- 1West China Medical School, West China Hospital, Sichuan University, Chengdu, China
- 2College of Life Sciences, Sichuan University, Chengdu, China
- 3College of Biomedical Engineering, Sichuan University, Chengdu, China
- 4State Key Laboratory of Oncology in South China, Department of Radiation Oncology, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou, China
Introduction: Cancer-associated malnutrition is a pervasive and under-recognized complication that profoundly impacts treatment tolerance, clinical outcomes, and quality of life. Despite the availability of multiple nutritional screening and assessment tools, these instruments differ widely in sensitivity, specificity, and ease of integration into clinical workflows, and no universally accepted standard exists. This review critically examines the current landscape of malnutrition assessment in oncology, summarizes tool performance across populations and cancer types, and proposes strategies—such as artificial intelligence–enabled models and internationally harmonized protocols—to improve diagnosis, treatment planning, and overall patient outcomes.
Methods: A comprehensive literature search was conducted across PubMed, Web of Science, Embase, and Elsevier databases, covering studies published up to 13 March 2025. Medical Subject Headings (MeSH) were used to identify terms including “malnutrition,” “cachexia,” “cancer,” “nutritional status assessment,” “nutritional screening,” and “nutritional screening tool.” Boolean operators refined the strategy, and a two-stage screening excluded studies with irrelevant populations, outcomes, or designs, as well as non-peer-reviewed sources.
Results: Significant heterogeneity was found in tool performance and applicability across cancer types, clinical settings, and demographic subgroups. General instruments such as the Malnutrition Universal Screening Tool (MUST) and Nutritional Risk Screening 2002 (NRS-2002) demonstrated strong predictive validity in broad clinical use, whereas condition-specific tools like Patient-Generated Subjective Global Assessment (PG-SGA) offered superior sensitivity in high-risk populations, including patients with gastric or head and neck cancers. However, variability in thresholds, assessment frequency, and validation approaches highlights the urgent need for standardization.
Discussion: Current assessment strategies are limited by subjectivity, static single-point evaluations, and inconsistent implementation. Future innovations should integrate artificial intelligence, dynamic longitudinal monitoring, and multimodal data analytics to develop objective and personalized evaluation systems. Establishing globally harmonized standards will be crucial to improving nutritional care, reducing malnutrition-related morbidity, and enhancing survival and quality of life for patients with cancer.
Highlights
• Rigorous head-to-head evaluation of nutritional tools: this review delivers a comprehensive, side-by-side assessment of widely used nutritional screening instruments, clarifying their diagnostic accuracy and clinical utility for identifying cancer-related malnutrition.
• Precision guidance for cancer-specific care: we provide actionable recommendations for selecting the most appropriate tools tailored to cancer type and patient demographics, supporting precision nutrition strategies in oncology.
• Exposing critical gaps in current practice: our analysis underscores major shortcomings—including subjectivity, static single-point assessments, and lack of standardized protocols—that limit real-world implementation.
• A roadmap for next-generation solutions: we call for AI-enabled predictive models, dynamic longitudinal monitoring, and internationally harmonized standards to transform nutritional assessment and optimize cancer outcomes.
Introduction
Malnutrition is a prevalent and clinically significant comorbidity among patients with cancer, affecting a substantial proportion of individuals across disease types and care settings (1, 2). Comprehensive assessments indicate that moderate to severe malnutrition is common, with prevalence rates reaching approximately 25% in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) (3). Similar trends are observed in gynecologic malignancies, particularly in advanced or recurrent disease, where malnutrition and sarcopenia are frequently documented (4).
Cancer-associated malnutrition profoundly influences treatment tolerance, recovery trajectories, and overall health status (5, 6). It is typically characterized by involuntary weight loss with significant depletion of skeletal muscle and adipose tissue (7, 8). This progressive tissue wasting disrupts organ function and induces a fragile yet metabolically stable state (9). Closely related to this process is cancer cachexia, a multifactorial syndrome defined by involuntary muscle and fat loss combined with systemic inflammation (7) (Other effects of cancer cachexia are shown in Figure 1). Cancer cachexia is commonly classified into three stages—pre-cachexia, cachexia, and refractory cachexia—reflecting the escalating severity of metabolic and functional impairment (10–12).
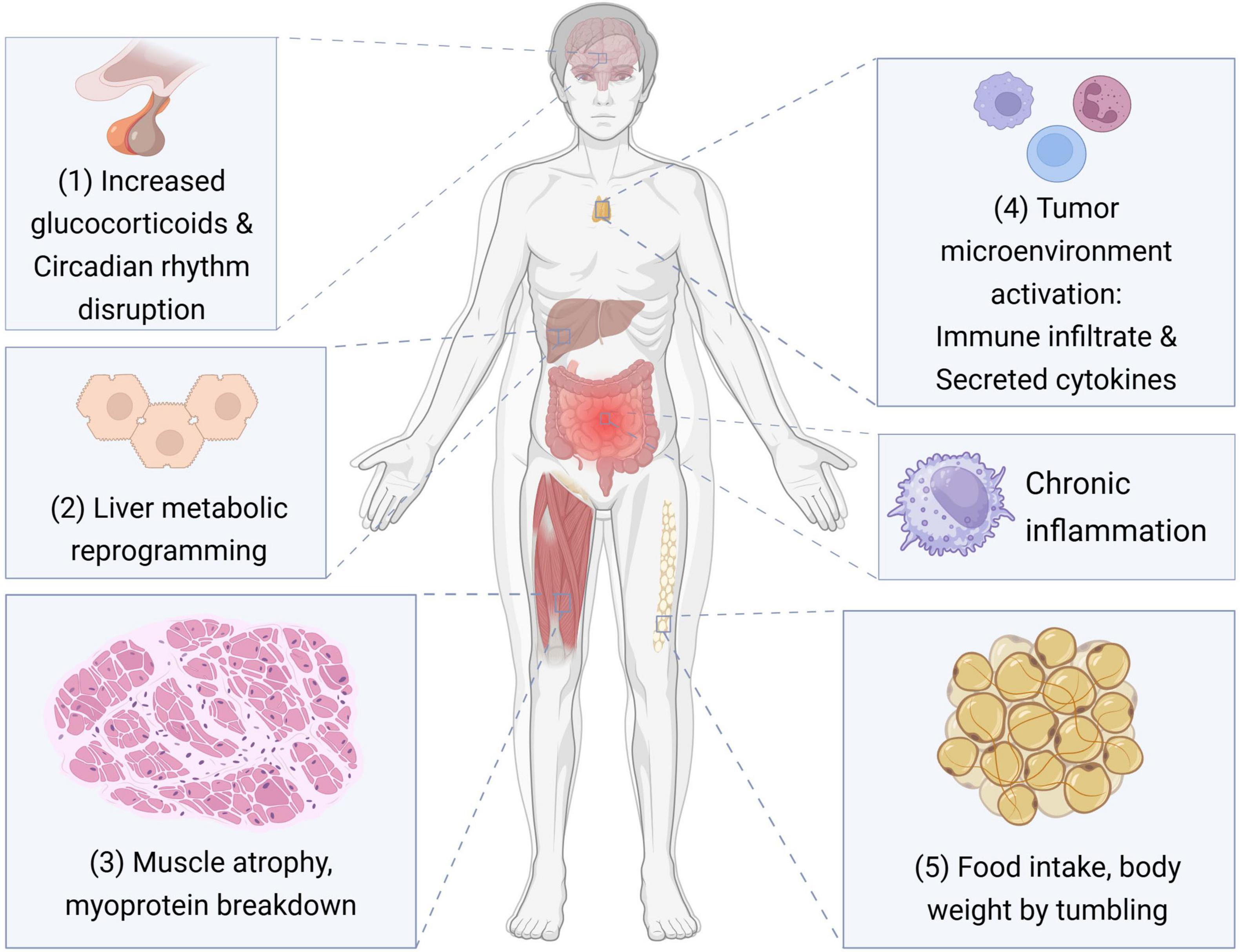
Figure 1. The impact of cancer cachexia at the tissue and organ level. This figure illustrates the key pathophysiological mechanisms through which cancer cachexia affects various tissues and organ systems. The processes include: (1) elevated glucocorticoid levels and disruption of circadian rhythms; (2) metabolic reprogramming in the liver; (3) skeletal muscle atrophy and enhanced myoprotein breakdown; (4) activation of the tumor microenvironment, characterized by immune cell infiltration and pro-inflammatory cytokine secretion; (5) systemic chronic inflammation. These factors collectively contribute to reduced food intake and progressive loss of body weight, hallmark features of cancer cachexia.
The consequences of malnutrition and cachexia extend beyond nutritional metrics, contributing to diminished quality of life, prolonged hospitalization, increased readmission rates, and higher mortality (5, 6). Early identification and systematic assessment of nutritional status have therefore become essential components of comprehensive cancer care, with the goal of improving treatment outcomes and patient wellbeing.
Multiple nutritional screening and assessment tools are currently employed in clinical practice, including the Patient-Generated Subjective Global Assessment (PG-SGA), Mini Nutritional Assessment (MNA), Nutritional Risk Screening 2002 (NRS-2002), and the Malnutrition Universal Screening Tool (MUST). Furthermore, in order to adapt to different clinical environments, nutritional Assessment tools will also extend many variations, such as Mini-Nutritional Assessment Short-Form (MNA-SF), which provides a more convenient, fast and low-cost assessment method (13). However, disparities in sensitivity, specificity, usability, and cultural adaptability remain, and the absence of universally accepted diagnostic criteria has hindered validation efforts, with many studies relying on suboptimal reference standards (e.g., alternative tools, biochemical markers, or composite scores) (14–16).
Given these limitations, a critical synthesis of available screening and assessment methods is urgently needed to guide clinicians in selecting tools tailored to specific cancer types, treatment stages, and patient populations. In addition, the integration of effective nutritional interventions has demonstrated the potential to improve clinical outcomes and quality of life (17, 18). This review provides a comprehensive comparison of existing malnutrition screening and assessment strategies, offering evidence-based guidance for optimizing nutritional management in oncology.
