- 1National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng, China
- 2College of Agriculture, Henan University, Kaifeng, China
- 3Department of Food Science, Rutgers University, New Brunswick, NJ, United States
- 4Joint International Research Laboratory of Food & Medicine Resource Function, Kaifeng, China
As one of the most widely consumed beverages worldwide, coffee has garnered increasing scientific interest due to its potential health benefit in recent decades. Epidemiological studies have consistently shown that regular coffee consumption significantly reduces the incidence risks of various chronic diseases, including type 2 diabetes mellitus (T2DM), Alzheimer's disease (AD), cardiovascular disorders, and nephropathies. Pharmacological research further supports these findings, linking the protective effects of coffee to its complex composition of bioactive compounds. Coffee beans contain over 1,000 such compounds, with caffeine, trigonelline, chlorogenic acids (CGAs), cafestol, kahweol, and melanoidins constituting the core functional components. These phytochemicals act through multi-target, synergistic mechanisms that regulate neurological functions, metabolic homeostasis, and inflammatory pathways. This review systematically explores the major bioactive constituents of coffee, focusing on the molecular mechanisms underlying four key biological activities associated with chronic disease prevention: neuroprotection, anti-diabetic/anti-obesity effects, antioxidant activity, and anti-inflammatory properties. By elucidating these pharmacological pathways, we aim to establish a molecular theoretical foundation for repositioning coffee from an empirical beverage into a targeted nutritional intervention agent.
1 Introduction
Coffee, a dark beverage derived from the processed fruits of Coffea species in the Rubiaceae family, native to tropical regions, has long transcended its geographical origins. Since its first documented use as a stimulant in 15th-century Yemeni Sufi monasteries, coffee has evolved into a central element of global dietary culture (1). The global coffee industry continues to demonstrate strong growth, with the International Coffee Organization (ICO) reporting that, as of 2023, over 125 million people are employed across the coffee value chain. Annual production exceeds 10 million metric tons of coffee beans, with the global market value surpassing US$495.5 billion (1, 2).
Beyond its economic significance, the appeal of this dark brew lies in its multifaceted health effects, unveiled through interdisciplinary advances in nutritional epidemiology, molecular medicine, and systems biology. It also conforms to the theory of medicinal food homology and has nutritional and medicinal value (3, 4). Large-scale prospective cohort studies have consistently shown significant associations between coffee consumption and reduced risks of diabetes mellitus, neurodegenerative disorders, and cardiovascular diseases (5–8). Far from serving solely as a “caffeine vehicle,” coffee is a chemically complex beverage rich in bioactive compounds. Coffee beans contain polyphenols (e.g., CGAs), alkaloids (e.g., caffeine, trigonelline), diterpenes (e.g., cafestol), and trace minerals (magnesium, potassium) among others. Additionally, the roasting process produces Maillard reaction products such as melanoidins, collectively encompassing over 1,000 identified chemical constituents (5, 6, 9).
Over the past decade, research paradigms have shifted from macro-level association analyses to investigations of molecular mechanisms: Caffeine (1,3,7-trimethylxanthine), a central nervous system (CNS) stimulant, enhances cognitive function and reduces Parkinson's disease (PD) risk through adenosine A2A receptor (A2AR) antagonism, though its dose-dependent effects may induce anxiety and disrupt calcium (Ca2+) homeostasis at high intake levels (6). Chlorogenic acid (5-O-caffeoylquinic acid, 5-CQA), the predominant polyphenol in coffee, activates the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway to suppress oxidative stress and inhibits α-glucosidase to modulate postprandial glycemia. Diterpenes such as cafestol exhibit dual effects—potentially elevating the levels of low-density lipoprotein cholesterol (LDL-C) while inducing glutathione-S-transferase (GST) for anticancer activity (7, 8). Melanoidins demonstrate metal-chelating properties, however, their concomitant formation with acrylamide (a Group 2A carcinogen per IARC classification) warrants caution (7).
The diverse chemical constituents of coffee collectively orchestrate health outcomes through intricate synergistic, antagonistic, or cascading interactions across multiple molecular targets, regulating oxidative stress, inflammatory responses, metabolic pathways, and neuroprotective mechanisms.
Current pharmacological research on coffee remains fragmented, often emphasizing isolated constituents while lacking a systematic understanding of multicomponent synergies. This review integrates molecular evidence linking key coffee bioactives to four primary health domains: neuroprotection, metabolic regulation, antioxidant activity, and anti-inflammatory effects. By mapping the target networks of CGAs, caffeine, and related compounds, we elucidate the biological basis of coffee's “multi-target regulation.” This integrated perspective advances a more holistic understanding of coffee as a functional food and provides a theoretical fondation for its health-promoting properties.
2 Principle bioactive compounds in coffee
Commercial coffee cultivation primarily involves three species: Coffea arabica L. (Arabica), Coffea canephora Pierre ex A. Froehner (Robusta), and Coffea liberica Hiern (Liberica). Of these, Arabica accounts for ~70% of the global market share, favored for its low bitterness and complex aromatic profile (5). Coffee processing systems encompass traditional methods such as dry (natural), wet (washed), and honey (pulped natural) processing. Brewing techniques are equally diverse, including espresso, French press, American drip, Turkish preparation, moka pot, and instant coffee preparations (5). The health impacts of coffee are closely tied to its complex chemical composition, which exhibits dynamic variations influenced by genetic differences, roasting degrees, and brewing methods.
Raw (unroasted) coffee beans are primarily composed of carbohydrates (59–61%), lipids (8–18%), and proteins (9–16%), supplemented by nitrogenous compounds (1–6%), minerals (4%), and acid-ester substances (6–15%) (10). Roasting induces significant compositional changes driven by Maillard reactions and pyrolysis. Carbohydrates contents decrease to 38–42%, lipids rise to 10–15%, proteins adjust to 7.5–10%, while nitrogenous compounds diminish to 1–2%. A notable outcome of roasting is the formation of melanoidins—characteristic Maillard reaction products—which comprise about 25% of the roasted bean mass. Meanwhile, mineral content stabilizes at 3.7–5%, and acid-ester compounds remain constant at 6% (11). This gradient transformation in chemical composition directly reflects the complex biochemical changes during thermal processing.
The bioactive constituents of coffee are broadly classified into four major categories: Alkaloids (e.g., caffeine, trigonelline); Polyphenols (e.g., CGAs); Diterpenes (e.g., cafestol); Maillard reaction products (e.g., melanoidins). These compounds interact through complex synergistic and antagonistic mechanisms, forming multidimensional regulatory networks that underpin coffee's diverse health effects-ranging from neuroprotection to metabolic homeostasis (6, 8, 9).
2.1 Alkaloids
Alkaloids were among the first bioactive constituents of coffee to be studied, with caffeine recognized as the primary compound due to its rapid physiological responses and dose-sensitive properties (12). Caffeine (Figure 1A), a methylxanthine derivative, possesses a planar, rigid molecular structural configuration that facilitates efficient transmembrane diffusion, allowing for swift accumulation in CNS (12). Significant interspecies variations exist in caffeine content: C. arabica contains 1.2–1.5% by dry weight, whereas C. canephora (Robusta) contains significantly more, ranging from 2.2 to 2.7% (13). Caffeine remains highly stable during thermal processing and is primarily metabolized in the liver through P450 1A2 (CYP1A2). Its metabolic clearance is modulated by genetic polymorphisms in cytochrome P450 enzymes (14).
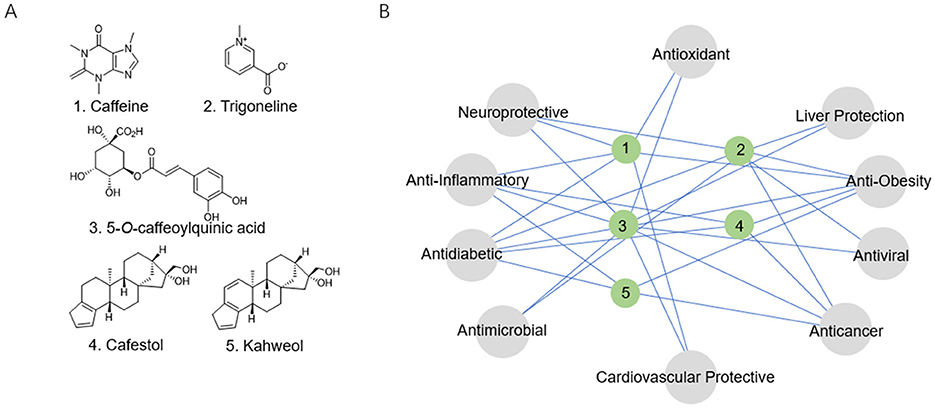
Figure 1. (A) Structural formulas of the coffee's core functional components. (B) The relationship network between the coffee's core functional components and biological activity.
Caffeine exerts its physiological functions primarily through mechanisms: competitive antagonism of adenosine A1/A2A receptors; and specific inhibition of phosphodiesterase 4/5 (PDE4/5) activity, leading to elevated intracellular cyclic adenosine monophosphate/cyclic guanosine monophosphate (cAMP/cGMP) levels, which in turn enhance Ca2+ release, caffeine behaves as a low-affinity benzodiazepine-site antagonist at γ-aminobutyric acid type A (GABA_A) receptors. While the latter interaction contributes to the vigilance-promoting properties of the methylxanthine, it may also underlie its anxiogenic potential and the observed reduction in seizure threshold (15, 16). Dose-response studies reveal beneficial that low to moderate caffeine intake—typically 50–200 mg/day (equivalent to 1–2 cups of coffee) or 200–400 mg/day (3–5 cups)—is associated with various health benefits. However, excessive intake (>500 mg/day, or more than six cups) may lead to adverse effects including heightened anxiety and sleep disturbances, a single serving of coffee, providing approximately 100 mg of caffeine, yields mean plasma caffeine concentrations of 10 and 19 μmol/L in male and female individuals, respectively (15, 17–19). Caffeine has been demonstrated to exhibit a range of pharmacological activities, including neuroprotective, anti-obesity, anti-inflammatory, and anti-diabetic effects (Figure 1B) (15, 17).
Trigonelline (Figure 1A), the second most abundant pyridine alkaloid in coffee, constitutes 1–3% of the dry mass of raw beans (20). Its distinctive chemical properties and metabolic transformations contribute to a range of health benefits, particularly in the management of diabetes and the prevention of neurodegenerative diseases (Figure 1B) (20, 21).
