- 1Tecnologico de Monterrey, School of Medicine and Health Sciences, Monterrey, Mexico
- 2School of Doctorate Studies and Research, Universidad Europea de Madrid, Madrid, Spain
- 3Tecnologico de Monterrey, School of Engineering and Sciences, Monterrey, Mexico
- 4Faculty of Biomedical and Health Sciences, Universidad Europea de Madrid, Madrid, Spain
Background and aims: Depression is a leading cause of disability worldwide; studies have described it as a multifactorial disease that involves biological, psychological, and environmental factors. This systematic review explores the role of vitamins B9, B12, and D in depression, particularly emphasizing their biological effects, genetic variant interactions, and potential treatment implications.
Methods: A systematic literature review was conducted in Web of Science (WOS) and PubMed up to 15th June 2025. This review included 24 studies from randomized controlled trials (RCTs), observational studies, and case reports and examined the associations between genetic variants involved in vitamins B9, B12, and D metabolism; their biological processes; and outcomes in depression. Following the PRISMA criteria, researchers analyzed and extracted data independently; this resulted in the inclusion of 24 eligible papers (14 of vitamins B9 and B12 and 10 of vitamin D).
Results: Studies varied widely in design and methodology. Deficiencies in vitamins B9 (folate) and B12 (cobalamin) are associated with decreased neurotransmitter biosynthesis, higher homocysteine levels, and increased depressive symptoms. Vitamin D deficiency has also been associated with mood regulation through its effects on neurotransmission. Genetic variants, particularly in the MTHFR gene, have been associated with significant influence on individual susceptibility to depression in some populations, highlighting the interaction between genetics and micronutrient bioavailability and the need for further studies with diverse populations, larger study samples, and the inclusion of more genetic variants.
Conclusion: This systematic review emphasizes the role of vitamins B9, B12, and D and genetic variants associated with the development of depression. Regardless of the encouraging findings that supplementation with vitamins B9, B12, and D could support depressive symptomatology, additional research is needed to propose therapeutic guidelines. Personalized strategies considering dietary, genetic and environmental factors could enhance treatment results for individuals with depression.
1 Introduction
Depression is a very common mental health disorder; it is characterized by persistent emotions of sadness and indifference in pleasurable activities (1). Depression has been recognized as a significant public health concern (2); its importance is emphasized by two factors: its widespread occurrence and its negative impact on the quality of life of individuals (3). According to the World Health Organization (WHO), an estimated 280 million people globally were affected by this disease, with a notably higher prevalence among women (4). In its most severe manifestations, depression may lead to suicide; symptoms such as persistent thoughts of death may appear, as well as suicidal ideation or attempts (5).
The etiology of depression has been described as a complex and multifactorial disease linking environmental, psychological, and biological factors (5). Research into the biological factors including the genetic component of depression vulnerability has received increased interest since the early 2000s, revealing a growing understanding of its role in the disorder (6). These research findings have revealed information on the heritability of depression, which is estimated to be between 31 and 42% (6). A meta-analysis estimated that the heritability for depression is 37% (95% CI: 31–42%) (7), and data from family studies reveal a twofold to threefold increase in the risk of illness in first-degree children of patients with depression (6). The latter indicates that genetic factors may account for a significant susceptibility of individuals to depression; thus, more research should be performed (8).
Several authors have identified and reported numerous potential genetic variants linked with depression (9). These variants are frequently linked to synaptic plasticity, stress response pathways, neurotransmitter synthesis, and activity (9). Nevertheless, individuals with depression show food intake patterns that are frequently decreased in critical micronutrients such as vitamin B12 (VB12) (10), vitamin B9 (VB9) (11), and vitamin D (VD) (12), which might affect the onset, duration, and severity of the disease (13). Various studies have demonstrated the prevalence of VB9 and VB12 lower serum levels among some individuals with depression (10, 11), while low VD concentrations have also been associated with this disease (14). In addition to being associated with a variety of mental diseases, both VB9 and VB12 deficiency could aggravate depressive symptoms owing to excitotoxic responses mediated by homocysteine (HCy) buildup (15), although the results are still conflicting and further research must be done.
Micronutrient research has historically assumed that everyone has the same fundamental biological functions. However, recent studies have discovered numerous genetic variants that alter an individual’s general response to dietary micronutrient consumption (16). The complicated interactivity between micronutrients, genetics, and depression highlights the importance of individualized approaches to depression prevention and treatment. Studies have linked the interaction of micronutrients, gene variants, and incidence and severity of depression. This systematic review aimed to synthesize and integrate the existing, yet fragmented, body of evidence regarding the complex interplay between vitamins B9, B12, and D and depression. By consolidating data from biological systems, clinical studies, and functional genomics, this review aimed to elucidate the mechanisms underlying these interactions. Consequently, it aimed to generate novel insights that can inform possible complementary strategies for the prevention and management of depressive symptoms.
2 Research method
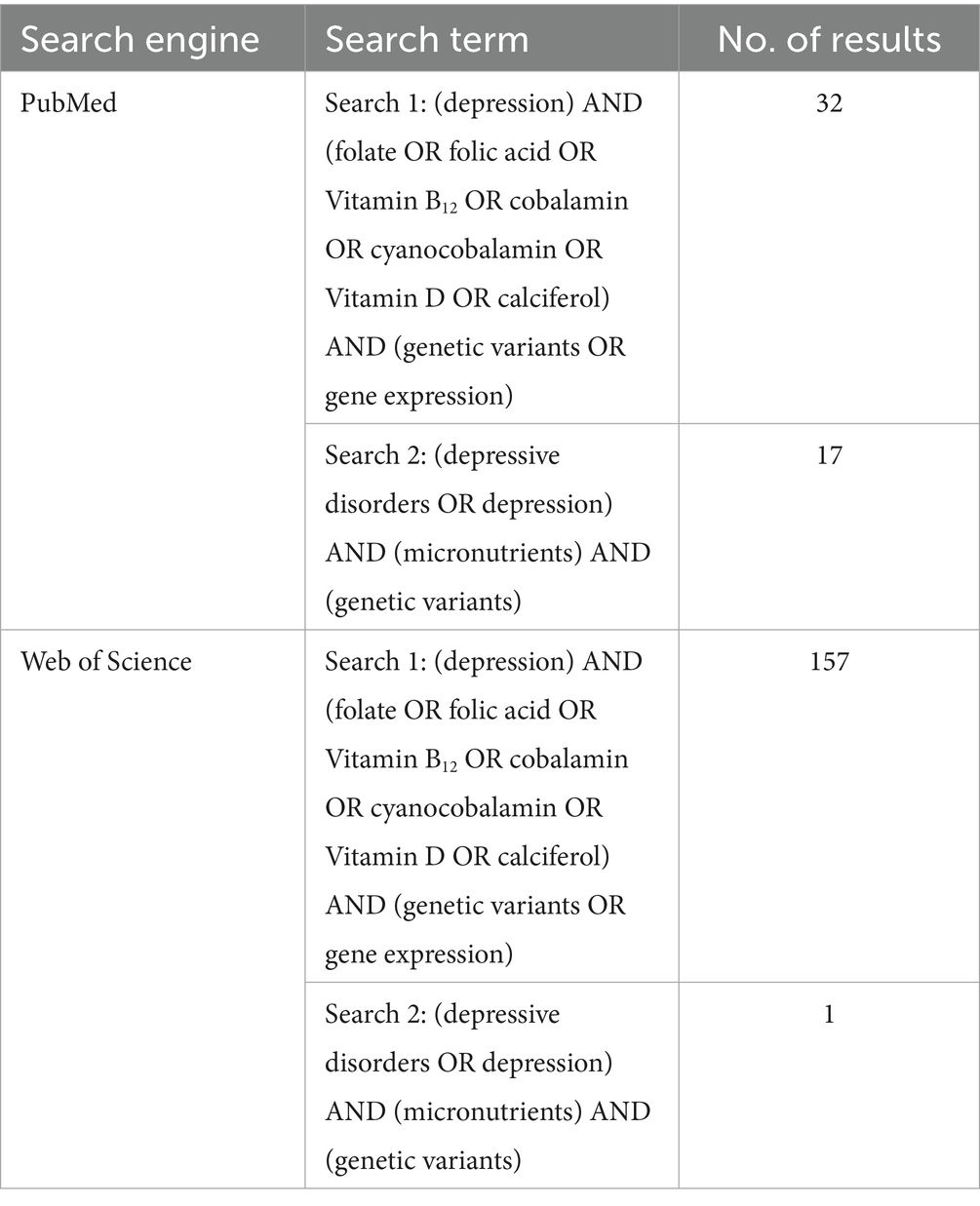
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria were used to report this systematic review. From inception until 15 June 2025, the electronic databases such as Web of Science (WOS) and PubMed were searched. The citations were downloaded and imported into Covidence software, where duplicates were automatically removed. The search strategy aimed to identify studies exploring the association between vitamin-related genetic variants (specifically for vitamins D, B9, and B12) and depression or depressive symptoms. To capture a broad yet relevant range of literature, multiple search terms were applied, as detailed in Table 1. These included the following terms:
• Population/Disease terms: “depression” and “depressive disorders.”
• Exposure terms: “folate,” “folic acid,” “vitamin B12,” “cobalamin,” “cyanocobalamin.” “vitamin D,” “calciferol,” and “micronutrients.”
• Genetic terms: “genetic variants” and “gene expression.”
The search was restricted to studies published in English, those conducted on human subjects, and those including adult participants (≥19 years old).