Methods
We conducted a systematic literature review to evaluate nutritional screening and assessment tools in oncology populations, employing a hybrid methodology that integrates the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines with established Systematic Literature Review (SLR) principles. The review process comprised four key stages: (1) formulation of research questions and objectives; (2) definition of review scope; (3) literature search, selection, and eligibility screening; and (4) quality appraisal and validation of included studies.
The primary research question guiding this review was: Which nutritional screening and assessment tools are commonly used in cancer care, and how do they perform in terms of sensitivity, specificity, and clinical feasibility across diverse healthcare settings? To address this question, we set the following objectives: (1) systematically identify studies reporting the use of nutritional screening and assessment tools in oncology; (2) classify and compare tools by design, intended application, and target population; (3) evaluate the methodological quality of included studies using appropriate appraisal frameworks; and (4) identify key gaps, challenges, and opportunities to inform future innovation.
A systematic search of PubMed, Web of Science, Embase, and Elsevier databases was performed, incorporating Medical Subject Headings (MeSH) terms from the United States National Library of Medicine (accessed 13 March 2025). Search descriptors included “malnutrition,” “cachexia,” “cancer,” “nutritional status assessment,” “nutritional screening,” and “nutritional screening tool,” combined with Boolean operators (AND and OR) to maximize sensitivity and specificity. No date restrictions were imposed; however, emphasis was placed on studies published within the past 5 years to ensure relevance.
The selection process adhered to PRISMA 2020 standards (Figure 2). A total of 317 records were retrieved. After removing duplicates (n = 47) and automated exclusions (n = 122), 146 articles were screened. Fourteen were excluded as irrelevant, and full-text retrieval was attempted for 132 articles, of which 13 could not be accessed. Ultimately, 119 articles were assessed for eligibility, and 22 met the inclusion criteria. Exclusion reasons included irrelevant focus (n = 3), unsuitable outcomes (n = 10), inappropriate settings (n = 6), and conference abstracts (n = 1). To ensure comprehensiveness, reference lists of relevant reviews were manually screened using Artificial intelligence-assisted literature tools.
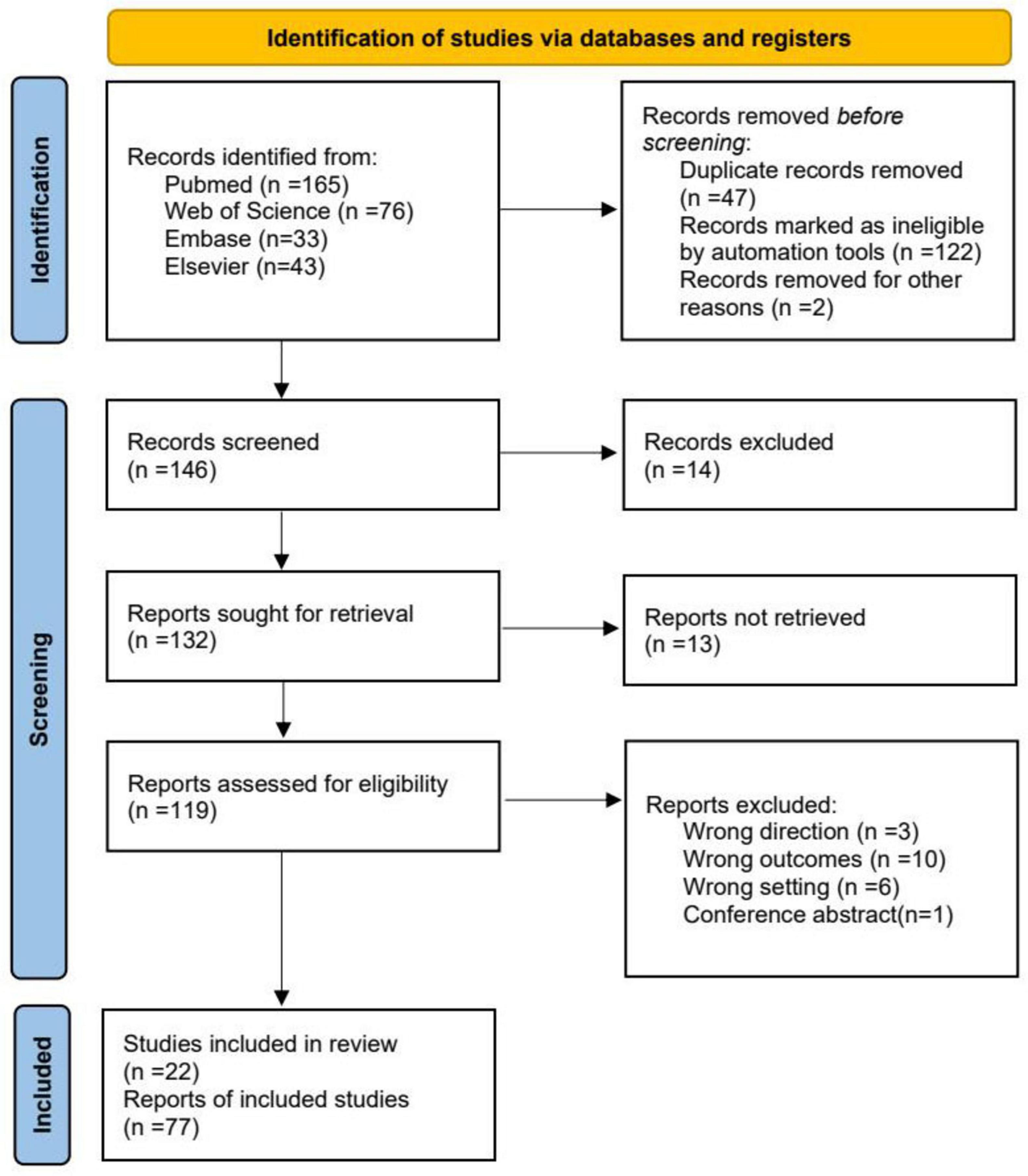
Figure 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the study selection process for the systematic review. PRISMA provides authors with guidance and examples of how to completely report why a systematic review was done, what methods were used, and what results were found.
This review focused exclusively on nutritional screening and assessment instruments validated or applied in oncology care. Both general-purpose tools (e.g., MUST, NRS-2002, PG-SGA) and cancer-specific instruments were included, spanning inpatient, outpatient, and home-based care settings. Studies involving adult and pediatric oncology patients were considered, with particular attention to tools demonstrating cross-context applicability.
Results
Nutritional screening and assessment tools demonstrated varied applications and performance in oncology settings. The Malnutrition Universal Screening Tool (MUST) showed high sensitivity (80.0%) and specificity (74.7%), outperforming tools such as NRS-2002 and MNA-SF in certain studies. The Nutritional Risk Screening 2002 (NRS-2002), widely adopted in hospitals, exhibited high sensitivity and reliability in identifying nutritional risk and predicting clinical outcomes such as complications and mortality. The Global Leadership Initiative on Malnutrition (GLIM) criteria offered a sensitive and specific framework for diagnosing disease-related malnutrition using phenotypic and etiologic criteria, applicable across diverse clinical and global settings. Meanwhile, the Patient-Generated Subjective Global Assessment (PG-SGA), tailored specifically for cancer patients, enabled detailed evaluation through patient-reported and clinician-assessed components, facilitating graded interventions based on total score. It demonstrated high clinical utility in identifying malnutrition and guiding nutritional support in oncologic populations.
Nutrition screening and assessment tools
Nutritional intervention is a critical component of cancer care, with preemptive assessment and management significantly improving surgical outcomes and quality of life (19, 20). Despite its importance, significant gaps exist in clinical implementation. Studies demonstrate that while technology-assisted systems can achieve high screening completion rates [e.g., 91% shortly after admission (21)], only a minority of healthcare professionals (14%) routinely use validated screening tools, despite most (65%) acknowledging the importance of nutrition (22). This gap underscores substantial implementation barriers.
According to ESPEN guidelines, nutritional management involves two key steps: initial screening to identify risk without etiological analysis, followed by a comprehensive assessment for those who screen positive to diagnose malnutrition and develop individualized interventions (23, 24). Table 1 outlines the ESPEN Cancer Nutrition Screening and Assessment Framework. The ESPEN 2003 Guidelines recommend Nutritional Risk Screening 2002 (NRS-2002) as the preferred screening tool for inpatients, where a score ≥ 3 indicates “nutritional risk” and necessitates further assessment (23).
