- 1College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Maternity and Child Health Hospital, Fujian Medical University, Fuzhou, Fujian, China
- 2Fujian Medical University, Fuzhou, Fujian, China
- 3Fujian Clinical Research Center for Maternal-Fetal Medicine, Fuzhou, Fujian, China
Objective: To study the potential correlation between pregnancy weight gain (WG) and the incidence of gestational diabetes mellitus (GDM).
Methods: Clinical records of women with singleton pregnancies who had a first visit at Fujian Maternity and Child Health Hospital before 14 weeks and delivered after 28 weeks were retrospectively analyzed. Based on the first trimester WG, the participants were grouped as inadequate (iWG-F), adequate (aWG-F), and excessive (eWG-F) WG groups. The outcomes of interest included GDM, gestational hypertension, preeclampsia, small for gestational age (SGA), LGA, low birth weight (LBW), preterm birth, macrosomia, primarily cesarean section (CS), and admission to the neonatal intensive care unit (NICU). Statistical analyses included logistic regression, interaction, and mediation analyses.
Results: A total of 16,824 pregnancies were analyzed. GDM incidences of the iWG-F, aWG-F, and eWG-F groups were 24.53%, 26.62%, and 29.46%, respectively, with a statistically significant difference (p < 0.001). Multivariable logistic regression showed that inadequate WG correlated with reduced risk of GDM when adjusted for pre-pregnancy body mass index (PPBMI) of below 18.5 kg/m2 [adjusted odds ratio (aOR) = 0.68], and 18.5–23.9 kg/m2 (aOR = 0.88). The association of inadequate WG and reduced risk of GDM persisted when adjusted for age <30.5 years (aOR = 0.81), fasting glucose ≥4.9 mmol/L (aOR = 0.74), triglycerides <1.4 mmol/L (aOR = 0.84), and HDL <1.63 mmol/L (aOR = 0.85). WG in the second trimester was associated with GDM (β = −0.003, p = 0.036) and partially mediated the effect of eWG-F (−3.7% of the total effect). WG before OGTT showed no association with GDM.
Conclusion: First trimester WG is significantly associated with the occurrence of GDM. In contrast, there is only a minimal association between second-trimester and pre-OGTT WG and the risk of GDM. Inadequate first-trimester weight gain reduces GDM risk, especially in younger women, women with normal or low PPBMI, elevated fasting glucose, and low HDL or triglycerides, without increasing abnormal neonatal birth weight. Early pregnancy represents a critical window for GDM prevention. Minimal weight gain during this period may be a feasible and acceptable approach to reducing GDM risk.
Introduction
Gestational diabetes mellitus (GDM) impacts around 14% of pregnant women worldwide (1, 2). A 2018 systematic review reported that a pooled GDM prevalence in mainland China was as high as 14.8% (3), rising to 21% in some regions by 2021 (4). GDM is associated with numerous short-term adverse maternal and offspring outcomes and long-term health risks, including pregnancy-related hypertension, preterm birth, cesarean delivery, large for gestational age (LGA), etc. (5–10). The diagnosis of GDM is typically established between 24 and 28 weeks, and the screening mostly focuses on late gestational diabetes (11). However, recent research suggests that the metabolic changes underlying GDM, such as insulin resistance (12), impaired insulin secretion, or relative insulin deficiency (13, 14), may be detectable as early as the first trimester (before 14 weeks of gestation). This early metabolic dysfunction indicates that early pregnancy could be a crucial period for reducing the incidence of late GDM and its associated complications. Randomized controlled trials suggest that lifestyle interventions with a mean weight gain (WG) reduction of −0.76 kg (95% CI: −1.55, 0.03) during early to mid-pregnancy can reduce the incidence of GDM (15–17). However, the impact of maternal WG during early and mid-pregnancy on GDM risk remains unclear, which prevents the development of effective early preventive strategies. This study aims to study the connection between maternal WG in the first trimester (WG-F), the second trimester (WG-S), and weight gain (WG) before the oral glucose tolerance test (OGTT) and the incidence of late gestational diabetes, providing evidence to guide interventions for improving maternal and neonatal health outcomes.
Methods
Study design
This retrospective cohort study, done at Fujian Maternity and Child Health Hospital, spanned two outpatient and inpatient locations in the Gulou and Jinan Districts of Fuzhou, China. Training personnel used electronic medical records to collect participants’ information from their first visit to 3 days postpartum, including weight, medical, and obstetrical data gathered from personal weight records, antenatal clinic visits, and delivery admissions. Medical data were extracted from the healthcare system in August 2024 for analysis. This study received approval from the Ethics Committee of the hospital (2024KY200) and adhered to the Declaration of Helsinki principles (World Medical Association Declaration of Helsinki). No informed consent was needed, as the study utilized anonymized historical medical records data.
Study population
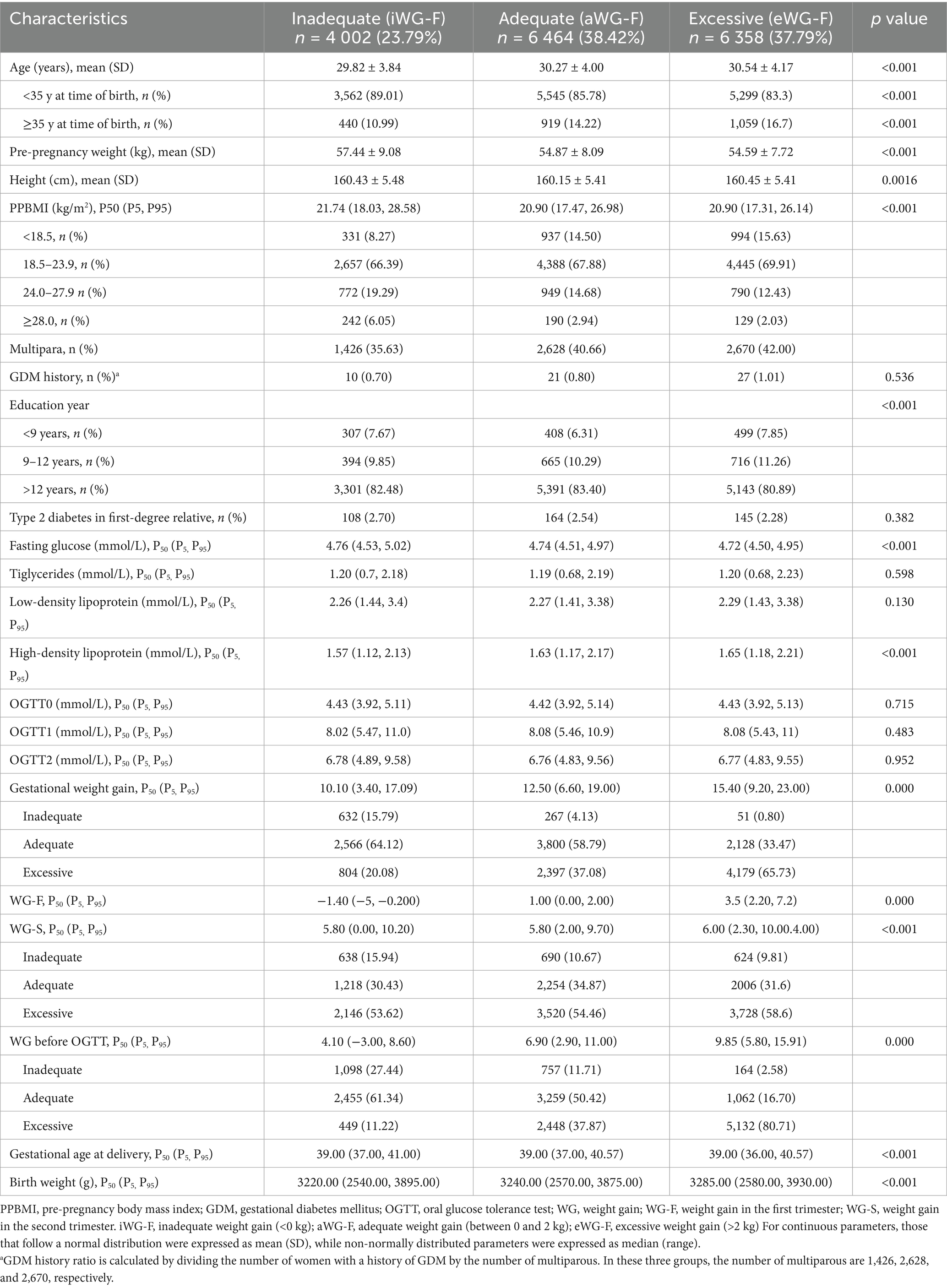
Patients were enrolled from January 2021 to December 2023. The inclusion criteria were: (1) first visit before 14 gestational weeks, (2) singleton pregnancy, and (3) delivery after 28 weeks. Exclusion criteria were: (1) pre-existing chronic conditions such as heart, kidney, or thyroid disease, type 1 or type 2 diabetes, hypertension, (2) non-compliance with weight monitoring, (3) incomplete documented medical records, (4) OGTT not performed between 24 and 28 weeks, and (5) missing blood test results in the first trimester. According to the weight monitoring and evaluation standards for pregnant women by the Chinese Nutrition Society (18), individuals were categorized into three groups based on WG-F: weight gain <0 kg as inadequate (iWG-F), weight gain between 0 and 2 kg as adequate (aWG-F), and weight gain >2 kg as excessive (eWG-F). (Figure 1).