2.1 Study selection
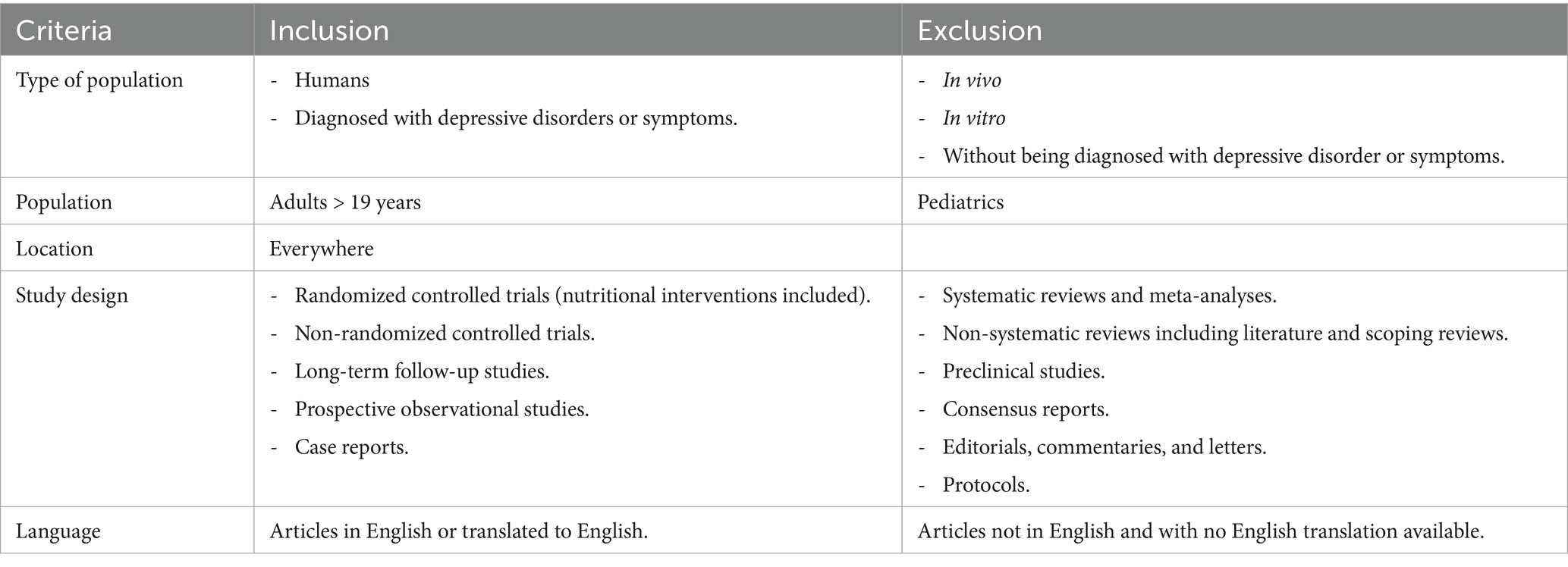
Studies were assessed if they (1) included VD, VB9, or VB12-associated genetic variants involved in vitamin metabolism and (2) had individuals who were depressed or had depressive symptoms. Table 2 details the full inclusion and exclusion criteria. Only original, peer-reviewed studies were considered.
Two researchers reviewed the titles and abstracts for relevance (RSG and MRGS). Subsequently, a third researcher (AC-S) reviewed and resolved the disagreements until consensus was reached. Then, successively, individually from each other, two researchers (RSG and MRGS) reviewed the full texts of potentially eligible articles. Any disagreements were discussed by the third researcher (AC-S) until consensus was reached. Data were extracted from the studies by two authors (RSG and MRGS) independently, and the disagreements were discussed until a consensus was reached.
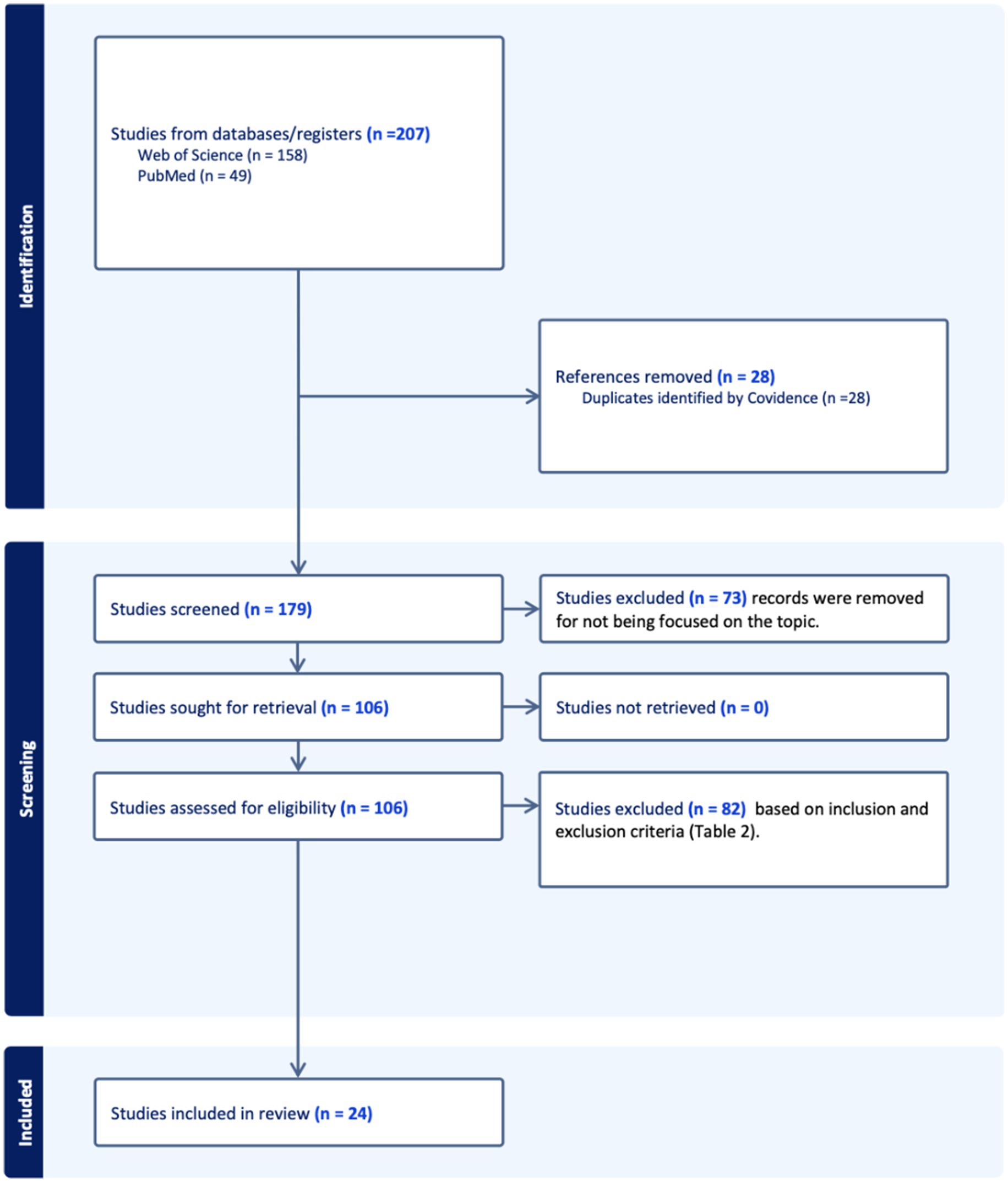
Overall, 207 records were identified through all database searches. After 28 duplicates had been removed, titles and abstracts from the remaining 179 records were screened, and the full text of 106 was assessed for eligibility. Finally, 24 studies were included in this systematic review. A comprehensive flow diagram following the PRISMA criteria of the study selection process is presented in Figure 1.
3 Results and discussion
3.1 Depression, etiology, and risk factors
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), states that depressive disorders (DD) violence and neglect in childhood have also been presented as risk factors for the onset of depression in adults (17). Genetic factors also play an important role, and family history remains one important risk factor, which includes major depressive disorder (MDD), also known as clinical depression or depression and other subtypes (1). In the late 2000s, the WHO placed MDD as the third most significant cause of the global burden of illness, and it is anticipated to be the primary cause of disability by 2030 (18). Depression is considered a complex pathology, where irregularities in neurotransmission are hypothesized as contributing factors in the development of MDD (19). Although the efficacy of different antidepressant medications suggests that this is not an invalid hypothesis (20), emerging theories include neuroregulatory systems and neural circuits in MDD complexity in GABA, glutamate, and glycine in the etiology of depression (17). Thyroid dysfunctions and growth abnormalities are also identified to be related to depressive symptoms (17), while estimates of heritability range between 31 and 42% (6). Depression can appear at any age but usually begins at young adulthood (21). Moreover, chronic degenerative disorders including diabetes, cancer, and Parkinson’s disease frequently interact with depression, intensifying the severity of both conditions (17). Some of the genetic studies identified a few polymorphisms associated with depression in the genes DRD4, HTR1A, MAOA, S LC6A4, PCLO, and 5-HTT (9). For example, variants in HTR1A—which encodes serotonin receptor 1A—affect serotonin signaling in relation to mood regulation (22). Other important genes may include SLC6A3, which has been implicated in several neuropsychiatric diseases, but the mechanism remains inconclusive (23). Another example is the serotonin transporter gene, 5-HTT, which has a specific variation called 5-HTTLPR, one of the most relevant genes studied in psychiatric genomics, and is known to moderate the relationship between stress and depression (24). As research on genetics increases, a more complex relationship between hereditary and environmental factors arises in the etiology of DP. Finally, life stressors such as interpersonal difficulties or serious illness or injury might trigger depression as well (25).
3.2 Biological systems of vitamin B9, vitamin B12, and vitamin D in depression
During the diagnosis of depression, clinicians are encouraged to rule out nutritional deficiencies, such as VB9, VB12, and VD (17). This might be due to the different biological mechanisms that micronutrients are involved in, which will be further discussed.
