, Q, et al. Effect of Lactobacillus fermentation on the structural feature, physicochemical property, and bioactivity of plant and fungal polysaccharides: a review. Trends Food Sci Technol. (2024) 148:104492. doi: 10.1016/j.tifs.2024.104492
91. Liu, Y, Li, Y, Ke, Y, Li, C, Zhang, Z, Wu, Y, et al. In vitro saliva-gastrointestinal digestion and fecal fermentation of Oudemansiella radicata polysaccharides reveal its digestion profile and effect on the modulation of the gut microbiota. Carbohydr Polym. (2021) 251:7041. doi: 10.1016/j.carbpol.2020.117041
92. Chen, M, Chen, X, Guo, Y, Liu, N, Wang, K, Gong, P, et al. Effect of in vitro digestion and fermentation of kiwifruit pomace polysaccharides on structural characteristics and human gut microbiota. Int J Biol Macromol. (2023) 253:253. doi: 10.1016/j.ijbiomac.2023.127141
93. Guo, D, Lei, J, He, C, Peng, Z, Liu, R, Pan, X, et al. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from Clitocybe squamulose. Int J Biol Macromol. (2022) 208:343–55. doi: 10.1016/j.ijbiomac.2022.03.126
94. Ge, Q, Hou, C-l, Rao, X-h, Zhang, A-q, Xiao, G-m, Wang, L-y, et al. In vitro fermentation characteristics of polysaccharides from Coix seed and its effects on the gut microbiota. Int J Biol Macromol. (2024) 262:9994. doi: 10.1016/j.ijbiomac.2024.129994
95. Hu, W, Di, Q, Liang, T, Zhou, N, Chen, H, Zeng, Z, et al. Effects of in vitro simulated digestion and fecal fermentation of polysaccharides from straw mushroom (Volvariella volvacea) on its physicochemical properties and human gut microbiota. Int J Biol Macromol. (2023) 239:124188. doi: 10.1016/j.ijbiomac.2023.124188
96. Yu, W, Wang, J, Xiong, Y, Liu, J, Baranenko, D, Zhang, Y, et al. In vivo absorption, in vitro simulated digestion, and fecal fermentation properties of Imperata cylindrica polysaccharides and their effects on gut microbiota. Food Chem. (2024) 461:773. doi: 10.1016/j.foodchem.2024.140773
97. Han, X, Zhou, Q, Gao, Z, Lin, X, Zhou, K, Cheng, X, et al. In vitro digestion and fecal fermentation behaviors of polysaccharides from Ziziphus jujuba cv. pozao and its interaction with human gut microbiota. Food Res Int. (2022) 162:162. doi: 10.1016/j.foodres.2022.112022
98. Fu, C, Ye, K, Ma, S, Du, H, Chen, S, Liu, D, et al. Simulated gastrointestinal digestion and gut microbiota fermentation of polysaccharides from Agaricus bisporus. Food Chem. (2023) 418:135849. doi: 10.1016/j.foodchem.2023.135849
99. Wu, D-T, Yuan, Q, Guo, H, Fu, Y, Li, F, Wang, S-P, et al. Dynamic changes of structural characteristics of snow Chrysanthemum polysaccharides during in vitro digestion and fecal fermentation and related impacts on gut microbiota. Food Res Int. (2021) 141:141. doi: 10.1016/j.foodres.2020.109888
100. Zhang, Y, Peng, Y, Zhao, L, Zhou, G, and Li, X. Regulating the gut microbiota and Scfas in the Faeces of T2dm rats should be one of antidiabetic mechanisms of Mogrosides in the fruits of Siraitia grosvenorii. J Ethnopharmacol. (2021) 274:274. doi: 10.1016/j.jep.2021.114033
101. Koh, A, De Vadder, F, Kovatcheva-Datchary, P, and Bäckhed, F. From dietary Fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
102. Liu, J, Liu, Y, Huang, C, He, C, Yang, T, Ren, R, et al. Quercetin-driven Akkermansia muciniphila alleviates obesity by modulating bile acid metabolism via an Ila/M6a/Cyp8b1 signaling. Adv Sci. (2025) 12:865. doi: 10.1002/advs.202412865