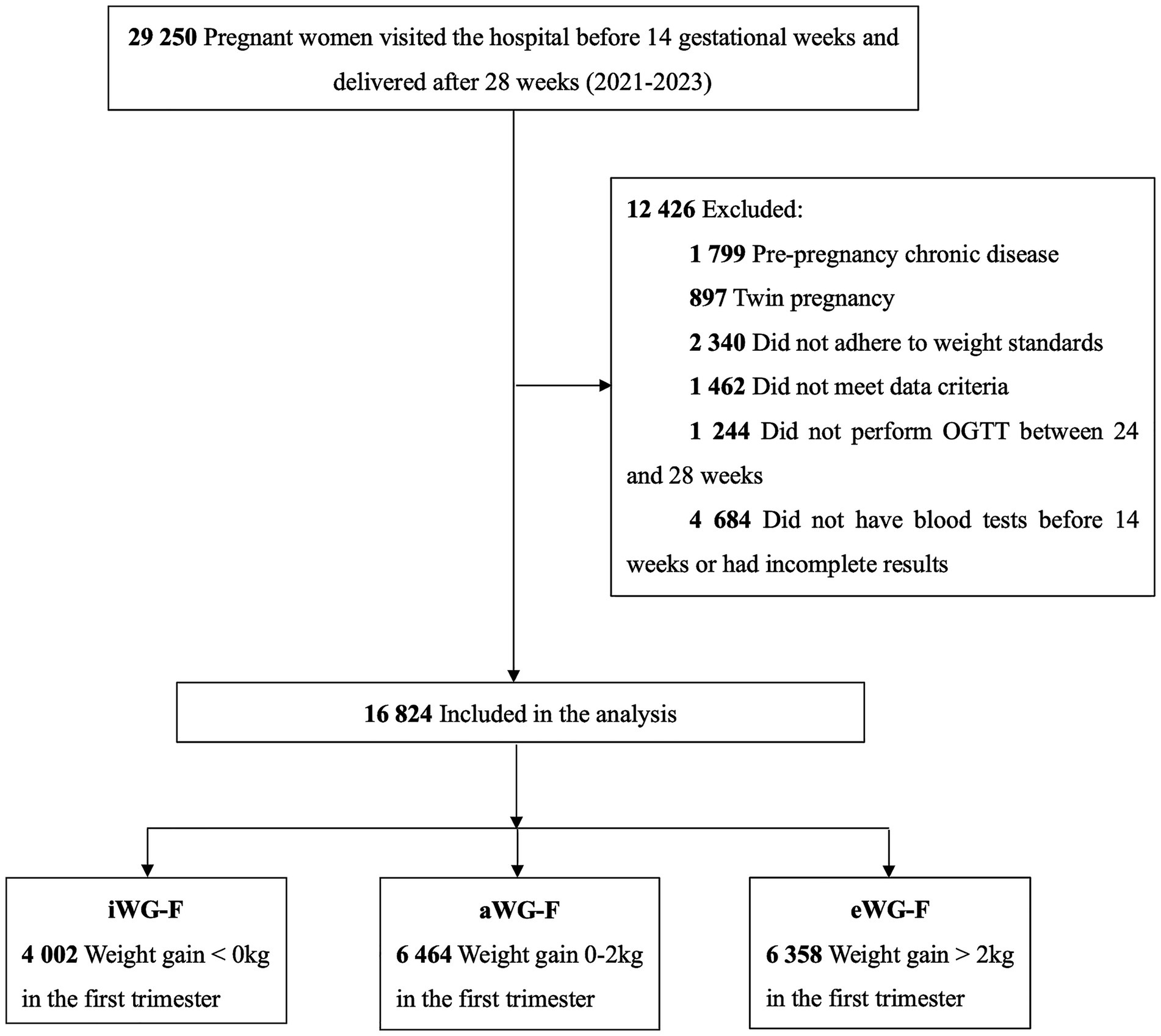
Figure 1. Flow diagram of study participants. iWG-F, inadequate weight gain in the first trimester; aWG-F, adequate weight gain in the first trimester; eWG-F, excessive weight gain in the first trimester.
Measurements
Weight before pregnancy was self-reported at the initial hospital visit, with concurrent height measurements. Throughout pregnancy, women self-recorded their weight at home on the last day of the 13th week and each subsequent gestational week during the second and third trimesters, following standardized procedures: taken in the morning, after voiding, on an empty stomach, using a precise electronic scale with 0.1 kg accuracy. Trained researchers entered these measurements into the electronic medical record system during subsequent prenatal visits. Pre-delivery weight was the last recorded weight measured at home before delivery or at the hospital at the time of delivery. Gestational weight gain (GWG), WG-F, WG-S, and WG before the OGTT were calculated as follows: GWG, weight just before the delivery - weight before pregnancy; WG-F, weight at the end of 13 weeks - pre-pregnancy weight; WG-S, weight at the end of the week before the OGTT - weight at the end of 13 weeks; and WG before the OGTT, weight at the end of the week before the OGTT - pre-pregnancy weight. The pre-pregnancy body mass index (PPBMI), pre-pregnancy weight (kg)/height (m) squared. For the weight gain, weight gain <0 kg was defined as inadequate (iWG-F), weight gain between 0 and 2 kg was defined as adequate (aWG-F), and weight gain >2 kg was considered excessive (eWG-F).
Outcomes
The main outcome was GDM. The secondary outcomes included gestational hypertension, preeclampsia, small for gestational age (SGA), large for gestational age (LGA), low birth weight (LBW), preterm birth, macrosomia, primarily cesarean section (CS), and hospitalization in the neonatal intensive care unit (NICU).
Lifestyle interventions
All pregnant women were guided to adopt healthy dietary and lifestyle habits during gestation. Women received routine prenatal care and dietary guidance from obstetricians and nutritionists at least three times: during the initial visit, mid-pregnancy, and late pregnancy, based on the 2016 Chinese Dietary Guidelines for Pregnant Women (19).