2.2 Polyphenols
Polyphenols constitute the most abundant antioxidants in coffee. CGAs, the predominant polyphenolic compounds, serve as core contributors to coffee's antioxidant and metabolic regulatory properties. CGAs are hydroxycinnamic acid derivatives (molecular formula C16H18O9) formed via ester bonds between caffeic and quinic acids (22). Their catechol moieties impart strong antioxidant properties, with 5-CQA (Figure 1A) comprising over >75% of total CGAs (23). Substantial varietal differences are observed: C. arabica beans contain 5-8% CGAs by dry weight, while C. canephora (Robusta) ranges from 7 to 10%. Thermal processing significantly degrades CGAs—Light roasting (180°C) retains 60–70%, Medium roasting (210°C) preserves 30–40%, Dark roasting (240°C) leaves only 10–15%. Degradation products include caffeic acid, quinic acid, and chlorogenic acid lactones, the latter contributing to coffee's bitterness (24). Despite low oral bioavailability (3–5%), CGAs exhibit multidimensional bioactivities encompassing antioxidant effects, glycemic regulation, anti-inflammatory actions, and neuroprotection (Figure 1B) (22, 24). A typical serving of coffee contains approximately 27.33–121.25 mg of chlorogenic acids, with 5-CQA accounting for 36–40% of this content. Following consumption, approximately one-third of the ingested 5-CQA enters the systemic circulation, reaching peak plasma concentrations within 0.5–1.5 h to exert its pharmacological activity (16, 25).
2.3 Diterpenes
Cafestol and kahweol (Figure 1A), unique furan diterpenes predominantly concentrated in coffee oils, constitute 19–22% of the lipid fraction in coffee beans (26). Their physiological effects are highly dependent on brewing method: paper-filtered preparations remove over 85% of these diterpenoids, whereas unfiltered brewing techniques such as French press and Turkish coffee retain their full content (6–12 mg per cup) (27). These compounds exhibit paradoxical biological activities. While they may raise low-density lipoprotein (LDL) cholesterol levels and potentially elevating cardiovascular disease risk, they also confer beneficial effects, including anti-inflammatory, hepatoprotective, and anti-carcinogenic properties (Figure 1B) (26, 27).
2.4 Maillard reaction products
The Maillard reaction, a key process in coffee roasting, generates a complex array of compounds, among which melanoidins are prominent characteristic high-molecular-weight polymers (5–100 kDa) formed through condensation reactions between reducing sugars and amino acids at 140–200°C. These melanoidins exhibit furan and pyrrole ring-enriched molecular structures, endowing them with transition metal ion chelation capacity and lipid peroxidation inhibitory activity (28). However, this reaction also produces potentially harmful substances such as acrylamide, a Group 2A carcinogen (IARC classification) derived from the Strecker degradation of asparagine and reducing sugars at temperatures exceeding 120 °C. Dark-roasted coffee may contain acrylamide levels up to 10 μg/kg, raising concerns due to its neurotoxic and carcinogenic concerns (28, 29).
3 Therapeutic potential and pharmacological mechanisms of coffee
3.1 Neuroprotective effects
With the rapidly global rise in the elderly population, neurodegenerative diseases have emerged as major public health challenges. Developing interventions to delay neuronal damage and preserve cognitive function is crucial for preventing and managing conditions such as AD and PD. AD, the most prevalent neurodegenerative disorder affecting over 24 million patients worldwide, is characterized by progressive cognitive decline encompassing memory loss, language deficits, and impaired executive dysfunction. Its pathogenesis involves extracellular β-amyloid (Aβ) plaque deposition and intraneuronal neurofibrillary tangles (NFTs) formed by hyperphosphorylated Tau protein (pTau), ultimately leading to neuronal toxicity and death (30). PD, the second most common neurodegenerative disease, exhibits age-related increases in prevalence and is characterized primarily by the selective degeneration of dopaminergic neuron in the substantia nigra pars compacta (31). In both AD and PD, mitochondrial dysfunction, reactive oxygen species (ROS)-mediated macromolecular damage, and microglial overactivation—leanding to the release of pro-inflammatory cytokines [e.g., tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β)] collectively exacerbate neurodegeneration in both AD and PD (32).
Recent studies demonstrate an inverse correlation between coffee consumption and the risk of neurodegenerative diseases. A 2022 multinational meta-analysis of 6,121 subjects found that moderate daily intake of 1–4 cups of coffee was associated with a reduced incidence of Alzheimer's disease, whereas excessive consumption (>4 cups/day) may yield counterproductive effects (33). Similarly, a large-scale cohort study involving 389,505 participants identified 2.5 cups per day as the optimal protective threshold against AD (34). Furthermore, a 126-month longitudinal study of 227 cognitively normal elder adults observed slower cerebral Aβ deposition rates in high coffee intake groups, suggesting that coffee may potentially attenuate cognitive decline through Aβ pathology suppression (35). A 9-year follow-up study of 1,696 AD-related dementia (ADRD), 1,093 PD, and 419 neurodegenerative disease death cases revealed that coffee consumption was significantly associated with reduced risks of ADRD and PD. Notably, a comparison between caffeinated and decaffeinated coffee consumers highlighted that specifically caffeinated coffee was linked to a lower risk of neurodegenerative diseases and associated mortality, underscoring the critical role of caffeine (36). Regarding caffeine, a Meta-Analysis indicated that it not only reduces the risk of Parkinson's disease in healthy individuals but also ameliorates disease progression in patients with established Parkinson's (37). Similarly, a separate double-blind, randomized controlled trial further confirmed the beneficial effect of caffeine on motor function in PD patients (38).
The neuroprotective effects of coffee arise from the synergistic actions of its bioactive components. Caffeine acts as an adenosine A2A receptor antagonist, modulating neurotransmission and promoting neuronal survival, while CGAs mitigate neural damage through potent antioxidant and anti-inflammatory mechanisms (32).
3.1.1 Caffeine
Caffeine exerts neuroprotective effects through the synergistic modulation of multiple pathways, including adenosine receptor antagonism, neuroinflammatory suppression, oxidative stress reduction, mitochondrial function enhancement, and synaptic plasticity regulation (Figure 2). In streptozotocin (STZ)-induced AD rat models, caffeine significantly decreased hippocampal oxidative stress markers, inhibited Aβ plaque deposition and tau hyperphosphorylation, while enhancing neural progenitor cell proliferation and improving spatial memory function (39). Rotenone-induced PD rat models, caffeine was shown to restore tyrosine hydroxylase (TH) expression and cytoplasmic NRF2 levels, thereby reversing acetylcholine esterase (AChE) depletion (40).
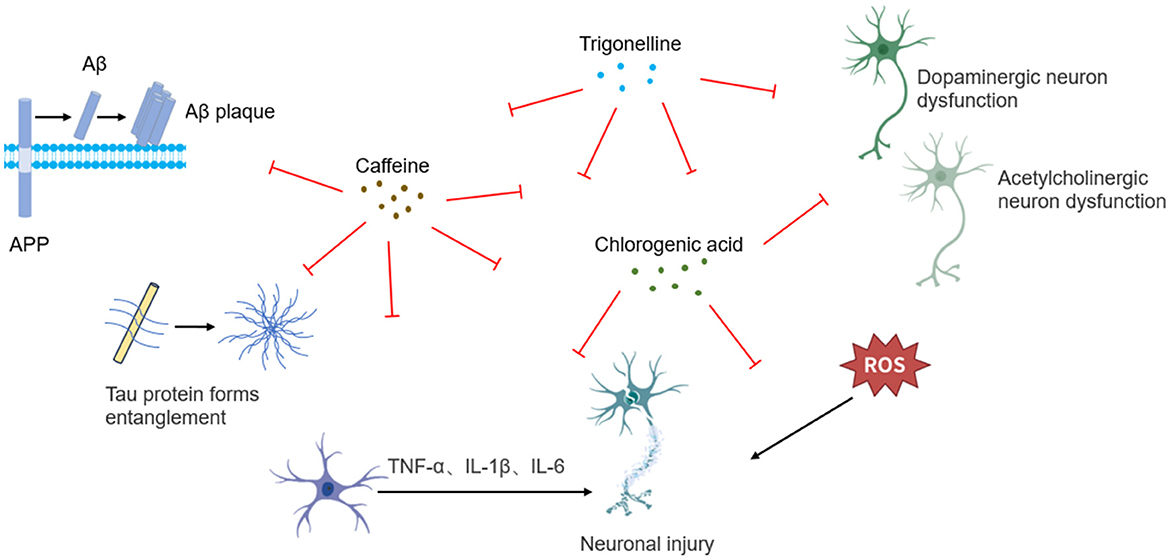
Figure 2. Schematic illustration of neuroprotective mechanisms underlying coffee's core functional components.
In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse models, caffeine administration reversed striatal dopamine (DA) depletion, improved motor coordination, and reduced the number of MPTP-induced glial fibrillary acidic protein (GFAP)-positive cells. This intervention also restored mitochondrial complex I activity inhibition, decreased ROS generation, and elevated glutathione (GSH) levels, highlighting its antioxidative, anti-inflammatory, and dopaminergic neuroprotective mechanisms (41). In aluminum chloride (AlCl3)-induced neurotoxicity models, it is revealed that caffeine reduced cortical lipid peroxidation and nitric oxide (NO) levels, restored GSH homeostasis, inhibited aberrant AChE and Na+/K+-ATPase activation, and downregulated pro-inflammatory cytokine TNF-α (42). In chronic hypoxia-induced cognitive impairment mouse models, caffeine modulated striatal DA metabolic homeostasis via adenosine A2A receptor blockade and TH expression upregulation in dopaminergic neurons (42).
3.1.2 Chlorogenic acid
The neuroprotective effects of CGA stem from its multidimensional regulatory capabilities, encompassing free radical scavenging, neuroinflammatory suppression, apoptotic pathway inhibition, and autophagy promotion, collectively maintaining neuronal homeostasis (Figure 2). In pentylenetetrazole (PTZ)-induced epileptic mouse models, CGA significantly improved behavioral abnormalities, enhanced AChE activity, and increased levels of brain-derived neurotrophic factor (BDNF), as well as monoamines including DA, norepinephrine (NE), and serotonin (5-HT). Its antioxidant effects were evidenced by elevated GSH levels, upregulation of Nrf2 gene expression, and reductions in malondialdehyde (MDA) and NO concentrations. Anti-inflammatory mechanisms of CGA involved suppression of pro-inflammatory cytokines—IL-1β, IL-6, and TNF-α, alongside inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) nuclear translocation (p65), and downregulation of inducible nitric oxide synthase (iNOS) expression. Furthermore, CGA significantly reduced pro-apoptotic protein Bax expression and caspase-3 activity, demonstrating multi-pathway synergistic protection (43). In lithium-pilocarpine-induced epilepsy models in rats, CGA similarly conferred neuroprotective effects by alleviating hippocampal oxidative stress and reducing neuronal apoptosis (44). In kainic acid-induced rat models, CGA alleviated excitatory neurotoxicity through glutamate homeostasis maintenance, AMP-activated protein kinase (AMPK)/sirtuin 1 (SIRT1)/ peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-mediated mitochondrial biogenesis activation, and PTEN-induced putative kinase 1 (PINK1)/Parkin-dependent mitophagy enhancement (45). In 6-hydroxydopamine (6-OHDA)-induced neuronal injury models, CGA decreased the levels of ROS, suppressed excessive extracellular signal-regulated kinases 1/2 (Erk1/2) phosphorylation, and prevented Akt signaling inactivation, thereby attenuating dopaminergic neuron apoptosis. In A53T α-synuclein transgenic mice, CGA further alleviated PD pathology through modulation of the Akt/Erk1/2 signaling cascade (46). In MPTP-induced zebrafish models, CGA demonstrated the ability to reverse dopaminergic neuron loss, improve motor dysfunction, and reduce α-synuclein aggregation (47). Complementary studies in MPTP-induced PD mice confirmed its neuroprotective effects through mitochondrial dysfunction amelioration, apoptotic signaling inhibition, and Akt/Erk1/2/ glycogen synthase kinase 3 beta (GSK3β) phosphorylation network regulation (48). In neonatal rat models of hypoxic-ischemic brain injury (HIE), CGA promoted Nrf2 nuclear translocation, suppressed NF-κB activation, and upregulated heme oxygenase-1 (HO-1), silent information regulator 1 (Sirt1), and IκBα. Subsequent in vitro studies using cortical neuron further validated its SIRT1-mediated regulation of the NRF2/NF-κB signaling axis in preserving neuronal viability (49). Additional models consistently corroborated CGA's broad neuroprotective efficac.