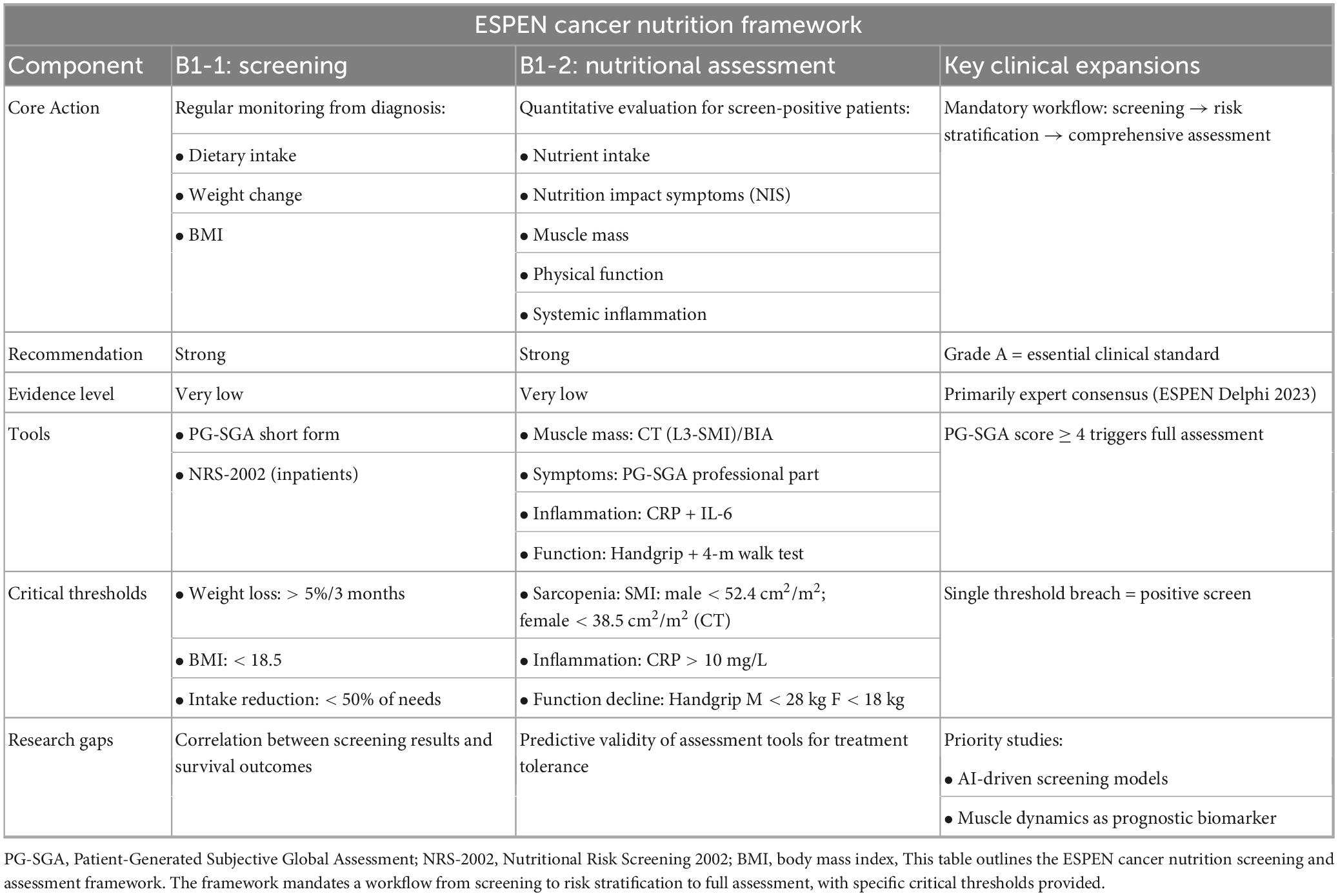
Table 1. European Society for Clinical Nutrition and Metabolism (ESPEN) cancer nutrition screening and assessment framework (2).
Nutrition screening: quickly and efficiently identify people at risk of malnutrition
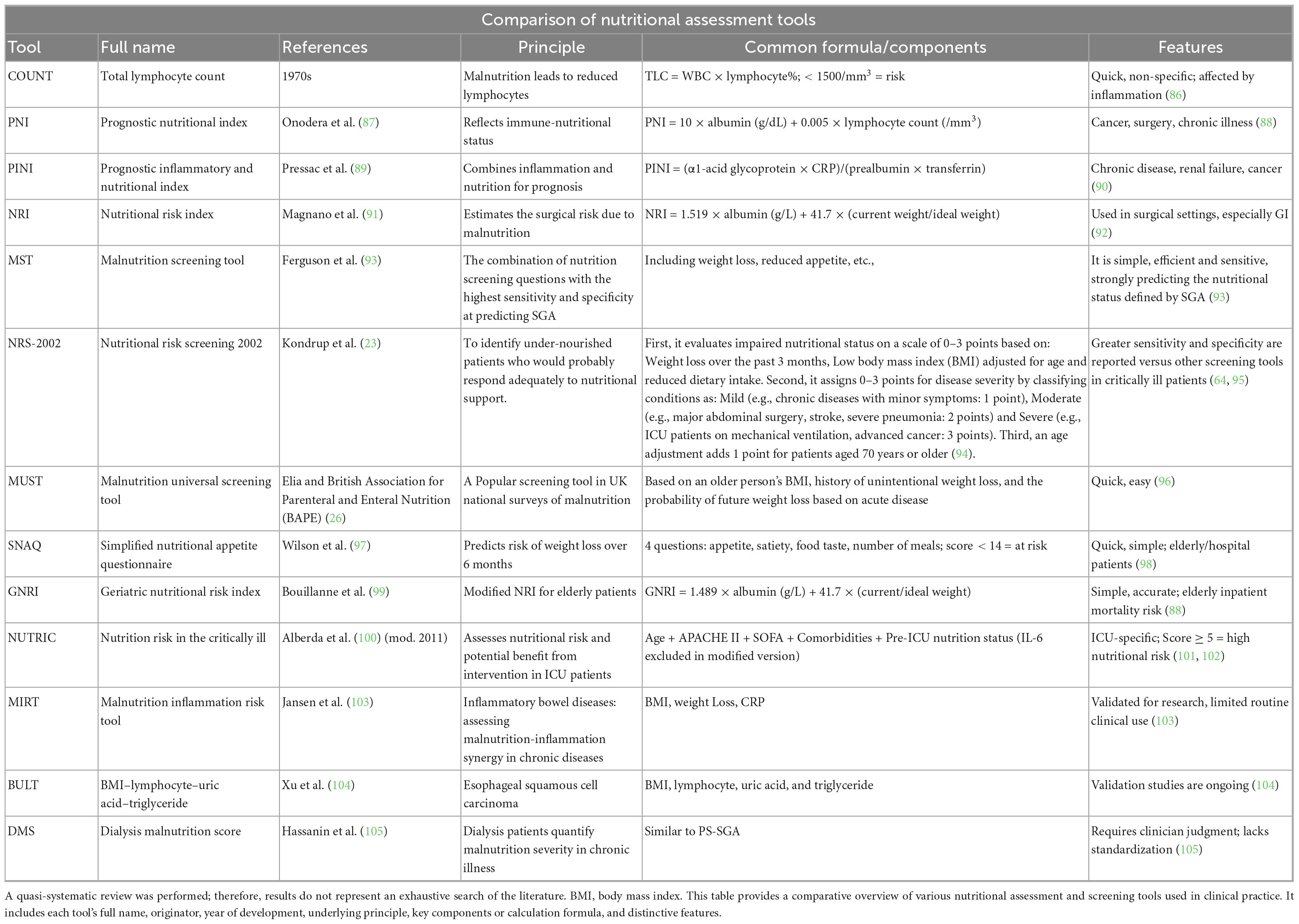
The Malnutrition Universal Screening Tool (MUST) is a rapid, repeatable universal screening tool for adult patients in different healthcare settings. The score is based on the following three indicators (24): BMI: 0 (> 20 kg/m2), 1 (18.5–20 kg/m2), 2 (< 18.5 kg/m2); Involuntary weight loss: 0 points (< 5%), 1 point (5%–10%), 2 points (> 10%); Effects of acute illness on food intake: 0 (none), 2 (presence of acute illness that prevents eating for the next 5 days). The total score classified patients as low risk (0), medium risk (1), and high risk (≥ 2) [Cortes et al. (25)]. MUST show the highest sensitivity (80.0%) and specificity (74.7%) in the study, with a positive predictive value of 44.4% (26). Compared to the ESPEN standard as the gold standard, MUST outperforms other tools such as NRS-2002, Malnutrition Screening Tool (MST), Simplified Nutritional Assessment Questionnaire (SNAQ), and MNA-SF on these indicators (27, 28). In addition, MUST is recommended for use in community and hospital settings to be able to predict length of stay and likelihood of readmission and to monitor changes in nutritional status (29, 30).
Kondrup et al. (23) developed the nutrition screening tool NRS-2002 based on 128 studies on the effectiveness of nutritional support (31). At present, it is the most commonly used malnutrition screening tool for hospital patients (32), with high sensitivity, consistent with the diagnosis of experienced physicians, and can also predict adverse outcomes such as complications, mortality, and prolonged hospital stay (33, 34). NRS-2002 is divided into the preliminary screening stage and the scoring stage (35). Initial screening stage: includes 4 questions (BMI < 20.5, weight loss in the past 3 months, recent reduction in intake, serious illness). If either answer is “yes,” formal screening is initiated. This was followed by a scoring stage: divided into nutritional status (0–3 points), disease severity (0–3 points), and age adjustment: patients ≥ 70 years old plus 1 point. Finally, the total score is calculated: nutritional status score + disease score + age score. A total score of ≥ 3 indicates a high risk or definite malnutrition and requires nutritional support (1). The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends that NRS-2002 be used in hospitalized patients, especially those with severe illness, and that NRS-2002 be used in combination with the modified NUTRIC Score (36, 37).
Furthermore, MUST and MST are widely used in various clinical settings. MUST is favored for its ease of use, particularly among emergency and newly diagnosed patients. Lima et al. (38) pointed out that MUST exhibit high sensitivity and accuracy in screening for nutritional risks, making it suitable for the early identification of malnutrition risks among hospitalized patients (38). In studies on cancer patients, MUST and NRS-2002 performed well in predicting clinical outcomes, especially in critical care settings (39). Table 2 provides a comparative overview of various nutritional assessment and screening tools used in clinical practice.