Energy intake was adjusted according to the woman’s pre-pregnancy BMI, physical activity levels, and GWG. In early pregnancy, women without significant morning sickness were advised to maintain a balanced pre-pregnancy diet. At the same time, those with poor appetite or vomiting were recommended to consume a daily minimum of 130 g of carbohydrates, allowing flexibility in food choices. Women with severe hyperemesis gravidarum were advised to seek medical attention. During mid-to-late pregnancy, a daily intake of 300–500 g of dairy products and 150–250 g of fish, poultry, eggs, or lean meat was recommended, with deep-sea fish consumption encouraged 2–3 times per week. Women were advised to take multivitamins containing 0.8 mg of folic acid, 60 mg of iron, and vitamin D as needed, and to engage in moderate-intensity aerobic exercise for 30–40 min, 3–5 times per week, which could be divided into shorter sessions throughout the day.
Statistical analysis
Studio version 2024.04.2 (Posit Software, PBC) was used for all analyses, including t-tests, chi-square tests, cut-off value calculations, logistic regressions, and interaction and mediation. Continuous parameters that follow a normal distribution were expressed as mean ± standard deviation (SD), while non-normally distributed parameters were expressed as medians (range). Forest plots were generated with Prism version 10.2.0 (GraphPad Software, San Diego, CA, USA). Baseline characteristics were compared across the three categories based on the weight gain in the first trimester. Logistic regression was used to analyze the associations between WG categories (iWG-F and eWG-F) and 10 short-term pregnancy outcomes, using aWG-F as the reference group. Covariates included PPBMI, maternal age, delivery weeks, parity, and GWG, except for preterm birth analysis, where delivery weeks were excluded. For GDM analysis, univariate logistic regression calculated odds ratios (ORs) and 95% confidence intervals (CIs) for 13 clinical characteristics. Multivariable logistic regression estimated adjusted odds ratios (aORs) and 95% CIs.
Logistic regression was used to assess the relationships between WG-F, WG-S, and WG before OGTT in patients with GDM. Interaction and mediation analyses explored potential pathways, adjusting for maternal age, PPBMI, parity, education level, gestational age at delivery, fasting glucose, triglycerides, and HDL levels in early pregnancy. Subgroup analyses were performed by stratifying participants by age, PPBMI, fasting glucose, triglycerides, and HDL, with subgroup-specific factors excluded from the confounding variables.
Results
Participants’ characteristics
In total, 16,824 pregnancies were enrolled in this study. Of them, 4,002, 6,464, and 6,358 pregnant women demonstrated iWG-F, aWG-F, and eWG-F, respectively, with median weight changes of −1.40, 1.00, and 3.50 kg. Women in the iWG-F group were the youngest, had the highest pre-pregnancy weight and PPBMI, and exhibited the smallest WG-S, WG before OGTT, and total gestational weight gain (all p < 0.001). Women in the eWG-F group had the most significant proportion of multiparous women and the lowest educational levels (<9 years) (p < 0.001). Early pregnancy blood test results indicated that women in the iWG-F group had the highest fasting glucose and the lowest HDL levels (all p < 0.001). No significant differences were observed among the three groups in the prevalence of a history of GDM, type 2 diabetes in first-degree relatives, triglyceride levels, low-density lipoprotein levels in the first trimester, or OGTT results. Detailed baseline characteristics are presented in Table 1.
First trimester WG and pregnancy outcomes
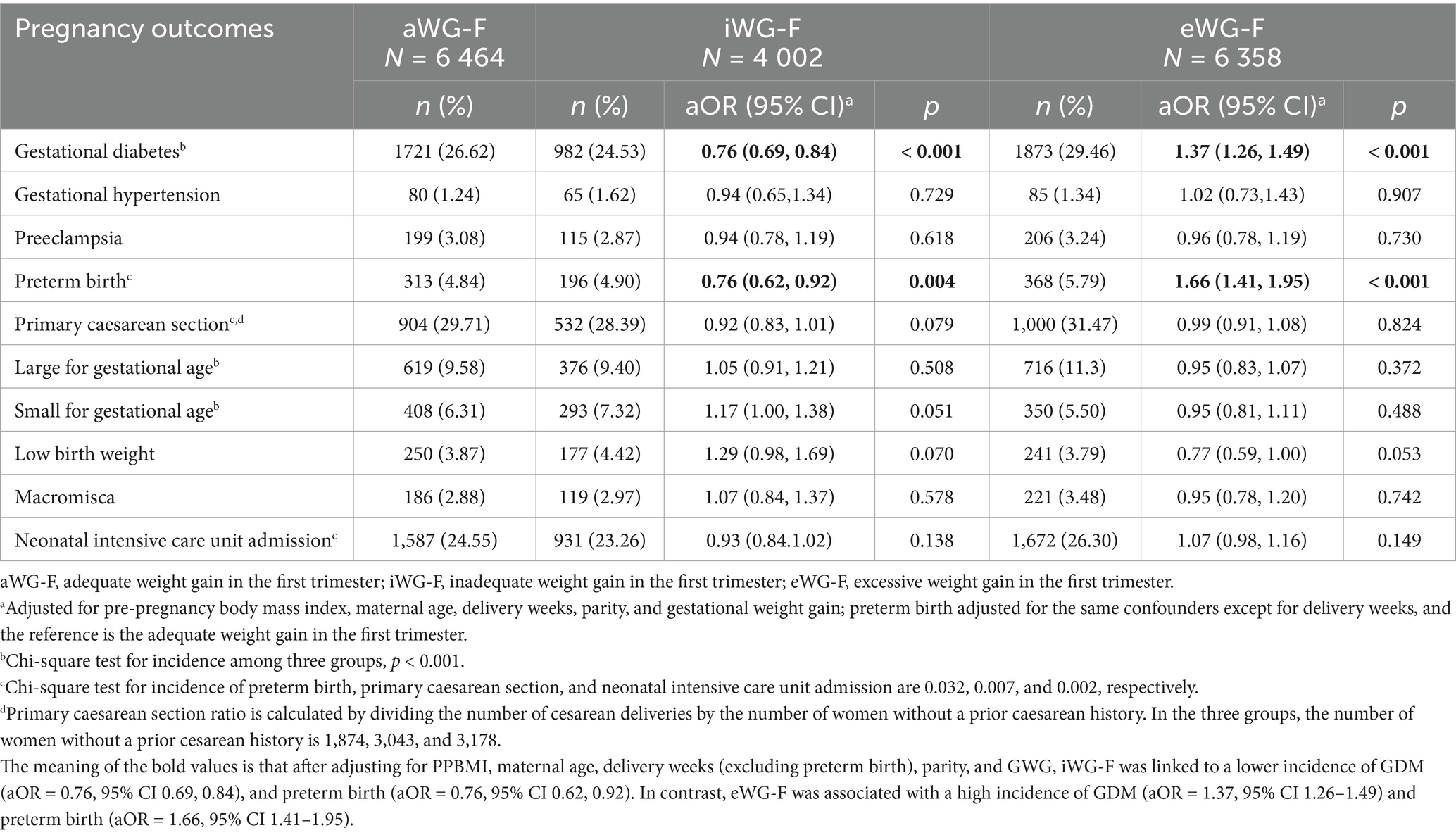
Among the three groups, iWG-F had the lowest incidence of GDM (24.53%), primary CS (28.39%), LGA (9.40%), and NICU admission (23.26%). In contrast, this group reported the highest incidence of SGA (7.32%). In contrast, eWG-F had the highest incidence of GDM (29.5%), preterm birth (5.79%), LGA (11.3%), and NICU admission (26.30%) but the lowest incidence of SGA (5.50%) (all p < 0.05). After adjusting for PPBMI, maternal age, delivery weeks (excluding preterm birth), parity, and GWG, iWG-F was linked to a lower incidence of GDM (aOR = 0.76, 95% CI 0.69, 0.84), and preterm birth (aOR = 0.76, 95% CI 0.62, 0.92). In contrast, eWG-F was associated with a high incidence of GDM (aOR = 1.37, 95% CI 1.26–1.49) and preterm birth (aOR = 1.66, 95% CI 1.41–1.95). WG-F had no significant association with gestational hypertension, preeclampsia, primary CS, NICU admission, or newborn weight (Table 2).