3.3 Vitamins B9 and B12
3.3.1 VB9 properties and deficiency
VB9, frequently known as folate or folic acid, is essential for multiple processes in biology, including neurotransmitter production and DNA methylation (11). It must be received from food or supplements because it cannot be synthesized by the body naturally. Green leafy vegetables, legumes, and fortified cereals or grains are natural sources of VB9 (26). Cereal fortification has been implemented worldwide as a measure to satisfy the needs of this nutrient and prevent its insufficiency (27); for example, in the USA, plasma levels of HCy were reduced (28). A VB9 deficiency may result in hematological, dermatological, and neuropsychiatric symptoms, such as irritability, insomnia, cognitive impairment, and depressive symptomatology (29). The oral route is usually preferred for correcting the deficiency; if it cannot be used, the intramuscular or parenteral route can be considered (29).
The Daily Recommended Intake (DRI) of VB9 varies according to age, sex, and stage of life. For adults, the DRI is 400 μg (29), whereas for pregnant and lactating women, the requirement increases to 600 μg and 500 μg (30), respectively. In individuals with depression, VB9 or its active form supplementation as adjuvant therapy might improve depressive symptoms (31).
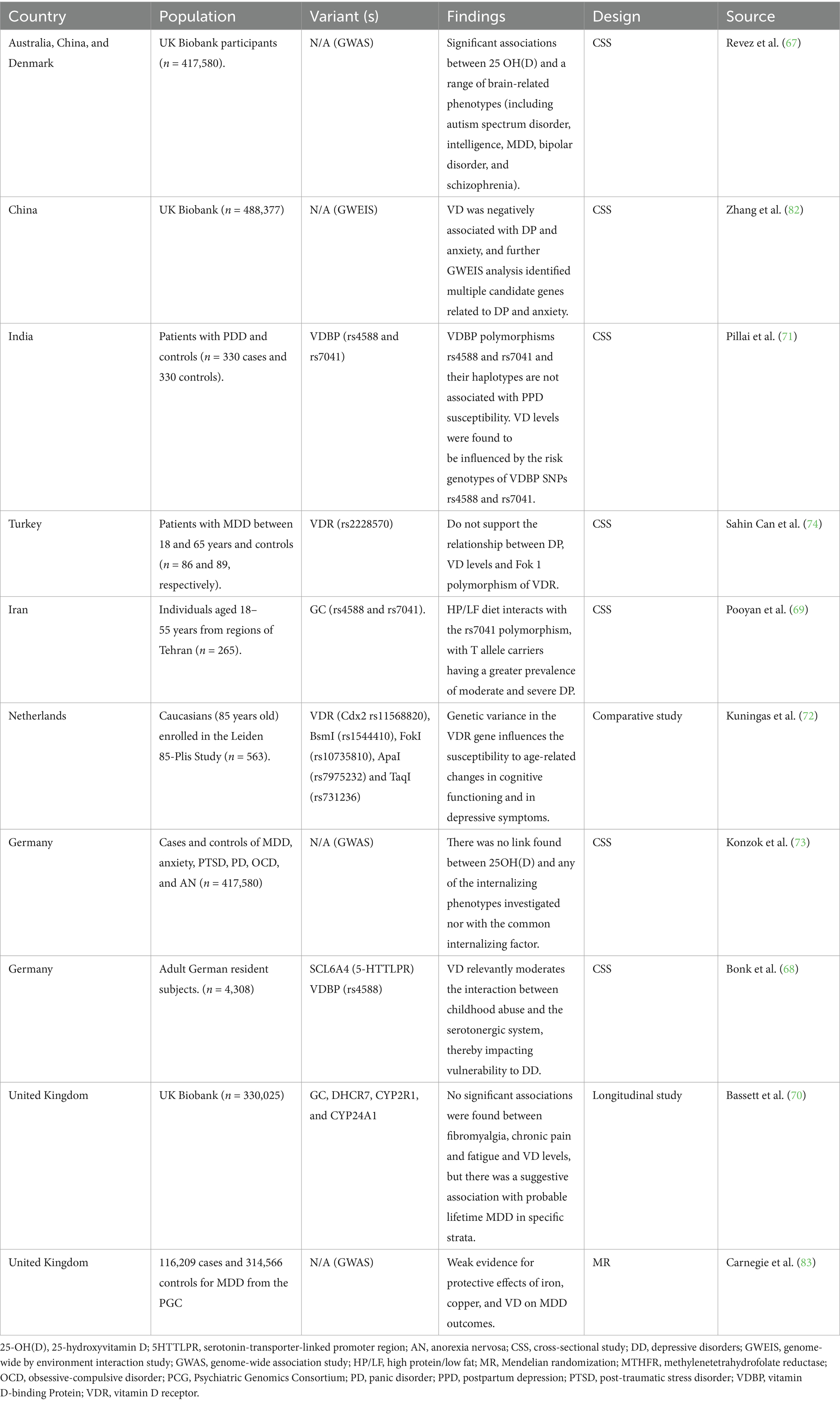
Numerous studies have linked VB9 deficiency to a higher incidence, prolonged episodes, and more severe presentations of depression (11), which may be due to its role in neurotransmission biosynthesis (32). As shown in Figure 2, serotonin, dopamine, adrenaline, and noradrenaline, all of which are fundamental in mood regulation, involve VB9 and VB12.
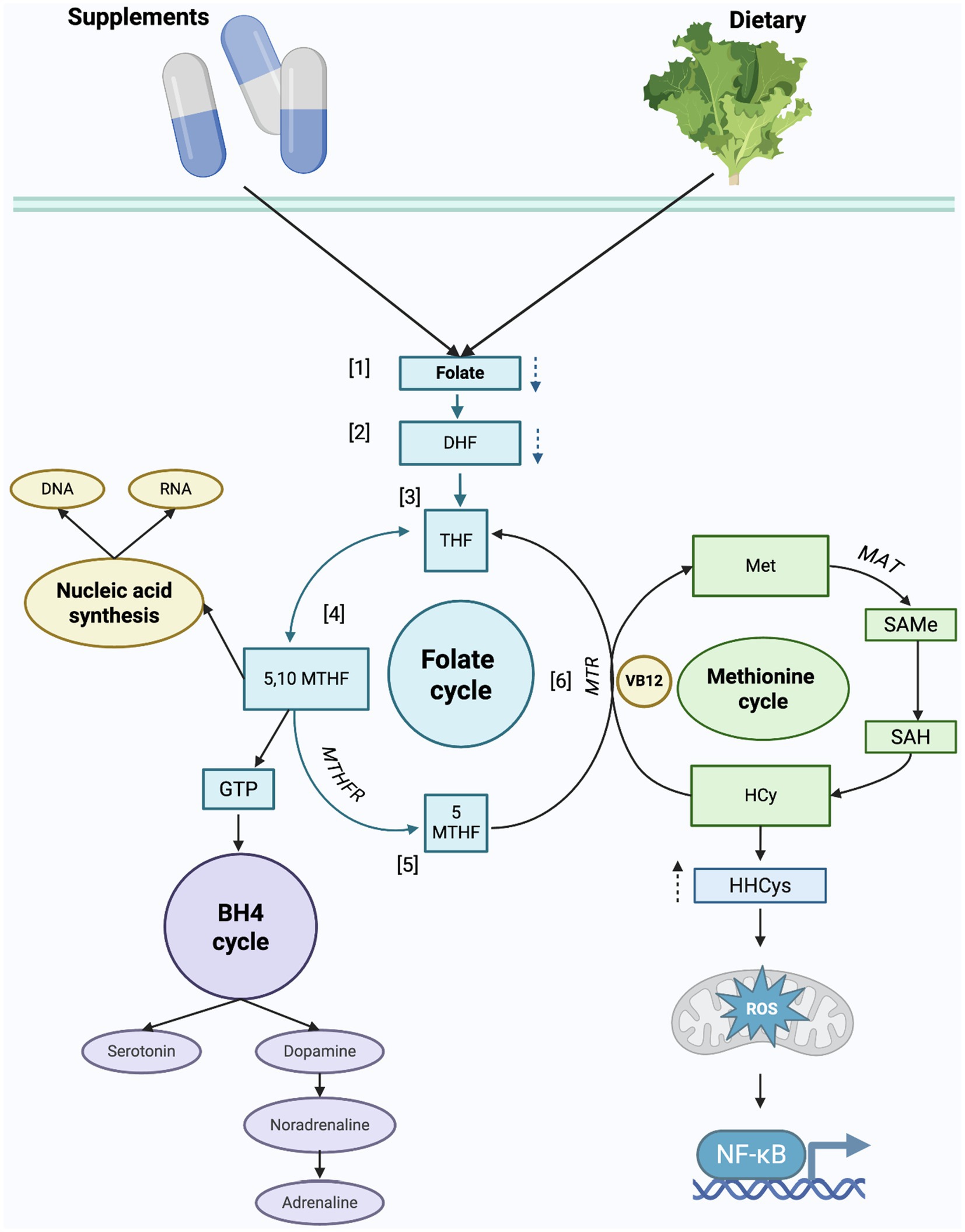
Figure 2. Biological roles of vitamins B9, B12 in folate and methionine cycles related to depression. [1]. Folic acid, the synthetic form of VB9, is reduced to [2] dihydrofolate (DHF) and subsequently reduced to [3] tetrahydrofolate (THF). THF is further catalyzed, and [4] 5,10-methylenetetrahydrofolate (5,10- MTHF) is obtained. 5,10-MTHF is predominantly used in the purine and thymidine synthesis, as mentioned above as the attributed functions of VB9 and VB12. For 5,-10 MTHF to convert to the active form of VB9, [5] methyltetrahydrofolate (5-MTHF), the enzyme methylenetetrahydrofolate reductase (MTHFR) must catalyze the reaction. 5-MTHF together with VB12 is required for the remethylation of homocysteine (HCy) to methionine (Met) in the methionine cycle. [6] The conversion to Met is due to the action of methionine synthase (MTR), which is a VB12-dependent enzyme. Subsequently, Met is converted to S-adenosylmethionine (SAMe) by the S-Adenosylmethionine synthetase (MAT) enzyme. SAMe is a methyl group donor involved in DNA and RNA methylation. In neurotransmitter synthesis, THF derivatives are involved in the regeneration of tetrahydrobiopterin (BH4) from dihydrobiopterin (BH2), an important cofactor in the conversion of amino acids to serotonin, dopamine, and norepinephrine.