103. Gao, K-X, Peng, X, Wang, J-Y, Wang, Y, Pei, K, Meng, X-L, et al. In vivo absorption, in vitro simulated digestion and fecal fermentation properties of polysaccharides from Pinelliae rhizoma praeparatum cum alumine and their effects on human gut microbiota. Int J Biol Macromol. (2024) 266:391. doi: 10.1016/j.ijbiomac.2024.131391
104. Zhou, X, Zhang, Z, Huang, F, Yang, C, and Huang, Q. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from flaxseed. Molecules. (2020) 25:354. doi: 10.3390/molecules25194354
105. Cheng, Y, Tian, S, Chen, Y, Xie, J, Hu, X, Wang, Y, et al. Structural characterization and in vitro fermentation properties of polysaccharides from Polygonatum cyrtonema. Int J Biol Macromol. (2024) 258:258. doi: 10.1016/j.ijbiomac.2023.128877
106. Chen, L, Liu, J, Ge, X, Xu, W, Chen, Y, Li, F, et al. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from Helicteres angustifolia L. Int J Biol Macromol. (2019) 141:1065–71. doi: 10.1016/j.ijbiomac.2019.09.073
107. Wu, D-T, Fu, Y, Guo, H, Yuan, Q, Nie, X-R, Wang, S-P, et al. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: dynamic changes in physicochemical properties and impacts on human gut microbiota. Int J Biol Macromol. (2021) 168:733–42. doi: 10.1016/j.ijbiomac.2020.11.130
108. Liu, Z, Li, L, Ma, S, Ye, J, Zhang, H, Li, Y, et al. High-dietary Fiber intake alleviates antenatal obesity-induced postpartum depression: roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J Agric Food Chem. (2020) 68:13697–710. doi: 10.1021/acs.jafc.0c04290
109. Su, L, Xin, C, Yang, J, Dong, L, Mei, H, Dai, X, et al. A polysaccharide from Inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus. Int J Biol Macromol. (2022) 214:312–23. doi: 10.1016/j.ijbiomac.2022.06.071
110. Chen, G, Wang, M, Zeng, Z, Xie, M, Xu, W, Peng, Y, et al. Fuzhuan brick tea polysaccharides serve as a promising candidate for remodeling the gut microbiota from colitis subjects in vitro: fermentation characteristic and anti-inflammatory activity. Food Chem. (2022) 391:133203. doi: 10.1016/j.foodchem.2022.133203
111. Bai, Y, Zhou, Y, Zhang, R, Chen, Y, Wang, F, and Zhang, M. Gut microbial fermentation promotes the intestinal anti-inflammatory activity of Chinese yam polysaccharides. Food Chem. (2023) 402:4003. doi: 10.1016/j.foodchem.2022.134003
112. Wang, Q, Chen, H, Yin, M, Cheng, X, Xia, H, Hu, H, et al. In vitro digestion and human gut microbiota fermentation of Bletilla striata polysaccharides and oligosaccharides. Front Cell Infect Microbiol. (2023) 13:335. doi: 10.3389/fcimb.2023.1105335
113. Chen, L, Wang, Y, Liu, J, Hong, Z, Wong, K-H, Chiou, J-C, et al. Structural characteristics and in vitro fermentation patterns of polysaccharides from boletus mushrooms. Food Funct. (2023) 14:7912–23. doi: 10.1039/d3fo01085f
114. Yuan, M, Ke, S, Wang, A, Wang, X, Zhuang, M, Ning, M, et al. Changes in physicochemical and gut microbiota fermentation property induced by acetylation of polysaccharides from Cyperus esculentus. Int J Biol Macromol. (2024) 267:1172. doi: 10.1016/j.ijbiomac.2024.131172
115. Chang, C-J, Lin, C-S, Lu, C-C, Martel, J, Ko, Y-F, Ojcius, DM, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. (2015) 6:8489. doi: 10.1038/ncomms8489
116. Li, H-Y, Zhou, D-D, Gan, R-Y, Huang, S-Y, Zhao, C-N, Shang, A, et al. Effects and mechanisms of probiotics, prebiotics, Synbiotics, and Postbiotics on metabolic diseases targeting gut microbiota: a narrative review. Nutrients. (2021) 13:211. doi: 10.3390/nu13093211
117. Huang, Y, Hu, J, Xia, Q, Tang, M, Wang, Y, Wang, G, et al. Amelioration of obesity and inflammation by polysaccharide from unripe fruits of raspberry via gut microbiota regulation. Int J Biol Macromol. (2024) 261:129825. doi: 10.1016/j.ijbiomac.2024.129825