3.1.3 Trigonelline
Evidence from in vitro studies, animal models, and molecular docking analyses suggests that trigonelline holds therapeutic potential for neurodegenerative disorders including AD, PD, and depression through mechanisms involving oxidative stress mitigation, neuroinflammatory suppression, and AChE inhibition (Figure 2) (50–52). In senescence-accelerated mouse prone 8 (SAMP8) models, trigonelline significantly reduced hippocampal pro-inflammatory cytokines TNF-α and IL-6, while increasing concentrations of neurotransmitters including DA, NE, and serotonin, thereby ameliorating cognitive dysfunction (50). In models of oxygen-glucose deprivation/reperfusion (OGD/R)-induced hippocampal neuronal injury, trigonelline conferred neuroprotection via modulating the phosphoinositide 3-kinase (PI3K)/Akt pathway, exerting antioxidative, anti-inflammatory, and anti-apoptotic effects to preserve neuronal structural integrity (53). Investigations using 5XFAD transgenic AD models carrying APP/PS1 mutations further demonstrated trigonelline's ability to activate creatine kinase B-type (CK-B), promote axonal regeneration, reverse β-Aβ-induced axonal degeneration, and enhance memory function (51). Additionally, in lipopolysaccharide (LPS)-induced cognitive impairment models, trigonelline alleviated hippocampal oxidative stress and inflammation by suppressing the NF-κB/ Toll-like receptor 4 (TLR4) signaling pathway and modulating AChE activity, ultimately improving cognitive performance (54).
3.2 Antidiabetic and anti-obesity effects
Diabetes mellitus (DM) and obesity are major global metabolic disorders that pose significant public health challenges due to their high prevalence and pathophysiological synergism. According to the World Health Organization (WHO), over 10% of adults worldwide suffer from diabetes (predominantly T2DM), while the obese population exceeds 650 million. These metabolic dysregulations frequently coexist and exert marked synergistic pathological effects. Obesity, a key risk factor for T2DM, contributes to metabolic dysfunction predominantly through adipose tissue dysregulation. Dysfunctional white adipose tissue (WAT) expansion leads to leptin resistance and decreased adiponectin secretion, while released free fatty acids (FFAs) induce insulin resistance (IR) in peripheral tissues including skeletal muscle and liver. Concurrently, chronic low-grade inflammation triggered by adipose tissue macrophage infiltration impairs insulin signaling pathways (e.g., insulin receptor substrate (IRS)-PI3K-AKT cascade) via the release of pro-inflammatory cytokines such as TNF-α and IL-6, thereby exacerbating glucolipid metabolic disturbances. Studies further confirm pathological links between obesity-associated mitochondrial dysfunction, endoplasmic reticulum stress, gut microbiota dysbiosis, and pancreatic β-cell decompensation in T2DM progression (55). The cross-sectional study of 2,556 adults revealed that coffee consumption was associated with more favorable body composition parameters, including lower body mass index (BMI) and waist girth, as well as reduced systemic inflammation, indicated by lower high-sensitivity C-reactive protein (hs-CRP) levels (56). In a 3-year follow-up study of a cohort characterized by advanced age, high cardiovascular risk, elevated obesity rates, dyslipidemia, and a high prevalence of T2DM, the new initiation of moderate caffeinated coffee consumption was associated with reductions in both total body fat and visceral adipose tissue (57). A randomized, controlled, crossover study revealed that lightly roasted coffee led to a greater reduction in body fat percentage in participants compared to roasted coffee, an effect attributable to its higher content of CGA and caffeine (58). Furthermore, additional studies have demonstrated that coffee consumption can reduce daily energy intake and increase energy expenditure (59, 60). Notably, evidence from cohort studies and Mendelian randomization analyses reveals inverse correlations between coffee consumption and T2DM/obesity risks, with coffee extracts exhibiting anti-obesity bioactivity (55, 61, 62).
3.2.1 Caffeine
Caffeine exerts anti-obesity and anti-diabetic effects through coordinated central-peripheral synergistic regulation mechanisms. It integrates energy metabolism and glucolipid homeostasis networks via hypothalamic appetite suppression, adipose tissue lipolysis activation, and insulin sensitization (Figure 3) (63). In diabetic Wistar rat models, caffeine intervention significantly reduced fasting blood glucose (FBG) levels and improved glucose tolerance through upregulating skeletal muscle glucose transporter 4 (GLUT4) expression, activating AMP-activated protein kinase (AMPK) to enhance peripheral glucose uptake, and antagonizing adenosine A1 receptor (A1R) to improve insulin sensitivity (64). In diet-induced obesity (DIO) mouse models, caffeine exerted dual regulatory effects through A1R-mediated suppression of oxytocin neurons in the paraventricular nucleus (PVN), leading to reducing food intake, increased energy expenditure, and ultimate body weight reduction. These effects were accompanied by improved plasma triglyceride (TG) levels and glucose tolerance (65). Combined studies using 3T3-L1 adipocytes and C57BL/6J mice revealed caffeine's metabolic regulatory actions through multi-target mechanisms by suppressing adipogenic proteins [FASN, p-acetyl-CoA carboxylase (pACC)] to reduce lipid droplet accumulation, activating the AMPK/SIRT1 axis to enhance autophagy, and promoting white adipose browning and mitochondrial biogenesis. These mechanisms collectively contributed to attenuated weight gain, improved insulin sensitivity, and optimized lipid profiles, supporting caffeine's metabolic benefits (66). Molecular further revealed that caffeine inhibited adipogenic enzyme activities (GPDH, FAS) while activating lipolytic enzymes [hormone-sensitive lipase (HSL)] in 3T3-L1 cells. This process involved downregulation of adipogenic transcription factors [PPAR-γ2, CCAAT/enhancer-binding protein alpha (C/EBPα)] mRNA expression and upregulation of fatty acid oxidation [ACO, carnitine acyltransferase (CAT)] and lipolysis [adipose triglyceride lipase (ATGL, HSL)] genes, suggesting AMPK-mediated reprogramming of lipid metabolism (67). Animal studies further confirmed caffeine's anti-obesity effects through modulation of hepatic AMPKα-liver X receptor alpha (LXRα)/sterol regulatory element-binding protein 1c (SREBP-1c) signaling pathway (68).
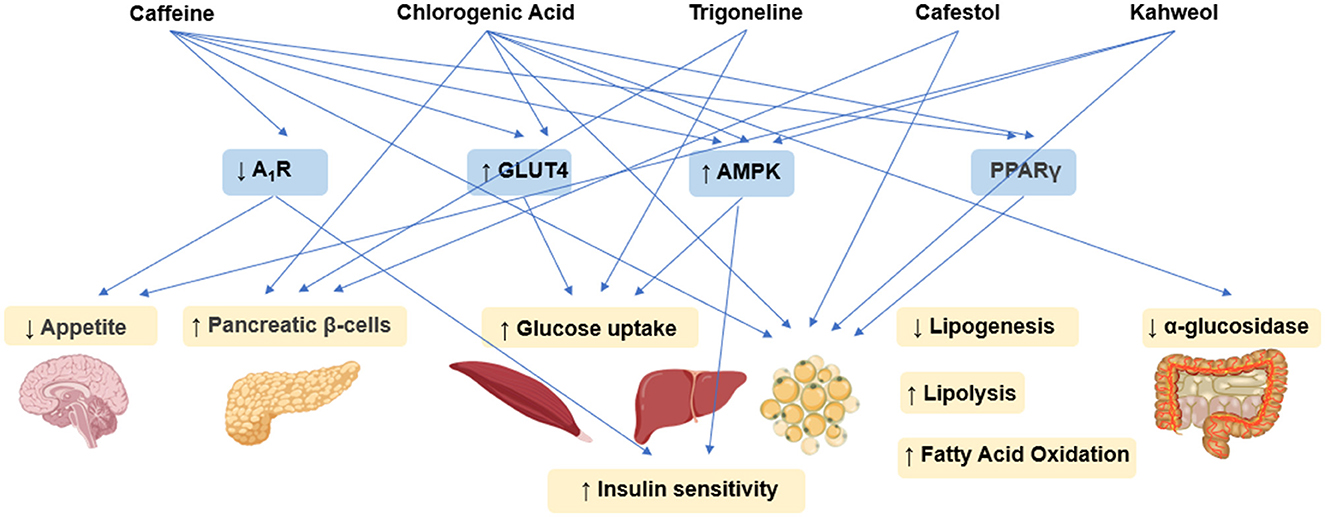
Figure 3. Schematic illustration of antidiabetic and anti-obesity effects mechanisms underlying coffee's core functional components.