Several authors have studied VB9 supplementation in individuals with depression (33). For example, in a case report, Jha et al. administered 15 mg of L-methylfolate (folate’s active form), as an adjunct to the pharmacological treatment of a 25-year-old male case of non-responding depression, with other psychiatric comorbidities and a polymorphism in the MTHFR gene, finding favorable results (34). Although excess VB9 is excreted through the urine, their high levels have been suggested to accelerate the development of preneoplastic conditions (35), impair cognitive function in the elderly (36), and disrupt immunological processes (35). When VB9 supplementation is given to patients with depression, other research designs with a greater degree of evidence have demonstrated favorable outcomes (33). Nevertheless, further research is required to determine the ideal supplement forms and amounts needed. Similarly, additional studies should aim to explain the underlying mechanisms of VB9 in depressive symptoms and explore potential interactions with treatments and genetic factors (33).
3.3.2 VB12 properties and deficiency
Cobalamin, also known as VB12, is a fundamental micronutrient belonging to the group of water-soluble vitamins (37). It is found in food sources such as beef and poultry, as well as in fortified cereals or in dietary supplements (38). VB12 is produced by bacteria and archaea present in predatory organisms, which is why it is primarily found in meat products. Similar to VB9, VB12 deficiency is multifactorial, and although its manifestations are similar, VB12 deficiency involves other symptoms such as memory loss, cognitive difficulty, and personality changes (39). VB12 is stored in the liver, lowering its possibility of deficiency (39). Depending on the underlying cause, the route of administration and duration of treatment varies (39). Deficiencies of VB12 can be treated with intramuscular injections or oral VB12 (39). The IOM established a DRI of 2.4 mcg/day of VB12 for male and female individuals over 14 years of age, a requirement that only increases during pregnancy and lactation to 2.6 mcg and 2.8 mcg, respectively (40). In individuals with depression, the relationship of this vitamin has been studied because its function, as in VB9, is strictly related to the synthesis of neurotransmitters (41). In general, the majority of authors study the function of both due to their proximity to their metabolic pathways, which will be further discussed.
3.4 Biological role of VB9 and B12 in depression
Biologically based evidence suggests a connection between low VB9 and VB12 status and depressive symptomatology. Circulating plasma levels of VB9 and VB12 can be influenced by various factors, including alterations in enzymatic activity, deficiency of other micronutrients, or dietary choices and lifestyle (19). Depression frequently causes appetite alterations (1), which could lead to limitations in the consumption of other critical micronutrients (42). A deficiency in VB9 results in lower concentrations of 5-MTHF, the biologically active form of VB9, due to its role in the VB9 and Met cycles, and may lead to increased HCy levels and impaired remethylation of HCy to Met; Met is key for the synthesis of S-adenosylmethionine (SAMe), a methyl donor compound involved in neurotransmitter synthesis, such as serotonin and dopamine (43). Additionally, VB9 and B12 are essential for DNA synthesis and cellular metabolism (43). These mechanisms may be disrupted by VB9 deficiency, leading to HHCy and impaired neurotransmitter production (43), which could contribute to depressive symptoms. Similarly, VB12 participates in mood modulation by maintaining the myelin sheaths of neurons and by its implication as a cofactor in the biosynthesis of SAMe (37, 43). A deficiency in VB12 may result in neurological and psychiatric manifestations, such as depressive symptoms (10). Since VB9 and VB12 participate in interconnected cycles, a deficiency in either may lead to increased HCy levels, which have been associated with mechanisms contributing to depression (15, 41). Clinical evidence has demonstrated that low levels of VB9 and VB12 are associated with higher depressive symptoms (10, 11). Moreover, some meta-analyses suggest that VB9 may improve symptoms when used as an adjunct with routine antidepressant agents (33). Although the biological pathways through which VB9 and VB12 exert their effects in the pathogenesis of depression have been proposed and described, the complex interactions of the factors involved in depression require further research to establish definitive therapeutic recommendations.
3.5 Homocysteine and depression
HCy is a non-dietary amino acid that can be converted via transsulfuration to cystathionine or recycled into methionine (Met) through the Met cycle (44, 45). Both processes depend on B complex vitamins, primarily vitamins B6, VB9, and VB12 (44). Deficiency in these vitamins impairs the Met cycle, leading to HHCy, which has been linked to several conditions, such as depression, chronic inflammation, neurotoxicity, and alterations in epigenetic regulation (44, 46, 47). HHCy is described as serum levels above 15 μmol/L (normal serum values range from 5 to 15 μmol/L) (45). Additionally, HHCy has been linked to the activation of pro-inflammatory pathways, including NF-κB and the overproduction of reactive oxygen species (ROS) (42). Although free radicals (especially ROS) are normal byproducts of cell metabolism and are essential for cell signaling and pathogen defense, increased levels that exceed cellular antioxidant defenses lead to oxidative stress (OS) (42). ROS also play an important role in many complex diseases, such as depression, and one of the proposed mechanisms associating ROS and depression includes the initiation of inflammatory pathways, which may interact with neurotransmitters and neural circuits in a chronic and sustained manner (42). Furthermore, ROS can interfere with neuroinflammation, neuroplasticity, biosynthesis, and the release of serotonin and dopamine, both crucially implicated in mood regulation (42). NF-κB is a transcriptional factor involved in inflammation, modulation of immune response, and promoting cell survival (48). In homeostatic conditions, NF-κB is sequestered in the cytoplasm inactivated due to its inhibitor IκB (48). When inflammatory cytokines activate the pathway, IκB is phosphorylated and degraded, allowing the translocation of NF-κB dimers to the cell nucleus, promoting the transcription of genes involved in inflammatory and stress responses (48). Studies in rodent models of depression have shown that the activation of NF-κB is a key mediator in neuroinflammation (49). Since NF-κB also regulates neuroplasticity and synaptic activity, its dysregulation may impair these processes and contribute to depressive symptomatology (49). Furthermore, the chronic activation of NF-κB due to prolonged stress may promote higher inflammatory states and increased OS (42). Certain pharmacological interventions, such as antidepressants, may affect NF-κB signaling, suggesting a potential therapeutic application by targeting this pathway (49).
3.6 Genetic variants of VB9 and VB12 associated with depression
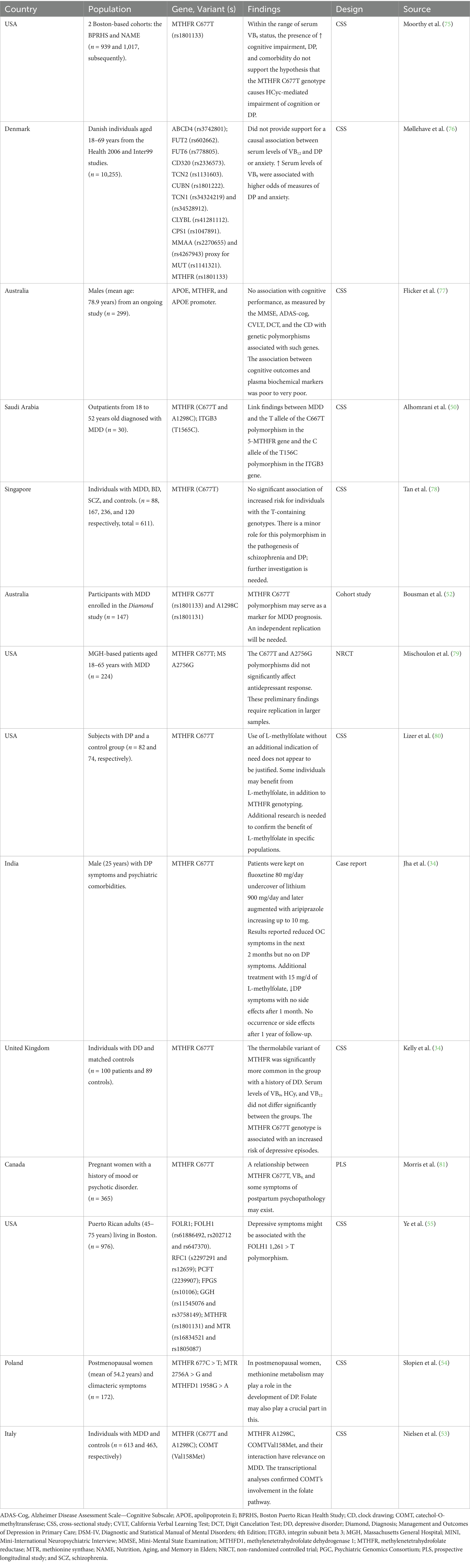
Current research reveals a complex interaction between genetic variations, metabolism of nutrients, and MDD. The MTHFR gene has been explored widely by several researchers due to its critical role for coding a limiting enzyme in the folate cycle and its connection to methylation, DNA, and neurotransmitter synthesis. Certain variants in this gene have been described and associated with depression, particularly the C677T variant. Alhomrani et al. explored this variation in 30 Saudi Arabian individuals from an outpatient clinic, obtaining results linking the T allele to MDD (50). In Northern Ireland, Kelly et al. similarly described an increased risk of DD among carriers of the 677 T allele (51). In Australia, Bousman et al. studied 147 participants with MDD from another study cohort, analyzing the same variant, but showing that the CC genotype revealed the most severe symptom severity course in this population (52). Nevertheless, the results regarding other variants, such as A1298C, were less clear. In the Italian population, Nielsen et al. reported that those women with the CC genotype for the MTHFR A1298C variant have a higher vulnerability for MDD, together with a genetic vulnerability to develop DO suitable to COMT Val158Met polymorphism (53). These results highlight the need for further studies to explain the role of the variants of MTHFR in diverse MDD populations.