Nutritional assessment: comprehensive diagnosis of nutritional status and development of personalized intervention programs
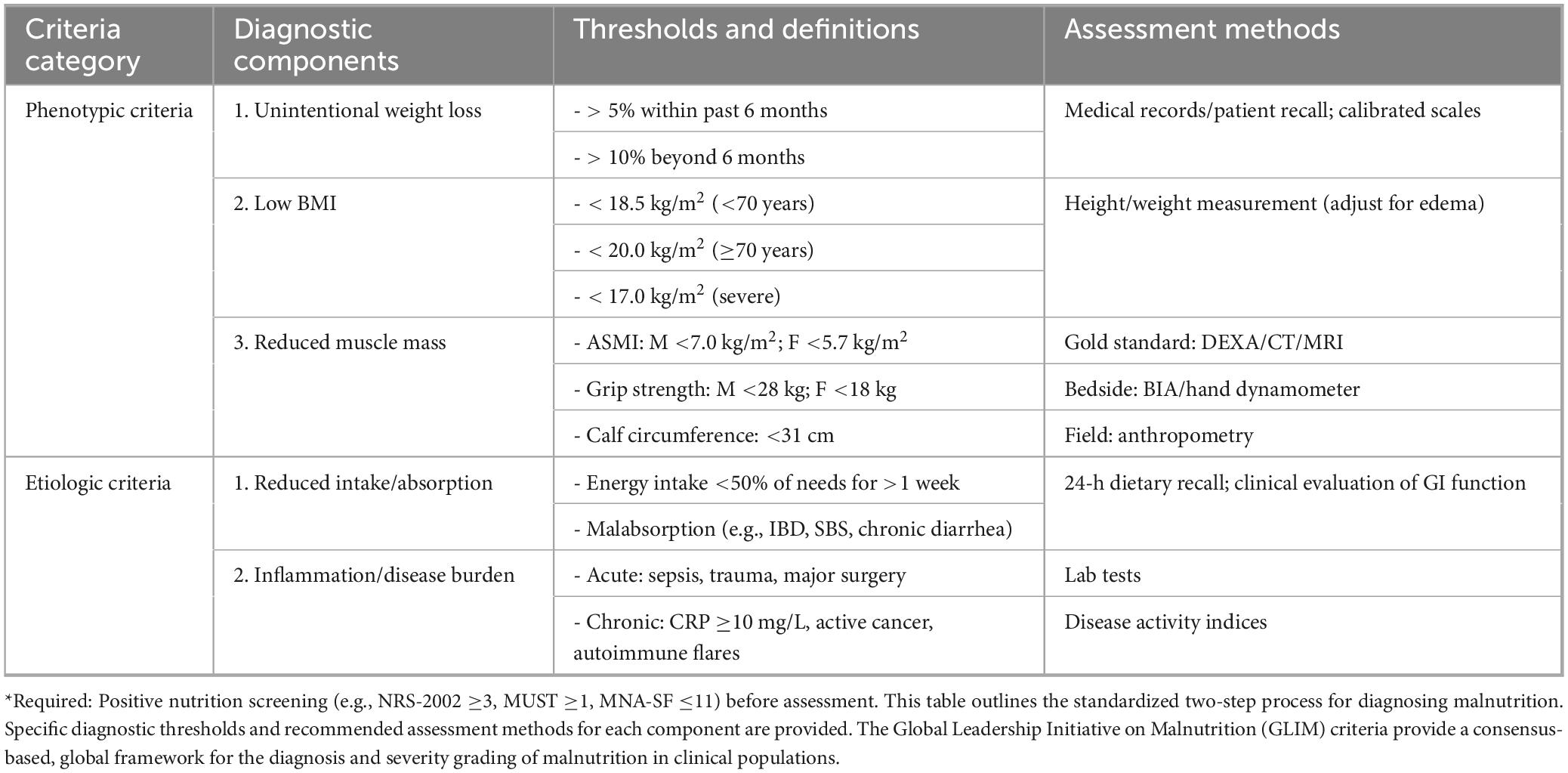
Global Leadership Initiative on Malnutrition (GLIM) is a relatively new, widely adaptable tool with higher sensitivity and specificity to disease-related malnutrition (40). Table 3 outlines the standardized two-step process for diagnosing malnutrition in GLIM. The diagnosis of GLIM is based on five criteria, including three phenotypic criteria and two pathological criteria. The three phenotypic criteria are involuntary weight loss (weight loss > 5% within six months or weight loss > 10% for more than six months), low BMI (adult < 18.5 kg/m2), muscle mass reduction (grip strength measurement, male < 28 kg, female < 18 kg); the two pathological criteria are reducing food intake or undernutrition (food intake < 50% requirement, lasting > 1 week), inflammation or disease burden (burns, severe infections and other systemic inflammatory reactions) (34). At the time of diagnosis, the patient should meet at least one phenotype standard and one pathological standard at the same time. At the same time, the phenotypical criteria of GLIM can also be used to assess the severity of malnutrition, which is divided into moderate (stage 1) and severe (stage 2) (41). In addition, the scope of application of GLIM is particularly wide and applicable to global clinical environments, including inpatients, community medical care, and chronic disease management (41).
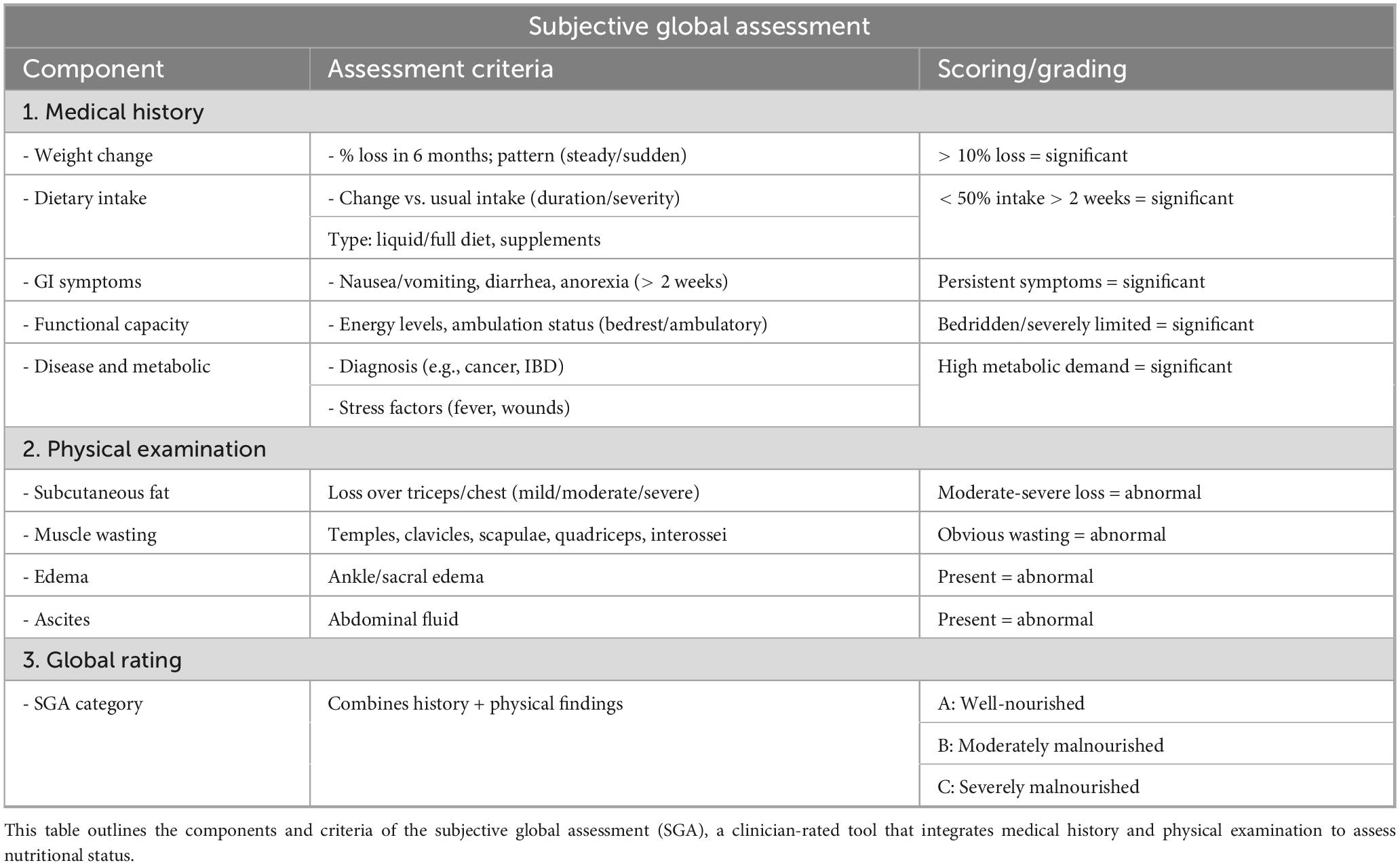
Patients-Generated Subjective Global Assessment (PG-SGA) is a further development of Subjective Global Assessment (SGA), a nutritional assessment tool specially tailored for tumor patients (42). Table 4 outlines the components and criteria of the Subjective Global Assessment (SGA). The evaluation of PG-SGA is based on two parts: patient self-assessment and professional evaluation. The patient’s self-assessment includes weight changes, dietary intake, and symptoms affecting eating and mobility, with a score range of 0–13 points; professional evaluation includes metabolic needs, disease-related stress, and physical examination with a total score of 0–7 (43, 44). The standard of testing is that when the total score is greater than or equal to 9 points, it is severe malnutrition. A total of 2–8 points prompt the need to adjust the diet or oral nutrition. Regular testing is enough when 0–1 point is enough. At the same time, it should be noted that some guidelines suggest that when the PG-SGA score in tumor patients is > 4, it indicates nutritional risks (45). It is characterized by hierarchical intervention based on the total score to assess health status, demonstrating high sensitivity and accuracy. Its effectiveness significantly exceeds that of GLIM in the treatment of certain tumors (46).
In summary, various nutritional screening tools have their advantages in clinical applications. Choosing the appropriate tool should consider the specific conditions of patients and the clinical environment. Future research should further explore the adaptability and effectiveness of these tools in different types of cancer patients to optimize nutritional management strategies for cancer patients.