3.2.2 Chlorogenic acid
CGA ameliorates glucolipid metabolic disorders through multi-target synergistic mechanisms (Figure 3). Its anti-diabetic and anti-obesity effects primarily contributed to α-glucosidase inhibition to delay carbohydrate absorption, AMPK/PPARγ pathway activation to enhance insulin sensitivity, and bidirectional regulation of lipolysis/adipogenesis-related genes for metabolic homeostasis. In high-fat diet/streptozotocin (STZ)-induced T2DM rat models, CGA intervention significantly reduced the levels of FBG, serum total cholesterol (TC), and TGs, while emhancing insulin expression in pancreatic β-cells. Transcriptomics and network pharmacology analyses identified modulation of the advanced glycation end products (AGE)-receptor for AGE (RAGE) signaling pathway as a key mechanism underlying its hypoglycemic effects (69). In obesity animal models, CGA activated AMPK signaling, thereby promoting lipolysis and fatty acid β-oxidation while suppressing key lipogenic enzyme activities (70). Experiments using 3T3-L1 preadipocytes further confirmed CGA enhances cell viability, reduces lactate dehydrogenase (LDH) release as an oxidative damage marker, and inhibits adipocyte differentiation through multiple mechanisms. These include: decreasing ROS generation while elevating superoxide dismutase (SOD), GSH, CAT levels; suppressing pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) with concomitant IL-10 upregulation; and reducing lipid droplet size and TG content via downregulation of adipogenic genes (PPARγ2, SREBP1, C/EBPα) and modulation of lipid metabolism genes (FAS, ACC, AMPK, ACO, HSL) (71). Additionally, In vitro enzyme kinetics revealed that CGA's α-glucosidase inhibitory pattern exhibits concentration-dependent mode-switching—competitive inhibition at low concentrations transitioning to noncompetitive inhibition at higher concentrations, demonstrating mixed-type inhibition characteristics (72). In db/db diabetic mice, CGA markedly decreased body weight and blood glucose by upregulating fatty acid oxidation genes (CPT1α, ACOX1) and lipolytic genes (ATGL, HSL), while downregulating lipogenic genes (MGAT1, DGAT1/2) and lipid transporter genes (CD36, FATP4), therby effectively alleviating hepatic lipid accumulation (73). Experiments using C3H10T1/2 cells demonstrated that CGA activates AMPK signaling, thereby promoting glucose the expressin of transporter GLUT2 and phosphofructokinase (PFK), enhancing glucose uptake and glycolytic efficiency, inducing mitochondrial biogenesis, ultimately upregulating uncoupling protein 1 (UCP1)-mediated thermogenesis (74).
3.2.3 Trigonelline
Trigonelline, a characteristic alkaloid in coffee, has demonstrated anti-diabetic potential through multi-dimensional mechanistic studies (Figure 3) (75). In HFD-STZ-induced diabetic rat models, Trigonelline synergistically supports pancreatic β-cell function by modulating glucose transporters and key glycolytic enzyme activities, significantly improving insulin sensitivity and reducing blood glucose and lipid profiles (76). Further studies in the same models revealed that trigonelline effectively suppresses β-cell apoptosis through the downregulation of the pro-apoptotic factor caspase-3 and enhancement of antioxidant enzyme activities. These anti-diabetic effects appear to be closely associated with inflammatory response modulation (77). Notably, trigonelline ameliorates dysregulated lipid absorption by activating key intestinal lipid-metabolizing enzymes, manifesting as reduced serum TGs and total TC levels alongside elevated high-density lipoprotein cholesterol (HDL-C) (78). In studies on complications associated with T2DM, trigonelline has been shown to significantly slow the progression of diabetic nephropathy and enhance insulin sensitivity, primarily through activation of the PPAR-γ signaling pathway (79).
3.2.4 Cafestol and Kahweol
Cafestol and kahweol, two characteristic diterpenoids found in coffee, exhibit dual regulatory effects on glucolipid metabolism (Figure 3) (80). Studies demonstrate that kahweol inhibits lipid accumulation in 3T3-L1 adipocytes and enhances glucose uptake through the activation of AMPK signaling pathway (81), while cafestol exerts hypoglycemic effects by stimulating insulin secretion in pancreatic β-cells and augmenting glucose transport in skeletal muscle cells (82). In Caenorhabditis elegans models, cafestol increases energy expenditure by activating key fat-oxidizing enzymes via the DAF-12-dependent pathway (83). In streptozotocin (STZ)-induced diabetic rat models, kahweol has been shown to enhancere insulin secretion in INS-1 cells through NF-κB inflammatory pathway modulation and p-AKT/B-cell lymphoma 2 (Bcl-2) apoptosis signaling regulation (84). Molecular investigations further demonstrate that kahweol suppresses 3T3-L1 preadipocyte differentiation by downregulating the core adipogenic transcription factor PPARγ (85).
3.3 Anti-inflammatory effects
Inflammation, an evolutionarily conserved defense mechanism in response to pathogen invasion, tissue injury, or endogenous danger signals, is characterized by pathological processes such as immune cell activation, the release of pro-inflammatory mediators, and changes in microvascular permeability (86). Clinically, inflammation is categorized into acute and chronic forms. Acute inflammation features neutrophil infiltration and cardinal clinical signs - rubor (redness), tumor (swelling), calor (heat), dolor (pain), mediated through precisely orchestrated molecular cascades involving NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways. Chronic inflammation manifests as prolonged monocyte/macrophage activation, aberrant secretion of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and tissue fibrotic remodeling. It plays a critical role in the pathogenesis of metabolic syndrome, neurodegenerative disorders, and malignancies (86). A meta-analysis of 11 cross-sectional studies involving 66,691 participants revealed that coffee consumption is associated with reduced C-reactive protein (CRP) levels (87). Furthermore, two additional studies further support an inverse association between coffee consumption and CRP levels (36, 88). A multi-omics analysis following coffee consumption revealed a significant downregulation of pro-inflammatory genes and concurrent upregulation of anti-inflammatory genes. This coordinated shift led to reduced serum levels of inflammatory cytokines, suggesting a potential anti-inflammatory effect of coffee (89).
3.3.1 Caffeine
Caffeine modulates inflammatory cascades through multi-target synergistic mechanisms, including inhibition of NF-κB and MAPK signaling pathways, blockade of NLRP3 inflammasome assembly, and downregulation of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β (Figure 4) (86, 90). In lipopolysaccharide (LPS)-induced neuroinflammatory models using male C57BL/6J mice, caffeine intervention significantly elevated BDNF levels, while concurrently suppressing pro-inflammatory cytokines including TNF-α, IL-6, IL-1β, and interferon-gamma (IFN-γ), and reducing lipid peroxidation (91). Further studies in neuroinflammatory-depressive comorbidity rat models have demonstrated caffeine's ability to regulate LPS-induced cortical p-AKT and NF-κB expression, indicating anti-inflammatory effects via AKT phosphorylation/NF-κB signaling axis modulation (92). Complementary experiments employing human retinal pigment epithelial cells (ARPE-19) and C57BL/6J mice confirmed that caffeine effectively inhibits LPS-triggered inflammation by reducing mRNA expression of TNF-α, IL-6, and IL-1β, inhibiting p-NF-κB p65 nuclear translocation, restoring monolayer integrity, and upregulating BDNF levels, revealing its potent protective anti-inflammatory role in retinal (93). In models of non-alcoholic steatohepatitis (NASH), caffeine ameliorated hepatic inflammation by downregulating TLR4/MAPK/NF-κB signaling and suppressing NLRP3 inflammasome activation (94).
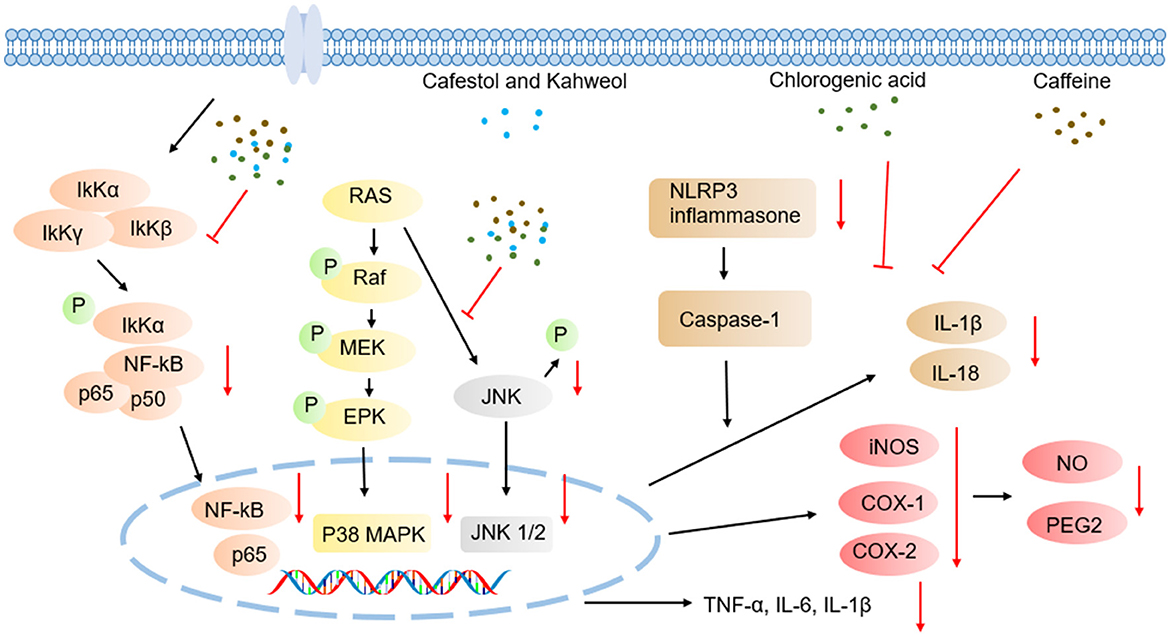
Figure 4. Schematic illustration of anti-inflammatory effects mechanisms underlying coffee's core functional components.
3.3.2 Chlorogenic acid
CGA exerts anti-inflammatory effects through multi-target synergistic mechanisms, including inhibition of NF-κB and MAPK signaling pathway activation, blockade of NLRP3 inflammasome assembly, and downregulation of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β (Figure 4). Additionally, CGA activates the Nrf2/ARE antioxidant pathway to alleviate oxidative stress-associated inflammation (95–100). In a cecal ligation and puncture (CLP)-induced septic acute liver injury (SALI) mouse model, CGA dose-dependently ameliorated hepatic tissue damage. qPCR and Western blot analyses confirmed its significant inhibition of pro-inflammatory cytokines in TNF-α and IL-1β expression, along with downregulation of TLR4 and NF-κB, indicating that its anti-inflammatory effects are closely linked to modulation of TLR4/NF-κB signaling axis (101). In LPS-stimulated RAW264.7 macrophage models, CGA concurrently inhibited inflammatory mediator secretion and signaling pathway activation by reducing IL-6, IL-1β, and TNF-α protein/mRNA expression, suppressing iNOS and cyclooxygenase-2 (COX-2) enzymes and their downstream products (NO and prostaglandin E2 (PGE2)), blocking p38 and c-Jun N-terminal kinase (JNK) phosphorylation, and inhibiting NF-κB p65 nuclear translocation, thereby validating its regulatory role in TLR4 downstream NF-κB/MAPK pathways (102). I. Further studies using LPS/ATP co-activated RAW264.7 cell models revealed that CGA suppresses inflammasome activation via miR-155-mediated epigenetic regulation. Specifically, CGA dose-dependently downregulated NLRP3 expression and caspase-1 activity, inhibited IL-1β/IL-18 maturation and release, and blocked NF-κB nuclear translocation while concurrently reducing microRNA-155 (miR-155) levels. Functional rescue experiments confirmed that miR-155 inhibitors replicated CGA's anti-inflammatory effects, whereas miR-155 mimics reverse these actions, elucidating CGA's cascade inhibitory effects through the miR-155/NF-κB/NLRP3 signaling axis (103).