Additionally, other variants implicated in VB9 and VB12 metabolism, beyond the MTHFR, have been described. One of them is the MTR, a gene that codes for an enzyme in the final step of methionine biosynthesis (54). The MTR GG genotype showed a significant increase in the risk for moderate and severe depression in a study conducted in postmenopausal Polish women; they also found that the MTHFR C677T contributes significantly to the depression risk (54). Moreover, other variants in the gene FOLH1, 1561C > T polymorphism, have been described as a factor linked to reduced depressive symptoms; this was studied in a cohort of the Puerto Rican population, though its lack of mediation through plasma VB9 or HCy indicates more investigation is needed to understand the mechanisms involved (55).
However, the potential therapeutic role of L-methylfolate supplementation as a treatment adjuvant, especially in individuals with SSRI-resistant depression, appears promising. However, clearer guidelines and long-term efficacy studies with large sample sizes and different types of VB9 supplementation are needed. While initial reports suggest it might be helpful in complex psychiatric conditions for some individuals, the lack of established protocols for addressing genetic variants such as MTHFR, as well as dosage and supplementation type, makes clinical application challenging. These findings contribute to the understanding of how VB9 and VB12 metabolism may impact depressive symptomatology.
Finally, the studies on VB9 and VB12 are currently emphasizing the importance of addressing the limitations in their methodologies, such as cross-sectional designs, exclusion of diverse populations, potential biases, and reliance on self-reported measures. Future research is expected to adopt longitudinal designs and larger sample sizes with diverse populations and explore additional genetic variants to understand the interaction between genetic variants associated with VB9 and VB12 metabolism and depression.
3.7 Vitamin D
3.7.1 VD properties and deficiency
VD belongs to the group of fat-soluble vitamins, can be endogenously synthetized, and can be obtained by supplementation or some dietary sources such as yeasts, mushrooms, fatty fish, egg yolk, and fortified products such as milk (56, 57). While its best-known function is its role in bone mineralization (57), an increasing number of studies have associated VD deficiency with increased depressive symptomatology (58). VD insufficiency is frequently asymptomatic; therefore, its screening has not been established as a general guideline, although populations such as African American individuals, Hispanic individuals, pregnant women, babies, and older adults have been considered to be at increased risk (59). VD deficiency is multifactorial and may result from inadequate sun exposure and insufficient dietary intake (56). The IOM established a DRI for VD of 600 IU for men and women over the age of 18 years and for adults aged 70 years and older, an additional 200 IU should be added (56). Some authorities have even suggested an intake of up to 1,500–2,000 IU in healthy adults (60). In cases of deficiency or in certain pathologies, higher doses of VD, by supplementation, may be recommended (56). To confirm an existing deficiency, the 25-hydroxyvitamin D blood test is advised (60).
3.7.2 Biological functions of VD associated with depression
Apart from the well-known function in calcium regulation and absorption (60), VD is also acknowledged for having multiple functions in human health such as immunomodulation, as well as mood disorders (57). One of the proposed mechanisms to explain the relationship between depression and VD is the impact it may have on neurotransmission, as can be observed in Figure 3. VD has been suggested to boost the activity of tryptophan hydroxylase 2, an enzyme responsible for converting tryptophan to serotonin in the brain (58), a major neurotransmitter involved in mood regulation. Similarly, in vitro studies have linked VD to the inhibition of MAO activity (61), an enzyme responsible for the breakdown of serotonin, dopamine, and norepinephrine (62), thereby increasing postsynaptic availability of these neurotransmitters and potentially alleviating depression-related symptomatology. VD has also been associated with depressive due to its anti-inflammatory properties, which may help modulate pro-inflammatory cytokine activity (63). In vivo, VD has been shown to enhance the expression of brain-derived neurotrophic factor (BDNF) (47), a protein involved in neuronal survival, plasticity, and cognitive functions. In individuals with depression, low levels of this protein have been described (64), suggesting that VD may have antidepressant effects.
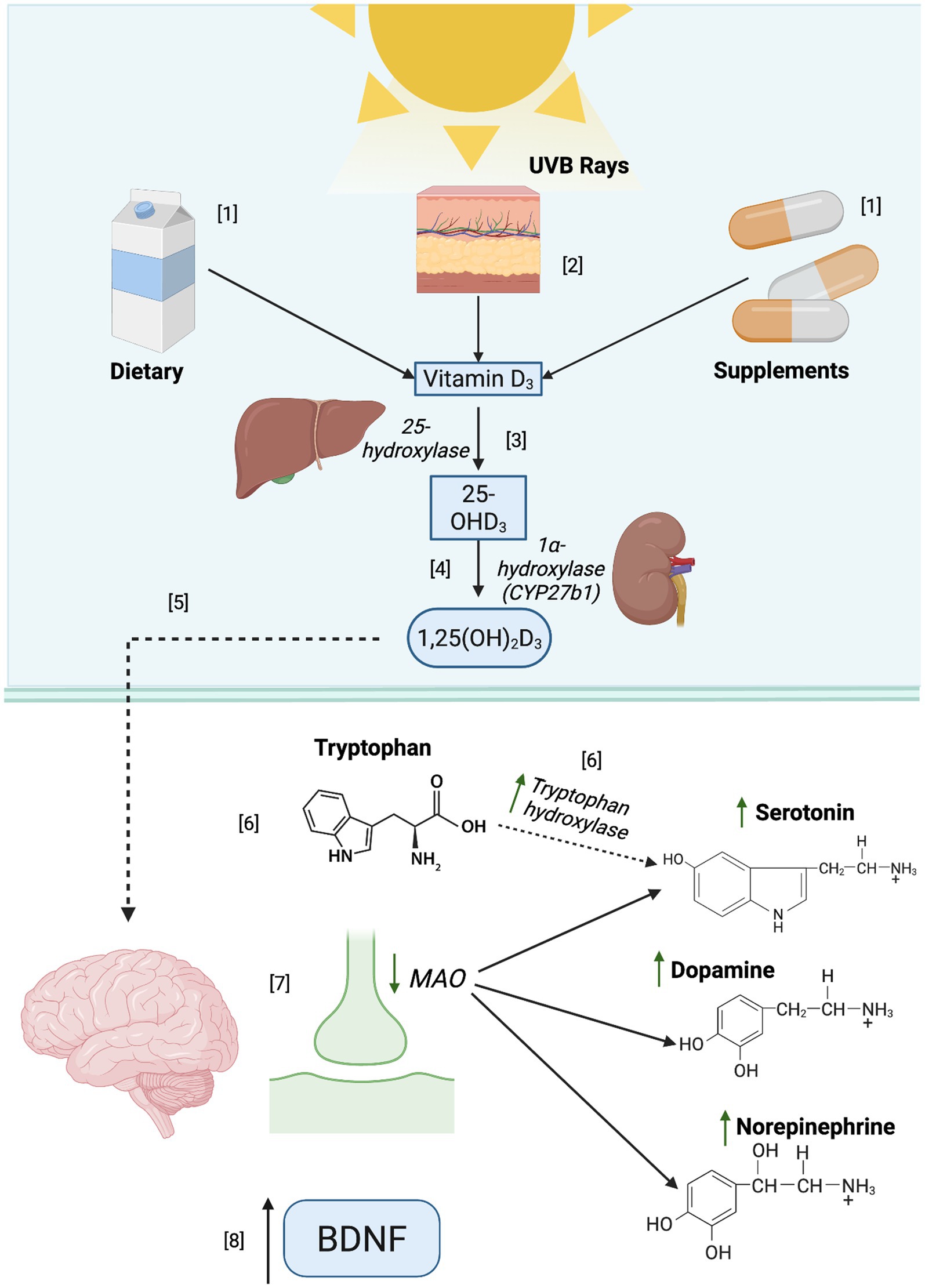
Figure 3. Biological role of vitamin D in neurotransmitter regulation and its implications in depression. [1] Cholecalciferol (D3) may be obtained from different sources, such as the diet and supplements. [2] It is synthesized naturally when the skin is exposed to ultraviolet B rays (UVB), leading to the synthesis of D3, the most potent form of VD. [3] In the liver, VD undergoes hydroxylation and forms calcidiol (25- hydroxyvitamin D or [25(OH)D3]), the major circulating form of VD. [4] In the kidneys, 25(OH)D is converted into a biologically active form of VD known as calcitriol (1, 25-dihydroxyvitamin D, [1,25 (OH)2 D]). [1,25(OH)2D] binds to the vitamin D receptor (VDR), which is widely distributed throughout the body and highly expressed in the brain and many other organs because it exerts effects on several critical biological processes. In the brain, [6] the activity of the enzyme tryptophan hydroxylase is augmented by [1,25(OH)2D], increasing serotonin synthesis. [7] Some studies suggest that VD may decrease the MAO activity, potentially increasing serotonin, dopamine, and norepinephrine availability. Additionally [8], BDNF expression could be increased due to VD.
Several clinical studies have reported the relationship between serum VD levels and depression symptoms (58). Low levels of serum 25(OH)D have been linked to higher depression symptomatology, according to some research (61). On the other hand, RCTs have demonstrated that VD supplementation, specifically with cholecalciferol (VD3), could significantly improve depressive symptoms in individuals with low levels of 25(OH) VD (58, 65). Although VD has a well-documented regulatory effect on many biological processes, and clinical findings are promising, the relationship between VD and depression is complex and influenced by various factors, including individual variability in response to supplementation, type, and even population. There is currently not enough research to recommend the therapeutic use of VD supplementation in the prevention or treatment of depression.