Discussion
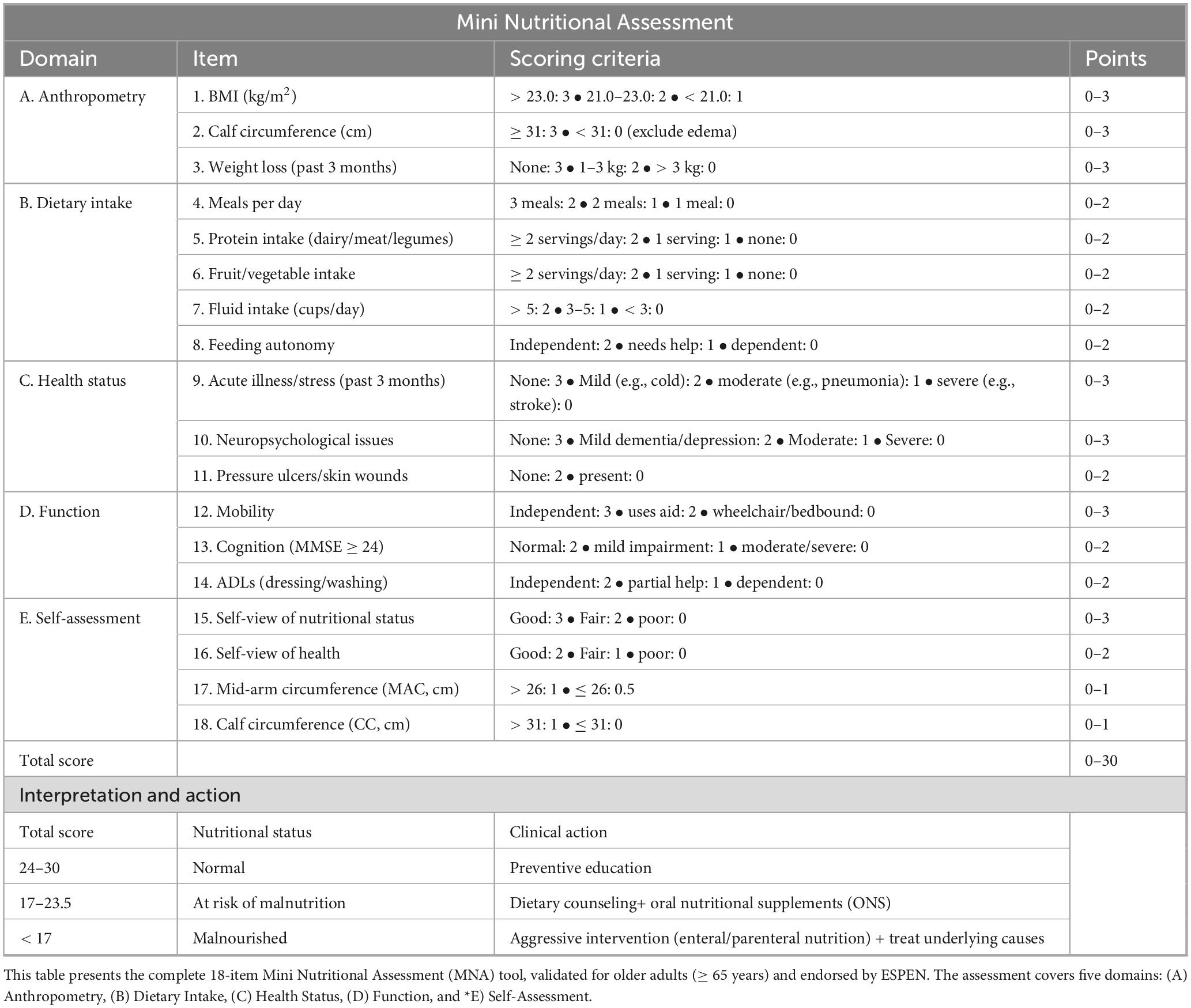
Current screening and assessment tools for cancer-related malnutrition show considerable variability in effectiveness and consistency across clinical contexts. Ruan et al. (47) employed hierarchical Bayesian latent class meta-analysis to compare three tools—MNA, NRS-2002, and PG-SGA—and demonstrated that PG-SGA had superior diagnostic accuracy, achieving sensitivity and specificity of 96.4% and 90.5%, respectively (2). In contrast, MNA achieved a sensitivity of 91.0% and specificity of 72.0%, while NRS-2002 exhibited lower sensitivity (74.7%) but higher specificity (85.4%) (47). Table 5 details the full 18-item MNA. These findings highlight PG-SGA’s value for early detection of malnutrition risk, particularly in patients requiring urgent intervention. Similarly, Gascón-Ruiz et al. (48) reported that the GLIM criteria significantly outperformed ESPEN standards in detecting malnutrition (46.7% vs. 21.2%) (48), reinforcing the importance of evidence-based tool selection tailored to patient conditions (49).
Cancer-specific applications
Tool performance varies by cancer type. In colorectal cancer, MUST demonstrated significant associations with body composition metrics and clinical outcomes (50). Its use of BMI (< 18.5 kg/m2) and weight loss (> 5%) aligns closely with common nutritional deterioration patterns. Xie et al. (51) further confirmed that NRS-2002 is effective for predicting short-term outcomes (51), making it a valuable adjunct to MUST.
For gastric cancer, patients are prone to Sarcopenia, which directly affects surgical tolerance, postoperative recovery and prognosis. Therefore, tools that go beyond conventional nutritional screening and specifically assess muscle mass and function are of vital importance. Lu et al. (52) found that SARC-CalF provided strong diagnostic value (AUC 0.896) (52). Although self-screening tools such as MUST and MST are practical and reliable (53, 54), limitations exist in gastric cancer populations. To address this, Chen et al. (55) developed the SNRSGC tool for home-based self-assessment following gastrectomy (55).
In pancreatic cancer, Yu et al. (56) identified Controlling Nutritional Status (CONUT) as a strong prognostic indicator, while NRS-2002 was particularly predictive of mortality (HR 1.248; 95% CI, 1.155–1.348; P < 0.001) (56). The CONUT score reflects albumin depletion, lymphopenia, and hypocholesterolemia—hallmarks of pancreatic cancer malnutrition—whereas NRS-2002 integrates systemic disease burden. Menozzi et al. (57) recommended complementing screening with CT-based body composition analysis for surgical candidates (57, 58).
For head and neck squamous cell carcinoma (HNSCC), Tu et al. (59) demonstrated that PG-SGA is more sensitive preoperatively due to its detailed symptom tracking (e.g., dysphagia, anorexia), whereas NRS-2002 offers rapid, practical assessment postoperatively (59). Before the operation, the tumor itself caused dysphagia and insufficient intake. PG-SGA can record the subjective symptoms of patients in detail, with high sensitivity, and is suitable for formulating preoperative nutrition plans. After the operation, the risks shift to surgical trauma, stress response and fasting. The concise structure and assessment of “disease severity” of NRS-2002 make it more convenient for rapid implementation in the busy clinical environment after surgery. In esophageal and pharyngeal cancers, PG-SGA’s patient-reported components provide unique insights into swallowing difficulties, pain, and other functional impairments, enabling precision nutrition planning (60, 61).
Critically ill and age-specific populations
In critically ill patients, the NUTRIC score demonstrated strong external validity for predicting mortality and nutritional intervention benefits (62). Further studies confirmed that mNUTRIC and NRS-2002 outperform other tools in predicting mortality and organ failure (63), although Rattanachaiwong et al. (64) highlighted their limited sensitivity for diagnosing severe malnutrition compared to ESPEN/ASPEN-based tools.
In pediatric oncology, Lovell et al. (65) identified significant resource constraints limiting nutrition care, while Gallo et al. (66) validated a novel pediatric screening tool with superior accuracy for detecting low muscle mass. These findings highlight an urgent need for age-specific and resource-adapted solutions.
In elderly cancer patients, a systematic evaluation identified six validated screening tools (MNA, MST, MUST, NRS-2002, NUTRISCORE, PG-SGA SF) with established cut-offs (67–69). The combination of MNA-SF (elderly-specific) and PG-SGA (oncology-specific) improved prediction of hospital stay length and readmission rates (69).
Resource considerations and implementation barriers
Tool applicability is influenced by healthcare resources. In low-resource settings, simple, low-cost instruments like MUST and MST are practical due to minimal equipment requirements (53, 54). High-resource centers can leverage comprehensive multimodal assessments, integrating PG-SGA, CONUT, and CT imaging for precision care. However, implementation gaps remain a persistent challenge. Despite strong awareness of malnutrition’s impact, clinician adoption rates of validated tools remain low, and training gaps hinder consistency (70–72).
Limitations of existing tools
Current instruments face three core limitations: (1) Subjectivity and recall bias: Tools like PG-SGA and MNA rely heavily on patient-reported intake and weight history, which may be inaccurate due to fatigue, cognitive decline, or treatment side effects (25). (2) Static, single-point evaluation: Tools such as NRS-2002 and MUST lack dynamic monitoring capacity, failing to capture rapid nutritional deterioration during therapy (73, 74). (3) Limited applicability in special populations: Obese cancer patients with sarcopenia often evade detection due to low-BMI thresholds (75, 76). Adjusted diagnostic cut-offs for weight and muscle loss in obesity have been proposed (77).
Future research directions
Future research should focus on tool innovation, clinical integration, and international harmonization: (1) AI/ML-Driven Models: Kiss et al. (78) found that machine learning models based on GLIM criteria maintained predictive accuracy for clinical outcomes even when excluding muscle mass (78). Meanwhile, Wu et al. (79) developed an online machine learning tool to effectively predict malnutrition in colorectal cancer patients without weight loss data, identifying NRS-2002 as the most suitable screening method (79). Besides, tools like MyCancerRisk (80) and ML-based colorectal cancer prediction models (81) demonstrate how artificial intelligence can enhance precision screening, even in patients lacking classic weight-loss markers. (2) Personalized Protocols: Screening should be stratified by cancer type, baseline BMI, sarcopenia, and systemic inflammation to refine clinical accuracy (82). (3) Clinical Implementation: Strategies must emphasize clinician training, simplified workflows, and multidisciplinary data integration (82, 83). (4) Global Standardization: The absence of unified criteria remains a critical barrier (56, 84); internationally validated frameworks would enable consistent application and research comparability.