Univariate analysis of clinical factors associated with GDM risk
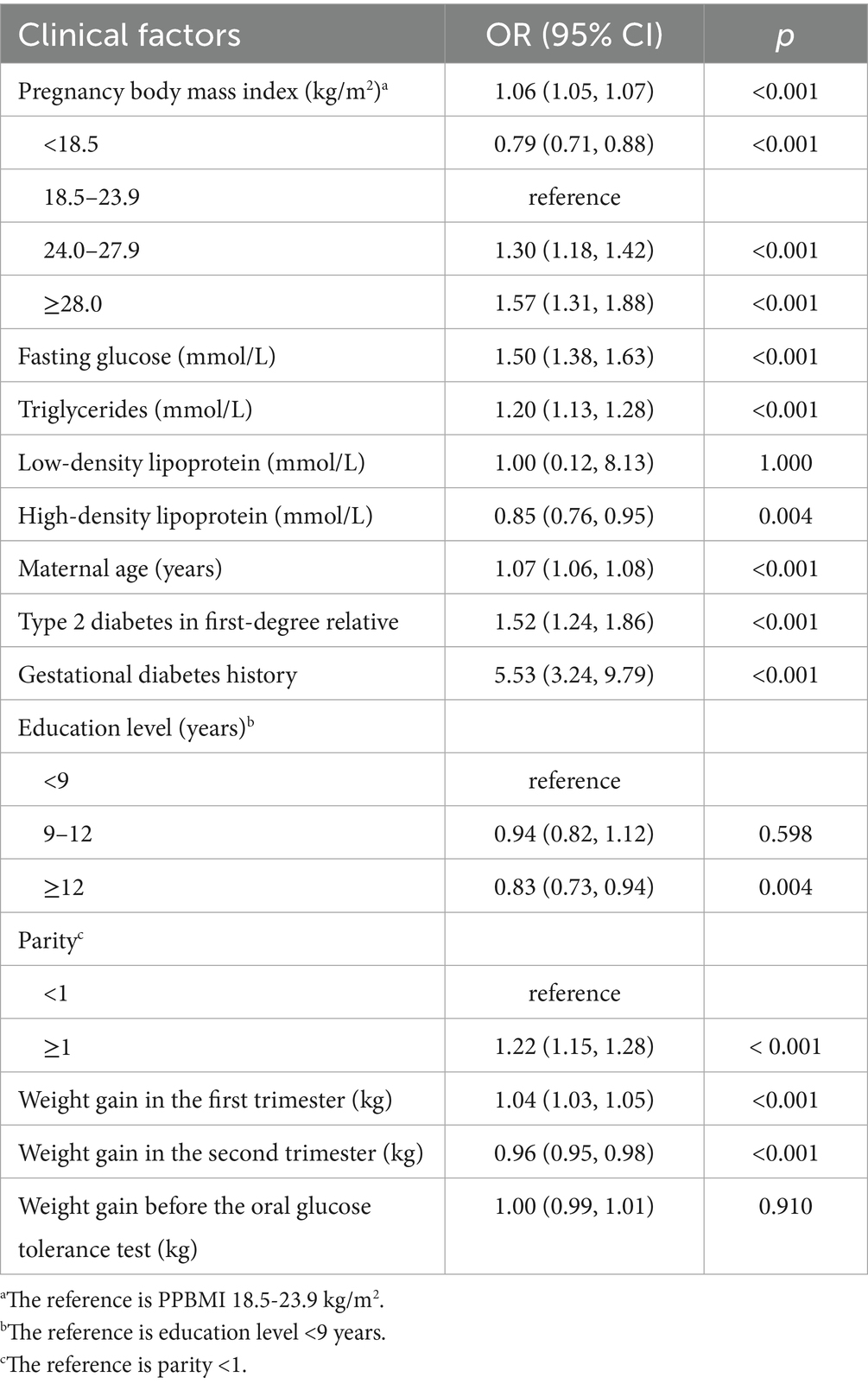
Factors, significantly associated with an elevated risk of GDM, included PPBMI (OR = 1.06, 95% CI 1.05, 1.07), maternal age (OR = 1.07, 95% CI 1.06, 1.08), parity ≥1 (OR = 1.22, 95% CI 1.15, 1.28), fasting glucose (OR = 1.50, 95% CI 1.38, 1.63) and triglycerides levels (OR = 1.20, 95% CI 1.13, 1.28) in early pregnancy, type 2 diabetes in first-degree relative (OR = 1.52, 95% CI 1.24, 1.86), a history of GDM (OR = 5.53, 95% CI 3.24, 9.79), and WG-F (OR = 1.04, 95% CI 1.03, 1.05). Conversely, WG-S (OR = 0.96, 95% CI 0.95, 0.98), HDL levels (OR = 0.85, 95% CI 0.76, 0.95), and having 12 or more years of education (OR = 0.83, 95% CI 0.73, 0.94) were linked to a reduced risk of GDM. WG before OGTT had no significant association with GDM (p = 0.910) (Table 3). The cutoff values were 30.5 years for maternal age, 1.4 mmol/L for triglycerides, 4.9 mmol/L for fasting glucose, and 1.63 mmol/L for HDL.
Association of trimester-specific weight gain with GDM incidence across subgroups
Among all participants, using adequate weight gain as the reference, iWG-F was associated with a 14% decrease in GDM incidence (aOR = 0.86, 95% CI 0.78, 0.94) after adjusting for clinical factors significantly associated with GDM identified in the univariate analysis. In contrast, the eWG-F group showed an 18% increase in GDM incidence (aOR = 1.18, 95% CI 1.08, 1.29). Neither WG before OGTT nor WG-S showed a significant association with GDM (p > 0.05).
In the subgroup analysis, iWG-F was associated with a 32%, 12%, 19%, 26%, 16%, and 15% reduction in GDM incidence among participants with a PPBMI <18.5 kg/m2 (aOR = 0.68, 95% CI 0.48, 0.97), 18.5–23.9 kg/m2 (aOR = 0.88, 95% CI 0.77, 1.00), age <30.5 years (aOR = 0.81, 95% CI 0.70, 0.92), fasting glucose ≥4.9 mmol/L (aOR = 0.74, 95% CI 0.63, 0.87), triglyceride <1.4 mmol/L (aOR = 0.84, 95% CI 0.75, 0.95), and HDL <1.63 mmol/L (aOR = 0.85, 95% CI 0.75, 0.97), respectively (Figure 2). eWG-F was associated with an 18%, 101%, and 23% increase in GDM incidence for participants with a PPBMI of 18.5–23.9 kg/m2 (aOR = 1.18, 95% CI 1.06, 1.31), ≥28.0 kg/m2 (aOR = 2.01, 95% CI 1.15, 3.70), and those aged ≥30.5 years (aOR = 1.23, 95% CI 1.09, 1.40), respectively. Regardless of fasting glucose, triglyceride, or HDL levels in early pregnancy, eWG-F consistently increased the risk of GDM (all p < 0.05) (Figure 2). WG-S was not significantly associated with GDM incidence in any subgroup. However, among participants with a PPBMI <18.5 kg/m2, excessive WG before OGTT was linked to a decreased incidence of GDM (aOR = 0.67, 95% CI 0.49, 0.91), and this association was not observed in other subgroups (Figure 2).