3.3.3 Cafestol and Kahweol
Studies have demonstrated the therapeutic potential of kahweol and cafestol across multiple inflammatory models (Figure 4). Kahweol significantly reduces the expression of cytokines such as IL-6 and TNF-α, and chemokine (CXCL8) in human keratinocyte HaCaT cells by suppressing MAPK, NF-κB, and signal transducer and activator of transcription (STAT) signaling cascades (104). In hepatic inflammation models, Kahweol effectively alleviates liver inflammatory damage through downregulation of NF-κB and STAT3 activation in primary Kupffer cells and hepatocytes (105). Further investigations revealed that kahweol suppresses IL-2 secretion in phytohemagglutinin (PHA)/phorbol myristate acetate (PMA)-activated Jurkat lymphocytes via ERK/c-Fos signaling axis blockade, further confirming its anti-inflammatory activity (106).
3.4 Antioxidant
Oxidative stress refers to a pathological imbalance wherein the production rate of ROS exceeds the clearance capacity of the antioxidant defense system (107, 108). Under normal physiological conditions, ROS generated via the mitochondrial electron transport chain, NADPH oxidase, and other metabolic pathways—such as superoxide anion (O2·−) and hydrogen peroxide (H2O2)—participate in cellular signal transduction and immune regulation. When exogenous stimuli (e.g., ultraviolet radiation, environmental pollutants) or endogenous metabolic disorders lead to excessive ROS accumulation, the compensatory capacity of antioxidant enzyme systems—including SOD, CAT, and GSH—is overwhelmed, triggering lipid peroxidation (reflected by elevated malondialdehyde levels), protein carbonylation (increased protein carbonyl content), and DNA oxidative damage (107, 108). This disruption of redox homeostasis activates pro-inflammatory signaling pathways such as NF-κB/NLRP3, inducing programmed cell death and contributing to the pathogenesis of major diseases including neurodegenerative disorders, atherosclerosis, and malignancies. Consequently, restoring the oxidant-antioxidant dynamic equilibrium has emerged as a critical therapeutic strategy for modulating disease progression (107).
3.4.1 Caffeine
Caffeine alleviates oxidative stress damage by activating the Nrf2/ARE pathway to enhance the expression of endogenous antioxidant enzymes such as SOD and CAT (Figure 5). In a hyaluronic acid-induced ocular hypertension rat model, caffeine significantly suppressed ocular oxidative stress—evidenced by reduced MDA levels and elevated SOD activity—effectively protecting visual pathway neurons from oxidative damage, despite having no intraocular pressure-lowering effect was observed (107). In hyperoxia-induced bronchopulmonary dysplasia (BPD) models using neonatal rats, caffeine restored oxidative balance by normalizing lipid peroxidation to normoxic levels, upregulating Nrf2 expression, and inhibiting its negative regulator Kelch-like ECH-associated protein 1 (Keap1), thereby reinstating SOD1 antioxidant function (108).
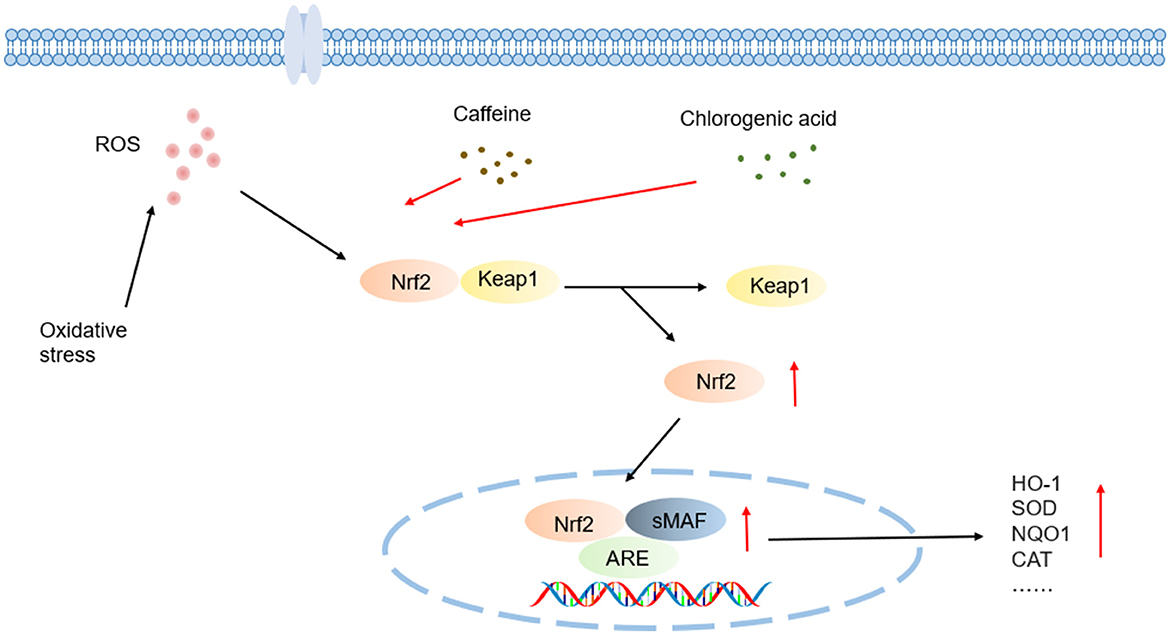
Figure 5. Schematic illustration of antioxidant effects mechanisms underlying coffee's core functional components.
Studies employing oxalate-treated Madin-Darby canine kidney (MDCK) epithelial cells demonstrated caffeine pretreatment inhibits oxidized protein accumulation via restoring Nrf2 pathway activity and downregulating the Snail1 transcription factor. Nrf2 gene silencing experiments confirmed this pathway as caffeine's core mechanism against epithelial-mesenchymal transition (EMT) (109). In models of hyperoxia-damaged type II alveolar epithelial cells (AEC II), caffeine suppressed ERK1/2 phosphorylation, apoptosis, and proliferation inhibition by antagonizing A2A receptors and disrupting the downstream cAMP/PKA/Src signaling cascade. A2A receptor knockout experiments further validated this pathway's central regulatory role, while A2A receptor agonists partially counteracted caffeine's protective effects (110).
3.4.2 Chlorogenic acid
Chlorogenic acid (CGA) exerts synergistic antioxidant effects through direct scavenge of free radicals such as ·OH, O2·−, activation of the Nrf2/Keap1-ARE pathway, and upregulation of antioxidant enzymes such as HO-1, SOD, and CAT (Figure 5). The catechol moiety of 3,4-dihydroxybenzoic acid unit in its caffeoyl group interacts specifically with free radicals via a hydrogen-donating mechanism: during radical quenching process, adjacent phenolic hydroxyl groups transfer protons to highly reactive radicals like superoxide anion (O2·−) and hydroxyl radical (·OH) through hydrogen atom transfer (HAT) or single electron transfer (SET) reactions, forming thermodynamically stable semiquinone radical intermediates. These intermediates, stabilized by an intramolecular conjugated system through electron delocalization, effectively block radical-mediated chain oxidation reactions. This electron delocalization significantly lowers the redox potential of phenoxyl radicals and enhances the kinetic stability of antioxidant reactions (111).
In H2O2-induced oxidative damage models using PC12 cells, CGA demonstrated significant cytoprotective effects by lowering intracellular ROS levels, reducing lactate dehydrogenase LDH release and decreasing apoptosis rates, while activating the Nrf2 signaling axis to induce phase II detoxifying enzymes—including HO-1, NAD(P)H:quinone oxidoreductase 1 (NQO1), GSH, thioredoxin reductase 1 (TrxR1), and thioredoxin 1 (Trx1). Nrf2 gene silencing experiments confirmed this transcription factor as the pivotal regulator of CGA's neuroprotective effects (112). Additionally, in doxorubicin (DOX)-induced cardiotoxicity rat models, CGA significantly suppressed oxidative stress damage and cardiomyocyte programmed death through Nrf2/HO-1 pathway modulation, further validating its multi-target antioxidant mechanisms (113).
4 Concluding remarks and prospects
As a complex system spanning cultural, scientific, and commercial domains, coffee health research has evolved from early “risk assessment” paradigms into a new era of “precision nutrition.” Investigating the health effects of coffee fundamentally represents a scientific exploration interrogating the interplay between its inherent natural chemical complexity and human biological diversity. This review systematically elucidates the chemobiological properties of key bioactive components in coffee—including polyphenols, alkaloids, and diterpenoids—and constructs molecular networks illustrating their multi-target mechanisms in regulating neuroprotection, metabolic homeostasis, oxidative stress, and inflammatory balance. Accumulating evidence repositions coffee not merely a “caffeine delivery vehicle,” but as a dynamic, biosynergistic system with diverse physiological benefits.
Current research paradigms encounter three critical limitations. First, the predominant focus on isolated components and linear pathway associations fails to replicate the synergistic/antagonistic interactions of multi-component systems under physiological consumption conditions. This underscores the need for systematic pharmacology approaches to unravel the compositional interplay of coffee. Second, the biological functions of minor constituents remain largely underexplored due to a disproportionate emphasis on caffeine and CGAs leaves, demanding functional omics technologies to unveil their regulatory potential of these lesser-known compounds. Third, an overreliance on in vitro experiments and rodent models constrains clinical translatability. To bridge this gap, an integrated validation framework encompassing in vitro, animal, and human studies is essential to advance coffee's transformation from an empirical consumed beverage to a precision-targeted nutritional intervention.
Author contributions
RP: Conceptualization, Writing – original draft. ML: Writing – original draft, Data curation. YZ: Data curation, Writing – original draft. SZ: Data curation, Writing – original draft. BY: Writing – original draft, Data curation. XY: Writing – original draft, Data curation. SL: Funding acquisition, Writing – review & editing, Project administration. ZL: Writing – review & editing, Funding acquisition. WK: Writing – review & editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Key R&D Program of China (2022YFF1100300).