However, AI and ML integration faces significant challenges, including fragmented electronic health records, missing data, heterogeneous nutritional metrics, and risk of algorithmic bias. Transparency (Explainable AI), data protection, and human oversight remain essential (85). Future efforts should not only develop objective, dynamic tools but also optimize usability to improve adherence and facilitate cross-disciplinary collaboration, ultimately enabling nutrition assessment to become a seamless component of oncology care.
Cancer-related malnutrition screening and assessment tools exhibit significant heterogeneity in sensitivity, specificity, and clinical usability. Tool selection should be context-driven, considering cancer type, age, and resource availability (Table 6). Future directions emphasize AI-driven innovation, personalization, and international standardization to improve patient outcomes and integrate nutritional assessment into routine oncology practice.
Conclusion
Malnutrition remains a prevalent and clinically significant complication in oncology, with profound implications for treatment tolerance, recovery, and overall survival. In recent years, the development and refinement of cancer-specific nutritional screening and assessment tools have become a focal point in clinical nutrition and oncologic care. As evidence continues to underscore the detrimental effects of cancer-related malnutrition, research efforts have increasingly shifted toward early detection strategies, integrating simplified yet comprehensive tools to optimize patient outcomes. Emerging trends emphasize the combination of multiple validated instruments with novel technologies—including artificial intelligence, machine learning, and digital health platforms—to improve diagnostic precision, enhance clinical applicability, and facilitate timely, personalized nutritional interventions.
Author contributions
LZ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ZD: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YZ: Writing – original draft, Writing – review & editing. ZC: Writing – original draft, Writing – review & editing. JH: Writing – original draft, Writing – review & editing. LH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by 1.3.5 Project for Artificial Intelligence (No. ZYAI24054), “Qimingxing” Research Fund for Young Talents (No. HXQMX0101), West China Hospital, Sichuan University, Key Research and Development Program of Sichuan Province (No. 2023YFS0306), and National Natural Science Foundation of China (No. 82204490).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AZ declared a shared affiliation with authors LZ, ZD, YZ, ZC, JH at time of review.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. We have thoroughly polished the language throughout the manuscript using professional editing tools such as Deepseek, ChatGPT 5.0, and Grammarly. Special attention was given to rewriting lengthy, complex, or awkwardly phrased sentences to improve clarity and fluency.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fatiregun OA, Bakewell N. Malnutrition after cancer surgery in low-income and middle-income countries. Lancet Glob Health. (2023) 11:e302. doi: 10.1016/s2214-109x00022-0
2. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. (2017) 36:1187–96. doi: 10.1016/j.clnu.2017.06.017
3. Laing E, Gough K, Krishnasamy M, Michael M, Kiss N. Prevalence of malnutrition and nutrition-related complications in patients with gastroenteropancreatic neuroendocrine tumours. J Neuroendocrinol. (2022) 34:e13116. doi: 10.1111/jne.13116
4. Morton M, Patterson J, Sciuva J, Jaya Perni, Backes F, Nagel C, et al. Malnutrition, sarcopenia, and cancer cachexia in gynecologic cancer. Gynecol Oncol. (2023) 175:142–55. doi: 10.1016/j.ygyno.2023.06.015
5. Bossi P, Delrio P, Mascheroni A, Zanetti M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients. (2021) 13:1980. doi: 10.3390/nu13061980
7. Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. (2016) 75:199–211. doi: 10.1017/s002966511500419x
8. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by special interest groups (SIG) ‘cachexia-anorexia in chronic wasting diseases’ and ‘nutrition in geriatrics’. Clin Nutr. (2010) 29:154–9. doi: 10.1016/j.clnu.2009.12.004
9. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Prim. (2018) 4:17105. doi: 10.1038/nrdp.2017.105
10. Arends J. Malnutrition in cancer patients: causes, consequences and treatment options. Eur J Surg Oncol. (2023) 50:107074. doi: 10.1016/j.ejso.2023.107074
11. Skipworth RJE, Stewart GD, Dejong CHC, Preston T, Fearon KCH. Pathophysiology of cancer cachexia: much more than host–tumour interaction? Clin Nutr. (2007) 26:667–76. doi: 10.1016/j.clnu.2007.03.011
12. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. (2011) 12:489–95. doi: 10.1016/S1470-204570218-7
13. Liu H, Jiao J, Zhu M, Wen X, Jin J, Wang H, et al. Nutritional status according to the short-form mini nutritional assessment (MNA-SF) and clinical characteristics as predictors of length of stay, mortality, and readmissions among older inpatients in China: a national study. Front Nutr. (2022) 9:815578. doi: 10.3389/fnut.2022.815578
14. Sánchez-Rodríguez D, Marco E, Ronquillo-Moreno N, Maciel-Bravo L, Gonzales-Carhuancho A, Duran X, et al. ASPEN-AND-ESPEN: a postacute-care comparison of the basic definition of malnutrition from the American society of parenteral and enteral nutrition and academy of nutrition and dietetics with the european society for clinical nutrition and metabolism definition. Clin Nutr. (2019) 38:297–302. doi: 10.1016/j.clnu.2018.01.017
15. Power L, Mullally D, Gibney ER, Clarke M, Visser M, Volkert D, et al. A review of the validity of malnutrition screening tools used in older adults in community and healthcare settings – a MaNuEL study. Clin Nutr ESPEN. (2018) 24:1–13. doi: 10.1016/j.clnesp.2018.02.005
16. Yang M, Xiao N, Tang L, Zhang Y, Wen Y, Yang X. Evaluating the accuracy of a nutritional screening tool for patients with digestive system tumors: a hierarchical Bayesian latent class meta-analysis. PLoS One. (2024) 19:e0316070. doi: 10.1371/journal.pone.0316070
17. Victoria-Montesinos D, García-Muñoz AM, Navarro-Marroco J, Lucas-Abellán C, Mercader-Ros MT, Serrano-Martínez A, et al. Phase angle, handgrip strength, and other indicators of nutritional status in cancer patients undergoing different nutritional strategies: a systematic review and meta-analysis. Nutrients. (2023) 15:1790. doi: 10.3390/nu15071790
18. Feinberg J, Nielsen EE, Korang SK, Halberg Engell K, Nielsen MS, Zhang K, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. (2017) 2017:CD011598. doi: 10.1002/14651858.CD011598.pub2
19. Baji DB, Patel JP, Konanur Srinivasa NK, Gande A, Anusha M, Dar H. Nutrition care in cancer surgery patients: a narrative review of nutritional screening and assessment methods and nutritional considerations. Cureus. (2022) 14:94. doi: 10.7759/cureus.33094
20. Håkonsen SJ, Pedersen PU, Bath-Hextall F, Kirkpatrick P. Diagnostic test accuracy of nutritional tools used to identify undernutrition in patients with colorectal cancer: a systematic review. JBI Database Syst Rev Implement Rep. (2015) 13:141–87. doi: 10.11124/jbisrir-2015-1673
21. Carrera-Gil F, Prieto I. Efficiency of a technology-assisted nutritional screening system: a retrospective analysis of 11,722 admissions in a tertiary hospital. Clin Nutr ESPEN. (2024) 64:51–6. doi: 10.1016/j.clnesp.2024.08.022
22. Almasaudi AS. An investigation of the clinical nutritional practices of oncologists and the management of cancer-related malnutrition in inpatient care. Eur Rev Med Pharmacol Sci. (2023) 27:9928–36. doi: 10.26355/eurrev_202310_34171
23. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. (2003) 22:415–21. doi: 10.1016/s0261-561400098-0
24. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
25. Cortes R, Yañez A, Capitán-Moyano L, Millán-Pons A, Bennasar-Veny M. Evaluation of different screening tools for detection of malnutrition in hospitalised patients. J Clin Nurs. (2024) 33:4759–71. doi: 10.1111/jocn.17170
26. Elia M, British Association For Parenteral And Enteral Nutrition. The ‘MUST’ Report: Nutritional Screening of Adults: A Multidisciplinary Responsibility: Development and Use of the ‘Malnutrition Universal Screening Tool’ (‘MUST’) for adults. Redditch: Bapen (2003).