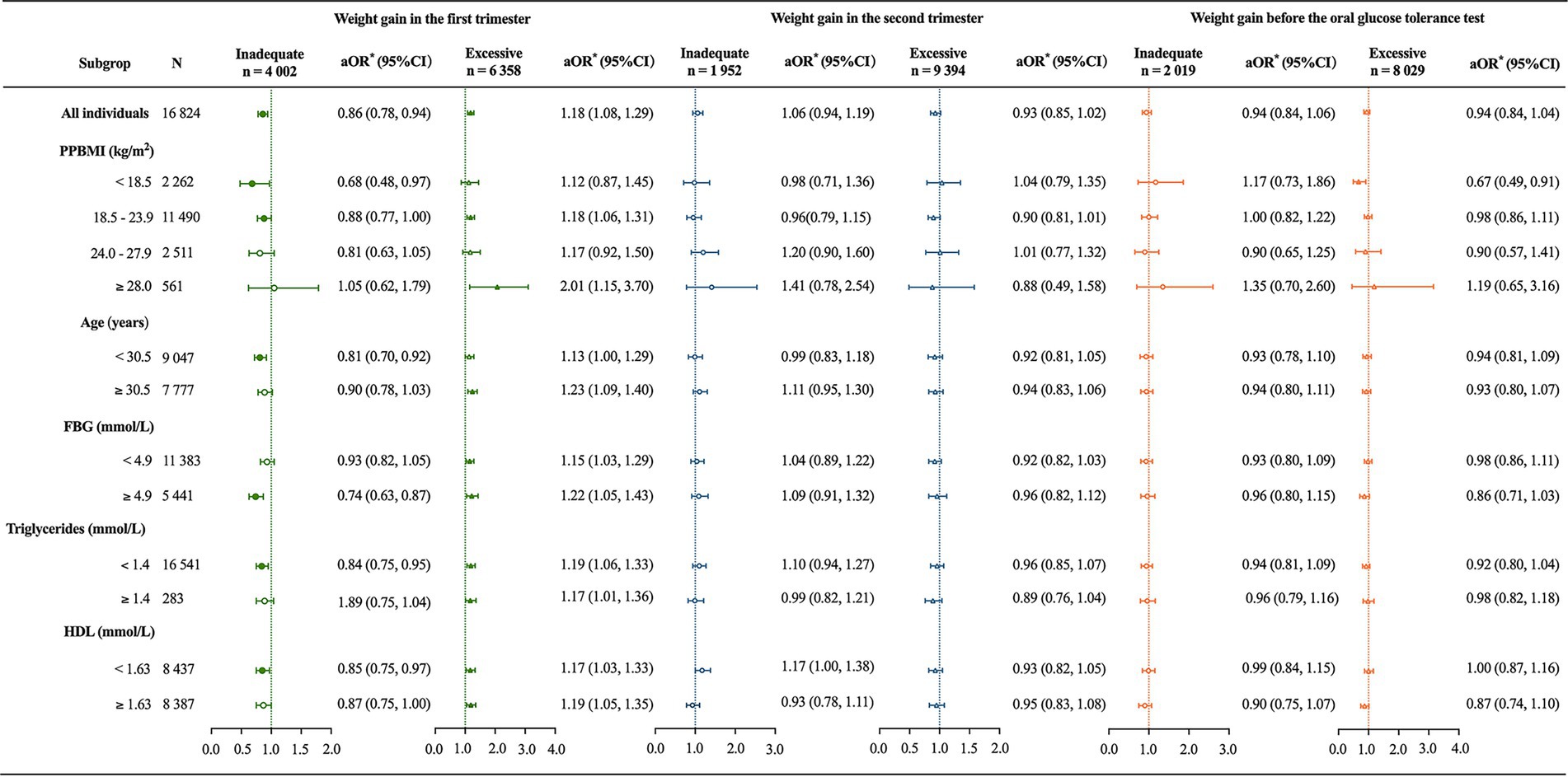
Figure 2. Association of trimester-specific weight gain with gestational diabetes incidence across subgroups. PPBMI, prepregnancy body mass index; FBG, fasting blood glucose; HDL, high-density lipoprotein. *The primary independent variable was categorized weight gain by trimester, and the outcome variable was gestational diabetes mellitus. For all individuals, confounding variables included maternal age, PPBMI, parity, educational level, gestational age at delivery, FBG, triglycerides, and HDL. Subgroup analyses excluded clinical factors specific to each subgroup from the list of confounding variables. Adequate weight gain served as the reference category.
Interaction and mediation analyses in the intermediate model
After adjusting for PPBMI, parity, fasting glucose, triglycerides, HDL, age, family history of diabetes, history of GDM, and education level, WG-F (β = 0.0418, p < 0.001) and WG-S (β = −0.0159, p = 0.02585) were both significantly associated with the occurrence of GDM. The interaction effect between WG-F and WG-S (β = 0.000595, p = 0.69680) was insignificant.
A mediation effect analysis revealed that the regression coefficient for the total effect for iWG-F was −0.027 (95% CI: −0.044, −0.009; p = 0.003). However, the indirect effect was insignificant (p = 0.198). For eWG-F, the mediation effect analysis reported regression coefficient of the total effect of 0.027 (95% CI: 0.012, 0.042; p = 0.001). The regression coefficient of the indirect effect was −0.001 (95% CI: −0.002), also significant (p = 0.000), indicating a partial mediating role of WG-S in the effect of eWG-F on GDM, with an effect proportion of −3.7% (Figure 3).