Acknowledgments
We thank all the staff members of the National R & D Center for Edible Fungus Processing Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang H, Speakman JR. The complexity of coffee and its impact on metabolism. J Endocrinol. (2024) 262:e240075. doi: 10.1530/JOE-24-0075
2. Gil-Gómez JA, Florez-Pardo LM, Leguizamón-Vargas YC. Valorization of coffee by-products in the industry, a vision towards circular economy. Discov Appl Sci. (2024) 6:480. doi: 10.1007/s42452-024-06085-9
3. Cong B. Perspectives in food & medicine homology. Food Med Homol. (2024) 1:9420018. doi: 10.26599/FMH.2024.9420018
4. Sun-Waterhouse D-X, Chen X-Y, Liu Z-H, Waterhouse GIN, Kang W-Y. Transformation from traditional medicine-food homology to modern food-medicine homology. Food Med Homol. (2024) 1:9420014. doi: 10.26599/FMH.2024.9420014
5. Carneiro SM, Oliveira MBPP, Alves RC. Neuroprotective properties of coffee: an update. Trends Food Sci Technol. (2021) 113:167–79. doi: 10.1016/j.tifs.2021.04.052
6. Farias-Pereira R, Park C-S, Park Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci Biotechnol. (2019) 28:1287–96. doi: 10.1007/s10068-019-00662-0
7. Machado F, Coimbra MA, Castillo MDD, Coreta-Gomes F. Mechanisms of action of coffee bioactive compounds – a key to unveil the coffee paradox. Crit Rev Food Sci Nutr. (2024) 64:10164–86. doi: 10.1080/10408398.2023.2221734
8. Saud S, Salamatullah AM. Relationship between the chemical composition and the biological functions of coffee. Molecules. (2021) 26:7634. doi: 10.3390/molecules26247634
9. Karagöz MF, Koçyigit E, Koçak T, Özturan Sirin A, Icer MA, Agagündüz D, et al. Decoding coffee cardiometabolic potential: chemical composition, nutritional, and health relationships. Comp Rev Food Sci Food Safe. (2024) 23:e13414. doi: 10.1111/1541-4337.13414
10. Açikalin B, Sanlier N. Coffee and its effects on the immune system. Trends Food Sci Technol. (2021) 114:625–32. doi: 10.1016/j.tifs.2021.06.023
11. Hu GL, Wang X, Zhang L, Qiu MH. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. (2019) 10:3113–26. doi: 10.1039/C9FO00288J
12. Marcinek K, Luzak B, Rozalski M. The effects of caffeine on blood platelets and the cardiovascular system through adenosine receptors. IJMS. (2024) 25:8905. doi: 10.3390/ijms25168905
13. Yan A, La Rosa A, Patil Chhablani P, Chhablani J. Caffeine and vision: effects on the eye. TJO. (2024) 54:291–300. doi: 10.4274/tjo.galenos.2024.43895
14. Wang M, Guo W, Chen J-F. Caffeine: a potential mechanism for anti-obesity. Purinerg. Signall. (2024) 21:893–909. doi: 10.1007/s11302-024-10022-1
15. Fortunato IM, Pereira QC, Oliveira FDS, Alvarez MC, Santos TWD, Ribeiro ML. Metabolic insights into caffeine's anti-adipogenic effects: an exploration through intestinal microbiota modulation in obesity. IJMS. (2024) 25:1803. doi: 10.3390/ijms25031803
16. Di Pietrantonio D, Pace Palitti V, Cichelli A, Tacconelli S. Protective effect of caffeine and chlorogenic acids of coffee in liver disease. Foods. (2024) 13:2280. doi: 10.3390/foods13142280
17. Abu-Hashem AA, Hakami O, El-Shazly M, El-Nashar HAS, Yousif MNM. Caffeine and purine derivatives: a comprehensive review on the chemistry, biosynthetic pathways, synthesis-related reactions, biomedical prospectives and clinical applications. Chem Biodivers. (2024) 21:e202400050. doi: 10.1002/cbdv.202400050
18. Song X, Singh M, Lee KE, Vinayagam R, Kang SG. Caffeine: a multifunctional efficacious molecule with diverse health implications and emerging delivery systems. IJMS. (2024) 25:12003. doi: 10.3390/ijms252212003
19. Pohanka M. Role of caffeine in the age-related neurodegenerative diseases: a review. MRMC. (2022) 22:2726–35. doi: 10.2174/1389557522666220413103529
20. Konstantinidis N, Franke H, Schwarz S, Lachenmeier DW. Risk assessment of trigonelline in coffee and coffee by-products. Molecules. (2023) 28:3460. doi: 10.3390/molecules28083460
21. Nguyen V, Taine EG, Meng D, Cui T, Tan W. Pharmacological activities, therapeutic effects, and mechanistic actions of trigonelline. IJMS. (2024) 25:3385. doi: 10.3390/ijms25063385
22. Yan Y, Zhou X, Guo K, Zhou F, Yang H. Use of chlorogenic acid against diabetes mellitus and its complications. J Immunol Res. (2020) 2020:1–6. doi: 10.1155/2020/9680508
23. Johal K, Jones DJW, Bell L, Lovegrove JA, Lamport DJ. Impact of coffee-derived chlorogenic acid on cognition: a systematic review and meta-analysis. Nutr Res Rev. (2024) 38:1−14. doi: 10.1017/S0954422424000209
24. Huang J, Xie M, He L, Song X, Cao T. Chlorogenic acid: a review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front Pharmacol. (2023) 14:1218015. doi: 10.3389/fphar.2023.1218015
25. Lu H, Tian Z, Cui Y, Liu Z, Ma X. Chlorogenic acid: a comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Comp Rev Food Sci Food Safe. (2020) 19:3130–58. doi: 10.1111/1541-4337.12620
26. Wuerges KL, Dias RCE, Viegas MC, Benassi MDT. Kahweol and cafestol in coffee brews: comparison of preparation methods. Rev Ciênc Agron. (2020) 51:5. doi: 10.5935/1806-6690.20200005
27. Eldesouki S, Qadri R, Abu Helwa R, Barqawi H, Bustanji Y, Abu-Gharbieh E, et al. Recent updates on the functional impact of kahweol and cafestol on cancer. Molecules. (2022) 27:7332. doi: 10.3390/molecules27217332
28. Strocchi G, Rubiolo P, Cordero C, Bicchi C, Liberto E. Acrylamide in coffee: what is known and what still needs to be explored. A review. Food Chem. (2022) 393:133406. doi: 10.1016/j.foodchem.2022.133406
29. Santanatoglia A, Angeloni S, Bartolucci D, Fioretti L, Sagratini G, Vittori S, et al. Effect of brewing methods on acrylamide content and antioxidant activity: studying eight different filter coffee preparations. Antioxidants. (2023) 12:1888. doi: 10.3390/antiox12101888
30. Mirzaei F, Agbaria L, Bhatnagar K, Sirimanne N, Omar A'amar N, Jindal V, et al. Coffee and Alzheimer's disease. In:Moradikor N, Chatterjee I, , editors. Progress in Brain Research. Manchester: Elsevier (2024). p. 21–55.
31. Asuku AO, Ayinla MT, Olajide TS, Oyerinde TO, Yusuf JA, Bayo-Olugbami AA, et al. Chapter one - coffee and Parkinson's disease,” In:Moradikor N, Chatterjee I, , editors. Progress in Brain Research. Neuroscience of Coffee Part B. Manchester: Elsevier (2024). p. 1–19.
32. Raza ML. Coffee and brain health: an introductory overview. In:Moradikor N, Chatterjee I, , editors. Progress in Brain Research. Manchester: Elsevier (2024). p. 1–22.
33. Nila IS, Villagra Moran VM, Khan ZA, Hong Y. Effect of daily coffee consumption on the risk of alzheimer's disease: a systematic review and meta-analysis. JLM. (2023) 13:83–9. doi: 10.15280/jlm.2023.13.2.83
34. Zhu Y, Hu C-X, Liu X, Zhu R-X, Wang B-Q. Moderate coffee or tea consumption decreased the risk of cognitive disorders: an updated dose–response meta-analysis. Nutr Rev. (2024) 82:738–48. doi: 10.1093/nutrit/nuad089
35. Gardener SL, Rainey-Smith SR, Villemagne VL, Fripp J, Doré V, Bourgeat P, et al. Higher coffee consumption is associated with slower cognitive decline and less cerebral aβ-amyloid accumulation over 126 months: data from the australian imaging, biomarkers, and lifestyle study. Front Aging Neurosci. (2021) 13:744872. doi: 10.3389/fnagi.2021.744872
36. Zhang T, Song J, Shen Z, Yin K, Yang F, Yang H, et al. Associations between different coffee types, neurodegenerative diseases, and related mortality: findings from a large prospective cohort study. Am J Clin Nutr. (2024) 120:918–26. doi: 10.1016/j.ajcnut.2024.08.012
37. Hong CT, Chan L, Bai C-H. The effect of caffeine on the risk and progression of Parkinson's disease: a meta-analysis. Nutrients. (2020) 12:1860. doi: 10.3390/nu12061860
38. Hamdan M, Suharto AP, Nugraha P, Islamiyah WR. Motor improvement in Parkinson's disease patients receiving caffeine adjuvants: a double-blind randomized controlled trial in Indonesia. Narra J. (2024) 4:e826. doi: 10.52225/narra.v4i2.826
39. Tiwari V, Mishra A, Singh S, Shukla S. Caffeine improve memory and cognition via modulating neural progenitor cell survival and decreasing oxidative stress in alzheimer's rat model. CAR. (2023) 20:175–89. doi: 10.2174/1567205020666230605113856
40. Adeyeye TA, Babatunde BR, Ehireme SE, Shallie PD. Caffeine alleviates anxiety-like behavior and brainstem lesions in a rotenone-induced rat model of Parkinson's disease. J Chem Neuroanat. (2023) 132:102315. doi: 10.1016/j.jchemneu.2023.102315
41. Karuppagounder SS, Uthaythas S, Govindarajulu M, Ramesh S, Parameshwaran K, Dhanasekaran M. Caffeine, a natural methylxanthine nutraceutical, exerts dopaminergic neuroprotection. Neurochem Int. (2021) 148:105066. doi: 10.1016/j.neuint.2021.105066
42. Zhong Z, Dong H, Zhou S, Lin C, Huang P, Li X, et al. Caffeine's neuroprotective effect on memory impairment: suppression of adenosine a2 a receptor and enhancement of tyrosine hydroxylase in dopaminergic neurons under hypobaric hypoxia conditions. CNS Neurosci Ther. (2024) 30:e70134. doi: 10.1111/cns.70134
43. Althagafi HA. Neuroprotective role of chlorogenic acid against hippocampal neuroinflammation, oxidative stress, and apoptosis following acute seizures induced by pentylenetetrazole. Metab Brain Dis. (2024) 39:1307–21. doi: 10.1007/s11011-024-01400-0