27. Trujillo EB, Kadakia KC, Thomson C, Zhang FF, Livinski A, Pollard K, et al. Malnutrition risk screening in adult oncology outpatients: an ASPEN systematic review and clinical recommendations. J Parent Enteral Nutr. (2024) 48:874–94. doi: 10.1002/jpen.2688
28. Poulia K-A, Klek S, Doundoulakis I, Bouras E, Karayiannis D, Baschali A, et al. The two most popular malnutrition screening tools in the light of the new ESPEN consensus definition of the diagnostic criteria for malnutrition. Clin Nutr. (2017) 36:1130–5. doi: 10.1016/j.clnu.2016.07.014
29. Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr. (2004) 92:799–808. doi: 10.1079/bjn20041258
30. Cheung HHT, Joynt GM, Lee A. Diagnostic test accuracy of preoperative nutritional screening tools in adults for malnutrition: a systematic review and network meta-analysis. Int J Surg. (2024) 110:1090. doi: 10.1097/JS9.0000000000000845
31. Calleja Fernandez A, Vidal Casariego A, Cano Rodriguez I, Ballesteros Pomar M. Original / valoración nutricional. Nutr Hosp. (2015) 31:6. doi: 10.3305/nh.2015.31.5.8606
32. Xu R, Chen X-D, Ding Z. Perioperative nutrition management for gastric cancer. Nutrition. (2022) 93:111492. doi: 10.1016/j.nut.2021.111492
33. Cortés-Aguilar R, Malih N, Abbate M, Fresneda S, Yañez A, Bennasar-Veny M. Validity of nutrition screening tools for risk of malnutrition among hospitalized adult patients: a systematic review and meta-analysis. Clin Nutr. (2024) 43:1094–116. doi: 10.1016/j.clnu.2024.03.008
34. Kondrup J. Nutritional risk screening (NRS-2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. (2003) 22:321–36. doi: 10.1016/s0261-561400214-5
35. Sun Z, Kong X-J, Jing X, Deng R-J, Tian Z-B. Nutritional risk screening 2002 as a predictor of postoperative outcomes in patients undergoing abdominal surgery: a systematic review and meta-analysis of prospective cohort studies. PLoS One. (2015) 10:e0132857. doi: 10.1371/journal.pone.0132857
36. Raslan M, Gonzalez MC, Gonçalves Dias MC, Nascimento M, Castro M, Marques P, et al. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition. (2010) 26:721–6. doi: 10.1016/j.nut.2009.07.010
37. Cattani A, Eckert IC, Brito JE, Tartari RF, Silva FM. Nutritional risk in critically ill patients: how it is assessed, its prevalence and prognostic value: a systematic review. Nutr Rev. (2020) 78:1052–68. doi: 10.1093/nutrit/nuaa031
38. Lima J, Brizola Dias AJ, Burgel CF, Bernardes S, Gonzalez MC, Silva FM. Complementarity of nutritional screening tools to GLIM criteria on malnutrition diagnosis in hospitalised patients: a secondary analysis of a longitudinal study. Clin Nutr. (2022) 41:2325–32. doi: 10.1016/j.clnu.2022.08.022
39. Gil-Andrés D, Cabañas-Alite L. A narrative review comparing nutritional screening tools in outpatient management of cancer patients. Nutrients. (2024) 16:752. doi: 10.3390/nu16050752
40. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement: academy of nutrition and dietetics and American society for parenteral and enteral nutrition. J Parent Enteral Nutr. (2012) 36:275–83. doi: 10.1177/0148607112440285
41. Chen L-K, Lee W-J, Peng L-N, Liu L-K, Arai H, Akishita M. Recent advances in sarcopenia research in asia: 2016 update from the asian working group for sarcopenia. J Am Med Dir Assoc. (2016) 17:767.e1–767.e7. doi: 10.1016/j.jamda.2016.05.016
42. Cederholm T, Jensen GL. To create a consensus on malnutrition diagnostic criteria: a report from the Global leadership initiative on malnutrition (GLIM) meeting at the ESPEN Congress 2016. Clin Nutr. (2017) 36:7–10. doi: 10.1016/j.clnu.2016.12.001
43. Isenring E, Cross G, Daniels L, Kellett E, Koczwara B. Validity of the malnutrition screening tool as an effective predictor of nutritional risk in oncology outpatients receiving chemotherapy. Support Care Cancer. (2006) 14:1152–6. doi: 10.1007/s00520-006-0070-5
44. Bruno K, Sobreira, da Silva M, Chaves G. Association of body composition with toxicity to first-line chemotherapy and three-year survival in women with ovarian adenocarcinoma. Acta Oncol. (2021) 60:1611–20. doi: 10.1080/0284186x.2021.1983210
45. Clemente G, Gallo M, Giorgini M. Modalities for assessing the nutritional status in patients with diabetes and cancer. Diabetes Res Clin Pract. (2018) 142:162–72. doi: 10.1016/j.diabres.2018.05.039
46. Sealy MJ, Haß U, Ottery FD, van der Schans CP, Roodenburg JLN, Jager-Wittenaar H. Translation and cultural adaptation of the scored patient-generated subjective global assessment: an interdisciplinary nutritional instrument appropriate for Dutch cancer patients. Cancer Nurs. (2018) 41:450–62. doi: 10.1097/ncc.0000000000000505
47. Ruan X, Nakyeyune R, Shao Y, Shen Y, Niu C, Zang Z, et al. Nutritional screening tools for adult cancer patients: a hierarchical Bayesian latent-class meta-analysis. Clin Nutr. (2020) 40:1733–43. doi: 10.1016/j.clnu.2020.09.033
48. Gascón-Ruiz M, Casas-Deza D, Torres-Ramón I, Zapata-García M, Alonso N, Sesma A, et al. GLIM vs ESPEN criteria for the diagnosis of early malnutrition in oncological outpatients. Clin Nutr. (2021) 40:3741–7. doi: 10.1016/j.clnu.2021.04.025
49. Skipper A, Coltman A, Tomesko J, Charney P, Porcari J, Piemonte TA, et al. Position of the academy of nutrition and dietetics: malnutrition (undernutrition) screening tools for all adults. J Acad Nutr Diet. (2019) 120:709–13. doi: 10.1016/j.jand.2019.09.011
50. Almasaudi AS, McSorley ST, Dolan RD, Edwards CA, McMillan DC. The relation between Malnutrition Universal Screening Tool (MUST), computed tomography–derived body composition, systemic inflammation, and clinical outcomes in patients undergoing surgery for colorectal cancer. Am J Clin Nutr. (2019) 110:1327–34. doi: 10.1093/ajcn/nqz230
51. Xie B, Sun Y, Sun J, Deng T, Jin B, Gao J. Applicability of five nutritional screening tools in Chinese patients undergoing colorectal cancer surgery: a cross-sectional study. BMJ Open. (2022) 12:e057765. doi: 10.1136/bmjopen-2021-057765
52. Lu J, Xu Q, Zhu S, Chen L, Ding L, Hua H, et al. Comparison of five sarcopenia screening tools in preoperative patients with gastric cancer using the diagnostic criteria of the European Working Group on Sarcopenia in Older People 2. Nutrition. (2021) 95:111553. doi: 10.1016/j.nut.2021.111553
53. Cawood AL, Walters ER, Sharp SKE, Elia M, Stratton RJ. ‘Self-screening’ for malnutrition with an electronic version of the Malnutrition Universal Screening Tool (‘MUST’) in hospital outpatients: concurrent validity, preference and ease of use. Br J Nutr. (2018) 120:528–36. doi: 10.1017/s000711451800185x
54. Di Bella A, Croisier E, Blake C, Pelecanos A, Bauer J, Brown T. Assessing the concurrent validity and interrater reliability of patient-led screening using the malnutrition screening tool in the ambulatory cancer care outpatient setting. J Acad Nutr Dietet. (2019) 120:1210–5. doi: 10.1016/j.jand.2019.10.015
55. Chen Z, Yu H, Yuan H, Wang J, Wang Q, Zhu M, et al. Development and validation of self-screening tool for nutrition risk in patients with gastric cancer after gastrectomy: a study protocol. Nurs Open. (2024) 11:e2104. doi: 10.1002/nop2.2104
56. Yu M, Li X, Chen M, Liu L, Yao T, Li J, et al. Prognostic potential of nutritional risk screening and assessment tools in predicting survival of patients with pancreatic neoplasms: a systematic review. Nutr J. (2024) 23:17. doi: 10.1186/s12937-024-00920-w
57. Menozzi R, Valoriani F, Ballarin R, Alemanno L, Vinciguerra M, Barbieri R, et al. Impact of nutritional status on postoperative outcomes in cancer patients following elective pancreatic surgery. Nutrients. (2023) 15:1958. doi: 10.3390/nu15081958
58. Mękal D, Sobocki J, Badowska-Kozakiewicz A, Sygit K, Cipora E, Bandurska E, et al. Evaluation of nutritional status and the impact of nutritional treatment in patients with pancreatic cancer. Cancers. (2023) 15:3816–3816. doi: 10.3390/cancers15153816
59. Tu Y, Chen F, Yu Q, Song L, Chen M. Application of NRS-2002 and PG-SGA in nutritional assessment for perioperative patients with head and neck squamous cell carcinoma: an observational study. Medicine. (2024) 103:e40025–40025. doi: 10.1097/md.0000000000040025
60. Jogiat UM, Sasewich H, Turner SR, Baracos V, Eurich DT, Filafilo H, et al. Sarcopenia determined by skeletal muscle index predicts overall survival, disease-free survival, and postoperative complications in resectable esophageal cancer: a systematic review and meta-analysis. Ann Surg. (2022) 276:e311–8. doi: 10.1097/SLA.0000000000005452
61. Antasouras G, Papadopoulou SK, Tolia M, Pandi A-L, Spanoudaki M, Tsoukalas N, et al. May nutritional status positively affect disease progression and prognosis in patients with esophageal and pharyngeal cancers? A scoping review of the current clinical studies. Med Sci. (2023) 11:64. doi: 10.3390/medsci11040064
62. Rahman A, Martin C, Agarwala R, Heyland DK. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the ‘modified nutric’ nutritional risk assessment tool. Clin Nutr. (2014) 33:S2. doi: 10.1016/s0261-561450004-0
63. Moghaddam O, Emam M, Irandoost P, Hejazi M, Iraji Z, Yazdanpanah L, et al. Relation between nutritional status on clinical outcomes of critically ill patients: emphasizing nutritional screening tools in a prospective cohort investigation. BMC Nutr. (2024) 10:69. doi: 10.1186/s40795-024-00869-3
64. Rattanachaiwong S, Zribi B, Kagan I, Theilla M, Heching M, Singer P. Comparison of nutritional screening and diagnostic tools in diagnosis of severe malnutrition in critically ill patients. Clin Nutr. (2020) 39:3419–25. doi: 10.1016/j.clnu.2020.02.035