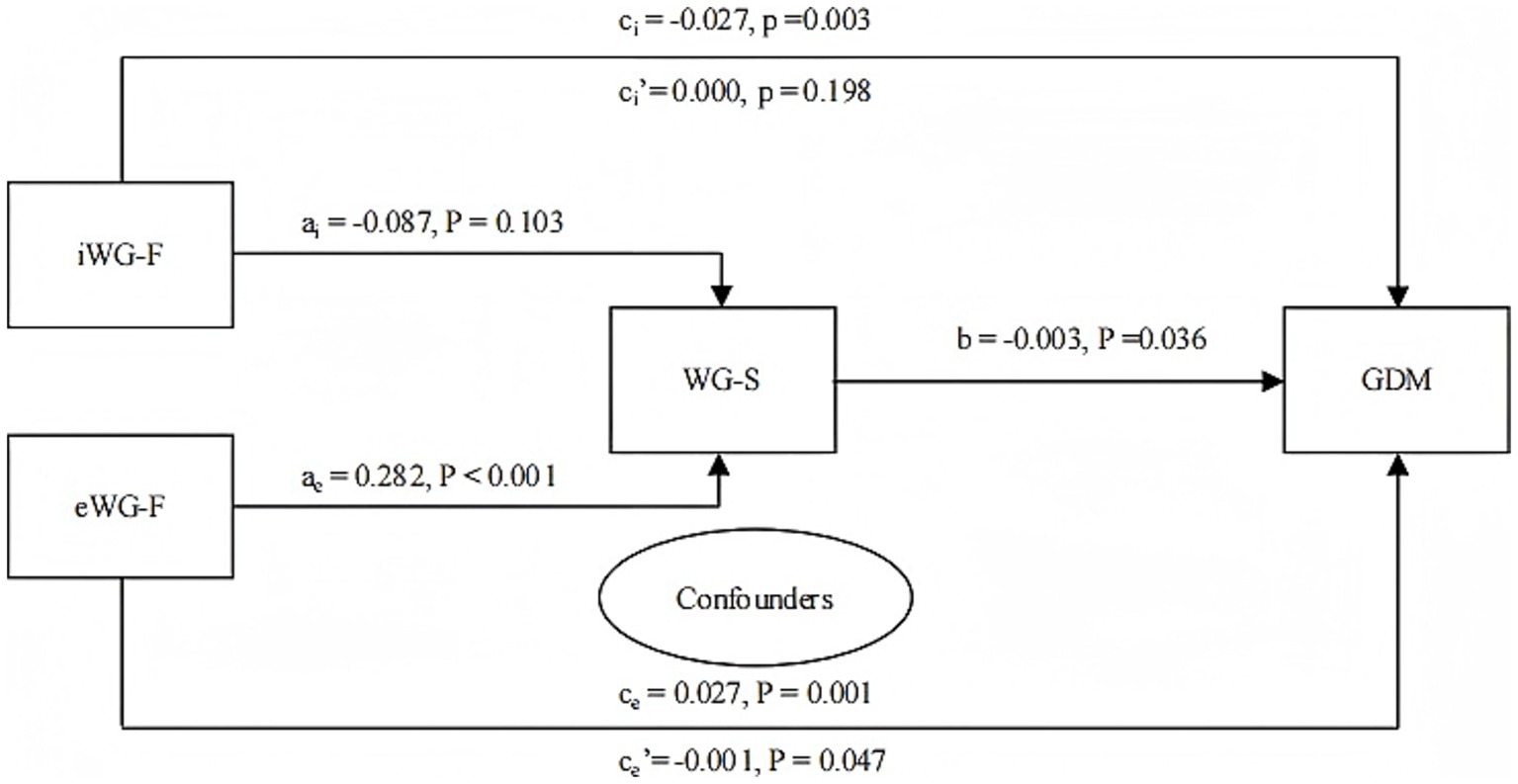
Figure 3. Mediation effect of WG-S on the relationship between iWG-F, eWG-F, and GDM (n = 16,824). iWG-F, inadequate weight gain in the first trimester; eWG-F, excessive weight gain in the first trimester; WG-S, weight gain in the second trimester. GDM, gestational diabetes mellitus. Confounders: Prepregnancy body mass index, parity, fasting glucose, triglycerides, high-density lipoprotein, age, family history of diabetes, history of gestational diabetes, and education level. ai: Regression coefficient of iWG-F vs. WG-S; ae: Regression coefficient of eWG-F vs. WG-S; b: Regression coefficient of WG-S vs. GDM; ci: Total effect, regression coefficient when iWG-F vs. GDM (no mediator variable WG-S in the model); ci’: Indirect effect, regression coefficient when iWG-F vs. GDM (mediator variable WG-S in the model); ce: Total effect, regression coefficient when eWG-F vs. GDM (no mediator variable WG-S in the model); ce’: Indirect effect, regression coefficient when eWG-F vs. GDM (mediator variable WG-S in the model).
Discussion
This study demonstrated a significant association of the first-trimester WG with the incidence of GDM, while no such association was found for the second-trimester and pre-OGTT WG. Inadequate first-trimester weight gain reduces GDM risk, especially in younger women, women with normal or low PPBMI, elevated fasting glucose, and low levels of HDL or triglycerides. This effect does not correlate with abnormal neonatal birth weight.
Excessive WG in the first trimester was shown to increase GDM risk in women with different pre-pregnancy BMI (20–22). However, as reported by Carreno et al. (23) this effect was only observed in women of normal weight. According to Morisset et al. (24) only WG during the first trimester was identified as an independent predictor of GDM, with no association observed for second-trimester weight gain, similarly to other reports (25). Brunner et al. (26) showed that excessive WG before a GDM screening test was linked to higher risk of GDM, while Gibson et al. (27) identified pre-diagnostic WG as a GDM risk factor in overweight or obese women, while no such impact was noted in women of normal BMI or underweight.
In this study, mediation analysis after adjusting for confounders identified eWG-F as a significant predictor of an increased risk of GDM (β = 0.027, p = 0.001). Subgroup analysis further showed a significance of this association in pregnant women with normal weight or obesity [aOR = 1.18 (95% CI 0.61, 1.31) and 2.01 (95% CI 1.15, 3.70), respectively], but not among underweight or overweight women. Excessive WG before OGTT did not lead to increased GDM risk, no matter maternal age, PPBMI, blood glucose, TG, or HDL levels, which confirms the results of Herring et al. (28).
Among women with lower PPBMI, excessive WG before OGTT correlated with lower GDM risk. Although WG-S was not directly associated with GDM incidence in multivariable logistic regression, there still was a significant negative correlation between WG-S and GDM incidence (β = −0.003, p = 0.036) in mediation analysis. Additionally, WG-S partly mediated the link between eWG-F and GDM, accounting for −3.7% of the total effect. Despite the minimal effect size, these findings suggest that although WG-S may not independently influence GDM risk, it can modify the impact of excessive early pregnancy WG on the incidence of GDM and potential differences in glucose metabolism across PPBMI categories and pregnancy stages, as well as the complex relationship between WG and GDM risk. Future studies should investigate the mechanisms of this effect.
Early pregnancy is accompanied by an increase in insulin secretory response that precedes changes in insulin sensitivity (12). Insulin resistance initially decreases before rising in mid-to-late pregnancy (29) to support fetal growth. Women who develop late GDM exhibit insufficient insulin secretion and resistance adaptations, potentially impairing hunger and satiety signals (30, 31), which may increase appetite and lead to excessive WG. Excessive WG in early pregnancy predominantly involves adipose tissue (32, 33), which may impair insulin sensitivity, exacerbate hyperglycemia, and disrupt lipid and protein metabolism (34–36). These findings suggest that excessive early pregnancy WG may be a high-risk factor for late-onset GDM.
Changes in lifestyle, such as dietary adjustment and physical exercise, were shown to reduce the risk of GDM by approximately 20% (37–39). Thangaratinam et al. (40) reviewed 44 randomized controlled trials (7,278 women) and demonstrated that lifestyle interventions significantly reduced gestational WG. This aligns with findings by Hill et al. (41) and Mudd et al. (42). In this study, while health education on balanced diets, weight management, and exercise was provided from early pregnancy, 23.79%, 38.42%, and 37.79% of participants experienced inadequate, adequate, and excessive WG-F, with GDM incidences of 24.53%, 26.6%, and 29.5%, respectively (p < 0.0001, Table 2). Mediation analysis indicated that iWG-F decreased the risk of GDM regardless of WG-S, consistent with other studies (25, 43, 44). Elevated fasting glucose and low HDL levels in early pregnancy are considered high-risk factors for GDM (45, 46). Subgroup analysis in this study revealed that iWG-F was strongly associated with a reduced incidence of GDM in women with elevated fasting glucose and low HDL, as well as in lower-risk women with normal or underweight PPBMI, younger women, and women with low triglyceride levels. For these low-risk women, weight loss may potentially lead to a higher loss of fat mass, decreasing regional adipose tissue depots, enhancing insulin sensitivity, alleviating insulin resistance (47–49), and potentially lowering the risk of late gestational diabetes. Nandita et al. (50) suggested that inadequate weight gain throughout pregnancy increases the incidence of SGA and LBW. Logistic regression demonstrated a significant association of WG with preterm birth but not with abnormal neonatal weight or NICU admission. These findings emphasize the importance of stage-specific weight management during pregnancy. Future research should explore the mechanisms underlying these associations to refine guidelines for optimizing maternal and neonatal outcomes.
Strengths and limitations
This study benefits from a large cohort, focusing on weight gain patterns in populations with varying risk during different stages of pregnancy. The findings offer insights for targeted interventions to prevent GDM and may apply to similar maternal health contexts. By combining logistic regression with mediation analysis, the study identified potential roles of the first and second trimesters WG in the development of GDM. Partial mediation of the relationship between second-trimester weight gain and subsequent outcomes provides a basis for future studies to explore underlying pathways.