118. Lai, Y, Yu, H, Deng, H, Fang, Q, Lei, H, Liu, L, et al. Three main metabolites from Wolfiporia cocos (F. A. Wolf) Ryvarden & Gilb regulate the gut microbiota in mice: a comparative study using microbiome-metabolomics. Front Pharmacol. (2022) 13:1140. doi: 10.3389/fphar.2022.911140
119. Zhu, L, Ye, C, Hu, B, Xia, H, Bian, Q, Liu, Y, et al. Regulation of gut microbiota and intestinal metabolites by Poria Cocos oligosaccharides improves glycolipid metabolism disturbance in high-fat diet-fed mice. J Nutr Biochem. (2022) 107:107. doi: 10.1016/j.jnutbio.2022.109019
120. Lai, Y, Deng, H, Chen, M, Fan, C, Chen, Y, Wang, F, et al. In vitro fermentation properties of grape seed polysaccharides and the effect on regulating gut microbiota in mice. J Food Meas Charact. (2023) 17:5506–17. doi: 10.1007/s11694-023-02058-5
121. Chen, P, Chen, X, Hao, L, Du, P, Li, C, Han, H, et al. The bioavailability of soybean polysaccharides and their metabolites on gut microbiota in the simulator of the human intestinal microbial ecosystem (Shime). Food Chem. (2021) 362:362. doi: 10.1016/j.foodchem.2021.130233
122. Liu, C, Du, P, Cheng, Y, Guo, Y, Hu, B, Yao, W, et al. Study on fecal fermentation characteristics of Aloe polysaccharides in vitro and their predictive modeling. Carbohydr Polym. (2021) 256:117571. doi: 10.1016/j.carbpol.2020.117571
123. Kanwal, S, Joseph, TP, Owusu, L, Xiaomeng, R, Meiqi, L, and Yi, X. A polysaccharide isolated from Dictyophora Indusiata promotes recovery from antibiotic-driven intestinal Dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients. (2018) 10:1003. doi: 10.3390/nu10081003
124. Li, S, Qi, Y, Chen, L, Qu, D, Li, Z, Gao, K, et al. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int J Biol Macromol. (2019) 124:931–7. doi: 10.1016/j.ijbiomac.2018.11.271
125. Chen, C, Guan, X, Liu, X, Zhuang, W, Xiao, Y, Zheng, Y, et al. Polysaccharides from bamboo shoot (Leleba oldhami Nakal) byproducts alleviate antibiotic-associated diarrhea in mice through their interactions with gut microbiota. Foods. (2022) 11:647. doi: 10.3390/foods11172647
126. Bie, N, Duan, S, Meng, M, Guo, M, and Wang, C. Regulatory effect of non-starch polysaccharides from purple sweet potato on intestinal microbiota of mice with antibiotic-associated diarrhea. Food Funct. (2021) 12:5563–75. doi: 10.1039/d0fo03465g
127. Lai, Y, Deng, H, Fang, Q, Ma, L, Lei, H, Guo, X, et al. Water-insoluble polysaccharide extracted from Poria Cocos alleviates antibiotic-associated diarrhea based on regulating the gut microbiota in mice. Foods. (2023) 12:3080. doi: 10.3390/foods12163080
128. Wang, G, Xie, L, Huang, Z, and Xie, J. Recent advances in polysaccharide biomodification by microbial fermentation: production, properties, bioactivities, and mechanisms. Crit Rev Food Sci Nutr. (2023) 64:12999–3023. doi: 10.1080/10408398.2023.2259461
129. Ren, Y, Sun, Q, Gao, R, Sheng, Y, Guan, T, Li, W, et al. Low weight polysaccharide of Hericium erinaceus ameliorates colitis via inhibiting the Nlrp3 Inflammasome activation in association with gut microbiota modulation. Nutrients. (2023) 15:739. doi: 10.3390/nu15030739
130. Su, J, Li, D, Chen, Q, Li, M, Su, L, Luo, T, et al. Anti-breast Cancer enhancement of a polysaccharide from spore of Ganoderma lucidum with paclitaxel: suppression on tumor metabolism with gut microbiota reshaping. Front Microbiol. (2018) 9:9. doi: 10.3389/fmicb.2018.03099