44. Carreño-González AJ, Liberato JL, Celani MVB, Lopes NP, Lopes JLC, Gobbo-Neto L, et al. Neuroprotective effects of chlorogenic acid against oxidative stress in rats subjected to lithium-pilocarpine-induced status epilepticus. Naunyn Schmiedeberg Arch Pharmacol. (2024) 397:6989–99. doi: 10.1007/s00210-024-03080-0
45. Pai M-S, Wang K-C, Yeh K-C, Wang S-J. Stabilization of mitochondrial function by chlorogenic acid protects against kainic acid-induced seizures and neuronal cell death in rats. Eur J Pharmacol. (2023) 961:176197. doi: 10.1016/j.ejphar.2023.176197
46. He S, Chen Y, Wang H, Li S, Wei Y, Zhang H, et al. Neuroprotective effects of chlorogenic acid: modulation of Akt/Erk1/2 signaling to prevent neuronal apoptosis in Parkinson's disease. Free Radic Biol Med. (2024) 222:275–87. doi: 10.1016/j.freeradbiomed.2024.06.018
47. Gao X, Zhang B, Zheng Y, Liu X, Rostyslav P, Finiuk N, et al. Neuroprotective effect of chlorogenic acid on Parkinson's disease like symptoms through boosting the autophagy in zebrafish. Eur J Pharmacol. (2023) 956:175950. doi: 10.1016/j.ejphar.2023.175950
48. Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Dilnashin H, et al. Neuroprotective effect of chlorogenic acid on mitochondrial dysfunction-mediated apoptotic death of da neurons in a parkinsonian mouse model. Oxid Med Cell Longev. (2020) 2020:1–14. doi: 10.1155/2020/6571484
49. Zheng Y, Li L, Chen B, Fang Y, Lin W, Zhang T, et al. Chlorogenic acid exerts neuroprotective effect against hypoxia-ischemia brain injury in neonatal rats by activating Sirt1 to regulate the Nrf2-NF-κB signaling pathway. Cell Commun Signal. (2022) 20:84. doi: 10.1186/s12964-022-00860-0
50. Aktar S, Ferdousi F, Kondo S, Kagawa T, Isoda H. Transcriptomics and biochemical evidence of trigonelline ameliorating learning and memory decline in the senescence-accelerated mouse prone 8 (Samp8) model by suppressing proinflammatory cytokines and elevating neurotransmitter release. GeroScience. (2023) 46:1671–91. doi: 10.1007/s11357-023-00919-x
51. Farid MM, Yang X, Kuboyama T, Tohda C. Trigonelline recovers memory function in Alzheimer's disease model mice: evidence of brain penetration and target molecule. Sci Rep. (2020) 10:16424. doi: 10.1038/s41598-020-73514-1
52. Feng J, Liu W, Feng D, Chitrakar B, Chen X, Sang Y, et al. Neuroprotective effects of trigonelline in eggplant on oxidative damage of PC12 cells and cognitive impairment in aging mice. J Funct Foods. (2024) 121:106441. doi: 10.1016/j.jff.2024.106441
53. Qiu Z, Wang K, Jiang C, Su Y, Fan X, Li J, et al. Trigonelline protects hippocampal neurons from oxygen-glucose deprivation-induced injury through activating the PI3K/Akt pathway. Chem Biol Interact. (2020) 317:108946. doi: 10.1016/j.cbi.2020.108946
54. Khalili M, Alavi M, Esmaeil-Jamaat E, Baluchnejadmojarad T, Roghani M. Trigonelline mitigates lipopolysaccharide-induced learning and memory impairment in the rat due to its anti-oxidative and anti-inflammatory effect. Int Immunopharmacol. (2018) 61:355–62. doi: 10.1016/j.intimp.2018.06.019
55. Wang Q, Hu G-L, Qiu M-H, Cao J, Xiong W-Y. Coffee, tea, and cocoa in obesity prevention: mechanisms of action and future prospects. Curr. Res. Food Sci. (2024) 8:100741. doi: 10.1016/j.crfs.2024.100741
56. Annunziata G, Frias-Toral E, Campa F, Antonieta Touriz Bonifaz M, Verde L, Galasso M, et al. Differential impact of coffee quantity and sweetening on body composition parameters and inflammation. Front Nutr. (2025) 12:1673677. doi: 10.3389/fnut.2025.1673677
57. Henn M, Babio N, Romaguera D, Vázquez-Ruiz Z, Konieczna J, Vioque J, et al. Increase from low to moderate, but not high, caffeinated coffee consumption is associated with favorable changes in body fat. Clin Nutr. (2023) 42:477–85. doi: 10.1016/j.clnu.2023.02.004
58. Fernández-Cardero Á, Sierra-Cinos JL, Bravo L, Sarriá B. Consumption of a coffee rich in phenolic compounds may improve the body composition of people with overweight or obesity: preliminary insights from a randomized, controlled and blind crossover study. Nutrients. (2024) 16:2848. doi: 10.3390/nu16172848
59. Gavrieli A, Karfopoulou E, Kardatou E, Spyreli E, Fragopoulou E, Mantzoros CS, et al. Effect of different amounts of coffee on dietary intake and appetite of normal-weight and overweight/obese individuals: coffee, dietary intake, and appetite. Obesity. (2013) 21:1127–32. doi: 10.1002/oby.20190
60. Acheson KJ, Zahorska-Markiewicz B, Pittet P, Anantharaman K, Jéquier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am J Clin Nutr. (1980) 33:989–97. doi: 10.1093/ajcn/33.5.989
61. Hosseini S, Bahadoran Z, Mirmiran P, Azizi F. Habitual coffee drinking and the chance of prediabetes remission: findings from a population with low coffee consumption. J Diabetes Metab Disord. (2024) 23:817–24. doi: 10.1007/s40200-023-01356-5
62. Mohamed AI, Erukainure OL, Salau VF, Islam MS. Impact of coffee and its bioactive compounds on the risks of type 2 diabetes and its complications: a comprehensive review. Diab Metab Syndr Clin Res Rev. (2024) 18:103075. doi: 10.1016/j.dsx.2024.103075
63. Liu C, Li Y, Song G, Li X, Chen S, Zou D, et al. Caffeine promotes the production of Irisin in muscles and thus facilitates the browning of white adipose tissue. J Funct Foods. (2023) 108:105702. doi: 10.1016/j.jff.2023.105702
64. Urzúa Z, Trujillo X, Huerta M, Trujillo-Hernández B, Ríos-Silva M, Onetti C, et al. Effects of chronic caffeine administration on blood glucose levels and on glucose tolerance in healthy and diabetic rats. J Int Med Res. (2012) 40:2220–30. doi: 10.1177/030006051204000620
65. Wu L, Meng J, Shen Q, Zhang Y, Pan S, Chen Z, et al. Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat Commun. (2017) 8:15904. doi: 10.1038/ncomms15904
66. Yinghao W, Wenyuan P, Chunfeng L, Jingning Y, Jun S, Chengting Z, et al. Caffeine promotes adipocyte autophagy through the ampk/sirt1 signaling pathway and improves high-fat diet–induced obesity and leptin resistance. J Food Biochem. (2025) 2025:8864899. doi: 10.1155/jfbc/8864899
67. Kong L, Xu M, Qiu Y, Liao M, Zhang Q, Yang L, et al. Chlorogenic acid and caffeine combination attenuates adipogenesis by regulating fat metabolism and inhibiting adipocyte differentiation in 3T3-L1 cells. J Food Biochem. (2021) 45:13795. doi: 10.1111/jfbc.13795
68. Xu M, Yang L, Zhu Y, Liao M, Chu L, Li X, et al. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. (2019) 10:7489–97. doi: 10.1039/C9FO00502A
69. He H, Wei Q, Chang J, Yi X, Yu X, Luo G, et al. Exploring the hypoglycemic mechanism of chlorogenic acids from Pyrrosia petiolosa (Christ) Ching on type 2 diabetes mellitus based on network pharmacology and transcriptomics strategy. J Ethnopharmacol. (2024) 322:117580. doi: 10.1016/j.jep.2023.117580
70. Tian D, Liu S, Lu Y, Zhang T, Wang X, Zhang C, et al. Low-methoxy-pectin and chlorogenic acid synergistically promote lipolysis and β-oxidation by regulating AMPK signaling pathway in obese mice. Int J Biol Macromol. (2024) 280:135552. doi: 10.1016/j.ijbiomac.2024.135552
71. Liu L, Zhang C, Zhai M, Yu T, Pei M, Du P, et al. Effect of chlorogenic acid on lipid metabolism in 3T3-L1 cells induced by oxidative stress. Food Biosci. (2023) 51:102330. doi: 10.1016/j.fbio.2022.102330
72. Abioye RO, Nwamba OC, Okagu OD, Udenigwe CC. Synergistic effect of acarbose–chlorogenic acid on α-glucosidase inhibition: kinetics and interaction studies reveal mixed-type inhibition and denaturant effect of chlorogenic acid. ACS Food Sci Technol. (2023) 3:1255–68. doi: 10.1021/acsfoodscitech.3c00146
73. Yan Y, Li Q, Shen L, Guo K, Zhou X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front Endocrinol. (2022) 13:1042044. doi: 10.3389/fendo.2022.1042044
74. Han X, Zhang Y, Guo J, You Y, Zhan J, Huang W. Chlorogenic acid stimulates the thermogenesis of brown adipocytes by promoting the uptake of glucose and the function of mitochondria. J Food Sci. (2019) 84:3815–24. doi: 10.1111/1750-3841.14838
75. Li M, Li S, Jiang S, Li W. The hepatoprotective effect of trigonelline in diabetic rat through insulin-related irs1-glut2 pathway: a biochemical, molecular, histopathological, and immunohistochemical study. Pharmacogn Mag. (2024) 20:1215–25. doi: 10.1177/09731296241247365
76. Wadhwa G, Krishna KV, Taliyan R, Tandon N, Yadav SS, Banerjee D, et al. Preclinical pharmacokinetics of trigonelline using ultra-performance liquid chromatography–tandem mass spectrometry and pharmacological studies targeting type 2 diabetes. Separ Sci Plus. (2021) 4:185–94. doi: 10.1002/sscp.202000118
77. Liu L, Du X, Zhang Z, Zhou J. Trigonelline inhibits caspase 3 to protect β cells apoptosis in streptozotocin-induced type 1 diabetic mice. Eur J Pharmacol. (2018) 836:115–21. doi: 10.1016/j.ejphar.2018.08.025
78. Hamden K. Inhibition of key digestive enzymes-related to diabetes and hyperlipidemia and protection of liver-kidney functions by trigonelline in diabetic rats. Sci Pharm. (2013) 81:233–46. doi: 10.3797/scipharm.1211-14