However, this study has several limitations that should be taken into consideration. First, it only collected short-term outcomes, thus preventing conclusions about the association between GWG and long-term maternal and offspring complications. Second, as an observational study, it cannot establish causal relationships between inadequate WG in the first trimester and maternal or neonatal complications; randomized controlled trials would be needed to determine causality. Third, the data were collected from a single hospital and involved only Chinese women, which limits the generalizability of the results. Additional studies involving diverse populations and multi-centre data are recommended. Fourth, pre-pregnancy weight was measured at home and self-reported, which may have introduced inaccuracies and biases in the data.
Conclusion
First-trimester WG is significantly linked to the incidence of GDM, while second-trimester and pre-OGTT weight gain show minimal or no significant association. Inadequate first-trimester weight gain reduces the risk of GDM, particularly in subgroups characterized by younger age, normal or underweight PPBMI, elevated fasting glucose, low HDL, or low triglyceride levels, without increasing abnormal neonatal birth weight. Early pregnancy may represent a critical window for GDM prevention. Encouraging minimal or no WG during the early gestation period may be a feasible and acceptable strategy for managing GDM risk.
Data availability statement
The data generated and analyzed during the current study are not publicly available due to privacy protection and ethical restrictions. Requests to access these datasets should be directed to LZ, MTgwNjAxMTc2NTZAMTYzLmNvbQ==.
Ethics statement
The studies involving humans were approved by this study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (2024KY200), and the research adhered to the principles outlined in the Declaration of Helsinki (World Medical Association Declaration of Helsinki). The Ethics Committee of Fujian Maternity and Child Health Hospital waived the requirement for informed consent due to the study’s nature, which utilized anonymized historical medical records data. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this approach posed minimal risk to participants and complied with the hospital Ethics Committee approval (2024KY200) and the Declaration of Helsinki principles.
Author contributions
ZC: Data curation, Formal analysis, Writing – original draft, Conceptualization, Writing – review & editing, Methodology, Validation. JL: Writing – review & editing, Formal analysis, Validation, Data curation, Methodology, Conceptualization. LL: Formal analysis, Data curation, Methodology, Writing – original draft, Project administration. JS: Formal analysis, Methodology, Writing – review & editing, Validation, Supervision, Data curation. HH: Investigation, Software, Supervision, Project administration, Writing – original draft, Formal analysis, Data curation, Validation. RC: Writing – review & editing, Methodology, Formal analysis, Resources. QL: Project administration, Formal analysis, Writing – review & editing, Resources, Methodology. LH: Methodology, Writing – review & editing, Supervision, Formal analysis, Validation, Data curation, Visualization. LZ: Validation, Project administration, Writing – review & editing, Resources, Methodology, Investigation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2021Y9164, No. 2023Y9392), and the Fujian Provincial Health Technology Project (No. 2020GGA021, No. 2020CXA017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang, H, Li, N, Chivese, T, Werfalli, M, Sun, H, Yuen, L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
2. Saeedi, M, Cao, Y, Fadl, H, Gustafson, H, and Simmons, D. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: a systematic review and meta-analysis. Diabetes Res Clin Pract. (2021) 172:108642. doi: 10.1016/j.diabres.2020.108642
3. Gao, C, Sun, X, Lu, L, Liu, F, and Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. (2019) 10:154–62. doi: 10.1111/jdi.12854
4. Zhu, H, Zhao, Z, Xu, J, Chen, Y, Zhu, Q, Zhou, L, et al. The prevalence of gestational diabetes mellitus before and after the implementation of the universal two-child policy in China. Front Endocrinol. (2022) 13:960877. doi: 10.3389/fendo.2022.960877
5. Ye, W, Luo, C, Huang, J, Li, C, Liu, Z, and Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. (2022) 377:e067946. doi: 10.1136/bmj-2021-067946
6. Cameron, NA, Yee, LM, Dolan, BM, O’Brien, MJ, Greenland, P, and Khan, SS. Trends in cardiovascular health counseling among postpartum individuals. JAMA. (2023) 330:359–67. doi: 10.1001/jama.2023.11210
7. Chan, AYL, Gao, L, Hsieh, MH-C, Kjerpeseth, LJ, Avelar, R, Banaschewski, T, et al. Maternal diabetes and risk of attention-deficit/hyperactivity disorder in offspring in a multinational cohort of 3.6 million mother-child pairs. Nat Med. (2024) 30:1416–23. doi: 10.1038/s41591-024-02917-8
8. Lowe, WL, Scholtens, DM, Lowe, LP, Kuang, A, Nodzenski, M, Talbot, O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. (2018) 320:1005–16. doi: 10.1001/jama.2018.11628
9. Scholtens, DM, Kuang, A, Lowe, LP, Hamilton, J, Lawrence, JM, Lebenthal, Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. (2019) 42:381–92. doi: 10.2337/dc18-2021
10. Green, JB. Cardiovascular consequences of gestational diabetes. Circulation. (2021) 143:988–90. doi: 10.1161/CIRCULATIONAHA.120.052995
11. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2018. Diabetes Care. (2018) 41:S13–27. doi: 10.2337/dc18-S002
12. Powe, CE, Huston Presley, LP, Locascio, JJ, and Catalano, PM. Augmented insulin secretory response in early pregnancy. Diabetologia. (2019) 62:1445–52. doi: 10.1007/s00125-019-4881-6
13. Buchanan, TA, Xiang, AH, and Page, KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. (2012) 8:639–49. doi: 10.1038/nrendo.2012.96
14. Nolan, CJ, Damm, P, and Prentki, M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. (2011) 378:169–81. doi: 10.1016/S0140-6736(11)60614-4
15. Koivusalo, SB, Rönö, K, Klemetti, MM, Roine, RP, Lindström, J, Erkkola, M, et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish gestational diabetes prevention study (RADIEL): a randomized controlled trial. Diabetes Care. (2016) 39:24–30. doi: 10.2337/dc15-0511
16. Wang, C, Wei, Y, Zhang, X, Zhang, Y, Xu, Q, Sun, Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. (2017) 216:340–51. doi: 10.1016/j.ajog.2017.01.037
17. Bain, E, Crane, M, Tieu, J, Han, S, Crowther, CA, and Middleton, P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. (2015) 3:CD010443. doi: 10.1002/14651858.CD010443.pub2
18. Chinese Nutrition Society. Weight monitoring and evaluation during pregnancy period of Chinese women: T/CNSS 009–2021. Beijing, China: People’s Health Publ House (2021).