131. Guo, C, Guo, D, Fang, L, Sang, T, Wu, J, Guo, C, et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in Colon. Carbohydr Polym. (2021) 267:118231. doi: 10.1016/j.carbpol.2021.118231
132. Sun, S-S, Wang, K, Ma, K, Bao, L, and Liu, H-W. An insoluble polysaccharide from the Sclerotium of Poria Cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in Ob/Ob mice via modulation of gut microbiota. Chin J Nat Med. (2019) 17:3–14. doi: 10.1016/s1875-5364(19)30003-2
133. Wang, T, Han, J, Dai, H, Sun, J, Ren, J, Wang, W, et al. Polysaccharides from Lyophyllum decastes reduce obesity by altering gut microbiota and increasing energy expenditure. Carbohydr Polym. (2022) 295:295. doi: 10.1016/j.carbpol.2022.119862
134. Rehman, AU, Siddiqui, NZ, Farooqui, NA, Alam, G, Gul, A, Ahmad, B, et al. Morchella esculenta mushroom polysaccharide attenuates diabetes and modulates intestinal permeability and gut microbiota in a type 2 diabetic mice model. Front Nutr. (2022) 9:9. doi: 10.3389/fnut.2022.984695
Glossary
AAD - Antibiotic-associated diarrhea
ACEPI - Acetylated Cyperus esculentus polysaccharide
ACP - Agrocybe cylindracea polysaccharide
AF4 - Asymmetric flow field-flow fractionation
ABP - Agaricus bisporus polysaccharide
BRP2-2 - Brassica rapa L. polysaccharide fraction 2–2
CAZymes - Carbohydrate-active enzymes
CEPI - Native Cyperus esculentus polysaccharide
C0X-2 - Cyclooxygenase-2
CYP - Chinese yam polysaccharide
DIP - Dictyophora indusiate polysaccharide
EPS - Extracellular polysaccharides
FBTPS-3 - Fuzhuan brick tea polysaccharide fraction 3
FVP - Flammulina velutipes polysaccharide
GLP - Ganoderma lucidum polysaccharide
GP - Grape seed polysaccharide
HEP10 - Hericium erinaceus low-molecular-mass polysaccharide
HPs - Herbal polysaccharides
ICP - Imperata cylindrica polysaccharide
IN - Inonotus obliquus polysaccharide
JPS - Ziziphus jujuba cv. Pozao polysaccharide
KFP - Kiwifruit pomace polysaccharide
LLP - Loquat leaf polysaccharide
LPF - Lactobacillus fermentum polysaccharide
LPS - Lipopolysaccharide
MALDI-TOF MS - Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
MALLS - Multi-angle laser light scattering
MEP - Morchella esculenta polysaccharide
NCVP - Nostoc commune Vauch. polysaccharide
ORP - Oudemansiella radicata polysaccharide
PCP - Polygonatum cyrtonema polysaccharide
PKPS - Polygonatum kingianum polysaccharide
PPA - Pinelliae Rhizoma Praeparatum Cum Alumine polysaccharide
PSPP - Purple sweet potato polysaccharide
RI - Refractive index
SC - Size-exclusion chromatography
SCFA - Short-chain fatty acid
SGP - Ganoderma lucidum spore polysaccharide
SHIME - Simulator of the human intestinal microbial ecosystem
THDP2 - Tetrastigma hemsleyanum polysaccharide fraction 2
TLR4 - Toll-like receptor 4
VVP - Volvariella volvacea polysaccharide
WGP - Panax ginseng polysaccharide
WIP - Water-insoluble Poria cocos polysaccharide
Keywords: dietary fiber, gut microbiota, short chain fatty acid, in vitro fermentation, prebiotic, functional food
Citation: Lai Y, Wang Y, Liu C, Lou G, Feng J and Li Y (2025) In vitro fermentation characteristics and prebiotic activity of herbal polysaccharides: a review. Front. Nutr. 12:1687766. doi: 10.3389/fnut.2025.1687766
Edited by:
Barbara E. Teixeira-Costa, Fluminense Federal University, BrazilReviewed by:
Jayvadan Jayantilal Vaishnav, Parul University, IndiaZichao Wang, Henan University of Technology, China
Carolyne Pimentel Rosado, Federal University of the State of Rio de Janeiro, Brazil
Copyright © 2025 Lai, Wang, Liu, Lou, Feng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Li, bGl5aW5nMTgzMjg1OUAxNjMuY29t
 Yong Lai
Yong Lai Yu Wang
Yu Wang