3.7.3 VD genetic variants associated with depression
Recent studies have demonstrated a complex relationship between VD, genetic variants, and depression. One example includes research conducted by Zhang et al. who analyzed the UK Biobank and found a negative association between VD levels and depression and anxiety, in which significant associations between blood VD and psychiatric traits were found (66). Another study analyzing the UK Biobank conducted by Revez et al. found a significant association between 25 OH D levels and brain-related phenotypes that included MDD (67). Furthermore, low VD levels were linked to a higher probability of depression among populations, including carriers of 5-HTTP gene variants and individuals with a history of traumatic childhood events (68). Additionally, recent studies have examined the relationship between depression and genetic variations in the VD pathway. Vitamin D levels might be influenced by certain polymorphisms; some studies have associated this with GC and CYP2R1 genes (69, 70). The GC gene encodes for VD-binding protein, involved in the transportation of VD to various tissues (70). Similarly, the gene CYP2R1 encodes the VD 25-hydroxylase, the liver enzyme involved in the 25-hydroxylation of VD, a key regulator in the synthesis of 25 (OH)D (70). Pooyan et al. studied 265 individuals in Tehran using convenience sampling and found that a high-protein/low-protein diet interacts among carriers of the T allele in the rs7041 polymorphism, having a greater prevalence of moderate and severe depression symptomatology (69). In addition, this polymorphism was also studied in 330 PPD patients and controls; the findings were that rs4588 and rs704 are not associated with PPD in this population; however, they concluded that VD levels are influenced by polymorphisms in the vitamin D-binding protein (VDBP) (71). These genetic variants may have a significant influence on an individual’s metabolism of VD, resulting in a substantial effect on mental health outcomes. However, the small sample sizes and non-diverse populations in these studies make it difficult to examine how these factors may interact. Additionally, Kuningas et al. studied a cohort of 599 men aged 85 years and older from Leiden in the Netherlands and found that variants in vitamin D receptor (VDR) may have an impact on an individual’s cognitive ability as they age (72). In contrast, Konzok et al. and Sahin Can et al. studied different populations and did not find significant results linking VD levels or variants with depression (73, 74). Finally, more research needs to be performed regarding the genetic variants associated with VD biotransformation across different populations, using larger sample sizes and exploring a broader range of genetic variants.
3.8 Narrative appraisal of risk of bias
The main strength of this review is that it has systematically mapped the current state of the scientific literature in this area, including studies on micronutrient supplementation, genetic variants, and diagnostic assessments of depressive disorders. However, the included studies varied considerably in sample size, ranging from single-case reports (e.g., (34), n = 1) to large population-based cohorts (e.g., UK Biobank studies with n > 400,000; 67, 73, 74, 82) Table 3. Many of the smaller cross-sectional and case–control studies, particularly those examining vitamin B9/B12 polymorphisms (e.g., Saudi Arabia, n = 30; Singapore, n = 611), may lack statistical power to detect subtle genetic effects. Representativeness also varied: several studies recruited population-based samples (e.g., Denmark, n = 10,255; USA Boston cohorts, n ~ 2,000), enhancing external validity, whereas clinical samples from psychiatric settings (e.g., India, n = 25; Australia, n = 147) may limit generalizability to broader populations with depressive disorders. Overall, the heterogeneity in sample size and recruitment context highlights potential bias in extrapolating findings across populations (Table 4).
Exposure to nutrients and assessment of depressive outcomes were generally well-characterized but not entirely standardized. Serum levels of vitamin B9 and B12 were measured in some cohorts (e.g., (51, 76), whereas other studies relied on genotype-based proxies (e.g., MTHFR C677T) without direct biochemical confirmation. Depression assessment varied from structured clinical interviews (e.g., Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria) to self-reported depressive symptoms and cognitive scales [e.g., MMSE and Alzheimer Disease Assessment Scale—Cognitive Subscale (ADAS-Cog)], creating potential inconsistencies in outcome ascertainment. For vitamin D studies, genome-wide association study (GWAS) and genome-wide by environment interaction study (GWEIS) approaches provided high-resolution genetic data but often lacked detailed nutrient exposure data, complicating interpretation of causal associations.
Several studies accounted for relevant confounders such as age, sex, comorbidities, and lifestyle factors (e.g., (55, 67)), although adjustment was inconsistent across studies. Smaller or single-center studies often reported limited adjustment, increasing susceptibility to residual confounding (e.g., (50, 71)). The diversity in covariate control underscores the challenge of comparing outcomes across observational designs and reduces the robustness of pooled inferences regarding genetic-nutrient interactions in depressive disorders.
Coverage of genetic variants ranged from single candidate polymorphisms (e.g., MTHFR C677T, rs1801133) to multi-locus panels and genome-wide approaches (e.g., UK Biobank GWAS/GWEIS; 67, 82). Candidate gene studies frequently focused on variants within key folate, vitamin B12, or vitamin D pathways, which provided mechanistic insight but may have missed additional genetic contributors. GWAS and GWEIS studies offered broader genomic coverage, enhancing discovery potential, but often lacked detailed phenotype stratification, limiting functional interpretation. In several studies, proxies were used for variants (e.g., MMAA rs4267943 as a proxy for MUT rs1141321), which may introduce measurement bias.
Given the heterogeneity in study designs and methodologies, findings should be interpreted with caution. Because of that, a single standardized risk of bias tool could not be uniformly applied. This approach allowed us to systematically consider factors such as sample size and representativeness, exposure and outcome ascertainment, confounder adjustment, genetic variant coverage, and reporting transparency. While this method provided a nuanced evaluation of the included randomized controlled trials (RCTs), observational studies, and case reports, it also highlighted the challenges of synthesizing evidence across such diverse designs. These limitations underscore the importance of developing standardized assessment frameworks for future research in this field.
3.9 Future directions
Future research in this field should prioritize well-designed longitudinal and interventional studies to clarify causal relationships between vitamin deficiencies, genetic variants, and depression with a focus on specific populations such as adolescents, older adults, postpartum women, and individuals living with chronic comorbidities. Further studies are required to establish optimal dosing thresholds for vitamin B9, B12, and D supplementation in these groups and to assess the potential long-term risks associated with over-supplementation.
4 Conclusion
This systematic review studied the complex relationship between genetic variants, VD, VB12, VB9, and depression. Evidence suggests that VB9 and VB12 play an essential role in neurotransmitter biosynthesis and DNA methylation and contribute to overall neural health. Their deficiencies have been described as potential exacerbating factors of depressive symptoms. Several genetic factors, such as genetic variants in the MTHFR gene and others involved in the vitamin’s metabolic pathway, may further aggravate this symptomatology, indicating that individual genetic predispositions could influence susceptibility to depression in the context of micronutrient availability. VD has also emerged as a significant micronutrient of interest. It explored the role of neurotransmission modulation, and neuroplasticity has increased the interest regarding its relationship with depressive symptomatology. Although clinical studies have shown a correlation between low VD levels and increased depressive symptoms, further research is needed to clarify its therapeutic potential and the individual factors influencing responses to supplementation. Despite the promising findings, existing research is still limited by methodological challenges such as small sample sizes and cross-sectional designs. Future research should employ rigorous methodologies such as longitudinal designs and larger and more diverse populations to acquire a better understanding of how these variables interact to influence symptoms of depression in different groups. Furthermore, large-scale clinical trials are necessary to develop therapeutic guidelines for the addition of VB9, VB12, and VD as an adjuvant to depression treatment, particularly in individuals who are resistant to standard antidepressant medications. This review emphasizes the importance of an individualized approach for depression prevention and management, which includes the use of personalized nutrition (nutrigenetics) to identify potential alterations in vitamin metabolism by genetic variation and other environmental factors. With further study of these complex interactions, future advances in treatment may enhance the quality of life for individuals affected by depression and its outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RS-G: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. HR-O: Data curation, Visualization, Writing – review & editing. RG-S: Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. RC-S: Data curation, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. RC-S acknowledges financial support provided by the Challenge-Based Research Funding Program at Tecnológico de Monterrey since this program funded the entire project in the grant with number E004 -TM-10-372.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
VD, vitamin D; VB9, vitamin B9; VB12, vitamin B12; HCy, homocysteine; MDD, major depressive disorder; Met, methionine; HHCy, hyperhomocysteinemia.
References
1. Chand, SP, and Arif, H. Depression: StatPearls. Treasure Island, FL: StatPearls Publishing (2023).
2. The Lancet. Ensuring care for people with depression. Lancet. (2022) 399:885. doi: 10.1016/s0140-6736(21)01149-1
3. Hofmann, SG, Curtiss, J, Carpenter, JK, and Kind, S. Effect of treatments for depression on quality of life: a meta-analysis. Cogn Behav Ther. (2017) 46:265–86. doi: 10.1080/16506073.2017.1304445
4. World Health Organization. (2025). Depressive disorder (depression). Available online at: https://www.who.int/news-room/fact-sheets/detail/depression [Accessed October 4, 2025]
5. Otte, C, Gold, SM, Penninx, BW, Pariante, CM, Etkin, A, Fava, M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2:16065. doi: 10.1038/nrdp.2016.65
6. Sullivan, PF, Neale, MC, and Kendler, KS. Genetic epidemiology of major depression: review and Meta-analysis. Am J Psychiatry. (2000) 157:1552–62. doi: 10.1176/appi.ajp.157.10.1552
7. Flint, J, and Kendler, KS. The genetics of major depression. Neuron. (2014) 81:484–503. doi: 10.1016/j.neuron.2014.01.027
8. Dunn, EC, Brown, RC, Dai, Y, Rosand, J, Nugent, NR, Amstadter, AB, et al. Genetic determinants of depression. Harv Rev Psychiatry. (2015) 23:1–18. doi: 10.1097/HRP.0000000000000054
9. Shadrina, M, Bondarenko, EA, and Slominsky, PA. Genetics factors in major depression disease. Front Psych. (2018) 9:334. doi: 10.3389/fpsyt.2018.00334
10. Sangle, P, Sandhu, O, Aftab, Z, Anthony, AT, and Khan, S. Vitamin B12 supplementation: preventing onset and improving prognosis of depression. Cureus. (2020) 12:e11169. doi: 10.7759/cureus.11169
11. Bender, A, Hagan, KE, and Kingston, N. The association of folate and depression: a meta-analysis. J Psychiatr Res. (2017) 95:9–18. doi: 10.1016/j.jpsychires.2017.07.019
12. Wang, R, Xu, F, Xia, X, Xiong, A, Dai, D, Ling, Y, et al. The effect of vitamin D supplementation on primary depression: a meta-analysis. J Affect Disord. (2024) 344:653–61. doi: 10.1016/j.jad.2023.10.021
13. Zielińska, M, Łuszczki, E, and Dereń, K. Dietary nutrient deficiencies and risk of depression (review article 2018–2023). Nutrients. (2023) 15:2433. doi: 10.3390/nu15112433
14. Anglin, RES, Samaan, Z, Walter, SD, and McDonald, SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:100–7. doi: 10.1192/bjp.bp.111.106666
15. Moradi, F, Lotfi, K, Armin, M, Clark, CCT, Askari, G, and Rouhani, MH. The association between serum homocysteine and depression: a systematic review and meta-analysis of observational studies. Eur J Clin Investig. (2021) 51:e13486. doi: 10.1111/eci.13486
16. van Ommen, B, El-Sohemy, A, Hesketh, J, Kaput, J, Fenech, M, Evelo, CT, et al. The micronutrient genomics project: a community-driven knowledge base for micronutrient research. Genes Nutr. (2010) 5:285–96. doi: 10.1007/s12263-010-0192-8