79. Li Y, Li Q, Wang C, Lou Z, Li Q. Trigonelline reduced diabetic nephropathy and insulin resistance in type-2 diabetic rats through peroxisome proliferator-activated receptor-γ. Exp Ther Med. (2019) 18:1331–7. doi: 10.3892/etm.2019.7698
80. Mellbye FD, Nguyen MD, Hermansen K, Jeppesen PB, Al-Mashhadi ZK, Ringgaard S, et al. Effects of 12-week supplementation with coffee diterpene cafestol in healthy subjects with increased waist circumference: a randomized, placebo-controlled trial. Nutrients. (2024) 16:3232. doi: 10.3390/nu16193232
81. Baek J-H, Kim N-J, Song J-K, Chun K-H. Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (Ampk). BMB Rep. (2017) 50:566–71. doi: 10.5483/BMBRep.2017.50.11.031
82. Mellbye FB, Jeppesen PB, Hermansen K, Gregersen S. Cafestol, a bioactive substance in coffee, stimulates insulin secretion and increases glucose uptake in muscle cells: studies in vitro. J Nat Prod. (2015) 78:2447–51. doi: 10.1021/acs.jnatprod.5b00481
83. Farias-Pereira R, Kim E, Park Y. Cafestol increases fat oxidation and energy expenditure in Caenorhabditis elegans via DAF-12-dependent pathway. Food Chem. (2020) 307:125537. doi: 10.1016/j.foodchem.2019.125537
84. El-Huneidi W, Anjum S, Bajbouj K, Abu-Gharbieh E, Taneera J. The coffee diterpene, kahweol, ameliorates pancreatic β-cell function in streptozotocin (Stz)-treated rat ins-1 cells through nf-kb and p-akt/bcl-2 pathways. Molecules. (2021) 26:5167. doi: 10.3390/molecules26175167
85. Kim JS, Lee SG, Kang YJ, Kwon TK, Nam J-O. Kahweol inhibits adipogenesis of 3T3-L1 adipocytes through downregulation of PPARγ. Nat Prod Res. (2018) 32:1216–9. doi: 10.1080/14786419.2017.1326039
86. Eichwald T, Solano AF, Souza J, De Miranda TB, Carvalho LB, Dos Santos Sanna PL, et al. Anti-inflammatory effect of caffeine on muscle under lipopolysaccharide-induced inflammation. Antioxidants. (2023) 12:554. doi: 10.3390/antiox12030554
87. Choi S, Je Y. Coffee consumption and C-reactive protein levels: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. (2024) 34:2425–39. doi: 10.1016/j.numecd.2024.06.024
88. Choi S, Je Y. Association between coffee consumption and high C-reactive protein levels in Korean adults. Br J Nutr. (2023) 130:2146–54. doi: 10.1017/S0007114523001241
89. Ruan P, Yang M, Lv X, Shen K, Chen Y, Li H, et al. Metabolic shifts during coffee consumption refresh the immune response: insight from comprehensive multiomics analysis. MedComm. (2024) 5:e617. doi: 10.1002/mco2.617
90. Zhang X, Cao R, Li C, Zhao H, Zhang R, Che J, et al. Caffeine ameliorates age-related hearing loss by downregulating the inflammatory pathway in mice. Otol Neurotol. (2024) 45:227–37. doi: 10.1097/MAO.0000000000004098
91. Basu Mallik S, Mudgal J, Hall S, Kinra M, Grant GD, Nampoothiri M, et al. Remedial effects of caffeine against depressive-like behaviour in mice by modulation of neuroinflammation and BDNF. Nutr Neurosci. (2022) 25:1836–44. doi: 10.1080/1028415X.2021.1906393
92. Zhang R, Zhang L, Du W, Tang J, Yang L, Geng D, et al. Caffeine alleviate lipopolysaccharide-induced neuroinflammation and depression through regulating p-AKT and NF-κB. Neurosci Lett. (2024) 837:137923. doi: 10.1016/j.neulet.2024.137923
93. Conti F, Lazzara F, Romano GL, Platania CBM, Drago F, Bucolo C. Caffeine protects against retinal inflammation. Front Pharmacol. (2022) 12:824885. doi: 10.3389/fphar.2021.824885
94. Vargas-Pozada EE, Ramos-Tovar E, Rodriguez-Callejas JD, Cardoso-Lezama I, Galindo-Gómez S, Talamás-Lara D, et al. Caffeine inhibits nlrp3 inflammasome activation by downregulating tlr4/mapk/nf-κb signaling pathway in an experimental nash model. IJMS. (2022) 23:9954. doi: 10.3390/ijms23179954
95. Chai X, Liang Z, Zhang J, Ding J, Zhang Q, Lv S, et al. Chlorogenic acid protects against myocardial ischemia–reperfusion injury in mice by inhibiting Lnc Neat1/NLRP3 inflammasome-mediated pyroptosis. Sci Rep. (2023) 13:17803. doi: 10.1038/s41598-023-45017-2
96. Gu T, Zhang Z, Liu J, Chen L, Tian Y, Xu W, et al. Chlorogenic acid alleviates lps-induced inflammation and oxidative stress by modulating cd36/ampk/pgc-1α in raw264. 7 macrophages IJMS. (2023) 24:13516. doi: 10.3390/ijms241713516
97. Jiao H, Zhang M, Xu W, Pan T, Luan J, Zhao Y, et al. Chlorogenic acid alleviate kidney fibrosis through regulating TLR4/NF-κB mediated oxidative stress and inflammation. J Ethnopharmacol. (2024) 335:118693. doi: 10.1016/j.jep.2024.118693
98. Orhan S, Turkmen R, Demirel HH, Akosman MS, Turkmen T, Firat F. Chlorogenic acid mitigates potassium dichromate-induced acute hepato-nephrotoxicity by attenuating the NF-κB signalling pathway. Mol Biol Rep. (2024) 51:798. doi: 10.1007/s11033-024-09717-w
99. Shah M-A, Kang J-B, Park D-J, Kim M-O, Koh P-O. Chlorogenic acid alleviates cerebral ischemia-induced neuroinflammation via attenuating nuclear factor kappa B activation. Neurosci Lett. (2022) 773:136495. doi: 10.1016/j.neulet.2022.136495
100. Wang Q, Liu T, Koci M, Wang Y, Fu Y, Ma M, et al. Chlorogenic acid alleviated afb1-induced hepatotoxicity by regulating mitochondrial function, activating nrf2/ho-1, and inhibiting noncanonical nf-κb signaling pathway. Antioxidants. (2023) 12:2027. doi: 10.3390/antiox12122027
101. Fang S, Su H, Liu J, Zhai K, Gao Y, Xiang Y, et al. Network pharmacology and molecular docking to explore the potential mechanism of chlorogenic acid in septic acute liver injury and experimental validation of TLR4/NF-κB pathway in vivo. Naunyn Schmiedeberg Arch Pharmacol. (2025) 398:7331–42. doi: 10.1007/s00210-024-03712-5
102. Ontawong A, Duangjai A, Vaddhanaphuti CS, Amornlerdpison D, Pengnet S, Kamkaew N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon. (2023) 9:e13917. doi: 10.1016/j.heliyon.2023.e13917
103. Zeng J, Zhang D, Wan X, Bai Y, Yuan C, Wang T, et al. Chlorogenic acid suppresses mir-155 and ameliorates ulcerative colitis through the nf-κb/nlrp3 inflammasome pathway. Mol Nutr Food Res. (2020) 64:2000452. doi: 10.1002/mnfr.202000452
104. Kwon YJ, Kwon HH, Leem J, Jang YY. Kahweol inhibits pro-inflammatory cytokines and chemokines in tumor necrosis factor-α/interferon-γ-stimulated human keratinocyte hacat cells. CIMB. (2024) 46:3470–83. doi: 10.3390/cimb46040218
105. Seo H-Y, Kim M-K, Lee S-H, Hwang JS, Park K-G, Jang BK. Kahweol ameliorates the liver inflammation through the inhibition of nf-κb and stat3 activation in primary kupffer cells and primary hepatocytes. Nutrients. (2018) 10:863. doi: 10.3390/nu10070863
106. Park JB. Kahweol found in coffee inhibits il-2 production via suppressing the phosphorylations of erk and c-fos in lymphocytic jurkat cells. J Diet Suppl. (2021) 18:433–43. doi: 10.1080/19390211.2020.1784347
107. Adekeye AO, Fafure AA, Jeje-Pius ST, Asuquo DO, Sanya JO, Ogundipe L. Caffeine abrogates oxidative stress imbalance: Its implication on lateral geniculate nucleus and visual cortex following hyaluronic acid exposure. J Chem Neuroanat. (2021) 117:101996. doi: 10.1016/j.jchemneu.2021.101996
108. Endesfelder S, Strauß E, Scheuer T, Schmitz T, Bührer C. Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respir Res. (2019) 20:88. doi: 10.1186/s12931-019-1063-5
109. Kanlaya R, Subkod C, Nanthawuttiphan S, Thongboonkerd V. Caffeine prevents oxalate-induced epithelial-mesenchymal transition of renal tubular cells by its anti-oxidative property through activation of Nrf2 signaling and suppression of Snail1 transcription factor. Biomed Pharmacother. (2021) 141:111870. doi: 10.1016/j.biopha.2021.111870
110. Wang X, Lv S, Sun J, Zhang M, Zhang L, Sun Y, et al. Caffeine reduces oxidative stress to protect against hyperoxia-induced lung injury via the adenosine A2A receptor/cAMP/PKA/Src/ERK1/2/p38MAPK pathway. Redox Report. (2022) 27:270–8. doi: 10.1080/13510002.2022.2143114
111. Miao M, Xiang L. Pharmacological action and potential targets of chlorogenic acid. In:Do G, , editor. Advances in Pharmacology. Beijing: Elsevier (2020). p. 71–88.
112. Yao J, Peng S, Xu J, Fang J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors. (2019) 45:616–26. doi: 10.1002/biof.1507
Keywords: coffee, caffeine, chlorogenic acids, neuroprotective activity, diabetes mellitus, obesity, anti-inflammatory, antioxidant
Citation: Peng R, Lan M, Zhang Y, Zhang S, Yu B, Yang X, Li S, Liu Z and Kang W (2025) Transforming coffee from an empirical beverage to a targeted nutritional intervention: health effects of coffee's core functional components on chronic diseases. Front. Nutr. 12:1690881. doi: 10.3389/fnut.2025.1690881
Received: 25 August 2025; Accepted: 28 October 2025;
Published: 17 November 2025.
Edited by:
Cliff Kelvin Riley, Hazardous Substances Regulatory Authority, JamaicaReviewed by:
Mabel Buelna-Chontal, National Institute of Cardiology, I.Ch., MexicoStefania Tacconelli, University of Studies G. d'Annunzio Chieti and Pescara, Italy
Copyright © 2025 Peng, Lan, Zhang, Zhang, Yu, Yang, Li, Liu and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiming Li, c2hpbWluZ0BydXRnZXJzLmVkdQ==; Zhenhua Liu, bGl1emhlbmh1YTYyM0AxNjMuY29t; Wenyi Kang, a2FuZ3dlbmd5QGhvdG1haWwuY29t
 Rui Peng
Rui Peng Mengqi Lan1,2
Mengqi Lan1,2 Shiming Li
Shiming Li Zhenhua Liu
Zhenhua Liu Wenyi Kang
Wenyi Kang