19. Chinese Nutrition Society. Dietary guidelines for pregnant women. J Clin Pediatr. (2016) 34:877–80.
20. Dong, B, Yu, H, Wei, Q, Zhi, M, Wu, C, Zhu, X, et al. The effect of pre-pregnancy body mass index and excessive gestational weight gain on the risk of gestational diabetes in advanced maternal age. Oncotarget. (2017) 8:58364–71. doi: 10.18632/oncotarget.17651
21. Hantoushzadeh, S, Sheikh, M, Bosaghzadeh, Z, Ghotbizadeh, F, Tarafdari, A, Panahi, Z, et al. The impact of gestational weight gain in different trimesters of pregnancy on glucose challenge test and gestational diabetes. Postgrad Med J. (2016) 92:520–4. doi: 10.1136/postgradmedj-2015-133816
22. Lan, X, Zhang, Y-Q, Dong, H-L, Zhang, J, Zhou, F-M, Bao, Y-H, et al. Excessive gestational weight gain in the first trimester is associated with risk of gestational diabetes mellitus: a prospective study from Southwest China. Public Health Nutr. (2020) 23:394–401. doi: 10.1017/S1368980019003513
23. Carreno, CA, Clifton, RG, Hauth, JC, Myatt, L, Roberts, JM, Spong, CY, et al. Excessive early gestational weight gain and risk of gestational diabetes mellitus in nulliparous women. Obstet Gynecol. (2012) 119:1227–33. doi: 10.1097/AOG.0b013e318256cf1a
24. Morisset, A-S, Tchernof, A, Dubé, M-C, Veillette, J, Weisnagel, SJ, and Robitaille, J. Weight gain measures in women with gestational diabetes mellitus. J Women's Health. (2011) 20:375–80. doi: 10.1089/jwh.2010.2252
25. MacDonald, SC, Bodnar, LM, Himes, KP, and Hutcheon, JA. Patterns of gestational weight gain in early pregnancy and risk of gestational diabetes mellitus. Epidemiology. (2017) 28:419–27. doi: 10.1097/EDE.0000000000000629
26. Brunner, S, Stecher, L, Ziebarth, S, Nehring, I, Rifas-Shiman, SL, Sommer, C, et al. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: a meta-analysis. Diabetologia. (2015) 58:2229–37. doi: 10.1007/s00125-015-3686-5
27. Gibson, KS, Waters, TP, and Catalano, PM. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet Gynecol. (2012) 119:560–5. doi: 10.1097/AOG.0b013e31824758e0
28. Herring, SJ, Oken, E, Rifas-Shiman, SL, Rich-Edwards, JW, Stuebe, AM, Kleinman, KP, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol. (2009) 201:61.e1–7. doi: 10.1016/j.ajog.2009.01.039
29. Hivert, M-F, Backman, H, Benhalima, K, Catalano, P, Desoye, G, Immanuel, J, et al. Pathophysiology from preconception, during pregnancy, and beyond. Lancet. (2024) 404:158–74. doi: 10.1016/S0140-6736(24)00827-4
30. Lain, KY, and Catalano, PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. (2007) 50:938–48. doi: 10.1097/GRF.0b013e31815a5494
31. Thaweethai, T, Soetan, Z, James, K, Florez, JC, and Powe, CE. Distinct insulin physiology trajectories in euglycemic pregnancy and gestational diabetes mellitus. Diabetes Care. (2023) 46:2137–46. doi: 10.2337/dc22-2226
32. Lewis, GF, Carpentier, A, Adeli, K, and Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. (2002) 23:201–29. doi: 10.1210/edrv.23.2.0461
33. Hedderson, MM, Gunderson, EP, and Ferrara, A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. (2010) 115:597–604. doi: 10.1097/AOG.0b013e3181cfce4f
35. Koeslag, JH, Saunders, PT, and Terblanche, E. A reappraisal of the blood glucose homeostat which comprehensively explains the type 2 diabetes mellitus-syndrome X complex. J Physiol. (2003) 549:333–46. doi: 10.1113/jphysiol.2002.037895
36. Aedh, AI, Alshahrani, MS, Huneif, MA, Pryme, IF, and Oruch, R. A glimpse into milestones of insulin resistance and an updated review of its management. Nutrients. (2023) 15:921. doi: 10.3390/nu15040921
37. Cantor, AG, Jungbauer, RM, McDonagh, M, Blazina, I, Marshall, NE, Weeks, C, et al. Counseling and behavioral interventions for healthy weight and weight gain in pregnancy: evidence report and systematic review for the US preventive services task force. JAMA. (2021) 325:2094–109. doi: 10.1001/jama.2021.4230
38. Guo, X-Y, Shu, J, Fu, X-H, Chen, X-P, Zhang, L, Ji, M-X, et al. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: a meta-analysis and meta-regression. BJOG Int J Obstet Gynaecol. (2019) 126:311–20. doi: 10.1111/1471-0528.15467
39. Song, C, Li, J, Leng, J, Ma, RC, and Yang, X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obes Rev. (2016) 17:960–9. doi: 10.1111/obr.12442
40. Thangaratinam, S, Rogozinska, E, Jolly, K, Glinkowski, S, Roseboom, T, Tomlinson, JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. (2012) 344:e 2088. doi: 10.1136/bmj.e2088
41. Hill, B, Skouteris, H, and Fuller-Tyszkiewicz, M. Interventions designed to limit gestational weight gain: a systematic review of theory and meta-analysis of intervention components. Obes Rev. (2013) 14:435–50. doi: 10.1111/obr.12022
42. Mudd, LM, Owe, KM, Mottola, MF, and Pivarnik, JM. Health benefits of physical activity during pregnancy: an international perspective. Med Sci Sports Exerc. (2013) 45:268–77. doi: 10.1249/MSS.0b013e31826cebcb
43. Robitaille, J. Excessive gestational weight gain and gestational diabetes: importance of the first weeks of pregnancy. Diabetologia. (2015) 58:2203–5. doi: 10.1007/s00125-015-3725-2
44. Yin, A, Tian, F, Wu, X, Chen, Y, Liu, K, Tong, J, et al. Excessive gestational weight gain in early pregnancy and insufficient gestational weight gain in middle pregnancy increased risk of gestational diabetes mellitus. Chin Med J. (2022) 135:1057–63. doi: 10.1097/CM9.0000000000001972
45. Wang, Y-Y, Liu, Y, Li, C, Lin, J, Liu, X-M, Sheng, J-Z, et al. Frequency and risk factors for recurrent gestational diabetes mellitus in primiparous women: a case control study. BMC Endocr Disord. (2019) 19:22. doi: 10.1186/s12902-019-0349-4
46. MacNeill, S, Dodds, L, Hamilton, DC, Armson, BA, and VandenHof, M. Rates and risk factors for recurrence of gestational diabetes. Diabetes Care. (2001) 24:659–62. doi: 10.2337/diacare.24.4.659
47. Kolnes, KJ, Petersen, MH, Lien-Iversen, T, Højlund, K, and Jensen, J. Effect of exercise training on fat loss-energetic perspectives and the role of improved adipose tissue function and body fat distribution. Front Physiol. (2021) 12:737709. doi: 10.3389/fphys.2021.737709
48. Heianza, Y, Xue, Q, Rood, J, Bray, G, Sacks, F, and Qi, L. Abstract MP30: circulating MicroRNA-128-1 and changes in energy metabolism and insulin sensitivity in response to weight loss diet interventions: the pounds lost trial. Circulation. (2022) 145:AMP30–10. doi: 10.1161/circ.145.suppl_1.MP30
49. Goodpaster, BH, Kelley, DE, Wing, RR, Meier, A, and Thaete, FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. (1999) 48:839–47. doi: 10.2337/diabetes.48.4.839
Keywords: weight gain, gestational diabetes mellitus, preeclampsia, small for gestational age, preterm birth
Citation: Chen Z, Liu J, Lin L, Shi J, Huang H, Chen R, Liao Q, Huang L and Zheng L (2025) The impact of weight loss in early pregnancy on the incidence of late gestational diabetes: a retrospective cohort study. Front. Nutr. 12:1688268. doi: 10.3389/fnut.2025.1688268
Edited by:
A. Seval Ozgu-Erdinc, University of Health Sciences Bilkent City Hospital, TürkiyeReviewed by:
Maria Christou, University Hospital of Ioannina, GreecePatrícia Rosinha, Unidade Local de Saúde de Entre o Douro e Vouga, Portugal
Copyright © 2025 Chen, Liu, Lin, Shi, Huang, Chen, Liao, Huang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianghui Zheng, emhlbmdsaWFuZ2h1aUBmam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Zihua Chen1,2,3†
Zihua Chen1,2,3† Lin Lin
Lin Lin Huihui Huang
Huihui Huang Qiuping Liao
Qiuping Liao Lianghui Zheng
Lianghui Zheng