17. Bains, N, and Abdijadid, S. Major depressive disorder. Treasure Island, FL: StatPearls Publishing (2022).
18. Malhi, GS, and Mann, JJ. Depression. Lancet. (2018) 392:2299–312. doi: 10.1016/S0140-6736(18)31948-2
19. Cosci, F, and Chouinard, G. The monoamine hypothesis of depression revisited: could it mechanistically novel antidepressant strategies? In: J Quevedo, AF Carvalho, and CA Zarate, editors. Neurobiology of depression. London: Elsevier (2019). 63–73.
20. Moncrieff, J, Cooper, RE, Stockmann, T, Amendola, S, Hengartner, MP, and Horowitz, MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry. (2023) 28:3243–56. doi: 10.1038/s41380-022-01661-0
21. Klein, DN, Glenn, CR, Kosty, DB, Seeley, JR, Rohde, P, and Lewinsohn, PM. Predictors of first lifetime onset of major depressive disorder in young adulthood. J Abnorm Psychol. (2013) 122:1–6. doi: 10.1037/a0029567
22. Donaldson, ZR, Piel, DA, Santos, TL, Richardson-Jones, J, Leonardo, ED, Beck, SG, et al. Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology. (2014) 39:291–302. doi: 10.1038/npp.2013.185
23. Reith, MEA, Kortagere, S, Wiers, CE, Sun, H, Kurian, MA, Galli, A, et al. The dopamine transporter gene SLC6A3: multidisease risks. Mol Psychiatry. (2022) 27:1031–46. doi: 10.1038/s41380-021-01341-5
24. Karg, K, Burmeister, M, Shedden, K, and Sen, S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression Meta-analysis revisited. Arch Gen Psychiatry. (2011) 68:444–54. doi: 10.1001/archgenpsychiatry.2010.189
25. Bjørndal, LD, Ebrahimi, OV, Røysamb, E, Karstoft, K-I, Czajkowski, NO, and Nes, RB. Stressful life events exhibit complex patterns of associations with depressive symptoms in two population-based samples using network analysis. J Affect Disord. (2024) 349:569–76. doi: 10.1016/j.jad.2024.01.054
26. Shulpekova, Y, Nechaev, V, Kardasheva, S, Sedova, A, Kurbatova, A, Bueverova, E, et al. The concept of folic acid in health and disease. Molecules. (2021) 26:3731. doi: 10.3390/molecules26123731
27. Shlobin, NA, LoPresti, MA, Du, RY, and Lam, S. Folate fortification and supplementation in prevention of folate-sensitive neural tube defects: a systematic review of policy. J Neurosurg Pediatr. (2021) 27:294–310. doi: 10.3171/2020.7.PEDS20442
28. Song, WO, Chung, C-E, Chun, OK, and Cho, S. Serum homocysteine concentration of US adults associated with fortified cereal consumption. J Am Coll Nutr. (2005) 24:503–9. doi: 10.1080/07315724.2005.10719496
29. Khan, KM, and Jialal, I. Folic acid deficiency. Treasure Island, FL: StatPearls Publishing (2024).
30. Kominiarek, MA, and Rajan, P. Nutrition recommendations in pregnancy and lactation. Med Clin North Am. (2016) 100:1199–215. doi: 10.1016/j.mcna.2016.06.004
31. Altaf, R, Gonzalez, I, Rubino, K, and Nemec, EC. Folate as adjunct therapy to SSRI/SNRI for major depressive disorder: systematic review & meta-analysis. Complement Ther Med. (2021) 61:102770. doi: 10.1016/j.ctim.2021.102770
32. Barnett, H, D’Cunha, NM, Georgousopoulou, EN, Kellett, J, Mellor, DD, McKune, AJ, et al. Effect of folate supplementation on inflammatory markers in individuals susceptible to depression: a systematic review. Explor Res Hypothesis Med. (2017) 2:1–15. doi: 10.14218/ERHM.2017.00025
33. Gao, S, Khalid, A, Amini-Salehi, E, Radkhah, N, Jamilian, P, Badpeyma, M, et al. Folate supplementation as a beneficial add-on treatment in relieving depressive symptoms: a meta-analysis of meta-analyses. Food Sci Nutr. (2024) 12:3806–18. doi: 10.1002/fsn3.4073
34. Jha, S, Kumar, P, Kumar, R, and Das, A. Effectiveness of add-on l-methylfolate therapy in a complex psychiatric illness with MTHFR C677 T genetic polymorphism. Asian J Psychiatr. (2016) 22:74–5. doi: 10.1016/j.ajp.2016.05.007
35. Fardous, AM, and Heydari, AR. Uncovering the hidden dangers and molecular mechanisms of excess folate: a narrative review. Nutrients. (2023) 15:4699. doi: 10.3390/nu15214699
36. Ding, Z, Luo, L, Guo, S, Shen, Q, Zheng, Y, and Zhu, S. Non-linear association between folate/vitamin B12 status and cognitive function in older adults. Nutrients. (2022) 14:2443. doi: 10.3390/nu14122443
37. Al Amin, ASM, and Gupta, V. Vitamin B12 (cobalamin). Treasure Island, FL: StatPearls Publishing (2023).
38. Fang, H, Kang, J, and Zhang, D. Microbial production of vitamin B12: a review and future perspectives. Microb Cell Factories. (2017) 16:15. doi: 10.1186/s12934-017-0631-y
39. Ankar, A, and Bhimji, SS. Vitamin B12 deficiency. Treasure Island, FL: StatPearls Publishing (2024).
40. National Academies of Sciences, Engineering and Medicine. Dietary reference intakes for Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academies Press (1998).
41. Lyon, P, Strippoli, V, Fang, B, and Cimmino, L. B vitamins and one-carbon metabolism: implications in human health and disease. Nutrients. (2020) 12:2867. doi: 10.3390/nu12092867
42. Correia, AS, Cardoso, A, and Vale, N. Oxidative stress in depression: the link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants. (2023) 12:470. doi: 10.3390/antiox12020470
43. Ducker, GS, and Rabinowitz, JD. One-carbon metabolism in health and disease. Cell Metab. (2016) 25:27. doi: 10.1016/j.cmet.2016.08.009
44. Zaric, BL, Obradovic, M, Bajic, V, Haidara, MA, Jovanovic, M, and Isenovic, ER. Homocysteine and hyperhomocysteinaemia. Curr Med Chem. (2019) 26:2948–61. doi: 10.2174/0929867325666180313105949
46. Bhatia, P, and Singh, N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundam Clin Pharmacol. (2015) 29:522–8. doi: 10.1111/fcp.12145
47. Khairy, EY, and Attia, MM. Protective effects of vitamin D on neurophysiologic alterations in brain aging: role of brain-derived neurotrophic factor (BDNF). Nutr Neurosci. (2021) 24:650–9. doi: 10.1080/1028415X.2019.1665854
48. Liu, T, Zhang, L, Joo, D, and Sun, SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. (2017) 2:1–9. doi: 10.1038/sigtrans.2017.23
49. Sokołowska, P, Seweryn Karbownik, M, Jóźwiak-Bębenista, M, Dobielska, M, Kowalczyk, E, and Wiktorowska-Owczarek, A. Antidepressant mechanisms of ketamine’s action: NF-κB in the spotlight. Biochem Pharmacol. (2023) 218:115918. doi: 10.1016/j.bcp.2023.115918
50. Alhomrani, M, Alamri, AS, Mabrouk, I, Al-hazmi, A, Hassan, MM, Abdallah, FB, et al. Association between the 5-methyl tetrahydrofolate reductase (5-MTHFR) and integrin Β3 subunit (ITGB3) genes polymorphism and major depressive disorder. J King Saud Univ. (2022) 34:102076. doi: 10.1016/j.jksus.2022.102076
51. Kelly, CB, McDonnell, AP, Johnston, TG, Mulholland, C, Cooper, SJ, McMaster, D, et al. The MTHFR C677T polymorphism is associated with depressive episodes in patients from Northern Ireland. J Psychopharmacol. (2004) 18:567–71. doi: 10.1177/0269881104047285
52. Bousman, CA, Potiriadis, M, Everall, IP, and Gunn, JM. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. (2014) 165:68–76. doi: 10.1002/ajmg.b.32209
53. Gabriela Nielsen, M, Congiu, C, Bortolomasi, M, Bonvicini, C, Bignotti, S, Abate, M, et al. MTHFR: genetic variants, expression analysis and COMT interaction in major depressive disorder. J Affect Disord. (2015) 183:179–86. doi: 10.1016/j.jad.2015.05.003
54. Słopien, R, Jasniewicz, K, Meczekalski, B, Warenik-Szymankiewicz, A, Lianeri, M, and Jagodziński, PP. Polymorphic variants of genes encoding MTHFR, MTR, and MTHFD1 and the risk of depression in postmenopausal women in Poland. Maturitas. (2008) 61:252–5. doi: 10.1016/j.maturitas.2008.08.002
55. Ye, X, Lai, C-Q, Crott, JW, Troen, AM, Ordovas, JM, and Tucker, KL. The folate hydrolase 1561C>T polymorphism is associated with depressive symptoms in Puerto Rican adults. Psychosom Med. (2011) 73:385–92. doi: 10.1097/PSY.0b013e31821a1ab4
56. Sizar, O, Khare, S, Goyal, A, and Givler, A. Vitamin D deficiency. Treasure Island, FL: StatPearls Publishing (2025).