65. Lovell AL, Laughton S, Wood A, Pugh G. Nutrition screening, assessment, and intervention practices for children with cancer in Aotearoa, New Zealand. Nutrition. (2023) 116:112218. doi: 10.1016/j.nut.2023.112218
66. Gallo N, Horvath K, Czuppon K, Tomsits E, Felegyhazi E, Kovacs GT. Different nutritional screening tools and recommended screening algorithm for pediatric oncology patients. Clin Nutr. (2021) 40:3836–41. doi: 10.1016/j.clnu.2021.05.013
67. Hooper L, Abdelhamid A, Attreed NJ, Campbell WW, Channell AM, Chassagne P, et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst Rev. (2015) 4:CD009647. doi: 10.1002/14651858.cd009647.pub2
68. Chen X, Liu X, Ji W, Zhao Y, He Y, Liu Y, et al. The PG-SGA outperforms the NRS-2002 for nutritional risk screening in cancer patients: a retrospective study from China. Front Nutr. (2023) 10:1272420. doi: 10.3389/fnut.2023.1272420
69. Stefani G, Crestani M, Scott L, Soares C, Steemburgo T. Complementarity of nutritional assessment tools to predict prolonged hospital stay and readmission in older patients with solid tumors: a secondary analysis of a cohort study. Nutrition. (2023) 113:112089. doi: 10.1016/j.nut.2023.112089
70. Gebbia V, Cuggino R, Spada M, Blasi L, Mezzatesta P, Marchesa P, et al. Nutritional management of the patient with pancreatic cancer: from the diagnostic and therapeutic pathway to an integrated hospital-territory approach. La Clin Ter. (2023) 174:203–10. doi: 10.7417/CT.2023.2520
71. Kirbiyik F, Ozkan E. Knowledge and practices of medical oncologists concerning nutrition therapy: a survey study. Clin Nutr ESPEN. (2018) 27:32–7. doi: 10.1016/j.clnesp.2018.07.004
72. Ottery F. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. (1996) 12:S15–9. doi: 10.1016/0899-900790011-8
73. Ludlow S, McKeown E, Squires K, Pullen S, Barnes P, Gibberd A, et al. A comparative study of malnutrition screening tools in advanced liver disease: sensitivity, specificity, and patient acceptability. Clin Nutr ESPEN. (2025) 68:557–66. doi: 10.1016/j.clnesp.2025.06.003
74. Yin L, Chong F, Huo Z, Li N, Liu J, Xu H. GLIM-defined malnutrition and overall survival in cancer patients: a meta-analysis. J Parent Enteral Nutr. (2022) 47:207–19. doi: 10.1002/jpen.2463
75. Dickerson RN, Andromalos L, Brown JC, Correia MITD, Pritts W, Ridley EJ, et al. Obesity and critical care nutrition: current practice gaps and directions for future research. Crit Care. (2022) 26:283. doi: 10.1186/s13054-022-04148-0
76. Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition –a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
77. Mwala N, Borkent J, van der Meij B, de van, der Schueren M. Challenges in identifying malnutrition in obesity; an overview of the state of the art and directions for future research. Nutr Res Rev. (2024) 38:1–27. doi: 10.1017/s095442242400012x
78. Kiss N, Steer B, de van, der Schueren M, Loeliger J, Alizadehsani R, et al. Machine learning models to predict outcomes at 30-days using global leadership initiative on malnutrition combinations with and without muscle mass in people with cancer. J Cachexia Sarcop Muscle. (2023) 14:1815–23. doi: 10.1002/jcsm.13259
79. Wu T, Xu H, Li W, Zhou F, Guo Z, Wang K, et al. The potential of machine learning models to identify malnutrition diagnosed by GLIM combined with NRS-2002 in colorectal cancer patients without weight loss information. Clin Nutr. (2024) 43:1151–61. doi: 10.1016/j.clnu.2024.04.001
80. Xiao X, Yi X, Soe NN, Latt PM, Lin L, Chen X, et al. A web-based tool for cancer risk prediction for middle-aged and elderly adults using machine learning algorithms and self-reported questions. Ann Epidemiol. (2024) 101:27–35. doi: 10.1016/j.annepidem.2024.12.003
81. Bakaloudi DR, Papaemmanouil A, Vadarlis A, Makrakis D, Germanidis G, Timotheadou E, et al. Critical evaluation and comparison of nutritional clinical practice guidelines for cancer patients. Clin Nutr. (2023) 42:670–86. doi: 10.1016/j.clnu.2023.03.009
82. Wang PP, Soh KL, Binti Khazaai H, Ning CY, Huang XL, Yu JX, et al. Nutritional assessment tools for patients with cancer: a narrative review. Curr Med Sci. (2024) 44:71–80. doi: 10.1007/s11596-023-2808-4
83. Gascón-Ruiz M, Casas-Deza D, Torres-Ramón I, Zapata-García M, Alonso N, Sesma A, et al. Comparison of different malnutrition screening tools according to GLIM criteria in cancer outpatients. Eur J Clin Nutr. (2021) 76:698–702. doi: 10.1038/s41430-021-01021-1
84. Hu Y, Yang H, Zhou Y, Liu X, Zou C, Ji S, et al. Prediction of all-cause mortality with malnutrition assessed by nutritional screening and assessment tools in patients with heart failure: a systematic review. Nutr Metab Cardiovasc Dis. (2022) 32:1361–74. doi: 10.1016/j.numecd.2022.03.009
85. Sguanci M, Palomares SM, Cangelosi G, Petrelli F, Sandri E, Ferrara G, et al. Artificial intelligence in the management of malnutrition in cancer patients: a systematic review. Adv Nutr. (2025) 16:100438. doi: 10.1016/j.advnut.2025.100438
86. Peng P, Chen L, Shen Q, Xu Z, Ding X. Prognostic nutritional index (PNI) and controlling nutritional status (CONUT) score for predicting outcomes of breast cancer: a systematic review and meta-analysis. Pak J Med Sci. (2023) 39:1535–41. doi: 10.12669/pjms.39.5.7781
87. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
88. Ellez H, Keskinkilic M, Semiz H, Arayici M, Kısa E, Oztop I. The prognostic nutritional index (PNI): a new biomarker for determining prognosis in metastatic castration-sensitive prostate carcinoma. J Clin Med. (2023) 12:5434–5434. doi: 10.3390/jcm12175434
89. Pressac M, Vignoli L, Aymard P, Ingenbleek Y. Usefulness of a prognostic inflammatory and nutritional index in pediatric clinical practice. Clin Chim Acta. (1990). 188:29–36. doi: 10.1016/0009-8981(90)90157-n
90. Ingenbleek Y. Revisiting PINI scoring in light of recent biological advances. Nutrients. (2023) 15:1846–1846. doi: 10.3390/nu15081846
91. Magnano M, Mola P, Machetta G, Maffeis P, Forestiero I, Cavagna R, et al. The nutritional assessment of head and neck cancer patients. Eur Arch Otorhinolaryngol. (2014). 272:3793–9. doi: 10.1007/s00405-014-3462-z
92. Kronvall G, Smith P. Normalized resistance interpretation, the NRI method. APMIS. (2016) 124:1023–30. doi: 10.1111/apm.12624
93. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. (1999) 15:458–64. doi: 10.1016/s0899-900700084-2
94. Foletto E, Bernardes S, Milanez D, Razzera E, Silva F. Complementarity of nutrition screening with global leadership initiative on Malnutrition criteria for diagnosing malnutrition in critically ill patients: a comparison study of nutritional risk screening 2002 and modified nutrition risk in the critically ill score. J Parent Enteral Nutr. (2024) 48:440–8. doi: 10.1002/jpen.2629
95. Martinuzzi ALN, Manzanares W, Quesada E, Reberendo MJ, Baccaro F, Aversa I, et al. Nutritional risk and clinical outcomes in critically ill adult patients with COVID-19. Nutr Hosp. (2021) 38:1119–25. doi: 10.20960/nh.03749
96. Dent E, Hoogendijk EO, Visvanathan R, Wright ORL. Malnutrition screening and assessment in hospitalised older people: a review. J Nutr Health Aging. (2019) 23:431–41. doi: 10.1007/s12603-019-1176-z
97. Wilson M-MG, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. (2005) 82:1074–81. doi: 10.1093/ajcn/82.5.1074
98. Lidoriki I, Schizas D, Frountzas M, Machairas N, Prodromidou A, Kapelouzou A, et al. GNRI as a prognostic factor for outcomes in cancer patients: a systematic review of the literature. Nutr Cancer. (2020) 73:391–403. doi: 10.1080/01635581.2020.1756350
99. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent J-P, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
100. Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. (2009) 35:1728–37. doi: 10.1007/s00134-009-1567-4
101. dos Reis AM, Fructhenicht AVG, Moreira LF. NUTRIC score use around the world: a systematic review. Rev Bras Ter Intensiva. (2019) 31:379–85. doi: 10.5935/0103-507X.20190061
102. Al-Dorzi HM, Arabi YM. Nutrition support for critically ill patients. J Parent Enteral Nutr. (2021) 45:47–59. doi: 10.1002/jpen.2228
103. Jansen I, Prager M, Valentini L, Büning C. Inflammation-driven malnutrition: a new screening tool predicts outcome in Crohn’s disease. Br J Nutr. (2016) 116:1061–7. doi: 10.1017/s0007114516003044
104. Xu J, Cao J, Wang Y, Yao X, Wang Y, He Z, et al. Novel preoperative nutritional assessment tool and prognostic model for ESCC patients. J Cancer. (2019) 10:3883–92. doi: 10.7150/jca.31286
Keywords: cancer, malnutrition, malnutrition assessment, malnutrition screening tools, targeted therapy
Citation: Zhang L, Ding Z, Zhao Y, Cheng Z, Hu J and Huo L (2025) Advances and challenges in nutritional screening and assessment for cancer patients: a comprehensive systematic review and future directions. Front. Nutr. 12:1688344. doi: 10.3389/fnut.2025.1688344
Received: 19 August 2025; Accepted: 26 September 2025;
Published: 16 October 2025.
Edited by:
Ailin Zhao, Sichuan University, ChinaReviewed by:
Yadong Song, The First Affiliated Hospital of Zhengzhou University, ChinaGuoyu Wu, Guangdong Pharmaceutical University, China
Yien Luo, Guangdong Provincial People’s Hospital, China
Copyright © 2025 Zhang, Ding, Zhao, Cheng, Hu and Huo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanqing Huo, aHVvbHFAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
 Luocheng Zhang
Luocheng Zhang Zibo Ding
Zibo Ding Yanfei Zhao
Yanfei Zhao Ziyao Cheng
Ziyao Cheng Jiahao Hu
Jiahao Hu Lanqing Huo
Lanqing Huo