57. Janoušek, J, Pilařová, V, Macáková, K, Nomura, A, Veiga-Matos, J, Silva, DD, et al. Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit Rev Clin Lab Sci. (2022) 59:517–54. doi: 10.1080/10408363.2022.2070595
58. Musazadeh, V, Keramati, M, Ghalichi, F, Kavyani, Z, Ghoreishi, Z, Alras, KA, et al. Vitamin D protects against depression: evidence from an umbrella meta-analysis on interventional and observational meta-analyses. Pharmacol Res. (2023) 187:106605. doi: 10.1016/j.phrs.2022.106605
59. Office of Dietary Supplements. (2024) Vitamin D - Health Professional Fact Sheet. Available online at: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ [Accessed December 8, 2024]
60. Chauhan, K, Shahrokhi, M, and Huecker, MR. Vitamin D. Treasure Island, FL: StatPearls Publishing (2023).
61. Akpınar, Ş, and Karadağ, MG. Is vitamin D important in anxiety or depression? What is the truth? Curr Nutr Rep. (2022) 11:675–81. doi: 10.1007/s13668-022-00441-0
62. Jones, DN, and Raghanti, MA. The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J Chem Neuroanat. (2021) 114:101957. doi: 10.1016/j.jchemneu.2021.101957
63. Grudet, C, Wolkowitz, OM, Mellon, SH, Malm, J, Reus, VI, Brundin, L, et al. Vitamin D and inflammation in major depressive disorder. J Affect Disord. (2020) 267:33–41. doi: 10.1016/j.jad.2020.01.168
64. Vyas, CM, Mischoulon, D, Chang, G, Reynolds, CF, Cook, NR, Weinberg, A, et al. Relation of serum BDNF to major depression and exploration of mechanistic roles of serum BDNF in a study of vitamin D3 and omega-3 supplements for late-life depression prevention. J Psychiatr Res. (2023) 163:357–64. doi: 10.1016/j.jpsychires.2023.05.069
65. Mikola, T, Marx, W, Lane, MM, Hockey, M, Loughman, A, Rajapolvi, S, et al. The effect of vitamin D supplementation on depressive symptoms in adults: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2023) 63:11784–801. doi: 10.1080/10408398.2022.2096560
66. Murray, CJL, Barber, RM, Foreman, KJ, Ozgoren, AA, Abd-Allah, F, Abera, SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. (2015) 386:2145–91. doi: 10.1016/S0140-6736(15)61340-X
67. Revez, JA, Lin, T, Qiao, Z, Xue, A, Holtz, Y, Zhu, Z, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun. (2020) 11:1647. doi: 10.1038/s41467-020-15421-7
68. Bonk, S, Hertel, J, Zacharias, HU, Terock, J, Janowitz, D, Homuth, G, et al. Vitamin D moderates the interaction between 5-HTTLPR and childhood abuse in depressive disorders. Sci Rep. (2020) 10:22394. doi: 10.1038/s41598-020-79388-7
69. Pooyan, S, Rahimi, MH, Mollahosseini, M, Khorrami-Nezhad, L, Nasir, Y, Maghbooli, Z, et al. A high-protein/low-fat diet may interact with vitamin D-binding protein gene variants to moderate the risk of depression in apparently healthy adults. Lifestyle Genom. (2018) 11:64–72. doi: 10.1159/000492497
70. Bassett, E, Gjekmarkaj, E, Mason, AM, Zhao, SS, and Burgess, S. Vitamin D, chronic pain, and depression: linear and non-linear mendelian randomization analyses. Transl Psychiatry. (2024) 14:274. doi: 10.1038/s41398-024-02997-7
71. Pillai, RR, Sharon, L, Wilson, AB, Premkumar, NR, Kattimani, S, Sagili, H, et al. Association of VDBP (rs4588 and rs7041) gene polymorphisms with susceptibility to postpartum depression in south Indian population: a cross-sectional study. Psychiatry Res. (2022) 316:114713. doi: 10.1016/j.psychres.2022.114713
72. Kuningas, M, Mooijaart, SP, Jolles, J, Slagboom, PE, Westendorp, RGJ, and van Heemst, D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. (2009) 30:466–73. doi: 10.1016/j.neurobiolaging.2007.07.001
73. Konzok, J, Baumeister, S-E, Winkler, TW, Leitzmann, MF, and Baurecht, H. Effect of 25-hydroxyvitamin D levels on the internalising dimension as a transdiagnostic risk factor: mendelian randomisation study. Br J Psychiatry. (2023) 222:257–63. doi: 10.1192/bjp.2023.32
74. Sahin Can, M, Baykan, H, Baykan, Ö, Erensoy, N, and Karlıdere, T. Vitamin D levels and vitamin D receptor gene polymorphism in major depression. Psychiatr Danub. (2017) 29:179–85. doi: 10.24869/psyd.2017.179
75. Moorthy, D, Peter, I, Scott, TM, Parnell, LD, Lai, C-Q, Crott, JW, et al. Status of Vitamins B-12 and B-6 but Not of Folate, Homocysteine, and the Methylenetetrahydrofolate Reductase C677T Polymorphism Are Associated with Impaired Cognition and Depression in Adults. J Nutr. (2012) 142:1554–60. doi: 10.3945/jn.112.161828
76. Møllehave, LT, Skaaby, T, Simonsen, KS, Thuesen, BH, Mortensen, EL, and Sandholt, CH. Association studies of genetic scores of serum vitamin B12 and folate levels with symptoms of depression and anxiety in two Danish population studies. Eur J Clin Nutr. (2017) 71:1054–60. doi: 10.1038/ejcn.2017.97
77. Flicker, L, Martins, RN, Thomas, J, Acres, J, Taddei, K, Norman, P, et al. Homocysteine, Alzheimer genes and proteins, and measures of cognition and depression in older men. J Alzheimers Dis. (2004) 6:329–36. doi: 10.3233/JAD-2004-6313
78. Tan, E-C, Chong, S-A, Lim, LCC, Chan, AOM, Teo, Y-Y, Tan, C-H, et al. Genetic analysis of the thermolabile methylenetetrahydrofolate reductase variant in schizophrenia and mood disorders. Psychiatr Genet. (2004) 14:227–31. doi: 10.1097/00041444-200412000-00012
79. Mischoulon, D, Lamon-Fava, S, Selhub, J, Katz, J, Papakostas, GI, Iosifescu, DV, et al. Prevalence of MTHFR C677T and MS A2756G polymorphisms in major depressive disorder, and their impact on response to fluoxetine treatment. CNS Spectr. (2012) 17:76–86. doi: 10.1017/S1092852912000430
80. Lizer, MH, Bogdan, RL, and Kidd, RS. Comparison of the frequency of the methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism in Depressed Versus Nondepressed Patients. J Psychiatr Pract. (2011) 17:404–9. doi: 10.1097/01.pra.0000407963.26981.a6
81. Morris, E, Hippman, C, Albert, A, Slomp, C, Inglis, A, Carrion, P, et al. A prospective study to explore the relationship between MTHFR C677T genotype, physiological folate levels, and postpartum psychopathology in at-risk women. PLoS One. (2020) 15:e0243936. doi: 10.1371/journal.pone.0243936
82. Zhang, Z, Yang, X, Jia, Y, Wen, Y, Cheng, S, Meng, P, et al. Vitamin D and the risks of depression and anxiety: an observational analysis and genome-wide environment interaction study. Nutrients. (2021) 13:3343. doi: 10.3390/nu13103343
Keywords: depression, vitamin D, folate, vitamin B12, genetic variants, MTHFR, nutrigenetics
Citation: Soriano-Gonzalez R, Ramirez-Olea H, Gonzalez-Soltero R and Chavez-Santoscoy RA (2025) The biological relationship among depression, vitamins B9, B12, and D, and genetic variants: a systematic review. Front. Nutr. 12:1690378. doi: 10.3389/fnut.2025.1690378
Edited by:
Patrick Noël Pallier, Queen Mary University of London, United KingdomReviewed by:
Syed Fahad Javaid, United Arab Emirates University, United Arab EmiratesIkhwan Rinaldi, RSUPN Dr. Cipto Mangunkusumo, Indonesia
Copyright © 2025 Soriano-Gonzalez, Ramirez-Olea, Gonzalez-Soltero and Chavez-Santoscoy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rocio Gonzalez-Soltero, bWFyaWFkZWxyb2Npby5nb256YWxlekB1bml2ZXJzaWRhZGV1cm9wZWEuZXM=; Rocio Alejandra Chavez-Santoscoy, Y2hhdmV6LnNhbnRvc2NveUB0ZWMubXg=
 Rosella Soriano-Gonzalez
Rosella Soriano-Gonzalez Hugo Ramirez-Olea
Hugo Ramirez-Olea Rocio Gonzalez-Soltero
Rocio Gonzalez-Soltero Rocio Alejandra Chavez-Santoscoy
Rocio Alejandra Chavez-Santoscoy